

Uncovering the hidden diversity of Paleogene sponge fauna of the East European Platform through reassessment of the record of isolated spicules
MAGDALENA ŁUKOWIAK, ANDRZEJ PISERA, and TETIANA STEFANSKA
Łukowiak, M., Pisera, A., and Stefanska, T. 2019. Uncovering the hidden diversity of Paleogene sponge fauna of the East European Platform through reassessment of the record of isolated spicules. Acta Palaeontologica Polonica 64 (4): 871–895.
Despite being reported from various localities and stratigraphic intervals, knowledge of the siliceous sponges from the Cenozoic of Eastern Europe remains surprisingly limited. Studies assessing their diversity are almost exclusively in Russian and rather hard to obtain. The most comprehensive elaboration of the sponge spicules from the Paleogene of the East European Platform was published in 2003 and deals with material from Ukraine, Russia, Belarus, and Lithuania. However, the classification in that paper is purely artificial and extremely difficult to interpret according to modern biological criteria. A reassessment of this material is carried out, with the aim of revising all morphotypes of spicules, and identifying them to the lowest possible taxonomic level. Results suggest that the assemblage is much more diverse than previously thought, including members of 24 demosponge families (class Demospongiae), one homoscleromorph (class Homoscleromorpha), and at least one hexactinellid (class Hexactinellida). Our improved understanding of the diversity of Paleogene sponge fauna of the East European Platform will have implications for the interpretation of the past and future ecological and paleobiogeographic studies.
Key words: Demospongiae, Homoscleromorpha, Hexactinellida, spicules, Ukraine, Russia, Eocene.
Magdalena Łukowiak [mlukowiak@twarda.pan.pl] and Andrzej Pisera [apis@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Science, Twarda 51/55 00-818 Warszawa, Poland.
Tetiana Stefanska [t.stefanska@ukr.net], Institute of Geological Sciences, National Academy of Science of Ukraine, O. Gonchar St. 55-B 02054 Kiev, Ukraine.
Received 20 February 2019, accepted 26 July 2019, available online 15 November 2019.
Copyright © 2019 M. Łukowiak et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Modern sponges are inherent components of marine benthic communities with notable ecological roles (Díaz and Rützler 2001; Bell 2008; de Goeij et al. 2013; Mueller et al. 2014; Łukowiak et al. 2018) and substantial share in terms of their biomass (Conway et al. 2001; Hooper et al. 2002; Pansini and Longo 2003; Samaai 2006; van Soest 2007; van Soest et al. 2012). As can be expected, the same is true for Paleogene and Neogene paleocenoses worldwide (Pisera 1999).
To infer the origins of Recent sponge associations, numerous studies have focused on Cenozoic assemblages which have proved to be of unexpectedly diverse and abundant character. These include, for example, rich sponge faunas from the Eocene of Australia and New Zealand (Hinde and Holmes 1892; Hinde 1910; Kelly and Buckeridge 2005; Buckeridge et al. 2013; Łukowiak 2015, 2016; Łukowiak and Pisera 2016) and the Eocene and Miocene of Europe (Pisera and Busquets 2002; Matteucci and Russo 2005; Pisera et al. 2006; Frisone et al. 2014, 2016).
Loose sponge spicules have been described from the Paleogene and Neogene of the Atlantic Ocean (Ivanik 1983; Palmer 1988) and current reports show spicule-rich deposits from the Paleogene of central Ukraine (e.g., Konenkova et al. 1996; Pisera 2000; Ivanova 2014; Stefanska 2014, 2015; Stefanska and Stefansky 2014). Neogene assemblages include spicules from the lower Miocene of the Vienna Basin (Łukowiak et al. 2014), middle Miocene of Poland (Hurcewicz 1991), Portugal (Pisera et al. 2006), Czech Republic (Pisera and Hladilová 2004), Bosnia and Herzegovina (Ivanik 2002), Croatia (Pezelj et al. 2016), and southern Ukraine (Ivanova and Olshtynska 2004).
The most comprehensive study of disassociated sponge spicules from the Paleogene of the East European Platform is that of Ivanik (2003). The study deals with sponge spicules from numerous outcrops and boreholes situated in Ukraine, Russia (the Volga region, Don River Basin, Kaliningrad region), Belarus, and Lithuania. Nevertheless, the morphotypes of spicules described in that study were assessed only artificially and classified within parataxonomic units that are based purely on geometrical characters of the spicules.
Despite the richness of the sponges studied by Ivanik (2003), the study remains virtually unknown and inaccessible as it was published in a book that is hard to obtain and, except for the summary, written in Russian. Moreover, Ivanik (2003) offers only very general and brief discussion of biological meaning of parataxonomically-described material.
Unfortunately, Ivanik’s (2003) biological conclusions are only postulative, not demonstrative, i.e., he refers to numerous sponge taxa that supposedly existed in the study area during the Paleogene but does not illustrate the indicated spicule types, preventing independent evaluation. For example, Ivanik (2003) refers to the presence of “lychniscosans” (order Lychniscosida Schrammen, 1903) or Thrombus Sollas, 1886, but does not illustrate spicules that belong to these taxa. As such, the results of the study cannot be used directly to interpret sponge evolution, ecology, or paleobiogeography: while the work is comprehensive and rich, the utility of the publication is limited.
The aim of our study is to reassess Ivanik’s (2003) material in the light of currently accepted poriferan systematics by revising all spicule morphotypes and classifying them to the lowest possible taxonomic level. We also compare the spicules in Ivanik (2003) to their modern counterparts, allowing inferences of increased diversity to be made about the Paleogene sponges of the East European Platform. The study is expected to serve as a baseline for future ecological and paleobiogeographic reconstructions of ancient sponge fauna of this region.
Assignment of spicules to taxa
The plates published by Ivanik (2003: pls. 1–13) were reassessed following currently accepted systematics (see Boury-Esnault and Rützler 1997; Morrow and Cárdenas 2015) and rearranged to Figs. 1–10, with respect to their taxonomic affinities. The illustrations were slightly edited to improve their quality. Possible taxonomic affinities are summarized in Table 1. The most characteristic spicules are re-described, and their taxonomic position discussed in some detail, below. Additional spicule illustrations of Ivanik (2003: pls. 14–24) are not reproduced or re-illustrated as they are of poor quality, rendering the determination of spicule difficult. However, some of these are referred to in the text, and a reassessment of these spicules is presented briefly in Table 2.
Table 1. Taxonomic affinity of spicules from the East-European Platform and adjacent regions illustrated by Ivanik (2003).
|
Taxonomical attribution |
Figure |
Plate, respectively |
Spicule |
Stratigraphical position |
|
Astrophorina |
1Q |
11: 1 |
centrotylote ?oxea |
upper Eocene |
|
1S |
11: 2 |
plagiotriaene |
middle Eocene |
|
|
1P, T, U |
11: 5, 6, 3 |
triaenes |
upper Eocene |
|
|
1R |
11: 4 |
?triaene |
lower Eocene |
|
|
1I |
11: 7 |
triaene |
middle Eocene |
|
|
7AB |
13: 26 |
?amphiaster |
middle Eocene |
|
|
?Astrophorina |
2N, O |
1: 3, 4 |
oxeas |
upper Eocene |
|
2S |
11: 9 |
?triaene |
middle Eocene |
|
|
Geodiidae |
1X |
7: 1 |
mesotriaene |
middle Eocene |
|
1K |
7: 10 |
mesotriaene |
upper Eocene |
|
|
1V |
12: 1 |
irregular triaene |
upper Eocene |
|
|
?Geodiidae |
2W–Y |
8: 8, 9, 7 |
pinakids |
upper Eocene |
|
Geodiidae |
1W |
7: 2 |
style |
middle Eocene |
|
Geodia or Caminus sp. |
1C, E, J, N |
12: 3, 7, 11, 8 |
sterrasters |
upper Eocene |
|
Geodia sp., Caminus sp. or Tethyida |
1B |
12: 12 |
spheraster |
Upper Cretaceous |
|
Geodiidae, Ancorinidae, or Pachastrellidae |
7V |
4: 10 |
orthotriaene |
middle Eocene |
|
Geodiidae, Vulcanellidae |
1M |
5: 13 |
dichotriaene |
upper Eocene |
|
Geodiidae, Ancorinidae, or Pachastrellidae |
1AB |
6: 1 |
dichotriaene |
upper Eocene |
|
Ancorinidae (O), Geodiidae,
Ancorinidae, |
1L, O |
5: 4, 3 |
anatriaenes |
middle–upper Eocene |
|
Geodiidae or Theneidae |
1Y, AA |
7: 5, 6 |
diaenes |
middle Eocene |
|
Erylus sp. |
1A, G, H |
12: 10, 6, 9 |
aspidasters |
upper Eocene |
|
?Dercitus sp. |
2Q |
4: 11 |
orthotriaene |
lower Oligocene |
|
?Penares sp. |
2Z |
8: 2 |
dichotriaene |
middle Eocene |
|
Ancorinidae |
2E |
4: 6 |
protriaene |
middle Eocene |
|
7S |
4: 17 |
anatriaene |
upper Eocene |
|
|
2R, 1Z |
7: 8, 7 |
diaenes |
middle Eocene |
|
|
2K, U, V |
6: 4, 5, 6 |
anadichotriaenes |
upper Eocene |
|
|
Stelletta sp. |
2C |
4: 4 |
protriaene |
middle Eocene |
|
2A |
4: 7 |
protriaene |
lower Oligocene |
|
|
2B, D |
4: 8, 9 |
protriaenes |
lower Eocene, lower Oligocene |
|
|
2F, J |
4: 14 5: 2 |
anatriaenes |
upper Eocene |
|
|
2G |
4: 16 |
protriaene |
upper Eocene |
|
|
2H |
5: 6 |
anatriaene |
middle Eocene |
|
|
2I |
5: 9 |
prodichotriaene |
upper Eocene |
|
|
2L, P |
5: 10, 12 |
prodichotriaenes |
Cretaceous, middle Eocene |
|
|
2T |
6: 2 |
orthodichotriaene |
upper Eocene |
|
|
?Stelletta sp. |
2M |
5: 1 |
anatriaene |
upper Eocene |
|
Pachastrellida |
3S |
13: 16 |
?plesiaster |
middle Eocene |
|
3L |
3: 18 |
tylostyle |
upper Eocene |
|
|
3T |
5: 7 |
calthrop |
middle Eocene |
|
|
3E |
5: 11 |
prodichotriaene |
upper Eocene |
|
|
3G |
5: 14 |
dichotriaene |
middle Eocene |
|
|
3O |
6: 9 |
mesotriaene |
middle Eocene |
|
|
3D, I–K, P |
6: 8, 7, 12, 10, 13 |
mesotriaenes |
lower Eocene |
|
|
3U |
12: 18 |
oxyaster |
upper Eocene |
|
|
?Pachastrellidae |
3A |
4: 15 |
anatriaene |
lower Oligocene |
|
3F |
5: 8 |
calthrop |
lower Eocene |
|
|
3C |
5: 5 |
anatriaene |
middle Eocene |
|
|
3B |
6: 3 |
orthodichotriaene |
upper Eocene |
|
|
Triptolemma sp. |
3M, N |
7: 17; 11: 12 |
mesodichotriaenes |
upper, lower Eocene |
|
Taxonomical attribution |
Figure |
Plate, respectively |
Spicule |
Stratigraphical position |
|
Pachastrellidae or Calthropellidae |
3H |
5: 15 |
dichotriaene |
middle Eocene |
|
3Q, R |
9: 12, 14 |
triods |
middle Eocene |
|
|
Theneidae |
4A |
4: 5 |
protriaene |
middle Eocene |
|
Alectona sp. |
4P, R, T |
1: 14, 16, 18 |
acanthoxeas |
upper Eocene |
|
4O |
2: 3 |
acanthoxea |
middle Eocene |
|
|
4I–K |
9: 9, 10, 8 |
acanthotriods |
middle–upper Eocene |
|
|
Theonellidae |
9E, R |
7: 15; 8: 13 |
phyllotriaenes |
middle Eocene |
|
9H |
7: 18 |
phyllotriaene |
upper Eocene |
|
|
9A, C |
8: 1, 3 |
phyllotriaenes |
Upper Cretaceous, middle Eocene |
|
|
9X, Z |
8: 17, 11 |
tetracrepid desmas |
lower Eocene, middle Eocene |
|
|
9L, O, P |
9: 4, 3, 6 |
phyllotriaenes with ornamented cladome |
Upper Cretaceous, middle–upper Eocene |
|
|
Theonellidae (?Discodermia sp.) |
9D, F, G |
8: 4, 5, 6 |
discotriaenes |
middle–upper Eocene |
|
Pleromidae |
9S |
8: 18 |
megaclone |
upper Eocene |
|
9T, U, V, Y |
8: 15, 16; 7: 16; 8: 12 |
megaclones |
Upper Cretaceous |
|
|
9W |
8: 14 |
?megaclone |
Paleocene |
|
|
9J, K, N, Q |
9: 1, 2, 7; 11: 15 |
megaclones |
Upper Cretaceous, lower Eocene |
|
|
Samus anonymus |
4E, G, H |
13: 27; 11: 14; 7: 11 |
amphitriaenes |
upper Eocene |
|
?Samus anonymus |
4F |
7: 12 |
broken ?amphitriaene |
upper Eocene |
|
Tetillidae |
4D |
1: 11 |
centrotylote oxea |
middle Eocene |
|
?Tetillidae |
4B, C |
4: 12, 13 |
anatriaene |
upper Eocene |
|
Agelas sp. |
4L, M |
2: 1, 2 |
verticillate oxeas |
upper Eocene |
|
4N |
3: 14 |
verticillate style |
upper Eocene |
|
|
Tethyida |
5Z |
12: 17 |
oxyaster |
middle Eocene |
|
5AE, AG |
12: 19, 20 |
oxyasters |
upper Eocene |
|
|
5AF |
12: 22 |
oxyaster |
upper Eocene |
|
|
?Tethyida |
5AH, AI |
12: 13, 14 |
spherasters |
middle Eocene |
|
5Y |
12: 15 |
oxyspheraster |
middle Eocene |
|
|
?Axinelliade |
5O, P |
13: 4, 3 |
ophirhabds |
middle–upper Eocene |
|
Axinellidae |
5Q–T |
1: 10, 9, 7, 8 |
flexuous oxeas |
middle–upper Eocene |
|
Plocamione sp. |
5F–K |
13: 18, 19, 20, 21 |
acanthostrongyles |
middle–upper Eocene |
|
?Plocamione sp. |
5L, N |
3: 7, 12 |
acanthostyles |
upper Eocene |
|
5E |
3: 17 |
exotyle |
upper Eocene |
|
|
5M |
4: 1 |
acanthostyle |
middle Eocene |
|
|
Janulum sp. |
5B–D |
2: 13, 14, 15 |
strongyles |
middle Eocene |
|
?Janulum sp. |
5A |
2: 12 |
strongyle |
upper Eocene |
|
Haplosclerida |
5AA–AD |
13: 7–10 |
microstrongyles |
middle–upper Eocene |
|
Petrosiidae |
5V–X |
2: 7, 11, 8 |
strongyles |
middle–upper Eocene |
|
Dotona sp. |
5U |
2: 18 |
acanthostrongyle |
upper Eocene |
|
Placospongia sp. |
1D, F |
12: 4, 5 |
selenasters |
middle Eocene |
|
Spirastrellidae |
6X |
12: 21 |
spheraster |
middle Eocene |
|
Sphaerostylus sp. |
6B, C |
3: 3a, 3b |
tylostyles |
middle–upper Eocene |
|
6A |
3: 2 |
spherostyle |
middle Eocene |
|
|
?Sphaerostylus sp. |
6D |
3: 4 |
tylostyle |
middle–upper Eocene |
|
?Mycale or ?Sphaerotylus sp. |
6G |
3: 22 |
exotyle |
upper Eocene |
|
Mycale sp. |
6H |
4: 2 |
exotyle |
middle Eocene |
|
Histodermella sp. |
6Y, Z |
2: 20, 21 |
tylotes |
middle Eocene |
|
6N, O |
1: 13, 12 |
acanthoxeas |
middle–upper Eocene |
|
|
Microcionidae |
6E, F, J, M |
3: 5, 6, 16, 13 |
acanthostyles |
upper Eocene |
|
Myxilla sp. |
6I, K |
3: 8, 15 |
acanthostyles |
upper Eocene |
|
6U, V |
13: 14, 15 |
anisochelaes |
upper Eocene |
|
|
Merlia sp. |
6L |
13: 25 |
clavidisc |
upper Eocene |
|
Taxonomical attribution |
Figure |
Plate, respectively |
Spicule |
Stratigraphical position |
|
Zyzzya sp. |
6P–R |
2: 19, 16, 17 |
acanthostrongyles |
upper Eocene |
|
?Crellidae |
4S |
1: 17 |
sanidaster |
upper Eocene |
|
Poecilosclerida |
6W |
13: 17 |
isochela |
middle Eocene |
|
?Poecilosclerida |
6S, T |
13: 1, 2 |
sigmas |
upper Eocene |
|
Demospongiae |
7C–E |
1: 1; 13: 13; 1: 2 |
oxeas |
middle–upper Eocene |
|
7G |
2: 4 |
acanthorhabd |
upper Eocene |
|
|
7L, O, Q |
2: 22; 3: 1; 7: 3 |
styles |
upper Eocene |
|
|
7K, M, N |
3: 11, 9, 10 |
acanthostyles |
upper Eocene |
|
|
7W, X |
3: 20, 19 |
tylostyles |
middle Eocene |
|
|
7Z |
3: 21 |
exotyle |
upper Eocene |
|
|
7P |
3: 23 |
style |
middle Eocene |
|
|
7R |
7: 9 |
reduced triaene |
middle Eocene |
|
|
7AC |
12: 16 |
spheraster |
upper Eocene |
|
|
7T, U |
13: 5,6 |
unknown |
upper, middle Eocene |
|
|
Demospongiae (?Heteroxyidae) |
7F, H |
2: 5, 6 |
acanthoxeas |
upper Eocene |
|
?Demospongiae |
7A, B |
1: 5, 6 |
oxeas |
middle–upper Eocene |
|
7I, J |
2: 9, 10 |
acanthostrongyles |
middle–upper Eocene |
|
|
7AA |
11: 13 |
fragment of a ?triaene |
upper Eocene |
|
|
Lithistid demosponge |
9M |
9: 5 |
siliceous plate |
middle Eocene |
|
9B |
12: 2 |
?sphaeroclone |
lower Eocene |
|
|
Demospongiae or homoscleromorph |
8P |
5: 16 |
calthrop |
lower Eocene |
|
8N |
6: 14 |
dichotriaene |
middle Eocene |
|
|
Placinolopha sp. |
8I, J |
7: 14, 13 |
lophocalthrops |
upper Eocene |
|
8H, O |
11: 8; 13: 24 |
broken ?lophodiactine |
middle Eocene |
|
|
8F, G |
13: 22, 23 |
amphiclads |
upper Eocene |
|
|
?Placinolopha sp. |
8K |
11: 11 |
?lophocalthrop |
lower Eocene |
|
Hexactinellida |
8C, 10L |
9: 16, 15 |
hexactines |
middle–upper Eocene |
|
10C–E |
10: 3, 2, 4 |
pinular hexactines |
upper Eocene |
|
|
10F, I, H |
10: 5, 7, 8 |
pinular hexactines |
middle Eocene |
|
|
10G |
10: 6 |
smooth hexactine |
middle Eocene |
|
|
?Hexasterophora |
10K |
10: 13 |
hexactine |
middle Eocene |
|
?Lyssacinosida |
8B, 10B |
9: 14, 13 |
pentactines |
middle Eocene |
|
10M |
9: 17 |
stauractine |
upper Eocene |
|
|
?Rossellidae |
10A |
10: 1 |
pentactine |
middle Eocene |
|
10J |
10: 9 |
tauactine |
upper Eocene |
|
|
?Pheronematidae |
9I |
10: 10 |
anchorate spicule |
upper Eocene |
|
?Hexactinellida |
8D, E |
10: 11, 12 |
?oxeas |
middle–upper Eocene |
|
8A |
11: 10 |
?fragment of dictyonal skeleton |
lower Eocene |
|
|
incerte sedis |
7Y, 8M |
7: 4; 4: 3 |
spicule fragment |
upper Eocene |
|
8L |
6: 11 |
spicule fragment |
lower Eocene |
|
|
9M |
11: 15 |
siliceous plate |
middle Eocene |
|
|
8Q |
8: 10 |
fragment of a diatom |
middle Eocene |
Table 2. Taxonomic affinity of spicules from the East-European Platform and adjacent regions illustrated by Ivanik (2003).
|
Taxonomical attribution |
Plate |
Spicule |
|
Geodiidae |
17: 1–3; 18: 1 |
sterrasters |
|
Geodiidae (?Erylus sp.) |
15: 2; 16: 1–3 |
aspidasters |
|
14: 2 |
sterraster (aspidaster) |
|
|
Geodia or Caminus sp. |
15: 1 |
sterraster |
|
?Geodia or Caminus sp. |
14: 1 |
sterraster |
|
?Geodiidae |
20: 2 |
?plesiaster |
|
Pachastrellidae? |
19: 5 |
oxyspheraster |
|
Astrophorid demosponge |
22: 2 |
prodichotriaene |
|
Demosponge (?Geodiidae) |
24: 2 |
pinakid |
|
Alectona sp. |
21: 3, 4 |
acanthotriods |
|
Lithistid demosponge (Corallistidae?) |
22: 3 |
dichotriaene |
|
Lithistid demosponge (?Theonellidae) |
22: 4 |
tetraclone desma |
|
23: 2 |
phyllotriaene |
|
|
Lithistid demosponge (Pleromidae) |
22: 5 |
megaclone |
|
Lithistid demosponge (?Pleromidae) |
23: 1 |
ornamented dichotriaene |
|
Lithistid demosponge |
23: 3 |
ornamented dichotriaene |
|
Tethyida |
19: 4 |
oxyaster |
|
Plocamione sp. |
19: 3 |
acanthostrongyle |
|
Petrosiidae sp. |
18: 2–4 |
strongyles |
|
?Mycale
or |
20: 7 |
spherotyle |
|
Histodermella sp. |
20: 8, 9; 21: 1 |
acanthoxeas |
|
Zyzzya sp. |
18: 5; 19: 1 |
acanthostrongyles |
|
21: 2 |
acanthostrongyle |
|
|
?Poecilosclerida |
20: 3 |
sigma |
|
Demospongiae |
20: 4 |
oxea |
|
20: 5 |
strongyle |
|
|
20: 6 |
style |
|
|
19: 2 |
acanthostyle |
|
|
Hexactinellida |
23: 4; 24: 1 |
pinular hexactines |
|
incerte sedis |
20: 1 |
spheraster or ascidian spicule |
|
22: 1 |
triod |
Class Demospongiae Sollas, 1885
Subclass Heteroscleromorpha Cárdenas, Pérez, and Boury-Esnault, 2012
Order Tetractinellida Marshall, 1876
Suborder Astrophorina Sollas, 1887
Family Geodiidae Gray, 1867
Geodiidae indet.
Figs. 1C, E, J, N, 2Z.
Material.—Upper and middle Eocene, south-central Ukraine.
Remarks.—The bean-shaped to round massive microscleres with small projections tightly arranged on the spicule surface are considered to be sterrasters (Ivanik 2003: pl. 14: 1, 2, pl. 15: 1, 2; Fig. 1C, E, J, N ). They are found in two geodiid genera, Geodia Lamarck, 1815 and Caminus Schmidt, 1862 (compare Cárdenas et al. 2011 and van Soest et al. 2014: fig. 9). The spherical microsclere (Fig. 1B) may also belong to one of these genera (but affinity with Placospongia Gray, 1867 or even Tethyidae cannot be ruled out as well due to poor preservation). Likewise, the big, slender mesotriaenes with a characteristic shaft protruding from the both sides (Fig. 1K, I, V) are probably of geodiid affinity (compare Geodia garoupa in Carvalho et al. 2016 or G. praelonga in Sim-Smith and Kelly 2015). The same is true for characteristic tetraxial spicule with a long shaft and reduced two clads (Fig. 1W) that may belong to some Geodia (former Isops) species. Monaxonic spicules with bulb-shaped ending called exotyles (Fig. 5E) could be assigned to Geodia (Isops) but their vulcanellid, hadromerid, or raspailiid affinity is also possible (see Cárdenas et al. 2011). The same is true for some anatriaenes (Fig. 1L) and orthodichotriaenes (Ivanik 2003: pl. 22: 3; Fig. 1M, AB) which may belong to Geodiidae, Ancorinidae, or Pachastrellidae.
Amongst the illustrated spicules the plesiaster (Ivanik 2003: pl. 20: 2) most likely belongs to a geodiid sponge as well. However, more accurate taxonomic assignment is not possible due to the lack of characteristic features of this spicule.
Moreover, the short-shafted dichotriaene with flat, long leaf-shaped clads (Fig. 2Z) resembles triaenes of modern Penares Gray, 1867, especially those of P. sclerobesa Topsent, 1904 (compare Topsent 1904: pl. 10: 13). However, also some astrophorid lithistid demosponges are characterized by similar spicule types.
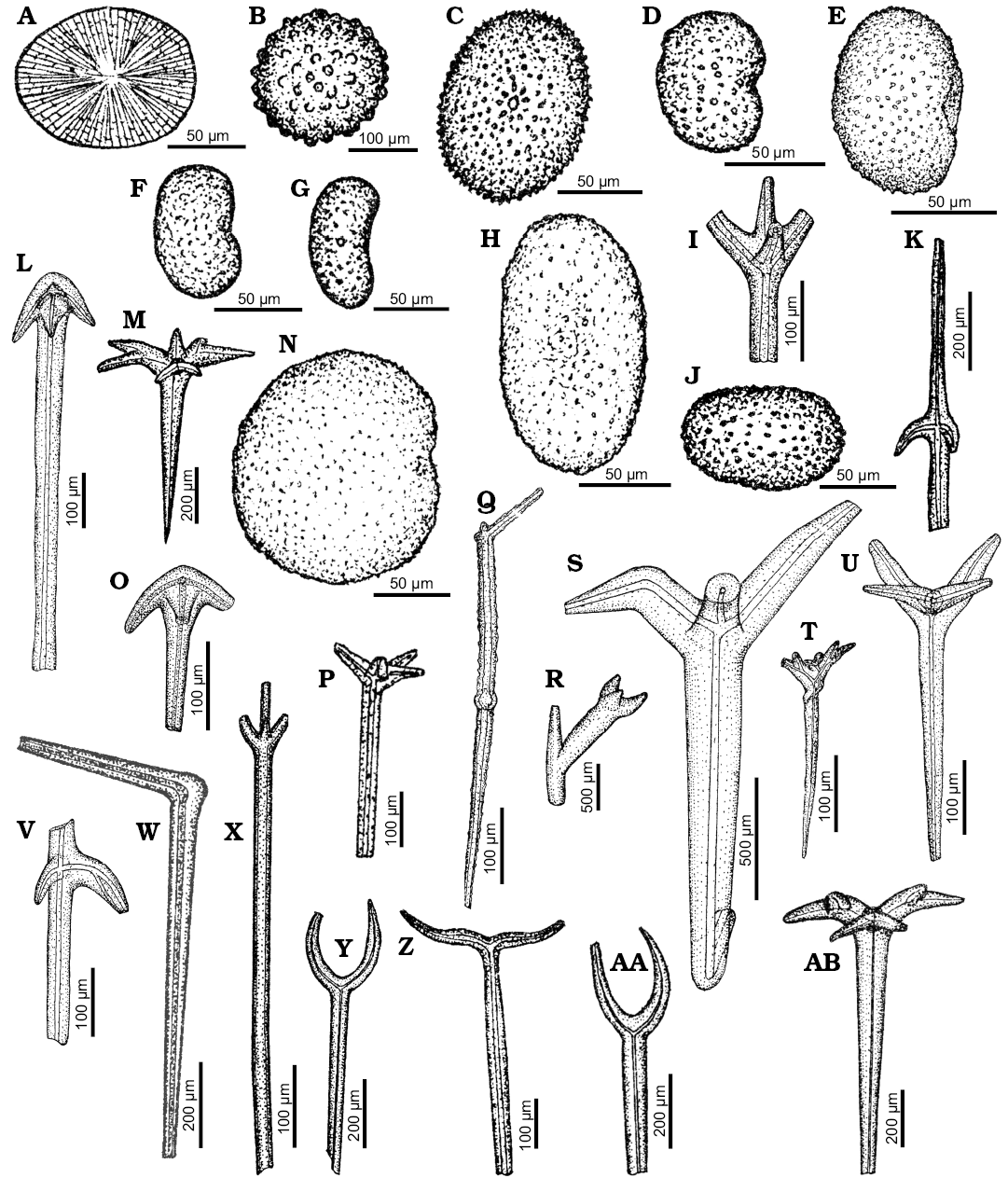
Fig. 1. Spicule morphotypes from south-central Ukraine; Grigorevka borehole, upper Eocene (B), Glâdov Âr, Upper Cretaceous (A, G, J), Pogonovka borehole, upper Eocene (C), Russkie Tiŝki, middle Eocene (D, F, I, O, W, X, Z, AA), Markovka, upper Eocene (E, H, N), Nikol’skoe, upper Eocene (K, L, Q, T, U, V), Pesčanoe, upper Eocene (M, AB), Kantemirovka, upper Eocene (P), Harkov region, middle Eocene (S), Verhnee, lower Eocene (R), and Staroverovka, middle Eocene (Y). A, G, H. Aspidasters. B. Spheraster. C, E, J, N. Sterrasters. D, F. Selenasters. I. Triaene. K, X. Mesotriaene. L, O. Anatriaenes. M, AB. Dichotriaenes. Q. Centrotylote ?oxea. R. ?Triaene. S. Plagiotriaene. P, T, U. Triaenes. V. Irregular triaene. W. Style. Y–AA. Diaenes. After Ivanik (2003); modified.
Genus Erylus Gray, 1867
Type species: Erylus mammillaris (Schmidt, 1862), Azores Canaries Madeira, Adriatic Sea, Recent.
Erylus sp.
Fig. 1A, G, H.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The flat, ellipsoidal spicules called aspidasters (Fig. 1A, G, H) are characteristic for geodiid Erylus Gray, 1867 (subfamily Erylinae Sollas, 1888; compare Adams and Hooper 2001: fig. 3I). Also other spicules (Fig. 5E, M) and some of the aspidasters (e.g., Fig. 1H), could belong to some other erylinin sponges e.g., Pachymatisma monaena Lendenfeld, 1907 (compare Lendenfeld 1907: pl. 35: 21–46). However, this assignment is highly speculative.
Family Ancorinidae Schmidt, 1870
Ancorinidae indet.
Figs. 1O, Z, 2E, H, K, R, U, V.
Material.—Lower, middle–upper Eocene, south-central Ukraine.
Remarks.—Spicules of ancorinid affinity are anatriaenes (Fig. 2H, K, U, V) and diaenes with wavy clads (Figs. 1Z, 2R). The latter ones resemble spicules of modern Tribrachium Weltner, 1882 (compare with van Soest 2017: fig. 51F). Also some of the pro- (Fig. 2E) and anatriaenes (Fig. 1O) could belong to ancorinids, e.g., Ecionemia Bowerbank, 1862 (compare van Soest and Beglinger 2009: fig. 7).
There are other spicules that could be assigned to Ancorinidae, e.g., those from the Fig. 2Q (?Dercitus) and Fig. 10B (?Stryphnus; compare Sollas 1888: pls. 15, 6: 4–6); however, the later one can be also a dermal pentactine of Hexactinellida.
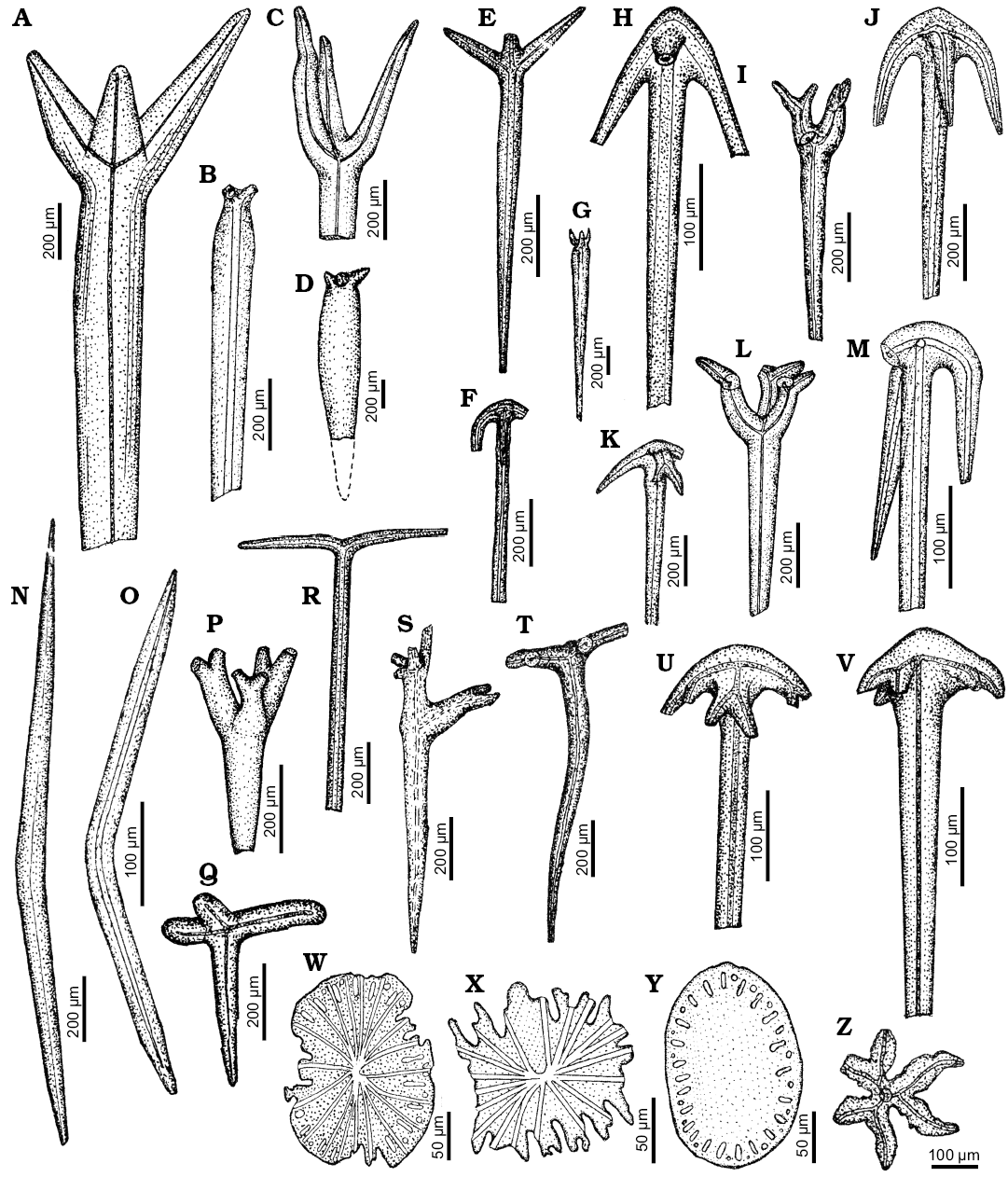
Fig. 2. Spicule morphotypes from south-central Ukraine; Čečva, lower Oligocene (A, B), Staroverovka, middle Eocene (C, L), Verhnee, lower Eocene (D), Russkie Tiŝki, middle Eocene (E, H, R, Z), Nikol’skoe, upper Eocene (F, I, J, K, M, T), Černoleska, upper Eocene (G), Markovka, upper Eocene (N), Melovoe, middle Eocene (O), Glâdov Âr, Cretaceous (P), Markovka, lower Oligocene (Q), Lipcy, middle Eocene (S), Pogonovka, upper Eocene (U), Melovoe, upper Eocene (V), Pesčanoe, upper Eocene (W, Y), and Kiselevka, upper Eocene (X). A–E, G. Protriaenes. F, H, J, K, M, U, V. Anatriaenes. I, L, P. Prodichotriaenes. N, O. Oxeas. Q. Orthotriaene. R. Diaene. S. ?Triaene. T. Orthodichotriaene. W–Y. Pinakids. Z. Dichotriaene. After Ivanik (2003); modified.
Genus Stelletta Schmidt, 1862
Type species: Stelletta grubii Schmidt, 1862 (type by subsequent designation), Adriatic Sea, Recent.
Stelletta spp.
Fig. 2A–D, F–J, L, P, T.
Material.—Cretaceous, lower Eocene, upper Eocene, lower Oligocene, south-central Ukraine.
Remarks.—Some of the big protriaenes (Fig. 2A, C), prodichotriaenes (Fig. 2I, L, P), and especially the protriaenes with massive shafts (Fig. 2B, D, G), resemble those of ancorinid Stelletta (compare Sim 1996: figs. 1–6). The same is true for incomplete anatriaenes (Fig. 2F, H, J, M) and orthodichotriaene (Fig. 2T) that resembles spicules of Stelletta (Myriastra) illustrated by Sollas (1888: pls. 12 and 14).
Family Pachastrellidae Carter, 1875
Pachastrellidae indet.
Figs. 3D, G–K, O–T, 7Y, 8L.
Material.—Lower Eocene, middle Eocene, upper Eocene, lower Oligocene, south-central Ukraine.
Remarks.—Pachastrellid affinity is apparent for numerous calthrops (Fig. 3T), mesotriaenes (Figs. 3D, I, K, O, P, 8L), mesodichotriaenes (Fig. 3J), and triods (Fig. 3Q, R) (compare Maldonado 2002; Łukowiak and Pisera 2016). A big plesiaster illustrated on the Fig. 3S may also be attributed to pachastrellids. It displays a great similarity to Characella pachastrelloides (Carter, 1876) (compare Lévi and Lévi 1989: fig. 33). The same is true for long club-shaped spicule (Fig. 7Y) whose morphology resembles that of the modern pachastrellid Ancorella paulini Lendenfeld, 1907 (compare with Lendenfeld 1907: pl. 12: 9). The pachastrellid affinity of some other spicules cannot be ruled out (e.g., Figs. 3A–C, F–H). However, these spicules might also belong to geodiids, calthropellids, ancorinids, or even tetillids.
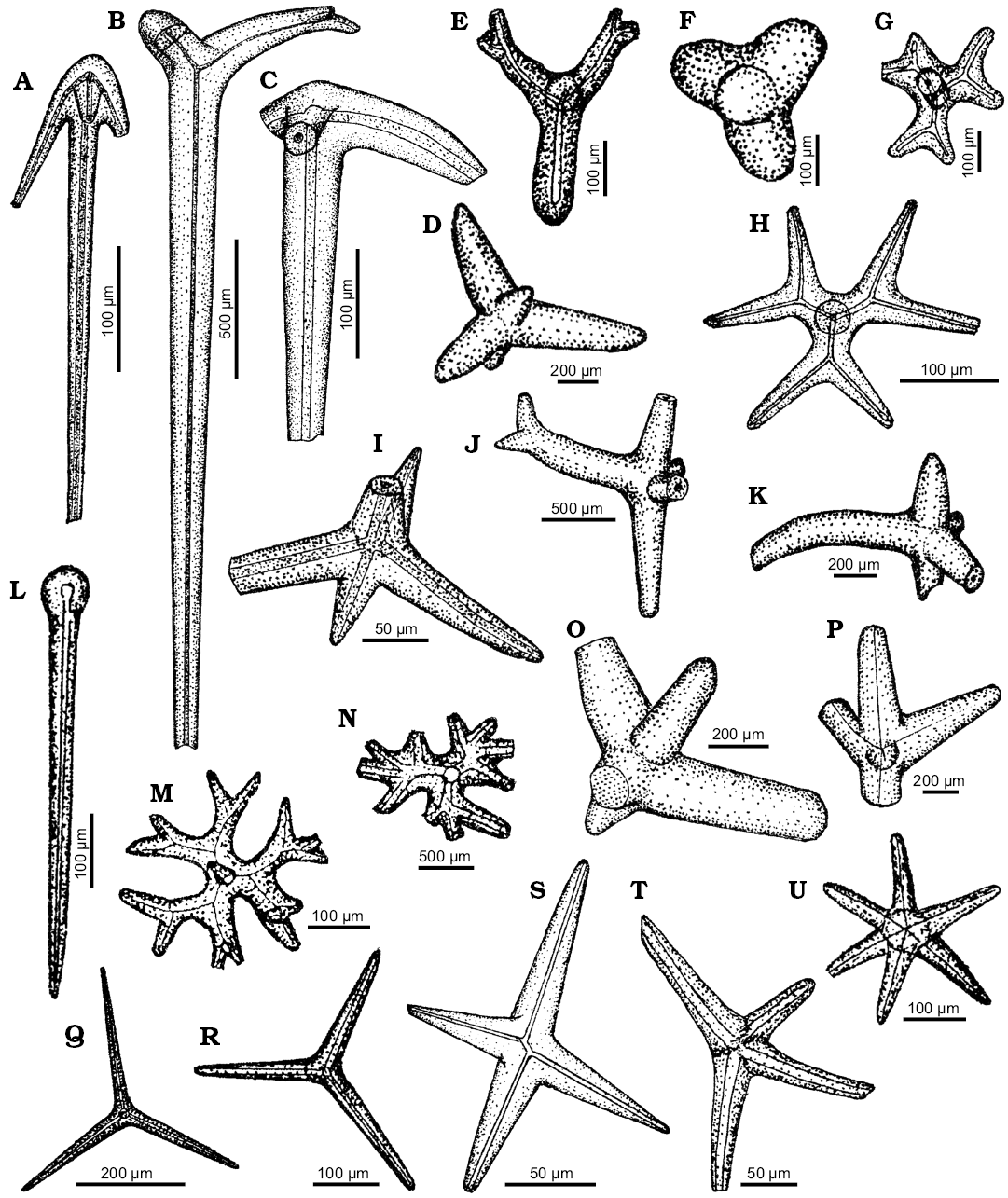
Fig. 3. Spicule morphotypes from south-central Ukraine; Černoleska, upper Eocene (A), Pesčanoe, upper Eocene (B), Russkie Tiŝki, middle Eocene (C, I, Q), Verhnee, lower Eocene (D, F, H, J, K, O, P), Nikol’skoe, upper Eocene (E, L), Staroverovka, middle Eocene (G), Markovka, upper Eocene (M), Kiselevka, lower Eocene (N), Grigorevka, middle Eocene (R), Kiselevka, middle Eocene (S), Markovka, middle Eocene (T), and Pesčanoe, upper Eocene (U). A, C. Anatriaenes. B. Orthodichotriaene. D, I–K, O, P. Mesotriaenes. E. Prodichotriaene. F, T. Calthrops. G, H. Dichotriaenes. L. Tylostyle. M, N. Mesodichotriaenes. Q, R. Triods. S. ?Plesiaster. U. Oxyaster. After Ivanik (2003); modified.
Genus Triptolemma Laubenfels, 1955
Type species: Triptolemma cladosum (Sollas, 1888) (by original designation), Banda Sea, Recent.
Triptolemma sp.
Fig. 3M, N.
Material.—Lower and upper Eocene, south-central Ukraine.
Remarks.—The mesodichotriaenes with variously divided clads (Fig. 3M, N) are identical with spicules of pachastrellid genus Triptolemma. They show great resemblance especially to mesodichotriaenes of T. cladosum (Sollas, 1888) (compare Sollas 1888: pl. 35: 23).
Family Theneidae Carter, 1883
Theneidae indet.
Fig. 4A.
Material.—Middle Eocene, south-central Ukraine.
Remarks.—The long, thin protriaene (Fig. 4A) may be assigned to Theneidae. This type of spicules appear, e.g., in Thenea megaspina Lendenfeld, 1907 (Lendenfeld 1907: pl. 21: 20). The same applies for the diaenes (Fig. 1Y, AA) which resemble spicules of T. megaspina Lendenfeld, 1907 (compare Lendenfeld 1907: pl. 21: 21). However, geodiid affinity of the latter spicules cannot be ruled out either.
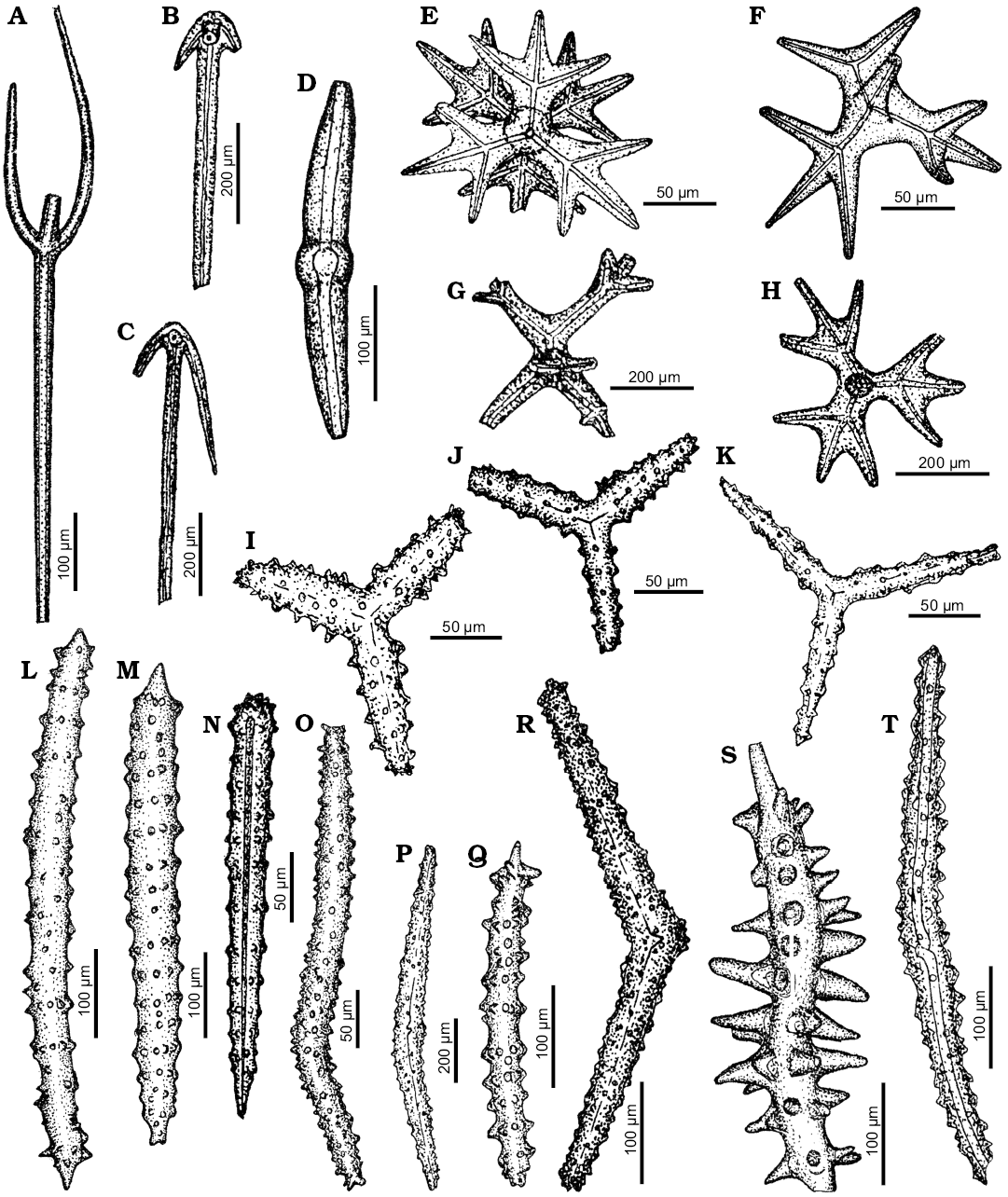
Fig. 4. Spicule morphotypes from south-central Ukraine; Russkie Tiŝki, middle Eocene (A, O), Nikol’skoe, upper Eocene (B, C), Kantemirovka, middle Eocene (D), Melovoe, upper Eocene (E), Staroverovka, middle Eocene (F), Kantemirovka, upper Eocene (G, I), Markovka, upper Eocene (H, N), Pogonovka borehole, upper Eocene (J, L, M), Markovka, middle Eocene (K, P, T), Kiselevka, middle Eocene (Q), Lipcy, middle Eocene (R), and Kiselevka, upper Eocene (S). A. Protriaene. B, C. Anatriaenes. D. Centrotylote oxea. E–H. Amphitriaenes. I–K. Acanthotriods. L, M. Verticillate oxeas. N. Verticillate style. O–R, T. Acanthoxeas. S. Sanidaster. After Ivanik (2003); modified.
Family Thoosidae Cockerell, 1925
Genus Alectona Carter, 1879
Type species: Alectona millari Carter, 1879 (by monotypy), Celtic Sea, Recent.
Alectona spp.
Fig. 4I–K, O, P, R, T.
Material.—Middle–upper Eocene, south-central Ukraine.
Remarks.—The spiny triactines (also called acanthotriods) with long rays covered with spines regularly arranged in whirls (Ivanik 2003: pl. 21: 3, 4; Fig. 4I–K) resemble those of Alectona triradiata Lévi and Lévi, 1983 (compare Bavestrello et al. 1998: fig. 2a–c). However, the spicules of modern Alectona possess very well developed spines also on the ray tips while those from Ukraine lack the spines on the tips. Although, this difference in spines arrangement may be due to the poor preservation state of the fossil spicules.
In turn, the long, bent oxeas that are rather irregularly covered by minute spines (Fig. 4P) show great resemblance to spicules of modern Alectona primitiva Topsent, 1932 (compare Vacelet and Vesseur 1971: fig. 22).
The smaller acanthoxeas (Fig. 4O, R, T) are less bent in the middle of its shaft and regularly covered with minute spines. They are almost identical with the spicules of modern Alectona millari Carter, 1879 (compare Carter 1879: pl. 17: 3). The acanthorhabds (Fig. 4S), in turn, might be of alectonid affinity because similar spicules (diactines) have been described by Bavestrello et al. (1998) and assigned to Alectona sp. (see Bavestrello et al. 1998: fig. 7). Still, these spicules also resemble sanidasters of Crellastrina alecto Topsent, 1898 and spicules of some species of Halicnemia Bowerbank, 1864 (Stelligeridae; van Soest 2017: fig. 34).
Family Theonellidae Lendenfeld, 1903
Theonellidae indet.
Fig. 9A, D–H, L, O, P, R, U, X, Z.
Material.—Lower Eocene, middle–upper Eocene, Upper Cretaceous, Paleocene, south-central Ukraine.
Remarks.—The most common lithistid spicules reported by Ivanik (2003) seem to belong to the family Theonellidae. These are mostly ectosomal phyllotriaenes (Fig. 9A, E, H, U), as well as discotriaenes (Fig. 9D, F, G). Such spicules are characteristic for Discodermia du Bocage, 1869 (discotriaenes) and Theonella Gray, 1868 (phyllotriaenes) and closely related genera. Presence of one or more of these genera is also supported by occurrence of rare fragmentary preserved tetraclone desmas (Fig. 9S, X, Z) that are characteristic for this family.
The ectosomal phyllotriaenes with complex morphology of the cladome (Fig. 9L, O, P) resemble those found in an undescribed theonellid sponge taxon from the Salomon Islands (AP unpublished material).
Family Pleromidae Sollas, 1888
Pleromidae indet.
Fig. 9J, K, N, Q, S–W, Y.
Material.—Lower Eocene, Upper Cretaceous, south-central Ukraine.
Remarks.—Pleromidae are commonly represented by smooth megaclones. Despite that some of the illustrated megaclones (Fig. 9J, K, N, Q, T–W, Y) are Late Cretaceous, the same type of spicules was also found in the Paleogene deposits (e.g., Fig. 9S, see figure caption).
Suborder Spirophorina Bergquist and Hogg, 1969
Family Samidae Sollas, 1888
Genus Samus Gray, 1867
Type species: Samus anonymus Gray, 1867; by monotypy.
Samus anonymus Gray, 1867
Fig. 4E, G, H.
Material.—Middle–upper Eocene, upper Eocene, south-central Ukraine.
Remarks.—The spicules illustrated on the Fig. 4E and the partially preserved spicules on the Fig. 4G, H are amphitriaenes. These highly diagnostic spicules are almost identical in size (clads about 50 µm long) and shape with spicules of modern spirophorid sponge Samus anonymus Gray, 1867 (compare van Soest and Hooper 2002: fig. 1). The broken amphitriaene from the Fig. 4F seems to belong to Samus as well.
Family Tetillidae Sollas, 1886
Tetillidae indet.
Fig. 4D.
Material.—Middle and upper Eocene, south-central Ukraine.
Remarks.—The stout centrotylote oxea of about 230 µm of length (Fig. 4D) is of great morphological resemblance, and the same size, as the spicules of tetillids (compare e.g., Acanthotetilla gorgonosclera, van Soest 1977: fig. 4e). However, similar spicules can be found in geodiids (van Soest 2017: fig. 55d) and thoosids (e.g., Carballo et al. 2004: fig. 21d). The long thin anatriaenes (Fig. 4B, C) and acanthoxea (Fig. 6N) could belong to tetillids as well. The acanthoxeas of similar morphology are found, e.g., in Acanthotetilla walteri Peixinho, Fernandez, Oliveira, Caires, and Hajdu, 2007 (Peixinho et al. 2007: fig. 7c), but their coelosphaerid affinity is more likely.
Order Agelasida Hartman, 1980
Family Agelasidae Verrill, 1907
Genus Agelas Duchassaing and Michelotti, 1864
Type species: Agelas dispar Duchassaing and Michelotti, 1864 (by subsequent designation; Burton and Rao 1932), Eastern Caribbean, Recent.
Agelas spp.
Fig. 4L–N.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The long verticillate oxeas illustrated on Fig. 4L, M belong to Agelas. These spicules are equipped with 18 whorls of short tubercles regularly arranged along the spicule. They are similar with acanthoxeas of modern species of Agelas, especially A. axifera Hentschel, 1911 and A. sansibarica Perino and Pronzato in Manconi et al., 2016. These two species have in their skeletons verticillated oxeas of almost identical morphology (compare Hentschel 1911: fig. 54 and Manconi et al. 2016: fig. 3). The spicules illustrated by Ivanik (2003) seem to exhibit a greater resemblance to spicules of A. sansibarica as they are of comparable length and possess a similar number of whorls.
There is also one verticillate style (Fig. 4N) with regular whorls of tubercles that could be assigned to one of the Agelas species (compare spicules of Agelas ceylonica Dendy, 1905, illustrated by Manconi et al. 2016: fig. 11e).
Order Tethyida Morrow and Cárdenas, 2015
Tethyida indet.
Fig. 5Y, AE, AF, AG.
Material.—Middle Eocene, upper Eocene, Upper Cretaceous, south-central Ukraine.
Remarks.—The oxyaster shown on the Fig. 5AF clearly belongs to Thetyida. This spherical spicule with divided ray tips is about 150 µm in diameter and might belong to some species of Timea Gray, 1867 (Timeidae; Timea diplasterina Rützler, Piantoni, van Soest, and Díaz, 2014: fig. 125c). However, it could also belong to Tethya Lamarck, 1815 (Tethyidae; compare Sarà 2002: fig. 9e). Also, various asters (~60–150 µm in diameter) might belong to Tethya (Figs. 1B, 5Y, AE, AG).
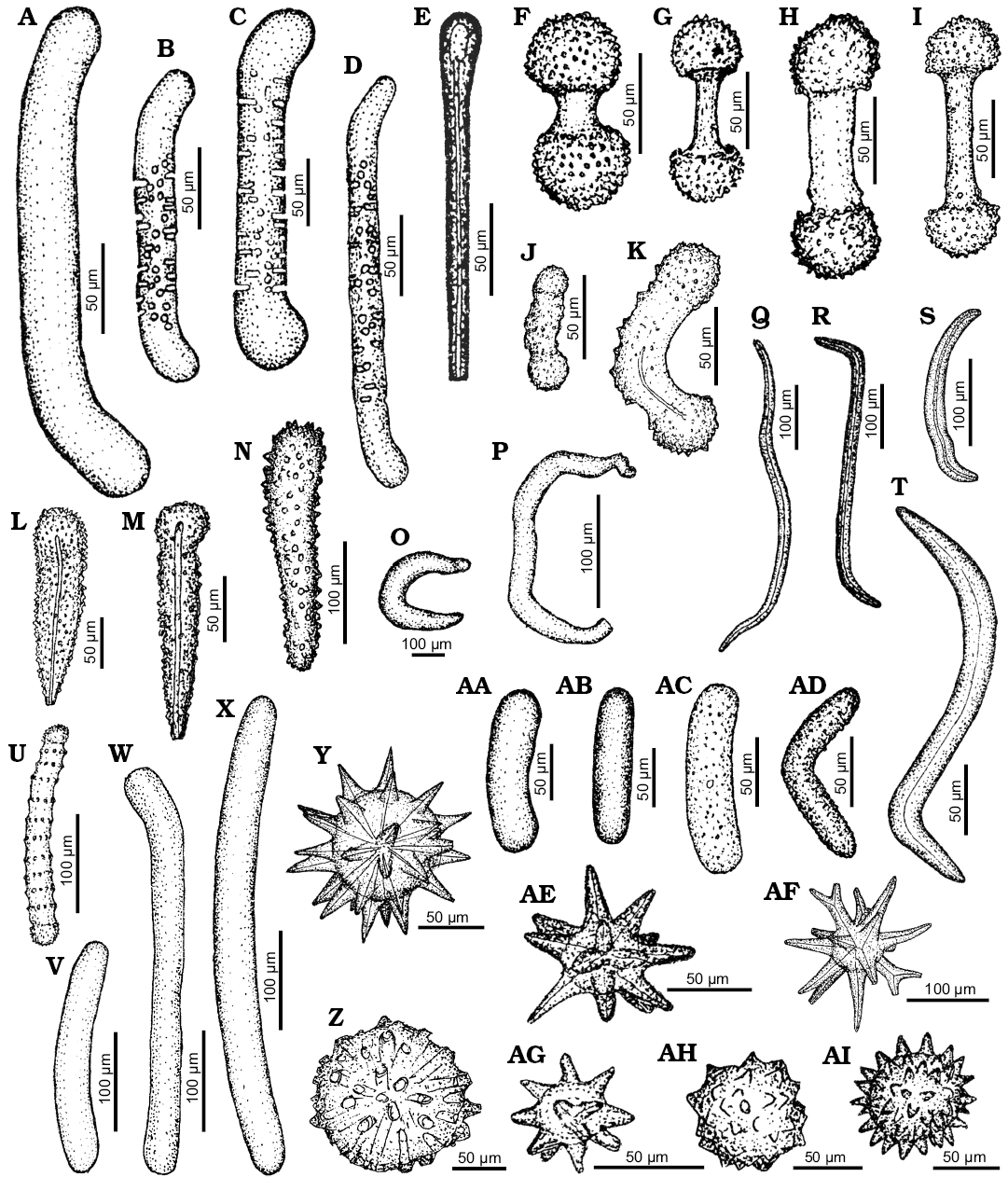
Fig. 5. Spicule morphotypes from south-central Ukraine; Kiselevka, upper Eocene (A, E, N, V, X), Russkie Tiŝki, middle Eocene (B, C, F, G, I, J, M, AA), Markovka, middle Eocene (D, H, Q, R, W), Melovoe, upper Eocene (K), Nikol’skoe, upper Eocene (L, P, AF), Staroverovka, middle Eocene (O, Y, AI), Markovka, upper Eocene (S, U, AD, AE, AG), Pesčanoe, upper Eocene (T), Harkov region, middle Eocene (Z), Kantemirovka, upper Eocene (AB), Russkie Tiŝki, upper Eocene (AC), and Kiselevka, middle Eocene (AH). A–D, V–X. Strongyles. E. Exotyle. F–K. Acanthostrongyles. L–N. Acanthostyles. O, P. Ophirhabds. Q–T. Flexuous oxeas. U. Acanthostrongyle. Y. Oxyspheraster. Z. Oxyaster. AA–AD. Microstrongyles. AE, AG. Oxyasters. AF. Oxyaster. AH, AI. Spherasters. After Ivanik (2003); modified.
Order Axinellida Lévi, 1953
Family Axinellidae Carter, 1875
Axinellidae indet.
Fig. 5Q–T.
Material.—Middle–upper Eocene, south-central Ukraine.
Remarks.—Among the illustrated spicules, the 200–480 µm long flexuous oxeas (Fig. 5Q–T) may show axinellid affinities. However, flexuous oxeas are also characteristic for the heteroscleromorph family Bubaridae (compare Alvarez and van Soest 2002). Still, owing to the general shape and thickness of the discussed spicules, their attribution to Axinellidae is more likely.
Family Raspailiidae Nardo, 1833
Genus Plocamione Topsent, 1927
Type species: Plocamione dirrhopalina Topsent, 1927 (by monotypy), Azores, Canaries, Madeira (geounit), Recent.
Plocamione spp.
Fig. 5F–K.
Material.—Middle–upper Eocene, south-central Ukraine.
Remarks.—The spicules illustrated on the Fig. 5J, K and in Ivanik (2003: pl. 19: 3) are identical with spicules of the two raspailiid species, Plocamione carteri (Ridley and Duncan, 1881) and P. dirrhopalina Topsent, 1927. The skeleton of these two species possesses choanosomal acanthostrongyles (compare Hooper 2002: fig. 20d) of comparable size (50 µm in living and 34 and 45 µm in fossil representatives; Ridley and Duncan 1881: pl. 23: 19). The morphologies of the other choanosomal acanthostrongyles, illustrated on the same figure are very similar too (Fig. 5F–I). The style from the Fig. 3Q may belong to some Plocamione species as well (compare Hooper 2002: fig. 20a). Nevertheless, its vulcanellid affinity cannot be ruled out either.
The same is true for the long acanthostyle illustrated on the Fig. 5M which resembles the spicules belonging to the recent taxon Plocamione pachysclera (Lévi and Lévi, 1983) (compare Raspailia pachysclera Lévi and Lévi, 1983: fig. 12.2; Hooper 2002: fig. 20j). They are identical in terms of their morphology and size. Still, similar spicules also appear in erylinid Pachymatisma monaena Lendenfeld, 1907 (Lendenfeld 1907: pl. 35: 35).
The spicules illustrated on the Fig. 5E, L, N may belong to Plocamione as their morphology and size is comparable with some spicules of this extant genus (compare with Hooper 2002: fig. 20d).
Genus Janulum Laubenfels, 1936
Type species: Janulum spinispiculum (Carter, 1876) (type by original designation), South European Atlantic Shelf, Recent.
Janulum sp.
Fig. 5B–D.
Material.—Middle Eocene, south-central Ukraine.
Remarks.—The strongyles (Fig. 5B–D) are similar in size (165–250 µm) and morphology to acanthostrongyles of the modern raspailiid genus Janulum (compare Kelly et al. 2015: figs. 2, 3). The fossil spicules seem to have some signs of erosion in places where the spines in the modern spicules originate.
Order Haplosclerida Topsent, 1928
Family Petrosiidae van Soest, 1980
Petrosiidae indet.
Fig. 5V–X.
Material.—Middle–upper Eocene, south-central Ukraine
Remarks.—The strongyles (Fig. 5V–X) may belong to one of the species of Petrosia Vosmaer, 1885 (compare Petrosia davilai Alcolado, 1979, Silva and Zea 2017: fig. 2) or Xestospongia Laubenfels, 1932 (X. deweerdtae, Silva and Zea 2017: fig. 5).
Order Clionaida Morrow and Cárdenas, 2015
Family Clionaidae d’Orbigny, 1851
Genus Dotona Carter, 1880
Type species: Dotona pulchella Carter, 1880 (type by monotypy), South India and Sri Lanka.
Dotona sp.
Fig. 5U.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The verticillate cylindrical acanthostrongyle (Ivanik 2003: pls. 18: 5, 19: 1; Fig. 5U) covered with spirally coiled rows of tubercles and with microspined heads can be found in the modern clionaid Dotona pulchella Carter, 1880 (compare Calcinai et al. 2001: fig. 2a, b). However, the spicules of Dotona do not exceed 100 µm in length, while the spicules described by Ivanik (2003) are twice as long (200 µm). This type of spicules resembles also acanthostrongyles of acarnid Zyzzya (compare Z. fuliginosa [Carter, 1879], van Soest et al. 1994: figs. 19, 20).
Family Placospongiidae Gray, 1867
Genus Placospongia Gray, 1867
Type species: Placospongia melobesioides Gray, 1867 (by original designation), geounit Palawan/North Borneo, Recent.
Placospongia spp.
Fig. 1D, F.
Material.—Middle Eocene, south-central Ukraine
Remarks.—The bean-shaped to round, massive, 70–85 µm long microsclere spicules with polygonal plates between the grooves arranged on the spicule surface (Fig. 1D, F) are selenasters. This type of spicules is characteristic for clionaid genus Placospongia.
The nearly spherical spicule (Fig. 5AH) might also belong to this genus, except that it is considerably bigger than the spherasters typically found in Placospongia. As such, this assignment should be treated with caution.
Family Spirastrellidae Ridley and Dendy, 1886
Spirastrellidae indet.
Fig. 6X.
Material.—Middle Eocene, south-central Ukraine.
Remarks.—The anthasters (Fig. 6X); that is, spherasters with complex ray tips, are similar to the spicules characterizing modern spirastrellid Diplastrella megastellata Hechtel, 1965 (compare Rützler et al. 2014: fig. 19b).
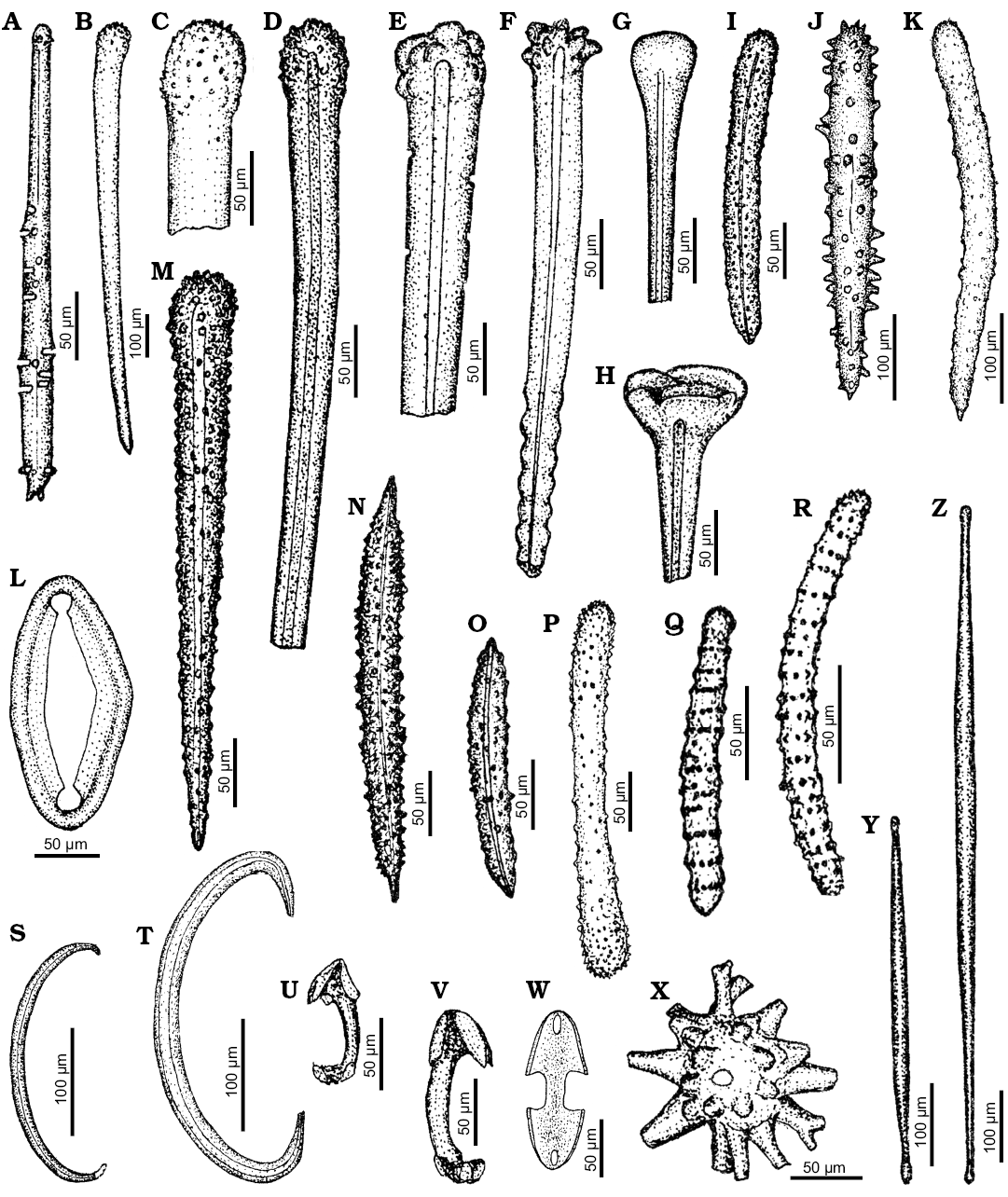
Fig. 6. Spicule morphotypes from south-central Ukraine; Russkie Tiŝki, Middle Eocene (A, H, O, Y, Z), Kiselevka, upper Eocene (B, C), Markovka, middle Eocene (D), Russkie Tiŝki, upper Eocene (E), Kiselevka, upper Eocene (F, G, M), Pogonovka borehole, upper Eocene (I), Markovka, upper Eocene (J, K, N), Nikol’skoe, upper Eocene (L, P), Grigorevka borehole, upper Eocene (Q, R, S, T), Melovoe, upper Eocene (U, V), and Kiselevka, middle Eocene (W, X). A. Spherostyle. B–D. Tylostyles. E, F, I–K, M. Acanthostyles. G, H. Exotyles. L. Clavidisc. N, O. Acanthoxeas. P–R. Acanthostrongyles. S, T. Sigmas. U, V. Anisochelae. W. Isochela. X. Spheraster. Y, Z. Tylotes. After Ivanik (2003); modified.
Order Polymastida Morrow and Cárdenas, 2015
Family Polymastiidae Gray, 1867
Genus Sphaerotylus Topsent, 1898
Type species: Polymastia capitatus Vosmaer, 1885 (by original designation), geounit Northern Norway and Finnmark, Recent.
Sphaerotylus spp.
Fig. 6A–C.
Material.—Middle Eocene, middle-upper Eocene, south-central Ukraine.
Remarks.—The club-shaped spicules (Fig. 6A, B) resemble exotyles of Recent Sphaerotylus, especially those of S. vanhoeffeni Hentschel, 1914 (Plotkin et al. 2017: fig. 29d). Also, the tylostyles (Fig. 6C, ?D) and a fragment of the club-shaped spicule (Fig. 6G) could be part of an exotyle and assigned to one of the Sphaerotylus species (compare Plotkin et al. 2017: fig. 18).
Order Poecilosclerida Topsent, 1928
Family Mycalidae Lundbeck, 1905
Genus Mycale Gray, 1867
Type species: Hymeniacidon lingua Bowerbank, 1866 (by subsequent designation; Thiele 1903), Celtic Seas, Recent.
Mycale spp.
Fig. 6H.
Material.—Middle Eocene, south-central Ukraine.
Remarks.—The nail-shaped spicule (Fig. 6H), even if incomplete and lacking a central button on its top, strongly resembles those of modern Mycale (Rhaphidotheca) loricata (Topsent, 1896) (compare van Soest and Hajdu 2002: fig. 13a, b). A club-like spicule (Fig. 6G) is similar to those in Mycale (R.) arctica Fristedt, 1887 (compare Koltun 1959: pl. 70: 25) and could be attributed to Mycale as well.
Family Coelosphaeridae Dendy, 1922
Genus Histodermella Lundbeck, 1910
Type species: Histodermella ingolfi Lundbeck, 1910 (by subsequent designation; Laubenfels 1936), South and West Iceland, Recent.
Histodermella spp.
Fig. 6N, O, Y, Z.
Material.—Middle Eocene, middle-upper Eocene, south-central Ukraine.
Remarks.—The long oxeas that are spined along their whole length except for their tips (Ivanik 2003: pls. 20: 8, 9, 21: 1; Fig. 6N, O) could probably be assigned to Histodermella. This genus includes 4 extant species (van Soest et al. 2018). Three of those species (Histodermella kagigunensis Lehnert, Stone, and Heimler, 2013, H. natalenisis [Kirkpatrick, 1903], and H. inglofi Lundbeck, 1910) possess this type of spicules (compare Kirkpatrick 1903: pl. 6: 18; Lehrnert et al. 2013: fig. 1; Lundbeck 1910: pl. 4: 4).
In addition, the fusiform tylotes (Fig. 6Y, Z) can be found in H. natalensis as well (compare Kirkpatrick 1903: pl. 7: 18a). However, a coelosphaerid or myxillid (see below) affinity of that spicule (compare Coelosphaera [Coelosphaera] tubifex Thomson, 1873, Boury-Esnault et al. 1994: fig. 80a) cannot be ruled out either but is less possible.
Family Myxillidae Dendy, 1922
Genus Myxilla Schmidt, 1862
Type species: Myxilla (Myxilla) rosacea (Lieberkühn, 1859), Adriatic Sea, Recent.
Myxilla spp.
Fig. 6I, K, U, V.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—Several spicules of different morphology may be assigned to the family Myxillidae, or even to the genus Myxilla. These are the acanthostyles (Fig. 6I, K), and anisochelae (Fig. 6U, V). However, this attribution is not unambiguous and their referral to mycalids is also possible. Still, the slender fusiform tylotes (Fig. 6Y, Z), that were here ascribed to Histodermella, might be also of myxillid affinity.
Family Microcionidae Carter, 1875
Microcionidae indet.
Fig. 6E, F, J, M.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The acanthostyles with massively sculptured heads (Fig. 6E, F), as well as other acanthostyles illustrated on the same figure (Fig. 6J, M), could be assigned to some of the microcionid genera. For instance, the former resemble the acanthostyles present in modern Clathria (Thalysias) zeai van Soest, 2017 (compare van Soest 2017: fig. 95b, e). Similar spicules also appear in Discorhabdella Dendy, 1924 (compare Boury-Esnault et al. 1994: fig. 83a).
Order Merliida Vacelet, 1979
Family Merliidae Kirkpatrick, 1908
Genus Merlia Kirkpatrick, 1908
Type species: Merlia normani Kirkpatrick, 1908 (type by original designation), Azores, Canaries, Madeira, Recent.
Merlia sp.
Fig. 6L.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The small oval, ring-shaped spicule called clavidisc shown on Fig. 6L can be assigned to one of the species of Merlia. Four recent species of Merlia are distinguished and three of them, M. normani Kirkpatrick, 1908, M. tenuis Hoshino, 1990, and M. deficiens Vacelet, 1980, possess clavidiscs. The spicule that is illustrated here can be assigned to one of these species or its close relatives. It is worth noting that Merlia is characterized by basal calcareous skeleton that was not found in these deposits. Similar calvidiscs, not associated with basal calcareous skeleton, are known since the Early Jurassic (Wiedenmayer 1994).
Family Acarnidae Dendy, 1922
Genus Zyzzya Laubenfels, 1936
Type species: Zyzzya fuliginosa (Carter, 1879) (type by original designation), Torres Strait Northern Great Barrier Reef, Recent.
Zyzzya sp.
Fig. 6P–R.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The verticillate acanthostrogyles (Fig. 6Q, R) and acanthostrongyle (Fig. 6P) can be assigned to one of the species of Zyzzya. Five species are currently distinguished within this genus (van Soest et al. 2018). Amongst them, Z. fuliginosa (Carter, 1879) is characterized by presence of both these spicule types (compare van Soest et al. 1994: figs. 19, 20). The length of the acanthostrongyles found in modern Z. fuliginosa is up to 250 µm (see van Soest et al. 1994: table 2), while the fossil spicule is 165 µm long, thus falling within the range observable in that taxon. There are also some other spicules of similar morphology (Fig. 5U). However, contrary to the verticillate acanthostrongyles of Zyzzya, these spicules are strongly sculptured also at the tips. Such morphology makes their attribution to Acarnidae less likely.
Family Crellidae Dendy, 1922
Crellidae indet.
Fig. 4S.
Material.—Upper Eocene, south-central Ukraine.
Remarks.—The long acanthorhabd with slightly spirally twisted rhabd and long spines along the whole spicule (Fig. 4S) may belong to some sponges of the family Crellidae. Today, there is a single species known in this family, Crellastrina alecto which is characterized by similar, though smaller (up to 120 µm long), curved acanthoxeas (compare Topsent 1904: pl. 15: 16). Besides, the acanthoxeas present in this species are characterized by rugose spine tips which distinguishes them from the fossil spicules described by Ivanik (2003). Thus, the fossil spicule that is illustrated here may belong to unknown species of Crellastrina.
Other demosponge spicules
Material.—Lower Eocene, middle–upper Eocene, upper Eocene, Upper Cretaceous, south-central Ukraine.
Remarks.—The long bent oxeas (Fig. 2N, O) might belong to sponges of the suborder Astrophorina.
Pinakid spicules (Fig. 2W–Y); that is, flat oval discs with peripherally arranged gaps (that can be marginally open), are difficult to assign to a family level taxon as no such spicules are known in living sponges. They probably belong to some demosponges related to the geodiid Erylus, e.g., E. monticularis Kirkpatrick, 1900. This species possesses aspidasters that resemble the pinakids. Pinakids are quite common in the fossil record (Carter 1871; Mostler 1986: fig. 35.12–15, Schrammen 1924: pl. 7.43).
Some of the spicules illustrated by Ivanik (2003), such as those on Fig. 5O, P, resemble ophirhabds present in bubarid sponges. However, the general morphology of these spicules make their assignment difficult.
The desma illustrated on Fig. 9L is also difficult to be attributed to a taxon with certainty. Still, its morphology resembles the triders of Phymaraphinidae. The lithistid ectosomal irregular phyllo/dichotriaene (Fig. 9C) cannot be easily attributed to any lower taxon as well. On the contrary, the object (Fig. 8Q) illustrated by Ivanik (2003), attributed by him to the category of discoidal spicules, is actually a fragment of a diatom.
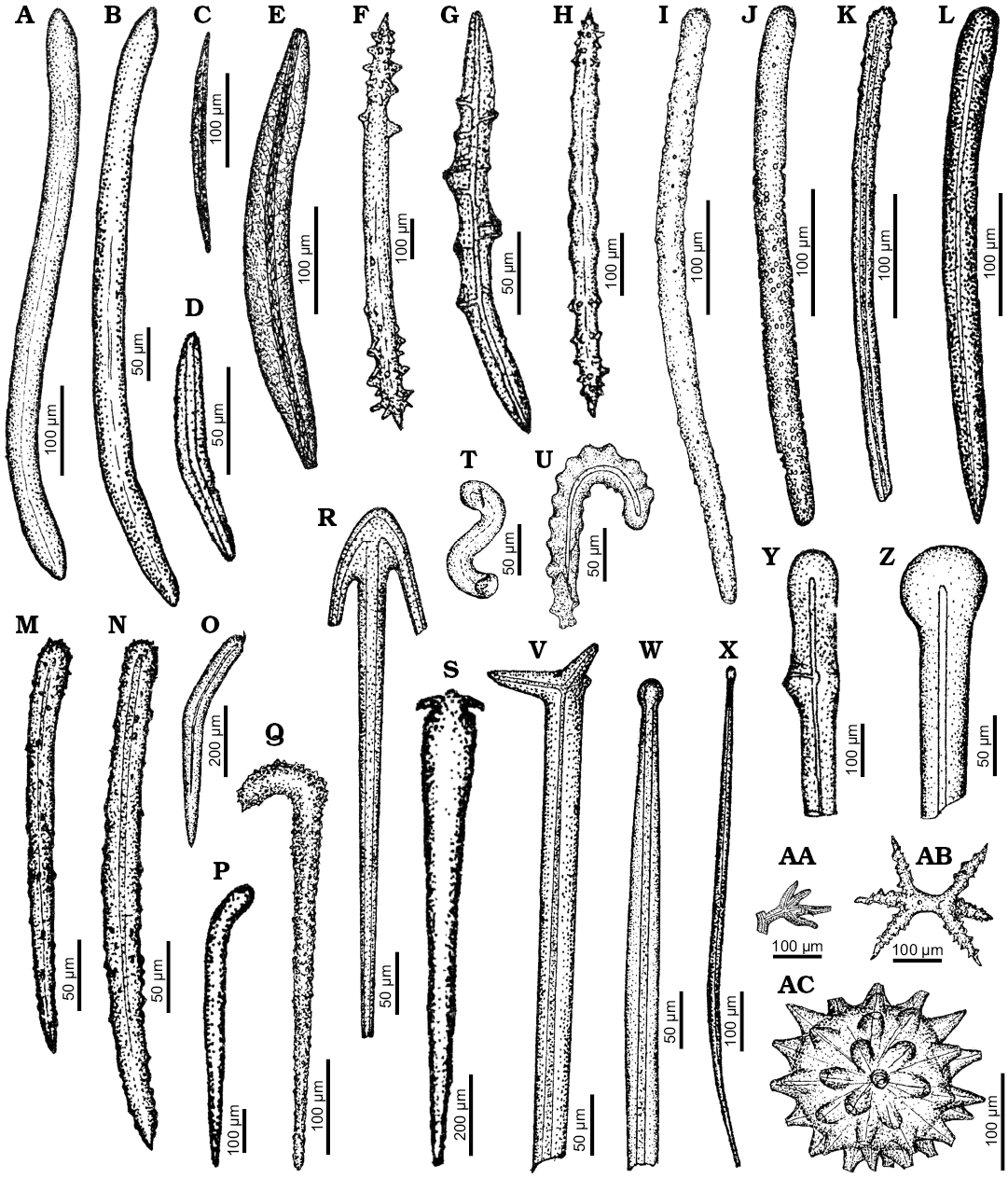
Fig. 7. Spicule morphotypes from south-central Ukraine; Markovka, upper Eocene (A, E, K), Kiselevka, middle Eocene (B, U), Lipcy, middle Eocene (C), Russkie Tiŝki, middle Eocene (D, J, P, R, V, W, X, AB), Nikol’skoe, upper Eocene (F, H, Y), Kantemirovka, upper Eocene (G, L, AA), Kiselevka, upper Eocene (I, O, Z), Pogonovka, upper Eocene (M, N), Melovoe, upper Eocene (Q, T), Černoleska, upper Eocene (S), and Staroverovka, middle Eocene (AC). A–E. Oxeas. F, H. Acanthoxeas. G. Acanthorhabd. I, J. Acanthostrongyles. K, M, N. Acanthostyles. L, O–Q. Styles. R. Reduced triaene. S. Anatriaene. T, U. Unknowns spicule. V. Orthotriaene. W, X. Tylostyles. Y. Spicule fragment. Z. Exotyle. AA. ?Fragment of a triaene. AB. Amphiaster. AC. Spheraster. After Ivanik (2003); modified.
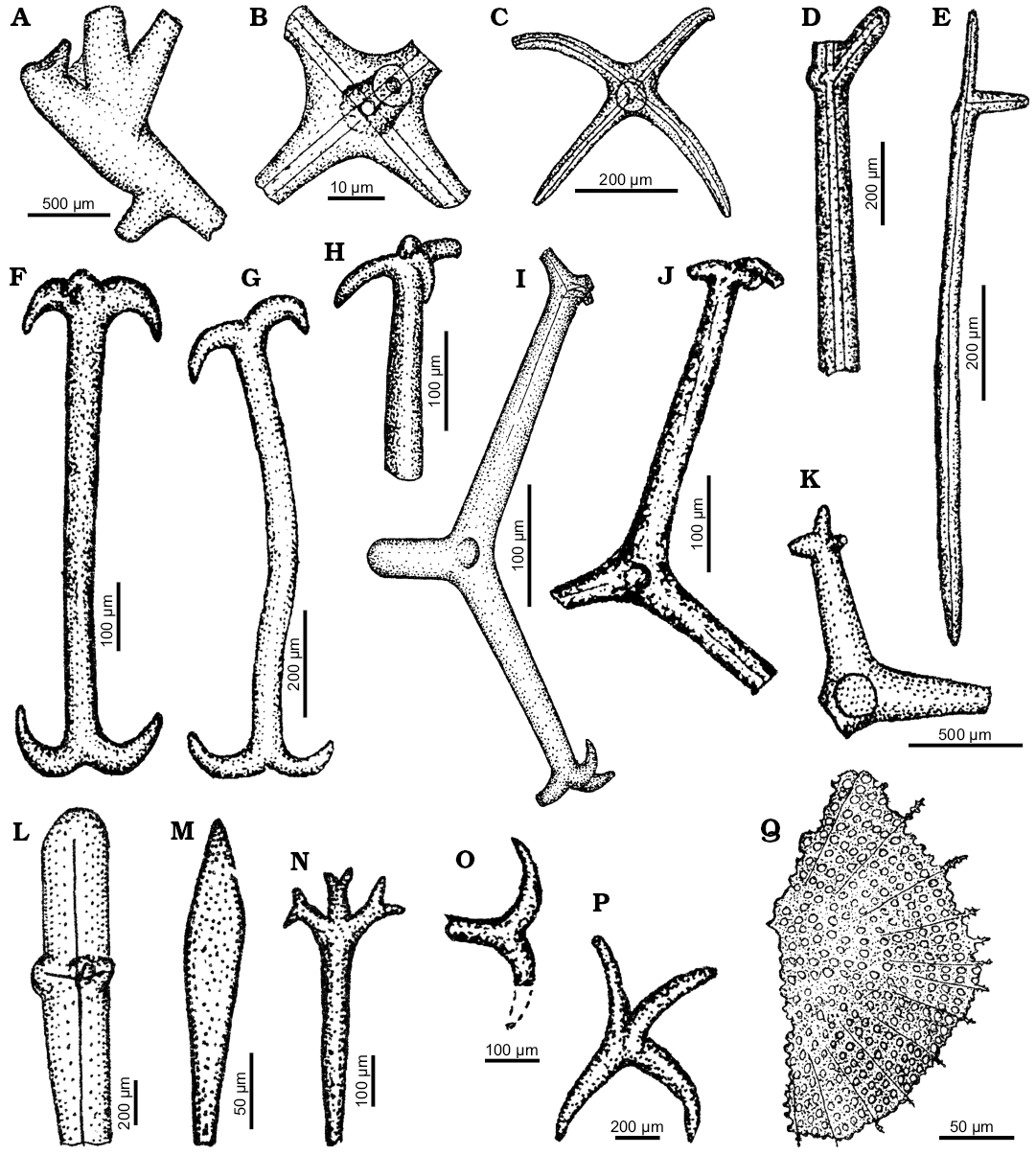
Fig. 8. Spicule morphotypes from south-central Ukraine; Verhnee, lower Eocene (A, K, L, P), Russkie Tiŝki, middle Eocene (B, D, N, Q), Nikol’skoe, upper Eocene (C, E, G, J), Markovka, upper Eocene (F, O), Lipcy, middle Eocene (H), Melovoe, upper Eocene (I), and Kantemirovka, upper Eocene (M). A. ?Fragment of dictional skeleton. B. Pentactine. C. Hexactine. D, E. ?Oxeas. F, G, O. Amphiclads. H. Broken lophodiactine. I, J. Lophocalthrops. K. ?Lophocalthrop. L. Spicule fragment. M. ?Spicule fragment. N. Dichotriaene. P. Calthrop. Q. Fragment of a diatom. After Ivanik (2003); modified.
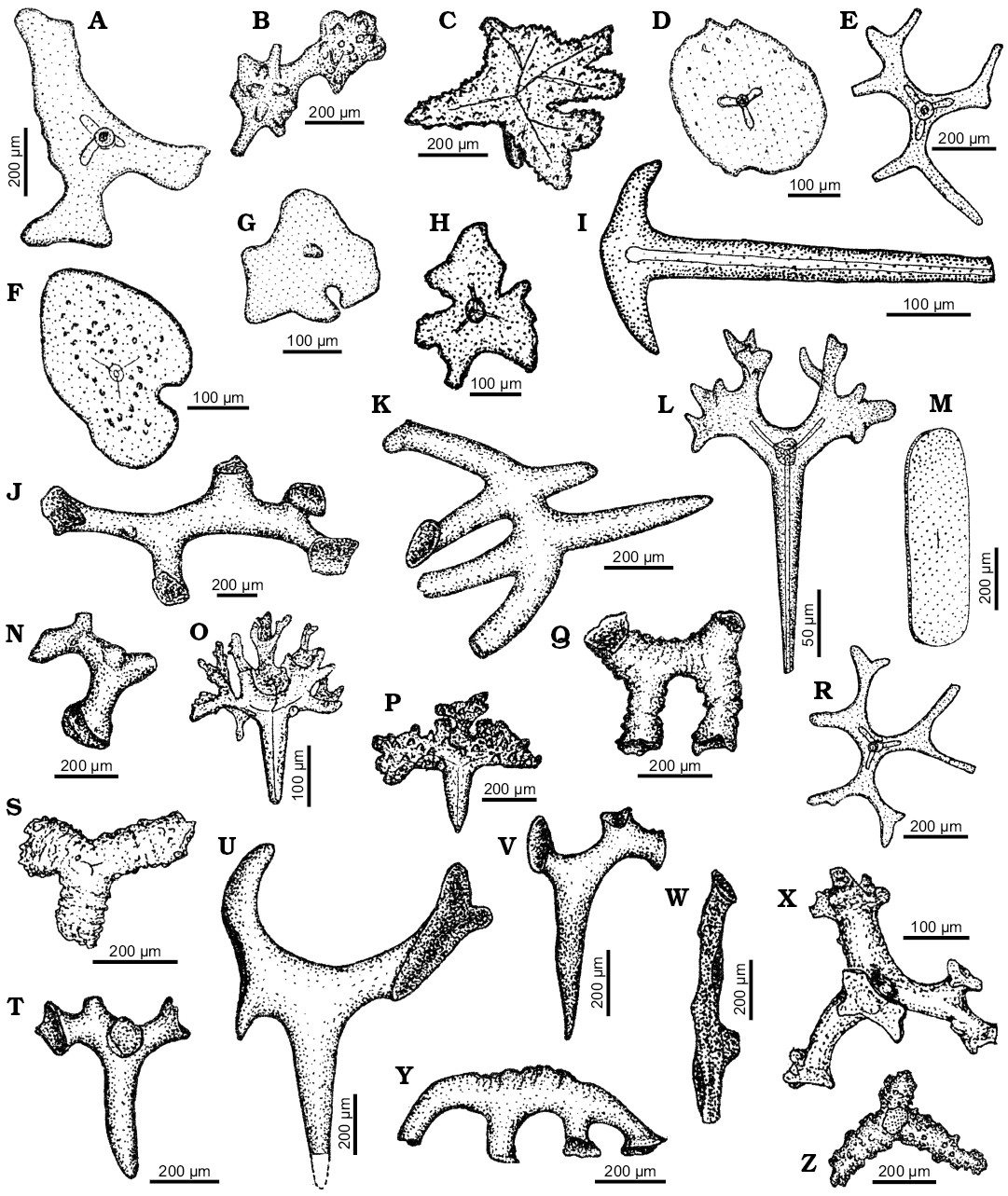
Fig. 9. Spicule morphotypes from south-central Ukraine (A–V, X–Z) and Lithuania (W); Markovka, middle Eocene (A, D, E), Verhnee, lower Eocene (B, Z), Glâdov Âr, Upper Creatceous (C, J, K, P, Q, T, U, V, Y), Pesčanoe, upper Eocene (F, O), Kiselevka, middle Eocene (G, L, R), Markovka, upper Eocene (H, X), Nikol’skoe, upper Eocene (I, S), Lipcy, middle Eocene (M), Monastyrek, lower Eocene (N), and Neravai borehole, Paleocene (W). A, C, E, H, L, O, P, R. Phyllotriaenes. B. Sphaeroclone. D, F, G. Discotriaenes. I. Anchorate spicule. J, K, N, Q, S–W, Y. Megaclones. M. Siliceous plate. X, Z. Tetracrepid desmas. After Ivanik (2003); modified.
Class Homoscleromorpha Bergquist, 1978
Order Homosclerophorida Dendy, 1905
Family Plakinidae Schulze, 1880
Genus Placinolopha Topsent, 1897
Type species: Placinolopha bedoti Topsent, 1897 (by original designation), Banda Sea, Recent.
Placinolopha spp.
Fig. 8F–K, O.
Material.—Lower, middle, upper Eocene, south-central Ukraine.
Remarks.—Many spicules display features characteristic for the sponges of the smallest modern sponge class, Homoscleromorpha. They are characterized by the lophate actine tips. There are several 500–600 µm long lophodiactines (Fig. 8F–H, O) that resemble amphiclads of the Recent species Placinolopha sarai Lévi and Lévi, 1989 (compare Lévi and Lévi 1989: fig. 13). In turn, some lophoclathrops (Fig. 8I, J, possibly K) with long lophate clads characterize modern Placinolopha moncharmonti Sarà, 1960 (compare Sarà 1960: fig. 1).
Class Hexactinellida Schmidt, 1870
Hexactinellida indet.
Figs. 8B–E, 10A, B, D, E–G, I–L.
Material.—Middle, middle–upper Eocene, south-central Ukraine.
Remarks.—The hexactines illustrated on Fig. 10C, E, F, I, H are most probably dermal spicules of rosselid sponges (compare Tabachnick 2002b: figs. 18, 19). However, similar spicules can be found also in other hexactinellid groups, e.g., Euretidae Zittel, 1877 (order Sceptrulophora), Aphrocallistidae Gray, 1867 (order Hexactinosida), or even in the amphidiscosid family Pheronematidae Gray, 1870 (see Reiswig 2002a; Reiswig and Wheeler 2002; Tabachnick and Menshenina 2002b). Yet, their rossellid affinity seems most likely because another spicule illustrated on the same figure (Fig. 10J), called dermal triactine, is also noted in the rossellid sponges (compare Tabachnick 2002b: fig. 40d). Besides, a fragment of the sparsely tuberculated pentactine (Fig. 10A) can be attributed to rossellids as well. It resembles especially the hypodermal pentactines of Crateromorpha (Neopsacas) Tabachnick, 2002b (see Menshenina et al. 2007: figs. 2k, 7h; as well as Tabachnick 2002b: fig. 18e).
In turn, the hexactine with a rough, weakly tuberous surface, slightly bent rays, and one ray fusing with a fragment of another spicule (Fig. 10K) seems to be a part of dictyonal framework of hexasterophorid sponges (see Reiswig 2002b: figs. 2b, 3c; 2002c: fig. 2b–d; Reiswig and Wheeler 2002: fig. 8B).
Some anchorate basalia (Fig. 10J) may belong to pheronematid sponges (compare Lévi and Lévi 1989: fig. 2).
Some pentactines (Figs. 8B, 10B) and stauractines (Fig. 10M) resemble dermalia of Symplectella Dendy, 1924 (Lyssacinosida, Euplectellidae) (see Tabachnick 2002a: fig. 27f–h).
The oxyhexactines (Figs. 8C, 10L) show some affinity to choanosomal spicules of Hyalonema Gray, 1832 (Amphidiscosida, Hyalonematidae) (see Tabachnick and Menshenina 2002a: fig. 1l). Amongst the spicules of unquestionable hexactinellid affinity are also those illustrated on Figs. 8B, D, E, 10K, but their more accurate attribution is impossible.
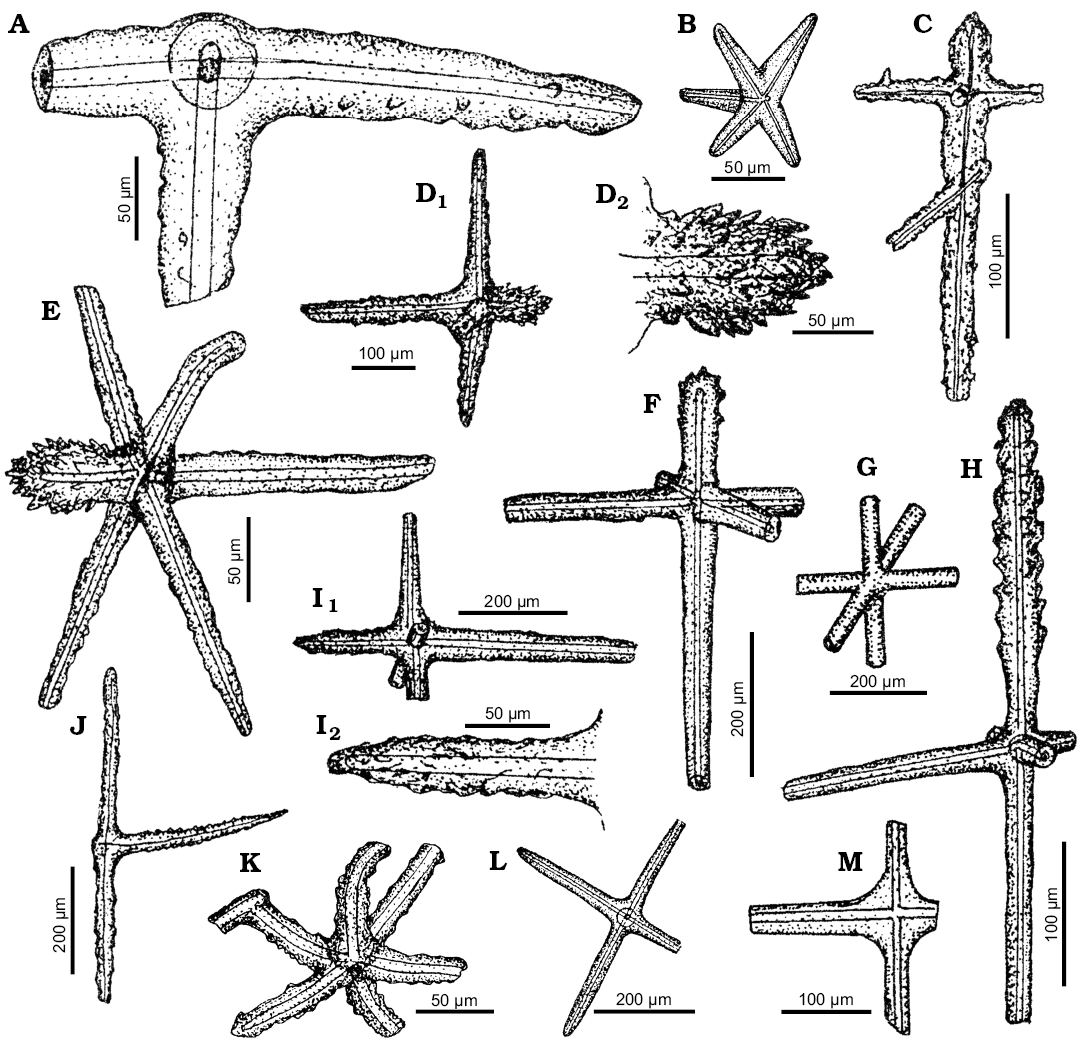
Fig. 10. Spicule morphotypes from south-central Ukraine; Kiselevka, middle Eocene (A, F, H, I, L), Staroverovka, middle Eocene (B), Nikol’skoe, upper Eocene (C, D, E, J, M), Russkie Tiŝki, upper Eocene (G), and Russkie Tiŝki, middle Eocene (K). A, B. Pentactines. C–F, H, I. Pinnular hexactines. G. Smooth hexactine. J. Tautactine. K, L, Hexactines. M. Stauractine. After Ivanik (2003); modified.
Concluding remarks
A reassessment of the disassociated Paleogene sponge spicules from the southwestern of the East European Platform studied by Ivanik (2003) revealed a diverse sponge fauna. The fauna appears to be dominated by Demospongiae (at least 24 families) while Hexactinellida were relatively rare but diverse. The majority of spicules attributable to hexactinellids appear to be dermal pentactines and their derivatives, most likely representing the family Rossellidae. Potentially, a single genus of Homoscleromorpha was recognized as well.
This reassessment, together with a new as yet unpublished material (MŁ, AP, and TS, unpublished data), will serve as a baseline for further taxonomic, ecological, and biogeographic studies of the poorly known Paleogene sponge fauna of this region.
Acknowledgements
We would like to thank Viviana Frisone (Museo di Archeologia e Scienze Naturali “G. Zannato”, Vicenza, Italy) , Michelle Kelly (NIWA, Wellington, New Zealand), and an anonymous reviewers whose suggestions helped to considerably improve this manuscript. We would like to thank Daniel Madzia (Institute of Paleobiology, PAS, Warsaw, Poland) for linguistic help. This study was financially supported by National Science Centre Grant No. 2016/21/B/ST10/02332 to AP.
References
Adams, C.L. and Hooper, J.N.A. 2001. A revision of australian Erylus (Porifera: Demospongiae: Astrophorida: Geodiidae) with a tabular review of worldwide species. Invertebrate Taxonomy 15: 319–340. Crossref
Alcolado, P.M. 1979. Nueva especie de porifero (genero Strongylophora) encontrada en Cuba. Poeyana 196: 1–5.
Alvarez, B. and van Soest, R.W.M. 2002. Family Bubaridae Topsent, 1894. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 748–754. Kluwer Academic/Plenum Publishers, New York. Crossref
Bavestrello, G., Calcinai, B., Cerrano, C., and Sarà, M. 1998. Alectona species from North-Western Pacific (Demospongiae: Clionidae). Journal of the Marine Biological Association of the UK 78: 59–73. Crossref
Bell, J.J. 2008. The functional roles of marine sponges. Estuarine, Coastal and Shelf Science 79: 341–353. Crossref
Bergquist, P.R. 1978. Sponges. 268 pp. Hutchinson, London and University of California Press, Berkeley.
Bergquist, P.R. and Hogg, J.J. 1969. Free amino acid patterns in demospongiae: a biochemical approach to sponge classification. Cahiers de Biologie Marine 10: 205–220.
du Bocage, J.V. Barboza 1869 [1870]. Eponges siliceuses nouvelles du Portugal et de l’île Saint-Iago (Archipel de Cap-Vert). Jornal de Sciencias mathematicas, physicas e naturaes 2: 159–162.
Boury-Esnault, N. and Rützler, K. (eds.) 1997. Thesaurus of Sponge Morphology. 55 pp. Smithsonian Institution Press, Washington. Crossref
Boury-Esnault, N., Pansini, M., and Uriz, M.-J. 1994. Spongiaires bathyaux de la mer d’Aloboran et du golfe ibéro-marocain. Collection. Mémoires du Muséum national d’histoire naturelle 160: 1–174.
Bowerbank, J.S. 1862. On the anatomy and physiology of the Spongiadae. Part III On the generic characters, the specific characters, and on the method of examination. Philosophical Transactions of the Royal Society 152: 1087–1135. Crossref
Bowerbank, J.S. 1864. A Monograph of the British Spongiadae. 290 pp. Ray Society, London. Crossref
Bowerbank, J.S. 1866. A Monograph of the British Spongiadae. Volume 2. i–xx, 1–388 pp. Ray Society, London.
Buckeridge, J.S., Kelly, M., Janussen, D., and Reiswig, H.M. 2013. New Palaeogene sponges from the Red Bluff Tuff, Chatham Island, New Zealand. New Zealand Journal of Geology and Geophysics 56: 171–185. Crossref
Burton, M. 1959. Sponges. In: The John Murray Expedition 1933–34. Scientific Reports, Vol. 10 (5), 151–281. British Museum (Natural History), London.
Burton, M. and Rao, H.S. 1932. Report on the shallow-water marine sponges in the collection of the Indian Museum.Part 1. Records of the Indian Museum 34: 299–358.
Cárdenas, P., Pérez, T., and Boury-Esnault, N. 2012. Sponge systematics facing new challenges. Advances in Marine Biology 61: 79–209. Crossref
Cárdenas, P., Xavier, J.R., Reveillaud, J., Schander, C., and Rapp, H.T. 2011. Molecular phylogeny of the Astrophorida (Porifera, Demospongiae) reveals an unexpected high level of spicule homoplasy. PLoS ONE 6: e18318. Crossref
Calcinai, B., Bavestrello, G., Cerrano, C., and Sarà, M. 2001. Boring sponges living into precious corals from the Pacific Ocean. Italian Journal of Zoology 68: 153–160. Crossref
Carballo, J.L., Cruz, J.A., and Gómez, P. 2004. Taxonomy and description of clionaid sponges (Hadromerida Clionaidae) fromthe Pacific Ocean of Mexico. Zoological Journal of Linnean Society 141: 353–387. Crossref
Carter, H.J. 1871. On fossil sponge-spicules of the Greensland compared with those of existing species. Annals and Magazine of Natural History 7 (38): 112–141. Crossref
Carter, H.J. 1875. Notes introductory to the study and classification of the Spongida. Part II. Proposed classification of the Spongida. Annals and Magazine of Natural History 4 (16): 126–145. Crossref
Carter, H.J. 1876. Descriptions and figures of deep-sea sponges and their spicules, from the Atlantic Ocean, dredged up on board H.M.S. “Porcupine”, chiefly in 1869 (concluded). Annals and Magazine of Natural History 18 (105): 226–240, 307–324, 388–410, 458–479. Crossref
Carter, H.J. 1879. On a new species of excavating sponge (Alectona millari); and on a new species of Rhaphidotheca (R. affinis). Journal of the Royal Microscopical Society 2: 493–499. Crossref
Carter, H.J. 1880. Report on specimens dredged up from the Gulf of Manaar and presented to the Liverpool Free Museum by Capt. W.H. Cawne Warren. Annals and Magazine of Natural History 6 (31): 35–61, 129–156. Crossref
Carter, H.J. 1883. Contributions to our knowledge of the Spongida. Annals and Magazine of Natural History 12 (71): 308–329. Crossref
Carvalho, M.S. de, Lopes, D.A., Cosme, B., and Hajdu, E. 2016. Seven new species of sponges (Porifera) from deep-sea coral mounds at Campos Basin (SW Atlantic). Helgoland Marine Research 70: 1–33. Crossref
Cockerell, T.D.A. 1925. The family Clionidae. Science 62: 567. Crossref
Conway, K.W., Krautter, M., Barrie, J.V., and Neuweiler, M. 2001. Hexactinellid sponge reefs on the Canadian continental shelf: A unique “living fossil”. Geoscience Canada 28: 71–78.
De Goeij, J.M., Oevelen, D., Vermeij, M.J., Osinga, R., Middelburg, J.J., de Goeij, A.F., and Admiraal, W. 2013. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342: 108–110. Crossref
Dendy, A. 1905. Report on the sponges collected by Professor Herdman at Ceylon, in 1902. In: W.A. Herdman (ed.), Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar, 57–246. Royal Society, London.
Dendy, A. 1922. Report on the Sigmatotetraxonida collected by H.M.S. “Sealark” in the Indian Ocean. In: Reports of the Percy Sladen Trust Expedition to the Indian Ocean in 1905. Transactions of the Linnean Society of London 18: 1–164. Crossref
Dendy, A. 1924. Porifera. Part I. Non-Antarctic sponges. Natural History Report. British Antarctic (Terra Nova) Expedition, 1910 (Zoology) 6: 269–392.
Díaz, M.C. and Rützler, K. 2001. Sponges: an essential component of Caribbean coral reefs. Bulletine of Marine Sciences 69: 535–546.
Duchassaing de Fonbressin, P. and Michelotti, G. 1864. Spongiaires de la mer Caraibe. Natuurkundige verhandelingen van de Hollandsche maatschappij der wetenschappen te Haarlem 21 (2): 1–124.
Frisone, V., Pisera, A., and Preto, N. 2016. A highly diverse siliceous sponge fauna (Porifera: Hexactinellida, Demospongiae) from the Eocene of northeastern Italy: systematics and palaeoecology. Journal of Systematic Palaeontology 14: 949–1002. Crossref
Frisone, V., Pisera, A., Hajdu, E., Preto, N., Zorzi, F., and Zorzin, R. 2014. Isolated spicules of Demospongiae from Mt. Duello (Eocene, Lesssini Mts, northern Italy): preservation, taxonomy and depositional environment. Facies 60: 883–904. Crossref
Fristedt, K. 1887. Sponges from the Atlantic and Arctic Oceans and the Behring Sea. Vega-Expeditionens Vetenskap. Iakttagelser (Nordenskiöld) 4: 401–471.
Gray, J.E. 1832. Synopsis of the Contents of the British Museum. 42nd Edition. 370 pp. G. Woodfall and Son, London.
Gray, J.E. 1867. Notes on the arrangement of sponges, with the descriptions of some new genera. Proceedings of the Zoological Society of London 1867: 492–558.
Gray, J.E. 1868. Note on Theonella, a new genus of coralloid sponges from Formosa. Proceedings of the Zoological Society of London 1868: 565–566.
Gray, J.E. 1870. Notes on anchoring sponges (in a letter to Mr. Moore). Annals and Magazine of Natural History, Series 4 6 (34): 309–312. Crossref
Hartman, W.D. 1980. Systematics of the Porifera. In: W.D. Hartman, J.W. Wendt, and F. Wiedenmayer (eds.), Living and Fossil Sponges, Notes for a Short Course. Sedimenta 8: 24–51.
Hechtel, G.J. 1965. A systematic study of the Demospongiae of Port Royal, Jamaica. Bulletin of the Peabody Museum of Natural History 20: 1–103.
Hentschel, E. 1911. Tetraxonida. 2. Teil. In: W. Michaelsen and R. Hartmeyer (eds.), Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905 3 (10): 279–393.
Hentschel, E. 1914. Monaxone Kieselschwämme und Hornschwämme der Deutschen Südpolar-Expedition 1901–1903. Deutsche Südpolar-Expedition 15: 35–141.
Hinde, G.J. 1910. On the fossil sponge spicules in a rock from deep lead at Princess Royal Township, Norseman District, Western Australia. Bulletin of Geological Survey of Western Australia 36: 7–24.
Hinde, G.J. and Holmes, W.M. 1892. On the sponge remains in the Lower Tertiary Strata near Oamaru, Otago, New Zealand. Journal of the Linnean Society 24: 177–262. Crossref
Hooper, J.N.A. 2002. Family Raspailiidae Hentschel, 1923. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 469–510. Kluwer Academic/Plenum Publishers, New York. Crossref
Hooper, J.N.A., Kennedy, J., and Quinn, R.J. 2002. Biodiversity “hotspots”, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera). Biodiversity and Conservation 11: 851–885. Crossref
Hoshino, T. 1990. Merlia tenuis n. sp. encrusting shell surfaces of gastropods Chicoreus, from Japan. In: K. Rützler (ed.), New Perspectives in Sponge Biology, 295–301. Smithsonian Institution Press, Washington, D.C.
Hurcewicz, H. 1991. Igły gąbek w utworach Badenu okolic Korytnicy. Przegląd Geologiczny 39: 11–12.
Ivanik, M.M. 1983. Paleogene and Neogene sponge spicules from Sites 511, 512, and 513 in the South Atlantic. In: W.J. Ludwig, V.A. Krasheninnikov et al. (eds.), Initial Reports. Deep Sea Drilling Project, 933–950. US Government Printing Office, Washington. Crossref
Ivanik, M.M. 2002. Spongiofauna from Badenian sediments near the Bogotovo Selo (Yugoslavia) [in Ukrainian]. Paleontological Collection 34: 7–17.
Ivanik, M.M. 2003. Paleogenovaâ Spongiofauna Vostočno-Èvropejskoj Platformy i Sopredel’nyx Regionov. 202 pp. Institut Geologičeskih Nauk, Nacional’naâ Akademiâ Nauk Ukrainy, Kiev.
Ivanova, T.A. 2014. Spicules of lithistid sponges from the Upper Eocene of the Verkhovtsevo Depression (Middle Dnieper Region) [in Russian]. Visnik Dnipropetrovskogo universitetu. Seriâ “Geologiâ. Geografiâ” 22 (3/2): 69–82.
Ivanova, T.A. and Olshtynska, A.P. [Olštynska, A.P] 2004. To the question of ecology and paleogeography of the Kartvelian basin of the Plain Crimea (middle Miocene, South Ukraine) [in Russian]. In: Problemi stratigrafii fanerozoû Ukraini, 160–164. Institut Geologičnih Nauk Nacionalnoi Akademii Nauk Ukraini, Kiiv.
Kelly, M. and Buckeridge, J.S. 2005. An early Paleogene sponge fauna, Chatham Island, New Zealand. New Zealand Journal of Marine and Freshwater Research 39: 899–914. Crossref
Kelly, M., Erpenbeck, D., Morrow, C., and van Soest, R.W.M. 2015. First record of a living species of the genus Janulum (class Demospongiae) in the Southern Hemisphere. Zootaxa 3980: 255–266. Crossref
Kirkpatrick, R. 1900. Description of sponges from Funafuti. Annals and Magazine of Natural History 6 (34): 345–362. Crossref
Kirkpatrick, R. 1903. Descriptions of South African Sponges. Part III. Marine Investigations in South Africa 2: 233–264.
Kirkpatrick, R. 1908. On two new genera of recent pharetronid sponges. Annals and Magazine of Natural History 2 (12): 503–514. Crossref
Koltun, V.M. 1959. Siliceous horny sponges of the northern and fareastern seas of the U.S.S.R. [in Russian]. Opredeliteli po Faune SSSR, Izdavaemye Zoologičeskim Institutom Akademii Nauk SSSR 67: 1–236.
Konenkova, I.D., Bogdanovich, E.M. [Bogdanovič, E.M.], Korallova, V.V., and Manyuk, V.V. [Manûk, V.V.] 1996. New data on the distribution of Upper Eocene sediments in the Middle Dnieper Region [in Russian]. Dopovidi Nacionalnoi Akademii Ukraini 6: 100–104.
Lamarck, J.B.P. de Monet, Comte de 1815 [1814]. Suite des polypiers empâtés. Mémoires du Muséum d’Histoire naturelle, Paris 1: 69–80, 162–168, 331–340.
Laubenfels, M.W. de 1932. The marine and fresh-water sponges of California. Proceedings of the United States National Museum 81: 1–140. Crossref
Laubenfels, M.W. de 1936. A discussion of the sponge fauna of the Dry Tortugas in particular and the West Indies in general, with material for a revision of the families and orders of the Porifera. Carnegie Institute of Washington Publication 467 (Tortugas Laboratory Paper 30): 1–225.
Laubenfels, M.W. de 1955. Porifera. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology. Part E, Archaeocyatha and Porifera, E21–E112. Geological Society of America and University of Kansas Press, Lawrence, Kansas.
Lehnert, H., Stone, R.P., and Heimler, W. 2013. Histodermella kagigunensis sp. nov. from the Gulf of Alaska and Aleutian Islands; first records of the genus from the North Pacific. Journal of the Marine Biological Association of the United Kingdom 93: 1245–1248. Crossref
Lendenfeld, R. von 1903. Porifera. Tetraxonia. In: F.E. Schulze (ed.), Das Tierreich. Vol. 19, vi–xv, 1–168. Friedländer, Berlin.
Lendenfeld, R. von 1907. Die Tetraxonia. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf der Dampfer Valdivia 1898–1899 11: 59–374.
Lévi, C. 1953. Sur une nouvelle classification des Démosponges. Compte rendu hebdomadaire des séances de l’Académie des sciences Paris 236: 853–855.
Lévi, C. and Lévi, P. 1983. Démosponges bathyales récoltées par le N/O “Vauban” au sud de la Nouvelle-Calédonie. Bulletin du Muséum National d’Histoire Naturelle (4A) 5: 931–997.
Lévi, C. and Lévi, P. 1989. Spongiaires (Musorstom 1 and 2). In: J. Forest (ed.), Résultats des Campagnes Musorstom. Mémoires du Muséum national d’Histoire naturelle, Series A (Zoologie) 4: 25–103.
Lieberkühn, N. 1859. Neue Beiträge zur Anatomie der Spongien. Archiv für Anatomie und Physiologie 30: 353–382, 515–529.
Lundbeck, W. 1905. Porifera. Part II. Desmacidonidae (pars). The Danish Ingolf-Expedition 6 (2): 1–219.
Lundbeck, W. 1910. Porifera. Part III) Desmacidonidae (pars). The Danish Ingolf-Expedition 6 (3): 1–124.
Łukowiak, M. 2015. Reconstruction of the Late Eocene “soft” sponge fauna of southern Australia. Zootaxa 3917: 1–65. Crossref
Łukowiak, M. 2016. Fossil and modern sponge fauna of southern Australia and adjacent regions compared: interpretation, evolutionary and biogeographic significance of the late Eocene “soft” sponges. Contributions to Zoology 85: 13–35. Crossref
Łukowiak, M. and Pisera, A. 2016. Bodily preserved
Eocene non-lithistid demosponge fauna from southern Australia: taxonomy
and affinities. Journal of Systematic
Palaeontology 15: 473–497. Crossref
Łukowiak, M., Cramer, K.L., Madzia, D., Hynes, M.G., Norris, R.D., and A. O’Dea. 2018. Historical change in a Caribbean reef sponge community and long-term loss of sponge predators. Marine Ecology Progress Series 601: 127–137. Crossref
Łukowiak, M., Pisera, A., and Schlögl, J. 2014. Bathyal sponges from the late Early Miocene of the Vienna Basin (central Paratethys, Slovakia). Paläontologische Zeitschrift 88: 263–277. Crossref
Maldonado, M. 2002. Family Pachastrellidae. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 141–162. Kluwer Academic/Plenum Publishers, New York. Crossref
Manconi, R., Perino, E., and Pronzato, R. 2016. A new species of Agelas from the Zanzibar Archipelago, western Indian Ocean (Porifera, Demospongiae). ZooKeys 553: 1–31. Crossref
Marshall, W. 1876. Ideen über die Verwandtschaftsverhältnisse der Hexactinelliden. Zeitschrift für Wissenschaftliche Zoologie 27: 113–136.
Matteucci, R. and Russo, A. 2005. The Middle Eocene siliceous sponges from Val di Chiampo (Lessini Mountains, northern Italy). Annali dell’Universita degli Studi di Ferrara Museologia Scientifica e Naturalistica 2005: 51–62.
Menshenina, L.L., Tabachnick, K.R., and Janussen, D. 2007. Revision of the subgenus Neopsacas (Hexactinellida, Rossellidae, Crateromorpha) with the description of new species and subspecies. Zootaxa 1463: 55–68. Crossref
Morrow, C. and Cárdenas, P. 2015. Proposal for a revised classification of the Demospongiae (Porifera). Frontiers in Zoology 12: 7. Crossref
Mostler, H. 1986. Beitrag zur stratigraphischen Verbreitung und phylogenetischen Stellung der Amphidiscophora und Hexasterophora (Hexactinellida, Porifera). Mitteilungen Der Ostereichischen Geologischen Gesellschaft 78: 319–359.
Mueller, B., De Goeij, J.M., Vermeij, M.J.A, Mulders, Y., Ent, E. van der, Ribes, M., and Duyl, F.C. van 2014. Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PLOS ONE: e90152-7. Crossref
Nardo, G.D. 1833. Auszug aus einem neuen System der Spongiarien, wonach bereits die Aufstellung in der Universitäts-Sammlung zu Padua gemacht ist. In: L. Oken (ed.), Isis, oder Encyclopädische Zeitung, 519–523. Oken, Jena.
d’Orbigny, A.D. 1851. Cours élémentaire de paléontologie et de géologie stratigraphiques. 382 pp. Masson, Paris. Crossref
Palmer, A.A. 1988. Paleoenvironmental significance of siliceous sponge spicules from Sites 627 and 628, Little Bahama Bank, Ocean Drilling Program Leg 101. In: J.A. Austin, W. Schlager et al. (eds.), Proceedings of the Ocean Drilling Program, Scientific Results 101: 159–168. Crossref
Pansini, M. and Longo, C. 2003. A review of the Mediterranean Sea sponge biogeography with, in appendix, a list of the demosponges hitherto recorded from this sea. Biogeography 24: 59–90. Crossref
Peixinho, S., Fernandez, J., Oliveira, M.V., Caíres, S., and Hajdu, E. 2007. Description of two new species of Acanthotetilla Burton, 1959 from NE Brazil, Southwestern Atlantic (Tetillidae, Spirophorida, Demospongiae). In: M.R. Custódio, G. Lôbo-Hajdu, E. Hajdu, G. Muricy (eds.), Porifera Research. Biodiversity, Innovation and Sustainability. Livros de Museu Nacional 28: 509–515.
Pezelj, D., Sremac, J., and Bermanec, V. 2016. Shallow-water benthic foraminiferal assemblages and their response to the palaeoenvironmental changes—example from the Middle Miocene of Medvednica Mt. (Croatia, Central Paratethys). Geologica Carpathica 67: 329–345. Crossref
Pisera, A. 1999. Postpaleozoic history of the siliceous sponges with rigid skeleton. Memoires of the Queensland Museum 44: 473–478.
Pisera, A. 2000. New species of lithistid sponges from the Paleogene of the Ukraine. Zoosystema 22: 285–298.
Pisera, A. and Busquets, P. 2002. Eocene siliceous sponges from the Ebro Basin (Catalonia, Spain). Geobios 35: 321–346. Crossref
Pisera, A. and Hladilová, S. 2004. Siliceous sponge spicules from Karpatian of the Carpathian Foredeep in Moravia. In: R. Brzobohaty, I. Cicha, M. Kovac, and F. Rogl (eds.), The Karpatian. A Lower Miocene Stage of the Central Paratethys, 189–191. Masaryk University, Brno.
Pisera, A., Cachao, M., and Da Silva, C.M. 2006. Siliceous sponge spicules from the Miocene Mem Moniz marls (Portugal) and their environmental significance. Rivista Italiana di Paleontologia e Stratigrafia 112: 287–299.
Plotkin, A., Morrow, C., Gerasimova, E., and Rapp, H.T. 2017. Polymastiidae (Demospongiae: Hadromerida) with ornamented exotyles: a review of morphological affinities and description of a new genus and three new species. Journal of the Marine Biological Association of the United Kingdom 97: 1351–1406. Crossref
Reiswig, H.M. 2002a. Family Aphrocallistidae Gray, 1867. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1294–1298. Kluwer Academic/Plenum Publishers, New York. Crossref
Reiswig, H.M. 2002b. Family Farreidae Gray, 1872. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1344–1352. Kluwer Academic/Plenum Publishers, New York. Crossref
Reiswig, H.M. 2002c. Family Uncinateridae Fam. nov. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1384–1388. Kluwer Academic/Plenum Publishers, New York. Crossref
Reiswig, H.M. and Wheeler, B. 2002. Family Euretidae Zittel, 1877. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1313–1343. Kluwer Academic/Plenum Publishers, New York. Crossref
Ridley, S.O. and Dendy, A. 1886. Preliminary report on the Monaxonida collected by H.M.S. Challenger. Part I. Annals and Magazine of Natural History 18: 325–351, 470–493. Crossref
Ridley, S.O. and Duncan, P.M. 1881. On the genus Plocamia, Schmidt, and on some other sponges of the order Echinonemata with descriptions of two additional new species of Dirrhopalum. Journal of the Linnean Society, Zoology 15: 476–497. Crossref
Rützler, K., Piantoni, C., van Soest, R.W.M., and Díaz, M.C. 2014. Diversity of sponges (Porifera) from cryptic habitats on the Belize barrier reef near Carrie Bow Cay. Zootaxa 3805: 1–129. Crossref
Sarà, M. 1960. Diactinolopha, genere nuovo di Plakinidae per D. moncharmonti sp. n. rinvenuta nel golfo di Napoli (Demospongiae). Annuario dell’Istituto e Museo de Zoologia dell’Università di Napoli 12: 1–7.
Sarà, M. 2002. Family Tethyidae Gray, 1848. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 245–267. Kluwer Academic/Plenum Publishers, New York. Crossref
Samaai, T. 2006. Biodiversity “hotspots”, patterns of richness and endemism, and distribution of marine sponges in South Africa based on actual and interpolation data: A comparative approach. Zootaxa 1358: 1–37.
Schmidt, O. 1862. Die Spongien des adriatischen Meeres. 88 pp. Wilhelm Engelmann, Leipzig.
Schmidt, O. 1870. Grundzüge einer Spongien-Fauna des atlantischen Gebietes. 88 pp. Wilhelm Engelmann, Leipzig.
Schrammen, A. 1903. Zur Systematik der Kieselspongien. Mitteilungen aus dem Roemer-Museum 19: 1–21.
Schrammen, A. 1924. Die Kieselspongien der oberen Kreide von Nordwestdeutschland. III und letzter Teil. Mit Beiträgen zur Stammesgeschichte. Monographien zur Geologie und Paläontologie 1: 1–159.
Schulze, F.E. 1880. Untersuchungen über den Bau und die Entwicklung der Spongien. Neunte Mittheilung. Die Plakiniden. Zeitschrift für wissenschaftliche Zoologie 34 (2): 407–451.
Silva, J.A. and Zea, S. 2017. New records of sponges of the genera Petrosia and Xestospongia (Demospongiae, Haplosclerida, Petrosiidae) from the Colombian Caribbean. Boletín de Investigaciones Marinas y Costeras 46: 113–136. Crossref
Sim, C.J. 1996. A new sponge species, Stelletta kundukensis (Demospongiae: Stellettidae) from Korea. Korean Journal of Systematic Zoology 12: 79–82.
Sim-Smith, C. and Kelly, M. 2015. The marine fauna of New Zealand: Sponges in the family Geodiidae (Demospongiae; Astrophorina). NIWA Biodiversity Memoir 188: 102.
Sollas, W.J. 1885. A classification of the sponges. Annals and Magazine of Natural History 16 (95): 395. Crossref
Sollas, W.J. 1886. Preliminary account of the tetractinellid sponges dredged by H.M.S. “Challenger” 1872–1876. Part I. The Choristida. Scientific Proceedings of the Royal Dublin Society (new series) 5: 177–199.
Sollas, W.J. 1887. Sponges. In: A. Black and C. Black (eds.), Encyclopaedia Britannica 9th Edition, Vol. 22, 412–429. A. and C. Black, Edinburgh.
Sollas, W.J. 1888. Report on the Tetractinellida collected by H.M.S. Challenger, during the years 1873–1876. In: Report on the Scientific Results of the Voyage of H.M.S. Challenger, 1873–1876. Zoology 25: 1–458.
Stefanska, T.A. [Stefanskaâ, T.A.] 2015. Sponge fossils of Middle Dnieper River Upper Eocenian deposits (geological survey sheet area “Kobelyaki”) [in Russian]. Visnik Dnipropetrovskogo universitetu. Seriâ “Geologiâ, Geografiâ” 23: 114–124.
Stefanska, T.A. [Stefanskaâ, T.A.] 2014. New sponge spicules from the Eocene deposits of paleodepressions of Ukrainian Shield (Middle Dnieper Region) [in Ukrainian]. Paleontologičnyj Zbirnik 46: 30–36.
Stefanska, T.A. [Stefanskaâ, T.A.] and Stefansky, V.L. [Stefanskij, V.L.] 2014. Composition and value of sponge fauna for biostratigraphy and paleoecology of Eocene of Middle Dnieper Region [in Russian]. Mieždunarodnyj Naučnyj Institut “Educatio” 5: 107–111.
Tabachnick, K.R. 2002a. Family Euplectellidae Gray, 1867. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1400–1446. Kluwer Academic/Plenum Publishers, New York. Crossref
Tabachnick, K.R. 2002b. Family Rossellidae Schulze, 1885. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1453–1517. Kluwer Academic/Plenum Publishers, New York. Crossref
Tabachnick, K.R. and Menshenina, L.L. 2002a. Family Hyalonematidae Gray, 1857. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1244–1275. Kluwer Academic/Plenum Publishers, New York. Crossref
Tabachnick, K.R. and Menshenina, L.L. 2002b. Family Pheronematidae Gray, 1870. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 1279–1292. Kluwer Academic/Plenum Publishers, New York. Crossref
Thiele, J. 1903. Kieselschwämme von Ternate. II. Abhandlungen herausgegeben von der Senckenbergischen naturforschenden Gesellschaft 25: 933–968.
Thomson, C.W. 1873. The Depths of the Sea. 527 pp. Macmillan and Co., London.
Topsent, E. 1896. Campagnes du Yacht Princesse Alice. Sur deux curieuses Espérellines des Açores. Bulletin de la Société Zoologique de France 21: 147–150.
Topsent, E. 1897. Spongiaires de la Baie d’Amboine (Voyage de MM. M. Bedot et C. Pictet dans l’Archipel Malais). Revue suisse de Zoologie 4: 421–487. Crossref
Topsent, E. 1898. Eponges nouvelles des Açores. (Première serie). Mémoires de la Société Zoologique de France 11: 225–255.
Topsent, E. 1904. Spongiaires des Açores. Résultats des campagnes scientifiques accomplies par le Prince Albert I Monaco 25: 1–280. Crossref
Topsent, E. 1927. Diagnoses d’Éponges nouvelles recueillies par le Prince Albert ler de Monaco. Bulletin de l’Institut océanographique Monaco 502: 1–19.
Topsent, E. 1928. Spongiaires de l’Atlantique et de la Méditerranée provenant des croisières du Prince Albert ler de Monaco. Résultats des campagnes scientifiques accomplies par le Prince Albert I Monaco 74: 1–376.
Topsent, E. 1932. Notes sur des clionides. Archives de Zoologie expérimentale et générale 74: 549–579.
Vacelet, J. 1979. Descriptions et affinités d’une éponge sphinctozoaire actuelle. In: C. Lévi and N. Boury-Esnault (eds.), Biologie des Spongiaires. Sponge Biology. Colloques Internationaux du Centre National de la Recherché Scientifique 291: 483–493.
Vacelet, J. 1980. Squelette calcaire facultatif et corps de régénération dans le genre Merlia, éponges apparentées aux Chaetétidés fossiles. Compte rendu hebdomadaire des séances de l’Académie des sciences, Paris (D) 290: 227–230.
Vacelet, J. and Vasseur, P. 1971. Éponges des récifs coralliens de Tuléar (Madagascar). Téthys 1: 51–126.
van Soest, R.W.M. 1977. A revision of the megacanthoxea-bearing tetillids (Porifera, Spirophorida), with a description of a new species. In: P.W. Hummelinck and L.J. van der Steen (eds.), Uitgaven van de Natuurwetenschappelijke Studiekring voor Suriname en de Nederlandse Antillen No. 89. Studies on the Fauna of Curaçao and other Caribbean Islands 53: 1–14.
van Soest, R.W.M. 1980. Marine sponges from Curaçao and other Caribbean localities. Part II. Haplosclerida. In: P.W. Hummelinck and L.J. van der Steen (eds.), Uitgaven van de Natuurwetenschappelijke Studiekring voor Suriname en de Nederlandse Antillen. No. 104. Studies on the Fauna of Curaçao and other Caribbean Islands 62 (191): 1–173.
van Soest, R.W.M. 2007. Sponge biodiversity. Journal of Marine Biological Association of the UK 87: 1345–1348. Crossref
van Soest, R.W.M. 2017. Sponges of the Guyana Shelf. Zootaxa 4217: 1–225. Crossref
van Soest, R.W.M. and Beglinger, E.J. 2009. New bioeroding sponges from Mingulay coldwater reefs, north-west Scotland. Journal of the Marine Biological Association of the United Kingdom 89: 329–335. Crossref
van Soest, R.W.M. and Hajdu, E. 2002. Family Mycalidae Lundbeck, 1905. In: J.N.A. Hooper and R.W.M. van Soest (eds.), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 669–690. Kluwer Academic/Plenum Publishers, New York. Crossref
van Soest, R.W.M. and Hooper, J.N.A. 2002. Family Samidae Sollas, 1886. In: J.N.A. Hooper and R.W.M. van Soest (eds), Systema Porifera. A Guide to the Classification of Sponges, Vol. 1, 99–101. Kluwer Academic/Plenum Publishers, New York. Crossref
van Soest, R.W.M., Boury-Esnault, N., Hooper, J.N.A., Rützler, K., de Voogd, N.J., Alvarez, B., Hajdu, E., Pisera, A., Manconi, R., Schönberg, C., Klautau, M., Picton, B., Kelly, M., Vacelet, J., Dohrmann, M., Díaz, M.-C., Cárdenas, P., Carballo, J.L., Ríos, P., and Downey, R. 2018. World Porifera Database [accessed at http://www.marinespecies.org/porifera on 2018-02-25]
van Soest, R.W.M., Boury-Esnault, N., Vacelet, J., Dohrmann, M., Erpenbeck, D., de Voogd, N.J., Santodomingo, N., Vanhoorne, B., Kelly, M., and Hooper, J.N.A. 2012. Global diversity of sponges (Porifera). PLoS ONE 7: e35105. Crossref
van Soest, R.W.M., Meesters, E.H., and Becking, L.E. 2014. Deep-water sponges (Porifera) from Bonaire and Klein Curaçao, Southern Caribbean. Zootaxa 3878: 401–443. Crossref
van Soest, R.W.M., Zea, S., and Kielman, M. 1994. New species of Zyzzya, Cornulella, Damiria, and Acheliderma (Porifera: Poecilosclerida), with a review of fistular genera of Iophonidae. Bijdragen tot de Dierkunde 64: 163–192. Crossref
Vosmaer, G.C.J. 1885. Porifera, Parts Vll–XI. In: H.G. Bronn (ed.), Die Klassen und Ordnungen des Thier-Reichs. Vol. 1, 177–368. C.F. Winter’sche, Leipzig.
Verrill, A.E. 1907. The Bermuda Islands: Part V. An account of the coral reefs (characteristic life of the Bermuda coral reefs). Porifera: Sponges. Transactions of the Connecticut Academy of Arts and Sciences 12: 330–344. Crossref
Wiedenmayer, F. 1994. Contribution of the knowledge of post-Paleozoic neritic and archibenthal sponges (Porifera). Schweizerische Paläontologische Mitteilungen 116: 1–147.
Weltner, W. 1882. Beiträge zur Kenntniss der Spongien. 62 pp. Inaugural Dissertation, Freiburg.
Zittel, K.A. 1877. Studien über fossile Spongien. I. Hexactinellidae. Abhandlungen der Mathematisch-Physikalischen Classe der Königlich-Bayerischen Akademie der Wisseneschaften 13: 1–63.
Acta Palaeontol. Pol. 64 (4): 871–895, 2019
https://doi.org/10.4202/app.00612.2019