

Eocene antiquity of the European nyctitheriid euarchontan mammal Darbonetus
JERRY J. HOOKER
Until now Darbonetus was represented by only one valid species, the type species D. aubrelongensis from the early Oligocene of the Quercy region, France. A late appearance of this genus and of its closest relative Amphidozotherium have been thought to result from dispersal from outside western Europe, rather than a local ancestry earlier in the late Eocene. Here, a new species, Darbonetus sigei sp. nov., is described from the middle Priabonian site of La Débruge, France. Although clearly closely related to D. aubrelongensis, D. sigei shows less reduction of its p2, p3, and m3 than in the more derived type species. The early age of D. sigei suggests that its origins were within the still isolated central European island and that it is unnecessary to invoke dispersal from another continent.
Introduction
Darbonetus aubrelongensis Crochet, 1974 was described from the earliest Oligocene fissure filling of Aubrelong 1, Quercy, France, based on three dentaries. The holotype (UP.Au1.705) shows p3–m3 and the alveolus of p2. One of the paratypes (UP.Au1.811) bears only one tooth (p4), but more mesial alveoli, which have been interpreted as representing three incisors, one canine, and one-rooted p1 and p2, with the possibility that the elongated alveolus of p2 might have housed two roots (Crochet 1974). Subsequently, the species has been recorded from other Quercy fissure fillings, namely Ravet, La Couaille, Mas de Got, Roqueprune 4, Pech Crabit 1, and Itardies (Remy et al. 1987), extending the range from Mammalian Paleogene reference level MP21 to younger Oligocene MP23 (Crochet 1995) (see BiochroM’97 1997 for explanation of MP reference levels). Crochet (2016), however, recognized Aubrelong 1 as the only locality for D. aubrelongensis until he recorded the species from a new Quercy locality, Valbro, attributed to MP22. Crochet (2016) noted that two specimens from Valbro show variation in the width of the m3 talonid, one slightly wider, the other slightly narrower than in the holotype.
A second species, Darbonetus tuberi Crochet, 1995 was described from the middle Oligocene (MP25) Quercy site of Le Garouillas. This species has been shown instead to belong to another nyctithere genus, Saturninia (Hooker and Weidmann 2000). This attribution is justified because its cristid obliqua meets the back of the trigonid buccal of the midline without ascending it and its m2–m3 entoconid is relatively mesially positioned, both features shared with the type species Saturninia gracilis Stehlin, 1941. Moreover, it lacks the major reduction in size of the p3 and m3 shown by D. aubrelongensis.
Preparation by the author of a small matrix block of sandy organic mud in the NHMUK 19th Century Forsyth Major Collection from the middle Priabonian (late Eocene) site of La Débruge, Vaucluse, France, has revealed a dentary of Darbonetus with four premolars and three molars, plus alveoli for i1–i3 (distal part only for i1) and the canine (Fig. 1A, C). The apparently associated lower canine was found separated in the same block (Fig. 1B). This specimen is described here as Darbonetus sigei sp. nov. Two other species of nyctithere have already been described from La Débruge: Saturninia gracilis (see Stehlin 1941; Bonis 1964) and Euronyctia saturninensis Smith, 2006a, bringing the total number of species for the family from this site to three.
Institutional abbreviations.—MNHN, Muséum National d’Histoire Naturelle, Paris, France; NHMUK, Earth Sciences Department, Natural History Museum, London, UK; UP, Laboratoire de Paléontologie des Vertébrés, Université de Paris VI, France.
Other abbreviations.—We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Nomenclatural acts.—The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The LSID for this publication is: urn:lsid:zoobank.org:pub:62963E94-0AF0-4E85-B1BF-6C4773815702. The electronic edition of this work was published in a journal with an ISSN 0567-7920, eISSN 1732-2421, and is available from the following digital repository: APP archive at http://www.app.pan.pl/article/item/app004572018.html
Systematic palaeontology
Class Mammalia Linnaeus, 1758
Superorder Euarchonta Waddell, Okada,
and Hasegawa, 1999
Family Nyctitheriidae Simpson, 1928
Subfamily Amphidozotheriinae Sigé, 1976
Remarks.—Manz and Bloch (2014) have suggested that this subfamily is paraphyletic. However, they demonstrated a clade containing all the previously recognized genera of the subfamily, but which also included the nyctitheriines Scraeva hatherwoodensis Cray, 1973 and Saturninia rigassii Hooker and Weidmann, 2000. They correctly recognized the genus Saturninia to be paraphyletic, but S. hatherwoodensis has subsequently been shown to be closely related to other accepted members of the Amphidozotheriinae, viz. Amphidozotherium Filhol, 1877, Darbonetus Crochet, 1974, Paradoxonycteris Revilliod, 1922, Euronyctia Sigé, 1997, and Sigenyctia Smith, 2006b (Hooker 2017). Recognition that Saturninia is paraphyletic does not mean that subfamily Amphidozotheriinae is also paraphyletic. It simply means that one species of Saturninia is an amphidozotheriine. Therefore, this subfamily is here regarded as monophyletic. North American Paleocene Limaconyssus Gingerich, 1987 was also included with European amphidozotheriines by Manz and Bloch (2014), but there is conflict between their character descriptions and the published illustrations of Limaconyssus. The latter and poorly known S. rigassii do not fit well within the subfamily (JJH unpublished data).
Genus Darbonetus Crochet, 1974
Type species: Darbonetus aubrelongensis Crochet, 1974; Rupelian, early Oligocene fissure filling, Aubrelong 1, Quercy, France.
Emended diagnosis.—Amphidozotheriine with: p3 small but two-rooted; p4 protoconid and metaconid separated to the base (shared with Paradoxonycteris and Euronyctia); p4 paraconid lies horizontally as seen in lingual view; p4 trigonid relatively low (shared with Euronyctia); ascending part of lower molar cristid obliqua weak (shared with Amphidozotherium); m1–m2 entoconid small (shared with Amphidozotherium); m1 paraconid not deflected mesially; hypoconulid central on m1, positioned more lingually on m2; m2–m3 paraconid basally extended mesially (shared with Amphidozotherium).
Other genera have an erect to slightly procumbent p4 paraconid as seen in lingual view. Amphidozotherium has: tiny single rooted p3; more exodaenodont lower canine and p1–p3; p4 trenchant with taller trigonid, protoconid and metaconid that merge basally and strongly exodaenodont talonid; mesially deflected m1 paraconid; m2 with median hypoconulid; and m1–m2 with greater difference in height between trigonid and talonid. Paradoxonycteris, Euronyctia, Sigenyctia, and Scraeva have stronger ascending part of lower molar cristid obliqua and m1–m2 with larger entoconid. Paradoxonycteris, Euronyctia, and Sigenyctia have more lingually positioned m1 hypoconulid. Euronyctia and Sigenyctia have a more deeply concave-edged cristid obliqua. Scraeva has less procumbent p2 and slightly less lingually positioned m2 hypoconulid.
Darbonetus sigei sp. nov.
Fig. 1.
ZooBank LSID: urn:lsid:zoobank.org:act:62963E94-0AF0-4E85-B1BF-6C4773815702.
Etymology: Named for Bernard Sigé for his substantial published work on nyctitheres.
Holotype: NHMUK.PV.M10044, left dentary with four premolars, three molars, alveoli for three incisors and canine, plus apparently associated left canine. There are no other specimens known.
Type locality: La Débruge, Vaucluse, France.
Type horizon: The sandy organic mud lithology of the block that contained the specimen and of what remains still attached to the specimen, shows that it almost certainly originates from the “marnes ligniteuses” horizon (Bonis 1964). The associated fauna is attributed to the Isoptychus pseudosiderolithicus–Palaeotherium muehlbergi thaleri Biozone (Hooker 1987) and reference level MP18 (Brunet et al. 1987); middle Priabonian, Eocene.
Diagnosis.—Two-rooted p2; p3 length 72% of p4; p4 with prominent entoconid; p4 and m1–m3 hypoconid tilted mesially (shared with other amphidozotheriine genera); m1–m2 with cuspate entoconid; length of m3 90% of m2; m3 with entoconid.
Darbonetus aubrelongensis has: p2 with one root; p3 length is 56% of p4; p4 with weaker entoconid; p4 and m1–m3 hypoconid erect; m1–m2 with crestiform entoconid; m3 length 79–84% of m2; m3 lacking entoconid.
Table 1. Maximum measurements (in mm) of the teeth of the holotype of Darbonetus sigei sp. nov.
| |
Length |
Width (single) |
Trigonid width |
Talonid width |
|
c1 |
1.15 |
0.53 |
|
|
|
dp1 |
0.82 |
0.49 |
|
|
|
p2 |
0.96 |
0.52 |
|
|
|
p3 |
0.84 |
0.51 |
|
|
|
p4 |
1.18 |
|
0.78 |
0.69 |
|
m1 |
1.55 |
|
1.01 |
0.99 |
|
m2 |
1.51 |
|
1.05 |
0.92 |
|
m3 |
1.27 |
|
0.88 |
0.62 |
Description.—The specimen is well preserved, although very fragile. The dentary is broken posteriorly, so that the ascending ramus is lost (Fig. 1C1). The mental foramen is situated below p3. The symphysis extends posteriorly to below p2 (Fig. 1C2). The teeth are only lightly worn and show all the necessary crown details. They are very close in size (Table 1) to those of D. aubrelongensis. Because of fragility, it has not been possible to remove matrix fully below all the tooth crowns, so that the roots of p3 cannot be observed (Fig. 1A3). It is assumed that, as the crown of this tooth is larger than that of the two-rooted p3 of D. aubrelongensis, it also has two roots. The p2, however, can be seen clearly to have two roots, unlike that of D. aubrelongensis, which, according to the even outline of the alveolus of the holotype, had one. The canine and the first premolar (judged to be dp1 as it has thinner enamel than the p2) have a single root each, confirming their likely root number also in D. aubrelongensis, which was based on their alveoli (Crochet 1974). The incisor alveoli are smaller than that of the canine (Fig. 1A1, A2). The canine is a fairly large simple procumbent tooth, but with a distal cuspule (Fig. 1B). It is less exodaenodont than that of Amphidozotherium cayluxi Filhol, 1877.
The dp1 is smaller and relatively shorter compared to p4 than in A. cayluxi (Sigé 1976). Like the canine it also has a distal cuspule, which leans slightly distally. It has a lingual cingulum that is interrupted adjacent to the main cusp. It is slightly obliquely implanted in the jaw. The p2 is larger than dp1, with a taller, sharper main cusp, a longer mesial slope, and two roots, but otherwise very similar in structure. Like dp1, p3 is implanted slightly obliquely in the jaw, presumably an individual trait. It is similar to p2 but slightly smaller, relatively shorter mesiodistally, with a more exodaenodont talonid and more continuous cingulum lingually.
The p4 is semimolariform, with a relatively low trigonid, exodaenodont talonid, and small entoconid. Apart from the diagnostic differences, the p4 is very similar in shape to that of D. aubrelongensis, although the latter’s paraconid is more lingually situated and does not project mesially (Fig. 1A2). The p4 of the holotype D. aubrelongensis is slightly damaged distolingually and it is difficult to judge if an entoconid was present. However, VBO148 from Valbro shows presence of an entoconid that is weaker than in D. sigei (Crochet 2016: fig. 1G).
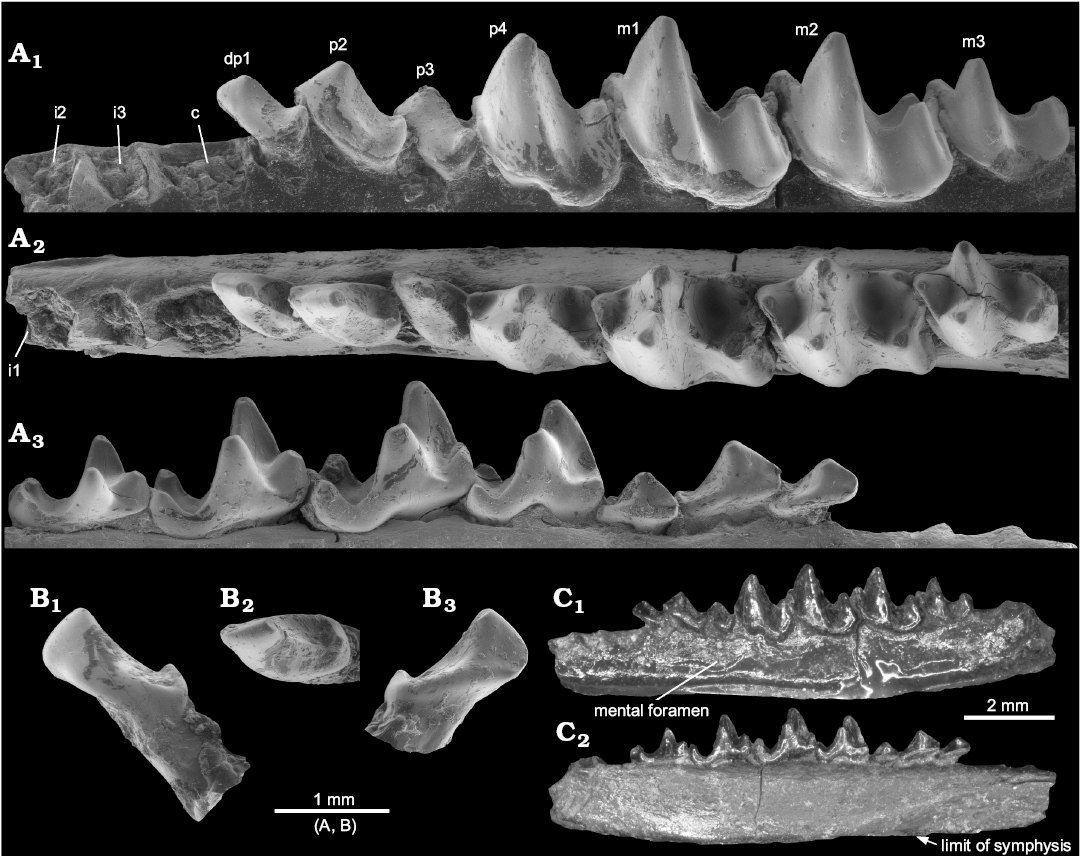
Fig. 1. Holotype of nyctitheriid euarchontan Darbonetus sigei sp. nov. (NHMUK.M10044) from the Priabonian, Eocene of La Débruge, Vaucluse, France. Left dentary with four premolars and three molars in situ, plus associated lower canine; the cheek teeth and alveoli in the dentary (A), the canine (B), the complete dentary (C); in buccal (A1, B1, C1), occlusal (A2, B2), and lingual (A3, B3, C2) views. Scanning electron micrographs of uncoated original (A, B), light micrographs (C).
The first and second molars have the typical amphidozotheriine structure with a lingual cingulum between the paraconid and metaconid and with a concave-edged cristid obliqua that rises part way up the distolingual wall of the metaconid. The talonid is slightly wider than the trigonid on m1 and slightly narrower on m2. The hypoconulid is more or less central on m1, but closer to the entoconid on m2. The entoconid is small and cuspate. Like p4, the m1 paraconid is also more lingually situated than in D. aubrelongensis. It is uncertain whether these additional differences of p4 and m1 are taxonomic or of an individual nature. The finding of more specimens of D. sigei would solve the problem.
The markedly smaller m3 has a much narrower talonid than on m2, with a lower hypoconid and a tiny entoconid mesiolingual of the hypoconulid. There is slight damage to the distal wall of the hypoconulid, but it appears not to have been distally salient. On D. aubrelongensis, m3 has an even narrower talonid and no entoconid.
Similar-sized Euronyctia saturninensis, also from La Débruge, can be distinguished from D. sigei by having: a longer p3; no p4 talonid exodaenodonty; m1 with more lingually positioned hypoconulid; m1–m2 with taller talonid cusps, especially the entoconid, a small entoconulid, a crestiform hypoconulid that partly extends behind the distal wall of the entoconid, a more deeply concave-edged cristid obliqua, and a paraconid that is more lingually positioned. The third nyctithere species from La Débruge, Saturninia gracilis, differs from D. sigei in having: smaller size; a p3, which is relatively larger with respect to p4; p4–m3 with a less concave-edged cristid obliqua that does not ascend the metaconid; lower molars with a cristid obliqua that meets the back of the trigonid buccal of the midline, without a lingual trigonid cingulum and with a larger entoconid; m2 with a centrally placed hypoconulid; and m3 with a projecting hypoconulid. Both have p3 with a more prominent paraconid, p4 with a longer talonid, m2–m3 paraconid that lacks the mesial deflection, and m3 that is less reduced.
Stratigraphic and geographic range.—Type locality and horizon only.
Discussion
In all diagnostic aspects, Darbonetus sigei appears primitive with respect to D. aubrelongensis. Thus the p2, p3, and m3 are less reduced in size whilst p4 has more talonid cusps and p2 has an additional root. The mesially tilted form of the m1–m2 hypoconid in D. sigei (Fig. 1A1) is shared with all other amphidozotheriines, the erect form in D. aubrelongensis being unique to this species. As D. sigei is older than any of the records of D. aubrelongensis, it is potentially ancestral to the latter, although this hypothesis would need to be corroborated by the finding of morphological intermediates in intermediate-aged strata.
Sigé (1976) thought that both Amphidozotherium and Darbonetus entered western Europe from outside the area in what was then considered to be the early Oligocene. In 1976, the Eocene–Oligocene boundary had not yet been formalized (this happened later: Premoli Silva and Jenkins 1993). Subsequently, Sigé’s “early Oligocene” has become latest Eocene (late Priabonian). The major faunal turnover known as the Grande Coupure (Stehlin 1910) affected European mammals a little later in what is now the earliest Oligocene, following dispersal of numerous new mammal taxa from Asia. Amphidozotherium cayluxi pre-dates the Grande Coupure, occurring at the sites of Escamps, Quercy, France, Headon Hill (Bembridge Limestone Formation), UK, and Eclépens C, Switzerland, belonging to the Palaeotherium medium medium–P. curtum curtum Biozone and reference level MP19 (Hooker and Weidmann 2000; Hooker et al. 2009). It has long been clear that A. cayluxi was not a Grande Coupure immigrant, but Sigé (1976) postulated that, as both Amphidozotherium and Darbonetus were morphologically distinct and had no known close relatives earlier in the late Eocene, both appeared by dispersal from another continent. The sites Aubrelong 1 and Valbro, where D. aubrelongensis occurs, postdate the Grande Coupure in the early Rupelian, Oligocene (MP21) (Hooker et al. 2009; Hooker 2010) and might be expected to have dispersed into Europe along with the other Grande Coupure immigrants. However, it can now be seen that Darbonetus also did not arrive in Europe either at the Grande Coupure or in the late Priabonian, as its record there now extends back to the middle Priabonian. The genus therefore evolved in Europe in the Eocene along with other members of the Amphidozotheriinae, which now include other genera, such as Euronyctia and Scraeva (Hooker and Weidmann 2000; Hooker 2017), which are more similar to non-amphidozotheriine nyctitheres than are A. cayluxi or D. aubrelongensis and whose record extends back as far as the Bartonian (late middle Eocene). Nyctitheres dispersed to Europe from North America in the latest Paleocene (Hooker 2015) and probably also in the earliest Eocene (Hooker 2018). There is no evidence to suggest that subsequently any European nyctithere taxa arrived in that continent from elsewhere. Instead, they evolved there as endemics.
Acknowledgements.—I thank Don Russell (MNHN) for access to a cast of the holotype of Darbonetus aubrelongensis, Alex Ball and Tomasz Goral (Electron Microscopy Unit, NHMUK) for SEM facility support and Pip Brewer (NHMUK) for help with photography. Comments by Carly Manz (Florida Museum of Natural History, Gainesville, USA) and Thierry Smith (Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium) have improved the paper.
References
BiochroM’97 1997. Synthèses et tableaux de correlations. In: J.-P. Aguilar, S. Legendre, and J. Michaux (eds.), Actes du Congrès BiochroM’97 Montpellier. Mémoires et Travaux de l’École Pratique des Hautes Études, Institut de Montpellier 21: 769–805.
Bonis, L. de 1964. Etude de quelques mammifères du Ludien de La Débruge (Vaucluse). Annales de Paléontologie 1964: 121–154.
Brunet, M., Franzen, J.L., Godinot, M., Hooker, J.J., Legendre, S., Schmidt-Kittler, N., and Vianey-Liaud, M. 1987. European reference levels and correlation tables. Münchner Geowissenschaftliche Abhandlungen A 10: 13–31.
Cray, P.E. 1973. Marsupialia, Insectivora, Primates, Creodonta and Carnivora from the Headon Beds (Upper Eocene) of southern England. Bulletin of the British Museum (Natural History), Geology 23: 1–102.
Crochet, J.-Y. 1974. Les insectivores des Phosphorites du Quercy. Palaeovertebrata 6: 109–159.
Crochet, J.-Y. 1995. Le Garouillas et les sites contemporains (Oligocène, MP 25) des Phosphorites du Quercy (Lot, Tarn-et-Garonne, France) et leurs faunes de vertébrés. 4. Marsupiaux et insectivores. Palaeontographica A 236: 39–75.
Crochet, J.-Y. 2016. Valbro: un nouveau site à vertébrés de l’Oligocène inférieur (MP 22) de France (Quercy). IV. Marsupialia, Insectivora. Annales de Paléontologie 102: 7–10. Crossref
Filhol, H. 1877. Recherches sur les phosphorites du Quercy. Étude des fossiles qu’on y rencontre et spécialement des mammifères [2]. Annales des Sciences Géologiques, Paris 8 (1): 1–340.
Gingerich, P.D. 1987. Early Eocene bats (Mammalia, Chiroptera) and other vertebrates in freshwater limestones of the Willwood Formation, Clark’s Fork Basin, Wyoming. Contributions from the Museum of Paleontology, the University of Michigan 27: 275–320.
Hooker, J.J. 1987. Mammalian faunal events in the English Hampshire Basin (late Eocene–early Oligocene) and their application to European biostratigraphy. Münchner Geowissenschaftliche Abhandlungen A 10: 109–116.
Hooker, J.J. 2010. The ‘Grande Coupure’ in the Hampshire Basin, UK: taxonomy and stratigraphy of the mammals on either side of this major Palaeogene faunal turnover. In: J.E. Whitaker and M.B. Hart (eds.), Micropalaeontology, Sedimentary Environments and Stratigraphy: a Tribute to Dennis Curry (1912–2001). The Micropalaeontological Society, Special Publications, 147–215. The Geological Society, London.
Hooker, J.J. 2015. A two-phase Mammalian Dispersal Event across the Paleocene–Eocene transition. Newsletters on Stratigraphy 48: 201–220. Crossref
Hooker, J.J. 2017. Occlusal and morphogenetic field evolution in the dentition of European Nyctitheriidae (Euarchonta, Mammalia). Historical Biology 30: 42–52. Crossref
Hooker, J.J. 2018. A mammal fauna from the Paleocene–Eocene Thermal Maximum of Croydon, London, UK. Proceedings of the Geologists’ Association [published online, doi: 10.1016/j.pgeola.2018.01.001] Crossref
Hooker, J.J. and Weidmann, M. 2000. The Eocene mammal faunas of Mormont, Switzerland. Systematic revision and resolution of dating problems. Mémoires Suisses de Paléontologie 120: 1–141.
Hooker, J.J., Grimes, S.T., Mattey, D.P., Collinson, M.E., and Sheldon, N.D. 2009. Refined correlation of the UK Late Eocene–Early Oligocene Solent Group and timing of its climate history. Geological Society of America Special Papers 452: 179–195. Crossref
Manz, C.L. and Bloch, J.I. 2014. Systematics and phylogeny of Paleocene–Eocene Nyctitheriidae (Mammalia, Eulipotyphla?) with description of a new species from the Late Paleocene of the Clarks Fork Basin, Wyoming, USA. Journal of Mammalian Evolution 22: 307–342. Crossref
Premoli Silva, I. and Jenkins, D.G. 1993. Decision on the Eocene–Oligocene boundary stratotype. Episodes 16: 379–382.
Remy, J.A., Crochet, J.-Y., Sigé, B., Sudre, J., Bonis, L. de, Vianey-Liaud, M., Godinot, M., Hartenberger, J.-L., Lange-Badré, B., and Comte, B. 1987. Biochronologie des phosphorites du Quercy: mise à jour des listes fauniques et nouveaux gisements de mammifères fossiles. Münchner Geowissenschaftliche Abhandlungen A 10: 169–188.
Revilliod, P. 1922. Contribution à l’étude des chiroptères des terrains tertiaires. Troisième partie et fin. Abhandlungen Schweizerischen Paläontologischen Gesellschaft 45: 133–195.
Sigé, B. 1976. Insectivores primitives de l’Eocène supérieur et Oligocène inférieur d’Europe occidentale. Nyctithériidés. Mémoires du Muséum National d’Histoire Naturelle (NS), C, Sciences de la Terre 34: 1–140.
Sigé, B. 1997. Les mammifères insectivores des nouvelles collections de Sossís et sites associés (Éocène supérieur, Espagne). Geobios 30: 91–113. Crossref
Simpson, G.G. 1928. A new mammalian fauna from the Fort Union of southern Montana. American Museum Novitates 297: 1–15.
Smith, R. 2006a. Le genre Euronyctia (Nyctitheriidae, Mammalia) en Europe occidentale. Strata, série 1 13: 229–241.
Smith, R. 2006b. Sigenyctia oligocaena n. gen. n. sp., nyctithère (Mammalia, Lipotyphla) de l’Oligocène inférieur de Belgique (Formation de Borgloon, MP21). Bulletin de l’Institut Royal des Sciences Naturelles de Belgique (Sciences de la Terre) 76: 131–136.
Stehlin, H.G. 1910. Remarques sur les faunules de mammifères des couches Eocènes et Oligocènes du Bassin de Paris. Bulletin de la Société Géologique de France, série 4 9: 488–520.
Stehlin, H.G. 1941. Zur Stammesgeschichte der Soriciden. Eclogae Geologicae Helvetiae 33: 298–306.
Waddell, P.J., Okada, N., and Hasegawa, M. 1999. Towards resolving the interordinal relationships of placental mammals. Systematic Biology 48: 1–5. Crossref
Jerry J. Hooker [j.hooker@nhm.ac.uk], Department of Earth Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK.
Received 12 January 2018, accepted 28 February 2018, available online 13 June 2018.
Copyright © 2018 J.J. Hooker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 63 (2): 235–239, 2018
https://doi.org/10.4202/app.00457.2018