

Pleistocene non-passeriform landbirds from Shiriya, northeast Japan
JUNYA WATANABE, HIROSHIGE MATSUOKA, and YOSHIKAZU HASEGAWA
Watanabe, J., Matsuoka, H., and Hasegawa, Y. 2018. Pleistocene non-passeriform landbirds from Shiriya, northeast Japan. Acta Palaeontologica Polonica 63 (3): 469–491.
Located on the eastern margin of Eurasia, the Japanese Archipelago hosts a unique modern fauna of terrestrial vertebrates including landbirds which show a high proportion of endemic species/subspecies. Despite its potential importance in taxonomy and biogeography, the Pleistocene landbird fossil record has been scarce on Japanese islands, providing little information on the history of the unique fauna in the region. In this study, fossil remains of non-passeriform landbirds from the Middle–Late Pleistocene (Marine Isotope Stages [MIS] 9 and 5e) of Shiriya, northernmost Honshu Island, Japan, are revised with extensive osteological comparisons. As a result, the presence of at least six non-passeriform landbird species, represented by 71 specimens, was confirmed: Syrmaticus sp., Coturnicini gen. et sp. indet., Columbidae gen. et sp. indet., Apus sp., Haliaeetus sp., and Accipitridae gen. et sp. indet. The Shiriya paleoavifauna is the first substantial Pleistocene landbird fauna reported from the central Japanese islands so far, and suggests that the overall landbird fauna in northern Honshu in the last interglacial period (MIS 5e) was not drastically different from the present one, in contrast to the presence of several extinct land mammals and seabirds in the local fauna. The occurrence of Syrmaticus despite the supposedly colder climate in that time than today suggests that the distribution of modern S. soemmerringii might not be totally defined by climatic factors, but probably affected by a biogeographic barrier at the strait between Honshu and Hokkaido islands.
Key words: Aves, Phasianidae, Columbidae, Apodidae, Accipitridae, biogeography, Pleistocene, Japan.
Junya Watanabe [watanabe-j@kueps.kyoto-u.ac.jp, ORCID http://orcid.org/0000-0002-9810-5286] and Hiroshige Matsuoka [maca@kueps.kyoto-u.ac.jp], Department of Geology and Mineralogy, Graduate School of Science, Kyoto University, Sakyoku Kitashirakawa Oiwakecho, Kyoto 606-8502, Japan.
Yoshikazu Hasegawa [hasegawa@gmnh.pref.gunma.jp], Gunma Museum of Natural History, 1674-1 Kamikuroiwa, Tomioka, Gunma 370-2345, Japan.
Received 30 May 2018, accepted 11 July 2018, available online 8 August 2018.
Copyright © 2018 J. Watanabe et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Japanese Archipelago, a chain of island arcs located on the eastern margin of Eurasia, hosts a remarkable vertebrate fauna due to its unique geographic location and geological history. Zoogeographically, it lies near the transition between the Palearctic and Oriental realms, which is sometimes referred to as the Sino-Japanese Realm to appreciate its distinctness (Holt et al. 2013a, b; but see also Kreft and Jetz 2013; Rueda et al. 2013). Concerning the modern landbird fauna, 47.2% of the currently recognized species/subspecies breeding in the Japanese Archipelago today is endemic to the region (Yamasaki 2017). In addition, Japanese islands might have been the origins of radiation in numbers of landbird lineages that now have broader geographic distributions (“reverse colonization”; Nishiumi and Kim 2015). As such, the Pleistocene landbird fossil record in the Japanese Archipelago is of potential interest from the taxonomic and biogeographic perspectives.
The Japanese vertebrate fauna has evolved under the complex geomorphologic history of the Japanese Archipelago. The central part of the archipelago consists of four relatively large islands (Hokkaido, Honshu, Shikoku, and Kyushu islands) and numerous smaller ones surrounding them (hereafter collectively referred to as central Japanese islands). Although the central Japanese islands had been formed by back-arc rifting in the early–middle Miocene (Jolivet et al. 1994; Otofuji 1996), they are considered to have been partially connected to the Eurasian Continent on their southwestern part by land bridges in some part of the middle Miocene–earliest Pleistocene (until ~1.7 Ma), after which the separation from the continent became more pronounced (Chinzei 1991; Kitamura et al. 2001; Kitamura 2008). In some glacial periods in the Pleistocene, however, the central Japanese islands were connected to the Eurasian Continent with two major pathways formed by land bridges, one through the Korean Peninsula and the other through Sakhalin Island, causing biotic interchanges with the continent (Kawamura 1998; Konishi and Yoshikawa 1999; McKay 2012; Nakazawa and Bae in press). In the Last Glacial Maximum, when the sea level was lower by ~120 m than in the present (e.g., Bintanja et al. 2005; Oba and Irino 2012), Honshu, Shikoku, and Kyushu islands were connected to each other (Paleo-Honshu–Shikoku–Kyushu Island) but probably separated from the continent, whereas Hokkaido Island was connected to the continent through Sakhalin Island (Paleo-Sakhalin–Hokkaido–Kuril Peninsula; Ota and Machida 1987; Ota and Yonekura 1987; Park et al. 2000; Chinzei and Machida 2001). Even in that time, the strait between Honshu and Hokkaido islands (Tsugaru Strait) remained, acting as a biogeographic barrier for terrestrial animals (Blakiston’s Line; Blakiston and Pryer 1880; Milne 1881; Blakiston 1883; Tokuda 1941; Dobson 1994), perhaps except for those with high dispersal ability (e.g., large mammals; Kawamura 2007; Sawaura et al. 2018).
In general, Pleistocene fossils provide material evidences of temporal and geographic distributions of modern species, and play crucial roles in investigating many aspects of evolutionary histories of modern taxa and faunas. Relevant topics include speciation events (Selander 1965; Stewart 2002), species longevity, extinction and turnover (Brodkorb 1960; Tyrberg 2002, 2008b; Olson 2010; Meijer et al. 2015), and paleoclimate inference (Tyrberg 1999; Finlayson et al. 2008; Holm and Svenning 2014). Meta-analysis of compiled avian fossil records might be an attractive approach to these topics (e.g., Tyrberg 1999; Holm and Svenning 2014), but one should be cautious in interpreting the Pleistocene avian fossil record because there is a number of obstacles that may cause serious flaws in such analysis (Stewart 2002). One major issue prevalent in previously reported Quaternary paleoavifaunas is that fossils were identified without sufficient justifications. Many previous works report occurrences of species only in the form of faunal list and do not give descriptions or designations of fossil or comparative material, making it impossible to critically evaluate occurrence records in the literature. As many factors, including ontogenetic variation, sexual dimorphism, and geographic or temporal variation, may potentially influence quantitative and qualitative features of the avian skeleton (Stewart 2007), one should be cautious in choosing comparative specimens. Disregarding geographic variation may lead to incorrect taxonomic assignment (Storer 1992; Olson 2005). Identifications based on the distributions of modern species may lead to misidentification (Stewart 2005), or, for the purpose of biogeography, circular reasoning (Bever 2005; Nesbitt and Stocker 2008). Temporal variation (Northcote 1981; Ericson 1987; Stewart 2002) can potentially confound identification. In order to enable critical evaluation of identifications by future studies, it is advisable to clearly state the nature of the comparative material used, including species examined, quantity, and sexual and geographic coverage.
Despite its potential importance, the landbird fossil record in the Japanese Archipelago has been scarce. Apart from those in the Ryukyu Islands (e.g., Ono and Hasegawa 1985; Matsuoka 2000; Matsuoka and Hasegawa 2018) and archaeological sites (e.g., Nokariya and Ono 1980), most known localities yield only a few species (Takai 1962; Hasegawa 1964; Tomida 1978; Ono 1980; Rich et al. 1986), providing little concrete evidence for the history of landbird fauna in the region. In this respect, the Shiriya local fauna (Hasegawa et al. 1988), a Middle–Late Pleistocene vertebrate fauna recovered from fissure-filling deposits in Shiriya, northeast Japan, is of much interest. It consists of diverse land and marine vertebrates, including marine fish, amphibians, large carnivorous and herbivorous mammals, small land mammals, chiropterans, marine mammals, and various land- and seabirds. Hasegawa et al. (1988) listed 15 non-passeriform avian species for the Shiriya local fauna, including three landbirds (Phasianus sp., Buteo buteo, and Haliaeetus cf. pelagicus), but most of the avian material was left undescribed. Also, Hasegawa et al. (1988) did not give justification for identifications or designations of specimens for all species, necessitating a comprehensive revision.
In this report, as an outcome of a revision of the avian material from the Shiriya local fauna (Watanabe and Matsuoka 2015; Watanabe et al. 2016; JW, HM, and YH unpublished material), the non-passeriform landbird fossils from the Pleistocene of Shiriya are described, which turned out to belong to the following four families: Phasianidae, Columbidae, Apodidae, and Accipitridae. Detailed osteological descriptions are presented, in order to facilitate critical evaluation of identifications and comparison by future studies.
Institutional abbreviations.—ICM, Iida City Museum, Iida, Japan; KUGM, Department of Geology and Mineralogy, Kyoto University, Japan; MVZ, Museum of Vertebrate Zoology, University of California, Berkeley, USA; NSMT AS, Avian Skeleton Collection, Department of Zoology, National Museum of Nature and Science, Tsukuba, Japan; NSMT PV, Vertebrate Paleontology Collection, Department of Geology and Paleontology, National Museum of Nature and Science, Tsukuba, Japan; USNM, Division of Birds, Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA; UWBM, Ornithology Collection, Burke Museum of Natural History and Culture, University of Washington, Seattle, USA; YIO, Yamashina Institute for Ornithology, Abiko, Japan.
Other abbreviations.—MIS, Marine Isotope Stage; lig., ligamentum/ligamenti; m., musculus/musculi; n., nervus/nervi.
Geological setting
The fossils described in this study came from fissure-filling deposits in Shiriya, Aomori Prefecture, northeastern Japan. Geological background of the localities has been reviewed by Hasegawa et al. (1988) and Watanabe and Matsuoka (2015), thus only a brief summary would suffice here.
Shiriya is located at the northeastern extremity of Honshu Island, Japan (41°24’N, 141°24’E; Fig. 1). The oldest rocks exposed in this area include slate, sandstone, chert, limestone, and limestone breccia (Murata 1962; Tsushima and Takizawa 1977; Matsuoka 1987; Sano et al. 2009), which comprise a pre-Tertiary tectonic complex (Kamada 2000). It is unconformably overlain by Miocene and Pliocene marine sedimentary rocks which yield no vertebrate remains (Tsushima and Takizawa 1977). On these basement rocks, a series of Middle–Late Pleistocene marine terraces is developed, each of whose age is correlated to a Marine Isotope Stage (MIS) based on dated volcanic ash layers interbedded in its deposits and relative altitudes to others (Koike and Machida 2001).
The fossils described here came from unconsolidated deposits that fill fissures or caves in limestone and limestone breccia bodies. Hasegawa et al. (1988) recognized four major vertebrate localities in the area: Loc. 1 (“Locality A” in Nakajima and Kuwano 1957); Loc. 2 (Shiriya Second Quarry); Loc. 3 (Shiriya Tunnel); and Loc. 4 (unnamed tunnel near Shikkari [also spelled Shitsukari]). The avian remains were recovered from Locs. 1–3.
No direct dating is available for the fossil localities, but the occurrence of marine vertebrates and the inclusion of marine-originated sediments indicate that the fossil-bearing layers had been deposited near the past sea level (Nakajima and Kuwano 1957; Nakajima 1958; Hasegawa et al. 1988). Therefore, the localities can be correlated to certain marine terrace surfaces; Loc. 2 (70–80 m above sea level) can be correlated to the Toei surface (65–80 m; MIS 9), and both Loc. 1 (~45 m above sea level) and Loc. 3 (~35 m) to the Tonamigaoka surface (35–45 m; MIS 5e; ages according to Koike and Machida 2001). These assignments are consistent with the occurrence of land mammals typical of the Pleistocene of the central Japanese islands (Hasegawa 1972; Kamei et al. 1988).
Fig. 1. Geographic location of Shiriya localities. Landmarks mentioned in the text are also indicated. Inset (41°24’N, 141°24’E) shows major fossil localities mentioned in the text (stars; after Hasegawa et al. 1988). Dark grey indicates distribution of limestone/limestone breccia bodies (after Tsushima and Takizawa 1977). Abbreviations: I., island; Is., islands.
Material and methods
The avian fossils from Shiriya temporarily stored in ICM, KUGM, and NSMT PV were examined, including those figured by Hasegawa et al. (1988). Bone scraps were also screened for identifiable specimens. The fossils from Loc. 1 were collected by Zenji Nakajima in 1950’s, whereas those from locs. 2 and 3 were collected by YH and others in 1960 and 1987. All specimens described in this study are now formally deposited in NSMT PV. All specimens had been recovered as isolated bones after water-sieving of sediments. All limb bones described are skeletally mature, as confirmed from inspection of surface textures and other features that are indicative of ontogenetic stages (Tumarkin-Deratzian et al. 2006; Watanabe and Matsuoka 2013; Watanabe 2018a, b).
Comparison was primarily made with modern avian skeletons stored in KUGM, MVZ, NSMT AS, UWBM, USNM, and YIO. A list of comparative material is given in Appendix 1. The status and distribution of modern birds in the Japanese Archipelago were largely taken from Check-list of Japanese Birds (The Ornithological Society of Japan 2012). Osteological terminology generally follows Baumel and Witmer (1993), but that of Howard (1929) is also used when appropriate. The term “omal” is used to designate the upper/cranial end of the coracoid. Measurements were taken with digital calipers (Mitutoyo, Kawasaki, Japan) and values rounded to the nearest 0.1 mm are reported. Size and proportion of fossils were compared with those of comparative specimens through visual inspection of scatter plots of dimensions.
Systematic palaeontology
Class Aves Linnaeus, 1758
Order Galliformes Temminck, 1820
Family Phasianidae Horsfield, 1821
Remarks.—For the comparative purpose, the classification of modern taxa within this family follows del Hoyo and Collar (2014). Hasegawa et al. (1988) included Phasianus sp. in their faunal list, but did not designate any specimens.
Subfamily Phasianinae Horsfield, 1821
Tribe Phasianini Horsfield, 1821
Genus Syrmaticus Wagler, 1832
Syrmaticus sp.
Fig. 2A, B, E, F, G–K.
Material.—Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24531, 24532, left omal and sternal coracoids, respectively; NSMT PV 24533–24535, two right and one left proximal humeri, respectively; NSMT PV 24536, right distal humerus; NSMT PV 24537 left humeral shaft; NSMT PV 24538, right ulnare; NSMT PV 24539, worn proximal phalanx of the right major wing digit. Locality unrecorded, Shiriya, Aomori Prefecture, Japan; Middle–Late Pleistocene (~MIS 9/5e): NSMT PV 24540, tip of premaxilla; NSMT PV 24541, 24542, proximal and distal fragments of right carpometacarpus, respectively.
Measurements.—See Table 1 and Fig. 3.
Table 1. Measurements (in mm) of Syrmaticus sp. from the upper Pleistocene of Shiriya compared with Recent Syrmaticus soemmerringii and Syrmaticus reevesii. Abbreviations: BFAH, breadth of the facies articularis humeralis; D, craniocaudal depth; DCH, craniocaudal depth of the caput humeri; GDB, greatest distal breadth; n, number of individuals; W, dorsoventral width; WSA, mediolateral width of the sternal articulation.
|
Elements |
Syrmaticus sp. |
Syrmaticus soemmerringii |
Syrmaticus reevesii |
||||
|
n |
range |
mean |
n |
range |
mean |
||
|
Coracoid |
|||||||
|
BFAH |
10.6, – |
18 |
9.7–13.3 |
11.7 |
9 |
10.9–13.1 |
11.9 |
|
WSA |
–, 10.4 |
19 |
9.6–13.9 |
11.6 |
9 |
10.8–13.7 |
11.9 |
|
Humerus |
|||||||
|
proximal W |
19.2, –, – |
20 |
16.6–19.9 |
17.8 |
10 |
18.6–21.8 |
20.3 |
|
DCH |
7.8, 7.5, 7.9 |
20 |
6.4–8.2 |
7.3 |
10 |
7.0–8.9 |
8.0 |
|
W midshaft |
7.1, 6.5 |
19 |
6.3–7.9 |
7.0 |
10 |
6.7–8.7 |
7.9 |
|
D midshaft |
5.8, 5.3 |
19 |
4.7–6.4 |
5.4 |
10 |
5.5–6.9 |
6.2 |
|
distal W |
13.9, – |
19 |
12.2–14.5 |
13.0 |
10 |
13.6–16.2 |
14.8 |
|
distal D |
8.4, – |
19 |
7.4–8.9 |
8.0 |
10 |
8.4–11.0 |
9.4 |
|
GDB |
14.6, – |
19 |
12.8–15.3 |
13.8 |
10 |
14.4–17.5 |
15.8 |
|
Carpometacarpus |
|||||||
|
distal W |
4.1 |
12 |
3.5–4.5 |
3.9 |
10 |
3.7–4.5 |
4.1 |
|
distal D |
6.7 |
12 |
6.1–7.2 |
6.6 |
10 |
6.8–8.0 |
7.4 |
Description.—One well preserved proximal end of the humerus was recovered (Fig. 2A). The fossil is characterized by a narrow dorsal tricipital fossa (the fossa dorsal to the crus dorsale fossae), which is pointed proximally with a faint dorsal margin and only slightly excavates the distal margin of the caput humeri in its ventral part. The combination of these states is apparently unique to Phasianini (the dorsal tricipital fossa is wider and excavates the caput humeri more deeply in Rollulinae and most other tribes within Phasianinae except Pavonini). The fossil differs from Pavonini in a proximodistally thinner tuberculum ventrale that lacks a distinct attachment scar just ventral to its tip (which is characteristically present at this position in Pavonini). Within Phasianini, the fossil agrees only with Syrmaticus (except S. reevesii) in weak development of the incisura capitis (in Chrysolophus, Phasianus, Crossoptilon, Catreus, Lophura, and S. reevesii, the incisure is wider and deeper, and excavates the proximal margin of the tuberculum ventrale; Fig. 2C1, D1). Two other fragmentary proximal humeri are tentatively referred to the same species, based on the consistency of size and observable features.
Another phasianid humerus preserving the distal half of the bone (Fig. 2B) is referred to the same species as the proximal end with which it is roughly consistent in size. In the fossil, the processus flexorius is well developed distally, as in Coturnicini, Lophophorini, Phasianini, and Tetraonini (except Meleagris). The fossil differs from Coturnicini in the absence of a concavity on the epicondylus ventralis just caudal to the pit for the m. pronator superficialis (see below), and from most members of Tetraonini (except Perdix and Meleagris) in less craniocaudal compression of the body.
An omal end of the coracoid preserving the processus acrocoracoideus (Fig. 2J) and a sternal end of the coracoid with a worn angulus lateralis (Fig. 2K) are tentatively referred to this species, with which they are consistent in size. In the fossil, the impressio lig. acrocoracohumeralis is dorsoventrally thick and developed as a deep groove with distinct margins. This state is characteristic to some members of Phasianini and Tetraonini (except Perdix, Meleagris, and Bonasa) among phasianids. The omal end differs from Tetraonini and agrees with Phasianini in a relatively straight profile of the tuberculum brachiale in medial view (in Tetraonini, its dorsal tip is produced sternally to give a concave profile to the tuberculum brachiale). The fossil differs from Phasianus and agrees with Syrmaticus in a relatively poorly developed ventral part of the processus acrocoracoideus (in Phasianus, it is strongly elevated from the shaft and produced medially, with the ventral margin of the tuberculum brachiale protruding as medially as the dorsal margin; Fig. 2C3, D3, J3). The sternal protrusion of the processus lateralis observed in the fossil sternal end, the most sternal point of which is lying even sternally than the lateral end of the crista articularis sternalis (Fig. 2K), is apparently unique to Phasianini (in most other phasianids, the latter is the most sternal point in the coracoid). This feature is most pronounced in Syrmaticus within Phasianini, with which the fossil agrees (Fig. 2C2, D2).
One carpometacarpus preserving the proximal symphysis and processus intermetacarpalis (Fig. 2E), another preserving the distal symphysis and articular surfaces (Fig. 2F), a proximal phalanx of major wing digit lacking the caudodistal part (Fig. 2H), one moderately worn ulnare (Fig. 2I), and a tip of premaxilla (Fig. 2G) are tentatively referred to this species. The fossils are too fragmentary for definitive identification, although they are consistent with modern species of the genus in size and proportions.
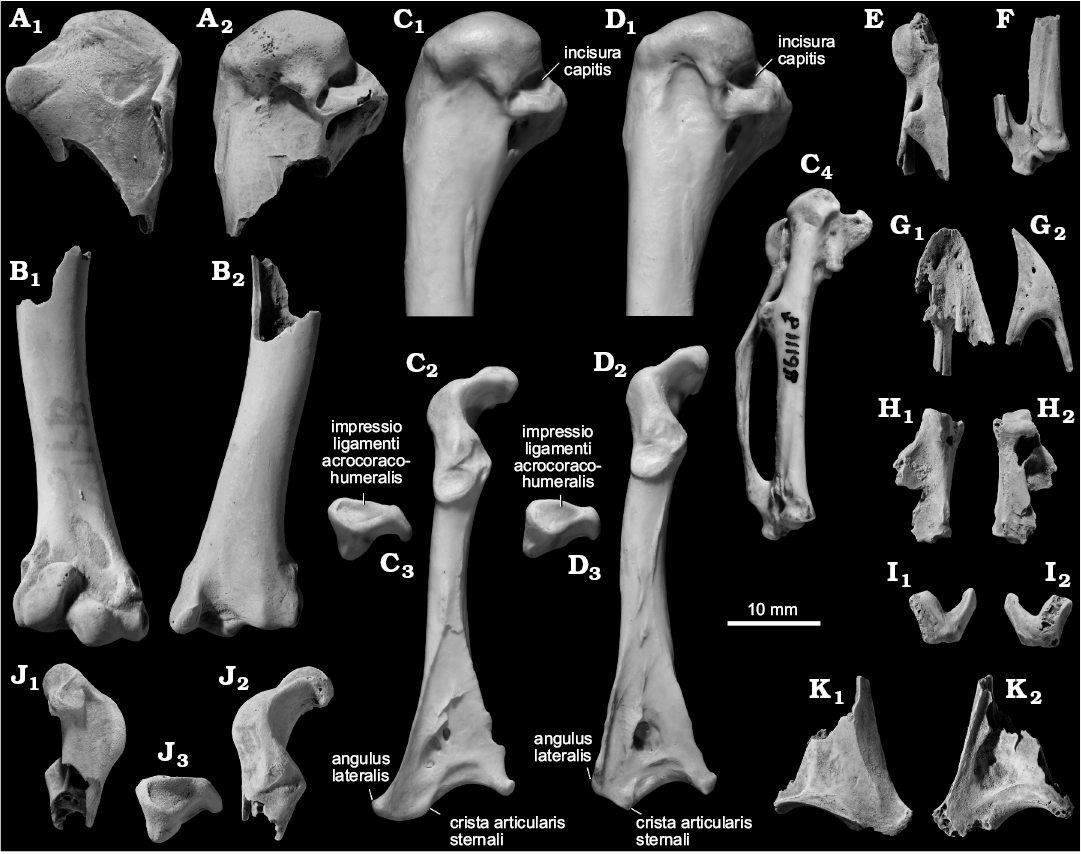
Fig. 2. Phasanid bird Syrmaticus sp. from the upper Pleistocene of Shiriya, Japan (A, B, E, F, H–L), compared with Recent Syrmaticus soemmerringii (Temminck, 1830) (C) and Recent Phasianus colchicus Linnaeus, 1758 (D). A. NSMT PV 24535, left humerus in cranial (A1) and caudal (A2) views. B. NSMT PV 24536, right humerus in cranial (B1) and caudal (B2) views. C. MVZ 49764, left humerus in caudal view (C1), left coracoid in dorsal (C2) and omal (C3) views, right carpometacarpus in dorsal view (C4). D. MVZ 68327, left humerus in caudal view (D1), left coracoid in dorsal (D2) and omal (D3) views. E, F. NSMT PV 24541 and NSMT PV 24542, respectively, right carpometacarpi in dorsal view. G. NSMT PV 24540, premaxilla in ventral (G1) and left lateral (G2) views. H. NSMT PV 24539, proximal phalanx of right major wing digit in dorsal (H1) and ventral (H2) views. I. Right ulnare (NSMT PV 24538), in distal (I1) and proximal (I2) views. J. Left coracoid (NSMT PV 24531), in ventral (J1), dorsal (J2), and omal (J3) views. K. Left coracoid (NSMT PV 24532), in ventral (K1) and dorsal (K2) views. Fossils coated with ammonium chloride.
Remarks.—This genus includes several modern species of pheasants which are distributed in China, northern Indochina, Taiwan, and most central Japanese islands (except for Hokkaido Island). As far as observed in this study, few qualitative features observable in the Shiriya material were found useful in distinguishing species within the genus, except for the development of the incisura capitis of the humerus noted above; the incisure is deep and wide in S. reevesii and the other genera of the tribe Phasianini, whereas it is narrower and shallower in the other modern species of Syrmaticus. Although it is tempting to speculate that this character reflects the phylogenetic relationship (e.g., the state observed in S. reevesii represents a primitive condition within Syrmaticus), the available evidence is inconclusive; most recent molecular phylogenetic analyses (Zhan and Zhang 2005; Wang et al. 2013; Jiang et al. 2014; Hosner et al. 2016) recovered either S. soemmerringii or S. reevesii as the most basal divergences within the genus, but the branching orders for these species have not been well resolved. In any way, the Shiriya fossils clearly agree with the group including S. soemmerringii, S. mikado, S. ellioti, and S. humiae. Although most dimensions of the Shiriya material are consistent with those of modern S. soemmerringii, the only species that occur on the central Japanese islands today, the sample size is too small to allow definitive assignment to any of the modern species (Fig. 3), let alone identification to the subspecific level.
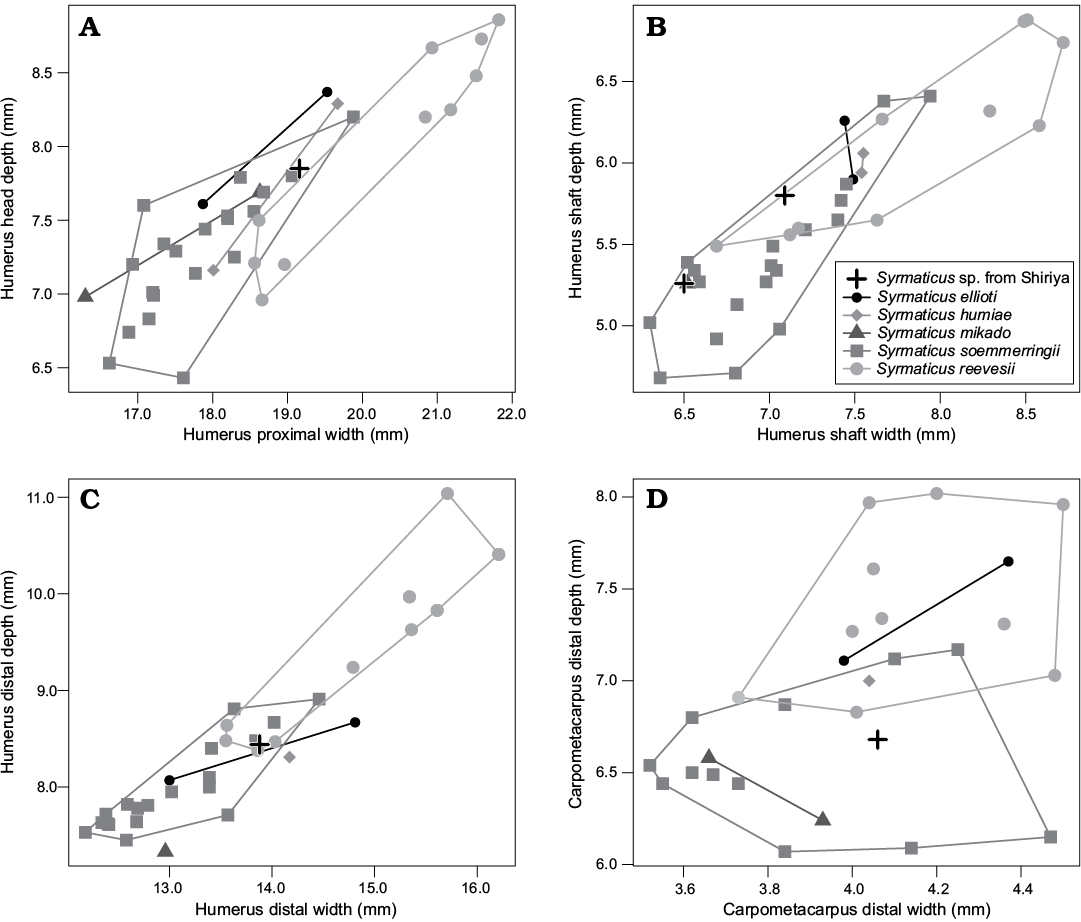
Fig. 3. Bivariate scatter plots of Recent and fossil species of Syrmaticus: proximal (A) and distal (C) humeral, humeral shaft (B), and carpometacarpal (D) measurements.
Two extinct species of Syrmaticus have been named so far: S. phasianoides (Jánossy, 1991) from the upper Miocene of Hungary (Jánossy 1991; Zelenkov 2016) and S. kozlovae Kurochkin, 1985, from the upper Miocene–lower Pliocene of Mongolia (Kurochkin 1985; Zelenkov and Kurochkin 2010). According to published dimensions (Zelenkov 2016), S. phasianoides is larger than any modern species of the genus, thus would be larger than the Shiriya material as well. According to published dimensions and descriptions (Zelenkov and Kurochkin 2010), S. kozlovae is slightly smaller than the Shiriya material, and further differs from it in certain osteological features of the distal humerus, including a less distally protruding processus flexorius (see Zelenkov and Kurochkin 2010).
The fossil record of the modern species of the genus is scanty. Syrmaticus soemmerringii has been identified from several Pleistocene localities in the central Japanese islands (Takai 1962; Nokariya and Ono 1980; Ono 1980), but little justifications were given in these previous reports. As S. soemmerringii had once been included in Phasianus in some past editions of the Check-list of Japanese Birds (e.g., The Ornithological Society of Japan 1974), this species may possibly be represented by identifications labeled as “Phasianus sp.” by Japanese authors (e.g., Ono in Rich et al. 1986). Although S. reevesii had been reported from the Pleistocene of Choukoutien (also known as Zhoukoudian), China (Shaw 1935), a later, more comprehensive report of the paleoavifauna (Hou 1993) did not mention this species.
Modern S. soemmerringii is the only species of the genus that naturally occurs in the central Japanese islands, where it is an endemic, resident breeder. Lying at the northeastern tip of Honshu Island, Shiriya is at the northern limit of the natural distribution of the species; it does not occur in Hokkaido Island except as an introduced breeder (The Ornithological Society of Japan 2012). If the Shiriya material represents that species, the occurrence would indicate that the species had spread throughout Honshu Island by the Late Pleistocene (MIS 5e), assuming that the genus originated in the continental Asia as indicated by the distribution and phylogeny of modern and fossil species (Zhan and Zhang 2005; Zelenkov and Kurochkin 2010).
Hasegawa et al. (1988) included Phasianus sp. in their faunal list for the Shiriya local fauna without designating any specimens. The examination of the entire avian material from Shiriya in this study could not identify specimens certainly referable to Phasianus rather than Syrmaticus. Although some fragmentary specimens within the material might possibly represent a species of the former genus, the absence of diagnostic specimens seems a sufficient reason to retract the occurrence of Phasianus sp. from the Shiriya local fauna.
Tribe Coturnicini Reichenbach, 1848
Coturnicini gen. et sp. indet.
Fig. 4.
Material.—Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24543, right distal humerus. Locality unrecorded, Shiriya, Aomori Prefecture, Japan; Middle–Late Pleistocene (~MIS 9/5e): NSMT PV 24544–24546, one right and two left distal tibiotarsi, respectively; NSMT PV 24547, right proximal tarsometatarsus.
Measurements.—See Table 2.
Description.—One distal end of the humerus was recovered (Fig. 4A). The fossil can be referred to Coturnicini (Coturnix, Synoicus, Tetraogallus, Alectoris, Ammoperdix, Perdicula, and Pternistis) by the following features that are apparently unique within Phasianidae: the concavity of the ventral surface of the epicondylus ventralis caudal to the pit for the m. pronator superficialis (Fig. 4A2), and the fossa m. brachialis being narrow and restricted to the ventral half of the shaft (the latter feature is exceptionally absent in Tetraogallus). Within Coturnicini, the fossil agrees with Coturnix and Synoicus and differs from the other genera examined in the presence of a marked depression on the cranioproximal margin of the condylus dorsalis. Furthermore, the fossil differs from Tetraogallus in the ventral position of the fossa m. brachialis mentioned above, and from Perdicula in a less abrupt dorsoventral expansion of the shaft toward the distal end. In these features, the fossil is consistent with Coturnix and Synoicus, which are osteologically rather similar to each other.
Three fragmentary distal ends of the tibiotarsus (Fig. 4C) and one proximal end of the tarsometatarsus (Fig. 4B) of a small phasianid were recovered. As few taxonomically useful characters can be observed in these fossils, they are tentatively assigned to the same species with the humerus.
Remarks.—The overall consistency of size suggests that only one species of Coturnicini is represented in the Shiriya material. Although the known elements could not be separated from modern Coturnix japonica, the only species of quail that occurs on the central Japanese islands today, it is not certain if it can be differentiated from other species of Coturnix or Synoicus, which are distributed throughout the Old World and Oceania.
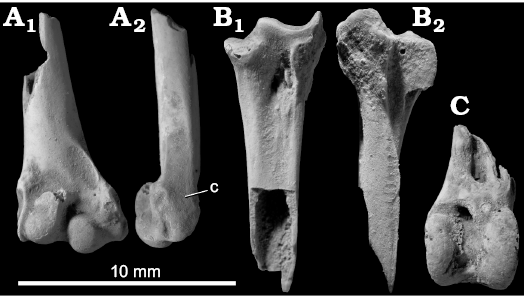
Fig. 4. Coturnicini gen. et sp. indet. from the upper Pleistocene of Shiriya, Japan. A. NSMT PV 24543, right humerus in cranial (A1) and ventral (A2) views. B. NSMT PV 24547, tarsometatarsus in dorsal (B1) and medial (B2) views. C. NSMT PV 24545, left tibiotarsus in cranial view. Fossils coated with ammonium chloride. Abbreviation: c, concavity on the ventral surface of epicondylus ventralis (see text).
Order Columbiformes Garrod, 1874
Family Columbidae Leach, 1820
Columbidae gen. et sp. indet.
Fig. 5.
Material.—Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24551, sternal fragment; NSMT PV 24552, left humeral shaft; NSMT PV 24553, right distal humerus.
Measurements.—See Table 2; sternum: no formal measurement possible.
Table 2. Measurements (in mm) of Coturnicini gen. et sp. indet. and Columbidae gen. et sp. indet. from the upper Pleistocene of Shiriya. Abbreviations: D, craniocaudal (for humerus) or dorsoventral (for leg elements) depth; GDB, greatest distal breadth; W, dorsoventral (for humerus) or mediolateral (for leg elements) width.
|
Elements |
Coturnicini |
Columbidae |
|
Humerus |
|
|
|
W midshaft |
|
4.9, – |
|
D midshaft |
|
3.6, – |
|
GDB |
5.2 |
|
|
distal W |
5.1 |
|
|
distal D |
3.0 |
|
|
Tibiotarsus |
|
|
|
distal W |
4.3, 4.3, 4.2 |
|
|
distal D |
4.4, 4.4, 4.1 |
|
|
Tarsometatarsus |
|
|
|
proximal W |
4.8 |
|
|
proximal D |
4.5 |
|
Description.—One moderately worn sternal fragment preserving the rostral area and the dorsal half of the pila carinae was recovered (Fig. 5C). The spina externa rostri is broken away, but at least was originally present, unlike in some genera of Raphinae, including Otidiphaps and Ptiliophaps. The spina interna rostri is moderately developed cranially, and is wider laterally than it is thick dorsoventrally.
One humeral shaft preserving proximally from the distal extents of the crista bicipitalis and tuberculum dorsalis and distally to the tuberculum supracondylare dorsale, and another distal fragment of the humerus preserving the area around the tuberculum supracondylare dorsale and condylus dorsalis were recovered (Fig. 5A, B).
Remarks.—Due to the rather fragmentary nature of the specimens, it was not possible to assign any of them to the subfamilial or generic level. The consistency of size suggests that only one species is represented, which would be similar in size to moderate-sized pigeons and turtle-doves, e.g., modern Streptopelia orientalis.
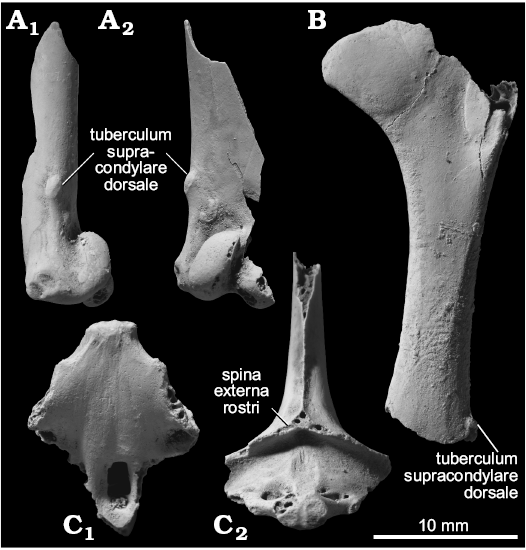
Fig. 5. Columbidae gen. et sp. indet. from the upper Pleistocene of Shiriya, Japan. A. NSMT PV 24551, right humerus in dorsal (A1) and cranial (A2) views. B. NSMT PV 24552, left humerus in cranial view. C. NSMT PV 24553 sternum in ventral (C1) and cranial (C2) views. Fossils coated with ammonium chloride.
Order Apodiformes Peters, 1940
Family Apodidae Olphe-Galliard, 1887 (1836)
Remarks.—The family Hemiprocnidae is sometimes treated as a subfamily of Apodidae (Hemiprocninae), but can be readily distinguished from Apodidae in numbers of osteological features, including weak medial protrusion of the processus acrocoracoideus of the coracoid (which is strongly protruded medially in Apodidae) and a caudally bowed os metacarpale minor of the carpometacarpus (straighter in Apodidae).
Subfamily Apodinae Olphe-Galliard, 1887 (1836)
Genus Apus Scopoli, 1777
Apus sp.
Fig. 6A, C, D.
Material.—Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24548, left coracoid; NSMT PV 24549, left carpometacarpus; NSMT PV 24550, proximal phalanx of right major wing digit.
Measurements.—See Table 3 and Fig. 7.
Table 3. Measurements (in mm) of Apus sp. from the upper Pleistocene of Shiriya, compared with Recent A. pacificus, A. apus, and A. nipalensis. “Diagonal length” of the proximal phalanx of major wing digit refers to the greatest distance from the proximal articular surface to the distocaudal end (not to the distal articular surface, which is lacking in the fossil). Abbreviations: D, craniocaudal (for wings bones) or dorsoventral (for coracoid) depth; n, number of individuals; PP, processus procoracoideus; W, dorsoventral (for wing bones) or mediolateral (for coracoid) width; WSA, width of sternal articulation.
|
Elements |
Apus sp. |
Apus pacificus |
Apus apus |
Apus nipalensis |
||||||
|
n |
range |
mean |
n |
range |
mean |
n |
range |
mean |
||
|
Coracoid |
||||||||||
|
medial length |
13.6 |
11 |
12.5–13.8 |
13.1 |
4 |
12.6–13.4 |
13.1 |
4 |
11.4–12.1 |
11.6 |
|
W below PP |
1.9 |
12 |
1.4–1.9 |
1.7 |
5 |
1.6–1.8 |
1.7 |
4 |
1.2–1.4 |
1.3 |
|
D below PP |
1.5 |
12 |
1.3–1.6 |
1.5 |
5 |
1.3–1.6 |
1.5 |
4 |
1.1–1.4 |
1.2 |
|
WSA |
4.3 |
12 |
4.0–4.9 |
4.4 |
5 |
4.2–4.4 |
4.3 |
4 |
3.6–3.8 |
3.7 |
|
Carpometacarpus |
||||||||||
|
length |
20.4 |
10 |
18.4–20.9 |
19.5 |
5 |
19.7–20.9 |
20.1 |
3 |
17.2–17.9 |
17.6 |
|
proximal W |
2.2 |
10 |
2.1–2.3 |
2.2 |
5 |
2.1–2.3 |
2.2 |
3 |
1.7–1.8 |
1.8 |
|
proximal D |
6.0 |
10 |
5.5–5.9 |
5.7 |
5 |
5.5–5.8 |
5.6 |
3 |
4.7–4.8 |
4.7 |
|
W midshaft |
2.4 |
10 |
2.1–2.3 |
2.2 |
5 |
1.9–2.2 |
2.1 |
3 |
1.5–1.7 |
1.6 |
|
D midshaft |
2.5 |
10 |
2.3–2.7 |
2.5 |
5 |
2.3–2.6 |
2.5 |
3 |
1.8–2.0 |
2.0 |
|
distal W |
3.0 |
10 |
2.7–3.2 |
2.9 |
5 |
2.6–2.9 |
2.7 |
3 |
2.2–2.4 |
2.3 |
|
Proximal phalanx of major wing digit |
||||||||||
|
diagonal length |
13.2 |
10 |
9.3–12.9 |
12.2 |
5 |
12.0–12.6 |
12.3 |
3 |
9.9–10.6 |
10.2 |
|
proximal W |
3.0 |
9 |
2.8–3.1 |
3.0 |
5 |
2.8–3.1 |
2.9 |
3 |
2.1–2.2 |
2.2 |
|
proximal D |
3.0 |
9 |
2.7–2.8 |
2.7 |
5 |
2.6–2.9 |
2.8 |
3 |
2.2–2.3 |
2.2 |
Description.—One left coracoid with a worn angulus lateralis was recovered (Fig. 6A). The fossil differs from the members of Cypseloidinae (Streptoprocne and Cypseloides) in the narrowness of a notch on the dorsal part of the sternal margin of the tuberculum brachiale (the notch being dorsoventrally broad and often deep in Cypseloidinae). It differs from Collocaliini (Collocalia and Aerodramus) and agrees with Apodini (Aeronautes, Tachornis, Cypsiurus, Tachymarptis, and Apus) and to a lesser extent with Chaeturini (Hirundapus and Chaetura) in an elongated and slit-like medioventral opening of the foramen n. supracoracoidei (which is omo-sternally narrower in Collocaliini). It also differs from Chaeturini in the absence of a strong constriction of the neck (in Chaeturini, the neck is constricted and the processus acrocoracoideus is slanted medially). Within Apodini, the fossil agrees with Tachornis, Tachymarptis, and Apus and differs from Aeronautes, Panyptila, and Cypsiurus in a relatively omal position of the dorsal opening of the foramen n. supracoracoidei (which is distinctly offset sternally from the omal margin of the processus supracoracoidei in the latter genera). The fossil has a characteristic profile of the sternal articulation surface, where the medioventral margin is strongly produced, the dorsal flange is lying rather medially (Fig. 6B2), and the dorsolateral margin is strongly concave. This condition is apparently unique to Tachymarptis and Apus within Apodidae. The single coracoid of Tachymarptis examined (T. melba, USNM 559068) is distinct from the fossil in the presence of a prominent ridge on the ventral margin of the shaft leading from the lateral part of the sternal end to the ventral margin of the sulcus supracoracoideus. The fossil is indistinguishable from A. apus and A. pacificus in both dimensions and qualitative characters.
One complete left carpometacarpus was recovered (Fig. 6C). The fossil differs from Cypseloidinae in a cranially lying sulcus tendinosus in dorsal view (Fig. 6C2, B3); which is lying on the caudal half of the distal shaft in Cypseloidinae) and the absence of a constriction of the distal shaft of the os metacarpale minor (the constriction is present around the distal one-fifth in Cypseloidinae). It differs from Collocaliini and Chaeturini and agrees with Apodini in a strong expansion of the os metacarpi minor near the distal symphysis. Collocaliini further differ in a deeper distal part of the fossa supratrochlearis, and Hirundapus differs in a cranially positioned processus pisiformis. Within Apodini, the fossil agrees with Tachymarptis and Apus in a relatively small processus alularis whose proximal margin is not slanted proximally. The fossil carpometacarpus is consistent in size with the coracoid described above, thus is referred to the same species with it.
A distal phalanx of the major wing digit lacking the distal articular surface was recovered (Fig. 6D). Although relatively few taxonomically useful characters were detected for this element, the fossil differs from Streptoprocne in the absence of a strong convexity on the cranial surface of the proximal shaft, from Cypseloides in the absence of a prominent swell on the middle part on the cranial surface, from Aerodramus in the absence of a strong constriction at the base of the distal extension of the caudal margin, from Chaeturini on one hand and Aeronautes, Tachornis, and Panyptila on the other in a moderately developed depression on the ventral surface (which is deeper and narrower in the former group and shallower in the latter), and from most genera of the family except Hirundapus, Tachymarptis, and Apus in the broadness of the tip of the distal extension of the caudal margin. Therefore, the fossil is consistent only with Tachymarptis and Apus among the genera compared. Its size is consistent with the carpometacarpus described above.
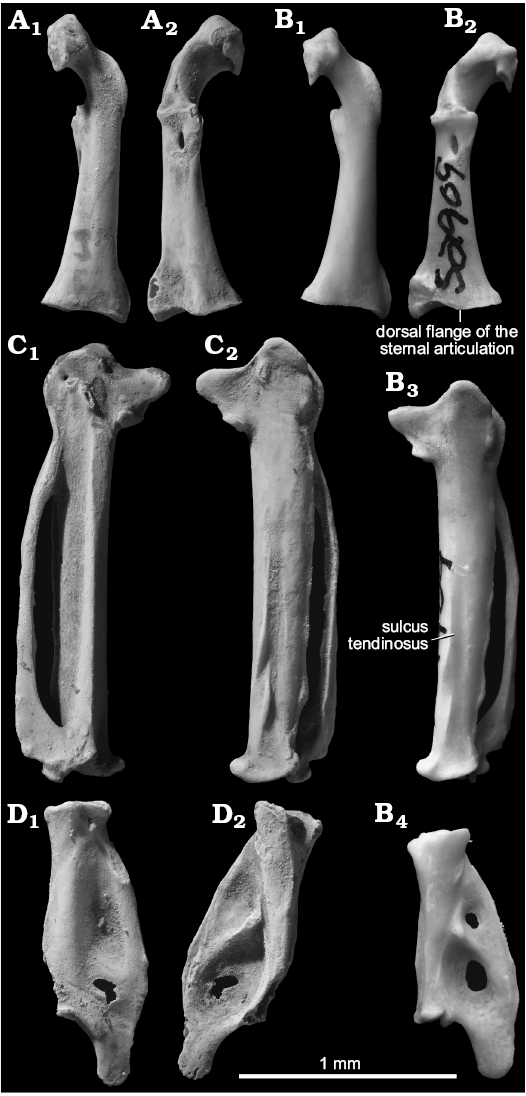
Fig. 6. Apodid bird Apus sp. from the upper Pleistocene of Shiriya, Japan (A, C, D), compared with Recent Apus pacificus (Latham, 1801) from Russia (B). A. NSMT PV 24548, left coracoids in ventral (A1) and dorsal (A2) views. B. UWBM 46958, left coracoid, in ventral (B1) and dorsal (B2) views; left carpometacarpus in dorsal view (B3); proximal phalanx of left major wing digit in dorsal view (B4). C. NSMT PV 24549, left carpometacarpus in ventral (C1) and dorsal (C2) views. D. NSMT PV 24550, proximal phalanx of right major wing digit in ventral (D1) and dorsal (D2) views. Fossils coated with ammonium chloride.
Remarks.—The genus Apus is distributed throughout the Old World (chiefly in Africa), and includes c. 15 modern species. Although recent authors tend to separate Tachymarptis from it (Päckert et al. 2012; Dickinson and Remsen 2013; del Hoyo and Collar 2014), there seems to be relatively few osteological differences between the two genera, as far as concerning the elements known for the Shiriya material (but see the feature of the coracoid mentioned above).
Only two species of the genus occur on Japanese islands today: A. pacificus and A. nipalensis. The Shiriya material is larger than the latter species and consistent in size with the former (Table 3; Fig. 7). However, the fossils cannot be distinguished from other modern species of the genus, including A. apus whose modern range spans over northern China.
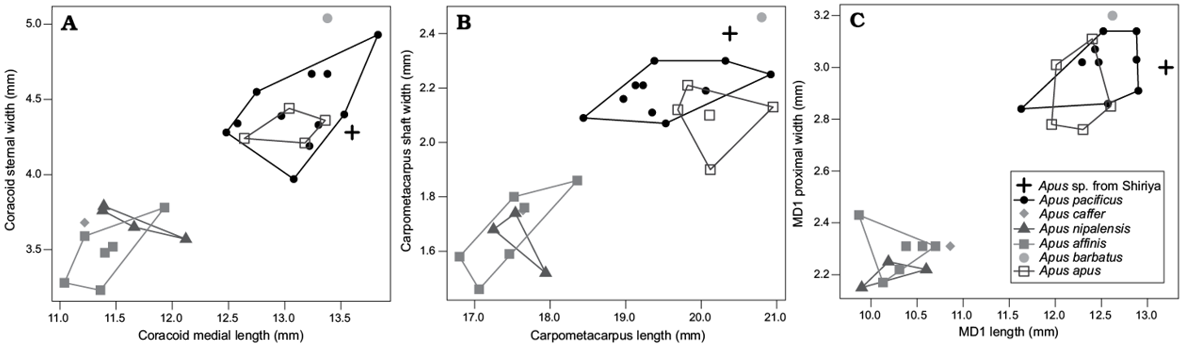
Fig. 7. Bivariate scatter plots of Recent and fossil species of Apus coracoidal (A), carpometacarpal (B), and proximal phalanx of major wing digit (C) measurements. MD1, proximal phalanx of major wing digit.
The fossil record of Apodidae was reviewed by Mlíkovský (1989, 2002) and Boev (2000). Several extinct species/subspecies of Apus have been described from Neogene deposits in Europe. Apus wetmorei Ballmann, 1976, and A. gaillardi (Ennouchi, 1930) are known from the upper Miocene of Italy and France, respectively. The former species was described as a species in size of modern A. caffer, and the latter was estimated to be smaller than modern A. apus (according to dimensions given by Harrison 1984). Therefore, these species would be smaller than the species represented by the Shiriya material, although no elements are known in common.
Apus apus palapus Jánossy, 1974, known from the Lower Pleistocene of Austria (Jánossy 1974) and possibly of Hungary (Jánossy 1978), is a temporal subspecies of A. apus which differs from the modern form in osteometric proportions. On its original description (Jánossy 1974), it was only compared with modern A. apus and A. pallidus. As such, despite Jánossy’s (1974) conjecture, it is not certain if that subspecies can be osteologically differentiated from modern A. pacificus. Apus baranensis Jánossy, 1978, is known from the upper Miocene–Lower Pleistocene of Hungary and Bulgaria (Jánossy 1978; Boev 2000; Kessler 2010). Being smaller than A. affinis, it is unlikely to be represented by the Shiriya material (see also Fig. 7). Although A. baranensis was synonymized with A. wetmorei by Mlíkovský (2002) based on the putative similarity of size alone, this act has been doubted by Zelenkov (2017) who mentioned the actual difference in size. Apus submelba Jánossy, 1972, known from several Middle–Late Pleistocene localities in Europe (Jánossy 1972, 1978; Mourer-Chauviré 1975; Tyrberg 2008a), is considered closely related to modern Tachymarptis (formerly Apus) melba, and indeed has been considered synonymous with the modern species by Mlíkovský (2002). The species might be better moved to Tachymarptis, if it is a distinct species and that genus deserves separation from Apus. In any way, published dimensions (Jánossy 1972) indicate that it was larger than the Shiriya material. Apart from these extinct forms, remains of modern species of the genus are known from numerous Quaternary localities throughout the Old World (e.g., Tyrberg 1998, 2008a; Harvati et al. 2013; Val 2016).
Order Accipitriformes Vieillot, 1816
Family Accipitridae Vigors, 1824
Remarks.—Hasegawa et al. (1988) included Haliaeetus cf. pelagicus and Buteo buteo in their faunal list, for each of which one specimen was figured. They are re-identified here as follows.
Genus Haliaeetus Savigny, 1809
Haliaeetus sp.
Fig. 8A, D, E–R.
1988 Haliaeetus cf. pelagicus (Pallas, 1811); Hasegawa et al. 1988: pl. 7: 13.
Material.—Loc. 1, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24554, right humeral shaft and NSMT PV 24555, terminal phalanx of left first toe (?). Loc. 2, Shiriya, Aomori Prefecture, Japan; middle Pleistocene (~MIS 9): NSMT PV 24556, left coracoid without sternal end; NSMT PV 24557, sternal end of right coracoid; NSMT PV 24558, left distal humerus; NSMT PV 24559–24561, right ulnar shafts; NSMT PV 24562, left distal ulna; NSMT PV 24563, 24564, left distal carpometacarpi; NSMT PV 24565, left alular phalanx; NSMT PV 24566, 24567, fragmentary right tibiotarsal shafts; NSMT PV 24568, right distal tibiotarsus, NSMT PV 24569–24580, terminal pedal phalanges. Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 24581, incomplete cranium; NSMT PV 24582, right humeral shaft; NSMT PV 24583, right distal humerus; NSMT PV 24584,left proximal ulna; NSMT PV 24585, left distal radius; NSMT PV 24586, left ulnare; NSMT PV 24587, left femoral shaft; NSMT PV 19010, right distal tibiotarsus; NSMT PV 24588,terminal phalanx of right first toe (?). Locality unrecorded, Shiriya, Aomori Prefecture, Japan; Middle–Late Pleistocene (~MIS 9/5e): NSMT PV 24589, left coracoidal shaft; NSMT PV 24590, 24591, left and right distal tibiotarsi, respectively; NSMT PV 24592–24597, terminal pedal phalanges.
Measurements.—See Table 4 and Fig. 9.
Table 4. Measurements (in mm) of Haliaeetus sp. from the middle–upper Pleistocene of Shiriya, compared with Recent Haliaeetus pelagicus, Haliaeetus albicilla, and Haliaeetus leucocephalus. Abbreviations: BFAH, breadth of the facies articularis humeralis; CNT, crista nuchalis transversa; D, depth perpendicular to width; IMB, impressio musculi brachialis; FT, fossa temporalis; n, number of individuals; PP, processus procoracoideus; PPO, processus postorbitalis; W, dorsoventral (for wing bones) or mediolateral (for the other elements) width.
|
Elements |
Haliaeetus
sp. |
Haliaeetus pelagicus |
Haliaeetus albicilla |
Haliaeetus leucocephalus |
||||||
|
n |
range |
mean |
n |
range |
mean |
n |
range |
mean |
||
|
Skull |
||||||||||
|
W PPO |
> 69 |
3 |
65.5–71.0 |
68.2 |
4 |
58.6–63.5 |
60.7 |
3 |
61.4–67.9 |
64.3 |
|
cranium W |
48.6 |
3 |
46.7–48.6 |
47.6 |
3 |
41.5–45.3 |
42.8 |
3 |
43.7–46.5 |
44.8 |
|
W across CNT |
58.7 |
3 |
56.6–57.5 |
57.1 |
4 |
50.6–57.3 |
53.4 |
3 |
54.8–60.4 |
57.4 |
|
Coracoid |
||||||||||
|
BFAH |
21.1, – |
9 |
21.3–25.1 |
23.0 |
9 |
18.3–23.2 |
20.8 |
3 |
18.9–22.2 |
20.6 |
|
W below PP |
10.4, 12.3 |
9 |
10.3–12.2 |
11.2 |
9 |
9.2–11.0 |
10.0 |
3 |
9.4–10.7 |
9.9 |
|
D below PP |
13.2, – |
9 |
13.3–16.8 |
14.5 |
9 |
10.9–14.0 |
12.5 |
3 |
12.0–13.3 |
12.5 |
|
Humerus |
||||||||||
|
W midshaft |
16.5, 15.5 |
4 |
16.6–17.9 |
17.2 |
6 |
13.2–16.3 |
14.7 |
3 |
14.9–16.4 |
15.5 |
|
D midshaft |
14.6, 12.9 |
4 |
14.0–16.2 |
14.8 |
6 |
11.9–14.7 |
13.4 |
3 |
13.3–14.9 |
13.9 |
|
Ulna |
||||||||||
|
proximal W |
24.2, –, – |
3 |
25.2–27.0 |
25.8 |
5 |
20.8–24.3 |
22.5 |
3 |
22.1–25.3 |
23.3 |
|
W below IMB |
13.8, 14.5, – |
3 |
13.2–14.3 |
13.9 |
5 |
11.4–13.8 |
12.4 |
3 |
12.3–13.4 |
12.9 |
|
D below IMB |
10.2, 10.8, – |
3 |
11.0–11.8 |
11.5 |
5 |
9.2–12.1 |
10.7 |
3 |
10.4–12.2 |
11.1 |
|
distal D |
–, –, 19.0 |
3 |
18.9–19.8 |
19.4 |
5 |
15.9–18.5 |
16.9 |
3 |
17.1–19.2 |
17.9 |
|
Radius |
||||||||||
|
distal W |
16.4 |
3 |
16.5–17.9 |
17.0 |
4 |
13.9–16.4 |
15.0 |
3 |
14.8–16.9 |
15.6 |
|
distal D |
8.0 |
3 |
7.9–9.2 |
8.5 |
4 |
6.7–8.0 |
7.2 |
3 |
6.9–8.1 |
7.6 |
|
Carpometacarpus |
||||||||||
|
distal W |
> 11.7 |
3 |
12.7–13.1 |
12.9 |
5 |
11.2–12.6 |
11.9 |
3 |
11.8–13.2 |
12.3 |
|
Femur |
||||||||||
|
W midshaft |
> 13.8 |
7 |
13.4–15.3 |
14.6 |
8 |
11.1–13.3 |
12.0 |
3 |
11.7–13.1 |
12.3 |
|
Tibiotarsus |
||||||||||
|
narrowest W |
13.9, 16.1, 13.2, –, – |
3 |
12.2–14.8 |
13.8 |
5 |
9.9–12.9 |
11.3 |
3 |
10.9–12.4 |
11.8 |
|
narrowest D |
8.1, 8.7, 8.9, –, – |
3 |
7.8–9.8 |
8.8 |
5 |
6.9–8.7 |
7.6 |
3 |
7.3–8.1 |
7.7 |
|
distal W |
22.0, 22.5, –, 27.0, –, 21.0 |
3 |
22.9–26.7 |
25.0 |
5 |
17.8–21.7 |
19.7 |
3 |
19.6–22.3 |
20.7 |
|
distal D |
14.9, 16.4, –, 18.4, – |
2 |
18.0, 21.6 |
– |
5 |
13.2–15.6 |
14.3 |
3 |
14.4–16.5 |
15.1 |
Description.—One cranium preserving a part of the frontals, the dorsal margin of the foramen magnum, and both tympanic cavities was recovered (Fig. 8A). In the fossil, there is only a shallow depression rostral to the tympanic cavity, ventral to the fossa temporalis, where a prominent excavation, from which the quadratic articulations are distinctly elevated, is present in most accipitrids (Fig. 8A3, B3, C3). This feature is apparently unique to Haliaeetus and Haliastur, as well as to some Gypaetinae (Pernis, Aviceda, Polyboroides, and Gypaetus). The crania in the latter group are much deeper dorsoventrally for its width (except Gypaetus) and characterized by a narrower fossa temporalis. In addition, the fossil differs from some other large-bodied accipitrid genera, including Aquila and Aegypius, in weaker lateroventral extension of the cranial roof over the laterocaudal margin of the cotyla quadratica squamosi.
Three coracoidal shafts, the most complete one of which preserves the dorsal part of the processus acrocoracoideus and entire processus procoracoideus (Fig. 8D, E), are referred to the same species as the cranium. The fossils have a stocky profile compared to most accipitrids, while lacking extensive pneumatization along the entire cranial margin of the sulcus supracoracoideus (which is seen in Gypini), agreeing with Harpia, Aquila, Haliastur, Milvus, Butastur, Haliaeetus, and Icthyophaga. Among those genera, the fossils differ from Harpia and Aquila and agree with the others in weak development of the sternal margin of the facies articularis clavicularis, which is developed as a prominent ridge on the medial surface of the processus acrocoracoideus in the former group (Fig. 8B4, C4). The fossils differ from the coracoid of Butastur in having a single pneumatic fovea caudal to the dorsal tip of the tuberculum brachiale (whereas Butastur has a pair of foveae in the same position).
One moderately worn proximal part of the ulna, with the olecranon broken away, was recovered (Fig. 8H). The shaft is relatively shallow craniocaudally, agreeing only with some species of Haliaeetus, including H. albicilla, H. leucocephalus, and H. pelagicus. Other qualitative features, including a strong constriction of the cotyla dorsalis in its ventral part (less apparent in, e.g., Harpia, Stephanoaetus, and Aquila) and the lack of pneumatic foramina in the proximal end (foramina pneumatica are present caudal to the proximal articular surfaces and/or around the impressio m. brachialis in Gypini), are consistent with the assignment to Haliaeetus.
Two distal ends of carpometacarpi, one of which preserves the distal part of the os metacarpi minor near the distal symphysis, are referred to this species (Fig. 8N). So far as observed in the better preserved specimen, the spatium intermetacarpale is craniocaudally narrow, as characteristically observed in Elanus, Kaupifalco, Butastur, and Haliaeetus (Fig. 8B9, C5). The fossils further differ from Gypini in the absence of foramina pneumatica at or around the distal symphysis.
Five distal ends of the tibiotarsus are referred to this species (Fig. 8O, P). In the fossils, the sulcus extensorius is lying near the lateral margin of the distal shaft, and almost restricted to the lateral half of the bone, agreeing with Circus, Accipiter, Haliaeetus (except H. leucogaster), Icthyophaga, and perhaps Aquila (the condition is less clear in the last genus; Fig. 8B10, C6). Among these genera, the fossils differ from Circus, Accipiter, and Aquila and agree with Haliaeetus and Icthyophaga in weak proximal extension of the medial margin of the condylus medialis on the caudal surface; in the former group, the margin clearly extends past the proximal margin of the condyle on the cranial side (Fig. 8C7), whereas it fades more distally in the latter group (Fig. 8B11). The tibiotarsus of Aquila further differs in a much deeper incisura intercondylaris (Fig. 8C6, C7). The tibiotarsus of Icthyophaga differs in a distinctly deeper, wider sulcus extensorius.
Two shafts and two distal fragments of the humerus (Fig. 8F, G), one distal end of the ulna (Fig. 8I), three ulnar shafts, one distal end of the radius (Fig. 8J), one complete ulnare (Fig. 8J), one alular phalanx lacking the distal tip (Fig. 8K), one distal shaft of the femur (Fig. 8L), one basal phalanx of the third toe, and 19 terminal phalanges of various toes (Fig. 8Q, R) are tentatively referred to this species based on their large size. Although the preserved parts carry little taxonomic information, some of these elements can be differentiated at least from other large accipitrids. In the fossil humerus, the proximal part of the condylus dorsalis does not possess a sharp, straight ventral margin (which is observed in Aegypius). The fossil distal ulna, radius, ulnare, and alar phalanx differ from the corresponding elements of Gypini in the absence of extensive pneumatization. From Aquila, the radius differs in a ventrally positioned caudal knob of the distal articular surface, the ulnare does in strongly convex distal margin of the facies articularis ulnaris, the alular phalanx in a relatively proximal position of the tubercle on the ventrocaudal margin near the proximal end (in Aquila, the tubercle is elongated distally), and the femur in a more strongly developed impressio ansae m. iliofibularis near the proximal extent of the condylus lateralis.
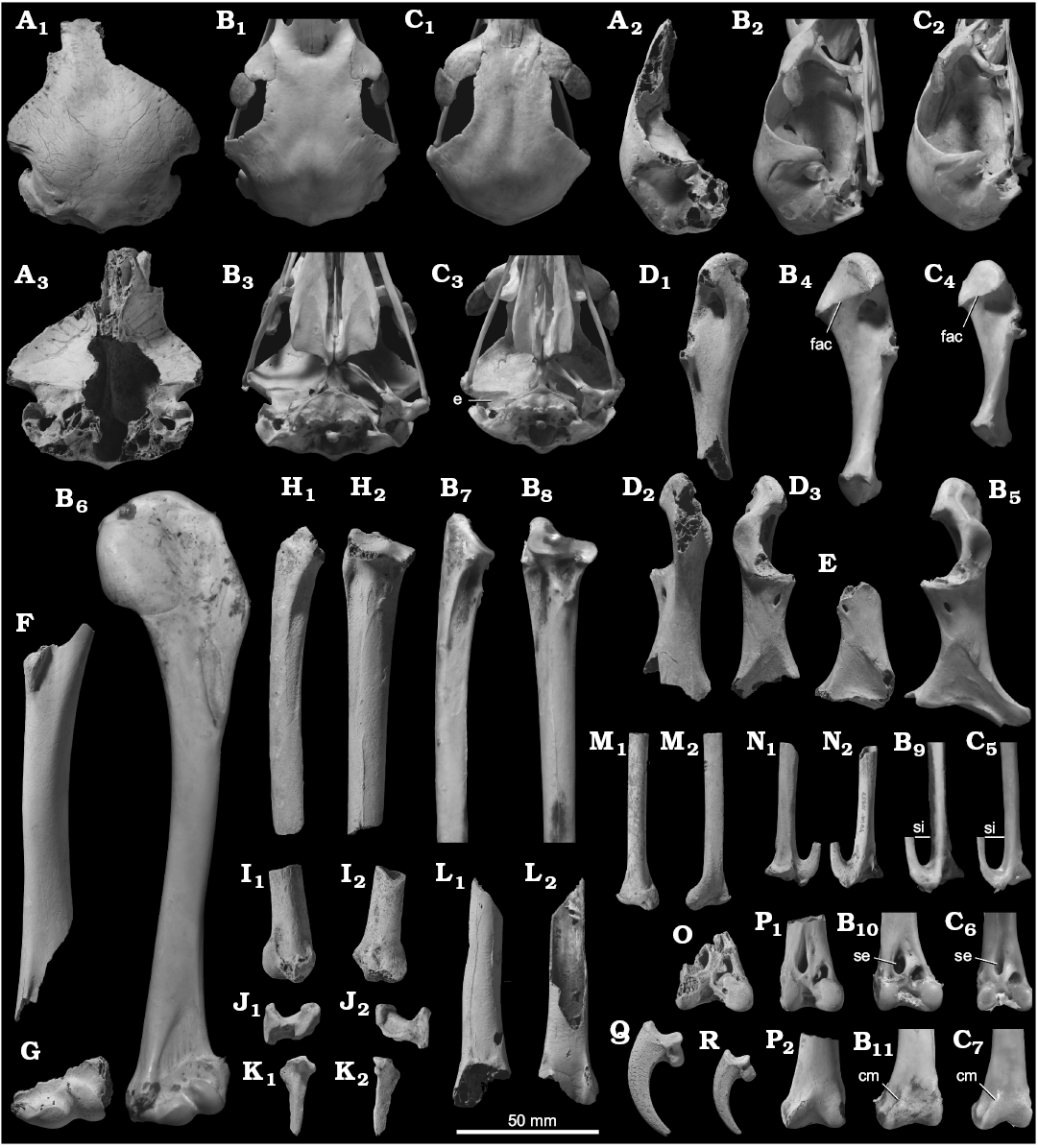
Fig. 8. Accipitrid bird Haliaeetus sp. from the middle–upper Pleistocene of Shiriya, Japan (A, D–R), compared with Recent Haliaeetus pelagicus (Pallas, 1811) from Japan (B) and Recent Aquila chrysaetos (Linnaeus, 1758) from Japan (C). A. NSMT PV 24581, cranium in dorsal (A1), right lateral (A2), and ventral (A3) views. B. NSMT AS 1344, cranium in dorsal (B1), right lateral (B2), and ventral (B3) views; right coracoid in medial (B4) and dorsal (B5) views; left humerus in cranial view (B6); left ulna in ventral (B7) and cranial (B8) views; left carpometacarpus in ventral view (B9); left tibiotarsus in cranial (B10) and caudal (B11) views (reversed for comparison). C. NSMT AS 1299, cranium in dorsal (C1), right lateral (C2), and ventral (C3) views; right coracoid in medial view (C4); left carpometacarpus in ventral view (C5); left tibiotarsus in cranial (C6) and caudal (C7) views (reversed for comparison). D. NSMT PV 24556, left coracoid in medial (D1), ventral (D2), and dorsal (D3) views. E. NSMT PV 24557, right coracoid in dorsal view. F, G. NSMT PV 24582 and NSMT PV 24558, respectively, right and left humeri in cranial view. H, I. NSMT PV 24584 and NSMT PV 24562, respectively, left ulnae in ventral (H1, I1), cranial (H2), and dorsal (I2) views. J. NSMT PV 24586, left ulnare in proximal (J1) and distal (J2) views. K. NSMT PV 24565, left alular phalanx in dorsal (K1) and ventral (K2) views. L. NSMT PV 24587, left femur in caudal (L1) and cranial (L2) views. M. NSMT PV 24585, left radius in caudal (M1) and cranial (M2) views. N. NSMT PV 24564, left carpometacarpus in dorsal (N1) and ventral (N2) views. O. NSMT PV 24591, right tibiotarsus in cranial view. P. NSMT PV 19010, left tibiotarsus in cranial (P1) and caudal (P2) views. Q, R. NSMT PV 24570 and NSMT PV 24571, respectively, terminal phalanges of right second and third pedal digits in medial view. Fossils coated with ammonium chloride. Abbreviations: cm, (proximal extent of the caudal margin of) condylus medialis; e, excavation rostral to the tympanic cavity (see text); fac, (sternal margin of) facies articularis clavicularis; se, sulcus extensorius; si, spatium intermetacarpale.
Remarks.—The material examined here includes the specimen figured as H. cf. pelagicus (NSMT PV 19010) by Hasegawa et al. (1988). The size variation within the material seems to be consistent with the presence of only one species, although the possibility that more species are represented cannot be ruled out. With 35 identifiable specimens, the species is the most common landbird species represented in the Shiriya material (minimum number of individuals being three for Loc. 2 and one for each of Locs. 1 and 3). As far as concerning the elements preserved, it was difficult to find unambiguous osteological features that allow assignment to certain clades within the family. Nevertheless, most elements of the Shiriya material could be differentiated from those of other large eagles, Aquila and Gypini, thus they most likely represent a sea eagle of the genus Haliaeetus. Three modern species of Haliaeetus occur on the central Japanese islands today (The Ornithological Society of Japan 2012): H. albicilla, H. pelagicus, and H. leucocephalus (although the last species has only been recorded as an accidental visitor). Partly due to the small size of the comparative material, it is at best difficult to assign the material to any of these three species (see Fig. 9). Hence, it seems justified to leave the specific identification open rather than designating a particular species as was done by Hasegawa et al. (1988), pending further study.
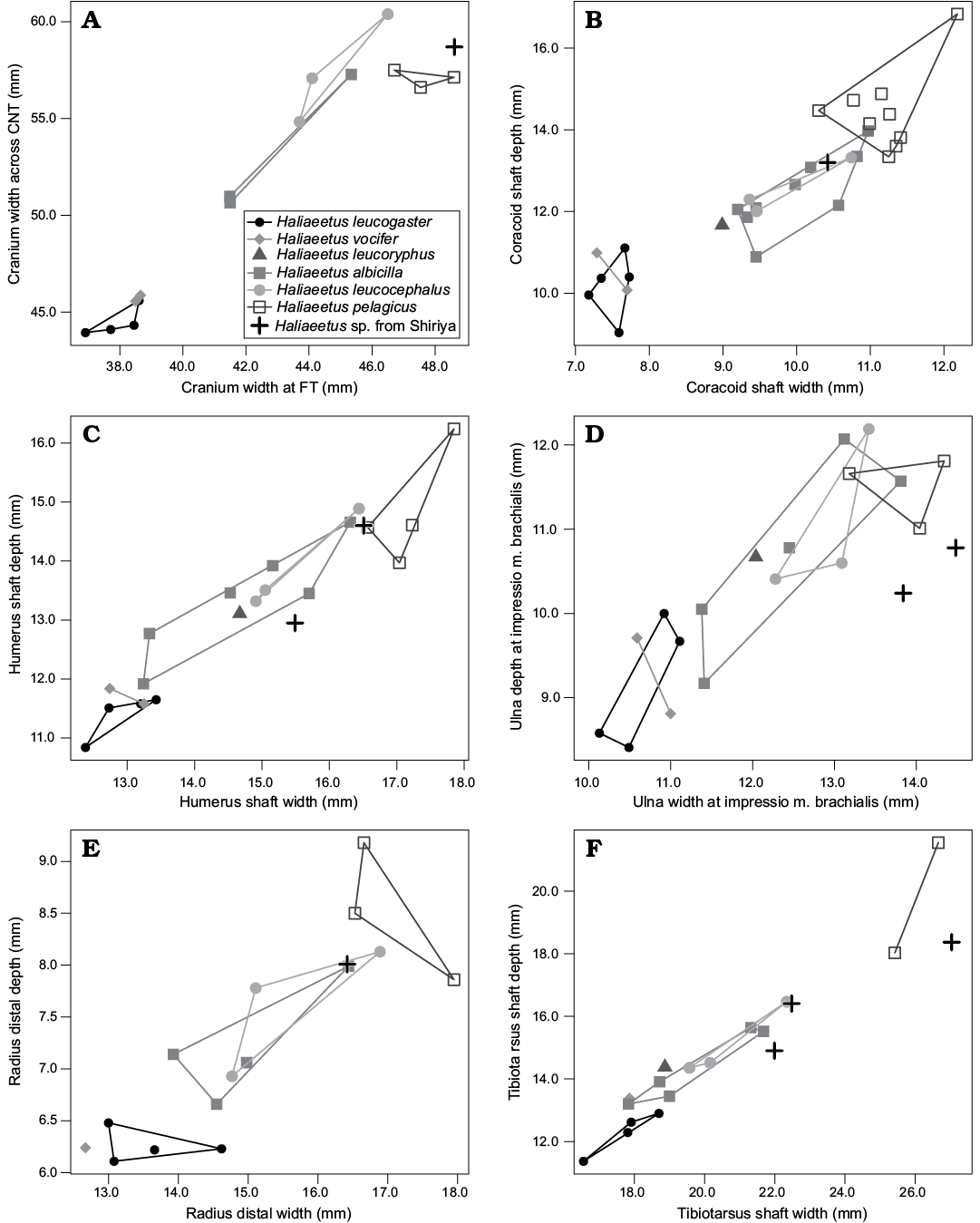
Fig. 9. Bivariate scatter plots of Recent and fossil species of Haliaeetus: cranial (A), coracoidal (B), humeral (C), ulnar (D), radial (E), and tibiotarsal (F) measurements. Abbreviations: CNT, crista nuchalis transversa; FT, fossa temporalis.
The oldest putative records of the genus include a large accipitrid from the lower Oligocene of Egypt (Accipitridae genus and species indeterminate, aff. Haliaeetus in Rasmussen et al. 1987) and Haliaeetus (?) sp. from the middle Miocene of Florida (Becker 1987a, b), whose true affinities are difficult to evaluate from the evidences provided. Neogene records include H. piscator Milne-Edwards, 1871, from the upper Miocene of France (Milne-Edwards 1867–1871), and H. fortis Kurochkin, 1985, from the upper Miocene–lower Pliocene of Mongolia (Kurochkin 1985; Zelenkov 2013). It should be noted that the placement of the former species within Haliaeetus has been doubted by Mlíkovský (2002). According to published dimensions and illustrations, both H. piscator and H. fortis seem to have been smaller than the species represented by the Shiriya material.
A putative Early Pleistocene species from Czech Republic, H. angustipes Jánossy, 1983, has been shown to be inseparable from modern H. albicilla by Mlíkovský (1997). An extinct species H. australis (Harrison and Walker, 1973) is known from the Holocene of the Chatham Islands (Dawson 1961; Harrison and Walker 1973; Olson 1984; Millener 1999). Published dimensions of that species (Harrison and Walker 1973) suggest that it might be comparable in size with the Shiriya material, but it is not feasible to make a comparison because the latter lacks the tarsometatarsus, the most intensively described element of H. australis (Harrison and Walker 1973; Olson 1984). A genetically distinct lineage which is most closely related to H. albicilla is known from Holocene deposits of Hawaiian Islands (Olson and James 1991; Fleischer et al. 2000; Hailer et al. 2015). Published dimensions of the Hawaiian material (Olson and James 1991) indicate that it is smaller than the Shiriya material.
Occurrence of modern species of the genus has been recorded from various Pleistocene localities. Haliaeetus albicilla and H. leucogaster are known from numerous localities in the Old World (e.g., Hou 1993; Tyrberg 1998, 2008a; Guerra et al. 2013; Huguet et al. 2017) and North America (e.g., Brodkorb 1964; Guthrie 1992; Emslie 1998), respectively. Haliaeetus vocifer has been recorded from a few localities in Africa, whereas H. vociferoides has been recorded from Madagascar (Lambrecht 1933; Phillips 1988). Baird (1991) mentioned the occurrence of cf. H. leucogaster from Quaternary cave deposits in Australia. Apparently, H. pelagicus does not have a definitive fossil record, apart from Holocene archaeological occurrences (e.g., Friedman 1935). Although Rich et al. (1986) cited the occurrence of H. cf. pelagicus from the Pleistocene of Yage, central Honshu Island, the original report of the site (Tomida 1978) designated the relevant species as Haliaeetus sp. indet. Further work with larger comparative material would be required to verify the identifications of the putative records of the last species, whose breeding range is largely restricted around the Sea of Okhotsk today.
Accipitridae gen. et sp. indet.
Fig. 10.
1988 Buteo buteo (Linnaeus, 1758); Hasegawa et al. 1988: pl. 7: 6.
Material.—Loc. 3, Shiriya, Aomori Prefecture, Japan; Late Pleistocene (~MIS 5e): NSMT PV 19003, right femoral shaft; NSMT PV 24598, terminal phalanx of pedal digit (right second?). Locality unrecorded, Shiriya, Aomori Prefecture, Japan; Middle–Late Pleistocene (~MIS 9/5e): NSMT PV 24599, left distal ulna.
Measurements.—See Table 5; no formal measurement was possible for the pedal phalanx.
Table 5. Measurements (in mm) of Accipitridae gen. et sp. indet. from the upper Pleistocene of Shiriya. Abbreviations: D, craniocaudal (for ulna) or craniocaudal (for femur) depth; W, dorsoventral (for ulna) or mediolateral (for femur) width.
|
Elements |
Measurements |
|
Ulna |
|
|
distal W |
8.6 |
|
distal D |
9.1 |
|
Femur |
|
|
W midshaft |
6.8 |
|
D midshaft |
6.6 |
Description.—One moderately worn distal ulna was recovered (Fig. 10B). In the fossil, the proximal margin of the trochlea carpalis is strongly elevated from the shaft, agreeing with Nisaetus, Kaupifalco, Melierax, Circus, Accipiter, and Buteo (and perhaps with Butastur, to a lesser extent). Concerning these genera, the fossil differs from Melierax in a proximally elongated trochlea carpalis, from Kaupifalco and Accipiter in the absence of a deep fossa ventral to the trochlea carpalis. In addition, the ulna of Accipiter differs in a long, sharp, and longitudinally oriented tip of the tuberculum carpale. Therefore, the fossil probably represents a species of Nisaetus, Circus, or Buteo, but further identification does not seem feasible.
One fragmentary shaft of the femur preserving a foramen pneumaticum (Fig. 10A) and one terminal pedal phalanx, probably of the right second toe (Fig. 10C), were recovered. The only notable feature of these fossils is the presence of a depression just proximal to the foramen pneumaticum medial to the crista trochanteris of the femur (Fig. 10A). This feature is observed in Macheiramphus, Kaupifalco, Melierax, Circus, and Buteo.
Remarks.—It is obvious that the three specimens represent one or more species much smaller than Haliaeetus sp. described above. Although the femur, NSMT PV 19003, was figured as Buteo buteo by Hasegawa et al. (1988), it is too fragmentary and not diagnostic enough to allow identification to the generic level. Even assuming that the ulna and femur represent the same species, they may possibly represent either Circus or Buteo, each of which includes several modern species distributed on the central Japanese islands today. Therefore, it seems reasonable to retract the record of B. buteo from the Shiriya local fauna.
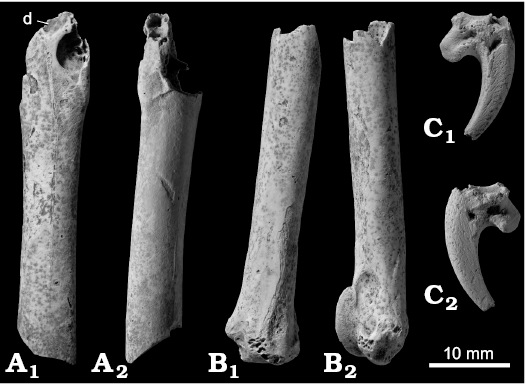
Fig. 10. Accipitridae gen. et sp. indet. from the upper Pleistocene of Shiriya, Japan. A. NSMT PV 19003, left femur in cranial (A1) and lateral (A2) views. B. NSMT PV 24599, left ulna in craniodorsal (B1) and ventral (B2) views. C. NSMT PV 24598, terminal phalanx of (right second?) pedal digit in lateral (C1) and medial (C2) views. Fossils coated with ammonium chloride. Scale bar 10 mm. Abbreviation: d, depression proximal to foramen pneumaticum (see text).
Concluding remarks
The present analysis of the avian remains from the Shiriya local fauna, northeastern Honshu Island, Japan, indicated the presence of six species of non-passeriform landbirds: Syrmaticus sp., Coturnicini gen. et sp. indet., Columbidae gen. et sp. indet., Apus sp., Haliaeetus sp., Accipitridae gen. et sp. indet. Species-level identification for the landbird remains was not feasible with the comparative materials examined in this study, unless it is assumed that only species occurring in this area today are represented. Such an assumption may potentially lead to circular reasoning in biogeographic and/or paleoclimate analyses, thus was not attempted here (see above). Indeed, when seabirds are taken into account, the Shiriya paleoavifauna includes an extralimital cormorant species (Watanabe et al. 2018) and at least three other extinct species (Hasegawa et al. 1988; Watanabe and Matsuoka 2015; Watanabe et al. 2016; JW, HM, and YH unpublished material). Nevertheless, the occurrences of the six landbird taxa are not unexpected, given that their relatives are members of the modern avifauna in the area. This is in contrast to the land mammal fauna recovered from the Shiriya localities (and Late Pleistocene localities on Honshu Island in general), which includes several extinct members of taxa that are not seen in the area today, including an elephant Palaeoloxodon naumanni, large deer Sinomegaceros yabei, and tiger Panthera tigris (e.g., Hasegawa 1972; Hasegawa et al. 1988; Iwase et al. 2012).
Although little is known about the Pleistocene terrestrial paleoclimate in northern Honshu Island, especially concerning the age of the Shiriya localities (MIS 9/5e), some information is available for central Honshu Island, where continuous records of lacustrine sediments exist (e.g., Tarasov et al. 2011; Kigoshi et al. 2017). Palynological analyses of such sediments have reconstructed that the climate in the last few interglacial periods, including MISs 9 and 5e, is slightly to considerably cooler (by up to ~4°C) than, and characterized by a comparable amount of precipitation to, the present (Tarasov et al. 2011; Kigoshi et al. 2017). If such conditions were also prevalent in northern Honshu Island, the Shiriya landbird fauna likely resided in such an environment. One remarkable element is Syrmaticus, whose modern species are generally associated with mountainous forests (Johnsgard 1999). The presence of Syrmaticus sp. in Shiriya may imply the presence of such a habitat in northern Honshu Island in the last interglacial period (MIS 5e) as is seen today. In addition, the presence of Syrmaticus may have some biogeographic implications. Modern S. soemmerringii, the only species of the genus occurring on the Japanese islands today, does not occur north to the Tsugaru Strait (i.e., on Hokkaido Island), the fact which was originally used to substantiate the biogeographic delineation now known as Blakiston’s line (Blakiston and Pryer 1880; Blakiston 1883). If Syrmaticus sp. from the Shiriya local fauna had similar climatic requirements to those of the modern species, it may follow that the northern distribution limit of the modern species is at least partly influenced by factors other than climatic ones (temperature), as Syrmaticus sp. from Shiriya could inhabit the northern end of Honshu Island in a colder climate condition than in the present. That is, the Tsugaru Strait may have acted as a biogeographic barrier that prevented northward dispersals to Hokkaido Island by Syrmaticus. If they could cross the strait to colonize Hokkaido Island in the last glacial period, as was apparently done by some large land mammals in the opposite direction (Kawamura 2007), they would have been able to persist there. Nevertheless, the possibility remains that they retreated southward in glacial periods. A more substantiated discussion may be possible after species-level identification could be made with a larger comparative material, and evidences from other fossil localities were accumulated.
Apart from species-level identifications, the landbirds identified from the Shiriya local fauna in this study add new pieces to the Pleistocene vertebrate community in northern Honshu Island. Syrmaticus sp., Coturnicini gen. et sp. indet., and Columbidae gen. et sp. indet. are likely to have been primarily granivorous or frugivorous birds as in the modern representatives of the taxa, and might have interacted with the diverse assemblage of rodents and shrews known from the same local fauna (Hasegawa et al. 1988). Accipitridae gen. et sp. was probably an aerial predator that preyed on such small vertebrates (possibly along with invertebrates). Haliaeetus sp. might have relied more on marine preys, such as a salmonid fish Oncorhychus sp. which was identified from Loc. 3 (Hasegawa et al. 1988). Apus sp. would have been an aerial insectivore, and in this respect an indirect interaction with insectivorous bats in the local fauna (Hasegawa et al. 1988) could be presumed, although direct competition would have been mitigated by the difference in diel activity patterns (diurnal swift versus nocturnal bats).
By describing six species of landbirds from the Pleistocene of Shiriya, this study formed a basis for comparison in future studies of the Pleistocene fossil birds in the Japanese Archipelago. Revision of avian materials from other Pleistocene localities in the region (Takai 1962; Hasegawa 1964; Tomida 1978; Nokariya and Ono 1980; Ono 1980; Rich et al. 1986) may shed more light on the biogeographic history of the landbird fauna. One pertinent problem is, however, the scarcity of avian skeletons from the eastern Eurasia and Japanese islands in the museum collections, which was partly responsible for the inconclusive identifications in this study. It is urgent to build larger skeletal collections of birds from the eastern Eurasia, in order to fully appreciate contemporary variation within and among modern forms, and to decipher history of the unique avifauna in the region.
Acknowledgements
The authors would like to thank the late Zenji Nakajima for providing the authors with specimens he collected. Sincere thanks are given to the following individuals who generously helped work with their collections during the research: Akihiro Koizumi and Takeshi Muramatsu (both ICM), Carla Cicero and Jessie A. Atterholt (both MVZ), Makoto Manabe, Chisako Sakata, and Yuri Kimura (all NSMT PV), Isao Nishiumi (NSMT AS), Christopher M. Milensky and Helen F. James (both USNM), Robert C. Faucett (UWBM), Takeshi Yamasaki and Tomoko Imamura (both YIO). The authors also appreciate the useful information provided by Naoki Kohno (NSMT PV), and the constructive comments by an anonymous reviewer.
References
Baird, R.F. 1991. Avian fossils from the Quaternary of Australia. In: P. Vickers-Rich, J.M. Monaghan, R.F. Baird, and T.H. Rich (eds.), Vertebrate Palaeontology of Australasia, 809–870. Pioneer Design Studio, Melbourne.
Ballmann, P. 1976. Fossile Vögel aus dem Neogen der Halbinsel Gargano (Italien), zweiter Teil. Scripta Geologica 38: 1–59.
Baumel, J.J. and Witmer, L.M. 1993. Osteologia. In: J.J. Baumel, A.S. King, J.E. Breazile, H.E. Evans, and J.C. Vanden Berge (eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd Edition. Publications of the Nuttall Ornithological Club 23, 45–132. The Nuttall Ornithological Club, Cambridge.
Becker, J.J. 1987a. Neogene Avian Localities of North America. 171 pp. Smithsonian Institution Press, Washington, D.C.
Becker, J.J. 1987b. The fossil birds of the late Miocene and early Pliocene of Florida. I. Geology, correlation, and systematic overview. In: C. Mourer-Chauviré (ed.), L’Évolution des Oiseaux d’Aprés le Témoignage des Fossiles. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon 99: 159–171.
Bever, G.S. 2005. Variation in the ilium of North American Bufo (Lissamphibia; Anura) and its implications for species-level identification of fragmentary anuran fossils. Journal of Vertebrate Paleontology 25: 548–560. Crossref
Bintaja, R., van de Wal, R.S.W., and Oerlemans, J. 2005. Modelled atmospheric temperatures and global sea levels over the past million years. Nature 437: 125–128. Crossref
Blakiston, T.W. 1883. Zoological indications of ancient connection of the Japan Islands with the continent. Transactions of the Asiatic Society of Japan 11: 126–140.
Blakiston, T.W. and Pryer, H. 1880. Catalogue of the birds of Japan. Transactions of the Asiatic Society of Japan 8: 172–241.
Boev, Z. 2000. The presence of Apus baranensis Janossy, 1977 (Aves: Apodidae) in the late Pliocene of Bulgaria. Acta Zoologica Bulgarica 52: 43–52.
Brodkorb, P. 1960. How many species of birds have existed. Bulletin of the Florida State Museum, Biological Science 5: 41–53.
Brodkorb, P. 1964. Catalogue of fossil birds, Part 2 (Anseriformes through Galliformes). Bulletin of the Florida State Museum, Biological Science 8: 195–335.
Chinzei, K. 1991. Late Cenozoic zoogeography of the Sea of Japan area. Episodes 14: 231–235.
Chinzei, K. and Machida, H. 2001. Historical development of geomorphology of the Japanese islands [in Japanese]. In: N. Yonekura, S. Kaizuka, M. Nogami, and K. Chinzei (eds.), Regional Geomorphology of the Japanese Islands, Volume 1: Introduction to Japanese Geomorphology, 297–322. University of Tokyo Press, Tokyo.
Dawson, E.W. 1961. An extinct sea eagle in the Chatham Islands. Notornis 9: 171–172.
Dickinson, E.C. and Remsen, J.V., Jr. (eds.) 2013. The Howard and Moore Complete Checklist of the Birds of the World, 4th Edition, Volume 1. l + 461 pp. Aves Press, Eastbourne.
Dobson, M. 1994. Patterns of distribution in Japanese mammals. Mammal Review 24: 91–111. Crossref
Emslie, S.D. 1998. Avian community, climate, and sea-level changes in the Plio-Pleistocene of the Florida Peninsula. Ornithological Monographs 50: 1–113.
Ennouchi, E. 1930. Contribution à l’étude de la faune du Tortonien de la Grive-Saint-Alban (Isère). 135 pp., 6 pls. Presses Modernes, Paris.
Ericson, P.G. 1987. Osteology of the Eider Somateria mollissima (L.). A Study of Sexual, Geographic and Temporal Morphometric Variation in the Eider Skeleton. 142 pp. Statens Historiska Museum, Stockholm.
Finlayson, G., Finlayson, C., Giles Pacheco, F., Rodriguez Vidal, J., Carrión, J.S., and Recio Espejo, J.M. 2008. Caves as archives of ecological and climatic changes in the Pleistocene—the case of Gorham’s cave, Gibraltar. Quaternary International 181: 55–63. Crossref
Fleischer, R.C., Olson, S.L., James, H.F., and Cooper, A.C. 2000. Identification of the extinct Hawaiian eagle (Haliaeetus) by mtDNA sequence analysis. Auk 117: 1051–1056. Crossref
Friedmann, H. 1935. Avian bones from prehistoric ruins on Kodiak Island, Alaska. Journal of the Washington Academy of Sciences 25: 44–51.
Garrod, A.H. 1874. On certain muscles [of the thigh] of birds and their value in classification. Part II. Proceedings of the Zoological Society of London 42: 111–124. Crossref
Guerra, C., McMinn, M., and Alcover, J.A. 2013. The Upper Pleistocene–Holocene raptorial bird guild from Eivissa Island (Pityusic Archipelago, Western Mediterranean Sea). Geobios 46: 491–502. Crossref
Guthrie, D.A. 1992. A Late Pleistocene avifauna from San Miguel Island, California. In: K.E. Campbell, Jr. (ed.), Papers in Avian Paleontology Honoring Pierce Brodkorb. Natural History Museum of Los Angeles County Science Series 36: 319–327.
Hailer, F., James, H.F., Olson, S.L., and Fleischer, R.C. 2015. Distinct and extinct: genetic differentiation of the Hawaiian eagle. Molecular Phylogenetics and Evolution 83: 40–43. Crossref
Harrison, C.J.O. 1984. A revision of the fossil swifts (Vertebrata, Aves, suborder Apodi), with descriptions of three new genera and two new species. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie 21: 157–177.
Harrison, C.J.O. and Walker, C.A. 1973. An undescribed extinct fish-eagle from the Chatham Islands. Ibis 115: 274–277. Crossref
Harvati, K., Darlas, A., Bailey, S.E., Rein, T.R., El Zaatari, S., Fiorenza, L., Kullmer, O., and Psathi, E. 2013. New Neanderthal remains from Mani peninsula, Southern Greece: the Kalamakia Middle Paleolithic cave site. Journal of Human Evolution 64: 486–499. Crossref
Hasegawa, Y. 1964. Preliminary report on the Gansuiji Formation and its fauna [in Japanese, with English summary]. Science Reports of the Yokohama National University, Section II: Biological and Geological Sciences 11: 71–78.
Hasegawa, Y. 1972. The Nauman’s elephant, Palaeoloxodon naumanni (Makiyama) from the Late Pleistocene off Shakagahama, Shodoshima Is. in Seto Inland Sea, Japan. Bulletin of the National Science Museum, Tokyo 15: 513–599.
Hasegawa, Y., Tomida, Y., Kohno, N., Ono, K., Nokariya, H., and Uyeno, T. 1988. Quaternary vertebrates from Shiriya area, Shimokita Peninsula, northeastern Japan [in Japanese, with English summary]. Memoirs of the National Science Museum (Tokyo) 21: 17–36.
Holm, S.R. and Svenning, J.-C. 2014. 180,000 years of climate change in Europe: avifaunal responses and vegetation implications. PLoS ONE 9: e94021. Crossref
Holt, B.G., Lessard, J.-P., Borregaard, M.K., Fritz, S.A., Araújo, M.B., Dimitrov, D., Fabre, P.-H., Graham, C.H., Graves, G.R., Jønsson, K.A., Nogués-Bravo, D., Wang, Z., Whittaker, R.J., Fjeldså, J., and Rahbek, C. 2013a. An update of Wallace’s zoogeographic regions of the world. Science 339: 74–78.
Holt, B.G., Lessard, J.-P., Borregaard, M.K., Fritz, S.A., Araújo, M.B., Dimitrov, D., Fabre, P.-H., Graham, C.H., Graves, G.R., Jønsson, K.A., Nogués-Bravo, D., Wang, Z., Whittaker, R.J., Fjeldså, J., and Rahbek, C. 2013b. Response to comment on “An update of Wallace’s zoogeographic regions of the world”. Science 341: 343. Crossref
Horsfield, T. 1821. Systematic arrangement and description of birds from the Island of Java. Transactions of the Linnean Society of London 13: 133–200. Crossref
Hosner, P.A., Faircloth, B.C., Glenn, T.C., Braun, E.L., and Kimball, R.T. 2016. Avoiding missing data biases in phylogenetic inference: an empirical study in the landfowl (Aves: Galliformes). Molecular Biology and Evolution 33: 1110–1125. Crossref
Hou, L. 1993. Avian fossils of Pleistocene from Zhoukoudian [in Chinese, with English summary]. Memoirs of Institute of Vertebrate Palaeontology and Palaeoanthropology, Academia Sinica 19: 165–297.
Howard, H. 1929. The avifauna of Emeryville Shellmound. University of California Publications in Zoology 32: 301–394.
del Hoyo, J. and Collar, N.J. 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World, Volume 1. 903 pp. Lynx Edicions, Barcelona.
Huguet, R., Vallverdú, J., Rodríguez-Álvarez, X.P., Terradillos-Bernal, M., Bargalló, A., Lombera-Hermida, A., Menéndez, L., Modesto-Mata, M., Van der Made, J., Soto, M., Blain, H.-A., García, N., Cuenca-Bescoós, G., Gómez-Merino, G., Pérez-Martínez, R., Expósito, I., Rofes, E.A.J., Burjachs, F., Canals, A., Bennaàsar, M., Nuñez-Lahuerta, C., Bermúdes de Castro, J.M., and Carbonell, E. 2017. Level TE9c of Sima del Elefante (Sierra de Atapuerca, Spain): a comprehensive approach. Quaternary International 433: 278–295. Crossref
Iwase, A., Hashizume, J., Izuho, M., Takahashi, K., and Sato, H. 2012. Timing of megafaunal extinction in the late Late Pleistocene on the Japanese Archipelago. Quaternary International 255: 114–124. Crossref
Jánossy, D. 1972. Die mittelpleistozäne Vogelfauna der Stránská Skála. Anthropos (Brno) 20: 35–64.
Jánossy, D. 1974. Die mittelpleistozäne Vogelfauna von Hundsheim (Niederösterreich). Sitzungsberichte (Österreichische Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse), Abteilung I: Biologie, Mineralogie, Erdkunde und verwandte Wissenschaften 182: 211–257.
Jánossy, D. 1978. Plio-Pleistocene bird remains from the Carpathian Basin III. Strigiformes, Falconiformes, Caprimulgiformes, Apodiformes. Aquila 84: 9–36.
Jánossy, D. 1983. Die mittelpleistozäne Vogelfauna von Přezletice bei Prag (ČSSR). In: W.-D. Heinrich (ed.), Wirbertier-Evolution und Faunenwandel in Känozoikum, 247–269. Akademie-Verlag, Berlin.
Jánossy, D. 1991. Late Miocene bird remains from Polgárdi (W-Hungary). Aquila 98: 13–35.
Jiang, L., Wang, G., Peng, R., Peng, Q., and Zou, F. 2014. Phylogenetic and molecular dating analysis of Taiwan blue pheasant (Lophura swinhoii). Gene 539: 21–29. Crossref
Johnsgard, P.A. 1999. The Pheasants of the World: Biology and Natural History, 2nd Edition. xviii + 398 pp. Smithsonian Institution Press, Washington, D.C.
Jolivet, L., Tamaki, K., and Fournier, M. 1994. Japan Sea, opening history and mechanism: a synthesis. Journal of Geophysical Research B: Solid Earth 99: 22237–22259. Crossref
Kamada, K. 2000. Shiriya complex: pre-Tertiary accretional complex at Cape Shiriya, Northern Japan [in Japanese]. Bulletin of the Faculty of Education, Hirosaki University 83: 39–47.
Kamei, T., Kawamura, Y., and Taruno, H. 1988. Mammalian stratigraphy of the Late Neogene and Quaternary in the Japanese islands [in Japanese, with English summary]. Memoirs of the Geological Society of Japan 30: 181–204.
Kawamura, Y. 1998. Immigration of mammals into the Japanese islands during the Quaternary [in Japanese, with English summary]. Quaternary Research (Japan Association for Quaternary Research) 37: 251–357. Crossref
Kawamura, Y. 2007. Last glacial and Holocene land mammals of the Japanese islands: their fauna, extinction and immigration. Quaternary Research (Japan Association for Quaternary Research) 46: 171–177. Crossref
Kessler, J. 2010. Új eredmények a Kárpát-medence neogén és negyedidőszaki madárvilágához III. Földtani Közlöny 140: 53–72.
Kigoshi, T., Kumon, F., Kawai, S., and Kanauchi, A. 2017. Quantitative reconstruction of paleoclimate in central Japan for the past 158,000 years based on a modern analogue technique of pollen composition. Quaternary International 455: 126–140. Crossref
Kitamura, A. 2008. Paleoceanographic changes of the Sea of Japan during 3.5–0.8 Ma. In: H. Okada, S.F. Mawatari, N. Suzuki, and P. Gautam (eds.), Origin and Evolution of Natural Diversity. Proceedings of the International Symposium held from 1–5 October 2007 in Sapporo, Japan, 187–194. 21st Century Center of Excellence Program for Neo-Science of Natural History, Hokkaido University, Sapporo.
Kitamura, A., Takano, O., Takata, H., and Omote, H. 2001. Late Pliocene–Early Pleistocene paleoceanographic evolution of the Sea of Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 172: 81–98. Crossref
Koike, K. and Machida, H. (eds.) 2001. Atlas of Quaternary Marine Terraces in the Japanese Islands [in Japanese]. 105 pp., 3 maps. University of Tokyo Press, Tokyo.
Konishi, S. and Yoshikawa, S. 1999. Immigration times of the two proboscidean species, Stegodon orientalis and Palaeoloxodon naumanni, into the Japanese islands and the formation of land bridge [in Japanese, with English summary]. Earth Science (Chikyu Kagaku) 53: 125–134.
Kreft, H. and Jetz, W. 2013. Comment on “An update of Wallace’s zoogeographic regions of the world”. Science 341: 343. Crossref
Kurochkin, E.N. 1985. Birds of the central Asia in Pliocene [in Russian]. Joint Soviet-Mongolian Paleontological Expedition, Transactions 26: 1–120.
Lambrecht, K. 1933. Handbuch der Palaeornithologie. 1024 pp. Gebründer Borntraeger, Berlin, Germany.
Latham, J. 1801. Supplementum Indicis ornithologici sive Systematis ornithologiae. 74 pp. Leigh Sotherby and Son, London.
Leach, W.E. 1820. Eleventh room. In: Synopsis of the Contents of the British Museum, 17th edition, 65–70. British Museum (Natural History), London.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus differentiis, synonymis, locis, Volume 1. 824 pp. Laurentii Salvii, Stockholm.
Matsuoka, A. 1987. Radiolarian age of the Shiriya Group in Aomori Prefecture, northeast Japan [in Japanese, with English summary]. Fossils (The Palaeontological Society of Japan) 42: 7–13.
Matsuoka, H. 2000. The Late Pleistocene fossil birds of the central and southern Ryukyu Islands, and their zoogeographical implications for the Recent avifauna of the archipelago. Tropics 10: 165–188. Crossref
Matsuoka, H. and Hasegawa, Y. 2018. Birds around the Minatogawa Man: the Late Pleistocene avian fossil assemblage of the Minatogawa Fissure, southern part of Okinawa Island, Central Ryukyu Islands, Japan. Bulletin of the Gunma Museum of Natural History 22: 1–21.
McKay, B.D. 2012. A new timeframe for the diversification of Japan’s mammals. Journal of Biogeography 39: 1134–1143. Crossref
Meijer, H.J.M., Tocheri, M.W., Due, R.A., Sutikna, T., Saptomo, E.W., and James, H.F. 2015. Continental-style avian extinctions on an oceanic island. Palaeogeography, Palaeoclimatology, Palaeoecology 429: 163–170. Crossref
Millener, P.R. 1999. The history of the Chatham Island’s bird fauna of the last 7000 years—a chronicle of change and extinction. In: S.L. Olson (ed.), Avian Paleontology at the Close of the 20th Century. Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution, Washington, D.C., 4–7 June 1996. Smithsonian Contributions to Paleobiology 89: 85–109.
Milne, J. 1881. Evidence of the glacial period in Japan. Transactions of the Asiatic Society of Japan 9: 53–86.
Milne-Edwards, M.A. 1867–1871. Recherches anatomiques et paléontologiques pour servir à l’histoire des oiseaux fossiles de la France, 4 volumes [2 texts + 2 plates]. 575 + 627 pp, 200 pls. Victor Masson, Paris.
Mlíkovský, J. 1989. A new swift (Aves: Apodidae) from the late Eocene of France. Annalen des Naturhistorischen Museums in Wien. Serie A: Mineralogie und Petrographie, Geologie und Palaontologie, Anthropologie und Prahistorie 90: 59–62.
Mlíkovský, J. 1997. Taxonomic identity of Haliaeetus angustipes Jánossy, 1983 (Aves: Accipitridae) from the Early Pleistocene of the Czech Republic. Buteo 9: 51–56.
Mlíkovský, J. 2002. Cenozoic Birds of the World, Part 1: Europe. 417 pp. Ninox Press, Praha.
Mourer-Chauviré, C. 1975. Les oiseaux du Pléistocène moyen et supérieur de France. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon 64: 1–624.
Murata, M. 1962. The Upper Jurassic of Cape Shiriya, Aomori Prefecture, Japan. Science Reports of the Tohoku University, 2nd Series (Geology) 5 (Special Volume): 119–126.
Nakajima, Z. 1958. On the occurrence of the Quaternary mammalian fauna from the limestone fissures near Shiriya-zaki, Shimokita Peninsula, Aomori Prefecture, Japan (No. 2) [in Japanese, with English summary]. Miscellaneous Reports of the Research Institute for Natural Resources, Tokyo 46–47: 37–39.
Nakajima, Z. and Kuwano, Y. 1957. On the occurrence of the Quaternary mammalian fauna from the limestone fissures near Shiriya-zaki, Shimokita Peninsula, Aomori Prefecture, Japan [in Japanese, with English summary]. Miscellaneous Reports of the Research Institute for Natural Resources, Tokyo 43–44: 153–159.
Nakazawa, Y. and Bae, C.J. (in press). Quaternary paleoenvironmental variation and its impact on initial human dispersals into the Japanese Archipelago. Palaeogeography, Palaeoclimatology, Palaeoecology. Crossref
Nesbitt, S.J. and Stocker, M.R. 2008. The vertebrate assemblage of the Late Triassic Canjilon Quarry (northern New Mexico, USA), and the importance of apomorphy-based assemblage comparisons. Journal of Vertebrate Paleontology 28: 1063–1072. Crossref
Nishiumi, I. and Kim, C.-H. 2015. Assessing the potential for reverse colonization among Japanese birds by mining DNA barcode data. Journal of Ornithology 156 (Supplement 1): S325–S331. Crossref
Nokariya, H. and Ono, K. 1980. Amphibian and avian remains from Taishaku-Kannondo cave site [in Japanese]. Annual Bulletin of Hiroshima University Taishaku-kyo Sites Research Centre 3: 75–84.
Northcote, E.M. 1981. Size differences between limb bones of Recent and subfossil Mute Swans Cygnus olor. Journal of Archaeological Science 8: 89–98. Crossref
Oba, T. and Irino, T. 2012. Sea level at the Last Glacial Maximum, constrained by oxygen isotopic curves of planktonic foraminifera in the Japan Sea. Journal of Quaternary Science 27: 941–947. Crossref
Olphe-Galliard, L. 1887. Contributions a la faune ornithologique de l’Europe occidentale: recueil comprenant les espèces d’oiseaux que se reproduisent dans cette région ou qui s’y montrent régulièrement de passage, augmenté de la description des principales espèces exotiques les plus voisines des indigènes ou susceptibles d’ètre confondues avec elles, ainsi que l’énumération des races domestiques, Fascicle 22. 110 pp. Veuve Cadoret, Bordeaux.
Olson, S.L. 1984. The relationships of the extinct Chatham Island eagle. Notornis 31: 273–277.
Olson, S.L. 2005. Correction of erroneous records of the cormorant from archeological sites in Alaska. Condor 107: 930–933. Crossref
Olson, S.L. 2010. Stasis and turnover in small shearwaters on Bermuda over the last 400 000 years (Aves: Procellariidae: Puffinus lherminieri group). Biological Journal of the Linnean Society 99: 699–707. Crossref
Olson, S.L. and James, H.F. 1991. Descriptions of thirty-two new species of birds from the Hawaiian Islands: Part I. Non-Passeriformes. Ornithological Monographs 45: 3–88. Crossref
Ono, K. 1980. Fossil birds from the Nojiri-ko Formation [in Japanese, with English summary]. Memoirs of the Geological Society of Japan 19: 161–166.
Ono, K. and Hasegawa, Y. 1985. Preliminary report on Late Pleistocene avifauna from Pinza-Abu Cave, Miyako Island, Okinawa, Japan [in Japanese]. In: Department of Education, Okinawa Prefectural Government (ed.), Pinaza-Abu, Reports on Excavations of the Pinza-Abu Cave, 115–137. Department of Education, Okinawa Prefectural Government, Naha.
Ota, Y. and Machida, H. 1987. Quaternary sea-level changes in Japan. In: M.J. Tooley and I. Shennan (eds.), Sea-level Changes, 182–224. Basil Blackwell, Oxford.
Ota, Y. and Yonekura, N. 1987. Coastlines [in Japanese]. In: Japan Association for Quaternary Research (ed.), Quaternary Maps of Japan, 70–72. University of Tokyo Press, Tokyo.
Otufuji, Y.-I. 1996. Large tectonic movement of the Japan Arc in late Cenozoic times inferred from paleomagnetism: review and synthesis. Island Arc 5: 229–249. Crossref
Pallas, P. 1811. Zoographia Rosso-Asiatica, sistens omnium animalium in extenso Imperio Rossico et adjacentibus maribus observatorum recesionem, domicilia, mores et descriptiones, anatomen atque icones plurimorum, Volume 1. xxii + 568 pp., 25 pls. Imperial Academy of Sciences, Petropoli.
Park, S.-C., Yoo, D.-G., Lee, C.-W., and Lee, E.-I. 2000. Last glacial sea-level changes and paleogeography of the Korea (Tsushima) Strait. Geo-Marine Letters 20: 64–71. Crossref
Peters, J.L. 1940. Check-list of Birds of the World, Volume 4. xii + 291 pp. Harvard University Press, Cambridge.
Phillips, J.L. 1988. The Upper Paleolithic of the Wadi Feiran, Southern Sinai. Paléorient 14: 183–200. Crossref
Päckert, M., Martens, J., Wink, M., Feigl, A., and Tietze, D.T. 2012. Molecular phylogeny of Old World swifts (Aves: Apodiformes, Apodidae, Apus and Tachymarptis) based on mitochondrial and nuclear markers. Molecular Phylogenetics and Evolution 63: 606–616. Crossref
Rasmussen, D.T., Olson, S.L., and Simons, E.L. 1987. Fossil birds from the Oligocene Jebel Qatrani Formation, Fayum Province, Egypt. Smithsonian Contributions to Paleobiology 62: 1–20.
Reichenbach, H.G.L. 1848 [1849]. Avium systema naturale das natürliche System der Vögel, Part 5, Die vollständigste Naturgeschichte des In- und Auslandes, Abteilung II. Vögel. Band III. Synopsis Avium. 10 pp. Expedition der vollständigsten naturgeschlichte, Dresden.
Rich, P.V., Hou, L.H., Ono, K., and Baird, R.F. 1986. A review of the fossil birds of China, Japan and Southeast Asia. Geobios 19: 755–772. Crossref
Rueda, M., Rodríguez, M.Á., and Hawkins, B.A. 2013. Identifying global zoogeographical regions: lessons from Wallace. Journal of Biogeography 40: 2212–2225. Crossref
Sano, S., Sugisawa, N., and Shimaguchi, T. 2009. Discovery of megalodontoid bivalves in the Shiriya area, northern Honshu, Northeast Japan, and its geological implications [in Japanese, with English summary]. Memoir of the Fukui Prefectural Dinosaur Museum 8: 51–57.
Savigny, J.-C. 1809. Système des oiseaux de l’Égypte et de la Syrie. In: Description de l’Égypte, ou recueil des observations et des recherches qui ont été faites en Égypte pendant l’expédition de l’armée Française, histoire naturelle, Volume 1, 63–114, 14 pls. Imprimerie impériale, Paris.
Sawaura, R., Sawada, J., Sato, T., Suzuki, T., and Sasaki, K. 2018. Late Pleistocene hares of the Japanese archipelago: paleobiogeographic implication at the Last Glacial Maximum. International Journal of Osteoarchaeology 28: 179–187. Crossref
Scopoli, G.A. 1777. Introductio ad historiam naturalem sistens genera lapidum, plantarum, et animalium: hactenus detecta, caracteribus essentialibus donata, in tribus divisa, subinde ad leges naturae. 506 pp. Wolfgangum Gerle, Prague. Crossref
Selander, R.K. 1965. Avian speciation in the Quaternary. In: H.E. Wright Jr. and D.G. Frey (eds.), The Quaternary of the United States: a Review Volume for the VII Congress of the International Association for Quaternary Research, 527–542. Princeton University Press, Princeton.
Shaw, T.-H. 1935. Preliminary observations on the fossil birds from Chou-Kou-Tien. Bulletin of the Geological Society of China 14: 77–81. Crossref
Stewart, J.R. 2002. The evidence for the timing of speciation of modern continental birds and the taxonomic ambiguity of the Quaternary fossil record. In: Z. Zhou and F. Zhang (eds.), Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing, 1–4 June 2000, 259–280. Science Press, Beijing.
Stewart, J.R. 2005. The use of modern geographical ranges in the identification of archaeological bird remains. In: G. Grupe and J. Peters (eds.), Feathers, Grit and Symbolism: Birds and Humans in the Ancient Old and New Worlds. Proceedings of the 5th Meeting of the ICAZ Bird Working Group in Munich. Documenta Archaeobiologiae 3: 43–54.
Stewart, J.R. 2007. An Evolutionary Study of Some Archaeologically Significant Avian Taxa in the Quaternary of the Western Palaearctic, BAR International Series, 1653. xx + 272 pp. Archaeopress, Oxford.
Storer, R.W. 1992. Intraspecific variation and the identification of Pliocene and Pleistocene grebes. In: K.E. Campbell, Jr. (ed.), Papers in Avian Paleontology Honoring Pierce Brodkorb. Natural History Museum of Los Angeles County, Science Series 36: 419–422.
Takai, F. 1962. Vertebrate fossils from the Tadaki Formation [in Japanese, with English summary]. Journal of the Anthropological Society of Nippon 70: 36–40.
Tarasov, P.E., Nakagawa, T., Demske, D., Österle, H., Igarashi, Y., Kitagawa, J., Mokhova, L., Bazarova, V., Okuda, M., Gotanda, K., Miyoshi, N., Fujiki, T., Takemura, K., Yonenobu, H., and Fleck, A. 2011. Progress in the reconstruction of Quaternary climate dynamics in the Northwest Pacific: a new modern analogue reference dataset and its application to the 430-kyr pollen record from Lake Biwa. Earth-Science Reviews 108: 64–79. Crossref
Temminck, C.-J. 1820. Manuel d’ornithologie, ou Tableau systématique des oiseaux qui se trouvent en Europe: précédé d’une analyse du sysème général d’ornithologie, et suivi d’une table alphabétique des espèces, 2nd Edition, Volume 1. cxv + 439 pp. H. Cousin, Paris.
Temminck, C.J. 1830. [Faison Soemmerring. Phasianus soemmerringii. Temm.]. In: C.J. Temminck and M. Laugier de Chartrouse (eds.), Nouveau recueil de planches coloriées d’oiseaux, part 82, [6–8], pls. 487–488. Levrault, Paris.
Temminck, C.-J. and Schlegel, H. 1844–1850. Aves. In: P.F. von Siebold (ed.), Fauna japonica, sive, Descriptio animalium, quae in itinere per Japoniam, jussu et auspiciis, superiorum, qui summum in India Batava Imperium tenent, suscepto, annis 1823–1830 collegit, notis observationibus et adumbrationibus illustravit, Volume 4. 141 pp, 91 pls. Lugduni Batavorum, Amsterdam.
The Ornithological Society of Japan 1974. Check-list of Japanese Birds, 5th Edition, 2 volumes + addenda. xiii + 364 pp., x + 120 pp. The Ornithological Society of Japan, Sanda.
The Ornithological Society of Japan. 2012. Check-list of Japanese Birds, 7th Edition. 438 pp. The Ornithological Society of Japan, Sanda.
Tokuda, M. 1941. Biogeography of Japan [in Japanese]. 201 pp. Kokon Shoin, Tokyo.
Tomida, S. 1978. On the Quaternary cave and fissure deposits and vertebrate fossils from Yagé Quarry, near lake Hamana, central Japan [in Japanese, with English summary]. Bulletin of the Mizunami Fossil Museum 5: 113–141.
Tsushima, K. and Takizawa, F. 1977. Geology of the Shiriyazaki District, Quadrangle Series Scale 1:50,000, Aomori (5) No. 4 [in Japanese, with English summary]. 36 pp., 3 pls., 1 map. Geological Survey of Japan, Kawasaki.
Tumarkin-Deratzian, A.R., Vann, D.R., and Dodson, P. 2006. Bone surface texture as an ontogenetic indicator in long bones of the Canada goose Branta canadensis (Anseriformes: Anatidae). Zoological Journal of the Linnean Society 148: 133–168. Crossref
Tyrberg, T. 1998. Pleistocene Birds of the Palearctic: a Catalogue. Publication of the Nuttal Ornithological Club 27. ix + 720 pp. The Nuttal Ornithological Club, Cambridge.
Tyrberg, T. 1999. Seabirds and Late Pleistocene marine environments in the Northeast Atlantic and the Mediterranean. In: S.L. Olson (ed.), Avian Paleontology at the Close of the 20th Century: Proceedings of the 4th International Meeting of the Society of Avian Paleontology and Evolution, Washington, D.C., 4–7 June 1996. Smithsonian Contributions to Paleobiology 89: 139–157.
Tyrberg, T. 2002. Avian species turnover and species longevity in the Pleistocene of the Palearctic. In: Z. Zhou and F. Zhang (eds.), Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing, 1–4 June 2000, 281–289. Science Press, Beijing.
Tyrberg, T. 2008a. Pleistocene birds of the Palearctic. Available at http://web.telia.com/~u11502098/pleistocene.pdf [accessed March 10, 2015]
Tyrberg, T. 2008b. The Late Pleistocene continental avian extinction—an evaluation of the fossil evidence. Oryctos 7: 249–269.
Val, A. 2016. New data on the avifauna from the Middle Stone Age layers of Sibudu Cave, South Africa: taphonomic and palaeoenvironmental implications. Quaternary International 421: 173–189. Crossref
Vieillot, L.P. 1816. Analyse d’une nouvelle ornithologie élémentaire. 70 pp. Deterville, Paris.
Vigors, N.A. 1824. Sketches in ornithology; or, observations on the leading affinities of some of the more extensive groups of birds. Zoological Journal 1: 308–346.
Wagler, J.G. 1832. Neue Sippen und Gattungen der Säugthiere und Vögel. Isis von Oken 1832: 1218–1235.
Wang, N., Kimball, R.T., Braun, E.L., Liang, B., and Zhang, Z. 2013. Assessing phylogenetic relationships among Galliformes: a multigene phylogeny with expanded taxon sampling in Phasianidae. PLoS ONE 8: e64312. Crossref
Watanabe, J. 2018a. Ontogeny of macroscopic morphology of limb bones in modern aquatic birds and their implications for ontogenetic ageing. In: C. Acosta Hospitaleche, F.L. Agnolin, N. Haidr, J.I. Noriega, and C.P. Tambussi (eds.), Paleontología y Evolución de las Aves, Contribuciones del MACN 7, 183–220. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires.
Watanabe, J. 2018b. Ontogeny of surface texture of limb bones in modern aquatic birds and applicability of textural ageing. Anatomical Record 301: 1026–1045. Crossref
Watanabe, J. and Matsuoka, H. 2013. Ontogenetic change of morphology and surface texture of long bones in the gray heron (Ardea cinerea, Ardeidae). In: U.B. Göhlich and A. Kroh (eds.), Paleornithological Research 2013. Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution, Vienna 2012, 279–306. Naturhistorisches Museum Wien, Vienna.
Watanabe, J. and Matsuoka, H. 2015. Flightless diving duck (Aves, Anatidae) from the Pleistocene of Shiriya, northeast Japan. Journal of Vertebrate Paleontology 35: e994745.
Watanabe, J., Matsuoka, H., and Hasegawa, Y. 2016. Two species of Uria (Aves: Alcidae) from the Pleistocene of Shiriya, northeast Japan, with description and body mass estimation of a new species. Bulletin of the Gunma Museum of Natural History 20: 59–72.
Watanabe, J., Matsuoka, H., and Hasegawa, Y. 2018. Pleistocene fossils from Japan show the recently extinct spectacled cormorant (Phalacrocorax perspicillatus) was a relict. Auk: Ornithological Advances 135: 895–907.
Yamasaki, T. 2017. Biogeographic pattern of Japanese birds: a cluster analysis of faunal similarity and a review of phylogenetic evidence. In: M. Motokawa and H. Kajihara (eds.), Species Diversity of Animals in Japan, 117–134. Springer Japan, Tokyo.
Zelenkov, N.V. 2013. New finds and revised taxa of early Pliocene birds from Western Mongolia. In: U.B. Göhlich and A. Kroh (eds.), Paleornithological Research 2013. Proceedings of the 8th International Meeting of the Society of Avian Paleontology and Evolution, Vienna 2012, 153–170. Naturhistorisches Museum Wien, Vienna.
Zelenkov, N.V. 2016. Revision of non-passeriform birds from Polgárdi (Hungary, Late Miocene): 2. Galliformes. Paleontological Journal 50: 623–634. Crossref
Zelenkov, N.V. 2017. Revision of non-passeriform birds from Polgárdi (Hungary, Upper Miocene): 3. Neoaves. Paleontological Journal 51: 203–213. Crossref
Zelenkov, N.V. and Kurochkin, E.N. 2010. Neogene phasianids (Aves: Phasianidae) of Central Asia: 3. Genera Lophogallus gen. nov. and Syrmaticus. Paleontological Journal 44: 328–336. Crossref
Zhan, X.-J. and Zhang, Z.-W. 2005. Molecular phylogeny of avian genus Syrmaticus based on the mitochondrial cytochrome b gene and control region. Zoological Science 22: 427–435. Crossref
List of comparative materials examined; specimens are listed by species/subspecies and then geographic regions. Sex composition and number of partial skeletons are given in parentheses (m, male; f, female, u, unsexed; p, partial skeleton).
Phasianidae.—Rollulus rouloul: aviary, MVZ 59088 (m). Arborophila brunneopectus: Thailand, USNM 343998 (f). Afropavo congensis: aviary, USNM 430171 (m). Pavo cristatus: aviary, MVZ 179393, USNM 289970, 321837, UWBM 116856 (3 m, 1 f). Argusianus argus: Thailand, USNM 19471 (u); aviary, UWBM 85092 (f). Polyplectron napoleonis: aviary, USNM 621671, 621673, UWBM 82965 (2 m, 1 f). Coturnix coturnix: aviary, MVZ 136488 (m). Coturnix japonica: Mongolia, UWBM 59843 (m); Korea, USNM 633328 (f); aviary, USNM 343450, 344514 (1 m, 1 f). Coturnix coromandelica: aviary, MVZ 134780 (m). Synoicus ypsilophorus: New Guinea, USNM 53359 (m). Synoicus chinensis: New Guinea, MVZ 149273 (m); Philippines, USNM 562143, 562145 (2 m). Tetraogallus himalayensis: aviary, USNM 430182, UWBM 65198 (1 m, 1 f). Alectoris chukar: Israel, USNM 605025, 605030 (2 f); aviary, MVZ 31125 (m). Ammoperdix heyi: Israel, USNM 605023 (f). Perdicula asiatica: aviary, USNM 491899 (u). Pternistis capensis: South Africa, USNM 558454, 558460 (1 m, 1 f). Pternistis afer: Zimbabwe, USNM 488838, 489335 (1 m, 1 f). Francolinus pintadeanus: Thailand, USNM 343198, 343199 (2 m). Dendroperdix sephaena: Tanzania, USNM 634713, 634721 (1 m, 1 f). Peliperdix lathami: Cameroon, USNM 291780 (m). Scleroptila levaillantii: South Africa, USNM 489466 (m). Scleroptila afra: South Africa, UWBM 53131 (m). Bambusicola thoracicus: China, USNM 318518, 318741 (1 m, 1 f); Japan (introduced), MVZ 128713 (m). Bambusicola sonorivox: Taiwan, USNM 611819 (f). Gallus gallus: Indonesia, USNM 560790 (f); aviary, MVZ 12495, 45503 (2 u). Lophophorus impejanus: aviary, MVZ 137892, USNM 631757, 638632, UWBM 84421 (2 m, 2 f). Tragopan temminckii: aviary, USNM 491867, 621027 (1 m, 1 f). Tragopan caboti: aviary, MVZ 45525, 53922 (2 f). Ithaginis cruentus: China, USNM 319182 (m); Tibet, USNM 319189 (f). Pucrasia macrolopha: aviary, UWBM 57088 (m). Syrmaticus ellioti: aviary, UWBM 33449, 82958 (2 m). Syrmaticus humiae: aviary, USNM 615083, UWBM 82957 (2 m). Syrmaticus mikado: aviary, USNM 346556, UWBM 58985 (1 m, 1 f). Syrmaticus soemmerringii: aviary, MVZ 46111, 49764, USNM 292907, 292968, 344667 (3 m, 1 f, 1 u). Syrmaticus reevesii: aviary, USNM 289572, 290302, 290303, 320867, 322767, 344402, 346350, 428249, 500369, 500710 (8 m, 3 f). Chrysolophus pictus: aviary, MVZ 138007, 136614, UWBM 119203 (3 m). Chrysolophus amherstiae: aviary, UWBM 73867 (f). Phasianus colchicus: Korea, USNM 633483, 633485 (1 m, 1 f); California (introduced), MVZ 51673, 51673A, 68327, 84651, 133528, 135071, 156120, 176024 (4 m, 3 f). Crossoptilon crossoptilon: China, USNM 319197, 319200 (1 m, 1 u); aviary, USNM 554966 (f). Crossoptilon mantchuricum: aviary, MVZ 53980, USNM 320973 (1 m, 1 f). Crossoptilon auritum: aviary, MVZ 137900 (m). Catreus wallichii: aviary, USNM 346852, 347598 (2 f). Lophura swinhoii: aviary, USNM 320294, 343428 (1 m, 1 f). Lophura erythrophthalma: aviary, MVZ 56240 (m). Perdix perdix: Canada, MVZ 140248, USNM 641454, 641462 (1 m, 2 f). Meleagris gallopavo: California, MVZ 183406, 183408, 183409 (2 m, 1 f); New York, USNM 556325 (f). Bonasa umbellus: Canada, MVZ 15546, 68068 (1 m, 1 f). Bonasa bonasia: Siberia, USNM 557496, 557499 (1 m, 1 f). Lagopus muta: Sweden, USNM 620108 (m); Alaska, MVZ 137293 (f). Dendragapus obscurus: Nevada, MVZ 151729, 151733 (2 f); California, USNM 630803 (f). Falcipennis falcipennis: Primorye, USNM 622432 (f). Falcipennis canadensis: Canada, MVZ 63067 (f); Idaho, MVZ 61793 (m). Lyrurus tetrix: Kalininskaya, USNM 500266 (m). Centrocercus urophasianus: Montana, USNM 17707 (f); Nevada, MVZ 151737, 151739 (1 m, 1 f). Tympanuchus phasianellus: South Dakota, USNM 559925 (m). Tympanuchus pallidicinctus: Kansas, MVZ 98474, 13175 (1 m, 1 f).
Columbidae.—Columba livia: California (introduced), MVZ 129295, 129298 (1 m, 1 f). Columba rupestris: Korea, MVZ 135053 (m). Columba leuconota: aviary, MVZ 30684 (f). Columba guinea: aviary, MVZ 29113 (m). Columba palumbus: France, MVZ 141896 (m). Columba arquatrix: South Africa, MVZ 158703 (m). Streptopelia orientalis: Japan, MVZ 123361, 128741 (1 m, 1 f). Streptopelia decaocto: Korea, MVZ 142609, 142610 (1 m, 1 u). Streptopelia semitorquata: South Africa, MVZ 158704 (m). Streptopelia capicola: South Africa, MVZ 158705 (f). Spilopelia chinensis: Australia, MVZ 143413 (m); aviary, MVZ 41184. Spilopelia senegalensis: Botswana, MVZ 158706 (f); South Africa, MVZ 158707 (m). Macropygia unchall: locality unknown, MVZ 43622, 46152 (2 f). Macropygia phasianella: Australia, MVZ 142187 (m); aviary, 54636 (m). Ectopistes migratorius: locality unknown, MVZ 84315 (u). Patagioenas leucocephala: aviary, MVZ 138015 (f). Patagioenas plumbea: Peru, MVZ 165113 (u). Patagioenas fasciata: California, MVZ 54522, 65175 (1 m, 1 f). Patagioenas cayennensis: Panama, MVZ 154938 (f). Patagioenas flavirostris: Mexico, MVZ 74760, 78703 (1 m, 1 f). Starnoenas cyanocephala: aviary, MVZ 41332 (f). Geotrygon chrysia: aviary, MVZ 45593 (f). Leptotila verreauxi: Mexico, MVZ 74761 (m); El Salvador, MVZ 85621 (f). Zentrygon frenata: Colombia, MVZ 141733 (m). Zenaida asiatica: Arizona, MVZ 68837 (f); Mexico, MVZ 51073 (m). Zenaida galapagoensis: Galapagos, MVZ 140769, 140958 (1 m, 1 f). Zenaida auriculata: Colombia, MVZ 94080, 94081 (2 m). Zenaida macroura: Arizona, MVZ 151848 (f); Kansas, MVZ 65118 (m). Zenaida graysoni: aviary, MVZ 46606 (f). Columbina inca: Mexico, MVZ 74762, 153894 (1 m, 1 f). Columbina picui: Paraguay, MVZ 168806 (f); Bolivia, MVZ 141731 (f). Columbina cruziana: aviary, MVZ 74908 (f). Claravis pretiosa: El Salvador, MVZ 85615, 85616 (1 m, 1 f). Henicophaps albifrons: aviary, MVZ 44278, 46012 (1 f, 1 u). Gallicolumba luzonica: aviary, MVZ 47109, 53804 (1 m, 1 f). Gallicolumba rufigula: aviary, MVZ 49759 (m). Alopecoenas.jobiensis: aviary, MVZ 47105, 51344 (1 m, 1 u). Alopecoenas xanthonurus: Mariana Islands, MVZ 115697 (m). Alopecoenas rubescens: aviary, MVZ 44500, 46017 (2 f). Alopecoenas canifrons: Palau Islands, MVZ 95122 (m). Alopecoenas stairi: aviary, MVZ 51708 (m). Leucosarcia melanoleuca: Australia, UWBM 62821, 62938 (2 f). Geophaps plumifera: aviary, MVZ 136616, 136617 (1 m, 1 f). Phaps chalcoptera: Australia, MVZ 154306 (m); aviary, MVZ 53809 (f). Phaps elegans: aviary, MVZ 30693, 133589 (1 f, u). Phaps histrionica: Australia, MVZ 141935 (m). Ocyphaps lophotes: Australia, MVZ 143420 (m); California, MVZ 88909 (f). Geopelia striata: Australia, MVZ 142189, 143414 (1 m, 1 f). Otidiphaps nobilis: aviary, MVZ 46124 (u). Goura cristata: aviary, MVZ 42673, 133590 (1 m, 1 f). Didunculus strigirostris: aviary, MVZ 27595 (f). Turtur afer: aviary, MVZ 40448 (f). Turtur brehmeri: Ghana, MVZ 164175 (f). Oena capensis: South Africa, MVZ 158709 (m). Treron axillaris: aviary, MVZ 27141 (m). Ducula aenea: aviary, MVZ 51705 (u). Ducula pacifica: Samoa, MVZ 65412, 65409 (u). Ducula latrans: Fiji, MVZ 51372, 51373 (1 m, 1 f). Ducula bicolor: aviary, MVZ 50566, 50570 (1 m, 1 f). Megaloprepia magnifica: New Guinea, MVZ 149287, 149288 (1 m, 1 f). Chrysoena viridis: Solomon Islands, MVZ 93619 (m). Ptilinopus solomonensis: Bismark Archipelago, MVZ 93389 (m). Ptilinopus ornatus: New Guinea, MVZ 149284 (m). Ptilinopus perlatus: New Guinea, MVZ 149285, 149286 (2 m). Ptilinopus insolitus: Bismark Archipelago, MVZ 93390 (f). Ptilinopus roseicapilla: Marianas Islands, MVZ 115695 (f). Ptilinopus porphyraceus: Samoa, MVZ 65418 (m). Hemiphaga novaeseelandiae: New Zealand, MVZ 80402 (f). Lopholaimus antarcticus: Australia, MVZ 154297 (f).
Apodidae.—Cypseloides niger: Alaska, MVZ 39795 (m); Washington, UWBM 19444, 21286, 33110, 91683 (3 m); Oregon, UWBM 59363 (f); Dominican Republic, USNM 290999 (m); locality unknown, UWBM 19444 (u). Streptoprocne rutila: Venezuela, USNM 614122 (m). Streptoprocne phelpsi: Guyana, USNM 622775 (m). Streptoprocne zonaris: El Salvador, MVZ 85724 (m); Colombia, MVZ 120732 (f). Streptoprocne semicollaris: Mexico, MVZ 152986, USNM 489351 (1 m, 1 f). Chaetura spinicaudus: Guyana, USNM 637197 (f). Chaetura cinereiventris: Costa Rica, MVZ 155742, 155743 (2 f). Chaetura vauxi: Washington, UWBM 48231, 48232, 48237 (1 m, 2 f); Oregon, UWBM 42223 (m); California, MVZ 41027 (f); El Salvador, MVZ 85721 (m). Chaetura pelagica: Florida, MVZ 53252–53256, 66685 (1 m, 5 f); Honduras, UWBM 32456 (u). Hirundapus caudacutus: Russia, UWBM 46916, 47128, 47230, 47231, 47345, 57445 (3 m, 3 f); Japan, USNM 500799, YIO 65584, 65584 (2 m, 1 f). Hirundapus cochinchinensis: Thailand, MVZ 156689 (u). Hirundapus giganteus: Thailand, MVZ 156688 (u). Collocalia troglodytes, Philippines, USNM 430517 (f). Collocalia esculenta, Philippines, USNM 635376 (f); Solomon Islands, UWBM 63237, 63239 (2 f). Aerodramus hirundinaceus: Papua New Guinea, MVZ 149295 (f). Aerodramus sawtelli: Cook Islands, UWBM 42487, 42488, 42541–42543, 42565–42567 (2 m, 5 f, 1 u). Aerodramus inquietus: Pohnpei Island (Caroline Islands), USNM 431409 (m). Aerodramus bartschi: Mariana Islands, MVZ 95143 (f); Guam, MVZ 115703, 115704, 115713–115717 (5 m, 2 f). Aerodramus spodiopygius: Fiji, MVZ 51721–51723 (1 m, 1 f, 1 u); New Caledonia, USNM 561598 (m). Aeronautes saxatalis saxatalis: Washington, UWBM 40650, 42303, 54112, 54113, 55981, 55982 (4 m, 2 f); California, MVZ 41907, 77721–77723 (3 m, 1 f); Montana, MVZ 28986 (m); Arizona, UWBM 33227, 34155, 34156 (1 m, 1 f, 1 u). Tachornis phoenicobia: Dominican Republic, USNM 555775 (m). Tachornis squamata: Guyana, USNM 621960 (m). Panyptila cayennensis: Panama, USNM 431346 (u); Brazil, USNM 321670 (u). Cypsiurus parvus: Alaska, MVZ 67569 (f); Gabon, USNM 64851 (f). Tachymarptis melba: Crete, USNM 559068 (u). Apus pacificus: Russia (ssp. pacificus), USNM 500258, UWBM 43827–43830, 44398, 44399, 46954–46956, 46958, 46959 (6 m, 6 f); Korea (ssp. pacificus), MVZ 148837 (m); Japan (ssp. pacificus?), YIO63553, 63946 (1 m, 1 f); Taiwan (ssp. kanoi or kurodae?), YIO 48 (f). Apus caffer: South Africa, UWBM 53219 (m). Apus nipalensis: Japan, YIO 63093 (u); Taiwan, USNM 611837 (m); Vietnam, USNM 431471, 431472 (2 m). Apus affinis: Cameroon, USNM 292415, 292416 (1 m, 1 f); São Tomé, USNM 614156, 614157 (2 u); Gabon, USNM 642847, 642848 (2 m). Apus barbatus: South Africa, UWBM 52770 (m). Apus apus, Spain, UWBM 1962 (u); France, UWBM 32988 (u); Russia, UWBM 56587, 56589 (1 m, 1 f); China, NSMT AS Morioka Collection unnumbered (u).
Accipitridae.—Pandion haliaetus (Pandionidae): Indonesia, USNM 560783 (f). Elanus caeruleus: South Africa, USNM 558445, 558447 (2 f). Elanus leucurus: California, USNM 19603 (u). Gampsonyx swainsonii: Guyana, USNM 623084, 623110 (1 m, 1 f). Leptodon cayanensis: Panama, USNM 613953 (m). Pernis ptilorhynchus: Nepal, USNM 620644 (u); Thailand, USNM 343983 (m). Aviceda subcristata: Indonesia, USNM 558306 (f); Australia, USNM 620187 (f). Polyboroides typus: Zimbabwe, USNM 430434, 430435 (1 m, 1 f). Gypohierax angolensis: Angola, USNM 18892 (u); French Congo, USNM 226143 (u). Gypaetus barbatus: Algeria, USNM 17834 (u); aviary, USNM 345684 (f). Neophron percnopterus: North Africa, USNM 17835 (u). Spilornis cheela: Thailand, USNM 19474, 343985 (1 m, 1 u). Terathopius ecaudatus: Tanzania, USNM 634509 (m). Pithecophaga jefferyi: Philippines, USNM 226900 (u, composite); aviary, USNM 499879 (m). Circaetus cinereus: Zimbabwe, USNM 430776 (m). Trigonoceps occipitalis: aviary, USNM 320859, 347358 (1 f, 1 u). Necrosyrtes monachus: Sudan, USNM 291440, 291443 (1 m, 1 f). Gyps himalayensis: Kasmir, USNM 19534 (m). Gyps africanus: Zimbabwe, USNM 431591 (f). Aegypius monachus: Spain, USNM 614152 (u); China, USNM 289569 (u). Macheiramphus alcinus: Malaysia, USNM 559816 (u). Harpia harpyja: Aviary, USNM 429223, 429721 (2 f). Stephanoaetus coronatus: aviary, USNM 345669, 346654 (1 m, 1 f). Nisaetus cirrhatus: aviary, USNM 344616 (m). Aquila gurneyi: Indonesia, USNM 554908 (u). Aquila chrysaetos: Tennessee, USNM 19394 (u). Aquila audax: Australia, USNM 620191, 620192 (1 m, 1 u). Aquila verreauxii: South Africa, USNM 612539 (u). Aquila spilogaster: Zimbabwe, USNM 430796, 490548 (1 m, 1 f). Kaupifalco monogrammicus: Liberia, USNM 347392, 347393 (1 m, 1 f). Melierax metabates: Zimbabwe, USNM 430326, 430381 (2 m). Circus aeruginosus: aviary, USNM 344419 (m). Circus cyaneus: California, USNM 610734 (f); Maryland, USNM 291684 (m). Circus melanoleucos: Russia, USNM 500257 (m). Accipiter soloensis: Indonesia, USNM 560782 (f). Accipiter nisus: Sweden, USNM 610363, 610366 (1 m, 1 f); China, USNM 290308 (f). Accipiter gentilis: Sweden, USNM 610353, 610358 (1 m, 1 f); Japan, USNM 499854 (u; p). Haliaeetus leucogaster: Indonesia, USNM 556992, 556993, 560784, 560785 (2 m, 2 f); aviary, YIO 60790 (m; p). Haliaeetus vocifer: Tanzania, USNM 488146 (m); Sudan, USNM 291449, 291450 (2 f). Haliaeetus leucoryphus: locality unknown, USNM 612540 (u). Haliaeetus albicilla: China, USNM 292774 (m); Korea, USNM 431363 (m); Japan, YIO 8974 (u; p); locality unknown, NSMT AS 578, 821, 874, 1197, NSMT PV 532, YIO 70778 (2 m, 3 u; 3 p). Haliaeetus leucocephalus: Alaska, USNM 489276, 615007, 615009 (2 m, 1 f). Haliaeetus pelagicus: Bering Island, USNM 17044 (u; p); Japan, YIO 8998, 8999 (1 m, 1 u; 2 p); aviary, YIO 9000, 15137 (1 m, 1 f; 2 p); locality unknown, NSMT AS 601, 620, 816, 1344, USNM 16959, YIO 70530 (6 u; 3 p). Icthyophaga ichthyaetus: Sulawesi, USNM 224807 (m); aviary, USNM 468555 (u). Haliastur sphenurus: Australia USNM 610563 (m). Haliastur indus: Borneo, USNM 18759 (u); Indonesia, USNM 558303, 558304 (1 m, 1 f). Milvus migrans: Pakistan, USNM 557810 (f); Cameroon, USNM 291317 (m); China, USNM 292039, 319228, 319448 (1 m, 3 f). Busarellus nigricollis: Brazil, USNM 345772, 345773 (1 m, 1 f); aviary, USNM 431130 (f). Geranospiza caerulescens: Mexico, USNM 18450 (u); Brazil, USNM 345774 (m). Butastur indicus: Korea, USNM 18577 (u); Philippines, 611775 (f). Ictinia plumbea: Panama, USNM 613355, 613356 (2 m). Buteo lagopus: Sweden, USNM 610370 (f). Buteo buteo: China, USNM 292701, 318559–318561, 318739 (1 m, 2 f, 2 u).
Acta Palaeontol. Pol. 63 (3): 469–491, 2018
https://doi.org/10.4202/app.00509.2018