

Thyasirid bivalves from Cretaceous and Paleogene cold seeps
KRZYSZTOF HRYNIEWICZ, KAZUTAKA AMANO, ROBERT G. JENKINS, and STEFFEN KIEL
Hryniewicz, K., Amano, K., Jenkins, R.G., and Kiel, S. 2017. Thyasirid bivalves from Cretaceous and Paleogene cold seeps. Acta Palaeontologica Polonica 62 (4): 705–728.
We present a systematic study of thyasirid bivalves from Cretaceous to Oligocene seep carbonates worldwide. Eleven species of thyasirid bivalves are identified belonging to three genera: Conchocele, Maorithyas, and Thyasira. Two species are new: Maorithyas humptulipsensis sp. nov. from middle Eocene seep carbonates in the Humptulips Formation, Washington State, USA, and Conchocele kiritachiensis sp. nov. from the late Eocene seep deposit at Kiritachi, Hokkaido, Japan. Two new combinations are provided: Conchocele townsendi (White, 1890) from Maastrichtian strata of the James Ross Basin, Antarctica, and Maorithyas folgeri (Wagner and Schilling, 1923) from Oligocene rocks from California, USA. Three species are left in open nomenclature. We show that thyasirids have Mesozoic origins and appear at seeps before appearing in “normal” marine environments. These data are interpreted as a record of seep origination of thyasirids, and their subsequent dispersal to non-seep environments. We discuss the age of origination of thyasirids in the context of the origin of the modern deep sea fauna and conclude that thyasirids could have deep sea origins. This hypothesis is supported by the observed lack of influence of the Cretaceous and Paleogene Oceanic Anoxic Events on the main evolutionary lineages of the thyasirids, as seen in several other members of the deep sea fauna.
Key words: Bivalvia, Thyasiridae, cold seeps, deep sea, ecology, evolution, Cretaceous, Paleogene.
Krzysztof Hryniewicz [krzyszth@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51/55, 00-818 Warszawa, Poland.
Kazutaka Amano [amano@juen.ac.jp], Department of Geoscience, Joetsu University of Education, 1Yamayashiki Joetsu City, Niigata 943-8512, Japan.
Robert G. Jenkins [robertgj@staff.kanazawa-u.ac.jp], School of Natural System, College of Science and Engineering, Kanazawa University, Kanazawa City, Ishikawa 920-1192, Japan.
Steffen Kiel [steffen.kiel@nrm.se], Swedish Museum of Natural History, Department of Palaeobiology, Box 500 07, 104 05 Stockholm, Sweden.
Received 23 May 2017, accepted 2 August 2017, available online 31 October 2017.
Copyright © 2017 K. Hryniewicz et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Marine chemosynthesis-based ecosystems comprise hydrothermal vents (e.g., Van Dover 2000), hydrocarbon seeps (e.g., Sibuet and Olu 1998; Levin 2005) and wood and nekton falls (Smith and Baco 2003; Bernardino et al. 2010). Hydrocarbon seeps are submarine edifices where low-temperature fluids are released to the water column (Judd and Hovland 2007). These environments are populated by faunas dominated by macroinvertebrate species living in symbiosis with chemoautotrophic bacteria (Levin 2005), among which the most iconic are epifaunal and semi-infaunal groups like vestimentiferan tubeworms (Bright and Lallier 2010), vesicomyid clams and bathymodiolin mussels (Taylor and Glover 2010) and large abyssochrysoid gastropods (Sasaki et al. 2010). Infaunal groups, among them lucinid and thyasirid bivalves, have received less attention, although often being dominant in some Recent cold seeps (Kharlamenko et al. 2016). Fossil lucinid bivalves are relatively well known, commonly comprising the main faunal element of Cretaceous and Paleogene seeps (Kiel 2013).
The focus of this study are the less well known fossil thyasirid seep faunas. Many species are known from Cretaceous to Pleistocene seep deposits worldwide (e.g., Van Winkle 1919; Yabe and Nomura 1925; Kauffman 1967; Squires and Gring 1996; Goedert et al. 2003; Kiel et al. 2008; Amano et al. 2013). Although thyasirids are rarely common in ancient seep settings, the genus Cretaxinus Hryniewicz, Little, and Nakrem, 2014, was reported to be restricted to ancient seep environments, and it is possible that one more thyasirid genus occurs at fossil seeps only (Nobuhara et al. 2008). Despite a growing volume of data on fossil seeps, the taxonomy of thyasirids in these habitats remains poorly understood. The purpose of this paper is to improve the current knowledge of fossil seep-inhabiting thyasirids using newly collected material and museum collections. Due to an overwhelming number of Neogene seep deposits, especially in Japan and the Russian Far East, the current study is restricted to more manageable Cretaceous and Paleogene occurrences.
Institutional abbreviations.—CSUN, California State University at Northridge, USA; GPIBo, Steinmann-Institut für Geologie, Mineralogie und Paläontologie, Universität Bonn, Germany; JUE, Joetsu University of Education, Japan; LACMIP, Natural History Museum of Los Angeles County, Los Angeles, USA; MMH; Natural History Museum of Denmark, Geological Museum, Copenhagen, Denmark; NRM, Swedish Museum of Natural History (Naturhistoriska riksmuseet), Stockholm, Sweden; PMO, Natural History Museum, University of Oslo, Norway; UCMP, University of California Museum of Paleontology, Berkeley, USA; UMUT, University Museum, University of Tokyo, Tokyo, Japan; USNM, United States National Museum of Natural History, Washington, USA; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Material
This study is based on a collection of specimens from Cretaceous–Oligocene localities worldwide (Figs. 1, 2). The majority of these localities have already been described; therefore we only briefly outline the localities and provide references to more detailed descriptions. The localities are listed below in an alphabetical order.
Bear River, southwestern Washington State, USA.—Large seep deposit in an abandoned quarry on the south side of Bear River in Pacific County, western Washington State, embedded in upper Eocene strata of “Siltstone of Cliff Point” (LACMIP loc. 5802). It contains a diverse fauna dominated by Bathymodiolus willapaensis; the thyasirids reported herein are quite rare (Goedert and Squires 1990; Squires and Goedert 1991; Goedert and Benham 2003; Kiel 2010).
Bullman Creek, northwestern Washington State, USA.— Cold seep limestone found as float on beach terrace approximately 1 km east of the mouth of Bullman Creek in Clallam County, northwestern Washington State, derived from the lower Oligocene Jansen Creek Member of the Makah Formation; coordinates: 48°20.798’ N, 124°31.223’ W.
Canyon River, western Washington State, USA.—Large cold seep carbonate block with large thyasirid bivalves exposed by natural etching. Found as float on gravel bar, east bank of Canyon River, approximately 100 m upstream from bridge, coordinates: 47°18.166’ N, 123°30.567’ W; SE ¼ of Section 13, T. 21 N., R. 7 W according to US public land description system, Grays Harbor County, Washington State. The outcrop is surrounded by uppermost Eocene to lower Oligocene deposits of the Lincoln Creek Formation.
Colesbukta area, Spitsbergen, Svalbard.—Thyasirids identified as Conchocele conradii by Rosenkrantz (1970) come from prodelta and offshore sandstone and siltstone of the Paleocene Basilika Formation cropping out in several localities on the western shore of Colesbukta. The majority of the specimens described herein comes from Fossildalen and were first mentioned by Hägg (1925), with some of those occurrences later identified as seep deposits and wood-rich sediments (Hryniewicz et al. 2016). The fauna is dominated by C. conradii associated with diverse background species, chiefly mollusks.
East Twin River, northwestern Washington State, USA.— Block of cold-seep limestone found as float along base of bluff approximately 110 m north and 490 m west of the southeast corner of Section 24, T. 31 N., R. 10 W according to US public land description system, approx. 1.6 km east of East Twin River, Clallam County, Washington State. The block comes from the upper part of the Pysht Formation and is of late Oligocene age (Nesbitt et al. 2013).
Humptulips Formation, western Washington State, USA.—Our material comes from two distinct localities from the middle Eocene Humptulips Formation in western Washington State, USA. LACMIP loc. 12385 is a large seep deposit in an abandoned meander on the East Fork of the Humptulips River in Grays Harbor County, hosting a diverse mollusk fauna including the bathymodiolin Vulcanidas? goederti, the vesicomyid “Pliocardia” cf. tschudi, and the thyasirid described herein, previously identified as Thyasira (Conchocele) folgeri (Goedert and Squires 1990; Kiel and Amano 2013). The second site is a small seep deposit found in the river bed of the Humptulips River (CSUN loc. 1583) with the main taxa being the lucinid bivalve Elongatolucina elassodyseides and the abyssochrysoid gastropod Humptulipsia raui (Goedert and Kaler 1996; Kiel 2008; Gill and Little 2013).
James Ross Basin, Western Antarctica.—Specimens of the large thyasirid “Thyasira” townsendi are common throughout Maastrichtian strata of Snow Hill and López de Bertodano formations of Snow Hill and Seymour islands, respectively (Wilckens 1910). Some of the sites have recently been identified as seep-related (Little et al. 2015). Here we re-illustrate two specimens previously figured by Wilckens (1910).
Kami-Atsunai, Hokkaido, Japan.—Large, lower Oligocene seep deposit found in deep-water strata of the Nuibetsu Formation along the Atsunai River, 1.5 km east of the Kami-Atsunai railway station in Urahoro-cho in eastern Hokkaido, Japan. The main taxa found at this locality are the vesicomyid Hubertschenckia ezoensis, the bathymodiolin Bathymodiolus inouei, and a species of Conchocele discussed herein (Amano and Jenkins 2011).
Kiritachi, Hokkaido, Japan.—Articulated specimens of Conchocele kiritachiensis sp. nov. described herein come from an upper Eocene seep deposit of Sakasagawa Formation, cropping out at the cliff along the Kotanbetsu River, 2 km west of Seiryu Bridge in Kiritachi, Tomamae Town, northwestern Hokkaido (Loc. SK 15 of Noda [1992]; 44°13’22” N, 141°53’8” E). The locality also contains buccinid gastropods (Amano and Oleinik 2016).
LACMIP loc. 16504, western Washington State, USA.—An upper Oligocene seep deposit, found in the bathyal siltstone of the Lincoln Creek Formation (Squires 1995). The deposit contains a diverse fauna of mollusks (Squires and Goedert 1995; Goedert and Benham 1999) and sponges (Rigby and Goedert 1996).
Maeshima, Kyushu, Japan.—Small blocks of heavily indurated, grey seep carbonates found at the beach within a Santonian submarine slump deposits of the Himenoura Group at Maeshima, Goshoura Island, Kumamoto Prefecture, southern Kyushu, Japan. The carbonates contain small molluscan fossils, among them low-spired gastropods and small thyasirid bivalves here identified as Thyasira tanabei. Details for this locality will be provided in a separate publication.
Montrose, Nebraska, USA.—The material described in this paper comes from the Upper Cretaceous (Campanian; Jamie Brezina, personal communication 2017) offshore Pierre Shale of the Western Interior Seaway. It was collected in 2014 by Andrzej Kaim (Institute of Paleobiology, Warsaw) near the Warbonnet Battlefield Memorial, close to Montrose, Nebraska, USA. The memorial is located on top of a seep deposit with numerous bivalves, mostly lucinids, and less common gastropods, including well preserved naticiform gastropods (Andrzej Kaim, personal communication 2017).
Murdock Creek, northwestern Washington State, USA.— Several small blocks of seep carbonate float found on the beach platform west of the mouth of Murdock Creek, Clallam County, Washington State, derived from the upper lower Oligocene deep-water strata of the lower part of the Pysht Formation (LACMIP loc. 6295). These blocks include a diverse but scattered fauna including protobranch, vesicomyid, and bathymodiolin bivalves, provannid gastropods, and serpulid worm tubes (Goedert and Squires 1993; Kiel and Amano 2013; Vinn et al. 2013).
SR4, western Washington State, USA.—Seep deposit with a diverse fauna found along the bank of Satsop River at 47°16’18.0” N, 123°28’56.2” W, in the upper Oligocene part of the Lincoln Creek Formation (Peckmann et al. 2002; Zwicker et al. 2015).
Tanami, Honshu, Japan.—A medium-sized seep deposit found in upper Eocene to lower Oligocene submarine fan deposits of the Tanamigawa Formation near Tanami, Kushimoto Town in Wakayama Prefecture, southern Honshu, Japan (Amano et al. 2013). The main invertebrate species at this locality is a species of Conchocele, accompanied by less numerous specimens of the bathymodiolin Bathymodiolus aff. inouei (Amano and Jenkins 2011), and the vesicomyid Hubertschenkia ezoensis. A complete list of invertebrate macrofauna from this locality is given by Amano et al. (2013).
Tappu, Hokkaido, Japan.—Four carbonate blocks derived from dark grey mudstone found 130 m and 150 m upstream from Eikou Bridge at Tappu, along the Shimokinebetsu River in Obira Town in northwestern Hokkaido. The fauna of these upper Eocene sites consists of the thyasirid Conchocele taylori documented herein, the vesicomyid Hubertschenckia ezoensis, the malletiid Malletia poronaica, and the carditid Cyclocardia elliptica, in association with some other taxodont bivalves and some turrid gastropods (Ohara and Kanno 1969, 1973; Amano et al. 2013).
Yayoi, Hokkaido, Japan.—Cold-seep deposit located in a high cliff along the Ikushunbetsu River to the west of Yayoi Town in Mikasa City in central Hokkaido, Japan, that is enclosed in dark grey mudstone of the upper Eocene Poronai Formation. The fauna of this site consists mainly of a species of Conchocele and the vesicomyid Hubertschenckia ezoensis, along with a few gastropods (Yokoyama 1890; Amano and Jenkins 2007).
West Fork of Grays River, western Washington State, USA.—Blocks of cold seep carbonate on gravel bar approximately 30 m upstream from the old bridge over the West Fork of Grays River, approximately 800 m east and 950 m south of the northwest corner of Section 28, T. 11 N., R. 7 W, according to US public land description system; Pacific County, Washington State; coordinates: 46.404230°N, 123.553867°W The rocks in this area were mapped as middle to upper Eocene “Siltstone of Unit B” (Wolfe and McKee 1968).
Whiskey Creek, northwestern Washington State, USA.— Large limestone boulders eroded from a landslide west of the mouth of Whiskey Creek in Clallam County on the north side of the Olympic Peninsula in Washington State, USA. They appear to be derived from upper Eocene strata of the Pysht Formation; the most common taxa are the lucinid bivalve Cryptolucina megadyseides and the vesicomyid Adulomya chinookensis, whereas the thyasirid reported here is uncommon (Goedert et al. 2003).
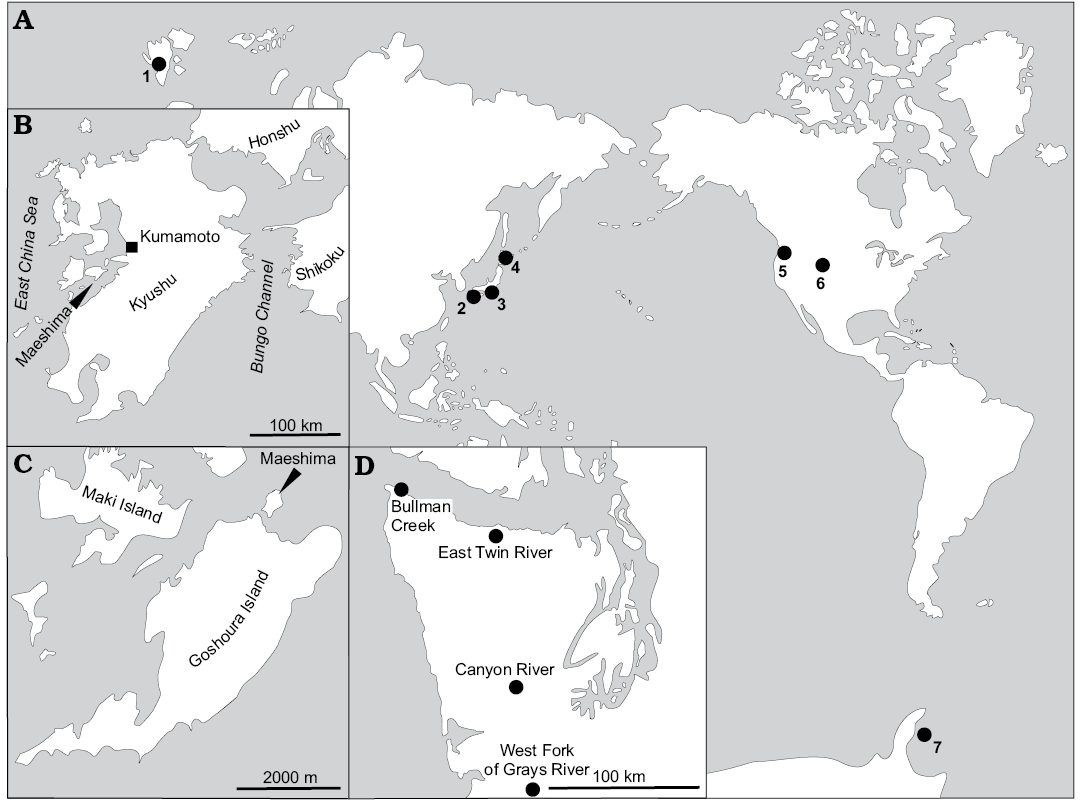
Fig. 1. A. Map showing some of the fossil seep localities bearing thyasirids examined in this study. Detailed maps of Amakusa area, Kyushu, Japan (B, C), Washington State, USA (D). 1, Colesbukta area, Spitsbergen, Svalbard; 2, Maeshima, Amakusa area, Kyushu, Japan; 3, Tanami, Honshu, Japan; 4, Hokkaido, Japan; 5, Washington State, USA; 6, Montrose, Nebraska, USA; 7, James Ross Basin, Seymour Island, Antarctica. After Campbell 2006 (A) and Goedert and Benham 1999 (D). For detailed list of localities discussed, the reader is refered the Material section, and references therein.
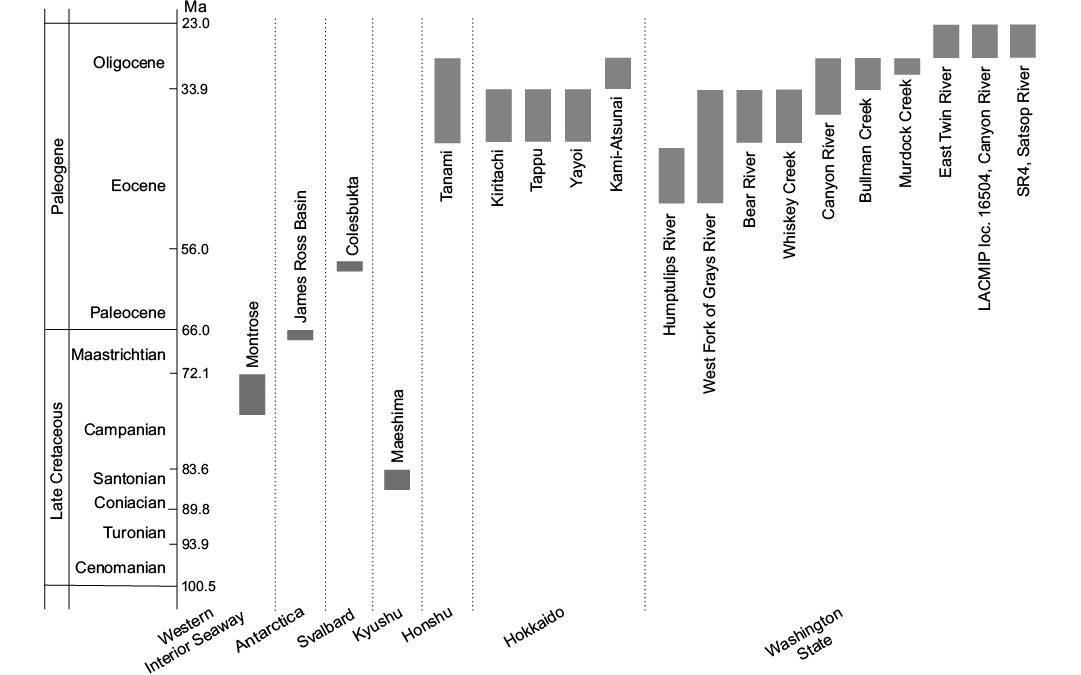
Fig. 2. Geological ages of the fossil seep localities bearing thyasirids examined in this study.
Systematic palaeontology
Class Bivalvia Linnaeus, 1758
Subclass Heterodonta Neumayr, 1884
Order Lucinida Gray, 1854
Family Thyasiridae Dall, 1900 (1895)
Genus Conchocele Gabb, 1866
Type species: Thyasira bisecta Conrad, 1849; Miocene, Astoria Formation, Astoria, Oregon, USA.
Remarks.—Various species concepts have been applied to the extant members of Conchocele. In some cases smaller and more rounded specimens were referred to as Conchocele bisecta (Conrad, 1849), whereas larger and more quadrangular specimens were referred to as Conchocele disjunta (Gabb, 1866) (e.g., Tegland 1928; Habe 1964; Boss 1967; Bernard 1972). The current approach is to treat both morphospecies as a variation of the same biological species, and to refer to the extant species as C. bisecta due to the rule of priority (Kamenev et al. 2001; Oliver and Frey 2014). The more extensive discussion of this problem is beyond the scope of this paper, however, we would like to point out that both C. bisecta and C. disjuncta are based on fossil rather than extant material, and each fossil species was erected based on material of different age (Miocene and Pliocene, respectively). Caution should be applied when synonymizing these two fossil species, and when applying these names to extant material. The only name for a North Pacific Conchocele that has been applied to a living species, as far as we are aware of, is Thyasira disjuncta var. ochotica Krishtofovich, 1936 (locality: sea shore). It might be worth to carefully check this “variation” for conchological differences to C. disjuncta; if there are notable differences, C. “ochotica” might be used for extant North Pacific species.
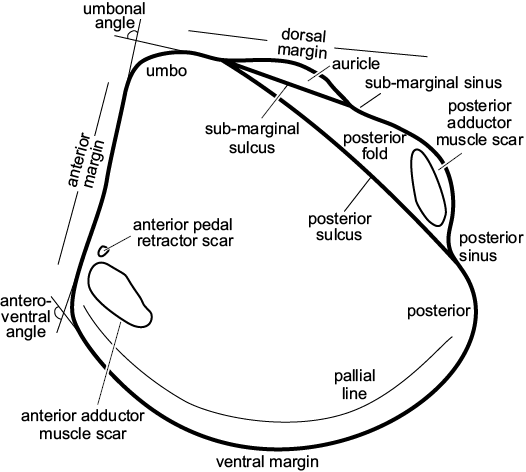
Fig. 3. Schematic drawing of a model thyasirid bivalve with explanations of the main morphological terms used herein.
Conchocele townsendi (White, 1890)
Fig. 4.
1890 Lucina? townsendi sp. nov.; White 1890: 14, pl. 3: 1, 2.
1903 Lucina? townsendi White, 1890; Weller 1903: 67, pl. 11: 2, 3.
1910 Thyasira townsendi (White, 1890); Wilckens 1910: 53–56, pl. 2: 31, 32, pl. 3: 1.
1988 Thyasira townsendi (White, 1990) (sic!): Zinsmeister and Macellari 1988: 273, 276, figs. 9.7, 9.8.
2008 “Thyasira” townsendi (White, 1890); Kiel et al. 2008: 535.
2015 “Thyasira” townsendi (White, 1890); Little et al. 2015: fig. 5G–I.
Type material: Holotype unknown; hypotypes NRM Mo 1560; USNM 405773 (Zinsmeister and Macellari 1988).
Type locality: Saint Paul’s and Saint Peter’s islands, Magellan Strait.
Type horizon: Cretaceous (details unknown).
Material.—Two specimens (NRM Mo 1552, 1560) from Maastrichtian cold seep carbonates of Seymour Island, Antarctica.
Remarks.—The species was initially described as a possible species of Lucina by White (1890) from St. Paul’s and St. Peter’s islands in the Straits of Magellan, both of which we could not locate. It was thereafter noted from Antarctic strata by Weller (1903), who mentioned it from outcrops of the Admiralty Sound area, Weddell Sea. The first precise locality information comes from the work of Wilckens (1910), who mentions numerous occurrences on Snow Hill and Seymour Islands, western Weddell Sea. Wilckens (1910) formally transferred the species to Thyasira. This interpretation was followed in a description of Zinsmeister and Macellari (1988), who noticed that the species is similar to Conchocele disjuncta Gabb, 1869, from the Pliocene of California. However, they considered a closer relationship between the two species unlikely due to the significant temporal and spatial distance between them.
Doubts about including “Thyasira” townsendi in Thyasira were expressed by Kiel et al. (2008) and Little et al. (2015). Here we formally transfer the species to Conchocele based on the beaks located close to the anterior of the shell, a steeply sloping anterior margin bound by a keel, the lack of a distinct submarginal and deep posterior sulcus, and a posterior margin indented by a deep sinus. This is the only Cretaceous thyasirid which we can confidently place in the genus Conchocele. The Coniacian “Thyasira” cretacea Whiteaves, 1874, from Enos Canyon, California (Anderson 1958) is not figured well enough for a more detailed comparison. The same applies to “Thyasira” cretacea from the Late Cretaceous of Vancouver Island (Whiteaves 1874), and to “Thyasira” collignoni from Campanian of New Caledonia (Freneix 1980), the figured specimens of which lack a well preserved posterior margin. “Aphrodina” hataii Katto and Hattori, 1965, from the Campanian–Maastrichtian Sada Limestone seep deposit in Shikoku, Japan (Nobuhara et al. 2008) has a deep posterior sulcus but lacks a characteristic posterior sinus (KH personal observation) as well as a truncated anterior margin and should not be included in Conchocele.
Stratigraphic and geographic range.—Maastrichtian of the James Ross Basin and possibly the Magellan Strait area.
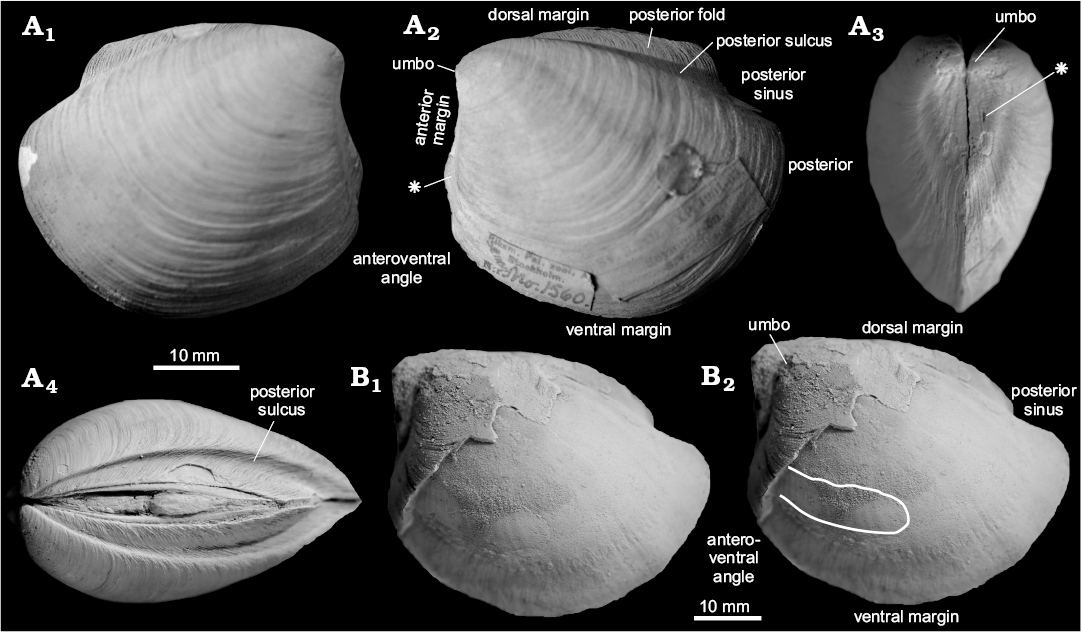
Fig. 4. Thyasirid bivalve Conchocele townsendi (White, 1890) from Maastrichtian cold seep carbonates of Seymour Island, James Ross Basin, Antarctica. A. NRM Mo 1560; a complete shell in right (A1) and left (A2) lateral view, showing outline and fine commarginal ornament; in anterior view (A3), showing two ridges running from the umbo towards the anteroventral angle; in dorsal view (A4), showing narrow and sharp posterior sulcus and posterior fold and weak furrow possibly representing accessorial ligament attachment surface. B. NRM Mo 1552; an internal mold in left lateral view with fragments of the shell adhering to the anterior and umbonal area (B1) and with outline of the anterior adductor muscle scar visible (B2).
Conchocele conradii (Rosenkrantz, 1942)
Fig. 5.
1925 Thyasira bisecta Conrad, 1849; Hägg 1925: 46–48, pl. 4: 14–17, pl. 5: 18, 19, non 20.
1942 Thyasira conradii n. sp.; Rosenkrantz 1942: 277–278.
1970 Thyasira (Conchocele) conradi Rosenkrantz, 1942 (sic!); Rosenkrantz 1970: 427, fig. 7.1, non fig. 7.2.
1970 Conchocele conradii (Rosenkrantz, 1942); Vonderbank 1970: pl. 10: 2a, b.
?1980 Conchocele conradii (Rosenkrantz, 1942); Thiedig et al. 1980: 143, pl. 1: 1, 3, non 4.
2016 Conchocele conradii (Rosenkrantz, 1942); Hryniewicz et al. 2016: fig. 12A.
Type material: Holotype unknown; lectotype MMH 10792 (Rosenkrantz 1970).
Type locality: South-western side of Colesbukta, Spitsbergen, Svalbard.
Type horizon: Paleocene, Basilika Formation.
Material.—All of the material studied comes from Paleocene Basilika Formation, Colesbukta area, Spitsbergen, Svalbard. Two specimens from GPIBo, including one figured by Vonderbank (GPIBo Nr. 159) (1970); 14 unnumbered specimens from PMO; 37 specimens from NRM, including one figured (NRM PZ Mo 182204; Fig. 5A), this comprises five specimens (Mo PZ 149148–152) illustrated by Hägg (1925) and one (NRM PZ Mo 182204) by Hryniewicz et al. (2016); 121 unnumbered specimens from the collections of ZPAL, and one numbered and figured specimen (ZPAL L.16/1). The material from GPIBo contains only specimens with sandstone matrix which are unlikely to be of seep origin. The specimens from PMO are all of seep origin; the material from NRM and ZPAL contains a mixture of seep and non-seep specimens, with seep specimens prevailing.
Remarks.—Conchocele conradii from the south-western side of Colesbukta on Spitsbergen, Svalbard, was first described as Thyasira bisecta Conrad, 1849, by Hägg (1925), who noticed that the specimens from Svalbard are larger than the largest specimens of C. bisecta from the Miocene Astoria Formation type locality in Oregon, USA (Moore 1963). He also noticed that the Svalbard specimens are closely related to C. townsendi from the Cretaceous of the James Ross Basin, Antarctica, discussed above. A new species name, Thyasira conradii, was thereafter proposed for the Svalbard species by Rosenkrantz (1942). Its generic affinity was later revisited by Rosenkrantz (1970) and by Vonderbank (1970), who formally moved it to Conchocele.
An allegedly similar species, Conchocele aff. conradii, was mentioned to occur in the lower Paleocene Kangilia Formation in West Greenland (Rosenkrantz 1970). The illustration provided therein (Rosenkrantz 1970: fig. 7.2) is insufficient for a more detailed comparison; the overall shell outline presented there is somewhat different from that of C. conradii, and is more reminiscent of C. townsendi. Therefore, until more material is available for study, we do not include the specimens from the Kangilia Formation in C. conradii. Conchocele conradii differs from C. townsendi by its somewhat shorter shell, more rounded ventral margin, broader posterior sulcus, and the lack of two ridges on the anterior margin of the shell. The specific significance of the latter character is unknown, however, as not all specimens of C. townsendi have the two ridges mentioned. All other Paleogene species of Conchocele that we have investigated have more quadrangular shells than C. conradii.
Stratigraphic and geographic range.—Paleocene cold seep carbonates and associated sunken driftwood environments of the Basilika Formation, Colesbukta, Spitsbergen, Svalbard. Possibly also upper Eocene–lower Oligocene strata of the Renardodden Formation, Renardoden, Belsund area, Spitsbergen, Svalbard (Thiedig et al. 1980). A similar species Conchocele aff. conradii was reported from lower Paleocene Kangilia Formation from West Greenland (Rosenkrantz 1942, 1970).
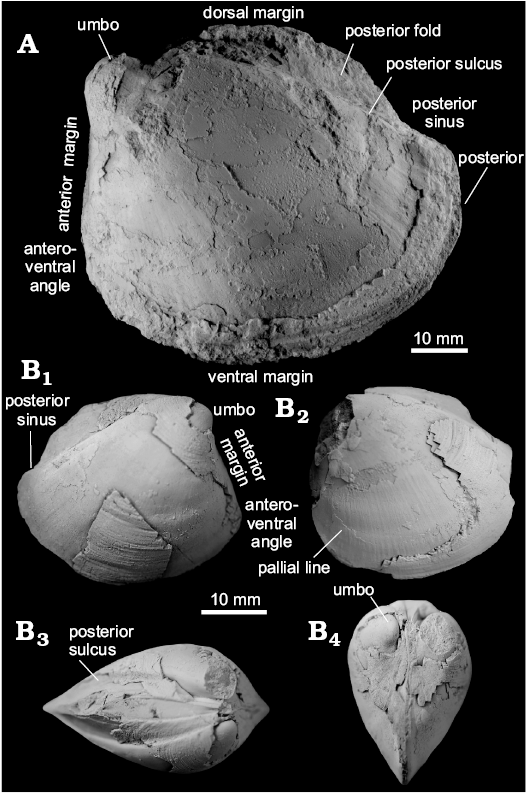
Fig. 5. Thyasirid bivalve Conchocele conradii (Rosenkrantz, 1942) from Paleocene strata of the Basilika Formation, Colesbukta, Spitsbergen, Svalbard. A. NRM PZ Mo 182204; a medium sized internal mold in left lateral view with no clear outline of an anterior adductor muscle scar visible. B. ZPAL L.16/1; an internal mold in right (B1) and left (B2) views with fragments of the shell adhering (no clear anterior adductor muscle scar visible); in dorsal view (B3) showing fragments of a posterior sulcus; anterior fragments of the shell (B4) showing flat anterior margin without ridges.
Conchocele taylori Hickman, 2015
Fig. 6, 7.
1979 Conchocele cf. nipponica (Yabe and Nomura, 1925); Katto and Masuda 1979: pl. 2: 12a, b, 13a, b.
2003 Conchocele bisecta (Conrad, 1849); Goedert et al. 2003: pl. 42: 9.
2007 Conchocele bisecta (Conrad, 1849); Amano and Jenkins 2007: fig. 2A, D–F.
2013 Conchocele bisecta (Conrad, 1849); Amano et al. 2013: fig. 6E, I.
2015 Conchocele taylori sp. nov.; Hickman 2015: 12, fig. 4E–G.
2016 Conchocele bisecta (Conrad, 1849); Nobuhara et al. 2016: figs. 2–15.
Type material: Holotype UCMP 110688, paratype UCMP 110689 (Hickman 2015).
Type locality.—Rock Creek seep carbonate (for details, see Hickman 2015: 14).
Type horizon.— Keasey Formation, upper Eocene.
Material.—The material examined comprises at least 13 specimens from Canyon River (uppermost Eocene–lower Oligocene, western Washington State, USA), including two figured specimens (ZPAL L.16/3–4; Fig. 7C, E); nine specimens from Bullman Creek (lower Oligocene, northwestern Washington State, USA), including one figured specimen (ZPAL L.16/5; Fig. 7F); three specimens from Murdock Creek (upper lower Oligocene, northwestern Washington State, USA); and three specimens from Whiskey Creek (upper Eocene, northwestern Washington State, USA), and three specimens from LACMIP loc. 16504 (upper Oligocene, western Washington State, USA). The collection from Japan comprises 66 specimens from Tanami (upper Eocene–early Oligocene, Honshu); 199 specimens from Tappu (upper Eocene, Hokkaido); and 82 specimens from Yayoi (upper Eocene, Hokkaido), all housed in JUE; 14 specimens from Kami-Atsunai (lower Oligocene, Hokkaido) housed at ZPAL, including two figured (ZPAL L.16/2 and ZPAL L.16/6; Fig. 7B, G); and 226 specimens from Kami-Atsunai housed at JUE. The original description of Hickman (2015) was based on small specimens < 25 mm long, in this study we figure and describe specimens up to 69 mm long.
Emended diagnosis.—Shell up to 69 mm long, equivalve. Smaller specimens ovate, larger specimens ovate-triangular; posterior sulcus on smaller specimens weak, progressively stronger on larger specimens; umbones prominent; anterior dorsal margin protruding and concave on specimens smaller than approximately 30 mm; anterior margin of larger specimens with protruding anteroventral angle. Shell surface with numerous, fine prominent growth lines; ligament sunken in deep groove; lunule broad and shallow; dentition unknown.
Description.—Umbo incurved, moderately prominent. Umbonal angle close to 90°. Lunule lanceolate, moderately deep. Anteroventral angle broad and convex, bound by two indistinct ridges. On largest specimens lunule absent, anterior margin weakly convex in dorsalmost part, straight in the remaining part. Ventral margin convex, broadly arcuate, with deepest point posterior to mid-line. Posterior of the shell moderately pointed in specimens, rounded in larger specimens. Posterior shell margin indented by a moderately deep sinus, posterior sulcus rather shallow and weakly convex. Posterior fold prominent and well visible, some specimens have an additional, weak ridge mid-fold. Dorsal margin arcuate. Ligament sunken, occupying approximately 2/3 of the dorsal margin. Anterior adductor muscle scar relatively well impressed, elongated but shorter than that of other Conchocele species, occupying ca. 1/4 of the shell length. Anterior pedal retractor muscle scar small, separated from anterior adductor muscle scar. Posterior adductor muscle scar small, elongated, located inside posterior fold.
Remarks.—Conchocele taylori differs from the late Eocene–early Oligocene C. bathyaulax Hickman, 2015, from several non-seep localities from the Keasey Formation in Oregon, USA, by its greater umbonal angle, a less prominent umbo, a more prominent posterior fold, and by having a concave anterior margin (Hickman 2015). The Miocene Conchocele bisecta (Conrad, 1849) from the Astoria Formation in Oregon differs from Conchocele taylori by its more pronounced umbo in smaller specimens, and more quadrangular shell shape in larger specimens. The anteroventral margin of smaller specimens of Conchocele taylori is less pointed than that of C. bisecta. The anterior adductor muscle scar of Conchocele taylori is shorter than that of C. bisecta. Conchocele townsendi has much more elongated shell, and deeper posterior sulcus as compared to C. taylori.
Stratigraphic and geographic range.—Late Eocene–Oligocene, North Pacific margin from Oregon to southern Honshu.
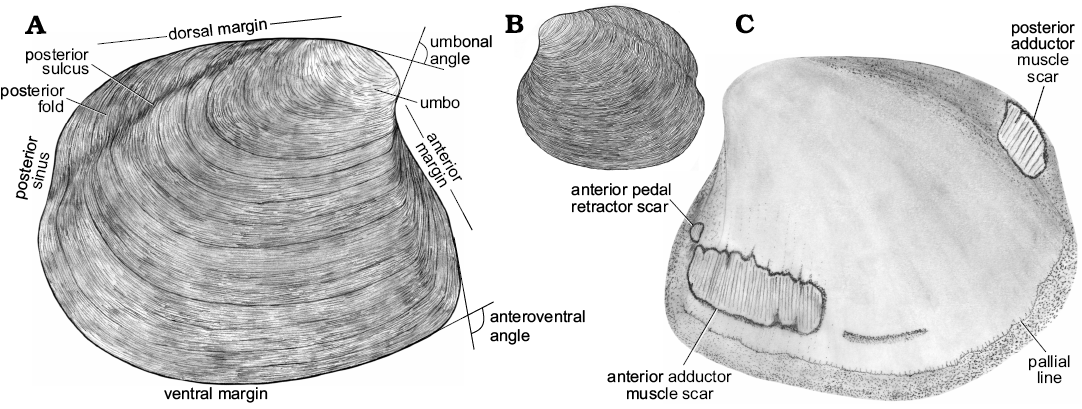
Fig. 6. Schematic illustration of the thyasirid bivalve Conchocele taylori Hickman, 2015, highlighting its main morphological features. A. Based on JUE 16038, a larger, presumably adult shell, exterior of the right valve. B. Based on ZPAL L.16/6, a small, presumably juvenile shell, exterior of the left valve. C. Based on ZPAL L.16/6, an internal mold of a larger presumably adult specimen, left valve. Not to scale.
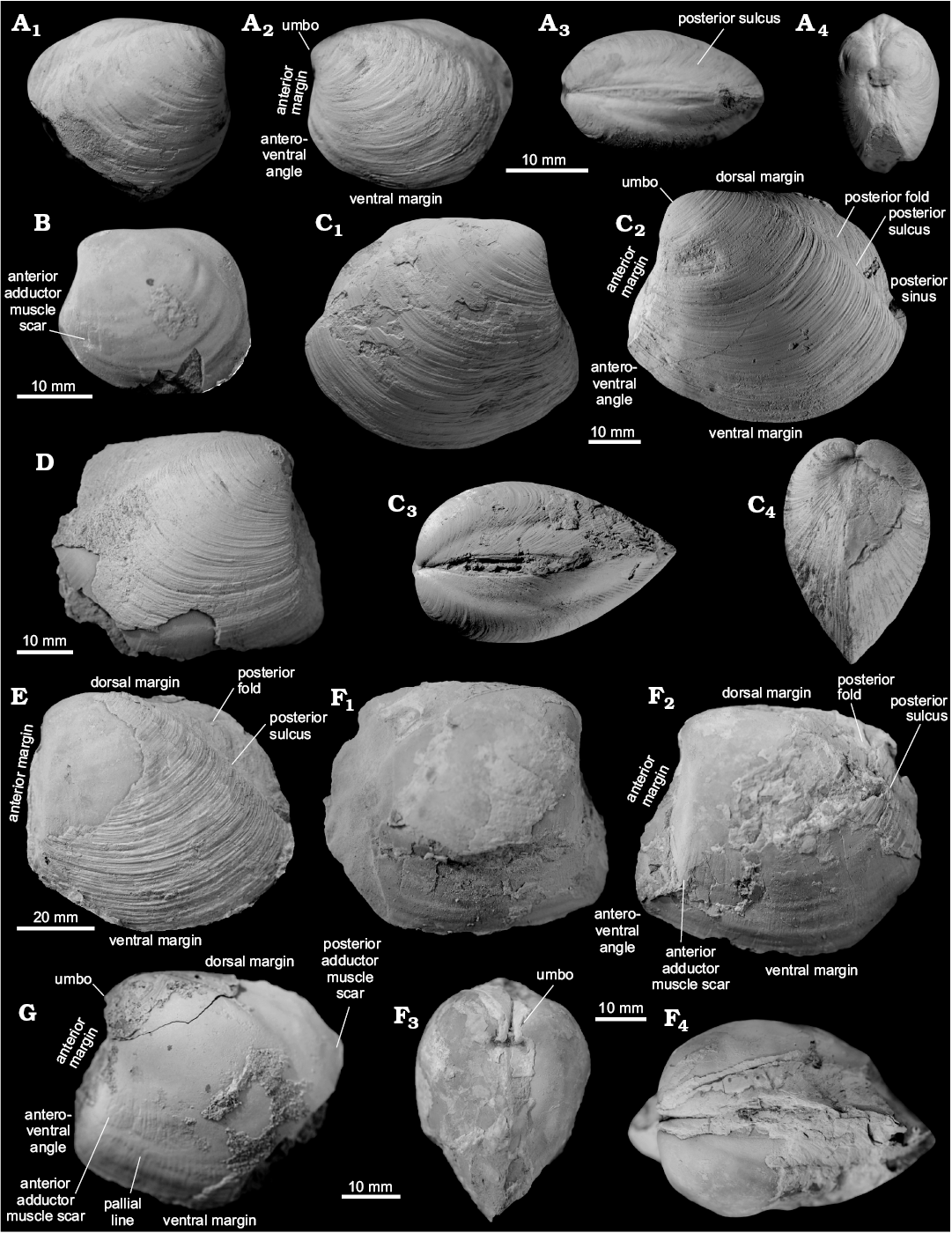
Fig. 7. Thyasirid bivalve Conchocele taylori Hickman, 2015. A. JUE 16038 from a upper Eocene seep carbonate of the Poronai Formation, Yayoi Town, Hokkaido, Japan; a partially preserved smaller specimen in right (A1) and left (A2) lateral views, showing a relatively rounded shell outline; dorsal aspect with relatively weak posterior sulcus (A3); an anterior view (A4), showing relatively flat anterior area with weak ridges running towards the anteroventral angle. B. ZPAL L16/2 from a lower Oligocene seep carbonate of the Nuibetsu Formation, Kami-Atsunai, Hokkaido, Japan; an internal mold of a smaller specimen in left lateral view, showing the anterior adductor muscle scar, and its more angular outline compared to the specimen illustrated in A. C. ZPAL L16/3 from an upper Eocene–lower Oligocene seep carbonate from Canyon River, Lincoln Creek Formation, western Washington State, USA; a medium-sized, well preserved shell in right (C1) and left (C2) lateral views, showing external ornament of fine commarginal growth lines and shell outline; dorsal view (C3) shows shallow posterior sulcus similar to that of JUE 16038; anterior view (C4) shows lunule. D. JUE 16039 from a lower Oligocene seep carbonate from Nuibetsu Formation, Kami-Atsunai, Hokkaido, Japan; a partially preserved shell of a medium-sized individual in right lateral view, showing the well-developed anteroventral angle similar to the specimen shown in C. E. ZPAL L.16/4 from an upper Eocene–lower Oligocene seep carbonate from Canyon River, Lincoln Creek Formation, western Washington State, USA; a large specimen in left lateral view, with poorly incurved umbo (the anteroventral angle was covered with matrix during the preparation of this figure). F. ZPAL L.16/5 from a lower Oligocene seep carbonate from Makah Formation, Bullman Creek, northwestern Washington State, USA; an internal mold with slightly damaged posterior showing right (F1) and left (F2) lateral views and left anterior adductor muscle scar; anterior view (F3) shows incurved umbones and shallow lunule; dorsal view (F4) shows shallow posterior sulcus. G. ZPAL L.16/6 from a lower Oligocene seep carbonate of the Nuibetsu Formation, Kami-Atsunai, Hokkaido, Japan; a partially preserved internal mold in left lateral view of showing anterior adductor muscle scar; note that the anteroventral angle, the curvature of the umbones, and the shape of the preserved dorsal and ventral margins is very similar to that of F.
Conchocele kiritachiensis sp. nov.
Figs. 8, 9.
Etymology: Named for the type locality (Kiritachi).
Type material: Holotype: JUE 16036; an articulated and undeformed specimen with most of the shell preserved. Other figured specimens designated as paratypes (JUE 16037-1 to 16037-7), all from the type locality.
Type locality: A hydrocarbon seep deposit at Kiritachi, Tomamae Town, Hokkaido, Japan.
Type horizon: Sakasagawa Formation, upper Eocene.
Material.—Type material and 10 unnumbered specimens from the type locality, all stored in JUE paleontological collections.
Dimensions.—The holotype is 45.5 mm long and 41.1 mm high.
Diagnosis.—Medium-size species of Conchocele with umbones displaced strongly towards anterior, straight anterior shell margin, pointed beaks and obtuse anteroventral angle. Posterior sinus deep and obtuse. Posterior fold broad but short.
Description.—Shell medium-sized, up to 62.1 mm long, equivalve, moderately inflated, rhomboidal in outline. External ornament composed of densely spaced, low commarginal growth lines. Umbones terminal, umbonal angle around 95°, beaks prosogyrous, not incurved, anteroventral angle around 120°. Lunule absent, but anterior area demarcated with crude ridge running from umbo toward anteroventral angle. Ventral margin nearly straight close to anteroventral angle, well rounded in median and posterior part, with deepest curvature located posterior to mid-line. Posterior angle around 95°, posterior sinus deep, forming an obtuse angle. Posterior sulcus dorsally convex, sharp. Posterior dorsal margin rounded. Anterior adductor muscle scar well-impressed, striated, elongate ovate. Posterior adductor muscle scar rather small, weakly impressed, visible in both side of posterior sulcus. Ligament sunken but hinge structure unknown.
Remarks.—This species belongs to a group of Conchocele species with rhomboidal shells with pointed beaks and straight anterior shell margin. A similar and coeval species from Eastern Pacific is Conchocele bathyaulax Hickman, 2015 from non-seep deposits of the upper Eocene–lower Oligocene Keasey Formation, Oregon, which has narrower and longer posterior fold than C. kiritachiensis. Conchocele cf. bathyaulax from upper Eocene–Oligocene seeps in Washington State is also similar to C. kiritachiensis, but differs by having a more inflated and shorter shell and an overall larger size.
There are several similar species known in the Cenozoic of the Northwestern Pacific. Yabe and Nomura (1925: pl. 23: 8, 10a) figured “Thyasira” bisecta from the Neogene of Sakhalin. Similar specimens from the upper Pliocene of south-western Sakhalin were later figured and designated by Kristofovich (1936: 29, pl. 1: 3, 4) as “Thyasira” bisecta var. alta. In the following work, Kristofovich (1964: 213, pl. 53: 5) mentions “Thyasira” disjuncta var. alta to occur in the upper Miocene of Sakhalin, Kamchatka and Pliocene of Sado Island. The age of the Sakhalin occurrence was later found by Khudik (1989) to be Miocene–Pliocene. This species is more elongated, has a smaller apical angle, and narrower posterior fold than C. kiritachiensis, but is otherwise similar and belongs to the same species group. Some specimens described by Kristofovich (1936) as new species “Thyasira” pervulgata (Kristofovich 1936: 45, pl. 6: 4, 6) from the Eocene? and “Thyasira” wajampolkana (Kristofovich 1936: 44, pl. 6: 3, 3a) from the Miocene of western Kamchatka could also belong to the same species group as Conchocele kiritachiensis. However, both species are not sufficiently well described and figured to allow a more detailed comparison.
Stratigraphic and geographic range.—Type locality and horizon only.
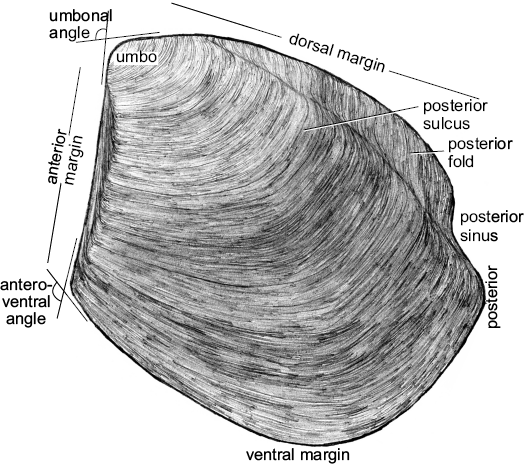
Fig. 8. Schematic illustration of the external shell of the thyasirid bivalve Conchocele kiritachiensis sp. nov. highlighting its main morphological features.
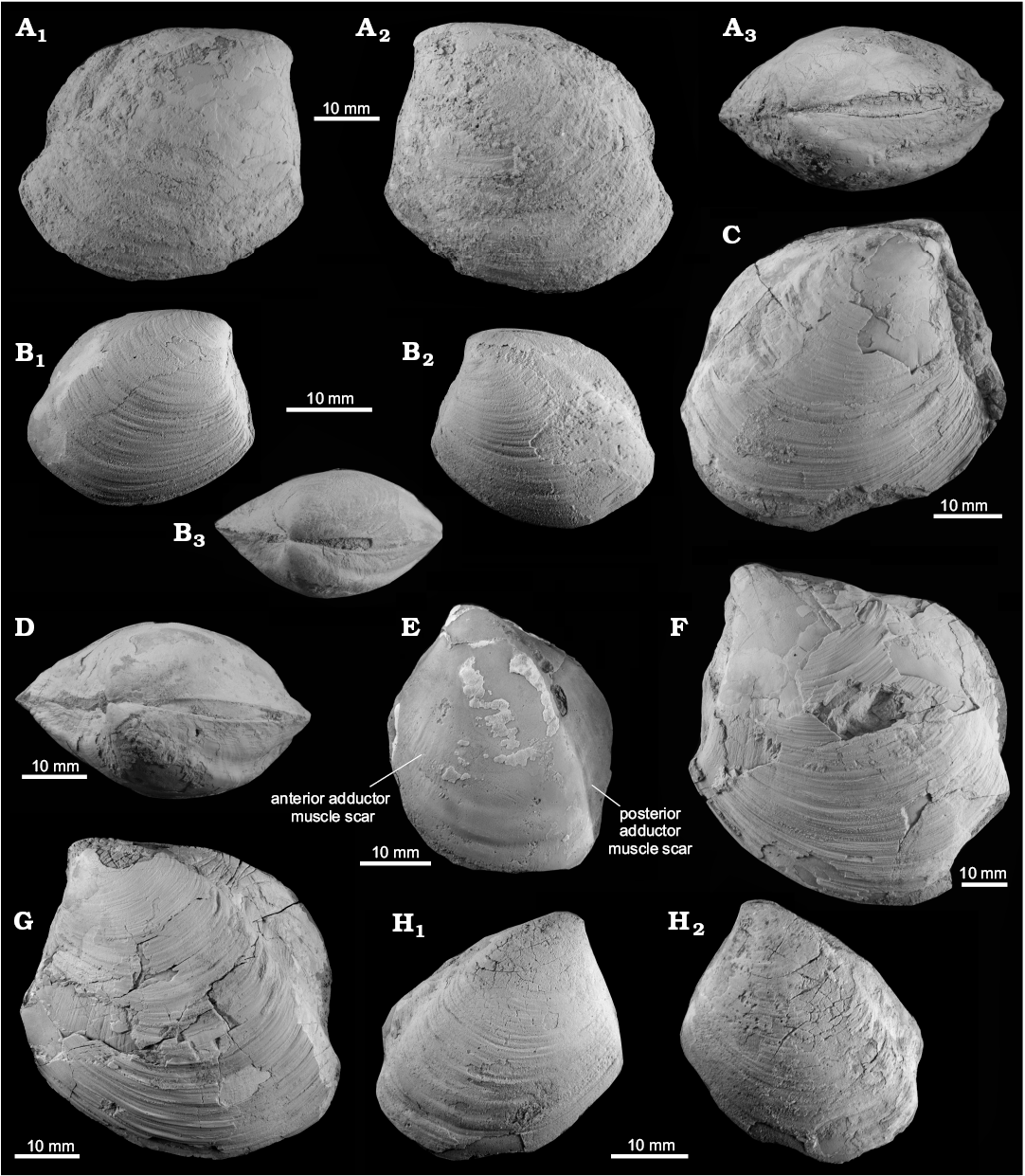
Fig. 9. Thyasirid bivalve Conchocele kiritachiensis sp. nov. from an upper Eocene seep carbonate of the Sakasagawa Formation, Kiritachi, Hokkaido, Japan. A. JUE 16036, holotype in right (A1) and left lateral (A2) views; dorsal view (A3) showing anterior flat area demarcated by rude ridge and a posterior fold. B. JUE 16037-3, paratype, young shell in right (B1) and left (B2) lateral and dorsal (B3) views. C. JUE 16037-2, paratype in right lateral view. D. JUE 16037-4, paratype in dorsal view, showing distinct anterior flat area demarcated by rude ridge and a distinct posterior sulcus. E. JUE 16037-7, inner side of paratype in left lateral view, showing both anterior and posterior adductor muscle scar. F. JUE 16037-6, paratype in left lateral view, the largest specimen of this species, having a pointed umbo and a wide posterior fold. G. JUE 16037-1, paratype, large specimen in left lateral view, showing a pointed umbo and a wide posterior fold. H. JUE 16037-5, paratype in right (H1) and left (H2) lateral views, showing narrow apical angle, instead posterior fold partly broken.
Conchocele cf. bathyaulax Hickman, 2015
Fig. 10.
Material.—Six specimens from Canyon River (upper Eocene–lower Oligocene, western Washington State, USA), including one figured (ZPAL L.16/8; Fig. 10B); and three specimens from East Twin River (upper Oligocene, northwestern Washington State, USA), including two figured (ZPAL L.16/7, 9; Fig. 10A, C).
Description.—Shell large, up to 80 mm long, thin, equivalve, inflated, subquadrate to rhomboidal. External ornament composed of densely spaced, commarginal growth lines. Umbones terminal and prosogyrous, not incurved. Lunule absent, anterior of shell flattened with a weak anterior ridge running from the umbo towards anteroventral shell angle. Anterior margin straight, anteroventral angle obtuse. Ventral margin rounded, with deepest point located posterior to mid-line. Posterior shell extremity weakly pointed. Posterior sulcus straight to weakly dorsally convex. Posterior fold high, with weak ridge running amidst. Inner shell surface covered with thin radial striae. Anterior adductor muscle scar well impressed, elongated, striated, covering ca. 1/3 of shell length, separated from pallial line. Anterior pedal retractor muscle scar medium-sized, striated. Posterior adductor muscle scar weaker than anterior one, striated, restricted to posterior fold. Ligament unknown.
Remarks.—Conchocele bathyaulax and Conchocele cf. bathyaulax share a characteristic inflated shell with projecting umbo and straight anterior margin. They differ by the width of the posterior fold, which is narrower in C. bathyaulax compared to C. cf. bathyaulax, and C. bathyaulax is shorter than C. cf. bathyaulax. Due to the large intraspecific morphological variability exhibited by many thyasirids (e.g., Oliver and Killeen 2002; Oliver and Sellanes 2005; Rodrigues et al. 2008), we prefer to leave C. cf. bathyaulax species in open nomenclature and compare it to C. bathyaulax until material warranting a more coherent systematic statement is available. Another similar fossil species is Conchocele kiritachiensis sp. nov., which is less inflated than C. cf. bathyaulax and has a longer shell with more deeply incised posterior sulcus (cf. Fig. 9). All previously mentioned species are similar to the extant C. scarlatoi Ivanova and Moskaletz, 1984, from shallow waters of the Sea of Japan (Kharlamenko et al. 2016), with which they share shells with projecting umbones and straight anterior margin lacking a lunular incision. Concocele taylori from uppermost Eocene–Oligocene seep carbonates of paleo-Northern Pacific margin has more rounded shell, with more dorsally convex posterior fold and protruding anteroventral angle (cf. Fig. 7). The Miocene C. bisecta has a more rounded shell with a concave anterior margin, a lunular incision, and a rectangular anteroventral margin (Moore 1963).
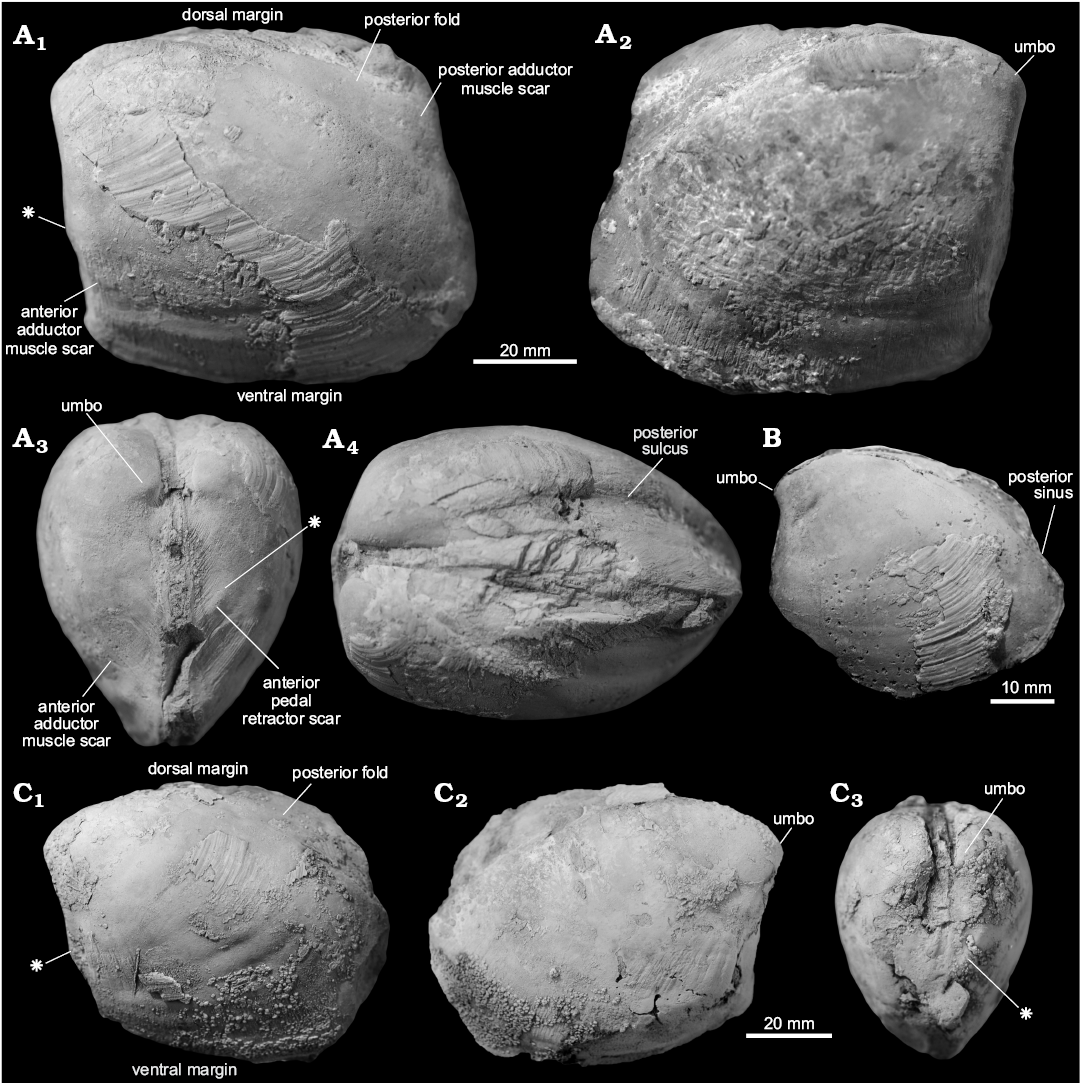
Fig. 10. Thyasirid bivalve Conchocele cf. bathyaulax Hickman, 2015. A. ZPAL L.16/7 from an upper Oligocene seep carbonate of the Pysht Formation, East Twin River, northwestern Washington State, USA; an articulated specimen in left (A1) and right (A2) views, showing characteristic rhomboidal shape with roughly parallel, convex dorsal and ventral margins, and roughly parallel anterior and posterior margins, asterisk shows a lateral aspect of a secondary ridge (A1); anterior view (A3), showing anterior pedal and adductor muscle scars, weakly incurved umbones and inflated shells, asterisk shows a frontal aspect of a secondary ridge shown in A1; dorsal view (A4), shows moderately deep posterior sulcus. B. ZPAL L.16/8 from an upper Eocene–lower Oligocene of the Canyon River, Lincoln Creek Formation, western Washington State, USA; a medium-sized specimen in left lateral view, showing incurved umbo. C. ZPAL L.16/9 from an upper Oligocene of the Pysht Formation, East Twin River, northwestern Washington State, USA; a large specimen in left (C1) and right (C2) lateral views with umbo more protruding than ZPAL L.16/7, 8 (A, B), asterisk shows a lateral aspect of a secondary ridge shown in C3; anterior view (C3) showing weak secondary ridge running from umbo towards the anteroventral angle (asterisked).
Genus Maorithyas Fleming, 1950
Type species: Maorithyas marama Fleming, 1950; Recent, Fiordland, New Zealand.
Species included: Maorithyas humptulipsensis sp. nov. from middle Eocene seep carbonates at LACMIP loc. 12385 and CSUN loc. 1583, Humptulips Formation, Humptulips River, Washington State, Maorithyas folgeri (Wagner and Schilling, 1923), from the San Emigdio (Oligocene) and Wagonwheel (Eocene) formations, Kern County, Southern California, USA; Maorithyas marama Fleming, 1950, Recent, southern New Zealand and Maorithyas flemingi Powell, 1955, Recent, southern New Zealand. Possibly also Maorithyas? sp. from the late Eocene Bear River seep deposit in western Washington State, USA.
Maorithyas humptulipsensis sp. nov.
Figs. 11, 12.
1990 Thyasira (Conchocele) folgeri Wagner and Schilling, 1923; Goedert and Squires 1990: fig 2a.
Etymology: Named after the Humptulips River.
Type material: Holotype: ZPAL L.16/10 (Fig. 12A); an articulated specimen with most of the shell preserved. Other figured (Fig. 12B–D)specimens designated as paratypes (NRM Mo 182388, ZPAL L.16/11, ZPAL L.16/12), all from the type locality.
Type locality: LACMIP loc. 12385, Humptulips River, Washington State, USA.
Type horizon: Seep carbonates of the Humptulips Formation, middle Eocene.
Material.—From the type locality: type material and 5 unnumbered specimens housed at ZPAL, 32 housed at NRM from CSUN loc. 1583 (middle Eocene, Humptulips River, western Washington State, USA): 10 unnumbered specimens, the largest of which reaches 45 mm in length, housed at NRM.
Dimensions.—The holotype is 35 mm long and 35 mm high.
Diagnosis.—Bulbous species of Maorithyas with pointed anteroventral angle and weak posterior fold not extending beyond outline of shell.
Description.—Shell up to 62 mm long, thin, equivalve, very inflated, subrounded to pentagonal. External ornament composed of very fine, commarginal growth lines. Umbones subcentral, anterior of midline of the shell, prosogyrous, strongly incurved. Lunule present, heart-shaped, shallow. Anterior margin straight and short, bound by two indistinct ridges running from umbo, anteroventral angle obtuse. Ventral margin rounded, with deepest point around mid-line. Posterior margin straight with very weak sinus. Posterior fold not projecting beyond posterior margin of the shell, posterior sulcus shallow and broad, with weak secondary sulcus sometimes visible amidst fold. Posterodorsal margin weakly concave. Escutcheon absent. Ligament narrow, sunken. Interior of shell covered with thinly spaced, radial striae, more common in anterior. Anterior adductor muscle scar weak and small, visible only in its anterior part. Anterior pedal retractor muscle scar shallow, small and oval. Posterior adductor muscle scar small and weak, restricted to posterior fold.
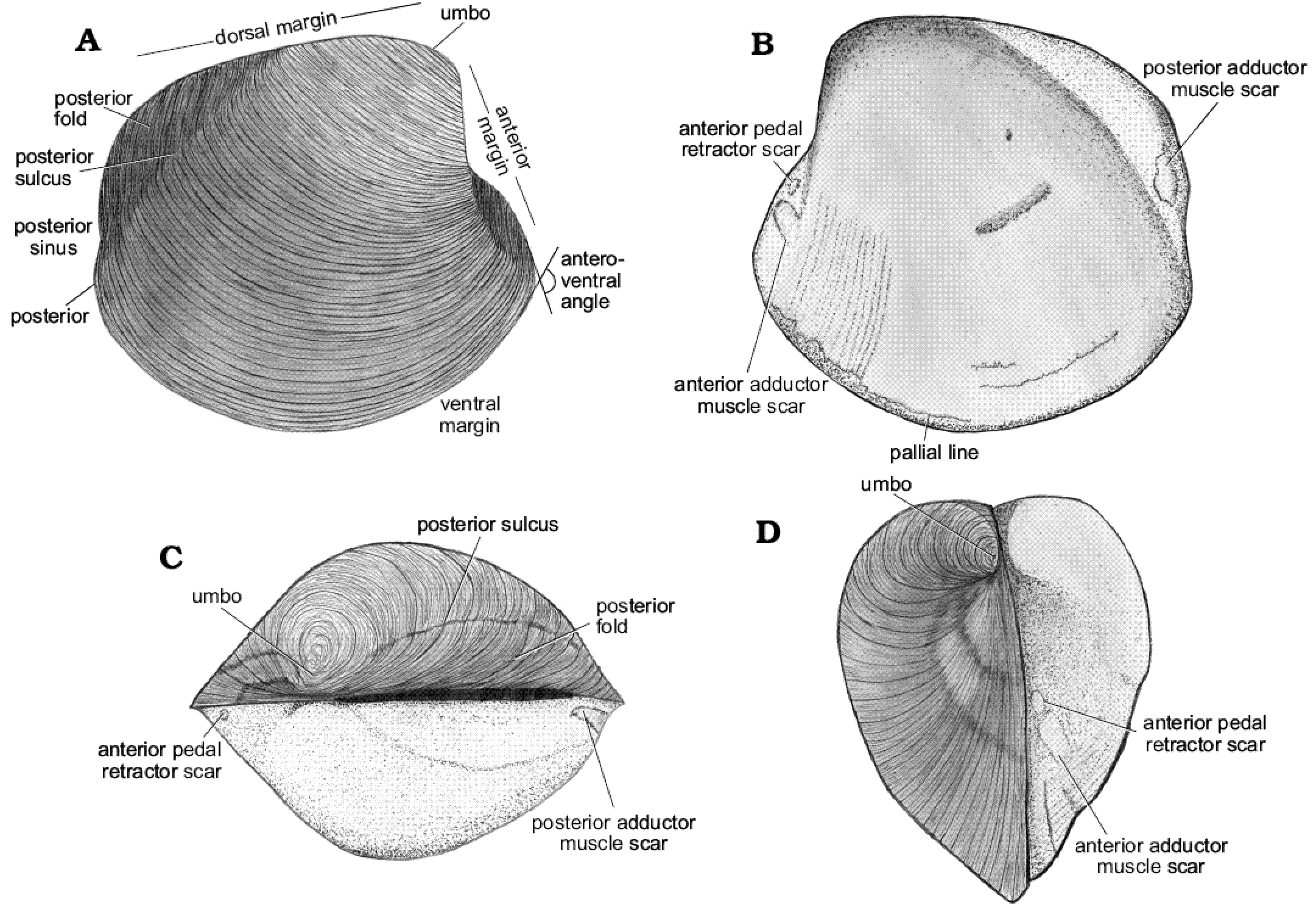
Fig. 11. Schematic illustration of the thyasirid bivalve Maorithyas humptulipsensis sp. nov., highlighting its main morphological features. A. Outer surface of the right valve in lateral view. B. Internal surface of the right valve in lateral view. C. Outer surface of the right valve in dorsal view and internal mold of the left valve view. D. Outer surface of the right valve in anterior view and internal mold of the left valve view.
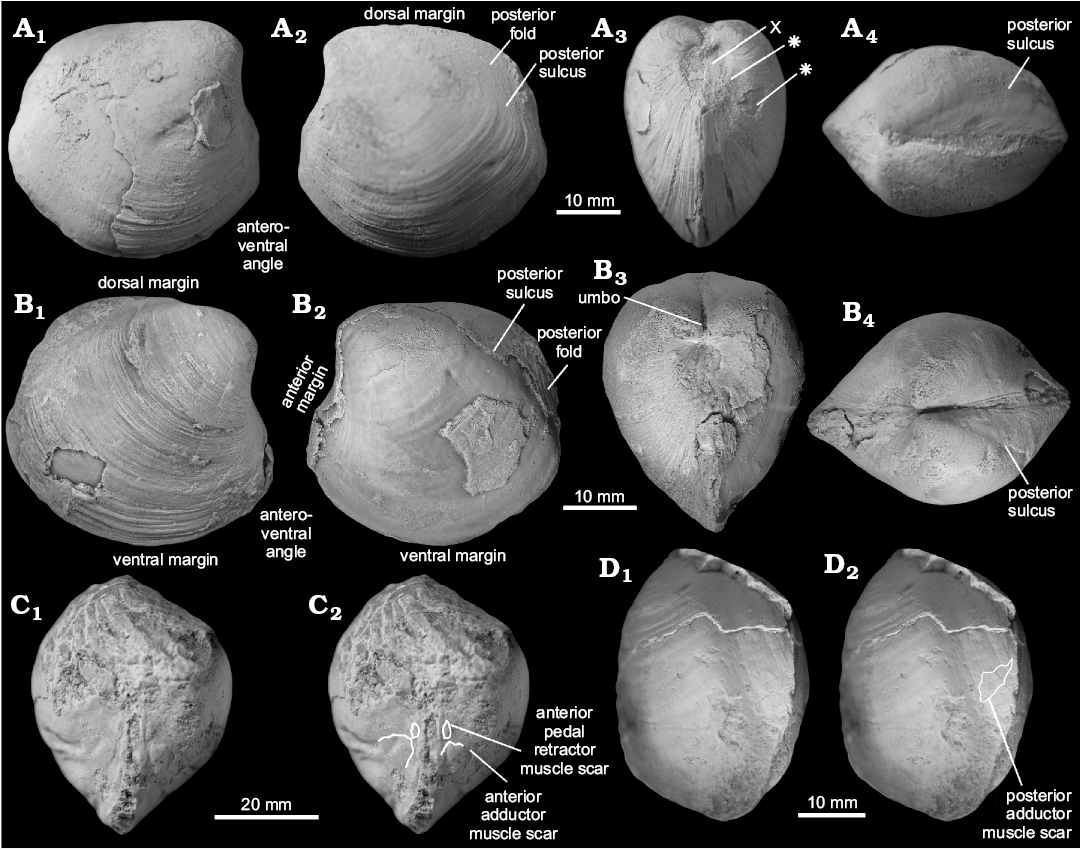
Fig. 12. Thyasirid bivalve Maorithyas humptulipsensis sp. nov. from a middle Eocene seep carbonate of the Humptulips Formation, LACMIP loc. 12385, Washington State, USA. A. ZPAL L.16/10, holotype in right (A1) and left (A2) lateral views; anterior view (A3), showing two ridges running from umbo towards the anterioventral angle (asterisks), bounding weak lunule (×). B. NRM Mo 182388, a partially preserved specimen in right (B1) and left (B2) lateral views; the shape similar to that of the specimen shown in A, although the dorsal margin is more convex; the anterior view (B3) shows inflated shell with incurved umbo; dorsal view (B4) shows weak posterior sulcus. C. ZPAL L.16/11, a partially preserved internal mold in anterior view (C1); the interpretative drawing (C2) shows the position of anterior adductor and anterior pedal retractor muscle scars. D. ZPAL L.16/12, specimen in oblique posterior view (D1), the interpretative drawing (D2) shows the posterior adductor muscle scar.
Remarks.—Maorithyas humptulipsensis differs from the Recent Maorithyas marama Fleming, 1950, and Maorithyas flemingi Powell, 1955, by its more rhomboidal shell, more anteriorly situated umbo and less pronounced posterior fold, which does not extend beyond the posterior margin of the shell (cf. Fleming 1950: pl. 25: 2; Powell 1955: pl. 1: 1). However, the degree of extension of the posterior fold seems to have some range of variation in the type species. For example, three specimens identified as M. marama, collected at Stewart Island and stored at the Museum of New Zealand Te Papa Tongarewa (M 26537) show a narrower posterior fold than the type. The Recent “Maorithyas” hadalis from the Japan Trench figured by Fujikura et al. (1999: fig. 3) and Okutani et al. (1999) differs in characters of the hinge plate, nymph and anterior adductor muscle scar from Maorithyas humptulipsensis, and may not even belong to Maorithyas (Valentich-Scott et al. 2014: 17).
Maorithyas is a poorly known genus, comprising only two named and one unnamed extant species (Fleming 1950; Powell 1955; Spencer 2009: 199). In addition to a single fossil species M. humptulipsensis, it is possible that “Thyasira” folgeri Wagner and Schilling, 1923, from upper Eocene seep carbonates in the Wagonwheel Mountains in central California (Squires and Gring 1996; Kiel and Peckmann 2007) belongs to Maorithyas. The holotype of “Thyasira” folgeri (UCMP 11434, UCMP loc. 3195; Oligocene, San Emigdio Formation, Devil’s Kitchen, Kern County, southern California, USA; Moore 1988: pl. 7: 9; Fig. 13) is similarly rounded and inflated as M. humptulipsensis, and also has a very weak to no distinct posterior sinus. It has, however, a less pronounced posterior fold different from that of M. humptulipsensis, and a less pronounced anteroventral angle. Thyasira folgeri from the Wagonwheel Mountain seep (Squires and Gring 1996: fig. 4.8) is very similar to the holotype in general outline and the lack of a posterior sinus, and is also unlikely to belong to Conchocele as classified by Squires and Gring (1996). In our opinion, “Thyasira” folgeri from both mentioned localities in Kern County should be considered a species of Maorithyas, thus being Maorithyas folgeri (Wagner and Schilling, 1923). The holotype of T. folgeri was found together with lucinids in “limy concretions in the black shale member” (Wagner and Schilling 1923: 243) and might hence also be from a seep deposit.
Stratigraphic and geographic range.—Middle Eocene seep deposits (LACMIP loc. 12385 and CSUN loc. 1583) from the Humptulips Formation, Washington State, USA.
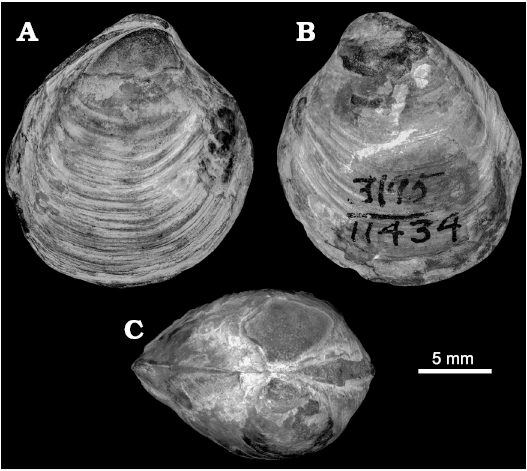
Fig. 13. Thyasirid bivalve Maorithyas folgeri (Wagner and Schilling, 1923) from Oligocene strata of the San Emigdio Formation, Devil’s Kitchen, California, USA. UCMP 11434, holotype in right (A), left (B) lateral, and dorsal (C) views.
Maorithyas? sp.
Fig. 14.
Material.—One specimen (ZPAL L.16/13) from the upper Eocene Bear River site from the “Siltstone of Cliff Point” in western Washington State, USA.
Remarks.—The specimen from the Bear River site is incomplete and weathered, therefore its systematic assignment is only tentative. Its inflated shell and very shallow posterior sulcus somewhat resembles that of Maorithyas humptulipsensis from the middle Eocene Humptulips River site in Washington State. However, the Bear River specimen is 21 mm long and shorter than the majority of the specimens of Maorithyas humptulipsensis available for comparison. Also, it has a somewhat less pronounced posterior fold than M. humptulipsensis, and a less pronounced umbo, suggesting that the Bear River specimen does not belong to M. humptulipsensis. Any further discussion of the Bear River species has to be postponed until more material from this locality is available.
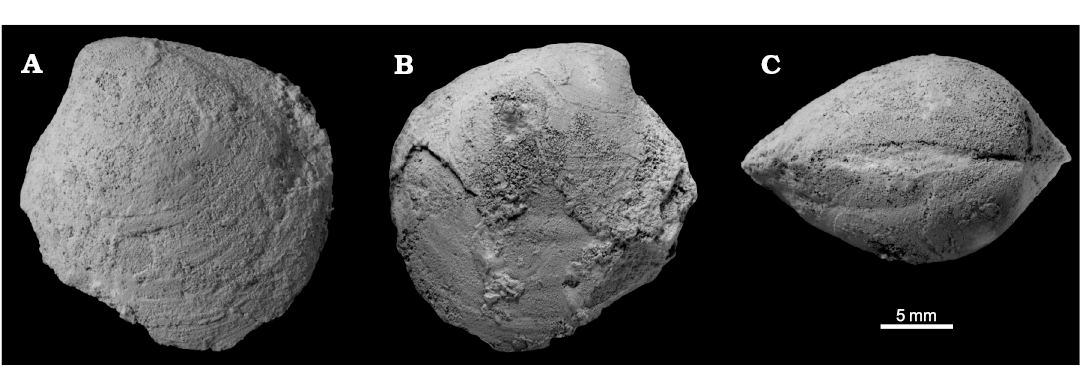
Fig. 14. Thyasirid bivalve Maorithyas? sp. from upper Eocene cold seep carbonates in the “Siltstone of Cliff Point”, Bear River, western Washington State, USA. ZPAL L.16/13, a poorly preserved specimen in left (A), right (B) lateral, and dorsal (C) views.
Genus Thyasira Lamarck, 1818
Type species: Thyasira flexuosa (Montagu, 1803); Recent, Falmouth Harbour, Cornwall, UK.
Thyasira tanabei Kiel, Amano, and Jenkins, 2008
Fig. 15.
1993 Conchocele? sp.; Kanie et al. 1993: fig. 2.
2008 Thyasira tanabei sp. nov.; Kiel et al. 2008: 530, fig. 5A–G, fig. 6A.
?2009 Thyasirid bivalve; Kiel et al. 2009: fig. 3L, M.
2017 Thyasira tanabei Kiel, Amano, and Jenkins, 2008; Jenkins et al. 2017: fig. 3H, I.
Type material: Holotype (UMUT MM 29533) and paratypes (UMUT MM 29534–29537, 29539) (Kiel et al. 2008).
Type locality: Yasukawa seep site in Nakagawa Town, Hokkaido.
Type horizon: Omagari Formation, Yezo Group, lower Campanian, Upper Cretaceous.
Material.—Three illustrated specimens (ZPAL L.16/14–17; Fig. 15) and 3 unnumbered from Santonian cold seep carbonates from Maeshima, Kyushu, Japan.
Remarks.—The specimens from Santonian cold seep carbonates of Himenoura Group, Maeshima, Kyushu, are similar in their general appearance to Thyasira tanabei specimens from Cretaceous cold seep, sunken leatherback turtle carcass and possibly sunken wood associations from Yezo Group, Hokkaido, previously described and figured by Kiel et al. (2008, 2009) and Jenkins et al. (2017). The specimens from Maeshima (max. 5 mm in length) are smaller than their conspecifics from cold seeps of Hokkaido, which approach 12 mm in length (Kiel et al. 2008: table 2), and are also less inflated and have more pronounced posterior folds. In shape, the Maeshima specimens resemble an undescribed thyasirid species from the Akita River wood fall in Hokkaido, which are in the size range of the Maeshima specimens (4 mm in length; Kiel et al. 2009: 78, fig. 3L, M) and are also only slightly inflated. However, due to poor preservation of the material from the Akita River wood fall, a more detailed comparison between the specimens of the two sites is currently not possible.
Stratigraphic and geographic range.—Cretaceous of Japan; known from Albian–Campanian cold seeps of Hokkaido (the Ponbetsu, Kanajirisawa, and Yasukawa sites; Kiel et al. 2008), Santonian cold seeps of Maeshima, Kyushu, Campanian turtle fall from Nio Creek, Hokkaido, and questionably the Coniacian Akita River wood-fall on Hokkaido (Kiel et al. 2009).
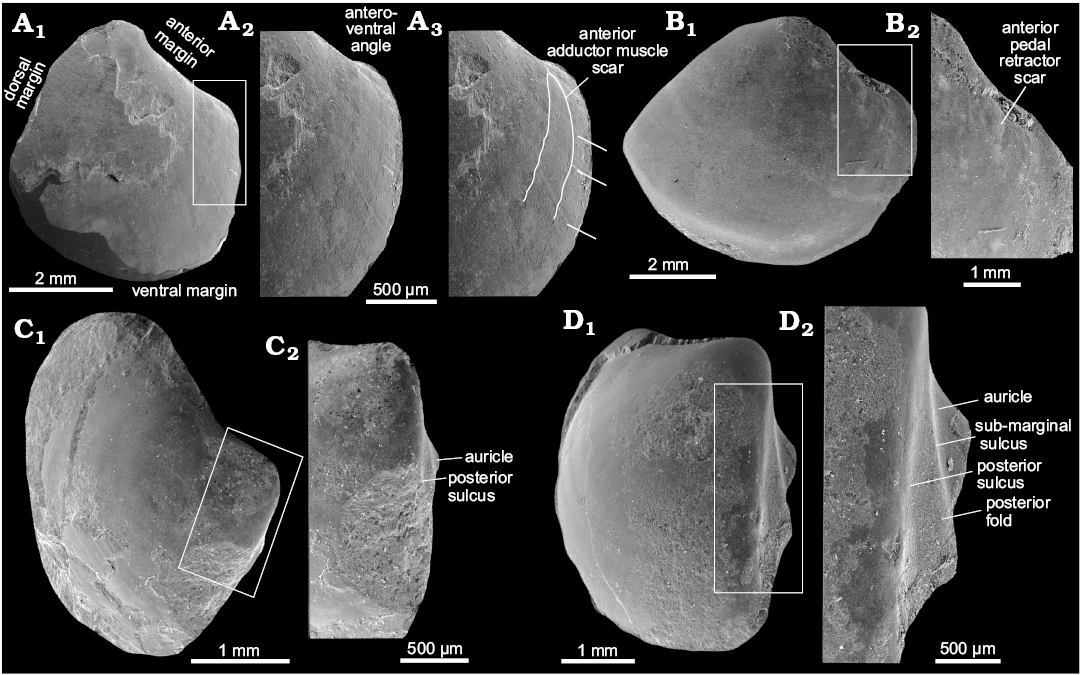
Fig. 15. Thyasirid bivalve Thyasira tanabei Kiel, Amano, and Jenkins, 2008, from Santonian seep carbonates from the Himenoura Group, Maeshima, Goshoura Island, Kumamoto Prefecture, southern Kyushu, Japan. A. ZPAL L.16/14, a partial internal mold in oblique right lateral view (A1); detail with indicated radial striae and anterior adductor muscle scar (A2, A3). B. ZPAL L.16/15, a partial internal mold in oblique right lateral view (B1); probably anterior pedal retractor muscle scar (B2). C. ZPAL L.16/16, a specimen in oblique dorsal view (C1); a very weak posterior sulcus and posterior fold, with minute auricle visible (C2). D. ZPAL L.16/17, a specimen in oblique posterodorsal view (D1); posterodorsal shell margin with posterior sulcus, posterior fold, submarginal sulcus and auricles indicated (D2).
Thyasira becca Kauffman, 1967
Fig. 16.
1967 Thyasira becca becca subsp. nov.; Kauffman 1967: 126, pl. 4:1–21, pl. 5: 28, 33, 37, 38.
1967 Thyasira becca cobbani subsp. nov.; Kauffman 1967: 132, pl. 1: 28, pl. 5: 34–36.
Type material: Holotype: USNM 153512; paratypes listed by Kauffman (1967).
Type locality: USGS Mesozoic locality D1410, Fall River County, South Dakota.
Type horizon: Middle Baculites scotti Zone, Pierre Shale, upper Campanian, Upper Cretaceous.
Material.—Three illustrated specimens (ZPAL L.16/18–20; Fig. 16) from Campanian seep carbonate at Warbonnet Battlefield Monument, Montrose, Nebraska.
Remarks.—The specimens from Montrose fit the description of Thyasira becca from the Campanian of the Western Interior Seaway (Kauffman 1967). Thyasira becca was initially subdivided into two subspecies: the rounded T. becca becca, and the subrectangular T. becca cobbani (Kauffman 1967: 126); however we hesitate to include our specimens in any of those subspecies due to the small number of available specimens. Thyasira becca is significantly larger and more rounded than T. tanabei from the Albian–Campanian cold seeps and sunken wood from Japan (Kiel et al. 2008). Thyasira xylodia from Eocene–Miocene whale and wood falls in Washington State is, at a length of approximately 10 mm, more triangular and higher than the Montrose specimens, and it also differs by having a much lower posterior fold (Kiel and Goedert 2007: fig. 7A).
Thyasirid species from the Western Interior Seaway belong to the Thyasira advena–Thyasira becca species complex, comprising large, rounded to oval species of Thyasira with protruding umbones. The group comprises T. advena sensu lato, T. becca sensu lato, and T. cantha (Kauffman 1967). The species complex is contrasted with the Thyasira rostrata complex, comprising smaller, thin-shelled and triangular species with weakly protruding umbones. This group is composed of T. beauchampi sensu lato, T. quadrula, T. rostrata, and T. triangulata. Shape and size-wise, the species from the T. advena–T. becca complex resemble Recent rounded species from the T. sarsi–T. southwardae–T. methanophila complex, comprising chemosymbiotic opportunists (T. sarsi) and seep-restricted specialists (T. methanophila) (Taylor et al. 2007; Amano et al. 2015).
Stratigraphic and geographic range.—Campanian of the Western Interior Seaway, North America. The species has been recorded from cold seep carbonates known as “Tepee Buttes” (Kauffman et al. 1996) and from non-seep localities in Colorado, South Dakota and potentially Canada (Kauffman 1967). Here we report the species from a Campanian “Tepee Butte” seep carbonate from Warbonnet Battlefield Monument, Montrose, northwestern Nebraska.
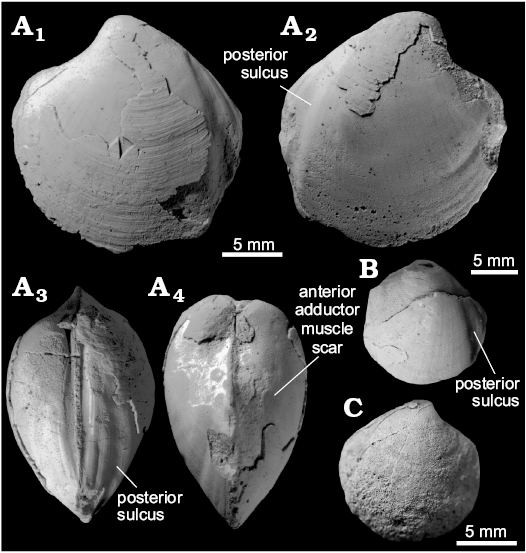
Fig. 16. Thyasirid bivalve Thyasira becca Kauffman, 1967, from a Campanian seep carbonate at Warbonnet Battlefield Monument, Montrose, Nebraska, USA. A. ZPAL L.16/18, a partial internal mold in left (A1) and right (A2) lateral views, with fragments of the shell preserved and weak radial striation on the surface of the mold; dorsal view (A3) shows broad posterior sulcus; anterior view (A4) shows poorly preserved anterior adductor muscle scar. B. ZPAL L.16/19, a partial internal mold in left lateral view, showing broad posterior sulcus and posterior part of the posterior adductor muscle scar. C. ZPAL L.16/20, of a complete internal mold in right lateral view showing the shell outline.
Thyasiridae gen. et sp. indet. A
Fig. 17A.
Material.—One illustrated specimen (ZPAL L.16/21; Fig. 17A) from middle to upper Eocene cold seep carbonates of the “Siltstone of Unit B” from the West Fork of Grays River, western Washington State, USA.
Description.—Shell ovate, the available specimen is 18.9 mm long and 19.3 mm high with preserved surface covered with fine commarginal growth lines. Sulcus well visible, moderately deep and straight. Umbo poorly incurved. Anterior and posterior adductor muscle scars unknown.
Remarks.—This species is in rather poor condition and we leave it in open nomenclature. It could represent a juvenile specimen of an unidentified species of Conchocele (e.g., Kharlamenko et al. 2016: fig. 4D–F), a specimen of an unidentified species of Maorithyas or of Thyasira.
Thyasiridae gen. et sp. indet. B
Fig. 17B.
Material.—One illustrated specimen (ZPAL L.16/22; Fig. 17B) from upper Oligocene cold seep carbonates (Lincoln Creek Formation) of SR4 locality, from the Satsop River area, western Washington State, USA.
Description.—Shell quadrangular-oval, 29 mm long, 26.8 mm high. Umbo poorly incurved. Posterior sinus shallow but present, posterior sulcus shallow, broad. Posterior fold high, sculptured by radial ridge running from the umbo towards the posterior end of fold. Surface sculptured by commarginal growth line. Muscle scars unknown.
Remarks.—This specimen resembles smaller individuals of Conchocele taylori in outline and the height of the posterior fold, and C. cf. bathyaulax in the presence of secondary radial ridge amidst posterior fold. However this assignment cannot be confirmed due to the damage of the only specimen available from SR4. More specimens are needed before this species can be assigned to any genus of thyasirids.
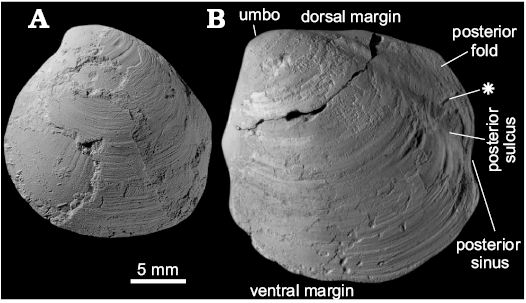
Fig. 17. Thyasiridae gen. et sp. indet A and B, respectively. A. ZPAL L.16/21 from middle to upper Eocene cold seep carbonates of West Fork of Grays River, “Siltstone of Unit B”, western Washington State, USA; a poorly preserved specimen in left lateral view with partially preserved shell. B. ZPAL L.16/22 from an upper Oligocene, SR4 seep carbonate, Satsop River area, Lincoln Creek Formation, western Washington State, USA; a specimen in left left lateral view showing shell outline, external ornament, and secondary ridge running amidst posterior fold (asterisk).
Discussion
The origins and evolutionary history of the thyasirids and their adaptation to the chemosymbiotic life style are arguably the most obscure among all chemosymbiotic bivalve families. In better-studied examples such as the bathymodiolins, there is a trend from a low degree to a high degree of structural integration of the symbionts (Lorion et al. 2013). Likewise, the pliocardiin vesicomyids, which rely almost exclusively on nutrition from their symbionts, appear to be derived from the asymbiotic vesicomyins (Johnson et al. 2017). Furthermore, bathymodiolins appear to have colonized low-sulfide habitats first and subsequently adapted to high-sulfide environments (Distel 2000). The current understanding of the phylogeny of the thyasirids, however, seems to indicate the opposite pattern: symbiotic species with highly integrated gill endosymbionts appear to be basal and live mostly in sulfide-rich environments such as pockmarks, cold seeps, and fjords rich in organic matter (e.g., Dando and Southward 1986; Dando and Spiro 1993; Dando et al. 1994; Fujiwara et al. 2001; Oliver and Killeen 2002; Dufour 2005; Batstone et al. 2014; Kharlamenko et al. 2016; Åstrom et al. 2017). There is some molecular evidence that smaller, asymbiotic thyasirids (i.e., Axinulus Verrill and Bush, 1898; Mendicula Iredale, 1924; Leptaxinus Verrill and Bush, 1898; Adontorhina Berry, 1947) may form a separate subfamily Axinopsidinae which is derived with respect to the genus Thyasira (i.e., Bernard 1972; Coan et al. 2000); these thyasirids are found mainly in colder waters in polar or deep sea settings (e.g., Payne and Allen 1991; Zelaya 2009, 2010) and do not show a particular association with seep environments.
The fossil record as outlined herein indicates that the thyasirids first appeared in the late Mesozoic, with the oldest species, Cretaxinus hurumi Hryniewicz, Little, and Nakrem, 2014, coming from the earliest Cretaceous cold seep carbonates in Spitsbergen (Berriasian; ca. 140 Ma; Fig. 18; Hryniewicz et al. 2014). This relatively young origin is surprising considering their basal position among heterobranch bivalves (Taylor et al. 2007)—the slightly more derived lucinids have a fossil record dating back to at least the Silurian (ca. 430 million years ago; Liljedahl 1992). Furthermore, as we will discuss below, most thyasirid genera with a fossil record first appear at hydrocarbon seeps and apparently colonized “normal” soft sediments only later in their geological history. It should be kept in mind, though, that the thyasirids could potentially have a much longer fossil record but due to their typically small and thin-shelled nature and their preference for deep water, they have a low fossilization potential (cf. Valentine et al. 2006) and may simply have been overlooked. In contrast, their apparent abundance at fossil seep deposits might be a result of the rapid in situ carbonate precipitation at seeps, which facilitates the preservation of shells, including small and thin-shelled ones (Kiel and Little 2006). Although several large species of modern thyasirids are known from seep environments, thyasirids generally peak in diversity in normal, deep sea benthic environments (Payne and Allen 1991), which likely reflects their deep sea origins.
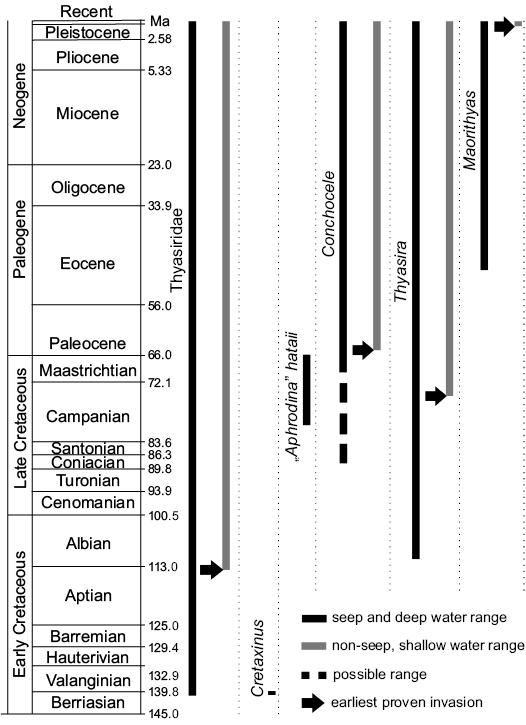
Fig. 18. Geological ranges of Thyasiridae and the discussed thyasirid genera at seeps and non-seep environments.
Even if earlier thyasirids have been overlooked for the reasons outlined above, or because they looked very different from the modern ones, we feel confident that the fossil record as outlined here reflects the history of the radiation of the modern thyasirids. Therefore it should be a valuable baseline for the interpretation of molecular phylogenetic data on this group. We will now go through the relevant clades:
–Cretaxinus hurumi is the oldest known thyasirid, occurs only in earliest Cretaceous seep deposits in Spitsbergen, and has not been seen in the surrounding mudstone (Hryniewicz et al. 2014).
–Thyasira clade 1 consists of the extant species Thyasira flexuosa Lamarck, 1818, Thyasira gouldii Philippi, 1845a, and Thyasira polygona (Jeffreys, 1864), all known chiefly from non-seep environments (e.g., López-Jamar et al. 1987; Oliver and Killeen 2002; Taylor et al. 2007; Batstone et al. 2014; Amano et al. 2015). Their shells are characterized by being higher than long, and by having a median flattened area (Amano et al. 2015). The oldest fossil species of this clade is T. tanabei from Cretaceous deep-water seep deposits in Japan (Albian–Campanian, 113–72 Ma) and possibly also from Cretaceous wood-fall environments from Japan (Kiel et al. 2008, 2009; this study). Thus the T. flexuosa–T. polygona–T. gouldii clade appears to have initially inhabited cold seeps from which they are now absent. A similar pattern is known from the protobranch bivalve Nucinella Wood, 1851, which has been reported from seeps as old as late Triassic and until Oligocene time (Peckmann et al. 2011; Amano et al. 2013; Hryniewicz et al. 2014), but is not known from such environments today. Alternatively, T. tanabei may represent a member of an extinct, seep-specialized Thyasira clade with a shell convergent to that of the T. flexuosa–T. polygona–T. gouldii clade.
–Thyasira clade 2, consisting of the extant Thyasira methanophila Oliver and Sellanes, 2005, T. sarsi (Philippi 1845b) and most likely also T. capitanea Åstrom and Oliver in Åstrom et al., 2017, T. oleophila Clarke, 1989, T. scotiae Oliver and Drewery, 2013; T. southwardae Oliver and Holmes, 2006 and T. volcolutre Rodrigues and Oliver in Rodrigues et al., 2008 (Taylor et al. 2007). A likely early Miocene member of this clade is Thyasira nakazawai from deep-water seep deposits in central Honshu, Japan (ca. 23–16 Ma; Amano et al. 2015) and we suggest that Thyasira becca Kauffman, 1967, from shallow-water seeps and non-seep environments in the Western Interior Seaway could potentially represent a Late Cretaceous member of the T. methanophila–T. sarsi clade. Even older species of this clade include an as-yet unnamed species of Thyasira from Albian (113–100 Ma) deep-water cold seeps from the Ellef Ringnes Island in Canadian Arctic Archipelago (Williscroft et al. 2017), and “Lucina” rouyana d’Orbigny, 1844, from deep-water cold seep carbonates of Czech Carpathians (Valanginian–Hauterivian, ca. 140–130 myr old; Ascher 1906), which has been interpreted as a species of Thyasira (Kiel et al. 2008; Kaim et al. 2013). The latter species need further taxonomic work, but it appears likely that the T. methanophila–T. sarsi clade has been inhabiting seeps since the Cretaceous.
–“Aphrodina” hataii from the Campanian–Maastrichtian “Sada Limestone” seep deposit (Katto and Hattori 1965; Nobuhara et al. 2008) may represent an independent genus (Takami Nobuhara, KH, and SK, unpublished material) and is only known from this seep locality.
–Conchocele Gabb, 1866, arguably represents the most successful radiation of a thyasirid bivalve at seeps, at least in the Pacific Ocean. The oldest documented occurrence of Conchocele is C. townsendi from Maastrichtian (latest Cretaceous; 72–66 Ma) shallow-water seep carbonates and other marine sediments in the James Ross Basin, Antarctica (Little et al. 2015). A potentially older record is “Thyasira” cretacea from Coniacian (89–86 Ma) possible seep carbonates from Enos Canyon, California (Anderson 1958). Within Conchocele, we distinguish two morphologically distinct subclades. The first comprises species with straight anterior margin, and pointed, anteriorly placed beaks, with C. kiritachiensis from the upper Eocene seep carbonates from eastern Pacific margin (Kiritachi, Tomamae-cho, Hokkaido) and Conchocele cf. bathyaulax from the upper Eocene–Oligocene seep carbonates and non-seep environments from western Pacific margin (Washington State). This subclade likely comprises also the extant C. scarlatoi from shallow waters of the Sea of Japan. The second subclade comprises species with protruding anterior margin and incurved, moderately pointed umbones. This group comprises C. taylori, broadly distributed in the seep environments of northern circum-Pacific area, and extant Conchocele species known from seep environments of northern circum-Pacific area and the Caribbean (Kharlamenko et. al 2016). Due to the scarcity of data especially on the younger taxa, the precise relationships between Cretaceous, Paleocene, Neogene, and extant seep Conchocele remain to be characterized.
–Maorithyas Fleming, 1950, is a poorly known genus with only two extant species known from rather shallow water environments off New Zealand (Fleming 1950; Powell 1955). The oldest fossil species is the middle Eocene Maorithyas humptulipsensis reported here from a deep-water (ca. 2000–2500 m; cf. Kiel and Amano 2013) seep carbonate in western Washington State, USA. A remarkably similar case of a deep-water origin of a modern shallow-water clade is the bathymodiolin mussel Vulcanidas, whose oldest fossil record is from the very same deep-water seep deposit as M. humptulipsensis (Kiel and Amano 2013).
–The genus Channelaxinus Valentich-Scott and Coan, 2012, was recently reported from a Miocene deep-water seep carbonate in Italy (Kiel and Taviani 2017).
In general, major evolutionary novelties are considered to have developed in shallow water throughout the Phanerozoic, and have then moved offshore over geologic time scales, making the deep sea a refuge for relict taxa (Jablonski et al. 1983; Jablonski and Bottjer 1991). However, some counter examples of deep-water origins and subsequent colonization of shallower water exist, indicating that the deep sea could also act as a source of biodiversity because it is larger, more stable and not (or at least less) affected by large extinction events as are shallower water habitats (Thuy et al. 2012). Examples of such offshore-onshore pattern include several groups of corals, mollusks and crustaceans (e.g., Hessler and Wilson 1983; Jablonski and Bottjer 1991; Jablonski 2005; Lindner et al. 2008), and, as outlined here, the modern thyasirids appear to be another such example.
The Cretaceous rise of the modern thyasirids might be linked to their sulfide requirements. Extant thyasirids show a preference for relatively low sulfide concentrations compared to other chemosymbiotic bivalve families (e.g., Oliver et al. 2013) and this appears to have also been the case for their Paleogene relatives (Kiel et al. 2016). Marine sulfate concentrations appear to control sulfide availability at methane seeps (Wortmann and Paytan 2012) and extremely low sulfate concentrations during the Cretaceous did indeed have a major impact on the evolution of the seep fauna (Kiel 2015). This low sulfide availability during the Cretaceous might have favored the adaptation of thyasirids to the methane seep environment.
There is a remarkable coincidence between the rise of large thyasirids at seeps and the disappearance of the semi-infaunal, seep-restricted bivalve Caspiconcha. Caspiconcha was abundant at seeps during the early Cretaceous and significantly decreased in abundance after the drop in marine sulfate concentrations about 120 million years ago (Kiel 2015). Its last record is from the Campanian (Omagari seep carbonate; Jenkins et al. 2013) roughly coeval with the appearance of the large thyasirids Conchocele and “Thyasira” hataii at seeps. A possible connection between the two events might be deduced from an observation made on extant chemosymbiotic mytilids and thyasirids colonizing piles of oil-rich (and hence sulfide-rich) drill-hole cuttings underneath oil rigs in the North Sea. The first colonizer of these piles is the epifaunal bathymodiolin mussel Idas, which relies on sulfide emitting from the cuttings. Idas declines in abundance after about a year when infaunal thyasirids become abundant within the cuttings and take up the majority of the sulfide (Dando and Hartley 2005). Analogously, we speculate that the large infaunal thyasirids that appeared at seeps during the late Cretaceous were essentially mining most of the available sulfide at seeps from within the sediment, and thus depriving the semi-infaunal Caspiconcha of its source of nutrition. With sulfide levels already being low during the late Cretaceous low-sulfide interval (cf. Kiel 2015), Caspiconcha was already in decline, and increased sulfide mining by the large thyasirids might have been the final blow that drove Caspiconcha to extinction.
The fossil history of the thyasirids as outlined here indicates that the so-called Oceanic Anoxic Events during the Cretaceous and early Cenozoic, including the Paleocene/Eocene Thermal Maximum, had little (if any) effect on the diversity and the taxonomic composition of thyasirids at seeps. Instead, they show a slow rise in diversity in terms of genera and clades within genera throughout the Cretaceous and Cenozoic (Fig. 18). The major rise in marine sulfate concentration after the early Eocene and the concomitant rise in sulfide availability at seeps (cf. Wortmann and Paytan 2012; Kiel 2015) likely created high sulfide niches at seeps, which the low sulfide-adapted thyasirids were unable to exploit. Therefore the thyasirids do not show a major radiation in the early Cenozoic, in contrast to bathymodiolin mussels (Lorion et al. 2013) and vesicomyid clams (Johnson et al. 2017). Remarkably, the genus Thyasira is the only seep-inhabiting genus that showed a decrease in mean body size after the Eocene rise in sulfide availability, in contrast to all others, which show an increase in mean body size (Kiel 2015).
Conclusions
This paper presents a study of thyasirid bivalves from Cretaceous and Paleogene cold seep deposits. Eleven species, belonging to three long-lasting genera (Conchocele Gabb, 1866, Thyasira Lamarck, 1818, and Maorithyas Fleming, 1950) have been identified. The fossil record suggests that modern thyasirids originated at seeps in the earliest Cretaceous and persisted in this environment for 140 Ma until the present day. The “deep-to-shallow” origination patterns exhibited by thyasirids is similar to that observed among some other invertebrate groups, such as corals and crustaceans, showing that the deep sea may be a source of some evolutionary novelties. Likewise, similar to other deep sea and seep-inhabiting invertebrates, thyasirids show no significant diversity changes around major Mesozoic and Cenozoic Oceanic Anoxic Events, analogously to various other members of the deep sea fauna and raises doubts about the global character of these events.
Acknowledgements
The specimens from Washington State, USA, were donated by James L. and Gail H. Goedert (Wauna, USA), whom we would like to sincerely thank. We would also like to thank Grażyna and Marian Dziewiński (ZPAL) for photography of the fossils, and Jamie Brezina (South Dakota School of Mines and Technology, Rapid City, USA) for information on the Western Interior Seaway seeps. Photos of the holotype of Maorithyas folgeri were provided by Carole Hickman (University of California, Berkeley, USA). Special thanks go to the referees Alan G. Beu (GNS Science, Lower Hutt, New Zealand) and P. Graham Oliver (Natural Museum of Wales, Cardiff, UK) for their comments and opinions that helped to improve this manuscript. The permission for fieldwork on Svalbard to KH was granted under RIS–10173 application: “The influence of Paleocene/Eocene Thermal Maximum on oceanic chemosynthesis-based ecosystems”. Financial support to KH was provided by the Polish National Science Centre (NCN) research grant 2014/15/B/ST10/04886 “The influence of Paleocene/Eocene Thermal Maximum on oceanic chemosynthesis-based ecosystems” (for field work on Svalbard), by the Institute of Paleobiology, Polish Academy of Sciences (for the visit to the collections in Vienna) and by SYNTHESYS grant SE-TAF-5990 (for a visit to collections in Stockholm). Financial support to SK for various field work was provided by the Deutsche Forschungsgemeinschaft (grant Ki802/6-1), by the European Commission through a Marie-Curie fellowship (MEIF-CT-2005-515420), and by the Joetsu University of Education. Field work in Hokkaido was financially supported by a Grant-in-aid for Scientific Research from the Japan Society for Promotion of Science (C, 26400500, 2014–2016; C, 17K05691, 2017–2019) to KA and RGJ. Material from Montrose, Nebraska, was collected thanks to the support of the Polish National Science Centre (NCN) research grant 2012/07/B/ST10/04189 to Andrzej Kaim (ZPAL).
References
Amano, K. and Jenkins, R.G. 2007. Eocene drill holes in cold seep bivalves of Hokkaido, northern Japan. Marine Ecology 28: 108–114. Crossref
Amano, K. and Jenkins, R.G. 2011. New fossil Bathymodiolus (sensu lato) (Bivalvia: Mytilidae) from Oligocene seep carbonates in Eastern Hokkaido, Japan, with remarks on the evolution of the genus. The Nautilus 125: 29–35.
Amano, K. and Oleinik, A. 2016. Ancistrolepidine gastropods (Buccinidae) from the upper Eocene hydrocarbon seep deposits in Hokkaido, northern Japan. The Nautilus 130: 158–163.
Amano, K., Jenkins, R.G., Sako, Y., Ohara, M., and Kiel, S. 2013. A Paleogene deep sea methane-seep community from Honshu, Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 387: 126–133. Crossref
Amano, K., Little, C.T.S., Campbell, K.A., Jenkins, R.G., and Saether, K.P. 2015. Paleocene and Miocene Thyasira sensu stricto (Bivalvia: Thyasiridae) from chemosynthetic communities from Japan and New Zealand. The Nautilus 129: 43–53.
Anderson, F.M. 1958. Upper Cretaceous of the Pacific Coast. Geological Society of America Memoir 71: 1–378. Crossref
Ascher, E. 1906. Die Gastropoden, Bivalven und Brachiopoden der Grödischter Schichten. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients 19: 135–167.
Åstrom, E.K.L., Oliver, P.G., and Carroll, M.L. 2017. A new genus and two new species of Thyasiridae associated with methane seeps off Svalbard, Arctic Ocean. Marine Biology Research 13: 402–416. Crossref
Batstone, R.T., Laurich, J.R., Salvo, F., and Dufour, S.C. 2014. Divergent chemosymbiosis-related characters in Thyasira cf. gouldi (Bivalvia: Thyasiridae). PLoS ONE 9 (3): e92856. Crossref
Berry, S.S. 1947. New Mollusca from the Pleistocene of San Pedro, California—III. Bulletins of American Paleontology 31: 256–275.
Bernard, F.R. 1972. The genus Thyasira in Western Canada (Bivalvia: Lucinacea). Malacologia 11: 365–389.
Bernardino, A.F., Smith, C.R., Baco, A., Altamira, I., and Sumida, P.Y.G. 2010. Macrofaunal succession in sediments around kelp and wood falls in the deep NE Pacific and community overlap with other reducing habitats. Deep sea Research I 57: 708–723. Crossref
Boss, K.J. 1967. Thyasira disjuncta (Gabb, 1866) in the Caribbean Sea. Bulletin of Marine Science 17: 387–388.
Bright, M. and Lallier, F.H. 2010. The biology of vestimentiferan tubeworms. Oceanography and Marine Biology: An Annual Review 48: 213–266. Crossref
Campbell, K. 2006. Hydrocarbon seep and hydrothermal vent paleonenvironments and paleontology: past developments and future research directions. Palaeogeography, Palaeoclimatology, Palaeoecology 232: 362–347. Crossref
Coan, E.V., Scott, P.V., and Bernard, F.R. 2000. Bivalve seashells of Western North America. Marine bivalve molluscs from Arctic Alaska to Baja California. Santa Barbara Museum of Natural History Monographs 2: iii–v, 1–764.
Conrad, T.A. 1849. Description of new fresh water and marine shells. Proceedings of the Academy of Natural Sciences of Philadelphia 4: 152–156.
Clarke, A.H. 1989. New mollusks from undersea oil seeps off Louisiana. Malacology Data Net 2: 122–134.
Dall, W.H. 1895. Contributions to the Tertiary fauna of Florida, with especial reference to the Miocene Silex-beds of Tampa and the Pliocene beds of the Caloosahatchie River. Part III, A new classification of the Pelecypoda. Wagner Free Institute of Science of Philadelphia, Transactions 3: 483–570.
Dando, P.R. and Hartley, J.P. 2005. Colonisation and species succession of a seep-type community on an oil-coated drill cuttings pile in the North Sea. In: Third International Symposium on Hydrothermal Vent and Seep Biology, Abstract Volume, 13. Scripps Institution of Oceanography, La Jolla.
Dando, P.R. and Southward, A.J. 1986. Chemoautotrophy in bivalve molluscs of the genus Thyasira. Journal of Marine Biological Association of the United Kingdom 66: 915–929. Crossref
Dando, P.R. and Spiro, B. 1993. Varying nutritional dependence of the thyasirid bivalves Thyasira sarsi and T. equalis on chemoautotrophic chemosymbiotic bacteria, demonstrated by isotope ratios of tissue carbon and shell carbonate. Marine Ecology Progress Series 92: 151–158. Crossref
Dando, P.R., Bussmann, I., Niven, S.J., O’Hara, S.C.M., Schmaljohann, R., and Taylor, L.J. 1994. A methane-seep area in the Skagerrak, the habitat of pogonophore Siboglinium poseidoni and the bivalve mollusc Thyasira sarsi. Marine Ecology Progress Series 107: 157–167. Crossref
Distel, D. 2000. Phylogenetic relationships among the Mytilidae (Bivalvia): 18S rRNA data suggest convergence in mytilid body plans. Molecular Phylogenetics and Evolution 15: 25–33. Crossref
d’Orbigny, A. 1844. Paléontologie française: description zoologique et géologique de tous les animaux mollusques et rayonnés fossiles de France. Terrains crétacés (3). Pelecypoda. 807 pp. Victor Masson, Paris.
Dufour, S.C. 2005. Gill anatomy and the evolution of symbiosis in the bivalve family Thyasiridae. Biological Bulletin 208: 200–212. Crossref
Fleming, C.A. 1950. New Zealand Recent Thyasiridae (Mollusca). Transactions of the Royal Society of New Zealand 78: 251–254.
Freneix, S. 1980. Bivalves néocrétacés du Nouvelle-Calédonie. Signification biogéographique, biostratigraphique, paléoécologique. Annales de Paléontologie (Invertébrés) 66: 67–134.
Fujikura, K., Kojima, S., Tamaki, K., Maki, Y., Hunt, J., and Okutani, T. 1999. The deepest chemosynthesis-based community yet discovered from the hadal zone, 7326 m deep, in the Japan Trench. Marine Ecology Progress Series 190: 17–26. Crossref
Fujiwara, Y., Kato, C., Masui, N., Fujikura, K., and Kojima, S. 2001. Dual symbiosis in the cold seep thyasirid clam Maorithyas hadalis from the hadal zone in the Japan Trench, western Pacific. Marine Ecology Progress Series 214: 151–159. Crossref
Gabb, W.M. 1866. Paleontology of California. Vol. 2. Cretaceous and Tertiary fossils. Sect. 1 (Tertiary Invertebrate Fossils). 124 pp. Geological Survey of California, Philadelphia.
Gill, F.L. and Little, C.T.S. 2013. A new genus of lucinid bivalve from hydrocarbon seeps. Acta Palaeontologica Polonica 58: 573–578.
Goedert, J.L. and Benham, S.R. 1999. A new species of Depressigyra? (Gastropoda: Peltospiridae) in Eocene and Oligocene rocks of Western Washington. The Veliger 41: 112–116. Crossref
Goedert, J.L. and Benham, S.R. 2003. Biogeochemical processes at ancient methane seeps: the Bear River site in southwestern Washington. Geological Society of America Field Guide 4: 201–208.
Goedert, J.L. and Kaler, K.L. 1996. A new species of Abyssochryssos (Gastropoda, Loxonematoidea) from middle Eocene cold-seep carbonate in the Humptulips Formation, western Washington. The Veliger 39: 65–70.
Goedert, J.L. and Squires, R.L. 1990. Eocene deep sea communities in localized limestone formed by subduction-related methane seeps, southwestern Washington. Geology 18: 1182–1185. Crossref
Goedert, J.L. and Squires, R.L. 1993. First Oligocene records of Calyptogena (Bivalvia: Vesicomyidae). The Veliger 36: 72–77.
Goedert, J.L., Thiel, V., Schmale, O., Rau, W.W., Michaelis, W., and Peckmann, J. 2003. The Late Eocene “Whiskey Creek” methane-seep deposit (western Washington State). Part I: geology, palaeontology and molecular geobiology. Facies 48: 223–240. Crossref
Gray, J.E. 1854. A revision of the arrangement of the families of bivalve shells (Conchifera). The Annals and Magazine of Natural History, Series 2 13: 408–418. Crossref
Habe, T. 1964. Shells of the Western Pacific in Colour. Vol. 2. 233 pp. Hoikusha Publishing, Osaka.
Hägg, R. 1925. A new Tertiary fauna from Spitsbergen. Bulletin of the Geological Institute of the University of Uppsala 20: 39–55.
Hessler, R.R. and Wilson, G.D.F. 1983. The origin and biogeography of malacostracan crustaceans in the deep sea. In: R.W. Sims, J.H. Price, and P.E.S. Whalles (eds.), Evolution, Time, and Space: The Emergence of the Biosphere, 227–254. Academic Press, London.
Hickman, C.S. 2015. Paleogene marine bivalves of the deep-water Keasey Formation in Oregon, Part III: The heteroconchs. PaleoBios 32: 1–44.
Hryniewicz, K., Bitner, M.A., Durska, E., Haström, J., Hjálmarsdóttir, H.R., Jenkins, R.G., Little, C.T.S., Miyajima, Y., Nakrem, H.A., and Kaim, A. 2016. Paleocene methane seep and wood-fall marine environments from Spitsbergen, Svalbard. Palaeogeography, Palaeoclimatology, Palaeoecology 462: 41–56. Crossref
Hryniewicz, K., Little, C.T.S., and Nakrem, H.A. 2014. Bivalves from the latest Jurassic–earliest Cretaceous hydrocarbon seep carbonates from central Spitsbergen, Svalbard. Zootaxa 3859: 1–66. Crossref
Iredale, T. 1924: Results from Roy Bell’s Molluscan collections. Proceedings of the Linnaean Society of New South Wales 49: 179–278.
Ivanova, M.B. and Moskaletz, I.P. [Moskalec, I.P] 1984. Two new species of bivalve molluscs of the family Thyasiridae from the aquatoria of the Far Eastern State Marine Nature Reserve [in Russian]. In: B.S. Levin (ed.), Životnyj mir Dal’nevostočnogo Morskogo Zapovednika, 45–50. Akademiâ Nauk SSSR, Dal’nevostočnyj Naučnyj Centr, Vladivostok.
Jablonski, D. 2005. Evolutionary innovations in the fossil record: the intersection of ecology, development and macroevolution. Journal of Experimental Zoology (Molecular Development and Evolution) 304B: 504–519. Crossref
Jablonski, D. and Bottjer, D.J. 1991. Environmental patterns in the origins of higher taxa: the post-Paleozoic fossil record. Science 252: 1831–1833. Crossref
Jablonski, D., Sepkoski, J.J., Bottjer, D.J., and Sheehan, P.M. 1983. Onshore-offshore patterns in the evolution of Phanerozoic shelf communities. Science 222: 1123–1125. Crossref
Jeffreys, J.G. 1864. British Conchology Vol. II. Marine Shells. 465 pp. John van Voorst, London.
Jenkins R.G., Kaim, A., Little, C.T.S., Iba, Y., Tanabe, K., and Campbell, K.A. 2013. Worldwide distribution of the modiomorphid bivalve genus Caspiconcha in late Mesozoic hydrocarbon seeps. Acta Palaeontologica Polonica 58: 357–382.
Jenkins, R.G., Kaim, A., Sato, K., Moriya, K., Hikida, Y., and Hirayama, R. 2017. Discovery of chemosynthesis-based association on the Cretaceous basal leatherback sea turtle from Japan. Acta Palaeontologica Polonica 62: 683–690. Crossref
Judd, A. and Hovland, M. 2007. Seabed Fluid Flow. The Impact on Geology, Biology and the Marine Environment. 475 pp. Cambridge University Press, Cambridge. Crossref
Johnson, S.B., Krylova, E.M., Audzijonyte. A., Sahling, H., and Vrijenhoek, R.C. 2017. Phylogeny and origins of chemosynthetic vesicomyid clams. Systematics and Biodiversity 15: 346–360. Crossref
Kaim, A., Skupien, P., and Jenkins, R.G. 2013. A new Lower Cretaceous hydrocarbon seep locality from the Czech Carpathians and its fauna. Palaeogeography, Palaeoecology, Palaeoclimatology 390: 42–51. Crossref
Kamenev, G.M., Nadtochy, V.A., and Kuznetsov, A.P. 2001. Conchocele bisecta (Conrad, 1849) (Bivalvia: Thyasiridae) from cold-water methane-rich areas of the Sea of Okhotsk. The Veliger 44: 84–94.
Kanie, Y., Yoshikawa, Y., Sakai, T., and Takahashi, T. 1993. The Cretaceous chemosynthetic cold water−dependent molluscan community discovered from Mikasa City, central Hokkaido. Science Report of the Yokosuka City Museum 41: 31–36.
Katto, J. and Hattori, M. 1965. Some Veneridae from the Shimantogawa Group in the Outer Zone of Shikoku, Japan. Research Reports of the Kochi University, Natural Science 13: 7–10.
Katto, J. and Masuda, K. 1979. Tertiary Mollusca from the southern part of Kii Peninsula, Wakayama Prefecture, Southwest Japan. Research Reports of the Kochi University, Natural Science 27: 97–111.
Kauffman, E.G. 1967. Cretaceous Thyasira from the Western Interior of North America. Smithsonian Miscellaneous Collections 152: 1–159.
Kauffman, E.G., Arthur, M.A., Howe, B., and Scholle, P.A. 1996. Widespread venting of methane-rich fluids in Late Cretaceous (Campanian) submarine springs (Tepee Buttes), Western Interior seaway, U.S.A. Geology 24: 799–802. Crossref
Kharlamenko, V.I., Kamenev, G.M., Kalachev, A.V., Kiyashko, S.I., and Ivin, V.V. 2016. Thyasirid bivalves from the methane seep community off Paramushir Island (Sea of Okhotsk) and their nutrition. Journal of Molluscan Studies 82: 391–402. Crossref
Khudik, V.D. [Hudik, V.D.] 1989. Dvustvorčatye mollûski miocena Ûgo-Zapadnogo Sahalina. 130 pp. Akademiă Nauk SSSR, Dal’nevostočnoe Otdelenie Dal’nevostočnyj Geologičeskiy Institut, Vladivostok.
Kiel, S. 2008. An unusual new gastropod from an Eocene hydrocarbon seep in Washington State. Journal of Paleontology 82: 188–191. Crossref
Kiel, S. 2010. On the potential generality of depth-related ecologic structure in cold-seep communities: Cenozoic and Mesozoic examples. Palaeogeography, Palaeoclimatology, Palaeoecology 295: 245–257. Crossref
Kiel, S. 2013. Lucinid bivalves from ancient methane seeps. Journal of Molluscan Studies 79: 349–363. Crossref
Kiel, S. 2015. Did shifting seawater sulfate concentrations drive the evolution of deep sea methane seep ecosystems? Proceedings of the Royal Society B 282: 20142908. Crossref
Kiel, S. and Amano, K. 2013. The earliest bathymodiolin mussels: an evaluation of Eocene and Oligocene taxa from deep sea methane seep deposits in western Washington State, USA. Journal of Paleontology 87: 589–602. Crossref
Kiel, S. and Goedert, J. 2007. New mollusks associated with biogenic substrates in Cenozoic deep-water sediments of Washington State. Acta Palaeontologica Polonica 52: 41–52.
Kiel, S. and Little, C.T.S. 2006.Cold seep mollusks are older than the general marine mollusk fauna. Science 313: 1429–1431. Crossref
Kiel, S. and Peckmann, J. 2007. Chemosymbiotic bivalves and stable carbon isotopes indicate hydrocarbon seepage at four unusual Cenozoic fossil localities. Lethaia 40: 345–357. Crossref
Kiel, S. and Taviani, M. 2017. Chemosymbiotic bivalves from Miocene methane-seep carbonates in Italy. Journal of Paleontology 91: 444–466. Crossref
Kiel, S., Amano, K., and Jenkins, R.G. 2008. Bivalves from Cretaceous cold seep deposits on Hokkaido, Japan. Acta Palaeontologica Polonica 53: 525–537. Crossref
Kiel, S., Amano, K., Hikida, Y., and Jenkins, R.G. 2009. Wood-fall associations from Late Cretaceous deep-water sediments of Hokkaido, Japan. Lethaia 42: 74–82. Crossref
Kiel, S., Amano, K., and Jenkins, R.G. 2016. Predation scar frequencies in chemosymbiotic bivalves at an Oligocene seep deposit and their potential relation to inferred sulfide tolerances. Palaeogeography, Palaeoclimatology, Palaeoecology 453: 139–145. Crossref
Kristofovich, L.V. [Krištofovič, L.V.] 1936. Shells of the group Thyasira bisecta (Conrad) from the Tertiary deposits of the west coast of Kamchatka [in Russian]. Trudy Neftânogo-Razvedočnogo Instituta, Seriâ A 88: 3–67.
Kristofovich, L.V. [Krištofovič, L.V.] 1964. Mollûski tretičnyh otloženij Sahalina. 230 pp. Nedra, Leningrad.
Lamarck, J.B. 1818. Histoire naturelle des animaux sans vertèbres: présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent: précédée d’une introduction offrant la détermination des caractères essentiels de l’Animal, sa distinction du végétal et des autres corps naturels, enfin, l’Exposition des Principes fondamentaux de la Zoologie. Tome Cinquiéme. 612 pp. Deterville et Verdiére, Paris.
Levin, L. 2005. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanography and Marine Biology: An Annual Review 43: 1–46. Crossref
Lindner, A., Cairns, S.D., and Cunningham, C.W. 2008. From offshore to onshore: multiple origins of shallow-water corals from deep sea ancestors. PLoS ONE 3(6): e2429. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis,locis. Editio decima. 824 pp. Laurentius Salvius, Holmiae.
Liljedahl, L. 1992. The Silurian Ilonia prisca, oldest known deep-burrowing suspension-feeding bivalve. Journal of Paleontology 66: 206–210. Crossref
Little, C.T.S., Birgel, D., Boyce, A.J., Crame, A.J., Francis, J.E., Kiel, S., Peckmann, J., Pirrie, D., Rollinson, and G.K., Witts, J.D. 2015. Late Cretaceous (Maastrichtian) shallow water hydrocarbon seeps from Snow Hill and Seymour Islands, James Ross Basin, Antarctica. Palaeogeography, Palaeoclimatology, Palaeoecology 418: 213–228. Crossref
Lorion, J., Kiel, S., Faure, B., Kawato, M., Ho, Y.W.S., Marshall, B., Tsuchida, S., Miyazaki, Y.-I., and Fujiwara, Y. 2013. Adaptative radiation of chemosymbiotic deep sea mussels. Proceedings of the Royal Society B 280: 20131243. Crossref
López-Jamar, E., González, G., and Mejuto, J. 1987. Ecology, growth and production of Thyasira flexuosa (Bivalvia, Lucinacea) from Ría de la Coruña, North-West Spain. Ophelia 27: 111–126. Crossref
Montagu, G. 1803. Testacea Britannica. 606 pp. J. White, London.
Moore, E.J. 1963. Miocene marine mollusks from the Astoria Formation in Oregon. United States Geological Survey Professional Paper 419: 1–119.
Moore, E.J. 1988. Tertiary marine pelecypods of California and Baja California: Lucinidae trough Chamidae. United States Geological Survey Professional Paper 1228-D: 1–46.
Nesbitt, E.A., Martin, R.A., and Campbell, K.A. 2013. New records of Oligocene diffuse hydrocarbon seeps, northern Cascadia margin. Palaeogeography, Palaeoclimatology, Palaeoecology 390:116–129. Crossref
Neumayr, M. 1884. Morphologie des Bivalvenschlosses. Kaiserlich-Königliche Akademie der Wissenschaften, Wien. Mathematisch-Naturwissenschaftliche Klasse, Sitzungsberichte Abteilung 1: 385–418.
Nobuhara, T., Hitomi, S., and Shiratori, Y. 2016. Predatory drill intensity on deep sea bivalves from the Eocene Poronai Formation, Hokkaido, Japan―comparison between the cold-seep site and its surrounding normal mud [in Japanese with English abstract]. Geoscience Reports of Shizuoka University 43: 13–20.
Nobuhara, T., Onda, D., Kikuchi, N., Kondo, Y., Matsubara, K., Amano, K., Jenkins, R.G., Hikida, Y., and Majima, R. 2008. Lithofacies and fossil assemblages of the Upper Cretaceous Sada Limestone, Shimanto City, Kochi Prefecture, Shikoku, Japan [in Japanese with English abstract]. Fossils, The Palaeontological Society of Japan 84: 47–60.
Noda, Y. 1992. Neogene molluscan faunas from the Haboro Coal-field, Hokkaido, Japan. Science Reports of the Tohoku University, Second Series (Geology) 62: 1–141.
Ohara, S. and Kanno, S. 1969. Mid-Tertiary molluscan faunas from the Uryu coal-field of central Hokkaido, Japan. Fossils, The Palaeontological Society of Japan 17: 41–49.
Ohara, S. and Kanno, S. 1973. Mid-Tertiary marine molluscan faunas of the Uryu coal-field. Science Reports of the Tohoku University, 2nd Series, Special Volume 6: 125–135.
Okutani, T., Fujikura, K., and Kojima, S. 1999. Two new hadal bivalves of the family Thyasiridae from the plate convergent area of the Japan Trench. Venus 58: 49–54.
Oliver, P.G. and Drewery, J. 2013. New species of chemosymbiotic clams (Vesicomyidae and Thyasiridae) from a putative “seep” in the Hatton-Rockall Basin, north-east Atlantic. Journal of the Marine Biological Association of the United Kingdom 94: 389–402. Crossref
Oliver, P.G. and Frey, M.A. 2014. Ascetoaxinus quatsinoensis sp. et gen. nov. (Bivalvia: Thyasiroidea) from Vancouver Island, with notes on Conchocele Gabb, 1866, and Channelaxinus Valentich-Scott and Coan, 2012. Zootaxa 3869: 452–468. Crossref
Oliver, P.G. and Killeen, I.J. 2002. The Thyasiridae (Mollusca: Bivalvia) of the British continental shelf and North Sea oilfields. Biomôr Reports 3: 1–73.
Oliver, P.G. and Holmes, A.M. 2006. New species of Thyasiridae (Bivalvia) from chemosynthetic communities in the Atlantic Ocean. Journal of Conchology 39: 175–183.
Oliver, P.G. and Holmes, A.M. 2007. A new species of Axinus (Bivalvia: Thyasiroidea) from the Baby Bare Seamount, Cascadia Basin, NE Pacific, with a description of the anatomy. Journal of Conchology 39: 363–376.
Oliver, P.G. and Sellanes, J. 2005. New species of Thyasiridae from the methane seepage area of Concepción, Chile. Zootaxa 1092: 1–20. Crossref
Oliver, P.G., Rodrigues, C.F., Carney, R., and Duperron, S. 2013. Spinaxinus (Bivalvia: Thyasiroidea) from sulfide biogenerators in the Gulf of Mexico and hydrothermal vents in the Fiji Back Arc: chemosymbiosis and taxonomy. Scientia Marina 77: 663–676. Crossref
Payne, C.M. and Allen, J.A. 1991. The morphology of deep sea Thyasiridae (Mollusca: Bivalvia) from the Atlantic Ocean. Philosophical Transactions: Biological Sciences 334: 481–562. Crossref
Peckmann, J., Goedert, J.L., Thiel, V., Michaelis, W., and Reitner, J. 2002. A comprehensive approach to study of methane seep deposits from the Lincoln Creek Formation, western Washington State, USA. Sedimentology 49: 855–873. Crossref
Peckmann, J., Kiel, Sandy, S.M.R., Taylor, D.G., and Goedert, J.L. 2011. Mass occurrences of the brachiopod Halorella in Late Triassic methane-seep deposits, Eastern Oregon. Journal of Geology 119: 207–220. Crossref
Philippi, R.A. 1845a. Bemerkungen über die Mollusken-Fauna von Massachusetts. Zeitschrift für Malakozoologie 1845: 68–79.
Philippi, R.A. 1845b. Kritische Bemerkungen über einige Trochus-arten und die Gattung Axinus. Zeitschrift für Malakozoologie 1845: 87–91.
6, A.W.B. 1955. Mollusca of the southern islands of New Zealand. Department of Scientific and Industrial Research Cape Expedition Series Bulletin 15: 1–151.
Rigby, K.J. and Goedert, J.L. 1996. Fossil sponges from a localized cold seep limestones in Oligocene rocks of the Olympic Peninsula, Washington. Journal of Paleontology 70: 900–908. Crossref
Rodrigues, C.F., Oliver, P.G., and Cunha, M.R. 2008. Thyasiroidea (Bivalvia: Mollusca) from the mud volcanoes of the Gulf of Cadiz. Zootaxa 1752: 41–56.
Rosenkrantz, A. 1942. Slægten Thyasiras geologiske Optræden. Meddelelser fra Dansk Geologisk Forening 10: 277–278.
Rosenkrantz, A. 1970. Marine Upper Cretaceous and lowermost Tertiary deposits in West Greenland. Investigations before and since 1938. Meddelelser fra Dansk Geologisk Forening 19: 406–453.
Sasaki, T., Warén, A., Kano, Y., Okutani, T., and Fujikura, K. 2010. Gastropods from Recent hot vents and cold seeps: systematics, diversity and life strategies. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 169–254. Springer, Dordrecht. Crossref
Sibuet, M. and Olu, K. 1998. Biogeography, diversity and fluid dependence of deep sea cold-seep communities at active and passive margins. Deep Sea Research II 45: 517–567. Crossref
Smith, C.R. and Baco, A.R. 2003. Ecology of whale falls at deep sea floor. Oceanography and Marine Biology: An Annual Review 41: 311–354.
Spencer, H.G., Marshall, B.A., Maxwell, P.A., Grant-Mackie, J.A., Stilwell, J.D., Willan, R.C., Campbell, H.J., Crampton, J.S., Henderson, R.A., Bradshaw, M.A., Waterhouse, J.B., and Pojeta, J. 2009. Phylum Mollusca. Chitons, clams, tusk shells, squids and kin. In: D. Gordon (ed.), New Zealand Inventory of Biodiversity, Volume One. Kingdom Animalia. Radiata, Lophotrochozoa, Deuterostomia, 161–254. Canterbury University Press, Christchurch.
Squires, R.L. 1995. First fossil species of the chemosynthetic community gastropod Provanna: localized cold-seep limestones in the upper Eocene and Oligocene rocks, Washington. The Veliger 38: 30–36.
Squires, R.L. and Goedert, J.L. 1991. New late Eocene mollusks from localized limestone deposits formed at subduction-related methane seeps, southwestern Washington. Journal of Paleontology 65: 412–416. Crossref
Squires, R.L. and Goedert, J.L. 1995. An extant species of Leptochiton (Mollusca: Polyplacophora) in Eocene and Oligocene cold-seep limestones, Olympic Peninsula, Washington. The Veliger 38: 47–53.
Squires, R.L. and Gring, M.P. 1996. Late Eocene chemosynthetic? bivalves from suspect cold seeps, Wagonwheel Mountain, Central California. Journal of Paleontology 70: 63–73. Crossref
Taylor, J.D. and Glover, E.A. 2010. Chemosymbiotic bivalves. In: S. Kiel (ed.), The Vent and Seep Biota. Aspects from Microbes to Ecosystems, 107–136. Springer, Dordrecht. Crossref
Taylor, J.D., Williams, S.T., and Glover E.A. 2007. Evolutionary relationships of the bivalve family Thyasiridae (Mollusca: Bivalvia), monophyly and superfamily status. Journal of the Marine Biological Association of the United Kingdom 87: 565–574. Crossref
Tegland, N.M. 1928. Thyasira disjuncta Gabb, not Thyasira bisecta Conrad, the Recent West Coast shell. The Nautilus 16: 129–131.
Thiedig, F., Pickton, C.A.G., Lehmann, U., Harland, U.B., and Anderson, H.J. 1980. Das Tertiär von Renardodden (Östlich Kapp Lyell, Westspitzbergen, Svalbard). Mitteilungen Geologisch-Paläontologisches Institut der Universität Hamburg 49: 135–146.
Thuy, B., Gale, A.S., Kroh, A., Kucera, M., Numberger-Thuy, L.D., Reich, M., and Stöhr, S. 2012. Ancient origin of the modern deep sea fauna. PLoS ONE 7 (10): e46913. Crossref
Valentich-Scott, P., Powell, C.L. II, Lorenson, T.D., Edwards, B.E. 2014. A new genus and species of Thyasiridae (Mollusca, Bivalvia) from deep-water Beaufort Sea, northern Alaska. Zookeys 462: 11–26. Crossref
Valentine, J.W., Jablonski, D., Kidwell, S.M., and Roy, K. 2006. Assessing the fidelity of the fossil record by using marine bivalves. Proceedings of the National Academy of Sciences of the USA 103: 6599–6604. Crossref
Van Dover, C.L. 2000. The Ecology of Deep Sea Hydrothermal Vents. 424 pp. Princeton University Press, Princeton.
Van Winkle, K. 1919. Remarks on some new species from Trinidad. Bulletins of American Paleontology 8: 19–27.
Verrill, A.E. and Bush, K.J. 1898. Revision of the deep water Mollusca of the Atlantic Coast of North America, with description of new genera and species, Pt. 1, Bivalvia. Proceedings of the United States National Museum 20: 775–901. Crossref
Vinn, O., Kupriyanova, E.K., and Kiel, S. 2013. Serpulids (Annelida, Polychaeta) at Cretaceous to modern hydrocarbon seeps: ecological and evolutionary patterns. Palaeogeography, Palaeoclimatology, Palaeoecology 390: 35–41. Crossref
Vonderbank, K. 1970. Geologie und Fauna der Tertiären Ablagerungen Zentral-Spitsbergens. Norsk Polarinstitutt Skrifter 153: 5–119.
Wagner, C.M. and Schilling, K.H. 1923. The San Lorenzo Group of the San Emigdio region, California. University of California Publications Bulletin of the Department of Geological Sciences 14: 235–276.
Weller, S. 1903. The Stokes collection of Antarctic fossils. Journal of Geology 11: 413–419. Crossref
White, C.A. 1890. Scientific results of explorations by the U.S. Fish Commission Steamer Albatross. No. 10. On certain Mesozoic fossils from the Islands of St. Paul’s and St. Peter’s in the Straits of Magellan. Proceedings of the United States National Museum 13: 13–14. Crossref
Whiteaves, G.F. 1874. Notes on the Cretaceous fossils collected by Mr. James Richardson, at Vancouver and the adjacent islands. Geological Survey of Canada Report of Progress for 1873–74: 5–18.
Wilckens, O. 1910. Die Anneliden, Bivalven und Gastropoden der antarktischen Kreideformation. Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903 3 (12): 1–132.
Williscroft, K., Grasby, S.E., Beauchamp, B., Little, C.T.S., Dewing K., Birgel, D., and Poulton, T. 2017. Extensive Cretaceous (Albian) methane seepage on Ellef Ringnes Island, Canadian High Arctic. Geological Society of America Bulletin 129: 788–805. Crossref
Wolfe, E.W. and McKee, E.H. 1968. Geologic Map GM-4. Geology of the Grays River quadrangle, Wahkiakum and Pacific Counties, Washington. Scale 1:62500. 6 pp. State Printing Plant, Olympia.
Wood, S.V. 1851. Monograph of the Crag Mollusca with descriptions of shells from the Upper Tertiaries of the British Isles. Part 2. Bivalves. Palaeontographical Society Monographs 4: 1–150.
Wortmann, U.G. and Paytan, A. 2012. Rapid variability of seawater chemistry over the past 130 million years. Science 337: 334–336. Crossref
Yabe, H. and Nomura, S. 1925. Notes on the Recent and Tertiary species of Thyasira from Japan. Science Reports of the Tohoku Imperial University. 2nd Series, Geology 4: 83–95.
Yokoyama, M. 1890. Versteinerungen aus der japanischen Kreide. Palaeontographica 36: 159–202.
Zelaya, D.G. 2009. The genera Thyasira and Parathyasira in the Magellan region and adjacent Antarctic waters (Bivalvia: Thyasiridae). Malacologia 51: 271–290. Crossref
Zelaya, D.G. 2010. The new species of Thyasira, Mendicula and Axinulus (Bivalvia; Thyasiroidea) from Sub-Antarctic and Antarctic waters. Polar Biology 33: 607–616. Crossref
Zinsmeister, W.J. and Macellari, C.E. 1988. Bivalvia (Mollusca) from Seymour Island, Antarctic Peninsula. In: R.M. Feldmann and M.O. Woodburne (eds.), Geology and Paleontology of Seymour Island, Antarctic Peninsula, 253–284. Geological Society of America, Boulder.
Zwicker, J., Smrzka, D., Gier, S., Goedert, J.L., and Peckmann, J. 2015. Mineralized conduits are part of the uppermost plumbing system of Oligocene methane-seep deposits, Washington State (USA). Marine and Petroleum Geology 66: 616–630. Crossref
Acta Palaeontol. Pol. 62 (4): 705–728, 2017
https://doi.org/10.4202/app.00390.2017