


The oldest “intermetamorphic” larva of an achelatan lobster from the Lower Jurassic Posidonia Shale, South Germany
JOACHIM T. HAUG, CAROLIN HAUG, and GÜNTER SCHWEIGERT
Haug, J.T., Haug, C., and Schweigert, G. 2019. The oldest “intermetamorphic” larva of an achelatan lobster from the Lower Jurassic Posidonia Shale, South Germany. Acta Palaeontologica Polonica 64 (4): 685–692.
Achelatan lobsters, also known as spiny and slipper lobsters, develop via a highly specialised larval form. This special larva, phyllosoma, is flat, translucent, possesses elongate legs and can grow to enormous sizes. Although these larvae may appear very fragile, they are well-known as fossils. Thousands of specimens have been found in the lithographic limestone of Southern Germany (Tithonian, Upper Jurassic, about 150 mya). At least three types of fossil, but modern-appearing phyllosoma larvae are known. Additionally, fossil larvae that possess only some of the characters of modern-day phyllosoma larvae are known from the same Lagerstätte, but also from the younger limestone beds of Lebanon. Here we report a new achelatan fossil from the older Posidonia Shale (Toarcian, Lower Jurassic, 175–183 mya). The specimen shows certain characters of a phyllosoma larva, but other characters appear like those of post-phyllosoma stages of achelatan lobsters. This specimen is therefore the oldest occurrence of an achelatan lobster larva. We compare the new specimen with other fossil larvae with such mixed or “intermetamorphic” morphologies.
Key words: Decapoda, Achelata, phyllosoma, zoea, Jurassic, Germany.
Joachim T. Haug [joachim.haug@palaeo-evo-devo.info] and Carolin Haug [carolin.haug@palaeo-evo-devo.info], Ludwig Maximilians University Munich, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany.
Günter Schweigert [guenter.schweigert@smns-bw.de] State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany.
Received 21 April 2019, accepted 31 July 2019, available online 14 October 2019.
Copyright © 2019 J.T. Haug et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Decapoda is a group of crustaceans that have a crucial impact on their ecosystems, but, in the case of edible forms, are also important for the economy of certain areas. While the many forms of adult representatives of Decapoda, such as prawns, shrimps, lobsters, and crabs, are well-known to most people, their larvae are less well-known to the public. This is surprising, given the fact that these larvae are at least as important as their adult forms. Only if the larva survives, it will be able to become an adult. Ecologically, larvae of Decapoda may even be more important than their adult counterparts given their specific role in the marine food web: they are part of the plankton, preying on smaller plankton and being prey for larger organisms. Some of these larvae reach enormous sizes, remain in the plankton for a longer time span and moult into different larval stages, in some cases more than ten distinguishable ones (Palero et al. 2014b). The probably most impressive examples are the larval stages of achelatan lobsters, spiny lobsters and slipper lobsters. These larvae are called phyllosoma and can reach up to 150 mm in leg span (Johnson 1951; Palero et al. 2014a). The larvae are very flat, translucent and possess long and thin legs (e.g., Palero et al. 2014a). All species have very long larval phases during which they reach these extreme sizes (Marinovic et al 1994; Mikami and Greenwood 1997; Matsuda and Yamakawa 2000; Abrunhosa et al. 2008; Kizhakudan and Krishnamoorthi 2014). To remain in the water column despite their unusually large size, these larvae ride on jellyfish and other gelatinous macro-plankton (Shojima 1963; Herrnkind et al. 1976; Ates et al. 2007).
While one might expect that fragile-appearing larval forms such as a phyllosoma are impossible to be found as fossils, quite the opposite is true. While we only have a handful of larval forms of other groups of Decapoda, we have literally thousands of fossils of phyllosoma larvae. Most of these immature achelatan fossils originate from the Solnhofen-type lithographic limestone beds of southern Germany and have an age of about 150 million years (Tithonian; Polz 1970, 1971, 1972, 1973, 1984, 1987, 1996; Haug et al. 2011, 2014). The Cretaceous lithographic limestone of Lebanon have a comparable preservation potential and have provided us with some fossil phyllosoma-like larvae of about 90 million years (Turonian) in age (Pasini and Garassino 2009; Haug et al. 2011). Some remains of compound eyes from the Lower Cretaceous of Brazil have also been interpreted as possible parts of phyllosoma larvae (Tanaka et al. 2009; see also Audo et al. 2019).
Yet, some of the fossil achelatan larvae from the lithographic limestone of Lebanon are not phyllosoma larvae in the strict sense (Haug et al. 2013a). Also in the lithographic limestone of Southern Germany some more unusual larval forms of achelatan lobsters have been found. These fossils have combinations of characters that occur in modern phyllosoma larvae and other characters that today only occur in post-phyllosoma stages of achelatan lobsters (Polz 1995; Haug and Haug 2013, 2016; Haug et al. 2009, 2013a). More importantly, there are different types of such larvae, each of them possessing different types of character combinations. This indicates that the diversity of larval morphologies of achelatan lobsters was higher in the past (Haug et al. 2013a). In an evolutionary frame, three possible explanations for these intermediate types of morphologies have been suggested. (i) Some specimens could represent ontogenetic stages between the last phyllosoma stage and the first true post-phyllosoma stage; hence, in these species the metamorphosis from the planktic phyllosoma to the benthic forms seems to have occurred in more moult stages than in modern forms; (ii) Some specimens could represent the larvae of early representatives of Achelata that do not possess the highly derived larval morphologies of extant phyllosoma larvae; (iii) Some forms could also represent paedomorphic adults. At least for case 1, likely candidates have been identified (Haug and Haug 2016). Case 2 might be more tricky to identify, as the modern-type phyllosoma larvae seem to have co-occurred with the larvae with intermediate morphologies (Haug et al. 2013a). Case 3 appears to be even more challenging to be identified.
Here we report a fossil larval achelatan lobster that is, to our knowledge, the stratigraphically oldest fossil of such a larva. Furthermore, it represents a larval form with an intermediate type of morphology. We discuss the implications of this find.
Institutional abbreviations.—SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany.
Material and methods
The single specimen in the focus of this study comes from the Posidonia Shale (Posidonienschiefer Formation, Toarcian, 175–183 mya) and was found in a now abandoned quarry at Gomaringen, Southern Germany. It is deposited in the Staatliches Museum für Naturkunde Stuttgart under repository number SMNS 70449.
The specimen was documented with a Keyence VHX-6000 digital microscope. Cross-polarised coaxial light was used for illumination, improving the contrast between fossil and matrix significantly (e.g., Hörnig et al. 2014; Audo et al. 2016 and references therein). The fossil was documented as a composite image: each image detail was recorded with a stack of images differing in focal planes and processed into one sharp image. Adjacent image details were stitched to a large panorama image. Additionally, unpolarised reflected light (ring illumination) was used to produce another composite image. Of this, the virtual surface (cf. Haug et al. 2013b) was reconstructed (based on depth-from-defocus principles; built-in software). This was used to record snapshots under different angles. These images were assembled to stereo-images to show the three-dimensional relief of the fossil.
Systematic palaeontology
Achelata Scholtz and Richter, 1995
Remarks.—Based on the discussed characters, the specimen can be recognised as an ingroup of Achelata, any further interpretation is currently not possible and thus we refrain from creating a new taxon on genus and species level for it.
Achelata gen. et sp. indet.
Figs. 1, 2.
Material.—SMNS 70449 (only known specimen), late larval stage with prominent exopods from Gomaringen near Tübingen, Southern Germany, Neth quarry, Unterer Stein Bed, Posidonienschiefer Formation (Posidonia Shale). Early Toarcian, Harpoceras falciferum Zone (Riegraf et al. 1984). The quarry is abandoned since decades and after renaturation the section is no longer exposed.
Description.—A late larval stage with prominent exopods, but already adult-type body, proximal region of antenna and endopods of posterior thoracopods. Rather small at this stage with only about 20 mm main body length (Fig. 3B).
Incompletely preserved euarthropodan specimen; total length of main body axis in anterior-posterior axis plus preserved parts of anterior projections slightly more than 20 mm (Fig. 1A). Body elongate, about five times as long as wide (maximum width). Body subdivided into three more or less distinct regions. Anterior region without laterally projecting appendages; middle region with prominent laterally projecting appendages; posterior region without appendages, but subdivided into seven more or less distinct sections (Fig. 1B).
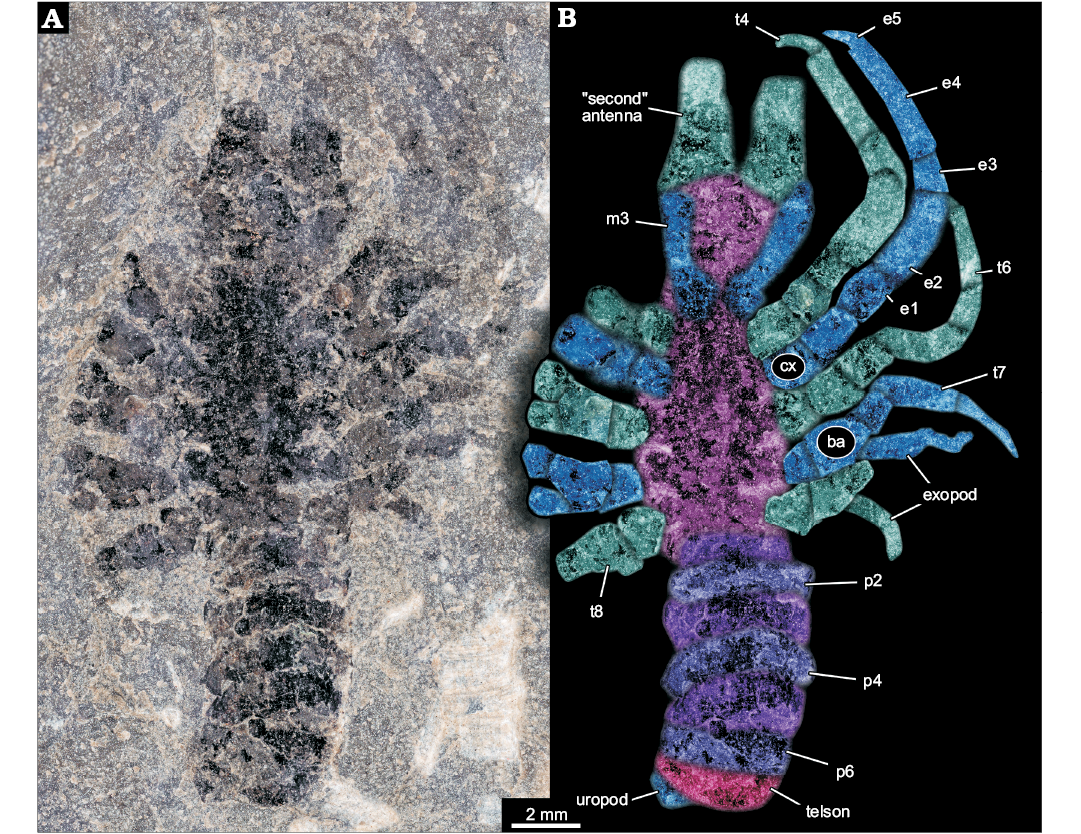
Fig. 1. Larva of Achelata gen. et sp. indet. (SMNS 70449), Toarcian, Lower Jurassic, Gomaringen, Southern Germany. A. Composite microscopic photograph under cross-polarised light. B. Colour-marked photograph indicating the visible structures. Abbreviations: ba, basipod; cx, coxa; e1–5, endopod element 1–5; m3, maxilliped 3; p2–6, pleon segment 2–6; t4–8, thoracic appendages 4–8 (“pereiopods” 1–5).
Anterior region of the body about as long as maximum width of the body. Anterior rim of the anterior region as wide as maximum body width, anterior region narrowing posteriorly. Posterior rim of the anterior region about 80% of the width of the anterior rim. Anteriorly with two projections directed forward, apparently broken off, must have been significantly longer. Proximal width of projections more than 30% of the body width. Furthermore, two more structures seen as faint impressions, most likely representing appendages, arise from close to the posterior rim of the anterior region. Thinner than the anterior projecting structures (hence smaller diameter), about 20% of the maximum body width. At least five times as long as wide. Proximally with a distinct square-shaped region, set off from the further distal one.
Middle region of the body about twice as long as anterior region. Narrower anteriorly, widening posteriorly to reach the maximum width in the middle (anterior-posterior axis), and remaining as wide towards the posterior. Middle region with five prominent appendages on each side, evenly distributed along the lateral rim. All appendages apparently sub-similar originally, yet in different states of preservation. Maximum length of an appendage about 70% of the entire body length excluding appendages; diameter about 25% of the body width.
Overall appendage morphology with a proximal main axis with two distinct elements (coxa and basipod). Coxa slightly longer along proximal-distal axis than wide. Basipod slightly longer than coxa. Basipod carrying two branches distally, the more medially placed one presumably being the endopod and the more laterally placed one presumably being the exopod. Endopod clearly subdivided into five elements. Proximal element of endopod (ischium) similar to basipod in size. Endopod element 2 (merus) significantly longer, about twice as long as preceding elements, more than twice as long as wide, slightly curved. Endopod element 3 (carpus) significantly shorter, slightly shorter than the coxa, also more slender, slightly tapering distally. Endopod element 4 (propodus) longest of the series, slightly shorter than the combined length of ischium and merus. About as slender as carpus, slightly tapering distally. Endopod element 5 (dactylus) short, slender, inward curved, about as long as carpus, but only 50% of the diameter. Exopod not well preserved, subdivision not apparent, slender. Maximum length of exopod at least as long as merus, but only about 50% of its width (diameter).
Prominent appendage 1 without exopod, either as original condition or not preserved/not visible . Prominent appendage 2 slightly longer than prominent appendage 1; also here the presence of an exopod is unclear. Prominent appendage 3 smaller than preceding, i.e,. slightly shorter and more slender. The propodus of prominent appendage 3 is more slender and tapering distally, the dactylus is almost as wide as the distal edge of the propodus. Exopod present. Prominent appendages 4 and 5 progressively smaller, exopods present.
Posterior region of the body with seven more or less distinct sections. First section slightly narrower than middle region, more or less rectangular in dorsal-ventral view, short, about 20% of the maximum body width. Second section wider, slightly longer and very gently curving backwards. Section 3 about as wide as section 2, slightly longer, even more curved. Section 4 with similar dimensions as 3, but even more curved. Section 5 longer, slightly narrower, also appearing curved. Section 6 similar in dimensions to 5, but appearing more rectangular again. Section 7 sub-similar to 6. A small lobe-like structure protrudes from under section 7; width about 30% of maximum body width, half as long as wide.
Three-dimensional relief of specimen shows that the region between the prominent appendages is subdivided into six structures (Fig. 2). Each appears as depression running from the insertion region of one appendage to the corresponding one on the other side. The anteriormost one appears to be corresponding to the smaller appendage visible in the anterior region.
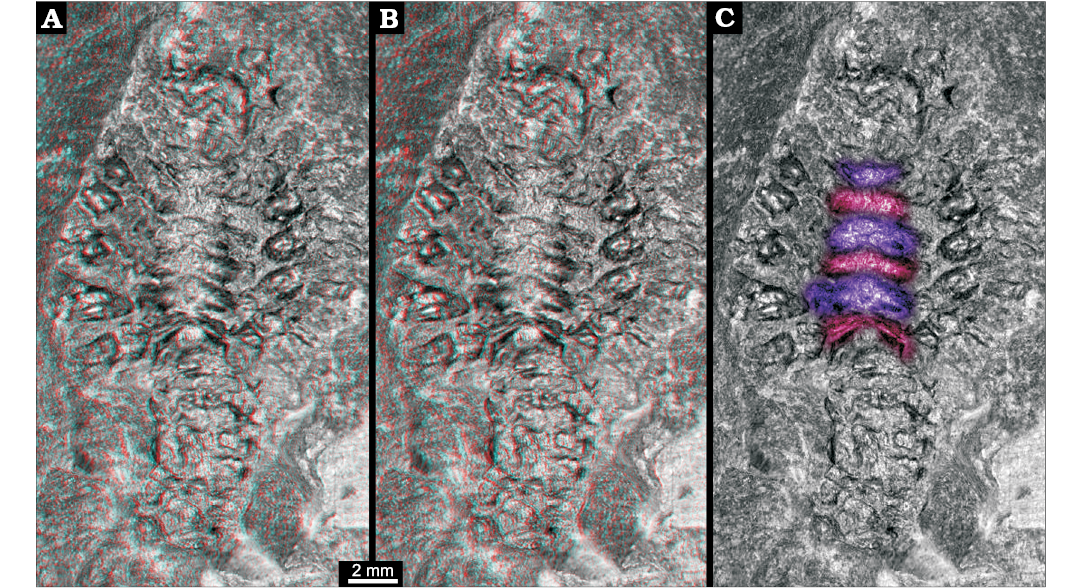
Fig. 2. Larva of Achelata gen. et sp. indet. (SMNS 70449), Toarcian, Lower Jurassic, Gomaringen, Southern Germany. A. Stereo-image showing original relief of the fossil; based on virtual surface. B. Depth-inverted stereo-image showing morphologically correct relief in ventral view. Please use red-cyan glasses to view A and B. C. Non-stereo image; colour-marked are the elevations of the thoracic sternum.
Remarks.—The available structures preserved on the specimen provide us with enough information to allow a sound systematic interpretation. The principle body organization with an anterior region, i.e., head region, a middle region with five pairs of prominent appendages and a posterior trunk region with seven distinct sections, is best compatible with the interpretation of the specimen as a representative of Decapoda.
In this context, the anterior projections are best interpreted as massive antennae, and the superimposed appendages as the maxillipeds three (= thoracopods 3). Five pairs of appendages of the middle region represent the posterior five thoracopods (= pereiopods). The seven sections of the posterior trunk are interpreted as the six pleon segments and the telson, the lobe-like structure as a part of the uropods.
The posterior five thoracopods appear like robust walking appendages and indicate a position within Reptantia (the group including lobsters and crab-like forms). This may be further supported by the fact that the specimen is preserved in dorso-ventral orientation, possibly indicating a certain original compression of the body in this direction. The depressions in the median region would correspond to elevations in the thoracic sternum still indicating the individual segmentation. There are six of these corresponding to the segments bearing maxillipeds three and the five prominent posterior thoracopods.
Among crustaceans of the group Decapoda the exopods, i.e., the outer branches of biramous appendages, become reduced during ontogeny when the organism settles to the ground (e.g., Williamson 1969). The fact that exopods are still present at least on some of the thoracic appendages at a size of about 20 mm indicates that the specimen is a larval representative of Achelata. Modern larvae of Achelata with biramous appendages can reach leg spans of 150 mm (Johnson 1951; Palero et al. 2014a).
An identity as an achelatan lobster is further supported by the fact that thoracopod 4 does not possess a chela, but a simple curved dactylus and no finger-like extension of the propodus (Scholtz and Richter 1995). An additional character supporting this interpretation is that of the five prominent thoracopods the second one is slightly larger than the first one (Scholtz and Richter 1995). The prominent anterior broken-off appendages, best understood as the proximal parts of the massive antennae, further support that the specimen is a representative of Achelata.
Overall, the specimen resembles non-phyllosoma-type fossil larvae from the Mesozoic such as Polzicaris sahelalmae from the lithographic limestone of Lebanon (Turonian?, about 90 million years old; Haug et al. 2011, 2013a) and especially “Palinurina” tenera Oppel, 1862 from the Solnhofen lithographic limestone (Tithonian, about 150 million years old; Haug and Haug 2016). This similarity accounts for the overall body organisation, appendage morphology with robust endopods, but retaining the exopods, and the arrangement of the thoracopod insertions, which is more oval/circular than triangular (as in adult achelatan lobsters).
Unlike many achelatan lobsters developing via a stage with intermediate morphology between phyllosoma-type morphology and juvenile/adult-type morphology. Differing from some other comparable forms by body shape. Differing from Polzicaris sahelalmae (Fig. 3A), which is much larger at a comparable stage and retains the specialised phyllosoma-type claw, which is absent in SMNS 70449. Differing from “Palinurina” tenera mainly through size (Fig. 3C1, C2), the smallest known stage of “Palinurina” tenera is only sclerotised at its thoracic appendages, hence less far sclerotised than SMNS 70449, but much larger (Fig. 3C1). Specimens of “Palinurina” tenera with a comparable morphology to SMNS 70449 are even larger.
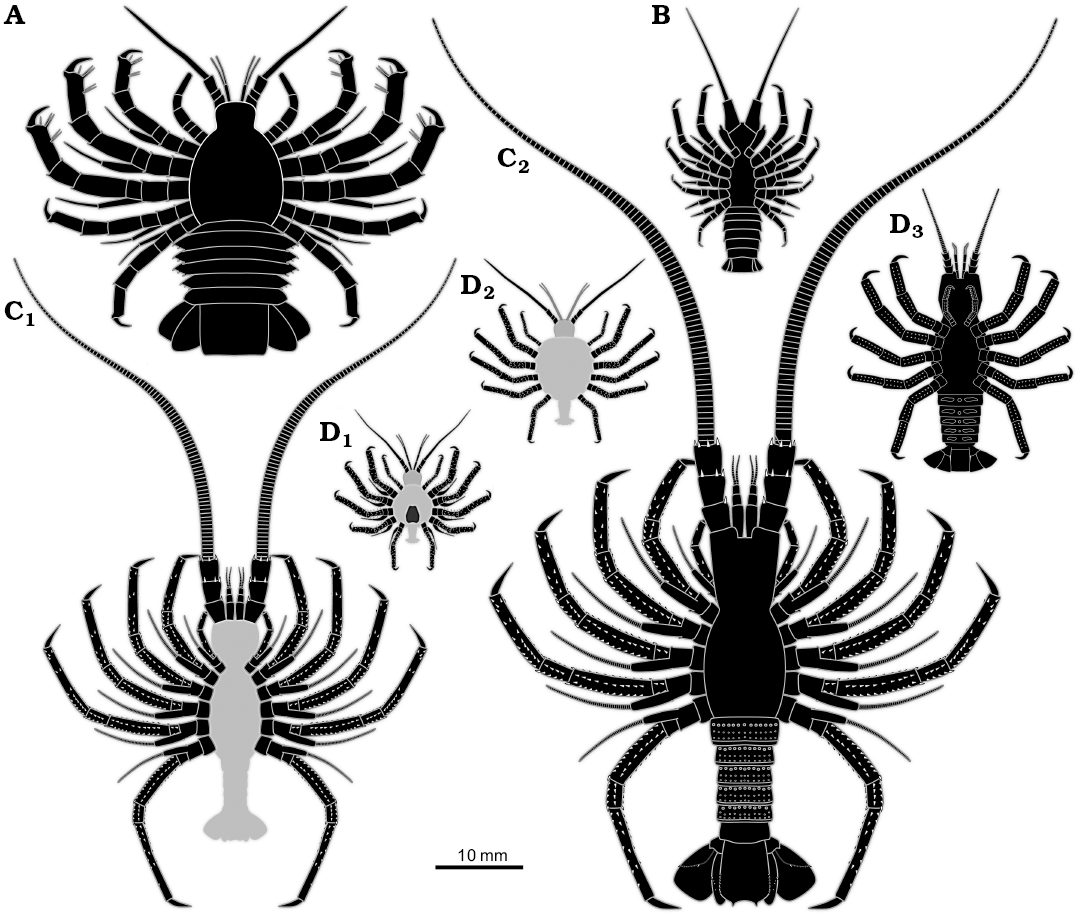
Fig. 3. Size comparison of different non-phyllosoma type achelatan larvae. All specimens as idealised restorations. The light grey areas represent body parts not being preserved, but inferred. A. Polzicaris sahelalmae (based on Haug et al. 2013a). B. SMNS 70449. C. “Palinurina” tenera, earliest (C1) and largest (C2) known stage (based on Haug and Haug 2016). D. Cancrinos claviger, earlier larva still possessing exopods (D1), later larva with exopods already absent (D2), possible juvenile, yet without triangular sternum (D3) (D1, D2, based on Haug et al. 2013a; D3, based on Haug and Haug 2015).
Discussion
The oldest larva of an achelatan lobster.—Based on the preserved details, we can identify the new specimen as the larva of an achelatan lobster. So far, fossil larvae of achelatan lobsters were only known from the lithographic limestone of Lebanon and Southern Germany and possibly from Brazil. Hence, the new find represents the oldest report of such a larval form.
The specimen has a morphology that is unknown from any extant achelatan lobster, as it combines characters that are in modern forms characteristic for phyllosoma-type larvae (e.g., presence of exopods, circular arrangement of posterior thoracopods) and post-phyllosoma stages (e.g., robust posterior thoracopod endopods, well developed and sclerotised pleon). In its overall morphology it resembles some of the intermediate stages known from the lithographic limestone of southern Germany. Most strongly it reminds of the stages of “Palinurina” tenera (Haug and Haug 2016), yet these are significantly larger. The leg arrangement and the rough body outline appear also quite similar to later immature stages that have been interpreted as Cancrinos claviger (Haug and Haug 2013, 2015; Fig. 3D1–D3). Yet, the latter has already lost ontogenetically its exopods at a comparable body size.
Specimen SMNS 70449 is a new addition to the growing group of fossil achelatan larvae with intermediate or “intermetamorphic” morphologies. It adds yet another subtle variation concerning the combination of characters and an additional size range, smaller than most of the known forms, but larger than the exopod-bearing stages of C. claviger. Given its age, the new larva is the oldest of these larvae, being about 30 million years older than any other known achelatan larva. It is also the second-oldest report of a eumalacostracan larva so far, the oldest candidate being of Triassic age (Hyžný et al. 2016).
Evolutionary background.—As the new find is a single specimen, it is not possible to clearly identify the evolutionary background explaining the intermetamorphic morphology of the larva. It is, of course, tempting that the oldest known larva is not of the typical phyllosoma-type, but of a mixed morphology type. Indeed, it could potentially represent a case of an evolutionary early larva that has only evolved some morphological characteristics of modern phyllosoma larvae, but not all of them. Yet, it will require more specimens, ideally representing an ontogenetic sequence, to support or reject such an assumption.
Life habits.—Similar to other intermetamorphic forms, it remains difficult to make any suggestion concerning the life habits of the new specimen. Its appendages might possess a certain ability to grab something, similar to a pseudochela, but lack the distinct type of phyllosoma grasping-type chela known for example in Polzicaris sahelalmae. The leg arrangement clearly speaks against a benthic life style, walking would have been rather ineffective. Given the quite small body size and the presence of exopods, the specimen might have indeed lived as part of the plankton. Yet, as we simply lack a comparable stage in modern forms, any assumptions remain speculative.
Associated crustacean fauna.—The crustacean fauna of the Posidonia Shale of Southern Germany is not very diverse; fossils of crustaceans have been considered to be rather rare (e.g., Fraaye and Jäger 1995). Among the known forms (Fraaye and Jäger 1995; Schweigert 2001, 2018; Schweigert et al. 2003; Audo 2016) are: (i) the lobster-like fossils of Uncina posidoniae, reaching large sizes of almost half a meter; (ii) numerous species of polychelidan lobsters (nowadays only known from the deep sea), among them species of Proeryon, Tonneleryon, and Coleia (note that Audo 2016 doubted that Coleia sinuata is a valid separate species, only its position in Polychelida was supported based on a single known specimen); (iii) rare specimens of glypheidan lobsters (a group nowadays only known by two genera from relict areas); (iv) unclear records, among them a supposed mantis shrimp and a supposed specimen of a hermit crab of Palaeopagurus.
It seems that the new specimen is the first record of the group Achelata in the Posidonia Shale of Southern Germany. It is also the first example among crustaceans, which possibly could have lived as part of the plankton. All other records appear to have been benthic or at least necto-benthic adults. With this, the new fossil also expands the range of possible ecological roles among the crustaceans from the Posidonia Shale.
Conclusions
The specimen reported here represents the oldest fossil record of an achelatan lobster larva. It possesses a mixture of typical phyllosoma characters and post-phyllosoma characters, which has in this combination not been found in any other achelatan larva to date. With this, it provides further important information on the different possible morphotypes of achelatan larvae, however, its evolutionary background can currently not be reconstructed reliably. As first representative of achelatan lobsters in the Posidinia Shale and as first possible planktonic crustacean from these deposits, it contributes important information to the systematic composition and ecological roles of the fauna in the Posidonia Shale.
Acknowledgements
Denis Audo (Yunnan University, Kunming, Chiny) and Francisco Vega (Universidad Nacional Autónoma de México, Mexico City, México) kindly provided helpful comments to improve the manuscript. Part of the project has been supported by the German Research Foundation in the project “Palaeo-Evo-Devo of Malacostraca” (Ha 6300/3-1, continued under Ha 6300/3-2). JTH is supported by the Volkswagen Foundation with a Lichtenberg professorship. CH and JTH are supported by J. Matthias Starck (Ludwig-Maximilians-Universität, Munich, Germany). People providing free computer software, in this case OpenOffice, are especially thanked.
References
Abrunhosa, F.A., Santiago, A.P., and Abrunhosa, J.P. 2008. The early phyllosoma stages of spiny lobster Panulirus echinatus Smith, 1869 (Decapoda: Palinuridae) reared in the laboratory. Brazilian Journal of Biology 68: 179–186. Crossref
Ates, R., Lindsay, D.J., and Sekiguchi, H. 2007. First record of an association between a phyllosoma larva and a prayid siphonophore. Plankton and Benthos Research 2: 67–69. Crossref
Audo, D. 2016. Tonneleryon, a new gregarious polychelidan lobster from the early Toarcian Posidonia Shale of Holzmaden (Germany). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 280: 285–298. Crossref
Audo, D., Haug, J.T., Haug, C., Charbonnier, S., Schweigert, G., Müller, C.H.G., and Harzsch, S. 2016. On the sighted ancestry of blindness—exceptionally preserved eyes of Mesozoic polychelidan lobsters. Zoological Letters 2: art. 13. Crossref
Audo, D., Robin, N., Luque, J., Krobicki, M., Haug, J.T., Haug, C., Jauvion, C., and Charbonnier, S. 2019. Palaeoecology of Voulteryon parvulus (Eucrustacea, Polychelida) from the Middle Jurassic of La Voulte-sur-Rhône Fossil-Lagerstätte (France). Scientific Reports 9: 5332. Crossref
Fraaye, R., and Jäger, M. 1995. Decapods in ammonite shells-examples of inquilinism from the Jurassic of England and Germany. Palaeontology 38: 63–75. Crossref
Haug, J.T., Ahyong, S., and Haug, C. 2014. Fossil malacostracan larvae. In: J.W. Martin, J. Olesen, and J.T. Høeg (eds.), Atlas of Crustacean Larvae, 176–179. The Johns Hopkins University Press, Baltimore.
Haug, J.T., and Haug, C. 2013. An unusual fossil larva, the ontogeny of achelatan lobsters, and the evolution of metamorphosis. Bulletin of Geosciences 88: 195–206. Crossref
Haug, J.T., and Haug, C. 2015. “Crustacea”: Comparative aspects of larval development. In: A. Wanninger (ed.), Evolutionary Developmental Biology of Invertebrates 4: Ecdysozoa II: Crustacea, 1–37. Springer, Wien. Crossref
Haug, J.T. and Haug, C. 2016. “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters—The case of the species tenera Oppel, 1862. Arthropod Structure and Development 45: 108–121. Crossref
Haug, J.T., Audo, D., Charbonnier, S., and Haug, C. 2013a. Diversity of developmental patterns in achelate lobsters—today and in the Mesozoic. Development Genes and Evolution 223: 363–373. Crossref
Haug, J.T., Haug, C., Waloszek, D., and Schweigert, G. 2011. The importance of lithographic limestones for revealing ontogenies in fossil crustaceans. Swiss Journal of Geosciences 104 (Supplement 1): S85–S98. Crossref
Haug, J.T., Haug, C., Waloszek, D., Maas, A., Wulf, M., and Schweigert, G. 2009. Development in Mesozoic scyllarids and implications for the evolution of Achelata (Reptantia, Decapoda, Crustacea). Palaeodiversity 2: 97–110.
Haug, J.T., Müller, C.H.G., and Sombke, A. 2013b. A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity and Evolution 13: 425–432. Crossref
Herrnkind, W., Halusky, J., and Kanciruk, P. 1976. A further note on phyllosoma larvae associated with medusae. Bulletin of Marine Science 26: 110–112.
Hörnig, M.K., Haug, C., Herd, K.J. and Haug, J.T. 2014. New insights into dictyopteran early development: smallest Palaeozoic roachoid nymph found so far. Palaeodiversity 7: 159–165.
Hyžný, M., Haug, C., and Haug, J.T. 2016. Mesoprosopon triasinum from the Triassic of Austria revisited: The oldest eumalacostracan larva known to date and its significance for interpreting fossil cycloids. Gondwana Research 37: 86–97. Crossref
Johnson, M.W. 1951. A giant phyllosoma larva of a loricate crustacean from the tropical Pacific. Transactions of the American Microscopical Society 70: 274–278. Crossref
Kizhakudan, J.K. and Krishnamoorthi, S. 2014. Complete larval development of Thenus unimaculatus Burton and Davie, 2007 (Decapoda, Scyllaridae). Crustaceana 87: 570–584. Crossref
Marinovic, B., Lemmens, J.W., and Knott, B. 1994. Larval development of Ibacus peronii Leach (Decapoda: Scyllaridae) under laboratory conditions. Journal of Crustacean Biology 14: 80–96. Crossref
Matsuda, H. and Yamakawa, T. 2000. The complete development and morphological changes of larval Panulirus longipes (Decapoda, Palinuridae) under laboratory conditions. Fisheries Science 66: 278–293. Crossref
Mikami, S. and Greenwood, J.G. 1997. Complete development and comparative morphology of larval Thenus orientalis and Thenus sp. (Decapoda: Scyllaridae) reared in the laboratory. Journal of Crustacean Biology 17: 289–308. Crossref
Pasini, G. and Garassino, A. 2009. A new phyllosoma form (Decapoda,? Palinuridae) from the Late Cretaceous (Cenomanian) of Lebanon. Atti della Società italiana di Scienze naturali e del Museo civico di Storia naturale di Milano 150: 21–28.
Palero, F., Clark, P.F., and Guerao, G. 2014a. Achelata. In: J.W. Martin, J. Olesen, and J.T. Høeg (eds.), Atlas of Crustacean Larvae, 272–278. The Johns Hopkins University Press, Baltimore.
Palero, F., Guerao, G., Hall, M., Chan, T.Y., and Clark, P.F. 2014b. The “giant phyllosoma” are larval stages of Parribacus antarcticus (Decapoda: Scyllaridae). Invertebrate Systematics 28: 258–276. Crossref
Polz, H. 1970. Zur Unterscheidung von Phalangites priscus Münster und Palpipes cursor Roth (Arthropoda) aus den Solnhofener Plattenkalken. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1970 (12): 705–722.
Polz, H. 1971. Eine weitere Phyllosoma-Larve aus den Solnhofener Plattenkalken. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1971 (8): 474–488.
Polz, H. 1972. Entwicklungsstadien bei fossilen Phyllosomen (Form A) aus den Solnhofener Plattenkalken. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1972 (11): 678–689.
Polz, H. 1973. Entwicklungsstadien bei fossilen Phyllosomen (Form B) aus den Solnhofener Plattenkalken. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1973 (5): 284–296.
Polz, H. 1984. Krebslarven aus den Solnhofener Plattenkalken. Archaeopteryx 2: 30–40.
Polz, H. 1987. Zur Differenzierung der fossilen Phyllosomen (Crustacea, Decapoda) aus den Solnhofener Plattenkalken. Archaeopteryx 5: 23–32.
Polz, H. 1995. Ein außergewöhnliches Jugendstadium eines palinuriden Krebses aus den Solnhofener Plattenkalken. Archaeopteryx 13: 67–74.
Polz, H. 1996. Eine Form-C-Krebslarve mit erhaltenem Kopfschild (Crustacea, Decapoda, Palinuroidea) aus den Solnhofener Plattenkalken. Archaeopteryx 14: 43–50.
Riegraf, W., Werner, G., and Lörcher, F. 1984. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias ε). 195 pp. Enke, Stuttgart.
Scholtz, G. and Richter, S. 1995. Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca). Zoological Journal of the Linnean Society 113: 289–328. Crossref
Schweigert, G. 2001. Hartmanns Eryon—ein Krebs aus dem Posidonienschiefer. Fossilien 2001: 279–282.
Schweigert, G. 2018. Neufund von Glypheopsis grandichela im Posidonienschiefer. Fossilien 35 (6): 56–57.
Schweigert, G., Garassino, A., Hall, R.L., Hauff, R.B., and Karasawa, H. 2003. The lobster genus Uncina Quenstedt, 1851 (Crustacea: Decapoda: Astacidea: Uncinidae) from the Lower Jurassic. Stuttgarter Beiträge zur Naturkunde, Serie B 332: 1–43.
Shojima, Y. 1963. Scyllarid phyllosomas’ habit of accompanying the jellyfish. Bulletin of the Japanese Society for the Science of Fish 29: 349–353. Crossref
Tanaka, G., Smith, R.J., Siveter, D.J., and Parker, A.R. 2009. Three-dimensionally preserved decapod larval compound eyes from the Cretaceous Santana Formation of Brazil. Zoological Science 26: 846–850. Crossref
Williamson, D.I. 1969. Names of larvae in the Decapoda and Euphausiacea. Crustaceana 16: 210–213. Crossref
Acta Palaeontol. Pol. 64 (4): 685–692, 2019
https://doi.org/10.4202/app.00627.2019