


Evolution of reproductive strategies in dictyopteran insects—clues from ovipositor morphology of extinct roachoids
MARIE K. HÖRNIG, CAROLIN HAUG, JOERG W. SCHNEIDER, and JOACHIM T. HAUG
Hörnig, M.K., Haug, C., Schneider, J.W., and Haug, J.T. 2018. Evolution of reproductive strategies in dictyopteran insects—clues from ovipositor morphology of extinct roachoids. Acta Palaeontologica Polonica 63 (1): 1–24.
Dictyoptera, which comprises cockroaches, termites and mantids, is a quite successful group of insects in evolutionary terms with a long fossil record—roachoid insects were already abundant 315 million years ago in the Carboniferous forests. One of the most remarkable autapomorphies of extant dictyopterans, and possibly a major factor for their persisting success, is the ability to produce oothecae. Despite the robustness of this sort of egg package, fossils of oothecae are very rare, the oldest direct evidences being from the Cretaceous Crato Formation in Brazil (115 mya). The ability to produce oothecae is presumably linked to a specific ovipositor morphology, including a significant length reduction. Hence, ovipositor morphology can indirectly inform about the reproductive strategy of a species. Herein we describe the ovipositor morphology of various fossil forms of dictyopteran insects. Early fossil roachoids, in contrast to the modern forms, possessed a very long and prominent ovipositor, reminiscent of the ovipositor in orthopterans (Ensifera), indicating that these forms laid individual, rather small eggs into a substrate. We present examples from different fossil deposits, which show the entire range of ovipositor morphologies, from very long forms over forms with ovipositors partly reduced in length to modern-appearing morphologies. Most remarkably, different shapes of ovipositors seem to be present in roachoids in the fauna of the 115 million years old Crato Formation—species with long prominent ovipositors co-existed with species with a reduced short and broad ovipositor. Additionally, females that carry oothecae attached to their abdomen indicate a third type of ovipositor: a further reduced ovipositor as seen in modern forms, which already allowed the internal production of oothecae.
Key words: Dictyoptera, Blattodea, reproductive behaviour, oviposition, evolutionary reconstruction, Palaeozoic.
Marie K. Hörnig [marie.hoernig@palaeo-evo-devo.info], Ernst-Moritz-Arndt-University of Greifswald, Zoological Institute and Museum, Cytology and Evolutionary Biology, Soldmannstr. 23, 17489 Greifswald, Germany.
Carolin Haug [carolin.haug@palaeo-evo-devo.info] and Joachim T. Haug [joachim.haug@palaeo-evo-devo.info], LMU Munich, Biocenter, Department of Biology II and GeoBio-Center, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany.
Joerg W. Schneider [joerg.schneider@geo.tu-freiberg.de], Technical University Bergakademie Freiberg, Geological Institute, Department of Palaeontology, Bernhard-von-Cotta Str. 2, 09599 Freiberg, Germany, and Kazan Federal University, Kremlyovskaya str. 18, 420008, Kazan, Russia.
Received 6 November 2016, accepted 12 January 2018, available online 5 March 2018.
Copyright © 2018 M.K. Hörnig et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
There are at least 7570 extant species of blattodeans reported (including 4641 species of cockroaches and 2929 species of termites) (Beccaloni and Eggleton 2013), which are almost ubiquitously distributed, and about 2500 species of mantodeans (2425 in 2013; Zhang 2013). Hence, Dictyoptera, the monophyletic group including these morphotypes, comprises more than 10,000 species, making it a comparatively successful group among non-holometabolous insects.
At present, the consensus scenario of the relationships between these three groups within extant dictyopterans contains Isoptera as sister group of Cryptocercus (wood roaches) deeply nested within Blattodea, and Mantodea as the sister group of Blattodea (Lo et al. 2003, 2007; Grimaldi and Engel 2005; ; Inward et al. 2007; Djernæs et al. 2012, 2015; Legendre et al. 2015). Early representatives of Dictyoptera were roach-like in appearance and are therefore referred to as “roachoids” (see Discussion). These latter forms possess a fossil record extending back to at least the Pennsylvanian (e.g., Scudder 1895; Schneider 1978a, b, 1984; Rasnitsyn and Quicke 2002; Grimaldi and Engel 2005; Hörnig et al. 2014).
It is assumed that mantodeans and termites evolved by the Jurassic or early in the Cretaceous (Rasnitsyn and Quicke 2002; Grimaldi 1997, 2003; Grimaldi and Engel 2005; Svenson and Whiting 2009; for different interpretations see Béthoux and Wieland 2009; Béthoux et al. 2010; discussed in Hörnig et al. 2013; Legendre et al. 2015), hence modern dictyopterans must have evolved before this time. The stem species (more or less equal to the “last common ancestor”) of extant dictyopterans evolved a very notable autapomorphy: the eggs were deposited in a kind of package, the so-called “ootheca”, which is made of secretions from asymmetrical true accessory glands of abdominal segment nine (e.g., McKittrick 1964; Klass 1998; Bohn and Klass 2003; see also Goldberg et al. 2015 for similarities of oothecae in different insects). This characteristic is shown by all representatives of Mantodea, Blattodea, and one species of Isoptera, Mastotermes darwinensis Froggatt, 1897, consensually regarded as sister group to the rest of termites. Indeed all other termites deviate secondarily from this strategy and produce single eggs (Nalepa and Lenz 2000; Courrent et al. 2008; Klass et al. 2008; Wieland 2013).
Genuine fossil oothecae are very rare, with only five specimens in total so far (Grimaldi and Engel 2005; Anisyutkin et al. 2008; Poinar 2010; Hörnig et al. 2013). Some further specimens of putative oothecae are described in the literature, but their interpretation is controversial (see discussion of ovipositor morphology and oothecae of Paleozoic roachoids). Additionally, the inference of reproductive strategies based on hatchling morphology of fossil species has been proposed, as it is known for extant species of cockroaches that the presence and form of broodcare can be mirrored in the developmental status of the nymphs (Arillo 2007; Hörnig et al. 2016). But this is currently not yet possible in a wide taxonomic range due to the lack of hatchlings, or better small nymphs in general (see discussions e.g., in Haug et al. 2013; Hörnig et al. 2014, 2016). Therefore, one might conclude that it is quite challenging to infer the reproductive strategies in the majority of extinct representatives of Dictyoptera based on fossil remains. Yet, for example, the ability to produce oothecae is presumably linked to a specific morphology of the posterior part of the abdomen, and especially to the length of the ovipositor. While insects with short ovipositors do not obligatorily produce oothecae, it seems likely that a long ovipositor can be seen as contraindication for producing this kind of egg package. Hence, aspects of ovipositor morphology represent a useful tool to infer details of reproductive strategies of extinct dictyopterans.
A relatively long ovipositor is likely the plesiomorphic condition of pterygotes (see e.g., Sharov 1966; Scudder 1971; Grimaldi and Engel 2005; discussion in Hädicke et al. 2014), which was reduced several times within Pterygota (Klass 2007), but seems to be retained in early dictyopterans. With such long ovipositors, it is generally admitted that early roachoids were not able to produce oothecae (Laurentiaux 1951; Grimaldi 1997; Grimaldi and Engel 2005; Hörnig et al. 2013). Extant representatives of Mantodea have an externally visible ovipositor (at least its distal part), reduced into a rather short and broad form, allowing the formation of oothecae and their attachment to the substrate (e.g., Levereault 1936; Preston-Mafham 1990). Ovipositors of modern species of Blattodea are further specialised, being reduced in length and covered by the so-called “subgenital plate”, which is formed by post-ocular segment 15 (abdominal sternite 7) (Marks and Lawson 1962; Bohn 2003; Deitz et al. 2003; Klass and Meier 2006; Bell et al. 2007). This results in the ability to internally produce the ootheca and carry it by attaching it to the abdomen (McKittrick 1964; Scudder 1971). In Mantodea the subgenital plate is in fact present but not that large (as in Blattodea; Levereault 1936).
Herein we describe ovipositor morphologies of different fossil dictyopterans and discuss possible conclusions about their reproductive strategies. Based on that, we provide an evolutionary reconstruction of the transformation of the ovipositor within Dictyoptera and, consequently, the presumed evolution of the reproductive strategies within this group.
Institutional abbreviations.—KUP, Institute of Geology and Palaeontology of the Charles University, Prague, Czech Republic; NHMS-WP, Naturhistorisches Museum Schloss Bertholdsburg, Schleusingen, Germany; ROMIP, Royal Ontario Museum Invertebrate Palaeontology, Toronto, Canada; YPM IP, Invertebrate Paleontology collection of the Yale Peabody Museum of Natural History, New Haven, USA.
Other abbreviations.—CuP, vein cubitus posterior.
Material and methods
We investigated several examples of dictyopteran fossils with preserved ovipositors from different localities and different geological periods: the late Carboniferous Mazon Creek locality, the early Permian Cabarz and Obora localities and the Early Cretaceous Crato Formation.
Carboniferous material (Mazon Creek).—The specimens from the Carboniferous Carbondale Formation of Mazon Creek, USA (YPM IP 000057, YPM IP 027870, YPM IP 027872) are preserved in ironstone concretions and determined to an age of ca. 309 mya (Moscovian, middle Pennsylvanian).
The selected specimens were first described and figured by Sellards (1904). He placed the nymphs into genera and species erected by Scudder in 1882 and 1895 based on isolated wings. As there is no further support so far which nymph correlates to which winged imago, we use collection numbers for specimen designation.
Permian material (Cabarz, Obora).—The specimen from the Cabarz quarry, Goldlauter Formation, Thuringian Forest Basin, Germany (NHMS-WP 5045) comes from lacustrine laminated claystone of early Permian (Cisuralian, Asselian) age, which is of about 295 mya (Schneider and Werneburg 2012; Schneider et al. 2013). This specimen is not described in detail in the present study, but included for comparison.
The specimen from the renowned Obora insect site, Bačov Horizon, Letovice Formation, Boskovice Graben, Czech Republic (KUP 114a,b) is preserved in a lacustrine claystone of early Permian (Cisuralian, Artinskian/Kungurian) age, which is about 285 to 280 mya (Schneider and Scholze 2016).
Cretaceous material (Crato Formation).—The specimens from the Cretaceous Crato Formation, Brazil, formerly addressed to as part of the Santana Formation (for details see Martill 2007) (ROMIP64634, ROMIP64635, ROMIP64636, ROMIP64637, ROMIP64638, ROMIP64639, ROMIP64640, ROMIP64641) are on long term loan to the Royal Ontario Museum, Toronto (ROM). They are preserved in a limestone, which is assigned to the Aptian stage (115 mya; Grimaldi 2003).
Data collection.—All specimens were photographed with a Canon Eos Rebel T3i camera equipped with a MP-E 65 mm objective and a MeiKe LED Macro Ring Flash FC 100 or a Canon Twin Flash MT-24. To reduce reflections, cross-polarised illumination was used (e.g., Haug et al. 2011a, b; Kerp and Bomfleur 2011; Hörnig et al. 2014, 2016). Different adjacent image details were stitched to panoramas with the photomerge-function of Adobe Photoshop CS3. To generate stereo images (red/cyan), two photographs from different angles were recorded, subsequently edited and arranged with Adobe Photoshop CS3 (e.g., Haug et al. 2011a).
Morphological terminology.—Terminology should represent an important issue to be thought of in scientific procedures, leading to well-founded, broadly applicable and unambiguous terminologies (e.g., Richter et al. 2010).
Insects appear to be especially challenging in this aspect. Many terms were coined for structures appearing only for specific ingroups. While this is not only the case in insects, the group, as being so morphologically diverse, is clearly vulnerable for such practices. One could argue that highly specialised terminology leads to less ambiguous descriptions. Yet, this comes at the cost of broader understandability. Moreover, many terminologies imply hidden meanings about the origins of certain structures (see e.g., discussion of Hädicke vs. Haug in Hädicke et al. 2014). Therefore, we opt for a neutral terminology in the following. This should allow also non-expert readers to access more easily specialised literature. Specialist terminology is given in brackets to keep the reference to ingroup specific literature. Descriptions use general arthropod terms and follow a strict pattern of describing segment by segment (Haug et al. 2012).
To assist the reader we provide data on the external morphology of selected extant dictyopterans (Figs. 1, 2). We selected females of the extant species Hierodula cf. grandis (Burmeister, 1838) and Periplaneta americana (Linnaeus, 1758) for documentation. Specimens of P. americana were taken from the breeding of the Zoological Institute and Museum of the Ernst-Moritz-Arndt-University of Greifswald. One female of H. cf. grandis came from a private breeding and was kindly provided by Marc Bierling, Munich.
The ovipositor of polyneopteran insects, including dictyopterans, is composed of 3 pairs of so-called “valves” (gonapophyses 8, 9/gonopod 8, 9 = valves 1, 2; gonoplaque = valves 3) and are transformed parts of appendages of abdominal segments 8 (valve 1) and 9 (valve 2) (Klass et al. 2012; Figs. 1, 2), but the exact identity of valves 3 is still unclear to some degree (in comparison to non-insect arthropods; e.g., Marks and Lawson 1962; Grimaldi and Engel 2005; Hädicke et al. 2014). In fossils it is not always possible to clearly identify which of these is preserved. In some cases the orientation of the fossil (dorsal versus ventral, lateral versus medial) can give some hints, but only if more than one pair can be seen. In case of doubt, we treat these structures under “open” terminology.
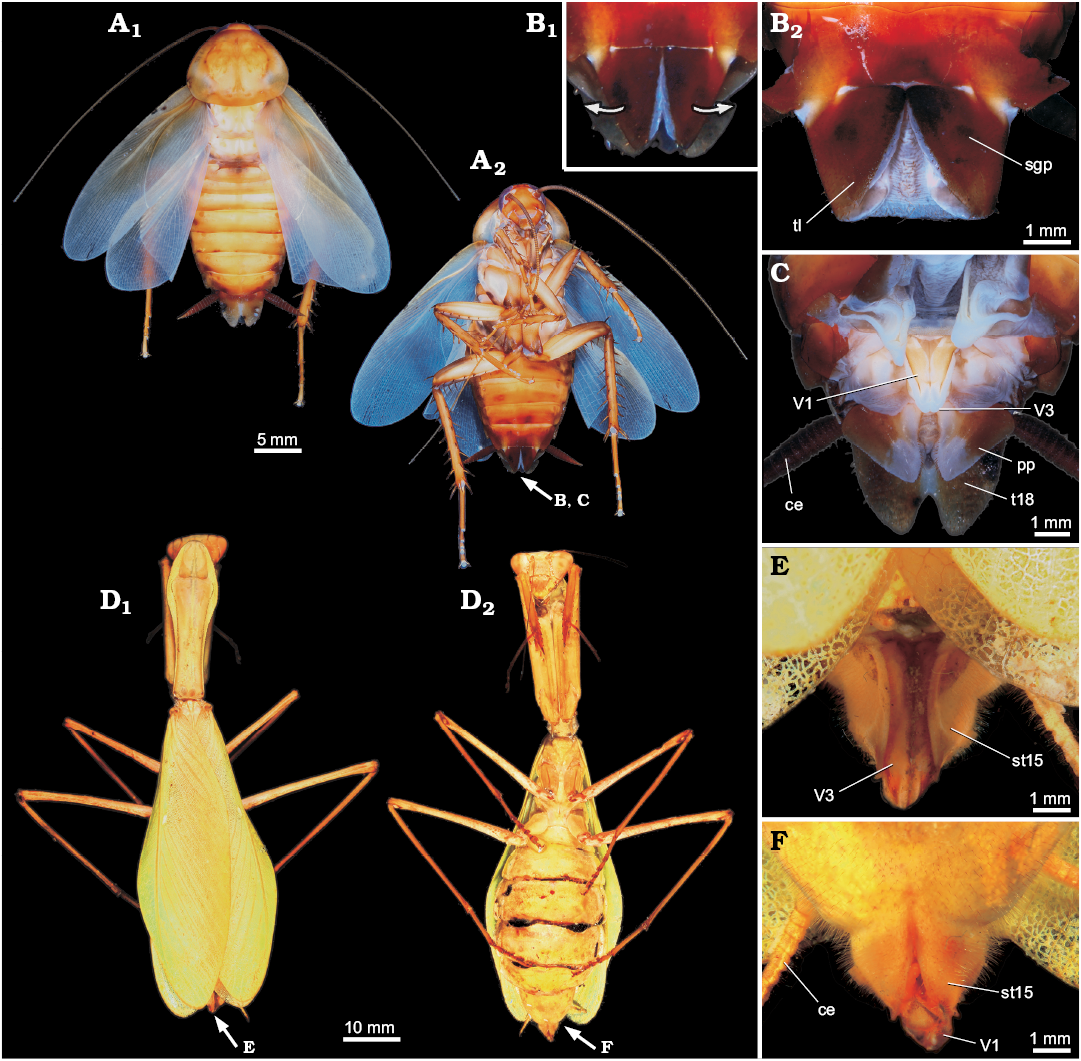
Fig. 1. General external morphology of extant Dictyoptera. A–C. American cockroach Periplaneta americana (Linnaeus, 1758). A. Overview in dorsal (A1) and ventral (A2) views. B. Close-up of subgenital plate in ventral view (B1, B2). C. Close-up of the ovipositor, subgenital plate ventrally bent for exposition of the ovipositor valves. D–F. Giant Asian mantis Hierodula cf. grandis (Burmeister, 1838). D. Overview in dorsal (D1) and ventral (D2) views. Close-up of ovipositor in dorsal (E) and ventral (F) views. Abbreviations: ce, cercus; pp, paraproct; sgp, subgenital plate; st15, sternite of post-ocular segment 15 (abdominal sternite 7; subgenital plate); tl, terminal lobes; t18, tergite of the post-ocular segment 18 (abdominal segment 10); V1, V3, valves 1, 3.
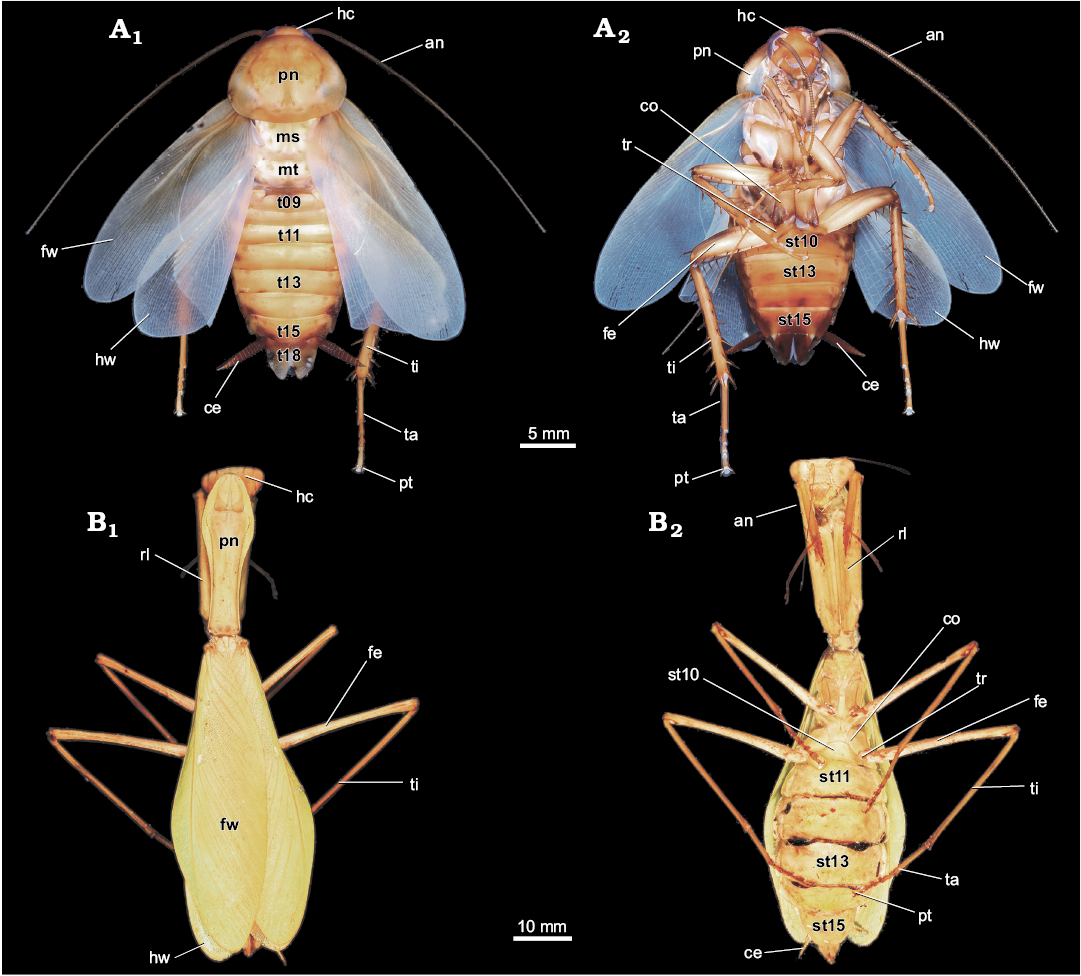
Fig. 2. General external morphology of extant Dictyoptera. A. Female of American cockroach Periplaneta americana (Linnaeus, 1758), in dorsal (A1) and ventral (A2) views. B. Female of giant Asian mantis Hierodula cf. grandis (Burmeister, 1838), in dorsal (B1) and ventral (B2) views. Abbreviations: an, antenna; ce, cercus; co, coxa of metathoracic leg; fe, femur of metathoracic leg; fw, forewing; hc, head capsule; hw, hindwing; ms, mesonotum; mt, metanotum; pn, pronotum; pt, pretarsus of metathoracic leg; rl, raptorial leg (prothoracic leg); st10–15, sternite of post-ocular segment 10–15 (abdominal sternite 2–7); t9–18, tergite of the post-ocular segment 9–18 (abdominal segment 1–10); ta, tarsus of metathoracic leg; ti, tibia of metathoracic leg; tr, trochanter of metathoracic leg.
Results
All specimens have a blattoid habitus. The body is divided into presumably 20 segments. The head capsule is formed by the ocular segment and post-ocular segments 1–5. The thorax with walking appendages includes post-ocular segments 6–8. The abdomen without walking appendages includes (presumably, as the fossil is an insect) post-ocular segments 9–19. As in all specimens at least parts of the ovipositor are preserved, we assume that all described specimens are females.
Carboniferous roachoid dictyopteran nymphs from Mazon Creek
Figs. 3–8.
Locality: Mazon Creek, Illinois, USA.
Horizon: Carbondale Formation, Moscovian, middle Pennsylvanian, upper Carboniferous.
YPM IP 000057 (?phylloblattoid type).—The specimen (Figs. 3, 5A, 8A), original to Sellards (1904: fig. 13, pl. 1: 2) is preserved in ventral position. The total body length is about 16 mm, and width (without appendages) is about 9 mm. Shape of head capsule in ventral view is relatively stout, about 3 mm wide. Exact length unclear as posterior part concealed by pronotum; visible part 1.5 mm. Compound eyes are not preserved.
Post-ocular segments 6–8 each with a separate, well sclerotised lateral extruded tergite (pro-, meso-, and metanotum). Pronotum is about 4 mm long and 6.5 mm wide and partly overhanging the posterior region of the head capsule. Mesonotum is strongly curved in dorsal view; medially 2 mm long, up to 6 mm long laterally, 9 mm wide. Metanotum is strongly curved in dorsal view; medially 2.5 mm long, up to 5.5 mm long laterally, 7 mm wide. The entire medial length of thorax (post-ocular segments 6–8) is about 8.5 mm. Appendages of post-ocular segments 6–8 developed as walking legs; only parts of legs preserved (Fig. 5A3, A4). Post-ocular segments 7 and 8 additionally bear dorsally anlagen of fore- and hindwings (continuous with the tergites).
The visible abdominal sternites are well sclerotised. The sternites of post-ocular segments 10–15 are clearly identifiable. Post-ocular segment 9 is not visible but it is unclear whether this is the original condition or due to the incomplete preservation. The visible abdominal sternites are about 0.5 mm long respectively, sternite of post-ocular segment 15 is about 1 mm in length.
Distal parts of appendages of post-ocular segment 16 or 17 (valves of ovipositor) are well developed (Fig. 5A1, A2); in ventral view, one pair of valves is visible which is about 2.7 mm long and 0.6 mm wide at the base and tapers to a fine point on the distal end. Appendages of post-ocular segment 19 (cerci) are not preserved.
YPM IP 027872 (part/counterpart).—The specimen (Figs. 4, 5B, 8B), original to Sellards (1904: fig. 11) is preserved in dorso-lateral view. The total body length is about 16 mm, body width (without appendages) is about 6.5 mm. The head is not visible (covered by pronotum or not preserved).
Post-ocular segments 6–8 dorsally each with a separate, well sclerotised tergite (pro-, meso-, and metanotum). Pronotum is about 4 mm long and 6 mm wide. Mesonotum is curved in dorsal view; medially 2 mm long, up to 3 mm long laterally, 6 mm wide. Metanotum is curved in dorsal view; medially 2 mm long, up to 3.5 mm long laterally, 6.5 mm wide. The entire medial length of thorax (post-ocular segments 6–8) is about 8 mm. Appendages of post-ocular segments 6–8 developed as walking legs; only parts of legs preserved (parts of the coxae are visible because of erosion of the median parts of the thoracic tergites). Post-ocular segments 7 and 8 appear to bear anlagen of fore- and hindwings (continuous with the tergites).
The visible abdominal tergites are well sclerotised, each about 1 mm long. Clearly identifiable are tergites of post-ocular segments 13–17. The tergites of post-ocular segment 9–12 and 18 are not visible but it is unclear in which cases this is the original condition or caused due to incomplete preservation.
Appendages of post-ocular segments 16 and 17 (ovipositor; Fig. 5B): one pair of valves is clearly recognisable (spread apart). The valves are about 4 mm long and 1 mm wide at the base. Appendages of post-ocular segment 19 (cerci) are not preserved.
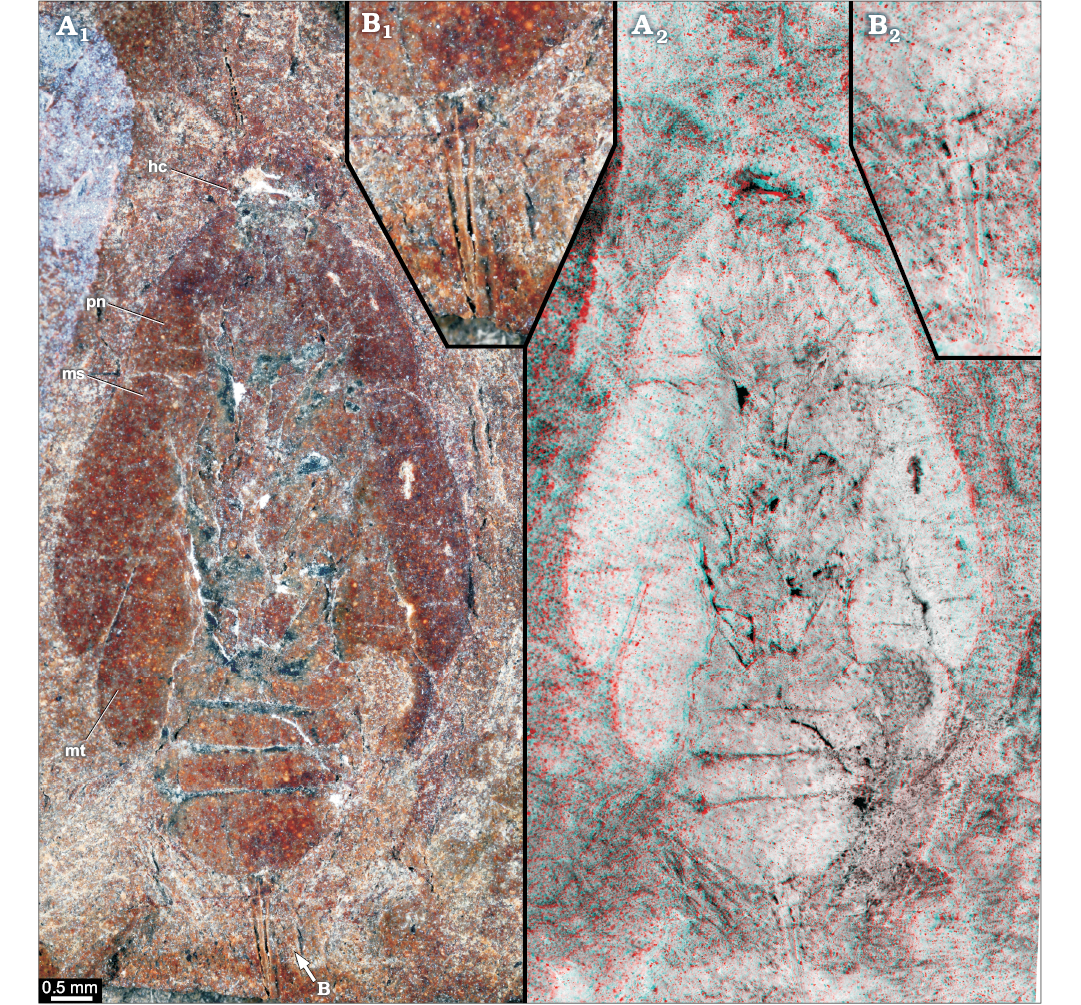
Fig. 3. Roachoid nymph YPM IP 000057 from the Carboniferous Carbondale Formation of Mazon Creek, USA; in ventral view. Overview (A) and close-up images of ovipositor (B); A1, B1, photographs without stereo information; A2, B2, red-cyan stereo-anaglyphs (please use red-cyan glasses to view). Abbreviations: hc, head capsule; ms, tergite of mesothorax; mt, tergite of metathorax; pn, tergite of prothorax.

Fig. 4. Roachoid nymph YPM IP 027872 from the Carboniferous Carbondale Formation of Mazon Creek, USA; in dorso-lateral view. Part (A) and counterpart (B). A1, B1, overview images; A2, B2, red-cyan stereo-anaglyphs (please use red-cyan glasses to view); A3, B3, close up images of ovipositor. Abbreviations: ms, tergite of mesothorax; mt, tergite of metathorax; pn, tergite of prothorax.
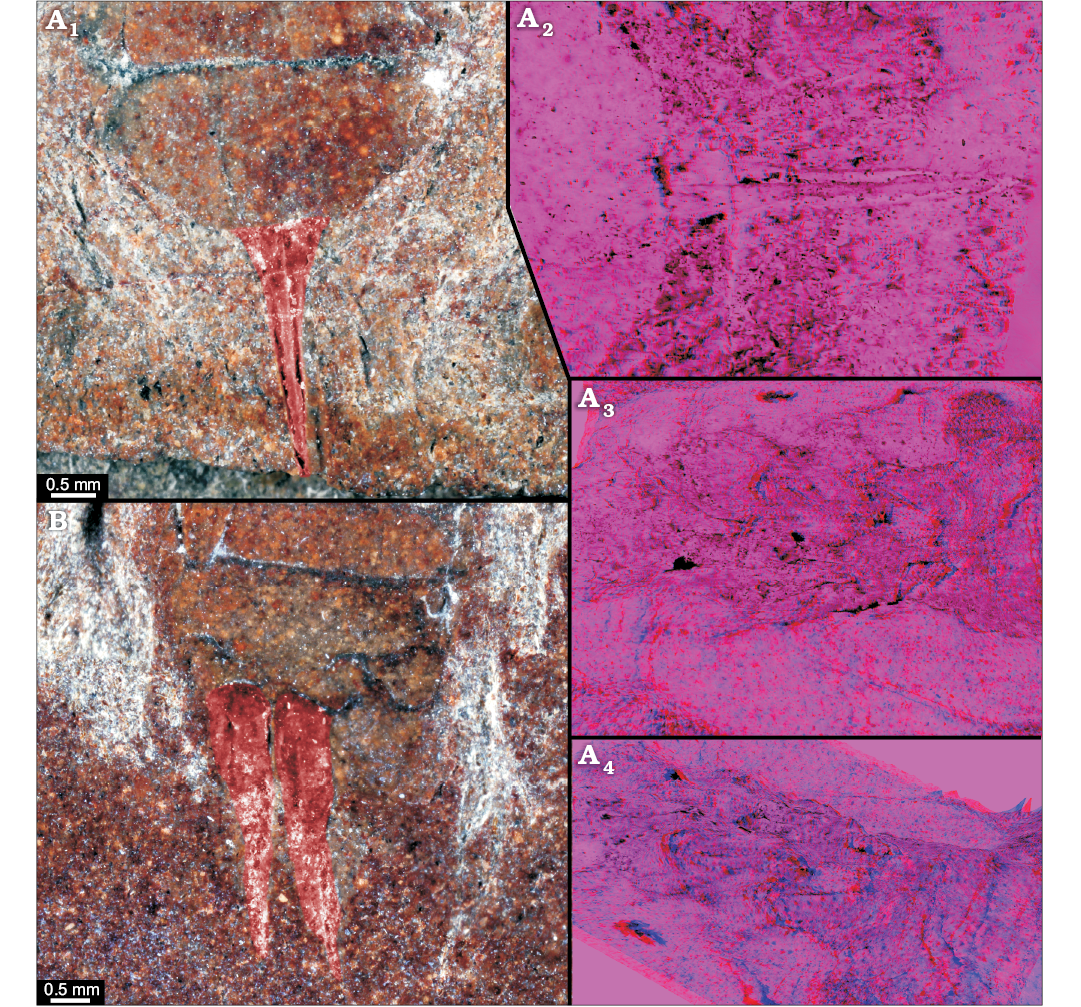
Fig. 5. Roachoid nymphs YPM IP 000057 (A) in ventral view and YPM IP 027872 in dorso-lateral view (B) with interpretations of preserved structures. A1, B, colour-marked close-up image of ovipositor; A2, stereo close-up image of the ovipositor; A3, A4, stereo close-up images of thorax with visible parts of the coxae (red-cyan stereo-anaglyphs; please use red-cyan glasses to view).
YPM IP 027870 (mylacrid type; part/counterpart).—The specimen (Figs. 6, 7, 8C), original to Sellards (1904: figs. 6, 7) is preserved in dorsal position. The total body length is about 25 mm and body width is about 15 mm (without appendages). The head is not visible (covered by pronotum or not preserved).
Post-ocular segments 6–8 dorsally each with a separate, well sclerotised tergite (pro-, meso-, and metanotum). Pronotum is about 8 mm long and 14 mm wide. Mesonotum is strongly curved in dorsal view; medially 2.5 mm long, up to 9 mm long laterally, 15 mm wide. Metanotum is curved in dorsal view; medially 3 mm long, up to 4 mm long laterally, 12 mm wide. The entire medial length of thorax (post-ocular segments 6–8) is about 13.5 mm. Appendages of post-ocular segments 6–8 developed as walking legs; only parts of legs preserved (parts of the coxae are visible because of erosion of the median parts of the thoracic tergites). Post-ocular segments 7 and 8 bear anlagen of fore- and hindwings (continuous with the tergites).
Post-ocular segments 9–18 dorsally with a separate, well sclerotised and about 1 mm long tergite each. On post-ocular segment 16 or 17 a deltoid-shaped dent is visible (see stereo images; Fig. 7), which could be interpreted as insertion point of an ovipositor. Appendages of post-ocular segment 19 (cerci) are only fragmentarily preserved.
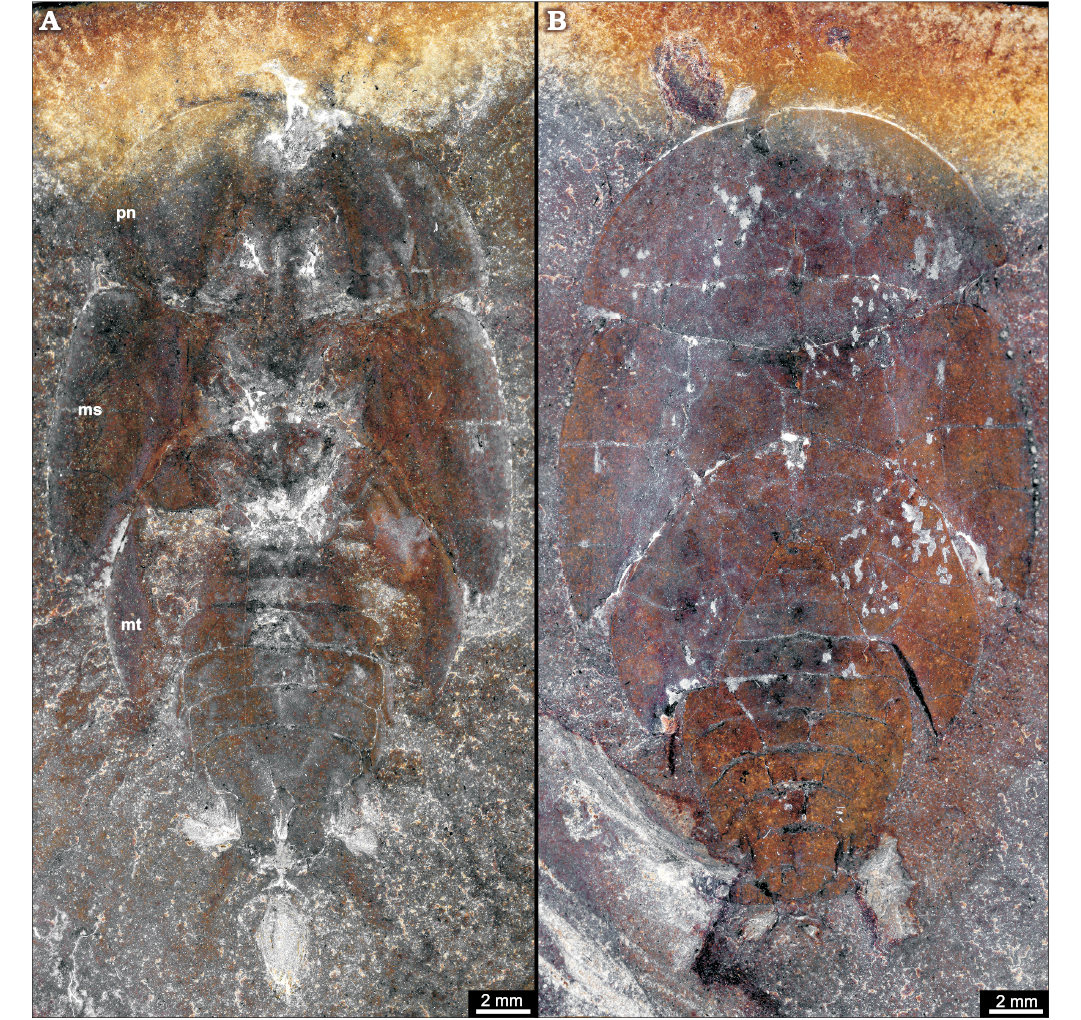
Fig. 6. Roachoid nymph YPM IP 027870 from the Carboniferous Carbondale Formation of Mazon Creek, USA; in dorsal view. Overview of part (A) and counterpart (B), thorax with visible parts of the coxa because of erosion of the median parts of thoracic tergites. Abbreviations: ms, tergite of mesothorax; mt, tergite of metathorax; pn, tergite of prothorax.
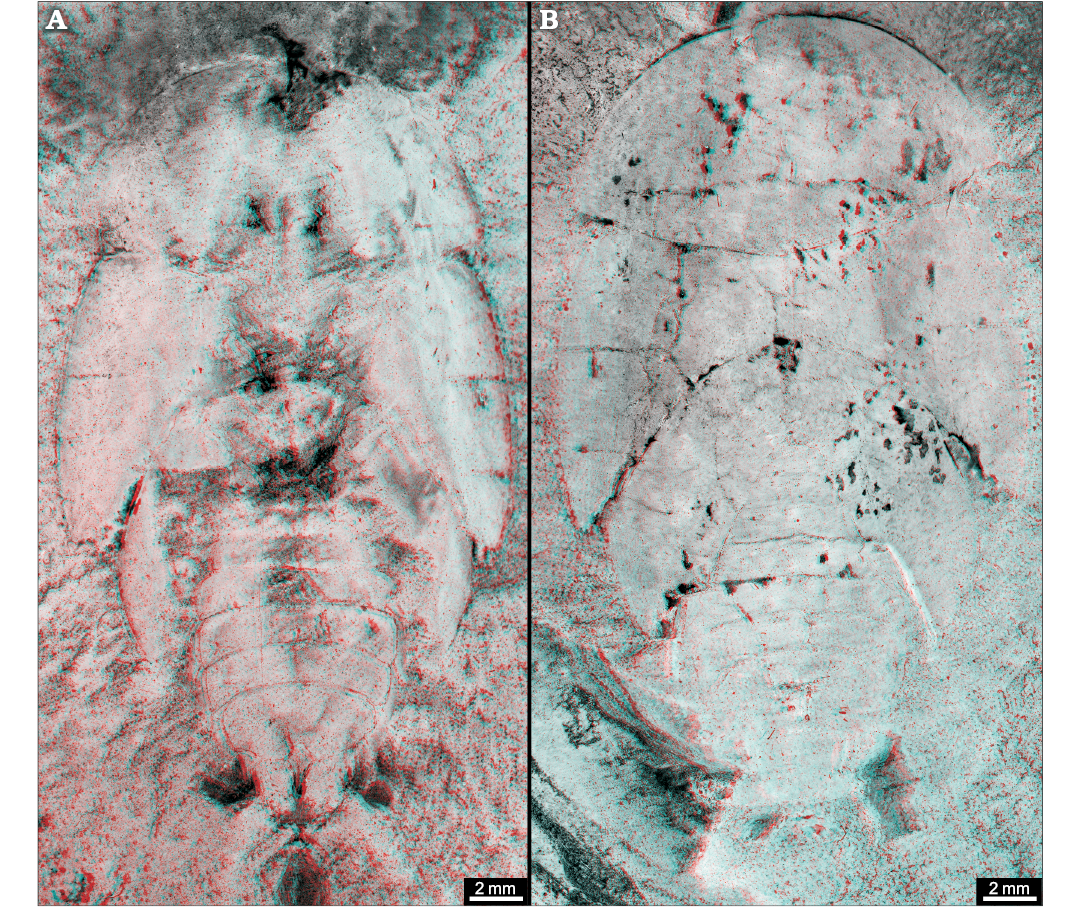
Fig. 7. Roachoid nymph YPM IP 027870 from the Carboniferous Carbondale Formation of Mazon Creek, USA; in dorsal view. Overview of part (A) and counterpart (B). Red-cyan stereo-anaglyphs (please use red-cyan glasses to view).
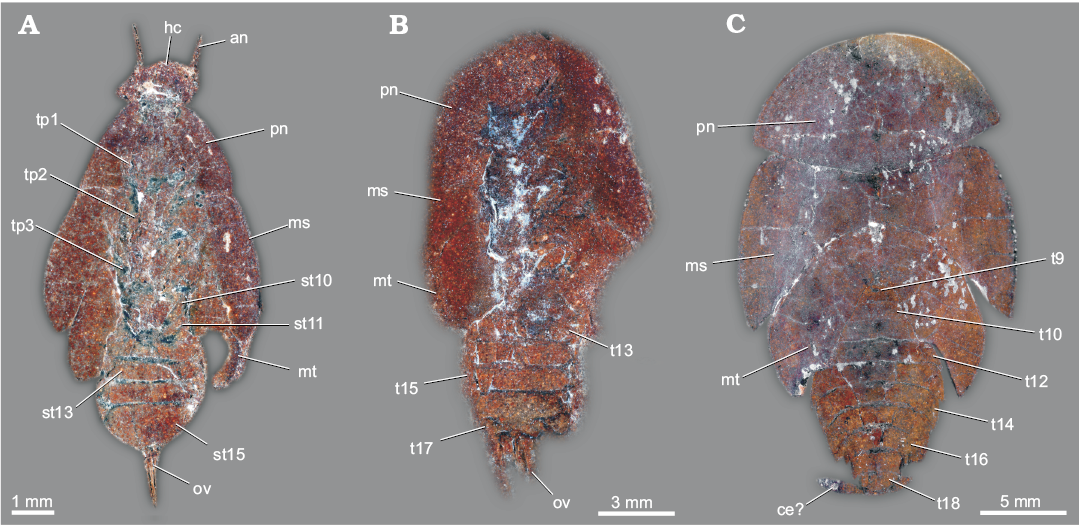
Fig. 8. Roachoid nymphs from the Carboniferous Carbondale Formation of Mazon Creek, USA; with interpretations of preserved structures. A. YPM 000057. B. YPM IP 027872. C. YPM IP 027870. Abbreviations: an, antenna; ce, cercus; hc, head capsule; ms, tergite of mesothorax; mt, tergite of metathorax; ov, ovipositor; pn, tergite of prothorax; st10–15, sternite of post-ocular segment 10–15 (abdominal segment 2–7); t9–18, tergite of post-ocular segment 9–18 (abdominal segment 1–10); tp1–3, thoracopod 1–3 (pro-, meso- and metathoracic legs).
Permian roachoid from Obora
Fig. 9.
Locality: Obora insect site, Boskovice Graben, Czech Republic.
Horizon: Lacustrine claystone, Letovice Formation, Bačov Horizon, Cisuralian, Artinskian/Kungurian, early Permian.
KUP 114a, b (part/counterpart), Syscioblatta sp.—The specimen (Fig. 9) is preserved in dorsal view and nearly complete. The total body length is about 15 mm (without appendages).
Shape of head capsule in dorsal view is drop-shaped and about 2 mm long. Compound eyes are not preserved. Post-ocular segment 6 dorsally with a separate, well sclerotised tergite (pronotum). Pronotum about 4.7 mm long and has a maximum width of about 5.2 mm. Pronotum is not overhanging the head capsule. Meso- and metanotum (post-ocular segments 7 and 8) are concealed by the wings. Appendages of post-ocular segments 6–8 (legs) are well preserved. Prothoracic leg (appendage of post-ocular segment 6): coxa is about 2.5 mm long and 1 mm wide, femur is about 2.5 mm long and 0.8 mm wide, tibia is about 3 mm long and 0.3 mm wide, tarsus is about 3.5 mm long and about 0.1 mm wide. Mesothoracic leg (appendage of post-ocular segment 7): coxa is not visible, femur is about 2.7 mm long and 0.5 mm wide, tibia is about 3.1 mm long and 0.4 mm wide, tarsus is about 3.7 mm long (including pretarsus with claw) and about 0.3 mm wide. Metathoracic leg (appendage of post-ocular segment 8): coxa is not visible, femur is about 2.5 mm long and 0.9 mm wide, tibia is about 7.7 mm long and 0.4 mm wide, tarsus is about 3.1 mm long and about 0.3 mm wide. Fore- and hindwings on post-ocular segments 7 and 8 protrude beyond the abdomen about one third of the entire body length. The forewings are about 14 mm long and about 5 mm wide (maximum width). The wings have a characteristic color pattern, schematically shown in Schneider et al. (2013: fig. 4, top right). Post-ocular segments 9–19 (abdominal segments) are not visible (concealed by wings).
Appendages of post-ocular segments 16 and/or 17 (valves of ovipositor) are well developed (Fig. 9A2), the visible part of the ovipositor is about 5 mm long and 1.2 mm wide at the base. Two parts of the ovipositor are identifiable, which is interpreted as valves 1 and 3. The surface seems to be partly structured, but we cannot rule out for sure that this is a preservation artefact. Appendages of post-ocular segment 19 (cerci) are not visible.
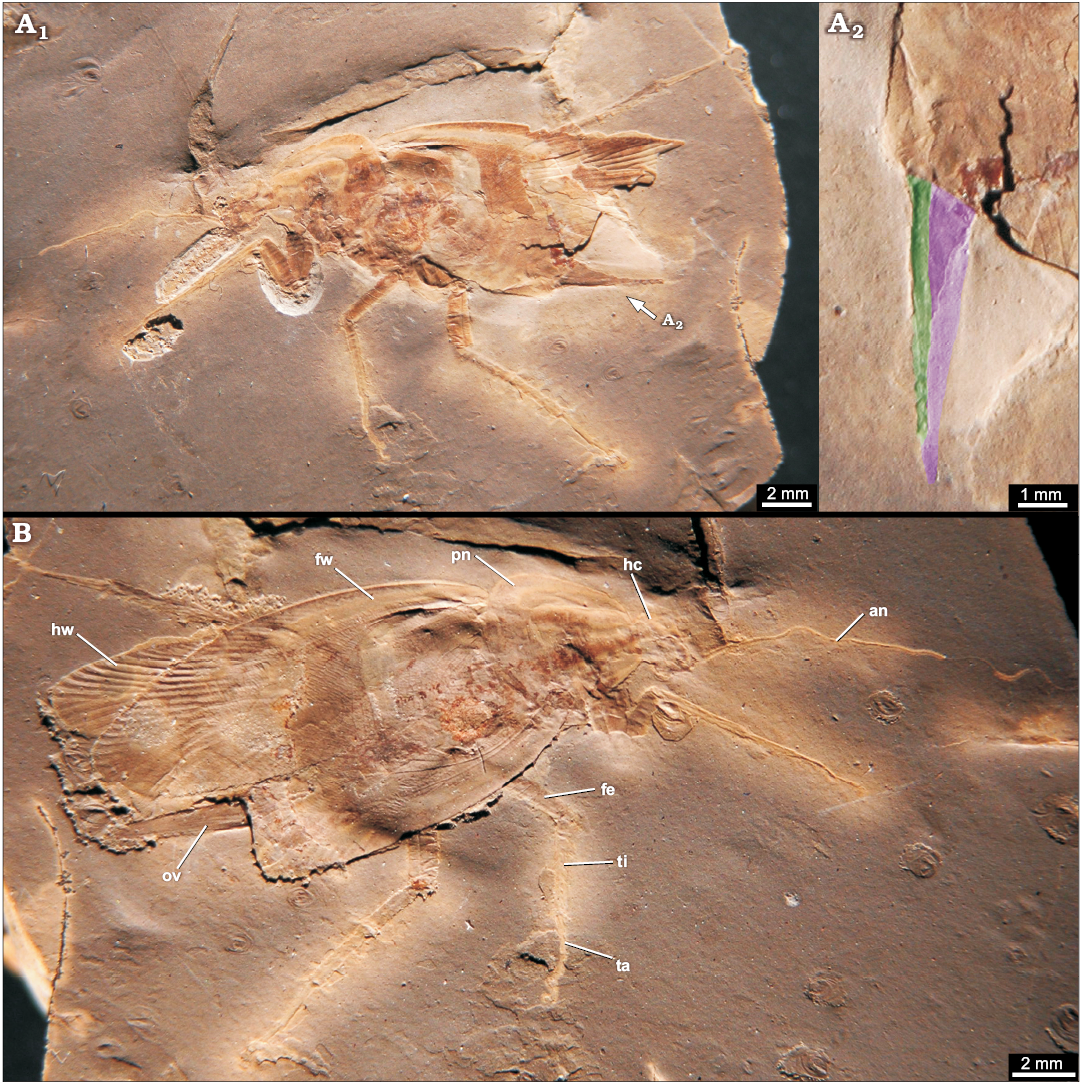
Fig. 9. Roachoid Syscioblatta sp. from early Permian of Obora, Czech Republic; in dorsal view. A. Part KUP 114a. B. Counterpart KUP 114b. A1, B, overviews; A2, close-up of the ovipositor (green, valve 1; purple, valve 3). Abbreviations: an, antenna; fe, femur of prothoracic leg; fw, fore wing; hc, head capsule; hw, hind wing; ov, ovipositor; ta, tarsus of prothoracic leg; ti, tibia of prothoracic leg.
Cretaceous blattodean-like dictyopterans from the Crato Formation
Figs. 10–13.
Locality: Araripe Basin, Brazil.
Horizon: Crato Formation (formerly part of Santana Formation), Aptian, lower Cretaceous.
ROMIP64635.—Specimen (Figs. 10A1, 11A1) is preserved in ventral position. The total body length is about 6.5 mm and body width is about 3.5 mm (without appendages).
Head is fragmentarily preserved. Post-ocular segments 6–7 (pro- and mesothorax) are not preserved. Post-ocular segment 8 (metathorax) ventrally with a separate, well sclerotised sternite. The individual lengths of pro-, meso-, metathorax and abdomen are undeterminable due to the incomplete preservation. Forewings on post-ocular segment 7 are about 8 mm long (insertion points are not visible because of incomplete preservation) and 2.5 mm wide. The vein cubitus posterior (CuP) is strongly curved. Hindwings on post-ocular segment 8 are about 7.5 mm long (insertion points are not visible) and about 3.5 mm wide. Fore- and hindwings protrude beyond the abdomen about one third up to one half of the entire body length.
The visible abdominal sternites are well sclerotised. Sternites of post-ocular segments 10–15 are clearly identifiable. Post-ocular segment 9 is not visible, but it is unclear whether this is the original condition or caused by the incomplete preservation. Appendages of post-ocular segments 16 or 17 (valves of ovipositor) are well developed (Figs. 10A2 and 11A2). One pair of valves of the ovipositor is visible (spread apart). Each valve is about 1.5 mm long and 0.1 mm wide at the base and tapers to a fine point on the distal end. Appendages of post-ocular segment 19 (cerci) are about 0.7 mm long and at the base 0.1 mm wide and subdivided into several elements.
ROMIP64634.—The specimen (Figs. 10B1, 11B1) is preserved in dorsal view. The total body length is about 8.3 mm and body width is about 4 mm (without appendages).
Shape of head capsule in dorsal view is relatively stout and about 2.2 mm wide. Exact length unclear as posterior part concealed by pronotum; visible part 0.6 mm. Compound eyes are well preserved with a diameter of about 1 mm. Post-ocular segment 6 dorsally with a separate, well sclerotised tergite (pronotum). Pronotum is about 2.5 mm long, 3 mm wide and partly overhanging the head capsule posteriorly. Meso- and metanotum (post-ocular segments 7 and 8) are concealed by the wings. Appendages of post-ocular segments 6–8 (legs) are not preserved. Fore- and hindwings on post-ocular segments 7 and 8 protrude beyond the abdomen about one quarter of the entire body length. Tergites of post-ocular segments 9–18 (abdominal segments) are not visible (concealed by wings).
Appendages of post-ocular segments 16 or 17 (valves of ovipositor) are well developed (Figs. 10B2, 11B2). One pair of valves is visible (spread apart due to preservation), each valve about 1.5 mm long and 0.1 mm wide at the base. Appendages of post-ocular segment 19 (cerci) are about 1.7 mm long and at the base 0.3 mm wide and taper to a fine point on the distal end. The cerci are subdivided into several elements and are preserved with numerous setae.
ROMIP64636.—The specimen (Figs. 10C2, 11C2) is preserved in ventro-lateral position. The total body length is about 6.2 mm without head (head not preserved) and total body width is about 3 mm (without appendages).
Post-ocular segments 6–8 (pro-, meso-, metathorax) and post-ocular segments 9–15 (abdominal segments) presumably ventrally with a separate, well sclerotised sternite (segments only partly preserved and not individually identifiable). The individual lengths of the pro-, meso-, metathorax and abdomen are undeterminable due to their incomplete preservation. Appendages of post-ocular segments 6–8 (legs) are not preserved. Fore- and hindwings on post-ocular segments 7 and 8 protrude beyond the abdomen about one quarter of the entire body length.
Appendages of post-ocular segments 16 and/or 17 (valves of ovipositor) are well developed (Figs. 10C2, 11C2), about 1.2 mm long and 1.2 mm wide at the base. Inner valves (valves 2) are about 0.2 mm wide and 1.2 mm long. Lateral valves (presumably valves 3) are about 0.5 mm wide at the base and about 1 mm long. Appendages of post-ocular segment 19 (cerci) are not preserved.
ROMIP64638.—The specimen (Figs. 10D1, 11D1) is preserved in ventral position. The total body length is about 14 mm and body width is about 6.5 mm (without appendages). The head is very weakly preserved (width presumably about 2.7 mm), but compound eyes are partly recognisable with a diameter of about 0.8 mm.
Post-ocular segments 6–8 (pro-, meso-, metathorax) each ventrally with separate, well sclerotised sternite. Prothorax is about 3 mm long. Lengths of meso- and metathorax are undeterminable due to their weak preservation. Appendages of post-ocular segments 6–8 (legs) are not preserved. Fore- and hindwings on post-ocular segments 7 and 8 protrude beyond the abdomen about one fifth of the entire body length.
Abdominal segments (post-ocular segments 9–15) are only partly identifiable due to the incomplete preservation. Appendages of post-ocular segments 16 and/or 17 (valves of ovipositor; Figs. 10D2, 11D2) are well developed. Two pairs of valves are visible. Inner valves (valves 2) are about 0.2 mm wide at the base and about 2.5 mm long. Lateral valves (presumably valves 3) have a maximum width of 0.7 mm and are about 2.5 mm long. Appendages of post-ocular segment 19 (cerci) are not preserved.
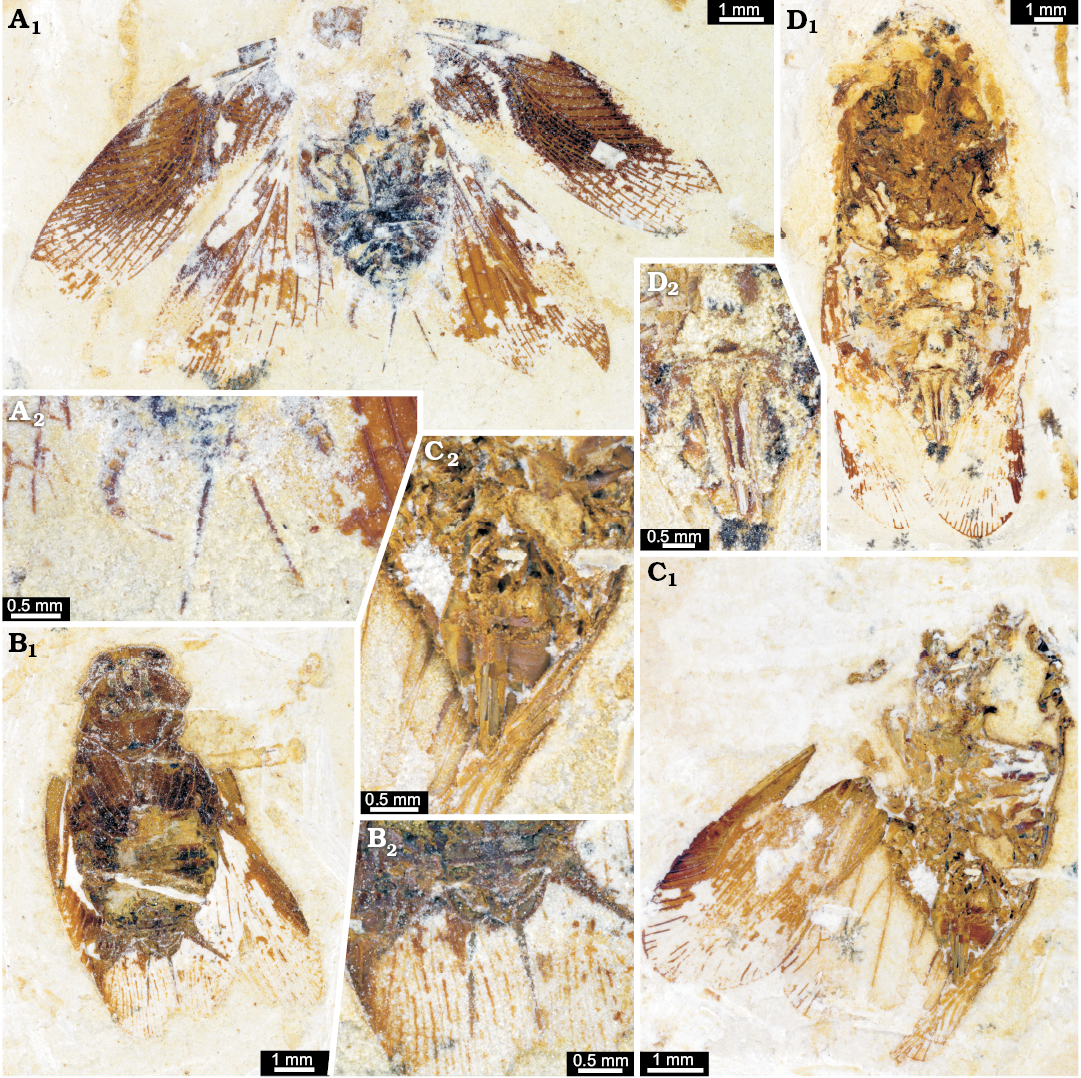
Fig. 10. Undescribed cockroaches or roachoids from the Aptian, Cretaceous Crato Formation of Brazil. A. ROMIP64635 in ventral view. B. ROMIP64634 in ventro-lateral view. C. ROMIP64636 in dorsal view. D. ROMIP64638 in ventral view. A1–D1, overviews; A2–D2, close ups of the ovipositor.
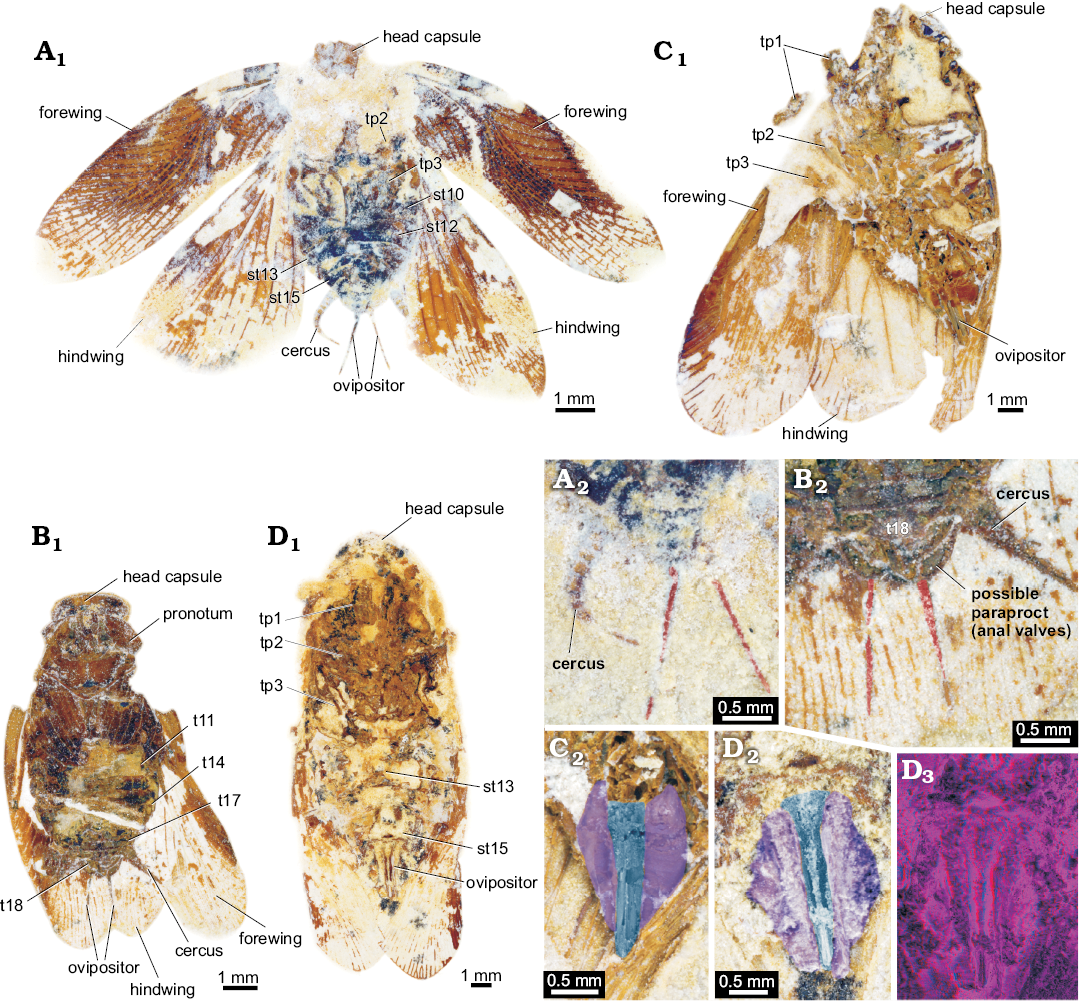
Fig. 11. Undescribed cockroaches or roachoids from the Aptian, Cretaceous Crato Formation of Brazil; with interpretations of preserved structures. A. ROMIP64635 in ventro-lateral view. B. ROMIP64634 in dorsal view. C. ROMIP64636 in ventral view. D. ROMIP64638 in ventral view. A1–D1, overviews; A2–D2, colour-marked close ups of the ovipositor (red, undeterminable valves; blue, valves 2; purple, valves 3); D3, red-cyan stereo-anaglyph (please use red-cyan glasses to view). Abbreviations: st10–15, sternite of post-ocular segment 10–15 (abdominal segment 2–7); t11–18, tergite of post-ocular segment 11–18 (abdominal segment 3–10); tp1–3, thoracopod 1–3 (pro-, meso-, and metathoracic legs).
ROMIP64637.—The specimen (Figs. 12A1, 13A1) is preserved in ventral position. The total body length is about 8 mm and body width is about 3.5 mm (without appendages). Head and compound eyes are fragmentarily preserved. The width of the head is about 2.2 mm (length not determinable due to the weak preservation). Compound eyes have a diameter of slightly more than 1 mm.
Sternites of post-ocular segments 6–8 (pro-, meso-, metathorax) are only fragmentarily preserved. Prothorax is about 1.3 mm long (width is not determinable due to incomplete preservation). Mesothorax about 1 mm, and metathorax about 0.8 mm long. Appendages of post-ocular segments 6–8 (legs) are fragmentarily preserved; parts of the tibia preserved with several spines. Forewings on post-ocular segments 7 are about 9 mm long and 3 mm wide. CuP (vein) is strongly curved. Hindwings on post-ocular segment 8 are about 7.5 mm long (insertion points are not visible) and about 3.8 mm wide. Fore- and hindwings protrude beyond the abdomen about one quarter up to one third of the entire body length.
The visible abdominal sternites are well sclerotised. The sternites of post-ocular segments 10–15 are clearly identifiable. The sternite of post-ocular segment 9 is not visible but it is unclear whether this is the original condition or caused by incomplete preservation, but putative parts of the tergite 9 are visible laterally. Post-ocular segment 15 (sternite 7) is drawn out posteriorly into two lobes.
Appendages of post-ocular segments 16 and or 17 (valves of ovipositor; Figs. 12A2 and 13A2) are well developed. One pair of valves is recognisable. Valves 1 protrude beyond post-ocular segment 15 (sternite 7) about 0.4 mm (insertion point on post-ocular segment 17 not visible) and are less than 0.1 mm wide. The visible part of the elongated post-ocular segment 15 (sternite 7) is about 1 mm wide at the base and about 0.6 mm long. Appendages of post-ocular segment 19 (cerci) are partly preserved; subdivided into several elements.
ROMIP64639, Ponopterix maxima Bechly, 2007.—The specimen (Figs. 12B1, 13B1) is preserved in ventral position. Total body length is about 10 mm and body width is about 4 mm (without appendages). Head has a diameter of 2.5 mm. Compound eyes are well preserved; diameter about 1 mm.
Post-ocular segments 6–8 (pro-, meso-, metathorax) each ventrally with separate, well sclerotised sternite. Prothorax is about 2 mm long and 2 mm wide. Mesothorax is about 2 mm and metathorax is about 1.5 mm long. Appendages of post-ocular segments 6–8 (legs) are not preserved. Fore- and hindwings (post-ocular segments 7 and 8) slightly protrude beyond the abdomen.
The visible abdominal sternites are well sclerotised. The sternites of post-ocular segments 11–15 are clearly identifiable. Post-ocular segments 9 and 10 are not visible but it is unclear whether this is the original condition or caused by incomplete preservation.
Appendages of post-ocular segments 16 or 17 (valves of ovipositor; Figs. 12B2, 13B2) are well developed, about 0.7 mm long and 0.5 mm wide at the base (no individual valves determinable; presumably valves 1 visible because of the fossils position). Appendages of post-ocular segment 19 (cerci) taper to a fine point, are subdivided into several elements, about 1.2 mm long and 0.3 mm wide at the base.
ROMIP64640, Ponopterix maxima Bechly, 2007.—The specimen (Figs. 12C1, 13C1) is preserved in ventral position. Total body length is about 11 mm and body width is about 4 mm (without appendages). Head has a diameter of 2.5 mm. Compound eyes and antennae are fragmentarily preserved.
Post-ocular segments 6–8 (pro-, meso-, metathorax) each ventrally with separate, well sclerotised sternite. Prothorax is about 2.5 mm long and 2 mm wide. Mesothorax is about 2 mm and metathorax is about 1.5 mm long. Appendages of post-ocular segments 6–8 (legs) are fragmentarily preserved; spines visible. Fore- and hindwings (post-ocular segments 7 and 8) not overhanging the abdomen, details are not visible (due to preserved position).
The visible abdominal sternites are well sclerotised. The sternites of post-ocular segments 10–15 are clearly identifiable. Post-ocular segment 9 is not visible, but it is unclear whether this is the original condition or caused due to incomplete preservation.
Appendages of post-ocular segments 16 or 17 (valves of ovipositor; Figs. 12C2, 13C2) are well developed. One pair of valves is visible (presumably valves 1); each valve is about 0.8 mm long and 0.5 mm wide at the base. Appendages of post-ocular segment 19 (cerci) taper to a fine point on the distal end, are subdivided into several elements and about 1.2 mm long and 0.2 mm wide at the base.
ROMIP64641 (undescribed).—The specimen (Figs. 12D1, 13D1) is preserved in ventral position. The total body length is about 8.5 mm and body width is about 3.5 mm (without appendages). The head has a diameter of about 2 mm. Compound eyes are not determinable.
Post-ocular segments 6–8 (pro-, meso-, metathorax) each ventrally with separate, well sclerotised sternite. Prothorax is about 1.2 mm long and 2.7 mm wide. Mesothorax is not well preserved, presumably about 1 mm long. Length of metathorax is undeterminable due to the incomplete preservation. Appendages of post-ocular segments 6–8 (legs) are fragmentarily preserved; spines visible. Forewings on post-ocular segment 7 are about 9.5 mm long and 2.7 mm wide. Insertion points of hindwings on post-ocular segment 8 are not visible. Fore- and hindwings protrude beyond the abdomen about one half of the entire body length. Sternites of abdominal segments (post-ocular segments 9–15) are not clearly identifiable due to the incomplete preservation. The supposed post-ocular segment 15 (sternite 7) is drawn out posteriorly into two lobes.
Appendages of post-ocular segments 16 or 17 (valves of ovipositor; Figs. 12D2, 13D2) are well developed. One pair of valves is determinable. The valves (presumably valve 1) are about 1.1 mm long and about 0.1 mm wide. The visible part of the elongated post-ocular segment 15 (sternite 7) is about 0.5 mm long (median part seems to lack, presumably due to incomplete preservation). Appendages of post-ocular segment 19 (cerci) taper to a fine point on the distal end and are subdivided into several elements. Cerci are about 2 mm long and about 0.3 mm wide at the base.

Fig. 12. Ponopterix maxima Bechly, 2007 (B, C) and undescribed cockroaches or roachoids (A, D) from the Aptian, Cretaceous Crato Formation of Brazil; in ventral views. A. ROMIP64637. B. ROMIP64639. C. ROMIP64640. D. ROMIP64641. A1–D1, overviews; A2–D2, close ups of the ovipositors.
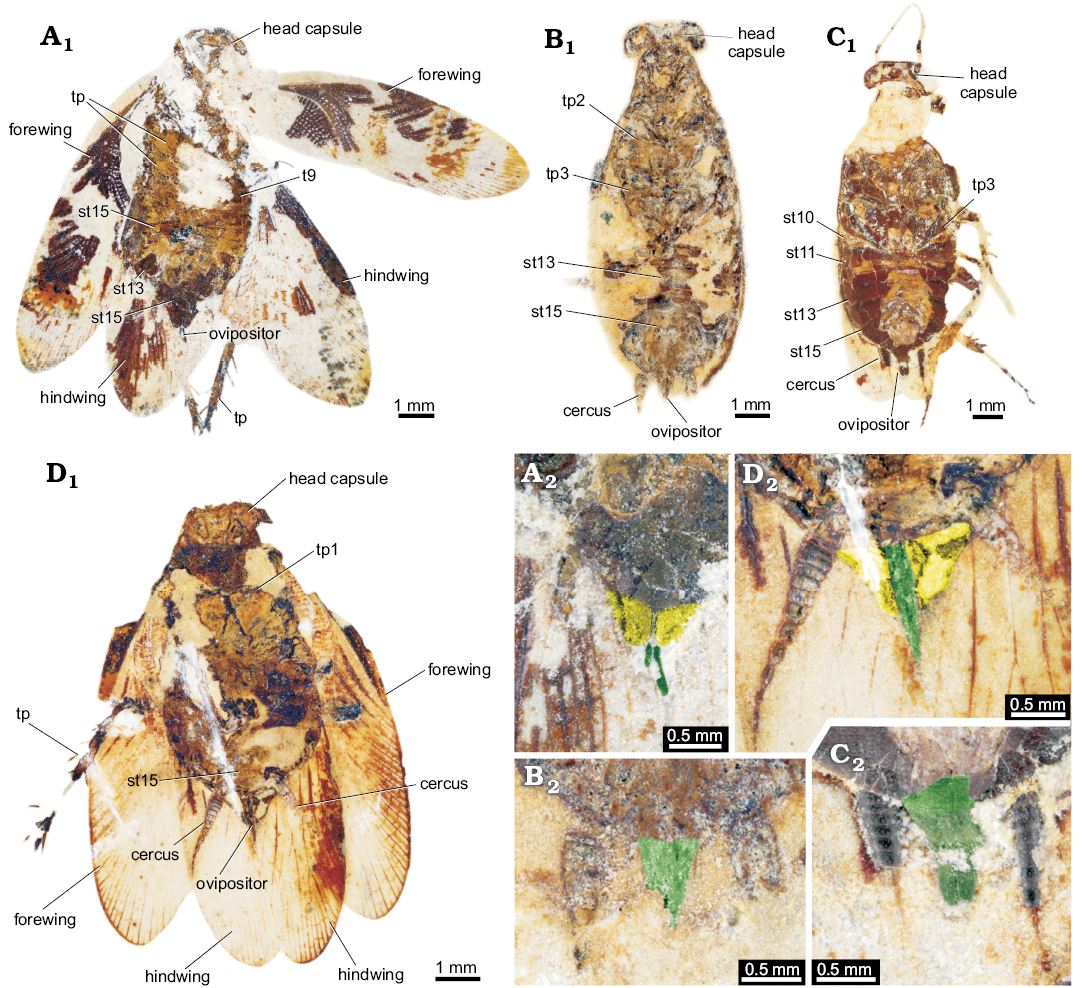
Fig. 13. Ponopterix maxima Bechly, 2007 (B, C) and undescribed roachoids (A, D) from the Aptian, Cretaceous Crato Formation of Brazil; in ventral views with interpretations of preserved structures. A. ROMIP64637. B. ROMIP64639. C. ROMIP64640. D. ROMIP64641. A1–D1, overviews; A2–D2, colour-marked close ups of the ovipositor (green, valves 1; yellow, part of post-ocular segment 15 [sternite 7; subgenital plate]). Abbreviations: st10–15, sternite of post-ocular segment 10–15 (abdominal segment 2–7); t9, tergite of post-ocular segment 9 (abdominal segment 1); tp1–3, thoracopod 1–3 (pro-, meso-, and metathoracic legs)
Discussion
Palaeozoic specimens
Identification.—There has been a debate about whether the Palaeozoic roachoids should be treated as blattodeans or “further down the tree” as non-blattodean dictyopterans (e.g., Sellards 1904; Hennig 1969; Bohn and Klass 2003; Grimaldi and Engel 2005). Apomorphies that characterise modern dictyopterans, such as a shortened ovipositor and presumably the production of oothecae appear to be clearly absent in Palaeozoic species. Thus, it is important to clearly differentiate between two different evolutionary levels:
(i) Dictyoptera sensu stricto (sensu Béthoux et al. 2009), with a short ovipositor and the ability to produce oothecae, including the two sister groups Blattodea (which itself also includes Isoptera as an ingroup) and Mantodea (Béthoux and Wieland 2009).
(ii) Dictyoptera sensu lato (sensu Béthoux et al. 2009), the larger group including Dictyoptera sensu stricto and the roachoids with (plesiomorphically) long ovipositors.
Several of the recognised apomorphies of Dictyoptera sensu lato are ambiguous or difficult to evaluate in fossils, as the preservational condition often only allows the identification of parts of these features. Characteristics for identifying a roachoid are flattened, splayed, and elongated coxae; a flattened body; tegminous forewings; in some instances, forewings with a distinctive and strongly curved CuP vein, or a claval furrow (Grimaldi and Engel 2005). An additional important feature characterising Dictyoptera sensu lato is the large pronotum (tergum of the first thoracic segment), which may partly conceal the head capsule. We consider this as a relevant character. Representatives of other groups with large pronota, such as Palaeodictyoptera, differ in their detailed morphology, as they possess a notch exposing the head. As all here treated specimens possess a large pronotum partly covering the head and, where preserved, rather long coxae, we consider all of them as ingroup representatives of Dictyoptera sensu lato. More exact phylogenetic placement of most specimens is hampered by different factors and will be discussed below.
Developmental status.—The specimens YPM IP 000057, YPM IP 027870, and YPM IP 027872 were already depicted by Sellards (1904; see above). A very remarkable feature on this specimens is the long ovipositor, which is especially well preserved in YPM IP 000057. Sellards (1904) assumed that these specimens represent roachoid nymphs and assigned them to specific species, for some of which he also described assumed adults. Reconstructing ontogenetic sequences of fossil arthropods, especially insects, is a challenging task (e.g., Haug et al. 2013) and usually depends on having a large sample available for reconstructing (more or less) continuous parts of ontogenetic sequences.
At the moment, we still have very few comparable Carboniferous specimens available. In most of these, only the large discoid pronotum allows the identification as Dictyoptera sensu lato because, apart from tergites and ovipositor, neither many details of wings, legs or further structures are preserved.
Hence, the ascription of fossil nymphs to adult-based species as done by Sellards (1903, 1904) is plausible, but not exclusive. Two interpretations can explain the morphological features of the herein described specimens:
(i) The Carboniferous specimens described here could represent paedomorphic adults not possessing fully functional wings. Such paedomorphic-appearing adults are known among extant cockroach species, such as Macropanesthia spp. or Cryptocercus spp. (Nalepa and Bandi 2000; Bell et al. 2007: 35–36). There are also rare cases of species with paedomorphic females and non-paedomorphic males in cockroaches, such as Nocticola termitophila, or at the population level e.g., in Paratemnopteryx howarthi and Alluaudellina cavernicola (Nalepa and Bandi 2000) (yet, it seems to be more often occurring in other extant insect groups, such as beetles, e.g., Lampyridae: McDermott 1964; Branham and Wenzel 2003; Jeng 2008; Duliticola (= Platerodrilus), Lycidae: Mjöberg 1925; Kazantsev 2002). However, in cases in which larger numbers of forewings of one species of Palaeozoic roachoids are known, a distinct dimorphism in the wing geometry is visible—a long slender type and a short wider type with identical venation patterns. This was interpreted by Laurentiaux (e.g., 1951, 1963) and Schneider (1978b) as an expression of sexual dimorphism, hence as fully developed wings of males and females, contradicting the lack of functional wings in one of the sexes (see also discussion below). However, we cannot fully exclude that already in the Carboniferous roachoid species occurred in which neither adult females nor adult males possessed wings.
(ii) If the specimens indeed represent nymphal stages of roachoids, their morphology is remarkable, as they possess a long external ovipositor. In this case, genital structures usually present at (and defining) the adult stage would be already developed in the nymph. This interpretation is also supported by modern counterparts, such as nymphs of Orthoptera already possessing a (long) external ovipositor (Ensifera: Sperber et al. 2003; Moon et al. 2009), as well as in nymphs of Grylloblattodea (Nagashima 1991). Given the extreme ovipositor length in definite adult roachoid specimens (e.g., Figs. 9, 14), the presence of the ovipositor at least in late nymphal stages is likely. In this case, these long valves would develop gradually, gaining length with each molt. Yet, we do not know the exact length of the valves of the nymphs as they all appear to be broken off distally. We can therefore only conclude that this second interpretation is rather plausible but cannot further validate it.
As externally visible ovipositors in nymphs are known from different groups of Polyneoptera (see above) and are possibly developed in early dictyopterans, this character might be considered a plesiomorphy. Alternatively, it is also possible that in this group the ovipositor development is pre-displaced (peramorphic heterochrony), resulting in a larger ovipositor in the adult, i.e., the large size of the ovipositor is achieved by an evolutionary change to an early onset of the development of the ovipositor.

Fig. 14. Roachoid Sysciophlebia balteata (Scudder, 1879) (NHMS-WP 5045) from the early Permian Goldlauter Formation, Thuringian Forest basin, Cabarz quarry, Germany. A. Overview (composite photograph of part and counterpart). B. Close-up of the ovipositor (red-coloured). Abbreviations: an, antenna; fe, femur of prothoracic leg; fw, forewing; hc, head capsule; hw, hind wing; mp, maxillary palpus; pn, pronotum; ti, tibia of prothoracic leg.
Ovipositor morphology.—The ovipositor of specimen YPM IP 000057 is preserved prominently and clearly recognisable, but also in specimens YPM IP 027870 and YPM IP 027872 structures are visible which can be interpreted as parts of an ovipositor. As the exact insertion areas are mostly difficult to assess, the identification of the valves remain ambiguous.
In specimen YPM IP 000057, the first pair of valves is visible thanks to the exposure of the ventral side in the fossil. In specimen YPM IP 027870 one pair of valves is almost exclusively visible in the stereo image, as the colour contrast to the matrix is rather low. In specimen YPM IP 027872, the situation is more complex. Sellards (1904) assumed the presence of more than a single pair of valves. Based on the specimens, this is difficult to evaluate. One pair can be verified. The presumed further lateral valves of Sellards (1903, 1904) are very incompletely preserved and in fact, may well represent a different structure. Sellards (1904) interpreted YPM IP 000057 and YPM IP 027872 as conspecific, representing different developmental stages. Although YPM IP 027872 is not too well preserved, it indeed resembles YPM IP 000057 in the outlines of the thoracic nota, hence, it is at least plausible that both specimens are conspecific. Yet, both specimens are of the same size and are therefore unlikely representing two successive instars. Hence, it can be considered as more likely that only a single pair of valves is represented in YPM IP 027872 and that the ovipositor morphology is similar to what we find in YPM IP 000057.
Carboniferous roachoids which are preserved with an ovipositor are rarely reported in the literature (e.g., Brongniart 1893; Laurentiaux 1951; Schneider 1978a, 1984; Wei et al. 2013). Meanwhile, numerous new finds are known (but are poorly described) which indicate that for nearly all Palaeozoic major groups of roachoids, an external long ovipositor was typical (Figs. 9, 14, 15).
It can be assumed that roachoids with long ovipositors did not have the ability to form oothecae. Compared to observations of modern polyneopterans, it is likely that these early dictyopterans laid single eggs into a substrate like many species of Orthoptera (about egg deposition of Orthoptera see e.g., Masaki 1986; Masaki and Walker 1987; Preston-Mafham 1990).
Direct evidences of the egg deposition mode cannot be provided yet, and fossil oothecae are very rare, with only a few definitive specimens in total so far (Grimaldi and Engel 2005; Anisyutkin et al. 2008; Poinar 2010; Hörnig et al. 2013). Beside these Cretaceous and Miocene specimens, five fossils from the Carboniferous are described in the literature as oothecae-like and are discussed to be remains of putative oothecae of Carboniferous roachoids by Sellards (1904: fig. 25), Schlechtendal (1912: pl. 4: 32, without description), Handlirsch (1906: 181, pl. 18: 47–49, the specimen of Sellards 1904 and Schlechtendal 1912), Pruvost (1919: text figs. 34, 35, pl. 22: 15, 16; 1930: 164, not figured). Yet, clear evidence cannot be provided that these fossils indeed represent oothecae or, even if that would be the case, that they were produced by dictyopterans.
Laurentiaux (1951, 1963) and Laurentiaux and Laurentiaux (1981) discussed possible links between sexual dimorphism of the geometry of the wings of Carboniferous representatives of Mylacridae, such as the presence of extremely long ovipositors in Anthracoblattina ensifera of Commentry, France, and the supposed presence of oothecae in the Carboniferous. Laurentiaux concluded a split into two evolutionary linages of Dictyopera in the early late Carboniferous (Laurentiaux 1959: fig. 3): the Eoblattodea, which possess long ovipositors, and the Neoblattodea, which are able to produce oothecae. He also argued that Eoblattodea, which includes Anthracoblattina (Phyloblattidae Schneider, 1983), became extinct in the Permian, and Mylacridae, as representatives of Neoblattodea, already became extinct in the late Carboniferous (Laurentiaux 1959). Representatives of Poroblattinidae of Laurentiaux’ (1959), i.e., “Série Poro-mesoblattinide”, occur up to the Triassic, while from his “Mesoblattinidea” arose the modern groups of Dictyoptera (Laurentiaux 1959).
Meanwhile, based on further fossil findings of representatives of all groups of Palaeozoic dictyopterans listed by Schneider (1983) except Archimylacridae und Mylacridae, an extremely long ovipositor can be observed in these groups (JS, personal observation). This includes also representatives of Poroblattinidae, which furthermore do not occur up to the Mesozoic, as assumed by Laurentiaux (1959) and Vršanský et al. (2002), but became extinct in the early Permian (Schneider 1983). New completely preserved fossil findings of roachoids from the Carboniferous and Permian (JS, personal observation) allow the conclusion that the males did not possess long and slender forewings, as discussed by Laurentiaux (1951, 1959, 1963), Laurentiaux and Laurentiaux (1981), and Schneider (1977) with comparison to modern groups. Instead, the described long and slender forewings belong to the females, which cover partially the long ovipositor with the wings; the broader forms of the fore wings belong to males (JS, personal observation). This relation of the length of the fore wings of males and females is first reversed in modern groups, in which females possess relatively broader forewings than visible in males.
Therefore, the dimorphism in the wing geometry of the Carboniferous to Jurassic roachoids was possibly misinterpreted in the context of the distinction between roachoid males and females with a reduced ovipositor. Hence, it cannot be seen as argument for a possible presence of the ability to produce oothecae of Palaeozoic representatives of Dictyoptera sensu lato.
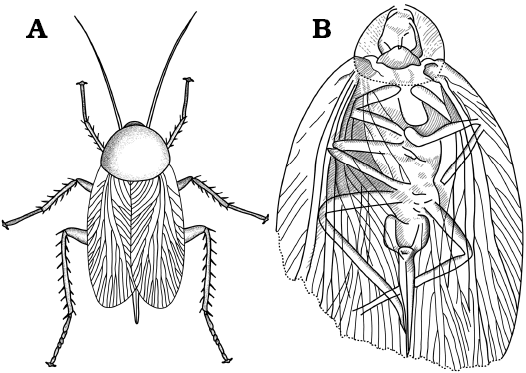
Fig. 15. Adult Carboniferous roachoids. A. Reconstruction of Etoblattina mazona Scudder, 1882, redrawn from Sellards (1904), primarily based on a specimen from Mazon Creek showing the pronotum, two forewings, and below them parts of the hindwings, but no ovipositor. Sellards (1904: figs. 10–14) assumed that the nymphs from Mazon Creek are conspecific with E. mazona. The here shown reconstruction based on his assumption is not provable. B. Drawing of Anthracoblattina ensifera Brongniart, 1894, redrawn from Laurentiaux (1951). Brongniart (1889) was the first who noted the presence of an external ovipositor in late Palaeozoic blattoids in his description of A. ensifera (hence the given specific epithet). Not to scale.
Cretaceous specimens
Ascription of the specimens to Dictyoptera/Blattodea.—Compared to the Carboniferous specimens, those from the Cretaceous Crato Formation appear much more similar to Recent species in terms of general morphology. The specimens with the elongate, but not far laterally extending pronotum can be determined to the species level as Ponopterix maxima Bechly, 2007. All other specimens are more problematic. They distantly resemble modern cockroaches, especially representatives of Blattellidae, but differ markedly in the morphology of the ovipositors. Therefore, we will treat these under open nomenclature as the aim of the present study was not taxonomic, but focused on the ovipositor morphology.
Ovipositor morphology.—Type 1: Among the specimens of the Cretaceous Crato Formation of Brazil, several principally different shapes of ovipositors are recognisable. Two types can be directly observed, a third type is indirectly inferred.
The ovipositor type 1 is represented by two specimens: ROMIP64635 (Figs. 10A1, 11A1) and ROMIP64634 (Figs. 10B1, 11B1). One pair of valves is apparent, which cannot be exactly identified. The valves are quite long and slender, but prominent, comparable to those of Carboniferous dictyopterans, although not as long. The two valves appear separated in both specimens, which is most likely caused by the incomplete preservation.
Until now, long and slender ovipositors have been recognised only in one case for Cretaceous roachoids; one representative of ?Mesoblattina from Cretaceous (Mongolia) is depicted by Vishniakova (1986: 167 and 203). The specimen was interpreted (see drawing in Vishniakova 1986: 167) to bear a long prominent ovipositor. The next oldest described roachoid specimens with long ovipositors are represented by the Jurassic species Karataublatta longicaudata, Rhipidoblattina maculata, R. brevivalvata (Vishniakova 1968; Liang et al. 2017), and Solemnia alexandri (Vršanský 2008). While not as long as in these older forms, ovipositor type 1 in the Crato specimens is significantly longer than that of type 2 (see below). This morphology indicates that these forms, despite their modern appearance, retained the plesiomorphic strategy of laying single eggs into a substrate.
Type 2: Specimens that have a protruding, but rather short and broad ovipositor (here, called type 2) co-occur with specimens featuring the long ovipositor type 1. Most likely, the second pair of valves is rather elongate, and the first and third pairs of valves are rather broad. In some specimens, the ovipositor valves are partly protruded by an elongated, but rather broad structure on the most distal part of the abdomen. In comparison with the ovipositor morphology of modern representatives of Mantodea, it is plausible that these structures represent an elongated part of post-ocular segment 15 (sternite 7).
This type of ovipositor is found in specimens of Ponopterix maxima Bechly, 2007 (Figs. 12B1, C1, 13B1, C1), but also in specimens which appear like modern forms in general morphology (ROMIP64636, Figs. 10C1 and 11C1; ROMIP64638, Figs. 10D1 and 11D1, ROMIP64637, Figs. 12A1 and 13A1; ROMIP64641, Figs. 12D1 and 13D1). While in the here described specimens of P. maxima, the first pair of valves is more difficult to spot, a specimen depicted in Bechly (2007) has a well preserved first and second pair of valves. Similar ovipositors have been described for most Cretaceous dictyopteran species (Rasnitsyn and Quicke 2002), including specimens of another species of the Crato Formation, Ponopterix axelrodi Vršanský and Grimaldi, 1999 (Vršanský 2003).
Bechly (2007) already compared this type of ovipositor to that of Mantodea. From a functional point of view, it is unlikely that these roach-like dictyopterans with ovipositor type 2 could produce oothecae like modern blattodeans. Yet, it could well be possible that a protruding ovipositor facilitated the production of an ootheca, presumably in a mode that is comparable to that of Mantodea. Thus, these early forms might well have produced oothecae attached to a substrate.
Type 3: A third type of ovipositor is indicated by specimens with preserved oothecae attached to the abdomen (Fig. 16; Grimaldi and Engel 2005; Hörnig et al. 2013). It must be assumed that these forms had a rather short ovipositor concealed by the elongated post-ocular segment 15 (abdominal sternite 7). Only this structure would allow roaches to “grab” the ootheca and carry it with them. Yet, it must be noted that these specimens strongly resemble most of the specimens with ovipositors of morphotype 1 and 2 in general body morphology.
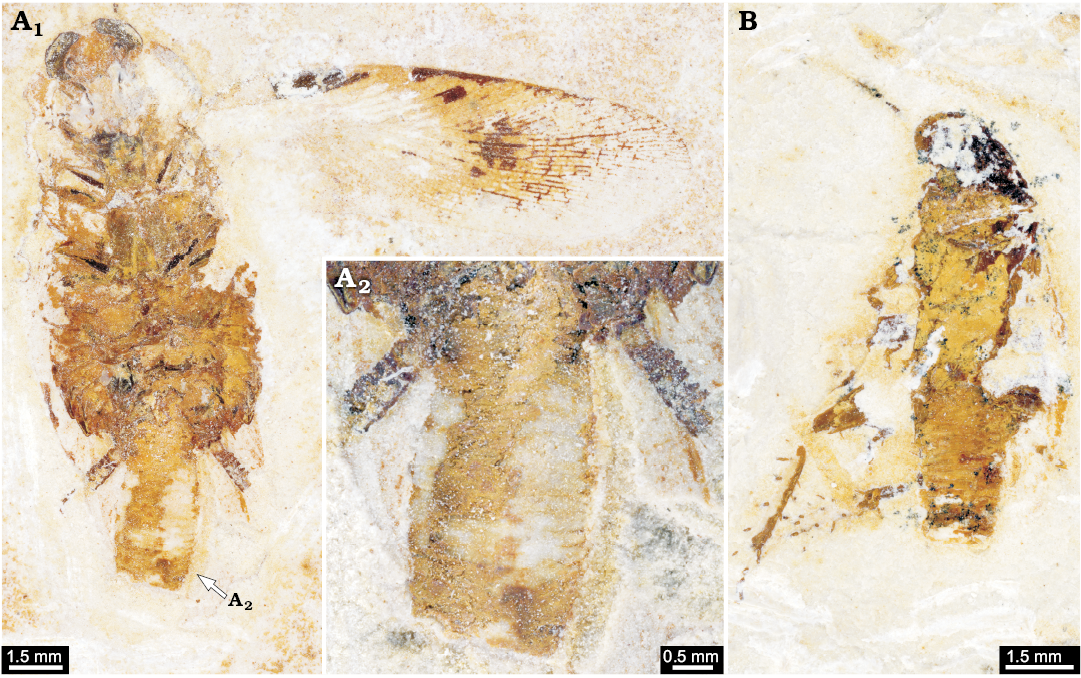
Fig. 16. Cockroaches with oothecae from the Aptian, Cretaceous Crato Formation of Brazil. A. AI 3208, “Mesoblattina” cf. limai Pinto and Purper, 1986. Overview (A1), close-up of ootheca (A2). B. Undescribed cockroach AI 292 (from Hörnig et al. 2013: fig. 4).
Evolutionary reconstruction
Although the present data cause some challenges concerning taxonomy and identity of the here described specimens, we can infer an evolutionary reconstruction of the transformation of the ovipositor. Coupled to this, it is also possible to infer the evolutionary changes of the reproductive strategies within Dictyoptera sensu lato.
Dictyoptera sensu lato.—The ancestral condition for Dictyoptera sensu lato is a long and slender ovipositor, which most likely already started to develop in the late nymphs. Whether this condition is (aut-)apomorphic for Dictyoptera sensu lato or a plesiomorphy is currently unclear. These early forms did not yet produce oothecae but laid single eggs, presumably into substrate. The long ovipositors must have caused evolutionary costs, as it constraints the female’s mobility. These costs were counterbalanced by the advantage of the protection of the eggs. The extremely long ovipositors at least persisted from the Carboniferous into the Jurassic (Vishniakova 1968, 1982; Schneider 1984); in the Cretaceous, still moderately long ovipositors were present, and the strategy to lay single eggs co-existed with further derived ones.
Dictyoptera sensu stricto.—Autapomorphic for Dictyoptera sensu stricto is a shorter ovipositor with a still rather elongate and slender second pair of valves and a broader first and third pair of valves; the second pair of valves could be protruded from the first and third pair of valves, but were at equal length in resting position.
This principal type of ovipositor is still found in modern mantodeans (Figs. 1D–F, 2B). The stem species of Dictyoptera sensu stricto might have produced oothecae attached to the substrate, e.g., branches or leaves. While the case material is still viscid, single eggs are deposited in a specific arrangement (as indicated in modern mantodeans; Preston-Mafham 1990; Grimaldi and Engel 2005; Leong 2009; Wieland 2013). If representatives of Mantodea were indeed already present in the Jurassic, this strategy, which persists until today in Mantodea and was further derived in the blattodean linage, must have been already developed before.
Blattodea.—A further shortened ovipositor concealed by the elongate post-ocular segment 15 (abdominal sternite 7; subgenital plate; Figs. 1A–C, 2A) is considered autapomorphic for Blattodea (Fig. 17). This facilitates the production of free (non-adhesive) oothecae and active maternal transport (at least for some time). This strategy was at least present since the Cretaceous and persists until today (and was further derived in representatives of ovoviviparic and viviparic blattodean species; see e.g., Bell et al. 2007).
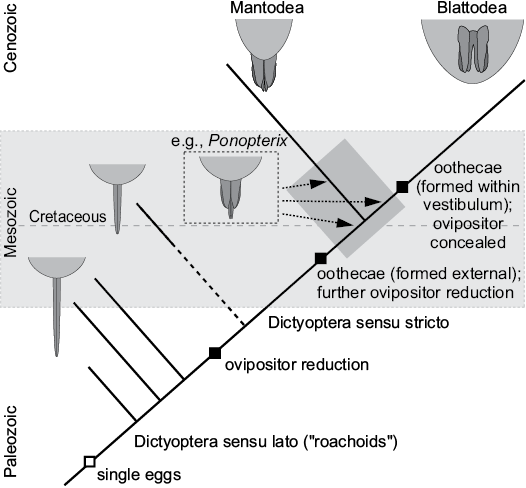
Fig. 17. Proposed evolution of ovipositor morphology in Dictyoptera.
Evolution of the subgenital plate.—An important structure in the genital apparatus of female modern representatives of Dictyoptera sensu stricto is the specialised sternite of post-ocular segment 15 (abdominal sternite 7), the so-called sub-genital plate. In all modern female representatives, it is elongated in anterior-posterior axis (compared to other sternites). It is furthermore drawn out posteriorly into two distinct lobes, which partly (Mantodea) or fully (Blattodea) cover the genitalia, forming the ventral border, the so-called vestibulum in the latter.
It has been suggested that this specialisation of the sternite of post-ocular segment 15 characterises a larger group within Polyneoptera including not only Dictyoptera but also Dermaptera (Ax 2000). This evolutionary interpretation clearly ignores fossil dictyopterans. Neither already known specimens from the Carboniferous, nor specimens re-investigated here show a specialisation of the sternite of post-ocular segment 15. From a functional point of view, this seems barely surprising. With an elongate ovipositor, a drawn out sternite of post-ocular segment 15 would not provide any support. Only in combination with a shortened ovipositor, such a specialisation of the sternite has advantages.
Therefore, it would be interesting if we could demonstrate that shortening of the ovipositor and specialisation of the sternite of post-ocular segment 15 was indeed also evolutionarily coupled, or which character evolved first. Unfortunately, our fossils are only of limited value for this discussion. Two specimens with a type 2 ovipositor (ROMIP64637, Figs. 12A, 13A; ROMIP64641, Figs. 12D, 13D) clearly possess already a specialised sternite of post-ocular segment 15, being drawn out into two distinct lobes. Other specimens are less informative in this region. At least, it seems that all specimens that have a longer ovipositor do not have a specialised sternite of post-ocular segment 15. However, due to the difficulties of preservation and interpretation in this region, this cannot be stated with certainty. With this type of data, we can only conclude that a specialised sternite of post-ocular segment 15 (sub-genital plate) evolved within Dictyoptera sensu lato.
Conclusions
The phylogenetic consequences from the data presented here are limited as we did not deal with other characters besides the ovipositor. Yet, we can point out possible character conflicts which will need to be addressed in future studies:
(i) The ovipositor morphology of type 1 makes it unlikely that species with such an ovipositor are an ingroup of Dictyoptera sensu stricto.
(ii) Specimens with ovipositor morphology of type 2 are (likely) representatives of ingroups of Dictyoptera sensu stricto. Yet, their exact position could be either before the branching off of Mantodea, i.e., below the “crown-group”; hence, Dictyoptera sensu stricto is not necessarily identical to the “crown-group”, on the lineage towards Mantodea, or on the lineage towards Blattodea (see also Fig. 17). This principal discussion has been put forward (at least in part) for Ponopterix, but also applies for other forms with ovipositor type 2 (see Vršanský 2003; Grimaldi and Engel 2005; Bechly 2007). We consider it as unlikely that these forms are ingroups of Blattodea as it would demand for a parallel evolution of the blattodean ootheca (Vršanský 2003; Bechly 2007).
(iii) Specimens with ovipositor type 3 are likely an ingroup of Blattodea.
(iv) A fossil ootheca from the Cretaceous of Israel (Anisyutkin et al. 2008) is not necessarily that of a mantodean. It might well be the product of a non-mantodean dictyopteran with an ovipositor of type 2.
(v) The Cretaceous is a time in which all three reproductive strategies (single eggs, substrate-attached ootheca, “mobile” ootheca) existed at the same time slice (and in the same fauna), all in otherwise very similar-appearing cockroach-like morphotypes.
Acknowledgements
This study would not have been possible without the help and support of numerous people and institutions. We want to heartily thank all these people. Jean-Bernard Caron, Peter Fenton, and Janet Waddington (all Royal Ontario Museum, Toronto, Canada), Paul Mayer (Field Museum, Chicago, USA), and Susan Butts and Jessica Utrup (both Yale Peabody Museum, New Haven, USA) provided access to the fossil material and technical equipment and helped with work in the collections. Ralf Werneburg (Naturhistorisches Museum Schloß Bertholdsburg, Schleusingen, Germany), and Stephan Brauner (Nationaler Geopark Thüringen Inselsberg-Drei Gleichen, Friedrichroda, Germany), delivered the Permian specimen from Cabarz insect site and made the excellent composite photograph. Rainer Jahnke (Greifswald, Germany) and Marc Bierling (Munich, Germany) are thanked for providing the extant specimens. Jakob Krieger, Andy Sombke, Christian W. Hädicke (all University of Greifswald, Germany), helped with literature search and discussions. Günter Schweigert (Staatliches Museum für Naturkunde, Stuttgart, Germany) is thanked for the allowance to reuse images published previously in Palaeodiversity. MKH, CH, and JTH would like to thank Steffen Harzsch (University of Greifswald, Germany), J. Matthias Starck (LMU, Munich, Germany), and Derek E.G. Briggs (Yale University, New Haven, USA) for their support. MKH was funded by a fellowship of the Studienstiftung des deutschen Volkes. CH was kindly funded by an Equal Opportunities Sponsorship of the LMU Munich. JWS acknowledges the support of the German Research Foundation (DFG), grant DFG Schn 408/21. JTH was supported by the Alexander von Humboldt-Foundation with a Feodor Lynen fellowship and a Feodor Lynen return fellowship and by Yale University. We additionally thank all people spending their free time for programming open source, open access or low cost software, such as Open Office and Blender. We thank Olivier Béthoux (Muséum National d’Histoire Naturelle, Paris, France) and one anonymous reviewer for their helpful comments on a previous version of this manuscript.
References
Anisyutkin, L.D., Grachev, V.G., Ponomarenko, A.G., Rasnitsyn, A.P., and Vršansky, P. 2008. Fossil insects in the Cretaceous mangrove facies of southern Negev, Israel. In: V. Krassilov and A.P. Rasnitsyn (eds.), Plant-arthropod Interactions in the Early Angiosperm History: Evidence From the Cretaceous of Israel, 190–223. Pensoft, Sofia.
Arillo, A. 2007. Paleoethology: fossilized behaviours in amber. Geologica Acta 5 (2): 159.
Ax, P. 2000. Multicellular Animals. The Phylogenetic System of the Metazoa. Volume II. 396 pp. Springer Verlag, Berlin.
Beccaloni, G. and Eggleton, P. 2013. Order Blattodea. In: Z.-Q. Zhang (ed.), Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa 3703: 46–48.
Bechly, G. 2007. “Blattaria”: cockroaches and roachoids. In: D.M. Martill, G. Bechly, and R.F. Loveridge (eds.), The Crato Fossil Beds of Brazil: Window into an Ancient World, 239–249. Cambridge University Press Cambridge.
Bell, W.J., Roth, L.M., and Nalepa, C.A. 2007. Cockroaches: Ecology, Behavior, and Natural History. 248 pp. Johns Hopkins University Press, Baltimore.
Béthoux, O. and Wieland, F. 2009. Evidence for Carboniferous origin of the order Mantodea (Insecta: Dictyoptera) gained from forewing morphology. Zoological Journal of the Linnean Society 156: 79–113. Crossref
Béthoux, O., Beckemeyer, R.J., Engel, M.S., and Hall, J.D. 2010. New data on Homocladus grandis, a Permian stem-mantodean (Polyneoptera: Dictyoptera). Journal of Paleontology 84: 746–753. Crossref
Béthoux, O., Klass, K.D., and Schneider, J.W. 2009. Tackling the “Protoblattoidea problem”: Revision of Protoblattinopsis stubblefieldi (Dictyoptera; Late Carboniferous). European Journal of Entomology 106: 145–152. Crossref
Bohn, H. 2003. Ordnung Blattoptera, Schaben. In: H.H. Dathe (ed.), Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil: Insecta, 197–223. Spektrum, Heidelberg.
Bohn, H. and Klass, K.D. 2003. Dictyoptera, Ordnungen 13–15. In: H.H. Dathe (ed.), Lehrbuch der Speziellen Zoologie, Band I: Wirbellose Tiere, 5. Teil: Insecta, 181–182. Spektrum, Heidelberg.
Branham, M.A. and Wenzel, J.W. 2003. The origin of photic behavior and the evolution of sexual communication in fireflies (Coleoptera: Lampyridae). Cladistics 19: 1–22.Crossref
Brongniart, C. 1889. Les blattes de l’époque houillère. Comptes-Rendus de l’Académie des Sciences 108: 252.
Brongniart, C. 1893. Recherches pour servir à l’histoire des insectes fossiles des temps primaires; précédées d’une etude sur la nervation des ailes des insectes. Thèses 494 pp., Atlas 44 pp., 37 pls. Imprimerie Théolier, Saint-Etienne.
Courrent, A., Quennedey, A., Nalepa, C.A., Robert, A., Lenz, M., and Bordereau, C. 2008. The fine structure of colleterial glands in two cockroaches and three termites, including a detailed study of Cryptocercus punctulatus (Blattaria, Cryptocercidae) and Mastotermes darwiniensis (Isoptera, Mastotermitidae). Arthropod Structure & Development 37: 55–66. Crossref
Deitz, L.L., Nalepa, C., and Klass, K.D. 2003. Phylogeny of the Dictyoptera re-examined (Insecta). Entomologische Abhandlungen 61: 69–91.
Djernæs, M., Klass, K.D., and Eggleton, P. 2015. Identifying possible sister groups of Cryptocercidae + Isoptera: A combined molecular and morphological phylogeny of Dictyoptera. Molecular Phylogenetics and Evolution 84: 284–303.Crossref
Djernæs, M., Klass, K.D., Picker, M.D., and Damgaard, J. 2012. Phylogeny of cockroaches (Insecta, Dictyoptera, Blattodea), with placement of aberrant taxa and exploration of out-group sampling. Systematic Entomology 37: 65–83.Crossref
Goldberg, J., Bresseel, J., Constant, J., Kneubühler, B., Leubner, F., Michalik, P., and Bradler, S. 2015. Extreme convergence in egg-laying strategy across insect orders. Scientific Reports 5: 7825. Crossref
Grimaldi, D.A. 1997. A fossil mantis (Insecta, Mantodea) in Cretaceous amber of New Jersey: with comments on the early history of the Dictyoptera. American Museum Novitates 3204: 1–11.
Grimaldi, D.A. 2003. A revision of Cretaceous mantises and their relationships, including new taxa (Insecta: Dictyoptera: Mantodea). American Museum Novitates 3412: 1–47. Crossref
Grimaldi, D.A. and Engel, M. 2005. Evolution of the Insects. 772 pp. Cambridge University Press, New York.
Hädicke, C.W., Hörnig, M.K., Haug, C., and Haug, J.T. 2014. New data on fossil Archaeognatha from Baltic amber and the origin of the insect ovipositor. Palaeodiversity 7: 167–183.
Handlirsch, A. 1906–1908. Die fossilen Insekten und die Phylogenie der rezenten Formen: Ein Handbuch für Paläontologen und Zoologen. 1430 pp. Verlag von Wilhelm Engelmann, Leipzig.
Haug, C., Mayer, G., Kutschera, V., Waloszek, D., Maas, A., and Haug, J.T. 2011a. Imaging and documenting gammarideans. International Journal of Zoology 2011: 380829. Crossref
Haug, J.T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., and Waloszek, D. 2011b. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy 244: 259–272. Crossref
Haug, J.T., Leipner, A., Wappler, T., and Haug, C. 2013. Palaeozoic insect nymphs: new finds from the Piesberg quarry (Upper Carboniferous, Germany). Bulletin of Geociences 88: 779–791. Crossref
Haug, J.T., Mayer, G., Haug, C., and Briggs, D.E.G. 2012. A Carboniferous non-onychophoran lobopodian reveals long-term survival of a Cambrian morphotype. Current Biology 22: 1673–1675. Crossref
Hennig, W. 1969. Die Stammesgeschichte der Insekten. 436 pp. Waldemar Kramer, Frankfurt am Main.
Hörnig, M.K., Haug, J.T., and Haug, C. 2013. New details of Santanmantis axelrodi and the evolution of the mantodean morphotype. Palaeodiversity 6: 157–168.
Hörnig, M.K., Haug, C., Herd, K.J., and Haug, J.T. 2014. New insights into dictyopteran early development: smallest Palaeozoic roachoid nymph found so far. Palaeodiversity 7: 159–165.
Hörnig, M.K., Sombke, A., Haug, C., Harzsch, S., and Haug, J.T. 2016. What nymphal morphology can tell us about parental investment—a group of cockroach hatchlings in Baltic amber documented by a multi-method approach. Palaeontologia Electronica 19.1.6A: 1–20.
Inward, D., Beccaloni, G., and Eggleton, P. 2007. Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biology Letters 3: 331–335. Crossref
Jeng, M.L. 2008. Comprehensive Phylogenetics, Systematics, and Evolution of Neoteny of Lampyridae (Insecta: Coleoptera). 395 pp. PhD Dissertation, University of Kansas, Lawrence.
Kazantsev, S.V. 2002. A generic review of Duliticolinae, new subfamily (Coleoptera: Lycidae). Elytron 16: 5–21.
Kerp, H. and Bomfleur, B. 2011. Photography of plant fossils—new techniques, old tricks. Review of Palaeobotany and Palynology 166: 117–151. Crossref
Klass, K.D. 1998. The ovipositor of Dictyoptera (Insecta): homology and ground-plan of the main elements. Zoologischer Anzeiger 236: 69–101.
Klass, K.D. 2007. Die Stammesgeschichte der Hexapoden: eine kritische Diskussion neuerer Daten und Hypothesen. Denisia 20: 413–450.
Klass, K.D. and Meier, R. 2006. A phylogenetic analysis of Dictyoptera (Insecta) based on morphological characters. Entomologische Abhandlungen 63: 3–50.
Klass, K.D., Matushkina, N.A., and Kaidel, J. 2012. The gonangulum: A reassessment of its morphology, homology, and phylogenetic significance. Arthropod Structure & Development 41: 373–394. Crossref
Klass, K.D., Nalepa, C., and Lo, N. 2008. Wood-feeding cockroaches as models for termite evolution (Insecta: Dictyoptera): Cryptocercus vs. Parasphaeria boleiriana. Molecular Phylogenetics and Evolution 46: 809–817. Crossref
Laurentiaux, D. 1951. Le problème des blattes paléozoïques à ovipositeur externe. Annales de Paléontologie 37: 185–196.
Laurentiaux, D. 1959. La reproduction chez les insectes blattaires du Carbonifère: facteurs du panchronisme et classification naturell de l’ordre. Bulletin de la Société géologique de France 7 (1): 759–766.
Laurentiaux, D. 1963. Antiquité du dimorphisme sexuel des Blattes. Comptes Rendus de l’Académie des Sciences 257: 3971–3974.
Laurentiaux-Vieira, F. and Laurentiaux, D. 1981. Mise en évidence d’un dimorphisme sexuel chez les Blattes Dictyomylacris du Stéphanian de Commentry (Allier). Annales Société Géologique du Nord 100: 175–182.
Legendre, F., Nel, A., Svenson, G.J., Robillard, T., Pellens, R., and Grandcolas, P. 2015. Phylogeny of Dictyoptera: dating the origin of cockroaches, praying mantises and termites with molecular data and controlled fossil evidence. PLOS ONE 10 (7): e0130127. Crossref
Leong, T.M. 2009. Oviposition and hatching in the praying mantis, Hierodula patellifera (Servile) in Singapore (Mantodea: Mantidae: Paramantinae). Nature in Singapore 2: 55–61.
Levereault, P. 1936. The morphology of the carolina mantis. The University of Kansas Science Bulletin 24: 205–259.
Liang, J., Shih, C., and Ren, D. 2017. New Jurassic predatory cockroaches (Blattaria: Raphidiomimidae) from Daohugou, China and Karatau, Kazakhstan. Alcheringa 42: 101–109. Crossref
Lo, N., Bandi, C., Watanabe, H., Nalepa, C., and Beninati, T. 2003. Evidence for cocladogenesis between diverse dictyopteran lineages and their intracellular endosymbionts. Molecular Biology and Evolution 20: 907–913. Crossref
Lo, N., Beninati, T., Stone, F., Walker, J., and Sacchi, L. 2007. Cockroaches that lack Blattabacterium endosymbionts: the phylogenetically divergent genus Nocticola. Biology Letters 3: 327–330. Crossref
Marks, E.P. and Lawson, F.A. 1962. A comparative study of the dictyopteran ovipositor. Journal of Morphology 111: 139–171. Crossref
Martill, DM. 2007. The age of the Cretaceous Santana Formation fossil Konservat Lagerstätte of north-east Brazil: a historical review and an appraisal of the biochronostratigraphic utility of its palaeobiota. Cretaceous Research 28 (6): 895920. Crossref
Masaki, S. 1986. Significance of ovipositor length in life cycle adaptations of crickets. In: F. Taylor and R. Karban (eds.), The Evolution of Insect Life Cycles, 20–34. Springer, New York. Crossref
Masaki, S. and Walker, T.J. 1987. Cricket life cycles. Evolutionary Biology 21: 349–423. Crossref
McDermott, F.A. 1964. The taxonomy of the Lampyridae (Coleoptera). Transactions of the American Entomological Society 90: 1–72.
McKittrick, F.A. 1964. Evolutionary study of cockroaches. Cornell University Agricultural Experiment Station, New York State College of Agriculture, Ithaca, NY, Memoir 389: 1–197.
Mjöberg, E. 1925. The Mystery of the so-called “Trilobite Larvae” definitely solved. Psyche 32: 119–154. Crossref
Moon, S.R., Noh, D.J., Yang, J.O., Yoon, C.M., Ahn, K.S., and Kim, G.H. 2009. Seasonal occurrence and developmental characteristics of ussur brown katydid, Paratlanticus ussuriensis Uvarov (Orthoptera: Tettigoniidae). Korean Journal of Applied Entomology 48: 11–19. Crossref
Nagashima, T. 1991. Postembryonic development and homology of external genitalia in Galloisiana nipponensis (Caudell et King) (Notoptera: Grylloblattidae). International Journal of Insect Morphology and Embryology 20: 157–168. Crossref
Nalepa, C.A. and Bandi, C. 2000. Characterizing the ancestors: paedomorphosis and termite evolution. In: T. Abe, D. Bignell, and M. Higashi (eds.), Termites: Evolution, Sociality, Symbioses, Ecology, 53–75. Springer, Dordrecht.
Nalepa, C.A. and Lenz, M., 2000. The ootheca of Mastotermes darwiniensis Frogatt (Isoptera: Mastotermitidae): homology with cockroaches oothecae. Proceedings of the Royal Society of London. Series B: Biological Sciences 267: 1809–1813. Crossref
Poinar, G. Jr. 2010. Palaeoecological perspectives in Dominican amber. Annales de la Société entomologique de France 46: 23–52. Crossref
Preston-Mafham, K. 1990. Grasshoppers and Mantids of the World. 192 pp. Blanford, London.
Pruvost, P. 1919. Introduction l’étude du terrain houiller du Nord et du Pas-de-Calais: La faune continentale du terrain du Nord de la France. 584 pp. Imprimerie Nationale, Paris.
Pruvost, P. 1930. La faune continentale du terrain huiller de Belgique. Mémoires du Musée royal d’histoire naturelle de Belgique 44: 142–166.
Rasnitsyn, A.P. and Quicke, D.L.J. 2002. History of Insects. 517 pp. Kluwer, Dordrecht. Crossref
Richter, S., Loesel, R., Purschke, G., Schmidt-Rhaesa, A., Scholtz, G., Stach, T., Vogt, L., Wanninger, A., Brenneis, G., Döring, C., Faller, S., Fritsch, M., Grobe, P., Heuer, C.M., Kaul, S., Møller, O.S., Müller, C.H.G., Rieger, V., Rothe, B.H., Stegner, M.E.J., and Harzsch, S. 2010. Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Frontiers in Zoology 7: 29. Crossref
Schlechtendal, D. von 1912. Untersuchungen über die carbonischen Insekten und Spinnen von Wettin unter Berücksichtigung verwandter Faunen. 1. Revision der Originale von Germar, Giebel und Goldenberg. Nova Acta Leopoldina Carolina 98: 1–186.
Schneider, J. 1977. Zur Variabilität der Flügel paläozoischer Blattodea (Insecta), Teil 1. Freiberger Forschungshefte C 326: 87–105.
Schneider, J. 1978a. Zur Taxonomie und Biostratigraphie der Blattodea (Insecta) aus dem Oberkarbon und Perm der DDR. Freiberger Forschungshefte C 340: 152.
Schneider, J. 1978b. Zur Variabilität der Flügel paläozoischer Blattodea (Insecta), Teil 2. Freiberger Forschungshefte C 334: 21–39.
Schneider, J. 1983. Die Blattodea (Insecta) des Paläozoikums, Teil 1: Systematik, Ökologie und Biostratigraphie. Freiberger Forschungshefte C 382: 106–145.
Schneider, J. 1984. Die Blattodea (Insecta) des Paläozoikums, Teil 2: Morphogenese der Flügelstrukturen und Phylogenie. Freiberger Forschungshefte C 391: 5–32.
Schneider, J.W. and Scholze, F. 2016. Late Pennsylvanian to Early Triassic conchostracan biostratigraphy—a preliminary approach. In: S.G. Lucas and S.Z. Shen (eds.), The Permian Timescale. Geological Society, London, Special Publications 450: 1–22.
Schneider, J.W. and Werneburg, R. 2012. Biostratigraphie des Rotliegend mit Insekten und Amphibien. In: Deutsche Stratigraphische Kommission (ed.), Stratigraphie von Deutschland X. Rotliegend. Teil I: Innervariscische Becken. Schriftenreihe Deutsche Gesellschaft für Geowissenschaften 61: 110–142.
Schneider, J.W., Lucas, S.G., and Barrick, J.E. 2013. The Early Permian age of the Dunkard Group, Appalachian basin, U.S.A., based on spiloblattinid insect biostratigraphy. International Journal of Coal Geology 119: 88–92. Crossref
Scudder, G.G.E. 1971. Comparative morphology of insect genitalia. Annual Review of Entomology 16: 379–406. Crossref
Scudder, S.H. 1895. Revision of the American fossil cockroaches, with descriptions of new forms. Bulletin of the United States Geological Survey 124: 1–176.
Sellards, E.H. 1903. Some new structural characters of Paleozoic cockroaches. American Journal of Science 88: 307–315. Crossref
Sellards, E.H. 1904. A study of the structure of Paleozoic cockroaches, with descriptions of new forms from the Coal Measures. American Journal of Science 18: 113–134, 213–227. Crossref
Sharov, A.G. 1966. Basic Arthropodan Stock with Special Reference to Insects. 271 pp. Pergamon Press, Oxford.
Simonetta, A.M. and Delle Cave, L. 1981. An essay in the comparative and evolutionary morphology of Palaeozoic arthropods. Accademia Nazionale dei Lincei 49: 389.
Sperber, C.F., Rocha, A.,
Lopes-Andrade, C., and Mesa, A. 2003. Izecksohniella
puri sp. n., a new Brazilian cricket species (Orthoptera:
Grylloidea: Phalangopsidae) from Atlantic Forest remnants. Zootaxa 244: 1–12. Crossref
Svenson, G.J. and Whiting, M.F. 2009. Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): the roles of Gondwanan vicariance and morphological convergence. Cladistics 25: 468–514. Crossref
Vishniakova, V.N. 1968. Mesozoic cockroaches with an external ovipositor and pecularities of their reproduction (Blattodea). In: B.B. Rohdendorf (ed.), Jurassic Insects of Karatau, 55–86. Nauka, Moscow.
Vishniakova, V.N. [Višniakova, V.N.] 1982. Jurassic cockroaches of the new family Blattulidae from Siberia [in Russian]. Paleontologičeskij žurnal 1982 (2): 69–79.
Vishniakova V.N. [Višniakova, V.N.] 1986. Blattida (= Blattodea) [in Russian]. In: A.P. Rasnitsyn (ed.), Nasekomye v rannemelovyh ekosistemah Zapadnoj Mongolii. Transactions of Joint Soviet-Mongolian Palaeontological Expedition 28: 166–169.
Vršanský, P. 2003. Umenocoleoidea—an amazing lineage of aberrant insects (Insecta, Blattaria). Amba Projekty 7: 1–32.
Vršanský, P. 2008. Late Jurassic Cockroaches (Insecta, Blattaria) from the Houtiyn-Hotgor locality in Mongolia. Paleontological Journal 42: 36–42.
Vršansky, P., Vishniakova, V.N., and Rasnitsyn, A.P. 2002. Order Blattida Latreille, 1810. In: A.P. Rasnitsyn and D.L.J. Quicke (eds.), History of Insects, 263–270. Kluwer Academic Publishers, Dodrecht.
Wei, D.D., Béthoux, O., Guo, Y.X., Schneider, J.W., and Ren, D. 2013. New data on the singularly rare “cockroachoids” from Xiaheyan (Pennsylvanian; Ningxia, China). Alcheringa 37: 547–557. Crossref
Wieland, F. 2013. The phylogenetic system of Mantodea (Insecta: Dictyoptera). Species, Phylogeny and Evolution 3: 3–222. Crossref
Zhang, A., Schneider, J.W., and Hong, Y. 2013. The most ancient roach (Blattodea): a new genus and species from the earliest Late Carboniferous (Namurian) of China, with a discussion of the phylomorphogeny of early blattids. Journal of Systematic Palaeontology 11: 27–40. Crossref
Zhang, Z.-Q. 2013. Phylum Arthropoda. In: Z.-Q. Zhang (ed.), Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa 3703: 17–26.
Acta Palaeontol. Pol. 63 (1): 1–24, 2018
https://doi.org/10.4202/app.00324.2016