

The enamel microstructure of Manidens condorensis: New hypotheses on the ancestral state and evolution of enamel in Ornithischia
MARCOS G. BECERRA and DIEGO POL
Becerra, M.G. and Pol, D. 2020. The enamel microstructure of Manidens condorensis: New hypotheses on the ancestral state and evolution of enamel in Ornithischia. Acta Palaeontologica Polonica 65 (1): 59–70.
Previous studies on enamel microstructure in Ornithischia have focused on derived lineages of this clade based on species from the northern hemisphere. Here we describe the enamel microstructure of Manidens condorensis from the late Early Jurassic of Argentina that belongs to Heterodontosauridae (interpreted as the basal-most clade of Ornithischia). Enamel microstructure in the cheek teeth lacks a basal unit layer, presents incipient divergent crystallite as the dominant enamel type and parallel crystallite enamel type (with or without incrementing lines). Enamel of maxillary and dentary teeth differs from each other in enamel distribution (asymmetric vs. symmetric), structure (presence vs. absence of tubules, and less vs. more abundant parallel crystallite enamel with incrementing lines) and ordering (regular ordering of enamel types vs. in patches). The enamel microstructure of Manidens is the simplest of all known Ornithischia, and is more similar to that of the sauropodomorph Plateosaurus than to the one reported for the basal theropod Coelophysis. Similarities within Ornithischia are present with pachycephalosaurids and, to a lesser extent, with ankylosaurs. Phylogenetic optimization of enamel characters in Ornithischia allows the inference of new ancestral states for the internal nodes of the major lineages and to highlight evolutionary transformations: (i) absence of a basal unit layer and presence of parallel crystallite and incipient divergent columnar enamel as the ancestral state for Ornithischia; (ii) the shared presence of incipient divergent columnar units or poorly developed divergent columnar enamel in Pachycephalosauridae and Thyreophora represents a retention of the plesiomorphic ornithischian condition; (iii) the wavy enamel of Dryomorpha evolved from the ancestral incipient divergent columnar units present in Ornithopoda and; (iv) enamel thickness and asymmetry has independently evolved at least four times in Ornithischia.
Key words: Dinosauria, Ornithischia, Heterodontosauridae, Manidens, enamel microstructure, evolution, Jurassic, Argentina.
Marcos G. Becerra [mbecerra@mef.org.ar] and Diego Pol [dpol@mef.org.ar], Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Museo Paleontológico Egidio Feruglio, Fontana 140, Trelew, Chubut U9100GYO, Argentina.
Received 27 July 2019, accepted 4 November 2019, available online 26 February 2020.
Copyright © 2020 M.G. Becerra and D. Pol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Studies of non-prismatic enamel in reptilians through Scanning Electron Microscope allowed the characterization of its micromorphology and organization in a functional, phylogenetic and evolutionary context (Sander 1999, 2000). In particular, the enamel morphology in Dinosauria was approached by several authors (Sander 1999, 2000; Hwang 2005, 2010, 2011; Stokosa 2005; Heckert and Miller-Camp 2013). Based on an extensive sampling and comparisons, Hwang (2005) aimed to test phylogenetically if similarities in enamel morphology of major dinosaur taxa reflect common ancestry or functional/ecomorphological convergences. Later works supported results of Sander (1999) in Dinosauria: (i) “schmelzmuster” (i.e., the three-dimensional arrangement of all enamel types and major discontinuities characterizing the enamel of a tooth crown) was recovered as synapomorphic at a family level of many clades (e.g., Hadrosauridae, Neoceratopsia, Ankylosauria); and (ii) functional restrictions modelled similar “schmelzmuster” in phylogenetically distant species but with similar diets/ecology and teeth morphology. Other conclusions of successive works of Hwang (2005, 2010, 2011) on Dinosauria indicated that complexity in enamel microstructure was correlated mostly with the complexity of the tooth crown and not with its phylogenetic position, and that the degree of homoplasy in enamel morphology of Theropoda was much higher than in Ornithischia. Current knowledge of enamel morphology in Ornithischia was provided by Sander (1999, 2000) and Hwang (2005, 2010, 2011). This is based on a broad sampling of derived taxa belonging to Stegosauria, Ankylosauridae, Nodosauridae, Pachycephalosauridae, Ceratopsia, Iguanodontia, and Hadrosauridae, lineages whose origin is not earlier than the Bajocian or more recent times (e.g., Spencer 2013). This sampling reunites species from the Northern Hemisphere (USA, Canada, Mongolia, England) and mostly Cretaceous (excepting Stegosaurus, Dryosaurus, and Camptosaurus, from the Kimmeridgian of USA). Hwang (2011) noted this sampling bias and highlighted the importance of acquiring information of early ornithischians to infer the ancestral state of this clade, the polarity of change in enamel microstructure, and the historical patterns that explain shared schmelzmuster between distant lineages.
Manidens condorensis, a heterodontosaurid ornithischian represented by skull, postcranial remains and isolated teeth, was found in the latest Early Jurassic Cañadón Asfalto Formation (Central Patagonia, Argentina) (Pol et al. 2011; Becerra et al. 2018). Heterodontosauridae are phylogenetically and chronologically the first diversification of Ornithischia, predating the diversification of Genasauria (Thyreophora + Neornithischia). The availability of many isolated teeth referred to Manidens condorensis allows studying the enamel microstructure in a representative of the most basal ornithischian clade (Butler et al. 2008b). We additionally discuss the new information within a phylogenetic framework to reconstruct major evolutionary changes in the enamel microstructure in Ornithischia.
Institutional abbreviations.—MPEF-PV, Museo Paleontológico Egidio Feruglio, colección de Paleontología de Vertebrados, Trelew, Chubut, Argentina.
Other abbreviations.—BUL, basal unit layer; DCE, divergent columnar enamel; DCU, divergent columnar units; EDJ, enamel-dentine junction; EES, external enamel surface; IDCE, incipient divergent columnar enamel; IDCU, incipient divergent columnar units; IL, incrementing lines; MC, mesial crest limiting the mesial cavity.
Material and methods
The following isolated teeth morphologically referred to Manidens were sectioned: dentary teeth MPEF-PV 10862, 10863, and 10865; maxillary teeth MPEF-PV 3821, 10823 and 10864 (Fig. 1A1–F1). Tooth morphology, as described by Pol et al. (2011), and Becerra et al. (2014, 2018), allows relating each isolated tooth to either the maxillary or dentary tooth rows (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Becerra_Pol_SOM.pdf). In addition, the degree of development of the cingular edges (maxillary teeth), the height/width proportion of each tooth (maxillary and dentary teeth), the presence of a mesial cavity (maxillary and dentary teeth excepting MPEF-PV 10823), and the variation of these features within both tooth rows allow relating all teeth to a mid-posterior position of their corresponding tooth rows (Becerra et al. 2014, 2018). However, the degree of damage of these teeth precludes a more accurate inference of their possible position, and thus the following enamel description, although based in mid-posterior teeth, is generalized to each dentition. All the sectioned specimens are housed at the collection of the Museo Paleontológico Egidio Feruglio (Trelew, Argentina). Sectioning and SEM imaging followed the protocol of Sander (1999). No sputtercoating with gold was needed. Three sectioning planes (longitudinal, transversal and tangential) were conducted to assess the three-dimensional structure and orientation of enamel crystallites (Fig. 1A2–F2). The ornamentation of the external enamel surface (EES) was explored in the cleaned surface (ethanol 70%) of well-preserved specimens (dentary tooth MPEF-PV 3812 and maxillary tooth MPEF-PV 10861). SEM imaging was made with a scanning microscope Jeol JSM-6460 equipped with a secondary and backscattered electron detector (15 kV and 20 Pa). Phylogenetic changes of enamel microstructure in Ornithischia was addressed, including the new information of Manidens and that of other 29 taxa (specific to suprageneric level depending on the case) in a data matrix of 11 characters, evaluated in a supertree based on the most parsimonious topologies from bibliography (see SOM). The supertree was used to optimize ancestral states using parsimony for additive (characters 1, 2, 4, 8) and non-additive characters in TNT 1.1 (Goloboff et al. 2008a, b). The description of features follows the terminology and hierarchies of enamel organization defined by Sander (1999).
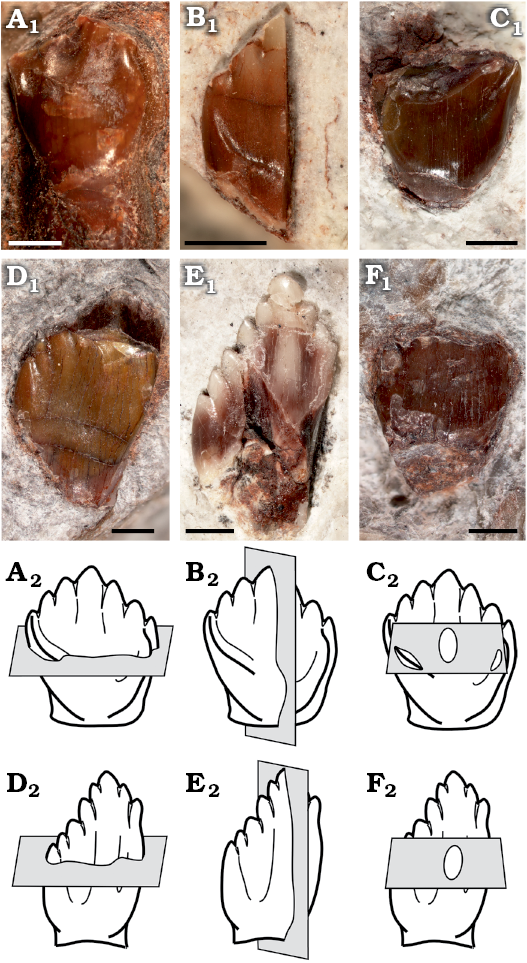
Fig. 1. Sectioned isolated teeth of a basal ornithischian Manidens condorensis Pol, Rauhut, and Becerra, 2011 from Cañadón Asfalto Formation (Queso Rallado locality, late Toarcian), Chubut province, Argentina. A. MPEF-PV 3821. B. MPEF-PV 10823. C. MPEF-PV 10864. D. MPEF-PV 10863. E. MPEF-PV 10862. F. MPEF-PV 10865. In labial (A–D) and lingual (E, F) views. Photographs (A1–F1), explanatory drawings of cross-section (transversal section) (A2, D2), longitudinal section (B2, E2), and tangential section (C2, F2). Sections as defined by Sander (1999). Scale bars 1 mm.
The enamel microstructure of Manidens condorensis represents the most basal ornithischian studied to date, and its enamel micromorphology is critical to (i) infer the plesiomorphic condition in Ornithischia and (ii) infer the ancestral enamel condition for several internal nodes within Ornithischia. This was explored through a parsimony optimization on a simplified phylogenetic tree of Ornithischia including the early ornithischian Manidens and the outgroup taxa Plateosaurus engelhardti (Sander 1999) and Coelophysis bauri (Hwang 2011). This topology includes only the small sample of ornithischians with known enamel microstructure and, therefore, the optimization of ancestral states may be biased by this limited taxon sampling. The optimization of ancestral states in the topology and the resulting synapomorphies were addressed following the hierarchical levels presented by Sander (1999) in the SOM, whereas most relevant results were here discussed.
Results
Maxillary dentition
Enamel surface, distribution and thickness.—The enamel surface MPEF-PV 10861 is smooth, lacks any micromorphological ornamentation and shows randomly oriented scratches that vary in length and width (Fig. 2A). The scratches in the studied area are not related to a planar occlusal wear surface, are distant to the crown edge, and in some cases their orientation seems to be mainly related to a combination of the hypothetic direction of the jaw motion (i.e., orthal; Becerra et al. 2014, 2018) and the heterogeneity of the tooth surface (e.g., the paracingular fossa, on the sides of the central and secondary ridges of each crown face). The combination of these features indicates that these scratches are likely the result of tooth-food interactions. Nevertheless, very small punctuations are randomly distributed along the entire surface, interpreted as enamel porosities (see below). Enamel is proportionally thicker in the labial (non-occlusal) face of the crown in both longitudinal (MPEF-PV 10823, Fig. 2C, excluding values of the cingular margin and below it) and transversal (MPEF-PV 3821, Fig. 3) sections (Table 1). In longitudinal section, enamel becomes thinner through the crown base more abruptly in the lingual face (changing along 11.37 μm of apicobasal length) than in the labial face (changing along 544.25 μm of apicobasal length) before disappearing. Enamel thickness reaches its highest value at the midpoint of the labial face and decreases through the crown apex down to 11.16 μm. Nevertheless, the cutting edges of the denticles are engrossed as identified by Becerra et al. (2018). In the lingual face, enamel shows a constant thickness along the crown face, becomes thinner at the paracingular fossa (e.g., Sereno 2012; Becerra et al. 2018) reaching a minimum value of 8.2 μm, becomes thicker through the edge of the cingular entoloph (10.9 μm), and maintains a constant thickness along the occlusal face of the cingulum (9.46 μm), until abruptly disappearing reaching the crown base. The average enamel thickness in the occlusal face of the crown does not significantly differ with that of the cingular entoloph of the same face (see SOM). The cross-section only gives information about enamel thickness and structure mesially and distally within the labial face, which varies in average in a mesiodistal direction within each face (becoming thicker distally and within the mesial cavity). Labially, the enamel at the mesial section is 27.07 μm thick in average. At this point and in a mesial direction, the enamel becomes thinner through the edge of the labial MC (22 μm), then becomes thicker inside the mesial cavity (average of 28.31 μm) and then gradually thins again through the edge of the lingual MC (12–14 μm). The enamel layer then thickens through the midpoint of the lingual face below the mesial entoloph, although reaching values lower than in the mesial cavity or the opposite face (average of 15.68 μm). At this midpoint, enamel stars becoming thinner distally through the end of the mesial entoloph (minimum of 11–12 μm). A slight increase in thickening occurs between the mesial and the distal entolophs (14–16 μm), which continues over the distal entoloph up to reaching the highest values of the face (average of 21.94 μm). This transitional thickening continues to the distal region of the opposing face, where the average reaches its maximum values of 31 μm.
Table 1. Thickness (in μm) of enamel. Abbreviations: pd, proportional difference of enamel thickness between compared faces; *, average value.
| |
Maxillary teeth |
Dentary teeth |
||||||
|
specimen number |
lingually |
labially |
labial/lingual pd (%) |
specimen number |
lingually |
labially |
labial/lingual pd (%) |
|
|
Cross-section |
MPEF-PV 3821 |
19.44 |
28.2 |
31 |
MPEF-PV 10862a, b |
22.9–26.28 |
23.6–26.94 |
2.69* |
|
Longitudinal section |
MPEF-PV 10823 |
9.83 |
13.17 |
29 |
MPEF-PV 10863 |
24.58 |
25.24 |
2.61 |
|
Intersample face pd (%) |
|
49.43 |
53.30 |
– |
|
0.04* |
0.12* |
– |
Enamel type.—Enamel at the crystallite level in maxillary teeth of Manidens is mainly composed by parallel and divergent crystallite (with and without forming clusters). The contact between crystallite is parallel in parallel crystallite, and the angle between divergent crystallite varies at the same cluster and also through the enamel. The divergent crystallites dominate over any other crystallite type. In cross-section (MPEF-PV 3821), parallel and/or slightly divergent crystallites without forming clusters are dominant near the EDJ (Fig. 3). The same sectioning plane shows extremely abundant divergent crystallite forming clusters at the middle region (Fig. 3) that reaches the EES in most of the enamel, whereas in some places these clusters reach to an outer layer of parallel crystallite. The longitudinal section (MPEF-PV 10823) differs with the cross-section in that its enamel is entirely dominated by divergent crystallite forming slender clusters, with parallel crystallite only seen in few regions near the EDJ (Fig. 2C). The tangential section (MPEF-PV 10864) shows an irregular disposition of enamel crystallite, with unclear boundaries between different crystallite divergence clusters (as in transversal and longitudinal sections). Consequently, these clusters cannot be identified as columnar units separated by convergence zones (Fig. 2B). The poorly defined limits between diverging units of enamel in Plateosaurus (Sander 1999) and pachycephalosaurids (Hwang 2005) was related to times of low depositional rates of enamel, possibly occurring the same in Manidens. Following Sander (1999), the lack of well-developed boundaries between slender divergent clusters is related to IDCU, and as such these are described at the crystallite level. Thus, the maxillary crowns lack a well-differentiated module level, showing enamel mostly formed by IDCU over the parallel crystallite. In cross-section, the presence of thin IL are confirmed, which are disposed near the EDJ in almost all its extension, and are scarce in the middle and outermost enamel (involving parallel and divergent crystallite for both cases). The IL are not identified in the longitudinal section. In cross-section, some regions show a complete development of IDCU from the EDJ to the EES, while in other regions this enamel rests on parallel crystallite layers with or without IL, and/or are below an external layer of parallel crystallite. In cross-section are frequent narrow spaces perpendicular to the EDJ, starting at the EDJ without crossing completely throughout the enamel, initiating at the middle region of the enamel and opening to the EES, or completely crossing the enamel. In oblique view of the base of the enamel layer exposed at longitudinal and transversal sections, some internal pores can be identified opening to the EDJ. In tangential section are also identified perforations perpendicular to the EDJ (Fig. 2B), and additionally were identified pores at the EES (Fig. 2A) and at the EDJ (Fig. 2C1). These perforations are randomly distributed and here identified as tubules, being a strong structural difference in enamel between dentary and maxillary teeth that can be added to the already contrasting dentitions of Manidens (Becerra et al. 2018). The presence of parallel/divergent crystallite as a distinctive layer above the EDJ allows considering it as a differentiated enamel type. In the longitudinal and cross-sections with oblique view of the enamel base, the absence of polygonal shapes at the EDJ related to the limits of columnar units also allows discarding the existence of a BUL, which contrasts to the condition of all described ornithischians (Sander 1999; Hwang 2004, 2011).
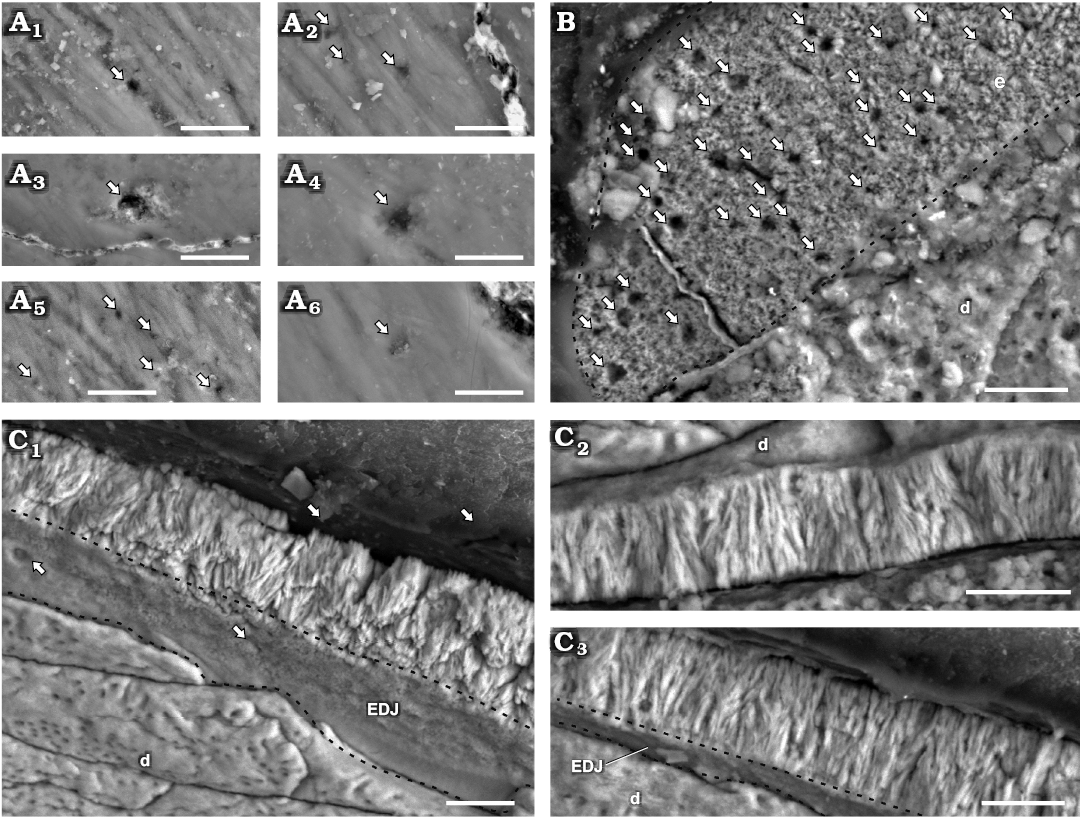
Fig. 2. Enamel in isolated maxillary teetornithischian Manidens condorensis Pol, Rauhut, and Becerra, 2011 from Cañadón Asfalto Formation (Queso Rallado locality, late Toarcian), Chubut province, Argentina. A. MPEF-PV 10861, details of different regions of the EES (A1–A6); arrows point to tubules reaching the EES. B. MPEF-PV 10864, tangential section; arrows point to tubules transversally sectioned, dashed line indicates the limit of enamel. C. MPEF-PV 10823, longitudinal section detailing different regions of the enamel layer (C1–C3); arrows point to tubules reaching the EDJ, dashed lines indicate the limits of the EDJ in oblique views. Abbreviations: d, dentine; EDJ, enamel-dentine junction; EES, external enamel surface. Scale bars 10 µm.
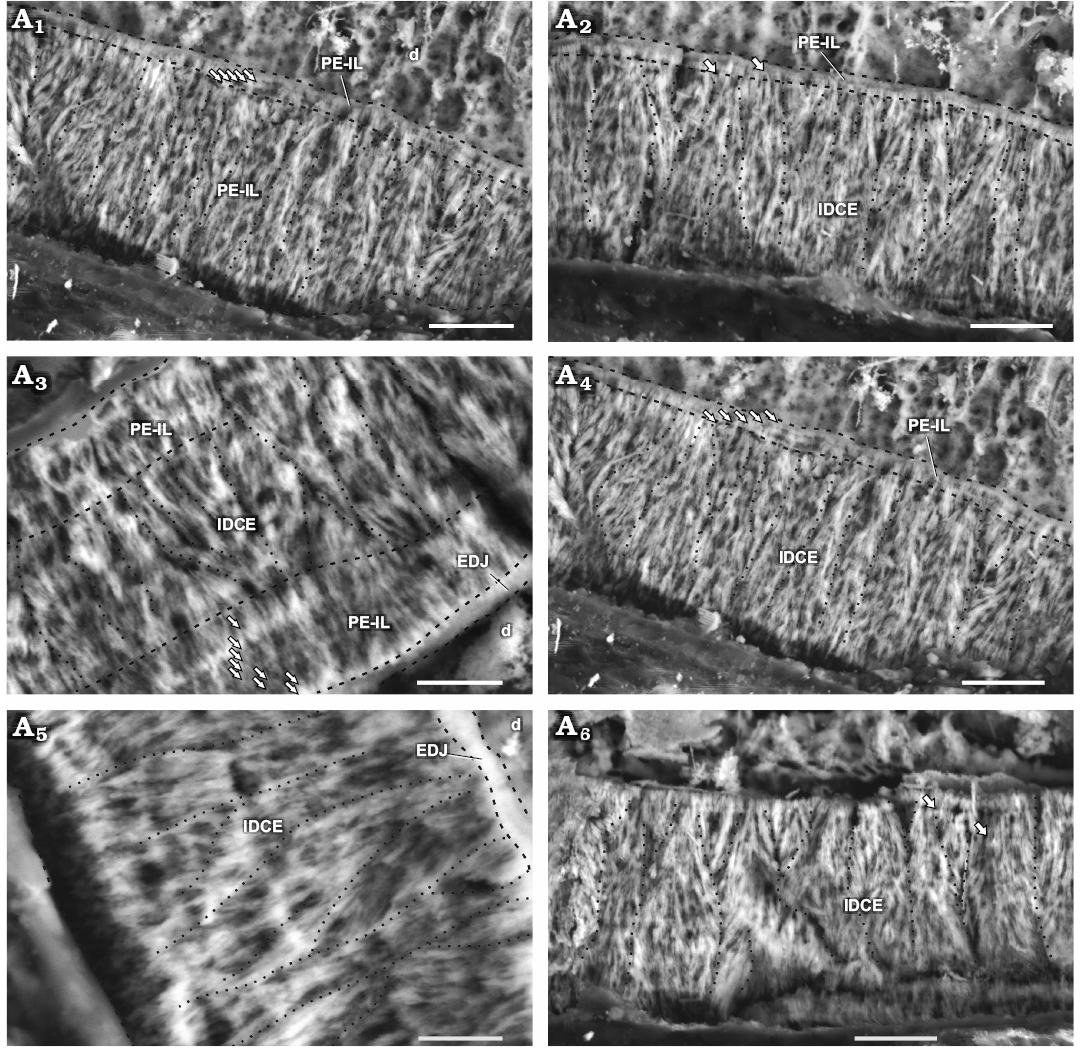
Fig. 3. Enamel in maxillary tooth MPEF-PV 3821 of a basal ornithischian Manidens condorensis Pol, Rauhut, and Becerra, 2011 from Cañadón Asfalto Formation (Queso Rallado locality, late Toarcian), Chubut province, Argentina. Details of the most common enamel in maxillary teeth (A1, A2, A4), basal PE-IL enamel followed by IDCE up to the EES. Details of different patches comprising a tri-layered enamel (A3) and only IDCE from the EDJ to the EES (A5, A6). Arrows point to IL, dot lines separate different IDCU, dashed lines indicate the possible limits of different enamel types and the EDJ. Abbreviations: d, dentine; EDJ, enamel-dentine junction; IDCE, incipient divergent columnar enamel; IDCU, incipient divergent columnar units; IL, incrementing lines; PE-IL, parallel/divergent crystallite enamel with incrementing lines. Scale bars 10 µm.
Schmelzmuster.—The enamel in the maxillary teeth of Manidens condorensis is disposed asymmetrically between faces. The type of dominant enamel corresponds to the IDCU. Parallel crystallite with or without IL frequently forms a basal enamel layer above the EDJ (Fig. 3A1, A2, A4) and less frequently as the outermost layer (Fig. 4C). Enamel appearing in tri-layered (parallel crystallite, IDCU enamel and parallel crystallite again; Fig. 3A3) and mono-layered patches (only IDCU enamel; Fig. 3A5, A6) are distributed along the enamel volume. Divergent crystallite without forming clusters is also identified adjacent to the EDJ and presenting IL, as composing the outermost enamel layer (with or without IL). This enamel lacks of BUL, external micro-ornamentation and empty enamel spaces, but presents randomly distributed tubules, although the evidences does not allow to discern if these are perpendicular, anastomosed or branching tubules, and if all these cross throughout the entire enamel layer. In addition, specimen MPEF-PV 10823 (longitudinal section) is the smallest in size of the sectioned maxillary teeth, and shows clear differences in enamel thickness and microstructure (Table 1; Fig. 3A3) if compared with MPEF-PV 3821 (Table 1; Fig. 4). Early ontogenetic stages of different reptilian species (e.g., Varanus niloticus, Sander 1999, Bactrosaurus johnsoni, Hwang 2005), show thinner enamel layer if compared with their advanced stages. Possibly the small size and thinner enamel of MPEF-PV 10823 is due to size or age differences, and might relate to a differential diet during ontogeny (see Discussion).
Dentary dentition
Enamel surface, distribution, and thickness.—The EES is smooth and lacks micro-ornamental features or porosities related to the enamel microstructure. As described for the enamel surface of the maxillary tooth, random scratches with varying length, width and orientation were identified in the evaluated enamel surface of the dentary tooth, likely related to tooth-food interaction as well (Fig. 4A). The differences of average enamel thickness in sectioned dentary teeth is not significant between faces (ranging between 22–27 μm), characterizing a symmetric disposition of enamel in the dentary dentition of Manidens (Table 1). Enamel thickens along the cutting margin of denticles (up to 39.47 μm), near the base of each denticle and between denticles (36.5–37.5 μm), and at the mesial cavity. Enamel gets thinner farther away from the denticles base (20–25 μm) at the point where the slope changes in the MC (12–12.5 μm), and through the crown base. In longitudinal section, enamel becomes thinner through the crown base more abruptly lingually (changing along 191.4 μm of apicobasal length) than labially (changing along 2191.4 μm of apicobasal length) before disappearing. The area included within the mesial cavity (MC and the mesial cavity itself) possess an enamel thickness similar to that of the crown faces (average of 21.98 μm).
Enamel type.—At the crystallite level and similar to maxillary teeth, the enamel in dentary teeth of Manidens possess parallel crystallite, crystals that diverge from each other without forming clusters, and abundant divergent crystallite forming clusters or bunches. IL are associated to parallel crystallite, being discontinuous in occurrence along the entire enamel and diffuse or absent in places with divergent crystallite. A thin layer of parallel crystallite with IL is located above the EDJ only in a few regions of the enamel layer (Fig. 4C1). As in the maxillary teeth, the contact between divergent enamel crystallite is angular rather than parallel. The divergent crystallites form slender units in which their angle to each other varies within the incipient unit and through the enamel (Fig. 4C, D). There is no clear separation between divergence units, which appear as diffuse contacts from their base through the EES in longitudinal and in cross-sections. In tangential section (Fig. 4B), there are no clear and continuous boundaries between the divergence units that form a polygonal pattern as in DCE (Sander 1999). Instead, the tangential section shows an irregular arrangement of crystallite without forming clear and well defined divergent clusters (Fig. 4B). The presence of IDCU at the enamel of dentary crowns indicates the absence of a well-differentiated module level, as in maxillary teeth. There are no tubules, empty spaces or voids. Three types of enamel are identified: abundant enamel of IDCU, and scarce regions of parallel crystallite and divergent crystallite without forming clusters. The longitudinal (Fig. 4D) and cross-sections (Fig. 4C) expose the EDJ in oblique inner view, which shows a basal layer with a continuous surface and lacking of boundaries between units forming polygonal shapes. The absence of polygonal shapes allows affirming that there is no BUL in the enamel of dentary teeth of Manidens, as in the maxillary dentition.
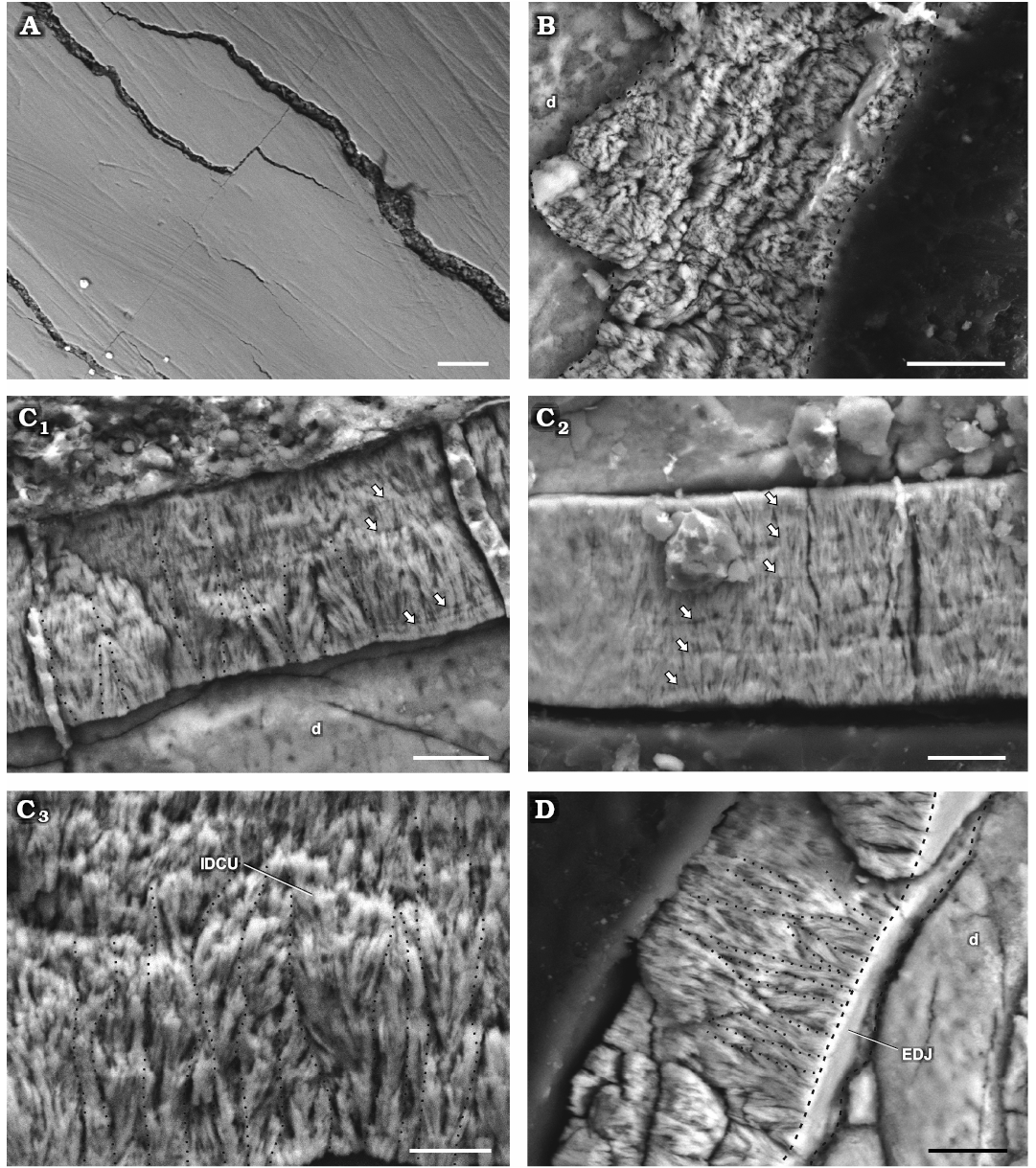
Fig. 4. Enamel in isolated dentary teeth of a basal ornithischian Manidens condorensis Pol, Rauhut, and Becerra, 2011 from Cañadón Asfalto Formation (Queso Rallado locality, late Toarcian), Chubut province, Argentina. A. MPEF-PV 3812, detail of the EES. B. MPEF-PV 10865, details of the tangential section. C. MPEF-PV 10863, cross-sections (C1–C3). D. MPEF-PV 10862, longitudinal section. Arrows point to IL, dot lines separate different IDCU, dashed lines indicate the limits of the EDJ in oblique view. Abbreviations: d, dentine; EDJ, enamel-dentine junction; EES, external enamel surface; IDCU, incipient divergent columnar units; IL, incrementing lines. Scale bars 10 µm.
Schmelzmuster.—The sectioned dentary teeth of Manidens show a thin enamel symmetrically distributed, structurally formed by IDCU dominating the entire layer. Regions of parallel and/or divergent crystallite (not forming IDCU) are identified, while these with IL appear sporadically in patches in the middle and external portions of enamel. The enamel lacks of any regular arrangement of enamel types, although is frequent the presence of parallel crystallite with IL above the EDJ. There are no BUL, tubules, empty spaces, or micro-ornamentation in the EES.
Comparisons with Dinosauria and Ornithischia
The enamel of Plateosaurus and “Gyposaurus” (Basal Sauropodomorpha, as described by Hwang 2011), besides being a thin layer symmetrically distributed (does not exceed 40 μm thick), combines parallel crystallite enamel type with crystallite grouped in incipient units (IDCU), lacks basal unit layer (BUL) and shows sporadic incrementing lines (IL). These features summarize with great similarity the “schmelzmuster” of the dentary dentition in Manidens. However, in Manidens the divergent crystallite enamel forming IDCU is more abundant if compared with Plateosaurus and “Gyposaurus” (Tang et al. 2001; Hwang 2011).
Within Ornithischia, the enamel in teeth of pachycephalosaurids is quite similar to Manidens. The enamel of premaxillary/anterior dentary teeth in the pachycephalosaurid specimens share with the dentary teeth of Manidens the presence of parallel crystallite, the common IDCU in bunches and the IL (Hwang 2005). The presence of a basal layer of parallel/divergent crystallite with IL (BUL in the pachycephalosaurid teeth), followed by divergent crystallite forming IDCU, and finishing with a layer of parallel/divergent crystallite with IL (without clustering) in some regions of the enamel of Manidens maxillary teeth structurally resembles the enamel of maxillary/posterior dentary teeth of indeterminate pachycephalosaurid specimens (Hwang 2005). Nonetheless, these dentitions differ from Manidens in having a less abundant IDCU in relation to the parallel crystallite, the presence of a BUL, and the outermost layer of parallel crystallite in the pachycephalosaurid specimens is firstly without IL and then with IL (Hwang 2005, 2011). Maxillary teeth of Manidens shows a tri-layered arrangement of enamel types only in some isolated patches, being far from dominating completely the enamel as occurs in the maxillary/posterior dentary teeth of the pachycephalosaurid specimens (Hwang 2005, 2011). Enamel in premaxillary/anterior dentary teeth of the pachycephalosaurid specimens shares it asymmetric enamel distribution with the maxillary teeth of Manidens, while the symmetric distribution of enamel in maxillary/posterior dentary teeth of the former specimens is shared with the dentary dentition of Manidens (Hwang 2005, 2011). The enamel types identified in Manidens, mainly including parallel/divergent crystallite (with or without IL) and IDCU, is similar to the pachycephalosaurid specimens (Hwang 2005, 2011), and also to Plateosaurus and “Gyposaurus” outside Ornithischia. However, as mentioned for the dentary dentition, the disposition and distribution of these enamel types is different between taxa. Parallel crystallite dominates the enamel in Plateosaurus and “Gyposaurus” over the IDCU appearing in patches, BUL and IDCU for premaxillary/anterior dentary teeth and a tri-layered enamel formed by the BUL with IL followed by IDCU enamel and finishing with parallel crystallite without and with IL in indeterminate pachycephalosaurid specimens. However, IDCU dominates enamel with patches of parallel/divergent crystallite (with or without IL) in dentary teeth of Manidens, enamel is mainly formed by a layer of parallel crystallite (with IL) and IDCU up to the EES (with patches of the aforementioned tri-layered enamel, and patches with complete IDCU) in maxillary teeth of Manidens. However, in the dentary teeth of Manidens, the IDCU dominates enamel with patches of parallel/divergent crystallite (with or without IL). Also, the enamel in maxillary teeth of Manidens is mainly formed by a layer of parallel crystallite (with IL) and IDCU up to the EES (with patches forming the aforementioned tri-layered enamel, and patches with complete IDCU).
The enamel in Manidens raises an interesting study case, because resembles the “schmelzmuster” of Pachycephalosauridae in some patches of the maxillary dentition, their enamel types are the same and shared with basal sauropodomorphs, and Manidens and the pachycephalosaurid specimens also resemble the enamel in Ankylosauria in other aspects. While the maxillary/posterior dentary teeth of the indeterminate pachycephalosaurid show a similar “schmelzmuster” but lacking of DCE, Manidens presents sporadic and randomly distributed tubules in its maxillary teeth as in specimens of Ankylosauria. Tubules together with the asymmetric disposition of enamel are shared with species of Ceratopsia and derived Ornithopoda, but these are incipiently developed in the maxillary teeth of Manidens (Sander 1999; Hwang 2005, 2010, 2011). Summarizing, the dentition of Manidens combines simple enamel features as those in basal sauropodomorphs with slightly differentiated enamel features as in pachycephalosaurids, and vaguely resembling features present in ankylosaurs and cerapods (Hwang 2011).
Discussion
Enamel variation across dentitions, ontogeny, and dietary implications.—The described “schmelzmuster” of the upper and lower dentitions of Manidens condorensis combines parallel crystallite enamel type (with and/or without IL), divergent crystallite without forming clusters (with and/or without IL), and divergent crystallite forming IDCU. This enamel lacks of BUL, enamel voids and external micro-ornamentations, and does not present a module level. Although the enamel types present in maxillary and dentary teeth Manidens condorensis summarize a structurally simple enamel (Sander 1999), these show evident structural differentiations. The abundance and structural ordering of these enamel types, the thickness variation and the tubules represent strong differences between dentitions. The enamel is symmetrically distributed between faces in the dentary teeth and lack tubules, while the maxillary teeth show an asymmetric distribution of enamel, and multiple tubules appear as randomly distributed. Although both dentitions the enamel is dominated in proportion by the IDCU, the enamel type of parallel/divergent crystallite with or without IL is seen in patches and more abundantly in dentary teeth, whereas in maxillary teeth these correspond to a minor component that appears regularly as layers near the EDJ and less frequently near the EES. Although the IL are present in both dentitions, these are sporadically developed in dentary teeth and regularly ordered in maxillary teeth, varying as mentioned for the parallel crystallite. The differences in structure are uncommon between the upper and lower dentition of the same species, but Sander (1999) and Hwang (2005) already related the complexity of crown morphology with the complexity of enamel. Manidens shows strong morphological differences between maxillary and dentary cheek dentitions (Becerra et al. 2018), these also explaining the structural differences in enamel between dentitions. Morphologic differences are common between premaxillary and cheek dentitions in early ornithischians, but the strong differentiation between maxillary and dentary dentitions is unique for Manidens in Ornithischia (Becerra et al. 2018). This strong heterodonty was demonstrated to improve the craniomandibular functioning without affecting the primitive anatomy of the skull and the plesiomorphic jaw motion (Becerra et al. 2018). A process of modular evolution over the opposing cheek dentitions and resulting from the selective pressure due to diet specialization explains this maxillary-dentary heterodonty, with the differences in enamel structure between dentitions due to these strong differences in morphology.
There are notable differences in size and enamel features (i.e., micromorphology and thickness) between MPEF-PV 10823 and other maxillary teeth, but this specimen preserves the same morphologic features proportional to its crown size (i.e., hypothetic number of denticles, size of cingular edges, crown proportions) than other crowns of the mid tooth row. Similar size-related variations of dentition in young individuals were described for Heterodontosaurus (Butler et al. 2008a; Norman et al. 2011), Dysalotosaurus (Hübner and Rauhut 2010), extant diapsids (Brown et al. 2015) and other reptiles (Berkovits and Sellis 2016). Specimen MPEF-PV 10823 is smaller than others, and its smaller size is also likely related to ontogeny, inferring that possibly belonged to a young individual and that the size of teeth increased during ontogeny of Manidens. The enamel of MPEF-PV 10823 is simpler and thinner than other specimens, which was possibly true for the entire cheek dentition of younger individuals. This also implies a lesser stress-resistant dentition if compared with that of later ontogenetic stages, with larger teeth and more complex and thicker enamel. This difference in enamel features and structure during ontogeny and the functional constraints ruled by the hardness and thickness of enamel may support a different diet between juvenile and adult forms. The enamel is more easily affected by wear in younger individuals, possibly leading to feed on softer and more energetic food items (omnivorous diet) than in adult stages (herbivorous/omnivorous diet), as occurs in extant reptiles (Berkovitz and Sellis 2016).
The “schmelzmuster” and enamel features of Manidens can be characterized in the context of the hypothetic adaptive complexes described by Sander (1999), which were erected using a broad sampling of non-mammalian fossil taxa. A thin enamel layer with parallel crystallite are features shared between Manidens and species grouped by Sander (1999) in the insectivore adaptive complex, a feeding behavior that might be supported by the small size of Manidens and its inherent metabolic requirements. However, the crown morphology and enamel types in the schmelzmuster of Manidens resemble features shared with species grouped in the non-oral processing herbivore adaptive complex, as in Plateosaurus (i.e., parallel crystallite and IDCU). The close packing of teeth in the cheek dentition of Manidens (Becerra et al. 2014, 2018) is the only shared feature with a third adaptative complex, those species grouped in the oral processing herbivore complex (Sander 1999). The comparison of enamel microstructure with other non-mammalian amniotes supports a feeding behavior with low intraoral processing of vegetation, not excluding the possibility of omnivory principally associated with insectivory. The diet inference supported by enamel microstructure adjusts with the previous hypothesis of craniomandibular functioning in Manidens based on skull morphology and wear facets (Becerra et al. 2014, 2018). A diet with low to none oral processing in Manidens has been also supported by (i) the moderate development of craniomandibular anatomy (regions for adductor jaw musculature, the retroarticular process, the jaw articulation ventrally offset, cheeks); (ii) the amount and restricted development of vertically oriented wear facets in apical and cingular cutting margins; and (iii) the plesiomorphic orthal jaw motion. All these features, together with enamel microstructure, allow considering that Manidens was likely herbivorous but not specialized for this diet, likely with different diets in young individuals and facultative omnivory if necessary (Weishampel and Norman 1989; Barrett et al. 2010; Becerra et al. 2014, 2018). In addition, other features present in the dentition of the species favored an herbivorous diet, likely compensating (at least in part) the deficiencies in the skull: (i) the closely-packed and slightly emarginated ordering of opposing cheek teeth; (ii) large denticles; and (iii) a complex interaction of opposing teeth resulting in a double occlusion per masticatory cycle (Pol et al. 2011; Norman et al. 2011; Sereno 2012; Becerra et al. 2016, 2018).
Enamel evolution in Ornithischia.—The addition of Manidens and outgroup taxa (Plateosaurus and Coelophysis) allows optimizing the absence of a BUL (Fig. 5F), the presence of IDCU as the dominant enamel type (Fig. 5E), and the parallel crystallite as a minor component (Fig. 5C), and sporadically developed IL (Fig. 5G) as the ancestral condition in Ornithischia. In addition, enamel surface (Fig. 5J) is optimized as smooth at the base of Ornithischia and with a thickness smaller than 100 µm (Fig. 5B, C) and lacking enamel voids (Fig. 5K). The ancestral state of enamel symmetry (symmetric/asymmetric, Fig. 5L) and tubules (absence/present but not dominant, Fig. 5I) are optimized as ambiguous in the node Ornithischia, mainly because these were coded has ambiguous in Manidens due to the presence of different conditions in the upper and lower dentition.
Within Ornithischia and regardless of the thickness and volume of the enamel layer, the appearance of a BUL possibly happened once in the evolution of Ornithischia, appearing at the base of Genasauria along with the derived craniomandibular anatomy related to herbivory (e.g., Ostrom 1966; Tanoue et al. 2009a, b; Sereno 2012; Ösi et al. 2014, 2017; Nabavizadeh 2016; Strickson et al. 2016). The optimization of IDCU enamel (Fig. 5E) in all internal nodes of Ornithischia (Genasauria, Marginocephalia, Cerapoda, Tyreophora) allows supporting a common ancestry between the IDCU (or poorly developed DCU) enamel type of Pachycephalosauridae and Tyreophora, contrasting with the convergence hypothesis proposed by Hwang (2005). The identification of tubules in the enamel of Manidens is present only in the maxillary dentition (i.e., ambiguous), but is unambiguously present in most other ornithischian lineages (Fig. 5I). Given the ambiguous presence of this feature in the dentition of Manidens, the condition at base of Ornithischia is also optimized ambiguously. This, plus the sporadic presence of tubules at some internal nodes of Genasauria makes uncertain the evolutionary history of this feature and further research and data are needed to understand it more completely.
The morphology of the enamel became increasingly complex along lineages such as Psittacosaurus and Neoceratopsia, non-hadrosaurian ornithopods, and Hadrosauriformes (Sander 1999; Hwang 2005, 2011). Enamel complexity shows homoplastic evolutionary patterns along these lineages, which seem to respond similarly to selective pressures related to masticatory efficiency (i.e., wrinkled enamel surface, Fig. 5J; enamel asymmetry, Fig. 5L; enamel thickness, Fig. 5B, C; a resistant, wavy or columnar, enamel, Fig. 5E, H; a more resistant outermost enamel layer, SOM). Functional or morphological constraints likely caused these convergences from the same ancestral condition during the adaptation of different lineages to herbivory.
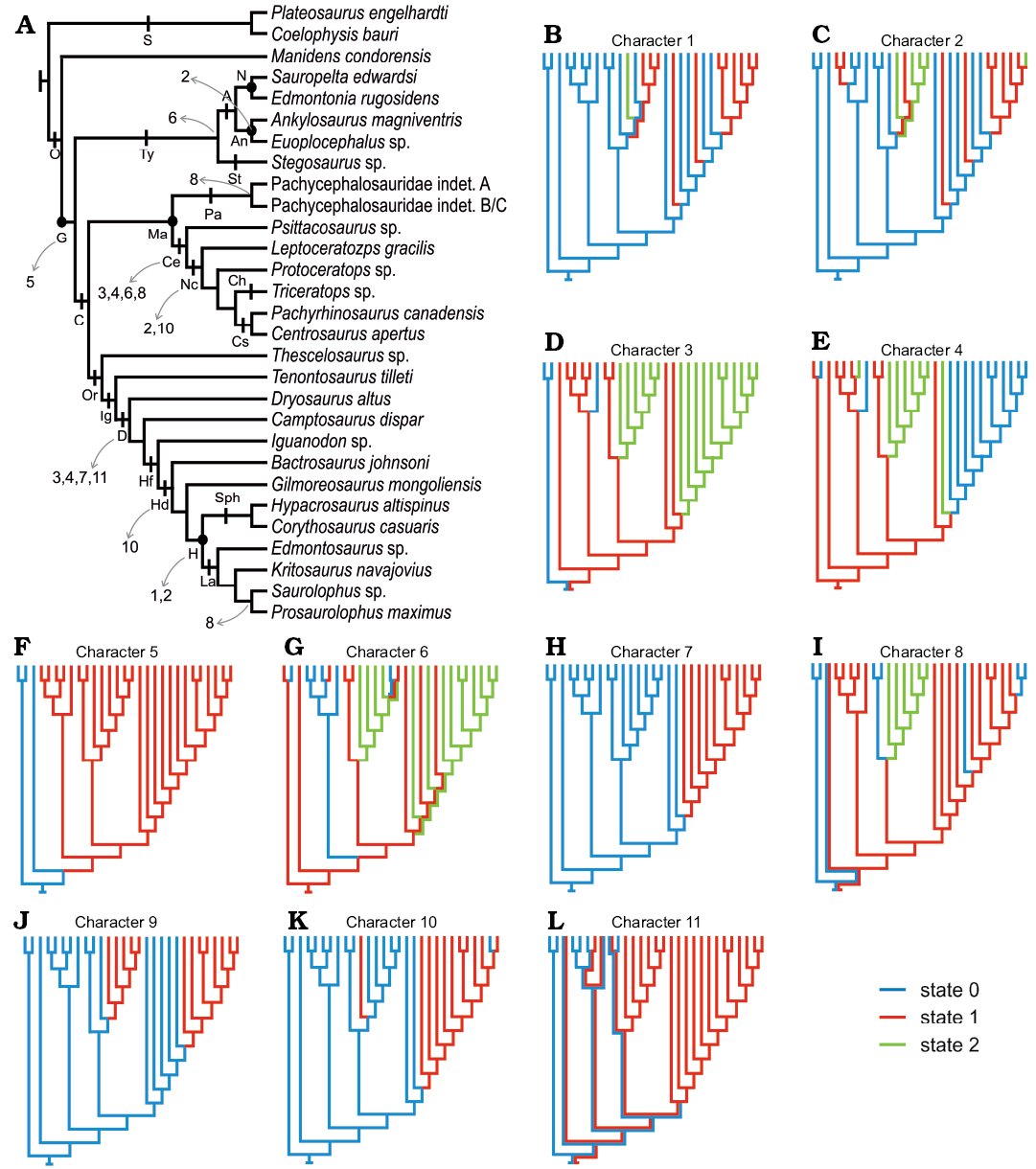
Fig. 5. Constructed topology based on the revised bibliography (see SOM). A. Phylogenetic topology comprising the ornithischian dinosaurs with known enamel micromorphology, indicating the represented major ornithischian lineages and the enamel characters recovered as synapomorphic of different groups (which can be optimized). B–L. Optimized character states in the topology expressed as different colours. Minimum enamel thickness (B), maximum enamel thickness (C), parallel crystallite enamel (D), columnar enamel (E), basal unit layer (F), incrementing lines (G), wavy enamel (H), tubules (I), micro-ornamentation on the enamel surface (J), enamel voids (K), enamel symmetry (L). Abbreviations: A, Ankylosauria; An, Ankylosauridae; C, Cerapoda; Ce, Ceratopsia; Ch, Chasmosaurinae; Cs, Centrosaurinae; D, Dryomorpha; G, Genasauria; H, Hadrosauridae; Hd, Hadrosauroidea; Hf, Hadrosauriformes; Ig, Iguanodontia; L, Lambeosaurinae; Ma, Marginocephalia; N, Nodosauridae; Nc, Neoceratopsia; O, Ornithischia; Or, Ornithopoda; S, Saurischia; Sph, Saurolophinae; St, Stegosauria.
The increase in enamel thickness shows in this study at least three cases of convergences within Ornithischia (Nodosauridae, Neoceratopsia, and Hadrosauridae). In addition, the enamel asymmetry is depicted as occurring in Ornithopoda, Ceratopsia, and Euoplocephalus, but the remaining internal nodes are optimized as ambiguous and the evolutionary pathway of enamel asymmetry is therefore unclear. Other descriptions (Norman et al. 2011; Sereno 2012; Becerra et al. 2014, 2018) and phylogenies (Butler et al. 2008b; Becerra et al. 2016) have addressed the evolution of enamel thickness and symmetry including more taxa than the ones considered here given these features can be determined in taxa for which the enamel microstructure is unknown (see SOM). These studies nonetheless support and complement the results we obtained. Sereno (2012), for instance, describes the independent occurrence of enamel thickening together with the trend to completely loose the enamel on the functional surface of the crown (i.e., enamel asymmetry) in the dentition of Heterodontosaurinae (Norman et al. 2011; Butler et al. 2012; Becerra et al. 2014). Similarly, in the phylogenies of Butler et al. (2008b) and Becerra et al. (2016), symmetric enamel is optimized at the base of Ornithischia, Genasauria, Tyreophora, and Cerapoda, and enamel asymmetry is depicted as independently acquired in Heterodontosaurinae, Iguanodontia, and Ceratopsia. However, these authors codified the enamel in the suprageneric taxa Stegosauria and Ankylosauria as symmetric (see SOM), ignoring the enamel asymmetry of Euoplocephalus. The combined results of these authors and our research allow considering at least four convergent events in the development of enamel asymmetry in Ornithischia: occurring in Euoplocephalus among Ankylosauria, Heterodontosaurinae, Iguanodontia among Ornithopoda, and within basal Ceratopsians.
Conclusions
The enamel micromorphology of Manidens condorensis represents the first study of this kind for Heterodontosauridae, the earliest and basal-most radiation within Ornithischia. In Manidens and as described in other dinosaurs (e.g., Hwang 2005), enamel complexity is correlated to the crown complexity, which allows relating the differences in enamel microstructure between maxillary (morphologically more complex crown) and dentary teeth (morphologically simpler crown) with the strong morphological heterodonty between these dentitions (Becerra et al. 2018). Additionally, the recovery of a possible young individual indicates that simpler enamel types appear in early ontogenetic stages for Manidens, and possibly resulted in dietary differences due to ontogenetic restrictions of enamel (Berkovitz and Shellis 2016). This study reinforces the hypothesis that phylogenetic constraints and dietary habits play an important role in shaping enamel microstructure in Ornithischia. Phylogenetic constraints determine the starting point from where enamel microstructure will subsequently develop in different enamel types, being a critical factor determining the evolution of enamel microstructure in Ornithischia. Most of the derived enamel types in this clade can be explained as the modification of IDCU enamel and parallel crystallite with sporadically developed IL, states present in Manidens and optimized in the ancestral node Ornithischia.
The description of enamel microstructure in Manidens provides critical new information as it is the first member of a basal branch of Ornithischia for which enamel microstructure is determined. This influences the ancestral reconstruction at the base of Ornithischia through the analysis of enamel characters in phylogenetic trees of this clade. Further research, including a larger sampling of taxa can contribute to better understanding the evolution of enamel in this clade and its relation with the adaptive changes in other features of the craniomandibular apparatus. Further description of enamel microstructure in basal branches of Ornithischia can provide a better assessment of the ancestral condition of the clade but, more importantly, information on outgroups (basal dinosaurs, sauropodomorphs, dinosauriforms) is deeply needed. Phylogenetic topologies including more taxa will allow testing our hypothesis of enamel evolution and describing the adaptation of the craniomandibular apparatus to different diets during the evolution of lineages that dominated the herbivore faunas during the Mesozoic.
Acknowledgements
The discussions and help of Oliver W.M. Rauhut (Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany), Jose L. Carballido (Museo Paleontológico Egidio Feruglio, Trelew, Chubut province, Argentina), and P. Martin Sander (Universität Bonn, Bonn, Germany) about enamel microstructure and dental anatomy in Archosauria and Ornithischia are thanked. Fieldwork was possible thanks to the authorities of the Secretaría de Cultura de la Provincia del Chubut. Fieldwork and research were supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina), under grants PICT 2017-1897 (MGB), 1288 and 0808 (DP); Deutsche Forschungsgemeinschaft (DFG, Germany), under grant RA1012/9-1 (Oliver W.M. Rauhut); and National Science Foundation (USA), under grants DEB 0946430 and DEB 1068089 (Guillermo W. Rougier). The authors thank to Aluar Aluminio Argentino SAIC for access to the SEM lab as well as the valuable help of Jaime Groizard and Mauricio Luquet (both Aluar Aluminio Argentino SAIC, Puerto Madryn, Chubut province, Argentina).
References
Barrett, P.M., Butler, R.J., and Nesbitt, S.J. 2010. The roles of herbivory and omnivory in early dinosaur evolution. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 101: 383–396. Crossref
Becerra, M.G., Gomez, K.L., and Pol, D. 2017. A sauropodomorph tooth increases the diversity of dental morphotypes in the Cañadón Asfalto Formation (Early–Middle Jurassic) of Patagonia. Comptes Rendus Palevol 16: 832–840. Crossref
Becerra, M.G., Pol, D., Marsicano, C.A., and Rauhut, O.W.M. 2014. The dentition of Manidens condorensis (Ornithischia; Heterodontosauridae) from the Jurassic Cañadón Asfalto Formation of Patagonia: morphology, heterodonty and the use of statistical methods for identifying isolated teeth. Historical Biology 26: 480–492. Crossref
Becerra, M.G., Pol, D., Rauhut, O.W.M., and Cerda, I.A. 2016. New heterodontosaurid remains from the Cañadón Asfalto Formation: cursoriality and the functional importance of the pes in small heterodontosaurids. Journal of Paleontology 90: 555–577. Crossref
Becerra, M.G., Pol, D., Rössner, G.E., and Rauhut, O.W.M. 2018. Heterodonty and double occlusion in Manidens condorensis: a unique adaptation in an Early Jurassic Ornithischia improving masticatory efficiency. Science of Nature 105: 41. Crossref
Berkovitz, B.K. and Shellis, R.P. 2016. Tooth Replacement and Ontogeny of the Dentition. The Teeth of Non-mammalian Vertebrates. 343 pp. Academic Press, Cambridge. Crossref
Brown, C.M., VanBuren, C.S., Larson, D.W., Brink, K.S., Campione, N.E., Vavrek, M.J., and Evans, D.C. 2015. Tooth counts through growth in diapsid reptiles: implications for interpreting individual and size-related variation in the fossil record. Journal of Anatomy 226: 322–333. Crossref
Butler, R.J., Porro, L.B., and Norman, D.B. 2008a. A juvenile skull of the primitive ornithischian dinosaur Heterodontosaurus tucki from the “Stormberg” of southern Africa. Journal of Vertebrate Paleontology 28: 702–711. Crossref
Butler, R.J., Porro, L.B., Galton, P.M., and Chiappe, L.M. 2012. Anatomy and cranial functional morphology of the small-bodied dinosaur Fruitadens haagarorum from the Upper Jurassic of the USA. PLoS One 7: e31556. Crossref
Butler, R.J., Upchurch, P. and Norman, D.B. 2008b. The phylogeny of the ornithischian dinosaurs. Journal of Systematic Palaeontology 6: 1–40. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008a. TNT, a free program for phylogenetic analysis. Cladistics 24: 1–13. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008b. TNT (Tree Analysis Using New Technology) ver. 1. 1. Published by the authors, Tucumán.
Heckert, A.B. and Miller-Camp, J.A. 2013. Tooth enamel microstructure of Revueltosaurus and Krzyzanowskisaurus (Reptilia: Archosauria) from the Upper Triassic Chinle Group, USA: Implications for function, growth, and phylogeny. Palaeontologia Electronica 16: 1–23. Crossref
Huebner, T.R. and Rauhut, O.W. 2010. A juvenile skull of Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia), and implications for cranial ontogeny, phylogeny, and taxonomy in ornithopod dinosaurs. Zoological Journal of the Linnean Society 160: 366–396. Crossref
Hwang, S.H. 2005. Phylogenetic patterns of enamel microstructure in dinosaur teeth. Journal of Morphology 266: 208–240. Crossref
Hwang, S.H. 2010. The utility of tooth enamel microstructure in identifying isolated dinosaur teeth. Lethaia 43: 307–322. Crossref
Hwang, S.H. 2011. The evolution of dinosaur tooth enamel microstructure. Biological Reviews 86: 183–216. Crossref
Nabavizadeh, A. 2016. Evolutionary trends in the jaw adductor mechanics of ornithischian dinosaurs. The Anatomical Record 299: 271–294. Crossref
Norman, D.B., Crompton, A.W., Butler, R.J., Porro, L.B., and Charig, A.J. 2011. The Lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: cranial anatomy, functional morphology, taxonomy, and relationships. Zoological Journal of the Linnean Society 163: 182–276. Crossref
Ösi, A., Barrett, P.M., Földes, T., and Tokai, R. 2014. Wear pattern, dental function, and jaw mechanism in the Late Cretaceous ankylosaur Hungarosaurus. The Anatomical Record 297: 1165–1180. Crossref
Ösi, A., Prondvai, E., Mallon, J., and Bodor, E.R. 2017. Diversity and convergences in the evolution of feeding adaptations in ankylosaurs (Dinosauria: Ornithischia). Historical Biology 29: 539–570. Crossref
Ostrom, J.H. 1966. Functional morphology and evolution of the ceratopsian dinosaurs. Evolution 20: 290–308. Crossref
Pol, D., Rauhut, O.W.M., and Becerra, M.G. 2011. A Middle Jurassic heterodontosaurid dinosaur from Patagonia and the evolution of heterodontosaurids. Naturwissenschaften 98: 369–379. Crossref
Sander, P.M. 1999. The microstructure of reptilian tooth enamel: terminology, function, and phylogeny. Münchner Geowissenschaftliche Abhandlungen, Reihe A, Geologie und Palaontologie 38: 1–103.
Sander, P.M. 2000. Prismless enamel in amniotes: terminology, function, and evolution. In: M.F. Teaford, M.M. Smith, and M.W. Ferguson (eds.), Development, Function and Evolution of Teeth, 92–106. Cambridge University Press, New York. Crossref
Sereno, P.C. 2012. Taxonomy, morphology, masticatory function and phylogeny of heterodontosaurid dinosaurs. ZooKeys 226: 1–225.
Spencer, M.R. 2013. Phylogenetic and Biogeographic Assessment of Ornithischian Diversity Throughout the Mesozoic: A Species-level Analysis from Origin to Extinction. 296 pp. Unpublished Ph.D. Thesis, University of Iowa, Iowa City.
Stokosa, K. 2005. Enamel microstructure variation within the Theropoda. In: K. Carpenter (ed.), The Carnivorous Dinosaurs, 163–178. Indiana University Press, Bloomington.
Strickson, E., Prieto-Márquez, A., Benton, M.J., and Stubbs, T.L. 2016. Dynamics of dental evolution in ornithopod dinosaurs. Scientific Reports 6: 28904. Crossref
Tang, F., Jing, X.-S., Kang, X.-M., and Zhang, G.-J. 2001. Omeisaurus maoianus: A Complete Sauropoda from Jinyan, Sichuan. 128 pp. China Ocean Press, Beijing.
Tanoue, K., Grandstaff, B.S., You, H.L., and Dodson, P. 2009a. Jaw mechanics in basal ceratopsia (Ornithischia, Dinosauria). The Anatomical Record 292: 1352–1369. Crossref
Tanoue, K., You, H.L., and Dodson, P. 2009b. Comparative anatomy of selected basal ceratopsian dentitions. Canadian Journal of Earth Sciences 46: 425–439. Crossref
Weishampel, D.B. and Norman, D.B. 1989. Vertebrate herbivory in the Mesozoic; jaws, plants, and evolutionary metrics. Geological Society of America Special Papers 238: 87–101. Crossref
Acta Palaeontol. Pol. 65 (1): 59–70, 2020
https://doi.org/10.4202/app.00658.2019