

A new vesselless angiosperm stem with a cambial variant from the Upper Cretaceous of Antarctica
M. LAURA PIPO, ARI IGLESIAS, and JOSEFINA BODNAR
Pipo, M.L., Iglesias A., and Bodnar, J. 2020. A new vesselless angiosperm stem with a cambial variant from the Upper Cretaceous of Antarctica. Acta Palaeontologia Polonica 65 (2): 261–272.
We sectioned a permineralized stem preserved in marine calcareous concretions from the Campanian (Upper Cretaceous) of James Ross Island, Antarctic Peninsula using the cellulose-acetate peel technique. The material is a slender stem displaying a combination of characters such as: (i) absence of vessels and axial parenchyma, (ii) presence of a cambial variant which produces axial vascular elements in segments (AVES pattern), and (iii) elongated upright ray cells. This character combination allows us to assign this fossil to family Chloranthaceae and to relate it to an extant genus Sarcandra. Consequently we describe Sarcandraxylon sanjosense gen. et sp. nov., representing the first vegetative fossil of Chloranthaceae—a basal angiosperm family with a fossil record extending back into the Early Cretaceous and dominated by pollen grains and a limited number of reproductive mesofossils. Besides doubtfully assigned leaves, there are no reported Cretaceous macrofossils of Chloranthaceae, which hinders our understanding of the overall pattern of morphological evolution for the family. The new fossil constitutes the first fossil occurrence of the Sarcandra clade in high latitudes of Western Gondwana. The particular wood anatomy and small diameter suggest a new plant habit (sub-shrub) for the physiognomy of the Cretaceous Antarctic floras.
Key words: Angiospermae, Chloranthales, Chloranthaceae, cambial variant, Campanian, Antarctica, James Ross Island.
M. Laura Pipo [laurapipo2@gmail.com] and Ari Iglesias [ari_iglesias@yahoo.com.ar], Instituto de Investigaciones en Biodiversidad y Medioambiente (INIBIOMA, CONICET-UNCOMA), Quintral 1250, (8400) San Carlos de Bariloche, Río Negro, Argentina.
Josefina Bodnar [jbodnar@fcnym.unlp.edu.ar], División Paleobotánica, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, CONICET, Paseo del Bosque s/n, (B1900FWA) La Plata, Buenos Aires, Argentina.
Received 28 October 2019, accepted 9 January 2020, available online 4 May 2020.
Copyright © 2020 M.L. Pipo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Chloranthaceae is a basal angiosperm family with a fossil record extending back into the Early Cretaceous, with a record dominated by pollen grains and a limited number of reproductive mesofossils. Besides doubtfully assigned leaves, there are no reported Cretaceous macrofossils of Chloranthaceae, what hinders our understanding of the overall pattern of morphological evolution for the family.
The phylogenetic position of Chloranthaceae among the basal angiosperm lineages is still unresolved. This family has received considerable attention because of its primitive morphology (i.e., monosulcate pollen, lack of vessels, tracheid-vessel transitional elements, scalariform vessel perforations, small flowers often without a perianth), which suggests a close relationship with Piperales, Magnoliales, Laurales, or Trochodendrales (Burger 1977; Cronquist 1981; Walker and Walker 1984; Verdcourt 1985; Endress 1986, 1987; Carlquist 1992a; Todzia 1993). Recent molecular analyses placed Chloranthales in a basal polytomy with the magnoliid and eudicots/monocots/Ceratophyllaceae clades (APG IV 2016).
Although the Chloranthaceae has a disjunct tropical distribution with four extant genera, it has a global pollen record since the Early Cretaceous (Couper 1958; Hughes and McDougall 1994; Eklund et al. 2004; Zhang et al. 2011) which highlights that the family had a long history, and the extant restricted distribution represents a relict of a much broader one in the past (Eklund et al. 2004). Besides the presence of Clavatipollenites and Asteropollis pollen-types, the fossil record of the family is based mostly of reproductive organs mostly from the Northern Hemisphere.
The oldest evidence of Chloranthaceae from Antarctica comes from pollen grains of Clavatipollenites hughesii Couper, 1958 from the lower to middle Albian and extends through the Late Cretaceous (Dettman and Thomson 1987; Dettman 1989; Askin 1994). Beside the pollen record, only putative chloranthaceous leaves were identified from the Albian of the Alexander Island (Cantrill and Nichols 1996).
Despite its important role in the early diversification of angiosperms, little is known about the evolution of morphology and anatomy in Chloranthaceae because of the sparse fossil record. Therefore, our understanding about the secondary growth and anatomical characters is poor, making the whole plant reconstructions hardly possible.
In this work, we describe a new permineralized stem from the Santa Marta Formation (Santonian–Campanian) in the James Ross Island (Antarctic Peninsula), which represents the first Chloranthaceae vegetative fossil from the Southern Hemisphere. This stem displays a combination of characters reminiscent of the extant Sarcandra, and sheds a new light on the morphological diversification of the family during the Late Cretaceous.
Institutional abbreviations.—IAA-Pb, Paleontological and Geological Collection of the Argentine Antarctic Institute, Buenos Aires, Argentina.
Other abbreviations.—AVES, axial vascular elements in segments.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in Plant Fossil Names Registry (PFNR) (https://www.plantfossilnames.org).
Geological setting
The Santa Marta Formation corresponds to the basal unit of the Marambio Group in the James Ross Basin (Elliot 1988; Del Valle et al. 1992), and is divided into two members: the lower Alpha Member and the upper Beta Member (Olivero et al. 1986; Olivero 2012). The studied fossil plant was found at the locality informally named as “Sitio Feliz” by Iglesias (2016), near the San José Pass (Nývlt and Šerák 2009) between the Monolithic Lake and Santa Marta Cove (Fig. 1). This locality corresponds to the upper part of the Beta Member, in the upper section of the N Sedimentary Sequence (Olivero and Medina 2000). The sedimentology of the Santa Marta Formation at the fossiliferous section consists of an alternation of thick bioturbated packages of fine-grained, well-sorted sandstone, silty very fine grained sandstone, and mudstone beds, with abundant plant debris and large tree trunks. Fossil leaves, wood, and marine macrofauna were found in calcareous concretions from a single thick fossiliferous layer. The exceptional fossil preservation in its massive and chaotic structure may be due to a rapid burial event in anoxic environment (Iglesias 2016).
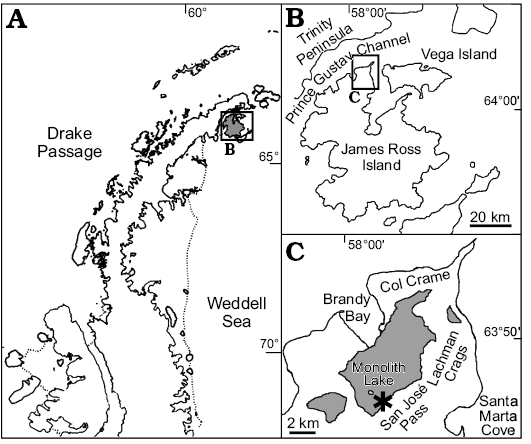
Fig. 1. A. Location of the new fossil locality in Antarctic Peninsula, James Ross Island (gray). B. Map of James Ross Island at the northern Antarctic Peninsula. C. Map with the Santa Marta Formation outcrops (area shaded in gray) at the northern James Ross Island, showing location of the new fossil locality (asterisk). Modified from Olivero 2012 and Carvalho et al. 2013.
Geological studies interpreted the Santa Marta Formation as facies of delta slopes and channel complexes of a progradational deepwater delta system in a marine shelf (Scasso et al. 1991; Olivero 2012). During its deposition, the sediments were delivered to deltaic systems by major rivers flowing to the east-southeast, being the main clastic source and preserved continental biota, drained by deltaic currents (Olivero et al. 2008). Geological and paleontological studies suggested that leaves and trunks were transported from western continental areas of the Antarctic Peninsula (Scasso et al. 1991).
Earlier contributions indicated that the Santa Marta Formation would have been deposited during the Santonian–Campanian interval on the basis of mollusk, foraminifer, and palynomorph associations (e.g., Crame 1983; Dettman and Thomson 1987; Keating 1992; Olivero 2012; Carvalho et al. 2013; Florisbal et al. 2013). Strontium isotopes (87Sr/86Sr ratio ~0.70745) analyses, indicated a late Coniacian–late Campanian age at the Santa Marta Formation (McArthur et al. 2000). Based on the stratigraphy, and according to the presence of the ammonoid association 5 (kossmaticeratid amonoids: Natalites cf. morenoi Riccardi, 1983 and Natalites taylori Spath, 1953) of Olivero (1992), the fossiliferous level from which the studied stem occurred would correspond to the early–middle Campanian (~83–77 Ma).
Palaeobotanical background
Palaeobotanical research from the Santa Marta Formation consists of several studies on permineralized woods (e.g., Poole and Francis 1999, 2000; Poole et al. 2000a, b; Poole and Gottwald 2001; Cantrill and Poole 2005; Pujana et al. 2017, 2018), pollen grains (e.g., Dettmann and Thomson 1987; Keating 1992; Barreda et al. 2019), and leaf compressions (e.g., Hayes 1999; Hayes et al. 2006; Kvacek and Sakala 2011; Iglesias 2016). Previous studies about the floral assemblages from this formation, suggested a mixed canopy forest with an increasing dominance of eudicots, although magnoliids (e.g., Cunoniaceae, Nothofagaceae, Laurales) and conifers (Araucariaceae and Podocarpaceae) were still frequent; that would developed in a back-arc basin of an emergent volcanic arc (Elliot 1988).
Particularly, from the upper part of the Beta Member, Iglesias (2016) described the first Campanian compression flora for Antarctica; composed of cycads (Zamiaceae), conifers (Araucaria, Araucarites, Brachyphyllum, and Pagiophyllum), several ferns (including Pteridaceae and ?Schizaeaceae), and angiosperms (including ?Cunoniaceae and Lauraceae). At the same locality, a new type of fossil preservation has been found, which consists of permineralized plant remains inside carbonate concretions, comprising vegetative and reproductive organs with three-dimensional organic connections. The complete flora has been described in the Ph.D. thesis of the senior author (MLP) who reported several leaves, seeds, wood, and twigs. The results have been only partially published yet (Pipo and Iglesias 2017; Pipo et al. 2018, 2019).
The calcified fossils contain also crystals of framboidal pyrite, interpreted as a proxy of anaerobic oxidation of methane by a consortium of archaea that reduce the dissolved sulfates and induce the precipitation of carbonates and iron sulphide (Hinrichs and Boetious 2002). Although the fossil angiosperm stem from this study is fragmentary, nevertheless it retained the original shape and the almost intact anatomy with good preservation of both xylem and bark. Nonetheless the pyritization poses a problem in more detailed studies of tracheid wall characters (e.g., pits, cross-fields, wall thickenings) at light microscopy or SEM.
Material and methods
Fossil preparation.—Anatomical sections (transverse, tangential, and radial) were obtained from the calcareous concretion using the cellulose-acetate peel technique (Joy et al. 1956), in this case using hydrochloric acid (5–10%). The peels were mounted using Trabasil® NR2 mounting medium on glass slides (Noetinger et al. 2017) and studied with Olympus CH30, Olympus BX50 epifluorescence microscope (long pass green filter) from the INIBIOMA sede Salmonicultura, Argentina; a compound microscope Nikon SMZ800 from the INIBIOMA; and a FEI Nova Nano SEM-230 from the Servicio de Microscopía, Centro Atómico Bariloche (CNEA), San Carlos de Bariloche, Argentina. Photographs were taken with a Leica DMC2900 and Nikon Digital Sight DS-Fi1 digital cameras. For SEM observation, a fragment of the stem was isolated from the sample rock using DremelR4000 rotary tool equipped with a diamond wheel, mounted in an aluminum SEM stub, and platinum/palladium coated.
The plant material is deposited in the Repositorio de colecciones paleontológicas y geológicas del Instituto Antártico Argentino, Ciudad Autónoma de Buenos Aires, Argentina.
Description and systematic treatment.—For the description of the wood anatomy we used IAWA List of Microscopic Features for Hardwood Identification (IAWA Committee 1989). A minimum of 30 measurements of each character were made, except for protoxylem cells that only 18 measurements could be taken. For systematic APG IV (2016) was followed; and for wood comparisons the InsideWood database (2004-onward; http://insidewood.lib.ncsu.edu/search) and the database of Japanese woods in the Forestry and Forest Products Research Institute web page (http://db.ffpri.affrc.go.jp/WoodDB/JWDB-E/home.php accessed in 2019) were used.
We apply the term “typical cambial activity” when the cambium is formed by one continuous ring of cambial cells (i.e., radial and fusiform initials) that produces both continued secondary xylem centripetally and phloem centrifugally. Every other cambial activity different from the previous is called as “cambial variant” (Carlquist 2001a). In the description of cambial variants, the terminology of Angyalossy et al. (2014) is used.
Systematic palaeobotany
Order Chloranthales Martius, 1835
Family Chloranthaceae Robert Brown ex Sims, 1820 nomen conservandus
Genus Sarcandraxylon nov.
PFNR: PFN001016.
Etymology: Combination of Sarcandra, a extant Chloranthaceae genus, and wood ending greek; in reference its similarity of stem anatomy.
Type species: Sarcandraxylon sanjosense sp. nov.; see below.
Diagnosis.—Stem has wide, circular and parenchymatous pith. The primary vascular system is formed by one cycle of collateral vascular bundles, with endarch protoxylem. Secondary vascular system developed by a cambial variant resulting in segments of vascular tissue separated by wide interfascicular multiseriate parenchyma rays. Secondary xylem is vesselless without axial parenchyma.
Stratigraphic and geographic range.—Upper Beta Member, Santa Marta Formation, Marambio Group, James Ross Basin at San Jose Pass, northern James Ross Island, Antarctic Peninsula, early–middle Campanian (~83–77 Ma), Late Cretaceous.
Sarcandraxylon sanjosense sp. nov.
PFNR: PFN001017.
Etymology: From the San José pass (James Ross Island), a nearest locality where the fossil was found.
Holotype: IAA-Pb 621, a stem 3 mm long and 5 mm wide in a rock sample mounted on SEM stub; six cellulose-acetate peels mounted in glass slides (three in transverse section; and three in tangential section).
Type locality: San Jose Pass (equivalent to CF cite from Iglesias 2016), northern James Ross Island, Antarctic Peninsula (63°54’14” S; 57°54’40” W; Fig. 1).
Type horizon: Early–middle Campanian (~83–77 Ma), Late Cretaceous, Upper Beta Member, Santa Marta Formation, Marambio Group, James Ross Basin.
Diagnosis.—Secondary vascular cylinder with interfascicular rays and axial elements in segments. Homocellular and heterogenous interfascicular rays consisting of square and procumbent parenchyma cells. The secondary xylem segments contain tracheids and uniseriate homocellular and homogeneous rays with elongated upright cells. Growth rings are indistinct. Secondary phloem with fibers arranged in caps. Bark composed by dead cells of secondary phloem and cork cells which are accumulated in a multilayered rhytidome.
Description.—The slender stem is 5 mm in diameter, circular in transverse section (Fig. 2A). Although its periphery is not completely preserved, the whole shape remains almost intact. The central circular pith is 1.87 mm in diameter. It is composed of large isodiametric parenchyma cells (40–70 µm; mean 50.36 µm). The primary vascular system is formed by one cycle of collateral vascular bundles, only 19 could be counted due to preservation (although may reach almost 30 in the whole), with endarch protoxylem (cell sizes 2.5–5 µm), and metaxylem cells size 5–7.5 µm (mean diameter 5.92 µm) (Fig. 2B). The secondary vascular cylinder is radially dissected, due to a cambial variant derived from one vascular cambium with differential activity, in which the interfascicular cambium only produces ray parenchyma cells resulting in axial vascular elements in segments (AVES pattern). The secondary xylem lacks vessels, and is composed by tracheids (cellular diameters range 12.5–25 µm; mean diameter 17.9 µm) and uniseriate homocellular and homogenous rays. These fascicular rays are two cells high and composed of elongated upright parenchyma cells (Figs. 2C, E, 3A, C, D). Growth rings and axial parenchyma are absent. The radial thickness of xylem segments is up to 202.5 µm. The interfascicular rays are broad (67 µm), multiseriate (5–6, up to 8 cells wide), homocellular, and heterogenous; consisting of square and procumbent parenchyma cells (5–10 µm; mean 7.33 µm) (Fig. 3B). The secondary phloem is arranged in half-moon shaped bundles and wide interfascicular rays (Fig. 2D). Phloem cells are poorly preserved; only some collapsed cells were identified, leaving a large central gap from the center to the base of bundles. Caps of phloem fibers are present externally in the bundles, with a mean size of 26.25 µm per 68.75 µm. The fibers are hard to measure because they are small and poorly preserved. The bark is composed by dead cells of secondary phloem, and cork cells which are accumulated in a multilayered rhytidome (mean bark growth 50 µm). It has a sinuous shape, with external shallow valleys in the interfascicular areas of phloem, and pronounced ribs over the phloem fiber caps (Fig. 2D).
Stratigraphic and geographic range.—Type locality and horizon only.
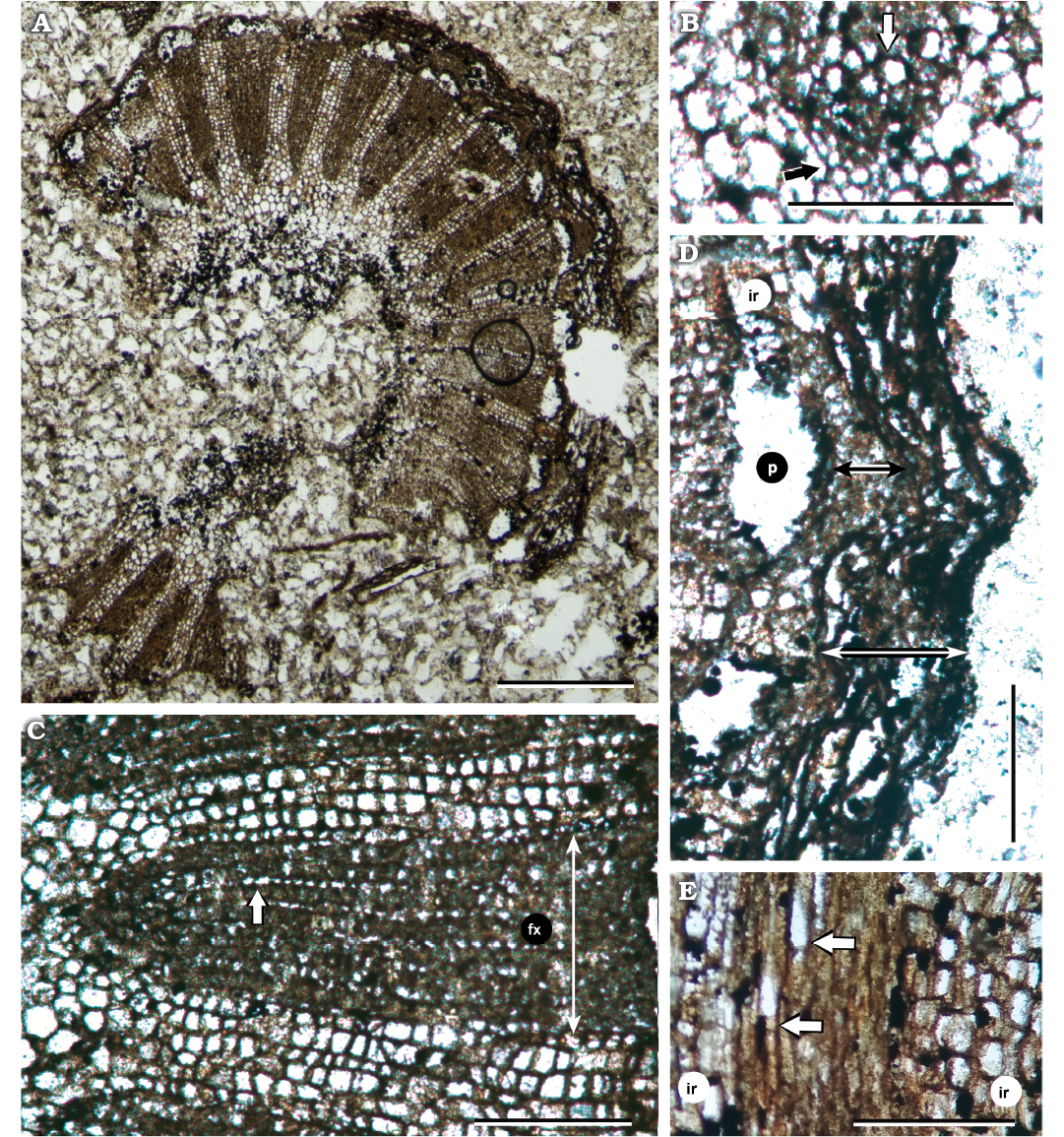
Fig. 2. Light micrographs of the stem of chloranthacean angiosperm Sarcandraxylon sanjosense gen. et sp. nov. (IAA-Pb 621), San José Pass, Antarctica, early–middle Campanian. A. Transverse section of the complete stem with pith, secondary xylem, phloem, and bark. B. Protoxylem (black arrow) and metaxylem (white arrow) in a vascular bundle. C. Secondary xylem in transverse section, pith in the left (white arrow), two interfascicular rays and fascicular secondary xylem (fx) intercalated. White arrow pointing uniseriate fascicular ray. D. Detail of bark and secondary phloem. Note phloem with AVES pattern as well. Phloem cells not preserved (p) and phloem fibers cap (black double head arrow) separated by interfascicular rays (ir). Bark (white double head arrow) with a continued thickened layer, forming shallow ribs. E. Tangential section of the stem, arrows pointing fascicular uniseriate rays; at both sides interfascicular rays (ir). Scale bars: 50 µm; except A, 1 mm.
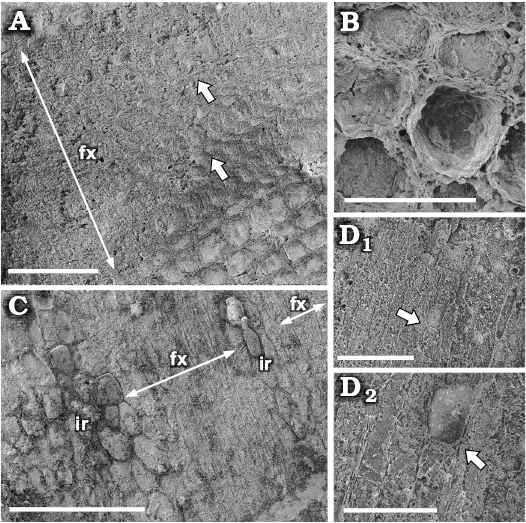
Fig. 3. SEM images of the secondary xylem of the chloranthacean angiosperm Sarcandraxylon sanjosense gen. et sp. nov. (IAA-Pb 621), San José Pass, Antarctica, early–middle Campanian. A. Transverse section of the stem, showing one vascular bundle in the middle, pith toward the right, white arrows pointing uniseriate fascicular rays. B. Parenchymatic cells of an interfascicular ray in transverse section showing the main shape and piritization of the cell walls. C. Secondary xylem in longitudinal tangential section, showing fascicular xylem (fx) and interfascicular rays (ir). D. Longitudinal section of fascicular xylem; D1, showing tracheids and a two cells tall uniseriate ray (white arrow) with elongate upright cells; D2, detail showing cell walls partially replaced with framboidal pyrite (white arrow). Scale bars: A, D, 100 µm; B, 40 µm; C, 200 µm; E, 50 µm.
Discussion
Systematic affiliation.—The stem of the new Antarctic taxon possesses a very distinctive anatomical combination of character states. The absence of vessels in the xylem is one of the unique features of the general gymnosperm and some basal angiosperm woods. However, among them, only few have cambial variants as the new Antarctic taxon in this study. Among gymnosperms, the groups that present both cambial variants and vesselless wood, are cycads (Greguss 1968) and some orders of seed ferns as Medullosales, Corystospermales, Pentoxylales, Hermanophytales (Arnold 1962; Bose et al. 1985; Tidwell and Ash 1990; Tidwell 2002; Bodnar 2012; Bodnar and Coturel 2012). Cycad stem anatomy does not resemble that of the new taxon, since cycads are characterized by the presence of mucilage canals in pith, rays, and bark; medullary bundles; also they possess fascicular multiseriate wood rays and cambial variants that produce different vascular patterns than AVES (e.g., successive cambia; Greguss 1968; Cantrill 2000; Martínez et al. 2012).
Seed ferns, on the other hand, developed a broad diversity of cambial variants, such as successive cambia, differential cambial activity, multiple cambia, remnant activity of the cambium, and centripetal cambium (Bodnar 2012, and references therein). Among them only two orders present similar AVES pattern as the one found in the new taxa here described: Hermanophytales and Umkomasiales (=Corystospermales). However, in Hermanophytales the interfascicular rays are heterocellular, including parenchyma, fibers, and vascular traces (Arnold 1962; Tidwell and Ash 1990; Tidwell 2002). Within the Umkomasiales, the genera Kykloxylon, Cuneumxylon, Tranquiloxylon, and Rhexoxylon show a stem anatomy with xylem wedges separated by wide interfascicular rays (Artabe and Brea 2003; Decombeix et al. 2014); but in the first three genera these rays are not connected to the pith (Herbst and Lutz 1995; Artabe and Brea 2003) differing from Sarcandraxylon gen. nov. In Rhexoxylon the secondary growth is far more complex, with two vascular systems (i.e., medullary and cylindrical) and frequently presence of successive cambia (Bodnar 2008, and references therein). Furthermore, in the Umkomasiales, the interfascicular rays contrast with the homocellular rays of the new Antarctic taxon, since they are composed of a variety of cell types (parenchyma, sclereids, idioblasts), and abundant secretory cavities (Bodnar 2008).
Within angiosperms, the AVES pattern is found in some representatives of the ANA-grade (Austrobaileyales), eumagnoliids (Piperales), Chloranthales, and certain families of the core eudicot as Apocynaceae, Asteraceae, Begoniaceae, Cucurbitaceae, Dilleniaceae, Menispermaceae, Ranunculaceae, and Vitaceae (Angyalossy et al. 2014; Trueba et al. 2015). However, the lack of vessels is an uncommon feature in angiosperms, and among all the previous groups indicated above, the only family that has representatives with both AVES pattern and vesselless wood is Chloranthaceae (see further discussions below in “Wood anatomy in an evolutionary perspective” section).
Chloranthaceae includes four extant genera (Ascarina J.R. Forster and G. Forster, 1777, Chloranthus Swartz, 1787, Hedyosmum Swartz, 1788, and Sarcandra Gardner, 1846), nonetheless only Chloranthus and Sarcandra possess the AVES pattern, and only the latter has been reported as having vesselless stems as well (Swamy and Bailey 1950).
The Sarcandra wood is known by having scanty axial parenchyma, and mostly composed of tracheids and transitional tracheid-vessels elements in stems (Fig. 4). It shares with the Antarctic stem the uniseriate and homogenous fascicular rays composed of elongated upright cells, but the multiseriate (4–5 cells wide) interfascicular rays are homogenous in the extant genus, composed by upright cells. Sarcandra has secondary phloem arranged in AVES pattern with wide interfascicular rays and phloem fibers arranged in caps (Swamy and Bailey 1950; Swamy 1953; Carlquist 1987) as in the new fossil.
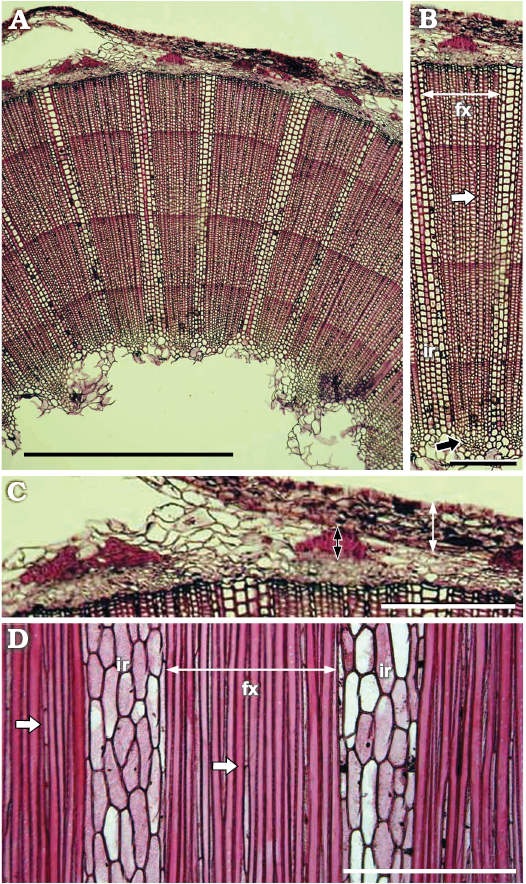
Fig. 4. Light micrographs of the stem of extant chloranthacean angiosperm Sarcandra glabra (Thunberg, 1794) Nakai, 1930. Images taken from the database of Japanese woods of the Forestry and Forest Products Research Institute (http://db.ffpri.affrc.go.jp/WoodDB/JWDB-E/home.php; accessed in 2019). A. Transverse section of the stem, pith almost entirely missing, secondary xylem with growth rings, secondary phloem and bark. B. Secondary xylem in transverse section, two interfascicular rays (ir) and fascicular secondary xylem (fx) intercalated. White arrow pointing uniseriate fascicular ray. Black arrow pointing protoxylem. C. Detail of bark and secondary phloem. Note phloem with AVES pattern as well. Phloem cells preserved and phloem fibers cap (black double headed arrow) separated by interfascicular rays. Bark (white double headed arrow) with a continued thickened layer. D. Tangential section of the stem, arrows pointing fascicular uniseriate rays in fascicular xylem (fx). Interfascicular rays (ir) intercalated. Scale bars 50 µm, except A, 1 mm.
Although sharing most anatomical characters with Sarcandra stems (Table 1), no transitional vessel elements were able to distinguish in the xylem of the Antarctic stem. Furthermore, the Antarctic specimen differs from Sarcandra due to the absence of distinct growth rings (even the fossil is larger than most of extant Sarcandra twigs), and the presence of square and procumbent parenchyma cells in the interfascicular rays. As the studied stem differs to all extant representatives of Chloranthaceae we designate it to a new genus and species.
Table 1. Summary of Sarcandraxylon sanjosense gen. et sp. nov. wood characters compared with some basal angiosperms. TDT, tangential diameter of tracheids; + present; – absent; ? unkown; * cambial variant in Piper betle produce two vascular boundle cycles; ** vessels in primary xylem of stem and secondary xylem in roots. Transitional tracheids were also noted. Fascicular rays include rays in taxa that not develop AVES pattern, and taxa with AVES pattern.
|
Species |
References |
AVES pattern |
Growth rings |
Vessels |
Imperforate tracheary elements |
TDT |
Pitting |
Axial |
Fascicular rays |
Interfascicular rays |
Ray |
Ray |
|
Amborella trichopoda |
– |
+ |
– |
+ |
? |
scalariform |
diffuse |
two distinct sizes |
– |
upright to square cells. Procumbent cells in wider rays |
multiseriate: |
|
|
Austrobaileya scandens |
+ |
– |
+ |
+ |
? |
fibers: bordered pits; vessels perforation plates scalariform, pits scalariform, opposite and alternate |
vasicentric |
uniseriate and narrow multiseriate |
wide multiseriate |
upright |
multiseriate: 2–3 cells wide |
|
|
Saururus cernuus |
+ |
– |
+ |
+ |
? |
fiber-tracheids with bordered pits; vessels with scalariform perforation plates |
? |
– |
wide multiseriate |
? |
? |
|
|
Piper betle |
+ * |
– |
+ |
+ |
10–15 |
bordered pits |
+ |
– |
wide multiseriate |
upright and procumbent |
? |
|
|
Aristolochia spp. |
+ |
– |
+ |
+ |
? |
bordered pits |
diffuse, diffuse in agregates, scantly vasicentric and banded apotracheal |
– |
wide multiseriate |
uniseriate: upright; multiseriate: upright and procumbent |
? |
|
|
Tasmannia spp. |
– |
+ |
– |
+ |
16–46 |
bordered and scalariform |
scarse, |
two distinct sizes |
– |
uniseriate: upright; multiseriate: upright and procumbent |
multiseriate: |
|
|
Chloranthus spp. |
+ |
– |
+ |
+ |
mean 21 |
fiber-tracheids: bordered pits; vessels: scalariform perforation plate, bordered pit |
– |
uniseriate and |
wide multiseriate |
upright, prominently elongated |
interfascicular multiseriate:
|
|
|
Sarcandra glabra |
see |
+ |
+ |
– ** |
+ |
15–20 (mean 17.5) |
bordered and scalariform |
– |
uniseriate |
wide multiseriate |
uniseriate: upright elongated; multiseriate: upright |
interfascicular multiseriate:
|
|
Sarcandraxylon
|
this paper |
+ |
– |
– |
+ |
12.5–25 (mean 17.9) |
? |
– |
uniseriate |
wide multiseriate |
uniseriate: upright elongated; multiseriate: square and procumbent |
interfascicular multiseriate: 5–6, up to 8 cells wide |
The fossil record of Chloranthaceae.—Chloranthaceous fossils have been found on almost all continents, with a continuous record since the Early Cretaceous. The origin of the family was inferred as Barremian–Hauterivian, based on the presence of Clavatipollenites-type pollen (Couper 1958; Kemp 1968; Doyle 1969; Hughes and McDougall 1987; Crane 1989; Friis et al. 1999).
Eklund et al. (2004), and Doyle and Endress (2014) summarized the fossil record with affinities to the family, which comprises pollen grains of Clavatipollenites and Asteropollis. Reproductive mesofossil genera come from the Northern Hemisphere (Fig. 4) and related to the Asteropollis plant and Canrightia from the Barremian–Aptian of the Western Portuguese Basin, Portugal (Hedlund and Norris 1968; Friis and Perdersen 2011); Chloranthistemon from the Santonian–Campanian of the Kristianstad Basin, Sweden, and the Turonian of the Potomac Group, USA (Crane et al. 1989); Couperites from the Cenomanian of the Potomac Group, USA (Pedersen et al. 1991); and Zlatkocarpus from the Cenomanian of the Bohemian Cretaceous Basin, Czech Republic (Kvacek and Friis 2010; Doyle and Endress 2014).
In the Southern Hemisphere the chloranthoid fossil record is abundant but not as diverse as in the Northern Hemisphere; and exclusively based on pollen grains from Australia, Argentina, and Antarctica (Fig. 5). Dettman (1994) recognized the presence of Clavatipollenites hughesii in the Barremian to the Campanian in the Eromanga and Gippsland basins, and Asteropollis asteoroids (Hedlund and Norris 1968) in the early Albian from the Eromanga and Surat basins all for Southern Australia. In the Cretaceous of Patagonia the pollen record is widespread (Archangelsky et al. 2009), although showing a broader morphological spectrum thus not necessarily closely related with Chloranthaceae (Perez-Loinaze et al. 2015). The oldest record for Clavatipollenites is from the late Barremian–Aptian, at the Deseado Massif and Austral basins; whereas the oldest record for Asteropollis is from the Aptian, in the Deseado Massif, Neuquén, and San Luis basins (Archangelsky et al. 2009, and references therein). Particularly, in the early Aptian of the Deseado Massif Basin, Archangelsky and Taylor (1993) reported small dispersed anthers with in situ Clavatipollenites pollen grains, being the first record of a chloranthoid reproductive mesofossil for the whole Southern Hemisphere (Fig. 5).
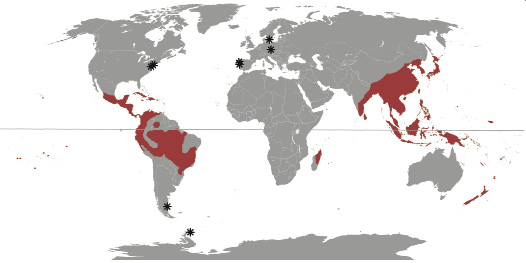
Fig. 5. World distribution of extant Chloranthaceae (red area) and sites where macro/meso fossils of the family were found (asterisks): Couperites (USA), Chloranthistemon (Sweden and USA), Asteropollis plant and Canrightia (Portugal), Zlatkocarpus (Czech Republic), loose anthers (Argentina), and Sarcandraxylon gen. nov. (Antarctic Peninsula).
In Antarctica the oldest chloranthoid pollen record was found in the early to middle Albian Kotick Point Formation, Gustav Group, at the James Ross Island; and corresponds to Clavatipollenites hughesii (Dettman and Thomson 1987; Dettman 1989). The Antarctic pollen record extends through the Upper Cretaceous, with Campanian records in the Marambio Group (Santa Marta Formation) at the James Ross Island (Dettman and Thomson 1987), and in the Maastrichtian Lopez de Bertodano Formation at the Seymour Island (Askin 1994). There are putative Antarctic macrofossils, based on leaves with chloranthoid teeth, found in the early Albian at the Alexander Island (Cantrill and Nichols 1996), although diagnostic leaf characters used are disputed, due that similar teeth and main leaf venation are present in others angiosperm groups (Hickey and Wolf 1975; Doyle and Endress 2000).
The fossil genera presented above were related to different extant Chlorantaceae genera: Clavatipollenites, Couperites, and Zlatkocarpus to Ascarina; Chloranthistemon to Chloranthus; and Asteropollis and Canrightia to Hedyosmum (Eklund et al. 2004, and references therein). The Pennipollis plant (Friis et al. 2000) was cautiously assigned to Chloranthaceae, but the phylogenetic analysis of Doyle (2014) related it to Ceratophyllaceae.
In this manner, the fossil studied herein corresponds to the first record of a vegetative organ unequivocally assignable to Chloranthaceae, and the first anatomically preserved stem assignable to the family. It also agrees with pollen interpretation, which indicates the presence of the family in the Cretaceous for Antarctica. Furthermore, the similar morphology to the extant genus reveals the first occurrence of a Sarcandra-related taxon by the Campanian in high latitudes from Western Gondwana; other than Ascarina clade-relationship due to the phylogenetic relationship claimed for the Clavatipollenites hughesii pollen grains, for the same formation.
Wood anatomy in an evolutionary perspective.—There are two unusual characters combined in the wood anatomy of Sarcandraxylon sanjosense gen. et sp. nov. that make it unusual among angiosperms: the absence of vessels and the presence of AVES pattern.
Although vessels are considered to be a hallmark of flowering plants, some angiosperm groups lack them and produce wood entirely composed of tracheids. Vessels are defined by the absence of pit membranes, short length and wide diameter, interconnection of a large number of vessel elements, and by differentiated perforation plates (Frost 1930). Tracheids, on the other hand, are characterized by great length, small diameter, angularity of outline, presence of primary wall in the pit membrane, and absence of a distinct end wall (Frost 1930). The extant woody vesselless angiosperm families are Amborellaceae, Winteraceae, Trochodendraceae, and Chloranthaceae (Fig. 6).
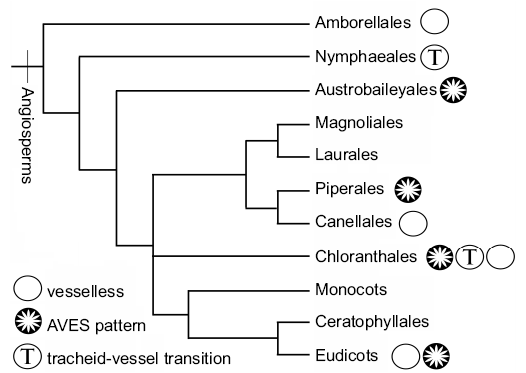
Fig. 6. Simplified phylogenetic representation (modified from APG VI 2016), with the three main atypical morphological characters discussed: presence of AVES pattern, absence of vessels, and presence of transitional tracheid-vessel elements.
Over the past century there has been much discussion about the evolution of secondary xylem in angiosperms, with respect to the origin of vessels (Thompson and Bailey 1916; Bailey 1944; Young 1981; Carlquist 1987) if it was primitively vesselless, in which case vessels were gained repeatedly in every group within angiosperms, or if vessels came first in the angiosperms stem-group and were lost in Amborellaceae, Winteraceae, Trochodendraceae, and Chloranthaceae. After this, a new element was found and added to the equation: the tracheid-vessel transitional elements. These tracheids show extensive pitting where the end of the cells overlap and the primary wall of the pits is partially degraded (Carlquist 1987; Takahashi 1988; Takahashi and Tamura 1990).
In the first anatomical researches of Sarcandra (Swamy and Bailey 1950; Swamy 1953) the secondary xylem was described as vesselless. Afterwards, Carlquist (1987) observed the presence of vessels in the secondary xylem of roots and in the primary xylem of stems, and transitional tracheid-vessel elements in the secondary xylem. Also, based on SEM studies of wide tracheary elements with scalariform patterns on end walls, Carlquist (1987) detected the presence of different amount of degradation in the pit membrane. This kind of porosity in the pit membranes was found in tracheids of several basal angiosperms as well as: Amborella, some representatives of Winteraceae, Tetracentron, Illicium, Schisandraceae, and in Austrobaileya (Carlquist 1987). Even though no transitional elements were able to distinguish in Sarcandraxylon gen. nov., the above discussion shows that several genera originally described as vesselless actually show transitional vessel elements, probably illustrating early stages in angiosperm vessel evolution (Carlquist and Schneider 2001, and references therein). Due the pyritization of the cell walls in the new fossil material, we cannot refuse the total absence of transitional elements in it.
The organization of secondary vascular system found in Sarcandraxylon sanjosense gen. et sp. nov. is the result of a cambial variant derived from one vascular cambium with differential activity which produces axial vascular elements in segments (AVES) pattern. In this cambial variant the interfascicular cambium produces only multiseriate rays and the fascicular cambium produces axial elements and, in some cases, also rays (Angyalossy et al. 2014).
This type of cambial variant is present in different groups within the flowering plants (Fig. 6), being quite well represented in basal angiosperms. The order Piperales is characterized by its presence of this cambial variant, producing one cycle of vascular bundles (Aristolochiaceae and Saururaceae) or several (Piperaceae) (Carlquist et al. 1995; Trueba et al. 2015). Within Austrobaileyales, the genus Austrobaileya, was described as having two kinds of rays: uniseriate and multiseriate (Bailey and Swamy 1949; Carlquist 2009), which is compatible with AVES pattern. In Chloranthales, the genera Sarcandra and Chloranthus differ from Hedyosmum and Ascarina by the presence of the cambial variant (Carlquist 1992a, b, 2009).
The AVES pattern is also present in several families within the eudicots core as: Apocynaceae, Asteraceae, Begoniaceae, Cucurbitaceae, Dilleniaceae, Menispermaceae, Ranunculaceae, and Vitaceae (Angyalossy et al. 2014). Given the variety of groups developing this cambial variant it seems that this kind of secondary growth is successful in different habitats and growth habits. For example, it is present in many groups of climbing plants. The proliferation of parenchyma is thought to be an adaptation to this growth habit, which requires flexibility at later stages of development, additionally the parenchyma provides torsional resistance to stems and, because of their latent meristematic activity, can repair stems that have become fractured or split (Spicer 2016, and references therein). The AVES pattern also occurs in some sub-shrubs (e.g., Sarcandra) with restricted wood production. There are not clear explanations of the anatomical benefits of this cambial variant for sub-shrub habit.
It has been proposed that early-diverging lineages of angiosperms possessed an active bifacial cambium being the reduction of secondary growth in sub-shrubs and strictly herbaceous habits a result from a reduction of cambial activity and the limitation of axial elements into the fascicular areas (Carlquist 2009; Trueba et al. 2015). At least in some seed plants, the formation of fascicular cambium precedes the interfascicular cambium (Nieminen et al. 2015); thus the delayed or decelerated activity of the interfascicular cambium could respond to heterochronic processes (Bodnar et al. 2018). The new taxon described in this study adds a new element to the flora that grew in the early–middle Campanian in the Antarctic Peninsula. The small stem diameter, the anatomical characters sharing with the extant Sarcandra, and the absence of growth rings, let us interpret that the new fossil genus corresponds to a sub-shrub, probably living in the understory. This discovery strengthens the hypotheses that the basal angiosperms would have presented different stages in the xylem evolution (Carlquist and Schneider 2001; Field et al. 2004) already by the Cretaceous.
Conclusions
Sarcandraxylon sanjosense gen. et sp. nov. is the first fossil of an anatomically preserved stem of Chloranthaceae. Although the presence of Clavatipollenites hughesii pollen in the Santa Marta Formation has already suggested the presence of this family in the Antarctic continent, that pollen species was related to the extant Ascarina clade. The new permineralized stem with preserved wood and bark has so similar wood anatomy to the extant genus that reveals the first occurrence of a Sarcandra-related taxon by the Campanian in high latitudes from Western Gondwana.
The finding of a cambial variant in a Late Cretaceous sub-shrub plant, proposes a revaluation about woodiness and cambial evolution among early angiosperms.
Acknowledegments
We would like to thank Instituto Antártico Argentino (IAA) and Fuerza Aérea Argentina for logistic in the Antarctic expedition. To Marcelo A. Reguero, José O´Gorman, Marta Fernández, Juan J. Moly (all Universidad Nacional de La Plata, La Plata, Argentina), and Rodolfo Coria (Museo Carmen Funes, Plaza Huincul, Argentina) for field assistance. We thank Eduardo Lucio (Museo de Piedras de El Bolsón, El Bolsón, Argentina) for the use of rock sectioning lab; the INIBIOMA sede Salmonicultura for the use of fluorescence microscopy, and Paula Troyon and Manuel Corte from the Servicio de Microscopía y Rayos X (Centro Atómico Bariloche, San Carlos de Bariloche, Argentina) for SEM imaging. We are grateful to Brian A. Atkinson (Kansas University, Lawrence, Kansas, USA) for the early review and comments on the manuscript. We also would like to thank Nathan Jud (William Jewell College, Liberty, MO, USA) and an anonymous reviewer for their comments that improved the manuscript. Research was founded by grant PICTO-2010-0093 to Marcelo A. Reguero, and CONICET Ph.D. grant to MLP.
References
Angyalossy, V., Pace, M.R., and Lima, A.C. 2014. Liana anatomy: a broad perspective on structural evolution of the vascular system. In: S.A. Schnitzer, F. Bongers, R.J. Burnham, and F.E. Putz (eds.), Ecology of Lianas, 253–287. John Wiley & Sons Ltd. Chichester. Crossref
APG IV 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society 181: 1–20. Crossref
Archangelsky, S. and Taylor, T.N. 1993. The ultrastructure of in situ Clavatipollenites pollen from Early Cretaceous of Patagonia. American Journal of Botany 80: 879–885. Crossref
Archangelsky, S., Barreda, V., Passalia, M.G., Gandolfo, M., Pramparo, M., Romero, E., Cuneo, R., Zamuner, A., Iglesias, A., Llorens, M., Puebla, G.G., Quattrocchio, M., and Volkheimer, W. 2009. Early angiosperm diversification: evidence from southern South America. Cretaceous Research 30: 1073–1082. Crossref
Arnold, C.A. 1962. A Rhexoxylon-like stem from the Morrison Formation of Utah. American Journal of Botany 49: 883–886. Crossref
Artabe, A.E. and Brea, M. 2003. A new approach to Corystospermales based on Triassic permineralized stems from Argentina. Alcheringa 27: 209–229. Crossref
Askin, R.A. 1994. Monosulcate angiosperm pollen from the Lopez de Bertodano Formation (Upper Campanian–Maastrichtian–Danian) of Seymour Island, Antarctica. Review of Palaeobotany and Palynology 81: 151–164. Crossref
Bailey, I.W. 1944. The development of vessels in angiosperms and its significance in morphological research. American Journal of Botany 31: 421–428. Crossref
Bailey, I.W. and Swamy, B.G.L. 1949. The morphology and relationships of Austrobaileya. Journal of The Arnold Arboretum 30: 211–226.
Barreda, V.D., Palazzesi, L., and Olivero, E.B. 2019. When flowering plants ruled Antarctica: evidence from Cretaceous pollen grains. New Phytologist 223: 1023–1030. Crossref
Bodnar, J. 2008. Rhexoxylon cortaderitaense (Menéndez) comb. nov., a species of permineralized stems newly assigned to the Corystospermaceae, from the Triassic of Argentina. Alcheringa 32: 171–190. Crossref
Bodnar, J. 2012. Estudios evolutivos-del desarrollo en tallos fósiles de Corystospermaceae (Corystospermales, Spermatopsida). Revista del Museo Argentina de Ciencias Naturales 14: 143–166. Crossref
Bodnar, J. and Coturel, E.P. 2012. El origen y diversificación del crecimiento cambial atípico en plantas fósiles: procesos del desarrollo involucrados. Boletín de la Sociedad Argentina de Botánica 47: 33–70.
Bodnar, J., Pipo, M.L., and Beltrán, M. 2018. Evolución del cámbium interfascicular en plantas fósiles y su relación con el hábito y el clima. XVII Simposio Argentino de Paleobotánica y Palinología (Paraná). Boletín de la Asociación Latinoamericana de Paleobotánica y Palinología 18: 93.
Bose, M.N., Pal, P.K., and Harris, T.M. 1985. The Pentoxylon plant. Philosophical Transactions of the Royal Society 310: 77–108. Crossref
Burger, W.C. 1977. The Piperales and the Monocots: Alternate hypotheses for the origin of monocotiledoneous flowers. Botanical Review 43: 345–393. Crossref
Cantrill, D.J. 2000. A petrified cycad trunk from the Late Cretaceous of the Larsen Basin, Antarctica. Alcheringa 24: 307–318. Crossref
Cantrill, D.J. and Nichols, G.J. 1996. Taxonomy and palaeoecology of Early Cretaceous (Late Albian) angiosperm leaves from Alexander Island, Antarctica. Review of Palaeobotany and Palynology 92: 1–28. Crossref
Cantrill, D.J. and Poole, I. 2005. Taxonomic turnover and abundance in Cretaceous to Tertiary wood floras of Antarctica: implications for changes in forest ecology. Palaeogeography, Palaeoclimatology, Palaeoecology, 215: 205–219. Crossref
Carlquist, S. 1987. Presence of vessels in wood of Sarcandra (Chloranthaceae); comments on vessel origins in angiosperms. American Journal of Botany 74: 1765–1771. Crossref
Carlquist, S. 1989. Wood anatomy of Tasmannia; summary of wood anatomy of Winteraceae. Aliso 12: 257–275. Crossref
Carlquist, S. 1992a. Wood anatomy and stem of Chloranthus; Summary of wood anatomy of Chloranthaceae, with comments on relationships, vessellessness, and the origin of monocotyledons. IAWA Journal 13: 3–16. Crossref
Carlquist, S. 1992b. Wood Anatomy of Hedyosmum (Chloranthaceae) and the Tracheid-vessel Element Transition. Aliso 13: 447–462. Crossref
Carlquist, S. 1993. Wood and bark anatomy of Aristolochiaceae; systematic and habital correlations. IAWA Journal 14: 341–357. Crossref
Carlquist, S. 2001a. Comparative Wood Anatomy. Systematic, Ecological, and Evolutionary Aspects of Dicotyledonous Wood. 460 pp. Springer-Verlag, Heidelberg.
Carlquist, S. 2001b. Observations on the vegetative anatomy of Austrobaileya: habital, organographic and phylogenetic conclusions. Botanical Journal of the Linnean Society 135: 1–11. Crossref
Carlquist, S. 2009. Xylem heterochrony: an unappreciated key to angiosperm origin and diversifications. Botanical Journal of the Linnean Society 161: 26–65. Crossref
Carlquist, S. and Schneider, E.L. 2001. Vegetative anatomy of the New Caledonian endemic Amborella trichopoda: relationships with the Illiciales and implications for vessels origin. Pacific Science 55: 305–312. Crossref
Carlquist, S., Dauer, K., and Nishimura, S.Y. 1995. Wood and stem anatomy of Saururaceae with reference to ecology, phylogeny, and origin of the monocotyledons. IAWA Journal 16: 133–150. Crossref
Carvalho, M.A., Cabral Ramos, R.R., Crud, M.B., Witovisk, L., Kellner, A.W.A., Silva, H.P., Grillo, O.N., Riff, O.D., and Romano, P.S.R. 2013. Palynofacies as indicators of paleoenvironmental changes in a Cretaceous succession from the Larsen Basin, James Ross Island, Antarctica. Sedimentary Geology 295: 53–66. Crossref
Couper, R.A. 1958. British Mesozoic microspores and pollen grains. A systematic and stratigraphic study. Palaeontographica Abteilung B 103: 75–179.
Crame, J.A. 1983. Cretaceous inoceramid bivalves from Antarctica. In: R.L. Oliver, P.R. James, and J.B. Jago (eds.), Antarctic Earth Science, 298–302. Australian Academy of Science, Canberra and Cambridge University Press, Cambridge.
Crane, P.R. 1989. Paleobotanical evidence on the early radiation of nonmagnoliid dicotyledons. Plant Systematics and Evolution 162: 165–191. Crossref
Crane, P.R., Friis, E.M., and Pedersen, K.R. 1989. Reproductive structures and function in Cretaceous Chloranthaceae. Plant Systematics and Evolution 165: 211–226. Crossref
Cronquist, A. 1981. An Integrated System of Classification of Flowering Plants. 1262 pp. Columbia University Press, Nueva York.
Decombeix, A.L., Bomfleur, B., Taylor, E.L., and Taylor, T.N. 2014. New insights into de anatomy, development, and affinities of corystosperm trees from the Triassic of Antarctica. Review of Paleobotany and Palinology 203: 22–34. Crossref
Del Valle, R.A., Elliot, D.H., and Macdonald, D.I.M. 1992. Sedimentary basins on the east flank of the Antarctic Peninsula: proposed nomenclature. Antarctic Science 4: 477–478. Crossref
Dettman, M.E. 1989. Antarctica: Cretaceous cradle of austral temprate rainforest? In: J.A. Crame (ed.), Origins and Evolution of the Antarctic Biota. Geological Society of London Special Publication 47: 89–105. Crossref
Dettmann, M.E. 1994. Cretaceous vegetation: the microfossil record. In: R.S. Hill (ed.), History of the Australian Vegetation: Cretaceous to Recent, 143–170. Cambridge University Press, Cambridge. Crossref
Dettmann, M.E. and Thomson, M.R.A. 1987. Cretaceous palynomorphs from the James Ross Island area, Antarctica—a pilot study. British Antarctic Survey Bulletin 77: 13–59.
Doyle, J.A. 1969. Cretaceous angiosperm pollen of the Atlantic Coastal Plain and its evolutionary significance. Journal of the Arnold Arboretum Harvard University 50: 1–35. Crossref
Doyle, J.A. 2014. Recognising angiosperm clades in the Early Cretaceous fossil record. Historical Biology: An International Journal of Paleobiology 27: 414–429. Crossref
Doyle, J.A. and Endress, P.K. 2000. Morphological phylogenetic analysis of basal angiosperms comparison and combination with molecular data. International Journal of Plant Sciences 161: S121–S153. Crossref
Doyle, J.A. and Endress, P.K. 2014. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. International Journal of Plant Sciences 175: 555–600. Crossref
Eklund, H. 1999. Big Survivors with Small Flowers: Fossil History and Evolution of Laurales and Chloranthaceae. 52 pp. Unpublished Ph.D. Thesis, Uppsala University, Uppsala.
Eklund, H., Doyle, J.A., and Herendeen, P.S. 2004. Morphological phylogenetic analysis of living and fossil Chloranthaceae. International Journal of Plant Sciences 165: 107–151. Crossref
Elliot, D.H. 1988. Tectonic setting and evolution of the James Ross Basin, northern Antarctic Peninsula. In: R.M. Feldmann and M.O. Woodburne (eds.), Geology and Paleontology of Seymour Island, Antarctic Peninsula. Geological Society of America Memoir 169: 541–555. Crossref
Endress, P.K. 1986. Reproductive structures and phylogenetic significance of extant primitive angiosperms. Plant Systematics and Evolution 152: 1–28. Crossref
Endress, P.K. 1987. The Chloranthaceae: reproductive structures and phylogenetic position. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 109: 153–226.
Field, T.S., Arens, N.C., Doyle, J.A., Dawson, T.E., and Donoghue, M.J. 2004. Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30: 82–107. Crossref
Florisbal, L.S., Kochhann, K.G.D., Baecker-Fauth, S., and Fauth, G. 2013. Benthic foraminifera, ostracods and radiolarians from the Lachman Crags Member (Santa Marta Formation), Upper Santonian–Lower Campanian (Upper Cretaceous) of James Ross Island, Antarctica. Revista Brasileira de Paleontologia 16: 181–196. Crossref
Friis, E.M. and Pedersen K.R. 2011. Canrightia resinifera gen. et sp. nov., a new extinct angiosperm with Retimonocolpites-type pollen from the Early Cretaceous of Portugal: missing link in the eumagnoliid tree? Grana 50: 3–29. Crossref
Friis, E.M., Pedersen, K.R., and Crane, P.R. 1999. Early angiosperm diversification: the diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Annals of the Missouri Botanical Garden 86: 259–296. Crossref
Friis, E.M., Pedersen, K.R., and Crane, P.R. 2000. Reproductive Structure and Organization of Basal Angiosperms from the Early Cretaceous (Barremian or Aptian) of Western Portugal. International Journal of Plant Sciences 161: S169–S182. Crossref
Frost, F.H. 1930. Specialization in secondary xylem of dicotyledons. II. Evolution of end wall of vessel segment. Botanical Gazette 90: 198–212. Crossref
Greguss, P. 1968. Xylotomy of the Living Cycads, with a Description of their Leaves and Epidermis. 260 pp. Akademiai Kiadó, Budapest.
Hayes, P.A. 1999. Cretaceous Angiosperm Leaf Floras from Antarctica. 310 pp. Ph.D. Thesis, University of Leeds, Leeds.
Hayes, P.A., Francis, J.E., Cantrill, D.J., and Crame, J.A. 2006. Palaeoclimate analysis of Late Cretaceous angiosperm leaf floras, James Ross Island, Antarctica. In: J.E. Francis, D. Pirrie, and J.A. Crame (eds.), Cretaceous–Tertiary High-latitude Palaeoenvironments, James Ross Basin, Antarctica. Geological Society of London, Special Publications 258: 49–62. Crossref
Hedlund R.W. and Norris, G. 1968. Spores and pollen grains from Fredricksburgian (Albian) strata, Marshall County, Oklahoma. Pollen Spores 10: 9–159.
Herbst, R. and Lutz, A.I. 1995. Tranquiloxylon petriellai nov. gen. et sp. (Pteridospermales) from the Upper Triassic Laguna Colorada Formation, Santa Cruz province, Argentina. Ameghiniana 32: 231–236.
Hickey, L.J. and Wolf, J.A. 1975. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden 62: 538–589. Crossref
Hinrichs, K.U. and Boetius, A. 2002. The anaerobic oxidation of methane: New insights in microbial ecology and biogeochemistry. In: G. Wefer, D. Billet, D. Hebbeln, B.B. Jorgensen, M. Schluter, and T. Van Weering (eds.), Ocean Margin Systems, 457–477. Springer-Verlag, Berlin. Crossref
Hughes, N.F. and McDougall, A.B. 1987. Records of angiospermid pollen entry into the English Early Cretaceous succession. Review of Paleobotany and Palynology 50: 255–212. Crossref
Hughes, N.F. and McDougall, A.B. 1994. Search for antecedents of Early Cretaceous monosulcate columellate pollen. Review of Palaeobotany and Palynology 83: 175–183. Crossref
IAWA Committee 1989. IAWA List of microscopic features for hardwood identification. IAWA Journal 10: 219–332.
Iglesias, A. 2016. New Upper Cretaceous (Campanian) flora from James Ross Island, Antarctica. Ameghiniana 53: 358–374. Crossref
Joy, K.W., Willis, A.J., and Lacey, W.S. 1956. A Rapid Cellulose Peel Technique in Palaeobotany. Annals of Botany 20: 635–637. Crossref
Keating, J.M. 1992. Palynology of the Lachman Crags Member, Santa Marta Formation (Upper Cretaceous) of northwest James Ross Island. Antarctic Science 4: 293–304. Crossref
Kemp, E.M. 1968. Probable angiosperm pollen from British Barremian to Albian strata. Palaeontology 11: 421–434.
Khaing, W.W. 2016. Morphological and Anatomical studies on Piper betle L. cultivated in three different localities in Myanmar. Journal of the Myanmar Academy of Arts and Science 14: 1–22.
Kvacek, J. and Friis, E.M. 2010. Zlatkocarpus gen. nov., a new angiosperm reproductive structure with monocolpate-reticulate pollen from the Late Cretaceous (Cenomanian) of the Czech Republic. Grana 49: 115–127. Crossref
Kvacek, J. and Sakala, J. 2011. Late Cretaceous flora of James Ross Island (Antarctica)—preliminary report. Czech Polar Reports 1: 96–103. Crossref
Martínez, L.C.A., Artabe, A.E., and Bodnar, J. 2012. A new cycad stem from the Cretaceous in Argentina and its phylogenetic relationships with other Cycadales. Botanical Journal of the Linnean Society 170: 436–458. Crossref
McArthur, J.M., Brame, J.A., and Thirlwall, M.F. 2000. Definition of the Late Cretaceous stage boundaries in Antarctica using strontium isotope stratigraphy. Journal of Geology 108: 623–640. Crossref
Nieminen, K., Blomster, T., Helariutta, Y., and Mähönen, A.P. 2015. Vascular cambium development. The Arabidopsis Book 13: 1–23. Crossref
Noetinger, S., Pujana, R.R., Burrieza, A., and Burrieza, H.P. 2017. Use of UV-curable acrylates gels as mounting media for palynological samples. Revista del Museo Argentino de Ciencias Naturales 19: 19–23. Crossref
Nývlt, D. and Šerák, L. 2009. James Ross Island—Northern Part. Topographic map 1 : 25000. Česká geologická služba, Praga.
Olivero, E.B. 1992. Asociaciones de ammonites de la Formación Santa Marta (Cretácico tardío), Isla James Ross. In: C. Rinaldi (ed.), Geología de la Isla James Ross, 45–75. Instituto Antártico Argentino, Buenos Aires.
Olivero, E.B. 2012. Sedimentary cycles, ammonite diversity and palaeoenvironmental changes in the Upper Cretaceous Marambio Group, Antarctica. Cretaceous Research 34: 348–366. Crossref
Olivero, E.B. and Medina, F.A. 2000. Patterns of Late Cretaceous ammonite biogeography in southern high latitudes: the family Kossmaticeratidae in Antarctica. Cretaceous Research 21: 269–279. Crossref
Olivero, E.B., Ponce, J.J., and Martinioni, D.R. 2008. Sedimentology and architecture of sharp-based tidal sandstones in the Upper Marambio Group, Maastrichtian of Antarctica. Sedimentary Geology 210: 11–26. Crossref
Olivero, E.B., Scasso, R.A., and Rinaldi, C.A. 1986. Revision of the Marambio Group, James Ross Island, Antarctica. Contribuciones del Instituto Antártico Argentino 331: 1–28.
Patel, R.N. and Bowles, A. 1980. Wood anatomy of the dicotyledons indigenous to New Zealand. Piperaceae. New Zealand Journal of Botany 18: 507–513. Crossref
Pedersen, K.R., Crane, P.R., Drinnan, A.N., and Friis, E.M. 1991. Fruits from the mid-Cretaceous of North America with pollen grains of the Clavatipollenites type. Grana 30: 577–590. Crossref
Perez-Loinaze, V.S., Barreda, V.D., Archangelsky, S., and Archangelsky, A. 2015. Cretaceous angiosperm pollen from the Kachaike Formation, south-western Santa Cruz Province, Argentina. Historical Biology 28: 941–951. Crossref
Pipo, M.L. and Iglesias, A. 2017. Permineralized plants in calcareous concretions from the Campanian, James Ross Basin, Antarctic Peninsula. Actas 11° Congreso de la Asociación Paleontológica Argentina, General Roca, Río Negro, 2016. Ameghiniana 54: R93.
Pipo, M.L., Iglesias, A., and Bodnar, J. 2018. Coníferas y angiospermas anatómicamente preservadas en Concreciones calcáreas del Cretácico Superior, Cuenca de James Ross, Península Antártica. Actas Reunión de Comunicaciones de la Reunión de comunicaciones de la Asociación Paleontológica Argentina 2018, Puerto Madryn, Chubut. Publicación Electrónica de la Asociación Paleontológica Argentina 19: R29–R30.
Pipo, M.L., Iglesias, A., and Bodnar, J. 2019. Permineralized fern petioles and roots from the Upper Cretaceous, James Ross Island, Antarctica. Actas Reunión de Comunicaciones de la Reunión de comunicaciones de la Asociación Paleontológica Argentina 2019, La Plata, Buenos Aires. Publicación Especial de la Asociación Paleontológica Argentina 20: R1.
Poole, I. and Francis, J.E. 1999. The first record of fossil atherospermataceous wood from the upper Cretaceous of Antarctica. Review of Palaeobotany and Palynology 107: 97–107. Crossref
Poole, I. and Francis, J.E. 2000. The first record of Winteraceae wood from the Cretaceous of Antarctica. Annals of Botany 85: 307–315. Crossref
Poole, I. and Gottwald, H. 2001. Monimiaceae sensu lato, an Element of Gondwanan Polar Forests: Evidence from the Late Cretaceous–Early Tertiary Wood Flora of Antarctica. Australian Systematic Botany 14: 207–230. Crossref
Poole, I., Cantrill, D.J., Hayes, P., and Francis, J.E. 2000a. The fossil record of Cunoniaceae: new evidence from Late Cretaceous fossil wood of Antarctica. Review of Palaeobotany and Palynology 111: 127–144. Crossref
Poole, I., Richter, H.G., and Francis, J.E. 2000b. Gondwanan origins for Sassafras (Lauraceae): evidence from Late Cretaceous fossil wood of Antarctica. IAWA Journal 21: 463–475. Crossref
Pujana, R.R., Raffi, M.E., and Olivero, E.B. 2017. Conifer fossil woods from the Santa Marta Formation (Upper Cretaceous), Brandy Bay, James Ross Island, Antarctica. Cretaceous Research 77: 28–38. Crossref
Pujana, R.R., Iglesias, A., Raffi, M.E., and Olivero, E.B. 2018. Angiosperm fossil woods from the Upper Cretaceous of Western Antarctica (Santa Marta Formation). Cretaceous Research 90: 349–362. Crossref
Scasso, R. A., Olivero, E.B., and Buatois, L.A. 1991. Lithofacies, biofacies and ichnoassemblage evolution of a shallow submarine volcaniclastic fan-shelf depositional system (Upper Cretaceous, James Ross Island, Antarctica). Journal of South American Earth Sciences 4: 239–260. Crossref
Spicer, R. 2016. Variation in angiosperm wood structure and its physiological and evolutionary significance. In: A.T. Groover and Q.C.B. Cronk (eds.), Comparative and Evolutionary Genomics of Angiosperm Trees, Plant Genetics and Genomics: Crops and Models, 19–60. Springer, Dordrecht. Crossref
Swamy, B.G.L. 1953. The morphology and relationships of the Chloranthaceae. Journal of the Arnold Arboretum 34: 375–411.
Swamy, B.G.L. and Bailey, I.W. 1950. Sarcandra, a vesselless genus of Chloranthaceae. Journal of the Arnold Arboretum 31: 117–129.
Takahashi, A. 1988. Morphology and ontogeny of stem xylem elements in Sarcandra glabra (Thunb.) Nakai (Chloranthaceae): Additional evidence for the occurrence of vessels. Botanical Magazine Tokyo 101: 387–395. Crossref
Takahashi, A. and Tamura, M. 1990. Ocurrence of vessel elements in stem of Sarcandra glabra. Journal of Japanese Botany 65: 81–86.
Thompson, W.P. and Bailey, I.W. 1916. Are Tetracentron, Trochodendron, and Drimys specialized or primitive types? Memoirs of the New York Botanical Garden 6: 27–32.
Tidwell, W.D. and Ash, S.R. 1990. On the Upper Jurassic stem Hermanophyton and its species from Colorado and Utah, USA. Palaeontographica Abteilung B 128: 77–92.
Tidwell, W.D. 2002. Hermanophyton—an enigmatic plant from the Jurassic. In: U. Dernbach and W.D. Tidwell (eds.), Secrets of Petrified Plants: Fascination from Millions of Years, 199–201. D’Oro Publishers, Heppenheim.
Todzia, C.A. 1993. Chloranthaceae. In: K. Kubitzki, J.G. Rohwer, and V. Bittrich (eds.), The Families and Genera of Vascular Plants, Vol 2, Flowering Plants. Dicotyledons, 281–287. Springer-Verlag, Berlin. Crossref
Trueba, S., Rowe, N.P., Neinhuis, C., Wanke, S., Wagner, S.T., and Isnard, S. 2015. Stem Anatomy and the Evolution of Woodiness in Piperales. International Journal of Plant Sciences 176: 468–485. Crossref
Verdcourt, B. 1985. Notes on Malesian Chloranthaceae. Kew Bulletin 40: 213–224. Crossref
Walker, J.W. and Walker, A.G. 1984. Ultrastructure of Lower Cretaceous Angiosperm pollen and the origin and early evolution of flowering plants. Annals of the Missouri Botanical Garden 71: 464–521. Crossref
Young, D.A. 1981. Are the angiosperms primitively vesselless? Systematic Botany 6: 313–330. Crossref
Zhang, Q., Antonelli, A., Feild, T.S., and Kong, H.Z. 2011. Revisiting taxonomy, morphological evolution, and fossil calibration strategies in Chloranthaceae. Journal of Systematics and Evolution 49: 315–329. Crossref
Acta Palaeontol. Pol. 65 (2): 261–272, 2020
https://doi.org/10.4202/app.00697.2019