

First endocranial description of a South American hadrosaurid: The neuroanatomy of Secernosaurus koerneri from the Late Cretaceous of Argentina
MARCOS G. BECERRA, ARIANA PAULINA-CARABAJAL, PENÉLOPE CRUZADO-CABALLERO, and JEREMÍAS R.A. TABORDA
Becerra, M.G., Paulina-Carabajal, A., Cruzado-Caballero, P., and Taborda, J.R.A. 2018. First endocranial description of a South American hadrosaurid: The neuroanatomy of Secernosaurus koerneri from the Late Cretaceous of Argentina. Acta Palaeontologica Polonica 63 (4): 693–702.
The endocranial morphology of Secernosaurus koerneri (= Kritosaurus australis junior synonym), a hadrosaurid from the Upper Cretaceous of Argentina, was studied using latex and digital endocasts based on three fragmentary braincases. This new information allowed describing and comparing the neuroanatomy of this South American representative of the clade for the first time. The endocast morphology is mostly complete (except for the pituitary and the inner ear regions), and most cranial nerves and some blood vessels were reconstructed. Also, some features of the inner ear were observed in the CT scans, nonetheless its incompleteness restricts further comparisons. Secernosaurus koerneri shares its overall endocranial morphology with saurolophinid hadrosaurids, indicating a conservative brain morphology for Cretaceous hadrosaurids worldwide. The novel cranial information increases the knowledge of the neuroanatomy in hadrosaurids by adding a southern perspective, since knowledge on the endocranial anatomy of the lineage is biased by species from North America.
Key words: Dinosauria, Hadrosauridae, Secernosaurus koerneri, endocast, neuroanatomy, Cretaceous, South America, Argentina.
Marcos G. Becerra [mbecerra@mef.org.ar], Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Museo Paleontológico Egidio Feruglio (MEF), Fontana 140, Trelew U9100GYO, Chubut, Argentina.
Ariana Paulina-Carabajal [a.paulinacarabajal@conicet.gov.ar], Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-UNCo), Quintral 1350, San Carlos de Bariloche 8400, Río Negro, Argentina.
Penélope Cruzado-Caballero[pccaballero@unrn.edu.ar], Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Rio Negro (CONICET-UNRN), Av. General Roca 1242, General Roca 8332, Río Negro, Argentina.
Jeremías R.A. Taborda [jemreias.taborda@conicet.gov.ar], Centro de Investigaciones en Ciencias de la Tierra (CICTERRA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad Nacional de Córdoba, FCEFyN, Vélez Sarsfield 1611, Ciudad Universitaria X5016GCA, Córdoba, Argentina.
Received 14 July 2018, accepted 3 September 2018, available online 19 September 2018.
Copyright © 2018 M.G. Becerra et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The endocranial morphology (and inferred brain anatomy) in hadrosaurids is known from natural or artificial endocasts, mostly from species of the Northern Hemisphere. The neuroanatomy was described in several species from North America including Corythosaurus sp., Edmontosaurus sp., Hypacrosaurus altispinus, Lambeosaurus sp., and Gryposaurus notabilis (e.g., Marsh 1893; Lambe 1920; Ostrom 1961; Hopson 1979; Serrano-Brañas et al. 2006; Evans et al. 2009), whereas there are few studied species from Asia and Europe, including Amurosaurus riabinini, Arenysaurus ardevoli, and Telmatosaurus transsylvanicus (Nopsca 1900; Saveliev et al. 2012; Lauters et al. 2013; Cruzado-Caballero et al. 2015). On the contrary, the record of hadrosaurids in the Southern Hemisphere is low and almost exclusive from South America (Horner et al. 2004; Cruzado-Caballero et al. 2017), with cranial bones being scarce and braincase elements even less frequently preserved (e.g., Coria 2014; Cruzado-Caballero 2017).
In this context, the cranial hadrosaurid remains from Los Alamitos Formation (late Campanian–early Maastrichtian of North Patagonia) referred to Secernosaurus koerneri Brett-Surman, 1979 (Bonaparte et al. 1984; Prieto-Márquez and Salinas 2010), although fragmentary, represent the most complete braincase material described for a hadrosaurid in South America to date. The aim of this study is to describe the endocranial morphology of this species based on three fragmentary braincases, and compare it with other studied hadrosaurids, highlighting its importance for future comparative analyses.
Institutional abbreviations.—MACN, Vertebrate Paleontology collection of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
Other abbreviations.—CN, cranial nerve; CT, computed tomography.
Material and methods
The studied specimens MACN-RN 02, MACN-RN 143, and MACN-RN 144 (Fig. 1) correspond to incomplete braincases recently assigned to Secernosaurus koerneri (Prieto-Marquez and Salinas 2010). These specimens were X-ray CT-scanned using a medical 64-channel multi-slicer tomographer Philips Brilliance CT 64 Channel-DS, in the Clínica La Sagrada Familia (Buenos Aires, Argentina). The CT data were obtained using a penetration power of 120 kV and 271 mA, and the reconstruction parameters for all CT image series are: field of view 451; slice thickness of 0,8 mm; and slice increment of 0,4 mm. The CT database is composed of three sets of slices of 591 images for MACN-RN 02, 348 images for MACN-RN 143 and 784 images for MACN-RN 144. The segmentation of each specimen was made using the software 3D Slicer v4.1.1 (Fedorov et al. 2012). Then, a single and more complete model was generated based on the isolated endocasts of the three specimens (see the 3D pdf in the SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app63-Becerra_etal_SOM.pdf) using the software DesignSpark Mechanical v2015.0. The endocasts of the three specimens were assembled following Prieto-Marquez and Salinas (2010) for MACN-RN 144 and MACN-RN 02, and by overlapping repeated braincase and endocranial regions between these and MACN-RN 143. Due to the similar braincase size of specimens, these and their endocasts were not scaled prior to be assembled. The anatomical information of the latex endocast of MACN-RN 144, and a fourth specimen MACN-RN 142, were also considered in this description (see below).
The specimens MACN-RN 144 and MACN-RN 02 easily fit together in the 3D space. In their communication, Prieto-Marquez and Salinas (2010) represent an anatomical continuation between MACN-RN 144 and MACN-RN 02 considering that belonged to the same individual, whereas MACN-RN 142 and MACN-RN 143 to different individuals to each other and with the former. Although is highly likely a natural continuation between MACN-RN 144 and MACN-RN 02, these were separately described. As previously mentioned, MACN-RN 143 belonged to a similarly sized individual than that for MACN-RN 144 and MACN-RN 02. A size difference due to ontogeny, however, is evident between MACN-RN 144 and MACN-RN 142, with the later being smaller than the former. No osteological differences were observed between the here addressed specimens that allow describing intraspecific or sex related dimorphic variation, although a proportionally more developed large venous sinus is present in MACN-RN 144 than in MACN-RN 142 (see Discussion).
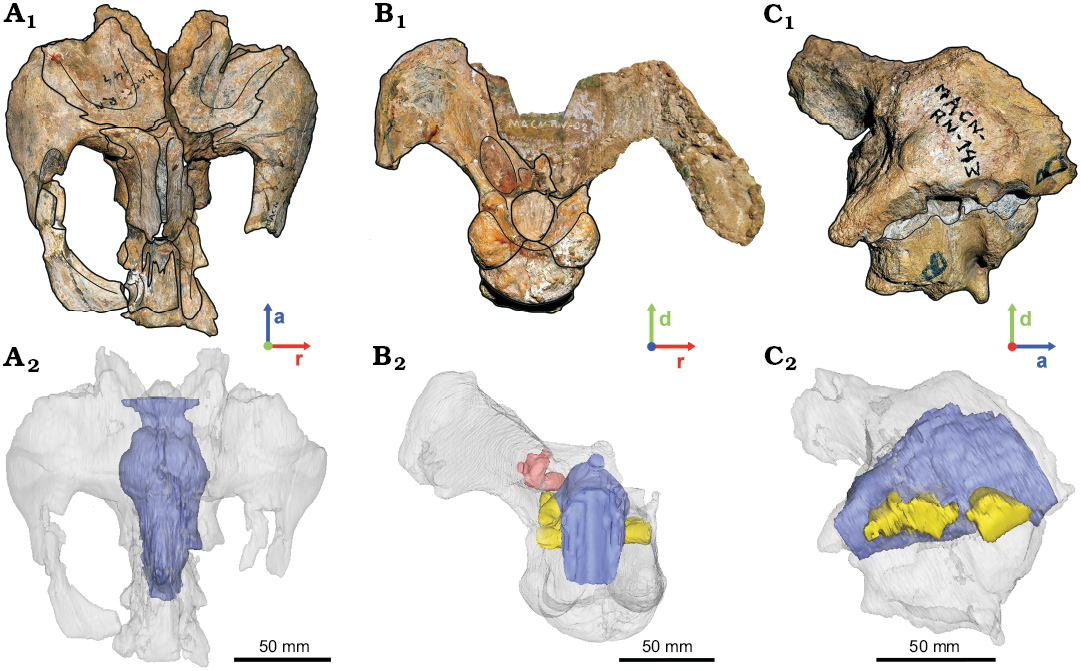
Fig. 1. Hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia, braincases and their reconstructed endocasts. A. MACN-RN 144 in dorsal view. B. MACN-RN 02 in posterior view. C. MACN-RN 143 in lateral view. Photographs (A1–C1), CT-scan rendition of endocasts (A2–C2). Transparent gray, braincase; blue, encephalon; pink, inner ear; yellow, foramina of the cranial nerves. Abbreviations: a, anterior; d, dorsal; r, right.
Results
MACN-RN 144.—The preserved braincase includes the frontals, fragmentary parietals, both orbitosphenoids, both laterosphenoids, and the presphenoids (= ethmoids in Gilmore 1937; = ethmoidal complex in other dinosaurs, see Ali et al. 2008 and references therein), in articulation with both postorbitals and a fragment of the left squamosal (e.g., Prieto-Márquez and Salinas 2010: figs. 4–6; Fig. 1A1). The basicranium, together with any record of the pituitary fossa and the passages for the CN VI are missing. Therefore, the reconstructed endocast corresponds to the forebrain and the mid-dorsal portion of the midbrain and hindbrain (Fig. 1A2). The olfactory bulbs are relatively small compared to the size of the cerebral hemispheres, as in other hadrosaurids (e.g., Hopson 1979; Evans et al. 2009). They are not clearly differentiated and the median separation between each other is shallow in the digital endocast. However, these features are better observed in the latex endocast, showing oval olfactory bulbs, slightly divergent from the midline (Figs. 2A, 3B). The olfactory tracts are short and transversely wide in dorsal view, separated from the cerebral hemispheres by a marked constriction in dorsal view (Figs. 2, 3B7, B8). The olfactory bulbs and tracts of Secernosaurus koerneri are similar to those described in other hadrosaurids such as Edmontosaurus sp. (Lambe 1920). Differences are observed, however, in the orientation of the olfactory bulbs, which are slightly projected anterodorsally in Secernosaurus koerneri, as in Gryposaurus notabilis (Ostrom 1961; Hopson 1979). In contrast, Corythosaurus sp. and Hypacrosaurus altispinus have marked anterodorsally projected olfactory bulbs (Evans et al. 2009: fig. 7). Nevertheless, the olfactory tracts are slender and relatively longer in non-hadrosaurid taxa such as Leaellynasaura amicagraphica, Dysalotosaurus lettowvorbecki, Tenontosaurus tilletti, Mantellisaurus atherfieldensis, and Iguanodon bernissartensis, supporting the tendency of the reduction of the olfactory system in hadrosaurids proposed by other authors (Lautenschlager and Hübner 2013; Lauters et al. 2012; Thomas 2015; Brasier et al. 2016; Sharp et al. 2017). As in other hadrosaurids, the cerebral hemispheres of Secernosaurus koerneri are laterally and dorsally wide (e.g., Hopson 1979; Fig. 2). Each cerebral hemisphere is dorsolaterally subdivided in two lobes, a feature of Secernosaurus koerneri that has not been described in other hadrosaurids (Figs. 2A, D, 3; see Discussion). The osteological correlate of this feature is a blunt ridge directed medially and anterolaterally from the interfrontal suture.
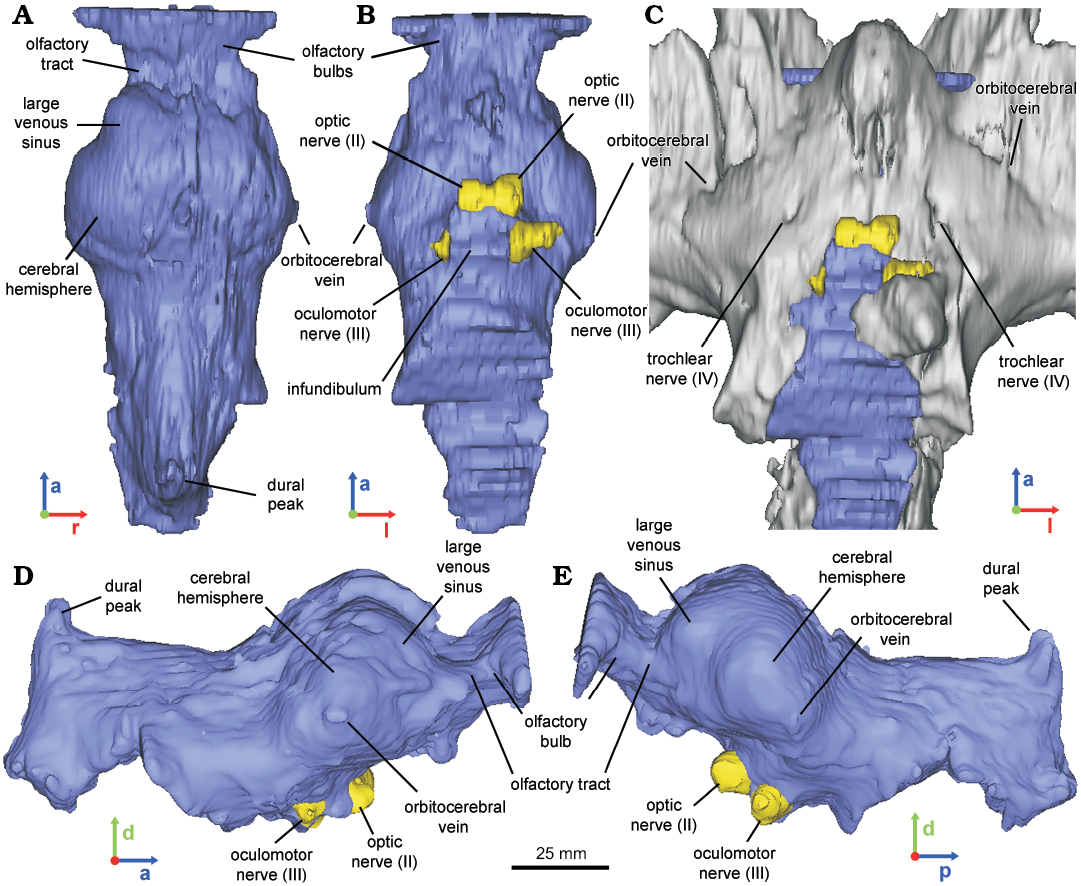
Fig. 2. Reconstructed endocast of braincase of the hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 (MACN-RN 144) from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia; in dorsal (A), ventral (B), left (D), and right (E) views, with a ventral view of both the fossil specimen and the endocast (C). Blue, encephalon; gray, braincase; yellow, foramina of the cranial nerves. Abbreviations: a, anterior; d, dorsal; l, left; p, posterior; r, right.
The latex endocast of the specimen allowed identifying the orbitocerebral vein lateroventrally to the cerebral lobes (also observed in MACN-RN 142, Fig. 3A3–A6), a blood vessel that is barely observed in the CT scan (Fig. 2). Posteroventrally to the cerebral hemispheres, the hindbrain is narrow with a subtriangular transversal section, as in Gryposaurus notabilis and other hadrosaurids (e.g., Ostrom 1961; Evans et al. 2009; Saveliev et al. 2012). There is no floccular process, an absence occurring in both the digital and latex endocasts of Secernosaurus koerneri and also in other studied hadrosaurids. A conspicuous dural peak ends the posteriormost portion of the endocast, indicating the presence of an important dorsal longitudinal venous sinus. The angle formed between the anterior and posterior margins of the dural peak is approximately 93º, closer to Parasaurolophus and smaller than in any other hadrosaurids (e.g., Farke et al. 2013; Cruzado-Caballero et al. 2015). The passage for CN II (optic nerve) is large, circular in section, and separated from its counterpart (Fig. 2B). Posteroventral to CN II, a partial passage for CN III (oculomotor nerve) is identified (Fig. 2B, D–E). In the braincase, a slight opening is observed at both sides between the orbitosphenoid and the laterosphenoid, and dorsal to CN III, corresponding probably to the external exit foramen for CN IV (trochlear nerve; Fig. 2C). Although the low contrast of the CT scans prevents the identification of the complete path for this nerve, this passage is reproduced by the latex endocast (Fig. 3B5, B6). The infundibulum is proximally circular in section and the inclination of its posterior wall indicates the presence of a low but thick dorsum sellae.
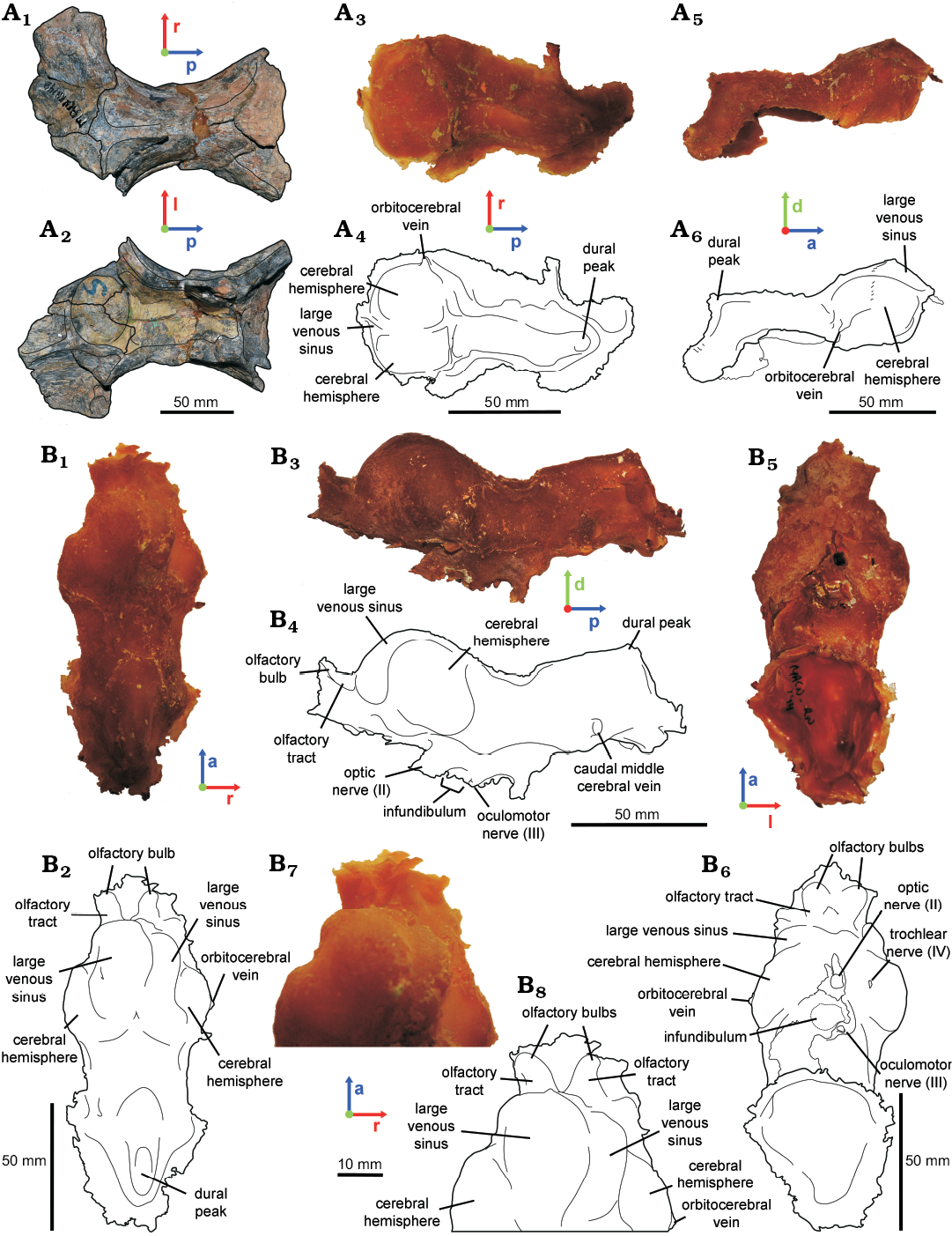
Fig. 3. Hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia. A. MACN-RN 142 (parietals and frontals). B. MACN-RN 144 (braincase). In dorsal (A1, A3, A4, B1, B2, B7, B8), ventral (A2, B5, B6), lateral right (A5, A6) and left (B3, B4) views. Photographs (A1, A2); latex endocasts (A3, A5, B1, B3, B5, B7); line drawings (A4, A6, B2, B4, B6, B8).
MACN-RN 02.—The preserved braincase includes the basioccipital, the damaged basisphenoid, the exoccipital-opisthotic complex (complete in the left side), the supraoccipital, and the left prootic (e.g., Prieto-Márquez and Salinas 2010: figs. 4–6; Fig. 1B). A fracture between the prootic-opisthotic and the basicranium affected the preservation of the inner ear and the base of the cranial nerves. The reconstructed endocast corresponds mostly to the hindbrain up to the foramen magnum, and an incomplete left inner ear (Fig. 4). Cranial nerves IX–XI (glossopharingeal–vagoaccessory nerves) leave the endocranial cavity through a single metotic foramen, together with the internal jugular vein. The metotic passage is large if compared to the opennings for the cranial nerves and is laterally projected, merging distally with the anterior branch of CN XII (hypoglossal nerve). The hypoglossal nerve has two branches. Whereas the posterior branch exists through a single external foramen enclosed by the exoccipitals, the anterior branch exits the endocranial cavity through a foramen that is distally merged with the metotic foramen, as occurring in other hadrosaurids as Hypacrosaurus altispinus and Corythosaurus sp. (Evans et al. 2009; see Discussion below). The caudal middle cerebral vein is recognized as a slender passage posterodorsally oriented and exiting the endocranial cavity through a foramen on the occipital region of the braincase, as in other archosaurs (Witmer et al. 2008). Two protuberances on the ventral side of the medulla oblongata may represent sinuses of the ventral longitudinal venous sinus, a structure reminiscent of the “basilar artery” illustrated for Anatosaurus sp. (= Edmontosaurus sp., AMNH 5236) by Ostrom (1961) (Fig. 4A–C).
The left inner ear could only be partially reconstructed, including segments of the anterior, posterior and lateral semicircular canals, possibly the anterior and posterior ampullae, and the proximal portion of the lagena (Fig. 4D–G). If paralleling the horizontal semicircular canal with the transversal plane in lateral view, the ventral region of the medulla oblongata orients obliquely at an angle of 45°, but not the dorsal region (Fig. 4A, B).
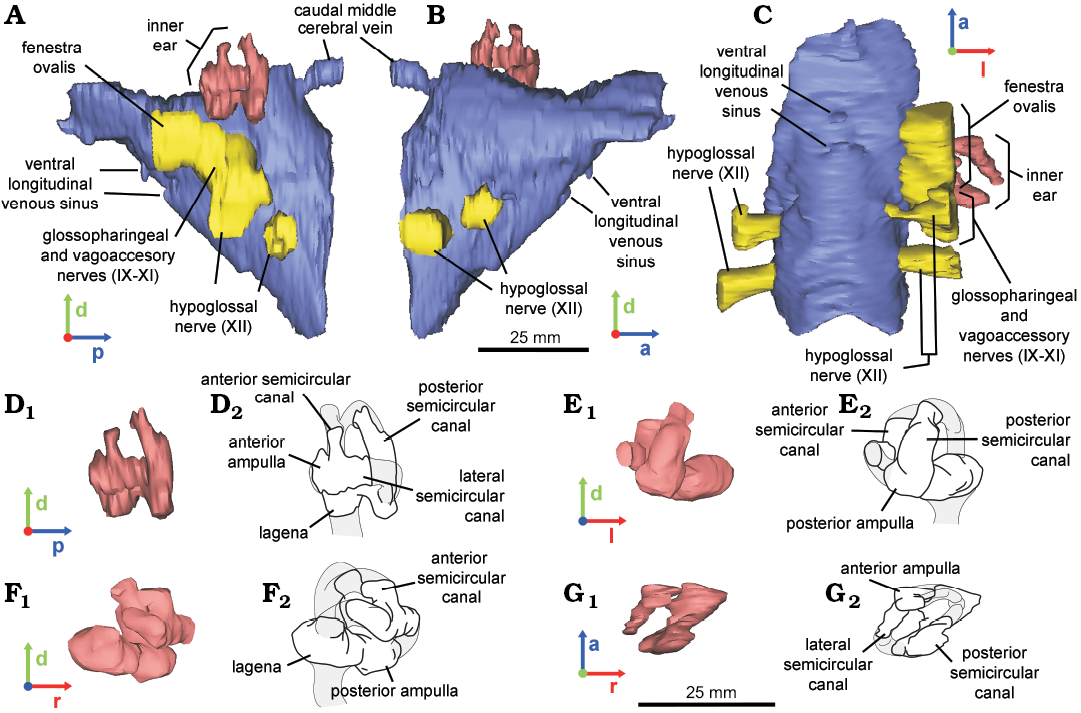
Fig. 4. Reconstructed endocast of braincase (A–C) and inner ear (D–G) of the hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 (MACN-RN 02) from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia; in left (A, D), right (B), ventral (C), posterior (E), anterior (F), and dorsal (G) views. Endocasts (A–C, D1–G1); line drawings (D2–G2). Blue, encephalon; pink, inner ear; yellow, foramina of the cranial nerves. Abbreviations: a, anterior; d, dorsal; l, left; p, posterior; r, right.
MACN-RN 143.—The braincase preserves part of the basisphenoid, the opisthotic–exoccipital complex, the prootics and the laterosphenoids (e.g., Prieto-Márquez and Salinas 2010: fig. 8; Fig. 1C). The endocast represents the right half of the midbrain–hindbrain, comprising from the infundibulum anteriorly to almost reaching the foramen magnum posteriorly (Fig. 1C), and preserves the canals and foramina for CNs V–XI. Nevertheless, in the endocast the passages of CNs VII–XI are confluent to each other at their base and with the fenestra ovalis since this region is internally damaged (Fig. 5). In the braincase, the foramen for CN V is the largest of all cranial foramina, having an irregular shape including an anterior lobe for the ophthalmic branch (V1), a ventral lobe for the maxillomandibular branches (V2,V3) and a posterior subcircular commissure probably for a vascular element. This “triangular” shape is reflected in the cast of the canal (Fig. 5). Posterior to the foramen for CN V, the next subcircular foramen corresponds to CN VII (facial nerve; Fig. 5). Prieto-Marquez and Salinas (2010: fig. 8D) identified two exits for the CN VII, possibly for the palatine and hyomandibular branches of the facial nerve, but although a slight bone prominence interrupts the subcircular shape of this foramen anteriorly, this study does not support their description. Three large openings posterior to the foramen for CN VII are confluent due to fractures. In anteroposterior order, they represent the fenestra ovalis, the metotic foramen (for CNs IX–XI), and the anterior branch of CN XII (Fig. 5A). The anterior face of the basisphenoid might form the posterior wall of the infundibulum (Fig. 5A, C), although no pathways or exits for CN VI (the abducens nerve) piercing the basisphenoid were identified, nor for the cerebral branch of the internal carotid artery.
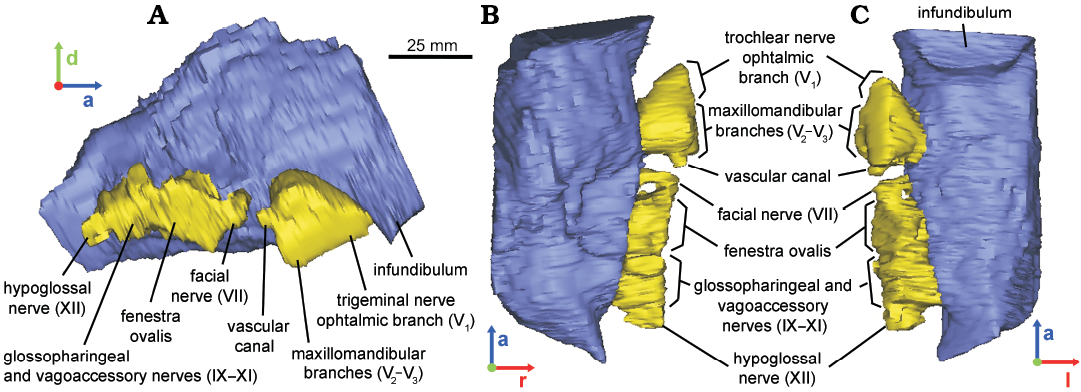
Fig. 5. Reconstructed endocast of braincase of the hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 (MACN-RN 143) from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia; in lateral right (A), dorsal (B), and ventral (C) views. Blue, encephalon; yellow, foramina of the cranial nerves. Abbreviations: a, anterior; d, dorsal; l, left; r, right.
Discussion
Although incomplete, the morphology of the endocast of Secernosaurus koerneri is informative. The three fragmentary braincases and their endocasts represent three different regions that can be combined in a single final reconstruction (Fig. 6A, B). The endocast of Secernosaurus koerneri (Fig. 6C), as in other hadrosaurids, shows its largest widening across the cerebral hemispheres. In addition, the anterior and middle cerebral regions meet without forming an angle ventrally in the endocast, a feature not observed in other saurolophine but present in lambeosaurine hadrosaurs (pontine flexure in Hopson 1979 and Cruzado-Caballero et al. 2015). An apparent subdivision of the anterolateral side of the cerebral hemispheres is observed in both specimens preserving the dorsal region of the forebrain, MACN-RN 142 and MACN-RN 144 (Fig. 3). Although this feature could be a product of deformation in MACN-RN 142 (latex endocast), the left side of MACN-RN 144 seems undistorted and bears these “lobes”, interpreted here as large venous sinuses on the anterodorsal region of the cerebral hemispheres (Figs. 2A, D, 3). Unlike lambeosaurines and the basal hadrosauroid Batyrosaurus rozhdestvenskyi, which show marked impressions of blood vessels on the ventral side of the frontals and the medial wall of the laterosphenoids (Evans 2005: fig. 1; Godefroit et al. 2012c), the endocasts of Secernosaurus koerneri (including the latex ones) do not exhibit clear blood vessels on the cerebral hemispheres. As mentioned, the olfactory bulbs in Secernosaurus koerneri are relatively small and poorly divergent. The size of the olfactory bulbs and the olfactory ratio (i.e., the ratio between the greatest diameter of the olfactory bulb and the greatest diameter of the cerebral hemisphere regardless of their orientation) have been used as indicators for olfactory acuity and breeding habits in modern animals (see Zelenitsky et al. 2009 and references therein). Although the olfactory ratio by itself would not be enough to calculate olfactory acuity (Zelenitsky et al. 2009), it would be a useful measurement in morphological comparisons (e.g., Paulina-Carabajal et al. 2016, 2017; Paulina-Carabajal and Filippi 2018). The olfactory ratio in Secernosaurus koerneri is approximately 22% of the cerebral hemispheres (greatest diameter of the olfactory bulb equals to 9 mm, greatest diameter of the cerebral hemisphere equals 42 mm), whereas in Alligator mississippiensis it ranges between 49.8–55.1% (Zelenitsky et al. 2009), and is under 20% for most groups of living birds (Zelenitsky et al. 2011). Based on the endocasts illustrated by Evans et al. (2009), the olfactory bulbs seem to be slightly larger in lambeosaurine hadrosaurids, suggesting a possible lower olfactory acuity for the South American species. The shape and size of the pituitary remains unknown for this species.
The general morphology of the cranial nerves in the endocast and the relative position of their external foramina in the braincase follow the same pattern described for other hadrosaurids (e.g., Hopson 1979; Evans et al. 2009; Cruzado Caballero et al. 2015). Particularly in Secernosaurus koerneri, the passages and cranial foramina for CNs III and IV are separate, as in Arenysaurus ardevoli and lambeosaurines (Evans et al. 2009), but unlike Edmontosaurus sp. (Lambe 1920). Although there are grooves running from the foramen for CN V of Secernosaurus koerneri, which indicate the orientation of the ophthalmic (V1) and maxillary and mandibular branches (V2, V3) outside the braincase, the branches leave the endocranial cavity through a single foramen, as in other hadrosaurs (e.g., Evans et al. 2009; Lauters et al. 2013; Cruzado-Caballero et al. 2015). Cranial nerve XII has two branches and the most anterior runs anterolaterally to join distally the metotic passage, which is the reason why there is a single external foramen on the exoccipital. This character seems to be highly variable in Hadrosauridae, because although the state in Secernosaurus koerneri is shared with Hypacrosaurus altispinus and Corythosaurus sp. (Evans et al. 2009), it differs from other hadrosaurids within the lineage. In A. ardevoli, A. riabinini, and Lambeosaurus sp. the anterior branch of CN XII shares the metotic passage with the CNs IX–XI throughout its entire length (Evans et al. 2009; Lauters et al. 2013; Cruzado-Caballero et al. 2015), a feature also present possibly in Edmontosaurus sp. (Lambe 1920). Considering the species Gryposaurus notabilis, which corresponds to the closest relative of Secernosaurus koerneri from which endocasts were described (e.g., Prieto-Marquez et al. 2016), this feature is remarkable different from the later as it shows three canals for CN XII (Hopson 1979; Evans et al. 2009). Three exits for the hypoglossal nerve were also figured for Lophorhothon atopus (Langston 1960: fig. 151), supporting its highly variable condition within Hadrosauroidea (e.g., Prieto-Marquez et al. 2016). As for the blood vessel foramina, the presence of a separate passage for the orbitocerebral vein and the caudal middle cerebral vein are confirmed. Unfortunately, the incompleteness of the inner ear morphology prevents further comparisons.
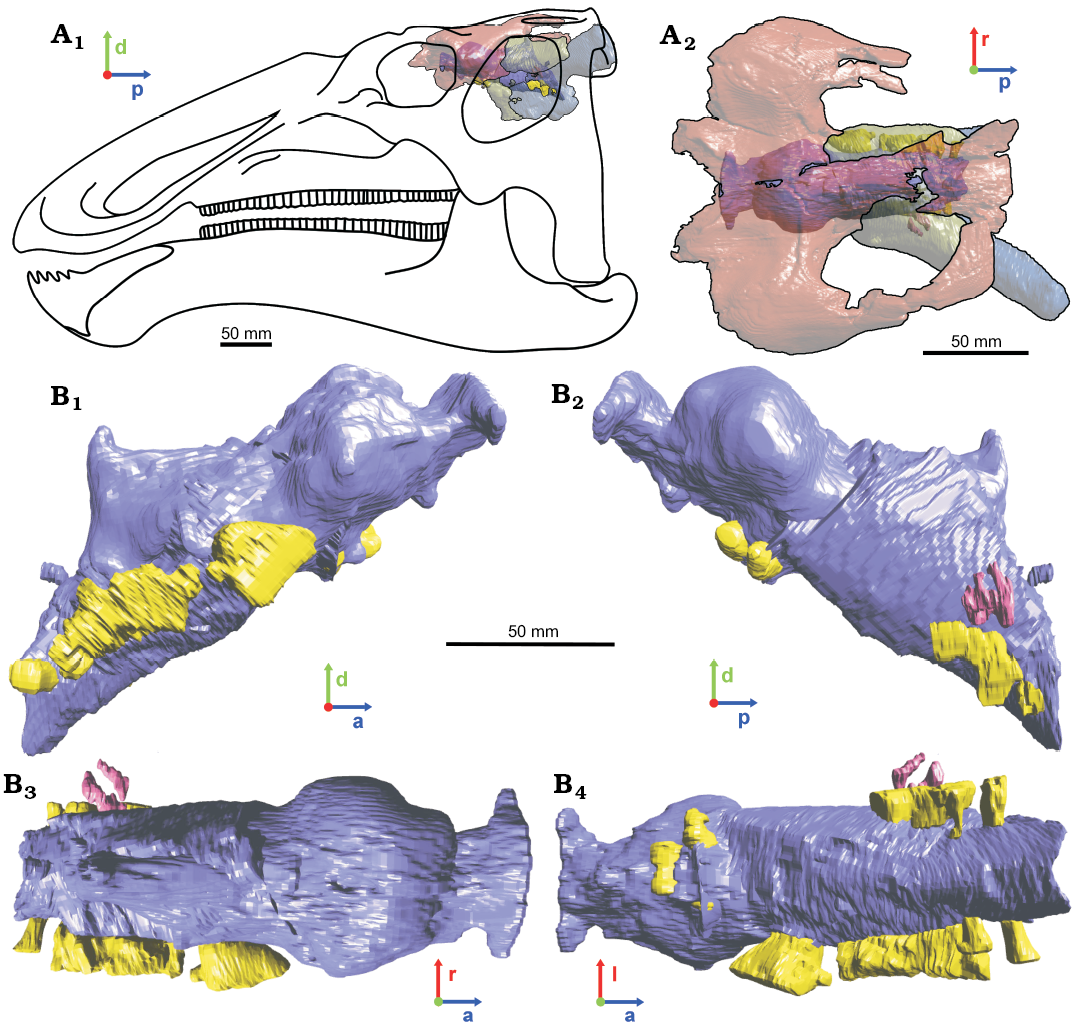
Fig. 6. 3D-reconstructed endocast based on the information of the studied specimens representing the brain morphology of the hadrosaurid dinosaur Secernosaurus koerneri Brett-Surman, 1979 from the late Campanian–early Maastrichtian, Los Alamitos Formation of North Patagonia. A. Braincase and endocasts in 3D positioned over a line drawing reconstruction of the skull in left lateral view; based on the mounted skeletons at the MACN (size for the line drawing is estimative). B. Dorsal view of the 3D specimens MACN-RN 144 (transparent red), MACN-RN 143 (transparent green) and MACN-RN 02 (transparent blue) in order from left to right and as they were positioned to compile the final endocranial reconstruction. C. Final endocast reconstruction in right (C1) and left (C2), dorsal (C3), and ventral (C4) views. The anatomical labelling was presented in former figures. Blue/darker gray, encephalon; pink/midtone gray, inner ear; yellow/brighter gray, foramina of the cranial nerves. Abbreviations: a, anterior; d, dorsal; l, left; p, posterior; r, right. All specimens with their 3D endocasts are presented as a 3D pdf in the SOM.
Conclusions
The comparative neuroanatomy of Secernosaurus koerneri within a phylogenetic framework (e.g., Prieto-Marquez 2010) including hadrosaurids from northern landmasses (e.g., A. riabinini, A. ardevoli, Edmontosaurus sp., G. notabilis) indicates that the former reunites similar features with other saurolophine species than to lambeosaurines. This study also supports a conservative brain morphology for Cretaceous hadrosaurids if compared with non-hadrosaurid ornithopods (e.g., Lautenschlager and Hübner 2013; Lauters et al. 2012). The last is not an unexpected result. These comparative conclusions are mainly related to two principal causes, evolutionary history and biased knowledge. The taxonomic affinity of Secernosaurus koerneri with Gryposaurus-related species from North America (and the resulting paleobiogeographic history of Hadrosauridae in South America) restricts the overall endocranial morphology of the former to that in its closer relatives in Kritosaurini (e.g., Prieto-Marquez 2010; Prieto-Marquez and Salinas 2010; Prieto-Marquez 2014; Prieto-Marquez et al. 2016). If the descriptions detailing the paleoneurology based solely in neurocranial features are excluded (e.g., Godefroit et al. 2008; 2012a, b), the known endocranial anatomy of hadrosaurids is mainly represented by species from North America. If considering the taxonomic diversity of Hadrosauridae, about 12 of near 50 species have at least a partial endocranial reconstruction (two from Asia, two from Europe, the one presented here from South America, and the remainder form North America), being less than 20 species within the more diverse Ornithopoda (Horner et al. 2004; Spencer 2013; Prieto-Marquez et al. 2016; Strickson et al. 2016; Cruzado-Caballero and Powell 2017; Xing et al. 2017; and references cited herein). Thus, the overall knowledge of the endocranial anatomy in Hadrosauridae is not only poorly represented if considering the known diversity and exceptionally rich fossil record of the lineage, but also is extremely biased to species from North America.
The novel anatomical information presented here increases our understanding of the cranial anatomy of Secernosaurus koerneri, in a first attempt to better understand the paleobiology of this South American representative of hadrosaurids and is a significant contribution to the brain anatomy of this clade of ornithopod dinosaurs. In addition, our understanding of the endocranial morphology of Hadrosauridae is far from being well represented. Further research is needed addressing the endocranial anatomy of species outside North America to clarify the neurocranial morphological variability and function within the clade. Future studies in the neuroanatomy of southern hadrosaurids will allow addressing if the anatomical differences between taxa of southern and northern hemispheres remained more strongly related to their phylogenetic history (supporting the conservative evolution of the brain) or followed different evolutionary pathways related to the vicariance event that leaded to the diversification of Hadrosauridae in South America. Additionally, more complete knowledge on the endocranial morphology of hadrosaurids will allow including endocranial characters in a phylogenetic framework and pair more accurately its morphological variation with the evolutionary history and the paleobiogeographic and phylogenetic patterns of diversification of the lineage.
Acknowledgements
The authors would like to thank Alejandro G. Kramarz (MACN) for the access to the specimens under his care and for allowing the CT scanning. We are thankful to the staff of the Clínica la Sagrada Familia (Buenos Aires, Argentina) for the access to the CT scanner, as well as the valuable help of Julia B. Desojo (Universidad Nacional de La Plata, Buenos Aires, Argentina) and Alejandro G. Kramarz to achieve the proposed objectives. The comments and revisions made by Albert Prieto-Marquez (University of Bristol, Bristol, UK) and Stephan Lautenschlager (University of Birmingham, Birmingham, UK) improved the content of this manuscript, and are here thanked. This research was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina), under the grants PICT 2016-0481 (to AP-C) and PICT 2016-0419 (to PC-C), CONICET under the grant PIP 0733 (to AP-C), the Universidad Nacional de Río Negro under the grant PI UNRN 40-A-572 (to PC-C), the Spanish Ministerio de Ciencia e Innovación and the European Regional Development Fund (CGL2017-85038-P; to PC-C), PUE-2016 CONICET to CICTERRA (JRAT), and The Jurassic Foundation (to MGB).
References
Ali, F., Zelenitsky, D.K., Therrien, F., and Weishampel, D.B. 2008. Homology of the “ethmoid complex” of tyrannosaurids and its implications for the reconstruction of the olfactory apparatus of non-avian theropods. Journal of Vertebrate Paleontology 28: 123–133. Crossref
Bonaparte, J.F., Franchi, M.R., Powell, J.E., and Sepulveda, E.G. 1984. La Formación Los Alamitos (Campaniano–Maastrichtiano) del sudeste de Río Negro, con descripción de Kritosaurus australis n. sp. (Hadrosauridae). Significado paleogeográfico de los vertebrados. Revista de la Asociación Geológica de Argentina 39: 284–299.
Brasier, M.D., Norman, D.B., Liu, A.G., Cotton, L.J., Hiscocks, J.E., Garwood, R.J., Antcliffe, J.B., and Wacey, D. 2016. Remarkable preservation of brain tissues in an Early Cretaceous iguanodontian dinosaur. Geological Society, London, Special Publications 448: 383–398. Crossref
Coria, R.A. 2014. South American hadrosaurs: considerations on their diversity. In: D.A. Eberth and D.C. Evans (eds.), Hadrosaurs, 332–339. Indiana University Press, Bloomington.
Cruzado-Caballero, P. 2017. New hadrosaurid remains from the Late Cretaceous of Río Negro Province (Argentina, Late Cretaceous). Journal of Iberian Geology 43: 307–318. Crossref
Cruzado-Caballero, P. and Powell, J. 2017. Bonapartesaurus rionegrensis, a new hadrosaurine dinosaur from South America: implications for phylogenetic and biogeographic relations with North America. Journal of Vertebrate Paleontology 37: e1289381. Crossref
Cruzado-Caballero, P., Filippi, L.S., Méndez, A.H., Garrido, A.C., and Díaz-Martínez, I. 2017. First ornithopod remains from the Bajo de la Carpa Formation (Santonian, Upper Cretaceous), northern Patagonia, Argentina. Cretaceous Research 83: 182–193. Crossref
Cruzado-Caballero, P., Fortuny, J., Llacer, S., and Canudo, J.I. 2015. Paleoneuroanatomy of the European lambeosaurine dinosaur Arenysaurus ardevoli. PeerJ 3: e802. Crossref
Evans, D.C. 2005. New evidence on brain-endocranial cavity relationships in ornithischian dinosaurs. Acta Palaeontologica Polonica 50: 617–622.
Evans, D.C., Ridgely, R., and Witmer, L.M. 2009. Endocranial anatomy of lambeosaurine hadrosaurids (Dinosauria: Ornithischia): a sensorineural perspective on cranial crest function. The Anatomical Record 292: 1315–1337. Crossref
Farke, A.A., Chok, D.J., Herrero, A., Scolieri, B., and Werning, S. 2013. Ontogeny in the tube-crested dinosaur Parasaurolophus (Hadrosauridae) and heterochrony in hadrosaurids. PeerJ 1: e182. Crossref
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J.C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F., Sonka, M., and Buatti, J. 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging 30: 1323–1341. Crossref
Gilmore, C.W. 1937. On the detailed skull structure of a crested hadrosaurian dinosaur. Proceedings of the United States National Museum 3027: 481–491. Crossref
Godefroit, P., Bolotsky, Y.L., and Bolotsky, I.Y. 2012a. Osteology and relationships of Olorotitan arharensis, a hollow-crested hadrosaurid dinosaur from the latest Cretaceous of Far Eastern Russia. Acta Palaeontologica Polonica 57: 527–560. Crossref
Godefroit, P., Bolotsky, Y.L., and Lauters, P. 2012b. A new saurolophine dinosaur from the latest Cretaceous of far Eastern Russia. PLoS One 7: e36849. Crossref
Godefroit, P., Escuillié, F., Bolotsky, Y.L., and Lauters, P. 2012c. A new basal hadrosauroid dinosaur from the Upper Cretaceous of Kazakhstan. In: P. Godefroit (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems, 335–358. Indiana University Press, Bloomington.
Godefroit, P., Shulin, H., Tingxiang, Y., and Lauters, P. 2008. New hadrosaurid dinosaurs from the uppermost Cretaceous of northeastern China. Acta Palaeontologica Polonica 53: 47–74. Crossref
Hopson, J.A. 1979. Paleoneurology. In: C. Gans (ed.), Biology of the Reptilian, Vol. IX., 39–146. Academic Press, New York.
Horner, J.R., Weishampel, D.B., and Forster, C.A. 2004. Hadrosauridae. In: D.B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria, 2nd edition, 438–463. University of California Press, Berkeley. Crossref
Lambe, L.M. 1920. The hadrosaur Edmontosaurus from the Upper Cretaceous of Alberta. Geological Survey of Canada, Memoir 120: 1–79. Crossref
Langston, Jr W. 1960. The vertebrate fauna of the Selma Formation of Alabama. Pt. VI. The Dinosaurs. Fieldiana Geology Memoirs 3: 313–361.
Lautenschlager, S. and Hübner, T. 2013. Ontogenetic trajectories in the ornithischian endocranium. Journal of Evolutionary Biology 26: 2044–2050. Crossref
Lauters, P., Coudyzer, W., Vercauteren, M., and Godefroit, P. 2012. The brain of Iguanodon and Mantellisaurus: perspectives on ornithopod evolution. In: P. Godefroit (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems, 213–224. Indiana University Press, Bloomington.
Lauters, P., Vercauteren, M., Bolotsky, Y.L., and Godefroit, P. 2013. Cranial endocast of the lambeosaurine hadrosaurid Amurosaurus riabinini from the Amur Region, Russia. PLoS One 8: e78899. Crossref
Marsh, O.C. 1893. The skull and brain of Claosaurus. American Journal of Science 265: 83–86. Crossref
Nopcsa, F. 1900. Dinosaurierreste aus Siebenbergen: Schadel von Limnosaurus transsylvanicus nov. gen. et specie. Denkschriften der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse 65: 555–591.
Ostrom, J.H. 1961. Cranial morphology of the hadrosaurian dinosaurs of North America. Bulletin of the American Museum of Natural History 122: 37–186.
Paulina-Carabajal, A. and Filippi, L. 2018. Neuroanatomy of the abelisaurid theropod Viavenator: The most complete reconstruction of cranial endocast and inner ear for a South American representative of the clade. Cretaceous Research 83: 84–94. Crossref
Paulina-Carabajal, A., Lee, Y.-N., and Jacobs, L.L. 2016. Neuroanatomy of the primitive nodosaurid dinosaur Pawpawsaurus campbelli and paleobiological implications of some endocranial features. PLoS One 11: e0150845. Crossref
Paulina-Carabajal, A., Sterli, J., Georgi, J., and Poropat, S. 2017. Comparative neuroanatomy of extinct horned turtles (Meiolaniidae) and extant terrestrial turtles (Testudinoidea), with comments on the paleobiological implications of selected endocranial features. Zoological Journal of the Linnean Society 180: 930–950. Crossref
Prieto-Marquez, A. 2010. Global phylogeny of Hadrosauridae (Dinosauria: Ornithopoda) using parsimony and Bayesian methods. Zoological Journal of the Linnean Society 159: 435–502. Crossref
Prieto-Márquez, A. 2014. Skeletal morphology of Kritosaurus navajovius (Dinosauria: Hadrosauridae) from the Late Cretaceous of the North American south-west, with an evaluation of the phylogenetic systematics and biogeography of Kritosaurini. Journal of Systematic Palaeontology 12: 133–175. Crossref
Prieto-Marquez, A. and Salinas, G.C. 2010. A re-evaluation of Secernosaurus koerneri and Kritosaurus australis (Dinosauria, Hadrosauridae) from the Late Cretaceous of Argentina. Journal of Vertebrate Paleontology 30: 813–837. Crossref
Prieto-Marquez, A., Erickson, G.M., and Ebersole, J.A. 2016. A primitive hadrosaurid from southeastern North America and the origin and early evolution of “duck-billed” dinosaurs. Journal of Vertebrate Paleontology 36: e1054495. Crossref
Saveliev, S.V., Alifanov, V.R., and Bolotsky, Y.L. 2012. Brain anatomy of Amurosaurus riabinini and some neurobiological peculiarities of duck-billed dinosaurs. Paleontological Journal 46: 79–91. Crossref
Serrano-Brañas, C.I., Hernández-Rivera, R., Torrez-Rodríguez, E., and Espinoza Chávez, B. 2006. A natural hadrosaurid endocast from the Cerro del Pueblo Formation (Upper Cretaceous) of Coahuila, Mexico. In: S.G. Lucas and R.M. Sullivan (eds.), Late Cretaceous Vertebrates from the Western Interior. New Mexico Museum of Natural History and Science Bulletin 35: 317–322.
Sharp, A.C., Siu, K., and Rich, T.H. 2017. Revealing the skeleton of the polar dinosaur Leaellynasaura amicagraphica using synchrotron computed tomography. In: A. Farke, A. MacKenzie, and J. Miller-Camp (eds.), Society of Vertebrate Paleontology 77th Annual Meeting, Meeting Program and Abstracts, 193. Society of Vertebrate Paleontology, Calgary.
Spencer, M.R. 2013. Phylogenetic and Biogeographic Assessment of Ornithischian Diversity Throughout the Mesozoic: A Species-level Analysis from Origin to Extinction. 296 pp. Unpublished Ph.D. Thesis, University of Iowa, Iowa City.
Strickson, E., Prieto-Márquez, A., Benton, M.J., and Stubbs, T.L. 2016. Dynamics of dental evolution in ornithopod dinosaurs. Scientific Reports 6: 28904. Crossref
Thomas, D.A. 2015. The cranial anatomy of Tenontosaurus tilletti Ostrom, 1970 (Dinosauria, Ornithopoda). Palaeontologia Electronica 18: 1–99. Crossref
Witmer, L.M., Ridgely, R.C., Dufeau, D.L., and Semones, M.C. 2008. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. In: H. Endo and R. Frey (eds.), Anatomical Imaging, Towards a New Morphology, 67–87. Springer, Tokyo.
Xing, H., Mallon, J.C., and Currie, M.L. 2017. Supplementary cranial description of the types of Edmontosaurus regalis (Ornithischia: Hadrosauridae), with comments on the phylogenetics and biogeography of Hadrosaurinae. PLoS One 12: e0175253. Crossref
Zelenitsky, D.K., Therrien, F., and Kobayashi, Y. 2009. Olfactory acuity in theropods: palaeobiological and evolutionary implications. Proceedings of the Royal Society of London B Series 276: 667–673. Crossref
Zelenitsky, D.K., Therrien, F., Ridgely, R.C., McGee, A., and Witmer, L. 2011. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proceedings of the Royal Society of London B Series 278: 3625–3634. Crossref
Acta Palaeontol. Pol. 63 (4): 693–702, 2018
https://doi.org/10.4202/app.00526.2018