

A new Beneziphius
beaked whale from the ocean floor off Galicia, Spain and
biostratigraphic
reassessment of the type species
ISMAEL MIJÁN, STEPHEN LOUWYE, and OLIVIER LAMBERT
Miján, I., Louwye, S., and Lambert, O. 2017. A new Beneziphius beaked whale from the ocean floor off Galicia, Spain and biostratigraphic reassessment of the type species. Acta Palaeontologica Polonica 62 (1): 211–220.
Although the fossil record of beaked whales (Cetacea, Odontoceti, Ziphiidae) is continuously improving, the geological age of new taxa is often poorly constrained. Based on a partial cranium from deep seafloor deposits off Galicia, Spain, we describe a new species of the stem beaked whale genus Beneziphius, B. cetariensis sp. nov. The latter differs from the type species B. brevirostris in the larger size, the rostrum being proportionally longer, the premaxillae being longer than the maxillae at the apex of the rostrum, the left premaxillary sac fossa being transversely concave, and the ascending process of the premaxilla reaching the vertical. Considering that the stratigraphic context of deep-sea deposits off the Iberian Peninsula is not precisely known, we provisionally propose an extended, middle Miocene to early Pliocene interval for the geological age of B. cetariensis. Nonetheless, the palynological analysis of sediment sampled from cavities in the cranium of the holotype of B. brevirostris, discovered during the second part of the nineteenth century in inland deposits of Antwerp (north of Belgium), yield an early to mid-Serravallian age (13.2–12.8 Ma, late middle Miocene). B. brevirostris is thus the oldest described species of the “Messapicetus clade”, a large clade of stem ziphiids in which most species are dated from the late Miocene. The description of the Galician species B. cetariensis broadens the biogeographic distribution of Beneziphius and confirms the strong ziphiid faunal affinities between the eastern coast of the North Atlantic and the southern margin of the North Sea Basin.
Key words: Mammalia, Cetacea, Ziphiidae, Miocene, North Atlantic, North Sea, Iberian Peninsula.
Ismael Miján [ismaelmijan@gmail.com], Sociedade Galega de Historia Natural, Praza de Canido s/n, E-15401 Ferrol, Spain.
Stephen Louwye [stephen.louwye@ugent.be], Research Unit Palaeontology, Department Geology and Soil Science, Ghent University, 281 Krijgslaan S8, B-9000 Ghent, Belgium.
Olivier Lambert [olivier.lambert@naturalsciences.be] (corresponding author), Institut royal des Sciences naturelles de Belgique, D.O. Terre et Histoire de la Vie, rue Vautier 29, B-1000 Brussels, Belgium.
Received 15 September 2016, accepted 9 November 2016, available online 8 December 2016.
Copyright © 2017 I. Miján et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The family Ziphiidae (beaked whales) is the second most diverse among cetaceans with more than 22 extant species (Dalebout et al. 2014; Morin et al. 2016). These deep-diving odontocetes (echolocating toothed whales) are characterized by a series of morphological features, including dental reduction, elevation of the vertex of the skull, enlargement of the pterygoid sinuses, and various skull specializations, some of them sexually dimorphic, both at the level of external morphology and bone histology, that stimulate debate about functional interpretations (Heyning 1984; Buffrénil et al. 2000; McLeod 2002; Lambert et al. 2011; Gol’din 2014). During the last years the ziphiid fossil record increased significantly, especially due to the discovery of many specimens on the seafloor, at great depth (Bianucci et al. 2007, 2013; see also Ramassamy 2016 for a new ziphiid from inland deposits). These deep-sea fossils generally consist of isolated rostra and partial skulls including the vertex, facial area, and rostrum.
This paper adds a new species to the list of four new fossil beaked whale species described based on cranial material from the seafloor off the Iberian Peninsula (Bianucci et al. 2013). This is also the first record of the stem ziphiid genus Beneziphius Lambert, 2005 outside the North Sea area.
The geological age of fossils from deep-sea deposits is unfortunately generally poorly constrained (Bianucci et al. 2007, 2013; but see Antunes et al. 2015). Furthermore, many ziphiid remains, including the type series of Beneziphius brevirostris Lambert, 2005, collected in the area of Antwerp (north of Belgium) during the second part of the nineteenth century could not be precisely dated up to now, due to limited information about localities and horizons (Lambert 2005). Therefore, we extracted a sediment sample from cavities in the rostrum of the holotype of B. brevirostris for palynological analysis, finding an early to mid-Serravallian age for the sediment.
Institutional abbreviations.—IRSNB, Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium; ML, Museu da Lourinhã, Lourinhã, Portugal; SGHN, Museo da Natureza da Sociedade Galega de Historia Natural, Ferrol, Spain.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank. lsid:zoobank.org:pub: C1885545-298D-42C9-B847-C616240B273C; urn:lsid:zoobank.org:act:6985C25B-2988-4A0C-9755-C06ABCD37188.
Material and methods
We examined a partial ziphiid skull, SGHNMF MA0953, recovered from the seafloor off the northwest of the Iberian Peninsula, on the Ortegal Spur, taken by bottom trawling in the fishing ground known as A Selva (see Bianucci et al. 2013 for more details). The fossil is strongly phosphoritized, similar to those described in previous works on deep-sea ziphiids (Bianucci et al. 2007, 2013). This specimen was compared to fossil ziphiid taxa from the Neogene of the North Sea curated at the IRSNB and from the North Atlantic seafloor off the Iberian Peninsula curated at the SGHN and ML.
A total of 5.23 g of sediment was extracted from the open mesorostral groove of the holotype skull of Beneziphius brevirostris IRSNB ED002-M.1885, Antwerp area, Belgium. The sediment sample was prepared for palynological analysis following a standard maceration technique as described by Quaijtaal et al. (2014).
For the cranial features we follow the terminology in Mead and Fordyce (2009).
Systematic palaeontology
Order Cetacea Brisson, 1762
Clade Pelagiceti Uhen, 2008
Clade Neoceti Fordyce and Muizon, 2001
Suborder Odontoceti Flower, 1867
Family Ziphiidae Gray, 1850
“Messapicetus clade” sensu Bianucci et al. 2016
Genus Beneziphius Lambert, 2005
Type species:
Beneziphius brevirostris Lambert, 2005; from
the Antwerp area (Belgium, southern margin of the North Sea Basin),
early to mid-Serravallian (Lambert 2005; this study).
Species included: Type species and Beneziphius
cetariensis sp. nov.
Emended diagnosis.—Beneziphius differs from all other members of the family in the following unique combination of characters: ankylosed thickened premaxillae dorsally roofing the mesorostral groove for most of its length (as in Choneziphius, Messapicetus, Ziphirostrum, and other members of the “Messapicetus clade” sensu Bianucci et al. 2016); presence of a moderately excavated prenarial basin; presence of a medial gap between premaxillary sac fossae; right premaxillary sac fossa barely transversely concave (the three latter characters being major differences with Choneziphius); dorsal surface of the maxillae lateral to the prenarial basin covered by excrescences (as in Choneziphius); premaxillary crests on the vertex being anterolaterally directed (as in Ziphiinae sensu Bianucci et al. 2016 and members of the “Messapicetus clade”); nasals being not anteriorly longer than the premaxillary crests (differing from Ziphiinae); and nasals lacking an anteromedial excavation (differing from Hyperoodontinae sensu Bianucci et al. 2016).
Beneziphius cetariensis sp. nov.
Figs. 1–4.
Etymology: From Latin cetarius, fishmonger; in reference to the Galician city Cedeira (Latin Cetaria), where many fishermen involved in the discovery of offshore fossil ziphiids come from.
Holotype: SGHNMF MA0953, a partial skull including rostrum, facial area (without the supraorbital regions) and part of the vertex.
Type locality: A Selva fishing ground, depth of approximately 500 m, off the Galician coast; approximate geographic coordinates: 44°10’ N, 8°40’ W.
Type horizon: Poorly constrained, middle Miocene to early Pliocene (see remarks below).
Diagnosis.—Beneziphius cetariensis differs from B. brevirostris in: the larger size; the rostrum being proportionally longer; the premaxillae being longer than the maxillae at the apex of the rostrum; the left premaxillary sac fossa being transversely concave; and the ascending process of the premaxilla reaching the vertical, with the posterodorsal portion of the ascending process slightly overhanging the bony nares.
Table 1. Measurements (in mm) of the cranium of
Beneziphius cetariensis sp. nov. holotype
(SGHNMF MA0953) compared to B. brevirostris.
e, estimate, + incomplete, – missing data. Part of the measurements
for B. brevirostris taken from Lambert (2005).
| |
Beneziphius cetariensis |
Beneziphius brevirostris |
|
|
SGHNMF MA0953 IRSNB ED002-M.1885 (holotype) |
|
IRSNB 3782-M.1886 |
|
|
Length of maxilla on rostrum |
e350 |
e295 |
– |
|
Maximum width of premaxillae on rostrum |
43 |
e40 |
43 |
|
Width of rostrum at base |
– |
– |
e127 |
|
Width of prenarial basin at level
of mid-length |
38 |
24 |
21.5 |
|
Width of premaxillary sac fossae |
117 |
111 |
+87 |
|
Maximum width right premaxillary sac fossa |
e74 |
56 |
– |
|
Maximum width left premaxillary sac fossa |
e51 |
39 |
38 |
|
Minimum transverse width right ascending process of premaxilla |
34 |
30 |
– |
|
Width of bony nares |
e57 |
53 |
– |
|
Width across transverse premaxillary crests |
e155 |
122 |
– |
|
Width of right premaxillary crest |
62 |
37 |
– |
|
Maximum total width of nasals |
e94 |
71 |
– |
|
Length of suture between nasals |
50 |
31 |
– |
|
Minimum distance between maxillae across vertex |
e67 |
57 |
– |
Description.—The nearly complete rostrum of this medium-size ziphiid is relatively short, less than 60% of the estimated condylobasal length (see Table 1 for cranial dimensions), but proportionally longer than in Beneziphius brevirostris (ratio between width of premaxillary sac fossae and length of maxilla on rostrum estimated to 0.33 and 0.38 in the holotypes of B. cetariensis and B. brevirostris, respectively). Transverse sections of the rostrum are higher than wide for the anterior half of rostrum length. The vertex of the skull is proportionally elevated and somewhat anterodorsally projected.
Premaxilla: Anteriorly, the premaxilla is longer than the maxilla, forming alone the tip of the rostrum for more than 50 mm (Figs. 1, 2), a difference with Beneziphius brevirostris and Choneziphius planirostris (both latter taxa having the maxilla reaching the end of the rostrum). In this anterior region, the lateral surface of the bone is pierced by a large foramen opening forwards. The thickened premaxillae are sutured to each other above a large part of the mesorostral groove; their suture is ankylosed for most of its extent (Fig. 1A). In lateral view, the maximum elevation of the premaxillae is approximately at half the rostrum length. The premaxillae are separated anteriorly for 120 mm, revealing an open mesorostral groove (transverse diameter 12 mm) as observed in B. brevirostris, Choneziphius leidyi, Messapicetus, and Ziphirostrum. Backwards, the premaxillae diverge abruptly 30 mm anterior to the premaxillary foramina, forming the U-shaped anterior limit of the moderately excavated prenarial basin. The prenarial basin is shallower than in Ziphirostrum marginatum, with a dorsoventral extent closer to Z. turniense and B. brevirostris.
The large premaxillary foramen is distinctly anterior to the level of the lost antorbital notch, at the bottom of the thick maxillary lateral wall of the prenarial basin; the foramen is followed posteriorly by a wide and deep groove (probably partly homologous to the posterolateral sulcus), which widens progressively backwards towards the anterior part of the corresponding premaxillary sac fossa. The right groove widens more than the left, leading to a wider right premaxillary sac fossa (ratio between maximum widths of left and right fossae 0.69). In addition to asymmetric dimensions, the left fossa is transversely concave (but not as much as in Choneziphius spp.), whereas the right fossa is barely excavated. As in B. brevirostris, the lateral margin of the left premaxillary sac fossa overhangs somewhat the adjoining maxilla, a condition not as developed as in Choneziphius, Globicetus, Imocetus, Izikoziphius, and Ziphius. The two premaxillary sac fossae are widely separated medially, a major difference with Choneziphius spp.
In anterior view, the right ascending process of the premaxilla is markedly transversely constricted (Fig. 3). The better-preserved right premaxillary crest is thin and nearly rectilinear in dorsal view (Figs. 1A, 4); it is directed anterolaterally and slightly laterally in its outer portion. This outer portion of the crest overhangs the ascending process, a condition related to the vertex being projected anterodorsally, more so than in B. brevirostris and Z. marginatum. Due to some degree of wear in the vertex region, the presence/absence of a contact between the posterior projection of the premaxilla and the frontal is difficult to assess.
Maxilla: The maxilla is visible in dorsal view for most of the length of the rostrum (Fig. 1A). This dorsal exposure increases progressively along the posterior two thirds of the rostrum. The oblique dorsolateral surface is slightly transversely concave. Although partly worn, multiple excrescences cover this surface, probably for the origin of rostral muscles in a way similar to Beneziphius brevirostris and Choneziphius spp. (though with a high intraspecific variation in C. planirostris; Lambert 2005; Bianucci et al. 2013). From a level shortly anterior to the premaxillary foramen, the lateral margin of the rostrum raises posterodorsolaterally, increasing the depth of the prenarial basin. At this level, the dorsomedial surface of the maxilla is barely transversely convex, much less than in Z. marginatum.
A posterior dorsal infraorbital foramen is preserved on the left side, partly overhung by the lateral margin of the left premaxillary sac fossa. Posterior to the ascending process of the premaxilla, the maxilla raises towards the vertex as a high wall; in dorsal view, this wall is much longer anteroposteriorly than in some species with a less anterodorsally projected vertex (e.g., C. planirostris and Z. marginatum). The minimum distance between the maxillae across the elevated vertex is lower than the maximum width of the nasals (Fig. 4).
The ventrolaterally facing alveolar groove is distinct and continuous in the maxilla until a level about 55 mm anterior to the premaxillary foramen (Figs. 1B, 2). Although the surface of the alveolar groove is lightly worn, undulations correspond to highly reduced alveoli. It is nevertheless not possible to determine the upper tooth count. Shallow alveoli are similarly observed in B. brevirostris, likely corresponding to small, non-functional teeth. At half the rostrum length, a foramen pierces the maxilla just dorsal to the alveolar groove; it is followed anteriorly by a deep sulcus.
Ventrally, a large foramen is present in the maxilla at the anterior end of the ventral exposure of the vomer (Fig. 2B). Additionally, a major palatine foramen is anteromedial to each pterygoid sinus fossa.
Vomer: On the ventral surface of the rostrum, the vomer is exposed as a thin stripe (maximum breadth 4.5 mm) for a length of 88 mm between the maxillae. At the level of the pterygoid sinus fossae, the exposure of the vomer between the palatines is artificially increased due to heavy wear of the palate region.
Nasal: In dorsal view, the joined nasals form a large, roughly triangular area of the vertex (Figs. 1A, 4). Each nasal is anteromedially pointed and longer than transversely wide. The anterior tip of the nasal nearly reaches the same anteroposterior level as the right premaxillary crest, only being slightly posterior. The left nasal is longitudinally shorter than the right; the suture with the corresponding frontal is shifted forwards compared to the right side. In addition, the suture between nasals is shifted to the left and directed anterolaterally towards the left side. The dorsal surface of the nasals is depressed medially and the top of the right nasal is markedly higher than the top of the left nasal (both features best seen in anterior view; Fig. 3). The posterolateral margin of the nasal displays a broad contact with the corresponding premaxillary crest.
Frontal: On the vertex, only the anteriormost portion of the frontals, along the nasals, is preserved. Therefore, we cannot assess the longitudinal extent of the frontals and the location of the frontal-supraoccipital suture. Considering the preserved maxillae along the lateral walls of the vertex, the minimum width of each frontal was much smaller than the maximum width of each nasal (Fig. 4).
Pterygoid/palatine: The palatine-maxilla suture could not be observed on the partly worn palate, and the palatine is only detected as a slightly thickened region anterior to the pterygoid. Part of the pterygoid-palatine suture is visible, turning along the anterolateral margin of the pterygoid sinus fossa in an anteromedial and then posteromedial direction (Fig. 1B, 2). The maximum longitudinal distance between the anterior margin of the pterygoid sinus fossa and the pterygoid-palatine suture is 15 mm. The wide pterygoid sinus fossa extends anteriorly to the level of the premaxillary foramen (distinctly anterior to the level of the antorbital notch).
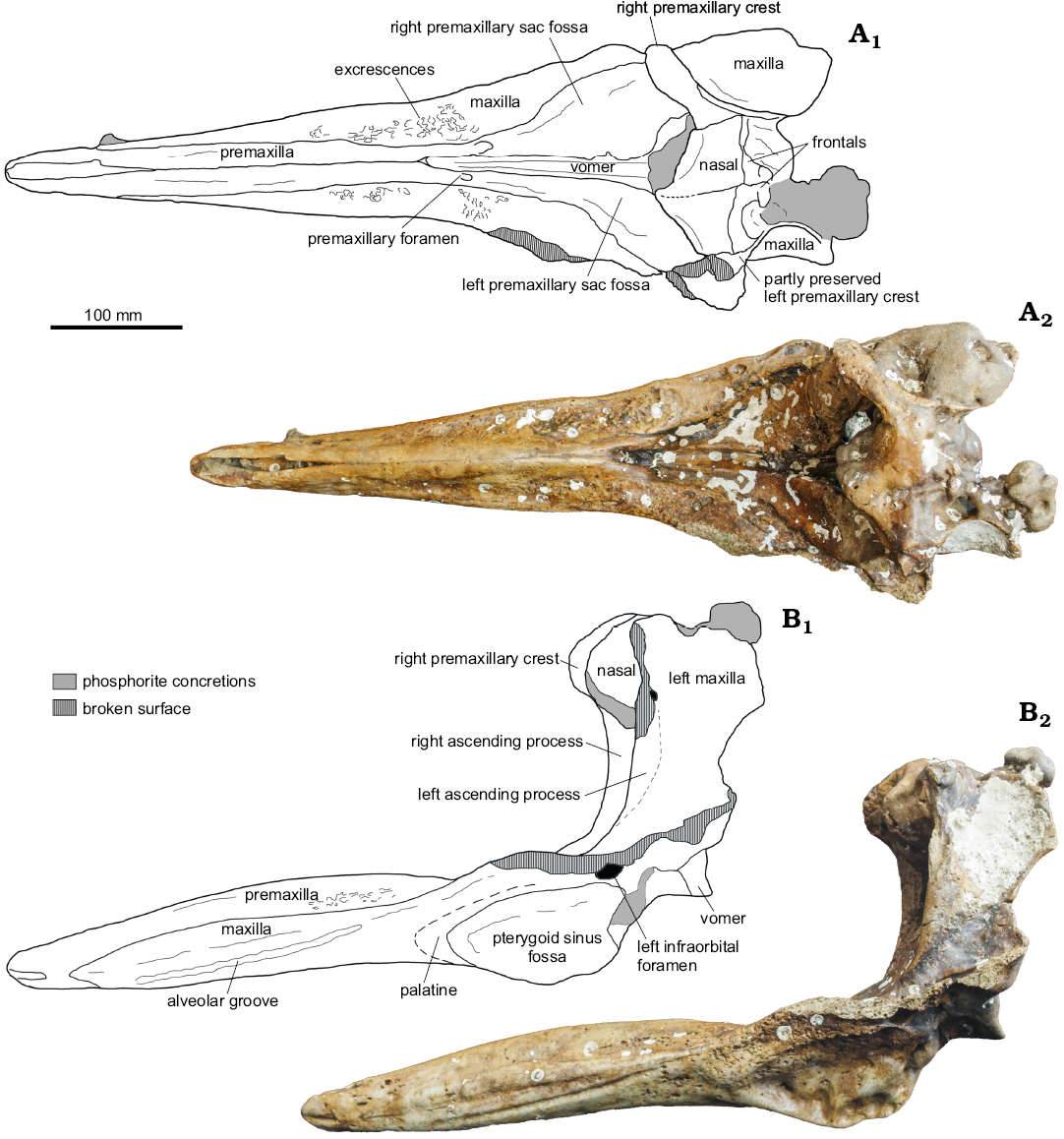
Fig. 1. Partial cranium of a beaked whale Beneziphius cetariensis sp. nov., holotype (SGHNMF MA0953) from the middle Miocene to early Pliocene of A Selva fishing ground, off the Galician coast, Spain, in dorsal (A) and left lateral (B) views. Interpretive drawings (A1, B1) and photographs (A2, B2).

Fig. 2. Partial cranium of a beaked whale Beneziphius cetariensis sp. nov., holotype (SGHNMF MA0953) from the middle Miocene to early Pliocene of A Selva fishing ground, off the Galician coast, Spain, in right lateral (A) and ventral (B) views.
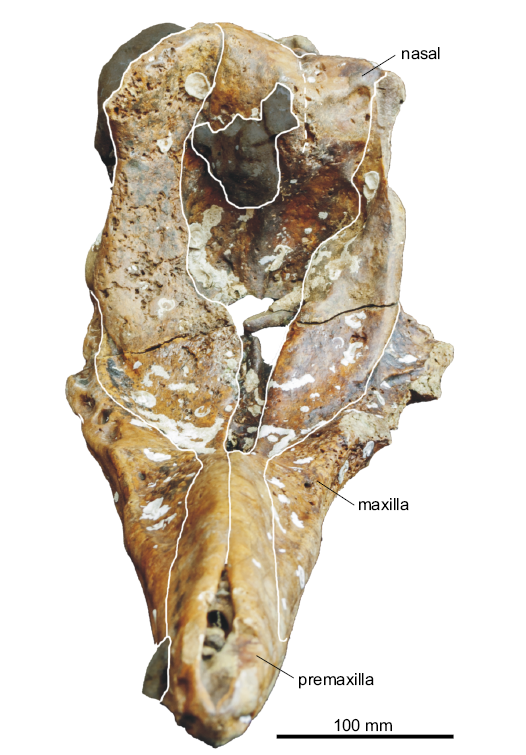
Fig. 3. Partial cranium of a beaked whale Beneziphius cetariensis sp. nov., holotype (SGHNMF MA0953) from the middle Miocene to early Pliocene of A Selva fishing ground, off the Galician coast, Spain, in anterior and slightly dorsal view.
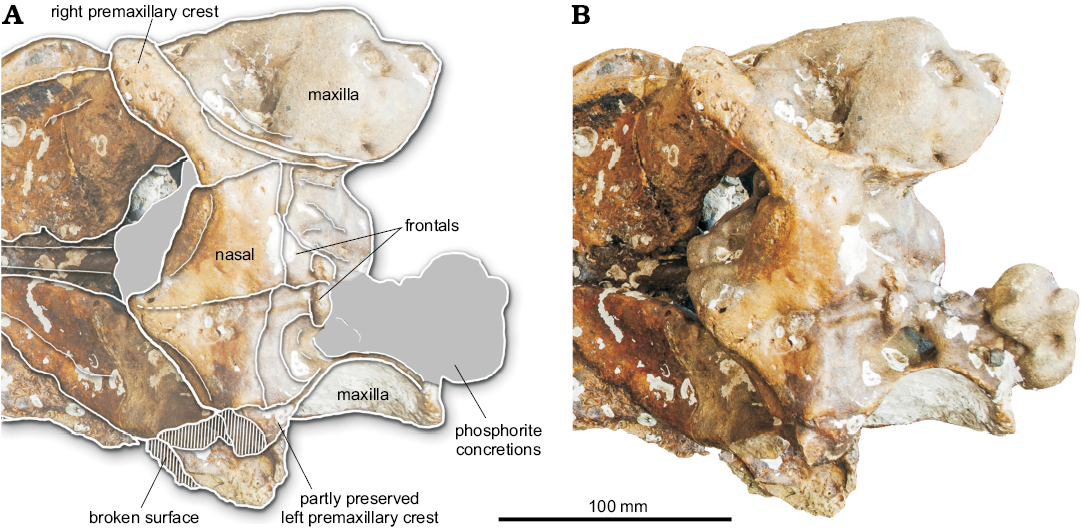
Fig. 4. Vertex of the partial cranium of a beaked whale Beneziphius cetariensis sp. nov., holotype (SGHNMF MA0953) from the middle Miocene to early Pliocene of A Selva fishing ground, off the Galician coast, Spain, in dorsal view. Interpretive drawing (A) and photograph (B).
Remarks.—Unfortunately no sediment was preserved with the specimen SGHNMF MA0953 (recovered by trawling); no micropalaeontological analysis could thus be performed.This specimen was found in the same locality as part of the ziphiids described by Bianucci et al. (2013), in the area of Ortegal Spur; specimens of Choneziphius leidyi and Tusciziphius atlanticus originate from the same area and possibly from the same layers. The stratigraphic context of this deep submarine region is unfortunately poorly known, with Oligo-Miocene marl and conglomerate irregularly covered with Plio-Pleistocene deposits. Associated with phosphorite pebbles, the skulls may correspond to a late early to middle Miocene phosphogenesis episode (Bianucci et al. 2013). However, specimens from a deep-sea locality off Portugal sharing several ziphiid species with the Galician assemblage were recently biostratigraphically dated from the latest Messinian to early Pliocene (Antunes et al. 2015). Furthermore, though the type species of Beneziphius is dated from the late middle Miocene (early to mid-Serravallian, Antwerp area, Belgium; this study), other species of the genera Choneziphius and Tusciziphius are reported from younger, late Miocene and early Pliocene deposits from Belgium and Italy (Bianucci 1997; Lambert 2005). Therefore, it seems more careful to provisionally retain a broad, middle Miocene to early Pliocene stratigraphic range for the Galician ziphiids of Ortegal Spur, pending a much-needed biostratigraphical or radiometric dating of specimens.
Stratigraphic and geographic range.—Type locality only.
Results
Phylogeny.—To investigate the phylogenetic relationships of Beneziphius cetariensis, we coded SGHNMF MA0953 in a recently published morphological matrix of fossil and extant ziphiids (Bianucci et al. 2016; codings for SGHNMF MA0953 in Appendix 1). With all characters equally weighted, the heuristic search produced 57 most parsimonious trees, with tree length 168, CI 0.49, and RI 0.78. In the strict consensus tree, the relationships of B. cetariensis with B. brevirostris and a clade including Choneziphius, Globicetus, Imocetus, and Tusciziphius are not resolved (detail of “Messapicetus clade” in Fig. 5A); in 38 shortest trees B. cetariensis is more stemward than B. brevirostris, whereas the latter is more stemward in the last 19 shortest trees. Following the same method as Bianucci et al. (2016), we downweighted homoplastic characters (Goloboff 1993; constant k = 3) and obtained a single most parsimonious tree. This tree follows the topology of the tree obtained by Bianucci et al. (2016: fig. 13), with the addition of B. cetariensis stemward to B. brevirostris in a paraphyletic genus Beneziphius (detail of “Messapicetus clade” in Fig. 5B).
Reassessment of the geological age of Beneziphius brevirostris.—Due to the absence of data on their precise locality and horizon, the holotype and the referred specimen of Beneziphius brevirostris from the Antwerp area, Belgium, were given a Neogene age range in the original publication (Lambert 2005).
The microscopic palynological analysis of the sediment sample extracted from the skull of the holotype IRSNB ED002-M.1885 revealed a rich and diverse assemblage; a total of 46 dinoflagellate cysts and eight acritarch species were recorded (see Appendix 2). The preservation of the palynomorphs is poor to moderate, with lots of torn and thus partial cysts, and the number of well-preserved dinoflagellate cysts (i.e., not folded) is limited. As a consequence the analysis comprised extended scanning under a transmitted light microscope. The presence of pre-Neogene reworked dinoflagellates was noted.
The relative dating of this sediment sample with dinoflagellate cysts relies on two key species with biostratigraphic value. The dinoflagellate cyst Operculodinium? borgerholtense has a restricted range from the late Burdigalian (Chron C5Cn2r; 16.5 Ma) to the mid-Serravallian (Chron C5Ar1n; 12.7 Ma) (Soliman et al. 2009). Habibacysta tectata has a first occurrence in the mid-Langhian (14.2 Ma) in the high latitudes of the northern hemisphere (Iceland Sea; Schreck et al. 2012), and a first occurrence in the mid-Langhian at 14.2 Ma is also recorded at lower latitutes (Porcupine Basin, off southwestern Ireland; Quaijtaal et al. 2014). The sediment recovered from the bone has a mid-Langhian to mid-Serravallian age according to both above-mentioned species.
The age of the sample can be even further refined. With an early to mid-Serravallian age (13.2–12.8 Ma), the Achomosphaera andalousiensis Zone was defined by Dybkjær and Piasecki (2010) onshore and offshore Denmark as the interval from the common first occurrence of the eponymous species to the first occurrence of Gramocysta verricula, the latter species being absent in the assemblage of the analysed sediment sample. Diagnostic events in this zone are the last occurrence of Cerebrocysta poulsenii and Cleistosphaeridium placacanthum. Both latter species are recorded in the assemblage, together with A. andalousiens.
In summary, the sediment sample recovered from the holotype of B. brevirostris has an early to mid-Serravallian age following the biozonation by Dybkjær and Piasecki (2010).
Discussion and conclusions
Systematic affinities of SGHNMF MA0953.—The partial skull SGHNMF MA0953 lacks the deep anteromedial excavation of the nasals of Hyperoodontinae and the nodular protuberance formed by the interparietal or frontals in Berardiinae (Bianucci et al. 2007). It shares with Ninoziphius, members of the “Messapicetus clade” (sensu Bianucci et al. 2016; including among others Aporotus, Beneziphius, Choneziphius, Messapicetus, and Ziphirostrum), and Ziphiinae the anterolateral direction of the premaxillary crests on the vertex. A prenarial basin is similarly observed in members of the “Messapicetus clade” and the ziphiine Ziphius cavirostris; the prenarial basin is shallower in SGHN MA0953 than in Ziphirostrum marginatum and adult males of Z. cavirostris. Whereas Choneziphius lacks any prenarial basin and differs from SGHNMF MA0953 in the medial contact between right and left premaxillary sac fossae, it shares with the latter and Beneziphius brevirostris the presence of excrescences on the dorsal surface of the maxillae in the proximal rostral portion. Additionally, the dorsal roofing of the mesorostral groove by the thickened and ankylosed premaxillae observed in SGHNMF MA0953 is present in Beneziphius and Choneziphius, but also in most other members of the “Messapicetus clade” (premaxillae not ankylosed in Aporotus spp.), constituting the key synapomorphy of this large group (Bianucci et al. 2016). However, contrasting with B. brevirostris and Choneziphius, other members of the “Messapicetus clade” do not display any excrescences on the dorsal surface of the maxillae. Despite (i) the lack of resolution for the relationships of Beneziphius spp. in the phylogenetic analysis with equally-weighted characters, and (ii) the paraphyly of this genus in the analysis with downweighted homoplastic characters (Fig. 5B), we think that the unique combination of characters diagnosing Beneziphius (especially the highly distinctive morphology of the prenarial basin and rostrum base) is sufficient to support the referral of SGHNMF MA0953 to the same genus as B. brevirostris in the “Messapicetus clade”, pending the discovery of more complete specimens for both species.
SGHNMF MA0953 differs from B. brevirostris in its larger size, the proportionally longer rostrum with premaxillae being anteriorly longer than the maxillae, the transversely concave left premaxillary sac fossa, and the posterodorsal portion of the ascending process of the premaxilla slightly overhanging the bony nares. These differences are judged sufficient to allow the description of a new species. As in other extant and extinct odontocetes, the difference in rostrum length can be tentatively correlated to a different degree of specialization for suction feeding; short-snouted species with a reduced oral opening are generally considered as more efficient for suction (Werth 2006). A similar reasoning has been proposed for a pair of fossil pontoporiids from the late Miocene of Peru, one with a shortened rostrum and the other with a considerably longer rostrum, also grouped in a same genus (Lambert and Muizon 2013). Noteworthily, although demonstrating a high degree of specialization for suction feeding, the nearly edentulous extant ziphiids retain a proportionally long rostrum, restricting instead the gape with large gums and extensive lower lips (Werth 2006).
Lacking the vertex, a more fragmentary skull from the Galician coast (SGHNMF MA0936; Ziphiidae aff. Ziphirostrum sp.) shares some similarities with SGHNMF MA0953: dorsal roofing of the mesorostral groove by the thickened and ankylosed premaxillae, prenarial basin, and excrescences on the maxillae in the proximal part of the rostrum (Bianucci et al. 2013). However, it differs from the latter in its larger size, proportionally longer rostrum, narrower dorsal exposure of the premaxilla along the prenarial basin, absence of a transverse concavity on the left premaxillary sac fossa, and less abrupt elevation of the left ascending process of the maxilla towards the rostrum.
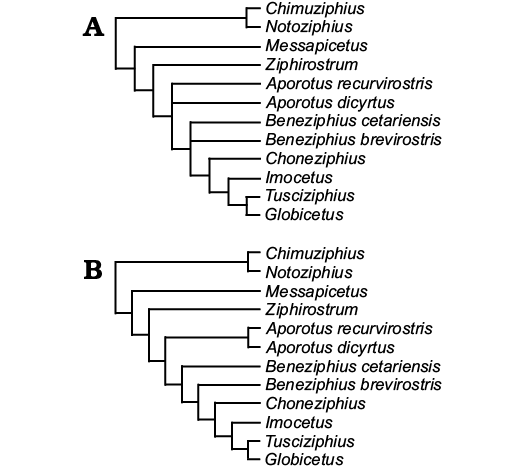
Fig. 5. Detail of the results of the phylogenetic analyses of the family Ziphiidae for the “Messapicetus clade”. A. Strict consensus of the heuristic search with equally-weighted characters, showing the unresolved relationships of Beneziphius cetariensis sp. nov. and B. brevirostris. B. Single most parsimonious tree of the heuristic search with downweighted homoplastic characters (k = 3), with B. cetariensis sp. nov. more stemward than B. brevirostris. Results for the other parts of the clade Ziphiidae follow Bianucci et al. (2016).
Geological range of Beneziphius and origin of the “Messapicetus clade”.—Except for the rostrum of an unnamed ziphiid most likely originating from middle Miocene deposits of the Calvert Formation, Maryland, USA (Lambert et al. 2010) and tentatively identified as a member of the “Messapicetus clade” due to the closure of the mesorostral groove by thickened premaxilla, all currently recognized members of the clade with stratigraphic information associated date from a late Miocene–early Pliocene interval (Bianucci et al. 2016; Ramassamy 2016). Biostratigraphically dated from the early to mid-Serravallian (late middle Miocene, 13.2–12.8 Ma), Beneziphius brevirostris is thus the oldest described species of this clade (Fig. 6), partly filling the gap between the bulk of the group and a probable early Miocene origin of the family Ziphiidae, as supported by an oldest ziphiid record dated from the Burdigalian (ca. 17 Ma; Wichura et al. 2015) and molecular divergence estimates for crown Ziphiidae ranging from 21.98 to 16.6 Ma (early Miocene; McGowen et al. 2009; Hassanin et al. 2012).
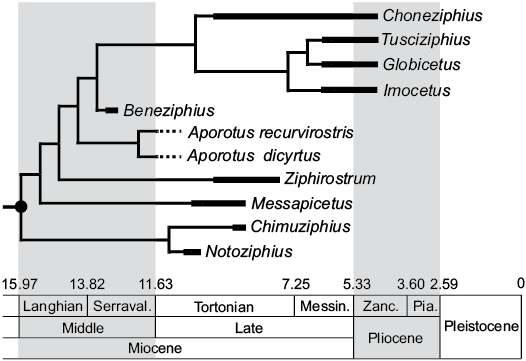
Fig. 6. Stratigraphically calibrated phylogenetic relationships within the stem ziphiid “Messapicetus clade” (black circle). Beneziphius is the oldest described member of the group, based on the biostratigraphic dating of sediment sampled from the holotype of Beneziphius brevirostris (early to mid-Serravallian, 13.2–12.8 Ma). The unresolved phylogenetic relationships of the two currently recognized species of Beneziphius are not illustrated (see comments in the text and Fig. 5). Messin., Messinian, Pia., Piazencian; Serraval., Serravallian; Zanc., Zanclean. Modified from Bianucci et al. (2016).
Biogeography.—The genus Beneziphius was previously only recorded from the southern margin of the North Sea Basin (Lambert 2005). The new record from the Atlantic Ocean off Galicia extends somewhat the biogeographic range of the genus (Fig. 7). Interestingly, the genus Choneziphius is similarly reported from both the North Sea Basin and the same locality off Galicia (A Selva) as SGHNMF MA0953 (Bianucci et al. 2013). Furthermore, as mentioned above a more fragmentary skull from A Selva (SGHNMF MA0936) was tentatively referred to Ziphirostrum, whereas another specimen from the Galician coast was identified as aff. Caviziphius sp.; genera Caviziphius and Ziphirostrum were both first described in the North Sea Basin (Bianucci and Post 2005; Lambert 2005; Bianucci et al. 2013). Highlighted in Fig. 7, these ziphiid faunal similarities at the genus level confirm a close connection between the North Sea and the Atlantic coast of the Iberian Peninsula during the middle Miocene to early Pliocene. Still, differences at the species level indicate either different geological ages for the faunas considered or/and different faunal compositions. Better-constrained ages for a large part of these species are much needed to allow more detailed analyses of the evolution of the relationships between North Atlantic and North Sea fossil ziphiid faunas.
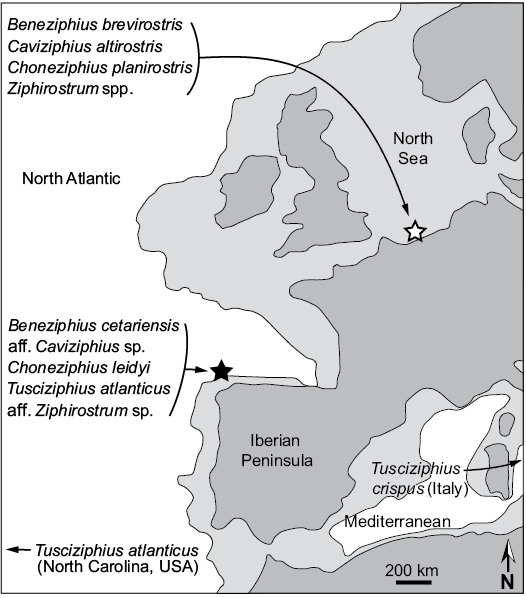
Fig. 7. Simplified palaeogeographic map of NW Europe, showing coastlines and the limits of the continental shelf (light grey) during the Serravallian–Tortonian (late middle to early late Miocene). Black star is the type locality of Beneziphius cetariensis sp. nov. (A Selva fishing ground) and white star is the type locality of B. brevirostris (Antwerp, Belgium). Other fossil ziphiid species are listed whose corresponding genera are found in at least two regions. Map redrawn from Scotese (2014). Note that the North Atlantic–Mediterranean connection was probably more complex than illustrated in this schematic map (e.g., Kouwenhoven and van der Zwaan 2006).
Acknowledgements
We are sincerely thankful the late Carmen Rodríguez and Gerardo González (Cedeira, Spain) for generously donating the specimen SGHNMF MA0953 studied here and the IES Punta Candieira for interceding with the donors. Thanks to Sébastien Bruaux and Georges Lenglet (both Institut royal des Sciences naturelles de Belgique, Brussels, Belgium), Octávio Mateus (Museu da Lourinhã, Portugal), James G. Mead and Charles W. Potter (both National Museum of Natural History, Washington DC, USA), and Adri Rol (Zoological Museum of Amsterdam, The Netherlands) for kindly providing access to the collections under their care, and to Giovanni Bianucci (Università di Pisa, Italy) and Klaas Post (Natuurhistorisch Museum Rotterdam, The Netherlands) for fruitful discussions about fossil ziphiid systematics, phylogeny, and biogeography. The two reviewers Giovanni Bianucci and James G. Mead provided useful comments that improved the quality of this work. Sabine Van Cauwenberghe (Ghent University, Ghent, Belgium) is thanked for assisting with the palynological maceration.
References
Antunes, M.T., Legoinha, P., and Balbino, A. 2015. Megalodon, mako shark and planktonic foraminifera from the continental shelf off Portugal and their age. Geologica Acta 13: 181–190.
Bianucci, G. 1997. The Odontoceti (Mammalia Cetacea) from Italian Pliocene. The Ziphiidae. Palaeontographia Italica 84: 163–192.
Bianucci, G. and Post, K. 2005. Caviziphius altirostris, a new beaked whale from the Miocene southern North Sea basin. Deinsea 11: 1–6.
Bianucci, G., Di Celma, C., Urbina, M., and Lambert, O. 2016. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ 4: e2479. Crossref
Bianucci, G., Lambert, O., and Post, K. 2007. A high diversity in fossil beaked whales (Odontoceti, Ziphiidae) recovered by trawling from the sea floor off South Africa. Geodiversitas 29: 5–62.
Bianucci, G., Mijan, I., Lambert, O., Post, K., and Mateus, O. 2013. Bizarre fossil beaked whales (Odontoceti, Ziphiidae) fished from the Atlantic Ocean floor off the Iberian Peninsula. Geodiversitas 35: 105–153. Crossref
Brisson, M.-J. 1762. Regnum Animale in classes IX distributum, sine synopsis methodica. 296 pp. Theodorum Haak, Paris.
Buffrénil, V. de, Zylberberg, L., Traub, W., and Casinos, A. 2000. Structural and mechanical characteristics of the hyperdense bone of the rostrum of Mesoplodon densirostris (Cetacea, Ziphiidae): summary of recent observations. Historical Biology 14: 57–65. Crossref
Dalebout, M.L., Baker, C.S., Steel, D., Thompson, K., Robertson, K.M., Chivers, S.J., Perrin, W.F., Goonatilake, M., Anderson, R.C., Mead, J.G., Potter, C.W., Thompson, L., Jupiter, D., and Yamada, T.K. 2014. Resurrection of Mesoplodon hotaula Deraniyagala, 1963: A new species of beaked whale in the tropical Indo-Pacific. Marine Mammal Science 30: 1081–1108. Crossref
Dybkjær, K. and Piasecki, S. 2010. Neogene dinocyst zonation for the eastern North Sea Basin, Denmark. Review of Palaeobotany and Palynology 161: 1–29. Crossref
Flower, W.H. 1867. Description of the skeleton of Inia geoffrensis and the skull of Pontoporia blainvillii, with remarks on the systematic position of these animals in the Order Cetacea. Transactions of the Zoological Society of London 6: 87–116. Crossref
Fordyce, R.E. and Muizon, C. de 2001. Evolutionary history of cetaceans: a review. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 169–233. Verlag Dr. Friedrich Pfeil, München.
Gol’din, P. 2014. “Antlers inside”: are the skull structures of beaked whales (Cetacea: Ziphiidae) used for echoic imaging and visual display? Biological Journal of the Linnean Society 113: 510–515. Crossref
Goloboff, P.A. 1993. Estimating character weights during tree search. Cladistics 9: 83–91. Crossref
Gray, J.E. 1850. Catalogue of the Specimens of Mammalia in the Collections of the British Museum. Part I Cetacea. 153 pp. Richard and John E. Taylor, London.
Hassanin, A., Delsuc, F., Ropiquet, A., Hammer, C., Jansen van Vuuren, B., Matthee, C., Ruiz-Garcia, M., Catzeflis, F., Areskoug, V., and Nguyen, T.T. 2012. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. Comptes Rendus Biologies 335: 32–50. Crossref
Heyning, J.E. 1984. Functional morphology involved in intraspecific fighting of the beaked whale, Mesoplodon carlhubbsi. Canadian Journal of Zoology 62: 1645–1654. Crossref
Kouwenhoven, T. and Van der Zwaan, G. 2006. A reconstruction of late Miocene Mediterranean circulation patterns using benthic foraminifera. Palaeogeography, Palaeoclimatology, Palaeoecology 238: 373–385. Crossref
Lambert, O. 2005. Systematics and phylogeny of the fossil beaked whales Ziphirostrum du Bus, 1868 and Choneziphius Duvernoy, 1851 (Cetacea, Odontoceti), from the Neogene of Antwerp (North of Belgium). Geodiversitas 27: 443–497.
Lambert, O. and Muizon, C. de 2013. A new long-snouted species of the Miocene pontoporiid dolphin Brachydelphis and a review of the Mio-Pliocene marine mammal levels in the Sacaco Basin, Peru. Journal of Vertebrate Paleontology 33: 709–721. Crossref
Lambert, O., Buffrénil, V. de, and Muizon, C. de 2011. Rostral densification in beaked whales: diverse processes for a similar pattern. Comptes Rendus Palevol 10: 453–468. Crossref
Lambert, O., Godfrey, S.J., and Fuller, A.J. 2010. A Miocene ziphiid (Cetacea: Odontoceti) from Calvert Cliffs, Maryland, USA. Journal of Vertebrate Paleontology 30: 1645–1651. Crossref
McGowen, M.R., Spaulding, M., and Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution 53: 891–906. Crossref
McLeod, C.D. 2002. Possible functions of the ultradense bone in the rostrum of Blainville’s beaked whale (Mesoplodon densirostris). Canadian Journal of Zoology 80: 178–184. Crossref
Mead, J.G. and
Fordyce, R.E. 2009. The therian skull: a lexicon with emphasis on the
odontocetes. Smithsonian Contributions to
Zoology 627: 1–248.
Crossref
Morin, P.A., Scott Baker, C., Brewer, R.S., Burdin, A.M., Dalebout, M.L., Dines, J.P., Fedutin, I., Filatova, O., Hoyt, E., and Jung, J.L. 2016. Genetic structure of the beaked whale genus Berardius in the North Pacific, with genetic evidence for a new species. Marine Mammal Science (published online).
Quaijtaal, W., Donders, T.H., Persico, D., and Louwye, S. 2014. Characterising the middle Miocene Mi-events in the Eastern North Atlantic realm: a first high-resolution marine palynological record from the Porcupine Basin. Palaeogeography, Palaeoclimatology, Palaeoecology 399: 140–159. Crossref
Ramassamy, B. 2016. Description of a new long-snouted beaked whale from the Late Miocene of Denmark: evolution of suction feeding and sexual dimorphism in the Ziphiidae (Cetacea: Odontoceti). Zoological Journal of the Linnean Society 178: 381–409. Crossref
Schreck, M., Matthiessen, J., and Head, M.J. 2012. A magnetostratigraphic calibration of Middle Miocene through Pliocene dinoflagellate cyst and acritarch events in the Iceland Sea (Ocean Drilling Program Hole 907A). Review of Palaeobotany and Palynology 187: 66–94. Crossref
Scotese, C.R. 2014. The PALEOMAP Project PaleoAtlas for ArcGIS, Version 2, Volume 1, Cenozoic Plate Tectonic, Paleogeographic, and Paleoclimatic Reconstructions. http://www.scotese.com.
Soliman, A., Head, M.J., and Louwye, S. 2009. Morphologogy and distribution of the Miocene dinoflagellate cysts Operculodinium? borgerholtense Louwye, 2001, emend. Palynology 33 (2): 73–84. Crossref
Uhen, M.D. 2008. New protocetid whales from Alabama and Mississippi, and a new cetacean clade, Pelagiceti. Journal of Vertebrate Paleontology 28: 589–593. Crossref
Werth, A.J. 2006. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. Journal of Mammalogy 87: 579–588. Crossref
Wichura, H., Jacobs, L.L., Lin, A., Polcyn, M.J., Manthi, F.K., Winkler, D.A., Strecker, M.R., and Clemens, M. 2015. A 17-My-old whale constrains onset of uplift and climate change in east Africa. Proceedings of the National Academy of Sciences 112: 3910–3915. Crossref
Appendix 1
Codings for Beneziphius
cetariensis SGHNMF MA0953 in the morphological matrix of
Bianucci et al. (2016).
Beneziphius cetariensis 20220 03221 ??110 0???? ????? ?1??2
01001 ????? ????? ???1? ?
Appendix 2
List of dinoflagellate cysts and acritarch species recorded in the sediment sample associated to the holotype of Beneziphius brevirostris IRSNB ED002-M.1885.
Dinoflagellate cysts
Achomosphaera andalousiensis
Barssidinium graminosoum
Barssidinium pliocenicum
Barssidinum sp.
Batiacasphaera minuta
Batiacasphaera complex
Bitectatodinium tepikiense
Corrudinium devernaliae
Cleistosphaeridium placacanthum
Cerebrocysta poulsenii
Dapsilidinium pseudocolligerum
Dinopterygium cladoides
Habibacysta tectata
Hystrichokolpoma rigaudiae
Hystrichosphaeropsis obscura
Impagidinium pallidum
Impagidinium patulum
Impagidinium spp.
Invertocysta lacrymosa
Invertocysta tabulatum
Labyrinthodinium truncatum
Lejeunecysta challengerensis
Lingulodinium machaerophorum
Melitasphaeridium choanophorum
Operculodinium? borgerholtense
Operculodinium centrocarpum
Operculodinium israelianum
Operculodinium longispinigerum
Operculodinium piaseckii
Operculodinium spp.
Operculodinium? eirikianum
Paucisphaeridium sp. B
Palaeocystodinium golzowense
Quinquecuspis concreta
Reticulatosphaera actinocoronata
Selenopemphix brevispinosa
Selenopemphix dionaeacysta
Selenopemphix nephroides
Spiniferites spp.
Tectatodinium pellitum
Trinovantedinium glorianum
Trinovantedinium ferugnomatum
Trinovantedinium harpagonium
Tuberculodinium vancampoae
Acritarchs
Cymatiosphaera aff. baffinensis
Cymatiosphaera spp.
Cyclopsiella elliptica-granosa complex (sensu de Verteuil and
Norris 1996)
Cometosphaera bullatio
Nannobarbophora gedlii
Paralecaniella indentata
Porcupinae indentata
Small spiny acritarch
Reworked dinoflagellate cysts
Pre-Neogene dinoflagellate cysts (26 dinocyst species)
Acta Palaeontol. Pol. 62 (1): 211–220, 2017
https://doi.org/10.4202/app.00309.2016