


A new aneuretine ant from the Paleocene Paskapoo Formation of Canada
JOHN S. LAPOLLA and PHILLIP BARDEN
While ants were present in the Cretaceous they were relatively rare. This changes dramatically from the Eocene onward when ants rise to the levels of global dominance seen in terrestrial ecosystems today. Their prevalence is well documented in various Eocene, Oligocene, and Miocene fossil deposits, but there is a conspicuous absence of ant-bearing fossil deposits around the Cretaceous–Paleogene boundary. In particular, until this study, there were no definitive ant-yielding deposits between 78 million and 55 million years ago, a 23-million-year gap. This gap is significant because, in addition to spanning a time when ant prevalence increases by an order of magnitude, the fossil rich deposits from the Eocene onward reveal a dramatically different ant fauna from that observed in the Mesozoic. The Paleocene Paskapoo Formation in Alberta, Canada has yielded a variety of fossil arthropod specimens, including a single worker ant fossil. Examination of this fossil indicates it is a species belonging to the now monotypic and relictual ant subfamily Aneuretinae. Napakimyrma paskapooensis gen et. sp. nov. is here described, revealing a species exhibiting a number of interesting and unique morphological features. Its placement as an aneuretine is supported by the presence of a long anterior peduncle of the petiole. A total of 11 fossil aneuretine taxa are now known, but for several, their placement within the subfamily is highly uncertain and needs further study. A discussion of all fossil aneuretine taxa is provided. Given the uncertainty of the apomorphies defining the Aneuretinae, and the fact that fossil species suggest a greater historic morphological diversity for the subfamily than is present now in the single, living species, it is important to consider the need for flexibility in defining synapomorphies for Aneuretinae and any such analysis will need to involve reevaluation of the fossil fauna.
Introduction
The ubiquity of ants today in diverse terrestrial habitats across the globe establishes them as one of the most dominant insect groups with broad implications for community and ecosystem functioning. Based on the existing fossil record, their rise to global dominance began in the Eocene with diversification and increases in abundance accelerating through the Oligocene and Miocene (Grimaldi and Agosti 2000; Dlussky and Rasnitsyn 2002; LaPolla et al. 2013). This is despite the fact that ants were present as far back as at least the mid-Cretaceous; ants appear to have been a relatively modest component of terrestrial ecosystems in the Mesozoic (Nel et al. 2004; Perrichot et al. 2008; Barden and Grimaldi 2014). Cretaceous ant communities were largely comprised of stem-group lineages, which filled surprisingly diverse ecological roles (Barden and Grimaldi 2016), but all of which presumably did not survive into the Cenozoic following the Cretaceous–Paleogene mass extinctions. While the ant fossil record is rich, with over 700 described species from nearly 70 deposits (Barden 2017), there is a conspicuous absence of windows into the Paleocene. Until now, no definitive ant-yielding deposits were known from any ages between ~78 million-year-old Canadian Medicine Hat amber, the last glimpse into the Cretaceous and stem-ant lineages (McKellar et al. 2013), and ~55 million-year-old Mø clay from the Fur Formation in Denmark (Rust and Anderson 1999). Including the single species described from the Fur Formation, all Cenozoic ant fossils are so-called crown-ants and possess the same synapomorphies found in modern lineages. A potential exception to this may be a Paleocene species from Menat, France currently classified as an eumenine wasp, but that Archibald et al. (2018) speculated was a putative ant. Any fossil ant evidence from the Paleocene is likely to yield important insights into a critical period of time following the close of the Mesozoic era when the composition of the ant fauna changed markedly, preceding a global rise to ecological dominance.
The Paskapoo Formation in Alberta, Canada is of Paleocene age, and has yielded a variety of fossil arthropod specimens (Wighton 1980; Kevan et al. 1981; Baker and Wighton 1984; Wighton and Wilson 1986). Mitchell and Wighton (1979) first reported on a possible ant specimen from the Paskapoo Formation, but over the subsequent decades no further study was completed on the fossil. Here we describe this ant specimen. The specimen is of particular interest with respect to fossil ants because the age of the Paskapoo Formation has been estimated to be of Tiffanian to late Paleocene (60.2–56.0 million years old) (Fox 1990; Lerbekmo and Sweet 2000), placing it well within a critical time period after the Cretaceous—within the 23-million-year long gap between the last window into ant evolution in the Cretaceous and the heretofore earliest Cenozoic ants known from the Fur Formation. The paleoclimate of the Alberta Plains of the Paleocene was likely warm-temperate, for which Fox (2011) speculated a similar modern habitat would be the current humid coastal plain of the southeastern United States. Based on a collection of largely aquatic adult and larval insect fossils, Mitchell and Wighton (1979) suggested the Blackfalds site where the fossil ant was discovered was a forested backswamp (Fig. 1).
Fig. 1. Map of Western Canada showing type locality (asterisked) of Napakimyrma paskapooensis.
Institutional abbreviations.—UAIPC, University of Alberta Invertebrate Paleontology Collection, Edmonton, Canada.
Other abbreviations.—APS, length of anterior face of petiolar scale: length of anterior portion of petiole from posterior portion of peduncle to tip of node; GL, maximum gaster length: maximum length of gaster from most anterior to most posterior point; GW, maximum gaster width: maximum length of gaster from most ventral to most dorsal point; HL, head length: maximum length of head in lateral view, excluding mandibles; LHW, lateral head width: maximum width of head in lateral view; ML, mesosoma length: in lateral view, the distance from the posteriormost border of the putative metapleural lobe to the anteriormost border of the pronotum, excluding the neck; PDH, propodeum height: height of the propodeum as measured in lateral view from the base of the metapleuron to the maximum height of the propodeum; PPS, length of posterior face of petiolar scale: length of posterior portion of petiole from tip of node to margin with gaster; PRH, pronotum height: in lateral view, the maximum height from the furthest ventral point of the pronotal margin to the furthest dorsal point of the pronotal margin; TL, total length: HL+ML+GL.
Material and methods
This study is based on a single compression fossil ant from fine-grained argillaceous limestone of the Paskapoo Formation (Alberta, Canada). The specimen was collected from the Blackfalds site of Mitchell and Wighton (1979; Fig. 1) and is deposited in the University of Alberta Fossil Insect Collection. The fossil specimen was immersed in 95% ethanol for examination and photography. All measurements were taken using a Leica MZ16 dissecting microscope with a stage micrometer, recorded to the nearest 0.001 mm, and rounded to two decimal places for presentation. Digital color images were created using a Q-imaging digital camera attached to a Leica MZ16 dissecting microscope and Syncroscopy Auto-Montage software.
Systematic palaeontology
Family Formicidae Latreille, 1809
Subfamily Aneuretinae Emery, 1913
Genus Napakimyrma nov.
Type species: Napakimyrma paskapooensis sp. nov.; by monotypy, see below.
Etymology: Combination of the Cree (indigenous people of Alberta) napaki, flat and the Greek myrma, ant.
Diagnosis.—As for the type species by monotypy.
Napakimyrma paskapooensis sp. nov.
Fig. 2.
Etymology: Toponym for the Paskapoo geological formation.
Holotype: UAIPC 6300, part (Fig. 2A–C) and counterpart (the part holds nearly the entire specimen; the counterpart consists of a small portion of the gaster and the faintest outline of part of a leg). Worker specimen laterally compressed.
Type locality: Blackfalds Insect and Plant Site, Dennis Wighton site 1, downstream from confluence with Blindman River on left bank of Red Deer River, Alberta, Canada.
Type horizon: Tiffanian to late Paleocene (Fox 1990; Lerbekmo and Sweet 2000).
Diagnosis.—Large worker ant (TL: 6.8 mm) with extended anterior petiolar peduncle comprising approximately 50% of total petiole length and distinct angular dorsal propodeal margin; dorsal face of the propodeum long; petiolar node very high, ~2/3 of petiole length; petiole with gradually rounded ventral margin.
Description.—Laterally compressed worker specimen; overall large (TL: 6.8 mm), but due to preservation details of the cuticle such as sculpturing or setal patterns are not visible. Most head features are not clear but head may have been longer than broad based on appearance in lateral view, antennae not preserved; distal end of mandible visible along with part of an apical and an immediate subapical tooth which appear to be equal in size; anterior face of pronotum rises steeply toward dorsum (at ca. 45° angle); pronotum with prominent rounded dorsum; propleuron appears faintly visible as preserved in lateral view; metanotal area distinctly “v-shaped” with sharp posterior declivity; anterior portion of propodeum with distinct right angle leading to long, flat dorsal face; junction of dorsal and declivitous posterior propodeal faces meet at an obtuse angle; declivitous margin long and steep; propodeum height and length approximately equal. Portions of a single putative foreleg preserved as disarticulated femur, tibia, and portion of the tarsus; femur wide, approximately 2× width of tibia; single tibial spur visible. Metacoxa present and articulated behind and underneath the petiole as preserved. Petiole with long anterior peduncle inclined ventrally; petiolar node large and triangular; ventral margin of petiole slightly concave underneath node; petiole height at its greatest approximately 2/3 petiole length; gaster large and rotund; G1–G3 are visible on specimen. Measurements (in mm): TL, 6.8; HL, 1.57; LHW, 0.55; ML, 2.42; PRH, 1.14; PDH, 0.72; APS, 0.33; PPS, 0.35; GW, 2.39; GL, 2.81.
Remarks.—Because the gastral cuticle is significantly less rigid than that of thoracic segments in ants, the large size of the gaster appears to be largely preservational. The femur is probably distorted too, appearing wider than it would have been in life. This species is diagnosable from all other North American fossil ants by an extended anterior peduncle, sinuous ventral margin of petiole and angular dorsal propodeal margin.
Stratigraphic and geographic range.—Type locality and horizon only.
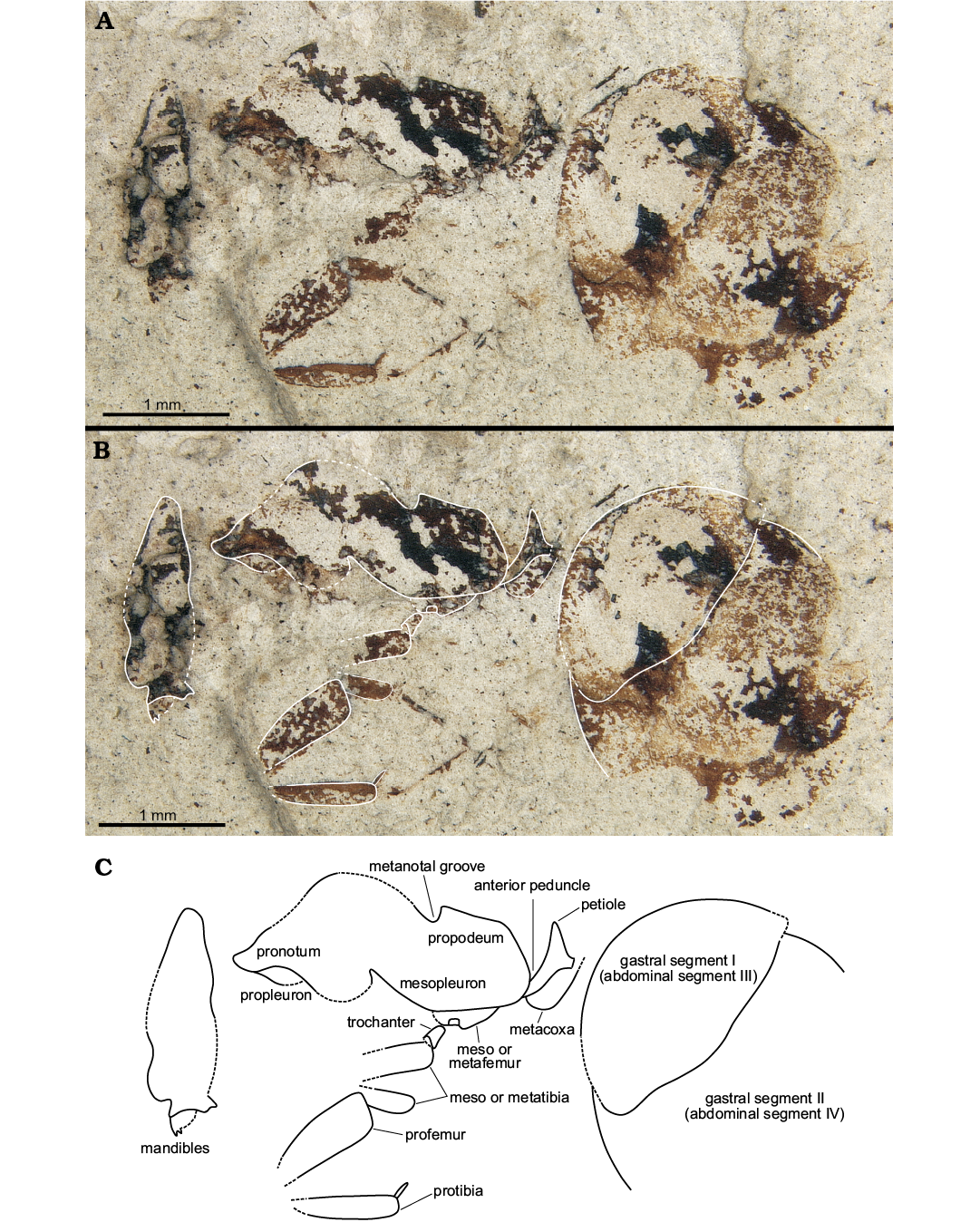
Fig. 2. Aneuretine ant Napakimyrma paskapooensis gen. et sp. nov. (holotype UAIPC 6300) from the Paleocene Paskapoo Formation at Dennis Wighton site 1 of Blackfalds Insect and Plant Site. Automontage generated photographs (A, B), line drawing (C).
Concluding remarks
There are three morphological apomorphies known for the Aneuretinae following Bolton (2003) and Boudinot (2015): (i) petiole with a long anterior peduncle; (ii) helcium attached high on anterior face of first gastral segment; (iii) postsclerites of first gastral segment (abdominal segment III) reduced. These apomorphies, however, are based largely on the extant Aneuretus simoni Emery, 1893, the only extant species known within the subfamily. For the discussion below we exclude Burmomyrma rossi Dlussky, 1996 (known from a single partial alate specimen from Burmese amber) from the Aneuretinae. Lucena and Melo (2018) provide convincing evidence that this species is not in fact an ant but rather belongs in the Falsiformicidae. Despite the fact there are now eight fossil genera formally placed within the Aneuretinae, there is disagreement regarding the validity of these extinct taxa, which may change the interpretation of apomorphies for aneuretines (Bolton 2003; Boudinot 2015). According to Boudinot (2015) only Paraneuretus and Protaneuretus, both known from Baltic amber, can be definitively ascribed to the Aneuretinae. If we consider the controversial placement of several of the fossil genera, apomorphies for the aneuretines become less certain, except perhaps for the petiole possessing a long anterior peduncle. In total, there are now 11 described aneuretine species from eight fossil deposits (Table 1) and indeed many of these assignments are not supported by the synapomorphies identified by Bolton (2003), particularly the elongated anterior peduncle. Cananeuretus occidentalis Engel and Grimaldi, 2005 from Campanian Medicine Hat amber of Canada appears to possess an elongate anterior peduncle however no clear lateral view is possible (Engel and Grimaldi 2005). Aneuretellus deformis Dlussky, 1988 from Russian Sakhalin amber possesses a slight or absent petiole node as figured, and so may possess an elongate peduncle, there are however, no images of the specimen itself (Dlussky 1988). It is worth noting that until recently Sakhalin amber was thought to be of Paleocene age, but a recent revaluation of the chironomid fauna of the deposit has suggested it may be of Eocene origin (Baranov et al. 2015). Mianeuretus eocenicus Dlussky and Rasnitsyn, 2002 from Green River is visible from above only preventing a clear assessment of petiole structure. From Baltic amber, Paraneuretus and Protaneuretus succineus Wheeler, 1915 appear as the clearest aneuretine taxa with definitively elongate peduncles. Pityomyrmex tornquisti Wheeler, 1915 from Baltic amber was originally placed within Dolichoderinae (Wheeler 1915) and later moved to Aneuretinae by Dlussky and Rasnisyn (2009), although the type is lost and neither the figure nor description indicate the structure of the petiole. Unlike its Baltic congener, Paraneuretus dubovikoffi Dlussky, Rasnitsyn, and Perfilieva, 2015 from Bol’shaya Svetlovodnaya in Russia is described as possessing a “short peduncle” and, while the figured specimen possesses an aneuretine-like petiole, there are no images of the specimen itself (Dlussky et al. 2015). Mianeuretus mirabilis Carpenter, 1930 from the Florissant Formation is preserved with a dorsal view only, restricting interpretations for petiole shape. Britaneuretus anglicus Cockerell, 1915 from the Bouldnor Formation was first described by Cockerell (1915) as Dolichoderus anglicus Cockerell, 1915 within the Dolichoderinae and recently placed within Aneuretinae by Dlussky and Perfilieva (2014) although described with “no petiolar node” and thus no peduncle. For our purposes here we will only consider Paraneuretus longicornis Wheeler, 1915, Paraneuretus tornquisti Wheeler, 1915, and Protaneuretus succineus Wheeler, 1915 plus Aneuretus as belonging to the Aneuretinae, while Aneuretellus deformis Dlussky, 1988 and Cananeuretus occidentalis Engel and Grimaldi, 2005 represent potential members of the subfamily that may be confirmed with further study.
Table 1. Described fossil anueretine taxa. * indicates large uncertainty regarding subfamily placement based on assumption of long anterior peduncle as a synapomorphy for Anueretinae; ** indicates plausible but unconfirmed placement.
|
Taxon |
Locality |
Age |
Reference |
|
Cananeuretus occidentalis** |
Medicine Hat Amber |
84.9–70.6 |
|
|
Napakimyrma paskapooensis |
Paskapoo Formation |
60.2–56.0 |
this study |
|
Mianeuretus eocenicus* |
Green River |
53.5–48.5 |
|
|
Aneuretellus deformis** |
Sakhalin Amber |
47.8–41.3 |
|
|
Paraneuretus longicornis |
Baltic Amber |
47.8–41.3 |
|
|
Paraneuretus tornquisti |
Baltic Amber |
47.8–41.3 |
|
|
Pityomyrmex tornquisti* |
Baltic Amber |
47.8–41.3 |
Wheeler 1915; Dlussky and Rasnitsyn 2002 |
|
Protaneuretus succineus |
Baltic Amber |
47.8–41.3 |
|
|
Paraneuretus dubovikoffi* |
Bol’shaya Svetlovodnaya |
37.2–33.9 |
|
|
Mianeuretus mirabilis* |
Florissant |
37.2–33.9 |
|
|
Britaneuretus anglicus* |
Bouldnor Formation, Isle of Wight |
37.2–33.9 |
Our placement of N. paskapooensis within the Aneuretinae is based primarily on the structure of the petiolar peduncle. There are no other subfamily placements that would accommodate the anterior peduncle coupled with an absence of other subfamilial synapomorphies (e.g., a constricted gaster indicating the specimen as potential member of the Ponerinae) observed in N. paskapooensis. Also, as is seen in Aneuretus, Paraneuretus, and Protaneuretus the peduncle is attached very low to the propodeum and is directed dorsally. The placement of the helcium is high in Aneuretus and Protaneuretus, but not in Paraneuretus. It appears the helcium is not placed high in N. paskapooensis either, but given the condition of the gaster it is difficult to be certain of its actual placement. Additionally, the overall shape of the mesosoma of N. paskapooensis resembles that of Aneuretus and Paraneuretus, in particular the angular anterior face of the propodeum is similar to A. simoni Emery, 1893. Napakimyrma paskapooensis has some unique morphological features worth noting. The dorsal face of the propodeum is unusually long, and this does not appear to be due to preservation but rather a reflection of its true shape. The node of the petiole is also very high, certainly the highest observed among any other aneuretines, although this could be exaggerated by preservational distortion. The high petiolar node and very large and round gaster initially suggested possible placement within the Formicinae, but the anterior petiolar peduncle combined with a petiole node height that is nearly equal to the total petiole length is unlike anything seen among the formicines. These unique features may hint at a greater historic diversity for the now monotypic Aneuretinae. As additional potential aneuretine fossil material is uncovered, it is important to consider the need for flexibility in defining synapomorphies for a presently relictual lineage that likely exhibited greater morphological variation in the past.
The placement of N. paskapooensis within Aneuretinae makes it the oldest known Cenozoic ant from any subfamily. There are at least four relatively diverse Cenozoic rock fossil deposits that contain ant fossils: Florissant (Carpenter 1930), Green River (Dlussky and Rasnitsyn 2002), Kishenehn (LaPolla and Greenwalt 2015), and Okanagan Highlands (Archibald et al. 2006, 2018). There are three other fossil aneuretines described from North American deposits: Cananeuretus occidentalis Engel and Grimaldi, 2005 from Canadian amber (Engel and Grimaldi 2005) and one species each of Mianeuretus from the Florissant (Carpenter 1930) and from the Green River (Dlussky and Rasnitsyn 2002) (but see above). Given the definitive presence of aneuretines from Baltic amber, their presence within the Eocene of North America remains plausible but the fossils warrant further investigation. Certainly, the discovery here of a Paleocene aneuretine increases the need to understand the diversity of the subfamily through time. Given the lack of Paleocene ant fossils, additional material from the Paskapoo will provide other windows into the fossil ant fauna which will ultimately provide a sharper picture of ant evolution at the beginning of the Cenozoic.
Acknowledgements.—We would like to thank Lindsey Leighton and Danny Shpeley (both University of Alberta, Edmonton, Canada) for their assistance in procuring the specimen for examination and study. Dale Greenwalt (Smithsonian Institution, Washington DC, USA) provided encouragement and contacts critical for initiation of the project. We thank Vincent Perrichot (Université de Rennes, France) and Alexander Radchenko (National Academy of Sciences of Ukraine, Kiev, Ukraine) for thoughtful and helpful reviews of the original manuscript. This project was supported in part by a grant from the American Philosophical Society awarded to JSL.
References
Antropov, A.V., Belokobylskij, S.A.,
Compton, S.G., Dlussky, G.M., Khalaim, A.I., Kolyada, V.A., Kozlov,
M.A., Perfilieva, K.S., and Rasnitsyn, A.P. 2014. The wasps, bees and
ants (Insecta: Vespida=Hymenoptera) from the Insect Limestone (Late
Eocene) of the Isle of Wight, UK. Earth and Environmental Science
Transactions of The Royal Society of Edinburgh 104 (3-4): 335–446.
Archibald, S.B., Cover, S.P., and Moreau, C.S. 2006. Bulldog ants of the Eocene Okanagan Highlands and history of the subfamily (Hymenoptera: Formicidae: Myrmeciinae). Annals of the Entomological Society of America 99: 487–523. Crossref
Archibald, S.B., Rasnitsyn, A.P., Brothers, D.J., and Mathewes, R.W. 2018. Modernisation of the Hymenoptera: ants, bees, wasps, and sawflies of the early Eocene Okanagan Highlands of western North America. The Canadian Entomologist 150: 205–257. Crossref
Baker, G. and Wighton, D. 1984. Fossil aquatic oribatid mites (Acari: Oribatida: Hydrozetidae: Hydrozetes) from the Paleocene of south-central Alberta, Canada. The Canadian Entomologist 116: 773–775. Crossref
Baranov, V., Andersen, T., and Perkovsky, E.E. 2015. Orthoclads from Eocene Amber from Sakhalin (Diptera: Chironomidae, Orthocladiinae). Insect Systematics & Evolution 46: 359–378. Crossref
Barden, P. 2017. Fossil ants (Hymenoptera: Formicidae): ancient diversity and the rise of modern lineages. Myrmecological News 14: 1–30.
Barden, P. and Grimaldi, D. 2014. A diverse ant fauna from the mid-Cretaceous of Myanmar (Hymenoptera: Formicidae). PLoS ONE 9: e93627. Crossref
Barden, P. and Grimaldi, D. 2016. Adaptive radiation in socially advanced stem-group ants from the Cretaceous. Current Biology 26: 515–521. Crossref
Bolton, B. 2003. Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute 71: 1–370.
Boudinot, B.E. 2015. Contributions to the knowledge of Formicidae (Hymenoptera, Aculeata): a new diagnosis of the family, the first global male-based key to subfamilies, and a treatment of early branching lineages. European Journal of Taxonomy 120: 1–62. Crossref
Carpenter, F.M. 1930. The fossil ants of North America. Bulletin of the Museum of Comparative Zoology 70: 1–66.
Cockerell, T.D.A. 1915. British fossil insects. Proceedings of the United States National Museum 49: 469–99. Crossref
Dlussky, G.M. 1988. Ants of (Paleocene?) Sakhalin amber. Paleontological Journal 22: 50–61.
Dlussky, G.M. 1996. Ants (Hymenoptera: Formicidae) from Burmese amber. Paleontological Journal 30: 449–454.
Dlussky, G.M. and Perfilieva, K.S. 2014. Superfamily Formicoidea Latreille, 1802. In: A.V. Antropov, S.A. Belokobylskij, S.G. Compton, G.M. Dlussky, A.I, Khalaim, V.A, Kolyada, M.A, Kozlov, K.S. Perfilieva, and A.P. Rasnitsyn (eds.), The Wasps, Bees and Ants (Insecta: Vespida = Hymenoptera) from the Insect Limestone (Late Eocene) of the Isle of Wight, UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 104: 335–446.
Dlussky, G.M. and Rasnitsyn, A.P. 2002. Ants (Hymenoptera: Formicidae) of formation Green River and some other Middle Eocene deposits of North America. Russian Entomological Journal 11: 411–436.
Dlussky, G.M. and Rasnitsyn, A.P. 2009. Ants (Insecta: Vespida: Formicidae) in the Upper Eocene amber of central and eastern Europe. Paleontological Journal 43: 1024–1042. Crossref
Dlussky, G.M., Rasnitsyn, A.P., and Perfilieva, K.S. 2015. The ants (Hymenoptera: Formicidae) of Bol’shaya Svetlovodnaya (Late Eocene of Sikhote-Alin, Russian Far East). Caucasian Entomological Bulletin 11: 131–152.
Emery, C. 1893. Untitled contribution introduced by: “M.C. Emery, de Bologne, envoie les diagnoses de cinq nouveaux genres de Formicides”. Bulletin Bimensuel de la Société Entomologique de France 20 (for 1892): cclxxv–cclxxvii.
Emery, C. 1913. Hymenoptera. Fam. Formicidae. Subfam. Dolichoderinae. Genera Insectorum 137: 1–50.
Engel, M.S. and Grimaldi, D.A. 2005. Primitive new ants in Cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera: Formicidae). American Museum Novitates 3485: 1–23. Crossref
Fox, R.C. 1990. The succession of Paleocene mammals in western Canada. In: T.M. Bown and K.D. Rose (eds.), Dawn of the Age of Mammals in the Northern Part of the Rocky Mountain Interior, North America. Geological Society of America, Special Paper 243: 51–70. Crossref
Fox, R.C. 2011. An unusual early primate from the Paleocene Paskapoo Formation, Alberta, Canada. Acta Palaeontologica Polonica 56: 1–10. Crossref
Grimaldi, D. and Agosti, D. 2000. A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proceedings of the National Academy of Sciences of the United States of America 97: 13678–13683. Crossref
Kevan, D., Keith M.E., and Wighton, D. 1981. Paleocene orthopteroids from south-central Alberta, Canada. Canadian Journal of Earth Sciences 18: 1824–1837. Crossref
LaPolla, J.S. and Greenwalt, D.E. 2015. Fossil ants (Hymenoptera: Formicidae) of the Middle Eocene Kishenehn Formation. Sociobiology 62: 163–174. Crossref
LaPolla, J.S., Dlussky, G.M., and Perrichot, V. 2013. Ants and the fossil record. Annual Review of Entomology 58: 609–630. Crossref
Latreille, P.A. 1809. Genera crustaceorum et insectorum secundum ordinem naturalem in familias disposita, iconibus exemplisque plurimus explicata. Tomus 4. 399 pp. A. Koenig, Parisiis et Argentorati [= Paris and Strasbourg].
Lerbekmo, J.F. and Sweet, A.R. 2000. Magnetostratigraphy and biostratigraphy of the continental Paleocene in the Calgary area, southwestern Alberta. Bulletin of Canadian Petroleum Geology 48: 285–306. Crossref
Lucena, D.A.A. and Gabriel, G.A.R. 2018. Chrysidid wasps (Hymenoptera: Chrysididae) from Cretaceous Burmese amber: Phylogenetic affinities and classification. Cretaceous Research 89: 279–291. Crossref
Lucena, D.A.A. and Melo, G.A.R. 2018. Chrysidid wasps (Hymenoptera: Chrysididae) from Cretaceous Burmese amber: Phylogenetic affinities and classification. Cretaceous Research 89: 279–291. Crossref
McKellar, R.C., Glasier, J.R.N., and Engel, M.S. 2013. A new trap-jawed ant (Hymenoptera: Formicidae: Haidomyrmecini) from Canadian Late Cretaceous amber. Canadian Entomologist 145: 454–465. Crossref
Mitchell, P. and Wighton, D. 1979. Larval and adult insect from the Paleocene of Alberta, Canada. The Canadian Entomologist 111: 777–782. Crossref
Nel, A., Perrault, G., Perrichot, V., and Néraudeau, D. 2004. The oldest ant in the lower Cretaceous amber of Charente-Maritime (SW France) (Insecta: Hymenoptera: Formicidae). Geologica Acta 2: 23–29.
Perrichot, V., Nel, A., Néraudeau, D., Lacau, S., and Guyot, T. 2008. New fossil ants in French Cretaceous amber (Hymenoptera: Formicidae). Naturwissenschaften 95: 91–97. Crossref
Rust, J. and Andersen, N.M. 1999. Giant ants from the Paleogene of Denmark with a discussion of the fossil history and early evolution of ants (Hymenoptera: Formicidae). Zoological Journal of the Linnean Society 125: 331–348. Crossref
Wheeler, W.M. 1915. The ants of the Baltic Amber. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg 55: 1–142.
Wighton, D. 1980. New species of Tipulidae from the Paleocene of central Alberta, Canada. The Canadian Entomologist 112: 621–628. Crossref
Wighton, D.C. and Wilson, M.V.H. 1986. The Gomphaeschninae (Odonata: Aeshnidae): new fossil genus, reconstructed phylogeny, and geographical history. Systematic Entomology 11: 505–522. Crossref
John S. LaPolla [jlapolla@towson.edu], Department of Biological Sciences, Towson University, Towson, MD 21252, USA.
Phillip Barden [barden@njit.edu], Department of Biological Sciences, New Jersey Institute of Technology, Newark, NJ 07102, USA.
Received 16 March 2018, accepted 22 May 2018, available online 27 July 2018.
Copyright © 2018 J.S. LaPolla and P. Barden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 63 (3): 435–440, 2018
https://doi.org/10.4202/app.00478.2018