

A new evidence of passing the Maastichtian–Paleocene boundary by larger benthic foraminifers: The case of Elazigina from the Maastrichtian Tarbur Formation of Iran
LORENZO CONSORTI and KOOROSH RASHIDI
Consorti, L. and Rashidi, K. 2018. A new evidence of passing the Maastichtian–Paleocene boundary by larger benthic foraminifers: The case of Elazigina from the Maastrichtian Tarbur Formation of Iran. Acta Palaeontologica Polonica 63 (3): 595–605.
We describe a new Maastrichtian species of the benthic foraminifer Elazigina siderea from Tarbur Formation. Its main characters are the presence of heavy feathered umbilical sutures, a wide umbilical plug, and umbilical piles. This species, formerly reported from Turkey as Smoutina cruysi, constitutes the oldest known record of the genus Elazigina. Elazigina siderea sp. nov. comes from the Arabian domain and its presence is probably related to the migration of the Cretaceous foraminifer Orbitokathina. Prior to this study, the oldest representatives of this genus were only known from the Paleocene. Therefore, the presence of the new taxon in the Maastrichtian suggests the genus Elazigina passed the Cretaceous–Paleogene boundary, and survived to the environmental crisis associated with a great biosphere mass extinction that wiped out most of the Late Cretaceous larger foraminifers. This is supported by shell features displayed by Elazigina siderea sp. nov., interpreted as adaptation to thrive under elevated trophic levels, like the species of another benthic foraminifer Laffitteina.
Key words: Foraminifera, Globothalamea, Rotaliida, extinction, Cretaceous, Iran.
Lorenzo Consorti [lorenzo.consorti.es@gmail.com], Department of Earth, Environmental and Resources Sciences, University Federico II, Complesso di Monte Sant’Angelo (Edificio L), Via Cinthia, 21, 80126 Naples, Italy.
Koorosh Rashidi [koo.rashidi@gmail.com], Department of Geology, Payame Noor University, Po Box 19395-3697, Tehran, Iran.
Received 16 April 2018, accepted 8 August 2018, available online 23 August 2018.
Copyright © 2018 L. Consorti and K. Rashidi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Cretaceous–Paleocene (K–Pg) boundary represents one of the most important biological crises in the Phanerozoic (Raup and Sepkoski 1986). Most Cretaceous planktonic foraminifers have been wiped out at the K–Pg boundary (Berggren and Norris 1997; Molina et al. 1998; Fuqua et al. 2008; Alegret and Thomas 2009 among others), whereas the small benthic foraminifers from bathyal or shallow-water settings were apparently less affected (Culver 2003; Alegret and Thomas 2013).
The complex-shelled symbiont bearing foraminifers (so-called larger foraminifers) also experienced severe changes at the K–Pg boundary (Brasier 1988; MacLeod et al. 1997). Several alveolinids, miliolids, rhapydioninids, loftusids, siderolitids, orbitolinids, rotaloineans, and orbitoids disappear toward the end of the Cretaceous, with 83% loss of the total Maastrichtian assemblage (Boudagher-Fadel 2008; Goldbeck and Langer 2009). This is arguably associated to changes in shallow shelves that have affected the photosynthetic potential or those environmental parameters critical for larger foraminifers existance (Leutenegger 1984; Hallock and Schlager 1986; Hohenegger 1995; Hallock 1999). The end-Maastrichtian lowstand and cooling (Keller et al. 1997; Hallam and Wignall 1999; Habib and Saeedi 2007; Haq 2014; Chenot et al. 2018) have possibly been the mechanisms reducing suitable larger foraminifers niches. Furthermore, the studies that have dealt with larger foraminifers in K–Pg carbonate platform series (Tewari et al. 2007; Ogorelec et al. 2007) report enhancing of terrestrial carbon input and possible shallow-water eutrophication. Nevertheless, demise of larger foraminifers seems not coincident with the Chicxulub asteroid impact and Deccan traps eruptions that are usually associated with the K–Pg mass extinction (Ogorelec et al. 2007; Font et al. 2018).
There are few examples of larger foraminifers, however, attesting the survival through K–Pg; one of them is the genus Laffitteina Marie, 1946. Widely present in the Maastrichtian from the Pyrenean basin to the Middle East platforms (Fig. 1), Laffitteina occurs also in the Paleocene of the Middle East (Rahaghi 1992; İnan et al. 2005) where its stratigraphic distribution through the Cretaceous and Paleocene carbonates is practically continuous (Hottinger 2014). Pararotalia Le Calvez, 1949, Rotorbinella Brandy, 1944, Daviesina Smout, 1954, and Rotalispira Hottinger, 2014, are also listed among the scarce survivors, but they re-appear later in the Paleogene or Eocene, after some million years long community recuperation period (Global Community Maturation Cycle [GCMC]; sensu Hottinger 2001). Notwithstanding, the list of survivors is not exhaustive.
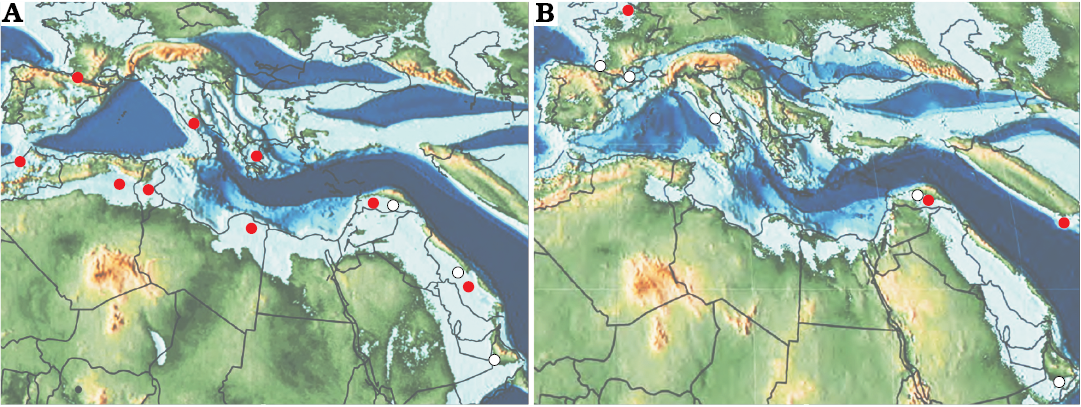
Fig. 1. Paleogeographic distribution of Elazigina (open circles) and Laffitteina (red circles) in latest Maastrichtian (A) and Paleocene (B). Records for the Maastrichtian are from Rahaghi (1992); Inan (1988, 2005); Schlüter et al. (2008); Goldbeck and Langer (2009); Hottinger (2014 and the references herein); and this work. Records for the Paleocene are from Rahaghi (1992); Inan (2005); Hottinger (2014 and the references herein); Serra-Kiel et al. (2016); and Benedetti et al. (2018). Maps are from Scotese (2013, 2014).
New sampling of the Maastrichtian Tarbur formation from the High Zagros zone (SW Iran) shows the occurrence of a rotalid morphotype surprisingly close to the genus Elazigina Sirel, 2012. Until now four species of Elazigina (informally referred to Hottinger 2014 as Plumokathina) have been described in the Paleocene of the Neotethys (Hottinger 2014): Elazigina dienii (Hottinger, 2014), E. lenticula (Hottinger, 2014), E. subsphaerica (Sirel, 1972) and E. harabekayisensis Sirel, 2012, from the Pyrenees to the Middle East (Serra-Kiel et al. 2016; Benedetti et al. 2018). However, no representatives of Elazigina have been reported from the Cretaceous.
Previously recorded in Turkey under the name Smoutina cruysi by İnan (1988), the Maastrichtian Elazigina morphotype is extremely abundant in Tarbur Formation, allowing a detailed architectural study that is here presented, resulting in the description of a new species: Elazigina siderea sp. nov. The new record here reported and the spreading of Elazigina before and after the K–Pg boundary shed light on the capability of some larger foraminifers to survive the environmental changes associated, in one way or another, to the end-Cretaceous mass extinction.
Institutional abbreviations.—APNU, Ardakan Payame Noor University, Collection Rashidi, Department of Geology, Teheran, Iran.
Other abbreviations.—GCMC, Global Community Maturation Cycle; K–Pg, Cretaceous–Paleogene.
Geological setting
The Tarbur Formation (James and Wynd 1965) crops out in the Zagros basin along the western limb of the Zagros zone, between the main Zagros fault and the Sabzposhan fault (Alavi 2004). The formation, composed of a thick rock pack (20–450 m) of marl and limestone, represents a Maastrichtian carbonate platform mainly characterized by rudists and foraminifers (Piryaei et al. 2010; Bakhtiar et al. 2011). The development of these carbonates ran parallel to the Late Cretaceous migration of the Zagros thrust emplacement deposited along the borders of foreland basin, on the top of forebulge areas and in vertical contact (or sometimes in etheropy) with the shale of Gurpi Formation that represents the foredeep sedimentation (Piryaei et al. 2010; Saura et al. 2011).
Outcrops of the Tarbur formation studied herein are from two localities of the Fars region nearby of Mandegan village (Fig. 2). Here, Tarbur Formation overlies the Gurpi Formation and is overlain by conglomerate deposits of the Bakhtiari Formation (Pliocene). Three lithostratigraphic units encompass the Mandegan section (Fig. 3). The first unit is mostly composed of thick-bedded limestone. The second one comprises medium-bedded limestone with marly limestone intercalations. The third unit, representing the end of Maastrichtian carbonate sedimentation in the area, is characterised by marly lenses. The new species of Elazigina appears in the middle part of the first unit. The Greenwich coordinates of the Mandegan section base are N 31°25’8.13” and E 51°24’34.58”.
The studied section bearing the type level of the new species of Elazigina is located in the Rod-Abad section. The section is positioned 6.5 km far to the NE of Mandegan village, very close to the village of Ab-Malakh, where the lower first unit could be sampled (Fig. 2). The Rod-Abad section (coordinates at the base: N 31° 8’32.46” and E 51°23’51.30”) is very rich in Elazigina. It records four short sea-level transgressive-regressive pulses represented by four marly levels rich in rudists in living-position intercalated with massive limestone. According to Bakhtiar et al. (2011), the marine paleoenvironment of Rod-Abad is shallower than in Mandegan.
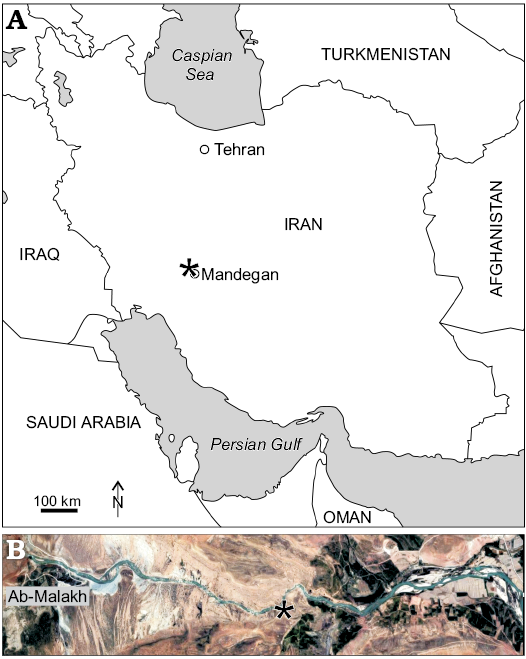
Fig. 2. Position (asterisked) of studied localities in the general map of Iran (A) and position of Rod-Abad section (B).
Material and methods
The foraminiers studied herein come from 17 samples of cemented carbonate rocks (labelled Tf1 to Tf15) of the Rod-Abad section (for sample distribution see Fig. 3). Carbonates were processed to obtain thin sections. Additional rocks, labelled Rt53 and Rt55, used for comparison come from the Mandegan section (see also Consorti et al. 2018: fig. 1C). More than 230 random and oriented sections of foraminifers, obtained and photographed from these samples, were taken into consideration for the present study. All the specimens are illustrated with a fixed enlargement in order to facilitate the comparison of the new taxa with other previously described. The specialised terms used for the architectural analysis of the rotaloidean foraminifers come from Hottinger (2006, 2014). The section orientation has been named according to Billman et al. (1980) and Hottinger (2014).
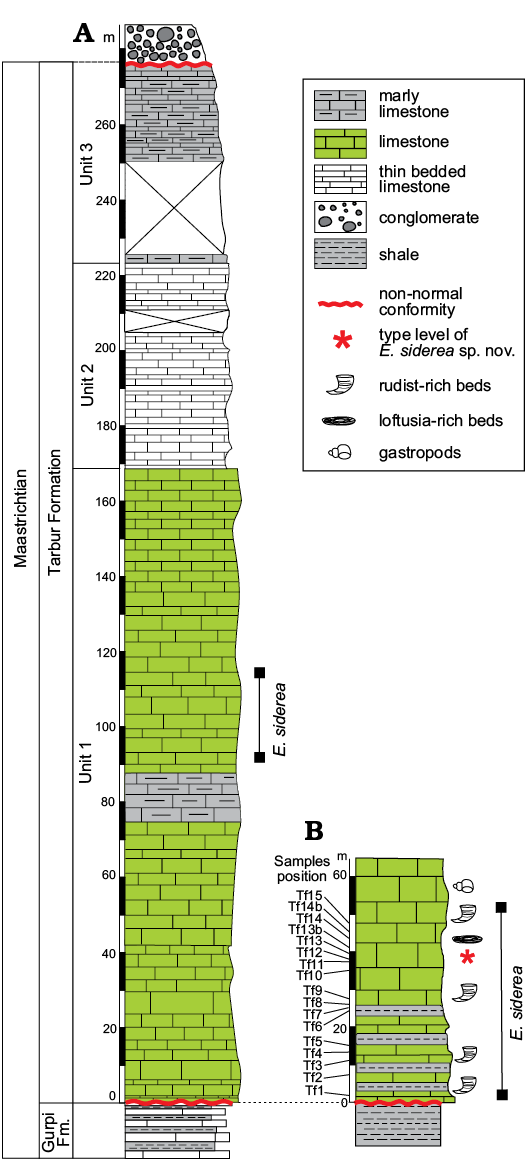
Fig. 3. Stratigraphic columns from Mandegan (A) and Rod-Abad (B) sections with position of the studied samples and distribution of Elazigina siderea sp. nov.
Systematic palaeontology
Phylum Foraminifera d’Orbigny, 1826
Class Globothalamea Pawlowski, Holzmann, and Tyszka, 2013
Order Rotaliida Delage and Hérouard, 1896
Superfamily Rotaloidea Ehrenberg, 1839
Remarks.—According to Consorti et al. (2017a) superfamily Rotaloidea includes families Rotaliidae and Pararotaliidae.
Family Rotaliidae Ehrenberg, 1839
Subfamily Kathininae Hottinger, 2014
Remarks.—This subfamily has been defined by Hottinger (2014: 17) as: “The folia are small, inclined foreward. Their tips are fused to each other and with the umbilical fill produced by the previous chambers to form a solid mass perforated by numerous parallel funnels. Some species may have a massive central umbo”. Therefore, the ventral structure in most representatives of this subfamily originates from folia vertical development, similar to what is observed in Lockhartiinae (Consorti et al. 2017a, b). Consequently, the subfamily Kathininae is here included in the Family Rotaliidae and considered sister group of the Lockartiinae.
Genus Elazigina Sirel, 2012
Type species: Kathina subsphaerica Sirel, 1972; Turkey, Paleocene.
Remarks.—The main characteristics of the genus are: (i) Low trochospire with smooth dorsal side. (ii) Ventral side occupied by a massive umbilical plug (= central umbo in Hottinger 2014) and surrounded by umbilical piles and funnels. The presence of umbo is not due to folia coalescence. (iii) Absence of a keel. (iv) Spiral interlocular space constrained between umbilical plates and the umbilical piles. (v) Massive feathering occupying the ventral intraseptal interlocular spaces. See Sirel (2012) and Hottinger (2014) for more information on the shell architecture. See Serra-Kiel et al. (2016) and Benedetti et al. (2018) for remarks on taxonomy.
Elazigina has sometimes been confused with the genus Smoutina Drooger, 1960 (e.g., Inan 1988; see Hottinger 2014 for a thorough discussion). The umbilical plug of Elazigina is massive, whereas that of Smoutina is composed by numerous superposed lamellae (probably folia, see Hottinger 2014: pl. 5.18: 3) and is perforated by funnels. Ventral chamber sutures are feathered in Elazigina, unlike Smoutina. Furthermore, Smoutina is reported only from the Caribbean Paleobioprovince (Hottinger 2014), whereas Elazigina comes from the central and eastern Tethys area. The genus Cideina Sirel, 1991 from the Maastrichtian of Turkey differs from Elazigina by having the flat morphology and no central plug.
Elazigina siderea sp. nov.
Figs. 4, 5.
1988 Smoutina cruysi Drooger, 1960; Inan 1988: 471, pl. 1: 1–9.
2008 indet. Foraminifera at upper left side of the picture; Schlüter et al. 2008: 518, fig. 4g.
2013 Rotalia skourensis Pfernder, 1938; Vaziri-Moghaddam et al. 2013: 154, fig. 14E.
2016 Pararotalia? sp.; Schlagintweit et al. 2016: 177, fig. 7G.
Etymology: From Latin sidera, star; due to the typical umbilical outline and from the heavy feathering.
Type material: Holotype: APNU-Tf12, complete specimen sectioned along the axial direction (Fig. 4A). Paratypes: APNU-Tf12, complete specimen sectioned along the subaxial direction (Fig. 4G, K); APNU-Tf11, oblique basal sections of complete specimen (Fig. 5D, H); APNU-Tf14, oblique centred section of a complete specimen (Fig. 5F); all from type locality.
Type locality: Rod Abad section, Fars, Iran.
Type horizon: Base of Tarbur Formation, Maastrichtian, Cretaceous.
Material.—About 150 oriented sections and 80 random sections.
Diagnosis.—Medium-size lamellar perforate shell of chambers arranged in a low trochospire. The dorsal side is low convex. Convexity of the ventral side may be sometimes exaggerated. Periphery unkeeled, slightly acute or somewhat rounded. A large massive umbilical plug occupies the central part of the ventral side. Piles present all around the umbilical plug. Ventral sutures heavy feathered. Folia small and slightly oblique. Presence of spiral, vertical (funnels) and intraseptal interlocular spaces.
Description.—A set of 10 to 14 piles bed circularly the umbilical plug, some of these piles are fused with the plug periphery forming a lobed outline. Chamber walls are thick, but the wall of the last chambers may appear thinner. The spiral canal bears between the piles and the umbilical plates. There may be funnels between the piles line and the umbilical plug, but it is sometimes difficult to distinguish the spiral canal from the funnels. The intraseptal interlocular space opens to the exterior and is partially subdivided by the branches of the feathering. The feathered space may be very wide, so much to reach the periphery the dorsal side. There are three whorls; the last one is sometimes incomplete. There are seven chambers in the first, 10–11 chambers in the second and 15–16 in the third whorl. Shell diameter of most complete specimens ranges 0.85–0.9 mm and its thickness may vary between 0.5 mm and 0.55 mm; the diameter on axial ratio is around 1.6. The plug diameter at the umbilicus of adult specimens is 0.23 mm, it extends longitudinally for more than 0.4 mm. Feather branches are up to 0.12 mm long and 0.05 mm thick. No microspheric specimens have been found, the diameter of the megalosphere is around 0.06 mm.

Fig. 4. Rotaliid foraminifer Elazigina siderea sp. nov. (A, holotype) from the Maastrichtian of Rod Abad section, Iran; axial (A, B, D, I, K), subaxial (E–H, J, L, N, O), oblique (C), and transversal-basal (M) sections. A–D, G, J–O. APNU-Tf12 (type-level). F, H, N. APNU-Tf11. E. APNU-Tf4. Abbreviations: f, feathers; pi, piles; pl, umbilical plug; sc, spiral canal.
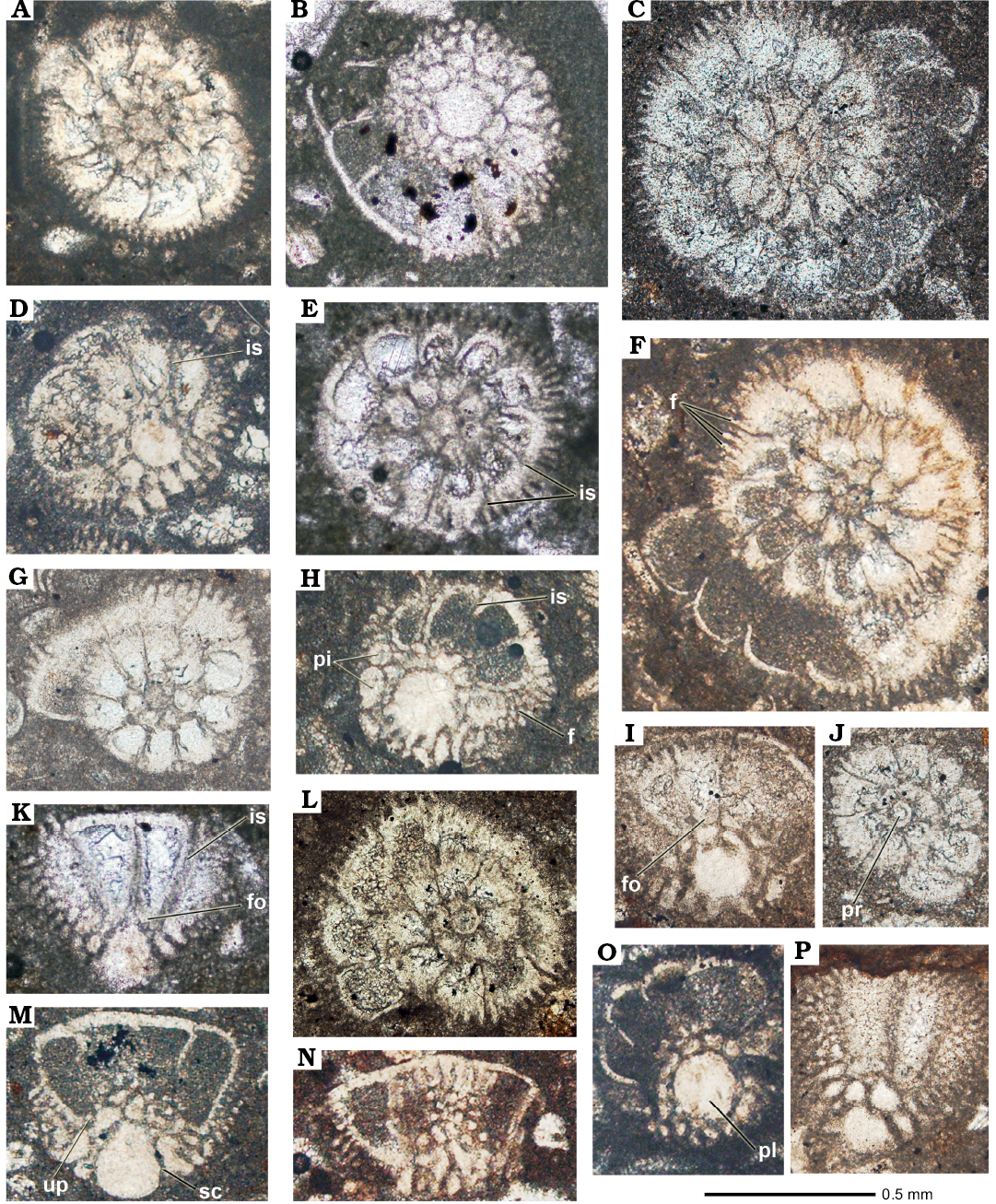
Fig. 5. Rotaliid foraminifer Elazigina siderea sp. nov. from the Tarbur Formation of Rod Abad (A, C, D, F–J) and Mandegan (B, E, K) sections, Iran; transversal (A, C, E, J), transversal-basal (B, O), transversal-oblique (D, F–I, L), and tangential (K, M, N, P) sections. C, J, L, M. APNU-Tf12 (type-level). D, G–I, P. APNU-Tf11. A, N. APNU-Tf10. F. APNU-Tf14. O. APNU-Tf4. B, E. APNU-Rt53. K. APNU-Rt55. Abbreviations: f, feathers; fo, folia; is, intraseptal canals; pi, piles; pl, umbilical plug; pr, proloculus; up, umbilical plate; sc, spiral canal.
Remarks.—Specimens from Turkey described in İnan (1988) display slightly higher diameter and axial values than the Iranian specimens (max. diameter: 1.1 mm; max. axial thickness: 0.67). These measurements are here taken into consideration as intraspecific variability. The last chambers appear frequently well rounded and their wall is thin. This may be due to poor calcification during the last shell growth stage.
Overall, the dimensions of E. siderea sp. nov. are reduced with respect to the Paleocene representatives, E. lenticula and E. subsphaerica. Hottinger (2014) does not provide complete measurements of E. dienii, but E. siderea appears smaller. E. dienii displays also a more acute periphery, less pronounced feathering and smaller umbo than E. siderea. The Middle East E. harabekayisensis has a thicker chamber wall, less piles, larger plug and less marked feathers than E. siderea. The piles surrounding the umbilical plug in E. siderea are well separated and clearly distinguishable in axial view, unlike most of the Paleocene allies, which piles frequently appear fused to the central plug or extremely reduced in dimension (see Hottinger 2014: pl. 6.8–11). A table comparing the measurements of all Elazigina species is presented in Table 1.
Table 1. Comparison of biometrical measurements (in mm) in the species of Elazigina. Abbraviations: ma, megalospheric form; mi, microspheric form.
|
Species |
Shell diameter |
Axial thickness |
Chambers in the last whorl |
Reference |
|
Elazigina subsphaerica |
ma: 1.04–1.48 mi: 1.68–2.40 |
~1.40 |
~20 |
|
|
Elazigina harabekayisensis |
1.52–1.88 |
1.00–1.32 |
~15 |
|
|
Elazigina lenticula |
2.00–2.40 |
0.90–1.00 |
~22 |
|
|
Elazigina dienii |
1.20 |
0.50–0.60 |
10–12 |
|
|
Elazigina siderea |
0.85–0.90 |
~0.50 |
10–16 |
this work |
Stratigraphic and geographic range.—Elazigina siderea sp. nov. is here described from the Maastrichtian of Iran and recognized from equivalent shallow-water carbonates of Turkey and Oman (Schlüter et al. 2008). Potentially, its presence can be extended along the whole Anatolian and Arabian sector. In this work, Elazigina siderea sp. nov. characterizes the Rod-Abad section and the upper part of the Mandegan section, beneath the first occurrence of Palaeoelphidium multiscissuratum (Smout, 1955) (see the column in Consorti et al. 2018: fig. 1C). Kathina sp. reported in Piryaei et al. (2010: fig. 12) at the lower part of Tarbur Formation is probably E. siderea sp. nov. and should be taken into consideration as a further record.
Discussion
The Late Cretaceous GCMC (see Hottinger 2001 for full explanation) represents a unit within the long evolutionary history of larger foraminifers (Hottinger 2001; Goldbeck and Langer 2009), characterized by a trend of increasing diversity through the Turonian–Maastrichtian time span. Cretaceous rotaloidean foraminifers, for example, follow such pattern (see Boix et al. 2009; Consorti et al. 2017a, b) and their acme of diversity in Central Tethys and Pyrenean gulf is recorded during the Santonian and the early Campanian (Consorti et al. 2017a). A subsequent pulse of foraminifers diversity was triggered by the middle–late Campanian highstand that generated new niches and the spreading of newcomer genera worldwide (Boudagher-Fadel 2008). This is especially true for the upper Campanian–Maastrichtian carbonate shelves of Arabian and Anatolian plates that represented hot spot of foraminifers diversity and endemism (Özcan 1993; Schlagintweit and Rashidi 2017; Consorti et al. 2018). Furthermore, several endemic foraminifers from these sediments have been described recently (İnan and İnan 2009; Görmüş et al. 2017; Schlagintweit and Rashidi 2017 and the references herein). High diversity in Middle East also continues in the Paleocene (Hottinger 2014). As the current central Indopacific realm (Langer and Hottinger 2000), high diversity of larger foraminifers in this area was most likely due to wide shelf areas and tropical climate (Scotese 2013) under the humid equatorial belt (Hay and Floegel 2012). The presence of both soft and hard bottoms would have further increased diversity (Hottinger 1988). Contrarily to the Indopacific, however, the shallow-water carbonate production in the upper Cretaceous Middle Eastern shelves did not contain coral frameworks, but was mainly characterized by foraminifers, echinoderms, rudists, red and green algae (see e.g., Schlüter et al. 2008; Piryaei et al. 2010; Bakhtiar et al. 2011). This may suggest possible fluctuation of nutrients in seawater or temperate waters (Simone and Carannante 1988; Ruberti et al. 2006; Carannante et al. 2008).
Notwithstanding the sea level fall trend recorded during the Maastrichtian and the Danian (Haq 2014) that could have reduced shallow-water niches, larger foraminifers in the Tarbur Formation and isochronous series in Iraq (Aqra Formation), Turkey (see e.g., Özcan 1993), Qatar and Oman (Simsima and Qahlah formations) show on-going high productivity and diversity. In this scenario, Elazigina siderea sp. nov. is associated with a very rich, mainly undescribed, rotaloidean assemblage, suggesting that its record coincides with the rotaliids (sensu lato) optimum at the final stage of the Late Cretaceous GCMC in Middle East. Among the abundant larger foraminifers of Tarbur Formation (see e.g., Rahaghi 1976; Schlagintweit et al. 2016), the assemblage studied in this work comprises Pseudomphalocyclus blumenthali Meriç, 1980, Fissoelphidium operculiferum Smout, 1955, Orbitokathina sp., and some species of the genus Loftusia (Fig. 6).
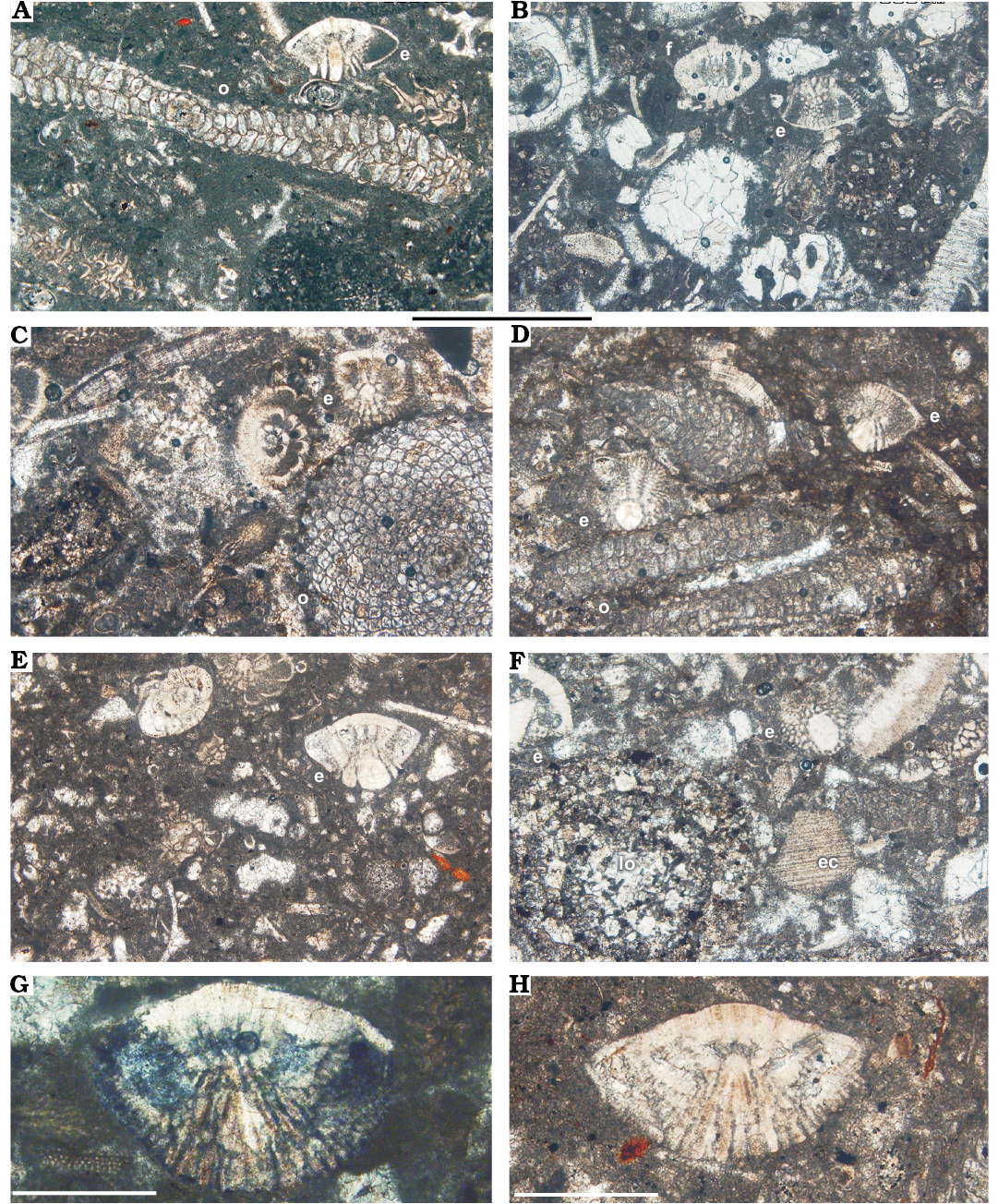
Fig. 6. A–F. Facies from the Tarbur Formation of Rod Abad section, Iran. A, C, D. Packstone with Pseudomphalocyclus blumenthali Meriç, 1980 (o) and Elazigina siderea sp. nov. (e). B, E. Packstone-wackestone with Fissoelphidium operculiferum Smout, 1955 (f), Elazigina siderea sp. nov. (e), and mollusc fragments. F. Packstone-grainstone with Loftusia (lo) and echinoderm fragments (ec). G, H. Orbitokathina sp. G. APNU-Tf14. H. APNU-Tf12. Scale bars A–F, 1 mm; G, H, 0.5 mm.
The shell of Elazigina siderea sp. nov., characterized by funnels and heavy feathers, does not display enveloping canals, or dimorphism. Enveloping canals helped Laffitteina to thrive in meso- to eutrophic environments, in analogy to the Neogene Pseudorotalia indopacifica (Thalmann, 1935) shells recovered in deltas of tropical rivers under the influence of organic matter and clay minerals inputs (Billman et al. 1980; Hottinger 2014). Meso- to eutrophic adaptation and occasional shallow infaunal life style allowed Laffitteina and small benthic r-strategists survival through K–Pg (Culver 2003; Hottinger 2014). This adaptation is arguably applicable to Elazigina siderea sp. nov. The latest Maastrichtian–Danian lowstand phase (Hallam and Wignall 1999; Alegret et al. 2001; Habib and Saeedi 2007; Haq 2014) may have increased the tropism in shallow seas through the establishment of “pools” restricted in water circulation on shelves (as e.g., Ogorelec et al. 2007: fig. 5). This would provide environment for few resistant foraminifers represented by Laffitteina, Elazigina, and probably Palaeoelphidium (Consorti et al. 2018).
The wide umbilical plug in Elazigina is interpreted to stabilize the shell immerged in soft substrates (Hottinger 2014). The ventral feathers could have further facilitated shell stability within carbonate mud and during motility, whereas umbilical orifices provided protoplasmic flux to screw in soft substrates, as in Ammonia catesbyana d’Orbigny, 1839 (see Langer et al. 1989). Following this interpretation, Elazigina would have been capable to withstand sunlight exposure and catch microalgae for photosynthesis or, if necessary, process the organic matter found within the substrate. The Paleocene Elazigina species were supposedly K-strategist (Hottinger 2014), but the Maastricthian Elazigina siderea sp. nov. was probably a r-strategist or a moderate K-strategist. This feature allowed the genus to survive during K–Pg and spread again during Paleocene, as testified by the rise of speciation rate and diversification all over the Neotethys (Fig. 7).
Elazigina siderea sp. nov. is most presumably linked to the Maastrichtian migration of the genus Orbitokathina Hottinger, 1966 from western Europe (Pyrenees) to Middle East (Fig. 7). Both taxa, belonging to the Paleocene group of Kathina, are closely related and are found together in the same samples. According to diameter measurement (≈ 0.7 mm), the specimens from Iran may be closely related to Orbitokathina cf. campaniana Boix, Villalonga, Caus, and Hottinger, 2009, but the latter material is not adequate for precise identification. In contrast to the Orbitokathina assemblages recovered in the Pyrenees, which comprise both large microspheric B-forms and small macrospheric A-forms, the assemblage from the Tarbur Formation contains only A-forms. The dispersal of Orbitokathina was most likely driven by the end-Cretaceous enclosure of the Pyrenean basin that affected the production of marine carbonates with larger foraminifers and promoted sedimentation of continental deposits (Rosell et al. 2001).

Fig. 7. Schematic representation of Elazigina evolution through time. Images not to scale, modified from Boix et al. (2009); Serra-Kiel et al. (2016); Hottinger (2014). Shallow Benthic Zones (SBZ) according Serra-Kiel et al. (1998).
Conclusions
The Maastrichtian rotaliid morphotypes of the Tarbur Formation from the Rod-Abad section in Fars region (Iran, Zagros Zone) are attributed to the genus Elazigina, and described as Elazigina siderea sp. nov. The new taxon is included in the subfamily Kathininae and is distinguished from the previously known species of Elazigina by the heavy feathered umbilical chamber sutures, by the presence of well-distinguished umbilical piles and reduced general dimensions.
Prior to this work, the genus Elazigina was only known from the Paleocene (SBZ 2–4). The record from the Maastrichtian is of interest because (i) it is the earliest occurrence in the geological record; and (ii) the genus survived across the Cretaceous–Paleogene mass extinction.
The demise of larger foraminifers at K–Pg boundary was most likely linked to the fluctuation of trophic levels in shallow seas, possibly due to Maastrichtian cooling and sea level fall that could have reduced the photosynthetic potential and the ecological niches of the full K-strategists. This would have favoured the survival of occasionally shallow-infaunal r-strategists taxa like Elazigina.
Acknowledgments
Comments by the reviewers Felix Schlagintweit (Munich, Germany) and Bruno Granier (University of Brest, France) are highly appreciated.
References
Alavi, M. 2004. Regional stratigraphy of the Zagros fold thrust belt of Iran and its proforeland evolution. American Journal of Science 304: 1‒20. Crossref
Alegret, L. and Thomas, E. 2009. Food supply to the seafloor in the Pacific Ocean after the Cretaceous/Paleogene boundary event. Marine Micropaleontology 73: 105–116. Crossref
Alegret, L. and Thomas, E. 2013. Benthic Foraminifera across the Cretaceous/Paleogene boundary in the Southern Ocean (ODP Site 690): Diversity, food and carbonate saturation. Marine Micropaleontology 105: 40–51. Crossref
Alegret, L., Molina, E., and Thomas, E. 2001. Benthic Foraminifera at the Cretaceous–Tertiary boundary around the Gulf of Mexico. Geology 29: 891–894. Crossref
Bakhtiar, H.A., Taheri, A., and Vaziri-Moghaddam, H. 2011. Maastrichtian facies succession and sea-level history of the Hossein-Abad, Neyriz area, Zagros Basin. Historical Biology 23: 145–153. Crossref
Benedetti, A., Marino, M., and Pichezzi, R.M. 2018. Paleocene to lower Eocene larger foraminiferal assemblages from Central Italy: new remarks on biostratigraphy. Rivista italiana di Paleontologia e Stratigrafia 124: 73‒90.
Berggren, W.A. and Norris, R.D. 1997. Biostratigraphy, phylogeny and systematics of Paleocene trochospiral planktic Foraminifera. Micropaleontology 43: 1–116. Crossref
Billman, H., Hottinger, L., and Oesterle, H. 1980. Neogene to recent Rotaliid Foraminifera from the Indopacific Ocean; their Canal system, their classification and their stratigraphic use. Schweizerische Palӓontologische Abhandlungen 101: 71‒113.
Boix, C., Villalonga, R., Caus, E., and Hottinger, L. 2009. Late Cretaceous rotaliids (Foraminiferida) from the Western Tethys. Neues Jahrbuch für Geologie und Palӓontologie-Abhandlungen 253: 197‒227. Crossref
BouDagher-Fadel, M.K. 2008. Evolution and geological significance of larger benthic Foraminifera. Developments in Palaeontology and Stratigraphy 21: 1‒544. Crossref
Brasier, M.D. 1988. Foraminiferid extinction and ecological collapse during global biological events. In: G.P. Larwood (ed.), Extinction and Survival in the Fossil Record. Systematics Association Special Volume 34: 37–64.
Carannante, G., Cherchi, A., Graziano, R., Ruberti, D., and Simone, L. 2008. Post-Turonian Rudist-Bearing Limestones of the Peri-Tethyan Region: Evolution of the Sedimentary Patterns and Lithofacies in the Context of Global Versus Regional Controls. SEPM Special Publication 89: 255–270.
Consorti, L., Frijia, G., and Caus, E. 2017a. Rotaloidean Foraminifera from the Upper Cretaceous carbonates of Central and Southern Italy and their chronostratigraphic age. Cretaceous Research 70: 226‒243. Crossref
Consorti, L., Schlagintweit, F., and Rashidi, K. 2018. Palaeoelphidium gen. nov. (type species: Elphidiella multiscissurata Smout, 1955): The oldest Elphidiellidae (benthic Foraminifera) from Maastrichtian shallow-water carbonates of the Middle East. Cretaceous Research 86: 163‒169. Crossref
Consorti, L., Villalonga, R., and Caus, E. 2017b. New Rotaliids (benthic Foraminifera) from the Late Cretaceous of the Pyrenees in northeastern Spain. Journal of Foraminiferal Research 47: 284‒293. Crossref
Chenot, C., Deconinck, J.-F., Pucéat, E., Pellenard, P., Guiraud, M., Jaubert, M., Jarvis, I., Thibault, N., Cocquerez, T., Bruneau, L., Razmjooeid, M.J., Boussaha, M., Richard, J., Sizun, J.-P., and Stemmerik, L. 2018. Continental weathering as a driver of Late Cretaceous cooling: new insights from clay mineralogy of Campanian sediments from the southern Tethyan margin to the Boreal realm. Global and Planetary Change 162: 292–312. Crossref
Culver, S.J. 2003. Benthic Foraminifera across the Cretaceous–Tertiary (K–T) boundary: a review. Marine Micropaleontology 47: 177–226. Crossref
Font, E., Adatte, T., Andrade, M., Keller, G., Bitchong, A.M., Carvallo, C., Ferreira, J., Diogo, F., and Mirão, J. 2018. Deccan volcanism induced high-stress environment during the Cretaceous–Paleogene transition at Zumaia, Spain: Evidence from magnetic, mineralogical and biostratigraphic records. Earth and Planetary Science Letters 484: 53–66. Crossref
Fuqua, L.M., Bralower, T.J., Arthur, M.A., and Patzkowsky, M.E. 2008. Evolution of calcareous nannoplankton and the recovery of marine food webs after the Cretaceous–Paleocene mass extinction. Palaios 23: 185–194. Crossref
Goldbeck, E.J. and Langer, M.R. 2009. Biogeographic provinces and patterns of diversity in selected Upper Cretaceous (Santonian–Maastrichtian) larger Foraminifera. In: T.D. Demchuk and A.C. Gray (eds.), Geologic Problem Solving with Microfossils: A Volume in Honour of Garry D. Jones. SEPM Special Publication 93: 187‒232. Crossref
Görmüş, M., Ameen Lawa, F.A., and Al Nuaimy, Q.A.M. 2017. Suraqalatia brasieri n.gen., n.sp. (larger Foraminifera) from the Maastrichtian of Sulaimani area in northern Iraq. Arabian Journal of Geosciences 10: 365. Crossref
Habib, D. and Saeedi, F. 2007. The Manumiella seelandica global spike: Cooling during regression at the close of the Maastrichtian. Palaeogeography, Palaeoclimatology, Palaeoecology 255: 87‒97. Crossref
Hallam, A. and Wignall, P.B. 1999. Mass extinctions and sea-level changes. Earth-Science Reviews 48: 217–250. Crossref
Haq, B.U. 2014. Cretaceous eustasy revisited. Global and Planetary Change 113: 44–58. Crossref
Hallock, P. 1999. Symbiont-bearing Foraminifera. In: B.K. Sen Gupta (ed.), Modern Foraminifera, 123‒139. Kluwer Academic, Dordrecht. Crossref
Hallock, P. and Schlager, W. 1986. Nutrient excess and the demise of coral reefs and carbonate platforms. Palaios 1: 389‒398. Crossref
Hay, W.W. and Floegel, S. 2012. New thoughts about the Cretaceous climate and oceans. Earth-Science Reviews 115: 262‒272. Crossref
Hohenegger, J. 1995. Depth estimation by proportions of living larger Foraminifera. Marine Micropaleontology 26: 31‒47. Crossref
Hottinger, L. 1988. Significance of diversity in shallow benthic foraminifera. In: R. Matteucci (ed.), Atti del Quarto Simposio di Ecologia e Paleoecologia delle Comunità Bentoniche, Sorrento, 35‒51. Museo Regionale di Scienze Naturali, Torino.
Hottinger, L. 2001. Learning from the past. In: R. Levi-Montalcini (ed.), Discovery and Spoliation of the Biosphere. Frontiers of Life 4, 449‒477. Academic Press, San Diego.
Hottinger, L. 2006. Illustrated glossary of terms
used in foraminiferal research. Carnets de Geologie
6 (M02): 1–126. http://paleopolis.rediris.es/cg/CG2006_M02/index.html
Hottinger, L. 2014. Paleogene Larger Rotaliid Foraminifera from the Western and Central Neotethys. 191 pp. Springer, Heidelberg. Crossref
İnan, N. 1988. Sur la presence de Smoutina cruysi Drooger dans le Maastrichtien supérieur de Sivas (est de la Turquie). Revue de Paléobiologie 7: 467‒475.
İnan, N. and İnan, S. 2009. Endemic Foraminifera of the Late Maastrichtian from the northern branch of the Neotethys, NE Turkey. Micropaleontology 55: 514‒522.
İnan, N., Tasli, K., and İnan, S. 2005. Laffitteina from the Maastrichtian– Paleocene shallow marine carbonate successions of the Eastern Pontides (NE Turkey): biozonation and microfacies. Journal of Asian Earth Sciences 25: 367–378 Crossref
James, G.A. and Wynd, J.G. 1965. Stratigraphic nomenclature of Iranian Oil Consortium Agreement Area. AAPG Bulletin 49: 2218‒2232.
Keller, G., López-Oliva, J.G., Stinnesbeck, W., and Adatte, T. 1997. Age, stratigraphy and deposition of near-K/T siliciclastic deposits in Mexico: Relation to bolide impact? Geological Society of America Bulletin 109: 410–428. Crossref
Langer, M.R. and Hottinger, L. 2000. Biogeography of selected “larger” Foraminifera. Micropaleontology 45: 105‒126.
Langer, M.R., Hottinger, L., and Huber, B. 1989. Funcional morphology in low-diverse benthic Foraminifera assemblages from tidal flat of the North Sea. Senckenbergiana Maritima 20: 81‒99.
Leutenegger, S. 1984. Symbiosis in benthic Foraminifera: specificity and host adaptations. Journal of Foraminiferal Research 14: 16‒35. Crossref
MacLeod, N., Rawson P.F., Forey P.L., Banner F.T., BouDagher-Fadel M.K., Bown P.R., Burnett J.A., Chambers P., Culver S., Evans S.E., Jeffrey C., Kaminski M.A., Lord A.R., Milner A.C., Milner A.R., Morris N., Owen E., Rosen B.R., Smith A.B., Taylor P.D., Urquhart, E., and Young, J.R. 1997. The Cretaceous–Tertiary biotic transition. Journal of the Geological Society 154: 265–292. Crossref
Molina, E., Arenillas, I., and Arz, J.A. 1998. Mass extinction in planktic Foraminifera at the Cretaceous/Tertiary boundary in subtropical and temperate latitudes. Bulletin de la Société Géologique de France 169: 351–363.
Ogorelec, B., Dolenec, T., and Drobne, K. 2007. Cretaceous/Tertiary boundary problem on shallow carbonate platform: Carbon and oxygen excursions, biota and microfacies at the K/T boundary sections Dolenja Vas and Sopada in SW Slovenia, Adria CP. Palaeogeography, Palaeoclimatology, Palaeoecology 255: 64‒76. Crossref
Özcan, E. 1993. Late Cretaceous benthic foraminiferal proliferation on the Arabian Platform: taxonomic remarks on the genus Orbitoides d’Orbigny 1848. Geological Journal 28: 309‒317. Crossref
Piryaei, A., Reijmer, J.J.G., van Buchem, F.S.P., Yazdi-Moghadam, M., Sadouni, J., and Danelian, T. 2010. The influence of Late Cretaceous tectonic processes on sedimentation patterns along the northeastern Arabian plate margin (Fars Province, SW Iran). Geological Society Special Publications 330: 211‒251. Crossref
Rahaghi, A. 1976. Contribution à l’étude de quelques grands foraminifères de l’Iran. Société National Iranienne des Pétroles, Laboratoire de Micropaléontologie 6: 1–78.
Rahaghi, A. 1992. The geographic and stratigraphic range of the genus Laffitteina Marie, 1946, in Iran and description of Laffitteina jaskii n. sp. Revista Española de Micropaleontología 3: 5‒11.
Raup, D.M. and Sepkoski, J.J. 1986. Periodic extinction of families and genera. Science 231: 833–836. Crossref
Rosell, J., Llompart, C., and Linares, R. 2001. El “garumniense” prepirenaico. Revista de la Sociedad Geológica de España 14: 47‒56.
Ruberti, D., Toscano, F., Carannante, G., and Simone, L. 2006. Rudist lithosomes related to current pathways in Upper Cretaceous temperate-type, inner shelves: A case study from the Cilento area, southern Italy. Geological Society London Special Publications 255: 179‒195. Crossref
Saura, E., Vergés, J., Homke, S., Blanc, E., Serra-Kiel, J., Bernaola, G., Casciello, E., Fernández, N., Romaire, I., Casini, G., Embry, J.C., Sharp, I.R., and Hunt, D.W. 2011. Basin architecture and growth folding of the NW Zagros early foreland basin during the Late Cretaceous and early Tertiary. Journal of the Geological Society 168: 235–250. Crossref
Scotese, C.R. 2013. Map Folio 17, Late Cretaceous, (Maastrichtian, 68 Ma). In: C.R. Scotese (ed.), PALEOMAP PaleoAtlas for ArcGIS, Volume 2, Cretaceous Paleogeographic, Paleoclimatic and Plate Tectonic Reconstructions. 31 pp. PALEOMAP Project, Evanston.
Scotese, C.R. 2014. Maps 8–15, The Cenozoic. In: C.R. Scotese (ed.), PALEOMAP PaleoAtlas for ArcGIS, Volume 1, Atlas of Paleogene Paleogeographic Maps (Mollweide Projection). 14 pp. PALEOMAP Project, Evanston.
Schlagintweit, F. and Rashidi, K. 2017. Persiella pseudolituus n. gen., n. sp., and Flabelloperforata tarburensis n. gen., n. sp., two new larger benthic Foraminifera from the upper Maastrichtian of Iran. Acta Paleontologica Romaniae 13: 3‒19.
Schlagintweit, F., Rashidi, K., and Barani, F. 2016. First record of Gyroconulina columellifera Schroeder & Darmoian, 1977 (larger benthic Foraminifera) from the Maastrichtian Tarbur Formation of SW Iran (Zagros Fold-Thrust-Belt). Geopersia 6: 169‒185.
Schlüter, M., Steuber, T., Parente, M., and Mutterlose, J. 2008. Evolution of a Maastrichtian–Paleocene tropical shallow-water carbonate platform (Qalhat, NE Oman). Facies 54: 513–527. Crossref
Serra-Kiel, J., Hottinger, L., Caus, E., Drobne, K., Ferrandez, C., Jauhri, A.K., Less, G., Pavlovec, R., Pignatti, J., Samsò, J.M., Schaub, H., Sirel, E., Strougo, A., Tambareau, Y., Tospquella, J., and Zakrebskaya, E. 1998. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bulletin de la Société Géologique de France 169: 281‒299.
Serra-Kiel, J., Vicedo, V., Razin, P., and Grélaud, C. 2016. Selandian–Thanetian larger Foraminifera from the lower Jafnayn Formation in the Sayq area (eastern Oman Mountains). Geologica Acta 14: 315‒333.
Simone, L. and Carannante, G. 1988. The fate of foramol (“temperate-type”) carbonate platforms. Sedimentary Geology 60: 347‒354. Crossref
Sirel, E. 2012. Seven new larger benthic foraminiferal genera from the Paleocene of Turkey. Revue de Paléobiologie 31: 267‒301.
Tewari, V.C., Stenni, B., Pugliese, N., Drobne, K., Riccamboni, R., and Dolenec, T. 2007. Peritidal sedimentary depositional facies and carbon isotope variation across K/T boundary carbonates from NW Adriatic platform. Palaeogeography, Palaeoclimatology, Palaeoecology 255: 64‒76. Crossref
Vaziri-Moghaddam, H., Safari, A., Shahriari, S., Khazaei, A., and Taheri, A. 2013. Biostratigraphy and Palaeoecology of the Maestrichtian Deposits (Tarbur and Gurpi Formations) at Gardbishe Area (South of Borojen). Scientific Quarterly Journal (Geosciences) 87: 143‒162.
Acta Palaeontol. Pol. 63 (3): 595–605, 2018
https://doi.org/10.4202/app.00487.2018