

Feeding convergence among ray-finned fishes: Teeth of the herbivorous actinopterygians from the latest Permian of East European Platform, Russia
MACIEJ PINDAKIEWICZ, MATEUSZ TAŁANDA, TOMASZ SULEJ, GRZEGORZ NIEDŹWIEDZKI, ANDREY G. SENNIKOV, ALEXANDR S. BAKAEV, VALERIY V. BULANOV, VALERIY K. GOLUBEV, and ALLA V. MINIKH
Pindakiewicz, M., Tałanda, M., Sulej, T., Niedźwiedzki, G., Sennikov, A.G., Bakaev, A.S., Bulanov, V.V., Golubev, V.K., and Minikh, A.V. 2020. Feeding convergence among ray-finned fishes: Teeth of the herbivorous actinopterygians from the latest Permian of East European Platform, Russia. Acta Palaeontologica Polonica 65 (1): 71–79.
A unique functional adaptation to herbivory within early ray-finned fishes is exemplified by the late Permian actinopterygians within the family Eurynotoidiidae with policuspid teeth strongly modified with respect to the primitive actinopterygian conditions. Here we report additional finds of multidenticulated teeth from the fluvial latest Permian deposits of Russia. The teeth belong to the members of endemic Eurynotoidiidae and show rather high morphological diversity. We confirm that the Russian forms are the earliest known ray-finned fishes with substantial modifications of teeth adapted to the processing of food. These finds confirm some previous suggestions that the adaptation to herbivory first developed in freshwater fishes, not marine. We found very similar dental adaptations in some groups of Recent freshwater teleosts, especially in characiforms and cichlids. It suggests that sympatric species of Permian Eurynotoidiidae explored various herbivorous niches like modern fish in East African lakes. Apparently, this first pulse of adaptive radiation in ray-finned fishes was probably caused by diversification of Permian aquatic vertebrate community.
Key words: Actinopterygii, Eurynotoidiidae, multicuspid teeth, herbivory, convergence, Permian, Russia.
Maciej Pindakiewicz [mpindakiewicz@twarda.pan.pl] and Tomasz Sulej [sulej@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warsaw, Poland.
Mateusz Tałanda [m.talanda@biol.uw.edu.pl] (corresponding author), Department of Palaeobiology and Evolution, Faculty of Biology, Biological and Chemical Research Centre, University of Warsaw, Warsaw, Poland.
Grzegorz Niedźwiedzki [grzegorz.niedzwiedzki@ebc.uu.se], Department of Organismal Biology, Evolutionary Biology Center, Uppsala University, Norbyvägen 18A, 752 36 Uppsala, Sweden.
Andrey G. Sennikov [sennikov@paleo.ru], Valeriy V. Bulanov [bulanov@paleo.ru], Valeriy K. Golubev [vg@paleo.ru], Borissiak Paleontological Institute of the Russian Academy of Sciences, Profsoyuznaya Str. 123, Moscow 117647, Russia; and Kazan Federal University, Institute of Geology and Petroleum Technologies, Kremlyovskaya Str. 4, Kazan 420008, Russia.
Alexandr S. Bakaev [alexandr.bakaev.1992@mail.ru], Borissiak Paleontological Institute of the Russian Academy of Sciences, Profsoyuznaya Str. 123, Moscow 117647, Russia.
Alla V. Minikh [a.v.minih@mail.ru], Saratov State University, Astrakhanskaya Str. 83, Saratov 410012, Russia.
Received 3 April 2019, accepted 21 November 2019, available online 27 January 2020.
Copyright © 2019 M. Pindakiewicz et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Actinopterygians, ray-finned fishes, are the most successful group of vertebrates on our planet (Near et al. 2012). They colonized almost every aquatic ecosystem around the world (with the exception of hypersaline lakes). Most fishes are predators exploiting multiple niches, from large apex predators to tiny plankton eaters. However, in some groups, bizarre, multidenticulate, specialized teeth have evolved, which are interpreted as an adaptation to herbivorous feeding, mainly benthic scraping of plants (Streelman et al. 2003; Gibson 2015).
Herbivores constitute only 8–20% of actinopterygians diversity in freshwater biota (Nimet et al. 2015) and 16–18% in coral reefs (Bellwood et al. 2014). Nowadays, two groups of derived actinopterygians, Cyprinidae, and Cichlidae, dominate among herbivores in freshwater ecosystems, while Siganidae, Acanthuridae, and Scaridae in marine realm.
The literature survey outlined below revealed that there are conflicting claims about the first appearance of fish herbivory. According to Bellwood (2003), the first herbivorous fishes evolved from marine durophagous forms as Perciformes, Anguiliformes, and Syngnathiformes, as suggested by the Cenozoic fossil record from the shallow marine sediments (Bellwood et al. 2014). Gibson (2015, 2016) suggested that adaptation to herbivory in actinopterygians evolved much earlier and in more basal forms, represented by the Late Triassic holostean Hemicalypterus from the Chinle Formation of Utah. Esin (1995, 1997) claims however, that freshwater herbivorous fishes existed already in the Permian. The teeth of most ray-finned fishes are morphologically simple, but some fishes have more complex, multidenticulate teeth for scrapping algae from the surface of hard objects (Streelman et al. 2003). The Late Triassic Hemicalypterus had such multicuspid teeth resembling those of Recent scat (Scatophagus argus), implying similar feeding behavior—grazing algae from surface of rocks (Gibson 2015). Gibson (2015, 2016) suggested that Hemicalypterus represents one of the oldest known ray-finned fish to have possibly exploited an herbivorous trophic feeding niche.
However, some paleoichthyologists claimed already in the 1990s that herbivory evolved even earlier in actinopterygians (Esin 1995, 1997). The middle to upper Permian deposits in East-European Russia yielded numerous and diverse fish remains, including those of possible herbivores. According to these findings the earliest herbivorous actinopterygians appear in the middle Permian, and diversified in the late Permian (Esin 1995, 1997; Minikh and Minikh 2009; Minikh et al. 2015).
This study focuses on material collected from uppermost Permian strata of the East European Platform (Russia). Many, probably endemic actinopterygians lived there in the middle–late Permian times (Esin 1997; Esin and Mashin 1998; Minikh and Minikh 1998a, b, 2009; Minikh et al. 2016). These are still poorly-known groups of late Palaeozoic ray-finned fishes, and among them Eurynotoidiformes are especially characteristic and widespread in the Permian deposits of East European Russia. These spindle-shaped forms with blunt snout and heterocercal caudal fin were morphologically very similar to holosteans like some members of Ospiida and Amiida (Minikh et al. 2015). First report on herbivory adaptations in the late Permian genus Isadia Minikh, 1986 was published by Esin (1997). His interpretation of trophic specialization of Eurynotoidiidae was additionally studied by Minikh and Minikh (2009), who first pointed on similarity of teeth of Isadia and Acanthuridae (two types of teeth were mentioned—for scrapping and filter feeding). Besides that, Bulanov (2006) suggests that herbivory in Eurynotoidiidae evolved parallely to specialized aquatic and probably algae feeding, herbivorous kotlassiid seymouriamorphs.
Here we employ scanning electron microscope (SEM) imaging to examine collected Permian fish teeth and compare them to modern herbivores. We confirm that herbivory was the most probable diet of Eurynotoidiidae, and the late Permian record from East European Russia provides the oldest potential evidence for key functional innovations in feeding that would become repeated motifs over the subsequent evolutionary history of ray-finned fishes (Esin 1997; Minikh and Minikh 2009; Minikh et al. 2015).
Institutional abbreviations.—SGU, Saratov State University, Saratov, Russia; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Material and methods
The studied material was collected at Sokovka site in Vyazniki (Fig. 1) in Vladimir Region (see Sennikov and Golubev 2006; Newell et al. 2010; Owocki et al. 2012; Bajdek et al. 2015, 2017; Lebedev et al. 2015; Niedźwiedzki et al. 2016). The fossil-bearing deposits were examined during fieldworks in 2008, 2010, and 2013 organized by Institute of Paleobiology, Polish Academy of Sciences, Poland; Faculty of Biology, University of Warsaw, Poland; and Borissiak Paleontological Institute, Russian Academy of Sciences, Moscow, Russia. The fossiliferous Sokovka outcrop represents the section of the uppermost Permian—Zhukov Member of Vokhma Formation (Lebedev et al. 2015; Scholze et al. 2019). Sediments are mostly unconsolidated alluvial sand and subordinate conglomerate and claystone layers (Fig. 1). Conglomerate beds occur within sandstone sequence (Sennikov and Golubev 2006; Newell et al. 2010; Owocki et al. 2012) in the Sokovka section. These deposits have unique and rich fossil content of terrestrial and aquatic vertebrates. Altogether they are part of the Vyazniki Biotic Assemblage (Sennikov and Golubev 2006, 2017). They represent the latest Permian fauna living there just before faunal turnover associated with the Permian–Triassic transition.
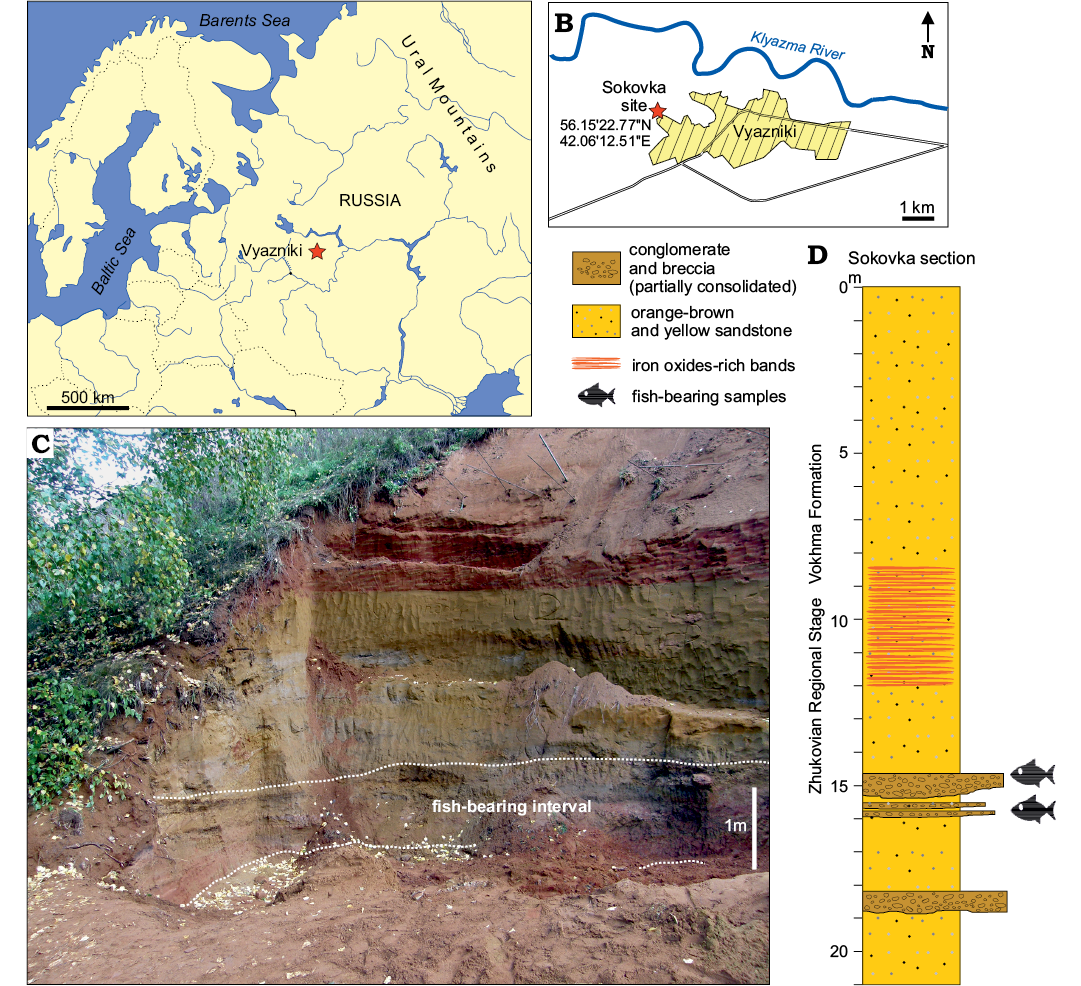
Fig. 1. Location of the fish-bearing site and details of the exposed section. A. Map of the Eastern Europe with position of Vyazniki (BY, Belarus, LV, Latvia; EST, Estonia; LT, Lithuania). B. The area around the town of Vyazniki with position of Sokovka site (star). C. Photograph of the Sokovka section from 2013 and exposure of the fish-bearing deposits. D. The simplified section from Sokovka site showing the fish-bearing layers. Modified from Newell et al. 2010, Owocki et al. 2012, and Bajdek et al. 2017.
The Vyazniki fauna includes various amphibians, dicynodonts, specialized therocephalians, and earliest large proterosuchid—Archosaurus rossicus (Ivakhnenko et al. 1997; Sennikov and Golubev 2006, 2017; Lebedev et al. 2015; Shishkin et al. 2018). The well characterized changes in the vertebrate assemblages through time are the main base for biostratigraphy in that region (Ivakhnenko et al. 1997; Esin and Mashin 1998; Minikh and Minikh 1998a, b, 2009; Golubev 2000, 2005; Sennikov and Golubev 2006, 2017; Newell et al. 2010; Minikh et al. 2014; Lebedev et al. 2015). The highly diversified ichthyofauna of Sokovka outcrop is represented by hybodont shark Lissodus sp., predatory paleoniscids of Discordichthyiformes, like Mutovinia sennikovi, smaller predators like Saurichthys, coelacanths (Pindakiewicz 2015), Strelnia sp. and Evenkia sp. (Minikh et al. 2014) and huge dipnoan Permoceratodus gentilis (Lebedev et al. 2015). Abundant remains of actinopterygian eurynotoidiform Isadia, mainly isolated teeth, represents herbivores (see Discussion).
The collection housed at the Institute of Paleobiology, PAS Warsaw, Poland was sieved in the field into three fractions: large (more than 3 mm), medium (1–3 mm) and small (under 1 mm). The residuum was separated from grains by additional strains and three other methods of separation: electromagnetic (for small fraction), manual (for large fraction) and density (for medium fraction). A heavy liquid: LST Fastfloat was used for the density separation. Scanning electron microscope (SEM) imaging of the specimens was conducted in the Institute of Paleobiology, PAS.
Systematic palaeontology
Class Actinopterygii Klein, 1885
Order Eurynotoidiformes Minikh and Minikh, 1990
Family Eurynotoidiidae Minikh and Minikh, 1990
Genus Isadia Minikh, 1986
Type species: Isadia suchonensis Minikh, 1986; Vologda region, Mutovino locality, late Permian, Severodvinian Stage, Poldarsa Formation.
Species included: Isadia aristoviensis Minikh, 1990, I. suchonensis Minikh, 1986, I. arefievi Minikh, 2015, I. opokiensis Minikh, 2017.
Isadia aristoviensis Minikh, 1990
Fig. 2A–I.
Material.—Isolated teeth (ZPAL V.51/1–9) and 208 unnumbered teeth: 124 maxillary and 84 mandibular from Sokovka outcrop, Vyazniki in Vladimir Region, Russia, late Permian (Upper Vyatkian).
Description.—The maxillary teeth have 3 cusplets of the crown with acrodine. The crown is flat and labially convex and lingually concave. The internal canal starts at the beginning of the root and ends in the base of crown. Morphotypes differ in size, length of base and shape of crown. The smaller morphotype is more straight, has short base and has slightly flat and labiolingually curved cusps. The acrodine tips are wider than in large morphotype. Length of the maxillary teeth estimates between 2.1–4.7 mm and 5.6–10 mm (with the crown). The width of the crown is placed between 0.6–1 mm (small morphotype) and 1.2–1.6 mm (large morphotype).
Stratigraphic and geographic range.—Late Permian of the East European Platform.
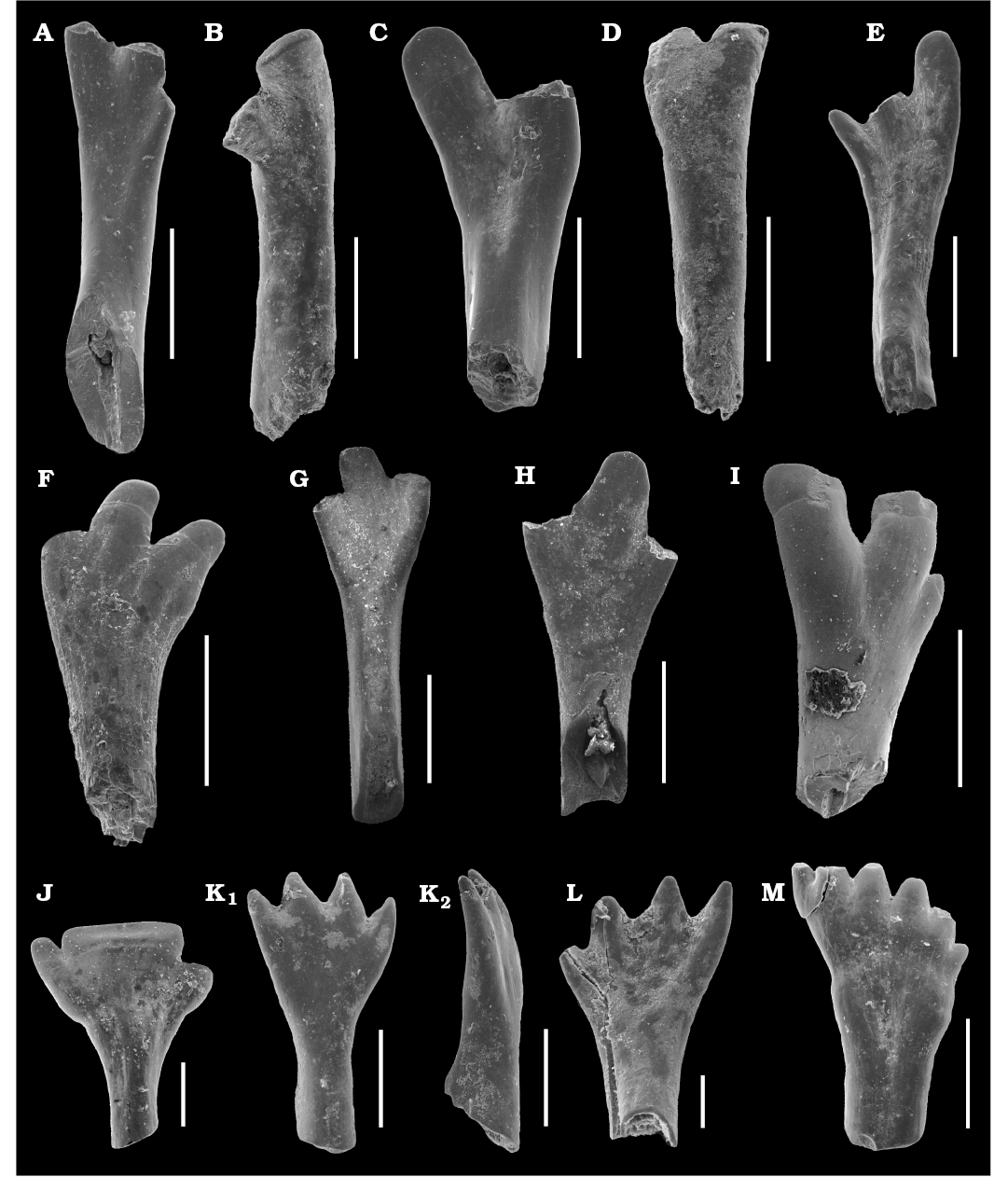
Fig. 2. The isolated teeth of actinopterygian fish Isadia from the Sokovka outcrop, Vyazniki, Russia, late Permian (Upper Vyatkian). A–D. Isadia aristoviensis Minikh, 1990, mandibulary teeth. A. ZPAL V.51/1, lingual view. B. ZPAL V.51/2, labial view. C. ZPAL V.51/3, lingual view. D. ZPAL V.51/4, labial view. E–I. Isadia aristoviensis Minikh, 1990, maxillary teeth. E. ZPAL V.51/6, lingual view. F. ZPAL V.51/7, labial view. G. ZPAL V.51/5, lingual view. H. ZPAL V.51/8, lingual view. I. ZPAL V.51/9, labial view. J. Isadia arefievi Minikh, 2015, ZPAL V.51/10, mandibular tooth, ?lingual view. K, L. Isadia suchonensis Minikh, 1986, mandibular teeth. K. ZPAL V.51/11, lingual (K1) and lateral (K2) views. L. ZPAL V.51/12, labial view. M. Isadia suchonensis Minikh, 1986, ZPAL V.51/13, maxillary teeth, ?labial view. Scale bars 1 mm (A–I), 0.5 mm (J, K, M), 0.2 mm (L).
Isadia arefievi Minikh, 2015
Fig. 2J.
Material.—Isolated tooth (ZPAL V.51/10) and two unnumbered mandibular teeth from Sokovka outcrop, Vyazniki in Vladimir Region, Russia, late Permian (Upper Vyatkian).
Description.—The base of the crown is labio-lingually curved, short and thick. These are characters unique for Isadia arefievi teeth (Minikh et al. 2015). In the collected teeth the whole crown is very wide and has three cusps (the cusp number of I. arefievi range up to 5, what it seen on specimens from Eleonora locality; see Minikh et al. 2015). The middle cusp is especially wide, making crown shovel-shaped. Flat crown is lightly labiolingually curved. The two outer cusplets are very small, thin, and occur on different heights of the tooth. The distal half of the crown is made of acrodine. Width of the crown is between 1.2–2 mm, teeth are smaller than in Isadia aristoviensis. The length is unknown because collected specimens are not well preserved. They represent mandibular teeth according to the number of cusplets in this species (Minikh et al. 2015).
Remarks.—The teeth of Isadia arefievi are rare in material from Sokovka. The same morphotype was found only in Vologda Region, Russia, Eleonora site (Salavyoro Formation) and Nizhny Novgorog Region, Russia, Lagernyi Ovrag-3 (Obnora Formation), dated as lower part of the uppermost Permian by ichthyozone Mutovinia sennikovi–Gnathorhiza otschevi (Minikh et al. 2015). This species of Isadia could have only one row of teeth, forming a tool for scrapping algae. Enlargement of the medium cusp suggests an adaptation to reduce pressure for each tooth while scrapping algae, creating a large abrasive surface. The same trend might be seen in durophagous hybodont teeth (Ginter 2012). Lack of wide, triangular labial roots of the base is caused probably by some taphonomic factors. The teeth described here suggest that I. arefievi survived to the terminal part of the late Permian in East European Platform.
Stratigraphic and geographic range.—Terminal late Permian of the Moscow Syneclise and southwestern Mezen Syneclise; Russia.
Isadia suchonensis Minikh, 1986
Fig. 2K–M.
Material.—Isolated teeth (ZPAL V.51/11–13) and 25 unnumbered teeth: 14 maxillary and 11 mandibular from Sokovka outcrop, Vyazniki in Vladimir Region, Russia, late Permian (Upper Vyatkian).
Description.—The teeth are curved in anterior view, with tips directed slightly lingually. A shallow row in the middle of the crown is visible on the labial side in some specimens. It is oriented parallel to the tooth axis. Teeth are small and have interior passage larger than Isadia aristoviensis and it ends close to the tips of the crown. The crown of the collected teeth has 4–7 cusps (in some specimens of this species collected from other outcrops show 3–9 cusps on the crown). The tips of the cusps are not covered by acrodine (in our investigations, the acrodine tips in discussed species are developed and clearly distinct from tooth bases in absence of tubercle microstructure of the external surface). The length of the teeth is 1.16–1.47 mm. Two morphotypes of these teeth were found. One with 4–5 cusps, and second with 6–7 smaller cusps. The mandibular teeth have straight base of the crown with less cusplets than crowns of the maxillary teeth (Minikh et al. 2015).
Remarks.—Isadia suchonensis Minikh, 1986 (holotype SGU 104 Б/P-2, Mutovino locality) is the type species of this genus. It is distinguished by elongated and narrow teeth on a short maxilla (Minikh and Minikh 1986). All maxillary teeth are morphologically similar to each other. The mandibular teeth differ by width of cusplets. All have wide crown with many small cusps. The teeth from Sokovka outcrop are not as large as in previously known specimens of I. suchonensis. In the holotype of I. suchonensis acrodine is very small but still noticeable. The small size of specimens from Sokovka suggests that it might be a subspecies of I. suchonensis that developed lack of acrodine or this is an effect of ontogeny. Likewise, this could be a preservational artifact. This requires further research.
Stratigraphic and geographic range.—Permian, East European Platform.
Discussion
The species of Isadia had unique dentition among early actinopterygians (Esin 1997). The crown of their teeth is multicuspid. Marginal teeth of Isadia are not capable of durophagus diet, because they are too thin and delicate to crush shells, also the number and morphology of the crown cusps are not adapted for filtrating. They are too large and wide to filter microflora and microfauna from water. Cusplets are small and wide similar to Hemicalypterus and herbivorous cichlids and characids (Fig. 3). Comparison with teeth of African cichlids and scats showed that Hemicalypterus was already a specialized herbivore (Gibson 2015). Isadia also shares this similarity and most likely diet. The teeth of different species of Isadia are similar to modern herbivorous fishes from different ecological niches.
Cichlids possess homodont dentition (Streelman et al. 2003). Adult individuals of the East African cichlid Labeotropheus fuelleborni Ahl, 1926, have three cusplets on each maxillary tooth and two on each mandibular tooth. The dentition of Isadia aristoviensis (ZPAL V.51/2) is similar to L. fuelleborni (Fig. 3A). L. fuelleborni and I. aristoviensis have teeth with their base rooted deeply in the jaws and multicuspid crown. L. fuelleborni is a highly specialized algae biter and scraper. This cichlid lives only in shallow water of the litoral zone (Streelman et al. 2003). Young fishes have one to two cusplets on each tooth and narrower jaws. The number of cusps of each tooth strongly depends on width of the jaw. Probably this expression suggests heterodentical stage in evolution of their ancestor (Streelman et al. 2003). Young fishes live in extremely shallow waters where they scrape surfaces of rocks, and when they grew older they swim to deeper but still shallow waters (Ribbink et al. 1983). We suggest that similar ontogeny had I. aristoviensis (ZPAL V.51/8). We found many maxillary teeth of different sizes and the degree of development of the third cusp. The cheek teeth were shorter, while the longer teeth with bases rooted deeply in the bone were located in the posterior side of the jaw. Mandibular teeth are similar to the maxillary, but they have only two apical cusps on the crown (Minikh et al. 2015). This could suggest that the maxilla of I. aristoviensis in its ontogeny had tendency to grow wider reflecting change in diet from scraping to biting algae like in some modern fish.
Isadia arefievi has a more derived dentition (Figs. 2J, 3K) compared to other Eurynotoidiidae. In its teeth the middle cusp is the largest in comparison to small, short lateral cusps. Shape of the middle cusp suggests that this species of Isadia was scrapping small fragments of algae from rocks like Eretmodini, and especially like African cichlid Eretmodus cyanostictus Boulenger, 1898. This cichlid is specialized in scrapping algae from surface of rocks in deeper parts of river (Burress 2015). But there is one difference, Eretmodus has monocuspid teeth (Vandervennet and Huysseune 2005), while I. arefievi is multicuspid. This difference has explanation in evolution of these fishes. In both the mechanism of developing spatula-shaped teeth could be very similar, from conical teeth (Vandervennet et al. 2006). Difference lies in ancestors of both fish. Eretmodus ancestors had monocuspid, conical teeth (Vandervennet et al. 2006), while Isadia ancestors had multicuspid teeth (Bakaev 2018). In both lineages the development of shovel like teeth is a result of lateral enlargement and labiolingual flattening.
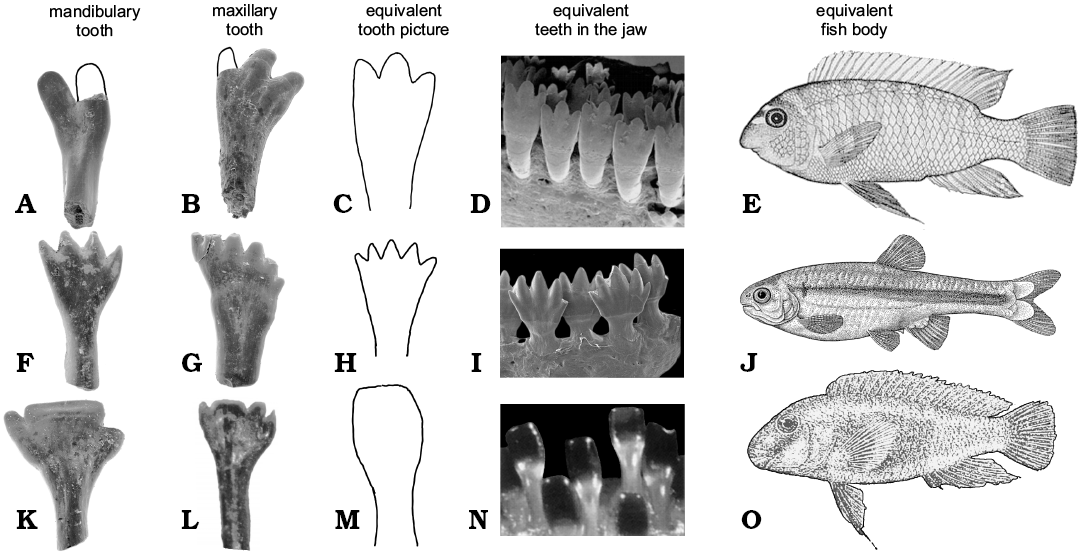
Fig. 3. Comparison of teeth of actinopterygian fish Isadia spp. from the Late Permian of Sokovka, Russia with their Recent equivalents. A, B. Isadia aristoviensis. C–E. Labeotropheus fuelleborni (C from Streelman et al. 2003; D, E from Abertson and Kocher 2006). F, G. Isadia suchonensis. H, J. Monotocheirodon kontos (from Menezes et al. 2013). I. Bryconamericus lethostigmus (from Hirschmann et al. 2017). K, L. Isadia arefievi. M–O. Eretmodus cyanosticus (M from Rüber et al. 1999; N, O from Boulenger 1915). Not to scale.
Teeth of Isadia suchonensis are morphologically similar to teeth of South American characid Monotocheirodon kontos Menezes, 2013. M. kontos has cutting bite. Species of this genus are algivorous fishes that live near bottom and pick algae from the sediment (Pouilly et. al 2006). The same situation is observed in some closely related genera, such as Bryconamericus (Fig. 3I; Hirschmann et al. 2017). Jaws of M. kontos bears only 7–9 teeth in one row (Menezes et al. 2013). In M. kontos like in I. suchonensis the maxillary and premaxillary teeth have more cusps (6–7, up to 9), than mandibulary ones (3–5 cusps). In both species the medium cusps of the mandibular teeth are slightly larger than others. In M. kontos and I. suchonensis teeth have more antero-posteriorly compressed crown than their base. Isadia suchonensis probably occupied similar niche like M. kontos. Like Monotocheirodon it could live in deeper parts of river and specialize in cutting and filtrating algae from sediment.
Acrodine on the cusp of teeth appeared early in the evolution of actinopterygians (Janvier 1996), and might be plesiomorphic for the whole group. Teleostei had teeth covered by acrodine. The lack of acrodine appeared independently in many fishes lineages (Ørvig 1978). Lack of acrodine in I. suchonensis (ZPAL V.51/11–13) might suggests that it belongs to Teleosteomorpha (Patterson 1982). But more likely this feature is autapomorphic for this species and it is a feeding adaptation as acrodine generates difference in compression between itself and rest of teeth (Ørvig 1978). In the holotype of I. suchonensis the acrodine is still visible, although to lesser degree than in other species of Isadia.
Multicuspid teeth of Isadia could have evolved from monocuspid, carnivorous ancestors like in the case of cichlids (Vandervennet et al. 2006). Carnivorous actinopterygian teeth more closely resemble Isadia teeth than teeth of typical durophagous forms because they have distinct acrodine cap and recognizable crown from its base. Carnivorous teeth are long and also labiolingually curved (Janvier 1996). Durophagous teeth are very short and rounded. In the latter group the acrodine cap becomes just a thin layer on upper part of teeth. It is an adaptation to crush invertebrates. Usually these teeth formed tooth plates (Fracasso and Hovorka 1987). Evolution from durophagy to herbivory would require much greater morphological transition in this case. That is why we consider as more probable that Isadia ancestors were carnivorous with long, conical teeth.
The teeth morphologies of herbivorous cichlids and characids is unique and very characteristic (Rüber et al. 1999). Long multicuspid teeth or short monocuspid spatula-shaped teeth are only a few examples of their diversity (Burress 2015), they reflect feeding specializations. Studying these morphologies shows how fish adapted to their environment (Delariva and Agostinho 2001). The teeth described here are not identical to cichlids but the differences are very small and probably their way of functioning was nearly the same. Analogies to Characidae and Cichlidae suggest that these multicuspid eurynotoidiids were diverse and specialized herbivores. The convergence of modern cichlids and characids to Isadia is very strong, showing that most of their adaptations have mechanical nature (Salzburger 2009; Schon and Marten 2004). It means that, herbivory is even older than the example of Hemicalypterus, and make Isadia and other Eurynotoidiidae potentially the oldest known herbivorous actinopterygian in the geological time. The species of Isadia were freshwater fishes, so it suggests that herbivory first appeared not in the marine, but rather in freshwater ecosystem.
Conclusions
Isadia dentition reveals considerable new anatomical data on the feeding apparatus of one of the earliest herbivorous actinopterygians. Their morphology shows that some late Permian actinopterygians were highly specialized fishes. We recognized three species of Isadia in the microvertebrate material collected from the uppermost Permian deposits from the Sokovka site at Vyazniki. All of them have specialized dentition for herbivory. Thus far, they are known only from freshwater sediments of the late Permian of Cis-Ural Foredeep and East European Platform. Some possibly herbivorous genera of Eurynotoidiidae were also found in the middle Permian (upper Urzhumian) of Russia (Bulanov and Minikh 2017; Esin 1997; Minikh and Minikh 2009; Bakaev 2018). Isadia shares many distinctive teeth specializations with the stratigraphically younger holostean Hemicalypterus and Recent characiforms and cichlids. It suggests, that herbivory in actinopterygians first appeared in the freshwater environment instead of marine realm.
Along with the results presented here for Isadia from Sokovka, emerging results for middle and late Permian fish fossils from Russia point to unanticipated and phylogenetically informative characters that might help to clarify the Late Paleozoic actinopterygian evolution in the freshwater ecosystems of northern Pangea. This record from late Permian of Russia indicates that ray-finned fishes were diversifying into different trophic niches and exploring different modes of feeding as early as the latest Paleozoic, thus altering our perception of the ecological roles of fishes during the late Paleozoic.
Acknowledgements
We thank Jerzy Dzik and Andrzej Kaim (both ZPAL) for helpful comments on the manuscript.We also thank Jeffrey T. Streelman (Georgia Institute of Technology, Atlanta, USA), and R. Craig Albertson (The Forsyth Institute), Naercio A. Menezes (University of São Paulo), Alice Hirschmann (Universidade Federal do Pampa), and Lukas Rüber (Natural History Museum Bern) for providing photographs and drawings used in Fig. 2. The reviewers, Małgorzata Bieńkowska-Wasiluk (University of Warsaw, Warsaw, Poland) and Sarah Gibson (St. Cloud State University, Minnesota, USA) provided very useful remarks and constructive comments that improved the article, which is greatly appreciated. The field study was supported by grant from the Polish Ministry of Science and Higher Education (7986/B/2011/40 to TS). AGS, VVB, and VKG were funded by RFBR according to the research projects 17-54-10013, 17-04-00410, 17-04-01937 and by the subsidy of the Russian Government to support the Program of “Competitive Growth of Kazan Federal University among World’s Leading Academic Centers”. ASB and AVM were funded by RFBR according to the research project 17-04-01937. GN is currently funded by grant from the Swedish Research Council (2017-05248).
References
Albertson, R.C. and Kocher, T.D. 2006. Genetic and developmental basis of cichlid trophic diversity. Heredity 97: 211–221. Crossref
Bajdek, P., Owocki, K., Niedźwiedzki, G., Sennikov, A.G., and Golubev, V.K. 2017. Residues from the Upper Permian carnivore coprolites from Vyazniki in Russia—key questions in reconstruction of feeding habits. Palaeogeography, Palaeoclimatology, Palaeoecology 482: 70–82. Crossref
Bajdek, P., Qvarnstrцm, M., Owocki, K., Sulej, T., Sennikov, A.G., Golubev, V.K., and Niedźwiedzki, G. 2015. Microbiota and food residues including possible evidence of pre-mammalian hair in Upper Permian coprolites from Russia. Lethaia 49: 455–477. Crossref
Bakaev, A.S. 2018. Systematics and evolution of the order Eurynotoidiformes M. Minikh et A. Minikh, 1990 (Pisces, Actinopterygii) [in Russian]. In: I.V. Novikov and A.V. Ivanov (eds.), Problemy paleoecologii i istoričeskoj geoecologi, 8–10. PIN RAN, Moskva.
Bellwood, D.R. 2003. Origins and escalation of herbivory in fishes: a functional perspective. Paleobiology 29: 71–83. Crossref
Bellwood, D.R., Goatley, C.H.R., Brandl, S.J., and Bellwood, O. 2014. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proceedings of the Royal Society B: Biological Sciences 281: 20133046. Crossref
Boulenger, G.A. 1915. Catalogue of the fresh-water fishes of Africa in the British Museum (Natural History). Vol. 3. Order of Trustees. i–xii + 526 pp. British Museum of Natural History, London.
Bulanov, V.V. 2006. Trophic adaptations of seymouriamorph Parareptiles and the position of this group in the structure of aquatic assemblages in Late Paleozoic [in Russian]. In: S.V. Rožnov (ed.), Evolûciâ biosfery i bioraznoobraziâ, 395–415. KMK Press, Moskva.
Bulanov, V.V. and Minikh, A.V. 2017. Convergent morphogenesis of the dental system of tetrapods and ray-finned fish of Middle to Late Permian of Eastern Europe [in Russian]. In: S.V. Rožnov (ed.), Morfogenez v individual’nom i istoričeskom: Ontogenez i formirovanie biologičeskogo raznoobraziâ, 9–11. Paleontologičeskij institut im. A.A. Borisâka, RAN, Moskva.
Burress, E.D. 2015. Cichlid fishes as models of ecological diversification: patterns, mechanisms, and consequences. Hydrobiologia 748: 7–27. Crossref
Delariva, R.L. and Agostinho, A.A. 2001. Relationship between morphology and diets of six neotropical loricariids. Journal of Fish Biology 58: 832–847. Crossref
Esin, D.N. 1995. Pozdnepermskie paleoniscidy Evropejskoj časti Rossii [in Russian]. 23 pp. Unpublished Ph.D. Thesis, Moskovskoj gosudarstvennyj universitet, Moskva.
Esin, D.N. 1997. Peculiarities of trophic orientation changes in palaeoniscoid assemblages from the Upper Permian of the European Part of Russia [in Russian]. Modern Geology 21: 185–195.
Esin, D.N. and Mashin, V.L. 1998. The zonal subdivision of the Upper Permian based on different faunal and floral group. 5.7. Ichthyolites. In: N.K. Esaulova, V.R. Lozovsky, and A.Y. Rozanov (eds.), Stratotypes and Reference Sections of the Upper Permian in the Regions of the Volga and Kama Rivers, 176–188. GEOS, Moscow.
Fracasso, M.A. and Hovorka, S.D. 1987. First occurrence of a phyllodont tooth plate (Osteichthyes, Platysomidae) from the permian San Andres Formation, subsurface, Texas Panhandle. Journal of Paleontology 61: 375–379. Crossref
Gibson, S.Z. 2015. Evidence of a specialized feeding niche in a Late Triassic ray-finned fish: evolution of multidenticulate teeth and benthic scraping in Hemicalypterus. The Science of Nature 102: 9–16. Crossref
Gibson, S.Z. 2016. Redescription and phylogenetic placement of †Hemicalypterus weiri Schaeffer, 1967 (Actinopterygii, Neopterygii) from the Triassic Chinle Formation, Southwestern United States: New insights into morphology, ecological niche, and phylogeny. PLoS ONE 11: e0163657. Crossref
Ginter, M. 2012. Ryby kopalne. 172 pp. Wydawnictwo Uniwersytetu Warszawskiego, Warszawa.
Golubev, V.K. 2000. Permian and Triassic chroniosuchians and biostratigraphy of the Upper Tatarian deposits of Eastern Europe based on tetrapods [in Russian]. Transactions of the Paleontological Institute of the Russian Academy of Sciences 276: 1–174.
Golubev, V.K. 2005. Permian tetrapod stratigraphy. New Mexico Museum of Natural History and Science Bulletin 30: 95–99.
Hirschmann, A., Fagundes, N.J., and Malabarba, L.R. 2017. Ontogenetic changes in mouth morphology triggers conflicting hypotheses of relationships in characid fishes (Ostariophysi: Characiformes). Neotropical Ichthyology 15: e160073. Crossref
Ivakhnenko, M.F. [Ivahnenko, M.F.], Golubev, V.K., Gubin, Y.M., Kalandadze, N.N., Sennikov, A.G., and Rautian, A.S. 1997. Permian and Triassic tetrapods of Eastern Europe [in Russian]. Trudy Paleontologičeskogo Instituta RAN 268: 1–216.
Janvier, P. 1996. Early Vertebrates. 396 pp. Clarendon Press, Oxford.
Lebedev, O.A., Sennikov, A.G., Golubev, V.K., Krupina, N.I., Niedźwiedzki, G., and Sulej, T. 2015. The first find of Permian ceratodontids (Dipnoi, Osteichthyes) in Russia. Paleontological Journal 49: 1112–1124. Crossref
Menezes, N.A., Weitzman, S.H., and Quagio-Grassiotto, I. 2013. Two new species and a review or the inseminating freshwater fish genus Monotocheirodon (Characiformes: Characidae) from Peru and Bolivia. Papéis Avulsos de Zoologia 53: 129–144. Crossref
Minikh, A.V. [Minih, A.V.] and Minikh, M.G. [Minih, M.G.] 1995. Pozdnepermskie ryby Prikazanskogo Povolž’â. 16 pp. VINITI, Moskva.
Minikh, A.V. and Minikh, M.G. 1998a. The zonal subdivision of the Upper Permian based on different faunal and floral group. 5.6. Fishes. In: N.K. Esaulova, V.R. Lozovsky, and A.Y. Rozanov (eds.), Stratotypes and Reference Sections of the Upper Permian in the Regions of the Volga and Kama Rivers, 173–176. GEOS, Moscow.
Minikh, A.V. [Minih, A.V.] and Minikh, M.G. [Minih, M.G.] 2009. Ihtiofauna permi Evropejskoj Rossii. 244 pp. Izdatel’skij Centr “Nauka”, Saratov.
Minikh, A.V., Arefiev, M.P., and Golubev, V.K. 2015. A new fish species of the genus Isadia (Actinopterygii, Eurynototoidiformes) from the new locality on the Malaya Northern Dvina River (terminal Permian, Vologda Region). Paleontological Journal 49 (6): 615–626. Crossref
Minikh, A.V. [Minih, A.V.], Minikh, M.G. [Minih, M.G.], and Andrushkevich, S.O. [Andruškevič, S.O.] 2014. Ichthyofauna from the terminal Permian in the vicinity of the town of Vyazniki, Vladimir Region [in Russian]. Izvestiâ Saratovskogo universiteta. Novaâ seriâ. Seriâ Nauki o Zemle 14 (2): 91–96.
Minikh, A.V. [Minih, A.V.], Minikh, M.G. [Minih, M.G.], and Yankevich, D.I. [Ânkevič, D.I.] 2016. Permian and Triassic holotypes kept in the Earth Sciences Museum at the Saratov State University. 2. Actinopterygian fishes [in Russian]. Izvestiâ Saratovskogo universiteta. Novaâ seriâ. Seriâ Nauki o Zemle 16 (1): 178–187. Crossref
Minikh, M.G. and Minikh, A.V. 1998b. Fishes. In: V.R. Lozovskij and N.K. Esaulova (eds.), Permian–Triassic boundary in Continental Series of Eastern Europe, 74–88. GEOS, Moscow.
Near, T.J., Eytan, R.I., Dornburg, A., Kuhn, K.L., Moore, J.A., Davis, M.P., Wainwright, P.C., Friedman, M., and Smith, W.L. 2012. Resolution of ray-finned fish phylogeny and timing of diversification. Proceedings of the National Academy of Science of the United States of America 109: 13698–13703. Crossref
Newell, A.J., Sennikov, A.G., Benton, M.J., Molostovskaya, I.I., Golubev, V.K., Minikh, A.V., and Minikh, M.G. 2010. Disruption of playa-lacustrine depositional systems at the Permo-Triassic boundary: Evidence from Vyazniki and Gorokhovets on the Russian Platform. Journal of the Geological Society London 167: 695–716. Crossref
Niedźwiedzki, G., Bajdek, P., Qvarnström, M., Sulej, T., Sennikov, A.G., and Golubev, V.K. 2016. Reduction of vertebrate coprolite diversity associated with the end-Permian extinction event in Vyazniki region, European Russia. Palaeogeography, Palaeoclimatology, Palaeoecology 450: 77–90. Crossref
Nimet, J., Delariva, R.L., Wolff, L.L., and Silva, J.C. 2015. Trophic structure of fish fauna along the longitudinal gradient of a first-order rural stream. Acta Limnologica Brasiliensa 27: 381–393. Crossref
Owocki, K., Niedźwiedzki, G., Sennikov, A.G., Golubev, V.K., Janiszewska, K., and Sulej, T. 2012. Upper Permian vertebrate coprolites from Vyazniki and Gorokhovets, Vyatkian regional stage, Russian Platform. Palaios 27: 867–877. Crossref
Ørvig, T. 1978. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Nephrotus and Colobodus, with remarks on the dentition in other forms. Zoologica Scripta 7: 297–326. Crossref
Patterson, C. 1982. Morphology and interrelationships of primitive actinopterygian fishes. American Zoology 22: 241–259. Crossref
Pindakiewicz, M.K. 2015. The latest Permian–earliest Triassic microvertebrate fauna from the Vyazniki, Russia. In: J.W.M. Jagt, G. Hebda, S. Mitrus, E.A. Jagt-Yazykova, A. Bodzioch, D. Konietzko-Meier, K. Kardynał, and K. Gruntmejer (eds.), European Annual Vertebrate Paleontology, Annual Meeting XIII, 67. European Association of Vertebrate Paleontology, Opole.
Pouilly, M., Barrera, S., and Rosales, C. 2006. Changes of taxonomic and trophic structure of fish assemblages along an environmental gradient in the Upper Beni watershed (Bolivia). Journal of Fish Biology 68: 137–156. Crossref
Ribbink, A.J., Marsh, A.C., Marsh, B.A., and Sharp, B.J. 1983. The zoogeography, ecology, and taxonomy of genus Labeotropheus AHL, 1927, of Lake Malawi (Pisces: Cichlidae). Zoological Journal of the Linnean Society 79: 223–243. Crossref
Rüber, L., Verheyen, E., and Meyer, A. 1999. Replicated evolution of trophic specializations in an endemic cichlid fish lineage from Lake Tanganyika. Proceedings of the National Academy of Science of the United States of America 96: 10230–10235. Crossref
Salzburger, W. 2009. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Molecular Ecology 18: 169–185. Crossref
Scholze, F., Golubev, V.K., Niedźwiedzki, G., Schneider, J.W., and Sennikov, A.G. 2019. Late Permian conchostracans (Crustacea, Branchiopoda) from continental deposits in the Moscow Syneclise, Russia. Journal of Paleontology 93: 72–97. Crossref
Schon, I. and Marten, K. 2004. Adaptive, pre-adaptive and non-adaptive components of radiation in ancient lakes: a review. Organisms, Diversity & Evolution 4: 137–154. Crossref
Sennikov, A.G. and Golubev, V.K. 2006. Vyazniki biotic assemblage of the terminal Permian. Paleontological Journal 40 (Supplement 4): S475–S481. Crossref
Sennikov, A.G. and Golubev, V.K. 2017. Sequence of Permian Tetrapod Faunas of Eastern Europe and the Permian–Triassic Ecological Crisis. Paleontological Journal 51: 600–611. Crossref
Shishkin, M.A., Sennikov, A.G., and Golubev, V.K. 2018. Comments on the paper of B.P. Vjuschkov “Locality of Permian Terrestrial Vertebrates in the Vicinities of the Town of Vyazniki”. Paleontological Journal 52: 175–187. Crossref
Streelman, J.T., Webb, J.F., Albertson, R.C., and Kocher, T.D. 2003. The cusp of evolution and development: a model of cichlid tooth shape diversity. Evolution & Development 6: 600–608. Crossref
Vandervennet, E. and Huysseune, A. 2005. Histological description of tooth formation in adult Eretmodus cf. cyanostictus (Teleostei, Cichlidae). Archives of Oral Biology 50: 635–643. Crossref
Vandervennet, E., Wautier, K., Verheyen, E., and Huysseune, A. 2006. From conical to spatulate: intra- and interspecific changes in tooth shape in closely related cichlids (Teleostei; Cichlidae: Eretmodini). Journal of Morphology 267: 516–525. Crossref
Acta Palaeontol. Pol. 65 (1): 71–79, 2020
https://doi.org/10.4202/app.00620.2019