

A new species of the neopterygian fish Enchodus from the Duwi Formation, Campanian, Late Cretaceous, Western Desert, central Egypt
Waymon L. Holloway, Kerin M. Claeson, Hesham M. Sallam, Sanaa El-Sayed, Mahmoud Kora, Joseph J.W. Sertich, and Patrick M. O’Connor
Holloway, W.L., Claeson, K.M., Sallam, H.M., El-Sayed, S., Kora, M., Sertich, J.J.W., and O’Connor, P.M. 2017. A new species of the neopterygian fish Enchodus from the Duwi Formation, Campanian, Late Cretaceous, Western Desert, central Egypt. Acta Palaeontologica Polonica 62 (3): 603–611.
The neopterygian fish Enchodus was a widespread, speciose genus consisting of approximately 30 recognized species that were temporally distributed from the late Early Cretaceous through the Paleocene. Many Enchodus specimens are fragmentary cranial remains or isolated dental elements, as is the case for previously reported occurrences in Egypt. Here, we present the most complete specimen of Enchodus recovered from the Late Cretaceous of northeast Africa. The specimen was collected from the upper Campanian Duwi Formation, near the village of Tineida (Dakhla Oasis, Western Desert, Egypt). The new species, Enchodus tineidae sp. nov., consists of right and left dentaries, a partial ectopterygoid, and other cranial bones. The size of the specimen places it into the upper body-size range for the genus. The palatine tooth, an element often useful for diagnosing Enchodus to the species level, is not preserved, but a combination of other cranial characters supports the referral of this specimen to Enchodus. In particular, the dentary preserves three symphysial rostroventral prongs and two tooth rows, the lateral of which consists of small denticles, whereas the medial row comprises large, mediolaterally-compressed teeth. The rostral-most tooth exhibits the highest crown, whereas the rest of the teeth are of lower, variable crown heights. The eight robust, caudal-most medial-row teeth are distributed in a cluster pattern never before observed in Enchodus. Additionally, the dentary and preopercle are both without dermal ornamentation, and the mandibular sensory canal is closed. Phylogenetic analysis recovers this new species as the sister species to E. dirus from North America. Along with previously described materials from Israel, Jordan, Syria, Lebanon, Italy, Morocco, and Libya, this specimen represents a thirteenth species from the northwestern Tethyan geographic distribution of Enchodus.
Key words: Actinopterygii, Enchodus, Cretaceous, Campanian, Egypt.
Waymon L. Holloway [wh332501@ohio.edu], Center for Ecology and Evolutionary Studies, Irvine Hall, Ohio University, Athens, Ohio 45701, USA; and Department of Biological Sciences, 107 Irvine Hall, Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio 45701, USA.
Kerin M. Claeson [kerincl@pcom.edu], Department of Anatomy, Philadelphia College of Osteopathic Medicine, 4170 City Avenue, Philadelphia, Pennsylvania, 19131, USA.
Hesham M. Sallam [sallam@mans.edu.eg], Sanaa El-Sayed [muvp.eg@gmail.com], and Mahmoud Kora [kora@mans.edu.eg], Mansoura University Vertebrate Paleontology Center, Department of Geology, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
Joseph J. W. Sertich [jsertich@dmns.org], Department of Earth Sciences, Denver Museum of Nature & Science, Denver, Colorado 80205l, USA.
Patrick M. O’Connor [oconnorp@ohio.edu], Center for Ecology and Evolutionary Studies, Irvine Hall, Ohio University, Athens, Ohio 45701, USA; and Department of Biomedical Sciences, 228 Irvine Hall, Heritage College of Osteopathic Medicine, Ohio University, Athens, Ohio 45701, USA.
Received 2 December 2016, accepted 11 July 2017, available online 8 September 2017.
Copyright © 2017 W.L. Holloway et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The genus Enchodus has a long history in the literature (e.g., Hay 1903a, b; Green 1911; Maury 1930; Arambourg 1954), having first been described by Agassiz (1835). A sizeable body of work on this genus exists, reflective of the approximately thirty known species (e.g., Fielitz and Gonzalez-Rodriguez 2010; Silva and Gallo 2011; Friedman 2012). Some of the more notable characteristics for the genus are palatine bones possessing a single, large fang, a corresponding dentary fang near the mandibular symphysis, and a large head, relative to overall body length (Goody 1969; Chalifa 1989; Friedma 2012). The temporal range for the genus is from the Barremian (ca. 127 Ma) to Danian (ca. 64 Ma). Perhaps best known from numerous North American localities (e.g., Cope 1872; Hay 1903b; Hussakof 1908; Goody 1976; Wilson and Chalifa 1989; Fielitz 1996, 2002; Shimada and Everhart 2003; Schein and Lewis 2007; Becker et al. 2010; Fielitz and Gonzalez-Rodriguez 2010; Schein et al. 2013), specimens referred to Enchodus have also been recovered from Central and South America, Europe, northern and western Africa, and southwest Asia (Cope 1886; Hay 1903a; Maury 1930; Siegfried 1954; Leonardi 1966; Goody 1968; Sorbini 1976; Yabumoto and Uyeno 1994; Kriwet and Gloy 1995; Chalifa 1996; Rigo 1999; Rana et al. 2005; Jacobs et al. 2006; Kear et al. 2009; Friedman 2012). At least twelve species have been described from the Late Cretaceous, northwestern Tethyan region that includes modern-day Israel, Jordan, Syria, Lebanon, Italy, Morocco, and Libya (e.g., Arambourg 1952, 1954; Goody 1969; Chalifa 1989, 1996; Forey et al. 2003; Silva and Gallo 2011, 2016). Previously recovered Egyptian material referred to Enchodus was noted by Allam (1986) but not formally described.
The preservation quality of previously described Enchodus material ranges from nearly complete specimens (e.g., Chalifa 1989; Fielitz and Gonzalez-Rodriguez 2010; Schein et al. 2013) to very fragmentary remains (e.g., Chalifa 1996; Cavin 1999), with much of the documented material from around the world comprising isolated teeth or fragmentary jaw material. Isolated palatine teeth and other isolated or fragmentary bones have often been used to identify material to the species level and as type material (e.g., Arambourg 1954; Case and Schwimmer 1988; Chalifa 1996; Fielitz 1996; Bardet et al. 2000; Becker et al. 2010; Silva and Gallo 2011). Additional cranial and post-cranial material of Enchodus is far less common than are isolated teeth, worldwide. Here, we present new cranial and jaw material of Enchodus from Egypt; the first such material to be described from the region.
Institutional abbreviations.—MUVP, Mansoura University Vertebrate Paleontology Center, Mansoura, Egypt.
Other abbreviations.—d1, rostral-most dentary tooth; d2–13, dentary tooth 2–13; E1–6, ectopterygoid tooth 1–6.
Nomenclatural acts.—The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be re-solved and the associated information viewed through any standard web browser by appending the LSID to the pre-fix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:2DAEC67C-970D-472A-BE12-BB5EC276FF2E. The electronic edition of this work was published in a journal with an eISSN 1732-2421, and has been archived and is available from the following digital repository: http://www.app.pan.pl/article/item/app003312016.html
Geological setting
Sequences of the Duwi Formation, together with the underlying Quseir Formation and the overlying Dakhla Formation, represent an early Campanian through early Danian succession exposed throughout central and southern Egypt (Klitzsch et al. 1979; Tantawy et al. 2001). The Duwi Formation is broadly exposed and accessible in multiple areas near the Dakhla Oasis, Western Desert, central Egypt (Fig. 1). Together, the three formations include a variety of depositional environments, ranging from fluviatile and estuarine to various marine facies, preserving vertebrate and invertebrate fossils (Klitzsch et al. 1979; Hendriks 1984; Hermina 1990; Klitzsch and Schandelmeier 1990; Tantawy et al. 2001; Mahmoud 2003; O’Connor et al. 2010; Claeson et al. 2014; Sallam et al. 2016). The type section of the Duwi Formation is located at the Gebel Duwi in the Quseir area, Red Sea Coast (Youssef 1957). A more thorough overview of the geology of the area and the Duwi Formation, specifically, was given by Sallam et al. (2016).
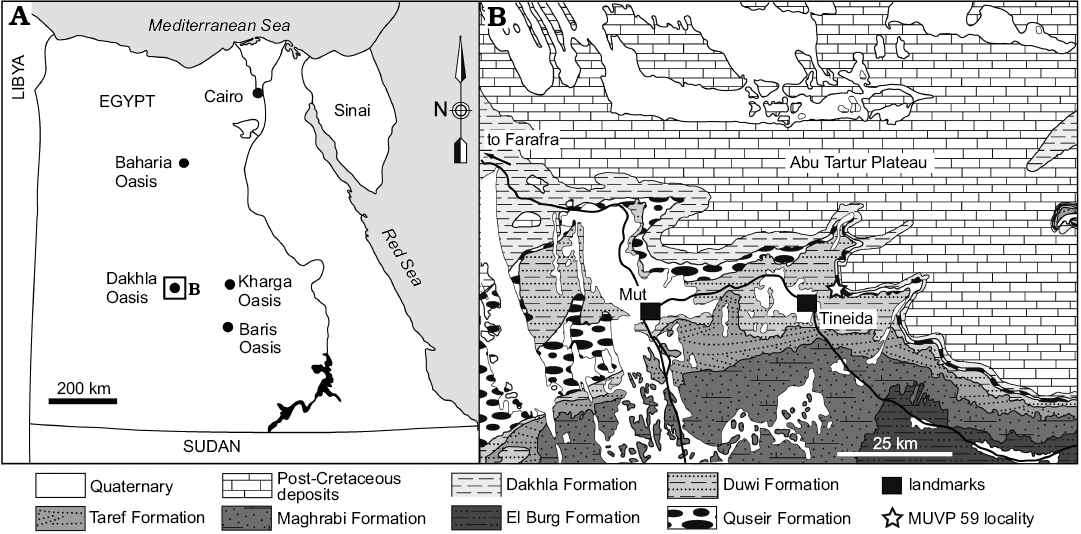
Fig. 1. Location and geological map of the Dakhla Oasis in the Western Desert, central Egypt, depicting outcrops of Upper Cretaceous rock units. MUVP 59 was recovered from the Duwi Formation exposed near the village of Tineida. Map of Egypt with dashed lines indicating the position of the field area (A), and map of studied area (B). Modified after El Khawaga et al. (2005).
Material and methods
Ongoing work by the Mansoura University Vertebrate Paleontology (MUVP) initiative includes field reconnaissance through the Quseir, Duwi, and Dakhla formations near the villages of Mut, Tineida, and Baris. The fish material (MUVP 59) described in this report was recovered from the Duwi Formation exposed near the village of Tineida and is accessioned into the MUVP collections. Data for this study and an interactive 3D PDF of a digital reconstruction of MUVP 59 are available in the SOM 1 (Supplementary Online Material available at http://app.pan.pl/SOM/app62-Holloway_etal_SOM.pdf) as well as in the Morphobank Digital Repository: http://www.morphobank.org/index.php/Projects/ProjectOverview/project_id/2428.
The exposed surfaces of the specimen were mechanically prepared from both sides to reveal the majority of the paired dentaries, an ectopterygoid, and other elements. Several additional elements are in close proximity and overlap one another. This spatial relationship of the elements and the delicate nature of the preserved material precluded complete three-dimensional preparation of the specimen. Subsequent to mechanical preparation, a latex mold of the surfaces was prepared. The specimen was also CT scanned on a Philips Brilliance CT 64-channel scanner using the following protocol: 120 kV, 200 mA, and a slice thickness of 0.67 mm. Processing of DICOM files, digital preparation, and visualization were completed in Avizo 7.1 (Visualization Science Group (VSG)/FEI, USA).
The phylogenetic position of Enchodus tineidae sp. nov., among the Enchodontidae was assessed based on the eighty-seven character matrix of Fielitz and González-Rodríguez (2010) with the addition of characters 31, 32, 40, 56, and 59 (SOM 2: characters 88–92) of Silva and Gallo (2011) and two taxa of Cavin et al. (2012), E. cf. dirus from Greece (Gavdos_fish_2/2010) and E. faujasi. The resultant 92 character, 29 taxon matrix was analyzed using PAUP* 4.0b10 (Swofford 2002) utilizing a heuristic search with stepwise addition and 1000 random addition replicates.
Systematic palaeontology
Aulopiformes Rosen, 1973
Alepisauroidei Baldwin and Johnson, 1996
Enchodontoidei Berg, 1940
Enchodontidae Lydekker, 1889 sensu Silva and Gallo, 2011
Genus Enchodus Agassiz, 1835
Type species: Enchodus lewesiensis (Mantell, 1822); the Chalk of Sussex, UK, Turonian.
Enchodus tineidae sp. nov.
Figs. 2, 3.
ZooBank LSID: urn:lsid:zoobank.org:pub:2DAEC67C-970D-472A-BE12-BB5EC276FF2E
Etymology: In reference to the type locality near the village of Tineida.
Holotype: MUVP 59; a disarticulated, three-dimensionally preserved, partial skull.
Type locality: Northeast of the village of Tineida, Dakhla Oasis, Western Desert, Egypt.
Type horizon: The middle–upper portion of the Duwi Formation, upper Campanian (73 Ma), Upper Cretaceous.
Material.—MUVP 59, right and left dentaries, left anguloarticular, left maxilla, right ectopterygoid, left preopercle, left opercle, and various unidentifiable skull fragments, branchiostegals, and other bone fragments.
Diagnosis.—Extinct enchodontid teleost characterized by the following combination of cranial, jaw, and dental characters: (i) closed mandibular sensory canal, (ii) mandibular symphysis that tapers rostrally, (iii) between six to eight ectopterygoid teeth that are neither curved nor of equal size, (iv) unornamented mandible and preopercular, and (v) opercular ornamentation consisting of radiating ridges.
Description.—Jaws: The dentaries of MUVP 59 are complete (Figs. 2, 3), except for the caudal-most portion of each element. The preserved length of the right dentary is 26.36 cm. The left dentary is broken and displaced in several places, but the combined preserved length of the fragments is roughly equal to that of the right dentary. There is no ornamentation on the lateral surface of the dentary. The dentary exhibits the weathered bases of three rostroventrally projecting dentary prongs and preserves a symphyseal surface with a slot-ridge assembly (Figs. 2D, 3B). There is no indication, through examination of either the actual specimen or CT scans of the specimen, of an open or partially open mandibular sensory canal. Along its medial aspect, the body of the dentary has a shallow fossa that delimits the caudal margin of the symphysis. At the bifurcation of the dentary into dorsal and ventral rami, there is a sharply incised fossa that continues caudally the length of the dentary and is slightly overhung by the ventral margin of the dorsal ramus that was presumably the site of articulation with the anguloarticular bone. The dorsal margin of the dentary is wide, preserving multiple teeth. The body and ventral ramus of the dentary consists of a thin plate of bone that terminates caudally in a gracile ventral rod.
Two tooth rows are preserved. The lateral row of dentition consists of a margin of small, evenly spaced denticles (2.2 mm in crown height). The medial row comprises eleven massive teeth exhibiting varying crown heights. The rostral-most tooth (d1) has the highest crown at 26.6 mm, with the second tooth (d2) having the lowest crown of the series at 7.9 mm. Caudal to d2, tooth crowns increase in height until position d6, with all succeeding teeth being approximately equal in height. The base of each medial-row tooth is nearly circular in cross-section and associated with a bulbous attachment to the dentary. The crowns of the medial-row teeth are highly rostro-caudally elongate, with convex lateral and medial surfaces. The six rostral-most preserved teeth are recurved. This morphology contrasts with the five caudal-most teeth, which are triangular in profile and have an apex that is centered above the tooth bases. In addition to the eleven preserved medial-row teeth of the right dentary, a gap between d6 and d9 appears to have accommodated two additional teeth that are not preserved. The eleven preserved teeth, in addition to the presumed missing teeth, are distributed across the dentary in an irregular pattern. The eight rostral-most teeth are spaced at regular intervals, whereas the five caudal-most teeth cluster in one triplet and one doublet, with the caudal margin of d9 nearly contacting the rostral margin of d10, the caudal margin of d10 nearly contacting the rostral margin of d11, and the caudal margin of d12 and rostral margin of d13 in contact with one another (Figs. 2, 3). This arrangement is symmetric on both dentaries, indicating that the distribution is not random. Furthermore, the teeth in each of these clusters are equally sized and well-developed, indicating those teeth are of the same ontogenetic stage, rather than some being mature and others being replacement teeth (Fink 1981).
The caudal portion of the left anguloarticular (Fig. 2) is partially preserved, including the quadrate facet. There is a weak ridge along the lateral surface of the anguloarticular, ventral to the quadrate facet, presumably the “ventral bar” of Fielitz (2004). The quadrate facet is defined rostrally by a partially preserved, long coronoid process and caudally by a partially preserved articular process. There is a small retroarticular process, caudal to the quadrate facet. The preserved portion of the maxilla (Fig. 2) is a straight, mediolaterally flat strip of bone that bears no teeth.
Hyopalatine series: The right ectopterygoid, excluding some of the rostral portion, is preserved (Figs. 2, 3). In cross-section, the ectopterygoid has a V-shaped trough for articulation with the dermopalatine (Fig. 3D). Ventral to this trough, the bases of five teeth are preserved. Similar to the medial-row teeth of the dentary, tooth bases on the ectopterygoid are circular in cross-section. In contrast to the dentary medial-row teeth, the preserved partial crown of the most complete ectopterygoid tooth is circular in cross-section and straight along its height, without any distal curvature. Furthermore, the bases and cylindrical partial tooth crown project at a ventrocaudal angle from the ectopterygoid. The rostral-most preserved ectopterygoid tooth base has the largest diameter at 9.4 mm, with each of the four succeeding teeth exhibiting smaller bases of approximately equal diameters of 7.25 mm. The distance between the bases of E2–E5 and those adjacent to each is approximately equal at 23 mm. The distance between E5–E6 tooth bases is nearly half of the distance between any other two adjacent ectopterygoid teeth at 13.5 mm. Based on the size and spacing of the preserved teeth, an estimated minimum ectopterygoid length of 183.15 mm would be required for the complete element to have possessed nine teeth, if the rostral-most preserved tooth in MUVP 59 represented the original E1 and each of the hypothetical non-preserved caudal teeth were sized and spaced the same as the two caudal-most preserved teeth. That ectopterygoid length would be over sixty-nine percent the length of the dentary, which is morphology inconsistent with known enchodontids. Instead, the rostral-most preserved ectopterygoid tooth in MUVP 59 probably represents E2, with the original E1 and rostral ectopterygoid region not preserved. Such morphology would be consistent with nearly all enchodontids in which six to eight ectopterygoid teeth are present, with the second of the series being the largest (SOM 2: character 12 (2)).
Opercular series: The dorsal ramus of the preopercle is preserved (Fig. 2). Though the ventral extent of the preopercle is incomplete, the preopercle was clearly much longer dorsoventrally than rostrocaudally and appears to have widened ventrally. There is no evidence of dermal ornamentation of the preopercle. The opercle is also partially preserved and also appears to have been longer dorsoventrally than rostrocaudally. The lateral aspect of the opercle is ornamented with rows of ridges that radiate from a central point along the rostral margin of the element to more widespread points along the caudal margin.
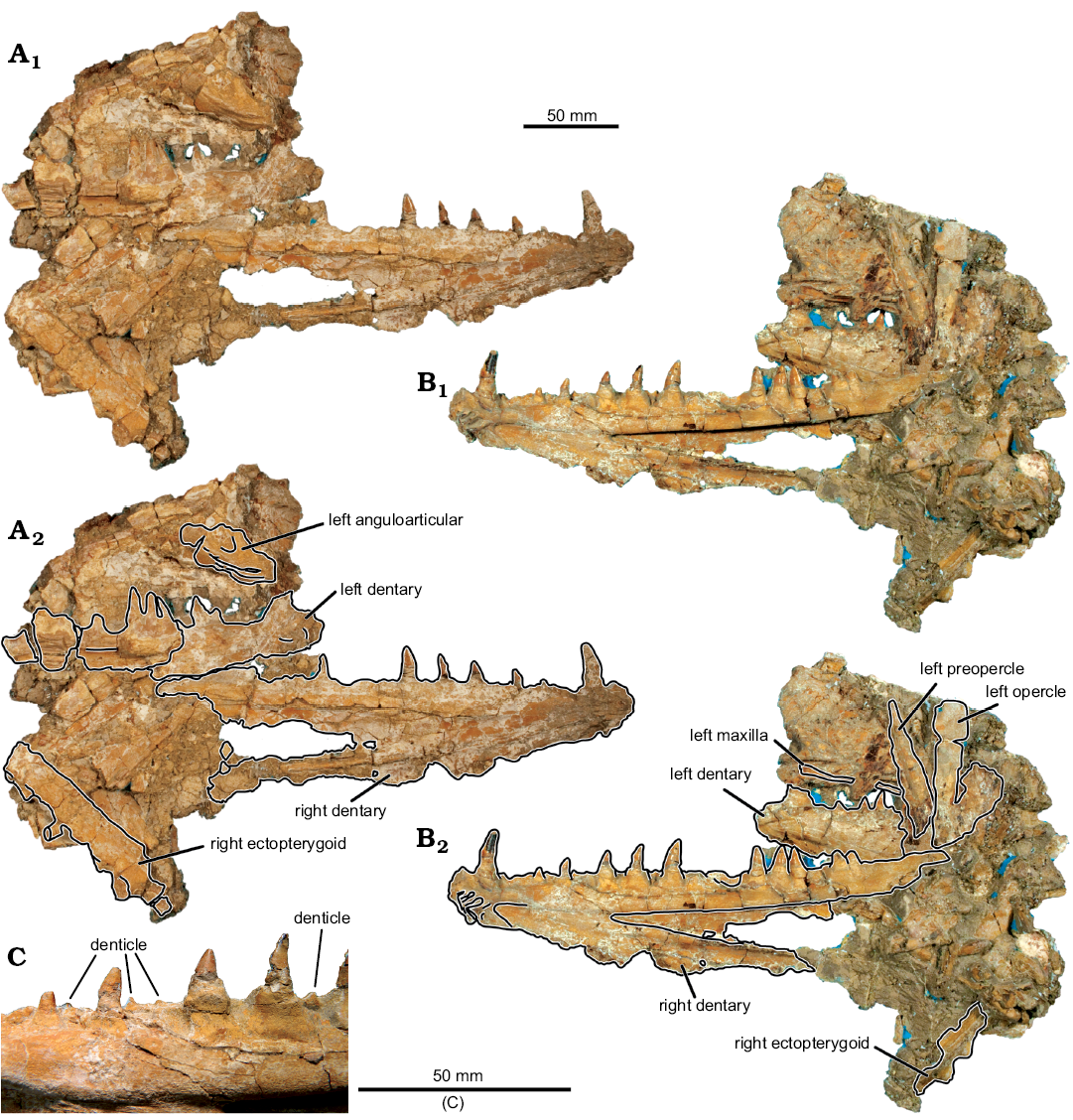
Fig. 2. Aulopiform teleost Enchodus tineidae sp. nov. holotype (MUVP 59) from the Campanian of central Egypt. A, B. Photographs of specimen in lateral (A1) and medial (B1) views; photographs with identifiable elements outlined, in lateral (A2) and medial (B2) views. C. Close up of denticles of the lateral tooth row.
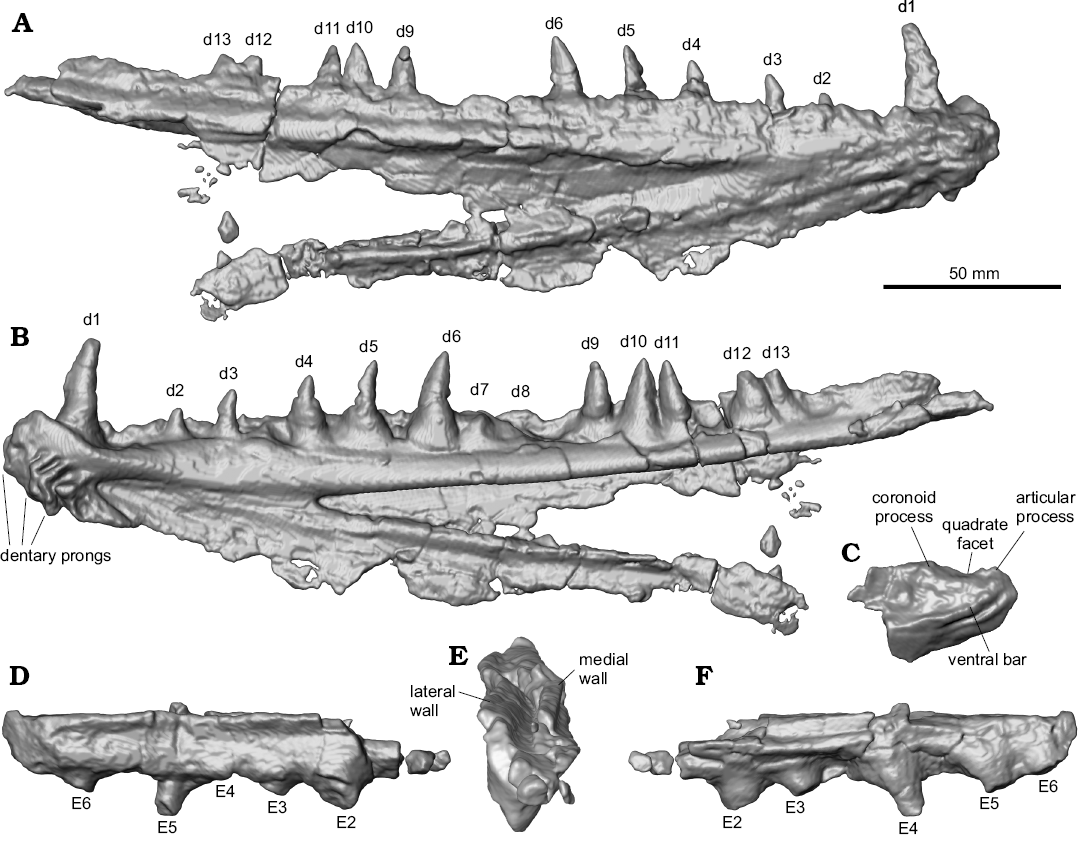
Fig. 3. Digital reconstructions of the aulopiform teleost Enchodus tineidae sp. nov. holotype (MUVP 59) from the Campanian of central Egypt. Right dentary showing its caudal extent, otherwise obscured by matrix, in lateral (A) and medial (B) views. Caudal portion of the left anguloarticular in lateral view (C), showing its dorsal aspect, otherwise obscured by matrix. Right ectopterygoid showing its caudal extent and dorsal aspect, otherwise obscured by matrix, in lateral (D), caudodorsal (E), showing V-shaped trough for articulation with the dermopalatine, and medial (F) views. Abbreviations: d, dentary tooth; E, ectopterygoid tooth; numbers 1–13 indicate tooth position in the respective bone.
Phylogenetic analysis
The phylogenetic analysis produced 48 equally parsimonious trees, with a tree length of 268 steps, consistency index (CI) of 0.52, homoplasy index (HI) of 0.48, retention index (RI) of 0.74, and rescaled consistency index (RC) of 0.39. The majority rule consensus tree, shown in Fig. 4, recovers Enchodus tineidae as highly nested among other species of Enchodus. The relationships among taxa largely remain unchanged from that of Fielitz and González-Rodríguez (2010), with the following exceptions: (i) Enchodus tineidae is the sister species to the clade of E. dirus and E. cf. dirus (Gavdos_fish_2/2010) of Cavin et al. (2012); (ii) rather than E. gladiolus and E. zimapanensis being recovered as sister species, E. gladiolus is now recovered as the sister species to the clade described above, in (i), and E. zimapanensis now forms a polytomy with this (((E. dirus + E. cf. dirus) + E. tineidae) + E. gladiolus) clade, E. petrosus, and E. faujasi; (iii) rather than being the sister taxon of the clade of (E. dirus + (E. gladiolus + E. zimapanensis)), E. petrosus is now recovered as part of the polytomy described above, in (ii); and (iv) rather than being the sister taxon to the clade of (E. petrosus + (E. dirus + (E. gladiolus + E. zimapanensis))), E. gracilis is now recovered as the sister taxon to the clade of (E. venator + E. shumardi).
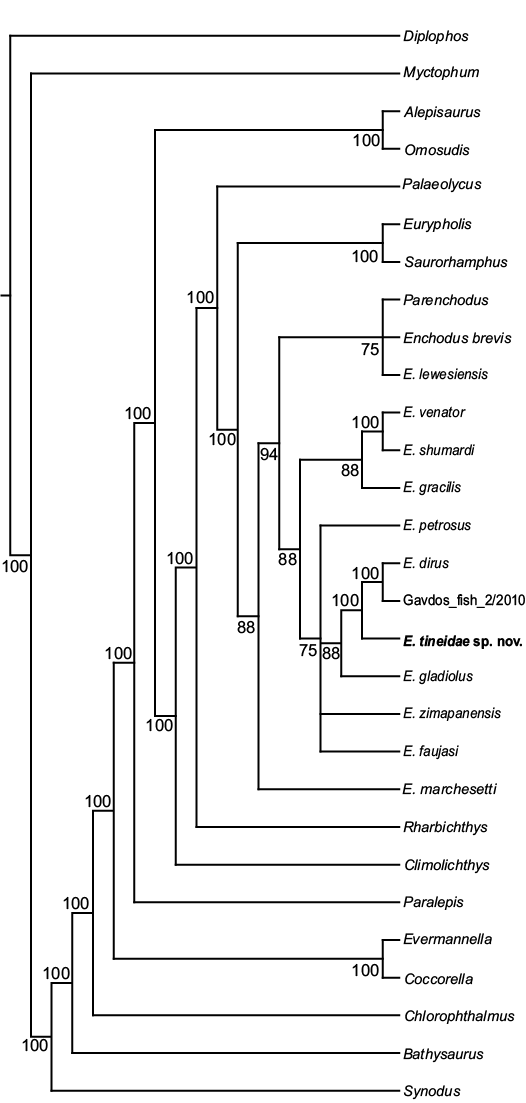
Fig. 4. Majority rule consensus of 222 equally parsimonious trees (TL = 263, CI = 0.53, HI = 0.47, RI = 0.75, and RC = 0.4). Numbers next to branches indicate the percentage of trees in which each clade is present.
Discussion
Taxonomic assessment.—The specimen presented here does not preserve the palatine teeth that are typically most informative for the diagnosis of Enchodus. However, other skull elements are well preserved and the analysis presented herein recovers MUVP 59 within the enchodontid (sensu Silva and Gallo 2011) ((Eurypholis + Saurorhamphus) + (Enchodus + (Palaeolycus + Parenchodus))) genus Enchodus (Fig. 4). MUVP 59 is a member of the Enchodontidae on the basis of 3 synapomorphies: (i) ectopterygoid with a single row of between six to eight teeth, the second of which is the largest (SOM 2: character 12(2)) and (ii) two dentary tooth rows (SOM 2: character 37(1), the medial of which consists of (iii) a single long tooth near the symphysis that is at least 33% longer than the teeth caudal to it (SOM 2: character 38(1)) (Fielitz 2004; Silva and Gallo 2011). The absence of maxillary teeth in MUVP 59 is consistent with all enchodontids (Fielitz 2004), though Silva and Gallo (2011) considered the maxilla of Eurypholis to be toothed. However, the maxilla of MUVP 59 cannot be determined to have been included in gape due to disarticulation of most of the preserved elements and the lack of a preserved premaxilla. As such, character 30 (SOM 2) was scored as missing for MUVP 59 in this analysis.
The presence of a mandibular symphysis that tapers rostrally (SOM 2: character 35(0)), rather than remaining as deep as the rest of the mandible, three rostroventrally-projecting prongs near the mandibular symphysis (SOM 2: character 36(1)), a closed mandibular sensory canal (SOM 2: character 40(2)), and the absence of preopercular dermal ornamentation exclude MUVP 59 from Palaeolycus (Fielitz 2004). MUVP 59 is excluded from Eurypholis and Saurorhamphus because it possesses an opercular dermal pattern consisting of ridges (SOM 2: character 50(1)), rather than tubercles, and no mandibular (SOM 1: character 41(0)) or preopercular (SOM 2: character 49(0)) dermal patterning is present. MUVP 59 is further excluded from Eurypholis because it possesses a single articular facet for the hyomandibula (SOM 2: character 90(0)) (Silva and Gallo 2011), rather than two facets, and from Saurorhamphus because it does not possess more than eight, equally-sized ectopterygoid teeth, the teeth of the upper jaw are straight (SOM 2: character 88(0)), rather than curved, and the dentary teeth are not of equal size. MUVP 59 is excluded from Parenchodus because the mandibular symphysis does not remain deep, it possesses a vertical bar on the caudal portion of the articular (SOM 2: character 42(1)), the teeth of the upper jaw are straight, it possesses a single articular facet for the hyomandibula and a closed mandibular sensory canal, it lacks either a mandibular or preopercular dermal pattern, and its opercular dermal pattern is present as ridges (SOM 2: character 50(1)), rather than ridges with tubercles along each ridge.
MUVP 59 belongs to Enchodus based on the presence of a ventral bar on the caudal portion of the articular. However, MUVP 59 lacks the mandibular, preopercular, or opercular dermal pattern of tubercles seen in E. marchesettii (Fielitz 2004) or ridges with tubercles along each ridge seen in nearly all other Enchodus species except for E. lewesiensis, a taxon that exhibits a mandibular dermal pattern of ridges. There are two other exceptions to such dermal patterns within Enchodus. Enchodus cf. dirus from Greece (Gavdos_fish_2/2010) of Cavin et al. (2012) shares with MUVP 59 an opercular dermal pattern consisting of ridges but also possesses mandibular and preopercular dermal patterns of ridges, unlike the absence of such patterns in MUVP 59. Like the condition seen in MUVP 59, E. dirus lacks any mandibular or preopercular dermal pattern, but the opercular dermal pattern of that species was scored as missing by Fielitz (2004). These two latter species form a small clade within Enchodus, with MUVP 59 as the sister taxon (Fig. 4). MUVP 59 is differentiated from all other species of Enchodus by the lack of an open or partially open mandibular sensory canal.
MUVP 59 can be confidently assigned to the enchodontid genus Enchodus on the basis of the morphological character states described above. Character state inconsistencies between MUVP 59 and other Enchodus species are here considered to be inadequate for either the exclusion of the specimen from Enchodus, particularly given the variability of states for some characters (e.g., dermal ornamentation) among species of Enchodus, or for the erection of a new genus. This assessment is supported by the results of the phylogenetic analysis, which recovered MUVP 59 nested within Enchodus as the sister taxon to E. dirus and material referred to E. dirus. Hence, MUVP 59 is here considered a new species, E. tineidae sp. nov., included in the genus Enchodus. Moreover, the unique suite of characters exhibited by MUVP 59, including the triplet and doublet clustering of dentary teeth d9–d11 and d12–d13, respectively, and the autapomorphic closed mandibular sensory canal and pattern of dermal ornamentation are diagnostic for E. tineidae.
Paleobiogeography.—Placing Enchodus tineidae within the paleobiogeographical context of Enchodus and other enchodontids yields expected results. Enchodus, Eurypholis, Saurorhamphus, Palaeolycus, and Parenchodus, genera that each share at least some character states with E. tineidae, are all western Tethyan in their distribution (Fielitz 2004; Silva and Gallo 2011). Species of Enchodus generally fall within one of two major clades that are structured geographically such that one is primarily known from North America and the other primarily from the modern Mediterranean region (Fielitz 2004; Cavin et al. 2012). Enchodus dirus is a species known from the Western Interior Seaway and the eastern coast of present-day North America (e.g., Cavin et al. 2012). Material referred to E. dirus is also known from present-day Greece, and the occurrence of this species in both the western Tethys and North America has been proposed to be the result of one in a series of Late Cretaceous vicariance events (Cavin et al. 2012; Silva and Gallo 2016). Based on some characters described here, such as overall size and dentary tooth count, E. tineidae bears a greater superficial similarity to some Tethyan species of Enchodus than to most North American species. Phylogenetic analysis and differential diagnosis, however, indicates an affinity of E. tineidae to E. dirus, possibly related to the vicariance event proposed by Cavin et al. (2012) or simply resulting from E. dirus and E. tineidae forming a subclade with a transatlantic distribution.
Conclusions
Enchodus tineidae sp. nov., is the first enchodontoid species from Egypt to be formally described. A number of morphological characters possessed by the new species are consistent with those unique to the genus Enchodus, among enchodontoids (Fielitz 2004; Silva and Gallo 2011), whereas others are inconsistent with any known species of Enchodus. Phylogenetic analysis grouped the new species with E. dirus of North America and European material referred to that latter species (Cavin et al. 2012). The sister taxon of this clade is a North American species, with more distantly-related taxa occupying a polytomy that includes three species known from North America, Central America, and western Africa, respectively. The phylogenetic relationships of E. tineidae to European and North American taxa provide new data for future biogeographic studies. Finally, ongoing exploration of fossil-bearing, Late Cretaceous strata of central Egypt will continue to provide novel data and perspectives on northern African ecosystems near the close of the Mesozoic (Claeson et al. 2014; Sallam et al. 2016) and shed more light on the ecology and paleogeography of a number of teleost and other groups.
Acknowledgements
We thank Salah N. Ayyad, Mahrous A. El-Enen, Haytham S. Al-Atfy, Yasin El Saay (all MUVP) for logistical support. Khalef Hassan and Moahmoud Borsat (both Egyptian Security Service) played a critical role during the 2008 field season. We also thank the Mansoura University Vertebrate Paleontology Center for helping with the field and laboratory work and the Cincinnati Museum of Natural History and Science for collections access. We thank Matthew Graham, Sebastian Egberts, Joseph Groenke, and Eric Lund (all Ohio University, Athens, USA) for mechanical preparation and molding/casting of MUVP 59; John Sattler (Ohio University, Athens, USA) for photography; and Joseph Sands, Cynthia Pugh, and Brooke Keener (all Holzer Clinic, Athens, USA) for assistance with CT scanning. Funding for the fieldwork portion of this project was provided by the National Geographic Society-Waitt Foundation (W88-10), the Ohio University Heritage College of Osteopathic Medicine, and the Ohio University Office of Research and Sponsored Programs.
References
Agassiz, L. 1835. Recherches sur les poisons fossils: Tome IV. i–xii + 1–296. Imprimerie de Petitpierre, Neuchâtel.
Allam, A.M. 1986. A regional and paleoenvironmental study on the Upper Cretaceous deposits of the Bahariy Oasis, Libyan Desert, Egypt. Journal of African Earth Sciences 5: 407–412. Crossref
Arambourg, C. 1952. Les vertébrés fossils des gisements de phosphates (Maroc-Algérie-Tunisie). Notes du Service Géologique du Maroc, Notes et Mémoires 92: 1–372.
Arambourg, C. 1954. Les poisons crétacés du Jebel Tselfat (Maroc). Notes du Service Géologique du Maroc, Notes et Mémoires 118: 1–188.
Bardet, N., Cappetta, H., Pereda Superbiola, X., Mouty, M., Al Maleh, A.K., Ahmad, A.M., Khrata, O., and Gannoum, N. 2000. The marine vertebrate faunas from the Late Cretaceous phosphates of Syria. Geological Magazine 137: 269–290. Crossref
Becker, M.A., Mallery, C.S. Jr., and Chamberlain, J.A. Jr. 2010. Osteichthyans from an Arkadelphia Formation—Midway Group Lag Deposit (Late Maastrichtian–Paleocene), Hot Spring County, Arkansas, U.S.A. Journal of Vertebrate Paleontology 30: 1019–1036. Crossref
Case, G.R. and Schwimmer, D.R. 1988. Late Cretaceous fish from the Blufftown Formation (Campanian) in Western Georgia. Journal of Paleontology 62: 290–301. Crossref
Cavin, L. 1999. Occurrence of a juvenila teleost, Enchodus sp., in a fish gut content from the Upper Cretaceous of Goulmima, Morocco. Special Papers in Palaeontology 60: 57–72.
Cavin, L., Alexopoulos, A., and Piuz, A. 2012. Late Cretaceous (Maastrichtian) ray-finned fishes from the island of Gavdos, southern Greece, with comments on the evolutionary history of the aulopiform teleost Enchodus. Bulletin de la Société Géologique de France 187: 561–572. Crossref
Chalifa, Y. 1989. New species of Enchodus (Pisces: Enchodontoidei) from the Lower Cenomanian of Ein-Yabrud. Journal of Paleontology 63: 356–364. Crossref
Chalifa, Y. 1996. New species of Enchodus (Aulopiformes: Enchodontidae) from the Northern Negev, Israel, with comments on evolutionary trends in the Enchodontoidei. In: G. Arratia and G. Viohl (eds.), Mesozoic Fishes—Systematics and Paleoecology, 349–367. Verlag Dr. Friedrich Pfeil, München.
Claeson, K.M., Sallam, H.M., O’Connor, P.M., and Sertich, J.J.W. 2014. A revision of the Upper Cretaceous lepidosirenid lungfishes from the Quseir Formation, Western Desert, Central Egypt. Journal of Vertebrate Paleontology 34: 760–766. Crossref
Cope, E.D. 1872. On the families of fishes of the Cretaceous formations in Kansas. Proceedings of the American Philosophical Society 12: 327–357.
Cope, E.D. 1886. A contribution to the vertebrate paleontology of Brazil. Proceedings of the American Philosophical Society 23: 1–21.
Davis, M.P. and Fielitz, C. 2010. Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations. Molecular Phylogenetics and Evolution 57: 1194–1208. Crossref
El Khawaga, M.L., Philobbos, E.R., and Riad, S. 2005. Stratigraphic Lexicon and Explanatory Notes on the Geological Map of the South Western Desert, Egypt. scale 1:250 000. 81 sheets. UNESCO, Cairo.
Fielitz, C. 1996. A Late Cretaceous (Turonian) ichthyofauna from Lac des Bois, Northwest Territories, Canada, with paleobiogeographic comparisons with Turonian and ichtyofaunas of the Western Interior Seaway. Canadian Journal of Earth Sciences 33: 1375–1389. Crossref
Fielitz, C. 2002. First record of endopterygoid teeth in the North American Late Cretaceous teleostean fish Enchodus gladiolus (Aulopiformes: Enchodontidae). Transactions of the Kansas Academy of Science 105: 27–32. Crossref
Fielitz, C. 2004. The phylogenetic relationships of the Enchodontidae (Teleostei: Aulopiformes). In: G. Arratia, M.V.H. Wilson, and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 619–634. Verlag Dr. Friedrich Pfeil, München.
Fielitz, C. and González-Rodríguez, K.A. 2010. A new species of Enchodus (Aulopiformes:Enchodontidae) from the Cretaceous (Albian to Cenomanian) of Zimapán, Hidalgo, México. Journal of Vertebrate Paleontology 30: 1343–1351. Crossref
Fink, W.L. 1981. Ontogeny and phylogeny of tooth attachment modes in Actinopterygian fishes. Journal of Morphology 167: 167–184. Crossref
Forey, P.L., Yi, L., Patterson, C., and Davies, C.E. 2003. Fossil fishes from the Cenomanian (Upper Cretaceous) of Namoura, Lebanon. Journal of Systematic Palaeontology 1: 227–330. Crossref
Friedman, M. 2012. Ray-finned fishes (Osterichthyes, Actinopterygii) from the type Maastrichtian, the Netherlands and Belgium. Scripta Geologica, Special Issue 8: 113–142.
Goody, P.C. 1968. The skull of Enchodus faujasi from the Maastricht of Southern Holland. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series B 71: 209–231.
Goody, P.C. 1969. The relationships of certain Upper Cretaceous teleosts with special reference to the myctophoids. Bulletin of the British Museum (Natural History) of Geology, Supplement 7: 1–259.
Goody, P.C. 1970. The Cretaceous teleostean fish Cimolichthys from the Niobrara Formation of Kansas and the Pierre Shale of Wyoming. American Museum Novitates 2434: 1–29.
Goody, P.C. 1976. Enchodus (Teleostei: Enchodontidae) from the Upper Cretaceous Pierre Shale of Wyoming and South Dakota with an evaluation of the North American enchodontid species. Palaeontographica, Abteilung A 152: 91–112.
Green, W.R. 1911. A description of the specimens of the teleostean genus Enchodus in the University of Kansas Museum. The University of Kansas Science Bulletin 7: 71–107.
Hay, O.P. 1903a. On a collection of Upper Cretaceous fishes from Mount Lebanon, Syria, with descriptions of four new genera and nineteen new species. Bulletin of the American Museum of Natural History 19: 395–452.
Hay, O.P. 1903b. On certain genera and species of North American Cretaceous actinopterous fishes. Bulletin of the American Museum of Natural History 19: 1–96.
Hussakof, L. 1908. Catalogue of types and figured specimens of fossil vertebrates in the American Museum of Natural History. Bulletin of the American Museum of Natural History 25: 1–104.
Hendriks, F., Luger, P., Kallenbach, H., and Schroeder, J.H. 1984. Stratigraphical and sedimentological framework of the Kharga-Sinn el Kaddab Strech (western and southern part of the Upper Nile Basin), Western Desert Egypt. Berliner Geowissenschaftliche Abhandlungen A 50: 117–151.
Hermina, M. 1990. The surroundings of Kharga, Dakhla, and Farafra oases. In: R. Said (ed.). The Geology of Egypt, 259–292. Balkema, Rotterdam.
Jacobs, L.L., Mateus, O., Polcyn, M.J., Schulp, A.S., Antunes, M.T., Morais, M.L., and da Silva Tavares, T. 2006. The occurrence and geological setting of cretaceous dinosaurs, mosasaurs, plesiosaurs, and turtles from Angola. Journal of the Paleontological Society of Korea 22: 91–110.
Kear, B.P., Rich, T.H., Ali, M.A., Al-Mufarrih, Y.A., Matiri, A.H., Al-Masary, A.M., and Attia, Y. 2009. An Upper Cretaceous (Campanian–Maastrichtian) actinopterygian fish assemblage from the marginal marine Adaffa Formation of Saudi Arabia. Cretaceous Research 30: 1164–1168. Crossref
Klitzsch, E. and Schandelmeier, H. 1990. South Western Desert. In: R. Said (ed.), The Geology of Egypt, 249–258. Balkema, Rotterdam.
Klitzsch, E., Harms, J.C., Lejal-Nicol, A., and List, F.K. 1979. Major subdividions and depositional environments of Nubia strata, Southwestern Egypt. American Association of Petroleum Geologists Bulletin 63: 967–974.
Kriwet, J. and Gloy, U. 1995. Zwei mesopelagische Raubfische (Actinopterygii: Euteleostei) aus dem Unterturon der Kronsberg-Mulde bei Hannover/Misburg (NW-Deutschland). Berliner Geowissenschaftliche Abhandlungen E 16: 335–355.
Leonardi, A. 1966. L’ittiofauna cenomaniana di Floresta-Messina. Palaeontographica Italia 60: 33–67.
Mahmoud, M.S. 2003. Palynology and paleoenvironment of the Quseir Formation (Campanian) from central Egypt. Journal of African Earth Sciences 36: 135–148. Crossref
Maury, C.J. 1930. O Cretaceo da Parahyba do Norte. Serviço Geologico E Mineralogico do Brasil 8: 1–305.
O’Connor, P.M., Sertich, J.J.W., Sallam, H., and Seiffert, E.R. 2010. Reconnaissance paleontology in the Late Cretaceous of Dakhla and Kharga Oases, Western Desert, Egypt. Journal of Vertebrate Paleontology 30 (Supplement 3): 141.
Rana, R.S., Kumar, K., Singh, H., and Rose, K.D. 2005. Lower vertebrates from the Late Paleocene–Earliest Eocene Akli Formation, Giral Lignite Mine, Barmer District, western India. Current Science 89: 1606–1613.
Rigo, D. 1999. The fossils of the Cretaceous Lagerstätte of Polazzo (Fogliano-Redipuglia, Gorizia, NE Italy). Natura Nascosta 19: 10–19.
Sallam, H.M., O’Connor, P.M., Kora, M., Sertich, J.J.W., Seiffert, E.R., Faris, M., Ouda, K., El-Dawoudi, I., and Saber, S. 2016. Vertebrate paleontological exploration of the Upper Cretaceous succession in the Dakhla and Kharga Oases, Western Desert, Egypt. Journal of African Earth Sciences 117: 223–234. Crossref
Schein, J.P. and Lewis, R.D. 2007. Actinopterygian fishes from Upper Cretaceous rocks in Alabama, with emphasis on the teleostean genus Enchodus. Paludicola 6: 41–86.
Schein, J.P., Parris, D.C., Poole, J.C., and Lacovara, K.J. 2013. A nearly complete skull of Enchodus ferox (Actinopterygii, Aulopiformes) from the Upper Cretaceous Ripley Formation of Lowndes County, Alabama. Bulletin of the Alabama Museum of Natural History 31: 78–83.
Shimada, K. and Everhart, M.J. 2003. Ptychodus mammillaris (Elasmobranchii) and Enchodus cf. E. shumardi (Teleostei) from the Fort Hays Limestone Member of the Niobrara Chalk (Upper Cretaceous) in Ellis County, Kansas. Transactions of the Kansas Academy of Science 106: 171–176. Crossref
Siegfried, P. 1954. Die Fisch-Fauna des Westfälischen Ober-Senons. Palaeontographica Abteilung A 106: 1–36.
Silva, H.M.A. and Gallo, V. 2011. Taxonomic review and phylogenetic analysis of Enchodontoidei (Teleostei: Aulopiformes). Annals of the Brazilian Academy of Sciences 83: 483–511. Crossref
Silva, H.M.A. and Gallo,V. 2016. Distributional patterns of enchodontoid fishes in the Late Cretaceous. Cretaceous Research 65: 223–231. Crossref
Slaughter, B.H. and Thurmond, J.T. 1974. A lower Cenomanian ichthyofauna from the Bahariya Formation of Egypt. Annals of the Geological Survey of Egypt 4: 25–40.
Sorbini, L. 1976. L’ittiofauna cretacea di Cinto Euganeo (Padova-Nord Italia). Bollettino del Museo Civico di Storia Naturale di Verona 3: 469–567.
Swofford, D.L., 2002. PAUP*. Phylogenetic Analysis Using Parsimony (and other methods). Version 4.0b10. Sinauer Associates, Sunderland.
Tantawy, A.A., Keller, G., Adatte, T., Stinnesbeck, W., Kassab, A., and Schulte, P. 2001. Maastrichtian to Paleocene depositional environment of the Dakhla Formation, Western Desert, Egypt: sedimentology, mineralogy, and integrated micro- and macrofossil biostratigraphies. Cretaceous Research 22: 795–827. Crossref
Willimon, E.L. 1973. An enchodontid skull from the Austin Chalk (Upper Cretaceous) of Dallas, Texas. The Southwestern Naturalist 18: 201–210. Crossref
Wilson, M.V.H. and Chalifa, Y. 1989. Fossil marine actinopterygian fishes from the Kaskapau Formation (Upper Cretaceous: Turonian) near Watino, Alberta. Canadian Journal of Earth Sciences 26: 2604–2620. Crossref
Yabumoto, Y. and Uyeno, T. 1994. Late Mesozoic and Cenozoic fish faunas of Japan. The Island Arc 3: 255–269. Crossref
Youssef, M.I. 1957. Upper Cretaceous rocks in Kosseir area. Bulletin Institute Désert Égypte 7: 35–54.
Acta Palaeontol. Pol. 62 (3): 603–611, 2017
https://doi.org/10.4202/app.00331.2016