


A new marrellomorph euarthropod from the Early Ordovician of Argentina
MARÍA J. ARIS, JOSE A. CORRONCA, SEBASTIÁN QUINTEROS, and PAOLO L. PARDO
Aris, M.J., Corronca, J.A.,
Quinteros, S., and Pardo, P.L. 2017. A new marrellomorph euarthropod
from the Early Ordovician of Argentina. Acta
Palaeontologica Polonica 62 (1): 1–8.
Marrellomorphs (class Marrellomorpha) are a group of Paleozoic arthropods with a very poor fossil record. Here we describe a new marrellomorph arthropod Mimetaster florestaensis sp. nov. from the Tremadocian (earliest Ordovician) of Argentina. The new species is characterized by the shape and direction of the three pairs of principal spines, and the existence of strong secondary spines only in the proximal two-thirds of the anterolateral spines. As a result of phylogenetic analysis the new species integrates a trichotomy with Mimetaster hexagonalis and a Moroccan unnamed marrellid as sister groups. This discovery increases the known diversity of Marrellomorpha and represents the first occurrence of this group in South America, expanding the spatial distribution of the clade.
Key words: Marrellida, Mimetasteridae, Tremadocian, Floresta Formation, Argentina, Salta.
María J. Aris [josefinaaris03@gmail.com], Cátedra de
Paleontología, Consejo de Investigación de la Universidad Nacional de
Salta (CIUNSa) and Instituto para el Estudio de la Biodiversidad de
Invertebrados (IEBI), Facultad de Ciencias Naturales de la Universidad
Nacional de Salta, Av. Bolivia 5150, CP 4400 Salta, Argentina.
Jose A. Corronca [jcoronca@gmail.com]
and Paolo L. Pardo [pardobio@gmail.com], Instituto para el Estudio de
la Biodiversidad de Invertebrados (IEBI) and Consejo Nacional de
Investigaciones Científicas y Técnicas (CONICET), Facultad de Ciencias
Naturales de la Universidad Nacional de Salta, Av. Bolivia 5150, CP
4400 Salta, Argentina.
Sebastián Quinteros [squint@unsa.edu.ar],
Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)
and Instituto de Bio y Geociencias del NOA (IBIGEO), Facultad de
Ciencias Naturales de la Universidad Nacional de Salta, Av. Bolivia
5150, CP 4400 Salta, Argentina.
Received
15 January 2016, accepted 17 September 2016, available online 4 November
2016.
Copyright © 2017 M.J. Aris
et al. This is an open-access article distributed under the terms of the
Creative Commons Attribution License (for details please see
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted
use, distribution, and reproduction in any medium, provided the original
author and source are credited.
Introduction
Since the discovery of Marrella splendens Walcott, 1912, the class Marrellomorpha has been the subject of many studies that have expanded the knowledge of the diversity of the group and, as a consequence of that, its spatial and temporal distribution. These studies have principally centred around the description of new taxa (Fritsch 1908; Gürich 1931; Lehman 1955; Van Roy 2006; Siveter et al. 2007; Van Roy et al. 2010, 2015; Haug et al. 2013; Legg 2016b), the reinterpretation of some genera and species (Whittington 1971; García Bellido and Collins 2006; Van Roy 2006), or phylogenetic relationships (Kühl et al. 2008; Kühl and Rust 2010; Rak et al. 2013). According to phylogenetic analysis, the marrellomorphs comprise two distinct groups: (i) Acercostraca, which includes the species Primicaris larvaformis Zhang, Han, Zhan, Liu, and Shu, 2003, Skania fragilis Legg, 2015 and S. sundbergi Lin, Gon, Gehling, Babcock, Zhao, Zhang, Hu, Yuan, Yu, and Peng, 2006, and the family Vachonisiidae (including Vachonisia rogeri Lehmann, 1955, Xylokorys chledophilia Siveter, Fortey, Sutton, Briggs, and Siveter 2007, and Enosiaspis hrungnir Legg, 2016a), and (ii) Marrellida (including Marrella splendens Walcott, 1912, Mimetaster hexagonalis Gürich, 1931, Furca bohemica Fritsch, 1908, and a Moroccan unnamed marrellid (Legg et al. 2013; Legg 2015, 2016b). The latter three species of marrellids belong to Mimetasteridae, a monophyletic family distributed during the Ordovician across Gondwana and Perunica, which expanded its palaeogeographic range to other regions during the Devonian.
Specimens recognized as Furca bohemica Fritsch, 1908 were poorly understood until the study by Perner (1919) who identified them as marrellomorph arthropods. Chlupáč (1999) reviewed this material and distinguished two morphotypes: the first one corresponds to the originally described species and the second, displaying long secondary spines, was described as a new species Furca pilosa Chlupáč, 1999. Rak et al. (2013) synonymized all known species of Furca from Bohemia arguing that the presence or absence of elongated secondary spines is a consequence of differential taphonomic processes at the time of fossilization.
Van Roy et al. (2010) reported an Ordovician marrellid from the lower part of Fezouata Shales (sensu Martin et. al 2015 and Gutierrez-Marco and Martin 2016). The authors assigned the specimen with doubts to the genus Furca, but did not identify the specimens down to the species level. However, based on the photographs supplied by the latter authors, it is very likely that these specimens represent a new species.
Mimetaster hexagonalis Gürich, 1931 has been discussed in several publications, beginning with the original description by Gürich (1931) later amended by Birenheide (1971) and Stürmer and Bergström (1976) based on several new specimens. The most recent reinterpretation of the species was published by Kühl and Rust (2010), who provided new evidence and discussed mutual and commensal relations with other fossil groups.
Here we describe a marrellomorph specimen found in the lowest level of the Floresta Formation in Salta, Argentina. Both the morphology and cladistic analysis of family relationships with other members of Mimetasteridae suggest that this specimen belongs to a new species of Mimetaster. This discovery provides new insights into paleogeography, species richness, and phylogenetic relationships within Marellomorpha.
Institutional abbreviations.—CNS-I, “Ciencias Naturales” Collection, Salta-Invertebrates, Universidad Nacional de Salta, Argentina.
Other abbreviations.—CI, Consistency Index (a measure of the relative amount of homoplasy of a tree); K, concavity (constant value which determines how strongly homoplasious characters are down-weighted); RI, Retention Index (a measure of the relative amount of homoplasy of a tree).
Geological setting
The specimen was collected from the Floresta Formation (Santa Victoria Group), which emerges from the Western flank of the mid-section of the Mojotoro Mountains (Eastern mountain range), in Salta Province, Argentina. (Fig. 1A).
The core of the mountain has an Ediacarian and lower Cambrian age and is formed by the Puncoviscana Complex (Aparicio González et al. 2011). These Precambrian units are covered by upper Cambrian sediments of the Meson Group (Turner 1960). Upper Cambrian–Ordovician sediments of the Santa Victoria Group (Turner 1960) unconformably overlie the Mesón Group. The Salta Group (Turner 1959), comprising Cretaceous to Eocene sediments, overlies the Santa Victoria Group only in the southern section of the mountain. The youngest deposits (Neogene) correspond to the Orán Group and overlie the basement or the Paleozoic sediments on the Eastern flank of the mountain range (Moya 1988, 1998).
The Santa Victoria Group in Mojotoro Mountains comprises eight lithostratigraphic units that, from the base to the top, are: La Pedrera, San José, Caldera, Floresta, Áspero, San Bernardo, Mojotoro, and Santa Gertrudis formations (Harrington 1957; Moya 1988, 1998). Deposition of the Floresta Formation was initiated during the earliest Tremadocian and ended in the late Tremadocian (Moya et. al. 1994; Aris and Malanca 2005). This lithostratigraphic unit is the most fossiliferous unit of the Santa Victoria Group (Aris 2005).
The section investigated in this work (Fig. 1B) consists of silty and muddy lutite, olive green, yellow-brown and grey-green siltstone, followed by sandy layers (Malanca 1996; Aris 2005; Aris and Malanca 2005). The fauna includes two characteristic elements of “Burgess Shale” type assemblages (Aris and Palomo 2014), including the marrellid, associated with lingulid and orthid brachiopods, amphigastropods, and trilobites. Considering the classical biozone scheme of Harrington (1957), many trilobites of this association belong to Parabolina (Neoparabolina) frequens argentina Biozone (late Cambrian–initial early Tremadocian), while others are representative of the Kainella meridionalis Biozone (early Tremadocian). For this reason, Aris (2005) and Aris and Malanca (2005) proposed the association as an intermediate fossil fauna between both biozones, considering the levels that contain them as the oldest in the Floresta Formation.
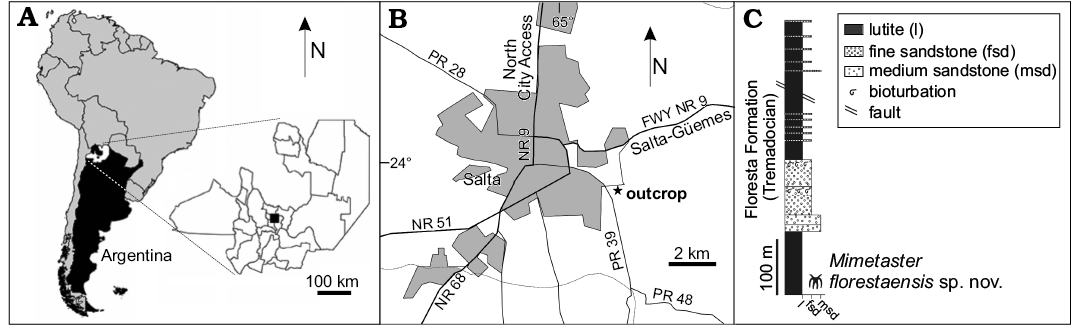
Fig. 1. Geographic map (A) and location of the studied area (B) and stratigraphic provenance of the material (C).
Material and methods
The specimen was recovered with its counterpart and is housed in the department of Paleontology in the School of Natural Sciences at the National University of Salta under the record numbers CNS-I 133/1-1 and CNS-I 133/1-1’. The specimen was examined under a binocular microscope Mikoba Series 745 with attached Cannon Power-Shot G10 digital camera. The specimen was described from the part and its counterpart, employing the terminology used by Rak et al. (2013) and Kühl and Rust (2010).
Phylogenetic analysis.—We include all characters proposed by Rak et al. (2013) and Legg (2016a) that are informative for our analysis. Since in those papers some characters are the same, we followed the information given by Legg (2016a), because this paper includes more taxa. Based on this publication, we have selected all taxa included in Marrellomorpha and, as out-group taxa, those which were the closest to Marrellomorpha in the phylogeny presented by Legg (2016a).
A total of sixteen taxa (including Agnostus, Oelandocaris, Henningmoenicaris, Sandtorpia, and Goticaris as outgroups), and fifty-three characters were analyzed (see Appendix 1). The matrix generated (see Appendix 2) was analyzed with TNT v. 1.1 (Goloboff et al. 2003), using parsimony as optimality criterion. Phylogenetic trees were searched under implicit enumeration (Branch and Bound) with equal and implied weight (K = 1, 3, 5). Support for equal weights was measured using Bootstrap. For trees under implied weighting, support was measured by Symmetric Re-sampling. Bootstrap and Symmetric Re-sampling employed 1000 addition sequences and 33% deletion probability
Characters and coding.—Characters and character states outlined by Rak et al. (2013) and Legg (2016a) were used; additional characters related to the form of the cephalic spines and the position and number of secondary spines were added (see Appendix 1).
Systematic palaeontology
Phylum Arthropoda Von Siebold, 1848
Class Marrellomorpha (Beurlen, 1930)
Order Marrellida (Raymond, 1920)
Family Mimetasteridae Birenheide, 1971
Genus Mimetaster Gürich, 1931
Type species: Mimetaster hexagonalis Gürich, 1931; Lower Devonian, Hunsrück Slate, Germany.
Mimetaster florestaensis sp. nov.
Fig. 2.
Etymology: In reference to the locality from where the specimen was collected.
Holotype: CNS-I 133/1-1, cephalic shield (mold).
Type locality: Mojotoro Mountains (24°48’27”S, 65°22’03”W), Villa Floresta, Salta. Argentina.
Type horizon: Santa Victoria Group, Floresta Formation, lower Tremadocian (Lower Ordovician).
Material.—Cephalic shield (CNS-I 133/1-1, part; CNS-I 133/1-1’, counterpart).
Diagnosis.—Cephalic field slightly inflated; short, anterolateral spines directed forward and outward (Fig. 2A–C). Anterolateral spines with small and strong secondary spines in the proximal inner and outer borders (Fig. 2B–D); distal portion smooth.
Description.—Cephalic shield has a central field with three pairs of primary spines (Fig. 2A1, C); each with a different length and orientation. Central cephalic field subtrapezoidal, slightly convex. In the central cephalic field the imprint of the posterior ventral margin is present (Fig. 2B, C). In addition, a delicate line is well marked in the posterior section of the field and seems to complete a circular route approaching the anterior edge. This structure could be interpreted as a suture line (Fig. 2B, C), but since only a single specimen is available, this cannot be confirmed unequivocally. The anterior margin curves forward slightly, elongating into a pair of short anterolateral spines (the shortest of the three pairs), which are directed forward and outward. The bases of secondary spines are present in the proximal section of the anterolateral spines on both the inner and outer margins. These secondary spines disappear distally, providing a smooth look to the remainder of the anterolateral spines. In the counterpart three secondary spines that are diminutive and well-defined (ca. 1 mm) are preserved (Fig. 2D).
Behind these spines, the cephalic field extends into a pair of mediolateral spines (the longest of the three pairs) that arch inward and backward. These are three times larger than the anterolateral spines, and extend considerably beyond the posterior margin of the cephalic field (Fig. 2A1, C). The widest point of the cephalic shield is measured between the most curved sections of the mediolateral spines. The base of these spines is relatively narrow (Fig. 2A, C), and extends from the base of the anterolateral spines to nearly the center of the central field of the cephalic shield.
A third pair of spines (the posterior pair) extends from the posterior border of the central field of the cephalic shield (Fig. 2A1, C), following a path similar to that of the mediolateral spines, though the length of these spines is uncertain because they are truncated. The internal borders are in contact medially, forming the posterior margin of the central part of the shield, which has a U-shape. The bases of the mediolateral and posterolateral spines do not touch, and both have triangular base of insertion of secondary spines. Some complete secondary spines are observed on the inner edge of the middle and posterolateral spines (Fig. 2A2, B, C). The secondary spines in the posterolateral pair are less numerous and have a wider base than those of the mediolateral secondary spines.
Remarks.—The presence of the secondary spines on the mediolateral and posterolateral spines is a shared character in Furca bohemica, Mimetaster hexagonalis, the Moroccan unnamed marrellid and Mimetaster florestaensis. sp. nov., but its position and number differs in the four species. The secondary spines on the anterolateral pair in the new species differs significantly not only in shape and length, but also in their unique position, occurring only in the proximal part of the spines. On the other hand, based on the phylogenetic analysis, Mimetaster, the Moroccan unnamed marrellid and the new taxon described here are more closely related to each other than to Furca bohemica, supporting the suggestion that this new taxon represents a new species of the genus Mimetaster.
Stratigraphic and geographic range.—Type locality and horizon only.
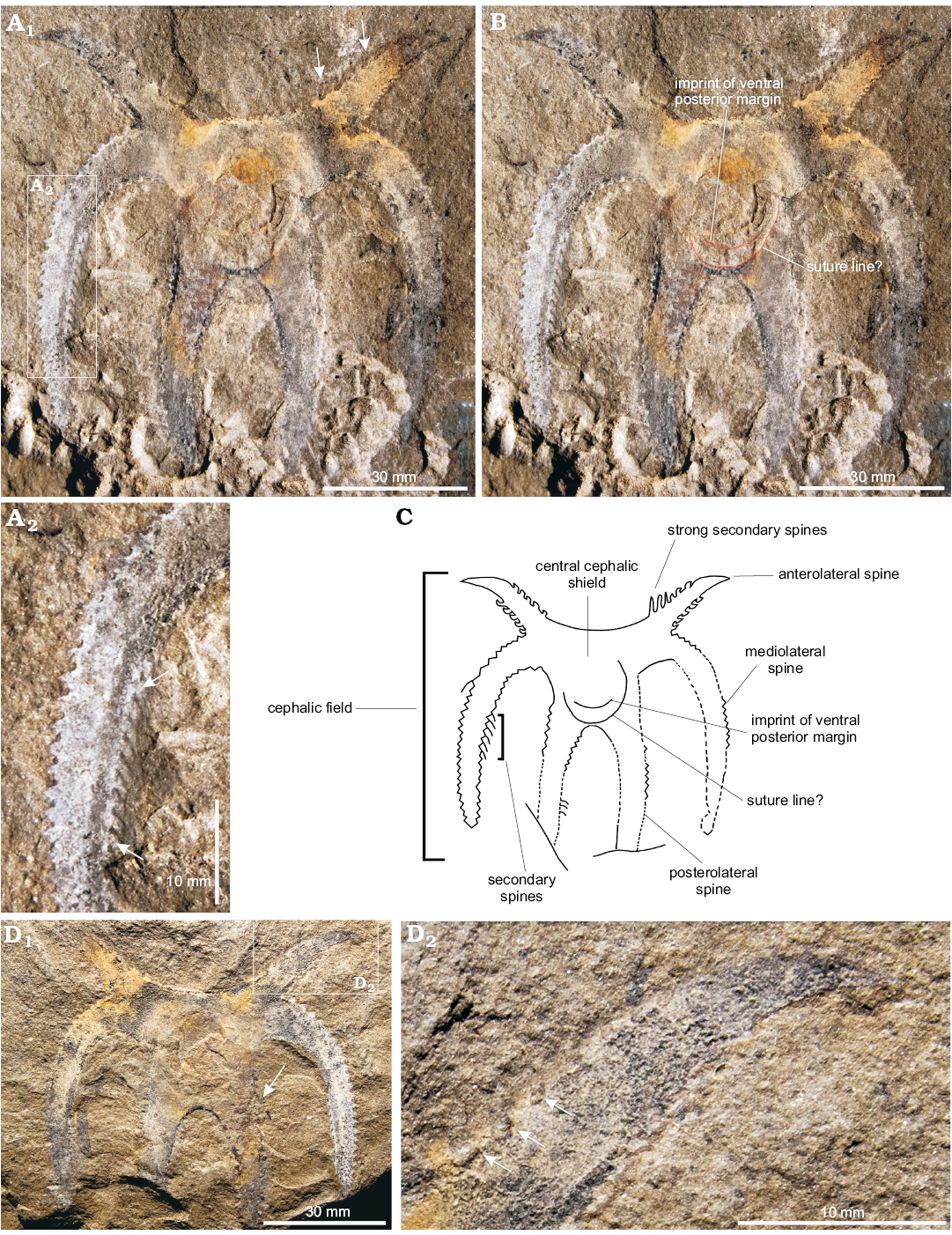
Fig. 2. Marellomorph arthropod Mimetaster florestaensis sp. nov. from Tremadocian of Mojotoro Mountains, Salta, Argentina. A–C. CNS-I 133/1-1, part. A. Cephalic shield and spines. Detail of the secondary spines on mediolateral spine (A2). B. View of the imprint of the ventral posterior margin of the cephalic shield. C. Explanatory drawing revealing the most important morphological characters. D. CNS-I 133/1-1´, counterpart showing detail of strong secondary spines on anterolateral spine. Arrows indicate the secondary spines.
Phylogenetic analysis
Rak et al. (2013) conducted a phylogenetic analysis of seven marrellomorph arthropod taxa, coding 16 trunk and cephalic characters. This analysis established the monophyly of the family Mimetasteridae, comprising Furca and Mimetaster, characterized by the presence of anterolateral spines and an inflated cephalic field (our characters 19 and 7, respectively) as synapomorphies. More recently, Legg (2015, 2016a) performed a phylogenetic analysis including 315 taxa and 752 characters. In those studies, this author recovers a terminal taxon (Moroccan unnamed marrellid) as sister taxon of Mimetaster hexagonalis, and this clade sister of Furca bohemica. We recover one most parsimonious tree (Fig. 3) with a length of 63 steps in the equally weighted analysis, and under implied weighting (Fit: K1, 4.833; K3, 2.550; K5, 1.500; CI, 0.825; RI, 0.857). Our cladogram recovers a similar topology to that of Rak et al. (2013), and Legg (2015, 2016a), but with the inclusion of the new taxon, Mimetaster florestaensis sp. nov., in a trichotomy with Mimetaster hexagonalis and the unnamed Moroccan marrellid of Legg (2016a). This clade is supported by “the presence of elongate anterior cephalic spines” (character 44[1]). Also, we recovered the monophyly of Mimetasteridae formed by the trichotomy mentioned above plus Furca bohemica. This family is supported by the same synapomorphies proposed by Rak et al. (2013) plus a new one found here: “presence of secondary spines on cephalic shield”. All other relationships inside Marrellomorpha found here are congruent with the topology recovered by Legg (2015, 2016a). Mimestaster florestaensis sp. nov. has as autapomorphies: “the presence of short and robust secondary spines” (character 5[1]), and by “the secondary spines in the anterolateral pair restricted to the proximal two-thirds of these spines” (character 6[1]). Based on this fact, we assigned the new taxon to a new species of the genus Mimetaster. We are aware that Mimetaster florestaensis is phenetically more close to Furca bohemica than with Mimetaster hexagonalis and the Moroccan unnamed marrellid. On the other hand, the knowledge of these taxa is poor; so its phylogenetic position could change if the knowledge of Mimetaster florestaensis and Furca bohemica is improved as a result of future discoveries.
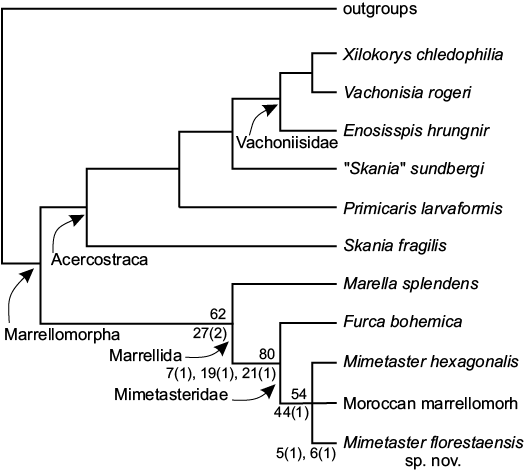
Fig. 3. Cladogram showing phylogenetic relationships of Marrellomorpha, incorporating the new taxon here described, CI = 0.825; RI = 0.857, 63 steps with characters of equal weight. Length of tree with implicit weighting equal to 1.500 (K = 5), 2.550 (K = 3) and 4.833 (K = 1). In the Marrellida clade, the numbers on the right represent apomorphies, with the character state between parentheses.
Conclusions
The finding of a new marrellid species in Floresta (Mojotoro Mountains, Salta) has different implications. This is the first occurrence of the Marrellomorpha in Argentina and South America, expanding the world-wide record of the group. This discovery increases the diversity of the class Marrellomorpha and family Mimetasteridae. In addition, the occurrence of the family Mimetasteridae is extended to the early Tremadocian. Finally, it corroborates the monophyly of the family established by Rak et al. (2013).
The data obtained so far for the class Marrellomorpha, despite its poor fossil record, suggest wide paleogeographic distribution of the group in early and middle Paleozoic times. Therefore, it is expected that in the future additional new species will be discovered, which should help in addressing currently unresolved questions regarding marrellomorph paleobiogeography, systematic position and paleoecology.
Acknowledgements
The authors would like to thank Franco Tortello (Universidad Nacional de La Plata, Argentina) for his critical review of the early draft of this manuscript and valuable suggestions. This work was funded through the Research Project (CIUNSa-2193/4) of the Board of Investigation of the Universidad Nacional de Salta. JAC, SQ, and PLP thank to CONICET for their support. In addition, the authors are very grateful with Štěpán Rak (Charles University, Prague, Czech Republic) for valuable comments and, especially, to Peter Van Roy (Yale University, New Haven, USA) who has contributed substantially to the improvement of this work.
References
Aris, M.J. 2005. Fauna trilobítica de la Formación Floresta (Tremadociano) en su localidad tipo y áreas vecinas (provincia, de Salta, República Argentina). 77 pp. Unpublished thesis, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Salta.
Aris, M.J. and Malanca, S. 2005. Asociaciones trilobíticas de la Formación Floresta (Tremadociano) en el sector medio de la sierra de Mojotoro, Salta, Argentina. In: E.J. Llambías, R.E. de Barrio, P. González, and P. Leal (eds.), XVI Congreso Geológico Argentino III. La Plata, Buenos Aires, Octubre 2005, 325–332. Asociación Geológica Argentina, Buenos Aires.
Aris, M.J. and Palomo, M. 2014. Primer registro de una fauna ordovícica tipo “Burgess Shale” en Argentina y Sudamérica. In: R.D. Martino, R. Lira, A. Guereschi, E. Baldo, J. Franzese, D. Krohling, M. Manassero, G. Ortega, and L. Pinotti (eds.), XIX Congreso Geológico Argentino. Córdoba, Junio 2014. Abstracts, S2–S4. Asociación Geológica Argentina, Buenos Aires.
Beurlen, K. 1930. Vergleichende Stammesgeschichte. Grundlagen, Methoden, Probleme unter besonderer Berücksichtigung der höheren Krebse. Fortschritte der Geologie und Palaeontologie 8: 317–586.
Birenheide, R. 1971. Beobachtungen am “Scheinstern” Mimetaster aus dem Hünsruck-Schiefer. Senckenbergiana lethaea 52: 77–91.
Chlupáč, I. 1999. Unusual arthropod from the Bohemian Ordovician. A review. Acta Universitatis Carolinae Geologica 43: 393–396.
Fritsch, A. 1908. Problematica silurica. In: J. Barrande (ed.), Système silurien du centre de la Bohème. 28 pp. Bellmann, Prague.
García Bellido, D.C. and Collins, D.H. 2006. A new study of Marrella splendens (Arthropoda, Marrellomorpha) from de Middle Cambrian Burgess Shale; British Columbia, Canada. Canadian Journal of Earth Sciences 43: 721–742. CrossRef
Gürich, G. 1931. Mimetaster hexagonalis, ein neuer Kruster aus dem unterdevonischen Bundenbacher Dachschiefer. Paläontologische Zeitschrift 13: 204–238. CrossRef
Gutiérrez-Marco, J.C. and Martin, E.L.O. 2016. Biostratigraphy and palaeoecology of Lower Ordovician graptolites from the Fezouata Shale (Moroccan Anti-Atlas). Palaeogeography, Palaeoclimatology, Palaeoecology 460: 35–39. CrossRef
Harrington, H.J. 1957. Ordovician Formations of Argentina. In: H.J. Harrington and A.F. Leanza (eds.), Ordovician Trilobites of Argentina. University of Kansas Special Publication 1: 1–39.
Haug, J.T., Castellani, C., Haug, C., Waloszek, D., and Maas, A. 2013. A Marrella-like arthropod from Cambrian of Australia: A new link between “Orsten”-type and Burgess Shale assemblages. Acta Paleontologica Polonica 58: 629–639.
Kühl, G. and Rust, J. 2010. Re-investigation of Mimetaster hexagonalis: A marrellomorph arthropod from the Lower Devonian Hunsrück Slate (Germany). Paläontologische Zeitschrift 84: 397–411. CrossRef
Kühl, G., Bergström, J., and Rust, J. 2008. Morphology, paleobiology and phylogenetic position of Vachonisia rogeri (Arthropoda) from the Lower Devonian Hunsrück Slate (Germany). Palaeontographica Abteilung A 286: 123–157.
Legg, D.A. 2015. The morphology and affinities of Skania fragilis (Arthropoda) from the middle Cambrian Burgess Shale. Bulletin of Geosciences 90: 509–518. CrossRef
Legg, D.A. 2016a. An acercostracan marrellomorph (Euarthropoda) from the Lower Ordovician of Morocco. The Science of Nature 103: 21. CrossRef
Legg, D.A. 2016b. A new marrellid artrhopod from the Ordovician of Wales. Acta Palaeontologica Polonica 61: 617–619. CorssRef
Legg, D.A., Sutton, M.D., and Edgecombe, G.D. 2013. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nature Communications 4: 2485. CrossRef
Lehmann, W.M. 1955. Vachonisia rogeri n. g. n. sp., ein Branchiopod aus dem unterdevonischen Hunsrückschiefer. Paläontologische Zeitschrift 29: 126–130. CrossRef
Lin, J.P., Gon III, S.M., Gehling, J.G., Babcock, L.E., Zhao, Y.L., Zhang, X.L., Hu, S.X., Yuan, J.L., Yu, M.Y., and Peng, J. 2006. A Parvancorina-like arthropod from the Cambrian of South China. Historical Biology 18: 33–45. CrossRef
Malanca, S. 1996. Morfología y ontogenia de un nuevo Shumardiidae (Trilobita) del Tremadociano de la sierra de Mojotoro, Salta, Argentina. In: S. Soruco (ed.), XII Congreso geológico de Bolivia I, Tarija, Bolivia, Octubre de 1996, 391–399. Sociedad Geológica Boliviana, Tarija.
Martin, E.L.O., Pittet, B., Gutiérrez-Marco, J.C., Vannier, J., El Hariri, K.H., Lerosey-Aubril, R., Masrour, M., Nowak, H., Servais, T., Vandenbroucke, T.R.A., Van Roy, P., Vaucher, R., and Lefebvre, B. 2015. The Lower Ordovician Fezouata Konservat-Lagerstätte from Morocco: Age, environment and evolutionary perspectives. Gondwana Research 34: 274–283. CrossRef
Moya, M.C. 1988. Estratigrafía del Tremadociano en el tramo austral de la Cordillera Oriental Argentina. 368 pp. Unpublished Ph.D. thesis, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Salta.
Moya, M.C. 1998. El Paleozoico Inferior en la sierra de Mojotoro, Salta-Jujuy. Revista de la Asociación Geológica Argentina 53: 55–69.
Moya, M.C., Malanca, S., Monteros, J., and Cuerda, A. 1994. Bioestratigrafía del Ordovícico Inferior en la Cordillera Oriental Argentina basada en graptolitos. Revista Española de paleontología 9: 91–104.
Perner, J. 1919. Furca bohemica – zástupce nové čeledi korýšů v českém siluru. Časopis Musea Království Českého 93: 32–33.
Rak, S., Ortega-Hernández, J., and Legg, D.A. 2013. A revision of the Late Ordovician marrellomorph arthropod Furca bohemica from Czech Republic. Acta Palaeontologica Polonica 58: 615–628.
Raymond, P.E. 1920. The appendages, anatomy, and relationships of trilobites. Connecticut Academy of Arts and Sciences 7: 1–169.
Siveter, D.J., Fortey, R.A., Sutton, M.D., Briggs, D.E.G., and Siveter, D.J. 2007. A Silurian “marrellomorph” arthropod. Proceeding of the Royal Society B, Biological Sciences 274: 2223–2229. CrossRef
Stürmer, W. and Bergström, J. 1976. The arthropods Mimetaster and Vachonisia from the Devonian Hunsrück Slate. Paläontologische Zeitschrift 50: 78–111. CrossRef
Turner, J. 1959. Estratigrafía de la Sierra de Narváez, Catamarca y La Rioja. Revista de la Asociación Geológica Argentina 12: 18–60.
Turner, J.C.M. 1960. Estratigrafía de la Sierra de Santa Victoria y adyacencias. Boletín de la Academia Nacional de Ciencias de Córdoba 41: 163–196.
Van Roy, P. 2006. Non-trilobite Arthropods from the Ordovician of Morocco. 230 pp. Unpublished Ph.D. thesis, Ghent University, Ghent.
Van Roy, P., Briggs, D.E.G., and Gaines, R.R. 2015. The Fezouata fossils of Morocco; an extraordinary record of marine life in the Early Ordovician. Journal of the Geological Society 172: 541–549. CrossRef
Van Roy, P., Orr, P.J., Botting, J.P., Muir, L.A., Vinther, J., Lefebvre, B., El Hariri K., and Briggs, D.E.G. 2010. Ordovician faunas of Burgess Shale type. Nature 465: 215–218. CrossRef
Von Siebold, C.T. 1848. Lehrbuch der vergleichenden Anatomie der Wirbellosen Thiere. In: C.T. von Siebold and H. Stannius (eds.), Lehrbuch der vergleichenden Anatomie, 1–679. Verlag von Veit and Company, Berlin. CrossRef
Walcott, C.D. 1912. Cambrian Geology and Paleontology 2, Nº 6. Middle Cambrian Branchiopoda, Malacostraca, Trilobita and Merostoma. Smithsonian Miscellaneous Collections 57: 145–229.
Whittington, H.B. 1971. Redescription of Marrella splendens (Trilobitoidea) from the Burgess Shales, Middle Cambrian; British Columbia. Bulletin of the Geological Survey of Canada 209: 1–24. CrossRef
Zhang, X.L., Han, J., Zhang, Z.F., Liu, H.Q., and Shu, D.G. 2003. Reconsideration of the supposed naraoiid larva from the Early Cambrian Chengjiang Lagerstätte, South China. Palaeontology 46: 447–465. CrossRef
Appendix 1
Characters considered in phylogenetic analysis.
Characters 1, 2 and 7–10 from Rak et al. (2013); characters 11–53 from Legg (2016a), characters 3–6 from this study.
Single dorsal cephalic shield with ventral shelf and median ridge: (0) absent, (1) present.
Posterolateral spines: (0) absent, (1) present.
Form of primary cephalic spines: (0) straight, (1) curved.
Quantity of secondary spines: (0) scarce, (1) numerous.
Size of secondary spines: (0) long and delicate, (1) short and robust.
Position of the secondary spines in the anterolateral pair: (0) along the length of the spine, (1) restricted to the proximal two-thirds of the spine
Inflated cephalic field: (0) absent, (1) present.
Compound eyes: (0) absent, (1) present.
Chelate cephalic endopods: (0) absent, (1) present.
Orientation of trunk exopod setae: (0) laterally directed, (1) medially directed.
Biramous cephalic appendages: (0) absent, (1) present.
Cephalic exopods much longer than endopods: (0) absent, (1) present.
Chelate cephalic endopods: (0) absent, (1) present.
Antenniform fifth appendage: (0) absent, (1) present.
Trunk exopod setae: (0) lamellate, (1)filamentous
Multisegmented trunk exopods with individual setae in each podomere: (0) absent, (1) present.
Cuticle calcification: (0) absent, (1) present.
Anterior margin of cephalon notched: (0) absent, (1) present.
Anterolateral spines: (0) absent, (1) present.
Mediolateral spines: (0) absent, (1) present.
Secondary spines on cephalic shield: (0) absent, (1) present.
Free head shield: (0) absent, (1) present.
Expanded cephalic doublure: (0) absent, (1) present.
Width of doublure: (0) narrow to moderately wide, (1) wide.
Cephalic tagmosis (number of limb-bearing segments): (0) two limb-bearing segments, (1) three limb-bearing segments, (2) four limb-bearing segments, (3) five limb-bearing segments.
Glabellar furrows or lobes: (0) absent, (1) present.
Genal spines (posterior corners of head shield extended): (0) absent, (1) present, short (2) present (as long as trunk).
Cephalic shield overlapping anterior trunk tergite(s): (0) overlap similar to that between adjacent thoracic segments, (1) cephalic shield covers multiple anterior tergites.
Tergal scutes extend laterally into paratergal folds: (0) absent, (1) present.
Trunk elongate (consisting of >25 somites): (0) absent, (1) present.
Raised axial region: (0) absent, (1) present.
Axial furrows: (0) absent, (1) present.
Telson: (0) absent, (1) present.
Telson fringed with setae: (0) absent, (1) present.
Antennal rami: (0) uniramous, (1) polyramous.
Appendage on third (tritocerebral) head segment: (0) unspecialised locomotory limb, (1) subchelate appendage, (2) “swimming paddle”.
Exopod on tritocerebral segment much longer than endopod: (0) absent, (1) present.
Exopod of post-mandibular limb multisegmented with inward pointing setae: (0) absent, (1) present
Mandible (gnathobasic appendage of third limb-bearing metamere is main feeding limb of adult head): (0) absent, (1) basipodite with elaboration of proximal endite.
Trunk endopod endites: (0) spiniferous, (1) rounded.
Rhabdomeric lateral eyes with new elements formed at a proliferation zone at side of developing eye field: (0) absent, (1) present.
Compound eyes medial margins: (0) separate, (1) medially contiguous.
Compound eye stalked, basally articulated: (0) absent (eye sessile), (1) present.
Anterior cephalic spines elongate: (0) absent, (1) present.
Dorsal marginal trunk rim: (0) absent, (1) present.
Elongate exopod tipped with setal “brush”: (0) absent, (1) present.
First post-antennal limbs with proximal endite in early larvae: (0) absent, (1) present.
First post-antennal limbs with proximal endites in later developmental stages: (0) absent, (1) present.
First post-antennal exopod with 3-2 setation pattern: (0) absent, (1) present.
Second post-antennal limb with proximal endite in early larvae: (0) absent, (1) present.
Third post-antennal limb with proximal endite in later developmental stage: (0) absent, (1) present.
Third post-antennal limb with setiferous multi-annulated exopod: (0) absent, (1) present.
Single enlarged lateral eye: (0) absent, (1) present.
Appendix 2
Character matrix used for the phylogenetic analysis.
Agnostus ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 1 0 0 0 0 0 1 0 2 1 1 0 1 0 1 1 0 0 0 0 0 1 0 0 0 ? ? ? ? ? 0 0 0 ? ? 0 ?
Oelandocaris ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 0 0 0 0 ? 3 0 0 0 ? 0 0 0 1 1 1 0 0 1 1 0 1 1 0 ? ? ? 0 0 1 0 0 0 0
Henningsmoenicaris ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 0 0 1 0 ? ? 0 0 0 ? 0 0 0 1 1 0 0 0 1 1 0 1 0 1 ? ? ? 0 1 1 0 1 0 0
Sandtorpia ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 0 0 1 0 ? ? 0 0 0 ? 0 0 0 ? ? 0 0 0 1 ? 0 0 ? ? ? ? ? 0 ? 1 0 ? 0 ?
Goticaris ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 0 0 0 0 ? 2 0 0 0 ? 0 0 0 1 0 0 0 0 1 1 0 1 ? 1 ? ? ? 1 1 0 1 0 1 1
Vachonisia 1 0 - - - - 0 0 1 1 1 1 1 1 1 1 0 1 0 0 0 1 0 ? 3 0 0 1 0 1 1 0 ? 0 0 1 1 0 0 1 0 ? ? ? 1 1 0 0 0 ? ? 0 ?
Xylokorys 1 0 - - - - 0 0 1 1 1 1 1 1 1 1 0 1 0 0 0 1 0 ? 3 0 0 1 0 1 1 0 ? 0 ? 1 1 0 0 1 0 ? ? ? 1 1 0 0 0 ? ? 0 ?
Marrella 0 1 1 - - - 0 0 0 1 ? 1 0 0 1 1 0 0 0 1 0 0 0 ? 0 0 2 ? 0 1 0 0 1 0 0 2 1 0 0 1 0 ? ? 0 0 0 0 0 0 ? ? 0 ?
Mimetaster 0 1 0 0 0 0 1 1 0 1 ? ? 0 0 1 1 0 0 1 1 1 0 0 ? 1 0 2 ? 0 1 0 0 1 0 0 0 1 0 0 1 ? ? ? 1 0 0 0 0 0 ? ? 0 ?
Furca 0 1 1 1 0 0 1 ? ? ? ? ? ? ? ? ? 0 0 1 1 1 0 0 ? ? 0 2 ? ? ? ? ? ? ? ? ? ? ? ? ? 0 ? ? 0 0 ? 0 0 0 ? ? 0 ?
Skania fragilis ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 1 0 1 1 1 ? 0 0 1 0 0 ? 0 ? 0 0 0 ? 0 0 1 0 ? ? ? 0 0 0 0 0 ? ? 0 ?
Skania sundbergi ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 1 0 1 1 1 ? 0 0 1 0 ? 1 0 ? 0 ? ? ? ? ? ? 0 ? ? ? 1 ? 0 0 0 ? ? 0 ?
Primicaris ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 0 0 1 0 1 1 1 ? 0 0 1 0 0 1 0 ? 0 0 0 1 0 0 1 0 ? ? ? 1 1 0 0 0 ? ? 0 ?
Enosiaspis hrugnir ? ? ? ? ? ? 0 ? ? ? ? ? ? ? ? ? 0 1 0 1 1 1 1 ? 3 0 0 1 0 1 1 0 ? 0 ? ? ? ? ? ? 0 ? ? ? 1 ? 0 0 0 ? ? 0 ?
Moroccan marrellomorph ? ? ? ? 0 0 ? ? ? ? ? ? ? ? ? ? 0 0 1 1 1 0 0 ? 1 0 2 ? 0 0 0 0 1 0 ? ? ? ? ? ? ? ? ? 1 ? ? 0 0 0 ? ? 0 ?
Floresta marrellomorph 0 1 1 1 1 1 1 ? ? ? ? ? ? ? ? ? 0 0 1 1 1 ? 0 ? - - - ? 0 ? ? - ? ? - - - - ? ? - - - 1 ? - - 0 ? ? ? ? ?
Acta Palaeontol. Pol. 62 (1): 1–8, 2017
http://dx.doi.org/10.4202/app.00240.2016