

Carcharocles-bitten odontocete caudal vertebrae from the Coastal Eastern United States
STEPHEN J. GODFREY, MIKE ELLWOOD, STEPHEN GROFF, and MICHAEL SCOTT VERDIN
A description and analysis is given of three Neogene odontocete caudal vertebrae that were bitten by the extinct megatooth sharks Carcharocles megalodon or Carcharocles chubutensis. The peduncular caudal vertebrae show bilateral gouge marks consistent with having been actively bitten and wedged between adjacent teeth of C. megalodon or C. chubutensis. None of the vertebrae show signs of healing. The occurrence of bite marks on distal caudals suggests active predation (vs. scavenging) in order to immobilize even relatively small prey prior to consumption.
Many cetacean bones exhibit shark bite traces (Deméré and Cerutti 1982; Bianucci et al. 1990; Cigala Fulgosi 1990; Purdy 1996; Renz 2002; Godfrey and Altman 2005; Aguilera et al. 2008; Ehret et al. 2009; Bianucci et al. 2010; Bianucci and Gingerich 2011; Kallal et al. 2012; Takakuwa 2014; Carrillo-Briceño et al. 2016; Collareta et al. 2017). Only a small fraction of these can be attributed to a specific shark, and fewer still to the feeding habits of the extinct mega-tooth shark, Carcharocles megalodon (Otodontidae) (Purdy 1996; Renz 2002; Godfrey and Altman 2005; Aguilera et al. 2008; Carrillo-Briceño et al. 2016; Collareta et al. 2017). Considered to have been the Neogene marine apex predator (Aguilera et al. 2008; Ehret 2010; Pimiento and Balk 2015; Kent in press), it is surprising how few fossils have been described showing trophic interactions between it and contemporary cetaceans. Here, three odontocete peduncular caudal vertebrae are described that show bilateral gouge marks consistent with having been actively bitten and wedged between adjacent teeth of C. megalodon or C. chubutensis.
Institutional abbreviations.—CMM-V, Calvert Marine Museum fossil vertebrate collection.
Geological and geographic settings
The vertebrae described herein were collected separately from Mio-Pliocene sediments along the Atlantic Coastal Plain. CMM-V-4360 (Fig. 1) was surface collected by ME from within Pliocene Yorktown Formation sediments in the PCS phosphate mine in Aurora, North Carolina, USA. The geology and paleontology of this site has been thoroughly described elsewhere (Ray 1983, 1987; Purdy et al. 2001; Ray and Bohaska 2001; Ray et al. 2008).

Fig. 1. A Neogene odontocete caudal vertebra, CMM-V-4360 from a spoil pile within the PCS phosphate mine in Aurora, North Carolina, USA. Peduncular vertebra in right (A) and left (B) lateral views and anteroventral (C) view. The deep diagonal gouges on the anteroventral margin of the vertebra were created when the vertebra was repeatedly wedged between two adjacent teeth.
CMM-V-8405 (Fig. 2) was collected by SG as beach float from Bayfront Park (formerly known as Brownie’s Beach), Calvert Cliffs, Maryland, USA. Although it was not found in situ, there is no reason to believe that it was not locally derived from the adjacent cliffs that comprise Miocene sediments from the Plum Point Member of the Calvert Formation. The local Miocene geology has been described most recently by Kidwell et al. (2015).

Fig. 2. A Miocene odontocete caudal vertebra, CMM-V-8405 from beach float at Bayfront Park, Calvert Cliffs, Maryland, USA. Peduncular vertebra in left (A) and right (B) lateral views and anteroventral (C) view. Multiple gouges are present on both sides of the vertebra.
CMM-V-8406 (Fig. 3) was collected by MSV as beach float from Willows Beach, Calvert Cliffs, Maryland, USA. Although it was also not found in situ, it is thought to have been locally derived from the adjacent cliffs that comprise Miocene sediments from the Plum Point Member of the Calvert Formation.
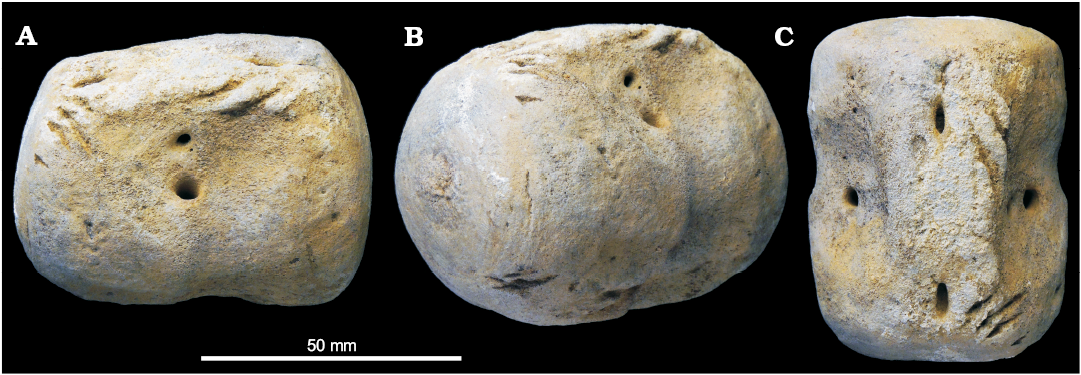
Fig. 3. A Miocene odontocete caudal vertebra, CMM-V-8406 from beach float at Willows Beach, Calvert Cliffs, Maryland, USA. Peduncular vertebra in right lateral (A), posterolateral (B), and dorsal (C) views. Multiple gouges are present on the top and bottom of the vertebra.
Description
Figures 1–3 illustrate three peduncular caudal vertebrae of eurhinodelphinid-size odontocetes (Table 1).
Table 1. Dimensions (in mm) of the odontocete peduncular vertebrae.
|
Specimen |
Length (L) |
Width (W) |
Height (H) |
L/W Ratio |
|
CMM-V-4360 |
80.9 |
59.7 |
73.7 |
1.36 |
|
CMM-V-8405 |
80.3 |
51.0 |
62.6 |
1.57 |
|
CMM-V-8406 |
73.0 |
57.3 |
59.5 |
1.27 |
In the details of their morphology, they match those of eurhinodelphinid-grade odontocetes (Fig. 4; Abel 1931: pls. 25–26, 29). These vertebrae are identified as distal caudals because of their lack of transverse processes and the diminutive size of their neural canal. Furthermore, they are peduncular vertebrae, characteristic of cetaceans, that occupy the region of the vertebral column immediately anterior to the fluke (Fig. 4). Peduncular vertebrae are those in which the body of the vertebra is higher than it is wide (Table 1) and the transverse processes, neural arch, and pedicles are very reduced or absent (Uhen 2004). The vertebrae illustrated in Figs. 1–3 derive from fully mature individuals; their circular epiphyses are fully fused. In all of these vertebrae, length is their greatest dimension, followed by their height, then width (Table 1). In the mysticetes from along Calvert Cliffs and from Aurora, and at least odontocetes more derived than Xiphiacetus and Zarhachis (Lambert et al. 2017: fig. 14) in which peduncular vertebrae are known, they are all both higher and wider than they are long.
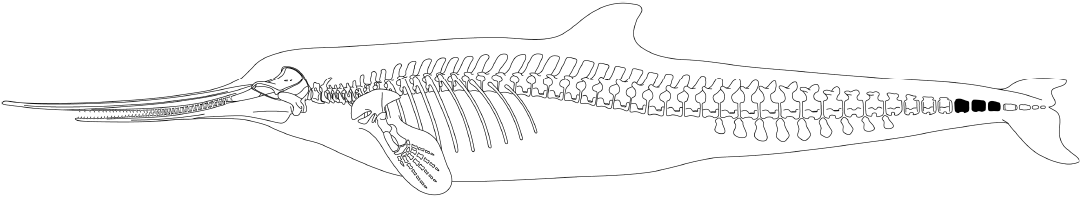
Fig. 4. Skeletal restoration and possible body outline of Xiphiacetus bossi in a left lateral view. The peduncular vertebrae in the caudal sequence are blackened. Body length is approximately 3.5 m. Inspired by Abel (1931).
Cylindrical best describes the shape of these vertebrae. Their neural arches are small (CMM-V-4360, Fig. 1) to virtually non-existent (CMM-V-8406, Fig. 3). The diameter of the neural canals in these vertebrae ranges from 2–5 mm. In all three vertebrae, at about the midpoint in the length of the centrum, the bilateral arterial foramen passes vertically through the body of the centrum.
In lateral views of CMM-V-4360 (Fig. 1), deep gouges are present on both antero-ventrolateral sides of the vertebra. The gouges begin at the anteroventral base of the centrum and pass diagonally across the body of the centrum where they end at about the midpoint in both length and height of the centrum. The longest gouge is approximately 46 mm. On the left side of the centrum, there is one major gouge whereas on the right side there are two; shorter and shallower incisions are also present on both ventrolateral sides of this vertebra.
In lateral views of CMM-V-8405 (Fig. 2), multiple gouges are present on both lateral sides of the vertebra. As in CMM-V-4360, the gouges also cross the body of the centrum diagonally in an anterodorsal-posteroventral direction. Both sides of this vertebra (mostly within the lower half of the centrum) are marked by six variably spaced and prominent gouges. The longest gouge on the right side of the centrum is approximately 37 mm (Fig. 2B).
CMM-V-8406 (Fig. 3), is also marked by multiple gouges but they are not as pronounced (as elongate or as deeply incised into the body of the centrum) as in the two aforementioned specimens. The longest gouge is approximately 19 mm (Fig. 3A). The anterior end of the vanishing neural arch is marked by two gouge marks whereas the right posterior end is marked by four bite traces. The right posteroventral end of the centrum is also scored by several short gouges (Fig. 3B).
Discussion and conclusions
Of the Neogene predators known, only Carcharocles megalodon and Carcharocles chubutensis had teeth large enough, with sufficient spacing (Fig. 5A) between adjacent teeth for the dolphin vertebrae described herein to have been gouged on both sides simultaneously in the manner in which they were. C. megalodon is known from both localities (Purdy et al. 2001; Kent in press). In the Miocene sediments along Calvert Cliffs and in the PCS phosphate mine in Aurora, C. chubutensis (which in Aurora, Purdy et al. (2001) list as Carcharodon subauriculatus) teeth occur in some of the same beds as teeth of C. megalodon. Individuals of C. chubutensis would also have been large enough to have created bite marks such as those preserved on the odontocete vertebrae described here. C. chubutensis is considered to have been the immediate predecessor and a chronomorph of C. megalodon (Perez et al. in press). Smaller predators like Carcharodon hastalis (Bianucci et al. 2010; Ehret et al. 2012; Kent in press), which occurs in both localities (in Aurora, Purdy et al. 2001 describe it as Isurus hastalis and its synonym Isurus xiphodon), were also considered. In C. hastalis, only the largest individuals, with teeth up to 75 mm in length, might have had tooth size and spacing large enough to make the gouges present on CMM-V-8406 (Fig. 3), but not on either CMM-V-4360 (Fig. 1) or CMM-V-8405 (Fig. 2).
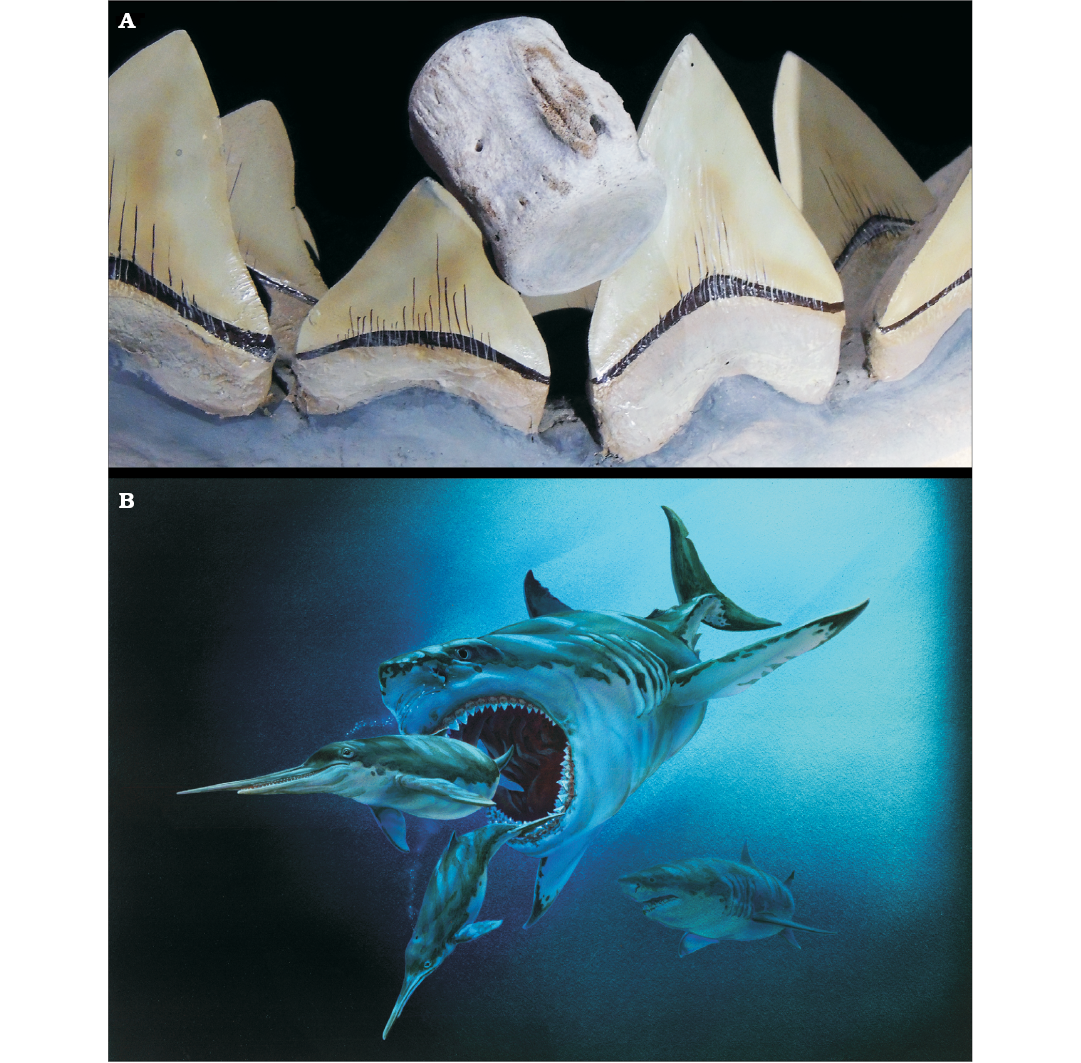
Fig. 5. A. CMM-V-4360 placed between two adjacent teeth in the reconstructed jaw of Carcharocles megalodon at the Calvert Marine Museum, Solomons, Maryland, USA. B. Life restoration of C. megalodon pursuing two eurhinodelphinids. Rendering by artist Tim Scheirer (CMM).
None of the bite traces show evidence of the cutting tooth having had serrations. However, the manner in which the teeth cut into the bone (i.e., parallel to the cutting edge), the teeth did not rake the surface of the bone, providing much opportunity for the preservation of serration marks had they been present. Furthermore, the spongy texture of the bone surface did not provide the necessary material resolution for serration marks to be preserved. Many other shark-tooth-marked bones are known but, to our knowledge, no other fossils are known where markings were made on single bones from having been wedged between two adjacent teeth.
Although we are confident that the gouges in CMM-V-4360 and CMM-V-8405 were made by Carcharocles spp. and that none of the vertebrae show signs of healing, unfortunately we do not know for sure if the tooth traces result from scavenging or active predation. Nevertheless, we think that a stronger case can be made for active predation vs scavenging. Extant great white sharks (Carcharodon carcharias) do not actively prey upon large adult baleen whales although they will scavenge their carcasses (Dickens 2008; Fallows et al. 2013; Collareta et al. 2017). Fallows et al. (2013) also observed that, during scavenging events, great white sharks generally show an initial preference for foraging on the caudal peduncle and fluke of a Bryde’s whale (Balaenoptera edeni) carcass before proceeding to blubber-rich regions of the body of the cetacean. Conversely, C. carcharias rarely scavenge smaller marine mammals (seals or diminutive odontocetes), however, they do actively prey upon these smaller prey items. Based on the feeding habits of extant great white sharks and cetacean and pinniped bones exhibiting large bite marks attributed to the activity of mega-toothed sharks, Collareta et al. (2017) proposed that megalodon preyed upon relatively small marine mammals (e.g., small-sized mysticetes) while also scavenging on the carcasses of larger whales. The odontocete vertebrae described here would have come from individual cetaceans no longer than 4 m, considerably smaller than large megalodon in the 15–18 m body-length range (Gottfried et al. 1996; Shimada 2003; Pimiento and Balk 2015; Grant et al. 2017). The reconstructed jaws shown in Fig. 5A accompany a reconstructed skeleton of a shark with a body length of about 11 m.
Modern large sharks attack small, echolocating toothed whales in such a way so as to avoid detection by both the lateral visual field and the anteriorly directed biosonar (Long and Jones 1996; Bianucci et al. 2010). Extant great white sharks are known to disable dolphins by biting their caudal peduncle (Long and Jones 1996: fig. 8). At a minimum, Carcharocles megalodon could certainly have done the same (Fig. 5B). That the caudal vertebrae show multiple gouges suggests that the peduncle of these odontocetes was jammed forcefully and repeatedly between adjacent teeth by powerful bite forces applied by teeth in the opposing jaw (Fig. 5A). The application of such repeated force seems more in keeping with the disabling of struggling prey rather than the dismembering of a small carcass so close to its fluke (Fig. 5B). Therefore, these Carcharocles-bitten odontocete caudal vertebrae suggest that this apex predator included this disabling tactic in its predatory repertoire, and that it also actively preyed upon relatively small odontocetes.
Acknowledgements
Tim Scheirer (CMM) skillfully created the life-restorations illustrated in Fig. 5B; thank you. We would also like to thank Giovanni Bianucci and Alberto Collareta (both Università di Pisa, Italy), and Victor Perez (University of Florida, Gainesville, USA) for their constructive reviews. Stephen Brusatte edited this contribution for Acta Palaeontologica Polonica; many thanks. Funding of this report came from the citizens of Calvert County, the Calvert County Board of County Commissioners, and the Clarissa and Lincoln Dryden Endowment for Paleontology at the Calvert Marine Museum.
References
Abel, O. 1931. Das skelett der eurhinodelphiden dem Oberen Miozän von Antwerpen. Memoires du Musée Royal d’Histoire Naturelle de Belgique 48: l9l–334.
Aguilera, O.A, García, L., and Cozzuol, M.A. 2008. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontologische Zeitschrift 82: 204–208. Crossref
Bianucci, G. and Gingerich, P.D. 2011. Aegyptocetus tarfa, n. gen. et sp. (Mammalia, Cetacea), from the middle Eocene of Egypt: clinorhynchy, olfaction, and hearing in a protocetid whale. Journal of Vertebrate Paleontology 31: 1173–1188. Crossref
Bianucci, G., Sorce, B., Storai, T., and Landini, W. 2010. Killing in the Pliocene: shark attack on a dolphin from Italy. Palaeontology 53: 457–470. Crossref
Carrillo-Briceño, J.D., Aguilera, O.A., De Gracia, C., Aguirre-Fernández, G., Kindlimann, R., and Sánchez-Villagra, M.R. 2016. An early Neogene elasmobranch fauna from the southern Caribbean (Western Venezuela). Palaeontologia Electronica 19: 27A.
Cigala Fulgosi, F. 1990 Predation (or possible scavenging) by a great white shark on an extinct species of bottlenosed dolphin in the Italian Pliocene. Tertiary Research 12: 17–36.
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology 469: 84–91. Crossref
Deméré, T.A. and Cerutti, R.A. 1982. A Pliocene shark attack on a cethotheriid whale. Journal of Paleontology 56: 1480–1482.
Dicken, M.L. 2008. First observations of young of the year and juvenile great white sharks (Carcharodon carcharias) scavenging from a whale carcass. Marine and Freshwater Research 59: 596–602. Crossref
Ehret, D.J. 2010. Paleobiology and Taxonomy of Extinct Lamnid and Otodontid Sharks (Chondrichthyes, Elasmobranchii, Lamniformes). 165 pp. Ph.D. Thesis, University of Florida, Gainesville.
Ehret, D.J., MacFadden, B.J., Jones, D.S., DeVries, T.J., and Salas-Gismondi, R. 2009. Caught in the act: trophic interactions between a 4-million-year-old white shark (Carcharodon) and mysticete whale from Peru. Palaios 24: 329–333. Crossref
Ehret, D.J., MacFadden, B.J., Jones, D.S., Devries, T.J., Foster, D.A., and Salas-Gismondi, R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology 55: 1139–1153. Crossref
Fallows, C., Gallagher, A.J., and Hammerschlag, N. 2013. White Sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PLOS ONE 8 (4): e60797. Crossref
Godfrey, S.J. and Altman, J. 2005. A Miocene cetacean vertebra showing a partially healed compression fracture, the result of convulsions or failed predation by the Giant White Shark, Carcharodon megalodon. Jeffersoniana 16: 1–12.
Gottfried, M.D., Compagno, L.J.V., and Bowman, S.C. 1996. Size and skeletal anatomy of the giant megatooth shark Carcharodon megalodon. In: A.P. Klimley and D.G. Ainley (eds.), Great White Sharks: the Biology of Carcharodon carcharias, 55–89. Academic Press, San Diego. Crossref
Grant, C., MacFadden, B., Antonenko, P., and Perez, V. 2017. 3-D fossils for K-12 education: a case example using the giant extinct shark Carcharocles megalodon. The Paleontological Society Papers 22: 197–209. Crossref
Kallal, R.J., Godfrey, S.J., and Ortner, D.J. 2012. Bone reactions on a Pliocene cetacean rib indicate short-term survival of predation event. International Journal of Osteoarchaeology 22: 253–260. Crossref
Kent, B.W. (in press). The cartilaginous fishes (chimaeras, sharks and rays) of Calvert Cliffs, Maryland, USA. In: S.J. Godfrey (ed.), Geology and Vertebrate Paleontology of Calvert Cliffs. Smithsonian Contributions to Paleobiology 100, 45–160. Smithsonian Institution Press, Washington, DC.
Kidwell, S.M., Powars, D.S., Edwards, L.E., and Vogt, P.R. 2015. Miocene stratigraphy and paleoenvironments of the Calvert Cliffs, Maryland. In: D.K. Brezinski, J.P. Halka, and R.A. Ortt, Jr., (eds.), Tripping from the Fall Line: Field Excursions for the GSA Annual Meeting, Baltimore 2015, Field Guide 40, 231–279. Geological Society of America.
Lambert, O., Bianucci, G., Urbina, M., and Geisler, J.H. 2017. A new inioid (Cetacea, Odontoceti, Delphinida) from the Miocene of Peru and the origin of modern dolphin and porpoise families. Zoological Journal of the Linnean Society 179: 919–946.
Long, D.J. and Jones, R.E. 1996. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. In: A.P. Klimley and D.G. Ainley (eds.), Great White Sharks: the Biology of Carcharodon carcharias, 293–307. Academic Press, San Diego. Crossref
Perez, V., Godfrey, S.J., Kent, B., Weems, R., and Nance, J. (in press). The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes); lateral cusplet loss through time. Journal of Vertebrate Paleontology.
Pimiento, C. and Balk, M.A. 2015. Body-size trends of the extinct giant shark Carcharocles megalodon: a deep-time perspective on marine apex predators. Paleobiology 41: 479–490. Crossref
Purdy, R.W. 1996. Paleoecology of fossil white sharks. In: A.P. Klimley and D.G. Ainley (eds.), Great White Sharks: the Biology of Carcharodon carcharias, 67–78. Academic Press, San Diego. Crossref
Purdy, R.W., Schneider, V.P., Applegate, S.P., McLellan, J.H., Meyer, R.L., and Slaughter, B.H. 2001. The Neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina. In: C.E. Ray and D.J. Bohaska (eds.), Geology and Paleontology at Lee Creek Mine, North Carolina, III, Smithsonian Contributions to Paleobiology 90, 71–202. Smithsonian Institution Press, Washington, DC.
Ray, C.E. (ed.) 1983. Geology and Paleontology of the Lee Creek Mine, North Carolina, I. Smithsonian Contributions to Paleobiology 53. 529 pp. Smithsonian Institution Press, Washington, DC.
Ray, C.E. (ed.) 1987. Geology and Paleontology of the Lee Creek Mine, North Carolina, II. Smithsonian Contributions to Paleobiology 61. 283 pp. Smithsonian Institution Press, Washington, DC.
Ray, C.E. and Bohaska, D.J. (eds.) 2001. Geology and Paleontology of the Lee Creek Mine, North Carolina, III. Smithsonian Contributions to Paleobiology 90. 365 pp. Smithsonian Institution Press, Washington, DC.
Ray, C.E., Bohaska, D.J., Koretsky, I.A., Ward, L.W., and Barnes, L.G. (eds.) 2008. Geology and Paleontology of the Lee Creek Mine, North Carolina, IV. Virginia Museum of Natural History Special Publication 14. 517 pp. Virginia Museum of Natural History Press, Martinsville.
Renz, M. 2002. Megalodon, Hunting the Hunter. 159 pp. PaleoPress, Lehigh Acres.
Shimada, K. 2003.The relationship between the tooth size and total body length in the white shark, Carcharodon carcharias (Lamniformes: Lamnidae). Journal of Fossil Research 35: 28–33.
Takakuwa, Y. 2014. A dense occurrence of teeth of fossil “mako” shark (“Isurus” hastalis: Chondrichthyes, Lamniformes), associated with a balaenopterid-whale skeleton of the Late Miocene Pisco Formation, Peru, South America. Bulletin of the Gunma Museum of Natural History 18: 77–86.
Uhen, M.D. 2004. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the Middle to Late Eocene of Egypt. University of Michigan Papers on Paleontology 34: 1–222.
Stephen J. Godfrey [Stephen.Godfrey@calvertcountymd.gov], Department of Paleontology, Calvert Marine Museum, PO Box 97, Solomons, Maryland, 20688, USA; National Museum of Natural History, Smithsonian Institution, Washington DC, 20560, USA.
Mike Ellwood [mellwood@comcast.net], 2234 Birch Road, Port Republic, Maryland, USA.
Stephen Groff [groff.sss@verizon.net], 11539 Tomahawk Trail, Lusby, Maryland, USA.
Michael Scott Verdin [thebeanpole65@yahoo.com], 3403 Willow St., Chesapeake Beach, Maryland, USA.
Received 17 April 2018, accepted 25 June 2018, available online 9 August 2018.
Copyright © 2018 S.J. Godfrey et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 63 (3): 463–468, 2018
https://doi.org/10.4202/app.00495.2018