

Feeding habits and habitat of herbivorous mammals from the Early–Late Hemphillian (Miocene) of Costa Rica
VÍCTOR ADRIÁN PÉREZ-CRESPO, CÉSAR A. LAURITO, JOAQUÍN ARROYO-CABRALES, ANA L. VALERIO, PEDRO MORALES-PUENTE, EDITH CIENFUEGOS-ALVARADO, and FRANCISCO J. OTERO
Pérez-Crespo, V.A., Laurito, C.A., Arroyo-Cabrales, J., Valerio, A.L. Morales-Puente, P., Cienfuegos-Alvarado, E., and Otero, F.J. 2018. Feeding habits and habitat of herbivorous mammals from the Early–Late Hemphillian (Miocene) of Costa Rica. Acta Palaeontologica Polonica 63 (4): 645–652.
Carbon and oxygen stable isotope values in the dental enamel of fossils were used to infer the diet and habitat of the extinct equids Calippus hondurensis, Dinohippus mexicanus, and Protohippus gidleyi, the gomphothere Gomphotherium hondurensis, and the llama Hemiauchenia vera of the Early–Late Hemphillian (Hh2) from San Gerardo de Limoncito, Puntarenas province, Costa Rica. The results suggest that these mammals fed mainly on C3 plants and lived in clearings of rainforests. This contrasts with previous studies from North America that indicated that the same species lived in forest savannas and fed mainly on C4 plants, but it is similar to the results obtained from the palynological record of the area, as well as with several vegetation models suggesting the presence of humid tropical forest during the Miocene in Central America.
Key words: Mammalia, carbon and oxygen stable isotopes, Neogene, Hemphillian, Costa Rica.
Víctor Adrián Pérez-Crespo [vapc79@gmail.com], Instituto de Geología, Universidad Nacional Autónoma de México, Circuito de la Investigación Científica S/N, Ciudad Universitaria, Del. Coyoacán, 04150, México.
César A. Laurito [clauritomora@ina.ac.cr], Instituto Nacional de Aprendizaje, La Uruca, San José, Costa Rica; Departamento de Historia Natural, Museo Nacional de Costa Rica, 749-1000, San José, Costa Rica.
Joaquín Arroyo-Cabrales [arromatu@hotmail.com], Laboratorio de Arqueozoología “M. en C. Ticul Álvarez Solórzano”, Subdirección de Laboratorios y Apoyo Académico, INAH, Moneda 16 Col. Centro, 06060, México.
Ana L. Valerio [avalerio@museocostarica.go.cr], Departamento de Historia Natural, Museo Nacional de Costa Rica. 749-1000, San José, Costa Rica.
Pedro Morales-Puente [mopuente@unam.mx], Edith Cienfuegos-Alvarado [edithca@unam.mx], and Francisco J. Otero [fotero@geologia.unam.mx], Instituto de Geología, Universidad Nacional Autónoma de México, Circuito de la Investigación Científica S/N, Ciudad Universitaria, Del. Coyoacán, 04150, México; Laboratorio de Isótopos Estables, Laboratorio Nacional de Geoquímica y Mineralogía, LANGEM-UNAM, Ciudad Universitaria, Coyoacán, 04150, México.
Received 22 June 2018, accepted 5 September 2018, available online 25 September 2018.
Copyright © 2018 V.A. Pérez-Crespo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The vertebrate fossil record of Central America is mainly confined to Cenozoic outcrops and particularly Miocene and Pleistocene ones (Lucas 2014). Studies in the region have focused on taxonomy (Cisneros 2005; Mead et al. 2012; MacFadden et al. 2015), paleobiogeography (MacFadden 2006; Kirby et al. 2008; Lucas and Alvarado 2010; Laurito and Valerio 2012), and biostratigraphy (Lucas 2014). The few studies with a paleoecological perspective have included analyses of carbon and oxygen stable isotope values in Pleistocene horses of several localities (MacFadden et al. 1999), in mammals from the Bartsovian (early Miocene) of Panama (MacFadden and Higgins 2004), and in some toxodonts from the Pleistocene of Honduras and Panama (MacFadden 2005).
This contrasts with North and South America, where a wide range of paleoecological studies using biogeochemical markers and morphofunctional methods have yielded far more information about Cenozoic mammals (MacFadden and Higgins 2004).
In the case of Costa Rica, several localities contain fossils from the Pleistocene (Lucas et al. 1997) and Miocene Epochs. Remains of fish, reptiles, birds, and terrestrial and marine mammals have been found in the Late Hemphillian North American Land Mammal Age (NALMA) outcrop of San Gerardo de Limoncito (Laurito and Valerio 2008, 2016; Laurito et al. 2005), and this has motivated taxonomic, biostratigraphical, and paleoecological studies (Laurito and Valerio 2010; Valerio 2010).
Morphofunctional aspects of three equid species found at San Gerardo, Calippus hondurensis, Dinohippus mexicanus, and Protohippus gidleyi, have been analyzed by extrapolation of information derived from specimens found in the USA (Laurito and Valerio 2010). However, such an approach is not ideal, since populations of a same species that lived in different geographic zones would have experienced different environmental conditions and would therefore have modified their feeding habits and the habitat itself (MacFadden 2005, 2008).
Laurito and Valerio (2010) concluded that the fossil mammal assemblage found in San Gerardo de Limoncito indicated that a wooded savanna existed there during the Miocene and that the equids and llamas fed mainly on grasses, whereas gomphotheres fed on leaves of trees and shrubs. The present study tests this view with carbon and oxygen stable isotope analyses of dental enamel for the same equid species, the gomphothere Gomphotherium hondurensis, and the llama Hemiauchenia vera, all from San Gerardo de Limoncito.
Other abbreviations.—CAM, Crassulacean Acid Metabolism; DF, degree of freedom; F, Fisher statistics; H, H statistic; NALMA, North American Land Mammal Age; p, probability level.
Carbon and oxygen stable isotopes
Carbon is incorporated into plants through photosynthesis in the three pathways C3, C4, and Crassulacean Acid Metabolism (CAM) (O’Leary 1988). The C3 photosynthetic pathway is present in trees and shrubs, and some temperate grasses, with δ13C values ranging between -34‰ and -22‰ (van der Merwe and Medina 1989; Cerling et al. 1997; Koch 1998; Drucker and Bocherens 2009). The C4 pathway is usually found in grasses as well as trees and shrubs from warm regions and has δ13C values between -14‰ and -10‰ (Smith and Epstein 1971; Koch 1998; Cerling 1999; Medrano and Flexas 2000). Several factors may affect the abundance of C3 and C4 plants in the ecosystem. At localities with temperatures lower than 25ºC, C3 plants increase in numbers while C4 plants decrease (Medrano and Flexas 2000). Also, C4 plants are able to cope with lower atmospheric CO2 and humidity levels than C3 plants (McInerney et al. 2011). In temperate areas, the two kinds of plants co-exist throughout the year, but at locations with different microhabitat conditions for temperature and humidity. Furthermore, the carbon isotope composition of C3 plants can be influenced by factors such as saline soils, low light intensity, water availability, efficient use of water and lack of nutrients, and the particular conditions in the habitat or microhabitat (Ehleringer et al. 1987; Mooney et al. 1989; Bocherens 2003; Codron et al. 2005).
The third photosynthetic pathway, CAM, is found in succulent plants such as cacti, bromeliads, and agaves, with δ13C values between -35‰ and -12‰. However, due to the carbon isotope values range, it is difficult to separate these plants from C3 and C4 plants based on δ13C values alone (Decker and De Wit 2005; Andrade et al. 2007).
When herbivores feed on plants, the carbon from those plants is incorporated into their tissues and structures such as dental enamel. Hence, the isotope values are correlated with those of the plants; nevertheless, there is a 14.1‰ increment (Cerling and Harris 1999). Animals that feed on C3 plants have carbon isotope values from -19‰ to -9‰, whereas those that have consumed C4 plants have values from -2‰ to +2‰. The C3/C4 mixed feeders show values from -9‰ to -2‰ (MacFadden and Cerling 1996).
In contrast to carbon, oxygen enters the body of animals during inhalation, in water derived from food, and in drinking water. It is in balance with oxygen that is lost through exhalation, feces, urine, and sweat (Koch et al. 1994; Koch 2007). The main source is drinking water, often derived from rain water that is affected by altitude, latitude, amount of precipitation and environmental temperature (Dansgaard 1964). The equilibrium between ingested and exhaled water could also be modified by the animals’ physiology and habitat (Sánchez 2005). However, herbivores inhabiting humid and closed (forest) zones generally show lower δ18O values than those living in arid and open (grassland, savannas, or prairies) zones (Ambrose and DeNiro 1986; Feranec and MacFadden 2006). Because of that situation, δ18O dental enamel can be used to infer past climatic conditions at a location and certain ecological characteristics of the analyzed species (Ayliffe et al. 1992; Sánchez-Chillón et al. 1994; Bryant and Froelich 1996; Kohn et al. 1996; Sponheirmer and Lee-Throp 1999; Schoeninger et al. 2000; Harris and Cerling 2002; Levin et al. 2006).
Material and methods
Location.—San Gerardo de Limoncito is in the Coto Brus Canton, the 4th district of Limoncito, Puntarenas province, Costa Rica, between 8º51’19.6” N and 83º 04’51.9” W and at 760 m above sea level (Fig. 1). Remains of fossil sharks, stingrays, turtles, crocodiles, birds, dolphins, whales, equids, gomphotheres, llamas, pampatheres, sloths, and peccaries have been recovered there. The sedimentological context of the fossil material considered in the present study encompasses littoral and fluvial environments, associated with a fan delta sequence, and characterized by reworked sedimentary intraclasts deposited as conglomerates from which most of the vertebrate remains have been obtained (Valerio 2010; Fig. 2). The vertebrate fauna, as well as scarce plant remains and sedimentological data, suggest a tropical estuary associated with ancient ecosystems of wooded savannas, with a predominance of grasslands in lowlands near the coast (Laurito and Valerio 2010).
The locality is part of the Curré Formation, from the middle–upper Miocene. Given the presence of Calippus hondurensis, Dinohippus mexicanus, and Protohippus gidleyi, Laurito and Valerio (2010) and Valerio and Laurito (2012) deduced that the site is from the Early–Late Hemphillian NALMA (6.57 Ma), corresponding to Stage Hh2 according to the chronology proposed by Tedford et al. (2004).
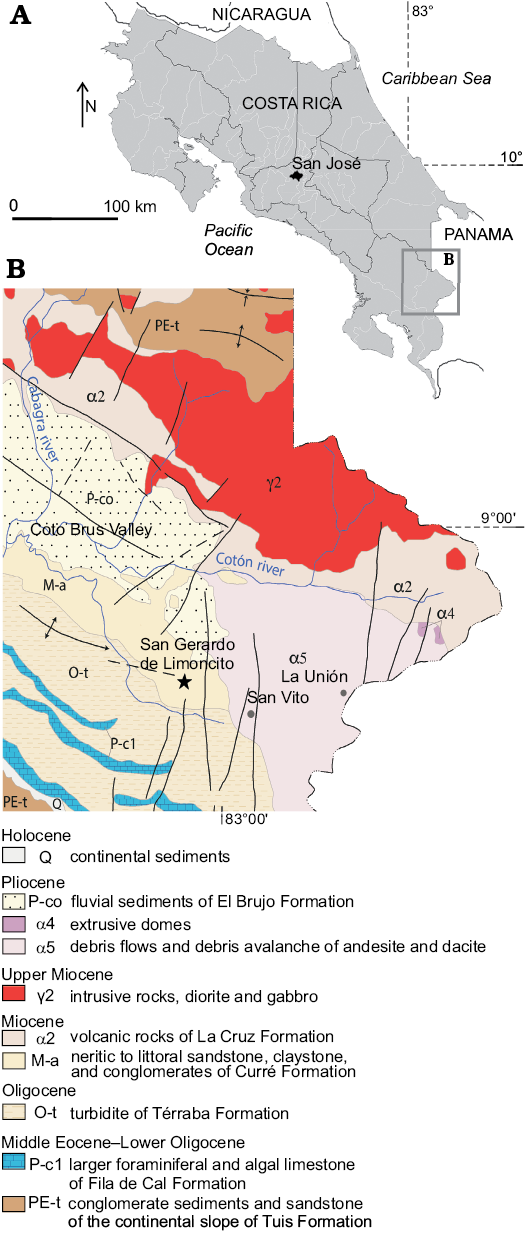
Fig. 1. A. Geographic location of studied area. B. Geological map of the San Gerardo de Limoncito area, modified from Denyer and Alvarado (2007).
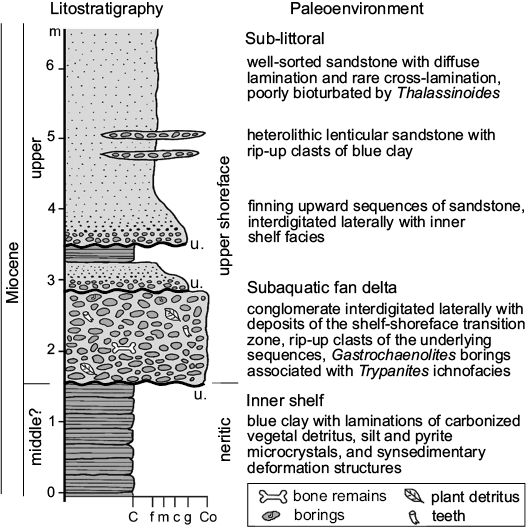
Fig. 2. Stratigraphic column of the fossil-bearing locality, San Gerardo de Limoncito, modified from Laurito and Valerio (2010). Abbreviations: C, clay; c, coarse sandstone; Co, conglomerate; f, fine; g, gravel; m, middle; u., unconformity.
Extraction and preparation of samples.—Samples were processed in the Stable Isotope Laboratory at the Instituto de Geología, Universidad Nacional Autónoma de México, México, using the method proposed by Koch et al. (1997). First, 20 mg of diagenetically unaltered tooth enamel was ground and sieved (125 μm mesh) to obtain a fine and uniform powder. Then 0.5 ml of hydrogen peroxide at 30% was added to eliminate the organic matter. After two hours, the samples were centrifuged and the hydrogen peroxide was decanted and washed again three times with type I water (grade HPLC 18.2 MΩ). Once the washing was finished, 5 ml of a buffer solution, Ca(CH3CO2)2 – CH3COOH 1.0 M, pH 4.75, was added and allowed to rest for nine hours. The buffer solution was decanted and the samples were washed again three times with type I water. Finally, to eliminate any remaining water, ethanol was added, and the solution was left for 20 hours in an oven at 90°C. Isotope ratios were determined with a Finnigan MAT 253 mass spectrometer with a dual inlet system and auxiliary Gas Bench equipment with a GC Pal auto-sampler with a temperature-controlled aluminum plate adjoined to the mass spectrometer (Révész and Landwehr 2002). Results were reported as δ18OVPDB and δ13CVPDB, and they were normalized using NBS-19, NBS-18, and LSVEC to the Vienna Pee Dee Belemnite (VPDB) scale in accordance with the corrections described by Coplen (1988), Werner and Brand (2001), and Coplen et al. (2006). For this technique, the standard deviation was 0.2‰ for oxygen and carbon.
Data analyses.—Average values were obtained for carbon and oxygen isotopes. For the δ13C values, reference was made to MacFadden and Cerling (1996) in order to infer the diet of the animal. The relationship between the δ13C and δ18O values was assessed by Analysis of Variance (ANOVA), a Kruskal-Wallis test, and a Tukey-Kramer test. Significance was set at p <0.05 and the utilized software was NCCS and PASS (Hintze 2004). The δ18O values were converted to Vienna Standard Mean Ocean Water (V-SMOW) using the Faure (1977) formula: δ18OV-SMOW: 01.030901 * δ18OV-PDB + 30.91, and transformed into δ18O of water through the Iacumin et al. (1996) formula: δ18Owater = δ18OV-SMOW – 33.63/0.998, to be compared with the rainwater δ18O values calculated for this locality (Bowen and Wilkinson 2002; Bowen and Revenaugh 2003; Bowen et al. 2005; Bowen 2008).
Results
Table 1 shows carbon and oxygen isotope values for the five species analyzed. There were no differences observed among δ13C values for the whole set of species (DF 17, F 0.94, p <0.471092; H 3.140302, p <0.534627) nor among δ18O values (DF 17, F 2.74, p <0.074511; H 7.57045, p <0.108643). The relationship between δ13C and δ18O values showed three groups: (i) most of the gomphotheres, Dinohippus mexicanus, and some Protohippus gidleyi specimens; (ii) Calippus hondurensis, one gomphothere and most of the Protohippus gidleyi specimens; and (ii) the llamas (Fig. 3).
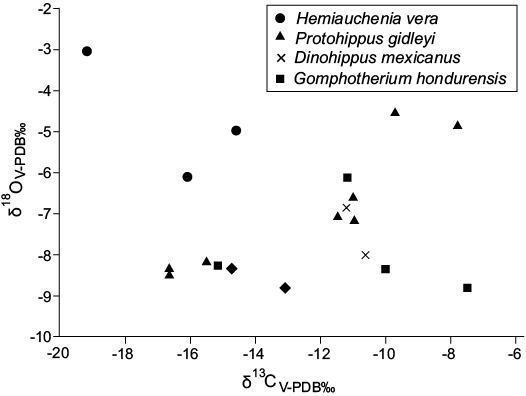
Fig. 3. Comparative analysis of carbon and oxygen stable isotope values in dental enamel from fossil herbivorous mammals of the San Gerardo de Limoncito locality.
The most negative δ13C value was in a llama that also had the most positive δ18O value; the highest positive δ13C value was from a gomphothere; and the lowest δ18O value was from a Protohippus gidleyi specimen (Table 1).
Table 1. Carbon and oxygen isotope values (‰) from dental enamel of fossil herbivorous mammals from the Early–Late Hemphillian of San Gerardo de Limoncito, Costa Rica (after Laurito and Valerio 2016).
|
Species |
δ13CV-PDB |
δ18OV-PDB |
δ18OV-SMOW Water |
|
Calippus hondurensis |
-14.7 |
-8.4 |
-11.4 |
|
Calippus hondurensis |
-13.1 |
-8.8 |
-11.8 |
|
Dinohippus mexicanus |
-11.0 |
-5.4 |
-8.3 |
|
Dinohippus mexicanus |
-11.6 |
-5.1 |
-8.0 |
|
Gomphoterium hondurensis |
-11.2 |
-6.6 |
-9.6 |
|
Gomphoterium hondurensis |
-15.2 |
-8.0 |
-11.0 |
|
Gomphoterium hondurensis |
-10.0 |
-6.9 |
-11.4 |
|
Gomphoterium hondurensis |
-7.4 |
-8.3 |
-11.8 |
|
Hemiauchenia vera |
-14.6 |
-5.0 |
-7.9 |
|
Hemiauchenia vera |
-16.1 |
-6.1 |
-9.0 |
|
Protohippus gidleyi |
-7.8 |
-4.9 |
-7.8 |
|
Protohippus gidleyi |
-16.7 |
-8.3 |
-11.3 |
|
Protohippus gidleyi |
-11.5 |
-7.1 |
-10.0 |
|
Protohippus gidleyi |
-9.7 |
-4.6 |
-7.4 |
|
Protohippus gidleyi |
-11.0 |
-7.2 |
-10.1 |
|
Protohippus gidleyi |
-11.1 |
-6.1 |
-9.1 |
|
Protohippus gidleyi |
-16.6 |
-8.5 |
-11.5 |
|
Protohippus gidleyi |
-15.5 |
-8.2 |
-11.2 |
Discussion
Diet.—The δ13C values from the equid Calippus hondurensis indicate that those animals fed on C3 plants. Because the wear on molars was only moderate, Laurito and Valerio (2010) concluded that this equid species was a browser; this is supported by the present data, since C3 plants are mainly trees and shrubs (Koch 1998).
In the case of the other equid Dinohippus mexicanus, data from studied individuals also suggest a diet based on C3 plants, while Laurito and Valerio (2010) indicated that D. mexicanus was more generalist in its diet, citing mesowear studies (Barrón-Ortíz and Guzmán-Gutiérrez 2008) that indicated that D. mexicanus at Rancho El Ocote, Mexico, were grazers, whereas individuals from Tecolotlán, Mexico, were mixed feeders or grazer-browsers. Isotope analyses of D. mexicanus populations from Rancho El Ocote and Yepomera (also in Mexico), and from Texas and Florida (USA), indicated that specimens from Mexican and Texan localities mainly fed on C4 plants, whereas those in Florida mainly fed on C3 plants (MacFadden 2008). Specimens from Tecolotlán had a C3/C4 mixed diet but with higher proportion of C3 plants (Pérez-Crespo et al. 2017). Overall it seems that populations of Dinohippus mexicanus varied widely in their feeding habits, depending upon the region where they lived.
The δ13C values of the three individuals of Gomphotherium hondurensis indicate that two of them fed only on C3 plants, whereas the third had a mixed diet of C3/C4 plants. Because gomphotheres had brachiodont molars, they have been thought to have eaten only leaves of trees and shrubs, i.e., mainly C3 plants; however, the Pleistocene gomphotheres Cuvieronius and Stegomastodon in North and South America (Sánchez et al. 2004; Pérez-Crespo et al. 2016) and Rhynchotherium in Florida and Mexico (MacFadden and Cerling 1996; Pérez-Crespo et al. 2015) had not specialized exclusively on either C3 or C4 plants; they mainly had a mixed C3/C4 diet, such as the one for G. hondurensis from San Gerardo de Limoncito.
The llama Hemiauchenia vera analyzed here specialized on C3 plants, whereas Laurito and Valerio (2016) indicated that this species from San Gerardo de Limoncito had a mixed C3/C4 diet. Analyses of several populations of camelids from the Eocene to Pleistocene of North America (Feranec 2003; Semprebon and Rivals 2010) indicated that Hemphillian llamas fed on C3 plants or were browsers, and that this habit was maintained in some specimens from two of the three most recent chronostratigraphic units NALMAs: Blancan and Rancholabrean. Among the latter, some llamas with mixed diets coexisted with others that specialized on C4 plants or were grazers, so that these animals can be considered more generalist than specialist in their diet.
In contrast, one of the eight Protohippus gidleyi specimens analyzed in the present study had a mixed diet of C3/C4 plants, with a high proportion of C3 plants, whereas most equids only fed on C3 plants. This contrasts with the conclusion based on cuspid morphology of molars (Laurito and Valerio 2010) that P. gidleyi was a browser-grazer with a mixed diet. This apparent difference can be explained by the following: (i) isotope analysis reveals the diet of an individual at the time when the molars were formed (Koch 2007); (ii) although most of the C3 plants were trees and shrubs, some were grasses (Koch 1998); and (iii) the wear at the tops of molars may be caused not only by food, but also by other abrasive material, such as sand particles (Sanson et al. 2007). Hence, neither the molar morphology (Laurito and Valerio 2010) nor the present isotope analyses eliminate the possibility that P. gidleyi fed on trees, shrubs, and also C3 grasses.
Habitat.—These herbivorous mammals lived in a closed zone (Fig. 3). Closed habitats include jungles or forests where C3 plants are abundant and animals that lived in this type of vegetation had δ13C and δ180 values more negative that those inhabited in grasslands (Feranec and MacFadden 2006). However, ecosystems where C3 plants are dominant are not necessarily wooded zones, but can be other types of habitat or diverse microhabitats (Drucker et al. 2008; Drucker and Bocherens 2009).
C3 habitats can be divided into tropical undergrowth, tropical rainforest, subtropical rainforest with closed canopy, forest with canopy and gaps, subtropical rainforest with open canopy, savanna with dense riparian vegetation, warm temperate forest, subtropical savanna, and tropical deciduous forest (Secord et al. 2008). δ13C values in enamel can indicate the habitat where animals lived (Zanazzi and Kohn 2008): < -21‰ to -15‰ for forest with a closed canopy, 15‰ to -8‰ for forest, and > -8‰ for xeric grasslands.
On this basis, since the most negative value found in the mammalian fauna of San Gerardo de Limoncito was -16.7‰ and the most positive was -7.6‰, some of these animals may have lived in a forest with a canopy and openings.
This contrasts with a suggestion by Laurito and Valerio (2010) that the area nearby San Gerardo de Limoncito probably included a wooded savanna with grasslands, where the size of Calippus hondurensis allowed it to feed on young leaves and buds in wooded zones, whereas Protohippus gidleyi inhabited the boundary of the savanna and the wooded zone, and Dinohippus mexicanus lived on the savanna; meanwhile, gomphotheres, llamas, peccaries, and sloths would have lived in the forest or on the savanna.
Herbivores currently living beneath the canopy in the Ituri forest in Africa have δ13C values (-26.0‰ to -20.2‰) more negative than mammals inhabiting forest gaps (-17.5‰ to -16.2‰) (Cerling et al. 2004). The negative δ13C values recorded in the present study for the three species of equids, the gomphotheres, and the llamas from the upper Miocene of southern Central America suggest that members of this megafaunal assemblage also inhabited forest gaps because of their large size, but fed on leaves of trees, fruits, and some herbaceous plants beneath the canopy.
However, one of the three gomphotheres in the present study, as well as one of the eight Protohippus gidleyi specimens, had isotope values that indicate mixed C3/C4 feeders. There are two possible explanations for this: the first is that these mammals used to feed on CAM plants, such as orchids, bromeliads, or cacti, which are found in tropical zones (Andrade et al. 2007; Lüttge 2010); the second is that these individuals were not natives of the area and came from other localities with C4 plants.
Two individuals of Protohippus gidleyi had isotope values that were more positive than for the others (δ13C -7.8‰ and -9.7‰; δ18O -4.4‰ and -4. 6‰) (Table 1). Variation in the δ18O of the water in a locality may influence the δ18O values in dental enamel (Hoppe et al. 2004a, b). Because some individuals may have drunk water in places other than those where they fed, these individuals may have accumulated δ18O values differing from those of the other animals from the same locality (Pellegrini et al. 2008; Widga et al. 2010).
The δ18O values of water obtained from the studied herbivorous mammals of San Gerardo de Limoncito are more negative than those of water calculated by Bowen and Wilkinson (2002), Bowen and Revenaugh (2003), Bowen et al. (2005), and Bowen (2008) for this site at the present day (Tables 1, 2). Variation in the δ18O values of water in Costa Rica may be due to variation in rainfall and, to a lesser extent, to variations of temperature and altitude, as well as aridity (Lachniet and Patterson 2002; Reynolds-Vargas and Fraile 2009; Sánchez-Murillo et al. 2013). The δ18O values in present day water are most positive from May to August, the dry season, and more negative from October to December, the season with the highest precipitation (Table 2).
Table 2. Oxygen isotopic values (δ18OV-SMOW‰) for water at San Gerardo de Limoncito at the present day. Data from Bowen and Wilkinson 2002; Bowen and Revenaugh 2003; Bowen et al. 2005; Bowen 2008.
|
January |
February |
March |
April |
May |
June |
July |
August |
September |
October |
November |
December |
Average |
|
-6.1 |
-5.5 |
-5.5 |
-5.6 |
-3.8 |
-3.3 |
-3.9 |
-3.2 |
-5.5 |
-6.1 |
-6.7 |
-7.0 |
-5.4 |
Comparison of the δ18O values from dental enamel of these fossils with the present day values for the same site suggests that during the Early–Late Hemphillian San Gerardo de Limoncito had a higher level of precipitation and was more humid than today. Humidity influences the global distribution of C3 and C4 plants (Medina et al. 1999; Medrano and Flexas 2000; Yamori et al. 2013); high humidity favors C3 plants over C4 plants. For example, in some desert areas of the southern USA and northern Mexico, where rain is more abundant in winter, C3 plants are more abundant at that season, while C4 plants are more abundant during summer (Ehleringer and Monson 1993).
However, carbon and oxygen isotopes data for some North American localities of same or close age indicate the abundance of C4 plants (MacFadden 2000). For example, at Rancho El Ocote (Guanajuato) and Tecolotlán (Jalisco), both from the Mexican Late Hemphillian (Hh3), the closest localities to Limoncito, carbon and oxygen isotope analyses indicates either abundance of C4 plants and grassland, with arid conditions at Rancho El Ocote, or humid conditions and higher abundance of C3 plants in Tecolotlán, including mixed forest and grassland (Pérez-Crespo et al. 2017). These results indicate that local environmental conditions may govern the abundance of C3 and C4 plants on a site more than the geographic parameters (latitude, elevation, and the like), as it was found at San Gerardo de Limoncito (Pérez-Crespo et al. 2017).
The pollen of 40 plant taxa recovered from sediments of the lower Miocene Uscari Formation indicated the presence of a tropical rainforest (Graham 1987). Although these outcrops of the Uscari Formation are relatively close to San Gerardo de Limoncito, they are allochronic with respect to the Curré Formation. Nevertheless, models for Central America during the late Miocene (Micheels et al. 2007; Pound et al. 2011) indicated that tropical rainforests were also present; this is consistent with the δ13C and δ18O values of the fossil herbivorous mammal fauna analyzed here and with these pollen records.
Conclusions
The horses Calippus hondurensis, Dinohippus mexicanus, and Protohippus gidleyi, the gomphothere Gomphotherium hondurensis, and the llama Hemiauchenia vera from San Gerardo de Limoncito, all fed on C3 plants; this is shown by the δ13C in the dental enamel from fossils, and it suggests that during the Early–Late Hemphillian the site was more humid than today, thus favoring the presence of C3 plants. Together with the δ13C and δ18O values of the dental enamel in these fossils, the paleovegetation reconstructions made for this geological period suggest that these herbivorous mammals lived in a tropical rainforest, but in clearings of the forest where they fed on plants that developed there.
Acknowledgements
Thanks to the Laboratorio Nacional de Geoquímica y Mineralogía/Laboratorio de Isótopos Estables at Instituto de Geología, Universidad Nacional Autónoma de México, México, as well as to Rafael Puente Martínez (Laboratorio de Isótopos Estables, Instituto de Geología, Universidad Nacional Autónoma de México, México) who helped to prepare the samples. Andrew Somerville (Iowa State University, Arnes, USA) very kindly reviewed the English. Bruce MacFadden (Florida Museum of Natural History, University of Florida, Gainesville, USA), Maria Teresa Alberdi (Departamento de Paleobiología, Museo Nacional de Ciencias Naturales, Madrid, Spain), and an anonymous reviewer provided important comments that improved the manuscript. Supportive grants were provided by Consejo Nacional de Ciencia y Tecnología (#132620) and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica-UNAM (#IN404714, IA104017).
References
Ambrose, S.H. and DeNiro, M.J. 1986. The isotopic ecology of East African mammals. Oecologia 69: 395–406. Crossref
Andrade, J.L., De La Barrera, E., Reyes-García, C., Ricalde, M.F., Vargas-Soto, G., and Cervera, C.J. 2007. El metabolismo ácido de las crasuláceas: diversidad, fisiología ambiental y productividad. Boletín de la Sociedad Botánica de México 87: 37–50.
Ayliffe, L.K., Lister, A.M., and Chivas, A.R. 1992. The preservation of glacial-interglacial climatic signatures in the oxygen isotopes of elephant skeletal phosphate. Paleogeography, Palaeoclimatology, Palaeoecology 99: 179–191. Crossref
Barrón-Ortiz, C.R. and Guzmán-Gutierrez, J.R. 2008. Hábitos alimentarios de los caballos Dinohippus mexicanusy Neohippario neurystile del Henfiliano Tardío de Tecolotán, Jalisco. Investigación y Ciencia 45: 24–29.
Bocherens, H. 2003. Isotopic biogeochemistry and the paleoecology of the mammoth steppe fauna. Deinsea 9: 57–76.
Bowen, G.J. 2008. The Online Isotopes in Precipitation Calculator, version 2.2. http://www.waterisotopes.org.
Bowen, G.J. and Revenaugh, J. 2003. Interpolating the isotopic composition of modern meteoric precipitation. Water Resources Research 39: 1299. Crossref
Bowen, G.J. and Wilkinson, B. 2002. Spatial distribution of δ18O in meteoric precipitation. Geology 30: 315–318. Crossref
Bowen, G.J., Wassenaar, L.I., and Hobson, K.A. 2005. Global applications of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 143: 337–348. Crossref
Bryant, J.D. and Froelich, P.N. 1996. A model of oxygen isotope fractionation in body water of large mammals. Geochimica et Cosmochimica Acta 59: 4523–4537. Crossref
Cerling, T.E. 1999. Paleorecords of C4 plants and ecosystems. In: R.F. Sage and and R.K. Monson (eds.), C4 Plant Biology, 445–469. Academic Press, London. Crossref
Cerling, T.E. and Harris, J.M. 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120: 347–363. Crossref
Cerling, T.E., Harris, J.M., Ambrose, S.H., Leakey, M.G., and Solounias, N. 1997. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. Journal of Human Evolution 33: 635–650. Crossref
Cerling, T.E., Hart, J.A., and Hart, T.T. 2004. Stable isotope ecology in the Ituri Forest. Oecología 138: 5–12. Crossref
Cisneros, J.C. 2005. New Pleistocene vertebrate fauna from El Salvador. Revista Brasileira de Paleontología 8: 239–255. Crossref
Codron, J., Codron, D., Lee-Throp, J.A., Sponheimer, M., Bond, W.J., de Ruiter, D., and Grant, R. 2005. Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from African savanna. Journal of Archaeological Science 32: 1757–1772. Crossref
Coplen, T. 1988. Normalization of oxygen and hydrogen isotope data. Chemical Geology (Isotope Geoscience Section) 72: 293–297. Crossref
Coplen, T., Brand, W.A., Gehre, M.M., Gröning, M., Meijer Harro, A.J., Toman, B., and Verkouteren, R.M. 2006. New Guidelines for δ13C measurements. Analytical Chemistry 78: 2439–2441. Crossref
Dansgaard, W. 1964. Stable isotopes in precipitation. Tellus 16: 436–468. Crossref
Decker, J.E. and De Wit, M.J. 2005. Carbon isotope evidence CAM photosynthesis in the Mesozoic. Terra Nova 18: 9–17. Crossref
Denyer, P. and Alvarado, G.E. 2007. Mapa Geológico de Costa Rica (1: 400000). Librería Francesa S.A., San José.
Drucker, D.G. and Bocherens, H. 2009. Carbon stable isotopes of mammal bone as tracer of canopy development and habitat use in temperate and boreal contexts. In: J.D. Creigthon and P.J. Roney (eds.), Forests Canopies: Forest Production, Ecosystem Health, and Climate Conditions, 2–8. Nova Science Publisher Inc., New York.
Drucker, D.G., Bridault, A., Hobson, K.A., Szuma, E., and Bocherens, H. 2008. Can carbon-13 in large herbivores reflect the canopy effect in temperate and boreal ecosystems? Evidence from modern and ancient ungulates. Paleogeography, Palaeoclimatology, Palaeoecology 266: 69–82. Crossref
Ehleringer, J.R. and Monson, R.L. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24: 411–439. Crossref
Ehleringer, J.R., Lin, Z.F., Field, C.D., Sun, G.L., and You, L.Y. 1987. Leaf isotope ratios of plants from a subtropical monsoon forest. Oecologia 72: 109–114. Crossref
Faure, G. 1977. Principles of Isotope Geology. 606 pp. John Wiley & Sons, New York.
Feranec, R.S. 2003. Stable isotopes, hypsodonty, and the paleodiet of Hemiauchenia (Mammalia: Camelidae): a morphological specialization creating ecological generalization. Paleobiology 29: 230–242. Crossref
Feranec, R.S. and MacFadden, B.J. 2006. Isotopic discrimination of resource partitioning among ungulates in C3-dominated communities from the Miocene of Florida and California. Paleobiology 32: 191–205. Crossref
Graham, A. 1987. Miocene communities and paleoenvironments of Southern Costa Rica. American Journal of Botany 74: 1501–1518. Crossref
Harris, J.M. and Cerling, T.E. 2002. Dietary adaptions of extent and Neogene suids. Journal of Zoology 266: 45–64.
Hintze, J. 2004. NCSS and PASS. Kaysville, UTHA: Number Cruncher Statistical System Available at htpp://.www.ncss.com.
Hoppe, K.A., Amundson, R., Vavra, M., McClaran, M.P., and Anderson, D.L. 2004a. Isotopic analyses of tooth enamel carbonate from modern North American feral horses: implications for paleoenvironmetal reconstruction. Paleogeography, Palaeoclimatology, Palaeoecology 203: 299–311. Crossref
Hoppe, K.A., Stover, S.M., Pascoe, J.R., and Amundson, R. 2004b. Patterns of tooth enamel biomineralization in modern domestic horses: Implications for isotopic microsampling. Paleogeography, Palaeoclimatology, Palaeoecology 206: 355–365. Crossref
Iacumin, P., Cominotto, D., and Longinelli, A. 1996. A stable isotope study of mammal skeletal remains of mid-Pleistocene age, Arago cave, eastern Pyrenees, France. Evidence of taphonomic and diagenetic effects. Paleogeography, Palaeoclimatology, Palaeoecology 126: 151–160. Crossref
Kirby, M.X., Jones, D.S., and MacFadden, B.J. 2008. Lower Miocene stratigraphy along the Panama Canal and its bearing on the Central American peninsula. PLoS ONE3: e2791. Crossref
Koch, P.L. 1998. Isotopic reconstruction of past continental environments. Annual Review of Earth and Planetary Science 26: 573–613. Crossref
Koch, P.L. 2007. Isotopic study of the biology of modern and fossil vertebrates. In: R.H. Michener and K. Lajtha (eds.), Stable Isotopes in Ecology and Environmental Science, 99–154. Blackwell Publishing, Boston. Crossref
Koch, P.L., Fogel, M.L., and Tuross, N. 1994. Tracing the diets of fossil animals using stable isotopes. In: K. Lajtha and R.H. Michener (eds.), Stable Isotopes in Ecology and Environmental Science, 63–92. Blackwell Scientific Publications, Boston.
Koch, P.L., Tuross, N., and Fogel, M.L. 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. Journal of Archaeological Science 24: 417–429. Crossref
Kohn, M.J., Schoeninger, M.J., and Valley, J.W. 1996. Herbivore tooth oxygen isotope compositions: effects of diet and physiology. Geochimica et Cosmochimica Acta 60: 3889–3896. Crossref
Lachniet, M.S. and Patterson, W.P. 2002. Stable isotopes values of Costa Rica surface waters. Journal of Hydrology 260: 135–150. Crossref
Laurito, C.A. and Valerio, A.L. 2008. Ictiofauna de la localidad de San Gerardo de Limoncito, Formación Curré, Mioceno Superior, Cantón de Coto Brus, Provincia de Puntarenas, Costa Rica. Revista Geológica de América Central 39: 65–85.
Laurito, C.A. and Valerio, A.L. 2010. Los caballos fósiles de la Formación Curré, Cantón de Coto Brus Costa Rica. 210 pp. Museo Nacional de Costa Rica, San José.
Laurito, C.A. and Valerio, A.L. 2012. Paleobiogeografía del arribo de mamíferos suramericanos al sur de América Central previo al gran intercambio biótico americano: Un vistazo al GABI en América Central. Revista Geológica de América Central 46: 123–144.
Laurito, C.A. and Valerio, A.L. 2016. Camellos laminos del Mioceno Tardío (Henfiliano Temprano) de la Formación Curré, San Gerardo de Limoncito, Cantón de Coto Brus, Provincia de Puntarenas, Costa Rica. Revista Geológica de América Central 54: 7–55. Crossref
Laurito, C., Valerio, A.L., Gómez, L., Mead, J., and Pérez, E. 2005. A Trionychidae (Reptilia: Testudines, Cryptodira) from the Pliocene of Costa Rica, Southern Central America. Revista Geológica de América Central 32: 7–11. Crossref
Levin, N.E., Cerling, T.E., Passey, B.H., Harris, J.M., and Ehleringer, J.R. 2006. A stable isotope aridity index for terrestrial environments. Proceedings of the National Academy Sciences 103: 11201–11205. Crossref
Lucas, S.G. 2014. Vertebrate paleontology in Central America: 30 years of progress. Revista Geológica de América Central Número Especial: 30 Aniversario: 139–155.
Lucas, S.G. and Alvarado, G.E. 2010. Fossil proboscidea from the Upper Cenozoic of Central America: Taxonomy, evolutionary and paleobiogeographic signficance. Revista Geológica de América Central 42: 9–42. Crossref
Lucas, S.G., Alvarado, G.E., and Vega, E. 1997. The Pleistocene mammals of Costa Rica. Journal of Vertebrate Paleontology 17: 413–427. Crossref
Lüttge, V. 2010. Ability of Crassulacean Acid Metabolism plants to overcome interacting stresses in tropical environments. AoB Plants 2010: plq005. Crossref
MacFadden, B.J. 2000. Cenozoic mammalian herbivores from the Americas: Reconstructing ancient diets and terrestrial communities. Annual Review of Ecology and Systematics 31: 33–59. Crossref
MacFadden, B.J. 2005. Diet and habitat of toxodonts megaherbivores (Mammalia, Notoungulata) from the late Quaternary of South and Central America. Quaternary Research 64: 113–124. Crossref
MacFadden, B.J. 2006. Extinct mammalian biodiversity of the ancient New World tropics. Trends in Ecology and Evolution 21: 157–165. Crossref
MacFadden, B.J. 2008. Geography variations in diets of ancient populations of 5-million-year-old (early Pliocene) horses from southern North America: Palaeogeography, Palaeoclimatology, Palaeoecology 266: 83–94. Crossref
MacFadden, B.J. and Cerling, T.E. 1996. Mammalian herbivore communities, ancient feeding ecology, and carbon isotopes: a 10 million-year sequence from the Neogene of Florida. Journal of Vertebrate Palaeontology 16: 103–115. Crossref
MacFadden, B.J. and Higgins, P. 2004. Ancient ecology of 15-million-year-old browsing mammals within C3 plant communities from Panama. Oecologia 140: 169–182. Crossref
MacFadden, B.J., Cerling, T.E., Harris, J.M., and Prado, J.L. 1999. Ancient latitudinal gradients of C3/C4 grasses interpreted from stable carbon isotopes of New World Pleistocene horses (Equus) teeth. Global Ecology and Biogeography 8: 137–149. Crossref
MacFadden, B.J., Morgan, G.S., Jones, D.S., and Rincón, A.F. 2015. Gomphothere proboscidean (Gomphotherium) from the Late Neogene of Panama. Journal of Paleontology 89: 360–365. Crossref
McInerney, F.A., Strömberg, C.A.E., and White, J.W.C. 2011. The Neogene transition from C3 to C4 grassland in North America: stable carbon isotope ratios of fossil phytoliths. Paleobiology 37: 23–49. Crossref
Mead, J.L., Baez, A., Swift, S.L., Lahse, J., and Paiz, L. 2012. Late Pleistocene mammals Chivicabé, Huehuetenango, Guatemala. Revista Mexicana de Ciencias Geológicas 29: 319–329.
Medina, E., Martinelli, L.A., Barbosa, E., and Victoria, R.L. 1999. Natural abundance of 13C in tropical grassesfrom INPA, Instituto Nacional de Pesquisas da Amazonia, herbarium. Brazilian Journal of Botany 22: 43–51. Crossref
Medrano, H. and Flexas, J. 2000. Fotorrespiración y mecanismos de concentración del dióxido de carbono. In: J. Azcón-Bieto and M. Talón (eds.), Fundamentos de Fisiología Vegetal, 187–201. McGraw-Hill Interamericana, Madrid.
Micheels, A., Brunch, A.A., Uhl, D., Utescher, T., and Mosbrugger, V. 2007. A Late Miocene climate model simulation with ECHAM4/ML and its quantitative validation with terrestrial proxy data. Palaeogeography, Palaeoclimatology, Palaeoecology 253: 251–270. Crossref
Mooney, H.A., Bullock, S.H., and Ehleringer, J.R. 1989. Carbon isotope ratios of plants of a tropical dry forest in Mexico. Functional Ecology 3: 137–142. Crossref
O’Leary, M.H. 1988. Carbon isotopes in photosynthesis. Bioscience 38: 328–336. Crossref
Pellegrini, M., Donahue, R.E., Chenery, C., Evans, J., Lee-Throp, J. Montgomery, J., and Mussi, M. 2008. Faunal migration in late-glacial in late-glacial central Italy: implications for human resource exploitation. Rapid Communications in Mass Spectrometry 22: 1714–1726. Crossref
Pérez-Crespo, V.A., Arroyo-Cabrales, J., Corona-M., E., Morales-Puente, P., Cienfuegos-Alvarado, E., and Otero, F.J. 2015. Diet of rinchothere (Proboscidea: Gomphotheriidae, Rynchoterium Species) of Taxco, Guerrero, México. The Southwestern Naturalist 60: 97–98. Crossref
Pérez-Crespo, V.A., Carranza y Castañeda, O., Arroyo-Cabrales, J., Morales-Puente, P., Cienfuegos-Alvarado, E., and Otero, F.J. 2017. Diet and habitat of unique individuals of Dinohippus mexicanus and Neohipparion eurystile (Equidae) from the late Hemphillian (Hh3) of Guanajuato and Jalisco, central Mexico: stable studies isotopes. Revista Mexicana de Ciencias Geológicas 34:38–44. Crossref
Pérez-Crespo, V.A., Prado, J.L., Alberdi, M.T., Arroyo-Cabrales, J., and Johnson, E. 2016. Diet and habitat for six American proboscidean species using carbon and oxygen stable isotopes. Ameghiniana 53: 39–51. Crossref
Pound, M.J., Haywood, A.M., Salzmann, U., Riding, J.B., Lunt, D.J., and Hunter, S.J. 2011. A Tortonian (Late Miocene, 11.61–7.25 Ma) global vegetation reconstruction. Palaeogeography, Palaeoclimatology, Palaeoecology 300: 29–45. Crossref
Reynolds-Vargas, J. and Fraile, J. 2009. Utilización de isótopos estables en la precipitación para determinar zonas de recarga del acuífero Barva, Costa Rica. In: Organización Internacional de Energía Atómica (ed.), Estudios de Hidrología Isotópica en América Latina 2006, 83–95. OEIA, Vienna.
Révész, K.M. and Landwehr, J.M. 2002. δ13C and δ18O isotopic composition of CaCO3 measured by continuous flow isotope ratio mass spectrometry: statistical? evaluation and verification by application to Devils Hole core DH-11 calcite. Rapid Communications in Mass Spectrometry 16: 2012–2114. Crossref
Sánchez, B. 2005. Reconstrucción del ambiente de mamíferos extintos a partir del análisis isotópico de los restos esqueléticos. In: P. Alcorno, R. Redondo, and J. Toledo (eds.), Nuevas técnicas metodológicas aplicadas al estudio de los sistemas ambientales: los isótopos estables, 49–64. Universidad Autónoma de Madrid, Madrid.
Sánchez, B., Prado, J.L., and Alberdi, M.T. 2004. Feeding ecology, dispersal, and extinction of South American Pleistocene gomphotheres (Gomphotheriidae, Proboscidea). Paleobiology 30: 146–161. Crossref
Sánchez-Chillón, B., Alberdi, M.T., Leone, G., Bonadonna, F.P., Stenni, B., and Longinelli, A. 1994. Oxygen isotopic composition of fossil equid tooth and bone phosphate: an archive of difficult interpretation. Palaeogeography, Palaeoclimatology, Palaeoecology 107: 317–328. Crossref
Sánchez-Murillo, R., Esquivel-Hernández, G., Welsh, K., Brooks, E.R., Boll, J., Alfaro-Solís, R., and Valdés-González, J. 2013. Spatial and temporal variations of stable isotopes in precipitation across Costa Rica: An analysis of historic GNIP records. Open Journal of Modern Hydrology 3: 226–240. Crossref
Sanson, G.D., Kerr, S.A., and Gross, K.A. 2007. Do silica phytoliths really wear mammalian teeth? Journal of Archaeological Science 34: 526–531. Crossref
Schoeninger, M.J., Kohn, M., and Valley, J.W. 2000. Tooth oxygen isotopes ratios as paleoclimate monitors in arid ecosystems. In: S.H. Ambrose and M.A. Katzemberg (eds.), Biogeochemical Approaches to Paleodietary Analysis, 117–140. Kluwer Academic/Plenum Publisher, New York.
Secord, R., Wing, S.L., and Chew, A. 2008. Stable isotopes in Early Eocene mammals as indicators of forest canopy structure and resource partitioning. Paleobiology 34: 282–300. Crossref
Semprebon, G.M. and Rivals, F. 2010.Trends in the paleodietary habits of fossil camels from Tertiary and Quaternary of North America. Palaeogeography Palaeoecology Palaeoclimatology 295: 131–145. Crossref
Smith, B. and Epstein, S. 1971. Two categories of 13C/12C ratios for higher plants. Plant Physiology 47: 380–384. Crossref
Sponheirmer, M. and Lee-Thorp, J.A. 1999. Oxygen isotopes in enamel carbonate and their ecological significance. Journal of Archaeological Science 26: 723–728. Crossref
Tedford, R.H., Albright, L.B., Barnosky, A.D. III., Ferrusquía-Villafranca, I., Hunt, R.M. Jr., Storer, J.E., Swisher, C.C. III., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (Late Oligocene through Early Pliocene epochs). In: M.O. Woodburne (ed.), Late Cretaceous and Cenozoic Mammals of North America, 169–231. Columbia University Press, New York.
Valerio, A.L. 2010. Paleontologia, bioestatigrafía, y paleoecología de los caballos fósiles de la Formación Curré, en el cantón de Coto Brus, Costa Rica (análisis basado en material dental). 353 pp. Tesis de Licenciatura, Universidad de San José, San José.
Valerio, A.L. and Laurito, C. 2012. Cetáceos fósiles (Mammalia, Odontoceti, Eurhinodelphinoidea, Inioidea, Physeteroidea) de la Formación Curré, Mioceno Superior, (Hemphilliano Temprano Tardío) de Costa Rica. Revista Geológica de América Central 46: 151–160.
Van der Merwe, N.J. and Medina, E. 1989. Photosynthesis and 12C/13C ratios in Amazonian rain forest. Geochimica et Cosmochimica Acta 53: 1091–1094. Crossref
Yamori, W., Hikosa, K., and Way, D. 2013. Temperature response of photosynthesis in C3, C4, CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119: 101–117. Crossref
Werner, R.A. and Brand, W.A. 2001. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Communications in Mass Spectrometry 15: 501–519. Crossref
Widga, C., Walker, J.D., and Stockli, L.S. 2010. Middle Holocene Bison diet and mobility in the eastern Great Plains (USA) based on δ13C, δ18O, and 87Sr/86Sr analyses of tooth enamel carbonate. Quaternary Research 73: 449–463. Crossref
Zanazzi, A. and Kohn, M.J. 2008. Ecology and physiology of White River mammals based on stable isotope ratios of tooth. Palaeogeography, Palaeoclimatology, Palaeoecology 257: 22–37. Crossref
Acta Palaeontol. Pol. 63 (4): 645–652, 2018
https://doi.org/10.4202/app.00517.2018