

Multispecies leatherback turtle assemblage from the Oligocene Chandler Bridge and Ashley formations of South Carolina, USA
BAILEY R. FALLON and ROBERT W. BOESSENECKER
Fallon, B.R. and Boessenecker, R.W. 2020. Multispecies leatherback turtle assemblage from the Oligocene Chandler Bridge and Ashley formations of South Carolina, USA. Acta Palaeontologica Polonica 65 (4): 763–776.
Paleogene dermochelyid species richness far exceeded that of today. Leatherback sea turtles were most species rich in the Paleogene, but their richness declined sharply during the Neogene with only one species existing today, Dermochelys coriacea. We describe the fossil remains of three leatherback genera (Natemys, Psephophorus, and Egyptemys) from the upper Oligocene Chandler Bridge Formation and two (Natemys and Psephophorus) from the lower Oligocene Ashley Formation of South Carolina, USA. The fossils consist of isolated and some associated carapacial ossicles. Several ossicles are referred to Natemys sp. because their scalloped edges are indicative of the carapacial sunflower pattern specific to this genus. Additionally, two Natemys morphotypes (Natemys sp. 1 and 2) are distinguished based on differences in ossicle thickness and internal structure. We refer two ossicles to cf. Psephophorus sp. because of their internal diploic structure and because one has a dorsal radial pattern while the other has a prominent ridge that exhibits strong visceral concavity. Finally, we refer one ossicle to cf. Egyptemys sp. because it has a shallow keel that shows little expression on the visceral surface, although we also acknowledge the ossicle’s similarity to some ridged ossicles of the genus Psephophorus. These ossicles represent the first multispecies assemblage of leatherback fossils reported worldwide. Furthermore, the specimens fill both temporal and geographic gaps for extinct leatherback genera and represent the first formally described dermochelyids from South Carolina and the Oligocene of the Atlantic Coastal Plain.
Key words: Chelonioidea, Natemys, Egyptemys, Psephophorus, Paleogene, Oligocene, North America.
Bailey R. Fallon [fallonbr@g.cofc.edu], Department of Geology and Environmental Geosciences, College of Charleston, Charleston, South Carolina 29424, USA.
Robert W. Boessenecker [boesseneckerrw@cofc.edu], Department of Geology and Environmental Geosciences, College of Charleston, Charleston, South Carolina 29424, USA; University of California Museum of Paleontology, University of California, Berkeley, California 94720, USA.
Received 25 February 2020, accepted 21 July 2020, available online 24 November 2020.
Copyright © 2020 B.R. Fallon and R.W. Boessenecker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The leatherback sea turtle Dermochelys coriacea Vandelli, 1761 is one of the most iconic marine organisms. With a carapace comprised of a mosaic of bony ossicles covered in a leathery dermis, D. coriacea is unique among all living marine turtles—the rest of which bear a rigid shell (Eckert and Luginbuhi 1988; Magwene and Socha 2013; Frazier et al. 2018). Dermochelys coriacea is the only surviving representative of the family Dermochelyidae and thus exploits niches that hard-shelled turtles cannot. It is known to dive over 1000 meters, thrive in high latitude (up to ~71°N and 47°S) waters, and feed principally on jellyfish, minimizing its competition with other sea turtles that cannot tolerate cold and low-resource environments (Eggleston 1971; Carriol and Vader 2002; Doyle et al. 2008; Fossette et al. 2010; Eckert et al. 2012; Heaslip et al. 2012; Curtis et al. 2015). Despite this, D. coriacea is listed as endangered in the United States and as vulnerable around the world, facing population declines resulting from bycatch and plastic pollution (Wallace et al. 2013).
As one of the largest, globally occurring reptiles, the modern leatherback is well studied but its ancestral forms are not (Matthews et al. 1994). Leatherback fossils have been reported from the margins of nearly all Cenozoic ocean basins including the Atlantic, Pacific, Indian, Southern Ocean, Paratethys, and Tethys (Andrews 1919; Gilmore 1937; de la Fuente et al. 1995; Köhler 1996; Wood et al. 1996; Tong et al. 1999; Karl 2002; Lynch and Parham 2003; Chesi et al. 2007; Karl et al. 2012). Leatherback richness has declined over time with the greatest richness occurring in the Eocene, and slowly diminishing throughout the Neogene, leaving only a single species today (Wood et al. 1996; Fallon and Boessenecker 2019).
Though leatherback fossils have been reported from throughout the Cenozoic, few have been described from the Oligocene (Wood et al. 1996; Köhler 1996; Karl 2002; Karl 2014). Karl (2002) reported leatherback fossils from Oligocene formations in western Europe, but no other dermochelyid fossils have been confidently reported from this epoch. Still, Wood et al. (1996) referred leatherback fossils from close to the Oligocene (upper Eocene of Egypt and lower Miocene of Oregon) to a new genus, Egyptemys Wood, Johnson-Gove, Gaffney, and Maley, 1996. They also proposed an Oligocene age for their newly named Natemys peruvianus Wood et al., 1996 from southern Peru, but it is possible that this specimen is actually Miocene in age (Cadena et al. 2018).
Oligocene strata in South Carolina have produced a diverse assemblage of marine vertebrates including four extinct hard-shelled sea turtle genera, Ashleychelys Weems and Sanders, 2014, Procolpochelys Hay, 1908, Carolinachelys Hay, 1923, and likely Euclastes Cope, 1870 but no leatherback fossils have been formally described from this assemblage (Weems and Sanders 2014; Weems and Brown 2017). The Oligocene Chandler Bridge and Ashley formations near Charleston, South Carolina, have produced fifteen leatherback carapacial ossicles that are described for the first time. The ossicles are referred to Natemys sp., cf. Psephophorus sp. Meyer, 1847, and cf. Egyptemys sp. and represent the first confidently-identified multispecies leatherback assemblage in the world.
Taxonomic note: Some confusion exists regarding the taxonomy of leatherback turtles from the Oligocene of Germany. A leatherback skull from an Oligocene (Chattian) formation in Doberg, Germany was first described as Chelonia ingens by Koenen (1891) and was later renamed Pseudosphargis ingens by Dames (1894). The skull was then referred to “Psephophorus” rupeliensis by Karl (1993). However, “Psephophorus” rupeliensis lacks a cranium and the holotype consists of vertebrae, a coracoid, an ilium, and five ossicles from a separate locality (Rupelian Clay near Rupelmonde) in Belgium (Van Beneden 1883; Köhler 1996). Karl (2014) later proposed that “Psephophorus” rupeliensis and Pseudosphargis ingens were conspecific, recombining them as Pseudosphargis rupeliensis. However, fossils from the German assemblage are still referred to “Psephophorus” rupeliensis by Zvonok and Danilov (2019) and Peters et al. (2019), while the Belgian holotype for this species is lost (Köhler 1996). Because the hypodigm of “Psephophorus” rupeliensis lacks a skull and the hypodigm of Pseudosphargis ingens lacks a shell, the two cannot be considered conspecific until overlapping material is discovered. Wood et al. (1996) cautioned that “Psephophorus” rupeliensis likely did not belong to the genus Psephophorus owing to many similarities with Natemys peruvianus (e.g., carapacial sunflower pattern consisting of enlarged ossicles with scalloped margins) and a sister taxon relationship between the two in their cladistic hypothesis (to the exclusion of Psephophorus). However, they stopped short of assigning “Psephophorus” rupeliensis to Natemys owing to the hypothesized presence of a plastron in Natemys peruvianus. Natemys peruvianus may not actually have a plastron, as the specimen may just be an empty flexible carapace that was folded over between decomposition and burial (Wood et al. 1996: 272). Until a more complete specimen of this taxon with a more clearly preserved plastron is discovered, this could be a taphonomic artifact. Owing to the similarity of the shells of Natemys peruvianus and “Psephophorus” rupeliensis as well as the sister taxon relationship between them (Wood et al. 1996), we provisionally consider the species as assignable to Natemys and use the binomial Natemys rupeliensis in this study. While this is justified based on present evidence, more complete specimens of either species are needed to test this hypothesis.
Institutional abbreviations.—CCNHM, Mace Brown Museum of Natural History, Charleston, South Carolina, USA; ChM, Charleston Museum, Charleston, South Carolina, USA.
Material and methods
Leatherback carapacial ossicles reported in this study were collected from the lower Oligocene Ashley Formation (Rupelian, 29.0–26.57 Ma) and the upper Oligocene Chandler Bridge Formation (Chattian, 24.7–23.5 Ma) from scattered localities (irrigation canals and active construction sites in the Coosaw Preserve, McKewn and Wescott Plantation subdivisions) in the vicinity of Ladson and Summerville, Dorchester County, South Carolina, USA (Fig. 1). Further detailed locality information is available upon request from CCNHM. The ossicles were prepared and curated at CCNHM. All specimens were measured using digital calipers and were photographed with a Canon Rebel EOS DSLR camera with a 100 mm macro lens.
Geological setting
Specimens were collected from the Oligocene Ashley and Chandler Bridge formations in the vicinity of Charleston, South Carolina (Fig. 1). The Ashley Formation consists of 10‒25 meters of lightly indurated, tan-olive, fossiliferous, massively bedded calcarenite; this unit overlies the Eocene Tupelo Bay and Harleyville formations and is in turn overlain by the Chandler Bridge Formation and younger strata within the vicinity of Summerville and Ladson, South Carolina. Several phosphatic intraformational bonebeds have permitted the subdivision of the Ashley Formation into three members: the Gettysville Member (recorded only in a single core), the Runnymede Marl Member, and the Givhan’s Ferry Member. The Runnymede Marl and Givhan’s Ferry members are frequently exposed by canal excavations and stormwater pond excavations at construction sites. The Runnymede Marl is virtually free of quartz while the Givhan’s Ferry Member is quartz rich (Weems et al. 2016). In pond excavations in the McKewn subdivision in Ladson, the contact between these upper members is exposed, and the Runnymede Marl consists of a massively bedded, pale grayish-green calcarenite with abundant vertical burrows up to 5 cm in diameter, occasionally infilled with pods (sensu Kidwell et al. 1986) of shell fragments, phosphate pebbles, and vertebrate skeletal material. This member is overlain by a patchy bonebed manifested as a horizon of abundant pods of phosphate pebbles, shells, shell fragments, and vertebrate skeletal elements, and occasionally as a shelly pavement dominated by oysters and barnacles. The Givhan’s Ferry Member locally consists of a relatively more fossiliferous, massively bedded olive-olive brown glauconitic calcarenite (RWB personal observations 2020). The Ashley Formation yields frequent invertebrates including the gastropod Epitonium Röding, 1798, the oyster Cubitostrea sp. Sacco, 1897, and the balanid barnacle Concavus sp. Newman, 1982. Other vertebrate fossils include sea turtles, whales, dolphins, and sea cows (Kellogg 1923; Domning 1997; Weems and Sanders 2014; Sanders and Geisler 2015; Boessenecker and Fordyce 2017; Geisler et al. 2017; Albright et al. 2019; Domning and Beatty 2019). The marine invertebrate, shark, and bony fish assemblages from this unit are virtually unstudied. Pervasive bioturbation, phosphatic bonebeds, and grain size suggest middle shelf deposition. 87Sr/86Sr ratios from the Ashley Formation indicate an age of 29–26.57 Ma for the entire unit (Boessenecker and Fordyce 2017).
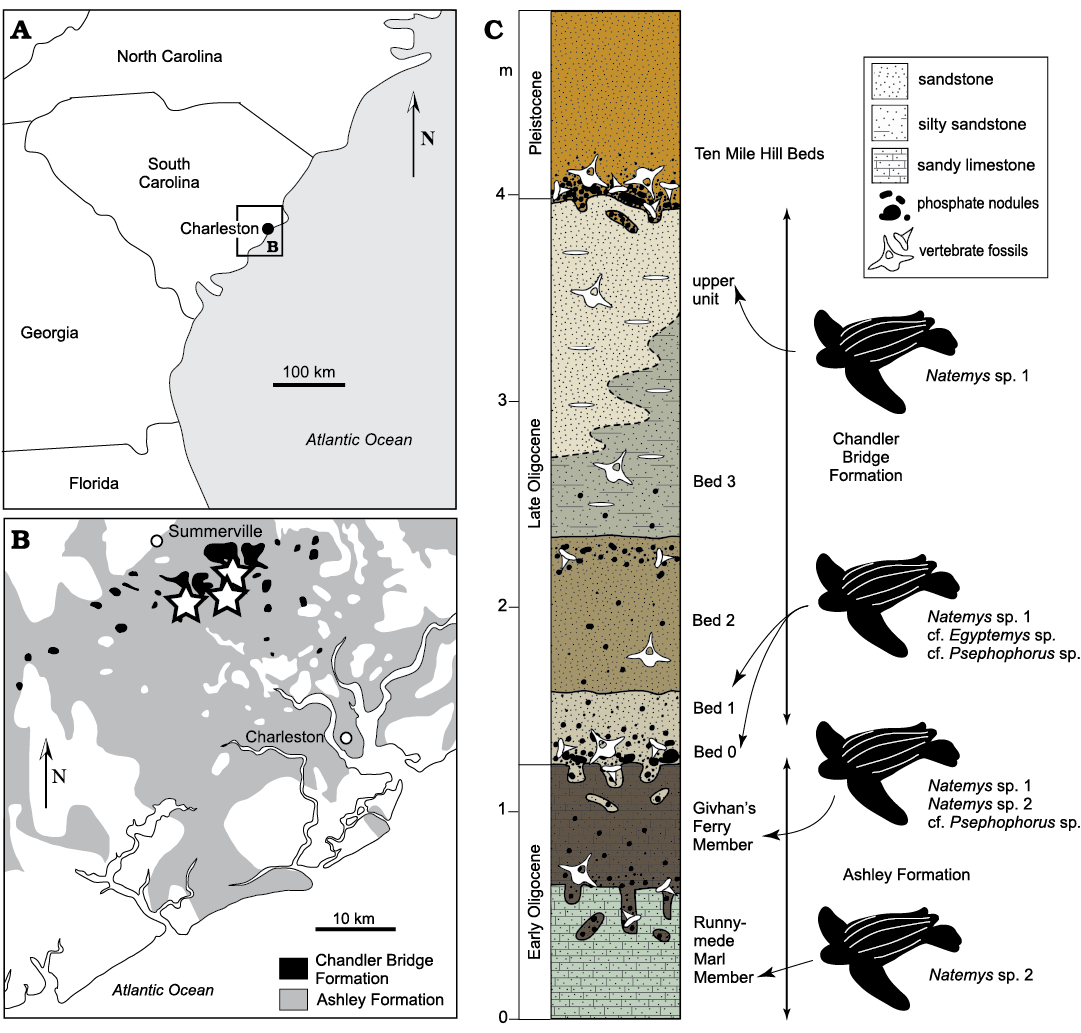
Fig. 1. The Ashley and Chandler Bridge formations and geologic context of CCNHM ossicles. A. Map of the southeastern United States. B. Map of Ashley and Chandler Bridge formation exposures on the coast of South Carolina. Stars denote Oligocene dermochelyid localities. C. Stratigraphic column of the Ashley and Chandler Bridge formations.
The Chandler Bridge Formation overlies the Ashley Formation and is thin (typically 30‒100 cm, but up to 2.5 m in the McKewn subdivision) and patchy, likely the result of Neogene erosion (Katuna et al. 1997). Unlike the Ashley Formation, the Chandler Bridge Formation is non-calcareous and consists of massively bedded and richly fossiliferous phosphatic siltstone and fine sandstone with occasional discoidal quartz pebbles. This unit is subdivided into four beds (Sanders et al. 1982; Katuna et al. 1997). Bed 0 (sensu Boessenecker and Geisler 2018) is patchy and infrequently preserved and consists of 10‒15 cm of unconsolidated olive-brown sand, silt and clay and is rich in phosphatic nodules and vertebrate fossils. Bed 1 is moderately well sorted and consists of unconsolidated light yellowish-brown silt with very fine-grained quartz sandstone and it is also rich in vertebrates. Bed 2 is a poorly sorted, brown to light tan unconsolidated silty sandstone that is rich in phosphate nodules and marine vertebrate fossils. Bed 3 is a compact, light olive-gray to dark bluish-gray, poorly sorted, silty fine-grained quartz sand with phosphate pebbles and quartz discoids. A rich vertebrate assemblage has been established including sharks, bony fish, an estuarine crocodile, sea turtles, marine birds, whales, dolphins, and sea cows (Sanders et al. 1982; Boessenecker and Geisler 2018). Dinoflagellates led to an interpretation that the Chandler Bridge Formation was initially deposited under shelf settings and transitioned towards estuarine and eventually nonmarine deposition, reflecting regressive deposition (Katuna et al. 1997). However, marine vertebrate fossils are common throughout the unit (Sanders et al. 1982; RWB personal obserwations 2020). Aside from plant debris (Sanders et al. 1982) and a single nonmarine turtle (Weems and Knight 2009), vertebrate fossil evidence (particularly sharks and fish) suggests continuous open marine deposition (Cicimurri and Knight 2009) with uncertain changes in relative sea level. The shark and ray assemblage from the Chandler Bridge Formation is indicative of inner to middle shelf environments with temperatures ranging from 20‒25°C (Cicimurri and Knight 2009). Similarly, the billfish Aglyptorhynchus Casier, 1966 suggests temperatures ranging from 20‒24°C (Fierstine and Weems 2009). 87Sr/86Sr ratios from oyster shells indicate an age of 24.7–23.5 Ma (Weems et al. 2016; Boessenecker and Fordyce 2017).
Systematic paleontology
Testudines Linnaeus, 1758 (sensu Joyce et al. 2004)
Cryptodira Duméril and Bibron, 1835
Chelonioidea Baur, 1893
Dermochelyidae Gray, 1825
Genus Natemys Wood, Johnson-Gove, Gaffney, and Maley, 1996
Type species: Natemys peruvianus Wood, Johnson-Gove, Gaffney, and Maley, 1996, southern coast of Peru, late Oligocene.
Natemys sp. 1
Fig. 2.
Material.—CCNHM 4405.1–4405.5, five associated non-ridged carapacial ossicles collected by Steve Hildenbrandt in July 2017 from an unnamed upper unit (potentially Bed 3 correlative) of the Chandler Bridge Formation, Coosaw Preserve Subdivision; CCNHM 4288, a non-ridged carapacial ossicle collected by RWB on June 14, 2018 from the Givhan’s Ferry Member of the Ashley Formation, McKewn Subdvision, Ladson, SC; CCNHM 5540, 5541, and 5542, three non-ridged carapacial ossicles collected by Steven Miller from Bed 1 (Fig. 1) of the Chandler Bridge Formation, locality uncertain. All Oligocene of South Carolina, USA.
Description.—CCNHM 4405.1 is elongate (40.9×23.0 mm) and tabular in cross section (see Table 1 for size dimensions of all ossicles). The dorsal surface is smooth with seven foramina near the center (Fig. 2C1). The visceral surface is scattered with minute pores and five foramina (Fig. 2C2). The sutural margins are straight with six shallow notches (Fig. 2C). The sutural surface reveals three distinct internal layers: a dense dorsal layer, a thick and highly vascularized middle layer, and a thin, compact visceral layer (Fig. 2C3). CCNHM 5540 is also elongate and has seven deep sutural notches. It has similar dimensions and surface textures compared to CCNHM 4405.1 and a roughly similar internal structure (Table 1, Fig. 2).
Table 1. Size dimensions (in mm) of leatherback sea turtle ossicles from the Ashley and Chandler Bridge formations, Charleston, South Carolina, USA.
|
Taxon |
Specimen |
Thickness |
Length |
Width |
|
Natemys sp. 1 |
CCNHM 4405.1 |
10.8 |
40.9 |
23.0 |
|
CCNHM 4405.2 |
8.7 |
36.5 |
27.5 |
|
|
CCNHM 4405.3 |
9.9 |
31.0 |
22.5 |
|
|
CCNHM 4405.4 |
6.3 |
29.9 |
18.0 |
|
|
CCNHM 4405.5 |
9.0 |
24.5 |
15.1 |
|
|
CCNHM 4288 |
5.8 |
23.2 |
17.9 |
|
|
CCNHM 5540 |
11.1 |
55.5 |
37.8 |
|
|
CCNHM 5541 |
8.8 |
21.9 |
18.4 |
|
|
CCNHM 5542 |
10.2 |
43.0 |
40.1 |
|
|
Natemys sp. 2 |
CCNHM 4287.1 |
18.0 |
74.9 |
44.1 |
|
CCNHM 4287.2 |
15.4 |
54.9 |
37.8 |
|
|
CCNHM 4910 |
14.8 |
36.8 |
28.4 |
|
|
cf. Egyptemys sp. |
CCNHM 4289 |
9.0 |
43.1 |
37.9 |
|
cf. Psephophorus sp. |
CCNHM 5460 |
8.9 |
44.7 |
34.7 |
|
CCNHM 5543 |
14.0 |
39.3 |
34.2 |
CCNHM 4405.2, 4405.3, 4405.4, 4405.5, 5541, and 5542 are all polygonal and approximately tabular in cross section, although CCNHM 4405.3 has a single rounded peak on the visceral surface (Fig. 2A2–I2). They have smooth dorsal surfaces with one to three scattered foramina (Fig. 2A1–I1). CCNHM 4405.2 and 4405.3 have smooth and round sutural margins, CCNHM 4405.4 has one crescent-shaped margin, and CCNHM 4405.5 has straight sutural margins (Fig. 2A1–I1). The six ossicles have visceral surfaces that are comparable to CCNHM 4405.1 and have one to sixteen scattered foramina (Fig. 2A2–I2). All have internal structures that are comparable to CCNHM 4405.1 as revealed by their sutural surfaces (Fig. 2A3–I3). CCNHM 5541 has a fractured edge that reveals its internal structure, which is indeed comprised of the three described layers. CCNHM 4288 is comparable to the CCNHM 4405.1–4405.5 ossicles, is the thinnest (5.8 mm) of all the ossicles reported here, and is one of the thinnest non-ridged fossil leatherback ossicles reported to date. The thicknesses of these ossicles do not exceed 11.1 mm.
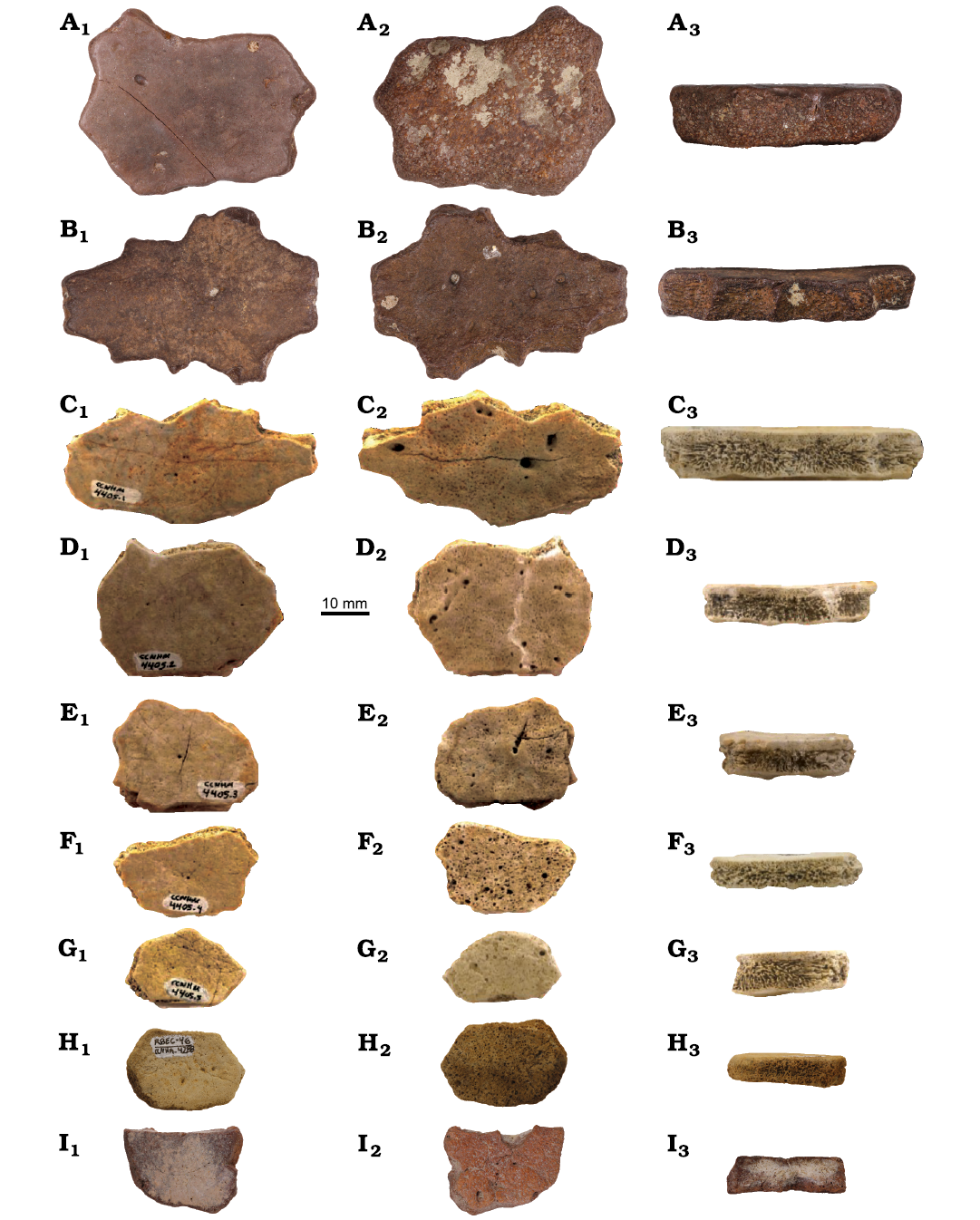
Fig. 2. Ossicles of leatherback turtle Natemys sp. 1 from Oligocene of South Carolina, USA. CCNHM 5542 (A), CCNHM 5540 (B), CCNHM 4405.1–4405.5 (C–G, respectively), CCNHM 4288 (H), and CCNHM 5541 (I), in dorsal (A1–I1), visceral (A2–I2), and sutural (A3–I3) views.
Remarks.— Specimens are assigned to Natemys sp. 1 owing to (i) the scalloped edges of some associated ossicles, (ii) to their triple-layered internal structure, and (iii) to their thinness.
The nine carapacial ossicles from the Chandler Bridge and Ashley formations (CCNHM 4405.1–4405.5, 4288, 5540, 5541, and 5542) are referred to the genus Natemys, which is historically represented by only one species, Natemys peruvianus. Wood et al. (1996) first identified this species based on a partial carapace that exhibits a distinct sunflower pattern. This pattern is defined by a linear series of enlarged ossicles with deeply scalloped edges that are surrounded by smaller, elongate “petal” ossicles arranged in a radial pattern (Wood et al. 1996). Natemys peruvianus is one of three leatherback species that exhibit such a pattern, the others being Natemys rupeliensis and Psephophorus polygonus Meyer, 1847 (Wood et al. 1996; Karl et al. 2012; Karl 2014; Peters et al. 2019; see taxonomic note in Introduction).
CCNHM 4405.1 and 5540 resemble the central ossicles of the sunflower pattern because they have deeply scalloped edges. As such, we refer these and their associated ossicles to Natemys sp. 1 (Peters et al. 2019; see taxonomic note). However, we differentiate these nine ossicles (Natemys sp. 1) from those we refer to Natemys sp. 2 based on ossicle thickness and internal structure. Natemys sp. 1 ossicles are thinner (5.8‒11.1 mm) than Natemys sp. 2 ossicles (14.8‒18.0 mm). Additionally, Natemys sp. 1 ossicles have a relatively thick middle internal layer whereas Natemys sp. 2 ossicles have a relatively thick visceral-most internal layer (see below for description).
Natemys sp. 2
Fig. 3.
Material.—CCNHM 4287.1 and 4287.2, a pair of associated non-ridged carapacial ossicles collected on different dates but within about a meter of each other by Shaun Coates on August 11, 2018 from the ?Givhan’s Ferry Member of the Ashley Formation, Chandler Bridge Creek; CCNHM 4910, a non-ridged ossicle collected by Shaun Coates on April 1, 2018 from the ?Runnymede Marl member of the Ashley Formation, Sawmill Branch Canal, Summerville. All Oligocene of South Carolina, USA.
Description.—CCNHM 4287.1 is the largest ossicle reported here, measuring 74.9 mm in length and 18.0 mm in maximum thickness. The fragment measures 44.1 mm in width and appears to be fractured in half, revealing its internal structure (Fig. 3A). The original dimensions may therefore be projected to be approximately 88×74.9 mm. CCNHM 4287.1 is slightly curved in cross section (Fig. 3A3). The dorsal surface is smooth and imperforate (Fig. 3A1). The visceral surface is also smooth, but has many small, dispersed pores (Fig. 3A2). The non-fractured sutural surfaces are highly vascularized and are heavily scalloped with four distinct notches (Fig. 3A1). The ossicle fragment is divided into three distinct internal layers that differ from the internal layers of Natemys sp. 1 ossicles (Figs. 2A3–I3, 3A3). The dorsal layer of CCNHM 4287.1 is thin (~3 mm) and compact with no discernable vascularization. The middle layer is thicker (~4 mm) and is moderately vascularized and the visceral-most layer is highly vascularized and is the thickest (~11 mm) internal layer (Fig. 3A3).
CCNHM 4287.2 is another large (54.9×37.8 mm) ossicle that is approximately tabular. Its dorsal, visceral and sutural surfaces are comparable to those of CCNHM 4287.1 (Fig. 3). These shared surface textures suggest CCNHM 4287.2 may also exhibit a stratified internal structure. CCNHM 4287.2 most notably differs from all other ossicles in having a fissure that runs along the visceral surface, splitting the ossicle approximately in half (Fig. 3B2). This fissure expands about halfway into the ossicle laterally (i.e., toward the dorsal surface) as revealed by its sutural surface (Fig. 3B3). CCNHM 4910 is a third, very thick (14.8 mm) carapacial ossicle. It is tabular in cross section, and has dorsal, visceral and sutural surfaces comparable to those of CCNHM 4287.1 and 4287.2 (Fig. 3).

Fig. 3. Ossicles of leatherback turtle Natemys sp. 2 from Oligocene of South Carolina, USA. CCNHM 4287.1 (A), CCNHM 4287.2 (B), and CCNHM 4910 (C), in dorsal (A1–C1), visceral (A2–C2), and sutural (A3–C3) views. Arrows indicate the fissure on the visceral and sutural surfaces of CCNHM 4287.2, and note the stratified internal structure of CCNHM 4287.1.
Remarks.— Specimens are assigned to Natemys sp. 2 owing to (i) the scalloped edges of some of the ossicles, (ii) to their triple-layered internal structure, and (iii) to their thickness.
CCNHM 4287.1 and 4287.2 have scalloped edges that are reminiscent of the central ossicles of the carapacial sunflower pattern unique to Natemys peruvianus and Natemys rupeliensis, leading us to refer these ossicles to the genus Natemys. We also refer CCNHM 4910 to Natemys sp. 2 because its thickness is most comparable to CCNHM 4287.1 and 4287.2. As mentioned, we distinguish these three Natemys sp. 2 ossicles from the Natemys sp. 1 ossicles because they are considerably thicker (14.8‒18.0 mm) than the Natemys sp. 1 ossicles and because they have a relatively thick visceral-most, not middle, internal layer.
Ossicle thickness, size and internal structure have historically been helpful in distinguishing ossicles of the genus Natemys from those of other leatherback genera. The triple-layered internal stratification of our Natemys sp. 1 and 2 ossicles is consistent with that of ossicles described by Köhler (1996: figs. 82, 83) and by Karl et al. (2012), which we consider to be Natemys (Fallon and Boessenecker 2019; see taxonomic note). Furthermore, Peters et al. (2019) noted that Natemys peruvianus and Natemys rupeliensis have considerably thickened ossicles when compared to other leatherback species like Cosmochelys dolloi Andrews, 1919, Egyptemys eocaenus Andrews, 1901, E. oregonensis Packard, 1940, Psephophorus polygonus, and “Psephophorus calvertensis” Palmer, 1909. CCNHM 4287.1, 4287.2, and 4910 are some of the thickest non-ridged ossicles reported to date (Fallon and Boessenecker 2019), which supports their identification as Natemys sp. 2. Peters et al. (2019) also used ossicle size to distinguish between leatherback genera even though this trait is ontogenetically variable and depends on the ossicle’s original position in the shell. They noted that N. peruvianus and N. rupeliensis ossicles tend to be larger than the ossicles of the genera Cosmochelys, Egyptemys and Psephophorus (Peters et al. 2019). This finding further supports our identification of CCNHM 4287.1 and 4287.2 as Natemys sp. 2 since they are some of the largest (88 and 54.9 mm, respectively) leatherback ossicles recorded (Wood et al. 1996;Peters et al. 2019). Moreover, CCNHM 4287.1 (Natemys sp. 2) is larger (88×74.9 mm) than the enlarged ossicles of Natemys peruvianus, which are 40‒65 mm in maximum length, and is more comparable to the enlarged ossicles of Natemys rupeliensis, which measure up to 102 mm in maximum length (Wood et al. 1996). Therefore, we note that Natemys sp. 2 compares well with N. rupeliensis in terms of having very large ossicles. Furthermore, Natemys sp. 2 and N. rupeliensis both date to the early Oligocene and both are found in the North Atlantic Ocean basin (Köhler 1996; see taxonomic note in the Introduction). In the future, it is possible that Natemys sp. 2 may prove to be synonymous with N. rupeliensis. However, we advise caution against such a determination until more complete specimens of Natemys sp. 2 are discovered and described. Still, the differences in thickness, size and internal structure between Natemys sp. 1 and 2 highlight the necessity in reporting such features in future work.
Interestingly, CCNHM 4287.2 preserves a fissure that runs along its mid-visceral surface. This is the first formal report of a leatherback ossicle exhibiting such a trait. The only other mentions of a visceral fissure-like feature on leatherback ossicles are from a “Psephophorus calvertensis” (synonymy P. polygonus sensu Peters et al. 2019) shell fragment found in the Calvert Formation of Maryland, USA, and from unpublished Belgian fossils (Köhler 1996; Roger Wood personal communication to Peters et al. 2019). While Köhler (1996) attributed this feature to an infection, we agree with Roger Wood that this is likely a fusion of two ossicles due to an individual’s ontogenetic aging process. The expansion of this fissure about halfway to the dorsal surface suggests the ossicle may have resulted from the incomplete fusion of two separate ossicles late in ontogeny. Such a fusion raises the possibility that the enlarged ossicles of Natemys (and perhaps Psephophorus) may have been formed by the coalescence of smaller ossicles.
cf. Egyptemys sp. Wood, Johnson-Gove, Gaffney, and Maley, 1996
Fig. 4.
Material.—CCNHM 4289, a ridged carapacial ossicle collected by RWB on June 26, 2018 from Bed 1 of the Chandler Bridge Formation, McKewn Subdivision, Ladson; Oligocene of South Carolina, USA.
Description.—CCNHM 4289 is one of two (see below) ossicles that exhibits a keel, or ridge. The ossicle has a shallow, transverse arch and a weak middorsal keel that is ~2 mm in height (Fig. 4A3). The dorsal surface is smooth but is scattered with shallow linear depressions (Fig. 4A1). The visceral surface shows little concavity corresponding to the dorsal keel, is weakly dimpled, and has several small pores (Fig. 4A2). The sutural margins are smooth and scalloped with eight notches (Fig. 4A1). The sutural surface suggests an internal structure that is comparable to that of the five CCNHM 4405.1–4405.5 ossicles (Natemys sp. 1).

Fig. 4. Ossicle of leatherback turtle cf. Egyptemys sp. (CCNHM 4289) from Oligocene of South Carolina, USA, in dorsal (A1), visceral (A2), and sutural (A3) views.
Remarks.—Specimen is assigned to cf. Egyptemys sp. owing to (i) its weakly keeled, dorsal ridge that (ii) shows little expression on the visceral surface, and (iii) to its thickness.
CCNHM 4289 is referred to cf. Egyptemys sp. Leatherback fossils now referred to this genus were originally referred to the species Psephophorus eocaenus and Psephophorus oregonensis by Andrews (1901) and Packard (1940). However, Wood et al. (1996) revised this classification based on carapacial ridge distinctions between the two genera. The genus Egyptemys is distinct in having weakly keeled ridges that lack a corresponding trough on the visceral surface, are semi-circular in cross section, and are confined to a narrow middorsal band on ridge-bearing ossicles (Wood et al. 1996). Köhler (1996) supported this anatomical distinction in his depiction of E. eocaenus keels. Parmley et al. (2006) noted that indeterminate dermochelyid ossicles from the late Eocene of Georgia (USA) share these ridge characteristics, and tentatively compared these ossicles to those of the genus Egyptemys. Furthermore, CCNHM 4289 does not compare well with most Psephophorus-type ossicles. The ridges on ossicles referred to the genus Psephophorus are very prominent, may or may not show a visceral concavity, and appear on ossicles that are usually anteroposteriorly elongated (Köhler 1996; Wood et al. 1996; Chesi et al. 2007; Delfino et al. 2013). CCNHM 4289 has a weak ridge that is semi-circular in cross section, is confined to a narrow middorsal band, is transversely wide, and mostly lacks visceral expression. This specimen is therefore best identified as cf. Egyptemys sp. based on its ridge characteristics. However, recent work suggests that weakly ridged ossicles with slight visceral concavity also exist on the accessory ridges of Psephophorus-type shells. Furthermore, the scalloped margins of CCNHM 4289 also mark it as a candidate for the sunflower pattern that has been reported in shells assigned to the genus Psephophorus (Peters et al. 2019). As such, our assignment of CCNHM 4289 to Egyptemys is tentative.
The geochronological age and thickness of CCNHM 4289 also support its identification as cf. Egyptemys sp. This genus is known from the late Eocene of northern Egypt and the early Miocene of Oregon and California, USA (Andrews 1901; Packard 1940; Mitchell and Tedford 1973; Köhler 1996; Wood et al. 1996). With an age of 24.7–23.5 Ma, CCNHM 4289 compares well with the geochronological ages of other Egyptemys fossils (Table 2). Although CCNHM 4289 falls within the thickness range recorded for Psephophorus ossicles, the substantial variation in Psephophorus ossicle thickness (4.8‒19.9 mm) offers little help in identification (Chesi et al. 2007; Delfino et al. 2013; Fallon and Boessenecker 2019). Still, Wood et al. (1996) noted that the ridged ossicles of Egyptemys eocaenus are no thicker than 12 mm, which is consistent with the 9.0 mm thickness of CCNHM 4289.
Table 2. All leatherback sea turtle fossils reported for the Cenozoic.
|
Taxon |
Geologic age |
Location |
Material |
References |
|
Arabemys crassiscutata |
late Paleocene–early Eocene |
Northern Saudi Arabia |
isolated bony ossicles |
|
|
Eosphargis gigas |
early-middle Eocene |
England |
humeri, skull, |
Owen 1850; Owen 1880; Köhler 1996 |
|
Eosphargis breineri |
Eocene |
Denmark |
humeri, skull, plastron |
Nielsen 1959, 1963 |
|
Cosmochelys
dolloi and |
Eocene |
Southern Nigeria; Southern Crimea; and Ukraine |
carapacial fragments and isolated ossicles |
|
|
Maorichelys wiffeni |
Eocene |
South Island, |
humerus fragment |
|
|
Psephophorus terrypratchetti |
Eocene |
South Island, New Zealand; and Seymour Island, Antarctica |
shell fragments, ribs, |
|
|
Psephophorus sp. |
Eocene |
South Carolina and |
carapacial fragments and isolated ossicles |
Müller 1847;
Thurmond and Jones 1981; de
la Fuente et al. 1995; Charleston Museum (Roger Wood,
personal |
|
Egyptemys
eocaenus |
late Eocene |
Northern Egypt |
humeri, carapacial |
|
|
Dermochelyidae indet. |
late Eocene |
Georgia, USA |
articulated and isolated ossicles |
|
|
Natemys rupeliensis |
Oligocene |
Niel and Terhaege, Belgium; and Doberg, Germany |
limb, rib, scapula, skull and plastron fragments, cervical centrum, ossicles, vertebrae |
|
|
Natemys sp. 1 |
early–late Oligocene |
South Carolina, USA |
CCNHM 4405, 4288, 5540, 5541, and 5542 |
this paper |
|
Natemys sp. 2 |
early–late Oligocene |
South Carolina, USA |
CCNHM 4287 and 4910 |
this paper |
|
cf. Egyptemys sp. |
late Oligocene |
South Carolina, USA |
CCNHM 4289 |
this paper |
|
cf. Psephophorus sp. |
early–late Oligocene |
South Carolina, USA |
CCNHM 5460 and 5543 |
this paper |
|
Psephophorus sp. |
late Oligocene |
South Carolina, USA |
ChM PV 4892 |
Charleston Museum |
|
Dermochelyidae indet. |
early Miocene |
East Pisco Basin, Peru |
carapacial fragment and partial forelimb |
|
|
Natemys peruvianus |
Oligocene or Miocene |
Pisco Basin, Peru |
partial carapace and |
|
|
Egyptemys oregonensis (sensu Wood et al. 1996) |
early Miocene |
Oregon and California, USA |
skull, small shell fragment |
|
|
Psephophorus
calvertensis |
early–middle Miocene |
Maryland, USA |
carapacial and scapular fragments, humerus |
|
|
“Psephophorus”
|
middle Miocene |
California, USA |
femur |
|
|
cf. Dermochelys sp. |
Miocene |
Virginia, USA |
juvenile humerus |
|
|
Psephophorus polygonus |
late Miocene |
Southern Italy; |
carapacial fragments and isolated ossicles |
|
|
Natemys (sensu this paper) |
late Miocene |
Denmark |
carapacial fragments, costal bone fragments |
|
|
cf. Psephophorus sp. |
early Pliocene |
California, USA |
isolated carapacial ossicle |
|
|
Psephophorus sp. |
early Pliocene |
Florida, USA |
isolated ossicle |
|
|
Psephophorus sp. |
early Pliocene |
North Carolina, USA |
one ridged and |
|
|
Dermochelys coriacea |
middle–late Holocene |
Coastal, Oman |
isolated ossicles |
cf. Psephophorus sp. Meyer, 1847
Fig. 5.
Material.—CCNHM 5460, an isolated non-ridged carapacial ossicle collected by Sarah J. Boessenecker on September 4, 2019 from the Givhan’s Ferry Member of the Ashley Formation, Wescott Plantation Subdivision, Summerville; CCNHM 5543, an isolated ridged ossicle collected by Steven Miller from Bed 1 of the Chandler Bridge Formation, locality uncertain. All Oligocene of South Carolina, USA.
Description.—CCNHM 5460 is a polygonal ossicle that is approximately tabular (Fig. 5A3). It is distinguished from the other ossicles in having shallow radial grooves on its otherwise smooth, slightly convex dorsal surface (Fig. 5A1). Its slightly concave visceral surface is ornamented with a woven lacework pattern of shallow ridges and grooves, and its sutural surface reveals an internal structure consisting of a dense dorsal layer and a vascularized visceral layer (Fig. 5A2, A3). CCNHM 5543 is thicker (14.0 mm) than CCNHM 5460 (8.9 mm), but is otherwise similar in length and width (Table 1). It has a smooth dorsal surface and a somewhat smooth visceral surface that is crossed by four deep grooves intersecting in the center of the ossicle (Fig. 5B2). CCNHM 5543 has a broad, low ridge that spans the entire width of the ossicle and is pronounced on the visceral surface (Fig. 5B3). Its internal structure is comparable to CCNHM 5460 as revealed by its sutural surface.
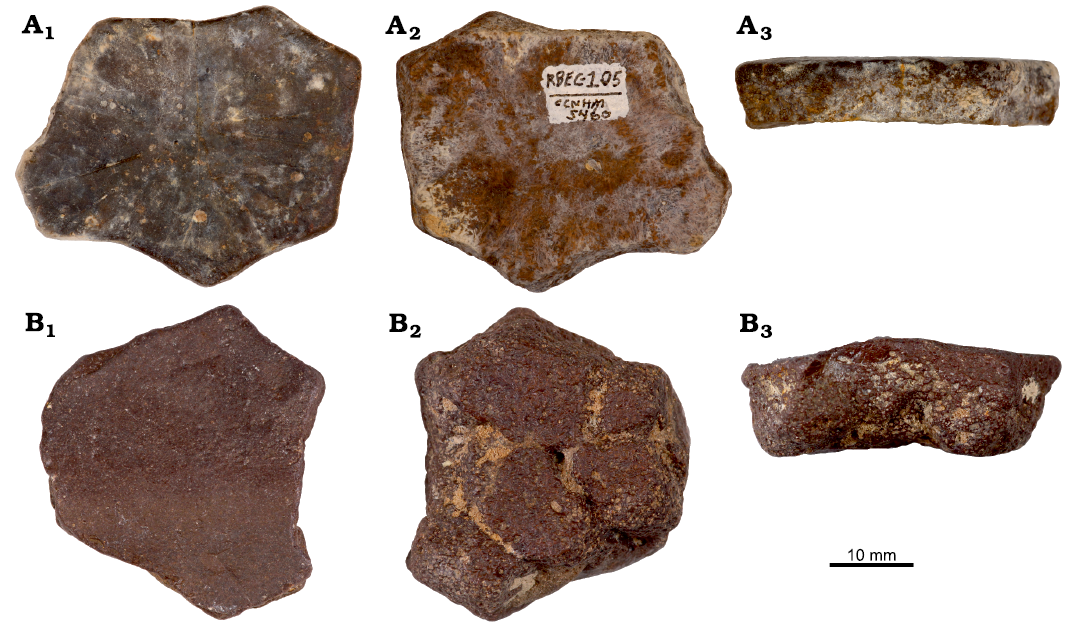
Fig. 5. Ossicles of leatherback turtle cf. Psephophorus sp. from Oligocene of South Carolina, USA. CCNHM 5460 (A) and CCNHM 5543 (B), in dorsal (A1, B1), visceral (A2, B2), and sutural (A3, B3) views.
Remarks.—Specimens are assigned to cf. Psephophorus sp. owing to (i) the dorsal, radial ornamentation on CCNHM 5460, (ii) the presence of a broad ridge with prominent visceral expression on CCNHM 5543, and (iii) to the ossicles’ diploic internal structure.
CCNHM 5460 is referred to the genus Psephophorus based on its dorsal surface texture and internal structure. Most notably, it has a dorsal, radial ornamentation that has also been described for the genera Arabemys Tong, Buffetaut, Thomas, Roger, Halawani, Memesh, and Lebret, 1999, Cosmochelys and Dermochelys, though the ornamentation is much less pronounced in CCNHM 5460 when compared to that of the former extinct genera (Andrews 1919; de la Fuente et al. 1995; Tong et al. 1999; Zvonok et al. 2013; Zvonok and Danilov 2019). Albright et al. (2003) also noted this distinction, and tentatively assigned Antarctic Eocene ossicles discussed in their study to cf. Psephophorus sp. Finally, the diploic internal structure of CCNHM 5460 also resembles ossicles of the genus Psephophorus as outlined by Delfino et al. (2013) and Fallon and Boessenecker (2019).
We assign CCNHM 5543 to cf. Psephophorus sp. based on the presence and structure of its ridge. Like other ridged ossicles referred to the genus Psephophorus, CCNHM 5543 has a ridge that is very prominent, demonstrates visceral concavity, and spans the entire width of the ossicle (Köhler 1996; Wood et al. 1996; Chesi et al. 2007; Delfino et al. 2013).
Discussion
South Carolina leatherbacks.—These newly reported ossicles represent the first published occurrences of leatherback sea turtle fossils from South Carolina. Their identifications are based on thickness, presence and structure of dorsal ridges, internal structure and geochronological age. CCNHM 4405.1–4405.5, 4288, 5540, 5541, 5542, 4287.1, 4287.2, and 4910 are referred to the genus Natemys because several of the associated ossicles exhibit heavily scalloped margins that are indicative of the sunflower pattern unique to the shells of this genus. There are differences in thickness and internal structure between some of these ossicles, however, leading us to recognize two morphotypes: Natemys sp. 1, and Natemys sp. 2, the latter of which resembles Natemys rupeliensis in size. CCNHM 4289 is tentatively referred to cf. Egyptemys sp. based on the presence of a weak dorsal ridge that is semi-circular in cross-section and is confined to a narrow band along the middle of the ossicle. Further, the ossicle is similar to those of the genus Egyptemys in thickness and geochronological age, but we also recognize its anatomical similarities to those of the genus Psephophorus. CCNHM 5460 and 5543 are referred to cf. Psephophorus sp. based on the radial ornamentation of CCNHM 5460 and on the dorsal ridge characteristics of CCNHM 5543.
First Oligocene leatherback record from the Atlantic Coastal Plain.—These ossicles are also the first formally described leatherback remains from the Oligocene of the Atlantic Coastal Plain. Other leatherback remains have been reported from the Atlantic Coastal Plain but they do not include fossils that date to the Oligocene. Wood et al. (1996) and Köhler (1996) mentioned Eocene Psephophorus sp. carapacial fragments from South Carolina. Müller (1847) and Thurmond and Jones (1981) reported hundreds of late Eocene Psephophorus sp. shell fragments from Alabama. There are also several articulated and isolated late Eocene dermochelyid ossicles from Georgia (Parmley et al. 2006), as well as “Psephophorus calvertensis” (synonymy P. polygonus sensu Peters et al. 2019) carapacial fragments, scapular fragments, and a humerus from the Miocene Calvert Cliffs in Maryland, USA (Palmer 1909; Weems 1974). Fabian et al. (2018) also described a juvenile cf. Dermochelys sp. humerus from the Miocene Calvert Formation of Virginia while Dodd and Morgan (1992) reported a Psephophorus sp. isolated ossicle from the Pliocene Bone Valley Formation in Florida. Four Psephophorus sp. and Dermochelys sp. shell fragments have also been recorded from the Pliocene Yorktown Formation of North Carolina (Köhler 1996; Zug 2001). Here, we report fossils that date to the Oligocene, thus filling a geochronological gap that existed for the leatherback fossil record of the Atlantic Coastal Plain.
As mentioned by Köhler (1996) and Wood et al. (1996, 2009), other leatherback remains from the Oligocene Atlantic Coastal Plain exist, but have not been formally described. A Psephophorus-like partial carapace is known from the Oligocene Chandler Bridge Formation, but the carapacial fragment (ChM PV 4892) currently housed at the Charleston Museum awaits study pending completion of preparation (Weems 1988; Wood et al. 1996, 2009). Additionally, a second Oligocene specimen consists of a partial carapace excavated from the Ashley Formation that is now housed at the South Carolina State Museum and awaits further study (Wood et al. 2009; Paul Bailey and Mark Bunce personal communication 2016).
First multispecies leatherback assemblage.—This paper reports the first multispecies leatherback assemblage reported in the fossil record. As Cenozoic dermochelyid richness was highest during the Eocene and Oligocene (Table 2), the existence of a multispecies assemblage is not surprising (Wood et al. 1996; Fallon and Boessenecker 2019). A similar assemblage may be mirrored in the Oligocene of western Europe, consisting of Belgian and German fossils of various leatherback remains, including vertebral, limb, cranial and shell specimens (Table 2). However, these fossils are from different localities and their taxonomic assignments are debatable as discussed in our taxonomic note (see above). Although a multispecies assemblage from the Oligocene of Europe would not be surprising, taxonomic uncertainty precludes such a conclusion and further study is needed.
Newly reported leatherback fossils from the Oligocene of South Carolina consist of carapacial ossicles representing three to four distinct leatherback morphs: Natemys sp. 1 and 2, cf. Psephophorus sp. and potentially cf. Egyptemys sp. Fossil remains of the genus Natemys have only been reported confidently from South America (and potentially Denmark, see Karl et al. 2012) while fossils of the Psephophorus have never been described from the Oligocene anywhere in the world (Wood et al. 1996; Table 2). Egyptemys fossils have only been formally reported from Eocene and Miocene formations in Africa and northwest North America, respectively (Table 2). As such, the South Carolina Oligocene fossils we describe fill a geographic gap for the genus Natemys and a temporal gap for the Psephophorus. Further, the ossicle we refer to cf. Egyptemys sp. potentially fills both a geographical and geochronological gap for this genus. Our findings thus suggest that extinct leatherbacks had a cosmopolitan distribution, not unlike their modern counterpart, D. coriacea (Wood et al. 1996; Karl 2002; Fossette et al. 2010; Karl 2014; Curtis et al. 2015).
Conclusions
Potentially three leatherback genera are represented in the Oligocene Ashley and Chandler Bridge formations of Charleston, South Carolina, USA. Identifications of fifteen carapacial ossicles as Natemys sp. 1 and 2, cf. Egyptemys sp. and cf. Psephophorus sp. are based on ossicle thickness, internal structure, presence and type of ridge, and geochronological age. These are the first leatherback sea turtle remains to be formally described from South Carolina and from the Oligocene of the Atlantic Coastal Plain. They represent the first confidently-identified multispecies leatherback assemblage known worldwide. They also fill geographical and temporal gaps in the leatherback fossil record as they date to the Oligocene and were found in eastern North America. While these ossicles offer insight into the evolutionary history of extinct dermochelyids, our findings highlight the need for formal study of abundant unpublished leatherback remains as well as the continued discovery of dermochelyid fossils.
Acknowledgements
Thanks to Shaun Coates, Steve Hildenbrandt and the late Steven Miller (Charleston, SC, USA) for donating fossils to the CCNHM collection for study in this paper. We would also like to thank Sarah Boessenecker (CCNHM) for field assistance. Thanks to Sarah Boessenecker, Matthew Gibson (ChM), and Jessica Peragine (ChM) for access to specimens under their care. This study benefitted from discussions with Jonathan Geisler (New York Institute of Technology, USA), Robert Weems (US Geological Survey, Reston, Virginia, USA), Mace Brown (CCNHM), and James Parham (California State University, Fullerton, USA), and from constructive comments by the reviewers James Parham and Dana Ehret (New Jersey State Museum, Trenton, USA) and the editor Daniel Barta (Oklahoma State University Center for Health Sciences, Tahlequah, USA).
References
Albright, L.B., Sanders, A.E., Weems, R.E., Cicimurri, D.J., and Knight, J.L. 2019. Cenozoic vertebrate biostratigraphy of South Carolina, U.S.A. and additions to the fauna. Bulletin of the Florida Museum of Natural History 57: 77‒236.
Albright, L.B., Woodburne, M.O., Case, J.A., and Chaney, D.S. 2003. A leatherback sea turtle from the Eocene of Antarctica: Implications for antiquity of gigantothermy in Dermochelyidae. Journal of Vertebrate Paleontology 23: 945‒949. Crossref
Andrews, C.A. 1901. Preliminary note on some recently discovered extinct vertebrates from Egypt. (Part II). Geological Magazine 8: 436‒444. Crossref
Andrews, C.A. 1919. A description of a new species of zeuglodont and of leathery turtle from the Eocene of southern Nigeria. Proceedings of the Zoological Society 18: 309‒19. Crossref
Baur, G. 1893. Notes on the classification of the Cryptodira. The American Naturalist 27: 672‒674.
Bianucci, G., Collareta, A., Bosio, G., Landini, W., Gariboldi, K., Gioncada, A., Lambert, O., Malinverno, E., de Muizon, C., Varas-Malca, R., Villa, I.M., Coletti, G., Urbina, M., and Di Celma, C. 2018. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeography, Palaeoclimatology, Palaeoecology 511: 256‒279. Crossref
Boessenecker, R.W. and Fordyce, R.E. 2017. A new eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). Journal of Systematic Palaeontology 15: 429‒469. Crossref
Boessenecker, R.W. and Geisler, J.H. 2018. New records of the archaic dolphin Agorophius (Mammalia: Cetacea) from the upper Oligocene Chandler Bridge Formation of South Carolina, USA. PeerJ 6: e529. Crossref
Cadena, E., Abella, J., and Gregori, M. 2018. The first Oligocene sea turtle (Pan-Cheloniidae) record of South America. PeerJ 6:e4554. Crossref
Carriol, R.P. and Vader, W. 2002. Occurrence of Stomatolepas elegans (Cirripedia: Balanomorpha) on a leatherback turtle from Finnmark, northern Norway. Journal of the Marine Biological Association of the United Kingdom 82: 1033‒1034. Crossref
Casier, E. 1966. Faune ichthyologique du London Clay. 510 pp. British Museum Natural History, London.
Chesi, F., Delfino, M., Varola, A., and Rook, L. 2007. Fossil sea turtles (Chelonii, Dermochelyidae and Cheloniidae) from the Miocene of Pietra Leccese (Late Burdigalian–Early Messinian) of southern Italy. Geodiversitas 29: 321‒33.
Cicimurri, D.J. and Knight, J.L. 2009. New record of an extinct fish, Fisherichthys folmeri Weems (Osteichthyes) from the lower Eocene of Berkeley County, South Carolina, USA. PaleoBios 29: 24‒28.
Cope, E.D. 1870. Synopsis of the extinct Batrachia, Reptilia and Aves of North America. Part II. Transactions of the American Philosophical Society, New Series 14: 105‒235. Crossref
Curtis, K.A., Moore, J.E., and Benson, S.R. 2015. Estimating limit reference points for western pacific leatherback turtles (Dermochelys coriacea) in the U.S. West Coast EEZ. PLoS ONE 10 (9): e0136452. Crossref
Dames, W. 1894. About zeuglodonts from Egypt and the relations of the archaeocetes to the remaining cetaceans. Paläontologische Abhandlungen 1 (5): 1‒36.
de la Fuente, M.S., Santillana, S.N., and Marenssi, S.A. 1995. An Eocene leatherback turtle (Cryptodira: Dermochelyidae) from Seymour Island, Antarctica. Stvdia Geològica Salmanticensia 31: 21‒34.
Delfino, M., Scheyer, T.M., Chesi, F., Fletcher, T., Gemel, R., Macdonald, S., Rabi, M., and Salisbury, S.W. 2013. Gross morphology and microstructure of type locality ossicles of Psephophorus polygonus Meyer, 1847 (Testudines, Dermochelyidae). Geological Magazine 150: 767‒782. Crossref
Dodd, C.K., Jr. and Morgan, G.S. 1992. Fossil sea turtles from the early Pliocene Bone Valley Formation, Central Florida. Journal of Herpetology 26: 1‒8. Crossref
Domning, D.P. 1997. Fossil Sirenia of the west Atlantic and Caribbean region. VI. Crenatosiren olseni (Reinhart, 1976). Journal of Vertebrate Paleontology 17: 397‒412. Crossref
Domning, D.P. and Beatty, B.L. 2019. Fossil Sirenia of the west Atlantic and Caribbean region. XII. Stegosiren macei, gen. et sp. nov. Journal of Vertebrate Paleontology 39: e1650369. Crossref
Doyle, T.K, Houghton, J.D.R, O’Súilleabháin, P.F., Hobson, V.J., Marnell, F., Davenport, J., and Hays, G.C. 2008. Leatherback turtles satellite-tagged in European waters. Endangered Species Research 4: 23‒31. Crossref
Duméril, C. and Bibron, G. 1835. Erpétologie Générale, ou, Histoire Naturelle Complète des Reptiles. 706 pp. Roret, Paris. Crossref
Eckert, K.L. and Luginbuhi, C. 1988. Death of a giant. Marine Turtle Newsletter 43: 2‒3.
Eckert, K.L., Wallace, B.P., Frazier, J.G., Eckert, S.A., and Pritchard, P.C.H. 2012. Synopsis of the biological data on the leatherback sea turtle (Dermochelys coriacea). U.S. Department of Interior, Fish and Wildlife Service. Biological Technical Publication BTP-R4015-2012, 160 pp.
Eggleston, D. 1971. Leathery turtle (Reptilia: Chelonia) in Foveaux Strait (Note). New Zealand Journal of Marine and Freshwater Research 5: 522‒523. Crossref
Fabian, G.A., Parris, D.C., and Nestor-Pasicznyk, W. 2018. A humerus of a juvenile dermochelyid turtle from the Miocene of Virginia. The Mosasaur: The Journal of the Delaware Valley Paleontological Society 10: 85‒90.
Fallon, B.R. and Boessenecker, R.W. 2019. First record of the leatherback sea turtle (Dermochelyidae) from the Mio-Pliocene Purisima Formation of northern California, USA. PaleoBios 36: 1‒8.
Fierstine, H.L. and Weems, R.E. 2009. Paleontology of the Oligocene Ashley and Chandler Bridge formations of South Carolina, 4: analysis and new records of billfishes (Perciformes: Xiphioidei). Palaeo Ichthyologica 11: 43‒88.
Fossette, S., Hobson, V.J., Girard, C., Calmettes, B., Gaspar, P., Georges, J., and Hays, G.C. 2010. Spatio-temporal foraging patterns of a giant zooplanktivore, the leatherback turtle. Journal of Marine Systems 81: 225‒234. Crossref
Frazier, J.G., Azzarà, V., Munoz, O., Marcucci, L.G., Badel, E., Genchi, F., Cattani, M., Tosi, M., and Delfino, M. 2018. Remains of leatherback turtles, Dermochelys coriacea, at mid-late Holocene archaeological sites in coastal Oman: Clues of past worlds. PeerJ 6: e6123. Crossref
Geisler, J.H., Boessenecker, R.W., Brown, M., and Beatty, B.L. 2017. The origin of filter feeding in whales. Current Biology 27: 2036‒2042. Crossref
Gilmore, C.W. 1937. A new marine turtle from the Miocene of California. Proceedings of the California Academy of Sciences, 4th Series 23 (10): 171‒174.
Gray, J.E. 1825. A synopsis of genera of Reptiles and Amphibia, with a description of some new species. Annals of Philosophy 2 (10): 193‒217.
Hay, O.P. 1908. The fossil turtles of North America. Carnegie Institution of Washington Publication 75: 1‒568.
Hay, O.P. 1923. Oligocene sea turtles of South Carolina. Pan-American Geologist 40: 29–31.
Heaslip, S.G., Iverson, S.J., Bowen, W.D., and James, M.C. 2012. Jellyfish support high energy intake of leatherback sea turtles (Dermochelys coriacea): Video evidence from animal-borne cameras. PLoS ONE 7 (3): e33259. Crossref
Joyce, W.G., Parham, J.F., and Gauthier, J. 2004. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. Journal of Paleontology 78 (5): 989‒1013. Crossref
Karl, H.-V. 1993. About two problematic fossil sea turtle remains (Testudines, Chelonioidea) in northern Germany. Mauritiana 14: 289‒296.
Karl, H.-V. 2002. Overview of the fossil marine turtle families in Central Europe (Reptilia, Testudines). Mauritiana 18: 171‒202.
Karl, H.-V. 2014. The fossil turtles from the Doberg near Bünde. In: M. Kaiser and R. Ebel. (eds.), Der Doberg bei Bünde: Eine klassische Fundstelle der Paläontologie, 90‒102. Verlag Dr. Friedrich Pfeil, Munchen.
Karl, H.-V. and Tichy, G. 2007. Maorichelys wiffeni n. gen. n. sp., a new sea turtle from the Eocene of New Zealand (Chelonii: Dermochelyidae). Studia Geologica Salmanticensia 43: 11‒24.
Karl, H.-V., Lindow, B.E.K., and Tütken, T. 2012. Miocene leatherback turtle material of the genus Psephophorus (Testudines: Dermochelyoidea) from the Gram Formation (Denmark). Studia Palaeocheloniologica 4 (9): 205‒216.
Katuna, M.P., Geisler, J.H., and Colquhoun, D.J. 1997. Stratigraphic correlation of Oligocene marginal marine fluvial deposits across the middle and lower coastal plain, South Carolina. Sedimentary Geology 108: 181‒194. Crossref
Kellogg, R. 1923. Description of an apparently new toothed cetacean from South Carolina. Smithsonian Miscellaneous Collections 76 (7): 1‒7.
Kidwell, S.M., Fursich, F.T., and Aigner, T. 1986. Conceptual framework for the analysis and classification of fossil concentrations. Palaios 1: 228‒238. Crossref
Koenen, A.V. 1891. Chelone ingens nov. sp. In: E. Lienenklaus (ed.), Die Ober-Oligocän-Fauna des Doberges. Jahresbericht Naturwissenschaftlichen Vereins Osnabrück 8: 43–174.
Köhler, R. 1996. Eocene Turtles and Whales from New Zealand. 359 pp. Ph.D. Dissertation University of Otago, Dunedin.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. 824 pp. Laurentii Salvii, Stockholm. Crossref
Lynch, S.C. and Parham, J.F. 2003. The first report of hard-shelled sea turtles (Cheloniidae sensu lato) from the Miocene of California, including a new species (Euclastes hutchisoni) with unusually plesiomorphic characters. PaleoBios 23: 21‒35.
Magwene, P.M. and Socha, J.J. 2013. Biomechanics of turtle shells: how whole shells fail in compression. Journal of Experimental Zoology 319: 86‒98. Crossref
Matthews, P., McCarthy, M.D., and Young, M. 1994. The Guinness Book of Records. 39 pp. Facts on File, New York.
Meyer, H. von 1847. Communication to Professor Bronn. Neues Jahrbuchfiir Mineralogie, Geognosie, Geologie und Petrefactenkunde 1847: 572‒580.
Mitchell, E. and Tedford, R.H. 1973. The Enaliarctinae: a new group of extinct aquatic Carnivora and a consideration of the origin of the Otariidae. Bulletin of the American Museum of Natural History 151: 203‒284.
Müller, J. 1847. Bericht tiber die von Dr. Koch in Alabama gesammelten Knochen Reste seines Hydrarchus. Neues Jahrbuchfiir Mineralogie, Geognosie, Geologie und Petrefaktenkunde 1847: 623‒631.
Newman, W.A. 1982. A review of the extant taxa of the “Group of Balanus concavus” (Cirripedia, Thoracica) and a proposal for genus-group ranks. Crustaceana 43 (1): 25–36. Crossref
Packard, E.L. 1940. A new turtle from the marine Miocene of Oregon. Oregon State College Studies in Geology 2: 1–31.
Palmer, W. 1909. Description of a new species of leatherback turtle from the Miocene of Maryland. Proceedings of the U.S. National Museum 36: 369‒373. Crossref
Parmley, D., Hutchinson, J.H., and Parham, J.F. 2006. Diverse turtle fauna from the late Eocene of Georgia including the oldest records of aquatic Testudinoids in southeastern North America. Journal of Herpetology 40: 343‒350. Crossref
Peters, M.E., Bosselaers, M.E.J., Post, K., and Reumer, J.W.F. 2019. A Miocene leatherback turtle from the Westerschelde (The Netherlands) with possible cetacean bite marks: identification, taphonomy and cladistics. Cainozoic Research 19: 121‒133.
Röding, P.F. 1798. Museum Boltenianum sive Catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat Joa. Fried Bolten, M.D.p.d. per XL. annos proto physicus Hamburgensis. Pars secunda continens Conchylia sive Testacea univalvia, bivalvia & multivalvia. viii, 199 pp. Trapp, Hamburg.
Sacco, F. 1897. Mollusks from the tertiary soils of Piedmont and Liguria, Part 24. Bollettino dei Musei di Zoologia ed Anatomia Comparata della R. Università di Torino 12 (298): 101‒102.
Sanders, A.E. and Geisler, J.H. 2015. A new basal odontocete from the upper Rupelian of South Carolina, U.S.A., with contributions to the systematics of Xenorophus and Mirocetus (Mammalia, Cetacea). Journal of Vertebrate Paleontology 35: e890107. Crossref
Sanders, A.E., Weems, R.E., and Lemon, E.M., Jr. 1982. The Chandler Bridge Formation; a new Oligocene stratigraphic unit in the lower Coastal Plain of South Carolina. In: Contributions to Stratigraphy, U.S. Geological Survey Bulletin 1529-H: 105‒124.
Thurmond, J.T. and Jones, D.E. 1981. Fossil Vertebrates of Alabama. 244 pp. The University of Alabama Press, University of Alabama.
Tong, H., Buffetaut, E., Thomas, H., Roger, J., Halawani, M., Memesh, A., and Lebret, P. 1999. A new dermochelyid turtle from the late Paleocene–early Eocene of Saudi Arabia. Earth & Planetary Sciences 329: 913‒19. Crossref
Van Beneden, P.J. 1883. Note on Sphargis bones found in the brick earth of the country of Waas. Bulletins de l’Académie Royale des Sciences, des Lettres et des Beaux-Arts de Belgique 3 (6): 665‒684.
Vandelli, D. 1761. Epistola de holothurio, et Testudine coriacea ad celeberrimum Carolum Linnaeum equitem naturae curiosum Dioscoridem II. 12 pp. Padua, Conzatti.
Wallace, B.P., Tiwari, M., and Girondot, M. 2013. Dermochelys coriacea, leatherback. The IUCN Red List of Threatened Species 2013: e.T6494A43526147.
Weems, R.E. 1974. Middle Miocene sea turtles (Syllomus, Procolpochelys, Psephophorus) from the Calvert Formation. Journal of Paleontology 48: 278‒303.
Weems, R.E. 1988. Palaeocene turtles from the Aquia and Brightseat formations, with discussions of their bearing on sea turtle evolution and phylogeny. Proceedings of the Biological Society of Washington 101: 109‒145.
Weems, R.E. and Brown, K.M. 2017. More-complete remains of Procolpochelys charlestonensis (Oligocene, South Carolina), an occurrence of Euclastes (upper Eocene, South Carolina), and their bearing on Cenozoic pancheloniid sea turtle distribution and phylogeny. Journal of Paleontology 91: 1228‒1243. Crossref
Weems, R.E. and Knight, J.L. 2009. A new species of Bairdemys (Pelomedusoides: Podocnemididae) from the Oligocene (Early Chattian) Chandler Bridge Formation of South Carolina, USA, and its paleobiogeographic implications for the genus. In: D.B. Brinkman, P.A. Holroyd, and J.D. Gardner (eds.), Morphology and Evolution of Turtles, 289‒303. Springer, Dordrecht. Crossref
Weems, R.E. and Sanders, A.E. 2014. Oligocene pancheloniid sea turtles from the vicinity of Charleston, South Carolina, USA. Journal of Vertebrate Paleontology 34: 80‒99. Crossref
Weems, R.E., Albright, L.B., Bybell, L.M., Cicimurri, D.J., Edwards, L.E., Harris, W.B., Lewis, W.C., Osborne, J.E., Sanders, A.E., and Self-Trail, J.M. 2016. Stratigraphic revision of the Cooper Group and the Chandler Bridge and Edisto Formations in the Coastal Plain of South Carolina. South Carolina Geology 49: 1‒24.
Wood, R.C., Johnson-Gove, J., Gaffney, E.S., and Maley, K.F. 1996. Evolution and phylogeny of leatherback turtles (Dermochelyidae), with descriptions of new fossil taxa. Chelonian Conservation and Biology 2: 266‒286.
Wood, R.C., Knight, J.L., Cicimurri, D., and Sanders, A.E. 2009. Fossil leatherback turtles (Dermochelyidae) from the Paleogene of South Carolina. In: D.R. Braman (compiler), Turtle Symposium, October 17–18, 2009. Abstracts and Program. Special Publication of the Royal Tyrrell Museum of Paleontology: 195‒198.
Zug, G.R. 2001. Turtles of the Lee Creek mine (Pliocene: North Carolina). In: C.E. Ray and D.J. Bohaska (eds.), Geology and Paleontology of the Lee Creek Mine, North Carolina, III. Smithsonian Contributions to Paleobiology 90: 203‒218.
Zvonok, E.A. and Danilov, I.G. 2019. Paleogene turtles of Crimea. Paleontological Journal 53: 62‒73. Crossref
Zvonok, E.A., Danilov, I.G., and Syromyatnikova, E.V. 2013. The first reliable record of fossil leatherback sea turtle (Dermochelyidae) in northern Eurasia (middle Eocene of Ukraine). Paleontological Journal 47: 199‒202. Crossref
Acta Palaeontol. Pol. 65 (4): 763–776,
2020 https://doi.org/10.4202/app.00740.2020