

Gyrochorte “highways” and their environmental significance in shallow-marine sediments
ANDREAS WETZEL, NOELIA B. CARMONA, and JUAN J. PONCE
Wetzel, A., Carmona, N.B., and Ponce, J.J. 2020. Gyrochorte “highways” and their environmental significance in shallow-marine sediments. Acta Palaeontologica Polonica 65 (1): 209–218.
The reworking of a trace by a subsequently following organism represents a so-called sequorichnial behavior and leads to formation of a “burrowing highway”. Burrowing highways occur more frequently than assumed in the fossil record. Their ichnological and sedimentological meaning is elucidated by using the trace fossil Gyrochorte. Gyrochorte producers exploiting sandy event beds tend to use “burrowing highways” in the same direction. Evidently, the Gyrochorte producers are thigmotactically highly sensitive as they can recognize a burrow produced by the same species because of the less densely packed grains, a somewhat increased mud content, and supposedly mucus segregated within the burrow. These changes make the reworking of pre-existing burrows energetically advantageous. However, in shallow-marine settings mucus is degraded rapidly and loose sediment consolidates in a short while. Therefore, the time to recognize a pre-existing burrow appears to be limited and a rather high number of organisms is a prerequisite for reutilization of a previous trace. “Burrow highways” potentially represent an additional criterion to characterize an opportunistic population strategy.
Key words: Polychaete, ichnology, behaviour, sequorichnia, Cretaceous, Argentina.
Andreas Wetzel [andreas.wetzel@unibas.ch], Departement Umweltwissenschaften, Geologie, Universität Basel, Bernoullistrasse 32, CH-4056 Basel, Switzerland.
Noelia B. Carmona [ncarmona@unrn.edu.ar ] and Juan J. Ponce [jponce@unrn.edu.ar], CONICET, Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Río Negro, General Roca. 8332, Rio Negro, Argentina.
Received 18 July 2019, accepted 8 October 2019, available online 23 January 2020.
Copyright © 2020 A.Wetzel et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Gyrochorte commonly occurs in nearshore and shallow-marine deposits formed in moderate to moderately high energy environments, including storm-dominated shelves, bars, shorefaces of beach complexes, and embayment areas (e.g., Gibert and Benner 2002). Typically, these traces are associated with fine-sand tempestites and represent post-event burrows suggesting that their producers exploited the seafloor during quiet periods between high-energy events, most commonly storms (e.g., Gibert and Benner 2002). The suggested worm-like producer is supposedly a polychaete (Seilacher 2007; Fürsich et al. 2017) that moved obliquely through the sediment and displaced sediment upward and backward while a plait-like ridge was formed atop a sandy bed (Weiss 1941; Heinberg 1973; Seilacher 2007; Fürsich et al. 2017). The resultant burrow represents a wall-like structure, being internally obliquely laminated (Seilacher 1955; Heinberg 1973). The corresponding trace fossils belong to the ichnotaxon Gyrochorte comosa Heer, 1865. The chevron-shaped ridges of the trace indicate the direction of movement towards their acute side (e.g., Weiss 1941; Seilacher 1955). During burrowing, the sediment was processed while the producers followed a sediment-feeding mode (e.g., Weiss 1941; Seilacher 2007; Fürsich et al. 2017). Gyrochorte producers may also exploit a nourishment-rich layer sideways and form oblique transverse pads, ridges or fan-like structures, recently grouped into Gyrochorte variabilis Fürsich, Alberti, and Pandey, 2017. This behaviour documents a rather sensitive response of Gyrochorte producers on the distribution of benthic food within the substrate.
The present study deals with Gyrochorte producers that optimized their burrowing efforts by following a previously formed trace, as expressed by converging and after some distance diverging ridges. For such multiply used burrows, Nara and Ikari (2011) introduced the term burrowing “highways” based on accurate observations on diverging Protovirgularia formed by at least two bivalves. Recently, Macaronichnus specimens occurring closely aside of each other were interpreted to document that one producer followed the other. For this type of behaviour the ethological category of sequorichnia was proposed by Nara and Seike (2019).
Lower Cretaceous shallow-marine sandstone beds in the Neuquén Basin (Argentina) contain abundant Gyrochorte and “highways” are commonly present. Additional examples of “highways” found in other formations and figured in literature demonstrate that a sequorichnial behaviour is not uncommon among Gyrochorte producers. It is the purpose of the present study to describe repeatedly used Gyrochorte “highways”, to interpret the behavioural aspects, and to decipher their environmental significance.
Institutional abbreviations.—NMB, Natural History Museum of Basel, Switzerland.
Geological setting
Gyrochorte “highways” have been studied in detail in shallow-marine deposits of the Lower Cretaceous (Valanginian) Mulichinco Formation in the Neuquén Basin (Argentina; Fig. 1). The studied material was found in the Puerto Curaco section where the Mulichinco Formation is well exposed along a cliff facing a gorge. The interval studied is limited by the points having the GPS coordinates 69°55’54.05’’W 37°22’19.95’’S and 69°55’51.66’’W 37°22’17.78’’S, respectively. The studied section is mainly composed of well-sorted fine-grained sandstone beds separated from each other by mudstone varying in thickness from subtle partings to strata being up to a few centimetres thick (Fig. 2). Invertebrate fossils, trace fossils and the dominance of small-scale wave- and current-ripple cross-lamination suggest deposition in a full-marine upper offshore to lower shoreface setting (e.g., Echevarría et al. 2012; Wesolowski et al. 2018). Recently, this section has been logged and interpreted as stacked parasequences (Wesolowski et al. 2018). Within the studied interval, lensoid sand bodies pinch-out over a distance of a few hundreds of meters and hence, imply to have formed in an upper offshore to shoreface bar complex (Schwarz 2012; Ponce et al. 2015).
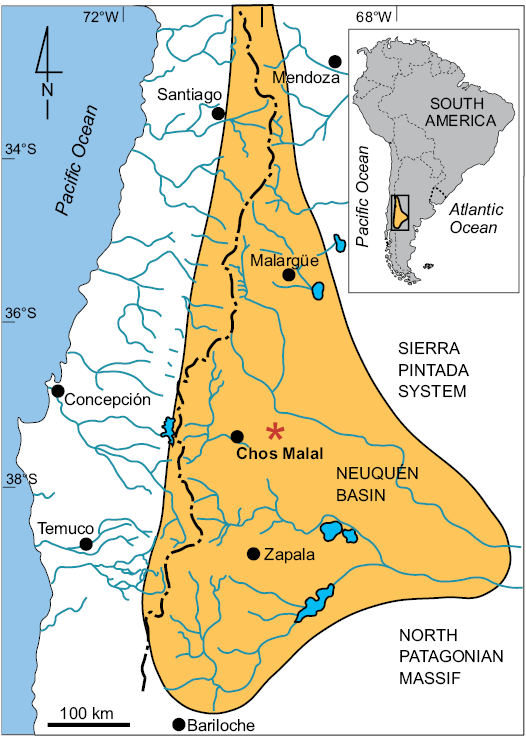
Fig. 1. Location map (based on Schwarz 2012), asterisk marks the study area within the Neuquén Basin (highlighted) while the inset refers to the location of the detailed map within South America.
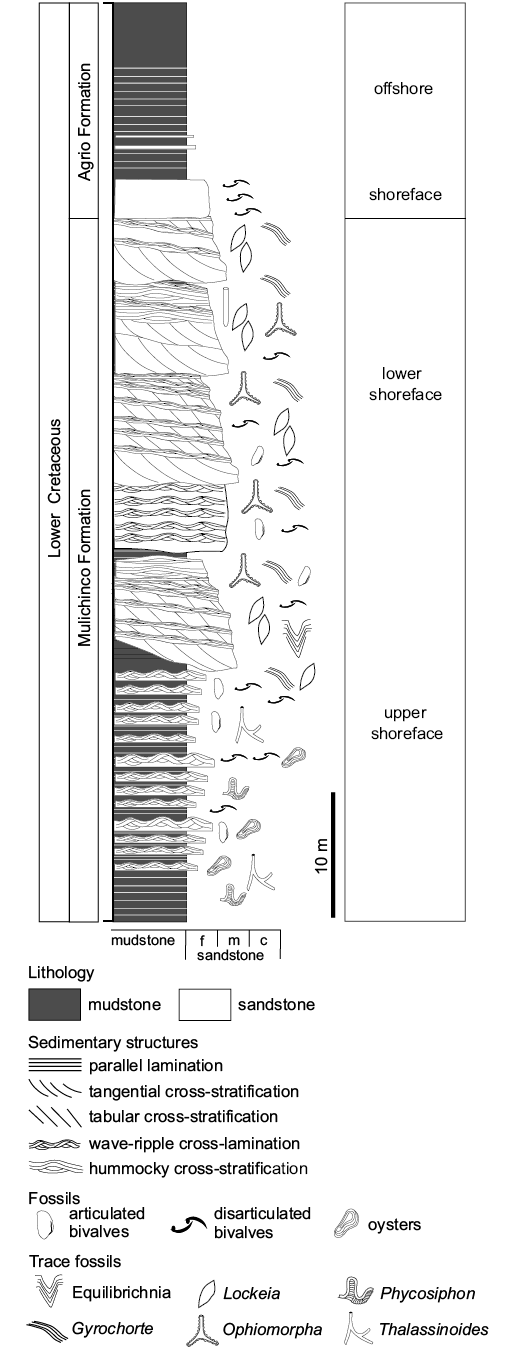
Fig. 2. Schematic section of the studied interval of the Mulichinco Formation exposed at Puerto Curaco locality. c, coarse; f, fine; m, middle.
Results
Observations.—In the Mulichinco Formation, Gyrochorte occurs at varying abundance mainly in well-sorted fine-sandstone beds (grain size 60–250 μm). The beds display a smooth, undulating base of long or short wavelength and a wave-rippled surface; interference ripples may also occur (Fig. 3). The wave ripples have an amplitude of 10–20 mm and a wavelength of 60–100 mm. Even if having parallel crests, the ripples tend to be slightly asymmetric; the ripple symmetry index (RSI = Ls/Ll; Ls, length of stoss side; Ll, length of lee side) is in the range of 1.3 to 2. The tops of the sandstone beds are very well preserved.

Fig. 3. Trace fossil Gyrochorte comosa Heer, 1865 on bed surfaces structured by wave ripples; Mulichinco Formation, Lower Cretaceous, Puerto Curaco, Neuquén Province, Argentina (field photographs). A. Gyrochorte comosa on bed surface exhibiting parallel-crested, symmetric ripples. B. Parallel-crested, slightly asymmetric ripples traversed by G. comosa being more frequent at the stoss-side and the crest of the ripples. C. Bed surface structured by interfering symmetric ripples documenting sequorichnial behaviour of G. comosa producers (black arrows, “converging” traces; white arrows, “diverging” traces); note acute angle between diverging or converging traces. D. Bed surface exhibiting parallel-crested, slightly asymmetric ripples; G. comosa is overtopped by sand at the lee side of the ripples (white arrows). All specimens left in the field.
Gyrochorte comosa Heer, 1865 and Gyrochorte variabilis Fürsich, Alberti, and Pandey, 2017 are both present (Fig. 4). The specimens within any one bed are of similar size. In positive epirelief, the ridges of Gyrochorte are 2–5 mm wide and have steep, nearly vertical flanks that jut above a smooth surface commonly by 1–2 mm, rarely up to 4 mm. There are no depressions on the upper bed surface along the Gyrochorte ridges. Similarly, the lower surface of thin sand beds containing, but not penetrated by Gyrochorte exhibits no deformation related to the trace. Recurrently up to several mm thick sand layers exhibit Gyrochorte only in negative hyporelief, while a positive epirelief expression is missing. In sandy beds of various thickness the vertical extent of the burrows is estimated to be commonly in the range of less than 2 to 4 mm, rarely 7 mm. In thin section, Gyrochorte burrows display a looser packing of quartz grains and the clay content is a little higher than in the adjacent host sediment, which consists of rather pure, densely packed quartz sand (Fig. 5). The proportion of sand and clay particles was estimated by point counting of thin sections (Weltje 2002). Host sediment and Gyrochorte contain on average ~86 ±2% and ~81 ±2% sand grains as well ~8 ±2% and ~12 ±2% clay particles, respectively, while the remaining volume is occupied by pores. The proportion of pores, however, could not be measured directly while located between irregular particle surfaces.
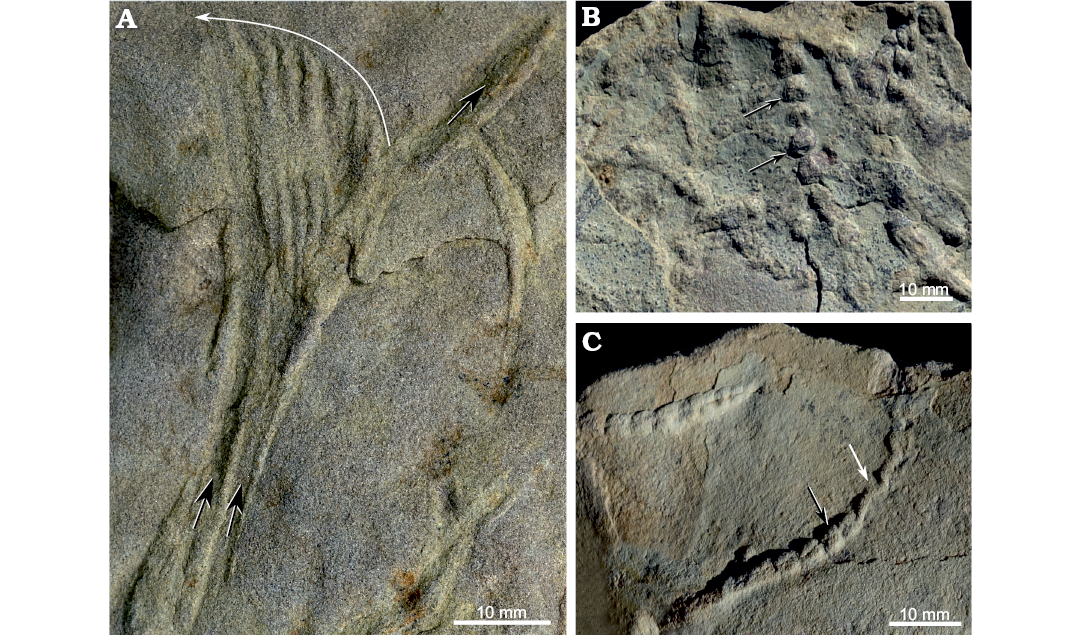
Fig. 4. Different ichnospecies of Gyrochorte encountered in the Mulichinco Formation, Lower Cretaceous, Puerto Curaco, Neuquén Province, Argentina (field photographs). A. Two parallel Gyrochorte comosa Heer, 1865 (black arrows, lower left), one continuing as such (black arrow, upper right), and one diverging from parallel course and becoming Gyrochorte variabilis Fürsich, Alberti, and Pandey, 2017 (shift of causative tube indicated by white arrow). B. Gyrochorte variabilis exhibiting circular, slightly bilobate pads (black arrows). C. Gyrochorte variabilis composed of elongate pads (black arrow) becoming oblique with slight zigzag pattern (white arrow). For details about G. variabilis see Fürsich et al. (2017). All specimens left in the field.
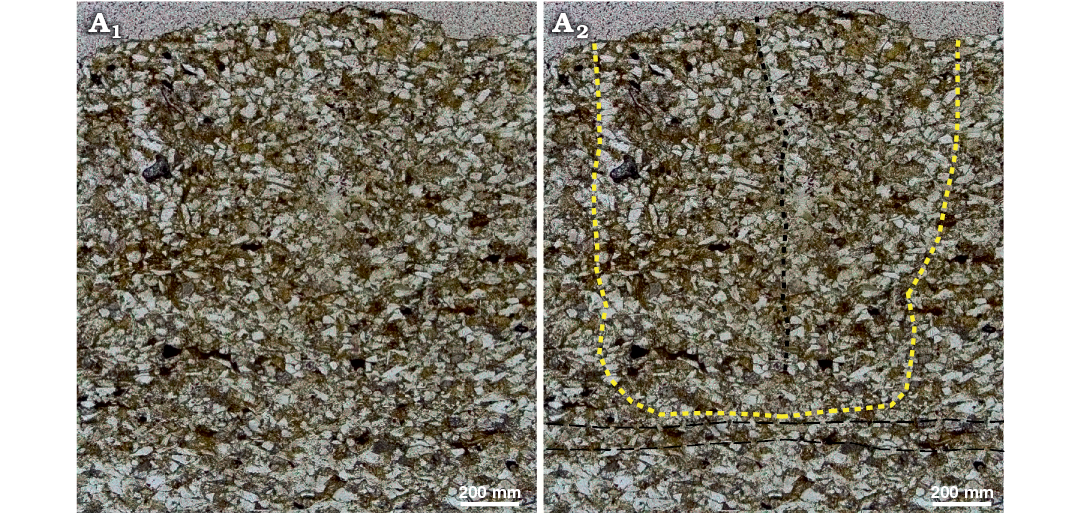
Fig. 5. Thin section of Gyrochorte comosa Heer, 1865 (NMB O156) from Mulichinco Formation, Lower Cretaceous, Puerto Curaco, Neuquén Province, Argentina. Original photomicrograph (A1); photomicrograph with overlay (A2); note displaced grains within the G. comosa in particular along its boundaries (yellow doted line), central divide within trace (black doted line), faint, undeformed lamination of sediment horizontal black dashed lines. Photo in plain light.
The surfaces of the strata containing Gyrochorte exhibit a low degree of bioturbation varying between 0% and ~30%. Gyrochorte may occur solely as single trace, but commonly several specimens are present. In some instances, Gyrochorte tends to occur preferably on the gently dipping sides and crests of ripples rather than in ripple troughs (Figs. 3, 4). Gyrochorte is the dominant ichnogenus within a trace-fossil assemblage of otherwise low diversity. Only 2–4 different ichnotaxa co-occur within the same bed, in addition to Gyrochorte, Arenituba, Lockeia, Ophiomorpha, Phycosiphon, Polykladichnus, Protovirgularia, Ptychoplasma, Siphonichnus, Skolithos, and Spongeliomorpha may be present. In many instances, the deeply penetrating trace fossils, for instance those produced by bivalves (Lockeia, Protovirgularia, Ptychoplasma, and Siphonichnus), overprint Gyrochorte. In some beds, Phycosiphon and Gyrochorte mutually crosscut (Fig. 6). This Phycosiphon appears as printed epirelief on sandstone-bed surfaces (Fig. 6).
Commonly, two or more Gyrochorte converge to form a “highway” while they overlap completely or only partly, but parallel burrows touching each other also occur (Fig. 7). In 60–80% of the observed cases, the chevron-shaped ornamentation is oriented in the same direction for the converging and diverging burrows (Fig. 7). The “highways” are commonly a few, up to about 100 mm long (Fig. 7). The angle between two Gyrochorte converging for a “highway” ranges 10–45°. Where the traces approach at larger angles they crosscut. In beds <2 mm thick, “highways” of Gyrochorte on the upper and lower surface match, while in beds >4 mm thick the overlap of successive burrows is only 60–80%.
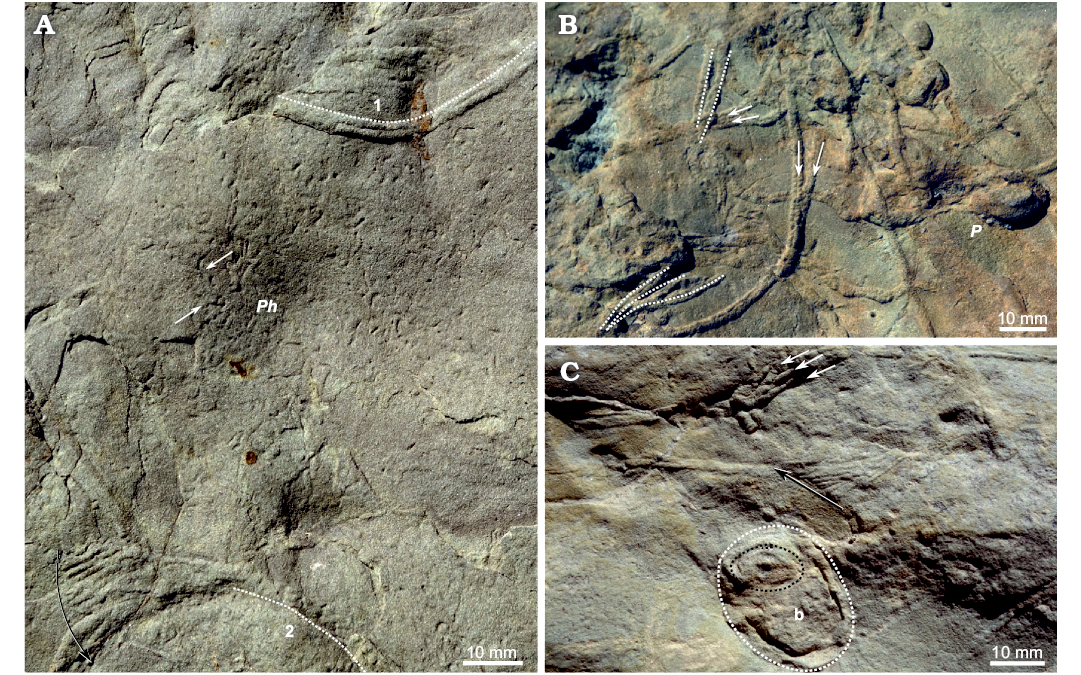
Fig. 6. Trace fossils co-occurring with “burrowing highways” of Gyrochorte ichsp. A. Mutual cross-cutting of Gyrochorte ichsp. (1, 2) and Phycosiphon incertum Fischer-Ooster, 1856 (Ph) exhibiting typical elbow-shaped turns (white arrows); Gyrochorte comosa Heer, 1865 overprinting Phycosiphon ichsp. (1, white doted line; bilobate crest of Gyrochorte well visible); Phycosiphon ichsp. overprinted by G. comosa (2, white doted line; bilobate crest of Gyrochorte ichsp. not clearly developed while bioturbated); G. comosa showing transition to Gyrochorte variabilis Fürsich, Alberti, and Pandey, 2017 expressed by fan-like appearance (shift of causative tube marked by black arrow). B. Gyrochorte comosa associated with Ptychoplasma ichsp. (P); sequorichnial behaviour of several Gyrochorte ichsp. producers is evidenced by parallel and/or overlapping traces (parallel traces, white doted lines; diverging/converging traces, white arrows). C. Parallel Gyrochorte comosa showing sequorichial behaviour (white arrows) associated with bivalve trace Siphonichnus ichsp. (b) (surrounded by black doted line) and cross-section of Lockeia ichsp. (surrounded by white doted line); G. comosa showing transition to G. variabilis expressed by fan-like appearance (shift of causative tube marked by black arrow). All specimens left in the field.
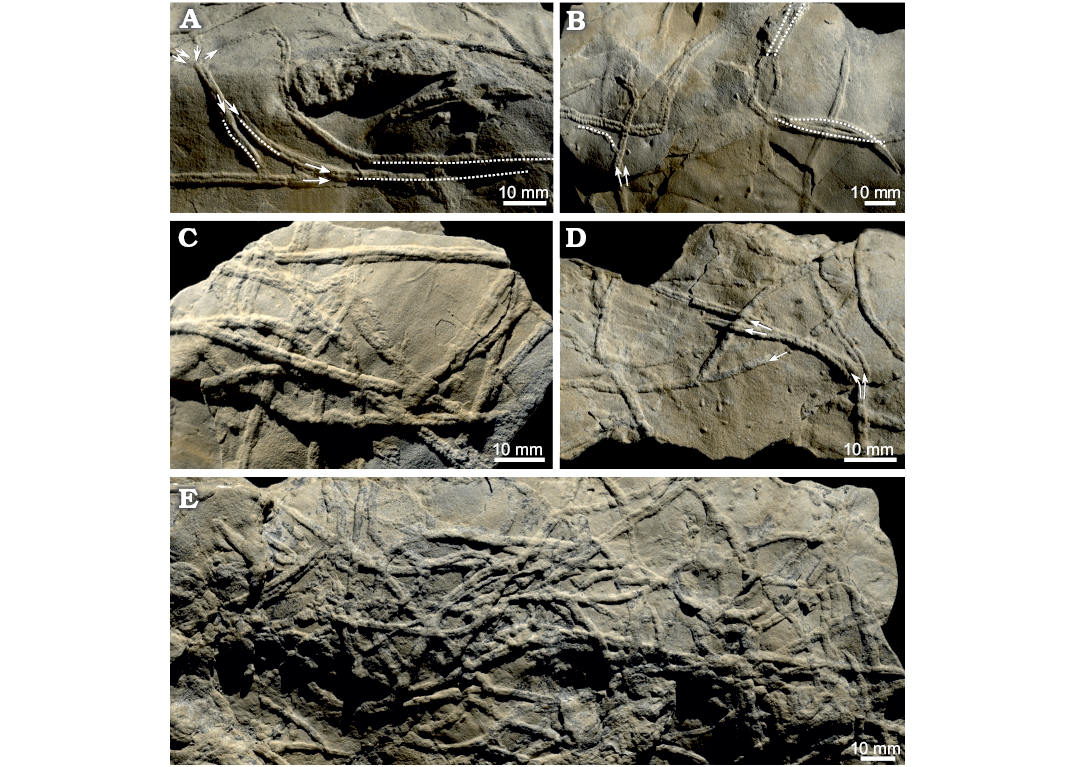
Fig. 7. “Burrowing highways” of Gyrochorte comosa Heer, 1865 in the Mulichinco Formation, Lower Cretaceous, Puerto Curaco, Neuquén Province, Argentina (field photographs). Crowded parallel and overlapping G. comosa concentrated at ripple crest (A–E). Note acute angle between sequorichnial Gyrochorte ichsp. (arrows, traces converging to and diverging from a parallel course; doted lines, parallel/overlapping traces). All specimens left in the field.
Interpretation.—The sandstone beds are interpreted to have been formed by storms and strong longshore currents and to have been subsequently reworked by weak currents and waves near the fair-weather wave base (e.g., Seilacher 1982; Myrow and Southard 1996; Dumas et al. 2005). This interpretation matches previous findings, as the beds containing abundant Gyrochorte represent facies association 7 (FA 7) of Wesolowski et al. (2018) “weakly storm-affected lower shoreface”. Currents and waves, however, reworked the beds hosting Gyrochorte only episodically. After such an episode, mud settled to the seafloor and covered it, otherwise the sediment surface itself and the steep flanks of the Gyrochorte ridges, which were formed after deposition of mud could not so sharply be preserved (Seilacher 2007).
In many beds, Gyrochorte occurs in high abundance in a low-diversity trace-fossil association, and hence matches the main criteria for an opportunistic population strategy (e.g., Ekdale 1985). Consequently, the observations in the Mulichinco Formation substantiate the hypothesis that Gyrochorte producers are classified as opportunistic post-event burrowers (e.g., Gibert and Benner 2002; Fürsich et al. 2017). Given that Gyrochorte was produced by polychaetes (Seilacher 2007; Fürsich et al. 2017) it is important that some polychaetes maximize their reproductive efforts when faced with the possibility of death, and also when settlement space is available (Barry 1989). Consequently, the settling by Gyrochorte-producers on tempestites may record that these organisms followed the above mentioned strategy, when facing physical disturbances. A short-term response is possible because many polychaetes have external fertilization (Rouse and Pleijel 2001). This, along with post-event settling, could explain the common occurrence of Gyrochorte producers. Furthermore, the reworking of the seafloor by storm events loosens the sandy substrate, facilitating the burrowing activities of these polychaetes as already mentioned for the sequorichnial behavior of Macaronichnus producers, which prefer to burrow in soft sediment because of their large body size (Nara and Seike 2019).
In positive epirelief, the plait-like ridges occur on a smooth surface showing no depressions along the burrows. Thin sections provide evidence that quartz grains are less densely packed in the burrow than in adjacent host sediment and that argillaceous material was introduced, supposedly originating from the mud covering the bed surface (see above; Fig. 5). There is no evidence that material was transferred into the burrow from beside or below when the Gyrochorte producer reworked the sediment. In addition, the Gyrochorte producers very likely secreted mucus as many burrowing polychaetes do (e.g., Kristensen and Kostka 2005). Consequently, the production of Gyrochorte changed the properties of the burrow fill compared to the unburrowed host sediment, at least for a short while after trace formation (see below). Similarly, Heinberg (1973) and Fürsich et al. (2017) reported a rearrangement of the grain fabric within Gyrochorte burrows. Polychaetes that are supposed to produce Gyrochorte (Seilacher 2007; Fürsich et al. 2017) are known to be equipped with an impressive array of sensory structures (Purschke 2005; Lindsey 2009) with which to gain information about their environment. They very likely have the capability to perceive changes in grain packing in their habitat and/or organic compounds derived from the same species.
Looser packing of grains and enhanced porosity (water content), a little additional mud, and probably some mucus reduce the resistance of the sediment against grain displacement (e.g., Mitchell 1993). Consequently, to burrow in such deposits requires less energy than in adjacent, densely packed pure siliciclastic sand.
In fact, the recognition of a trace produced by an organism of the same species implies that some mucus or at least species-specific organic compounds occur within a trace. Furthermore, mucus and irrigation of the burrow during production may stimulate the growth of microbes that can represent an additional food source (e.g., Kristensen and Kostka 2005). Easy-to-burrow sediment seems to be also present close to the trace as indicated by parallel Gyrochorte in contact with each other or partly overlapping ones. Burrow irrigation could have changed the properties of the nearby sediment temporarily. The content of nutritious material within Gyrochorte is supposedly lower than in the adjacent sediment because on average about one-tenth of the organic matter is removed when sediment is digested once by a sediment-feeding organism (e.g., Lopez and Levinton 1987: 241, 5–15%). Even if the concentration of nutritious particles was lower in a Gyrochorte because of changed sediment properties, presence of mucus (and subsequently, microbes), and/or mud, the trace could be reworked at lower energetic expenses and hence, it would be attractive to re-burrow a pre-existing trace and apply a sequorichnial behaviour.
The use of “highways” is energetically advantageous only as long as the burrow fill does not consolidate again or the secreted mucus is degraded. Non-leathery mucus can be readily degraded within some days to a few weeks (e.g., Aller 1994; Kristensen and Kostka 2005). Similarly, (re-)arrangement of loosely packed sand, and hence compaction, can occur in a rather short time due to pressure fluctuations induced by waves or currents (e.g., Kruithof and Westfreid 2008).
Obviously, Gyrochorte producers possessed high thigmotactic capabilities. These are nicely documented by fan-shaped Gyrochorte variabilis composed of parallel burrows in touch with one another (Fürsich et al. 2017: fig. 10B). Therefore, it is not surprising that in the case of “highways” the subsequent burrower mainly moved in the same direction as the first one, as indicated by the chevron-shaped pattern of the converging and diverging ridges (Fig. 7). Direction of movement is towards the tip of the V-shaped pads (see discussion in Schlirf 2000). In contrast, moving in the opposite direction is energetically not so favourable because the internal lamination of the burrow is inclined against the direction of movement. Therefore, it is argued that energy-efficient reworking of the sediment was of major importance for the Gyrochorte producer when applying sequorichnial behaviour, even where the supply of nourishing material was lower. If a subsequent burrowing organism approached a pre-existing trace at a too obtuse angle (>45°) it just crossed the pre-existing trace (Fig. 7). In this case, the looser sediment was apparently not recognized as advantageous because it occupied a too small interval, while the intersection angle between both burrows was too obtuse and the body of the trace producer was considerably inclined when moving through the substrate (Heinberg 1973; Seilacher 2007).
Besides taking advantage of easy-to-burrow sediment, the Gyrochorte producers may have followed each other for a short distance for reproduction, as envisaged by Nara and Seike (2019). However, many polychaetes possibly including the Gyrochorte producers, have external fertilization (e.g., Rouse and Pleijel 2001). Furthermore, the similar size of the studied Gyrochorte specimens favors an opportunistic settlement strategy and, therefore, reproduction appears not to be the reason for the observed sequorichnial behaviour.
Gyrochorte occurring only at the base of a thin sandstone layer, but having no counterpart on the bedding surface, imply either that these burrows were produced within the sand or that the upper part of the sand layer was reworked by waves. The latter case is true for sand layers thinner than the vertical extent of the burrows.
Discussion
Sequorichnial behaviour of the Gyrochorte producers and the resultant “highways” is rather common as their occurrence is not restricted to the studied deposits. Highways were also found in outcrops of the Upper Jurassic Lajas Formation (GPS coordinates 70°20’85.9’’W 38°80’71.5’’S) and Agrio Formation (GPS coordinates 70°01’33.7’’W 38°35’04.6’’S), both within the Neuquén Basin (Argentina), as well as in Mesozoic sediments in Switzerland (Fig. 8). Furthermore, “highways” have been figured in the literature without being described as such by, for instance Hallam (1970: pl. 1A, right side), Schlirf (2000: pl. 9: 1, 7), Gibert and Benner (2002: fig. 6A, centre), Fürsich et al. (2017: fig. 3B, centre), and Seilacher (2007: 102). The specimens from the Agrio Formation are rather similar to those of the Mulichinco Formation as the Gyrochorte “highways” occur in fine-sand layers having slightly asymmetric wave ripples on top (Fig. 8). Producers of Chondrites reworked the mud covering the ripple surfaces and crosscut Gyrochorte (Fig. 8), implying a considerable organic matter content in the mud cover. The Gyrochorte “highways” found in the Lajas Formation occur in fine-sandstone beds having interference ripples on the surface (Fig. 8). Therefore, these beds formed above wave base (e.g., Reineck and Singh 1980). In such a case, wave pumping of pore water may accelerate mucus degradation and re-consolidation of less densely packed sediment (Kruithof and Westfreid 2008).
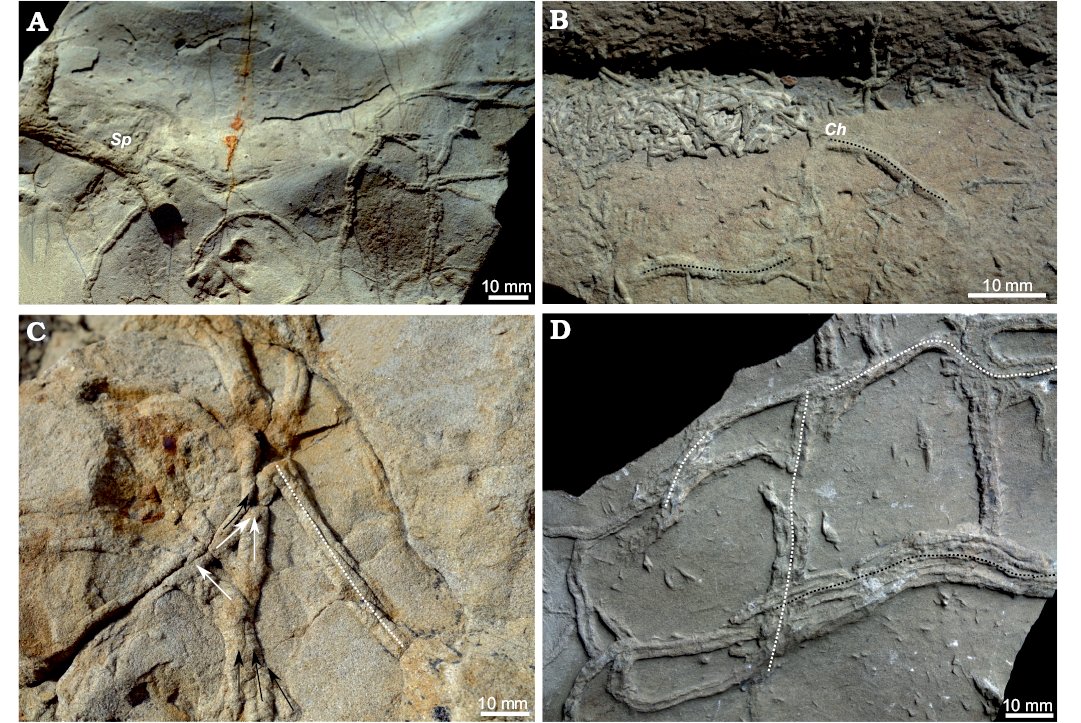
Fig. 8. Gyrochorte “highways” documenting a sequorichnial behaviour of the producers from different localities. A, B. Agrio Formation, Cretaceous, Neuquén Basin, Argentina. A. Bed surface showing interference ripples cross-cut by Spongeliomorpha ichsp. (Sp). B. Ripple-trough fill reworked by Chondrites ichsp. (Ch) producers (black doted lines). C. Lajas Formation, Upper Jurassic, Neuquén Basin, Argentina; converging (black arrows), diverging (white arrows), and overlapping Gyrochorte ichsp. (white doted line). D. Middle Jurassic, Helvetic realm, exact location not recorded, Switzerland (NMB O157) showing parallel Gyrochorte ichsp., 2 parallel traces (white doted line between them), 3 parallel traces (black doted line between them). Specimens A–C left in the field.
On foreshore bars, during phases of hydraulic activity, sediment is sorted and clean sand becomes enriched, while fine-grained material including organic matter is washed out. It settles out of suspension when conditions become calm again. Therefore, when just deposited the sand should contain little benthic food, but during waning hydraulic activity particulate organic matter and fine fraction sinking to the seafloor can be incorporated into sand due to wave- and current-pumping effects (e.g., Huettel et al. 1996; Shum and Sundby 1996; Bourke et al. 2017). The infiltrated organic material represents a potential food source for the Gyrochorte producers. The preferential presence of Gyrochorte on the gently dipping side or crest of ripples may document infiltration of benthic food by wave pumping as observed in experiments (Huettel et al. 1996; Shum and Sundby 1996).
Sequorichnial behaviour becomes increasingly recognized in the trace fossil record. After the seminal observations on Protovirgularia by Nara and Ikari (2011), “highway” traces of earthworms were observed (Wetzel et al. 2016). The earthworms take advantage of using an already mucus-covered trail, while the polarity of movement does not matter. Similarly, more than 30 modern snail taxa follow previous traces lubricated by mucus because of different reasons such as to return to specific locations, to move in an energy conserving mode, to self-organize in groups, or to search/attract a mate (Ng et al. 2013). In addition, many species including the marine mud snail Ilyanassa obsoleta can recognize the polarity of movement of the previous trace producer and move in the same direction (e.g., Trott and Dimock 1978; Bretz and Dimock 1983). In the fossil record, Protovirgularia “highways” document movement of the users in the same direction (Nara and Ikari 2011). In fact, the internal structure of Protovirgularia is supposedly asymmetric as evidenced by the V-shaped basal part and the asymmetric body of the burrowing bivalves (e.g., Seilacher and Seilacher 1994; Ekdale and Bromley 2001). Hence, reworking in the same direction appears to save energy and is therefore attractive for the burrowing organisms despite the lower benthic food content. In turn, sequorichnial behaviour implies that Protovirgularia producers are also thigmotactically highly sensitive and can react to the consistency of the substrate. Arthrophycus paralleling each other for several centimeters, but avoiding overlap have been described by Rindsberg and Martin (2003). This phobotactic behaviour documents that the tracemakers could sense other burrows at a short distance through the substrate. In addition, in the studied outcrop Ptychoplasma has been found to coincide with and overprint Gyrochorte (Fig. 6B). However, such a coincidence has been observed only rarely, and so it remains an open question if it represents a random case or a causal relation.
To form a highway, a subsequent burrower needs to come quite close to a trace to recognize it. This is possible as long as the looser sediment is not dewatered and mucus is fresh. Because consolidation and mucus degradation can occur in a short time, the number of burrowers has to be so large that a subsequent burrower meets a previous trace during this time span. Because Gyrochorte producers appear to follow an opportunistic population strategy it should not be a limiting factor. It appears that “use of highways” could represent an additional criterion for opportunistic population strategy of burrowing organisms that are present in reasonable abundance.
In a few examples, Phycosiphon and Gyrochorte mutually crosscut each other. The Gyrochorte producers evidently reworked the sand, while the Phycosiphon producers exploited the mud. As Phycosiphon producers in muddy event beds are suggested to follow an opportunistic population strategy (e.g., Goldring et al. 1991 [for taxonomic assignment see Wetzel and Bromley 1994]; Wetzel and Balson 1992; Wetzel and Uchman 2001), the same is likely for the Gyrochorte producer. However, it is possible that the Gyrochorte-producing organisms lived within the area from that the sand of the event bed originated.
Conclusions
Following the trace of a previous burrower and constituting a burrowing “highway”, so-called sequorichnial behaviour, occurs more frequently than recognized yet. Gyrochorte producers tend to re-burrow a previously formed trace of the same species while its fill is less densely packed than the adjacent sediment and contains some mud and probably mucus. Therefore, it is easier to burrow along a previous trace than in undisturbed sediment. The lower benthic food content within the older trace appears to be of subordinate importance.
The following, overlapping to paralleling Gyrochorte is mainly produced in the same direction as the pre-existing one. To rework a burrow in the opposite direction has no advantage because lamination within Gyrochorte is inclined against the direction of movement.
Gyrochorte producers were evidently thigmotactically highly sensitive, as they could recognize a burrow produced by another organism of the same species and the direction of movement. The Gyrochorte producers, supposedly polychaetes, very likely segregated some mucus or other organic compounds as other endobenthic polychaetes do.
The time span to join an existing burrow after its production for a “highway” was supposedly rather short because mucus degrades and loosely packed sand dewaters due to pumping effects of waves and currents rather rapidly. Therefore, Gyrochorte producers should be present in considerably high numbers for this life strategy to work.
Sequorichnial behavior documented by burrow “highways” potentially represents an additional criterion for organisms following an opportunistic population strategy.
Where Gyrochorte occurs only in negative hyporelief at the base of thin sand layers, but not at their top surface it may indicate recurrent reworking by waves and/or currents and represent a valuable tool to characterize sediment dynamics.
Acknowledgements
Careful and constructive reviews by Masakazu Nara (Kochi University, Japan) and Andrew Rindsberg (University of West Alabama, Livingston, USA) improved the manuscript. Field work was supported by Swiss National Science Foundation (SNF grant 200021_169042) to AW and Universidad Nacional de Río Negro grants PI-UNRN 2015 40-A-468 and PI-UNRN 2017 40-A-616 to NC and JJP. All these contributions are gratefully acknowledged.
References
Aller, R.C. 1994. Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Organic Geochemistry 114: 331–345. Crossref
Barry, J.P. 1989. Reproductive response of a marine annelid to winter storms: an analog to fire adaptation in plants? Marine Ecology Progress Series 54: 99–107. Crossref
Bourke, M.F., Marriott, P.J., Glud, R.N., Hasler-Sheetal, H., Kamalanathan, M., Beardall, J., Greening, C., and Cook, P.L.M. 2017. Metabolism in anoxic permeable sediments is dominated by eukaryotic dark fermentation. Nature Geoscience 10: 30–35. Crossref
Bretz, D.D. and Dimock, R.V., Jr. 1983. Behaviorally important characteristics of the mucous trail of the marine gastropod Ilyanassa obsoleta (Say). Journal of Experimental Marine Biology and Ecology 71: 181–191. Crossref
Dumas, S., Arnott, R.W.C., and Southard, J.B. 2005. Experiments on oscillatory-flow and combined-flow bedforms: implications for interpreting parts of the shallow-marine sedimentary record. Journal of Sedimentary Research 75: 501–513. Crossref
Echevarría, J., Damborenea, S.E., and Manceñido, M.O. 2012. Palaeodemecological analysis of infaunal bivalves “Lebensspuren” from the Mulichinco Formation, Lower Cretaceous, Neuquén Basin, Argentina. Ameghiniana 49: 47–59. Crossref
Ekdale, A.A. 1985. Paleoecology of the marine endobenthos. Palaeogeography, Palaeoclimatology, Palaeoecology 50: 63–81. Crossref
Ekdale, A.A. and Bromley, R.G. 2001. A day and a night in the life of a cleft-foot clam: Protovirgularia–Lockeia–Lophoctenium. Lethaia 34: 119–124. Crossref
Fischer-Ooster, C. von 1858. Die fossilen Fucoiden der Schweizer Alpen, nebst Erörterung über deren geologisches Alter. 74 pp. Huber u. Comp., Bern.
Fürsich, F.T., Alberti, M., and Pandey, D.K. 2017. Behavioural variants of the trace fossil Gyrochorte. Zitteliana 89: 13–22.
Gibert, J.M. de and Benner, J.S. 2002. The trace fossil Gyrochorte: ethology and paleoecology. Revista Española de Paleontología 17: 1–12.
Goldring, R., Pollard, J.E., and Taylor, A.M. 1991. Anconichnus horizontalis: a pervasive ichnofabric-forming trace fossil in post-Paleozoic offshore siliciclastic facies. Palaios 6: 250–263. Crossref
Hallam, A. 1970. Gyrochorte and other trace fossils in the Forest Marble (Bathonian) of Dorset, England. In: T.P. Crimes and J.C. Harper (eds.), Trace Fossils. Geological Journal, Special Issue 3: 189–200.
Heer, O. 1865. Die Urwelt der Schweiz. 662 pp. Friedrich Schulthess, Zürich.
Heinberg, C. 1973. The internal structure of the trace fossils Gyrochorte and Curvolithus. Lethaia 6: 227–238. Crossref
Huettel, M., Ziebis, W., and Forster, S. 1996. Flow-induced uptake of particulate matter in permeable sediments. Limnology and Oceanography 41: 309–322. Crossref
Kristensen, E. and Kostka, J.E. 2005. Macrofaunal burrows and irrigation in marine sediment: Microbiological and biogeochemical interactions. In: E. Kristensen, R.R. Haese, and J.E. Kostka (eds.), Macro- and Microorganisms in Marine Sediments. Coastal and Estuarine Studies 60: 125–157. Crossref
Kruithof, J. and Westfreid, J.E. 2008. Compaction process in underwater sand ripples in pulsed flow. In: D.R. Parsons, T. Garlan, and J.L. Best (eds.), Third International Workshop on Marine and River Dune Dynamics, MARID 2008, 203–205, SHOM, Brest.
Lindsay, S.M. 2009. Ecology and biology of chemoreception in polychaetes. Zoosymposia 2: 339–367. Crossref
Lopez, G.R. and Levinton, J.S. 1987. Ecology of deposit-feeding animals in marine sediments. Quarterly Review of Biology 62: 235–260. Crossref
Mitchell, J.K. 1993. Fundamentals of Soil Behavior. Second Edition. 437 pp. Wiley & Sons, New York.
Myrow, P.M. and Southard, J.B. 1996. Tempestite deposition. Journal of Sedimentary Research 66 A: 875–887. Crossref
Nara, M. and Ikari, Y. 2011. “Deep-sea bivalvian highways”: An ethological interpretation of branched Protovirgularia of the Palaeogene Muroto-Hanto Group, southwestern Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 305: 250–255. Crossref
Nara, M. and Seike, K. 2019. Palaeoecology of Macaronichnus segregatis degiberti: Reconconstructing the infaunal lives of the travisiid polychaetes. Palaeogeography, Palaeoclimatology, Palaeoecology 516: 284–294. Crossref
Ng, T.P.T., Saltin, S.H., Davies, M.S., Johannesson, K., Stafford, R., and Williams, G.A. 2013. Snails and their trails: the multiple functions of trail-following in gastropods. Biological Reviews 88: 683–700.Crossref
Ponce, J.J., Carmona, N., Montagna, A.O., and Canale, N. 2015. Sedimentología e Icnología de los Sistemas Petroleros no Covencionales de la Cuenca Neuquina. 122 pp. Universidad de Río Negro, General Roca.
Purschke, G. 2005. Sense organs in polychaetes (Annelida). Hydrobiologia 535/536: 53–78. Crossref
Reineck, H.-E. and Singh, I.B. 1980. Depositional Sedimentary Environments. 549 pp. Springer, Berlin. Crossref
Rindsberg, A.K. and Martin, A.J. 2003. Arthrophycus in the Silurian of Alabama (USA) and the problem of compound trace fossils. Palaeogeography, Palaeoclimatology, Palaeoecology 192: 187–219. Crossref
Rouse, G.W. and Pleijel, F. 2001. Polychaetes. 353 pp. Oxford University Press, Oxford.
Schlirf, M. 2000. Upper Jurassic trace fossils from the Boulonnais (northern France). Geologica and Palaeontologica 34: 145–213.
Schwarz, E. 2012. Sharp-based marine sandstone bodies in the Mulichinco Formation (Lower Cretaceous), Neuquén Basin, Argentina: remnants of transgressive offshore sand ridges. Sedimentology 59: 1478–1508. Crossref
Seilacher, A. 1955. Spuren und Fazies im Unterkambrium. In: O.H. Schindewolf and A. Seilacher (eds.), Beiträge zur Kenntnis des Kambriums der Salt Range. Akademie der Wissenschaften und der Literatur in Mainz, Abhandlungen der mathematisch-naturwissenschaftlichen Klasse 10: 373–399.
Seilacher, A. 1982. Distinctive features of sandy tempestites. In: G. Einsele and A. Seilacher (eds.), Cyclic and Event Stratification, 333–349. Springer, Berlin. Crossref
Seilacher, A. 2007. Trace Fossil Analysis. 226 pp. Springer, Berlin.
Seilacher, A. and Seilacher, E. 1994. Bivalvian trace fossils: a lesson from actuopaleontology. Courier Forschungs-Institut Senckenberg 169: 5–15.
Shum, K.T. and Sundby, B. 1996. Organic matter processing in continental shelf sediments—the subtidal pump revisited. Marine Chemistry 53: 81–87. Crossref
Trott, T.J. and Dimock, R.V., Jr. 1978. Intraspecific trail following by the mud snail Ilyanassa obsoleta. Marine and Freshwater Behaviour and Physiology 5: 91–101. Crossref
Weiss, W. 1941. Enstehung der ‹Zöpfe› im Schwarzen und Braunen Jura. Natur und Volk 71: 179–184.
Weltje, G. 2002. Quantitative analysis of detrital modes: statistically rigorous confidence regions in ternary diagrams and their use in sedimentary petrology. Earth-Science Reviews 57: 211–253. Crossref
Wesolowski, L.J.N., Buatois, L.A., Mángano, M.G., Ponce, J.J., and Carmona, N.B. 2018. Trace fossils, sedimentary facies and parasequence architecture from the Lower Cretaceous Mulichinco Formation of Argentina: The role of fair-weather waves in shoreface deposits. Sedimentary Geology 367: 146–163. Crossref
Wetzel, A. and Balson, P. 1992. Sedimentology of fine-grained turbidites inferred from continuously recorded physical properties data. Marine Geology 104: 165–178. Crossref
Wetzel, A. and Bromley, R.G. 1994. Phycosiphon incertum revisited: Anconichnus horizontalis is its junior subjective synonym. Journal of Paleontology 68: 1396–1402. Crossref
Wetzel, A. and Uchman, A. 2001. Sequential colonization of muddy turbidites in the Eocene Beloveža Formation, Carpathians, Poland. Palaeogeography, Palaeoclimatology, Palaeoecology 168: 171–186. Crossref
Wetzel, A., Uchman, A., and Bromley, R.G. 2016. Underground miners come out to the surface—trails of earthworms. Ichnos 23: 99–107. Crossref
Acta Palaeontol. Pol. 65 (1): 209–218, 2020
https://doi.org/10.4202/app.00655.2019