

Three shell types in Mardinella daviesi indicate the evolution of a paratrimorphic life cycle among late Paleocene soritid benthic foraminifera
LORENZO CONSORTI, FELIX SCHLAGINTWEIT, and KOOROSH RASHIDI
Consorti, L., Schlagintweit, F., and Rashidi, K. 2020. Three shell types in Mardinella daviesi indicate the evolution of a paratrimorphic life cycle among late Paleocene soritid benthic foraminifera. Acta Palaeontologica Polonica 65 (3): 641–648.
Soritids are a successful group of larger Foraminifera with several extant representatives that display a complex alternation of generations, the so-called paratrimorphic life cycle. Fossil soritids are abundantly widespread through the geological record starting from the Albian. The oldest Soritidae possessing annular chambers and cross-wise oblique chamber communications is represented by the Paleocene species Mardinella daviesi. The present paper provides a description of the morphology and anatomy of the three reproductive generations recovered in a Mardinella-rich assemblage from a lower Paleogene shallow-water carbonate succession in SW Iran (Zagros Zone). Comparison among proportion of schizonts vs. gamonts with extant soritids shows that there is an easy match. This would confirm that the paratrimorphic life cycle was successfully adopted by the early soritids to maintain fitness with the environment and to keep a stable mutual relationship with a certain group of endosymbionts.
Key words: Foraminifera, Soritidae, reproduction cycle, endosymbionts, Paleogene, Iran.
Lorenzo Consorti [lorenzo.consorti.es@gmail.com; lconsorti@units.it], Department of Mathematics and Geosciences, University of Trieste. Via Weiss 2, Trieste, Italy.
Felix Schlagintweit [felix.schlagintweit@gmx.de], Lerchenauerstr. 167, 80935 München, Germany.
Koorosh Rashidi [kooroshrashidi@yazd.ac.ir], Department of Geology, Yazd University, 89195-741 Yazd, Iran.
Received 18 November 2019, accepted 23 March 2020, available online 4 June 2020.
Copyright © 2020 L. Consorti et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Alternation of reproductive generations in large benthic foraminifera is usually represented by the synchronous occurrence of different morphotypes in a monospecific coenosis. Among K-strategy taxa, the reproduction cycle shows alternating sexually and asexually produced generations that are sometimes related to seasonality (Zohary et al. 1980; Hottinger 2001b), lunar cycles (Hohenegger et al. 2019) or to the preference of a certain ecological gradient (Leutenegger 1977). The alternation of reproduction in the microspheric agamont is by means of meiosis. The multiple fission produces haploid and uninucleate megalospheric gamonts. Gamonts generate gametes and zygotes that mature into agamonts (Goldstein 1999; Hottinger 2001b, 2006). During the end of ontogenesis, mature agamonts produce several morphologically distinct brood chambers that are marked by a rapid increase in the irregularity and volume of the cavity. These chambers usually lack most of their inner skeletal structures and host a relatively high number of embryos, also called offspring, that are released once the prolocular shell formation is completed (Ross 1972; Hottinger 2006; Langer et al. 2009). The alternated reproduction often produces a clear dimorphism in the first growth stages expressed by the presence of two morphologically distinct prolocular chambers (Leutenegger 1977; Goldstein 1999; Hohenegger et al. 1999; Hohenegger 2011). In a restricted number of symbiont-bearing larger Foraminifera, e.g., Heterostegina depressa d’Orbigny, 1826, Amphistegina gibbosa d’Orbigny, 1839, Amphisorus hemprichii Ehrenberg, 1839, Sorites orbiculus (Forskål, 1775) and Nummulites venosus (Fichtel and Moll, 1798) among others (see e.g., Hohenegger et al. 1999, 2000, 2019), reproduction is paratrimorphic and becomes complicated by the addition of a further asexual generation. In this case, the schizont, which is produced by the agamont, may generate several successive asexual megalospheric generations (Harney et al. 1998; Dettmering et al. 1998; Goldstein 1999). This becomes well evident when comparing the test sizes among adult individuals (Hottinger 2000). In fact, paratrimorphic taxa show the occurrence of three classes of shells (Goldstein 1999; Hottinger 2006; Hohenegger 2011). However, to distinguish both megalospheric gamont and schizont on a pure morphological basis is not always straightforward. Furthermore, complete life cycle is known in a few numbers of extant species (about 30) and is hardly observable in the fossil record, with few exceptions including nummulitids and soritids (see e.g., Hottinger 2006).
In this paper we aim to report some evidences of a paratrimorphic life cycle in a Paleocene shallow-water foraminiferal assemblage dominated by the soritid Mardinella daviesi (Henson, 1950). Based on the exquisite preservation, we consider these shells few reworked. The morphological data extracted from this study reveal the occurrence of the three categories of shells that belong to distinct reproduction phases. This important finding helps to shed light on the paleobiology of the Soritoidea. In particular, these new data were recovered from the genus Mardinella, which is considered the oldest soritid foraminifer with cross-wise oblique communications (Hottinger 2001a; Vicedo and Serra-Kiel 2015).
Institutional abbreviations.—Gmm, Geoscience Museum of Mashhad, Mashhad, Iran.
Other abbreviations.—SBZ, Shallow Benthic Zones.
Historical background
A comprehensive morphological characterization of this species can be found in the diagnosis of Azzarolina provided by Vicedo and Serra-Kiel (2015). The shell architecture includes a porcelaneous discoidal shape composed by an embryonic apparatus (protoconch and deuteroconch), which is followed by several annular chambers with multiple aligned aperture. The chamber lumen is subdivided by radial partitions (beams) and oblique central pillars. The stolon axes signature is cross-wise oblique.
The genus Mardinella was first described by Meriç and Çoruh (1991) from the Paleocene of Turkey with the type-species of Orbitolites shirazensis Rahaghi, 1983 from the Paleocene of Iran (Rahaghi 1983). Orbitolites shirazensis was later recognized by Vicedo and Serra-Kiel (2015) as a junior synonym of Taberina daviesi Henson, 1950 and used to erect the genus Azzarolina. The morphological arguments proposed to establish Azzarolina are based on the occurrence of cross-wise stolon axes in contrast to the radial pattern of Taberina Keijzer, 1945. This resulted in a new combination of Azzarolina daviesi (Henson, 1950). The architectural constraints chosen to discern Taberina from Azzarolina are solidly based (Hottinger 1978) and are not discussed herein. Nonetheless, the description of Mardinella in Meriç and Çoruh (1991) also includes an identical statement; “diagonal stolons connecting the chambers” (Meriç and Çoruh 1991: 168) that is, in our point of view, the equivalent constrain used by Vicedo and Serra-Kiel (2015). Therefore, the nominal genus Mardinella seems to have priority, resulting in the combination of Mardinella daviesi (Henson, 1950) (Schlagintweit et al. 2019). Mardinella and Azzarolina were, however, established referring to different type-species that, according to the ICZN, define a “subjective synonymy” (see also Hayward et al. 2019) with Mardinella being the senior synonym. A complete revision of the original Taberina daviesi type-material and comparison with Mardinella from Turkey deserve detailed study, but this falls beyond the scope of the present contribution.
Geological setting, material, and methods
Four Mardinella-rich levels are studied from the Qorban member of the Sachun Formation (Paleocene–early Eocene; James and Wynd 1965). The succession mainly consists of thick-bedded gray limestone beds intercalated by a few yellowish sandy limestone levels and dolomitic limestone. The geographic coordinates of the samples section base are 29°19’28.08” N 52°87’54.21” E; located about 65 km southeast of the city of Shiraz in the western flank of the Mozaffari Anticline, 4 km from the road Shiraz-Jahrom (Fig. 1A, B). The Qorban Member (Heibati et al. 2014; Bavi et al. 2016) is a carbonate unit with no evaporite levels that are usually widespread at the Sachun Formation type locality (James and Wynd 1965; Shabafrooz et al. 2013). This Qorban Member section (Fig. 1) falls into the Shallow Benthic Zones (SBZ) 3 and 4 of Thanetian age (Serra-Kiel et al. 1998). Larger foraminifera are abundant throughout the section. Based on their vertical distribution, the approximate boundary between SBZ 3 and SBZ 4 is placed around 135 m (Fig. 1). SBZ 3 (Serra-Kiel et al. 1998; Hottinger 2014) is recognized by the occurrence of Elazigina cf. lenticula (Hottinger, 2014), Miscellanea cf. juliettae Leppig, 1988, Miscellanites iranicus (Rahaghi, 1983), Miscellanites cf. minutus (Rahaghi, 1983), Dictyoconus? turriculus Hottinger and Drobne, 1980, Assilina cf. yvettae (Schaub, 1981), Mardinella daviesi (Henson, 1950), Kathina delseota Smout, 1954, and Dictyokathina simplex Smout, 1954. SBZ 4 shows the vertical distribution of Idalina sinjarica Grimsdale, 1952, Lockhartia retiata Sanders, 1962, Daviesina langhami Smout, 1954, Daviesina intermedia Smout and Haque, 1956 and Dictyokathina simplex Smout, 1954. More details on the stratigraphy and fauna from this section can be found in Schlagintweit et al. (2019, 2020) and Benedetti et al. (2020).
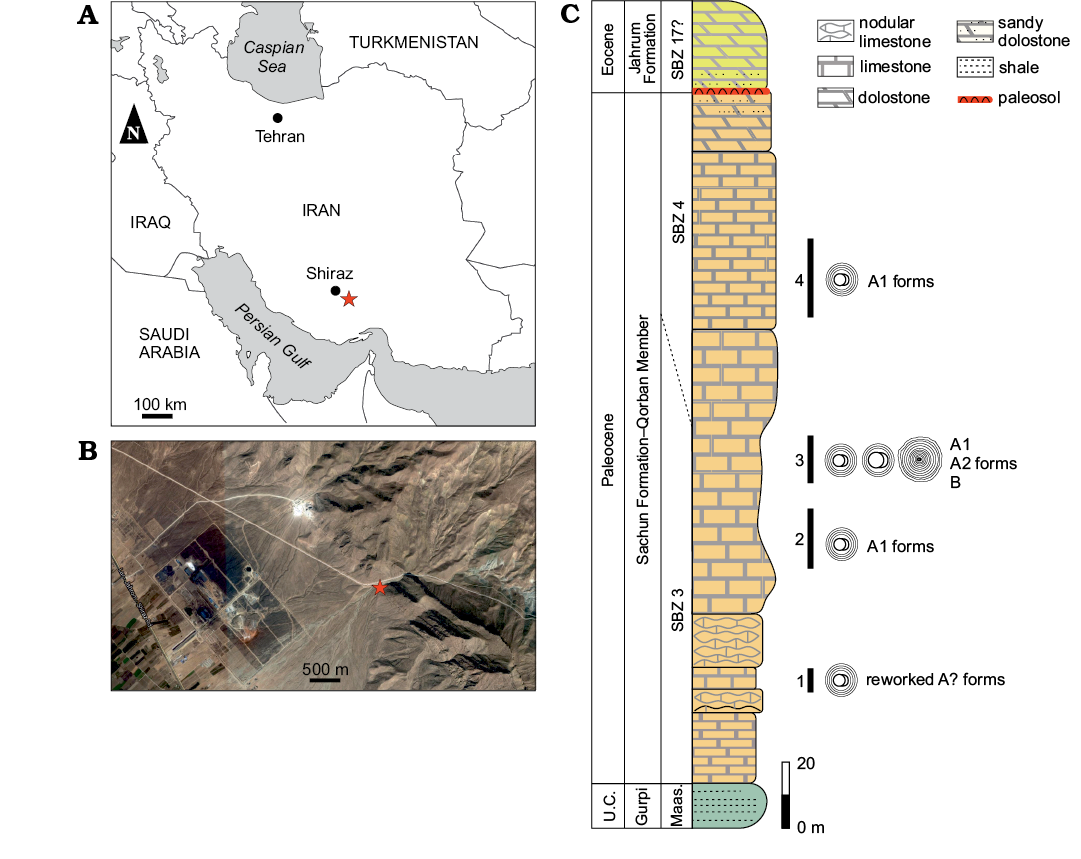
Fig. 1. Measured column of the Qorban member in the study locality. A. Position of the Qorban section in the general map of Iran. B. Satellite image with the position of the section base (star). C. Qorban Member stratigraphy with indication of the four Mardinella-rich levels studied in this work. Abbreviations: Fm., Formation; Maas., Maastrichtian; SBZ, Shallow Benthic Zones; U.C., Upper Cretaceous.
The study relies on 83 thin sections of strongly lithified limestone that host nearly 180 well-preserved Mardinella specimens. The specimens figured in this work are deposited in the paleontological collection of the Geoscience Museum of Mashhad (Iran) under the label Gmm13980F39 to Gmm13890F41. Although their orientation has not intentionally been driven, we had the chance to study several centered axial and equatorial sections, which clearly show some critical shell features such as the dimensions of the nepionic stage and the brood chambers.
Shell preservation varies depending on the textural character of the hosting carbonate. Level 1 is represented by fine-grained, often recrystallized, packstone-grainstone textures with miliolids and bivalve shells. Grains are heavily fragmented and medium to severely oxidized arguably by subaerial reworking. Level 1 is in fact sandwiched between two nodular bodies that possibly represent a proximal marine or a continental-transitional system. Benthic foraminifera are represented by fragmented, transported and decorticated specimens. In contrast hereto, facies recovered in levels 2, 3, and 4 display packstone-wackestone textures with a well-diversified bioclast assemblage dominated by dasycladalean green algae, lamellar perforated foraminifera, large bivalve fragments, gastropods, miliolids, and scattered ostracods with articulated valves. The recovered Mardinella shells show no traces of decortication processes. Fragile brood chambers, which are very rarely preserved in fossil assemblages (Hottinger 2000, 2001b), appear as well. All these features indicate that the shells suffered neglectable reworking or size election size, suggesting a relatively fast lithification nearby to the living community.
Results
Microspheric generation (B form).—Two substages of the ephebic development are clearly observable: adult and adult reproductive (Fig. 2). The adult one is characterized by the normal development of successive annular chambers (about 30) possessing both beams and oblique pillars. The adult reproductive shell growth is marked by an anomalous increase in volume of the chamber lumen (see arrows in Fig. 2B). The first couple of chambers of this substage host few scattered and irregular skeletal elements. In some cases, the only elements observed are purely represented by disordered growing beams. These could be interpreted as the ultimate remaining elements dividing the chamber lumen and functioning as egg-holders (see Hottinger 2006: fig. 24B and C). A set of empty chambers, free of any skeletal structures, are also observed. These represent the development of brood chambers that were arguably entirely filled by a dense accumulation of offspring during asexual reproduction. Comparable cases have been recorded in extant soritids (Marginopora; see Ross 1972).
The maximum diameter measured among these morphotypes ranges from 8.6 mm to 7.2 mm. The adult reproductive stage occupies the least 1.4 mm of the total shell radius. Chamber height of the very first measurable chamber is around 0.034 mm whereas their width is around 0.1 mm. The last normal-growing ephebic chambers are 0.46 mm in width and 0.09 mm in height. Height of the adult reproductive chambers varies between 0.13 mm and 0.24 mm, whereas their width ranges from 0.48 mm to 0.65 mm.
These shells occur only in level 3 of the four levels examined for this study. Abundance of Microspheric B forms is relatively low; among the undamaged well-preserved 43 specimens recovered from this level, just 4 whole shells and one fragment can be attributed to this group with certainty.
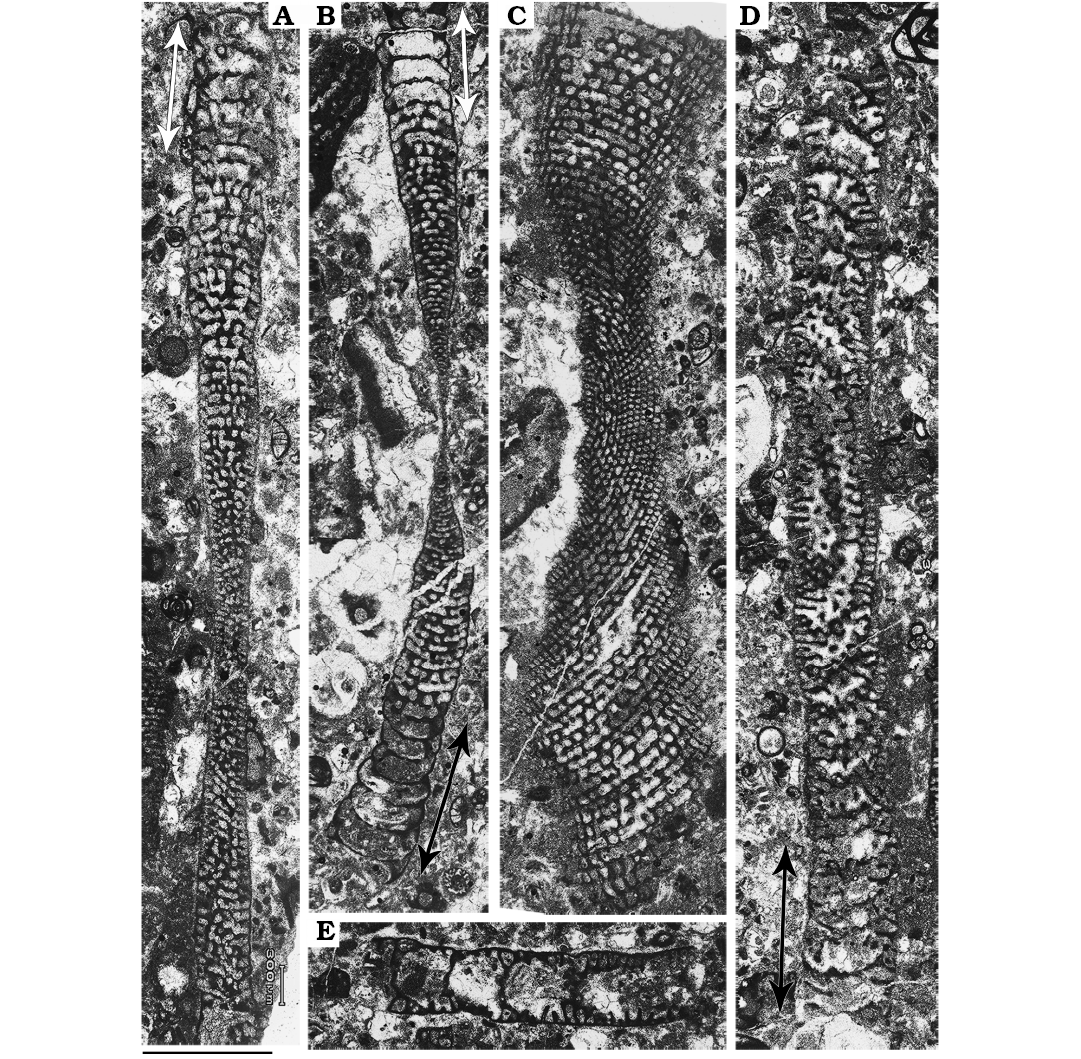
Fig. 2. Microspheric B forms (agamonts) of soritid foraminifer Mardinella daviesi (Henson, 1950) from the Thanetian, Paleocene of Iran. A. Gmm13980F40a, subaxial oblique section; note the brood chambers in the adult reproductive stage of growth (arrows). B. Gmm13980F40b, axial section showing empty brood chambers (two-headed arrow) on both sides of the specimen. C. Gmm13980F40c, subequatorial section. D. Gmm13980F40d, oblique section with some brood chambers (two-headed arrow). E. Gmm13980F41a, fragment of shell with five brood chambers; note the irregularly disposed beams. Scale bar 1 mm.
Megalospheric generations (A1 and A2 forms).—The group of foraminifers composed by shells with large proloculi are usually referred to the megalospheric generations (Hohenegger et al. 2000; Hohenegger 2000). The assemblage here recovered displays both juvenile and adult specimens (Fig. 3). Juveniles are composed by a set of 6–10 annular chambers. The axis of maximum diameter of the embryonic stage varies between 0.53 and 0.58 mm. The mean diameter of the juvenile shells is about 1.5 mm. Adult individuals show around 40 annular chambers of a total diameter of approximately 4.5 mm. The embryonic stage measured at the maximum diameter in adult specimens, comprising both protoconch and deuteroconch, displays two size groups. One is distinguished by initial chambers with a mean of 1.4 mm diameter, the other falls between 0.44 mm and 0.77 mm. Such a discrepancy could indicate the occurrence of two megalospheric generations (Hohenegger et al. 2000; Hohenegger 2000). Wider measurement is arguably associated to the gamont (A2), whereas the reduced one to the (A1) schizont.
Shells associated to schizont generation occur abundantly in levels 2, 3, and 4, whereas the gamont generation appears just in level 3. The frequency of A1 individuals is very high in the three levels, whereas A2 occur only in the level 3 with two certain recovered individuals. Abundance of A1 forms is high among all the samples recovered, showing concentrations around 85% of the total Mardinella assemblage in level 3, and 100% in levels 2 and 4.
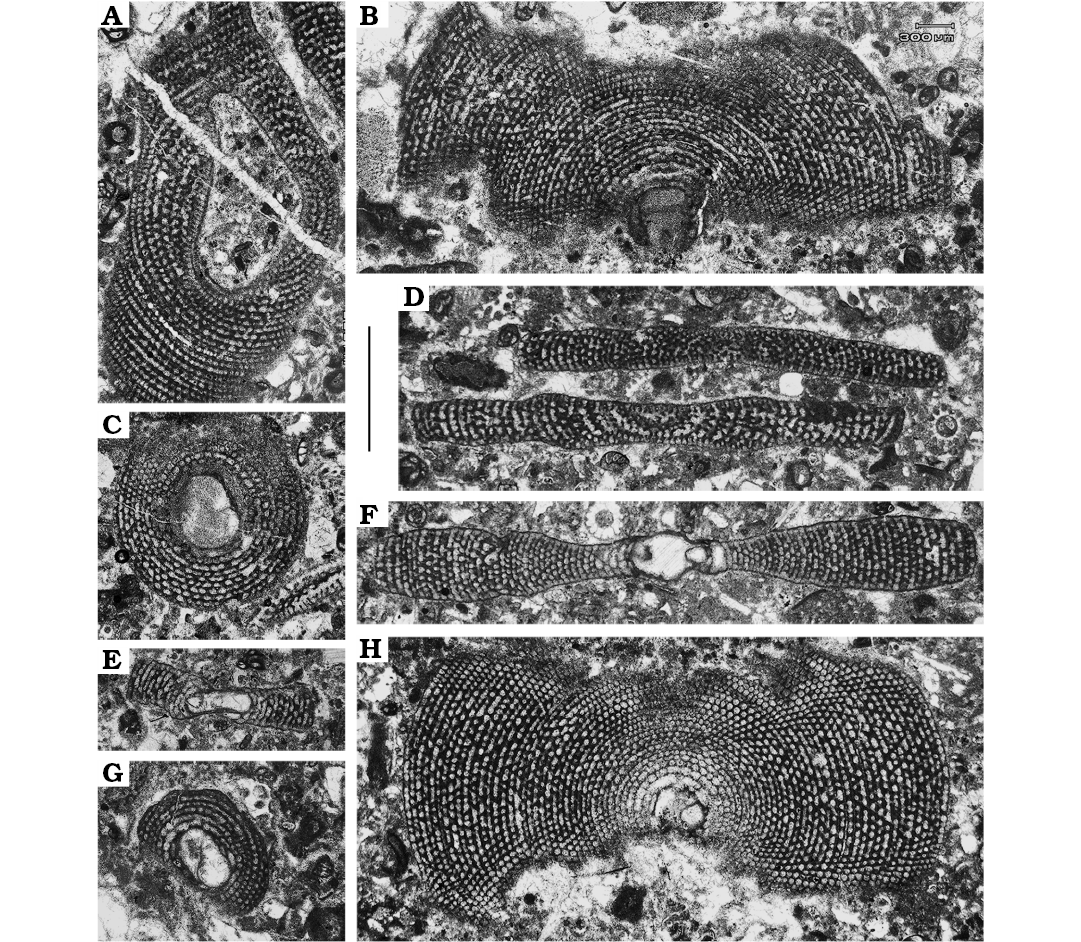
Fig. 3. Megalospheric forms of soritid foraminifer Mardinella daviesi (Henson, 1950) from the Thanetian, Paleocene of Iran. A. Gmm13980F41b, centred section of a gamont (A2) individual. C, E, G. Gmm13980F39a, Gmm13980F39c, Gmm13980F41c, respectively, juvenile schizonts (A1) in equatorial (C, G) and axial (E) views. B, H. Gmm13980F40e, Gmm13980F41d, respectively, equatorial section of an adult schizont. D. Gmm13980F39b, subaxial sections of two adult schizonts. F. Gmm13980F39d, equatorial section of an adult schizont; note the crosswise oblique disposition of pillars. Scale bar 1 mm.
Discussion
Alternation of generations in K-strategist symbiont bearing Foraminifera successfully promote evolution, allowing a permanent fitness over time (Hottinger 2001b). The mechanism maintains the genetic variability through sexual reproductive phases. For those larger Foraminifera that generally depend in terms of natural selection and environmental adaptation on symbiotic algae, the alternated generations help to maintain a stable mutual relationship. The most effective manner to obtain this is by producing a large number of cloned individuals, through repeated asexual reproduction phases, with the advantage to inherit symbionts directly from the agamont mother cell (Hottinger 2000; Pochon et al. 2007). This is the reason why, in Recent assemblages, megalospheric shells dominate the fauna (Hottinger 1982) and, in paratrimorphic species, they are almost entirely represented by schizonts (Zohary et al. 1980; Hohenegger et al. 1999, 2000). Lutenegger (1977) postulated that the distribution of the megalospheric shizonts is higher at shallower depth. This helps to maximize light resources extending the depth niche ranges (Hohenegger 2004), to maintain a stable relation with certain groups of endosymbionts (Hallock 1999), and to maintain high fitness in case of loss of favourable environmental conditions (Hottinger 2000; Hohenegger 2000).
Paratrimorphic life strategy is observed in several extant soritids with seasonal epiphytic behaviour (Hottinger 1977; Zohary et al. 1980; Debenay and Pairy 2010). Since the basic architectural design of these shells is functional in hosting symbionts within the alcoves and in remaining attached to sea grass leaves (Hottinger 2000), the attribution of these features to Mardinella is predictable. The lateral chamber partitions (beams) arguably operated as recent loci for symbionts, indicating shallow depths (0–20 m; Hottinger 1977; Hohenegger 2000, 2004) in order to catch sunlight for photosynthesis. This is also supported by the presence of green algae and miliolids in the Mardinella-rich facies. The widespread occurrence of three shell types thus reinforces with high confidence the evidence of a paratrimorphic reproduction in the first true soritid known so far. Furthermore, the dominance of megalospheric schizonts could be considered as a further clue towards a strict comparison with Recent taxa. Analogues can be found in some Eocene Orbitolites, with distinct brood chambers, figured by Hottinger (2006: fig. 24) and in several soritids (probably Mardinella) from the Paleocene of Iraq (Salih 2012: fig. 6.13). The existence of two reproductive generations in Reticulotaberina Nafarieh, Consorti, and Caus, 2019 as well as the existence of alveolar loci (Nafarieh et al. 2019), might represent another case of paratrimorphism driven by endosymbiosis with algae. Based on molecular studies, the relationship between soritids and the dinoflagellate Symbiodinium lineage supposedly started by the early Eocene (Pochon and Pawlowski 2006). Therefore, an earlier occurrence, in the late Paleocene, cannot be excluded. However, the mechanism as well as the timing of endosymbiosis acquisition remain vague. For instance, there are no traces of polymorphism through the Cretaceous Soritoidea with peneropliform habit (see Consorti et al. 2015, 2016, 2018; Cruz-Abad et al. 2017), suggesting that they were epibenthic detritivores or forms that occasionally acquired chlorophytes. This might support the view of Richardson (2001) in which the group evolved from nonphytal to phytal habitats by changing the type of endosymbionts (chlorophyte to dinophyte), and leads to suppose that this likely occurred once the cyclical arrangement becomes a stable character. The oldest, true, seagrass meadow with some evidence of epiphytic organisms was reported from the latest Cretaceous (Hart et al. 2016; Van der Ham et al. 2017). Therefore, an interaction between the soritids and seagrass leaves during the Paleocene could be reasonably expected. Understanding the coevolutionary dynamics of symbioses and its timing through the geological record represents however a big challenge as many aspects remain poorly known and some key approaches, such as molecular studies, still need a deep implementation (Pochon and Pawlowski 2006).
Conclusions
Mardinella daviesi represents the oldest soritid with cyclical chambers and crosswise oblique communications known so far. The three fossil shell types recovered from the Paleocene of Iran help shed light on the history of soritids. Here we conclude that (i) similarly to their Recent counterparts, brood chambers in microspheric individuals are marked by an increase of growth anomaly and shell volume, (ii) the simultaneous occurrence of megalospheric (A1 and A2 forms) and microspheric (B form) generations suggests that the paratrimorphic cycle was possibly the life strategy of Mardinella, (iii) symbiosis with unicellular algae was taking place during the late Paleocene, (iv) the evolutionary step from the peneropliform to the cyclical chamber arrangement suggests that, in soritids, the acquisition of epiphytic behaviour was arguably accompanied by a stable endosymbiosis with dinophytes.
Acknowledgements
Comments and suggestions made by the reviewers Mike Kaminski (Department of Geosciences, King Fahd University, Dhahran, Saudi Arabia) and Katica Drobne (Research Centre of the Slovenian Academy of Sciences and Arts, Ljubliana, Slovenia) are highly appreciated.
References
Bavi, O. A., Adabi, M. H., Sadeghi, A., and Amir-Bakhtiar, H. 2016. Diagenetic processes of the Ghorban Member of the Sachun Formation in type section (Tang-e-Mehdi, southern flank of the Ghareh Anticline south east Shiraz) [in Persian]. Journal of Stratigraphy and Sedimentology Researches 63: 1–22.
Benedetti, A., Consorti, L., Schlagintweit, F., and Rashidi, K. 2020. Ornatorotalia pila n. sp. from the late Palaeocene of Iran: ecological, evolutionary and paleobiogeographic inferences. Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1741572]. Crossref
Consorti, L., Boix, C., and Caus, E. 2016. Pseudorhapydionina bilottei sp. nov., an endemic foraminifera from the post-Cenomanian/Turonian boundary (Pyrenees, NE Spain). Cretaceous Research 59: 147–154. Crossref
Consorti, L., Caus, E., Frijia, G., and Yazdi-Moghadam, M. 2015. Praetaberina new genus (type species: Taberina bingistani Henson, 1948): a stratigraphic marker for the late Cenomanian. Journal of Foraminiferal Research 45: 378–389. Crossref
Consorti, L., Navarro-Ramirez, J.P., Bodin, S., and Immenhauser, A. 2018. The architecture and associated fauna of Perouvianella peruviana, an endemic larger benthic foraminifer from the Cenomanian–Turonian transition interval of central Peru. Facies 64: 2. Crossref
Cruz-Abad, E., Consorti, L., Di Lucia, M., Parente, M., and Caus, E. 2017. Fissumella motolae n. gen. n. sp., a new soritoidean (Foraminifera) from the lowermost Albian carbonate platform facies of central and southern Italy. Cretaceous Research 78: 1–7. Crossref
Debenay, J.P. and Payri, C.E. 2010. Epiphytic foraminiferal assemblages on Macroalgae in reefal environments of New Caledonia. Journal of Foraminiferal Research 40: 36–60. Crossref
Dettmering, C., Rӧttger, R., Hohenegger, J., and Schmaljohann, R. 1998. The trimorphic life cycle in Foraminifera: observations from cultures allow new evaluation. European Journal of Protistology 34: 363–368. Crossref
Goldstein, S. 1999. Foraminifera: A biological overview. In: K. Sen Gupta (ed.), Modern Foraminifera, 37–55. Kluwer, Dordrecht. Crossref
Hallock, P. 1999. Symbiont-bearing Foraminifera. In: K. Sen Gupta (ed.), Modern Foraminifera, 123–139. Kluwer, Dordrecht. Crossref
Harney, J.N., Hallock, P., and Talge, H.K. 1998. Observations on a trimorphic life cycle in Amphistegina gibbosa populations from the Florida Keys. Journal of Foraminiferal Research 28: 141–147. Crossref
Hart, M.B., FitzPatrick, M.E.J., and Smart, C.W. 2016. The Cretaceous/Paleogene boundary: Foraminifera, sea grasses, sea level change and sequence stratigraphy. Palaeogeography, Palaeoclimatology, Palaeoecology 441: 420–429. Crossref
Hayward, B.W., Le Coze, F., Vachard, D., and Gross, O. 2019. World Foraminifera Database. Azzarolina Vicedo and Serra-Kiel, 2015. http://www.marinespecies.org/foraminifera/aphia.php?p=taxdetails&id =874710
Heibati, Z., Shahvaran, S., and Kargar S. 2014. Introduction of Sachun member in intermediate subzone of Zagros [in Persian]. In: A. Shahidi (ed.), 33rd National Geosciences Congress of Iran, Abstracts, 1–8. Geological Survey of Iran, Tehran.
Hohenegger, J. 2000. Coenoclines of larger foraminifera. Micropaleontology 46 (Supplement 1): 127–151.
Hohenegger, J. 2004. Depth coenoclines and environmental considerations of Western Pacific larger Foraminifera. Journal of Foraminiferal Research 34: 9–33. Crossref
Hohenegger, J. 2011. Large Foraminifera—Greenhouse Constructions and Gardeners in the Oceanic Microcosm. 81 pp. The Kagoshima University Museum, Kagoshima.
Hohenegger, J., Kinoshita, S., Briguglio, A., Eder, W., and Wöger, J. 2019. Lunar cycles and rainy seasons drive growth and reproduction in nummulitid foraminifera, important producers of carbonate buildups. Scientific Reports 9: 8286. Crossref
Hohenegger, J., Yordanova, E., and Hatta, A. 2000. Remarks on West Pacific Nummulitidae (Foraminifera). Journal of Foraminiferal Research 30: 3–28. Crossref
Hohenegger, J., Yordanova, E., Nakano, Y., and Tatzreiter, Y. 1999. Habitats of larger foraminifera on the upper reef slope of Sesoko Island, Okinawa, Japan. Marine Micropaleontology 36: 109–168. Crossref
Hottinger, L. 1978. Comparative anatomy of elementary shell structures in selected larger foraminifera. In: R.H. Hedley and C.G. Adams (eds.), Foraminifera, 203–266. Academic Press, London.
Hottinger, L. 1977. Distribution of larger Peneroplidae, Borelis and Nummulitidae in the Gulf of Elat, Red Sea. Utrecht Micropaleontological Bulletins B 15: 35–110.
Hottinger, L. 1982. Larger foraminifera, giant cells with a historical backgroud. Naturwissenschaften 69, 361–371. Crossref
Hottinger, L. 2000. Functional morphology of benthic foraminiferal shells, envelopes of cells beyond measure. Micropaleontology 46 (Supplement 1): 57–86.
Hottinger, L. 2001a. Archaiasinids and related Porcelaneous larger Foraminifera from the late Miocene of the Dominican Republic. Journal of Paleontology 75: 475–512. Crossref
Hottinger, L. 2001b. Learning from the past. In: R. Levi-Montalcini (ed.), Discovery and Spoliation of the Biosphere. Frontiers of Life, Vol. 4, 449‒477. Academic Press, San Diego.
Hottinger, L. 2006. Illustrated glossary of terms used in foraminiferal research. Notebooks on Geology, Brest, Memoir 2006/2: 1–126 [available online at http://paleopolis.rediris.es/cg/uk_index.html_MO2]. Crossref
Hottinger, L. 2014. Paleogene Larger Rotaliid Foraminifera from the Western and Central Neotethys. 191 pp. Springer, Heidelberg. Crossref
James, G.A. and Wynd, J. G. 1965. Stratigraphic nomenclature of Iranian Oil Consortium Agreement Area. AAPG Bulletin 49: 2218–2232. Crossref
Langer, M., Makled, W.A., Pietsch, S.J., and Weinmann, A.E. 2009. Asynchronous calcification in juvenile megalospheres: An ontogenetic window into the life cycle and polymorphism of Peneroplis. Journal of Foraminiferal Research 39: 8–14. Crossref
Leutenegger, S. 1977. Reproduction cycles of larger foraminifera and depth distribution of generations. Utrecht Micropaleontological Bulletins 15: 27–34.
Meriç, E. and Çoruh, T. 1991. Mardinella, A new genus and discussion on Orbitolites shirazensis Rahaghi, 1983. Journal of Islamic Academy of Sciences 4: 166–169.
Nafarieh, E., Consorti, L., Ghasemi-Nejad, E., and Caus, E. 2019. Reticulotaberina n. gen. jahrumiana n. sp., a new soritoidean (Praerhapydioninidae) from the Eocene of Iran. Revue de Micropaléontologie 65: 100382. Crossref
Pochon, X. and Pawlowski, J. 2006. Evolution of the soritids-Symbiodinium symbiosis—a review. Symbiosis 42: 77–88.
Pochon, X., Garcia-Cuetos, L., Baker, A.C., Castella, E., and Pawlowski, J. 2007. One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in soritid foraminifera. Coral Reefs 26: 867–882. Crossref
Rahaghi, A. 1983. Stratigraphy and faunal assemblage of Paleocene–Lower Eocene in Iran. Ministry of Oil, National Iranian Oil Company, Geological laboratories, Teheran, Publication 10: 1–73.
Richardson, S.L. 2001. Endosymbiont change as a key innovation in the adaptive radiation of Soritida (Foraminifera). Paleobiology 27: 262–289. Crossref
Ross, C.H. 1972. Biology and ecology of Marginopora vertebralis (Foraminiferida), Great Barrier Reef. Journal of Protozoology 19: 181–192. Crossref
Salih, H.D. 2012. Larger benthic foraminiferal assemblages from Sinjar Formation, SW Sulaimaniyah City Kurdistan Region, Iraq. Iraqi Bulletin of Geology and Mining 8: 1–17.
Schlagintweit, F., Rashidi, K., and Kohkan, H. 2020. Coscinospira prima n. sp., a new peneroplid foraminifer from the Paleocene of Iran and its bearing on the phylogeny of the Peneroplidae. Journal of Mediterranean Earth Sciences 12: 31–42.
Schlagintweit, F., Rashidi, K., Yarahmadzahi, H., Habibimood, S., Amirshahkarmi, M., Ahmadi H., and Mirjalili, M. 2019. New data on some type species of Maastrichtian–Paleocene Dasycladales (Green algae) from Iran, Part 2 Hamulusella Elliott in Deloffre and Granier 1992. Micropaleontology 65: 407–423.
Serra-Kiel, J., Hottinger, L., Caus, E., Drobne, K., Ferrandez, C., Jauhri, A.K., Less, G., Pavlovec, R., Pignatti, J., Samsò, J.M., Schaub, H., Sirel, E., Strougo, A., Tambareau, Y., Tospquella, J., and Zakrebskaya, E. 1998. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bulletin de la Société Géologique de France 169: 281‒299.
Shabafrooz, R., Mahbobi, A., Mossavi-Harami, R., and Amiri-Bakhtiar, R. 2013. Facies analysis and sequence stratigraphy of the evaporite bearing Sachun Formation at the type locality, South East Zagros Basin, Iran. Carbonates and Evaporites 28:457–474. Crossref
Van der Ham, R.W.J.M., van Konijnenburg-van Cittert, J.H.A., and Indeherberge, L. 2007. Seagrass foliage from the Maastrichtian type area (Maastrichtian, Danian, NE Belgium, SE Netherlands). Review of Palaeobotany and Palynology 144: 301–321. Crossref
Vicedo, V. and Serra-Kiel, J. 2015. The new genus Azzarolina (Foraminifera): the false Taberina from the Paleocene of the Middle East. Journal of Foraminiferal Research 45: 369–377. Crossref
Zohary, T., Reiss, Z., and Hottinger, L. 1980. Population dynamics of Amphisorus hemprichii (Foraminifera) in the Gulf of Elat (Aqaba), Red Sea. Eclogae geologicae Helvetiae 73: 1071–1094.
Acta Palaeontol. Pol. 65 (3): 641–648,
2020 https://doi.org/10.4202/app.00703.2019