

The first green lacewings from the late Eocene Baltic amber
VLADIMIR N. MAKARKIN, SONJA WEDMANN, and THOMAS WEITERSCHAN
Makarkin, V.N., Wedmann, S., and Weiterschan, T. 2018. The first green lacewings from the late Eocene Baltic amber. Acta Palaeontologica Polonica 63 (3): 527–537.
Pseudosencera baltica gen. et sp. nov. of Chrysopinae (Chrysopidae, Neuroptera) is described from Baltic amber. Additionally, another species, Nothochrysa? sp. (Nothochrysinae), is left in the open nomenclature. Pseudosencera baltica gen. et sp. nov. represents the oldest confident record of Chrysopinae. The new genus lacks the apparent forewing intramedian cell, and possesses three character states not found in other Chrysopinae: the simple AA1, the short basal crossvein between M and Cu, and 5‒6 rings of setae on the antennal flagellomeres. This genus is probably a specialised form in a basal branch of Chrysopinae, that could not be attributed to any of the known tribes. The specimen of Nothochrysa? sp. consists only of fragments of the forewings. The late Eocene Baltic amber represents the oldest horizon where Chrysopinae and Nothochrysinae are found to coexist. It is highly likely that Chrysopidae were extremely rare in these forests.
Key words: Neuroptera, Chrysopinae, Nothochrysinae, Cenozoic, Baltic amber.
Vladimir N. Makarkin [vnmakarkin@mail.ru], Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok 690022, Russia.
Sonja Wedmann [Sonja.Wedmann@senckenberg.de], Senckenberg Forschungsstation Grube Messel, Markstrasse 35, D-64409 Messel, Germany.
Thomas Weiterschan [thomas.weiterschan@web.de], Forsteler Strasse 1, 64739 Höchst Odw., Germany.
Received 16 May 2018, accepted 5 July 2018, available online 23 July 2018.
Copyright © 2018 V.N. Makarkin et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The family Chrysopidae (green lacewings) today is one of the most speciose neuropteran groups with a nearly wordwide distribution. It is divided into four subfamilies: Limaiinae (Middle Jurassic to Paleogene), Nothochrysinae, and Chrysopinae (both Paleogene to Recent), and Apochrysinae (Recent). The fossil Mesochrysopidae and Corydasialidae were recently considered as chrysopid subfamilies by Engel et al. (2018), but these should be considered as separate families, because they are distinguished from Chrysopidae by many characters (see Makarkin and Menon 2005; Liu et al. 2017). The fossil record of green lacewings is rich, consisting of 64 named species known from the Middle Jurassic to the Pliocene (Archibald and Makarkin 2015; Khramov et al. 2016; Khramov 2018; Lu et al. 2018).
Three rather distinct phases may be identified in the evolutionary history of Chrysopidae: (i) the Mesozoic, when the extinct subfamily Limaiinae dominated (at least from the Middle Jurassic to at least the mid-Cretaceous); (ii) the Paleogene, when Nothochrysinae were dominant among chrysopids; and (iii) the Neogene to Recent, when Chrysopinae became the most dominant group (Archibald et al. 2014). In the Cenozoic, the late Eocene to the Oligocene was the most interesting period in chrysopid evolution when the transition from the “nothochrysine” to “chrysopine” phase occurred. However, chrysopids from this interval are poorly studied; nearly all of them belong to Nothochrysinae (e.g., Carpenter 1935; Adams 1967; Nel and Séméria 1986; CoBabe et al. 2002). Only one species from the late Eocene of France is an alleged chrysopine (Séméria and Nel 1990). Therefore, the discovery of other chrysopine species from the late Eocene is of high interest.
Chrysopids occur very rarely in the late Eocene Baltic amber, and hitherto no species had been described. The first mention of a chrysopid specimen in Baltic amber was by Carl Berendt in Göppert and Berendt (1845). Since then this information has been repeated many times (e.g., Hagen 1852; Giebel 1856; Scudder 1885, 1890, 1891; Handlirsch 1907, 1921; Krüger 1923). However, this specimen has never been described (or even illustrated), and it is probably lost now. The first documented record of Chrysopidae from Baltic amber was a photographed adult specimen in Weitschat and Wichard (1998: pl. 55d). The depository of this specimen is unknown, and it has also not been described. Several chrysopid larvae are also known from Baltic amber, but again none of these have been described (see Scheven 2004; Weitschat 2009; Makarkin and Archibald 2013).
In this paper, we describe the first chrysopids from Baltic amber, a new genus and species and species in open nomenclature. These chrysopids belong to two different subfamilies, Chrysopinae and Nothochrysinae, respectively.
Institutional abbreviations.—SMF, Senckenberg Research Institute and Natural History Museum Frankfurt, Germany.
Other abbreviations.—AA1–3, first to third anterior analis; CuA, anterior cubitus; CuA1, first (proximal-most) branch of CuA; CuP, posterior cubitus; M, media; MA and MP, anterior and posterior branches of the media; Psc, pseudocubitus; Psm, pseudomedia; RA, anterior radius; RP, posterior radius; RP1–5, first (proximal-most) to fifth branches of RP; ScP, posterior subcosta; 1aa1-aa2, 1aa2-aa3, first (basal) crossvein between AA1 and AA2, AA2 and AA3, respectively; 1im, first crossvein between MA and MP; 1r-m, first (basal) crossvein between R and M; 1scp-r, first (proximal-most) crossvein connecting ScP and R/RA; 1icu, 2icu, first (basal) and second crossveins between CuA and CuP, respectively; 2m-cu, second crossvein between M/MP and Cu/CuA.
Material and methods
Specimens.—This study is based on two specimens from Baltic amber, deposited in the amber collection of the Senckenberg Museum Frankfurt am Main (SF). Line drawings were prepared by Thomas Weiterschan (Figs. 3B, 4) and Vladimir Makarkin (Fig. 6), while photographs were taken by Sonja Wedmann and Thomas Weiterschan using a Leica MZ12.5 stereomicroscope and an attached Nikon D300 digital camera. Extension of depth of focus was achieved by stacking several photos using Helicon Focus, version 5.3 X 64.
Terminology.—We use the general venational terminology of Kukalová-Peck and Lawrence (2004) in the interpretation of Yang et al. (2012, 2014). The venation of Chrysopinae is highly specialized, with numerous fusions (Fig. 1). Correct interpretation of the venation is mainly inferred from the pupal wing tracheation of Mallada signatus (Schneider, 1851) and Chrysopa nigricornis Burmeister, 1839 (see Tillyard 1916: fig. 5; Comstock 1918: fig. 184). The most important transformations in the venation of Chrysopinae are listed below. (i) In both wings, the aligned sections of MA, MP (only in the forewing of most species), the branches of RP, and the crossveins connecting them form the pseudomedia, and the aligned sections of CuA, MP, MA, the branches of RP, and the crossveins connecting them form the pseudocubitus. (ii) In the forewings of most species of Chrysopinae, MA and MP are fused for some distance to form the intramedian cell (i.e., the proximal-most cell between MA and MP) (see Breitkreuz et al. 2017: fig. 16). However, this cell appears to be absent in several species of Chrysopinae (including Pseudosencera baltica gen. et sp. nov.) and all Apochrysinae. We believe that the intramedian cell is actually present but its posterior side (i.e., proximal part of MP) is fused with CuA and 2m-cu is reduced. In particular, this can be supported by that fact in the extant Leptochrysa prisca Adams and Penny, 1992 (which may be the only living Limaiinae; Makarkin and Archibald 2013), the proximal part of MP within the intramedian cell is very close to CuA and 2m-cu is extremely short or reduced (see Adams and Penny 1992: fig. 10). Therefore, we follow Adams (1967: fig. 44), not Breitkreuz et al. (2017: fig. 15B), in the interpretation of the intramedian cell of Apochrysinae and Pseudosencera baltica gen. et sp. nov. (iii) The chrysopine CuA in the hind wing is interpreted to be partially fused with MP. Similarly configured CuA in Apochrysinae is also treated as partially fused with MP (see e.g., Breitkreuz et al. 2017: fig.15B), although the pupal wing tracheation of no species in this subfamily have been examined. (iv) In all Chrysopinae, MA in the hind wing is basally fused with RP.
Crossveins are designated after the longitudinal veins which they connect and are numbered in sequence from the wing base, e.g., 1scp-r, first (proximal-most) crossvein connecting ScP and R/RA; 2icu, second crossvein between CuA and CuP; 1im, proximal crossvein between MA and MP. Terminology of wing spaces and details of venation (e.g., veinlets) follows Oswald (1993).
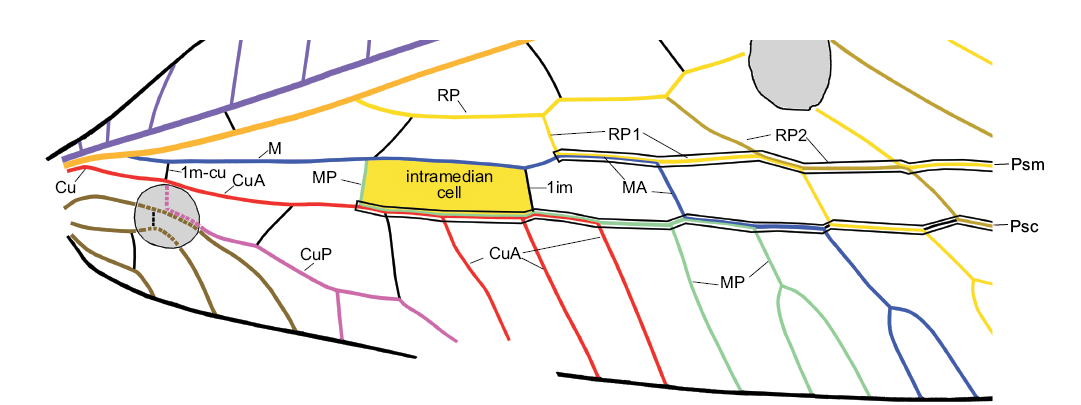
Fig. 1. The intramedian cell, pseudomedia and pseudocubitus in the forewing of green lacewing Pseudosencera baltica gen. et sp. nov. from the late Eocene Baltic amber. Psm and Psc are outlined black in this figure. Abbreviations: 1im, first crossvein between MA and MP; 1m-cu, first (basal) crossvein between M and Cu; CuA, anterior cubitus; CuP, posterior cubitus; M, media; MA and MP, anterior and posterior branches of the media; Psc, pseudocubitus; Psm, pseudomedia; RP, posterior radius; RP1, proximal-most branch of RP; ScP, posterior subcosta.
Systematic palaeontology
Order Neuroptera Linnaeus, 1758
Family Chrysopidae Schneider, 1851
Subfamily Chrysopinae Schneider, 1851
Genus Pseudosencera nov.
Type species: Pseudosencera baltica sp. nov.; by monotypy, see below.
Etymology: From the Greek adjective pseudes [ψευδής], false, and Sencera, a genus-group name.
Diagnosis.—As for the type species by monotypy.
Remarks.—Pseudosencera gen. nov. may be easily distinguished from all other Chrysopinae by the simple AA1 and the short 1m-cu. Of the genera lacking the apparent intramedian cell (see discussion below), the new genus resembles the subgenus Sencera Navás, 1925 (of the genus Ankylopteryx Brauer, 1864) more than the genus Nesochrysa Navás, 1910 as their hind wings are narrow and have similar venation (see Breitkreuz et al. 2015: fig. 10; New 1980: fig. 78), and the antennae are not longer than the forewing.
Pseudosencera baltica sp. nov.
Figs. 2–4.
Etymology: In reference to Baltic amber.
Holotype: SMF-Be-2518; an incompletely preserved specimen.
Type locality: Baltic amber (precise collecting locality unknown).
Type horizon: Late Eocene.
Diagnosis.—Antennal flagellomeres with 5‒6 rings of setae. In forewing, RA relatively short, entering margin far before wing apex; basal subcostal crossvein (1scp-r) located far proximad origin of RP; single distal subcostal crossvein; apparent intramedian cell absent; Psm well developed, straight; Psc well developed, slightly zigzagged; AA1 simple; basal crossvein 1m-cu short; distal crossvein 2icu very long. In hind wing, MA fused with RP proximally; Psm well developed, straight; CuA partially fused with MP.
Description.—Head: vertex not elevated; eyes large; ventral border of clypeus slightly concave. Frontal sutures and epistomal suture well developed. Anterior margin of labrum deeply concave. Galea enlarged (long, broad) with short digitus (finger-like apical process). Maxillary palpus five-segmented: two basal segments appear short; third to fifth (terminal) segments elongate (relative length of third to fifth segments: 1.5–1–2.4); terminal segment longest, fusiform. Labial palpus three-segmented; all segments elongate, terminal segment fusiform. Antennae incompletely preserved: scapus rather broad, but short; pedicellus elongate (ca. 1.4 as long as wide), probably very slightly constricted medially (poorly visible); proximal flagellomeres elongate, each probably with 5‒6 rings of setae (difficult to count).
Details of thorax not clearly discernible; pronotum dorsally covered with rare long setae. Forelegs and mid-legs shorter than hind legs; all legs covered (especially femur and tibia) with dense, relatively long, fine setae.
Abdomen: complete, but poorly preserved.
Forewing: 13.2 mm long, 4.6 mm wide (length/width ratio 2.9). Costal space moderately broad for most part, basally and apically narrowed; with simple subcostal veinlets relatively widely spaced, and closely spaced apically. ScP relatively short, entering margin far before wing apex. Subcostal space moderately broad; proximal subcostal crossvein (1scp-r) located very basally, nearly at mid-way from wing base to origin of RP; only one distal crossvein in area of pterostigma detected. Tympanum on basal portion of R not detectable with confidence. RA relatively short, entering margin before wing apex, with 4‒5 short, simple distal branches. Origin of RP located relatively close to wing base (at 0.18 of complete length), slightly proximad 2m-cu. Length of RP proximad 1r-m and 1r-m nearly equal. RA space (between RA and RP) relatively narrow, with 13 crossveins. RP strongly zigzagged, with 12 branches, four of them entering Psm; two distal-most branches simple, other branches forked. Basal crossvein 1r-m long, inclined at acute angle to M, connecting RP, M slightly distad fork of M. M appears to originate from R near wing base; forked at nearly a right angle and at level of origin of RP. Apparent intramedian cell absent (actually, it is formally represented by a square formed by the basal crossvein-like part of MP, the proximal part of MP+CuA, the crossvein 1im and the proximal part of MA, see below for details). MA proximally fused with RP1; distally (distad Psc) with two simple branches (left wing) or once forked branches (right wing). MP basally crossvein-like, then fused with CuA; distally (distad Psc) with two simple branches (left wing) or one simple, one forked branch (right wing). Psm well developed, nearly straight. Psc well developed, slightly zigzagged distally. Basal crossvein 1m-cu (“M5”) short, located opposite origin of CuP; 2m-cu absent. CuA partially fused with MP, probably with three long simple branches. CuP once forked, with widely-spaced branches (anterior branch more than twice longer than posterior branch). Two intracubital crossveins: 1icu long, located far proximad basal part of MP; 2icu very long, located distad posterior branch of CuP fork; length of CuP from 2icu to fork nearly equal to branch of CuP fork. Crossvein between CuP, AA1 very short. All anal veins short, simple; AA1 arched; AA2 incurved proximad 1aa1-aa2, arched after; AA3 incurved proximad 1aa2-aa3, nearly straight after. Two crossveins between anal veins: 1aa1-aa2 rather short; 1aa2-aa3 long. Two complete gradate series of crossveins, slightly divergent distally; 8 crossveins in inner series anterior to Psm; 10 crossveins in outer series anterior to Psc (i.e., RP3). Wing membrane apparently hyaline throughout.
Hind wing: markedly narrower than forewing; 11.0 mm long as preserved (estimated complete length ca. 11.8‒12.0 mm), ca. 3.1 mm wide. Costal space narrow, with simple subcostal veinlets relatively widely spaced before pterostigmal region (basally relatively closely spaced). Termination of ScP, RA not preserved. Subcostal space narrow; subcostal crossveins not detected. RP originates relatively near wing base, strongly zigzagged, with 11 preserved branches, four of them entering Psm. Psm well developed, straight. M basally not fused with R, forked slightly distad origin of RP. MA basally crossvein-like (oblique), then fused with RP for considerable distance; probably simple. MP probably simple. Psc well developed, slightly zigzagged. CuA fused with MP for some distance; one preserved branch (CuA1) originated before fusion with MP. Two gradate series of crossveins (incompletely preserved).
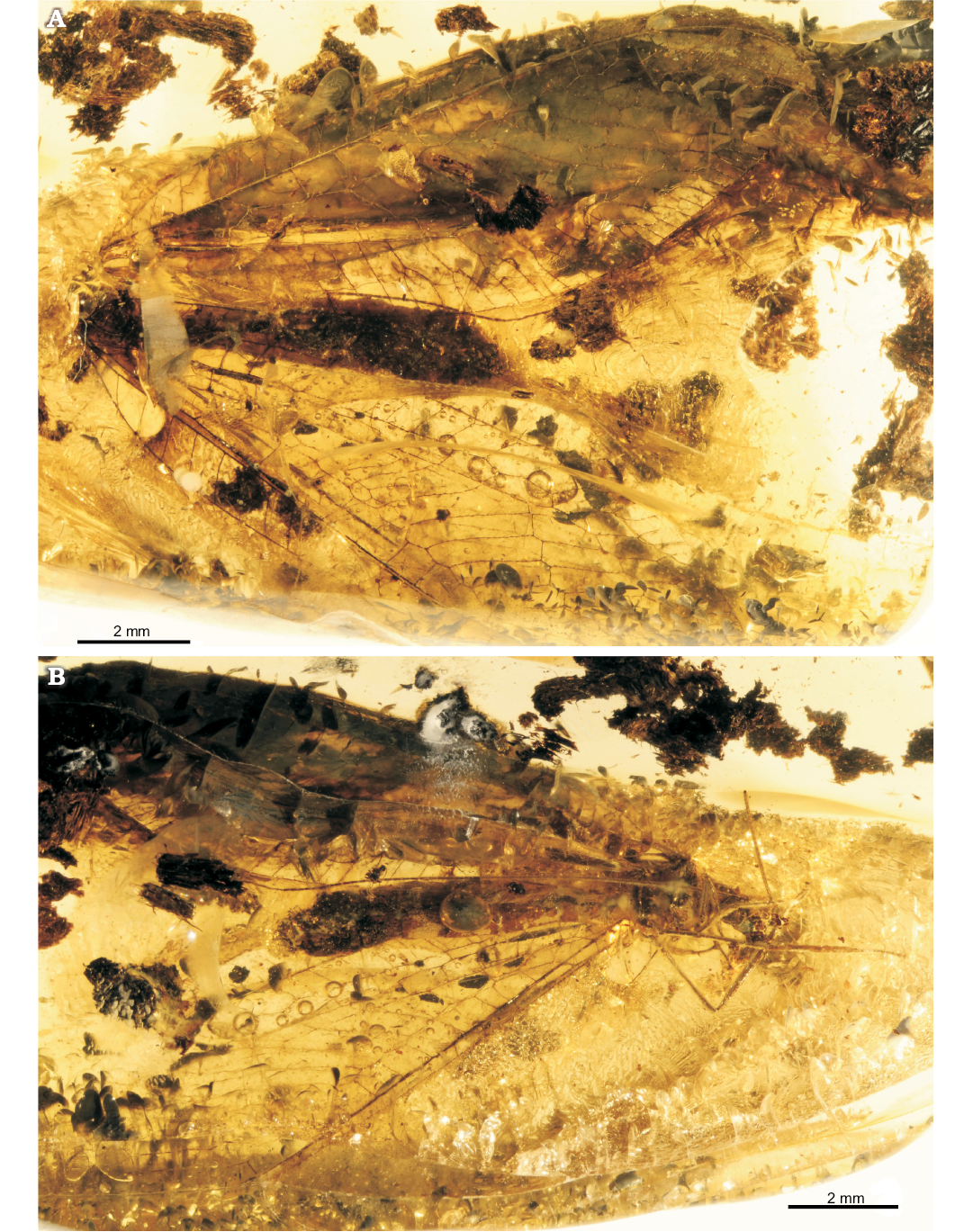
Fig. 2. Green lacewing Pseudosencera baltica gen. et sp. nov. from the late Eocene Baltic amber. Holotype (SMF-Be-2518) in dorsal (A) and lateral (B) views.
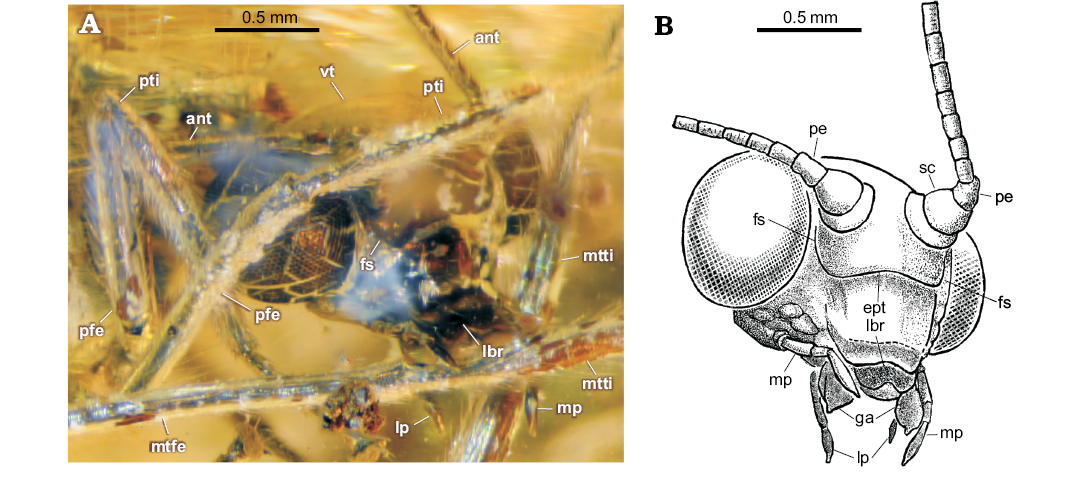
Fig. 3. Green lacewing Pseudosencera baltica gen. et sp. nov. from the late Eocene Baltic amber. Head and legs of the holotype SMF-Be-2518. A. Photograph, fronto-lateral view. B. Line drawing of the head, latero-frontal view. Abbreviations: ant, antenna; ept, epistomal suture; fs, frontal suture; ga, galea; lbr, labrum; lp, labial palpus; mp, maxillary palpus; mtfe, metafemur; mtti, metatibia; pe, pedicellus; pfe, profemur; pti, protibia; sc, scapus; vt, vertex.
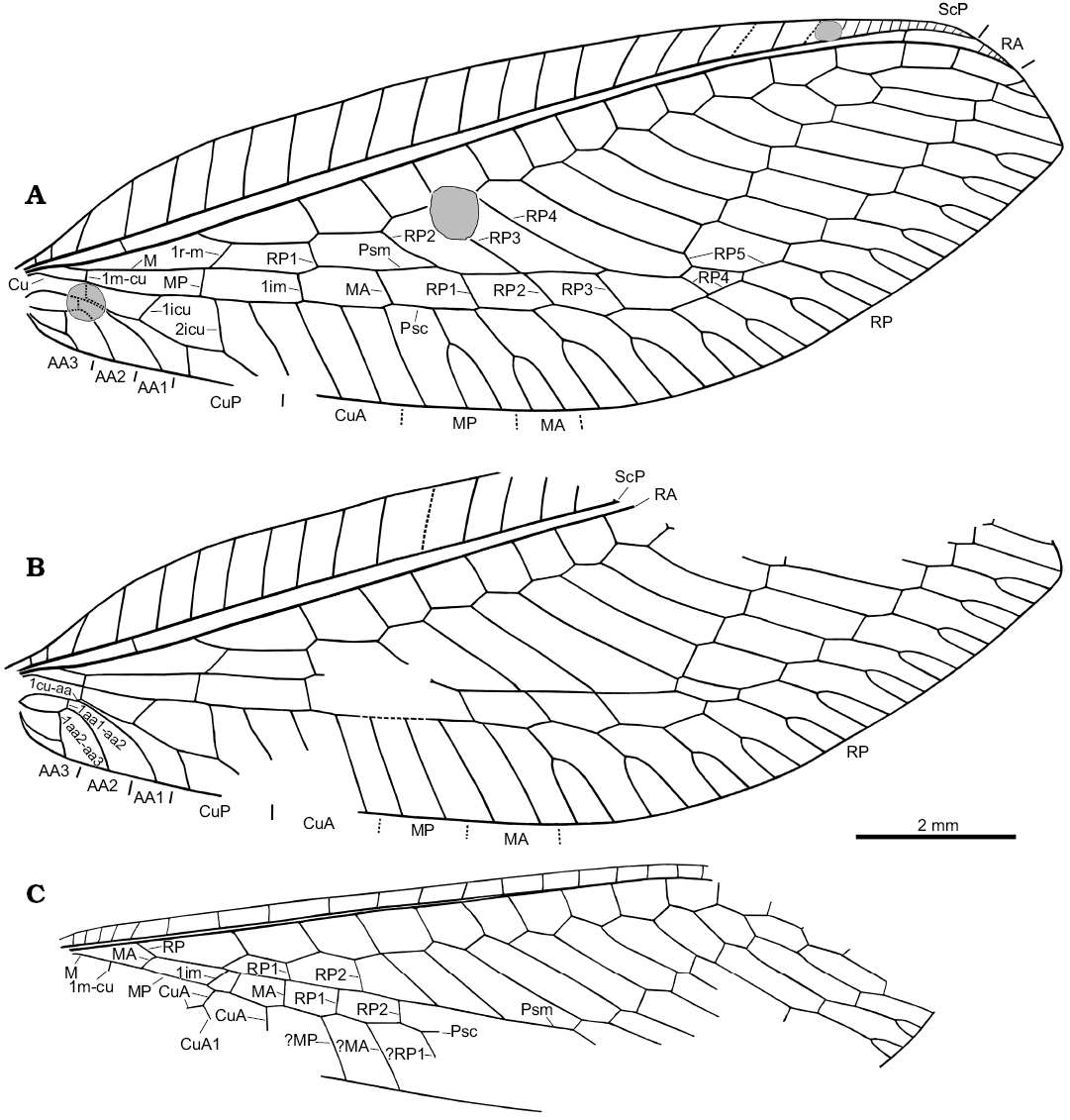
Fig. 4. Green lacewing Pseudosencera baltica gen. et sp. nov. from the late Eocene Baltic amber. Wing venation of the holotype SMF-Be-2518, right (A) and left (B) forewing, left hind wing (C). B, C converted to right dorsal view. Abbreviations: 1aa1-aa2, first crossvein between AA1 and AA2; 1aa2-aa3, first crossvein between AA2 and AA3; 1icu, first (basal) crossvein between CuA and CuP; 2icu, second crossvein between CuA and CuP; 1cu-aa, fist crossvein between CuP and AA1; 1im, first crossvein between MA and MP; 1m-cu, first (basal) crossvein between M and Cu; AA1–3, first to third anterior analis; CuA, anterior cubitus; CuA1, first (proximal-most) branch of CuA; CuP, posterior cubitus; M, media; MA and MP, anterior and posterior branches of the media; Psc, pseudocubitus; Psm, pseudomedia; RA, anterior radius; RP, posterior radius; RP1–5, first (proximal-most) to fifth branches of RP; ScP, posterior subcosta.
Remarks.—A chrysopid specimen figured by Weitschat and Wichard (1998: pl. 55d) appears to be conspecific with Pseudosencera baltica gen. et sp. nov. as its venation is nearly identical with that of the holotype. This specimen was previously treated as probably belonging to Belonopterygini (a tribe of Chrysopinae) by Makarkin and Archibald (2013) and Archibald et al. (2014), but the phylogenetic relationships of Pseudosencera baltica gen. et sp. nov. are obscure, what is discussed below. Its antennae (if they are completely preserved, what is rather likely) are markedly shorter than the forewing (nearly two-thirds).
Stratigraphic and geographic range.—Late Eocene Baltic amber.
Subfamily Nothochrysinae Navás, 1910
Nothochrysa? sp.
Figs. 5, 6.
Material.—SMF-Be-2464; two fragmentarily preserved forewings from late Eocene of Baltic amber (precise collecting locality unknown).
Description.—Forewing ca. 10.5 mm long as preserved (ca. 15–16 mm estimated). Distal part of costal space narrow. ScP long. Distal subcostal veinlets closely spaced, several forked. Subcostal space distally rather broad, with several distal crossveins (five detected). RA long, almost reaching wing apex; with four distal veinlets. RA space relatively narrow, with 16 preserved crossveins. RP with 19 preserved branches; of these, five entering into Psm. Psm, Psc probably well developed, slightly zigzagged. Space between Psm, Psc very broad distally. Psm continues into inner gradate series, which consists of 12 crossveins (left wing). Psc continues into outer gradate series, which consists of 15 crossveins (left wing).
Remarks.—The new species is assigned to Nothochrysinae due to the presence of two features characteristic of the subfamily: Psm continues into the inner gradate series of crossveins, and ScP and RA are long. In Chrysopinae, Psm continues distad the inner gradate series, and ScP and RA are much shorter. It is tentatively assigned to the genus Nothochrysa for the following reasons: the wings are rather large possessing numerous RP branches (about 20); the well-developed Psm and Psc; two series of gradate crossveins; and the long ScP and RA. Of the known nothochrysine genera, this set of character states is only present in Nothochrysa.
The genus Nothochrysa, with five extant species, is distributed in Europe, North Africa, Turkey, China, and North America (Adams 1967; Aspöck et al. 2001; Yang et al. 2005; Kovanci and Canbulat 2007). Two fossil species are known: Nothochrysa stampieni Nel and Séméria, 1986 from the Oligocene (late Rupelian/basal Chattian) of Aix-en-Provence (France), and N. praeclara Statz, 1936 from the early Miocene (Aquitanian) or latest Oligocene (Chattian) of Rott (Germany). The forewing venation of these fossil species is rather similar to that of the Baltic amber species.
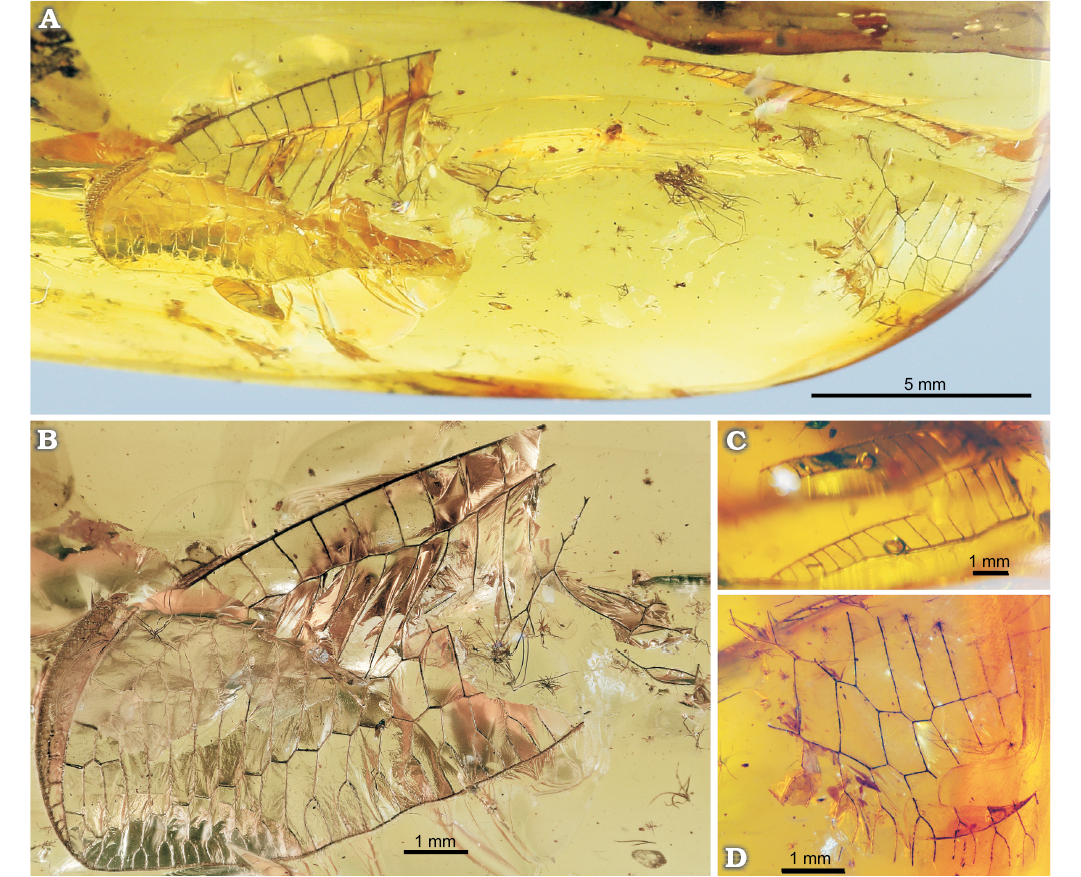
Fig. 5. Green lacewing Nothochrysa? sp. from the late Eocene Baltic amber. SMF-Be-2464, general view (A), left forewing (B), costal space of right forewing (C), fragment of right forewing (D).
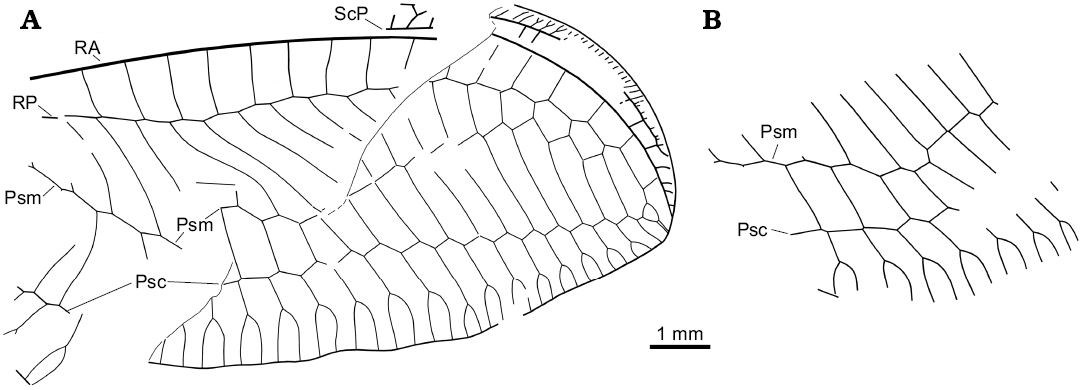
Fig. 6. Green lacewing Nothochrysa? sp. from the late Eocene Baltic amber. Wing venation of the SMF-Be-2464, left (A, converted to right dorsal view) and right (B) forewing. Abbreviations: Psc, pseudocubitus; Psm, pseudomedia; RA, anterior radius; RP, posterior radius; ScP, posterior subcosta.
Discussion
Imaginal chrysopids occur extremely rarely in Baltic amber. We have information only on five specimens: four Chrysopinae and one Nothochrysinae. Three of them most probably belong to Pseudosencera baltica gen. et sp. nov.: the holotype, the specimen reported by Weitschat and Wichard (1998: pl. 55d) mentioned above, and an unpublished specimen (in a private collection; VM personal observation). A fourth chrysopine specimen is in general similar to P. baltica gen. et sp. nov. but its wings are incomplete, and therefore its congeneric and conspecific status cannot be confirmed (in a private collection; VM personal observation). A single known nothochrysine specimen is described above; it is too fragmentary to discuss further. However, Pseudosencera baltica does possesses features that allow it to be discussed in detail. It is noteworthy that all the extant chrysopids, to which Pseudosencera gen. nov. is most similar, occur in tropical regions (see below). Therefore, we may presume that this genus is a thermophilic element in the Baltic amber neuropteran assemblage.
An analysis of characters of Pseudosencera gen. nov. shows that this genus cannot be assigned to Limaiinae and Nothochrysinae from its venation. Its most striking feature is the absence of the apparent forewing intramedian cell (actually, the intramedian cell is present, but MP is fused with CuA within it, see above). This character state is obviously apomorphic within Chrysopidae (see Brooks and Barnard 1990: 120), and occurs in two subfamilies: in all species of Apochrysinae and in four extant species of Chrysopinae: Belonopteryx arteriosa Gerstaecker, 1863 from Brazil and Argentina and two species of the genus Nesochrysa from Madagascar, i.e., Nesochrysa seyrigi (Navás, 1934) and N. grandidieri (Navás, 1913) (all Belonopterygini), and a single species of Sencera from southeastern Asia to Australia (Ankylopterygini) (see Navás 1913; Brooks and Barnard 1990; Breitkreuz et al. 2015).
Although Pseudosencera gen. nov. shares with Apochrysinae the absence of the apparent forewing intramedian cell, the venation of the latter otherwise strongly differs from that of this genus. Firstly, the basal crossvein 1scp-r is present in Pseudosencera gen. nov. (and in the vast majority of other Chrysopinae), however it is lost in all Apochrysinae as well as in the enigmatic genus Nothancyla Navás, 1910, which was recently confirmed to belong to Chrysopinae (Dai et al. 2016). Also, other forewing character states of Apochrysinae are not present in the new genus: e.g., the costal space is broad distally; the subcostal veinlets are closely spaced; Psm continues into the inner gradate series of crossveins; numerous crossveins are present anterior of the inner gradate series (often arranged into one or several additional gradate series); RP originates very close to the wing base. Further, MA in the hind wing in most Apochrysinae is not fused with the stem of RP (at most only touches it) (see e.g., Kimmins 1952: figs. 4–7, 9–11; Winterton and Brooks 2002: figs. 9, 10, 15), and the antennae are longer than the forewing in all Apochrysinae.
Of the four apomorphies of Apochrysinae proposed by Brooks (1997), i.e., Psm and Psc close together; the basal subcostal crossvein absent; the pedicel constricted, and antennal setae arranged in five rings, only the latter is found in Pseudosencera gen. nov. However, this condition occurs also in many Nothochrysinae and is probably plesiomorphic. Of the numerous synapomorphies of Apochrysinae (exclusive of Nothancyla) proposed by Winterton and Brooks (2002), only one character is detected in Pseudosencera gen. nov., i.e., the elongate setae on the femur and tibia. This feature, however, is too weak to support its assignment to this subfamily. Therefore, an apochrysyne affinity of Pseudosencera gen. nov. is very unlikely.
The general wing venation of Pseudosencera gen. nov. is similar to that of Chrysopinae. In particular, this affinity is supported by the location of the crossvein 1scp-r in the forewing: this crossvein is situated more basally in Chrysopinae (as found in Pseudosencera gen. nov.) than in Limaiinae and Nothochrysinae. The arrangement of the inner gradate series in the forewing of the new genus is also characteristic of Chrysopinae. The venation of the hind wing of Pseudosencera gen. nov. is typical for Chrysopinae.
However, the following three character states of Pseudosencera gen. nov. are not found in other Chrysopinae.
(i) The simple (non-forked) AA1. This condition often occurs in Mesozoic Limaiinae (e.g., Mesypochrysa Martynov, 1927 and Parabaisochrysa Lu, Wang, Ohl, and Liu, 2018; Makarkin 1997: figs. 1, 2; Khramov et al. 2016: fig. 3; Lu et al. 2018: fig. 2), and many Nothochrysinae, from the Eocene (e.g., Palaeochrysa Scudder, 1885; Asiachrysa Makarkin, 2014; some Tribochrysa Scudder, 1885; some Archaeochrysa Adams, 1967) and extant (all genera except Nothochrysa and Dictyochrysa Esben-Petersen, 1917). On the other hand, AA1 is forked in some other Mesozoic Limaiinae (e.g., Ren and Guo 1996: figs. 7, 8), some Nothochrysinae, all species of Chrysopinae, and all genera of Apochrysinae (however, the simple AA1 occurs in some species of the latter). It should be noted that AA1 is also forked in the closely related Mesozoic family Mesochrysopidae (see Makarkin and Menon 2005). Therefore, the forked AA1 is very probably plesiomorphic at the level of Chrysopoidea and Chrysopidae, but the simple AA1 evolved several times, possibly independently.
(ii) The short basal crossvein 1m-cu in the forewing is another characteristic feature of Pseudosencera gen. nov. Such a short 1m-cu occurs in more basal Limaiinae and Nothochrysinae, but it is long to very long in all extant Chrysopinae. All “crossveins” between Psm and Psc in Apochrysinae are short to very short as these veins are closely running over the entire length (see e.g., Winterton and Brooks 2002: figs. 5, 8, 10, 12, 14). Therefore, it seems to be incorrect to compare the length of 1m-cu in the specialised apochrysine venation with that in the more “normal” venation of other subfamilies. The condition found in Pseudosencera gen. nov. is very probably plesiomorphic in Chrysopidae.
(iii) Five to six rings of setae are found on the antennal flagellomeres of Pseudosencera gen. nov., where four rings are present in other Chrysopinae (Brooks 1997). But this condition, as found in Pseudosencera gen. nov., is plesiomorphic: setae on flagellar segments are arranged in five to six rings in more basal Nothochrysinae, and in five rings in Apochrysinae (Brooks and Barnard 1990); the setation of flagellomeres is unknown in the Mesozoic Limaiinae.
These three character states hamper the determination of the tribal affinity of Pseudosencera gen. nov. Molecular analyses of the tribal phylogeny of Chrysopinae are inconclusive, they support different relationships among the four tribes: “(Belonopterygini + (Ankylopterygini + Leucochrysini)) + Chrysopini” (Winterton and de Freitas 2006) or “(Belonopterygini + Leucochrysini) + (Ankylopterygini + Chrysopini)” (Jiang et al. 2017). The latter relationship of tribes was supported also by morphological data (Brooks 1997).
The absence of the apparent forewing intramedian cell is known in species of two tribes, Belonopterygini and Ankylopterygini. Judging from the proposed phylogenies mentioned above, this character state may have evolved independently. The character states of Pseudosencera gen. nov. are more similar to those of Ankylopterygini, but we cannot assign it to this tribe due to the presence of the above discussed three character states. Because two of these are plesiomorphic and not present in other Chrysopinae, we may assume that this new genus represents a basal branch of this subfamily. On the other hand, the absence of the apparent forewing intramedian cell and the simple AA1 are apomorphic features indicating that Pseudosencera gen. nov. is a specialised genus of a basal branch of Chrysopinae.
Hitherto, the only described Paleogene species of a presumed Chrysopinae was Paleochrysopa monteilsensis Séméria and Nel, 1990 from the late Eocene Monteils Formation, France. The species is known from an incomplete forewing. Its chrysopine affinity is based on the arrangement of the inner gradate series of crossveins, which is typical for this subfamily. But the long ScP and RA and the rectangular shape of the intramedian cell are more characteristic of Nothochrysinae. Therefore, the subfamily affinity of P. monteilsensis should be considered as preliminary.
All other known fossil Chrysopinae are known from the Miocene (Handschin 1937; Barbu 1939; Makarkin 1991; Engel and Grimaldi 2007). Most of these species were assigned to the extant genus Chrysopa Leach, 1815, sensu lato. It means that their venation generally resembles that of Chrysopa sensu stricto and similar chrysopine genera which are distinguished from one another mainly by their genitalia. However, there are small differences in the venation between these genera, which allow assignment of three species of Chrysopa sensu lato from the middle Miocene of Stavropol (southern Russia) to three extant genera of Chrysopini: Chrysoperla Steinmann, 1964, Suarius Navás, 1914, and Chrysopa sensu stricto (Makarkin 1991). Of course, these assignments are questionable as they cannot be supported by the study of genitalia.
One species of Leucochrysa McLachlan, 1868 has been described from the Miocene Dominican amber (Engel and Grimaldi 2007). So, fossil representatives of two tribes of Chrysopinae are known, Chrysopini and Leucochrysini, but no species of Belonopterygini and Ankylopterygini are known, to which Pseudosencera gen. nov. is most similar.
Conclusions
The late Eocene Baltic amber is the oldest locality where Chrysopinae and Nothochrysinae are found to coexist. Chrysopinae are represented by a species of specialised genus, which is the oldest confident record of the subfamily, possessing at least two plesiomorphic conditions not recorded in the extant taxa. In general, it is puzzling that green lacewings are so rare in Baltic amber, especially considering their habit of resting on plants (trees, in particular). Even the aquatic larvae of the Neuropteran family Nevrorthidae, which also pupate in water, occur more frequently in Baltic amber than chrysopids. Therefore, we may reasonably assume that Chrysopidae were indeed rare in the late Eocene ecological communities of the Baltic amber forest.
Acknowledgements
We thank Mónica Solorzano-Kraemer and Claudia Franz (both SMF) for the loan of the specimens; James E. Jepson (University College Cork, Ireland) for editing the English; S. Bruce Archibald (Simon Fraser University, Burnaby, Canada) and Xingyue Liu (China Agricultural University, Beijing, China) for providing helpful comments to improve the manuscript. The study is supported by a Grant No. 14-04-00649 of the Russian Foundation for Basic Research for VM.
References
Adams, P.A. 1967. A review of the Mesochrysinae and Nothochrysinae (Neuroptera: Chrysopidae). Bulletin of the Museum of Comparative Zoology 135: 215–238.
Adams, P.A. and Penny, N.D. 1992. New genera of Nothochrysinae from South America (Neuroptera: Chrysopidae). Pan-Pacific Entomologist 68: 216–221.
Archibald, S.B. and Makarkin, V.N. 2015. A new species of Archaeochrysa Adams (Neuroptera: Chrysopidae) from the Early Eocene of Driftwood Canyon, British Columbia, Canada. Canadian Entomologist 147: 359–369. Crossref
Archibald, S.B., Makarkin, V.N., Greenwood, D.R., and Gunnell, G.F. 2014. The Red Queen and Court Jester in green lacewing evolution: bat predation and global climate change. Palaios 29: 185–191. Crossref
Aspöck, H., Hölzel, H., and Aspöck, U. 2001. Kommentierter Katalog der Neuropterida (Insecta: Raphidioptera, Megaloptera, Neuroptera) der Westpaläarktis. Denisia 2: 1–606.
Barbu, I.Z. 1939. Insectes fossiles du tertiaire de l’Olténie. Buletinul Societatii romane de Geologie (Bulletin de la Société roumaine de Géologie) 4: 119–128.
Brauer, F. 1864. Entomologische Beiträge. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien 14: 891–902.
Breitkreuz, L.C.V., Winterton, S.L., and Engel, M.S. 2015. Revision of the green lacewing subgenus Ankylopteryx (Sencera) (Neuroptera, Chrysopidae). ZooKeys 543: 111–127. Crossref
Breitkreuz, L.C.V., Winterton, S.L., and Engel, M.S. 2017. Wing tracheation in Chrysopidae and other Neuropterida (Insecta): A resolution of the confusion about vein fusion. American Museum Novitates 3890: 1–44. Crossref
Brooks, S.J. 1997. An overview of the current status of Chrysopidae (Neuroptera) systematics. Deutsche Entomologische Zeitschrift (Neue Folge) 44: 267–275. Crossref
Brooks, S.J. and Barnard, P.C. 1990. The green lacewings of the world: a generic review (Neuroptera: Chrysopidae). Bulletin of the British Museum of Natural History (Entomology) 59: 117–286.
Burmeister, H.C.C. 1839. Handbuch der Entomologie. Zweiter Band. Besondere Entomologie. Zweite Abtheilung. Kaukerfe. Gymnognatha. (Zweite Hälfte; vulgo Neuroptera). xii + 294 pp. [i‒xii + 757–1050]. Enslin, Berlin.
Carpenter, F.M. 1935. Tertiary insects of the family Chrysopidae. Journal of Paleontology 9: 259–271.
CoBabe, E.A., Chamberlain, K.R., Ivie, M.A., and Giersch, J.J. 2002. A new insect and plant Lagerstätte from a Tertiary lake deposits along the Canyon Ferry Reservoir, southwestern Montana. Rocky Mountain Geology 37: 13–30. Crossref
Comstock, J.H. 1918. The Wings of Insects. ix + 430 pp. Comstock Publishing Company, Ithaca.
Dai, Y.T., Winterton, S.L., Garzón-Orduña, I.J., and Liu, X.Y. 2016. Mitochondrial phylogenomic analysis resolves the subfamily placement of enigmatic green lacewing genus Nothancyla (Neuroptera: Chrysopidae). Austral Entomology 56: 322–331. Crossref
Engel, M.S. and Grimaldi, D. 2007. The Neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates 3587: 1–58. Crossref
Engel, M.S., Winterton, S.L., and Breitkreuz, L.C.V. 2018. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us? Annual Review of Entomology 63: 531–551. Crossref
Esben-Petersen, P. 1917. Australian Neuroptera. Part iii. Proceedings of the Linnean Society of New South Wales 42: 203–219.
Gerstaecker, C.E.A. 1863. Ueber einige neue Planipennien aus den familien der Hemerobiiden und Panorpiden. Stettiner Entomologische Zeitung 24: 168–188.
Giebel, C.G. 1856. Fauna der Vorwelt mit steter Berücksichtidung der lebenden Thiere. Monographisch dargestellt von Dr. C.G. Giebel. Zweiter Band: Gliederthiere. Erste Abtheilung: Insekten und Spinnen. viii + 511 pp. F.A. Brodhaus, Leipzig.
Göppert, H.R. and Berendt, G.C. 1845. Der Bernstein und die in ihm befindlichen Pflanzenreste der Vorwelt, bearbeitet von Professor Dr. H.R. Goeppert in Breslau und Dr. G.C. Berendt in Danzig. In: G.C. Berendt (ed.), Die im Bernstein befindlichen organischen reste der Vorwelt, gesammelt in verbindung mit mehreren bearbeitet und herausgegeben von Dr. Georg Carl Berendt. Band 1, Abt. 1. iv + 125 pp., 7 pls. Buchhandlung, Berlin.
Hagen, H.A. 1852. Uebersicht der neueren Literatur, betreffend die Neuroptera Linn. Stettiner Entomologische Zeitung 13: 90–95.
Handlirsch, A. 1906–1908. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch für Paläontologen und Zoologen. ix + 1430 pp., 51 pls. [1906: 1–640, 1907: 641–1120, 1908: 1120–1430]. W. Engelmann, Leipzig.
Handlirsch, A. 1920–1921. Palaeontologie. In: C. Schröder (ed.), Handbuch der Entomologie. 3. Band, 117–306. [1920: 117–208, 1921: 209–306]. Gustav Fischer, Jena.
Handschin, E. 1937. Fossile Insekten aus Siebenbürgen. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 17: 25–29.
Jiang, Y.L., Garzón-Orduña, I.J., Winterton, S.L., Yang, F., and Liu, X.Y. 2017. Phylogenetic relationships among tribes of the green lacewing subfamily Chrysopinae recovered based on mitochondrial phylogenomics. Scientific Reports 7: 7218. Crossref
Khramov, A.V. 2018. A new assemblage of Early Cretaceous green lacewings (Chrysopidae: Neuroptera) from Transbaikalia. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107: 195‒202. Crossref
Khramov, A.V., Liu, Q., Zhang, H.C., and Jarzembowski, E.A. 2016. Early green lacewings (Insecta: Neuroptera: Chrysopidae) from the Jurassic of China and Kazakhstan. Papers in Palaeontology 2: 25–39. Crossref
Kimmins, D.E. 1952. Some new Australian Chrysopidae. Annals and Magazine of Natural History (Ser. 12) 5: 69–81.
Kovanci, B. and Canbulat, S. 2007. A new species of the genus Nothochrysa McLachlan 1868 from northwestern Turkey (Neuroptera: Chrysopidae) with a key to western Palaearctic species. Annales de la Société Entomologique de France (Nouvelle Série) 43: 165–168. Crossref
Krüger, L. 1923. Neuroptera succinica baltica. Die im baltischen Bernstein eingeschlossenen Neuroptera des Westpreussischen Provinzial-Museums (heute Museum für Naturkunde und Vorgeschichte) in Danzig. Stettiner Entomologische Zeitung 84: 68–92.
Kukalová-Peck, J. and Lawrence, J.F. 2004. Relationships among coleopteran suborders and major endoneopteran lineages: evidence from hind wing characters. European Journal of Entomology 101: 95–144. Crossref
Leach, W.E. 1815. Entomology. In: D. Brewster (ed.), The Edinburgh Encyclopaedia, Vol. 9, Part 1, 57–172. John Murray Baldwin & Cradocle, Edinburgh.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis,locis. Vol. 1. Editio decima. 824 pp. Laurentius Salvius, Holmiae.
Liu, X.Y., Lu, X.M., and Zhang, W.W. 2017. Phylogenetic position of Corydasialidae (Insecta: Neuropterida) revisited based on a significant new fossil in Cretaceous amber of Myanmar. Journal of Systematic Palaeontology 15: 571–581. Crossref
Lu, X.M., Wang, B., Ohl, M., and Liu, X.Y. 2018. The first green lacewing (Insecta: Neuroptera: Chrysopidae) from the mid-Cretaceous amber of Myanmar. Zootaxa 4399: 563‒570. Crossref
Makarkin, V.N. 1991. Miocene Neuroptera from North Caucasus and Sikhote-Alin [in Russian]. Paleontologičeskij žurnal 1991 (1): 57–68.
Makarkin, V.N. 1997. Fossil Neuroptera of the Lower Cretaceous of Baisa, East Siberia. Part 3. Chrysopidae. Spixiana 20: 107–118.
Makarkin, V.N. 2014. A new fossil green lacewing (Neuroptera: Chrysopidae) from the Eocene Tadushi Formation, eastern Sikhote-Alin. Far Eastern Entomologist 272: 1–7.
Makarkin, V.N. and Archibald, S.B. 2013. A diverse new assemblage of green lacewings (Insecta: Neuroptera: Chrysopidae) from the Early Eocene Okanagan Highlands, western North America. Journal of Paleontology 87: 122–145. Crossref
Makarkin, V.N. and Menon, F. 2005. New species of the Mesochrysopidae (Insecta, Neuroptera) from the Crato Formation of Brazil (Lower Cretaceous), with taxonomic treatment of the family. Cretaceous Research 26: 810–812. Crossref
Martynov, A.V. 1927. Jurassic fossil insects from Turkestan. 7. Some Odonata, Neuroptera, Thysanoptera. Bulletin de l’Academie des Sciences de l’URSS 21: 757–768.
McLachlan, R. 1868. A monograph of the British Neuroptera-Planipennia. Transactions of the [Royal] Entomological Society of London 16: 145–224. Crossref
Navás, L. 1910. Crisópidos (Ins. Neur.) nuevos. Brotéria (Zoológica) 9: 38–59.
Navás, L. 1913. Les Chrysopides (Ins. Névr.) du Musée de Londres [Ia]. Annales de la Société Scientifique de Bruxelles 37 (2): 292–330.
Navás, L. 1914. Les Chrysopides (Ins. Névr.) du Musée de Londres. Conclusion. Annales de la Société scientifique de Bruxelles 38 (2): 73–114.
Navás, L. 1925. Comunicaciones entomológicas. 7. Neurópteros del Museo de Berlín. Revista de la [Real] Academia de Ciencias Exactas Fisico-Quimicas y Naturales de Zaragoza, Ser. 1 9 (for 1924): 20–34.
Navás, L. 1934. Comunicaciones entomológicas. 17. Insectos de Madagascar. Primera [I] serie. Revista de la [Real] Academia de Ciencias Exactas Fisico-Quimicas y Naturales de Zaragoza, Ser. 1 17 (for 1933): 49–76.
Nel, A. and Séméria, Y. 1986. Une nouvelle espece de chrysopide fossile du Stampien Superieur (Oligocene) d’Aix-en-Provence Notochrysa stampieni n. sp. (Neuroptera, Chrysopidae, Notochrysinae). Neuroptera International 4: 23–30.
New, T.R. 1980. A revision of the Australian Chrysopidae (Insecta: Neuroptera). Australian Journal of Zoology (Supplementary Series) 77: 1–143.
Oswald, J.D. 1993. Revision and cladistic analysis of the world genera of the family Hemerobiidae (Insecta: Neuroptera). Journal of New York Entomological Society 101: 143–299.
Ren, D. and Guo, Z.G. 1996. On the new fossil genera and species of Neuroptera (Insecta) from the Late Jurassic of northeast China. Acta Zootaxonomica Sinica 21: 461–479.
Scheven, J. 2004. Bernstein-Einschlüsse: Eine untergegangene Welt bezeugt die Schöpfung. Erinnerungen an die Welt vor der Sintflut. 160 pp. Kuratorium Lebendige Vorwelt, Hofheim a.T.
Schneider, W.G. 1851. Symbolae ad monographiam generis Chrysopae, Leach. 178 pp. Hirt, Vratislaviae.
Scudder, S.H. 1885. Systematische Übersicht der fossilen Myriopoden, Arachnoideen und Insekten. In: K.A Zittel (ed.), Handbuch der Palaeontologie. Abt. 1. Palaeozoologie. Bd 2. Mollusca und Arthropoda, 721–831. Verlag von R. Oldenbourg, München.
Scudder, S.H. 1890. The Tertiary insects of North America. Report of the United States Geological Survey of the Territories 13: 1–734.
Scudder, S.H. 1891. Index to the known fossil insects of the world including myriapods and arachnids. Bulletin of the United States Geological Survey 71: 1–744.
Séméria, Y. and Nel, A. 1990. Paleochrysopa monteilsensis gen. et sp. nov., a new fossil of Chrysopidae from the Upper Eocene Formation of Monteils (France), with a review of the known chrysopid fossils (Insecta: Neuroptera). In: M.W. Mansell and H. Aspöck (eds.), Advances in Neuropterology. Proceedings of the Third International Symposium on Neuropterology, 27–32. South African Department of Agricultural Development, Pretoria.
Statz, G. 1936. Ueber neue Funde von Neuropteren, Panorpaten und Trichopteren aus den tertiären Schiefern von Rott am Siebengebirge. Decheniana 93: 208–255.
Steinmann, H. 1964. The Chrysopa species (Neuroptera) of Hungary. Annales Historico-Naturales Musei Nationalis Hungarici (Zoologica) 56: 257–266.
Tillyard, R.J. 1916. Studies in Australian Neuroptera. No. 3. The wing venation of the Chrysopidae. Proceedings of the Linnean Society of New South Wales 41: 221–248.
Weitschat, W. 2009. Jäger, Gejagte, Parasiten und Blinde Passagiere – Momentaufnahmen aus dem Bernsteinwald. Denisia 26: 243–256.
Weitschat, W. and Wichard, W. 1998. Atlas der Pflanzen und Tiere im Baltischen Bernstein. 256 pp. Dr. Friedrich Pfeil Verlag, München.
Winterton, S.L. and Brooks, S.J. 2002. Phylogeny of the Apochrysine green lacewings (Neuroptera: Chrysopidae: Apochrysinae). Annals of the Entomological Society of America 95: 16–28. Crossref
Winterton, S.L. and de Freitas, S. 2006. Molecular phylogeny of the green lacewings (Neuroptera: Chrysopidae). Australian Journal of Entomology 45: 235–243. Crossref
Yang, Q., Makarkin, V.N., and Ren, D. 2014. Two new species of Kalligramma Walther (Neuroptera: Kalligrammatidae) from the Middle Jurassic of China. Annals of the Entomological Society of America 107: 917–925. Crossref
Yang, Q., Makarkin, V.N., Winterton, S.L., Khramov, A.V., and Ren, D. 2012. A remarkable new family of Jurassic insects (Neuroptera) with primitive wing venation and its phylogenetic position in Neuropterida. PLoS ONE 7 (9): e44762. Crossref
Yang, X.K., Yang, C.K., and Li, W.Z. 2005. Neuroptera, Chrysopidae. In: Fauna Sinica. Insecta. Vol. 39. xiii + 398 pp. Science Press, Beijing.
Acta Palaeontol. Pol. 63 (3): 527–537, 2018
https://doi.org/10.4202/app.00504.2018