

Silurian myodocope ostracods from Poland
VINCENT PERRIER, EWA OLEMPSKA, DAVID J. SIVETER, MARK WILLIAMS, and NICOLAS LEGIOT
Perrier, V., Olempska, E., Siveter, D.J., Williams, M., and Legiot, N. 2019. Silurian myodocope ostracods from Poland. Acta Palaeontologica Polonica 64 (2): 379–397.
Newly collected material reveals that the Silurian myodocope ostracods from the Holy Cross Mountains, Poland comprise ten species (one new to science) belonging to four families: Bolbozoidae, Entomozoidae, Rhomboentomozoidae, and Cypridinidae. Biostratigraphic control using graptolites indicates that all three Polish outcrops investigated are of about the same chronostratigraphical level: middle Gorstian, lower Ludlow. The new occurrences in Poland extend the known distribution of several species and reinforce data that show many Silurian myodocope species with wide dispersal. Our new observations on the Holy Cross Mountains material confirm that the occurrences of Silurian myodocopes are mostly associated with pelagic animals and with rocks ranging from mudstone, siltstone or shale deposited in open- or deep-shelf marine settings. The cosmopolitan distribution of these ostracods, coupled with their facies and faunal associations, supports the notion of an ostracod (myodocope) ecological shift from benthic to planktonic habitats during the late Wenlock and Ludlow.
Key words: Ostracoda, Myodocopa, Silurian, Ludlow, Poland, Holy Cross Mountains.
Vincent Perrier [vincent.perrier@univ-lyon1.fr] and Nicolas Legiot [nicolas.legiot@etu.univ-lyon1.fr], Université de Lyon, UCBL, ENSL, CNRS, LGL-TPE, 69622 Villeurbanne, France.
Ewa Olempska [olempska@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warsaw, Poland.
David J. Siveter [djs@leicester.ac.uk] and Mark Williams [mri@leicester.ac.uk], School of Geography, Geology and the Environment, University of Leicester, Leicester LE1 7RH, UK.
Received 24 September 2018, accepted 5 December 2018, available online 18 March 2019.
Copyright © 2019 V. Perrier et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
This paper is part of series of works, initiated during the 1980s, that focuses on the (Silurian) origin of zooplanktonic ostracods. The idea that some Silurian ostracods represented pioneer zooplankton was developed when taxonomic, distributional and facies studies of British and other European myodocope faunas demonstrated that some had undergone a benthic to pelagic ecological shift during Silurian times (Siveter 1984; Siveter et. al. 1987, 1991; Siveter and Vannier 1990; Vannier and Abe 1992; Perrier et al. 2015). More recent studies have endorsed the idea regarding the shift in the mode of life of some Silurian myodocopes and have strengthened and expanded knowledge of their systematics, habitats, lifestyles, and their biostratigraphical and palaeogeographical utility (Perrier et al. 2007, 2011, 2014a–c, 2019; Perrier 2012; Perrier and Siveter 2013). These studies are underpinned by the discovery of five exceptionally preserved myodocope ostracod species from the Silurian Herefordshire Lagerstätte (Welsh Borderland, UK), which provide unrivalled data on the soft-anatomy and palaeobiology of ancient representatives of the group (Siveter et al. 2003, 2007, 2010, 2013, 2015, 2018).
Research on Silurian myodocope ostracods from Poland began at the end of the 19th century, when Gürich (1896) recorded two species of “Entomis” and Bolbozoe in the Silurian of the Holy Cross Mountains, Poland, from the so-called “Interrupta-Schiefern”. For more than 100 years, these faunas remained essentially unstudied. They were only mentioned briefly by Stupnicka et al. (1991), who identified four different species of bolbozoids and entomozoids at a new locality. The material studied for the present paper was collected over the last 30 years by Polish scientists and by our new field work that yielded additional material from the localities of Gürich (1896) and Stupnicka et al. (1991); see Geological Setting. Ten myodocope species are identified herein from the Silurian of Poland.
Institutional abbreviations.—FSL, Université Claude Bernard Lyon 1, Lyon, France; GSM, British Geological Survey, Keyworth, UK; I, Museo de Storia Natural e del Territorio, Calci, Italy; LPB, University of Brest, Brest, France; NM, Národní Museum, Prague, Czech Republic; OUMNH, Oxford University Museum of Natural History, Oxford, UK; UMC-IP-VP, University of Montpellier, Montpellier, France; ZPAL, The Polish Academy of Science, Warsaw, Poland.
Other abbreviations.—H, height; L, length.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:act:01C7B8C0-33F5-4067-939B-1568F17AB8C9
Material and methods
The Polish myodocopes occur as internal and external moulds in laminated brownish-grey shaly limestone beds. Rock matrix was removed from the specimens mechanically using fine needles. Casts of external moulds of the ostracods recovered were made with silicone rubber (Silicoset 105) using the techniques of Siveter (1982). Specimens and casts were coated with a thin layer of ammonium chloride and photographed using a Leitz Aristophot mounted with a Canon EOS 5D camera following the methods of Siveter (1990). Stereo-pairs were used for the description of a new species Nudator elegantulus sp. nov. and for the revision of Rhomboentomozoe rhomboidea. For scanning electron microscopy, specimens were mounted on aluminum stubs and coated with gold/aluminum alloy using an “Emitech K500X” and photographed with a HITACHI S-3600N scanning electron microscope at Leicester University. Specimens were measured using a micrometer under a Leica M80 binocular microscope.
Silurian myodocopes possess a bivalved carapace that is typically presumed to have been lightly calcified, with a probable ligamentous dorsal connection (see Perrier 2012). The moulds of each valve in many specimens preserves an adductor muscle scar/spot sub-centrally, corresponding to the site of internal attachment of the adductor muscle. The surface of the carapace, preserved via the external mould, may be smooth or have a range of types of ornament, including reticulation, corrugation and punctuation. The carapace surface shows, in some cases, post-mortem diagenetic features (“rosettes” of Siveter et al. 1987). The morphological terminology and measurements (including the adductorial muscle position) follow Siveter et al. (1987) and Perrier et al. (2019).
Geological settings
Silurian myodocopes from Poland were originally documented by Gürich (1896) from “Interrupta-Schiefern” layers at several sites in the Holy Cross Mountains, central Poland, such as Niestachów, Kleczanów, Zalesie, and Brzezinki. Most of these localities are now lost or inaccessible and the material has been lost during World War II. We had access to recently collected material and made new collections (2014) of Silurian myodocopes from three localities in the Holy Cross Mountains (Fig. 1).
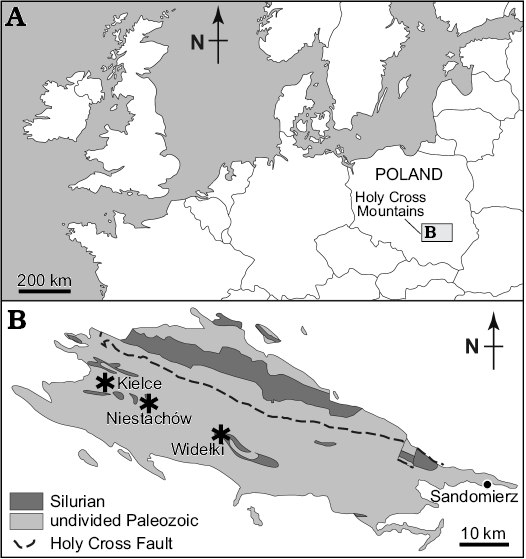
Fig. 1. Distribution of Silurian myodocope-bearing localities in the Holy Cross Mountains, Poland. A. Location of the Holy Cross Mountains. B. Myodocope-bearing localities (asterisks) sampled in the Holy Cross Mountains (geological map modified from Kozłowski et al. 2014).
Widełki.—Two exposures, 10 m apart, along a small track about 400 m from the road at Widełki village, 20 km SE of Kielce, Holy Cross Mountains. Together with material obtained in 2014, we studied specimens collected from Widełki by Barbara Żbikowska (Polish Geological Institute; 1991) and from a trench by Jan Malec (Holy Cross Division of the Geological Institute; 2005). Fossiliferous, laminated brownish-grey shaly limestone; Niewachlów Beds, lower part of the Ludlow Series based on graptolites (Stupnicka et al. 1991). Ostracods occur as flattened and disarticulated valves. The myodocope fauna includes Bolbozoe anomala, Bolbozoe psittaca, Nudator angiportatus, Nudator elegantulus sp. nov., Parabolbozoe bohemica, Richteria migrans, Rhomboentomozoe rhomboidea, and Silurocypridina calva. Associates include graptolites, cephalopods, and bivalves.
Niestachów.—Four field-side exposures several 10s of metres apart, about 500 m from the road at Niestachów Village, c. 1.5 km SE of Kielce, Holy Cross Mountains. Together with material collected in 2014, we studied specimens obtained from Niestachów by EO in 2014. Fossiliferous, laminated brownish-grey shaly limestone; Niewachlów Beds, lower part of the Ludlow Series based on graptolites (probably Cucullograptus (Lobograptus) scanicus Biozone; see Tomczyk 1956: fig. 7 upper). Ostracods are dominant and occur as flattened and disarticulated valves. The myodocope fauna includes Bolbozoe anomala, Bolbozoe psittaca, Nudator angiportatus, Nudator elegantulus sp. nov., Nudator inflatus, Richteria migrans, Rhomboentomozoe rhomboidea, Silurocypridina calva, and Silurocypridina retroreticulata. Associates include graptolites, cephalopods and bivalves. Part of Gurich’s (1896) material is from Niestachów, but precise localities are unknown.
Kielce.—Material collected by Jan Malec in 2014 (Malec 1993: fig. 2) during road work in Skrajna Street, Kielce, Holy Cross Mountains. Fossiliferous, laminated brownish-grey shaly limestone; Niewachlów Beds, lower part of the Ludlow Series based on graptolites (Cucullograptus [Lobograptus] scanicus Biozone; see Malec 1993: fig. 4 upper, locality A). Ostracods are abundant and occur as flattened and disarticulated valves. The myodocope fauna includes Bolbozoe anomala and Parabolbozoe bohemica. Associates include graptolites and cephalopods.
Systematic palaeontology
Class Ostracoda Latreille, 1802
Subclass Myodocopa Sars, 1866
Order Myodocopida Sars, 1866
Suborder Myodocopina Sars, 1866
Remarks.—The overwhelming majority of Myodocopa are Recent species that are defined on soft-part morphology. All known fossil myodocopes are assigned to the order Myodocopida; halocyprid myodocopes are unknown from the fossil record. The suborder Paleomyodocopina Kornicker and Sohn, 2000 was established for those fossil myodocopids that were known only from their carapaces in which a sub-central to anterodorsal node/bulb was present: namely, the Cypridinelliformacea, Nodophilomedacea, and Swainellacea. The Bolbozoidea (Silurian–Devonian) also have an anterodorsal bulb and therefore qualify for inclusion within that suborder. However, new knowledge of the appendages and other soft-parts of fossil myodocopids from the Ordovician and Silurian (Siveter et al. 2003, 2007, 2010, 2013, 2014, 2015, 2018) has fundamentally questioned the utility of carapace-based taxonomy alone and therefore the suborder Paleomyodocopina is not used here.
Stratigraphic and geographic range.—Ordovician to Recent; worldwide.
Superfamily Bolbozooidea Bouček, 1936 (sensu Bolbozoacea Bouček, 1936)
Family Bolbozoidae Bouček, 1936
1936 Bolbozoidae n.f.; Bouček 1936: 62.
1951 Entomozoidae nov. nom.; Přibyl 1951: 4 (= Entomidae Jones, 1873).
1990 Bolbozoidae Bouček, 1936; Siveter and Vannier 1990: 48.
2019 Bolbozoidae Bouček, 1936; Perrier et al. 2019: 27.
Included genera: Bolbozoe Barrande, 1872; Entomozoe Přibyl, 1951 (pro Entomis Jones, 1861; not Herrich-Schaffer, 1856); Oculparva Perrier et al. 2019; Parabolbozoe Přibyl, 1988.
Stratigraphic and geographic range.—Lower Silurian (Entomozoe tuberosa, Llandovery Series, Scotland; Siveter and Vannier 1990) to Lower Devonian (Bolbozoe largiglobosa, Emsian Stage, China; Wang and Zhang 1983).
Genus Bolbozoe Barrande, 1872
Type species: Bolbozoe anomala Barrande, 1872; subsequent designation by Bassler and Kellett, 1934. Ludlow Series, Silurian, Prague, Czech Republic.
Species included: Type species and Bolbozoe acuta Perrier, Vannier, and Siveter, 2011, Bolbozoe beccata Perrier, Siveter, Williams, Strusz, Steeman, Verniers, and Vandenbroucke, 2014c, Bolbozoe largiglobosa Wang and Zhang, 1983, Bolbozoe parvafraga Perrier, Vannier, and Siveter, 2011, Bolbozoe psittaca Perrier, Siveter, Williams, and Palmer, 2019, Bolbozoe rugosa Perrier, Vannier, and Siveter, 2011, Bolbozoe sp. nov. A of Perrier et al. 2019, Bolbozoe sp. nov. B of Perrier et al. 2019, and possibly Bolbozoe jonesi Barrande, 1872.
Stratigraphic and geographic range.—Upper part of the Wenlock Series, Silurian, to Emsian Stage, Devonian; Czech Republic (Perrier et al. 2011), France (Perrier et al. 2011), Sardinia (Gnoli et al. 2009), China (Wang 2009), Australia (Perrier et al. 2014c), Wales and England (Perrier et al. 2019) and Poland.
Bolbozoe anomala Barrande, 1872
Fig. 2A, B.
1861 E. divisa MSS.; Jones (in Salter) in Howell and Geikie 1861: 137 (GSM 36876).
1868 Entomis divisa Jones; Bigsby 1868: 74.
1868 Bolbozoe anomala Barr.; Bigsby 1868: 200.
1870 Entomis divisa Jones; Jones 1870: pl. 61: 12 (GSM 36876).
1872 Bolboz. anomala Barr.; Barrande 1872: 501, pl. 24: 27, 28 (NM-L 13993), 29, 30 (NM-L 23572).
1872 Bolboz. Jonesi Barr.; Barrande 1872: 503, pl. 27: 8 (NM-L 23650 [ex. NM-1600]); pl. 31: 4–6 (NM-CF 1242).
1873 Entomidella divisa Jones; Jones 1873: 416 (GSM 36876).
1878 Entomis divisa Jones; Salter 1878: 130 (GSM 36876).
1884 Bolbozoe divisa Jones; Jones 1884: 401, pl. 15: 4 (GSM 36876).
1900 Bolbozoe (?) italica n. f.; Canavari 1900: 208, pl. 26: 16 (I 97a), 17 (I 97b), 18 (I 98).
1900 Bolbozoe (?) capellinii n. f.; Canavari 1900: 208, pl. 26: 19.
1900 Bolbozoe (?) lanceolata n. f.; Canavari1900: pl. 26: 20 (I 99).
1934 Bolbozoe anomala Barrande; Bassler and Kellett 1934: 215.
1934 Bolbozoe (?) lanceolata Canavari; Bassler and Kellett 1934: 215.
1934 Bolbozoe (?) capellinii Canavari; Bassler and Kellett 1934: 215.
1934 Bolbozoe (?) italica Canavari; Bassler and Kellett 1934: 215.
1936 Bolbozoe anomala Barrande; Bouček 1936: 62.
1937 Bolbozoe (?) lanceolata Canavari; Chaubet 1937: pl. 7: 19 (UMC-IP-VP 7).
1954 Bolbozoe anomala Barrande; Hughes 1954: 42.
1958 Bolbozoe anomala Barrande; Pokorný 1958: 315.
1961 Bolbozoe anomala Barrande; Sylvester-Bradley 1961: 395, text-fig. 316.1c–f.
1985 gen. nov. A sp. nov. 1; Lethiers et al. 1985: 57, pl. 5: 1.
1985 gen. nov. A sp. nov. 2; Lethiers et al. 1985: 57, pl. 5: 3.
1985 gen. nov. C sp. nov. 1; Lethiers et al. 1985: 57, pl. 5: 4.
1987 Bolbozoe cf. anomala Barrande; Siveter et al. 1987: pl. 85: 3, 8–11; pl. 86: 1–6, pl. 87: 8, text-fig. 7.
1988 Bolbozoe (Bolbozoe) anomala Barrande; Přibyl 1988: 119.
1988 Bolbozoe (Bolbozoe) jonesi Barrande; Přibyl 1988: 118.
1990 Bolbozoe cf. anomala Barrande, 1872; Siveter and Vannier 1990: text-fig. 7c–d.
1990 “Bolbozoe” cf. anomala Barrande; Siveter and Vannier 1990: text-figs. 13C, 13F, 23.
1991 Bolbozoe cf. anomala Barrande; Siveter et al. 1991: pl. 1: 1; pl. 2: 2, 5.
1992 “Bolbozoe” cf. anomala Barrande; Vannier and Abe 1992: pl. 2: 1; text-fig. 1A.
2009 Bolbozoe anomala Barrande, 1872; Gnoli et al. 2009: pl. 2: 1–15.
2011 Bolbozoe anomala Barrande, 1872; Perrier et al. 2011: pl. 1: 1–20; text-figs. 6, 7H, 8, 9B, 10.
2012 Bolbozoe anomala Barrande, 1872; Perrier 2012: text-fig. 7C, D.
2013 Bolbozoe anomala Barrande, 1872; Perrier and Siveter 2013: text-fig. 22.6C.
2019 Bolbozoe anomala Barrande, 1872; Perrier et al. 2019: pl. 3: 1–18.
Type material: Lectotype: a right valve, NM-L 23572 (ex. CE1194); Barrande (1872: pl. 24: 29, 30); Perrier et al. (2011: pl. 1: 1); designated by Přibyl (1988: 119). Paralectotype: a left valve, NM-L 13993; Barrande (1872: pl. 24: 27, 28); Perrier et al. (2011: pl. 1: 2); designated by Přibyl 1988: 119. All from type locality.
Type locality: Lochkov suburb of Prague, Czech Republic.
Type horizon: Požáry Formation, Pridoli Series, stratigraphical division e2 of Barrande 1872 (Kříž 1992).
Material.—Tens of valves, from three localities (Widełki, Niestachów, and Kielce), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 1.8, H = 1.25 (ZPAL O.63/24) to L = 8.25, H = 6 (ZPAL O.63/25). Lectotype: L = 8.50, H = 5.95 (NM-L 23572). Paralectotype: L = 7.10, H = 4.95 (NM-L 13993). L/H ratio and adductor muscle position for four specimens from two localities (Fig. 3).

Fig. 2. Selected Silurian myodocope species, Holy Cross Mountains, Poland; Ludlow Series, Silurian. A, B. Bolbozoe anomala Barrande, 1872. A. ZPAL O.63/1, incomplete left valve. B. ZPAL O.63/2, juvenile right valve. C. Bolbozoe psittaca Perrier, Siveter, Williams, and Palmer, 2019, ZPAL O.63/3, left valve. D, E. Parabolbozoe bohemica (Barrande, 1872). D. ZPAL O.63/4, juvenile left valve. E. ZPAL O.63/5, right valve. F. Richteria migrans (Barrande, 1872), ZPAL O.63/6, left valve. G, H. Nudator angiportatus Perrier, Siveter, Williams, and Palmer, 2019. G. ZPAL O.63/7, juvenile left valve. H. ZPAL O.63/8, left valve. I, J. Nudator inflatus Perrier, Siveter, Williams, and Palmer, 2019. I. ZPAL O.63/9, right valve showing post-mortem diagenetic structures. J. ZPAL O.63/9, right valve. K. Silurocypridina retroreticulata Perrier, Vannier, and Siveter, 2011, ZPAL O.63/10, incomplete right valve. L. Silurocypridina calva Perrier, Vannier, and Siveter, 2011, ZPAL O.63/11, right valve. M. Cypridinid?, ZPAL O.63/12, right valve. All light photographs in lateral view. A–H, J, M, silicone casts of external moulds; I, K, L, internal moulds. Scale bars 1 mm, except M 2 mm.
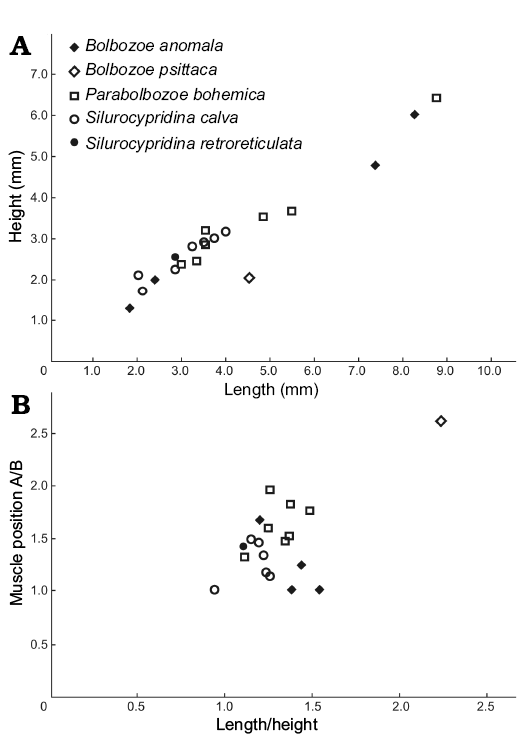
Fig. 3. Valve length/height plot (A) and position of the adductor muscle scar (A/B, where A and B are the distances between the muscle scar and the posterior end of the valve and the ventral margin, respectively) against length/height (B) of complete specimens of bolbozoid and cypridinid species from the Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Description.—Adult valve sub-ovoid, slightly tapering posteriorly. Anterior third of valve mostly occupied by a large hemispherical bulb forming c. 20–25% of valve area; bulb-centre lies just above valve mid-height and well above other surface areas of valve. Maximum valve length is just above bulb mid-height; maximum valve height is at mid-length and maximum valve width is at central part of bulb. Right valve overlaps left valve along ventral margin. A deep, narrow sulcus surrounds the bulb posteriorly and ventrally. An adductor muscle scar consisting of a single series of 7–8 inclined, almost parallel individual scars including about seven furrows, occurs at mid-length in the sulcus. Rostrum is very small, in some cases beak-like or absent. Rostral incisure slit-like, as seen in 3D preserved carapaces in anterior view. Early growth stages are more rounded in valve outline and the bulb is relatively larger than in adults.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains. Also known from the upper part of the Wenlock to at least the middle part of the Pridoli Series; the Czech Republic, France, Sardinia (Perrier et al. 2011), Wales and England (Perrier et al. 2019). If Bolbozoe jonesi is conspecific with Bolbozoe anomala the range of the species would extend into the Lochovian Series, Lower Devonian (see Perrier et al. 2011).
Bolbozoe psittaca Perrier, Siveter, Williams, and Palmer, 2019
Fig. 2C.
2019 Bolbozoe psittaca sp. nov.; Perrier et al. 2019: pl. 4: 1–13.
Holotype: A laterally compressed left valve (OUMNH 35080); Perrier et al. 2019: pl. 4: 1–4.
Type locality: Radnorshire (loc. 12 of Perrier et al. 2019), Powys, Wales, UK.
Type horizon: “Striped Flags” of Kirk 1947, Cucullograptus (Lobograptus) scanicus Biozone, Gorstian Stage, Ludlow Series (Perrier et al. 2019).
Material.—Five valves from two localities (Widełki and Niestachów), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 4.5, H = 2 (ZPAL O.63/3). Holotype: L = 5.15, H = 2.5 (OUMNH 35080). L/H ratio and adductor muscle position for one complete specimen (Fig. 3).
Description.—Lateral outline of adult valve is elongate ovoid, strongly tapered posteriorly. Anterior third of valve mostly occupied by large hemispherical bulb that forms the most prominent feature of valve; its diameter in adults is about one-third of valve length and its center is at valve mid-height. Maximum valve length is at about mid-height; maximum valve height is at about mid-length; maximum valve width is at the central part of bulb. A narrow sulcus surrounds the bulb almost entirely except anteriorly where bulb merges into rostrum without clear demarcation. Sulcus is widest at and above presumed site of (unknown) adductor muscle scar. Valve surface smooth.
Stratigraphic and geographic range.—Ludlow Series of the Holy Cross Mountains, Poland, England, and Wales (Perrier et al. 2019).
Genus Parabolbozoe Přibyl, 1988
Type species: Bolbozoe bohemica Barrande, 1872; by monotypy. Ludlow Series, Silurian, Prague, Czech Republic.
Species included: Type species and Parabolbozoe armoricana Perrier, Vannier, and Siveter, 2011 and Parabolbozoe britannica Perrier, Siveter, Williams, and Palmer, 2019.
Stratigraphic and geographic range.—Upper part of the Wenlock to Ludlow or Pridoli Series; Czech Republic (Perrier et al. 2011), France (Perrier et al. 2011), Sardinia (Gnoli et al. 2009), Wales and England (Perrier et al. 2019), and Poland.
Parabolbozoe bohemica (Barrande, 1872)
Fig. 2D, E.
1868 Bolbozoe Bohemica Barr.; Bigsby 1868: 200.
1872 Bolbozoe Bohemica Barr.; Barrande 1872: 502, pl. 27: 14 (= same specimen as fig. 17 upper) and 17 lower (= 2 specimens on slab NM-CE1192), 15, 16, 18, 20 (= NM original specimen numbers 1608, 1609, 1611, 1613), 19 (holotype, specimen NM-L 23658 [ex. CE1193]).
1896 Bolbozoe polonica nov. sp.; Gürich 1896: 378, pl. 15: 12a–c.
1898 Cypridina? polonica (Bolbozoe, Gürich); Jones1898: 339, pl. 17: 16.
1900 Bolbozoe (?) bohemica Barr.; Canavari 1900: 205, text-fig. 15a–c (specimen I 96).
1934 Bolbozoe bohemica Barrande; Bassler and Kellett 1934: 215.
1936 Bolbozoe bohemica Barrande; Bouček 1936: 63, text-fig. 4.
1937 Bolbozoe (?) bohemica Barr.; Chaubet 1937: 188, pl. 7: 18 a, 18b (specimens UMC-IP-VP 1, UMC-IP-VP 6).
1954 Bolbozoe bohemica Barrande; Hughes 1954: 42.
1958 Bolbozoe bohemica Barrande; Pokorný 1958: 315, text-fig. 1044.
1961 Bolbozoe bohemica Barrande; Sylvester-Bradley 1961: 396, text-figs. 316.1a, 18b; ?316.1g–i.
1984 Bolbozoe bohemica Barrande; Siveter 1984: pl. 2: 7–11; text-figs. 2, 3.
1987 “Bolbozoe” cf. bohemica Barrande; Siveter et al. 1987: pl. 84: 6–8, pl. 87: 6, 7; text-fig. 5E, F.
1988 Bolbozoe (Parabolbozoe) bohemica Barrande; Přibyl 1988: 120.
1990 “Bolbozoe” bohemica Barrande; Siveter and Vannier 1990: text-fig. 7g–i, 17, 23.
1991 Parabolbozoe cf. bohemica Barrande; Siveter et al. 1991: pl. 2: 9, 11, pl. 3: 2, 6.
1992 “Bolbozoe” cf. bohemica Barrande; Vannier and Abe 1992: text-fig. 1B.
2009 Parabolbozoe bohemica (Barrande, 1872); Gnoli et al. 2009: pl. 2: 18, 22–27, ?16, ?17, ?19–21.
2009 Parabolbozoe bohemica (Barrande, 1872); Siveter 2009: 54, pl. 8: 9.
2011 Parabolbozoe bohemica (Barrande, 1872); Perrier et al. 2011: pl. 3: 1–9, 18, 19, text-figs. 6, 7F, 8A.
2013 Parabolbozoe bohemica Barrande, 1872; Perrier and Siveter 2013: text-fig. 22.6B.
2015 Parabolbozoe bohemica? Barrande, 1872; Williams et al. 2015: text-fig. 2k–l.
2019 Parabolbozoe bohemica (Barrande, 1872); Perrier et al. 2019: pl. 6: 1–13; pl.7: 1–10.
Lectotype: A left valve, NM-L 23658 (ex. CE1193); Barrande (1872: pl. 27: 19, in reverse); Perrier et al. (2011: pl. 3: 1); designated by Bouček (1936: 63).
Type locality: Praha-Malá Chuchle, Vyskočilka, Czech Republic.
Type horizon: Kopanina Formation, Ludlow Series (stratigraphical division e2 of Barrande 1872; horizon with the trilobite “Cromus” beaumonti).
Material.—Tens of valves from two localities (Niestachów and Kielce), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2.95, H = 2.35 (ZPAL O.63/26) to L = 8.75, H = 6.35 (ZPAL O.63/5; Fig. 2E). Lectotype: L = 11.25, H = 8.10 (NM-L 23658). L/H ratio and adductor muscle position for seven specimens from one locality (Fig. 3).
Description.—Adult valve is sub-ovoid in lateral outline, gently curved dorsally and ventrally, posteriorly tapering slightly. Bulb is large, anterodorsal, has centre above valve mid-height, extends posteriorly to almost line of maximum valve height and ventrally to below valve mid-height; outline is sub-circular, with a slight indentation opposite the rostrum. Rostrum is large, hook-like, generally with a pointed end. Rostral incisure is well developed, below which the valve has a forward pointing projection. Caudal process well developed, pointed in early instars, subdued and rounded in late instars. Maximum valve length is at about the level of end of rostrum; maximum valve height is at about valve mid-length; maximum valve width is at the crest of bulb. A narrow, fairly deep sulcus flanks the bulb posteriorly and ventrally, is widest dorsally and at its mid-length site of adductor muscle scar. In posterior one-third of valve a narrow S-shaped sulcus skirts around dorsal and anterior base of caudal process and projects forward near valve ventral margin. Adductor muscle scar prominent, consisting of about 18–24 sub-parallel, radiating and alternating ridges and furrows arranged in a double series feather-like pattern. Reticulation covers external valve surface, including ventral part of bulb; consists of elongate polygons (muri 80–200 µm wide; fossae 100–500 µm wide), many hexagonal. Posteriorly and centrally in some large valves some polygons are extremely elongate, almost resembling corrugation. Centrodorsally in some valves 3–5 pore-bearing tubercles (diameter about 100 µm) occur atop the muri. In other valves 8–10 pore-bearing tubercles (diameter about 100 µm) occur atop the muri arranged in two rows ventrally. During ontogeny, size of bulb becomes relatively smaller and valve shape changes from almost circular to ovoid and density of reticulations decreases.
Remarks.—Bolbozoe polonica Gürich, 1896 was established on a single specimen, assumed lost during World War II. Gürich’s (1896) description and drawings indicate that the species had two sulci, a feather-like adductorial muscle scar and reticulated ornament like that of P. bohemica and it is probably its junior synonym.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the upper part of the Wenlock and lower part of the Ludlow series of the Czech Republic, France, Sardinia (Perrier et al. 2011), England and Wales (Perrier et al. 2019).
Suborder Entomozocopina Gründel, 1969
Superfamily Entomozoidea sensu Entomozoacea Přibyl, 1951
1951 Entomozoacea nov. nom.; Přibyl 1951: 103.
2019 Entomozoidea; Perrier et al. 2019: 51.
Remarks.—“Entomozoaceans” (= Entomozoacea sensu Přibyl, 1951) have long been regarded as myodocopes (e.g., Sylvester-Bradley 1961), but by their signature “finger-print” ornament and in generally lacking a rostrum and rostral incisure they are considered to be the most atypical of all of the ostracod groups that have traditionally been assigned to the Myodocopa (see discussion in Siveter and Vannier 1990; Kornicker and Sohn 2000). However, the discovery of a Palaeozoic myodocope with preserved soft-parts, Colymbosathon ecplecticos Siveter Sutton, Briggs, and Siveter, 2003 from the Silurian of England, has yielded new evidence to support a myodocope affinity for “entomozoaceans”. Colymbosathon ecplecticos has a very similar valve outline and lobal/sulcal morphology to that of Richteria migrans and both taxa lack a rostrum and rostral incisure.
Herein the concept of the “Entomozoacea” is used in its widespread and traditional sense, even though the type species of the type genus of the “Entomozoidae”, namely Entomozoe tuberosa (Jones, 1861) from the Silurian of Scotland, is known to be an entirely different form of ostracod (assigned to the myodocope superfamily Bolbozoidea Bouček, 1936; see Siveter and Vannier 1990 for discussion and recommendations).
Family Entomozoidae Přibyl, 1951
Emended diagnosis.—“Entomozoaceans” with bean-shaped or symmetrically to asymmetrically ovoid lateral outline, rarely with a weak anteroventral indentation; dorsal margin curved or straight, shorter than carapace length. Adductorial sulcus concave toward anterior end or straight; posteroventral sulcus present (subfamily Bouciinae) or absent. Adductor muscle scar characteristically a smooth patch (in some cases composed of many tiny scars), a pit or absent. Ornament usually present, consisting of transverse, longitudinal, spiral, concentric or irregular pattern of ribs, in some cases reduced to minute spines and reticulations (modified after Perrier et al. 2019).
Genus Richteria Jones, 1874
Type species: Cypridina serrate-striata Sandberger, 1845; by subsequent designation by Kegel 1934: 413. Lower Famennian, Upper Devonian of Germany.
Stratigraphic and geographic range.—Richteria is known from the Silurian and Devonian. Supposed records of its occurrence in younger and older strata (all the unrevised “Entomis” species, see Bassler and Kellett 1934) have not been confirmed by the present authors. The genus is known from Europe (Czech Republic, Germany, Poland, Belgium, France, Italy, UK; Perrier et al. 2007), Arctic Russia (Perrier et al. 2014a), Central Asia, and China (Perrier et al. 2007).
Richteria migrans (Barrande, 1872)
Fig. 2F.
1872 Entomis migrans Barr.; Barrande 1872: 514, pl. 24: 10–14, pl. 27: 22.
1873 Entomis migrans Barr., 1872; Jones 1873: 416.
1896 Entomis migratoria n. sp.; Gürich 1896: 374.
1899 Entomis migrans Barr.; Canavari 1899: 151.
1900 Entomis migrans Barr.; Canavari 1900: 193, pl. 25: 1, 2.
1900 Entomis Lamarmorai n. f.; Canavari 1900: 195, pl. 25: 3–5.
1900 Entomis n. f.?; Canavari 1900: 196, pl. 25: 6.
1900 Entomis Meneghinii n. f.; Canavari 1900: 196, pl. 25: 7–11.
1934 Entomis (Richteria) migrans Barr.; Kegel 1934: 413.
1934 Entomis migrans Barrande; Bassler and Kellett 1934: 304 (pars).
1936 Entomis (Richteria) migrans Barrande, 1872; Bouček 1936: 57, pl. 6: 5, 6; text-fig. 2a, 2b.
1937 Entomis (Richteria) migrans Barrande; Chaubet 1937: 186, pl. 4: 8a–c.
1951 Entomozoe (Richteria) migrans (Barrande); Přibyl 1951: 11, pl. 1: 4.
1952 Entomozoe migrans Barrande, 1872; Kupfahl 1952: 178.
1958 Entomozoe (Richteria) migrans (Barrande); Pokorný 1958: 311, text-fig. 1032.
1961 Entomozoe meneghinii Canavari; Sylvester-Bradley 1961: 389, text-fig. 312.2g–j.
1961 Richteria lamarmorai Canavari; Sylvester-Bradley 1961: 390, text-fig. 313.1a–e.
1984 “Entomis” migrans Barrande; Siveter 1984: 78, pl. 2: 12, 13, text-fig. 3.
1988 Richteria migrans (Barrande, 1872); Přibyl 1988:113, text-fig. 5.1.
1990 “Entomis” migrans; Siveter and Vannier 1990: 64, text-fig. 23.
1995 “Entomis” aff. lamarmorai Canavari, 1900; Kříž and Bogolepova 1995: 580, pl. 70: 12.
2000 E. migrans Barrande, 1872; Groos-Uffenorde et al. 2000: text-fig. 2.
2003 Richteria migrans taimyrica Abushik, subsp. nov.; Abushik et al. 2003: 122, pl. 27: 1, 2.
2007 Richteria migrans (Barrande, 1872); Perrier et al. 2007: 156, text-figs. 6–10.
2009 “Entomis” migrans Barrande, 1872; Siveter 2009: pl. 9: 14.
2009 Richteria migrans (Barrande, 1872); Gnoli et al. 2009: pl. 1: 1–11.
2012 Richteria migrans (Barrande, 1872); Perrier 2012: text-fig. 7G, H.
2013 Richteria migrans; Perrier and Siveter 2013: text-fig. 22.6D.
2014a Richteria migrans (Barrande, 1872); Perrier et al. 2014a: 56, pl. 1: A–N., text-figs. 2, 3.
2019 Richteria migrans (Barrande, 1872); Perrier et al. 2019: pl. 12: 1–3, 11, 12.
Lectotype: An incomplete right valve; on slab NM-L 22944; subsequently designated by Přibyl (1951: 111); figured by Barrande (1872: pl. 24: 12–14); Bouček (1936: text-fig. 2a); Perrier et al. (2007: text-fig. 6a, b).
Type locality: Former Dvorce quarry (today Podolí swimming pool), Podolí district of Prague, Czech Republic.
Type horizon: Kopanina Formation, Ludlow Series.
Material.—Tens of valves, from two localities (Widełki and Niestachów), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 1.4, H = 1.1 (ZPAL O.63/27) to L = 3.05, H = 2 (ZPAL O.63/28). Lectotype: L = 2.25, H = 1.50 (NM-L 22944). L/H ratio and adductor muscle position for seven specimens from two localities (Fig. 4).
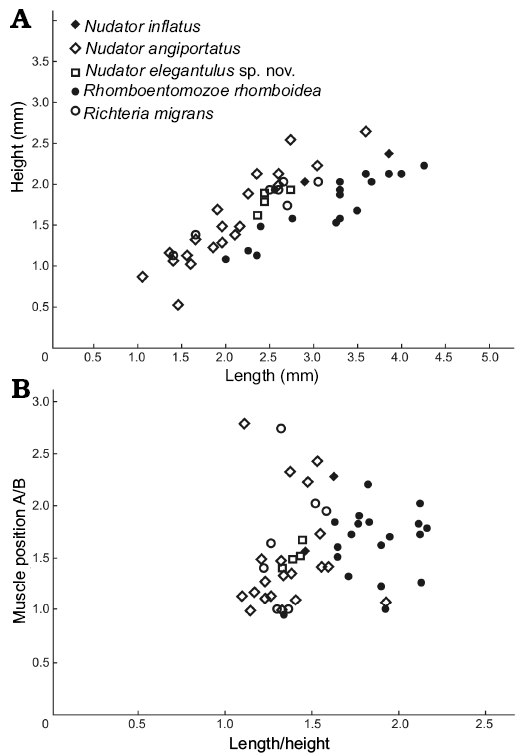
Fig. 4. Valve length/height plot (A) and position of the adductor muscle scar (A/B, where A and B are the distance between the muscle scar and the posterior end of the valve and the ventral margin, respectively) against length/height (B) of complete specimens of entomozoid species from the Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Description.—Valve large, bean-shaped in lateral outline. Greatest length is slightly above mid height; greatest height is at the adductorial sulcus. Dorsal margin shorter than valve length. Preadductorial and postadductorial areas very slightly curved dorsally; ventral, anterior and posterior margins convex. Right valve slightly larger and overlaps left valve along entire free margin. Contact list in left valve is narrow, ornamented by very fine striation. Anterior valve margin shows a faint notch in some large specimens. Adductorial sulcus long, deep, crescent shaped, extends two-thirds of valve height from in front of mid length near dorsal margin to below the preadductorial node. A simple elliptical adductor muscle scar is present at the slightly widened ventral extremity of the sulcus. Dorsal part of sulcus is deeper and narrower, ending at a small “bridge” (height about 50 µm) connecting preadductorial and postadductorial areas. Preadductorial node is generally unornamented but can be weakly ribbed, and is less developed in some specimens. External valve surface has up to 25 longitudinal ribs, including in some specimens short, intercalated and bifurcated ribs. The alignment of ribs is not disturbed by the adductorial sulcus. Ribs merge posteriorly, converge on a triangular smooth area anteriorly and curve slightly away from the valve margin ventrally. Some specimens show what seems to be very weak secondary reticulate ornament between the ribs. No evidence for presence of hinge structure; conjoined open valves are consistently connected along dorsal margin.
Remarks.—From its description and occurrence Entomis migratoria Gürich, 1896 (nomen nudum), is likely to be conspecific with Richteria migrans.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the upper part of the Ludlow Series in the Czech Republic, France, Sardinia, Poland, Central Asia, Arctic Russia, England, Wales, and possibly Germany and Sweden (see Perrier et al. 2007, 2014a, 2019).
Genus Nudator Perrier, Siveter, Williams, and Palmer, 2019
Type species: Nudator inflatus Perrier, Siveter, Williams, and Palmer, 2019. Ludlow Series, Silurian, Powys, Wales, UK.
Species included: Type species and Nudator angiportatus Perrier, Siveter, Williams, and Palmer, 2019, Nudator artumatus Perrier, Siveter, Williams, and Palmer, 2019, N. elegantulus sp. nov. and Nudator sp. nov. A of Perrier et al. 2019.
Emended diagnosis.—Entomozoidae with bean-shaped to ovoid lateral outline, dorsal margin slightly rounded. Adductorial sulcus narrow, well developed, extending up to 3/4 of valve height. Prominent postadductorial node present in type species only. Faint notch in anterior valve margin. Valve smooth or bearing minute ornament (modified after Perrier et al. 2019).
Remarks.—Nudator differs from other entomozoid genera in having unornamented or very faintly ornamented valves. Sineruga Perrier, 2012 and Nudator differ mainly in valve outline in lateral view.
Stratigraphic and geographic range.—Wenlock and Ludlow Series, Silurian of England, Wales (Perrier et al. 2019), Poland, and possibly France (Perrier et al. 2019).
Nudator inflatus Perrier, Siveter, Williams, and Palmer, 2019
Fig. 2I, J.
2019 Nudator inflatus sp. nov.; Perrier et al. 2019: 43, pl. 13: 1–14.
Holotype: A laterally compressed right valve (OUMNH 35000); Perrier et al. (2019: pl. 13: 4, 5).
Type locality: Radnorshire (loc. 10 of Perrier et al. 2019), Powys, Wales, UK.
Type horizon: “nilssoni-scanicus blue Mudstones” of Kirk (1947), Cucullograptus (Lobograptus) scanicus Biozone, Gorstian Stage, Ludlow Series.
Material.—Tens of valves, from two localities (Widełki and Niestachów), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2.9, H = 2 (ZPAL O.63/9, Fig. 2I, J) to L = 3.85, H = 2.35 (ZPAL O.63/29). Holotype: L = 2.00/H = 1.70 (OUMNH 35000). L/H ratio and adductor muscle position for two specimens from two localities (Fig. 4).
Description.—Valve large, bean-shaped in lateral outline. Greatest length is just above mid height; greatest height is at the adductorial sulcus. Valve outline in lateral view is almost straight dorsally, gently curved ventrally, posteriorly and anteriorly is evenly rounded. Valve surfaces gently inflated overall, lacking distinct or discrete lobes. Anterior valve margin in some cases has a faint indentation. Adductorial sulcus narrow, occurs just in front of mid-length, extends almost vertically from dorsal margin to just below mid-height ending with the adductor muscle spot. Prominent postadductorial tubercle variable in shape and size, diameter 15–25% of valve length. Adductorial sulcus generally curves around the front of the tubercle, in some specimens the sulcus appears to bifurcate and border both sides of tubercle. A simple elliptical adductor muscle scar occurs at the slightly widened ventral extremity of the sulcus. Valves smooth. No evidence for presence of hinge structure, but open valves in “butterfly position” are consistently to be connected along dorsal margin.
Remarks.—Nudator inflatus is distinguished from all other Nudator species by the presence of its large postadductorial node.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the Gorstian Stage, Ludlow Series of England and Wales (Perrier et al. 2019).
Nudator angiportatus Perrier, Siveter, Williams, and Palmer, 2019
Fig. 2G, H.
2019 Nudator angiportatus sp. nov.; Perrier et al. 2019: 44, pl. 14: 1–15.
Holotype: A laterally compressed left valve (OUMNH 35267); Perrier et al. (2019: pl. 14: 5–8, 14, 15).
Type locality: Loc. 56 of Perrier et al. (2019), Long Mountain, Wales, UK.
Type horizon: Lower part of the Irfon Formation, Cucullograptus (Lobograptus) scanicus Biozone, Gorstian Stage, Ludlow Series.
Material.—Tens of valves, from two localities (Widełki and Niestachów), Holy Cross Mountains, Poland; Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 1.05, H = 0.85 (ZPAL O.63/30) to L = 3.6, H = 2.6 (ZPAL O.63/8, Fig. 2H). Holotype: L = 2.70, H = 2.35 (OUMNH 35267). L/H ratio and adductor muscle position for 19 specimens from two localities (Fig. 4).
Description.—Valve large, bean-shaped in lateral outline. Greatest length is just above mid height; greatest height just in front of the adductorial sulcus at the site a small anterodorsal inflation of the carapce. Valve outline in lateral view is slightly concave dorsally, gently curved ventrally, posteriorly is evenly rounded, anteriorly above mid-height is more sharply rounded, and anteriorly has a weak indentation. Valve surfaces gently inflated overall, lacking distinct or discrete lobes. Adductorial sulcus narrow, occurs just in front of mid-length, originates just posteriorly of the small anterodorsal protuberance and curves forward from dorsal margin to just below mid-height ending with the adductor muscle spot. Anterior-anteroventral valve margin appears slightly concavely indented in lateral view in some specimens and has an apparent incisure (gape). A simple elliptical adductor muscle scar occurs at the slightly widened ventral extremity of the sulcus. Valves smooth. No evidence for presence of hinge structure, but conjoined open valves but open valves in “butterfly position” are connected along dorsal margin. During ontogeny valve shape changes from sub-triangular to more rounded/ovoid.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the Wenlock and Ludlow series of England and Wales (Perrier et al. 2019).
Nudator elegantulus sp. nov.
Figs. 5–7.
ZooBank LSID: urn:lsid:zoobank.org:act:01C7B8C0-33F5-4067-939B-1568F17AB8C9
Etymology: From Latin elegantulus, fine, elegant.
Holotype: A laterally compressed left valve (ZPAL O.63/15); Fig. 5C.
Type locality: Niestachów, Holy Cross Mountain, Poland.
Type horizon: Niewachlów Beds, Lower part of the Ludlow Series, based on graptolites, Cucullograptus (Lobograptus) scanicus Biozone.
Material.—Four valves from two localities (Widełki and Niestachów), Holy Cross Mountain, Poland, Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2.35, H = 1.6 (ZPAL O.63/13, Fig. 5A) to L = 2.75, H = 1.9 (holotype, ZPAL O.63/15, Fig. 5C). L/H ratio and adductor muscle position for four specimens from two localities (Fig. 4).

Fig. 5. Myodocope ostracod Nudator elegantulus sp. nov., Holy Cross Mountains, Poland; Ludlow Series, Silurian. A. ZPAL O.63/13, left valve. B. ZPAL O.63/14, right valve. C. Holotype ZPAL O.63/15, left valve. Light stereo-pair photographs in lateral view (of silicone casts of external moulds).
Diagnosis.—Nudator with ornament consisting of very delicate reticulation to backward oriented “chevrons”, excluding the triangular smooth area in front of carapace.
Description.—Valve large, bean-shaped in lateral outline. Greatest length at mid height; greatest height at the level of the adductorial sulcus. Valve outline in lateral view is straight dorsally, gently curved ventrally and rounded posteriorly and anteriorly. Valve surfaces gently inflated overall, lacking distinct or discrete lobes. Adductorial sulcus narrow, occurs just in front of mid-length, originates in the first third of the dorsal margin to just below mid-height ending with the adductor muscle spot. A simple elliptical adductor muscle scar occurs at the slightly widened ventral extremity of the sulcus. The triangular smooth area in front of the carapace has a slightly negative relief and covers an area representing 1/4 of the maximum length and 1/3 of the maximum height. The valve surface is ornamented apart from the anterior triangular smooth area. This ornament consists mainly of small backward oriented “chevrons” that are possibly the remains of a delicate reticulation (Fig. 6). There are about 200–250 chevrons per mm2, each about 20–50 μm across. During ontogeny, valve lateral outline changes from rounded to more ovoid.
Remarks.—Nudator elegantulus differs from N. angiportatus by its more distinct triangular smooth anterior area and by its ornament.
Stratigraphic and geographic range.—Known only from the type locality.
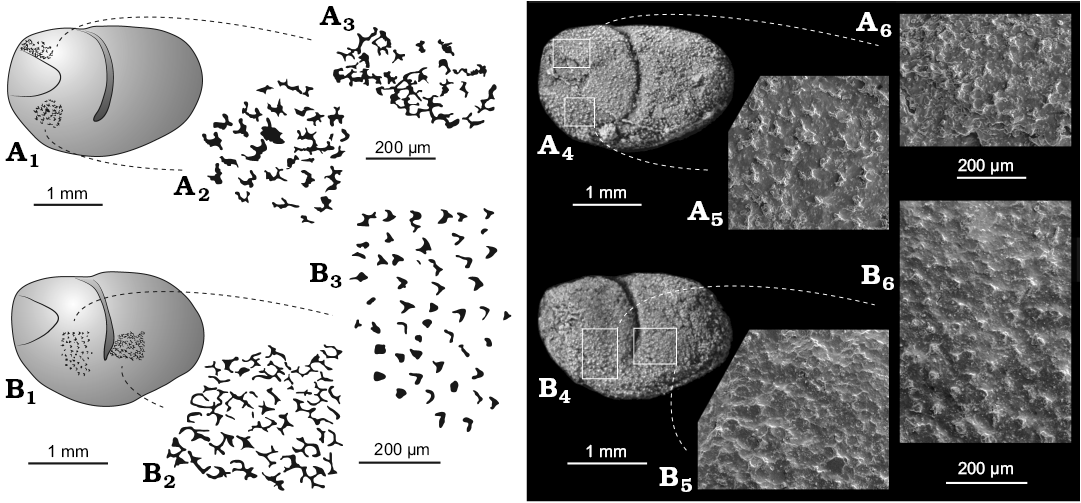
Fig. 6. Myodocope ostracod Nudator elegantulus sp. nov., details of the ornament, Holy Cross Mountains, Poland; Ludlow Series, Silurian. A. Holotype ZPAL O.63/15. Left valve (A1): detail of the ornament anterodorsally (A3, A6); detail of the ornament anteroventrally (A2, A5). B. ZPAL O.63/13. Left valve (B1): detail of the ornament anteriorly (B3, B6); detail of the ornament posterior to the adductorial sulcus (B2, B5). A4, B4, light photographs; A5, A6, B5, B6, SEM photographs; A1–A3, B1–B3, explanatory drawings; all lateral views of silicone casts of external moulds.
Family Rhomboentomozoidae Gründel, 1962
Type genus: Rhomboentomozoe Přibyl, 1951.
Emended diagnosis.—Left and right valves of apparently equal size. Lateral outline “triangular” to subquadrate, sometimes broadly egg-shaped. Straight dorsal margin usually well developed, sometimes corresponding to greatest carapace length; cardinal angles distinct or rounded. Sulcus always present, usually strongly developed, often extending across more than half of valve surface. Muscle attachment area absent or represented by a simple elliptical area at the ventral end of the sulcus. Spines may be developed near margin. Valve ornament absent or consisting of longitudinal ribs arranged parallel to dorsal margin; some outer ribs may be parallel to ends of valve (modified after Gooday 1983).
Remarks.—In addition to the type genus, conventionally the family includes Franklinella Stewart and Hendrix, 1945, Pseudoentomozoe Přibyl, 1951, Paraungerella Wang, 1986, Yulinentomozoe Wang, 1989, and possibly Vltavina Bouček, 1936. It is distinguished from the Entomozoidae by the generally triangular to subquadrate lateral outline and long, straight dorsal margin of the valves.
Subfamily Rhomboentomozoinae Gründel, 1962
Genus Rhomboentomozoe Přibyl, 1951
Type species: Rhomboentomozoe rhomboidea (Barrande, 1872), designated by Přibyl (1951: 111). Upper Silurian of Kozel near Beroun, Bohemia, Czech Republic.
Species included: Type species and possibly Rhomboentomozoe? sp. of Wang 2009.
Emended diagnosis.—Same as species.
Remarks.—Přibyl (1951: 115) assigned Isochilina armata pygmaea Ruedemann, 1901, from the Upper Ordovician Mohawkian of New York State, USA, to Rhomboentomozoe. Following Přibyl’s (1951) opinion, the Treatise on Invertebrate Paleontology (Sylvester-Bradley 1961: Q390) lists one “myodocopid” genus from the Ordovician, namely Rhomboentomozoe Přibyl, 1951. This supposed Ordovician record of Rhomboentomozoe is not accepted herein. Isochilina armata pygmaea is clearly very different from the Bohemian Silurian type-species of Rhomboentomozoe, namely Cryptocaris rhomboidea Barrande, 1872. The specimen from China of Rhomboentomozoe? sp. of Wang 2009 (15: pl. 1: 9) is incomplete and cannot be assigned to Rhomboentomozoe with certainty. If it is a Rhomboentomozoe species it would extend the range of the genus into the Lower Devonian (Pragian).
Siveter and Vannier (1990) considered that the genus Rhomboentomozoe is, “at best, a very atypical myodocope and most likely belongs to another group”. However, evidence from new material (e.g., muscle scar, rostrum) indicates its myodocope affinities. The species from Sardinia described by Canavari (1900: 201, pl. 26: 3–5) as Entomozoe preriodes shows marked resemblance to R. rhomboidea.
Stratigraphic and geographic range.—Ludlow Series, Silurian of the Czech Republic (Barrande 1872) and Poland, and possibly Sardinia (Canavari 1900). Also possibly Pragian, Lower Devonian of China (Wang 2009).
Rhomboentomozoe rhomboidea (Barrande, 1872)
Figs. 7, 8.
1872 Cryptoc. ? rhomboidea Barr.; Barrande 1872: 464, pl. 31: 14, 15.
1936 Entomis (Richteria) rhomboidea (Barrande, 1872); Bouček 1936: 58, pl. 6: 3, 4.
1951 Rhomboentomozoe rhomboidea (Barrande); Přibyl 1951: 15, pl. 1: 2, pl. 2: 5.
1954 Rhomboentomozoe rhomboidea (Barrande); Pokorný 1954: 462, text-fig. 680.
1958 Rhomboentomozoe rhomboidea (Barrande); Pokorný 1958: 311, text-fig. 1035.
1960 Rhomboentomozoe rhomboidea (Barrande); Zanina and Polenova 1960: 331, text-fig. 834.
1961 Rhomboentomozoe rhomboidea (Barrande); Sylvester-Bradley 1961: 390, text-fig. 315.2.
1962 Rhomboentomozoe; Gründel 1962: 1193, 1198, text-fig. 9.
1988 Rhomboentomozoe rhomboidea (Barrande); Přibyl 1988: 114, text-fig. 5.2.
1991 Rhomboentomozoe rhomboidea (Barrande); Stupnicka et al. 1991: 391.
1994 Rhomboentomozoe rhomboidea (Barrande, 1872); Whatley et al. 1994: 351.
Holotype: A carapace in butterfly position (NM-L 23322; CE 1222, lnv. No. 1648); Barrande (1872: pl. 31: 14, 15) and Bouček (1936: pl. 6: 4); herein Fig. 8G.
Type locality: Kozel, 3 km east of Beroun, Bohemia, Czech Republic.
Type horizon: Lower part of the Kopanina Formation, Ludlow Series, Silurian, horizon with the trilobite “Cromus” beaumonti.
Material.—Type specimens plus tens of valves from two localities (Widełki and Niestachów), Holy Cross Mountain, Poland, Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2, H = 1.05 (ZPAL O.63/16, Fig. 8A) to L = 4.25, H = 2.2 (ZPAL O.63/31). Holotype: L = 2.07, H = 1.67 (NM-L 23322; CE 1222, lnv. No. 1648). L/H ratio and adductor muscle position for 19 specimens from two localities (Fig. 4).
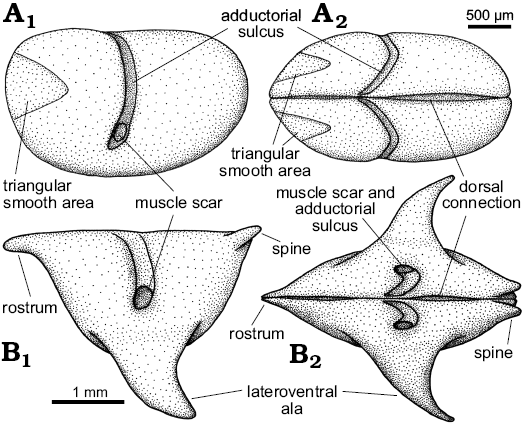
Fig. 7. Idealized reconstructions and terminology of Nudator elegantulus sp. nov. (A) and Rhomboentomozoe rhomboidea (B) from the Holy Cross Mountains, Poland; Ludlow Series, Silurian. In left lateral (A1, B1) and dorsal (A2, B2) views.
Emended diagnosis.—Entomozoidae with a triangular lateral valve outline, and a large, posterolaterally projecting ventral ala. Dorsal margin straight or slightly concave, ventral margin gently curved. Anterior and posterior cardinal corners pointed. Adductorial sulcus narrow, well developed, extending up to 1/2 of valve height, ending ventrally at a simple muscle spot. Faint notch in anterior valve margin. Valves smooth or weakly ribbed (modified after Gooday 1983).
Description.—Valve large, having an inverted triangular lateral outline. Maximum length at the dorsal margin; maximum height at the level of the lateroventral ala. Valve lateral outline is straight to weakly concave dorsally, gently curved posteriorly and anteriorly and more strongly curved ventrally. Valve surface is gently inflated overall. Adductorial sulcus narrow, concave forward, occurs just in front of mid-length and extends from the dorsal margin to just below mid-height ending with a simple adductorial muscle spot. The anterodorsal frontal projection resembles the straight rostrum of Recent halocyprid ostracods, and bears a well-developed notch on its valve margin and very weakly developed rostral incisure. Immediately adjacent to the posterodorsal cardinal corner a short posterodorsally projecting spine extends just above valve margin. Lateroventral ala is large, projects posteroventrally from the ventral part of the valve; in lateral view its anterior margin is convex anteriorly, its posterior margin is concave. During ontogeny, the ala becomes larger and projects more posteroventrally, and the rostrum and posterodorsal spine become more distinct.
Remarks.—The type specimen from Bohemia (Fig. 8G) was originally described as a phyllopod by Barrande (1872: 464, pl. 31: 14, 15). The Polish material is very similar to this type specimen, differing only by its seemingly flatter ala. The unique ribbed specimen figured by Bouček (1936: pl. 6: 3) and redrawn by Přibyl (1951: pl. 1: 2) is one of a kind: it has ribs that are absent in both the type specimen and the Polish material. More material would be needed to determine if it belongs to a separate species.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the Ludlow Series, Silurian of the Czech Republic: rarely in the lower part of the Kopanina Formation, “Cromus” beaumonti horizon, together with Richteria migrans, Vltavina perneri and other ostracodes and trilobites. Praha-Podolí (old quarry Dvorce, now the swimming pool) and Kozel (below Listice) near Beroun (Barrande 1872).
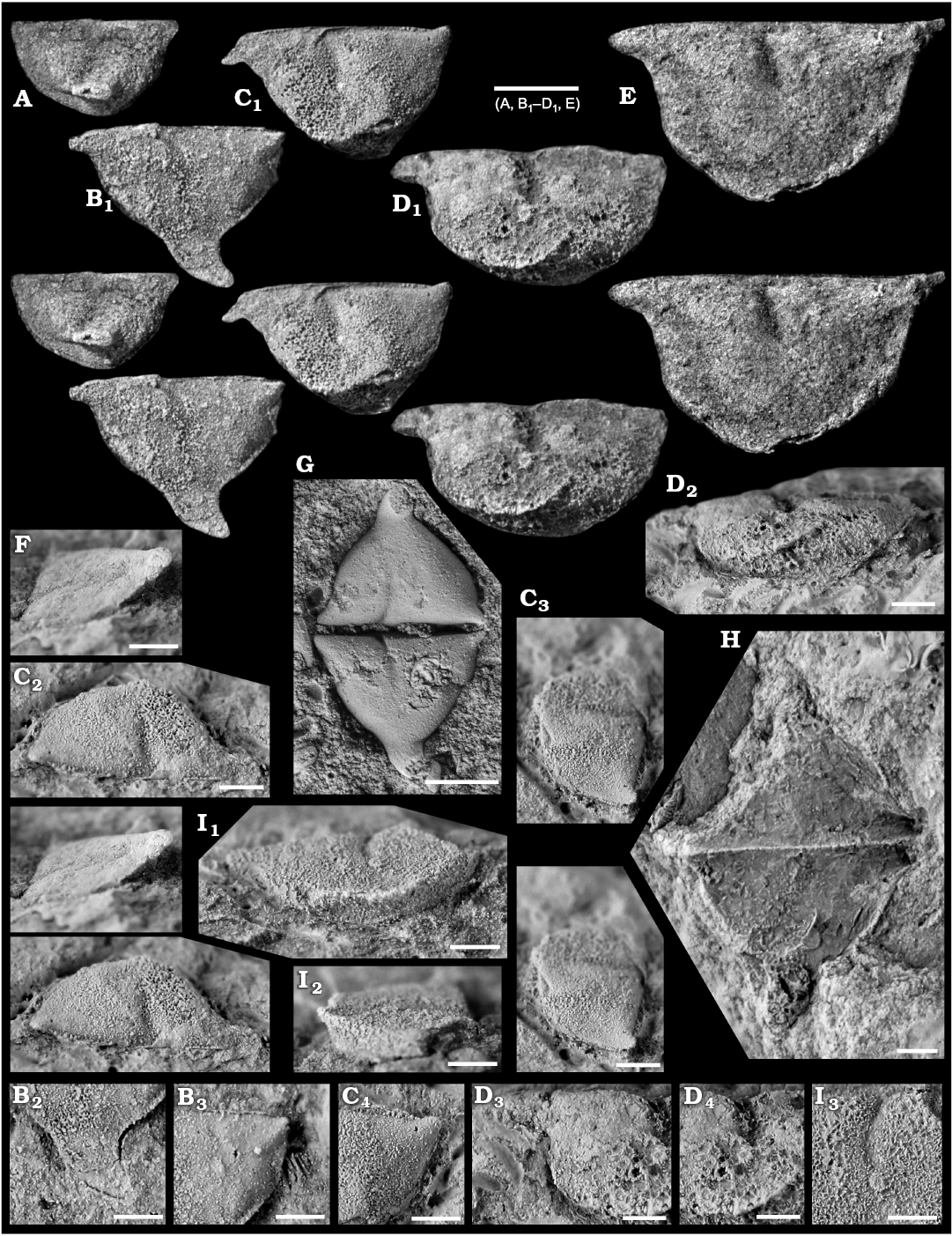
Fig. 8. Myodocope ostracod Rhomboentomozoe rhomboidea (Barrande, 1872), Holy Cross Mountains and Bohemia; Ludlow Series, Silurian. A. ZPAL O.63/16, left valve with incomplete ala, in lateral view. B. ZPAL O.63/17, left valve, in lateral view (B1), detail of the ala (B2), detail of the posterodorsal part of the valve (B3). C. ZPAL O.63/18, left valve with incomplete ala, in lateral (C1), dorsal (C2), and posterior (C3) views, detail of the posterodorsal part of the valve (C4). D. ZPAL O.63/19, left valve with incomplete ala, in lateral (D1), ventral (D2), and anterior (D3) views, detail of the rostrum (D4), detail of the adductorial muscle scar (D5). E. ZPAL O.63/20, left valve with incomplete ala, in lateral view. F. ZPAL O.63/21, right valve, in anterior view showing the ala. G. Holotype, L23322, open carapace in “butterfly” position. H. ZPAL O.63/22, open carapace in “butterfly” position. I. ZPAL O.63/23, right valve, in ventral view showing the proximal part of the ala on the ventrolateral part of the valve (I1), frontal view (I2), detail of the muscle scar (I3) view. All light photographs; A, B1, C1, C3, D1, E, F, stereo-pairs. A, E, F, silicone casts of external moulds; B–D, G–I, internal moulds. Scale bars: A, B1, C1, D1, E, G, 1 mm; B2, B3, C2–C4, D2–D4, F, H, I, 500 μm.
Superfamily Cypridinoidea sensu Cypridinacea Baird, 1850
Family Cypridinidae Baird, 1850
Remarks.—The Silurian genera Calocaria Vannier, 1987 and Silurocypridina Perrier, Vannier, and Siveter, 2011 are more similar in carapace morphology to typical Recent cypridinids than to typical living cylindroleberidid or sarsiellid myodocopids (see e.g., Kornicker 1975, 1981; Horne et al. 2002). The Silurian genera differ from typical cylindroleberidids in their less elongate shape. They are like sarsiellids in general shape but lack the caudal process characteristic of that family. Moreover, typically, the females of sarsiellids lack a well-developed rostrum and rostral incisure, thus contrasting with all known specimens of the two Silurian genera, in which those features are prominently developed. Verification of the familial assignment of the two Silurian genera depends on knowledge of their soft-part morphology.
Stratigraphic and geographic range.—Silurian to Recent; possibly also Ordovician (Myodoprimigenia fistuca from the Ashgill of South Africa; Gabbott et al. 2003).
Genus Silurocypridina Perrier, Vannier, and Siveter, 2011
Type species: Silurocypridina retroreticulata Perrier, Vannier, and Siveter, 2011. Upper Silurian of France.
Species included: Silurocypridina variostriata Perrier, Vannier, and Siveter, 2011 and Silurocypridina calva Perrier, Vannier, and Siveter, 2011.
Remarks.—Some of the Silurian myodocopid material from Europe that was provisionally referred to as “Cypridinid” and “cypridinid-like” by Siveter et al. (1987, 1991), an assignment supported by Kornicker and Sohn (2000), was subsequently assigned to Silurocypridina (Perrier et al. 2011). The tiny crescent-shaped adductor muscle scar of Silurocypridina is similar to that of the Silurian myodocope Calocaria but has no counterpart in other fossil or extant cypridinids. The simple dome-like valve morphology of Silurocypridina is similar to that of several late Palaeozoic and Mesozoic myodocopid genera (see Kornicker and Sohn 2000; e.g., Eocypridina Kesling and Ploch, 1960, see Wilkinson et al. 2004). Without information about their soft-anatomy, the classification of such relatively featureless cypridinaceans will remain unresolved.
Stratigraphic and geographic range.—Upper part of the Silurian; France, Czech Republic, England, Wales, Poland, and possibly Sardinia (see Perrier et al. 2011, 2019).
Silurocypridina retroreticulata Perrier, Vannier, and Siveter, 2011
Fig. 2K.
1987 “Cypridinid” gen. et sp. nov. A; Siveter et al. 1987: pl. 84: 1, 3, 5, pl. 87:1, 5.
1991 “Cypridinid” gen et. sp. nov. A of Siveter et al. 1987; Siveter et al. 1991: pl. 1: 4, pl. 2:10.
1992 “Cypridinid” sp. nov. A of Siveter et al. 1987; Vannier and Abe 1992: text-fig. 2B.
1995 “Cypridinid” sp. nov. A of Siveter et al. 1987; Vannier and Abe 1995: text-fig. 16A, B.
2000 “Cypridinid” Genus A, sp. A, Siveter et al. 1987; Kornicker and Sohn 2000: 21.
2011 Silurocypridina retroreticulata sp. nov.; Perrier et al. 2011: pl. 4: 1–7, text-figs. 6, 8A, 9H.
2012 Silurocypridina retroreticulata Perrier, Vannier and Siveter, 2011; Perrier 2012: text-fig. 7E.
2019 Silurocypridina retroreticulata Perrier, Vannier and Siveter, 2011; Perrier et al. 2019: pl. 17: 6, 7, 12, 13.
Holotype: A laterally compressed right valve (FSL 710505); Perrier et al. (2011: pl. 4: 4, text-fig. 9H).
Type locality: Les Buardières, Mayenne, France (Perrier et al. 2011).
Type horizon: La Lande Murée Formation; the type horizon is within the range of the upper part of the Wenlock Series to middle part of the Ludlow Series, and currently cannot be further resolved.
Material.—Three valves, all from locality Niestachów, Holy Cross Mountain, Poland, Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2.85, H = 2.55 (ZPAL O.63/10, Fig. 2K). Holotype: L = 3.46, H = 2.53 (FSL 710505). L/H ratio and adductor muscle position for one specimen (Fig. 3).
Description.—Valve dome-like, with nearly ovoid lateral outline; hinge short, located dorsally. Rostrum well developed, is about 15% of valve length, protrudes distinctly forward beyond the anteroventral margin of the valve. Rostral incisure well developed, rounded or angular in lateral outline. Adductor muscle scar is small, sited subcentrally, crescent-shaped convex anteriorly. Behind the adductor muscle scar and ventrally valves are reticulate, consisting of polygons with muri 50–100 µm wide and sola 100–250 µm wide. Anterodorsal part of valve (some 25% of lateral valve area) lacks ornament. A fine, narrow ridge occurs along ventral margin of the valve.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the Silurian of France, England and Wales (see Perrier et al. 2011, 2019).
Silurocypridina calva Perrier, Vannier, and Siveter, 2011
Fig. 2L.
1899 Cypridina sp. aff. C. Tosterupi Mob; Canavari 1899: 152.
1900 Cypridina tyrrhenica n.f.; Canavari 1900: 204, pl. 26: 10–12 (specimens I91, I92).
1987 “Cypridinid” sp.; Siveter et al. 1987: 801, pl. 85: 1, 2, 4, 5.
1987 “Cypridinid” sp. nov. B; Siveter et al. 1987: 805, pl. 87: 2–4.
1990 “Cypridinid” sp. nov.; Siveter and Vannier 1990: 64, text-fig. 23 (number 6).
1991 “Cypridinid” sp.; Siveter et al. 1991: 157, pl. 1: 3.
1992 “Cypridinid”-like myodocopes; Vannier and Abe 1992: 489, 509, pl. 2: 2, text-fig. 1.
1992 “Cypridinid” sp.; Vannier and Abe 1992: 494, text-fig. 6I, J.
2000 “Cypridinid” Genus B, sp. B of Siveter et al. 1987; Kornicker and Sohn 2000: 21.
2003 “Cypridinid” sp. nov. B of Siveter et al.; Gabbott et al. 2003: 157, text-fig. 4B.
2009 “Cypridinid” gen. and sp. nov.; Gnoli et al. 2009: 1, text-figs. 12–22.
2011 Silurocypridina calva sp. nov.; Perrier et al. 2011: pl. 5: 1–9, 12–14, text-figs. 6, 8A, 10.
2012 Silurocypridina calva Perrier, Vannier and Siveter, 2011; Perrier 2012: text-fig. 7F.
2019 Silurocypridina calva Perrier et al., 2011; Perrier et al. 2019: pl. 17: 1–5, 8–11.
Holotype: A three-dimensionally preserved left valve (LPB 18926); Perrier et al. (2011: pl. 5: 8, 9).
Type locality: Les Chevrolières, near St Denis-d’Orques, Sarthe, France (Perrier et al. 2011).
Type horizon: Le Val Formation; the type horizon is within the upper part of the Ludlow Series or lower part of the Pridoli Series, and currently cannot be further resolved.
Material.—Tens of valves, from two localities (Widełki and Niestachów), Holy Cross Mountain, Poland, Ludlow Series, Silurian.
Measurements (in mm).—Size range: L = 2, H = 2.1 (ZPAL O.63/32) to L = 4, H = 3.15 (ZPAL O.63/33). Holotype: L = 2.38, H = 1.48 (LPB 18926). L/H ratio and adductor muscle position for seven specimens from two localities (Fig. 3).
Description.—Valve dome-like, with sub-ovoid lateral outline; hinge short. Rostrum well developed, is about 10–15% of valve length, protrudes distinctly forward beyond anteroventral margin of valve. Rostral incisure well developed, rounded to angular in lateral outline. Adductor muscle scar is small, subcentral, crescent-shaped convex anteriorly. Valves smooth. During ontogeny valve shape changes from almost circular to sub-ovoid.
Remarks.—Silurocypridina calva displays considerable variation in valve outline and shape of the rostrum. Besides material from France and the Czech Republic other, less well preserved and limited material from Sardinia (Cypridina tyrrhenica Canavari, 1900; types in Museo de Storia Natural e del Territorio, Calci) is possibly conspecific. One very large incomplete specimen from Widełki (ZPAL O.63/12, Fig. 2M) shares some characteristics with “cypridinid” ostracods (i.e., valve outline and rostrum); however, its size (L = 15.25, H = 10.75 mm) and the absence of a crescent shape muscle scar are not consistent with the genus Silurocypridina. Either this specimen represents a deformed fossil belonging to a completely different group or it may belong to a new group of fossil “cypridinids”.
Stratigraphic and geographic range.—Ludlow Series, Holy Cross Mountains, Poland. Also known from the Silurian of the Czech Republic, France, England, Wales, and possibly Sardinia (see Perrier et al. 2011, 2019).
Discussion
Biostratigraphy.—Biostratigraphic control using graptolites and the uniformity of the myodocope assemblage of the Holy Cross Mountains succession indicates that, although they might not be exactly synchronous (Stupnicka et al. 1991), all Polish outcrops investigated herein are of about the same chronostratigraphical level (see Fig. 9): that is, middle Gorstian (lower part of the Ludlow) and more precisely within the Cucullograptus (Lobograptus) scanicus graptolite biozone sensu Zalasiewicz et al. (2009) or early part of the Angochitina elongata chitinozoan biozone sensu Steeman et al. (2016). This is consistent with the results of Perrier et al. (2019) who also identified Bolbozoe anomala, Bolbozoe psittaca, Parabolbozoe bohemica, Nudator angiportatus, Nudator inflatus, Silurocypridina calva, and Silurocypridina retroreticulata in the Cucullograptus (Lobograptus) scanicus Biozone of the UK (Fig. 9). The Polish myodocope assemblage corresponds to the assemblage present in the S. retroreticulata myodocope biozone of the UK (Perrier et al. 2019), with the exception of the presence of Richteria migrans. The S. retroreticulata Biozone is also recognized in France (Armorican Massif; Perrier et al. 2011). The new Polish occurrences of species restricted to the S. retroreticulata myodocope Biozone, that is N. inflatus and S. retroreticulata, endorses their position as widespread biostratigraphical markers of the middle Gorstian.
The Polish occurrence of Richteria migrans is the first record of that species at stratigraphical levels seemingly below the Ludfordian Stage (below the interval of the Saetograptus leintwardinensis and Monograptus formosus graptolite biozones). Richteria migrans is restricted to strata of the Ludfordian Stage in the UK (Long Mountain, Montgomery area and Ludlow area; Perrier et al. 2019), France (Armorican Massif and Montagne Noire; Perrier et al. 2007), the Czech Republic (Prague Basin; Perrier et al. 2007), Sardinia (Gnoli et al. 2009), Arctic Russia (Kotel’ny Island and Taimyr Peninsula; Perrier et al. 2014a), Uzbekistan (Nura-Tau Range; Perrier et al. 2014a) and possibly Germany and Gotland (Perrier et al. 2014a).
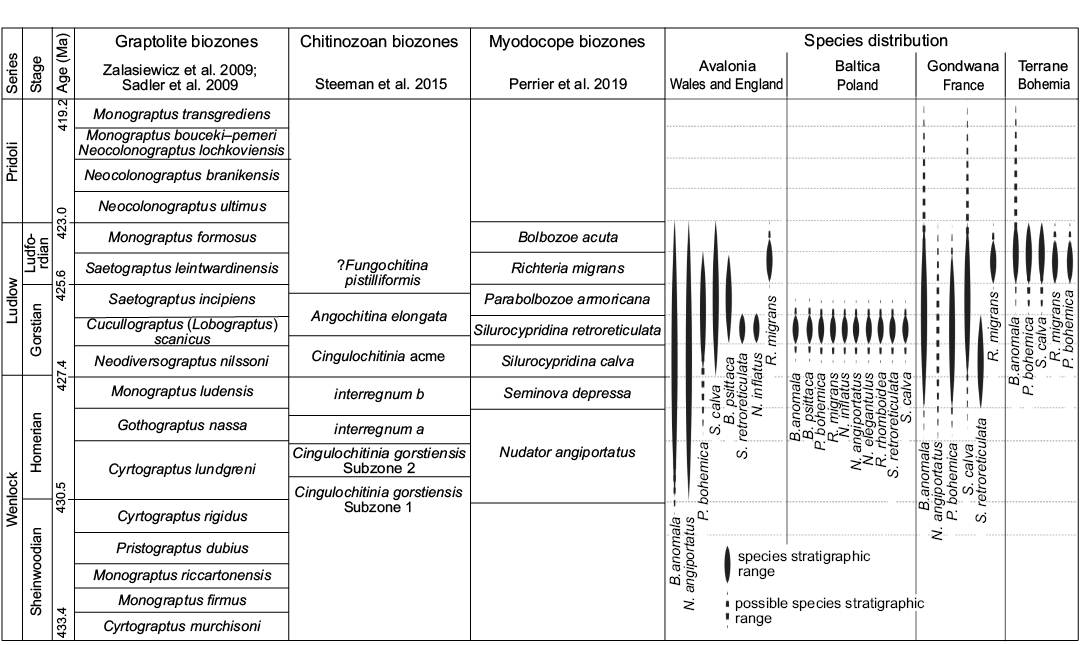
Fig. 9. Stratigraphical distribution of myodocope species in the Silurian of Poland compared to England, Wales, France, and Bohemia.
Biogeography, lifestyle, and the origin of ostracod zooplankton.—The widespread palaeogeographical distribution of several myodocope species at tropical to mid palaeo-latitudes in both hemispheres during the Silurian has been noted extensively (e.g., Siveter et al. 1991; Perrier et al. 2007, 2011, 2014a, c, 2019; Fig. 10). The new occurrences in Poland extend the known distribution of several species and reinforce data that show many Silurian myodocope species with wide dispersal. According to current knowledge, the northern part of the Tornquist Ocean, which separated Avalonia from Baltica, closed in the latest Ordovician Hirnantian (Torsvik and Rehnström 2003) and during the late Silurian the south part of the Holy Cross Mountains was situated along the Trans-European Suture Zone margin of Baltica (Nawrocki et al. 2007; Kozłowski et al. 2014). This region was located in southerly subtropical palaeolatitudes (15–20°; Fig. 10). Torsvik and Cocks (2011, 2013) placed Baltica east of the amalgamated Laurentia and Avalonia continents. The closest upper Silurian myodocope-bearing regions are Gotland (south Baltica), Germany, Wales and the Welsh Borderland (Avalonia). South Baltica shows strong myodocope faunal links with the Welsh Basin (eight species in common; Perrier et al. 2019). Other regions show similar assemblages, with the following number of myodocope species in common with the Holy Cross Mountains: Bohemia (terrane situated in the Rheic Ocean, Fig. 10; five species), the Armorican Massif (North Gondwana; six species), the Montagne Noire (North Gondwana; five species), Sardinia (North Gondwana; five species) and Uzbekistan (probably situated between North and South China terranes; four species; VP, DJS, and MW unpublished material). Both localities from Arctic Russia, Taimyr, and New Siberian Islands have been little studied and bear only one myodocope species namely the highly cosmopolitan R. migrans (Perrier et al. 2014a), a species also present in Poland.
Our new observations on the Holy Cross Mountains confirm that the occurrences of Silurian myodocopes are mostly associated with mudstone, siltstone or shale beds deposited in open- or deep-shelf marine settings. In addition to ostracods, these assemblages are dominated by other pelagic animals, namely cephalopods and graptolites, and rare bivalves, preserved in laminated sediments that lack bioturbation (Siveter et al. 1991; Perrier et al. 2011). The cosmopolitan distribution of the ostracods, coupled with their facies and faunal associations, supports the notion of an ostracod (myodocope) ecological shift from benthic to planktonic habitats during the late Wenlock and Ludlow (Siveter 1984; Siveter et al. 1987, 1991; Siveter and Vannier 1990; Vannier and Abe 1992; Perrier et al. 2007, 2011, 2014a, c, 2019).
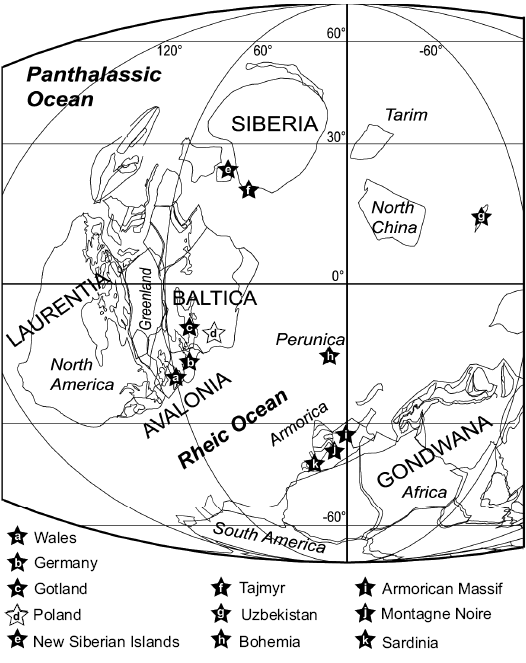
Fig. 10. Palaeogeographical distribution of Silurian myodocope bearing regions; map for Ludow (420 Ma, after Torsvik and Cocks 2013), but species span from late Wenlock to Pridoli. The position of the occurrence in Uzbekistan is poorly constrained palaeogeographically.
Conclusions
Silurian myodocope ostracods from the Holy Cross Mountains, Poland comprise ten species (including one new, Nudator elegantulus sp. nov.) that belong to four families: Bolbozoidae, Entomozoidae, Rhomboentomozoidae, and Cypridinidae. Biostratigraphic control, Cucullograptus (Lobograptus) scanicus graptolite Biozone, Angochitina elongata chitinozoan Biozone and Silurocypridina retroreticulata myodocope Biozone, indicates that the three Polish outcrops investigated are of mid-Gorstian age. The new occurrences in Poland extend the known distribution of several species and reinforce data that show many Silurian myodocope species with wide dispersal. Poland shows links at the species level with other regions on Baltica, Avalonia, Siberia, Gondwana, and two Rheic Ocean terranes. Our observations on the Holy Cross Mountains confirm that the occurrences of Silurian myodocopes are mostly associated with pelagic animals and with depositional environments ranging from open- to deep-shelf. The cosmopolitan distribution of these ostracods, coupled with their facies and faunal associations, supports the notion of an ostracod (myodocope) ecological shift from benthic to planktonic habitats during the late part of the Wenlock and continuing into the Ludlow.
Acknowledgements
We thank Vojtèch Turek and Lenka Váchová (both National Museum, Prague, Czech Republic) for the picture of Rhomboentomozoe rhomboidea type specimen, Marie-Béatrice Forel (Muséum National d’Histoire Naturelle, Paris, France) and Giles Miller (Natural History Museum, London, UK) for their helpful comments on the manuscript. This research was funded by a Leverhulme Trust grant (RPG-324: Pioneer ostracod zooplankton).
References
Abushik, A.I., Modzalevskaya, T.L., Tolmacheva, T.Y., Melnikova, L.M., Sobolev, N.N., and Sobolevskaya, R.F. 2003. Atlas of the Palaeozoic Fauna of Taimyr, Part I, Brachiopods, Ostracods, Conodonts [in Russian]. 240 pp. VSEGEI, St Petersburg.
Baird, W. 1850. The Natural History of the British Entomostraca. 364 pp. The Ray Society, London. Crossref
Barrande, J. 1872. Système Silurien du centre de la Bohème, 1. 647 pp. Chez l’auteur, Prague.
Bassler, R.S. and Kellett, B. 1934. Bibliographic index of Paleozoic Ostracoda. Special Paper of Geological Society of America 1: 1–500.
Bigsby, J.J. 1868. Thesaurus siluricus. The Flora and Fauna of the Silurian Period; with Addenda (from Recent Acquisitions). 214 pp. John Van Voorst, London. Crossref
Bouček, B. 1936. Die Ostracoden des böhmischen Ludlows (Stufe eß). Neues Jahrbuch für Mineralogie, Geologie und Paläontologie 76: 31–98.
Canavari, M. 1899. Ostracodi Siluriano di Sardegna. Atti della Societa Toscana di Scienze Naturali Residente in Pisa, Processi Verbali 11: 150–153.
Canavari, M. 1900. Fauna dei calcari nerastri con Cardiola ed Orthoceras di Xea San Antonio in Sardegna. Palaeontographica Italica 5: 187–210.
Chaubet, M.C. 1937. Contribution à l’étude du Gothlandien du versant méridional de la Montagne Noire. 213 pp. Unpublished Ph.D. thesis, Faculté des sciences de Montpellier, Montpellier.
Gabbot, S.E., Siveter, David J., Aldridge, R.J., and Theron, J.N. 2003. The earliest myodocopes: ostracodes from the late Ordovician Soom Shale Lagerstätte of South Africa. Lethaia 36: 151–160. Crossref
Gnoli, M., Perrier, V. and Serventi, P. 2009. The state of research on Sardinian Silurian Crustacea. In: C. Corradini, A. Ferretti, and P. Storch (eds.), The Silurian of Sardinia. Rendiconti della Societa Paleontologica Italiana 3 (1): 143–155.
Gooday, A.J. 1983. Entomozoacean Ostracods from the Lower Carboniferous of south-western England. Palaeontology 26: 755–88.
Groos-Uffenorde, H., Lethiers, F., and Blumenstengel, H. 2000. Ostracodes and Devonian Stratigraphy. Courier Forschungsinstitut Senckenberg 220: 99–111.
Gründel, J. 1962. Zur Phylogenetik und Taxionomie der Entomozoidae (Ostracoda) unter Ausschluss der Bouciinae. Geologie 11: 1184–1203.
Gründel, J. 1969. Neue taxonomische Einheiten der Unterklasse Ostracoda (Crustacea). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1969 (6): 353–361.
Gürich, G. 1896. Das Paläeozoicum im Polnischen Mittelgebirges. Verhand-lungen der Russisch-kaiserlichen mineralogischen Gesellschaft zu St.Petersburg Serie 2 32: 374–391.
Herrich-Schaffer, G.A.W. 1856. Sammlung neuer, oder wenig bekannter Außereuropäischer Schmetterlinge 1. 52 pp. GJ Manz., Regensburg.
Horne, D.J., Cohen, A., and Martens, K. 2002. Taxonomy, morphology and biology of Quaternary and living Ostracoda. In: J.A. Holmes and A. Chivas (eds.), The Ostracoda: Applications in Quaternary Research. Geophysical Monograph 131: 5–36. Crossref
Howel, H.H. and Geikie, A. 1861. The geology of the neighbourhood of Edinburgh. Memoirs of the Geological Survey of Great Britain 32: 137.
Hughes, D.D. 1954. Barrande’s ostracode localities. Micropaleontology 8: 41–47. Crossref
Jones, T.R. 1861. Entomis. In: H.H. Howel and A. Geikie (eds.), The Geology of the Neighbourhood of Edinburgh. Memoirs of the Geological Survey of Great Britain 32: 137.
Jones, T.R. 1870. On ancient water-fleas of the ostracodous and phyllopodous tribes (bivalved Entomostraca). The Monthly Microscopical Journal 4 (4): 184–193. Crossref
Jones, T.R. 1873. Notes on the Palaeozoic bivalved Entomostraca, No. 10. Entomis and Entomidella. Annals and Magazine of Natural History 11 (4): 413–417. Crossref
Jones, T.R. 1874. Uber Entomis und ein neues Genus Richteria. Neues Jahrbuch für Mineralogie Geologie und Paläontologie 2: 180. [in form of a letter to Prof. Geinitz; title given in the table of contents.]
Jones, T.R. 1884. Notes on the Palaeozoic bivalved Entomostraca, No. 18. Some species of the Entomididae. Annals and Magazine of Natural History 14 (5): 391–403. Crossref
Jones, T.R. 1898. On the fossil Cyprinidae and some allied Ostracoda. Annales and Magazine of Natural History, Series 7 1 (5): 333–344. Crossref
Kegel, W. 1934. Zur Kenntnis paläozoischer Ostracoden 4. Über die Gattung Entomis und ihre mittel-devonischen Arten. Jahrbuch der preußischen geologischen Landesanstalt zu Berlin 54 (for 1933): 409–420.
Kesling, R.V. and Ploch, R.A. 1960. New Upper Devonian cypridinacean ostracod from southern Indiana. Contributions from the Museum of Paleontology, University of Michigan 15: 281–292.
Kirk, N. 1947. Geology of the Anticlinal Disturbance of Breconshire and Radnorshire: Pont Faen to Presteign. Unpublished Ph.D. thesis, University Cambridge, Cambridge.
Kornicker, L.S. 1975. Antarctic Ostracoda (Myodocopina). Smithsonian Contributions to Zoology 163: 1–720. Crossref
Kornicker, L.S. 1981. Revision, distribution, ecology, and ontogeny of the ostracode subfamily Cyclasteropinae (Myodocopina: Cylindroleberididae). Smithsonian Contributions to Zoology 319: 1–548. Crossref
Kornicker, L.S. and Sohn, I.G. 2000. Myodocopid Ostracoda from the Late Permian of Greece and a basic classification for Paleozoic and Mesozoic Myodocopida. Smithsonian Contributions to Paleobiology 91: 1–33. Crossref
Kozłowski, W., Domańska-Siuda, J., and Nawrocki, J. 2014. Geochemistry and petrology of the Upper Silurian greywackes from the Holy Cross Mountains (central Poland): implications for the Caledonian history of the southern part of the Trans-European Suture Zone (TESZ). Geological Quarterly 58: 311–336. Crossref
Kříž, J. and Bogolepova, O.K. 1995. Cardiola signata community (Bivalvia) in Cephalopod limestones from Tajmyr (Gorstian, Silurian, Russia). Geobios 28: 573–583. Crossref
Kříž, J. 1992. Silurian Field Excursions: Prague Basin (Barrandian), Bohemia. 111 pp. National Museum of Wales Geological Series, Cardiff.
Kupfahl, H.-G. 1952. Paläontologische Untersuchungen zur Grenze Gotlandium/Devon im Kellerwald und bei Marburg. Paläontologische Zeitschrift 25: 160–180. Crossref
Latreille, P.A. 1802. Histoire naturelle générale et particulière des Crustacés et des Insectes, Familles naturelles des genres. Third edition. 467 pp. Dupuis, Paris.
Lethiers, F., Le Fèvre, J., Vannier, J., and Weyant, M. 1985. Paléozoïque. In: Oertli, H.L. (ed.), Atlas des ostracodes de France. Mémoires Elf-Aquitaine, Pau, 9: 3–87.
Malec, J. 1993. Upper Silurian and Lower Devonian in the western Holy Cross Mountains. Geological Quarterly 37: 501–536.
Nawrocki, J., Dunlop, J., Pecskay, Z., Krzemiński, L., Żylińska, A., Fanning, M., Kozłowski, W., Salwa, S., Szczepanik, Z., and Trela, W. 2007. Late Neoproterozoic to Early Palaeozoic palaoegeography of the Holy Cross Mountains (Central Europe): an integrated approach. Journal of the Geological Society 164: 405–423. Crossref
Perrier, V. 2012. An atypical Silurian myodocope ostracod from the Armorican Massif, France. Acta Palaeontologica Polonica 57: 363–373. Crossref
Perrier, V. and Siveter, David J. 2013. The use of “European” ostracode faunas in testing Silurian stratigraphy and palaeogeography. In: D.A.T. Harper and T. Servais (eds.), Early Palaeozoic Biogeography and Palaeogeography. Geological Society, London, Memoirs 38: 347–356. Crossref
Perrier, V., Bogolepova, O.K., Gubanov, A.P., Siveter, David J., and Williams, M. 2014a. A pelagic myodocopid ostracod from the Silurian of Arctic Russia. Journal of Micropalaeontology 34: 51–57. Crossref
Perrier, V., Siveter, David J., Williams, M., and Lane, P.D. 2014b. An Early Silurian “Herefordshire” myodocope ostracod from Greenland and its palaeoecological and palaeobiogeographical significance. Geological Magazine 151: 591–599. Crossref
Perrier, V., Siveter, David J., Williams, M., and Palmer, D. 2019. British Silurian Myodocopes Ostracods. Monograph of the Palaeontographical Society 172 (651): 1–99. Crossref
Perrier, V., Siveter, David J., Williams, M., Strusz, D.L., Steeman, T., Verniers, J., and Vandenbroucke, T.R. 2014c. Myodocope ostracods from the Silurian of Australia. Journal of Systematic Palaeontology 13: 727–739. Crossref
Perrier, V., Vannier, J., and Siveter, David J. 2007. The Silurian pelagic myodocope ostracod Richteria migrans. Transactions of the Royal Society of Edinburgh, Earth Sciences 98: 151–163. Crossref
Perrier, V., Vannier, J., and Siveter, David J. 2011. Silurian bolbozoids and
cypridinids (Myodocopa) from Europe: pioneer pelagic ostracods. Palaeontology
54: 1361–1391. Crossref
Perrier, V., Williams, M., and Siveter, David J. 2015. The fossil record and palaeoenvironmental significance of marine arthropod zooplankton. Earth-Science Reviews 146: 146–162. Crossref
Pokorný, V. 1954. Zaklady zoologicke mikropaleontologie. 650 pp. Akademia, Prague.
Pokorný, V. 1958. Grundzüge der zoologischen Mikropaläontologie Bd. 2. 582 pp. Deutscher Verlag der Wissenschaften, Berlin.
Přibyl, A. 1951. On the Bohemian Ostracoda of the families Entomozoidae and Entomoconchidae. Bulletin international de l’Académie tchèque des Sciences 1949: 1–28.
Přibyl, A. 1988. Ostracodes from the Silurian of central Bohemia. Sbornik geologicky vĕd Paleontologie 29: 49–143.
Ruedemann, R. 1901. Trenton conglomerate of Rysedorph Hill, Rensselaer Co., NY, and its fauna. New York State Museum, Bulletin 49: 3–114.
Salter, J. W. 1878. A Catalogue of the Cambrian and Silurian Fossils in the Museum of Practical Geology. 144 pp. Museum of Practical Geology, London.
Sandberger, G. 1845. Die erste Epoche der Entwickelungsgeschichte der Erdkörpers. Jahrbücher des Vereins für Naturkunde im Herzogthum Nassau 2: 89–124.
Sars, G.O. 1866. Oversigt af Norges marine Ostracoder. Norske Videnskcaps-Akademien Forhandlingar 1865: 1–130.
Siveter, David J. 1982. Casts illustrating fine ornament of a Silurian ostracod. In: R.H. Bate, E. Robinson, and L.M. Sheppard (eds.), Fossil and Recent Ostracods, 105–122. British Micropalaeontological Society, Ellis Horwood, Chichester.
Siveter, David J. 1984. Habitats and modes of life of Silurian ostracods. In: M.G. Bassett and J.D. Lawson (eds.), The Autecology of Silurian Organisms. Special Papers in Palaeontology 32: 71–85.
Siveter, David J. 2009. Silurian. In: J.E. Whittaker and M.B. Hart (eds.), Ostracods in British Stratigraphy. Micropalaeontological Society, London, Special Publications 3: 45–90. Crossref
Siveter, David J. and Vannier, J. 1990. The Silurian myodocope Entomozoe from the Pentland Hills, Scotland: its taxonomic, ecological and phylogenetic significance and the affinity of bolbozoid Myodocopes. Transactions of the Royal Society of Edinburgh, Earth Sciences 81: 71–76. Crossref
Siveter, David J., Briggs, D.E.G., Siveter, Derek J., and Sutton, M.D. 2010. An exceptionally preserved myodocopid ostracod from the Silurian of Herefordshire, UK. Proceedings of the Royal Society of London, Series B 277: 1539–1544. Crossref
Siveter, David J., Briggs, D.E.G., Siveter, Derek J., and Sutton, M.D. 2015. A 425-million-year-old Silurian pentastomid parasitic on ostracods. Current Biology 25: 1632–1637. Crossref
Siveter, David J., Briggs, D.E.G., Siveter, Derek J., and Sutton, M.D. 2018. A well preserved respiratory system in a Silurian ostracod. Biology Letters: 20180464. [published online, https://doi.org/10.1098/rsbl.2018.0464]. Crossref
Siveter, David J., Briggs, D.E.G., Siveter, Derek J., Sutton, M.D., and Joomun, S.C. 2013. A Silurian myodocope with preserved soft-parts: cautioning the interpretation of the shell-based ostracod record. Proceedings of the Royal Society of London, Series B: 280. [published online, https://doi.org/10.1098/rspb.2012.2664]. Crossref
Siveter, David J., Siveter, Derek J., Sutton, M.D., and Briggs, D.E.G. 2007. Brood care in a Silurian ostracod. Proceedings of the Royal Society of London, Series B 274: 465–469. Crossref
Siveter, David J., Sutton, M.D., Briggs, D.E.G., and Siveter, Derek J. 2003. An ostracode crustacean with soft-parts from the Lower Silurian. Science 302: 1749–1751. Crossref
Siveter, David J., Tanaka, G., Farrell, Ú.C., Martin, M.J., Siveter, Derek J., and Briggs, D.E.G. 2014. Exceptionally preserved 450-million-year-old Ordovician ostracods with brood care. Current Biology 24: 801–806. Crossref
Siveter, David J., Vannier, J., and Palmer, D. 1987. Silurian myodocopid ostracodes: their depositional environment and the origin of their shell microstructures. Palaeontology 30: 783–813.
Siveter, David J., Vannier, J., and Palmer, D. 1991. Silurian myodocopes: pioneer pelagic ostracods and the chronology of an ecological shift. Journal of Micropalaeontology 10: 157–173. Crossref
Siveter, Derek J. 1990. Photography. In: D.E.G. Briggs and P.R. Crowther (eds.), Palaeobiology: A Synthesis, 505–508. Blackwell, Oxford.
Steeman, T., Vandenbroucke, T.R., Williams, M., Verniers, J., Perrier, V., Siveter, David J., Wilkinson, J., Zalasiewicz, J., and Emsbo, P. 2016. Chitinozoan biostratigraphy of the Silurian Wenlock–Ludlow boundary succession of the Long Mountain, Powys, Wales. Geological Magazine 153: 95–109. Crossref
Stewart, G.A. and Hendrix, W.E. 1945. Ostracoda of the Olentangy Shale, Franklin and Delaware counties, Ohio. Journal of Paleontology 19: 96–115.
Stupnicka, E., Przybyłowicz, T., and Żbikowska, B. 1991. The age of the Niewachlów greywackes and shales from Widełki near Bardo (Holy Cross Mountains). Przegląd Geologiczy 9: 389–393.
Sylvester-Bradley, P.C. 1961. Order Myodocopida Sars, 1866. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part Q, Arthropoda 3, Crustacea, Ostracoda, 387–406. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Tomczyk, H. 1956. Wenlock and Ludlow in the Kielce syncline of the Święty Krzyż Mountains. Instytut Geologiczny Prace 16: 1–129.
Torsvik, T.H. and Cocks, L.R.M., 2011. The Palaeozoic palaeogeography of central Gondwana. Geological Society, London, Special Publications 357: 137–166. Crossref
Torsvik, T.H. and Cocks, L.R.M. 2013. New global palaeogeographical reconstructions for the Early Palaeozoic and their generation. In: D.A.T. Harper and T. Servais (eds.), Early Palaeozoic Biogeography and Palaeogeography. Geological Society, London, Memoirs 38: 5–24. Crossref
Torsvik, T.H. and Rehnström, E.F. 2003. The Tornquist Sea and Baltica–Avalonia docking. Tectonophysics 362: 67–82. Crossref
Vannier, J. 1987. On Calocaria maurae Vannier gen. et sp. nov. Stereo-Atlas of Ostracod Shells 14: 45–48.
Vannier, J. and Abe, K. 1992. Recent and early Palaeozoic myodocope ostracodes: functional morphology, phylogeny, distribution and lifestyles. Palaeontology 3: 485–517.
Vannier, J. and Abe, K. 1995. Size, body plan and respiration in the Ostracoda. Palaeontology 38: 843–873.
Wang, S.-Q. 1986. Devonian Rhomboentomozoinae (Ostracoda) from Guangxi [in Chinese with English summary]. Acta Palaeontologica Sinica 25: 155–168.
Wang, S.-Q. 1989. Early Devonian ostracodes from Zhangmu of Yulin, Guangxi [in Chinese with English summary]. Acta Palaeontologica Sinica 28: 249–268.
Wang, S.-Q. 2009. Palaeozoic Entomozoacea and Leperditicopida (Ostracoda) of China [in Chinese, English summary]. Fossil Ostracoda of China 3: 1–251.
Wang, S.-Q. and Zhang, X.-B. 1983. Ostracodes from Lower and Middle Devonian of the Luofu and other areas, Guangxi Province [in Chinese, English summary]. Acta Palaeontologica Sinica 22: 551–565.
Whatley, R.C., Siveter, David J., and Boomer, I.D. 1994. Arthropoda (Crustacea: Ostracoda). In: M.J. Benton (ed.), The Fossil Record 2, 344–356. Chapman and Hall, London.
Wilkinson, I.P., Williams, M., Siveter, David J., and Wilby, P.R. 2004. A Carboniferous necrophagous myodocopid ostracod from Derbyshire, England. Revista Espanola de Micropaleontologia 36: 187–198.
Williams, M., Vandenbroucke, T.R., Perrier, V., Siveter, David J., and Servais, T. 2015. A link in the chain of the Cambrian zooplankton: bradoriid arthropods invade the water column. Geological Magazine 152: 923–934. Crossref
Zalasiewicz, J.A., Taylor, L., Rushton, A.W.A., Loydell, D.K., Rickards, R.B., and Williams, M. 2009. Graptolites in British stratigraphy. Geological Magazine 146: 785–850. Crossref
Zanina, I.E. and Polenova, E.N., 1960. Subclass Ostracoda [in Russian]. In: N.E. Černyševa (ed.), Členistonogie. Trilobitoobraznye i rakoobraznye, 264–421. Gosudarstvennoe naučno-tekhničeskoe izdatel’stvo literatury po geologii i ohrane nedr, Moskva.
Acta Palaeontol. Pol. 64 (2): 379–397, 2019
https://doi.org/10.4202/app.00552.2018