

The fossil record of camelids demonstrates a late divergence between Bactrian camel and dromedary
DENIS GERAADS, GILLES DIDIER, W. ANDREW BARR, DENNE REED, and MICHEL LAURIN
Geraads, D., Didier, G., Barr, W.A., Reed, D., and Laurin, M. 2020. The fossil record of camelids demonstrates a late divergence between Bactrian camel and dromedary. Acta Palaeontologica Polonica 65 (2): 251–260.
A new compilation of the Old World fossil record of Camelidae and a recent phylogenetic analysis allow a new assessment of the timing of the clade’s diversification. Using a recent implementation of the fossilized birth-death process, we show that the divergence between Bactrian camel and dromedary has a peak probability density around 1 Ma and probably occurred less than 2 million years ago. These dates are much younger than molecular estimates, which place the divergence between the dromedary and the Bactrian camel between 4 and 8 million years ago. Calibration problems in molecular dating seem to explain much of this difference.
Key words: Mammalia, Camelidae, phylogeny, divergence time, fossil record, birth-and-death models, Pleistocene, Africa.
Denis Geraads [denis.geraads@mnhn.fr] and Michel Laurin [michel.laurin@mnhn.fr, ORCID ID: http://orcid.org/ 0000-0003-2974-9835] (corresponding author), CR2P-UMR 7207, CNRS, MNHN, UPMC, Sorbonne Université, CP 38, 8 rue Buffon, 75231 Paris Cedex 05, France.
Gilles Didier [gilles.didier@umontpellier.fr], IMAG, Univ Montpellier, CNRS, Montpellier, France.
W. Andrew Barr [wabarr@gwu.edu], Center for the Advanced Study of Human Paleobiology, Department of Anthropology, The George Washington University, Washington DC 20052, USA.
Denné Reed [reedd@austin.utexas.edu], Department of Anthropology, University of Texas at Austin. Austin, TX 78712, USA.
Received 22 January 2020, accepted 4 March 2020, available online 16 April 2020.
Copyright © 2020 D. Geraads et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Dating the Tree of Life has become an important goal in systematics because timetrees are now routinely used in comparative analyses that study character correlation and hence impact functional biology, in fields as diverse as biomechanics (e.g., Almécija et al. 2015), physiology (e.g., Legendre et al. 2016; Uyeda et al. 2017) and genomics (e.g., Liedtke et al. 2018; Organ et al. 2011). Timetrees are also used to study the evolution of biodiversity through time, using birth-and-death models (Nee et al. 1994; Didier et al. 2017). It is relatively easy to obtain average diversification rates over time (e.g., Höhna et al. 2011), but studying phenomena such as mass extinction events (e.g., Soul and Friedman 2017) and evolutionary radiations (Quental and Marshall 2009), is difficult to do without incorporating fossil data (Rabosky 2009; Sanmartín and Meseguer 2016). Timetrees are even used in conservation biology, through the use of the Phylogenetic Diversity Index (Faith 1992), which allows a better quantification of biodiversity than taxon counts (Bertrand et al. 2006).
Dating the Tree of Life was initially an essentially paleontological enterprise (Hennig 1981; Laurin 2012), given that fossils provide the only direct evidence of past biodiversity. However, with the development of molecular dating methods, efforts on that front have progressively shifted increasingly towards molecular phylogenetics, which has benefited from the development of many sophisticated methods (see review in Sauquet 2013) that take into consideration variations in molecular evolutionary rates and can use a variety of time constraints with detailed prior information (e.g., Guindon 2018) or incorporate extinct taxa from the fossil record into the analysis (Pyron 2011; Ronquist et al. 2012a, b). These sophisticated developments and the intensive sequencing efforts of the last decades have resulted in such an explosion of the number of available molecular sequences that the limiting factor in dating the Tree of Life accurately is arguably the lack of reliable time constraints, most of which are derived from the fossil record (e.g., Benton and Donoghue 2007; Parham et al. 2012). Indeed, recent studies suggest that the calibration constraints have great influence over the resulting molecular dates (e.g., Marjanović and Laurin 2007; Warnock et al. 2015) and that insufficient effort has been made to obtain reliable constraints (e.g., Parham et al. 2012; Sterli et al. 2013).
This relative paucity in good dating constraints may explain the frequent discrepancies between molecular and paleontological estimates of clade ages, with molecular ages typically being significantly older than paleontological ages (e.g., Marjanović and Laurin 2007). It is tempting to attribute these discrepancies to the incompleteness of the fossil record, which, after all, only directly provides minimal age estimates. While this may be the right explanation in some cases, this phenomenon is so pervasive that it is unlikely to be the whole answer, and other factors such as variations in generation time can influence molecular rates of evolution and explain some of these discrepancies (e.g., Springer et al. 2017). In any case, palaeontologists often state that some clades must be significantly more recent than their purported molecular estimates, even when taking into consideration the incompleteness of the fossil record (e.g., Fountaine et al. 2005; Wible et al. 2007; Marjanović and Laurin 2008) and uncertainties about the systematic position of some fossils (e.g., Sterli et al. 2013).
To resolve this controversy, improved methods are required to obtain less biased estimates of clade ages based on the fossil record. Such methods should ideally produce probability densities of nodal ages, rather than only the minimal ages that have long been available. Fortunately, important progress has been made on this front recently, using birth-and-death models (Heath et al. 2014; Zhang et al. 2016). Below, we use such a method (Didier and Laurin 2020) to reassess the age of the divergence between two charismatic camelids, the Bactrian camel (Camelus bactrianus) and the dromedary (Camelus dromedarius), for which fieldwork by several of the authors (DG, WAB, and DR) has produced important new data with well constrained geochronological ages.
Camelidae is an ideal taxon to carry out this study because recent molecular dating studies suggest an age for the Bactrian camel/dromedary divergence of about 4 Ma (Wu et al. 2014; Heintzman et al. 2015), or as much as 8 Ma (Cui et al. 2007). Recently, to calibrate their molecular tree documenting the diversification of wild and domestic populations of Bactrian camels, Ming et al. (2020) assumed a camel/dromedary divergence age of 5.73 Ma based on the TimeTree database (Kumar and Hedges 2011), which summarizes the published molecular ages. However, the fossil record suggests a much younger date of 1–2 Ma (Geraads et al. 2019). Given the two- to eight-fold difference between these two sets (molecular and paleontological) of estimates, an attempt at refining the paleontological estimate of this divergence seems timely. Fortunately, the Neogene fossil record of Camelidae has been well-studied, and several diagnostic characters allow us to recognize unambiguously stem-members of various taxa, including those of the Bactrian and the dromedary camels. Our recent phylogenetic analysis of Neogene Old World Camelidae (Geraads et al. 2019) allows us to reappraise the timing of diversification of this clade.
Material and methods
Geraads et al. (2019) produced three equiparsimonious trees using a total of 22 characters, all ordered, analysed using parsimony in TNT (Goloboff et al. 2003; Goloboff and Catalano 2016) and PAUP*4 (Swofford 2003), which provide the topologies that we use to date the evolutionary radiation of Old World Camelini. In addition to Camelus species, the analysis included Paracamelus gigas Zdansky, 1926, P. alexejevi Khaveson, 1950, and P. alutensis (Ştefănescu, 1895). The North American Megacamelus merriami (Frick, 1921), which is the best-known close relative of Old World camels, was used as an outgroup.
To estimate the timing of the camelid evolutionary radiation, we used a recently-developed computer program (DateFBD) that implements a method that rests on the fossilized birth-and-death model (Didier 2019). Didier and Laurin (2020) provided a method able to compute the distribution of any divergence time of the tree given the speciation (equated with cladogenesis, in this case), extinction and fossilization rates (designated collectively as “rates” below), the tree topology and the (exact) ages of the fossils. In practical applications, the uncertainty on the rates, on the fossil ages and on the tree topologies has to be handled. In the case where there are a great number of equiparsimonious trees (as in Didier and Laurin 2020), the divergence time distributions over all these trees can be summed. Since we have only three equiparsimonious trees in this dataset, we analysed each tree separately. In order to deal with uncertainty on the rates and on the fossil ages, the approach sums the probability densities of nodal ages over all the possible values of rates and fossil ages by using an importance sampling procedure (Didier and Laurin 2020). We assume an improper uniform prior distribution over [0,∞] for the speciation, extinction and fossilization rates. Each fossil age is entered as a range of possible ages (Table 1; SOM 1 and 2, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Geraads_etal_SOM.pdf), and we assume a flat distribution within that range to sample each occurrence within that range. The biased density used in the importance sampling procedure weights each combination of possible fossil ages and rates proportionally to the probability of the tree with these fossil ages under the fossilized birth-and-death model with these speciation, extinction and fossilization rates (Didier and Laurin 2020). Sampling this distribution requires a Monte Carlo Markov chain approach and provides the posterior distributions of all the parameters sampled, notably of the speciation, extinction and fossilization rates, which are displayed in Fig. 1.
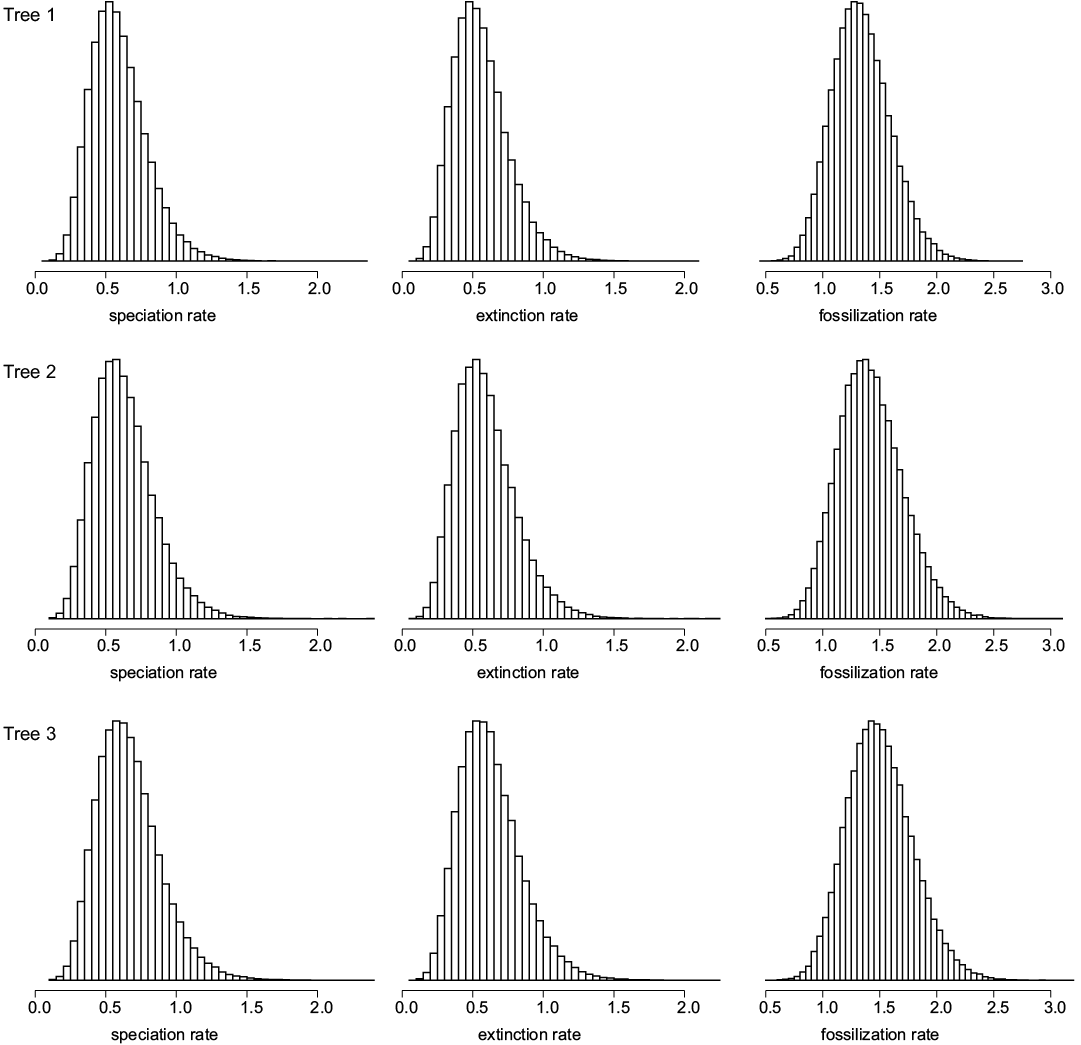
Fig. 1. Probability density histograms of speciation (cladogenesis), extinction and fossilization rates for the three equiparsimonious trees. All rates are in events per lineage and per million years. The height of each box of the plots is proportional to the posterior probability for the corresponding rate to be in the interval delineating its base.
Table 1. List of the records used in “DateFBD”. We list only those records that can be identified to species with good confidence; this explains why a number of Camelus records, even if identified to species by other authors, are left aside. Note that several of the dates are only biochronological estimates. The data that are actually used in the analysis (see SOM 1) are in the second column. Notes: 1 the type-specimen is hardly fossilized; 2 identified as Paracamelus gigas, but certainly incorrect; 3 species identification likely, but not quite certain; 4 the earliest and most reliable record for the living species; 5 no associated fauna reported; age probably within the 6.2–3.45 Ma range; 6 Plio-Pleistocene (= early Pleistocene in modern terminology) for Teilhard de Chardin and Trassaert (1937), but with little support; contemporaneous with Dinofelis abeli, dated by Werdelin and Peigné (2010) 4.5–3.5 Ma; 7 Chron C2An; 8 stratigraphic origin (“Pontian”) very uncertain; associated with zygolophodont mastodont (i.e., “Mammut” borsoni), but this species survives until well into the Pliocene; 9 said to be of middle Pliocene age, but age unsupported—we discard this report; 10 this occurrence would be earlier than 4 Ma, but Khaveson (1954) clearly stated that the fossil was not found in situ—we discard this report; 11 the purported stratigraphic origin is very doubtful, because there are very few large mammals in the Odessa limestone, and the preservation of the reported fossil suggests a karstic filling rather than a limestone—we discard this report. Abbreviations: AMNH, American Museum of Natural History, New York, USA; CCEC, Centre de Conservation et d’Etude des Collections, Lyon, France; INSAP, Institut National des Sciences de l’Archéologie et du Patrimoine, Rabat, Morocco; KNM, National Museums of Kenya, Nairobi, Kenya; MEUU, Museum of Evolution, Uppsala University, Sweden; MNHN, Muséum National d’Histoire Naturelle, Paris, France; MNCN, Museo Nacional de Ciencias Naturales, Madrid, Spain; MTA, Sehit Cuma Dag Natural History Museum, Ankara, Turkey; NHMUK, Natural History Museum, London, UK; NME, National Museum of Ethiopia, Authority for Research and Conservation of Cultural Heritage, Addis-Ababa, Ethiopia; NMNHU, National Museum of Natural History, Kiev, Ukraine; SVCP, Service de Valorisation des Collections de Paléontologie, N’Djamena, Chad; UCBL, Université Claude Bernard, Lyon, France; ZIN, Zoological Institute, Saint Petersburg, Russia.
|
Taxa, age range, and localities (type locality first) |
Age range of the locality (Ma) |
Material |
Housed in |
References |
Seen by the authors |
|
Camelus sivalensis Falconer and Cautley, 1836 (2.8–0.6 Ma) |
|||||
|
Pinjor Stage, |
2.6–0.6 |
about 120 cranial and post-cranials specimens |
NHMUK |
yes |
|
|
Boulder Conglomerate (India) |
1.7–0.6 |
partial cranium and other
cranial |
AMNH |
yes |
|
|
Quranwala (India) |
2.8–2.6 |
cranium |
Panjab University, Chandigarh, India |
no |
|
|
Chandigarh, Pinjor Formation (India) |
2.6–0.6 |
partial maxilla and mandible |
Panjab University, Chandigarh, India |
no |
|
|
Camelus thomasi Pomel, 1893 (1.2–0.5 Ma) |
|||||
|
Tighennif (Algeria) |
1.2–0.8 |
complete cranium, 45 other cranial and postcranial elements |
MNHN |
yes |
|
|
Grotte des Rhinocéros (Morocco) |
0.6–0.5 |
isolated teeth, metatarsal |
INSAP |
yes |
|
|
Camelus grattardi Geraads, 2014 (2.9–2.2 Ma) |
|||||
|
Omo Shungura Member G4 (Ethiopia) |
2.25–2.1 |
maxilla |
NME |
yes |
|
|
Omo Shungura Member G3 (Ethiopia) |
2.25–2.1 |
partial mandible |
NME |
yes |
|
|
Omo Shungura Member D5 (Ethiopia) |
2.5–2.4 |
phalanx |
NME |
yes |
|
|
Mille Logya, Seraitu Beds (Ethiopia) |
2.9–2.4 |
partial cranium, isolated
teeth, |
NME |
yes |
|
|
West Turkana, Upper Lomekwi (Kenya) |
2.7–2.6 |
mandible |
KNM |
yes |
|
|
Camelus knoblochi Nehring, 1901 (0.8–0.01 Ma) |
|||||
|
Luchka, Volga River (Russia) |
0.2–0.01 |
partial cranium 1 |
ZIN |
yes |
|
|
Sjara-osso-gol (China) |
0.05–0.03 |
metatarsal, partial cranium,
|
MNHN |
yes |
|
|
Wulanmulan (China) |
0.07–0.03 |
maxilla |
Ordos Museum, Ordos, China |
no |
|
|
Lakhuti-2 (Tajikistan) |
0.8–0.6 |
no details |
? |
no |
|
|
Koshkurgan (Kazakhstan) |
0.4–0.3 |
teeth and postcranials 2 |
? |
no |
|
|
Tiraspol (Moldova) |
0.8–0.6 |
no details |
? |
no |
|
|
Russia, Ukraine |
0.4–0.1 |
several crania |
various |
no |
|
|
Taxa, age range, and localities (type locality first) |
Age range of the locality (Ma) |
Material |
Housed in |
References |
Seen by the authors |
|
Camelus bactrianus Linnaeus, 1758 (Holocene and Recent) |
|||||
|
various localities |
0 |
17 Recent skulls |
MNHN; CCEC; ZIN |
|
yes |
|
Shahr-i-Sokhta (Iran) |
0.004–0.003 |
a few post-cranials 3 |
? |
no |
|
|
Sialk (= Siyalk) (Iran) |
0.005–0.004 |
engravings 3 |
? |
no |
|
|
Anau (Turkmenistan) |
0.005–0.004 |
no details 3 |
? |
no |
|
|
Camelus dromedarius Linnaeus, 1758 (Holocene and Recent) |
|||||
|
various localities |
|
34 Recent skulls |
MNHN; CCEC |
|
yes |
|
Al Gharbia (Abu Dhabi) |
0.006–0.007 |
numerous skeletons 4 |
mostly uncollected? |
Beech et al.
2009; |
no |
|
Paracamelus gigas Schlosser, 1903 (6.2–3.45 Ma) |
|||||
|
Loc. 102, Henan (China) |
? 5 |
partial skeleton |
MEUU |
no |
|
|
Gaozhuangian Mammal Age (China) |
4.9–3.6 |
no details |
? |
no |
|
|
Locs. 11, 17, 26, |
4.5–3.5 6 |
partial jaws |
Natural History Museum, Tianjin, China |
no |
|
|
Venta del Moro, Librilla (Spain) Paracamelus sp. |
6.2–5.7 |
isolated teeth, a few postcranials |
MNCN |
Morales 1984;
|
no |
|
Kossom Bougoudi (Chad) Paracamelus sp. |
6–5 |
mandible and metapodials |
SVCP |
yes |
|
|
Paracamelus alutensis Ştefănescu, 1895 (3.5–1.5 Ma) |
|||||
|
Milcovul de Jos (Romania) |
2.5–1.5 |
mandible |
cast in UCBL |
yes (cast) |
|
|
Khapry-Liventsovka (Russia) |
2.5–2.2 |
more than 200 bones |
? |
no |
|
|
Fratesti (Romania) |
2.5–1.5 |
partial mandible |
Fratesti school ? |
no |
|
|
Oltet Valley (Romania) |
2–1.5 |
no details |
? |
no |
|
|
Dolinskoe (Moldova) |
2–1.5 |
no details |
? |
no |
|
|
Cherevichnoe (Ukraine) |
2.5–2.2 |
a few findings |
? |
no |
|
|
Sarikol Tepe (Turkey) |
2.5–2.3 |
maxilla, partial mandible, metapodials |
MTA |
no |
|
|
karstic fillings of the Odessa catacombs (Ukraine) |
3.5–3 |
partial mandible |
NMNHU |
no |
|
|
Paracamelus alexejevi Khaveson, 1950 (6–3 Ma) |
|||||
|
karstic fillings of the Odessa catacombs (Ukraine) |
3.5–3 7 |
abundant material, but fragmentary and partly reconstructed |
Paleontological Museum, Odessa, Ukraine |
no |
|
|
Cherkassy (Ukraine) |
6–3 8 |
partial mandible |
? |
no |
|
|
Kagul district (Moldova) |
? 9 |
partial cranium |
? |
no |
|
|
Pavlodar (Kazakhstan) |
? 10 |
phalanx |
? |
no |
|
|
Odessa limestone (Ukraine) |
? 11 |
partial mandible |
Geological Institute, Russian Academy of Sciences, Moscow, Russia |
no |
|
|
Megacamelus merriami (Frick, 1921) (6.7–4.8 Ma) |
|||||
|
Mount Eden Formation, Keams Canyon, Santee and Devil’s Nest (Hemphillian 3–4; USA) |
6.7–4.8 |
abundant cranial |
AMNH |
yes |
|
Results
The density of the speciation (cladogenesis) rate peaks at 0.51, 0.55, and 0.57 events per lineage and per My, with 95% confidence intervals of [0.23, 0.98], [0.25, 0.99], and [0.26, 1.11] for trees 1, 2, and 3, respectively. The extinction rate density has modes at 0.47, 0.53, and 0.54 events per lineage and per My, with 95% confidence intervals of [0.22, 0.95], [0.21, 0.94], and [0.23, 1.06] for trees 1, 2, and 3, respectively. The fossilization rate density has peaks at 1.29, 1.35, and 1.43 fossiliferous horizons per lineage and per My with 95% confidence intervals of [0.88, 1.85], [0.86, 1.84], and [0.95, 2.06] for trees 1, 2, and 3, respectively.
According to our age estimates, most clades have a peak probability density at an age that is compatible with a fairly literal reading of the fossil record (Fig. 2), which suggests that this record is less incomplete than it has been claimed by molecular systematists (Cui et al. 2007; Wu et al. 2014; Heintzman et al. 2015). The Camelus crown-group has a peak density at an age just under 1 Ma (0.95 Ma for trees 1 and 2 and 0.93 Ma for tree 3), and its density dwindles to near 0 between 2 and 3 Ma (95% confidence intervals are [1.82, 0.75] for trees 1 and 2 and [1.73, 0.74] for tree 3; Fig. 3). The more inclusive Camelus clade (whose oldest members are all extinct) has a peak density at 3. Ma with 95% confidence interval [4.78, 2.77] for trees 1 and 2 and at 2.97 Ma with 95% confidence interval [4.57, 2.75] for tree 3. The probability densities dwindle to near 0 around 6.5 Ma for the three trees (Fig. 3).
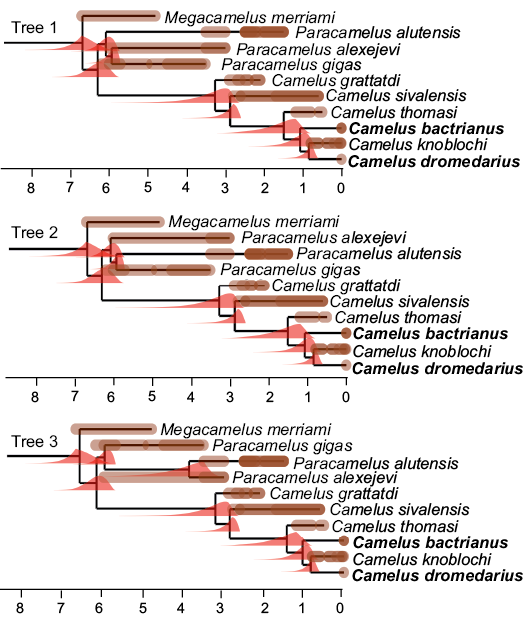
Fig. 2. Time-calibrated equiparsimonious trees. At each node, the probability density computed by diversification is shown (in red, all displaying a left skew). The age of each fossil record (in million years) is shown as a brown bar along each branch, which extends from the oldest to the youngest plausible age for each record. Darker shades represent overlapping possible age ranges, whereas brown dots represent very well-dated fossils. Extant taxa are in bold. A monophyletic Camelus is diagnosed by the loss of p3 and a smaller P3. The Paracamelus clade is diagnosed by a long muzzle. Camelus grattardi lacks derived characters of other representatives of the Camelus clade, the paraglenoid process, a shallower infra-orbital shelf, an oblique ascending ramus of the mandible, a thickened corpus, a broader P4 relative, and long ligament scars on the phalanges. The position of the poorly studied Camelus knoblochi relative to extant forms rests only on the morphology of the choanae.
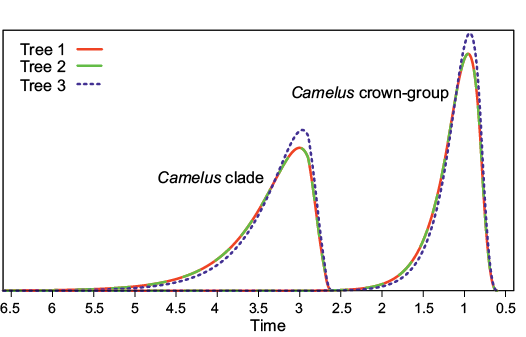
Fig. 3. Probability density of the age of crown (extant) Camelus and of Camelus itself (including older stem-species, namely Camelus grattardi, Camelus sivalensis, and Camelus thomasi) for the three equiparsimonious trees. These were computed by DateFBD (Didier 2019). The curves for trees 1 (red) and 2 (green) overlap (curve with long dashes). Only tree 3 (blue) yields a different probability density. The probability for a divergence time to be in a given time interval is proportional to the surface below its probability density curve for this interval of time (it is exactly the probability of being in the interval if one assumes that the total surface below the probability density curve is equal to one).
Discussion
The relatively narrow probability densities for the rates (Fig. 1) suggest that our results are fairly reliable. All the simulations passed the convergence tests implemented in the coda package with effective sizes above 10 000 samples (Plummer et al. 2006). Estimated speciation and extinction rates for Neogene camelids are about twice as high as those obtained by Didier and Laurin (2020) on Permo-Carboniferous amniotes, whereas the fossilization rates are about 40 times as high. The latter discrepancy presumably reflects the much greater collection effort in Neogene sediments than in those of the late Paleozoic, as well as their greater availability (Bouysse et al. 2000). Whether the more modest discrepancies in speciation and extinction rates reflect genuine differences in evolutionary dynamics between Permo-Carboniferous amniotes and Neogene camelids is less certain because this is only the second empirical application of our method (the first one being found in Didier and Laurin 2020), but the simulations performed so far (Didier and Laurin 2020) suggest that rate estimates should improve with stratigraphic sampling density. This suggests that the rates for Neogene camelids should be reliable, despite the relatively low number of sampled taxa.
The differences in rates and ages between the three trees are modest, especially between trees 1 and 2. The slightly greater differences between results of the third tree and those of the other two trees can probably be explained by the fact that tree 3 places Paracamelus gigas, which is geologically older than the two other species of Paracamelus, in a basal position within Paracamelus. This results in younger ages for Paracamelus, for the node subtending Paracamelus and Camelus, and even for the Camelus crown. Nevertheless, these differences remain subtle and do not affect our main conclusions because the molecular ages with which we can compare our results differ greatly from our estimates.
This estimated divergence date between the lineages leading to both extant Camelus species (0.94 Ma) is only slightly older than the minimal age of 0.6–0.8 Ma that we had recently reported (Geraads et al. 2019); it is in fairly good agreement with the discovery in Syria at 450 ky of a camel said to be intermediate (unfortunately, no details have been published yet) between C. bactrianus and C. dromedarius (Martini et al. 2015). Indeed, the 95% confidence intervals on our estimate of the divergence date between Bactrian camel and dromedary extends until 0.75 Ma, and after this divergence, the phenotypes of both lineages would have diverged gradually. Back at 0.5 Ma, the phenotypes of both lineages should be markedly closer to each other than today. By contrast, our age estimate is much more recent than the estimates provided by molecular dating: c. 4.4 Ma according to Wu et al. (2014) using the whole genome, and c. 4.1 Ma by Heintzmann et al. (2015), based partly on the genome of the late Middle Pleistocene American Camelops. Based on mtDNA sequence, Cui et al. (2007) even suggested that this divergence occurred around 8 Ma, which is before the Camelidae immigrated into the Old World, where its earliest securely dated record is at Librilla, Spain, c. 6.3 Ma (Alberdi et al. 1981). Our results strengthen the conclusions of Geraads et al. (2019) and are incompatible with the molecular ages mentioned above. However, these strong discrepancies with recent molecular estimates should be explained.
Cui et al. (2007) calibrated their tree using two calibration constraints: a cow/pig divergence, set at 65 Ma, and a mouse/rat divergence, set at 14 Ma. Both constraints deserve comments. The first (cow/pig divergence) was taken from Springer et al. (2003), a molecular study of placental mammal diversification. As such, that constraint is a secondary calibration, a practice that has been criticized (Shaul and Graur 2002) for adding uncertainty that is not necessarily incorporated into subsequent estimates. This is unfortunately the case here, to the extent that Cui et al. (2007) do not report credibility or confidence intervals for the estimated ages. In addition, a cow/pig divergence at 65 Ma implies a substantial cryptic biodiversity of Mesozoic placentals, which analyses of the fossil record refute (Davies et al. 2017). The other calibration, between mouse and rat, was set at an older age than suggested by Benton and Donoghue (2007: table 1), who suggested an age of 11–12.3 Ma, and Kimura et al. (2015), who suggested an age of about 12 Ma. More importantly, the phylogenetic analysis of Patnaik (2014), which is probably the most thorough paleontological study of the Mus/Rattus divergence, suggests an even younger minimal age of only 7.3 Ma. However, comparisons between the results of Patnaik (2014) and Kimura et al. (2015) are complicated by differences in taxonomic sample and topology. Nevertheless, it seems fair to state that the fossil record indicates that the minimal age of the Mus/Rattus divergence is less than 14 Ma, given that Patnaik (2014) and Kimura et al. (2015) agreed on this point. Thus, Cui et al. (2007) probably overestimated the age of the mouse/rat divergence by 15–90% (an admittedly large bracket that reflects considerable uncertainty in the implications of the murine fossil record). In addition, the assumption of a constant rate of change was rejected for that dataset (Cui et al. 2007: 5), and all the problems evoked here, combined with the lack of confidence or credibility intervals, may explain much of the discrepancy between the age of 8 Ma reported by Cui et al. (2007) and our estimate.
The study by Wu et al. (2014) similarly displays calibration problems. It used four constraints: Homo sapiens/Mus musculus (61.5–100.5 Ma), Canis lupus familiaris/Equus caballus (62.3–71.2 Ma), Bos taurus/H. sapiens (95–113 Ma), all from Benton and Donoghue (2007), and H. sapiens/Monodelphis domestica (124.3–138.4 Ma), reportedly from a web site (http://www.fossilrecord.net/) that seems to no longer work. The three constraints taken from Benton and Donoghue (2007) appear much too old. Like the constraints used by Cui et al. (2007), they imply a substantial cryptic biodiversity of Mesozoic placentals, which are refuted by the analyses of Davies et al. (2017). They also rely on taxonomic affinities of mostly fragmentary Cretaceous mammals that have been refuted by subsequent research. For instance, the Bos taurus/H. sapiens divergence calibration set at 95–113 Ma relies on the age of Bobolestes, Paranyctoides, and Batodon, which Benton and Donoghue (2007: 34) suggest fit within this divergence. However, the phylogenetic analyses of Wible et al. (2007), O’Leary et al. (2013) and Carrillo and Asher (2017) placed these taxa on the placental stem and suggested that crown-placentals were known only from the Cenozoic. These problems with calibration constraints are serious enough to cast doubt about the reliability of the ages reported by Wu et al. (2014).
Heintzman et al. (2015) used three constraints. One is a prior on the root of crown-group Artiodactyla placed at 59 Ma with a lognormal distribution, with 90% of the prior distribution placed between 52.5 and 66 Ma, based on the first occurrence of Himalayacetus in the fossil record. Benton et al. (2015: 67) reported that Himalayacetus was known only from a partial dentary and two molars and that it may be related to ambulocetids or pakicetids, so this constraint appears to be correct. Another constraint is a normal prior with a mean of 17.5 Ma and a standard deviation of 1.52 Ma for the divergence between Camelini and Lamini, based on the first appearance of Aepycamelus (Lamini), and this seems to reflect the current consensus (Gasparini et al. 2017). The third constraint is a prior with a mean of 4.4 Ma and a standard deviation of 1.43 Ma for the Camelus bactrianus/Camelus dromedarius divergence taken from Wu et al. (2014), which is subject to the caveats mentioned above. Thus, while two of the three constraints appear to be appropriate based on current knowledge, the node of interest (divergence between C. bactrianus and C. dromedarius) had been constrained on the age obtained by Wu et al. (2014), which is most likely inflated, as explained above.
To sum up, the differences between our paleontological results and those of recent molecular studies do not appear to result from conflicting signals from these two types of data. Rather, they result from a suboptimal use of the fossil record to constrain molecular dating analyses. This is not surprising because the paleontological literature is rich and widely scattered. We suggest that future endeavors in this field would be best undertaken by research groups incorporating both molecular systematists and paleontologists, as has been done for some of the best works in this field (e.g., Ronquist et al. 2012a).
Conclusions
Our reasonably well-constrained divergence dates (Figs. 2, 3) show that the recently-developed paleontological dating method based on the fossilized birth-death process (Didier and Laurin 2020) works well with datasets of a modest size (here, with 10 species) and in clades with a moderately-rich fossil record (here, a total of 38 stratigraphic occurrences). Thus, this method could profitably be used in a wide variety of taxa with a reasonably rich fossil record and a sufficiently complex morphology that allows placing fossils in a phylogeny. Such taxa range in time at least from the Cambrian (e.g., trilobites) to the present. An extensive use of such methods in palaeontology should significantly improve the accuracy and reliability of our calibration of the Tree of Life, both by directly estimating divergence dates of clades that are well-represented in the fossil record, and through the use of the resulting probability density distributions of divergence times to better constrain molecular clocks of clades with a poor fossil record. This should significantly enhance the integration of palaeontologists into the rapidly-growing community of systematists involved in dating the Tree of Life.
Acknowledgements
This project relies to a large extent on data collected through the Mille-Logya Project (MLP) led by Zeresenay Alemseged (University of Chicago, USA), whom we thank for his work which has made this study possible. We thank both reviewers, Marcelo Sánchez-Villagra (University of Zurich, Switzerland) and John Rowan (University of Massachusetts, Amherst, USA), for their constructive comments on an earlier version of the paper, and the editor Olivier Lambert (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium), for his efficient handling of the draft.
References
Alberdi, M.T., Morales, J., Moya, S., and Sanchiz, B. 1981. Macrovertebrados (Reptilia y Mammalia) del yacimiento finimioceno de Librilla (Murcia). Estudios geológicos 37: 307–312
Almécija, S., Orr, C.M., Tocheri, M.W., Patel, B.A., and Jungers, W.L. 2015. Exploring phylogenetic and functional signals in complex morphologies: The hamate of extant anthropoids as a test-case study. The Anatomical Record 298: 212–229. Crossref
Baigusheva, V.S. 1971. Fossil theriofauna of the Liventzovka sand-pit [in Russian]. Trudy Zoologičeskogo instituta 49: 5−29.
Beech, M., Mashkour, M., Huels, M., and Zazzo, A. 2009. Prehistoric camels in south-eastern Arabia: the discovery of a new site in Abu Dhabi’s Western Region, United Arab Emirates. Papers from the forty-second meeting of the Seminar for Arabian Studies held in London, 24–26 July 2008. Proceedings of the Seminar for Arabian Studies 39: 17–30.
Benecke, N. 1994. Der Mensch und seine Haustiere: die Geschichte einer jahrtausendealten Beziehung. 470 pp. Theiss, Stuttgart.
Benton, M.J. and Donoghue, P.C.J. 2007. Paleontological evidence to date the tree of life. Molecular Biology and Evolution 24: 26–53. Crossref
Benton, M.J., Donoghue, P.C., Asher, R.J., Friedman, M., Near, T.J., and Vinther, J. 2015. Constraints on the timescale of animal evolutionary history. Palaeontologia Electronica 18: 1–107. Crossref
Bertrand, Y., Pleijel, F., and Rouse, G.W. 2006. Taxonomic surrogacy in biodiversity assessments, and the meaning of Linnaean ranks. Systematics and Biodiversity 4: 149–159. Crossref
Boule, M., Breuil, H. Licent, E., and Teilhard de Chardin, P. 1928. Le Paléolithique de la Chine. Archives de l’Institut de Paléontologie humaine 4: 1–138.
Bouysse, P., Acharyya, S.K., Brezhnev, V.D., et al. 2000. Geological Map of the World. Commission for the Geological Map of the World and the UNESCO, Paris.
Bulliett, R.W. 1975. The Camel and the Wheel. 327 pp. Harvard University Press, Cambridge.
Carrillo, J.D. and Asher, R.J. 2017. An exceptionally well-preserved skeleton of Thomashuxleya externa (Mammalia, Notoungulata), from the Eocene of Patagonia, Argentina. Palaeontologia Electronica 20.2.34A: 1–33. Crossref
Colbert, E.H. 1935. Siwalik Mammals in the American Museum of Natural History. Transactions of the American Philosophical Society 26: 1–401. Crossref
Compagnoni, B. and Tosi, M. 1978. The camel: its distribution and state of domestication in the Midle East during the third millenium B.C. in light of finds from Shahr-I Sokhta. In: R.H. Meadow and M.A. Zeder (eds.), Approaches to Faunal Analysis in the Middle East. Peabody Museum Bulletin 2: 91–98.
Cui, P., Ji, R., Ding, F., Qi, D., Gao, H., Meng, H., Yu, J., Hu, S., and Zhang, H. 2007. A complete mitochondrial genome sequence of the wild two-humped camel (Camelus bactrianus ferus): an evolutionary history of Camelidae. BMC Genomics 8: 241. Crossref
Davies, T.W., Bell, M.A., Goswami, A., and Halliday, T.J. 2017. Completeness of the eutherian mammal fossil record and implications for reconstructing mammal evolution through the Cretaceous/Paleogene mass extinction. Paleobiology 43: 521–536. Crossref
Didier, G. 2019. DateFBD [software]. https://github.com/gilles-didier/DateFBD
Didier, G. and Laurin, M. 2020. Exact distribution of divergence times from fossil ages and tree topologies. Systematic Biology syaa021 [published online, https://doi.org/10.1093/sysbio/syaa021]. Crossref
Didier, G., Fau, M., and Laurin, M. 2017. Likelihood of tree topologies with fossils and diversification rate estimation. Systematic Biology 66: 964–987. Crossref
Dong, W., Hou, Y., Yang, Z., Zhang, L., Zhang, S., and Liu, Y. 2014. Late Pleistocene mammalian fauna from Wulanmulan Paleolithic Site. Quaternary International 347: 139–147. Crossref
Faith, D.P. 1992. Conservation evaluation and phylogenetic diversity. Biological Conservation 61: 1–10. Crossref
Falconer, H., and Cautley, P.T. 1836. Note on the fossil camel of the Sivalik Hills. Asiatic Researches 19: 115–134.
Fountaine, T.M.R., Benton, M.J., Dyke, G.J., and Nudds, R.L. 2005. The quality of the fossil record of Mesozoic birds. Proceedings of the Royal Society of London, Series B 272: 289–294. Crossref
Frick, C. 1921. Extinct vertebrate faunas of the badlands of Bautista creek and San Timoteo cañon, Southern California. University of California Publications, Bulletin of the Department of Geology 12: 277–424.
Gasparini, G.M., De los Reyes, M., Francia, A., Scherer, C.S., and Poiré, D.G. 2017. The oldest record of Hemiauchenia Gervais and Ameghino (Mammalia, Cetartiodactyla) in South America: Comments about its paleobiogeographic and stratigraphic implications. Geobios 50: 141–153. Crossref
Gaur, R., Raghavan, P., and Chopra, S.R.K. 1984. On the remains of Camelus sivalensis (Artiodactyla, Camelidae) from Pinjor Formation of Upper Sivaliks. Indian Journal of Earth Sciences 11: 158–165.
Geraads, D. 2014. Camelus grattardi nov. sp., a new camel from the Shungura Formation, Omo valley, Ethiopia, and the relationships of African fossil Camelidae (Mammalia). Journal of Vertebrate Paleontolology 34: 1481–1485. Crossref
Geraads, D. and Bernoussi, R. 2016. Suidae and Camelidae. In: J.-P. Raynal and A. Mohib (eds.), Préhistoire de Casablanca. 1. La Grotte des Rhinocéros (Fouilles 1991 et 1996). Villes et Sites Archéologiques du Maroc 6: 133–134.
Geraads, D., Barr, W.A., Reed, D., Laurin, M., and Alemseged, Z. 2019. New remains of Camelus grattardi (Mammalia, Camelidae) from the Plio-Pleistocene of Ethiopia and the phylogeny of the genus. Journal of Mammalian Evolution [published online, https://doi.org/10.1007/s10914-019-09489-2]. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221−238. Crossref
Goloboff, P.A., Farris, J., and Nixon, K. 2003. TNT, Tree Analysis Using New Technology [software]. www.lillo.org.ar/phylogeny/tnt
Guindon, S. 2018. Accounting for calibration uncertainty: Bayesian molecular dating as a “doubly intractable” problem. Systematic Biology 67: 651–661. Crossref
Harris, J. M. 1991. Camelidae. In: J.M. Harris (ed.), Koobi Fora Research Project. Volume 3: The Fossil Ungulates: Geology, Fossil Artiodactyls and Palaeoenvironments, 86–91. Clarendon Press, Oxford.
Harrison, J.A. 1985. Giant camels from the Cenozoic of North America. Smithsonian contributions to Paleobiology 57: 1–29. Crossref
Heath, T.A., Huelsenbeck, J.P., and Stadler, T. 2014. The fossilized birth-death process for coherent calibration of divergence-time estimates. Proceedings of the National Academy of Sciences 111: E2957–E2966. Crossref
Heintzman, P.D., Zazula, G.D., Cahill, J.A., Reyes, A.V., MacPhee, R.D., and Shapiro, B. 2015. Genomic data from extinct North American Camelops revise camel evolutionary history. Molecular Biology and Evolution 32: 2433–2440. Crossref
Hennig, W. 1981. Insect Phylogeny. xi + 514 pp. John Wiley and Sons, Chichester.
Höhna, S., Stadler, T., Ronquist, F., and Britton, T. 2011. Inferring speciation and extinction rates under different sampling schemes. Molecular Biology and Evolution 28: 2577–2589. Crossref
Honey, J.G., Harrison, J.A., Prothero, D.R., and Stevens, M.S. 1998. Camelidae. In: C.M. Janis, K.M. Scott, and L.L. Jacobs (eds.), Evolution of Tertiary Mammals of North America, 439–462. Cambridge University Press, Cambridge. Crossref
Khaveson, I.I. 1950. Camels of the genus Paracamelus [in Russian]. Doklady Akademi Nauk SSSR 70: 917–920.
Khaveson, I.I. 1954. Camels from the Tertiary of the Oriental hemisphere (genus Paracamelus) [in Russian]. Trudy Paleontologičeskogo instituta 47: 100–162.
Khisarova, G.D. 1963. Fossil mammal bones from Koshkurgan, South Kazakhstan [in Rusiian]. In: Materialy pro istorii fauny i flory Kazahstana 4, 50–67. Akademiâ Nauk, Alma Ata.
Kimura, Y., Hawkins, M.T., McDonough, M.M., Jacobs, L.L., and Flynn, L.J. 2015. Corrected placement of Mus-Rattus fossil calibration forces precision in the molecular tree of rodents. Scientific Reports 5: 14444. Crossref
Kostopoulos, D.S. and Sen, S. 1999. Late Pliocene (Villafranchian) mammals from Sarikol Tepe, Ankara, Turkey. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und historische Geologie 39: 165–202.
Kozhamkulova, B.S. 1986. The late Cenozoic two-humped (Bactrian) camels of Asia. Quartärpaläontologie 6: 93–97.
Kumar, S. and Hedges, S.B. 2011. Timetree2: Species Divergence Times on the Iphone. Bioinformatics 27: 2023–2024. Crossref
Laurin, M. 2012. Recent progress in paleontological methods for dating the Tree of Life. Frontiers in Genetics 3: 1–16. Crossref
Legendre, L.J., Guénard, G., Botha-Brink, J., and Cubo, J. 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Systematic Biology 65: 989–996. Crossref
Liedtke, H.C., Gower, D.J., Wilkinson, M., and Gomez-Mestre, I. 2018. Macroevolutionary shift in the size of amphibian genomes and the role of life history and climate. Nature Ecology and Evolution 2: 1792–1799. Crossref
Likius, A., Brunet, M., Geraads, D., and Vignaud, P. 2003. Le plus vieux Camelidae (Mammalia, Artiodactyla) d’Afrique: limite Mio-Pliocène, Tchad. Bulletin de la Société géologique de France 174: 187–193. Crossref
Logvynenko, V.N. 2000. The camels (Camelidae, Tylopoda) from the Pliocene and Eopleistocene of Ukraine [in Ukrainian]. Vestnik zoologii, Supplement 14: 120–127.
Logvynenko, V.N. 2001. Paracamelus minor (Camelidae, Tylopoda)—a new camelid species from the Middle Pliocene of Ukraine [in Ukrainian]. Vestnik zoologii 35: 39–42.
Marjanović, D. and Laurin, M. 2007. Fossils, molecules, divergence times, and the origin of lissamphibians. Systematic Biology 56: 369–388. Crossref
Marjanović, D. and Laurin, M. 2008. Assessing confidence intervals for stratigraphic ranges of higher taxa: the case of Lissamphibia. Acta Palaeontologica Polonica 53: 413–432. Crossref
Martini, P. and Geraads, D. 2018. Camelus thomasi (Mammalia, Camelidae) from the type-locality Tighennif, Algeria. Geodiversitas 40: 115–134. Crossref
Martini, P., Costeur, L., Le Tensorer, J.-M., and Schmid, P. 2015. Pleistocene camelids from the Syrian desert: the diversity in El Kowm. Anthropologie 119: 687–693. Crossref
Ming, L., Yuan, L., Yi, L., et al. 2020. Whole-genome sequencing of 128 camels across Asia reveals origin and migration of domestic bactrian camels. Communications Biology 3 (1): 1–9. Crossref
Morales, J. 1984. Venta del Moro: su macrofauna de mamíferos, y bioestratigrafía continental del Mioceno terminal Mediterraneo. 327 pp. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid.
Nagel, D., Pronin, K., Pytlik, I., Urbanek, C., Ivanoff, D., and Semenov, Y. 2004. Die Pliozäne Fauna Der Katakomben Von Odessa (Ukraine). Berichte der Institut für Erdwisenschaften der Universität Graz 9: 278–280.
Nee, S., May, R.M., and Harvey, P.H. 1994. The reconstructed evolutionary process. Philosophical Transactions of the Royal Society of London, Series B 344: 305–311. Crossref
O’Leary, M.A., Bloch, J.I., Flynn, J.J., et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339: 662–667.
Organ, C.L., Canoville, A., Reisz, R.R., and Laurin, M. 2011. Paleogenomic data suggest mammal-like genome size in the ancestral amniote and derived large genome size in amphibians. Journal of Evolutionary Biology 24: 372–380. Crossref
Parham, J.F., Donoghue, P.C.J., Bell, C.J., et al. 2012. Best practices for justifying fossil calibrations. Systematic Biology 61: 346–359. Crossref
Patnaik, R. 2014. Phylogeny of Siwalik murine rodents: Implications for Mus-Rattus divergence time. Journal of the Paleontological Society of India 59: 15–28.
Pérez-Lorente, F., Herrero, C., Herrero, E., and Montoya, P. 2009. Paracamelichnum jumillensis, n. ichnogen. n. ichnosp., Upper Miocene Camelidae ichnites from the Hoya de la Sima site (Murcia, Spain). Ichnos 16: 208–219. Crossref
Pickford, M., Morales, J., and Soria, D. 1995. Fossil camels from the upper Miocene of Europe: implications for biogeography and faunal change. Geobios 28: 641–650. Crossref
Plummer, M., Best, N., Cowles, K., and Vines, K. 2006. CODA: Convergence diagnosis and output analysis for MCMC. R News 6: 7–11.
Pyron, R.A. 2011. Divergence-time estimation using fossils as terminal taxa and the origins of Lissamphibia. Systematic Biology 60: 466–481. Crossref
Quental, T.B. and Marshall, C.R. 2009. Extinction during evolutionary radiations: reconciling the fossil record with molecular phylogenies. Evolution 63: 3158–3167. Crossref
Qiu, Z.-X., Qiu, Z.-D., Deng, T., Li, C.-K., Zhang, Z.-Q., Wang, B.-Y., and Wang, X. 2013. Neogene land mammal stages/ages of China. In: X. Wang, L.J. Flynn, and M. Fortelius (eds.), Fossil Mammals of Asia. 30–83. Columbia University Press, New York. Crossref
Rabosky, D.L. 2009. Heritability of extinction rates links diversification patterns in molecular phylogenies and fossils. Systematic Biology 58: 629–640. Crossref
Radulescu, C. and Burlacu, D. 1993. On the presence of Paracamelus alutensis (Gr. Stefanescu) (Camelidae, Mammalia) at Fratesti (Giurgiu Dept., Romania). Analele Universitătii Bucuresti, Geologie 42: 65–68.
Ronquist, F., Klopfstein, S., Vilhelmsen, L., Schulmeister, S., Murray, D.L., and Rasnitsyn, A. 2012a. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology 61: 973–999. Crossref
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., and Huelsenbeck, J.P. 2012b. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. Crossref
Rowan, J., Martini, P., Likius, A., Merceron, G., and Boisserie, J.-R. 2018. New Pliocene remains of Camelus grattardi (Mammalia, Camelidae) from the Shungura Formation, Lower Omo Valley, Ethiopia, and the evolution of African camels. Historical Biology 31: 1123–1134. Crossref
Sahni, M.R. and Khan, E. 1988. Pleistocene Vertebrate Fossils and Prehistory of India. 90 pp. Books and Books, New Delhi.
Sanmartín, I. and Meseguer, A.S. 2016. Extinction in phylogenetics and biogeography: From timetrees to patterns of biotic assemblage. Frontiers in Genetics 7 [published online, https://doi.org/10.3389/fgene. 2016.00035]. Crossref
Sauquet, H. 2013. A practical guide to molecular dating. Comptes rendus Palevol 12: 355–367. Crossref
Shaul, S. and Graur, D. 2002. Playing chicken (Gallus gallus): Methodological inconsistencies of molecular divergence date estimates due to secondary calibration points. Gene 300: 59–61. Crossref
Soul, L.C. and Friedman, M. 2017. Bias in phylogenetic measurements of extinction and a case study of end-Permian tetrapods. Palaeontology 60: 169–185. Crossref
Springer, M.S., Emerling, C.A., Meredith, R.W., Janečka, J.E., Eizirik, E., and Murphy, W.J. 2017. Waking the undead: Implications of a soft explosive model for the timing of placental mammal diversification. Molecular Phylogenetics and Evolution 106: 86–102. Crossref
Springer, M.S., Murphy, W.J., Eizirik, E., and O’Brien, S.J. 2003. Placental mammal diversification and the Cretaceous–Tertiary boundary. Proceedings of the National Academy of Sciences 100 (3): 1056–1061. Crossref
Ştefănescu, G. 1895. Le chameau fossile de Roumanie. Anuarulŭ Museului de Geologiă şi de Paleontologia 1894: 91–123.
Sterli, J., Pol, D., and Laurin, M. 2013. Incorporating phylogenetic uncertainty on phylogeny-based paleontological dating and the timing of turtle diversification. Cladistics 29: 233–246. Crossref
Svistun, V.I. 1971. New findings of camel (Tylopoda, Camelidae) remains in Pontian deposits of the South of the USSR European part [in Russian]. Vestnik zoologii 1: 64–68.
Svistun V.I., David, A.I., and Nesin, V.A. 1989. Camel’s cranium Paracamelus alexejevi Havesson (Camelidae) found in mid-pliocene deposits in Moldavia [in Russian]. In: A.L. Ânšin (ed.), Četvertičnyj period. Paleontologiâ i arxeologiâ, 52–61. Štiintsa, Kišinev.
Swofford, D.L. 2003. PAUP* Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.01 (build 163). Sinauer Associates, Sunderland.
Teilhard de Chardin, P. and Trassaert, M. 1937. The Pliocene Camelidae, Giraffidae, and Cervidae of South-Eastern Shansi. Palaeontologia Sinica C 1 (102): 1–68.
Titov, V.V. 2003. Paracamelus from the late Pliocene of the Black Sea region. In: A. Petculescu and E. Ştiucă (eds.), Advances in Vertebrate Paleontology “Hen to Panta”, 17–24. Emil Racoviţǎ Institute of Speleology, Bucharest.
Titov, V.V. 2008. Habitat conditions for Camelus knoblochi and factors in its extinction. Quaternary International 179: 120–125. Crossref
Titov, V.V. and Logvynenko, V.N. 2006. Early Paracamelus (Mammalia, Tylopoda) in Eastern Europe. Acta Zoologica Cracoviensia 49A: 163–178. Crossref
Titov, V.V. and Tesakov, A.S. 2013. Late Miocene (Turolian) vertebrate faunas from Southern European Russia. In: X. Wang, L.J. Flynn, and M. Fortelius (eds.), Fossil Mammals of Asia, 538–545. Columbia University Press, New York. Crossref
Uyeda, J.C., Pennell, M.W., Miller, E.T., Maia, R., and McClain, C.R. 2017. The evolution of energetic scaling across the vertebrate tree of life. The American Naturalist 190: 185–199. Crossref
Warnock, R.C., Parham, J.F., Joyce, W.G., Lyson, T.R., and Donoghue, P.C. 2015. Calibration uncertainty in molecular dating analyses: there is no substitute for the prior evaluation of time priors. Proceedings of the Royal Society of London B: Biological Sciences 282: 20141013. Crossref
Werdelin, L. and Peigné, S., 2010. Carnivora. In: L. Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 603–658. University of California Press, Berkeley. Crossref
Wible, J.R., Rougier, G.W., Novacek, M.J., and Asher, R.J. 2007. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature 447: 1003–1006. Crossref
Wu, H., Guang, X., Al-Fageeh, M. B., Cao, J., Pan, S., Zhou, H., Zhang, L, Abutarboush, M. H., Xing, Y., Xie, Z., and Wang, J. 2014. Camelid genomes reveal evolution and adaptation to desert environments. Nature Communications 5 (5188): 1–9. Crossref
Yuan, S., Chen, T., and Gao, S. 1983. Uranium series dating of “Ordos man” and Sjara-Osso-Gol culture. Acta Anthropologica Sinica 2: 90–94.
Zdansky, O. 1926. Paracamelus gigas Schlosser. Palaeontologia Sinica C 2 (4): 1–44.
Zhang, C., Stadler, T., Klopfstein, S., Heath, T.A., and Ronquist, F. 2016. Total-evidence dating under the fossilized birth-death process. Systematic Biology 65: 228–249. Crossref
Acta Palaeontol. Pol. 65 (2): 251–260, 2020
https://doi.org/10.4202/app.00727.2020