

Unusual shell anatomy and osteohistology in a new Late Cretaceous panchelid turtle from northwestern Patagonia, Argentina
MARCELO S. DE LA FUENTE, IGNACIO MANIEL, JUAN MARCOS JANNELLO, JULIANA STERLI, ALBERTO C. GARRIDO, RODOLFO A. GARCIA, LEONARDO SALGADO, JOSÉ I. CANUDO, and RAÚL BOLATTI
De la Fuente, M.S., Maniel, I., Jannello, J.M., Sterli, J., Garrido, A.C., Garcia, R.A., Salgado, L., Canudo, J.I., and Bolatti, R. 2017. Unusual shell anatomy and osteohistology in a new Late Cretaceous panchelid turtle from northwestern Patagonia, Argentina. Acta Palaeontologica Polonica 62 (3): 585–601.
Rionegrochelys caldieroi de la Fuente, Maniel, and Jannello gen. et sp. nov. is a Late Cretaceous turtle from Rio Negro Province, Argentina. The holotype and the referred specimens of this new species show an unusual shell morphology and microanatomy. The proportion between the carapace and plastron and the peculiar morphology of the shell such as the heart shaped carapace, a very deep nuchal notch, peripheral bones 2–11 with strongly gutter, the first vertebral scute twice as wide as long and subrectangular in shape, the posterior margin of vertebral scute 5 is three lobe shaped, and the unexpected osteohistology characterized by a massive structure, with higher compactness (80.6%) than other chelids, suggests beyond doubt that this turtle may be considered a new taxon. A semi-aquatic habitat with tendency towards terrestrial environments is inferred for Rionegrochelys caldieroi similar to that of the extant pelomedusid Pelomedusa subrufa among the extant pleurodires. Rionegrochelys caldieroi is recovered as a stem chelid. This new species seems to be closely related to Bonapartemys bajobarrealis and the clade formed by Lomalatachelys neuquina plus Mendozachelys wichmanni.
Key words: Testudines, Pleurodira, post-cranial morphology, paleohistology, Cretaceous, Argentina, Río Negro.
Marcelo S. de la Fuente [mdelafuente1910@gmail.com], Ignacio Maniel [nachomaniel@gmail.com], Juan Marcos Jannello [marcosjannello@hotmail.com], Grupo Vinculado al IANIGLA CCT-Mendoza, Museo de Historia Natural de San Rafael, Av. Balloffet S/N° frente al Parque Mariano Moreno, 5600 San Rafael, Mendoza, Argentina.
Juliana Sterli [jsterli@mef.org.ar], Museo Paleontológico Egidio Feruglio, Av. Fontana 140, 9100 Trelew, Chubut, Argentina.
Alberto C. Garrido [albertocarlosgarrido@gmail.com], Museo Provincial de Ciencias Naturales “Prof. Dr. Juan Olsacher”, Dirección General de Minería Elena de Vega 472, 8340 Zapala, Neuquén, Argentina.
Rodolfo A. García [rodosnow@yahoo.com.ar], Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Rio Negro, Museo Provincial Carlos Ameghino, Belgrano 1700, Paraje Pichi Ruca (predio Marabunta) R8324CZH Cipolletti, Río Negro, Argentina.
Leonardo Salgado [lsalgado@unrn.edu.ar], Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Rio Negro, Gral Roca, Rio Negro, Argentina.
José I. Canudo [jicanudo@unizar.es] Grupo Aragonsaurus-IUCA, Facultad de Ciencias, Universidad de Zaragoza 50009 Zaragoza, Spain.
Raúl Bolatti [rbolatti@hotmail.com], Unidad de Gestión Margen Sur, Municipalidad de Cipolletti, R8324CZH Cipolletti, Río Negro, Argentina.
Received 10 January 2017, accepted 18 March 2017, available online 6 July 2017.
Copyright © 2017 M.S. de la Fuente et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
“El Anfiteatro” is an area located in northwestern Patagonia with expansive outcrops of five different Cretaceous horizons of the Neuquén Group (i.e., Huincul, Cerro Lisandro, Portezuelo, Plottier, and Bajo de la Carpa formations). Its significant abundance of vertebrate remains has only been explored in recent years (Canudo et al. 2004; Salgado et al. 2009). At the beginning of the 21st century, a state institution of the Province of Río Negro (Argentina) (Endemas, “Ente para el Desarrollo de la Margen Sur”), the National University of the Comahue (Argentina), and the University of Zaragoza (Spain) organized a series of field trips headed by one of us (LS) to the area of the “El Anfiteatro” in search of fossil vertebrates. The remains recovered from these five different lithostratigraphic units were described by Salgado et al. (2009). The Plottier Formation in particular has yielded remains of panchelid turtles, crocodyliforms, titanosaur sauropods, theropods (cf. Carcharodontosauridae, Coelurosauria indet., cf. Unenlagiinae), and ornithopods. Among them, the most complete specimen is the shell of a pleurodire turtle (Panchelidae) referred by Salgado et al. (2009) to Chelidae gen. et sp. indet. The term Panchelidae is herein used to refer to the total group that includes the crown chelids and the stem (Joyce et al. 2004). Panchelidae is the sister group of Panpelomedusoides within Pleurodira but has an opposing geographic distribution and evolutionary history (see Maniel and de la Fuente 2016, and references therein, for most recent summary of the fossil record of the group).
The main goals of this paper are to describe the macro and microanatomy of this new taxon, to point out its morphological and paleohistological peculiarities, and to explore its paleoecology and the relationships of this new chelonian species within the panchelid clade.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA; MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MHNSR, Museo de Historia Natural de San Rafael, Argentina; MPA, Museo Municipal de Ciencias Naturales “Carlos Darwin” de Punta Alta, Argentina; MPCA, Museo Provincial “Carlos Ameghino”, Cipolletti, Argentina; MZUSP, Museu de Zoologia da Universidade de Sao Paulo, Brazil; NHMUK, Natural History Museum, London, UK; QM, Queensland Museum, Brisbane, Australia; SMF, Senckenberg Museum Frankfurt, Germany; USNM, National Museum of Natural History, Smithsonian Institution, Washington DC, USA.
Other abbreviations.—MPT, most parsimonious tree; SE, standard error.
Material and methods
Cladistic analysis.—A cladistic analysis was performed to evaluate the phylogenetic relationships of the new species of turtle presented here. A dataset of 23 taxa and 62 morphological characters (see SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app62-Fuente_etal_SOM.pdf) based on de la Fuente et al. (2017) was enlarged for the present analysis by adding five new taxa (extant species Chelydra serpentina and Pelomedusa subrufa, and extinct Upper Cretceous species Bonapartemys bajobarrealis, Lomalatachelys neuquina, and Prochelidella portezuelae) and one character (character 3, contact frontals-nasals). However, one species was also removed (Notoemys laticentralis) due to its scarce availability of characters. Four taxa (Chelydra serpentina, Araripemys barretoi, Pelomedusa subrufa, and Podocnemis sextuberculata) form the outgroup and 19 taxa of extinct and extant turtles the ingroup. Chelydra serpentina was the taxon selected to root the analysis. All characters were weighted equally and twelve characters were ordered (characters 4, 5, 28, 30, 36, 41, 43, 45, 54, 55, 58, 61, and 62). In order to evaluate the phylogenetic relationships of turtles and find the most parsimonious tree (MPT), two cycles of heuristic search were performed as implemented in the phylogenetic program TNT (Goloboff et al. 2008a, b). If more than one MPT was found, a strict consensus tree was calculated. Furthermore, if the strict consensus tree had polytomies in the region of interest, the IterPcR script, written by Pol and Escapa (2009) was run. The script presented by these authors looks for the least stable taxa within the trees, prunes them from the strict consensus, and shows the source of instability (due to contradicted characters or missing data). Clade support was calculated using Bremer support as well as jackknife and bootstrap analyses. In the figure for the cladistic analysis we only show the jackknife and bootstrap values that exceed 50%.
Paleohistology.—We performed five thin sections on five elements, in particular three costal and two peripheral plates of the referred specimen (MPCA-AT 26). The planes of sectioning varied among the different shell bone elements: the costal elements were cut parallel to the anteroposterior axis (perpendicular to the incorporated ribs) and the peripheral elements were cut perpendicular and parallel to the anteroposterior axis of the turtle shell. All thin sections were prepared in accordance with the methodology outlined in Chinsamy and Raath (1992). The bone microstructure of the thin sections was documented using a petrographic polarizing microscope (Nikon ECLIPSE E200). Nomenclature and definitions of structures follow Francillon-Vieillot et al. (1990). The terms “external” and “internal” are used throughout the text instead of “dorsal” and “ventral” to prevent confusion between the dorsal carapacial and ventral plastral bones of the turtle shell (i.e., the “dorsal” surface of a neural plate corresponds to the external surface of the bone, whereas the “dorsal” surface of a plastral plate corresponds to the visceral surface of the bone). The terms “inner/outer” refer to the relative position within the cortical bone, toward the core or toward the surface of the plate, respectively (Scheyer and Sánchez-Villagra 2007). To infer the lifestyle of Rionegrochelys caldieroi de la Fuente, Maniel, and Jannello gen. et sp. nov. on the basis of its shell bone histology, compactness parameters were calculated using the Windows version 4.5.5 of BONE PROFILER Software (Girondot and Laurin 2003; Laurin et al. 2004). Since several of the sampled plates are incomplete or crushed, we performed the compactness analysis only on two costal elements. For image analysis, the picture of the sectioned bone was transferred into black and white (black for bone and white for vascular spaces).
Geological setting
The landscape in the area of “El Anfiteatro” comprises a natural depression at the foot of the northern escarpment of the Meseta de Rentería (Fig. 1), which displays an completeness sequence of continental sediments of the Upper Cretaceous assigned to the Neuquén Group (Cazau and Uliana 1973; Hugo and Leanza 2001; Leanza et al. 2008). This sequence includes a set of predominantly fluvial deposits and scarce aeolian and lacustrine deposits. The sequence was deposited between the Cenomanian and the lower Campanian, during the beginning of the foreland basin stage of the Neuquén Basin (Legarreta and Gulisano 1989; Franzese et al. 2003). According to Salgado et al. (2009), in the area of El Anfiteatro, vertebrate-bearing fossiliferous levels have been found in deposits of the Cerro Lisandro (lower Turonian), Portezuelo (upper Turonian–lower Coniacian?), and Plottier (upper Coniacian–lower Santonian), formations included recently by Garrido (2010) as part of the redefined Río Neuquén Subgroup (Figs. 1, 2).
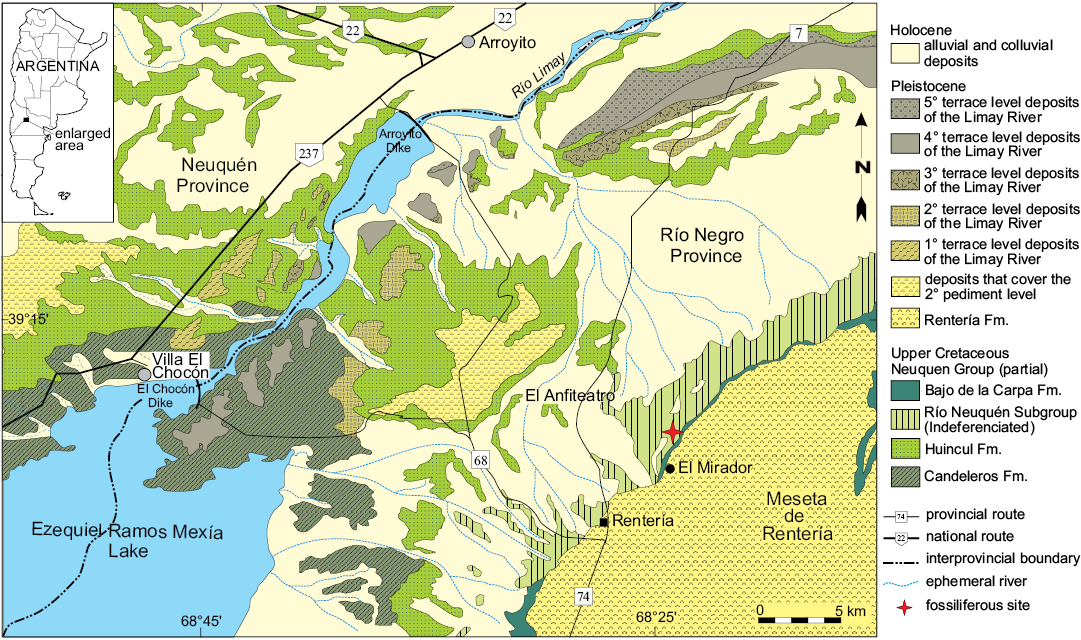
Fig. 1. Geologic map of the “El Anfiteatro” area. Modified from Salgado et al. (2009).

Fig. 2. Stratigraphic profile of the Plottier Formation outcrop, showing the levels where Rionegrochelys caldieroi de la Fuente, Maniel, and Jannello gen. et sp. nov. was recovered. Colours of deposits are indicated according to Rock-Color Chart Committee (1991). Modified from Salgado et al. (2009).
In general, the deposits of the Río Neuquén Subgroup exhibit extensive outcrops along the topographic fronts of the plateau relief that characterizes the extra-Andean area of the Neuquén Basin. Stratigraphically and sedimentologically this subgroup is characterized by the alternation of meandering fluvial deposits with different sediment-carrying capacities, yielding interstratified intervals of sand-bed and fine-grained meandering streams that define each lithostratigraphic unit (the formations) mainly regulated by climate control (Garrido 2010, 2011). In this context, fluctuating paleoclimatic regimes have been established for the deposits of the Neuquén Group, ranging from humid and subhumid to semiarid conditions, with marked seasonal dry periods (Garrido 2000).
In the area of El Anfiteatro (Fig. 3A), the dominant fine-grained intervals, such as the Cerro Lisandro Formation, form a characteristic relief of badlands (called “huayquerías” in Argentina). By contrast, the sandy-dominated units form high-stepped walls exhibiting their best outcrops in the ravines that cross the region occupying the topographic projections that form the erosive front of the Meseta de Rentería (to the east of El Anfiteatro viewpoint, El Mirador, on Provincial Road 7) (Hugo and Leanza 2001; Leanza et al. 2008).
Several fossils have been reported for these units. In the Cerro Lisandro Formation, bivalves with both valves preserved, teeth and cranial fragments of fishes, and turtles (poorly preserved plates) have been found. In the Plottier Formation (Fig. 3B–D), well-preserved and highly abundant remains of turtles and crocodiles (osteoderms and cranial remains) have been recovered. Dinosaurs are known in all these formations, while abundant petrified trunks are known in the Portezuelo and Plottier formations (Salgado et al. 2009). In general terms, the fish and bivalve remains were found in deposits originating in abandoned channels of an ox-bow type, whereas numerous isolated teeth and other small bony remains are mainly associated with sheet flood deposits formed on the flood plain. The channel-filling deposits usually contain sizeable remains of silicified wood, as well as large isolated bones of sauropod dinosaurs, most of which are poorly preserved and show evidence of transport. In contrast, the best-preserved and/or most associated bone remains, have been recovered from crevasse deposits. Among them the holotype of Rionegrochelys caldieroi was found in a yellowish mudstone level where no other specimens were retrieved (Fig. 3B–D).

Fig. 3. A. General view of the Upper Cretaceous outcrops of the “El Anfiteatro” area, northern Patagonia, Argentina, where the holotype of Rionegrochelys caldieroi gen. et sp. nov. was found. B. In situ photograph of the holotype of Rionegrochelys caldieroi gen. et sp. nov. C. Levels of the Plottier Formation (arrow indicates the location of specimen). D. Transportation of the holotype.
Systematic palaeontology
Testudines Batsch, 1788
Pleurodira Cope, 1865
Panchelidae Joyce, Parham, and Gauthier, 2004
Genus Rionegrochelys de la Fuente, Maniel, and Jannello nov.
Type species: Rionegrochelys caldieroi sp.nov.; see below.
Etymology: From Río Negro Province (Argentina) and from Greek chelys, aquatic turtle.
Diagnosis.—As for the type species by monotypy.
Rionegrochelys caldieroi de la Fuente, Maniel, and Jannello sp. nov.
Figs. 4–6.
Etymology: In honour of the late Ing. Victorino Caldiero (1945–1999), for his contribution to the project of developing the southern margin of Cipolletti, Rio Negro Province, Argentina.
Holotype: MPCA-AT 258; carapace and plastron of an adult specimen.
Type locality: Northern flank of the Parrita site (39º18ʹ13ʺ S; 68º24ʹ35ʺ W), El Anfiteatro, Río Negro Province, Argentina.
Type horizon: Upper Cretaceous, Plottier Formation, according to Musacchio (2006) and Musacchio and Vallati (2007), this horizon is Coniacian–Santonian in age, but Garrido (2010, 2011) more recently referred this horizon to the upper Coniacian–lower Santonian.
Material.—MPCA-AT 30, right lateral edge of carapace with plastral bridge suture; MPCA-AT 29, right hypoplastral bone; MPCA-AT 26, isolated plates of a carapace. All from type locality and horizon.
Diagnosis.—Rionegrochelys caldieroi can be diagnosed as a panchelid pleurodire by the suturing of the pelvic girdle to the shell, the presence of a cervical scute, only one suprapygal bone, and vertebral scutes that are narrower than the pleural scutes. It shares with South American panchelids and Chelodina: vertebral 1 wider than vertebral 2 and axillary buttresses parallel or adjacent to costal rib 1. It differs from other panchelids by the following unique combination of characters: the first vertebral scute is twice as wide as long and subrectangular in shape, a prominent and discontinuous mid-carapace crest, a strong nuchal notch, peripheral bones 2–11 with strongly gutter ends, posterior margin of the vertebral 5 in the carapace is three lobe shaped, the plastron longer than the carapace, an anteriorly wide thoracic vertebra 1 that is tapered posteriorly in ventral view, in its heart-shaped carapace, and in its wide and short cuneiform mesoplastra crossed by a humeropectoral sulcus.
Description.—Carapace: The carapace of the holotype of Rionegrochelys caldieroi is low and wide (length/width ratio 1.03) with a strong nuchal notch (reaching peripheral 1), moderately large in size (carapace length 430 mm), and equivalent in size to Lomalatachelys neuquina (Lapparent de Broin and de la Fuente 2001). Contrary to other Cretaceous panchelid species (e.g., Bonapartemys bajobarrealis, Yaminuechelys gasparinii) and extant South American and Australasian chelids (e.g., Phrynops hilarii, MHNSR H-1550, NHMUK 86-3-10-1; Emydura macquarii, QMJ 61586, QMJ 78979), where the maximum width is at the margin of the seventh and eight peripheral bones, in Rionegrochelys caldieroi the greatest width is at the margin of the fourth peripheral bones (MPCA-AT 258 carapace maximum width 410 mm). The nuchal notch, the carapace width/length ratio of 1.03, and the curved lateral margins of the posterior peripherals produce a heart-shaped carapacial outline. Although this carapace shape is a characteristic of this species, intraspecific variation in carapace outline is recognized in some chelid species (e.g., Chelus fimbriatus, Pritchard and Trebbau 1984; Sánchez Villagra et al. 1995). These authors point out that the carapace outline of this extant species can be oval, subquadrangular with straight lateral sides, or with expanded anterior or posterior margins. However, the heart-shaped outline of the Rionegrochelys caldieroi carapace is an unusual shape for both extant and extinct panchelid turtles (Maniel and de la Fuente 2016).
The carapace of Rionegrochelys caldieroi is decorated with relatively sharp longitudinal crests on the costal bones, as in some Australasian chelids (e.g., Elseya novaeguineae, USNM 313654). Along the midline, a discontinuous keel is recognized from the first neural bone to the suprapygal bone. Although the carapace decoration partially obscured sutures between bones in several sectors, the shell seems to be formed, as in crown pleurodires, by one nuchal, eight neurals, 16 costals, 22 peripherals, one suprapygal, and one pygal bone (Maniel and de la Fuente 2016).
The strong nuchal emargination is formed by the first peripheral and the nuchal bone. This emargination is deeper than the condition recognized in Yaminuechelys gasparinii and Y. maior (de la Fuente et al. 2001; Bona and de la Fuente 2005). Although the nuchal notch of Rionegrochelys caldieroi is strongly developed, it is not reaching the first costal as in Araripemys barretoi (Meylan 1996). The anterior peripherals, the bridge peripherals, and the posterior peripherals show a strong gutter. Each gutter end is coincident with a marginal scute that extends over half of two consecutive peripheral bones. The posterior free margin of the peripheral bones is slightly indentation in coincidence with the marginal scutes. A more extreme condition can be recognized in the panpleurodire Platychelys oberndorferi (Bräm 1965) and in the chelid Chelus fimbriatus (Sánchez-Villagra et al. 1995) which is formed by a strong indentation of the posterior peripheral bones.
The nuchal bone is twice as wide as long, with a wide cervical scute. Although the costal-neural sutures of the first and second neural bones are not apparent on the left side in dorsal view, they are recognized in visceral view, these neural bone are subquadrangular in shape. The third to fifth neurals are also sub quadrangular, while the sixth and seventh neural bones are hexagonal. The anterior neural bones are relatively wide as in the extant Chelus fimbriatus. This neural pattern with predominant sub quadrangular neural bones deviates from the more frequent chelid condition characterized by narrow quadrangular or hexagonal neural bones (Pritchard 1988). The suprapygal bone is quite large and subpentagonal in shape, while the pygal is nearly trapezoidal. The eighth pair of costal bones contacts one another along the carapace midline. The first to fourth costals have parallel lateral sides, while the lateral end of the fifth and seventh costal bones is much narrower than the lateral end of the adjacent elements.
Costals 1–8 are preserved on both sides of the holotype. As is typical in turtles, costal 1 is the largest costal bone. It is also wider than long. This bone contacts anteromedially the nuchal, anterolaterally peripherals 1–3, laterally peripheral 4, medially neural 1 and 2, and posteriorly costal 2. Costal 2 contacts anteriorly costal 1 and laterally peripherals 4 and 5. Both costals 2 contact posteriorly costals 3. Both costals 3 contact laterally peripherals 5 and 6, medially neurals 3 and 4, and posteriorly costal 4. Costals 4 contact laterally peripherals 6 and 7, medially neurals 4 and 5, and anteriorly and posteriorly costals 3 and 5, respectively. Costals 5 contact anteriorly and posteriorly costals 4 and 6, respectively, laterally peripherals 6 and 7, and medially neurals 5 and 6. Costals 6 contact laterally peripherals 8 and 9, medially neurals 6 and 7, anteriorly costal 5, and posteriorly costal 7. Costals 7 contact laterally peripherals 9 and 10, and medially neurals 7 and 8. Costals 8 contact each other in the midline of the carapace and anteriorly contact neural 8 and costal 7, laterally peripherals 10 and 11, and posteriorly the suprapygal. The suprapygal is pentagonal and contacts anterolaterally costal 8 and posterolaterally peripheral 11. The pygal is trapezoidal in shape and contacts anteriorly the suprapygal and laterally both peripherals 11.
The peripheral bones are relatively narrow in the anterior free margin and slightly wider in the posterior one. Peripheral 1 is the largest and, like the other peripherals of the anterior margin, is trapezoidal in outline. This bone contacts the nuchal medially, peripheral 2 laterally, and costal 1 posteriorly, as is usual in turtles. Peripheral 2 contacts peripheral 1 medially, peripheral 3 laterally and costal 1 posteriorly. The third to seventh peripheral bones contribute to the bridge. As well as the second peripheral, the bridge peripherals, and the eighth to eleventh peripheral bones are characterized by strongly dorsally curved, gutter-like margins. The axillary buttress fits between the third peripheral and the first costal, while the inguinal buttress is attached between the seventh peripheral and the lateral extreme of the fifth costal. As is typical in pleurodires, the ilia in Rionegrochelys caldieroi are sutured to the carapace (Gaffney and Meylan 1988). The iliac suture extends over the posterior area of the seventh costal, the eighth costal, and the anterolateral edge of the suprapygal. A different condition is seen in Yaminuechelys gasparinii and Y. maior where this suture extends over the eighth costal and the anterolateral edge of the suprapygal.
The cervical scute is wider than long. The vertebral scutes are strongly marked on the carapace. The first and fifth vertebral scutes are the widest. The first vertebral scute is twice as wide as long and subrectangular in shape. This scute covers most of the nuchal bone, the caudal-medial portions of both peripherals 1, the medial part of both costals 1, and probably half of neural 1. A slightly sinuous intervertebral sulcus between vertebral scutes 1 and 2 crosses the caudal portion of neural 1. The second and third vertebral scutes are slightly wider than long and subquadrangular in outline. Vertebral scute 2 covers the caudal portion of neural 1, neural 2, and most of neural 3, a small mediocaudal portion of costal 1, the medial part of both costals 2, and a portion of the anteromedial part of costal 3. The intervertebral sulcus between vertebrals 2 and 3 crosses at least neural 3 and both costals 3 in their medial part. Vertebral scute 3 covers the caudal medial portion of both costals 3, the medial part of both costals 4, neural 4, and most of the medial portion of both costals 5. The intervertebral sulcus between vertebrals 3 and 4 with an anteriorly marked inflection crosses both costals 5 in their medial portion, and the posterior part of neural 5. By contrast, vertebral 4 is slightly longer than wide and pentagonal in shape. This vertebral scute covers neurals 6–8, the caudal medial part of costals 5, the medial part of costals 6 and 7, and the cranial medial part of costals 8. The intervertebral sulcus between vertebrals 4 and 5 crosses both costals 8. The fifth vertebral scute is slightly wider than long and characterized by a three lobed posterior margin. This vertebral scute covers part of the caudal portion of both costals 8, most of the suprapygal, and small portion of the proximal margins of the pygal and peripheral bones 11. Four pairs of pleural scutes are recognized in Rionegrochelys caldieroi as is usual in turtles. Pleural 1 is slightly longer than wide and covers the posterolateral portion of peripheral 1, the medial part of peripherals 2–4, the central and lateral part of costal 1, and the cranial centrolateral portion of costal 2. Pleural 2 is wider than long and covers costals 2–4. Pleural 3 is also wider than long and covers costals 4–6. Pleural 4 is as wide as long and covers costals 6–8. The interpleural sulci between pleural scutes cross costals 2, 4, and 6 as usual in turtles. The sulcus between pleural 4 and vertebral 5 crosses costals 8.
On the anterior margin of the carapace, marginal scutes 1–4 are recognized. Marginal scutes 4–8 are also identified on the right and left peripheral bridge. Although the intermarginal sulcus between marginal 1 and 2 is scarcely discernible, marginal scute 1 is subrectangular in shape, as does marginal scute 2. Marginal scute 1 covers the proximolateral sector of the nuchal and only the cranial medial part of peripheral 1. Marginal scutes 2–4 also cover two peripheral bones each without extending onto costal bone 1. From marginal scutes 5–11 (with the exception of marginal 10), the scutes cover the costal bone and show an alternating pentagonal and rectangular outline. Marginal 12, pentagonal in shape, covers the pygal, peripheral 11, and the suprapygal.
Plastron: The plastron of the holotype of Rionegrochelys caldieroi is preserved in good condition. It is medium sized (450 mm length along the midline) and slightly longer than the carapace (430 mm length along the midline). The anterior and posterior lobes are nearly equal in length. The bridge (= axillo-inguinal distance) is about 70% of the anteroposterior length of the posterior plastral lobe. The relative length of the bridge of Rionegrochelys caldieroi is greater than in long necked chelids such as Yaminuechelys and Hydromedusa or short necked chelids such as Prochelidella portezuelae (de la Fuente 2003) and Phrynops geoffroanus (AMNH 79048, USNM 306646), but it is shorter than the bridge of other extinct species (e.g., Bonapartemys bajobarrealis; see Lapparent de Broin and de la Fuente 2001).
The broad anterior plastral lobe (width/length ratio 1.71) in Rionegrochelys caldieroi is U-shaped. As in the panpleurodire Platychelys oberndorferi (Bräm 1965) and the chelid Chelus fimbriatus (AMNH 70638, USNM 64154), the anterior lobe extends beyond the anterior margin of the carapace. The posterior plastral lobe of Rionegrochelys caldieroi is as long as wide. The lateral margins of the posterior lobe are curved and have a strong femoral-anal notch, as is usual in species of the Phrynops geoffroanus complex (see Rhodin and Mittermeier 1983). The anal notch in Rionegrochelys caldieroi is U-shaped as in Bonapartemys bajobarrealis and Lomalatachelys neuquina, and unlike the V-shaped condition of Yaminuechelys gasparinii among extinct chelids. However, intraspecific variation of this feature has been pointed out in some fossil and extant pleurodires (Wood and Díaz de Gamero 1971; Cadena et al. 2008; Ferreira et al. 2016).
The epiplastra in Rionegrochelys caldieroi are long and broad with a relatively short medial contact. These bones are partially separated by a large, rounded entoplastron. Between the large hyo and hypoplastra, the mesoplastra are located. They are cuneiform and they are relatively long in comparison to those of Yaminuechelys spp. The xiphiplastra show slight interdigitating sutures with the hypoplastra. They are broad, but deeply notched at the limit imposed of the femoral-anal sulcus. On their visceral surface, the pubis and ischium are attached by a suture to the xiphiplastron. The pubic scar is lateral in position and obliquely oriented at an obtuse angle with respect to the plastral midline, whereas the ischial scar is L-shaped with the short branch of the L extended on the xiphiplastral tips and the longer one oriented slightly obliquely towards the shorter. The xiphiplastral tips end in nearly blunted margins.
As in other pleurodires, Rionegrochelys caldieroi has six pairs of plastral scutes (extragular, humeral, pectoral, abdominal, femoral, and anal) and one odd scute (gular). The plastron has a simple extragular-gular scheme with small extragular scutes on the epiplastra and a small, subtriangular gular scute over the anterior margin of the entoplastron. The large entoplastron is crossed posteriorly by the humero-pectoral sulcus, which is unusual among South American panchelids. An entoplastron crossed by this sulcus can otherwise be seen in some Australasian species, such as Elseya novaeguineae (USNM 313654), E. schultzei and E. rhodini (Thompson et al. 2015), E. flaviventralis (Thompson and Georges 2016), Emydura macquarii (NHMUK 86-8-26-5), and Rheodytes leukops (QM J 85198). The interfemoral sulcus is longer than any of the other median sulci of the plastron. The interanal sulcus and gular scutes are the shortest, whereas the interpectoral sulcus is longer than the interhumeral or interabdominal sulci. This peculiar plastral formula is recognized in some specimens of extant Chelus fimbriatus (e.g., USNM 64154). Although many authors have used these midline contact lengths of plastral scutes to characterize species of turtles, Lovich and Ernst (1989) and Sánchez-Villagra et al. (1995) reported a great amount of intraspecific variation in extant turtles such as Mauremys reevesii, Graptemys pulchra, Platemys platycephala, and Chelus fimbriatus. The lateral cuneiform mesoplastra are crossed by the pectoro-abdominal sulcus. A similar condition can be seen in other extinct Patagonian species of panchelid turtles (e.g., Bonapartemys bajobarrealis, Linderochelys rinconensis, Lomalatachelys neuquina, Prochelidella portezuelae, Yaminuechelys gasparinii, Y. maior) and platychelyid panpleurodire such as Notoemys oxfordiensis, Notoemys laticentralis, and Platychelys oberndorferi (see Bräm 1965; de la Fuente and Iturralde-Vinent 2001; de la Fuente et al. 2001, 2007; Lapparent de Broin and de la Fuente 2001; de la Fuente 2003; Bona and de la Fuente 2005; Lapparent de Broin et al. 2007; Cadena and Joyce 2015).
Pelvic girdle: The pelvic girdle is represented by both right and left halves in the holotype (Figs. 4, 5). Each one is tri-radiate in lateral view. As is typical in pleurodires, the pubis and ischium of each half are attached by suture to the xiphiplastron. The iliac blade of the ilium expands dorsally to be attached by suture to the carapace. The pubis is sutured only by the lateral process, whereas the ischium is suturally attached by a broad surface extending from its symphysis to a lateral process. As is typical in pleurodires, the pubis and ischium are separated from each other by a large and confluent thyroid fenestra.
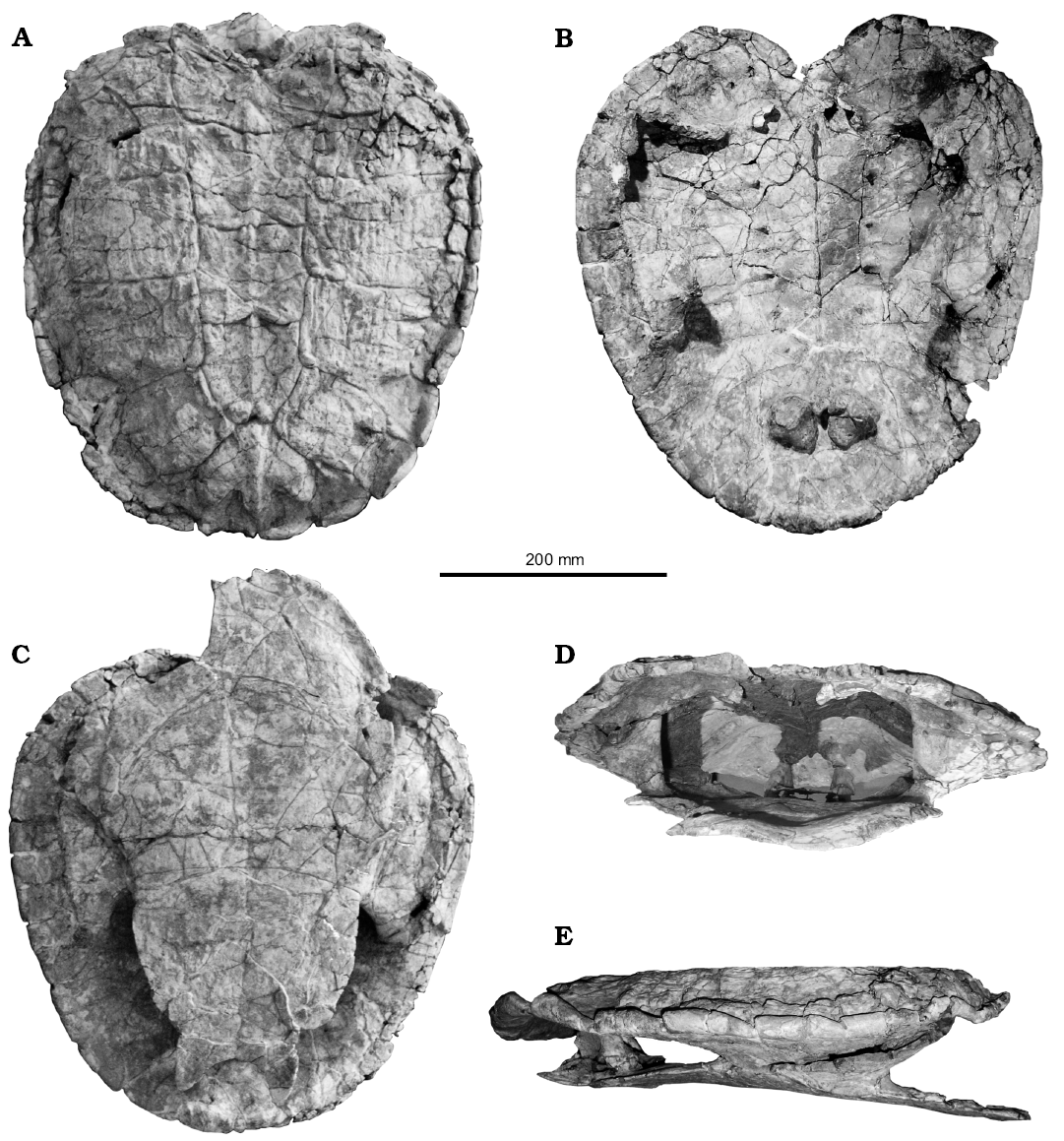
Fig. 4. Photographs of the holotype of the panchelid turtle Rionegrochelys caldieroi gen. et sp. nov. (MPCA-AT 258) from Parrita site, Upper Cretaceous. Carapace in dorsal (A) and visceral (B) views. Plastron in ventral view (C). Carapace and plastron in anterior (D) and lateral (E) views. Note suturally attached pelvic girdle.

Fig. 5. Explanatory drawings of the holotype of the panchelid turtle Rionegrochelys caldieroi gen. et sp. nov. (MPCA-AT 258) from Parrita site, Upper Cretaceous. Carapace in dorsal (A) and visceral (B) views. Plastron in ventral view (C). Carapace and plastron in anterior (D) and lateral (E) views. Note suturally attached pelvic girdle.
Thoracic vertebrae. At least six dorsal vertebrae are preserved in the holotype of Rionegrochelys caldieroi. Among them, the first, second, and third are in articulation and in good condition, allowing detailed description (Fig. 6). All contacts of the preserved thoracic vertebrae are platycoelous synchondroses. Thoracic vertebra 1 is well preserved and is as long as wide. The anterior articular end is concave facing anteriorly and slightly ventrally. It is also as wide as long. In ventral view, thoracic vertebra 1 is wide anteriorly and tapered posteriorly. A different condition is seen in other extant and extinct crown chelids (Fig. 7), where an hourglass shape is the most common pattern recognized in the ventral view of the first thoracic vertebra. The well-developed prezygapophyses face medio-dorsally and are laterally strongly divergent. Thoracic rib 1 is articulated with the anterior end of thoracic vertebra 1, whereas thoracic rib 2 is articulated between the posterior end of thoracic vertebra 1 and the anterior end of thoracic vertebra 2. Thoracic vertebra 2 is longer than the first one, and longer than wide. Its anterolateral end contacts thoracic rib 2, whereas its posterolateral end contacts thoracic rib 3. Thoracic vertebra 3 is also slightly longer than wide like the second one. The anterolateral margin of this thoracic vertebra contacts thoracic rib 3, whereas the posterolateral margin contacts thoracic rib 4.
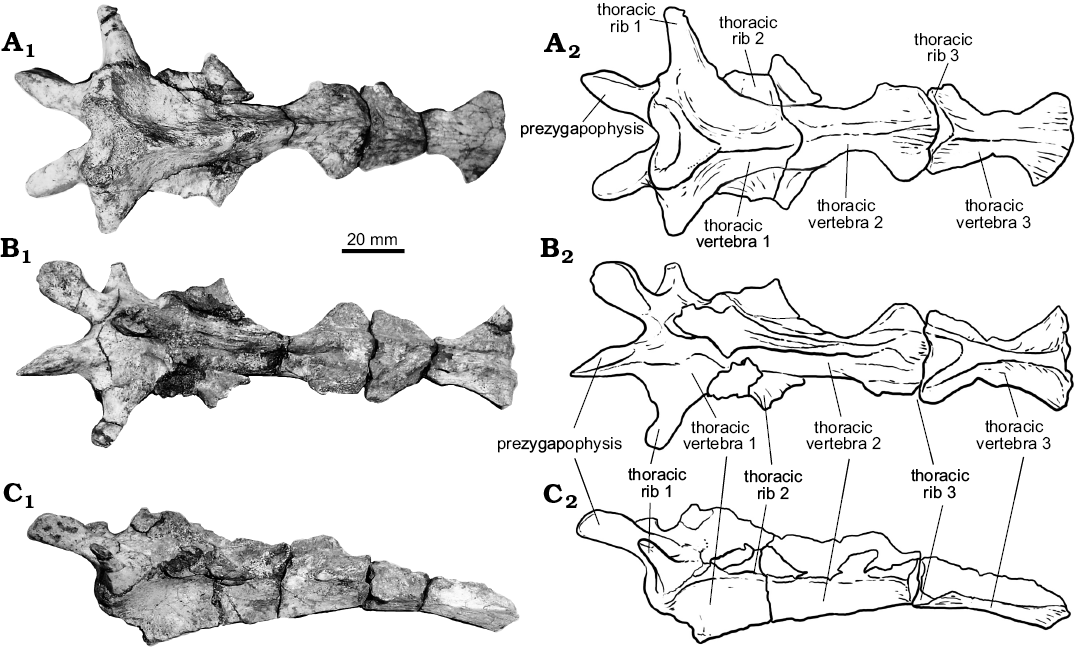
Fig. 6. The holotype of the panchelid turtle Rionegrochelys caldieroi gen. et sp. nov. (MPCA-AT 258) from Parrita site, Upper Cretaceous. Thoracic vertebrae 1–3 in dorsal (A), ventral (B), and lateral (C) views. Photographs (A1–C1) and explanatory drawings (A2–C2).
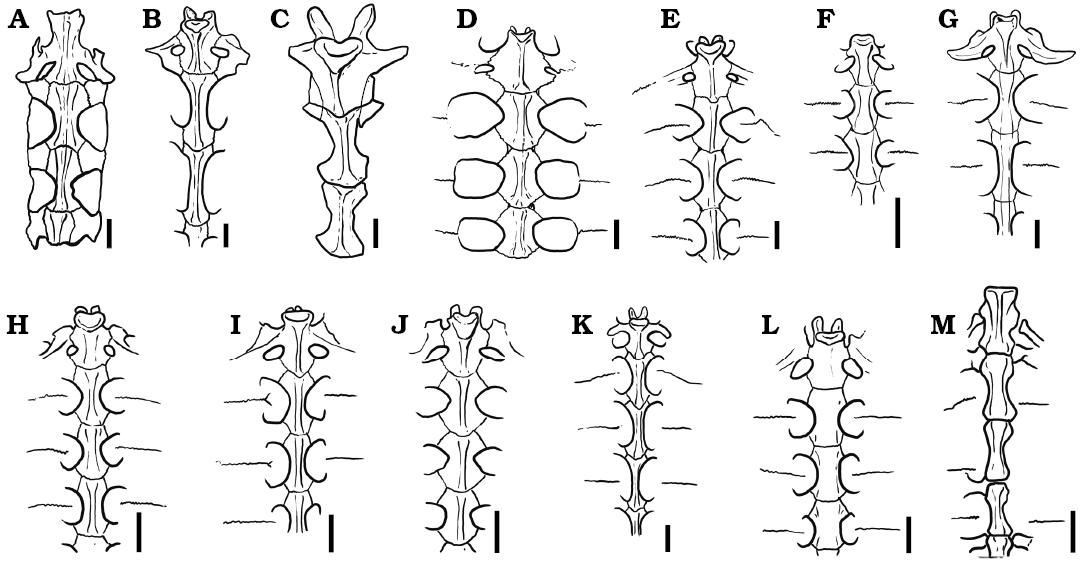
Fig. 7. Comparison of thoracic vertebrae 1–3 in some extinct and extant pleurodiran turtles. A. Chelodina colliei Gray, 1856 (NHMUK 64-12-22-66). B. Elseya dentata (Gray, 1863) (NHMUK 76-5-19-27). C. Rionegrochelys caldieroi gen. et sp. nov. (MPCA-AT 258). D. Chelus fimbriatus (Schneider, 1783) (MZUSP 2619). E. Phrynops hilarii (Duméril and Bibron, 1835) (MHNSR H-1550). F. Platemys platycephala (Schneider, 1792) (MHNSR H-1554). G. Rheodytes leukops Legler and Cann, 1980 (QMJ 7693). H. Acanthochelys macrocephala (Rhodin, Mittermeier, and McMorris, 1984) (MACN H-8288). I. Hydromedusa tectifera Cope, 1870 (MHNSR-H 1615). J. Mesoclemmys nasuta (Schweigger, 1812) (MACN H-11967). K. Podocnemis sextuberculata Cornalia, 1849 (MZUSP 2501). L. Pelomedusa subrufa (Bonnaterre, 1789) (SMF 7953). M. Yaminuechelys gasparinii de la Fuente, Lapparent de Broin, and Manera de Bianco, 2001 (MPA 86-86-IC). Scale bars 20 mm.
Paleohistology.—The microanatomy shows a compact diploe structure with the external and internal cortex framing in the interior area of cancellous bone (Fig. 8A). The external cortex is thicker, double in size compared with the internal cortex. The cancellous bone is well developed, occupying 70% of the cross-sectional area in peripheral elements and less than 50% in costal elements. The osteohistology of all the shell elements is described together. Variation among samples is mentioned if applicable.
External cortex: The external cortex is mostly composed of primary bone. In the peripheral plates, primary bone consists of interwoven bundles of structural collagenous fibers. On the other hand, costal plates are formed by mineralized collagenous fibers arranged parallel/sub-parallel to the external surface. These fibers exhibit two main orientation, which are parallel and transversal to the progression of the element (Fig. 8B, C). Sharpey´s fibers are present in the costal plates. They are short and oriented mostly perpendicularly or oblique to the outer surface. Resorption lines associated with successive episodes of bone resorption and deposition are clearly observed in the outer (ornamented) portion of the cortex of one peripheral and one costal plate (Fig. 8B, D). Vascularization is mostly formed by simple vascular canals that run in parallel or perpendicular to the anteroposterior axis. Simple vascular canal openings to the external surface can be clearly made out (Fig. 8E). Some primary osteons are also observed and these commonly anastomose. Secondary osteons are more commonly distributed in the inner (i.e., perimedulary) portion of the cortex. These osteons are more abundant in some costal plates, in which they even reach the outer cortex (Fig. 8B). Secondary osteons have bone composed of alternating lamellae (Fig. 8B). Bone cell lacunae are commonly arranged following the orientation of the structural fibers in which they are embedded. Short canaliculi are preserved. Growth marks are present, mostly discontinuous, and not easily discerned.
Cancellous bone: The cancellous bone is quite massive and strongly remodeled. Short trabeculae appear in the costal elements and long ones in the peripheral elements. The trabeculae themselves are composed of centripetally deposited secondary lamellar bone formed over different growth generation with variation in the orientation of each lamella. Intertrabecular spaces are generally small and circular or sub-circular in section. Interstitial areas within the trabecular bone are composed by primary and secondary bone, as seen in the external cortex. Flattened bone cell lacunae follow the centripetally deposited lamellar bone linings (Fig. 8F).
Internal cortex: The internal cortex is composed mainly of parallel-fibered bone matrix that can locally form lamellar bone. In one costal element, the fibers are parallel to the latero-medial axis in the central area (which corresponds to the rib), and gradually change their arrangement toward the suture margins, where they become parallel to the anteroposterior axis (Fig. 8G). This is made visible by the monorefringent properties of the bone in the center and the birefringent properties toward the margins, as well as by the change in the shape of the bone cell lacunae, which are circular/sub-circular in section in the center and strongly elongated and flattened toward the suture margins. Vascularization is typically poor (mainly avascular in several cases). Some simple vascular canals and primary osteons appear to be longitudinally oriented. At least five lines of arrested growth are visible (Fig. 8G).
Sutures: The structure of sutures could be studied in the three costal elements. The microanatomical structure is typically “peg and socket”. The matrix principally consists of woven bone. Dense Sharpey´s fibers are oriented parallel to the protrusions in all samples. In some samples, Sharpey´s fibers continue into the cancellous bone.
The results of the compactness parameters calculated for a costal element of Rionegrochelys caldieroi using BONE PROFILER Software were: modeled compactness 80.6%, lowest compactness at the center 11.1% (SE 0%), modeled compactness at the center 70.5%, compactness at the maximum periphery 87% (SE 0%), modeled compactness at the periphery 87.2%, compactness at the transition between cancellous bone and compact bone (point P) 36% (SE 0.6%), and the slope of compactness change at point P 1.15 (SE 0.008%).
Stratigraphic and geographic range.—El Anfiteatro, Río Negro Province, Argentina. Plottier Formation (upper Coniacian–lower Santonian), Rio Neuquén Subgroup, Neuquén Basin.
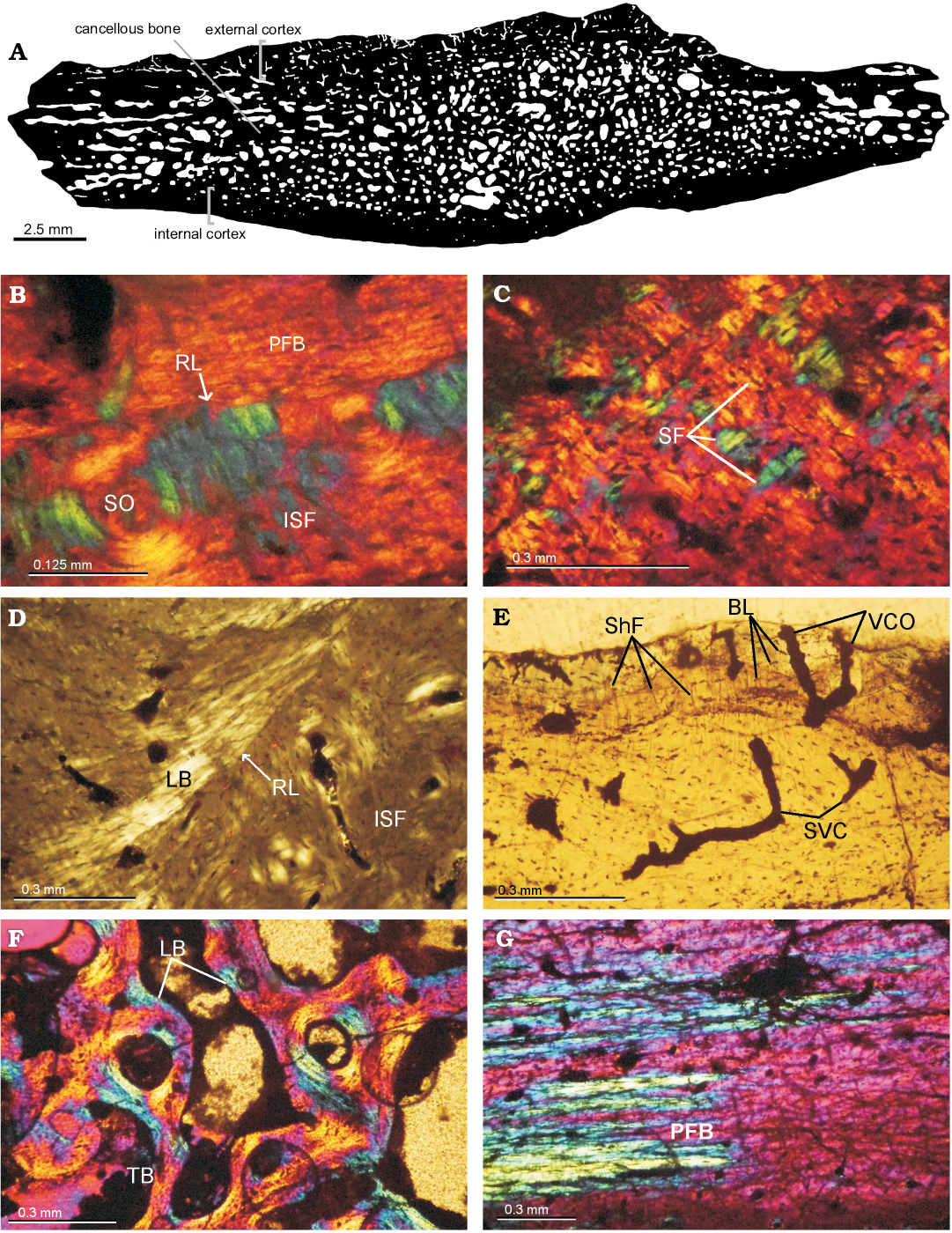
Fig. 8. Shell bone histological section of the panchelid turtle Rionegrochelys caldieroi gen. et sp. nov. (MPCA-AT 26) from Parrita site, Upper Cretaceous. A. Black and white processed image from costal element. B. Close view of external cortex in costal element showing a resorption line. External cortex of peripheral element showing structural fibers (C), a resorption line and lamellar bone (D). E. Vascularization in external cortex of costal element. F. General view of cancellous bone of peripheral element. G. Transition in the orientation of PFB (parallel-fibered bone) in the internal cortex of costal element. Abbreviations: BL, bone lacunae; ISF, interwoven structural collagenous fibre bundles; LB, lamelar bone; PFB, parallel-fibred bone; RL, resorption line; SF, structural fiber; ShF, Sharpey’s fibres; SO, secondary osteon; SVC, simple vascular canal; TB, bone trabeculae; VCO, vascular canal opening to the external surface.
Phylogenetic analyses
Results.—Four MPTs of 209 steps (CI: 0.421; RI: 0.598) were found after the two cycles of the two cycles of heuristic search in TNT (Goloboff et al. 2008a, b) and a strict consensus tree was calculated (Fig. 9). The list of synapomorphies common to the MPTs is shown in SOM 2. In the strict consensus tree, there is a basal polytomy involving three taxa: Prochelidella portezuelae, the crown-clade Chelidae, and a clade formed by Rionegrochelys caldieroi + Bonapartemys bajobarrealis, Lomalatachelys neuquina, and Mendozachelys wichmanni. Two main groups are recovered within crown Chelidae. One formed by the short-necked Australian species and the other formed by the short and long-necked South American species plus the Australian long-necked Chelodina colliei. Yaminuechelys maior + Yaminuechelys gasparinii are recovered as the sister group to Hydromedusa tectifera. The total clade Panchelidae (Joyce et al. 2004; Maniel and de la Fuente 2016) was supported by two synapomorphies in the strict consensus (character 22, state 0, splenial present; character 25, state 0, a cervical vertebrae series with only the 6th cervical vertebra with procoelous centrum). The presence of a splenial bone is a state observed in all extant chelids, except for Rheodytes leukops. This condition is also seen in Yaminuechelys maior and Mendozachelys wichmanni. A procoelous centrum in 6th cervical vertebra is a condition seen in all extant chelids, although this condition is also recognized in fossil taxa such as Yaminuechelys maior, Yaminuechelys gasparinii, Prochelidella portezuelae, and Mendozachelys wichmanni. The monophyletic group formed by Rionegrochelys caldieroi, Bonapartemys bajobarrealis, Lomalatachelys neuquina, and Mendozachelys wichmanni was supported by two synapomorphies in the strict consensus: pubic scar lateral, located on the margin of the xiphiplastron (character 56, state 0) and ischial scar extending onto the xiphiplastral tips (character 57, state 1). The position of pubic scar; lateral, located in the margin of the xiphiplastron, could not be recognized in M. wichmanni, but it is present in remaining taxa, and this condition is also present in Chelus fimbriatus and Rheodytes leukops. The position of ischial scar, extending onto the xiphiplastral tips, could be recognized in all the species of the clade. This condition is also present in Chelus fimbriatus and some Australasian species (such as Elseya dentata, Emydura macquarii, and Myuchelys latisternum). The crown clade Chelidae was diagnosed in all MPTs by two synapomorphies: lack of a neural bone series (character 36, state 3) and cervical scute longer than wide (character 50, state 1). The lack of neural bones as a synapomorphy of chelids can be often recognized in Australasian species (Chelodina colliei is the only species among the taxa included in this analysis in which neural bones are present in dorsal view), Platemys platycephala and Acanthochelys macrocephala. The remaining chelid species present two conditions. Phrynops hilarii, Chelus fimbriatus, Hydromedusa tectifera, Yaminuechelys gasparinii, and Y. maior present a continuous series which does not reach the suprapygal bone. This condition is also present in Prochelidella portezuelae, Mendozachelys wichmanni, Bonapartemys bajobarrealis, Lomalatachelys neuquina, and Rionegrochelys caldieroi as well as in the outgroup (Pelomedusa subrufa and Podocnemis sextuberculata). By contrast, Mesoclemmys nasuta and Chelodina colliei present a discontinuous series of neural bones lacking the first one and also not reaching the suprapygal. The cervical scute that is longer than wide is a condition present in some Australasian chelids (such as Emydura macquarii, Rheodytes leukops, and Myuchelys latisternum) and South American chelids (such as Platemys platycephala, Mesoclemmys nasuta, and Phrynops hilarii), the rest of the extant chelids presenting a cervical scute that is wider than long. In Hydromedusa tectifera the cervical scute does not even reach the anterior margin. Two characters (characters 34 and 61) were recovered as autapomorphies of Rionegrochelys caldieroi: the development of an enlarged costal-vertebral tunnel along the entire thoracic vertebrae series (character 34, state 0), and the presence of a pectoral scute on the middle of the entoplastron (character 61, state 1). The first one is shared with Chelydra serpentina and Chelus fimbriatus. This condition was probably present in the Yaminuechelys clade, but it has not been recognized yet. The pectoral scute extending over the middle of the entoplastron is also present in Podocnemis sextuberculata, Elseya dentata, Emydura macquarii, Chelus fimbriatus, and Chelodina colliei. The taxon list is shown in SOM 3.

Fig. 9. Strict consensus tree from the four MPT’s obtained from the morphological cladistic analysis of Rionegrochelys caldieroi gen. et sp. nov. The stars show the alternative positions of Pseudemydura umbrina. The numbers in the left of the lines are Bremer support values, whereas the numbers in the right of the lines are Jackknife and Bootstrap support values, respectively, that exceed 50%. Abbreviations: Af, Africa, SAm, South America, NAm, North America, Au, Australia; l-n, long-necked panchelids, s-n, short-necked panchelids.
Discussion
Taxonomic and phylogenetic remarks.—The carapace, plastron, and pelvic girdle of Rionegrochelys caldieroi fit well with those of panchelid pleurodiran turtles. The elements of the pelvic girdle (pubis, ischium and ilium) are suturally attached to the shell, a synapomorphy recognized for Pleurodira (see Gaffney and Meylan 1988, and references therein). Although the skeletal elements that yield panchelid synapomorphies, such as the skull or the cervical vertebrae are not preserved in the holotype of the new species, the type of pelvic-shell connection, in conjunction with an assemblage of shell characters, such as the presence of a cervical scute, short and wide mesoplastra crossed by a pectoro-abdominal sulcus, the presence of a single suprapygal bone, vertebral scutes narrower than the pleural ones, and inguinal buttress extending over peripherals 7 and 8, allow Rionegrochelys caldieroi to be assigned to the clade Panchelidae. The unusual proportion between carapace and plastron (plastron longer than carapace) and the peculiar morphology of the shell such as the heart-shaped carapace, a very deep nuchal notch, peripheral bones 2–11 with strongly dorsally curved margins, the first vertebral scute being twice as wide as long and subrectangular in shape, the trilobate posterior margin of vertebral scute 5, the development of a wide costal-vertebral tunnel along the entire thoracic vertebral series, and a pectoral scute extending over the middle of the entoplastron together with the results of the phylogenetic analysis shows that this taxon is a new species.
Some Cretaceous panchelid species such as Prochelidella portezuelae, Lomalatachelys neuquina, and Bonapartemys bajobarrealis, that are only known from postcranial remains, mainly the shell, were added to this phylogenetic analysis. When the analysis was performed, some of the cranial characters recovered previously as synapomorphies of Chelidae (Gaffney 1977; Bona and de la Fuente 2005; de la Fuente et al. 2017), such as the absence of the quadratojugal, the lateral cheek emargination or the presence of nasals, were neither recovered as Panchelidae nor as Chelidae, due to the low percentage of characters scored in the matrix by these taxa. Indeed, Mendozachelys wichmanni is the only fossil taxon with cranial and post-cranial remains with over 50% of characters scored in the matrix, whereas other extinct Patagonian species known mainly by shells do not reach this percentage (Bonapartemys bajobarrealis 43%, Lomalatachelys neuquina 37%, Prochelidella portezuelae 42%, and Rionegrochelys caldieroi 46%).
It is noteworthy that Mendozachelys wichmanni and Lomalatachelys neuquina were recovered as stem chelid in contrast with previous morphological analyses (Cadena et al. 2008; de la Fuente et al. 2015b, 2017) in which they appear within the chelid crown clade. The absence of skulls in most of the extinct South American species including in this analysis, and the amount of the plesiomorphic condition present on their post-cranial remains not allow us clarified the position of these taxa. De la Fuente et al. (2017) realized a constrained analyses following a molecular topology leading to two topologies showing a different position of Mendozachelys wichmanni, This taxon was recovered in one of the two topologies as a sister taxa of the crown Chelidae (de la Fuente et al. 2017: fig. 11B), a position more closely related to the topology recovered here.
Paleohistological remarks.—The general histology of Rionegrochelys caldieroi shows plesiomorphic characters for turtles, including the diploe structure of the shell with external and internal compact bone framing interior cancellous bone, the metaplastic incorporation of dermal interwoven structural fiber bundles, the presence of Sharpey’s fibers in the external cortex, and parallel-fibered bone in the internal cortex (e.g., Scheyer et al. 2007; Cerda et al. 2016). Within chelids, the presence of canal openings as small foramina on the surface of the external cortex has only been reported for Phrynops geoffroanus and Yaminuechelys ssp. (Scheyer 2007; Jannello et al. 2016)
The characteristic resorption from the outside in the external cortex has been reported in some lineages of turtles in recent years (de la Fuente et al. 2015a; Buffrénil et al. 2016; Jannello et al. 2016). Buffrénil et al. (2016) reported a superficial ornamented layer separated from the subjacent bone strata by a reversion line, with discordant bone deposits above and under this line in members of Trionychidae (Amyda cartilaginea, Trionyx triunguis, Aspideretoides cf. riavinini, and Cyclanorbis senegalensis) and in Araripemydidae (Araripemys barretoi). Specimens of other taxa with resorption lines in the external cortices, such as Condorchelys antiqua, Bothremys barberi, and Podocnemis erythrocephala, have been interpreted as pathological conditions (e.g., Cerda et al. 2016). Within Chelidae, Yaminuechelys spp. are the only taxa for which resorption processes have been reported to date (Jannello et al. 2016). Buffrénil et al. (2016) proposed two distinct modalities for the formation and maintenance of ornamentation in turtles: (i) local bone surface flattened by resorption, sculpturing relief resulting from slight differences in bone accretion rates between the top of the ridges and the floor of the pits, and (ii) the surface of the bone not entirely flattened by resorption, with the subsequent accretion of bone enhancing the preexisting reliefs. The second modality, previously reported for Yaminuechelys spp. (Jannello et al. 2016), seems to correspond with that observed in Rionegrochelys caldieroi.
Paleoecology.—Several authors (e.g., Scheyer and Sander 2007; Scheyer et al. 2014) have studied the correlation between the shell microstructure and the lifestyle of extant and extinct turtles and concluded that it is a valuable tool for inferring different ecological habits in the group. In this respect, those bones with a low degree of compactness and homogenization of the compact cortical and cancellous shell areas are correlated with aquatic specializations. The bone shell microanatomy of Rionegrochelys caldieroi has a massive structure, with higher compactness (80.6%) than other chelids such as Yaminuechelys spp. (68%). The high compactness of the shell bones of Rionegrochelys caldieroi is comparable with that reported in terrestrial turtles (e.g., Solemydidae; Scheyer et al. 2014). These results are rather unexpected, since chelids are characterized by living in freshwater aquatic to semiaquatic environments (de la Fuente et al. 2014). Taphonomic data also contradict fully terrestrial habits for Rionegrochelys caldieroi. The relationship between the stylopodium, zeugopodium, and autopodium of the forelimb is a good indicator of habitat as well (Joyce and Gauthier 2004), but this parameter could not be applied to Rionegrochelys caldieroi because the limbs were not preserved. Although the terrestrial interpretation needs to be tested with further histological sections, we provisionally suggest semi-aquatic habits with a tendency to terrestrial ones, similar to the extant pelomedusid Pelomedusa subrufa (Pritchard 1979; Ernst and Barbour 1989; Boycott and Bourquin 2008) and the extant chelid Platemys platycephala, a poor swimmer that frequently roams the forest floor (Ernst and Barbour 1989). The presence of an enlarged costo-vertebral tunnel in Rionegrochelys caldieroi are recognized within suction feeders turtles as Chelydra serpentina, Macrochelys temminckii, and Chelus fimbriatus could be suggest this kind of alimentation patterns to Rionegrochelys caldieroi. Indeed, these last species (Chelydra serpentina, Macrochelys temminckii, and Chelus fimbriatus), are bottom walkers (Joyce and Gauthier 2004) and sometimes could be found outside the freshwater (Ernst and Barbour 1989).
Conclusions
Rionegrochelys caldieroi de la Fuente, Maniel, and Jannello gen. et sp. nov. is assigned to Panchelidae on the basis of a combination of characters such as the pelvis shell attached by suture, the presence of a cervical scute, the first vertebral scute wider than the second one, short and wide mesoplastral bones crossed by a pectoro-abdominal sulcus, the presence of a single suprapygal bone, and vertebral scutes narrower than pleural ones. In a preliminary analysis, Rionegrochelys caldieroi is recovered as a stem chelid and as a sister taxon of Bonapartemys bajobarrealis and the clade formed by Lomalatachelys neuquina and Mendozachelys wichmanni. The unusual proportion between carapace and plastron and the peculiar morphology of the shell (such as the heart-shaped carapace, the very deep nuchal notch, peripheral bones 2–11 with strongly dorsally curved margins, vertebral scute 1 subrectangular in shape and twice as wide as long, and the posterior margin of vertebral scute 5 three lobe shaped) suggest that this specimen represents a new taxon.
The bone shell microanatomy of Rionegrochelys caldieroi has a massive structure, with higher compactness (80.6%) than other chelids, comparable to that reported for terrestrial turtles. These results are rather unexpected, since chelids are characterized by living in freshwater aquatic to semiaquatic environments. Taking into account that the taphonomic data and the geological context suggest fluvial deposits, semi-aquatic habits with terrestrial tendencies, similar to the extant pelomedusid Pelomedusa subrufa, are proposed for Rionegrochelys caldieroi.
Acknowledgements
Adán Pérez García (Universidad Nacional de Educación a Distancia, Madrid, Spain) and Ian Smales (Biosis, Melbourne, Australia) provided photographs of Chelydra serpentina and Pseudemydura umbrina. Ignacio Cerda (MPCA) provided assistance in paleohistological studies. The illustration of turtle materials was made by Jorge González (La Plata, Argentina) and grammar reviewed by Cecilia Deschamps (Universidad Nacional de La Plata, Argentina) and Rupert Glasgow (Zaragoza, Spain). Endemas and the Agencia Cultura Rio Negro promoted and authorized the fieldwork conducted by LS, JIC, and their crew. We are grateful to Walter Joyce (University of Fribourg, Switzerland) and Adán Pérez Garcia for the helpful and constructive comments. This study was supported partially by PICT 2013-0095 ANPCyT (MSdelaF) and by UNRN PI 40-A-312 (LS). Repsol-YPF financed the field campaign. This paper forms part of the project CGL2014-53548 and is subsidized by the Spanish Ministry of Economy and Competitiveness, the European Regional Development Fund. The Willi Hennig Society sponsored the TNT cladistics software.
References
Batsch, A.J.C.K. 1788. Versuch einer Anleitung, zur Kenntnis und Geschichte der Thiere und Mineralien, 528 pp. Akademische Buchhandlung, Jena.
Bona, P. and de la Fuente, M.S. 2005. Phylogenetic and paleobiogeographic implications of Yaminuechelys maior (Staesche, 1929) new comb., a large long-necked chelid turtle from the early Paleocene of Patagonia, Argentina. Journal of Vertebrate Paleontology 25: 569–582. Crossref
Boycott, R.C. and Bourquin, O. 2008. Pelomedusa subrufa (Lacépède 1788) helmeted terrapin. In: A.G.J Rhodin, P.C.H. Pritchard, P.P. Van Dijk, R.A. Saumure, K.A. Buhlman, and J.B. Iverson (eds.), Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoises and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5: 007.1–007.6.
Bräm, H. 1965. Die Schildkröten aus dem oberen Jura (Malm) der Gegend von Solothurn. Schweizerische Paläontologische Abhandlungen 83: 1–190.
Buffrénil, V., Clarac, F., Canoville, A., and Laurin, M. 2016. Comparative data on the differentiation and growth of bone ornamentation in gnathostomes (Chordata: Vertebrata). Journal of Morphology 277: 634–670. Crossref
Cadena, E.A. and Joyce, W. 2015. A review of the fossil record of turtles of the clades Platychelyidae and Dortokidae. Bulletin of the Peabody Museum of Natural History 56: 3–20. Crossref
Cadena, E.A., Jaramillo, C.A., and Paramo, M.E. 2008. New material of Chelus colombiana (Testudines; Pleurodira) from the lower Miocene of Colombia. Journal of Vertebrate Paleontology 28: 1206–1212. Crossref
Canudo, J.I., Salgado L., Barco, J.L., Bolatti, R., and Ruiz-Omeñaca, J.L. 2004. Dientes de dinosauriosterópodos y saurópodos de la Formación Cerro Lisandro (Cenomaniense superior–Turoniense inferior, Cretácico superior) en Río Negro (Argentina). Geo-Temas 6: 31–34.
Cazau, L.B.A. and Uliana, M.A.1973. El Cretácico superior continental de la Cuenca Neuquina. Actas del 5° Congreso Geológico Argentino 3: 131–163.
Cerda, I.A., Sterli J., and Scheyer, T.M. 2016. Bone shell microstructure of Condorchelys antiqua Sterli, 2008, a stem turtle fromthe Jurassic of Patagonia. Comptes Rendus Palevol 15: 133–146. Crossref
Cope, E.D. 1865. Third contribution to the herpetology of tropical America. Proceedings of the Academy of Natural Sciences of Philadelphia 1865: 185–198.
Chinsamy, A. and Raath, M.A., 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Ferreira, G.S., Rincon, A.D., Solórzano, A., and Langer, M.C. 2016. Review of the fossil matamata turtles: earliest well-dated record and hypotheses on the origin of their present geographical distribution. Science of Nature 103: 28. Crossref
Francillon-Vieillot, H., de Buffrénil, V., Castanet, J., Géraudie, J., Meunier, F.J., Sire, J.Y., Zylberberg, L., and de Ricqlès, A., 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, 471–530. Van Nostrand Reinhold, New York.
Franzese, J., Spalletti, L., Gómez Pérez, I., and Macdonald, D. 2003. Tectonic and paleonvironmental evolution of Mesozoic sedimentary basins along the Andean foothills of Argentina (32°–54°S). Journal of South American Earth Sciences 16: 81–90.
de la Fuente, M.S. 2003. Two new pleurodiran turtles from the Portezuelo Formation (Upper Cretaceous) of northern Patagonia, Argentina. Journal of Paleontology 77: 559–575. Crossref
de la Fuente, M.S. and Iturralde-Vinent, M. 2001. A new pleurodiran turtle from the Jagua formation (Oxfordian) of western Cuba. Journal of Paleontology 75: 860–869. Crossref
de la Fuente, M.S., Calvo, J., and González Riga, B.J. 2007. A new Cretaceous chelid turtle from Northern Neuquén Basin, Neuquén Province, Argentina. Ameghiniana 44: 485–492.
de la Fuente, M.S., de Lapparent de Broin, F., and Manera de Bianco, T. 2001. The oldest and first nearly complete skeleton of a chelid, of the Hydromedusa sub-group (Chelidae, Pleurodira), from the Upper Cretaceous of Patagonia. Bulletin de la Société Géologique de France 17: 237–244. Crossref
de la Fuente, M.S., Maniel, I., Jannello, J.M., Filippi, L.S., and Cerda, I. 2015a. Long-necked chelid turtles from the Campanian of northwestern Patagonia with comments on K/P survivorship of the genus Yaminuechelys. Comptes Rendus Palevol 14: 563–576. Crossref
de la Fuente, M.S., Maniel, I., Jannello, J.M., Sterli, J., González Riga, B.J., and Novas, F.E. 2015b. A new and large short-necked chelid turtle from the Loncoche Formation (Late Campanian–Early Maastrichtian) Mendoza Province, Argentina: macro-, microanatomy, and preliminary phylogenetic relationships. In: P.S.R. Romano and G. Ribeiro de Oliveira (eds.), 5th Turtle Evolution Symposium, Julio 2015, Rio de Janeiro. PeerJ Preprints 3: e1104.
de la Fuente, M.S., Maniel, I., Jannello, J.M., Sterli, J., González Riga, B.J., and Novas, F.E. 2017. A new large panchelid turtle (Pleurodira) from the Loncoche Formation (upper Campanian–lower Maastrichtian) of the Mendoza Province (Argentina): Morphological, osteohistological studies, and a preliminary phylogenetic analysis. Cretaceous Research 69: 147–168. Crossref
de la Fuente, M.S., Sterli, J., and Maniel, I. 2014. Origin, Evolution, and Biogeographic History of South American Turtles. 168 pp. Springer Earth System Sciences, Springer Cham, New York. Crossref
Ernst, C.H. and Barbour, R.W. 1989. Turtles of the World. 313 pp. Smithsonian Institution Press, Washington D.C.
Gaffney, E.S. 1977. The side-necked turtle family Chelidae: a theory of relationships using shared derived characters. American Museum Novitates 2681: 1–23.
Gaffney, E.S. and Meylan, P. 1988. A phylogeny of turtles. In: M.J. Benton (ed.), The Phylogeny and Classification of the Tetrapods 1: Amphibians, Reptiles, Birds. Systematics Association, Special Volume 35A: 157–169. Clarendon Press, Oxford.
Garrido, A.C. 2000. Estudio estratigráfico y reconstrucción paleoambiental de las secuencias fosilíferas continentales del Cretácico Superior en las inmediaciones de Plaza Huincul, provincia del Neuquén. 78 pp. Unpublished Ph.D. Thesis, Escuela de Geología de la Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba.
Garrido, A.C. 2010. Estratigrafía en el Grupo Neuquén, Cretácico Superior de la Cuenca Neuquina (República Argentina): Nueva propuesta de ordenamiento estratigráfico. Revista del Museo Argentino de Ciencias Naturales, Nueva Serie 12: 121–177.
Garrido, A.C. 2011. El Grupo Neuquén (Cretácico Tardío) en la Cuenca Neuquina. Relatorio del 18° Congreso Geológico Argentino 1: 231–244.
Girondot, M. and Laurin, M. 2003. Bone profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. Journal of Vertebrate Paleontology 23: 458−461. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K. 2008a. A free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K. 2008b. TNT: Tree Analysis Using New Technology, version 1.1 (Willi Hennig Society Edition). Program and documentation available at http://www.zmuc.dk/public/phylogeny/tnt.
Hugo, C.A. and Leanza, H.A. 2001. Hoja Geológica 3069-IV General Roca (escala 1:250.000). Provincias de Río Negro y Neuquén. Servicio Geológico Minero Argentino Boletín 308: 1–65.
Jannello, J.M., Cerda, I., and de la Fuente, M.S. 2016. Shell bone histology of the long-necked chelid Yaminuechelys (Testudines: Pleurodira) from the late Cretaceous–early Paleocene of Patagonia with comments on the histogénesis of bone ornamentation. The Science of Nature 103: 26. Crossref
Joyce, W.G. and Gauthier, J.A. 2004. Palaeoecology of Triassic stem turtles sets new light on turtle origins. Proceedings Royal Society of London B 271: 1–5. Crossref
Joyce, W.G., Parham J.F., and Gauthier, J.A. 2004. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. Journal of Paleontology 78: 989–1013. Crossref
de Lapparent de Broin, F. and de la Fuente, M.S. 2001. Oldest world Chelidae (Chelonii, Pleurodira), from the Cretaceous of Patagonia. Comptes Rendues Académies des Sciences de Paris 333: 463–470. Crossref
de Lapparent de Broin, F., de la Fuente, M.S., and Ferrnández, M.S. 2007. Notoemys (Chelonii, Pleurodira), Late Jurassic of Argentina: new examination of the anatomical structures and comparisons Revue de Paléobiologie 26: 99–136.
Laurin, M., Girondot, M., and Loth, M.M. 2004. The evolution of long bone microstructure and lifestyle in lissamphibians. Paleobiology 30: 589–613. Crossref
Leanza, H., Apesteguía, S., and Garrido, A.C. 2008. El Anfiteatro de Rentería: escenario de gigantes. In: A. Ardolino and H. Lema (eds.), Sitios de Interés Geológico de la República Argentina. Servicio Geológico Minero Argentino Anales 46: 593–601.
Legarreta, L. and Gulisano, C.A. 1989. Análisis estratigráfico secuencial de la Cuenca Neuquina (Triásico Superior–Terciario inferior). In: G. Chebli and L. Spalletti (eds.), Cuencas Sedimentarias Argentinas. 10° Congreso Geológico Argentino. Serie Correlación Geológica 6: 221–243.
Lovich, J.E. and Ernst, C.H. 1989. Variation in the plastral formulae of selected turtles with comments on taxonomic utility. Copeia 1989: 304–318. Crossref
Maniel, I. and de la Fuente, M.S. 2016. A Review of the Fossil Record of Turtles of the Clade Pan-Chelidae. Bulletin of the Peabody Museum of Natural History 57: 191–227. Crossref
Meylan, P.A. 1996. Skeletal morphology and relationships of the Early Cretaceous side necked turtle, Araripemys barretoi (Testudines: Pelomedusoides: Araripemydidae), from the Santana Formation of Brazil. Journal of Vertebrate Paleontology 16: 20–33. Crossref
Musacchio, E.A. 2006. Charophyta del Cretácico Tardío y el Paleoceno del centro oeste de Argentina. Revista Brasileira de Paleontología 9: 93–100. Crossref
Musacchio, E.A. and Vallati, P. 2007. Late Cretaceous non marine microfossil of the Plottier Formation (Cretaceous) at Zampal, Argentina. In: E. Diaz Martinez and I. Rabano (eds.), 4° European Meeting on the Paleontology and Stratigraphy of Latin America. Instituto Geológico y Minero de España, Madrid. Cuadernos del Museo Geominero 8: 273–278.
Pol, D. and Escapa, J.H. 2009. Unstable taxa in cladistics analysis: identification and the assessment of relevant characters. Cladistics 25: 1–13. Crossref
Pritchard, P.C.H. 1979. Encyclopedia of Turtles. 895 pp. TFH Publications Inc., Neptune Township.
Pritchard, P.C.H. 1988. A survey of neural bones among recent chelonian species, with functional interpretations. Acta Zoologica Cracoviensia 31: 625–686.
Pritchard, P.C.H. and Trebbau, P. 1984. The turtles of Venezuela. Society for the Study of Amphibians and Reptiles Contribution to Herpetology 2: 1–403.
Rhodin, A.G.J. and Mittermeier, R.A. 1983. Description of Phrynop williamsi a new species of chelid turtle of the South American P. geoffroanus complex. In: A.G. Rhodin and K. Miyata (eds.), Advances in Herpetology and Evolutionary Biology: Essays in Honor of Ernest H. Williams, 58–73. Harvard Museum of Natural History, Museum Comparative Zoology, Cambridge.
Rock-Color Chart Committe. 1991. Rock Color Chart. 16 pp. Geological Society of America, Special Publication. Boulder.
Salgado, L., Canudo, J.I., Garrido A.C., Ruiz-Omeñaca, J.I., García, R.A., de la Fuente, M.S., Barco, J.L., and Bollati, R. 2009. Upper Cretaceous vertebrates from “El Anfiteatro” (Río Negro, Patagonia, Argentina). Cretaceous Research 30: 767–784. Crossref
Sánchez-Villagra, M.R., Pritchard, P.C.H, Paolillo, A., and Linares, O.J. 1995. Geographic variation in the mata mata turtle, Chelus fimbriatus, with observations on its shell morphology and morphometry. Chelonian Conservation and Biology 1: 293–300.
Scheyer, T.M. 2007. Comparative Bone Histology of the Turtle Shell (Carapace and Plastron): Implications for Turtle Systematics, Functional Morphology, and Turtle Origins. 343 pp. Doctoral Thesis, Mathematisch-Naturwissenschaftliche Fakultät, Universität Bonn, Bonn.
Scheyer, T.M. and Sánchez-Villagra, M.R. 2007. Carapace bone histology in the giant pleurodiran turtle Stupendemys geographicus: phylogeny and function. Acta Palaeontologica Polonica 52: 137−154.
Scheyer, T.M. and Sander, P.M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society of London B 274: 1885–1893. Crossref
Scheyer, T.M., Mors, T., and Einarsson, E. 2012. First record of soft shelled turtles (Cryptodira, Tryonychidae) from the Late Cretaceous of Europe. Journal of Vertebrate Paleontology 32: 1027–1032. Crossref
Scheyer, T.M., Pérez-García, A., and Murelaga X .2014. Shell bone histology of solemydid turtles (stem Testudines): palaeoecological implications. Organisms Diversity & Evolution 15: 199–212. Crossref
Scheyer, T.M., Sander, P.M., Joyce, W.G., Bohme, W., and Witzel, U. 2007. Unique plywood structure in the shell of fossil and recent soft shelled turtles (Trionychidae) revealed by soft tissue and bone histology: A key adaptation? Organisms Diversity & Evolution 7: 136–144. Crossref
Thompson, S.A. and Georges, A. 2016. A new species of freshwater turtle of the genus Elseya (Testudinata: Pleurodira: Chelidae) from the Northern Territory Australia. Zootaxa 4061: 18–28. Crossref
Thompson, S.A., Amepou, Y., Anamiato, J., and Georges, A. 2015. A new species and subgenus of Elseya (Testudines: Pleurodira: Chelidae) from New Guinea. Zootaxa, 4006: 59–82. Crossref
Wood, R.C. and Diaz de Gamero, M.L. 1971. Podocnemis venezuelensis a new fossil pelomedusid (Testudines, Pleurodira) from the Pliocene of Venezuela and a review of the history of Podocnemis in South America. Breviora 376: 1–23.
Acta Palaeontol. Pol. 62 (3): 585–601, 2017
https://doi.org/10.4202/app.00340.2017