

Ecomorphological and taphonomic gradients in clypeasteroid-dominated echinoid assemblages along a mixed siliciclastic-carbonate shelf from the early Miocene of northern Sardinia, Italy
ANDREA MANCOSU and JAMES H. NEBELSICK
Mancosu, A. and Nebelsick, J.H. 2017. Ecomorphological and taphonomic gradients in clypeasteroid-dominated echinoid assemblages along a mixed siliciclastic-carbonate shelf from the early Miocene of northern Sardinia, Italy. Acta Palaeontologica Polonica 62 (3): 627–646.
Clypeasteroid echinoids are widespread and abundant within Miocene sedimentary sequences of the Mediterranean area within both siliciclastic and carbonate deposits. Herein, three clypeasteroid-dominated echinoid assemblages from the mixed siliciclastic-carbonate succession of the Mores Formation (lower Miocene) cropping out within the Porto Torres Basin (northern Sardinia) are described. These assemblages were compared to previously described clypeasteroid-bearing deposits from the Miocene of northern Sardinia with the purpose of investigating their palaeoecology and taphonomy along a shelf gradient. These goals are accomplished by various methods including (i) logging sedimentary facies, (ii) analysing the functional morphology of sea urchin skeletons, (iii) comparing the relative abundance of taxa and taphonomic features, and (iv) studying associated fauna, flora, and trace fossils. The clypeasteroid-bearing deposits differ greatly with respect to echinoid diversity, accompanying fauna and flora, sedimentological signatures, and taphonomic features. They also show variations in depositional environments and the mechanism of formation of the deposits. Three different shelf settings are distinguished: littoral, inner sublittoral, and outer sublittoral environments. Furthermore, an ecomorphological gradient along the shelf is recognized with respect to echinoid taxa and their morphologies. This gradient ranges from shallow water to a moderately deep shelf and is interpreted with respect to both abiotic and biotic factors as well as the taphonomy of the echinoid tests.
Key words: Echinoidea, taphonomy, functional morphology, palaeoecology, Miocene, Sardinia.
Andrea Mancosu [andrea.mancosu@gmail.com], Dipartimento di Scienze Chimiche e Geologiche, Università degli studi di Cagliari, Via Trentino 51, 09127 Cagliari, Italy.
James H. Nebelsick [nebelsick@uni-tuebingen.de], Institut für Geowissenschaften, Universität Tübingen, Sigwartstraße 10, D-72076 Tübingen, Germany.
Received 20 February 2017, accepted 15 May 2017, available online 26 July 2017.
Copyright © 2017 A. Mancosu and J.H. Nebelsick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Since their appearance in the Early Jurassic, irregular echinoids, with their morphological innovations and feeding habits that have allowed for the exploitation of new ecological niches (Kier 1982; Smith 1984; Saucède et al. 2007; Barras 2008), have represented an important component of marine benthic communities in shallow, as well as deeper water environments. Clypeasteroid echinoids are of more recent origin compared to other irregular echinoids evolving from cassiduloids in the late Paleocene (Kier 1982; Smith 1984, 2001; Kroh and Smith 2010). Clypeasteroids rapidly diversified, reaching a cosmopolitan distribution in the middle Eocene and are today a highly successful and morphologically diverse group including such well-known and highly derived forms as sand dollars, keyhole urchins, sea biscuits, pea urchins, and cake urchins (Seilacher 1979; Kier 1982; Ghiold 1984; Ghiold and Hoffmann 1986; Mooi 1989; Smith 2001; Nebelsick and Kroh 2002).
Clypeasteroid echinoids are common, if not very abundant, within Oligo-Miocene sedimentary sequences in the Mediterranean area, and have left an extensive fossil record of genera such as Clypeaster, Echinocyamus, Scutella, Parascutella, and Amphiope. These clypeasteroids show a widespread distribution within both carbonate and siliciclastic deposits from shallow to deeper shelf settings. This distribution allows for palaeoenvironmental reconstructions and provides opportunities for investigating echinoid palaeoecology and taphonomy along environmental gradients (see Néraudeau et al. 2001; Nebelsick and Kroh 2002; Kroh and Nebelsick 2003; Tsaparas et al. 2007; Belaústegui et al. 2012, 2013; Mancosu and Nebelsick 2013, 2015; Grun and Nebelsick 2016 and references therein).
Herein, three clypeasteroid-dominated echinoid assemblages from the early Miocene of northern Sardinia were studied with the aim of reconstructing palaeoecological and associated palaeoenvironmental conditions. In addition, an overview of the clypeasteroid echinoid assemblages from the Miocene of northern Sardinia is provided in order to discuss ecomorphological and taphonomic variations along an environmental gradient in a mixed siliciclastic-carbonate shelf.
Clypeasteroids from the Miocene of Sardinia.—Sardinia has a rich Miocene echinoid fauna with both historical monographs and recent publications describing a large number of clypeasteroid species (e.g., see Comaschi Caria 1972 and references cited therein; Stara and Borghi 2014) with for instance, Comaschi Caria (1972) listed thirty different species of Clypeaster. More recently, Stara and Borghi (2014) identified five Amphiope species in the Oligo-Miocene of Sardinia. A number of studies have addressed the sedimentological setting, taphonomy, and subsequent palaeoenvironmental interpretation of Miocene echinoids, including clypeasteroids from Sardinia (Stara et al. 2012; Mancosu and Nebelsick 2013, 2015, 2016, 2017).
Taxonomic classification to the species level is notoriously difficult for clypeasteroids, especially Clypeaster (see Imbesi Smedile 1958; Kroh 2005). This is due to high phenotypic plasticity, the fact that adaptive strategies are commonly repeated, and varying taxonomic concepts (e.g., Poddubiuk 1985; Rose and Poddubuik 1987; Kroh 2005; Rahman et al. 2015). These factors have led to the designation of subgenera (see Mortensen 1948b and discussion in Mihaljević et al. 2011) and numerous nominal species (see discussion in Kroh 2005; Mihaljević et al. 2011). Identification to species level was attempted whenever possible in the present study.
Taphonomic processes affecting echinoids.—Echinoids, in general, with their intricate multi-plated skeleton, are potentially good indicators of taphonomic processes and can be used as tools to reconstruct ambient ecological conditions (e.g., Lewis 1980; Donovan 1991; Gordon and Donovan 1992; Brett el al. 1997; Ausich 2001; Nebelsick 2004). A wide range of taphonomic processes influence their preservation. These processes can be separated into those affecting the test on the sediment surface, such as spine and plate disarticulation, fragmentation, abrasion, encrustation, bioerosion, and corrosion, and those affecting the skeleton after final burial including radial cracking by sediment loading, implosion of the test, grain indentation, and diagenesis (Nebelsick 1999, 2008).
Actualistic studies based on laboratory and field observations have helped to clarify the taphonomic processes influencing echinoid skeletons (Allison 1990; Kidwell and Baumiller 1990; Greenstein 1991, 1993a, b, 1995; Nebelsick 1992a, b, 1995, 1996, 1999, 2008; Nebelsick and Kampfer 1994; Schein and Lewis 2000; Banno 2008; Dynowski 2012). Preservation is related to both intrinsic factors, including the architecture of test and nature of connective tissues, and extrinsic factors, such as temperature, oxygen levels, bacterial activity and transport mechanisms. Furthermore, echinoids can potentially offer favourable substrates for skeletozoan colonization. Encrustation and bioerosion occur on a wide variety of both recent and fossil echinoid tests (e.g., Santos et al. 2003; Borszcz 2012 and references cited therein; Belaustegui et al. 2013; Mancosu and Nebelsick 2015; Rahman et al. 2015; Thompson et al. 2015) particularly in soft bottom environments where biogenic particles provide the most important substrate for the settlement of epibionts.
As discussed by Nebelsick et al. (1997), Santos and Mayoral (2008), Belaùstegui et al. (2013) and Rahman et al. (2015), clypeasteroids with their rather sturdy tests are suitable substrates for skeletozoan colonization in soft-bottom marine environments forming benthic islands (sensu Seilacher 1982), which can be frequently encrusted and bioeroded.
Institutional abbreviations.—MDLCA, Museo di Geologia e Paleontologia Domenico Lovisato, Università di Cagliari, Italy.
Geological setting
The Oligo-Miocene sedimentary succession of Sardinia is subdivided into three main sedimentary cycles and located in the SSE-NNW oriented Sardinian Basin. This is a trough extending from Cagliari in the south to the Gulf of Sassari in the northwest and comprises a number of minor sub-basins (Fig. 1B). The origin of the Sardinian Basin is due to the subduction of Neotethyan oceanic crust to the east of Sardinia and opening of the Western Mediterranean back-arc basin (Cherchi and Montandert 1982; Thomas and Gennesseaux 1986; Facenna et al. 2002; Speranza et al. 2002).
The northern part of the Sardinian Basin consists of a generally N to S striking halfgraben system, the western branch of which consists of the Porto Torres Basin in the North, which is separated from the southern Logudoro Basin by the E-W oriented Ittiri Fault (Thomas and Gennesseaux 1986; Funedda et al. 2000; Benisek et al. 2009; Murru et al. 2015; Reuter et al. 2016). Magmatic activity accompanied the halfgraben formation resulting in andesitic and basaltic flows and pyroclastic and ignimbritic events, Aquitanian to middle Burdigalian in age (Lecca et al. 1997; Funedda et al. 2000). Continuous subsidence led to marine conditions from the Burdigalian onward forming a mixed siliciclastic-carbonate succession (Funedda et al. 2000; Casula et al. 2001).
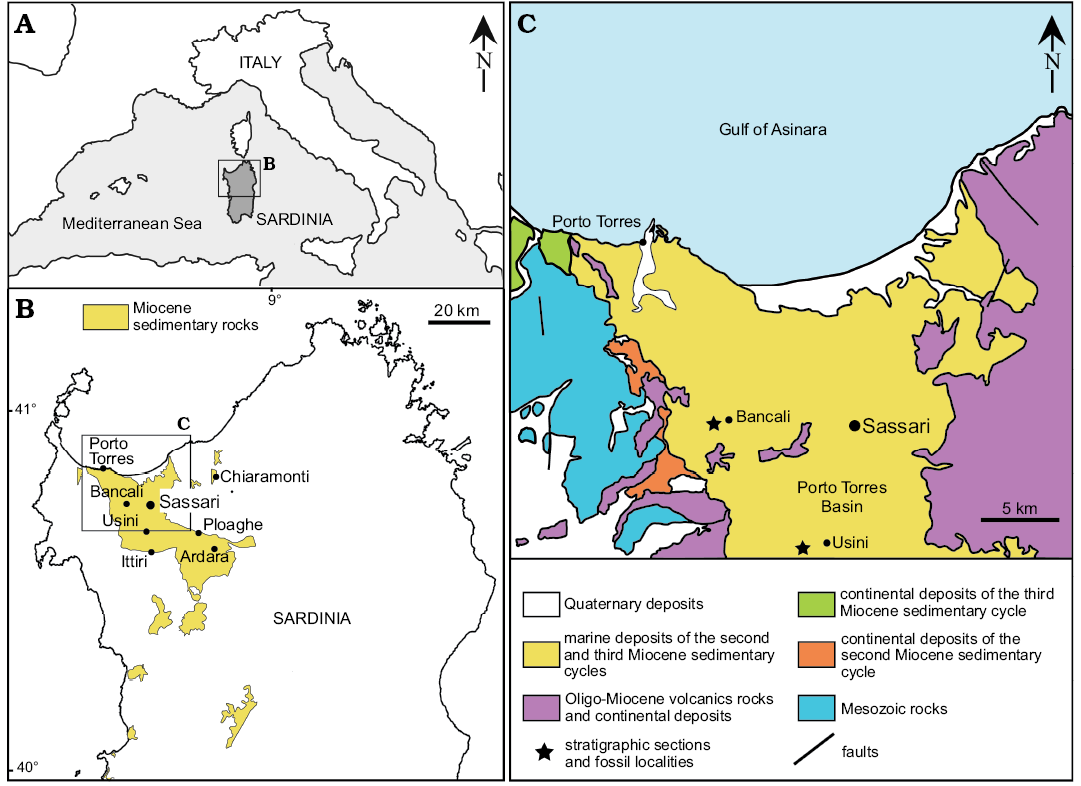
Fig. 1. A. Map of the Mediterranean showing location of studied area B. Distribution of Miocene sedimentary rocks in northern Sardinia and location of echinoid assemblages cited within the text. C. Simplified geological map of the northern part of the Porto Torres Basin with the location of the clypeasteroid-echinoid assemblages of Bancali and Usini.
The tectono-sedimentary development and the stratigraphic framework of the Porto Torres Basin is partially comparable to that of the well-known, adjacent Logudoro Basin (Martini et al. 1992; Funedda et al. 2000; Vigorito et al. 2006; Murru et al. 2015). In both basins, the second and third Miocene sedimentary cycles were recognized by Mazzei and Oggiano (1990) and Funedda et al. (2000, 2003). The sedimentary sequence is subdivided into five main lithostratigraphic units (Mazzei and Oggiano 1990; Funedda et al. 2000; Fig. 2) and starts with fluvio-deltaic conglomerate and litharenitic sand of the Oppia Nuova Formation (?middle–upper Burdigalian) (Funedda et al. 2000). The overlying Mores Formation (late Burdigalian), to which the studied sedimentary successions belong, consists of calcarenite, bioclastic limestone and subordinate shallow water, fossiliferous coarse-grained sandstone and conglomerate (Mazzei and Oggiano 1990; Funedda et al. 2000). The Borutta Formation follows which is assigned to the Late Burdigalian–Langhian following calcareous nannoplankton (biozones NN4/NN5 of Martini 1971) by Mazzei and Oggiano (1990) and Francolini (1994) and planktonic foraminifera (biozone N7 of Blow 1969) by Bossio et al. (2006). It contains calcareous siltstone, fine-grained sandstone, and marl representing deeper shelf environments. The Borutta Formation is overlain by the Florinas Formation (Langhian–Serravallian) following an unconformity. This formation mainly contains quartz-rich, coarse sandstone representing fluvial, lacustrine and brackish coastal environments (Mazzei and Oggiano 1990; Funedda et al. 2000). The Monte Santo Formation (Serravallian to ?Tortonian–lower Messinian), which pertains to the third sedimentary cycle, overlies with a transgressive contact the Florinas Formation and consists mainly of bioclastic limestone, in part enriched in rhodolith deposits accumulated within channels, with slumps and olistoliths possibly indicating a platform slope setting (Funedda et al. 2000).
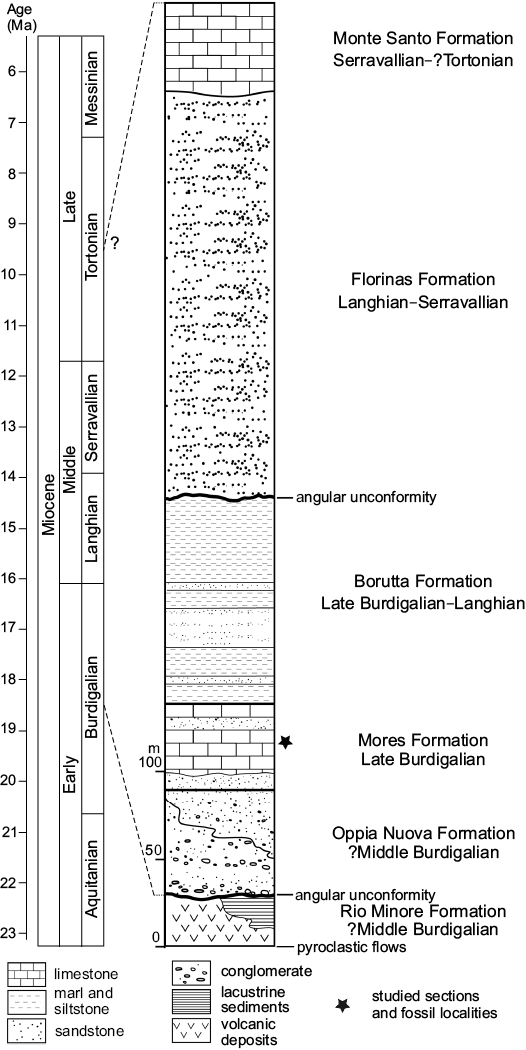
Fig. 2. Stratigraphy of the Miocene volcano-sedimentary succession of the Porto Torres Basin (based on Mazzei and Oggiano 1990; Martini et al. 1992; Francolini 1994; Funedda et al. 2000, 2003; Bossio et al. 2006).
Material and methods
Field and laboratory investigations were conducted with respect to palaeontology, taphonomy, and sedimentology. Echinoid tests, both complete and fragmented, were collected throughout the successions in 2016. Classification at and above genus level follows Kroh and Smith (2010) and Smith and Kroh (2011). Echinoid fragments were identified after comparison to complete specimens (e.g., Nebelsick 1992a, b; Donovan 2003). Two stratigraphic sections were measured. The frequency of fragments and complete tests was estimated per rock surface. The degree of close-packing determined as dense, loose, or dispersed follows Kidwell and Holland (1991). The orientation of complete specimens was recorded relative to bedding planes. Various taphonomic signatures (disarticulation, fragmentation, abrasion, encrustation, and bioerosion) were evaluated in the field and laboratory. The preservation of tubercles on the test surfaces was used to evaluate abrasion. Palaeoecological interpretations of echinoid faunas followed actualistic comparisons to closely related extant taxa.
Results
Bancali Section.—The first and second clypeasteroid-dominated echinoid assemblages (Bancali Assembalge 1 and Bancali Assemblage 2) occur within a sedimentary succession which crops out near to the village of Bancali (40°43ʹ56ʺ N, 8°26ʹ55ʺ E) (Fig. 1C), and belongs to the Mores Formation (Figs. 2, 3A).
Bancali Assemblage 1 occurs within an approximately 1.4 m-thick conglomerate deposit consisting of abundant, well-rounded, up to 5 cm large quartz pebbles, floating in a medium- to coarse-grained sandstone. Within this matrix-supported conglomerate, remains of the clypeasteroid echinoids Amphiope (Fig. 4A–E) and Clypeaster (both highly- and moderately-domed) as well as bivalves (Aequipecten submalvinae and Spondylus) occur in large numbers. Reworked barnacles can also be observed and calcareous algae are also present.
The clypeasteroid echinoids all lack spines and, although complete tests are present (Fig. 4A), they consist mainly of test fragments (Fig. 4B–E). The echinoid remains range from loosely packed and dispersed to very densely packed (sensu Kidwell and Holland 1991). Imbricated specimens are not present. The orientation of echinoids ranges from concordant to perpendicular (Figs. 4A, B, 5A). Test fragments are also present and include small fragments, pie-shaped segments and larger fragments up to half test size (Fig. 6: states 6–10). Both inter and intra-plate fragmentation is present.
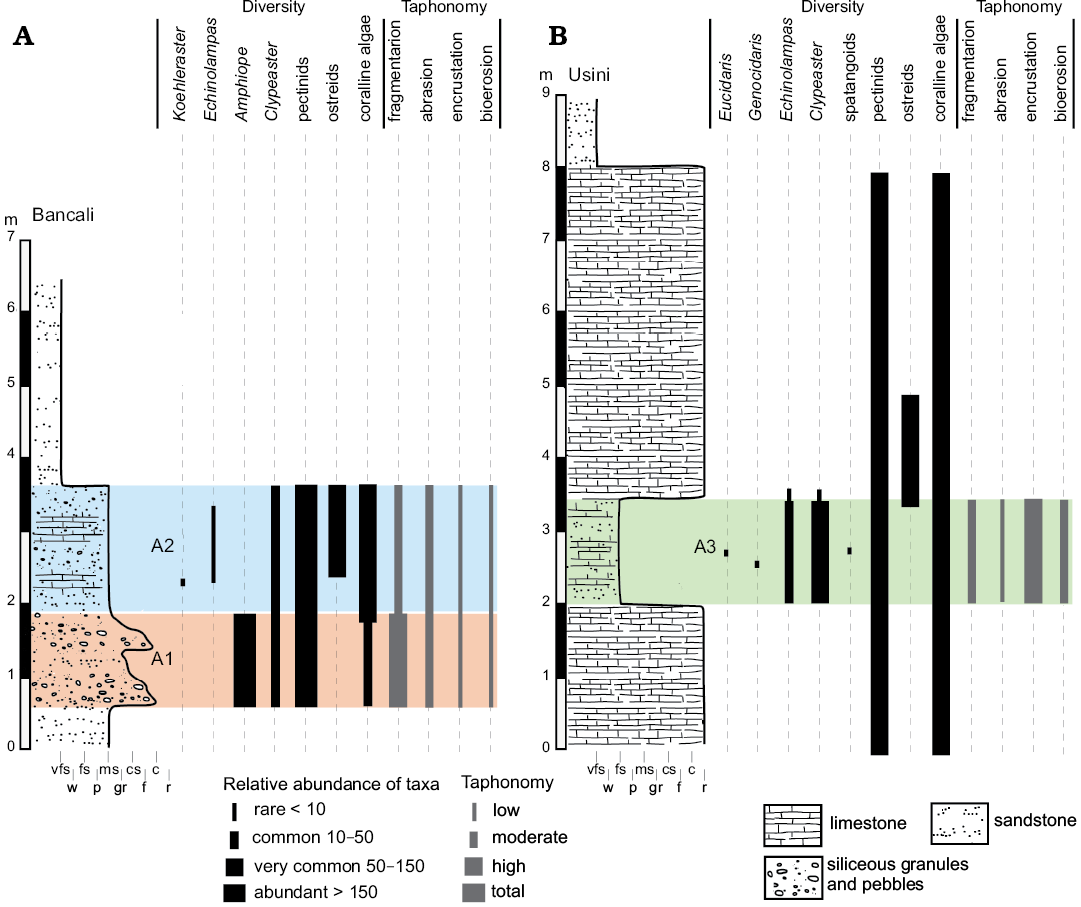
Fig. 3. Stratigraphic sections of Bancali (A) and Usini (B) with distribution and relative abundance of recognized echinoids and associated macrofauna and flora. Abbreviations: A1, Bancali Assemblage 1; A2, Bancali Assemblage 2; A3, Usini Assemblage; c, conglomerate; cs, coarse sandstone; f, floatstone; fs, fine sandstone; gr, grainstone; ms, medium sandstone; p, packstone; r, rudstone; vfs, very fine sandstone; w, wackestone.
Different degrees of abrasion were observed, ranging from specimens showing well preserved surface details to highly abraded fragments with rounded margins (Fig. 4E). Fragments show evidence of encrustation by barnacles (Fig. 4C), serpulids, and coralline algae; skeletozoan colonizations can affect both the oral and aboral surface of the test. Bioerosion is present as circular to sub-circular Oichnus-like holes (sensu Wisshak et al. 2015; Fig. 4D). Collapsed central areas of the test (Fig. 4A) and radial cracking can also be present.
The deposit containing the second clypeasteroid-dominated echinoid assemblage (Bancali Assemblage 2) is found within a ca. 1.8 m-thick poorly sorted medium- to coarse-grained mixed siliciclastic-carbonate sandstone with abundant, scattered quartz granules and pebbles and disarticulated and densely packed bivalves. Bancali Assemblage 2 consists of Clypeaster (Fig. 4F), with different morphotypes, and Echinolampas. A single complete specimens of the echinoneid Koehleraster was also found (Fig. 4G). Other major biotic constituents are the pectinid Aequipecten submalvinae, the spondylid Spondylus and large oysters. Reworked barnacles also occur in large numbers.
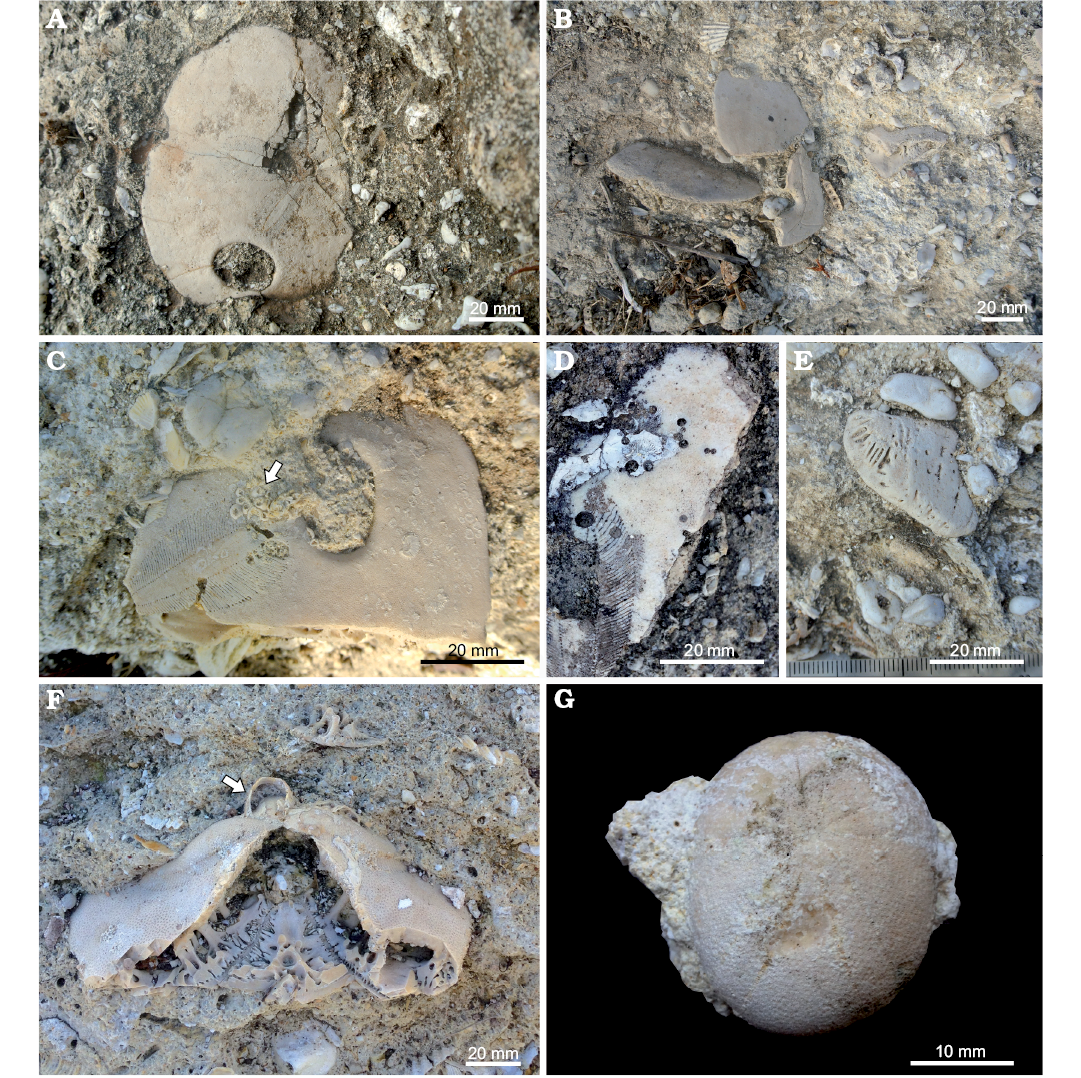
Fig. 4. Bancali Assemblage 1 (A–E) and Bancali Assemblage 2 (F, G); early Miocene, Bancali, Sardinia, Italy. A. Amphiope sp. showing collapse of the central area of the test. B. Chaotically oriented test fragments of Amphiope sp. C. Encrustation by barnacles (arrow) on Amphiope sp. remains. D. Circular holes on Amphiope sp. fragment. E. Highly abraded fragment of Amphiope sp. F. Clypeaster (C. intermedius morphotype) encrusted by barnacles (arrow). G. Koehleraster sp. (MDLCA 23583).
Complete tests and fragments all lack spines. Echinoid remains are dispersed showing parallel to perpendicular orientations both in cross section and plan view (Fig. 5B). Complete tests are usually well preserved (Fig. 6: states 1–3). Fragments, from small test remains to half skeletons, show a wide spectrum of preservation ranging from well-preserved surfaces to highly abraded fragments with rounded margins (Fig. 6: states 4–6). Both inter- and intraplate fragmentation occurs. Complete tests and fragments are encrusted by barnacles (Fig. 4E) and coralline algae. Bioerosion is present as circular Oichnus-like holes. The rest of the section is composed of a ca. 3 m-thick fine-grained sandstone with pectinid bivalve remains.
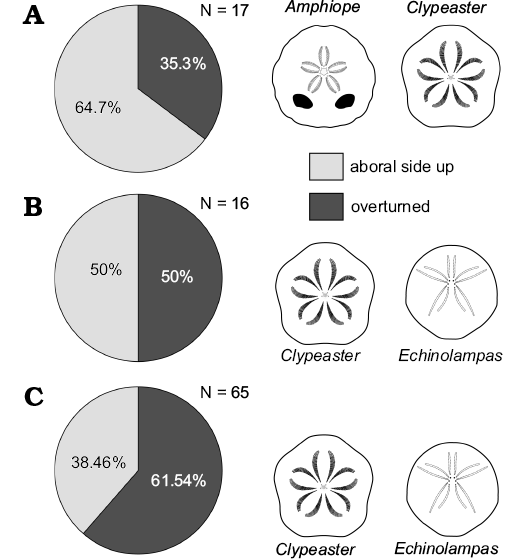
Fig. 5. Orientation data of complete tests. Early Miocene echinoids within Bancali Assemblage 1 (A), Bancali Assemblage 2 (B), and Usini Assemblage (C). N, number of counted specimens.
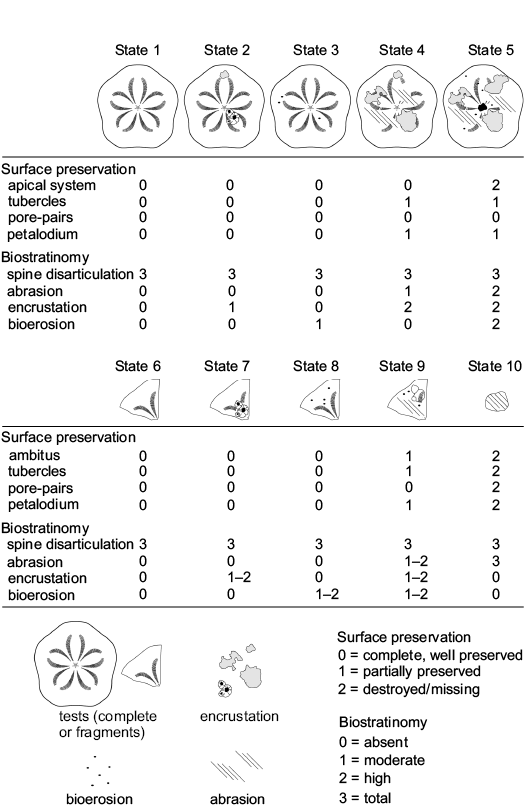
Fig. 6. Taphonomic gradient recognized on complete tests and fragments of clypeasteroid echinoids by using the qualitative analysis of the surface characters.
Usini Section.—The third clypeasteroid-dominated echinoid assemblage (Usini Assemblage) occurs within a sedimentary succession which crops out along the road from Sassari to Ittiri near the village of Usini (40°39ʹ30ʺ N, 8°31ʹ00ʺ E) (Fig. 1C) and belongs to the Mores Formation (Fig. 3B).
The echinoid assemblage occurs within an approximately 1.5 m-thick fine-grained sandstone (mixed siliciclastic-carbonate) with abundant bivalves, coralline algal fragments and rhodoliths lying on a 2 m-thick rudstone also composed of coralline-algal branches, rhodoliths and bivalves. Abundant remains of the pectinid Aequipecten submalvinae and the spondylid Spondylus were observed within both deposits.
Echinoids are abundant and dominated by different morphotypes of Clypeaster (Fig. 7A–D). In addition, a single specimen of a poorly-preserved flat and relatively thin-shelled Clypeaster was found. The echinolampadoid Echinolampas (Fig. 7F) occurs subordinately. Fragments of spatangoids are rare (Fig. 7E). Regular echinoids consist of small spines of the cidaroid Eucidaris and a single specimen of the small trigonocidarid echinoid Genocidaris. The echinoids consist mainly of complete tests lacking spines although test fragments also occur. The echinoid remains are dispersed within the matrix (sensu Kidwell and Holland 1991) and with orientations ranging from perpendicular to parallel to the bedding plane (Figs. 5C, 7).
A wide range of preservation states from complete specimens with exquisitely preserved surface details to highly abraded tests is present for both complete tests and fragments (Fig. 6: states 1–10). Fragment size varies from smaller test remains to half skeletons. Both inter- and intraplate fragmentation occurs. Encrustation by coralline algae and bryozoans (Fig. 7A, D) is found on both complete tests and fragments. High degree of encrustation on complete tests can be present with skeletozoan colonization affecting both the oral and aboral surface of the same specimens. Bioerosion occurs as 8-shaped and circular borings (Fig. 7D).
The echinoid-bearing sandstone deposit is overlapped by an approximately 4.5 m-thick bioclastic limestone (floatstone to rudstone) with coralline-algae and accumulations of pectinid and oyster shells.
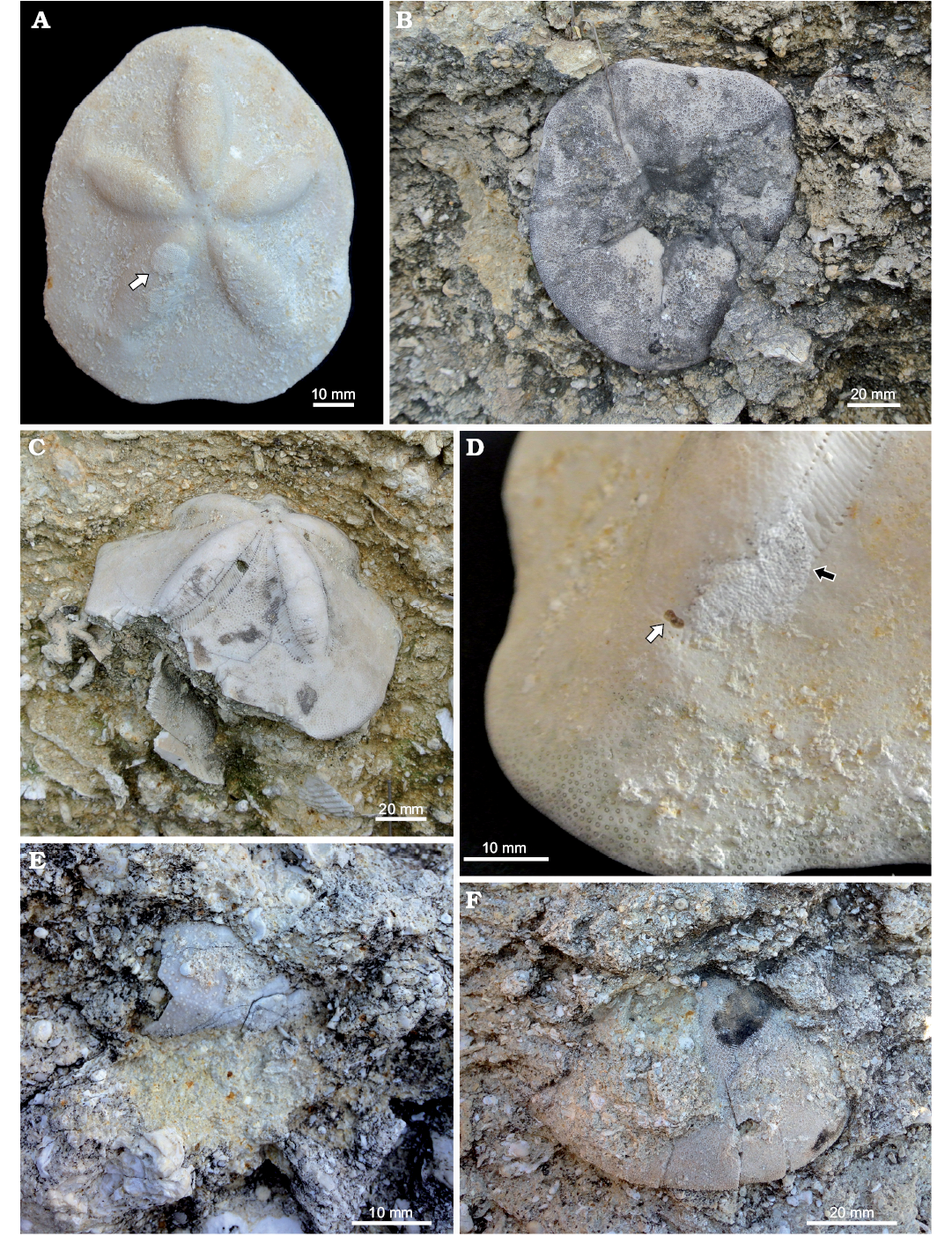
Fig. 7. Usini Assemblage; early Miocene, Usini, Sardinia, Italy. A. Complete test of Clypeaster (morphotype Clypeaster scillae) encrusted by (arrow) bryozoans (MDLCA 23584). B. Clypeaster (morphotype C. scillae) perpendicularly oriented. C. Clypeaster (morphotype C. calabrus). D. Encrustation by bryozoans (black arrow) and figure of 8-shaped boring (white arrow) on Clypeaster sp. (MDLCA 23585). E. Spatangoid remains. F. Echinolampas sp.
Discussion
Functional morphology of echinoid test and actualistic comparison.—Clypeasteroids: Amphiope from Bancali Assemblage 1, which has been described by Stara and Borghi (2014) as Amphiope sp. 2, is a large lunulate sand dollar interpreted as a shallow burrowing, deposit feeder, based on its flat test and the presence of branched well-developed food grooves, which reach from the ambital areas of the test to the peristome and serve to transport food particles (e.g., Ellers and Telford 1984).
The robust test construction of Amphiope, with thick test walls and internal supports, and the presence of broad lunules, is interpreted as an adaptation to high energy, wave-swept, sandy environments (Seilacher 1979; Telford and Mooi 1987). There has been an intense debate as to the function of lunules. As discussed by Smith and Ghiold (1982) and Telford (1981, 1983) ambulacral lunules are mainly involved in food gathering and hydrodynamic stability since they reduce lift.
Living sand dollars inhabit tropical to temperate environments in both exposed and protected soft-bottom coastal areas (e.g., Salsman and Tolbert 1965; Weihe and Gray 1968; Bell and Frey 1969; Ebert and Dexter 1975; Lane and Lawrence 1980; Steimle 1990; Bentley and Cockcroft 1995; Pomory et al. 1995; Guilherme et al. 2015) although the sand dollar Echinarachnius parma has an extended bathymetric range to depths of about 1600 m (Mortensen 1948b; Ellers and Telford 1984). These clypeasteroids are mainly shallow burrowing deposit feeders living just below the sediment surface (e.g., Bell and Frey 1969; Ebert and Dexter 1975; Telford and Mooi 1987; Bentley and Cockcroft 1995), though semi-infaunal suspension feeders such as Dendraster and more rarely Encope also occur (e.g., Timko 1976; Mooi 1997; Lawrence et al. 2004; Fodrie et al. 2007).
Sand dollars are common bioturbators (Stanley and James 1971; Reidenauer 1989; Li et al. 2013; Brustolin et al. 2014, 2016) and are known to ingest sediment particles and a variety of small micro-organisms and organic particles (Telford and Mooi 1986; Challener et al. 2009; Hilber and Lawrence 2009). These clypeasteroids can reach very high population densities as a result of gregarious behavior and commonly show patchy distributions related to sediment grain-size, hydrodynamic regime, availability of food resources (Bell and Frey 1969; Pomory et al. 1995; Swigart and Lawrence 2008; Guilherme et al. 2015; Brustolin et al. 2016). Mass accumulations of hundreds or even thousands of individuals per m2 are documented for the sand dollars Mellita quinquiesperforata, Encope grandis, Echinarachnius parma, Dendraster excentricus, and Scaphechinus mirabilis (Salsman and Tolbert 1965; Chia 1969; Merril and Hobson 1970; Stanley and James 1971; Ebert and Dexter 1975; Seilacher 1979; Steimle 1990; Nebelsick and Kroh 2002; Takeda 2008). In addition to wave exposure, which is seen as a major factors shaping benthic community structure and diversity in shallow water environments (Smith 1981; Bentley and Cockcroft 1995), sand dollars can be an important factor in structuring benthic communities by dominating habitat and detrital food resources (Steimle 1990), by reducing the primary productivity of the microphytobenthos (Li et al. 2013) and by disturbing and excluding some invertebrates with their burrowing activity (Smith 1981; Creed and Coull 1984; Morin et al. 1985; Reidenauer 1989).
Clypeaster shows a high morphological variability within the three studied assemblages. Four morphotypes (named C. scillae morphotype, C. calabrus morphotype, C. intermedius morphotype, and C. marginatus morphotype), were recognized based upon major test features as summarized in Table 1.
Table 1. Major test features of the four Clypeaster morphotypes recognized within the echinoid assemblages from early Miocene sections at Bancali and Usini, Sardinia, Italy.
|
Morphotype |
Test size |
Ambital outline |
Marginal tumidity |
Test profile |
Assemblage |
|
Clypeaster scillae |
medium to large |
subpentagonal /elongated |
high |
higly inflated concave oral
surface large |
Bancali 2, Usini |
|
Clypeaster calabrus |
large |
pentagonal |
relatively high |
highly inflated/elevated petals flat oral surface relatively small infundibulum |
Bancali 1, |
|
Clypeaster intermedius |
large |
pentagonal |
relatively low |
moderately inflated slightly concave to flat oral surface relatively small infundibulum |
Bancali 1, |
|
Clypeaster marginatus |
small to medium |
pentagonal to subcircular |
low thin and sharp margin |
very depressed flat to slightly concave oral surface large to relatively small infundibulum |
Usini |
The genus Clypeaster has a number of specialized morphological features common to clypeasteroids and a non-protrusible Aristotle’s lantern (Mooi 1989) which is used for crushing rather than scraping (Smith 1984; Telford et al. 1987; Ellers and Telford 1991). Rose and Poddubiuk (1987) explored the morphological variations in fossil Clypeaster and considered several features of the test, such as the ambital outline, test profile, petal shape and tuberculation in order to infer the mode of life of fossil species. Six different morphotypes were recognized ranging from the Eocene to the Miocene present either in the Caribbean or Mediterranean areas (Rose and Poddubiuk 1987). Corresponding morphotypes can be in part recognized in the studied assemblages.
Thick-shelled and highly-domed Clypeaster, represented by C. scillae and C. calabrus morphotypes, are interpreted as epibenthic to ploughing forms, comparable to the Recent Clypeaster rosaceus, which feeds by ingesting biogenic particulate material, such as fragments of corals and coralline algae, and dead leaves of Thalassia (Telford et al. 1987; Hendler et al. 1995; Kampfer and Ott 1995). C. rosaceus lives epibenthically and is rarely found buried up to its ambitus only (Telford et al. 1987). Its thick skeleton is interpreted as an adaptation to high energy environments (Kampfer and Ott 1995; Néraudeau et al. 2001). Clypeaster with flat profiles, thin margins and flat ventral surfaces, represented by the C. marginatus morphotype, are interpreted as shallowly buried deposit feeders such as the extant C. humilis and C. subdepressus. C. humilis is a shallowly buried echinoid living in sand patches within seagrass meadows (Nebelsick 1992b) and sand filled lagoons of the Indo-West-Pacific (James and Pearse 1969). C. subdepressus occurs commonly at depths 1–50 m (Serafy 1979; Hopkins 1988; Hendler et al. 1995; Rodríguez-Barreras 2014) living in sandy shelly substrates, but also in muddy sediments and seagrass meadows in the warm water of the Caribbean Sea and Atlantic Ocean (Hendler et al. 1995). It lives epibenthically to shallowly buried (Chesher 1969; Seilacher 1979; Telford et al. 1987; Velluttini and Bigotto 2010) feeding on organic material such as diatoms. Accessory podia of the oral surface collect sand particles, which are transported to the mouth and crushed by the Aristotle’s lantern (Telford et al. 1987). Moderately high test profiles with relatively flat ventral surface with a small infundibulum and relatively thick and tumid margins are represented by the C. intermedius morphotype. These are interpreted as partially burrowed deposit feeders.
Living Clypeaster species inhabit tropical and subtropical regions (e.g., Ghiold and Hoffman 1984, 1986; Nebelsick 1992b; Hopkins 1988) living in mobile substrates down to ca. 500 m, although most species are confined to shallow waters (e.g., Mortensen 1948b; Endean and Pope 1964; Serafy 1970; Hopkins 1988; Nebelsick 1992a). Modern representatives of the genus Clypeaster do not seem to form such dense aggregations described for sand dollars (see references cited above). Field studies on the Caribbean echinoids C. rosaceous and C. humilis from the Red Sea, have reported maximum population densities of 27 individuals per 100 m2 and 2 individuals per m2, respectively (Kampfer and Ott 1995; Nebelsick 2008). This may explain the lower density observed in Bancali Assemblage 2 and Usini Assemblage compared to that of the sand dollar-dominated Bancali Assemblage 1.
Echinolampadoids: Echinolampas barcinensis, which occurs both in assemblages Bancali Assemblage 2 and the Usini Assemblage, is interpreted as a partially buried deposit feeder. It has a large test, low arched in profile, with long petals and conjugate anisopores associated with respiratory tube feet (see Smith 1980b), and a slightly concave oral surface with phyllodes and weakly inflated bourrelets as a means to facilitate deposit feeding.
Echinolampadoid echinoids have not been observed to a great extent in their natural habitats (e.g., Mortensen 1948a; Higgins 1974; Thum and Allen 1975; Gladfelter 1978; Mooi 1990a). They have been described as bulk sediment swallowers feeding on organic material coatings of coarse sediment grains (De Ridder and Lawrence 1982; Mooi 1990a).
Modern representatives of Echinolampas live epifaunally to shallowly burrowed in subtropical to tropical environments (e.g., Mortensen 1948a; Thum and Allen 1975; Mooi 1990b). Most Echinolampas species occur between 8 and 400 m, with the exception of E. rangii found between 1570 and 1670 m (Mooi 1990a). The Caribbean E. depressa is typically found living in relatively coarse coralline algal sands from 30 to over 310 m (Mooi 1990a). E. ovata occurs in the littoral zone of the Red Sea and the Indian Ocean from 9 to 75 m depth (Mortensen 1948a; Mooi 1990b) living partially to shallowly burrowed in carbonate sands. Echinolampas crassa, reported at depths from 12 to 500 m off South Africa, is a shallow burrowing, deposit-feeder which lives in biogenic substrates (Thum and Allen 1975; Mooi 1990b). McNamara and Philip (1980) suggested that some fossil species of Echinolampas may have lived buried into the sediment similar to the extant Rhyncholampas pacificus which lives buried only below the petals.
Echinoneoids: Koehleraster from Bancali Assemblage 2 is a small to medium-sized ovoid echinoneoid with a concave oral surface and is interpreted to be a shallow burrower in sandy sediments. This interpretation is due to its strong morphological affinity to the Recent echinoneoids Koehleraster abnormalis and the very similar Echinoneus cyclostomus (see Smith and Kroh 2011). As noted by Rose (1976) and Smith (1980b) suckered tube feet in Echinoneus are primarily used in feeding and burial activity. Oral tube feet are also used to adhere to rocks or large shell fragments, possibly for stability. Koehleraster abnormalis is rare and its ecology is poorly known. This echinoid (previously attributed to the genus Echinoneus) occurs in the Indo-Pacific and exhibits a highly disjunct geographical distribution (Ghiold 1989). It is reported to inhabit sands under coral heads in lagoon grass flats and under boulders in shallow sublittoral environments (Sloan et al. 1979; Lane et al. 2000). Its occurs from the intertidal to 85 m (Mortensen 1948a; Lane et al. 2000).
Koehleraster can be compared to the much better known Echinoneus cyclostomus which is nearly ubiquitous throughout the tropics from the Atlantic Ocean to the Indo-Pacific (Mortensen 1948a; Rose 1976; Ghiold 1989; Hendler et al. 1995). It can be found underneath rocks or corals or be shallowly burrowed in sandy sediments (Westergren 1911; Rose 1976; Liao 1978; Sloan et al. 1979; Smith 1980a; Chao 2000; Rodríguez-Barreras et al. 2012). Echinoneus cyclostomus is reported from shallow waters to 570 m of depth (Liao 1978; Hendler et al.1995; Chao 2000; Lane et al. 2000; Rodríguez-Barreras et al. 2012). Rose (1976) reported E. cyclostomus from a low-energy microenvironment within an otherwise high energy sublittoral tropical reef. Fontaine (1953) and Rose (1976) interpreted E. cyclostomus as indicative of reef proximity. The co-occurrence of Echinoneus with Brissus has been recorded in reef associated bioclastic sediments in extant (Kier and Grant 1965) and fossil environments (Challis 1980; Donovan and Veale 1996; Kroh 2004).
Cidaroids: Eucidaris from Usini Assemblage is a small cidaroid echinoid with short spines which is interpreted here as living in sandy substrates with coralline algae. Extant species of Eucidaris, such as E. galapagensis, E. metularia, E. thouarsii, and E. tribuloides, are particularly abundant from the intertidal zone to depths of 20–30 m (Mortensen 1928; Kier and Grant 1965; Nebelsick 1992a, b; Samyn 2003; Sciberras and Schembri 2007), although their distribution can reach 450 m of depth (Mortensen 1928; Phelan 1970). E. tribuloides is found in a variety of substrates including sands, rocky bottoms, and within Thalassia meadows (Kier and Grant 1965; Smith 1978) but also in muddy substrates and detrital bottoms (Tanti and Schembri 2006; Sciberras and Schembri 2007). This cidaroid wedges itself in crevices or takes shelter under coral or rocks (Hendler et al. 1995; Rodríguez-Barreras 2014). E. metularia occupies a variety of substrates, most commonly coral reefs and carpets as well as sand with coral patches (Smith 1978; Nebelsick 1992a, b), but is also found in lagoons with sea-grass beds (Samyn 2003).
Eucidaris is an opportunistic omnivore which consumes mostly corals, non- and coralline algae, sponges, sea grasses, and comatulid crinoids (McPherson 1968; Glynn et al. 1979; Santos et al. 2002; Sonnenholzner et al. 2009; Baumiller et al. 2010; Cabanillas-Terán et al. 2016; Stevenson et al. 2017). Although this genus has a pantropical distribution (Lessios et al. 1999), E. tribuloides has only recently been observed in the shallow water of the Maltese Islands (Tanti and Schembri 2006).
Camarodonts: The palaeoenvironmental interpretation of the small camarodont echinoid Genocidaris is described in detail in Mancosu and Nebelsick (2016). Recent occurrences are found down to 500 meters (Tortonese 1965; Grubelic 1998; Como et al. 2008; Sciberras et al. 2009; Smith and Gale 2009; Hernández et al. 2013). The Recent Mediterranean G. maculata is found on a variety of soft and hard substrates in moderate depths (Pérès and Picard 1964; Tortonese 1965; Koukouras et al. 2007) including seagrass (Como et al. 2008) and coralline algal dominated sediments (Sciberras et al. 2009) consuming bryozoans and other bioclastic fragments (Mortensen 1943; De Ridder and Lawrence 1982).
Palaeoenvironmental interpretation.—The three clypeasteroid-rich echinoid assemblages studied herein show clear differences with respect to taxonomic composition as well as sedimentological and taphonomic signatures (Table 2).
Bancali Assemblage 1 indicates a littoral environment dominated by soft substrates (Fig. 8A). The clypeasteroid assemblage, dominated by the sand dollar Amphiope, occurs in coarse-grained sediments suggesting high energy conditions. The chaotically arranged orientation of the echinoid remains and the wide spectrum of preservation suggest multiple episodes of shell reworking.
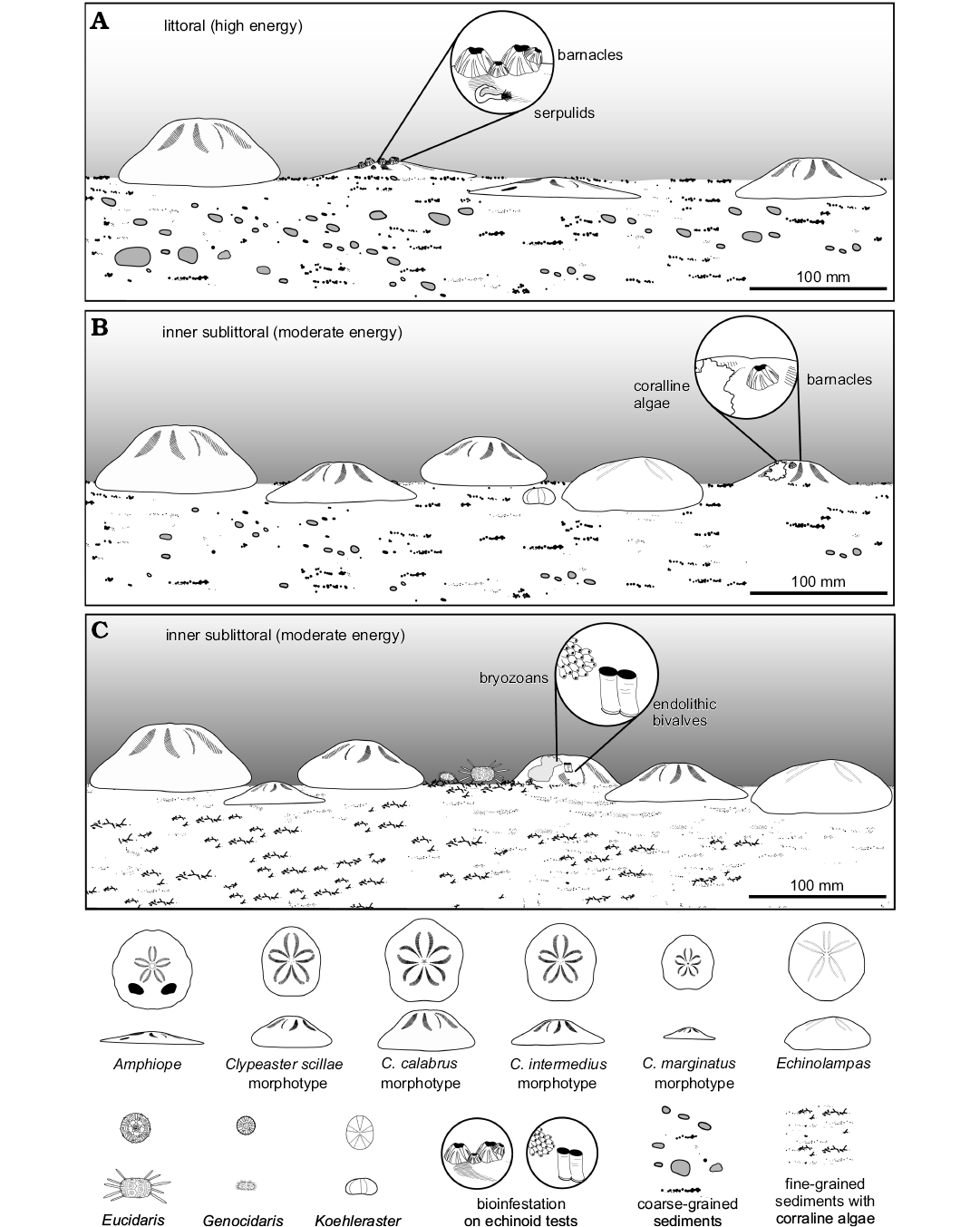
Fig. 8. Palaeoecological reconstruction of the echinoid assemblages recognized within early Miocene sections at Bancali and Usini, Sardinia, Italy. A. Bancali Assemblage 1. B. Bancali Assemblage 2. C. Usini Assemblage.
Circular to sub-circular borings (Oichnus-like holes) could potentially be the result of either predation or post-mortem bioerosion. Similar boreholes are described on the clypeasteroids Mellita, Leodia, and Clypeaster (Moore 1956; Lindsay 1996; Tewfik and Scheuer 2013; Tewfik 2014) and the much smaller Echinocyamus and Fibularia (Nebelsick and Kowalewski 1999; Ceranka and Złotnik 2003; Grun et al. 2014, 2017). These have been interpreted as the result of the lethal predation of cassid gastropods, which prey upon echinoids by drilling into the test and leaving circular to subcircular bore holes. In the Bancali Assemblage 1, however, circular holes occur in large number on the same specimens (Fig. 4D), which is highly unusual for cassid predation on echinoids, suggesting post-mortem colonization by macro-endolithic organisms. This is consistent with the fact that the bored echinoid remains are also affected by encrustation by barnacles, serpulids, and coralline algae. Post mortal encrustation and bioerosion suggest extended residence times before final burial (see Kidwell and Baumiller 1990; Nebelsick et al. 1997). Repeated episodes of shell reworking can also be inferred since encrustation and bioerosion are present on all sides of the same specimens. Radial cracking and the central test collapse are post-burial features (Nebelsick 1999) resulting from sediment loading (see Müller 1951 for the same effect on spatangoids).
Sedimentological, palaeontological and taphonomic features as recognized in Bancali Assemblage 2 and Usini Assemblage, respectively, suggest an inner sublittoral environment (Fig. 8B, C). The chaotically arranged orientation of the echinoid remains and the wide spectrum of preservation suggest multiple episodes of in situ reworking. The depositional environment is inferred as a moderate energy setting in which echinoid tests would have been moved about and overturned, buried and exposed again by episodic storms.
The exquisitely preserved test surface of some complete specimens, even in those affected by encrustation and bioerosion, suggests that dead echinoids were present on the sediment surface for a longer period of time without being affected by severe and/or long transport. This is also consistent with the occurrence in the Usini Assemblage of some large and thick-shelled Clypeaster showing highly abraded aboral surfaces, affected by encrustation and bioerosion, and exquisitely preserved oral surfaces. These features suggest that echinoid tests remained on the substrate in a position of maximum hydrodynamic stability under lower transport velocities for an appreciable period of time. Repeated episodes of shell reworking in situ can be inferred since encrustation and bioerosion occurs on both the aboral and oral side of the same specimens.
The 8-shaped boring apertures observed on Clypeaster are possibly made by endolithic gastrochaenid bivalves which bore in hard substrates and secret an aragonitic chimney showing an 8-shaped form (Schiapparelli et al. 2005; Belaústegui et al. 2013, 2017; Rahman et al. 2015; Casoli et al. 2016).
Table 2. Summary of taxonomic, sedimentological, and taphonomic features of the clypeasteroid assemblages from early Miocene sections at Bancali and Usini, Sardinia, Italy.
| |
Bancali Assemblage 1 |
Bancali Assemblage 2 |
Usini Assemblage |
|
Sedimentary environment |
siliciclastic/carbonate |
siliciclastic/carbonate |
siliciclastic/carbonate |
|
Accumulation thickness (cm) |
140 |
180 |
150 |
|
Taxonomic composition |
|||
|
Eucidaris |
absent |
absent |
rare |
|
Genocidaris |
absent |
absent |
rare |
|
Koehleraster |
absent |
rare |
absent |
|
Echinolampas |
absent |
rare |
common |
|
Amphiope |
abundant |
absent |
absent |
|
Clypeaster |
common |
common |
very common |
|
Spatangoids indet. |
absent |
absent |
rare |
|
Sedimentary fabric |
|||
|
Density |
densely packed to dispersed |
loosely packed to dispersed |
loosely packed to dispersed |
|
Imbrication |
absent |
absent |
absent |
|
Orientation of complete specimens |
chaotic |
chaotic |
chaotic |
|
Detailed taphonomy |
|||
|
Spine disarticulation |
total |
total |
total |
|
Fragmentation |
high |
moderate |
moderate |
|
Surface abrasion |
moderate |
low |
moderate |
|
Encrustation |
low |
low |
high |
|
Bioerosion |
low |
low |
moderate |
|
Post-depositional features |
|
|
|
|
Grain indentation |
absent |
absent |
absent |
|
Radial cracking |
present |
absent |
absent |
|
Implosion of the central area of the test |
present |
absent |
absent |
|
Paleoenvironment |
littoral (high energy) |
inner sublittoral (moderate energy) |
inner sublittoral (moderate energy) |
|
Genesis of the accumulation |
multiple in situ reworking events |
multiple in situ reworking events |
multiple in situ reworking events |
Ecomorphological and taphonomic gradient along a mixed siliciclastic-carbonate shelf.—A wide variety of echinoid-bearing deposits from the Miocene of northern Sardinia studied recently (Mancosu and Nebelsick 2013, 2015, 2017) differ greatly with respect to taxonomic makeup, sedimentary facies and fabric, density of the occurrences, taphonomic signatures, size frequency distributions and accompanying fauna and flora. These differences are interpreted with respect to both biotic and abiotic factors including life habit and population ecology, energy conditions and substrate relationship. These factors can be correlated to an onshore-offshore gradient. Further factors are related to the architecture of the test and taphonomic processes, including transport and time-averaging which determine the origin of accumulations and the preservation potentials of echinoid tests.
Three different habitats with differing echinoid faunas can be distinguished pertaining to the littoral, inner sublittoral, and outer sublittoral environments (Fig. 9). In littoral environments with mobile substrates and higher energy conditions, represented by assemblages of Bancali Assemblage 1, Chiaramonti and Ardara, echinoid diversity appears low. The echinoid assemblages are characterized by few genera including the shallow infaunal deposit feeding sand dollars Amphiope, with different species (see Stara and Borghi 2014), Parascutella and various Clypeaster morphotypes (see Mancosu and Nebelsick 2013, 2015 and herein) which lived shallow infaunally to epifaunally in coarse-grained siliciclastic substrates. Sand dollars were well adapted to soft-bottom habitats with high energy conditions with little competition from other deposit feeding macroinvertebrates, particularly irregular echinoids other than clypeasteroids.
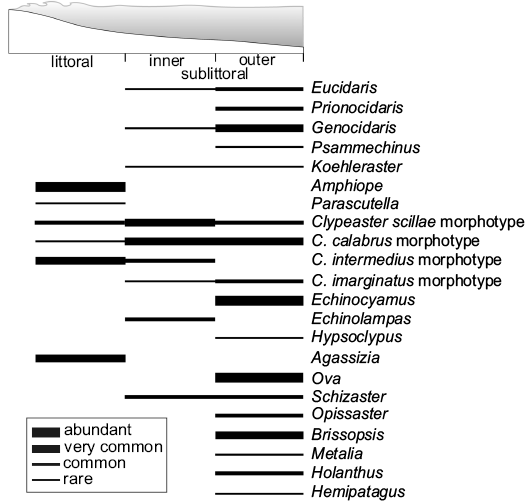
Fig. 9. Distribution of clypeasteroid echinoids and associated echinoid taxa along a depth gradient as recognized in the Miocene sedimentary succession of northern Sardinia (based on Mancosu and Nebelsick 2013, 2015, 2017, and this paper).
The infaunal spatangoid Agassizia is a significant component of the echinoid fauna within the sand dollar assemblage of Chiaramonti (Stara et al. 2012; Mancosu and Nebelsick 2013) which has been interpreted as an in situ accumulation of a shallow water environment with relatively low energy conditions (Mancosu and Nebelsick 2013).
A similar association of clypeasteroids and spatangoids was reported by Bentley and Crockcroft (1995) with the co-occurrence of the sand dollar Echinodiscus bisperforatus and the spatangoid Echinocardium cordatum which dominate the benthic communities in sheltered, sandy subtidal settings along the South African south coasts. In the Northern Red Sea, the shallow burrowing clypeasteroids Sculpsitechinus auritus and Clypeaster humilis similarly share sandy, shallow water substrates with the burrowing spatangoid Lovenia elongata (Nebelsick 1992b).
Inner sublittoral environments, represented by assemblages Bancali Assemblage 2 and Usini Assemblage, respectively, are characterised by sandy substrates and moderate water energy, and appear to be dominated by different morphotypes of Clypeaster living epibenthically to shallow infaunally and the deposit feeding echinolampadoid Echinolampas. The epibenthic regular echinoids Eucidaris and Genocidaris, which lived on secondary hardgrounds (coralline algal carpet), are also present. The echinoneoid Koehleraster and spatangoids rarely occur.
A similar situation occurs at Padru Monzu (SE of Ploaghe, northern Sardinia) where Miocene fine-grained sandstones with abundant coralline algae remains are dominated by different morphotypes of Clypeaster and associated Echinolampas and the spatangoid Schizaster (AM personal observation).
In outer sublittoral habitats at moderate depth, more diverse echinoid-dominated benthic communities occurred. The echinoid assemblage of Ittiri (Mancosu and Nebelsick 2015) occurs within fine-grained sandstone and was dominated by Clypeaster calabrus and C. marginatus morphotypes which lived epifaunally and shallow infaunally respectively. The deep burrower spatangoid Ova and the echinolampadoid Hypsoclypus, which ploughed the sediment surface, also occurred (Mancosu and Nebelsick 2015).
A comparable outer sublittoral environment was recognized in the Porto Torres area (Mancosu and Nebelsick 2017). Here, fine-grained sandy substrates are dominated by mostly infaunal spatangoids together with the minute clypeasteroid Echinocyamus having a shallow infaunal lifestyle. The shallow- to semi-infaunal flat Clypeaster marginatus also occurs sporadically. Thick-shelled Clypeaster (Clypeaster scillae morphotype) and Echinocyamus were also observed within the heteropic rhodolith beds associated with the regular echinoids, Prionocidaris, Eucidaris, Psammechinus; trigonocidarids and diadematoids were also found (Mancosu and Nebelsick 2017).
The co-occurrence of clypeasteroid species was observed in all assemblages. Interspecific competition may have been limited by different feeding strategies and food selection among deposit-feeding clypeasteroid echinoids which developed complex feeding strategy (e.g., Telford and Mooi 1986; Barras 2008) utilizing their morphologically specialized Aristotle’s lantern (Mooi 1989). Differences in selective feeding behaviour based on different feeding mechanisms and grain selection provide a basis for resource partitioning in clypeasteroid echinoids, such as Echinocyamus, Clypeaster and sand dollars (De Ridder and Lawrence 1982; Findlay and White 1983; Telford et al. 1983, 1987; Ghiold 1984; Telford and Mooi 1986; Hilber and Lawrence 2009; Guilherme et al. 2015; Brustolin et al. 2016).
The sympatric occurrences of different Clypeaster species have been described in different tropical environments. In shallow-water environments of the Florida coast and the Gulf of Mexico, two Clypeaster species with very different morphologies are present (Kier and Grant 1965; Kier 1975; Telford et al. 1987): the inflated C. rosaceus, an epibenthic deposit feeder (see Kampfer and Ott 1995), and the flattened C. subdepressus, a shallow infaunal burrower. In the Northern Bay of Safaga, Red Sea, three sympatric Clypeaster species with differing morphologies occur (Nebelsick 1992b). A further example for the differentiation of forms and possible burial depths is given by Kroh (2005) with the sympatric Clypeaster campanulatus, C. scillae and C. calabrus from Miocene assemblages.
Sedimentological and taphonomic overprinting of preservation potentials.—The ecomorphological gradient discussed above could be biased by sedimentological and taphonomic overprinting which affects the preservation of the various echinoid taxa. The preservation potential of echinoids is related to a complex interplay of factors including test architecture, life habits and population ecologies, environmental conditions affecting taphonomic processes as well as and other factors including time averaging (e.g., Donovan 1991; Nebelsick 1995; Brett et al. 1997; Ausich 2001).
Regular echinoids such as echinothurioids, cidaroids, diadematoids and most Paleozoic echinoids (Thompson and Ausich 2016; Thompson and Denayer 2017) dissociate rapidly when subjected to post-mortem transportation and reworking due to the fact that they have imbricate or only slightly interlocking plates. Complete tests of these taxa are thus rare (see discussion in Mancosu et al. 2015 and references cited therein). Clypeasteroid echinoids with plate interlocking, internal supports and thick shells show, in contrast, high preservation potentials (Seilacher 1979; Nebelsick and Kroh 2002; Belaústegui et al. 2012; Mancosu and Nebelsick 2013, 2015). Spatangoid echinoids fall between these extremes, having a thin-shelled and rigid corona with sutured plates (McNamara 1987).
Apart from skeletal architecture, further factors influencing the preservation potential of echinoids are environmental conditions and life habit (e.g., Greenstein 1993b, 1995; Nebelsick 1995). Regular echinoids have a relatively poor fossil record compared to that of irregular sea urchins (Kier 1977). Furthermore, regular echinoids diversified on hard substrates in areas dominated by erosion while irregular echinoids diversified within soft sediments where active sedimentation takes place (Smith 1984). Hard substrates may show higher diversities of epifaunal regular echinoids than soft substrates dominated by irregular echinoids. The fauna of the former, however, is often preserved as fragments while complete test can be preserved in the latter (e.g., Nebelsick 1996).
The origin of high density clypeasteroid assemblages in higher energy, shallow environments is promoted by concentration of skeletal material and rapid burial (Moffat and Bottjer 1999; Nebelsick and Kroh 2002; Belaústegui et al. 2012; Mancosu and Nebelsick 2013, 2015). Different possibilities for the generation of the studied clypeasteroid dominated accumulations range from proximal storm deposits, to multiple reworked and in situ accumulations. Thus, in addition to reflecting ecological relationships of the living benthic communities, the low diversity in littoral environments is interpreted to reflect high energy environmental conditions to which clypeasteroids are adapted. Taphonomic loss is difficult to evaluate, but fragmented tests of echinoid taxa, other than clypeasteroids, whether regular or irregular forms, are not present in the studied littoral environments with the exception of the infaunal spatangoid Agassizia in the assemblage of Chiaramonti.
The echinoid assemblages which occur in inner and outer sublittoral environments are interpreted as parautochthonous in origin. These settings, with moderate to low energy and only sporadic major storms allowed for the preservation of echinoid remains other than clypeasteroids, such as regular echinoids and irregular echinolampadoids and thin-shelled spatangoids.
Conclusions
Three clypeasteroid-rich echinoid assemblages from two different mixed siliciclastic-carbonate sedimentary successions from the Miocene of northern Sardinia show clear differences in taxonomic composition as well as in sedimentological and taphonomic signatures.
A low-diversity assemblage (Bancali Assemblage 1), points to a shallow water, high energy environments and consists exclusively of the clypeasteroid echinoids Amphiope and Clypeaster. A slightly higher diversity is found in the inner sublittoral communities, represented by the Bancali Assemblage 2 and Usini Assemblage, which are dominated by different morphotypes of the clypeasteroid Clypeaster and the echinolampadoid Echinolampas. Associated minor echinoid taxa include the echinoneoid Koehleraster (Bancali Assemblage 2) and the cidaroid Eucidaris, the trigonocidarid Genocidaris and spatangoids (Usini Assemblage). The co-occurrence of different irregular echinoids within each assemblage suggests resource partitioning among deposit feeders. The clypeasteroid assemblages studied herein originated by multiple in situ reworking events.
The echinoid-bearing deposits described in this paper represent only three of a wider variety of clypeasteroid-bearing deposits from the Miocene of northern Sardinia, which differ greatly with respect to taxonomic make up, sedimentary fabrics, bed morphology, density of occurrence, taphonomic signatures, size frequency distributions and accompanying fauna and flora.
An ecomorphological gradient has been recognized. The sand dollar Amphiope is restricted to and often dominates littoral environments with coarse-grained mainly siliciclastic sediments, where it forms mass occurrences. Clypeaster, with its thick-shelled morphotypes, had a wider tolerance of habitat and substrate preference occurring both in littoral environments with high water energy characterised by coarse-grained siliciclastic substrates, as well as in low energy, inner and outer sublittoral environments with fine-grained mixed siliciclastic-carbonate substrates. The flat, relatively thin-shelled Clypeaster forms, such as C. marginatus, and the minute fibulariid Echinocyamus seem to be restricted to fine-grained sediments of moderate to low energy, outer sublittoral environments.
With respect to accompanying non-clypeasteroid taxa, the echinolampadoids Echinolampas and Hyspsoclypus are a minor components within the inner and outer sublittoral clypeasteroid assemblages. The spatangoid echinoid Agassizia occurs in large number in littoral environments dominated by sand dollars. Spatangoids also occur in inner and outer sublittoral environments in association with the clypeasteroids Clypeaster and Echinocyamus and regular echinoids. The echinoneoid Koehleraster occurs rarely in littoral and outer sublittoral environments with mobile substrates.
Echinoid preservation within specific environments is determined by numerous factors including ecological preferences, gregarious behavior, differential preservation potentials and the nature of the sediments which they occur. These factors lead to different genetic mechanisms underlying the formation of echinoid-bearing deposits as well as the respective representation of echinoid taxa.
Acknowledgements
The authors are grateful to Andreas Kroh (Natural History Museum, Vienna, Austria) and Jeffery R. Thompson (University of Southern California, Los Angeles, USA) whose critical comments and constructive suggestions helped to improve the paper. JHN thanks the SFB-TRR141 Project A07 of the German Science Foundation.
References
Allison, P.A. 1990. Variation in rates of decay and disarticulation of Echinodermata: implications for the application of actualistic data. Palaios 5: 432–440. Crossref
Ausich, W.I. 2001. Echinoderm taphonomy. In: M. Jangoux and J.M. Lawrence (eds.), Echinoderm Studies 6, 171–227. Balkema, Rotterdam.
Banno, T. 2008. Ecological and taphonomic significance of spatangoid spines: relationship between mode of occurrence and water temperature. Paleontological Research 12: 145–157. Crossref
Barras, C.G. 2008. Morphological innovation associated with the expansion of atelostomate irregular echinoids into fine-grained sediments during the Jurassic. Palaeogeography, Palaeoclimatology, Palaeoecology 263: 44–77. Crossref
Baumiller, T.K., Salamon, M.A., Gorzelak, P., Mooi, R., Messing, C.G., and Gahn, F.J. 2010. Post-Paleozoic crinoid radiation in response to benthic predation preceded the Mesozoic marine revolution. Proceedings of the National Academy of Sciences USA 107: 5893–5896. Crossref
Belaústegui, Z., de Gibert, J.M., Nebelsick, J.H., Domènech, R., and Martinell, J. 2013. Clypeasteroid tests as a benthic island for gastrochaenid bivalve colonization: evidence from the Middle Miocene of Tarrragona (NE Spain). Palaeontology 56: 783–796. Crossref
Belaústegui, Z., Muñiz, F., Nebelsick, J.H., Domènech, R., and Martinell, J. 2017. Echinoderm ichnology: bioturbation, bioerosion and related processes. Journal of Paleontology [published online]. Crossref
Belaústegui, Z., Nebelsick, J.H., De Gibert, J.M., Domènech, R., and Martinell, J. 2012. A taphonomic approach to the genetic interpretation of clypeasteroid accumulations from Tarragona (Miocene, NE Spain). Lethaia 45: 548–565. Crossref
Bell, M. and Frey, R.W. 1969. Observations on ecology and the feeding and burrowing mechanism of Mellita quinquiesperforata (Leske). Journal of Paleontology 43: 553–560.
Benisek, M.F., Betzler, C., Marcano, G., and Mutti, M. 2009. Coralline-algal assemblages of a Burdigalian platform slope: implications for carbonate platform reconstruction (northern Sardinia, western Mediterranean Sea). Facies 55: 375–386. Crossref
Bentley, A.C. and Cockcroft, A.C. 1995. Sublittoral sand dollar (Echinodiscus bisperforatus) communities in two bays on the South African coast. South African Journal of Zoology 30: 5–18. Crossref
Blow, W.H. 1969. Late Middle Eocene to Recent planktonic foraminferal biostratigraphy. In: P. Brönnimann and H.H. Renz (eds.), Proceedings of the First International Conference on Planktonic Microfossils, 199–422. E.J. Brill, Leiden.
Borszcz, T. 2012. Echinoids as substrates for encrustation—review and quantitative analysis. Annales Societatis Geologorum Poloniae 82: 139–149.
Bossio, A., Dall’ Antonia, B., Da Prato, S., Foresi, L.M., and Oggiano, G. 2006. Preliminary stratigraphical investigations of the Miocene successions of the Porto Torres Basin (northern Sardinia, Italy). Atti della Società Toscana di Scienze Naturali, Memorie Serie A 111: 67–74.
Brett, C.E., Moffat, H.A., and Taylor, W.L. 1997. Echinoderm taphonomy, taphofacies, and Lagerstätten. In: J.A. Waters and C.G. Maples (eds.), Geobiology of Echinoderms. Paleontological Society Papers 3: 147–190.
Brustolin, M.C., Thomas, M.C., Mafra, L.L. Jr., and Lana, P.C. 2014. Does Encope emarginata (Echinodermata: Echinoidea) affect spatial variation patterns of estuarine subtidal meiofauna and microphytobenthos? Journal of Sea Research 91: 70–78. Crossref
Brustolin, M.C., Thomas, M.C., Mafra, L.L. Jr., and Lana, P.C. 2016. Bioturbation by the sand dollar Encope emarginata (Echinoidea, Mellitidae) changes the composition and size structure of microphytobenthic assemblages. Hydrobiologia 779: 183–192.
Cabanillas-Terán, N., Loor-Andrade, P., Rodríguez-Barreras, R., and Cortés, J. 2016. Trophic ecology of sea urchins in coral-rocky reef systems, Ecuador. PeerJ 4: e1578. Crossref
Casoli, E., Ricci, S., Antonelli, F., Sacco Perasso, C., Belluscio, A., and Ardizzone, G. 2016. Impact and colonization dynamics of the bivalve Rocellaria dubia on limestone experimental panels in the submerged Roman city of Baiae (Naples, Italy). International Biodeterioration and Biodegradation 108: 9–15. Crossref
Casula, A., Cherchi, A., Montadert, L., Murru, M., and Sarria, E. 2001. The Cenozoic Graben System of Sardinia (Italy): geodynamic evolution from new seismic and field data. Marine and Petroleum Geology 18: 863–888. Crossref
Ceranka, T. and Złotnik, M. 2003. Traces of cassid snails predation upon the echinoids from the Middle Miocene of Poland. Acta Palaeontologica Polonica 48: 491–496.
Challener, R.C., Miller, M.F., Furbish, D.J., and McClintock, J. 2009. Evaluation of sand grain crushing in the sand dollar Mellita tenuis (Echinoidea: Echinodermata). Aquatic Biology 7: 261–268. Crossref
Challis, G.R. 1980. Palaeoecology and Taxonomy of Mid-Tertiary Maltese Echinoids. 401 pp. Unpublished Ph.D. Thesis, University of London, London.
Chao, S.M. 2000. The irregular sea urchins (Echinodemata: Echinoidea) from Taiwan, with descriptions of six new records. Zoological Studies 39: 250–285.
Cherchi, A. and Montandert, L. 1982. Il sistema di rifting oligo-miocenico del Mediterraneo occidentale e sue conseguenze paleogeografiche sul Terziario sardo. Memorie della Società Geologica Italiana 24: 387–400.
Chesher, R.H. 1969. Contributions to the biology of Meoma ventricosa (Echinoidea: Spatangoida). Bulletin of Marine Science 19: 72–110.
Chia, F.S. 1969. Some observations on the locomotion and feeding of the sand dollar Dendraster excentricus. Journal of Experimental Marine Biology and Ecology 3: 162–170. Crossref
Comaschi Caria, I. 1972. Gli echinidi del Miocene della Sardegna. 95 pp. Stabilimento Tipografico Editoriale Fossataro, Cagliari.
Como, S., Magni, P., Baroli, M., Casu, D., De Falco, G., and Floris, A. 2008. Comparative analysis of macrofaunal species richness and composition in Posidonia oceanica, Cymodocea nodosa and leaf litter beds. Marine Biology 153: 1087–1101. Crossref
Creed, E.L. and Coull, B.C. 1984. Sand dollar, Mellita quinquiesperfortata (Leske), and sea pansy, Renilla reniformis (Cuvier) effects on meiofaunal abundance. Journal of Experimental Marine Biology and Ecology 84: 225–234. Crossref
De Ridder, C. and Lawrence, J.M. 1982. Food and feeding mechanisms: Echinoidea. In: M. Jangoux and J.M. Lawrence (eds.), Echinoderm Nutrition, 499–519. Balkema, Rotterdam.
Donovan, S.K. 1991. The taphonomy of echinoderms: calcareous multi-element skeletons in the marine environment. In: S.K. Donovan (ed.), The Processes of Fossilisation, 241–269. Belhaven Press, London.
Donovan, S.K. 2003. Completeness of a fossil record: the Pleistocene echinoids of the Antilles. Lethaia 36: 1–7. Crossref
Donovan, S.K. and Veale, C. 1996. The irregular echinoids Echinoneus Leske and Brissus Gray in the Cenozoic of the Antillean Region. Journal of Paleontology 70: 632–640. Crossref
Dynowski, J. 2012. Echinoderm remains in shallow-water carbonates at Fernandez Bay, San Salvador Island, Bahamas. Palaios 27: 181–189. Crossref
Ebert, T.A. and Dexter, D.M. 1975. A natural history study of Encope grandis and Mellita grantii, two sand dollars in the northern Gulf of California, Mexico. Marine Biology 32: 397–407. Crossref
Ellers, O. and Telford, M. 1984. Collection of food by oral surface podia in the sand dollar, Echinarachnius parma (Lamarck). Biological Bulletin 166: 574–582. Crossref
Ellers, O. and Telford, M. 1991. Forces generated by the jaws of Clypeasteroids (Echinodermata: Echinoidea). Journal of Experimental Biology 155: 585–603.
Endean, R. and Pope, E.C. 1964. Rediscovery of the echinoid Clypeaster tumidus (Tenison-Woods) and an emended description. Records of the Australian Museum 26: 275–281. Crossref
Facenna, C., Speranza, F., D’Ajello Caracciolo, F., Mattei, M., and Oggiano, G. 2002. Extensional tectonics on Sardinia (Italy): insights into the arc-back-arc transitional regime. Tectonophysics 356: 213–232. Crossref
Findlay, R.H. and White, D.C. 1983. The effects of feeding by the sand dollar Mellita quinquiesperforata (Leske) on the benthic microbial community. Journal of Experimental Marine Biology and Ecology 72: 25–41. Crossref
Fodrie, J.F., Herzka, S.Z., Lucas, A.J., and Francisco, V. 2007. Intraspecific density regulates positioning and feeding mode selection of the sand dollar Dendraster excentricus. Journal of Experimental Marine Biology and Ecology 340: 169–183. Crossref
Fontaine, A. 1953. The shallow water echinoderms of Jamaica. Part III. The sea urchins (Class Echinoidea). Natural History Society of Jamaica, Natural History Notes 61: 3–9.
Francolini, L. 1994. Sull’età di un ciclo miocenico dell’area di Sorso e Sennori, a Nord di Sassari (Sardegna Settentrionale). Atti della Società Toscana di Scienze Naturali, Memorie Serie A 101: 137–144.
Funedda, A., Oggiano, G., and Pasci, S. 2000. The Logudoro basin: a key area for the tertiary tectono-sedimentary evolution of North Sardinia. Bollettino della Società Geologica Italiana 119: 31–38.
Funedda, A., Oggiano, G., and Pascucci, V. 2003. I depositi Miocenici della Sardegna settrentionale: il bacino Logudoro. In: V. Pascucci (ed.), Atti del Convegno GEOSED 2003, 381–414. Editoria e Stampa, Sassari.
Ghiold, J. 1984. Adaptive shifts in clypeasteroid evolution—feeding strategies in the soft-bottom realm. Neues Jahrbuch fur Geologie und Paläontologie Abhandlungen 169: 41–73.
Ghiold, J. 1989. Species distributions of irregular echinoids. Biological Oceanography 6: 79–162.
Ghiold, J. and Hoffman, A. 1984. Clypeasteroid echinoids and historical biogeography. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 9: 529–538.
Ghiold, J. and Hoffman, A. 1986. Biogeography and biogeographic history of clypeasteroid echinoids. Journal of Biogeography 13: 183–206. Crossref
Gladfelter, W.B. 1978. General ecology of the echinolampadoid urchin Cassidulus caribbearum. Marine Biology 47: 149–160. Crossref
Glynn, P.W., Wellington, G.M., and Birkeland, C. 1979. Coral reef growth in the Galapagos: Limitation by sea urchins. Science 203: 47–49. Crossref
Gordon, C.M. and Donovan, S.K. 1992. Disarticulated echinoid ossicles in paleoecology and taphonomy: The last interglacial Falmouth Formation of Jamaica. Palaios 7: 157–166. Crossref
Greenstein, B.J. 1991. An integrated study of echinoid taphonomy: Predictions for the fossil record of four echinoid Families. Palaios 6: 519–540. Crossref
Greenstein, B.J. 1992. Taphonomic bias and evolutionary history of the family Cidaridae (Echinodermata: Echinoidea). Paleobiology 18: 50–79. Crossref
Greenstein, B.J. 1993a. Is the fossil record of regular echinoids so poor? A comparison of living and subfossil assemblages. Palaios 8: 587–601. Crossref
Greenstein, B.J. 1993b. The effect of life habit on the preservation potential of echinoids. In: B.N. White (ed.), Proceedings of the 6th Symposium on the Geology of The Bahamas, 55–74. Bahamian Field Station, San Salvador.
Greenstein, B.J. 1995. The effects of life habit and test microstructure on the preservation potential of echinoids in Graham’s Harbour, San Salvador Island, Bahamas. Geological Society of America, Special Paper 300: 177–188. Crossref
Grubelic, I. 1998. Presence of the species Genocidaris maculata Agassiz, 1869, Echinoidea, Echinodermata in the Adriatic Sea. Periodicum Biologorum 100: 39–42.
Grun, T. and Nebelsick, J.H. 2016. Taphonomy of a clypeasteroid echinoid using a new quasimetric approach. Acta Palaeontologica Polonica 61: 689–699.
Grun, T., Kroh, A., and Nebelsick, J.H. 2017. Comparative drilling predation on time-averaged phosphatized and nonphosphatized assemblages of the minute clypeasteroid echinoid Echinocyamus stellatus from Miocene offshore sediments (Globigerina Limestone Formation, Malta). Journal of Paleontology [published online]. Crossref
Grun, T., Sievers, D., and Nebelsick, J.H. 2014. Drilling predation on the clypeasteroid echinoid Echinocyamus pusillus from the Mediterranean Sea (Giglio, Italy). Historical Biology 26: 745–757. Crossref
Guilherme, P.D.B., Brustolin, M.C., and De L. Bueno, M. 2015. Distribution patterns of ectosymbiont crabs and their sand dollar hosts in a subtropical estuarine sandflat. Revista de Biologiá Tropical 63: 209–220.
Hendler, G., Miller, J.E., Pawson, D.L., and Kier, P.M. 1995. Sea Stars, Sea Urchins, and Allies: Echinoderms of Florida and the Caribbean, 390 pp. Smithsonian Institution Press, Washington DC.
Hernández, J.C., Clemente, S., Tuya, F., Pérez-Ruzafa, A., Sangil, C., Moro-Abad, L., and Bacallado-Aránega, J.J. 2013. Echinoderms of the Canary Islands, Spain. In: J.J. Alvarado and F.A. Solís-Marín (eds.), Echinoderm Research and Diversity in Latin America, 471–510. Springer Berlin, Heidelberg.
Higgins, R.C. 1974. Observations on the biology of Apatopygus recens (Echinoidea: Echinolampadoida) around New Zealand. Journal of Zoology 173: 505–516. Crossref
Hilber, S.E. and Lawrence, J.M. 2009. Analysis of sediment and gut contents of the sand dollar Mellita tenuis, Encope michelini, and Encope aberrans off the Central Florida Gulf Coast. Gulf of Mexico Science 27: 74–81.
Hopkins, T.S. 1988. A review of the distribution and proposed morphological groupings of extant species of the genus Clypeaster in the Caribbean Sea and Gulf of Mexico. In: R.D. Burke, P.V. Mladenov, P. Lambert, and R.L. Parseley (eds.), Echinoderm Biology: Proceedings of the 6th International Echinoderm Conference, 337–345. Balkema, Rotterdam.
Imbesi Smedile, M. 1958. Clypeasteri aquitaniani, elveziani e tortoniani della Calabria. Palaeontographia Italica 53: 1–47.
James, D.B. and Pearse, J.S. 1969. Echinoderms from the Gulf of Suez and the Northern Red Sea. Journal of the Marine Biological Association of India 11: 78–12.
Kampfer, S. and Ott, J. 1995. Nutrition and feeding preferences in the Caribbean echinoid Clypeaster rosaceus (Echinodermata: Echinoidea). In: A. Eleftherion, A.D. Ansell and C.J. Smith (eds.), Biology and Ecology of Shallow Coastal Waters. Proceedings of the 28th European Marine Biology Symposium, 309–313. Olsen and Olsen, Fredensborg.
Kidwell, S.M. and Baumiller, T. 1990. Experimental disintegration of regular echinoids: roles of temperature, oxygen and decay thresholds. Paleobiology 16: 247–271. Crossref
Kidwell, S.M. and Holland, S.M. 1991. Field description of coarse bioclastic fabric. Palaios 6: 426–434. Crossref
Kier, P.M. 1975. The echinoids of Carrie Bow Cay, Belize. Smithsonian Contribution to Zoology 206: 1–45. Crossref
Kier, P.M. 1977. The poor fossil record of the regular echinoid. Palaeobiology 3: 168–174. Crossref
Kier, P.M. 1982. Rapid evolution in echinoids. Palaeontology 25: 1–9.
Kier, P.M. and Grant, R.E. 1965. Echinoid distribution and habits, Key Largo Coral Reef Preserve, Florida. Smithsonian Miscellaneous Collection 149: 1–68.
Koukouras, A., Sinis, A.I., Bobori, D., Savas, K., and Miltiadis-Spyridon, K. 2007. The echinoderm (Deuterostomia) fauna of the Aegean Sea, and comparison with those of the neighbouring seas. Journal of Biological Research 7: 67–92.
Kroh, A. 2004. Echinoneus & Echinometra—two new records of tropical echinoids from the Miocene of Austria and their palaeoclimatic implications. Berichte des Institutes für Erdwissenschaften der Karl-Franzens-Universität Graz 9: 229–230.
Kroh, A. 2005. Catalogus Fossilium Austriae. Band 2. Echinoidea Neogenica. 210 pp. Österreichische Akademie der Wissenschaften, Wien.
Kroh, A. and Nebelsick, J.H. 2003. Echinoid assemblages as a tool for palaeoenvironmental reconstruction—an example from the Early Miocene of Egypt. Palaeogeography, Palaeoclimatology, Palaeoecology 201: 157–177. Crossref
Kroh, A. and Smith, A.B. 2010. The phylogeny and classification of post-Palaeozoic echinoids. Journal of Systematic Palaeontology 8: 147–212. Crossref
Lane, J.E.M. and Lawrence, J.M. 1980. Seasonal variation in body growth, density and distribution of a population of sand dollars, Mellita quinquiesperforata (Leske). Bulletin of Marine Science 30: 871–882.
Lane, D.J.W., Marsh, L.M., VandenSpiegel, D., and Rowe, F.W.E. 2000. Echinoderm fauna of the South China Sea: an inventory and analysis of distribution patterns. The Raffles Bullettin of Zoology (Supplement) 8: 459–493.
Lawrence, J.M., Herrera, J., and Cobb, J. 2004. Vertical posture of the clypeasteroid sand dollar Encope michelini. Journal of the Marine Biological Association of the United Kingdom 84: 407–406. Crossref
Lecca, L., Lonis, R., Luxoro, S., Melis, F., Secchi, F., and Brotzu, P. 1997. Oligo-Miocene volcanic sequences and rifting stages in Sardinia: a review. Periodico di Mineralogia 66: 7–61.
Lessios, H.A., Kessing, B.D., Robertson, D.R., and Paulay, G. 1999. Phylogeography of the pantropical sea urchin Eucidaris in relation to land barriers and ocean currents. Evolution 53: 806–817. Crossref
Lewis, R.D. 1980. Taphonomy. In: T.W. Broadhead and J.A. Waters (eds.), Echinoderms, notes for a short course. University of Tennessee Studies in Geology 3: 27–39.
Li, B., Keesing, J.K., Lourey, M., and McLaughlin, J. 2013. Feeding and bioturbation effects of the sand dollar Peronella lesueuri (L. Agassiz, 1841) (Echinodermata) on microphytobenthos and sediment fluxes. Marine and Freshwater Behaviour and Physiology 46: 431–446. Crossref
Liao, Y.L. 1978. The echinoderms of Xisha Islands, Guangdong Province, China. 3. Echinoidea. Studia Marina Sinica 12: 102–127.
Lindsay, W.J. Jr. 1996. Changing abundances of Cassis tuberosa and its echinoid prey on San Salvador, 1973–1995. Proceedings of the 6th Symposium on the Natural History of the Bahamas: 121–125.
Mancosu, A. and Nebelsick, J.H. 2013. Multiple routes to mass accumulations of clypeasteroid echinoids: A comparative analysis of Miocene echinoid beds of Sardinia. Palaeogeography, Palaeoclimatology, Palaeoecology 374: 173–186. Crossref
Mancosu, A. and Nebelsick, J.H. 2015. The origin and paleoecology of clypeasteroid assemblages from different shelf setting of the Miocene of Sardinia, Italy. Palaios 30: 273–387. Crossref
Mancosu, A. and Nebelsick, J.H. 2016. Echinoid assemblages from the early Miocene of Funtanazza (Sardinia): A tool for reconstructing depositional environments along a shelf gradient. Palaeogeography, Palaeoclimatology, Palaeoecology 454: 139–160. Crossref
Mancosu, A. and Nebelsick, J.H. 2017. Palaeoecology and taphonomy of spatangoid-dominated echinoid assemblages: A case study from the Early–Middle Miocene of Sardinia, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology 466: 334–352.
Mancosu, A., Nebelsick, J.H., Kroh, A., and Pillola, G.L. 2015. The origin of echinoid shell beds in siliciclastic shelf environments: three examples from the Miocene of Sardinia, Italy. Lethaia 48: 83–99. Crossref
Martini, E. 1971. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In: A. Farinacci (ed.), Proceedings of the Second Planktonic Conference, 739–785. Tecnoscienza, Roma.
Martini P., Oggiano G., and Mazzei R. 1992. Siliciclastic-carbonate sequences of Miocene grabens in northern Sardinia, Western Mediterranean Sea. Sedimentary Geology 76: 63–78. Crossref
Mazzei, R. and Oggiano, G. 1990. Messa in evidenza di due cicli sedimentari nel Miocene dell’area di Florinas (Sardegna Settentrionale). Atti della Società Toscana di Scienze Naturali, Memorie, Serie A 97: 119–147.
McNamara, K. 1987. Plate translocation in spatangoid echinoids: its morphological, functional and phylogenetic significance. Paleobiology 13: 312–325. Crossref
McNamara, K.J. and Philip, G.M. 1980. Tertiary species of Echinolampas (Echinoidea) from southern Australia. Memoirs of the National Museum of Victoria 41: 1–14. Crossref
McPherson, B.F. 1968. Contributions to the biology of the sea urchin Eucidaris tribuloides (Lamarck). Bulletin of Marine Science 18: 400–403.
Merril, R.J. and Hobson, E.S. 1970. Field observations of Dendraster excentricus, a sand dollar of western North America. American Midland Naturalist 83: 595–624. Crossref
Mihaljević, M., Jerjen, I., and Smith, A.B. 2011. The test architecture of Clypeaster (Echinoidea, Clypeasteroida) and its phylogenetic significance. Zootaxa 2983: 21–38.
Moffat, H.A. and Bottjer D.J. 1999. Echinoid concentration beds: two examples from the stratigraphic spectrum. Palaeogeography, Palaeoclimatology, Palaeoecology 149: 329–348. Crossref
Mooi, R. 1989. Living and fossil genera of the Clypeasteroida (Echinoidea: Echinodermata): an illustrated key and annotated checklist. Smithsonian Contributions to Zoology 488: 1–51. Crossref
Mooi, R. 1990a. A new “living fossil” echinoid (Echinodermata) and the ecology and paleobiology of Caribbean Echinolampadoids. Bulletin of Marine Science 46: 688–700.
Mooi, R. 1990b. Living cassiduloids (Echinodermata: Echinoidea): a key and annotated list. Proceedings of the Biological Society of Washington 103: 63–85.
Mooi, R. 1997. Sand dollars of the genus Dendraster (Echinoidea: Clypeasteroida): phylogenetic systematics, heterochrony, and distribution of species. Bulletin of Marine Science 61: 343–375.
Moore, D.R. 1956. Observations of predation on echinoderms by three species of cassididae. Nautilus 69: 73–76.
Morin, J.G., Kastendiek, J.E., Harrington, A., and Davis, N. 1985. Organization and patterns of interactions in a subtidal sand community on an exposed coast. Marine Ecology 27: 163–185. Crossref
Mortensen, T. 1928. A Monograph of the Echinoidea, I. Cidaroidea, 551 pp. C.A. Reitzel, Copenhagen.
Mortensen, T. 1943. A Monograph of the Echinoidea, III, 2. Camaradonta. I. Orthopsidae, Glyphocyphidae, Temnopleuridae, and Toxopneustidae. 553 pp. C.A. Reitzel, Copenhagen.
Mortensen, T. 1948a. A Monograph of the Echinoidea, IV, 1. Holectypoida, Cassiduloida. 371 pp. C.A. Reitzel, Copenhagen.
Mortensen, T. 1948b. A Monograph of the Echinoidea, IV, 2. Clypeasteroida. Clypeasteridæ, Arachnoidæ, Fibulariidæ, Laganidæ and Scutellidæ. 471 pp. C.A. Reitzel, Copenhagen.
Müller, A. H. 1951. Grundlagen der Biostratonomie. Abhandlungen der deutsche Akademie der Wissenschaft Berlin 3: 1–147.
Murru, M., Bassi, D., and Simone, L. 2015. Displaced/re-worked rhodolith deposits infilling part of a complex Miocene multistorey submarine channel: A case history from Sassari area (Sardinia, Italy). Sedimentary Geology 326: 94–108. Crossref
Nebelsick, J.H. 1992a. Echinoid distribution by fragment identification in the Northern Bay of Safaga, Red Sea, Egypt. Palaios 7: 316–328. Crossref
Nebelsick, J.H. 1992b. The Northern Bay of Safaga (Red Sea, Egypt): An actuopalaeontological approach. III Distribution of echinoids. Beiträge zur Paläontologie von Österreich 17: 5–79.
Nebelsick, J.H. 1995. Uses and limitations of actuopalaeontological investigations on echinoids. Geobios 18 : 329–336. Crossref
Nebelsick, J.H. 1996. Biodiversity of shallow-water Red Sea echinoids: implications for the fossil record. Journal of the Marine Biological Association United Kingdom 76: 185–194. Crossref
Nebelsick, J.H. 1999. Taphonomic comparison between recent and fossil sand dollars. Palaeogeography, Palaeoclimatology, Palaeoecology 149: 349–358. Crossref
Nebelsick, J.H. 2004. Taphonomy of echinoderms: introduction and outlook. In: T. Heinzeller, and J.H. Nebelsick (eds.), Echinoderms München. Proceedings of the 11th International Echinoderm Meeting, 471–478. Taylor & Francis, Rotterdam. Crossref
Nebelsick, J.H. 2008. Taphonomy of irregular echinoid Clypeaster humilis from the Red Sea: implications for taxonomic resolution along taphonomic grades. In: W.I. Ausich and G.D. Webster (eds.), Echinoderm Paleobiology, 115–128. Indiana University Press, Bloomington.
Nebelsick, J.H. and Kampfer, S. 1994. Taphonomy of Clypeaster humilis and Echinodiscus auritus from the Red Sea. In: B. David, A. Guille, J.P. Féral, and M. Roux (eds.), Echinoderms Through Time, 803–808. Balkema, Rotterdam.
Nebelsick, J.H. and Kowalewski, M. 1999. Drilling predation on recent clypeasteroid echinoids from the Red Sea. Palaios 14: 127–144. Crossref
Nebelsick, J.H. and Kroh, A. 2002. The stormy path from life to death assemblages: The formation and preservation of mass accumulation of fossil sand dollars. Palaios 17: 378–393. Crossref
Nebelsick, J.H., Schmid, B., and Stachowitsch, M. 1997. The encrustation of fossil and recent sea-urchins tests: ecological and taphonomical significance. Lethaia 30: 271–284. Crossref
Néraudeau, D., Goubert, E., Lacour, J.M., and Rouchy, J.M. 2001. Changing biodiversity of Mediterranean irregular echinoids from the Messinian to present-day. Palaeogeography, Palaeoclimatology, Palaeoecology 175: 43–60. Crossref
Pérès, J.M. and Picard, J. 1964. Nouveau manuel de Bionomie Bentique de la Mer Méditerranèe. Recueil des travaux de la Station Marine d’Endoume 31: 5–137.
Phelan, T. 1970. A field guide to the cidaroid echinoids of the Northweastern Atlantic Ocean, Gulf of Mexico, and the Caribbean Sea. Smithsonian Contributions to Zoology 40: 1–67. Crossref
Poddubiuk, R.H. 1985. Evolution and adaptation in some Caribbean Oligo-Miocene Clypeaster. In: B.F. Keegan and B.D.S O’Connor (eds.), Proceedings of 5th International Echinoderm Conference, 75–80. Balkema, Rotterdam.
Pomory, C.M., Robbins, B.D., and Lares, M.T. 1995. Sediment grain size preference by the sand dollar Mellita Tenuis Clark, 1940 (Echinodermata: Echinoidea): A laboratory study. Bulletin of Marine Science 56: 778–783.
Rahman, I.A., Belaústegui, Z., Zamora, S., Nebelsick, J.H., Domènech, R., and Martinell, J. 2015. Miocene Clypeaster from Valencia (E Spain): insights into taphonomy and ichnology of bioeroded echinoids using X-ray micro-tomography. Palaeogeography, Palaeoclimatology, Palaeoecology 438: 168–179. Crossref
Reidenauer, J.A. 1989. Sand-dollar Mellita quinquiesperforata (Leske) burrow trails: sites of harpacticoid disturbance and nematode attraction. Journal of Experimental Marine Biology and Ecology 130: 223–235. Crossref
Reuter, M., Auer, G., Brandano, M., Harzhauser, M., Corda, L., and Piller, W.E. 2016. Post-rift sequence architecture and stratigraphy in the Oligo-Miocene Sardinia Rift (Western Mediterranean Sea). Marine and Petroleum Geology 79: 44–63. Crossref
Rodríguez-Barreras, R. 2014. The shallow-water echinoids (Echinodermata: Echinoidea) of Cuba. Marine Biodiversity Records 7: 1–8. Crossref
Rodríguez-Barreras, R., Calzada-Marrero, J.R., and Sabat, A.M. 2012. A new record of the echinoid Echinoneus cyclostomus and additional information on Cassidulus caribaearum for Puerto Rico. Marine Biodiversity Records 5: e112. Crossref
Rose, E.P.F. 1976. Some observations on the Recent holectypoid echinoid Echinoneus cyclostomus and their palaeoecological significance. Thalassia Jugoslavica 12: 299–306.
Rose, E.P.F. and Poddubiuk, R.H. 1987. Morphological variation in the Cenozoic echinoid Clypeaster and its ecological and stratigraphical significance. Annales Instituti Geologici Publici Hungarici 70: 463–469.
Salsman, G.G. and Tolbert, W.H. 1965. Observation on the sand dollar Mellita quinquiesperforata. Limnology and Oceanography 10: 152–155. Crossref
Samyn, Y. 2003. Shallow-water regular echinoids (Echinodermata: Echinoidea) from Kenya. African Zoology 38: 193–212.
Santos, C.P., Coutinho, A.B., and Hajdu, E. 2002. Spongivory by Eucidaris tribuloides from Salvador, Bahia (Echinodermata: Echinoidea). Journal of Marine Biological Association of the United Kingdom 82: 295–297. Crossref
Santos, A.G. and Mayoral, E.J. 2008. Colonization by barnacles on fossil Clypeaster: an exceptional example of larval settlement. Lethaia 41: 317–332. Crossref
Santos, A.G., Mayoral, E., Muñiz, F., Bajo, I., and Adriaensens, O. 2003. Bioerosión en erizos irregulares (Clypeasteroidea) del Mioceno Superior en el sector suroccidental de la cuenca del Guadalquivir (Provincia de Sevilla). Revista Española de Paleontología 18: 131–141.
Saucède, T., Mooi, R., and David, B. 2007. Phylogeny and origin of Jurassic irregular echinoids (Echinodermata: Echinoidea). Geological Magazine 144: 333–359. Crossref
Schein, J.P. and Lewis, R.D. 2000. The relationship between living echinoid populations and their skeletal remains in the sea-floor sediment, San Salvador, Bahamas. In: B.J. Greenstein and C.J. Carney (eds.), Proceedings of the 10th Symposium on the Geology of The Bahamas, and Other Carbonate Regions, 163–174. Bahamian Field Station, San Salvador.
Schiaparelli, S., Franci, G., Albertelli, G., and Cattaneo-Vietti, R. 2005. A nondestructive method to evaluate population structure and bioerosion activity of the boring bivalve Gastrochaena dubia. Journal of Coastal Research 21: 383–386. Crossref
Schneider, C.L. 2003. Hitchhiking on Pennsylvanian Echinoids: Epibionts on Archaeocidaris. Palaios 18: 435–444. Crossref
Sciberras, M., Rizzo, M., Mifsud, J.R., Camilleri, K., Borg, J.A., Lanfranco, E., and Schembri, P.J. 2009. Habitat structure and biological characteristic of a maerl bed off the northeastern coast of the Maltese Island (central Mediterranean). Marine Biodiversity 39: 251–264. Crossref
Seilacher, A. 1979. Constructional morphology of sand dollars. Palaeobiology 5: 191–221. Crossref
Seilacher, A. 1982. Ammonite shells as a habitat in the Posidonia Shales of Holzmaden—floats or benthic islands? Neues Jahrbuch für Geologie und Paläontologie Monatshefte 2: 98–114.
Serafy, D.K. 1970. A new species of Clypeaster from the Gulf and Caribbean and a key to the species in the tropical Northwestern Atlantic (Echinodermata: Echinoidea). Bulletin of Marine Science 20: 662–677.
Serafy, D.K. 1979. Echinoids (Echinodermata: Echinoidea). Memoirs of the Hourglass Cruises 5: 1–120.
Sloan, N.A., Clark, A.M., and Taylor, J.D. 1979. The echinoderms of Aldabra and their habitats. Bulletin of the British Museum of Natural History, Zoology 37: 81–128.
Smith, A.B. 1978. A functional classification of the coronal pores of regular echinoids. Palaeontology 21: 81–84.
Smith, A.B. 1980a. The structure and arrangement of echinoid tubercles. Philosophical Transaction of the Royal Society of London B 289: 1–54. Crossref
Smith, A.B. 1980b. The structure, function, and evolution of tube feet and ambulacral pores in irregular echinoids. Palaeontology 23: 39–83.
Smith, A.B. 1984. Echinoid Palaeobiology. 190 pp. George Allen and Unwin Ltd., London.
Smith, A.B. 2001. Probing the cassiduloid origins of clypeasteroid echinoids using stratigraphically restricted parsimony analysis. Paleobiology 27: 392–404. Crossref
Smith, A.B. and Gale, A.S. 2009. The pre-Messinian deep-sea Neogene echinoid fauna of the Mediterranean: Surface productivity controls and biogeographical relationships. Palaeogeography, Palaeoclimatology, Palaeoecology 281: 115–125. Crossref
Smith, A.B. and Ghiold, J. 1982. Roles for holes in sand dollar (Echinodermata): a review of lunules function and evolution. Paleobiology 8: 242–253. Crossref
Smith, A.B. and Kroh, A. (eds.) 2011. The Echinoid Directory. World Wide Web electronic publication. http://www.nhm.ac.uk/research-curation/projects/echinoid-directory [accessed 16/02/2017]. Natural History Museum, Vienna.
Smith, A.L. 1981. Comparison of macrofaunal invertebrates in sand dollar (Dendraster excentricus) beds and in adjacent areas. Marine Biology 65: 191–198. Crossref
Speranza, F., Villa, I.M., Sagnotti, L., Florindo, F., Cosentino, D., Cipollari, P., and Mattei, M. 2002. Age of the Corsica and Sardinia rotation and Liguro-Provencal Basin spreading: new paleomagnetic and Ar/Ar evidences. Tectonophysics 347: 231–251. Crossref
Sonnenholzner, J.I., Ladah, L.B., and Lafferty, K.D. 2009. Cascading effects of fishing on Galapagos rocky reef communities: reanalysis using corrected data. Marine Ecology Progress Series 375: 209–218. Crossref
Stanley, D.J. and James, N.P. 1971. Distribution of Echinarachnius parma (Lamarck) and associated fauna on Sable Island Bank, southeast Canada. Smithsonian Contributions to the Earth Sciences 6: 1–24. Crossref
Stara P., Rizzo R., Sanciu L., and Fois D. 2012. Note di geologia e paleoecologia relative ad alcuni siti ad Amphiope (Echinoidea: Clypeasteroidea) in Sardegna. Parva Naturalia 9: 121–171.
Stara, P. and Borghi, E. 2014. The echinoid genus Amphiope L. Agassiz, 1840 (Echinoidea Astriclypeidae) in the Oligo-Miocene of Sardinia (Italy). Biodiversity Journal 5: 245–268.
Steimle, F.W. 1990. Population dynamics, growth and reproduction estimates for the sand dollar Echinarachnius parma. Fishery Bulletin 88: 179–189.
Stevenson, A., Gahn, F.J., Baumiller, T.K., and Sevastopulo, G.D. 2017. Predation on feather stars by regular echinoids as evidenced by laboratory and field observations and its paleobiological implications. Paleobiology 43: 274–285. Crossref
Swigart, J.P. and Lawrence, J.M. 2008. Small-scale distribution of the sand dollar Mellita tenuis and Encope michelini (Clypeasteroida, Echinodermata) off the Central Florida Gulf Coast. Gulf of Mexico Science 26: 46–56.
Takeda, S. 2008. Mechanism maintaining dense beds of the sand dollars Scaphechinus mirabilis in northern Japan. Journal of Experimental Marine Biology and Ecology 363: 21–27. Crossref
Tanti, C.M. and Schembri, P.J. 2006. A synthesis of the echinoderm fauna of the Maltese Islands. Journal of the Marine Biological Association of the United Kingdom 86: 163–165. Crossref
Telford, M. 1981. A hydrodynamic interpretation of sand dollar morphology. Bulletin of Marine Science 31: 605–622.
Telford, M. 1983. An experimental analysis of lunule function in the sand dollar Mellita quinquiesperforata. Marine Biology 76: 125–134. Crossref
Telford, M. and Mooi, R. 1986. Resource partitioning by sand dollars in carbonate and siliceous sediments: evidence from podial and particle dimensions. Biological Bulletin 171: 197–207. Crossref
Telford, M. and Mooi, R. 1987. The art of standing still. New Scientist 1556: 30–35.
Telford, M., Harold, A., and Mooi, R. 1983. Feeding structures, behavior, and microhabitat of Echinocyamus pusillus (Echinoidea: Clypeasteroida). Biological Bulletin 165: 745–757. Crossref
Telford, M., Mooi, R., and Harold, A. 1987. Feeding activities of two species of Clypeaster (Echinoides, Clypeasteroida): further evidence of clypeasteroid resource partitioning. Biological Bulletin 172: 324–336.
Tewfik, A. 2014. Losing the shell game: Consequences of seascapes without predatory gastropods. In: Proceedings of the 67th Gulf and Caribbean Fisheries Institute November 3–7, 2014, Christ Church, Barbados, 331–338. Bridgetown, Barbados.
Tewfik, A. and Scheuer, B. 2013. Ecology of the King Helmet, Cassis tuberosa (L.), in South Caicos. Caribbean Naturalist 2: 1–10.
Timko, P.L. 1976. Sand dollars as suspension feeders: a new description of feeding in Dendraster excentricus. Biological Bulletin 151: 247–259. Crossref
Thomas, B. and Gennesseaux, M. 1986. A two-stage rifting in the basins of the Corsica-Sardinian straits. Marine Geology 72: 225–239. Crossref
Thompson, J.R. and Ausich, W.I. 2016. Facies distribution and taphonomy of echinoids from the Fort Payne Formation (late Osagean, early Viséan, Mississippian) of Kentucky. Journal of Paleontology 90: 239–249. Crossref
Thompson, J.R. and Denayer, J. 2017. Revision of echinoids from the Tournaisian (Mississippian) of Belgium and the importance of disarticulated material in assessing palaeodiversity. Geological Journal 52: 529–538. Crossref
Thompson, J.R., Crittenden, J., Schneider, C.L., and Bottjer, D. 2015. Lower Pennsylvanian (Bashkirian) echinoids from the Marble Falls Formation, San Saba, Texas, USA. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 276: 79–89. Crossref
Thum, A.B. and Allen, J.C. 1975. Distribution and Abundance of the Lamp Urchin Echinolampas crassa (Bell) 1880 in False Bay Cape. Transactions of the Royal Society of South Africa 41: 359–373. Crossref
Tortonese, E. 1965. Echinodermata: Fauna d’Italia. 450 pp. Calderini, Bologna.
Tsaparas, N., Drinia, H., Antonarakou, A., Marcopoulou-Diakantoni, A., and Dermitzakis, M.D. 2007. Tortonian Clypeaster fauna (Echinoidea: Clypeasteroida) from Gavdos Island (Greece). Bulletin of the Geological Society of Greece 40: 225–237.
Velluttini, B.C. and Bigotto, A.E. 2010. Embryonic, larval, and juvenile development of the sea biscuit Clypeaster subdepressus (Echinodermata: Clypeasteroida). PloS ONE 5: 1–5. Crossref
Vigorito, M., Murru, M., and Simone, L. 2006. Architectural patterns in a multistorey mixed carbonate-siliciclastic submarine channel, Porto Torres Basin, Miocene, Sardinia, Italy. Sedimentary Geology 186: 213–236. Crossref
Weihe, S.C. and Gray, I.E. 1968. Observation on the biology of the sand dollar Mellita quinquiesperforata (Leske). Journal of the Elisha Mitchell Scientific Society 84: 315–327.
Westergren, A.M. 1911. Reports on the scientific results of the Expedition “Albatross”. Echini—Echinoneus and Micropetalon. Memoirs of the Museum of Comparative Zoology 39: 35–68.
Wisshak, M., Kroh, A., Bertling, M., Knaust, D., Nielsen, J.K., Jagt, J.W.M., Neumann, C., and Nielsen, K.S.S. 2015. In defence of an iconic ichnogenus—Oichnus Bromley, 1981. Annales Societatis Geologorum Poloniae 85: 445–451. Crossref
Acta Palaeontol. Pol. 62 (3): 627–646, 2017
https://doi.org/10.4202/app.00357.2017