

The first pipizine hoverfly from the Oligocene of Céreste, France
VALENTIN NIDERGAS, JIŘÍ HADRAVA, ROMAIN GARROUSTE, JAKUB PROKOP, THOMAS SCHUBNEL, and ANDRÉ NEL
Nidergas, V., Hadrava, J., Garrouste, R., Prokop, J., Schubnel, T., and Nel, A. 2018. The first pipizine hoverfly from the Oligocene of Céreste, France. Acta Palaeontologica Polonica 63 (3): 539–548.
The Oligocene Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov. is the first fossil Pipizinae found in the lacustrine outcrop of Céreste (South-East of France). It differs from the other Pipizinae in the male genitalia, with a surstylus without tooth and shorter than epandrium, and a long epandrium with a very deep and narrow median theca. It is compared to other extant and fossil Pipizinae. Its position in this clade is supported by its inclusion in previous morphological phylogenetic analysis of the Syrphidae. Palaeoecological inferences for the paleobiota of Céreste are made based on this taxon and point to the presence of a mixed forest. The taphonomy of these flies is discussed. They were probably embedded in surface microbial mats. The pollinator role of Oligopipiza quadriguttata is also discussed on the basis of the presence of pollen surrounding the fossil flies.
Key words: Insecta, Syrphidae, Pipizinae, palaeoecology, Paleogene, France.
Valentin Nidergas [valentin.nidergas@gmail.com], Romain Garrouste [garroust@mnhn.fr], Thomas Schubnel [thomas.schubnel@wanadoo.fr], and André Nel [anel@mnhn.fr] (corresponding author), Institut Systématique Evolution Biodiversité (ISYEB), Muséum national d’Histoire naturelle, CNRS, Sorbonne Université, EPHE, 57 rue Cuvier, CP 50, F-75005 Paris, France.
Jiří Hadrava [hadravaj@natur.cuni.cz] and Jakub Prokop [jprokop@natur.cuni.cz], Department of Zoology, Faculty of Science, Charles University, Viničná 7, CZ-128 43, Praha 2, Czech Republic; and Institute of Entomology, Biological Centre, Czech Academy of Science, Branišovská 31, CZ–370 05 České Budějovice, Czech Republic.
Received 2 May 2018, accepted 15 June 2018, available online 20 July 2018.
Copyright © 2018 V. Nidergas et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
With more than 6000 described extant species living in all climatic regions (except Antarctica, Spitsbergen, and some remote oceanic islands), the Syrphidae are one of largest families of Diptera in number of species. They have important roles in ecosystems as pollinators (Ssymank et al. 2008; Glaum 2017; Lefèbvre et al. 2018) and as aphid predators (Rojo et al. 2003; Bičík and Láska 2011). Grimaldi (1999) considered that the Syrphidae radiated during the Cretaceous. Wiegmann et al. (2003) dated the clade Schizophora between 107 and 84 Myrs and considered the Syrphidae as their sister group, suggesting the same minimum age for this family. Wiegmann et al. (2011) dated the Syrphidae from ca. 100 Myrs. The “mid” Cretaceous corresponds to the period of radiation and diversification of the angiosperms (Barba-Montoya et al. 2018). Young et al. (2016) and Pauli et al. (2018) recently proposed the Syrphidae as sister group of the Pipunculidae + Schizophora. Nevertheless, the oldest accurate fossil members of the Syrphidae and Pipunculidae are dated from the Eocene (Dirickxs 2009; Archibald et al. 2014). Kovalev (1979) recorded what may be the oldest example of Syrphidae from the Late Cretaceous resins of the Taimyr in Siberia, but this fossil has never been described or re-examined (Evenhuis 1994). Fossil Syrphidae are poorly known compared to extant hoverflies, with ca. 106 described species all over the world (Hull 1945; Evenhuis 1994; Kotthoff and Schmid 2005; Dirickx 2009). The last description of a fossil syrphid dates back thirteen years ago (Kotthoff and Schmid 2005).
The extant Pipizinae are widely spread in all world ecozones except in the Polar ecozones and the Afrotropical region (Thompson 1972; Vujić et al. 2013; Mengual et. al. 2015). It contains approximately 180 species distributed in eight genera: Claussenia Vujić and Ståhls, 2013, Cryptopipiza Mutin, 1998, Heringia Rondani, 1856, Neocnemodon Goffe, 1944, Pipiza Fallén, 1856, Pipizella Rondani, 1856, Trichopsomyia Williston, 1888, and Triglyphus Loew, 1840. Pipizines have long been considered as a tribe of the subfamilies Syrphinae or Eristalinae (Ståhls et al. 2003; Hippa and Ståhls 2005). They have strong morphological similarity with the Eristalinae (e.g., pilose postpronotum), but they share ecological and biological characteristics with the Syrphinae (Mengual et al. 2015). Larvae are aphid predators on several species of plants, like Rumex Linnaeus, 1753, Prunus Linnaeus, 1753 or Ulmus Linnaeus, 1753 according to the “Syrph The Net” database (Speight and Castella 2016).
Only few fossils are currently attributed to the Pipizinae, viz., Pipiza melanderi Hull, 1945 (lowermost Oligocene of Florissant, USA), Pipiza venilia Heyden, 1870 (Oligocene of Rott, Germany), Pseudopipiza antiqua Hull, 1945, Pseudopipiza europa Hull, 1945, and Palaeopipiza xenos Meunier, 1902, (Eocene, Baltic amber) (Heyden 1870; Meunier 1902; Hull 1945; Evenhuis 1994). Théobald (1937) described and listed all the known fossil Syrphidae from France, but none was described from the middle Oligocene of Lubéron. In this paper, we describe one new genus and species of the Pipizinae from the middle Oligocene of Céreste, Lubéron, in Southern France. We also discuss the systematic position of the newly described pipizine fossil based on a morphology phylogenetic analysis.
Institutional abbreviations.—RNGL, Public Museum of the Réserve Naturelle Géologique du Lubéron, Apt, France.
Other abbreviations.—BSE, back-scattered electrons; dm-cu, distal median cubital crossvein; EDS, energy-dispersive X-ray spectroscopy; M1, first branch of median vein; R1, R2+3, R4+5, branches of radius; r-m, radial median crossvein; Sc, subcostal vein; sv, supplementary pseudovein.
Material and methods
In total, 15 fossil specimens of the new genus and new species were collected, by digging and splitting the laminites with a hammer and a spatula, during the last 25 years in the National Geological Reserve of Lubéron, Cereste, France, from middle Oligocene clay-limestone laminite (base of late Rupelian) (Cautru and Gigot 1982; Ducreux et al. 1985). The 15 specimens of the type series, described herein, are deposited at the public collection of the Réserve Naturelle Géologique du Lubéron at Apt, France. All the specimens were chosen to form the type material.
The Oligocene paleolatitude of the outcrop of Céreste was very close to the modern one. The paleoclimate in the area was cooler than during the Eocene but still relatively warm (Pound and Salzmann 2017). The layers that contain insects at Céreste are very thin clay-limestone laminite rich in Silica and calcium carbonates. The fossil insects contain a great quantity of carbon and sulfur, unlike the sediment (SOM: fig. 1 in Supplementary Online Material available at http://app.pan.pl/SOM/app63-Nidergas_etal_SOM.pdf). The good 3D preservation of very tiny morphological structures (setae on eyes, body and wings) suggests that the insects are compressed “mummies”. These animals were probably embedded in microbial mats that were originally floating at the water surface (Nel 1991).
Drawings were made with a drawing tube attached to a Nikon SMZ1500 stereomicroscope and digitized using Adobe ® Photoshop-Elements 12. Then, drawings were vectorized and reworked with Inkscape v0.92. Photographs were made with a reflex camera Nikon D800 mounted on a Nikon SMZ25 stereomicroscope, edited with DxO PhotoLab v1.1.2 and then focus-stacked with the “D-map” algorithm on Zerene Stacker v1.04. We used environmental scanning electron microscopy (ESEM) to obtain microstructural details of these fossils, with the back-scattered electrons (BSE) mode. We used Energy-dispersive X-ray spectroscopy (EDS) in order to analyze the element composition of the fossils and its matrix. Then the spectra were plotted in R and reworked on Inkscape v.0.92. Electron microscopy in BSE mode (SEM BSE) makes it possible to highlight differences in chemical composition (atomic number contrast) between the mineral matrix and the fossilized residues, in addition to the microtopographic differences revealed by the conventional mode. EDS spectrometry makes it possible to know the chemical nature of these differences and to identify the elements involved in the fossilization process, which can provide information on the sedimentary conditions, the taphonomic process and the initial nature of the fossilized organic elements. These two methods are mainly allowed by the use of the environmental mode to overcome the need to metallize the samples.
We followed Thompson (1999) for morphology nomenclature. The systematics of hoverflies follows Mengual et al. (2015). A new phylogenetic analysis to assess the systematic position of this new fossil was performed using 111 characters and 96 species, including the new fossil taxon, based on the morphological matrix used by Mengual et al. (2015), based on 111 characters and 96 species including the new fossil taxon (see SOM; Mengual et al. 2015; Hippa and Ståhls 2005). The tree research was performed using Wagner’s parsimony in PAUP* 4.0 b10 (Swofford 2002), with the following parameters: heuristic search 100 replicates, non-ordered characters, and TBR recombination.
Systematic palaeontology
Order Diptera Linnaeus, 1758
Family Syrphidae Latreille, 1802
Subfamily Pipizinae Williston, 1885
Genus Oligopipiza Nidergas, Hadrava, and Nel nov.
Etymology: Combination of Oligo in reference to Oligocene and Pipiza, the type genus of the subfamily.
Type species: Oligopipiza quadriguttata sp. nov., by monotypy, see below.
Diagnosis.—Same as for the type species.
Oligopipiza quadriguttata Nidergas, Hadrava, and Nel sp. nov.
Figs. 1–7.
Etymology: Combination of Latin quadric, four and guttata, drop; in reference to the shape of the yellow maculae on tergites II and III.
Type material: Holotype: RNGL-S01 (male). Paratypes: RNGL-S10 (female allotype), RNGL-S03, RNGL-S07, RNGL-S08 (males), RNGL-S02, RNGL-S04–06, RNGL-S09–15 (females). Nearly complete specimens with wings and legs preserved as imprint and counter-imprint; all from the type locality; coll. AN.
Type locality: Céreste, Lubéron, South-East France.
Type horizon: Middle Oligocene.
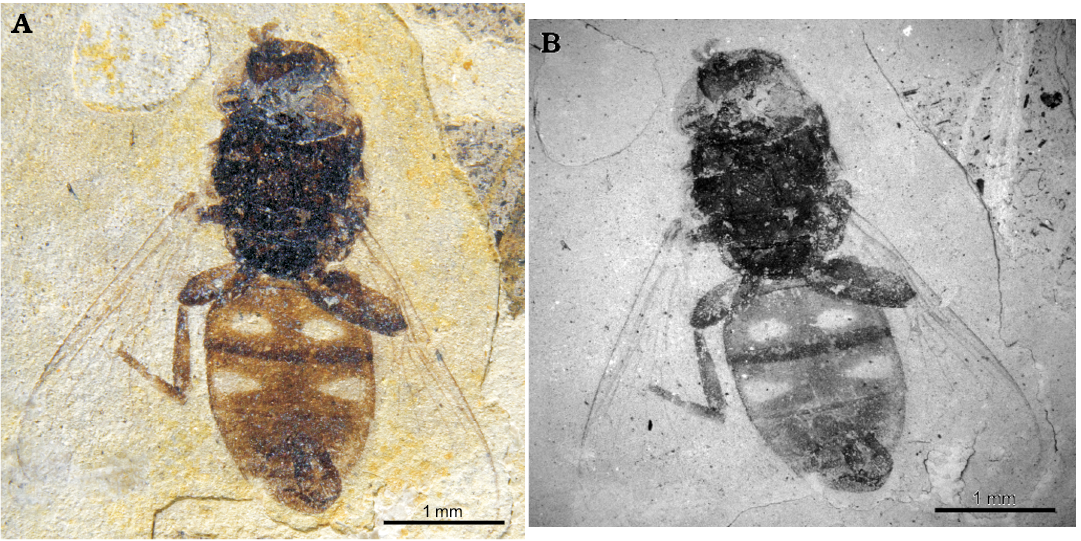
Fig. 1. Pipizine hoverfly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., holotype RNGL-S01; Rupelian, Céreste, France. Imprint of male habitus. Normal light photograph (A), ESEM photograph (B).
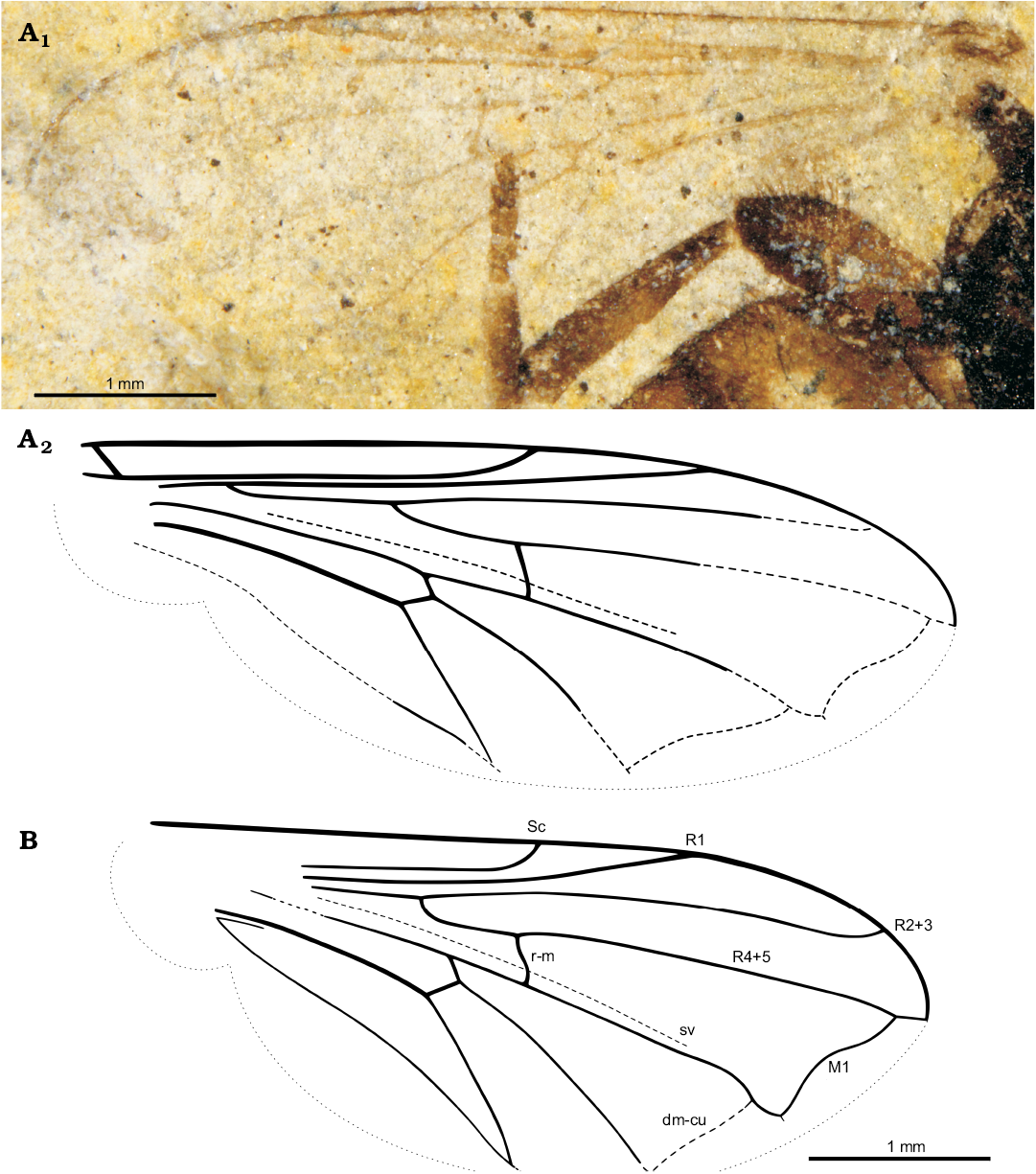
Fig. 2. Pipizine hoverfly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., Rupelian, Céreste, France. Wing venations. A. Holotype RNGL-S01, normal light photograph (A1), reconstruction (A2). B. Allotype RNGL-S10, reconstruction. Abbreviations: dm-cu, distal median-cubital crossvein; M1, first branch of median vein; R1, R2+3, R4+5, branches of radius; r-m, radial median crossvein; Sc, subcostal vein; sv, supplementary pseudovein.
Material.—Type material only.
Diagnosis.—Medium-sized species, ca. 6–8 mm long; face simple, flat; basoflagellomere rounded; hind femora 4× longer than wide; abdomen ovoid, 1.5× longer than wide, dark; tergites II and III with two yellow maculae on each.
Head without frontal prominence; face simple, not protruding, and apparently concave below antennal fossa; antenna shorter than head, arista bare; no pronounced gibbosity above antennal bases; eyes pilose (Fig. 3B), holoptic in male, dichoptic in female; anterior mesopleuron possibly pilose; posterior anepisternum and anterior anepimeron hairy; metanotum (subscutellum) bare; no bristles on posterior margin of scutellum; no spurs on mid and hind coxae and hind trochanters; crossvein r-m slightly curved and ending before middle of cell dm; R2+3 perpendicular to costa; R4+5 making a strong curve basal of r-m, straight distal of r-m, and slightly curved just before crossvein M1 insertion; cell R4+5 acute apically because M1 is making an acute angle with the vein R4+5; R4+5 pedicel straight and short; abdomen emarginated, not petiolate, oval; four visible abdominal tergites in male, five in female; tergites II to IV entirely pilose; male genitalia very large, about 0.4× abdomen length; surstylus without tooth, shorter than epandrium, epandrium long, with a very deep and narrow median theca, from cerci to basal 3/10 of theca.
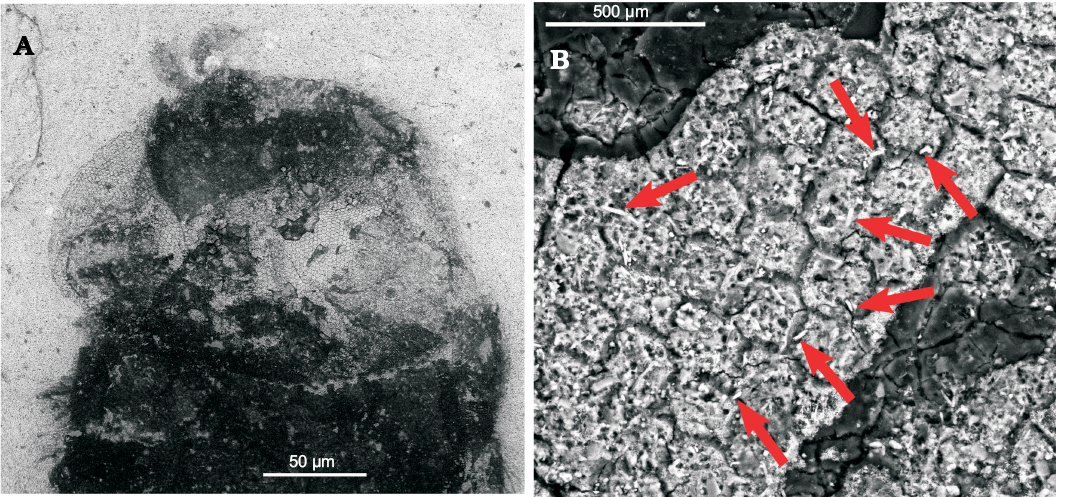
Fig. 3. Pipizine hoverfly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., holotype RNGL-S01, Rupelian, Céreste, France. A. Head, ESEM photograph. B. Eye’s omatidia, arrows point to microtrichia.
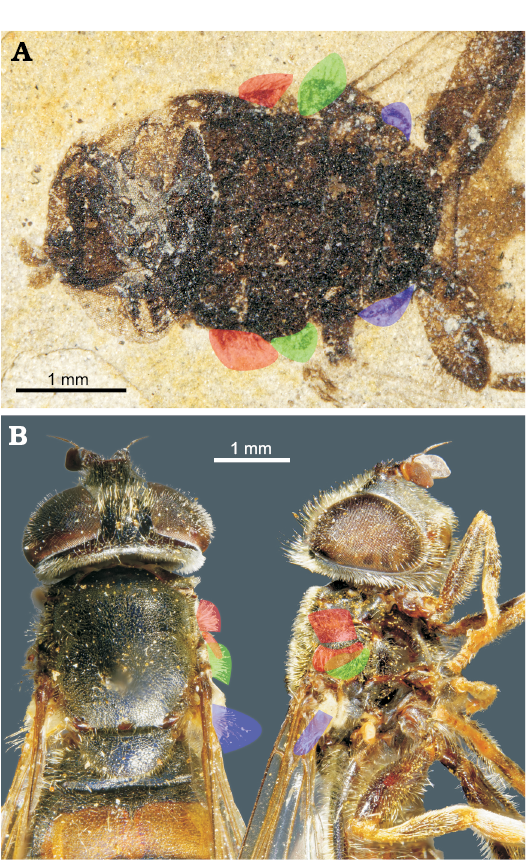
Fig. 4. Dorso-lateral pilosity of thoracic pleura of pipizine hoverflies. A. Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., holotype RNGL-S01, Rupelian, Céreste, France. B. Extant Pipiza festiva Meigen, 1822. Red, posterior anepisternal setae; green, anepimeral setae; blue, calypter setae.
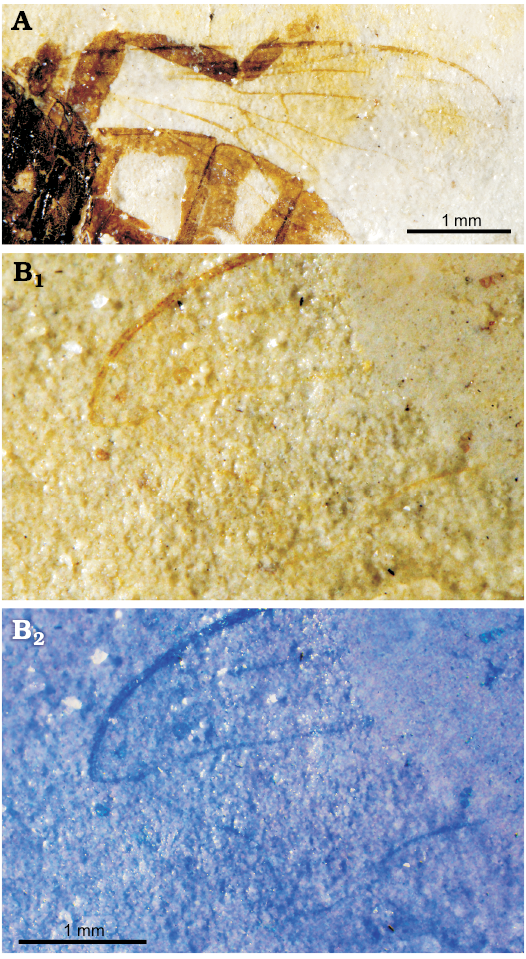
Fig. 5. Pipizine hoverfly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., allotype RNGL-S10, Rupelian, Céreste, France. A. Wing. B. Wing apex, normal light photograph (B1), treated to better show veins (B2).
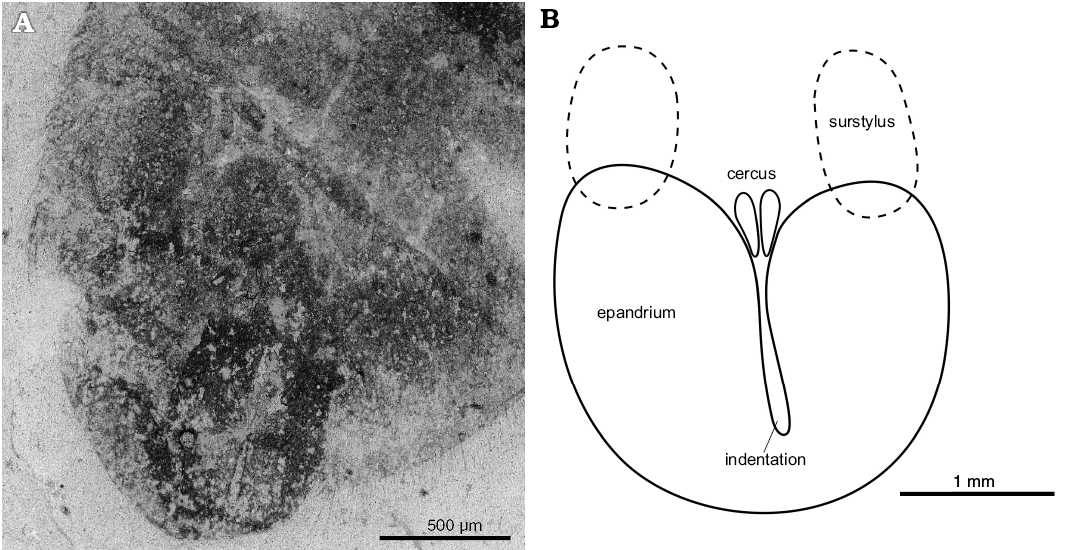
Fig. 6. Pipizine hovefly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., holotype RNGL-S01, Rupelian, Céreste. Male epandrium. ESEM photograph (A), reconstruction (B).
Description.—The abdominal ornamentation pattern on tergites II and III is subject to variations in size and shape (Fig. 7), but the general pattern is the same, viz. two ovoid to sub-rectangular yellow maculae on tergite II and two “water drop-shaped” to sub-triangular maculae on tergite III. The asymmetry of these spots is probably due to taphonomical artefacts.
Holotype (male RNGL-S01): Head: face simple, not protruding, apparently concave below antennal fossa; frons rounded; eyes holoptic, suture 0.15× tergite III length, anterior angle about 100–110°, size of omatidia uniform all over eyes, size 22.5 µm, about 0.45× maximum width of epandrium theca indentation; antenna very short, less than 1/5 as long as head length, scape and basoflagellomere rounded, arista at basal third of basoflagellomere, apparently bare.
Thorax: mesoscutum and scutellum apparently completely dark; at least posterior anepisternum and anterior anepimeron hairy.
Legs: covered with pale short bristles; hind femora 4× as long as wide, locally covered with long hairs between apical 1/5 and apical 3/5, about 2× longer than hind tibia basal width; apex of hind tibia 1.8× as large as base; length of hind metatarsus about 0.56× hind tibia length; hind tarsomeres II, III, IV, and V of equal lengths; claws basally pale and black in its apical third.
Wing: hyaline, with pale veins; R4+5 and R2+3 straight, crossvein r-m before middle of cell dm, spurious vein sv distinct.
Abdomen entirely covered with hairs, as long as basal width of hind tibia, apparently erected as it is visible for those situated on the sides of tergites; tergite I dark; tergites II and III dark, each with two pale maculae, probably originally yellow; tergite IV completely dark.
Genitalia: theca of epandrium about 0.5× as long as tergite III, about 1.1× as long as wide, with a deep and narrow indentation opened from cerci to basal 3/10, 7.1× as long as wide; cerci about 3× times as long as maximum width of epandrium indentation; surstyli short without tooth; hypandrium not visible.
Allotype (female RNGL-S10): It completes the description as follows: frons pilose; wing 4.5 mm long; crossvein r-m ending at basal fourth of cell dm, cell R4+5 acute apically, upper crossvein M1 curved at its center; tergite I with posterior margin wave-shaped. Terminalia not visible. Sexual dimorphism: eyes dichoptic; tergite V visible; absence of hairs on hind femur (but this character could be a fossilization artefact).
Dimensions (taken on all specimens): body lengths vary between 6.8–8.1 mm (average value 7.4 mm); abdomen lengths vary between 2.7–4.5 mm (average value 3.6 mm); widths vary between 2.1–3.1 mm (average value 2.5 mm).
Stratigraphic and geographic range.—Rupelian (33.9–28.4 MYR BP), middle Oligocene; Céreste, at the boundary between the Vaucluse and the Alpes-de-Haute-Provence (43.9°N, 5.6°E), France.
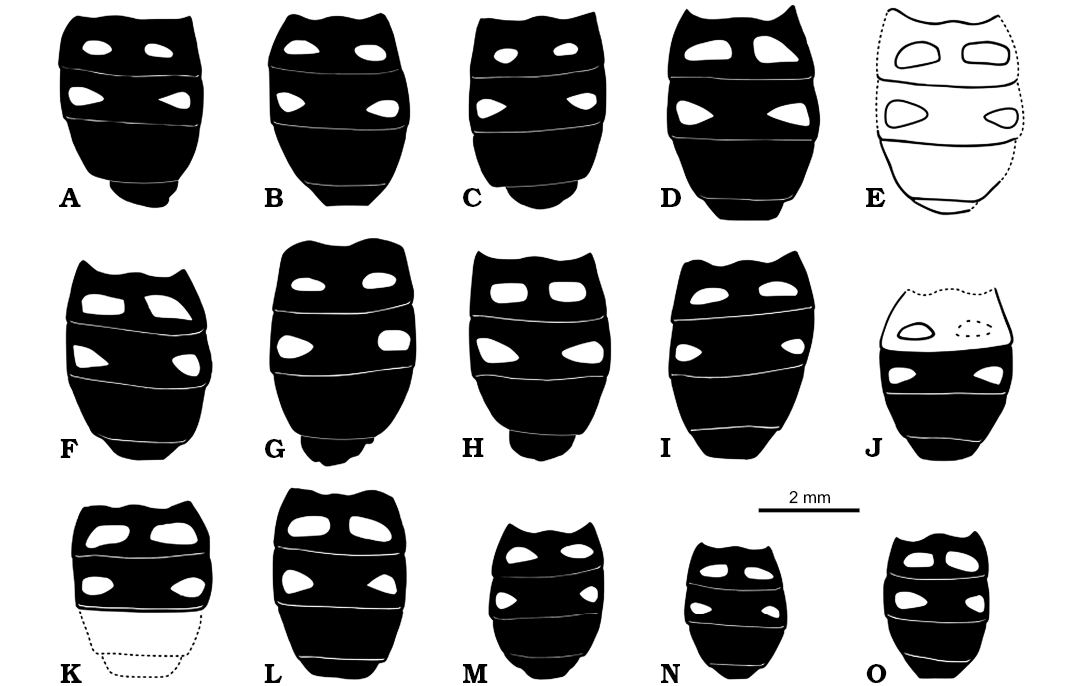
Fig. 7. Pipizine hoverfly Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov., Rupélian, Céreste, France. Reconstructions of abdomens of all type specimens. A. RNGL-S1 (holotype, male). B. RNGL-S2 (female). C. RNGL-S3 (male). D. RNGL-S4 (female). E. RNGL-S5 (female). F. RNGL-S6 (female). G. RNGL-S7 (male). H. RNGL- RNGL-S8 (male). I. RNGL-S9 (female). J. RNGL-S10 (female). K. RNGL-S11 (female). L. RNGL-S12 (female). M. RNGL-S13 (female). N. RNGL-S14 (female). O. RNGL-S15 (female).
Discussion
Oligopipiza gen. nov. is indisputably attributed to the Syrphidae due to the characteristic wing venation, and can be attributed to the Pipizinae because of the following characters (after Hippa and Ståhls 2005; Vujić et al. 2013; and Mengual et al. 2015): shape of face simple (reversal, present in Pipizinae, but also in Microdon [Microdontinae] and Eumerus [Merodontini]); arista bare; metanotum (subscutellum) bare (present in Pipizinae, but also Microdon and Ubristes [Microdontinae]); microtrichia retrolaterally on apical part of metatibia absent (character of Pipizinae but also in some genera of other tribes); wing vein R4+5 straight, not sinuate; crossvein r-m sub-perpendicular to R4+5 and ending before middle of discal cell; vein M1 making an acute angle with R4+5; antetergite fused to tergite I (general shape of tergite I identical to that of Pipizella) (character of the Pipizinae but also is? present in other tribes); 1st abdominal spiracle and metasternum of pipizine type (character of the Pipizini but also in some genera of other tribes; Thompson 1972: fig. 2.3); abdominal terga laterally bordered (presence of a thicker sclerotized line at margins of tergites). These characters are putative synapomorphies of the Pipizinae but they are subject to convergencies in some other tribes. Nevertheless their combination is found only in Pipizinae.
Oligopipiza differs from the extant genera of Pipizinae as follows: Cryptopipiza Mutin, 1998 (replacement name for Pseudopipiza Violovitsh, 1985), has a surstylus longer than the epandrium, itself very broad and short, while the surstylus is shorter than epandrium in Oligopipiza (Violovitsh 1985). Triglyphus Loew, 1840 is excluded because Oligopipiza has more than three visible abdominal tergites (three in Triglyphus). The median indentation between the two lobes of male epandrium is very deep and narrow, so that the basal part of epandrium is very narrow, which is not the case for Heringia Róndani, 1856 and Pipiza Fallén, 1810 (Vujić et al. 2008, 2013). The head of Pipiza is also angular between the frons and the face, at the lunule, unlike Oligopipiza, which does not have this angle. Oligopipiza could share with Trichopsomyia Williston, 1888 the possibly pilose anterior mesopleuron, but its median indentation between the two lobes of male epandrium is very deep and narrow, unlike in this extant genus (Thompson 1981). Neocnemodon Goffe, 1944 is excluded because of the absence of spurs on mid and hind coxae and hind trochanters in Oligopipiza (Thompson 1972; Speight and Smith 1975). Affinities with Claussenia Vujić and Ståhls, 2013 are excluded because the female of Oligopipiza has no bristles on posterior margin of scutellum while Claussenia has 4–6 such long back bristles (Claussen et al. 1994; Vujić et al. 2013). Additionaly Claussenia has a tooth on the surstylus unlike Oligopipiza. Pipizella Róndani, 1856 is excluded because the cell R4+5 of Oligopipiza is acute apically as upper crossvein M1 is not perpendicular to R4+5 and the basoflagellomere is rounded (not elongated as in Pipizella or Heringia).
Among the fossil syrphids attributed to the Pipizinae, Oligopipiza has the eyes not densely pilose unlike Palaeopipiza xenos Meunier, 1902, and their ornamentations of the abdomen are different. Hull (1945) suggested placing Palaeopipiza in the Eumerini. After Meunier (1902) and Hull (1945), the type of Pipiza venilia should be restudied; its generic attribution is uncertain. Pipiza melanderi has pale spots on all abdominal segments unlike Oligopipiza. The genus Pseudopipiza Hull, 1945 is supposed to differ from Pipiza “in the face and in the confluence point of the apical cross vein M1 being practically at wing tip” (Hull 1945: 294), which is clearly the case in the type species Pseudopipiza antiqua but not in Pseudopipiza europa (see Hull 1945: pl. 13: 113 and 121). The later has its confluence point between R4+5 and M1 very far from wing margin. Pseudopipiza europa should be revised. Pseudopipiza differs from Oligopipiza as follows: a very short crossvein r-m (long in Oligopipiza), part of M basal of bm-cu making a strong angle with part of M distal of it, part of R4+5 basal of r-m straight (making a strong curve in Oligopipiza), and crossvein dm-cu far from the posterior wing margin (close to it in Oligopipiza).
Phylogenetic placement of Oligopipiza and its implications.—The phylogenetic analysis gave 650 equally most parsimonious cladograms, of length = 945 steps. The strict consensus and the semi-strict consensus cladograms (Fig. 8) differ only in the hierarchy between the three species of Triglyphus. The obtained phylogeny is similar to the combined morphological and molecular one of Mengual et al. (2015: fig. 2). The Eristalinae appear non monophyletic with the Microdontinae between “inner” and ”outer” Ersitalinae. The Pipizinae and the Syrphinae are sister groups. The Pipizinae are monophyletic, but their phylogeny is clearly less solved than in Mengual et al. (2015). Oligopipiza falls in this clade, supporting the attribution of this taxon.
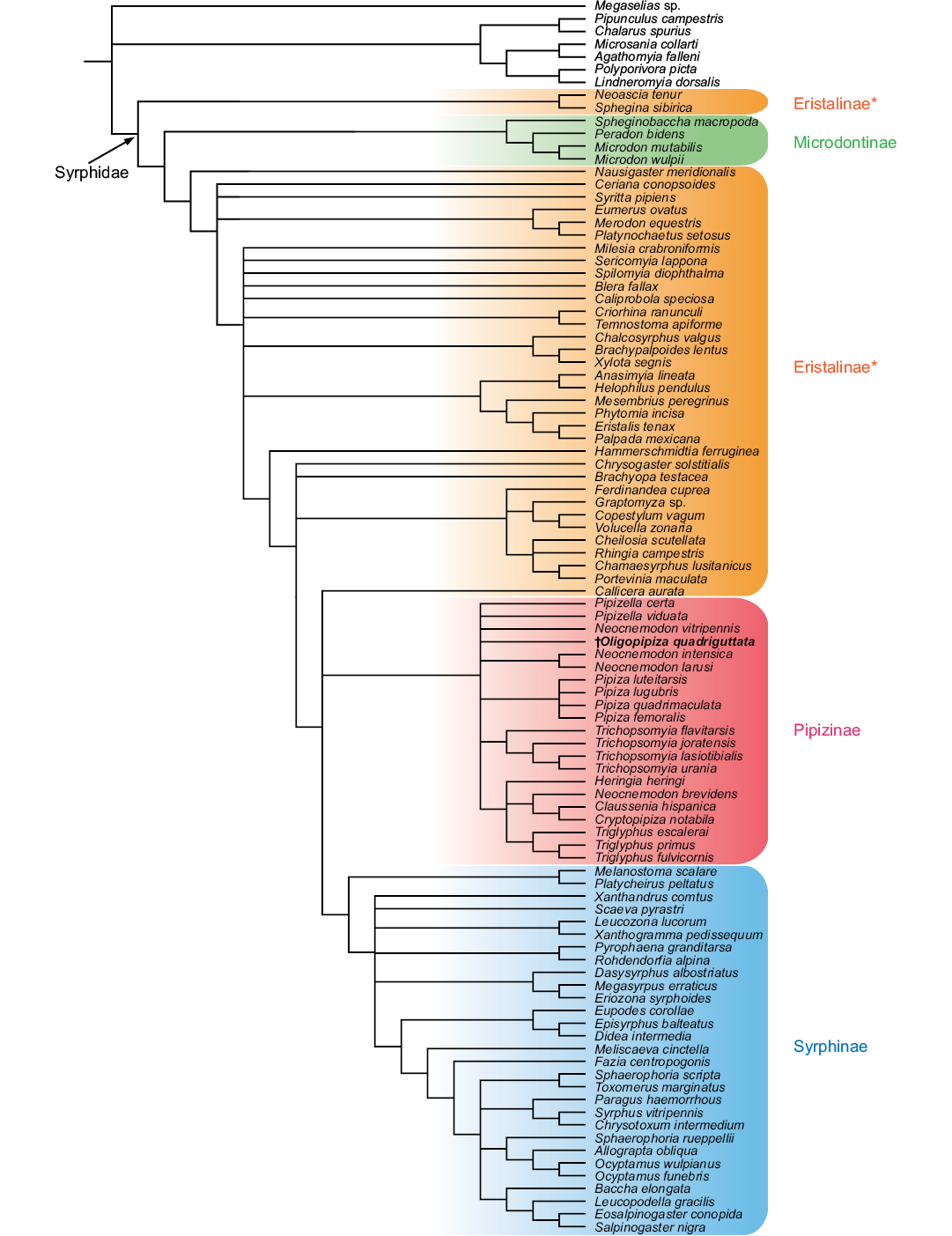
Fig. 8. Syrphid semi-strict consensus cladogram based on morphological characters after Mengual et al. (2015), with Oligopipiza added.
Palaeoecological inferences for Oligopipiza quadriguttata.—About 23% of extant European species of pipizine hoverflies are associated with coniferous/mixed forests, with 57% for Neocnemodon species, 66% for Trichopsomyia, and 16% for Pipizella (Speight and Castella 2016). The paleoflora from Céreste corresponds to a mixed mesophytic forest with Pinus spp., under a tropical warm climate (Thiébaut 1999; Gregor 2002). We found numerous pollen grains around the majority of the specimens of Oligopipiza quadriguttata, which have two aerial bags, about 60 µm in size (SOM: fig. 2), so they probably are pollens of Pinaceae. Even if only macrofossil of Pinus sp. are recorded at Céreste (Gregor 2002), some palynological analyses by extraction in these sediments need to be done. This observation suggests that O. quadriguttata was living in a mixed forest, confirming the results of Gregor (2002), but we cannot confirm it was a pollinator of conifer because there is no extant species of European pipizine pollinators of conifers. Fossil Aphididae and Psylloidea are known from the same outcrop (Heie and Lutz 2002), Oligopipiza was probably a predator of these insects, as for the extant taxa (Mengual et al. 2015).
Conclusions
We described Oligopipiza quadriguttata Nidergras, Hadrava, and Nel gen. et sp. nov. representing the first known Syrphidae from the Oligocene of the lacustrine outcrop of Céreste (Lubéron, France). It belongs to the subfamily Pipizinae, confirming that this clade was already rather diverse during the Eocene–Oligocene. It also supports the presence of a mixed forest in the corresponding palaeoenvironment.
Acknowledgements
We sincerely thank an anonymous referee and Ximo Mengual (Zoologisches Forschungsmuseum Alexander Koenig, Leibniz Institut für Biodiversität der Tiere, Bonn, Germany) for their useful comments on the first version of the paper. We also thank Ximo Mengual (Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany) for his help providing us with the matrix of characters used in Mengual et al. (2015) for the phylogenetic analysis. We also thank Emmanuel Delfosse and Christophe Daugeron (both Muséum national d’Histoire naturelle, Paris, France) for their courtesy and help in the consultation in collections of extant Syrphidae. We thank Sylvain Pont (Muséum national d’Histoire naturelle, Paris, France) for the acquisitions of the environmental SEM and spectroscopy X-ray imaging (MNHN Microscopy Platform facilities). The work of JH was supported by the Institutional Research Support grant of the Charles University, Prague (No. SVV 260 434/2018).
References
Archibald, S.B., Kehlmaier, C., and Mathewes, R.W. 2014. Early Eocene big headed flies (Diptera: Pipunculidae) from the Okanagan Highlands, western North America. The Canadian Entomologist 146: 429–443. Crossref
Barba-Montoya, J., dos Reis, M., Schneider, H., Donoghue, P.C.J., and Yang, Z.-H. 2018. Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution. New Phytologist 218: 819–834. Crossref
Bičík, V. and Láska, P. 2011. Comparison of the importance of aphid predators and parasitoids based on field samples. Acta Societatis Zoologicae Bohemicae 75: 1–6.
Cautru, J.P. and Gigot, P. 1982. L’Oligocène lignitifère du bassin de Manosque-Forcalquier (Alpes-de-Haute-Provence). Etude géologique de la partie nord du bassin (synclinal d’Apt-Forcalquier). Inventaire des Ressources Nationales de Charbon. 180 pp. BRGM, Paris.
Claussen, C., Goeldlin de Tiefenau, P., and Lucas, J.A.W. 1994. Zur Identität von Pipizella heringii (Zetterstedt) var. hispanica Strobl, 1909 – mit einer Typenrevision der paläarktischen Arten der Gattung Heringia Róndani, 1856, sensu stricto (Diptera, Syrphidae). Mitteilungen der Schweizerischen Entomologischen Gesellschaft 67: 309–326.
Dirickx, H. 2009. Les Syrphidae (Diptera) revisités. L’héritage du passé. Bulletin de la Société Royale Belge d’Entomologie 145: 49–86.
Ducreux, J.L., Hugueney, M., and Truc, G. 1985. La formation des calcaires et lignites de Sigonce (Oligocène moyen, bassin de Forcalquier, Alpes-de-Haute-Provence): datation à l’aide des mammifères; reconstitution des milieux de dépôts. Geobios 18: 109–114. Crossref
Evenhuis, N.L. 1994. Catalogue of the Fossil Flies of the World (Insecta: Diptera). 600 pp. Backhuys Publishers, Leiden.
Glaum, P. 1997. A theoretical basis for the study of predatory syrphid fly ecology. Theoretical Ecology 10: 391–402. Crossref
Gregor, H.-J. von 2002. Die fossile Megaflora von Cereste in der Provence. Flora Tertiaria Mediterranea 4: 1–55.
Grimaldi, D. 1999. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden 86: 373–406. Crossref
Heie, O.E. and Lutz, H. 2002. Fossil aphids from Early Oligocene deposits near Céreste, France, with descriptions of new genera and species (Hemiptera, Sternorrhyncha, Aphidoidea). Mainzer Naturwissenchaften Archiv 40: 113–122.
Heyden, L. von 1870. Fossile Dipteren aus der Braunkohle von Rott im Siebengebirge. Palaeontographica 17: 237–266.
Hippa, H. and Ståhls, G. 2005. Morphological characters of adult Syrphidae: descriptions and phylogenetic utility. Acta Zoologica Fennica 215: 1–72.
Hull, F.M. 1945. A revisional study of the fossil Syrphidae (Diptera). Bulletin of the Museum of Comparative Zoology 95: 215–355.
Kotthoff, U. and Schmid, U. 2005. A new fossil hoverfly (Insecta, Diptera: Syrphidae) from the Randeck Maar (Early Miocene, Sout-west Germany). Palaeontology 48: 1091–1096. Crossref
Kovalev, V.G. 1979. The main aspects in the evolution of Diptera Brachycera in the Mesozoic Era [in Russian]. In: O.A. Skarlato, K.V. Skufjin, E.P. Narchuk, O.P. Negrobov, and M.N. Kandybina (eds.), Èkologičeskie i morfologičeskie principy sistematiki dvukrylyh (Insecta), 35–37. Akademiâ Nauk SSSR, Zoologičeskij Institut, Leningrad.
Lefebvre, V., Villemant, C., Fontaine, C., and Daugeron, C. 2018. Altitudinal, temporal and trophic partitioning of flower-visitors in Alpine communities. Scientific Reports 8 (4706): 1–12. Crossref
Mengual, X., Ståhls, G., and Rojo, S. 2015. Phylogenetic relationships and taxonomic ranking of pipizine flower flies (Diptera: Syrphidae) with implications for the evolution of aphidophagy. Cladistics 31: 491–508. Crossref
Meunier, F. 1902. Description de quelques diptères de l’ambre. Annales de la Société Scientifique de Bruxelles 26: 94–104.
Nel, A. 1991. Analyse d’entomofaunes cénozoiques. Intérêt de la Paléoentomologie pour les Sciences de la Terre et de la Vie. 882 pp. Thèse de Doctorat (nouveau régime), Université de Reims-Champagne-Ardenne, Reims.
Pauli, T., Burt, T.O., Meusemann, K., Bayless, K., Donath, A., Podsiadlowski, L., Mayer, C., Kozlov, A., Vasilikopoulos, A., Liu, S., Zhou, X., Yeates, D., Misof, B., Peters, R.S., and Mengual, X. 2018. New data, same story: phylogenomics does not support Syrphoidea (Diptera: Syrphidae, Pipunculidae). Systematic Entomology 43: 447–459. Crossref
Pound, M.J. and Salzmann, U. 2017. Heterogeneity in global vegetation and terrestrial climate change during the late Eocene to early Oligocene transition. Scientific Reports 7 (43386): 1–12. Crossref
Rojo, S., Gilbert, F., Marcos-García, M.A., Nieto, J.M., and Mier Durante, M.P. 2003. A World Review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and their Prey. 319 pp. CIBIO Ediciones, Alicante.
Speight, M.C.D. and Smith, K.G.V. 1975. A key to males of the British species of Neocnemodon Goffe (Dipt.: Syrphidae). Entomologist’s Record 87: 150–153.
Speight, M.C.D. and Castella, E. 2016. StN Content and Glossary of Terms 2016. Syrph the Net, the database of European Syrphidae (Diptera) 94: 1–89.
Ståhls, G., Hippa, H., Rotheray, G., Muona, J., and Gilbert, F. 2003. Phylogeny of Syrphidae (Diptera) inferred from combined analysis of molecular and morphological characters. Systematic Entomology 28: 433–450. Crossref
Swofford, D.L. 2002. PAUP* Phylogenetic Analysis Using Parcimony (*and Other Methods). Version 4.0 b10. Sinauer Associates.
Ssymank A., Kearns C.A., Pape T., and Thompson F.C. 2008. Pollinating flies (Diptera): a major contribution to plant diversity and agricultureal production. Biodiversity 9 (1–2): 86–89. Crossref
Théobald, N. 1937. Les insectes fossiles des terrains oligocènes de France. Bulletin Mensuel (Mémoires) de la Société des Sciences de Nancy 1: 1–473.
Thiébaut, M. 1999. A new locality of Raskya vetusta (Ettingshausen) Manchester & Hably from France. Revue de Paléobiologie 18: 509–515.
Thompson, F.C. 1972. A contribution to a generic revision of the Neotropical Milesinae (Diptera: Syrphidae). Arquivos de Zoologia 23: 73–215.
Thompson, F.C. 1981. The flower flies of the West Indies (Diptera: Syrphidae). Memoirs of the Entomological Society of Washington 9: 1–200.
Thompson, F.C. 1999. A key to the genera of the flower flies (Diptera: Syrphidae) of the Neotropical region including descriptions of new genera and species and a glossary of taxonomic terms. Contributions on Entomology, International 3: 321–378.
Violovitsh, N.A. 1985. New flower flies (Diptera, Syrphidae) of the Palaearctic fauna [in Russian]. Novye i Maloizvestnye Vidy Fauny Sibiri (Supplement) 18: 80–96.
Vujić, A., Radenković, S., and Polić, D. 2008. A review of the luteitarsis group of the genus Pipiza Fallén (Diptera: Syrphidae) with description of a new species from the Balkan Peninsula. Zootaxa 1845: 33–46.
Vujić, A., Ståhls, G., Ačanski, J., Bygebjerg, R., and Stefanović, A. 2013. Systematics of Pipizini and taxonomy of European Pipiza Fallen: molecular and morphological evidence (Diptera, Syrphidae). Zoologica Scripta 42: 288–305. Crossref
Wiegmann, B.M., Trautwein, M.D., Winkler, I.S., Barr, N.B., Kim, J.-W., Lambkin, C., Bertone, M.A., Cassel, B.K., Bayless, K.M., Heimberg, A.M., Wheeler, B.M., Peterson, K.J., Pape, T., Sinclair, B.J., Skevington, J.H., Blagoderov, V., Caravas, J., Kutty, S.N., Schmidt-Ott, U., Kampmeier, G.E., Thompson, F.C., Grimaldi, D.A., Beckenbach, A.T., Courtney, G.W., Friedrich, M., Meier, R., and Yeates, D.K. 2011. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences of the United States of America 108: 5691–5695. Crossref
Wiegmann, B.M., Yeates, D.K., Thorne, J.L., and Kishino, H. 2003. Time flies, a new molecular time-scale for brachyceran fly evolution without a clock. Systematic Biology 52: 745–756. Crossref
Young, A.D., Lemmon, A.R., Skevington, J.H., Mengual, X., Ståhls, G., Reemer, M., Jordaens, K., Kelso, S., Lemmon, E.M., Hauser, M., De Meyer, M., Misof, B., and Wiegmann, B.M. 2016. Anchored enrichment dataset for true flies (order Diptera) reveals insights into the phylogeny of flower flies (family Syrphidae). BMC Evolutionary Biology 16: 1–13. Crossref
Acta Palaeontol. Pol. 63 (3): 539–548, 2018
https://doi.org/10.4202/app.00500.2018