

Variability of conch morphology in a cephalopod species from the Cambrian to Ordovician transition strata of Siberia
JERZY DZIK
Dzik, J. 2020. Variability of conch morphology in a cephalopod species from the Cambrian to Ordovician transition strata of Siberia. Acta Palaeontologica Polonica 65 (1): 149–165.
A block of stromatolitic limestone found on the Angara River shore near Kodinsk, Siberia, derived from the exposed nearby Ust-kut Formation, has yielded a sample of 146 ellesmeroceratid nautiloid specimens. A minor contribution to the fossil assemblage from bellerophontid and hypseloconid molluscs suggests a restricted abnormal salinity environment. The associated shallow-water low diversity assemblage of the conodonts Laurentoscandodus triangularis and Utahconus(?) eurypterus indicates an age close to the Furongian–Tremadocian boundary. Echinoderm sclerites, trilobite carapaces, and hexactinellid sponge spicules were found in another block from the transitional strata between the Ust-kut and overlying terrigenous Iya Formation; these fossils indicate normal marine salinity. The conodont L. triangularis is there associated with Semiacontiodus iowensis and Cordylodus angulatus. This means that the stromatolitic strata with cephalopods are older than the early Tremadocian C. angulatus Zone but not older than the Furongian C. proavus Zone. The sample of nautiloid specimens extracted from the block shows an unimodal variability in respect to all recognizable aspects of their morphology. The material is probably conspecific with the poorly known Ruthenoceras elongatum from the same strata and region.
Key words: Cephalopoda, Nautiloidea, Endoceratida, Ellesmeroceratina, evolution, Furongian, Tremadocian, Russia.
Jerzy Dzik [dzik@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warszawa, Poland and Faculty of Biology, Biological and Chemical Centre, University of Warsaw, Żwirki i Wigury 101, 02-096, Warszawa, Poland.
Received 4 September 2019, accepted 13 November 2019, available online 26 February 2020.
Copyright © 2020 J. Dzik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The crucial apomorphy of the Cephalopoda is their hydrostatic organ, the phragmocone, likely representing an adaptation to pelagic life. One would thus expect that they should be associated with open-sea environments from the beginning of their evolution. The oldest known nautiloid fossils of the genus Plectronoceras were first found associated with numerous trilobites in a thin-bedded limestone interbedded with an intraformational conglomerate indicating deposition in turbulent but fully marine waters, seemingly supporting such inference. Ironically, the majority of the latest Cambrian nautiloids come from extremely shallow-water, mostly stromatolitic environments (Chen and Teichert 1983: 13, 37; Landing and Kröger 2009; Wu et al. 2017; Xiao et al. 2018). Truly pelagic nautiloids appeared much later (Kröger et al. 2009). The question emerges: were those most ancient nautiloids adapted to restricted environments with abnormal salinity or, rather, were they thrown into a hostile environment by catastrophic factors? Moreover, how strong were selective forces acting on the conch morphology and structure in early stages of the cephalopod evolution. These questions can be answered by estimating the limits of population variability of the early nautiloid conch. The finding of an unusually rich accumulation of ellesmeroceratid nautiloids material in the Ust-kut Formation on the Angara River in Siberia offers the rare opportunity to address both these questions. In the present paper the stratigraphic, taphonomic and palaeoecological context of this new nautiloid occurrence is discussed, the ontogeny and morphology of mature conchs, as well as data on variability of measurable and graphically traceable traits of their morphology, are presented.
Institutional abbreviations.—ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Other abbreviations.—S, S0, M, P, denote conodont elements locations in the apparatus.
Geological setting
The nautiloid specimens being a subject of the present paper come from a loose block (No. 1) of a light pink limestone, about 1.5 m in diameter. It has been found on the left bank of the Angara River about 25 km upstream of Kodinsk and about 3 km upstream of the mouth of Verkhnaya Kezhma Creek, where formerly the village of Pashino was located (Fig. 1A, B, E). At this place the marls underlying the stromatolites of the Ust-kut Formation are of more than 1.5 m in thickness. It is likely that the block comes from the base of the limestone part of the formation. Nautiloid conchs are abundant in the rock matrix of the block. In most part they are chaotically oriented, but locally many conchs are vertical. By contrast, on the presumably upper surface and in the middle of the bed their longitudinal axes tend to be horizontal (Fig. 1C, D). Some conchs are overgrown by stromatolite that may form vertical columns. Cavities between columns and some nautiloid conchs are filled with a red marly sediment, empty, or partially filled with calcite crystals.

Fig. 1. Loose block No. 1 of a stromatolitic limestone with abundant nautiloid conchs of the Ust-kut Formation (latest Furongian or earliest Tremadocian) found on the left bank of the Angara River at the former village Pashino. A. The block partially exploited for fossils. B. Stromatolite columns with empty cavities and a laminar cover above. C. A piece of the rock with exposed nautiloids. D. Polished rock surface (note similarity of the specimen in the middle to the holotype of Ruthenoceras elongatum Korde, 1949). E. Naturally abraded upper surface of the stromatolite columns.
Another block (No. 2) found nearby shows the contact of stromatolites with the overlying bedded grey organodetrital limestone, about 5 cm thick, a nodular red glauconitic limestone of similar thickness and the base of a sandstone unit with pieces of reworked nautiloid phragmocones (Fig. 2D). Below the limestone bed, there is a cupular stromatolite covered with limestone containing numerous iron ooids of up to 2 mm diameter. Such oolite intercalations are common within the stromatolite buildups of variable and frequently highly ordered pinnate morphology exposed about 2 km upstream of the Angara River. In other places the spaces between stromatolite columns are a couple of centimeters wide and are filled with chaotically oriented flat pebbles, frequently with vertical orientation. The depositional environment was apparently turbid. Somewhat more of the sandstone stratum with reworked nautiloids, about 20 cm thick, is seen in yet another loose block (No. 3; Fig. 2D).
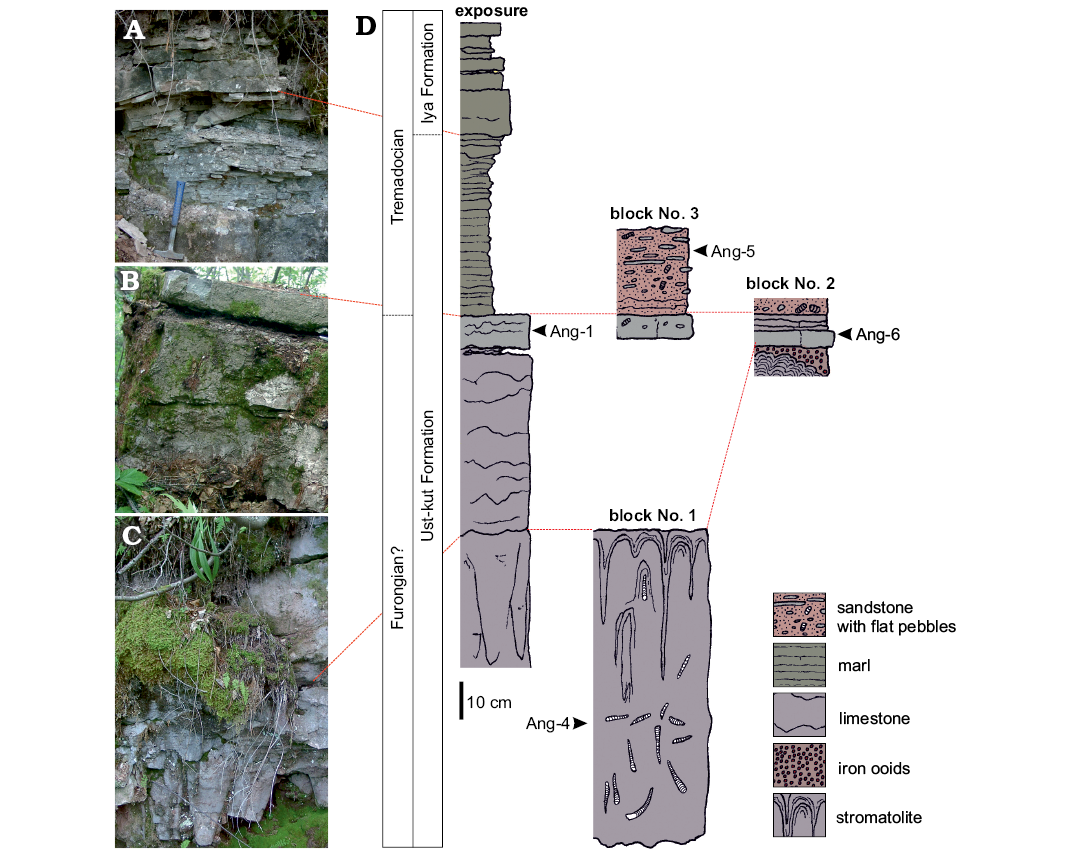
Fig. 2. Exposure of the source strata for the blocks with nautiloids found a few kilometers upstream the Angara River. A. Transition from the Ust-kut to Iya Formation. B. Top of the limestone succession of the Ust-kut Formation. C. Columnar stromatolite and limestone of the Ust-kut Formation. D. Field sketch of the section showing position of conodont samples and probable correspondence between the strata exposed and the loose blocks.
Columnar stromatolites similar to those in block No. 1, with cones of about 10 cm in diameter, are exposed on the left bank of the Angara River at a distance of a few kilometers upstream (Fig. 2C). Although nautiloids have not been found there, this seems to be a continuation of the same stratum. It is covered with a 52 cm thick bed of a red variegated limestone with obolid brachiopods and bellerophontid molluscs followed by 9 cm thick nodular dark grey limestone that marks the top of the Ust-kut Formation (Fig. 2B). The sequence of yellowish weathering dolomitic marls above, with exposed 85 cm of its thickness (Fig. 2A), changes into a coarse-grained, diagonally laminated sandstone of the Iya Formation exposed a few meters above.
The transition between the Ust-kut and Iya formations may differ in exposures on the Angara River. Stromatolites of variable structure may form layers covering irregular surfaces cut in the bedded marl by erosion. They may also develop domes of variable size composed of minute, centimetre-size columns. The columns may form regular structures with a pennate arrangement, as seen in vertical sections. In other places the Iya Formation sandstone contacts directly the massive stromatolite domes of the Ust-kut Formation several meters wide and high (Dronov et al. 2009: fig. 4). Some of them were overturned and embedded in the marl. In 2007, while sampling those sections and collecting fossils, I had access neither to GPS coordinates nor to any topographic map. Therefore locations of particular exposures given above are approximate, thus hampering the reconstruction of their spatial disposition in the field and requiring correction by future research. Unfortunately, these outcrops are today flooded with waters of an artificial lake since the completion of the Boguchany Dam in Kodinsk in 2015.
Taphonomy, material and methods
The chaotic orientation of nautiloid conchs within the pink rock matrix filling spaces between stromatolitic columns and their fragmentation suggests a tempestitic origin of their accumulation. There was less sediment than conchs deposited, as indicated by empty cavities within the rock, partially filled with calcitic cement. Some conchs are overgrown by stromatolite, which shows that the accumulation developed within an episode of the stromatolite growth. There are no fully marine benthic faunal elements like trilobites, rhynchonelliform brachiopods, or echinoderms in these Ust-kut Formation deposits on the Angara River.
146 nautiloid specimens of various completeness are subjects of the study. All were fragmented before burial but some of them are complete enough to enable restoration of the whole conch.
The nautiloid specimens were mechanically removed from the rock matrix of block No. 1 with hammer and pincers. They tend to split along septa rather than the conch wall and require frequent mounting with cyanoacrylate glue. Small pieces of the rock matrix were then removed from the conch surface with a needle under the microscope. Some specimens were sectioned in the medial plane. Subsequently, they were polished or etched and acetate peels were taken. The original aragonite of the shell wall and septa had been recrystallized or removed during diagenesis usually enabling to trace them in sections only as discontinuities in coloration. This makes acetate peels less informative than ground and polished specimens. Fortunately, the original correspondence between septal necks and connecting rings is visible under the scanning electron microscope on pieces of phosphatised siphuncles extracted from the conodont sample Ang-6 taken from block No. 2.
Fossils were scanned wet in 600 or 1200 dpi resolution with an office flatbed scanner to avoid angular deformation of photographs taken with a wide lens camera. The specimens height is small enough to yield satisfactorily focused scans. They were then measured with ImageJ software and the conch contours and septa were drawn with a graphic tablet in Adobe Photoshop. The most complete specimens were coated with ammonium chloride to enhance contrast for photography with a camera.
The conodont samples were dissolved in acetic acid, decanted and dried. The residuum was enriched in the electromagnetic Frantz separator and the phosphatic or secondarily phosphatized fossils were picked with a hair mounted on a handle. These specimens were mounted on SEM stubs and coated with gold.
Age of the fauna
The acetic acid resistant residue of sample Ang-4 taken from block No. 1 yielded 53 elements of Laurentoscandodus triangularis (Furnish, 1938). The Drepanoistodus-like morphology of S0 elements associated with non-geniculate M elements (Fig. 3C, E) make the identification of the species rather safe (see Landing et al. 1996). Seven specimens of S elements that may represent Utahconus(?) eurypterus (Abaimova, 1971) or a less derived species from the same lineage were also found. Their morphology does not show diagnostic aspects of the species. However, several S0 elements with a laterally extending base characteristic of U.(?) eurypterus (Fig. 3L; see Abaimova 1971) are represented in sample Ang-1 from the topmost limestone layer of the Ust-kut Formation that has yielded 161 specimens of U.(?) eurypterus and 106 specimens of L. triangularis. Some S elements in the apparatus of U.(?) eurypterus are of small size and simple morphology, with a lot of morphological transitions. The locations of such elements in the apparatuses are hard to determine. It cannot be excluded that other conodont species (e.g., Semiacontiodus sp.) with unspecific morphology are represented in the sample but this does not seem likely. In any case, the conodont fauna is of low taxonomic diversity. The significant difference in contribution of particular species to the examined samples suggests their somewhat different geological age (together with the change in lithology).
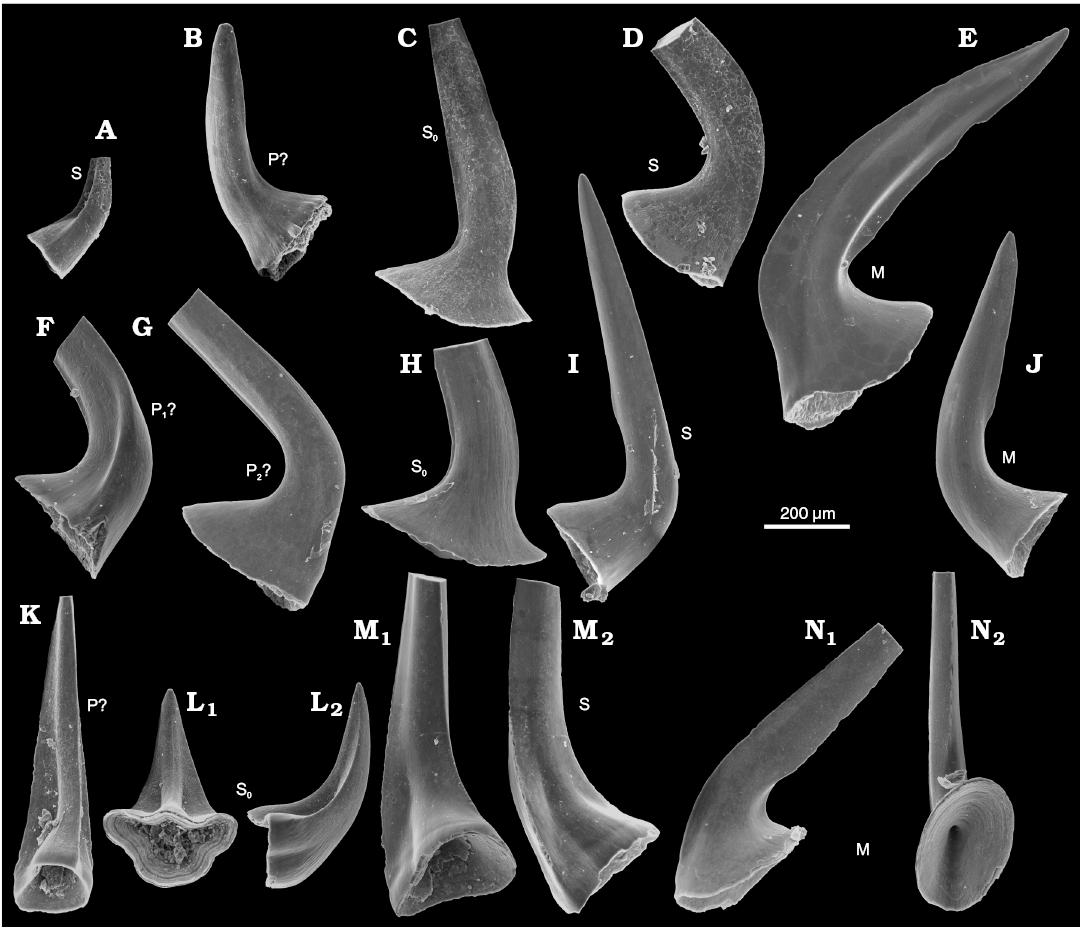
Fig. 3. Conodonts from the probably latest Furongian Ust-kut Formation from the exposure at Pashino on the Angara River, Siberia, Russia, samples Ang-4, block No. 1 (A–E; Fig. 1) and Ang-1, topmost limestone layer (F–N; Fig. 2B). A, K–N. Utahconus(?) eurypterus (Abaimova, 1971), ZPAL N. IV/163, 168, 169, 170, and 172, respectively. B–J. Laurentoscandodus triangularis (Furnish, 1938), ZPAL N. IV/165, 166, 167, 173, 174, 175, 177, and 176, respectively; in posterior views, except for medial view in L1 and occlusals view in M1 and N2. Tentative identification of elements locations indicated S, S0, M, P.
According to Landing et al. (1996) L. triangularis appeared in the latest Cambrian Cordylodus proavus Zone in China and continued into the Ordovician Rossodus manitouensis Zone in Laurentia. Geniculate M elements characteristic for advanced ozarkodinid or prioniodontid conodonts are missing in all investigated samples from the Ust-kut Formation, its age thus probably precedes, the R. manitouensis Zone.
Sample Ang-5 from block No. 3 of a 7 cm thick grey limestone bed and 25 cm of a red sandstone with reworked nautiloids has yielded 206 large specimens of L. triangularis, 14 specimens of Semiacontiodus iowensis (Furnish, 1938), and 8 specimens of Cordylodus angulatus Pander, 1856. This seems to correspond to the Ust-kut Formation topmost limestone bed and the basal marly to sandy Iya Formation beds exposed a few kilometers upstream the Angara River from the place with loose blocks.
Thus, the succession of conodonts in the Cambrian–Ordovician succession on the Angara River upstream of Kodinsk shows that the stromatolitic strata with cephalopods are older than the early Tremadocian C. angulatus Zone but not older than the Furongian C. proavus Zone. In fact, the nautiloid fauna from Tribes Hill Formation in eastern New York of C. angulatus Zone age (Kröger and Landing 2007) is taxonomically more diverse than that from the Ust-kut stromatolites. The exact position of the Cambrian–Ordovician boundary in its stratotype section remains a matter of controversy (Cooper et al. 2001; Terfelt et al. 2012) but this does not matter with respect to the Angara section, where the guide conodont species of the genus Iapetognathus are not present. Likely, the Siberian stromatolitic rock unit is more or less coeval with the nautiloid-bearing strata of similar sedimentary facies in northern China and the North American Midcontinent, i.e., latest Furongian.
Paleoecological context
The abundant nautiloids and relatively rare conodonts are the only pelagic organisms represented in block No. 1. 14 specimens of the probable bellerophontid Sinuitopsis(?) sp. have been extracted from block No. 1 (Fig. 4A, B). Also five specimens of the enigmatic probable monoplacophoran Hypseloconus(?) sp. from the same block are in the collection (Fig. 4D). Additional small specimens of these two molluscan species were present but these fossils are difficult to remove from the rock matrix; their true frequency is thus likely underestimated. Among fossils of benthic animals neither rhynchonelliform brachiopods nor echinoderms co-occur with the molluscs, which suggests abnormal environmental condition preventing invertebrates depending on fully marine, normally-saline conditions to survive.
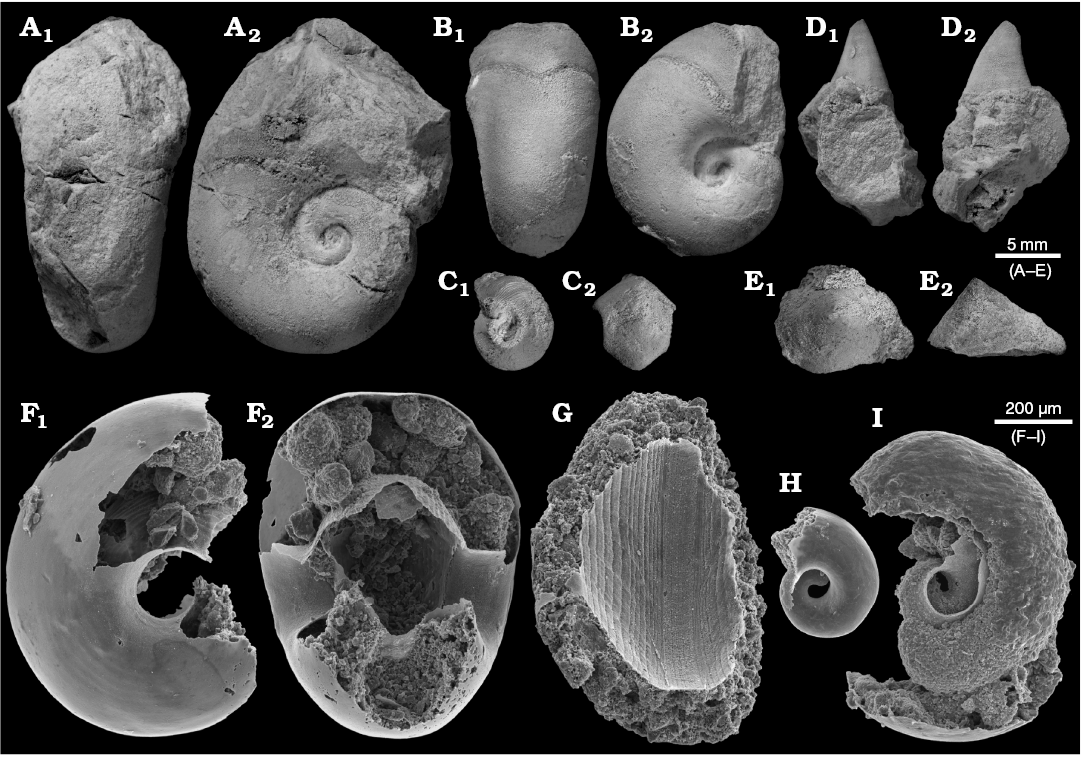
Fig. 4. Benthic bellerophontid and monoplacophoran molluscs from the probably latest Furongian Ust-kut Formation from the exposure at Pashino on the Angara River, Siberia, Russia; samples Ang-4, block No. 1 (A, B, D) and Ang-1, topmost limestone bed (C, E–I). A, B. Sinuitopsis sp. nov., ZPAL N. IV/154 and 155, in external (A1, B1) and lateral (A2, B2) views. C. Bellerophontid gen. et sp. nov. ZPAL N. IV/156, in lateral (C1) and external (C2) views. D. Hypseloconid ZPAL N. IV/157, in anterior (D1) and lateral (D2) views. E. Monoplacophoran? ZPAL N. IV/158, in dorsal (E1) and lateral (E2) views. F–I. Phosphatised conchs of juvenile individuals probably representing the same species as that on C; ZPAL N. IV/162, 161, 160, and 159, respectively, in lateral (F1, H, and I) and apertural (F2, G) views.
A somewhat more diverse molluscan assemblage has been collected from the topmost limestone bed of the Ust-kut Formation with three juvenile bellerophontids probably conspecific with Sinuitopsis(?) sp., two specimens of a low conical monoplacophoran(?) (Fig. 4E), and four specimens of a tightly coiled bellerophontid (Fig. 4C). Conodont sample Ang-1 from the same bed contains numerous phosphatised larval and early juvenile conchs of a bellerophontid presumably representing a single species (or at least a genus; Fig. 4F–I). Most likely these are larval and juvenile conchs of the associated tightly coiled bellerophontids. A compact mass of phosphate within one of the conchs may represent mineralized soft tissue (Fig. 4I). Only one microscopic specimen of a loosely coiled bellerophontid, possibly representing Sinuitopsis(?) sp. has been encountered in the sample (unfortunately destroyed while mounting on the SEM stub). This may mean that the tightly-coiled bellerophontid had planktotrophic larvae and increased mortality during metamorphosis whereas the Sinuitopsis(?) sp. development was protected by the egg covers against the hostile anoxic environment of phosphatization.
The apparent increase in benthic species diversity near the top of the Ust-kut Formation probably reflects a change towards normal marine conditions. This has been completed with deposition of calcareous red sandstone with flat pebbles and reworked nautiloid phragmocones present in block No. 3. Sample Ang-5 from this block yielded numerous phosphatised echinoderm sclerites, fragmentary trilobite carapaces, hexactinellid sponge spicules, and Sphenothallus phosphatic tubes, thus a rather standard Early Ordovician fossil assemblage. Fully marine conditions continued to govern the Siberian basin until the end of Dapingian (Dronov et al. 2009: fig. 2; Kanygin et al. 2010).
Morphology of the Ust-kut Formation nautiloid
The fossil assemblage extracted from block No. 1 shows a diversity of conch shape that at first glance suggests the presence of numerous nautiloid genera, if not families. The most complete, presumably mature, specimens are strongly curved near the base of the living chamber with the phragmocone gently bent or almost straight (Fig. 5A, B) while other specimens of similar size remain straight up to their aperture (Fig. 5C).
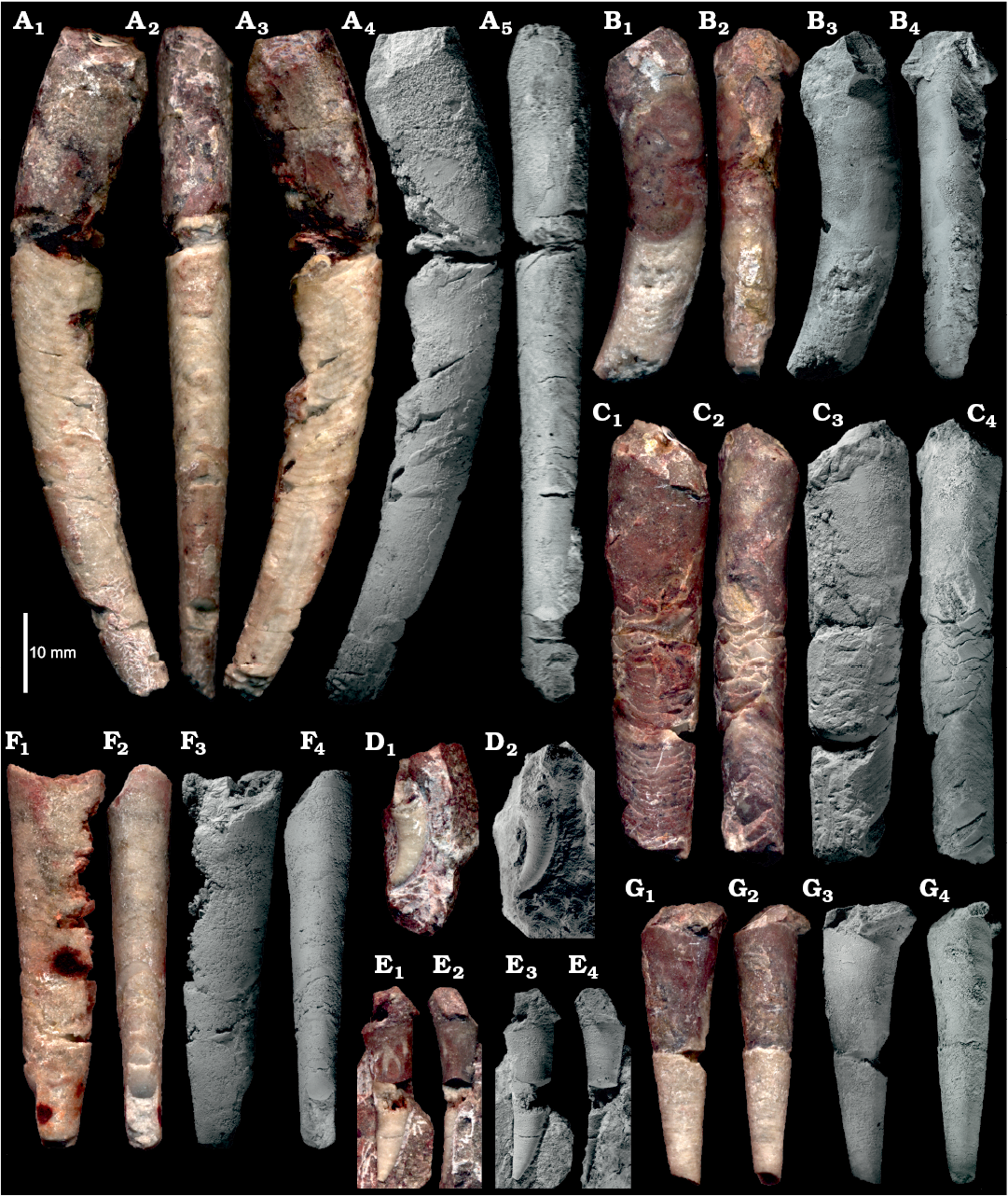
Fig. 5. Representative specimens of ellesmeroceratid nautiloids interpreted below as Ruthenoceras elongatum Korde, 1949, from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. A. The most complete specimen ZPAL N. IV/4. B. Strongly curved specimen ZPAL N. IV/42. C. Straight specimen ZPAL N. IV/91. D. Strongly curved apex of specimen ZPAL N. IV/104. E. Relatively straight apex with a high expansion rate of specimen ZPAL N. IV/101. F. Straight and compressed specimen ZPAL N. IV/68. G. Straight and rounded specimen ZPAL N. IV/100 showing a high expansion rate. In lateral (A1, A3, A4, B1, B3, C1, C3, F1, F3, D, E1, E3, G1, G3) and dorsal (A2, A5, B2, B4, C2, C4, F2, F4, E2, E4, G2, G4) views. Note the ontogenetic increase in inclination of septa. Photographs of wet specimens (A1–A3, B1, B2, C1, C2, F1, F2, D1, E1, E2, G1, G2) and whitened with ammonia chloride (A4, A5, B3, B4, C3, C4, F3, F4, D2, E3, E4, G3, G4).
Juvenile specimens and most of the phragmocone of adults tend to be straight but a striking difference in the conch cross section as well as the conch expansion rate (“apical angle”) is visible (Fig. 5F, G). Even greater differences are exhibited by the conch apices, some strongly curved (Fig. 5D), while others are almost straight (Fig. 5E). Among other distinctions between individuals, the most surprising morphological feature is the inclination of septa and their convexity. Relatively complete specimens show that this aspect of the phragmocone structure changes in ontogeny (Fig. 5A) and specimens of similar size may also strongly differ in this aspect. Consequently, the contact of septum with the phragmocone wall (suture line) is unusually diverse for straight nautiloid conchs (but see Dzik 1984: fig. 37e). Various aspects of morphological conch diversity in the block No. 1 sample, both those easily measurable and those that can be represented in graphics, are reviewed below.
Suture line.—As explained by Seilacher (1975) with his “balloon model”, the contact of septa with the conch wall (suture line) depends mostly on the cross section of the conch. In cephalopod conchs with circular cross sections the suture line is usually straight; compression (or depression) of the conch results in the development of a more or less deep sinus (or lobe and the corresponding saddles). This is well exemplified by the Siberian ellesmeroceratids. Their compressed conchs show a wide sinus on the flanks whereas in conchs of similar size with a more cylindrical shape the suture is usually straight (Figs. 5F, G, 6A–F). This changes to some degree through ontogeny, with juveniles being less compressed than adults. An additional complication is added to this picture by the increasing inclination of the septa during development. Some specimens develop a small lobe within the wide lateral lobe on both sides of the conch in its ventral region. Such lobes occur in cephalopods by local retention of the body during its migration towards the conch aperture as proposed by the “tie point model” of Seilacher (1975). They modify the septal geometry only near its flanks (Fig. 6G, H). Presumably, the retractor muscles attached there. The small saddles develop mostly in the last few septa of adult and subadult phragmocones. The extent of such additional lobes is extremely variable within the sample and it is hard to find two specimens with a similar septal geometry that changes also within the conch. This makes the suture line morphology useless in attempts to separate different taxa within this sample.
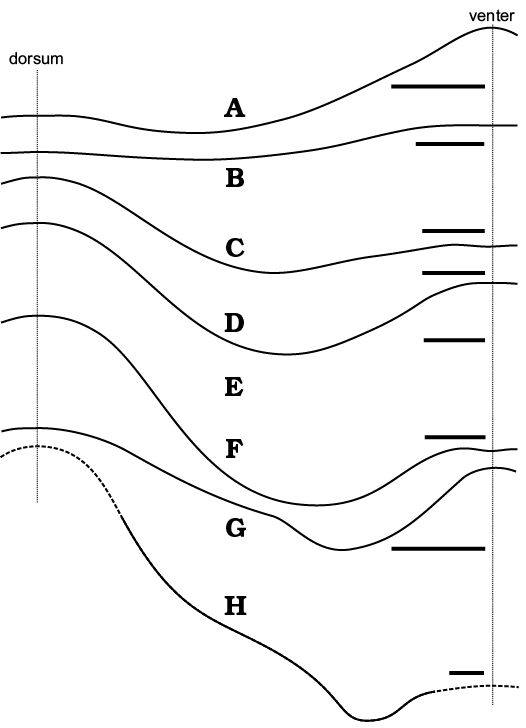
Fig. 6. Suture lines of ellesmeroceratid nautiloids from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. A–H. ZPAL N. IV/109, 111, 10, 27, 56, 51, 48, and 9, respectively. Scale bars 2 mm.
Siphuncle structure.—Particular specimens strongly differ in the disposition of septa and the angle of their contact with the siphuncle (Fig. 7). This is related to the inclination of septa described above and depends mostly on the stage of ontogeny. The diversity of shape of diaphragms within the siphuncle is even more chaotic. They may be regularly convex (Fig. 7D, F), conical (Fig. 7C), or oblique (Fig. 7I). Apparently the tip of the siphon’s soft parts was rather disorderly truncated while being withdrawn from the siphuncle. The almost complete juvenile phragmocone ZPAL N. IV/92 (Fig. 7C) shows that diaphragms may continue to almost half of the phragmocone length.
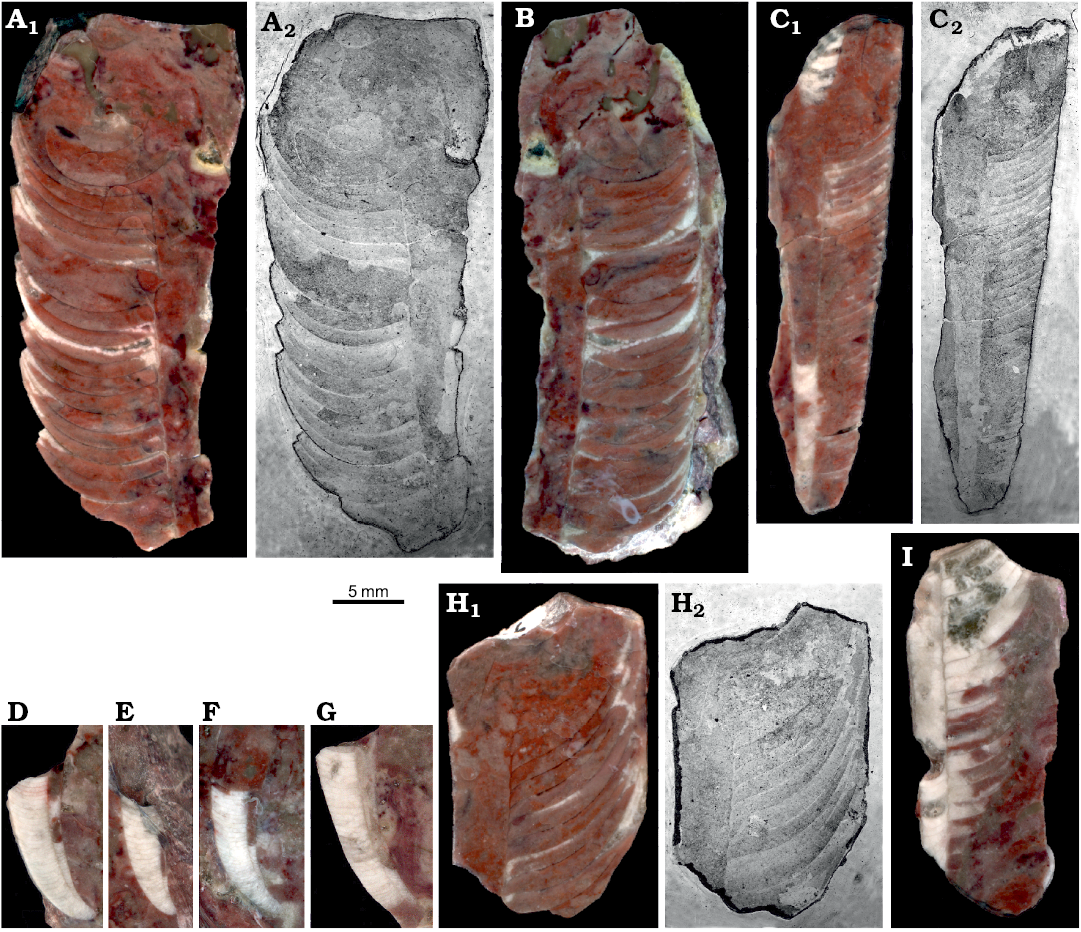
Fig. 7. Medial sections of ellesmeroceratid nautiloids interpreted below as Ruthenoceras elongatum Korde, 1949, from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. A, B. Mature phragmocones with moderately oblique septa, ZPAL N. IV/14 and 15, respectively. C. Almost complete juvenile phragmocone ZPAL N. IV/92 showing extend of diaphragms in the siphuncle. D–G. Apical parts of phragmocones (not strictly medial sections), ZPAL N. IV/115, 118, 121, and 125, respectively. H. Mature phragmocone ZPAL N. IV/16 with extremely oblique septa. I. Straight part of the phragmocone ZPAL N. IV/17 with oblique diaphragms. Wet ground surfaces (A1, B, C1, D–G, H1, I) and acetate peels (A2, C2, H2).
The mode of preservation of nautiloid conchs in block No. 1 is too poor to identify all morphological details of the siphuncle; nevertheless it is visible that the septal necks are short and the connecting rings are relatively thick (Fig. 7B). Fortunately, the conodont sample Ang-6 taken from block No. 2 has yielded highly informative phosphatised connecting rings. Presumably at the time of phosphatization the septa still had their original aragonitic matrix and their morphology was delimited by a thin phosphatic film covering them from both sides. This is the most common preservation mode of calcareous fossils in the ‘small shelly fossils’ assemblages (Dzik 1994). Unlike later cephalopods (Mutvei 2002, but see also Kulicki et al. 2007), but similar to the Late Ordovician Bactroceras (Hewitt and Stait 1985), the septal necks do not continue into the calcified-perforate layer but terminate within a homogenous connecting ring. Its whole volume appears to be homologous to the spherulitic-prismatic layer of advanced nautiloids but it is unclear whether it was originally calcified. The phosphatized organic fibres (or aragonitic spicules according to Hewitt and Stait 1985) are of a rather chaotic and loose disposition, although arranged in bundles in places (Fig. 8B). Expansion of the connecting rings into the camerae in the plectronoceratids suggests that they were also relatively elastic in the closely related ellesmeroceratids. Their calcification, if it occurred at all, happened late in phragmocone development.
The smallest siphuncle piece is about 150 µm in diameter, which implies that the conch diameter was much less than 1 mm at this stage. Also in his description of randomly cut specimens of his Muriceras murus from the base of the Threadgill member of the Tanyard formation in Texas Flower (1964: 89) reported the smallest apical conch diameter measuring 0.4 mm. This suggests the presence of a subspherical embryonic conch although it is generally incorrectly assumed that a large embryonic conch is plesiomorphic for the nautiloids (e.g., Mutvei and Stumbur 1971).
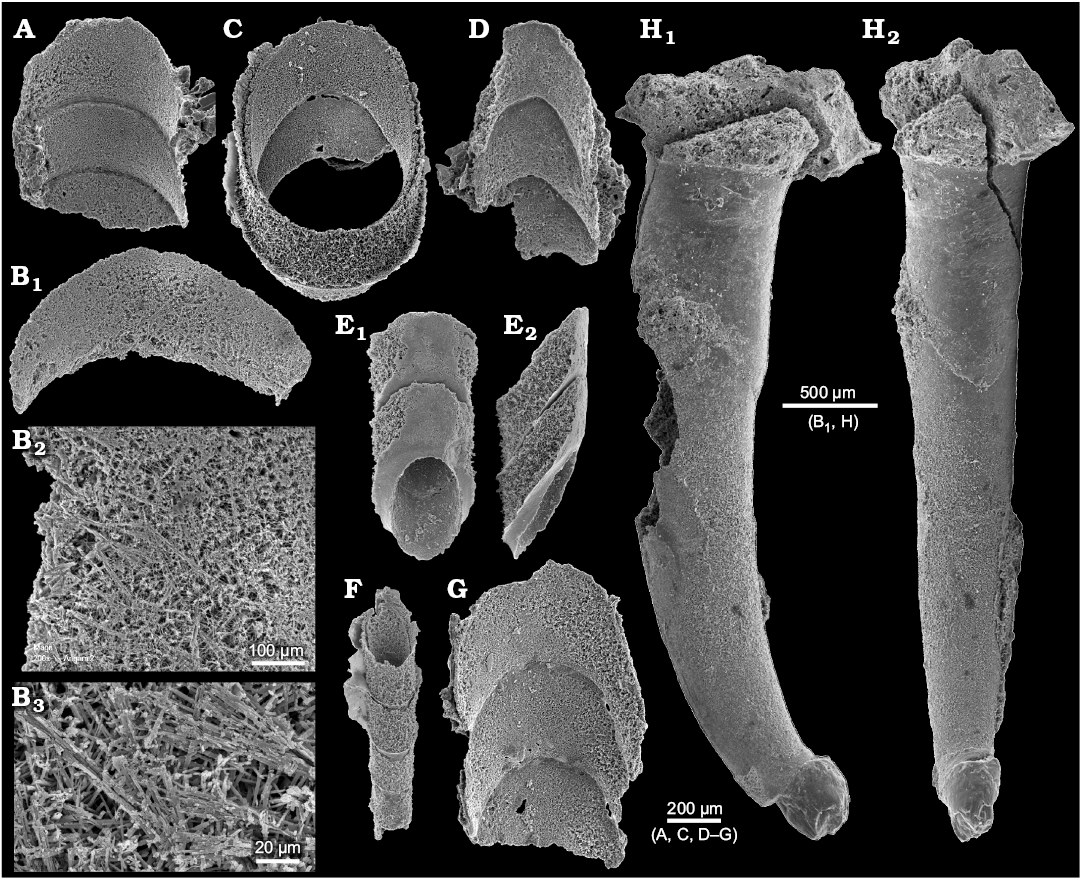
Fig. 8. A–G. Phosphatised siphuncle connecting rings of ellesmeroceratid nautiloids (interpreted below as Ruthenoceras elongatum Korde, 1949) from sample Ang-6, block No. 2, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. ZPAL N. IV/152, 148, 147, 153, 151, 149, 150, respectively. Specimens in dorsal views, also details in higher magnification (B2 and B3; see also Dzik 2010: fig. 7f), except ventral (E1) and lateral (E2) views. ZPAL N. IV/148 and 150 (see also Dzik 2010: fig. 7b and c). H. For comparison the earliest Cambrian (Tommotian) “hyolith” Turcutheca crassaecochlia Syssoiev, 1962, ZPAL MoXX/7 from the Tommotian (Dokidocyathus lenaicus Zone) at Bydyangaia on the Lena River, central Yakutia of possible distant cephalopod affinity, probably mature specimen with displaced embryonic part preserved as a glauconitic internal mold, lateral (H1) and posterior (“ventral”) (H2) views (see also Dzik 2010: fig. 7b and c).
Conch apex.—Several specimens preserve much of the conch apex (Figs. 5, 7, and 9) but no one has its very tip preserved. This is consistent with the material illustrated by other authors (e.g., Flower 1964; Chen and Teichert 1983) and suggests that the ellesmeroceratid embryonic conch was poorly calcified or even purely organic. It underwent destruction at an early stage of the ontogeny or diagenesis and this was probably the reason for the withdrawal of the soft parts from the siphuncle from the phragmocone tip. In ellesmeroceratids the hole was filled by diaphragms, while in the endoceratids they were supplemented with massive calcareous endocones of possibly hydrostatic function (e.g., Collins 1967). It may be worthy to note that in the Early Cambrian laterally compressed “hyolith” Turcutheca conch the embryonic part shows a different mode of preservation suggestive of being poorly calcified (Fig. 8H).
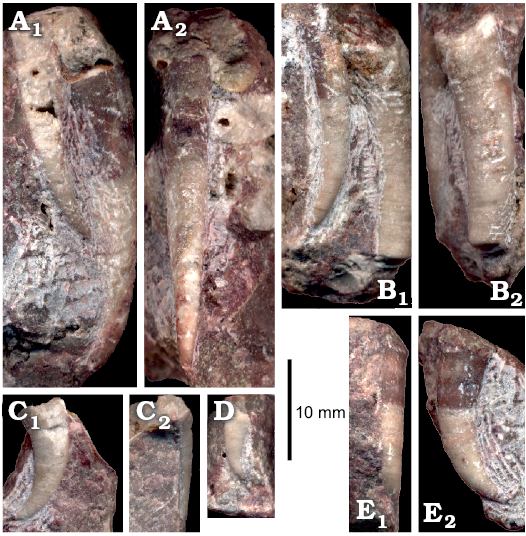
Fig. 9. Apical parts of ellesmeroceratid nautiloid conchs (interpreted below as Ruthenoceras elongatum Korde, 1949) from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. A, B. ZPAL N. IV/11 and 117, respectively; conchs with low expansion rate rate in lateral (A1, B1) and dorsal (A2, B2) views. C–E. ZPAL N. IV/103, 18, and 114, respectively; conchs with high expansion rate rate in lateral (C1, D, E2), ventral (C2), and dorsal (E1) views.
The nautiloid conchs from the Ust-kut Formation do not show ornamentation of their external surface except for some indistinct growth increments on living chambers (e.g., Fig. 3B). It is thus not possible to state whether the change in the conch ornamentation connected around the end of the larval stage is marked there, as in some orthoceratids (Dzik 1981: fig. 1c; also Ristedt 1968; Kiselev 1971). Some indistinct irregularity in the conch geometry at the stage when its strong bending and high expansion rate ended may correspond to the metamorphosis but this is hardly convincing and remains to be proven with a better material. The strong curvature and high expansion rate make juveniles from the Ust-kut Formation similar to the oldest ellesmeroceratid Plectronoceras. Possibly this is a case of “recapitulation of phylogeny in ontogeny”.
The conch geometry change appears to be a characteristic aspect of the morphology of the nautiloids from the Ust-kut Formation. It is rather difficult to quantify such traits based exclusively on fragmentary specimens. Here, I am attempting to overcome this difficulty using contours of particular specimens to superimpose them on each other starting from specimens that are the most complete. Specimens are fit according to the diameter in the middle of their length. The resulting picture (Fig. 10) shows that there is a great diversity of forms in the sample. They differ in curvature of the apical part, its expansion rate, extend of virtually straight later stages in the conch development, as well as the onset of mature growth, when the conch curves again. Despite these prominent differences it is not possible to discern any distinct groups of specimens that would suggest to assign them to different species. There appears to be a continuity in variation of this aspect of the conch morphology.
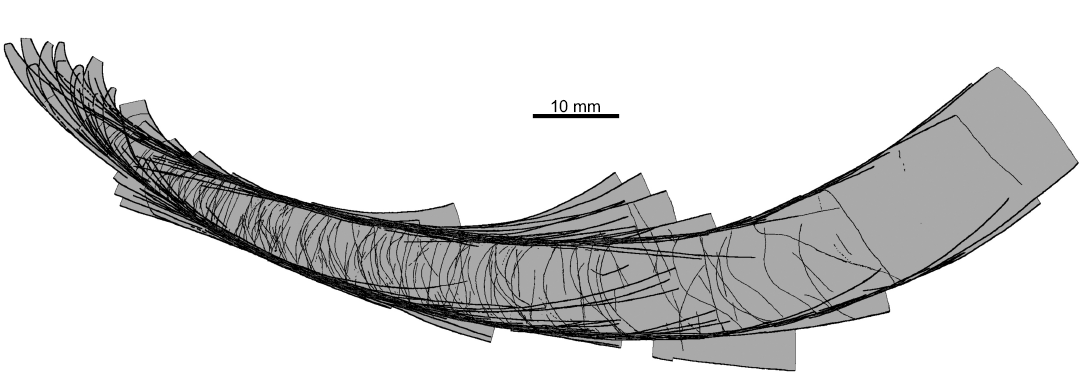
Fig. 10. Contours of all the ellesmeroceratid nautiloid conchs (interpreted below as Ruthenoceras elongatum Korde, 1949) from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia, superimposed on the most complete specimen ZPAL N. IV/4 (Fig. 5A).
The question of how many species are represented in the material requires more rigorous quantitative approach. To answer this question, I measured the aspect of the conch morphology that are commonly used to diagnose nautiloid species, i.e., the aperture width and height, siphuncle diameter, length of the living chamber, apical angle, suture depth and inclination of the septa.
Discussion on species delimitation
Plots of bivariate relationships between particular measured characters show that some aspects of the conch geometry did not change during ontogeny. For example, this applies to the conch compression (cross section of the conch quantified as the ratio between the conch width and height at the aperture; Fig. 11A). The distribution of such ratio values appears to be unimodal and does not allow to distinguish in the sample more than one species on this basis.
Also the ratio between the body chamber length and aperture height (the living chamber elongation) shows a linear relationship in the ontogeny (Fig. 11B). This distribution is unimodal within the sample and does not point at the presence of more than one species as well.
Another character of potential taxonomic value is the relative siphuncle width. Unfortunately, it is visible only in a small number of specimens without destroying the fossils. Anyway, the change of this character through ontogeny is linear and the plot shows unimodal distribution of the ratio in the nautiloids from the Ust-kut Formation (Fig. 11C).
A different pattern is revealed by the relationship of the septum depth to the stage of ontogeny as expressed by the aperture height. As it has appeared, septa remained relatively shallow in juveniles to increase their depth in most of the ontogeny (Fig. 11D). No separate routes of changes of the septal morphology can be identified with confidence in the Pashino sample although increase of variation was tremendous. So great variability suggests a rather low selection pressure on the convexity of septa. The functional value of their geometry was thus not significant in this particular species. Apparently, there was a factor other than the implosion risk that controlled the shape of the septa. Despite a great variability, no separate ontogenies can be identified on the plot.
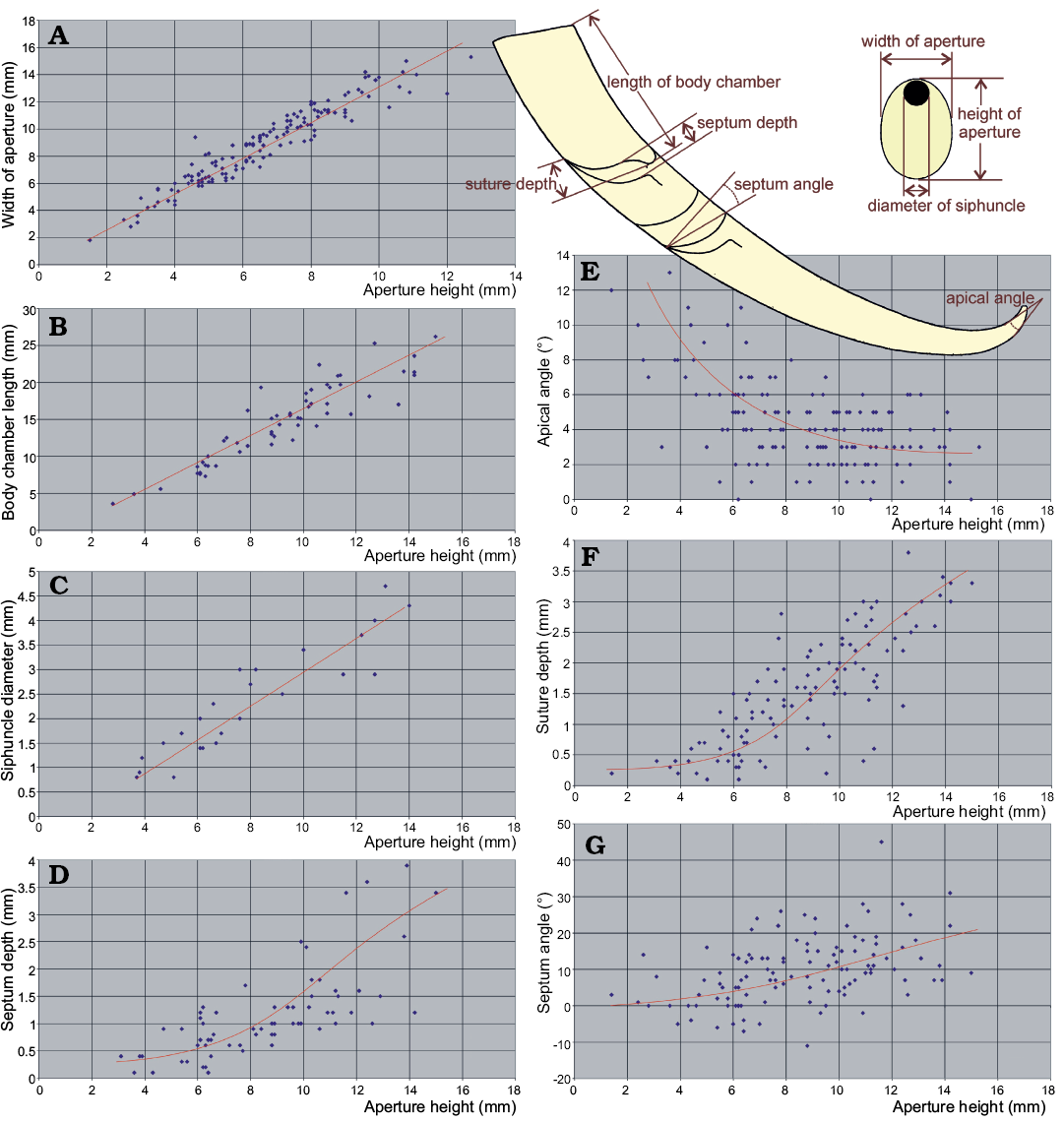
Fig. 11. Ontogenetic change of conch geometry aspects of ellesmeroceratid nautiloids from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. Aperture height is used as a measure of an individual age. A–C. Characters with linear growth pattern. D–G. Characters with non-linear growth pattern. The regression lines are intuitive (drawn by hand and not computed) because only one dimension (aperture height) is measurable in smallest conchs.
The depth of the suture lobe (Fig. 11F) shows a pattern of ontogenetic change similar to that of the septum depth (Fig. 12B), initially increasing very slowly and then changing strongly the slope of regression and increasing variability. This is somewhat less apparent in change of the obliquity of septa (Fig. 11G). It increases slowly but consistently in later stages of ontogeny.
These characters change in ontogeny because of the same cause. The septum depth correlates with the inclination of septa (Fig. 12A) although the relationship is not linear. The septum depth increases faster than its obliquity.
The apical angle similarly depends on the ontogenetic stage. It decreases strongly during early ontogeny (Fig. 11E).
The strong link between these indices and ontogeny prevents the use of the principal component analyses because the pattern of mortality (population dynamics) may seriously distort the frequency distribution of characters. The indices characterizing stages with higher mortality rate would dominate the others. The real pattern of variability would then be biased. Anyway, in the case of the Pashino sample the mortality rate seems to not change drastically through ontogeny, as shown by the size frequency distribution. Presuming a low significance of the resulting bias, I plotted some indices against each other in search of clusters that would substantiate more than one species presence in the sample. This attempt has failed.
The plots of the living chamber elongation vs. the conch compression (Fig. 12C), septum inclination versus the conch compression (Fig. 12D), and septum concavity versus the conch compression (Fig. 12E) all show clearly unimodal distribution of data points.
To conclude: at the moment there is no evidence that more than one nautiloid species is represented in the sample collected from block No. 1 of the Ust-kut Formation stromatolitic limestone. The species is very variable, which is not an unusual feature of Paleozoic nautiloids (e.g., Stridsberg 1985). However, if this sample is taken as a reference standard of species variability range, taxonomy of at least the Late Cambrian and Early Ordovician nautiloids should be critically re-evaluated.
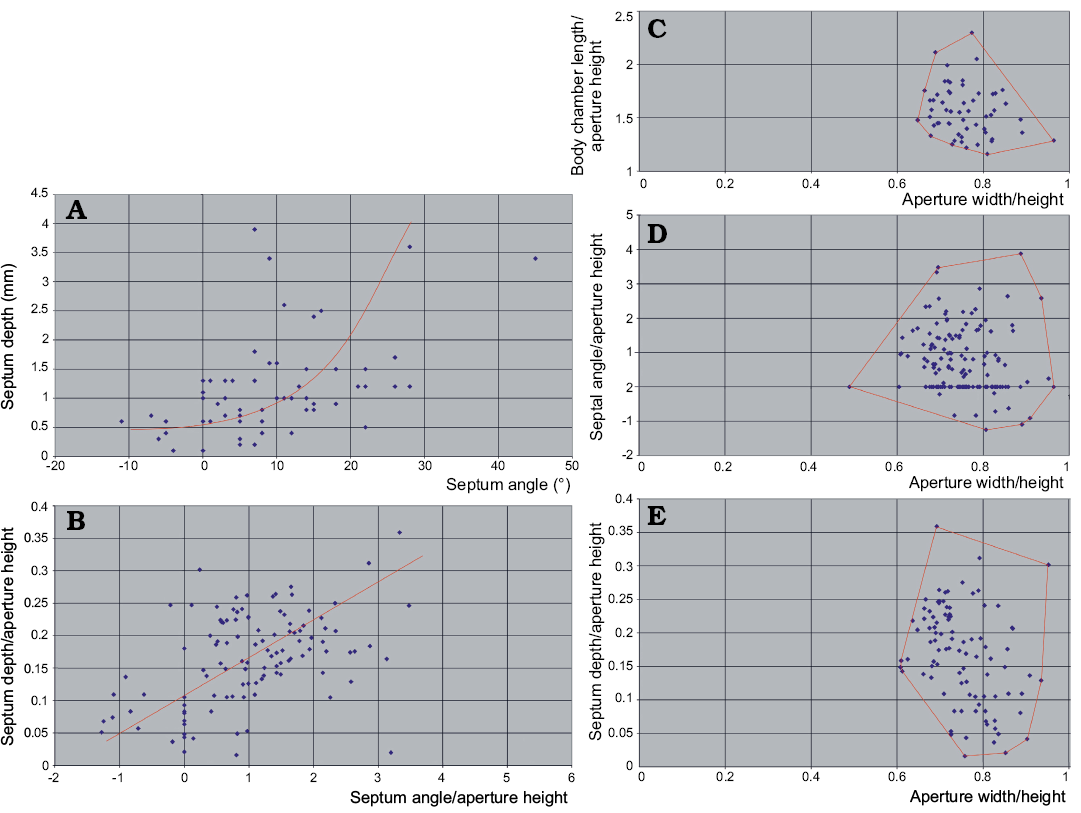
Fig. 12. Relationships between the basic conch geometry aspects of ellesmeroceratid nautiloids from sample Ang-4, block No. 1, probably latest Furongian Ust-kut Formation found at Pashino on the Angara River, Siberia, Russia. A. With an increase of septum depth its obliquity increases even stronger but the correlation is rather loose. B. If non-linear correspondence to ontogeny of these phragmocone aspects is ignored, the pattern of variability appears roughly unimodal. C–E. Also the distribution of indices of the living chamber elongation, septum inclination and depth does not reveal any multimodality. The regression lines in A and B are intuitive (drawn by hand and not computed) because only one dimension (aperture height) is measurable in smallest conchs.
Systematic palaeontology
Order Endoceratida Teichert, 1933
Suborder Ellesmeroceratina Flower in Flower and Kummel, 1950
Remarks.—Teichert (1969) insisted on using the stem cer- instead to cerat- in suprafamilial rank cephalopod taxa, contrary to the old tradition and despite recommendation of the International Code of Zoological Nomenclature. He argued that both endings are allowed by the Greek grammar. In result, different stems are applied by many authors to endings of families (e.g., Ellesmeroceratidae) and orders (e.g., Ellesmerocerida). I did not found his attitude substantiated while preparing a review of the nautiloid phylogeny (Dzik 1984) and I continue to use the traditional endings also in this paper having support in King and Evans (2019).
Chen and Teichert (1983) elevated suborders Plectronoceratina Flower, 1964 and Ellesmeroceratina Flower in Flower and Kummel, 1950 to the ordinal rank based on the presence of expanded connecting rings and breviconic conchs in the former. As commented above, this may have been caused by the fibrous structure of the connecting rings lacking the additional firm calcified-perforate layer characteristic for Cochlioceras and more advanced nautiloids (Mutvei 2002). In this respect the plectronoceratids do not seem to be different from the ellesmeroceratids, as exemplified by the Angara material, even if their siphuncle remained tubular.
Family Ellesmeroceratidae Kobayashi, 1934
Remarks.—Korde (1949) introduced the family name Ruthenoceratidae without any comments. Chen and Teichert (1983) proposed to separate longiconic ellesmeroceratids with supposedly thin connecting rings and diaphragms restricted to apical part of the phragmocone into the family Acaroceratidae. I find both these taxa redundant at the present stage of knowledge.
Genus Ruthenoceras Korde, 1949
Type species: Ruthenoceras elongatum Korde, 1949, Boguchany on the Angara River, Siberia, Ust-Kut Formation, probably latest Cambrian.
Emended diagnosis.—Moderately elongated compressed conch strongly endogastrically bent near the apex and near the base of the living chamber but with gently curved phragmocone showing a change from septa arranged transversely to their strongly oblique orientation.
Remarks.—Korde (1949), while studying thin petrographic sections of the “algal limestone” collected from an exposure located 4 km from the village of Boguchany on the Angara River (that time considered to be late Cambrian age), noticed two oblique sections of nautiloid conchs, each a few millimetres long. She misinterpreted the sediment-filled siphuncle as the living chamber and considered her two new genera and species, R. elongatum and Angaroceras globosum, to represent ascoceratids (“Mixochoanites”). Balashov (1962: 124) pointed out that the “algal limestone” in the area represents the Ust-kut Formation of early Ordovician age. Although the Korde’s (1949) locality is about twenty kilometres away from the place where the here described material was discovered, the strata in the area are virtually horizontal and little doubt remains that her material, as well as the Balashov’s (1962) Clarkoceras angarense come from the same rock unit. This introduces a serious nomenclatorial problem. Korde’s (1949) thin sections do not show diagnostic characters of the species, but there is nothing that could contradict its conspecificity with my material that comes from the same formation in the same region. At first glance the siphuncle appears to be wider than in specimens from the Pashino block No. 1 sample but the Korde’s (1949) specimens were apparently cut obliquely close to the transverse plane near the conch venter, like the centrally located specimens on the polished slab from the Pashino block No. 1 (Fig. 1D). The most parsimonious solution would thus be to choose one of Korde’s (1949) names and interpret the genus and species based on the new material as well as on data of Balashov (1962).
The material from the loose block No. 1 fits well the morphology of the only nautiloid species identified by Balashov (1962) from the Ust-Kut Formation on Angara. A few other nautiloids from the same Formation on Chunya, Lena, and Podkamennaya Tunguska rivers were reported by him, but whether they are strictly coeval with the Angara material remains to be proven by conodont studies. Their conchs are straight, except for Levisoceras from Chunya.
Balashov (1962) attributed his new species from the Ust-kut Formation to Clarkoceras Ruedemann, 1905, but the type species of this genus, C. newtonwinchelli (Clarke, 1897) is breviconic and quite different from the Siberian species. Possibly, the genus Ectenolites Ulrich and Foerste, 1936, with the type species E. subgracilis from the earliest Tremadocian Gasconade Dolostone in Missouri, is a senior synonym of Ruthenoceras. It shows a similar morphology of the phragmocone but the living chamber is much more elongated in some of its species (Kröger and Landing 2007) although it is not preserved completely in the holotype of E. subgracilis (Ulrich et al. 1943: pl. 58: 1, 2). The taxonomic value of this character has to be determined. It may be noteworthy that its fossils come from a stromatolite-rich rock unit (Overstreet et al. 2003). Among other North American type species of ellesmeroceratid genera, that of Albertoceras Ulrich and Foerste, 1936, i.e., A. walcotti, is similar to the Siberian nautiloids except for its narrow siphuncle that is somewhat departed from the conch wall and for a tendency to exogastric curvature (Ulrich et al. 1944: pl. 23: 14). It is known from a minute, probably juvenile specimen from the Mons Formation of Alberta, together with equally small specimens classified in A. gracillimum. Despite its small size, its living chamber is somewhat constricted but there is no sign of maturity in septal crowding. Some similarity to the Siberian species is noticeable in Eremoceras pergracile (Ulrich, Foerste, and Miller, 1943) from the Ellenburger limestone in Texas (but not the type species of Eremoceras Hyatt, 1884, which is breviconic) and E. obliquum (Ulrich, Foerste, and Miller, 1943) from the Oneota dolomite in Wisconsin.
Among the latest Cambrian ellesmeroceratids from China, the type species of Acaroceras Chen, Qi, and Chen, 1979, A. endogastrum Chen, Qi, and Chen, 1979, based on a single specimen from the upper part of the Fengshan Formation in Inner Mongolia, fits probably the general slightly endogastric conch shape and inclined septa of the Siberian species, although it is known only from fragmentary longitudinal sections of the phragmocone (Chen et al. 1979; Chen and Teichert 1983). The Siberian species differs from the Chinese one in a wider siphuncle and more densely distributed septa. Altogether eleven species of this genus and 122 of other genera were named by Chinese authors based on a rather limited material from the Wanwankou Member of the Fengshan Formation.
The only other Chinese Cambrian ellesmeroceratid with similarly inclined septa and a wide siphuncle, Qiushugouceras inclinatum Chen and Teichert, 1983, differs from the Siberian species in an orthoconic conch appearance (but only two longitudinally sectioned, probably juvenile, specimens are available) and in longer, gently curved (loxochoanitic) septal necks (Chen and Teichert 1983).
Stratigraphic and geographic range.—Latest Furongian or earliest Tremadocian near Kodinsk, Siberia.
Ruthenoceras elongatum Korde, 1949
Fig. 13.
Holotype: The only specimen available to Korde (1949) was a phragmocone in a petrographic thin section (Korde 1949: fig. 1); its repository was not specified but it is likely the Paleontological Institute of the Russian Academy of Sciences, Moscow.
Type locality: Angara River shore 4 km from the former village Boguchany near the Kodinsk dam on the Angara River.
Type horizon: Stromatolitic limestone of the Ust-kut Formation, probably latest Furongian.
Material.—146 specimens collected from a loose block of stromatolitic limestone at the shore of Angara River at the former village Pashino near Kodinsk.
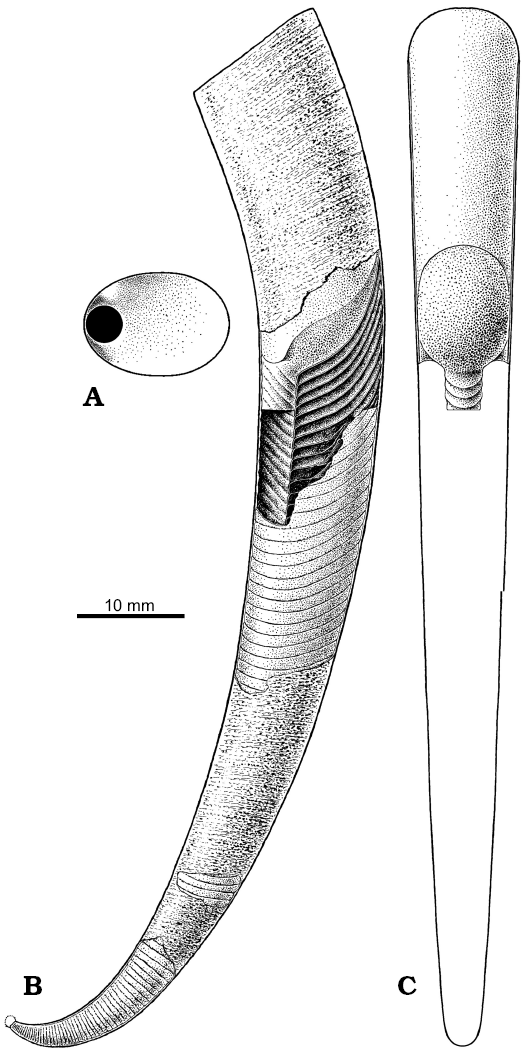
Fig. 13. Restoration of the conch of Ruthenoceras elongatum Korde, 1949 from the Ust-kut Formation of Siberia, with hypothetical subspherical apex based mostly on ZPAL N. IV/4 (Fig. 5A). A. Septum in proximal view. B. Conch in lateral view with the body and proximal part of sipho exposed. C. The body and a portion of sipho in dorsal view.
Diagnosis.—As for the genus.
Remarks.—Balashov (1962) based his Clarkoceras angarense on seven specimens collected by V.P. Maslov in 1952 at his exposure 236 and by K.G. Ginsburg 1955 in 1955 at his exposure 55, probably from the upper Ust-Kut Formation on the left bank of Angara, 1.2 km downstream of Pashennaya Kochegda and from the right bank of Angara downstream of the Bryansk constriction. Some of the three specimens from the Chunya river collected by G.F. Lungershausen in 1949 were also illustrated. The holotype consists of an incomplete living chamber bent near the base and the proximal portion of its gently curved phragmocone showing a change from only slightly to strongly oblique septa. The three paratypes exhibit even more oblique septa. All those specimens are laterally compressed and they are virtually straight. Another paratype shows a conch with a curvature increase near the apex and towards the living chamber base (Balashov 1962: pl. 5: 10). Balashov’s (1962) material fits the modal morphology of the ellesmeroceratid from Pashino and little doubt remains that they are conspecific. No other species shows a similar disposition of septa and shape of the conch.
Concluding remarks
Cephalopods originated from septate conchiferans by developing a chambered buoyancy regulation apparatus (Kröger et al. 2011). Such chambers occur widely in various conical shells as a result of the soft body being withdrown from the narrowest portion of the shell. The crucial apomorphy of the cephalopods is the sipho—a cord of soft tissues penetrating the septa and enabling removal of liquid from chambers and its replacement with gas (Dzik 1981). Unfortunately, no “connecting link” between early conchiferans and the cephalopods that could enlighten the question of their origin was identified yet (Dzik 2010).
A peculiar aspect of the order Endoceratida (including Ellesmeroceratina) is the withdrawal of the sipho from the apical part of the conch. This prevented the exchange of cameral liquid from a significant portion of the phragmocone. The lack of the embryonic conch in all known ellesmeroceratid specimens, may mean that it was destroyed before deposition, probably during life of the animal. The truncation of such organic or poorly mineralized protoconch would have opened the siphuncle apically. This apparently forced the sipho to withdraw and seal the opening with a calcareous diaphragm. The sipho then migrated stepwise with the growth of the phragmocone, leaving a series of diaphragms behind. In subsequent evolution of the Endoceratina the diaphragms were supplemented with massive calcareous siphuncular deposits (endocones; probably their oldest report is in Huaiheceras, Chen et al. 1979: pl. 2: 2). A large centimetre-sized larval (or embryonic) conch developed. No such embryonic structures occurred in the Ellesmeroceratina, as convincingly proven by findings of apical conch parts much less than 1 mm in diameter (e.g., Flower 1964). This means that the embryonic conch of the ellesmeroceratids and plectronoceratids was of size comparable to that of the orthoceratids, bactritids, and ammonoids (Dzik 1981; Kröger 2006). Such protoconch was probably present in Cochlioceras (Dzik 1981) considered to be closely related to orthoceratids by Mutvei (2002) and classified in the Orthoceratida by Kröger et al. (2007), although orthoceratids had thin connecting rings already in the mid Tremadocian (Kröger 2008).
The siphuncle structure of the plectronoceratid nautiloids is surprisingly complex as for their geological age (Landing and Kröger 2009; Fang et al. 2019). Moreover, its particular aspects are contradictory in functional effects. An elongation of septal necks, although strengthening the siphuncle, reduces the contact of soft tissue with the phragmocone chamber. Eventually, in the endoceratids, they completely covered the sipho. Eventually, the cameral liquid exchange stopped that resulted in the lack of cameral deposits in the endoceratids. The bulbous expansion of plectronoceratid connecting rings was interpreted as a measure to increase the surface of soft tissue contact with the cameral liquid (Mutvei et al. 2007). An alternative interpretation would be that the elastic connecting rings were sucked into the chambers by lowered pressure of the gas released from the cameral liquid under action of the sodium pump in the siphonal epithelium. Anyway, the unresolved question remains: which of the siphuncular structures represents the ancestral state, the complex one of plectronoceratids or the generalised one of ellesmeroceratids?
Acknowledgements
I am grateful to Aleksandr Kanygin (Institute of Petroleum Geology and Geophysics, Novosibirsk, Russia) and Andrey Dronov (St. Petersburg State University, Russia) for introducing me into the Irkutsk Amphitheatre Geology. My participation in the expedition to the Angara and upper Lena Rivers in 2007 (led by Taras Gonta, Institute of Petroleum Geology and Geophysics, Novosibirsk, Russia) was possible owing to the exchange program between the Polish Academy of Sciences and the Russian Academy of Sciences. Grażyna Dziewińska (Institute of Paleobiology PAS, Warsaw, Poland) took photographs of whitened specimens. Peer reviews by Björn Kröger (University of Helsinki, Finnland), to whom I thank for constructive criticism, and by Christian Klug (University of Zürich, Switzerland), who greatly improved clarity of the text, are greatly appreciated.
References
Abaimova, G.P. 1971. New Early Ordovician conodonts from the southeastern part of the Siberian Platform [in Russian]. Paleontologičeskij žurnal 1971 (5): 486–492.
Balashov, Z.G. 1962. Nautiloidei ordovika Sibirskoi Platformy. 206 pp. Izdatelstvo Leningradskogo Universiteta, Leningrad.
Chen, J.-Y. and Teichert, C. 1983. Cambrian Cephalopoda of China. Palaeontographica A 181: 1−102.
Chen, J.-Y., Zou, X.-P., Chen, T.-E., and Qi, D.-L. 1979. Late Cambrian Ellesmerocerida (Cephalopoda) of north China. Acta Palaeontologica Sinica 18: 103−119.
Clarke, J.M. 1897. The Lower Silurian Cephalopoda of Minnesota. Minnesota. Geology and Natural History Survey 3: 761–812.
Collins, D.H. 1967. Endocone diaphragms and the “phragmocone of Ecdyceras” (Nautiloidea. Journal of Paleontology 41: 1101−1112.
Cooper, R.A., Nowlan, G.S., and Williams, S.H. 2001. Global Stratotype Section and Point for base of the Ordovician System. Episodes 24: 19–28. Crossref
Dronov, A.V., Kanygin, A.V., Timokhin, A.V., Tolmacheva, T.Y., and Gonta, T.V. 2009. Correlation of eustatic and biotic events in the Ordovician paleobasins of the Siberian and Russian Platforms. Paleontological Journal 43: 1477–1497. Crossref
Dzik, J. 1981. Origin of the Cephalopoda. Acta Palaeontologica Polonica 26: 161–191.
Dzik, J. 1984. Phylogeny of the Nautiloidea. Palaeontologia Polonica 45: 1–255.
Dzik, J. 1994. Evolution of “small shelly fossils” assemblages of the early Paleozoic. Acta Palaeontologica Polonica 39: 247–313.
Dzik, J. 2010. Brachiopod identity of the alleged Late Cambrian monoplacophoran ancestors of cephalopods. Malacologia 52: 97–113. Crossref
Fang, X., Kröger, B., Zhang, Y.-D., Zhang, Y.-B., and Chen, T.-E. 2019. Palaeogeographic distribution and diversity of cephalopods during the Cambrian–Ordovician transition. Palaeoworld 28: 51–57. Crossref
Flower, R.H. 1964. The nautiloid order Ellesmeroceratida (Cephalopoda). New Mexico Bureau of Mines and Mineral Resources, Memoir 12: 1–164.
Flower, R.H. and Kummel, B. 1950. A Classification of the Nautiloidea. Journal of Paleontology 24: 604–616.
Furnish, W.M. 1938. Conodonts from the Prairie du Chien (Lower Ordovician) beds of the upper Mississippi valley. Journal of Paleontology 12: 318–340.
Hewitt, R.A. and Stait, B.A. 1985. Phosphatic connecting rings and ecology of an Ordovician ellesmeroceratid nautiloid. Alcheringa 9: 229−243. Crossref
Hyatt, A. 1884. Genera of fossil cephalopods. Proceedings of the Boston Society of Natural History 22: 253–338.
Kanygin, A., Dronov, A., Timokhin, A., and Gonta, T. 2010. Depositional sequences and palaeoceanographic change in the Ordovician of the Siberian craton. Palaeogeography, Palaeoclimatology, Palaeoecology 296: 285−296. Crossref
King, A.H. and Evans, D.H. 2019. High-level classification of the nautiloid cephalopods: a proposal for the revision of the Treatise Part K. Swiss Journal of Palaeontology 138: 65−85. Crossref
Kiselev, G.N. 1971. Embryonic conchs of the Silurian michelinoceratids [in Russian]. Voprosy Paleontologii 6: 41−50, 107−110.
Kobayashi, T. 1934. The Cambro-Ordovician formations and faunas of South Chosen. Palaeontology. Pt. II, Lower Ordovician faunas. Journal of the Faculty of Sciences of the Imperial University of Tokyo, Section II, Geology, Mineralogy, Geography, Seismology 3: 249–328.
Korde, K.B. 1949. Nautiloids of the Upper Cambrian of Angara [in Russian]. Doklady Akademii Nauk SSSR 69 (5): 671–673
Kröger, B. 2006. Early growth-stages and classification of orthoceridan cephalopods of the Darriwillian (Middle Ordovician) of Baltoscandia. Lethaia 39: 129–139. Crossref
Kröger, B. 2008. A new genus of middle Tremadocian orthoceratoids and the Early Ordovician origin of orthoceratoid cephalopods. Acta Palaeontologica Polonica 53: 745–749. Crossref
Kröger, B. and Landing, E. 2007. The earliest Ordovician cephalopods of eastern Laurentia—ellesmerocerids of the Tribes Hill Formation, eastern New York. Journal of Paleontology 81: 841–857. Crossref
Kröger, B., Beresi, M.S., and Landing, E. 2007. Early orthoceratoid cephalopods from the Argentine Precordillera (Lower−Middle Ordovician). Journal of Paleontology 81: 1266−1283. Crossref
Kröger, B., Servais, T., and Zhang, Y. 2009. The origin and initial rise of pelagic cephalopods in the Ordovician. PLoS ONE 4: e7262. Crossref
Kröger, B., Vinther, J., and Fuchs, D. 2011. Cephalopod origin and evolution: A congruent picture emerging from fossils, development and molecules. Bioessays 33: 602−613. Crossref
Kulicki, C., Tanabe, K., and Landman, N.H. 2007. Primary structure of the connecting ring of ammonoids and its preservation. Acta Palaeontologica Polonica 47: 157–168.
Landing, E. and Kröger, B. 2009. The oldest cephalopods from east Laurentia. Journal of Paleontology 83: 123–127. Crossref
Landing, E., Westrop, S.R., and Knox, L.A. 1996. Conodonts, stratigraphy, and relative sea-level changes of the Tribes Hill Formation (Lower Ordovician, east-central New York). Journal of Paleontology 70: 656–680. Crossref
Mutvei, H. 2002. Connecting ring structure and its significance for classification of the orthoceratid cephalopods. Acta Palaeontologica Polonica 52: 823–827.
Mutvei, H. and Stumbur, H. 1971. Remarks on the genus Pictetoceras (Cephalopoda; Ellesmerocerida). Bulletin of the Geological Institutions of the University of Uppsala, New Series 2 13: 117−122.
Mutvei, H., Zhang, Y.-B., and Dunca, E. 2007. Late Cambrian plectronocerid nautiloids and their role in cephalopod evolution. Palaeontology 50: 1327–1333. Crossref
Overstreet, R.B., Oboh-Ikuenobe, F.E., and Gregg, J. M. 2003. Sequence stratigraphy and depositional facies of Lower Ordovician cyclic carbonate rocks, Southern Missouri, U.S.A. Journal of Sedimentary Research 73: 421–433. Crossref
Ristedt, H. 1968. Zur Revision der Orthoceratidae. Abhandlungen der mathematisch-naturwissenschaftliche Klasse 1968 (4): 212–287.
Ruedemann, R. 1905. The structure of some primitive cephalopods. New York State Museum Bulletin 80 (10): 269–341.
Seilacher, A. 1975. Mechanische Simulation und funktionelle Evolution des Ammoniten-Septums. Paläontologische Zeitschrift 49: 268–286. Crossref
Stridsberg, S. 1985. Silurian oncocerid cephalopods from Gotland. Fossils and Strata 18: 1−65.
Teichert, C. 1933. Der Bau der actinoceroiden Cephalopoden. Palaeontographica A 78: 111–230.
Teichert, K. 1969. Names and authorship of some cephalopod orders. Journal of Paleontology 43: 561−562.
Terfelt, F, Bagnoli, G., and Stouge, S. 2012. Re-evaluation of the conodont Iapetognathus and implications for the base of the Ordovician System GSSP. Lethaia 45: 227–237. Crossref
Ulrich, E.O. and Foerste, A.F. 1936. New genera of Ozarkian and Canadian cephalopods. Denison University Journal of Scientific Laboratories, Bulletin 30: 259–290.
Ulrich, E.O., Foerste, A.F., and Miller, A.K. 1943. Ozarkian and Canadian cephalopods. Part II: Brevicones. Geological Society of America Special Papers 49: 1−240. Crossref
Ulrich, E.O., Foerste, A.F., Miller, A.K., and Unklesbay, A.G. 1944. Ozarkian and Canadian cephalopods. Part III: Longicones and Summary. Geological Society of America Special Papers 58: 1−226. Crossref
Xiao, E.-Z., Latif, K., Riaz, M., Qin, Y., and Wang, H. 2018. Calcified microorganisms bloom in Furongian of the North China Platform: Evidence from microbialitic-bioherm in Qijiayu Section, Hebei. Open Geosciences 10: 250–260. Crossref
Wu, Y.-Y., Zhang, T.-S., Lü, J.-L., and Liu, Y. 2017. The sedimentological characteristics of microbialites of the Cambrian in the vicinity of Beijing, China. Journal of Palaeogeography 6 (2): 117e131. Crossref
Acta Palaeontol. Pol. 65 (1): 149–165, 2020
https://doi.org/10.4202/app.00674.2019