

Palaeobiology and evolutionary context of Angulithes mermeti, a streamlined early Late Cretaceous shallow-water nautiloid
MARKUS WILMSEN and EMAD NAGM
Wilmsen, M. and Nagm, E. 2019. Palaeobiology and evolutionary context of Angulithes mermeti, a streamlined early Late Cretaceous shallow-water nautiloid. Acta Palaeontologica Polonica 64 (4): 831–849.
Cretaceous nautiloids are commonly characterized by inflated shells and prolonged stratigraphic ranges. In the Albian, the species of Angulithes appeared and compressed, short-lived forms with narrow venters emerged during the Cenomanian age. Based on a new description, the late Cenomanian nautiloid Angulithes mermeti is discussed with its palaeobiological background and placed in an evolutionary context of the Cenomanian lineage of Angulithes, considering contemporaneous palaeoenvironmental changes and inferred functional traits. A. mermeti is characterized by a nearly oxycone shell with sharp venter and narrow umbilicus, a fairly sinuous suture, low inter-septal distances, and an almost dorsal siphuncle. Its palaeobiogeographical occurrence was latitudinally restricted to shallow tropical–subtropical shelf seas with a preferred habitat depth between 5–50 m. Several morphological trends reflected by the Cenomanian species of the genus culminated in the late Cenomanian species A. mermeti, i.e., (i) increasing shell compression and sharpening of the venter, (ii) increasing folding of the septa, (iii) reduction of inter-septal distances, and (iv) dorsally directed migration of the siphuncle. The hydrodynamically efficient form was favorable to successfully populate the wide and shallow epicontinental seas that formed during the Cenomanian age. The increasing sutural sinuosity and the dense septal spacing aimed to buttress the shells against shell-breaking predators while the functional reason for the dorsal-directed migration of siphuncle is more elusive; it may have improved the efficiency of the hydrostatic apparatus and its internal position is beneficial in the case of predation, too. The gradual morphological change in the Cenomanian lineage of the genus Angulithes provides a well-constrained case study of rapid evolutionary response to major environmental pressure, i.e., the opening of newly available niches in the course of the great early Late Cretaceous transgression, in an otherwise rather bradytelic biotic group.
Key words: Cephalopoda, Nautilida, palaeobiogeography, autecology, functional morphology, evolutionary patterns, Cenomanian, Egypt.
Markus Wilmsen [markus.wilmsen@senckenberg.de], Senckenberg Naturhistorische Sammlungen Dresden, Museum für Mineralogie und Geologie, Sektion Paläozoologie, Königsbrücker Landstraße 159, 01109 Dresden, Germany.
Emad Nagm [emad.nagm@yahoo.com], Department of Geology, College of Science, Taibah University, Madinah 41411, Saudi Arabia and Department of Geology, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt.
Received 10 May 2019, accepted 10 August 2019, available online 26 November 2019.
Copyright © 2019 M. Wilmsen and E. Nagm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Recent nautilids are iconic externally shelled cephalopods and a well-studied group, today being restricted to a few species populating relatively small relict habitats in deeper waters (e.g., Saunders and Landman 1987; Ward et al. 2016). During the Cretaceous nautiloids were common and both environmentally and palaeobiogeographically much more widespread (e.g., Wiedmann 1960). However, Cretaceous nautiloids are fairly meagre in external shell characters and tend to be rather conservative in terms of evolutionary change, commonly resulting in prolonged stratigraphical ranges, especially when compared to fast-evolving contemporaneous ammonites (e.g., Ward and Signor 1983). Furthermore, homeomorphies, common in nautiloids in general (e.g., Kummel 1956; Dzik 1984), and taphonomical constraints (often restriction to commonly poorly preserved internal moulds in carbonate facies and/or selective preservation of ribbing; e.g., Wilmsen 2016; Malchyk et al. 2017) complicate the understanding of systematic relationships and the elaboration of comprehensive taxonomical revisions. Furthermore, when evaluating the general shell forms of Cretaceous nautiloids it is fairly obvious that many representatives of the typical genera such as Eutrephoceras Hyatt, 1894, Paracenoceras Spath, 1927, Cymatoceras Hyatt, 1884 or Cimomia Conrad, 1866 are characterized by inflated shells with wide whorl cross-sections and broadly rounded or even flattened venters (e.g., see Kummel 1956; Dzik 1984: text-fig. 71), suggesting, in analogue to ammonites, an overall low hydrodynamic efficiency (e.g., Westermann 1996, 1999; see also Peterman et al. 2019). However, in the latest Early Cretaceous, a genus of compressed forms with predominantly narrow, tapered venters arose, i.e., Angulithes de Montfort, 1808, some representatives of which acquired disco- to oxycone shell shapes and were also characterized by fairly short-lived stratigraphical ranges. The scope of the present contribution is a detailed description as well as a taxonomic, autecological, stratigraphical, and palaeobiogeographical discussion of the strongly compressed nautiloid species Angulithes mermeti (Coquand, 1862) based on new material from the upper Cenomanian of Egypt. Furthermore, evolutionary trends in Cenomanian species of Angulithes are evaluated in the light of contemporaneous palaeoenvironmental changes and inferred functional traits.
Institutional abbreviations.—MMG, Museum for Mineralogy and Geology, Senckenberg Naturhistorische Sammlungen Dresden, Germany: African (AFK) and Spanish (SpK) Cretaceous sub-collections; EN-WI/WH, collection from Wadi Irkas/Wadi Hawashiya sections, Department of Geology, Al-Azhar University, Assiut, Egypt; MB.C., Museum für Naturkunde, Berlin, Germany.
Other abbreviations.—D, diameter; Dmax, maximum diameter; Wb, whorl breadth at Dmax; Wh, whorl height at Dmax; Th, shell inflation; w, whorl expansion; U, umbilical exposure.
Geological setting
West of the Gulf of Suez, Wadi Araba is the main geomorphological feature represented by a low and wide southwest–northeast-directed valley that dissects the northern part of the Eastern Desert of Egypt into two high and large plateaus, i.e., the Northern and Southern Galala plateaus (Fig. 1A). To the south, Wadi Qena forms a north-northwest–east–southeast-oriented geomorphological element parallel to the Gulf of Suez/Red Sea rift system. The Galala plateaus and the southwestern slopes of Wadi Qena consist of clastic upper Paleozoic–Lower Cretaceous strata and mainly carbonate Upper Cretaceous–Paleogene successions (Bandel and Kuss 1987; Kuss et al. 2000). At their foothills, lower Upper Cretaceous rocks are well-exposed and widely distributed (Fig. 1A).
Structurally, Said (1962) subdivided the continental platform area of Egypt into two tectonic domains, the unstable shelf in the north and the stable shelf in the south. Most sedimentary exposures in the northern and central parts of the study area are following a NE-SW direction, reflecting the course of the Syrian Arc System (the best-known structural feature in the northern unstable part of the shelf; Said 1990), the tectonic activity of which began during the Early Cretaceous and intensified during the Late Cretaceous (Aal and Lelek 1994; Kuss et al. 2000; Rosenthal et al. 2000). To the south at Wadi Qena (Fig. 1A), the stable shelf area is represented by a nearly horizontal sedimentary cover with less deformation.
During the early Late Cretaceous, the study area was situated at the southern margin of the Neotethys at about 5–10° northern palaeo-latitudes (e.g., Philip and Floquet 2000; Barrier and Vrielynck 2008). In response to the major Cenomanian sea-level rise, a widespread peri-continental shelf sea covered large areas in Sinai and the Eastern Desert during the late Cenomanian. Transgression proceeded from north to south and the facies sequence shows an overall retrogradational stacking (Said 1990; Wilmsen and Nagm 2012, 2013). Marine onlap started in the study area with the mixed siliciclastic to carbonate strata of the Galala Formation, representing an open lagoonal to shallow-marine shelf environment (Wilmsen and Nagm 2012; Fig. 1B). In the northern part of the study area, the Galala Formation comprises the lower upper Cenomanian, being overlain by the offshore strata of the uppermost Cenomanian to Turonian Maghra El Hadida Formation, while the Galala Formation ranges into the Turonian in the southern part (Wadi Qena; Fig. 1B), reflecting the time-transgressive onlap of marine facies belts during the Late Cretaceous transgression. The Galala Formation in Wadi Araba and its lower upper Cenomanian segment in Wadi Qena correspond to the depositional sequence Cenomanian 5 (DS Ce 5) in the Cenomanian standard sequence stratigraphy (Robaszynski et al. 1998; Wilmsen 2003; Fig. 1C). The nautiloids of this study stem from a carbonate-dominated interval in the upper part of the depositional sequence (the Neolobites Bioevent of Nagm 2019; Fig. 1B) and were collected from measured sections logged in detail at three sites, i.e., at Wadi Ghonima, Wadi Irkas (acronym EN-WI), and Wadi Hawashiya (acronym EN-WH) (Fig. 1A, C). The lower two thirds of the sections show overall retrogradational facies stacking patterns by means of decreasing siliciclastic content and grain size as well as increasing carbonate contents, reflecting the transgressive systems tract of DS Ce 5 (Fig. 1C). The Neolobites Bioevent marks the maximum flooding zone of the sequence, overlain by highstand deposits that are erosionally truncated along sequence boundary SB Ce 5 (see Wilmsen and Nagm 2012, 2013 and Nagm 2019 for further stratigraphical and sedimentological details).
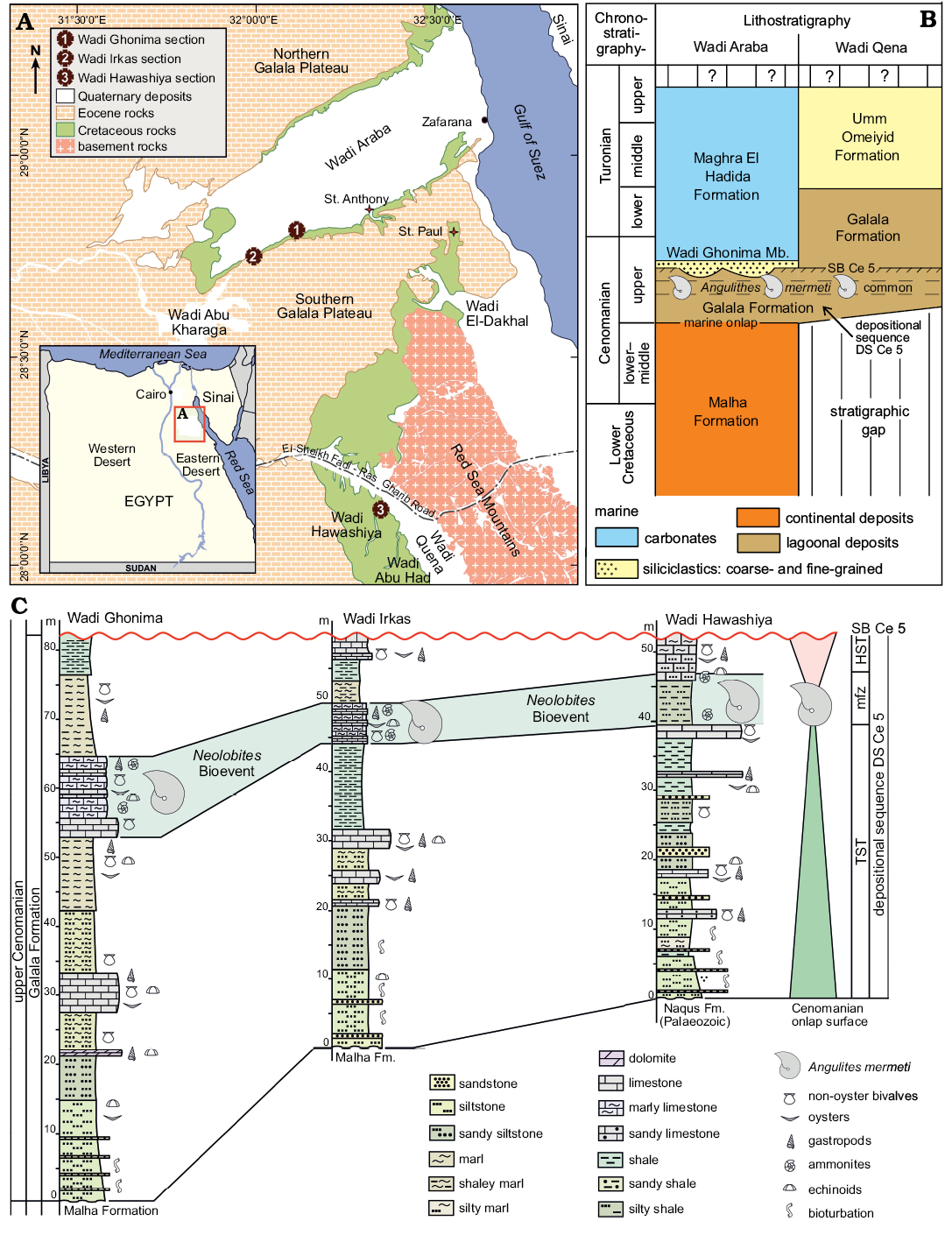
Fig. 1. A. Location of the studied sections in Wadi Araba (1, Wadi Ghonima section 28°51’19.00’’ N, 32°8’48.00’’ E; 2, Wadi Irkas section 28°49’44.40’’ N, 32°3’38.70’’ E) and the northern part of Wadi Qena (3, Wadi Hawashiya section 28°7’51.00’’ N, 32°22’0.00’’ E). B. Stratigraphical framework of the lower Upper Cretaceous in the study area; the stratigraphical position of the nautiloids described herein is indicated. C. Correlation and sequence stratigraphic interpretation of the studied sections; the Neolobites Bioevent sensu Nagm (2019) represents the maximum flooding zone (mfz) of depositional sequence DS Ce 5. Abbreviations: HST, highstand systems tract; TST, transgressive systems tract.
Material and methods
Herein, we follow the nautiloid classification of Shimansky (1975) with the exception that we regard the genus Deltoidonautilus Spath, 1927 as a synonym of Angulithes Montfort, 1808 (see also Kummel 1956; Wilmsen 2000). Morphological descriptions and terms are used according to Teichert (1964). Biometrical measurements were obtained using a Vernier Caliper and are also given in mm and percent of maximum diameter (in brackets; Fig. 2, Table 1). The data were also used to plot the shell form of A. mermeti in the Westermann morphospace diagram (cf. Ritterbush and Bottjer 2012), commonly used for comparing planispiral ammonoid shells. First, raw values of the key shape parameters of shell inflation (Th), whorl expansion (w), and umbilical exposure (U) are calculated (see Table 2). Scaling followed the procedures given in Ritterbush and Bottjer (2012) and Yacobucci (2018):
Thscaled = (Thraw – 0.14) / 0.54
wscaled = (wraw – 1.00) / 0.77
Uscaled = Uraw / 0.52
Scaling is necessary in order to bring the values within the range of values for common ammonoids. The scaled values where finally normalized to range from 0 to 1:
Thnorm = Thscaled / (Thscaled + wscaled + Uscaled)
wnorm = wscaled / (Thscaled + wscaled + Uscaled)
Unorm = Uscaled / (Thscaled + wscaled + Uscaled)
The normalized values are plotted in the Westermann morphospace diagram (see Yacobucci 2018 for a detailed protocol).

Fig. 2. Shell parameters, suture terminology, and biometric factors of the planispiral nautiloid shell (modified after Wilmsen 2016). Abbreviations: Dmax, maximum diameter; U, umbilical width at Dmax; Wb, whorl breadth at Dmax; Wh, whorl height at Dmax.
Table 1. Measurements (in mm; in brackets % of Dmax) of the studied specimens of Angulithes mermeti (Coquand, 1862), see also Fig. 2. Abbreviations: Dmax, maximum diameter; U, size of umbilicus at Dmax; Wb, whorl breadth at Dmax; Wh, whorl height at Dmax; Wh-180°, whorl height at -180° from Dmax.
|
Specimen No. |
Dmax |
Wb |
Wh |
Wb/Wh |
U |
Wh-180° [= Dmax – (Wh+U)] |
|
AFK 202 |
120.9 (100%) |
45.3 (37.5%) |
75.2 (62.2%) |
0.60 |
~6 (5.0%) |
39.7 |
|
AFK 218 |
– |
42.5 |
72.0 |
0.59 |
– |
– |
|
AFK 225 |
180.4 (100%) |
61.3 (34.0%) |
105.2 (58.3%) |
0.58 |
9.0 (5.0%) |
66.2 |
|
AFK 272 |
– |
25.9 |
48.1 |
0.54 |
– |
– |
|
EN-WI-1 |
131.6 (100%) |
47.0 (35.7%) |
76.6 (58.2%) |
0.61 |
~7 (5.3%) |
48.0 |
|
EN-WI-2 |
57.3 (100%) |
20.0 (34.9%) |
33.6 (58.6%) |
0.59 |
~4 (6.9%) |
19.7 |
|
EN-WI-5 |
– |
39.1 |
72.2 |
0.54 |
– |
– |
|
EN-WH-1 |
113.0 (100%) |
33.3 (29.4%) |
68.5 (60.6%) |
0.49 |
~6 (5.3%) |
38.5 |
|
type of Coquand (1862: pl. 2) |
~200 (100%) |
~57.5 (28.8%) |
~121 (60.5%) |
0.48 |
~11 (5.5%) |
~68 |
|
Nautilus munieri Choffat (1886: pl. 2) |
137.6 (100%) |
50.2 (36.5%) |
83.2 (60.5%) |
0.60 |
~7 (5.1%) |
47.4 |
Table 2. Raw values of shell inflation (thickness ratio; Th = Wb/Dmax), whorl expansion rate (w = Wh/Wh at -180°) and umbilical exposure U (= width of umbilicus in % of Dmax) of Angulithes mermeti (Coquand, 1862) for illustration in Westermann morphospace (Fig. 8A); see Table 1 for initial measurements and Ritterbush and Bottjer (2012) and Yacobucci (2018) for methodology. Abbreviations: Dmax, maximum diameter; U, size of umbilicus at Dmax; Wb, whorl breadth at Dmax; Wh, whorl height at Dmax.
|
Specimen No. |
shell |
whorl expansion rate (w) |
umbilical exposure (U) |
|
AFK 202 |
0.37 |
1.89 |
0.05 |
|
AFK 225 |
0.34 |
1.59 |
0.05 |
|
EN-WI-1 |
0.35 |
1.60 |
0.05 |
|
EN-WI-2 |
0.35 |
1.71 |
0.07 |
|
EN-WH-1 |
0.29 |
1.78 |
0.05 |
|
type of Coquand (1862: pl. 2) |
0.29 |
1.78 |
0.06 |
|
Nautilus munieri Choffat (1886: pl. 2) |
0.37 |
1.76 |
0.05 |
|
mean |
0.34 |
1.73 |
0.054 |
|
scaled mean |
0.37 |
0.94 |
0.104 |
|
normalized mean |
0.26 |
0.67 |
0.07 |
Systematic palaeontology
Order Nautilida Agassiz, 1847
Superfamily Nautiloidea Blainville, 1825
Family Hercoglossidae Spath, 1927
Genus Angulithes Montfort, 1808
Type species. Angulithes triangularis Montfort, 1808, from the Cenomanian of France.
Angulithes mermeti (Coquand, 1862)
Figs. 3–5, 6A, B, 10A.
1862 Nautilus Mermeti H. Coq., Coquand 1862: 166, pl. 2: 1, 2.
1886 Nautilus Munieri; Choffat 1886: 1, pl. 1: 2, pl. 2: 1.
1907 Nautilus Mermeti Coquand; Pervinquière 1907: 46.
1910 Nautilus Mermeti Coquand; Eck 1910: 11.
1912 Nautilus munieri Choffat; Schlagintweit 1912: 99, pl. 6: 9.
1914 Nautilus Mermeti Coquand; Eck 1914: 183, pl. 9: 1.
non 1914 Nautilus Mermeti Coquand var. Munieri Choffat; Eck 1914: 184, pl. 9: 2–4 (=Angulithes triangularis Montfort, 1808).
1956 Lissoniceras mermeti (Coquand); Benavides-Cáceres 1956: 435, pl. 40: 1–3.
1960 Angulithes (Angulithes) triangularis mermeti (Coquand); Wiedmann 1960: 188, pl. 22: h; pl. 25: 8, 9; pl. 26: 4; pl. 27: 1, 2; text-figs. 16–20.
1989 Angulithes mermeti (Coquand); Luger and Gröschke 1989: 358.
1992 Deltoidonautilus mermeti (Coquand); Abdel-Gawad et al. 1992: 326, pl. 1: 1.
2000 Angulithes mermeti (Coquand, 1862); Wilmsen 2000: pl. 5: 3; text-fig. 5.
2002 Angulithes sp.; Meister and Rhalmi 2002: 768, pl. 5: 1, 3; text-fig. 13b, c.
2004 Angulithes mermeti (Coquand, 1862); Abdel-Gawad et al. 2004: pl. 1: 1.
2006 Angulithes mermeti (Coquand, 1862); El Qot 2006: 114, pl. 24: 1, 2.
2006 Angulithes mermeti (Coquand); Abdel-Gawad et al. 2006: pl. 3: 4, 7.
2009 Angulithes mermeti (Coquand, 1862); Nagm 2009: 52, text-figs. 5.1A, B, 5.2A.
2010 Angulithes sp.; Cavin et al. 2010: text-fig. 7X.
2011 Angulithes mermeti (Coquand, 1862); Ayoub Hannaa 2011: 205, pl. 21: 1; text-fig. 3.36.
2012 Angulithes sp.; Benyoucef et al. 2012: pl. 1: 1.
2012 Angulithes mermeti (Coquand, 1862); Ayoub Hannaa and Fürsich 2012: 64, text-fig. 7.
2015 Angulithes mermeti (Coquand); Nagm 2015: text-fig. 7D.
2015 Angulithes mermeti (Coquand, 1862); Meister and Piuz 2015: 19, pl. 1: 1–4.
2017 Angulithes mermeti (Coquand, 1862); Meister et al. 2017: 5, pl. 1: 1a, b.
2019 Angulithes mermeti (Coquand, 1862); Nagm 2019: text-fig. 6C.
Material.—Eight internal moulds (listed in Table 1) from the lower upper Cenomanian Neolobites vibrayeanus Zone of the Galala Formation in Wadi Araba, north Eastern Desert of Egypt (Wadi Ghonima section, Wadi Irkas section), and northern Wadi Qena (Wadi Hawashiya; see Fig. 1A for location), ranging from complete adult specimens to small septate fragments. AFK 225 (Fig. 3) is an excellently preserved complete adult specimen from Wadi Ghonima, Egypt.
Measurements.—See Table 1.
Description.—Involute, almost oxycone and large-sized nautiloid with strongly compressed whorl cross-section (Wb/Wh = 0.5–0.6). The small and shallow umbilicus (5–7% of Dmax) has a rounded wall with a gradual transition onto the lower flanks where the maximum whorl breadth occurs. From there, the flanks converge to a venter which is acute in juvenile and adolescent stages, becoming very narrowly rounded in the adult stage. Maximum diameter is around 200 mm as indicated by septal approximation, e.g., in AFK 225. Ribbing has not been observed on any part of the internal moulds. The external suture is rather sinuous with a faint umbilical saddle and a low lobe on the umbilical wall rising to a prominent, knee-like saddle on the lower flank. The mid- and outer flanks are characterized by a wide lateral lobe that rises to a narrowly pointed saddle at the venter. The septal spacing is rather low (8–10 mm at D = 120 mm, ~12 mm at D = 180 mm), resulting in a high number of septa per whorl (e.g., 14 septa/half a whorl in specimen EN-WI-1 at D ~130 mm). The siphuncle has a subdorsal position, at about 1/5 of the distance from the dorsum to the venter. The nearly complete and mature specimen AFK 225 suggests that the length of the adult body chamber was relatively short and did not exceed 150° of the last whorl.
Stratigraphic and geographic range.—All specimens described herein from Wadi Araba and Wadi Qena (Eastern Desert, Egypt) are from the lower upper Cenomanian Neolobites vibrayeanus Zone that is broadly contemporaneous to the Calycoceras (C.) naviculare–C. (Proeucalycoceras) guerangeri Biozone of the NW European standard biostratigraphy (see Nagm et al. 2010a, b and Nagm and Wilmsen 2012 for details). This stratigraphic range seems to correspond to the records from literature which almost all fall into the late Cenomanian. The palaeogeographic distribution is confined to occurrences south and north of the (western) Neotethys in northern Africa and southern Europe with additional records from Peru and Oman.

Fig. 3. Hercoglossid nautiloid Angulithes mermeti (Coquand, 1862), AFK 225 from the Cenomanian of Wadi Ghonima, Egypt, in apertural (A1) and lateral (A2) views.
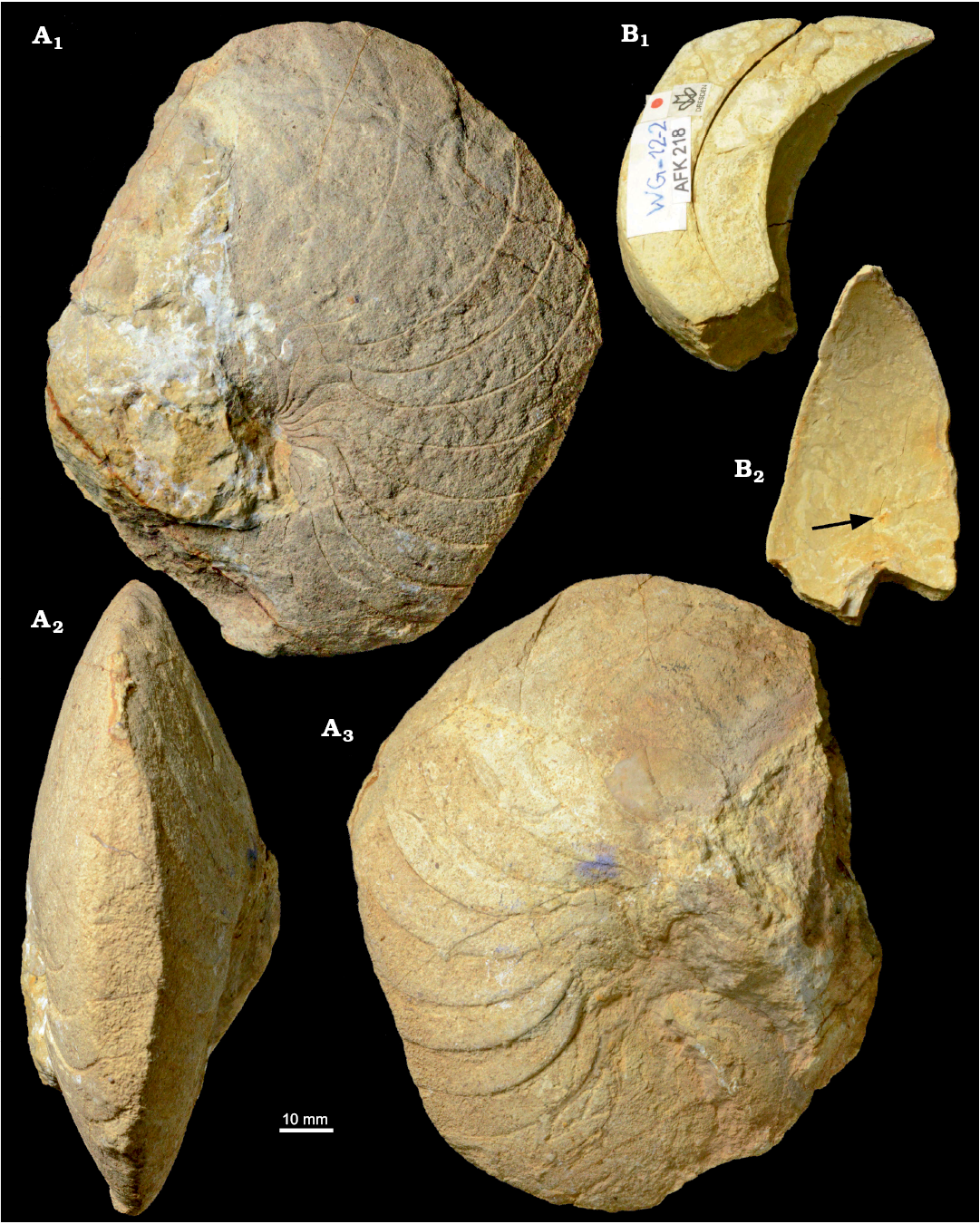
Fig. 4. Hercoglossid nautiloid Angulithes mermeti (Coquand, 1862) from the Cenomanian of Wadi Ghonima, Egypt. A. AFK 202 in lateral (A1, A3) and ventral (A2) views. B. AFK 218 in lateral (B1) and apertural (B2) views; arrow shows the position of the siphuncle.
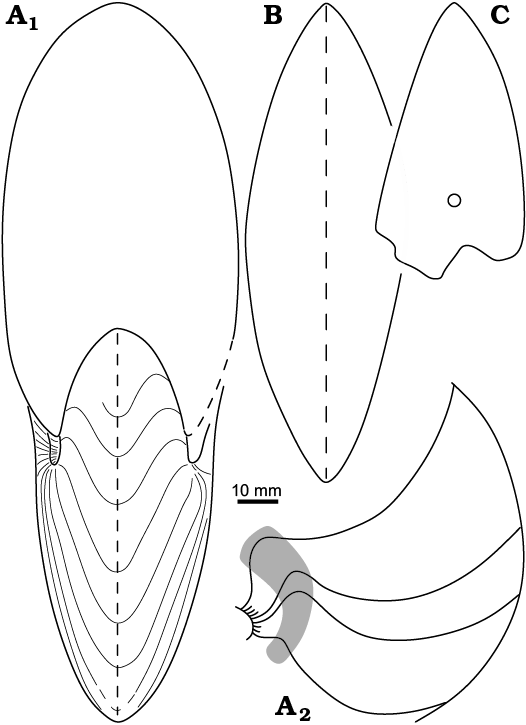
Fig. 5. Cross-sections and external sutures of the hercoglossid nautiloid Angulithes mermeti (Coquand, 1862) from the Cenomanian of Wadi Ghonima, Egypt. A. AFK 225, shell shape in apertural view (A1), external sutures (A2); grey shading indicates position of the umbilical saddle. B. AFK 202, shell shape in ventral view. C. AFK 218, whorl shape, showing the position of the siphuncle.
Discussion
Taxonomical remarks.—The smooth, strongly compressed, nearly oxycone form with sharp venter, the sinuous external suture line with conspicuous saddles on the lower flank and the venter, the high density of septa, and the subdorsal position of the siphuncle are characteristic features of Angulithes mermeti (Coquand, 1862) as described and illustrated in the literature (see synonymies above). The type specimen is from Ténoukla, near Tébassa (northeast Algeria), from the uppermost beds of the “étage rhotomagien” (Coquand 1862: 167; the “étage rhotomagien” broadly corresponds to the Cenomanian). In the original illustration (Coquand 1862: pl. 2: 1, 2), the sutures have been displayed in a simplified, less sinuous pattern as already remarked by Pervinquère (1907), Schlagintweit (1912) and Eck (1914) (see Fig. 6A). This led Choffat (1886) to the erection of a new species when he compared his Nautilus munieri (Fig. 7B) to the illustration of Coquand (1862). However, Coquand’s (1862) species has carefully been described and can unequivocally be recognized, and both taxa are contemporaneous. Thus, the two taxa are conspecific and Nautilus mermeti Coquand, 1862 has priority over Nautilus munieri Choffat, 1886. Nevertheless, the specimens recorded as “Nautilus Mermeti Coquand var. Munieri Choffat” by Eck (1914: 184, pl. 9: 2–4) correspond to Angulithes triangularis Montfort, 1808 based on their more inflated shell form and less sharp venter. Wiedmann (1960), on the other hand, followed yet another taxonomical approach and considered A. mermeti and A. triangularis as conspecific and regarded the former as a subspecies of the latter. However, A. triangularis differs from A. mermeti by its less compressed whorl section (Wb/Wh = 0.65–0.80), less sinuous suture, larger inter-septal distances and a more central position of the siphuncle (Fig. 6C, D; see Wilmsen 2000). Furthermore, the two taxa seem to be stratigraphically separate, A. triangularis being a typical late early to middle Cenomanian species (e.g., Wilmsen 2000; Debris 2006) while A. mermeti is slightly younger (see subchapters on Stratigraphy and Evolutionary trends during the Cenomanian below).

Fig. 6. Shell form (A1–C1), external sutures (A2–C2), and whorl cross-section (D) of selected hercoglossid nautiloids. A. Nautilus mermeti (Coquand, 1862) from the Cenomanian of Ténoukla near Tébassa, northeast Algeria (after Coquand 1862: pl. 2: 1, 2). B. Nautilus munieri Choffat, 1886 from the upper Cenomanian of Villa Nova d’Ourem, Portugal (after Choffat 1886: pl. 2: 1). C. Angulithes triangularis Montfort, 1808 (MB.C.2052) from the lower Sardinero Formation, lower Middle Cenomanian of Langre, Cantabria, northern Spain (after Wilmsen 2000: pl. 1: 2b, pl. 5: 15). D. Nautilus triangularis Montfort, 1808 from the Cenomanian of Île de Madame, Charente-Maritime, France (after d’Orbigny 1840: pl. 12: 2).
Palaeobiogeography.—Angulithes mermeti has been recorded from the upper Cenomanian of southern America (Peru: Schlagintweit 1912; Benavides-Cáceres 1956), Portugal (Choffat 1886; Barroso-Barcenilla et al. 2011; Segura et al. 2014), Spain (Wiedmann 1960, 1964; Barroso-Barcenilla et al. 2017), the Maghreb area (Morocco, Algeria and Tunisia: Coquand 1862; Pervinquière 1907; Meister and Rhalmi 2002; Cavin et al. 2010; Benyoucef et al. 2012; Meister et al. 2017), Egypt (Western Desert: Blanckenhorn 1900; Eastern Desert and Sinai: Eck 1910, 1914; Luger and Gröschke 1989; Abdel-Gawad et al. 1992, 2004, 2006; El Qot 2006; Nagm 2009, 2015, 2019; Ayoub Hannaa 2011; Ayoub Hannaa and Fürsich 2012) and Oman (Meister and Piuz 2015). This palaeobiogeographical distribution suggests an affinity to tropical–subtropical palaeolatitudes north and south of the palaeo-equator and a general warm-water preference of the species (latitudinal restriction; cf. Fig. 7). The reports from northern Spain are so far the northernmost records of A. mermeti, reaching into the Northern Transitional Subprovince of Ernst et al. (1996) which was characterized by a mingling of Boreal and Tethyan faunal elements throughout the Late Cretaceous. Other Late Cretaceous representatives of the genus are also known from the warm-temperate part of the Boreal Realm (e.g., Angulithes westphalicus Schlüter, 1872; cf. Wilmsen 2000) but the genus appears to be absent from high palaeo-latitudes.
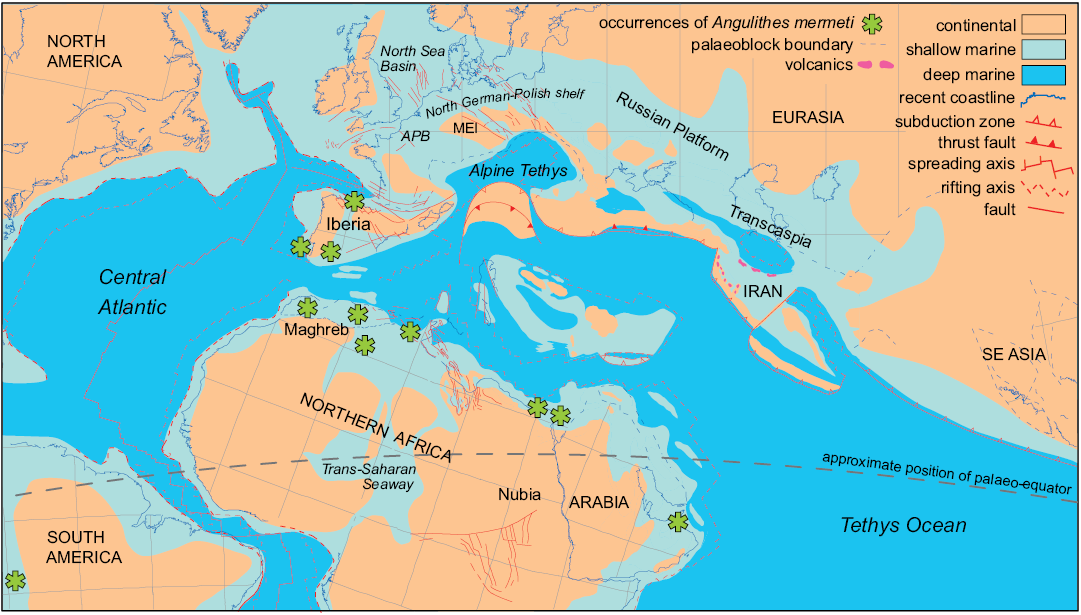
Fig. 7. Palaeobiogeographical distribution of the hercoglossid nautiloid Angulithes mermeti (Coquand, 1862). Cenomanian palaeogeographical and plate tectonic situation modified after Barrier and Vrielynck (2008); nautiloid occurrences are indicated by asterisks (see text for literature sources). Abbreviations: APB, Anglo-Paris Basin; MEI, Mid-European Island,
Autecology of Angulithes mermeti.—The smooth shell and streamlined, nearly oxycone form (mean umbilical exposure: 0.054; mean whorl expansion over the last 180°: 1.73; mean overall inflation: 0.34; see Table 2) indicate hydrodynamic efficiency and suggest, analogue to the basic planispiral ammonoid shell shapes (Jacobs and Chamberlain 1996; Westermann 1996; Ritterbush and Bottjer 2012; Ritterbush et al. 2014; Lukenender 2015), adaptation for continuous fast swimming combined with excellent acceleration of A. mermeti. In the Westermann (1996: fig. 1) morphospace diagram developed for planispiral ammonites (Fig. 8), the oxycone shell form caps the nekton field and A. mermeti plots into the nekton field between oxycones and discocones. In direct comparison, Nautilus pompilius has a significantly higher shell inflation ratio and a smaller whorl expansion (Fig. 8). Increased acceleration, speed and steerage (maneuverability combined with directional stability) by reducing drag is indicated by the smooth shell, small umbilicus, compression of the whorl section, and by the narrowly rounded to acute venter (Chamberlain 1993; Westermann 1996, 1999). According to Ritterbush (2016), such oxycone shells produce minimal drag at larger sizes and/or faster speeds (low drag coefficient of 0.25 compared to 0.7–1.1 in cadicones and gyrocones, respectively; cf. Chamberlain 1993). In contrast to the limited steering that restricts the Recent Nautilus to demersal bottom feeding (Chamberlain 1992), A. mermeti almost certainly was shaped for better hydrodynamic performance and may thus have been able to pursuit small nektonic prey.
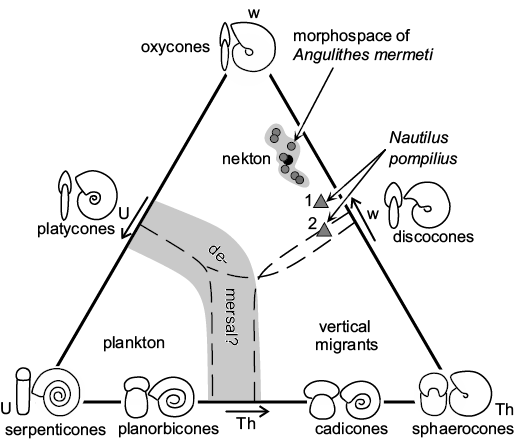
Fig. 8. Westermann morphospace diagram, simplified and modified after Ritterbush et al. (2014), with placement of the hercoglossid nautiloid Angulithes mermeti (Coquand, 1862) (see Table 2 for raw data; black circle indicates mean value); for comparison, two specimens of Nautilus pompilius Linnaeus, 1758 are plotted in the diagram, too (1, specimen 17 of Tajika et al. 2015; 2, an early Pleistocene specimen from Wani et al. 2008). Abbreviations: Th, shell inflation; U, umbilical exposure; w, whorl expansion.
Furthermore, the ability for rapid acceleration and sustained fast swimming was certainly beneficial to escape shell-breaking predators (see below). The brevidome shell (body chamber length of about 145°, similar in length to that of the Recent Nautilus pompilius) aided in stability and indicates a similar living position and orientation of the aperture (30±15° from the vertical) as in the modern ectocochleates (Fig. 9; Chamberlain 1987; Westermann 1999; Peterman et al. 2019). The compressed form of A. mermeti is shared by the co-occurring ammonite Neolobites vibrayeanus that corresponds to morphotype group 11 of Batt (1989). According to this latter study from the upper Albian to middle Turonian of the Western Interior Seaway (USA), ammonites of this group were almost always associated with nearshore and proximal offshore facies, thus being reliable indicators of relatively shallow conditions (≤ 40 m water depth; Batt 1989). However, the shell shape of N. vibrayeanus is more platycone due to the high and sub-parallel flanks and the venter is flattened (tabulate) to weakly concave, the sharp umbilical shoulders being ornamented by numerous small clavi (e.g., Wiese and Schulze 2005). This shell form certainly produced considerable drag at the leading edge during backward swimming (cf. Westermann 1996) and was definitely less well optimized (streamlined) for fast locomotion and improved steerage (Chamberlain 1993; Westermann 1999).
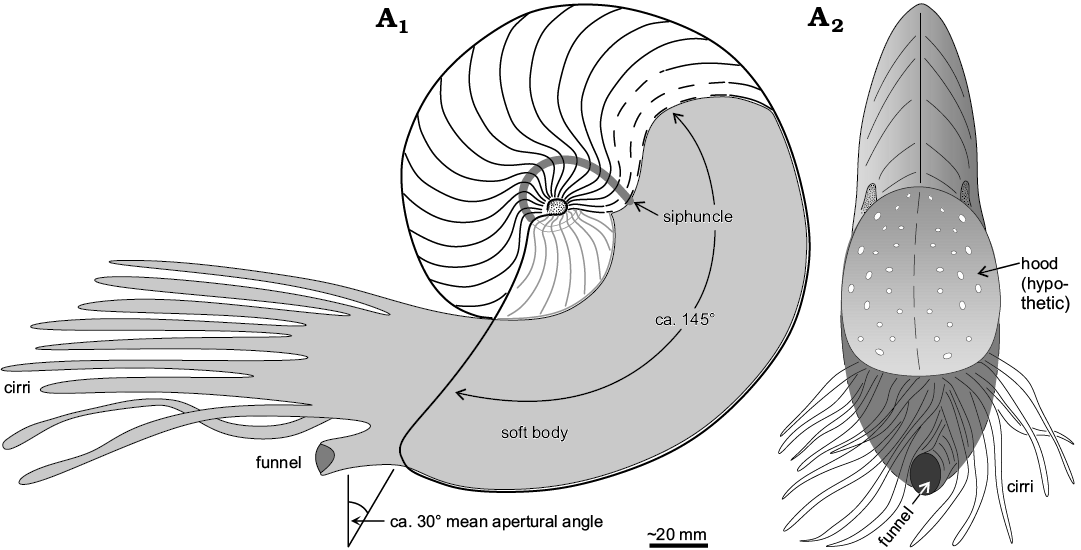
Fig. 9. Reconstruction of the life position of the hercoglossid nautilid Angulithes mermeti (Coquand, 1862) in sagittal cross-section (A1) and apertural view (A2) (approximately 1/2 of natural size).
Based on the carbonate facies of the host strata and palaeogeographical constraints, the habitat of A. mermeti was a relatively shallow, wide epicontinental sea and the species sometimes even invaded open-lagoonal environments where it was recorded from dasycladalean algae-bearing wacke- and floatstone with rudists and corals (Fig. 10; see Wilmsen and Nagm 2012 for facies analysis of the Galala Formation). Wiese and Schulze (2005) also reconstructed a preferential distribution of the co-occurring ammonite N. vibrayeanus in shallow-marine shelf settings in predominantly less than 30 m water depth (see also Batt 1989, for inferred palaeobathymetry of ammonites of morphotype group 11). Based on random size distribution of the ammonites and taphonomical observations, Wiese and Schulze (2005: 944) also concluded that the depositional setting corresponds to the original habitat and that post-mortem transport played no role. Nautiloids, on the other hand, are conventionally thought to be prone for significant post-mortem drift (e.g., House 1987). However, extensive post-mortem transport will commonly result in the removal of shells from the fossil record under most geological circumstances (Hewitt 1988) and the inferred drift shells of Nautilus pompilius along the eastern African coast, the classical “smoking gun” for this process, have recently been suggested to rather indicate the existence of a modern, or recently extirpated, living population in the western Indian Ocean (Yacobucci 2018). Furthermore, only those shells at all with relatively high inflation are likely to float for a significant time after death of the animal whereas limited post-mortem buoyancy of compressed shells argues against substantial dispersal by (surface) currents (Yacobucci 2018). We also do not see any evidence of heavy fouling by encrusting epibenthos suggesting prolonged periods of floating (cf. Reyment 2008) and thus conclude that the strata in which A. mermeti has been found corresponding to the original habitat of the species. Habitat depth was thus indeed very shallow, in the case of dasycladalean algae-bearing strata the upper, light-saturated parts of the euphotic zone where the species lived between rudist/coral thickets and above green algae meadows in open lagoons of at a maximum 5–20 m water depth (Fig. 10A–C) (e.g., Liebau 1984). The fact the larger study area was part of a structured, predominantly shallow shelf system (e.g., Bauer et al. 2002; Schulze et al. 2005; Wilmsen and Nagm 2012) argues against significant daily vertical movements of A. mermeti (as documented for the Recent Nautilus; e.g., Saunders and Ward 1987, see Ward et al. 2016 for a current synopsis) because such regular ascents and descents at the magnitude of the Nautilus (between 130–700 m; Dunstan et al. 2011) would have always been associated with extensive horizontal migrations. We thus conclude for a preferred habitat depth of A. mermeti between 5–50 m, corresponding to the depth range of the Galala Formation (Wilmsen and Nagm 2012).
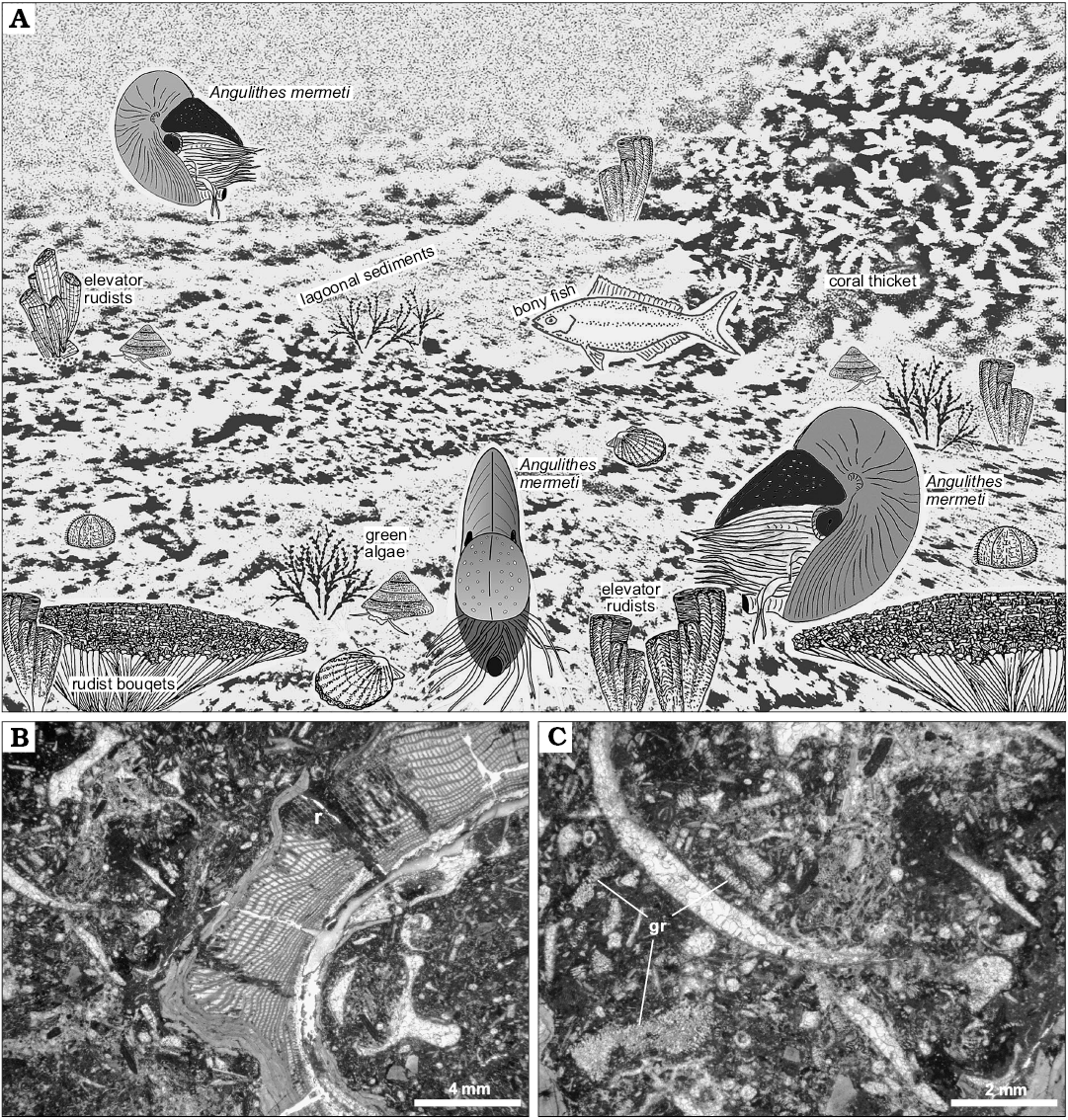
Fig. 10. Palaeoecology of the hercoglossid nautiloid Angulithes mermeti (Coquand, 1862). A. Reconstruction of A. mermeti (Coquand, 1862) in the lagoonal shallow-water environment of the Galala Formation (background after a subaqueous photograph in the property of MW from a lagoonal site in the present-day Red Sea near Hughhada taken in 1999, treated by greyscale-filtering in Photoshop CS2); rudist illustrations from Mitchell (2002). B. Bioclastic rudist (r) floatstone, the lagoonal host sediment in which A. mermeti has been found in the Wadi Ghonima section (thin-section photomicrograph of sample 080217-18). C. Close-up of Fig. 9B showing the bioclastic packstone matrix in detail, including numerous fragments of dasycladalean algae (gr).
Stratigraphy.—Where well-dated, A. mermeti has been recorded exclusively from lower upper Cenomanian strata (e.g., Luger and Gröschke 1989; Nagm 2009, 2019; Ayoub-Hannaa and Fürsich 2012; Kennedy and Gale 2015; Meister et al. 2017), often associated with the ammonite Neolobites vibrayeanus (d’Orbigny, 1841) that is very widespread in the western Tethyan Realm from northern Africa and Iberia into the Middle East (e.g., Lefranc 1981; Luger and Gröschke 1989; Kennedy and Simmons 1991; Wiese and Schulze 2005; “assemblage faunique à N. vibrayeanus [A]” of Meister and Abdallah 2005). N. vibrayeanus characterizes the eponymous lower upper Cenomanian ammonite biozone that is broadly contemporaneous to the Calycoceras (C.) naviculare/C. (Proeucalycoceras) guerangeri Biozone of the NW European standard biostratigraphy (Charrière et al. 1998; Meister and Rhalmi 2002; Nagm et al. 2010a, b; Nagm and Wilmsen 2012; Meister et al. 2017; Wright and Kennedy 2017). In terms of absolute duration, the early late Cenomanian corresponds to the interval between 95.5–94.5. Ma (Ogg and Hinnov 2012). Thus, the range of A. mermeti is rather short, corresponding to about 1 myr of geological time. The common co-occurrence of A. mermeti and N. vibrayeanus was early recognized (Basse and Choubert 1959; Luger and Gröschke 1989) and the correlatable character of the assemblage has led some authors to define a bioevent, i.e., the Neolobites bioevent of Cavin et al. (2010), the cephalopod assemblage 2 of Barroso-Barcenilla et al. (2011), the Neolobites vibrayeanus bioevent (A1) of Meister and Piuz (2013), the N. vibrayeanus–A. mermeti assemblage of Segura et al. (2014) and the Neolobites bioevent of Nagm (2019). Based on these records it becomes apparent that this bioevent is related to maximum flooding conditions of Cenomanian depositional sequence DS Ce 5 (see Fig. 1B, C; Wilmsen and Nagm 2013; UZA-2.4 in Segura et al. 2014), forming a maximum flooding bioevent sensu Ernst et al. (1996) and Wilmsen (2012). Such events commonly have an overlapping character and the Neolobites bioevent can be correlated from Iberia via northern Africa into the Middle East (Meister et al. 2017; Nagm 2019). For a detailed stratigraphical, palaeontological and sedimentological description of the event, see Nagm (2019).
Evolutionary trends during the Cenomanian.—Starting with the first appearance of the genus Angulithes de Montfort, 1808 in the Albian (A. arcuatus [Deshayes in Leymerie, 1842]), there are a number of directed morphological trends reflected by the Cenomanian species of the genus that appear to document gradual evolutionary changes during the course of the stage (c. 100–94 Ma). These have in part already been noted by Wilmsen (2000) and pertain to A. fleuriausianus (d’Orbigny, 1840), A. triangularis Montfort, 1808, A. mermeti (Coquand, 1862) and Angulithes vascogoticus Wiedmann, 1960, broadly tracing a chronological sequence characterized by (Fig. 11): (i) an increasing compression of the shell associated with a sharpening of the venter; (ii) an increasing sinuosity of the suture lines; (iii) a reduction of inter-septal distances; (iv) a dorsally directed migration of the siphuncle.
The Cenomanian age witnessed one of the greatest Phanerozoic transgressions (e.g., Hallam 1992) and it is thus very tempting to relate the observed changes in the successive species of Angulithes to the concomitant sea-level rise and the expansion of shallow epicontinental seas in the early Late Cretaceous. A. fleuriausianus is still very similar to its basic Albian predecessor, A. arcuatus, by means of a relatively inflated whorl shape with only slight ventral angling, a low septal sinuosity combined with wide septal spacing and central position of the siphuncle (note that siphuncle position is unknown in A. arcuatus). This general similarity to A. fleuriausianus (d’Orbigny, 1840) has already been noted in the original description of Nautilus arcuatus by Deshayes in Leymerie (1842: 15) and more material is needed to evaluate if the two taxa are in fact conspecific (d’Orbigny’s [1840] species would have priority). Nevertheless, it is quite clear that these two taxa form the basic stock of the morphological development in Angulithes during the Cenomanian. In the late early to middle Cenomanian, the more compressed and angular-ventered A. triangularis appeared, also featuring a slightly more dorsally oriented siphuncle and a more sinuous suture. These trends continued into the early late Cenomanian, when A. mermeti represents a culmination of these morphological developments. In the late late Cenomanian, A. vascogoticus sustained the septal differentiation by means of a strongly sinuous suture transitional to Hercoglossa (Wiedmann 1960) and an almost dorsal siphuncle, but the shell of this species is less compressed compared to A. mermeti and the distance of the septa is larger (Fig. 11, Table 3; Wilmsen 2000).
Table 3. Septal distances (in mm, measured at venter) of different Cenomanian species of Angulithes.
|
Species |
Diameter (D) |
Distance of septa |
|
A. fleuriausianus (d’Orbigny, 1840), early to middle Cenomanian |
D = 120 (SpK 206) |
c. 20 |
|
A. triangularis de Montfort, 1808, late early to middle Cenomanian |
D = 130 (SpK 211) |
c. 17 |
|
A. mermeti (Coquand, 1862), early late Cenomanian |
D = 120 (AFK 202) |
10–12 |
|
A. vascogoticus Wiedmann, latest Cenomanian |
D = 125–138 (SpK 214, 216, 223) |
c. 18–20 |
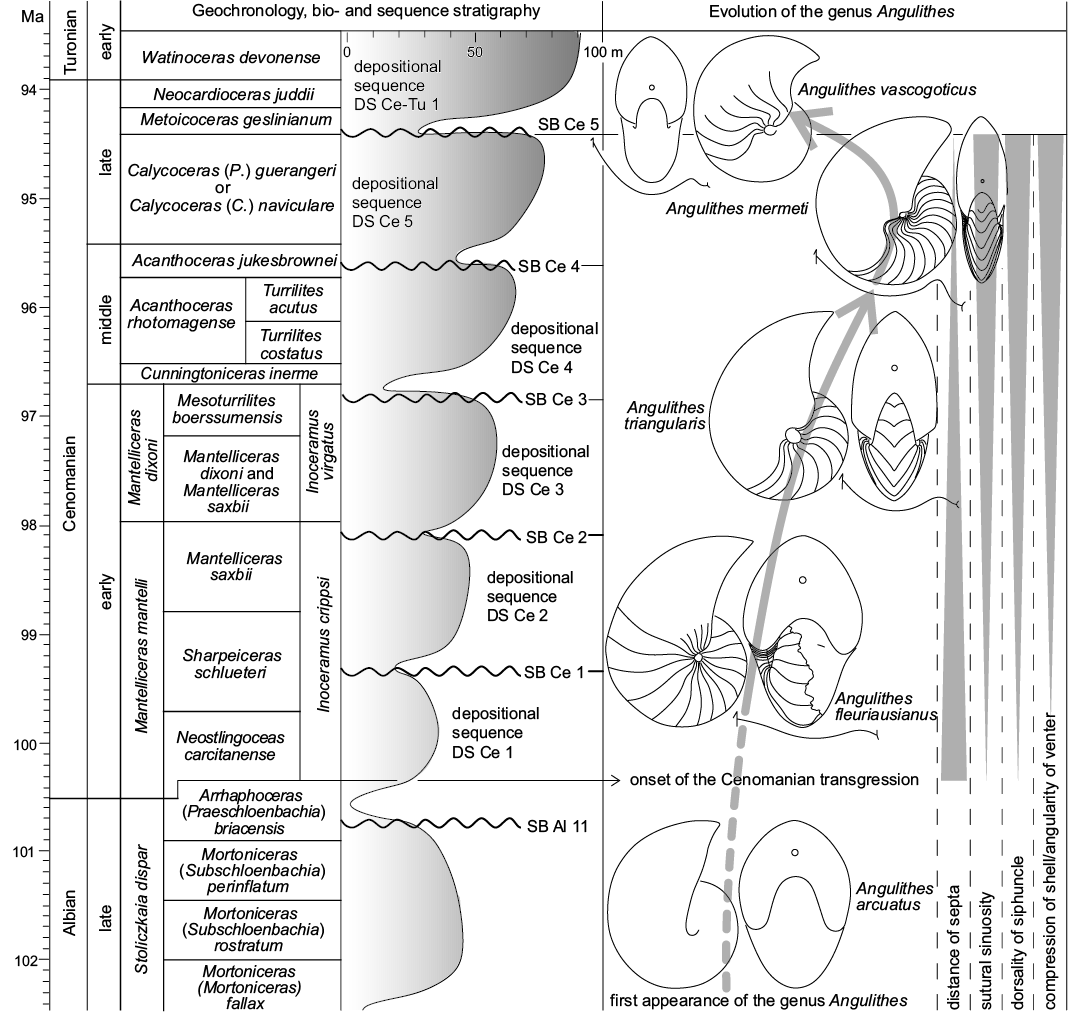
Fig. 11. Geochronology (Ogg and Hinnov 2012), ammonite biostratigraphy (Wright and Kennedy 2017) and sequence stratigraphy (Robaszynski et al. 1998; Wilmsen 2003) of the Cenomanian Stage plotted against evolutionary trends in the hercoglossid nautiloid genus Angulithes. See text for further explanations.
The increasingly more compressed forms, culminating in the nearly oxycone shell of A. mermeti, certainly reflect improved swimming capabilities and steerage (see discussion on autecology of A. mermeti above). Such adaptations were necessary to successfully occupy new niches in the wide, shallow epicontinental seas that formed during the early Late Cretaceous and are mirrored by the morphology of many contemporaneous ammonoids found in such settings (e.g., Batt 1989). The increasing sutural sinuosity and the denser spacing of the septa are probably buttressed the shells but a simple relation to increasing water depths falls short of a meaningful explanation given the inferred shallow habitat depth of A. mermeti (see above). The reason for the dorsal-directed migration of siphuncle is likewise not straightforward. Both aspects are detailed below.
An important function of the septa in ectocochleate cephalopods, apart from forming the hydrostatic apparatus, i.e., the phragmocone, is the buttressing of shell against water pressure that may ultimately result in shell implosion (e.g., Westermann 1973). For Cretaceous ammonites, Batt (1991) developed a “Sutural Amplitude Index” (SAI) based on maximum height of sutural elements and the length of the suture that offers a useful proxy for the relative living depth of ammonoids with comparable shell morphologies, with inferred deeper water dwellers being characterized by higher SAI indices. This observation suggests that a greater septal support for the shell wall under increased hydrostatic pressures is effective against implosion. Even if this relationship was questioned by some authors (e.g., Olóriz and Palmqvist 1995; Daniel et al. 1997; Olóriz et al. 2002), Hassan et al. (2002) demonstrated by means of finite-element analysis of simulated ammonoid septa that the marginal differentiation in fact improved the dispersion of stress in the shell walls. This line or argument was also applied to nautiloids and Westermann and Ward (1980) concluded that septal curvature and thickness in orthocone nautiloids are useful proxies to bathymetry. Following this suggestion, Tintant and Kabamba (1985: 60) also proposed that the shape of the suture line in planispiral nautiloids is an adaptive response to variations in water depth, i.e., a plicate septum improved mechanical resistance to implosion. Studies on Recent nautilids showed that their (in comparison to most ammonites relatively thick) shells implode at a water depth of around 800 m, that the maximum depth range comprises about 100–700 m and that the preferred depth range is between 100–300 m (Hewitt and Westermann 1987; Saunders and Ward 1987; Dunstan et al. 2011; Ward et al. 2016). The suture line of Nautilus pompilius is relatively strongly differentiated (see Wani et al. 2008), the external part of which showing similar amplitudes as in A. mermeti, but the distance of the septa is relatively high compared to the Cenomanian species (Fig. 12A, B). According to the autecological discussion above, the habitat depth of A. mermeti was much shallower than the one of N. pompilius, i.e., a relatively shallow, wide epicontinental shelf sea, and considerable vertical migrations were largely excluded. Thus, habitat depth can be omitted as trigger for increasing sutural differentiation and denser septal spacing in Angulithes. A similar conclusion has been reached for the sutural differentiation in Jurassic ammonites (e.g., Olóriz et al. 2002; Pérez-Claros 2005).

Fig. 12. Comparison of whorl sections, position of siphuncles, sutural sinuosity, and septal spacing of Angulithes mermeti (Coquand, 1862) (A) and a specimen illustrated as Nautilus pompilius Linnaeus, 1758 in the Treatise (Kummel 1964: fig. 329) (B). A. AFK 225 from the upper Cenomanian of Egypt in lateral (A1) and apertural (A2) views; external suture (A3). B. Specimen from Tagnan (Philippines) at D = 130 mm from the SW Pacific (drawn from an artificial internal mould) (B1, B2); suture line (B3) (modified from Wani et al. 2008).
An interesting explanation for the generally simple septum design in many nautiloids put forward by Westermann (1971) is that wall implosion of the rounded ovate whorls of nautiloids is prevented largely by the vaulted shell-surface design of the walls themselves, the septum functioning mainly against pressure transmitted through the soft body. A deviation from this inflated design to more compressed forms (for the sake of better swimming capabilities and steerage) weakens the shell and may necessitate a stronger vaulting and/or denser spacing of the septa even if the habitat depth is relatively shallow. A thickening of the outer shell and septa may fulfill the same function but increases weight (as does denser septal spacing to some extent) and thus counteracts the need for better swimming skills; in any case, (nearly) neutral buoyancy acts as a constraint on the total weight of the shell.
Another factor influencing the need for increased rigidity of nautiloid shells in shallow waters is the greater stress by shell-breaking predators in such settings compared to deeper waters (Vermeij 1977; Ward 1996; Kelley and Hansen 2001); the complex vertical migration patterns of modern Nautilus are also dictated by avoidance of daytime visual predators (e.g., Dunstan et al. 2011). The number of durophagous predators during the Mesozoic Marine Revolution increased significantly from the Early into the Late Cretaceous (Vermeij 1977) and important Cretaceous durophages known to attack ectocochleate cephalopods were mosasaurid lizards and (ptychodontoid) sharks (e.g., Tsujita and Westerman 2001; Kauffman 2004). Considering this, Wilmsen and Yazykova (2003) suggested that the preferential shallow-water distribution of ribbed cymatoceratid nautiloids reflects increased predation pressure, their coarse ornament apparently being defensive in nature (other sculptural defensive elements such as horns and spines that commonly occur in contemporaneous ammonites were obviously missing from the genetic toolkit of Cretaceous nautiloids). Ribbing is unknown in Angulithes (only some ventral folds are known from the adult body chambers of A. vascogoticus; Wilmsen 2000) and, thus, septal folding and approximation may be the generic evolutionary response in this lineage in order to increase shell resistance against predation. Furthermore, in case of a shell injury, the reduced individual cameral volume due to the approximated septa and the compressed shell may have facilitated survival because only a limited segment of the phragmocone would have been affected. Interestingly, Lemanis et al. (2016) proposed that sutural complexity does not really aid in greater resistance to hydrostatic pressure but it seems to enhance resistance of the shell to point loads such as those exerted by pointed-toothed shell-breaking predators (e.g., mosasaurs, ptychodontoid sharks). We thus speculate that the septal folding and approximation in Angulithes was predominantly an “act of defense” against predation with the hydrodynamically efficient shell form providing additional means for possible successful escape (according to Vermeij 1977, locomotion may be more important than armor as an antipredatory measure). We suggest that these adaptations were clues for the successful occupation of the dynamic and perilous shallow-water habitats by A. mermeti during the early late Cenomanian that previously were not natural nautiloid territory.
The functional interpretation for the dorsal-directed migration of the siphuncle is difficult to elucidate. Without going into any detail, Tintant and Kabamba (1985) proposed that the dorsal position of the siphuncle represents an adaptation to a relative deep-water mode of life, facilitating the pumping of cameral liquids necessary for vertical movements. However, as shown above, A. mermeti lived in shallow waters, most likely did not migrate vertically and nevertheless developed a fairly dorsal siphuncle; thus, this explanation cannot be applied here. Densely spaced septa and compression of the shell reduce the volume of the chambers, facilitating their emptying/filling by the siphuncle. The dorsal position of the siphuncle, on the other hand, minimizes the arc length relative to a median position and thus reduces siphuncular surface area. However, Chamberlain (1978) and Chamberlain and Moore (1982) have shown that the permeability of the siphuncle of the living Nautilus is three times higher than the actual rates of osmotic flow. Thus, the transport capacity of the siphonal tube is no limiting factor for short-term buoyancy regulations (Kröger 2002) and its dorsal position may therefore fulfill another function in the hydrostatic apparatus of A. mermeti (and A. vascogoticus). The pellicle, an organic membrane with hydrophilic properties that lines the septal faces and inner shell walls plays an important role in the fluid balance within the chambers and buoyancy changes. In partially emptied chambers, it transports the liquid to the siphuncle by capillary forces, acting as blotting-paper (Ward 1987). The pellicle surface area in Nautilus chambers is significantly smaller than in ammonoids (Kröger 2002), suggesting that the direct contact of the cameral liquid with the siphuncle may be more important for (short-term) buoyancy regulations in nautiloids. This is where the dorsal position of the siphuncle has advantages: in living position, it prevents early decoupling of the cameral liquid and the siphuncle in the last-formed chamber and allows a rapid and nearly complete emptying (Fig. 9); due to the median position of the siphuncle in Recent Nautilus, the decoupling already occurs after approximately half of the cameral liquid has been removed (Ward et al. 1981). This late decoupling may be a benefit in the shallow-water habitat and guarantees the maintenance of buoyancy of the relatively heavy shells buttressed by numerous, densely spaced septa. Interestingly, the dorsal siphuncle in Late Devonian clymeniid ammonoids may have served a similar function: in inferred life-position, the dorsal clymeniid siphuncle is located at or near the bottom of the most recently formed chambers, seemingly an ideal location for an effective complete draining of liquid and maintaining buoyancy of the relatively thick-walled (= heavy) shells (Gottobrio and Saunders 2005). Another explanation may be the fact that the internal position provides a better protection of the siphuncular tube in the case of predation that, in conjunction with the low individual cameral volumes (see above), facilitates survival from phragmocone shell injuries.
Conclusions
Cretaceous nautiloids are commonly characterized by inflated shells with broad whorl cross-sections and round or flattened venters. Furthermore, they tend to be rather long-ranging compared to co-occurring ammonites. However, in the Albian, the genus Angulithes Montfort, 1808 with compressed forms and predominantly narrow venters arose, some representatives of which acquired nearly oxycone shell shapes and were also characterized by fairly short-lived stratigraphical ranges. Herein, the strongly compressed nautiloid species Angulithes mermeti (Coquand, 1862) is treated in detail and evolutionary trends among Cenomanian species of Angulithes are evaluated in the light of contemporaneous palaeoenvironmental changes and inferred functional traits.
Angulithes mermeti is characterized by a compressed, nearly oxycone shell form with a sharp venter and narrow, shallow umbilicus, a fairly sinuous external suture line, a low distance of septa, and a subdorsal position of the siphuncle. The studied nautiloids stem from a carbonate-dominated interval roughly in the middle part of the early late Cenomanian depositional sequence Cenomanian 5 in which they commonly co-occur with the ammonite Neolobites vibrayeanus (d’Orbigny, 1841), forming the “Neolobites Bioevent”. The strata correspond to a maximum flooding interval that can be correlated from Iberia via the southern Tethyan margin along northern Africa into the Middle East; the species has also been reported from South America (Peru). Its latitudinally restricted palaeobiogeographical distribution suggests an affinity to tropical–subtropical warm-water areas. Based on facies and palaeogeography, the environment of A. mermeti was a relatively shallow, wide epicontinental sea with a preferred habitat depth between 5–50 m. The smooth shell, small and shallow umbilicus, compression of the whorl section, and the narrowly rounded to acute venter indicate (relative to most other nautiloids) pronounced acceleration, high speed and steerage (maneuverability combined with directional stability) by reducing drag. A. mermeti may thus have been able to successfully pursuit small nektonic prey in shallow-water areas structured by reefs and shoals. Furthermore, the hydrodynamically efficient shell form was probably beneficial in order to escape shell-breaking predators.
Starting with the appearance of the genus Angulithes in the Albian, several gradual morphological changes are reflected by the Cenomanian species of the genus, i.e., A. fleuriausianus (d’Orbigny, 1840), A. triangularis Montfort, 1808, A. mermeti Coquand, 1862 and A. vascogoticus Wiedmann, 1960, i.e., (i) an increasing compression of the shell and angulation of the venter, (ii) an increasing folding of the suture, (iii) a reduction of inter-septal distances, and (iv) a dorsally directed migration of the siphuncle. The early late Cenomanian species A. mermeti reflects a culmination of these morphological trends. The hydrodynamically efficient form was favourable to successfully occupy new niches in the wide, shallow epicontinental seas that formed during the early Late Cretaceous. The increasing sutural sinuosity and the denser spacing of the septa may be related to the buttressing of the shells. However, in contrast to conventional interpretations, these adaptations are not related to a greater septal support for the shell wall under increased hydrostatic pressures against implosion as evident from the shallow-water habitat of A. mermeti. We speculate that the septal folding and approximation in Angulithes aimed at an improved resistance of the shell to point loads such as exerted by (pointed-toothed) shell-breaking predators (e.g., ptychodontoid sharks) which were common in the conquered shallow warm-water habitats. The functional reason for the dorsal-directed migration of siphuncle is more difficult to explain. In living position it prevents early decoupling of the cameral liquid and the siphuncle in the last-formed chambers and allows a nearly complete emptying directly by the siphuncle which may be beneficial for the maintenance of buoyancy. Furthermore, the internal position provides a better protection of the siphuncular tube in the case of predation and non-lethal shell injuries.
The herein presented directed morphological change in the Cenomanian representatives of the genus Angulithes provides a well-constrained case study of gradual (Darwinian) evolutionary response in a nautiloid lineage triggered by the opening of newly available niches in the course of the expansion of widespread shallow epeiric seas during the great early Late Cretaceous transgression. It also suggests that strong environmental pressure obviously acting during the Cenomanian age was able to induce rapid evolutionary response in otherwise bradytelic biotic groups such as nautiloids.
Acknowledgements
The manuscript benefited from constructive reviews by Marcela Cichowolski (Universidad de Buenos Aires, Argentina), Jiří Frank (National Museum, Prague, Czech Republic) and an anonymous referee. Furthermore, we thank Ronald Winkler and Manuel Röthel (both Senckenberg Naturhistorische Sammlungen Dresden, Germany) for digital photography and collection work, respectively. Daniela Erler (Senckenberg Naturhistorische Sammlungen Dresden, Germany) helped to obtain important literature.
References
Aal, A. and Lelek, J. 1994. Structural development of the northern Sinai, Egypt and its implications on the hydrocarbon prospectivity of the Mesozoic. GeoArabia 1: 15–30.
Abdel-Gawad, G.I., Aboulela, N.M., and Gameil, M. 1992. Mollusca biostratigraphy of the Cenomanian–Turonian strata of Gebel Nezzazat area, West Central Sinai, Egypt. In: Proceedings of the First International Conference on the Geology of the Arab World, 321–332. Cairo University, Giza.
Abdel-Gawad, G.I., El Qot, G.M., and Mekawy, M.S. 2006. Cenomanian–Turonian macrobiostratigraphy of Abu Darag area, Northern Galala, Eastern Desert, Egypt. In: Proceedings of the eighth International Conference on the Geology of the Arab World, 553–568. Cairo University, Giza.
Abdel-Gawad, G.I., El Sheikh, H.A., Abdelhamid, M.A., El Beshtawy, M.K., Abed, M.M., Fürsich, F.T., and El Qot, G. 2004. Stratigraphic studies on some Upper Cretaceous successions in Sinai, Egypt. Egyptian Journal of Paleontology 4: 263–303.
Agassiz, L. 1847. An Introduction to the Study of Natural History, In a Series of Lectures Delivered in the Hall of the College of Physicians and Surgeons. 58 pp. Greeley and McElrath, New York.
Ayoub-Hannaa, W.S. 2011. Taxonomy and Palaeoecology of the Cenomanian–Turonian Macro-invertebrates from Eastern Sinai, Egypt. 410 pp. Ph.D. thesis, University of Würzburg, Würzburg.
Ayoub-Hannaa, W.S. and Fürsich, F.T. 2012. Cenomanian–Turonian ammonites from eastern Sinai, Egypt, and their biostratigraphic significance. Beringeria 42: 57–92.
Bandel, K. and Kuss, J. 1987. Depositional environment of the pre-rift sediments of the Galala Heights (Gulf of Suez, Egypt). Berliner geowissenschaftliche Abhandlungen A78: 1–48.
Barrier, E. and Vrielynck, B. 2008. Map 6: Cenomanian (99.6–93.5 Ma). In: E. Barrier and B. Vrielynck (eds.), Palaeotectonic Maps of the Middle East Tectono-Sedimentary Palinsspastic Maps from the Late Norian to Pliocene. Commission for the Geological Map of the World (CGMW/CCGM), Paris.
Barroso-Barcenilla, F., Audije-Gil, J., Berrocal-Casero, M., Callapez, P., Carenas, B., Comas-Rengifo, M.J., García Joral, F. García-Hidalgo, J.F., Gil-Gil, J., Goy, A., Ozkaya De Juanas, S.A., Rodríguez García, S., Faria Dos Santos, V., Segura, M., and Sevilla, P. 2017. El Cenomaniense–Turoniense de Tamajón (Guadalajara, España): contexto geológico, contenido fósil e interpretación paleoambiental. Boletín de la Real Sociedad Española de Historia Natural, Sección Geológica 111: 67–84.
Barroso-Barcenilla, F., Callapez, P.M., Ferreira Soares, A., and Segura, M. 2011. Cephalopod assemblages and depositional sequences from the upper Cenomanian and lower Turonian of the Iberian Peninsula (Spain and Portugal). Journal of Iberian Geology 37: 9–28.
Basse, E. and Choubert, G. 1959. Les faunes d’ammonites du Cénomanien–Turonien de la partie orientale du domaine atlasique marocain et de ses annexes sahariennes. In: 20eme Congres Géologique International de Mexico, 1956. Symposium Crétace 2: 58–82.
Batt, R.J. 1989. Ammonite shell morphotype distribution in the Western Interior Greenhorn Sea (Cretaceous) and some paleoecological implications. Palaios 4: 32–42. Crossref
Batt, R.J. 1991. Sutural amplitude of ammonite shells as a paleoenvironmental indicator. Lethaia 24: 219–225. Crossref
Bauer, J., Kuss, J., and Steuber, T. 2002. Platform environments, microfacies and systems tracts of the Upper Cenomanian–Lower Santonian of Sinai, Egypt. Facies 47: 1–25. Crossref
Benavides-Cáceres, V.E. 1956. Cretaceous system in northern Peru. Bulletin of the American Museum of Natural History 108: 1–493.
Benyoucef, M., Meister, C., Bensalah, M., and Malti, F. Z. 2012. La plateforme préafricaine (Cénomanien supérieur–Turonien inférieur) dans la région de Béchar (Algérie): stratigraphie, paléoenvironnements et signification paléobiogéographique. Revue de Paléobiologie 31: 205–218.
Blainville, H.M.D. de 1825–1827. Manuel de malacologie et de conchyliologie. 664 pp. (1825), 87 pls. (1827). Levrault, Paris.
Blanckenhorn, M. 1900. Neues zur Geologie und Paläontologie Aegyptens. Zeitschrift der Deutschen Geologischen Gesellschaft 52: 21–47.
Cavin, L., Tong, H., Boudad, L., Meister, C., Piuz, A., Tabouelle, J., Aarab, M., Amiot, R., Buffetaut, E., Dyke, G., Hua, S., and Le Loeuff, J. 2010. Vertebrate assemblages from the early Late Cretaceous of southeastern Morocco. Journal of African Earth Sciences 57: 391–412. Crossref
Chamberlain, J.A. 1978. Permeability of the siphuncular tube of Nautilus: its geologic and paleoecologic implications. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1978 (3): 29–142.
Chamberlain, J.A. 1987. Locomotion of Nautilus. In: W.B. Saunders and N.H. Landman (eds.), Topics in Geobiology 6: 489–525. Plenum Press, New York. Crossref
Chamberlain, J.A. 1992. Cephalopod locomotor design and evolution: the constraints of jet propulsion. In: J.M.V Rayner and R.J. Wootton (eds.), Biomechanics in evolution. Society for Experimental Biology, Seminar Series 36, 57–98. Cambridge University Press, Cambridge.
Chamberlain, J.A. 1993. Locomotion in ancient seas: constraint and opportunity in cephalopod adaptive design. Geobios, Mémoir Spécial 15: 49–61. Crossref
Chamberlain, J.A. and Moore, W.A. 1982. Rupture strength and flow rate of Nautilus siphuncular tube. Paleontology 8: 408–425. Crossref
Charrière A., Andreu, B., Ciszak, R., Kennedy, W.J., Rossi, A., and Vila, J.-M. 1998. La transgression du Cénomanien supérieur clans la Haute Moulouya et le Moyen Atlas meridional, Maroc. Geobios 31: 551–569. Crossref
Choffat, P.L. 1886. Recueil d´études paléontologiques sur la Faune Crétacique du Portugal, I. 40 pp. Section des Travaux Géologiques du Portugal, Lisbonne. Crossref
Conrad, T.A. 1866. Observations on recent and fossil shells, with proposed new genera and species. American Journal of Conchology 2: 101–103.
Coquand, H. 1862. Géologie et Paléontologie de la région Sud de la province de Constatine. Mémoires de la Société d’Émulation de la Provence 2: 1–342.
Daniel, T.L., Helmuth, B.S., Saunders, W.B., and Ward, P.D. 1997. Septal complexity in ammonoid cephalopods increased mechanical risk and limited depth. Paleobiology 23: 470–481. Crossref
Debris, J.-P. 2006. Détermination pratique des nautiles Kimméridgiens et Crétacés de la région du Havre (Haute Normandie). Bulletin de la Société Géologique de Normandie et des Amis du Muséum du Havre 92: 5–18.
Dunstan, A.J., Ward, P.D., and Marshall, N.J. 2011. Vertical distribution and migration patterns of Nautilus pompilius. PLoS ONE 6 (2): e16311. Crossref
Dzik, J. 1984. Phylogeny of the Nautiloidea. Palaeontologia Polonica 45: 1–220.
Eck, O. 1910. Die Cephalopoden der Schweinfurth‘schen Sammlung aus der oberen Kreide Ägyptens. 43 pp. Inaugural dissertation, Philosophical Faculty, Friedrich-Wilhelms-University, Berlin.
Eck, O. 1914. Die Cephalopoden der Schweinfurth‘schen Sammlung aus der oberen Kreide Ägyptens. Zeitschrift der Deutschen Geologischen Gesellschaft 66: 179–216.
El Qot, G. 2006. Late Cretaceous macrofossils from Sinai, Egypt. Beringeria 36: 3–163.
Ernst, G., Niebuhr, B., Wiese, F., and Wilmsen, M. 1996. Facies development, basin dynamics, event correlation and sedimentary cycles in the Upper Cretaceous of selected areas of Germany and Spain. In: J. Reitner, F. Neuweiler, and F. Gunkel (eds.), Global and regional controls on biogenic sedimentation. II. Cretaceous Sedimentation. Research Reports. Göttinger Arbeiten zur Geologie und Paläontologie, Sonderband 3: 87–100.
Gottobrio, W.E. and Saunders, W.B. 2005. The clymeniid dilemma: functional implications of the dorsal siphuncle in clymeniid ammonoids. Paleobiology 31: 233–252. Crossref
Hallam, A. 1992. Phanerozoic Sea-level Changes. 266 pp. Columbia University Press, New York.
Hassan, M.A., Westermann, G.E.G., Hewitt, R.A., and Dokainish, M.A. 2002. Finite-element analysis of simulated ammonoid septa (extinct Cephalopoda): septal and sutural complexities do not reduce strength. Paleobiology 28: 113–126. Crossref
Hewitt, R.A. 1988. Nautiloid shell taphonomy: interpretations based on water pressure. Palaeogeography, Palaeoclimatology, Palaeoecology 63: 15–25. Crossref
Hewitt, R.A. and Westermann, G.E.G. 1987. Nautilus shell architecture. In: W.B. Saunders and N.H. Landman (eds.), Topics in Geobiology 6: 435–461. Plenum Press, New York. Crossref
House, M.R. 1987. Geographic distribution of Nautilus shells. In: W.B. Saunders and N.H. Landman (eds.), Topics in Geobiology 6: 53–64. Plenum Press, New York. Crossref
Hyatt, A. 1884. Genera of fossil cephalopods. Proceedings of the Boston Society of Natural History 22: 273–338.
Hyatt, A. 1894. Phylogeny of an acquired characteristic. Proceedings of the American Philosophical Society 32 (for 1893): 349–647. Crossref
Jacobs, D.K. and Chamberlain, J.A. 1996. Buoyancy and hydrodynamics in ammonoids. In: N.H. Landmann, K. Tanabe, and R.A. Davies (eds.), Ammonoid Paleobiology. Topics in Geobiology 13: 169–224. Crossref
Kauffman, E.G. 2004. Mosasaur predation on Upper Cretaceous nautiloids and ammonites from the United States Pacific Coast. Palaios 19: 96–100. Crossref
Kelley, P.H. and Hansen, T.A. 2001. Mesozoic marine revolution. In: D.E.G. Briggs and P.R. Crowther (eds.), Palaeobiology II, 94–97. Blackwell Science, Oxford. Crossref
Kennedy, W.J. and Gale, A.S. 2015. Upper Albian and Cenomanian ammonites from Djebel Mrhila, Central Tunisia. Revue de Paléobiologie 34: 235–361.
Kennedy, W.J. and Simmons, M. 1991. Mid-Cretaceous ammonites and associated microfossils from the Central Oman Mountains. Newsletters on Stratigraphy 25: 127–154. Crossref
Kröger, B. 2002. On the efficiency of the buoyancy apparatus in ammonoids: Evidences from sublethal shell injuries. Lethaia 35: 61–70. Crossref
Kummel, B. 1956. Post-Triassic Nautiloid genera. Bulletin of the Museum of Comparative Zoology at Harvard College in Cambridge 114: 319–494.
Kummel, B. 1964. Nautiloidea–Nautilida. In: Moore, R.C. (ed.), Treatise on Invertebrate Paleontology, Part K, Mollusca 3, K383–K457. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Kuss, J., Scheibner, C., and Gietl, R. 2000. Carbonate platform to basin transition along an upper Cretaceous to lower tertiary Syrian Arc uplift, Galala Plateaus, Eastern Desert, Egypt. GeoArabia 5: 405–424.
Lefranc, J.P. 1981. Études de Neolobites vibrayeanus, ammonite Cénomanienne du Sahara Algérien. 106. Congrès national des Sociétés Savantes, Perpignan, sciences fascicule 1: 155–199.
Lemanis, R., Zachow, S., and Hoffmann, R. 2016. Comparative cephalopod shell strength and the role of septum morphology on stress distribution. PeerJ 4: e2434.Crossref
Leymerie, A. 1842. Suite du mémoire sur le terrain Crétacé du département de l’Aube. Seconde partie (partie paléontologique). Mémoires de la Société géologique de France 5: 1–34.
Liebau, A. 1984. Grundlagen der Ökobathymetrie. Paläontologische Kursbücher 2: 149–184.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Volume 1. 824 pp. Laurentii Salvii, Holmiae. Crossref
Luger, P. and Gröschke, M. 1989. Late Cretaceous ammonites from the Wadi Qena area in the Egyptian Eastern Desert. Palaeontology 32: 355–407.
Lukeneder, A. 2015. Ammonoid habitats and life history. In: C. Klug, D., Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology. Topics in Geobiology 43: 689–791. Crossref
Malchyk, O., Machalski, M., Waksmundzki, B., and Duda, M. 2017. Shell ornament, systematic position and hatching size of Epicymatoceras vaelsense (Nautilida): New insights based on specimens in mould preservation from the Upper Cretaceous of Poland. Cretaceous Research 80: 1–12. Crossref
Meister, C. and Abdallah, H. 2005. Précision sur les successions d’ammonites du Cénomanian–Turonien dans la région de Gafsa, Tunisie du centre-sud. Revue de Paléobiologie 24: 111–199.
Meister, C. and Piuz, A. 2013. Late Cenomanian–Early Turonian ammonites of the southern Tethys margin from Morocco to Oman: biostratigraphy, paleobiogeography and morphology. Cretaceous Research 44: 83–103. Crossref
Meister, C. and Piuz, A. 2015. Cretaceous ammonites from the Sultanate of Oman (Adam Foothills). GeoArabia 20: 17–74.
Meister, C. and Rhalmi, M. 2002. Quelques ammonites du Cénomanien–Turonien de la région d’Errachidia-Boudnid-Erfoud (partie méridionale du Haut Atlas Central, Maroc). Revue de Paléobiologie 21: 759–779.
Meister, C., Piuz, A., Cavin, L., Boudab, L., Bacchia, F., Ettachfina, E.M., and Benyoucef, M. 2017. Late Cretaceous (Cenomanian–Turonian) ammonites from southern Morocco and south western Algeria. Arabian Journal of Geosciences 10: 1–46. Crossref
Mitchell, S.F. 2002. Palaeoecology of corals and rudists in mixed volcaniclastic–carbonate small-scale rhythms (Upper Cretaceous, Jamaica). Palaeogeography, Palaeoclimatology, Palaeoecology 186: 237–259. Crossref
Montfort, D. de 1808. Conchyliologie systématique et classification méthodique des coquilles; offrant leurs figures, leur arrangement générique, leurs descriptions caractéristiques, leur noms. Ainsi que leur synonymie en plusieurs langues, Volume 1. 409 pp. F. Schoell, Paris.
Nagm, E. 2009. Integrated stratigraphy, palaeontology and facies analysis of the Cenomanian–Turonian (Upper Cretaceous) Galala and Maghra el Hadida formations of the western Wadi Araba, Eastern Desert, Egypt. 213 pp. Ph.D. thesis, University of Würzburg, Würzburg.
Nagm, E. 2015. Stratigraphic significance of rapid faunal change across the Cenomanian–Turonian boundary in the Eastern Desert, Egypt. Cretaceous Research 52: 9–24. Crossref
Nagm, E. 2019. The late Cenomanian maximum flooding Neolobites bioevent: A case study from the Cretaceous of northeast Egypt. Marine and Petroleum Geology 102: 740–750. Crossref
Nagm, E. and Wilmsen, M. 2012. Upper Cenomanian–Turonian (Cretaceous) ammonoids from Wadi Qena, central Eastern Desert, Egypt: taxonomy, biostratigraphy and palaeobiogeographic implications. Acta Geologica Polonica 62: 63–89. Crossref
Nagm, E., Wilmsen, M., Aly, M., and Hewaidy, A. 2010a. Biostratigraphy of the Upper Cenomanian–Turonian (lower Upper Cretaceous) successions of the western Wadi Araba, Eastern Desert, Egypt. Newsletters on Stratigraphy 44: 17–35. Crossref
Nagm, E., Wilmsen, M., Aly, M., and Hewaidy, A. 2010b. Cenomanian–Turonian (Cretaceous) ammonoids from the western Wadi Araba area, Eastern Desert, Egypt. Cretaceous Research 31: 473–499. Crossref
Ogg, J.G. and Hinnov, L.A. 2012. Cretaceous. In: F.M. Gradstein, J.G. Ogg, M. Schmitz, and G.M. Ogg (eds.), The Geologic Time Scale 2012, Vol. 2, 793–853. Elsevier, Amsterdam. Crossref
Olóriz, F. and Palmqvist, P. 1995. Sutural complexity and bathymetry in ammonites: fact or artifact? Lethaia 28: 167–170. Crossref
Olóriz, F., Palmqvist, P., and Pérez-Claros, J. 2002. Morphostructural constraints and phylogenetic overprint on sutural frilling in Late Jurassic ammonites. Lethaia 35: 18–168. Crossref
d’Orbigny, A. 1840–1842. Paléontologie Française. Terrain Crétacé I, Céphalopodes. 662 pp. (1840: 1–120; 1841: 121–430; 1842: 431–662). Masson, Paris.
Pérez-Claros, J. 2005. Allometric and fractal exponents indicate a connection between metabolism and complex septa in ammonites. Paleobiology 31: 221–232. Crossref
Pervinquière, L. 1907. Études de paléontologie tunisienne. 1. Céphalopodes des terrains secondaires. 438 pp. Carte Géologique Tunisie, Paris.
Peterman, D.J., Barton, C.C., and Yacobucci, M.M. 2019. The hydrostatics of Paleozoic ectocochleate cephalopods (Nautiloidea and Endoceratoidea) with implications for modes of life and early colonization of the pelagic zone. Palaeontologia Electronica 22.2.24A: 1–29. Crossref
Philip, J. and Floquet, M. 2000. Late Cenomanian (94.7–93.5). In: J. Dercourt, M. Gaetani, B. Vrielynck, E. Barrier, B. Biju-Duval, M.F. Brunet, J.P. Cadet, S. Crasquin, and M. Sandulescu (eds.), Atlas Peri-Tethys Palaeogeographical Maps, 129–136. CCGM/CGMW, Paris.
Reyment, R.A. 2008. A review of the post-mortem dispersal of cephalopod shells. Palaeontologia Electronica 11: 11.3.12A.
Ritterbush, K.A. 2016. Interpreting drag consequences of ammonoid shells by comparing studies in Westermann Morphospace. Swiss Journal of Palaeontology 135: 125–138. Crossref
Ritterbush, K.A. and Bottjer, D.J. 2012. Westermann Morphospace displays ammonoid shell shape and hypothetical paleoecology. Paleobiology 38: 424–446. Crossref
Ritterbush, K.A., Hoffmann, R., Lukeneder, A., and DeBaets, K. 2014. Pelagic palaeoecology: the importance of recent constraints on ammonoid palaeobiology and life history. Journal of Zoology 292: 229–241. Crossref
Robaszynski, F., Juignet, P., Gale, A.S., Amédro, F., and Hardenbol, J. 1998. Sequence stratigraphy in the Cretaceous of the Anglo-Paris Basin, exemplified by the Cenomanian stage. In: T. Jaquin, P. De Graciansky, and J. Hardenbol (eds.), Mesozoic and Cenozoic Sequence Stratigraphy of European Basins. Society of Economic Paleontologists and Mineralogists, Special Publication 60: 363–385. Crossref
Rosenthal, E., Weinberger, G., Almogi-Labin, A., and Flexer, A. 2000. Late Cretaceous–early Palaeogene development of depositional basins in Samaria as a reflection of eastern Mediterranean tectonic evolution. Bulletin of the American Association of Petroleum Geologists 84: 997–1114. Crossref
Said, R. 1962. The Geology of Egypt. 377 pp. Elsevier, Amsterdam.
Said, R. 1990. The Geology of Egypt. 721 pp. Balkema, Rotterdam.
Saunders, W.B. and Landman, N.H. 1987 (eds.). Nautilus: the Biology and Paleobiology of a Living Fossil. Topics in Geobiology 6. 623 pp. Plenum Press, New York. Crossref
Saunders, W.B. and Ward, P.D. 1987. Ecology, distribution, and population characteristics of Nautilus. In: W.B. Saunders and N.H. Landman (eds.), Topics in Geobiology 6: 137–162. Crossref
Schlagintweit, O. 1912. Die Fauna des Vracon und Cenoman in Perú. Neues Jahrbuch für Geologie und Paläontologie, Beilagen-Band 33: 43–135.
Schlüter, C. 1872. Die Spongitarienbänke der oberen Quadraten- und unteren Mucronaten-Schichten des Münsterlandes 20. Hauptversammlung der Deutschen Geologischen Gesellschaft, September 1872: 1–38.
Schulze, F., Kuss, J., and Marzouk, A. 2005. Platform configuration, microfacies and cyclicities of the upper Albian to Turonian of west-central Jordan. Facies 50: 505–527. Crossref
Segura, M., Barroso-Barcenilla, F., Callapez, P., García-Hidalgo, J.F., and Gil-Gil, J. 2014. Depositional sequences and ammonoid assemblages in the upper Cenomanian–lower Santonian of the Iberian Peninsula (Spain and Portugal). Geologica Acta 12: 19–27.
Shimansky, V.N. [Šimanskij, V.N.] 1975. Cretaceous Nautiloids [in Russian]. Trudy Paleontologičeskogo Instituta Akademii Nauk SSSR 150: 1–208.
Spath, L.F. 1927. Revision of the Jurassic cephalopod fauna of Kachh (Cutch). Memoir of the Geological Survey of India, New Series 9 (2): 1–84.
Tajika, A., Morimoto, N., Wani, R., Naglik, C., and Klug, C. 2015. Intraspecific variation of phragmocone chamber volumes throughout ontogeny in the modern nautilid Nautilus and the Jurassic ammonite Normannites. PeerJ 3: e1306. Crossref
Teichert, K. 1964. Morphology of hard parts. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part K, Mollusca 3, K13–K53. Geological Society of America, Boulder, and University of Kansas Press, Lawrence.
Tintant, H. and Kabamba, M. 1985. The role of the environment in the Nautilaceae. In: U. Bayer and G.M. Friedman (eds.), Sedimentary and Evolutionary Cycles: 58–66. Springer, Berlin. Crossref
Tsujita, C.J. and Westermann, G.E.G. 2001. Were limpets or mosasaurs responsible for the perforations in the ammonite Placenticeras? Palaeogeography, Palaeoclimatology, Palaeoecology 169: 245–270. Crossref
Vermeij, G.J. 1977. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology 3: 245–258. Crossref
Wani, R., De Ocampo, R.S.P., Aguilar, Y.M., Zepeda, M.A., Kurihara, Y., Hagino, K., Hayashi, H., and Kase, T. 2008. First discovery of fossil Nautilus pompilius Linnaeus, 1758 (Nautilidae, Cephalopoda) from Pangasinan, northwestern Philippines. Paleontological Research 12: 89–95. Crossref
Ward, P.D. 1987. The Natural History of Nautilus. 267 pp. Allen and Unwin, London.
Ward, P.D. 1996. Ammonoid extinction. In: N.H. Landman, K. Tanabe, and R.A. Davies, (eds.), Ammonoid Paleobiology. Topics in Geobiology 13: 815–824. Crossref
Ward, P.D. and Signor, P.W. 1983. Evolutionary tempo in Jurassic and Cretaceous ammonites. Paleobiology 9: 183–198. Crossref
Ward, P.D., Dooley, F., and Barord, G.J. 2016. Nautilus: biology, systematics, and paleobiology as viewed from 2015. Swiss Journal of Palaeontology 135: 169–185. Crossref
Ward, P., Greenwald, L., and Magnier, Y. 1981. The chamber formation cycle in Nautilus macromphalus. Paleobiology 7: 481–493. Crossref
Westermann, G.E.G. 1971. Form, structure and function of shell and siphuncle in coiled Mesozoic ammonoids. Royal Ontario Museum Life Science Contributions 78: 1–39. Crossref
Westermann, G.E.G. 1973. Strenght of concave septa and depth limits of fossil cephalopods. Lethaia 6: 383–403. Crossref
Westermann, G.E.G. 1996. Ammonoid life and habitat. In: N.H. Landman, K. Tanabe, and R.A. Davies (eds.), Ammonoid Paleobiology. Topics in Geobiology 13: 607–707. Crossref
Westermann, G.E.G. 1999. Life habits of nautiloids. In: E. Savazzi (ed.), Functional Morphology of the Invertebrate Skeleton, 263–298. John Wiley and Sons, Chichester.
Westermann, G.E.G. and Ward, P.D. 1980. Septum morphology and bathymetry in cephalopods. Paleobiology 6: 48–50. Crossref
Wiedmann, J. 1960. Zur Stammesgeschichte jungmesozoischer Nautiliden unter besonderer Berücksichtigung der iberischen Nautilinae d’Orb. Palaeontographica, Abteilung A 115 (Lieferung 1–6): 144–206.
Wiedmann, J. 1964. Le Crétacé supérieur de l’Espagne et du Portugal et ses Céphalopodes. Estudios Geologicos 20: 107–148.
Wiese, F. and Schulze, F. 2005. The upper Cenomanian (Cretaceous) ammonite Neolobites virbrayeanus (d’Orbigny, 1841) in the Middle East: taxonomic and palaeoecologic remarks. Cretaceous Research 26: 930–946. Crossref
Wilmsen, M., 2000. Late Cretaceous nautilids from northern Cantabria, Spain. Acta Geologica Polonica 50: 29–43.
Wilmsen, M. 2003. Sequence stratigraphy and palaeoceanography of the Cenomanian Stage in northern Germany. Cretaceous Research 24: 525–568. Crossref
Wilmsen, M. 2012. Origin and significance of Upper Cretaceous bioevents: Examples from the Cenomanian. Acta Palaeontologica Polonica 57: 759–771. Crossref
Wilmsen, M. 2016. Nautiliden. In: B. Niebuhr and M. Wilmsen (eds.), Kreide-Fossilien in Sachsen, Teil 2. Geologica Saxonica 62: 59–102.
Wilmsen, M. and Yazykova, E.A. 2003. Campanian (Late Cretaceous) nautiloids from Sakhalin, Far East Russia. Acta Palaeontologica Polonica 48: 481–490.
Wilmsen, M. and Nagm, E. 2012. Depositional environments and facies development of the Cenomanian–Turonian Galala and Maghra el Hadida formations of the Southern Galala Plateau (Upper Cretaceous, Eastern Desert, Egypt). Facies 58: 229–247. Crossref
Wilmsen, M. and Nagm, E. 2013. Sequence stratigraphy of the lower Upper Cretaceous (Upper Cenomanian–Turonian) of the Eastern Desert, Egypt. Newsletters on Stratigraphy 46: 23–46. Crossref
Wright, C.J. and Kennedy, W.J. 2017. The Ammonoidea of the Lower Chalk. Part 7. The Palaeontographical Society Monographs 648 (171): 461–561. Crossref
Yacobucci, M.M. 2018. Postmortem transport in fossil and modern shelled cephalopods. PeerJ 6: e5909. Crossref
Acta Palaeontol. Pol. 64 (4): 831–849, 2019
https://doi.org/10.4202/app.00637.2019