

Paleocene of Menat Formation, France, reveals an extraordinary diversity of orthopterans and the last known survivor of a Mesozoic Elcanidae
THOMAS SCHUBNEL, LAURE DESUTTER-GRANDCOLAS, ROMAIN GARROUSTE, SOPHIE HERVET, and ANDRÉ NEL
Schubnel, T., Desutter-Grandcolas, L., Garrouste, R., Hervet, S., and Nel, A. 2020. Paleocene of Menat Formation, France, reveals an extraordinary diversity of orthopterans and the last known survivor of a Mesozoic Elcanidae. Acta Palaeontologica Polonica 65 (2): 371–385.
The orthopteran fauna of the Paleocene of Menat Formation (France) is revised. It comprises at least 12 species in the following clades: Grylloidea (an undescribed species, Menatgryllus longixiphus gen. et sp. nov.); Tettigoniidae (Prophasgonura lineatocollis); Elcanidae (Cenoelcanus menatensis gen. et sp. nov.); two Eumastacoidea (Paleochina duvergeri gen. et sp. nov. and Paleochina minuta sp. nov., tentatively placed in the extant family Chorotypidae). These two last taxa are compared to the other described fossil Eumastacoidea. As all these Eumastacoidea are represented by tegmina or hindwings, their previous attributions to the Eumastacidae sensu stricto are questionable. All previously described fossil Caelifera from Menat are considered of uncertain position. Those that were previously considered as Acridoidea are excluded from this clade. In consequence, the oldest described Acridoidea are Oligocene, at the time of diversification of the grasses on which these insects predominantly live, in accordance with the most recent molecular dating of the Acrididae. Cenoelcanus menatensis is the youngest and first Cenozoic representative of the Mesozoic Elcanidae, showing that this family survived the Cretaceous–Paleocene extinction and became extinct during the Paleogene.
Key words: Insecta, Ensifera, Elcanoidea, Acridoidea, Eumastacoidea, Paleogene, France.
Thomas Schubnel [thomas.schubnel@wanadoo.fr], Laure Desutter-Grandcolas [desutter@mnhn.fr], Romain Garrouste [garrosut@mnhn.fr], and André Nel [anel@mnhn.fr] (corresponding author), Institut Systématique Evolution Biodiversité (ISYEB), Muséum national d’Histoire naturelle, CNRS, Sorbonne Université, Université des Antilles, EPHE, 57 rue Cuvier, CP 50, 75005 Paris, France.
Sophie Hervet [sophie.hervet@yahoo.fr], Association Paléovergne, Musée de Paléontologie de Menat, Place de la Mairie, F-63560 Menat, France.
Received 5 September 2019, accepted 25 November 2019, available online 28 February 2020.
Copyright © 2020 T. Schubnel et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
One of the major problems in paleontology is the incompleteness of the fossil record, complicating the studies in phylogeny and palaeoecology. It is especially the case for the Paleocene insects for which the number of recorded species is less than 300 vs. more than 7000 for the Eocene (internet site Fossilworks at http://fossilworks.org/). The current knowledge on the Paleocene Orthoptera is especially very scarce. Kevan and Wighton (1981, 1983) described some Prophalangopsidae from the Paleocene of Alberta (Canada). Petrulevičius (1999) cited the presence of Orthoptera in the Paleocene from Argentina. Ensiferan Gryllidae and Tettigoniidae and Caeliferan Eumastacidae and Tetrigidae have been described from the Paleocene–Eocene Fur and Olst formations in Denmark (Rust 1999; Zessin 2017a, b). Lin and Huang (2006) described a Paleocene representative of Prophalangopsidae from the Northern Tibet in China, recently transferred into the Stenopelmatidae by Wang et al. (2019). Therefore, the Paleocene Konservat Lagerstätte of Menat is of great interest because it has led an abundant and diverse orthopteran fauna. From this locality Piton (1936, 1937, 1940) described Prophasgonura lineatocollis Piton, 1940 and Conocephalus martyi Piton, 1940, two taxa he attributed to the tettigoniids, plus five “Caelifera”, viz. “Oedipoda spec.”; Menatacridium eocenicum Piton, 1936 (in Acridiidae); a fossil genus “Eremobiites” without type species; Orthacanthacris incertus Piton, 1940 (in “Cyrthacanthacrinae”, sic. for Cyrtacanthacridinae); and Ochrilidia lineata Piton, 1940 (in “Truxalinae”). Zeuner (1944) discussed on these fossils but they were never revised. New recent field investigations have given a series of new fossils of great interest, including the youngest Elcanidae and the first accurate Eumastacoidea from this outcrop that we describe herein. We also revise the taxa previously described by Piton (1936, 1937, 1940).
The Elcanoidea is currently a strictly Mesozoic clade, probably of the stem group Caelifera (Béthoux and Nel 2002). The orthopteran superfamily Eumastacoidea Burr, 1899 is one of the most “basal” clades of the extant Caelifera (Song et al. 2015). This group appeared in the fossil record during the Late Jurassic, well before the oldest accurate records of the Acridoidea, dated from the Oligocene (Gorochov and Rasnitsyn 2002; Song et al. 2015; Ataabadi et al. 2017). Nevertheless, only eleven fossil species are currently attributed to the Eumastacoidea (internet sites Fossilworks; Cigliano et al. 2019), ranging between the Upper Jurassic and the Oligocene. Thus these new discoveries of Paleocene Elcanidae and Eumastacoidea are of great interest to determine their evolutionary history, age, and past diversity.
Institutional abbreviations.—MNHN, Muséum national d’Histoire naturelle, Paris, France; MNT, Musée de Paléontologie de Menat¸ Menat, Puy-de-Dôme, France.
Other abbreviations.—C, costa; CuA/P, cubitus anterior/posterior; CuPaα, most anterior branch of CuP; CuPaβ, median branch of CuP; CuPb, posterior branch of CuP; M, median vein; RA/P, radius anterior/posterior; ScA/P, subcostal anterior/posterior.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:D395B22A-F1ED-4CD9-862B-384E60BD8B4C
Material and methods
The middle Paleocene Menat fossil site, small outcrop near the southeast of the village of Menat (46°06’ N; 2°54’ E, Menat Basin, Puy-de-Dôme, France), is a volcanic maar containing a rather small paleolake ca. 1 km in diameter, filled with sedimentary rocks (spongo-diatomites) with remains of diverse aquatic and terrestrial flora and fauna (Piton 1940; Nel 1989, 2008; Nel and Roy 1996). The composition of faunal and floral remains suggests that this lake was surrounded by a forest and the palaeoenvironment was warm and humid (Wedmann et al. 2018). Following the pollen, mammalian stratigraphic, and radiometric K/Ar analyses, the age of the Menat outcrop was estimated as 59 Ma. (Kedves and Russel 1982; Nel 2008). However, the new estimate based on macroflora postulated its age within 60–61 Ma (Wappler et al. 2009). Orthoptera are diverse but infrequent at Menat, representing only 1% of the very rich and diverse entomofauna, dominated by Coleoptera (78%).
The specimens were studied using a stereomicroscope Olympus SCX9 in MNHN. Photographs were taken with a Nikon D800 and the images treated with graphic software.
We follow the wing venation nomenclature of Béthoux and Nel (2002) for the Orthoptera, as confirmed by Desutter-Grandcolas et al. (2017).
Systematic palaeontology
Orthoptera Latreille, 1793
Ensifera Chopard, 1920
Grylloidea Laicharting, 1781
Grylloidea gen. et sp. indet.
Fig. 1.
Material.—MNT NEL 1681 (small adult female with well-developed ovipositor), Menat, Paleocene.
Measurements.—Fore femur 2.8 mm long; fore tibia 3.0 mm long; hind femur 5.0 mm long, 2.0 mm wide; hind tibia 4.8 mm long; ovipositor 7.5 mm long.
Description.—Body densely covered with strong setae (Fig. 1A1). Head triangular with small, protruding eyes; last three joints of maxillary palpi about equal in length; joint 5 slightly, but regularly widened toward apex; apex truncated apically; scapes and antennae not visible; pronotum small, with shape not clear; wings and tegmina apparently lacking; right fore leg and both hind legs present; mid legs lacking; right fore tibia with an outer tympanum, elongate, probably obliterate, not slit-like; inner tympanum not visible; at least two apical spurs, one ventral and one dorsal; basitarsomere I long, but less than half tibia I length; both hind legs well-preserved; hind femora thick at base, not filiform at apex; hind tibiae shorter than hind femora; with three inner apical spurs, the dorsal the longest (Fig. 1A2); outer apical spurs not visible; at least two inner and two outer subapical spurs, short; no spine above and between subapical spurs; basitarsomeres III longer than half hind tibiae; with several dorsal (inner, outer?) spines; tarsomeres II cylindrical, not flattened; tarsomeres III with a pair of claws; abdomen. Cerci long and thin, with very long setae; ovipositor longer than hind femora; apex short, triangular, no ornamentation visible.

Fig. 1. Orthopteran Grylloidea sp., MNT NEL 1681 from Paleocene of Menat, France. A. Habitus. B. Apex of hind tibia.
Remarks.—The shape of the hind legs, with three-joint tarsi, that of the ovipositor and the presence of acoustic tympanum on fore tibia attest that this fossil belongs to Grylloidea.
?Gryllidae Laicharting, 1781
Genus Menatgryllus nov.
Zoobank LSID: urn:lsid:zoobank.org:act:42A274A8-2443-4D8B-A496-598AFBD44B0F
Type species: Menatgryllus longixiphus sp. nov., by monotypy, see below.
Etymology: Named after the Menat locality and Gryllus, the generic name for a cricket.
Diagnosis.—As for the type species by monotypy.
Remarks.—The presence of forewings and acoustic tympana in females indicates that males possibly had forewings with a stridulatory apparatus.
Menatgryllus longixiphus sp. nov.
Fig. 2.
Zoobank LSID: urn:lsid:zoobank.org:act:DFC23D67-3E7E-4F55-854 A-AEBC4F01B39C
Etymology: Named after its very long ovipositor.
Holotype: MNT-1053, female with legs and ovipositor well-preserved, abdomen and hind legs in ventral view, head and thorax in lateral position.
Type locality: New quarry, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Diagnosis.—Acoustic tympanum present on fore tibia; forewings and hindwings both present in females; no serrulation on hind tibiae; tarsomeres 2 flattened; strong setae on the fastigium.
Measurements.—Fore femur 4.2 mm long; fore tibia 2.7 mm long; hind femur 8.0 mm long, 2.9 mm wide; hind tibia 7.5 mm long; ovipositor 17.5 mm long.
Description.—Body densely covered with short setae; head long and narrow in lateral view; scape longer than wide; left eye present but hardly visible, possibly protruding; fastigium distinct, but its shape unclear; with some strong setae (apical?); maxillary palpi hardly distinct; pronotum present in lateral position; lateral lobe probably longer than high, anterior angle longer than posterior one; legs all present; tarsi short with flattened second tarsomere (Fig. 2); left fore leg with a well-developed outer tympanum, elongate and obliterate; right fore leg possibly with a smaller elongate inner tympanum; left tibia with three apical spurs; both legs with complete three-joint tarsi; basitarsomere I with two long and subequal apical spurs; mid legs complete, from coxae to tarsi; mid tibiae with four apical spurs (seen in ventral view: Fig. 2); hind femora thick and short, without a filiform apical part; hind tibiae slightly shorter than hind femora; with three inner apical spurs, the dorsal the longest and longer than half basitarsomere, also with three outer apical spurs, shorter than inner spurs, and with at least four inner and four outer subapical spurs, with no discrepancy between inner and outer subapical spurs; no serrulation between and above subapical spurs; hind basitarsomeres with several dorsal spines in addition to apical ones; forewings close to left hind femur; wings plicated, colored, except along veins; venation unclear; hind wings much longer than forewings and much longer than body; abdomen complete; subgenital plate small and transverse; distal margin concave; cerci broken, but right only long; ovipositor very long, more than twice hind tibia length; apex small; ornamentation not visible.
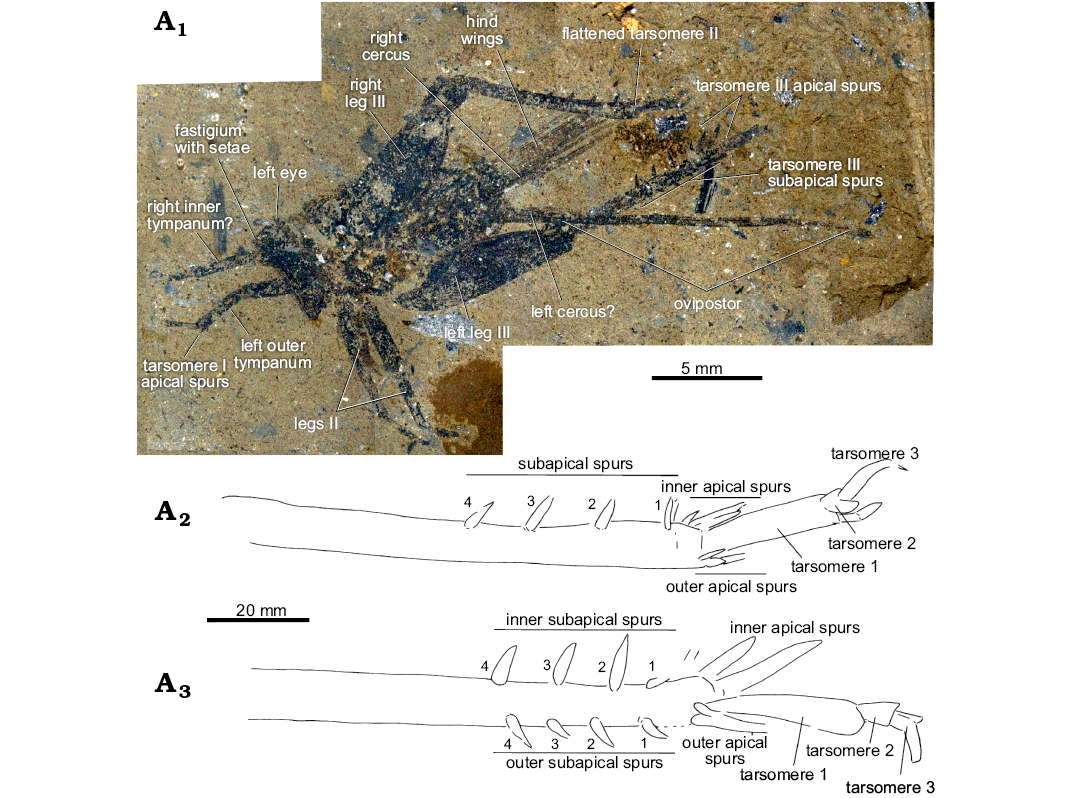
Fig. 2. Grylloidean orthopteran Menatgryllus longixiphus gen. et sp. nov., holotype, MNT-1053 from Paleocene of Menat, France. Photograph of habitus (A1), reconstruction of hind tibiae and tarsus (A2, left; A3, right).
Remarks.—The shape of the hindlegs, with three-joint tarsi, the shape of the ovipositor and the presence of acoustic tympanum on tibia I attest that this fossil belongs to Grylloidea. The presence of three apical spurs on fore tibia could fit the synapomorphy of the family Gryllidae (sensu Chintauan-Marquier et al. 2016), but the absence of serrulation on hind tibiae, the flattened shape of tarsomeres 2, and the presence of strong setae on the fastigium make an original combination of characters, supporting a new genus. Menatgryllus gen. nov. shares with the Baltic amber genus Eopentacentrus Gorochov, 2010 the presence of four pairs of subapical spurs on the hind tibiae but it differs from this genus in the fore tibia with three apical spurs instead of two (Gorochov 2010). Some other fossil gryllid genera are described from the Paleocene or the Eocene, but they are based on isolated wings (Gorochov 1992; Rust 1999), impossible to compare to Menatgryllus gen. nov.
Stratigraphic and geographic range.—Type locality and horizon.
Tettigoniidae Krauss, 1902
Genus Prophasgonura Piton, 1940
Type species: Prophasgonura lineatocollis Piton, 1940; Paleocene, Menat, France.
Prophasgonura lineatocollis Piton, 1940
Fig. 3A.
Holotype: MNHN.F. R07050 (coll. Louis Piton), female with wings attached to body.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Remarks.—This poorly preserved fossil is a Tettigoniidae as shown by its long antennae, long curved ovipositor, and long tegmina, but its exact affinities are impossible to establish because the body structures are poorly preserved and the wing venation cannot be deciphered. Prophasgonura lineatocollis is a Tettigoniidae of uncertain affinities.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Genus Conocephalus Thunberg, 1815
Type species: Conocephalus conocephalus (Linnaeus, 1767), Recent.
Conocephalus martyi Piton, 1940
Fig. 3B.
Holotype: MNHN.F.R07019 (coll. Louis Piton), an adult Orthoptera with hind legs and wings attached to thorax.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Remarks.—The body structures are very poorly preserved and the venation of tegmen is impossible to decipher. It is only possible to see that there is a dense net of cells covering the tegmen. It is a Tettigoniidae of uncertain affinities, impossible to accurately attribute to the extant genus Conocephalus.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.

Fig. 3. Habitus of Ensifera from Paleocene of Menat, France. A. Prophasgonura lineatocollis Piton, 1940, holotype, MNHN.F.R07050. B. Conocephalus martyi Piton, 1940, holotype, MNHN.F.R07019.
Caelifera Ander, 1936
Acridoidea MacLeay, 1821
Acrididae MacLeay, 1821
Genus Oedipoda Latreille, 1829
Type species: Oedipoda caerulescens (Linnaeus, 1758); Recent, Europe, Northern Africa, Asia.
Oedipoda sp.
1940 Oedipoda spec.; Piton, 1940: 140–141, fig. 16.
Material.—No repository number, currently lost (coll. Louis Piton); basal half of a tegmen from Menat, Paleocene.
Remarks.—This tegmen is very narrow, with a very narrow area between C and ScP, unlike the extant genus Oedipoda Latreille, 1829. This pattern is quite strange for an Orthoptera as Piton (1940: fig. 16) figured because the putative M+CuA would diverge very basally from R and the cubital and anal veins are not distally fused as in the Caelifera. It is a Polyneoptera of uncertain affinities.
Family uncertain
Genus Orthacanthacris Karsch, 1896
Type species: Orthacanthacris humilicrus (Karsch, 1896), Recent, Afrotropics.
Orthacanthacris incertus Piton, 1940
Fig. 4A.
Holotype: MNHN.F.R07017 (coll. Louis Piton); a very poorly preserved specimen, with fragments of three wings, and dubious remains of thorax and head.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Remarks.—It is even not possible to determine which wings are forewing(s) vs. hindwing(s). It is either a Mantodea or an Orthoptera of uncertain affinities.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Family uncertain
Genus Ochrilidia Stål, 1873
Type species: Ochrilidia tryxalicera Stål, 1873, Recent, Eastern Africa.
Ochrilidia lineata Piton, 1940
Fig. 4B.
Holotype: MNHN.F.R07043 (coll. Louis Piton); a nearly complete but very poorly preserved specimen.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Remarks.—The extant genus Ochrilidia belongs to the Acrididae: Gomphocerinae. This fossil has narrow tegmina, with apparently few cells, with a narrow area between C and R and a darkened area between RA and C in distal part of wing Its hind legs are elongate and with a rather thin femur; the head is orthognathous. The rather reduced number of cells in distal part of tegmina would exclude this fossil from the Acrididae. It could be an Eumastacoidea. Its attribution to the genus Ochrilidia is not supported by any argument. It is a Caelifera of uncertain affinities.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.

Fig. 4. Habitus of Caelifera from Paleocene of Menat, France. A. Orthacanthacris incertus Piton, 1940, holotype MNHN.F.R07017. B. Ochrilidia lineata Piton, 1940, holotype, MNHN.F.R07043.
Family uncertain
Genus Menatacridium Piton, 1936
Type species: Menatacridium eocenicum Piton, 1936; Menat, Paleocene.
Menatacridium eocenicum Piton, 1936
Holotype: No repository number, currently lost (coll. Louis Piton); a nearly complete tegmen.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Remarks.—This taxon was very poorly described and figured by Piton (1936, 1937, 1940: pl. 19: 1). We can only say that it is a Polyneoptera of uncertain affinities, possibly an Orthoptera, but probably not an Acridoidea.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Orthoptera indet.
Material.—No repository number, currently lost (coll. Louis Piton); a hind femur from Menat, Paleocene.
Remarks.—A hind femur has been ascribed to the new taxon name Eremobiites by Piton (1940), but this genus is preoccupied by Eremobiites Suassure, 1884. Furthermore, there is no named type species for the genus Eremobiites Piton, 1940, thus this genus name, that is based on a taxon in open nomenclature, should be considered invalid. This fossil is an Orthoptera indet.
Caelifera superfamily incertae sedis
Caelifera genus and sp. indet. A
Fig. 5.
Material.—MNHN.F.A71197 (coll. Philippe Olivier); a body with legs and wings attached from Menat, Paleocene.
Description.—Head visible but poorly preserved, 2.0 mm wide; antenna short, 3.0 mm long. Thorax very poorly preserved. Abdomen missing. Fore, mid and hind legs preserved. Hind femur elongate and rather narrow, 15.0 mm long, 1.5 mm wide; hind tibia very long and narrow, with two series of spines, the outer ones of proximal half very short and acute while those of distal half of femur broad and not acute; the inner series of spines all short and acute. Tarsi not preserved. Forewing 17.0 mm long, poorly preserved, hyaline except for two dark longitudinal zones, one along R and one along posterior wing margin, plus two transverse bands. Venation hardly visible, except for the costal area that is very broad, 2.0 mm wide with a series of ca. 10 curved crossveins. Hind wing hyaline, narrow but partly folded, 16.0 mm long; C very weakly curved basal of apex of ScA; area between C and ScA narrow, 0.4 mm wide, but poorly preserved; area between C and ScP narrow, with a series of short parallel crossveins; ScP and R strongly approximate but not touching; area between C, ScA, ScP, and RA darkened; M simple; a series of broad cells posterior to M; area between RA and RP narrow, with one row of simple crossveins between them; RP with four posterior branches, with one row of cells between them; part of wing posterior to CuP poorly preserved.

Fig. 5. Habitus of Caelifera genus and sp. indet. A, MNHN.F.A71197 from Paleocene of Menat, France.
Remarks.—This fossil, although nearly complete, has its body and wings poorly preserved, rendering its affinities very uncertain. The presence of a very broad forewing costal area would suggest affinities with the Acridoidea, but some Eumastacoidea Chorotypidae (Hemicharianthus spp.) have also such a very broad area. The very particular outer spines of the hind femora are quite unusual among the Caelifera, and remind those of the fossil Elcanidae and the extant Tridactylidae. We cannot accurately name this fossil, even if certainly corresponds to a new taxon.
Eumastacoidea Burr, 1899
Chorotypidae Stål, 1873 (?)
Genus Paleochina nov.
Zoobank LSID: urn:lsid:zoobank.org:act:F95A0640-E6B9-41D1-BF8 B-667066B2F7E2
Etymology: Named after Paleocene and the extant genus China.
Type species: Paleochina duvergeri sp. nov. (see below).
Species included: Type species and Paleochina minuta sp. nov.
Diagnosis.—Tegmen narrow; few intercalary cells between main veins; narrow area between radial vein and costa; darkened area between RA and C; five branches of RP with two rows of cells between them; C nearly straight along anterior margin; ScA ending on costa well basal of base of RP; narrow area between RA and RP.
Remarks.—This taxon is only tentatively placed in the Chorotypidae because the current classification of the Eumastacoidea is mainly based on the genital structures.
Paleochina duvergeri sp. nov.
Figs. 6, 7A.
Zoobank LSID: urn:lsid:zoobank.org:act:AD7F0C52-16A3-4CDB-A5 02-C676C84A7F7E
Etymology: Named after M. Bernard Duverger, for his authorization of collect of fossils in the area of Menat.
Holotype: MNT NEL 3267, two fore- and two hind wings, two hind legs.
Type locality: New quarry, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Diagnosis.—M+CuA separating from R well distal of apex of ScA; two rows of cells between CuA+CuPaα and CuPaβ.
Description.—Forewing hyaline, very narrow, 23.3 mm long, 2.7 mm wide in widest part, 1.5 mm in narrowest; posterior margin straight; C very weakly curved basal of apex of ScA; area between C and ScA narrow, 0.3 mm wide, with a series of ca. 10 short parallel crossveins; apex of ScA 6.0 mm from wing base; area between ScA and ScP 0.3 mm wide; area between C and ScP narrow, 0.3 mm wide, with a series of short parallel crossveins; ScP and R strongly approximate but not touching; apex of ScP 6.0 mm distal of that of ScA; area between C and RA darkened, 0.25 mm wide, without visible crossvein, except near apex of RA; apex of RA 1.3 mm from wing apex; M+CuA appressed to R in basal half of wing, separating from R 8.5 mm from wing base; independent stem of M+CuA 2.3 mm long; M simple; CuPaα weak, ending into CuA 0.7 mm from point of separation between M and CuA; CuA+CuPaα simple; two rows of irregular cells between M and CuA+CuPaα; base of RP 12.3 mm distal of wing base; area between RA and RP narrow, 0.3 mm wide, with one row of simple crossveins between them; RP with five posterior branches, with two rows of cells between them; two rows of cells between CuA+CuPaα and CuPaβ; one row of cells between M+CuA and CuPa, and between CuPa and CuPb; CuPb straight, area between CuPb and anal vein very narrow.
Hindwing 22.0 mm long, narrow, with the anal area folded along first anal vein; hyaline, except for a darkened area between C and RA, as in forewing; part of wing anterior to the anal area nearly identical to forewing.
Hind leg long and rather thin: femur 16.7 mm long, 1.3 mm wide, with longitudinal anterior and posterior crests, without spines; tibia 13.2 mm long, 0.3 mm wide; with a series of ca. 28 strong spines, becoming more and more broad distally, apical spines poorly preserved; tarsus also poorly preserved.

Fig. 6. Wings and hind legs of chorotypid orthopteran Paleochina duvergeri gen. et sp. nov., holotype, MNT NEL 3267 from Paleocene of Menat, France.
Remarks.—The forewings, hindwings, and legs are grouped on a surface of 8 × 7 cm. They clearly belong to the same individual. The body is missing. This locust may have been attacked by a predator that has eaten the body. Wings and legs remained grouped because they have fallen in a surface algal mat (diatoms), and was transported at the lake bottom when the mat has sunk. This scenario is in accordance with what is generally observed in diatom paleolake (Nel 1991).
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Paleochina minuta sp. nov.
Fig. 7B, C.
Zoobank LSID: urn:lsid:zoobank.org:act:C33D57DF-9413-4C75-A312-906D99EC2532
Etymology: Named after the minute size of the tegmen.
Type material: Holotype: MNT NEL 1928. Paratype: MNT BDL 1043, both isolated tegmina.
Type locality: New quarry, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype and paratype only.
Diagnosis.—M+CuA separating from R well basal of apex of ScA; one row of cells between CuA+CuPaα and CuPaβ.
Description.—Holotype MNT NEL 1928 (Fig. 7B). Forewing hyaline, very narrow, 14.0 mm long, 2.2 mm wide in widest part; posterior margin straight; C very weakly curved basal of apex of ScA; area between C and ScA narrow, 0.3 mm wide, but poorly preserved; apex of ScA 4.3 mm from wing base; area between ScA and ScP 0.2 mm wide; area between C and ScP narrow, 0.2 mm wide, with a series of short parallel crossveins; ScP and R strongly approximate but not touching; apex of ScP 2.5 mm distal of that of ScA; area between C and RA darkened, 0.2 mm wide, without visible crossvein, except near apex of RA; apex of RA 1.5 mm from wing apex; M+CuA appressed to R in basal half of wing, separating from R 4.0 mm from wing base; independent stem of M+CuA 1.2 mm long; M simple; CuPaα weak, ending into CuA 0.4 mm from point of separation between M and CuA; CuA+CuPaα simple; one row of cells between M and CuA+CuPaα; base of RP 7.3 mm distal of wing base; area between RA and RP narrow, 0.4 mm wide, with one row of simple crossveins between them; RP with five posterior branches, with two rows of cells between them; one row of cells between CuA+CuPaα and CuPaβ; one row of cells between M+CuA and CuPa, and between CuPa and CuPb; CuPb straight, area between CuPb and anal vein very narrow.
Paratype MNT BDL 1043 (Fig. 7C). Same pattern of coloration and venation as for holotype, tegmen ca. 14.5 mm long, 2.8 mm wide.

Fig. 7. Tegmina of chorotypid orthopteran Paleochina spp. from Paleocene of Menat, France. A. Paleochina duvergeri gen. et sp. nov., holotype, MNT NEL 3267; A1, left wing; A2, right wing (reversed view). B. Paleochina minuta sp. nov., holotype, MNT NEL 1928. C. Paleochina minuta sp. nov., paratype, MNT BDL 1043. Scale bars 5 mm. Abbreviations: C, costa; CuA/P, cubitus anterior/posterior; CuPaα, most anterior branch of CuP; CuPaβ, median branch of CuP; CuPb, posterior branch of CuP; M, median vein; RA/P, radius anterior/posterior; ScA/P, subcostal anterior/posterior.
Remarks.—Paleochina duvergeri sp. nov. and Paleochina minuta sp. nov. have very similar tegmina, that of P. duvergeri being longer than that of P. minuta and with few differences indicated in the respective diagnoses. We consider that they belong to different species within the same genus. Sexual dimorphism that concern the size and shape of the tegmina can occur in the Caelifera. As we do not know the sex of these insects, it is not totally excluded that they could correspond to male and female of the same species but differences in the venation support a specific non-identity. These tegmina are typical of the Eumastacoidea, viz. reduction of vein CuPaα (Sharov 1968; Dirsh 1975), tegmen very narrow, with few intercalary cells between main veins and a narrow area between radial vein and costa. Such structures can be found in some Chorotypidae Stål, 1873 and Eumastacidae Burr, 1899 (Dirsh 1975; internet site www.orthopteran.speciesfile.org). Among the Chorotypidae, Paleochina gen. nov. differs from Erucius Stål, 1875 (Eruciinae Burr, 1899) in the broader radial area and broader and darkened area between RA and C (Dirsh 1975: fig. 2). This last character of Paleochina gen. nov. is present in the genus China Burr, 1899, but China mantispoides (Walker, 1870), unique species of this genus, has only three branches of RP instead of five in Paleochina gen. nov. (Descamps 1974: fig. 30). Eupatrides Brunner von Wattenwyl, 1898, second genus of the subfamily Chininae Burr, 1899, has a broader area between C and ScA, with C making a strong curve instead of being straight. Among the winged Chorotypinae Stål, 1873, Burrinia Bolívar, 1930 has two rows of cells in area between ScA and C and less developed area of RP (Bolívar 1930). Chorotypus Bolívar, 1930, Orchetypus Brunner von Wattenwyl, 1898, Phyllochoreia Westwood, 1839, and Hemierianthus Saussure, 1903 have broader tegmina, with numerous rows of cells between main veins. Pseudorchetypus Descamps, 1974 and Scirtotypus Brunner von Wattenwyl, 1898 has C making a strong curve instead of being straight in its basal part (Descamps 1974). The extant Erianthinae Karsch, 1889 have a broader area between C and ScP.
Among the extant winged Eumastacidae, several genera, e.g., Eumastax Burr, 1899, Homeomastax Descamps, 1979, Hysteromastax Descamps, 1979, Helicomastax Rowell and Bentos-Pereira, 2001, Paramastax Burr, 1899, and Temnomastax Rehn and Rehn, 1942, among others, have much reduced venation of the tegmina, with three or less branches of RP (Dirsh 1975; Descamps 1974, 1979; internet site www.orthopteran.speciesfile.org). Paleochina gen. nov. is probably not a Eumastacidae and more likely a Chorotypidae, but a more complete specimen with genitalia at least partly preserved will be necessary to better support its exact relationships.
Among the fossil Eumastacoidea, Promastax archaicus Handlirsch, 1910 (type species of the fossil family Promastacidae Kevan and Wighton, 1981, Paleocene–Eocene, Canada, the distal two-third of a tegmen) has only three branches of RP, ScA ending on costa distal of base of RP, a broad area between RA and RP, unlike Paleochina gen. nov. (Handlirsch 1910: fig. 1). Notice that this fossil family is based on few, poor characters (Kevan and Wighton 1981), and should be revised. The second genus and species of this family, Promastacoides albertae Kevan and Wighton, 1981 (same outcrop) is based on a nearly complete tegmen. The original description and figure are problematic, but it seems to have M with three branches, and a quite broad wing, unlike the Eumastacoidea. Its phylogenetic relationships are uncertain.
All the described fossil taxa currently placed in Eumastacidae are based on wings, thus their exact positions in the Eumastacoidea are tentative. The Jurassic genus Archaeomastax Sharov, 1968 differs from Paleochina gen. nov. in the broader area between ScP and RA (Sharov 1968). The Eocene Eoerianthus Gorochov in Gorochov and Labandeira, 2012, has only three branches of RP and apices of ScP and RA very close to wing apex (in the type species E. eocaenicus Gorochov, 2012). The second species E.? multispinosa (Scudder, 1890), originally placed in the fossil genus Tyrbula Scudder, 1885 (type species Tyrbula russelli Scudder, 1885), and Truxalidae, is rather poorly preserved. The Paleocene–Eocene Eozaenhuepfer Zessin, 2017 has a two-branched median vein (Zessin 2017b), while it is simple in Paleochina gen. nov. The fossil genus Taphacris Scudder, 1890 is rather poorly defined (Zeuner 1944; Rehn 1948). Its type species Taphacris reliquata Scudder, 1890 (Eocene–Oligocene of Florissant, USA) has a simple M but only four branches of RP, unlike Paleochina gen. nov. (Scudder, 1890). Taphacris bitttaciformis Cockerell, 1909 (same outcrop) is poorly described and figured, nevertheless it has a three-branched RP (Cockerell 1909, 1926), unlike Paleochina gen. nov. Taphacris bitttaciformis tillyardi Cockerell, 1926 (same outcrop) is even based on a more fragmentary fossil. The Chinese Paleocene Taphacris stenosis Lin, 1977 has also four branches of RP and a simple M, but a distinctly longer ScA than in Paleochina gen. nov., reaching mid wing level (Lin 1977). The Chinese Early Cretaceous Taphacris turgis Lin, 1980 has apparently the same characters but it would need a revision (Lin 1980). The Miocene Paleomastacris ambarinus Pérez-Gelabert, Hierro, Dominici, and Otte, 1997 (from the Dominican amber) is based on an apterous fossil, not comparable to Paleochina gen. nov. (Pérez-Gelabert et al. 1997; Pérez-Gelabert 2002). Lastly Lewis et al. (1990) cited a Eumastacidae from the Miocene of Idaho. Orthacanthacris rhenana Theobald, 1937 (Oligocene, Germany) could also be an Eumastacoidea for the narrow tegmina and thin and long hind legs; it differs from Paleochina gen. nov. in the more numerous branches of RP (Théobald 1937). This taxon needs to be revised. Lewis (1974) figured undescribed specimens from the Oligocene of Ruby River Basin (Montana) that are attributable to the Eumastacoidea. Lewis (1976) also figured an undescribed eumastacoid tegmen (either in Eumastacidae or Chlorotypidae) from the same outcrop. Its area between C and ScA is broader than in Paleochina gen. nov. Martins-Neto (1991a) cited the presence of an Eumastacoidea in the Cretaceous of the Crato Formation in Brazil, that was never described.
Among the other Caelifera described from Menat, only “Oedipoda spec.” and Ochrilidia lineata are comparable to Paleochina gen. nov. “Oedipoda spec.” differs from Paleochina gen. nov. in the presence of three dark spots on the tegmen. Ochrilidia lineata differs from Paleochina gen. nov. is the absence of secondary zigzagged longitudinal veins between branches of RP. It also differs from Paleochina duvergeri in the distinctly shorter tegmina (ca. 16–17 mm long). The “genus and species A” described above differs from Paleochina gen. nov. in the very broad costal area in forewing. Heads (2008) described an apterous Eumastacoidea Proscopiidae from the Lower Cretaceous of the Crato formation in Brazil, showing the antiquity of this superfamily.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Elcanoidea Handlirsch, 1906
Elcanidae Handlirsch, 1906
Remarks.—The exact phylogenetic relationships of the Elcanoidea within the Orthoptera remain debatable. If Gorochov and Rasnitsyn (2002) considered them as sister group to all other Orthoptera, Béthoux and Nel (2002) considered them as Caelifera on the basis of a phylogenetic analysis.
Genus Cenoelcanus nov.
Zoobank LSID: urn:lsid:zoobank.org:act:0745091A-8429-45A2-9B26-A3AF46952577
Type species: Cenoelcanus menatensis sp. nov., by monotypy, see below.
Etymology: Named after Cenozoic and genus Elcanus.
Diagnosis.—As for the type species by monotypy.
Cenoelcanus menatensis sp. nov.
Fig. 8.
Zoobank LSID: urn:lsid:zoobank.org:act:E1F797A4-4027-472F-A4A8-7622B2EBB123
Etymology: Named after the village of Menat.
Holotype: MNHN.F.A71198 (coll. Philippe Olivier); an incomplete tegmen.
Type locality: Historical quarry of Menat, Menat, Puy-de-Dôme, France.
Type horizon: Middle Paleocene, Menat Basin.
Material.—Holotype only.
Diagnosis.—Forewing characters only. Area between R and M+CuA with a series of short crossveins; distal fusion of of CuPaβ, CuPb, and A; base of RP very close to point of separation of M and CuA; M+CuA and RP seem to be aligned; only four branches of RP.
Description.—Distal two-third of a forewing with dark areas along costal part and mid part of tegmen, preserved part 13.0 mm long, 4.2 mm wide in widest part, 3.0 mm in narrowest; posterior margin straight; only distal part of area between C and ScP preserved, 0.7 mm wide, with a series of parallel crossveins; ScP and R not touching, with a series of very short crossveins between them; apex of ScP 8.0 mm distal of that of ScA; area between C and RA 0.3 mm wide, without crossveins; apex of RA 1.3 mm from wing apex; M+CuA not appressed to R, with a series of short crossveins between them, M simple; CuPaα very weak; CuA+CuPaα simple; one row of cells between M and CuA+CuPaα; base of RP as a short oblique veinlet very close to point of separation between M and CuA, 0.15 mm apart; stem of M+CuA aligned with RP; area between RA and RP narrow, 0.4 mm wide, with one row of simple crossveins between them; RP with four posterior branches, with one row of cells between them; one row of cells between CuA+CuPaα and CuPaβ; one row of cells between M+CuA and CuPa, and between CuPa and CuPb; CuPaβ distinctly curved, and parallel to posterior wing margin in distal part; CuPb straight, area between CuPb and first anal vein very narrow; anal area not preserved.
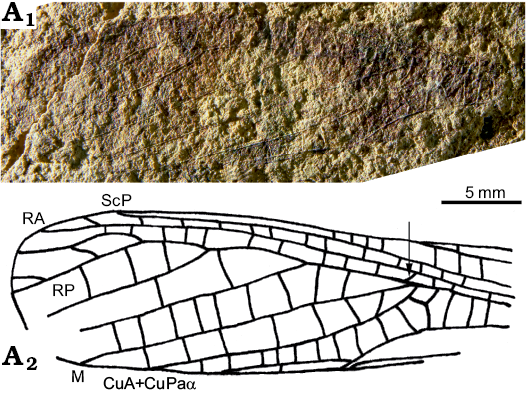
Fig. 8. Tegmen of Elcanid orthopteran Cenoelcanus menatensis gen. et sp. nov., holotype, MNHN.F.A71198 from Paleocene of Menat, France. Photograph (A1), reconstruction (A2). Abbreviations: CuA/P, cubitus anterior/posterior; CuPaα, most anterior branch of CuP; M, median vein; RA/P, radius anterior/posterior; ScP, subcostal posterior.
Remarks.—The broad area between ScP and costal margin indicates that this fossil is a tegmen and not a hindwing. The presence of long veins parallel to posterior wing margin, the truncate wing apex, the veins M and CuA separating very far from wing base, and the weak CuPaα are characters present in Caelifera. Cenoelcanus menatensis gen. et sp. nov. has a very particular character, viz. the alignment of RP with stem of M+CuA so that RP seems to be a distal branch of M+CuA. This structure is only present in the pre-Cenozoic superfamily Elcanoidea Handlirsch, 1906 (= Permelcanidae Sharov, 1962 + Elcanidae Handlirsch, 1906), and considered as the main synapomorphy of this group by Gorochov and Rasnitsyn (2002: fig. 430). The Permelcanidae would differ from the Elcanidae by the presence of a better defined origin of RP, which is clearly a plesiomorphic state of character (Sharov 1968). Cenoelcanus gen. nov. better falls in the Elcanidae because the base of RP is reduced to a short oblique veinlet. Gorochov et al. (2006) divided the Elcanidae into Elcaninae and Archelcaninae Gorochov et al. 2006, and characterized the Elcaninae on the basis of the more or less narrowed area between tegminal RA and RP, the fused (with each other) distal parts of CuPaβ, CuPb, and A, and “possibly the rather small spines of the hind tibiae”. As Cenoelcanus gen. nov. has two most important venation characters of the Elcaninae, we consider it belongs to this subfamily. The Archelcaninae would be characterized by the presence of large spines on hind tibia, unknown in our fossil.
Cenoelcanus gen. nov. differs from all the Mesozoic elcanid genera (in which the tegmina are known) by the combination of the two characters: presence of only four branches of RP and a very short vein between M+CuA and RP (Gorochov 1986; Martins-Neto 1991b; Gorochov et al. 2006; Poinar et al. 2007; Fang et al. 2015, 2018a, b). The very long ScP is a character only present in the Permo-Triassic Meselcana Sharov, 1968, which could look strange to find in the youngest representative of the Elcanidae. But a reversal is always possible. Burmelcana Peñalver and Grimaldi, 2010 and Hispanelcana Peñalver and Grimaldi, 2010 are based on nymphs in amber, impossible to compare to Cenoelcanus gen. nov. (Peñalver and Grimaldi 2010; Fang et al. 2015). A structure analogous to the “elcanoid” alignment of M+CuA and RP is present in the extant genus Charilaus Stål, 1875 (Pamphagodidae Bolívar, 1884) but in this genus, M+CuA is making a thick complex vein together with R, from which CuA, M, and RP successively separate. Thus it is certainly not homologous to the structure of the Elcanoidea and Cenoelcanus.
Stratigraphic and geographic range.—Paleocene, Menat Formation, Menat, France.
Discussion
Paleochina duvergeri sp. nov., Paleochina minuta sp. nov., and Cenoelcanus menatensis sp. nov. are the first Caelifera found in the outcrop of Menat that can be accurately placed in superfamilies. The other fossil Caelifera described by Piton (1940) are all incertae sedis, and cannot be placed in the superfamily Acridoidea. They cannot be used for future dating of this clade. Song et al. (2015) used Tyrbula russelli Scudder, 1885 (lowermost Oligocene of Florissant, Colorado; Scudder 1885, 1890) to calibrate the dating of Acrididae, but Zeuner (1944: 361) indicated that: “Scudder considered Tyrbula as an acridine genus on account of the general proportions and the clubbed antennae. Clubbed antennae, however, also occur in the Catantopinae. The smallness of the eyes speaks most emphatically against the Acridinae. The venation, which would settle the point, has not been described.” In fact, this fossil would need a complete revision.
Song et al. (2015) dated the Acrididae origin from the Latest Cretaceous and their diversification from the Paleocene–Eocene, but Song et al. (2018) dated the origin of the family from the Paleocene and the diversification of the majority of the subfamilies from the late Eocene and the Oligocene, which is more in accordance with the Oligocene age of the oldest known Acridoidea (Ataabadi et al. 2017). As these insects frequently live on grasses (Pooideae), these relatively young fossils are in accordance with the major diversification of this clade of plants that happened during the late Eocene–Oligocene (Schubert et al. 2019). The Eumastacoidea, more related to trees, are less and less frequent in the fossil record at the same time. Nevertheless, true grasses of bambusoid type are known in the “mid” Cretaceous Burmese amber (Poinar 2004), thus Acridoidea could have been Cretaceous too. They remain to be discovered. Menat paleolake is a good candidate to search after them.
Cenoelcanus menatensis sp. nov. is the youngest and first Cenozoic representative of the Mesozoic family Elcanidae. The previously youngest Elcanoidea were dated from ca. 100 Myrs in Burmese amber. This discovery shows that this clade survived the Cretaceous/Paleogene extinction event and became extinct during the Paleogene, maybe after the Paleocene. Such situations occur also in other animal clades, such as the multituberculates mammals or some Chrysopoidea (Nel et al. 2005). Li et al. (2019) supposed that some Cretaceous Elcanidae were able to swim under water using leaf-like metatibial spurs. The extant pygmy mole crickets of the genus Xya also swim under water using similar structures (Burrows and Sutton 2012). The Elcanidae and Tridactylidae co-existed during the Cretaceous and are both recorded in the “mid” Cretaceous Burmese amber. The reasons of the Cenozoic extinction of the Elcanidae and of the survival of the Tridactylidae remain unknown. Unfortunately the body and leg morphology of Cenoelcanus menatensis sp. nov. are still unknown. Future discoveries will possibly help to determine if this Paleocene “survivor” were also able to swim.
Conclusions
The orthopteran fauna of the Menat can be considered as the richest for the Paleocene period. It seems to be dominated by the Caelifera, which could correspond to a fossilization artifact because the locusts are generally more robust with thicker cuticle than the crickets and katydids. The more remarkable facts concerning this assemblage is the diversity of the Eumastacoidea and the presence of the youngest representative of the extinct supermfamily Elcanoidea, while the Acridoidea remain unrecorded. This fauna has an old still “Mesozoic” influence. Further field researches will be necessary to verify these preliminary results.
Acknowledgements
We thank two anonymous referees for their useful remarks on the first version of this paper. We sincerely thank Bernard Duverger, president of the Communauté de Commune du Pays de Menat for his kind authorization to collect fossils.
References
Ataabadi, M.M., Bahrami, A., Yazdi, M., and Nel, A. 2017. A locust witness of a trans-oceanic Oligocene migration between Arabia and Iran (Orthoptera: Acrididae). Historical Biology 31: 574–580. Crossref
Béthoux, O. and Nel, A. 2002. Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera sensu nov. Zootaxa 96: 1–88. Crossref
Bolívar, I. 1930. Monografia de los Eumastacidos (Orth. Acrid.). Trabajos del Museo Nacional de Ciencias Naturales Serie Zoológica 46: i–xxxii + 1–380.
Burr, M. 1899. Essai sur les Eumastacides, tribu des Acridoidea. Anales de la Sociedad Española de Historia Natural, Madrid 28: 75–112.
Burrows, M. and Sutton, G.P. 2012. Pygmy mole crickets jump from water. Current Biology 22: R990–R991. Crossref
Chintauan-Marquier, I.C., Legendre, F., Hugel, S., Robillard, T., Grandcolas, P., Nel, A., Zuccon, D., and Desutter-Grandcolas, L. 2016. Laying the foundations of evolutionary and systematic studies in crickets (Insecta, Orthoptera): a multilocus phylogenetic analysis. Cladistics 32: 54–81. Crossref
Cigliano, M.M., Braun, H., Eades, D.C., and Otte, D. 2019. Orthoptera Species File. Version 5.0/5.0. http://Orthoptera.SpeciesFile.org (consulted 05/09/2019) Crossref
Cockerell, T.D.A. 1909. Description of Tertiary insects. Part 6. American Journal of Science (4) 27: 381–387. Crossref
Cockerell, T.D.A. 1926. A fossil orthopterous insect formerly referred to Mecoptera. Proceedings of the Entomological Society of Washington 28: 142.
Descamps, M. 1974. Les Mnesicleines des Philippines (Orthoptera, Eumastacoidea). Bulletin du Muséum d’Histoire Naturelle (3) 184: 1653–1996.
Descamps, M. 1979. Eumastacoidea néotropicaux: diagnoses, signalisations, notes biologiques. Annales de la Société Entomologique de France (N.S.) 15: 117–155.
Desutter-Grandcolas, L., Jacquelin, L., Hugel, S., Boistel, R., Garrouste, R., Henrotay, M., Warren, B.H., Chintauan-Marquier, I.C., Nel, P., Grandcolas, P., and Nel, A. 2017. 3-D imaging reveals four extraordinary cases of convergent evolution of acoustic communication in crickets and allies (Insecta). Scientific Reports 7 (1) (7099): 1–8. Crossref
Dirsh, V.M. 1975. Classification of the Acridomorph Insects. v–vii + 1–171. E.W. Classey, Oxford.
Fang, Y., Heads, S.W., Wang, H., Zhang, H., and Wang, B. 2018a. The first Archelcaninae (Orthoptera, Elcanidae) from the Cretaceous Jehol biota of Liaoning, China. Cretaceous Research 86: 129–134. Crossref
Fang, Y., Muscente, A.D., Heads, S.W., Wang, B., and Xiao, S. 2018b. The earliest Elcanidae (Insecta, Orthoptera) from the Upper Triassic of North America. Journal of Paleontology 92: 1028–1034. Crossref
Fang, Y., Wang, B., Zhang, H., Wang, H., Jarzembowski, E.A., Zheng, D., Zhang, Q., Li, S., and Liu, Q. 2015. New Cretaceous Elcanidae from China and Myanmar (Insecta, Orthoptera). Cretaceous Research 52: 323–328. Crossref
Gorochov, A.V. [Gorohov, A.V.] 1986. Gryllida (= Orthoptera) [in Russian]. In: A.P. Rasnicyn (ed.), Nasekomye v rannemelovyh ekosistemah zapadnoj Mongolii. Trudy Sovmestnaâ Sovetsko-Mongol’skaâ Paleontologičeskaâ Èkspeditsiâ 28: 1–213.
Gorochov, A.V. 1992. New and little-known fossil crickets (Orthoptera, Grylloidea) from Eurasia. Paleontological Journal 26: 96–102.
Gorochov, A.V. 2010. New and little known orthopteroid insects (Polyneoptera) from fossil resins: Communication 3. Paleontological Journal 44: 434–450. Crossref
Gorochov, A.V. and Labandeira, C.C. 2012. Eocene Orthoptera from Green River Formation of Wyoming (USA). Russian Entomological Journal 21: 357–370. Crossref
Gorochov, A.V. and Rasnitsyn, A.P. 2002. Superorder Gryllidea Laicharting, 1781 (= Orthopteroidea Handlirsch, 1903). In: A.P. Rasnitsyn and D.L.J. Quicke (eds.), History of Insects, 293–303. Kluwer Academic, Dordrecht. Crossref
Gorochov, A.V., Jarzembowski, E.A., and Coram, R.A. 2006. Grasshoppers and crickets (Insecta: Orthoptera) from the Lower Cretaceous of Southern England. Cretaceous Research 27: 641–662. Crossref
Handlirsch, A. 1910. Canadian fossil insects. Insects from the Tertiary lake deposits of the southern interior of British Columbia, collected by Mr. Lawrence M. Lambe, in 1906. Contributions to Canadian Palaeontology 2. Canada Department of Mines, Geological Survey Branch, Memoir 12-P: 93–129. Crossref
Heads, S.W. 2008. The first fossil Proscopiidae (Insecta, Orthoptera, Eumastacoidea) with comments on the historical biogeography and evolution of the family. Palaeontology 51: 499–507. Crossref
Kedves, M. and Russell, D.E. 1982. Palynology of the Thanetian layers of Menat. The geology of the Menat Basin, France. Palaeontographica B 182 (4–6): 87–150.
Kevan, D.K.McE. and Wighton, D.C. 1981. Paleocene orthopteroids from South-Central Alberta, Canada. Canadian Journal of Earth Sciences 18: 1824–1837. Crossref
Kevan, D.K.McE. and Wighton, D.C. 1983. Further observations on North American Tertiary orthopteroids (Insecta, Grylloptera). Canadian Journal of Earth Sciences 20: 217–224. Crossref
Lewis, S.E. 1974. Four specimens of fossil grasshoppers (Orthoptera: Caelifera) from the Ruby River basin (Oligocene) of Southwestern Montana. Annals of the Entomological Society of America 67: 523–524. Crossref
Lewis, S.E. 1976. A new specimen of fossil grasshopper (Orthoptera: Caelifera) from the Ruby River basin (Oligocene) of Southwestern Montana. Annals of the Entomological Society of America 69: 120. Crossref
Lewis, S.E., Heikes, P.M., and Lewis, K.L. 1990. Entomofauna from the Clarkia site (P-33) (Miocene) near Clarkia, Idaho. Occasional Papers in Paleobiology of the St Cloud State University 4: 1–8.
Li, C.-T., Fang, Y., Zhang, H.-C., and Qi, H. 2019. Application of analytic hierarchy process in swimming ability test on metatibial spurs of Cretaceous Elcanidae (Insecta, Orthoptera). Acta Palaeontologica Sinica 2019: 114–121.
Lin, Q.-B. 1977. Insect fossils from Yunnan Province, China [in Chinese, with English summary]. In: Mesozoic Fossils from Yunnan Province, China, 2, 373–382. Academia Sinica, Science Press, Beijing.
Lin, Q.-B. 1980. Fossil insects from the Mesozoic of Zhejiang and Anhui provinces [in Chinese, with English summary]. In: Divisions and Correlations of the Mesozoic Volcano-Sedimentary Strata in Zhejiang and Anhui Provinces, 211–234. Academia Sinica Science Press, Beijing.
Lin, Q.-B. and Huang, D.-Y. 2006. Discovery of Paleocene Prophalangopsidae (Insecta, Orthoptera) in the Jiangtang Basin, Northern Tibet, China. Alcheringa 30: 97–102. Crossref
Martins-Neto, R.G. 1991a. Primeiro registrado de Eumastacoidea (Insecta, Caelifera) da Formação Santana, Cretáceo Inferior do Nordeste do Brasil. Anais da Academia Brasiliera de Ciências 63: 91–92.
Martins-Neto, R.G. 1991b. Sistemática dos Ensifera Insecta, (Orthopteroidea) da Formação Santana (Cretáceo inferior do Nordeste do Brasil). Estudos Tecnologicos 14. Acta Geologica Leopoldensia 32: 5–160.
Nel, A. 1989. Les Gyrinidae fossiles de France (Coleoptera). Annales de la Société Entomologique de France (N.S.) 25: 321–330.
Nel, A. 1991. Analyse d’Entomofaunes cénozoiques. Intérêt de la Paléoentomologie pour les Sciences de la Terre et de la Vie. 882 pp. Thèse de Doctorat (nouveau régime), Université de Reims-Champagne-Ardenne, Reims.
Nel, A. 2008. The oldest bee fly in the French Paleocene (Diptera: Bombyliidae). Comptes Randus Palevol 7 (7): 401–405. Crossref
Nel, A. and Roy, R. 1996. Revision of the fossil “mantid” and “ephemerid” species described by Piton from the Palaeocene of Menat (France) (Mantodea: Chaeteessidae, Mantidae; Ensifera: Tettigonioidea). European Journal of Entomology 93: 223–234.
Nel, A., Martinez-Delclòs, X., and Hutin, A. 2005. Mesozoic chrysopid-like Planipennia: a phylogenetic approach (Insecta, Neuroptera). Annales de la Société Entomologique de France (N.S.) 41: 29–68. Crossref
Peñalver, E. and Grimaldi, D.A. 2010. Latest occurrences of the Mesozoic family Elcanidae (Insecta: Orthoptera), in Cretaceous amber from Myanmar and Spain. Annales de la Société Entomologique de France (N.S.) 46: 88–99. Crossref
Pérez-Gelabert, D.E. 2002. Further characterization of Paleomastacris ambarinus Perez et al. (Orthoptera: Eumastacidae) from Dominican amber. Proceedings of the Entomological Society of Washington 104: 330–334.
Pérez-Gelabert, D.E., Hierro, B., Dominici, G.O., and Otte, D. 1997. New eumastacid grasshopper taxa (Orthoptera: Eumastacidae: Episactinae) from Hispaniola, including a fossil new genus and species from Dominican amber. Journal of Orthoptera Research 6: 139–151. Crossref
Petrulevičius, J.F. 1999. Insectos del Cenozoico de la Argentina. Revista de la Sociedad Entomologica Argentina 58: 95–103.
Piton, L. 1936. Les Orthoptères tertiaires d’Auvergne. Miscellanea Entomologica 37: 77–79.
Piton, L. 1937. Note complémentaire sur Menatacridium eocenium Piton. Miscellanea Entomologica 38: 5–6.
Piton, L. 1940. Paléontologie du gisement éocène de Menat (Puy-de-Dôme), flore et faune. Mémoire de la Société d’Histoire Naturelle d’Auvergne 1: 1–303.
Poinar, G.O. Jr. 2004. Programinis burmitis gen. et sp. nov., and P. laminatus sp. nov., Early Cretaceous grass-like monocots in Burmese amber. Australian Systematic Botany 17: 497–504. Crossref
Poinar, G.O. Jr., Gorochov, A.V., and Buckley, R. 2007. Longioculus burmensis, n. gen., n. sp. (Orthoptera: Elcanidae) in Burmese amber. Proceedings of the Entomological Society of Washington 109: 649–655.
Rehn, J.A.G. 1948. The acridoid family Eumastacidae (Orthoptera); a review of our knowledge of its components, featueres and systematics, with a suggested new classification of its major groups. Proceedings of the Academy of Natural Sciences of Philadelphia 100: 77–139.
Rust, J. 1999. Oldest known pteroplistine cricket and other Gryllidae (Orthoptera) from the Paleogene Fur and Olst formations of Denmark. Entomologica Scandinavica 30: 35–45. Crossref
Schubert, M., Marcussen, T., Meseguer, A.S., and Fjellheim, S. 2019. The grass subfamily Pooideae: Cretaceous–Palaeocene origin and climate-driven Cenozoic diversification. Global Ecology and Biogeography 28: 1168–1182. Crossref
Scudder, S.H. 1885. Systematische Übersicht der fossilen Myriapoden, Arachnoiden und Insekten. In: K.A. Zittel (ed.), Handbuch der Paläontologie, Abtheilung 1, Paläozoologie 2, 721–831. R. Oldenbourg, München. Crossref
Scudder, S.H. 1890. The fossil insects of North America (with notes on some European species). The Tertiary insects. Report of the United States Geological Survey of the Territories 13: 1–734. Crossref
Sharov, A.G. [Šarov, A.G.] 1968. Phylogeny of the Orthopteroidea [in Russian, translated into English in 1971: Israel program for scientific translations, Keter Press, Jerusalem]. Trudy Paleontologičeskogo Instituta, Akademiâ Nauk S.S.S.R. 118: 1–216.
Song, H.-J., Amédégnato, C., Cigliano, M.M., Desutter-Grandcolas, L., Heads, S.W., Huang, Y., Otte, D., and Whiting, M.F. 2015. 300 million years of diversification: elucidating the patterns of orthopteran evolution based on comprehensive taxon and gene sampling. Cladistics 31: 621–651. Crossref
Song, H.-J., Mariño-Pérez, R., Woller, D.A., and Cigliano, M.M. 2018. Evolution, diversification, and biogeography of grasshoppers (Orthoptera: Acrididae). Insect Systematics and Diversity 2: 1–25. Crossref
Théobald, N. 1937. Les insectes fossiles des terrains oligocènes de France. Bulletin Mensuel (Mémoires) de la Société des Sciences de Nancy 1: 1–473.
Wang, H., Fang, Y., Li, S., Hou, X., Wang, B., and Zhang, H. 2019. Restudy of the Paleocene orthopteran insect Hylophalangopsis chinensis Lin and Huang, 2006 in northern Tibet. Journal of Asian Earth Sciences 175: 93–98. Crossref
Wappler, T., Currano, E.D., Wilf, P., Rust, J., and Labandeira, C.C. 2009. No post-Cretaceous ecosystem depression in European forests? Rich insect-feeding damage on diverse middle Palaeocene plants, Menat, France. Proceedings of the Royal Society, London B276: 4271–4277. Crossref
Wedmann, S., Uhl, D., Lehman, T., Garrouste, R., Nel, A., Gomez, B., Smith, K., and Schaal, S.F.K. 2018. The Konservat-Lagerstätte Menat (Paleocene; France)—an overview and new insights. Geologica Acta 16: 1–31.
Zessin, W. 2017a. Neue Insekten aus dem Moler (Paläozän/Eozän) von Dänemark Teil 2 (Orthoptera: Ensifera: Tettigoniidae) und Bilder von den Fundstellen auf der Insel Mors, Dänemark. Virgo Mitteilungsblatt des Entomologischen Vereins Mecklenburg 19: 65–76.
Zessin, W. 2017b. Neue Insekten aus dem Moler (Paläozän/Eozän) von Dänemark Teil 3 (Orthoptera: Caelifera: Eumastacidae, Tetrigidae). Virgo Mitteilungsblatt des Entomologischen Vereins Mecklenburg 19: 77–83.
Zeuner, F.E. 1944. The fossil Acrididae (Orth. Salt.). Part 4. Acrididae incertae sedis and addendum to Catantopinae. Annals and Magazine of Natural History 11 (11): 359–383. Crossref
Acta Palaeontol. Pol. 65 (2): 371–385, 2020
https://doi.org/10.4202/app.00676.2019