

A new endemic genus of eomyid rodents from the early Miocene of Japan
YURI KIMURA, YUKIMITSU TOMIDA, DANIELA C. KALTHOFF, ISAAC CASANOVAS-VILAR, and THOMAS MÖRS
Kimura, Y., Tomida, Y., Kalthoff, D.C., Casanovas-Vilar, I., and Mörs, T. 2019. A new endemic genus of eomyid rodents from the early Miocene of Japan. Acta Palaeontologica Polonica 64 (2): 303–312.
Fossil rodents are generally scarce in the Miocene of Japan. However, as much as three taxa of eomyid rodents had been reported from the early Miocene Nakamura Formation (ca. 18.5 Ma) in Gifu Prefecture, central Japan. In this study, we revisit one of them—the small-sized taxon—and assign this material to a new genus, Japaneomys, which is so far known only from the type locality. The new genus is closely related to but distinguished from Asianeomys, which is more widely distributed in Central and East Asia, by having: (i) more bunodont cheek teeth with lower lophids, yet complete transverse lophids; (ii) two-rooted p4; (iii) anterior lobe narrower than posterior lobe on m1, correspondingly synclinid I shorter and shallower than synclinid IV; (iv) hypolophid anteriorly concave on m1. Japaneomys shows a derived but peculiar four-layered enamel microstructure with longitudinally oriented Hunter-Schreger bands, compared to three-layered microstructure in typical eomyid rodents. A phylogenetic analysis indicates that Japaneomys is more basal than Asianeomys and likely diverged in the late Oligocene when Japan was still part of the eastern margin of continental Asia, suggesting that certain small mammal populations could have undergone allopatric speciation isolated from inner-continental regions of Asia.
Key words: Mammalia, Rodentia, Eomyidae, enamel microstructure, dental morphology, time-scaled phylogeny, paleogeography, Miocene, Japan.
Yuri Kimura [ykimura.research@gmail.com] and Yukimitsu Tomida [y-tomida@kahaku.go.jp], Department of Geology and Paleontology, National Museum of Nature and Science, 4-1-1 Amakubo, Tsukuba, Ibaraki, 305-0005, Japan.
Daniela C. Kalthoff [daniela.kalthoff@nrm.se], Department of Zoology, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden.
Isaac Casanovas-Vilar [isaac.casanovas@icp.cat], Grup de Faunes del Neogen i Quaternari, Institut Català de Paleontologia Miquel Crusafont, ICTA-ICP; Edifici Z. Carrer de les Columnes, s/n., Campus de la Universitat Autònoma de Barcelona, E-08193 Cerdanyola del Vallès, Barcelona, Spain.
Thomas Mörs [thomas.moers@nrm.se], Department of Palaeobiology, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden.
Received 17 October 2018, accepted 8 January 2019, available online 1 April 2019.
Copyright © 2019 Y. Kimura et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The study of the Eomyidae dates back to the late 19th century (Cope 1884; Schlosser 1884). However, these rodents were not discovered in Asia until one century later with the first discovery of the Asian species of Leptodontomys from the late Miocene of North China (Zheng and Li 1982). Later, Wang and Emry (1991) identified fossils from the late Oligocene deposits in Inner Mongolia, China, as new species of Eomys and Pseudotheridomys, both genera of which had been discovered from Europe and North America. Qiu (1994) pointed out that Leptodontomys from North China possesses dental features intermediate between European and North American species, suggesting the geographic importance of the region for eomyid rodents. Concurrently, a small eomyid rodent was discovered from Gifu Prefecture, central Japan, and was preliminarily assigned to Pseudotheridomys sp. (Tomida and Setoguchi 1994). However, further taxonomic studies had been restricted due to its limited material. A breakthrough for the fossil was made by recent findings of Asianeomys from various localities in Central and East Asia. The genus has been discovered from North China (Wu et al. 2006; Gomes Rodrigues et al. 2014; Qiu and Li 2016), Mongolia (Maridet et al. 2015), and Kazakhstan (Lopatin 2000). With richer eomyid materials found in Asia, the Asian species of Pseudotheridomys (P. asiaticus from Inner Mongolia, P. yanshini from Kazakhstan) and those of Eomyodon (E. dangheensis from Gansu Province, E. bolligeri from Kazakhstan and Mongolia) were all transferred to Asianeomys (Wu et al. 2006; Maridet et al. 2015). Accordingly, the preliminary identification of the Japanese eomyid is questioned because its taxonomic assignment to Pseudotheridomys sp. was due to morphological similarities to “Pseudotheridomys” asiaticus.
Here, we report that the Japanese eomyid, which was once considered to be Pseudotheridomys sp., is a new genus endemic to the coastal margin of continental Asia and discuss that its evolution implies some level of geographic barriers in the region for small mammals.
Institutional abbreviations.—NMNS, National Museum of Nature and Science (formerly NSM), Tokyo, Japan.
Other abbreviations.—Tooth terminology: M, molar; P, premolar; upper case letters indicate upper teeth, and lower case letters indicate lower teeth. EDJ, enamel-dentine-junction; FAD, first appearance datum; HSB, Hunter-Schreger bands; IPM; interprismatic matrix; LAD, last appearance datum; MN, Mammal Neogene Zone for European land mammal age; MPT, most parsimonious tree; PE, Portio externa; PI, Portio interna.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:525E9D08-16F7-42B8-BCE4-6DBB1AC14B14
Geological setting
The specimens described here were recovered from outcrops on the left bank of the Kiso River in Dota, Kani City, Gifu Prefecture, central Japan (Fig. 1). It is stratigraphically located near the uppermost level of the Nakamura Formation, Mizunami Group. The Nakamura Formation, about 150 m in thickness, consists mainly of fluvio-lacustrine conglomerate, sandstone, and mudstone in the lower part and alternation of fluvio-lacustrine sandstone and mudstone with occasional lignite in the middle and upper part. At the Dota locality, the outcrops represent about 10-meter-thick sediments near the uppermost of the Nakamura Formation. Terrestrial vertebrate fossils are concentrated in the alternation of fine sandstone, siltstone, and mudstone close to the top of the formation. To date, the Dota locality has yielded a handful of small mammal taxa (Tomida et al. 2013) along with an isolated cervoid tooth (Nishioka and Ando 2016). Besides terrestrial mammals, many fossils of freshwater fish (numerous fragmentary bones and isolated/partially articulated pharyngeal teeth), including amiid fish (Yabumoto and Grande 2013) and cyprinid fish (Yasuno 1982), and freshwater bivalves have been found in the alternated thin beds. The Nakamura Formation is dated to range ~19.8–18.4 Ma on the basis of fission-track dating (Shikano 2003). Thus, the fossil-bearing horizon is estimated to be around 18.5 Ma. The Dota fauna is more likely correlated to MN3 (i.e., 20–16.9 Ma) by a general faunal comparison (Tomida 2011), which is concordant with the absolute age estimate. For more details about the geologic age and biochronological correlation, see Tomida et al. (2013) and references therein.
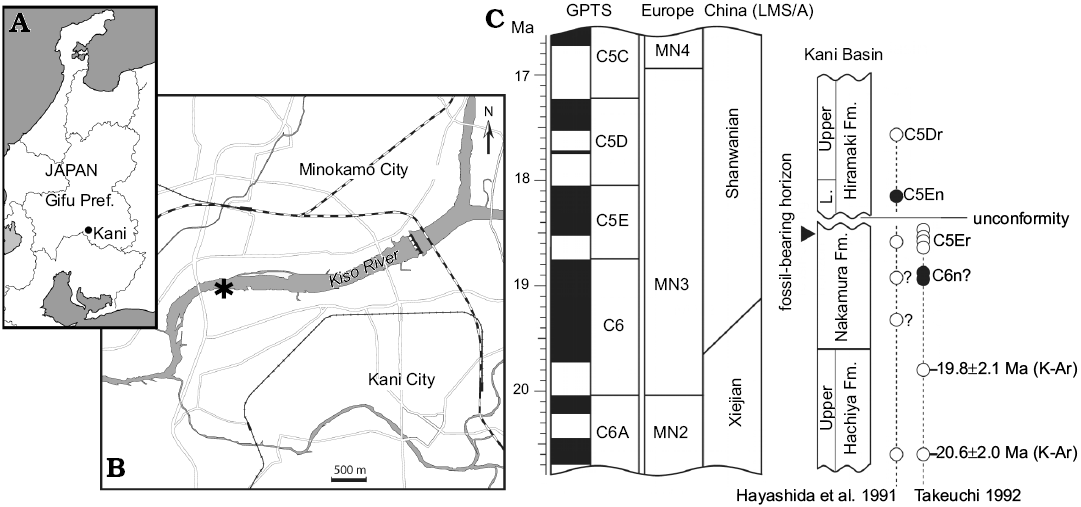
Fig. 1. Type locality and geologic age of Japaneomys yasunoi gen. et sp. nov. A, B. Location of Dota (asterisk) in Kani City, Gifu Prefecture, Japan (after Miyata and Tomida 2010). C. Stratigraphic position of the fossil bearing horizon and chronological relationship of the Miocene strata in Kani Basin (after Tomida et al. 2013). Absolute dates of the magnetozone boundaries follow Hilgen et al. (2012). The correlations of European MN zones and Chinese land mammal stage/age (LMS/A) follows Qiu et al. (2013). The black and white circles respectively indicate normal and reversed polarities of paleomagnetic samples from the sediments. Abbreviation: GPTS, Geomagnetic polarity timescale; L., Lower; Pref., Prefecture.
Material and methods
Tooth morphology and enamel structure.—Wang and Emry (1991) is followed for tooth terminology. Measurements were taken using a Leica DVM6 digital microscope with the instrumental precision of < 0.01 mm. In the isolated teeth, length was measured along a virtual axis in the maximum dimension, and width was measured perpendicular to this axis in the maximum dimension. The morphology of the p4 root was checked in MicroCT scanning images, which were obtained by the ScanXmate-E090 (Comscantechno) with a spatial resolution of 4.36 µm in the School of Dentistry at Tohoku University. Scanning Electron Microscope (SEM) images were taken at an acceleration voltage of 3 kV using the JSM-6510 (Jeol) scanning electron microscope at NMNS.
For the analysis of tooth enamel microstructure, a small portion of the lower incisor of NMNS-PV19994 was prepared, following the procedure detailed in Koenigswald (1980) and Kalthoff (2000). The enamel microstructure was studied and documented with a cold field emission scanning electron microscope Hitachi S-4300 at an acceleration voltage of 15 kV and at magnifications 90–2500× at the Swedish Museum of Natural History, Stockholm. The prepared material will be stored together with the type specimens at NMNS.
Phylogenetics.—To assess the relationship of Japanese eomyid with close relatives, we used cladistic analysis under maximum parsimony. As an underlying problem, we should note that phylogenetic analysis is challenging for the Eomyidae because most genera are diagnosed solely based on dental characters. Despite that character independence is assumed in morphology-based analyses, developmental studies have revealed that manipulation of developmental pathways can change the shape of teeth (e.g., Kangas et al. 2004; Harjunmaa et al. 2014; Marangoni et al. 2015), suggesting some level of character interdependency in tooth morphology. Not surprisingly, dental traits perform more poorly than osteological traits in resolving phylogeny of mammals (Sansom et al. 2017). Nevertheless, these findings do not necessarily reject dental morphology as phylogenetic characters, but they rather highlight the importance of rigorous tests for homoplasy ideally prior to phylogenetic analyses. In our analysis, we optimized the taxonomic selection of the ingroup based on our prior finding that a combination of dental characters which the Japanese eomyid possesses (four-rooted, hypolophid extending anteriorly) is present only in two other genera (Asianeomys and Keramidomys) and a descendant genus of Keramidomys across currently known 46 genera of the Eomyidae. Therefore, we consider the rarity of the dental traits to be related to the phylogenetic closeness among these genera.
The Japanese eomyid and 12 other ingroup taxa were scored for 19 dental characters (SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app64-Kimura_etal_SOM.pdf). We chose the dental characters based on the criteria shown in Gomes Rodrigues et al. (2009): minimal variation within a taxon; higher variation among taxa; not controlled by the effect of size; some degree of independence between upper and lower dentitions. As the criteria of homology, we used topological correspondence and connectivity of loph(id)s as in Candela and Rasia (2012). The data matrix is provided in SOM 2. For Asianeomys, three out of six species (A. fahlbuschi, A. asiaticus, and A. dangheensis) were included and scored based on direct observation. For Keramidomys, both of Chinese species (K. fahlbuschi and K. magnus) were included and scored based on direct observation, and four European species were chosen for analysis based on the availability of illustrations, SEM images, and detailed description from literature. Late Eocene Metanoiamys paradoxus, which is more basal than the studied genera at the subfamilial level (Flynn 2008), was defined as the outgroup. Further details for species selection are provided in SOM 1.
The matrix was built in Mesquite version 3.04 (Maddison and Maddison 2017a) and was run for cladistic analysis in TNT 1.5 (Goloboff and Catalano 2016). The Zephyr package (Maddison and Maddison 2017b) in Mesquite was additionally used for visual help. The exact search algorithm (Implicit enumeration) was performed with all characters equally weighted and unordered. The bootstrap value was calculated with 10 000 replicates under the same search algorithm, and the Bremer support was calculated with the default setting of aquickie.run. Two uninformative characters were excluded from the statistical calculation.
Time-scaled phylogenetic trees.—To compare the estimated nodal age of the Japanese eomyid with the geologic history of Japan, the resultant phylogenetic trees were time-scaled in two ways. The “equal” method was applied by the function DatePhylo in the R package strap (Bell and Lloyd 2015) using a root length (i.e., length of the base of the tree) of 1 million years, and the “minimum branch length (mbl)” method was applied by the function timePaleoPhy in the R package paleotree (Bapst 2012), using a fixed minimum branch length of 1 million years. Both time-scaling methods were applied to each most parsimonious tree (8 MPTs) separately, and the average age of the node between the Japanese eomyid and its sister was calculated for both methods. The 50% majority-rule consensus tree was also time-scaled for visualization purpose. For time calibration, the first appearance datum (FAD) and the last appearance datum (LAD) of the studied taxa were compiled (SOM 3) as stratigraphic occurrences from primary literatures supplemented by the Paleobiology Database (http://paleobiodb.org/#/) and the NOW Community (http://www.helsinki.fi/science/now/). We accessed both databases in July 2017. Because biostratigraphically inferred ages are often given as ranges, the median age of each range was taken for both FAD and LAD. If a species is known only from one locality, FAD and LAD were set as a given age ±1.0 Ma.
Systematic palaeontology
Order Rodentia Bowdich, 1821
Family Eomyidae Depéret and Douxamani, 1902
Genus Japaneomys nov.
Type species: Japaneomys yasunoi sp. nov., by monotypy, see below.
Etymology: A combination of Japan and Eomys, implying the occurrence of an eomyid genus in Japan.
Diagnosis.—As for the type and only known species.
Japaneomys yasunoi sp. nov.
Figs. 2–4.
ZooBank LSID: urn:lsid:zoobank.org:pub:525E9D08-16F7-42B8-BCE4-6DBB1AC14B14
1994 Pseudotheridomys sp.; Tomida and Setoguchi 1994: 191.
2011 Eomyidae gen. et sp. indet.; Tomida 2011: 2.
Etymology: Named after Toshikatsu Yasuno, who discovered the fossils while removing the matrix in search of fish fossils.
Type material: Holotype: NMNS-PV19995, isolated right m1. Paratype: NMNS-PV19994 fragmentary right dentary with incisor and p4 (Fig. 2).
Type locality: Dota Town, Kani City, Gifu Prefecture, central Japan.
Type horizon: Near the uppermost level of the Nakamura Formation, Mizunami Group, early Miocene (~18.5 Ma).
Diagnosis.—Japaneomys yasunoi possesses a combination of dental characters that are rare in the Eomyidae (Fig. 2): the presence of four roots in m1 and the hypolophid extending anteriorly to connect to the posterior ectolophid (≈ the anterior arm of hypoconid) on p4 and m1. These characters are present only in Asianeomys, Keramidomys, and Estramomys, the descendant genus of Keramidomys.
Differing from Asianeomys, Japaneomys yasunoi has a combination of the following characters: more bunodont pattern with lower lophids (i.e., very weak metalophid of p4 merged into the posterior wall of the metaconid and protoconid; mesoconid and ectolophid lower than hypoconid on p4; metalophid and hypolophid of m1, which run along pointy metaconid and entoconid, respectively, becoming very low in the talonid basin; hypolophid of m1 lower than the ectolophid), yet transverse lophids are complete, extending to the edge of the tooth; two rooted p4 (rather than three roots as in all species of Asianeomys but A. junggarensis); anterior lobe narrower than posterior lobe on m1 (correspondingly, synclinid I of m1 much shallower and shorter than half the length of synclinid IV); hypolophid anteriorly concave on m1.
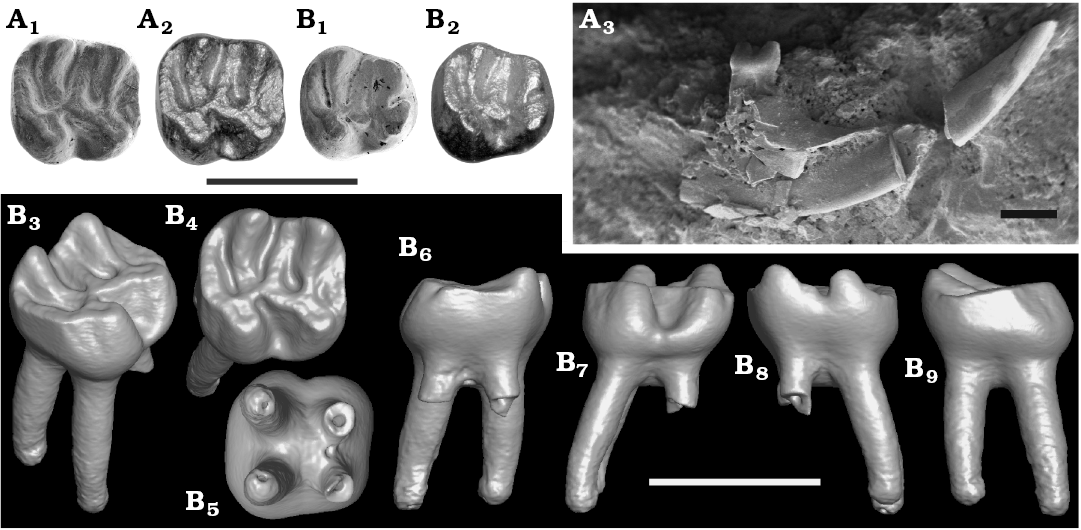
Fig. 2. Eomyid rodent Japaneomys yasunoi gen. et sp. nov. from the Dota locality, early Miocene. A. NMNS-PV19994, paratype, right p4. B. NMNS-PV19995, holotype, m1. In occlusal view (A1, A2, B1, B2); SEM images (A1, B1), HDR images (A2, B2). Labial view of the right mandible with p4 (A3). Surface model of m1: isometric (B3), occlusal (B4), bottom (B5), anterior (B6), labial (B7), lingual (B8), and posterior (B9) views. Scale bars 1 mm.
Shared with Asianeomys but differing from Keramidomys: absence of anteroconid on p4; anterior ectolophid connecting to the posterior wall of the protoconid rather than the occlusal surface of the protoconid on p4. Similar in size to Keramidomys, smaller than Asianeomys (Fig. 3).
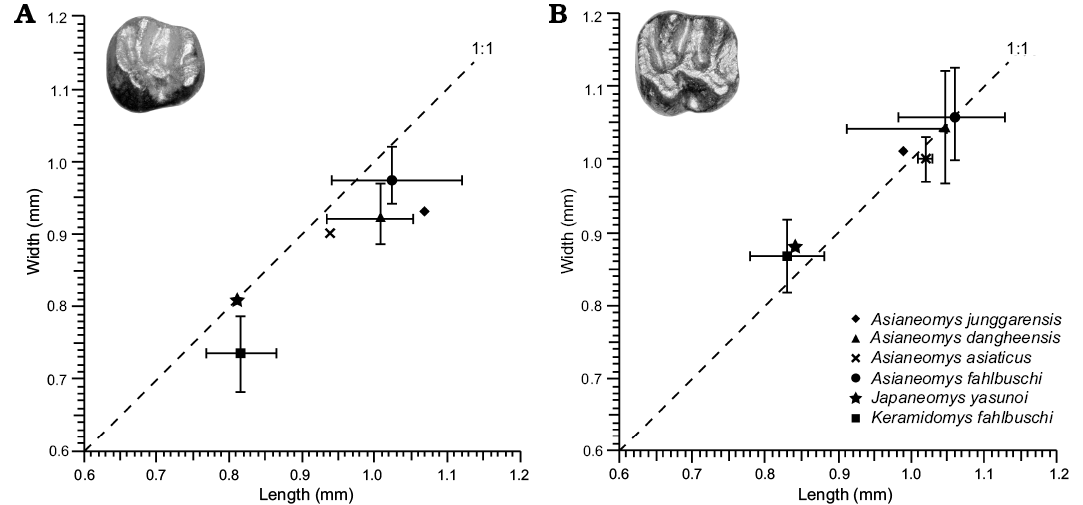
Fig. 3. Tooth measurements of Japaneomys, Asianeomys, and Keramidomys. Each symbol represents the mean of measurements, which were taken from the primary literature (Wang and Emry 1991; Wang 2002; Wu et al. 2006; Maridet et al. 2015; Qiu and Li 2016). Error bars represent the range of the measurements.
Description.—The right mandible is heavily damaged, with only the anterior half of the corpus preserved, retaining complete p4 in the tooth socket and a portion of the incisor. The lower incisor is oval in cross section (Fig. 4B). The outer enamel surface is smooth and shows a shallow groove towards the labial side of the tooth. The lower m1 was isolated from the jaw probably during the preparation work, but the tooth sockets of m1 and m2 are preserved. The m2 and m3 were not discovered. The diastema is relatively shallow. The mental foramen is not preserved. The eomyid mandible is very uniform (Engesser 1999), and that of Japaneomys yasunoi appears to be similar to that of other genera such as Eomys.
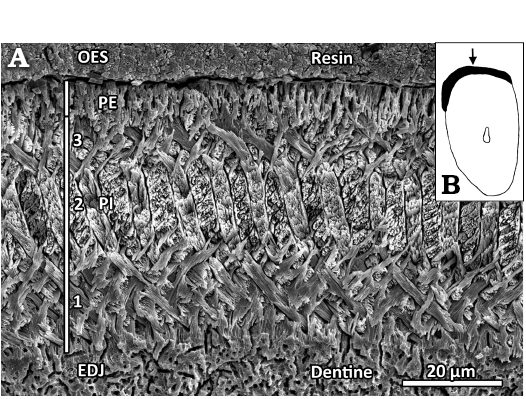
Fig. 4. Lower incisor (NMNS-PV19994, paratype) of eomyid rodent Japaneomys yasunoi gen. et sp. nov. A. SEM image of the enamel microstructure. Transverse section showing the four-layered schmelzmuster with a thick, tripartite Portio interna (PI) and a thin Portio externa (PE); EDJ, enamel-dentine-junction; OES, outer enamel surface. This section is located directly right (medial) to the arrow in B. B. Schematic drawing of the incisor cross section. The enamel band is shown in black, the solid arrow points to the shallow groove. The magnification is about ×26.
Lower fourth premolar (p4): Slightly worn specimen. Length 0.81 mm, width 0.63 mm in the anterior lobe, and 0.80 mm in the posterior lobe (Fig. 3). Bunodont tooth with two roots, which are not bifurcated even at the apex of each root as confirmed in CT image. Trapezoidal outline with round corners in occlusal view. The width of the posterior lobe is almost equal to the length of the tooth. There is no indication of the anteroconid. The metaconid and protoconid are of the same size and are aligned transversely without relative displacement of either cusp. These cusps are very weakly connected posteriorly by an extremely weak and low metalophid that is merged into the posterior wall of the metaconid and the protoconid (Fig. 2). The posterior side of the metaconid and protoconid form a single wall without being individualized. The anterior ectolophid is connected to the base of the protoconid at the posterolingual corner. The sinusid is nearly M-shaped. A long mesolophid is directed anterolingually and reaches the posterolingual corner of the metaconid, but it is extremely low. A mesostylid is present. The mesoconid has a bulbous base, which leaves the anterior and posterior ectolophid extremely short. The occlusal surface of the mesoconid and the ectolophid are lower than that of the hypoconid. The hypolophid runs downward steeply along the anteroposteriorly-compressed entoconid and becomes flat and low in the talonid basin, weakly connecting to the posterior ectolophid. The hypoconid is less compressed than the entoconid and is lower than the level of the metaconid and protoconid. A robust posterolophid is connected with the posterolingual corner of the hypoconid without narrowing its width and extends along the tooth edge to the posterolingual corner of the entoconid. All synclinids are narrow, forming V-shaped valleys. The synclinids II and IV are closed by the mesolophid and posterolophid, respectively. The synclinid IV is deeper than the synclinids II and III due to the strong posterolophid.
Lower first molar (m1): Slightly worn specimen. Length 0.84 mm, width 0.77 mm in the anterior lobe, and 0.88 mm in the posterior lobe (Fig. 3). Bunodont tooth with four roots. Quadrate outline in occlusal view, the longitudinal axis of the tooth is in the same length as the width of the tooth. The anterior lobe (formed by the metaconid, protoconid, and anterolophid) is transversely narrower than the posterior lobe (formed by the entoconid, hypoconid, and posterolophid) by ~10% (Fig. 2). The metaconid and entoconid are slightly displaced anteriorly compared to the protoconid and hypoconid, respectively. Due to the slight displacement of metaconid and the narrow anterior lobe, the synclinid I is significantly shallower and shorter than the synclinid IV by 67%, and the anterolophid is only half the length of the posterolophid although it reaches to the lingual side of the tooth, closing the synclinid I. The anterolophid is separated from the protoconid by an extremely shallow and narrow groove, making the labial side of the synclinid I closed. The anteroposteriorly-compressed metaconid and entoconid are high and pointy, resulting in the metalophid and hypolophid running steeply downward along the cusps and becoming flat and low on the talonid basin before they connect to the center of the protoconid and the posterior ectolophid, respectively. In the talonid basin, the hypolophid is lower than the level of the ecotolophid and the labial cusps, whereas the metalophid is at the same level as them (Fig. 2C). The metalophid is straight, whereas the hypolophid is anteriorly concave, directed posterolabially on the slope of the hypocone and anterolabially on the talonid basin, leaving the syncinid III narrower and shallower than the synclinid II on the labial side. The synclinid II is about half the length of the synclinid III because a low mesolophid is directed anterolingually to the posterior wall of the metaconid and closes the synclinid II with the mesostylid. A mesoconid is present but does not have a bulbous base. A well-developed posterolophid slightly lowers its height lingually but reaches the lingual side of the tooth, attaching to the posterior base of the hypocone and closing the synclinid IV.
Incisor enamel microstructure: A small fragment of the lower incisor of the paratype specimen of Japaneomys yasunoi (NMNS-PV19994) was sectioned transversally (Figs. 2B, 4B). The enamel band is thin and measures about 52 µm thick in its center. The enamel band slightly thickens laterally, thus forming a very shallow groove along the long axis of the tooth (arrow in Fig. 4B). As it is typical for eomyid incisors, the Portio interna (PI) consists of longitudinally oriented uniserial Hunter-Schreger bands (HSB), and the thin Portio externa (PE) is composed of radial enamel (about 9 µm in thickness, 17% of the enamel band thickness). The PI is split into three well-separated layers: the interprismatic matrix (IPM) runs perpendicular to the prisms in the innermost layer (about 20 µm, 38%) at the enamel-dentine-junction (EDJ) as well as in the outermost layer (about 9 µm, 17%) at the PI/PE junction, while the IPM is oriented parallel to the prisms in the middle layer (about 14 µm, 27%). Although being uniserial, the HSB frequently show double bands. The individual prisms are mostly round in the innermost and outermost layer of the PI, oval-shaped in the middle layer of the PI and lancet-shaped in the PE. No starting zone is present at the EDJ.
Stratigraphic and geographic range.—Type locality and horizon only.
Phylogenetic analysis
The analysis of 14 taxa with 17 informative dental characters found eight most parsimonious trees (consistency index = 0.78, retention index = 0.87, tree length = 32). The strict and 50% majority-rule consensus trees are shown respectively in SOM 3. Weak Bremer supports in the resultant trees would reflect the availability of a low number of dental traits to resolve phylogenetic relationships of the closely related taxa. Keramidomys is more strongly supported than other genera and possesses four synapomorphies: lophodont teeth (character 0); anterior ectolophid of p4 connecting to the occlusal surface of the protoconid (character 5); metaconid and protoconid of p4 connected through a long metalophid (character 6); entoloph of M1 open to syncline II due to the lack of the anterior entoloph in more than 50% of specimens (character 17). In Keramidomys, European species (K. carpathicus, K. ermannorum, K. mohleri, K. thaleri) are more derived than Asian species with two synapomorphic characters: mesoconid of m1 absent (character 10); synclinid II forming an anterior ring (character 12). This supports the hypothesis that Keramidomys originated in East Asia (Mein 2009) in comparison to an older hypothesis that Keramidomys is locally descended from Pseudotheridomys (Fahlbusch 1975, 1979; Hartenberger 1966). The scarcity of Asian endemic eomyid fossils resulted in the collapse of the branches for Japaneomys and Asianeomys in the strict consensus tree.
Incisor enamel microstructure
Japaneomys retains plesiomorphic traits in the lower cheek teeth, whereas the incisor enamel microstructure of the genus can be regarded as highly derived, showing multi-layered, longitudinally oriented HSB in the PI, making up most of the entire enamel thickness and showing only a thin layer of radial enamel in the PE. The thickening of the enamel band toward the lateral side is known as a unique character for eomyid lower incisors (Wahlert and Koenigswald 1985). However, Japaneomys yasunoi shows a hitherto unknown four-layered schmelzmuster instead of a three-layered schmelzmuster which was described by Wahlert and Koenigswald (1985) as being typical for all eomyids. A manuscript exploring this new character within a broad sample of the eomyid family is in preparation by some of the authors (DCK, YK, TM). The meaning of the relative frequent double bands in the PI cannot be evaluated at present.
Discussion
Miocene micromammals and paleogeography of Japan.—Japaneomys is a rodent component of the Dota fauna, which consist mostly of micromammal fossils. Table 1 lists continental-scale geographic distributions of the Dota micromammals at the generic level. Among them, five out of seven genera have been discovered outside Japan, and three of the five genera are known both from Europe and North America. The Dota Plesiosorex fejfari represents the first discovery of the genus in East Asia (Oshima et al. 2017). Other holarctically-distributed taxa are two rodents, eomyid Megapeomys and castorid Euroxenomys. For both genera, the Dota species represents the first discoveries of the genera from Asia (Tomida 2011; Mörs and Tomida 2018). The Dota Megapeomys repenningi possesses dental features more basal to European species, M. lavocati and M. lindsayi (Fejfar et al. 1998; Tomida 2011; Mörs and Flink 2018), and to North American M. bobwilsoni (Morea and Korth 2002). The Dota Euroxenomys nanus is one of the oldest records of the genus (Mörs and Tomida 2018). Thus, the Dota micromammals are significant for the chronological occurrence and geographic location to consider evolutionary pathways of the lineages and intercontinental dispersals. To date, eomyid Japaneomys and castorid Minocastor are the only genera endemic to the early Miocene of central Japan (i.e., the Dota locality).
Based on accumulated evidence of geological and paleomagnetic studies (e.g., Otofuji et al. 1985; Takahashi 1994; Kano et al. 2002; Martin 2011), tectonic processes of back-arc rifting opened the Japan Sea and rotated the southwest Japan Arc clockwise and the northeast Japan Arc counterclockwise in a double-door opening manner. The drifting initiated in the latest Oligocene/early Miocene and ceased in the middle Miocene with the climax of rifting at ~16 Ma (Fig. 5B). This means that, at the time of the Dota micromammals, their habitats were located in the coastal margin of continental Asia rather than paleo-islands of Japan. In fact, terrestrial deposits in the Kani Basin are interbedded with volcaniclastic sediments (Shikano 1995), associated with tectonic activities related to back-arc rifting during the opening of the Japan Sea. The wide biogeographic distributions of many Dota micromammals indicate feasible accessibility to the region without critical geographic barriers from inner-continental regions. This is congruent with the geologic history of Japan and with biogeographic distributions of large mammal fossils (e.g., Gomphotherium, Anchitherium, Plesiaceratherium, cf. Brachypotherium) found from the studied area (Table 1; Tassy 1994; Miyata and Tomida 2010; Fukuchi and Kawai 2011). Nevertheless, the presence of two endemic genera suggests that paleogeography of Japan would have allowed some degree of isolation for certain rodent lineages to evolve independently from populations in inner-continental regions. The influence of dynamic geographic change during the evolution of the Japanese islands on mammals in different body sizes with various dispersal abilities will be of further interest in future studies.
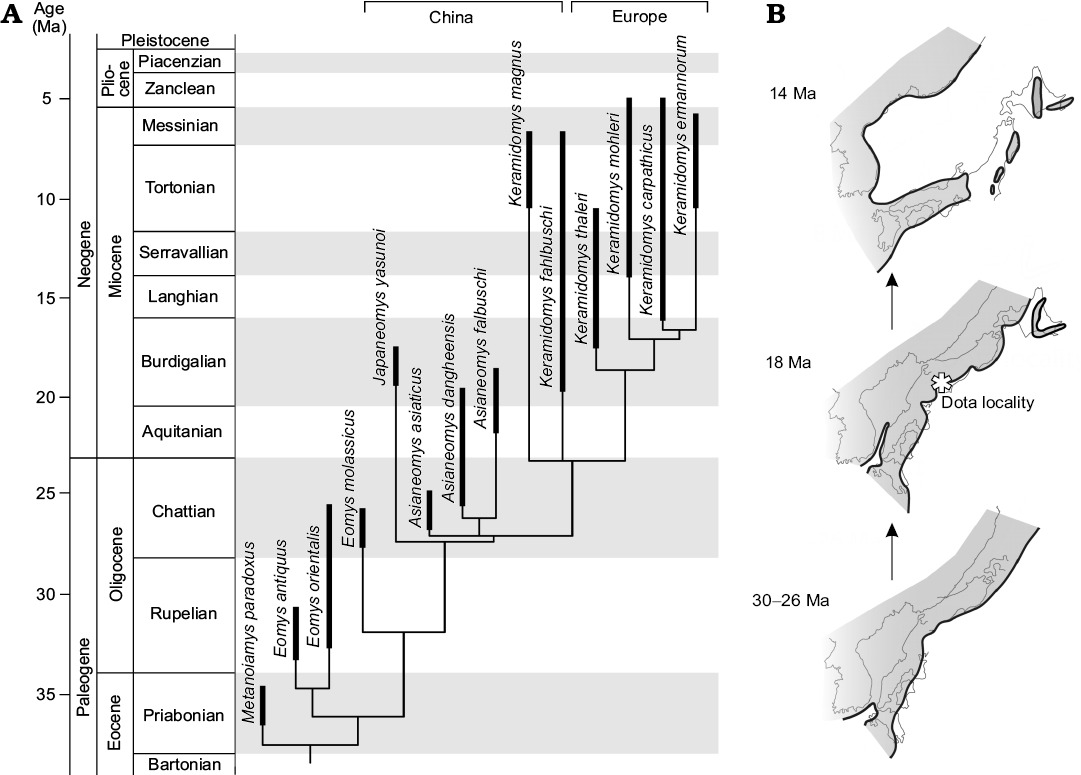
Fig. 5. Time-scaled phylogeny of eomyid rodents and paleogeography of Japan. A. Time-scaled 50% majority-rule tree of eomyid rodents, using the “equal” scaling method. B. Schematic paleogeography of the eastern margin of Asia from the Oligocene to the middle Miocene. Noda and Goto (2004) and NUMO (2004) were referred for the schematic figure, both of which compiled paleogeographic and tectonic information from the primary literatures cited therein. Black lines with shaded area indicate shorelines and land masses. Light gray lines show current coastlines of both Japan and east Asia. Note that Japan was part of the continent during the late Oligocene and progressively drifted during the Miocene. More details are provided in the text.
Endemism of Japaneomys.—Based on progressive change of dental traits from the early Oligocene to the early Miocene and the finding of intermediate Asianeomys cf. A. bolligeri in Mongolia, it is suggested that Asianeomys was to be a local Asian descendant that evolved from Eomys (Maridet et al. 2015). In our study, Japaneomys was positioned to be sister to the more lophodont genera, Asianeomys and Keramidomys. The nodal age between Japaneomys and its sister branch was estimated from time-scaled MPTs to be 27.3 ± 0.05 Ma (1σ) in the “equal” scaling method and to be 29.7 ± 0.4 (1σ) Ma in the “mbl” scaling method (Fig. 5A). Despite that different algorithms and parameters give different results, the estimated ages predate the opening of the Japan Sea and the formation of the Japanese islands (Ogasawara 1994; Takahashi 1994; Taira 2001; Kano et al. 2002). Therefore, the divergence between Japaneomys and its sister taxa occurred in the late Oligocene of East Asia, when Japan was part of the coastal margin of continental Asia. Japaneomys retained plesiomorphic dental features even afterwards, compared to Asianeomys and especially to contemporaneous species of Keramidomys.
Table 1. Continental-scale geographic distribution of mammals of the Dota and Hiramaki faunas at the generic level.
|
Order |
Family |
Genus |
Japan (Gifu Prefecture) |
continental Asia |
Europe |
North America |
References for the Japan record |
|
|
Dota fauna |
Hiramaki fauna |
|||||||
|
Soricomorpha |
Plesiosoricidae |
Plesiosorex |
+ |
|
+ |
+ |
+ |
|
|
Lagomorpha |
Ochotonidae |
cf. Amphilagus |
? |
|
+ |
+ |
|
undescribed |
|
Rodentia |
Castoridae |
Euroxenomys |
+ |
|
|
+ |
+ |
|
|
Youngofiber |
+ |
|
+ |
|
|
|||
|
Minocastor |
+ |
|
|
|
|
|||
|
Eomyidae |
Megapeomys |
+ |
|
|
+ |
+ |
||
|
Japaneomys |
+ |
|
|
|
|
this study |
||
|
Proboscidea |
Gomphotheriidae |
Gomphotherium |
|
+ |
+ |
+ |
+ |
|
|
Perissodactyla |
Equidae |
Anchitherium |
|
+ |
+ |
+ |
+ |
|
|
Rhinocerotidae |
Brachypotherium |
|
+ |
+ |
+ |
|
||
|
Plesiaceratherium |
|
+ |
+ |
+ |
|
|||
|
Artiodactyla |
Cervoidea |
Gen. et sp. indet. |
+ |
+ |
+ |
+ |
+ |
|
Conclusions
The small-sized eomyid from central Japan, which was originally assigned to Pseudotheridomys sp., is indeed a new endemic genus, named Japaneomys herein. The genus combines a number of plesiomorphic features in the cheek teeth with derived incisor enamel. Although dental morphology of the upper teeth of the new genus is unknown, a direct comparison of the genus with rich collections of Asianeomys fahlbuschi and Keramidomys fahlbuschi (a basal species of Keramidomys) from Inner Mongolia confirmed the validity of the new genus, separate from Asianeomys and more derived Keramidomys species. A phylogenetic analysis found that it diverged earlier than Asianeomys and Keramidomys, likely in the late Oligocene, which is well before the opening of the Japan Sea. This indicates that, in the eastern margin of continental Asia, some degree of geographic isolation allowed allopatric speciation of certain small mammals apart from inner continental taxa. The Dota fauna continues to be a remarkable Miocene fossil assemblage in understanding the role of intercontinental dispersal of small mammals as many represent the first record of the taxa in Asia, and also in evaluating the role of geographic barriers during the evolution of the Japanese islands and its interplay with mammals in different body sizes.
Acknowledgements
The authors deeply thank Qiu Zhuding and Li Qiang (both Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China) for their constructive advice, and Jiangzuo Qigao (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China) for his help in accessing the collections. We are grateful to the following people: Osamu Sasaki (The Tohoku University Museum, Sendai, Japan), Li Lüzhou (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China), Yoshinori Arai and Toshihiko Amemiya (both Nihon University, Tokyo, Japan) for CT scanning; Oldřich Fejfar (Charles University, Prague, Czech Republic) for access to the collection of Merkur North in Bohemia and discussion as well as for sampling and preparing the incisor fragment for microstructure analysis; Megumi Saito and Mika Yagishita (both NMNS, Tokyo, Japan) for imaging; Yusuke Ando (Mizunami Fossil Museum, Japan) and Takahisa Goda (Konan, Japan) for their help in the field. This work was mainly funded by the National Museum of Nature and Science, JSPS KAKENHI Grant Number JP18K13650 (Grant-in-Aid for Early-Career Scientists), and the Fujiwara Natural History Foundation (2018). Financial supports by Grant-in-Aid for Scientific Research (c) (1995–1997) and the Fujiwara Natural History Foundation (1998) were provided to YT. DCK acknowledges the Deutsche Forschungsgemeinschaft (DFG, Bonn, Germany) for various grants to study mammalian enamel microstructure. IC-V has received financial support from the Spanish Ministerio de Economía, Industria y Competitividad, the Agencia Estatal de Investigación and the European Regional Development Fund of the European Union (projects CGL2016-76431-P, AEI/FEDER EU, CGL2017-82654-P MINECO/FEDER EU; and research contract RYC-2013-12470), and the Generalitat de Catalunya (CERCA Programme). I C-V belongs to consolidated research group SGR 2017 116 of the Generalitat de Catalunya. TM was financially supported by the Royal Swedish Academy of Sciences (Stockholm, Sweden) and by the Japan Society for the Promotion of Science. We thank the editor Oliver Lambert, the reviewer Paloma López-Guerrero, and an anonymous reviewer for providing us constructive criticism and helpful comments for earlier drafts of this manuscript.
References
Bapst, D.W. 2012. Paleotree: An R package for paleontological and phylogenetic analyses of evolution. Methods in Ecology and Evolution 3: 803–807. Crossref
Bell, M.A. and Lloyd, G.T. 2015. Strap: An R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58: 379–389. Crossref
Candela, A.M. and Rasia, L.L. 2012. Tooth morphology of Echimyidae (Rodentia, Caviomorpha): Homology assessments, fossils, and evolution. Zoological Journal of the Linnean Society 164: 451–480. Crossref
Cope, E.D. 1884. The Vertebrata of the Tertiary formations of the West. U.S. Geological Survey of the Territories 3: 1–1044.
Emry, R.J., Wang, B.-Y., Tjutkova, L.A., and Lucas, S.G. 1997. A late Eocene eomyid rodent from the Zaysan Basin of Kazakhstan. Journal of Vertebrate Paleontology 17: 229–234. Crossref
Engesser, B. 1999. Family Eomyidae. In: G.E. Rossner, and K. Heissig (eds.), The Miocene Land Mammals of Europe, 319–335. Verlag Dr Friedrich Pfeil, Münich.
Fahlbusch, V. 1975. Die Eomyiden (Rodentia, Mammalia) der Oberen Süßwasser-Molasse Bayerns. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie 15: 63–90.
Fahlbusch, V. 1979. Eomyidae – Geschichte einer Säugetierfamilie. Paläontologische Zeitschrift 53: 88–97. Crossref
Fejfar, O., Rummel, M., and Tomida, Y. 1998. New eomyid genus and species from the early Miocene (MN zones 3–4) of Europe and Japan related to Apeomys (Eomyidae, Rodentia, Mammalia). National Museum of Nature and Science Monographs 14: 123–143.
Flynn, L.J. 2008. Eomyidae. In: C.M. Janis, G.F. Gunnell, and M.D. Uhen (eds.), Evolution of Tertiary Mammals of North America: Volume 2, Small Mammals, Xenarthrans, and Marine Mammals, 415–427. Cambridge University Press, New York. Crossref
Fukuchi, A. and Kawai, K. 2011. Revision of fossil rhinoceroses from the Miocene Mizunami Group, Japan. Paleontological Research 15: 247–257. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Gomes Rodrigues, H., Marivaux, L., and Vianey-Liaud, M. 2009. Phylogeny and systematic revision of Eocene Cricetidae (Rodentia, Mammalia) from Central and East Asia: on the origin of cricetid rodents. Journal of Zoological Systematics and Evolutionary Research 48: 259–268. Crossref
Gomes Rodrigues, H., Marivaux, L., and Vianey-Liaud, M. 2014. Rodent paleocommunities from the Oligocene of Ulantatal (Inner Mongolia, China). Palaeovertebrata 38 [published online, https://doi.org/ 10.18563/pv.38.1.e3]. Crossref
Hartenberger, J.L. 1966. Les rongeurs du Vallésien (Miocène supérieur) de Can Llobateres (Sabadell, Espagne); Gliridae et Eomyidae. Bulletin de la Société géologique de France 7: 596–604.
Harjunmaa, E., Seidel, K., Häkkinen, T., Renvoisé, E., Corfe, I.J., Kallonen, A., Zhang, Z.-Q., Evans, A.R., Mikkola, M.L., Salazar-Ciudad, I., Klein, O.D., and Jernvall, J. 2014. Replaying evolutionary transitions from the dental fossil record. Nature 512: 44–48. Crossref
Hilgen, F.J., Lourens, L.J., and Van Dam, J.A. 2012. The Neogene Period. In: F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G. Ogg (eds.), The Geological Time Scale 2012, 923–978. Elsevier, Amsterdam. Crossref
Kalthoff, D.C. 2000. Die Schmelzmikrostruktur in den Incisiven der hamsterartigen Nagetiere und anderer Myomorpha (Rodentia, Mammalia). Palaeontographica A 259: 1–193.
Kangas, A.T., Evans, A.R., Thesleff, I., and Jernvall, J. 2004. Nonindependence of mammalian dental characters. Nature 432: 211–214. Crossref
Kano, K., Yoshikawa, T., Yanagisawa, Y., Ogasawara, K., and Danhara, T. 2002. An unconformity in the early Miocene syn-rifting succession, northern Noto Peninsula, Japan: Evidence for short-term uplifting precedent to the rapid opening of the Japan Sea. Island Arc 11: 170–184. Crossref
Koenigswald, W. von 1980. Schmelzstruktur und Morphologie in den Molaren der Arvicolidae (Rodentia). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 539: 1–129.
Lopatin, A. V. 2000. New Early Miocene Aplodontidae and Eomyidae (Rodentia, Mammalia) from the Aral Formation of the Altynshokysu. Paleontological Journal 34: 198–202.
Maddison, W.P. and Maddison, D.R. 2017a. Mesquite: a Modular System for Evolutionary Analysis. Version 3.04 [http://mesquiteproject.org]
Maddison, D.R. and Maddison, W.P. 2017b. Zephyr: A Mesquite Package for Interacting with External Phylogeny Inference Programs. Version 2.01 [https://mesquitezephyr.wikispaces.com].
Marangoni, P., Charles, C., Tafforeau, P., Laugel-Haushalter, V., Joo, A., Bloch-Zupan, A., Klein, O.D., and Viriot, L. 2015. Phenotypic and evolutionary implications of modulating the ERK-MAPK cascade using the dentition as a model. Scientific Reports 5: 11658. Crossref
Maridet, O., Daxner-Höck, G., Badamgarav, D., and Göhlich, U.B. 2015. The eomyid rodents (Mammalia) from the Oligocene and Miocene of the Valley of Lakes (Central Mongolia). Palaontologische Zeitschrift 89: 207–228. Crossref
Martin, A.K. 2011. Double saloon door tectonics in the Japan Sea, Fossa Magna, and the Japanese Island Arc. Tectonophysics 498: 45–65. Crossref
Mein, P. 2009. The Miocene Keramidomys (Rodentia, Eomyidae) from the Sandelzhausen locality (Germany). Paläontologische Zeitschrift 83: 141–150. Crossref
Miyata, K. and Tomida, Y. 2010. Anchitherium (Mammalia, Perissodactyla, Equidae) from the Early Miocene Hiramaki Formation, Gifu Prefecture, Japan, and its implication for the early diversification. Journal of Paleontology 84: 763–773. Crossref
Morea, M.F. and Korth, W.W. 2002. A new eomyid rodent (Mammalia) from the Hemingfordian (Early Miocene) of Nevada and its relationship to Eurasian Apeomyinae (Eomyinae). Paludicola 4: 10–14.
Mörs, T. and Flink, T. 2018. Large apeomyine rodents (Mammalia, Eomyidae) from the early Miocene of Echzell, Germany. Historical Biology 30 (8): 1102–1111. Crossref
Mörs, T. and Tomida, Y. 2018. Euroxenomys nanus sp. nov., a minute beaver (Rodentia, Castoridae) from the Early Miocene of Japan. Paleontological Research 22: 145–149. Crossref
Mörs, T., Tomida, Y., and Kalthoff, D.C. 2016. A new large beaver (Mammalia, Castoridae) from the early Miocene of Japan. Journal of Vertebrate Paleontology 36: e1080720. Crossref
Nishioka, Y. and Ando, Y. 2016. A cervoid tooth from the lower Miocene Nakamura Formation of the Mizunami Group in Kani City, Gifu Prefecture, central Japan. Bulletin of the Mizunami Fossil Museum 42: 39–44.
Noda, Y. and Goto, M. 2004. Paleogeographic maps of the Japanese Islands and their application to exhibition of the Fukui Prefectural Dinosaur Museum. Memoir of the Fukui Prefectural Dinosaur Museum 3: 47–63.
NUMO 2004. Evaluating Site Suitability for a HLW Repository. Scientific Background and Practical Application of NUMO’s Siting Factors. Nuclear Waste Management Organization of Japan, NUMO-TR-04-04. Tokyo.
Ogasawara, K. 1994. Neogene paleogeography and marine climate of the Japanese Islands based on shallow-marine molluscs. Palaeogeography, Palaeoclimatology, Palaeoecology 108: 335–351. Crossref
Oshima, M., Tomida, Y., and Orihara, T. 2017. A new species of Plesiosorex (Mammalia, Eulipotyphla) from the Early Miocene of Japan: First record of the genus from East Asia. Fossil Imprint 73: 1–8. Crossref
Otofuji, Y., Matsuda, T., and Nohda, S. 1985. Opening mode of the Japan Sea inferred from the paleomagnetism of the Japan Arc. Nature 317: 603–604. Crossref
Qiu, Z.-D. 1994. Eomyidae in China. In: Y. Tomida, C.K. Li, and T. Setoguchi (eds.), Rodent and Lagomorph Families of Asian Origins and Diversification. National Science Museum Monographs 8: 49–55.
Qiu, Z.-D. and Li, Q. 2016. Neogene rodents from central Nei Mongol, China. Palaeontologia Sinica 198 (New Series C): 1–676.
Qiu, Z.-D., Wang, X.-M., and Li, Q. 2013. Neogene faunal succession and biochronology of Central Nei Mongol (Inner Mongolia). In: X. Wang, L.J. Flynn, and M. Fortelius (eds.), Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology, 155–186. Columbia University Press, New York. Crossref
Sansom, R.S., Wills, M.A., and Williams, T. 2017. Dental data perform relatively poorly in reconstructing mammal phylogenies: morphological partitions evaluated with molecular benchmarks. Systematic Biology 66 (5): 813–822. Crossref
Schlosser, M. 1884. Die Nager des europäischen Tertiärs nebst Betrachtungen über die Organisation und die geschichtliche Entwicklung der Nager überhaupt. Palaeontographica 21: 19–162.
Shikano, K. 1995. Stratigraphy of the Nakamura Formation [in Japanese]. In: Minokamo City Education Board (ed.), Stratigraphy and Fossils of the Nakamura Formation in the Minokamo Basin, 2–18. Minokamo City Education Board, Minokamo.
Shikano, K. 2003. Fission track ages of the Lower Miocene Mizunami Group in the Minokamo Basin, Gifu Prefecture, central Japan. [in Japanese]. Memoirs of the Minokamo City Museum 2: 1–8.
Taira, A. 2001. Tectonic evolution of the Japanese island arc system. Annual Review of Earth and Planetary Sciences 29: 109–134. Crossref
Takahashi, M. 1994. Miocene lateral bending of central Japan: Intra-arc deformation at arc-arc collision zone. Bulletin of the Geological Survey of Japan 45: 477–495.
Tassy, P. 1994. Gaps, parsimony, and early Miocene elephantoids (Mammalia), with a re-evaluation of Gomphotherium annectens (Matsumoto, 1925). Zoological Journal of the Linnean Society 112: 101–117. Crossref
Tassy, P. 1996. The earliest gomphotheres. In: J. Shoshani and P. Tassy (eds.), The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives, 89–91. Oxford University Press, Oxford.
Tomida, Y. 2011. A new species of the genus Megapeomys (Mammalia, Rodentia, Eomyidae) from the Early Miocene of Japan. Palaeontologia Electronica 14 (3): 25A.
Tomida, Y. and Setoguchi, T. 1994. Tertiary rodents from Japan. In: Y. Tomida, C.-K. Li, and T. Setoguchi (eds.), Rodent and Lagomorph Families of Asian Origins and Diversification. National Science Museum Monographs 8: 185–195.
Tomida, Y., Nakaya, H., Saegusa, H., Miyata, K. and Fukuchi, A. 2013. Miocene land mammals and stratigraphy of Japan. In: X.-M. Wang, L.J. Flynn, and M. Fortelius (eds.), Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology, 314–333. Columbia University Press, New York. Crossref
Wahlert, J.H. and Koenigswald, W. von 1985. Specialized enamel in incisors of eomyid rodents. American Museum Novitates 2832: 1–12.
Wang, B.-Y. 2002. Discovery of late Oligocene Eomyodon (Rodentia, Mammalia) from the Danghe area, Gansu, China. Vertebrata PalAsiatica 40: 139–145.
Wang, B.-Y. and Emry, R.J. 1991. Eomyidae (Rodentia: Mammalia) from the Oligocene of Nei Mongol, China. Journal of Vertebrate Paleontology 11: 370–377. Crossref
Wu, W.-Y., Meng, J., Ye, J., and Ni, X.-J. 2006. The first finds of eomyids (Rodentia) from the Late Oligocene–Early Miocene of the Northern Junggar Basin, China. Beitträge zur Paläontologie 30: 469–479.
Yabumoto, Y. and Grande, L. 2013. A new Miocene amiid fish, Amia godai from Kani, Gifu, Central Japan. Paleontological Research 17: 113–126. Crossref
Yasuno, T. 1982. Fossil pharyngeal teeth of sub-family Cyprininae fishes collected from the Miocene Mizunami Group in Kani Basin, Gifu Prefecture, Japan [in Japanese]. Bulletin of the Mizunami Fossil Museum 9: 15–23.
Zheng, S.-H. and Li, Y. 1982. Some Pliocene lagomorphs and rodents from Loc. 1 of Songshan, Tianzu Xian, Gansu Province. Vertebrata PalAsiatica 20: 35–44.
Acta Palaeontol. Pol. 64 (2): 303–312, 2019
https://doi.org/10.4202/app.00558.2018