
Parasitic gastropod bioerosion trace fossil on
Cenomanian oysters from Le Mans, France
and its ichnologic and taphonomic context
GÉRARD BRETON, MAX WISSHAK, DIDIER NÉRAUDEAU, and NICOLAS MOREL
Breton, G., Wisshak, M., Néraudeau, D., and Morel, N. 2017. Parasitic gastropod bioerosion trace fossil on Cenomanian oysters from Le Mans, France and its ichnologic and taphonomic context. Acta Palaeontologica Polonica 62 (1): 45–57.
We describe and name Loxolenichnus stellatocinctus Breton and Wisshak igen. et isp. nov., a bioerosion trace fossil on an Upper Cenomanian oyster from Le Mans (France). This trace is attributed here to a parasitic gastropod. The characteristics of this ichnospecies are a combination of one or several, vertical or oblique, complete penetrations, and an asymmetrical attachment etching (fixichnion) with a diagnostic set of stellate grooves increasingly distinct towards the margin of the trace. By including two former Oichnus ichnospecies, Loxolenichnus halo comb. nov. and Loxolenichnus taddei comb. nov., Oichnus, is now constrained to pure predation traces (praedichnia). The numerous oysters collected from the Marnes à Pycnodonte biauriculata Formation show associated epibionts and encrusters as well as borers and scrapers. Encrusters comprise 24 taxa while bioerosion trace fossils comprise 17 ichnotaxa ranging from very rare (< 0.1%) to quite abundant (81%). The taphonomic history leading to an ex-situ condensation of these oysters is complex. Both the Gnathichnus and Entobia ichnofacies are represented on the shellgrounds, presumably alternatingly.
Key words: Gastropoda, Ostreidae, trace fossil, taphonomy, bioerosion, Cenomanian, Paris Basin.
Gérard Breton [gerard-breton@orange.fr], Géosciences, Université de Rennes I, Campus de Beaulieu, F-35042 Rennes Cedex, France; correspondence address: 6 rue des Réservoirs, 76600 Le Havre, France.
Max Wisshak [Max.Wisshak@senckenberg.de], Marine Research Department, Senckenberg am Meer, 26382 Wilhelmshaven, Germany.
Didier Néraudeau [didier.neraudeau@univ-rennes1.fr], Géosciences, Université de Rennes I, Campus de Beaulieu, F-35042 Rennes Cedex, France.
Nicolas Morel [musee.vert@ville-lemans.fr], Muséum d’histoire naturelle “Musée Vert”, 204 avenue Jean-Jaurès, F-72100 Le Mans, France.
Received 26 July 2016, accepted 25 October 2016, available online 16 November 2016.
Copyright © 2017 G. Breton et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Fossil oysters are an important component of Mesozoic and Cenozoic shallow water marine communities. Their shells serve also as a substrate for encrusters and for other bioeroding organisms both during the oyster life and post-mortem. Boring, scraping, and etching organisms use oyster shells as any carbonate substrate that they “eat [...] for fun and profit” (Bromley 1992: 121). The high preservation potential of this bioerosion, and in some cases the epibiosis by encrusters, make such shells of a great significance for taphonomic and environmental interpretations.
The aim of this paper is to draw attention to a rare new trace fossil produced on an oyster shell by a parasitic gastropod, to describe briefly the taphonomic history of some Cenomanian oysters, and to provide an illustrated list of their encrusters and borers.
Institutional abbreviations.—MHNLM, Natural History Museum “Musée Vert”, Le Mans, France.
Other abbreviations.—LV, left valve; RV, right valve.
Geological setting
The Marnes à Pycnodonte biauriculata Formation is a 1–12 m thick layer of decimetric beds, alternations of marly limestone and sandy grey marl, containing a lot of oysters of the Upper Cenomanian (Calycoceras guerangeri Biozone) outcropping mainly in the Cenomanian stratotype (Sarthe Department, Paris Basin) around the town of Le Mans (Juignet 1974, 1980). A vast majority of the myriads of oysters in this formation belongs to the species Pycnodonte (Pycnodonte) biauriculata (Lamarck, 1819) while only a few to Rhynchostreon suborbiculatum (Lamarck, 1801). The occurrence of Ceratostreon flabellatum (Goldfuss, 1833) and Pycnodonte (Phygraea) vesicularis (Lamarck, 1806), subspecies P. (P.) vesicularis parvula Freneix and Viaud, 1986 remains anecdotic, whereas Amphidonte obliquata (Pulteney, 1813) occurs only as an epibiont. Several levels of the Marnes à Pycnodonte biauriculata Formation have a high specific diversity as inferred from the body fossils (e.g., Guillier 1886; Juignet 1974).
Material and methods
A construction and earthmoving works, near the “Lycée Bellevue” within the town of Le Mans, draw to the surface a great amount of oyster-rich marl dug from the Marnes à Pycnodonte biauriculata Formation. Patrice Rabœuf (MHNLM) collected thousands of oysters from a heap of marl resulted from these activities, which served as a basis of this paper.
No sedimentary section was accessible during the present study. The different levels were mixed-up, and are thought to represent strata of about 5 m thickness. Therefore, the oysters in this study do not belong to a single biocoenosis and not even to a single taphocoenosis as they have not been collected from a single horizon or individually logged. They must be considered as a random sampling from different levels of the Marnes à Pycnodonte biauriculata Formation.
More than one thousand specimens have been examined individually after cleaning. We did not cast the borings in order to preserve the maximum of oysters (except for two specimens, but the try was unsuccessful). From a random sample of 234 oyster shells, an evaluation of the frequency of the encrusters and ichnotaxa was undertaken by quantifying their abundance in percent (Table 1).
In order to increase the depth of field of the macrophotographs, zetagraphies were obtained by stacking 2–20 pictures combined under Zerene Stacker ©. One X-ray picture of one boring was taken by a dental X-ray device.
Table 1. Encrusters (A) and ichnotaxa (B) inventory, in order of decreasing relative abundance, recorded from a random sample of 234 oyster shells, collected in the Marnes à Pycnodonte biauriculata (Upper Cenomanian, Le Mans, France), with their known or inferred trace maker, and relative abundance. Percentages rounded to the nearest integer. Since no resin casts were available (see text), the list gives the ichnogenera when the ichnospecies are not recognizable. Likewise, most of the bryozoans are eroded, making their identification unsure, even at the generic level: they are divided into morphological categories.
A
|
Taxon or morphological group |
Informal group |
Abundance (%) |
Figures |
|
Amphidonte obliquata (Pulteney, 1813) |
oyster |
36 |
1A, C |
|
Glomerula Brünnich Nielsen, 1931 |
sabellid polychaete |
24 |
1C |
|
Monostromatic sheet-like bryozoan |
bryozoan |
21 |
|
|
Branched bryozoan with thin branches, incl. Voigtopora cf. calypso (d’Orbigny, 1850) |
bryozoan |
20 |
1D |
|
Leptopora subelegans (d’Orbigny, 1852) |
bryozoan |
17 |
1E |
|
Pyrgopolon (Septenaria) cenomanensis Kočí, Jäger, and Morel, 2016 |
serpulid polychaete |
8 |
|
|
Neovermilia laevis (Goldfuss, 1831) |
serpulid polychaete |
7 |
|
|
Spondylid |
bivalve |
5 |
1B |
|
Truncated cone-shaped bases of cyclostomes |
bryozoan |
2 |
|
|
Placopsilina cenomana d’Orbigny, 1850 |
foraminiferan |
2 |
1D |
|
Berenicea Lamouroux, 1821 |
bryozoan |
<1 |
1F |
|
Other oysters (Ceratostreon
flabellatum (Goldfuss, 1833) |
oyster |
<1 |
|
B
|
Ichnotaxon |
Inferred trace producer |
Abundance (%) |
Figures |
|
Entobia Bronn, 1837 |
clionaid sponges |
81 |
2A, B |
|
Gnathichnus pentax Bromley, 1975 |
echinoids |
66 |
2C, D, 3C |
|
Gastrochaenolites
lapidicus Kelly and Bromley, 1984 |
endolithic bivalves |
62 |
2 E, F |
|
Maeandropolydora sulcans Voigt, 1965 |
polychaetes, e.g., spionid |
46 |
3B |
|
Rogerella mathieui de Saint-Seine, 1956 |
acrothoracic cirripedes |
29 |
3A |
|
Talpina ramosa von Hagenow, 1840 |
phoronid |
20 |
3C |
|
Oichnus Bromley, 1981 incl. Oichnus simplex Bromley, 1981 |
predatory gastropod |
19 |
4A |
|
Dendrinid perforations incl. Dictyoporus
nodosus Mägdefrau, 1937 and |
foraminiferans? |
4 |
3D,E |
|
Igen. et isp. indet. |
? |
1 |
4B |
|
Podichnus centrifugalis Bromley and Surlyk, 1973 |
attachment scar of a brachiopod pedicle |
<1 |
4C |
|
?Radulichnus Voigt, 1977 |
grazing trace of a mollusk radula |
<<1 |
|
|
Loxolenichnus stellatocinctus igen. et isp. nov. |
attachment scar and penetration hole of a parasitic gastropod |
<<1 |
5 |
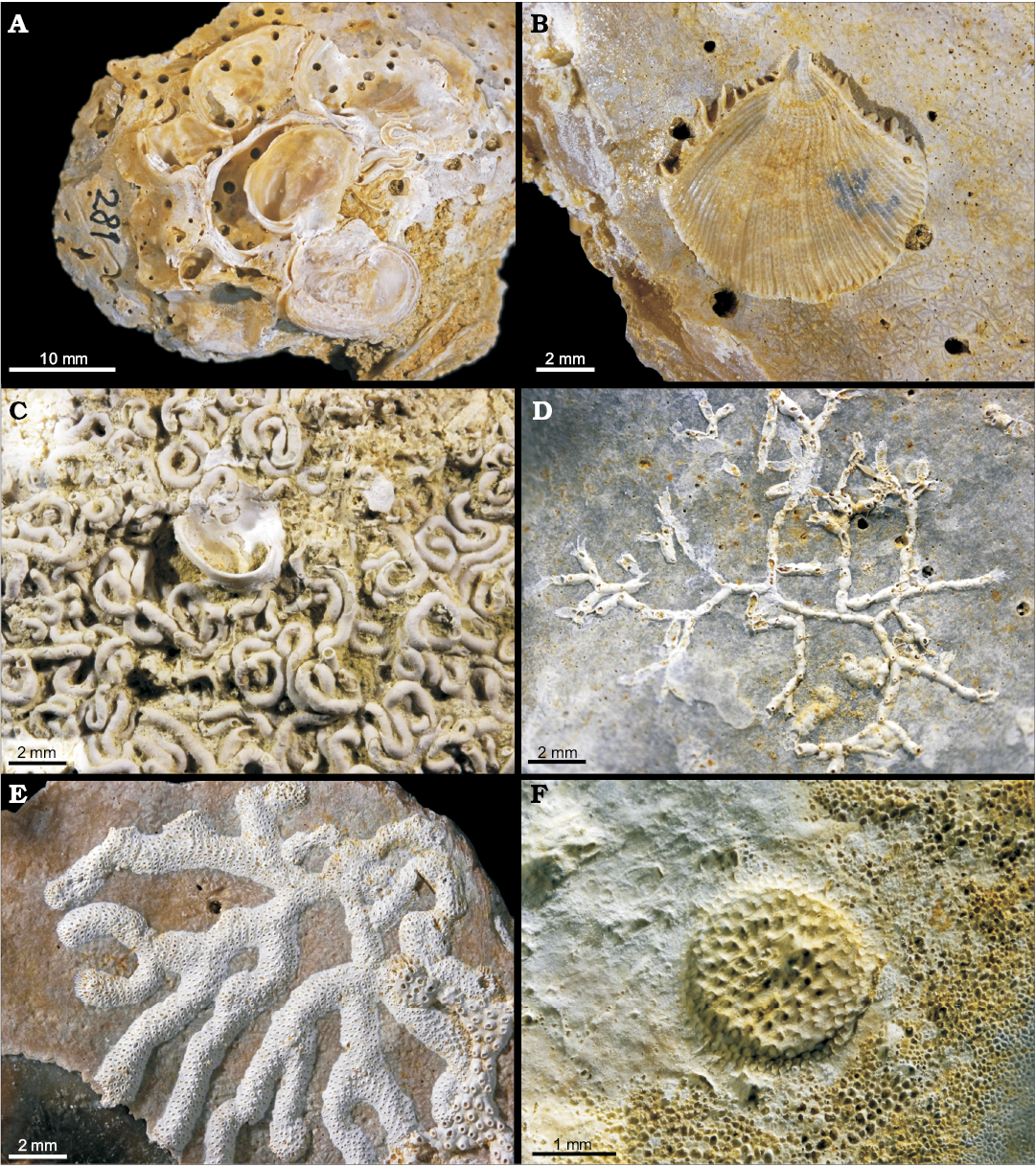
Fig. 1. Epibiosis on oyster Pycnodonte biauriculata (Lamarck, 1819), unless otherwise specified, Marnes à Pycnodonte biauriculata Formation, Upper Cenomanian, Lycée Bellevue earthmoving works, Le Mans, Sarthe Department, France. A. Amphidonte obliquata (Pulteney, 1813), MHNLM 2015.2.281; note that both the substrate as well as the fixed valves are perforated by the sponge boring Entobia isp.; one of the A. obliquata shells preserved two articulated valves. B. The fixed valve of an unidentified spondylid, MHNLM 2015.2.285. C. Polychaete Glomerula plexus (de Sowerby, 1829), MHNLM 2015.2.274; a heavy settlement anterior to the fixation of an A. obliquata. D. Bryozoan Voigtopora cf. calypso (d’Orbigny, 1850), MHNLM 2015.2.248; with some foraminifers Placopsilina cenomana d’Orbigny, 1850, between the branches. E. Bryozoan Leptopora cf. subelegans (d’Orbigny, 1852), MHNLM 2015.2.245. F. Bryozoan Berenicea sp. preserved through a bioimmuration on a large surface of fixation of a Pycnodonte (P.) biauriculata, MHNLM 2015.2.258.
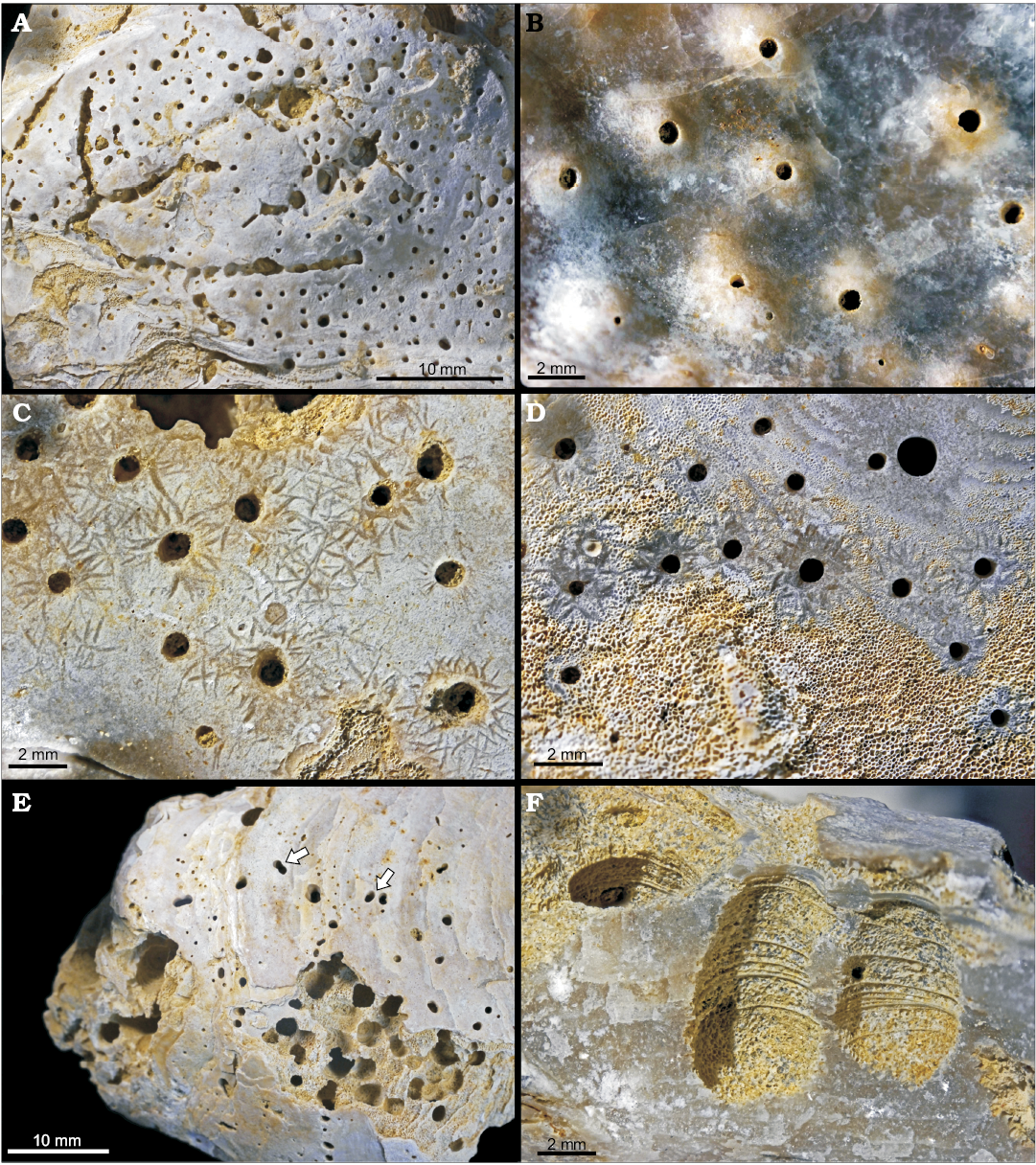
Fig. 2. Traces of scrapers and borers on oyster Pycnodonte biauriculata (Lamarck, 1819), unless otherwise specified, Marnes à Pycnodonte biauriculata Formation, Upper Cenomanian, Lycée Bellevue earthmoving works, Le Mans, Sarthe Department, France. A. Sponge boring Entobia cretacea Portlock, 1843, outer side of a LV, MHNLM 2015.2.207; identified by its long, straight camerate threads with aligned apertures and the roughly equidiametric and evenly spaced chambers. B. Sponge boring Entobia isp., inner side of a LV, MHNLM 2015.2.209; note the swellings and the discoloured areoles around the apertures, indicating a syn-vivo entobian infestation. C. Echinoid scraping traces Gnathichnus pentax Bromley, 1975, MHNLM 2015.2.182; these rather large G. pentax tend to concentrate around the entobian apertures. D. Echinoid scraping traces Gnathichnus pentax Bromley, 1975, MHNLM 2015.2.177; a rather small G. pentax, only present around the entobian apertures, the abrasion of the external layer of the shell brings the vesicular structure of the shell to light (lower part of the picture). E. Bivalve boring Gastrochaenolites cf. dijugus Kelly and Bromley, 1984, MHNLM 2015.2.223; most of the borers are concentrated on the “bump” of the LV, the arrows show the 8-shaped or geminate apertures, characteristic of G. dijugus. F. Bivalve boring Gastrochaenolites lapidicus Kelly and Bromley, 1984, MHNLM 2015.2.158; longitudinal section of two perforations showing a xenomorphic structure due to the heterogeneous shell.
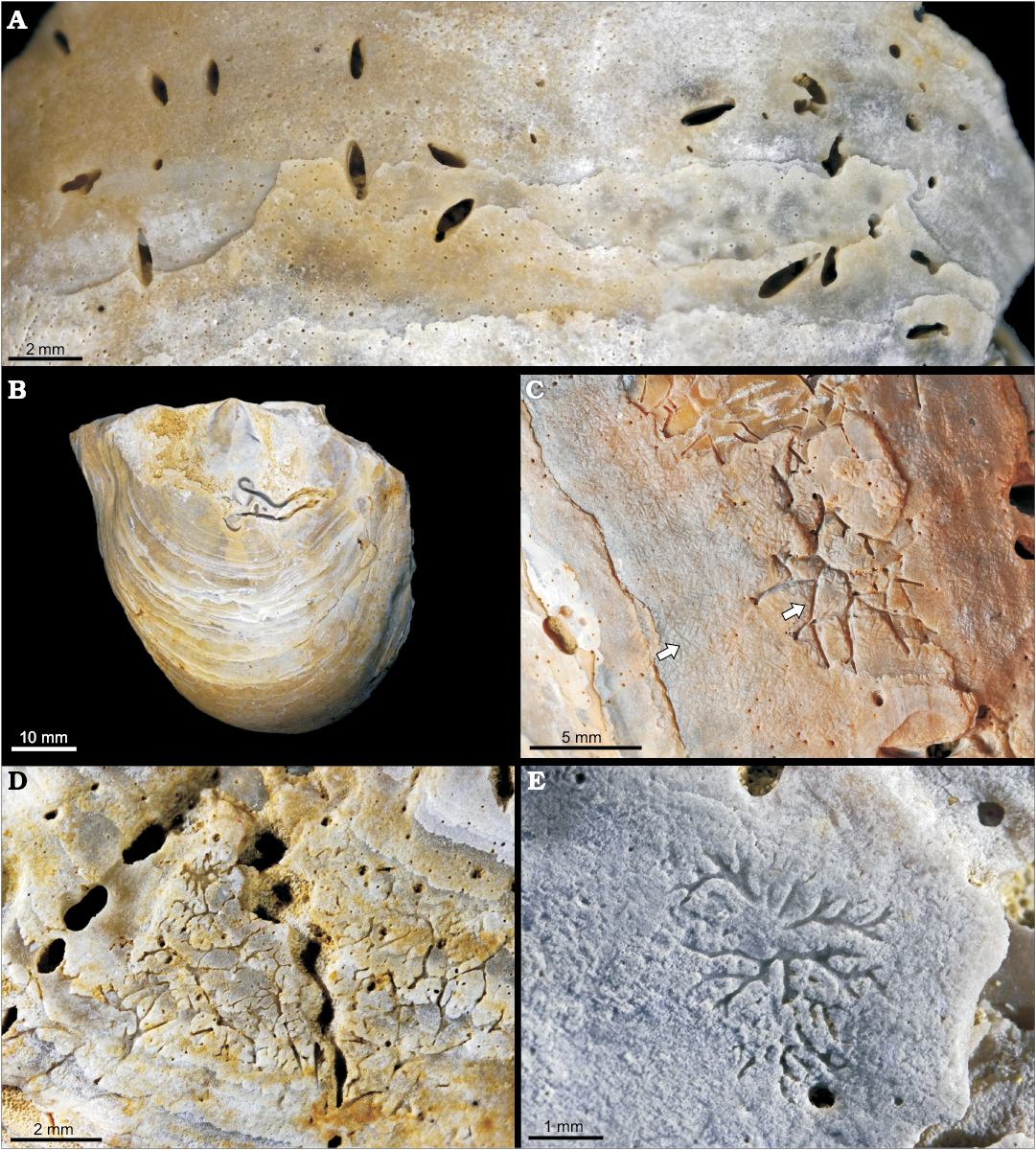
Fig. 3. Traces of borers on oyster Pycnodonte biauriculata (Lamarck, 1819), unless otherwise specified, Marnes à Pycnodonte biauriculata Formation, Upper Cenomanian, Lycée Bellevue earthmoving works, Le Mans, Sarthe Department, France. A. Acrothoracican barnacle traces Rogerella mathieui de Saint-Seine, 1956, MHNLM 2015.2.201; group of perforations on the outer side of a LV. B. Polychaete trace Maeandropolydora sulcans Voigt, 1975, MHNLM 2015.2.237. C. Phoronid trace Talpina ramosa von Hagenow, 1840, MHNLM 2015.2.345 (centered arrow); the arrow on the left side shows the extensive scraping of the surface of the shell by echinoids (Gnathichnus pentax). D. Trace fossil of Dictyoporus nodosus Mägdefrau, 1937, MHNLM 2015.2.141; note the aligned apertures of Entobia cretacea. E. Possible foraminiferan boring Dendrina anomala Mägdefrau, 1937, MHNLM 2015.2.142.
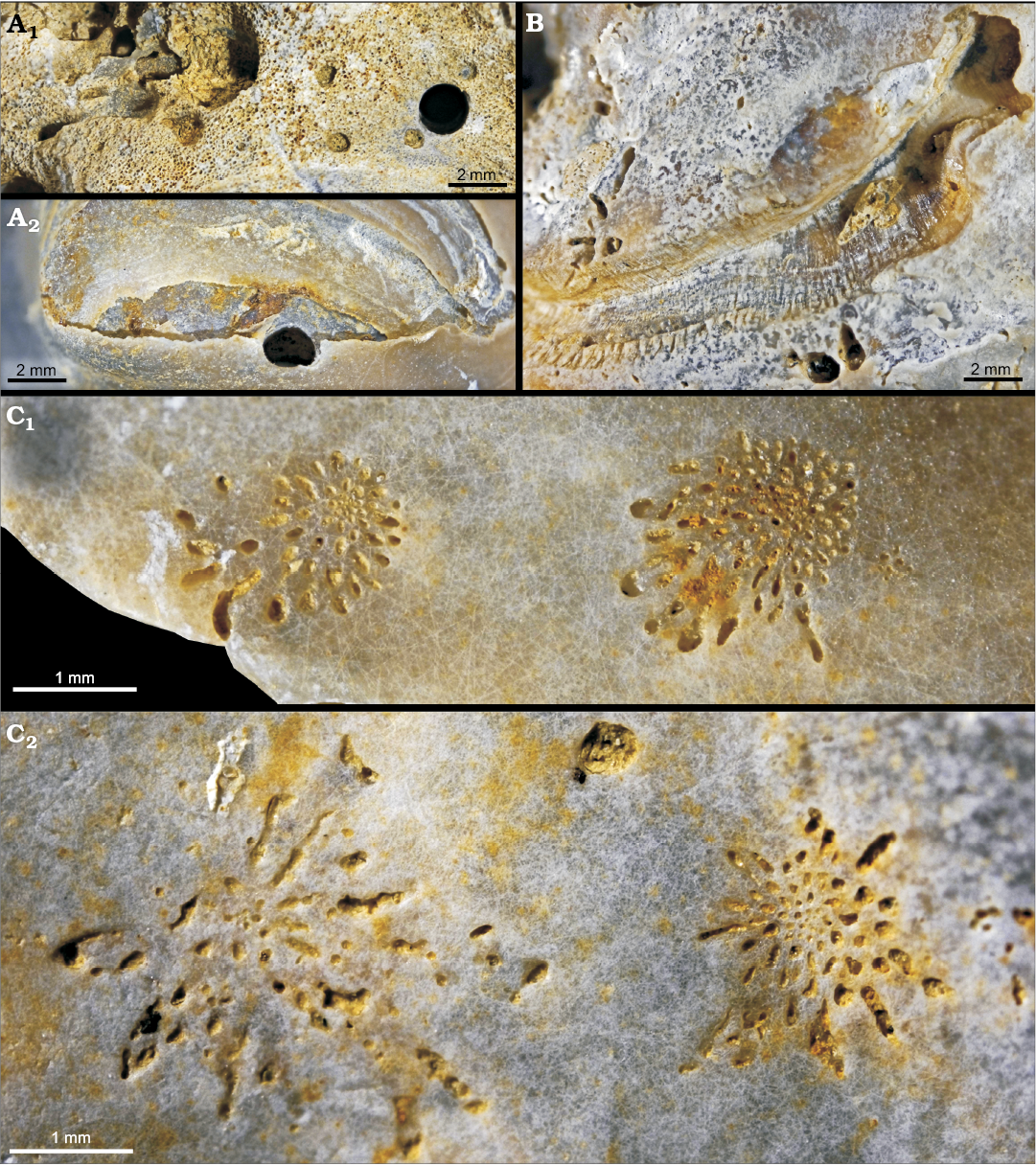
Fig. 4. Traces of borers on oyster Pycnodonte biauriculata (Lamarck, 1819), unless otherwise specified, Marnes à Pycnodonte biauriculata Formation, Upper Cenomanian, Lycée Bellevue earthmoving works, Le Mans, Sarthe Department, France. A. Gastropod drill hole Oichnus simplex Bromley, 1981, MHNLM 2015.2.195. The perforation on the outer (A1) and inner (A2) sides of the shell reaching the edge of the adductor muscle scar. B. Unidentified bioerosion trace, MHNLM 2015.2.203, on the outer side of the LV of Rhynchostreon suborbiculatum (Lamarck, 1801). C. Brachiopod pedicle attachment scar Podichnus centrifugalis Bromley and Surlyk, 1973, MHNLM 2015.2.143, two groupings (C1, C2) of two individual attachment scars on the inner side of the same shell.
Autecology of Pycnodonte biauriculata
Dhondt (1984) stated that the Pycnodonte (P.) biauriculata must have lived unattached all their life. This was only possible if they “floated” on what was probably an oozy substrate. She interprets the morphological change during their ontogeny, that is the acquisition of a “keel” (i.e., a heavy bump on the left valve acting as a ballast) and of large wings acting as “snow-shoes”, as an adaptation to life on muddy, soft bottom; the LV allows the oyster to “float” above the ooze surface and preventing its pallial cavity to get clogged up by mud. This can only be done in an environment without strong currents. Juignet (1974: 691) states that the oysters from the Marnes à Pycnodonte biauriculata Fm were fossilized where they lived, i.e., they form in situ assemblage.
Systematic palaeoichnology (by Gérard Breton and Max Wisshak)
Ichnogenus Loxolenichnus Breton and Wisshak nov.
Type ichnospecies: Loxolenichnus stellatocinctus Breton and Wisshak isp. nov.; see below.
Included ichnospecies: The type ichnospecies, Loxolenichnus halo (Neumann and Wisshak, 2009) comb. nov., and Loxolenichnus taddei (Ruggiero and Raia, 2014) comb. nov.
Figs. 5, 6.
Etymology: Contraction of the ancient Greek words loxós (λοξός), oblique, and solen (σωλήν), canal, pipe; allusion to the central oblique penetration hole in the holotype of the type ichnospecies. Ichnus is a common ending for trace fossils names, referring to ancient Greek ichnos, track. Gender masculine.
Diagnosis.—A single or rarely several cylindrical holes, completely cutting through calcareous shelly substrates, surrounded by a circular to elliptical depression with concentrical grooves.
Remarks.—The ichnogenus Oichnus Bromley, 1981, except the ichnospecies Oichnus halo Neumann and Wisshak, 2009 and Oichnus taddei Ruggiero and Raia, 2014 which are herein transferred to Loxolenichnus, lack the attachment scar and peripheral groove. The ichnogenera Anellusichnus Santos, Mayoral, and Muñiz, 2005, Lacrimichnus Santos, Mayoral, and Muñiz, 2003 and Centrichnus Bromley and Martinell, 1991 in turn are pure attachment scars and lack the penetration hole(s) passing through the substrate. Kardopomorphos, with its sole ichnospecies Kardopomorphos polydioryx Beuck, López Correa, and Freiwald, 2008 is a rounded or spiral-shaped pit, from which many whip-shaped, branched canals including sometimes one or few central larger canals penetrate deeply in the substrate. By including two former Oichnus ichnospecies, Oichnus is now constrained to pure predation traces (Praedichnia). The new ichnogenus, in contrast, comprises three ichnospecies that are all combinations of complete penetrations (parasitic in nature and thus unlike Praedichnia sensu stricto) and an attachment scar (Fixichnia), and this combination of two different modes of bioerosion is characteristic of the new ichnogenus.
Key to the three ichnospecies of Loxolenichnus:
1a Peripheral groove more or less stellate ................................................................................................................................................. Loxolenichnus stellatocinctus
1b Peripheral groove not stellate, rounded or oval ................................................................................................................................... 2
2a Peripheral groove rounded, the drill hole in the middle of the area surrounded by a quite distinct peripheral groove....................... Loxolenichnus halo
2b Peripheral groove oval, the drill hole tangential or close to the less distinct peripheral groove ........................................................ Loxolenichnus taddei
Loxolenichnus stellatocinctus Breton and Wisshak isp. nov.
Figs. 5, 6.
Etymology: From the Latin stellatus, star-shaped, and cinctus, encircled; allusion to the stellate groove surrounding the central perforation(s).
Type material: Holotype: MHNLM 2015.2.244 (Fig. 5). Paratypes: MHNLM 2015.2.346 and MHNLM 2015.2.347, ex collection Peter Girod. Two oyster valves from the lower Campanian Inoceramus lingua–Goniotheuthis quadrata Zone, found in a quarry near Höver, Germany (Fig. 6).
Type locality: Excavation of the earth working site of the Lycée Bellevue, town of Le Mans, France (48°00’53” N; 0°12’21” E Google Earth ©).
Type horizon: Cretaceous, Upper Cenomanian, Calycoceras guerangeri Biozone, Marnes à Pycnodonte biauriculata Formation.
Diagnosis.—One or several, vertical or oblique penetrations, irregularly cylindrical, surrounded on the outer substrate surface by an asymmetrical depression with a set of stellate grooves increasingly distinct towards the margin of the trace.
Description.—Holotype: Substrate: An incomplete left valve of the oyster R. suborbiculatum (preserved length ca. 6 cm) with ventral and ventro-posterior margins broken. The colour pattern (longitudinal brown flames) is preserved near the umbo. Entobian perforations near the posterior margin and on the centre of the shell. The holotype is located near the ventro-anterior margin (Fig. 5A).
Trace: The penetration hole is 8 mm long, roughly cylindrical, with a diameter of ca. 1 mm. It plunges very obliquely towards the centre of the valve into the shell which is 2.5 mm thick at this place. Its course is slightly sinuous (Fig. 5A) and the opening on the inner side of the valve is a trench of ca. 4 × 1 mm (Fig. 5B). The aperture on the outer side of the valve is an irregular depression ca. 3 × 1.5 mm. It is located in the middle of a roughly circular attachment scar on the shell, but slightly eccentric. Except for a recent partial mechanical etching, the surface of the shell is intact between the penetration hole and the peripheral stellate groove. The attachment scar is delineated by a stellate groove. There are 13 main rays, longer in the ventro-anterior direction (of the oyster shell) where they are up to 2.2 mm long, than in the opposite direction where they are reduced to small notches 0.5 mm deep. The transition between the shortest notch and the longest ray is progressive. The longest rays are sharply triangular, but their extremity is blunt and rounded. The groove is deepest along the side of the rays, so that there is an axial ridge in the axis of each ray. Between the eight main rays on the ventro-anterior side come in seven accessory rays, 3–5 times shorter than the main rays but with an axial ridge too. The accessory rays become progressively shorter—as do the main rays—in the dorso-posterior direction, where the groove becomes deeper, obliquely sunk into the shell, and the rays are there less clearly marked. Dorso-posteriorly, the inner side of the groove is a smooth surface, oblique, joining the outer surface of the shell with an angle (estimated) of 45°, 1.5 mm high which becomes progressively lower and fades, mainly in the anterior direction (Fig. 5C–E).
Paratypes: The paratype MHNLM 2015.2.346 (Fig. 6A–C) accommodates two Loxolenichnus stellatocinctus igen. et isp. nov., located directly next to each other, each surrounded by a stellate groove, the diameter of which are 8 mm in maximum. The first trace shows two penetration holes, one perpendicular and one oblique. The second trace shows, within the marginal stellate groove, two partial (1/4 circumference) grooves and there are two approximately central and one marginal penetration holes, one of them being superposed to one of the partial grooves. At least two penetration holes reach the adductor muscle scar. Callus formations as a host reaction are visible on the inner side of the valve and indicate a penetration during the life of the oyster. The second paratype MHNLM 2015.2.347 (Fig. 6D–F) is a right valve with two semi-circular concentric stellate grooves centred on the ventral margin of the valve. The grooves display alternately long and short rays. A possible penetrative way is a notch on the ventral margin of the valve, located at the centre of the grooves.
Remarks.—By including two former Oichnus ichnospecies, Oichnus is now constrained to pure predation traces (Praedichnia). The new ichnogenus, in contrast, comprises three ichnospecies that are all combinations of complete penetrations (parasitic in nature and thus unlike Praedichnia sensu stricto) and an attachment scar (Fixichnia), and this combination of two different modes of bioerosion is characteristic of the new ichnogenus.
Stratigraphic and geographic range.—Upper Cretaceous, France and Germany. Known only from oysters.
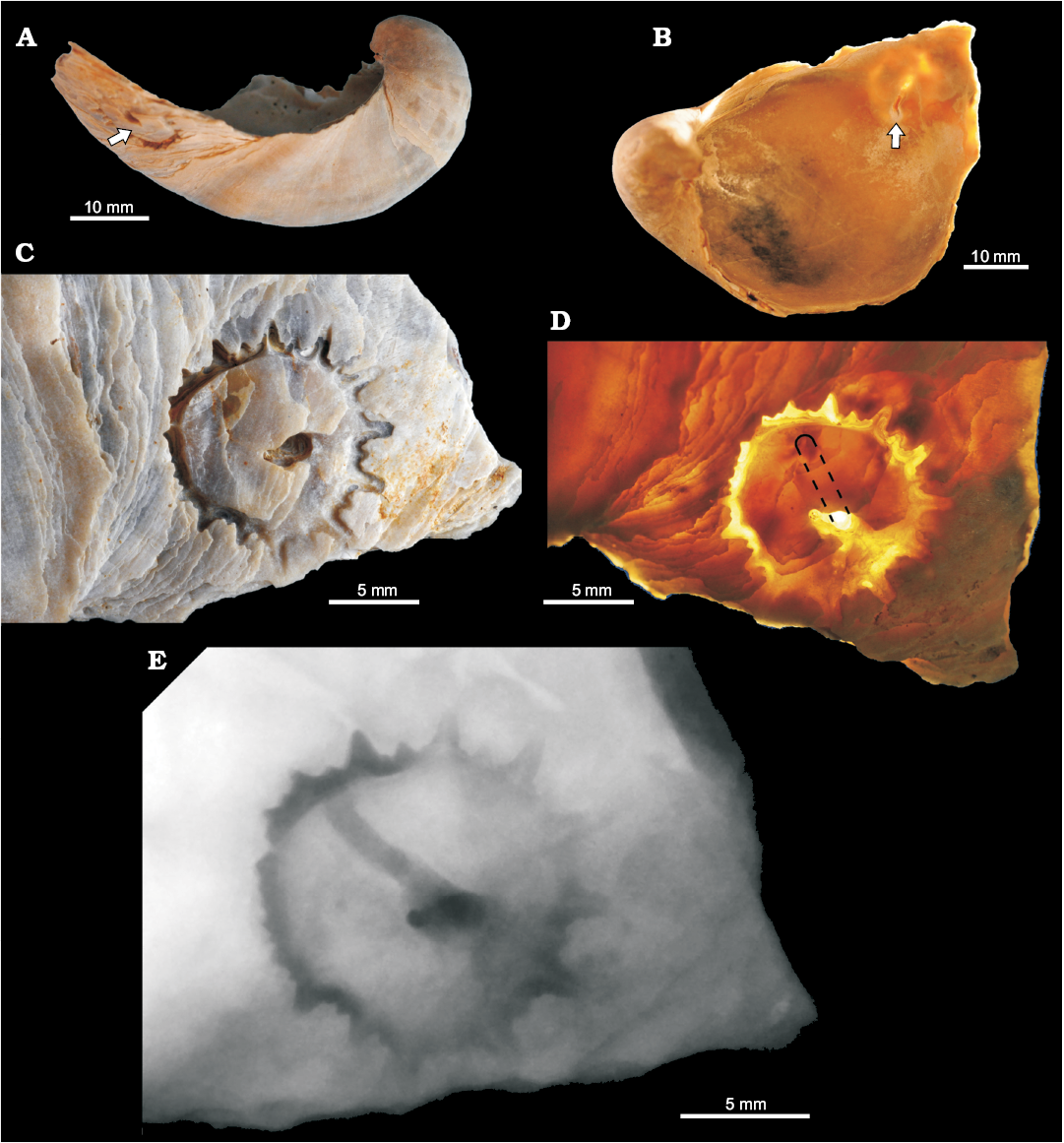
Fig. 5. Parasitic gastropod bioerosion and perforation trace Loxolenichnus stellatocinctus igen. et isp. nov., MHNLM 2015.2.244, holotype, Marnes à Pycnodonte biauriculata Formation, Upper Cenomanian, Lycée Bellevue earthmoving works, Le Mans, Sarthe Department, France; on LV of Rhynchostreon suborbiculatum (Lamarck, 1801). A. Entire LV shell with the arrow showing the perforation. B. LV (viewed from inside), the arrow shows the opening of the perforation on the inner side of the shell, diascopic illumination. Outer (C) and inner (D) sides of the shell, close-ups of the perforation, the dashed line delimitates approximately the course of the perforation through the shell, diascopic illumination. E. Positive X-ray print of the perforation.
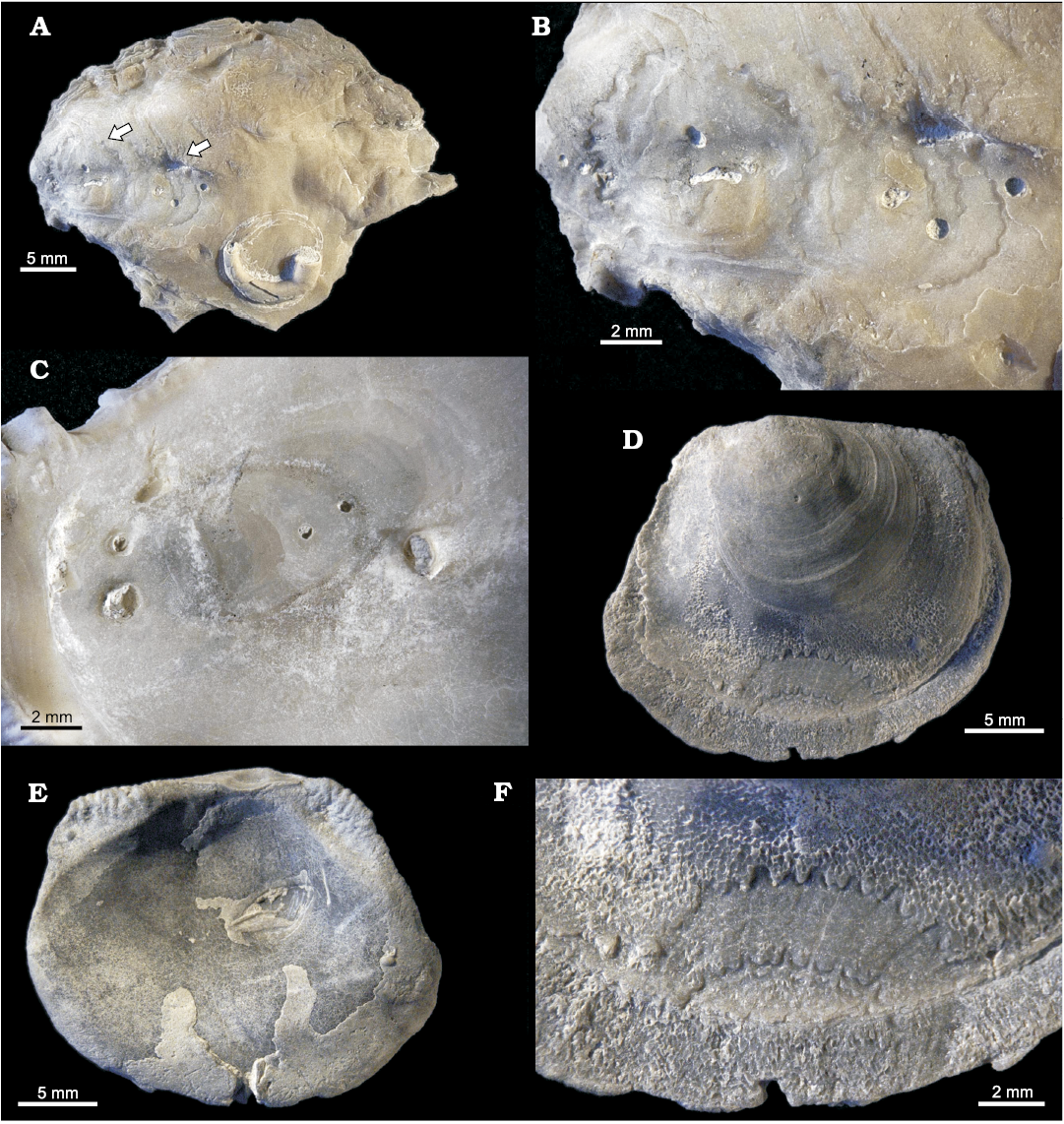
Fig. 6. Parasitic gastropod bioerosion trace Loxolenichnus stellatocinctus igen. et isp. nov., MHNLM 2015.2.346 and MHNLM 2015.2.347, paratypes; lower Campanian Inoceramus lingua–Goniotheuthis quadrata Zone, quarry near Höver, Germany. A. Outer side of an oyster valve, accommodating two specimens of L. stellatocinctus (arrows). B. Close-up of the two specimens and the multiple perforations. C. Inner side of the oyster valve showing two of the perforations reaching the adductor muscle pad. Outer (D) and inner (E) sides of an oyster valve with a marginal L. stellatocinctus. F. Close-up of D, note the two concentric stellate rims and the marginal notch.
Discussion
Trace-maker.—The trace-maker of Loxolenichnus stellatocinctus igen. et isp. nov. is thought to be a parasitic gastropod. The stellate groove corresponds to the fixation scar of the pseudopallium (= enlarged snout), the penetration hole giving way to the proboscis. An alternative interpretation (Christian Neumann, written communication 2016) would be to consider the stellate outline as the work of glands situated at the mantle margin, comparable to the attachment traces produced by Hipponicidae (Vermeij 1998) and Anomiidae (Neumann et al. 2015). Both hypotheses seem admissible, and we have no argument to favour one over the other. Predation of gastropods (naticids, muricids) on molluscs, brachiopods or echinoderms lead to a single hole, without fixation scar, and are addressed as Oichnus ispp. (Bromley 1981; see Wisshak et al. 2015 for the latest revision of this ichnogenus). On the contrary, the permanent settling of parasitic gastropods, such as eulimids which feed on echinoderms, or capulids among which a few species are parasitic on molluscs (Matsukuma 1978; Kabat 1990; Bongrain 1995), can leave a fixation scar on the shell of the host. Bongrain (1995) describes attachment scars of different species of Capulidae on the shell of Serravallian Gigantopecten. She notes that the trophic relations between Capulidae and Pectinidae vary from commensalism to parasitism.
In the adult female of Thyca spp., the foot is reduced and the snout is strongly enlarged in a pseudopallium. This attachment disc is permanently fused with the tissues of the host (Neumann and Wisshak 2009). “The ectoparasitic Capulidae also drill holes that resemble those of muricids, but these can be recognized by the accompanying attachment scar preserved” (Kelley and Hansen 2003: 116). Bromley and Heinberg (2006: 435) state: “The capulid gastropods are parasites and remain almost stationary throughout life and chiefly parasite bivalves […] Some species dissolve a notch at the margin of the host shell, whilst other bore a hole penetrating the shell thus leaving a preserveable trace fossil as evidence of the parasitism (e.g., Matsukuma 1978). The long-term presence of the capulid [pseudopallium] may etch the host shell where it is attached”. The capulids so fixed to their hosts obtain “…small amounts of fluids from the host’s feeding current for nutrition” (Kabat 1990: 156). Though this term is more often applied to birds or insects, we think that this is a kind of kleptoparasitism (= antagonistic symbiosis; Orr 1963).
Kabat (1990: 156–158, figs. 5, 6) illustrates an example of a Recent borehole made by Capulus danieli (Crosse, 1858) (= Capulus dilatatus Adams, 1860) on the shell of the bivalve Comptopallium vexillum (Reeve, 1856). The borehole is surrounded by a circular etching scar with several small rays. The borehole is located close to the periphery of the scar. For instance, Capulus danieli has an attachment area 4.2–33 mm in diameter surrounding the hole (1–1.67 mm in diameter) they pierce near the umbo of Pectinidae (Matsukuma 1978).
The size, structure and host of Loxolenichnus stellatocinctus igen. et isp. nov. is coherent with a taxonomic attribution to Capulidae for the trace maker. From the perspective of a kleptoparasitic mode of life, the boreholes which reach the adductor muscle are inefficient for feeding. This could explain the fixation scars encompassing multiple boreholes of the first lower Campanian specimen from Höver. The semi-circular stellate groove of the second lower Campanian specimen from Höver, centred on the ventral margin of a right valve, corresponds to a parasitic capulid, the proboscis of which enters the cavity of the oyster by a marginal notch (Bromley and Heinberg 2006).
Therefore, the trace left by the foot of the muricids Vitularia spp. on the shell of their hosts (oysters or Spondylus) is quite similar to Loxolenichnus taddeii (Ruggiereo and Raia, 2014). The very oblique tunnel (and its opening on the inner side of the oyster shell) drilled by Vitularia salebrosa (King and Broderip, 1832) (Herbert et al. 2009) is completely identical to Loxolenichnus stellatocinctus isp. nov. This brings into play muricids as potential trace makers for L. taddeii and possibly Loxolenichnus stellatocinctus, the shape of the gastropod attachment scar (stellate or circular) perhaps depending on the species.
Among the 86 gastropod species from all the Cenomanian stratotype, ?Lunatia varusensis (d’Orbigny, 1850) and ?Polinices difficilis (d’Orbigny, 1842) are considered by Kollmann (2015: CD: 68) as Naticidae and could then be good applicants for the borers of the trace Oichnus simplex of our oysters. Their systematic position was discussed and Kase and Ishikawa (2003) who showed that such gastropods assigned to the Naticidae belong actually to Ampullinidae, represented by the living Cernina fluctuata (Sowerby, 1825) which is an algal grazer. So the Oichnus ispp. trace producers in the Cenomanian from Le Mans remain unknown. In the same way, the three gastropods ?Atresius cenomanense (d’Orbigny, 1843), ?Atresius coloniae (Guéranger, 1867) and ?Atresius incertus (Guéranger, 1867) were assigned with doubt to Capulidae by Kollmann (2015: CD: 68). According to Kiel et al. (2008) the systematic position of these species is unclear as they possess cerithiform rather than cap-shaped shells. The genus Atresius itself belongs to Abyssochrysoidea (Kaim et al. 2014), a group of grazers rather than predators. Therefore, the question of the potential trace-makers of Loxonelichnus stellatocinctus igen. et isp. nov. remains open.
Settlement timing.—Entobia ispp. began to settle syn-vivo since the inner side of the shells sometimes shows a reaction (Fig. 2B). For the other borers, we lack any convincing argument for a settlement ante- or post-mortem, presumably both for many borers, with large majority of post-mortem borings since most traces can be found also, though less numerous, on the inner side of the shells. The grazing trace Gnathichnus pentax is present on both sides of many individual valves, and in direct association with other borers (Entobia or Rogerella holes) or encrusters (bryozoans). They are thus late traces. Contrary to MacEachern et al. (2007) who consider invalid the Gnathichnus ichnofacies, Gibert et al. (2007: 794) argue that “despite the discrete nature of the components, a shellground constitutes a continuous substrate available for skeletobiont colonization” and that “[the Gnathichnus ichnofacies] records short periods of exposure of hard substrates before their burial […] in contrast with that recorded by the Entobia ichnofacies that corresponds to long colonization windows, in stable substrate with low sedimentation rates” and we concur with this interpretation.
On the individual shells we studied, both Gnathichnus and Entobia are present, the mode of sampling is thus not responsible for the mixture of Entobia/Gnathichnus ichnofacies which must have been recurrent in all the Marnes à Pycnodonte biauriculata Formation from Le Mans.
Though Gnatichnus pentax is a widespread bioerosion trace fossil in our material, the echinoids seem poorly represented in the fossil record, though Obert (1988) argues that the echinoids are less rare than they seem to be, because their abundance is masked by the huge quantity of oyster shells. Anyhow, only Tiaromma michelini (Agassiz, 1840) and Salenia petalifera (Desmarest, 1825), among the Cenomanian echinoids from Le Mans are potential producers for the trace Gnathichnus pentax. In this case, there is a contradiction between the rarity and the low diversity of the body fossil (Néraudeau et al. 2015) and the ubiquitous trace fossils.
Remarks on oyster shell bed taphonomy.—Most of the oysters were found disarticulated. The valves of P. biauriculata are moderately to severely attacked by borers and scrapers and accommodate frequently encrusters. Only two shells over more than one thousand are devoid of any boring, scraping or encruster. Before their burial, the shells suffered not only bioerosion, but also mechanical breaking and/or dissolution. This dissolution could have been initiated on pre-existing borings and frequently affected the vesicular structure of the shell, well developed in the thickest part of the LV, i.e., the “bump” or “keel” sensu Dhondt (1984). The bottom of the cavities created by this dissolution is partly covered by encrusters. The taphonomic history of these shells was complemented by recent, meteoric dissolution if they stayed near the soil surface and by a pseudo-encrustation of a calcitic lining on tiny roots. During the attempt to get epoxy casts of some perforations, Paul Taylor (written communication 2016) noticed that the shells could not be dissolved by dilute hydrochloric acid because they were silicified.
Taking into account the fact that oysters have not been collected in stratigraphical order, our taphonomical remarks must be considered as provisional. Future investigations should study oysters in situ as did Videt (2004) for the P. biauriculata beds from the Charentes, in order to have a better understanding of the successive taphocoenoses of the oysters of the Marnes à Pycnodonte biauriculata Formation from Le Mans.
The “floating” life position of P. biauriculata inferred by Dhondt (1984) from its functional morphology concerns only those oysters growing on a soft ground. Later, they grow on the top of each other, so that they escape from the contact with substratum (Videt 2004: 174). Videt (2004: 114) states that fossil exogyres are most often in coquina beds stemming from a quick and catastrophical burial (“census assemblage”); their assemblages are not or little affected by condensation and are thus rather well preserved, and constituted of individuals coming from the same biosedimentary palaeoenvironment. That is not the case for the oysters that we studied herein, because this scenario does not leave any time for encrustation or perforation; their assemblages are thus allochthonous or at least para-autochthonous. Actually, most encrusters and borers settled post-mortem and colonized shells which have been disarticulated and transported, and were not buried in the sediment.
More generally, encrusters and borers of various populations of oysters are similar (e.g., Bottjer 1982; Farinati and Zavala 2002; Gibert et al. 2007; El-Hedeny 2007; Lopes 2011; Matteucci et al. 2012), whatever the age or the geographic localization. It means that the oyster shells act as any carbonate substrate and are encrusted and bored as would be any carbonate substrate in the same conditions. They are suitable hard substrate for several generations of borers/encrusters, though it does not seem possible to understand unequivocal sequences of colonization which “run a predictable course” (Bottjer 1982: 82).
Oysters are suspension-feeders: when they were alive, a good primary productivity in their environment allowed their growth and multiplication. After death, the oyster shells disarticulated and then were transported (by storm currents?) to their final site of deposition, where they were densely settled by suspension-feeding epibionts and/or sclerobionts. It indicates that the primary productivity of the environment has been continuously good. Encrustation and bioerosion by suspension-feeders is negatively correlated with hydrodynamics (energy) and sedimentation rate (Bottjer 1982): at the time when the oysters were encrusted and bored, the environment had a calm hydrodynamic regime and a low sedimentation rate.
Conclusions
The new ichnotaxon described herein complements the fossil record of parasitism by gastropods.
With the ichnotaxa recognized and described in the Cenomanian Marnes à Pycnodonte biauriculata Fm from Le Mans, our study adds to the specific diversity previously known from the body fossils and shed a new light on the palaeobiodiversity of the formation.
Further research about taphonomy, palaeoecology and ichnology of these oysters will need to work on stratigraphically sampled material.
Acknowledgements
Our warmest thanks to Patrice Rabœuf (Le Mans, France) who collected and prepared thousands of oyster shells, Christian Tailliez and Yann Boschet (both Lycée Bellevue, Le Mans, France), Paul Taylor (The Natural History Museum, London, UK) and Manfred Jäger (Rosenfeld, Germany) who helped us with the identification of epibionts, Helmut Zibrowius (Marseille, France) who provided documentation in an early stage of this work, Anders Warén (Naturhistoriska Riksmuseet, Stockholm, Sweden) who sent us documentation on parasitic gastropods, Géraldine Le Maoût (Le Havre, France) who took the X-ray picture of the holotype and Peter Girod (Berlin, Germany) who provided a number of specimens of the new ichnospecies Loxolenichnus stellatocinctus. We thank the journal reviewers Alfred Uchman (Jagiellonian University, Kraków, Poland), Tomoki Kase (National Museum of Nature and Science, Tsukuba, Japan), and Christian Neumann (Museum für Naturkunde, Berlin, Germany) for their constructive and pertinent comments on the manuscript.
References
Adams, A. 1860. On a new genus and some new species of Mollusca from Japan. Annals and Magazine of Natural History 3: 414–422.
Agassiz, L. 1840–1845. Etudes critiques sur les mollusques fossiles. 58+287 pp. De Petitpierre and de H. Wolfrath, Neuchâtel.
Beuck, L., López-Correa, M., and Freiwald, A. 2008. Biogeographical distribution of Hyrrokkin (Rosalinidae, Foraminifera) and its host-specific morphological and textural trace variability. In: M. Wisshak and L. Tapanila (eds.), Current Developments in Bioerosion. Erlangen Earth Conference Series, 329–360. Springer, Berlin.
Bongrain, M. 1995. Traces de bioérosion sur un Pectinidae (Bivalvia) du Miocène d’Aquitaine (SO France): un cas possible de commensalisme entre Pectinidae et Capulidae. Geobios 28: 347–358. Crossref
Bottjer, D.J. 1982. Paleoecology of epizoans and borings on some Upper Cretaceous chalk oysters from the Gulf Coast. Lethaia 15: 75–84. Crossref
Bromley, R.G. 1975. Comparative analysis of fossil and recent echinoid bioerosion. Palaeontology 18: 725–739.
Bromley, R.G. 1981. Concepts in ichnotaxonomy illustrated by small round holes in shells. Acta Geológica Hispánica 16: 55–64.
Bromley, R.G. 1992. Bioerosion: Eating rocks for fun and profit. In: C.G. Maples and R.R. West (eds.), Trace Fossils. Short Courses in Paleontology No. 5, 121–129. Paleontological Society, Knoxville.
Bromley, R.G. and Heinberg, K. 2006. Attachment strategy of organisms on hard substrates: A palaeontological view. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 429–453. Crossref
Bromley, R.G. and Martinell, J. 1991. Centrichnus, new ichnogenus for centrically patterned attachment scars on skeletal substrates. Bulletin of the Geological Society of Denmark 38: 243–252.
Bromley, R.G. and Surlyk, F. 1973. Borings produced by brachiopod pedicles, fossil and Recent. Lethaia 6: 349–365. Crossref
Bronn, H.G. 1837–1838. Lethea Geognostica oder Abbildungen und Beschribung der für die Gebirgs-Formationen bezeichnendsten Versteinerungen. II: Das Kreide und Molassen-Gebirge [plates: 1837; text: 1838], 545–1350. Schweitzerbart, Stuttgart.
Brünnich Nielsen, K. 1931. Serpulidae from the Senonian and Danian deposits of Denmark. Meddelelser fra Geologisk Forening 8: 71–113.
Crosse, M.H. 1858. Diagnoses de coquilles nouvelles. Revue et Magasin de Zoologie Pure et Appliquée 2 (10): 81.
Desmarest, A.G. 1825. Oursin. In: J.L.M. Defrance (ed.), Dictionnaire des Sciences Naturelles, Tome 37, 59–102. F.G. Levrault, Strasbourg.
Dhondt, A.V. 1984. The unusual Cenomanian oyster Pycnodonte biauriculatum. Geobios (Mémoire Spécial) 8: 53–61. Crossref
El-Hedeny, M. 2007. Encrustation and bioerosion on Middle Miocene bivalve shells and echinoid skeletons: paleoenvironmental implications. Revue de Paléobiologie 26: 381–389.
Farinati, E. and Zavala, C. 2002. Trace fossils on shelly substrate. An example from the Miocene of Patagonia, Argentina. Acta Geologica Hispanica 37: 29–36.
Freneix, S. and Viaud, J.-M. 1986. Huîtres du Crétacé supérieur du bassin de Challans Commequiers (Vendée). Biostratigraphie, taxinomie, paléobiologie. Bulletin Trimestriel de la Société Géologique de Normandie et des Amis du Muséum du Havre 73: 13–79.
de Gibert, J.M., Domènech, R., and Martinell, J. 2007. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro)bioerosion ichnofacies model. Acta Palaeontologica Polonica 52: 738–798.
Goldfuss, A. 1826–1844. Petrefacta Germaniae. Vol. 1 (1826–1833) viii+ 252 pp.; Vol. 2 (1834–1840) iii+312 pp.; Vol. 3 (1841–1844) iv+128 pp. Arnz and Co., Dusseldorf.
Guéranger, E. 1867. Album paléontologique du département de la Sarthe, représentant au moyen de la photographie les fossiles recueillis dans cette circonscription. 20 pp. De Beauvais et Vallienne, Le Mans.
Guillier, A. 1886. Géologie du Département de la Sarthe. 430 pp. Monnoyer, Le Mans.
von Hagenow, F. 1840. Monographie der Rügenschen Kreideversteinerungen, II. Radiarien und Annulaten, nebst Nachträgen zur ersten Abtheilung. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 1840: 629–672.
Juignet, P. 1974. La Transgression crétacée sur la bordure Orientale du Massif Armoricain. Aptien, Albien, Cénomanien de Normandie et du Maine. Le Stratotype du Cénomanien. 806 pp. Thèse de Doctorat d’État, University Caen, Caen [CNRS AO 9.643].
Juignet, P. 1980. Cénomanien. In: C. Mégnien and F. Mégnien (eds.), Synthèse géologique du Bassin de Paris, Vol. 1: Stratigraphie et paléogéographie. Mémoire BRGM 101: 292–297.
Kabat, A.R. 1990. Predatory ecology of naticid gastropods with review of shell boring predation. Malacologia 32: 155–193.
Kaim, A., Jenkins, R.G., Tanabe, K., and Kiel, S. 2014. Mollusks from late Mesozoic seep deposits, chiefly in California. Zootaxa 3861: 401–440. Crossref
Kase, T. and Ishikawa, M. 2003. Mystery of naticid predation history solved: evidence from a “living fossil” species. Geology 31: 403–406. Crossref
Kelley, P.H. and Hansen, T.H. 2003. The fossil record of drilling predation on bivalves and gastropods. In: P.H. Kelley, M. Kowalewski, and T.H. Hansen (eds.), Predator—Prey Interactions in the Fossil Record. Topics in Geobiology 20: 113–139. Plenum Press, New York
Kelly, S.R.A. and Bromley, R.G. 1984. Ichnological nomenclature of clavate borings. Palaeontology 27: 793–807.
Kiel, S., Campbell, K.A., Elder W.P., and Little C.S.T. 2008. Jurassic and Cretaceous gastropods from hydrocarbon seeps in forearc basin and accretionary prism settings, California. Acta Palaeontologica Polonica 53: 679–703. Crossref
King, P.P. and Broderip, W.J. 1835. Description of the Cirrhipeda, Conchifera and Mollusca, in a collection formed by the Officers of H.M.S. Adventure and Beagle employed between years 1826 and 1830 in surveying the Southern Coasts of South America, including the Straits of Magalhaens and the Coast of Tierra del Fuego. The Zoological Journal 5: 332–349.
Kočí, T., Jäger, M., and Morel, N. 2016. Sabellid and serpulid worm tubes (Polychaeta, Canalipalpata, Sabellida) from the historical stratotype of the Cenomanian (Late Cretaceous; Le Mans region, Sarthe, France). Annales de Paléontologie [published online].
Kollmann, H.A. 2015. Les gastéropodes. In: N. Morel (ed.), Stratotype Cénomanien. Patrimoine géologique 6, 172–178, CD: 64–69. Muséum national d’Histoire naturelle, Paris.
Lamarck, J.B. 1801. Système des animaux sans vertèbres ou tableau général des classes, des ordres et des genres de ces animaux. 432 pp. Déterville, Paris.
Lamarck, J.B. 1806. Mémoire sur les fossiles des environs de Paris. Annales du Muséum d’Histoire naturelle, Paris 8: 156–166.
Lamarck, J.B. 1819. Histoire naturelle des animaux sans vertèbres, présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres et la citation des principales espèces qui s’y rapportent. Vol. 6. 343 pp. Verdière, Paris.
Lamouroux, J.V.F. 1821. Exposition méthodique des genres de l’ordre des polypiers avec leur description et celles des principales espèces figurées dans 74 planches, les 63 premières appartenant à l’histoire naturelle des Zoophytes d’Ellis et Solander. 115 pp. Mme Veuve Agasse, Paris.
Lopes, R.P. 2011. Ichnology of fossil oysters (Bivalvia, Ostreidae) from the southern Brazilian coast. Journal of Geosciences 7: 94–103. Crossref
MacEachern, J.A., Pemberton, S.G., Gingras, M.K., and Bann, K. 2007. The ichnofacies paradigm: a fifty-year retrospective. In: W. Miller III (ed.), Trace Fossils. Concept, Problems, and Prospects, 52–77. Elsevier, Amsterdam.
Mägdefrau, K. 1937. Lebensspuren fossiler “Bohr”-Organismen. Beiträge zur Naturkundlichen Forschung in Südwestdeutschland 2: 54–67.
Matsukuma, A. 1978. Fossil boreholes made by shell-boring predators or commensals. Part 1: Boreholes of capulids gastropods. Venus, Japan Journal of Malacology 37: 29–45.
Matteucci, R., Manni, R., and Di Bella, L. 2012. Echinoid grazing traces on ostreid shell-ground from the Pliocene Ficulle quarry (central Italy). Journal of Mediterranean Earth Science 4: 71–79.
Néraudeau, D., Pineau, J.-P., and Dudicourt, J.-C. 2015. Les oursins cénomaniens de l’Ouest de la France (Sarthe et Charentes). In: N. Morel (ed.), Stratotype Cénomanien. Patrimoine Géologique 6, 209–228. Muséum national d’Histoire naturelle, Paris.
Neumann, C. and Wisshak, M. 2009. Gastropod parasitism on Late Cretaceous to Early Paleocene holasteroid echinoids. Evidence from Oichnus halo isp. n. Palaeogeography, Palaeoclimatology, Palaeoecology 284: 115–119. Crossref
Neumann, C., Wisshak, M., Aberhan, M., Girod, P., Rösner, T., and Bromley, R.G. 2015 Centrichnus eccentricus revisited. A new view on anomiid bivalve bioerosion. Acta Palaeontologica Polonica 60: 539–549.
Obert, D. 1988. Notice explicative de la feuille Bouloire. Carte géologique de la France à 1/50000, 359. 32 pp. BRGM éd., Orléans.
d’Orbigny A. 1842–1843. Paléontologie française. Description des Mollusques et Rayonnés fossiles de France. Terrains Crétacés. Tome II: Gastéropodes. Texte (1842): 456 pp. Atlas (1842–1843): pls. 149–236. Arthus Bertrand, Paris.
d’Orbigny, A. 1850. Prodrome de paléontologie stratigraphique universelle des animaux Mollusques et Rayonnés, Volume 1. ix + 392 pp. Victor Masson, Paris.
d’Orbigny, A. 1850–1852. Paléontologie française. Description des Mollusques et Rayonnés fossiles. Terrains crétacés, tome cinquième. Bryozoaires. 1192 pp. Victor Masson, Paris.
Orr, V. 1963. The drilling habit of Capulus danieli (Crosse) (Mollusca: Gastropoda). The Veliger 5: 63–67.
Portlock, J.E. 1843. Report on the Geology of the County of Londonderry and of Parts of Tyrone and Fermanagh. xxxi + 748 pp. Her Majesty’s Stationery Office, Dublin.
Pulteney, R. 1813. Catalogues of the Birds, Shells and Some of the More Rare Plants of Dorsetshire from the New Enlarged Edition of Mr. Hutchin’s History of That County. 92 pp. J. Nichols, London.
Reeve, L.A. 1852–1853. Monograph of the Genus Pecten. Conchologia Iconica, Vol. 8. 35 pp. Published by the author, London.
Ruggiero, E. and Raia, P. 2014. Oichnus taddei, a new fossil trace produced by capulids on brachiopod shells. Spanish Journal of Palaeontology 29: 15–24.
de Saint-Seine, R. 1956. Les cirripèdes acrothoraciques échinicoles. Bulletin de la Société Géologique de France 5: 299–303.
Santos, A., Mayoral, E., and Muñiz, F. 2003. New trace fossils produced by etching molluscs from the Upper Neogene of the Southern Iberian Peninsula. Acta Geologica Polonica 53: 181–188.
Santos, A., Mayoral, E., and Muñiz, F. 2005. Bioerosion scars of acorn barnacles from the southwestern Iberian Peninsula, Upper Neogene. Rivista Italiana di Paeontologia e Statigrafia 111: 181–189.
de Sowerby, J.C. 1829. The Mineral Conchiology of Great Britain, Part 1. vii + 234 pp. B. Meredith et al., London.
Stenzel, H.B. 1971. Oysters. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part N, Volume 3: Mollusca 6, Bivalvia, N953–1224. The Geological Society of America, Boulder.
Vermeij, G.J. 1998. Sabia on shells: a specialized pacific-type commensalism in the carribean Neogene. Journal of Paleontology 72: 465–472. Crossref
Videt, B. 2004. Dynamique des paléoenvironnements à huitres du Crétacé Supérieur nord-aquitain (SO France) et du Mio-Pliocène andalou (SE Espagne): biodiversité, analyse séquentielle, biogéochimie. Mémoires Géosciences Rennes 108: 1–261.
Voigt, E. 1965. Über parasitische Polychaeten in Kreide-Austern sowie einige andere in Muschelschalen bohrende Würmer. Paläontologische Zeitschrift 39: 193–211. Crossref
Voigt, E. 1975. Tunnelbaue rezenter und fossilier Phoronidea. Paläontologisches Zeitschrift 49: 135–167.
Wisshak, M., Kroh, A., Bertling, M., Knaust, D., Nielsen, J.K., Jagt, J.W.M., Neumann, C., and Nielsen, K.S.S. 2015. In defence of an iconic ichnogenus—Oichnus Bromley, 1981. Annales Societatis Geologorum Poloniae 85: 445–451. Crossref