

The osteoderm microstructure in doswelliids and proterochampsids and its implications for palaeobiology of stem archosaurs
DENIS A. PONCE, IGNACIO A. CERDA, JULIA B. DESOJO, and STERLING J. NESBITT
Ponce, D.A., Cerda, I.A., Desojo, J.B., and Nesbitt, S.J. 2017. The osteoderm microstructure in doswelliids and proterochampsids and its implications for palaeobiology of stem archosaurs. Acta Palaeontologica Polonica 62 (4): 819–831.
Osteoderms are common in most archosauriform lineages, including basal forms, such as doswelliids and proterochampsids. In this survey, osteoderms of the doswelliids Doswellia kaltenbachi and Vancleavea campi, and proterochampsid Chanaresuchus bonapartei are examined to infer their palaeobiology, such as histogenesis, age estimation at death, development of external sculpturing, and palaeoecology. Doswelliid osteoderms have a trilaminar structure: two cortices of compact bone (external and basal) that enclose an internal core of cancellous bone. In contrast, Chanaresuchus bonapartei osteoderms are composed of entirely compact bone. The external ornamentation of Doswellia kaltenbachi is primarily formed and maintained by preferential bone growth. Conversely, a complex pattern of resorption and redeposition process is inferred in Archeopelta arborensis and Tarjadia ruthae. Vancleavea campi exhibits the highest degree of variation among doswelliids in its histogenesis (metaplasia), density and arrangement of vascularization and lack of sculpturing. The relatively high degree of compactness in the osteoderms of all the examined taxa is congruent with an aquatic or semi-aquatic lifestyle. In general, the osteoderm histology of doswelliids more closely resembles that of phytosaurs and pseudosuchians than that of proterochampsids.
Key words: Archosauria, Doswelliidae, Proterochampsidae, palaeoecology, microanatomy, histology, Triassic, USA.
Denis A. Ponce [denispunrn@yahoo.com.ar], Universidad Nacional de Río Negro, Estados Unidos 750, General Roca, 8332, Río Negro, Argentina.
Ignacio A. Cerda [nachocerda6@yahoo.com.ar], Instituto de Investigación en Paleobiología y Geología (IIPG), Universidad Nacional de Río Negro, Av. Roca 1242, 8332, General Roca, Río Negro, Argentina; Museo “Carlos Ameghino”, Belgrano 1700, Paraje Pichi Ruca (predio Marabunta), Cipolletti, 8324, Río Negro, Argentina.
Julia B. Desojo [julideso@fcnym.unlp.edu.ar], División Paleontología Vertebrados, Museo de La Plata, Paseo del Bosque s/n, La Plata, B1900FWA, Buenos Aires, Argentina.
Sterling J. Nesbitt [sjn2104@vt.edu], Department of Geosciences, Virginia Tech, Blacksburg, 24061, Virginia, USA.
Received 9 May 2017, accepted 21 July 2017, available online 27 October 2017.
Copyright © 2017 D.A. Ponce et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Archosauriformes is a clade of diapsid reptiles that originated by the latest Permian and are extant as birds and crocodylians. Early in archosauriform evolution, osteoderms appeared superficial to the vertebrae and, in some taxa, within the dermis surrounding the limbs. These skeletal elements consist of mineralized organs entrenched within the dermis (Francillon-Vieillot et al. 1990; Vickaryous and Hall 2008). The function, size, shape, external ornamentation, and arrangement of osteoderms vary among archosauriforms (Vickaryous and Sire 2009). Among non-archosaurian archosauriforms, or stem archosaurs, osteoderms are commonly reported in the Doswelliidae and Proterochampsidae (Sues et al. 2013; Trotteyn et al. 2013). Doswelliids were medium size archosauriforms (Doswellia kaltenbachi, ~2 m length) and had a wide distribution across Pangaea (America and Europe) during the Middle and Late Triassic (Sues et al. 2013). The osteoderm arrangement consisted of several rows of osteoderms with pronounced external ornamentation and smooth anterior edges (Sues et al. 2013). The phylogenetic relationship of Vancleavea campi among other non-archosaurian archosauriforms has been historically difficult to assess because of a relatively few identifiable parsimony-informative characters (Long and Murry 1995; Parker and Barton 2008; Nesbitt et al. 2009). Vancleavea has been considered as an aquatic animal with a body (1.2 m in length) completely covered by five different osteoderms morphotypes (Nesbitt et al. 2009). Proterochampsids, which inhabited South America during the Late Triassic (Trotteyn et al. 2013), resemble crocodylians in general morphology (Trotteyn et al. 2013), and their osteoderms consist of a single row on the midline that fused to neural spines (Trotteyn et al. 2013).
Most studies of the histology and microanatomy of archosauriform osteoderms have concentrated on archosaurian groups, such as Phytosauria (Scheyer et al. 2014), Aetosauria (Parker et al. 2008; Cerda and Desojo 2011; Taborda et al. 2013, 2015; Scheyer et al. 2014), “Rauisuchia” (Scheyer and Desojo 2011; Cerda et al. 2013) and Dinosauria (e.g., Scheyer and Sander 2004; Cerda and Powell 2010; Hayashi et al. 2012; Burns and Currie 2014). Osteoderms of Doswelliidae and Proterochampsidae species have been only recently analysed (Scheyer et al. 2014; Cerda et al. 2015).
Here, we further explore and analyse the microstructure of the osteoderms of doswelliids (D. kaltenbachi and V. campi) and one proterochampsid (the holotype of C. bonapartei). The goals of this contribution are to recognize the general histological pattern in osteoderms of non-archosaurian archosauriforms, provide information about the origin and development of the osteoderms in doswelliids and proterochampsids by testing previous hypothesis (e.g., Cerda et al. 2015) and to demonstrate the value of osteoderms for future skeletochronological studies. Finally, we perform the first quantitative analysis of microanatomy in osteoderms of non-archosaurian archosauriforms to provide testable hypotheses about the palaeoecology of stem archosaurs.
Institutional abbreviations.—CPEZ, Coleção Municipal, São Pedro do Sul, Brasil; GR, Ruth Hall Museum of Paleontology at Ghost Ranch, USA; PULR, Museo de Ciencias Naturales de la Universidad Nacional de La Rioja, Argentina; PVL, Instituto Miguel Lillo, Tucumán, Argentina; PVSJ, División Paleontología de Vertebrados, Museo de Ciencias Naturales, Universidad Nacional de San Juan, Argentina; USNM, National Museum of Natural History (formerly United States National Museum), USA.
Other abbreviations.—LAGs, lines of arrested growth.
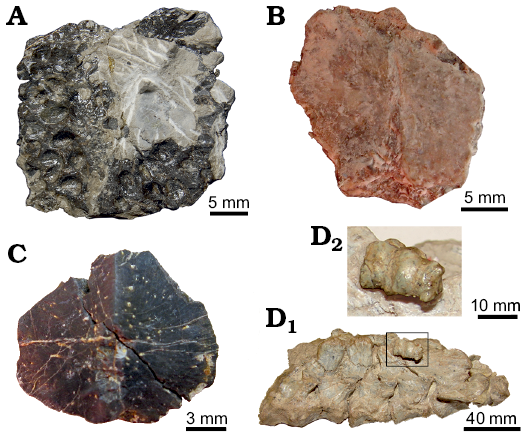
Fig. 1. Osteoderms examined in this study. A. Doswelliid Doswellia kaltenbachi Weems, 1980, USNM PAL 244214 from Near Doswell, Virginia, USA, Carnian. B, C. Doswelliid Vancleavea campi Long and Murry, 1995 from Coelophysis Quarry, “Siltstone Member” (GR 138) and Hayden Quarry, Petrified Forest Member (H4-102-08), Chinle Formation, New Mexico, USA, Early Norian. B. GR 138 (V1). C. H4-102-08 (V2). D. Proterochampsid Chanaresuchus bonapartei Romer, 1971, PULR 07 from La Rioja province, Ladinian–Carnian; D1, general view of a single row of osteoderms associated with presacral vertebrae; D2, detailed view of the sampled osteoderm (box inset in D1). All elements in external view (except D, lateral-medial view), with the anterior margin facing the upper part of the figure.
Table 1. Histological sections of osteoderms referred in the the current study.
|
Taxon |
Specimen |
Section type |
Locality |
Horizon |
References |
|
Doswellia kaltenbachi |
USNM PAL 2214 (holotype) |
transversal, parasagital |
Near Doswell, Virginia, USA |
Poor Farm Member, Falling Creek Formation; Carnian |
Weems 1980; Long and Murray 1995; Desojo et al. 2011; Sues et al. 2013 |
|
Vancleavea campi |
GR 138 and |
transversal |
Coelophysis Quarry (GR 138) and Hayden Quarry (H4-102-08), Ghost Ranch, New Mexico, USA |
“Siltstone Memeber” (GR 138) and Petrified Forest Member (H4-102-08), Chinle Formation; Early Norian |
Long and Murray 1995; Parker and Burton 2008; Nesbitt et al. 2009 |
|
Chanaresuchus bonapartei |
PULR 07 |
transversal, longitudinal |
La Rioja province, |
Chañares Formation; |
Material and methods
For this study osteoderms of Doswellia kaltenbachi, Vancleavea campi, and Chanaresuchus bonapartei were sampled. The identification, locality, and age of these specimens are compiled in Table 1, and the photographs of most osteoderms prior to sectioning are presented in Fig. 1. There is no photographic material of the osteoderms 1 and 2 of the GR 138 specimen. The specimens were to provide into thin sections based on the methodology of Chinsamy and Raath (1992). Transverse sections were obtained from all osteoderms, in addition to some longitudinal and parasagittal sections (Table 1). Two specimens of V. campi were sectioned and, therefore, the successive cross sections are identified as 1, 2 and V1 for GR 138 specimen and V2 for H4-102-08 (uncatalogued, Hayden Quarry, Ghost Ranch, New Mexico, USA) specimen. In this survey, we follow the recent phylogenetic analysis of Ezcurra (2016), in which this taxon is falls within Doswelliidae. The histological sections were made in the Departamento de Geología from Universidad Nacional de San Luis(Argentina), the Centro Regional de Investigaciones de La Rioja (CRILaR) (Argentina), and the American Museum of Natural History from New York (USA). The sections were analysed under a petrographic microscope (Nikon E200 Pol) under plane and cross-polarized light (for the latter, different types of filters where also employed). We estimated the percentage of area occupied by compact and cancellous bone in the cross sections. For this, we use the Windows version of ImageJ (Rasband 2003). Osteoderm compactness was calculated using the Windows version 4.5.5 of Bone Profiler Software (Girondot and Laurin 2003). Compactness observed (CO) makes reference to proportion (values between 0–1) of cross-sectional area of an osteoderm that includes compact bone. To prepare the images for the Bone Profiler Software, images of the sectioned bones were transformed into black and white (black for bone and white for vascular spaces) (Fig. 2). In addition, the newly sampled osteoderms of Doswellia kaltenbachi, Vancleavea campi, and Chanaresuchus bonapartei, we perform a compactness analysis of previously sectioned proterochampsid and doswellid osteoderms studied by Cerda et al. (2015), including: Archeopelta arborensis (CPEZ 239a), Tarjadia ruthae (PULR 063), Pseudochampsa ischigualastensis (PVSJ 567), and Chanaresuchus bonapartei (PVL 6244). The results of these analyses are compiled in Table 2.
Table 2. Comparison of observed compactness (CO) and life-styles among Archosauriformes and several Reptilia taxa.
|
Taxon |
Specimen |
Life-style |
CO |
References |
Age |
|
Doswelliidae |
|||||
|
Doswellia kaltenbachi |
USNM PAL 244214 |
aquatic-semiaquatic |
0.82 |
this study |
Carnian |
|
Vancleavea campi |
GR 138 (1) |
aquatic-semiaquatic |
0.85 |
Early Norian |
|
|
Vancleavea campi |
GR 138 (2) |
aquatic-semiaquatic |
0.5-0.54 |
||
|
Vancleavea campi |
GR 138 (V1) |
aquatic-semiaquatic |
0.75 |
||
|
Vancleavea campi |
H4-102-08 (V2) |
aquatic-semiaquatic |
0.72 |
||
|
Archeopelta arborensis |
CPEZ 239a |
aquatic-semiaquatic |
0.86 |
Ladinian– |
|
|
Tarjadia ruthae |
PULR 063 |
aquatic-semiaquatic |
0.88 |
||
|
Proterochampsidae |
|||||
|
Chanaresuchus bonapartei |
PULR 07 |
aquatic-semiaquatic |
0.93 |
this study |
Ladinian– |
|
Chanaresuchus bonapartei |
PVL 6244 |
aquatic-semiaquatic |
0.76 |
||
|
Pseudochampsa ischigualastensis |
PVSJ 567 |
aquatic-semiaquatic |
1 |
Carnian |
|
|
Pareiasauria |
|||||
|
Bradysaurus sp. |
SAM-PK-12140 |
? |
0.64 |
Permian |
|
|
Pareiasaurus serridens |
SAM-PK-10036 |
? |
0.70 |
||
|
Pareiasaurus sp. (smaller osteoderm) |
SAM-PK-1058 |
? |
0.69 |
Late Permian |
|
|
Pareiasaurus sp. (larger osteoderm) |
SAM-PK-1058 |
? |
0.64 |
||
|
Anthodon serrarius (thick osteoderm) |
SAM-PK-10074 |
? |
0.73 |
||
|
Anthodon serrarius (thin osteoderm) |
SAM-PK-10074 |
? |
0.74 |
||
|
Lepidosauria |
|||||
|
Pseudopus apodus |
PIMUZ A ⁄ III 1280 |
terrestrial |
0.98 |
Recent |
|
|
Tiliqua scincoides |
PIMUZ A ⁄ III 1281 |
terrestrial |
0.97 |
||
|
Testudines |
|||||
|
cf. Hesperotestudo (flat osteoderm) |
TMM 30967-1010.1 |
terrestrial |
0.92 |
Miocene– |
|
|
cf. Hesperotestudo (spiked osteoderm) |
TMM 30967-1010.2 |
terrestrial |
0.79 |
||
|
Crocodylomorpha |
|||||
|
Steneosaurus sp. |
NMS 7152 |
marine |
0.97 |
Upper Jurassic |
|
|
Alligator mississippiensis |
SMNS 10481b |
aquatic-semiaquatic |
0.84 |
Recent |
|
|
cf. Diplocynodon |
IPB R144 ⁄ 1 |
aquatic-semiaquatic |
0.77 |
Eocene–Miocene |
|
|
Ankylosauria |
|||||
|
Ankylosauridae indet. |
TMP 85.36.218 ⁄ 1 |
terrestrial |
0.89 |
Upper Cretaceous |
|
|
Nodosauridae indet. |
TMP 67.10.29 |
terrestrial |
0.71 |
||
We use the term “external” to refer the portion of the osteoderm oriented toward the body surface and “basal” for the portion that is oriented toward the interior of the organism (Scheyer and Sander 2004). These terms are synonyms of “distal/proximal” (Main et al. 2005), “superficial/deep” (Hill and Lucas 2006; Hill 2010). Also, we use the term “marginal” cortex to refer the lateral and medial regions of the osteoderms. For the terminology of growth marks, we follow the nomenclature proposed by Francillon-Vieillot et al. (1990). In this regard, the term “zone” refers to thick layers of woven or parallel fibered bone tissue. If the bone is vascularised, vascular spaces are more abundant or exclusively present in the zones. The term “annuli” refers to avascular or poorly vascularised layers of bone tissue, commonly narrower than the zones. The intrinsic fibres of the annuli always exhibit a higher degree of arrangement than in the zones, forming usually a parallel fibered or lamellar matrix.
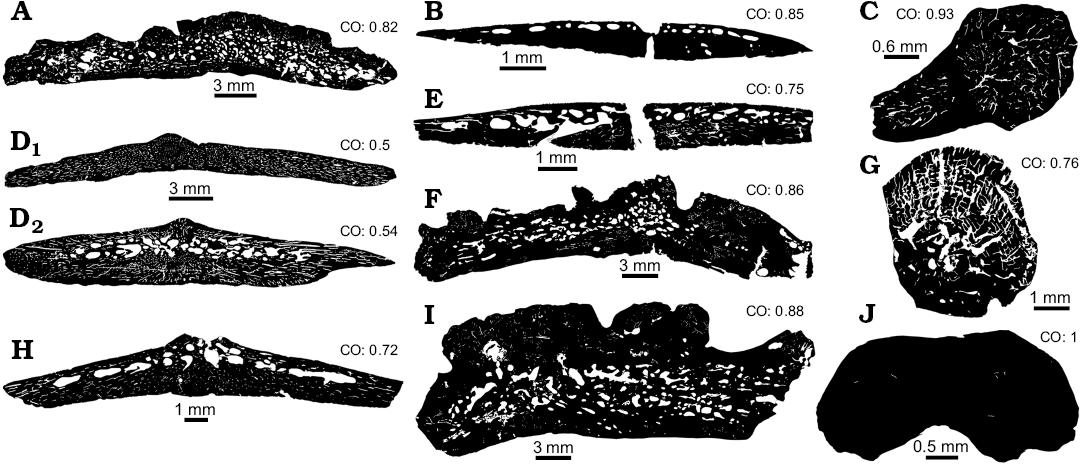
Fig. 2. Compactness degree obtained from all osteoderms of examined taxa and comparison with others Doswellidae and Proterochampsidae taxa. A. Doswelliid Doswellia kaltenbachi Weems, 1980, USNM PAL 244214 from Near Doswell, Virginia, USA, Carnian. B, D, E, G. Doswelliid Vancleavea campi Long and Murry, 1995 from Coelophysis Quarry, “Siltstone Member” (GR 138) and Hayden Quarry, Petrified Forest Member (H4-102-08), Chinle Formation, New Mexico, USA, Early Norian. B. GR 138 (1). D. GR 138 (2A, D1; 2B, D2). E. GR 138 (V1). G. H4-102-08 (V2). C, F. Proterochampsid Chanaresuchus bonapartei Romer, 1971 from La Rioja province, Ladinian–Carnian. C. PULR 07. F. PVL 6244. H. Doswelliid Archeopelta arborensis Desojo, Escurra, and Schultz, 2011, CPEZ 239a from São Pedro do Sul, Ladinian–Carnian. I. Proterochampsid Pseudochampsa ischigualastensis Trotteyn, Martínez, and Alcober, 2012, PVSJ 567 from San Juan province, Carnian–Norian. J. Doswelliid Tarjadia ruthae Arcucci and Marsicano, 1998, PULR 063 from La Rioja province, Ladinian–Carnian. As seen, all indexes are equal or upper to 0.5, which means all osteoderms are composed by at least 50% of compact bone. CO, compactness observed.
Results
Doswelliidae.—Doswellia kaltenbachi: The elements are wider than thick (~1/5) and have a trilaminar structure (diplöe), in which two distinct cortices (external and basal) of compact bone can be differentiated from an internal core of cancellous bone (Fig. 3A). The basal surface texture is smooth and convex in transverse section. The external surface exhibits a distinct pattern of valleys (concave surface) and ridges (convex surface). The internal core region of cancellous bone is thicker than the basal and external regions. The obtained compactness for this element is 0.82.
The basal cortex consists mostly of zonal bone, in which annuli and zones are observed in the complete extension of basal surface. A maximum of 13 annuli/zones are observed in the basal region (Fig. 3B). The zones consist of poorly vascularized parallel-fibred bone whereas the annuli are composed by avascular lamellar bone. Vascular canals in zones are longitudinally and circumferentially oriented and they become less abundant toward the outer surface. Sharpey’s fibres are absent in the basal cortex (Fig. 3B).
The internal core is composed of cancellous bone with short and thin trabeculae in comparison with other doswelliids (Archeopelta arborensis, Tarjadia ruthae; see Cerda et al. 2015) (Fig. 3C, D). The intertrabecular spaces exhibit a roughly constant diameter. The trabeculae are formed by secondary lamellar bone tissue surrounding the intertrabecular spaces. Remains of primary bone tissue are present within some trabeculae and they are mainly composed of woven-fibered bone (Fig. 3C, D).
The external cortex is almost entirely composed of primary bone tissue and consists of zonal bone (Fig. 3E). A total of 13 annuli are distributed throughout the compacta, and follow the sculpturing pattern of the external surface (Fig. 3E, F). A resorption line is only observed in a small portion of the anterior edge of the plate. Sharpey’s fibres are absent.
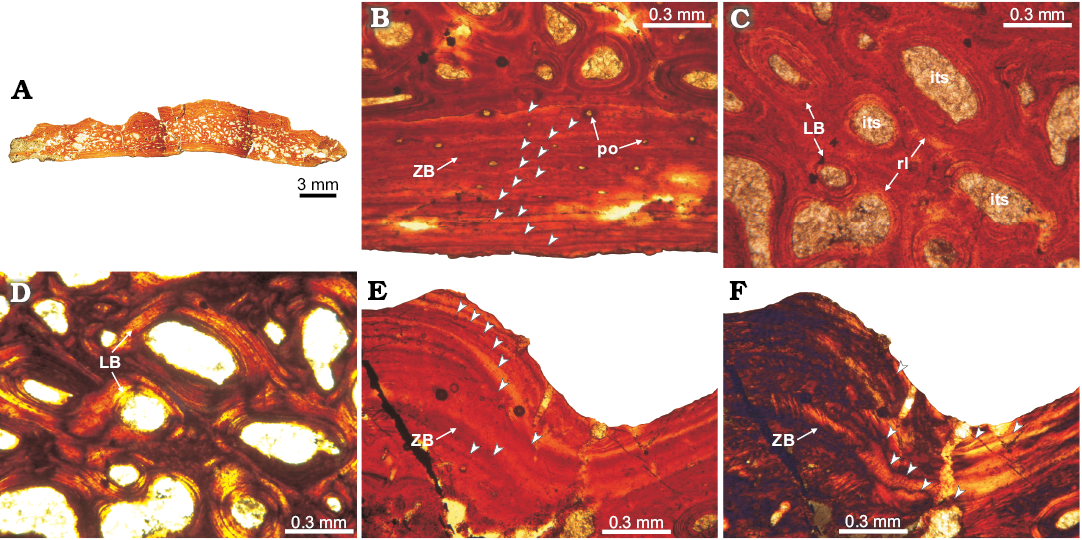
Fig. 3. Osteoderm histology of doswelliid Doswellia kaltenbachi Weems, 1980, USNM PAL 244214 from near Doswell, Virginia, USA, Carnian. A. Complete transversal section view; the deep excavations correspond with natural fractures of the element. B. Detail of the basal cortex; exhibits poorly vascularized primary zonal bone (arrowheads pointing to areas of parallel-fibered bone and annuli of lamellar bone). C, D. View of the internal core region; showing abundant intertrabecular spaces and vascular cavities, surrounded by secondary lamellar tissue and remains of woven-fibered bone of primary origin. E, F. Detail of the ornamentation in external cortex; consisting of poorly vascularized zonal bone; arrowheads pointing to growth marks (annuli) following the sculpted shape of the external surface. Note the absence of Sharpey’s fibres. A, B, C, E, normal light; D, F, cross-polarized light. Abbreviations: ZB, zonal bone; LB, lamellar bone; its, intertrabecular space; po, primary osteon; rl, resorption line.
Vancleavea campi: Because several osteoderms of at least two individuals of V. campi have been sampled, we describe them together and point out the peculiarities of each specimen. All of the elements are wider than thick (~1/5). Some transverse cross sections have different shapes because the shapes of the osteoderms vary. The osteoderms exhibit a trilaminar structure (diplöe), with a predominance of compact bone tissue (Figs. 4A, F, 5A1, A2, 6A). The external surface of some elements exhibits a small dorsal eminence in the center of the osteoderm (Figs. 5A1, A2, 6A). The basal cortex is thicker than the external one, and, in some cases, this cortex occupies more than half of the complete osteoderm thickness. The osteoderms are more vascularized compared to those of those of D. kaltenbachi. In general, the sampled bones exhibit high values of compactness. The compactness values obtained from the samples of GR 138 are: 0.82 (1), 0.5–0.54 (2) and 0.75 (V1). In specimen H4-102-08 (V2), the obtained compactness is 0.72.
The basal cortices exhibit variable microstructure among the sampled osteoderms. In osteoderms 1 and 2 of GR 138 specimen, the basal cortex is entirely composed of parallel-fibered bone (Figs. 4G, H, 5B, D). Although the intrinsic fibres of the parallel fibered bone are commonly oriented parallel to the basal surface, their arrangement is variable with regard to the sagittal axis of the osteoderm. In this regard, intrinsic fibres are parallel to the osteoderm sagittal axis along its midline, but their orientation changes nearer their lateral margins where they are oriented perpendicular to the sagittal axis of the element. This change is not only evidenced by a variation in the optical properties of the bone (monorefringent near the core, birefringent toward the margins), but also by a change in the shapes of the lacunae, which are roughly circular near the core and elongated toward the margins (Fig. 4H, J). Specimens GR 138 (V1) and H4-102-08 (V2) exhibit bundles of mineralized structural fibres (Figs. 4C, D, 6B, C, E, F). Where the bundles of mineralized structural fibres are present, they are either parallel or perpendicular to the basal surface. Those bundles arranged parallel to the surface are distributed in layers, which form a stratified pattern. In general, the basal cortex in all the sampled osteoderms is poorly vascularized, exhibiting a few radial canals and some primary osteons, which become more abundant toward both margins. Sharpey’s fibres are only clearly recorded in the basal cortex of specimen GR 138 (2) (Fig. 5C, D). These extrinsic fibres are oriented perpendicular to the basal surface at the midline of the element, but they become parallel to the surface toward the margins (Fig. 5C, D). Growth marks are not present.
The internal core exhibits large vascular spaces (usually greater than 0.05 mm), which are most abundant in GR 138 (2) (Fig. 5F). The intertrabecular spaces in this sample have an irregular shape and are continuous with vascular spaces oriented from the core to the margins. The tissue of the core in all the samples consists mainly of primary lamellar or parallel-fibered bone. Lamellar bone is surrounding vascular canals and resorption cavities (Figs. 4B, 5F–H, 6D, E). Interstitial remains of fibrolamellar tissue are visible in some osteoderms (Fig. 6E). The non-remodelled areas of the core exhibits small (usually smaller than 0.03 mm), simple canals (mostly radially oriented), and primary osteons. Also, near the external surface, a set of larger vascular spaces (some remodelled) and intertrabecular spaces are found, and these extend within the osteoderm towards the margins. In GR 138 (2), circular annuli are present in the central region of the core and also toward the marginal regions (Fig. 5E, F). We count a maximum of 13 annuli in the internal core region (Fig. 5G, H).
There is not a distinct boundary between the core and the external cortex in the osteoderms of Vancleavea campi (Figs. 4B, G, 6D). The external cortex appears to be only a thin layer (less than 0.3 mm) (Figs. 4B, G, 6D), except in GR 138 (2) in which this layer is thicker than in other osteoderms (Fig. 5G, H). The external cortex consists of poorly vascularized lamellar bone. Sharpey’s fibres in the external cortex are only present in GR 138 (2), and these fibres are oriented vertically to external surface (Fig. 5C, D). No growth marks are recorded in the external cortex.
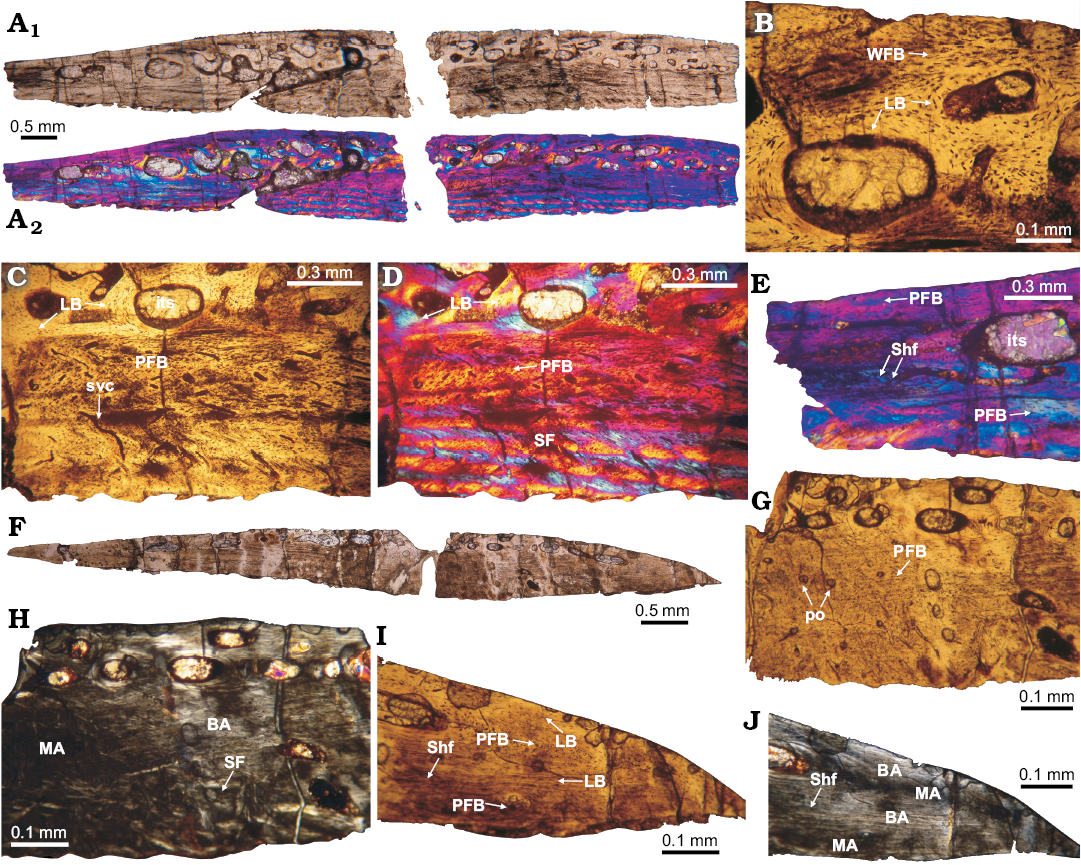
Fig. 4. Histology of osteoderms V1 (A–E) and 1 (F–J) of doswelliid Vancleavea campi Long and Murry, 1995, GR 138 from Coelophysis Quarry, “Siltstone Member”, Chinle Formation, New Mexico, USA, Early Norian. A. Complete transversal section under normal light (A1) and cross-polarized with lambda compensator (A2). Note the differences in color layers on the osteoderm with the lambda filter, indicating the presence of structural fibers. B, C. Details of the basal cortex constituted by structural fibers. D. Inner core region, close to external cortex, showing intertrabecular spaces and fibrolamellar bone matrix. E. Detail of the marginal region, which is mostly composed of parallel-fibered bone with abundant Sharpey’s fibers are inserted. F. Complete transversal section view. G, H. Details of the medial region; some structural fibers are faintly visible in the basal cortex, mixed with parallel-fibered bone. Parallel-fibered bone is present in the internal region. I, J. View of the marginal region osteoderm; showing some isolated Sharpey fibers and compact bone composed by both lamellar and parallel-fibered bone. Note how clearly differentiated monorefringent and birefringent area are present. A–C, F, G, I, normal light; A, D, E, normal light and cross-polarized light with lambda compensator; H, J, cross-polarized light. Abbreviations: BA, birefringent area; its, intertrabecular space; LB, lamellar bone; MA, monorrefringent area; PFB, parallel-fibered bone; po, primary osteon; SF, structural fibers; Shf, Sharpey fibers; svc, simply vascular canal.
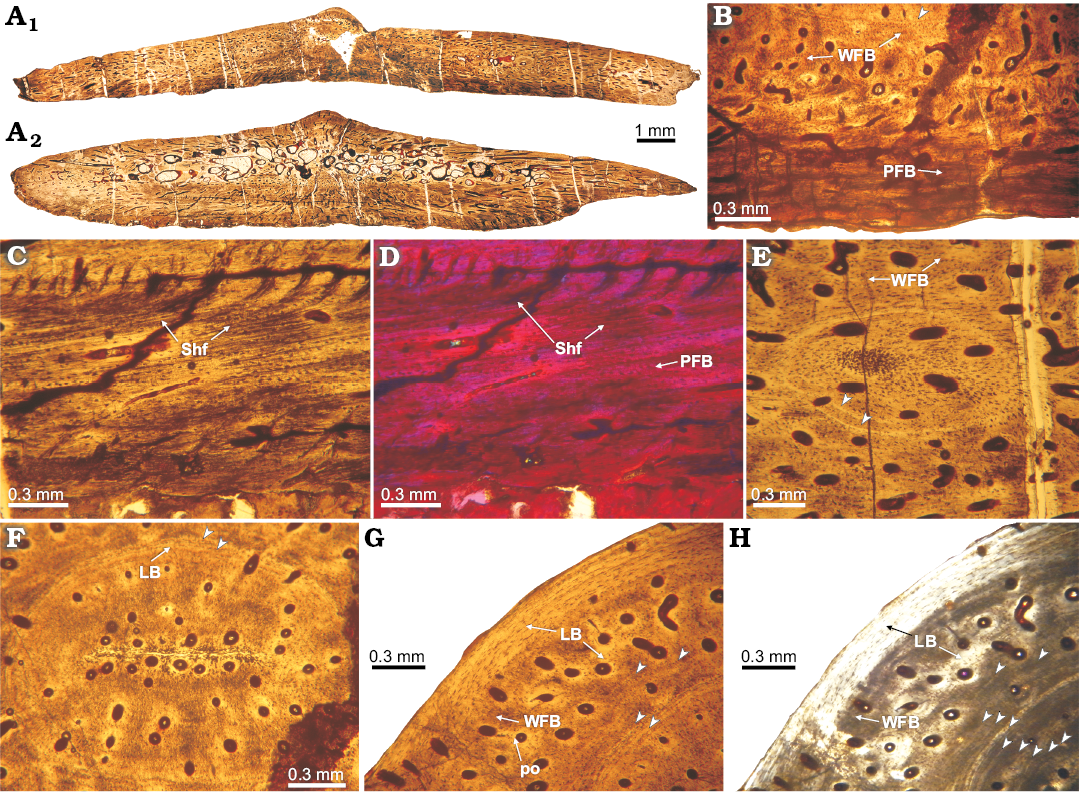
Fig. 5. Histology of osteoderm 2 of doswelliid Vancleavea campi Long and Murray 1995, GR 138 from Coelophysis Quarry, “Siltstone Member”, Chinle Formation, New Mexico, USA, Early Norian. A. Complete transversal section (2C, A1; 2B, A2). B. Detail of basal cortex, constituted by parallel-fibered bone; showing some lines of arrested growth (LAGs) and fibrolamellar bone toward core region. C, D. Detail of the basal cortex (close to the margins); with multiple Sharpey fibers in the parallel-fibered bone. E. View to the annuli with circular shape, on the lateral portions of the osteoderm, composed by lamellar tissue. F. Detail of the inner core region; showing the presence of a concentric annulus (formed by lamellar tissue) and highly vascularized primary tissue enclosed by the annuli. G, H. Detail of the external cortex on the central (i.e., midline) portion of the osteoderm, whereas the outermost portion consists of lamellar bone, the inner part is formed by fibrolamellar. Note arrowheads pointing to growth marks (annuli). A–C, F, G, normal light; H, cross-polarized light; D, cross-polarized light with lambda compensator. Abbreviations: LB, lamellar bone; PFB, parallel-fibered bone; Shf, Sharpey’s fibers; WFB, woven-fibered bone.
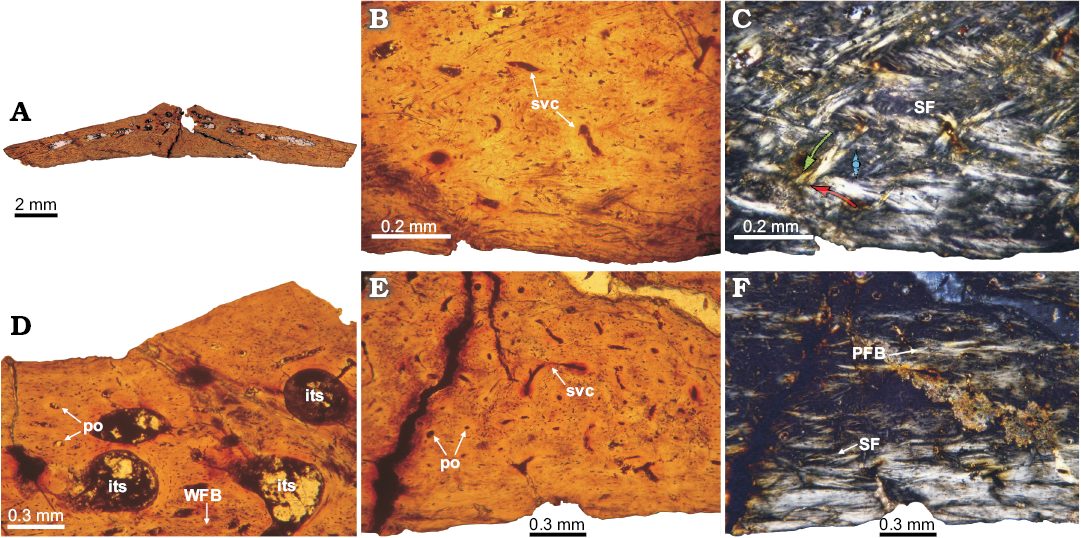
Fig. 6. Histology of osteoderm V2 of doswelliid Vancleavea campi Long and Murray 1995, H4-102-08 from Hayden Quarry, Petrified Forest Member, Chinle Formation, New Mexico, USA, Early Norian. A. Complete transversal section view. B, C. Detail of the basal cortex, which is poorly vascularized with some simple vascular spaces. Structural fibers are best observed under cross-polarized light. The arrows in C indicate the orientation of the fiber bundles. D. Detail of the external cortex and vascular spaces of the inner region. E, F. Detail of the basal cortex toward the marginal zones. In these areas, the presence of structural fibers is not as clear. A, B, D, E, normal light; C, F, cross-polarized light. Abbreviations: LB, lamellar bone; PFB, parallel fibered bone; po, primary osteon; re, resorption space; SF, structural fibers; svc, simply vascular canal; WFB, woven-fibered bone.
Proterochampsidae.—Chanaresuchus bonapartei: The sampled osteoderm was extracted from the midline, just dorsal to third cervical vertebra. Overall the osteoderm is small (length 3.8 mm) with a distinctive globular shape. A distinct groove on the basal surface, similar to the described for other proterochampsids (e.g., Cerda et al. 2015), is present in transverse section. The element exhibits a compact structure (compactness equal 0.76) and is poorly vascularized with circumferential canals (Fig. 7). The longitudinal section (Fig. 7A) is the best preserved and this section and exhibits a remnant of another osteoderm, which lies on the external surface of the completely preserved osteoderm.
The bone tissue is homogeneous and there is no a distinction between external, core, and basal regions. Compact bone is composed mainly of parallel-fibred bone tissue. In some areas, especially the core of the osteoderm, woven-fibred bone is present. This primary bone is poorly vascularized with simple canals, which are circumferentially arranged. Vascular spaces are usually smaller than 0.02 mm in diameter. We counted a maximum of 8 lines of arrested growth (LAGs) (Fig. 7B, C). Sharpey’s fibres are abundant and consist of large (larger than 1 mm in some instances) and compact sets (extending from the outermost portion toward the center of element) mostly of them oriented radially. In some cases, the extrinsic fibres are so abundant that they tend to blur the arrangement of the intrinsic fibres (Fig. 7D–F). At this point, we need to make a distinction between a complete osteoderm and a piece or rest of another osteoderm. As showed in the Fig. 7A, the incomplete osteoderm is located over the posterior half of the complete osteoderm, above its external surface (i.e., imbricated). We note that Sharpey’s fibres tend to be more abundant in the area of contact between osteoderms (Fig. 7D, E).
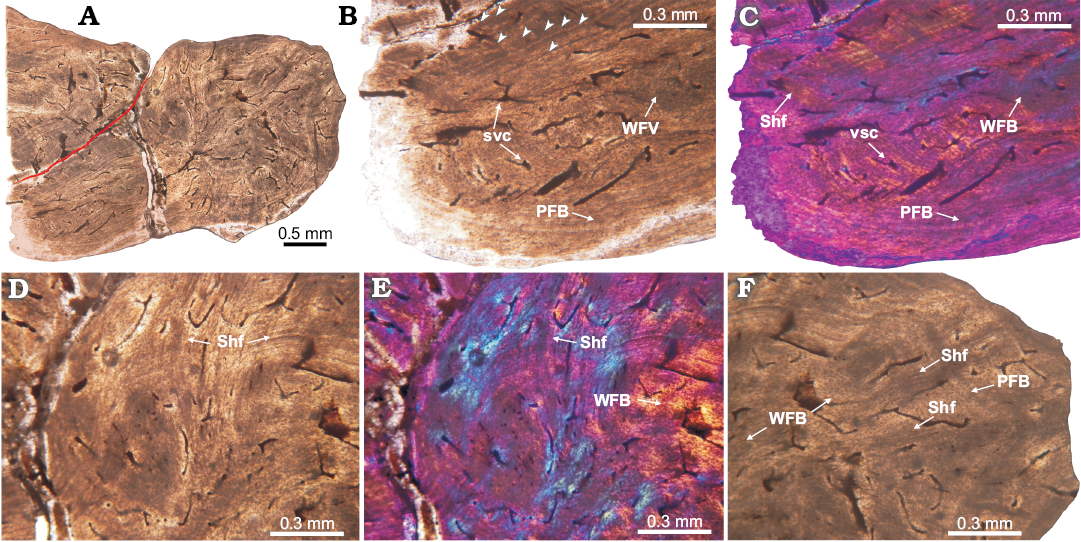
Fig. 7. Histology of proterochampsid Chanaresuchus bonapartei Romer, 1971, PULR 07 osteoderm from La Rioja province, Ladinian–Carnian. A. Complete longitudinal section view. Note the poorly vascularization. Red line indicates separation between the complete osteoderm and the rest of another. B, C. Detail of basal cortex. It is constituted in the outermost cortex by parallel-fibered bone and toward the interior of osteoderm, it acquires the features of woven-fibered bone. Multiple growth marks and some Sharpey fibers are observed. D, E. View of the upper portion of osteoderm. The abundance of Sharpey fibers prevents clear observance of the bone matrix, which appears to be parallel-fibered bone. Some LAGs can be tracked also. F. Detail of the marginal region. It is formed in its outer region by lamellar bone and in the perimedular portion by woven-fibered bone. Large and dense bundles of Sharpey fibers are observed. Some LAGs are also present. Arrowheads point to growth marks (LAGs). A, B, D, F, normal light; C, E, cross-polarized light with lambda compensator. Abbreviations: PFB, parallel-fibered bone; Shf, Sharpey fibers; svc, simple vascular canal; WFB, woven-fibered bone.
Histogenesis.—Several ossification mechanisms for osteoderms of extinct and extant archosauriforms have been proposed. The most common mechanism among these taxa is metaplastic ossification (Haines and Mohuiddin 1968). In this process, the ossification carries on a particular differenced tissue (e.g., tendinous tissue), which transform into bone tissue. Metaplastic ossification has been reported in temnospondyl amphibians (Witzmann and Soler-Gijon 2008), extant anurans (Ruibal and Shoemaker 1984), extant squamates (Zylberberg and Castanet 1985; Levrat-Calviac and Zylberberg 1986), extinct and extant archosaurs (Reid 1996; Scheyer and Sander 2004; Main et al. 2005; Vickaryous and Hall 2008; Cerda et al. 2015) and dermal bones of the turtle shell (Barrett et al. 2002; Scheyer and Sanchez-Villagra 2007; Scheyer and Sander 2007; Scheyer et al. 2007, 2008; Sterli et al. 2013). Metaplastic ossification in osteoderms can be identified by the presence of bundles of mineralized collagen bundles, which represent the primary (unmineralized) tissue and interweave in different orientations (i.e., structural fibres; Scheyer and Sander 2004, 2007; Main et al. 2005; Witzmann and Soler-Gijón 2008; Cerda and Powell 2010).
Intramembranous ossification is another type of ossification mechanism. This process includes a periosteal layer replacing existing non-cartilage connective tissue. This mechanism is recognized by the absence of structural fibres and has been proposed as a process for osteoderm development in extinct taxa, including some basal tetrapods (Buchwitz et al. 2012), pareiasaurs (Scheyer and Sander 2009), aetosaurs (Cerda and Desojo 2011; Scheyer et al. 2014), Revueltosaurus (Scheyer et al. 2014), and doswelliids and proterochampsids (Scheyer et al. 2014; Cerda et al. 2015).
A third process of ossification is present only in placodont osteoderms and consists of the formation of osseous tissue from a fibro-cartilaginous precursor tissue (Scheyer 2007). It is possible that certain taxa could have a combination of two or more different kind of ossification systems (Reid 1996; Vickaryous and Hall 2008; Buffrénil et al. 2011; Scheyer and Desojo 2011; Cerda et al. 2013).
Intramembranous ossification is proposed as the main mechanism of osteoderm formation in Doswellia kaltenbachi given the absence of structural fibres, as in other doswelliids such as Archeopelta arborensis and Tarjadia ruthae (Cerda et al. 2015). Nevertheless, because remodelling is present in the core, possible metaplastic tissue (i.e., structural fibres) formed early during the ossification process of the element could have been resorbed. Therefore, a metaplastic process, at least during the early phases of osteoderm formation, can not be entirely discarded as a possibility based on the current data. A possible intramembranous process of formation is consistent with the previous hypothesis for doswelliid osteoderm origin (Scheyer et al. 2014; Cerda et al. 2015). On the other hand, the presence of structural fibres in the basal cortex of some osteoderms of Vancleavea campi suggests a metaplastic process of ossification that occurred during the growth of some elements. Because structural fibres of V. campi are only found in a particular region of some osteoderms (i.e., basal cortex), it is possible that the development of these osteoderms includes intramembranous ossification, with later incorporation of collagenous fibres from the dermis by metaplasia. These results suggest that if V. campi belongs within Doswelliidae (sensu Ezcurra 2016), this mode of osteoderm formation exhibits more variation than that reported for other members of the clade (i.e., Tarjadia, Archeopelta, Jaxtasuchus, and Doswellia).
In Chanaresuchus bonapartei, the absence of structural fibres indicates that metaplastic ossification is not involved in the development of the osteoderms (Vickaryous and Hall 2008). Moreover, we infer an intramembranous origin for these osteoderms because the element is entirely composed of primary bone tissue (i.e., the bone tissue that formed first during the formation of the element is still preserved). In other words, secondary remodelling (that could erode hypothetical metaplastic bone) is absent in all sampled osteoderms. This interpretation agrees with the results of previous studies of proterochampsid (Cerda et al. 2015). Evaluating this interpretation in a broader phylogenetic context, an intramembranous origin of osteoderms could be the plesiomorphic condition for archosaurs because the metaplastic origin of the osteoderms has only been reported for pseudosuchians (e.g., Crocodylia and “Raiusuchia”) and dinosaurs (e.g., Thyreophora and Titanosauria) (Scheyer and Sander 2004; Vickaryous and Hall 2008; Cerda and Powell 2010; Scheyer and Desojo 2011).
Skeletochronology.—Growth marks (i.e., annuli/LAGs) within the osteodermal tissues indicate a slowdown or a complete interruption of bone deposition during the growth of the individual. Because the formation of these growth marks correspond with annual cycles, they have been used to estimate relative or absolute age in extinct and extant tetrapod osteoderms (e.g., Erickson and Brochu 1999; Ricqlés et al. 2003; Hill and Lucas 2006; Parker et al. 2008; Witzmann and Soler-Gijón 2008; Hill 2010; Witzmann 2009; Scheyer and Sander 2009; Witzmann 2010; Cerda and Desojo 2011; Scheyer and Desojo 2011; Taborda et al. 2013; Scheyer et al. 2014; Cerda et al. 2015).
Because of the presumption that growth marks have been deposited annually, the estimated minimum age at death for the studied specimens is 13 years old for Doswellia kaltenbachi, 13 years old for Vancleavea campi (osteoderm 2 of GR 138), and 8 years for Chanaresuchus bonapartei (PULR 07). In comparison with other doswelliids osteoderms, the age of D. kaltenbachi (USNM PAL 2214; 13 years) and V. campi (GR 138 (2); 13 years) is similar to the estimate for Archeopelta arborensis (CPEZ 239a; 11 years) (Cerda et al. 2015), older than Tarjadia ruthae (PULR 063; 20 years) (Cerda et al. 2015) and longer than Jaxtasuchus salomoni (SMNS 81902; 8 years) (Scheyer et al. 2014). The estimated age at death of the holotype of Chanaresuchus bonapartei (PULR 07; 8 years) is younger than other sampled proterochampsids, such as Pseudochampsa ischigualastensis (PVSJ 567; 20 years), but the same as C. bonapartei (PVL 6244; 8 years) (Cerda et al. 2015).
An unexpected growth mark distribution was recorded in one of the Vancleavea campi specimens (GR 138, 2A, C; Fig. 5E–H). Although the growth marks in some parts of the osteoderm exhibits the typical concentric arrangement, such arrangement is not maintained in all the samples (Fig. 5E, F). It is possible that the particular distribution of growth marks on some V. campi osteoderms is the result of the specific shape of each osteoderm morphotype, as with histology of some alligatoroid osteoderms (Burns et al. 2013). As previously reported, five different types of osteoderms cover the body of V. campi (Nesbitt et al. 2009). Several of the morphotypes have pointed projections and these projections could have been presented throughout much of the growth history of the osteoderm. Thus, is likely the annuli observed in the central area and lateral regions of these osteoderms may represent growth marks formed around the projections during the element formation.
Ornamentation in Doswellia kaltenbachi.—Ornamentation (or sculpturing) on the external surface of osteoderms is a common feature found in many tetrapods (e.g., Vickaryous and Sire 2009; Witzmann 2009; Cerda et al. 2015). Usually, the anatomy of this ornamentation consists of ridges, tubercles, and pits (e.g., Buffrénil et al. 2016). Two main mechanisms have been hypothesized to be responsible for the development and maintenance of external ornamentation in osteoderms. The first invokes local resorption and partial redisposition of the cortical bone. In extinct taxa (e.g., Archeopelta arborensis CPEZ 239a; Cerda et al. 2015), this process is inferred by the presence of distinct resorption lines within external cortex. This mechanism has been described in several archosauriforms such as dinosaurs, some doswelliids, pseudosuchians (e.g., Aetosauria, Phytosauria, Crocodylomorpha) (Buffrénil 1982; Hua and Buffrénil 1996; Scheyer and Sander 2004; Cerda and Desojo 2011; Scheyer et al. 2014; Cerda et al. 2015), and testudines (Buffrénil et al. 2016; Jannello et al. 2016). The second proposed mechanism consists of bone deposition external to areas with ridges or tubercles, without much resorption. This process is recognized by the presence of growth marks which follow the external sculpturing of the external cortical tissue, formed by primary bone, and also for the absence of reabsorbed areas. This type of ornamentation is observed in basal tetrapods, but also in some archosaurs such as early diverging loricatans (e.g., Prestosuchus chiniquensis) and the notosuchian crocodyliform Simosuchus clarki (Witzmann and Soler-Gijón 2008; Witzmann 2009; Buffrénil et al. 2016).
The condition of osteoderms from Doswellia kaltenbachi is peculiar, because of clear differences with other known doswelliids. Archeopelta arborensis, Tarjadia ruthae, and Jaxtasuchus salomoni developed their osteoderm ornamentation by resorption and redeposition processes (Cerda et al. 2015). Instead, the osteoderms of D. kaltenbachi (Fig. 3E, F) lack resorption lines and exhibit growth marks, which follow the ridge and pit ornamentation of the external cortex. These features are congruent with a preferential deposition process, unique among doswelliid osteoderms described so far. This observation contrasts with Scheyer et al. (2014), who claimed that preferential deposition is only observed in osteoderms with a smooth external ornamentation (e.g., “Rauisuchia”). However, evidence of two sculpturing mechanisms is present in doswelliid osteoderms. Vancleavea campi has no ornamentation on the external cortex, even though it has been grouped within the Doswelliidae (Ezcurra 2016; but see Desojo et al. 2011 for a different hypothesis).
Bone compactness and lifestyle.—The study of the compactness of bony elements has been usually employed to infer lifestyle, as in appendicular bones and osteoderms (e.g., Ricqlés and Buffrénil 2001; Houssaye 2009). For example, osteoderms of aquatic organisms exhibits an increase in the relative amount of compact bone tissue, having more dense and massive elements (pachyostotic sensu lato; Houssaye 2009) than those of terrestrial organisms. This morphology was interpreted as a functional feature to reduce buoyancy control and hydrostatic regulation of body trim (Taylor 2000; Ricqlés and Buffrénil 2001; Houssaye 2013).
The osteoderms of the doswelliids Doswellia kaltenbachi and Vancleavea campi exhibits a trilaminar structure, but are compact structures. Comparison with other doswelliids (Table 2, Fig. 7) reveals that this clade is characterized by high degrees of compactness in their osteoderms and this is what we also found here with the sampling of new taxa and specimens. This inference agrees with a previously proposed hypothesis for doswelliids palaeoecology, which was based on morphological features in the skull and postcrania and in appendicular histological tissues (e.g., femur of V. campi; Nesbitt 2011; Sues et al. 2013).
In the case of proterochampsids, all the sampled osteoderms are characterized by a high compactness, which is highest in Pseudochampsa ischigualastensis. Such high compactness can be interpreted as evidence for an aquatic or semi-aquatic lifestyle for this taxa following previous interpretation of other aquatic taxa (Trotteyn et al. 2013). Previous studies based on morphological analyses arrived at the same inference (Trotteyn et al. 2013). However, and according to Cerda et al. (2015), the small size and number of osteoderms (i.e., a single dorsal row) suggests that a buoyancy function for these elements seems rather unlikely. Also, it was proposed that high compactness in small osteoderms can be the result of the absence of space to develop cancellous bone (Scheyer and Sander 2009). This hypothesis can be used to explain the high compactness reported in proterochampsids osteoderms, for the anteroposterior length is commonly less than 10 mm.
It is worth to note that the high degree compactness of doswelliids and proterochampsids is also similar to some terrestrial reptiles, such as some lepidosaurs, testudines, and ankylosaurs (Table 2; Scheyer and Sander 2009). According to Scheyer and Sander (2009), the association of osteoderms compactness and lifestyle is still controversial in vertebrates and all the inferences obtained on this regard are only tentative.
Comparison with other Archosauriformes.—Recently, the osteoderms histology and microanatomy of several archosauriforms was reported and abundant data is available for comparison (e.g., Scheyer and Sander 2004; Hill and Lucas 2006; Parker et al. 2008; Vickaryous and Hall 2008; Hill 2010; Klein et al. 2009; Vickaryous and Sire 2009; Cerda and Desojo 2011; Scheyer and Desojo 2011; Cerda et al. 2013; Filippi et al. 2013; Scheyer et al. 2014; Cerda et al. 2015). The general microstructure and histological features of Doswellia kaltenbachi are similar to that reported for Archeopelta arborensis, Tarjadia ruthae, and Jaxtasuchus salomoni (Cerda et al. 2015). In this regard, a trilaminar structure with thick cortical bone is common in the four taxa. However, D. kaltenbachi represents a different mode in the origin of the external ornamentation. Such a mechanism consists of preferential deposition of bone tissue in the ridges of the external sculpturing. Conversely, A. arborensis, T. ruthae, and J. salomoni exhibit features in sculpturing compatible with local resorption and redeposition (Cerda et al. 2015). Other features recorded in D. kaltenbachi are similar to aetosaurs and phytosaurs, including trilaminar structure, strong ornamentation, and basal cortex formed of parallel-fibered bone. Variation occurs with respect to histology of the internal core, which is composed of different types of bone, including woven-fibred, parallel-fibred, fibro-lamellar bone and, in some taxa, mineralized structural fibre bundles (Scheyer and Sander 2004; Hill and Lucas 2006; Vickaryous and Hall 2008; Klein et al. 2009; Vickaryous and Sire 2009; Scheyer and Desojo 2011; Cerda et al. 2013; Scheyer et al. 2014).
The combination of histological features reported here for Vancleavea campi are different from other doswelliids and even other archosauriforms published to date. A thick basal cortex of structural fibres bundles, a group of non-concentric growth marks in the inner core, a set of large canals extended laterally and a thin layer of external cortex, are unique to this taxon. The observed differences reported in V. campi with regard to other doswelliids are not entirely unexpected if we considered that the phylogenetic position of this taxon is not firmly established. Because some phylogenetic analyses (e.g., Desojo et al. 2011) recovered V. campi as an archosauriform outside of Doswelliidae and Proterochampsidae, the mentioned histological differences may be simply differences related to phylogenetic position.
Chanaresuchus bonapartei has similarities with others proterochampsids such as Pseudochampsa ischigualastensis. The small size and complete compact structure are features shared between both taxa. But also, unexpected differences were found when compared with others osteoderms of the same species. Previously, Cerda et al. (2015) observed that osteoderms from another C. bonapartei specimen (PVL 6244) where highly vascularized internally. It is possible that this variation is the result of differences of age, sex, and/or the position of the osteoderm across the body. The current data does not allow us to support any of these possible explanations. Despite intraspecific variation, proterochampsid osteoderms exhibit a general pattern in which there is no differentiation among basal, inner, and external regions. Such a pattern far departs from that reported in other archosauriforms and it could be considered a derived condition of Proterochampsidae.
Conclusions
No evidence exists for a complete metaplastic origin of osteoderm tissues for Doswelliidae and Proterochampsidae. Metaplastic mineralization of the collagenous fibres from the dermis appears, however, to be involved only in the development of some osteoderms of Vancleavea campi. The ornamentation of Doswellia kaltenbachi (i.e., preferential deposition) differs from that of other doswelliids taxa and instead is similar to that found in basal tetrapods and the archosaur group “Rauisuchia”. The compact structure of the microanatomy of the examined stem archosaurs is congruent with an aquatic or semi-aquatic life style. However, the functional implications of proterochampsids are difficult to assess because of the very size of the elements, which are few in number in comparison with doswelliids. The histological features of D. kaltenbachi resemble those of other doswelliids and other archosauriforms (e.g., Phytosauria, Aetosauria, “Rauisuchia”). Despite their close phylogenetic relationship, osteoderms of Doswelliidae and Proterochampsidae do not share many histological features in their histology. Proterochampsid osteoderms, including those of Chanaresuchus bonapartei and Pseudochampsa ischigualastensis appear to be unique and derived within Archosauriformes clade.
Acknowledgements
We would like to thank Hans-Dieter Sues (National Museum of Natural History, Smithsonian Institute, Washington, D.C., USA) for providing material of Doswellia and Gabriela Cisterna (PULR) for providing material of the Chanaresuchus holotype. We also thank the CRILaR laboratory from Anillaco, La Rioja, Argentina for producing the thin sections of Chanaresuchus. Additionally we are thankful for the Instituto de Investigación en Paleobiología y Geología (IIPG) from General Roca, Argentina for providing the space for the Laboratorio de Microscopia, in which the final observations and photographs were taken. Also we want to thank William Parker (Jackson School of Geosciences, University of Texas at Austin, USA) and Michel Burns (Department of Biological Sciences, University of Alberta, Canada) for reviewing the paper. Finally we want to thank Malaquias Ponce (Universidad Nacional del Comahue, General Roca, Argentina), by helping us with thin sections measurements. This project was part of the M.Sc. Thesis of DP. Funding: PICT 2012-925 and PICT 2014-0609 (to JBD and IC).
References
Arcucci, A. and Marsicano, C.A. 1998. A distinctive new archosaur from the Middle Triassic (Los Chañares Formation) of Argentina. Journal of Vertebrate Paleontology 18: 228–232. Crossref
Barrett, P.M., Clarke, J.B., Brinkman, D.B., Champman, S.D., and Ensom, P.C. 2002. Morphology, histology, and identification of the “granicones” from the Purbeck Limestone Formation (Lower Cretaceous: Berriasian) of Dorset, southern England. Cretaceous Research 23: 279–295. Crossref
Buchwitz, M., Witzmann, F., Voigt, S., and Golubev, V. 2012. Osteoderm microstructure indicates the presence of a crocodylian-like trunk brancing system in a group of armoured basal tetrapods. Acta Zoologica 93: 260–280. Crossref
Buffrénil, V. de 1982. Morphogenesis of bone ornamentation in extant and extinct crocodilians. Zoomorphology 99: 155–166. Crossref
Buffrénil, V. de, Clarac, F., Canoville, A., and Laurin, M. 2016. Comparative data on the differentiation and growth of bone ornamentation in gnathostomes (Chordata: Vertebrata). Journal of Morphology 277: 634–670. Crossref
Buffrénil, V. de, Dauphin, Y., Rage. J.C., and Sire, J.-Y. 2011. An enamel like tissue, osteodermine, on the osteoderms of a fossil anguid (Glyptosaurinae) lizard. Comptes Rendus Palevol 10: 427–437. Crossref
Burns, M.E. and Currie, P.J. 2014. External and internal structure of ankylosaur (Dinosauria; Ornithischia) osteoderms and their systematic relevance. Journal of Vertebrate Paleontology 34: 835–851. Crossref
Burns, M.E., Vickaryous, M.K., and Currie, P.J. 2013. Histological variability in fossil and recent alligatoroid osteoderms: systematic and functional implications. Journal of Morphology [published online]. Crossref
Cerda, I.A. and Desojo, J.B. 2011. Dermal armour histology of aetosaurs (Archosauria: Pseudosuchia), from the Upper Triassic of Argentina and Brazil. Lethaia 44: 417–428. Crossref
Cerda, I.A. and Powell, J.E. 2010. Dermal armor histology of Saltasaurus loricatus, an Upper Cretaceous sauropod dinosaur from Northwest Argentina. Acta Palaeontologica Polonica 55: 389–398. Crossref
Cerda, I.A., Desojo, J.B., and Scheyer, T.M. 2015. Osteoderm histology of Proterochampsia and Doswelliidae (Reptilia: Archosauriformes) and their evolutionary and paleobiological implications. Journal of Morphology 276: 385–402. Crossref
Cerda, I.A., Desojo, J.B., Scheyer, T.M. and Schultz, C.L. 2013. Osteoderm microstructure of “rauisuchian” archosaurs from South America. Geobios 46: 273–283. Crossref
Chinsamy, A. and Raath, M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Desojo, J.B., Ezcurra, M.D., and Schultz, C.L. 2011. An unusual new archosauriform from the Middle–Late Triassic of southern Brazil and the monophyly of Doswelliidae. Zoological Journal of the Linnean Society 161: 839–87. Crossref
Erickson, G.M. and Brochu, C.A. 1999. How the “terror crocodile” grew so big. Nature 398: 205–206. Crossref
Ezcurra, M.D. 2016. The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms. PeerJ 4: e1778. Crossref
Filippi, L.S., Cerda, I.A., and Garrido, A.C. 2013. Morfología e histología de osteodermos de un Peirosauridae de la Cuenca Neuquina. Ameghiniana 50: 3–13. Crossref
Francillon-Vieillot, H., Buffrénil, V. de, Castanet. J., Géraudie. J., Meunier, F.J., Sire, J.-Y., Zylberberg, L., and Ricqlès, A. de 1990. Microstructures and mineralization of vertebrate skeletal tissues. In: J. Carter (ed.), Skeletal Biomineralizations: Patterns, Processes, and Evolutionary Trends, Vol. 1, 471–530. Van Nostrand Reinhold, New York.
Girondot, M. and Laurin, M. 2003. Bone profiler: a tool to quantify, model, and statistically compare bone-section compactness profiles. Journal of Vertebrate Paleontology 23: 458–461. Crossref
Haines, R.W. and Mohuiddin, A. 1968. Metaplastic bone. Journal of Anatomy 103: 527–538.
Hayashi, S., Carpenter, K., Watabe, M., and McWhinney, L. 2012. Ontogenetic histology of Stegosaurus plates and spikes. Palaeontology 55: 145–161. Crossref
Hill, R.V. 2010. Osteoderms of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 30 (6, supplement): 154–176. Crossref
Hill, R.V. and Lucas, S.G. 2006. New data on the anatomy and relationships of the Paleocene crocodilian Akanthosuchus langstoni. Acta Palaeontologica Polonica 51: 455–464.
Houssaye, A. 2009. “Pachyostosis” in aquatic amniotes: a review. Integrative Zoology 4: 325–340.
Houssaye, A. 2013. Palaeoecological and morphofunctional interpretation of bone mass increase: an example in Late Cretaceous shallow marine squamates. Biological Reviews of the Cambridge Philosophical Society 88: 117–139. Crossref
Hua, S. and Buffrénil, V. de 1996. Bone histology as clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). Journal of Vertebrate Paleontology 16: 703–717. Crossref
Long, R.A. and Murry, P.A. 1995. Late Triassic (Carnian and Norian) tetrapods from the southwestern United States. New Mexico Museum of Natural History and Science Bulletin 4: 1–254.
Jannello, J.M., Cerda, I.A., and Fuente, M. de la 2016. Shell bone histology of the long-necked chelid Yaminuechelys (Testudines: Pleurodira) from the late Cretaceous early Palaeocene of Patagonia with comments on the histogenesis of bone ornamentation. The Science of Nature 103: 1–26. Crossref
Klein, N., Scheyer, T.M. and Tütken, T. 2009. Skeletochronology and isotopic analysis of a captive individual of Alligator mississippiensis Daudin, 1802. Fossil Record 12: 121–131. Crossref
Levrat-Calviac, V. and Zylberberg, L. 1986. The structure of the osteoderms in the gekko: Tarentola mauritanica. American Journal of Anatomy 176: 437–466. Crossref
Main, R.P., Ricqlès, A. de, Horner, J.R., and Padian, K. 2005. The evolution and function of thyreophoran dinosaur scutes: implications for plate function in stegosaurs. Paleobiology 31: 291–314. Crossref
Nesbitt, S.J. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History 352: 1–292. Crossref
Nesbitt, S.J., Stocker, M.R., Small, B.J., and Downs, A. 2009. The osteology and relationships of Vancleavea campi (Reptilia: Archosauriformes). Zoological Journal of the Linnean Society 157: 814–864. Crossref
Parker, W.G. and Barton, B.J. 2008. New Information on the Upper Triassic archosauriform Vancleavea campi based on new material from the Chinle Formation of Arizona. Palaeontologia Electronica 11:1–20.
Parker, W.G., Stocker, M.R., and Irmis, R.B. 2008. A new Desmatosuchine aetosaur (Archosauria: Suchia) from the Upper Triassic Tecovas Formation (Dockum Group) of Texas. Journal of Vertebrate Paleontology 28: 692–701. Crossref
Rasband, W. 2003. Image J. National Institutes of Health, Bethesda, available at http://rsb.info.nih.gov/ij/
Reid, R.E.H. 1996. Bone histology of the Cleveland-Lloyd dinosaurs and of dinosaurs in general. Part I: Introduction to bone tissues. Brigham Young University Geological Studies 41: 25–72.
Ricqlès, A. de and Buffrénil, V. de 2001. Bone histology, heterochronies and the return of the tetrapods to life in water: Where are we? In: J. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 289–310. Verlag Dr. Friedrich Pfeil, München.
Ricqlès, A. de, Padian, K., and Horner, J.R. 2003. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Annales de Paléontologie 89: 67–101. Crossref
Romer, A.S. 1971. The Chañares (Argentina) Triassic reptile fauna. XI. Two new long-snouted thecodonts, Chanaresuchus and Gualosuchus. Breviora 379: 1–22.
Ruibal, R. and Shoemaker, V. 1984. Osteoderms in anurans. Journal of Herpetology 18: 313–328. Crossref
Scheyer T.M. 2007. Skeletal histology of the dermal armor of Placodontia: the occurrence of “postcranial fibro-cartilaginous bone” and its developmental implications. Journal of Anatomy 211: 737–753. Crossref
Scheyer, T.M. and Desojo, J.B. 2011. Palaeohistology and microanatomy of rauisuchian osteoderms (Archosauria: Pseudosuchia). Palaeontology 54: 1289–1302. Crossref
Scheyer, T.M. and Sánchez-Villagra, M.R. 2007. Carapace bone histology in the giant pleurodiran turtle Stupendemys geographicus: phylogeny and function. Acta Palaeontologica Polonica 52: 137–154.
Scheyer, T.M. and Sander, M.P. 2004. Histology of ankylosaur osteoderms: implications for systematics and function. Journal of Vertebrate Paleontology 24: 874–893. Crossref
Scheyer, T.M. and Sander, P.M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society B 274: 1885–1893. Crossref
Scheyer, T.M. and Sander, P.M. 2009. Bone microstructures and mode of skeletogenesis in osteoderms of three pareiasaurs taxa from the Permian of South Africa. Journal of Evolutionary Biology 22: 1153–1162. Crossref
Scheyer, T.M., Brüllmann, B., and Sánchez-Villagra, M.R. 2008. The ontogeny of the shell in side-necked turtles, with emphasis on the homologies of costal and neural bones. Journal of Morphology 269: 1008–1021. Crossref
Scheyer, T.M., Desojo, J.B., and Cerda, I.A. 2014. Bone histology of phytosaur, aetosaur, and other archosauriform osteoderms (Eureptilia, Archosauromorpha). The Anatomical Record 297:240–260. Crossref
Sterli, J., Fuente, M.S. de la, and Cerda, I.A. 2013. A new species of meiolaniform turtle and a revision on the Upper Cretaceous meiolaniforms of southern South America. Ameghiniana 50: 240–256. Crossref
Sues, D.-H., Desojo, J.B., and Ezcurra, M.D. 2013. Doswelliidae: A clade of unusual armoured archosauriforms from the Middle and Late Triassic. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny, and Palaeobiology of Early Archosaurs and their Kin. Geological Society of London Special Publication 379: 49–58. Crossref
Taborda, J.R.A., Cerda, I.A., and Desojo, J.B. 2013. Growth curve of Aetosauroides scagliai Casamiquela 1960 (Pseudosuchia: Aetosauria) inferred from osteoderm histology. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny, and Palaeobiology of Early Archosaurs and their Kin. Geological Society of London Special Publication 379: 413–424. Crossref
Taborda, J.R.A., Heckert, A.B., and Desojo, J.B. 2015. Intraspecific variation in Aetosauroides scagliai Casamiquela (Archosauria: Aetosauria) from the Upper Triassic of Argentina and Brazil: an example of sexual dimorphism? Ameghiniana 52: 173–187. Crossref
Taylor, M.A. 2000. Functional significance of bone ballast in the evolution of buoyancy control strategies by aquatic tetrapods. Historical Biology 14: 15–31. Crossref
Trotteyn, M.J., Arcucci, M.A., and Raugust, T. 2013. Proterochampsia: an endemic archosauriform clade from South America. In: S.J. Nesbitt, J.B. Desojo, and R.B. Irmis (eds.), Anatomy, Phylogeny, and Palaeobiology of Early Archosaurs and their Kin. Geological Society of London Special Publication 379: 59–90. Crossref
Trotteyn, M.J., Martínez, R.N., and Alcober, O.A. 2012. A new proterochampsid Chanaresuchus ischigualastensis (Diapsida, Archosauriformes) in the early Late Triassic Ischigualasto Formation, Argentina. Journal of Vertebrate Paleontology 32: 485–489. Crossref
Vickaryous, M.K. and Hall, B.K. 2008. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. Journal of Morphology 269: 398–422. Crossref
Vickaryous, M.K. and Sire, J.-Y. 2009. The integumentary skeleton of tetrapods: origin, evolution, and development. Journal of Anatomy 214: 441–464. Crossref
Weems, R.E. 1980. An unusual newly discovered archosaur from the Upper Triassic of Virginia, USA. Transactions of the American Philosophical Society 70: 1–53. Crossref
Witzmann, F. 2009. Comparative histology of sculptured dermal bones in basal tetrapods, and the implications for the soft tissue dermis. Palaeodiversity 2: 233–270.
Witzmann, F. 2010. Morphological and histological changes of dermal scales during the fish-to-tetrapod transition. Acta Zoologica 92: 281–302. Crossref
Witzmann, F. and Soler-Gijón, R. 2008. The bone histology of osteoderms in temnospondyl amphibians and in the chroniosuchian Bystrowiella. Acta Zoologica 89: 1–19.
Zylberberg, L. and Castanet, J. 1985. New data on the structure and the growth of the osteoderms in the reptile Anguis fragilis L. (Anguidae, Squamata). Journal of Morphology 186: 327–342. Crossref
Acta Palaeontol. Pol. 62 (4): 819–831, 2017
https://doi.org/10.4202/app.00381.2017