

Clonal colony in the Early Devonian cnidarian Sphenothallus from Brazil
HEYO VAN ITEN, JULIANA DE MORAES LEME, MARCELLO G. SIMÕES, and MARIO COURNOYER
Van Iten, H., Leme, J.M., Simões, M.G., and Cournoyer, M. 2019. Clonal colony in the Early Devonian cnidarian Sphenothallus from Brazil. Acta Palaeontologica Polonica 64 (2): 409–416.
The fossil record of polypoid cnidarians includes a number of taxa that were incorrectly identified as either tubiculous worms or plants. The holotype of the putative alga Euzebiola clarkei (Ponta Grossa Formation, Lower Devonian, Brazil), originally described under the name Serpulites sica, is re-described and re-figured as a species of Sphenothallus, a medusozoan cnidarian. Unlike Sphenothallus from other localities, the black, organic-walled Ponta Grossa specimen consists of a single parent tube that is confluent with the apical ends of at least 18 daughter tubes. The pattern of arrangement of the daughter tubes, which are arrayed in single file along the exposed face and the two thickened margins of the parent tube, partly resembles the whorl-like pattern of arrangement of colonial polyps of certain scyphozoan cnidarians. For these reasons, the Ponta Grossa Formation material figures prominently in the argument that Sphenothallus was a medusozoan cnidarian capable (in at least one species) of clonal budding.
Key words: Cnidaria, Medusozoa, Scyphozoa, Hydrozoa, clonal budding, Devonian, Brazil.
Heyo Van Iten [vaniten@hanover.edu], Department of Geology, Hanover College, Hanover, IN 47243, USA and Research Associate, Cincinnati Museum Center, Department of Invertebrate Paleontology, 1301 Western Avenue, Cincinnati, OH 45203, USA.
Juliana de Moraes Leme [jleme.usp@gmail.com], Department of Sedimentary and Environmental Geology, University of São Paulo, 05508-080, SP, Brazil.
Marcello G. Simões [profmgsimoes@gmail.com], Department of Zoology, São Paulo State University, Botucatu campus, 18618-689, Botucatu, SP, Brazil.
Mario Cournoyer [paleovision@videotron.ca], Musée de Paléontologie et de l’Évolution, 541 Congrégation Street, Montréal, Québec, Canada H3K 2J1.
Received 28 November 2018, accepted 4 February 2019, available online 3 April 2019.
Copyright © 2019 H. Van Iten et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Previous reports extending the known stratigraphical and paleogeographical ranges of the Paleozoic cnidarian Sphenothallus Hall, 1847 have increased the paleobiological significance of this extinct genus substantially (see Chang et al. 2018; Landing et al. 2018 and references cited therein). In addition to occurring in both oxic nearshore and dysoxic offshore facies (e.g., Van Iten et al. 1996; Chang et al. 2018), in such widely separated Cambrian and Ordovician terranes as Laurentia (e.g., Van Iten et al. 1996, 2002), South China (e.g., Zhu et al. 2000; Li et al. 2004; Peng et al. 2005; Van Iten et al. 2013; Muscente and Xiao 2015; Chang et al. 2018) and South Polar Gondwana (Fatka et al. 2012; Fatka and Kraft 2013; Van Iten et al. 2016b), Sphenothallus ranges downward into Cambrian Stage 3 (e.g., Chang et al. 2018), which hosts part of the record of the Cambrian Explosion (Yang et al. 2014). Together with the conulariid Paraconularia sp. from the latest Ediacaran Tamengo Formation of Brazil (Van Iten et al. 2014, 2016a; Parry et al. 2017), Sphenothallus from Cambrian Stage 3 are among the oldest, polypoid medusozoan cnidarians in the fossil record (Van Iten et al. 2014; Landing et al. 2018).
Van Iten et al. (1992) first documented a suite of anatomical similarities uniquely shared by Sphenothallus, conulariids and extant medusozoans, noting as well that similarities shared by Sphenothallus and various non-cnidarian groups are also shared with conulariids and extant medusozoans. The similarities thought to support assignment of both Sphenothallus and conulariids to the subphylum Medusozoa Peterson, 1979 include: (i) soft body fully covered by a tubular or steeply pyramidal, very finely lamellar, organic or organo-phosphatic periderm; (ii) periderm terminates aborally in a sub-conical expansion that (in some cases) is closed and floored by a very thin basal membrane; (iii) periderm exhibits (rarely) a transverse internal wall (schott) that extends adorally along the inner surface of the periderm; and (iv) soft body includes two or more oral tentacles. Most recently, Dzik et al. (2017) documented the presence in S. ruedemanni (Lower Ordovician, China) of eight radially arranged, seriated internal carinae similar to those present in the periderm of certain coronate polyps but not known to occur in any other taxa except several species of conulariids (e.g., Van Iten et al. 1996).
Another similarity, shared not only by Sphenothallus and extant medusozoans but also other invertebrates such as graptolites and pterobranchs, is the capacity for asexual budding. In support of their argument that at least some Sphenothallus formed clonal colonies, Van Iten et al. (1992: figs. 3, 4) presented two pieces of evidence: (i) a branching specimen of Sphenothallus sp. from the Mississippian Bear Gulch Formation (Montana, USA); and (ii) Clarke’s (1913: pl. 26: 16–18) shaded line drawings of a branching specimen of Serpulites sica Salter, 1856 from the Lower Devonian Ponta Grossa Formation of southern Paraná State, Brazil. Clarke’s (1913) drawings depict what he interpreted as a single parent tube bearing at least 16 daughter tubes arrayed along the two margins of the parent, with the apical end of the daughter tubes apparently lacking the basal holdfast and, instead, merging smoothly into the parent tube. Clarke (1913: 319) classified this specimen as a “problematum.” Later, Sommer (1954) reexamined Clarke’s fossil, referring it to the new algal genus and species Euzebiola clarkei. Interestingly, prior to 1913 the same specimen had been inspected by a paleobotanist, who declared that is was “positively not a plant but possibly a hydroid (Clarke 1913: 319).” Moreover, Sommer (1954: 178) characterized his assignment of Clarke’s (1913) specimen to the plant kingdom as merely a “taxonomic adventure”. Most recently, both this and the Bear Gulch Formation specimen were discussed by Landing et al. (2018) in their review paper on the early evolution of coloniality in eumetazoans. These authors added a third specimen, originally assigned to the genus Torellella Linnarsson, 1871 (Popov et al. 1989), to the list of known or probable specimens of Sphenothallus showing evidence of coloniality.
Importantly, Van Iten et al. (1992) stated that the current whereabouts of Clarke’s (1913) branching specimen were unknown. We here re-describe and re-illustrate Clarke’s (1913) material, highlighting places on the branching specimen where diagnostic features of the genus Sphenothallus and evidence of clonal budding are best preserved. We also argue that both the branching specimen and similar, unbranched tubes from the Ponta Grossa Formation belong in the genus Sphenothallus.
Material and methods
Previously illustrated fossil specimens assigned by Clarke (1913) to Serpulites sica occur on two cm-long slabs of massive siltstone that are housed in the paleontological collection of the National Mineral Agency (formerly the Department of Mineral Production, DNPM) in Rio de Janeiro, southeastern Brazil, under catalogue numbers DNPM 329 (the branching specimen; Clarke 1913: pl. 26: 16–18; Sommer 1954: pl. 15: 2) and DNPM 1 (five partial unbranched tubes; Clarke 1913: pl. 26: 15). Specific locality information is not provided in Clarke’s (1913) monograph and does not accompany the reposited specimens. Thus, locating precisely the sites that yielded these specimens is almost impossible. Nevertheless, information available in Clarke (1913: 62–67) indicates that his material comes from the Ponta Grossa Formation (Lower Devonian) near the city of Ponta Grossa (Ponta Grossa County, Paraná State, southern Brazil). We examined Clarke’s (1913) material under reflected light, using a Leitz binocular microscope equipped with a digital camera. Unfortunately, Clarke’s (1913) specimens are now coated with a clear lacquer-like substance, and this fact, together with the size of the host slabs, prevented us from ascertaining the elemental composition of the specimens through x-ray microbeam analysis or any other techniques. Finally, comparison specimens of Sphenothallus sp. from the Ordovician of Quebec, illustrated here (Fig. 6), are housed in the Museum of Paleontology and Evolution, Montreal, Quebec, Canada, under registration prefix MPEP.
Institutional abbreviation.—DPNM, National Department of Mineral Production, Rio de Janeiro, RJ, southeastern Brazil.
Geological setting
The pre-Carboniferous (Silurian–Devonian) rocks of Ponta Grossa County occur in the Apucarana sub-basin of the Paraná Basin (Figs. 1, 2), where they have been assigned collectively to the Silurian–Devonian Furnas Formation (Llandoverian–Lochkovian; Sedorko et al. 2017) and the Devonian Ponta Grossa Formation (Petri 1948) of the Paraná Group (Fig. 2). The sedimentology, stratigraphy and paleontology of these units have been discussed in detail elsewhere (see Assine and Simões 2018 and references therein for a recent review). The contact between the two formations is transitional, with strata above and below the contact (= transitional beds of Petri 1948) consisting of fine-grained sandstone with hummocky cross-stratification and wavy cross-lamination that was originally deposited in lower shoreface settings (Assine et al. 1994; Assine and Simões 2018; see however Bergamaschi 1999 for a different interpretation). Traditionally, the Ponta Grossa Formation has been subdivided into three members (Fig. 2), namely (in ascending order) the Pragian–Emsian Jaguariaíva Member, the Eifelian Tibagi Member, and the Givetian São Domingos Member (Lange and Petri 1967). Although these members can be traced laterally, both at the surface and in the subsurface, for hundreds of kilometers (Assine 1996), the thickness of the Ponta Grossa Formation is highly variable across the basin owing to the Late Devonian–Early Carboniferous regional discordance (Assine et al. 1994).
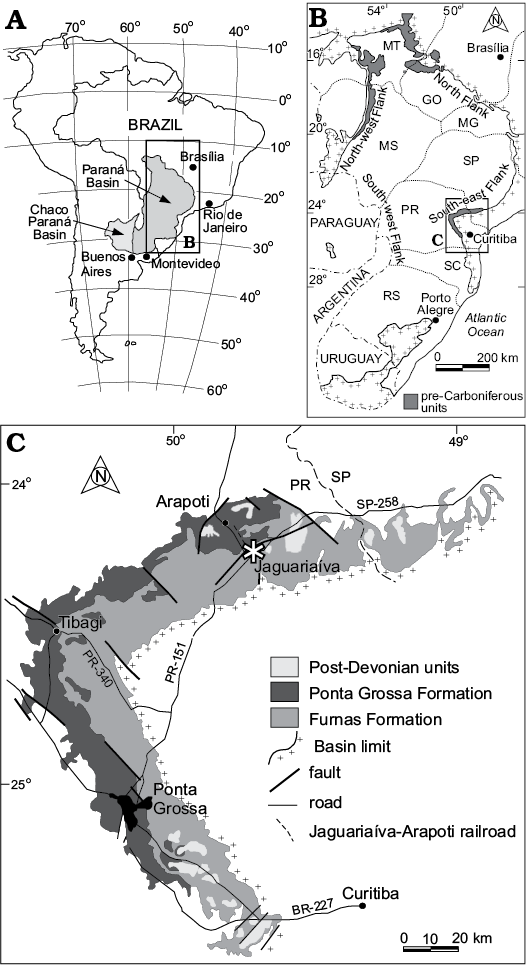
Fig. 1. A. Geographic location of the relevant geological sections within Paraná State, Brazil. B. Map showing the outcrop belt of the Lower Devonian Ponta Grossa Formation on the eastern margin of the Paraná Basin (from Simões et al. 2009). C. Geological map of the study area with Jaguariaíva asterisked.
The sedimentary succession of the Jaguariaíva and São Domingos members consists of marine shale, while fine-grained sandstone with hummocky cross-stratification forms the Tibagi Member. Shale of the Jaguariaíva and São Domingos members contain two maximum flooding surfaces (Assine et al. 1994; Bergamaschi 1999), both of which are tied to the global Devonian sea-level high (Assine et al. 1994). Tectonic reactivation of source areas on the northeastern border of the Paraná Basin was accompanied by the northeastward advance of deltaic sandstone of the Tibagi Member, recording a brief progradational regression between two transgressive successions (Assine et al. 1994, 1998; Assine 1996; Assine and Simões 2018).
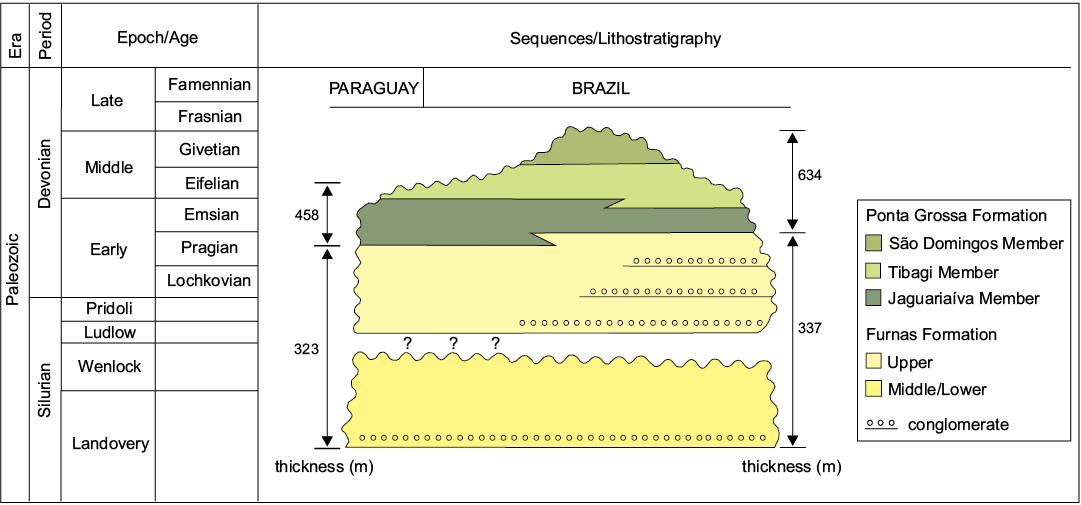
Fig. 2. Stratigraphic chart of the Silurian–Devonian interval in the Paraná Basin (modified from Assine et al. 1994; Sedorko et al. 2017). The exact level in the Jaguariaíva Member at which Clarke’s (1913) specimens of Sphenothallus were found is not known.
In the vicinity of Ponta Grossa (Fig. 3), the Ponta Grossa Formation consists of ~80 meters of highly fossiliferous shale and concretion-bearing siltstone of the Jaguariaíva Member (Fig. 3), which was deposited in shoreface and shallow marine platform settings (Bergamaschi 1999), mainly near or below storm wave base (Simões et al. 2000). Therefore, Clarke’s (1913) material most likely comes from the Jaguariaíva Member, which is composed in part of siltstone similar in color and texture to the rock matrix hosting his specimens.
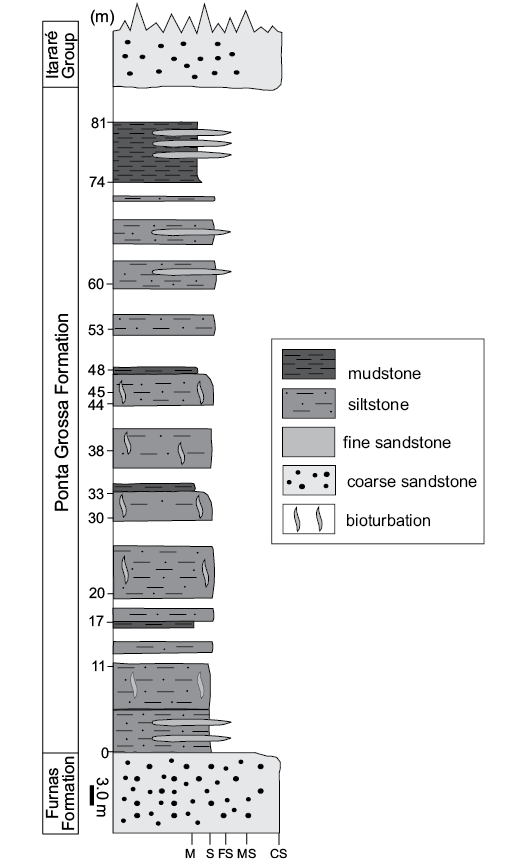
Fig. 3. Measured stratigraphical column of the Ponta Grossa Formation, Jaguariaíva section (from Simões et al. 2009). Abbreviations: M, mudstone, S, siltstone, FS, fine sandstone, MS, medium sandstone, CS, coarse sandstone.
Systematic palaeontology
Phylum Cnidaria Verrill, 1865
Subphylum Medusozoa Peterson, 1979
Class, order, and family uncertain
Genus Sphenothallus Hall, 1847
Type species: Sphenothallus angustifolius Hall, 1847, New York, Middle Ordovician.
Remarks.—Specimens belonging in the genus Sphenothallus generally consist of a single, slender, gently tapered hollow tube constructed of numerous, very thin (approximately 1–2 μm) lamellae that parallel the exterior surface of the tube (e.g., Van Iten et al. 1992, 2002; Muscente and Xiao 2015; Vinn and Kirsimäe 2015). Most specimens contain substantial amounts of the mineral apatite, but others are largely or entirely organic (see Muscente and Xiao 2015 and references cited therein). In transverse cross-sections the tube is sub-elliptical (except near the apical end, where it is more or less circular), with the total thickness of the tube increasing to its maximum at the ends points of the tube’s greatest diameter, thus forming two continuous, marginal thickenings having a crescent-shaped transverse cross-section. The relatively thin, frequently crumpled walls between the two thickenings are referred to here as faces (though without implying homology with the faces of conulariids). The pair of marginal thickenings, which owing to compaction are readily discernable as raised, levee-like berms or trough-like external molds, partially distinguishes Sphenothallus from all other tubular fossils such as Byronia Matthew, 1899, Torellela Linnarson, 1871 and Annulitubus Vinn, Zabini, Sene-Silva, Kirsimäe, and Susan-Marcos, 2016 (Zhu et al. 2000; Vinn et al. 2016; Landing et al. 2018). The wide or oral end of the tube is open, and the oral margin of each face is gently curved. The narrow, aboral (apical) end of the tube exhibits a small, sub-conical expansion floored by a thin membrane cemented in life to hard substrates including hardgrounds (Bodenbender et al. 1989) and shell material. Finally, the soft body of Sphenothallus, preserved in pyrite in certain specimens from the Lower Devonian Hunsrück Slate, Germany (Fauchald et al. 1986), includes a manubrium-like central mouth structure flanked by a pair of elongate tentacles situated at the end points of the greatest diameter of the tube.
Sphenothallus sica (Salter, 1856)
Figs. 4, 5.
1856 Serpulites sica; Salter 1856: 222, pl. 25, 19.
1913 Serpulites sica Salter, 1856; Clarke 1913: 85–86, pl. 26: 15–18.
1954 Euzebiola clarkei; Sommer 1954: 178, pl. 15: 2.
1992 Sphenothallus sica (Salter); Van Iten et al. 1992: fig. 3.
Material.—DPNM 329, the branching specimen (Figs. 4, 5). DPNM 1, association of five partial tubes; from the Jaguariaíva Member (Pragian–Emsian) of the Lower Devonian Ponta Grossa Formation near Ponta Grossa, Paraná State, Brazil.
Description.—Periderm fragments measuring up to about 145 mm long (DPNM 1) and a partial branching periderm measuring about 55 mm long and 32 mm wide (DPNM 329). Branching periderm consists of a single, very gently tapered parent tube (labelled “P” in Fig. 4A, B1) measuring about 40 mm long and bearing at least 16 variably curved daughter tubes (Fig. 4: 1–16); two of the daughter tubes (1, 2) originate at the wide of the parent, while the remaining daughter tubes (3–16) are arrayed in single file along each of the two lateral margins of the parent, in a manner resembling opposite or sub-opposite branching in plant stems. Additional tube fragments, at least some of which probably belong to certain of the numbered daughter tubes, are also present. Guyot-like basal portion of at least two additional daughter tubes (Fig. 5A1) present on the exposed face of the parent tube near its wide end. Periderm black, apparently organic and possibly laminated, in transverse cross-sections sub-elliptical. Periderm of the daughters confluent with that of the parent, the apical end of which has been truncated. Gently curved apertural margin may be preserved at the distal end of three of the daughter tubes (1–3). Marginal thickenings preserved as narrow, levee-like berms or as trough-like external molds. Internal schotts and basal attachment discs not observed.
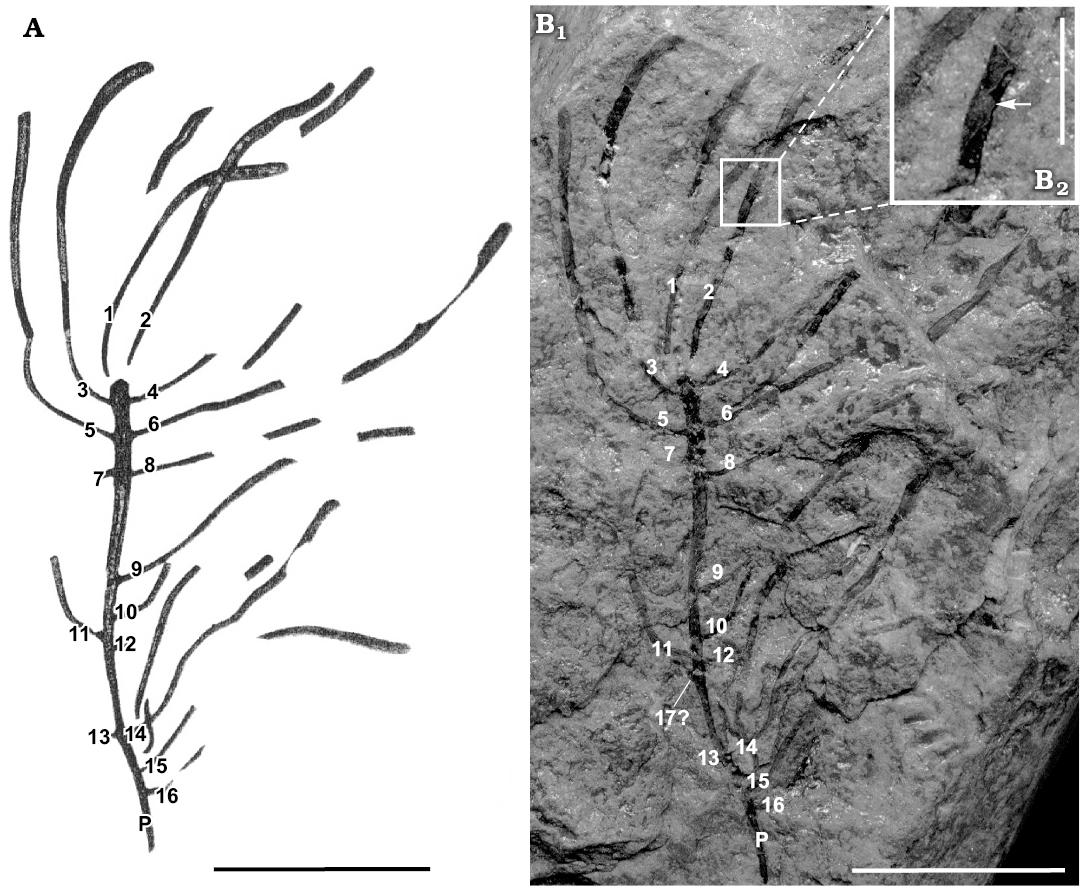
Fig. 4. Medusozoan cnidarian Sphenothallus sica Salter, 1856 (DNPM 329) from the Early Devonian Ponta Grossa Formation, Paraná State, southern Brazil. The numbers 1–16 indicate the evident, marginal daughter tubes, while the letter P indicates the parent tube. A. Reproduction of Clarke’s (1913) drawing. B. Light photographs. B1, general view; 17?, the possible basal portion of a seventeenth daughter tube; B2, detail, the arrow indicates a short longitudinal cross section through one of the marginal thickenings. Also present, near this site, is apparent spalling of fine lamellae. Scale bars: A, B1, 16 mm; C, 4 mm.
Remarks.—In agreement with the present description of DPNM 1 and DPNM 329, Clarke (1913: 85–86) states that these specimens consist of “chitinous [organic] tubes which may in their original state have been flat, as they are always reinforced by two chitinous cords at the margins, the connecting tissue being relatively thin.” Clarke (1913: 320) further states that the “cords are often found alone, the more tenuous parts having been torn away.” Re-examination of Clarke’s (1913) material (Figs. 4, 5) confirms most of these statements. Thus, inspection of broken ends and missing portions of the exposed face (e.g., Figs. 4B2, 5A1) shows that the tubes are hollow, with the former cavity now being filled with rock matrix, and that the transverse cross-section of the tubes is sub-elliptical. The “cords” noted by Clarke (1913) appear on external molds as a pair of shallow troughs, and in places where skeletal material of the exposed face is preserved, they are expressed as one or two low, narrow, levee-like berms (Fig. 5A3). This preservational feature likely developed during compaction when the more resistant marginal thickenings retained their relief in contrast to the thinner and more readily deformable faces. Moreover, in some of the places (e.g., Fig. 4B2) where the tube has been broken and is thus visible in cross-sections, the marginal portion of the tube is noticeably thicker than across the faces. Finally, Salter (1856: 222) stated that material from the Devonian Bokkeveld Group of South Africa exhibited “striae of growth” that “are conspicuous and oblique”; however, and as previously suggested by Clarke (1913: 86), we think that these features may actually be secondary wrinkles.

Fig. 5. Medusozoan cnidarian Sphenothallus sica Salter, 1856 (DNPM 329) from the Early Devonian Ponta Grossa Formation, Paraná State, southern Brazil. A1, marginal daughter tubes 5 and 6 (black arrows) and the isolated guyot-like feature (white arrow) between them; A2, side view of the guyot-like basal portion of marginal daughter tube 4 (arrow); A3, detail of daughter tubes 6 and 8, arrows indicate places where compaction has caused the intact marginal thickenings to appear as narrow, levee-like berms. Scale bars: A1, A2, 500 µm; A3, 1 mm.
Present near the wide and, presumably, youngest end of the parent tube of DPNM 329 are at least two, guyot-like protuberances that occur between the raised margins, in the middle of the thin exposed face (Fig. 5A1). Clarke (1913: pl. 26: 18) shows two additional guyot-like structures, both located on the exposed face between daughter tubes 3 and 4, but we did not see such features on that part of the parent tube. One of the guyot-like features we did see is situated between daughter tubes 5 and 6 (Fig. 5A1), while the second such structure occurs between tubes 7 and 8. Again in agreement with Clarke (1913), we suspect that a fourth, similar feature occurs at these same two levels on the still covered, opposite face of the parent tube. If this hypothesis is true, then at least some of the daughter tubes are arranged in whorls or “verticil[s]” (Clarke 1913: 319) consisting of four iso-latitudinal daughters. All of the tubes are incomplete, and some of the daughter tubes (7, 12 and 13) consist only of the basalmost portion. The narrow apical end of the parent tube is truncated, and there is no evidence of a holdfast or stolon. The free wide end of daughter tubes 1–3 is gently curved and approximately perpendicular to the lateral margins; thus, these three tubes may preserve the apertural margin. Unfortunately, the counterpart of DPNM 329 either was not collected or is missing, and as noted above the entire specimen is coated with a transparent lacquer-like substance that cannot be removed without destroying the specimen. Most of the tubes, including the parent, consist of short stretches of complete periderm (preserving the marginal thickenings and, probably, both of the two thin walls between them) alternating with stretches missing the exposed face (presumably present on the missing counterpart specimen) or consisting of an external mold (e.g., Fig. 4B2). Daughter tubes 1–8 and 12–13 are arranged in opposition or near opposition, with three of these pairs (comprising daughters 3–8) being separated from each other by approximately 3 mm. Furthermore, daughter tubes 1 and 2 originate at the wide end of the parent, which thus resembles a plant stem exhibiting dichotomous branching at its tip. The external surface of the expanded apical end of daughter tubes that preserve black peridermal material is confluent with the external surface of black peridermal material of the parent tube (Fig. 5A1, A2). Nowhere on the parent tube is there any evidence of isolated basal attachment discs such as those exhibited by fossils bearing epibiontic specimens of Sphenothallus (Fig. 6).
Stratigraphic and geographic range.—Early Devonian Ponta Grossa Formation, Paraná State, southern Brazil.
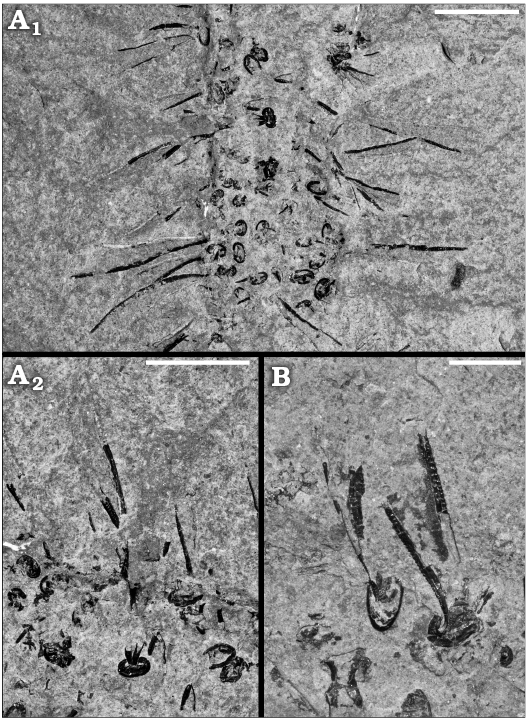
Fig. 6. Medusozoan cnidarian Sphenothallus sp. on a possible orthoconic cephalopod; from the Upper Ordovician (Katian 1–2) Utica Shale, Cap Santé, Québec, Canada. Light photographs of epibiontic tubes. A. MPEP1144.1, A2 detail of A1. B. MPEP1144.2. Scale bars A1, 5 mm; A2, B, 3 mm.
Discussion
Regarding branching specimen DPNM 329, Clarke (1913: 319–320) rejected the hypothesis of affinity with graptolites or plants, noting instead that the specimen “has features suggestive of hydroid structure.” Admittedly, certain macroscopic algae, for example the extant green alga Caulerpa sertularioides (Gmelin, 1768) Howe, 1905 and the extinct genus Callithamnopsis Ruedemann, 1909, resemble Clarke’s (1913) branching S. sica specimen in showing opposite and/or sub-opposite branching (C. sertularioides), or arrangement of branches in whorls (Callithamnopsis) (see for example Eubank 1946). However, more detailed comparisons show that these similarities probably are superficial. In particular, no known macroscopic alga consists of branches that are hollow and open at their tips, or that exhibit a pair of marginal thickenings. In contrast, the hypothesis that DPNM 329 is a colonial hydrozoan polyp is supported by similarities in gross morphology with species such as Eudendrium ramosum Linnaeus, 1758, colonies of which exhibit multiple branches bearing a single opening located at the distal end of the tube (see for example Marques et al. 2000). DPNM 329 also resembles the extant scyphozoan Stephanoscyphus racemosus Komai, the polyps of which exhibit a whorl-like arrangement, though with each “whorl” arising from a member of the preceding (ontogenetically) “whorl” (see for example Werner 1979: fig. 5b).
The partial tubes arrayed along the edges and the exposed face of the parent tube of DPNM 329 are not epibionts, but rather are best interpreted as clonal buds originally connected to the soft body of the specimen along which they are arrayed. We base this conclusion on three independent lines of evidence (see also Van Iten et al. 1992). First, the outer surface of the daughter tubes is continuous with the outer surface of the parent tube. Second, the parent tube lacks isolated basal attachment discs, which normally are present on fossils encrusted by multiple Sphenothallus (Fig. 6). Third, unlike more or less randomly arranged, epibiontic Sphenothallus specimens (Fig. 6), the daughter tubes in the S. sica specimen exhibit a regular, in most cases opposite or sub-opposite arrangement, including evident dichotomous branching at the wide end of the parent tube.
Conclusions
The ability of at least one species of Sphenothallus to produce clonal colonies constitutes yet another similarity shared with colonial hydrozoan and scyphozoan polyps. Although other invertebrates, for example pterobranchs, also produce clonal colonies, none of these non-cnidarian taxa shares with Sphenothallus the full suite of similarities enumerated above. Among currently known specimens of Sphenothallus, only Clarke’s (1913) branching specimen from the Ponta Grossa Formation, the branching specimen from the Bear Gulch Formation (Van Iten et al. 1992), and (apparently) the specimen noted by Landing et al. (2018), show compelling evidence of clonal budding. All of these specimens are thus of great importance to understanding an extinct genus of special interest to studies of the early diversification of Kingdom Metazoa and the origins of coloniality.
Acknowledgments
The authors are indebted to Rita de Cássia Tardin Cassab of the Geological Survey of Brazil (CPRM) in Rio de Janeiro for granting access to Clarke’s original fossil collection. Corrections and constructive comments by reviewers Petr Kraft (Charles University, Czech Republic) and Olev Vinn (University of Tartu, Estonia) are greatly appreciated. Financial support for this research was provided by a grant from the Hanover College Faculty Development Committee to HVI, and by grants (99/10823-5, 99/10824-1, 00/14903-2, 00/14904-9, and 01/12835-2) from the FAPESP (The State of São Paulo Research Foundation) and the CNPq (301023/94-8) to MGS.
References
Assine, M.L. 1996. Aspectos da estratigrafia das sequências pré-carboníferas da Bacia do Paraná no Brasil. 207 pp. Ph.D. Thesis, Universidade de São Paulo, Instituto de Geociências, São Paulo.
Assine, M.L. and Simões, M.G. 2018. Revisitando o devoniano paranaense. In: R. Machado, M.C. Moraes, and A. Bartorelli (eds.), Setembrino Petri, do Proterozoico ao Holoceno, 118–149. Sociedade Brasileira de Geologia, São Paulo.
Assine, M.L., Perinotto, J.A.J., Fúlfaro, V.J., and Petri, S. 1998. Progradação deltaica Tibagi no Devoniano Médio da Bacia do Paraná. Revista Brasileira de Geociências 28: 125–134. Crossref
Assine, M.L., Soares, P.C., and Milani, E.J. 1994. Sequências tectono-sedimentares mesopaleozóicas da Bacia do Paraná, sul do Brasil. Revista Brasileira de Geociências 24: 77–89. Crossref
Bergamaschi, S. 1999. Análise estratigráfica do Siluro-Devoniano (Formação Furnas e Ponta Grossa) da sub-bacia de Apucarana, Bacia do Paraná, Brasil. 167 pp. Ph.D. Thesis, Universidade de São Paulo, Instituto de Geociências, São Paulo.
Bodenbender, B., Wilson, M.A., and Palmer, T.J. 1989. Paleoecology of Sphenothallus on an Upper Ordovician hardground. Lethaia 22: 217–225. Crossref
Chang, S., Clausen, S., Zhang, L., Feng, Q., Steiner, M., Bottjer, D.J., Zhang, Y., and Shi, M. 2018. New probable cnidarian fossils from the lower Cambrian of the Three Gorges area, South China, and their ecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology 505: 150–166. Crossref
Clarke, J.M. 1913. Fósseis devonianos do Paraná. Monografia do Serviço Geológico e Mineralógico do Brasil 1: 1–353. Crossref
Dzik, J., Baliński, A., and Sun, Y. 2017. The origin of tetraradial symmetry in cnidarians. Lethaia 50: 306–321. Crossref
Eubank, L.L. 1946. Hawaiian representatives of the genus Caulerpa. University of California Publications in Botany 18: 409–432.
Fatka, O. and Kraft, P. 2013. Sphenothallus Hall, 1847 from Cambrian Skryje-Tyrovice Basin (Barrandian area, Czech Republic). Annales Societatis Geologorum Poloniae 83: 309–315.
Fatka, O., Kraft, P., and Szabad, M. 2012. A first report of Sphenothallus Hall, 1847 in Cambrian of Europe. Comptes Rendus Palevol 11: 539–547. Crossref
Fauchald, K., Stürmer, W., and Yochelson, E.L. 1986. Sphenothallus “Vermes” in the Early Devonian Hunsrück Slate, West Germany. Paläontologische Zeitschrift 60: 57–64. Crossref
Gmelin, S.G. 1768. Historia fucorum. 342 pp. Petropoli: Academia Scientiarum, Saint Petersburg.
Hall, J. 1847. Palaeontology of New York. Volume 1. Containing Descriptions of the Organic Remains of the Lower Division of the New York System. 339 pp. C. Van Benthuysen, Albany.
Howe, M.A. 1905. Phycological studies II. New Chlorophyceae, new Rhodophyceae and miscellaneous notes. Bulletin of the Torrey Botanical Club 32: 563–586. Crossref
Landing, E., Antcliffe, J.B., Geyer, G., Kouchinsky, A., Bowser, S.S., and Andreas, A. 2018. Early evolution of colonial animals (Ediacaran Evolutionary Radiation–Cambrian Evolutionary Radiation–Great Ordovician Biodiversification Interval). Earth-Science Reviews 178: 105–135. Crossref
Lange, F.W. and Petri, S. 1967. The Devonian of the Paraná Basin. In: J.J. Bigarella (ed.), Problems in Brazilian Devonian Geology. Boletim Paranaense de Geociências 21/22: 133–151.
Li, G.X., Zhu, M.Y., Van Iten, H., and Li, C. 2004. Occurrence of the earliest known Sphenothallus Hall in the Lower Cambrian (Qiongzhusian and Canglanpuan stages) of southern Shaanxi Province, South China. Geobios 37: 229–237. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Salvius, Stockholm. Crossref
Linnarsson, J.G.O. 1871. Om nagra förstengingar fran Sveriges och Norges “Primordialzon”. Öfver. Kong. Vetenskaps-Akademiens Forhandlingar 6: 789–796.
Marques, A.C., Höinhaus, R., and Mergner, H. 2000. Morphological study and taxonomical notes on Eudendridae (Cnidaria; Hydrozoa; Athecatae/Anthomedusae). Zoologische Meddelingen 74: 75–118.
Matthew, G.F. 1899. Studies on Cambrian faunas. No. 3. Upper Cambrian fauna of Mount Stephen, British Columbia. The trilobites and worms. Transactions of the Royal Society of Canada, Section 4 5: 39–66.
Muscente, A.D. and Xiao, S. 2015. New occurrences of Sphenothallus in the lower Cambrian of South China: implications for its affinities and taphonomic demineralization of small shelly fossils. Palaeogeography, Palaeoclimatology, Palaeoecology 437: 141–164. Crossref
Parry, L., Boggiani, P.C., Condon, D.J., Garwood, R.J., Leme, J.M., McElroy, D., Brasier, M.D., Trindade, R., Campanha, G.A.C., Pacheco, M.L., Diniz, C.Q.C., and Liu, A.G. 2017. Ichnological evidence for meiofaunal bilaterians from the terminal Ediacaran and earliest Cambrian of Brazil. Nature Ecology and Evolution 1: 1455–1464. Crossref
Peng, J., Babcock, L.E., Zhao, Y., Wang, P., and Yang, R. 2005. Cambrian Sphenothallus from Guizhou Province, China: early sessile predators. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 119–127. Crossref
Peterson, K.W. 1979. Development of coloniality in Hydrozoa. In: G. Larwood and B.R. Rosen (eds.), Biology and Systematics of Colonial Organisms, 105–139. Academic Press, New York.
Petri S. 1948. Contribuição ao estudo do Devoniano paranaense. Boletim do Departamento Nacional de Produção Mineral, Divisão de Geologia e Mineralogia, 129: 1–125.
Popov, L.E., Khazanovitch, K.K. [Khazanovič, K.K.], Borovko, N.G., Sergejeva, S.P., and Sobolevskaya, R.F. [Sobolevskaâ, R.F.] 1989. Opornye razrezy i stratigrafiâ kembro-ordovikskoj fosforitonosnoj obolovoj tolŝi na severo-zapade Russkoj platformy. 222 pp. Nauka, Leningrad.
Ruedemann, R. 1909. Some marine algae from the Trenton Limestone of New York. Bulletin of the New York State Museum 133: 194–210.
Salter, J.W. 1856. Description of Palaeozoic Crustacea and Radiata from South Africa. Transactions of the Geological Society of London, Series 2 7: 215–224.
Sedorko, D., Netto, R.G., Savrda, C.E., Assine, M.L., and Tognoli, F.M.W. 2017. Chronostratigraphy and environment of Furnas Formation by trace fossil analysis: calibrating the lower Paleozoic Gondwana realm in the Paraná Basin (Brazil). Palaeogeography, Palaeoclimatology, Palaeoecology 487: 307–320. Crossref
Simões, M.G., Leme, J.M., and Soares, S.P. 2009. Systematics, taphonomy, and paleoecology of homalonotid trilobites (Phacopida) from the Ponta Grossa Formation (Devonian), Paraná Basin, Brazil. Revista Brasileira de Paleontologia 12: 27–42. Crossref
Simões, M.G., Mello, L.H.C., Rodrigues, S.C., Leme, J.M., and Marques, A.C. 2000. Conulariid taphonomy as a tool in paleoenvironmental analysis. Revista Brasileira de Geociências 30: 757–762. Crossref
Sommer, F.W. 1954. Contribuição à paleofitografia do Paraná. In: F.W. Lange (ed.), Paleontologia do Paraná Volume Comemorativo do 1° Centenário do Estado do Paraná, 175–194. Comissão de Comemorações do Centenário do Paraná, Curitiba.
Van Iten, H., Cox, R.S., and Mapes, R.H. 1992. New data on the morphology of Sphenothallus Hall: implications for its affinities. Lethaia 25: 135–144. Crossref
Van Iten, H., Fitzke, J.A., and Cox, R.S. 1996. Problematical fossil cnidarians from the Upper Ordovician of the north-central USA. Palaeontology 39: 1037–1064.
Van Iten, H., Leme, J.M. Pacheco, M.L.A.F., Simões, M.G., Fairchild, T.R., Galante, F.R.D.G., Boggiani, P.C., and Marques, A.C. 2016a. Origin and early diversification of phylum Cnidaria: key macrofossils from the Ediacaran System of North and South America. In: S. Goffredo and Z. Dubinsky (eds.), The Cnidaria, Past, Present and Future, 31–40. Springer International Publishing, Switzerland. Crossref
Van Iten, H., Marques, A.C., Leme, J. M., and Simões, M.G. 2014. Origin and early evolution of the phylum Cnidaria Verrill: major developments in the analysis of the taxon’s Proterozoic–Cambrian history. Palaeontology 3: 1–14.
Van Iten, H., Muir, L.A., Botting, J.P., Zhang, Y., and Li, J. 2013. Conulariids and Sphenothallus (Cnidaria, Medusozoa) from the Tonggao Formation (Lower Ordovician, China). Bulletin of Geosciences 88: 713–722. Crossref
Van Iten, H., Muir, L.A., Simões, M.G., Leme. J.M.L., Marques, A.C., and Yoder, N. 2016b. Palaeobiogeography, palaeoecology and evolution of Lower Ordovician conulariids and Sphenothallus (Medusozoa, Cnidaria), with emphasis on the Fezouata Shale of southeastern Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 170–178. Crossref
Van Iten, H., Zhu, M., and Collins, D. 2002. First report of Sphenothallus Hall, 1847 in the Middle Cambrian. Journal of Paleontology 76: 902–905. Crossref
Verrill, A.E. 1865. Classification of polyps (extract condensed from Synopsis of the Polyps and Corals of the North Pacific Exploring Expedition under Commodore C. Ringgold and Captain John Rogers, U.S.N.). Communications of the Essex Institute 4: 145–152.
Vinn, O. and Kirsimäe, K. 2015. Alleged cnidarian Sphenothallus in the Late Ordovician of Baltica, its mineral composition and microstructure. Acta Palaeontologica Polonica 60: 1001–1008. Crossref
Vinn, O., Zabini, C., Sene-Silva, G., Kirsimäe, K., and Susan-Marcos, L. 2016. Possible polychaete tubeworms from the Late Emsian (Early Devonian) of the Parana Basin, Brazil. Acta Palaeontologica Polonica 61: 627–632.
Werner, B. 1979. Coloniality in the Scyphozoa: Cnidaria. In: G. Larwood and B.R. Rosen (eds.), Biology and Systematics of Colonial Organisms. Systematics Association Special Volume No. 11, 81–103. Academic Press, London.
Yang, J., Smith, M.R., Lan, T., Hou, J., and Zhang, X. 2014. Articulated Wiwaxia from the Cambrian Stage 3 Xiaoshiba Lagerstätte. Scientific Reports 4: 4643. Crossref
Zhu, M., Van Iten, H., Cox, R.S., Zhao, Y., and Erdtmann, B.D. 2000. Occurrence of Byronia Matthew and Sphenothallus Hall in the Lower Cambrian of China. Paläontologische Zeitschrift 74: 227–238. Crossref
Acta Palaeontol. Pol. 64 (2): 409–416, 2019
https://doi.org/10.4202/app.00576.2018