

Comparative bone microstructure of three archosauromorphs from the Carnian, Late Triassic Chañares Formation of Argentina
JORDI ALEXIS GARCIA MARSÀ, FEDERICO L. AGNOLÍN, and FERNANDO E. NOVAS
Marsà, J.A.G., Agnolín, F.L., and Novas, F.E. 2020. Comparative bone microstructure of three archosauromorphs from the Carnian, Late Triassic Chañares Formation of Argentina. Acta Palaeontologica Polonica 65 (2): 387–398.
The Chañares Formation exhibits one of the most important archosauriform records of early Carnian ecosystems. Here we present new data on the palaeohistology of Chañares archosauriforms and provide new insights into their paleobiology, as well as possible phylogenetically informative traits. Bone microstructure of Lagerpeton chanarensis and Tropidosuchus romeri is dominated by fibro-lamellar tissue and dense vascularization. On the other hand, Chanaresuchus bonapartei is more densely vascularized, but with cyclical growth characterized by alternate fibro-lamellar, parallel-fibered and lamellar-zonal tissues. Dense vascularization and fibro-lamellar tissue imply fast growth and high metabolic rates for all these taxa. These histological traits may be tentatively interpreted as a possible adaptative advantage in front of Chañares Formation environmental conditions.
Key words: Archosauromorpha, Lagerpeton, Tropidosuchus, paleobiology, paleohistology, Mesozoic, South America.
Jordi Alexis Garcia Marsà [jagmdarwinista@gmail.com] and Fernando E. Novas [fernovas@yahoo.com.ar], Laboratorio de Anatomía Comparada y Evolución de los Vertebrados, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, 470 Ángel Gallardo, 1405DJR, Buenos Aires, Argentina; CONICET, Av. Ángel Gallardo, 470, 1405DJR, Buenos Aires, Argentina.
Federico L. Agnolín [fedeagnolin@yahoo.com.ar], Laboratorio de Anatomía Comparada y Evolución de los Vertebrados, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, 470 Ángel Gallardo, 1405DJR, Buenos Aires, Argentina; Área de Paleontología, Fundación de Historia Natural “Félix de Azara”, Departamente de Ciencias Naturales y Antropología, Universidad Maimónides, 775 Hidalgo piso 7, 1405BDB, Buenos Aires, Argentina.
Received 27 May 2019, accepted 11 February 20120, available online 7 April 2020.
Copyright © 2020 J.A.G. Marsà et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The taxonomic and ecological diversity of Chañares Formation vertebrates, combined with its relatively short temporal span of deposition (236–234 Ma) (Marsicano et al. 2016) and its well-documented paleoenvironmental setting provide an ideal framework for investigating disparity growth dynamics of related taxa that span a pivotal stage in the evolution of terrestrial vertebrates, which represent the best record of tetrapod evolution immediately prior to the Late Triassic diversification of dinosaurs, advanced cynodonts, and mammals (Romer and Jensen 1966; Romer 1973; Bonaparte 1982).
Lagerpeton chanarensis and Chanaresuschus bonapartei were described by Romer (1971a, b) on the basis of partially articulated skeletons. Tropidosuchus romeri was described on the basis of several articulated and nearly complete individuals by Arcucci (1990). All available specimens from Lagerpeton, Chanaresuchus, and Tropidosuchus specimens come from the Late Triassic Chañares Formation (Carnian), at La Rioja province, in Argentina (Marsicano et al. 2016).
Romer (1971a) described Lagerpeton chanarensis as a new slender-limbed archosaur, based on an articulated right hind-limb that exhibited remarkable dinosaur-like traits. Subsequent expeditions recovered new Lagerpeton specimens, which reinforced its similarities to dinosaurs (Bonaparte 1984; Arcucci 1986; Sereno and Arcucci 1993), being currently considered the sister taxon of Dinosauriformes (Sereno and Arcucci 1993; Novas 1996; Ezcurra 2006; Nesbitt 2011; Cabreira et al. 2016). Lagerpeton chanarensis was the only known lagerpetid for decades until the discovery of Dromomeron and Ixalerpeton (Irmis et al. 2007; Nesbitt et al. 2009a; Small 2009; Martínez et al. 2012; Cabreira et al. 2016). In contrast, Chanaresuchus and Tropidosuchus have been included within Proterochampsidae, a group of crocodile-like basal archosauriforms (Sereno and Arcucci 1990; Sereno 1991; Dilkes and Sues 2009; Ezcurra et al. 2010). This clade is endemic to the Late Triassic beds of South America (Trotteyn et al. 2013).
Arcucci (1986) noted that several anatomical traits of Lagerpeton may be indicative of possibe proterochampsid affinities. In this sense, Arcucci (1990) remarked that the pelvis of Tropidosuchus possesses characters that are similar to Lagerpeton and Herrerasaurus (presence of a supracetabular crest, a ventral opening between pubis and ischium, a posterodorsal notch in acetabulum) and tarsal adaptations similar to basal dinosauromorphs as Lagosuchus and Lagerpeton. More recently, Novas and Agnolín (2015) indicated similarities between Lagerpeton and proterochampsids, particularly with Tropidosuchus. Lagerpeton resembles proterochampsids in several features, having a proximal pubis with a robust ambiens process, a pubic margin of pubis sigmoid in anterior view, a cup-like and ellipsoidal-shaped acetabulum, transverse processes on caudal vertebrae that a long and narrow, a femoral 4th trochanter that is proximodistally expanded, a middle tubercle surrounded by two shallow concavities on the caudal surface of the distal end of the tibia, an astragalus with an acute anteromedial corner, and a transversely thick metatarsal II. Furthermore, Lagerpeton and Tropidosuchus share an elongate and compact metatarsus with a metatarsal V that is reduced and devoid of phalanges, with articular surface for distal tarsal 4 subparallel to the longitudinal axis of shaft, and metatarsal IV longer than III. If we follow this proposal, lagerpetids and proterochampsids may be more closely related than previously thought.
Some authors made previous histological descriptions of selected proterochampsians from Northwestern Argentina. Ricqlès et al. (2008) described an indeterminate long bone of Chanaresuchus (MCZ 4036). This bone shows a fibro-lamellar tissue, but toward the periphery, the tissue progressively changes to lamellar-zonal, indicating active growth during a great part of early ontogeny. Cerda et al. (2015) described osteoderm histology of Chanaresuchus and Pseudochampsa. The osteoderms of Pseudochampsa are avascular and consist of parallel-fibered bone, which suggests that these elements grew at a constant, low rate. Conversely, the osteoderms of Chanaresuchus are well-vascularized structures composed of zones of woven-fibered bone and annuli of parallel-fibered bone. Arcucci et al. (2019) analyzed indeterminate proterochampsian specimens that revealed a predominance of fibro-lamellar tissue, suggesting fast bone growth.
The aim of the present contribution is to describe in detail and make comparissons between the bone histology of several archosauriforms from the Los Chañares Formation. These analyses may be useful to infer details on the behavior, ecology, and the growth patterns of roughly coeval proterochampsids and lagerpetids. On this basis, the disparity in growth strategies and the diverse ecological strategies carried out by several archosauromorphs of the Los Chañares Formation are compared in some detail. We also analyze the paleohistological data in the light of the varied phylogenetic proposals performed by different authors (Bonaparte 1984; Arcucci 1986; Sereno and Arcucci 1990, 1993; Sereno 1991; Dilkes and Sues 2009; Ezcurra et al. 2010; Novas and Agnolin 2015).
Institutional abbreviations.— MCZ, Museum of Comparative Zoology, Harvard University, Massachusetts, USA; PULR, Universidad Nacional de La Rioja, La Rioja, Argentina; PVL, Fundacion Miguel Lillo, San Miguel de Tucuman, Argentina.
Other abbreviations.—CCCB, Compact Coarse Cancellous Bone; EFS, External Fundamental System; ICL, Inner Circumferential Layer; LAG, Lines of Arrested Growth.
Material and methods
Bone tissue samples were extracted from mid-shaft of long bones (Fig. 1) belonging to: Lagerpeton chanarensis, femur (PULR-V 124) and femur and tibia (PVL-4625); one femur of Chanaresuchus bonapartei (PULR-V 125) and one femur of Tropidosuchus romeri (PVL-4604), all from the Los Chañares Formation, in the Rioja Province, Argentina. The specimens are confidently referred to the respective taxa carrying out a bibliographic review of Romer (1971a, b), Sereno and Arcucci (1993) and Arcucci (1990).
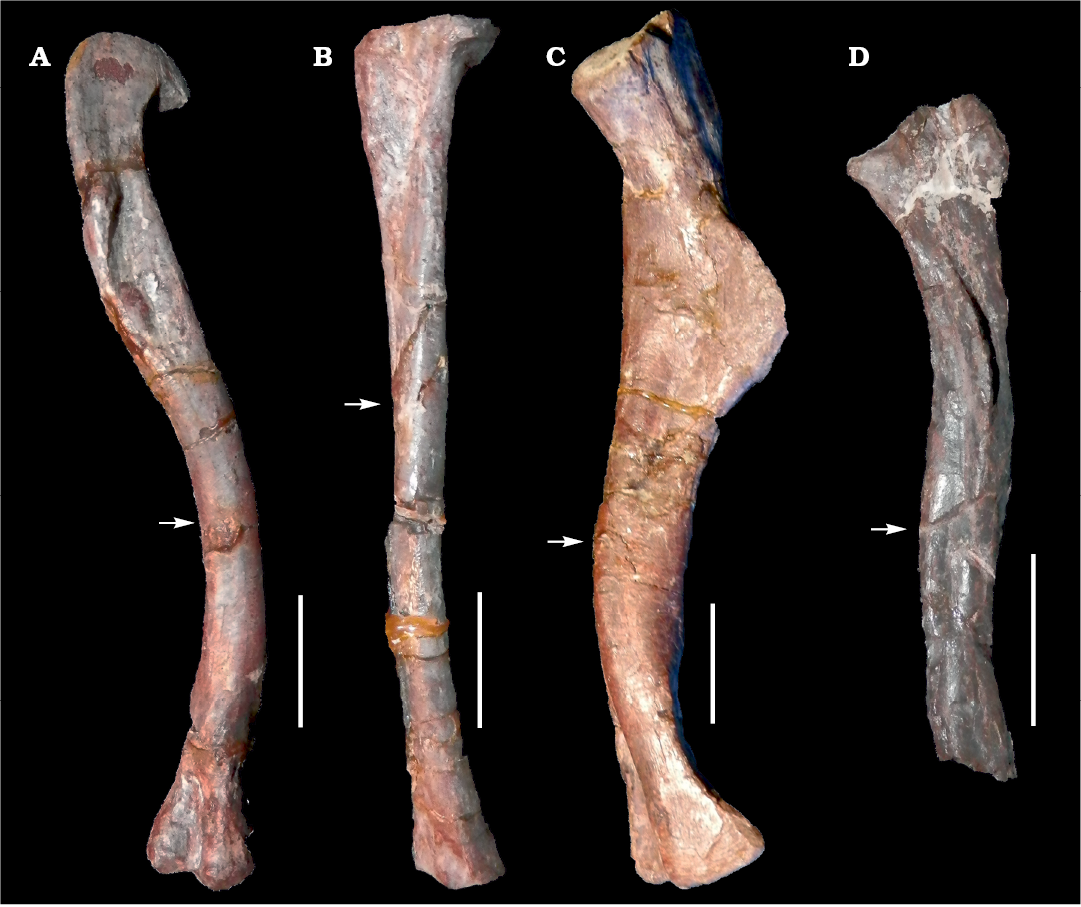
Fig. 1. Long bones of archosauromorphs from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). A, B. Lagerpeton chanarensis Romer, 1971 (PVL-4625) femur in posteromedial view (A) and tibia in lateral view (B). C. Chanaresuchus bonapartei Romer, 1971 (PULR-V 125), femur in posteromedial view. D. Tropidosuchus romeri Arcucci, 1990 (PVL-4604), femur in posteromedial view. Arrows indicate place of the mid-shaft extraction of bone tissue samples. Scale bars 10 mm.
PVL-4625 is an incomplete Lagerpeton skeleton, previously reported by Sereno and Arcucci (1993). This specimen is referred to Lagerpeton chanarensis on the basis of a unique combination of characters, including posterior dorsal vertebrae with anterodorsally inclined neural spines, first sacral vertebra with fan-shaped rib extending anterodorsally to the tip of the preacetabular process of the ilium, iliac blade with sinuous dorsal margin, preacetabular process laterally convex with anterior end directed anteromedially, ischial peduncle of ilium recessed, band-shaped eminence passing posterodorsally across lateral surface of postacetabular process, ischium with broad convex ventromedial flange and vertically deep puboischial suture, distal ischial blades horizontal, proximal end of pubis with subtriangular lateral fossa, pubic shaft deflected medially distal to ambiens process (Romer 1971a; Sereno and Arcucci 1993). The femur PULR-V 124 is referred to Lagerpeton are characterized by proximal end of femur with flat anteromedial surface, deep femoral head with hook-shaped medial extension, elongate aliform fourth trochanter, distal end of femur with large fibular condyle, among other traits (Müller et al. 2018).
PVL-4604 is a nearly complete skeleton previously described by Arcucci (1990), as belonging to Tropidosuchus romeri. The specimen shares with Tropidosuchus a unique combination of derived features, including relatively large orbitd, curved premaxilla at the distal tip, quadrate shaft subvertically oriented, skull roof with ornamentation formed by longitudinal crests, cervical vertebrae notably elongate and distinct from dorsal elemets, being parallelogram-shaped in lateral view (see Trotteyn et al. 2013).
PULR-V 125 is represented by a nearly complete skeleton belonging to Chanaresuchus bonapartei. The specimen shares with Chanaresuchus skull with reduced antorbital fenestra, slit-like external nares placed close together dorsally and distant from the tip of the snout; parietals swing sharply outwards posteriorly, suspensorium far back from the posterior margin of the occiput, lateral fenestra anteroposteriorly elongate, and notably long choanae (Romer 1971b; Trotteyn et al. 2013).
Thin sections were prepared following the method outlined by Chinsamy and Raath (1992). The bones were embedded in a clear epoxy resin (Araldite© GY 279, catalyzed with Aradur® hY 951) and left for 24 hours to set. They were cut into smaller blocks perpendicular to the long axis of the bone using a cut-off diamond tipped saw within a Ken 9025 grinding machine. One surface of each resin block was then affixed to a frosted petrographic glass slide using the same resin that was used for embedding and left to set for a further 24 hours. The sections were wet-ground to approximately 60 µm thick and polished using a Prazis APL-S polishing machine with abrasive papers of increasing grit size (P80, P120, P320, P400, P600, P1200, P1500, P2000, P3000). Samples were studied using a Zeiss Axio Scope.A1 petrographic polarizing microscope under normal, polarized, and lambda light regimes.
Vascularization in the samples have not been quantified. Therefore, the relative densities of vascular canals are assessed visually and described qualitatively, we follow the terminology of Warshaw (2008), with modifications. The following three terms are used: (i) sparse vascularization, vascular canals are irregularly distributed, with avascular stretches between them that generally exceed three times the diameter of the canals; (ii) moderate vascularization, vascular canals are more regularly distributed, with the distance between canals frequently less than three times the diameter of the canals; (iii) dense vascularization, vascular canals are separated from each other by less than the diameter of two canals.
Results
Lagerpeton chanarensis Romer, 1971.—Femur (PULR-V 124): Two samples were extracted from this femur. The first sample is eroded on its anterolateral surface and its posterior edge is broken off. However, this has not affected the main features of bone microstructure and medullar cavity (Fig. 2A1). The second sample corresponds to a small broken portion of a femur in oblique section.
In the first sample, the compacta is densely vascularized is composed of fibro-lamellar tissue, with an annulus in the compacta midway. The vascular canals are longitudinally oriented, and there are some primary osteons in the inner cortex. The medullary cavity is surrounded by a thin layer of endosteally deposited lamellar bone tissue that forms the inner circumferetial layer (ICL). An external fundamental system (EFS) was not observed. A high density of circular osteocyte lacunae are present throughout the cortex. In the inner circumferential lamellae the osteocyte lacunae are elongated.
Restricted to the medial portion of the shaft of the femur, there is an area where the perimedullar region is sharply separated of the compacta by a thin layer of compact coarse cancellous bone (CCCB). The CCCB does not expand beyond this limited area situated between the inner-middle cortex (Fig. 2A2). CCCB is formed through the compaction of trabeculae in the metaphysis, subsequently incorporated into the diaphyseal cortex during longitudinal growth and presents a characteristic structure of compacted lamellar trabeculae (Enlow 1962).
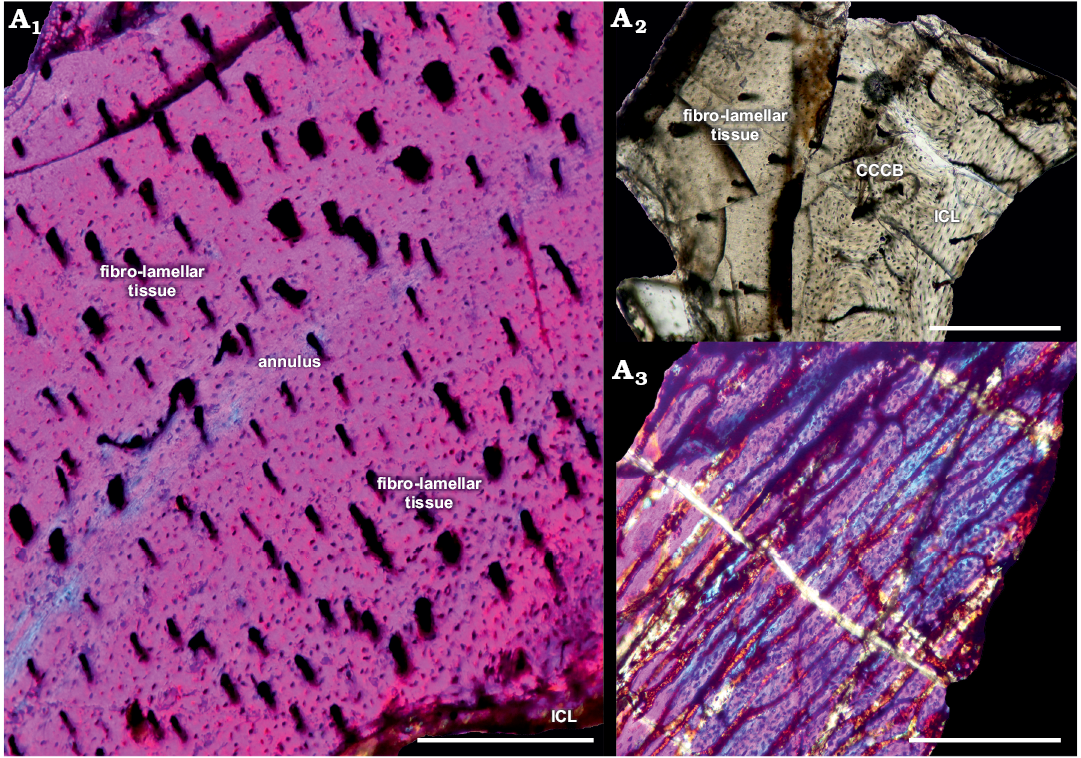
Fig. 2. Femur of archosauromorph Lagerpeton chanarensis Romer, 1971 (PULR-V 124, in lambda light) from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). Section showing the inner circumferential layer and the cortex (A1), detail of the compact coarse cancellous bone (A2), oblique section of the same femur (A3). Abbreviations: CCCB, Compact Coarse Cancellous Bone; ICL, Inner Circumferential Layer. Scale bars 300 µm.
In the second sample corresponds to a small broken portion of a femur. There is dense vascularization in the cortex, preserved in oblique section (Fig. 2A3). The vascular canals, conformed as primary osteons, are interconnected by Volkman’s canals.
Femur (PVL-4625): This femur is broken and partially collapsed on its anterior surface, whereas the posterior half is totally broken. This does not affect the main features of the preserved bone microstructure as well as the medullary cavity (Fig. 3A1)
The compacta is densely vascularized and is constituted of fibro-lamellar tissue with an annulus in the inner compacta. Vascular canals are conformed as primary osteons and are longitudinally oriented, with some anastomoses present. There are some Volkman’s canals between primary osteons. The medullary cavity is surrounded by a thin layer of endosteally deposited lamellar bone tissue that forms the ICL. The subperiosteum is composed by parallel-fibered tissue. A high density of subcircular osteocyte lacunae are present throughout the cortex. In the inner circumferential lamellae the osteocyte lacunae are elongated. Restricted to the anterior portion of the shaft of the femur the perimedullar region is sharply separated of the compacta by a thin layer of CCCB (Fig. 3A2), which presents a characteristic structure of compacted lamellar trabeculae. The CCCB does not expand beyond of this limited area situated between the inner-middle cortex.
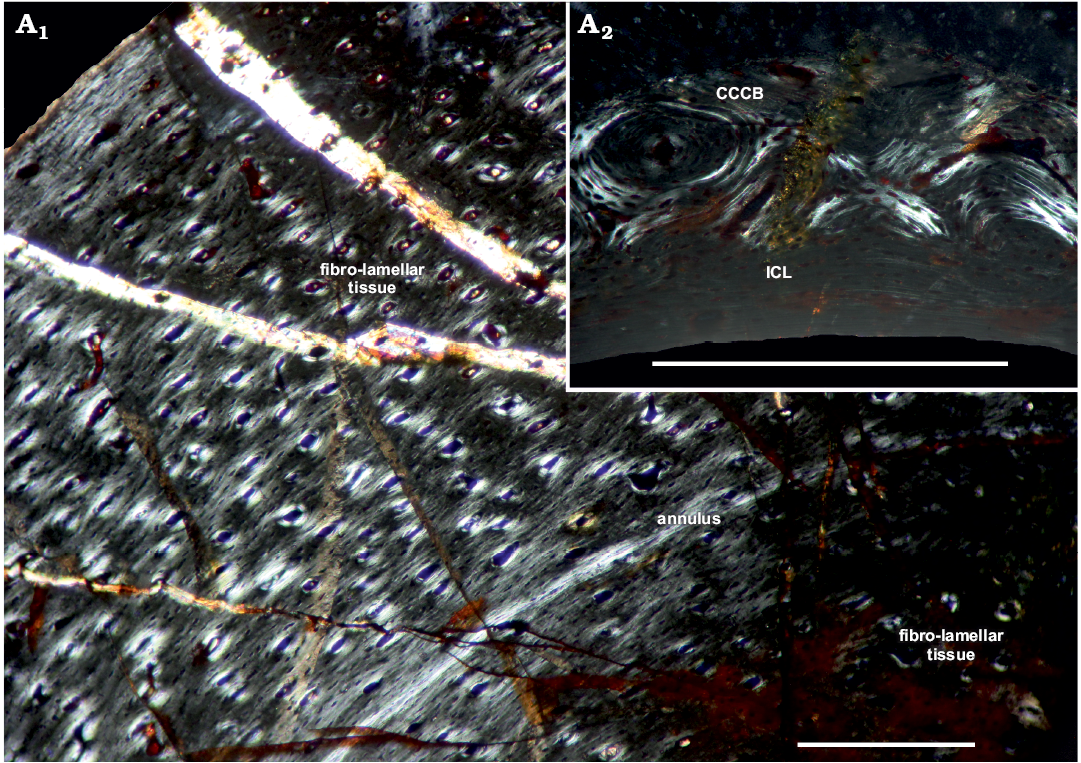
Fig. 3. Femur of archosauromorph Lagerpeton chanarensis Romer, 1971 (PVL-4625, in polarized light) from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). Section showing the inner circumferential layer and the cortex (A1), detail of the compact coarse cancellous bone (A2). Abbreviations: CCCB, Compact Coarse Cancellous Bone; ICL, Inner Circumferential Layer. Scale bars 300 µm.
Tibia (PVL-4625): The tibia of this specimen is partially broken on its posteromedial surface. This does not affect the main features of bone microstructure as well as the medullar cavity (Fig. 4).
The compacta is densely vascularized and is constituted of fibro-lamellar tissue, with the presence of a LAG in the compacta midway. The vascular canals are radially oriented, some of these canals are primary osteons in the inner cortex. Longitudinal and reticular canals are sparse, but they are scattered throughout the cortex. There are some Volkman’s canals. The medullary cavity is surrounded by a layer of endosteally deposited lamellar bone tissue, which forms the ICL. An EFS was not observed. A high density of subcircular osteocyte lacunae are present throughout the cortex. In the inner cortex some osteocyte lacunae have canaliculi. In the inner circumferential lamellae, the osteocyte lacunae are elongated.
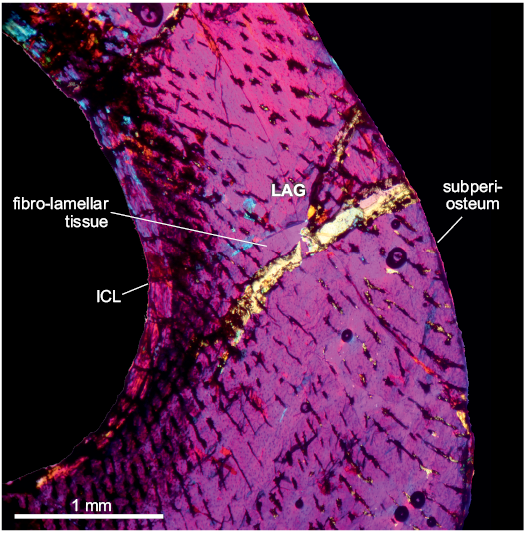
Fig. 4. Tibia of archosauromorph Lagerpeton chanarensis Romer, 1971 (PVL-4625, in lambda light) from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). Section showing the main features of the tibia bone microstructure. Abbreviations: ICL, Inner Circumferential Layer; LAG, line of arrestred growth.
Chanaresuchus bonapartei Romer, 1971.—Femur (PULR-V 125): The transverse section of the bone has been partially deformed by diagenetic processes and the medullary cavity is filled with sediment and bone cortex fragments. The outer cortex and subperiosteum are partially broken. This does not affect the main features of bone microstructure and bone marrow (Fig. 5).
The compacta is densely vascularized. The fibro-lamellar compacta shows a cyclical growth with the presence of a LAG and annulus. In the first zone, from the ICL to the LAG the tissue is conformed as parallel-fibered. In a second zone, there are two subdivisions: from the LAG to the middle of the cortex the tissue is parallel-fibered. In contrast, from the middle of the cortex to the annulus the tissue is fibro-lamellar. At the third zone, starting from the avascular lamellar-zonal annulus, the tissue is parallel-fibered, comprising a thin layer compared with the previous zone, which ends in a thin avascular lamellar layer. Vascular canals are reticular and longitudinal oriented, there are some primary osteons in the inner cortex. The medullary cavity is surrounded by a layer of endosteally deposited lamellar bone tissue, that forms the ICL. The subperiosteum is composed of lamellar-zonal tissue. A high density of circular osteocyte lacunae are present throughout the cortex. In the inner circumferential lamellae the osteocyte lacunae are elongated.
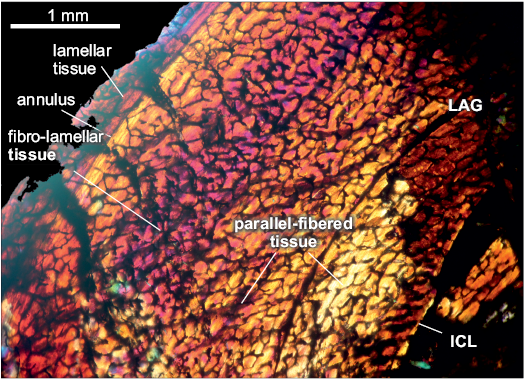
Fig. 5. Femur of archosauromorph Chanaresuchus bonapartei Romer, 1971 (PULR-V 125, in lambda light) from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). Section showing the main features of the femur bone microstructure. Abbreviations: ICL, Inner Circumferential Layer; LAG, line of arrestred growth.
Tropidosuchus romeri Arcucci, 1990.—Femur (PVL-4604): The cross-section of the bone is well preserved (Fig. 6).
The compacta is densely vascularized and conformed into fibro-lamellar tissue. The cortex is uninterrupted by growth marks. Vascular canals are represented by primary osteons and are mainly disposed as longitudinal canals, though more scarce reticular canals are present. The medullary cavity is surrounded by a layer of endosteally deposited lamellar bone tissue that forms the ICL.
Towards the outer cortex the fibro-lamellar tissue changed in some places by patches of parallel-fibered tissue. The subperiosteum is composed of parallel-fibered tissue.
The osteocyte lacunae are less abundant than in Chanaresuchus and Lagerpeton, and are subcircular in contour and present throughout the cortex and the inner circumferential lamellae.
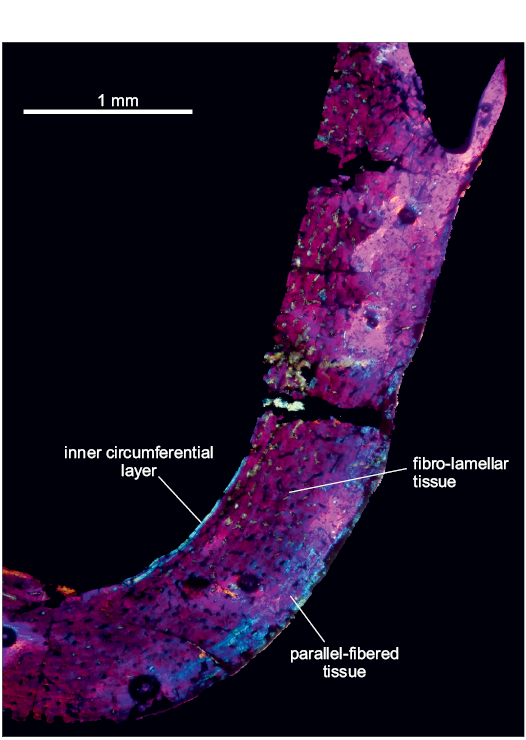
Fig. 6. Femur of archosauromorph Tropidosuchus romeri Arcucci, 1990 (PVL-4604, in lambda light) from La Rioja province, NW Argentina; Chañares Formation, Carnian (Late Triassic). Section showing the main features of the femur bone microstructure.
Discussion
Ontogenetic age of the studied specimens.—In bone tissues of tetrapods, reduced vascularization, predominance of longitudinal canals and lack of anastomoses, narrowing of zones deposited between successive LAGs, and formation of an EFS at the bone perimeter are all indicative of a slowing growth rate in an individual (e.g., Horner et al. 1999; Horner et al. 2000; Padian and Horner 2004; Fostowicz-Frelik and Sulej 2010; Knoll et al. 2010; Marsà et al. 2017). Some of these traits are present in the material here reported.
The absence of an EFS suggests that the growth had not completely ceased in Lagerpeton individuals, indicating that the described specimens had not reached somatic maturity. The annulus in the two available femora (PVL-4625 and PULR-V 124) and the LAG in the tibia appear not to be related to an overall decrease in growth rate. The vascularization in Lagerpeton does not decrease in external portions of the cortex and the bone bears a fast-growing aspect, lacking lamellar or pseudo-lamellar tissues. Annuli and LAGs in Lagerpeton specimens could represent an annual/seasonal pattern of growth cessation, triggered whether the environment happened to be stressful that year.
In contrast with Lagerpeton, in the Chanaresuchus hindlimb the vascularization decreases towards the periphery. Growth zones of the cortex decreases in width towards the subperiosteum. The subperiosteum is composed of a thin, avascular lamellar-zonal layerlayer without internal LAGs, but in a similar position of an EFS. These features indicate a strong decrease in growth rate, which would suggest that the specimen has nearly reached its somatic maturity.
In Tropidosuchus, towards the outer cortex the fibro-lamellar tissue is replaced in some places by parallel-fibered tissue patches, indicating a decrease in growth rate. In the subperiosteum a thin, avascular parallel-fibered layer is present in a similar position of an EFS. This also indicates a decrease in growth rate, suggesting that the specimen had nearly reached somatic maturity.
The absence of an EFS is shared with indeterminate proterochampsian specimens studied by Arcucci et al. (2019), indicating that these specimens were still growing, and were not fully-grown adults, at the time of death.
In some archosaurs, although the closure of the neurocentral suture is not an unambiguous indicator of maturaty, for many groups of archosaurs it has been shown be a morphological evidence of adult age (Brochu 1996; Irmis 2007). In all studied specimens, with the exception of PULR-V 124 that consists of an isolated femur, the neurocentral sutures are closed in the available cervical, dorsal and caudal vertebrae, indicating at least some degree of somatic maturity if this taxon follows pseudosuchian fusion pattern (Brochu 1996; Irmis 2007).
The EFS is an informative feature in palaeohistological studies because it indicates skeletal maturation and slowing down in the rate of bone deposition (e.g., Chinsamy-Turan 2005; Woodward et al. 2011). It is typical of taxa with determinate growth and consists of completely avascular lamellar or parallel-fibered bone, that may or may not have annuli or LAGs (Chinsamy-Turan 2005). In our sample, although the arrangement of the collagen fibers in the outer cortex of some specimens becomes parallel, the bone tissue is never avascular. Therefore, no fully-grown specimens are present in our sample of Chañares Formation archosauriforms. The well-vascularized nature of the external cortex (which also contains vascular spaces open to the sub-periosteal surface) is indicative of active bone deposition, suggesting that even our largest individuals were actively growing at the time of death (e.g., Chinsamy-Turan 2005; Erickson 2005; Cerda and Chinsamy-Turan 2012).
Proterochampsid growth patterns.—The evolution of bone growth rates and metabolic rates in Archosauromorpha represents an area of active research (e.g., Horner et al. 2000; Starck and Chinsamy 2002; Padian et al. 2004; Ricqlès et al. 2003, 2008; Erickson 2005; Fostowicz-Frelik and Sulej 2010; Griffin and Nesbitt 2016; Marsà et al. 2017; Griffin et al. 2019; Veiga et al. 2019). Bone microstructure reflects growth rate, in agreement with the Amprino’s Rule (Padian and Horner 2004; Ricqlès et al. 2008). Bone tissues of Lagerpeton and Tropidosuchus suggest a fast growth rate, significantly higher than most crurotarsans. The fibro-lamellar tissue of these taxa is characterized by dense vascularization mainly with longitudinal vascular canals, with radial and reticular canals also present, and zonation of the cortex is not evident. This combination of traits also occurs in some crurotarsans, including Terrestrisuchus gracilis Crush, 1984, Postosuchus kirkpatricki Chatterjee, 1985, as well as dinosauromorphs (Ricqlès et al. 2003, 2008; Padian and Horner 2004). Notably, the samples of Lagerpeton and Tropidosuchus show less vascularized tissues than in dinosaurs (Horner et al. 2000; Starck and Chinsamy 2002; Ricqlès et al. 2003; Padian and Horner 2004), being most comparable to basal dinosauriforms (e.g., Silesaurus, Asilisaurus, Lewisuchus; Fostowicz-Frelik and Sulej 2010; Griffin and Nesbitt 2016; Marsà et al. 2017).
In the proterochampsid Chanaresuchus, the compacta is more densely vascularized than in Lagerpeton and Tropidosuchus and fibro-lamellar, parallel-fibered and lamellar-zonal tissues are present. Contrary to Lagerpeton and Tropidosuchus, the cyclical growth of Chanaresuchus shows the presence of LAG, annuli and a thin avascular layer (in an EFS-equivalent position), which would indicate a notable decrease in the growth rate. In concordance, Ricqlès et al. (2008) studied a section of an indeterminate long bone shaft attributed to Chanaresuchus. This bone shows a well-developed reticulum of short vascular anastomoses uniting longitudinally oriented vascular canals. These authors noted an early rapid growth rate (based on an inner thick region of fibro-lamellar bone), which dramatically decreased later in ontogeny (based on the presence of peripheral lamellar-zonal bone; Ricqlès et al. 2008). The histology of the specimen described by Ricqlès et al. (2008) is very similar to the femur of Chanaresuchus here described, but in the latter the vascularization is even greater. Previously, Arcucci et al. (2019) conducted a histological examination of indeterminate proterochampsian specimens that revealed a predominance of fibrolamellar bone tissue, suggesting rapid periosteal osteogenesis and therefore overall fast bone growth.
The absence of an EFS in Chanaresuchus, Tropidosuchus, and Lagerpeton, as well as the proterochampsians studied by Arcucci et al. (2019) indicates that they were still growing, and that they were not fully-grown adults (Klein and Sander 2008). Fibro-lamellar tissue is shared between Chanaresuchus, Tropidosuchus, and Lagerpeton, whereas the presence of LAGs is shared by Chanaresuchus and Lagerpeton. The abundance of fibrolamellar cortical tissue and the existence of LAGs was documented in the indeterminate proterochampsian analyzed by Arcucci et al. (2019). An exception to this fibro-lamellar tissue is the proterochampsid Pseudochampsa ischigualastensis Trotteyn and Ezcurra, 2014, which shows a low growth rate (Cerda et al. 2015). These diverse growth patterns and rates suggest a deep variation of metabolism within Proterochampsia, a condition previously unknown in any non-archosaurian archosauriform clade. Along the same line of thought, the bone histology of Dromomeron romeri (Griffin et al. 2019) is similar to that of Lagerpeton chanarensis (woven-bone tissue, moderate to dense vascular canals in longitudinal configuration), but with higher LAGs numbers in Dromomeron.
Growth patterns here described differ from that seen in other archosauromorphs, as for example, in archosauromorphs from the Triassic faunas of Karoo, South Africa (see Botha-Brink and Smith 2011; e.g., Prolacerta brommi Parrington, 1935, Proterosuchus fergusi, Broom, 1903, Erythrosuchus africanus Broom, 1905, Euparkeria capensis Broom, 1913). The growth pattern of the early Triassic Prolacerta broomi exhibits a vascular pattern with longitudinally-oriented primary osteons, with anastomoses in some vascular canals, and with the prevalence of a mixture of weakly developed fibrolamellar and parallel-fibered tissue (Botha-Brink and Smith 2011). These features suggest notably lower growth rates than in Chañares archosauromorphs. Similar-aged Proterosuchus fergusi, a medium-sized proterosuchid, possesses radiating vascular canals within fibrolamellar bone tissue during early ontogeny, but its growth decreases dramatically during late ontogeny, with the onset of poorly vascularized lamellar-zonal bone tissue (Botha-Brink and Smith 2011). This contrasts with Chañares archosauromorphs which shows active growth.
The early Middle Triassic Erythrosuchus africanus (Gross 1934; Ricqlès 1976; Ricqlès et al. 2008; Botha-Brink and Smith 2011) exhibits uninterrupted growth and a variety of vascular patterns including laminar, reticular and radial, all within fibro-lamellar bone tissue, indicating rapid growth rates, similar to those found in fast-growing dinosaurs. The growth rates of Lagerpeton and Tropidosuchus are less than those of Erythrosuchus. Chanaresuchus shows a fast growth with a complex pattern of vascular canals, but with cyclical growth. Euparkeria capensis exhibits sparse to moderate vascular canals embedeed in parallel-fibered bone (Botha-Brink and Smith 2011). Euparkeria shows low growth rates compared with Chañares Formation archosauromorphs.
The histology of Teleocrater rhadinus Nesbitt, Butler, Ezcurra, Barrett, Stocker, Angielczyk, Smith, Sidor, Niedźwiedzki, and Charig, 2017, sister taxon of Ornithodira, resembles Lagerpeton and Tropidosuchus. In these taxa the cortex consists of woven bone tissue with longitudinal vascular canals and primary osteons, but in Teleocrater the number of anastomoses is higher (Nesbitt et al. 2017). Chanaresuchus shows growth patterns similar to Teleocrater, with a high number of anastomosis, but in Teleocrater the growth is uninturrupted.
The histology of hindlimb bones described here suggests that Lagerpeton and Tropidosuchus show similar growth rates, and together with Chanaresuchus exhibited a fast growth. This implies that these taxa possessed a relatively high metabolic rate, comparable to that seen in other archosaurs, including non-dinosaurian Dinosauriformes (Fostowicz-Frelik and Sulej 2010; Griffin and Nesbitt 2016; Marsà et al. 2017; Veiga et al. 2019). The osteohistology of Sacisaurus, Silesaurus, Asilisaurus, and Lewisuchus exhibit predominantly longitudinally-oriented primary osteons with few or no anastomoses. This simple vascular pattern is common to all silesaurids studied to date and indicates relatively slower growth rates when compared to most Dinosauria (Fostowicz-Frelik and Sulej 2010; Griffin and Nesbitt 2016; Marsà et al. 2017; Veiga et al. 2019).
The bone histology of the dinosauriform Nyasasaurus parringtoni Nesbitt, Barrett, Werning, Sidor, and Charig, 2012 is constituted by vascular canals configured as primary osteons, many of these canals are longitudinal canals, but at least half of these anastomose with other canals (in all directions), and locally, short radial canals may dominate. The outermost cortex shows transition from woven-fibred to paralleled-fibred bone. The bone histology of Nyasasaurus is similar to that of early dinosaurs, with plexiform and laminar vascular patterns (e.g., Herrerasaurus ischigualastensis Reig, 1963; Ricqlès et al. 2003; Padian et al. 2004; Tawa hallae Nesbitt, Smith, Irmis, Turner, Downs, and Norell 2009; Werning 2013; Coelophysis bauri Cope, 1887; Colbert 1995; Nesbitt et al. 2006; Megapnosaurus rhodesiensis Ivie, Slipinski, and Wegrzynowicz, 2001; Chinsamy 1990; Werning 2013; Lesothosaurus diagnosticus Galton, 1978; Knoll et al. 2010; Plateosaurus engelhardti Meyer, 1837; Klein and Sander 2007; Mussaurus patagonicus Bonaparte and Vince, 1979; Cerda et al. 2014; Massospondylus carinatus Owen, 1854; Chinsamy 1993). These features indicate that early saurischian dinosaurs had higher growth rates than Chañares archosauromorphs.
In contrast, the growth rate and levels of tissue disorganization are higher in Chañares archosauromorphs than in early ornithischians such as Fruitadens haagarorum Butler, Richard, Galton, Porro, Chiappe, Henderson, and Erickson, 2010 and Scutellosaurus lawleri Colbert, 1981, which exhibit slower rates of tissue deposition (Butler et al. 2010; Padian et al. 2004). In this sense, Fruitadens exhibits parallel-fibered bone tissue (Butler et al. 2010) and Scutellosaurus contains few fibrolamellar bone during early ontogeny and entirely lamellar-zonal bone tissue during adulthood (Padian et al. 2004).
The Late Triassic pterosaur Eudimorphodon ranzii Zambelli, 1973 shows moderate vascularization and a woven-fibered tissue cortex to parallel-fibered bone tissue later in ontogeny, with some anastomoses in few vascular canals (Padian et al. 2004; Werning 2013). Dimorphodon Owen, 1859, an Early Jurassic pterosaur, possesses fibro-lamellar bone tissue with some areas of more parallel-fibered bone, but with anastomosing vascular canals (Ricqlès et al. 2000; Padian et al. 2004; Werning 2013). The Early Jurassic pterosaur Dorygnathus banthensis Wagner, 1860 (Gross 1934), has more typical fibro-lamellar cortical tissues. Tropidosuchus and Lagerpeton show growth patterns similar to Dimorphodon and Dorygnathus, but with a lesser vascularity and anastomosis. Eudimorphodon shows a low growth pattern when compared with Chañares archosauromorphs.
Behavioral implications of paleohistological analysis.—Reig (1959) proposed semiaquatic habits for the proterochampsid Proterochampsa barrionuevoi Reig, 1959 based on the elongated and triangular snout and dorsally flattened head, a distinctive pattern of dermal ornamentation of the skull, and dorsally located orbits and external nares. These morphological characteristics resemble those of Recent crocodilians, which led several authors to suggest a probable aquatic lifestyle for proterochampsians, a hypothesis that was accepted by later authors and extrapolated to other members of the clade (Sill 1967; Romer 1971b, 1972; Bonaparte 1978). Cerda et al. (2015) studied osteoderm histology of proterochampsids, and concluded that the compact nature of their osteoderms fits with an increase in bone mass (sensu Houssaye 2013) supporting aquatic or semiaquatic lifestyle. However, Cerda et al. (2015) also indicated that given the diminutive size and low number of osteoderms in each individual (just a single row of osteoderms dorsal to the vertebral column), it is improbable that osteoderm compactness actually modifies the whole skeletal mass.
More recently, Arcucci (2011) noted that the proterochampsids Chanaresuchus bonapartei, Gualosuchus reigi Romer, 1971, and Tropidosuchus romeri, may have had a more terrestrial lifestyle than the genus Proterochampsa. Arcucci’s (2011) proposal rests on the fact that Chañares proterochampsids (i.e., Chanaresuchus, Gualosuchus, Tropidosuchus) lack anatomical features typical of aquatic taxa (e.g., absence of secondary palate, laterally compressed teeth, limbs long and slender, lacking signs of digit reduction or enlargement of the distal elements, subvertically positioned limbs, and tail not dorsoventrally tall, but transversely wide). In contrast, Arcucci et al. (2019) argued than anatomical features recognized in proterochampsians, such as marginal dentition, palatal teeth, morphology of the tail, limb modification, and dermal armor suggest a more terrestrial life style.
Several studies have demonstrated that modifications in bone microanatomy in tetrapods are correlated with lifestyle (Ricqlès and Buffrénil 2001; Houssaye 2009). In this regard, adaptation to aquatic life may be inferred on the basis of bone microstructure (Houssaye 2009; Canoville and Laurin 2010). Extant and extinct vertebrates secondarily adapted to aquatic life show four major bone types: osteoporotic, pachyostotic, osteosclerotic and pachy-osteosclerotic (Ricqlès and Buffrénil 2001; Houssaye 2009). Osteosporotic-like type consists of a lightening of the bones by decreasing the thickness of the compact cortical bone and increasing the osseous porosity. Conversely, pachyostosis (sensu stricto) corresponds to a hyperplasy (increase of deposit) of periosteal cortices that leads to an alteration of the bone morphology by increasing its volume (Ricqlès and Buffrénil 2001; Houssaye 2009). Osteosclerosis is a increase of bone inner compactness either as a result of incomplete endochondral ossification, inhibition of secondary remodelling and/or the filling of inner cavities, with no effect on the external dimensions of the bone. Finally, pachyosteosclerosis corresponds to the combination of the pachyostotic and osteosclerotic states in the same bone (Ricqlès and Buffrénil 2001; Houssaye 2009).
Chanaresuchus shows a pachyostotic femur, with a relatively reduced medullary cavity and wide cortex, being consistent with semiaquatic habits proposed by previous authors (in fact, the increase of the skeletal mass in pachyostotic bones is interpreted as an adaptation to reduce buoyancy; Taylor 2000). The pachyostotic condition of Chanaresuchus contrasts with Tropidosuchus, which shows a very thin cortex and wide medullar cavity, consistent with terrestrial habits. Conversely, Lagerpeton has been invariably considered as having terrestrial habits (Sereno and Arccuci 1993).
The cortex compactness and reduced medullary cavity recorded for the indeterminate proterochampsians studied by Arcucci et al. (2019), support an amphibious/terrestrial life style.
Palaeoecological implications.—The paleohistologiccal analysis of proterochampsids and Lagerpeton show two different growth strategies: on one hand a rapid growth with one or any growth mark in Lagerpeton and Tropidosuchus; on the other hand, Chanaresuchus was characterized by a marked cyclical growth, with fast growth intercalated with seasonal interruptions (sensu Chinsamy and Hurum 2006), showing a decrease in the growth rate to the subperiosteum.
The depositional environment in which the specimens of Lagerpeton, Tropidosuchus, and Chanaresuchus were found suggests a warm climate with variable rainfall, with humid periods alternating with extensive and harsh drier periods (Tucker and Benton 1982; Rogers et al. 2001; Mancuso et al. 2014). Their rapid early growth reflects a plesiomorphic growth strategy for archosauriforms (Ricqlès et al. 2008), including proterochampsids and Lagerpeton. Fast growth rates at early age may have improved juvenile survivorship in Chañares Formation environmental conditions (e.g., Tinkle 1969; Gasser et al. 2000; Curtin et al. 2009). The growth strategy observed in Tropidosuchus and Lagerpeton contrasts with that of Chanaresuchus, which is characterized by seasonal interruptions. In agreement with de Ricqlès et al. (2008), we posit that Chanaresuchus possesses progressively increased growth rates, exhibiting rapid early growth, but slow and cyclical late growth.
Phylogenetic implications.—Some authors have asserted that histological features may express “individual histories” but do not shed light on phylogenetic relationships (Castanet et al. 2001; Cubo et al. 2005; Ricqlès et al. 2004). However, recent authors (e.g., Padian et al. 2001, 2004; Padian and Horner 2004; Ricqlès et al. 2003, 2008; Fostowicz-Frelik and Sulej 2010; Legendre et al. 2014; Marsà et al. 2017; Veiga et al. 2019) agree in that histological characters may reflect phylogenetic signal.
Most non-avian archosauriforms show relatively slow growth rates (e.g., Ricqlès et al. 2003; Botha and Smith 2011). Some taxa, as Terrestrisuchus, erythrosuchians and Proterosuchus show a growth rate that is higher than in most crurotarsans, with bone microstructure that is highly similar to that observed in small dinosaurs (Ricqlès et al. 2003, 2008; Botha and Smith 2011). Ricqles et al. (2008) suggested that the ability to reach and maintain rapid growth rates during at least early and mid-ontogenetic stages is plesiomorphic for archosauriforms and that the Triassic was a time of experimentation in growth strategies. Pseudosuchians more derived than sphenosuchian Terrestrisuchus, secondarily reverted to a poorly vascularized matrix, and an indeterminate cyclical growth that persists in living crocodiles (Ricqlès 1978; Schweitzer and Marshall 2001; Botha and Smith 2011).
In this sense, the bone microstructure of Lagerpeton, Tropidosuchus and Chanaresuchus indicates notably high growth rates, congruent with previous studies indicating fast growth rates is plesiomorphic for archosauriforms (Ricqlès et al. 2003, 2008; Fostowicz-Frelik and Sulej 2010; Marsà et al. 2017).
Lagerpeton and Tropidosuchus resemble each other in the distribution and abundance of vascular canals composed by longitudinal primary osteons, some anastomoses, and the fibro-lamellar matrix throughout the cortex. This pattern differs from other basal archosauromorphs, and resemble non-dinosaurian dinosauriforms (Fostowicz-Frelik and Sulej 2010; Griffin and Nesbitt 2016; Marsà et al. 2017). Similar histological features suggest that these two taxa shared similar life history strategies and could indicate a possible close phylogenetic relationships, as was previously proposed by Novas and Agnolín (2015).
Conclusions
Lagerpeton and Tropidosuchus show a rapid growth rate, and the sampled individuals both lack evidence of a decrease in their growth rate. These two taxa resemble each other in the distribution of primary osteons, presence of anastomosis and fibro-lamellar bone tissues throughout the cortex. This suggests that these two taxa shared similar life history strategies (as growth rates, growth patterns, and ontogenetic stages) and also possible close phylogenetic relationships (Novas and Agnolín 2015; Fechner 2009; Arcucci 1986, 1990). Chanaresuchus, with a reduced medullary cavity related to a terrestrial/amphibious life style, exhibits a denser vascularization with a reticular pattern, with a cyclical growth conformed by an alternate fibro-lamellar, parallel-fibered and lamellar-zonal tissues. This is strongly different from the condition exhibited by Lagerpeton and Tropidosuchus. It is remarkable the disparity in growth strategies evolved among archosaurmorphs of Chañares at the same paleonvironment. However, it is not particularly surprising that different growth strategies exist within the same environment—all through vertebrate history there have been a diversity of successful growth strategies in the same environment.
Fast early growth and acquisition could represent an advantageous plesiomorphic trait to the low juvenile survivorship hypothesized for the Chañares Formation environment, as previously suggested for the coeval basal dinosauriform Lewisuchus (Marsà et al. 2017).
Acknowledgements
We thank Ignacio Cerda (Museo Carlos Ameghino, Cipolletti, Río Negro province, Argentina) for his advice, assistance, and comments on paleohistoligcal techniques. We thank Federico Brisson Egli, Sebastian Rozadilla, Mauricio Alexis Rolando, Mattia Motta, Julia Soledad D’Angelo, Nicolas Chimento and Gabriel Lio (all Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Autonomous City of Buenos Aires, Argentina) for comments and discussion. Special thanks to Mirttha González (Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Autonomous City of Buenos Aires, Argentina) for your assistance in the use of petrographic microscope. The research here presented has been supported by CONICET.
References
Arcucci, A.B. 1986. Nuevos materiales y reinterpretación de Lagerpeton chanarensis Romer (Thecodontia, Lagerpetonidae nov.) del Triásico Medio de La Rioja, Argentina. Ameghiniana 23: 233–242.
Arcucci, A.B. 1990. Un nuevo Proterochampsidae (Reptilia–Archosauriformes) de la fauna local de Los Chañares (Triásico Medio), La Rioja, Argentina. Ameghiniana 27: 365–378.
Arcucci, A.B. 2011. Sistemática y filogenia de los proterochampsidos (Amniota, Diápsida, Archosauriformes) del Triásico de América del Sur, y sus implicancias en el origen de Archosauria. 336 pp. PhD. dissertation, Universidad Nacional de San Luis, San Luis.
Arcucci, A., Previtera, E., and Mancuso, A.C. 2019. Ecomorphology and bone microstructure of Proterochampsia from the Chañares Formation. Acta Palaeontologica Polonica 64: 157–170. Crossref
Bonaparte, J.F. 1978. El Mesozoico de América del Sur y sus Tetrápodos. Opera Lilloana 26: 1–596.
Bonaparte, J.F.1982. Faunal replacement in the Triassic of South America. Journal of Vertebrate Paleontology 2: 362–371. Crossref
Bonaparte, J.F. 1984. Locomotion in rauisuchid thecodonts. Journal of Vertebrate Paleontology 3: 210–218. Crossref
Botha-Brink, J. and Smith, M.H. 2011. Osteohistology of the Triassic archosauromorphs Prolacerta, Proterosuchus, Euparkeria, and Erythrosuchus from the Karoo Basin of South Africa. Journal of Vertebrate Paleontology 31: 1238–1254. Crossref
Brochu, C.A. 1996. Closure of neurocentral sutures during crocodilian ontogeny: implications for maturity assessment in fossil archosaurs. Journal of Vertebrate Palaeontology 16: 49–62. Crossref
Butler, R.J., Galton, P.M., Porro, L.B., Chiappe, L.M., Henderson, D.M., and Erickson, G.M. 2010. Lower limits of ornithischian dinosaur body size inferred from a new Upper Jurassic heterodontosaurid from North America. Proceedings of the Royal Society B 277: 375–381. Crossref
Cabreira, S.F., Kellner, A.W.A., Dias-da-Silva, S., da Silva, L.R., Bronzati, M., de Almeida Marsola, J.C., Müller, R.T., de Souza Bittencourt, J., Batista, B.J., Raugust, T., Carrilho, R., Brodt, A., and Langer, M.C. 2016. A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Current Biology 26: 3090–3095. Crossref
Canoville, A. and Laurin, M. 2010. Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on palaeobiological inferences. Biological Journal of the Linnean Society 100: 384–406. Crossref
Castanet, J., Cubo, J., and Margerie, E. 2001. Signification de l’histodiversité osseuse: le message de l’os. Biosystema 19: 133–147.
Cerda, I.A. and Chinsamy-Turan, A. 2012. Biologial implications of the bone microstructure of the late Cretaceous ornithopod dinosaur Gasparinisaura cincosaltensis. Journal of Vertebrate Paleontology 32: 355–368. Crossref
Cerda, I.A., Pol, D., and Chinsamy-Turan, A. 2014. Osteohistological insight into the early stages of growth in Mussaurus patagonicus (Dinosauria, Sauropodomorpha). Historical Biology 26: 110–121. Crossref
Cerda, I.A., Desojo, J.B., Trotteyn, M.J., and Scheyer, T.M. 2015. Osteoderm histology of Proterochampsia and Doswelliidae (Reptilia: Archosauriformes) and their evolutionary and paleobiological implications. Journal of Morphology 276: 385–402. Crossref
Chinsamy, A. 1993. Bone histology and growth trajectory of the prosauropod dinosaur Massospondylus carinatus Owen. Modern Geology 18: 319–329.
Chinsamy. A. 1990. Physiological implications of the bone histology of Syntarsus rhodesiensis (Saurischia: Theropoda). Palaeontologia Africana 27: 77–82.
Chinsamy, A. and Hurum, J. 2006. Bone microstructure and growth patterns of early mammals. Acta Palaeontologica Polonica 51: 325–338.
Chinsamy, A. and Raath, M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Chinsamy-Turan, A. 2005. The Microstructure of Dinosaur Bone. 195 pp. The Johns Hopkins University Press, Baltimore.
Colbert, E.H. 1995. The Little Dinosaurs of Ghost Ranch. 232 pp. Columbia University Press, New York.
Cubo, J., Ponton, F., Laurin, M., Margerie, E., and Castanet, J. 2005. Phylogenetic signal in bone microstructure of Sauropsids. Systematic Biology 54: 562–574. Crossref
Curtin, A.J., Zug, G.R., and Spotila, J.R. 2009. Longevity and growth strategies of the desert tortoise (Gopherus agassizii) in two American deserts. Journal Arid Environments 73: 463–471. Crossref
Dilkes, D. and Sues, H. 2009. Redescription and phylogenetic relationships of Doswellia kaltenbachi (Diapsida: Archosauriformes) from the Upper Triassic of Virginia. Journal of Vertebrate Paleontology 29: 58–79. Crossref
Enlow, D.H. 1962. A study of post-natal growth and remodeling of bone. American Journal of Anatomy 110: 79–102. Crossref
Erickson, G. 2005. Assessing dinosaur growth patterns: a microscopic revolution. Trends in Ecology and Evolution 20: 677–684. Crossref
Ezcurra, M.D. 2006. A review of the systematic position of the dinosauriform archosaur Eucoelophysis baldwini Sullivan and Lucas, 1999 from the Upper Triassic of New Mexico, USA. Geodiversitas 28: 649–684.
Ezcurra, M.D., Lecuona, A., and Martinelli, A. 2010. A new basal archosauriform diapsid from the Lower Triassic of Argentina. Journal of Vertebrate Paleontology 30: 1433–1450. Crossref
Fechner, R. 2009. Morphofunctional Evolution of the Pelvic Girdle and Hindlimb of Dinosauromorpha on the Lineage to Sauropoda. 197 pp. Ph.D. Dissertation, Ludwigs Maximilians Universität, München.
Fostowicz-Frelik, L. and Sulej, T. 2010. Bone histology of Silesaurus opolensis Dzik, 2003 from the Late Triassic of Poland. Lethaia 43: 137–148. Crossref
Gasser, M., Kaiser, M., Berrigan, D., and Stearns, S.C. 2000. Life-history correlates of evolution under high and low adult mortality. Evolution 54: 1260–1272. Crossref
Griffin, C.T. and Nesbitt, S.J. 2016. The femoral ontogeny and long bone histology of the Middle Triassic (?late Anisian) dinosauriform Asilisaurus kongwe and implications for the growth of early dinosaurs. Journal of Vertebrate Paleontology 36: e1111224. Crossref
Griffin, C.T., Bano, L.S., Turner, A.H., Smith, N.D., Irmis, R.B., and Nesbitt, S.J. 2019. Integrating gross morphology and bone histology to assess skeletal maturity in early dinosauromorphs: new insights from Dromomeron (Archosauria: Dinosauromorpha). PeerJ 7:e6331. Crossref
Gross, W. 1934. Die Typen des mikroskopischen Knochenbaues bei foslen Stegocephalen und Reptilien. Zeitschrift für Anatomie 103: 731–764. Crossref
Horner, J.R., de Ricqlès, A., and Padian, K. 1999. Variation in dinosaur skeletochronology indicators: implications for age assessment and physiology. Paleobiology 25: 295–304. Crossref
Horner, J.R., Ricqlès, A. de, and Padian, K. 2000. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: growth dynamics and physiology based on an ontogenetic series of skeletal elements. Journal of Vertebrate Paleontology 20: 115–129. Crossref
Houssaye, A. 2009. Pachyostosis in aquatic amniotes: a review. Integrative Zoology 4: 325–340. Crossref
Houssaye, A. 2013. Palaeoecological and morphofunctional interpretation of bone mass increase: An example in Late Cretaceous shallow marine squamates. Biological reviews of the Cambridge Philosophical Society 88: 117–139. Crossref
Irmis, R.B. 2007. Axial skeleton ontogeny in the Parasuchia (Archosauria: Pseudosuchia) and its implications for ontogenetic determination in archosaurs. Journal of Vertebrate Paleontology 27: 350–361. Crossref
Irmis, R.B., Nesbitt, S.J., Padian, K., Smith, N.D., Turner, A.H., Woody, D., and Downs, A. 2007. A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science 317: 358–361. Crossref
Klein, N. and Sander, P.M. 2008. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology 34: 247–263. Crossref
Knoll, F., Padian, K., and Riqclès de, A. 2010. Ontogenetic change and adult body size of the early ornithischian dinosaur Lesothosaurus diagnosticus: Implications for basal ornithischian taxonomy. Gondwana Research 17: 171–179. Crossref
Legendre, L.J., Bourdon, E., Scofield, R.P., Tennyson, A.J.D., Lamrous, H., Ricqlès de, A., and Cubo, J. 2014. Bone histology, phylogeny, and palaeognathous birds (Aves: Palaeognathae). Biological Journal of the Linnean Society 112: 688–700. Crossref
Mancuso, A.C., Gaetano, L.C., Leardi, J.M., Abdala, F., and Arcucci, A.B. 2014. The Chanares Formation: a window to a Middle Triassic tetrapod community. Lethaia 47: 244–265. Crossref
Marsà, J.A.G., Agnolín, F.L., and Novas, F.E. 2017. Bone microstructure of Lewisuchus admixtus Romer, 1972 (Archosauria, Dinosauriformes). Historical Biology 31: 157–162. Crossref
Marsicano, C.A., Irmis, R.B., Mancuso, A.C., Mundil, R., and Chemale, F. 2016. The precise temporal calibration of dinosaur origins. Proceedings of the National Academy of Sciences of the United States of America 113: 509–513. Crossref
Martínez, R.N., Apaldetti, C., Colombi, C.E., Alcober, O.A., Sereno, P.C., Fernandez, E., Santi Malnis, P., Correa, G.A., and Abelín, D. 2012. Vertebrate succession in the Ischigualasto Formation. Journal of Vertebrate Paleontology 32: 10–30. Crossref
Müller, R.T., Langer, M.C., and Dias-Da-Silva, S. Ingroup relationships of Lagerpetidae (Avemetatarsalia: Dinosauromorpha): a further phylogenetic investigation on the understanding of dinosaur relatives. Zootaxa 4392: 149–158. Crossref
Nesbitt, S.J. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History 353: 1–292. Crossref
Nesbitt, S.J., Butler, R.J., Ezcurra, M.D., Barrett, P.M., Stocker, M.R., Angielczyk, K.D., Smith, R.M.H., Sidor, C.A., Niedźwiedzki, G., Sennikov, A.G., and Charig, A.J. 2017. The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544: 484–487. Crossref
Nesbitt, S.J., Irmis, R.B., Parker, W.G., Smith, N.D., Turner, A.H., and Rowe, T. 2009a. Hindlimb osteologyand distribution of basal dinosauromorphs from the Late Triassic of North America. Journal of Vertebrate Paleontology 29: 498–516. Crossref
Nesbitt, S.J., Stocker, M.R., Small, B.J., and Downs, A. 2009b. the osteology and relationships of Vancleavea campi (reptilia: Archosauriformes). Zoological of the Journal Linnean Society 157: 814–864. Crossref
Nesbitt, S.J., Turner, A.H., Erickson, G.M., and Norell, M.A. 2006. Prey choice and cannibalistic behavior in the theropod Coelophysis. Biology Letters 2: 611–614. Crossref
Novas, F.E. 1996. Dinosaur monophyly. Journal of Vertebrate Paleontology 16: 723–741. Crossref
Novas, F.E. and Agnolín, F.L. 2015. Lagerpeton chanarensis Romer, 1971 (Archosauriformes): A derived proterochampsian from the Middle Triassic of NW Argentina. In: V Congreso Latinoamericano de Paleontología de Vertebrados, Arroyo del Vizcaíno, 21–23 September 2015. Libro de resúmenes, 48. Departamento de Canelones, Colonia del Sacramento.
Padian, K. and Horner, J.R. 2004. Physiology. In: D.B. Weishampel, P. Dodson, and H. Osmolska (eds.), The Dinosauria. 2nd Edition, 660–671. University of California Press, Berkeley. Crossref
Padian, K., Horner, J.R., and Ricqlès, A. de 2004. Growth in small dinosaurs and pterosaurs: the evolution of archosaurian growth strategies. Journal of Vertebrate Paleontology 24: 555–571. Crossref
Padian, K., Ricqlès, A. de, and Horner, J. 2001. Dinosaurian growth rates and bird origins. Nature 412: 405–408. Crossref
Reig, O.A. 1959. Primeros datos descriptivos sobre nuevos reptiles arcosaurios del Triásico de Ischigualasto (San Juan, Argentina). Revista de la Asociación Geológica Argentina 13: 257–270.
Ricqles, A. de 1976. On bone histology of fossil and living reptiles, evolutionary significance. In: A. d’A. Bellairs and C.B. Cox (eds.), Morphology and Biology of Reptiles. Academic Press London 1: 123–150.
Ricqlès, A. de 1978. Sur la classification, la signification fonctionnelle et l’histoire des tissus osseux des tétrapodes. Troisième partie: Évolution. Annales de Paléontologie 64: 85–111.
Ricqlès, A. de and Buffrénil, V. de 2001. Bone histology, heterochronies and the return of the tetrapods to life. In: J.M. Mazin and V. de Buffrénil (eds.), Water Where Are We?, 95–117. Verlag Dr Friedrich Pfeil, Munchen.
Ricqlès, A. de, Castanet, J., and Francillon-Vieillot, H. 2004. The “message” of bone tissue in paleoherpetology. Italian Journal of Zoology 71: 3–12. Crossref
Ricqlès, A. de., Padian, K., and Horner, J. 2003. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Annales de Paléontologie 89: 67–101. Crossref
Ricqlès, A. de, Padian, K., Horner, J., and Francillon-Vieillot, H. 2000. Palaeohistology of the bones of pterosaurs (Reptilia: Archosauria): anatomy, ontogeny, and biomechanical implications. Zoological Journal of the Linnean Society 129: 349–385. Crossref
Ricqlès, A. de, Padian, K., Knoll, F., and Horner, J. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology. Annales de Paléontologie 94: 57–76. Crossref
Rogers, R.R., Arcucci, A.B., Abdala, F., Sereno, P., Forster C., and May, C.L. 2001: Paleoenvironment and taphonomy of the Chañares Formation tetrapod assemblage (Middle Triassic), northwestern Argentina: spectacular preservation in volcanogenic concretions. Palaios 16: 461–481. Crossref
Romer, A.S. 1971a. The Chañares (Argentina) Triassic reptile fauna X. Two new but incompletely known long-limbed pseudosuchians. Breviora 378: 1–10.
Romer, A.S. 1971b. The Chañares (Argentina) Triassic reptile fauna XI. Two new long-snouted thecodonts, Chanaresuchus and Gualosuchus. Breviora 379: 1–22.
Romer, A.S. 1972. The Chañares (Argentina) Triassic reptile fauna XII. The postcranial skeleton of the thecodont Chanaresuchus. Breviora 385: 1–21.
Romer, A.S. 1973. The Chañares (Argentina) Triassic reptile fauna XX. Breviora 413: 1–20.
Romer, A.S. and Jensen, J.A. 1966. The Chañares (Argentina) Triassic reptile fauna II. Sketch of the geology of the Río Chañares-Río Gualo region. Breviora 252: 1–20.
Schweitzer, M.H. and Marshall, C.L. 2001. A molecular model for the evolution of endothermy in the dinosaur-bird lineage. Journal of Experimental Zoology 291: 317–338. Crossref
Sereno, P.C. 1991. Basal archosaurs: phylogenetic relationships and functional implications. Society of Vertebrate Paleontology 2: 1–53. Crossref
Sereno, P.C. and Arcucci, A.B. 1990. The monophyly of the crurotarsal archosaurs and the origin of birds and crocodile ankle joints. Neues Jahrbuch fur Geologie und Paläontologie Abhandlungen 180: 21–52.
Sereno, P.C. and Arcucci, A.B. 1993. Dinosaurian precursors from the Middle Triassic of Argentina: Lagerpeton chanarensis. Journal of Vertebrate Paleontology 13: 385–399. Crossref
Sill, W.D. 1967. Proterochampsa barrionuevoi and the early evolution of the Crocodilia. Bulletin of the Museum of Comparative Zoology 135: 415–446.
Small, B.J. 2009. A Late Triassic dinosauromorph assemblage from the Eagle Basin (Chinle Formation), Colorado, USA. Journal of Vertebrate Paleontology 29: 182.
Starck, J.M. and Chinsamy, A. 2002. Bone microstructure and developmental plasticity in birds and other dinosaurs. Journal of Morphology 254: 232–246. Crossref
Taylor, M.A. 2000. Functional significance of bone ballast in the evolution of buoyancy control strategies by aquatic tetrapods. Historical Biology 14: 15–31. Crossref
Tinkle, D.W. 1969. The concept of reproductive effort and its relation to the evolution of life histories of lizards. The American Naturalist 103: 501–516. Crossref
Trotteyn, M.J., Arcucci, A.B., Raugust, T. 2013. Proterochampsia: an endemic archosauriform clade from South America. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny and Palaeobiology of EarlyArchosaurs and their Kin. Geological Society, London, Special Publications 379: 50–90. Crossref
Tucker, M.E. and Benton, M.J. 1982. Triassic environments, climates and reptile evolution. Palaeogeography, Palaeoclimatology and Palaeoecoly 40: 361–379. Crossref
Marsicano, C., Irmis, B.R., Mancuso, C., Roland, M., and Chemale, F. 2016. The precise temporal calibration of dinosaur origins. Proceedings of the National Academy of Sciences of the United States of America 113: 509–513. Crossref
Veiga, F.H., Botha-Brink, J., Ribeiro, A.M., Ferigolo, J., and Soares, M.B. 2019. Osteohistology of the silesaurid Sacisaurus agudoensis from southern Brazil (Late triassic) and implications for growth in early dinosaurs. Anais da Academia Brasileira de Ciências 91: e20180643. Crossref
Warshaw, J. 2008. Comparative primate bone microstructure: records of life history, function, and phylogeny. In: E.J. Sargis and M. Dagosto (eds.), Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay, 385–425. Springer, Dordrecht. Crossref
Werning, S. 2013. Evolution of Bone Histological Characters in Amniotes, and the Implications for the Evolution of Growth and Metabolism. 445 pp. Ph.D. Dissertation, University of California, Berkeley.
Woodward, H.N., Horner, J.R., and Farlow, J.O. 2011. Osteohistological evidence for determinate growth in the American alligator. Journal of Herpetology 45: 339–342. Crossref
Acta Palaeontol. Pol. 65 (2): 387–398, 2020
https://doi.org/10.4202/app.00644.2019