


Like phoenix from the ashes: How modern baleen whales arose from a fossil “dark age”
FELIX G. MARX, ERICH M.G. FITZGERALD, and R. EWAN FORDYCE
Marx, F.G., Fitzgerald, E.M.G., and Fordyce, R.E. 2019. Like phoenix from the ashes: How modern baleen whales arose from a fossil “dark age”. Acta Palaeontologica Polonica 64 (2): 231–238.
The evolution of baleen whales (Mysticeti), the largest animals on Earth, was punctuated by a pivotal turnover event. Following their emergence around 36 million years (Ma), mysticetes diversified into a disparate range of toothed and toothless species until 23 Ma, but then nearly vanished from the global fossil record for the next five million years. Following this early Miocene “dark age”, toothless mysticetes spectacularly reappeared around 18–17 Ma, whereas toothed mysticetes had gone entirely extinct. Here, we suggest that this turnover event reflects a change in mysticete habitat occupancy. Using the well-sampled record of Australasia as a case study, we show that Oligocene pre-“dark age” mysticetes formed distinct coastal and offshore assemblages, dominated by small (2–4 m), ecologically disparate toothed species, and larger (5–6 m) toothless filter feeders, respectively. Environmental change around the Oligocene–Miocene boundary led to the decline of the endemic coastal assemblages, leaving nearshore deposits virtually devoid of mysticetes. Filter feeders persisted offshore and subsequently re-invaded coastal habitats during the mid-Miocene Climatic Optimum, thus establishing the modern, cosmopolitan mysticete fauna.
Key words: Mammalia, Mysticeti, evolution, Oligocene, Miocene, Zealandia, Australia.
Felix G. Marx [felix.marx@monash.edu], Directorate Earth and History of Life, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, Brussels 1000, Belgium; Department of Geology, University of Liège, Belgium; School of Biological Sciences, Monash University, Clayton, Vic., Australia; Museums Victoria, Melbourne, Vic., Australia.
Erich M.G. Fitzgerald [efitzgerald@museum.vic.gov.au], Museums Victoria, GPO Box 666, Melbourne, Vic. 3001, Australia; Department of Life Sciences, Natural History Museum, London, UK; Departments of Vertebrate Zoology and Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
R. Ewan Fordyce [ewan.fordyce@otago.ac.nz], Department of Geology, University of Otago, 360 Leith Walk, Dunedin 9054, New Zealand; Departments of Vertebrate Zoology and Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Received 28 November 2018, accepted 1 February 2019, available online 29 April 2019.
Copyright © 2019 F.G. Marx et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Baleen whales (Mysticeti) are the largest animals on Earth. Unlike all other cetaceans, extant mysticetes are completely toothless, and instead rely on a comb-like keratinous filter, baleen, to strain small prey directly from seawater (Pivorunas 1979). Filter feeding enables whales to tap into vast food sources at a relatively low trophic level, and has turned them into major consumers and ecosystem engineers (Roman et al. 2014). In the North Pacific, for example, pre-whaling populations of extant mysticetes consumed as much as 10% of total net primary productivity (Croll et al. 2006).
The evolution of mysticetes from their archaeocete ancestors is becoming increasingly better documented, and reveals a stepwise morphological shift from toothed raptorial feeders to toothless suction and/or filter feeders (Deméré et al. 2008; Marx et al. 2016a; Geisler et al. 2017). During this transition, lasting from the origin of mysticetes around 36 Ma (Lambert et al. 2017) to the end of the Oligocene (23 Ma), filter feeders were just one of several ecomorphs, and by no means dominant. Instead, mysticetes evolved into a wide range of toothed and toothless forms (Barnes et al. 1995; Fitzgerald 2006, 2010; Tsai and Fordyce 2015; Marx et al. 2016a; Boessenecker and Fordyce 2017a; Geisler et al. 2017), leading to high levels of morphological disparity that have never been matched since (Marx and Fordyce 2015). This disparate golden age of baleen whale evolution was not to last.
Baleen whales abruptly vanish from the fossil record near the Oligocene–Miocene boundary, marking the beginning of an as yet unexplained 5 million-year-long “dark age” in their evolution. During this time, mysticetes are virtually absent even from deposits where other cetaceans (Odontoceti) abound, such as (i) the Chilcatay Formation of Peru (Di Celma et al. 2018); (ii) the Pungo River and lower Calvert formations of the United States East Coast (Gottfried et al. 1994; Whitmore and Kaltenbach 2008); and various formations across (iii) the Mediterranean basin (Bianucci and Landini 2002), the (iv) western and central Paratethys (Pilleri 1986a, b; Grigorescu and Kazár 2006); and (v) the western United States (Barnes 1977; Crowley et al. 1999; Goedert et al. 2007) (Fig. 1). This pattern is striking, considering that mysticetes are common in, and sometimes even dominate, Oligocene assemblages (Barnes et al. 1995; Fordyce 2006; Goedert et al. 2007).
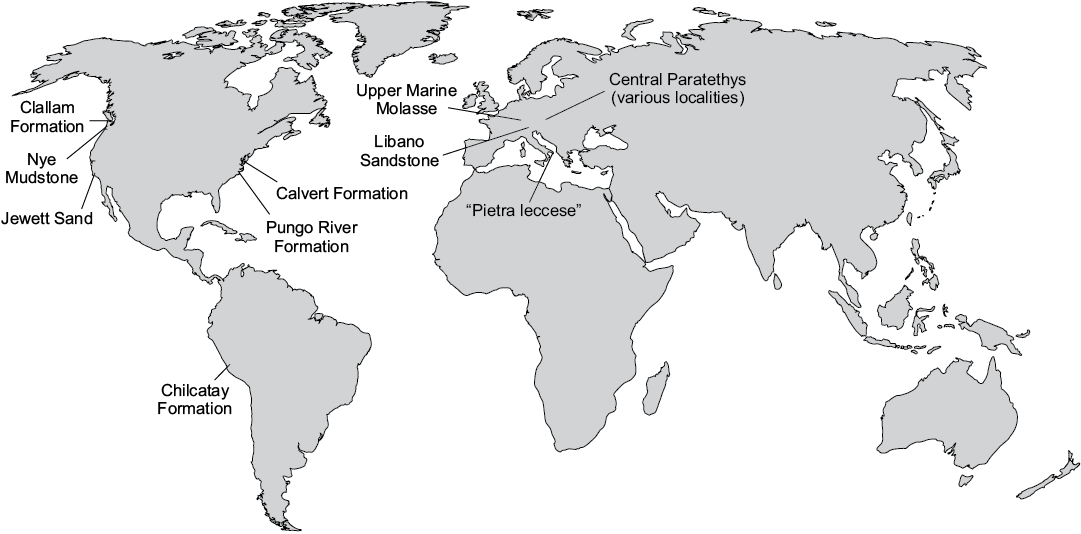
Fig. 1. Location of major formations that have yielded diverse odontocete assemblages, but few or no mysticetes. Sources: Clallam Formation, Nye Mudstone, and Jewett Sand (Barnes 1977; Crowley et al. 1999); Calvert Formation (Gottfried et al. 1994); Pungo River Formation (Whitmore and Kaltenbach 2008); Chilcatay Formation (Bianucci et al. 2018); Upper Marine Molasse (Pilleri 1986a, b); Libano Sandstone and ”Pietra leccese” (Bianucci and Landini 2002); Central Paratethys (Grigorescu and Kazár 2006).
Toothless baleen whales (Chaeomysticeti) finally re-appeared around 18–17 Ma, in the form of abundant, widespread, and comparatively large taxa that dominate middle Miocene assemblages across the Atlantic and Pacific basins (Gottfried et al. 1994; Otsuka and Ota 2008; Bisconti et al. 2013; Di Celma et al. 2017). Toothed mysticetes, by contrast, had gone entirely extinct. The early Miocene “dark age” thus marks a major turning point in baleen whale evolution: prior to it, mysticetes were relatively small, disparate, and included raptorial, suction and filter feeders; after it, only large, cosmopolitan filter feeders remained—a situation that has remained constant to this day, and defines the modern baleen whale fauna.
Here, we suggest that the early Miocene “dark age”, and the ensuing dominance of filter feeders, reflect a concurrent shift in mysticete habitat occupancy. Specifically, we predict that Oligocene toothed mysticetes retained closer ties to the coast than their toothless cousins, which in turn would have limited their geographical range and increased their susceptibility to environmental perturbations. Selective extinction of nearshore assemblages may explain the temporary absence of baleen whales from the early Miocene fossil record, with pelagic filter feeders persisting, but being poorly represented owing to their remote habitat. Modern mysticetes would ultimately have arisen from this pelagic stock, as reflected in the dominance of filter feeders following the end of the “dark age”.
Geological setting
To test the idea that Oligocene toothed mysticetes were primarily coastal, we focus on a globally informative case study: the late Oligocene fossil records of Victoria (Australia) and southern New Zealand. Both regions were located at similar palaeolatitudes, and preserve substantial coeval, well-dated (Graham et al. 2000; McLaren et al. 2009; Tanaka and Fordyce 2016; Korasidis et al. 2018) and richly fossiliferous late Oligocene deposits (Fitzgerald 2004; Boessenecker and Fordyce 2017a; Tsai and Fordyce 2018). The latter primarily include the Jan Juc Marl, Point Addis Limestone, and Waurn Ponds Limestone of Australia (Holdgate and Gallagher 2003; Piper et al. 2006); and the Otekaike Limestone and Kokoamu Greensand of New Zealand (Thompson et al. 2014).
Whereas the Australian strata formed in a relatively warm, restricted bay along a continental margin (Holdgate and Gallagher 2003), the nearby continental block of Zealandia was largely submerged during the late Oligocene. The remaining archipelago was far removed from any major landmass, and surrounded by cool- to temperate-water carbonate ramps that were broadly exposed to the open ocean (Thompson et al. 2014). This unique situation—extensive sediment accumulation in an offshore setting, now broadly exposed on land—singles out New Zealand from all other coeval cetacean localities globally, every one of which was located close to a major landmass. Taken together, Australia and New Zealand thus permit an unparalleled comparison of nearshore vs. open marine late Oligocene assemblages.
Material and methods
Both southern Victoria and the South Island of New Zealand have been consistently prospected as part of long-term fieldwork programmes at the University of Otago (OU; Dunedin, New Zealand) and Museums Victoria (NMV; Melbourne, Australia). Both are currently led by two of the authors (REF and EMGF), with a focus on the fossil-rich regions of North Otago, South Canterbury, and coastal Victoria. Tympanic bullae (ear bones), in particular, have always been collected when encountered, irrespective of their association with other elements or state of preservation. Both programmes have amassed a substantial number of specimens, many of which have recently been (re)described (Fitzgerald 2006, 2010; Boessenecker and Fordyce 2015a–c, 2017a; Tsai and Fordyce 2015, 2016, 2018; Fordyce and Marx 2016). Nevertheless, the majority have so far remained unreported, thus preventing a comprehensive species-level assessment of the two assemblages.
We quantified the number of mysticete fossils from both regions by counting the total number of tympanic bullae currently held at public institutions (see SOM: table 1, Supplementary Online Material available at http://app.pan.pl/SOM/app64-Marx_etal_SOM.pdf). We focussed on bullae because of their high diagnostic value and relative abundance. Most of the specimens were isolated finds and counted as a single data point, irrespective of whether they were left or right. This approach maximises the use of available material, but carries the risk of recording individuals twice. Given the considerable time scales and areas involved, however, we judge double-counting to be minimal. Where bullae formed part of articulated specimens, only one side was counted.
To determine assemblage structure, we used diagnostic morphological characters to identify all bullae to one of three higher taxa: (i) Mammalodontidae, a family of small, tooth-bearing raptorial and/or suction feeders with a total body length of around 3 m (Fitzgerald 2010); (ii) Eomysticetidae, a basal clade of toothless suction and/or filter feeders reaching 4–6 m (Boessenecker and Fordyce 2017a); and (iii) other, more crownward chaeomysticetes, all of them likely filter feeders and generally reaching lengths of 5–7 m (Tsai and Kohno 2016; Slater et al. 2017; Fordyce and Marx 2018). See SOM for details.
Finally we calculated the contribution (in %) of each group to the Australian and New Zealand records, and then tested for differences in the relative counts by means of a two-way chi-square test, using PAST 3.14 (Hammer et al. 2001). This approach focuses solely on proportions, rather than absolute abundance or taxonomic diversity, and should therefore be robust to potential sampling biases. Crucially, the basic nearshore/offshore contrast between New Zealand and Australia persisted throughout the late Oligocene, thus also minimising the effects of time averaging.
Results and discussion
Coastal vs. offshore assemblages.—We counted 67 tympanic bullae from Australia and 48 bullae from New Zealand (SOM: table 1). The Australian late Oligocene record is dominated by mammalodontids, with eomysticetids a distant second, and other chaeomysticetes being barely detectable (Fig. 2). By contrast, chaeomysticetes comprise most of the New Zealand assemblage, with eomysticetids contributing roughly one quarter, and mammalodontids only 4%. This pronounced difference in composition is statistically significant (χ2 = 77.744, p <0.001), and suggests the presence of distinct regional assemblages.
Given the geographical proximity of Australia and New Zealand, the marked divergence of their late Oligocene mysticete assemblages is best explained by environmental variation, namely, exposure to the open ocean and distance from the nearest major landmass. Specifically, the small-bodied mammalodontids seemingly were restricted to nearshore settings around Australia, where heterogeneous habitats allowed them to diversify into a disparate assemblage of raptorial and suction feeders. By contrast, larger filter feeding chaeomysticetes inhabited pelagic waters, allowing them to range around and beyond the archipelago of Zealandia.
The small number of chaeomysticetes from Australia likely represent occasional visitors, or else carcasses washed in from the open sea. Their low proportion is consistent with extant cetacean stranding records, which are generally dominated by nearshore species. Thus, in the North Pacific, pelagic taxa like balaenopterids and ziphiids constitute ≤2% of local death assemblages (Pyenson 2010). Eomysticetids occurred in both coastal and offshore settings, but were more abundant in the latter (15% vs. 23%). Unlike the purely Australasian mammalodontids (but see Bianucci et al. 2011; Shipps et al. 2018), they also enjoyed a global distribution (Boessenecker and Fordyce 2017a). Eomysticetids thus may have been pelagic, albeit somewhat intermediate in terms of their geographical range and habitat preference.
There are no comparable Oligocene offshore records that would allow a similar analysis elsewhere. Nevertheless, assemblages consistent with our Australasian scenario occur worldwide. Thus, other small toothed mysticetes (aetiocetids and Coronodon) inhabited the margins of the major landmasses of Japan (Barnes et al. 1995), western North America (Barnes et al. 1995; Marx et al. 2015), and the United States East Coast (Geisler et al. 2017). Most of these localities have also yielded either eomysticetids (Okazaki 2012; Boessenecker and Fordyce 2017a; Hernández Cisneros et al. 2017), or similar species (e.g., Maiabalaena, Sitsqwayk, Tlaxcallicetus) resembling eomysticetids in their size, archaic morphology, and basal phylogenetic position (Peredo and Uhen 2016; Hernández Cisneros 2018; Peredo et al. 2018). Other, more crownward, chaeomysticetes are generally rare. We therefore suggest that marked divisions between coastal and offshore assemblages existed globally, even though the composition of each assemblage varied between ocean basins.
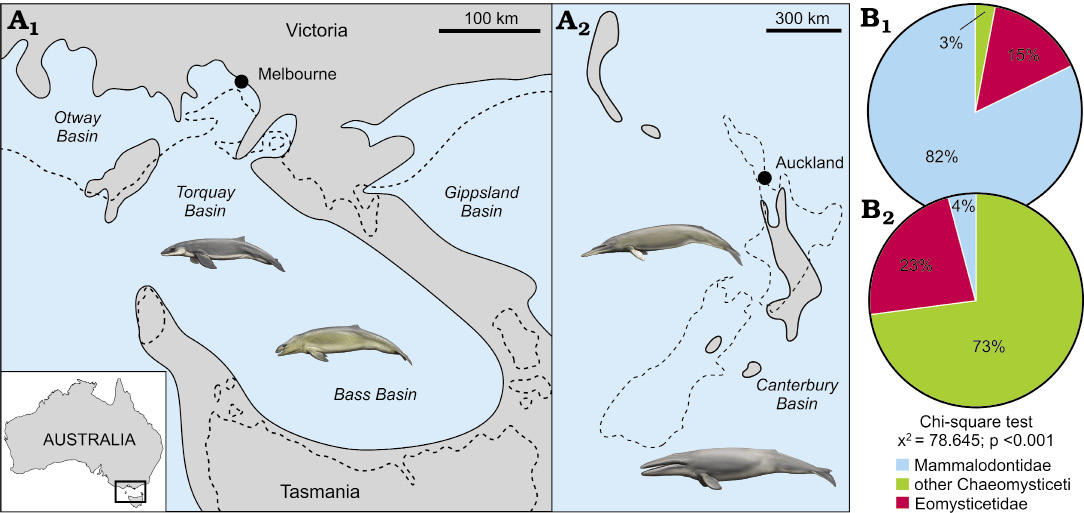
Fig. 2. Comparison of Australasian late Oligocene baleen whale assemblages: south-eastern Australia (A1, B1) and New Zealand (A2, B2). A. Late Oligocene palaeogeographical reconstruction. B. Differences in mysticete assemblage composition. Note the widespread inundation of New Zealand. Dashed lines in A delineate the modern coastline without compensating for late Neogene Alpine Fault movement. Palaeogeographical reconstructions are based on King et al. (1999), King (2000), Holdgate and Gallagher (2003). Drawings of whales by Carl Buell.
The early Miocene “dark age”.—Around the Oligocene–Miocene boundary (ca. 24–23 Ma), baleen whales seemingly vanish from the global fossil record, heralding a 5-million-year long global “dark age” in their evolution (Bianucci et al. 2018). In the few places where mysticetes do occur, such as the ca. 20 Ma Gaiman Formation (Patagonia, Argentina), only chaeomysticetes located crownward of Eomysticetidae are present (Cabrera 1926). The only exception to this pattern is a single, fragmentary eomysticetid from the earliest Miocene of New Zealand (Boessenecker and Fordyce 2017b).
Unlike mysticetes, odontocetes never disappear, which rules out taphonomy as a likely explanation for the “dark age”. Likewise, a previously suggested decrease in global diatom-based productivity (Bianucci et al. 2018) seemingly occurred too late (ca. 22–21 Ma) to have acted as a trigger (Renaudie 2016). Instead, we suggest that the demise of archaic mysticetes had an abiotic cause, such as the >0.5 shift in δ18O coinciding with the Oligocene–Miocene boundary (Zachos et al. 2008), and/or the pronounced 40 m drop in global sea level around 25–24 Ma (Miller et al. 2005) (Fig. 3). The latter seems especially significant, as it reached levels not previously experienced by any cetacean, nor subsequently seen again until the latest Miocene.
Restriction to the coast likely promoted endemism, which, along with foraging at a comparatively higher trophic level (Clementz et al. 2014), limited the population sizes of toothed mysticetes, and increased their susceptibility to environmental perturbations. Loss of shallow-water habitats in response to falling sea levels would furthermore have directly impacted coastal species, and may have been a major factor in their decline. The disappearance of the more pelagic eomysticetids is harder to explain. Their occurrence in coastal waters may hint at a somewhat limited geographical distribution, plausibly increasing their sensitivity to environmental change. Alternatively, their demise may simply have been a result of their relatively low overall abundance (15–23%).
As coastal (toothed) mysticetes declined, vacant niches were filled by a variety of diversifying odontocetes, resulting in marked faunal turnover. Both direct competition and opportunistic replacement may have contributed to this pattern, with echolocation plausibly providing odontocetes with a competitive advantage. Nevertheless, we consider opportunistic replacement to be more likely, given that (i) toothed mysticetes and odontocetes had previously existed and diversified alongside each other for some 13 million years (Marx et al. 2016b); (ii) this long period of coexistence was possible despite the early emergence of sophisticated echolocation (Geisler et al. 2014); and (iii) certain odontocete lineages, such as xenorophids, also seemingly disappeared during the “dark age”.
In light of these observations, we suggest that early Miocene environmental change equally affected both mysticetes and odontocetes, with endemic coastal assemblages being under the greatest pressure. Toothed mysticetes were the first to disappear in response, perhaps because they were fewer in number, or less ecologically versatile. By contrast, echolocating odontocetes were diverse and/or adaptable enough to weather the changes, and subsequently (re)invaded the newly vacant regions of nearshore ecospace. Unlike their coastal counterparts, pelagic mysticetes could range more widely, and thus benefitted from access to more varied habitats and scattered, but richly concentrated, prey. They persisted through the early Miocene, but, with few exceptions, are poorly represented in the mostly nearshore sedimentary record. Where they do occur, they seem to be associated with relatively open-water facies, consistent with a more pelagic habitat (Cuitiño et al. 2019).
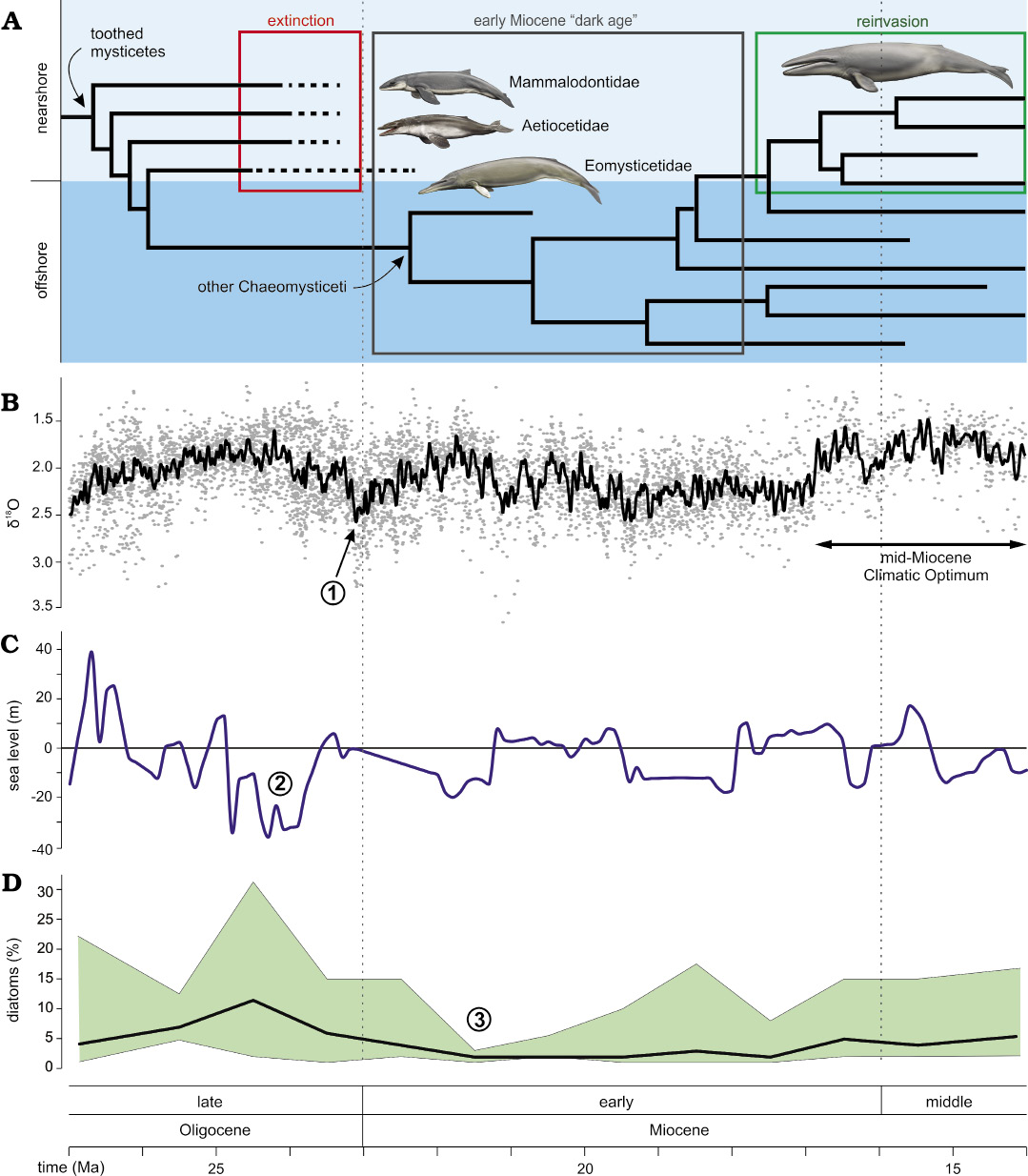
Fig. 3. Patterns and potential drivers of the early Miocene “dark age”. A. Extinction of toothed mysticetes and eomysticetids at the beginning of the early Miocene “dark age”, and subsequent reinvasion of coastal habitats by larger, toothless filter feeding mysticetes during the middle Miocene. The occurrence of eomysticetids in the earliest Miocene is based on a single, fragmentary specimen from New Zealand (Boessenecker and Fordyce 2017b). B. Global oxygen isotope curve, showing the timing of the mid-Miocene Climatic Optimum (Zachos et al. 2008). C. Global sea-level change (Miller et al. 2005). D. Global diatom abundance as derived from Deep-Sea Drilling Programme/Ocean Drilling Programme smear slides (Renaudie 2016). Highlighted events potentially relevant to the “dark age”: 1, a 0.5 shift in δ18O; 2, a 40 m sea-level fall; and 3, an early Miocene decrease in global diatom abundance. Reconstructions of mysticetes by Carl Buell.
The origin of the modern mysticete fauna.—Baleen whales spectacularly re-emerge in the fossil record close to the early–middle Miocene boundary, around 18–17 Ma (Gottfried et al. 1994; Kimura and Ozawa 2002; Otsuka and Ota 2008; Bisconti et al. 2013; Di Celma et al. 2017). The abruptness of this reappearance is best seen in locations with sequential early and middle Miocene exposures, such as the Calvert Formation of the eastern United States (Vogt and Parrish 2012; Vogt et al. 2018), and the Chilcatay–Pisco formations of Peru (Di Celma et al. 2017; DeVries and Jud 2018; Bianucci et al. 2018). In both cases, early Miocene strata virtually devoid of mysticetes are followed by middle Miocene beds in which baleen whales are abundant, or even dominant (Gottfried et al. 1994; Di Celma et al. 2017; Bianucci et al. 2018). Crucially, all middle Miocene baleen whales are toothless filter feeders, with no trace of the ecologically disparate coastal assemblages that existed during the Oligocene.
The sudden abundance of middle Miocene mysticetes might be explained by a reinvasion of coastal habitats by pelagic filter feeders, perhaps associated with the onset of the mid-Miocene Climatic Optimum (Zachos et al. 2008). Because of their offshore origins, these new arrivals were not only larger (5–8 m long) than their Oligocene predecessors, but also more uniform in their feeding style and widespread in their distribution. In particular, globally occurring taxa like Parietobalaena (Kellogg 1968; Otsuka and Ota 2008; Bisconti et al. 2013) and Pelocetus (Kellogg 1965; Kimura et al. 2007; Di Celma et al. 2017) seem to herald the onset of mysticete cosmopolitanism. Considering the profound ecological impact of extant whales (Roman et al. 2014), it is tempting to speculate that this proliferation of large filter feeders also had a lasting effect on ocean ecology, e.g., in the form of increased prey consumption (Croll et al. 2006), more numerous whale falls (Smith and Baco 2003; Pyenson and Haasl 2007), and elevated rates of vertical nutrient transfer (the “whale pump”) (Roman and McCarthy 2010).
The reinvasion of coastal habitats may have triggered a renewed diversification of mysticetes, perhaps giving rise to the speciose and relatively small-bodied cetotheriids (Gol’din 2018). Nevertheless, baleen whales never again attained the high levels of ecomorphological disparity that existed during the Oligocene (Marx and Fordyce 2015). Instead, the basic characteristics of this new cosmopolitan fauna—obligate filter feeding, comparatively large body size, and wide geographical range—remained largely constant, and continue to define mysticetes to this day. In this light, the early Miocene “dark age” represents a crucial turning point, which marks the rise of the modern mysticete fauna from the ashes of its disparate Oligocene past.
Conclusions
The evolution of baleen whales was punctuated by a major turnover event that resulted in a 5 million-year-long “dark age” in their global fossil record. This turnover event was plausibly driven by environmental change, and led to the disappearance of coastal assemblages composed of small, ecologically disparate species. Offshore assemblages dominated by larger filter feeders persisted, but remain cryptic in the fossil record owing to their remote habitat. Around the time of the mid-Miocene Climatic Optimum, pelagic species reinvaded nearshore waters, thereby establishing the modern, cosmopolitan mysticete fauna.
Acknowledgements
We thank Philip Mullaly, Brian Crichton (both Melbourne, Australia) and generations of amateur naturalists for finding and donating specimens to NMV; Alistair Evans, David Hocking (both Monash University, Melbourne, Australia), Giulia Bosio (University of Milano-Bicocca, Milan, Italy), Giovanni Bianucci (University of Pisa, Italy), and Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for helpful discussions; Carl Buell (Clifton Park, USA) for providing life reconstructions of extinct mysticetes; and G. Bianucci and an anonymous reviewer for their constructive comments. Fieldwork in Victoria by EMGF and associates with permission from: Victorian Department of Environment, Land, Water and Planning; Surf Coast Shire Council; and the Great Ocean Road Coast Committee. FGM was funded by an EU Marie Skłodowska-Curie Global Fellowship (656010/ MYSTICETI) and a postdoctoral fellowship from the Fonds de la Recherche Scientifique-FNRS (32795797). EMGF was supported by Australian Research Council Linkage Project LP150100403. REF was supported by the National Geographic Society, Washington DC (grants 3542-87, 3657-87, 4846-92, 5381-94, 6164-98).
References
Barnes, L.G. 1977. Outline of Eastern North Pacific fossil cetacean assemblages. Systematic Zoology 25: 321–343. Crossref
Barnes, L.G., Kimura, M., Furusawa, H., and Sawamura, H. 1995. Classification and distribution of Oligocene Aetiocetidae (Mammalia; Cetacea; Mysticeti) from western North America and Japan. Island Arc 3: 392–431. Crossref
Bianucci, G. and Landini, W. 2002. Change in diversity, ecological significance and biogeographical relationships of the Mediterranean Miocene toothed whale fauna. Geobios 35 (Supplement 1): 19–28. Crossref
Bianucci, G., Collareta, A., Bosio, G., Landini, W., Gariboldi, K., Gioncada, A., Lambert, O., Malinverno, E., de Muizon, C., Varas-Malca, R., Villa, I.M., Coletti, G., Urbina, M., and Di Celma, C. 2018. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeography, Palaeoclimatology, Palaeoecology 511: 256–279. Crossref
Bianucci, G., Gatt, M., Catanzariti, R., Sorbi, S., Bonavia, C.G., Curmi, R., and Varola, A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios 44: 549–585. Crossref
Bisconti, M., Lambert, O., and Bosselaers, M. 2013. Taxonomic revision of Isocetus depauwi (Mammalia, Cetacea, Mysticeti) and the phylogenetic relationships of archaic “cetothere” mysticetes. Palaeontology 56: 95–127. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2015a. Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: a new genus and species of Eomysticetidae (Mammalia: Cetacea) from the Oligocene of New Zealand. PeerJ 3: e1129. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2015b. A new eomysticetid (Mammalia: Cetacea) from the Late Oligocene of New Zealand and a re-evaluation of “Mauicetus” waitakiensis. Papers in Palaeontology 1: 107–140. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2015c. A new genus and species of eomysticetid (Cetacea: Mysticeti) and a reinterpretation of ‘Mauicetus’ lophocephalus Marples, 1956: transitional baleen whales from the upper Oligocene of New Zealand. Zoological Journal of the Linnean Society 175: 607–660. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2017a. A new eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). Journal of Systematic Palaeontology 15: 429–469. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2017b. Cosmopolitanism and Miocene survival of Eomysticetidae (Cetacea: Mysticeti) revealed by new fossils from New Zealand. New Zealand Journal of Geology and Geophysics 60: 145–157. Crossref
Cabrera, A. 1926. Cetáceos fósiles del Museo de La Plata. Revista del Museo de la Plata 29: 363–411.
Clementz, M.T., Fordyce, R.E., Peek, S.L., and Fox, D.L. 2014. Ancient marine isoscapes and isotopic evidence of bulk-feeding by Oligocene cetaceans. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 28–40. Crossref
Croll, D.A., Kudela, R., and Tershy, B.R. 2006. Ecosystem impact of the decline of large whales in the North Pacific. In: J.A. Estes, D.P. DeMaster, D.F. Doak, T.M. Williams, and R.L. Brownell (eds.), Whales, Whaling and Ocean Ecosystems, 202–214. University of California Press, Berkeley. Crossref
Crowley, B., Barnes, L.G., and Goedert, J.L. 1999. An early Miocene cetacean assemblage from Washington State, and comparisons with other West Coast assemblages. Journal of Vertebrate Paleontology 19 (Supplemant 3): 40A.
Cuitiño, J.I., Buono, M.R., Viglino, M., Farroni, N.D., and Bessone, S. 2019. Factors affecting the preservation and distribution of cetaceans in the lower Miocene Gaiman Formation of Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology [published online, https://doi.org/10.1016/j.palaeo.2019.03.013]. Crossref
Deméré, T.A., McGowen, M.R., Berta, A., and Gatesy, J. 2008. Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Systematic Biology 57: 15–37. Crossref
DeVries, T.J. and Jud, N.A. 2018. Lithofacies Patterns and Paleogeography of the Miocene Chilcatay and lower Pisco Depositional Sequences (East Pisco Basin, Peru). Boletin de la Sociedad Geologica del Peru, Volumen Jubilar 8: 124–167.
Di Celma, C., Malinverno, Elisa, Collareta, A., Bosio, G., Gariboldi, K., Lambert, O., Landini, W., Pierantonia, P.P., Gioncadac, A., Villa, I.M., Coletti, G., de Muizon, C., Urbina, M., and Bianucci, G. 2018. Facies analysis, stratigraphy and marine vertebrate assemblage of the lower Miocene Chilcatay Formation at Ullujaya (Pisco basin, Peru). Journal of Maps 14: 257–268. Crossref
Di Celma, C., Malinverno, E., Bosio, G., Collareta, A., Gariboldi, K., Gioncada, A., Molli, G., Basso, D., Varas-Malca, R., Pierantoni, P.P., Villa, I.M., Lambert, O., Landini, W., Sarti, G., Cantalamessa, G., Urbina, M., and Bianucci, G. 2017. Sequence stratigraphy and paleontology of the Upper Miocene Pisco Formation along the western side of the lower Ica Valley (Ica Desert, Peru). Rivista Italiana di Paleontologia e Stratigrafia 123: 255–274.
Fitzgerald, E.M.G. 2004. A review of the Tertiary fossil Cetacea (Mammalia) localities in Australia. Memoirs of Museum Victoria 61: 183–208. Crossref
Fitzgerald, E.M.G. 2006. A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales. Proceedings of the Royal Society B 273: 2955–2963. Crossref
Fitzgerald, E.M.G. 2010. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zoological Journal of the Linnean Society 158: 367–476. Crossref
Fordyce, R.E. 2006. A southern perpective on cetacean evolution and zoogeography. In: J. Merrick, M. Archer, G. Hickey, and M. Lee (eds.), Evolution and Biogeography of Australasian Vertebrates, 755–778. Auscipub, Oatlands.
Fordyce, R.E. and Marx, F.G. 2016. Mysticetes baring their teeth: a new fossil whale, Mammalodon hakataramea, from the Southwest Pacific. Memoirs of Museum Victoria 74: 107–116. Crossref
Fordyce, R.E. and Marx, F.G. 2018. Gigantism precedes filter feeding in baleen whale evolution. Current Biology 28: 1670–1676.e2. Crossref
Geisler, J.H., Boessenecker, R.W., Brown, M., and Beatty, B.L. 2017. The origin of filter feeding in whales. Current Biology 27: 2036–2042.e2. Crossref
Geisler, J.H., Colbert, M.W., and Carew, J.L. 2014. A new fossil species supports an early origin for toothed whale echolocation. Nature 508: 383–386. Crossref
Goedert, J.L., Barnes, L.G., and Furusawa, H. 2007. The diversity and stratigraphic distribution of cetaceans in early Cenozoic strata of Washington State, U.S.A. Geological Society of Australia Abstracts 85: 44.
Gol’din, P. 2018. New Paratethyan dwarf baleen whales mark the origin of cetotheres. PeerJ 6: e5800. Crossref
Gottfried, M.D., Bohaska, D.J., and Whitmore, F.C. 1994. Miocene cetaceans of the Chesapeake Group. Proceedings of the San Diego Society of Natural History 29: 229–238.
Graham, I.J., Morgans, H.E.G., Waghorn, D.B., Trotter, J.A., and Whitford, D.J. 2000. Strontium isotope stratigraphy of the Oligocene–Miocene Otekaike Limestone (Trig Z section) in southern New Zealand: Age of the Duntroonian/Waitakian Stage boundary. New Zealand Journal of Geology and Geophysics 43: 335–347. Crossref
Grigorescu, D. and Kazár, E. 2006. A new Middle Miocene odontocete (Mammalia: Cetacea) locality and the Sarmatian Marine Mammal Event in the Central Paratethys. Oryctos 6: 53–68.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: art. 4.
Hernández Cisneros, A.E. 2018. A new group of late Oligocene mysticetes from México. Palaeontologia Electronica 21: 7A. Crossref
Hernández Cisneros, A.E., González Barba, G., and Fordyce, R.E. 2017. Oligocene cetaceans from Baja California Sur, Mexico. Boletín de la Sociedad Geológica Mexicana 69: 149–173. Crossref
Holdgate, G.R. and Gallagher, S.J. 2003. Tertiary: a period of transition to marine basin environments. Geological Society of Australia Special Publication 23: 289–335.
Kellogg, R. 1965. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia, part 1: a new whalebone whale from the Miocene Calvert Formation. United States National Museum Bulletin 247: 1–45.
Kellogg, R. 1968. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia, part 8: supplement to the description of Parietobalaena palmeri. United States National Museum Bulletin 247: 175–197.
Kimura, T. and Ozawa, T. 2002. A new cetothere (Cetacea: Mysticeti) from the early Miocene of Japan. Journal of Vertebrate Paleontology 22: 684–702. Crossref
Kimura, T., Hasegawa, Y., Ohzawa, H., Yamaoka, T., Furukawa, Y., Ueda, T., Kiyoshi, T., Sugihara, M., and Sakuda, M. 2007. A mysticete whale (Cetacea) skeleton from the middle Miocene Bihoku Group, Shobara, Hiroshima, Japan Miscellaneous Reports of the Hiwa Museum for Natural History 48: 1–10.
King, P.R. 2000. Tectonic reconstructions of New Zealand: 40 Ma to the Present. New Zealand Journal of Geology and Geophysics 43: 611–638. Crossref
King, P.R., Naish, T.R., Browne, G.H., Field, B.D., and Edbrooke, S.W. 1999. Cretaceous to Recent sedimentary patterns in New Zealand. Institute of Geological & Nuclear Sciences Folio Series 1: 1–35.
Korasidis, V.A., Wallace, M.W., Wagstaff, B.E., Gallagher, S.J., McCaffrey, J.C., Allan, T., Rastogi, S., and Fletcher, M.-S. 2018. New age controls on Oligocene and Miocene sediments in southeastern Australia. Review of Palaeobotany and Palynology 256: 20–31. Crossref
Lambert, O., Martínez-Cáceres, M., Bianucci, G., Di Celma, C., Salas-Gismondi, R., Steurbaut, E., Urbina, M., and de Muizon, C. 2017. Earliest mysticete from the Late Eocene of Peru sheds new light on the origin of baleen whales. Current Biology 27: 1535–1541.e2. Crossref
Marx, F.G. and Fordyce, R.E. 2015. Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. Royal Society Open Science 2: 140434. Crossref
Marx, F.G., Hocking, D.P., Park, T., Ziegler, T., Evans, A.R., and Fitzgerald, E.M.G. 2016a. Suction feeding preceded filtering in baleen whale evolution. Memoirs of Museum Victoria 75: 71–82. Crossref
Marx, F.G., Lambert, O., and Uhen, M.D. 2016b. Cetacean Paleobiology. 319 pp. Wiley Blackwell, Chichester. Crossref
Marx, F.G., Tsai, C.-H., and Fordyce, R.E. 2015. A new Early Oligocene toothed “baleen” whale (Mysticeti: Aetiocetidae) from western North America—one of the oldest and the smallest. Royal Society Open Science 2: 150476. Crossref
McLaren, S., Wallace, M.W., Gallagher, S.J., Dickinson, J.A., and McAllister, A. 2009. Age constraints on Oligocene sedimentation in the Torquay Basin, southeastern Australia. Australian Journal of Earth Sciences 56: 595–604. Crossref
Miller, K.G., Kominz, M.A., Browning, J.V., Wright, J.D., Mountain, G.S., Katz, M.E., Sugarman, P.J., Cramer, B.S., Christie-Blick, N., and Pekar, S.F. 2005. The Phanerozoic record of global sea-level change. Science 310: 1293–1298. Crossref
Okazaki, Y. 2012. A new mysticete from the upper Oligocene Ashiya Group, Kyushu, Japan and its significance to mysticete evolution. Bulletin of the Kitakyushu Museum of Natural History and Human History, Series A (Natural History) 10: 129–152.
Otsuka, H. and Ota, Y. 2008. Cetotheres from the early Middle Miocene Bihoku Group in Shobara District, Hiroshima Prefecture, West Japan. Miscellaneous Reports of the Hiwa Museum for Natural History 49: 1–66.
Peredo, C.M. and Uhen, M.D. 2016. A new basal chaeomysticete (Mammalia: Cetacea) from the Late Oligocene Pysht Formation of Washington, USA. Papers in Palaeontology 2: 533–554. Crossref
Peredo, C.M., Pyenson, N.D., Marshall, C.D., and Uhen, M.D. 2018. Tooth loss precedes the origin of baleen in whales. Current Biology 28: 3992–4000.e2. Crossref
Pilleri, G. 1986a. The Cetacea of the Western Paratethys (Upper Marine Molasse of Baltringen). 70 pp. Gehirnanatomisches Institut, Universität Bern, Ostermundingen.
Pilleri, G. 1986b. The Denticeti of the Western Paratethys (Upper Marine Molasse of Switzerland). Investigations on Cetacea 19: 11–114.
Piper, K.J., Fitzgerald, E.M.G., and Rich, T.H. 2006. Mesozoic to early Quaternary mammal faunas of Victoria, south-east Australia. Palaeontology 49: 1237–1262. Crossref
Pivorunas, A. 1979. The feeding mechanisms of baleen whales. American Scientist 67: 432–440.
Pyenson, N.D. 2010. Carcasses on the coastline: measuring the ecological fidelity of the cetacean stranding record in the eastern North Pacific Ocean. Paleobiology 36: 453–480. Crossref
Pyenson, N.D. and Haasl, D.M. 2007. Miocene whale-fall from California demonstrates that cetacean size did not determine the evolution of modern whale-fall communities. Biology Letters 3: 709–711. Crossref
Renaudie, J. 2016. Quantifying the Cenozoic marine diatom deposition history: links to the C and Si cycles. Biogeosciences 13: 6003–6014. Crossref
Roman, J. and McCarthy, J.J. 2010. The whale pump: marine mammals enhance primary productivity in a coastal basin. PLOS ONE 5: e13255. Crossref
Roman, J., Estes, J.A., Morissette, L., Smith, C., Costa, D., McCarthy, J., Nation, J.B., Nicol, S., Pershing, A., and Smetacek, V. 2014. Whales as marine ecosystem engineers. Frontiers in Ecology and the Environment 12: 377–385. Crossref
Shipps, B.K., Peredo, C.M., and Pyenson, N.D. 2018. An unexpected northerner with burrowed bones: a new mammalodontid (Mysticeti) from the Pacific Northwest with Osedax bores provides insight into Oligocene marine taphonomy and mysticete evolution. Journal of Vertebrate Paleontology: Program and Abstracts, 215.
Slater, G.J., Goldbogen, J.A., and Pyenson, N.D. 2017. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proceedings of the Royal Society B: Biological Sciences 284: 20170546. Crossref
Smith, C.R. and Baco, A.R. 2003. Ecology of whale falls at the deep-sea floor. Oceanography and Marine Biology: an Annual Review 41: 311–354. Crossref
Tanaka, Y. and Fordyce, R.E. 2016. Awamokoa tokarahi, a new basal dolphin in the Platanistoidea (late Oligocene, New Zealand). Journal of Systematic Palaeontology 15: 365–386. Crossref
Thompson, N.K., Bassett, K.N., and Reid, C.M. 2014. The effect of volcanism on cool-water carbonate facies during maximum inundation of Zealandia in the Waitaki-Oamaru region. New Zealand Journal of Geology and Geophysics 57: 149–169. Crossref
Tsai, C.-H. and Fordyce, R.E. 2015. The earliest gulp-feeding mysticete (Cetacea: Mysticeti) from the Oligocene of New Zealand. Journal of Mammalian Evolution 22: 535–560. Crossref
Tsai, C.-H. and Fordyce, R.E. 2016. Archaic baleen whale from the Kokoamu Greensand: earbones distinguish a new late Oligocene mysticete (Cetacea: Mysticeti) from New Zealand. Journal of the Royal Society of New Zealand 46: 117–138. Crossref
Tsai, C.-H. and Fordyce, R.E. 2018. A new archaic baleen whale Toipahautea waitaki (early Late Oligocene, New Zealand) and the origins of crown Mysticeti. Royal Society Open Science 5: 172453. Crossref
Tsai, C.-H. and Kohno, N. 2016. Multiple origins of gigantism in stem baleen whales. The Science of Nature 103: 89. Crossref
Vogt, P.R., Eshelman, R.E., and Godfrey, S.J. 2018. Calvert Cliffs: eroding mural escarpment, fossil dispensary, and paleoenvironmental archive in space and time. Smithsonian Contributions to Paleobiology 100: 3–44.
Vogt, P.R. and Parrish, M. 2012. Driftwood dropstones in Middle Miocene Climate Optimum shallow marine strata (Calvert Cliffs, Maryland Coastal Plain): Erratic pebbles no certain proxy for cold climate. Palaeogeography, Palaeoclimatology, Palaeoecology 323–325: 100–109. Crossref
Whitmore, F.C., Jr. and Kaltenbach, J.A. 2008. Neogene Cetacea of the Lee Creek Phosphate Mine, North Carolina. Virginia Museum of Natural History Special Publication 14: 181–269.
Zachos, J.C., Dickens, G.R., and Zeebe, R.E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. Crossref
Acta Palaeontol. Pol. 64 (2): 231–238, 2019
https://doi.org/10.4202/app.00575.2018