

The endocranial anatomy of the stem turtle Naomichelys speciosa from the Early Cretaceous of North America
ARIANA PAULINA-CARABAJAL, JULIANA STERLI, and INGMAR WERNEBURG
Paulina-Carabajal, A., Sterli, J., and Werneburg, I. 2019. The endocranial anatomy of the stem turtle Naomichelys speciosa from the Early Cretaceous of North America. Acta Palaeontologica Polonica 64 (4): 711–716.
Fossil turtles are one of the least studied clades in regard to endocranial anatomy. Recently, the use of non-invasive technologies, such as radiographic computed tomography (CT), increased the knowledge of the neuroanatomy of several extinct and extant taxa. Here, we provide the description of the nasal cavity, cranial endocast, and inner ear of the stem turtle Naomichelys speciosa based on digital 3D reconstructions. This terrestrial form is characterized by a nasal cavity with anteroposteriorly elongated vestibulum and a large cavum nasi proprium, traits typically related to terrestrial habits. The large olfactory region of the cavum nasi proprium suggests that olfaction was probably the most important sense for this species. Our description of N. speciosa adds novel information to the knowledge of endocranial anatomy in early turtle evolution and provides an important foundation for future analyses and comparisons.
Key words: Reptilia, Helochelydridae, cranial endocast, paleoneurology, Mesozoic, North America.
Ariana Paulina-Carabajal [a.paulinacarabajal@conicet.gov.ar], INIBIOMA, Universidad Nacional del Comahue, CONICET, Quintral 1250, 8400 San Carlos de Bariloche, Argentina.
Juliana Sterli [jsterli@mef.org.ar], CONICET-Museo Paleontológico Egidio Feruglio, Av. Fontana 140, 9100 Trelew, Argentina.
Ingmar Werneburg [ingmar.werneburg@senckenberg.de], Senckenberg Centre for Human Evolution and Palaeoenvironment (HEP) an der Eberhard Karls Universität, Sigwartstraße 10, 72076 Tübingen, Germany; Fachbereich Geowissenschaften, Eberhard-Karls-Universität, Hölderlinstraße 12, 72074 Tübingen, Germany.
Received 5 February 2019, accepted 9 May 2019, available online 16 September 2019.
Copyright © 2019 A. Paulina-Carabajal et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Members of Helochelydridae are large-bodied stem turtles with terrestrial habits, characterized by their noticeable dermatocranial bone ornamentation, formed by tubercles (Fig. 1A1, A2). Helochelydrids are present in North America and in Europe since the Tithonian and up to the Maastrichtian (Joyce et al. 2011). In particular, Naomichelys speciosa Hay, 1908 is the only known helochelydrid species present in North America and it has been recognized in different localities ranging from the Aptian to the Campanian (Joyce et al. 2011; Joyce 2017). Hay (1908) originally named N. speciosa based on an isolated entoplastron. Recently, Joyce et al. (2014) provided a detailed description of a complete specimen (FMNH PR273) assigned to N. speciosa and provided some endocranial descriptions based on CT-scans. However, different researchers partially studied this specimen before. Hirayama et al. (2000) illustrated the carapace of FMNH PR273. Barrett et al. (2002) described a limb with osteoderms, and Scheyer and Anquetin (2008) presented its shell bone histology. Recently, for comparative purposes, Lautenschlager et al. (2018) presented an endocranial reconstruction of N. speciosa in lateral view. Here, we provide new anatomical information about this species with a detailed description of its endocranial anatomy based on a digital 3D model, which includes a partially preserved endocast, a right inner ear, and the nasal cavity.
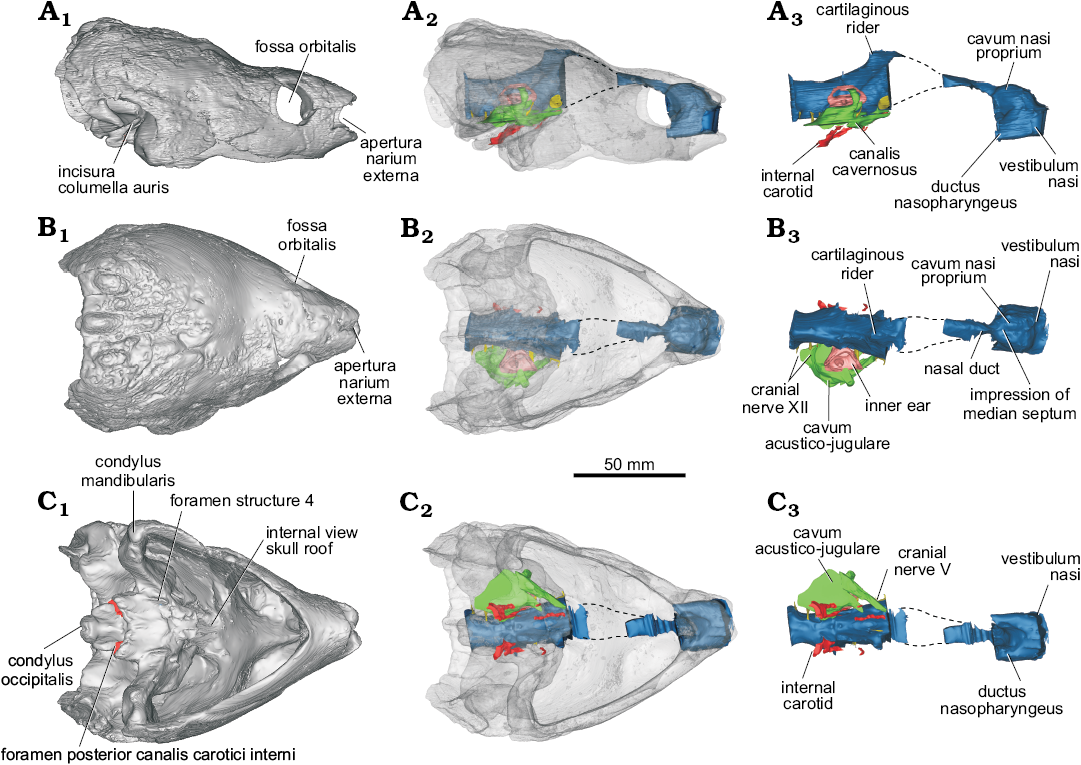
Fig. 1. Digitally rendered skull and endocranial anatomy of helochelydrid turtle Naomichelys speciosa Hay, 1908 (FMNH PR273) from the Early Cretaceous (Aptian/Albian) Trinity Group, Texas; in lateral (A), dorsal (B), and ventral (C) views. Surface reconstruction of the skull (A1–C1), cranial endocast including brain, inner ear, canalis cavernosus, and nasal cavity in situ with the bone rendered semi-transparent to allow observation of internal structures (A2–C2), isolated endocasts (A3–C3).
The endocranial morphology in turtles is still underexplored (see Paulina-Carabajal et al. 2017 for a detailed history). Some neuroanatomical studies have been conducted on extant turtles, but they are isolated attempts and the taxon sampling is not yet inclusive (e.g., Bojanus 1819; Ogushi 1913; Edinger 1929, 1934; Zangerl 1960; Gaffney and Zangerl 1968; Wyneken 2001; Deantoni et al. 2012). The situation is similar when extinct taxa are considered, although this has been changing in recent years with the use of CT-scans (e.g., Georgi 2008; Paulina-Carabajal et al. 2013a, b; Ferreira et al. 2018). The use of this non-invasive technique for the study of the neuroanatomy in fossil reptilian taxa dates back to the late 1990’s (e.g., dinosaur brain studies by Rogers 1998; Knoll et al. 1999), but only recently it has been used to explore the endocranial cavities (particularly those occupied by brain and inner ear) of extinct turtles (Paulina-Carabajal et al. 2013a, 2017; Lautenschlager et al. 2018; Evers and Benson 2019; Evers et al. 2019).
Our main goal was to describe in detail the endocranial anatomy of N. speciosa, which was only preliminarily studied so far (Paulina-Carabajal et al. 2013b; Lautenschlager et al. 2018: fig. 2). Special emphasis is devoted to the nasal cavity due to its particularly large development in terrestrial turtles.
Institutional abbreviation.—FMNH, Field Museum of Natural History, Chicago, USA.
Material and methods
Specimen FMNH PR273 of Naomichelys speciosa was found in the Early Cretaceous (Aptian/Albian) Trinity Group in Texas, USA. The µCT scan of the skull of Naomichelys speciosa (FMNH PR273) was made at the Department of Prehistory and Archeological Sciences of the University of Tübingen (Tübingen, Germany) using a medical tomographer (Phoenix v|tome|x). In total, 675 slices were obtained using a voltage of 150 kV and a current of 170 mA. The voxel size is x = 1068 pixels and y = 1382 pixels.
Since the anteroventral region of the snout is ossified, a complete turtle cranial endocast includes the space occupied by the encephalic structures proper, plus most of the cast of the nasal duct and the nasal cavity anteriorly (Paulina-Carabajal et al. 2019).
Although the skull of N. speciosa is more or less complete and preserved in 3D (Joyce et al. 2014; Fig. 1), it has some missing regions that had been reconstructed with plaster, which is clearly distinguishable from the bone in the µCT scan. These regions include parts of the skull roof and, therefore, the osseous correlates of the olfactory bulbs. Also the cerebral hemispheres are not observed in the cranial endocast, such as the forebrain and the nasal canal connecting the olfactory bulbs posteriorly and the nasal cavity anteriorly (Fig. 1A3).
Anatomical nomenclature follows Gaffney (1979) for skull, and non-preserved soft tissue anatomy observed in the cranial endocast follows Witmer et al. (2008).
Descriptions and comparisons
Endocast of the nasal cavity.—The three portions of the vertebrate nasal cavity (blue in Figs. 1, 2) (see Parsons 1959, 1970) are recognized in the endocast of Naomichelys speciosa: the vestibulum nasi, the cavum nasi proprium, and the ductus nasopharyngeus (Figs. 1A3, 2A1). Worth mentioning is the clear separation of the vestibulum nasi from the cavum nasi proprium and the relatively large development of the former (Figs. 1A2, A3, B2, B3, 2A1). A well-developed vestibulum nasi is associated with an elongate nose, as in Chelus fimbriata, trionychids, and Carettochelys insculpta (Parsons 1970). The vestibulum nasi is also large in the extinct terrestrial meiolaniids (Paulina-Carabajal et al. 2017), and in the extant terrestrial Gopherus berlandieri (Paulina-Carabajal et al. 2017), Macrochelys temminckii (Lautenschlager et al. 2018: fig. 3) and Malacochersus tornieri (Maunter et al. 2017). Since the external nasal gland in extant reptiles arises from the posterior portion of the vestibulum nasi (Parsons 1970), the large vestibulum nasi in certain extinct turtles (e.g., meiolaniids) could suggest the presence of an even larger external nasal gland than that in the extant forms mentioned above. On the other hand, the anteroposterior elongation of the vestibulum nasi of the nasal cavity in other extant terrestrial reptiles (e.g., iguanids) has been correlated with a specialization to desert life, since it limits the possibility of sand particles entering the nose (Parsons 1959).
In Naomichelys speciosa, the endocast of the cavum nasi proprium is subdivided—in dorsal view—into two well-developed lobes, indicating the presence of a low median septum inside the cavity (Figs. 1B3, 2A3). These lobes are almost parallel, unlike the markedly divergent lobes in the nasal cavity of Plesiochelys etalloni (Paulina-Carabajal et al. 2013a: fig. 2A), or Chelonoidis chilensis (Paulina-Carabajal et al. 2017: fig. 9A). In other studied turtles, there is no clear separation of the cavum nasi proprium. The dorsal region of the cavum nasi proprium corresponds to the olfactory region of the nasal cavity, where the sensitive epithelium is situated together with olfactory glands (Parsons 1970). As mentioned above, the external nasal gland, the largest of the nasal glands in reptiles, lies dorsally or laterally within the cavum nasi proprium but outside the cartilaginous nasal capsule, and these lobes may indicate the shape and size of those glands in N. speciosa. There are no publications regarding the osteological correlates of the soft tissues within the cavum nasi proprium, preventing further comparisons. Posteriorly, a marked constriction indicates the end of the cavum nasi proprium, followed backwards by the nasal duct/canal (for the cranial nerve I) that runs posteriorly to reach the olfactory bulbs (which were not reconstructed in the cranial endocasts due the damage of the original cranial roof).
The ductus nasopharyngeus is short but relatively large in diameter, particularly in the proximal portion (Fig. 2A2).
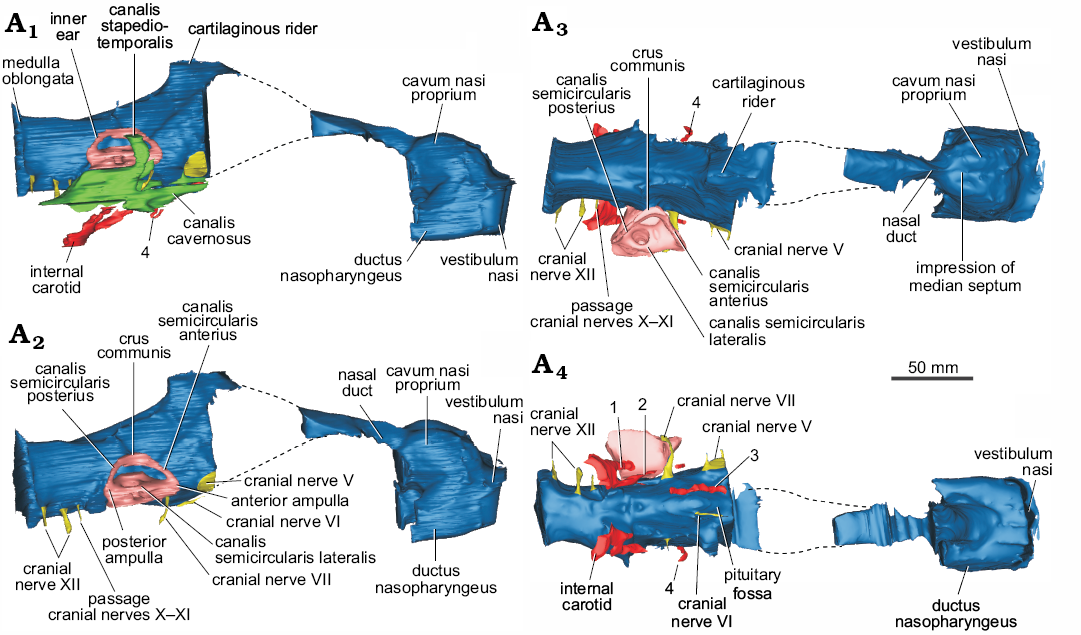
Fig. 2. Cranial endocast, nasal cavity, and inner ear (plus cavum acustico-jugulare, A1 and blood vessels) of helochelydrid turtle Naomichelys speciosa Hay, 1908 (FMNH PR273) from the Early Cretaceous (Aptian/Albian) Trinity Group, Texas; in right lateral (A1), right posterolateral (A2), dorsal (A3), and ventral (A4) views. 1, lateral branch of the internal carotid artery; 2, anteromedial branch of the carotid artery; 3, cerebral artery; 4, ?unnamed branch of the nervi vidiani.
Endocast of the brain.—Due to the poor preservation of the frontals and the lack of ossification of the anterior walls of the braincase, the cranial endocast (blue in Figs. 1–3) of N. speciosa misses most of the forebrain (Figs. 1A2, A3, B, C, 2). As in all turtles, the anterior walls of the braincase are not ossified preventing the reconstruction of cranial nerves I–IV. The hindbrain and midbrain are disposed sub-horizontally and there are no discernible optic lobes (Fig. 1B2, B3). This anatomy is similar to that observed in other turtles (Paulina Carabajal et al. 2017 and references therein; Ferreira et al. 2018; Lautenschlager et al. 2018), in which the cranial endocast is mostly tubular, with poorly marked angles between forebrain, midbrain, and hindbrain (Figs. 1A2, A3, 2A1, A2). The dorsal protuberance, known as “cartilaginous raider” (Zangerl 1960), is well-marked and triangular in lateral view in N. speciosa. This section corresponds to the tallest region of the cranial endocast and was probably occupied by a cartilaginous region of the skull roof (i.e., taenia marginalis) and by venous sinuses, structures located dorsally to the epiphysis (Figs. 1A2, A3, B2, B3, 2A1, A2).
Cranial nerve V (trigeminal nerve) has a larger passage in diameter, but is short and laterally oriented (Figs. 1C3, 2A2–A4). Cranial nerve VI (abducens nerve) is small in diameter and close ventrally to its counterpart at the midline (Fig. 2A4). The passage for this nerve is anteroposteriorly short and runs anteriorly to enter the pituitary (i.e., the pituitary fossa) posteriorly (Fig. 2A4). The pituitary is incomplete and its size and shape remain unknown. The root of cranial nerve VII (facial nerve) is just anteroventral to the inner ear in lateral view of the cranial endocast (Figs. 1C3, 2A2–A4). This nerve has a long passage of small diameter that projects posteroventrally reaching the canalis cavernosus laterally (Fig. 2A). The vidian nerve, a palatine branch of cranial nerve VII, is recognized in the endocast (Fig. 2A1, A3, A4) and runs through the canalis nervi vidiani, which connects the canalis carotici interni with the canalis cavernosus (Gaffney 1979). Cranial nerve VIII (statoacustic nerve) is not observed in our µCT scan. This nerve is small and is only observed in high-resolution μCT scans (e.g., Paulina-Carabajal et al. 2013a).
The medulla oblongata, which is low but anteroposteriorly long, has a flat ventral side in lateral view (Fig. 1A2, A3, 2A1, A2). There are three separated roots for the cranial nerves associated to the lateroventral side of the medulla. The posterior two passages correspond to branches of cranial nerve XII (hypoglossal nerve), whereas the anterior one corresponds to the foramen jugulare anterius for cranial nerve X (vagus nerve), cranial nerve XI (accessory nerve), and for the internal jugular vein (Figs. 1B3, 2A2, A4).
Endocast of the blood vessels.—The passages for blood vessels, such as internal carotid sub-divisions, are not clearly observed in our µCT-scan. Therefore, only partial sections of these passages were reconstructed, as shown in Figs. 2A1, A4, 3. There are two branches for the internal carotid artery that diverge just after entering the basicranium in the pterygoid/basisphenoid at the level of the basioccipital/basisphenoid suture (Joyce et al. 2014). The most lateral passage (number 1 in Figs. 2A4, 3) connects the canalis carotici interni with the canalis cavernosus. This description and position could correspond to the canalis pro ramo nervi vidiani (sensu Rollot et al. 2018). Another interpretation could be that this most lateral passage could be the arteria stapedialis (as it was interpreted for Plesiochelys etalloni by Paulina-Carabajal et al. 2013). However, as it was noticed by several authors, the split of the internal carotid and the stapedial artery is extracranial, and consequently, that bifurcation does not leave trace in bone (e.g., Albrecht 1967; Miyashita 2013). If this is the case, the interpretation of that structure in Plesiochelys etalloni should be re-evaluated. The anteromedial branch (number 2 in Figs. 2A4, 3) runs laterally to the endocast of the brain and probably corresponds to the palatine carotid artery. More anteromedially, there is a canal running below the endocast of the brain with an anteromedial direction. This canal pierces the basisphenoid and exits near the place where the pituitary fossa is. We interpret this branch as the cerebral artery (number 3 in Figs. 2A4, 3). The exact location where the cerebral and palatine arteries bifurcate cannot be reconstructed for this species due to the preservation of this specimen. Anteriorly to the exit of cranial nerve VII there is a short canal running dorso-ventrally (number 4 in Figs. 2A4, 3). This canal pierces the pterygoid and connects the canalis cavernosus with the exterior of the skull. We are not sure about the identity of this structure. Joyce et al. (2014) identified the opening of this canal in the ventral view of the pterygoid as the foramen pro ramo nervi vidiani, based on previously published works (e.g., Evans and Kemp 1975; Gaffney 1979). However, based on the reconstruction of the nervi vidiani for Eubaena cephalica, this canalis would not represent the canalis pro ramo nervi vidiani, but another, unnamed branch of the nervi vidiani (Rollot et al. 2018; Yann Rollot personal communication 2019). More endocast reconstructions of extinct and extant taxa will help to better understand these internal structures in turtles.
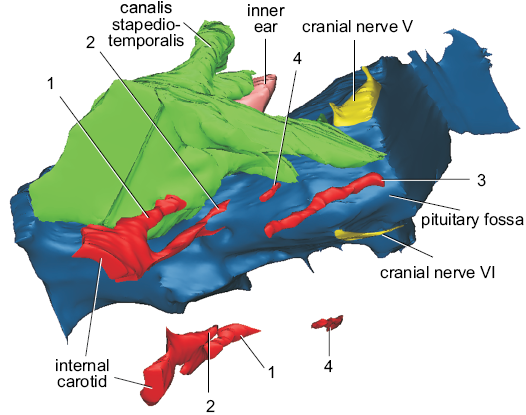
Fig. 3. Detail of the cranial endocast and blood vessels of helochelydrid turtle Naomichelys speciosa Hay, 1908 (FMNH PR273) in right lateroventral view. The image also includes the cavum acustico-jugulare, right inner ear, and cranial nerves V and VI. 1, lateral branch of the internal carotid artery; 2, anteromedial branch of the carotid artery; 3, cerebral artery; 4, ?unnamed branch of the nervi vidiani. Not to scale.
Endocast of the cavum acustico-jugulare.—In N. speciosa, the endocast of the cavum acustico-jugulare is well-preserved, mainly on the right side of the skull (Figs. 1A2, A3, B2, B3, C2, C3). It is a structure found in turtles (Gaffney 1979). It is an L-shaped cavity located in the posteroventral part of the skull (Gaffney 1979) that houses vessels, nerves, and parts of the middle ear (Gaffney 1979). The cavum acustico-jugulare is located between the cavum tympani and the cavum labyrinthicum. It is formed by the cranioquadrate space anteriorly and the recessus scalae tympani posteriorly (Gaffney 1979). The cranioquadrate space is anteriorly connected to the canalis cavernosus (Gaffney 1979) and the dorsal region is formed by the canalis stapedio-temporalis. The canalis stapedio-temporalis and the canalis cavernosus are confluent lateral to the inner ear (Fig. 2A1). The canalis cavernosus runs anteromedially inside the basicranium, connecting anteriorly with the base of the endocranial cavity, a situation reflected in the endocasts of both structures (Fig. 1A3, B3, C3). Through this space, the vena capitis lateralis would run (Gaffney 1979). The canalis stapedio-temporalis runs posterodorsally, lateral to the inner ear (Fig. 2A1, A3, A4). It ends in a foramen on the dorsal margin of the temporal fossa, the foramen stapedio-temporalis. The arteria stapedialis runs inside this canal in life (Gaffney 1979). The posteriormost part of the cavum acustico-jugulare is the recessus scalae tympani (Fig. 2A1, A3, A4). This space contains cranial nerves X and XI, the jugular vein, and the vena cerebralis posterior (Gaffney 1979).
Cast of the inner ear.—The complete structures of the inner ear are not clearly visible in our µCT-scan. The most complete reconstruction corresponds to the vestibule and the semicircular canals of the right inner ear (Figs. 1A2, A3, B, C, 2A2, A3), lacking the lagena. The general morphology of the inner ear is, however, a reminiscent of that in other turtles, being triangular, and with low—and subequal in size—anterior and posterior semicircular canals. The angle formed between the anterior and posterior semicircular canals in dorsal view is approximately 80º, which is less than the angle observed in the terrestrial meiolaniids and some testudinids where the same angle is closer to 100º (Paulina-Carabajal et al. 2017). In N. speciosa, the anterior and posterior semicircular canals are shorter than the crus communis. The top of the crus communis is flat and wide and is not taller than the anterior and posterior semicircular canals. Without forming a rounded loop, they descend to reach the anterior and posterior ampulae, respectively. On the contrary, semicircular canals taller than the crus communis are present in the inner ears of the chelid Chelodina, Pelumedusoides, Trionychidae, Cheloniidae, Emydidae, and in the testudinid Malacochersus tornieri (Lautenschlager et al. 2018). This is also seen in some extinct turtles (Neenan et al. 2017). The crus communis of N. speciosa is robust and wide, being approximately three to four times wider than the diameter of the tube of the semicircular canals. A similar, wide crus communis is present in other turtles such as in testudinids (Chelonoidis chilensis, Gopherus berlandieri, Kinixys belliana, Testudo graeca, T. hermanni), the geomydid Rhinoclemmys funerea (Paulina-Carabajal et al. 2017: figs. 9, 10), and Chelidae (Emydura subglobosa, Chelodina reimanni; Lautenschlager et al. 2018: fig. 2). In other turtles like Trionychidae (Pelodiscus sinensis), Cheloniidae (Chelonia mydas), Platysternidae (Platysternon megacephalum), and the testudionid M. tornieri (Neenan et al. 2017; Lautenschlager et al. 2018), the crus communis is wide but not as much as in N. speciosa. The region where the anterior semicircular canal joins the lateral semicircular canal (anterior ampulla) is extremely large when compared with the posterior ampulla (Fig. 2A2). We were not able to reconstruct the lagena in N. speciosa, although the illustration published by Lautenschlager et al. (2018: fig. 2) shows a short lagena, similar in size to that present in other studied turtles.
Concluding remarks
Although incomplete, the cranial endocast of Naomichelys speciosa adds novel anatomical information to the scarce knowledge of testudinatan’s endocast. Some paleobiological inferences can be derived from the relative proportions of certain regions of the brain and sense organs: this terrestrial species is characterized by a nasal cavity with anteroposteriorly elongated vestibulum and a large cavum nasi proprium (longer than the width of the inner ear). Both traits are typically related to terrestrial, and even arid habitats. In N. speciosa, the large olfactory region of the cavum nasi proprium plus other neurocranial traits such as the small optic lobes (which leave no impression on the walls of the endocranial cavity) might suggest that the olfaction was probably an important sense for this species.
Acknowledgements
The authors thank William Simpson and Oliver Rieppel (both, FMNH) who granted the access to the specimen. We also thank Heike Sherf (University of Tübingen, Germany) who scanned the skull. The skull was scanned while Walter G. Joyce (University of Fribourg, Switzerland) was studying it, consequently we would like to thank him as well. The reviewers Serjoscha Evers (University of Fribourg) and Marco Sales (Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil) are also deeply thanked for their comments, which improved this ms. The editor Stephen Brusatte (University of Edinburgh, UK) is thanked for handling this manuscript. AP-C was supported by PICT-2016-0481 and IW was supported by SNF-fund P300PA_164720 and DFG-fund WE 5440/6-1.
References
Albrecht, P.W. 1967. The cranial arteries and cranial arterial foramina of the turtle genera Chrysemys, Sternotherus, and Trionyx: a comparative study with analysis of possible evolutionary implications. Tulane Studies in Zoology 14: 81–99.
Barrett, P.M., Clarke, J.B., Brinkman, D.B., Chapman, S.D., and Ensom, P.C. 2002. Morphology, histology and identification of the “granicones” from the Purbeck Limestone Formation (Lower Cretaceous: Berriasian) of Dorset, southern England. Cretaceous Research 23: 279–295. Crossref
Bojanus, L.H. 1819. Anatome Testudinis Europaeae. Vol. 1 and 2. 178 pp. Impensis auctoris, typis Josephi Zawadzki, Vilnae.
Edinger T. 1929. Die fossilen Gehirne. Ergebnisse der Anatomie und Entwicklungsgeschichte, Band 28. 249 pp. Julius Springer-Verlag. Berlin.
Edinger T. 1934. Anton Fritsch’s “Großhirn von Polyptychodon“ ist der Steinkern eines Schildkrötenschädels. Psychiatrische en neurologische bladen 3–4: 396–404.
Evers, S.W. and Benson, R.B. 2019. A new phylogenetic hypothesis of turtles with implications for the timing and number of evolutionary transitions to marine lifestyles in the group. Palaeontology 62: 93–134. Crossref
Evers, S.W., Neenan, J.M., Ferreira, G.S., Werneburg, I., Barrett, P.M., and Benson, R.B.J. 2019. Neurovascular anatomy of the protostegid turtle Rhinochelys pulchriceps and comparisons of membranous and endosseous labyrinth shape in extant turtles. Zoological Journal of the Linnean Society zlz063 [published online, https://academic.oup.com/zoolinnean/advance-article/doi/10.1093/zoolinnean/zlz063/5552592] Crossref
Deantoni, F.O., Romano P.S.R., Azevedo, S.A.K. 2012. Analysis of internal structure of the skull of pelomedusoides (Testudines, Pleurodira) based on three-dimensional helical tomography. In: W.G. Joyce, J.A. Corsini, I. Werneburg, and M. Rabi (eds.), Symposium on Turtle Evolution, Tübingen, Germany. Abstracts, 17. University of Tübingen, Tübingen.
Evans, J. and Kemp, T.S. 1975. The cranial morphology of a new Lower Cretaceous turtle from southern England. Palaeontology 18: 25–40.
Ferreira, G.S., Iori, F.V., Hermanson, G., and Langer, M.C. 2018. New turtle remains from the Late Cretaceous of Monte Alto-SP, Brazil, including cranial osteology, neuroanatomy and phylogenetic position of a new taxon. Paläontologische Zeitschrift 92 (3): 481–498. Crossref
Gaffney, E.S. 1979. Comparative cranial morphology of recent and fossil turtles. Bulletin of the American Museum of Natural History 164: 63–376.
Gaffney, E.S., Zangerl, R. 1968. A revision of the chelonian genus Bothremys (Pleurodira: Pelomedusidae). Fieldiana Geolology 16: 193–239. Crossref
Georgi, J.A. 2008. Semicircular Canal Morphology as Evidence of Locomotor Environment in Amniotes. 235 pp. Unpublished Ph.D. thesis, Stony Brook University, Stony Brook.
Hay, O.P. 1908. The fossil turtles of North America. Carnegie Institution of Washington 75: 1–568.
Hirayama, R., Brinkman, D.B., and Danilov, I.G. 2000. Distribution and biogeography of non-marine Cretaceous turtles. Russian Journal of Herpetology 7: 181–198.
Joyce, W.G. 2017. A review of the fossil record of basal Mesozoic turtles. Bulletin of the Peabody Museum of Natural History 58: 65–114. Crossref
Joyce, W.G., Chapman, S.D., Moody, R.T., and Walker, C.A. 2011. The skull of the solemydid turtle Helochelydra nopcsai from the Early Cretaceous of the Isle of Wight (UK) and a review of Solemydidae. Special Papers in Palaeontology 86: 75–97.
Joyce, W.G., Sterli, J. and Chapman, S.D. 2014. The skeletal morphology of the solemydid turtle Naomichelys speciosa from the Early Cretaceous of Texas. Journal of Paleontology 88: 1257–1287.
Knoll, F., Buffetaut, E., and Bulow, M. 1999. A theropod braincase from the Jurassic of the Vaches Noires cliffs (Normandy, France): osteology and paleoneurology. Bulletin de la Société Géologique de France 170: 103–109.
Lautenschlager, S., Ferreira, G.S., and Werneburg, I. 2018. Sensory evolution and ecology of early turtles revealed by digital endocranial reconstructions. Frontiers in Ecology and Evolution 6: 7. Crossref
Mautner, A.K., Latimer, A.E., Fritz, U., and Scheyer, T.M. 2017. An updated description of the osteology of the pancake tortoise Malacochersus tornieri (Testudines: Testudinidae) with special focus on intraspecific variation. Journal of Morphology 278: 321–333. Crossref
Miyashita, T. 2013. Geometric and developmental perspectives on the evolution of the skull and internal carotid circulation in turtles. In: D.B. Brinkman, P.A. Holroyd, and J.D. Gardner (eds.), Morphology and Evolution of Turtles, 71–101. Springer, Dordrecht. Crossref
Neenan, J.M., Reich, T., Evers, S., Druckenmiller, P.S., Voeten, D.F.A.E., Choiniere, J.N., Barrett, P.M., Pierce, S.E. and Benson, R.B.J. 2017. Evolution of the sauropterygian labyrinth with increasingly pelagic lifestyles. Current Biology 27: 3852–3858. Crossref
Ogushi, K. 1913. Zur Anatomie der Hirnnerven und des Kopfsympathicus von Trionyx japonicus nebst einigen kritischen Bemerkungen. Morphologisches Jahrbuch 45: 441–480.
Parsons, T.S., 1959. Nasal anatomy and the phylogeny of reptiles. Evolution 13: 175–187. Crossref
Parsons, T.S. 1970. The nose and Jacobson’s organ. In: C. Gans and T.S. Parsons (eds.), Biology of the Reptilia, Vol. 2, Morphology B, 99–191. Academic Press, London.
Paulina-Carabajal, A., Sterli, J., and Werneburg, I. 2019. 3D model related to the publication: The endocranial anatomy of the stem turtle Naomichelys speciosa from the Early Cretaceous of North America. MorphoMuseuM 5:e99 [published online, https://doi.org/10.18563/journal.m3.99] Crossref
Paulina-Carabajal, A., Sterli, J., Georgi, J., Poropat, S.F., and Kear, B.P. 2017. Comparative neuroanatomy of extinct horned turtles (Meiolaniidae) and extant terrestrial turtles (Testudinidae), with comments on the palaeobiological implications of selected endocranial features. Zoological Journal of the Linnean Society 180: 930–950. Crossref
Paulina-Carabajal, A., Sterli, J., Müller, J., and Hilger, A. 2013a. Neuroanatomy of the marine Jurassic turtle Plesiochelys etalloni (Testudinata, Plesiochelyidae). PLoS ONE 8 (7): e69264. Crossref
Paulina-Carabajal, A., Sterli, J., Müller, J., Joyce, W.G., and Werneburg, I. 2013b. Endocranial reconstructions of extinct turtles using μ-CT scans: new insights into brain and inner ear anatomy. In: K.H. Albertine and J.T. Laitman (eds.), 10th International Congress of Vertebrate Morphology, Barcelona, España. The Anatomical Record 296 (Special Feature): 207.
Rogers, S.W. 1998. Exploring dinosaur neuropaleobiology: computed tomography scanning and analysis of an Allosaurus fragilis endocast. Neuron 21: 673–679. Crossref
Rollot, Y., Lyson, T.R., and Joyce, W.G. 2018. A description of the skull of Eubaena cephalica (Hay, 1904) and new insights into the cranial circulation and innervation of baenid turtles. Journal of Vertebrate Paleontology 38: e1474886. Crossref
Scheyer, T.M. and Anquetin, J. 2008. Bone histology of the Middle Jurassic turtle shell remains from Kirtlington, Oxfordshire, England. Lethaia 41: 85–96. Crossref
Witmer, L.M., Ridgely, R.C., Dufeau, D., and Semones, M.C. 2008. Using CT to peer into the past: 3D visualization of the brain and inner ear regions of birds, crocodiles, and nonavian dinosaurs. In: H. Endo and R. Frey (eds.), Anatomical Imaging: Towards a New Morphology, 67–88. Springer-Verlag, Tokyo. Crossref
Wyneken, J. 2001. The anatomy of sea turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470: 1–172.
Zangerl, R.1960. The vertebrate fauna of the Selma Formation of Alabama. V. An advanced cheloniid sea turtle. Fieldiana Geology Memoir 3: 281–312. Crossref
Acta Palaeontol. Pol. 64 (4): 711–716, 2019
https://doi.org/10.4202/app.00606.2019