
An integrated approach to understanding the role of the long neck in plesiosaurs
LESLIE F. NOÈ, MICHAEL A. TAYLOR, and MARCELA GÓMEZ-PÉREZ
Noè, L.F., Taylor, M.A., and Gómez-Pérez, M. 2017. An integrated approach to understanding the role of the long neck in plesiosaurs. Acta Palaeontologica Polonica 62 (1): 137–162.
The evolution and function of the long neck in plesiosaurs, and how the problems associated with stiffness or flexibility were overcome during feeding, or rapid swimming during predator avoidance, are explored, and a new interpretation for the function of the plesiosaur neck is presented. Based on the anatomy of the articular faces of contiguous cervical vertebral centra, neural arches, and cervical ribs, the plesiosaur neck was mainly adapted for ventral bending, with dorsal, lateral and rotational movements all relatively restricted. Predominant ventral bending indicates the neck was adapted for use beneath the body, suggesting feeding in the water column, close to the sea floor, or within soft sediments on the sea floor. A new model is proposed for the plesiosaur bauplan, comprising the head as a filter, straining, sieve feeding or sediment raking apparatus, mounted on a neck which acted as a stiff but ventrally flexible feeding tube, attached to the body which acted as a highly mobile feeding platform. Numerous features of plesiosaurs, including cranial and dental form, cervical vertebral morphology, body shape and limb-based propulsion, conform to this model. Comparative data from modern organisms support this novel explanation for the structure and function of the plesiosaur long neck. This integrative analysis offers an explanation for the evolution of the plesiosaur long neck as a key evolutionary novelty, and why this apparently enigmatic feature remained a prominent feature of plesiosaurs throughout their long evolutionary history.
Key words: Sauropterygia, Plesiosauria, long neck, functional anatomy, filter feeding, palaeoecology, evolution.
Leslie F. Noè [l.noe@uniandes.edu.co], Departamento de Geociencias, Universidad de los Andes, Cra 1 No 18A-10, AA 4976, Bogotá D.C., Colombia (ORCID http://orcid.org/0000-0003-2676-3316).
Michael A. Taylor [mat22@le.ac.uk], Department of Natural Sciences, National Museums Scotland, Chambers St., Edinburgh EH1 1JF, Scotland, and School of Museum Studies, University of Leicester, England (ORCID http://orcid.org/0000-0002-1495-8215).
Marcela Gómez-Pérez [mgomezperez@cantab.net], Departamento de Geociencias, Universidad de los Andes, Cra 1 No 18A-10, AA 4976, Bogotá D.C., Colombia; current address: Museo Geológico José Royo y Gómez, Servicio Geológico Colombiano, Diagonal 53 No. 34-53, Bogotá D.C., Colombia (ORCID http://orcid.org/0000-0002-0630-2435).
Received 21 December 2016, accepted 16 January 2017, available online 1 March 2017.
Copyright © 2017 L.F. Noè et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Members of the extinct order Plesiosauria (Reptilia, Sauropterygia), the plesiosaurians, are intriguing and fascinating animals to study, and an extraordinary challenge to interpret in the absence of direct living relatives or close modern analogues (e.g., Welles 1943; Wilkinson and Ruxton 2012). Plesiosauria have been traditionally divided into two groups: “plesiosauromorphs”, the long-necked plesiosaurians (Gray 1825) and “pliosauromorphs”, the short-necked plesiosaurians (Seeley 1874; Welles 1943; Brown 1981b). However, recent phylogenetic analyses (e.g., O’Keefe 2001a; Ketchum and Benson 2010; Benson and Druckenmiller 2014) have confirmed the long-standing suspicion that this dichotomy is too simplistic (Bakker 1993; Cruickshank 1994; Bardet 1995; Carpenter 1999). The “plesiosauromorph”, “pliosauromorph”, and intermediate morphotypes that characterise Plesiosauria therefore represent ecological or functional grades sensu Olson (1971), and not phylogenetic clades (Bakker 1993; Cruickshank 1994; Massare 1997).
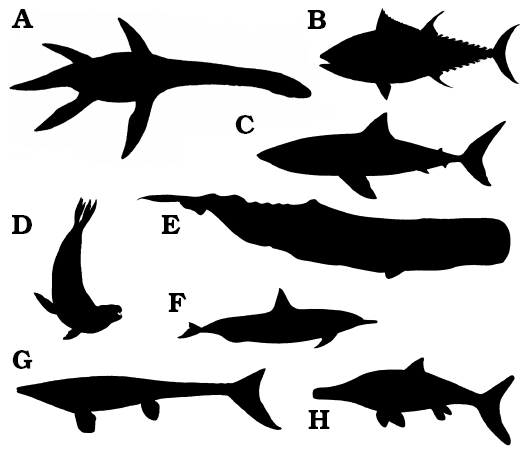
Irrespective of phylogenetic position, some animals within Plesiosauria exhibit a small head mounted on a long neck, a streamlined body powered by four hydrofoil-shaped limbs, and a moderately short tail (e.g., Robinson 1975; Massare 1987, 1988, 1994, 1997; Storrs 1993; Rieppel 1997); these animals are here termed “plesiosaurs”, following traditional, pre-cladistic terminology. The plesiosaur bauplan is fundamentally different from the vast majority of extant and extinct large (> 1 m body length) marine vertebrates, such as teleost “fish”, sharks, ichthyosaurs, mosasaurs, marine iguanas, sea turtles, penguins, pinnipeds, sirenians, and whales and dolphins (Fig. 1). These non-plesiosaurian, marine organisms, whether primarily or secondarily adapted to life in water, are generally characterised by a fusiform body with a large head; a short or absent neck; fins or fore-limbs anteriorly placed for steering and stabilization; a dorsomedial stabilizing fin frequently present; hind-limbs or fins that are anteriorly placed, reduced, absent or incorporated into the tail; and a large surface area tail powered by lateral or dorsoventral undulations of the body or elongate fins and flippers to provide the main propulsive thrust (e.g., Williston 1902, 1914; Watson 1924, 1951; Carroll 1985; Braun and Reif 1985). The plesiosaur bauplan, with its long neck, is so profoundly different from this general paradigm for large predaceous aquatic vertebrates, that the differences demand exploration and explanation.
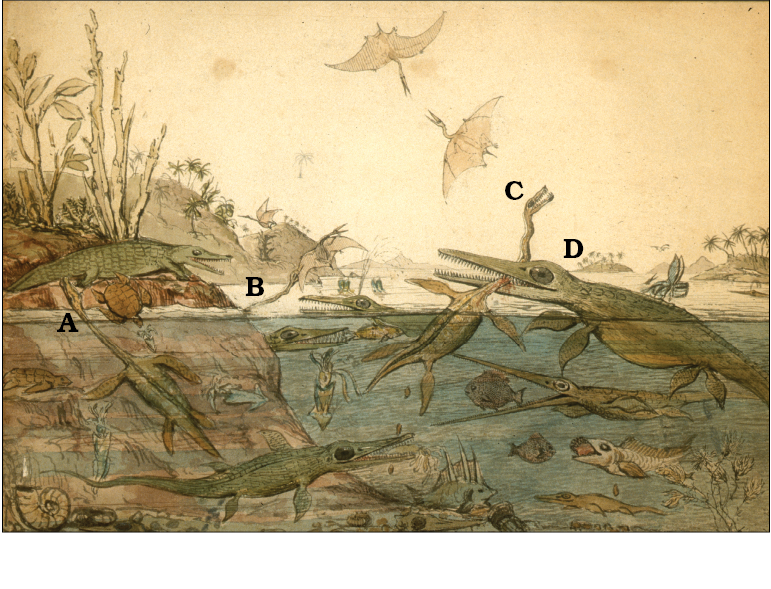
In some plesiosaurs, the neck has a higher cervical vertebral count than in any other known vertebrate taxon (Buckland 1836; Williston 1906, 1914; Sachs 2005; Kubo et al. 2012; Sachs et al. 2013). Indeed, when the genus Plesiosaurus de la Beche and Conybeare, 1821 was first published (de la Beche and Conybeare 1821; Conybeare 1822) the remarkable elongation of the cervical region was so startling that Georges Cuvier initially suspected the plesiosaur to be a spurious composite (either deliberate or accidental) of different animals (Torrens 1995). The specific epithet Plesiosaurus dolichodeirus Conybeare, 1824, was derived from the Homeric Greek for a long and graceful neck (Conybeare 1824; Hawkins 1834; Owen 1854), emphasising the uniqueness of this feature. In addition, it may have been the improbably long neck of the holotype of Elasmosaurus that led to the head being incorrectly placed on the tail (Anonymous 1868; Cope 1869; Davidson 2002), thereby giving the neck an artificially short appearance. Since those early descriptions, the functional and ecological significance of the long neck in plesiosaurs has provoked much speculation but little consensus, although the neck is usually considered to be an adaptation either for feeding or breathing (e.g., Conybeare 1824; Andrews 1910; Williston 1914; Brown 1981b; McHenry et al. 2005). In the original description of Plesiosaurus dolichodeirus Conybeare, 1824 the neck was variously envisaged as: an impediment to aquatic locomotion; a vulnerable point for attack by predators such as the giant Ichthyosaurus (now Temnodontosaurus) (Fig. 2); an adaptation for feeding when held in a swan-like pose above the sea surface; providing an extended reach or a swift strike at prey; or allowing breathing whilst the animal lay concealed at depth (Conybeare 1824). Subsequent attempts have revisited these original concepts in full or in part (e.g., Buckland 1836; Owen 1854; Hutchinson 1897; Williston 1914; North 1933; Taylor 1981; O’Keefe 2001b). However, despite nearly 200 years of research (e.g., Taylor 1997), the function and evolutionary significance of the plesiosaur long neck remain enigmatic.
The plesiosaur long neck, given its cost and significance to the living animal, is likely to have had a role in both feeding and locomotion. Feeding is essential for survival (Shuler 1950), and a major determinant of head, tooth and neck structure (Taylor 1987, 1989). However, the neck was also involved in respiration and had major implications for locomotion: the neck increased drag due to its form and large surface area, but was also potentially part of an integrated locomotor system, for instance affecting steering (as it lies in front of the locomotor apparatus) and because the rear of the neck acted as anchorage for musculature from the anterior limb girdles (Robinson 1975, 1977; Brown 1981b). Hence, any explanation of neck function should consider both slow speed locomotion and more rapid movement during respiration, feeding and predator avoidance.
In this paper, we take published anatomical studies of the head, neck and post-cervical body of typical long-necked plesiosaurians, and use this to interpret the possible roles of the neck. We combine this analysis with biomechanical interpretation to present a new model for the functional interpretation of the plesiosaur neck.
 |
|
|
Fig. 1. Body outlines of large (> 1 m total body length) extinct and extant vertebrates, primarily or secondarily adapted to the marine environment. A. Plesiosaurus sp., an extinct Jurassic plesiosauromorph plesiosaur. B. Thunnus thynnus, the extant Atlantic bluefin tuna, a teleost “fish”. C. Carcharodon carcharias, the extant great white shark. D. Hydrurga leptonyx, the extant leopard seal. E. Physeter macrocephalus, the extant sperm whale. F. Tursiops truncatus, the extant common bottlenose dolphin. G. Platecarpus sp., an extinct Cretaceous mosasaur. H. Ichthyosaurus sp. an extinct Jurassic ichthyosaur. Note how the plesiosaur is the only large marine vertebrate with a long neck. Images not to scale.
|
Fig. 2. One of the potential problems of a long neck in plesiosaurs, as envisaged by Henry de la Beche (painted in 1830) in Duria Antiquior. The large ichthyosaur (now Temnodontosaurus platydon) (D), bites the neck of the plesiosaur Plesiosaurus dolichodeirus (C), a common weak point for predatory attacks. Notice also the second plesiosaur (A) swimming within the water column, which together with the third (B, neck and head only shown), which are both attacking prey above the air-water interface (biting the tail of a crocodile and catching a flying pterosaur, respectively). Image courtesy and copyright of the National Museum of Wales (UK).
|
Institutional abbreviations.—NHMUK, The Natural History Museum, London, UK.
Other abbreviations.—C, cervical vertebra (followed by number within the cervical sequence, starting with the atlas and axis, so C3 is the first postaxial cervical vertebra); H, height of centrum posteriorly; L, length of centrum along ventral midline; W, maximum width of centrum posteriorly.
Material and methods
This study uses three genera, Muraenosaurus Seeley, 1874, Cryptoclidus Seeley, 1892 and Tricleidus Andrews, 1909 as exemplars of long-necked plesiosaurians. All three genera have been recovered from the Oxford Clay Formation (Callovian and Oxfordian, Jurassic), approximately 166–157 Ma BP (Cohen et al. 2013), principally from around Peterborough, England, UK. These plesiosaurs were chosen for the quantity of preserved specimens, their quality of preservation, and their well-known associated fauna (e.g., Hudson and Palframan 1969; Martill and Hudson 1991; Martill et al. 1994). Muraenosaurus and Cryptoclidus are known from numerous substantially complete specimens, whereas Tricleidus is known from relatively few specimens (Andrews 1913; Brown 1981b); key publications for each genus are cited in the text. Definitions of vertebral dimensions (L, W, H) and ontogenetic terminology (“juvenile”, “adult” and “old adult”) follow Brown (1981b).
Former hypotheses of neck function
Here we review the various hypotheses proposed to explain the evolution, function, and persistence of the long neck in plesiosaurs (Fig. 3). This acts as a precursor to presenting the anatomical evidence available to understand the form and function of the plesiosaur neck.
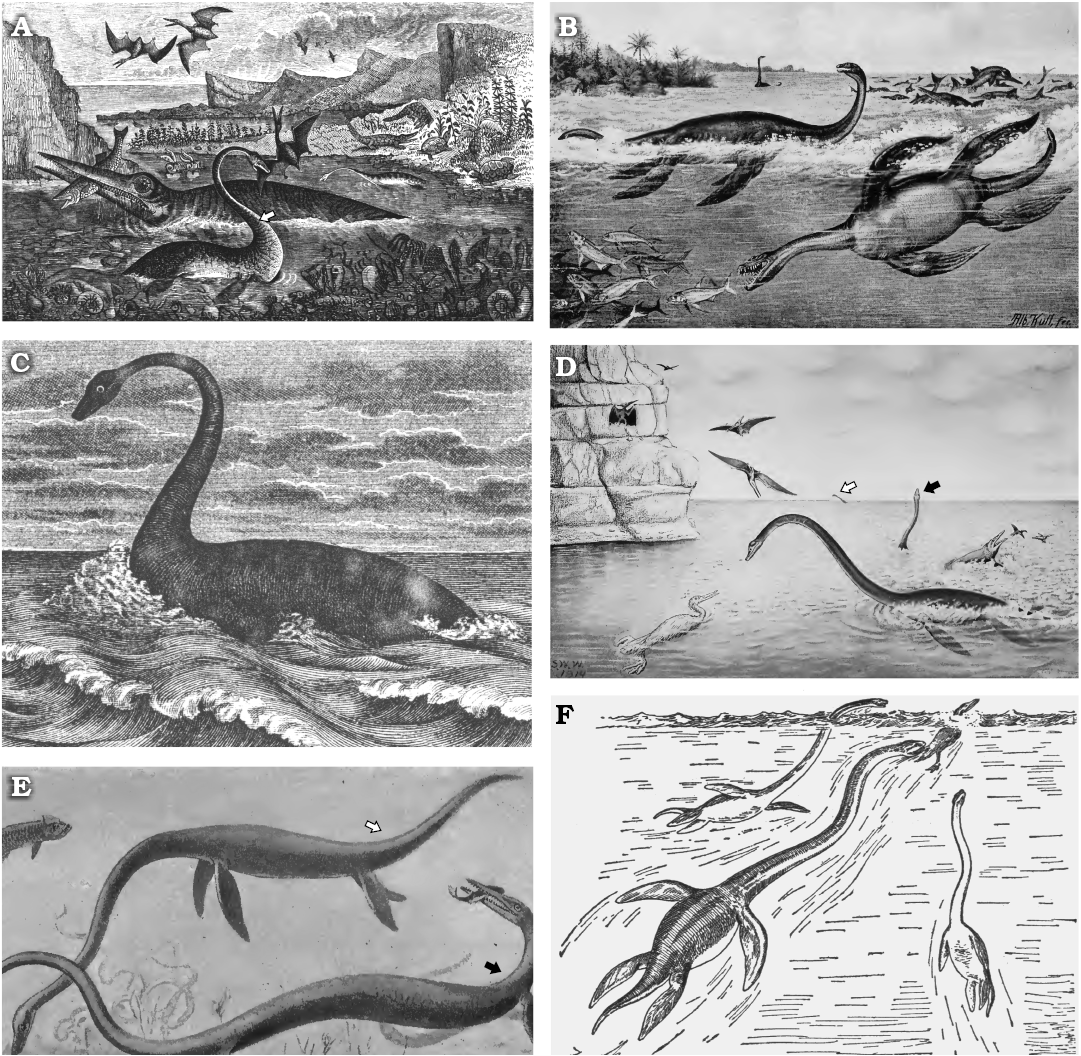
Fig. 3. Previous hypotheses of neck function of plesiosaurs. A. An unnamed plesiosaur (possibly Plesiosaurus dolichodeirus) swimming at the sea surface and preying on a pterosaur (note this duplicates the concept illustrated in Fig. 2B). B. Plesiosaurus guilelmi imperatoris (now genus Seeleyosaurus) with neck curved dorsally and swimming at the surface, and Thaumatosaurus victor (now genus Meyerasaurus) with neck held straight and chasing fish underwater. C. Plesiosaurus dolichodeirus swimming at the water surface, possibly scanning for submerged prey. D. Elasmosaurus platyurus uses its neck to hunt fish close to the ocean surface. Note a similar pose in the individual in the far distance (white arrow), but the almost vertical position of the neck in the individual in the centre right (black arrow). E. Elasmosaurus (white arrow) swimming beneath the surface and apparently feeding on benthic prey, with Clidastes (a mosasaur) catching a fish (black arrow). F. Three individuals of Elasmosaurus chasing down the flightless marine bird Hesperornis. Images from: A, Richardson (1851: frontispiece, “designed, drawn and engraved by Mr Nibbs”); B, Winkler (1873: “Le plésiosaure”); C, Hutchinson (1897: pl. 13, detail); D, E, Williston (1914: fig. 31 and 33, respectively); F, from Osborn (1918: fig. 86, upper part, drawn by W.K. Gregory from data by Williston 1914).
A neck with no adaptive value.—It has been suggested that the plesiosaur neck had no biomechanical function and was of little or no adaptive value (Williston 1902; Shuler 1950); however, this would burden the living animal with unnecessary biological costs (Conybeare 1824; Robinson 1975; Massare 1988, 1994, 1997; Alexander 1989). The neck was energetically expensive to build and maintain, and added drag during locomotion in water compared to a fusiform profile (Massare 1988; Alexander 1989). However, the long neck was a seemingly successful adaptation (Welles and Bump 1949; Colbert 1966), its value presumably demonstrated by its continued presence over a wide geographical range and throughout the long evolutionary history of Plesiosauria (Smith 2008). Hence it seems reasonable to assume the plesiosaur long neck did have a positive function, and was therefore of genuine evolutionary and adaptive value.
Phylogenetic inertia.—A variant of the above is the concept of phylogenetic baggage: that the plesiosaur neck was simply inherited from a relatively long-necked ancestor (Watson 1951; Taylor 1981), the animal surviving in spite of the neck rather than because of it (cf. Taylor 1989). However, this simply moves the question back in time without providing a satisfactory answer. Also, in some plesiosaurian lineages such as the Elasmosauridae, neck length increases over evolutionary time (Kubo et al. 2012; Sachs et al. 2013), suggesting positive selection pressure for a long neck. In addition, the short-necked polycotylid plesiosaurians are interpreted to have evolved short necks from longer-necked ancestors (Williston 1906; Bakker 1993; Carpenter 1997; O’Keefe 2001a). Hence there appears to be no reason to infer phylogenetic retention of a costly, but functionally useless, long neck.
Species-specific interactions.—The long neck might have been involved in intraspecific or interspecific interactions with other plesiosaurs, or other taxa, in the three overlapping roles of: (i) sexual selection (Darwin 1871; Taylor 1989), with longer-necked plesiosaurs attracting more or better-quality mates and therefore producing more viable offspring; (ii) status (Számadó 2011), with longer-necked plesiosaurs the dominant individuals within a population and thereby accessing more essential or rare resources (whether mates, nutriment, or other requirements); and/or (iii) signalling (Számadó 2011), with the length of the neck acting as a species-specific identifier, threat display, or other communication device.
Sexual recognition is necessary in all organisms with two genders, but there is no evidence for intraspecific hard-part variation in the plesiosaur neck or body, which is also true for sauropod dinosaurs (Taylor et al. 2011). However, a long neck appears to be expensive and maladaptive if solely for signalling, when much less costly modalities (such as pigment patterning or a dorsal crest) are potentially available (cf. e.g., Kellner and Campos 2002; Kear et al. 2006; Hone et al. 2012). It remains possible that the neck was used in antagonistic interactions, such as the neck-wrestling observed in giraffes (Simmons and Scheepers 1996) or the whole body combat of some snakes (e.g., Duvall et al. 1992; Shine 2002). However, as these suggestions concern behavioural interactions, they cannot easily be tested in the fossil record. In any case, the prevalence of the long neck in numerous plesiosaur genera over millions of years of geological time implies a fundamental biomechanical role.
Respiration.—To allow breathing whilst the body remained under water, thereby using the neck as a snorkel (e.g., Conybeare 1824; Shuler 1950). As air-breathing vertebrates, plesiosaurs were required to raise their breathing apparatus above water, but respiration seems unlikely as the sole function for the long neck. Using the neck as a snorkel (Shuler 1950), whilst maintaining the body submerged at depth (Conybeare 1824), can be discounted on the basis of hydrostatic pressure differences, using the same arguments as for sauropod necks (Alexander 1989; Taylor 1992). The air in the lungs is at surface atmospheric pressure, but the pressure on the body at depth, and therefore the blood capillaries in the lung, is greater by a factor of about 0.1 atmospheres per metre. Therefore, the elasmosaurid neck is far longer than is practical for sub-vertical snorkelling. A gentle slope of the neck is perfectly possible for normal breathing when at the surface, but the use of this breathing method for a life lived at the surface implies long periods of energetically expensive swimming in the immediate sub-surface zone (Alexander 1989), which seems highly improbable.
A greatly increased neck length also increases tracheal length, and therefore the volume of stagnant tidal air anterior of the lungs, and this additional dead space may require the animal to have larger lungs, or to breathe more deeply or frequently. Alternatively, the trachea may have been narrow reducing dead space but requiring a slower respiratory rate, as in the giraffe (Hugh-Jones et al. 1978), when surfaced. Either way, the air in the trachea would provide some additional buoyancy (although less so in the second case) that would help compensate for the weight of bone and muscle forming the neck; however, the relative importance of these two components (buoyancy vs weight) in plesiosaur palaeobiology has yet to be worked out, and changes in buoyancy with depth require further exploration.
Prey capture above or below the air-water interface.—It has been suggested that the neck was adapted for habitually holding the head above water for feeding (e.g., Brown 1981b; Halstead 1989), either to capture airborne prey such as pterosaurs from below (Brown 1904), or to plunge the head into water to catch fish from above (Williston 1914; Brown 1981b). However, long-term bouts of swimming at the surface in order to feed would lose the advantage of the streamlined body and the efficiency of the hydrofoil limbs due to enhanced drag near the surface (Alexander 1990). In addition, hunting airborne prey from beneath the water surface, or aquatic prey from above the surface, requires the eyes to be able to compensate for refraction at the air-water interface (Horváth and Varjú 1995), and unevenness (e.g., due to wave action) also reduces visibility. If the eyes were used in both media, they would have required special adaptations to have allowed vision both in air and water (Hanke et al. 2009). Hence habitual feeding from the surface, like surface breathing, seems improbable.
The plesiosaur neck has been proposed as an adaptation for underwater prey capture: using rapid sideways swipes (e.g., Romer 1955; Colbert 1958; Rieppel 1997), either for keeping the body away from prey (e.g., Massare 1987, 1994), or for feeding on the seafloor (e.g., Andrews 1910; McHenry et al. 2005). Employing the head to catch prey underwater using the mobility of the entire neck in any direction can be rejected, as considerable water resistance would be generated when trying to move the head rapidly whilst holding the body relatively still (Watson 1951; Halstead 1969; Robinson 1975). Alternatively plesiosaurs could have caught prey within the water column with sudden bursts of acceleration (Welles and Bump 1949; Massare 1988; Fig. 2F). However, the problems of wielding a neck with sufficient flexibility to chase prey at speed in water makes this mode of prey capture highly unlikely (Williston 1914; Watson 1951; Alexander 1989). A long neck in front of the propulsive apparatus would be hydrodynamically destabilizing, so if some or all was flexible to enable food procurement, how did the animal swim sufficiently fast to avoid predators without losing control of the head and neck? If the neck was stiff and rod-like to permit rapid movement during predator avoidance, how was the head employed during prey capture?
The plesiosaur neck has also been considered as an apparatus to dart the head forwards rapidly to surprise relatively fast-moving prey (Conybeare 1824; Hutchinson 1897; Shuler 1950; Romer 1955; Colbert 1958, 1966; Taylor 1981, 1992; Alexander 1989; Bakker 1993). In this way, the long neck would compensate for the low manoeuvrability of the body (Mazin 2001). However, this would require considerable flexibility, which is often considered absent from the plesiosaur neck (Buckland 1836; Williston 1914).
Despite the above considerations, plesiosaurs have frequently been considered to have been slow moving ambush predators; waiting for, or slowly swimming amongst, schools of prey; or relying on the flexibility of the neck to swing the head within reach of their food (Taylor 1981, 1987; Massare 1988; Martill et al. 1994). However, a lack of flexibility in the plesiosaur neck in most directions has been used as evidence against this active fish or cephalopod catching lifestyle (Robinson 1975). In the following section we review the anatomical evidence available for interpreting the function of the plesiosaur neck based on preserved morphology, and subsequently propose a new model for the roles of the plesiosaur head, neck and body.
Anatomical evidence
Here we provide the anatomical evidence that will constrain our model of plesiosaur head, neck and body function. We concentrate on gross similarities between plesiosaur taxa, rather than the numerous, relatively minor features separating members of the group which are emphasised in taxonomic and phylogenetic studies. However, it is important to take account intraspecific (including age-related) variation, and taphonomic effects (Welles 1952; Brown 1981b; O’Keefe and Hiller 2006). In the living animal, the neck was constructed from bone, connective tissue and muscles, arranged in regular metameric segments, and other tissues such as blood vessels, nerves and viscera. However, as palaeontologists we are forced to rely on the bony remains, and can only interpret soft part anatomy from osteological correlates on the preserved bones.
Anatomy of the head.—The plesiosaurian head is small, streamlined and lightly built (Taylor 1987) with much of the cranium formed from a series of bars and struts (Evans 1993; Brown and Cruickshank 1994), thereby minimizing cranial mass (Fig. 4). The skull is entirely akinetic, with a simple open-and-shut hinge-like motion between cranium and mandible, and a range of movement possible at the cranio-cervical ball-and-socket joint.
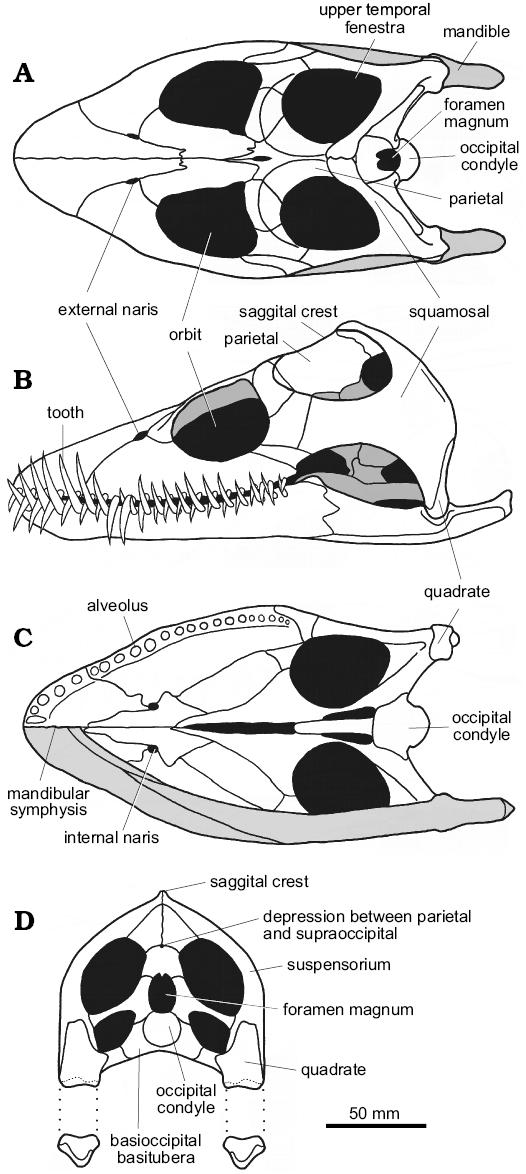
In the three Oxford Clay genera Muraenosaurus, Cryptoclidus, and Tricleidus, the skull is relatively broad, sub-triangular in dorsal and ventral views, and relatively high posteriorly (Figs. 4, 5A–C). The snout is blunt, although more pointed in Cryptoclidus than Muraenosaurus (Andrews 1910), and proportionally shorter and higher in Tricleidus than in Cryptoclidus (Brown 1981b). In plesiosaurs generally, the external nares lie well posterior of the snout tip and close to the orbits (Owen 1861) with the internal nares anterior to, or almost directly beneath, the external nares, possibly indicating a water-filled flow-through olfaction system (Cruickshank et al. 1991). The orbits are large, dorsally positioned and somewhat anteriorly directed, and lie approximately midway along the length of the cranium (Owen 1861; Massare 1987). The upper temporal fenestrae are large openings, located high on the back of the skull, that housed well-developed jaw adductor musculature in life (Araújo and Polcyn 2013). The two upper temporal fenestrae are separated along the dorsal midline by a high, narrow sagittal crest formed largely by the parietals. The parietal-squamosal junction lies close to the rear of the skull, with the squamosals typically forming the highest point of the cranium.
In posterior view (Fig. 4D), the skull is slightly higher than wide with the suspensorium forming a strong arch that slopes gently posteroventrally towards the quadrates. In all three Oxford Clay plesiosaurs, the posterior of the skull preserves, or can be inferred to preserve, a median pit above the foramen magnum, at the junction between the parietals and supraoccipital, which appears to be present in all members of Plesiosauria (Brown 1981b). Ventral of the foramen magnum, the occipital condyle acts as the hemispherical ball articulation with the strongly concave socket formed by the anterior of the atlas-axis complex; the suspensorium typically conceals the occipital condyle in lateral view (Andrews 1910). More ventrally, the basioccipital basitubera form stout ventral braces against the rear of the palate. Ventrally the quadrates lie well below the level of the palate, with the spacing between the jaw articulations of the order of 100–200 mm maximum width (Brown 1981b; Massare 1987).
The plesiosaur mandible is lightly built, with a wide gape (Evans 1999). The slender, intermeshing teeth do not occlude tip-to-tip, but may make contact along the sides of the crowns (Fig. 5A–C), although this varies between individuals. The mandibular rami are generally long and slender (Massare 1987), and immovably fused anteriorly at the mandibular symphysis. In Muraenosaurus the mandibular symphysis is short and shallow, with approximately five functional tooth pairs adjacent to the suture (Fig. 4B, C); in Cryptoclidus the symphysial region contains four pairs of teeth. The region around the mandibular symphysis is slightly expanded in Muraenosaurus, but unexpanded in Cryptoclidus; the mandible of Tricleidus is very similar in form to that of Muraenosaurus (Andrews 1910; Brown 1981b).
In Muraenosaurus each mandibular ramus contains 24–26 tooth positions; in Cryptoclidus 19–22; and Tricleidus 17. In Muraenosaurus and Tricleidus the dentition is weakly heterodont with relatively larger posterior teeth (Andrews 1910; Brown 1981b), as in most Cretaceous elasmosaurs (e.g., Shuler 1950; Welles 1952, 1962; Brown 1993). Cryptoclidus has more regularly sized teeth, the dentition comparable to the Kimmeridgian genus Kimmerosaurus Brown, 1981b and the Cretaceous southern ocean form Aristonectes Cabrera, 1941 (see also Brown 1993; Gasparini et al. 2003). The relatively high degree of homodonty in the three Oxford Clay genera (Fig. 5A–C) contributes to a tight (but not occluding) intermesh of the upper and lower teeth, usually on a one-to-one basis (Brown 1981b); this intermesh of teeth is true for the vast majority of plesiosaurs (e.g., Shuler 1950; Welles 1952; Brown 1993; Carpenter 1997; Storrs 1997).
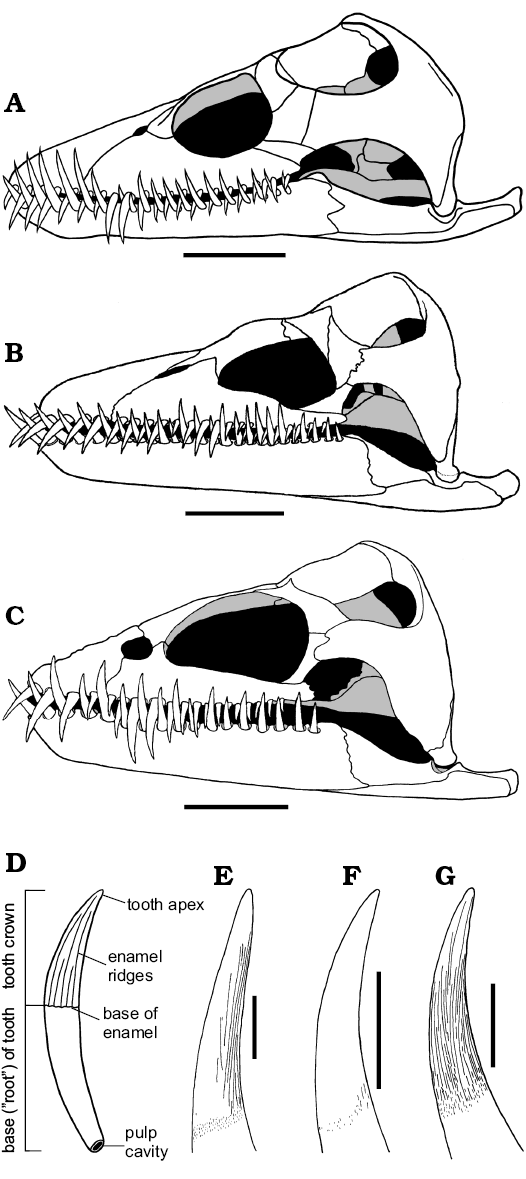
Each individual tooth in the Oxford Clay plesiosaurs (Fig. 5D–G) is sub-circular in cross-section, with a long, slender, curved and sharply-pointed crown, and a deeply rooted base with a large open pulp cavity (Andrews 1910; Massare 1987; Fig. 5D). The tooth crown is variably ornamented with fine enamel ridges of different lengths: in Muraenosaurus the crown is ornamented with ridges concentrated on the lingual side, a number reaching the apex (tip of the crown); in Tricleidus the crown is similar, but with just one or two ridges reaching the apex; in Cryptoclidus the ornamentation is reduced to just a few ridges on the basal part of the lingual surface (Andrews 1910; Brown 1981b), although there may be some tip-ward flattening of the tooth (however, see discussion below). In all three genera the teeth are rarely abraded or broken on their tips (Brown 1981b; Massare 1987); this contrasts with the situation in the more stoutly toothed and evidently sarcophagous pliosaurids, such as Liopleurodon and Simolestes, which frequently exhibit worn and/or broken teeth (Andrews 1913; Noè 2001). The spacing of the teeth within plesiosaurs is relatively regular, in the order of 5–10 mm (Massare 1987). Tooth form is similar in all plesiosaurs, with relatively minor differences in crown ornamentation and form.
 |
 |
|
Fig. 4. Reconstructions of the cranium and mandible of the plesiosaur Muraenosaurus leedsi from the Oxford Clay Formation, Callovian and Oxfordian, showing overall proportions and construction, and highlighting areas mentioned in the text. A. Dorsal view. B. Left lateral view. C. Ventral view with mandible removed (upper half) and mandible shaded grey (lower half). D. Posterior view with mandible shown disarticulated. Images modified from: A, Evans (1999); B, Evans (1999) with dentition added based on information from Andrews (1913) and Brown (1981b); C, D, compiled from LFN drawings and Andrews (1910), Brown (1981b), and Evans (1999). |
Fig. 5. Reconstructions of skulls (A–C, in left lateral view) and teeth (D–G, in axial view, with lingual surface to the right) of plesiosaurs from the Oxford Clay Formation, Callovian and Oxfordian. A, E. Muraenosaurus leedsi. B, F. Cryptoclidus oxoniensis. C, G. Tricleidus seeleyi. D. Generalized plesiosaur tooth showing overall morphology. Images from: A, Evans (1999); B, Brown and Cruickshank (1994: fig. 5); C, Brown (1981b: fig. 22); D, LFN drawings; E, Brown (1981b: fig. 19a, modified); F, Brown (1981b: fig. 5) with additional information from Andrews (1910); G, Brown (1981b: fig. 24a). Scale bars A–C, 50 mm, E–G, 10 mm, D not to scale. |
Anatomy of the neck.—The structure of the neck in plesiosaurs is unique within tetrapods, and yet remarkably conservative across Plesiosauria (Fig. 6). The neck tapers anteriorly (Williston 1914) and consists of a tightly conjoined atlas-axis complex (vertebrae C1 and C2, co-ossified in older individuals) followed by numerous cervical segments (C3+). The body of the atlas and axis each comprises a centrum and intercentrum (the “sub-vertebral wedge bones” of Owen 1847; Barrett 1858; Andrews 1910), whereas the body of each postaxial vertebra comprises a single spool-shaped centrum. All cervical vertebrae, including the atlas and axis, possess a dorsally placed neural arch (in paired sections on the atlas), and two cervical ribs mounted ventrally or laterally on the centrum. When seen in anterior or posterior view, each postaxial vertebra with its ribs forms a broadly triradiate structure, the details of which vary along the cervical series (Fig. 6B). The articular surfaces between contiguous cervical centra are flat or slightly concave (Brown 1981b), except for the strongly concave craniocervical joint on the anterior of the atlas, and the fused atlas-axis junction. The neck is deemed to terminate posteriorly at the last vertebra where the rib facets are positioned entirely on the sides of the centrum, rather than impinging on the neural arch (Seeley 1876); in plesiosaurs generally, the last few cervical vertebrae are not typical of the rest of the cervical series (Storrs 1997).
Neck length and vertebral proportions: The length of the neck can be defined in terms of absolute length (by counting the number of cervical vertebrae, or by adding up their lengths) or relative length compared with some other feature, for instance the length of the head. Each measure has its problems. Simple vertebral counts ignore the absolute length of each vertebra, whereas absolute neck length depends on the size of the individual, in terms of ontogenetic development, the maximum size attained by the species, and the volume of cartilage between the cervical vertebrae (Brown 1981b). The latter two are often uncertainly known and this may be confounded by taphonomic modification. Relative neck length can be defined by comparison to the length of the head, the body, or presacral length. Head-to-neck length proportions vary between plesiosaur taxa: in the Oxford Clay Formation plesiosaurs, Muraenosaurus has a skull less than one-fifth as long as the neck; Cryptoclidus a skull about a quarter of neck length; and in Tricleidus the skull is at least three times neck length based on the incompletely known cervical series (Andrews 1910; Brown 1981b). Moreover, these relative metrics are partly confounded by covariance during ontogeny (e.g., O’Keefe and Hiller 2006). For instance, in Muraenosaurus and Cryptoclidus, the neck shows positive allometry during ontogeny, with neck length relative to presacral length being approximately 20% less in “juvenile” Cryptoclidus than in “old adult” individuals (Brown 1981b).
In plesiosaurs generally, elongation of the neck is brought about through an increased number of cervical vertebrae (Williston 1914; Watson 1924). The number of cervical vertebrae varies between plesiosaur genera, but is always high compared to primitive reptiles (~4–6), basal diapsids (~6–9), ancestral sauropterygians (~8–25), and most extinct or extant tetrapods (almost invariably 7 in mammals, and up to 25 in modern birds) (Müller et al. 2010; Varela-Lasheras et al. 2011). In long-necked plesiosaurs, the cervical count ranges from the presumed primitive number of 28–32 (Brown 1981b) to more than 70 in Elasmosaurus platyurus Cope in Anonymous, 1868 (see Sachs 2005; Sachs et al. 2013) and Albertonectes vanderveldei Kubo, Mitchell, and Henderson, 2012, with different genera exhibiting various figures in between (e.g., 37 cervicals in Brancasaurus brancai Wegner, 1914; 47 in Morenosaurus stocki Welles, 1943; 57 in Aphrosaurus furlongi Welles, 1943; and 63 in Hydralmosaurus serpentinus [Cope, 1877]). In some plesiosaur taxa, increased neck length is also brought about by elongation of individual cervical vertebrae (Williston 1914; Watson 1951; O’Keefe and Hiller 2006). For instance, in Oxford Clay taxa, the absolute length of the atlas-axis complex and postaxial vertebrae is greater in Muraenosaurus than in Cryptoclidus or Tricleidus (Andrews 1910). Long cervical vertebrae also typify most Cretaceous elasmosaurs.
The relative proportions of length (L), width (W), and height (H) of the cervical centra have long been considered of taxonomic importance (Welles 1943; Brown 1981b), but also contain much functional information. Within plesiosaurs there is a general trend for postaxial cervical vertebrae to show an absolute increase in L posteriorly (Seeley 1874), but at the same time a decrease in L relative to H or W (Brown 1981b). In addition, the value of L for any given cervical vertebra within the series varies with ontogeny (with the vertebrae of younger animals being shorter and less well ossified). In Muraenosaurus the cervical centra are relatively long: L is always greater than H and usually greater than W in the anterior neck, although W is greater than L more posteriorly (Andrews 1910; Brown 1981b). Moreover, in Muraenosaurus the individual cervical centra are proportionally longer than in Cryptoclidus or Tricleidus; in the latter two genera L only rarely exceeds H but is never greater than W, so the vertebrae cannot be considered elongated (Brown 1981b). This results in a greater absolute length of the cervical vertebrae in Muraenosaurus than in Cryptoclidus or Tricleidus (Andrews 1910), so although the cervical region of Muraenosaurus contains just 37.5% more segments than Cryptoclidus (normally 44: 32), the neck of Muraenosaurus is proportionally twice as long because the relative length of each centrum is greater (Brown 1981b). There is also slight variation between individuals, and between species included within a genus (e.g., Muraenosaurus; Brown 1981b).
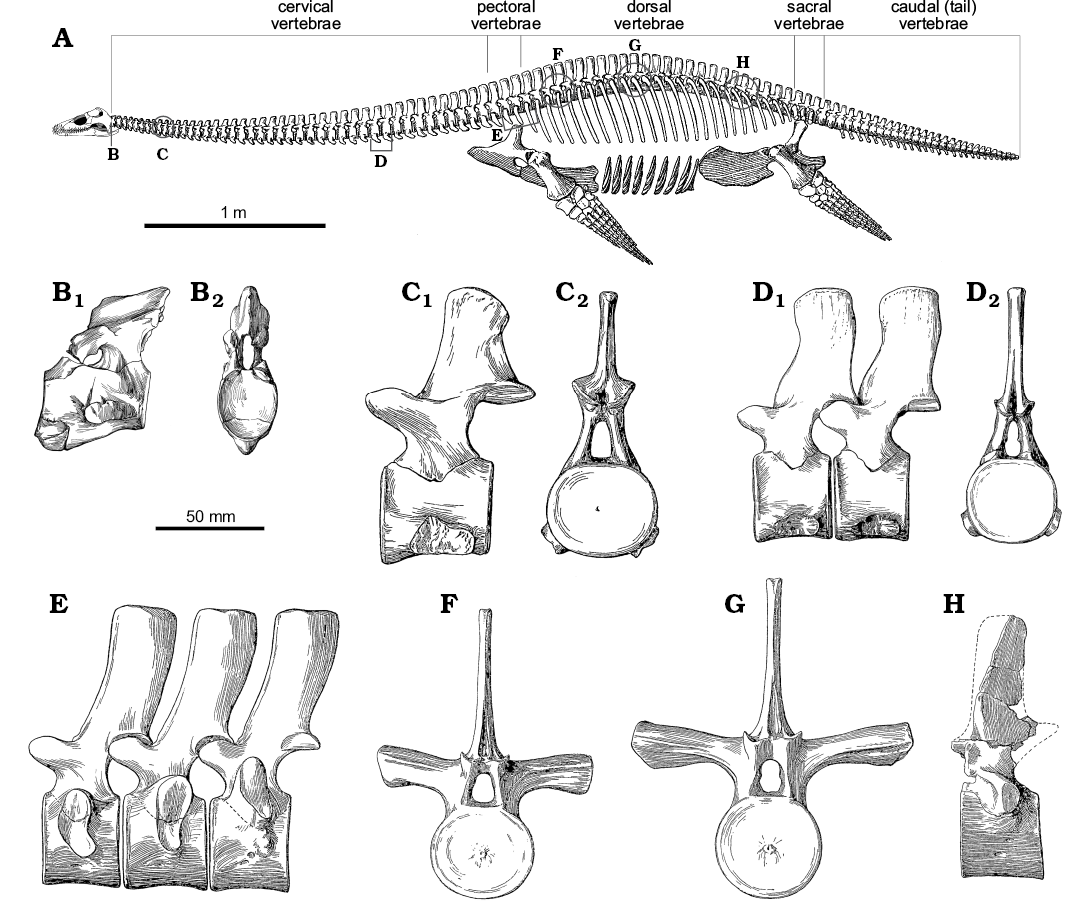
Fig. 6. Skeletal reconstruction and vertebrae of plesiosaur Muraenosaurus from the Oxford Clay Formation, Callovian-Oxfordian near Peterborough, UK. A. Skeleton showing regions of the vertebral column and approximate locations of illustrated vertebrae (B–H). B. Atlas-axis complex in left lateral (B1) and anterior (B2) views. C. Anterior cervical vertebrae in left lateral (C1) and posterior (C2) views. D. Posterior cervical vertebrae (possibly C34 and C35) in left lateral (D1) and posterior (D2) views. E. The last two pectoral and first dorsal vertebrae in left lateral view. F. Anterior dorsal vertebra in anterior view. G. Mid-dorsal vertebra in anterior view. H. Posterior dorsal vertebra in left lateral view. Note: the skeletal reconstruction is inaccurate: Andrews (1910) indicates the presence of 43–44 cervicals, 2–3 pectorals, 20 dorsals, 3–4 sacrals and an unknown number of caudals, whereas Brown (1981b) counted 43–44 cervicals, 3 pectorals, 19–20 dorsals, 4 sacrals and 24 caudals, however, Andrews (1910) illustrates the presence of 44 cervicals, 3 pectorals, 25 dorsals, 3 sacrals, and 30 caudals, thereby making both the body and the tail too long; no attempt has been made to correct this. Abbreviations: circles, indicate approximate position of figured vertebrae, as the serial position is not indicated; brackets, indicate known position of vertebrae figured. Images from: A, Andrews (1910: fig. 66) with the newly reconstructed head based on Evans (1999); B, Andrews (1910: fig. 49) with lateral view reversed from original to match skeletal reconstruction; C–H, Andrews (1910: fig. 50–55).
Cervical centra: The anterior face of the atlas centrum forms the deep cup-shaped articulating surface to receive the basioccipital condyle of the skull (Fig. 6B). In Muraenosaurus the posterior articular face of the axis and both articular faces of the postaxial cervical centra are relatively platycoelous, but develop a shallow V-shape in cross-section (Fig. 7C) with sharply defined borders with increasing age (Andrews 1910; Brown 1981b). In Cryptoclidus and Tricleidus, the vertebrae are amphicoelous (Evans 1993), especially in old individuals, with more rounded margins (Fig. 7D), producing a double sigmoidal curve in cross-section (Andrews 1910; Smellie 1915, 1916; Brown 1981b). The more rounded margins of the cervical centra in Cryptoclidus may indicate that relatively more movement was possible between the vertebrae than in Muraenosaurus (Brown 1981b).
In all three Oxford Clay plesiosaurs, the anterior face of the atlas is deeper dorsoventrally than laterally (Seeley 1874; Fig. 6B), whereas the posterior face of the axis is noticeably wider than high (Andrews 1910). This change in the articular faces of the atlas-axis complex from a dorsoventrally elongated anterior face to a laterally expanded posterior face is seen in all plesiosaurs (e.g., Owen 1847; Barrett 1858; Brown 1981b). The anterior and posterior articular faces of the postaxial cervical centra (C3+) of Muraenosaurus and Cryptoclidus are transversely expanded, rounded ovals (Figs. 6C, D, 7B, F), with W exceeding H throughout the length of the neck (Andrews 1910; Smellie 1916; Brown 1981b). This pattern of wider-than-high vertebral proportions is seen in all plesiosaurs (Watson 1924), irrespective of how the measurements are taken (e.g., Welles 1943, 1952; Brown 1981b). However, the broadening of cervical centra is most clearly expressed in Cretaceous elasmosaurs (Fig. 7E), where the vertebrae are commonly butterfly- or dumb-bell shaped (e.g., O’Keefe 2001a; O’Keefe and Hiller 2006).
Cervical neural arches: The dorsomedial surface of each cervical centrum exhibits a concave excavation for the neural canal between the facets for the neural arch (Andrews 1910). Viewed dorsally, the neural canal is hourglass-shaped, constricted medially, and widest posteriorly (Fig. 7G). Bounding the neural canal on each side are large diamond-shaped facets for reception of the pedicles of the neural arch. The neural arch extends almost the full length of the centrum (Andrews 1910; Brown 1981b) and thereby forms the lateral and dorsal margins of the neural canal. In Muraenosaurus, the neural canal and neural arch facets are longer than in Cryptoclidus and Tricleidus, reflecting the greater absolute length of the cervical centra.
The atlas bears a low, divided, strongly posteriorly-sloping neural arch with well-developed posterior zygapophyses; the axis neural arch is larger, with both anterior and posterior zygapophyses. In the postaxial cervical vertebrae (Fig. 7A), the neural arch narrows dorsally before widening to bear the dorsomedially directed anterior, and the ventrolaterally facing posterior, zygapophyses which are strong and well-developed throughout the cervical series in all plesiosaurs (Brown 1981b). In Oxford Clay genera, the zygapophyses are oval and overhang the articular surfaces of the centrum, less so anteriorly than posteriorly (Andrews 1910; Smellie 1916; Brown 1981b). The zygapophysial facets are flat in the anterior of the neck, but posteriorly the anterior zygapophyses become increasingly concave, whilst the posterior zygapophyses become more convex. In Muraenosaurus from C33 onwards, the left and right anterior zygapophyses on each neural arch are sufficiently close to form a semi-circular depression into which the preceding posterior zygapophyses fit, forming a peg-and-socket like arrangement (Seeley 1874).
Above the zygapophyses projects the neural spine. The atlas neural spine is low, whereas the axis neural spine is better developed, with its greatest height posteriorly, although lower in Cryptoclidus and Tricleidus than in Muraenosaurus. Anteriorly within the postaxial series, each neural spine is relatively low and strongly laterally compressed; the anterior of the neural spine lies so it is level with the posterior of the anterior zygapophyses (Seeley 1874; Andrews 1910; Brown 1981b). Posteriorly along the cervical series, the neural arches become high, especially in Muraenosaurus (Fig. 8), and the base lengthens, extending anteriorly between the anterior zygapophyses. In all three Oxford Clay genera, the neural spines are posteriorly inclined in the anterior of the neck, but become more upright along the length of the neck (Andrews 1910; Brown 1981b).
In Muraenosaurus from about C15 to C30, the neural spine has the anterior border nearly vertical and the posterior border inclined obliquely anterodorsally (Fig. 6C, D), except on the last cervical where it is inclined posteriorly (Seeley 1874). From C33 in Muraenosaurus, a medial slip of bone extends between the posterior zygapophyses, into which the lower anterior margin of the succeeding neural arch is wedged (Fig. 6D), thus forming an additional tongued-and-grooved vertebral joint (Seeley 1874). In Cryptoclidus and Tricleidus the neural spines are of similar construction, but are narrower anteroposteriorly and do not reach the same maximum height posteriorly along the neck (Andrews 1910; Brown 1981b). In Tricleidus the neural spines are narrower than in the other two genera, but overall relatively higher than in Cryptoclidus.
Cervical ribs: The cervical ribs have a similar form in all three Oxford Clay genera (Andrews 1910; Brown 1981b). In the atlas-axis complex the atlantal rib is small, although smaller in Muraenosaurus, than in Cryptoclidus and Tricleidus. In all three genera the axial rib is larger than the atlantal rib, and more similar in form to those on the succeeding vertebrae; in Cryptoclidus and Tricleidus the atlas rib is relatively larger than in Muraenosaurus.
In the three Oxford Clay genera, the postaxial cervical rib facets are prominent and single-headed. In Muraenosaurus the cervical rib head is higher than wide, with a slight dorsal projection, and is somewhat inclined posteriorly (Seeley 1874). In Cryptoclidus, the rib facets are about as long as high, and in the anterior of the neck the rib facets extend along the whole length of the centrum, but posteriorly are separated by a small gap from the anterior border (Andrews 1910; Smellie 1916). The cervical ribs are attached to the centra ventrolaterally in the anterior and middle of the neck, whereas posteriorly they begin to rise up onto the sides of the centrum; in Muraenosaurus this occurs at about C40, and by C44 the ribs are partly on the neural arch and partly on the centrum (the “pectoral” vertebrae; Seeley 1865; Fig. 6E); a similar pattern is seen in all plesiosaurs, although details vary.
The postaxial ribs project ventrolaterally, and are dorsoventrally compressed, becoming curved and rod-like posteriorly (Andrews 1910; Brown 1981b). Each cervical rib typically has an anterior projection or flange (the “hatchet” or “hammerhead” shape of Andrews 1910), particularly in the anterior of the neck (Seeley 1874; Andrews 1910). The anterior flange is variably developed, but is an ontogenetic feature that is more prominently developed in “old adults” of Cryptoclidus (Andrews 1910; Brown 1981b). The anterior cervical ribs of Muraenosaurus have roughened tips, indicating cartilage capping (Seeley 1874), and suggesting that individuals lacking clear osteological flanges may have possessed a cartilaginous extension in life. In various plesiosaur taxa (e.g., Plesiosaurus) the ossified cervical rib extensions are closely apposed, and almost touch (Storrs 1997). Posteriorly the cervical ribs become more rounded and longer, resembling the typical bent rod-like morphology of the dorsal ribs (Andrews 1910; Smellie 1915).
Vertebral centra surface ornamentation: On the postaxial cervical vertebrae, the anterior and posterior margins of the centra, particularly the lateral and ventral surfaces, exhibit ornamentation in bands adjacent to the articular faces (Fig. 7A, G). This ornamentation becomes more pronounced with increased age (Brown 1981b): for instance, in “juvenile” individuals of Muraenosaurus the ornamentation is relatively regular, closely-spaced longitudinal ridges (“plications” of Andrews 1910), which in “adults” ossifies to become more strongly developed irregular rugosities, although there may be marked intraspecific variability (Seeley 1874; Andrews 1910; Brown 1981b). In Cryptoclidus, the bone surface is generally smoother and neater in “juveniles”, and rougher and more wrinkled in older individuals (Brown 1981b). The ornamentation is most strongly developed on the cervical vertebrae of Muraenosaurus, but continues onto the “pectoral” and dorsal vertebrae with decreasing prominence caudally.
In Muraenosaurus the atlas is raised into a strong hypophysial ventromedian ridge which extends posteriorly onto the middle of the axis; in Cryptoclidus there is a distinct anteroventral prominence, but the ridge only extends from the atlas onto the anterior of the axis; in Tricleidus the hypophysial ridge is less well-developed anteriorly, but extends to the rear of the axis (Andrews 1910). In plesiosaurs generally, the ventral surfaces of the postaxial centra are gently concave anteroposteriorly and normally exhibit paired nutritive foramina (Fig. 7G), although these foramina may be variably expressed: duplicated, coalesced or absent. The nutritive foramina lie close together anteriorly, but are gradually separated by a low mid-ventral ridge which becomes less prominent posteriorly where the foramina are more widely spaced (Andrews 1910; Smellie 1916; Brown 1981b). The nutritive foramina communicate through the body of the vertebra with the foramina exposed on the centre of the neural canal.
In Muraenosaurus, the cervical centra exhibit a lateral longitudinal ridge (Fig. 7A, G) midway between the base of the neural arch and the top of the cervical rib (Seeley 1874; Andrews 1910). The ridge is generally absent in juveniles where the cervical vertebrae are less well ossified, but is often especially prominent in the anterior half of the neck of older individuals; a similar lateral crest is present in various Cretaceous elasmosaurs (Welles 1943, 1952, 1962). There is no lateral longitudinal ridge in Cryptoclidus or Tricleidus (Brown 1981b).
The neural spines exhibit ornamentation of the bone surface. In Cryptoclidus the base of the axis neural spine is developed into a strong ridge, not seen in Muraenosaurus. In the anterior cervical vertebrae of Cryptoclidus, the zygapophyses are connected by a ridge on the lateral surface of the neural spine (Fig. 7A), which disappears in the posterior of the neck (Andrews 1910; Brown 1981b), allowing the sides of the pedicles to pass uninterrupted into the neural spines. In addition, some specimens of Cryptoclidus exhibit an oblique, roughened ridge (Fig. 7A) sloping anteroventrally approximately halfway between the line of the zygapophyses and the tip of the neural spine (Smellie 1916). In Muraenosaurus, Tricleidus and Cryptoclidus the summits of the neural spines are abruptly truncated by a roughened, subtly V-shaped, indented dorsal surface (Seeley 1874; Andrews 1910; Brown 1981b).
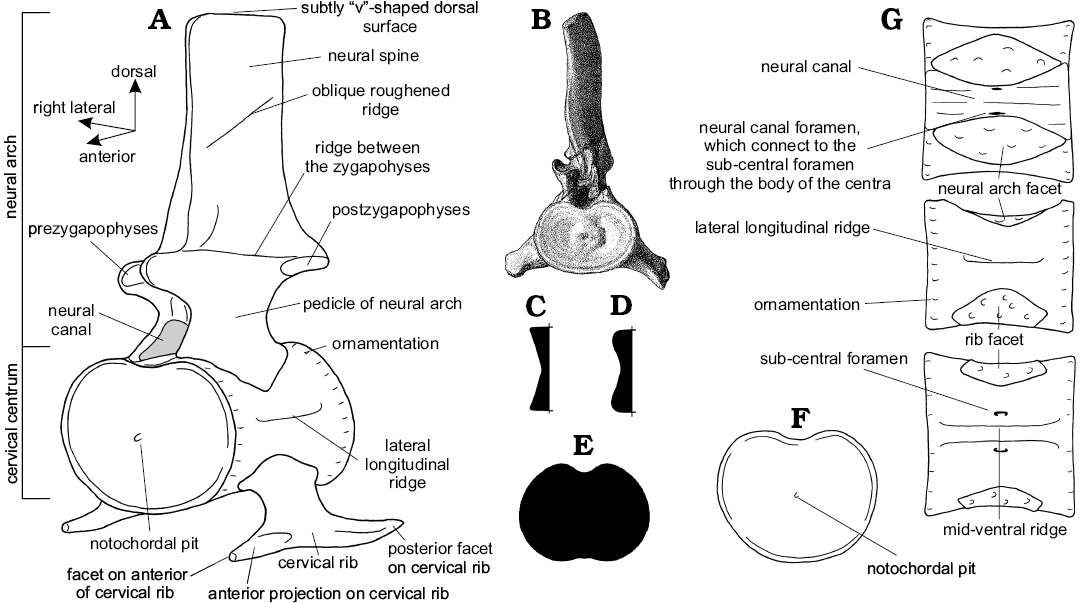
Fig. 7. Cervical vertebrae and cervical centra of plesiosaurs. A. Generalized plesiosaur cervical vertebra showing features mentioned in the text in oblique antero-left lateral view (note: not all features are present in all species). B. Posterior cervical vertebra of Muraenosaurus beloclis illustrating the form as preserved in anterior view (note the wider than high form of the centrum, typical of all plesiosaurs). Illustrative vertical cross-sections through the articular surfaces (to the left) of Muraenosaurus (C) and Cryptoclidus (D), showing variation in cross-sectional shape between genera, which may have affected the range of movement available at contiguous cervical joints (data from Brown 1981b). E. Illustrative articular face of a Cretaceous elasmosaur cervical vertebra, showing the laterally expanded butterfly- or dumbbell-shape. F–I. Views of a generalized plesiosaur cervical centrum (anterior face to the left), showing features mentioned in the text, in anterior (F), dorsal (G), left lateral (H), and ventral (I) views. Images from: B, Andrews (1910: pl. 7: 4); A, C–I, LFN drawings.
Anatomy of the body.—All three Oxford Clay plesiosaurs each had a short, compact, barrel-shaped body, which was somewhat dorsoventrally flattened (Brown 1981b; Fig. 6A). In terms of the vertebral column, the body is defined as commencing where the rib facets first impinge on the base of the neural arch (Fig. 6E). These transitional vertebrae, where the rib facets are partly on the centrum and partly on the lateral processes of the neural arches, have been termed “pectoral” (Seeley 1876). There are usually three pectoral vertebrae in plesiosaurs, the anteriormost of which arbitrarily marks the transition between neck and body (Seeley 1877). The “pectoral” region is followed by the trunk, formed by the dorsal vertebrae (Fig. 6E–H), which commences with the first vertebra where the rib facets are entirely placed on the lateral processes of the neural arches (Owen 1840a, b).
Ventrally, the gastralia are well developed, typically consisting of five interlocking elements per row, closely packed, and presumably tightly bound by ligaments to the ventrally positioned pectoral and pelvic girdles anteriorly and posteriorly, and the dorsal ribs laterally. This arrangement of interlocking gastralia, limb girdles and ribs produced a stiff and relatively inflexible body which must have precluded undulatory motion or compression of the body during swimming (Robinson 1977).
The limbs were the main propulsive organs in plesiosaurs (Andrews 1910; Halstead 1969; Robinson 1975, 1977; Radinsky 1987), and were placed laterally and ventrally at the four “corners” of the body. The limbs were strongly attached to the ventrally placed and typically strongly keeled limb girdles (Andrews 1910). All four limbs were of similar form: hydrofoil-shaped and hyperphalangic (Williston 1902; Carpenter et al. 2010), with tapering tips (Robinson 1975; Massare 1994) and covered with a sheath of integument (Owen 1861). The limbs thus differ considerably from the oar-like extremities of organisms typically living on or close to the water surface (Robinson 1975). Although all limbs were of similar shape, the fore-limbs in plesiosaurs are typically larger and longer than the hind-limbs, in contrast to pliosaurians where the reverse is generally true (e.g., Brown 1981b), although variation within Plesiosauria is more complex than this simple dichotomy (O’Keefe 2002). The limbs were heavily muscled (Colbert 1958), as evidenced by the prominence of muscle scars on the propodials (Robinson 1975). Thus it is generally agreed that plesiosaurs, uniquely amongst aquatic organisms, were propelled by all four limbs to bring about movement in water (e.g., Robinson 1975, 1977; Fig. 3).
The plesiosaur tail was moderately short and tapering, and typically shorter than the trunk (Williston 1914). The tail was not primarily involved in propulsion, although there might have been a small tail fin which was possibly used as a rudder (Storrs 1993; Smith 2013).
Discussion
We now consider functional interpretations of the role of the plesiosaur head, neck, and body, in relation to our new model. The most fruitful approach appears to be to consider the plesiosaur bauplan as simultaneously crucial for both feeding and locomotion.
Functional analysis of the neck.—Plesiosaurs have a cervical region constructed in a very specific and remarkably consistent manner (Fig. 8). The numerous cervical segments, sometimes individually elongated, but largely lacking osteological stiffening mechanisms, indicate that neck length was functionally important. Most obviously, elongation of the neck increases the animals’ reach by moving the head away from the body, analogous to the giraffe’s neck, and broadly comparable to the elephant’s trunk. The consistent presence of numerous cervical segments that lack bony stiffening adaptations, however, is also strong evidence that flexibility was an important functional element in plesiosaur necks (Evans 1993), and gives the potential for a considerable range of movement in the living animal (cf. Zarnik 1925–1926; Fig. 9). If flexibility was unimportant, bony and ligamentous constraints and a smaller number of longer cervical vertebrae would have been more efficient functional solutions in terms of mass and energy consumption by postural and locomotor musculature. This occurs, for instance, in the neck of Tanystropheus where the cervical region is constructed from just 12 elongated segments and stiffened by overlapping cervical ribs (Wild 1980; Tschanz 1988; Taylor 1989; Nosotti 2007). This suggests a relative lack of neck flexibility in Tanystropheus, when compared to the numerously segmented and unsupported cervical system of plesiosaurs.
Neck flexibility: Previous workers have considered the degree of neck flexibility in plesiosaurs to range from: extreme mobility (Hawkins 1840; Zarnik 1925–1926; Welles 1943; Welles and Bump 1949), including the ability to arch the neck like a swan (Conybeare 1824; Andrews 1910; Brown 1981b); through relative inflexibility (Hutchinson 1897; Williston 1914; North 1933; Shuler 1950; Storrs 1997); to almost complete rigidity (Buckland 1836; Watson 1924, 1951; Cruickshank and Fordyce 2002; Figs. 3, 9); although some of this variation in interpretation may be due to differences between the species studied (Watson 1924, 1951). It has also been proposed that flexibility varied along the neck, with more movement possible in the anterior third (Williston 1906; Shuler 1950) and much less, or almost none at the neck-body junction (Owen 1861; Andrews 1910; Robinson 1977). Some authors have argued dorsal flexure was restricted by the height and close apposition of the neural spines, especially posteriorly (Fig. 8), with only limited flexure available anteriorly (Williston 1914; Watson 1924; Storrs 1997). Others have considered flexibility in the horizontal plane to be considerable (Evans 1993; Bakker 1993), whilst others have argued lateral movement was limited by the overlap or contact of the contiguous “hatchet-shaped” cervical ribs (Buckland 1836; Williston 1914; Watson 1924, 1951). In Cretaceous elasmosaurs lateral mobility has been considered strictly limited by the closely apposed, wide and flat-faced posterior cervical centra (Welles 1943; Watson 1951; Storrs 1993). Ventral movement in the plesiosaur neck has been considered relatively unrestricted (e.g., Shuler 1950), with the cervical ribs providing attachment for strong hypaxial muscles to depress the neck (Watson 1924).
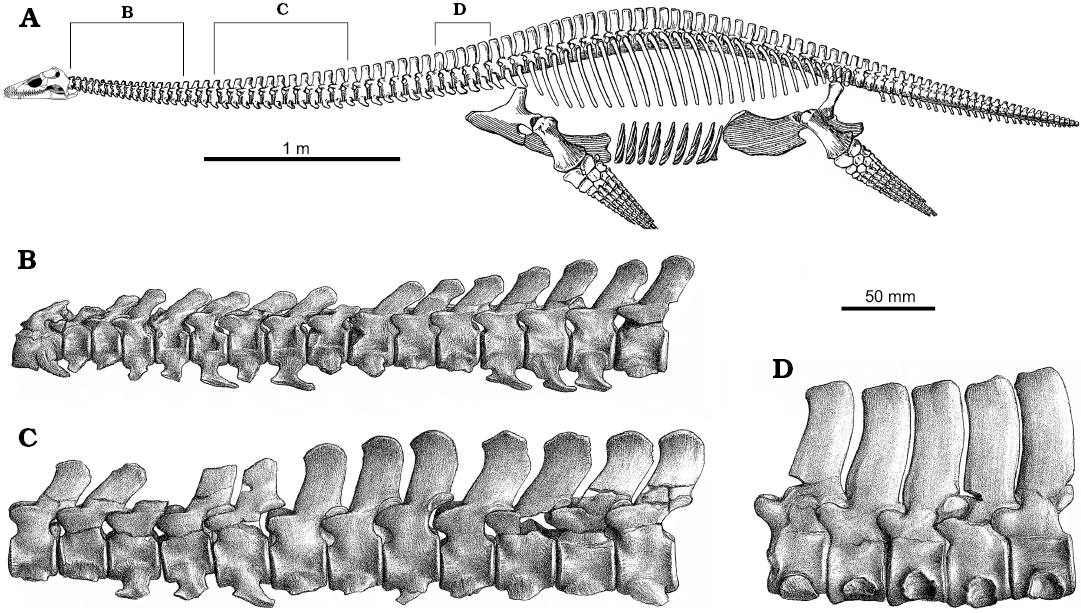
Fig. 8. Skeletal reconstruction of Muraenosaurus and articulated vertebrae of Muraenosaurus beloclis in right lateral view. A. Skeleton showing the approximate locations of the vertebral series (B–D). B. Articulated atlas-axis complex and C3–C17. C. Articulated series of 12 mid-cervical vertebrae. D. Posterior cervical vertebrae. B–D reversed to match skeleton above. Images from: A, Andrews (1910: fig. 66) with the newly reconstructed head based on Evans (1999); B–D, Andrews (1910: pl. 7: 5, 3).
In order to determine the range of flexibility available along the plesiosaur neck, it is necessary to carefully separate out the relevant anatomical factors. For instance, absolute and relative neck lengths, and the length of the individual cervical vertebrae, primarily used for taxonomic purposes (Welles 1943, 1952; Brown 1981b), are important for understanding reach, but are not particularly useful for understanding flexibility. The most important and easily observable parameter controlling neck flexibility is the shape of the articular surfaces of contiguous cervical vertebrae, as this can be expected to affect the range of movement available to the living animal (Zarnik 1925–1926; Evans 1993; Fig. 3).
To simplify the problem, the range of movement available at any point along the neck of a plesiosaur can be resolved into three components: dorsoventral pitch, pivoting about the transverse axis of the body; lateral yaw, about the vertical axis; and rotational roll, about the longitudinal axis (Fig. 10). As all movement along the neck occurs at the joints between contiguous vertebrae, any action, such as darting the neck in and out from the body (Bakker 1993), holding the neck in a swan-like pose (Conybeare 1824; Andrews 1910), or throwing the neck into coils to crush fish (e.g., Zarnik 1925–1926) is simply a more or less complex summation of the interactions of each of these three components of movement for the many intervertebral joints along the length of the neck (Figs. 3, 9).
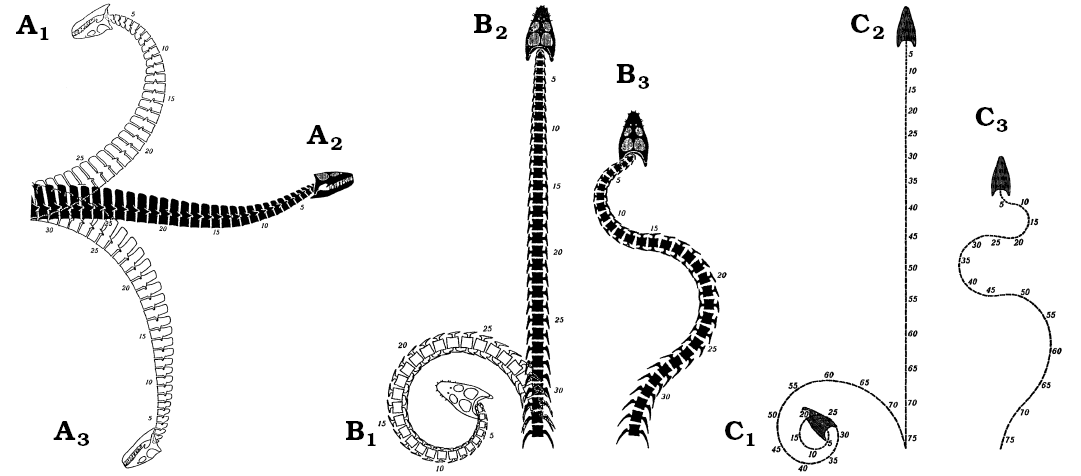
Fig. 9. The range of movement available to the plesiosaur neck in Seeleyosaurus (formerly Plesiosaurus guilelmi imperatoris) (A, B) and Elasmosaurus (C) as illustrated by Zarnik (1925–1926), which is radically different from that proposed in the present paper. A. Right lateral view showing neutral position (A2), interpreted maximum dorsal (A1) and ventral (A3) flexion. B, C. Dorsal view, showing the neutral position (B2, C2), extreme left lateral flexion (B2, C2), and maximum sinusoidal alternating lateral flexion (B3, C3). Images from Zarnik (1925–1926: figs. 18–20).
In plesiosaurs, the articular faces of the cervical centra are consistently wider than high (Figs. 6, 7) which suggests preferential dorsoventral flexion of the neck (Fig. 11A, B). A terminal articular face that is shallow from top to bottom can be expected to facilitate dorsoventral bending (pitch), whereas an articular face that is broad from side-to-side will restrict lateral bending (yaw). Rotation (roll) between contiguous vertebrae will not be greatly affected by the form of the articular faces, but will be limited by other soft tissues such as ligamentous ties and muscles. Hence, the enhanced width of the cervical centra seen in all plesiosaurs (Brown 1981b) suggests the neck was preferentially adapted for dorsoventral flexion with lateral movement somewhat restricted; this is in contrast to the subcircular vertebral centra found in “fish” and ichthyosaurs which suggests, based solely on the shapes of the contiguous articular surfaces of the centra, that movement was equally permissible in all directions. Rotational movement along the vertebral column is affected less by the shape of the articular faces than by the constraints imposed by the zygapophyses, the shape of the neural spines, and the relationships between contiguous cervical ribs.
The well-developed zygapophyses in plesiosaurs have been used to suggest the neck was relatively stiff (Buckland 1836; Williston 1914) but also relatively flexible (Brown 1981b). However, both conclusions appear to contain valid elements: the zygapophyses in general, and the peg-and-socket morphology of the posterior zygapophyses in Muraenosaurus, severely restrict and perhaps even preclude rotational movement about the long axis of the neck. This would also restrict dorsal and probably lateral movement, particularly when the neck was held out straight (Buckland 1836; Williston 1914). However, it is unlikely that the form of the zygapophyses much affected ventral flexion.
The height and tilt of the neural spines and their close apposition, especially toward the rear of the neck, severely limited dorsal flexion (Fig. 8), although a small amount of freedom of movement was possible, particularly in the anterior segments of the neck. The interlocking accessory articulations of the posterior neural spines in Muraenosaurus not only precluded rotation about the long axis, but also only permitted strictly limited lateral movement, especially when the neck was held out straight, as previously inferred for Cretaceous elasmosaurs (Welles 1943). Ventral movement of the neck was unaffected by the morphology of the neural spines.
It is not entirely clear how the cervical ribs interacted in life, but the hatchet-shaped anterior cervical ribs are likely to have restricted lateral bending by their close apposition (Buckland 1836; Williston 1914; Watson 1924, 1951; Storrs 1997). In the posterior of the neck, the rod-like cervical ribs overlap (Owen 1861), but sliding between the elements was severely limited by the intercostal muscles and connective tissues. However, the triradiate form of the cervical ribs and neural spines, projecting from the centrum in anterior or posterior views, suggests bending would preferentially occur between the cervical ribs, or cervical ribs and neural spine (Fig. 10C), much as a tripod will fall over between two of its legs, and not over them. Assuming a completely circular articular face, this three-point system would preferentially permit bending dorsolaterally both left and right, or ventrally. This available movement would be modified towards the rear of the neck as the positions of the cervical ribs rise towards the neural arch, enhancing the potential for ventral bending, but further restricting components of lateral movement as the cervical ribs act to widen the vertebral complex. Dorsoventral or rotational movement along the neck was probably minimally restricted by the cervical ribs, especially posteriorly. However, taken together, this combination of anatomical features indicates neck function was based primarily on ventral flexion, with significant stiffening and resistance to movement in other directions, especially posteriorly.
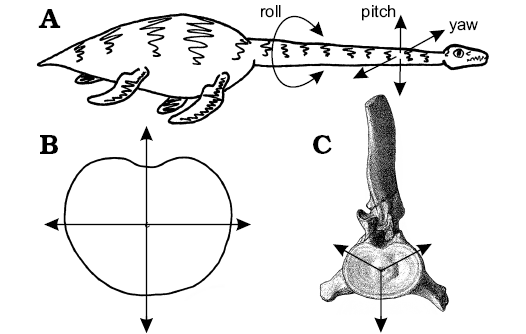 |
Fig. 10. The range of movement available to the plesiosaur neck. A. Sketch of living or plesiosaur illustrating the three senses of motion (roll, pitch, and yaw) potentially available at each vertebral junction. B. Enhanced dorsoventral movement produced by the laterally expanded articular face of a post-axial cervical vertebrae. Note how more of the same length arrows project dorsoventrally than laterally, indicating greater potential for flexibility in dorsoventral pitch that lateral yaw. C. The potential range of movement available at a plesiosaur posterior-cervical vertebra, where freedom of movement is greatest dorsolaterally between the neural spine and cervical ribs, or ventrally between the cervical ribs, in the same way a tripod is more likely to fall over between two of the legs than over one of them. Once the anatomy of the zygapophyses, neural spines, and cervical ribs are taken into account, the movement is restricted to the ventral direction. Images from: C, Andrews (1910: pl. 7: 4); A, B, LFN drawings. |
In addition to the neck, movement was also possible at the craniocervical joint between head and neck. In principle the craniocervical ball-and-socket joint permits a wide range of movement (Shuler 1950; Cruickshank and Fordyce 2002), however, the posteroventral slope of the squamosal-quadrate arch, and the orientation of the posterior braincase elements (Fig. 4), effectively place the atlas-axis complex inside the rear of the skull, covered by the suspensorium in lateral view. This suggests preferential dorsoventral movement, and somewhat reduced lateral flexibility. However, this is not corroborated by the dorsoventrally elongated shape of the atlantal cup (Shuler 1950), which indicates preferential lateral movement at the head-neck joint. Hence the head was probably relatively mobile in all directions on the anterior of the neck.
Overall, the range of movement available to the plesiosaur neck was strictly limited. The articular faces of the vertebrae imply enhanced dorsoventral bending ability over lateral flexibility. The form of the zygapophyses indicates severely restricted rotation about the long axis (Buckland 1836; Williston 1914). Dorsal bending was severely restricted by the position, height and form of the neural spines (Williston 1914; Watson 1924; Storrs 1997), and the cervical ribs restricted lateral movement of the neck (Buckland 1836; Williston 1914; Watson 1924, 1951), especially posteriorly. The osteological evidence thereby clearly indicates the plesiosaur neck was not capable of the S-shaped, swan-like postures (Andrews 1910), or elaborate twists and bends (Zarnik 1925–1926), often described or depicted for plesiosaurs (e.g., Hutchinson 1897; Storrs 1993; Cruickshank and Fordyce 2002; Figs. 3, 9). Rather, it was principally adapted for ventroflexion (Fig. 11). Indeed, the distribution of vertebral pathologies (Schmorl’s nodes and vertebral wedging; Hopley 2001) in a Lower Jurassic plesiosaur is consistent with compressive stresses resulting from ventral bending of the neck. In conclusion, neck anatomy indicates a function which lay predominantly in ventroflexion. This restricted flexibility also precludes the considerable bending of the neck required to shoot the head at speed after prey (see Zarnik 1925–1926: fig. 26). However, preferential ventral movement of the neck does not preclude limited dorsal, lateral or rotational flexion, for instance sufficient to raise the head to the surface to allow breathing (Zarnik 1925–1926: fig. 21).
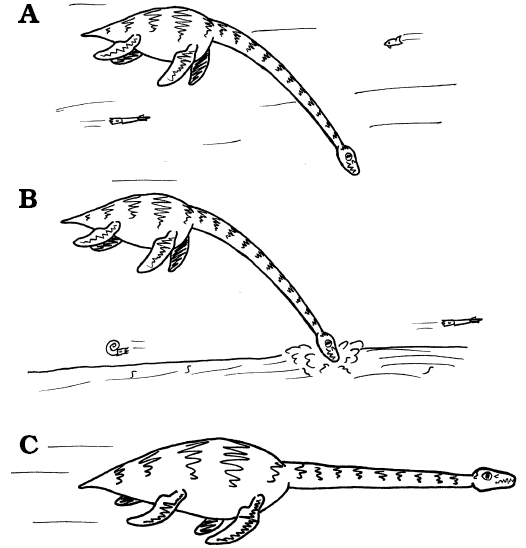 |
Fig. 11. Lifestyle strategies in plesiosaurs. A. Feeding beneath the body on mesoscopic prey (average diameter 5–10 mm and 100–200 mm), probably shoaling within the water column. B. Feeding on similar sized prey from within soft sediments. C. The escape response, with the neck approximately straight in front of the body, and held rigid by the presence of a dorsal ligament system, thereby permitting relatively rapid motion with minimal muscular effort to hold the head and neck anterior of the locomotor apparatus. Images from LFN drawings. |
Neck musculature: The osteological analysis is supported by what can be deduced about the neck musculature. The triradiate shape of the postaxial cervical vertebral segments (Fig. 10C), and the presence of roughened surfaces on the centra, neural spines and cervical ribs, all indicate the presence of strong cervical musculature and ligamentous ties. The anterior and posterior areas of ornamentation on the cervical vertebrae (Fig. 7A, G) have been interpreted as the attachment points for ligaments tying adjacent vertebrae together strongly (Brown 1981b). Bony ridges indicate the presence of strong neck musculature (Hawkins 1840), well-supplied with blood vessels (some passing through the subcentral foramina) and nerves (passing between the vertebrae) to feed and innervate the soft tissues of the neck. The strong basioccipital basitubera and the fused atlas-axis complex, found in all plesiosaurs, also allowed for the origin and insertion of powerful muscles to actuate the head (Bakker 1993). The lateral longitudinal ridge, developed in the anterior of the neck of longer-necked plesiosaurian taxa such as Muraenosaurus and the Cretaceous elasmosaurids (Welles 1943, 1952, 1962; Brown 1981b, 1993), and the roughening on the neural spines in taxa such as Cryptoclidus (Brown 1981b), further indicate the strength of muscle attachments. Subtle osteological differences between taxa suggest the muscles were arranged somewhat differently in, for instance, Cryptoclidus and Muraenosaurus.
The placement of the neural spines and cervical ribs thereby provided a firm three-point attachment system to flex the neck (Fig. 10C). Dorsally, the upper two portions would have provided leverage for the epaxial musculature to raise the neck and stiffened it against ventral and lateral bending moments when swimming. Anteriorly, the cervical ribs were shorter and more ventrolaterally located, suggesting that they were insertion points for muscles controlling ventral flexion of the neck, working antagonistically against the dorsal muscles, as well as giving some control over lateral bending. The powerful neck musculature would thus have allowed fine control of the head and neck in all available components of motion.
Dorsal nuchal ligament system: There was seemingly present in all plesiosaurs a strong nuchal, supraspinous or dorsomedian ligament extending from the rear of the skull posteriorly and over the cervical neural spines (Brown 1981b; Brown et al. 1986; Carpenter 1997; Storrs 1997; Gasparini 2009). The sloping ridge midway between the zygapophyses in Cryptoclidus, and the roughened and subtly V-shaped dorsal surfaces of the neural spines in virtually all Plesiosauria (Brown 1981b), are strongly indicative of attachments for ligamentous slips from a substantial nuchal ligament system along the dorsal surface of the vertebral column (Seeley 1874; Andrews 1910; Brown 1981b; Brown et al. 1986). The midline pit on the rear of the skull of many plesiosaurs has also been interpreted as the attachment point for a nuchal ligament system attached to the rear of the cranium (Brown et al. 1986). This ligamentous system may also have spread anteriorly onto the well-developed sagittal crest.
In most modern terrestrial tetrapods, the nuchal ligament system runs from the occiput or the anterior cervical vertebrae, along and above the dorsal surface of the cervical neural spines, to insert onto the thoracic neural spines (McGowan 1983, 1992; Gellman and Bertram 2002a; Wang et al. 2008). The ligament system is highly developed in animals such as ruminants with large, heavy heads, but is generally reduced or absent in birds, carnivores and primates (McGowan 1992; Gellman and Bertram 2002a, b). In herbivorous terrestrial mammals, the nuchal ligament system helps support the weight of the heavy head against gravity, whilst allowing the neck a wide range of ventral movement and some lateral flexibility. When the cervical muscles are relaxed, the head is held off the ground by the taut nuchal ligament, which may be aided by the epaxial cervical musculature (Dimery et al. 1985). During feeding, the head is brought into contact with food on the ground by the hypaxial musculature, working against the nuchal ligament (McGowan 1983; Gellman and Bertram 2002a). Energy is stored by stretching the elastin-rich ligament, and when feeding ceases, the head is raised, partially by the energy stored within the ligament, and partly by the epaxial musculature (McGowan 1983, 1992). During walking or running, tension in the nuchal ligament helps hold the head clear of the ground (McGowan 1992).
In plesiosaurs, the nuchal ligament was not so much needed to act against gravity, as the weight of the neck was presumably largely supported by the buoyancy of water (Taylor 1989). However, the plesiosaur neck was so long that it acted as a cantilever holding the head out from the body. This put a premium on reducing unnecessary distal (i.e., cranial) mass (Fig. 4). With the head cantilevered out from the body, the load on the neck (a beam) increased towards the rear as each vertebra had to carry an increasing number of cervical vertebrae anteriorly to prevent the neck from drooping along its length (due to its construction from muscles and bones with a density greater than that of water), or rising (due to buoyancy from air in the trachea and from fatty deposits). In engineering, this tendency is compensated for by deepening the beam. The presence of deepening along the plesiosaur neck towards the body (Williston 1914), and the light construction of the head, are thus strong evidence that the weight (or buoyancy) of the neck in water was a significant determinant of plesiosaur functional anatomy. A nuchal ligament as an integral part of this system would minimize the need for postural muscles which would have increased the mass of the neck and required costly materials and energy consumption for construction, movement and maintenance.
Here we assume the neck was negatively buoyant relative to sea water, as the neck was constructed from dense muscle and very dense bone; hence we consider positive buoyancy of the neck to be highly unlikely. This implies the body would have to support the weight of the neck, and also stop the animal from rotating head-downwards. It is not entirely clear how much effect the positive buoyancy of the air in the trachea would have had in counteracting the weight of the neck. It is likely the air-filled tracheal lumen would only compensate for a small percentage of the weight of muscle and bone in the neck. However, as the plesiosaur neck deepens posteriorly (Williston 1914), and assuming the tracheal lumen was of approximately constant diameter, the relative impact of any buoyancy provided by air would reduce with proximity to the body. This would be in addition to any contribution from partly oil-filled cervical centra, variation in buoyancy with depth and any tracheal collapse.
Functional analysis of the body.—We now consider the locomotor adaptations of plesiosaurs, which were large mobile organisms, highly adapted to life within the water column (Andrews 1910; Taylor 1987; Massare 1987; Collin and Janis 1997; Mazin 2001). The body was short, stiff, compact and dorsoventrally flattened (Buckland 1836; Hawkins 1840; Williston 1902, 1914; Colbert 1966; Bakker 1993) with no possibility of lateral undulation (Robinson 1975, 1977; Massare 1988). The tail was short and compact (Hawkins 1840; Williston 1902; Andrews 1910; Halstead 1969), and although this has been considered a powerful organ of propulsion (Welles and Bump 1949), the consensus is that the tail was not used for swimming (e.g., Hutchinson 1897; Williston 1914; Alexander 1989). However, it is possible a small tail-fin was present (Dames 1895; Halstead 1969; Smith 2013) which might have acted as a rudder (Buckland 1836; Hutchinson 1897; Robinson 1975), stabilizer (Taylor 1981), or both.
In plesiosaurs all four limbs were the organs of propulsion. They were heavily muscled, and the bones of the flippers were tightly-interlocking and hyperphalangic with tapering tips. This morphology indicates the limbs were used as relatively inflexible, high aspect ratio hydrofoils (Robinson 1977; Storrs 1993; O’Keefe 2001b). Hence, the limbs functioned as stiff, wing-like appendages (Robinson 1975; Massare 1988) for an efficient, if modified, version of lift-based underwater flight (see Robinson 1975, 1977; Tarsitano and Reiss 1982; Godfrey 1984; Halstead 1989; Storrs 1993; Massare 1997). Although the fore-limbs are generally larger than the hind-limbs in plesiosaurs (Robinson 1975; Halstead 1989; O’Keefe 2002; O’Keefe and Carrano 2005), all four extremities are of a very similar shape and construction, so it is likely locomotion was undertaken using all four limbs (Watson 1924; Robinson 1975; Halstead 1989; Massare 1994). The exact action of the limbs is not known (Tarsitano and Reiss 1982; Halstead 1989; Lingham-Soliar 2000), although it is likely the fore- and hind-limbs worked simultaneously but the two sets probably acted independently on either side of the body (Long et al. 2006), as each hind-limb needed to work within the vortex wake produced by the forelimb in such a way that its own operation was promoted rather than obviated (Halstead 1969; Storrs 1993); this would have required changes in frequency and/or phase depending on swimming speed, the details of which remain unknown. Reorganisation of the reptilian central nervous system would have been necessary to allow independent control of the fore- and hind-limbs for more subtle control of the body within water (Taylor 1981; Carroll 1985).
Streamlining: Plesiosaurs have been considered both perfectly adapted for aquatic life (Williston 1914) and not ideally streamlined (Watson 1924; Taylor 1981; Storrs 1993). The apparent sudden widening and deepening of the trunk at the neck-body junction (Watson 1951) is associated with posterior cervical vertebrae not representative of the rest of the cervical series (Storrs 1997). Hence it is likely the neck-body transition was affected by both the posterior cervical and “pectoral” vertebrae, both of which have been postulated to act as anchorage for a considerable volume of muscles arising from the anterior locomotor complex of the forelimbs (Williston 1914; Robinson 1975; Brown 1981b). This musculature would have provided substantial soft-part streamlining at the rear of the neck and anterior of the trunk, and thereby would have considerably enhanced the streamlined form of the body exhibited by plesiosaurs. Similarly, the muscles around the hip girdle and connected to the tail, such as the caudofemoralis musculature, would have smoothed body contours at the base of the tail. Skeletal shape in plesiosaurs might not have been ideally adapted to minimise total drag (Massare 1988, 1997; Storrs 1997); nevertheless the streamlined shape of the head (Taylor 1987; Rieppel 1997), the long tapering neck (Williston 1914), the cervical region merging smoothly into the “pectoral” and dorsal regions (Brown 1981b), the streamlined body (Welles and Bump 1949) and the smooth transition from body to tail all suggest a fully aquatic lifestyle (Olson 1971). Therefore plesiosaurs can be confidently reconstructed as primarily adapted for life within the water column (Robinson 1975; de Buffrénil and Mazin 2001), and not living close to the air-water interface, or as having a semi-aquatic lifestyle.
Nevertheless, overall streamlining was apparently badly spoilt by the large surface area, and hence profile and parasite drag, of the long neck (Massare 1988, 1994). Swimming efficiency was further impaired by the mass of the neck, and the stomach stones commonly preserved in plesiosaurs (Williston 1902; Brown 1904; Shuler 1950; Darby and Ojakangas 1980; Martin and Kennedy 1988; Everhart 2000; Cicimurri and Everhart 2001). This stone ballast was probably needed to establish trim control and longitudinal stability to enable the animal to swim slowly horizontally and to hover (e.g., Taylor 1987; Henderson 2006), especially when diving in shallow water when the animal was positively buoyant. This combination of features shows that plesiosaurs were animals in which swimming efficiency was important, at least in terms of energy consumption per unit cost of locomotion, but which were probably habitually slow swimmers, as indicated by the use of stone ballast (Taylor 1987). This suggests that high acceleration and high sustained swimming speeds were much less important in plesiosaurs than in the short-necked pliosauroids. This combination of slow swimming and high locomotor efficiency may seem improbable; however, Rhincodon (whale sharks) and Cetorhinus (basking sharks) are examples of slow swimmers which are also extremely efficient at cruising as shown by their high aspect ratio propulsive caudal fins, and steering pectoral fins (Sambilay 1990). In sharks, there is a trade-off between speed and efficiency, which require high aspect ratio fins, and manoeuvrability, which requires lower aspect ratio fins. However, this polarity does not exist in plesiosaur swimming, because all four limbs were used in propulsion and steering, as shown by their common structure and implied by the large ventral limb-girdles bearing well-developed actuator musculature. Rather, with limbs at the four corners of the body, plesiosaurs could potentially produce vectored thrust from different limbs, to provide fine control of movement in all directions, and around all axes. This is more useful in slow swimming or hovering animals than simple shark-like control fins, which require movement in order to generate a current over the control surfaces (Taylor 1981). This reinforces data from neck length and buoyancy control strategies for slow swimming in plesiosaurs, and provides the additional possibility of hovering within the water column whilst feeding.
Swimming speed: Plesiosaurs have generally been considered relatively slow swimmers, with the long neck impeding movement through water (Conybeare 1824; Buckland 1836; Williston 1902; Andrews 1910; Watson 1924, 1951; Shuler 1950; Colbert 1966; Taylor 1981; Massare 1988, 1994, 1997; Alexander 1989). A few authors, however, have considered plesiosaurs to be fast swimmers (Hutchinson 1897; Halstead 1969; Bakker 1993). Analysis of body shape in marine reptiles indicates that plesiosaurs were slower moving in water than pliosauroids, for a given body length, but faster than crocodilians or mosasaurs (Massare 1988; Motani 2002), with swimming speeds estimated at 2 ms-1 for a 3 m long animal (Alexander 1989). Plesiosaurs have been considered highly manoeuvrable (Watson 1924; Olson 1971), capable of rapid turns using their paddles as efficient water brakes (Welles and Bump 1949) or for “backwatering” (Watson 1924, 1951; Halstead 1969; Taylor 1981); however, this has been rejected on anatomical grounds (Robinson 1975). In any case, the drag generated by the length of the neck, whilst making such manoeuvres, would almost certainly have been greater than the muscular strength available to the animal. A study of the high aspect ratio flippers of plesiosaurs concluded they were specialized for slow but energetically efficient cruising, but were not very manoeuvrable (O’Keefe 2001b), corroborating suggestions based on a combination of an efficient locomotor system associated with a long neck (Robinson 1975).
The long plesiosaur neck has been regarded as a potential target for predators in a slow-moving organism unable to move rapidly when required (Conybeare 1824; Buckland 1836; Andrews 1913; Watson 1951; Fig. 2). One solution to this dilemma is for the neck to be held free of the water (Halstead 1969; Brown 1981b), but this can be discounted as the neck could not have been held up sufficiently high (Henderson 2006; this work), and would have had an unbalancing effect on the body (Shuler 1950). Moreover, near subsurface locomotion is exceedingly inefficient due to the production of a “bow wave” (Fig. 3C), even if moving slowly (Alexander 1989), assuming the flippers could have remained wholly underwater for efficient swimming.
Buoyancy control: Control of buoyancy within water is critical for swimming tetrapods (Shuler 1950; Taylor 1987; Storrs 1993). Neutral buoyancy in water would allow the plesiosaur body to act as a feeding platform, by permitting the animal to hover in, or move slowly through, the water column whilst minimising limb action, and maximising the efficiency of locomotion. The dorsoventral flattening of the body might also have provided additional stabilization during slow underwater swimming (Brown 1981b).
Numerous plesiosaurs have been discovered with exotic sand, gravel or pebbles, sometimes in considerable quantities, in a position in the body cavity which suggest in life they were contained within the gut, presumably in or close to the stomach (Williston 1902; Brown 1904; Shuler 1950; Darby and Ojakangas 1980; Martin and Kennedy 1988; Everhart 2000; Cicimurri and Everhart 2001). These gastroliths, or stomach stones, would undoubtedly have had an impact on buoyancy in water during underwater flight (Taylor 1993). Weight (not mass) in water is important for buoyancy control, especially as stomach stones are typically 2.6 times the density of water, whereas the body of a plesiosaur, on average, was probably slightly less dense than sea water. On the other hand, it has also been suggested that gastroliths functioned as a gastric mill to break down food in lieu of oral processing (Welles 1949; Shuler 1950; Taylor 1987; Massare 1987; McGowan 1992), possibly in the stomach or a muscular crop-like structure (Andrews 1910) by a process termed “gut chewing” (Reilly et al. 2001). However, it seems highly likely that gastroliths in the digestive tract of a plesiosaur, even if primarily for buoyancy control, would also have been co-opted for gut chewing.
Bone ballast (more or denser bone) is also seen in some plesiosaurians, and both gastroliths and bone ballast have been regarded as more efficient buoyancy control mechanisms for slow swimming organisms than hydrodynamic systems (Taylor 1981, 1992; Storrs 1993; Taylor 1993, 1994, 2000; Houssaye 2009). Stone has a greater impact on buoyancy than bone, and usually can be more easily collected and discarded, and at less metabolic cost (Taylor 1993, 1994, 2000). Gastroliths should therefore probably be seen as part of an overall buoyancy control system, allowing for adjustments of buoyancy in the water column, in conjunction with the air in the lungs (controlled through the degree of exhalation on or during diving). This produced a temporally layered system of buoyancy control in plesiosaurs, including the bone forming the body (slow: changed only at rates determined by physiology), gastroliths (intermediate: changed by ingestion, regurgitation or trituration), and volume of air in the lungs (rapid: potentially changed for every dive). Moreover, the buoyancy provided by air in the lungs would decrease, initially very rapidly, with depth; a 10 m overlying water column adds the equivalent of one atmosphere of pressure, compressing air in the lungs and trachea by 50%, assuming no exhalation. A plesiosaur could therefore have adjusted the depth at which it became neutrally buoyant to that most appropriate for feeding (Taylor 1993, 1994, 2000). It has been argued, from analysis of mathematical models of floating plesiosaurs (Henderson 2006), that gastroliths had little impact on buoyancy. However, a role in buoyancy control is strongly indicated by the taxonomic distribution of gastroliths (Taylor 1993), which shows a gross correlation with locomotor mode rather than diet amongst non-herbivorous aquatic tetrapods, and is supported by the frequent presence of intact (i.e., largely unbroken) cephalopod hooklets intermixed with the gastroliths, suggesting gastrolith function was primarily to control buoyancy in water.
One way to resolve this dichotomy of opinion is to note that there were potentially three static states for a plesiosaur which had some positive buoyancy at the surface: (i) static and stable, whilst floating at the surface; (ii) neutrally buoyant at a particular depth but unstable, maintained by body mass, gastroliths and lung inflation, and reached by active swimming; and (iii) static and stable, whilst resting on the sea floor, the position maintained by negative buoyancy. State (i) is dealt with by Henderson (2006) where an improbably large volume of gastroliths would be required to produce neutral buoyancy at the surface, whereas our analysis deals with state (ii), with plesiosaurs swimming and feeding fully submerged at depth, as a result of reduced volume air in the lungs, possibly modified by exhalation on or during diving; state (iii) has been inferred as a possible mode of feeding for the pachyostotic pliosaurid Pachycostasaurus (Cruickshank et al. 1996).
Hence a living plesiosaur had a margin of positive buoyancy at the surface (“freeboard” in nautical terminology), whereas a neutrally buoyant animal did not. This can be compared to a modern submarine, floating at the surface in dock, where loading consumables will make very little difference to its trim, yet it will make a great deal of difference to its submerged state, the weight of which needs to be carefully compensated for before safe diving can occur. Hence, based on the above, Henderson (2006) provides valuable evidence for plesiosaur positive buoyancy (freeboard) at the surface for respiration (especially at times of oxygen debt), but also argues against surface living in plesiosaurs. In addition, the Henderson (2006) model assumes a static, unmoving animal, (mostly) with fully inflated lungs whereas the living plesiosaur would have been actively or passively moving towards the surface when breaching, adding locomotor and inertial forces to the static model, and may or may not have had lungs full of air. The streamlining of the plesiosaur body, and the hydrofoil form of the flippers, provide ample evidence for life within the water column, where even a small percentage of gastrolith ballast would have had a significant impact on buoyancy control for the living plesiosaur.
Functional analysis of the head.—Plesiosaurs are usually considered as large, obligate aquatic predators that swallowed live prey whole within the water column (Andrews 1910; Taylor 1987; Massare 1987, 1997). Plesiosaur teeth generally lack well-marked cutting edges or serrations (Massare 1987); a description of Cryptoclidus teeth as markedly carinate is based on metriorhynchid crocodilian teeth found with NHMUK R8621 (Brown 1981b: 265, 267, fig. 5; Noè 2001). In addition, the simple open-and-shut jaws and the elongate dentition were incapable of complex oral processing (Shuler 1950; Taylor 1987; Massare 1987). Prey had to be swallowed whole, as shown by the inability to reduce large prey items into smaller pieces. The small, akinetic skull limited the size of prey relative to the plesiosaur (Williston 1902, 1914; North 1933; Shuler 1950; Massare 1987; Bakker 1993). This emphasis on small prey is reinforced by the light construction of the skull and the short, shallow mandibular symphysis. However, the wide gape of the jaws (Welles and Bump 1949; Evans 1999) maximised the size range of prey, especially long thin organisms, within this overall constraint.
The short jaws of plesiosaurs produced a relatively slow bite (Bakker 1993), unlike the rapid bite of modern long-jawed fish- or cephalopod-eating crocodilians and dolphins (Langston 1973; Iordansky 1973; Busbey 1995). Thus, fish and cephalopods have been considered too fast-moving to have been regular plesiosaur prey (Taylor 1981; Alexander 1989), except perhaps for heavily armoured, and presumably slower moving, “holostean” fishes (Halstead 1969). However, the long slender shape of plesiosaur teeth (Fig. 5), and the almost complete absence of wear on the tooth tips (Brown 1981b; Massare 1987; Storrs 1997), are inconsistent with a diet of: slow-moving, externally armoured or large, hard-boned fish; externally shelled cephalopods; or internally guarded teuthoids (Massare 1987; Brown and Cruickshank 1994; Martill et al. 1994). Moreover, such teeth are relatively weak (Conybeare 1824) especially against bending, and poorly adapted to resist the struggles of relatively large or powerful prey. In addition, the vast majority of plesiosaurs have strongly procumbent anterior teeth (e.g., Brown 1981b; Fig. 5A–C), which strongly argues against a piercing function: prey caught on the anterior teeth would have to be moved away from the gullet during subsequent jaw opening, risking prey loss. Also, although the teeth of plesiosaurs are somewhat variable in size, they are relatively homodont (Massare 1987); with only slight variation in tooth size along the jaws. This reduces the degree of functional specialisation along the tooth row compared to pliosauromorphs, where large caniniform teeth were used to grasp and penetrate prey, and hooked and ratchet-like posterior dentition retained and directed prey towards the gullet for swallowing (Taylor and Cruickshank 1993; Noè 2001). Hence piercing of prey by plesiosaurs, even lightly-boned fish or unarmoured cephalopods, seems improbable, with the anatomical evidence pointing to a diet of small, soft and/or easily subdued prey.
What is less clear is exactly how this range of prey was caught. Teeth in modern aquatic vertebrates, which have forms broadly similar to those of plesiosaurs, have functions ranging from puncture harpoons to sieves and strainers, with teeth capable of piercing also being used for sieving (e.g., the leopard seal, Hydrurga leptonyx; Taylor 1987). The numerous, curved, and interlocking teeth of plesiosaurs (Owen 1861; Williston 1914; Shuler 1950; Watson 1951; Brown 1981a, b; Radinsky 1987; Mazin 2001) have often been considered as a highly efficient fish or invertebrate filter trap (Welles and Bump 1949; Watson 1951; Colbert 1958, 1966; Brown 1981b; Taylor 1987; Halstead 1989). However, it has also been noted that plesiosaur teeth are too short and too widely spaced to have filtered plankton (Taylor 1987; Collin and Janis 1997), and plesiosaurs were therefore not filter- or suspension-feeders (sensu Sanderson and Wassersug 1993: 37). An approximate minimum prey width in the order of 10–20 mm for adult plesiosaurs is suggested by tooth spacing, and a maximum width in the order of 200 mm by the space between the jaw articulations (Brown 1981b; Massare 1987). In addition, the ventral location of the jaw joints, well beneath the level of the tooth row, indicates the teeth would have intermeshed all at the same time as the jaws closed, rather than leaving a gap at the front as in a traditional pair of scissors. This is seen in Stratesaurus (Benson et al. 2015) and is comparable to the parallel jaw closing system seen in flamingos (Sanderson and Wassersug 1993) and some herbivorous dinosaurs (e.g., Norman et al. 2011). Hence, with a piercing function unlikely, the interlocking teeth indicate plesiosaurs occupied a filter-, sieve-, strain-, or rake-feeding niche, and most likely fed on mesoscopic invertebrates and small fish (Brown 1981b; Brown et al. 1986; Rieppel 1997).
The skull size and tooth form of plesiosaurs therefore indicates specialization for feeding on a range of mesoscopic prey, predominantly non-selectively. The evidence from the slender intermeshing teeth, the weak or absent heterodonty, and the relative lack of tooth wear in most plesiosaurs, points to small, unarmoured, possibly shoaling prey captured by sieve, strain or rake feeding and trapped behind the teeth, rather than by puncture, and then swallowed whole. Hence, although plesiosaurs were not strictly suspension feeders, they did have the anatomical features that permitted feeding as non-selective mesophagous sieve-feeders, possibly concentrating on shoaling organisms: the Mesozoic equivalents of organisms such as modern sardines or sand-eels. This feeding strategy has hitherto been regarded as not present in diapsid marine reptiles (Collin and Janis 1997).
The numerous intermeshing teeth of plesiosaurs were therefore excellently adapted for sieving, straining or filtering invertebrates, small fish or cephalopods in open water. However, plesiosaur dentition, with its procumbent but recurved anterior teeth and interlocking dentition, would also have served well to rake and sieve shallow infauna from within sediments, as well as to capture small, live prey close to the sediment water interface. Although it has usually been assumed that the preferred prey of plesiosaurs was active and pelagic, and therefore caught within the water column (Massare 1987, 1997), cranio-dental morphology offers the possibility that some or all plesiosaurs also fed on benthic organisms, or animals burrowing in soft sediments (Andrews 1913; McHenry et al. 2005). Sediment straining is corroborated by the presence of sand and occasional small molluscs in some plesiosaur stomach contents.
Conclusions and future work
The analysis presented here indicates that the plesiosaur neck was strongly functionally adapted when examined using an integrated approach to improve understanding of the role of the plesiosaur neck by taking into consideration cranial, cervical, trunk, and limb anatomy. Our model uniquely proposes the plesiosaur neck was interchangeably flexible and stiff for two key elements of plesiosaur lifestyle: feeding and locomotion during predator avoidance. The long neck is thereby shown to be an integral part of the plesiosaur feeding and locomotor apparatus, and an essential element of the wider plesiosaur bauplan. The head and neck are seen as adapted to consume relatively small, soft-bodied or shoaling organisms beneath the body. Those prey organisms could have been within the water column, close to the sediment-water interface, or concentrated within soft sediment. Prey would have been caught behind the tightly intermeshing dentition, which acted as a sieve, filter, strainer or rake in water or sediment, utilizing the mobility of the head and anterior neck. The neck itself was a flexible “feeding tube” connecting the head to the body, and predominantly adapted for ventral flexion, with dorsal, lateral and rotational movement all relatively restricted. This ventroflexion of the neck was brought about by strong cervical musculature working against an extensible, elastin-rich dorsal nuchal ligament system. However, the animal could escape from danger: the streamlined body was powered by the four hydrofoil-shaped limbs and the neck was held stiff and approximately straight out in front of the body by the nuchal ligament system, aided by the epaxial muscles, and locked by well-developed zygapophyses and, when present, accessory articulations on the neural spines. This system enabled rapid swimming without the disadvantages of a flexible neck anterior to the locomotor apparatus, and left maximum muscular effort available for predator evasion. More generally, the neck also acted as part of an integrated locomotor apparatus, tightly integrated into the wider plesiosaurian bauplan. The body was well-adapted for life within the water column, as a hovering or slowly moving, but mobile, “feeding platform”. This did not restrict the depth of foraging (unlike feeding from the surface), and allowed rapid but controlled motion during times of danger. Such an integrated scenario recognises that the plesiosaur neck cannot be considered in isolation, and that any explanation of neck function must take into account associated adaptations of head and body, interpreting their interrelationships as part of an integrated whole (Taylor 1989; Wilkinson and Ruxton 2012). We now consider some wider implications of the model and possible avenues for future research.
Functional considerations.—Previous workers have tended to stress either the flexibility or rigidity of the plesiosaur neck, whereas our model includes elements of both, allowing plesiosaurs to respond to different imperatives at different times. The neck is here envisaged as flexible enough to permit efficient food gathering, and yet stiff enough to avoid problems of hydrodynamic destabilization as a result of rapid locomotion during predator avoidance. Indeed, it is remarkable that, although plesiosaurs have a radically different bauplan from terrestrial herbivorous ungulates and sauropod dinosaurs, a nuchal ligament system apparently permitted efficient feeding and locomotion in all three groups (Dimery et al. 1985; Schwarz et al. 2007; this work). However, there were undoubtedly functional compromises between the constraints of locomotion and feeding, and these require future investigation. Insights into plesiosaur ecology and evolution might be gained from quantitative study of buoyancy and tidal volumes in plesiosaur necks, and another fruitful line of investigation appears to be the anterodorsal orientation of the orbits, which seem more suited to hunting prey (or spotting predators) from below against the brightly lit surface, than seeking small, shoaling prey.
Niche separation and partitioning within and between plesiosaurs and other marine vertebrates.—The new model offers a striking new way of interpreting plesiosaur lifestyle as a unique specialization, able to be co-opted for niche partition between taxa (Fig. 11). Our interpretation proposes a radically different ecological niche from coeval Mesozoic marine vertebrates, and one that would clearly separate plesiosaurs from all other predatory marine reptiles and filter feeding “fish”. Organisms such as pliosaurians, polycotylid plesiosaurians, ichthyosaurs, mosasaurs, crocodilians and sharks were all to some extent specialists consuming large, hard-boned prey, fish or cephalopod molluscs (Massare 1987; Sato and Tanabe 1998; Noè 1999); marine turtles were grazers on small prey; and fish such as the giant Leedsichthys were pelagic suspension feeders (Liston 2004). Plesiosaurs on the other hand were specialist non-selective filter-, sieve-, or strain-feeders, taking mesoscopic prey from within the water column or close to the sediment-water interface, or by raking soft sediments. The lifestyle here proposed for plesiosaurs thereby acts as a form of competitive exclusion, thereby avoiding competition with other niche specialists (e.g., Brown 1993; Martill et al. 1994).
Assuming the Oxford Clay plesiosaurs Muraenosaurus, Cryptoclidus, and Tricleidus all coexisted sympatrically, then their relatively minor anatomical differences in head, neck and post-cervical body structure might be related to niche partitioning between these genera. This has been previously suggested for the plesiosaur fauna from the lowermost Hettangian of Somerset and elsewhere in England (Benson et al. 2015). The inevitable exceptions from our anatomical overview, far from weakening the argument, represent fruitful stimuli to further research on the deeper ecological and evolutionary variations in neck function and usage between plesiosaur genera and species. Some of this variation might have led to the observed differences (often taxonomically important) between the necks of plesiosaur taxa. It is also likely to reflect variations in foraging technique and niche partitioning within plesiosaurs, especially where coeval taxa are found in the same deposits.
In terms of prey, small organisms are typically more abundant and form a greater biomass than larger organisms in marine ecosystems. Smaller prey items are also typically at lower levels within trophic networks (Massé 2001), and are often concentrated in discrete spatial aggregations or patches (Benoit-Bird et al. 2013). Hence, a relatively large plesiosaur specializing on small prey compared to body size would need to range widely and eat more individual items in order to obtain sufficient energy (Robinson 1975) for growth, locomotion and other metabolic activities.
Evolutionary origins.—Our model of plesiosaur neck function sees the cervical region as part of an integrated feeding and locomotor complex, which suggests that the long neck was a key evolutionary novelty, and responsible in part for the longevity of Plesiosauria. The long neck appears to be part of a wider evolutionary strategy within Plesiosauria as they evolved from nothosaur-grade ancestors, with progressive adaptation to life in the offshore, relatively shallow, open water, photic zone of Mesozoic epicontinental seas (Storrs 1991, 1993; Rieppel 1997), and also probably into deeper water habitats that are typically poorly represented in the fossil record. It might have been, for instance, that the long neck was the evolutionary adaptation that resulted in a strikingly high diversity of plesiosaurs in the earliest Jurassic (see Benson et al. 2012, 2015).
This leads to broader questions regarding the evolutionary trajectory of Plesiosauria during their secondary adaptation to life in water. In an analysis of land-to-water transitions in secondarily aquatic tetrapods (Taylor 2002), a number of grades of adaptation were identified. Members of Plesiosauria were mostly assigned to inshore/coastal predators using underwater flight and stone and/or bone ballast (grade 4b in Taylor 2000; see also Taylor 2002); in the light of our analysis, this now seems an oversimplification, and the plesiosaurs may require to be allocated to their own unique adaptive grade. Analyses aimed at understanding and integrating data from feeding, buoyancy control and locomotion during the evolutionary transition of plesiosaurs from ancestral sauropterygians, as well as between members of Order Plesiosauria, such as plesiosaurs and pliosauromorphs, are likely to prove highly productive.
Many individual elements of our model of plesiosaur head, neck and body function have been proposed previously, for instance: the head as a strainer (Brown 1981b), the neck as a feeding tube (Shuler 1950; Mazin 1987) predominantly adapted for ventral bending (Shuler 1950), the body as a hunting platform within the water column rather than at the surface (Robinson 1975; Storrs 1993), and bottom feeding (Hutchinson 1897; Andrews 1910; McHenry et al. 2005). However, no previous interpretation has brought these elements together into an integrated whole. Our work suggests that the neck was a key evolutionary novelty, permitting the widespread radiation of plesiosaurs into Mesozoic shelf seas and beyond. Watson (1951: 181) was seemingly entirely correct when he wrote “plesiosaurs caught prey in some quite exceptional manner”.
Acknowledgements
We particularly thank Don Smith and Derek Walton (both University of Derby, UK) and the late Arthur Cruickshank (formerly Leicester City Museum and University of Leicester, UK) from amongst the many colleagues with whom we have discussed these ideas over more than a decade. The digital version of Duria Antiquior was kindly provided by Cindy Howells (National Museum of Wales, Cardiff, UK), the translation of Zarnik (1925–1926) was kindly provided by Glenn Storrs (Cincinnati Museum Center, Cincinnati, USA), and a copy of his unpublished M.Sc. (although now made available on-line) was kindly provided by Mark Evans (New Walk Museum and Art Gallery, Leicester, UK), all of whom we thank warmly. LFN acknowledges the staff at the Library and Learning Resource Centre (University of Derby, UK) and Ruth Banger, Libby Tilley, and Sarah Humbert (all Earth Science Department Library, University of Cambridge, UK). This study was partly funded by a scholarship from the University of Derby, and a FAPA grant from Universidad de los Andes permitted production of the final manuscript. MAT acknowledges the support of the libraries of the University of Leicester and National Museums Scotland. MGP acknowledges a Geociencias Postdoctoral Teaching Fellowship (Facultad de Ciencias, Universidad de los Andes), during part of which this work was undertaken. We gratefully acknowledge an anonymous reviewer and Peter Falkingham (Liverpool John Moores University, UK), both of whom provided us with extremely useful comments and feedback on the original version of this manuscript, and extend our thanks to Stephen Brusatte for editorial support.
References
Anonymous 1868. Untitled [Prof. Cope exhibited ...]. Proceedings of the Academy of Natural Sciences of Philadelphia 20: 92–93.
Alexander, R.M. 1989. Dynamics of Dinosaurs and Other Extinct Giants. 167 pp. Columbia University Press, New York.
Alexander, R.M. 1990. Size, speed, and buoyancy adaptations in aquatic animals. American Zoologist 30: 189–196. Crossref
Andrews, C.W. 1909. On some new Plesiosauria from the Oxford Clay of Peterborough. Annals and Magazine of Natural History Series 8 4 (23): 418–429. Crossref
Andrews, C.W. 1910. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay—Based on the Leeds Collection in the British Museum (Natural History), London, Part I. xxiii + 10 pp. British Museum (Natural History), London.
Andrews, C.W. 1913. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay—Based on the Leeds Collection in the British Museum (Natural History), London, Part II. xix + 13 pp. British Museum (Natural History), London.
Araújo, R. and Polcyn, M.J. 2013. A biomechanical analysis of the skull and adductor chamber muscles in the Late Cretaceous plesiosaur Libonectes. Palaeontologia Electronica 16 (2): 1–25.
Bakker, R.T. 1993. Plesiosaur extinction cycles—events that mark the beginning, middle, and end of the Cretaceous. In: W.G.E. Caldwell and E.G. Kaufman (eds.), Evolution of the Western Interior Basin. Geological Association of Canada, Special Paper 39: 641–664. St. John’s, Newfoundland.
Bardet, N. 1995. Evolution et extinction des reptiles marins au cours du Mésozoïque. PalaeoVertebrata 24: 177–283.
Barrett, L. 1858. On the atlas and axis of the Plesiosaurus. Annals and Magazine of Natural History Series 3 2 (11): 361–364.
Benoit-Bird, K.J., Battaile, B.C., Heppell, S.A., Hoover, B., Irons, D., Jones, N., Kuletz, K.J., Nordstrom, C.A., Paredes, R., Suryan, R.M., Waluk, C.M., and Trites, A.W. 2013. Prey patch patterns predict habitat use by top marine predators with diverse foraging strategies. PLoS ONE 8 (1): e53348. Crossref
Benson, R.B.J. and Druckenmiller, P.S. 2014. Faunal turnover of marine tetrapods during the Jurassic–Cretaceous transition. Biological Reviews of the Cambridge Philosophical Society 89: 1–23. Crossref
Benson, R.B.J., Evans, M., and Druckenmiller, P.S. 2012. High diversity, low disparity and small body size in plesiosaurs (Reptilia, Sauropterygia) from the Triassic–Jurassic boundary. PLoS ONE 7 (3): e31838. Crossref
Benson, R.B.J., Evans, M., and Taylor, M.A. 2015. The anatomy of Stratesaurus (Reptilia, Plesiosauria) from the lowermost Jurassic of Somerset, United Kingdom. Journal of Vertebrate Paleontology 34: e933739. Crossref
Braun, J. and Reif, W.-E. 1985. A survey of aquatic locomotion in fishes and tetrapods. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 169: 307–332.
Brown, B. 1904. Stomach stones and food of plesiosaurs. Science 20 (501): 184–185. Crossref
Brown, D.S. 1981a. Dental morphology and function in plesiosaurs. Journal of Dental Research 60B: 146.
Brown, D.S. 1981b. The English Upper Jurassic Plesiosauroidea (Reptilia) and a review of the phylogeny and classification of the Plesiosauria. Bulletin of the British Museum (Natural History), Geology Series 35: 253–347.
Brown, D.S. 1993. A taxonomic reappraisal of the families Elasmosauridae and Cryptoclididae (Reptilia: Plesiosauroidea). Revue de Paléobiologie Spéciale 7: 9–16.
Brown, D.S. and Cruickshank, A.R.I. 1994. The skull of the Callovian plesiosaur Cryptoclidus eurymerus, and the sauropterygian cheek. Palaeontology 37: 941–953.
Brown, D.S., Milner, A.C., and Taylor, M.A. 1986. New material of the plesiosaur Kimmerosaurus langhami Brown from the Kimmeridge Clay of Dorset. Bulletin of the British Museum (Natural History), Geology Series 40: 225–234.
Buckland, W. 1836. Geology and Mineralogy Considered with Reference to Natural Theology. xvi + 599, vii + 131 pp. William Pickering, London.
Busbey, A.B. 1995. The structural consequences of skull flattening in crocodilians. In: J. Thomason (ed.), Functional Morphology in Vertebrate Paleontology, 174–192. Cambridge University Press, Cambridge.
Cabrera, A. 1941. Un plesiosaurio nuevo del Cretáceo del Chubut. Revista del Museo de la Plata, Nueva Serie (Paleontología) 2: 113–130.
Carpenter, K. 1997. Comparative cranial anatomy of two American Cretaceous plesiosaurs. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 191–216. Academic Press, San Diego. Crossref
Carpenter, K. 1999. Revision of North American elasmosaurs from the Cretaceous of the Western Interior. Paludicola 2: 148–173.
Carpenter, K., Sanders, F., Reed, B., Reed, J., and Larson, P. 2010. Plesiosaur swimming as interpreted from skeletal analysis and experimental results. Transactions of the Kansas Academy of Science 113: 1–34. Crossref
Carroll, R.L. 1985. Evolutionary constraints in aquatic diapsid reptiles. In: J.C.W. Cope and P. W. Skelton (eds.), Evolutionary Case Histories from the Fossil Record. Special Papers in Palaeontology 33, 145–155. The Palaeontological Society, London.
Cicimurri, D.J. and Everhart, M.J. 2001. An elasmosaur with stomach contents and gastroliths from the Pierre Shale (Late Cretaceous) of Kansas. Transactions of the Kansas Academy of Science 104: 129–143. Crossref
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.-X. 2013. The ICS International Chronostratigraphic Chart. Episodes 36: 199–204 [updated 2016–12]; http://stratigraphy.org/index.php/ics-chart-timescale.
Colbert, E.H. 1958. Tetrapod extinctions at the end of the Triassic period. Proceedings of the National Academy of Sciences of the United States of America 44: 973–977. Crossref
Colbert, E.H. 1966. Evolution of the Vertebrates, a History of the Backboned Animals Through Time. xii + 479 pp. John Wiley and Sons, Inc., New York.
Collin, R. and Janis, C.M. 1997. Morphological constraints on tetrapod feeding mechanisms: Why were there no suspension-feeding marine reptiles? In: J.M. Callaway, and E.L. Nicholls (eds.), Ancient Marine Reptiles, 451–466. Academic Press, San Diego. Crossref
Conybeare, W.D. 1822. Additional notices on the fossil genera Ichthyosaurus and Plesiosaurus. Transactions of the Geological Society of London 2 (1): 103–123. Crossref
Conybeare, W.D. 1824. On the discovery of an almost perfect skeleton of the Plesiosaurus. Transactions of the Geological Society of London 2 (1): 381–389. Crossref
Cope, E.D. 1869. Synopsis of the extinct Batrachia, Reptilia and Aves of North America (first preprint). Transactions of the American Philosophical Society (New Series) 14: 1–252. Crossref
Cope, E.D. 1877. Report on the geology of the region of the Judith River, Montana, and on vertebrate fossils obtained on or near the Missouri River. Bulletin of the United States Geological and Geographical Survey 3 (3): 565–597.
Cruickshank, A.R.I. 1994. A juvenile plesiosaur (Plesiosauria: Reptilia) from the Lower Lias (Hettangian: Lower Jurassic) of Lyme Regis, England: A pliosauroid-plesiosauroid intermediate? Zoological Journal of the Linnean Society 112: 151–178. Crossref
Cruickshank, A.R.I. and Fordyce, R.E. 2002. A new marine reptile (Sauropterygia) from New Zealand: further evidence for a Late Cretaceous Austral radiation of cryptoclidid plesiosaurs. Palaeontology 45: 557–575. Crossref
Cruickshank, A.R.I., Martill, D.M., and Noè, L.F. 1996. A pliosaur (Reptilia, Sauropterygia) exhibiting pachyostosis from the Middle Jurassic of England. Journal of the Geological Society, London 153: 873–879. Crossref
Cruickshank, A.R.I., Small, P.G., and Taylor, M.A. 1991. Dorsal nostrils and hydrodynamically driven underwater olfaction in plesiosaurs. Nature 352: 62–64. Crossref
Dames, H.W. 1895. Die plesiosaurier der Süddeutschen Liasformation. Abhandlungen der Königlich Preussischen Akademie der Wissenschaften zu Berlin 1895 (2): 1–83.
Darby, D.G. and Ojakangas, R.W. 1980. Gastroliths from an Upper Cretaceous plesiosaur. Journal of Paleontology 54: 548–556.
Darwin, C. 1871. The Descent of Man, and Selection in Relation to Sex. vii + 423, vii + 475 pp. John Murray, London.
Davidson, J.P. 2002. Bonehead mistakes: The background in scientific literature and illustrations for Edward Drinker Cope’s first restoration of Elasmosaurus platyurus. Proceedings of the Academy of Natural Sciences of Philadelphia 152: 215–240. Crossref
de Buffrénil, V. and Mazin, J.-M. 2001. What is an aquatic tetrapod? Some introductory remarks. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water. Proceedings of International Meeting, Poitiers, 1996, 91–93. Verlag Dr. Friedrich Pfeil, München.
de la Beche, H.T. and Conybeare, W.D. 1821. Notice of the discovery of a new fossil animal, forming a link between the Ichthyosaurus and crocodile, together with general remarks on the osteology of the Ichthyosaurus. Transactions of the Geological Society of London 5: 559–594. Crossref
Dimery, N.J., Alexander, R.M., and Deyst, K.A. 1985. Mechanics of the ligamentum nuchae of some artiodactyls. Journal of Zoology, London A206: 341–351. Crossref
Duvall, D., Arnold, S.J., and Schuett, G.W. 1992. Pitviper mating systems: ecological potential, sexual selection, and microevolution. In: E.D.J. Brodie and J.A. Campbell (eds.), Biology of the Pitvipers, 321–336. Selva, Tyler.
Evans, M. 1993. An Investigation Into the Neck Flexibility of Two Plesiosauroid Plesiosaurs: Cryptoclidus eurymerus and Muraenosaurus leedsii. 63 + 12 pp. Unpublished M.Sc. Thesis, University College, London.
Evans, M. 1999. A new reconstruction of the skull of the Callovian elasmosaurid plesiosaur Muraenosaurus leedsi Seeley. Mercian Geologist 14 (4): 191–196.
Everhart, M.J. 2000. Gastroliths associated with plesiosaur remains in the Sharon Springs Member of the Pierre Shale (Late Cretaceous), Western Kansas. Transactions of the Kansas Academy of Science 103: 64–75. Crossref
Gasparini, Z. 2009. A new Oxfordian pliosaurid (Plesiosauria, Pliosauridae) in the Caribbean seaway. Palaeontology 52 (3): 661–669. Crossref
Gasparini, Z., Bardet, N., Martin, J.E., and Fernández, M. 2003. The elasmosaurid plesiosaur Aristonectes Cabrera from the latest Cretaceous of South America and Antarctica. Journal of Vertebrate Paleontology 23: 104–115. Crossref
Gellman, K.S. and Bertram, J.E.A. 2002a. The equine nuchal ligament 1: structural and material properties. Veterinary and Comparative Orthopaedics and Traumatology 15: 1–6.
Gellman, K.S. and Bertram, J.E.A. 2002b. The equine nuchal ligament 2: passive dynamic energy exchange in locomotion. Veterinary and Comparative Orthopaedics and Traumatology 15: 7–14.
Godfrey, S.J. 1984. Plesiosaur subaqueous locomotion: a reappraisal. Neues Jahrbuch für Geologie und Paläontologie (Munich) 11: 661–672.
Gray, J.E. 1825. A synopsis of the genera of reptiles and amphibia, with a description of some new species. Annals of Philosophy (London) 26: 193–217.
Halstead, L.B. 1969. The Pattern of Vertebrate Evolution. xii + 209 pp. Oliver & Boyd, Edinburgh.
Halstead, L.B. 1989. Plesiosaur locomotion. Journal of the Geological Society, London 146: 37–40. Crossref
Hanke, F.D., Hanke, W., Scholtyssek, C., and Dehnhardt, G. 2009. Basic mechanisms in pinniped vision. Experimental Brain Research 199: 299–311. Crossref
Hawkins, T. 1834. Memoirs of Ichthyosauri and Plesiosauri, Extinct Monsters of the Ancient Earth. 28 pp. Relfe and Fletcher, London.
Hawkins, T. 1840. Book of the Great Sea-Dragons, Ichthyosauri and Plesiosauri, Gedolim Taninim, of Moses. Extinct Monsters of the Ancient Earth. 30 pp. William Pickering, London.
Henderson, D.M. 2006. Floating point: a computational study of buoyancy, equilibrium, and gastroliths in plesiosaurs. Lethaia 39: 227–244. Crossref
Hone, D.W.E., Naish, D., and Cuthill, I.C. 2012. Does mutual sexual selection explain the evolution of head crests in pterosaurs and dinosaurs? Lethaia 45: 139–156. Crossref
Hopley, P.J. 2001. Plesiosaur spinal pathology: the first fossil occurrence of Schmorl’s nodes. Journal of Vertebrate Paleontology 21: 253–260. Crossref
Horváth, G. and Varjú, D. 1995. Underwater refraction-polarization patterns of skylight perceived by aquatic animals through Snell’s Window of the flat water surface. Vision Research 35: 1651–1666. Crossref
Houssaye, A. 2009. “Pachyostosis” in aquatic amniotes. Integrative Zoology 4: 325–340.
Hudson, J.D. and Palframan, D.F.B. 1969. The ecology and preservation of the Oxford Clay fauna at Woodham, Buckinghamshire. Quarterly Journal of the Geological Society of London 124: 387–418.
Hugh-Jones, P., Barter, C.E., Hime, J.M., and Rusbridge, M.M. 1978. Dead space and tidal volume of the giraffe compared with some other mammals. Respiration Physiology 35: 53–58. Crossref
Hutchinson, H.N. 1897. Extinct Monsters. A Popular Account of Some of the Larger Forms of Ancient Animal Life. xiv + 270 pp. Chapman & Hall, I.D., London.
Iordansky, N.N. 1973. The skull of the Crocodilia. In: C. Gans and T.S. Parsons (eds.), Biology of the Reptilia, 201–262. Academic Press, London.
Kear, B.P., Schroeder, N.I., and Lee, M.S.Y. 2006. An archaic crested plesiosaur in opal from the Lower Cretaceous high-latitude deposits of Australia. Biology Letters: Proceedings of the Royal Society of London B 2: 615–619. Crossref
Kellner, A.W.A. and Campos, D.A. 2002. The function of the cranial crest and jaws of a unique pterosaur from the Early Cretaceous of Brazil. Science 297: 389–392. Crossref
Ketchum, H.F. and Benson, R.B.J. 2010. Global interrelationships of Plesiosauria (Reptilia, Sauropterygia) and the pivotal role of taxon sampling in determining the outcome of phylogenetic analyses. Biological Reviews of the Cambridge Philosophical Society 85: 361–392. Crossref
Kubo, T., Mitchell, M.T., and Henderson, S.M. 2012. Albertonectes vanderveldei, a new elasmosaur (Reptilia, Sauropterygia) from the Upper Cretaceous of Alberta. Journal of Vertebrate Paleontology 32: 557–572. Crossref
Langston, W. 1973. The crocodilian skull in historical perspective. In: C. Gans and T.S. Parsons (eds.), Biology of the Reptilia, 263–284. Academic Press, London.
Lingham-Soliar, T. 2000. Plesiosaur locomotion: is the four-wing problem real or merely an atheoretical exercise? Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 217: 45–87.
Liston, J.J. 2004. An overview of the pachycormiform Leedsichthys. In: G. Arratia and A. Tintori (eds.), Mesozoic Fishes. 3—Systematics, Paleoenvironments and Biodiversity, 379–390. Verlag Dr. Friedrich Pfeil, München.
Long, J.H. Jr, Schumacher, J., Livingston, N., and Kemp, M. 2006. Four flippers or two? Tetrapodal swimming with an aquatic robot. Bioinspiration & Biomimetics 1: 20–29. Crossref
Martill, D.M. and Hudson, J.D. (eds.) 1991. Fossils of the Oxford Clay. 286 pp. The Palaeontological Association, London.
Martill, D.M., Taylor, M.A., and Duff, K.L. 1994. The trophic structure of the biota of the Peterborough Member, Oxford Clay Formation (Jurassic), UK. Journal of the Geological Society, London 151: 173–194. Crossref
Martin, J.E. and Kennedy, L.E. 1988. A plesiosaur with stomach contents from the Pierre Shale (Late Cretaceous) of South Dakota: a preliminary report. Proceedings of the North Dakota Academy of Science 42: 13.
Massare, J.A. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. Journal of Vertebrate Paleontology 7: 121–137. Crossref
Massare, J.A. 1988. Swimming capabilities of Mesozoic marine reptiles: implications for method of predation. Paleobiology 14: 187–205. Crossref
Massare, J.A. 1994. Swimming capabilities of Mesozoic marine reptiles: a review. In: L. Maddock, Q. Bone, and J.M.V. Rayner (eds.), Mechanics and Physiology of Animal Swimming, 133–149. Cambridge University Press, Cambridge. Crossref
Massare, J.A. 1997. Faunas, behavior, and evolution: introduction. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 401–421. Academic Press, San Diego.
Massé, H. 2001. On some marine trophic networks. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water. Proceedings of International Meeting, Poitiers, 1996, 39–54. Verlag Dr. Friedrich Pfeil, München.
Mazin, J.-M. 1987. Marine reptile groups and the late Triassic extinctions. Mémoires de la Société Géologique de France, Nouvelle Serie 150: 33–36.
Mazin, J.-M. 2001. Mesozoic marine reptiles: an overview. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water. Proceedings of International Meeting, Poitiers, 1996, 95–117. Verlag Dr. Friedrich Pfeil, München.
McGowan, C. 1983. The Successful Dragons, A Natural History of Extinct Reptiles. 263 pp. Samuel Stevens, Toronto.
McGowan, C. 1992. Dinosaurs, Spitfires And Sea Dragons. 365 pp. Harvard University Press, Massachusetts.
McHenry, C.R., Cook, A.G., and Wroe, S. 2005. Bottom-feeding plesiosaurs. Science 310: 75. Crossref
Motani, R. 2002. Swimming speed estimation of extinct marine reptiles: energetic approach revisited. Paleobiology 28: 251–262. Crossref
Müller, J., Scheyer, T.M., Head, J.J., Barrett, P.M., Werneburg, I., Ericson, P.G.P., Pol, D., and Sánchez-Willagra, R. 2010. Homeotic effects, somitogenesis and the evolution of vertebral numbers in recent and fossil amniotes. PNAS 107: 2118–2123. Crossref
Noè, L.F. 1999. The Callovian pliosaurs of the Oxford Clay—evidence and implications for the consumption of marine invertebrates. In: E. Hoch and A.K. Brantsen (eds.), Secondary Adaptation to Life in Water, September 13–17, 1999, Abstract volume, 39–41. Geologisk Museum, Copenhagen.
Noè, L.F. 2001. A Taxonomic and Functional Study of the Callovian (Middle Jurassic) Pliosauroidea (Reptilia, Sauropterygia). 347 + 182 pp. Unpublished Ph.D. Thesis, University of Derby, Derby.
Norman, D.B., Crompton, A.W., Butler, R.J., Porro, L.B., and Charig, A.J. 2011. The Lower Jurassic ornithischian dinosaur Heterodontosaurus tucki Crompton & Charig, 1962: cranial anatomy, functional morphology, taxonomy, and relationships. Zoological Journal of the Linnean Society 163: 182–276. Crossref
North, F.J. 1933. Dean Conybeare, Geologist. Cardiff Naturalist’s Society 66: 15–68.
Nosotti, S. 2007. Tanystropheus longobardicus (Reptilia, Protorosauria): re-interpretation of the anatomy based on new specimens from the Middle Triassic of Besano (Lombardy, northern Italy). Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 35 (3): 1–88.
O’Keefe, F.R. 2001a. A cladistic analysis and taxonomic revision of the Plesiosauria (Reptilia: Sauropterygia). Acta Zoologica Fennica 213: 1–63.
O’Keefe, F.R. 2001b. Ecomorphology of plesiosaur flipper geometry. Journal of Evolutionary Biology 14: 987–991. Crossref
O’Keefe, F.R. 2002. The evolution of plesiosaur and pliosaur morphotypes in the Plesiosauria (Reptilia: Sauropterygia). Paleobiology 28: 101–112. Crossref
O’Keefe, F.R. and Carrano, M.T. 2005. Correlated trends in the evolution of the plesiosaur locomotor system. Paleobiology 31 (4): 656–675. Crossref
O’Keefe, F.R. and Hiller, N. 2006. Morphologic and ontogenetic patterns in elasmosaur neck length, with comments on the taxonomic utility of neck length variables. Paludicola 5: 206–229.
Olson, E.C. 1971. Vertebrate Paleozoology. xv + 839 pp. Wiley-Interscience, New York.
Osborn, H.F. 1918. The Origin And Evolution of Life, On the Theory of Action Reaction and Interaction of Energy. xxxi + 322 pp. G. Bell and Sons, Ltd., London.
Owen, R. 1840a. A description of a specimen of the Plesiosaurus macrocephalus, Conybeare, in the collection of Viscount Cole, M.P., D.C.L., F.G.S., etc. Transactions of the Geological Society of London 2: 515–535.
Owen, R. 1840b. Report on British Fossil Reptiles. Report of the Ninth Meeting of the British Association for the Advancement of Science; Held at Birmingham in 1839: Reports of Researches in Science, 43–126. John Murray, London.
Owen, R. 1847. Description of the atlas, axis, and subvertebral wedge bones in the Plesiosaurus, with remarks on the homologies of those bones. The Annals and Magazine of Natural History 20: 217–225. Crossref
Owen, R. 1854. Geology and Inhabitants of the Ancient World. 39 pp. Crystal Palace Library, London.
Owen, R. 1861. Palaeontology, or a Systematic Summary of Extinct Animals and Their Geological Relations. xvi + 463 pp. Adam and Charles Black, Edinburgh.
Radinsky, L.B. 1987. The Evolution of Vertebrate Design. xi + 188 pp. University of Chicago Press, Chicago.
Reilly, S.M., McBrayer, L.D., and White, T.D. 2001. Prey processing in amniotes: biomechanical and behavioral patterns of food reduction. Comparative Biochemistry and Physiology Part A 128: 397–415. Crossref
Richardson, G.F. 1851. An Introduction to Geology and Its Associate Sciences, Mineralogy, Fossil Botany and Palaeontology. xvi + 508 pp. H.G. Bohn, London.
Rieppel, O. 1997. Sauropterygia: Introduction. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 107–119. Academic Press, San Diego.
Robinson, J.A. 1975. The locomotion of plesiosaurs. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 149: 286–332.
Robinson, J.A. 1977. Intracorporal force transmission in plesiosaurs. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 153: 86–128.
Romer, A.S. 1955. Vertebrate Paleontology. viii + 687 pp. University of Chicago Press, Chicago.
Sachs, S. 2005. Redescription of Elasmosaurus platyurus Cope, 1868 (Plesiosauria: Elasmosauridae) from the Upper Cretaceous (Lower Campanian) of Kansas, U.S.A. Paludicola 5: 92–106.
Sachs, S., Kear, B.P., and Everhart, M.J. 2013. Revised vertebral count in the “longest-necked vertebrate” Elasmosaurus platyurus Cope, 1868, and clarification of the cervical-dorsal transition in Plesiosauria. PLoS ONE 8 (8): e70877. Crossref
Sambilay, V.C. Jr 1990. Interrelationships between swimming speed, caudal fin aspect ratio and body length of fishes. Fishbyte 8 (3): 16–20.
Sanderson, S.L. and Wassersug, R. 1993. Convergent and alternative designs for vertebrate suspension feeding. In: J. Hanken and B.K. Hall (eds.), The Skull, 37–112. The University of Chicago Press, Chicago.
Sato, T. and Tanabe, K. 1998. Cretaceous plesiosaurs ate ammonites. Nature 394: 629–630.
Schwarz, D., Frey, E., and Meyer, C.A. 2007. Pneumaticity and soft-tissue reconstructions in the neck of diplodocid and dicraeosaurid sauropods. Acta Palaeontologica Polonica 52: 167–188.
Seeley, H.G. 1865. On two new plesiosaurs, from the Lias. Annals and Magazine of Natural History 16 (3) 352–359.
Seeley, H.G. 1874. On Muraenosaurus leedsii, a plesiosaurian from the Oxford Clay, Part I. Quarterly Journal of the Geological Society of London 30: 197–208. Crossref
Seeley, H.G. 1876. Similitudes of the bones in the Enaliosauria. Journal of the Linnean Society, London 12: 296–329. Crossref
Seeley, H.G. 1877. On Mauisaurus gardneri (Seeley), an elasmosaurian from the base of the Gault at Folkestone. Quarterly Journal of the Geological Society of London 33: 541–547. Crossref
Seeley, H.G. 1892. The nature of the shoulder girdle and clavicular arch in Sauropterygia. Proceedings of the Royal Society of London 51: 119–151. Crossref
Shine, R. 2002. Reproductive strategies in snakes. Proceedings of the Royal Society of London B 270: 995–1004. Crossref
Shuler, E.W. 1950. A new elasmosaur from the Eagle Ford Shale of Texas, the elasmosaur and its environment. Fondren Science Series 1 (2): 1–32.
Simmons, R.E. and Scheepers, L. 1996. Winning by a neck: sexual selection in the evolution of Giraffe. The American Naturalist 148 (5): 771–786. Crossref
Smellie, W.R. 1915. On a new plesiosaur from the Oxford Clay. Geological Magazine 52: 341–343. Crossref
Smellie, W.R. 1916. Apractocleidus teretipes: a new Oxfordian plesiosaur in the Hunterian Museum, Glasgow University. Transactions of the Royal Society of Edinburgh 51: 609–629. Crossref
Smith, A.S. 2008. Fossils explained 54. Plesiosaurs. Geology Today 24 (2): 71. Crossref
Smith, A.S. 2013. Morphology of the caudal vertebrae in Rhomaleosaurus zetlandicus and a review of the evidence for a tail fin in Plesiosauria. Paludicola 9: 144–158.
Storrs, G.W. 1991. Anatomy and relationships of Corosaurus alcovensis (Diapsida: Sauropterygia) and the Triassic Alcova Limestone of Wyoming. Bulletin of the Peabody Museum of Natural History 44: xii, 151.
Storrs, G.W. 1993. Function and phylogeny in sauropterygian (Diapsida) evolution. American Journal of Science 293 A: 63–90.
Storrs, G.W. 1997. Morphological and taxonomic clarification of the genus Plesiosaurus. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 145–190. Academic Press, San Diego. Crossref
Számadó, S. 2011. The cost of honesty and the fallacy of the handicap principle. Animal Behaviour 81: 3–10. Crossref
Tarsitano, S. and Reiss, J. 1982. Plesiosaur locomotion—underwater flight versus rowing. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 164: 188–192.
Taylor, M.A. 1981. Plesiosaurs—rigging and ballasting. Nature 290: 628–629. Crossref
Taylor, M.A. 1987. How tetrapods feed in water: a functional analysis by paradigm. Zoological Journal of the Linnean Society 91: 171–195. Crossref
Taylor, M.A. 1989. Neck and neck. Nature 341: 688–689. Crossref
Taylor, M.A. 1992. [Letter about elasmosaur necks]. The Lancet 340: 1228.
Taylor, M.A. 1993. Stomach stones for feeding or buoyancy? The occurrence and functional significance of gastroliths in marine tetrapods. Philosophical Transactions of the Royal Society of London B, Biological Sciences 341: 163–175. Crossref
Taylor, M.A. 1994. Stone, bone or blubber? Buoyancy control strategies in aquatic tetrapods. In: L. Maddock, Q. Bone, and J.M.V. Rayner (eds.), Mechanics and Physiology of Animal Swimming, 151–161. Cambridge University Press, Cambridge. Crossref
Taylor, M.A. 1997. Before the dinosaurs: the historical significance of the fossil marine reptiles. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, xix–xlxv. Academic Press, San Diego.
Taylor, M.A. 2000. Functional significance of bone ballast in the evolution of buoyancy control strategies by aquatic tetrapods. Historical Biology 14: 15–31. Crossref
Taylor, M.A. 2002. Origins of marine mammals. In: W.F. Perrin, B. Würsig, and J.G.M. Thewissen (eds.), Encyclopedia of Marine Mammals, 833–837. Academic Press, San Diego.
Taylor, M.A. and Cruickshank, A.R.I. 1993. Cranial anatomy and functional morphology of Pliosaurus brachyspondylus (Reptilia: Plesiosauria). Philosophical Transactions of the Royal Society of London B, Biological Sciences 341: 399–418. Crossref
Taylor, M.P., Hone, D.W.E., Wedel, M.J., and Naish, D. 2011. The long necks of sauropods did not evolve primarily through sexual selection. Journal of Zoology 285: 150–161. Crossref
Torrens, H. 1995. Mary Anning (1799–1847) of Lyme; “the greatest fossilist the world ever knew”. The British Journal for the History of Science 28: 257–284. Crossref
Tschanz, K. 1988. Allometry and heterochrony in the growth of the neck of Triassic prolacertiform reptiles. Palaeontology 31: 997–1011.
Varela-Lasheras, I., Bakker, A.J., van de Mije, S.D., Metz, J.A.J., van Alphen, J., and Galis, F. 2011. Breaking evolutionary and pleiotropic constraints in mammals: on sloths, manatees and homeotic mutations. EvoDevo 2 (11): 1–27. Crossref
Wang, X., Tedford, R.H., and Antón, M. 2008. Anatomy and function. How the parts work. In: X. Wang, R.H. Tedford, and M. Antón (eds.), Dogs, Their Fossil Relatives and Evolutionary History, 69–101. Colombia University Press, New York.
Watson, D.M.S. 1924. The elasmosaurid shoulder-girdle and fore-limb. Proceedings of the Zoological Society, London: 885–917. Crossref
Watson, D.M.S. 1951. Paleontology and Modern Biology. xii + 216 pp. Yale University Press, New Haven.
Wegner, T. 1914. Brancasaurus brancai n. g. n. sp., ein Elasmosauride aus dem Wealden Westfalens. In: F. Schoendorf (ed.), Festschrift für Wilhelm Branca zum siebzigsten geburtstage, 235–305. Verlag von Gebrüder Borntraeger, Leipzig.
Welles, S.P. 1943. Elasmosaurid plesiosaurs with a description of new material from California and Colorado. Memoir of the University of California, Berkeley 13: 125–215.
Welles, S.P. 1949. A new elasmosaur from the Eagle Ford Shale of Texas, systematic description. Fondren Science Series 1 (1): 1–28.
Welles, S.P. 1952. A review of the North American Cretaceous elasmosaurs. University of California Publications in Geological Sciences 29 (3): 47–144.
Welles, S.P. 1962. A new species of elasmosaur from the Aptian of Colombia and a review of the Cretaceous plesiosaurs. University of California Publications in Geological Sciences 44: 1–96.
Welles, S.P. and Bump, J.D. 1949. Alzadasaurus pembertoni, a new elasmosaur from the Upper Cretaceous of South Dakota. Journal of Paleontology 23: 521–535.
Wild, R. 1980. Neue funde von Tanystropheus (Reptilia, Squamata). Die Triasfauna der Tessiner Kalkalpen XXIV. Schweizerische Paläontologische Abhandlungen 102: 1–43.
Wilkinson, D.M. and Ruxton, G.D. 2012. Understanding selection for long necks in different taxa. Biological Reviews of the Cambridge Philosophical Society 87: 616–630. Crossref
Williston, S.W. 1902. On certain homoplastic characters in aquatic air-breathing vertebrates. Kansas University Science Bulletin 9: 259–266.
Williston, S.W. 1906. North American plesiosaurs: Elasmosaurus, Cimoliasaurus, and Polycotylus. American Journal of Science 21: 221–236. Crossref
Williston, S.W. 1914. Water Reptiles of the Past and Present. vi + 251 pp. University of Chicago Press, Chicago.
Winkler, T.C. 1873. Le Plesiosaurus dolichodeirus Conyb. du Musée Teyler. Archives du Musee Teyler 3 (3): 1–15.
Zarnik, B. 1925–1926. On the ethology of plesiosaurs with contributions to the mechanism of the cervical vertebrae of recent sauropsids [in Croatian]. Societas Scientiarum Naturalium Croatica, Hrvatsko Prirodoslovno Društvo 37–38: 424–479.