


Large palaeophiid and nigerophiid snakes from Paleogene Trans-Saharan Seaway deposits of Mali
JACOB A. MCCARTNEY, ERIC M. ROBERTS, LEIF TAPANILA, and MAUREEN A. O’LEARY
McCartney, J.A., Roberts, E.M., Tapanila, L., and O’Leary, M.A. 2018. Large palaeophiid and nigerophiid snakes from Paleogene Trans-Saharan Seaway deposits of Mali. Acta Palaeontologica Polonica 63 (2): 207–220.
The Paleogene was a time of high diversity for snakes, and was characterized by some of the largest species known to have existed. Among these snakes were pan-Tethyan marine species of Nigerophiidae and Palaeophiidae. The latter family included the largest sea snake, Palaeophis colossaeus, known from the Trans-Saharan Seaway of Mali during the Eocene. This paper describes new material collected from Malian Trans-Saharan Seaway deposits, including additional material of Palaeophis colossaeus, a new, large species of nigerophiid, Amananulam sanogoi gen. et sp. nov., and a medium-sized snake of indeterminate affinities. The material provides new information on the intracolumnar variation of the vertebral column in Palaeophis colossaeus. We estimate the total length of each species by regression of vertebral measurements on body size. Both Palaeophis colossaeus and Amananulam sanogoi gen. et sp. nov. are the largest or among the largest members of their respective clades. The large size of Tethyan snakes may be indicative of higher temperatures in the tropics than are present today.
Key words: Serpentes, body size, Paleogene, Teberemt Formation, Tamaguelelt Formation, Tilemsi Valley, Gao Trench, Mali.
Jacob A. McCartney [mccartneyj@geneseo.edu], Department of Biology, State University of New York College at Geneseo, Geneseo, New York, 14423, USA.
Eric M. Roberts [eric.roberts@jcu.edu.au], Department of Earth and Oceans, James Cook University, Townsville, Queensland, 4811 Australia.
Leif Tapanila [tapaleif@isu.edu], Department of Geosciences and Idaho Museum of Natural History, Idaho State University, Pocatello, Idaho, 83209, USA.
Maureen A. O’Leary [maureen.oleary@stonybrook.edu], Department of Anatomical Sciences, Stony Brook University, Stony Brook, New York, 11794, USA.
Received 13 November 2017, accepted 12 February 2018, available online 7 May 2018.
Copyright © 2018 J.A. McCartney et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Beginning in the Late Cretaceous, the Tethys Ocean was a region of high diversity for marine snakes (Rage 2013). Included in this diversity are the extinct lineages Palaeophiidae and Nigerophiidae, both of which ranged from the Late Cretaceous (Rage and Wouters 1979; Lingham-Soliar 1991; Rage and Werner 1999) to the Eocene (e.g., Rage 1980, 1983b, Rage et al. 2008; Snetkov 2011; Smith et al. 2016). They also occurred across the Tethys, including Europe (Owen 1841, 1850; Rage 1980; Averianov 1997; Kristensen et al. 2012), Asia (Tatarinov 1963; Nessov and Udovitschenko 1984; Rage and Prasad 1992; Averianov 1997; Rage et al. 2003, 2008; Bajpai and Head 2007), and Africa (Andrews 1901, 1924; Antunes 1964; Rage 1975, 1983b; Rage and Wouters 1979; Rage and Werner 1999; Rage and Dutheil 2008). In addition to their occurrences in Eurasia and Africa, palaeophiids are known from North and South America (Cope 1868; Marsh 1869; Lynn 1934; Hoffstetter 1958; Holman 1982; Parmley and Case 1988; Erickson 1998; Parmley and DeVore 2005), and nigerophiids are known from Madagascar (LaDuke et al. 2010; Pritchard et al. 2014). Because of their often poor preservation (typically disarticulated vertebral elements) and highly modified morphology compared to other snakes, these families have been difficult to place phylogenetically relative to living species and to each other, although palaeophiids and nigerophiids share some similarities in morphology (Rage 1975). Members of both families are often found in near-shore marine or marine sediments (e.g., Hoffstetter 1955; Rage 1975, 1983b; Rage et al. 2008), and their vertebral and rib morphology suggests they had transversely compressed bodies, thought to be an adaptation for swimming (e.g., Owen 1850; Hoffstetter 1955; Rage 1984).
Palaeophiidae contains into two subfamilies: Archaeophiinae, represented by two species of Archaeophis from Europe and Central Asia (Janensch 1906b; Tatarinov 1963), and Palaeophiinae, which includes 18 to 20 species in the genera Palaeophis and Pterosphenus. The species within the latter two genera form a morphological continuum, with snakes showing morphologies interpreted as aquatic adaptations (e.g., narrow vertebrae, ventrally situated synapophyses; as argued by Owen 1850; Hoffstetter 1955; Rage 1984; and others) referred to Pterosphenus, and those with what have been considered more generalized morphology (broader vertebrae, ventrolaterally facing synapophyses) to Palaeophis instead (Rage 1983b; Rage et al. 2003). The boundary between the genera is not distinct, and some species of Palaeophis are only slightly differentiated from species of Pterosphenus (Rage 1983a; Zouhri et al. 2017). Although palaeophiids have been hypothesized to be completely aquatic (Rage 1984), there is still a good deal of morphological variation present in the family suggesting some diversity of habits. For example, overall size is highly variable in this family: the smallest palaeophiid known, Palaeophis casei, was probably less than 0.5 m in total length (Holman 1982), while the largest, Palaeophis colossaeus, has previously been suggested to exceed nine meters (Rage 1983b). Palaeophiids have been recovered from a variety of aquatic environments as well, including shallow marine (Hoffstetter 1958; Hutchison 1985), near shore marine (Parmley and DeVore 2005), lagoons (Rage 1983a), estuarine (Westgate and Ward 1981; Holman 1982), mangrove forests (Westgate and Gee 1990; Houssaye et al. 2013), and near-coastal fluvial sediments (McCartney and Seiffert 2016). This known distribution, as well as the absence of palaeophiids from inland deposits, strongly suggests marine habits for the clade.
The largest of the palaeophiids, Palaeophis colossaeus, is among the species most morphologically distinct from the other palaeophiine genus Pterosphenus, in having mediolaterally broad vertebrae, zygapophyses that project laterally, and synapophyses that are not ventrally shifted from the typical position in snakes (Fig. 1), suggesting a body that is not transversely compressed (Rage 1983b). However, the synapophyses do face ventrally, suggesting there was a narrowing of the body as seen in aquatic snakes (Rage 1983b; Graham et al. 1987; Pattishall and Cundall 2008). Palaeophis colossaeus is known exclusively from the middle Eocene of Mali, having previously only been reported from one locality, Tamaguélelt, Mali (Hoffstetter 1955; Rage 1983b).
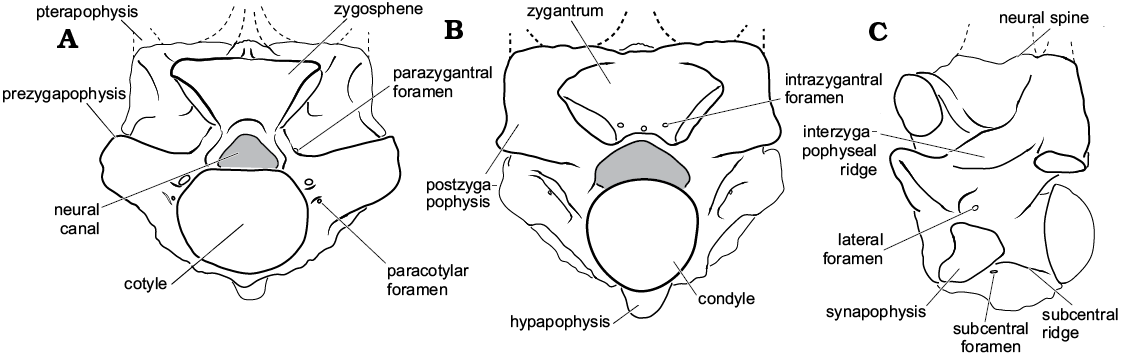
Fig. 1. Basic snake vertebral anatomy based on Palaeophis colossaeus (CNRST-SUNY 294) from the Eocene of Mali; in anterior (A), posterior (B), and lateral (C) views.
Nigerophiids are also thought to be aquatic based on similar vertebral morphology, but in contrast with palaeophiids they are more restricted in size, in some cases only reaching total lengths estimated at about 0.5 m (Pritchard et al. 2014). Nigerophiidae is also a less taxonomically diverse family, consisting of only seven named species; of those species, several are only tentatively referred to the family. Even less is known of the paleobiology of these species due to the relative paucity of material.
Both palaeophiid (Andrews 1924; Rage 1983b) and nigerophiid (Rage 1975) fossil snakes have been collected from localities that record the passage of the ancient Trans-Saharan Seaway, a subtropical epeiric sea that was an arm of the southern Tethys Ocean. It extended through West Africa to the Gulf of Guinea episodically between the Late Cretaceous and the Early Paleogene (e.g., Greigert 1966; Reyment 1980; Bellion et al. 1989). Here, we describe new snake material we have collected from Early Paleogene seaway deposits of Mali. Field expeditions conducted jointly by Stony Brook University and the Centre National de Recherche Scientifique et Technologique of Mali in 1999 and 2003 have resulted in the recovery of crocodyliform (Brochu et al. 2002; Hill et al. 2008), turtle (Gaffney et al. 2007), dinosaur (O’Leary et al. 2004), and fish (Claeson et al. 2010) remains from the Trans-Saharan Seaway rock from the Late Cretaceous through middle Eocene of Mali. Among this material are new specimens of Palaeophis colossaeus recovered from near the type locality of Tamaguélelt, allowing for a redescription of the species, and for the first assessment of the intracolumnar variation in the precloacal portion of the vertebral column. Additionally, material of a new nigerophiid and a second, indeterminate species were also recovered and are described here.
Institutional abbreviations.—CM, Carnegie Museum of Natural History, Pittsburgh, PA; NHMUK, Natural History Museum, London, UK; CNRST-SUNY, Centre National de la Recherche Scientifique et Technologique du Mali, Stony Brook University, USA; MNHN, Muséum national d’Histoire naturelle, Paris, France; YPM, Yale Peabody Museum of Natural History, New Haven, USA.
Nomenclatural acts.—This paper and the nomenclatural acts within have been registered in ZooBank. The LSID for this publication is: urn:lsid:zoobank.org:pub:3BE7FCB7-C50B-4C22-98B1-BA7AF508F91A.
Geological setting
Joint expeditions between the Centre National de Recherche Scientifique et Technologique of Mali and the Department of Anatomical Sciences, Stony Brook University in 1999 and 2003 identified over twenty fossil-producing localities in Cretaceous and Paleogene rocks of northern Mali (O’Leary et al. 2006; Hill et al. 2008; Claeson et al. 2010). The sites are located on the margins of the Iullemmeden and Taoudeni basins, as well as in the narrow Gao Trench (Détroit Soudanais), a small east-west rift basin, that connects the two large intracratonic basins (Fig. 2). Far-field stress associated with the opening of the south Atlantic Ocean due to rifting of South America and Africa resulted in widespread subsidence within these intracratonic basins during the late Mesozoic–early Cenozoic (Petters 1979; Reyment and Dingle 1987; Burke 1996). Globally high sea levels during this time also contributed to the formation of the epicontinental Trans-Saharan Seaway that inundated West Africa episodically during the Cretaceous–Paleogene (Greigert 1966; Reyment 1980) and resulted in the deposition of extensive near shore marine-marine rocks. Upper Cretaceous–Paleogene Trans-Saharan Seaway strata in the Tilemsi Valley overlie a deformed Pan-African suture belt, which defines the eastern boundaries of the Taoudeni Basin, and underlying basement rocks of the West African Craton. The thickest sequence of Trans-Saharan Seaway deposits is preserved to the south and east of the Tilemsi Valley within the Gao Trench (Fig. 3; see also Radier 1959).
Trans-Saharan Seaway strata have produced important vertebrate fossils (e.g., Brochu et al. 2002; Claeson et al. 2006; O’Leary et al. 2006; Hill et al. 2008). Two of the localities that yielded the fossil snakes described here, Mali-19, a Paleocene locality in the Teberemt Formation and Mali-20, a predominantly Eocene locality in the Tamaguélelt Formation, are located on the west and east extremes respectively of the Gao Trench (Fig. 2), with Mali-20 at the southernmost tip of the Tilemsi Valley. Mali-20 has two localities of phosphatic rocks that yielded snake fossils, and we refer to these as the “lower phosphates” and the “upper phosphates”.
The cycles of marine transgression and regression left behind thin sequences of sedimentary rocks that have been described in detail and interpreted as near shore marine to brackish water deposits (Tapanila et al. 2008). Tapanila et al. (2008) described the presence of repeated sequences of carbonates and shales that are punctuated by phosphatic conglomerates, the latter of which tend to be highly fossiliferous. In particular, Mali-20 (studied in detail in Tapanila et al. 2008), one of the localities that yielded snake fossils, contains two layers of dense deposits of coprolites and disassociated bone (the lower and upper phosphates). Tapanila et al. (2008) argued that these localities were very time-averaged.
The eastern and older locality, Mali-19, consists of 15 m of section that spans the Paleocene and possibly both the Cretaceous–Paleogene the Paleocene–Eocene boundaries, although complete boundary sections have not yet been identified. The 20 m thick section at Mali-20 (also known as “Tamaguélelt”) spans much of the Eocene (Tapanila et al. 2008), and both sections have been dated and correlated based on detailed biostratigraphy (O’Leary et al. 2006; Gaffney et al. 2007; Hill et al. 2008; Tapanila et al. 2008; Claeson et al. 2010). A key index fossil for dating these snake-producing localities is the echinoid Oriolampas michelini, which indicates Paleocene rocks (often Thanetian; Smith and Jeffery 2000). Biostratigraphic control above the snakes, however, remains very poor.
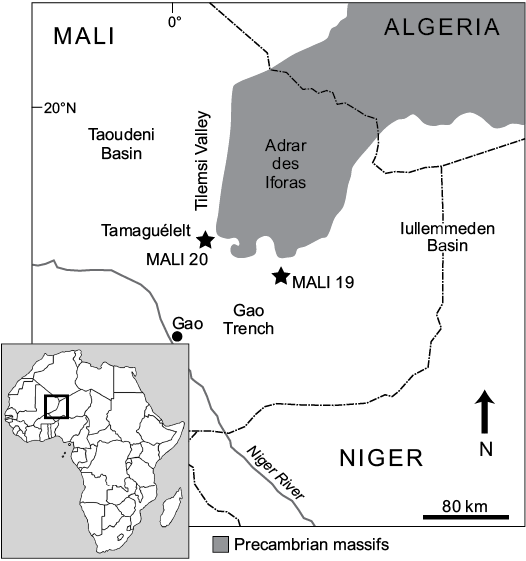
Fig. 2. Map of the field area in eastern Mali with the relative locations of the snake bearing localities.
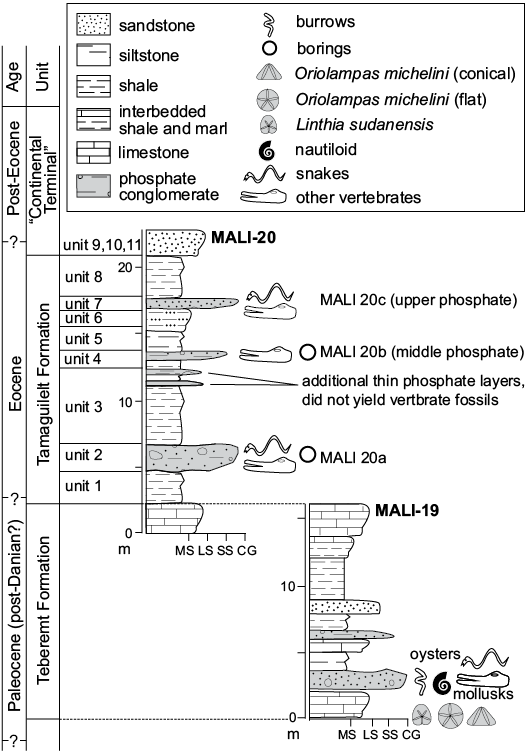
Fig. 3. Correlated stratigraphic sections of the Mali-19 and Mali-20 localities that have yielded snake vertebrae.
Material and methods
The fossils were surface collected from two localities in northwestern Mali. The specimens required little mechanical preparation, other than limited removal of accreted material on the surface of some specimens. Preparation was completed at the Stony Brook University Vertebrate Fossil Preparation Laboratory. The specimens are held in collections at Stony Brook University.
Estimating the body size of extinct snakes from isolated vertebrae is difficult due to uncertainties of vertebral position and number, but such estimations are commonly done in the literature (e.g., Marsh 1869; Rage 1983b; Head et al. 2009; LaDuke et al. 2010). Maximum likelihood methods exist for the estimation of body length in extinct snakes, but constructing such models requires precise knowledge of how vertebral morphology varies within the column, so that isolated vertebrae may be placed in their proper context. This kind of estimate has been done for extinct species that belong to extant clades for which models may be constructed (Head et al. 2009). Because no complete trunk of any palaeophiid or nigerophiid has been described, the exact pattern of variation, and even the total number of vertebrae, is unknown. Additionally, the phylogenetic relationships of both families are unknown, so there is no justification for producing a model for any particular extant family. We are thus unable to construct a model of the intracolumnar variation in the vertebrae of palaeophiids, and cannot precisely place isolated vertebrae within the column. Therefore, the body length estimates provided here are based on standard major axis regression of vertebral measurements on body length in a variety of extant snakes.
The total length (including tail) of Palaeophis colossaeus was estimated using standard major axis regressions of vertebral measurements on body length in a sample of extant snakes for which body size is known (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app63-McCartney_etal_SOM.pdf). The vertebra measured in the extant snakes was the largest in that individual’s vertebral column. Measurements made include the width across the prezygapophyses (not including the prezygapophyseal accessory process, which is absent in Palaeophiidae), as well as the transverse width of the cotyle (see Fig. 1). In larger specimens the measurements were made using digital calipers accurate to 0.1 mm. Smaller specimens were photographed with an Axiocam MRc camera coupled to a Zeiss Discovery.V12 stereo dissecting microscope, and measurements were subsequently made using Zeiss AxioVision (v. 4.4.1.0) or ImageJ (v. 1.50i; Schneider et al. 2012) software. The standard major axis regressions were performed using the package lmodel2 (v. 1.7-2; Legendre 2014) in the statistical software R (v. 3.4.0; R Core Team 2017). Each measurement was separately regressed against body size, producing two different models for estimating body length. This was done to accommodate the preservation of the materials, and permit estimation using the largest specimens of each species in the study. Standard major axis regression was performed because both variables (total body length and vertebral metrics) were measured with error, and because Palaeophis colossaeus lies beyond the data and is therefore an extrapolation (Ricker 1973).
Systematic palaeontology
Squamata Oppel, 1811
Serpentes Linnaeus, 1758
Palaeophiidae Lydekker, 1888
Genus Palaeophis Owen, 1841
Type species: Palaeophis toliapicus Owen, 1841; Isle of Sheppey, England; Ypresian, Eocene.
Palaeophis colossaeus Rage, 1983
Figs. 4, 5.
1983 Palaeophis colossaeus sp. nov.; Rage 1983b: 1029.
2003 Palaeophis colossaeus Rage, 1983; Rage et al. 2003: 698.
Holotype: MNHN.F.TGE 614, a mid-trunk vertebra (Rage 1983b).
Type locality: Tamaguélelt, Mali (Rage 1983b).
Type horizon: Early Eocene.
Material.—CNRST-SUNY 306, 308, 309, 323, anterior trunk vertebrae; CNRST-SUNY 297, 299, 300, 301, 313, 316, 321, anterior or mid-trunk vertebrae; CNRST-SUNY 261, 288, 291, 292, 294, 295, 304, 305, 310, 315, 325, mid-trunk vertebrae; CNRST-SUNY 289, 296, 302, 311, 318, 322, mid-trunk or posterior trunk vertebrae; CNRST-SUNY 293, 320, posterior trunk vertebrae; CNRST-SUNY 290, 298, 303, 307, 312, 314, 317, 319, trunk vertebrae. From the Eocene Tamaguilelt Formation, locality Mali-20, near Tamaguélelt, Mali; specimens were recovered from thick, phosphatic conglomerate beds (Tapanila et al. 2008). CNRST-SUNY 288, 289, 290, 291, 292, 293, 294, 295, 307, 308, 309, 310 from Unit 7 (Mali 20c in Tapanila et al. 2008); the remaining specimens were recovered from Unit 2 (Mali 20a in Tapanila et al. 2008).
Emended diagnosis.—Distinct from all other snakes by the following combination of characters: robust, large vertebrae lacking prezygapophyseal accessory processes, with a parazygosphenal foramen in the mid- and posterior trunk. Differentiated from all other palaeophiids by its greater size and the presence of parazygosphenal foramina in the mid- and posterior trunk. Further distinct from Pterosphenus and all Palaeophis except Palaeophis maghrebianus and Palaeophis virginianus by lacking any lateral compression in the vertebrae. Separated from Palaeophis maghrebianus by having a greater width of the zygosphene relative to that of the cotyle, and from Palaeophis virginianus by having a neural spine that arises from immediately posterior to the zygosphene.
Description.—Palaeophis colossaeus is known only from isolated vertebrae. There is substantial variation in the size of the new material, with the smallest vertebra (CNRST-SUNY 306) measuring 13.6 mm in neural arch width, and the largest (CNRST-SUNY 289) 47.3 mm across the same. To facilitate comparisons with other species, the mid-trunk vertebrae are described first, and variation in morphology of the subsequent regions is provided thereafter.
Mid-trunk and probable mid-trunk vertebrae make up the bulk of the collection. Throughout the collection, there are no complete neural spines, pterapophyses, or synapophyses. The best-preserved specimens are CNRST-SUNY 294, 310, and 325, and the description is primarily based on these specimens, with variations noted.
The base of the neural spine is triangular in cross-section, and extends from the posterior border of the neural arch to the posterior portion of the zygosphene. The zygosphene is massive, and is transversely wider than the cotyle. The dorsal surface of the zygosphene is highly variable, ranging from weakly concave (e.g., CNRST-SUNY 325), to flattened or even slightly convex dorsally (e.g., CNRST-SUNY 294). The anterior surface is deeply concave in all well-preserved specimens, and bears a weak arched ridge extending from the anterior edge of the neural arch pedicle on each side. An indistinct, thick ridge extends ventrally from the inferoposterior edge of the zygosphenal facet to the interzygapophyseal ridge. Lying between the zygosphenal facet and the prezygapophysis there is a single, large parazygosphenal foramen. On the posterior surface, the zygantrum is broad and tall. The anterior wall is smooth and vertical, and contains a variable number of foramina in a varied arrangement. Most frequently there are three foramina, one set low and close to each zygantral facet, and a median foramen that may be set in line with the other two, or superiorly.
The neural canal is trifoliate in appearance, with low ridges extending along the length of the dorsolateral sides of the canal. The walls of the neural canal are thick, but tapered anteriorly into a sharp crest continuous superiorly with the arched ridge of the zygosphene.
The pre- and postzygapophyses project strongly laterally. The facets are oval in outline, and their long axis is oriented at about 50–60° from the main vertebral axis; based on differences from the anterior trunk vertebrae (see below), higher values suggest a more posterior position in the column. The prezygapophyses are inclined superolaterally at about 20–25° from horizontal. There is no prezygapophyseal accessory process; instead, the thick prezygapophyseal buttress tapers to a ridge that extends ventrally toward the synapophyses. The superior portion of the buttress is convex laterally, such that it projects slightly beyond the lateral extent of the prezygapophyseal facet. The inferior portion of the buttress is weakly concave laterally. Throughout the collection, the height and lateral projection of the superior portion is variable, either narrower and more strongly projecting (exemplified by CNRST-SUNY 310), or deeper and flatter (exemplified by CNRST-SUNY 325). Comparison with anterior trunk vertebrae (see below) suggests the buttress transitions from projecting to flattened. Extending posteriorly from the prezygapophysis is a weakly projecting, thick interzygapophyseal ridge, which expands posteriorly toward the postzygapophysis and upward to the base of the pterapophysis. The lateral surface dorsal to the postzygapophyseal facet is gently concave. Nothing beyond the bases of the pterapophyses are preserved in any specimen, thus their height is unknown.
The cotyle is slightly wider than tall, and its thick lip is occasionally incomplete just inferior to the neural canal, causing its upper edge to appear flat or even slightly concave. The paracotylar fossa is divided into superior and inferior portions by a thickened ridge situated about one quarter of the distance from the top of the cotylar height. In several specimens this ridge bears a single foramen. Another single large foramen sits in the superior part of the paracotylar fossa, (doubled in CNRST-SUNY 288). The inferior half of the paracotylar fossa is occupied by a series of minute foramina surrounding the cotyle. The condyle is best preserved in CNRST-SUNY 310, and it shares the same general shape as the cotyle and is not upturned.
The synapophyses are damaged in all the specimens, but preservation on some (including the holotype, MNHN TGE 614) indicates the diapophyseal portion faces laterally, and the parapophyseal portion ventrally (Rage 1983b). Posterodorsal to the remnants of the synapophysis is a rugose patch, probably a site of muscular attachment. The scar consists of an anteroventrally oriented ridge offset by depressions above and below, with a variable number of other bumps also present. The superior depression bears the lateral foramen; a variable number of small foramina occur in the inferior depression as well. In CNRST-SUNY 302, the morphology of the rugosity is interrupted by an extremely deep depression.
Mid-trunk vertebrae bear a single, vertically directed hypapophysis. It is most completely preserved in CNRST-SUNY 290, and partly present in CNRST-SUNY 292. In all specimens retaining traces of the hypapophysis, the base is thick. In CNRST-SUNY 290, it is dorsoventrally short and extends anteriorly for approximately two-thirds of the centrum. As in the holotype, the hypapophysis tapers distinctly near its tip. Extending anteriorly from the hypapophysis to the inferior cotylar lip is a moderately thick ridge. The ridge is strongly arched dorsally in CNRST-SUNY 310 and CNRST-SUNY 325, and weakly arched in CNRST-SUNY 292. A shallow fossa lies between the ridge and the synapophysis. At the posterior edge of the synapophysis there are one or two foramina.
Anterior trunk vertebrae preserved in the collection include only four specimens, CNRST-SUNY 306, 308, 309, and 323. As in the mid-trunk, there are no complete neural spines or synapophyses, and the hypapophyses are incomplete. The anterior trunk vertebrae are similar to the mid-trunk ones in many respects; the anterior trunk vertebrae are more elongated, and have a smaller second, anterior hypapophysis. Additional differences in morphology are noted below.
The base of the neural spine extends over only approximately two-thirds of the length of the neural arch in all but CNRST-SUNY 323. In that specimen, the spine extends to the posterior edge of the zygosphene, probably an indication of a more posterior position in the vertebral column (see above). In CNRST-SUNY 308, the zygosphene has a notable midline notch, but this is absent in the other specimens. To either side of the notch, the superior edge of the zygosphene is convex dorsally, giving it bilobed appearance in anterior view. There is no parazygosphenal foramen in any of the anterior trunk vertebrae in the collection. The pre- and postzygapophyses do not project strongly laterally as compared to the mid-trunk, with the long axis oriented at about 32° from the main vertebral axis, and they are not as strongly inclined superolaterally (15° from horizontal). The facets are also narrower than they are in most of the mid-trunk vertebrae. The prezygapophyseal buttress has a morphology similar to that of CNRST-SUNY 325, with a dorsoventrally taller, but less projecting superior portion.
On the centrum, the rugose area posterodorsal to the prezygapophysis is most highly developed in the largest specimen (CNRST-SUNY 323) and only weakly developed in the smaller specimens. On the ventral surface of the centrum in the anterior trunk, palaeophiids bear two median hypapophyses in series. The anterior of these is broken off on each specimen, but a specimen in the British Museum (NHMUK PV R 9531) shows it to be short, narrow, and anteriorly projecting. The degree to which the size of the anterior hypapophysis changes through the anterior trunk is unclear. Lying between the anterior and posterior hypapophysis is a ridge of variable thickness, highest and narrowest in CNRST-SUNY 306, possibly indicating a more anterior position in the trunk by comparison with the British Museum specimen. In the other vertebrae, the ridge is low and thick. The subcentral foramina are largely similar to those in the mid-trunk, but in CNRST-SUNY 309, there is a single paramedian foramen on the left side of the subcentral ridge.
The posterior trunk is poorly represented in the collection. Only CNRST-SUNY 293 and CNRST-SUNY 320 can be definitively assigned to that region. Both are poorly preserved, with very few complete features. They are recognized as belonging to the posterior trunk by the complete absence of a subcentral keel anterior to the hypapophysis. All other features preserved are similar to the mid-trunk vertebrae.
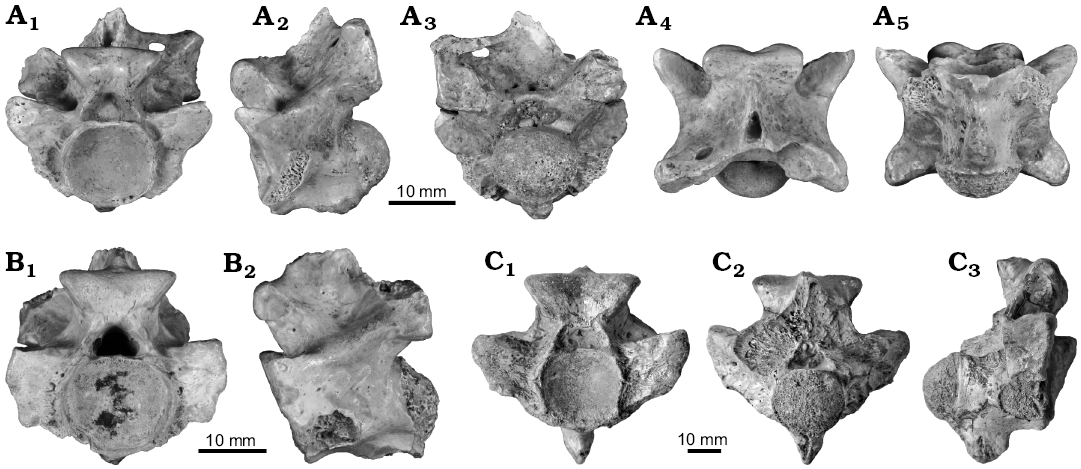
Fig. 4. Mid-trunk vertebrae of palaeophiid snake Palaeophis colossaeus Rage, 1983 from the Eocene of Tamaguélelt Formation, Mali. A. CNRST-SUNY 310; in anterior (A1), lateral (A2), posterior (A3), dorsal (A4), and ventral (A5) views. B. CNRST-SUNY 325; in anterior (B1) and lateral (B2, reversed to facilitate comparisons) views. C. CNRST-SUNY 290; in anterior (C1), lateral (C2), and ventral (C3) views.
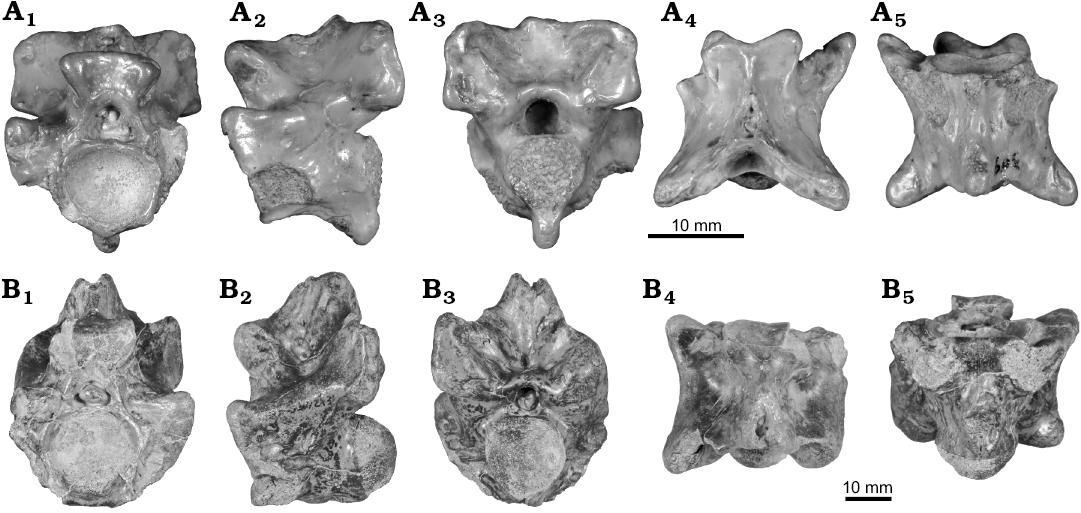
Fig. 5. Anterior (A) and posterior (B) trunk vertebrae of palaeophiid snake Palaeophis colossaeus Rage, 1983 from the Eocene of Tamaguélelt Formation, Mali. A. CNRST-SUNY 309; in anterior (A1), lateral (A2, reversed to facilitate comparisons), posterior (A3), dorsal (A4), and ventral (A5) views. B. CNRST-SUNY 293; in anterior (B1), lateral (B2), posterior (B3), dorsal (B4), and ventral (B5) views.
Remarks.—Intracolumnar variation in palaeophiid snakes is relatively poorly understood, with the only detailed discussions provided for Palaeophis maghrebianus (see Houssaye et al. 2013), Palaeophis nessovi (see Snetkov 2011), Palaeophis vastaniensis (see Bajpai and Head 2007), Pterosphenus kutchensis (see Rage et al. 2003), and Pterosphenus schweinfurthi (see Janensch 1906a; McCartney and Seiffert 2016). Anterior vertebrae of Palaeophis colossaeus are characterized by several features. The zygapophyses are small, and not laterally projecting; the zygosphenes are relatively wide; and for at least part of the anterior trunk, the neural spines are anteroposteriorly restricted in length. These characters appear to be common in many major clades of snakes (LaDuke 1991), although their evolutionary polarity is currently unknown. Anterior trunk vertebrae are further characterized in palaeophiids by the presence of an anterior hypapophysis, posited to be pleurocentrally derived (Hoffstetter 1955; Rage 1983b, 1984), and by vertebrae that are more anteroposteriorly elongate (Houssaye et al. 2013). In addition, in Palaeophis colossaeus there is a shape change in the prezygapophyseal buttress that appears to occur gradually from the anterior trunk through part of the mid-trunk, resulting in an increasingly elongate and projecting prezygapophyseal process. In the mid-trunk region, there is also a trend toward decreasing height of the keel extending anteriorly from the hypapophysis, resulting in its absence in the posterior trunk (also noted to occur in Pterosphenus; McCartney and Seiffert 2016). Of note is the absence of caudal vertebrae in this and other collections of Palaeophis colossaeus. Caudals are rare for palaeophiids generally, but those that are known are typical for snakes (Rage 1983a; Parmley and Case 1988), so it is unlikely that they are simply misidentified for this species.
Palaeophis colossaeus is morphologically distinguished from other palaeophiids by several features. It is the largest known species, exceeding slightly the greatest reported size for Palaeophis maghrebianus from Morocco (Arambourg 1952). Most of its vertebrae also bear bilateral parazygosphenal foramina, a feature found only in a few distantly related snakes, including two species of Acrochordus (see Hoffstetter and Gayrard 1964; Head 2005), and the colubroid snakes Synophis (see Bogert 1964), Renenutet (see McCartney and Seiffert 2016), and an unnamed species from the Eocene of India (Rage et al. 2003). Palaeophis colossaeus also differs from most other palaeophiids by having relatively broad, robust vertebrae with laterally-projecting zygapophyses, features shared with Palaeophis maghrebianus from Morocco and Palaeophis virginianus from North America (Rage 1983b). Nevertheless, the latter two species have vertebrae that are slightly narrower and more elongate than vertebrae from similar regions of the trunk in Palaeophis colossaeus; the zygosphene is additionally transversely broader in Palaeophis colossaeus, in some cases exceeding the cotylar width.
Stratigraphic and geographic range.—Known only from the early Eocene of Tamaguélelt, Mali.
Nigerophiidae Rage, 1975
Genus Amananulam nov.
Type species: Amananulam sanogoi gen. et sp. nov., see below.
Etymology: From the Tuareg language Tamasheq, aman, water, and anulam, snake.
Diagnosis.—As for type and only species.
Amananulam sanogoi sp. nov.
Fig. 6.
Etymology: Named in honor of Mamadou Sanogo, for his outstanding collaboration on the CNRST-SBU expeditions work in Mali.
Holotype: CNRST-SUNY 462, a mid-trunk vertebra.
Type locality: Locality Mali-19, in Northeastern Mali.
Type horizon: Paleocene.
Material.—Known only from the holotype.
Diagnosis.—Nigerophiid snake with the autapomorphic restriction of the hemal keel to the posterior quarter of the centrum. Additionally differentiated from other nigerophiid snakes by the combination of a dorsoventrally tall zygosphene, a dorsally concave neural arch, and a neural spine that is restricted to the posterior one-third of the neural arch.
Description.—Amananulam sanogoi gen. et sp. nov. is known from only a single, mid-trunk vertebra. The vertebra is small at only 10 mm in preserved length, although damage to the condyle shortens it somewhat. It preserves much of the centrum, a partial zygosphene, and portions of the neural arch. No zygapophyses or synapophyses are completely preserved.
The neural spine is incompletely preserved, but it is clearly restricted to the posterior quarter of the neural arch. It has a laterally broad base that tapers superiorly; the anterior surface sweeps steeply posterodorsally. Anterior to the neural spine the neural arch is concave dorsally, bounded anteriorly by a dorsally-projecting zygosphene. The zygosphene is tall, with superiorly projecting facets giving it a gently w-shaped dorsal edge. The facets also project anteriorly, producing a concave anterior surface. The superior portion of the left zygosphenal facet is damaged, but enough is preserved to indicate that the zygosphene is narrower than the cotyle. The zygantrum is tall to accommodate the height of the zygosphene, but it is otherwise poorly preserved.
The neural canal is subtriangular in shape. The walls of the arch are thick, and taper anteriorly to a blunt ridge. The zygapophyses are entirely absent except for a small portion of the right prezygapophysis. Along the inferolateral border of this portion is the remnant of the synapophysis, which appears to face ventrally. The interzygapophyseal ridge is so weakly projecting that it is nearly absent.
The cotyle is large and slightly wider than tall. The condyle is incompletely preserved, but it lacks a precondylar constriction. Subcentral ridges are absent, giving the ventral surface a smooth appearance, interrupted only by a pair of subcentral foramina set slightly posterior to the midpoint of the vertebra. The hypapophysis or hemal keel is broken, but is restricted to the very posteriormost portion of the centrum, projecting as a small nubbin. There is no ridge or crest extending anteriorly from the hypapophysis toward the cotyle. The ventral surface of the vertebra is concave in lateral view.
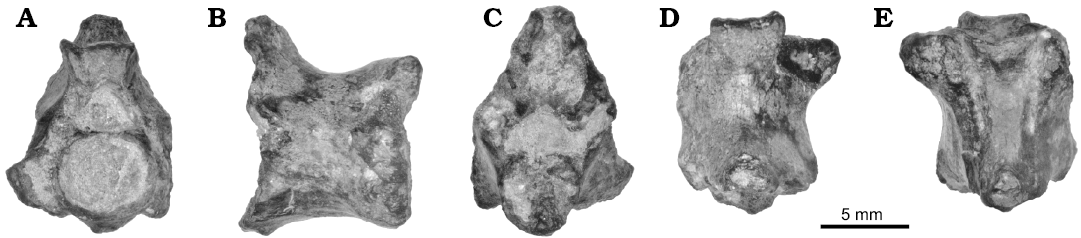
Fig. 6. Vertebra of the nigerophiid snake Amananulam sanogoi gen. et sp. nov. (CNRST-SUNY 426) from the Paleocene Teberemt Formation (phosphate of Mali-19), Mali; in anterior (A), lateral (B), posterior (C), dorsal (D), and ventral (E) views.
Remarks.—Amananulam sanogoi gen. et sp. nov. is referable to Nigerophiidae on the basis of several features, including the restricted anteroposterior length of the neural spine, and the weakness of the interzygapophyseal and subcentral ridges. Nigerophiids also have ventrally facing synapophyses, as in Amananulam sanogoi gen. et sp. nov., although that feature is thought to be an adaptation to an aquatic lifestyle found in other aquatic snakes (e.g., Lucas 1898; Rage and Werner 1999). Amananulam sanogoi gen. et sp. nov. is unique among nigerophiids in lacking a hemal keel that extends anteriorly to the cotyle, having instead a low knob-like process only. It also differs from almost all nigerophiids in having a dorsoventrally deep zygosphene and zygantrum, a feature that is only present in the presumed nigerophiid Woutersophis novus from Belgium (Rage 1980). The great height of the zygosphene gives the neural arch a swaybacked appearance with a deep concavity separating the zygosphene and neural spine; this morphology is shared with other species (Indophis sahnii, “Nessovophis” zhylga, and Kelyophis hechti; Rage and Prasad 1992; Averianov 1997; LaDuke et al. 2010), but in none is the depth of the concavity as great as in Amananulam sanogoi gen. et sp. nov. The near absence of the interzygapophyseal ridge and absence of subcentral ridges are features shared with Nigerophis and Nubianophis to the exclusion of other species referred to Nigerophiidae (Rage and Werner 1999).
Stratigraphic and geographic range.—Known only from the Paleocene locality Mali-19, eastern Mali.
Incertae sedis
Indeterminate genus and species
Fig. 7.
Material.—CNRST-SUNY 324, a possible anterior trunk vertebra from Early Eocene locality Mali-20, near Tamaguélelt, Mali. The specimen was recovered from Unit 2.
Description.—There is a single, fragmentary specimen of a snake not referable to either Palaeophis colossaeus or Amananulam sanogoi gen. et sp. nov., or to Palaeophiidae or Nigerophiidae. The specimen preserves portions of the neural arch, including both postzygapophyses and part of the zygantrum, and parts of the centrum including a hypapophysis. The neural spine, prezygapophyses, and synapophyses are unpreserved.
The neural spine is not preserved, but the base is wide posteriorly, and tapers quickly anteriorly to become thin. The anterior extent of the neural spine is unclear due to damage to the neural arch. The zygosphene is also completely missing. A low ridge extends posteroinferiorly from the broken zygosphene toward the interzygapophyseal ridge. This produces a deep fossa on the neural arch bounded posteriorly by an expansion of the arch accommodating the zygantrum. The full height of the zygantrum is uncertain, but what is preserved is somewhat tall suggesting an at least moderately robust zygosphene. There is a single midline intrazygantral foramen preserved in the anterior wall, which is otherwise smooth. The postzygapophyses are poorly preserved, and bear oval facets inclined at an angle of about 8° from horizontal. The interzygapophyseal ridge is thick and prominent. Posterior to the broken base of the synapophysis is a pair of minute lateral foramina.
The centrum bears a cotyle that is slightly wider than it is tall. The condyle is subcircular, and is turned upward. The subcentral ridges are thick, and bound a shallow subcentral fossa on the ventral aspect of the vertebra. The ridges are directed posteromedially and converge with the hypapophysis rather than reaching the condyle, giving the vertebra an almost Y-shaped appearance. The hypapophysis is thick and covers slightly more than half of the centrum. It is separated posteriorly from the inferior lip of the condyle by a gap. There is no ridge extending anteriorly to the cotyle. The height of the hypapophysis is unknown due to breakage of the tip. Anterior to the hypapophysis along the subcentral ridge on each side there is an elongate subcentral foramen.
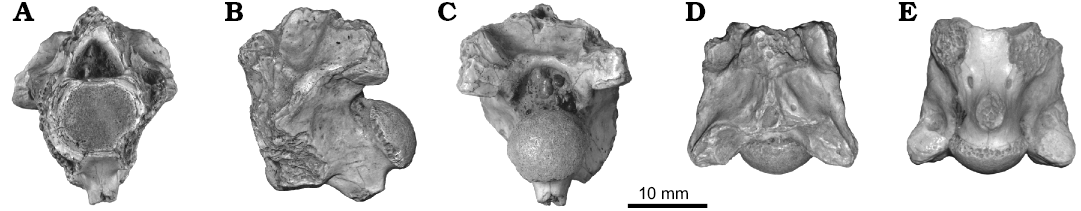
Fig. 7. Vertebra of an indeterminate snake CNRST-SUNY 324 from the Eocene Tamaguélelt Formation, Mali; in anterior (A), lateral (B), posterior (C), and ventral (D) views.
Remarks.—Unfortunately, the preservation of CNRST-SUNY 324 provides few clues as to its affinities, although it presents some unusual morphology. The vertebra is quite robust, reminiscent of many booid-grade snakes, as well as several extinct clades. The absence of a parazygantral foramen on the posterior surface of the neural arch suggests against madtsoiid affinities. The absence of a midline ridge extending anteriorly from the hypapophysis is uncommon among snakes, but is known to occur in some nigerophiids, the posterior trunk vertebrae of at least some palaeophiids (discussed above; also see McCartney and Seiffert 2016), and in the enigmatic snake Tuscahomaophis from the Paleocene of North America (Holman and Case 1992). However, both palaeophiids and Tuscahomaophis lack the upturn seen in the condyle of CNRST-SUNY 324. This morphology therefore differentiates it from all palaeophiids including Palaeophis colossaeus, with which it co-occurs. The gap between the hypapophysis and condyle is also unusual, and not known to occur in palaeophiids. The specimen additionally differs from Palaeophis colossaeus by having less strongly inclined zygapophyseal facets, and a fossa on the neural arch. Affinity with Nigerophiidae also seems unlikely, due to the prominent subcentral and interzygapophyseal ridges, as well as the anteriorly extensive neural spine; in any case, CNRST-SUNY 324 differs from Amananulam sanogoi gen. et sp. nov. in those features.
The position of the vertebra in the column cannot be readily determined from the anatomy preserved. The presence of a large hypapophysis is suggestive of the anterior trunk, but several lineages of snakes retain hypapophyses throughout the trunk (e.g., Palaeophiidae, Acrochordidae, Viperidae; Malnate 1972; Rage 1984). The angle at which the postzygapophyses project (estimated at ~37° from the main vertebral axis) is also reminiscent of the anterior trunk, but the facets are badly damaged making determination of this angle difficult.
Body length estimation
The regression models of both vertebral metrics against total length (including tail) revealed a highly significant (p ≤ 0.01; Fig. 8) relationship with total length of snakes. The models also had high explanatory power (r2 = 0.95 for cotylar width, r2 = 0.96 for trans-prezygapophyseal width), indicating that the relationship is neither spurious, nor strongly affected by other variables. The estimated total lengths for Palaeophis colossaeus range from 8.1 m using the trans-prezygapophyseal width of CNRST-SUNY 290 and up to 12.3 m using the cotylar width of NHMUK PV R 9870 (Table 1). Amananulam sanogoi is considerably smaller, with an estimated length of 2 m determined from cotylar width (Table 1). The indeterminate snake was intermediate in size between Palaeophis colossaeus and Amananulam sanogoi at 5.4 m (Table 1).
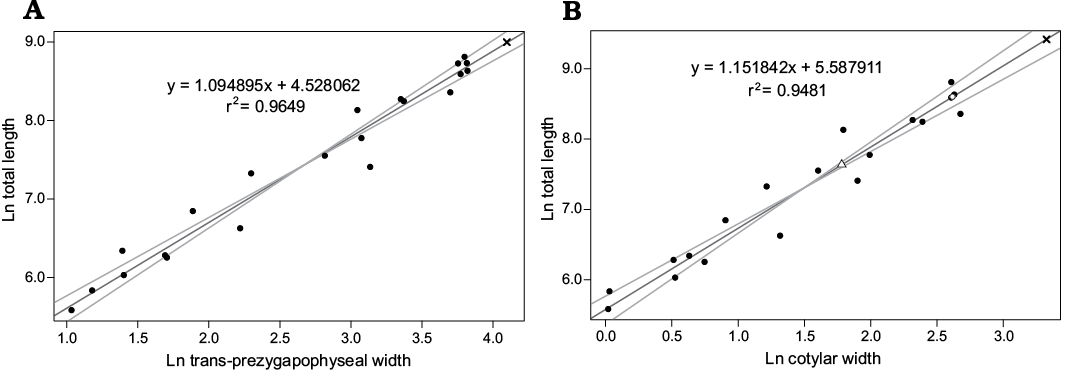
Fig. 8. Standard major axis regressions of vertebral metrics on total length in snakes. A. Regression of the width across the prezygapophyses on total length. Palaeophis colossaeus (CNRST-SUNY 290) is plotted as an × B. Regression of the width of the cotyle on total length. Palaeophis colossaeus (CNRST-SUNY 290) is plotted as an ×, Amananulam sanogoi gen. et sp. nov. (CNRST-SUNY 426) as an open triangle, and indeterminate snake CNRST-SUNY 324 as an open circle. Width across prezygapophyses is unavailable in the latter species due to damage.
Table 1. Measurements of fossil snakes from the Trans-Saharan Seaway in Mali. Estimated total length based on linear regression of vertebral measurements.
|
Species |
Specimen |
Transprezygapophyseal width (mm) |
Cotylar width (mm) |
Estimated total length (m) |
|
Palaeophis colossaeus |
CNRST-SUNY 290 |
60.2 |
– |
8.1 |
|
Palaeophis colossaeus |
NHMUK PV R 9870 |
– |
27.8 |
12.3 |
|
Amananulam sanogoi |
CNRST-SUNY 426 |
– |
5.9 |
2.1 |
|
gen. et sp. indet |
CNRST-SUNY 324 |
– |
13.7 |
5.4 |
Discussion
Body size.—Palaeophis colossaeus is a very large snake with vertebrae that are larger than any known living species, and are comparable in size to those of Gigantophis garstini from the upper Eocene of Egypt (Andrews 1901; McCartney and Seiffert 2016) and are smaller only than those of Titanoboa cerrejonensis from the Paleocene of Colombia (Head et al. 2009). The estimates of total length differ based on the metric used (8.1 m vs 12.3 m; Table 1), but in each case Palaeophis colossaeus is found to be larger than any other snake in the sample. Differences in estimates of total length can be partly attributed to the fact that different specimens preserved the largest example of each metric, and thus were probably derived from individuals of different actual length. Both specimens used (CNRST-SUNY 290 and NHMUK PV R 9870) were similar in overall size, although in measurements that could be taken on both specimens, NHMUK PV R 9870 was the larger. This result is in agreement with the higher estimate derived for that specimen.
The size of Amananulam sanogoi gen. et sp. nov. is substantially smaller than that of Palaeophis colossaeus, but it is nonetheless a surprisingly large nigerophiid. Its vertebral metrics exceed all species except “Nessovophis” zhylga from the Eocene of Kazakhstan, with which it is approximately equal in size (Averianov 1997). The remaining nigerophiids have vertebrae that are half as large or less than those of Amananulam sanogoi. The total length of Amananulam sanogoi is estimated here at approximately 2 m, placing it near the upper limit of the size range of extant sea snakes. The unidentified snake CNRST-SUNY 324 is intermediate in size between Palaeophis colossaeus and Amananulam sanogoi, estimated to be 5.4 m in total length. This length is well within the size range of extant large boids and pythonids (Murphy and Henderson 1997), and is probably an underestimate of length given that CNRST-SUNY 324 may be an anterior trunk vertebra, and thus would not be the largest vertebra within the column.
The size of an extinct snake has previously been used as a biological proxy for ancient temperatures (Head et al. 2009). The maximum body size within ectothermic clades is known to scale with average annual temperature, reflecting the need of ectothermic organisms to draw heat from their environment to drive metabolic reactions (Makarieva et al. 2005a, b). Increased ambient temperatures permit a higher basal metabolic rate in ectotherms; this increase can compensate for a drop in basal metabolic rate that occurs with increasing body size. The compensation results in a metabolic rate above the minimum value required to sustain life, even in extremely large individuals (Makarieva et al. 2005a). Head et al. (2009) used this relationship in snakes to estimate paleotemperature in the Paleocene Neotropics. The occurrence of the largest known snake, Titanoboa cerrejonensis, in the Paleocene Neotropics lead those authors to conclude that the average annual temperature was quite high (between 30–34°C), supporting hypotheses of warmer tropics during the global greenhouse of the early Paleogene (Kobashi et al. 2001; Pearson et al. 2001; Shellito et al. 2003; Zachos et al 2003).
The presence of three large snakes, including the largest known palaeophiid and a nigerophiid near that family’s maximum recorded size, would also suggest a warmer climate for the Paleogene tropics than today, albeit not as hot as the estimates of Head et al. (2009) for the Colombian Paleocene. However, there are several factors that make determining temperature from the vertebral size of the Malian species difficult. First is the uncertainty in the estimated body size of each species, resulting primarily from uncertainty in the vertebral position of the isolated fossils. For our estimates, the vertebrae are assumed to be the largest within an individual’s column, as is the case in the extant data set. It is unlikely that any of the vertebrae recovered happen to be the largest vertebra from an individual of each species, particularly in the cases of smaller sample sizes. This would tend to result in underestimates of size, and by extension would result in underestimates of temperature. Additionally, the number of vertebrae in the trunk of these fossil snakes is unknown, a variable that could potentially result in considerable under- or overestimates of size. Also of concern is the fact that these species were likely aquatic. There are no data specifically addressing the relationship between the size of the largest species of a clade and average temperature for aquatic tetrapods.
With these caveats, we tentatively suggest that the large size of snakes in the Trans-Saharan Seaway generally supports the hotter tropics hypotheses for early Paleogene climate (Kobashi et al. 2001; Pearson et al. 2001; Shellito et al. 2003; Zachos et al 2003), albeit to a lesser extent than in Head et al. (2009), that the Paleogene world was warmer than the present time. The Trans-Saharan Seaway has been reconstructed as a shallow tropical ecosystem near the paleo-equator (Petters 1979; Tapanila et al. 2008), and it would have provided a stable environment warm enough to permit the snakes to grow to a greater size than any snakes today.
Paleobiology.—Palaeophis species have historically been divided into two groups based on morphology, called the “primitive” grade, and the “advanced” grade (Rage et al. 2003). The “advanced” grade is distinguished by the greater lateral vertebral compression, taller pterapophyses, relatively small prezygapophyses, and more ventrally situated synapophyses. In the “primitive” grade, these features are less well developed or absent. The presence of these characters has been interpreted as indicating greater adaptation to aquatic life: the greater lateral compression and reduction in prezygapophyses serve to decrease the width of the vertebrae, and the shift of the synapophyses ventrally combined with their ventrally-facing facets permits the ribs to lie directly below the vertebrae. The effect of this morphology is to produce a narrow and deep body that is ideal for swimming, as in the tails of extant sea snakes. The increased height of the pterapophyses presumably accommodates an increase in muscle size without requiring the body to become wider. The implication is that snakes of the “primitive” grade were less well adapted to aquatic life, and had a more generalized body morphology (Rage and Wouters 1979). The polarity of these features, whether primitive or derived, awaits future phylogenetic studies.
Palaeophis colossaeus belongs to the “primitive” grade, and indeed shows the fewest features of the “advanced” grade characteristics of any species of Palaeophis. The vertebrae of Palaeophis colossaeus are extraordinarily broad, with neural arch width typically exceeding the length of the centrum in the mid-trunk region. The zygapophyses project strongly laterally, and in some cases the vertebrae have a ratio of trans-prezygapophyseal width to centrum length greater than 1.5. These features do not suggest a laterally compressed body. However, one feature present in Palaeophis colossaeus that does hint at aquatic living is the more ventrally-facing synapophysis (Rage 1983b: fig. 1). This morphology permits the ribs to lie more ventrally under the vertebrae rather than projecting directly laterally, thus producing a somewhat narrower habitus (Lucas 1898). Because no ribs are known for Palaeophis colossaeus it is impossible to know whether they were straight, and thus narrower, or curved outward, and thus somewhat broader. The presence of pterapophyses, albeit of unknown height, also suggest changes in the soft tissues related to swimming.
Based on the evidence we therefore suggest that Palaeophis colossaeus was at least somewhat adapted to life in water, and probably spent much time in or near water. The species has only been recorded from Tamaguélelt (Rage 1983b; this study) in phosphate beds indicative of highly productive, near-shore marine environments (Tapanila et al. 2008). The snake could therefore have been a nearshore marine species, a riverine or estuarine species that washed out, or flexible in its use of coastal waters.
In either fresh- or saltwater environments, the diet of Palaeophis colossaeus would only be restricted by what prey could be fit in its mouth. The question of how large that mouth was is not currently testable due to the complete dearth of cranial material for palaeophiine snakes. The skulls of two putatively closely related archaeophiine snakes are known, however. The skull of “Archaeophis” turkmenicus was described by Tatarinov (1988) as less kinetic than in many living snakes, potentially limiting the size of prey ingested. However, he also noted the elongation of the supratemporal and quadrate bones, leading to an enlarged gape that would permit consumption of large diameter food. In contrast, the skull of Archaeophis proavus was reconstructed by Janensch (1906b) as similarly kinetic to modern snakes, and thus presumably able to eat relatively large food. He also noted that the quadrates are short and anteriorly directed, limiting the gape size. The differences between these species render it difficult to infer anything about the skull of palaeophiids in general, and consequently their ability to consume relatively large prey. If the skull was relatively immobile, as in Archaeophis turkmenicus, then the substantial size attained by Palaeophis colossaeus may have been an adaptation that permitted consumption of large prey, simply by increasing the total size of the mouth. A similar adaptation has been suggested for some madtsoiid snakes (Wilson et al. 2010). If the skull of Palaeophis colossaeus was highly kinetic, the size of food consumed could be so large as to permit consumption of almost any known contemporaneous species. Regardless of the relative size of food consumed, there are many species that co-occur in the same beds or in other regional localities that would be potential prey for a giant snake, including a variety of pycnodont and dipnoan fish in both freshwater and marine realms (Patterson and Longbottom 1989; Longbottom 2010), dyrosaurid crocodylians (Hill et al. 2008), turtles (Gaffney et al. 2007), and mammals (Lavocat 1953; O’Leary et al. 2006), depending on the degree of terrestriality.
Extant giant snakes have few predators as adults. Reliable reports of lone predators capable of killing and eating very large snakes are limited to crocodylians and big felids; other smaller predators can succeed as well if they can hunt cooperatively (Pope 1961). Based on the estimated size of Palaeophis colossaeus, it is likely that there were very few predators of adult individuals. Among known contemporaneous animals, possible predators were probably limited to large dyrosaurids and sharks.
The vertebral morphology of nigerophiids shares several features with palaeophiids, and nigerophiids are also generally considered to be highly adapted aquatic snakes: they have narrow vertebrae, and synapophyses that face ventrally and in some cases are shifted ventrally as well. The vertebra of Amananulam sanogoi does not clearly preserve either of these features, but the width of the neural arch (NAW) is substantially less than the preserved centrum length (CL) (NAW:CL < 0.75), suggesting a relatively narrow vertebra. This narrowness combined with the great height of the vertebra produced by the upward sweep of the neural spine, is suggestive of aquatic habits (Rage and Werner 1999). Amananulam sanogoi was recovered from beds deposited in a shallow marine setting, similar to that of Palaeophis colossaeus. This suggests that it was a nearshore species, either marine, riverine, or some combination of these.
The challenges of considering diet are increased in Amananulam sanogoi because of the lack of cranial material for any nigerophiid, and the uncertainty of its phylogenetic position. It is impossible to say with any certainty whether nigerophiids were macrophagous, and thus possible inferences about the diet are limited. This extinct species was presumably carnivorous, and may have fed on an assortment of small vertebrates or invertebrates in its environment. In size it is substantially smaller than Palaeophis colossaeus, and as a consequence would have had a much greater risk of predation by other organisms.
Conclusions
The results of the study have expanded our understanding of the snake faunas of the Trans-Saharan Seaway by the addition of a new species of nigerophiid, Amananulam sanogoi gen. et sp. nov., and an indeterminate, non-palaeophiid species. The number of species is lower than some contemporaneous faunas (one species from the Paleocene, two from the Eocene), and include large, probably marine snakes. Smaller species, and those with stronger terrestrial tendencies are presumably less likely to be preserved in these settings, so it is to be hoped that further research in the Trans-Saharan Seaway will explore more terrestrial environments to expand our knowledge of the snake faunas from this time in Africa.
The size of these snakes, as estimated by vertebral measurements regressed on total body length, is notable: one of the largest known nigerophiids, and one of the largest known snakes lived in the Trans-Saharan Seaway at different times. The large size in these and other species naturally raises questions as to the reason for such regional gigantism. It is possible that the large size of these species is indicative of higher average temperatures in the region than are found in the tropics today.
Acknowledgements
We thank the members of the joint CNRST-SUNY expeditions to Mali for their work in collecting the fossil material. We thank Joseph Groenke (Stony Brook University, USA) for preparation of fossil material. Ronan Allain (MNHN), Sandra Chapman (NHMUK), David Kizirian (American Museum of Natural History, New York, USA), Stephen Rogers (CM), and Greg Watkins-Colwell (YPM) provided access to specimens in their care. Luci Betti-Nash (Stony Brook University) assisted with figure construction. Nathan Kley (Stony Brook University) and David Krause (Denver Museum of Nature and Science, USA) provided helpful discussions in the early stages of this research. The manuscript was improved by the insightful reviews of Jean-Claude Rage (MNHN) and Nick Longrich (University of Bath, UK). Funding for this research came in part from the L.S.B. Leakey Foundation, the Saurus Institute and the National Geographic Society, all of which funded fieldwork, as well as National Science Foundation grant NSF-EAR 0622359 to MAO’L and David Ferguson.
References
Andrews, C.W. 1901. Preliminary notes on some recently discovered extinct vertebrates from Egypt. (Part II.). Geological Magazine 8: 436–444. Crossref
Andrews, C.W. 1924. Note on some ophidian vertebrae from Nigeria. Geological Survey of Nigeria 7: 39–43.
Antunes, M.T. 1964. O Neocretácico e o Cenozóico do litoral de Angola. 254 pp. Junta de Investigações do Ultramar, Lisboa.
Arambourg, C. 1952. Les vertébrés fossiles des gisements de phosphates (Maroc, Algérie, Tunisie). Notes et Memoires du Service géologique du Maroc 92: 1–372.
Averianov, A.O. 1997. Paleogene sea snakes from the eastern part of Tethys. Russian Journal of Herpetology 4: 128–142.
Bajpai, S. and Head, J.J. 2007. An early Eocene palaeopheid snake from Vastan Lignite Mine, Gujarat, India. Gondwana Geological Magazine 22: 85–90.
Bellion, Y., Saint-Marc, P., and Damotte, R. 1989. Contribution à la connaissance des dépôts marins au passage Crétacé–Tertiaire dans la vallée du Tilemsi (Nord-Mali). Journal of African Earth Sciences 9: 187–194. Crossref
Bogert, C.M. 1964. Snakes of the genera Diaphorolepis and Synophis and the colubrid subfamily Xenoderminae. Senckenbergiana Biologica 45: 509–531.
Brochu, C.A., Bouare, M.L., Sissoko, F., Roberts, E.M., and O’Leary, M.A. 2002. A dyrosaurid crocodyliform braincase from Mali. Journal of Paleontology 76: 1060–1071. Crossref
Burke, K. 1996. The African Plate. South African Journal of Geology 99: 341–409.
Claeson, K.M., O’Leary, M.A., Roberts, E.M., Sissoko, F., Bouaré, M., Tapanila, L., Goodwin, D., and Gottfried, M.D. 2010. First Mesozoic record of the stingray Myliobatis wurnoensis from Mali and a phylogenetic analysis of Myliobatidae incorporating dental characters. Acta Palaeontologica Polonica 55: 655–674. Crossref
Cope, E.D. 1868. On some Cretaceous reptilia. Proceedings of the Philadelphia Academy of Natural Science 20: 233–242.
Erickson, B.R. 1998. A palaeophid from the Late Paleocene of South Carolina. In: A.E. Sanders (ed.), Transactions of the American Philosophical Society, New Series 88 (4): 215–220. Crossref
Gaffney, E.S., Roberts, E., Sissoko, F., Bouare, M.L., Tapanila, L., and O’Leary, M.A. 2007. Acleistochelys, a new side-necked turtle (Pelomedusoides: Bothremydidae) from the Paleocene of Mali. American Museum Novitates 3549: 1–24. Crossref
Graham, J.B., Lowell, W.R., Rubinoff, I., and Motta, J. 1987. Surface and subsurface swimming of the sea snake Pelamis platurus. Journal of Experimental Biology 127: 27–44.
Greigert, J. 1966. Description des formations Crétacées et Tertiaires du bassin des Iullemmeden (Afrique occidentale). Direction des Mines et de la Géologie, Niger 2: 1–273.
Head, J.J. 2005. Snakes of the Siwalik Group (Miocene of Pakistan): systematics and relationship to environmental change. Palaeontologia Electronica 8 (1): 1–33.
Head, J.J., Bloch, J.I., Hastings, A.K., Bourque, J.R., Cadena, E.A., Herrera, F.A., Polly, P.D., and Jaramillo, C.A. 2009. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature 457: 715–717. Crossref
Hill, R.V., McCartney, J.A., Roberts, E., Bouaré, M., Sissoko, F., and O’Leary, M.A. 2008. Dyrosaurid (Crocodyliformes: Mesoeucrocodylia) fossils from the Upper Cretaceous and Paleogene of Mali: implications for phylogeny and survivorship across the K/T boundary. American Museum Novitates 3631: 1–19. Crossref
Hoffstetter, R. 1955. Les Serpents marins de l’Eocène. Compte Rendu Sommaire des Séances de la Société Géologique de France 1–2: 16–19.
Hoffstetter, R. 1958. Un serpent marin du genre Pterosphenus (Pt. sheppardi nov. sp.) dans l’Éocène superieure de l’Équateur (Amérique du Sud). Bulletin de la Société Géologique de France 8: 45–50.
Hoffstetter, R. and Gayrard, Y. 1964. Observations sur l’ostéologie et la classification des Acrochordidae (Serpentes). Bulletin du Muséum National d’Histoire Naturelle 36: 677–696.
Holman, J.A. 1982. Palaeophis casei, new species, a tiny palaeophid snake from the early Eocene of Mississippi. Journal of Vertebrate Paleontology 2: 163–166. Crossref
Holman, J.A. and Case, G.R. 1992. A puzzling new snake (Reptilia: Serpentes) from the Late Paleocene of Mississippi. Annals of the Carnegie Museum 61: 197–205.
Houssaye, A., Rage, J.-C., Bardet, N., Vincent, P., Amaghzaz, M., and Meslouh, S. 2013. New highlights about the enigmatic marine snake Palaeophis maghrebianus (Palaeophiidae; Palaeophiinae) from the Ypresian (Lower Eocene) phosphates of Morocco. Palaeontology 56: 647–661. Crossref
Hutchison, J.H. 1985. Pterosphenus cf. P. schucherti Lucas (Squamata, Palaeophidae) from the Late Eocene of Peninsular Florida. Journal of Vertebrate Paleontology 5: 20–23. Crossref
Janensch, W. 1906a. Pterosphenus schweinfurthi Andrews und die Entwicklung der Palaeophiden. Archiv für Biontologie 1: 307–350.
Janensch, W. 1906b. Über Archaeophis proavus Mass., eine Schlange aus dem Eocän des Monte Bolca. Beiträge zur Paläontologie und Geologie Österreich-Ungarns und des Orients 19: 1–33.
Kobashi, T., Grossman, E.L., Yancey, T.E., and Dockery III, D.T. 2001. Reevaluation of conflicting Eocene tropical temperature estimates: molluskan oxygen isotope evidence for warm low latitudes. Geology 29: 983–986. Crossref
Kristensen, H.V., Cuny, G., Rasmussen, A.R., and Madsen, H. 2012. Earliest record of the fossil snake Palaeophis from the Paleocene/Eocene boundary in Denmark. Bulletin de la Société Géologique de France 183: 621–625. Crossref
LaDuke, T.C. 1991. The fossil snakes of Pit 91, Rancho La Brea, California. Contributions in Science, Natural History Museum of Los Angeles County 424: 1–28.
LaDuke, T.C., Krause, D.W., Scanlon, J.D., and Kley, N.J. 2010. A Late Cretaceous (Maastrichtian) snake assemblage from the Maevarano Formation, Mahajanga Basin, Madagascar. Journal of Vertebrate Paleontology 30: 109–138. Crossref
Lavocat, R. 1953. Sur la présence de quelques restes de mammifères dans le bone-bed Éocène de Tamaguilel (Soudan Français). Société géologique de France. Compte rendu sommaire des Séances 7–8: 409–410.
Legendre, P. 2014. lmodel2: Model II Regression, Version R Package Version 1.7-2.
Lingham-Soliar, T. 1991. Mosasaurs from the Upper Cretaceous of Niger. Palaeontology 34: 653–670.
Longbottom, A. 2010. A new species of the catfish Nigerium from the Palaeogene of the Tilemsi Valley, Republic of Mali. Palaeontology 53: 571–594. Crossref
Lucas, F.A. 1898. A new snake from the Eocene of Alabama. Proceedings of the United States National Museum 21: 637–638. Crossref
Lynn, W.G. 1934. A new snake (Palaeophis virginianus) from the Eocene of Virginia. Johns Hopkins University Studies in Geology 11: 245–249.
Makarieva, A.M., Gorshkov, V.G., and Li, B.-L. 2005b. Gigantism, temperature and metabolic rate in terrestrial poikilotherms. Proceedings of the Royal Society B 272: 2325–2328. Crossref
Makarieva, A.M., Gorshkov, V.G., and Li, B.-L. 2005a. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos 111: 425–436. Crossref
Malnate, E.V. 1972. Observations on the vertebral hypapophyses and associated musculature in some snakes, with special reference to the Colubridae. Zoologische Mededelingen 47: 225–239.
Marsh, O.C. 1869. Description of a new and gigantic fossil Serpent (Dinophis grandis), from the Tertiary of New Jersey. American Journal of Science, 2nd Series 48: 397–400.
McCartney, J.A. and Seiffert, E.R. 2016. A late Eocene snake fauna from the Fayum Depression, Egypt. Journal of Vertebrate Paleontology 36: e1029580. Crossref
Murphy, J.C. and Henderson, R.W. 1997. Tales of Giant Snakes: A Historical Natural History of Anacondas and Pythons. 221 pp. Krieger Publishing, Malabar, FL.
Nessov, L.A. and Udovitschenko, N.I. [Udoviŝenko, N.I.] 1984. Paleogene sea snakes and elasmobranch fishes of south Kazakhstan [in Russian]. Paleontologičeskij Sbornik 21: 69–74.
O’Leary, M.A., Roberts, E.M., Bouaré, M., Sissoko, F., and Tapanila, L. 2006. Malian Paenungulata (Mammalia: Placentalia): new African afrotheres from the early Eocene. Journal of Vertebrate Paleontology 26: 981–988. Crossref
O’Leary, M.A., Roberts, E.M., Head, J.J., Sissoko, F., and Bouaré, M.L. 2004. Titanosaurian (Dinosauria: Sauropoda) remains from the “Continental Intercalaire” of Mali. Journal of Vertebrate Paleontology 24: 923–930. Crossref
Owen, R. 1841. Description of some Ophidiolites (Palaeophis toliapicus) from the London Clay at Sheppey, indicative of an extinct species of serpent. Transactions of the Geological Society 6: 209–210. Crossref
Owen, R. 1850. Monograph on the Fossil Reptilia of the London Clay. Part II. Crocodilia, Ophidia. 68 pp. Palaeontographical Society, London. Crossref
Parmley, D. and Case, G.R. 1988. Palaeopheid snakes from the Gulf Coastal region of North America. Journal of Vertebrate Paleontology 8: 334–339. Crossref
Parmley, D. and DeVore, M. 2005. Palaeopheid snakes from the Late Eocene Hardie Mine local fauna of central Georgia. Southeastern Naturalist 4: 703–722. Crossref
Patterson, C. and Longbottom, A.E. 1989. An Eocene amiid fish from Mali, West Africa. Copeia 1989 (4): 827–836. Crossref
Pattishall, A. and Cundall, D. 2008. Dynamic changes in body form during swimming in the water snake Nerodia sipedon. Zoology 111: 48–61. Crossref
Pearson, P.N., Ditchfield, P.W., Singano, J., Harcourt-Brown, K.G., Nicholas, C.J., Olsson, R.K., Shackleton, N.J., and Hall, M.A. 2001 Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature 413: 481–487. Crossref
Petters, S.W. 1979. Stratigraphic history of the south-central Saharan region. Geological Society of America Bulletin 90: 753–760. Crossref
Pope, C.H. 1961. The Giant Snakes. 296 pp. Alfred A. Knopf, New York.
Pritchard, A., McCartney, J.A., Krause, D.W., and Kley, N.J. 2014. New snakes from the Upper Cretaceous (Maastrichtian) Maevarano Formation, Mahajanga Basin, Madagascar. Journal of Vertebrate Paleontology 34: 1080–1093. Crossref
R Core Team 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Radier, H. 1959. Contribution à l’étude géologique du Soudan oriental (A.O.F.), 2: Le Bassin Crétacé et Tertiaire de Gao, le Détroit Soudanais. Bulletin du Service de Géologie et de Prospection Minière 26: 309–556.
Rage, J.-C. 1975. Un serpent du Paléocène du Niger. Etude préliminaire sur l’origine des Caenophidiens (Reptilia, Serpentes). Comptes rendus de l’Académie des sciences de Paris 281: 515–518.
Rage, J.-C. 1980. Un Serpent marin nouveau de l’Éocène de Belgique. Le problème des Serpents marins du Paléogène. Comptes rendus de l’Académie des sciences de Paris 291: 469–471.
Rage, J.-C. 1983a. Les serpents aquatiques de l’Eocène européen. Définition des espèces et aspects stratigraphiques. Bulletin du Muséum national d’histoire naturelle, 4e séries, section C 5: 213–241.
Rage, J.-C. 1983b. Palaeophis colossaeus nov. sp. (le plus grand serpent connu?) de l’Éocène du Mali et le problème du genre chez les Palaeopheinae. Comptes rendus des séances de l’Académie des sciences de Paris 296: 1029–1032.
Rage, J.-C. 1984. Serpentes. Handbuch der Paläoherpetologie, Vol. 11. 80 pp. Gustav Fischer Verlag, Stuttgart.
Rage, J.-C. 2013. Mesozoic and Cenozoic squamates of Europe. Palaeobiodiversity and Palaeoenvironments 93: 517–534. Crossref
Rage, J.-C. and Dutheil, D.B. 2008. Amphibians and squamates from the Cretaceous (Cenomanian) of Morocco. Palaeontographica Abteilung A 285: 1–22. Crossref
Rage, J.-C. and Prasad, G.V.R. 1992. New snakes from the late Cretaceous (Maastrichtian) of Naskal, India. Neues Jahrbuch für Geologie und Paläontologie. Abhandlungen 187: 83–97.
Rage, J.-C. and Werner, C. 1999. Mid-Cretaceous (Cenomanian) snakes from Wadi Abu Hashim, Sudan; the earliest snake assemblage. Palaeontologia Africana 35: 85–110.
Rage, J.-C. and Wouters, G. 1979. Decouverte du plus ancien palaeopheide (Reptilia, Serpentes) dans le Maestrichtien du Maroc. Geobios 12: 293–296. Crossref
Rage, J.-C., Bajpai, S., Thewissen, J.G.M., and Tiwari, B.N. 2003. Early Eocene snakes from Kutch, Western India, with a review of the Palaeophiidae. Geodiversitas 24: 695–716.
Rage, J.-C., Folie, A., Rana, R.S., Singh, H., Rose, K.D., and Smith, T. 2008. A diverse snake fauna from the early Eocene of Vastan Lignite Mine, Gujar5at, India. Acta Palaeontologica Polonica 53: 391–403. Crossref
Reyment, R.A. 1980. Biogeography of the Saharan Cretaceous and Paleocene epicontinental transgressions. Cretaceous Research 1: 299–327. Crossref
Reyment, R.A. and Dingle, R.V. 1987. Palaeogeography of Africa during the Cretaceous Period. Palaeogeography, Palaeoclimatology, Palaeoecology 59: 93–116. Crossref
Ricker, W.E. 1973. Linear regressions in fishery research. Journal of the Fisheries Research Board of Canada 30: 409–434. Crossref
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. Crossref
Shellito, C.J., Sloan, L.C., and Huber, M. 2003. Climate model sensitivity to atmospheric CO2 levels in the early–middle Paleogene. Palaeogeography, Palaeoclimatology, Palaeoecology 193: 113–123. Crossref
Smith, A.B. and Jeffery, C.H. 2000. Maastrichtian and Palaeocene echinoids of the world: an illustrated key. Special Papers in Palaeontology 63: 1–404.
Smith, T., Kumar, K., Rana, R.S., Folie, A., Solé, F., Noiret, C., Steeman, T., Sahni, A., and Rose, K.D. 2016. New early Eocene vertebrate assemblage from western India reveals a mixed fauna of European and Gondwana affinities. Geoscience Frontiers 7: 1–33. Crossref
Snetkov, P.B. 2011. Vertebrae of the sea snake Palaeophis nessovi Averianov (Acrochordoidea, Palaeophiidae) from the Eocene of Western Kazakhstan and phylogenetic analysis of the superfamily Acrochordoidea. Palaeontological Journal 45: 305–313. Crossref
Tapanila, L., Roberts, E.M., Bouaré, M.L., Sissoko, F., and O’Leary, M.A. 2008. Phosphate taphonomy of bone and coprolite conglomerates: a case study from the Eocene of Mali, NW Africa. Palaios 23: 139–152. Crossref
Tatarinov, L.P. 1963. First discovery of ancient sea snakes in the USSR [in Russian]. Paleontologičeskij žurnal 1963 (2): 109–115.
Tatarinov, L.P. 1988. The structure of the skull of the sea snake “Archaeophis” turkmenicus from the Lower Eocene of Turkmenistan [in Russian]. Paleontologičeskij žurnal 1988 (1): 75–82.
Westgate, J.W. and Gee, C.T. 1990. Paleoecology of a middle Eocene mangrove biota (vertebrates, plants, and invertebrates) from southwest Texas. Palaeogeography, Palaeoclimatology, Palaeoecology 78: 163–177. Crossref
Westgate, J.W. and Ward, J.F. 1981. The giant aquatic snake Pterosphenus schucherti (Palaeophidae) in Arkansas and Mississippi. Journal of Vertebrate Paleontology 1: 161–164. Crossref
Wilson, J.A., Mohabey, D.M., Peters, S.E., and Head, J.J. 2010. Predation upon hatchling dinosaurs by a new snake from the Cretaceous of India. PLoS Biology 8 (3): 1–10. Crossref
Zachos, J.C., Wara, M.W., Bohaty, S., Delaney, M.L., and Petrizzo, M.R. 2003. A transient rise in tropical sea surface temperature during the Paleocene–Eocene thermal maximum. Science 302: 1551–1554. Crossref
Zouhri, S., Khalloufi, B., Bourdon, E., de Lapparent de Broin, F., Rage, J.-C., M’haïdrat, L., Gingerich, P.D., and Elboudali, N. 2017. Marine vertebrate fauna from the Late Eocene Samlat Formation of Ad-Dakhla, southwestern Morocco. Geological Magazine [published online, doi: 10.1017/S0016756817000759]. Crossref
Acta Palaeontol. Pol. 63 (2): 207–220, 2018
https://doi.org/10.4202/app.00442.2017