

Late Cretaceous mega-, meso-, and microfloras from Lower Silesia
ADAM T. HALAMSKI, JIŘÍ KVAČEK, MARCELA SVOBODOVÁ, EWA DURSKA, and ZUZANA HEŘMANOVÁ
Halamski, A.T., Kvaček, J., Svobodová, M., Durska, E., and Heřmanová, Z. 2020. Late Cretaceous mega-, meso-, and microfloras from Lower Silesia. Acta Palaeontologica Polonica 65 (4): 811–878.
Late Cretaceous plants from the North Sudetic Basin (Lower Silesia, south-western Poland) are reviewed on the basis of megaflora from 17 localities (270 identifiable specimens), mesoflora from two localities, and microflora from four localities. Major sites are Rakowice Małe and Bolesławiec. Eight megafloral assemblages are distinguished (Assemblage 1, Turonian; Assemblages 2, 3, lower–middle Coniacian; Assemblages 4, 5, upper Coniacian?–lower Santonian?; Assemblages 6–8, lower–middle Santonian); the bulk of the palaeoflora is from Assemblages 4–6 and 8. Megaflora consists of 29 taxa (6 ferns, 4 conifers, and 19 angiosperms). Geinitzia reichenbachii is the most common species. Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. is a trifoliolate leaf re-interpreted as a representative of Fagales. Three species of Dewalquea are distinguished: Dewalquea haldemiana, Dewalquea insignis, and Dewalquea aff. gelindenensis. Platanites willigeri Halamski and Kvaček sp. nov. is characterised by trifoliolate leaves, the median leaflet of which is ovate, unlobed, with a serrate margin, and cuneate base. Palaeocommunities inferred from the megafossil record include: a back swamp forest dominated by Geinitzia, with abundant ferns; a Dryophyllum-dominated riparian forest; a forest with Dewalquea and Platanites willigeri possibly located in the marginal part of the alluvial plain; dunes with D. haldemiana and Konijnenburgia; a fern savanna with patches of Pinus woodlands. Palynoassemblage A from the Nowogrodziec Member, studied mostly at Rakowice Małe and Żeliszów, consists of 126 taxa, including 105 terrestrial palynomorphs (54 bryophyte, lycophyte, and pteridophyte spores, 16 gymnosperms, 35 angiosperms). The mega- and mesofossil records are dominated by angiosperms; the palynoassemblages are dominated by ferns. Palaeocommunities represented solely by the microfossil record are halophytic (with Frenelopsis and unconfirmed presence of Nypa) and pioneer vegetation. Palaeocommunities are intermediate in general character between those pre-dating the Cretaceous Terrestrial Revolution and modern, angiosperm-dominated vegetation. In comparison to older plant assemblages from contiguous areas laurophylls are much rarer; this might correspond to a real phenomenon of exclusion of lauroids from Santonian riparian forests. The studied assemblage is more similar to younger palaeofloras than to older ones; this might be interpreted as stabilisation of communities after a period of pronounced change related to the rise to dominance of the angiosperms. In contrast to widespread endemism among vertebrates of the European Archipelago, the plant cover consists mostly of species that are widely distributed.
Key words: Angiospermae, Leptosporangiatae, palaeobotany, palynology, taxonomy, Coniacian, Santonian, Poland.
Adam T. Halamski [ath@twarda.pan.pl], Institute of Paleobiology, Polish Academy of Sciences, ul. Twarda 51/55, 00-818 Warszawa, Poland.
Jiří Kvaček [jiri.kvacek@nm.cz] and Zuzana Heřmanová [zuzka.hermanova@gmail.com], National Museum, Václavské náměstí 68, 115 79 Praha 1, Czech Republic.
Marcela Svobodová [msvobodova@gli.cas.cz], Institute of Geology of the Czech Academy of Sciences, Department of Paleobiology and Paleoecology, Rozvojová 269, 165 00 Praha 6, Czech Republic.
Ewa Durska [edurska@uw.edu.pl], Faculty of Geology, University of Warsaw, ul. Żwirki i Wigury 93, 02-089 Warszawa, Poland.
Received 4 March 2020, accepted 6 August 2020, available online 13 November 2020.
Copyright © 2020 A.T. Halamski et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The region of Lower Silesia (Dolny Śląsk, Niederschlesien in former German literature, south-western Poland) was important in the early days of palaeobotany (Goeppert 1844: 161–163). The first fossil vegetal remains ever figured as such may have been Carboniferous plants from Silesia (Volkmann 1720) and plant fossils from Lower Silesia were discussed by Schlotheim (1820), Sternberg (1821), and Rhode (1821–1823). Late Cretaceous plant assemblages from Silesia range from the Cenomanian (Goeppert 1841; Niebuhr 2019) through the Turonian (Macko 1963; Płachno et al. 2018), Coniacian (Velenovský 1883; Langenhan and Grundey 1891; Frič 1897; Halamski and Kvaček 2015) to the Santonian (Roemer 1889; Halamski et al. 2018b).
The aim of the present paper is the systematic description of plant fossils and palaeoecological reconstruction of communities found in outcrops situated in the North Sudetic Basin (study area ca. 300 km2) and ranging from Turonian to Santonian in age. The assemblages consist of mega-, meso- and microfossils; a complete treatment of the megaflora and a synopsis of the microflora are provided, whereas mesofossils have been treated separately (Heřmanová et al. 2019; ZH, JK, ATH, and Jiřina Dašková, unpublished material), so only a general account of the mesoassemblage is given here.
Institutional abbreviations.—BGR, Bundesanstalt für Geowissenschaften und Rohstoffe, Berlin-Spandau, Germany; MB, Museum für Naturkunde, Berlin, Germany; MGUWr, Muzeum Geologiczne Uniwersytetu Wrocławskiego, Wrocław, Poland; MMG, Museum of Mineralogy and Geology, Senckenberg Natural History Collections Dresden, Germany; NMP, National Museum, Prague, Czech Republic; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
Other abbreviations.— ≡, homotypic synonym (ICN, Art. 14.4); auct., auctorum (Latin: of authors), for a taxon of uncertain authorship; coll., collection (referring to an owner, not necessarily the collector); ICN, International Code of Nomenclature for algae, fungi, and plants; leg. legit (Latin: collected), indicating the collector; s.n., sine numero (Latin: without number, for a specimen belonging to an established collection but lacking an inventory number; ubi syn., ubi synonymia (Latin: where the synonimy [can be found]), for a reference containing an extensive synonymy list.
Historical background
If one disregards data on the geology of the area along the Kwisa river forming the easternmost extremity of Lusatia then belonging to the state of Saxony, provided by Charpentier (1778), the earliest geological description of the North Sudetic Basin was given by von Raumer (1819: 128–130), but the first author to deal systematically with Cretaceous plants therefrom was Heinrich Robert Goeppert (Göppert). He described a tree fern from Żeliszów (Goeppert 1836b: 449) and signalled conifer wood and fruits from the vicinity of Lwówek Śląski (Goeppert 1844: 170). Heinrich Robert Goeppert assembled an important collection of Cretaceous plants (kept in MGUWr) and studied and annotated other specimens (like those from the secondary school at Lwówek Śląski, now in MB, see below). A short overview of “nearly finished works” on that subject was published as a summary of a lecture given on 1st November 1865 (Goeppert 1866a). A paper on Cretaceous index plants (Goeppert 1886b) included data from a few localities in Lower Silesia (“Geinitzia cretacea” Ulina, Nowogrodziec, Bolesławiec, Rakowice Małe; “Cunninghamites oxycedrus” Rakowice Małe; “Credneria sp.” Wartowice Nowe (erroneously as Cenomanian); “Debeya serrata” Ołdrzychów, probably erroneously for Ulina; “Gleichenia dresleriana” environs of Lwówek). However, no systematic treatment has ever appeared, so Goeppert’s identifications cited by Geinitz (1849–1850), Williger (1882: 84, 86–89, 94, 102, 104–108), and after him by Scupin (1913: 65) and Milewicz (1997: 31), are in some cases nomina nuda. They are discussed in the systematic part of the present paper.
Cretaceous angiosperms from the North Sudetic Basin were first recorded in print by Drescher (1863) from the “Schichten von Neu-Warthau” (Assemblage 2 herein). Among several dozens of animal taxa he mentioned also “Credneria denticulata Z.” and the conifer “Geinitzia cretacea Endl.” (Drescher 1863: 311). Geinitzia was also reported from Ołdrzychów by Klocke (1864: 262).
The paper by Williger (1882), although devoted primarily to stratigraphy and the fossil fauna of the North Sudetic Basin, is nonetheless of special importance, insofar as in several cases it has been the only source providing detailed lists of plant taxa coming from single outcrops, allowing thus to supplement, or even to correct, laconic or partly false labels. The debt of the present authors to Gustav Williger is expressed in naming Platanites willigeri Halamski and Kvaček sp. nov. in his honour.
Roemer (1886, 1889) studied fossil plants from the ceramic clays mined in the suburbs of Bolesławiec. He described seven taxa in total, including one new species (Menispermites bunzlavensis Roemer, 1889). Unfortunately, only a small part of his collection could be found in MGUWr, whereas the rest (including types and most of figured specimens) seems to be lost.
An important collection of plant fossils from the North Sudetic Basin was assembled in the Löwenberger Gymnasium (secondary school at Lwówek Śląski), at least in part by the teacher and cantor Ernst Friedrich Dresler, the author of a local flora (Dresler 1883). These specimens are the basis of lists given by Goeppert (1866a), Williger (1882) and Scupin (1913), but, as mentioned previously, no systematic treatment of them has ever been published. The collection was acquired by MB (probably partly in 1909 and partly in 1926) and was studied by the present authors.
It is of interest to note that the area was intensely mined for coal from the second half of the eighteenth century to the second half of the nineteenth century (Maciejak and Maciejak 2013) and some historical specimens do indeed come from coal mines. Active and former sandstone quarries (Żeliszów, Rakowice Małe) are at present the best localities for the fossil flora. Details on history of existing collections and on collectors are given by Mohr (2009). Cretaceous fauna, palaeogeography, and stratigraphy were dealt with, among others, by Scupin (1913) and Andert (1934).
Detailed maps (1:25 000) of the studied area and/or corresponding explanations were published by Kühn and Zimmermann (1918), Baraniecki et al. (1955), Milewicz (1955, 1956a, 1964), and Berezowska and Berezowski (1982). An important cartographic work made by Georg Berg (sheets Bunzlau and Kaiserswaldau) was never published, but its manuscript is kept in the BGR. A synthesis of the Cretaceous stratigraphy of the North Sudetic Basin was given by Milewicz (1997), and a short overview in English was published by Walaszczyk (2008). Sedimentological studies of interest for the present study were published by Milewicz (1956b), Górniak (1986), Leszczyński (2010, 2018), and Chrząstek and Wypych (2018).
Palynomorphs from Rakowice Małe were studied by Thiergart (1942) who described a new species, Sporites appendicifer (now Appendicisporites appendicifer; Schizaeaceae) from that locality (see also Thiergart 1949). In 1957 Wilfried Krutzsch collected at Rakowice Małe and included these strata in his palynological zoning of the Central European Cretaceous: the “Löwenberger Bild” encompasses uppermost Coniacian to lower Santonian strata (Krutzsch 1958: 512, table 1), but details were not provided. This is also the case for two further publications in which the “Löwenberger Bild” is used as an equivalent of the lower Santonian (Krutzsch 1959a: pl. 4: 12–13, 1966a: 96–97). A few spores and pollen grains from Rakowice Małe were illustrated by Pacltová and Krutzsch (1970: pls. 100: 11, 101: 9–13, 28–32).
A palynoflora from the North Sudetic Basin was studied by the Polish palaeobotanist Jadwiga Raniecka-Bobrowska (1904–1990; see biographic note by Grabowska 1992), culminating in an internal report (Raniecka-Bobrowska 1968) with cursory descriptions of over seven hundred palynotaxa (see below, Material and methods, Microflora) based on material from nine boreholes. It was intended to form the basis of a future monograph (Raniecka-Bobrowska 1968: 8), which, however, was never prepared. Results were published only in abridged form (Raniecka-Bobrowska 1984, 1989), with generalised data and description of a single new species, Catinipollis lwowekensis Raniecka-Bobrowska, 1984 from a borehole near Bolesławiec. A few illustrations from the above-mentioned report were also published in a short account of Late Cretaceous palynofloras from Poland by Grabowska (2003), but without detailed provenance data. The palynological part of the present paper is partly based on Raniecka-Bobrowska (1968).
In the monograph of Cretaceous fruits and seeds from Central Europe Knobloch and Mai (1986) described two mesofossil species from Rakowice Małe, namely Calathiocarpus octocostatus (Knobloch, 1971) Knobloch and Mai, 1986 and Walbeckia guttaeformis (Knobloch, 1971) Knobloch and Mai, 1986.
Studies of the Cretaceous megaflora of this region, which were abandoned for more than a century, except for a short literature-based overview by Lilpop and Kostyniuk (1957), were resumed starting with Mohr (2009) who provided an account of the palaeobotanical collections in MB from a historical point of view. In the revision of the Cretaceous tree fern stems from Bohemia and Silesia Greguš et al. (2013) described Protopteris punctata (Sternberg, 1820) Presl in Sternberg, 1838, from the environs of Lwówek Śląski and Protopteris singeri (Goeppert, 1836b) Presl in Sternberg, 1838, from Żeliszów. Preliminary results of the present work were presented by Halamski and Kvaček (2018, 2019), Heřmanová et al. (2018), and Halamski et al. (2018b, 2019).
Geological setting
The Cretaceous plant fossils described in the present paper come from 17 localities (described in the Appendix 1) situated in an area between Nowogrodziec and Bolesławiec in the north and Lwówek Śląski in the south (Fig. 1). From a geographic point of view (Kondracki 1998; Bína and Demek 2012), this region belongs to the West Sudetic Foothills (Pogórze Zachodniosudeckie). More precisely, five localities are situated in the Pogórze Izerskie (German: Isergebirgs-Vorland, Czech: Frýdlantská pahorkatina) and twelve in the Pogórze Kaczawskie (German: Bober-Katzbach-Vorgebirge), the two subregions being separated by the valley of the Bóbr river.
The North Sudetic Basin or North Sudetic Synclinorium (Niecka Północnosudecka) is a geological structure situated on the northern foreland of the Sudetes, the latter forming the north-eastern edge of the Bohemian Massif. The strata belonging to the North Sudetic Basin lie unconformably upon the Variscan basement and consist of Upper Carboniferous, Permian, Lower to Middle Triassic, and Upper Cretaceous sedimentary and volcanic rocks (Śliwiński et al. 2003; Żelaźniewicz 2005). The present tectonic structure of the basin is due to a wide-scale inversion related to multiphase convergence between Africa–Iberia and Western-Central Europe (e.g., Kley and Voigt 2008) that began in the Cretaceous and continued in the Paleogene (Solecki 2011), the detailed timing of which being, however, poorly constrained (Sobczyk et al. 2019 and references therein).
The Cretaceous part of the North Sudetic Basin, which may be considered as the south-eastern prolongation of the East Brandenburg Basin, consists of limestone, marl and marly sandstone, sandstone, mudstone, and claystone, locally with coal intercalations (Milewicz 1997: fig. 6). The age of the Cretaceous succession in the North Sudetic Basin ranges from the Cenomanian to the Santonian (Milewicz 1997; Walaszczyk 2008). The Cenomanian to middle Coniacian rocks are of marine origin, whereas younger deposits are of marine origin in the western part of the Basin and of brackish to alluvial origin in its central and eastern parts, including the study area.
The outcrops dealt with in the present paper are situated in the central part of the North Sudetic Basin. No Cretaceous plant megafossils are known from either the western part of the North Sudetic Basin as it is mostly covered by Tertiary deposits, or from Cenomanian to Santonian rocks cropping out in the eastern and south-eastern extremities of the basin (Wleń Trough, Świerzawa Trough; Milewicz 1997: fig. 1).
During the first half of the Late Cretaceous, more precisely starting with the Cenomanian transgression during which the major part of Central Europe was flooded (Voigt et al. 2008: 924) and ending with the middle Santonian regression due to tectonic uplift (Walaszczyk 2008: 960), the present territory of Lower Silesia belonged to a seaway between the Tethys and the Northern Ocean with scattered islands, the so-called European Archipelago (Csiki-Sava et al. 2015 and references therein).
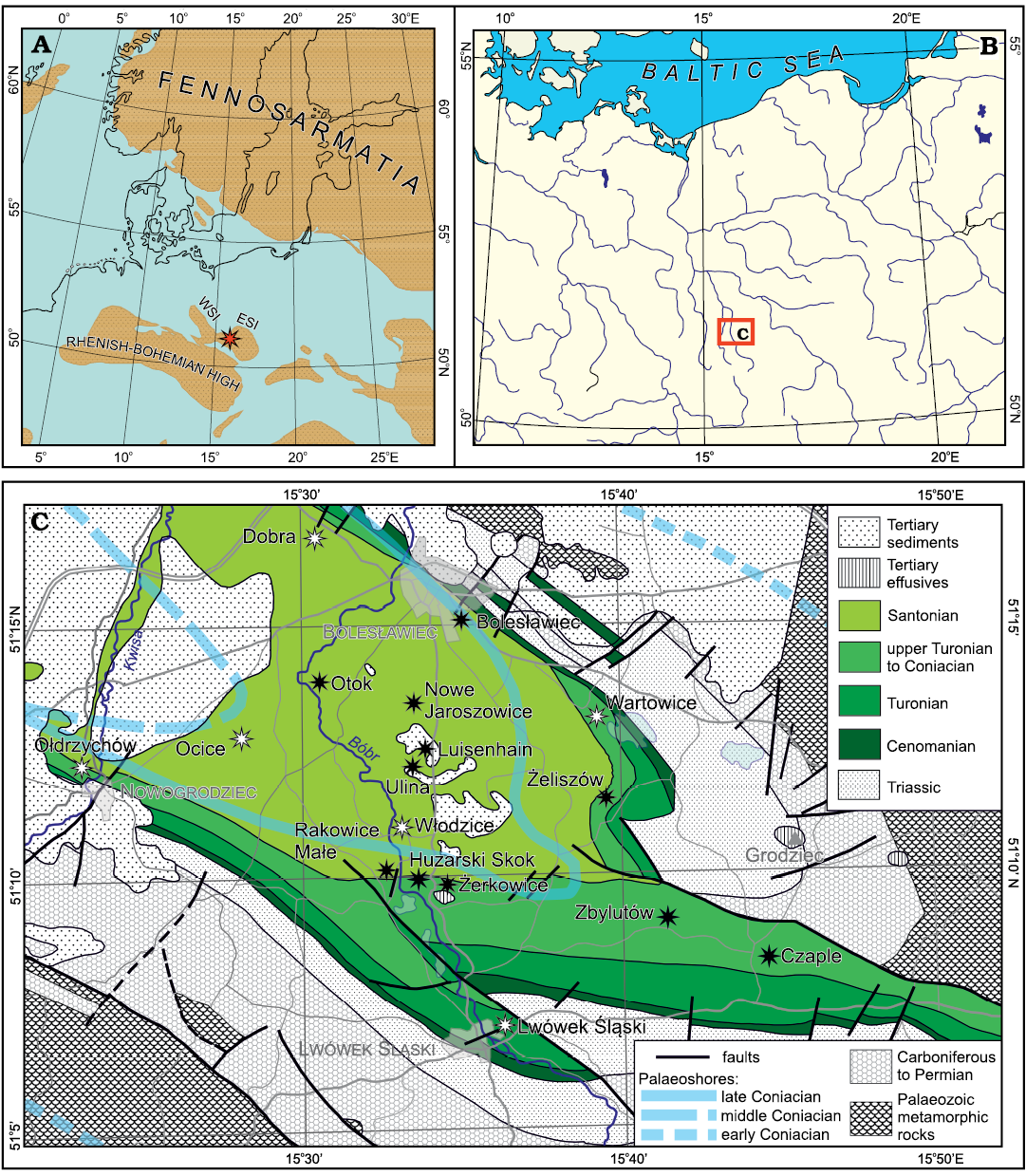
Fig. 1. Geographic, palaeogeographic, and geologic context of the studied flora. A. Late Cretaceous palaeogeography of Central and Northern Europe (after Ron Blakey from Csiki-Sava et al. 2015, modified after data in Chatziemmanouil 1982; Surlyk in Voigt et al. 2008; Halamski 2013; Halamski et al. 2016). The red asterisk shows the presumed position of the studied flora. ESI, East Sudetic Island; WSI, West Sudetic Island. B. Geographical location of the study area in Central Europe. C. Bedrock geological map of northern Silesia (pre-Cenozoic redrawn, simplified and partly corrected after Kozdrój et al. 2001; Cenozoic simplified after Sawicki 1966, 1995) with approximate locations of early, early middle, and late Coniacian palaeoshores (after Leszczyński 2018: fig. 40A, B, D). The studied localities (see Appendix 1) are shown by asterisks, black for those whose location is known in detail, white for those for which a precise location cannot be given.
Scupin (1911, map re-published in Scupin 1913, 1937) may have been the first to reconstruct two major islands, the “Riesen-Gebirgs-Insel” (now usually referred to as the West Sudetic Island) and the “Ost-Sudetische Landmasse” (East Sudetic Island), with the “Löwenberger Becken” (North Sudetic Basin) situated between them. It is generally agreed that the size and the extent of the two islands changed during their existence, but the precise position of coastlines remains a subject of controversy (Kędzierski 2005 and references therein; Biernacka and Józefiak 2009; Biernacka 2012). The landscape of the more southerly situated parts of the European Archipelago (namely the present-day Nysa Kłodzka Graben) is reconstructed as having been smoothly hilly (Migoń and Lidmar-Bergström 2001; Uličný 2001; August and Wojewoda 2004; Sobczyk et al. 2019). By way of contrast, the landscape of the area considered herein may have been flat to weakly undulating (Raniecka-Bobrowska 1968: 1 and references therein).
According to Leszczyński (2018: 810–811), the two above-mentioned islands existed as separate landmasses up to the early Coniacian. The regression that took place in the middle Coniacian resulted in a retreat of the sea to the west and in the merging of the two islands with emergence of the study area and sedimentary hiatus (Milewicz 1956b). A consequence of the late Coniacian transgression was the formation of an embayment aligned in an approximately NW-SE direction, ca. 35 km long and 15 km wide (Leszczyński 2018: fig. 40D; middle and late Coniacian coastlines are shown in Fig. 1C). This palaeogeographical disposition is supposed to have persisted until the final retreat of the sea from the study area in the middle Santonian.
A short survey of the Cretaceous strata of the North Sudetic Basin follows in stratigraphic order. Details on single outcrops are given in the Appendices 1 and 2.
Cenomanian.—A major marine transgression took place in the middle (perhaps even early) Cenomanian (Skelton 2003; Torsvik and Cocks 2017: 235; Voigt et al. 2008). These transgressive deposits lie mostly on Triassic rocks, less commonly on Permian or Paleozoic rocks (Milewicz 1997: 9); their thickness is quite varied, but usually about 100 m (Walaszczyk 2008). Cenomanian palaeofloras are rich and diversified in Bohemia (e.g., Heer 1869; Velenovský 1889; Eklund and Kvaček 1998; Kvaček and Friis 2010; Kvaček et al. 2012; Greguš and Kvaček 2015) and known also in southernmost Lower Silesia (Niebuhr 2019), but no plant fossils have ever been found in Cenomanian strata of the study area.
Turonian.—The Turonian deposits are ca. 150 m thick (Walaszczyk 2008). A single specimen of Geinitzia reichenbachii is described as coming from Turonian marl (lower part of the Rakowice Wielkie Formation) cropping out in an unidentified quarry near Lwówek. This is separated herein as Assemblage 1.
Lower–middle Coniacian.—Lower to middle Coniacian sandstone belongs to the upper part of the Rakowice Wielkie Formation.
According to Williger (1882: 84), the lowest part of Coniacian sandstone can be separated as the Wartowice beds (Neuwarthauer Schichten), including outcrops of which having yielded fossil plants at Wartowice and Zbylutów. Independently of the question of the detailed stratigraphic position of the discussed beds within the Coniacian sandstone, the plant assemblage may be distinguished on account of preservation of leaves coated by a brown ferrugineous substance and by its floristic composition (Ettingshausenia sp. 1, Pinus). This is termed herein Assemblage 2.
The Żerkowice Member (obere Quadersandstein sensu Williger 1882; uppermost part of the Rakowice Wielkie Formation) consists of sandstone beds and is ca. 100 m thick (Leszczyński 2018: 789). Plants are preserved as poor leaf imprints in sandstone, unidentifiable wood fragments, and tree fern stems (their stratigraphic position within this level is presumed) coming from Czaple and Żerkowice are termed herein Assemblage 3.
Coniacian–Santonian.—Unlike the previously described Cenomanian to Coniacian succession, which is entirely of marine origin (Walaszczyk 2008), the rocks forming the Nowogrodziec Member of the Czerna Formation were deposited in a mixed (coastal, lacustrine, paludal and lagoonal) environment (Leszczyński 2010). The period of non-deposition corresponds to the hiatus between the Żerkowice Member of the Rakowice Wielkie Formation and the Nowogrodziec Member of the Czerna Formation and was sufficiently long to allow the erosion of the lower to middle Coniacian sandstone (Berezowska and Berezowski 1982: 43 and references therein).
This part of the succession, which has yielded most of the plant fossils described in the present paper, is best exposed in the quarry at Rakowice Małe (see Appendix 1) and could also be observed at Żeliszów. Former outcrops include Żerkowice, Huzarski Skok, and Ołdrzychów (see also Leszczyński 2018). At Rakowice Małe it is up to 10 m thick (usually less); the basal part (ca. 70 cm) is made up of light multi-coloured clayey mudstone (Leszczyński 2018: fig. 16B; Fig. 2B) with subordinate flat pebbles of iron claystone, on surfaces of which plant imprints occur (Assemblage 4 herein), whereas the remaining part consists of dark claystone with irregular coal intercalations (Assemblage 5 herein; Leszczyński 2018: fig. 16C–G; Fig. 2F). The presence of amber in the coal (Fig. 2C) was noted by Goeppert (1836a) and Halamski et al. (2018b).
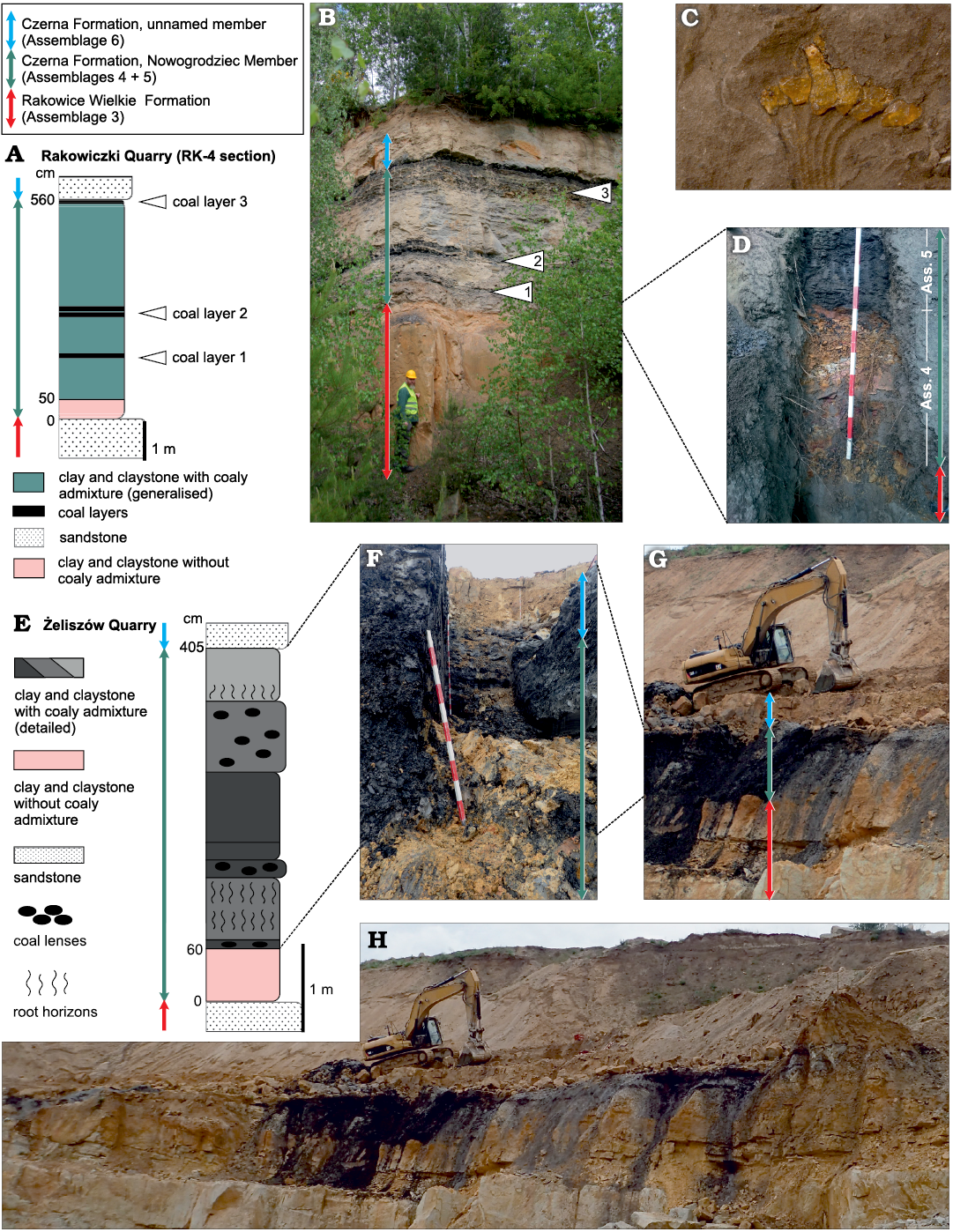
Fig. 2. Stratigraphic context of Assemblages 3–6. A–D. Rakowice Małe, Rakowiczki quarry. Generalised lithostratigraphy (A) of the section RK-4 situated in the central part of the northern wall at 51°9.943’N, 15°32.591’E; clay and claystone undifferentiated (white arrowheads indicate positions of the three coal layers serving as correlation levels). General view of the section RK-2 (B) located in the central-western part of the northern wall of the quarry at 51°9.958’N, 15°32.528’E (ATH, bottom left, is 187 cm tall). Enlargement of the basal part of the Nowogrodziec Member in the section RK-1 (D) located in the western part of the northern wall of the quarry at 51°9.963’N, 15°32.470’E (each coloured section of the stick is 10 cm long). C. Protodammara sp., partial view of a cone scale with amber fragments (see the entire specimen in Fig. 5F). E–H. Żeliszów quarry. General (H) and partial views (G) of the southern wall of the quarry during the excavations; trench located at 51°11.049’N, 15°38.892’E with middle and upper parts of the Nowogrodziec Member exposed (F) (stick as in D); detailed lithostratigraphic scheme of the Nowogrodziec Member (E) (colours stand for natural colour variants of clay and claystone). Photograph B taken in late spring 2020, D, in summer 2017, F–H, in spring 2019.
Santonian.—The part of the Czerna Formation overlying the Nowogrodziec Member is composed mostly of sandstone with subordinate intercalations of white clay used for the ceramic industry at the famous factory in Bolesławiec. It was interpreted as a deltaic deposit (Milewicz 1997, 2006). In more detail, the sedimentary environment may have consisted of a mosaic of deltaic plains, alluvial plains, wetlands, and shallow lakes (Górniak 1986: 128).
The sandstone is fine- to medium-grained, seldom coarse-grained with beds about 0.5–3 m thick (Berezowska and Berezowski 1982: 45). A brackish fauna consisting of bivalves and gastropods may be locally abundant (Scupin 1913; Milewicz 1964: 30). Fossil plants from the sandstone beds are preserved as mostly colourless imprints of isolated leaves without cuticles or fine venation details (Assemblage 6 herein). The most abundant fossils come from Ulina; other localities include Ocice, Otok, Rakowice Małe, and Żerkowice.
Plants from a single collection at Nowe Jaroszowice differ from Assemblage 6 both by systematic composition (Platanites sp. 1, otherwise unknown) and preservation (ferrugineous crusts) and so are separated herein as the Assemblage 7.
Megafloral remains from white “ceramic clays” are termed herein the Assemblage 8. Claystone and mudstone beds form intercalations, lenses and beds up to 6 m thick within sandstone (Berezowska and Berezowski 1982: 46). Plant remains (angiosperm leaves and Geinitzia twigs) form mass accumulations, in which majoritarily fragmented leaves recover one another (see Roemer 1889). Compound leaves of Dewalquea insignis and Dewalquea aff. gelindenensis are preserved with leaflets in organic connection, although no cuticles or fine venation details can be observed. Plants from the outcrop at Bolesławiec were described by Roemer (1889). Another locality was noted on labels as Luisenhain (name of a pre-1945 restaurant, no Polish equivalent; see Appendix 1 for a more detailed description and for the explanation of confusion of specimens coming from these two localities).
Stratigraphic distribution of plant fossils.—The stratigraphic distribution of the plant assemblages studied in the present paper is as follows: (i) Turonian: Rakowice Wielkie Formation—Assemblage 1; (ii) lower–middle Coniacian: Rakowice Wielkie Formation—Assemblages 2 and 3; (iii) upper Coniacian? or lower Santonian?: Czerna Formation, Nowogrodziec Member—Assemblages 4 and 5; (iv) lower to middle Santonian: Czerna Formation, unnamed member—Assemblages 6–8.
It is worth to underline that the units (i) to (iv) are considered to form a stratigraphic succession, whereas the ordering of assemblages within units, except for the directly observed succession of 4 and 5, is either tentative (2, 3) or represents facies variants (6–8). The exact position of the Coniacian–Santonian boundary is unknown, it might run as low as between the Rakowice Wielkie and Czerna formations or as high as in the basal part of the unnamed member of the Czerna Formation.
Finally, it might be of interest to note that due to lithological similarity Tertiary (Oligocene) plant-bearing sandstone beds from northern Silesia were several times mistaken for Cretaceous. This error was corrected already by Williger (1882: 124; see also Milewicz 1976: 24; Raniecka-Bobrowska 1977: 173), but Lilpop and Kostyniuk (1957: 144) mistakenly listed the Oligocene palm Flabellaria chamaeropifolia Goeppert, 1836b from Parowa (Tiefenfurth) near Bolesławiec as Cretaceous. This is also the origin of the erroneous information on the presence of palm macroremains in the Cretaceous of Silesia in Cieśliński (1989: 326).
Detailed stratigraphy of the Nowogrodziec Member.—The main purpose of excavations led by the present authors at Rakowice Małe (2017–2018) and at Żeliszów (2018–2019) was to investigate mesofossils and palynomorphs from Assemblage 5/Palynoassemblage A, i.e., the part of the Nowogrodziec Member with coaly intercalations.
The Nowogrodziec Member of the Czerna Formation consists of clay and claystone, either with (upper part) or without (basal part; Fig. 2D) coaly intercalations, and is thus sharply separated from underlying and overlying sandstone (Fig. 2). At Rakowice Małe it is up to 10 m thick (less than 6 m in the section RK-4; Fig. 2A, B), whereas at Żeliszów it is about 4 m thick (Fig. 2E, F). At Rakowice Małe the easily observable top of the sandstone of the Rakowice Wielkie Formation was taken as a reference level (“0”). In both quarries strong lateral variations of thickness and lithology of the Nowogrodziec Member were evident. For example, no distinct root horizon could be found in the measured section RK-4, whereas about 10 m eastwards such a horizon was easily observable. At Żeliszów, some 30 m westwards from the trench (Fig. 2H), the Nowogrodziec Member is altogether absent and the sandstone of the Rakowice Wielkie Formation is in direct contact with the sandstone of the Czerna Formation.
The stratigraphy of the Nowogrodziec Member at Rakowice Małe was discussed by Leszczyński (2010). At Żeliszów, we were able to examine a single trench (Fig. 2F) and its stratigraphic interpretation is provided in Fig. 2E. It may be noted that, as far as it could be observed, coaly intercalations at Rakowice Małe are decametre-long layers (Fig. 2B), whereas at Żeliszów they instead consist of decimetre-scale lenses.
The age of the Nowogrodziec Member was given as early Santonian on the basis of inoceramid bivalves from the Węgliniec IG-1 borehole (Mitura et al. 1969). This dating was accepted by Milewicz (1997). Walaszczyk (2008) briefly commented on these data as requiring reinterpretation, resulting in the age of the strata being viewed as late Coniacian. Such an assignment was repeated by Leszczyński (2010).
The micropalaeobotanical data assembled during the present investigations seem to indicate a Santonian age, yet it would be imprudent to consider this dating as decisive. Dinocysts are rare in the investigated samples. Concerning the pollen grains, the genus Neotriangulipollis, present in the material studied, is usually reported to occur from the middle Santonian to the lower Campanian (Góczán et al. 1967). It is uncertain to what extent the vertical distribution this seldom reported genus may be considered as fully known (see discussion in the Systematic palaeontology). Interporopollenites turgidus Tschudy, 1975, was found in the section RK-4 (depth 110–130 cm). Given its oldest reported occurrences are from the Santonian of Vendée (France; Azéma et al. 1981), and from the Santonian–Campanian deposits of Portugal (Kedves and Hegedus 1975), it is also suggestive of the Santonian rather than Coniacian.
Material and methods
Megaflora.—Lower Silesia is by no means an exception to the general situation in much of Europe, where the significant deterioration of quality of outcrops has occurred over the last decades. Small quarries are most often no more used and disappear, which is not compensated by the growth of a smaller number of large quarries. This is why the description of the megaflora is based mostly on historical specimens. The richest collections are those in MB which originate partly from the secondary school at Lwówek Śląski (see above), and partly from the Prussia Geological Survey (transferred from the Survey to MB by Walther Gothan). Another important collection is that in MGUWr, which dates back in part to Heinrich Robert Goeppert and partly to subsequent workers. Unfortunately, the specimens from Bolesławiec studied by Roemer (1889) could be traced only in part; the remaining ones are most likely lost.
Most of the material consists of leaves. Those belonging to Assemblages 2–4 and 6–8 are imprints, whereas those from Assemblages 1 and 5 contain some original carbonaceous matter, but no interpretable results were obtained from micromorphological studies so far. The entire palaeoflora is thus studied according to the methods developed for the study of poorly preserved (i.e., without either cuticles or fine venation details) Cretaceous assemblages, the most important element of which are morphographic systematics and widely, but precisely defined, fossil-genera (Halamski and Kvaček 2015: 102–103; Halamski et al. 2018a: 128). In accordance with editorial practice, plant taxon names are given with fully spelt authors’ names and year, following the zoological convention.
Subordinately, the presence of conifer cones and isolated cone scales, as well as of a single macroscopically recognisable platanoid infructescence, was noted in the Assemblage 5. Together with tree fern stems described in a previous paper (Greguš et al. 2013), plant fossil-taxa totalled 29 species.
Land plants from Assemblages 2 and 3 co-occur with an abundant marine fauna, similar to the situation in the Coniacian of the Idzików beds (Halamski and Kvaček 2015: pl. 1: 1, 2) or in the Campanian of eastern Poland and western Ukraine (Halamski 2013: fig. 2A).
Microflora.—For palynological study, mainly fine-grained, grey mudstone samples were selected at 20 or 40 cm intervals (more densely if changes of lithology occurred) and treated using a standard palynological extraction technique involving HCl-HF-HCl and acetolysis or heavy liquid ZnCl2 used for gravity separation. Some samples were sieved through 10 µm sieves. The residues were mounted in glycerine jelly; 5–10 slides were studied of each sample. Most samples contained enough spores and pollen admixed with marine microfossils for quantitative analysis. Counts are based on 5–10 slides, with totals of 170–220 grains per count. The slides were examined with a Leica DM2500 light microscope and for the microflora photos, the programme IM50 was used. Residues and slides are stored in the paleobotanical collection of the Institute of Geology, Department of Paleobiology and Paleoecology, Prague, Czech Republic.
Numerous palynomorphs were recovered by the present authors from the coaly (upper) part of the Nowogrodziec Member, i.e., from claystone corresponding to Assemblage 5 in terms of the megaflora. More precisely, 26 samples from Rakowice Małe (mainly from the section RK-4, see Fig. 2) and 14 from Żeliszów (see Fig. 2) were analysed for palynological content. This is termed herein Palynoassemblage A. Moreover, we studied historical samples from, and nearby, Rakowice Małe, including the former coal mines (MB.Pb.s.n., Wenig-Rackwitz, Andreashof, Zimmer 6/50: sample 1334; MB.Pb.s.n., Wenig-Rackwitz, Koniferen-Zweige: sample 1338; both prepared by Barbara Mohr) from Ołdrzychów (MGUWr 5638p), and from an uncertain location (MB.Pb.2008/0362, “Lwówek”, see that entry in the Appendix 1). They are tentatively referred to the Palynoassemblage A.
The MB.Pb.2008/0372 of Konijnenburgia cf. galleyi from Assemblage 4 at Żerkowice yielded poorly preserved spores (see Systematic palaeontology).
Concerning the strata overlying the Nowogrodziec Member, Raniecka-Bobrowska (1968, 1984, 1989, see also Grabowska 2003) was able to extract numerous palynomorphs from boreholes drilled through the upper part of the Santonian succession south of Bolesławiec. Given the importance of these unpublished data, they are summarised here and in some cases quoted in extenso. Raniecka-Bobrowska (1968) provides information on depths but not on lithologies, so it is impossible to translate these indications into the scheme of eight assemblages presented here and we refer to them collectively under Palynoassemblage B corresponding thus to Assemblages 6–8 in terms of megaflora.
Three samples from Assemblage 8 (MGUWr 2880p from Bolesławiec, MB.Pb.s.n. from Luisenhain, and MB.Pb. 2008/0331 from an unknown locality) were analysed for palynological content during the present study, but invariably only plant detritus without any identifiable palynomorphs was recovered.
As far as possible, in the systematic part palynomorphs are arranged according to a natural system, after Cavalier-Smith (1981), Wingate (1981), Friis et al. (2011), and Williams et al. (2017).
Systematic palaeontology
Megaflora
Kingdom Plantae Linnaeus, 1753 sensu Cavalier-Smith (1981)
Division Filicophyta Boureau, 1970
Class Leptosporangiatae von Goebel, 1881
Order Matoniales Pichi Sermolli ex Reveal, 1993
Family Matoniaceae Presl, 1848
Genus Konijnenburgia Kvaček and Dašková, 2010
Type: Konijnenburgia latifolia (Nathorst, 1908) Kvaček and Dašková, 2010 ≡ Nathorstia latifolia Nathorst, 1908; Cenomanian, Upper Cretaceous; Atanekerdluk, Greenland.
Konijnenburgia cf. galleyi (Miner, 1935) Kvaček and Dašková, 2010
Figs. 3, 4D–G.
Material.—Rakowice Małe, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2008/0365a, 2018/0062, MGUWr 6154p; Żerkowice, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2008/0372, 0373, 0374.
Description.—The available material consists of accumulations of fragments, the largest of which (Fig. 4F) shows the rachis 6 cm long and two pinnules in organic connection to the rachis.
Pinnules linear, 7–14.5 mm wide, integrimarginate, with neuropteridic base; median nerve 0.6–0.9 mm wide, subtransversely striated. Sori circular or elliptic, 1.2–1.7 mm wide, 1.5–2.0 mm long, the distance between adjacent ones 2–3 mm; sorus consisting of a median depression and 14–17 radially arranged sporangia.
Sporangia wedge-shaped (Fig. 3A1), 30–40 µm wide and 100 µm long, in some cases showing fragments of an annulus with thickly cutinised cells (Fig. 3A3) in marginal part. Fractured sporangia show a sporangial wall that is 20–40 µm thick (Fig. 3A2), it cannot be ruled out that the sporangium wall is covered by additional tissues, the unusually great apparent wall thickness resulting from the presence of a greater number of laminar structures, either a poorly preserved indusium or a leaf lamina. Spores smooth.
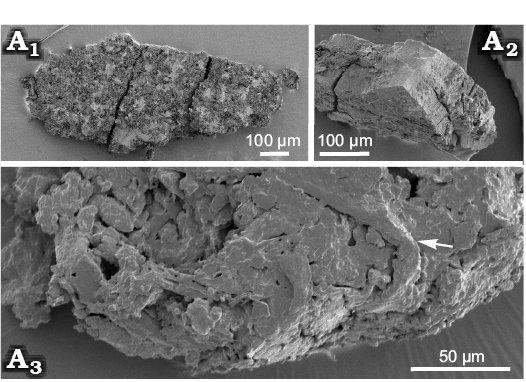
Fig. 3. SEM micrographs of sporangia and spores of Konijnenburgia cf. galleyi (Miner, 1935) Kvaček and Dašková, 2010; MB.Pb.2008/372, Żerkowice, North Sudetic Basin, Lower Silesia, Poland, Assemblage 4 (upper Coniacian?–lower Santonian?). A. Complete sporangium (A1), transverse section of a sporangium showing sporangial walls surrounding spores (A2), sporangium fragment showing thickly cutinised cells of the annulus (arrow) (A3).
Remarks.—The studied material is included into Konijnenburgia on account of the general organisation of the frond and the form of the sporangia. In quantitative characters (pinnule width and number of sporangia per sorus) it agrees best with K. galleyi (Miner, 1935) Kvaček and Dašková, 2010, known from a single fragmentary, but well-preserved specimen coming from the Cenomanian Dakota Sandstone of Kansas (Miner 1935: 288–289; pl. 1: 1–4). It differs from K. bohemica from the Bohemian Cenomanian in possessing a higher number of sporangia per sorus (12–14 in K. bohemica), in the apparent absence of any indusium, and in having pinnules distinctly separated and attached to the rachis through petiolules. Thickly cutinised cells of the annulus (Fig. 3A3) are similar to those of Konijnenburgia bohemica (Kvaček and Dašková 2010). The seemingly lacking indusium is considered here as a weak distinguishing character due to its tendency to fall off during the ripening of spores in modern species of Matoniaceae (van Konijnenburg-van Cittert 1993). Separated pinnules with petiolules occur in the type species K. latifolia from the Cenomanian of Greenland (Kvaček and Dašková 2010); however, this character can be variable within a single frond. Due to poor preservation, especially of spores, an open nomenclature is used.
The discussed fern is sometimes labelled as “Gleichenia dresleriana Goeppert” in the collections; the name appears also in floristic lists in Williger (1882) and Milewicz (1997). However, the name Gleichenia dresleriana Goeppert is a nomen nudum because no diagnosis has ever been published.
Order Cyatheales Frank, 1877
Family unknown
Genus Protopteris Sternberg, 1838
Type: Protopteris punctata (Sternberg, 1820) Presl in Sternberg, 1838 ≡ Lepidodendron punctatum Sternberg, 1820; Peruc-Korycany Formation, Cenomanian, Upper Cretaceous; Kounice, Bohemia.
Protopteris punctata (Sternberg, 1820) Presl in Sternberg, 1838
1820 Lepidodendron punctatum; Sternberg 1820: 20, 23, pl. 4: 1.
2013 Protopteris punctata (Sternberg) C. Presl in Sternberg; Greguš et al. 2013: 72–73, pl. 1: 1–8; pl. 2: 1 [ubi syn.].
Material.—Unknown locality in the Lwówek Śląski area, Assemblage 3, Coniacian: MGUWr 7398p.
Description.—Sandstone cast of a stem showing helically arranged leaf scars with typical traces of vascular bundles and adventitious roots among them. For a detailed description see Greguš et al. (2013).
Remarks.—The exact provenance of the stem fragment MGUWr 7398p, which is the only specimen of P. punctata from Silesia, is uncertain. It is labelled “Löwenberg” (now Lwówek Śląski), but possibly this should be understood rather as an indication of a region, and not of a precise locality. It is interesting to note, however, that it is quite unlikely that the age of MGUWr 2885p be Cenomanian, but rather either Turonian or Coniacian (Greguš et al. 2013); it is thus younger than all Bohemian material of this species.
Stratigraphic and geographic range.—Cenomanian, Bohemia; Coniacian, Silesia (Greguš et al. 2013: 72).
Protopteris singeri (Goeppert, 1836b) Presl in Sternberg, 1838
1836 Caulopteris singeri; Goeppert 1836b: 449, pl. 41: 1, 2.
2013 Protopteris singeri (Göppert) C. Presl in Sternberg; Greguš et al. 2013: 73–74; pl. 2: 2–5 [ubi syn.].
Material.—Żeliszów, Assemblage 3, Coniacian: MGUWr 2885p.b (holotype), MGUWr 2885p.a.
Description.—Sandstone casts of stems covered by helically arranged leaf scars. For a detailed description see Greguš et al. (2013).
Remarks.—This species is known solely from two specimens. According to the results of a more detailed field investigation, their age, given as Turonian–Coniacian by Greguš et al. (2013), is more probably Coniacian.
Stratigraphic and geographic range.—Type locality only.
Order and family unknown
Genus Cladophlebis Brongniart, 1849
Type: Cladophlebis albertsii (Dunker, 1846) Brongniart, 1849 ≡ Neuropteris albertsii Dunker, 1846; Lower Cretaceous, northern Germany.
Remarks.—It should be noted that C. albertsii, the type of the genus, has petiolulate (“neuropteridic”) pinnules. The original specific diagnosis is somewhat confused on that matter, referring to “pinnulis… sessilibus” (Dunker 1846: 8). It is clear, however, that this should not be understood as meaning sessile pinnules, as the description of the shape of the base follows: “basi rotundatis vel subcordatis”. The figure showing single points of attachment of pinnules to the rachis (Dunker 1846, pl. 7: 6a) is also clear, despite the very short petiolule.
It follows that the genus name Cladophlebis should not be applied to fernlike foliage with broad-based pinnules, as was done, among others, by Harris (1961) and Miller and Hickey (2008). A complete revision of Cladophlebis is, however, beyond the scope of the paper.
Cladophlebis sp. 1
Fig. 4B.
Material.—Rakowice Małe, Assemblage 5, late Coniacian–early Santonian?: MB.Pb.2018/0067.
Description.—The available material consists of two specimens, one (Fig. 4B) relatively small-sized but sufficient to partly decipher the architecture of the frond, the other showing solely two pinnules.
Frond bipinnate (preserved length ca. 20 mm, preserved width ca. 17 mm), rachis of the (n – 1)-th order ca. 1.5 mm thick, pinnulae inserted on rachises of both n-th and (n – 1)-th order; the two inserted on the rachis of the (n – 1)-th order lanceolate, the single one entirely preserved among those on the rachis of n-th order with rounded apex. Angle between the midvein of the pinnule and the rachis 50–90° (the difference between two pinnules on either side of the rachis). Pinnules shortly petiolulate, with a broad base, straight midvein and forking secondaries, 2.5–3.5 times as long as wide.
Remarks.—The fragmentary character of the studied material precludes any detailed comparison. Cladopteris albertsii is generally similar to the material from Rakowice Małe, with the exception of apparently possessing more curved midveins. Cladophlebis frigida (Heer, 1882) Seward, 1926, from the Lower Cretaceous of Greenland has longer pinnules (Heer 1922: pl. 10: 1–4). Cladophlebis sp. from the lower Maastrichtian? of the southern border of the Holy Cross Mountains (Central Poland) has elliptic pinnules (Halamski 2013). In Cladopteris gosauensis Kvaček and Herman in Herman and Kvaček, 2010, from the Campanian of Grünbach pinnules are sessile and their length-to-width ratio does not exceed 2 (Herman and Kvaček 2010).
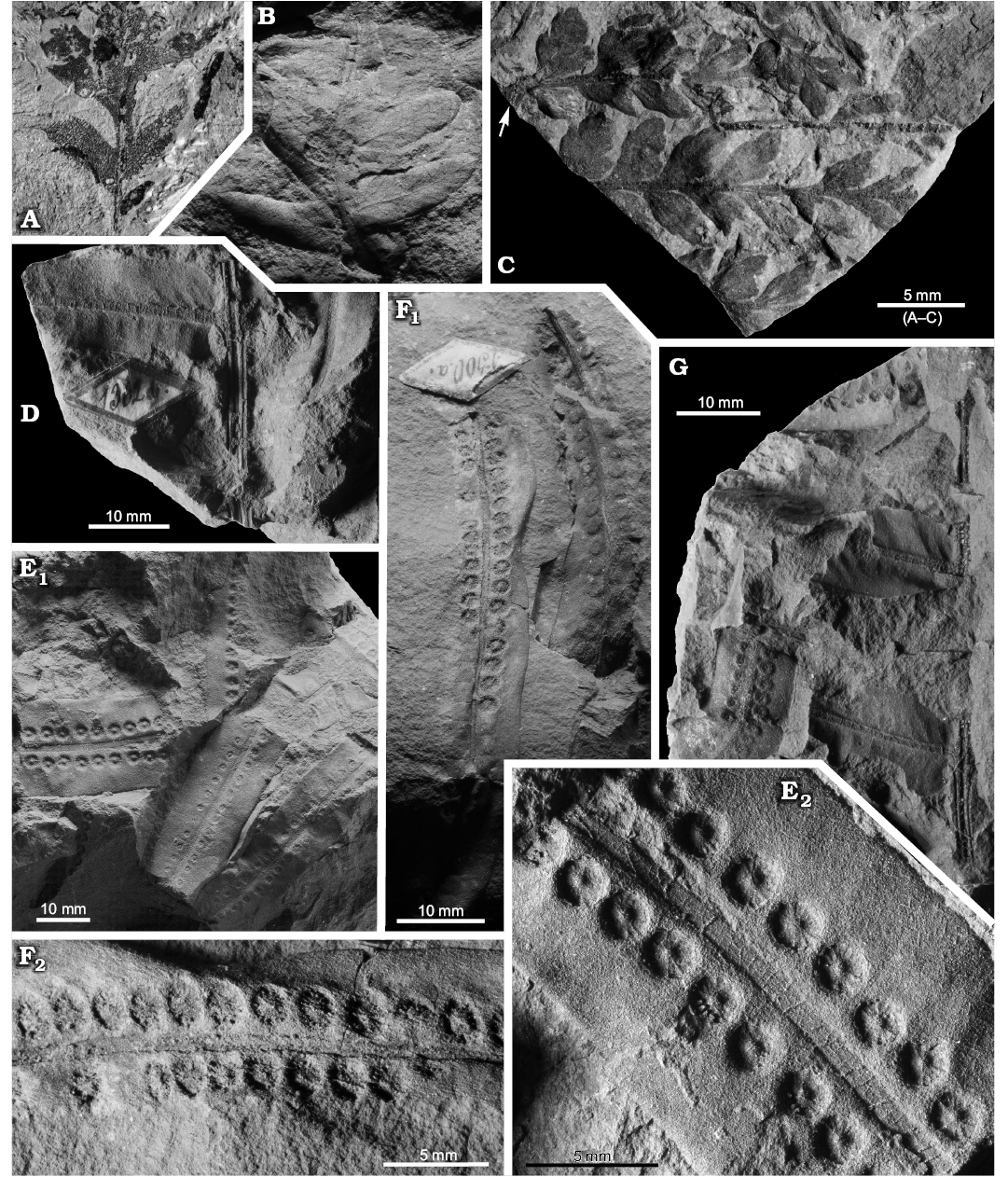
Fig. 4. Late Coniacian?–early Santonian? ferns from the North Sudetic Basin, Lower Silesia, Poland. A. Cladophlebis? sp. 2, fragment of a pinna MGUWr 7556p, Żeliszów, Assemblage 5. B. Cladophlebis sp. 1, fragment of a pinna MB.Pb.2018/0067, Rakowice Małe, Assemblage 5. C. Coniopteris? sp., fragment of a pinna MB.Pb.2018/0006 (rachis arrowed), Rakowice Małe, Assemblage 5. D–G. Konijnenburgia cf. galleyi (Miner, 1935) Kvaček and Dašková, 2010, Assemblage 4. D. Fragment of a pinna MB.Pb.2018/0062, Rakowice Małe. E. Accumulation of isolated pinnules MB.Pb.2008/373.2a (E1) and enlargement of a pinnule with sori (E2), Żerkowice. F. Two isolated pinnules MB.Pb.2008/373.1 (F1) and enlargement of the longest pinnule in the studied material with sori (F2), Żerkowice. G. Fragment of a pinna MB.Pb.2008/373.2b, Żerkowice.
Cladophlebis? sp. 2
Fig. 4A.
Material.—Żeliszów, Assemblage 5, upper Coniacian?–lower Santonian?: MGUWr 7556p.
Description.—The available pinna fragment consists of two nearly oppositely arranged pinnules. Each pinnule is lanceolate in shape, ca. 8 mm long and 2–3 mm wide, with broad slightly decurrent base and acute apex. Venation obscured by carbonaceous matter.
Remarks.—Cladophlebis? sp. 2 differs from Cladophlebis sp. 1 in having acute apices of pinnulae, decurrent bases, and suboppositely arranged pinnulae. The fragmentary character of the studied material precludes any detailed comparison like in the case of Cladophlebis sp. 1. Similarities with fronds of “Sphenopteris” dubia (Velenovský, 1888) Kvaček and Halamski in Halamski and Kvaček, 2016, and Anemia fremontii Knowlton, 1917, can be noted, but in Cladophlebis? sp. 2 pinnulae arrangement on the rachis is subopposite.
Genus Coniopteris Brongniart, 1849
Type: Coniopteris murrayana (Brongniart, 1835) Brongniart, 1849 ≡ Pecopteris murrayana Brongniart, 1835; Jurassic, Yorkshire, United Kingdom.
Remarks.—Coniopteris was interpreted as a representative of the Dicksoniaceae by Harris (1961). Kvaček and Manum (1993) applied the fossil-genus name Coniopteris to their specimen because of difficulties in distinguishing between species belonging to the Dennstaedtiaceae and the Dicksoniaceae. Li et al. (2020) interpreted Coniopteris as a stem group of Polypodiales.
Coniopteris? sp.
Fig. 4C.
Material.—Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2018/0006.
Description.—The only available specimen consists of three pinnae of last order arranged parallel to each other in a way suggesting they were attached to a common axis; the organic connection with a tiny fragment of rachis of the (n – 1)-th order is preserved, but is so small, that the direction of branching cannot be verified.
Frond at least bipinnate, branching catadromous?, preserved length of pinnae of the n-th order up to 25 mm. Pinnules small, trilobately dissected, 3–5 mm long and 2–3 mm wide; the terminal lobe of each pinnule always larger than the lateral ones. The first (catadromous) pinnule at the base of a pinna of the n-th order subcircular in shape. Venation difficult to observe.
Remarks.—The pinnules of the described fern are intermediate in shape between those of Coniopteris hymenophylloides (Brongniart, 1828) Seward, 1900, and C. murrayana from the Middle Jurassic of Yorkshire (Harris 1961). However, both have first pinnules of a pinna developed into aphlebioid filiform processes (van Konijnenburg-van Cittert and Morgans 1999), which is not the case in the studied material.
Division Gymnospermae (Lindley, 1830) Prantl, 1874
Class Coniferae (Jussieu, 1789) Engler, 1892
Order Pinales Gorožankin, 1904
Family Pinaceae Lindley, 1836
Genus Pinus Linnaeus, 1753
Type: Pinus sylvestris Linnaeus, 1753; Recent, Eurasia.
Pinus longissima Velenovský, 1885
Fig. 5G.
1882 Pinus Quenstedti Heer; Williger 1882: 84.
1885 Pinus longissima m.; Velenovský 1885: 29–30, pl. 1: 14–17.
Material.—Wartowice, Assemblage 2, Coniacian: MB.Pb. 2008/0337.
Description.—Ovuliferous cone 180 mm long and up to 25 mm broad, consisting of numerous helically arranged cone-scales, each scale bearing a distally diamond-shaped apophysis (escutcheon) with umbo. Some cone-scales apparently with two seeds on the adaxial side.
Remarks.—The described specimen bears a manuscript label by Heinrich R. Goeppert saying “Pinus longissima Goepp.”; this name, however, has never been validly published. Velenovský (1885) used the epithet longissimus (“the longest” in Latin) for the description of a Cenomanian ovuliferous cone from Kralupy nad Vltavou in Bohemia (Velenovský 1885: pl. 1: 14–17). The Coniacian cone from Wartowice belongs to the species described from the Bohemian Cenomanian. The oldest representatives of the modern genus Pinus are known from the Cretaceous (Miller 1988; Ryberg et al. 2012, Kvaček 2013b; Falcon-Lang et al. 2016).
Stratigraphic and geographic range.—Cenomanian, Bohemia (Dijkstra 1973: 665 and references therein); Coniacian, Silesia (this paper). A poorly preserved specimen from the Lower Greensand (Aptian) of Maidstone, England was compared to this species by Stopes (1915: 141); this was misindexed as an indication of the presence of this species in England by Seward (1919: 385).
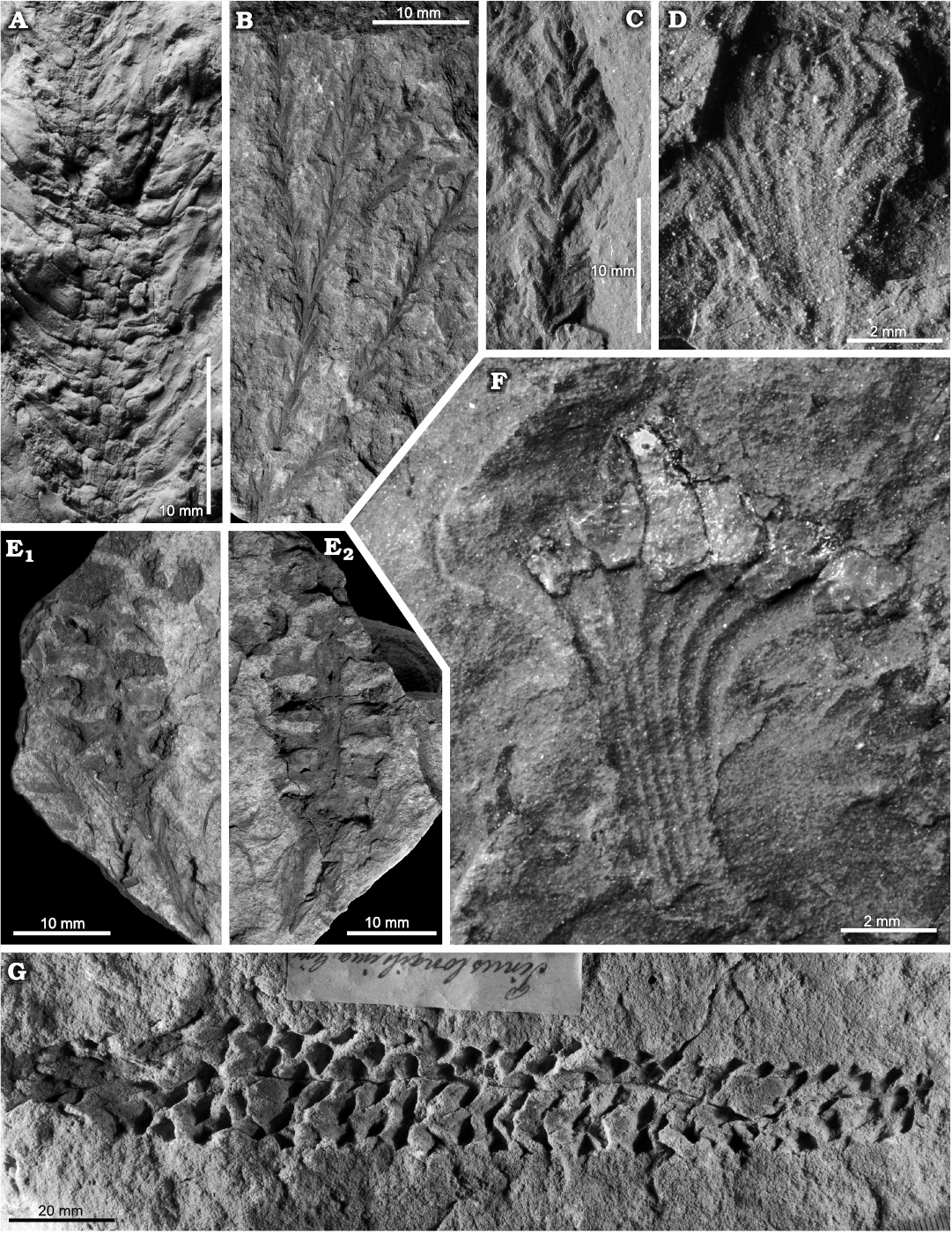
Fig. 5. Late Cretaceous conifers from the North Sudetic Basin, Lower Silesia, Poland. A–C. Twig fragments of Geinitzia reichenbachii (Geinitz, 1842) Hollick and Jeffrey, 1909. A. MB.Pb.2008/0240, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?). B. MB.Pb.2018/0049, Bolesławiec, Assemblage 8 (Santonian). C. MB.Pb.2008/0363, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?). D, F. Cone scales of Protodammara sp. D. MB.Pb.2018/0078, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?). F. MB.Pb.2018/005 with amber pieces, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?); see colour photograph in Fig. 2C. E. Geinitzia cf. formosa Heer, 1871. Ovuliferous cone longitudinally broken (part and counterpart) MB.Pb.2008/250a, b, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?). G. Pinus longissima Velenovský, 1885. Ovuliferous cone MB.Pb.2008/0337, Wartowice, Assemblage 2 (Coniacian).
Family Cupressaceae Gray, 1821
Genus Protodammara (Hollick and Jeffrey, 1906) Mays and Cantrill, 2018
Type: Protodammara speciosa Hollick and Jeffrey, 1906; Raritan Formation, Turonian?, Upper Cretaceous; Kreischerville, Staten Island, New York, USA.
Protodammara sp.
Figs. 2C, 5D, F.
Material.—Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2008/0249, 2018/0078.
Description.—Several ovuliferous cone scales on a bedding plane. Each cone scale 10–12 mm broad and 10–15 mm long, peltate, with resin ducts, 7–9 per cone scale. Head of each scale roundish, adaxially bearing 7–9 scar-like structures.
Remarks.—In the studied material no seed is attached to the cone scale, so it is not clear whether the scar-like structures are genuine seed attachment scars or only ridges of the cone scale surface. Similar isolated cone scales from the Bohemian Cenomanian were reported by Velenovský (1885) as Dammara borealis Heer, 1882 (JK, unpublished data). Morphology of the cone scales, particularly the arrangement of resin ducts, resembles that in the genus Doliostrobus Marion, 1884 (Bůžek et al. 1968; Kvaček 1971), a member of the family Doliostrobaceae Kvaček, 2002. The present material differs from Doliostrobus in lacking an apical spiny process.
Family unknown
Genus Geinitzia (Endlicher, 1847) Harris, 1979
Type: Geinitzia reichenbachii (Geinitz, 1842) Hollick and Jeffrey, 1909 ≡ Araucarites reichenbachii Geinitz, 1842; Cretaceous, Saxony, Germany.
Geinitzia reichenbachii (Geinitz, 1842) Hollick and Jeffrey, 1909
Fig. 5A–C.
1842 Araucarites reichenbachii; Geinitz 1842: 98. pl. 24: 4.
1909 Geinitzia reichenbachii; Hollick and Jeffrey 1909: 38; pl. 5: 7–10, pl. 8: 3, 4, pl. 16: 2–4; pl. 17: 1–4, pl. 18: 1–4.
2009 Geinitzia reichenbachii (Geinitz 1842) Hollick et Jeffrey 1909; Bosma et al. 2009: 489–490, figs. 3D, 4G, H [ubi syn.]
2018 Geinitzia reichenbachii (Geinitz, 1842) Hollick et Jeffrey 1909; Halamski et al. 2018a: 128; pl. 2: 6, 9.
Material.—Lwówek Śląski, Assemblage 1, Turonian: MB.Pb. 2018/0028 (Lettengrube bei Löwenberg, Slg. Dresler 1909, Turonmergel 29. Juli 1898). Huzarski Skok, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2018/0064. Rakowice Małe, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2008/0240, 0252, 0254. Ołdrzychów, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb. 2008/0265–0267, 2018/0024.1–3, 0025.1–2; MGUWr 5638p, 5615p; MMG PnK 36, 37. Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2008/0239, 0241 (coll. Dresler?), 0242 (coll. Schäfer), 0246 (coll. Dresler 1909), 0248, 0253, 0363, 2018/0069.1–10, 2018/0070.3–4, 0071.1–12, 0073.1–31, 0074.1–6, 0075.1–20. Żeliszów, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2008/0251a; Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb. 2008/0316, 317 (coll. Klotzsch). Rakowice Małe, Assemblage 6, lower–middle Santonian: MB.Pb.2008/0244. Włodzice, Assemblage 6, lower–middle Santonian: MGUWr 5594p, 5649p. Bolesławiec, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0049. Luisenhain, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0043, 0044. Dobra, Assemblage 8, lower–middle Santonian: MGUWr 5614p. Uncertain locality, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0042.
Description.—The available material consists of numerous twig fragments ca. 1 mm thick and up to ca. 5 cm in length (Fig. 5C), but usually shorter. Leaves helically arranged, spreading from axes at an angle of 20–40°, basally not contracted, falcate, up to 13 mm long (Fig. 5B), with acute apices.
Remarks.—Geinitzia reichenbachii is one of the most common conifers in the Upper Cretaceous of Europe, possibly representing more than one biological species. In the studied material it is the most abundant taxon in terms of the number of specimens and is present in the Turonian, Coniacian, and Santonian. In adjacent areas it is known from the Turonian of Silesia (Roemer 1886; Płachno et al. 2018), Coniacian of the Kłodzko region and Bohemia (Halamski and Kvaček 2015, 2016; Halamski et al. 2018a), Campanian of Grünbach in Austria (Herman and Kvaček 2010), and Campanian and Maastrichtian of eastern Poland and western Ukraine (Halamski 2013; see also Kunzmann 2010).
Stratigraphic and geographic range.—Late Cretaceous; Europe, North America.
Geinitzia cf. formosa Heer, 1871
Fig. 5E.
Material.—Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2008/0245 (coll. W. Zimmer 1912), MB.Pb.2008/0247 (coll. W. Zimmer 1919), MB.Pb.2008/0250 (coll. W. Zimmer 1911), MB.Pb.2018/0070.1–2.
Description.—The available material consists of longitudinal sections of female cones appearing on broken rock slabs.
Ovuliferous cones incompletely preserved, subcylindrical in shape, in the best preserved specimen (Fig. 5D) width ca. 13 mm, preserved length ca. 25 mm. Cone scales arranged helically, of conical shape. Seeds not observed.
Remarks.—The described ovuliferous cones are similar in architecture and shape to Geinitzia formosa described from the Santonian of Quedlinburg (Kunzmann 1999). The diagnosis of G. formosa includes seed shape, a character that cannot be checked in the studied material, hence open nomenclature is applied. Geinitzia schlotheimii Heer, 1871, from the Santonian of Aachen has ovoid (less elongate) cones (Kunzmann et al. 2003) and peltate to truncate scales (Halamski and Kvaček 2015). Fricia nobilis Velenovský, 1885, from the Turonian of Bohemia has larger ovoid cones (Kvaček 2013a).
Division Angiospermae Braun and Doell ex Doell, 1857
Class Dicotyledoneae de Candolle, 1817
Supersubclass Eudicotyledoneae Doyle and Hotton ex Halamski, 2013
Subclass Ranunculidae Takhtajan ex Reveal, 1992 emend. nov.
Remarks.—The paraphyletic group of eudicots consisting of the Ranunculales, Proteales (incl. Platanales), and Buxales is usually referred to by various informal names, for example “non-core eudicots”. An available name under the ICN is the subclass Ranunculidae Takhtajan ex Reveal, 1992.
Order Proteales Jussieu ex Berchtold and Presl, 1820
Family Platanaceae Lestiboudois, 1826
Genus Platananthus Manchester, 1986
Type: Platananthus synandrus Manchester, 1986, middle–upper Eocene, Oregon, USA.
Platananthus sp.
Fig. 6.
Material.—Żerkowice, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2008/0251a.
Description.—Globular reproductive structure 10×12 mm consisting of radially arranged units interpreted here as staminate flowers ca. 5 mm long, with well-developed tepals, each up to 1.5 mm in width. Stamens are poorly preserved.
Remarks.—In shape and size the described specimen resembles isolated reproductive units from the Bohemian Cenomanian described as Platanathus sp. (Kvaček 2003). Knobloch and Mai (1986, 1991) described male and female reproductive structures of Platanus richteri Knobloch and Mai, 1986, from the Santonian of Quedlinburg, Saxony-Anhalt, Germany, both of which are, however, significantly smaller than the studied material.

Fig. 6. Platanoid inflorescence Platananthus sp. from Żerkowice, North Sudetic Basin, Lower Silesia, Poland Assemblage 5 (upper Coniacian?–lower Santonian?). MB.Pb.2008/251a co-occurring with a Geinitzia twig (bottom left).
Subclass Rosidae Takhtajan, 1967 sensu Soltis et al. (2018)
Order Fagales Engler, 1892 sensu Soltis et al. (2018)
Family unknown
Genus Dryophyllum (Debey in de Saporta, 1865) Jones, Manchester, and Dilcher, 1988
Type: Dryophyllum subcretaceum Debey ex de Saporta, 1865; Eocene, Sézanne, France.
Remarks.—This genus is used here as a fossil genus of the Fagales sensu lato, as argued in detail in Halamski and Kvaček (2015: 109–110).
Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov.
Figs. 7–9, 15C.
1904 Bignonia Westerhausiana n. sp.; Richter 1904: 20, pl. 2: 1–6.
non 1977 Dewalquea westerhausiana; Rüffle and Knappe 1977: 279, pl. 4: 1.
Basionym.—Bignonia westerhausiana Richter, 1904 (Richter 1904: 20; pl. 2: 1–5; see Appendix 4).
Material.—Otok, Assemblage 6, lower–middle Santonian: MB.Pb.2008/0262, 2018/0039. Rakowice Małe, Assemblage 6, lower–middle Santonian: MB.Pb.2008/0256. Ulina, Assemblage 6, lower–middle Santonian: MGUWr 1699p.b, 1715p, 2707p, 2709p, 5593p, 5616p.a, 5617–5619p, 5637p, 5651p (mostly coll. Goeppert), MB.Pb.2008/0346, 0360, 0368, 2018/0029, 0045.
Description.—Leaf compound, trifoliolate (Fig. 8E). Leaflets notophyll to mesophyll (maximal recorded length ca. 10 cm, Fig. 9C; estimated total length over 15 cm), petiolulate, blade attachment marginal; base shape decurrent; apex poorly preserved, straight (Fig. 8A, C). Blade shape oblong, seldom ovate, length-to-width ratio ca. (3.5–)4–5(–6). Margin unlobed, serrate. Teeth regularly spaced, ca. 1–2 per cm, sinus shape rounded, proximal flank flexuous, distal flank concave (Fig. 9A, B).
Primary venation pinnate, midvein strong. Major secondaries craspedodromous, spacing irregular, 1–4 per 2 cm, attachment excurrent, angle to midvein variable, 50–70(–80°). Intersecondaries none. Tertiaries percurrent, straight to sinuous (Fig. 9B). Quaternaries poorly preserved, reticulate?
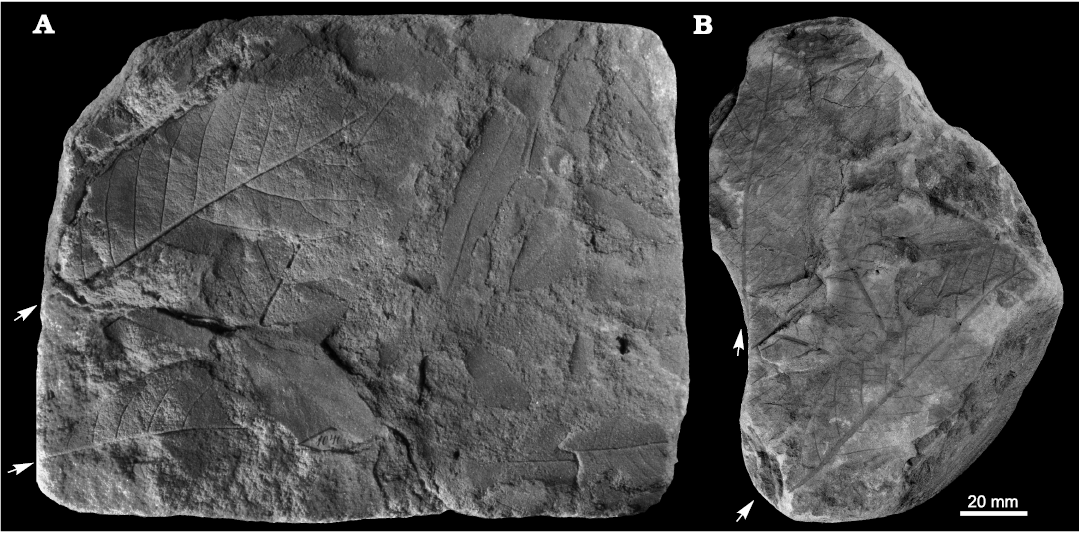
Fig. 7. Taphonomy of the fagalean angiosperm Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. from Ulina, North Sudetic Basin, Lower Silesia, Poland, Assemblage 6 (lower–middle Santonian). A. Three leaflets (arrows indicate the two largest ones possibly from the same leaf) and several incomplete or fragmentary Laurophyllum? sp. MB.Pb.2008/347. B. Four fragmentarily preserved leaflets (arrows indicate the two largest ones possibly belonging to the same leaf) MGUWr 5617p.

Fig. 8. Fagalean angiosperm Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. from the North Sudetic Basin, Lower Silesia, Poland, Assemblage 6 (lower–middle Santonian). A. Enlargement of the incomplete leaflet MB.Pb.2008/256 showing venation and serration of the margin, Rakowice Małe. B. Incomplete leaflet MB.Pb.2008/370, Ocice. C. Probable trifoliolate leaf MB.Pb.2008/339 with three fragmentary leaflets (see enlargement in Fig. 9B), Ulina. D. Probable trifoliolate leaf MGUWr 5618p with two incompletely preserved leaflets, Ulina. E. Incomplete trifoliolate leaf MB.Pb.2008/346, Ulina.
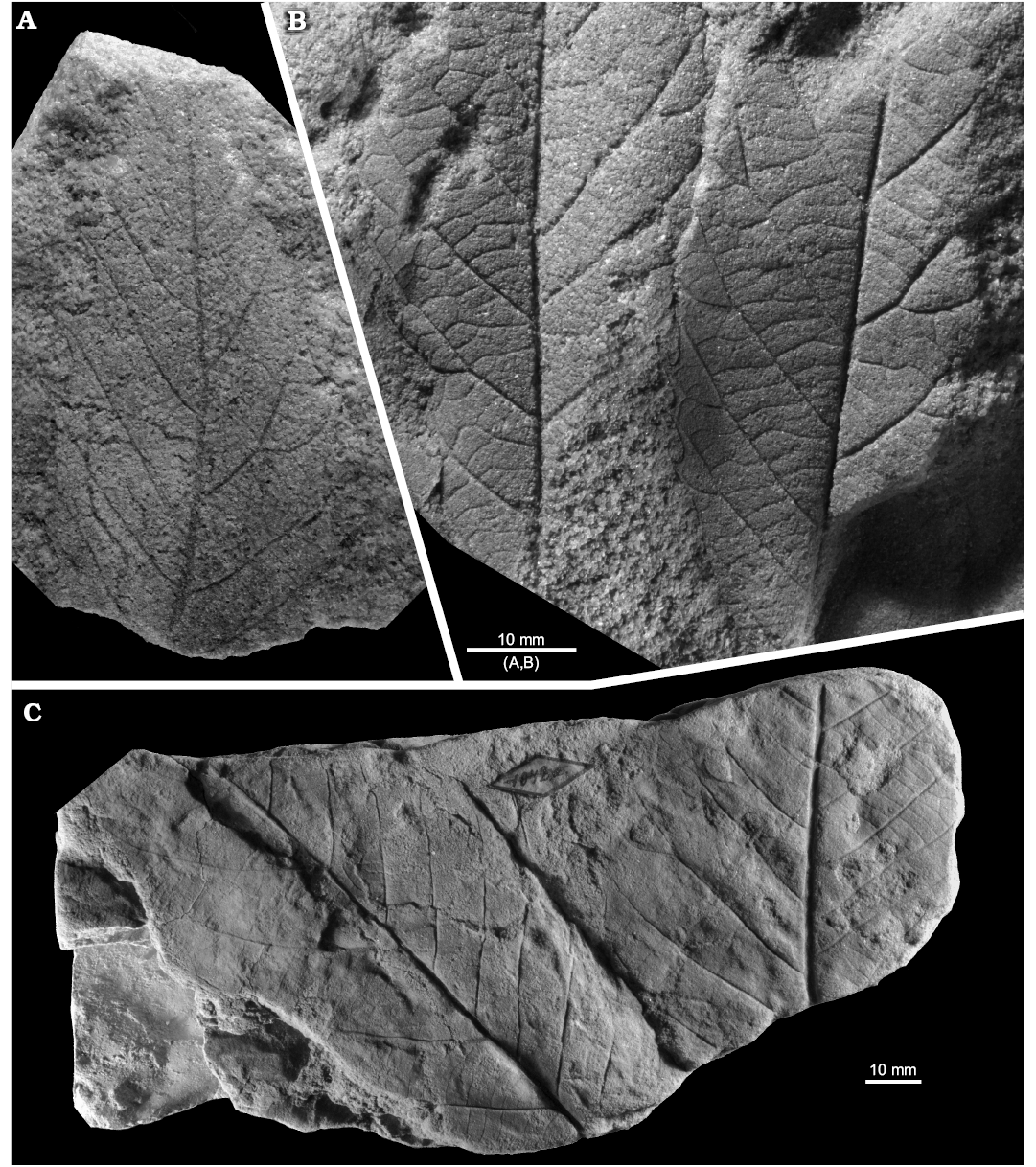
Fig. 9. Fagalean angiosperm Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. from Ulina, North Sudetic Basin, Lower Silesia, Poland, Assemblage 6 (lower–middle Santonian). A. Fragmentary leaflet MGUWr 5619p showing venation and serration. B. Enlargements of venation of fragments of two leaflets MB.Pb.2008/339 (see the entire specimen in Fig. 8C). C. Probable trifoliolate leaf MB.Pb.2008/0342 with two fragmentarily preserved leaflets.
Remarks.—The discussed species is included in Dryophyllum on account of several architectural features, among which marked asymmetry of lateral leaflets (Halamski and Kvaček 2015: pl. 4: 1, 3, 4) and lack of intersecondaries allow the distinction with Debeya Miquel, 1853. The type species of the latter, Debeya serrata Miquel, 1853, from the Maastrichtian of Kunrade (Limburg, Netherlands), possesses numerous intersecondaries (Miquel 1853: pl. 1: 1) and lateral leaflets differing only weakly from the median one.
Rüffle and Knappe (1977) interpreted Bignonia westerhausiana Richter, 1904, as a platanoid. However, such an interpretation is based on a specimen from another locality (not given, but not Westerhausen; Rüffle and Knappe 1977: 279) and of manifestly different architecture (pentafoliolate and not trifoliolate; Rüffle and Knappe 1977: pl. 4: 1), so is likely to be erroneous.
Coniacian Dryophyllum geinitzianum (Goeppert, 1844) Halamski and Kvaček, 2015, is very similar to the discussed species in leaf architecture, venation pattern, and serration of the margin; the main difference is in the leaflets of the former being less elongate (length-to-width ratio 2.5–4; Halamski and Kvaček 2015). The similarity of D. westerhausianum to D. geinitzianum was stressed already by Richter (1904: 20).
Ternstroemites longifolius (Friedrich, 1883) Rüffle and Krutzsch, 2005, from the Maastrichtian of Eisleben has similar serration of the margin, but secondaries are much denser and brochidodromous. It is possible that Aralia veatchii Knowlton, 1917, from the Frontier Formation of Wyoming should be interpreted as a trifoliolate leaf (see especially Knowlton 1917: pl. 39) analogous to Dryophyllum westerhausianum.
“Dryandroides” quercinea Velenovský, 1883, described from the Coniacian of Česká Lípa is distinguished from Dryophyllum by narrower leaves with stronger teeth (Halamski and Kvaček 2016) and the presence of intersecondaries. For a discussion on the ecological segregation of the two species, see below (group Fagofolia, Dryandroides).
Stratigraphic and geographic range.—Saxony, Westershausen (Santonian); Lower Silesia, Assemblage 4 (a single specimen; upper Coniacian?–lower Santonian?) and Assemblage 6 (lower–middle Santonian).
Subclass, order, and family unknown
Remarks.—As shown by Halamski (2013), compound leaves are typical of many eudicots. However, this feature alone does not allow any closer systematic distinction, even between basal and core eudicots, and says nothing about subclass- or order-level placement.
Debeya group sensu Krassilov et al. (2005)
Remarks.—Similarities among Late Cretaceous and Paleogene pedate, palmate, and trifoliolate compound leaves were observed by several authors (see historical account in Krassilov et al. 2005: 124–125), but taxonomic treatments and palaeobiological interpretations diverged widely. Rather than using a widely understood and manifestly artificial grouping, we follow Krassilov et al. (2005) in recognising an informal Debeya group encompassing a few fossil-genera with several species, but excluding species of globally similar morphology, yet distinguished by minute morphological details believed to be of taxonomic significance.
A palaeobiological interpretation of Cretaceous leaves without attached reproductive structures is largely hypothetical, but two suppositions on the systematic placement of the Debeya group that have been argued for in most detail (Platanales: Crane 1989; Ranunculales: Krassilov et al. 2005: 126) are as a matter of fact easily reconcilable, insofar as the two above-mentioned orders are phylogenetically very close to each other. It might thus be supposed that the Debeya group belongs to extinct representatives of basal eudicots (herein subclass Ranunculidae). It would, however, be premature, to formally propose such a systematic treatment. The species to be excluded from the Debeya group as understood herein are those interpreted as members of the Chloranthaceae (Cenomanian of Bohemia; JK, unpublished data; see also Halamski 2013: 419) or of the Fagales (see above).
The oldest undisputed representatives of the Debeya group are from the Turonian of Negev (Israel; Krassilov et al. 2005) and the youngest uncontested ones are from the Paleocene. However, similar plants in need of revision were reported from strata as old as Cenomanian and as young as Oligocene (Crane 1989 and references therein).
Genus Dewalquea de Saporta and Marion, 1873
Type: Dewalquea haldemiana Debey ex de Saporta and Marion, 1873; Campanian, Upper Cretaceous, Haldem, Westphalia.
Remarks.—Dewalquea was treated either as a separate genus (de Saporta and Marion 1873) or subgroup of Debeya (Knobloch 1964; Halamski 2013). Krassilov et al. (2005) stressed the necessity of fine distinctions within the Debeya group, for which reason the genus rank is adopted here.
The tertiaries of D. haldemiana, the type species, are ramified (Halamski and Kvaček 2013). Species with similar leaf architecture but with percurrent tertiaries, like Dewalquea paulinae (Halamski, 2013) Halamski comb. nov. [basionym: Debeya (Dewalquea) paulinae Halamski, 2013; Halamski 2013: 419, fig. 2B; see Appendix 4] from the upper Campanian of eastern Poland should possibly be segregated into another genus. The tertiary venation of Dewalquea insignis Hosius and von der Marck, 1880, is unknown.
The representatives of Dewalquea in the studied material are segregated into three species on account on the characters summarised in Table 1 (with the reservation that the architecture of the leaf of D. haldemiana is described on the basis of the better preserved type material, not of that from Lower Silesia)
Stratigraphic and geographic range.—Turonian, Near East (Krassilov et al. 2005); Coniacian to Paleocene, Europe; Late Cretaceous, North America (Berry 1910, 1916b; see also Crane 1989: 177). The genus (see also van der Burgh 2008: 11–12) lacks a comprehensive revision.
Table 1. Comparison of Dewalquea species present in the studied material.
|
Taxon Character |
Dewalquea haldemiana |
Dewalquea insignis |
Dewalquea aff. gelindenensis |
|
Margin |
entire |
serrate |
entire |
|
Leaflet width-to-length ratio |
>10 |
5–8 |
4–5 |
|
Leaflet width |
≤ 10 mm |
11–18 mm |
≤ 25 mm |
|
Width of leaflets within a leaf |
approximately the same |
median one widest, |
|
Dewalquea haldemiana Debey ex de Saporta and Marion, 1873
Fig. 10A–D.
1873 Dewalquea haldemiana (Araliophyllum haldemianum Deb. Ms.); de Saporta and Marion 1873: 60–61, pl. 7: 1–2.
1889 Dewalquea haldemiana; Roemer 1889: 143, in part: non fig. 12: 3.
non 2009 Debeya haldemiana; Mohr 2009: text-fig. 11.
2013 Debeya haldemiana (Debey ex de Saporta and Marion, 1873) Knobloch, 1964; Halamski 2013: 422, figs. 7B, G, 8, 9C, D, E, 10C.
2013 Debeya (Dewalquea) haldemiana (Debey ex de Saporta et Marion 1873) Halamski, 2013; Halamski and Kvaček 2013: 83–84; text-fig. 1 [ubi syn.].
2016 Debeya (Dewalquea) haldemiana (Debey ex de Saporta & Marion, 1873) Halamski, 2013; Halamski et al. 2016: 217–221; figs. 2a, e, f, 5 [ubi syn.].
Material.—Rakowice Małe, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2008/0256, 0320, 0365, 2018/0059 (coll. Schaefer), MB.Pb.2018/0060, 0063a, 0065, 0066, 0080.1–3, 0084, 0088. Żerkowice, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2018/0079; Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb. 2018/0058; Assemblage 7, lower–middle Santonian: MB.Pb. 2008/0344. Bolesławiec, Assemblage 8, lower–middle Santonian: MGUWr 1699p.a.
Description.—Leaf compound, pedate, consisting of seven subsessile leaflets, petiole most probably present but not preserved in the studied material. Leaflet width never exceeding 10 mm, maximum preserved length 9 cm, estimated length ca. 15 cm. Base cuneate, apex not preserved. Margin unlobed, untoothed. Venation pattern pinnate, probably brochidodromous (Fig. 10D), secondaries departing at ca. 45°.
Remarks.—The material from Assemblage 4 described herein as D. haldemiana is fragmentary. Nonetheless, the coriaceous, oblong leaflets with length-to-width ratios exceeding 10 and thickened entire margins are sufficiently characteristic to be identified, like in the case of mass accumulations of leaflets of the same species in the Campanian of Scania (Halamski et al. 2016: fig. 5). The material from Bolesławiec figured by Roemer as D. haldemiana (1889) has wider leaflets and is described below as D. aff. gelindenensis; that figured by Mohr (2009) from the same outcrop has serrate margins (Fig. 10F4, F5) and is decribed below as D. insignis; however, a single genuine specimen of D. haldemiana (Fig. 10C) was found in the same outcrop as well.
Stratigraphic and geographic range.—Late Coniacian to Santonian: Lower Silesia (this work); Campanian: Westphalia (Hosius and von der Marck 1880), Scania (Halamski et al. 2016), Roztocze (Halamski 2013); Maastrichtian: Volhynia-Podolia Upland (Halamski 2013).

Fig. 10. Late Cretaceous representatives of the eudicot angiosperm Dewalquea from the North Sudetic Basin, Lower Silesia, Poland. A–D. Dewalquea haldemiana Debey ex de Saporta and Marion, 1873. A. Apical part of an isolated leaflet MB.Pb.2018/0084, Rakowice Małe, Assemblage 4 (upper Coniacian?–lower Santonian?). B. Fragment of a compound leaf MB.Pb.2008/066, Rakowice Małe, Assemblage 4 (upper Coniacian?–lower Santonian?). C. Incomplete compound leaf MGUWr 1699p.a, Bolesławiec, Assemblage 8 (Santonian). D. Isolated leaflet MB.Pb.2008/0320 showing venation, Rakowice Małe, Assemblage 4 (upper Coniacian?–lower Santonian?). E, F. Dewalquea insignis Hosius and von der Marck, 1880, Bolesławiec, Assemblage 8 (Santonian). E. Incomplete leaf MGUWr 1699p.b, enlargement of a leaflet showing venation. F. Incomplete leaf MB.Pb.2008/323 (specimen figured by Mohr 2009: fig. 11 as Debeya haldemiana); general view (F1), enlargement of a leaflet under different lightning to show venation (F2), basal part (F3), two leaflets (F4, F5) showing teeth (arrowed).
Dewalquea insignis Hosius and von der Marck, 1880
Figs. 10E–F, 11, 15E.
1880 Dewalquea insignis Hos. & v. d. Marck; Hosius and von der Marck 1880: 172–173, pls. 32: 111–113, 33: 109, 34: 110.
2009 Debeya haldemiana; Mohr 2009: text-fig. 11.
2013 Debeya insignis (Hosius and von der Marck, 1880) Knobloch, 1964; Halamski 2013: in part: 421–422, figs. 7A, C, 8?, 9A?, F?
Material.—Bolesławiec, Assemblage 8, lower–middle Santonian: MB.Pb.2008/0323, 0325; MGUWr 1699p.b, 5639p.
Description.—Leaf compound, pedate, consisting of up to presumably nine subsessile to shortly petiolulate leaflets; leaf most probably petiolate but petiole not preserved in the studied material. Leaflets lanceolate, 11–18 mm wide, in the only subcomplete specimen median leaflet 18 mm wide, then width gradually diminishing towards the external leaflets 11 mm wide; preserved length up ca. 8 cm, estimated length up to ca. 12 cm, estimated length-to-width ratio ca. 5–8. Margin serrate, teeth ca. 2 per cm (Figs. 10F4, F5, 11A2), but often not observable, proximal side straight, distal side concave. Venation pattern pinnate (Fig. 11B), camptodromous or craspedodromous (interpretation uncertain); midvein thick, secondaries departing at an angle of ca. 45–60°, then curving towards the apex, possibly sometimes entering the teeth.
Remarks.—Dewalquea insignis was described from the Campanian of Haldem (Westphalia) by Hosius and von der Marck (1880) who interpreted the original material as having leaves with serrate margins and this condition should be retained, despite the reservations expressed by Halamski (2013) when selecting the lectotype. The venation pattern of the type material is pinnate craspedodromous according to Hosius and von der Marck (1880); this character was not observable during its examination by ATH in 2012 (Halamski 2013: fig. 7A, C). The venation pattern of leaves from Lower Silesia is similar to that described by Hosius and von der Marck (1880). Dewalquea aquisgranensis de Saporta and Marion, 1873, originally described from the Aachen Formation (sables d’Aix-la-Chapelle, Robaszynski et al. 2002), the age of which is middle Santonian to probable earliest Campanian (Batten and Li 1987; Batten et al. 1988; Streel et al. 1994; the confusion resulting from dealing with a Cretaceous plant in a monograph devoted to Paleocene flora was stressed already by Stockmans 1946: 28–29), has the same leaf organisation and similarly shaped leaflets, but the venation pattern is very different, with secondaries forming numerous anastomoses (de Saporta and Marion 1873: pl. 8: 5–7). Dewalquea pulchella Knowlton, 1917, from the Frontier Formation of Wyoming (USA) is similar in shape, with the exception of leaflets being subsessile and 5(–6) in number.
The plant material from the Campanian to Maastrichtian of eastern Poland reported under D. insignis by Halamski (2013: fig. 9A, F) is entire-margined and probably represents a different species. D. haldemiana, the most common representative of Dewalquea in the studied material, has narrower and entire-margined leaflets.
Stratigraphic and geographic range.—Santonian of the Liège-Limburg Basin, Belgium and Lower Silesia, Poland.
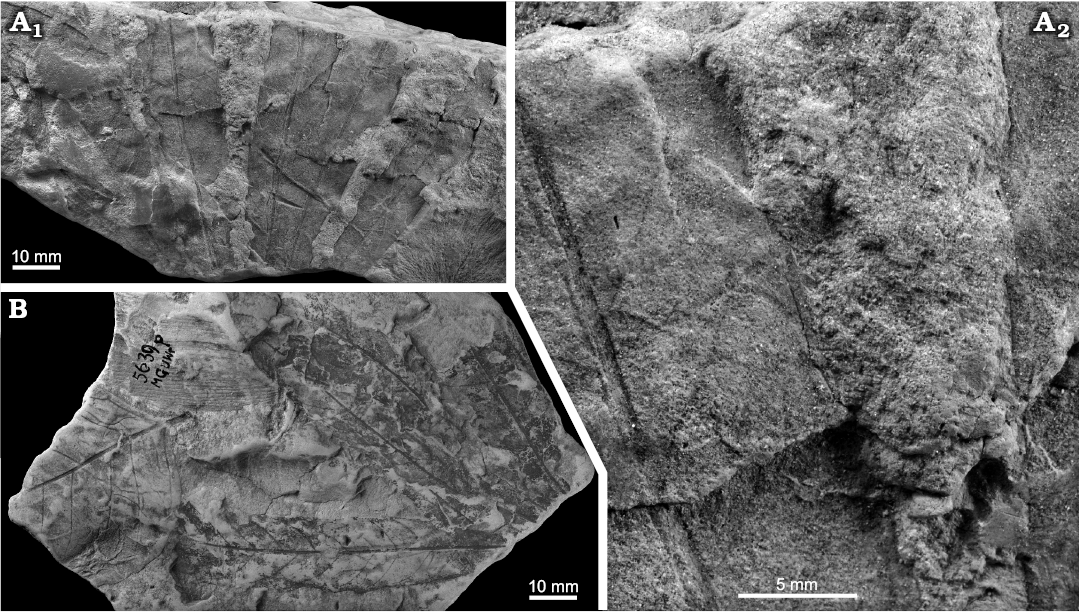
Fig. 11. Taphonomy of the eudicot angiosperm Dewalquea insignis Hosius and von der Marck, 1880 from the North Sudetic Basin, Lower Silesia, Poland. A. Fragmentary compound leaf MB.Pb.2008/325.1, Bolesławiec, Assemblage 8 (Santonian); general view (A1) and enlargement of fragments of two leaflets to show venation and teeth (A2). B. Accumulation of leaflets (presumably from the same compound leaf) MGUWr 5639p accompanied by Salicites? sp. and unidentified vegetal remains, Ulina?, Assemblage 8? (Santonian?).
Dewalquea aff. gelindenensis de Saporta and Marion, 1873
Figs. 12, 15A.
Material.—Bolesławiec, Assemblage 8, lower–middle Santonian: MGUWr 2880p (coll. Jonas 1885; figured by Roemer 1889: pl. 12: 3 as Debeya haldemiana), MGUWr 2881p (coll. F. Roemer 1886), MGUWr 6150p.
Description.—Leaves compound, pedate, petiolate, of (five to?) seven petiolulate leaflets. Petiole distally widened (Fig. 10A3). Petiolules short (never exceeding 5 mm). Leaflets symmetric, with marginal blade attachment, notophyll, up to ca. 25 mm in width, preserved length ca. 7 cm, estimated length >10 cm, estimated length-to-width ratio 4–5. The median leaflet always the widest, the lateral ones narrower; sometimes, but apparently not always, leaflet width gradually decreasing from the median leaflet towards the external ones. Base cuneate, apex possibly straight. Margin unlobed, untoothed (Fig. 10A2, XB3). Venation pattern pinnate camptodromous (Fig. 10A2); midvein stout; secondaries excurrent, departing at (45–)60–90°, gently curving, quite irregularly spaced, 1–3 per cm; finer venation not preserved.
Remarks.—The described material is distinguished from both co-occurring representatives of Dewalquea in having wider leaflets, and from D. aquisgranensis in lacking marginal serration (see Table 1).
To date, three entire-margined species of Dewalquea with more than three leaflets per leaf have been described: D. gelindenensis de Saporta and Marion, 1873, from the Paleocene of Belgium, as well as D. reniformis Krassilov in Krassilov et al., 2005, and D. gerofitica (Dobruskina, 1997) Krassilov in Krassilov et al., 2005 from the Turonian of Negev (Krassilov et al. 2005). The former is relatively close to the material described herein in leaflet shape and venation; however, the Paleocene species has always slightly emarginate apices (de Saporta and Marion 1873: pl. 9: 1, 4, 5). This character is difficult to check in plants from Lower Silesia, but a single leaflet seems to show a straight apex; one cannot be certain, however, that this is not a preservational artefact. In all other leaflets apices are not preserved. The two Turonian species differ from the material described herein in terms of leaf organisation (high divergence angle of leaflets for the former, leaf composed of four, more seldom five leaflets for the latter). To sum up, the material described here might represent a new species, but is described under open nomenclature due to insufficient preservation.
Leaves from eastern Poland described as Debeya insignis by Halamski (2013: figs. 8, 9A, F), but differing in having entire margins (see above), may represent the same species, although their preservation is rather fragmentary.
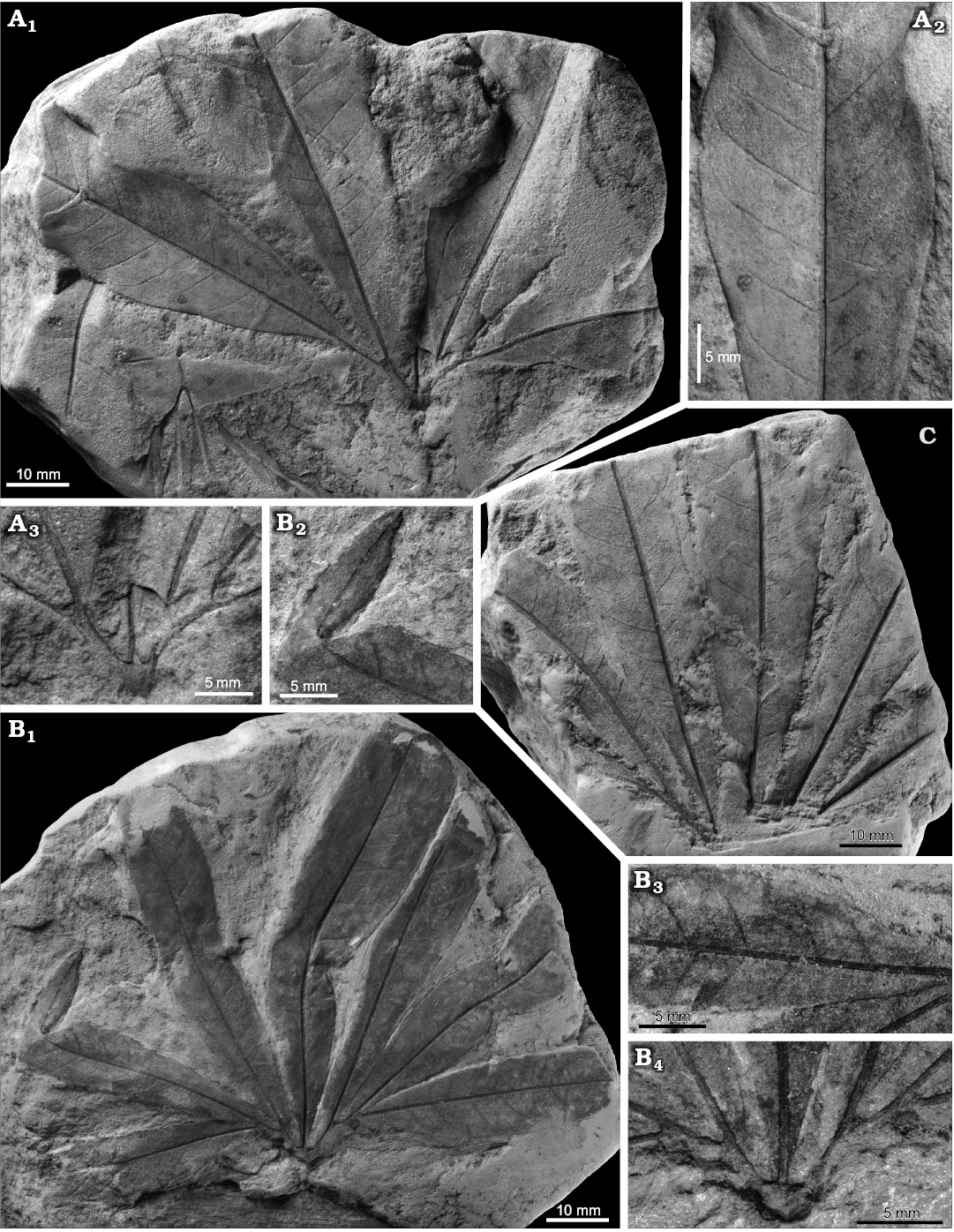
Fig. 12. Eudicot angiosperm Dewalquea aff. gelindenensis de Saporta and Marion, 1873 from Bolesławiec, North Sudetic Basin, Lower Silesia, Poland, Assemblage 8 (lower–middle Santonian). A. Incomplete compound leaf MGUWr 2881p; general view (A1), note a small fragmentary compound leaf of Dewalquea haldemiana in the bottom left corner; enlargement of the middle part a leaflet showing venation (A2) and of the basal part with petiole and petiolules (A3). B. Subcomplete compound leaf MGUWr 2880p (specimen figured by Roemer 1889: pl. 12: 3 as Debeya haldemiana); general view (B1), enlargement of the distal part of a leaflet (B2; interpretation uncertain, apex or taphonomic bias); enlargement of the middle part of a leaflet showing venation (B3) and of the basal part with petiole and petiolules (B4). C. Fragmentary compound leaf MGUWr 6150p.
Genus Dalbergites (Velenovský, 1885) Halamski and Kvaček, 2015
Type: Dalbergites atavius (Velenovský, 1885) Halamski and Kvaček, 2015 ≡ Cassia atavia Velenovský, 1885; Coniacian, Upper Cretaceous; Idzików, Kłodzko region, Poland.
Dalbergites sp.
Fig. 17F.
Material.—Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2018/0068. Żerkowice, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb. 2018/0023.
Description.—Lamina incompletely preserved, ca. 20 mm long and 13 mm wide, medially asymmetrical. Base rounded? Apex not preserved. Venation pinnate, secondaries poorly preserved, proximally decurrent, distally excurrent(?), departing at an angle of 50–80°. Margin unlobed, untoothed.
Remarks.—Small asymmetric phyllomes are interpreted as leaflets of pinnately compound leaves (Halamski and Kvaček 2015).
Supersubclass, class, order, and family unknown
Remarks.—As argued in detail in Halamski and Kvaček (2015: 102–103) and Halamski et al. (2018a: 128), Cretaceous angiosperm leaves are mostly described under a morphographic system, an inherently temporary, yet necessary solution (Bengtson 1985).
Form group Cinnamomophylls (Crabtree, 1987) Halamski, 2013
Fossil-genus Cinnamomoides Seward, 1925
Type: Cinnamomoides newberryi Berry, 1911 sensu Seward (1925); Upper Cretaceous, Atanekerdluk, Greenland. See Halamski (2013: 428) for a detailed discussion on the type and Halamski and Kvaček (2015: 113) for an emended diagnosis.
Cinnamomoides sp.
Fig. 17B.
Material.—Huzarski Skok, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb.2018/0079. Rakowice Małe, Assemblage 4, upper Coniacian?–lower Santonian?: MB.Pb. 2008/0364.
Description.—Leaf simple, blade shape elliptic. Base shape decurrent, apex not preserved. Venation pattern acrodromous with three main veins. Margin unlobed, untoothed.
Remarks.—The material described herein under Cinnamomoides sp. is poorly preserved, yet easily separable from other similar taxa in the studied flora on account of trifurcate venation.
Form group Platanofolia Krassilov, 1979
Remarks.—Platanoids are relatively frequent and diversified in the studied material. They are present in Assemblages 2, 6, 7, and 8 and Platananthus sp. in Assemblage 5 (four or five out of a total of eight assemblages). Platanoid foliage is represented by four species (out of a total of 18 angiosperm foliage taxa). It is worth noting, however, that all reports of representatives of Credneria Zenker, 1833 from the Upper Cretaceous of Lower Silesia (Drescher 1863; Williger 1882; Lilpop and Kostyniuk 1957) are shown herein to be based on misidentified specimens of Ettingshausenia.
Genus Ettingshausenia Stiehler, 1857
Type: Ettingshausenia cuneifolia (Bronn, 1837) Stiehler, 1857 ≡ Credneria cuneifolia Bronn, 1837. Cenomanian, Upper Cretaceous; Niederschöna, Saxony.
Remarks.—Ettingshausenia may be understood either broadly (Maslova et al. 2005; Herman and Kvaček 2010) or narrowly (Golovneva 2011). Following arguments given by Halamski and Kvaček (2015: 119) the former intepretation is used herein.
Ettingshausenia cf. superstes (Velenovský, 1882) Kvaček and Halamski in Halamski and Kvaček, 2015
Fig. 13A–C, E.
Material.—Czaple, Assemblage 2, Coniacian: MB.Pb.2008/ 0369. Wartowice, Assemblage 2, Coniacian: MB.Pb.2008/ 0332, 0333, 0334. Zbylutów, Assemblage 2, Coniacian: MB.Pb.2008/0336.2.
Description.—The available material consists of fragments of medium-sized leaves with weakly developed lobes.
Lamina probably notophyll, in the most complete specimen (Fig. 13B2) observed length about 6.7 cm and estimated laminar length-to-width ratio about 1.5. Base sharply cuneate (Fig. 13B); lateral lobes weakly developed, incised to about one tenth of the blade (Fig. 13C, E); a single observed large lobe (Fig. 13E) interpreted as the terminal one. Venation palmately pinnate brochidodromous to camptodromous with strong basal veins emerging from leaf base. Tertiary veins percurrent (Fig. 13B1).
Remarks.—This leaf type is similar to Ettingshausenia superstes, from the Coniacian of the Bohemian Cretaceous Basin (Velenovský 1882: 15–16 [8–9], pl. 4 [2]: 7–9; Halamski and Kvaček 2015: 311–312, fig. 6I–K), but differs in having small lobes which are not preserved in the holotype. Ettingshausenia onomasta (Bayer, 1896) Kvaček and Halamski in Halamski and Kvaček, 2015, from the Coniacian of Idzików (Halamski and Kvaček 2015) differs from the studied material in having well-developed lobes, which are acute-attenuate. Ettingshausenia gruenbachiana Herman and Kvaček, 2010, from the Campanian of Austria and Ettingshausenia senonensis (Knobloch, 1964) Kvaček and Váchová, 2006, are entire-margined, yet both differ from the studied material in having well-pronounced lobes.
Ettingshausenia sp. 1
Figs. 13D, F, G, 15F, G.
Material.—Ulina, Assemblage 6, lower–middle Santonian: MGUWr 5616p.b (coll. Goeppert), MB.Pb.2008/0264, 0338.
Description.—The available material consists of subcomplete to incomplete relatively large, trilobed leaves.
Lamina notophyll to mesophyll, maximal observed length about 9 cm, laminar length-to-width ratio about 1.1 (Fig. 13G) to 1.6 (Fig. 13D). Base cuneate. Lobes incised to one seventh to one sixth of the length. Venation palmately pinnate with robust basal veins emerging from the leaf base. Secondaries excurrent, departing at 30° (Fig. 13D) to 60° (Fig. 13G). Percurrent tertiary veins poorly preserved.
Remarks.—Ettingshausenia sp. 1 differs from Ettingshausenia cf. superstes in displaying stronger lobes. Similarities with Ettingshausenia onomasta from the Coniacian of Idzików (Halamski and Kvaček 2015) should be noted; however, the fragmentary character of the specimen, including in particular a poorly preserved leaf margin, precludes further comparison, so open nomenclature is used here. Variability of leaves classified here as Ettingshausenia sp. 1 is significant; it may correspond to polymorphism within a single tree, to a single variable species that cannot be further subdivided, to a single species with geographical races or ecotypes (Sell and Murrell 2018: xi), or finally to several biological species.
The specimen MGUWr 5616p (Fig. 13F) shows the co-occurrence of Ettingshausenia sp. 1 (5616p.b, lower leaf) and Dryophyllum westerhausianum (upper leaf, 5616p.a).
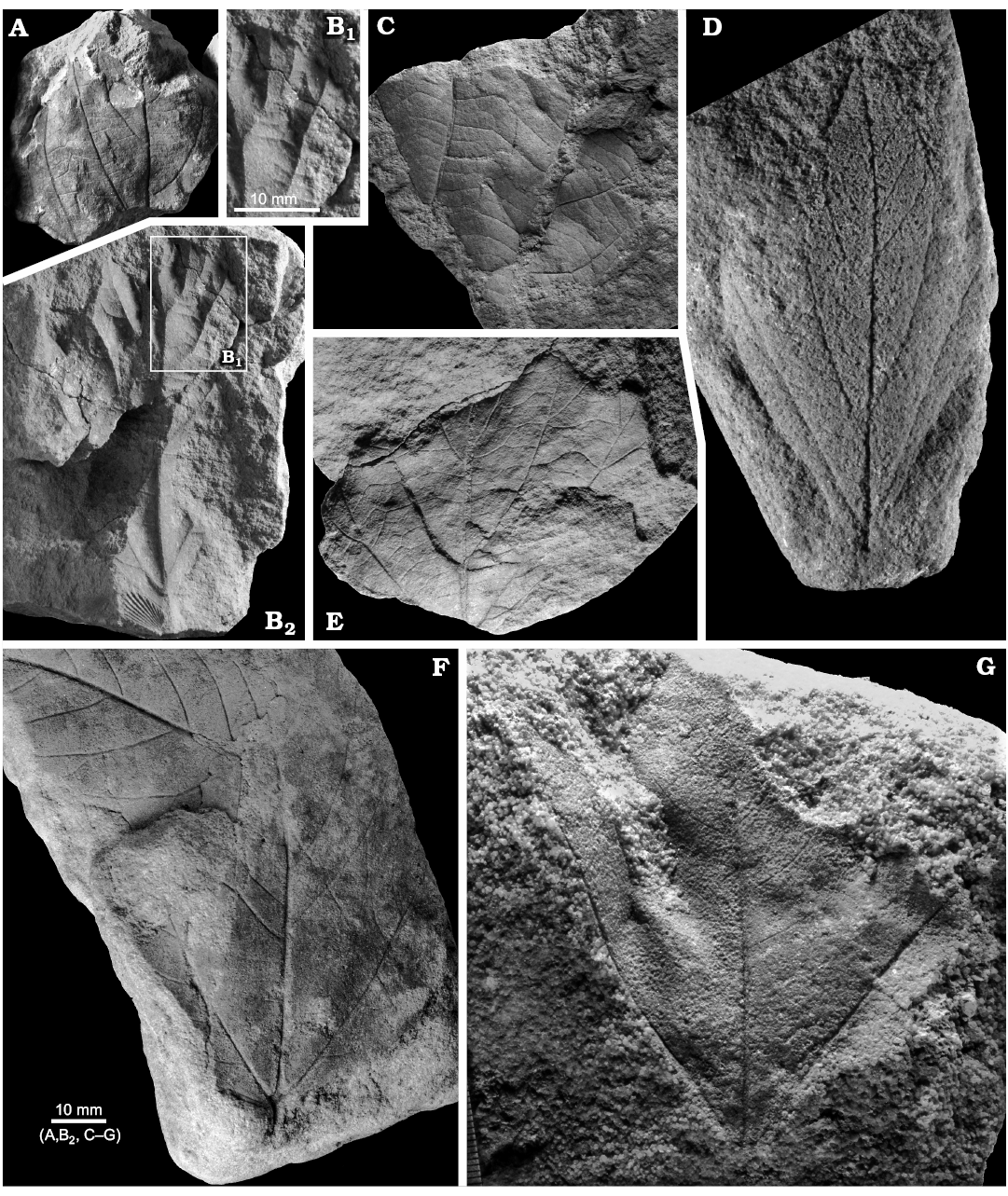
Fig. 13. Representatives of the platanoid Ettingshausenia from the North Sudetic Basin, Lower Silesia, Poland. A–C, E. Ettingshausenia cf. superstes (Velenovský, 1882) Kvaček and Halamski in Halamski and Kvaček, 2015, Assemblage 2 (Coniacian). A. Leaf fragment MB.Pb.2008/336, Zbylutów. B. Incomplete leaf MB.Pb.2008/333b, Wartowice; general view (B2), enlargement of the apical part (B1). C. Leaf fragment MB.Pb.2008/334, Zbylutów. E. Leaf fragment MB.Pb.2008/332, Zbylutów. D, F, G. Ettingshausenia sp. 1, Ulina, Assemblage 6 (Santonian?). D. Subcomplete leaf MB.Pb.2008/264. F. Incomplete leaf MGUWr 5616p. G. Incomplete leaf MB.Pb.2008/338.
Genus Platanites Forbes, 1851
Type: Platanites hebridicus Forbes, 1851; Paleocene, Isle of Mull, Inner Hebrides, Scotland, UK.
Remarks.—Platanites hebridicus is a trifoliolate platanoid leaf (see Boulter and Kvaček 1989 for a detailed description). Other species, described in much detail, are the similarly trifoliolate P. marginatus (Lesquereux, 1873) Johnson, 1996, from the Upper Cretaceous and lower Tertiary of South and North Dakotas, Montana, Wyoming, Colorado, Utah, and Arizona (Johnson 1996) and P. raynoldsii (Newberry, 1868) Manchester, 2014, from the Paleocene of North America (Manchester 2014). Platanites resembles Ettingshausenia and extant Platanus, but has compound leaves. However, discriminating between compound and simple leaves can be difficult in incomplete fossils. Fragmentary specimens of Platanites can be distinguished by basal secondaries connecting between the diverging primary veins displaying a chevron pattern. Such a pattern is usually absent in Ettingshausenia and even if it does occur, the chevrons are blunt, never sharp. The poorly preserved available material does not offer any traces of the trifoliolate condition of the original leaves, which anyway is seldom preserved in the fossil record and not necessarily constant in the living plant (Manchester 2014: 166). Erlingdorfia Johnson, 1996, is another trifoliolate platanoid distinguished by strongly trilobate median leaflets and bilobate lateral leaflets.
As far as it could be checked, this is the oldest record of the genus Platanites.
Platanites willigeri Halamski and Kvaček sp. nov.
Figs. 14, 15B, 16A, B.
Etymology: In honour of Gustav Williger (1856–1937; for biographic notes see Springer 1914: 114–117, Anonymous 1937), in recognition of his contribution to the study of the Cretaceous of Lower Silesia (Williger 1882).
Type material: Holotype: MB.Pb.2008/0335, basal part of a median leaflet; designated herein (Fig. 14B). Paratype 1: MB.Pb.2008/0328, incomplete median leaflet (about two thirds of the blade, with margin, lacking base and apex) (Figs. 14A, 16A). Paratype 2: MB.Pb.2018/0054.1, a complete lateral leaflet (Fig. 14C). Both paratypes from the same horizon and probably the same locality.
Type locality: Probably Luisenhain, temporary excavations in the first half of the 20th century. In the collection the type material is labelled “Bunzlau”, but due to recent mislabelling of some specimens (see Appendix 1, entry on Luisenhain) it is unfortunately impossible to be certain about the original locality.
Type horizon: Czerna Formation, most probably lower or middle Santonian.
Material.—Luisenhain, Assemblage 8, lower–middle Santonian: MB.Pb.2008/0356 (coll. W. Zimmer 1913), MB.Pb. 2018/0056 (coll. W. Zimmer 1912); Assemblage 8, lower–middle Santonian: MB.Pb.2008/0321, 0329 (counterpart of the paratype 1); MB.Pb.2018/0046, 0048, 0050, 0051.
Diagnosis.—Leaf interpreted as trifoliolate (Fig. 15D); terminal leaflet ovate, base cuneate; margin serrate, teeth rarely spaced; venation palmately pinnate, palinactinodromous; midvein stout, two basal veins emerging from leaf base, secondary veins forming chevron pattern by interconnected secondary veins between adjacent primaries in the lower part of the lamina. Presumed lateral leaflets unlobed with pinnate venation.
Description.—The available material consists of isolated, most often incomplete laminae, interpreted as terminal (median) leaflets and in a single case a lateral leaflet belonging to a compound trifoliolate leaf, petiolate and with petiolulate leaflets.
Terminal leaflet: blade attachment marginal, laminar size probably microphyll to notophyll; laminar length-to-width ratio probably ca. 1.5–1.6; laminar shape ovate, unlobed, medially asymmetric. Base symmetrical, cuneate (Fig. 14B; concave sensu Ellis et al. 2009). Apex not preserved.
Venation of the median leaflet approximately pinnate with mostly a single primary vein (Fig. 14A), but sometimes the first secondary vein being nearly as thick as the midvein (Fig. 16A) resulting in a quasi-palmate pattern. Compound agrophic veins present. Major secondaries craspedodromous, seldom semicraspedodromous, attachment decurrent, angle approximately uniform, 40–50°, spacing apparently decreasing distally. Marginal veins probably absent. Intersecondaries mostly absent (see Remarks). Tertiaries percurrent, mostly convex, relatively sparsely spaced, angles between them and the midvein mostly acute.
Margin of the median leaflet toothed, tooth spacing irregular. Teeth 1–2 per cm, varying in size, but forming not two distinct size classes; most often supplied by a single principal vein. Sinus shape rounded. Proximal flank weakly convex, distal flank straight to concave.
Presumed lateral leaflet (Fig. 14C): lamina lanceolate in shape, laminar length-to-width ratio ca. 1.7; apex shape rounded, probably retuse; teeth regular, blunt; venation pinnate.
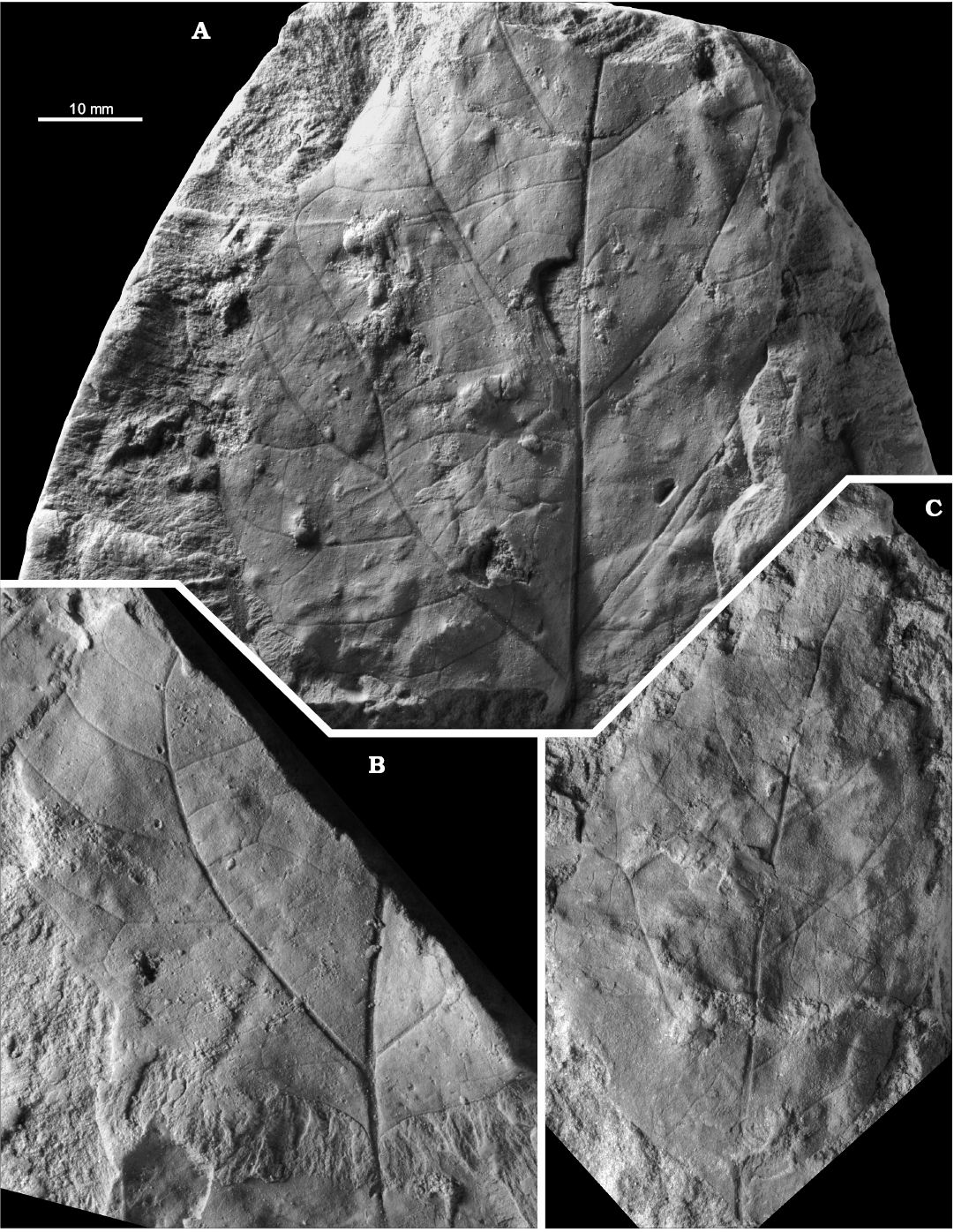
Fig. 14. Platanoid angiosperm Platanites willigeri Halamski and Kvaček, sp. nov. probably from Luisenhain, North Sudetic Basin, Lower Silesia, Poland, Assemblage 8 (lower–middle Santonian). A. Incomplete median leaflet MB.Pb.2008/0328, paratype (see also Fig. 16A). B. Basal part of a median leaflet MB.Pb.2008/0335, holotype. C. Complete lateral leaflet MB.Pb.2018/0054.1, paratype.
Remarks.—Platanoid affinities of the described leaves are undeniable on account of the venation pattern and tooth characters. Its architecture and shape show notable similarities to those of Platanites raynoldsii and Platanites marginatus of North America. From those leaves P. willigeri differs in having a less decurrent base. From Platanites hebridicus it differs in having more pronounced lobes and in the presence of lateral leaflets. However, the preservation of our material is fragmentary and no specimen shows the physical connection of leaflets.
Similarities of the presumed median leaflet to Platimeliphyllum palanense (Budantsev, 1983) Maslova, 2002, from the upper Paleocene–lower Eocene of Kamchatka (Maslova 2002: text-fig. 1; see also Maslova 2008: 1399–1400) should be noted. However, the generic diagnosis of Platimeliphyllum Maslova, 2002, includes cuticular features (see also Kodrul and Maslova 2007), so this name cannot be used for the material from the North Sudetic Basin. Another similar leaf is Protophyllum zaissanicum Romanova, 1963, from the uppermost Cretaceous (to lower Paleogene?) of Kyin-Kerish Hill in Kazakhstan (Romanova 1963; see also Kutuzkina 1974: 149, pl. 72: 1); it differs in more closely spaced leaf margin teeth.
Further leaves showing some characters in common with the laminae interpreted herein as median leaflets of a trifoliolate leaf include Late Cretaceous–Paleogene representatives of Platimelis Golovneva, 1994 that have relatively densely spaced small, symmetric teeth and cordate bases (Golovneva 1994; Moiseeva 2012). Leaves of Evaphyllum Maslova, 2003 (type and only known species E. kamchaticum Maslova, 2003, from the upper Paleocene–lower Eocene of Kamchatka (Russia); see also Maslova 2010: 1448–1449) are elliptic in shape. Leaves reported from the Coniacian to Campanian Valižgenian taphoflora of Kamchatka by Budancev (1983) as Platanus crednerioides Budancev, 1983 (Budancev 1983: pl. 22: 4), and Pseudoprotophyllum boreale (Dawson, 1883) Hollick, 1930 (Budancev 1983: pl. 23: 2), differ from our species, the former in having larger teeth, the latter in having agrophic veins forming a more complex system. Both Pseudoprotophyllum Hollick, 1930 and Pseudoaspidiophyllum Hollick, 1930, from the Creatceous of Alaska have peltate bases. In Viburniphyllum de Saporta, 1868, and Grewiopsis de Saporta, 1868, secondaries are more branched.
Platanophyllum Fontaine, 1889 was used by Arens and Allen (2014: 188) to accommodate simple, unlobed platanoid leaves found in the basal part of the Hell Creek Formation (Maastrichtian) in Montana (USA). Such a treatment seems inappropriate given the fragmentary character of the type material of Platanophyllum (P. crassinerve Fontaine, 1889; Potomac Group, Lower Cretaceous, Virginia, USA).
Stratigraphic and geographic range.—Assemblage 8 (lower–middle Santonian) at Luisenhain, Poland. Several specimens in MB are labelled “Bunzlau”, but this locality does not appear on the original labels (see Appendix 1, Luisenhain); all material of this species possessing original labels is from Luisenhain. It is uncertain whether P. willigeri is present at Bolesławiec, Poland.
Platanites sp. 1
Figs. 15D, 16C, E.
Material.—Nowe Jaroszowice, Assemblage 7, lower–middle Santonian: MB.Pb.2008/0268, 0344.
Description.—Leaf interpreted as trifoliolate, but in the available material only simple blades (presumably corresponding to median leaflets) are preserved. Blade attachment marginal, petiol(ul)e very long. Venation palmately pinnate. Secondaries connecting primaries in a way similar to a chevron pattern, with the angle of the secondaries being very wide (over 120°).
Remarks.—Interpretation of this fragmentary material is tentative. The similarity of the shape and architecture of the described phyllomes to those of the central leaflets of Platanites marginatus and P. hebridicus is striking. The long petiol(ul)e is also consistent with such an interpretation. However, it cannot be excluded that all specimens are distorted taphonomically in a similar way, or, in other words, they are lacking analogous parts, and that the original shape of the blade was different.
Viburnum vitifolium de Saporta and Marion, 1878, was described on the basis of a single specimen from Gelinden (de Saporta and Marion 1878: 73–75, pl. 12: 1) which is early Paleocene (Danian) in age (Kvaček 2010), and is also similar to Platanites sp. 1 described herein; differences include the margin, which is entire in our material and dentate in the Paleocene species.
Comparable leaves were described by Wolfe and Wehr (1987) from the Eocene of Republic (Washington state, USA) as Macginitiea gracilis (Lesquereux, 1872) Wolfe and Wehr, 1987. They have a similar arrangement of the basal veins, but are distinguished from the studied material by a sharp characteristic chevron pattern formed by interconnected secondaries (Wolfe and Wehr 1987; Manchester 2014) which is absent in the described material (compare Fig. 16C3 and Manchester 1986: fig. 5A–C). Members of Recent Diploclisia Miers, 1851 (Menispermaceae) are similar in shape and in venation; the main veins, however, are craspedodromous (Yu and Chen 1991: pl. 22: 5, 13).
Poorly preserved MB.Pb.2008/0340, 0341, and s.n. from the same outcrop might belong here.
Form group Magnoliaephylls Crabtree, 1987
Genus Laurophyllum Goeppert, 1854
Type: Laurophyllum beilschmiedioides Goeppert, 1854; Eocene, Java.
Remarks.—We use this genus as emended by Halamski and Kvaček (2015: 114–116). The emendation of this genus by Hill (1986) is rejected as manifestly different from the intention of the first author (cuticles instead of impressions).
Fig. 15. Semi-schematic reconstructions of the presumed main forest-forming angiosperms in the Coniacian–Santonian of Lower Silesia, Poland. A. Dewalquea aff. gelindenensis de Saporta and Marion, 1873 (compare Fig. 12A, B). B. Platanites sp. 1 (compare Fig. 16C; lateral leaflets hypothetical, supposed similar to those of Platanites marginatus, see Johnson 1996: fig. 86). C. Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. (compare Fig. 8C, E). D. Platanites willigeri Halamski and Kvaček, sp. nov. (compare Fig. 14A, C). E. Dewalquea insignis Hosius and von der Marck, 1880 (compare Fig. 10F). F, G. Ettingshausenia sp. 1 (compare Fig. 13G, D). Not to scale. Drawings by Bogusław Waksmundzki.
Laurophyllum? sp.
Fig. 16D.
Material.—Czaple, Assemblage 2, lower–middle Coniacian: MB.Pb.2018/0022. Otok, Assemblage 6, lower–middle Santonian: MB.Pb.2018/0021. Ulina, Assemblage 6, lower–middle Santonian: MB.Pb.2008/0263, 2018/0034, 0036. Bolesławiec, Assemblage 8, lower–middle Santonian: MGUWr 5607p (coll. O. Tornier). Luisenhain, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0057.1–4. Assemblage 8, lower–middle Santonian: MB.Pb.2008/0322, 0326, 0358, 2018/0047.
Description.—Leaves simple, microphyll to notophyll (possibly up to 10 cm long), petiolate. Blade shape ovate. Base shape convex, rounded, possibly concavo-convex? Apex not preserved, possibly convex to straight with acute angle. Venation pattern pinnate, midvein stout, secondaries excurrent, angle to midvein 50–70°, their course nearly straight (weakly curving towards the apex). Margin unlobed, untoothed.
Remarks.—Rather variable, but mostly poorly preserved, long entire-margined ovate leaves are described here under the fossil-genus Laurophyllum, similarly to previous taxonomic treatments of Late Cretaceous Central European floras (Halamski and Kvaček 2015, 2016). If secondaries are preserved in the material studied, they differ from those in L. acuminatum (Goeppert, 1844) Kvaček and Halamski in Halamski and Kvaček, 2015, in their less curved course.

Fig. 16. Santonian platanophylls and laurophylls from the North Sudetic Basin, Lower Silesia, Poland. A, B. Platanites willigeri Halamski and Kvaček, sp. nov. probably Luisenhain, Assemblage 8 (Santonian). A. Enlargement of a part of the paratype MB.Pb.2008/0328 (see Fig. 14A) showing teeth and venation. B. Incomplete leaf MB.Pb.2018/0050 showing venation. C, E. Platanites sp. 1, Nowe Jaroszowice, Assemblage 7 (Santonian). C. Incomplete presumed median leaflet MB.Pb.2008/344, general view (C1), enlargements of the base and of the margin and venation under different lightning (C2, C3). E. Incomplete median leaflet MB.Pb.2008/268. D. Laurophyllum sp., leaf MB.Pb.2008/263, Ulina, Assemblage 6 (Santonian).
Genus Araliaephyllum Fontaine, 1889
Type: Araliaephyllum obtusilobum Fontaine, 1889; Lower Cretaceous, Virginia, USA.
Araliaephyllum? sp.
Fig. 17D.
Material.—Assemblage 6?, lower–middle Santonian: MGUWr 2704p (locality uncertain; labelled “Bunzlau”, but the specimen is in sandstone; coll. Goeppert).
Description.—The single available leaf is incomplete, preserved width ca. 41 mm, estimated width <50 mm, preserved length ca. 71 mm, estimated length ca. 78 mm. Blade attachment marginal, size notophyll; base convex, apex unknown; blade symmetrical, ovate, probably palmately lobed (trilobate), lobes probably small. Midvein stout, two main secondaries departing near the base at an angle of ca. 40°, excurrent, slightly curved admedially, interpreted as entering the lobes; in the distal part of the median lobe poorly preserved traces of weaker excurrent secondaries departing at an angle of ca. 60°. Margin entire.
Remarks.—The interpretation of this single incomplete specimen is based on the assumption that the missing parts formed lateral lobes; given the size of the parts of the leaf with incompletely preserved margin, the lobes must have been narrow and arguably relatively short. Trilobate leaves from the Turonian–Santonian of Klikov in Bohemia were described by Němejc (1961) under Araliophyllum Ettingshausen, 1868, and from the Cenomanian of Maletín in Moravia by Greguš and Kvaček (2015) under Araliaephyllum Fontaine, 1889 (for discussion of relationships between both genera see Greguš and Kvaček 2015: 330); however, in these forms the secondaries are straight and the lateral lobes are larger. Similar forms were also described by Lesquereux (1892) and by Newberry (1895) from the Upper Cretaceous of North America. Smilacites panartius (Bayer, 1896) Halamski and Kvaček, 2015, from the Coniacian of Idzików is superficially similar in having the proximal part of the blade much wider than the distal one, but the base is hastate and there are no lobes; the venation pattern consists of seven primaries.
Form group Fagofolia Krassilov, 1979
Genus Dryandroides Unger, 1850
Type: Not selected.
Remarks.—The generic placement of the discussed species is purely conventional (Halamski and Kvaček 2016).
“Dryandroides” quercinea Velenovský, 1883
Fig. 17E.
1883 Dryandroides quercinea sp. n.; Velenovský 1883: 8 [33], pl. 2 [10]: 8a–15.
2016 “Dryandroides” quercinea Velenovský, 1883; Halamski and Kvaček 2016: 312–313; fig. 4A, B, D, E [ubi syn.].
Material.—Bolesławiec, Assemblage 8, lower–middle Santonian: MB.Pb.2008/0360.
Description.—The available specimen is a fragment of a leaf ca. 2 cm wide and 4.5 cm long, representing the distal part without the tip of the apex, the general form of which can, however, be reconstructed. Blade shape possibly ovate, apex acuminate. Venation pattern pinnate craspedodromous, secondaries departing at ca. 45–70°. Margin serrate.
Remarks.—This single incomplete specimen is similar to Dryophyllum westerhausianum, from which it differs, however, in the apex being acuminate and not straight. It is accommodated in the poorly known species “Dryandroides” quercinea described from the Coniacian of Bohemia (see Halamski and Kvaček 2016 for revision of the type material).
Halamski and Kvaček (2016) noted that “Dryandroides” quercinea and Dryophyllum geinitizianum do not co-occur in Bohemian Upper Cretaceous localities. In Lower Silesia the situation is similar: the only specimen of “Dryandroides” quercinea is known from the Assemblage 8, whereas Dryophyllum westerhausianum is common in Assemblage 6.
From group Rosifolia Krassilov, 1979
Genus Ternstroemites Berry, 1916a
Type: Ternstroemites eoligniticus Berry, 1916a; lower Eocene; Tennessee, USA (by subsequent designation of Andrews 1955: 249).
Remarks.—Ternstroemites was originally interpreted (Berry 1916a) as a form genus of the family Ternstroemiaceae Mirbel, 1816 (now included in the Pentaphylacaceae Engler in Engler and Prantl, 1897; at a time included in the Theaceae Mirbel, 1813, as e.g., by Wolfe and Wehr 1987: 16; see also Prince 2007). Krassilov (1979: 62) synonymised Ternstroemites with Celastrinites de Saporta, 1865. We follow the usage of Herman and Lebedev (1991: 87–88) who consider it as a valid form genus of dicots.
Ternstroemites? sp.
Fig. 17C.
Material.—Bolesławiec, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0045.
Description.—The single available specimen consists of two laminae (Fig. 17C1), one incomplete (preserved length ca. 45 mm, preserved width ca. 20 mm) and one fragmentary, interpreted herein as leaflets of a compound leaf. Blade shape possibly elliptic, neither base nor apex preserved. Margin serrate; teeth ca. 2 per cm, acute, anteriorly directed, proximal side concave to straight, distal side concave.
Venation pattern (Fig. 17C2) pinnate brochidodromous (rarely semicraspedodromous?). Midvein stout. Secondaries rather densely and somewhat irregularly spaced, excurrent, departing at 70–90°; intersecondaries ca. one per intercostal area, departure similar to that of secondaries, disappearing about halfway to margin; tertiaries not preserved.
Remarks.—This leaf fragment is included into Ternstroemites Berry, 1916a, on account of a serrate margin and a brochidodromous venation pattern with numerous intersecondaries. Four Cretaceous representatives of this genus, namely T. harwoodensis (Dawson, 1883) Bell, 1957, from the Campanian of Vancouver Island (Bell 1957) and the Coniacian of Kamchatka (Herman and Lebedev 1991), T. ripleyensis Berry, 1925, T. cretaceus Berry, 1925, and T. tennesseensis Berry, 1925, the latter three from the Ripley Formation of eastern United States (Berry 1925) are more finely serrate. Ternstroemites longifolius (Friedrich, 1883) Rüffle and Krutzsch, 2005, from the Maastrichtian of Eisleben (Saxony-Anhalt, Germany) has similar serration of the margin, but secondaries are much denser. It should, however, be kept in mind that such a fragment might have belonged to a leaf of very different architecture, like for example that of Araliopsoides kiensis (Baikovskaya, 1957) Golovneva in Golovneva and Nosova, 2012, from the Cenomanian of Siberia illustrated by Golovneva and Nosova (2012: fig. 8.7).
Ficonium silesiacum (Velenovský, 1885) Halamski and Kvaček, 2015, known from a single specimen from the Coniacian of Idzików, has a venation pattern with similarly numerous intersecondaries, but secondaries are camptodromous rather than brochidodromous and the margin is entire (Halamski and Kvaček 2015: text-fig. 10).
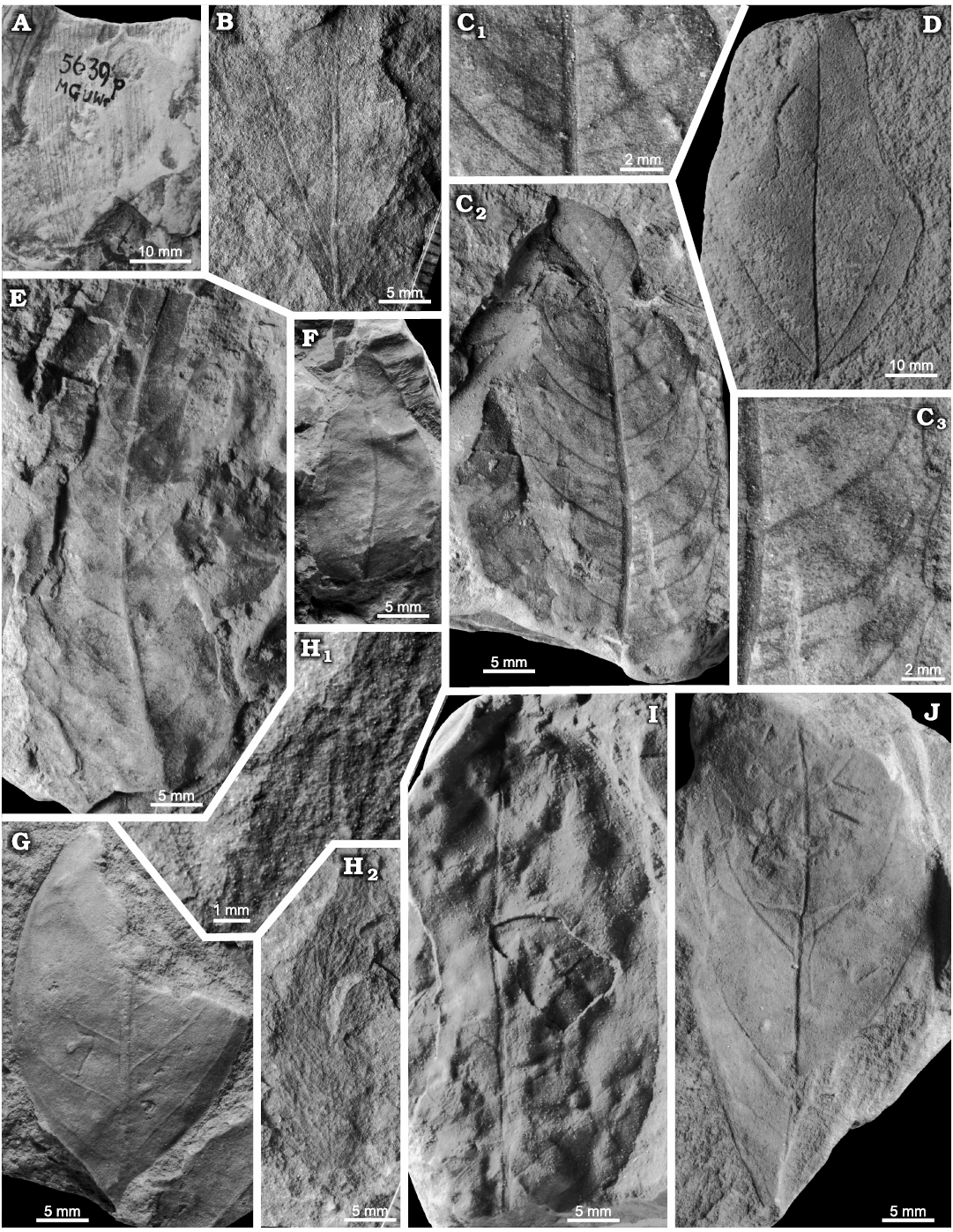
Fig. 17. Late Cretaceous angiosperms from the North Sudetic Basin, Lower Silesia, Poland. A. Monocotylophyllum sp., leaf fragment MGUWr 5639p.b, “Ulina”, Assemblage 8 (lower–middle Santonian). B. Cinnamomoides sp., incomplete leaf MB.Pb.2008/364, Rakowice Małe, Assemblage 4 (upper Coniacian?–lower Santonian?). C. Ternstroemites? sp., incomplete leaf MB.Pb.2018/0045, Bolesławiec, Assemblage 8 (lower–middle Santonian), general view (C2), enlargements showing margin (C3), and venation (C1). D. Araliaephyllum? sp., incomplete leaf MGUWr 2704p, uncertain locality, Assemblage 6 (lower–middle Santonian). E. Dryandroides quercinea Velenovský, 1883, incomplete leaf MB.Pb.2008/360, Bolesławiec, Assemblage 8 (lower–middle Santonian). F. Dalbergites sp., leaf MB.Pb.2018/0023, Żerkowice, Assemblage 5 (upper Coniacian?–lower Santonian?). G, I. Salicites petzeldianus Goeppert, 1844, leaves MB.Pb.2018/0054, 2018/0052, respectively, uncertain locality, Assemblage 8 (lower–middle Santonian). H. Dicotylophyllum sp. 1, leaf MB.Pb.2018/0076.1, Rakowice Małe, Assemblage 5 (upper Coniacian?–lower Santonian?), general view (H2) and enlargement of the margin (H1). J. Dicotylophyllum sp. 2, leaf MB.Pb.2018/0053, uncertain locality, Assemblage 8 (lower–middle Santonian).
Form group unknown
Genus Salicites (Hisinger, 1837) Halamski and Kvaček, 2015
Type: Salicites? wahlbergii (Nilsson, 1832) Hisinger, 1837 ≡ Phyllites (Salix?) wahlbergii Nilsson, 1832; Campanian, Upper Cretaceous; Köpinge, Scania, Sweden.
Remarks.—The emended diagnosis of Salicites was given by Halamski and Kvaček (2015: 122); the type species was revised by Halamski et al. (2016: 222).
Salicites petzeldianus Goeppert, 1844
Fig. 17G, I.
1844 Salicites Petzeldianus; Goeppert 1844: 220.
2015 Salicites petzeldianus Goepp. 1844; Halamski and Kvaček 2015: 122–123; text-fig. 14; pl. 10: 1, 4, 6–8.
Material.—Uncertain, Bolesławiec or Luisenhain, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0052.1–2, 0054.2–3.
Description.—Leaf simple, microphyll (ca. 3–6 cm long), petiolate. Blade attachment marginal, blade symmetrical, shape elliptic; base decurrent, asymmetric; apex rounded. Venation pattern pinnate, midvein stout; secondaries probably camptodromous, their attachment excurrent, spacing decreasing distally. Margin unlobed, untoothed.
Remarks.—The described material agrees with S. petzeldianus Goeppert, 1844, in the observable characters of shape (blade and base) and venation pattern. This generalised form may, in fact, correspond to more than one biological species.
Stratigraphic and geographic range.—Coniacian, Kłodzko region; Santonian, Lower Silesia.
Genus Dicotylophyllum auct.
Type: See discussion in Halamski (2013: 429–430).
Dicotylophyllum sp. 1
Fig. 17H.
Material.—Rakowice Małe, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb.2018/0076.1. Żerkowice, Assemblage 5, upper Coniacian?–lower Santonian?: MB.Pb. 2018/0058.
Description.—The better preserved specimen, MB.Pb.2018/ 0076.1 (Fig. 17H), is an incomplete microphyll leaf 26 mm long and 9 mm wide. Laminar shape approximately elliptic, blade medially symmetrical, base most probably rounded, apex straight. Primary venation pinnate; midvein stout, keeping approximately the same width from the base to the apex; secondaries excurrent, angle to midvein from 40–50° in the basal region to ca. 30° in apical region, densely spaced (ca. 9 per cm in the basal region, spacing weakly increasing distally), their course probably brochidodromous. Margin serrate, teeth ca. 9 per cm, regularly spaced, their proximal flank convex to straight, distal flank convex to concave. The other specimen is a leaf fragment with a similar margin.
Remarks.—This leaf differs from all other described herein in the tooth form. Dicotylophyllum sp. 3 sensu Halamski and Kvaček (2015) from the Coniacian of Krosnowice (Idzików beds) is similar in having closely spaced teeth, but ovate in shape.
Dicotylophyllum sp. 2
Fig. 17J.
Material.—Uncertain, Bolesławiec or Luisenhain, Assemblage 8, lower–middle Santonian: MB.Pb.2018/0053.
Description.—The single available leaf is incomplete, preserved length 44 mm, estimated length 80 mm?, preserved width 22 mm. Blade elliptic in shape, base straight, apex not preserved. Margin entire. Venation pinnate camptodromous: proximal secondaries decurrent, distal secondaries excurrent; poorly preserved tertiaries departing from midvein between the leaf base and the first secondaries, otherwise tertiaries not observable.
Remarks.—Dicotylophyllum sp. 2 differs from Cinnamomoides sp. in the basal venation pattern (the departure of the proximal secondaries is closer to the base in the latter) and from Salicites sp. in the variable attachment of secondaries (excurrent and decurrent within the same leaf) and greater length-to-width ratio.
Class Monocotyledoneae de Candolle, 1817
Order and family unknown
Genus Monocotylophyllum Reid and Chandler in Reid et al., 1926
Type: Not designated (see Andrews 1955: 190).
Monocotylophyllum sp.
Fig. 17A.
Material.—Uncertain, Bolesławiec or Luisenhain, Assemblage 8?, lower–middle Santonian: MGUWr 5639p.b.
Description.—The available material is a leaf fragment ca. 2.5 cm wide and ca. 3.5 cm long, suggestive of a linear leaf blade. It is uncertain whether the margin is preserved. Venation consisting of continuous parallel veins, ca. 10–17 per cm.
Remarks.—Monocotylophyllum is a form genus for monocot leaves not unlike in general form as those of Recent Acorus Linnaeus, 1753 (see Bogner and Mayo 1998: fig. 1C), Typha Linnaeus, 1753 (see Mikulska 1977: pl. 595: 2), or Iris Linnaeus, 1753 (see Skwirzyńska 1990: pls. 235, 238).
Palynology
Kingdom Alveolata Cavalier-Smith, 1981
Division Dinophyta Dillon, 1963 (= Dinoflagellata Bütschli, 1885)
Genus Chatangiella Vozzhennikova, 1967 emend. Lentin and Williams, 1976
Type: Chatangiella niiga Vozzhennikova, 1967; Santonian, Upper Cretaceous; river Romanicha, Khatanga Depression, West Siberia, Russia.
Chatangiella verrucosa (Manum, 1963) Lentin and Williams, 1976
Fig. 18M.
Remarks.—A single specimen found in sample 1338 from Rakowice Małe. Known from the Cenomanian–Turonian boundary to the late Campanian (Williams et al. 2004), from the middle Cretaceous according to Williams et al. (2017: 167).
Genus Dinogymnium Evitt, Clarke, and Verdier, 1967 emend. Lentin and Vozzhennikova, 1990
Type: Dinogymnium acuminatum Evitt, Clarke and Verdier, 1967; Uhalde Formation, Maastrichtian, Upper Cretaceous; Stanislaus County, California, USA.
Dinogymnium cretaceum (Deflandre, 1936) Evitt, Clarke, and Verdier, 1967
Fig. 18L.
Remarks.—Known from the Cenomanian (Boltenhagen 1980) to the Maastrichtian (M’Hamdi et al. 2015), “?Senonian” according to Williams et al. (2017: 273). Dinogymnium sp. (Fig. 18A) has been found in samples RK-4–110, 230.
Kingdom Plantae Linnaeus, 1753 sensu Cavalier-Smith (1981)
Division Marchantiophyta Stotler and Crandall-Stotler in Crandall-Stotler and Stotler, 2000
Genus Zlivisporis Pacltová, 1961
Type: Zlivisporis blanensis Pacltová, 1961; Turonian–Santonian, Upper Cretaceous; Zliv-Blana, South Bohemia, Czech Republic.
Zlivisporis simplex (Cookson and Dettmann, 1958) Braman, 2001
Fig. 18X.
Remarks.—Known from the Lower Cretaceous to the ?lowest Campanian (Braman 2001).

Fig. 18. Dinocysts (A, L–M), pollen (B–K, N–P, R–V, Y, CC), and spores (Q, W–X, Z–BB, DD–HH) from the North Sudetic Basin, Lower Silesia, Poland, Palynoassemblage A (upper Coniacian?–lower Santonian?). A. Dinogymnium sp., RK-4, depth 550–570 cm. B. Complexiopollis complicatus Góczán, 1964, RK-4, depth 130–150 cm. C. Complexiopollis sp., Żeliszów, depth 0–10 cm. D. Minorpollis minimus Krutzsch, 1959, RK-4, depth 90–110 cm. E. Plicapollis serta Pflug, 1953, RK-4, depth 90–110 cm. F. Trudopollis sp., four-pore form, RK-4, depth 90–110 cm. G. Interporopollenites turgidus Tschudy, 1975, RK-4, depth 110–130 cm. H. Pseudoplicapollis peneserta (Pflug, 1953) Krutzsch, 1967, Żeliszów, depth 230–250 cm. I. Neotriangulipollis sp., RK-4, depth 70–90 cm. J. Spinizonocolpites sp., RK-4, depth 90–110 cm. K. Cycadopites sp., RK-4, depth 110–130 cm. L. Dinogymnium cretaceum (Deflandre, 1936) Evitt, Clarke, and Verdier, 1967. RK-4, depth 90–110 cm. M. Chatangiella verrucosa (Manum, 1963) Lentin and Williams, 1976, Rakowice Małe, sample 1338. N. Pecakipollis sernoensis Pacltová and Krutzsch, 1970, Żeliszów, depth 150–170 cm. O. Triangulipollis sp., RK-4, depth 190–210 cm. P. Taxodiaceaepollenites hiatus (Potonié, 1931) Kremp, 1949, RK-4, depth 110–130 cm. Q. Echinatisporis varispinosus (Pocock, 1962) Srivastava, 1975, Żeliszów, depth 230–250 cm. R. Pecakipollis sp., RK-4, depth 110–130 cm. S. Trudopollis fossulotrudens (Pflug in Thomson and Pflug, 1953) Pflug, 1953, RK-4, depth 230–250 cm. T. Oculopollis sp., RK-4, depth 90–110 cm. U. Oculopollis sp. RK-4, depth 430–450 cm. V. Trudopollis sp., RK-4, depth 90–110 cm. W. Glecheniidites circiniidites (Cookson, 1953) Brenner, 1963, RK-4, depth 130–150 cm. X. Zlivisporis simplex (Cookson and Dettmann, 1958) Braman, 2001, Żeliszów, depth 210–230 cm. Y. Tricolporites sp., RK-4, depth 170–190 cm. Z. Appendicisporites foveolatus (Deák, 1962) Wingate, 1981, RK-4, depth 130–150 cm. AA. Appendicisporites appendicifer (Thiergart, 1942) Krutzsch, 1959. Incomplete spore from sample 1334, Rakowice Małe area. AB. Camarozonosporites insignis Norris, 1967, RK-4, depth 70–90 cm. AC. Classopollis cf. martinotii Reyre, 1970, RK-4, depth 130–150 cm. AD. Corrugatisporites sp., RK-4, depth 90–110 cm. AE. Verrucatosporites sp., RK-4, depth 550–570 cm. AF. Cyathidites minor Couper, 1953, RK-4, depth 130–150 cm. AG. Appendicisporites cristatus (Markova in Ivanova and Markova, 1961) Pocock, 1964, RK-4, depth 110–130 cm. AH. Biretisporites cf. deltoideus (Rouse, 1949) Dettmann, 1963, RK-4, depth 90–110 cm. AI. Corniculatisporites alekhinii (Bolkhovitina, 1953) Kuvaeva, 1972, Żeliszów, depth 70–90 cm.
Division Bryophyta Schimper, 1876 sensu stricto
Genus Cingutriletes Pierce, 1961 emend. Dettmann, 1963
Type: Cingutriletes clavus (Balme, 1957) Dettmann, 1963 ≡ Sphagnites clavus Balme, 1957; Donnybrook Sandstone, ?Lower Cretaceous; Murphy’s Shaft near Donnybrook, Perth Basin, Western Australia.
Cingutriletes clavus (Balme, 1957) Dettmann, 1963
Fig. 19A.
Remarks.—We use this genus as emended by Dettmann (1963) (see Jameossanaie 1987: 12).
Division Lycophyta Scott, 1909
Genus Camarozonosporites Pant ex Potonié, 1956 emend. Klaus, 1960
Type: Camarozonosporites cretaceus (Weyland and Krieger, 1953) Potonié, 1956 ≡ Rotaspora cretacea Weyland and Krieger, 1953; Carnian, Triassic; Eastern Alps, Austria.
Camarozonosporites insignis Norris, 1967
Fig. 18AB.
Remarks.—Known from the Lower Triassic (Song et al. 2000) to the Miocene (Barss et al. 1979). Representatives of Camarozonosporites Pant in Potonié, 1956, are spores of isosporous lycophytes (order Lycopodiales de Candolle ex Berchtold and Presl, 1820).
Genus Echinatisporis Krutzsch, 1959b
Type: Echinatisporis longechinus Krutzsch, 1959b; middle Eocene; Geiseltal, Saxony-Anhalt, Germany.
Echinatisporis varispinosus (Pocock, 1962) Srivastava, 1975
Fig. 18Q.
Remarks.—Known from the Berriasian (Ricketts and Sweet 1986) to the late Maastrichtian (Nichols et al. 1990). Another species from Palynoassemblage B is reported under open nomenclature as Echinatisporis sp. (Fig. 19B). Representatives of Echinatisporis Krutzsch, 1959b are microspores of heterosporous lycophytes belonging to the order Selaginellales Prantl, 1874.
Division Filicophyta Boureau, 1970
Class Leptosporangiatae von Goebel, 1881
Order Osmundales Link, 1833b
Family Osmundaceae Berchtold and Presl, 1820
Genus Biretisporites Delcourt and Sprumont, 1955 emend. Delcourt, Dettmann, and Hughes, 1963
Type: Biretisporites potoniaei Delcourt and Sprumont 1955; Wealdian, Lower Cretaceous; Hainaut, Belgium.
Biretisporites cf. deltoideus (Rouse, 1959) Dettmann, 1963
Fig. 18AH.
Remarks.—Known from the Albian (Brideaux 1968) to the Oligocene (Singh 1982).
Genus Baculatisporites Pflug and Thomson in Thomson and Pflug, 1953
Type: Baculatisporites primarius (Wolff, 1934) Thomson and Pflug, 1953 ≡ Sporites primarius Wolff, 1934; Pliocene, Freigericht mine near Dettingen a.M., Bavaria, Germany.
Baculatisporites parvopunctatus Weyland and Greifeld, 1953
Fig. 19C.
Remarks.—Reported from the Albian (Burger 1980) to the Eocene (Krutzsch 1959b). Spores belonging to Baculatisporites Thomson and Pflug, 1953 were probably produced by osmundaceous ferns.
Order Gleicheniales Link, 1833a
Family Gleicheniaceae Presl, 1825
Genus Glecheniidites Cookson, 1953 emend. Brenner, 1963
Type: Glecheniidites senonicus Ross, 1949; upper Santonian or lower Campanian, Upper Cretaceous; Åsen, Kristianstad Basin, Scania, Sweden (see Skarby 1964).
Glecheniidites circiniidites (Cookson, 1953) Brenner, 1963
Fig. 18W.
Remarks.—Found in sample RK-4, almost all depths. Reported from the Toarcian (Tchoumatchenco et al. 1992) to the Quaternary (Truswell 1987).
Genus Clavifera Bolkhovitina, 1953 emend. Bolkhovitina, 1966
Type: Clavifera triplex (Bolkhovitina, 1953) Bolkhovitina, 1966 ≡ Gleichenia triplex Bolkhovitina, 1953; Aptian, Lower Cretaceous; Moscow region, Russia.
Clavifera triplex (Bolkhovitina, 1953) Bolkhovitina, 1966
Fig. 19K.
Remarks.—Known from Cretaceous to Paleogene (Bolkhovitina 1966).
Genus Foveogleicheniidites Burger, 1976
Type: Foveogleicheniidites confossus (Hedlund, 1966) Burger in Norvick and Burger, 1976 ≡ Gleicheniidites confossus Hedlund, 1966; Woodbine Fm., Cenomanian, Upper Cretaceous; Bryan County, Oklahoma, USA.
Foveogleicheniidites sp.
Fig. 19O.
Remarks.—Representatives of Foveogleicheniidites Burger in Norvick and Burger, 1976 are known from the Barremian (Burger 1988) to the early Miocene (Macphail and Truswell 2004).
Order Schizaeales Schimper, 1869
Family Schizaeaceae Kaulfuss, 1827
Genus Appendicisporites Weyland and Krieger, 1953
Type: Appendicisporites tricuspidatus Weyland and Greifeld, 1953; Santonian, Upper Cretaceous; Aachen area, Nordrhein-Westfalen, Germany.
Appendicisporites appendicifer (Thiergart, 1942) Krutzsch, 1959b
Fig. 18AA.
Remarks.—Described from Rakowice Małe (Thiergart 1942), most probably from levels corresponding to our Assemblage 5 in which we have found, however, only fragmentary material of this characteristic species. The best specimen, illustrated herein, is from sample 1334. Slightly younger specimens (our Assemblages 6–8) were illustrated by Raniecka-Bobrowska (1984, 1989: pl. 205: 3) and by Grabowska (2003: pl. 28: 27). This species is rarely, but consistently, found in various Cenomanian deposits of the Bohemian Cretaceous Basin (Svobodová 1997) and by Góczán and Siegl-Farkas (1990) in Santonian or Campanian Oculopollis zaklinskaiae–Tetracolporopollenites (Brecolpites) globosus Zone of Hungary.
Appendicisporites foveolatus (Deák, 1962) Wingate, 1980
Fig. 18Z.
Remarks.—The oldest occurrences are from the upper Albian (Wingate 1980).
Appendicisporites cristatus (Markova in Ivanova and Markova, 1961) Pocock, 1964
Fig. 18AG.
Remarks.—Found in almost all samples from section RK-4. Known from the Lower Triassic (Song et al. 2000) to the Danian (Shugaevskaya 1978).
Genus Cicatricosisporites Potonié and Gelletich, 1933
Type: Cicatricosisporites dorogensis Potonié and Gelletich, 1933; Eocene, Dorog, Hungary.
Cicatricosisporites potomacensis Brenner, 1963
Fig. 19D.
Remarks.—Originally described from the Lower Cretaceous of Maryland (USA), but reported since from the Valanginian–Barremian of Israel (Brenner and Bickoff 1992), the Campanian (Srivastava 1975; Miki 1977) and even the Danian (Hargrove and Engelhardt 1997).
Cicatricosisporites dorogensis Potonié and Gelletich, 1933
Fig. 19N.
Remarks.—Originally described from the Eocene, but subsequently reported from the Valanginian–Barremian of Israel (Brenner and Bickoff 1992).
Genus Klukisporites Couper, 1958
Type: Klukisporites variegatus Couper, 1958; Lower Deltaic subseries, Bathonian, Middle Jurassic; Yorkshire, England.
Klukisporites pseudoreticulatus Couper, 1958
Fig. 19E.
Remarks.—Reported by Kemp (1970) from the Albian of Southern England (UK). The oldest occurrence might be in the Aalenian (Boulter and Windle 1993) or even the Toarcian (Goryacheva et al. 2018) and the youngest in the Campanian (Gies 1972).
Genus Distaltriangulisporites Singh, 1964 emend. Singh, 1971
Type: Distaltriangulisporites perplexus (Singh, 1964) Singh, 1971; Lower Cretaceous; Mannville, Alberta, Canada.
Distaltriangulisporites
perplexus (Singh, 1964)
Singh, 1971
Fig. 19G.
Remarks.—Originally described from the Lower Cretaceous of Alberta (Singh 1964); present in the same area in the Santonian–Campanian (Payenberg et al. 2002).
Genus Trilobosporites Pant ex Potonié, 1956
Type: Trilobosporites hannonicus (Delcourt and Sprumont, 1955) Potonié, 1956 ≡ Concavisporites hannonicus Delcourt and Sprumont, 1955; Wealdian, Lower Cretaceous; Hainaut, Belgium.
Trilobosporites sp.
Fig. 19F.
Remarks.—Representatives of this genus are reported from the Berriasian (Batten 1973) to the Santonian (Knauer and Siegl-Farkas 1992).
Genus Corniculatisporites Bolkhovitina, 1953 emend. Kuvaeva, 1972
Type: Corniculatisporites alekhinii (Bolkhovitina, 1953) Kuvaeva, 1972; Campanian, Upper Cretaceous; Ural Mts.
Corniculatisporites alekhinii (Bolkhovitina, 1953) Kuvaeva, 1972
Fig. 18AI.
Remarks.—A monolete schizaeaceous spore, originally described from Campanian strata of northern Urals, then by Kuvaeva (1972) from the upper Albian of Caucasus and Crimea and by Juhász (1977) from upper “Vraconian” (upper Albian to lower Cenomanian; Gignoux 1950; Czaplicka et al. 1968) of Bákony Mountains, Hungary.
Order Cyatheales Frank, 1877
Genus Cyathidites Couper, 1953
Type: Cyathidites australis Couper, 1953; Ohika beds, Jurassic; Buller Gorge, West Coast, New Zealand.
Cyathidites minor Couper, 1953
Fig. 18AF.
Remarks.—Originally described from Jurassic Ohika beds of New Zealand (Couper 1953: 28), known from the Upper Triassic to the Upper Cretaceous (Ziaja 2006: 13).
Order Polypodiales Link, 1833b
Genus Verrucatosporites Pflug and Thomson in Thomson and Pflug, 1953
Type: Verrucatosporites alienus (Potonié, 1931) Thomson and Pflug, 1953 ≡ Sporonites alienus Potonié, 1931; Oligocene to lower Miocene; Beisselsgrube, Ville, Nordrhein-Westfalen, Germany (Potonié 1931b).
Verrucatosporites sp.
Fig. 18AE.
Remarks.—Spores belonging to Verrucatosporites Pflug and Thomson in Thomson and Pflug, 1953, were mostly produced by representatives of Polypodiales (van Uffelen 1991).
Division unknown
Genus Corrugatisporites Weyland and Greifeld, 1953
Type: Corrugatisporites toratus Weyland and Greifeld, 1953; Santonian, Upper Cretaceous; Quedlinburg, Sachsen-Anhalt, Germany.
Corrugatisporites sp.
Fig. 18AD.
Remarks.—The botanical affinity of plants producing spores belonging to Corrugatisporites Weyland and Greifeld, 1953 is unknown.
Division Gymnospermae (Lindley, 1830) Prantl, 1874
Class Coniferae (Jussieu, 1789) Engler, 1892
Genus Classopollis Pflug, 1953
Type: Classopollis classoides Pflug, 1953; Lias, Siegelsum, Holstein, Germany.
Classopollis cf. martinotii Reyre, 1970
Fig. 18AC.
Remarks.—Pollen of representatives of Cheirolepidiaceae Takhtajan, 1963. Classopollis classoides Pflug, 1953, produced by species of Frenelopsis (Schenk, 1869) Reymanówna and Watson, 1976, was found in samples RK-4-90–130, 170, 350, 410, 430, 450, 490, 530, 570.
Genus Cerebropollenites Nilsson, 1958
Type: Cerebropollenites mesozoicus (Couper, 1958) Nilsson, 1958 ≡ Tsugaepollenites mesozoicus Couper, 1958; Upper Deltaic subseries, Bathonian, Middle Jurassic; Gristhorpe, Yorkshire, England.
Cerebropollenites mesozoicus (Couper, 1958) Nilsson, 1958
Fig. 19I.
Remarks.—Produced by representatives of Pinaceae Lindley, 1836 close to modern Tsuga Carrière, 1855 (Shang and Zavada 2003).
Genus Taxodiaceaepollenites (Potonié, 1931) Kremp ex Potonié, 1958
Type: Taxodiaceaepollenites hiatus (Potonié, 1931) Kremp ex Potonié, 1958 ≡ Pollenites hiatus Potonié, 1931; Oligocene to lower Miocene; Beisselsgrube, Ville, Nordrhein-Westfalen, Germany (Potonié 1931a).
Taxodiaceaepollenites hiatus (Potonié, 1931) Kremp ex Potonié, 1958
Fig. 18P.
Remarks.—Found in almost all samples, known from the Jurassic (Wang et al. 1984) to the Miocene (Weems and McCartan 1990).
Genus Podocarpidites Cookson, 1947b emend. Potonié, 1958
Type: Podocarpidites ellipticus Cookson, 1947b; Tertiary, Waterfall Gorge, Kerguelen Islands.
Remarks.—Podocarpidites was proposed by Cookson (1947b: 131) who did not selected a type species, which was first done by Couper (1953: 35). However, before 1958 proposing a genus name without indicating the type species did not invalidate the publication (ICN, Art. 40.1), so the spelling “Podocarpidites Cookson ex Couper” (e.g., Couper 1953: 35) is incorrect. Couper (1953) and Potonié (1958) emended the diagnosis.
Podocarpidites sp.
Fig. 19P.
Remarks.—Podocarpidites Cookson, 1947b, is a genus accommodating pollen of the Podocarpaceae Endlicher, 1847. Bisaccate pollen is very rare in Palynoassemblage A and infrequent in Palynoassemblage B.
Class Gnetopsida Thomé, 1886
Genus Ephedripites Bolkhovitina, 1953
Type: Ephedripites mediolobatus Bolkhovitina, 1953; Hauterivian, Lower Cretaceous; Kazakhstan.
Ephedripites virginiaensis Brenner, 1963
Fig. 19M.
Remarks.—Representatives of Ephedripites Bolkhovitina, 1953 are present in Palynoassemblages A and B; they were produced by species of Ephedraceae Dumortier, 1829 (Ruiz and Quattrochio 1997).
Class unknown
Genus Cycadopites Wodehouse, 1933
Type: Cycadopites follicularis Wilson and Webster 1946; Maastrichtian to Paleogene, Montana, USA.
Remarks.—Cycadopites was proposed by Wodehouse (1933) without selecting the type species, the material from the Eocene Green River Fm. at Parachute Creek, Colorado, being described as Cycadopites sp. The type species from the Fort Union Fm. of Montana was selected by Wilson and Webster (1946). However, similarly as in the case of Podocarpidites described above, the authorship of the genus should be described to Wodehouse (1933), following Andrews (1955: 141) not to “Wodehouse ex Wilson and Webster”, as done, among others, by Potonié (1958: 92) or Leffingwell (1970: 28).
Cycadopites sp.
Fig. 18K.
Remarks.—Cycadopites Wodehouse, 1933, is a pollen type that can be derived from Bennettitales, Ginkgoales, Cycadales, Pentoxylales, and some Peltaspermales (Zavialova and van Konijnenburg-van Cittert 2012: 15 and references therein). Found in almost all samples from the section RK-4.
Division Angiospermae Braun and Doell ex Doell, 1857
Class Dicotyledoneae de Candolle, 1817
Order Fagales Engler, 1892 sensu Soltis et al. (2018)
Normapolles group sensu Polette and Batten (2017)
Genus Complexiopollis Krutzsch, 1959a emend. Tschudy, 1973b
Type: Complexiopollis praeatumescens Krutzsch, 1959a; marls with Inoceramus labiatus, lower Turonian, Upper Cretaceous; Pirna, Saxony, Germany.
Remarks.—The original spelling of the epithet is praeatumescens (by reference to the “atumescens group”; Krutzsch 1959a: 135); the spelling praetumescens (e.g., Néraudeau et al. 2017) is incorrect.
Complexiopollis complicatus Góczán, 1964
Fig. 18B.
Remarks.—Reported from the upper Cenomanian (Kedves 1980; uncertain); otherwise known in the Oculopollis–Trilobosporites Dominance Zone (Góczán and Siegl-Farkas 1990) to the lower Campanian (Siegl-Farkas 1988). Another representative of this genus is reported under open nomenclature as Complexiopollis sp. (Fig. 18C). Four other species were found by Raniecka-Bobrowska (1984, 1989: pl. 216: 6–11, pl. 217: 1–2) in Palynoassemblage B.
Genus Emscheripollis Krutzsch, 1959a
Type: Emscheripollis inflatius Krutzsch, 1959a; Coniacian?, Upper Cretaceous; Weißwasser-Rietschen 19 borehole, Lusatia, Saxony, Germany (Krutzsch 1959a: 137, 139).
Emscheripollis sp.
Remarks.—Three taxa from Rakowice Małe belonging to Emscheripollis Krutzsch, 1959a were illustrated by Pacltová and Krutzsch (1970: pl. 101: 9–13, 28–32). Raniecka-Bobrowska (1984, 1989: pl. 217: 3–7) found these three taxa and two other species in Palynoassemblage B. Representatives of this genus are known from the Turonian (Krutzsch 1958) to the Maastrichtian (Olaru 1974).
Genus Interporopollenites Weyland and Krieger, 1953
Type: Interporopollenites proporus Weyland and Krieger, 1953; lower Campanian, Upper Cretaceous; Aachen area, Nordrhein-Westfalen, Germany.
Interporopollenites turgidus Tschudy, 1975
Fig. 18G.
Remarks.—Originally described from the Paleocene of Kentucky (USA), known in the Turonian and Santonian of Vendée, France (Azéma et al. 1981).
Genus Minorpollis Krutzsch, 1959a
Type: Minorpollis minimus Krutzsch, 1959a; Coniacian, Upper Cretaceous; Weisswasser-Rietschen, Lusatia, Saxony, Germany.
Minorpollis minimus Krutzsch, 1959a
Fig. 18D.
Remarks.—Found in almost all samples in the section RK-4 and in Palynoassemblage B (Raniecka-Bobrowska 1984, 1989: pl. 217: 8, 9). Known from the Coniacian (Góczán et al. 1967) to the Eocene (Krutzsch 1959a).
Genus Plicapollis Pflug, 1953
Type: Plicapollis serta Pflug, 1953; middle Senonian, Upper Cretaceous; Aachen area, Nordrhein-Westfalen, Germany.
Plicapollis serta Pflug, 1953
Fig. 18E.
Remarks.—Present in Palynoassemblages A and B (Raniecka-Bobrowska 1984, 1989: pl. 218: 4, 5). Known from the Coniacian (Krutzsch 1966a) to the Eocene (Góczán et al. 1967).
Genus Pseudoplicapollis Krutzsch in Góczán et al., 1967
Type: Pseudoplicapollis palaeocaenicus Krutzsch in Góczán et al., 1967; middle Paleocene, Wehmingen near Sarstedt, Lower Saxony, Germany.
Pseudoplicapollis peneserta (Pflug, 1953) Krutzsch, 1967
Fig. 18H.
Remarks.—Found in almost all samples in the section RK-4 and in Palynoassemblage B (Raniecka-Bobrowska 1984, 1989: pl. 218: 7). Known from the Coniacian (Hradecká et al. 1999) through the Santonian (Góczán and Siegl-Farkas 1990) to the Maastrichtian (Góczán et al. 1967).
Genus Neotriangulipollis Góczán, Groot and Krutzsch in Góczán et al., 1967
Type: Neotriangulipollis piolencensis Groot and Krutzsch in Góczán et al., 1967; Santonian, Upper Cretaceous; Piolenc, Vaucluse, southern France.
Neotriangulipollis sp.
Fig. 18I.
Remarks.—Representatives of Neotriangulipollis were known from the middle Santonian to the lower Campanian (Góczán et al. 1967). However, Néraudeau et al. (2017) recently reported a Neotriangulipollis co-occurring with Cenomanian–Turonian forms like Atlantopollis microreticulatus Krutzsch in Goczán et al., 1967 or Complexiopollis praeatumescens Krutzsch, 1959a.
Genus Pecakipollis Krutzsch and Pacltová in Góczán et al., 1967
Type: Pecakipollis bohemiensis Krutzsch and Pacltová in Góczán et al., 1967; Turonian–Santonian, Upper Cretaceous; borehole Pecák, Southern Bohemia, Czech Republic.
Pecakipollis sernoensis Pacltová and Krutzsch, 1970
Fig. 18N.
Remarks.—Two another species are reported under open nomenclature as Pecakipollis sp. (Fig. 18R) and aff. Pecakipollis sp. (Fig. 19J). Representatives of Pecakipollis possibly occur from the upper Coniacian (Góczán et al. 1967) to the middle Santonian (Pecák borehole, South Bohemian Basins; Pacltová 1981).
Genus Triangulipollis Krutzsch in Góczán et al., 1967
Type: Triangulipollis turonicus Krutzsch in Góczán et al., 1967; middle Turonian, Upper Cretaceous; Zeichen near Pirna, Saxony, Germany.
Triangulipollis sp.
Fig. 18O.
Remarks.—Representatives of Triangulipollis are known possibly from the Cenomanian (Durand and Louail 1976: 1721, under open nomenclature) and certainly from the Turonian (Batten 1989: 13) to the Paleocene (Mikhelis 1981).
Genus Trudopollis Pflug, 1953 emend. Krutzsch in Góczán et al., 1967
Type: Trudopollis pertrudens Pflug, 1953; Danian; Wehmingen near Sarstedt, Hannover, Germany.
Trudopollis fossulotrudens (Pflug in Thomson and Pflug, 1953) Pflug, 1953
Fig. 18S.
Remarks.—Known from the Coniacian to the Santonian.
Trudopollis sp.
Fig. 18F, V.
Remarks.—Species belonging to this genus are found in almost all samples in the section RK-4. We have found also atypical four-pored forms (Fig. 18F) similar to those reported by Pacltová (1981) from the Santonian of the borehole Pecák in the South Bohemian Basin. Representatives of Trudopollis Pflug, 1953, are known from the Turonian (Krutzsch 1958) to the Eocene–Oligocene boundary beds (Aleksandrova et al. 1985).
Genus Oculopollis Pflug, 1953
Type: Oculopollis concentus Pflug, 1953; middle Senonian, Upper Cretaceous; Aachen area, Nordrhein-Westfalen, Germany.
Oculopollis sp.
Fig. 18T, U.
Remarks.—Representatives of Oculopollis Pflug, 1953 are known from the Coniacian to the Paleocene.
Genus Piolencipollis Groot and Krutzsch in Góczán et al., 1967
Type: Piolencipollis piolencensis Groot and Krutzsch in Góczán et al., 1967; Santonian, Upper Cretaceous; Piolenc, Vaucluse, southern France.
Piolencipollis sp.
Fig. 19L.
Remarks.—Due to the mediocre quality of printing in Raniecka-Bobrowska (1984, 1989) we illustrate again a specimen from Palynoassemblage B. Representatives of the genus Piolencipollis are diagnostic for the Santonian.
Order unknown
Genus Tricolporites Cookson, 1947a
Type: Tricolporites sphaerica Cookson, 1947a; late Eocene–Miocene, Maryvale, Victoria, Australia.
Remarks.—We follow Stover and Partridge (1973: 258) and de Villiers and Cadman (1997: 90) in considering Tricolporites as a valid genus introduced by Cookson (1947a: 195; publication date September 1947). Alternatively it has been considered either as a valid genus introduced by Cookson (1947b: 134) with Tricolporites prolata Cookson, 1947b (Tertiary, Waterfall Gorge near Port Jeanne d’Arc, Kerguelen Islands) as the type (Cookson 1947b, publication date December 1947; Tschudy 1973a: 30), a genus invalidly proposed by Cookson (1947a, b) but validated by Srivastava (1972: 10) with T. prolata as the type species (Potonié 1960: 154, 1975: 116), or a nomenclaturally valid genus with T. sphaerica as the type species but synonymous with Rhoipites Wodehouse, 1933 (Kemp and Harris 1977: 36; Pocknall and Crosbie 1982: 7). Revision of this genus is out of scope of the present paper.
Tricolporites sp.
Fig. 18Y.
Remarks.—Tricolporites is a form genus of dicotyledonous pollen. Detailed investigation is often conducted using LM and SEM studies on the same specimen (Zetter 1989; Zetter et al. 2002).
Class Monocotyledoneae de Candolle, 1817
Order Arecales Bromhead, 1840
Family Palmae Jussieu, 1789
(= Arecaceae Berchtold and Presl, 1820)
Genus Spinizonocolpites Muller, 1968
Type: Spinizonocolpites echinatus Muller, 1968; Engkilili Formation, Cretaceous–Paleocene (after Muller 1968; upper Paleocene to middle Eocene according to Hutchinson 2005: 48–49 and references therein); Lupar river area, Sarawak, Borneo, Malaysia.
Remarks.—Spinizonocolpites is the original spelling (Muller 1968: 11) and must be retained; frequently used Spinozonocolpites (e.g., Graham 2011: 337) is incorrect.
Spinizonocolpites sp.
Fig. 18J.
Remarks.—The genus Spinizonocolpites is used for dispersed pollen of the palm genus Nypa Steck, 1757. Up to now, the oldest confirmed reports of Nypa were from the Maastrichtian (possibly upper Campanian; see review in McLoughlin et al. 2018).
Spermatophytes, division, class, order, or family unknown
Genus Catinipollis Krutzsch, 1966b
Type: Catinipollis geiseltalensis Krutzsch, 1966b; middle Eocene; Geiseltal, Sachsen-Anhalt, Germany.
Catinipollis lwowekensis Raniecka-Bobrowska, 1984
Fig. 19H.
Remarks.—This species was described from borehole KB-13, at a depth of 94.5 m. Due to the mediocre quality of print in the protologue we are reproducing two of the original photographs herein. The holotype is poorly preserved. We have not found any similar pollen in Palynoassemblage A. The only other species of this genus is the type species. The botanical affinities of these pollen grains are unknown (Krutzsch 1966b: 43–44).
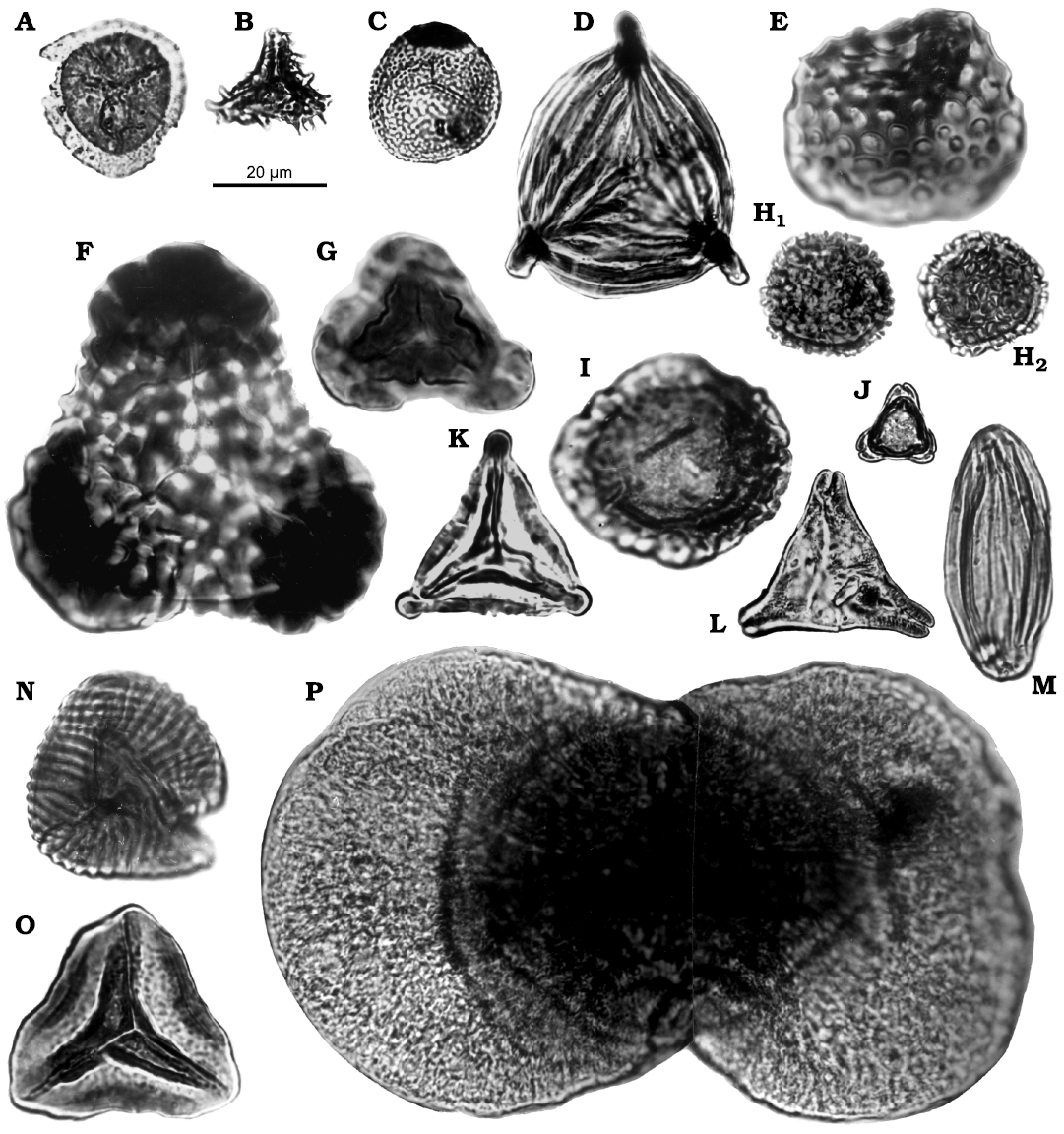
Fig. 19. Spores (A–G, K, N–O) and pollen (H–J, L–M, P) from the North Sudetic Basin, Lower Silesia, Poland, Palynoassemblage B (lower–middle Santonian). A. Cingutriletes clavus Dettmann, 1963, borehole KB18, depth 42.3 m. B. Echinatisporis sp., borehole KB18, depth 42.3 m. C. Baculatisporites parvopunctatus Weyland and Greifeld, 1953, borehole KB18, depth 29.8 m. D. Cicatricosisporites potomacensis Brenner, 1963, borehole KB13, depth 88.6 m. E. Klukisporites pseudoreticulatus Couper, 1958, borehole KB13, depth 94.5 m. F. Trilobosporites sp., borehole KB18, depth 38.3 m. G. Distaltriangulisporites perplexus (Singh, 1964) Singh, 1971, borehole KB13, depth 94.5 m. H. Catinipollis lwowekensis Raniecka-Bobrowska, 1984, holotype (slide 13/11, 48.5/110.2; repository unknown), borehole KB18, depth 35.0 m; equatorial focal plane (H1), focal plane on the surface (H2). I. Cerebropollenites mesozoicus (Couper, 1958) Nilsson, 1958, borehole KB17, depth 47.5 m. J. aff. Pecakipollis sp., borehole KB8, depth 77.5 mm. K. Clavifera cf. triplex (Bolkhovitina, 1953) Bolkhovitina, 1966, borehole KB13, depth 28.6 m. L. Piolencipollis sp., borehole KB16, depth 99 m; specimen illustrated by Raniecka-Bobrowska (1984, 1989: pl. 218: 2). M. Ephedripites virginiaensis Brenner, 1963, borehole KB18, depth 39.3 m. N. Cicatricosisporites dorogensis Potonié and Gelletich, 1933, borehole KB17, depth 38.0 m. O. Foveogleicheniidites sp., borehole KB18, depth 38.3 m. P. Podocarpidites sp., borehole KB8, depth 84.3 m. Photographs from the unpublished report by Raniecka-Bobrowska (1968).
Palaeoecology
Composition of the megaflora
Quantitative data on the fossil megaflora studied are given in Table 2. It should be noted that the assemblages as defined herein represent a wide spectrum of time-averaging (Behrensmeyer et al. 1992), were not sampled equally, and come from layers displaying different preservational potential, so only a general interpretation can be given.
The flora is dominated by angiosperms in terms of both taxa (19/29 ≈ 66%) and specimens (157/270 ≈ 58%). However, the most abundant single species is the conifer Geinitzia reichenbachii which accounts for 38.5% of the specimens of the studied material (Halamski and Kvaček 2019), whereas the second most abundant species, the dicot Dewalquea haldemiana, is represented by 17 specimens (6.3% of the material). G. reichenbachii must clearly have been common, even if the numerical value of its dominance may be positively biased by the fact that even small fragments of conifer twigs are identifiable, whereas similar-sized scraps of angiosperm leaves mostly are not. As has been commented several times already, fossil species G. reichenbachii may have corresponded to more than one biological species.
Out of 29 identified megafossil taxa, 13 are represented by single specimens and additional five by two specimens each. An elementary rarefaction analysis indicates that the original diversity is far from being sampled. As far as assemblages are concerned, four of them (Assemblages 1, 2, 3, 7) are represented by less than ten specimens, which means that their analysis can be made only in very general terms. Assemblages 4–6 and 8 are represented by numbers of specimens varying between 25 and 108.
Table 2. Summary of distribution of the studied plant species within the assemblages. Numbers in single cells are numbers of specimens. These are added within rows, representing the total number of specimens of a species and percentages referring to the total number of specimens (270), and within columns, representing the total number of specimens within an assemblage (percentages as above). The totals in bold in the last column indicate in how many assemblages a species is present; those in bold in the last row indicate of how many species an assemblage is composed; the percentages in italics refer to the total number of species (29).
|
Assemblages Taxa |
Turonian |
lower–middle |
upper Coniacian?–lower Santonian? |
(upper Coniacian? to) |
Total |
|||||
|
Ass. 1 |
Ass. 2 |
Ass. 3 |
Ass. 4 |
Ass. 5 |
Ass. 6 |
Ass. 7 |
Ass. 8 |
|||
|
Ferns |
Konijnenburgia cf. galleyi |
– |
– |
– |
6 |
– |
– |
– |
– |
1; 6 (2.2%) |
|
Protopteris punctata |
– |
– |
1 |
– |
– |
– |
– |
– |
1; 1 (0.4%) |
|
|
Protopteris singeri |
– |
– |
2 |
– |
– |
– |
– |
– |
1; 2 (0.7%) |
|
|
Cladophlebis sp. 1 |
– |
– |
– |
– |
1 |
– |
– |
– |
1; 1 (0.4%) |
|
|
Cladophlebis? sp. 2 |
– |
– |
– |
– |
1 |
– |
– |
– |
1; 1 (0.4%) |
|
|
Coniopteris? sp. |
– |
– |
– |
– |
1 |
– |
– |
– |
1; 1 (0.4%) |
|
|
Conifers |
Pinus longissima |
– |
1 |
– |
– |
– |
– |
– |
– |
1; 1 (0.4%) |
|
Protodammara sp. |
– |
– |
– |
– |
2 |
– |
– |
– |
1; 2 (0.7%) |
|
|
Geinitzia reichenbachii |
1 |
? |
– |
4 |
91 |
3 |
– |
5 |
5(6); 104 (38.5%) |
|
|
Geinitzia formosa |
– |
– |
– |
– |
6 |
– |
– |
– |
1; 6 (2.2%) |
|
|
Angiosperms |
Platananthus sp. |
– |
– |
– |
– |
1 |
– |
– |
– |
1; 1 (0.4%) |
|
Dryophyllum westerhausianum |
– |
– |
– |
– |
– |
19 |
– |
– |
1; 19 (7.0%) |
|
|
Dewalquea haldemiana |
– |
– |
– |
14 |
1 |
– |
1 |
1 |
4; 17 (6.3%) |
|
|
Dewalquea insignis |
– |
– |
– |
– |
– |
– |
– |
2 |
1; 2 (0.7%) |
|
|
Dewalquea aff. gelindenensis |
– |
– |
– |
– |
– |
– |
– |
3 |
1; 3 (1.1%) |
|
|
Dalbergites sp. |
– |
– |
– |
– |
2 |
– |
– |
– |
1; 1 (0.4%) |
|
|
Cinnamomoides sp. |
– |
– |
– |
2 |
– |
– |
– |
– |
1; 1 (0.4%) |
|
|
Ettingshausenia cf. superstes |
– |
5 |
– |
– |
– |
– |
1? |
– |
1(2); 6 (2.2%) |
|
|
Ettingshausenia sp. 1 |
– |
– |
– |
– |
– |
3 |
– |
? |
1(2); 3 (1.1%) |
|
|
Platanites willigeri |
– |
– |
– |
– |
– |
– |
– |
14 |
1; 14 (5.2%) |
|
|
Platanites sp. 1 |
– |
– |
– |
– |
– |
– |
2 |
– |
1; 2 (0.7%) |
|
|
Laurophyllum sp. |
– |
1 |
– |
– |
– |
4 |
– |
9 |
3; 14 (5.2%) |
|
|
Araliaephyllum? sp. |
– |
– |
– |
– |
– |
– |
– |
1 ? |
1; 1 (0.4%) |
|
|
Dryandroides quercinea |
– |
– |
– |
– |
– |
– |
– |
1 |
1; 1 (0.4%) |
|
|
Ternstroemites? sp. |
– |
– |
– |
– |
– |
– |
– |
1 |
1; 1 (0.4%) |
|
|
Salicites petzeldianus |
– |
– |
– |
– |
– |
– |
– |
4 |
1; 4 (1.5%) |
|
|
Dicotylophyllum sp. 1 |
– |
– |
– |
– |
2 |
– |
– |
– |
1; 2 (0.7%) |
|
|
Dicotylophyllum sp. 2 |
– |
– |
– |
– |
– |
– |
– |
1 |
1; 1 (0.4%) |
|
|
Monocotylophyllum sp. |
– |
– |
– |
– |
– |
– |
– |
1 |
1; 1 (0.4%) |
|
|
Total |
1 (3%) 1 (0.4%) |
3(4) (14%) 7 (2.6%) |
2 (7%) 4 (1.5%) |
4 (17%) 25 (9.6%) |
8 (28%) 108 (40%) |
4 (14%) 29 (10.7%) |
2 (4%) 4 (1.5%) |
10(11) (34%) 44 (15.9%) |
29 270 (100%) |
|
General characteristics of the mesofossil assemblage
Mesofossils were recovered solely from Assemblage 5 at Rakowice Małe and from one layer (150–200 cm) at Żeliszów. Mesoassemblages at both localities are different, the former being dominated by Normapolles fruits and corresponding to alluvial plain and “upland” vegetation, the latter being rich in conifers and representing a swamp forest (ZH, JK, ATH, and J. Dašková, unpublished material).
The fruits belonging to the Normapolles complex are small-sized reproductive structures, interpreted as corresponding mostly to extinct fagalean dicots (Friis et al. 2006, 2011, and references therein), are an important part of Late Cretaceous floras of the Northern hemisphere. From Rakowice Małe locality nine species of Normapolles affinity are currently known (enumeration based both on specimens collected during the present study and by previous authors): Calathiocarpus octocostatus (Knobloch, 1970) Knobloch and Mai, 1986, Walbeckia guttaeformis (Knobloch, 1970) Knobloch and Mai, 1986, Caryanthus communis Knobloch and Mai, 1986, Caryanthus trebecensis Knobloch and Mai, 1983, Caryanthus triasseris (Knobloch, 1964) Knobloch and Mai, 1986, Caryanthus sp., Zlivifructus microtriasseris (Knobloch and Mai, 1986) Heřmanová, Kvaček and Halamski in Heřmanová et al., 2019, Zlivifructus vachae Heřmanová, Dašková, and Kvaček in Heřmanová et al., 2017, and one unknown taxon. All of them represent small flowers and fruits. The flowers are bisexual, bisymmetrical, and have an inferior ovary with a hypanthium. The tepals are attached on top of the hypanthium. A detailed reinterpretation of Zlivifructus microtriasseris was given by Heřmanová et al. (2019). Unfortunately the present authors did not succeed in finding the most productive layer sampled by Ervín Knobloch with more diverse Normapolles inflorescences and lycophyte megaspores Ricinospora sp. (ZH, JK, ATH, and J. Dašková, unpublished material).
The Żeliszów locality provided mesofossils mostly in form of coniferous shoots (Cunninghamites cf. squamosus Heer, 1871, Geinitzia formosa and seeds (Alapaya sp., Seletya sp.). Angiosperm mesofossils were represented by reproductive units of plants belonging to the Normapolles group, either with a single bract bearing three male flowers or with female unisexual flowers. A cluster of Minorpollis pollen, probably a fragment of an anther, was found in a staminate flower. Among other mesofossils may be noted: rare clitellate cocoons assigned to Dictyothylakos sp. and and the problematic mesofossil Costatheca striata (Dijkstra, 1949) Hall, 1967.
Microflora
General characteristics of the palynoassemblages.—In a very general way the palynoassemblages investigated both by the present authors and by Raniecka-Bobrowska (1968) may be described as rich. The Normapolles group is represented by numerous taxa, which is quite typical for Central Europe, particularly in the Santonian–Campanian which is a period of great diversity for this grouping (Friis et al. 2011: 339). Fern spores are another abundantly represented group, the Gleicheniaceae especially in Palynoassemblage A and the Schizeaceae especially in Palynoassemblage B (Raniecka-Bobrowska 1984: 463). In Palynoassemblage A dinocysts and prasinophytes are relatively rare compared to non-marine palynomorphs; Raniecka-Bobrowska (1968) does not provide data on marine palynoflora. Both palynoassemblages contain reworked spores: in the Palynoassemblage B Raniecka-Bobrowska (1968) identified Carboniferous megaspores Cingulizonates radiatus Dybová and Jachowicz, 1957, and a representative of “Hymenozonosporites” (nomen nudum; see Potonié 1956: 66), as well as Triassic to Liassic Zebrasporites cf. interscriptus (Thiergart, 1949) Klaus, 1960. The presence of reworked sporomorphs was noted also in Palynoassemblage A, but they have not been documented in detail.
Palynoassemblage A.—From the Assemblage 5 we recovered 126 taxa of palynomorphs (see Appendix 3) and moreover two species of foraminiferal linings. They can be divided as follows: fungal spores, 2 taxa; land plants, 105 palynotaxa (spores, 54 palynotaxa; gymnosperm pollen, 16 palynotaxa; angiosperm pollen, 35 palynotaxa); non-marine algae, 4 palynotaxa; marine organisms, 15 palynotaxa (dinocysts, 11 palynotaxa; prasinophytes, 4 palynotaxa).
No significant vertical variation among successive samples was noted (Fig. 20). In particular, in RK-4 there was no overall trend of either increasing or decreasing salinity, as samples with no marine palynomorphs occur in all parts of the analysed section. In terms of terrestrial palynomorphs, the samples from Rakowice Małe contain more taxa than those from Żeliszów.
Quantitative data for two samples illustrate the typical composition of the palynoassemblage: Rakowice Małe (MB.Pb.2018/0005): fern spores 30%, gymnosperm pollen 30%, angiosperm pollen 32%, dinoflagellate cysts and foraminifers 8%; Ołdrzychów (MGUWr 5638p): fern and bryophyte spores 36%, Taxodiaceaepollenites hiatus (the only gymnosperm present) 27%, Normapolles 27%, dinoflagellate cysts and foraminifers 10%.
The Palynoassemblage A belongs to the Oculopollis–Complexiopollis Dominance Zone, encompassing the Coniacian and early Santonian (Siegl-Farkas and Wagreich 1996: 131).
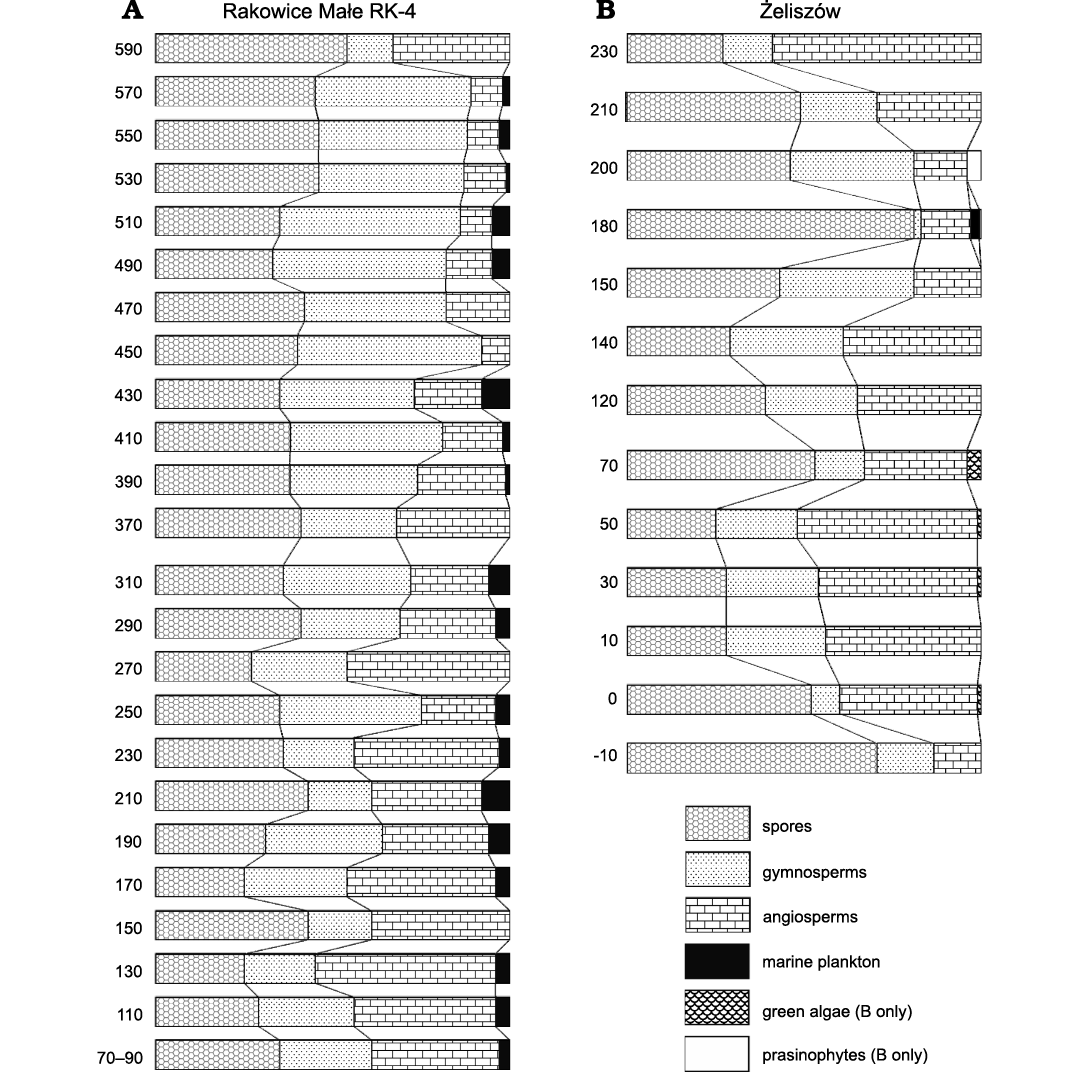
Fig. 20. Relative proportions of palynomorphs groups in the section RK-4, Rakowice Małe (A) and Żeliszów (B). Numbers on the left-hand side of the diagrams refer to distances (in cm) of samples from reference levels (see text for further explanation).
Marine microflora from the Palynoassemblage A.—Marine palynomorphs are rather rare, both in terms of taxa (15 species) and of specimens, the latter accounting for up to 10% of assemblages (see above), but it may be that dinocysts are altogether absent (MB.Pb.2008/0362; RK-4-150, 370, 470, 590). If present, dinoflagellate cysts are mostly broken. At Żeliszów, only a single dinocyst was found (Subtilisphaera sp., depth 180 cm); foraminifer linings and prasinophytes occur only at a depth of 180–200 cm and are otherwise absent.
Peridinioid dinocysts Alterbia, Chatangiella, Isabelidinium, Spinidinium, and Subtilisphaera as well as the gonyaulacoid Spiniferites tend to occur in water that is eutrophic because of significant nutrient supply due to either upwelling or terrigenous input (Wall et al. 1977; Arai and Viviers 2013). Dinogymnium is an estuarine genus (May 1977). The species Spiniferites ramosus is known in recent environments from regions where upper water salinity can be reduced permanently, or seasonally, by river discharge or snow melt. Open sea species, like Surculosphaeridium longifurcatum, are rare.
Among prasinophycean algae, Crassosphaera and Tasmanites are characteristic for marine environments. On the contrary, representatives of Leiosphaeridia and Pterospermella belong to so-called “disaster species” (Tappan 1980) able to thrive under conditions hazardous to other species (Guy-Ohlson 1996), such as reduced salinity.
To sum up, marine influence during the time of deposition of the upper part of the Nowogrodziec Member was relatively limited, more so at Żeliszów due to its greater distance from the open sea (see palaeogeography in Fig. 1C).
Land microflora of Palynoassemblages A and B.—The global compositions of the land microflora from Palynoassemblages A and B are similar. The relative ratios of spores vs. pollen taxa are identical (in both cases spores 54%, pollen 44%; see Raniecka-Bobrowska 1984). The ratios of gymnosperm to angiosperm pollen are also close: gymnosperms 31%, angiosperms 69% in Palynoassemblage A, one third to two thirds in Palynoassemblage B (Raniecka-Bobrowska 1984). This may be interpreted as testifying to a relative overall stability of regional vegetation pattern during the later half of the period studied herein, even if the megafloral record contains plant fossils corresponding to various single palaeocommunities.
In Palynoassemblage A, among the 105 palynotaxa corresponding to land plants (Appendix 3), the following ones are the most frequent: spores (Costatoperforosporites cf. foveolatus, Cyathidites minor, Deltoidospora sp., Dictyophyllidites harrisii, Gleicheniidites apilobatus, G. circiniidites, G. senonicus, Laevigatosporites ovatus, Stereisporites antiquasporites, Stereisporites sp.), gymnosperm pollen (Cycadopites sp., Taxodiaceaepollenites hiatus, Taxodiaceaepollenites sp.), angiosperm pollen (Complexiopollis sp., Minorpollis minimus, Pecakipollis sp., Plicapollis serta, Plicapollis sp., Pseudoplicapollis peneserta, Retitricolporites sp., Tricolpites sp., Trudopollis sp., Vacuopollis sp.).
From the Palynoassemblage B, Raniecka-Bobrowska (1968) assembled data on occurrences of over 700 morphotypes but they are not always identified, some are reworked (see above), and some are probably duplicated. In total 153 taxa of palynomorphs are sufficiently common to be included in a synoptic table. Taxa in common between Palynoassemblages A and B include: Appendicisporites appendicifer, Gleicheiniidites senonicus, Minorpollis minimus, and Pseudoplicapollis peneserta. Notable taxa absent in Palynoassemblage A are, among others: Trilobozonosporites exoticus Pacltová, 1961, Foveasporites budejovicensis (Pacltová, 1961) Pacltová and Krutzsch, 1970, Taurocusporites europaeus Krutzsch, 1973, Densoisporites velatus Weyland and Krieger, 1953, Podocarpidites sp., Semioculopollis praedicatus (Weyland and Krieger, 1953) Krutzsch in Góczán et al., 1967 and Piolencipollis sp. (Raniecka-Bobrowska 1984, 1989). Palynoassemblage B is richer in gymnosperm pollen in the lower part and in angiosperm pollen in the upper part (Raniecka-Bobrowska 1968: 27–28).
According to Krutzsch (1966a: 97), the “Löwenberger Bild” is characterised by the presence of Normapolles genera Pecakipollis, Papillopollis, Pseudotrudopollis, Extratriporopollenites, Capipollis, Santonipollis, and ?Magnoporopollis. Out of these seven genera, only representatives of Pecakipollis were found during the present investigation. However, in the investigated material from Palynoassemblage A we found numerous representatives of Trudopollis, Complexiopollis, Interporopollenites, and Neotriangulipollis cf. piolencipollis. We have no satisfactory explanation for such a marked difference in the composition of the Normapolles group from the same strata.
Comparisons.—According to Raniecka-Bobrowska (1968), Santonian Palynoassemblage B is very similar but richer than that described by Weyland and Greifeld (1953) from coeval strata from Quedlinburg. There are also similarities with Turonian–Santonian palynofloras from Bohemia (Pacltová 1961), but in this case differences are also notable: several representatives of Cicatricosisporites, Appendicisporites, and Gleicheniidites common in Lower Silesia are absent in Bohemia; in contrast, Papillopollis and Santonipollis occur in Bohemia, but are not known from Lower Silesia.
A Turonian–Santonian palynoflora from the Klikov Formation at Gmünd (Austria) was analysed by Zetter et al. (2002). This palynoassemblage is dominated by angiosperms (65 taxa, 60%), mainly by the Normapolles group (37%); bryophyte and pteridophyte spores are also numerous (25 taxa, 30%), whereas gymnosperms are rare (10%). The general aspect of the palynoassemblage in which Normapolles and ferns are the major groups is similar; there are, however, many differences in details: Araucariacidites is the most common gymnosperm at Gmünd, whereas it is absent in Lower Silesia; sulcate angiosperm pollen grains are more diverse at Gmünd.
Differences with a Santonian palynoassemblage from Romania, consisting of 45 pteridophyte, 8 gymnosperm, and 13 angiosperm taxa (Ţabără and Slimani 2019), are notable, even if a detailed comparison is hampered by the fact that many palynomorphs are identified only at genus level. The only species in common is Cicatricosisporites dorogensis. There are numerous representatives of Vadaszisporites Deák and Combaz, 1967 which is entirely absent in Lower Silesia.
Compared to slightly older (Coniacian) assemblages of Idzików (lower Idzików beds; Kvaček et al. 2015) and Březno (Halamski et al. 2018), Palynoassemblage A studied herein is characterised by fewer, less well preserved, and less diverse marine elements, but land plant palynomorph assemblages are globally similar. The presence of Pecakipollis and Neotriangulipollis, absent at Idzików and Březno, should, however, be noted. All in all, the microfossil record analysed herein should be understood as corresponding to broadly similar plant palaeocommunities preserved in marginal marine environment, as opposed to a fully marine setting represented by the Idzików and Březno beds.
An important difference from the slightly younger (Oculopollis zaklinskaiae–Tetracolporopollenites globosus Zone, late Santonian) palynoassemblage from Iharkút (Hungary) is that in the latter ferns are only a minor component (Bodor and Baranyi 2012). Overall, however, the palaeocommunity reconstructed as a Normapolles-related forest with herbaceous angiosperm and fern-dominated undergrowth (Siegl-Farkas and Wagreich 1996; Bodor and Baranyi 2012; Botfalvai et al. 2016) is of the same type as in Lower Silesia. Complexiopollis complicatus is a species in common. Late Santonian palynoassemblages from Bulgaria (Pavlishina et al. 2019) differ in the presence of numerous Krutzschipollis and Vadaszisporites grains.
Reconstruction of palaeocommunities
Palaeoecological studies are based on studies of museum specimens, freshly collected mega-, meso-, and microfloristic material, as well as on geological observations in the field. The available information allows a general scheme of plant palaeocommunities to be reconstructed.
Turonian and early to middle Coniacian.—The available plant material belonging to the Assemblages 1–3 is scarce and, as said above, can be interpreted only in very general terms.
The single Turonian plant in the studied material is Geinitzia reichenbachii. It may be noted that this species is also present in a small assemblage of Turonian plants known from the Opole Basin (Goeppert 1866b: 644; Płachno et al. 2018: 164). It is impossible to say whether it grew in the same type of community as in the Assemblage 5 (see below).
As far as the Assemblages 2 and 3 (treated jointly for the purpose of palaeoecological interpretation) are concerned, the notable feature is the presence of Pinus longissima and of two species of Protopteris, otherwise absent in the studied material. The tree ferns are an element of relatively well understood hygrophilous vegetation (Kvaček 2017: 45) On the other hand, the pine probably dwelt in an upland area (Kvaček 2017: 52), the vegetation of which is usually only imprefectly represented in the fossil record. This means that the Assemblages, as presently constituted, are heterogenous, i.e. consisting of a taphonomically mixed land plants having lived in different environments. For palynological record of upland vegetation, see below.
Late Coniacian?–early Santonian?—According to Leszczyński (2010), the depositional environment of the basal part of the Nowogrodziec Member (Assemblage 4 herein) was a lake, whereas that of the remaining part (Assemblage 5 herein) showed some marine influence, either as a marsh, a lagoon, or a (brackish) sea embayment. Fossil plant assemblages preserved in these two different sedimentary environments are different as well.
The most common plants of the Assemblage 4 are the fern Konijnenburgia and the angiosperm Dewalquea haldemiana. Geinitzia reichenbachii is relatively rare here (only four specimens out of 26, compared to 91 out of 108 in Assemblage 5). Dewalquea haldemiana is most often found in marine sediments (Hosius and von der Marck 1880; Halamski 2013; and especially Halamski et al. 2016) and may have been a species growing on coastal dunes (see Krassilov 2003: 94 for an analogous interpretation of single-species accumulations of Debeya pachyderma). Konijnenburgia, possessing a strongly cutinised epidermis and large indusia, is interpreted to have been a xerophytic fern living in an open habitat known as a fern savanna (Crane 1987: 124; fern steppe sensu Kvaček 2017: 46, see also Batten 1974; fern prairie sensu Friis et al. 2011: 468; see Krassilov 2003: 94 for an opposite view), that is, an open palaeocommunity dominated by ferns (see also below). Konijnenburgia may have grown both in a fern savanna and on dunes.
Assemblage 5 represents back and coast swamps, in some cases coal-forming. It is dominated by cupressoid conifers Geinitzia, Protodammara, Alapaya, and abundant ferns (Cladophlebis, Coniopteris) and lycophytes. Such communities are relatively well known in the fossil record; they have been reported from the Cenomanian of western France (Peyrot et al. 2019), from the Coniacian Idzików beds (Kvaček et al. 2015) and Březno Formation (Halamski et al. 2018a), and from the Campanian of Grünbach (Herman and Kvaček 2010) and Utah (Parker 1976). It is worth noting that Konijnenburgia, known in Assemblage 4, is absent. This conifer–fern–lycophyte palaeocommunity is also well represented in the palynoassemblage, with Taxodiaceaepollenites (the nearest living relatives thereof being the swamp trees Taxodium and Glyptostrobus), Cycadopites (pollen of cycads from lowland freshwater swamps, possibly drought tolerant; Abbink 1998; Barrón et al. 2006: 195), Biretisporites and Dictyophyllidites (two fern spore genera characteristic for swamps; Hampson et al. 2017), as well as Stereisporites (spores of the peat moss Sphagnum). Among non-marine algae, Ovoidites parvus dwelt in open waters of peat marshes.
However, several palynomorphs from Palynoassemblage A are suggestive of a different environment, not recorded in the megafossil record, namely salt-tolerant mangrove vegetation (Uličný et al. 1997; Kvaček 2000; Kvaček et al. 2006). This is especially evident for Classopollis classoides (pollen of cheirolepidiaceous Frenelopsis; Abbink 1998; Batten and MacLennan 1984; Riding et al. 2013), but several fern spore genera may have been produced by plants dwelling in this environment (Deltoidospora and several spores of schizeaceaous ferns; see also the situation described by Polette et al. 2018: 219). Megafossil remains from such communities were recorded from the Coniacian of the Idzików beds (Kvaček et al. 2015) and Březno Formation (Halamski et al. 2018a: 137).
It is well known that the mangrove-palm Nypa has been forming this kind of vegetal communities since the latest Cretaceous. The oldest known true mangroves were those formed by Nypa (Maastrichtian or possibly latest Campanian; El-Soughier et al. 2011; McLoughlin et al. 2018 and references therein) and by Palaeowetherellia (Euphorbiaceae; Maastrichtian, Egypt; Mazer and Tiffney 1982). The presumed Santonian mangroves from Scania were revealed to be based on a misinterpretation (McLoughlin et al. 2018), but palms are known already from Turonian swamps (Coiffard and Gomez 2010: 166). The presence of Spinizonocolpites sp. in the studied material is suggestive of the existence of Nypa (possibly in association with the mangrove-forming conifer Frenelopsis) in the Coniacian–Santonian of Lower Silesia, but at present only a single pollen grain has been found, so such an inference must be treated as unconfirmed.
The mesofossil record of Normapolles plants (Caryanthus, Zlivifructus) might correspond to forests occurring in well drained parts of aluvial plains and on the lower parts of slopes, with schizaeaceous ferns (Cicatricosisporites, Appendicisporites) forming the understory. It might have been tempting to associate the fagalean foliage Dryophyllum with Normapolles-producing trees, but the association of Normapolles with riparian settings has never been noted before.
The upland flora growing on slopes and further inland is preserved only fragmentarily. This palaeocommunity is tentatively reconstructed as a fern savanna with the matoniaceous fern Konijnenburgia (macrorests in Assemblage 4), co-occurring with producers of Ephedripites pollen and of matoniaceous and schizaeaceous thick-walled spores. Fern savannas with matoniaceous ferns are known from the English Wealden (Phlebopteris, Harris 1981) and from the Barremian of Europe (Coiffard et al. 2007, de la Fuente and Zetter 2016, Martín-Closas et al. 2016); matoniaceous and other drought-tolerant ferns inhabited xeric habitats in the Cenomanian of western France (Peyrot et al. 2019). It is uncertain whether fern savannas were replaced by other communities already in the Cretaceous (Crane et al. 1987: 124) or persisted into the Paleocene (Coe et al. 1987: 136). In the Lower Silesian Cretaceous there may have been patches of Pinus woodlands (macrorests in Assemblage 2). It must be noted that given the relatively small size of the island and the presumably subdued topography, “uplands” should be understand more in relative than in absolute way.
Early–middle Santonian.—The sandstone, mudstone, and claystone beds and rare coal seams of the Czerna Formation are interpreted as having been deposited in a mosaic of deltaic plains, alluvial plains, wetlands, and shallow lakes (Górniak 1986). The preservation of compound leaves with articulated leaflets is a strong argument for pre-burial transport having been relatively short in Assemblage 8 (Figs. 10F, 12) and Assemblage 6 (Figs. 8C–E, 9B, C).
Assemblage 6 is preserved in sandstone. They may contain a brackish macrofauna, although the investigated material was collected in a period when sedimentological details were not recorded, so the precise relationships of the fauna- and flora-bearing beds is not known. Sandstone may represent either deltaic or river channel deposits, so Assemblage 6 should be interpreted as representing trees forming riparian forests (Fig. 21), possibly on relatively coarse clastic substrates. It may be that the tree ferns Protopteris (known from Assemblage 3) may have formed a part of this assemblage as well. Betulaceae-like trees-dominated forests with diversified ferns in the understory were present in the Maastrichtian of Isona (Spain; Villalba-Breva et al. 2015; Marmi et al. 2016).
Assemblage 7 is very small in terms of the number of specimens it contains, but a meaningful interpretation is possible in the context of Assemblage 6 and, to a lesser extent, Assemblage 8. The absence of Platanites sp. 1 from Assemblages 6 (preserved in a similar lithology) and 8 is a hint of local variation among the palaeocommunities of the deltaic-alluvial plain of the investigated area. Possibly this species belonged to a different variant of riparian forests (Fig. 21) than that recorded in Assemblage 6. Given the position of the locality Nowe Jaroszowice in the central part of the marine embayment (Fig. 1C), as compared with that of Bolesławiec, it may be that the Platanites willigeri forest occupied a more humid setting, but a levee cannot be excluded either.
Assemblage 8 is preserved in claystone. Mass accumulation of leaves and subcomplete compound leaves are suggestive of parautochthonous character of this assemblage (Figs. 11A, 12A–C). In this case the original community may be hypothetically reconstructed to have dwelt in marginal parts of the alluvial plain (Górniak 1986: 128), for example in lakes having only limited contact with the marine embayment (Alexandrowicz 1976: 193). However, it is difficult to say whether the actual habitat of Assemblage 8 was drier or wetter than that of Assemblage 6.
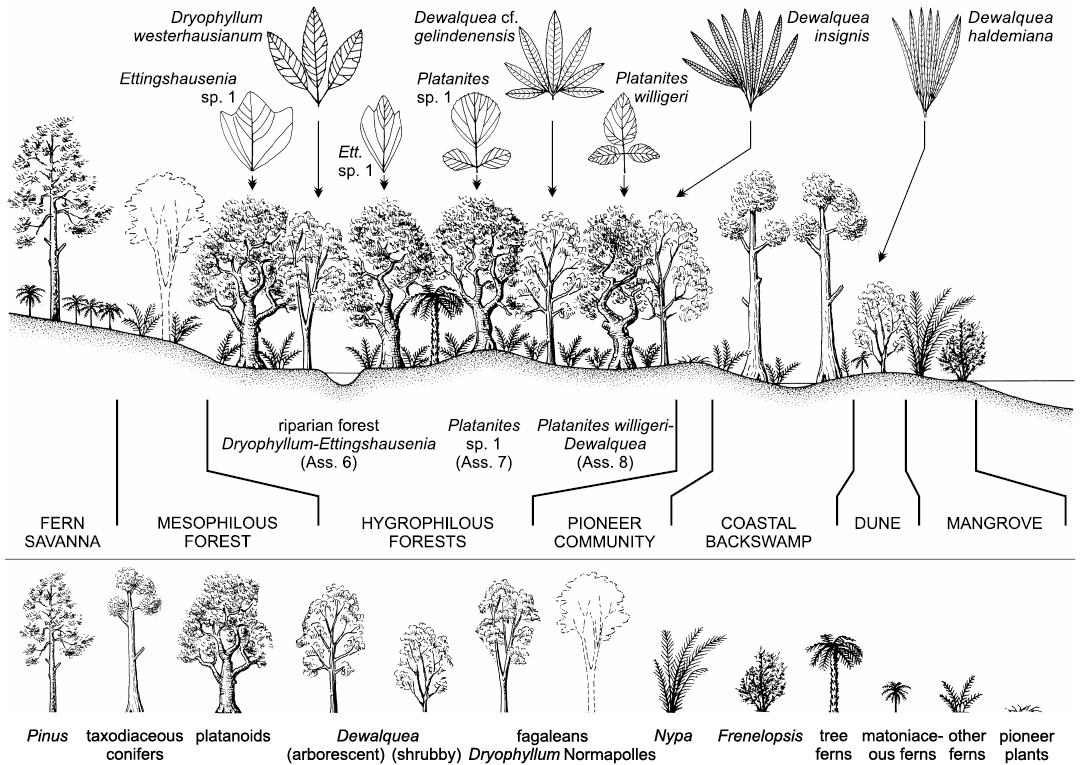
Fig. 21. Hypothetical reconstruction of vegetal palaeocommunities in the Coniacian–Santonian of Lower Silesia (palaeocatena sensu Krassilov 2003: 65). Among fagaleans, hygrophilous Dryophyllum preserved in the megafossil record (pollen unknown, even if possibly belonging to the Normapolles group) is distinguished from presumably mesophilous Normapolles trees known only from the microfossil record (dashed line, megafossils unknown). See text for further explanations. Drawings by Bogusław Waksmundzki. Abbreviations: Ass., Assemblage; Ett., Ettingshausenia.
General character of the communities.—The abundance and diversity of ferns, a group represented mostly in the palynological record, is a feature of the studied plant assemblages not found in today’s world. A similar situation was noted by Wing et al. (2012) in an upper Campanian site in Wyoming (USA) and interpreted as a palaeocommunity intermediate in general character between pre-angiosperm and modern vegetation. In other words, the so-called Cretaceous Terrestrial Revolution (Lloyd et al. 2008) had still not been completed.
However, the abundance and diversity of angiosperms in mega-, meso-, and microfossil records are unlike the situation in Wyoming where flowering plants were presumably growing only in patches and occupying mostly disturbed habitats (Wing et al. 2012). At least some Cretaceous Lower Silesian forests must have been angiosperm-dominated.
It is also worth noting that both in Lower Silesia and in Wyoming the most abundant ferns belong to the early-diverging orders Gleicheniales and Schizaeales, whereas the Polypodiales are much less frequent. This situation corresponds to a period before, or at the very beginning of, the major radiation of modern ferns (Schneider et al. 2004; Schuettpelz and Pryer 2009). It may be asked whether these ferns were already adapted to low-level light intensity in closed-canopy forests through the acquisition of the neochrome (Li et al. 2014; see also Watkins and Cardelús 2012), but the dating of this event is poorly constrained and the data at hand are insufficient to estimate the ecological form of the Cretaceous forests of Lower Silesia.
The aquatic communities, described for example in the Cretaceous of southern France (e.g., de Saporta 1890; Samuel and Gaillard 1984) or Spain (Marmi et al. 2016), are represented in Lower Silesia by lycophyte megaspores Ricinospora sp. (ZH, JK, ATH, and J. Dašková, unpublished material).
Comparison of mega-, meso-, and microfossil records
The megafossil assemblage from the North Sudetic Basin consists of 29 taxa and the mesofossil flora of 14 taxa (ZH, JK, ATH, and J. Dašková, unpublished material). Palynoassemblage A is composed of 105 taxa of land plants, over three times more than the number of megafossil taxa, and if pollen and spores known from the Palynoassemblage B are added, even more.
A meaningful comparison of the three kinds of plant fossil record can be done in a twofold way. First, comparison may bear on a single assemblage (palaeocommunity), which is the most obvious approach from a taphonomical point of view. Such a comparison of mega- and microfossil records from a Jurassic bed in Yorkshire was conducted by Slater and Wellman (2015). Secondly, given the well-known fact that most palynological records reflect regional vegetation and the megafossil record is biased towards particular, more local palaeocommunities, one may compare the total assemblages to draw inferences on regional scale.
As far as major plant groups across all assemblages are concerned, bryophytes (sensu lato) and lycophytes are known solely from the microfossil record and ferns are underrepresented as megafossils. This is analogous to the situation described for the Jurassic of Yorkshire by Slater and Wellman (2015). In contrast to the situation in Yorkshire, conifers are very well represented in the megafossil record and no single plant group seems underrepresented in the microfossil record.
In the mega- and mesofossil record the vegetation appears dominated by angiosperms and this is probably true as far as the forest-forming species are concerned. The microfossil record is dominated by ferns and corresponds to understory vegetation, vines, and possibly other non-forest palaeocommunities.
As outlined above, Assemblage 5 in the megafossil record corresponds to a backswamp forest (a single palaeocommunity), the mesofossil record to the backswamp forest, as well as the alluvial plain and “upland” vegetation (ZH, unpublished material), whereas the microfossil record documents the three above-mentioned palaeocommunities, but also the halophilous vegetation and fern savannas. A similar situation occurs in Assemblages 6–8, that correspond to variants of climax forest associations, whereas some heliophilous ferns and lycophytes known solely from the microflora might have belonged to pioneer communities (Raniecka-Bobrowska 1968; see also Spicer 2003: 138), like Lygodium, Pityrogramma, or Lycopodium cernuum in the modern flora (Verdoorn 1938; Spicer et al. 1985). Such palaeocommunities are also not reflected in the megafossil record here studied.
Comparison to previously described palaeofloras
It is interesting to compare the mainly Coniacian–Santonian palaeoflora of Lower Silesia described herein with other assemblages of plant fossils from the same palaeogeographic region, i.e., from the European Archipelago. The most striking feature in common with Coniacian palaeofloras (Idzików beds: Halamski and Kvaček 2015; Chlomek beds: Halamski and Kvaček 2016; Březno beds: Halamski et al. 2018a) is perhaps the abundance of trifoliolate fagaleans belonging to Dryophyllum, D. geinitzianum at Idzików and D. westerhausianum described here, arguably dominating one of the palaeocommunities. Other taxa in common are not numerous, including Dryandroides quercinea, Salicites petzeldianus, Geinitzia reichenbachii, Laurophyllum spp., and Konijnenburgia (the same as or closely related to the fern described under Nathorstia by Halamski and Kvaček 2015 and Halamski et al. 2018a). One should also note the general similarity of the palynoassemblages (see above).
Another approximately coeval palaeoflora is that from the Harz Mountains (Quedlinburg and environs; Richter 1904, 1905; Mägdefrau 1968: 350–358). There is one characteristic species in common, Dryophyllum westerhausianum, but the emblematic Credneria is altogether absent in Silesia. There are some similarities in the palynofloras (Weyland and Greifeld 1953), but an overall estimation of similarity cannot be made, as the German flora is in need of revision.
The comparison with the slightly younger flora of the Aachen Formation (sables d’Aix-la-Chapelle, Robaszynski et al. 2002; middle Santonian to probably earliest Campanian according to Batten and Li 1987; Batten et al. 1988; and Streel et al. 1994) is hampered by its poor preservation, which motivated Stockmans (1946) to describe most of the taxa under open nomenclature, and with schematic illustrations. One may wonder whether Dicotylophyllum sp. (Stockmans 1946: pl. 4: 10) be not an isolated leaflet of Dewalquea haldemiana or whether Dicotylophyllum sp. (Stockmans 1946: pl. 4: 14) or might be related to our Dicotylophyllum sp. 2. In very general terms, Lower Silesian and Belgian assemblages seem quite different, as coarsely serrate leaves (“Quercus” sensu Hosius and von der Marck 1880) are numerous there (Stockmans 1946: pl. 3: 1, 8, 20–27) and altogether absent in the material studied herein.
Finally, as noted above, the palaeoflora described herein shows some similarities with the plant fossil assemblage from the Frontier Formation (eastern North America). For historical reasons, palaeobotanists were only seldom attempted a direct comparison of coeval Cretaceous assemblages from Europe and North America, although similarities between European and American conifer floras were listed by Bosma et al. (2009). Such a collation for angiosperms is likely to be a fruitful subject of future research.
Temporal trends observed in the Late Cretaceous vegetation of Central Europe
Generally speaking, the angiosperm-dominated mega- and mesoassemblages and fern-dominated microassemblages do broadly correspond to general trends recorded in the northern temperate zone, both in Europe and in North America. Generally speaking, in North America palynofloras start being dominated by angiosperms between the Turonian and the Campanian, which is the case of megafossil assemblages since the Santonian (Upchurch and Wolfe 1987: 88), although major differences may be due to latitude, climate, and facies (Spicer 1990; Wing and Sues 1992; Wolfe 1997; Spicer et al. 2002). For example, conifers were favoured in cooler climates (Doyle et al. 1982) and in swamp forests (Saward 1992).
More in detail, it should be observed first that Assemblages 4–8 (late Coniacian? to early–middle Santonian) are chronologically relatively close to each other, all belonging to a time interval spanning about four million years (base of the Coniacian 89.8 Ma, Coniacian/Santonian boundary 86.3 Ma, top of the Santonian 83.6 Ma; Gradstein et al. 2012). Assemblages 4–5 are nearly coeval with the flora from the upper Idzików beds (see Halamski and Kvaček 2015: 100, 140 for details of the ammonite-based dating). For the purpose of establishing a general panorama of the vegetation we have treated Assemblages 4–8 as approximately coeval.
A striking difference between Coniacian and Santonian riparian forest palaeocommunities of the Central European Archipelago (Coniacian, Idzików beds: Halamski and Kvaček 2015; Kvaček et al. 2015; Chlomek beds: Halamski and Kvaček 2016; Santonian, present study) is the role of laurophylls. This morphotype, probably representing at least in part the magnolioid angiosperms belonging to the Lauraceae (and perhaps a few related families), is a major element of Coniacian palaeofloras, accounting for ca. 25–40% of the material according to localities of the Idzików beds; (Halamski and Kvaček 2015: 128) and for over 15% in the Chlomek beds (Halamski and Kvaček 2016: 299, 310). In the studied material the laurophylls are represented by 14 poorly preserved specimens (6% of the material). This is all the more striking given the similarity of the other dominant elements in the three above-mentioned cases (Dryophyllum, see above).
The platanoid-laurophyll riparian forests were a common element of latest Early and early Late Cretaceous vegetation in Europe (Kvaček et al. 2006; Coiffard et al. 2009) and analogous communities may still be found, for example in Mexico (Brand 1936). However, in the later part of the Late Cretaceous laurophylls are relatively rare in European palaeofloras (Herman and Kvaček 2010; Halamski 2013). In the Aachen sands palaeofloras the situation seems analogous to that in the approximately coeval material described here: only a few of the morphotypes illustrated by Stockmans (1946) belong to laurophylls. It seems that the exclusion of laurophylls from communities of riparian forests in Central Europe might be quite precisely dated to the Santonian, but evidently such a conclusion is based on a scarce factual material and should be confirmed by studies based on a greater number of palaeofloras.
It may also be noted that, if the Santonian vegetation described herein is broadly similar to Campanian palaeofloras from Central and Northern Europe (Halamski 2013; Halamski et al. 2016), among the three possibilities invoked by Halamski and Kvaček (2018: 192) to explain the peculiar character of the early Campanian Grünbach palaeoflora (Herman and Kvaček 2010), namely facies, climate, and age, at least age may be definitively discarded.
Another notable feature of the studied palaeoflora is the presence of long-ranging plant taxa. Dewalquea haldemiana is known from the late Coniacian to the Maastrichtian. Perhaps more interestingly, some of the described plant fossils represent species that are identical or closely related, to Paleocene taxa. This may concern Platanites willigeri similar to upper Paleocene–lower Eocene Platimeliphyllum palanense, Platanites similar to congeneric species known from the Maastrichtian of Montana and the Paleocene of the Isle of Mull, and Dewalquea aff. gelindenensis closely related to Dewalquea gelindenensis from the Paleocene of Belgium. Longevity of plant species may be interpreted as correlated with stability of communities (Paczoski 1933, DiMichele et al. 2004; but see Levin 2000: 171–173 and references therein). Consequently, it may be hypothesised that after a period of pronounced changes in the vegetation of Europe related to the establishment of angiosperm-dominated assemblages (e.g., Coiffard and Gomez 2010), plant communities in the later part of the Late Cretaceous become stabilised and thus more resilient to structural modifications. The major change of plant communities would thus have occurred during a period of stable greenhouse climate (Kaufmann 1995 and references therein), whereas the stabilisation of communities would have taken place simultaneously with a major cooling trend having started in the Campanian and continuing until the end of the Mesozoic (Linnert et al. 2014 and references therein).
It is at present unclear how this might relate to the conclusion made by Bosma et al. (2009: 494) about a distinct turnover in conifer floras between the Santonian and Campanian in the Netherlands. This turnover might, however, be more local phenomenon rather than a global one, insofar as, for example, Geinitzia reichenbachii is altogether absent in the Maastrichtian in the Netherlands (Bosma et al. 2009), but present in both the Campanian and Maastrichtian in Poland (Halamski 2013). Dutch Cretaceous angiosperms lack a comprehensive modern treatment.
Ecosystems of the European Archipelago: comparison of palaeobotanical and palaeozoological data
A recent synthesis of Cretaceous vertebrate faunas from the European Archipelago (Csiki-Sava et al. 2015) showed a peculiar zoogeographic pattern demonstrating both a uniqueness of the whole zooprovince on the one hand and high degree of endemicity between islands on the other. In other words, there are high-level taxa endemic for Europe, but not a single vertebrate species confirmed from more than one region (Csiki-Sava et al. 2015).
It should, however, be stressed that calling the above-mentioned distribution a ‘biogeographic’ repartition, as done by Csiki-Sava et al. (2015), might be misleading, insofar as a vertebrate-based zoogeographic pattern is not necessarily representative for the biosphere as a whole. Such is the present-day situation in South Africa, which in terms of zoogeographic regionalisation belongs to the Ethiopian region along with the major part of the continent (Darlington 1957), but forms a separate phytogeographic unit of the highest rank (South African Kingdom or Capensis; Engler 1910: 479–510; Takhtajan 1986).
Indeed, the above-mentioned Cretaceous palaeozoogeographic pattern is in stark contrast to the palaeophytogeographic one. There is no high-rank taxon peculiar to Europe, and most species for which a detailed identification was possible have been documented from several localities, like Dryophyllum westerhausianum, Dewalquea haldemiana, D. insignis, and others. The closest relatives of Konijnenburgia cf. galleyi and of Platanites willigeri are from North America. The Cretaceous “Quercus” described from Haldem (Hosius and von der Marck 1880) and Aachen region (Stockmans 1946) are known also from Morocco (Carpentier 1950). This conclusion is consistant with on the observation that only minor differences existed between European and Potomac provinces (Vakhrameev 1991: 223) and is still more evident in palynological analyses: the studied area belongs to the Normapolles Province with globally similar palynoassemblages spread between eastern North America, Central Europe, and a part of Asia (Vajda and Bercovici 2014: 32–33 and references therein). Some taxa, like Distaltriangulisporites perplexus, are common to both the studied area and Canada.
Conclusions
The megaflora of the North Sudetic Basin, studied on the basis of 270 specimens from 17 localities (study area ca. 300 km2), is represented by 29 species (6 ferns, 4 conifers, 19 angiosperms), grouped in eight assemblages (Assemblage 1, Turonian; Assemblages 2, 3, lower–middle Coniacian; Assemblages 4, 5, upper Coniacian?–lower Santonian?; Assemblages 6–8, lower–middle Santonian).
Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov. is reinterpreted as a member of the Fagales.
The following new combination is introduced: Dewalquea paulinae (Halamski, 2013) Halamski comb. nov. [basionym: Debeya paulinae Halamski, 2013].
Platanites willigeri Halamski and Kvaček sp. nov. is described. It is represented by trifoliolate leaves with ovate, unlobed median leaflets. Along with Platanites sp. 1, these are the oldest (Santonian) records of the genus.
The studied plants are interpreted to have dwelt in the following palaeocommunities: (i) back swamp forest dominated by Geinitzia, with ferns; (ii) Dryophyllum-dominated riparian forest with Ettingshausenia (Santonian); possibly Normapolles-dominated (as recorded by mesofossils) on the lower parts of slopes (hinterland); (iii) another variant of riparian forest with Platanites sp. 1 (Santonian); (iv) forest with Dewalquea spp. and Platanites willigeri in the marginal part of the alluvial plain; (v) fern savanna with patches of Pinus woodlands (documented in the Coniacian); (vi) dunes with Dewalquea haldemiana and Konijneburgia aff. galleyi; (vii) halophytic vegetation (mangroves) with Frenelopsis; a single grain of Spinizonocolpites sp. is suggestive of Nypa as a part of this community, but this requires confirmation; (viii) pioneer vegetation with lycophytes and ferns.
Out of these, i–vi, are known from the megafossil record, i, ii, also from the mesofossil record, and vii, viii, solely from the microfossil record.
The mega- and mesofossil records are dominated by angiosperms (canopy-forming species), whereas the microfossil record is dominated by fern spores (understory, vines, pioneer vegetation). Palaeocommunities are intermediate in general character between those pre-dating the Cretaceous Terrestrial Revolution and modern, angiosperm-dominated vegetation.
Microfossils from the Nowogrodziec Member were examined in detail. Palynoassemblage A (corresponding to Assemblage 5) consists of 105 taxa, largely fern spores (especially Gleicheniaceae) and representatives of the Normapolles group. Palynoassemblage B (corresponding to Assemblages 6–8; data after an unpublished report by Raniecka-Bobrowska 1968) is overall similar, but the dominant fern group is the Schizaeaceae.
A striking similarity among Coniacian floras from European Archipelago is the abundance of trifoliolate fagaleans (Dryophyllum westerhausianum in the North Sudetic Basin, D. geinitzianum at Idzików and Březno). On the other hand, a striking difference is the rarity of laurophylls. It is possible that exclusion of lauroids from riparian forests began about the Santonian.
Several species present in the palaeoflora described here are are the same as, or are related to, those that occur in younger (including Paleocene) assemblages. This might be interpreted as stabilisation of communities after a period of pronounced change related to the rise of dominance of angiosperms.
In contrast to the vertebrate-based palaeozoological pattern showing the Cretaceous European Archipelago as a zone of high endemism with strong differentiations between single islands, the plant cover of the same area and period is mostly comprised of widely distributed species, some of which are similar to, or possibly identical with, those found in North America.
Acknowledgements
This study could not be completed without the friendly help of Paweł Raczyński (MGUWr), both in the field and in the museum collections. Access to the quarries at Rakowice Małe and at Żeliszów was kindly authorised by Alfred Sroka (Chairman of the Board, Kwarcyty Bolesławieckie S.A.). The following curators and staff are thanked for granting the access to the collections and various help: Angela Ehling (BGR), Barbara Mohr, Catrin Puffert, and Eva-Maria Sadowski (all MB), Lutz Kunzmann (MMG), Dario De Franceschi (Muséum national d’Histoire naturelle, Paris, France), Emmanuel Robert (Université Claude Bernard-Lyon 1, Lyon, France), Yves Dutour, Nicolas Vialle, and Séverine Berton (all Muséum d’Histoire naturelle d’Aix-en-Provence, Aix-en-Provence, France). Markus Wilmsen (Senckenberg Naturhistorische Sammlungen Dresden, Germany) and Markus Fengler (Moritzburg, Germany) helped in finding biographic data on Gustav Williger. Aurelio J. Patierno (British Library, London) provided publication months needed for checking priority between two names published in the same year. Ireneusz Walaszczyk and Mariusz Niechwedowicz (both Faculty of Geology, University of Warsaw, Poland) commented on inoceramid and dinocyst data, respectively. Irena Grabowska (formerly Polish Geological Institute, Warsaw, Poland) kindly supplied unpublished data of the late Jadwiga Raniecka-Bobrowska. Drawings were made by Bogusław Waksmundzki (Faculty of Geology, University of Warsaw, Poland) and Andrzej Baliński (ZPAL) helped with the plates. Maceration of palynological samples was made by Alena Tichá in the Laboratory of the Czech Geological Survey (Prague, Czech Republic). The text benefited from detailed reviews by Robert Spicer (The Open University, Milton Keynes, UK) and Steven Manchester (Florida Museum of Natural History, Gainesville, USA). Research was financed through the grant “The Late Cretaceous flowering plant expansion in the context of the transgression of the Central European Sea” (2016/21/B/NZ8/02443) of the National Science Centre, Poland to ATH. Additional palynological analyses at Żeliszów by MS were financed by institutional support DKRVO 2019-2023 2.I.b of the Czech Ministry of Culture and RVO6785831 of the Institute of Geology of the Czech Academy of Sciences. All the above-mentioned persons and institutions are gratefully acknowledged.
References
Abbink, O.A. 1998. Palynological investigations in the Jurassic of the North Sea region. LPP Contribution Series 8: 1–192.
Aleksandrova, A.N., Bratceva, G.M., and Kulkova, I.A. 1985. Palynoflora of Central Siberia at the Eocene–Oligocene border. Izvestiâ Akademii nauk SSSR, Seriâ geologičeskaâ 1985 (11): 64–75.
Alexandrowicz, S.W. 1976. Foraminifera from the brackish Santonian deposits in the North Sudetic Basin. Annales de la Société géologique de Pologne 46: 183–195.
Andert, H. 1934. Die Fazies in der sudetischen Kreide unter besonderer Berücksichtung des Elbsandsteingebirges. Zeitschrift der deutschen geologischen Gesellschaft 86: 617–637.
Andrews, H.N., Jr. 1955. Index of generic names of fossil plants, 1820–1950. United States Geological Survey, Bulletin 1013: 1–262.
Anonymous 1937. Geheimer Bergrat Dr.-Ing. h.c. Gustav Williger. Neues lausitzisches Magazin 113: 226–227.
Arai, M. and Viviers, M.C. 2013. Dinoflagellate cyst superdominance assemblages from the Upper Cretaceous of the Santos Basin, offshore SE Brazil, and their palaeoecological significance. In: J.M. Lewis, F. Marret, and L. Bradley (eds), Biological and Geological Perspectives of Dinoflagellates, 285–292. The Micropalaeontological Society, Special Publications, Geological Society, London. Crossref
Arens, N.C. and Allen, S.E. 2014. A florule from the base of the Hell Creek Formation in the type area of eastern Montana: Implications for vegetation and climate. Geological Society of America, Special Paper 503: 173–203. Crossref
August, C. and Wojewoda, J. 2004. Late Carboniferous weathering and regolith of the Kudowa Trough: Palaeogeographic, palaeoclimatic and structural implications. Geologia Sudetica, 36: 53–66.
Azéma, C., Fauconier, D., and Viaud, J. 1981. Microfossils from the Upper Cretaceous of Vendée (France). Review of Palaeobotany and Palynology 35: 237–281. Crossref
Badura, J., Chrząstek, A., Hałuszczak, A., and Szynkiewicz, A. 2011. Wycieczki B1–B2: opis stanowisk. In: A. Żelaźniewicz, J. Wojewoda, and W. Ciężkowski (eds.), Przewodnik do wycieczek LXXXI Zjazdu Polskiego Towarzystwa Geologicznego, Żagań, 23–25 września 2011, 15–53. Wind, Wrocław.
Baikovskaya, T.N. [Bajkovskaâ, T.N.] 1957. On the Upper Cretaceous Floras of the Chulym-Yenisey Basin [in Russian]. In: P.I. Dorofeev (ed.), Sbornik pamâti A.N. Krištofoviča, 65–99. Izdatel’stvo AN SSSR, Moskva.
Balme, B.E. 1957. Spores and pollen grains from the Mesozoic of Western Australia. CSIRO Coal Research Section, Technical Communication 25: 1–48.
Baraniecki, L., Grocholski, A., and Mydlarski, T. 1955. Szczegółowa Mapa Geologiczna Sudetów 1:25000, arkusz M 33–32 Ad Iwiny. Instytut Geologiczny, Warszawa.
Barrón, E., Gómez, J.J., Goy, A., and Pieren, A.P. 2006. The Triassic–Jurassic boundary in Asturias (northern Spain): Palynological characterisation and facies. Review of Palaeobotany and Palynology 138: 187–208. Crossref
Batten, D.J. 1973. Palynology of Early Cretaceous soil beds and associated strata. Palaeontology 16: 399–424.
Batten, D.J. 1974. Wealden palaeoecology from the distribution of plants fossils. Proceedings of the Geologists’ Association 85: 433–458. Crossref
Batten, D.J. 1989. Systematic relationships between Normapolles pollen and the Hamamelidae. In: P.R. Crane and S. Blackmore (eds.), Evolution, Systematics, and Fossil History of the Hamamelidae, Volume 2: “Higher” Hamamelidae. Systematic Association Special Volume 40B, 9–21. Clarendon Press, Oxford.
Batten, D.J. and Li, W. 1987. Aspects of palynomorph distribution, floral provinces and climate during the Cretaceous. Geologisches Jahrbuch A 96: 219–237.
Batten, D.J. and MacLennan, A.M. 1984. The palaeoenvironmental significance of the conifer family Cheirolepidiaceae in the Cretaceous of Portugal. In: W.-E. Reif and F. Westphal (eds.), Third Symposium on Mesozoic Terrestrial Ecosystems, Short Papers, 7–12. Attempto, Tübingen.
Batten, D.J., Dupagne-Kievits, J., and Lister, J.K. 1988. Palynology of the Upper Cretaceous Aachen Formation of northeast Belgium. In: M. Streel and M.J.M. Bless (eds.), The Chalk District of the Euregio Meuse-Rhine, 95–103. Natuurhistorisch Museum Maastricht, The Netherlands and Laboratoires de Paléontologie de l’Université d’État à Liège.
Bayer, E. 1896. Die Flora der Chlomeker Schichten. Sitzungsberichte der Königlich böhmischen Gesellschaft der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse 27: 1–36.
Behrensmeyer, A.K., Hook, R.W., Badgley, C.E., Boy, J.A., Chapman, R.E., Dodson, P., Gastaldo, R.A., Graham, R.W., Martin, L.D., Olsen, P.E., Spicer, R.E., Taggart, R.E., and Wilson, M.V.H. 1992. Paleoenvironmental contexts and taphonomic modes. In: A.K. Behrensmeyer, J.D. Damuth, W.A. DiMichele, R. Potts, H.-D. Sues, and S.L. Wing (eds.), Terrestrial Ecosystems Through Time: Evolutionary Paleoecology of Terrestrial Plants and Animals, 15–136. The University of Chicago Press, Chicago.
Bell, W.A. 1957. Flora of the Upper Cretaceous Nanaimo Group of Vancouver Island, British Columbia. Geological Survey of Canada, Memoir 293: 1–84. Crossref
Bengtson, S. 1985. Taxonomy of disarticulated fossils. Journal of Paleontology 59: 1350–1358.
Berchtold, B.W. von and Presl, J.S. 1820. O přirozenosti rostlin, aneb Rostlinář, obsahugjej i gednanj o žiwobytj rostlinném pro sebe a z ohledu giných žiwotů podlé stawu nyněgssjho znánj; w potaženj na užitečnost w rolnictwj, hospodářstwj, řemeslech, uměnj i obchodu a w wztahowánj obzwlásstnjm na lekařstwj. 322 pp. K.W. Enders, Praha.
Berezowska, B. and Berezowski, Z. 1982. Objaśnienia do szczegółowej mapy geologicznej Sudetów. Arkusz Bolesławiec. 1:25 000. 130 pp. Instytut Geologiczny, Warszawa.
Berry, E.W. 1910. A new species of Dewalquea from the American Cretaceous. Torreya 10 (2): 34–38.
Berry, E.W. 1911. The flora of the Raritan Formation. Geological Survey of New Jersey, Bulletin 3: iii–v, 1–233. Crossref
Berry, E.W. 1916a. The lower Eocene floras of southeastern North America. United States Geological Survey, Professional Paper 91: 1–481. Crossref
Berry, E.W. 1916b. The Upper Cretaceous floras of the worls. In: W.B. Clark (ed.), Maryland Geological Survey, Upper Cretaceous, 183–313. The Johns Hopkins Press, Baltimore.
Berry, E.W. 1925. The flora of the Ripley Formation. United States Geological Survey, Professional Paper 136: 1–94. Crossref
Biernacka, J. 2012. Provenance of Upper Cretaceous quartz-rich sandstones from the North Sudetic Synclinorium, SW Poland: Constraints from detrital tourmaline. Geological Quarterly 56: 315–332. Crossref
Biernacka, J. and Józefiak, M. 2009. The Eastern Sudetic Island in the early-to-middle Turonian: Evidence from heavy minerals in the Jerzmanice sandstones, SW Poland. Acta Geologica Polonica 59: 545–565.
Bína, J. and Demek, J. 2012. Z nížin do hor. Geomorfologické jednotky České republiky. 343 pp. Academia, Praha.
Bodor, E.R. and Baranyi, V. 2012. Palynomorphs of the Normapolles group and related plant mesofossils from the Iharkút vertebrate site, Bakony Mountains (Hungary). Central European Geology 55: 259–292. Crossref
Bogner, J. and Mayo, S.J. 1998. Acoraceae. In: K. Kubitzki (ed.), The Families and Genera of Vascular Plants. Volume IV: Monocotyledons—Alismatanae and Commelinanae (except Gramineae), 7–11. Springer, Berlin. Crossref
Bolkhovitina, N.A. [Bolhovitina, N.A.] 1953. Characteristics of the Cretaceous sediments of central regions of the USSR on the basis of spores and pollen [in Russian]. Trudy Geologičeskogo Instituta AN SSSR 145: 1–183.
Bolkhovitina, N.A. 1966. The fossil spores of the ferns of the family Gleicheniaceae (taxonomy and distribution). In: M.I. Nejshtadt (ed.), The Importance of Palynological Analysis for the Stratigraphic and Paleofloristic Investigations. Papers for the Second International Palynology Conference, Utrecht 1966, 65–75. Nauka, Moscow.
Bolkhovitina, N.A. [Bolhovitina, N.A.] 1968. The spores of the fern family Gleicheniaceae and their importance for the stratigraphy [in Russian]. Trudy Geologičeskogo Instituta AN SSSR 186: 1–116.
Boltenhagen, E. 1980. Palynologie du Crétacé supérieur du Gabon. Comité des Travaux historiques et scientifiques, Mémoires de la Section des Sciences 7: 1–191.
Bosma, H., van Konijnenburg-van Cittert, J.H.A., van der Ham, R.W.J.M., van Amerom, H.W.J., and Hartkopf-Fröder, C. 2009. Conifers from the Santonian of Limburg, The Netherlands. Cretaceous Research 30: 483–495. Crossref
Botfalvai, G., Haas, J., Bodor, E.R., Mindszenty, A., and Ősi, A. 2016. Facies architecture and palaeoenvironmental implications of the upper Cretaceous (Santonian) Csehbánya formation at the Iharkút vertebrate locality (Bakony Mountains, Northwestern Hungary). Palaeogeography, Palaeoclimatology, Palaeoecology 441: 659–678. Crossref
Boulter, M.C. and Kvaček, Z. 1989. The Palaeocene flora of the Isle of Mull. Special Papers in Palaeontology 42: 1–49.
Boulter, M.C. and Windle, T. 1993. A reconstruction of some Middle Jurassic vegetation in northern Europe. In: M.E. Collinson and A.C. Scott (eds.), Studies in Palaeobotany and Palynology in Honour of Professor W.G. Chaloner, F.R.S. Special Papers in Palaeontology 49: 125–154.
Boureau, E. (ed.) 1970. Traité de Paléobotanique. Tome IV, fascicule I: Filicophyta. 519 pp. Masson et Cie, Paris.
Brand, D.D. 1936. Notes to accompany a vegetation map of northwest Mexico. The University of New Mexico Bulletin, Biological Series 4 (4): 3–27.
Braman, D.R. 2001. Terrestrial palynomorphs of the upper Santonian– ?lowest Campanian Milk River Formation, southern Alberta, Canada. Palynology 25: 57–107. Crossref
Brenner, G.J. 1962. The Spores and Pollen of the Potomac Group of Maryland. 302 pp. Unpublished Ph.D. Thesis, Pennsylvania State University.
Brenner, G.J. 1963. The spores and pollen of the Potomac Group of Maryland. Maryland Department of Geology, Mines and Water Resources, Bulletin 27: 1–215.
Brenner, G.J. and Bickoff, I.S. 1992. Palynology and age of the Lower Cretaceous basal Kurnub Group from the coastal plain to the northern Negev of Israel. Palynology 16: 137–185. Crossref
Brideaux, W.W. 1968. Palynology of the Lower Colorado Group (Late Lower Cretaceous) and Its Lithological Equivalents in Central and West-central Alberta, Canada. 533 pp. Unpublished Ph.D. Thesis, McMaster University, Hamilton, ON.
Bromhead, E.F. 1840. Remarks on the botanical system of Professor Perleb. Magazine of Natural History, new series 4: 329–338. Crossref
Brongniart, A.T. 1828. Prodrome d’une histoire des végétaux fossiles. viii + 223 pp. F.G. Levrault, Paris. Crossref
Brongniart, A.T. 1828–1838. Histoire des végétaux fossiles, ou recherches botaniques et geologiques sur les végétaux renfermés dans les diverses couches du globe. 488 pp. G. Dufour et D’Ocagne, Paris. Crossref
Brongniart, A.T. 1849. Tableau des genres de végétaux fossiles considérés sous le point de vue de leur classification botanique et de leur distribution géologique. In: A.C.V.D. d’Orbigny (ed.), Dictionnaire universel d’histoire naturelle, vol. 13, 52–176 [1–127]. L. Martinet, Paris.
Bronn, H.G. 1837. Lethaea geognostica oder Abbildung und Beschreibung der für die Gebirgs-Formationen bezeichnendsten Versteinerungen, Vol. 1. 560 pp. Schweizerbart’sche Verlagshandlung und Druckerei, Stuttgart.
Buch, L. von 1797. Versuch einer mineralogischen Beschreibung von Landeck. 53 pp. J. Korn, Breslau.
Budancev, L.Yu. [Budancev, L.Û.] 1983. Istoriâ arktičeskoj flory epohi rannego kajnofita. 156 pp. Nauka, Leningrad.
Burger, D. 1976. Some Early Cretaceous plant fossils from Queensland. Department of Natural Resources of Australia, Bureau of Mineral Resources, Geology and Geophysics, Bulletin 160: 1–22.
Burger, D. 1980. Palynological studies in the Lower Cretaceous of the Surat Basin, Australia. Australia, Bureau of Mineral Resources, Geology and Geophysics, Bulletin 189: 1–106.
Burger, D. 1988. Early Cretaceous environements in the Eromanga Basin; palynological evidence from GSQ Wyandra-1 corehole. In: P.A. Jell and G. Playford (eds.), Palynological and Palaeobotanical Studies in Honour of Basil E. Balme. Memoir of the Association of Australasian Palaeontologists 5: 173–186.
Burgh, J. van der 2008. Fossilium catalogus. II: Plantae. Pars 109: Index of angiosperm leaf species names: D–G, 1823–2006. 154 pp. Backhuys Publishers, Leiden.
Bütschli, O. 1880–1889. Erster Band. Protozoa: Mit einem Beitrag: Palaeontologische Entwicklung der Rhizopoda, von C. Schwager (3 vols.) In: Dr. H.G. Bronn’s Klassen und Ordnungen des Thier-Reichs, wissenschaftlich dargestellt in Wort und Bild. 2035 pp. C.F. Winter’sche Verlagshandlung, Leipzig. Crossref
Bůžek, Č., Holý, F., and Kvaček, Z. 1968. Die Gattung Doliostrobus Marion und ihr Vorkommen im nordböhmischen Tertiär. Palaeontographica, Abteilung B 123: 153–172.
Candolle, A.P. de 1817 (“1818”). Regni vegetabilis systema naturale, sive ordines, genera et species plantarum secundum methodi naturalis normas digestarum et descriptarum. Volumen primum. 564 pp. Treuttel & Würtz. Paris. Crossref
Carpentier, A. 1950. Flore sénonienne de Sidi Hajaj, précédé d’une Introduction stratigraphique de H. Salvan. Protectorat de la République française au Maroc, Service géologique, Notes et Mémoires 76: 149–154.
Carrière, E.A. 1855. Traité général des Conifères ou Description de toutes les espèces et variétés aujourd’hui connues, Avec leur synonymie, l’indication des procédés de culture et de multiplication qu’il convient de leur appliquer. xv + 656 pp. Chez l’auteur, Paris. Crossref
Cavalier-Smith, T. 1981. Eukaryote kingdoms: seven or nine? Biosystems 14: 461–481. Crossref
Charpentier, J.F.W. 1778. Mineralogische Geographie der Chursächsischen Lande. xliv + xvi + 432 pp. S.L. Crusius, Lepizig.
Chatziemmanouil, J.P. 1982. The Upper Cretaceous of the Vomb Trough, southern Sweden. Stockholm Contributions in Geology 38: 57–161.
Chrząstek, A. and Wypych, M. 2018. Coniacian sandstones from the North Sudetic Synclinorium revisited: palaeoenvironmental and palaeogeographical reconstructions based on trace fossil analysis and associated body fossils. Geologos 28: 29–53. Crossref
Cieśliński, S. 1989. Macroflora: Divisions Pteridophyta and Spermatophyta. In: L. Malinowska (ed.), Geology of Poland. Volume III: Atlas of Guide and Characteristic Fossils. Part 2c: Mesozoic, Cretaceous, 326–327. Wydawnictwa Geologiczne, Warsaw.
Clarke, R.T. 1965. Fungal spores from Vermejo Formation Coal Beds (Upper Cretaceous) of Central Colorado. The Mountain Geologist 2: 85–93.
Coe, M.J., Dilcher, D.L., Farlow, J.O., Jarzen, D.M., and Russell, D.A. 1987. Dinosaurs and land plants. In: E.M. Friis, W.G. Chaloner, and P.R. Crane (eds.), The Origins of Angiosperms and Their Biological Consequences, 225–258. Cambridge University Press, Cambridge.
Coiffard, C. and Gomez, B. 2010. The rise to dominance of the angiosperm kingdom: dispersal, habitat widening and evolution during the Late Cretaceous of Europe. Lethaia 43: 164–169. Crossref
Coiffard, C., Gomez, B., and Thévenard, F. 2007. Early Cretaceous angiosperm invasion of Western Europe and major environmental changes. Annals of Botany 100: 545–553. Crossref
Coiffard, C., Gomez, B., Thiébaut, M., Kvaček, J., Thévenard, F., and Néraudeau, D. 2009. Intramarginal veined Lauraceae leaves from the Albian–Cenomanian of Charente-Maritime (western France). Palaeontology 52: 323–336. Crossref
Cookson, I.C. 1947a. On fossil leaves (Oleaceae) and a new type of fossil pollen grain from Australian brown coal deposits. Proceedings of the Linnean Society of New South Wales 72: 183–197.
Cookson, I.C. 1947b. Plant microfossils from the lignites of Kerguelen Archipelago. British, Australian, and New Zealand Antarctic Research Expedition 1929/1931 2: 127–142.
Cookson, I.C. 1953. Difference in microspore composition of some samples from a bore at Comaum, South Australia. Australian Journal of Botany 1: 462–473. Crossref
Cookson, I.C. and Dettmann, M.E. 1958. Some trilete spores from Upper Mesozoic deposits in the eastern Australian Region. Proceedings of the Royal Society of Victoria 70: 95–128.
Cookson, I.C. and Dettmann, M.E. 1961. Reappraisal of the Mesozoic microspore genus Aequitriradites. Palaeontology 4: 425–427.
Cookson, I.C. and Eisenack, A. 1958. Microplankton from Australian and New Guinea Upper Mesozoic sediments. Proceedings of the Royal Society of Victoria 70: 19–79.
Couper, R.A. 1953. Upper Mesozoic and Cainozoic spores and pollen grains from New Zealand. New Zealand Geological Survey, Paleontological Bulletin 22: 1–77.
Couper, R.A. 1958. British Mesozoic microspores and pollen grains. A systematic and stratigraphic study. Palaeontographica B 103: 75–179.
Crabtree, D.R. 1987. Angiosperms of the Northern Rocky Mountains: Albian to Campanian (Cretaceous) Megafossil Floras. Annals of the Missouri Botanical Garden 74: 707–747. Crossref
Crandall-Stotler, B.J. and Stotler, R.E. 2000. Morphology and classification of the Marchantiophyta. In: A.J. Shaw and B. Goffinet (eds.), Bryophyte Biology, 21–70. Cambridge University Press, Cambridge. Crossref
Crane, P.R. 1987. Vegetational consequnces of angiosperm diversification. In: E.M., Friis, W.G. Chaloner, and P.R. Crane (eds.), The Origins of Angiosperms and Their Biological Consequences, 107–144. Cambridge University Press, Cambridge.
Crane, P.R. 1989. Paleobotanical evidence on the early radiation of nonmagnoliid dicotyledons. Plant Systematics and Evolution 162: 165–191. Crossref
Csiki-Sava, Z., Buffetaut, E., Ősi, A., Pereda-Suberbiola, X., and Brusatte, S.L. 2015. Island life in the Cretaceous—faunal composition, biogeography, evolution, and extinction of land-living vertebrates on the Late Cretaceous European archipelago. ZooKeys 469: 1–161. Crossref
Czaplicka, J., Juskowiak, O., Ryka, W., Kilian, Z., Tomczykowa, E., Tomczyk, H., Pajchlowa, M., Stopa, S.Z., Kozłowski, S., Kotański, Z., Kopik, J., Malinowska, L., Cieśliński, S., Woźny, E., Głodek, J., and Krajewski, S. 1968. Słownik stratygraficzny. 530 pp. Wydawnictwa Geologiczne, Warszawa.
Darlington, P.J., Jr. 1957. Zoogeography: the Geographical Distribution of Animals. xiii + 675 pp. John Wiley & Sons, Inc., New York.
Davey, R.J. and Williams, G.L. 1966. The genera Hystrichosphaera and Achomosphaera. In: R.J. Davey, C. Downie, W.A.S. Sarjeant, and G.L. Williams (eds.), Studies on Mesozoic and Cainozoic dinoflagellate cysts. Bulletin of the British Museum (Natural History), Geology Supplement 3: 28–52.
Davey, R.J., Downie, C., Sarjeant, W.A.S., and Williams, G.L. 1966. Fossil dinoflagellate cysts attributed to Baltisphaeridium. In: R.J. Davey, C. Downie, W.A.S. Sarjeant, and G.L. Williams (eds.), Studies on Mesozoic and Cainozoic dinoflagellate cysts. Bulletin of the British Museum (Natural History), Geology Supplement 3: 157–175.
Dawson, J.W. 1883. On the Cretaceous and Tertiary floras of British Columbia and the Northwest Territory. Proceedings and Transactions of the Royal Society of Canada 1 (4): 15–34. Crossref
Deák, M.H. 1962. Deux nouveaux genres de spore de la série d’argiles et de marnes aptiennes. Földtani Közlöny 92: 230–235.
Deák, M.H. 1963, Quelques spores striées de l’étage aptien. Revue de Micropaléontologie 5: 251–256.
Deák, M.H. 1964. Contribution à l’étude palynologique du Groupe d’Argiles à Munieria de l’étage Aptien. Acta Botanica Academiae Scientiarum Hungaricae 10: 95–126.
Deák, M.H. and Combaz, A. 1967. “Microfossiles organiques” du Wealdien et du Cénomanien dans un sondage de Charente-Maritime. Revue de Microplaéontologie 10: 69–96.
Deflandre, G. 1936. Microfossiles des silex crétacés. Première partie. Généralités. Flagellés. Annales de paléontologie 25: 151–191.
Delcourt, A. and Sprumont, G. 1955. Les spores et grains de pollen du Wealdien du Hainaut. Mémoires de la Société belge de Géologie, de Paléontologie et d’Hydrologie, nouvelle série 5: 1–73.
Delcourt, A.F., Dettmann, M.E., and Hughes, N.F. 1963. Revision of some Lower Cretaceous microspores from Belgium. Palaeontology 6: 282–292.
Dettmann, M.E. 1963. Upper Mesozoic microfloras from southeastern Australia. Proceedings of the Royal Society of Victoria 77: 1–148.
Dettmann, M.E. 1973. Angiospermous pollen from Albian to Turonian sediments of eastern Australia. Geological Society of Australia, Special Publication 4: 3–34.
Diéguez, C. and Meléndez, N. 2000. Early Cretaceous ferns from lacustrine limestone at Las Hoyas, Cuenca Province, Spain. Palaeontology 43: 1113–1141. Crossref
Dijkstra, S.J. 1949. Megaspores and some other fossils from the Aachenian (Senonian) in South Limburg, Netherlands. Mededelingen van de Geologische Stichting, Nieuwe Serie 3: 19–33.
Dillon, L.S. 1963. A reclassification of the major groups of organisms based upon comparative cytology. Systematic Biology 12 (2): 71–82. Crossref
DiMichele, W.A., Behrensmeyer, A.K., Olszewski, T.D., Labandeira, C.C., Pandolfi, J.M., Wing, S.L., and Bobe, R. 2004. Long-term stasis in ecological assemblages: evidence from the fossil record. Annual Review of Ecology, Evolution, and Systematics 35: 285–322. Crossref
Dobruskina, I.A. 1997. Turonian plants from the southern Negev, Israel. Cretaceous Research 18: 87–107. Crossref
Doell, J.C. 1857. Flora des Grossherzogthums Baden. Erster Band. vi + 482 pp. G. Braun’sche Hofbuchhandlung, Carlsruhe.
Doyle, J.A., Jardine, S., and Doerenkamp, A. 1982. Afropollis, a new genus of early angiosperm pollen. Bulletin du Centre de Recherches Exploration-Production Elf-Aquitaine 6: 39–117.
Drescher, R. 1863. Ueber die Kreide-Bildungen der Gegend von Löwenberg. Zeitschrift der deutschen geologischen Gesellschaft 15: 291–366.
Dresler, E.F. 1883. Flora von Löwenberg in Schlesien. Nach dem natürlichen System bearbeitet. Jahresberichte des Realprogymnasiums zu Löwenberg in Schlesien 13. 162 pp. Paul Holtsch Verlag, Löwenberg.
Dunker, W. 1846. Monographie der norddeutschen Wealdenbildung: Ein Beitrag zur Geognosie und Naturgeschichte der Vorwelt. Nebst einer Abhandlung über die in dieser Gebirgsbildung bis jetzt gefundenen Reptilien von H. von Meyer. xxxi + 83 pp. Verlag von Oehme und Müller, Braunschweig.
Dumortier, B.-C. 1829. Analyse des familles des plantes avec l’indication des principaux genres qui s’y rattachent. 104 pp. Imprimerie de J. Casterman, Aîné, Tournay. Crossref
Durand, S. and Louail, J. 1976. Intérêt stratigraphique du sondage de Loudun (Vienne) pour l’étude de Cénomanien de l’ouest de la France. Comptes rendus hebdomadaires des séances de l’Académie des sciences, Série D Sciences naturelles 283: 1719–1722.
Dybová, S. and Jachowicz, A. 1957. Mikrospory górnośląskiego karbonu produktywnego. Prace Instytutu Geologicznego 23: 1–328.
Ehrenberg, C.G. 1838. Über das Massenverhältniss der jetzt lebenden Kiesel-Infusorien und über ein neues Infusorien-Conglomerat als Polirschiefer von Jastraba in Ungarn. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, aus dem Jahre 1836, Physikalische Klasse 1838: 109–135.
Eisenack, A. 1972. Kritische Bemerkung zur Gattung Pterospermopsis (Chlorophyta, Prasinophyceae). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1972 (10): 596–601.
Eklund, H. and Kvaček, J. 1998. Lauraceous inflorescences and flowers from the Cenomanian of Bohemia (Czech Republic, Central Europe). International Journal of Plant Sciences 159: 668–686. Crossref
Ellis, B., Daly, D.C., Hickey, L.J., Johnson, K.R., Mitchell, J.D., Wilf, P., and Wing, S.L. 2009. Manual of Leaf Architecture. 190 pp. The New York Botanical Garden Press, New York
El-Soughier, M.I., Mehrotra, R.C., Zhoua, Z.-Y., and Shi, G.-L. 2011. Nypa fruits and seeds from the Maastrichtian–Danian sediments of Bir Abu Minqar, South Western Desert, Egypt. Palaeoworld 20: 75–83. Crossref
Endlicher, S.L. 1847. Synopsis Coniferarum. iv + 368 pp. Scheitlin & Zollikofer, Sangalli. Crossref
Engler, A. 1892. Syllabus der Vorlesungen über specielle und medicinisch-pharmaceutische Botanik: eine Übersicht über das Pflanzensystem mit Berücksichtigung der Medicinal-und Nutzpflanzen. 143 pp. Gebrüder Borntraeger, Berlin. Crossref
Engler, A. and Prantl, K.A.E. 1897. Die natürlichen Pflanzenfamilien, nebst ihren Gattungen und wichtigeren Arten. Nachträge zum II.–IV. Teil. 380 pp. Verlag von Wilhelm Engelman, Leipzig.
Engler, A. 1910. Die Pflanzenwelt Afrikas, insbesondere seiner tropischen Gebiete. Grundzüge der Pflanzenverbreitung in Afrika und die Charakterpflanzen Afrikas. I. Band: Allgemeiner Überblick über die Pflanzenwelt Afrikas und ihre Existenzbedingungen. 1029 pp. Wilhelm Engelmann, Leipzig. Crossref
Ettingshausen, C. 1868. Die fossile Flora der älteren Braunkohlenformation der Wetterau. Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der kaiserlichen Akademie der Wissenschaften 57: 807–893.
Evitt, W.R., Clarke, R.F.A., and Verdier, J.-P. 1967. Dinoflagellate studies III. Dinogymnium acuminatum n. gen., n.sp. (Maastrichtian) and other fossils formerly referable to Gymnodinium Stein. Stanford University Publications, Geological Sciences 10 (4): 1–27.
Falcon-Lang, H.J., Mages, V., and Collinson, M. 2016. The oldest Pinus and its preservation by fire. Geology 44: 303–306. Crossref
Firtion, F., 1952. Le Cénomanien inférieur du Nouvion-en-Thiérache: examen micropaléontologique. Annales de la Société géologique du Nord 72: 150–163.
Fontaine, W.M. 1889. The Potomac or younger Mesozoic flora. Monographs of the U.S. Geological Survey 15: 1–377.
Fradkina, A.F. 1967. Spore-pollen complexes of the Mesozoic of Western Yakutiya (Vilyuysk Syneclise and Priverkhoyansk Depression) [in Russian]. In: A.I. Tomskaâ and V.F. Vozin (eds.), Sporovo-pyl’cevye kompleksy Mezozoâ Zapadnoj Âkutii (Vilûjskaâ Sinekliza i Priverhnoânskij Progib). 124 pp. Âkuckoe Territorialnoe Geologičeskoe Upravlenie, Centralnaâ Kompleksnaâ Laboratoriâ, Leningrad.
Frank, A.B. 1877. Synopsis der Pflanzenkunde: Ein Handbuch für höhere Lehranstalten und für alle, welche sich wissenschaftlich mit der Naturgeschichte der Pflanzen beschäftigen wollen, von Dr. Johannes Leunis, weiland Professor der Naturgeschichte am Jospehinum in Hildesheim. Zweite gänzlich umgearbeitete Auflage mit 1229 Holzschnitten und dem Portrait des Professor Dr. Leunis. Nach den Tode des Verfassers fortgesetzt und gezüglich der Kryptogamen neu bearbeitet von Dr. A.B. Frank. 2052 pp. Hahn’sche Buchhandlung, Hannover.
Frič, A. 1897. Studien im Gebiete der böhmischen Kreideformation. Palaeontologische Untersuchungen der einzelnen Schichten. VI. Die Chlomeker Schichten. Archiv für naturwissenschaftliche Landesdurchforschung von Böhmen 10 (4): 3–84.
Friedrich, P. 1883. Beiträge zur Kenntnis der Tertiärflora der Provinz Sachsen. Abhandlungen zur geologische Spezialkarte von Preussen u. den Thüringischen Staaten 4 (3): 159–463.
Friis, E.M., Crane, P.R., and Pedersen, K.R. 2011. Early Flowers and Angiosperm Evolution. 586 pp. Cambridge University Press, Cambridge. Crossref
Friis, E.M., Pedersen, K.R., and Schönenberger, J. 2006. Normapolles plants: a prominent component of the Cretaceous rosid diversification. Plant Systematics and Evolution 260: 107–140. Crossref
Fuente, M. de la and Zetter, R. 2016. Palynomorphs. In: F.J. Poyato-Ariza and Á.D. Buscalioni (eds.), Las Hoyas: A Cretaceous Wetland. A Multidisciplinary Synthesis After 25 Years of Research on an Exceptional Fossil Lagerstätte from Spain, 31–42. Verlag Dr. Friedrich Pfeil, München. Crossref
Geinitz, H.B. 1839–1842. Characteristik der Schichten und Petrefacten des sächsisch-böhmischen Kreidegebirges. 166 + xxv pp. Arnoldische Buchhandlung, Dresden. Crossref
Geinitz, H.B. 1849–1855. Das Quadersandsteingebirge oder Kreidegebirge in Deutschland. iv + 292 pp. Craz & Gerlach, Freiberg. Crossref
Gies, T.F. 1972. Palynology of Sediments Bordering Some Upper Cretaceous Strand Lines in Northwestern Colorado. 356 pp. Ph.D. Thesis, Michigan State University, East Lansing, MI.
Gignoux, M. 1950. Géologie stratigraphique. 4e éd. viii + 735 pp. Masson & Cie, Paris.
Góczán, F. 1964. Stratigraphic palynology of the Hungarian Upper Cretaceous. Acta Geologica 8 (1–4), 229–264.
Góczán, F. and Siegl-Farkas, Á. 1990. Palynostratigraphical zonation of Senonian sediments in Hungary. Review of Palaeobotany and Palynology 66: 361–377. Crossref
Góczán, F., Groot, J.J., Krutzsch, W., and Pacltová, B. 1967. Die Gattungen des “Stemma Normapolles Pflug 1953b” (Angiospermae). Neubeschreibungen und Revision europäischer Formen (Oberkreide bis Eozän). Paläontologische Abhandlungen, Abteilung B – Paläobotanik 2: 427–633.
Goebel, K. von 1881. Beiträge zur vergleichenden Entwickelungsgeschichte der Sporangien. Botanische Zeitung 39: 97–706, 713–720.
Goeppert, H.R. 1836a. Bemerkungen über die Abstammung des Bernsteins. Annalen der Physik und Chemie 38: 624–625. Crossref
Goeppert, H.R. 1836b. Die fossilen Farrnkräuter. Systema filicum fossilium. Nova Acta Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum 17 (Supplement): vii–xxxii, 1–487.
Goeppert, H.R. 1841. Über die fossile Flora der Quadersandsteinformation in Schlesien, als erster Beitrag zur Flora der Tertiärgebilde. Nova Acta Academiae Caesareae Leopoldinae Naturae Curiosorum 19 (2): 99–134.
Goeppert, H.R. 1844. Uebersicht der fossilen Flora Schlesiens. In: F. Wimmer (ed.), Flora von Schlesien, preussischen und österreichischen Antheils. Zweiter Band, 157–225. Ferdinand Hirt, Breslau.
Goeppert, H.R. 1854. Die Tertiärflora auf der Insel Java nach den Entdeckungen des Herrn Fr. Junghuhn beschrieben und erörtert in ihrem Verhältnisse zur Gesammtflora der Tertiärperiode. 162 pp. C.W. Mieling, ’s, Gravenhage.
Goeppert, H.R. 1866a. Über die Flora der Kreideformation, insbesondere über die von Schlesien. Jahres-Bericht der Schlesischen Gesellschaft für vaterländische Cultur 23: 51–52.
Goeppert, H.R. 1866b. Ueber die fossile Kreideflora und ihre Leitpflanzen. Zeitschrift der deutschen geologischen Gesellschaft 17: 638–646.
Golovneva, L.B. 1994. A new genus Platimelis in Late Cretaceous–Early Paleogene Arctic floras [in Russian]. Botaničeskij žurnal 79 (1): 98–107.
Golovneva, L.B. 2011. The genus Ettingshausenia (Platanaceae) in Cenomanian–Turonian floras of Eurasia [in Russian]. In: L.B. Golovnëva, A.V. Goman’kov, D.B. Gromyko, and N.V. Nosova (eds.), Paleobotanika, Tom 2, 127–163. Marafon, Sankt Peterburg.
Golovneva, L.B. and Nosova, N.B. 2012. Al’b-senomanskaâ flora zapadnoj Sibiri. 436 pp. Marafon, Sankt Peterburg. Crossref
Górniak, K. 1986. Sedimentation of the Santonian rocks of the North-Sudetic Syncline and their potential for clay mineral deposits. Archiwum Mineralogiczne 41: 123–134.
Gorožankin, I.N. 1904. Lekcìi po morfologìi i sistematikě arhegonial’nyh” rastenìj. II: Pteridophyta. 93 pp. A.I. Mamontov, Moskva.
Goryacheva, A.A., Zorina, S.O., Ruban, D.A., Eskin, A.A., Nikashin, K.I., Galiullin, B.M., Morozov, V.P., Mikhailenko, A.V., Nazarenko, O.V. and Zayats, P.P. 2018. New palynological data for Toarcian (Lower Jurassic) deep-marine sandstones of the Western Caucasus, southwestern Russia. Geologos 24: 127–136. Crossref
Grabowska, I. 1992. Prof. dr Jadwiga Raniecka-Bobrowska (1904–1990). Wiadomości Botaniczne 36 (3/4): 57–59.
Grabowska, I. 2003. Kreda górna. In: S. Dybova-Jachowicz and A. Sadowska (eds.), Palinologia, 169–170, 174–175. Instytut Botaniki PAN, Kraków.
Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G.M. 2012. The Geologic Time Scale 2012. xviii + 1144 pp. Elsevier, Amsterdam.
Graham, A. 2011. The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. American Journal of Botany 98: 336–351. Crossref
Gray, S.F. 1821. A natural arrangement of British plants, according to their relations to each other as pointed out by Jussieu, De Candolle, Brown, &c., including those cultivated for use; with an Introduction to Botany, in which the terms newly introduced and explained; illustrated by figures. Vol. 1. xxviii + 824 pp. Baldwin, Cradock, and Joy, London. Crossref
Greguš, J. and Kvaček, J. 2015. Revision of Cenomanian flora from the Maletín Sandstone. Acta Musei Naturalis Pragae, Series B, Historia Naturalis 71: 315–364.
Greguš, J., Kvaček, J., and Halamski, A.T. 2013. Revision of Protopteris and Oncopteris tree fern stem casts from the Late Cretaceous of Central Europe. Acta Musei Nationalis Pragae, Series B – Historia Naturalis 69: 69–82. Crossref
Groot, J.J. and Groot, C.R. 1962. Plant microfossils from Aptian, Albian and Cenomanian deposits of Portugal. Comunicações dos Serviços Geológicos de Portugal 46: 133–171.
Groot, J.J., Penny, J.S., and Groot, C.R. 1961. Plant microfossils and age of the Raritan, Tuscaloosa, and Magothy formations of the eastern United States. Palaeontographica, Abteilung B 108: 121–140.
Guy-Ohlson, D. 1996. Prasinophycean algae. In: J. Jansonius and D.C. McGregor (eds.), Palynology: Principles and Applications, Vol. 1, 181–189. American Association of Stratigraphic Palynologists Foundation, Dallas, Texas.
Haacke, O. 1924. Das Steinkohlenvorkommen in der Kreide formation im Kreise Löwenberg-Bunzlau in Schlesien. Kohle und Erz 21: 625–632.
Halamski, A.T. 2013. Latest Cretaceous leaf floras from south-eastern Poland and western Ukraine. Acta Palaeontologica Polonica 58: 407–443.
Halamski, A.T. and Kvaček, J. 2013. The type specimen of Debeya (Dewalquea) haldemiana rediscovered. Acta Musei Nationalis Pragae, Series B Historia Naturalis 69: 83–86. Crossref
Halamski, A.T. and Kvaček, J. 2015. The Late Cretaceous (Coniacian) leaf and cone flora from the Sudetes. Palaeontographica, Abteilung B 292: 95–171. Crossref
Halamski, A.T. and Kvaček, J. 2016. The Coniacian leaf flora from the north-eastern part of the Bohemian Cretaceous Basin. Bulletin of Geosciences 91: 297–318. Crossref
Halamski, A.T. and Kvaček, J. 2018. Trends and regional diversification of Late Cretaceous vegetation as evidenced by Central European megafloras. In: 10th European Palaeobotany & Palynology Conference, Program & Abstracts University College Dublin, Ireland, 12–17 August 2018, 191–192. University College, Dublin.
Halamski, A.T. and Kvaček, J. 2019. The most common species in the Cretaceous of the Lwówek Syncline. In: G. Skrzyński and M. Badura (eds.), Sympozjum Sekcji Paleobotanicznej Polskiego Towarzystwa Botanicznego, 7 grudnia 2019 r., 24. Polska Akademia Nauk, Muzeum Ziemi, Warszawa.
Halamski, A.T., Kvaček, J., and Vajda, V. 2016. Late Cretaceous (Campanian) leaf and palynoflora from southern Skåne, Sweden. In: B.P. Kear, J. Lindgren, J.H. Hurum, J. Milàn, and V. Vajda (eds.), Mesozoic Biotas of Scandinavia and its Arctic Territories. Geological Society, London, Special Publications 434: 207–229. Crossref
Halamski, A.T., Kvaček, J., and Svobodová, M. 2018a. Fossil mega- and microflora from the Březno beds s.s. (Bohemian Cretaceous Basin, Coniacian). Review of Palaeobotany and Palynology 253: 123–138. Crossref
Halamski, A.T., Kvaček, J., Heřmanová, Z., Durska, E., Svobodová, M., and Raczyński, P. 2018b. Nowe dane o makro-, mezo- i mikroflorze późnokredowej Niecki Północnosudeckiej. In: G. Skrzyński, M. Badura, and A.M. Noryśkiewicz (eds.), Sympozjum Sekcji Paleobotanicznej Polskiego Towarzystwa Botanicznego, 8 grudnia 2018 r., 22–23. Polska Akademia Nauk, Muzeum Ziemi, Warszawa.
Halamski, A.T., Kvaček, J., Heřmanová, Z., Durska, E., and Svobodová, M. 2019. Mega-, meso-, and microflora from Coniacian–Santonian boundary beds in Lower Silesia. In: L. Frey (ed.), 58th Congress of the Polish Botanical Society: Botany without borders. Abstracts of Lectures and Posters of the 58th PBS Congress. Kraków, 1–7 July 2019, 220. Polish Botanical Society, Kraków.
Hall, J.W. 1967. Invalidity of the name Chrysotheca Miner for microfossils. Journal of Palaeontology 41: 1298–1299.
Hargrove, D.C. and Engelhardt, D.W. 1997 Palynology of the Maestrichtian/Danian boundary at Savannah River Site, South Carolina. Sedimentary Geology 108: 121–140. Crossref
Harris, T. M. 1961. The Yorkshire Jurassic flora. I. Thallophyta–Pteridophyta. 212 pp. British Museum (Natural History), London. Crossref
Harris, T.M. 1979. The Yorkshire Jurassic Flora. V. Coniferales. 166 pp. British Museum (Natural History), London.
Harris, T.M. 1981. Burnt ferns from the English Wealden. Proceedings of the Geologists’ Association 92: 47–58. Crossref
Hedlund, R.W. 1966. Palynology of the Red Branch Member of the Woodbine Formation, (Cenomanian), Bryan County, Oklahoma. Oklahoma Geological Survey, Bulletin 112: 1–69.
Heer, O. 1869. Beiträge zur Kreideflora, I. Flora von Moletein in Mähren. Neue Denkschriften der Allgemeinen Schweizerischen Gesellschaft für die Gesammten Naturwissenschaften 23: 1–24.
Heer, O. 1871. Beiträge zur Kreideflora II. Zur Kreideflora von Quedlinburg. Neue Denkschriften der Allgemeinen Schweizerischen Gesellschaft für die gesammten Naturwissenschaften 24 (2): 1–15.
Heer, O. 1882. Flora fossilis Arctica. Vol. VI, Part 2: Die fossile flora Grönlands, erster Theil. 12 pp. Friedrich Schultess, Zürich.
Heer, O. 1922. Flora fossilis Grønlandica. Afbildninger af Grønlands fossile Flora. Meddeelelser om Grønland, Tillæg til 5. Hefte, 2. 27 pp. Udgave, Kjøbenhavn.
Herman, A.B. and Kvaček, J. 2010. Late Cretaceous Grünbach Flora of Austria. 224 pp. Naturhistorisches Museum, Wien.
Herman, A.B. [German, A.B.] and Lebedev, E.L. 1991. Stratigrafiâ i flora melovyh otloženij severo-zapadnoj Kamčatki. 189 pp. Nauka, Moskva.
Heřmanová, Z., Dašková, J., Ekrt, B., and Kvaček, J. 2017. Zlivifructus gen. nov., a new member of the Normapolles complex. Review of Palaeobotany and Palynology 246: 177–184.
Heřmanová, Z., Kvaček, J., and Halamski, A.T. 2018. Preliminary report on plant reproductive structures from Rakowice Małe (Late Cretaceous, south-western Poland). In: J. Pšenička, J. Frojdová, A. Svobodová, and J. Dašková (eds.), 19th Czech-Slovak-Polish Palaeontological Conference & MIKRO 2018 workshop. Folia Musei Rerum Naturalium Bohemiae Occidentalis, Geologica et Paleobiologica Special Volume, 27. West Bohemian Museum, Pilsen.
Heřmanová, Z., Kvaček, J., Halamski, A.T., Zahájská, P., and Šilar, J. 2019. Reinterpretation of fossil reproductive structures Zlivifructus microtriasseris (Normapolles complex, Fagales) from the Czech and Polish Late Cretaceous. Review of Palaeobotany and Palynology 268: 88–94. Crossref
Hisinger, W. 1837. Lethæa Suecica, seu Petrificata Sueciæ, iconibus et characteribus illustrata. 124 pp. P.A. Norstedt et filius, Holmiæ.
Hill, R.S. 1986. Lauraceous leaves from the Eocene of Nerriga, New South Wales. Alcheringa 10: 327–351. Crossref
Hollick, A. 1930. The Upper Cretaceous floras of Alaska. With a description of the plant-bearing beds by G.C. Martin. United States Geological Survey, Professional Paper 159: iii–v, 1–123. Crossref
Hollick, A. and Jeffrey, E.C. 1906. Affinities of certain Cretaceous plant remains commonly referred to the genera Dammara and Brachyphyllum. The American Naturalist 40: 189–215. Crossref
Hollick, A. and Jeffrey, E.C. 1909. Studies of Cretaceous coniferous remains from Kreischerville, New York. Memoirs of the New York Botanical Garden 3: 1–76.
Hosius, A. and von der Marck, W. 1880. Die Flora der Westfälischen Kreideformation. Palaeontographica 26: 127–241.
Hradecká, L., Lobitzer, H., Ottner, F., Švábenická, L., and Svobodová, M. 1999. Biostratigraphy and facies of selected exposures in the Grünbach-Neue Welt Gosau Group (Coal-bearing series, Inoceramus-Marl and Zweiersdorf-Formation, Late Cretaceous and Paleocene, Lower Austria). Abhandlungen der Geologischen Bundesanstalt 56: 519–551.
Hughes, N.F. 1958. Paleontological evidence for the age of the English Wealden. Geological Magazine 95: 41–49. Crossref
Hutchinson, E.S. 2005. Geology of North-West Borneo: Sarawak, Brunei, and Sabah. xxii + 421 pp. Elsevier, Amsterdam. Crossref
Ivanova, E.A. and Markova, L.T. 1961. Schizaeaceae. In: S.R. Samojlovitch and N.D. Mtchedlischvili (eds.), Pollen and spores of western Siberia. Jurassic to Palaeocene. Trudy VNIGRI 177: 64–112.
Jameossanaie, A. 1987. Palynology and age of South Hospah coal-bearing deposits, McKinley County, New Mexico. New Mexico Bureau of Mines & Mineral Resources, Bulletin 112: 1–65.
Johnson, K.R. 1996. Description of seven common fossil leaf species from the Hell Creek Formation (Upper Cretaceous: Upper Maastrichtian), North Dakota, South Dakota, and Montana. Proceedings of the Denver Museum of Natural History, Series 3 12: 1–48.
Jones, J.H., Manchester, S.R., and Dilcher, D.L. 1988. Dryophyllum Debey ex Saporta, juglandaceous not fagaceous. Review of Palaeobotany and Palynology 56: 205–211. Crossref
Jongmans, W.J. and Dijkstra, S.J. 1973. Fossilium Catalogus. II: Plantae. Pars 84: Gymnospermae (Ginkgophyta et Coniferae) VI, 559–686. Uitgeverij Dr. W. Junk N.V., ’s-Gravenhage.
Jończy, D. 2016. Mapa turystyczna – Dolina Bobru od Jeleniej Góry do Bolesławca. Skala 1 : 50 000. Wydawnictwo Turystyczne PLAN, Jelenia Góra.
Jussieu, A.L. de 1789. Genera plantarum secundum ordines naturales disposita, juxta methodum in Horto Regio Parisiensi exaratam, anno M. DCC. LXXIV. (24) + lxxij + 498 pp. Apud viduam Herissant, typographum, et Theophilum Barrois, Parisiis. Crossref
Juhász, M. 1977. Monolete spores of Schizaeaceae from Hungarian Albian deposits. Acta Biologica Hungarica 23: 19–38.
Juhász, M. 1979. Dispersed Matoniaceae spores from the Hungarian Lower and Middle Cretaceous sediments. Acta Universitatis Szegediensis, Acta Biologica 25: 33–47.
Kara-Murza, E.N. 1954. Spores and pollen from the Mesozoic sediments of the Yenisei-Lena region (Jurassic–Cretaceous) [in Russian]. Trudy naučno-issledovatel’skogo Instituta Arktiki 4: 1–191.
Kaufmann, E.G. 1995. Global change leading to biodiversity crisis in a greenhouse world: The Cenomanian–Turonian (Cretaceous) mass extinction. In: National Research Council (eds.), Effects of Past Global Change on Life. National Research Council (US) Panel on Effects of Past Global Change on Life, 47–71. National Academies Press, Washington D.C.
Kaulfuss, G.W. 1827. Das Wesen der Farrnkräuter, besonders ihre Fruchtteile, zugleich mit Rücksicht auf systematische Anordnung betrachtet und mit einer Darstellung der Entwickelung der Pteris serrulata aus dem Samen begleitet. xxiv + 117 pp. C. Cnobloch, Leipzig.
Kedves, M. 1980. Palynological investigations on sediments of Lower Danian (Fish Clay, Denmark). Acta Universitatis Szegediensis, Acta Mineralogica-Petrographica 24: 167–186.
Kedves, M. 1986. Introduction to the Palynology of Pre-Quarternary Deposits, Part I. 164 pp. Akademiai Kiado, Hungarian Academy of Sciences, Budapest.
Kedves, M. and Hegedus, M. 1975. Pollen grains of the Interporopollenites fgen. from sediments of the Upper Cretaceous period of Portugal. Acta Universitatis Szegediensis, Acta Biologica 21: 48–62.
Kemp, E.M. and Harris, W.K. 1977. The palynology of early Tertiary sediments Ninetyeast Ridge Indian Ocean. Special Papers in Palaeontology 19: 1–70.
Kędzierski, M. 2005. Paleogeografia wschodniej części basenu wokółsudeckiego w cenomanie, turonie i koniaku. In: J. Skoczylas (ed.), Referaty. Tom XIV, 49–58. Uniwersytet im. A. Mickiewicza i Polskie Towarzystwo Geologiczne, Oddział w Poznaniu, Poznań.
Kemp, E.M. 1970. Aptian and Albian miospores from Southern England. Palaeontographica Abteilung B 131: 73– 143.
Klaus, W. 1960. Sporen der karnischen Stufe der ostalpinen Trias. Jahrbuch des Geologischen Bundesanstalt, Sonderband 5: 107–183.
Kley, J. and Voigt, T. 2008. Late Cretaceous intraplate thrusting in central Europe: Effect of Africa-Iberia-Europe convergence, not Alpine collision. Geology 36: 839–842. Crossref
Klocke, B. 1864. Die thonigen Schichten, welche die Kohle der Kreideformation bei Ullersdorf am Queis begleiten. Neues Lausitzisches Magazin 41: 260–265.
Knauer, J. and Siegl-Farkas, A. 1992. Palynostratigraphic position of the Senonian Beds overlying the Upper Cretaceous Bauxite Formations of the Bakony Mts. A Magyar Allami Fóldtani Intézet ávi Jelentése 1990: 463–471.
Knobloch, E. 1964. Neue Pflanzenfunde aus dem südböhmischen Senon. Jahrbuch der staatlichen Museums für Mineralogie und Geologie 1964: 133–201.
Knobloch, E. 1970. Ermöglichkeit die Paläokarplogie Aussagen zur Genese und Biostratigraphie der jungen Flyschsedimente? Mitteilungen der bayerischen Staatssammlung für Paläontologie und historische Geologie 10: 297–308.
Knobloch, E. 1971. Fossile Früchte und Samen aus der Flyschzone der mährischen Karpaten. Sborník geologických věd, Paleontologie 13: 7–46.
Knobloch, E. and Mai, D.H. 1983. Carbonized seeds and fruits from the Cretaceous of Bohemia and Moravia and their stratigraphic significance. Knihovnička Zemního plynu a nafty 4: 305–332.
Knobloch, E. and Mai, D.H. 1986. Monographie der Früchte und Samen in der Kreide von Mitteleuropa. Rozpravy Ústředního ústavu geologického 47: 1–219.
Knobloch, E. and Mai, D. 1991. Evolution of middle and Upper Cretaceous floras in Central and Western Europe. Jahrbuch der Geologischen Bundesanstalt 134: 257–270.
Knowlton, F.H. 1917. A fossil flora from the Frontier Formation of Southwestern Wyoming. United States Geological Survey Professional Paper 108: 73–94. Crossref
Kodrul, T.M. and Maslova, N.P. 2007. A new species of the genus Platimeliphyllum N. Maslova from the Paleocene of the Amur Region, Russia. Paleontological Journal 41: 1108–1117. Crossref
Kondracki, J. 1998. Geografia regionalna Polski. 441 pp. Wydawnictwo Naukowe PWN, Warszawa.
Konijnenburg-van Cittert, J.H.A. van 1993. A review of the Matoniaceae based on in situ spores. Review of Palaeobotany and Palynology 78: 235–267.
Konijnenburg-van Cittert, J.H.A. van and Morgans, H.S. 1999. Palaeontological Association Field Guides to Fossils: Number 8. The Jurassic Flora of Yorkshire. 134 pp. The Palaeontological Association, London. Crossref
Kozdrój, W., Krentz, O., and Opletal, M. (eds.) 2001. Comments on the Geological Map Lausitz-Jizera-Karkonosze (Without Cenozoic Sediments) 1:100 000. 64 pp. Państwowy Instytut Geologiczny, Warszawa.
Krassilov, V.A. 1979. Melovaâ flora Sahalina. 183 pp. Nauka, Moskva.
Krassilov, V.A. 2003. Terrestrial Palaeoecology and Global Change. 464 pp. Pensoft Publishers, Sofia.
Krassilov, V.A., Lewy, Z., Nevo, E., and Silantieva, N. 2005. Late Cretaceous (Turonian) Flora of Southern Negev, Israel. 252 pp. Pensoft Publishers, Sofia. Crossref
Kremp, G.O.W. 1949. Pollen analysis of the Miocene Brown Coal Bed of Konin on the Warthe River. Palaeontographica Abteilung B 90: 53–93.
Krutzsch, W. 1958. Sporen und Pollengruppen aus der Oberkreide und dem Tertiär Mitteleuropas und ihre stratigraphische Verbreitung. Zeitschrift für angewandte Geologie 3: 509–548.
Krutzsch, W. 1959a. Einige neue Formgattungen und -Arten von Sporen und Pollen aus der mitteleuropäischen Oberkreide und dem Tertiär. Palaeontographica Abteilung B 105: 125–157.
Krutzsch,W. 1959b. Mikropaläontologische (sporenpaläontologische) Untersuchungen in der Braunkohle des Geiseltales. Geologie: Beihefte 21–22: 1–425.
Krutzsch, W. 1963. Atlas der Mittel-Und Jungtertiaren Dispersen sporen- und pollen-Sowie der Mikroplanktonformen des Nordlichen Mittel-Euroas. Abhandlungen des Zentralen Geologischen Institutes 2: 1–141.
Krutzsch, W. 1966a. Die sporenstratigraphische Gliederung der Oberkreide in nördlichen Mittel-Europa. Abhandlungen des Zentralen Geologischen Institutes 8: 79–111.
Krutzsch, W. 1966b. Zur Kenntnis der präquartären periporaten Pollenformen. Geologie 55: 16–71.
Krutzsch, W. 1967. Atlas der der mittel- und jungtertiären dispersen Sporen- und Pollen- sowie der Mikroplanktonformen des nördlichen Mitteleuropas. Lieferung IV und V. Weitere azonotrilete (apiculate, murornate), zonotrilete, monolete und alete Sporenformen. 232 pp. VEB Gustav Fischer Verlag, Jena.
Krutzsch, W. 1973. Über Taurocusporites Stover und neue oberkretazische Arten vor allem aus Europa. Abhandlungen des Zentralen Geologischen Institutes 18: 77–98.
Kühn, B. and Zimmermann, E. 1918. Erläuterungen zur geologischen Karte von Preussen. Lfg. 202. Blatt Gröditzberg. Gradabteilung 61, Nr. 51. 63 pp. Königliche Preussische Geologische Landesanstalt, Berlin.
Kunzmann, L. 1999. Koniferen der Oberkreide und ihre Relikte im Tertiär Europas. Abhandlungen der Staatlichen Museums für Mineralogie und Geologie zu Dresden 45: 1–191.
Kunzmann, L. 2010. Geinitzia reichenbachii (Geinitz, 1842) Hollick and Jeffrey, 1909 and Sedites rabenhorstii Geinitz, 1842 (Pinopsida; Late Cretaceous) reconsidered and redescribed. Review of Palaeobotany and Palynonogy 159: 123–140. Crossref
Kunzmann, L., Knoll, H., and Gaipl, R. 2003. Neue Untersuchungen an Geinitzia Endl. 1847 aus den Aachener Schichten von Belgien und Deutschland (Oberes Santon, Oberkreide). Feddes Repertorium 114: 1–24. Crossref
Kutuzkina, E.F. 1974. Protophyllum Lesq. [in Russian]. In: A. Takhtajan (ed.), Magnoliophyta fossilia URSS. Volumen 1: Magnoliaceae–Eucommiaceae, 145–150. Nauka, Leninopolis.
Kuvaeva, S.B. 1972. Spores of a new genus Corniculatisporites from Cretaceous deposits of the Caucasus and the Crimea. Journal of Palynology of India 7: 20–25.
Kvaček, J. 2000. Frenelopsis alata and its microsporangiate and ovuliferous reproductive structures from the Cenomanian of Bohemia (Czech Republic, Central Europe). Review of Palaeobotany and Palynology 112: 51–78. Crossref
Kvaček, J. 2006. Platanoid staminate inflorescence and its associated foliage from the Bohemian Cenomanian (Czech Republic). Acta Universitatis Carolinae Geologica 47: 67–72. Crossref
Kvaček, J. 2013a. Fricia nobilis from the Turonian of the Czech Republic reinterpreted as an ovuliferous cone of a cupressoid conifer. Acta Musei Nationalis Pragae, Series B Historia Naturalis 69: 123–128. Crossref
Kvaček, J. 2013b. Pinus landsbergensis sp. nov., new pine from the Cenomanian of the Czech Republic. Bulletin of Geosciences 88: 829–836. Crossref
Kvaček, J. 2017. Late Cretaceous floras in Central Europe and Their Palaeoenvironment. 84 pp. Habilitační práce, Ústav geologie a paleontologie, Přírodovědecká fakulta, Univerzity Karlovy, Prague.
Kvaček, J. and Dašková, J. 2010. Konijnenburgia, a new genus of the fern family Matoniaceae. Review of Palaeobotany and Palynology 158: 308–318. Crossref
Kvaček, J. and Friis, E.M. 2010. Zlatkocarpus gen. nov., a new angiosperm reproductive structure with monocolpate-reticulate pollen from the Late Cretaceous (Cenomanian) of the Czech Republic. Grana 49: 115–127. Crossref
Kvaček, J. and Váchová, Z. 2006. Revision of platanoid foliage from the Cretaceous of the Czech Republic. Časopis Národního muzea, Řada přírodovědná 175: 77–89.
Kvaček, J., Gomez, B., and Zetter, R. 2012. The early angiosperm Pseudoasterophyllites cretaceus from Albian–Cenomanian of Czech Republic and France revisited. Acta Palaeontologica Polonica 57: 437–443. Crossref
Kvaček, J., Halamski, A.T., Svobodová, M., and Durska, E. 2015. Coniacian flora of the Sudetes (south-western Poland): Palaeoecological and palaeoclimatic interpretations. Palaeogeography, Palaeoclimatology, Palaeoecology 436: 178–187. Crossref
Kvaček, J., Uličný, D., Svobodová, M., and Špičáková, L. 2006. Cretaceous of Central Bohemia. In: O. Fatka and J. Kvaček (eds.), Excursion Field Guide of the 7th EPPC, Prague September 6–12, 2006, 58–65. Národní Muzeum, Praha.
Kvaček, Z. 1971. Supplementary notes on Doliostrobus Marion. Palaeontographica, Abteilung B 135: 115–126.
Kvaček, Z. 2002. Novelties on Doliostrobus (Doliostrobaceae), an extinct conifer genus of the European Palaeogene. Časopis Národního muzea 171: 131–175.
Kvaček, Z. 2010. Forest flora and vegetation of the European early Palaeogene—a review. Bulletin of Geosciences 85: 63–76. Crossref
Kvaček, Z. and Manum, S.B. 1993. Ferns in the Spitsbergen Palaeogene. Palaeontographica, Abteilung B 230:169–181.
Langenhan, H. and Grundey, M. 1891. Das Kieslingswalder Gestein und seine Versteinerungen. Jahresbericht nebst Mitglieder-Verzeichnis des Glatzer Gebirgs-Verein 10: 3–12.
Leffingwell, H.A. 1970. Palynology of the Lance (Late Cretaceous) and Fort Union (Paleocene) formations of the type Lance area, Wyoming. In: R.M. Kosanke and A.T. Cross (eds.), Symposium on Palynology of the Late Cretaceous and Early Tertiary. Geological Society of America, Special Paper 127: 1–64. Crossref
Lentin, J.K. and Williams, G.L. 1976. A monography of fossil peridinioid dinoflagellate cysts. Bedford Institute of Oceanography Report, Series BI-R-75-16: 1–237.
Lentin, J.K. and Williams, G.L. 1981. Fossil dinoflagellates. Index to genera and species. Bedford Institute of Oceanography, Report Series 12: 1–845.
Lentin, J.K. and Vozzhennikova, T.F. 1990. Fossil dinoflagellates from the Jurassic, Cretaceous and Paleogene deposits of the USSR—a re-study. American Association of Stratigraphic Palynologists, Contributions Series 23: 1–221.
Lesquereux, L. 1872. I. Enumeration and description of the fossil plants from the specimens obtained in the explorations of Dr. F.V. Hayden, 1870 and 1871. II. Remarks on the Cretaceous species described above. III. Tertiary flora of North America. United States Geographical and Geological Survey of the Territories, Annual Report 1871: 283–318.
Lesquereux, L. 1873. Enumeration and description of fossil plants from the western Tertiary formations. Descriptions of species of fossil plants from the Cretaceous of Kansas. United States Geographical and Geological Survey of the Territories, Annual Report 1872: 371–427.
Lesquereux, L. 1893. Cretaceous fossil plants from Minnesota. Minnesota Geology Natural History Survey Final Report 3 (1): 1–22. Crossref
Lestiboudois, T. 1826. Botanographie élémentaire, ou Principes de botanique, d’anatomie et de physiologie végétale; contenant la description des organes des plantes; la définition des termes usités en botanique; une théorie nouvelle sur l’anatomie et la physiologie végétale; l’art de décrire les plantes; l’exposé des méthodes les plus emploýees en botanique et la description des familles naturelles. xx + 559 pp. Vanackere, père and fils, Paris.
Leszczyński, S. 2010. Coniacian–?Santonian paralic sedimentation in the Rakowice Małe area of the North Sudetic Basin, SW Poland: sedimentary facies, ichnological record and palaeogeographical reconstruction of an evolving marine embayment. Annales Societatis Geologorum Poloniae 80: 1–24.
Leszczyński, S. 2018. Integrated sedimentological and ichnological study of the Coniacian sedimentation in North Sudetic Basin, SW Poland. Geological Quarterly 62: 767–816. Crossref
Levin, D.A. 2000. The Origin, Expansion, and Demise of Plant Species. 230 pp. Oxford University Press, Oxford.
Li, C., Miao, X., Zhang L.-B., Ma, J., and Hao, J. 2020. Re-evaluation of the systematic position of the Jurassic–Early Cretaceous fern genus Coniopteris. Cretaceous Research 105: 104–136. Crossref
Li, F.-W., Villarreal, J.C., Kelly, S., Rothfels, C.J., Melkonian, M., Frangedakis, E., Ruhsam, M., Sigel, E.M., Der, J.P., Pittermann, J., Burge, D.O., Pokorny, L., Larsson, A., Chen, T., Weststrand, S., Thomas, P., Carpenter, E., Zhang, Y., Tian, Z., Chen, L., Yan, Z., Zhu, Y., Sun, X., Wang, J., Stevenson, D.W., Crandall-Stotler, B.J., Shaw, A.J., Deyholos, M.K., Soltis, D.E., Graham, S.W., Windham, M.D. Langdale, J.A., Wong, G. K.-S., Mathews, S., and Pryer, K.M. 2014 Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proceedings of the National Academy of Sciences 111: 6672–6677. Crossref
Lindley, J. 1830. An Introduction to the Natural System of Botany: or, A Systematic View of the Organisation, Natural Affinities, and Geographical Distribution, of the Whole Vegetable Kingdom: Together with the Uses of the Most Important Species in Medicine, the Arts, and Rural or Domestic Economy. 374 pp. Longman, Rees, Orme, Brown, and Green, London.
Lindley, J. 1836. A natural system of botany; or, A systematic view of the organisation, natural affinities, and the geographical distribution, of the whole vegetal kingdom; together with the uses of the most important species in medicine, the arts, and rural or domestic economy. Second edition. xxvi + 526 pp. Longman, Rees, Orme, Brown, Green, and Longman. London. Crossref
Link, H.F. 1833a. Handbuch zur Erkennung der nutzbarsten und am häufigsten vorkommenden Gewächse. Dritter Theil. xviii + 536 pp. Haude und Spenersche Buchhandlung, Berlin.
Link, H.F. 1833b. Hortus Regius Botanicus Berolinensis, descriptus ab. Tomus 2. iv + 376 pp. Apud G. Reimer, Berolini.
Linnaeus, C. 1753. Species plantarum, exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. Tomus 1. 560 pp. Laurentii Salvii, Holmiae. Crossref
Linnert, C., Robinson, S.A., Lees, J.A., Bown, P.R., Pérez-Rodríguez, I., Petrizzo, M.R., Falzoni, F., Littler, K., Arz, J.A., and Russell, E.E. 2014. Evidence for global cooling in the Late Cretaceous. Nature Communications 5: 4194. Crossref
Lilpop, J. and Kostyniuk, M. 1957. Roślinność Polski w epokach minionych. Wydanie 2. 319 pp. Wydawnictwa Geologiczne, Warszawa.
Lloyd, G.T., Davis, K.E., Pisani, D., Tarver, J.E, Ruta, M., Sakamoto, M., Hone, D.W.E., Jennings, R., and Benton, M.J. 2008. Dinosaurs and the Cretaceous Terrestrial Revolution. Proceedings of the Royal Society B 275: 2483–2490. Crossref
Maciejak, K. and Maciejak, M. 2013. Coal mining in the Lwówek Śląski area in the 18th–19th ct. In: P.P. Zagożdżon and D. Madziarz (eds.), Dzieje górnictwa – element europejskiego dziedzictwa kultury, Tom 5, 195–207. Oficyna Wydawnicza Politechniki Wrocławskiej, Wrocław.
Macko, S. 1963. Sporomorphs from Upper Cretaceous near Opole (Silesia) and from the London Clays. Prace Wrocławskiego Towarzystwa Naukowego, Seria B 106: 1–136.
Macphail, M.K. and Truswell, E.M. 2004. Palynology of Neogene slope and rise deposits from ODP Sites 1165 and 1167, East Antarctica. Proceedings of the Ocean Drilling Program, Scientific Results 188: 1–20. Crossref
Mägdefrau, K. 1968. Paläobiologie der Pflanzen. Vierte, neubearbeitete Auflage. 549 pp. Gustav Fischer Verlag, Jena.
Manchester, S.R. 1986. Vegetative and reproductive morphology of an extinct plane tree (Platanaceae) from the Eocene of Western North America. Botanical Gazette 147: 200–226. Crossref
Manchester, S.R. 2014. Revisions to Roland Brown’s North American Paleocene flora. Acta Musei Nationalis Pragae, Series B Historia Naturalis 70: 153–210. Crossref
Manum, S.B. 1963. Some new species of Deflandrea and their probable affinity with Peridinium. Norsk Polarinstitutt, Årbok 1962: 55–67.
Marion, A.-F. 1884. Sur les caractères d’une conifère tertiaire, voisine des Dammarées (Doliostrobus sternbergi). Comptes rendus de l’Académie des Sciences 99: 821–823.
Marmi, J., Martín-Closas, C., Fernández-Marrón, M.T., Vicente, A., and Gomez, B. 2016. Latest Cretaceous plant landscapes from Northern Iberia. New Mexico Museum of Natural History and Science, Bulletin 71: 215–229.
Martín-Closas, C., Gomez, B., and Daviero-Gomez, V. 2016. Plants and their landscapes. In: F.J. Poyato-Ariza and Á.D. Buscalioni (eds.), Las Hoyas: A Cretaceous Wetland. A multidisciplinary Synthesis After 25 Years of Research on an Exceptional Fossil Lagerstätte from Spain, 43–56. Verlag Dr. Friedrich Pfeil, München.
Maslova, N.P. 2002. А new plant of the family Platanaceae from the early Paleogene reconstructed on the basis of leaves and inflorescences [in Russian, with English summary]. Palaeontologičeskij žurnal 2002 (2): 89–101.
Maslova, N.P. 2008. Association of vegetative and reproductive organs of platanoids (Angiospermae): Significance for systematics and phylogeny. Paleontological Journal 42: 1393–1404. Crossref
Maslova, N.P. 2010. Systematics of fossil platanoids and hamamelids. Paleontological Journal 44: 1379–1466. Crossref
Maslova, N.P., Moiseeva, M.G., Herman, A.B., and Kvaček, J. 2005. Did plane trees exist in the Cretaceous? Paleontological Journal 39 (4): 98–110.
May, F.E. 1977. Functional morphology, paleoecology, and systematics of Dinogymnium tests. Palynology 1: 103–121. Crossref
Mays, C. and Cantrill, D.J. 2018. Protodammara reimatamoriori, a new species of conifer (Cupressaceae) from the Upper Cretaceous Tupuangi Formation, Chatham Islands, Zealandia. Alcheringa 43: 114–126. Crossref
Mazer, S.J. and Tiffney, B.H. 1982. Fruits of Wetherellia and Palaeowetherellia (?Euphorbiaceae) from Eocene sediments in Virginia and Maryland. Brittonia 34: 300–333. Crossref
McLoughlin, S., Haig, D.W., Siversson, M., and Einarsson, E. 2018. Did mangrove communities exist in the Late Cretaceous of the Kristianstad Basin, Sweden? Palaeogeography, Palaeoclimatology, Palaeoecology 498: 99–114. Crossref
M’Hamdi, A., Slimani, H., Louwye, S., Soussi, M., Ben Ismail-Lattrache, K., and Ben Ali, W. 2015. Les kystes de dinoflagellés et palynofacies de la transition Maastrichtien–Danien du stratotype El Kef (Tunisie). Comptes rendus Palevol 14: 167–180. Crossref
Miers, J. 1851. A few remarks on the Menispermaceæ. Annals and Magazine of Natural History, Second Series 7: 33–45.
Migoń, P. and Lidmar-Bergström, K. 2001. Weathering mantles and their significance for geomorphological evolution of central and northern Europe since the Mesozoic. Earth-Science Reviews 56: 285–324. Crossref
Mikhelis, A.A. 1981. Normapolles pollen in Cretaceous/Palaeogene boundary deposits of the Priazov’ye, (Azov Sea Area). Review of Palaeobotany and Palynology 35: 209–229. Crossref
Miki, A. 1977. Late Cretaceous pollen and spore floras of northern Japan composition and interpretation. Journal of the Faculty of Science, Hokkaido University, Series 4, Geology and Mineralogy 17: 399–436.
Mikulska, J. 1977. Orchidaceae (pars 2), Sparganiaceae, Typhaceae, Araceae, Lemnaceae. In: J. Mądalski (ed.), Florae Polonicae terrarumque adiacentium iconographia. Tom V, Zeszyt 2, 1–57. Państwowe Wydawnictwo Naukowe, Warszawa.
Milewicz, J. 1955. Szczegółowa mapa geologiczna Sudetów 1:25000, arkusz M 33–31 Bd Nowogrodziec. Instytut Geologiczny, Warszawa.
Milewicz, J. 1956a. Szczegółowa mapa geologiczna Sudetów 1:25000, arkusz M 33–32 Ca Lwówek Śląski. Instytut Geologiczny, Warszawa.
Milewicz, J. 1956b. Zaburzenie utworów kredowych w Rakowicach Małych. Przegląd Geologiczny 4: 361–364.
Milewicz, J. 1964. Objaśnienia do szczegółowej mapy geologicznej Sudetów. Arkusz Lwówek Śląski (M 33–32 Ca). 1:25 000. 52 pp. Instytut Geologiczny, Warszawa
Milewicz, J. 1969. Objaśnienia do szczegółowej mapy geologicznej Sudetów. Arkusz Nowogrodziec (M 33–31 Bd). 1:25 000. 53 pp. Instytut Geologiczny, Warszawa.
Milewicz, J. 1976. Objaśnienia do szczegółowej mapy geologicznej Sudetów. Arkusz Tomisław. 1:25 000. 43 pp. Instytut Geologiczny, Warszawa.
Milewicz, J. 1997. Upper Cretaceous of the North-Sudetic depression (litho- and biostratigraphy, paleogeography, tectonics and remarks on raw materials). Acta Universitatis Wratislaviensis, Prace Geologiczno-Mineralogiczne 61: 5–59.
Milewicz, J. 2006. O osadach santońskich na obszarze basenu północnosudeckiego. Przegląd Geologiczny 54: 693–694.
Miller, C.N., Jr. 1988. The origin of modern conifer families. In: C.B. Beck (ed.), Origin and Evolution of Gymnosperms, 448–486. Columbia University Press, New York.
Miller, I.M. and Hickey, L.J. 2008. The fossil flora of the Winthrop Formation (Albian–Early Cretaceous) of Washington State, USA. Part I: Bryophyta and Pteridophytina. Bulletin of the Peabody Museum of Natural History 49: 135–180. Crossref
Miner, E.I. 1935. A new Laccopteris from the Cretaceous of Kansas. Contributions from the Museum of Paleontology, University of Michigan 4: 287–290.
Miquel, F. 1853. De fossiele Planten van het krijt in het hertogdom Limburg. Verhandelingen uitgegeven door de Commissie voor de Geologische Beschrijving en Kaart van Nederland 1: 35–56.
Mirbel, M. 1813. Notes pour servir à l’histoire naturelle de la famille des Orangers de M. A.-L. de Jussieu. Nouveau Bulletin des Sciences, publié par la Société philomatique de Paris 75: 377–383.
Mitura, J., Cieśliński, S., and Milewicz, J. 1969. Upper Cretaceous inoceramids from the North Sudetic Basin. Biuletyn Instytutu Geologicznego 217: 169–177.
Mohr, B. 2009. A truly European forest: A historic Lower Silesian Palaeobotanical Collection (Late Cretaceous) at the Museum of Natural History (Berlin). Earth Sciences History 28: 276–292. Crossref
Moiseeva, M.G. 2012. The Maastrichtian flora of the Amaam Lagoon area (Northeastern Russia). Stratigraphy and Geological Correlation 20: 579–679. Crossref
Muller, J. 1968. Palynology of the Pedawan and Plateau sandstone Formations (Cretaceous-Eocene) in Sarawak, Malaysia, Micropaleontology 14: 1–37. Crossref
Nakoman, E. 1966. Contribution to the palynology of the Tertiary formations of the Thrace Basin. 1. Qualitative study. Annales de la Société géologique du Nord, 65–107.
Nathorst, A.G. 1908. Paläobotanische Mitteilungen 5: Über Nathorstia Heer. Kongliga Svenska vetenskapsakademiens handlingar 43 (6): 14–19.
Němejc, F. 1961. Fossil plants from Klikov in S. Bohemia (Senonian). Rozpravy Československé akademie věd, Řada matematických a přirodních věd 71: 1–47.
Néraudeau, D., Perrichot, V., Batten, D.J., Boura, A., Girard, V., Jeanneau, L., Nohra, Y.A., Polette, F., Martin, S.S., Saint Martin, J.-P., and Thomas, R. 2017. Upper Cretaceous amber from Vendée, north-western France: Age dating and geological, chemical, and palaeontological characteristics. Cretaceous Research 70: 77–95. Crossref
Newberry, J.S. 1868. Notes on the later extinct floras of North America, with descriptions of some new species of fossil plants from the Cretaceous and tertiary strata. Annals of the Lyceum of Natural History of New York 9: 1–76. Crossref
Newberry, J.S. 1895. The flora of the Amboy clays, edited by Arthur Hollick. United States Geological Survey, Monographs 26: 1–260.
Nichols, D.J., Fleming, R.F., and Frederiksen, N.O. 1990. Palynological evidence of effects of the terminal Cretaceous event on terrestrial floras in Western North America. In: E.G. Kauffman and O.H. Walliser (eds.), Extinction Events in Earth History. IGCP, Proceeding of Project 216. Lecture Notes in Earth Sciences 30: 351–364. Crossref
Niebuhr, B. 2019. From animal to plant kingdom: the alleged sponge Siphonia bovista Geinitz from the Cretaceous of Saxony (Germany) in fact represents internal moulds of the cone-like plant fossil Dammarites albens Presl in Sternberg. Bulletin of Geosciences 94: 221–234. Crossref
Nilsson, S. 1832. Fossila Växter funna i Skåne och beskrifne. Kongliga Svenska Vetenskaps-Akademiens Handlingar (ser. 3) 19: 340–351.
Nilsson, T. 1958. Uber das vorkommen eines mesozoischen Sapropelgesteins in Schonen. Lunds universitets årsskrift, N.S. 54: 1–111.
Norris, G. 1967. Spores and pollen from Lower Colorado Group (Albian– ?Cenomanian) of central Alberta. Palaeontographica, Abteilung B 120: 72–115.
Norvick, M.S. and Burger, D. 1976. Palynology of the Cenomanian of Bathurst Island, Northern Territory, Australia. Bureau of Mineral Resources, Geology and Geophysics, Bulletin 151: 1–169.
Olaru, L. 1974. Palynological study of the Upper Cretaceous–Paleocene deposits of the East Carpathian Flysch deposits, (Romania). Analele Ştiinţifice ale Universităţii “Al. I. Cuza” din Iaşi, Serie Nouă, Geologie-Geografie 20: 83–88.
Pacltová, B. 1961. On some plant microfossils from freshwater sediments of the Upper Cretaceous, (Senonian), in the South Bohemian Basis. Part 1. Sbornik Ústředního Ústavu geologického, Paleontologie 26: 47–102.
Pacltová, B. 1968. Some new pollen grains from the Bohemian Cenomanian. Review of Palaeobotany and Palynology 7: 99–106. Crossref
Pacltová, B. 1971. Palynological study of Angiospermae from the Peruc Formation (?Albian–Lower Cenomanian) of Bohemia. Sborník geologických věd, Paleontologie B 13: 105–141.
Pacltová, B. 1981. The evolutiona and distribution of Normapolles pollen during the Cenophytic. Review of Palaeobotany and Palynology 35: 175–208. Crossref
Pacltová, B. and Krutzsch, W. 1970. Neue Pollen- und Sporenarten aus der mittleren Oberkreide, insbesondere Mitteleuropas. Paläontologische Abhandlungen, Abteilung B Paläobotanik 3: 573–598.
Paczoski, J. 1933. Podstawowe zagadnienia geografji roślin. 273 pp. Oddział Poznański Polskiego Towarzystwa Botanicznego, Poznań.
Parker, L.R. 1976. The paleoecology of the fluvial coal-forming swamps and associated floodplain environments in the Blackhawk Formation (Upper Cretaceous) of central Utah. In: A.T. Cross and E.B. Maxfield (eds.), Aspects of Coal Geology, Northwest Colorado Plateau: Some Geologic Aspects of Coal Accumulation, Alteration, and Mining in Western North America, 99–116. Brigham Young University, Provo, UT.
Pavlishina, P., Dochev, D., Nikolov, V., Yaneva, M., and Konyovska, R. 2019. Palynostratigraphy of dinosaur bone-bearing deposits from the Upper Cretaceous of Western Bulgaria. Acta Geologica Polonica 69: 617–626.
Payenberg, T.H.D., Braman, D.R., Davis, D.W., and Miall, A.D. 2002. Litho- and chronostratigraphic relationships of the Santonian–Campanian Milk River Formation in southern Alberta and Eagle Formation in Montana utilising stratigraphy, U-Pb geochronology, and palynology. Canadian Journal of Earth Sciences 39: 1553–1577. Crossref
Peyrot, D., Barrón, E., Polette, F., Batten, D., and Néraudeau, D. 2019. Early Cenomanian palynofloras and inferred resiniferous forests and vegetation types in Charentes (southwestern France). Cretaceous Research 94: 168–189. Crossref
Pflug, H.D. 1953. Zur Entstehung und Entwicklung des angiospermiden Pollens in der Erdgeschichte. Palaeontographica Abteilung B 95: 61–171.
Pierce, R.L. 1961. Lower Upper Cretaceous plant microfossils from Minnesota. Minnesota Geological Survey, Bulletin 42: 1–86.
Płachno, B.J., Jurkowska, A., Pacyna, G., Worobiec, E., Gedl, P., and Świerczewska-Gładysz, E. 2018. Plant assemblage from Opole, southern Poland: New data on Late Cretaceous vegetation of the northern part of the European Province. Proceedings of the Geologists’ Association, 129: 159–170. Crossref
Playford, G. 1971. Palynology of the Basal Cretaceous (Swan River) strata of Saskatchewan and Manitoba. Palaeontology 14: 533–565.
Pocknall, D.T. and Crosbie, Y.M. 1982. Taxonomic revision of some Tertiary tricolporate and tricolpate pollen grains from New Zealand. New Zealand Journal of Botany 20: 7–15. Crossref
Pocock, S.A.J. 1962. Microfloral ananlysis and age determination of strata at the Jurassic–Cretcaeous boundary in the western Canada plains. Palaeontographica, Abteilung B 111: 1–95.
Pocock, S.A.J. 1964. Pollen and spores of Chlamydospermidae and Schizaeaceae from Upper Manville strata of the Saskatoon area of Saskatchewan. Grana 5: 129–209. Crossref
Pocock, S.A.J. 1970. Palynology of the Jurassic sediments of Western Canada. Part 1. Terrestrial species. Palaeontographica, Abteilung B 130: 12–136.
Pocock, S.A.J. and Jansonius, J. 1961. The genus Classopollis Pflug, 1953. Micropaleontology 7: 439–449. Crossref
Polette, F. and Batten, D.J. 2017. Fundamental reassessment of the taxonomy of five Normapolles pollen genera. Review of Palaeobotany and Palynology 243: 47–91. Crossref
Polette, F., Batten, D.J., and Néraudeau, D. 2018. Re-examination of the palynological content of the Lower Cretaceous deposits of Angeac, Charente, south-west France: Age, palaeoenvironment and taxonomic determinations. Cretaceous Research 90: 204–221. Crossref
Potonié, R. 1931. Pollenformen aus tertiären Braunkohle. Jahrbuch der Preussischen geologischen Landesanstalt 52: 1–7.
Potonié, R. 1956. Synopsis der Gattungen der Sporae dispersae I. Teil: Sporites. Beihefte zum geologischen Jahrbuch 23: 3–103.
Potonié, R. 1958. Synopsis der Gattungen der Sporae dispersae II. Teil: Sporites (Nachträge), Saccites, Aletes, Praecolpates, Polyplicates, Monocolpates. Beihefte zum geologischen Jahrbuch 31: 3–114.
Potonié, R. 1960. Synopsis der Gattungen der Sporae dispersae III. Teil: Nachträge Sporites, Fortsetzung Pollenites, mit Generalregister zu Teil I–III. Beihefte zum geologischen Jahrbuch 39: 3–189.
Potonié, R. 1975. Synopsis der Gattungen der Sporae dispersae VII. Teil: Nachträge zu allen Gruppen (Turmae). Fortschritte in der Geologie von Rheinland und Westfalen 25: 23–151.
Potonié, R. and Gelletich, J. 1933. Ueber Pteridophytensporen einer eozänen Braunkohle aus Dorog in Ungarn. Sitzungsberichte der Gesellschaft naturforschender Freunde zu Berlin 33: 317–328.
Prantl, K. 1874. Lehrbuch der Botanik für Mittelschulen. viii + 240 pp. W. Engelmann, Leipzig.
Presl, C.B. 1825. Reliquiae Haenkeanae seu Descriptiones et icones plantarum, quas in America meridionali et boreali, in insulis Philippinis et Marianis collegit Thaddaeus Haenke. xv + 356 pp. J.G. Calve, Praga. Crossref
Presl, K.B. 1848. Die Gefässbündel im Stipes der Farrn. Abhandlungen der Königlichen Böhmischen Gesellschaft der Wissenschaften 5: 309–356.
Prince, L.M. 2007. A brief nomenclatural review of genera and tribes in Theaceae. Aliso 24: 105–121. Crossref
Pysiewicz-Jędrusik, R., Pustelnik, A., and Konopska, B. 1998. Granice Śląska: zmiany granic Śląska w czasie i przestrzeni, Śląsk na dawnej mapie, obraz Sudetów w dawnej kartografii. 105 pp. Rzeka, Wrocław.
Raniecka-Bobrowska, J. 1968. Santon przekroju geologicznego Krystyna-Bolesławiec w świetle badań palynologicznych. 34 pp. Unpublished typescript dated 12th Dec., 1968. Państwowy Instytut Geologiczny, Warszawa.
Raniecka-Bobrowska, J. 1977. Makroflora. In: J. Czermiński (ed.), Geology of Poland. Volume 2: Catalogue of Fossils. Part 3a: Cainozoic, Tertiary, 161–188. Wydawnictwa Geologiczne, Warsaw.
Raniecka-Bobrowska, J. 1984. Flora: Mikroflora: Miospory. In: L. Malinowska (ed.), Budowa geologiczna Polski. Tom III: Atlas skamieniałości przewodnich i charakterystycznych. Część 2c: Mezozoik, kreda, 462–478. Wydawnictwa Geologiczne, Warszawa.
Raniecka-Bobrowska, J. 1989. Flora: Microflora: Miospores. In: L. Malinowska (ed.), Geology of Poland. Volume 3: Atlas of Guide and Characteristic Fossils. Part 2c: Mesozoic, Cretaceous, 316–326. Wydawnictwa Geologiczne, Warsaw.
Raumer, K. von 1819. Das Gebirge Nieder-Schlesiens, der Graffschaft Glatz und eines Theils von Böhmen und der Ober-Lausitz, geognostisch dargestellt. xvi + 182 pp. G. Reimer, Berlin.
Reid, E.M., Chandler, M.E.J., and Groves, J. 1926. Catalogue of Cainozoic plants in the Department of Geology, British Museum, Natural History —The Bembridge Flora. 206 pp. British Museum (Natural History), London. Crossref
Reveal, J.L. 1992. Validation of subclass and superordinal names in Magnoliophyta. Novon 2: 235–237. Crossref
Reveal, J.L. 1993. New ordinal names for extant vascular plants. Phytologia 74: 173–177.
Reymanówna, M. and Watson, J. 1976. The genus Frenelopsis Schenk and the type species Frenelopsis hoheneggeri (Ettingshausen) Schenk. Acta Palaeobotanica 17: 17–26.
Reyre, Y. 1970. Stereoscan observations on the pollen genus Classopollis Pflug 1953. Palaeontology 13: 303–322.
Rhode, J.G. 1821–1823. Beiträge zur Pflanzenkunde der Vorwelt: nach Abdrücken im Kohlenschiefer und Sandstein aus schlesischen Steinkohlenwerken. iv + 40 pp. Grass, Breslau.
Richter, P. 1904. Über die Kreidepflanzen der Umgebung Quedlinburgs. Teil I (Beilage zum Programm des königl. Gymnasiums zu Quedlinburg, Ostern 1904). 20 pp. H. Klöppel, Quedlinburg a. H.
Richter, P. 1905. Über die Kreidepflanzen der Umgebung Quedlinburgs. Teil II. (Beilage zum Programm des königl. Gymnasiums zu Quedlinburg, Ostern 1905). 20 pp. H. Klöppel, Quedlinburg a. H.
Ricketts, B.D. and Sweet, A.R. 1986 Stratigraphy, sedimentology and palynology of the Kootenay-Blairmore transition in southwestern Alberta and southeastern British Columbia. Geological Survey of Canada, Paper 84 (15): 1–41. Crossref
Riding, J.B., Leng, M.J., Kender, S., Hesselbo, S.P., and Feist-Burkhardt, S. 2013. Isotopic and palynological evidence for a new Early Jurassic environmental perturbation. Palaeogeography, Palaeoclimatology, Palaeoecology 374: 16–27. Crossref
Robaszynski, F., Dhondt, A.V., and Jagt, J.W.M. 2002. Cretaceous lithostratigraphic units (Belgium). In: P. Bultynck and L. Dejonghe (eds.), Guide to a revised lithostratigraphic scale of Belgium. Geologica Belgica 4: 121–134. Crossref
Roemer, F. 1886. Einige neue Arten von Versteinerungen. Jahres-Bericht der Schlesischen Gesellschaft für vaterländische Cultur 63: 119.
Roemer, F. 1887. Fossile Flora in Thonen der Kreideformation bei Bunzlau. Jahres-Bericht der Schlesischen Gesellschaft für vaterländische Cultur 64: 117.
Roemer, F. 1889. Ueber Blattabdrücke in senonen Thonschichten bei Bunzlau in Niederschlesien. Zeitschrift der deutschen geologischen Gesellschaft 41: 139–147.
Romanova, E.V. 1963. New species angiosperm’s from the Upper Cretaceous of the Kyin-Kerish Hill (The Zaissan Depression) [in Russian]. In: I.G. Galuzo, B.S. Bažanov, V.S. Kornilova, and E.R. Orlovskaâ (eds.), Materialy po istorii fauny i flory Kazakhstana, Tom IV, 100–112. Izdatel’stvo Akademii nauk Kazahskoj SSR, Alma-Ata.
Ross, N.E. 1949. Investigations of the Senonian of the Kristianstad District, S. Sweden. I. On a Cretaceous pollen and spore bearing clay deposit of Scania, a preliminary report. Bulletin of the Geological Institute of Uppsala 34: 25–43.
Rouse, G.E. 1959. Plant microfossils from Kootenay coal-measures strata of British Columbia. Micropaleontology 5: 303–324. Crossref
Ruiz, L.C. and Quattrocchio, M.E. 1997. Estudio palinológico de la Formación Pedro Luro (?Maastrichtiano-Paleoceno) en la Cuenca del Colorado, República Argentina. Parte 2: Turma Saccites, Plicates, Poroses e incertae Sedis. Revista Española de Micropaleontología 29: 115–137.
Rüffle, L. and Knappe, H. 1977. Entwicklungsgeschichtlische und ökologische Aspekte zur Oberkreide-Flora, besonders einiger Fagaceae (Hamamelididae). Zeitschrift für geologische Wissenschaften 5: 269–303.
Rüffle, L. and Krutzsch, W. 2005. Blattreste aus dem mitteldeutschen Maastricht. Documenta naturae 155 (2): 5–27.
Ryberg, P.E., Rothwell, G.W., Stockey, R.A., Hilton, J., Mapes, G., and Riding, J.B. 2012. Reconsidering relationships among stem and crown group Pinaceae: Oldest record of the genus Pinus from the Early Cretaceous of Yorkshire, United Kingdom. International Journal of Plant Sciences 173: 917–932. Crossref
Rymut, K. (ed.) 1999. Nazwy miejscowe Polski. Historia – pochodzenie – zmiany. Tom 3: E–I. 543 pp. Polska Akademia Nauk, Instytut Języka Polskiego, Kraków.
Samuel, E. and Gaillard, M.G. 1984. Les gisements à flore fossile d’âge crétacé supérieur en France: localisation, stratigraphie et essai de corrélations des données macro et microfloristiques. Bulletin mensuel de la Société linnéenne de Lyon 53: 213–223. Crossref
Saporta, G. de 1865. Études sur la végétation du sud-est de la France à l’époque tertiaire. Deuxième partie. Annales des Sciences naturelles, Botanique, 5e série 3: 5–152.
Saporta, G. de 1868. Prodrome d’une flore fossile des travertins anciens de Sézanne. Mémoires de la Société géologique de France, 3e série 8: 289–436.
Saporta, G. de 1890. Le Nelumbium provinciale des lignites crétacés de Fuveau en Provence. Mémoires de la Société géologique de France, Paléontologie 5: 1–12.
Saporta, G. de and Marion, A.-F. 1873. Essai sur l’état de la végétation à l’époque des marnes heersiennes de Gelinden. Mémoires couronnés et mémoires des savants étrangers publiés par l’Académie royale des sciences, des lettres et des beaux-arts de Belgique 37: 3–94.
Saporta, G. de and Marion, A.-F. 1878. Révision de la flore heersienne de Gelinden: d’après une collection appartenant au comte G. de Looz. Mémoires couronnés et mémoires des savants étrangers publiés par l’Académie royale des sciences, des lettres et des beaux-arts de Belgique 41: 3–112.
Saward, S.A. 1992. A global view of Cretaceous vegetation patterns. In: P.J. McCabe and J.T. Parrish (eds.), Controls on the Distribution and Quality of Cretaceous Coals. Geological Society of America, Special Paper 267: 17–35. Crossref
Sawicki, L. (ed.) 1966. Mapa geologiczna regionu dolnośląskiego. Wydawnictwa Geologiczne, Warszawa.
Sawicki, L. (ed.) 1995. Geological Map of Lower Silesia with Adjacent Czech and German Territories (without Quaternary Deposits) 1:100 000. Państwowy Instytut Geologiczny, Warszawa.
Schenk, A. 1869. Beiträge zur Flora der Vorwelt. 3. Die fossilen Pflanzen der Wernsdorfer Schichten in den Nordkarpathen. Palaeontographica 19: 1–34.
Schimper, W.P. 1869. Traité de paléontologie végétale, ou, La flore du monde primitif dans ses rapports avec les formations géologiques et la flore du monde actuel. Tome premier. iv + 738 pp. J.B. Baillière et Fils, Paris. Crossref
Schlotheim, E.F. von 1820. Die Petrefactenkunde auf ihrem jetzigen Standpunkte: durch die Beschreibung seiner Sammlung versteinerter und fossiler Überreste des Thier- und Pflanzenreichs der Vorwelt erläutert. lxiii + 437 pp. Becker’sche Buchhandlung, Gotha.
Schneider, H., Schuettpelz, E., Pryer, K.M., Cranfill, R., Magallón, S., and Lupia, R. 2004. Ferns diversified in the shadow of angiosperms. Nature 428: 553–557. Crossref
Schuettpelz, E. and Pryer, K.M. 2009. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proceedings of the National Academy of Sciences of the USA 106: 11200–11205. Crossref
Scott, D.H. 1909. Studies in Fossil Botany. 2nd Edition. xi + 683 pp. Adam and Charles Black, London. Crossref
Scupin, H. 1911. Über sudetische, prätertiäre junge Krustenbewegungen und die VErteilung von Wasser und Land zur Kreidezeit in der Umgebung der Sudeten und des Erzgebirges. Eine Studie zur Geschichte der Kreidetransgression. Zeitschrift für Naturwissenschaften 82: 321–344.
Scupin, H. 1913. Die Löwenberger Kreide und ihre Fauna. Palaeontographica Supplement-Band 6: 1–276.
Scupin, H. 1937. Zur Paläogeographie des sudetischen Kreidemeeres. Zeitschrift der deutschen geologischen Gesellschaft 88: 309–325.
Sell, P. and Murrell, G. 2018. Flora of Great Britain and Ireland. Volume 1: Lycopodiaceae–Salicaceae. lxii + 787 pp. Cambridge University Press, Cambridge.
Seward, A.C. 1900. Catalogue of the Mesozoic Plants in the Department of Geology, British Museum (Natural History). The Jurassic Flora. I. The Yorkshire Coast. 341 pp. Printed by Order of the Trustees, London.
Seward, A.C. 1919. Fossil Plants. A text-book for students of botany and geology. Volume IV: Ginkgoales, Coniferales, Gnetales. xvi + 543 pp. University Press, Cambridge.
Seward, A.C. 1925. Notes sur la flore crétacique du Groenland. Étude critique. Traduit sur le texte anglais par Suzanne Leclercq. In: Société géologique de Belgique (eds.), 50me anniversaire, Livre jubilaire 1874–1924. Tome I, Fascicule I, 227–263. Imprimerie H. Vaillant−Carmanne, Liège.
Seward, A.C. 1926. The Cretaceous plant-bearing rocks of western Greenland. Philosophical Transactions of the Royal Society of London, Series B 215: 57–175. Crossref
Shang, Y. and Zavada, M.S. 2003. The ultrastructure of Cerebropollenites from the Jurassic and Cretaceous of Asia. Grana 42: 102–107. Crossref
Shugaevskaya, O.V. [Šugaevskaâ, O.V.] 1978. Characteristic spores and pollen of the Cretaceous of the Far East [in Russian]. In: G.B. Levašev (ed.), Geologiâ, magmatism i rudogenez zony perehoda ot kontinenta k okeanu, 22–24. Akademiâ nauk SSSR, Dalnevostočnyj naučnyj central’nyj biologo-počvennyj Institut, Vladivostok.
Siegl-Farkas, Á. 1982. Palynostratigraphy of the Upper Cretaceous in the Uppony Mountains. A Magyar Allami Fóldtani Intézet ávi Jelentése 1982: 101–117.
Siegl-Farkas, Á. 1988. Az Ajkai kőszén formáció palynosztratigráfiája és fejlődéstörténete. A Magyar Allami Fóldtani Intézet ávi Jelentése 1986: 179–209.
Siegl-Farkas, Á. and Wagreich, M. 1996. Correlation of palyno- (spores, pollen, dinoflagellates) and calcareous nannofossil zones in the Late Cretaceous of the Northern Calcareous Alps (Austria) and the Transdanubian Central Range (Hungary). In: H. Lobitzer and E. Dudich (eds.), Advances in Austrian-Hungarian Joint Geological Research, 127–135. Geologisches Bundesanstalt, Wien.
Singh, C. 1964. Microflora of the Lower Cretaceous Manville Group, east-central Alberta. Research Council of Alberta, Bulletin 15: 1–239.
Singh, C. 1971. Lower Cretaceous microfloras of the Peace River area, northwestern Alberta. Research Council Alberta, Bulletin 28: 1–542.
Singh, C. 1983. Cenomanian microfloras of the Peace River area Northwestern Alberta. Research Council of Alberta, Bulletin 44: 1–322.
Singh, R.K. 1982. Development of Palaeogene palynostratigraphy in northeast India. In: Cenozoic Stratigraphy and Palynology in India. Palaeontological Society of India, Special Publication 1: 37–49.
Skarby, A. 1964. Revision of Gleicheniidites senonicus Ross. Acta Universitatis Stockholmiensis, Stockholm Contributions in Geology 11: 59–77.
Skelton, P.W. 2003. Fluctuating sea-level. In: P.W. Skelton (ed.), The Cretaceous World, 67–83. Cambridge University Press, Cambridge.
Skwirzyńska, E. 1990. Liliaceae (pars 2), Amaryllidaceae, Iridaceae. In: J. Mądalski (ed.), Florae Polonicae terrarumque adiacentium iconographia. Tom II, Zeszyt 5, 1–53. Państwowe Wydawnictwo Naukowe, Warszawa.
Slater, S.M. and Wellman, C.H. 2015. A quantitative comparison of dispersed spore/pollen and plant megafossil assemblages from a Middle Jurassic plant bed from Yorkshire, UK. Paleobiology 41: 640–660. Crossref
Śliwiński, W., Raczyński, P., and Wojewoda, J. 2003. Sedimentation of the epi-Variscan cover in the North-Sudetic Basin. In: W. Ciężkowski, J. Wojewoda, and A. Żelaźniewicz (eds.), Sudety zachodnie od wendu do czwartorzędu, 119–126. Wind, Wrocław.
Sobczyk, A., Sobel, E.R., and Georgieva, V. 2019. Meso-Cenozoic cooling and exhumation history of the Orlica-Śnieżnik Dome (Sudetes, NE Bohemian Massif, Central Europe): Insights from apatite fission-track thermochronometry. Terra Nova 2019: 1–12.
Soltis, D., Soltis, P., Endress, P., Chase, M., Manchester, S., Judd, W., Majure, L., and Mavrodiev, E. 2018. Phylogeny and Evolution of the Angiosperms. Revised and updated edition. 590 pp. University of Chicago Press, Chicago. Crossref
Solecki, A.T. 2011. Structural development of the epi-Variscan cover in the North Sudetic Synclinorium area [in Polish, with English abstract]. In: A. Żelaźniewicz, J. Wojewoda, and W. Ciężkowski (eds.), Mezozoik i kenozoik Dolnego Śląska, 19–36. Wind, Wrocław.
Song, Z.-C., Shang, Y., Liu, Z., Huang, P., Wang, X., Qian, L., Du, B., and Zhang, D. 2000. Fossil Spores and Pollen of China. Volume 2: The Mesozoic Spores and Pollen [in Chinese]. 710 pp. Science Press, Beijing.
Spicer R.A. 1990. Reconstructing high-latitude Cretaceous vegetation and climate: Arctic and Antarctic compared. In: T.N. Taylor and E.L. Taylor (eds.), Antarctic Paleobiology, 27–36. Springer, New York. Crossref
Spicer, R.A. 2003. Changing climate and biota. In: P.W. Skelton (ed.), The Cretaceous World, 85–162. Cambridge University Press, Cambridge.
Spicer, R.A., Ahlberg, A., Herman, A.B., Kelley, S.P., Raikevich, M.I., and Rees, P.M. 2002. Palaeoenvironment and ecology of the middle Cretaceous Grebenka flora of northeastern Asia. Palaeogeography, Palaeoclimatology, Palaeoecology 184: 65–105. Crossref
Spicer, R.A., Burnham, R.J., Grant, P., and Glicken, H. 1985. Pityrogramma calomelanos, the primary, post-eruption colonizer of Volcán Chichonal, Chiapas, Mexico. American Fern Journal 75: 1–5. Crossref
Springer, R. 1914. Oberschlesische Bergmanns-Poesie und Prosa. Teil II. Gewidmet den Mitgliedern des Vereins technischer Bergbeamten Oberschlesiens und den Freunden des heimischen Bergbaus als Festschrift anläßlich des 75-jährigen Bestehens der oberschlesischen Bergschule zu Tarnowitz mit herzlichem “Glück auf”. 216 pp. Robert Springer, Borsigwerk O.-S.
Srivastava, S.K. 1972. Taxonomic notes on pollen genera Callistopollenites, Tricolporites, and Carpinipites. Canadian Journal of Botany 50: 9–12. Crossref
Srivastava, S.K. 1975. Microspores from the Fredericksburgh Group (Albian) of the southern United States. Paléobiologie continentale 6 (2): 1–119.
Staffa, M., Mazurski, K.R., Pisarski, G., and Czerwiński, J. 2002. Słownik geografii turystycznej Sudetów. Tom 7: Pogórze Kaczawskie. 703 pp. Wydawnictwo I-BiS, Wrocław.
Staffa, M., Mazurski, K.R., Czerwiński, J., and Pisarski, G. 2003. Słownik geografii turystycznej Sudetów. Tom 2: Pogórze Izerskie. 486 + 520 pp. Wydawnictwo I-BiS, Wrocław.
Steck, A. 1757. Dissertatio inauguralis medica de Sagu, quam sub Auspiciis Divinis ex decreto gratiosæ Facultatis Medicæ pro licentia summos in medicina honores et privilegia doctoralia legitime consequendi solenni eruditorum examini submittit. 44 pp. Typis Johannis Henrici Heitzii, Argentorati.
Sternberg, K.M. von 1821. Versuch einer geognostisch-botanischer Darstellung der Flora der Vorwelt. Zweites Heft. 33 pp. Bei Christoph Ernst Brenck’s Witwe, Regensburg.
Sternberg, K.M. von 1838. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt. Heft 7–8. 81–220 pp. Gotlieb Haase Söhne, Prag.
Stiehler, A.W. 1857. Beiträge zur Kenntniss der vorweltlichen Flora des Kreidegebirges im Harz. Palaeontographica 5: 47–80.
Stockmans, F. 1946. Végétaux des sables d’Aix-la-Chapelle récoltés en Belgique (Sénonien inférieur). Mémoires du Musée royal d’Histoire naturelle de Belgique 105: 3–51.
Stopes, M.C. 1915. Catalogue of the Mesozoic Plants in the British Museum (Natural History). The Cretaceous Flora: Part II. Lower Greensand (Aptian) Plants of Britain. xxxvi + 360 pp. Trustees of British Museum, Dulau & Co., London.
Stover, L.E. and Partridge, A.D. 1973. Tertiary and Late Cretaceous spores and pollen from the Gippsland Basin, southeastern Australia. Proceedings of the Royal Society of Victoria 85: 237–286.
Streel, M., Felder, P.J., and Bless, M.J.M. 1994. Excursion 2: Upper Cretaceous, Thursday 22 September 1994. In: Fourth European Palaeobotanical Palynological Conference, 1–10. Heerlen.
Svobodová, M. 1997. Mid-Cretaceous palynomorphs from the Blansko Graben (Czech Republic): affinities to both Tethyan and Boreal bioprovinces. Mededelingen Nederlands Institut voor Toegepaste Geowetenschappen TNO 58: 149–155.
Ţabără, D. and Slimani, H. 2019. Palynological and palynofacies analyses of Upper Cretaceous deposits in the Haţeg Basin, southern Carpathians, Romania. Cretaceous Research 104: 104–185. Crossref
Takhtajan, A.L. [Tahtajan, A.L.] (ed.) 1963. Osnovy Paleontologii. Spravočnik dlâ paleontologov i geologov SSSR. Golosemennye i pokrytosemennye. 743 pp. Gosudarstvennoe naučno-tehničeskoe Izdatel’stvo literatury po geologii i ohrane nedr, Moskva.
Takhtajan, A.L. [Tahtadžân, A.L.] 1967. Sistema i filogeniâ cvetkovyh rastenij. 610 pp. Nauka, Moskva.
Takhtajan, A. 1986. Floristic Regions of the World (translated by T.J. Crovello and A. Cronquist). 522 pp. University of California Press, Berkeley.
Tappan, H. 1980. The Paleobiology of Plant Protists. xxiv + 1028 pp. W.H. Freeman and Company, San Francisco.
Tchoumatchenco, P., Peybernes, B., Chernyavska, S., Lachkar, G., Surmont, J., Dercourt, J., Ivanov, Z., Rolando, J.-P., Sapunov, I., and Thierry, J. 1992. Étude d’un domaine de transition Balkan-Moesie; évolutions paléogeographique et paléotectonique de Sillon du Flysch Jurassique inférieur et moyen dans la Stara Planina Orientale (Bulgarie Orientale). Bulletin de la Société géologique de France, 8e série 163: 49–61.
Thiergart, F. 1942. Mikropaläobotanische Mitteilungen 1–3. Jahrbuch der Reischsamtes für Bodenforschung 62: 109–116.
Thiergart, F. 1949. Die stratigraphische Wert mesozoischer Pollen und Sporen. Palaeontographica, Abteilung B 89: 1–34.
Thomé, O.W. 1886. Prof. Dr. Thomé’s Flora von Deutschland, Österreich und der Schweiz, in Wort und Bild, für Schule und Haus. Band I. viii + 366 pp. Verlag von Fr. Eugen Köhler, Gera-Untermhaus. Crossref
Thomson, P.W. and Pflug, H.D. 1953. Pollen und Sporen des Mitteleuropäischen Tertiärs. Palaeontographica, Abteilung B 94: 1–138.
Torsvik, T. and Cocks, R.L.M. 2017. Earth History and Palaeogeography. x + 317 pp. Cambridge University Press, Cambridge. Crossref
Truswell, E.M. 1987. The palynology of core samples from the S.P. Lee Wilker land Cruise. In: M.A. Hampton (ed.), The Antarctic Continental Margin-Geology and Geophysics of offshore Wilkes Land. Circum-Pacific Council for Energy and Mineral Resources, Earth Science Series 5: 215–218.
Tschudy, B.D. 1973a. Palynology of the Upper Campanian, (Cretaceous) Judith River Formation, north-central Montana. United States Geological Survey, Professional Paper 770: iii–iv, 1–40. Crossref
Tschudy, R.H. 1973b. The Complexiopollis pollen lineage in Mississippi embayment. United States Geological Survey, Professional Paper 743-C: 1–15. Crossref
Tschudy, R.H. 1975. Normapolles pollen from the Mississippi embayment. United States Geological Survey, Professional Paper 865: 1–42. Crossref
Uffelen, G.A. van 1991. Fossil Polypodiaceae and their spores. Blumea 36: 253–272.
Uličný, D. 2001. Depositional systems and sequence stratigraphy of coarse-grained deltas in a shallow-marine, strike-slip setting: The Bohemian Cretaceous Basin, Czech Republic. Sedimentology 48: 599–628. Crossref
Uličný, D., Kvaček, J., Svobodová, M., and Špičáková, L. 1997. High-frequency sea-level fluctuations and plant habitats in Cenomanian fluvial to estuarine succession: Pecínov quarry, Bohemia. Palaeogeography, Palaeoclimatology, Palaeoecology 136: 165–197. Crossref
Unger, F. 1850. Genera et species plantarum fossilium. xl + 627 pp. Sumptibus Academiae Caesareae Scientiarum, apud Wilhelmum Braumüller, Vindobona. Crossref
Upchurch, G.R., Jr. and Wolfe, J.A. 1987. Mid-Cretaceous to Early Tertiary vegetation and climate: evidence from fossil leaves and woods. In: E.M. Friis, W.G. Chaloner, and P.R. Crane (eds.), The Origins of Angiosperms and Their Biological Consequences, 75–105. Cambridge University Press, Cambridge.
Vajda, V. and Bercovici, A. 2014. The global vegetation pattern across the Cretaceous–Paleogene mass extinction interval: A template for other extinction events. Global and Planetary Change 122: 29–49. Crossref
Vakhrameev, V.A. 1991. Jurassic and Cretaceous Floras and Climates of the Earth (translated by Ju.V. Litvinov, edited by Norman F. Hughes). 318 pp. Cambridge University Press, Cambridge.
Velenovský, J. 1882. Die Flora der böhmischen Kreideformation. I. Theil. Credneriaceae und Araliaceae. Beiträge zur Paläontologie Österreich-Ungarns 2 (1): 8–32.
Velenovský, J. 1883. Die Flora der böhmischen Kreideformation. II. Theil. Proteaceae, Myricaceae, Cupuliferae, Moreae, Magnoliaceae, Bombaceae. Beiträge zur Paläontologie Österreich-Ungarns und des Orients 3 (1): 26–47.
Velenovský, J. 1885. Die Gymnospermen der Böhmischen Kreideformation. 34 pp. E. Gregr, Prag. Crossref
Velenovský, J. 1888. Die Farne der böhmischen Kreideformation. Abhandlungen der königlichen böhmischen Gesellschaft der Wissenschaften, VII. Folge 2: 3–32.
Velenovský, J. 1889. Květena českého cenomanu. Sitzungsberichte der königlich-böhmischen Gesellschaft der Wissenschaften in Prag, mathematisch-naturwissenschaftlische Classe 3 (3): 1–75.
Verdoorn, F. 1938. Manual of Pteridology. xx + 640 pp. M. Nijhoff, The Hague.
Villalba-Breva, S., Marmi, J., Gomez, B., Daviero-Gomez, V., Martín-Closas, C., and Fernández-Marrón, M.F. 2015. Plant taphonomy and palaeoenvironment from the Upper Cretaceous of Isona, Tremp Basin, southern Pyrenees, Catalonia, Spain. Cretaceous Research 54: 34–49. Crossref
Villiers, S.E. de and Cadman, A. 1997. The palynology of Tertiary sediments from a palaeochannel in Namaqualand, South Africa. Palaeontologia Africana 34: 69–99.
Voigt, S., Wagreich, M., Surlyk, F., Walaszczyk, I., Uličný, D., Čech, S., Voigt, T., Wiese, F., Wilmsen, M., Niebuhr, B., Reich, M., Funk, H., Michalík, J., Jagt, J.W.M., Felder, P.J., and Schulp, A.S. 2008. Cretaceous. In: T. McCann (ed.), Geology of Central Europe. Volume 2: Mesozoic and Cenozoic, 923–998. Geological Society, London. Crossref
Volkmann, G.A. 1720. Silesia subterranea, oder Schlesien, mit seinen Unterirrdischen Schätzen, Seltsamheiten, welche dieses Land mit andern gemein, oder zuvoraus hat, als Edelen, und Unedelen, ohne und mit Figuren sich præsentirenden und seltsam gebildetem Steinen, auch ehemals theils durch die allgemeinen, theils Particulair-Fluthen hierher verschwemmten, und durch die Versteinerung Krafft in und ausser der Steinen in Stein verwandelten Holz, Kräuter und Blumen, Früchten, Erd- und Wasser-Thieren, ingleichen Metallen, Mineralien, unterschiedlichen Arten, so wohl in der Medicin als Mechanic dienlicher Erde, Sauer- Heil- und Gesund-Brunnen und Bädern. 344 pp. Moritz Georg Weidmann, Leipzig,
Vozzhennikova, T.F. [Vozžennikova, T.F.] 1967. Iskopaemye peridinei ûrskih, melovyh i paleogenovyh otloženij SSSR. 347 pp. Nauka, Moskva.
Walaszczyk, I. 2008. North Sudetic Basin (Outer Sudetic Cretaceous). In: T. McCann (ed.), The Geology of Central Europe. Volume 2: Mesozoic and Cenozoic, 959–961. Geological Society, London.
Wall, D., Dale, B., Lohmann, G.P., and Smith, W.K. 1977. The environmental and climatic distribution of dinoflagellate cysts in Modern marine sediments from regions in the North and South Atlantic Oceans and adjacent seas. Marine Micropaleontology 2: 121–200. Crossref
Wang, C.-F., Tian, M.-Q., Zhang, Z.-R., and Guo, S.-Y. 1984 The Mesozoic of the Plain area in Middle Hebei [in Chinese with English summary]. Acta Stratigraphica Sinica 8: 301–307.
Watkins, J.E., Jr. and Cardelús, C.L. 2012. Ferns in an angiosperm world: Cretaceous radiation into the epiphytic niche and diversification on the forest floor. International Journal of Plant Sciences 173: 695–710. Crossref
Weems, R.E. and McCartan, L. 1990. A summary of selected stratigraphic occurrences of Neogene and Quaternary invertebrate faunas and microfloras in the Charleston, South Carolina, area. In: Studies Related to the Charleston, South Carolina, Earthquake of 1886 Neogene and Quaternary Lithostratigraphy and Biostratigraphy. U.S. Geological Survey Professional Paper 1367: G1–G31.
Weyland, H. and Greifeld, G. 1953. Über strukturbietende Blätter und pflanzliche Mikrofossilien aus der untersenon Tonen der Gegend von Quedlinburg. Palaeontographica, Abteilung B 95: 30–52.
Weyland, H. and Krieger, W. 1953. Die Sporen und Pollen der Aachener Kreide und ihre Bedeutung für die Charakterisierung des mittleren Senons. Palaeontographica, Abteilung B 95: 2–29.
Williams, G.L., Fensome, R.A., and MacRae, R.A. 2017. The Lentin and Williams Index of fossil dinoflagellates, 2017 Edition. American Association Of Stratigraphic Palynologists, Contributions 48: 1–1097.
Williger, G. 1882. Die Löwenberger Kreidemulde, mit besonderer Berücksichtigung ihrer Fortsetzung in der preussischen Ober-Lausitz. Jahrbuch der Königlich Preussischen geologischen Landesanstalt und der Bergakademie zu Berlin für das Jahr 1881: 55–124.
Wilson, L.R. and Webster, R.M. 1946. Plant microfossils from a Fort Union Coal of Montana. American Journal of Botany 33: 271–278. Crossref
Wing, S.L. and Sues, H.-D. 1992. Mesozoic and Early Cenozoic terrestrial ecosystems. In: A.K. Behrensmeyer, J.D. Damuth, W.A. DiMichele, R. Potts, H.-D. Sues, and S.L. Wing (eds.), Terrestrial Ecosystems Through Time: Evolutionary Paleoecology of Terrestrial Plants and Animals, 327–416. The University of Chicago Press, Chicago.
Wing, S.L., Strömberg, C., Hickey, L.J., Tiver, F., Willis, B., Burnham, R.J., and Behrensmeyer, A.K. 2012. Floral and environmental gradients on a Late Cretaceous landscape. Ecological Monographs 82: 23–47. Crossref
Wingate, F.H. 1980. Plant microfossils from the Denton Shale Member of the Bokchito Formation, (Lower Cretaceous, Albian) in Southern Oklahoma. Bulletin of the Oklahoma Geological Survey 130: 1–93.
Wodehouse, R.P. 1933. Tertiary pollen II. The oil shales of the Eocene Green River Formation. Bulletin of the Torrey Botanical Club 60: 479–524 . Crossref
Wolfe, J.A. 1997. Relations of environmental change to angiosperm evolution during the Late Cretaceous and Tertiary. In: K. Iwatsuki and P.H. Raven (eds.), Evolution and Diversification of Land Plants, 269–290. Springer-Verlag, Tokyo. Crossref
Wolfe, J.A. and Wehr, W.C. 1987. Middle Eocene dicotyledonous plants from Republic, Northeastern Washington. United States Geological Survey, Bulletin 1597: 1–25.
Wolff, H. 1934. Mikrofossilien des pliozänen Humodils der Grube Freigericht bei Dettingen a. M. und Vergleich mit alteren Schichten des Tertiärs sowie post-Tertiären Ablagerungen. Arbeiten aus dem Institut für Paläobotanik und Petrographie der Brennsteine 5: 55–86.
Yu, C.H. and Chen, Z.L. 1991. Leaf Architecture of Woody Dicotyledons From Tropical and Subtropical China. 414 pp. Printing House of China Building Industry Press, Beijing.
Zavialova, N. and van Konijnenburg-van Cittert, J.H.A. 2012. Exine ultrastructure of in situ pollen from the cycadalean cone Androstrobus prisma Thomas et Harris 1960 from the Jurassic of England. Review of Palaeobotany and Palynology 173: 15–22. Crossref
Żelaźniewicz, A. 2005. Przeszłość geologiczna. In: J. Fabiszewski (ed.), Przyroda Dolnego Śląska, 61–134. Polska Akademia Nauk, Wrocław.
Zenker, J.C. 1833. Beiträge zur Naturgeschichte der Urwelt. Organische Reste (Petrefacten) aus der Altenburger Braunkohlen-Formation, dem Blankenburger Quadersandstein, jenaischen bunten Sandstein und böhmnischen Übergangsgebirge. 67 pp. Druck und Verlag von Friedrich Mauke, Jena.
Zetter, R. 1989. Methodik und Bedeutung einer routinemäßig kombinierten lichtmikroskopischen und rasterelektronenmikroskopischen Untersuchung fossiler Mikrofloren. Courier Forschungsinstitut Senckenberg 190: 41–50.
Zetter, R., Hesse, M., and Huber, K.H. 2002. Combined LM, SEM and TEM studies of Late Cretaceous pollen and spores from Gmünd, Lower Austria. Stapfia 80: 201–230.
Ziaja, J. 2006. Lower Jurassic spores and pollen grains from Odrowąż, Mesozoic margin of the Holy Cross Mountains, Poland. Acta Palaeobotanica 46: 3–83.
Description of localities.
All the localities listed below are situated in the territory of Poland and belong to the present administrative unit called województwo dolnośląskie; before 1945 they were all parts of the German Provinz Schlesien (or Provinz Niederschlesien). Up to 1815, however, the border between Lusatia (Łužyca, Łužica, Łużyce, Lausitz) and Silesia was situated on the Kwisa (Queis) river, so the localities Ołdrzychów and Osiecznica are not in the historical territory of Silesia (Pysiewicz-Jędrusik et al. 1998); this is why they are included in the geological description of Saxony (Charpentier 1788).
Polish toponyms not found on official 1:25 000 maps (451.23 Bolesławiec, published 1984; 451.32 Nowogrodziec, published 1976; 451.41 Gościszów, published 1984; 451.42 Raciborowice Górne, published 1992; 451.43 Lwówek Śląski, published 1976; 451.44 Pielgrzymka, published 1984) are after Staffa et al. (2002, 2003) and Jończy (2016). Corresponding German maps are the 1:25 000 Meßtischblätter: 4758 Siegersdorf (published 1943); 4759 Bunzlau (published 1943); 4858 Naumburg a.Q. (published 1937); 4859 Löwenberg (published 1937); 4860 Gröditzberg (published 1939).
Bolesławiec (Bunzlau).—The locality yielding Cretaceous (Santonian) plant fossils was described by Roemer (1887, 1889) as located a quarter of a mile south-east from the town centre along the road to Łosice (then Looswitz or Losswitz), so at about 51°15’8”N 15°34’57”E (southwards from the present streets Starzyńskiego and Kosiby, now partly covered by a forest, partly constructed). All specimens are imprints in white ceramic clays, but in a few cases some organic matter is preserved as well. This is the type locality of Menispermites bunzlavensis Roemer, 1889. Assemblage 8.For confusion in respect of specimens coming from Bolesławiec and from Luisenhain, see the entry on the latter locality.
Czaple (Hockenau).—The village is situated ca. 8 km E from Lwówek. Two specimens come from a hill called Kopka (formerly Hockenberg; Williger 1882: 86). Assemblage 2.
Dobra (Doberau, Dobrau).—The village is situated ca. 3 km NW from Bolesławiec. A single specimen (MGUWr 5638p) of Geinitzia reichenbachii is labelled “Doberau, coll. Goeppert”.
Gaszów (Gähnsdorf, Gehnsdorf).—Williger (1882: 89) lists “Caulopteris sp. ind.” from that locality (5.5 km NE from Lwówek). Material not extant.
Hochkirch.—Williger (1882: 94) listed “Credneria sp. ind.” from “Hochkirch”. This toponym may correspond to Kościelec 6.5 km south of Legnica or to Przesieczany 10 km NE from Zgorzelec (Rymut 1999). However, this may also be a misprint for Holzkirch (Kościelnik 4 km south of Lubań). Material not traced.
Huzarski Skok (Husarensprung).—This toponym, referring to an episode reported variously as having taken place during either the Silesian wars in 1761 or the Napoleonic wars in 1813 (Staffa et al. 2002: 215), denotes a rock overhanging an abandoned channel of the Bóbr river west of Żerkowice. A single collect made in 1925 by E. Zimmermann is labelled “ca. 300 m W von Km 7,7 des Chaussee Löwenbg–Bunzlau (NW vom Husarensprung)” and consists of plant imprints in iron claystone. Assemblage 4.
Luisenhain.—Several plant fossils in white ceramic clays are labelled “Luisenhain” (MB). According to an undated short letter from [E.] Zimmermann to W. Gothan kept in MB, this name refers to a restaurant situated along the road from Lwówek Śl. to Bolesławiec. Its detailed situation is shown on a handwritten map by Berg made in 1936 and kept in BGR. The fossil locality is a (former) clay pit situated ca. 300 m south from the restaurant, thus very close to Ulina (see that entry). Assemblage 8. Unfortunately new MB labels had been prepared incautiously and some of them saying “Luisenhain bei Bunzlau” were transcribed solely as “Bunzlau”. The present author (ATH) was able to see that for some specimens that have both old and new labels. However, specimens having only new labels saying “Bunzlau” may come either indeed from Bolesławiec or from Luisenhain. Such specimens are described as coming from an uncertain locality in the Assemblage 8, among them are the types of Platanites willigeri.
Lwówek Śląski (Löwenberg in Schlesien).— Six previously unpublished fossil plant specimens, all collected before 1900, are labelled as coming from near Lwówek. Apparently, such an indication should be understood quite broadly.The specimen of Geinitzia reichenbachii (MB 1.1.1.) collected on 29th July, 1898 (presumably by E. Dresler) from light Turonian claystone is especially important because it is the only Turonian plant fossil from the studied area. Unfortunately, the label (“Lettengrube b. Löwenbg. Turonmergel.”) is insufficiently precise. According to Williger (1882), clay pits were situated east from the town. Assemblage 1. Five further specimens (MB.Pb.2008/0316–0319, 0362) labelled “im hangend. Thon d. Kohlen in der Quadersandsteinform; coll. Klotzsch” are in dark clays; today the nearest outcrop of the corresponding strata is at Rakowice Małe. Assemblage 5.
Nowe (Neuen).—Williger (1882: 107) listed Muensteria schneideriana and Cylindrites spongioides. Both are trace fossils and not plants.
Nowe Jaroszowice (Neu-Jäschwitz).—The village (formerly also Jasiowice) is located ca. 4 km south from Bolesławiec. A clay mine situated east from Gröbel-Vorwerk (itself located NW from the village; Staffa et al. 2002: 361) yielded a few plant fossils found between 1912 and 1916 and kept in MB. Assemblage 7.
Ocice (Ottendorf).—The village is situated approximately halfway between Bolesławiec and Nowogrodziec. Two specimens of Dryophyllum westerhausianum come from the quarry (details unknown). Williger (1882: 108) lists a Geinitzia specimen from iron claystone (specimen not found).
Ołdrzychów (Ullersdorf am Queis).—Five Geinitzia twigs (collected in 1863, 1874, and 1877) come from this locality (now part of the town Nowogrodziec; coordinates of the church in Ołdrzychów: 51°11’46”N 15°23’7”E). The precise origin of the plant fossils is unknown. According to the lithology, they belong to the Assemblage 5.Confusion between Ullersdorf am Queis (Ołdrzychów) and Ullersdorf am Bober (Ulina) was taking place already among German-speaking palaeontologists, as testified by MB labels on which the name of either locality was corrected into the other (in both directions). According to Williger (1882), the only plant fossils found at Ołdrzychów are Geinitzia in clays. On the contrary, a more diversified angiosperm assemblage is known from Ulina; some of these specimens are mislabelled at MB [for example “Salicites dubius m. foliis latioribus Göppert” reported from Ulina by Williger (1882) but labelled as coming from Ołdrzychów]. Milewicz (1997: 31) misread Williger’s data and erroneously listed five species of angiosperms from Ulina as coming from Ołdrzychów.
Osiecznica (Wehrau).—This locality (the birthplace of the famous geologist A.G. Werner) is situated in the valley of Kwisa north from Nowogrodziec. The geological setting is relatively complicated, because similar lithologies occur in the Cretaceous and in the Oligocene (see Badura et al. 2011: 28–32). According to Williger (1882: 113–114), the palm Flabellaria chamaeropifolia Goeppert, 1836b, is Oligocene in age, but the seeds described from the environs of Osiecznica and nearby Kliczków (Klitschdorf) as Palmacites legitimus Goeppert, 1878 are Cretaceous. The material of the latter could not be traced.
Otok (Uttig).—The village is situated ca. 4 km SW from Bolesławiec. Three plant specimens come from here, one of which is located “am Bober, südl. v. Uttig”. It is possible that all the specimens labelled as “Otok” come from the quarries in the Bóbr valley. Assemblage 6.
Rakowice Małe (Wenig-Rackwitz).—The main locality is a large quarry (Rakowiczki mine; entrance: 51°9’58”N 15°32’48”E, western end: 51°9’58”N 15°32’25”E) situated on the southern flank of the Ostrzyca (Steinberg) hill. It was abandoned about 2009. Rocks that can be observed in the quarry include Coniacian sandstone belonging to the Żerkowice Member of the Rakowice Wielkie Formation, upper Coniacian or lower Santonian coaly clays belonging to the Nowogrodziec Member of the Czerna Formation, and Santonian sandstone belonging to the Czerna Formation; a detailed sedimentological account was given by Leszczyński (2010). Most of the plant fossils come from the Nowogrodziec Member that can be further subdivided into a multicoloured basal part with plant-bearing iron clays (Assemblage 4; the richest level according to Williger 1882: 104) and dark clays with coal intercalations (Assemblage 5; Williger 1882: 104–105). Santonian sandstone yielded rare imprints grouped as Assemblage 6. Besides the quarry, plant fossils were collected in other outcrops and in coal mines. Neither the outcrops nor the mines exist anymore. The outcrops are described on MB labels as located between the Ostrzyca hill and the Bóbr river. The mines were active from about 1789 to the second half of the nineteenth century (Haacke 1924, Maciejak and Maciejak 2013). The mines Gottes Segen and Georg Wilhelm were located west from the present quarry (Maciejak and Maciejak 2013: fig. 4), Entremonia was on the riverbank of the Bóbr, whereas Tremonia was located a few kilometres further northwest, near Andrzejówka (formerly Andreasthal, now a part of Kotlice, see Staffa et al. 2003: 87; erroneously given as a part of Włodzice Małe by Maciejak and Maciejak 2013). “Rakowice Małe” (without further details) is the type locality of the fern spore Sporites appendicifer Thiergart, 1942 (now Appendicisporites). One may presume that both the palynological material of Thiergart (1942) and Krutzsch (1958) as well as mesofossils of Knobloch and Mai (1986) came from the quarry, as the mining activity in the area is supposed to have stopped earlier.
Ulina (Ullersdorf am Bober).—Formerly a village (the name does not appear on the official Polish 1 : 25 000 map), now a part of Kraszowice. A tile factory was functioning there till the 1990-ies. Ceramic clays were exploited on a hill north-east from the village (ca. 51°11’57”N 15°33’36”E) in pits up to 5 m deep (information gathered from habitants in April 2018); as of 2018, these former excavations are completely overwhelmed. One may presume that rich collections of fossil angiosperms labelled as Ulina (Williger 1882: 103) come from sandstone found in such clay pits. Assemblage 6.
See also the entry on Luisenhain.
For the explanation of confusion between Ołdrzychów (Ullersdorf am Queis) and Ulina (Ullersdorf am Bober), see the entry on the former locality.
Wartowice (Neu-Warthau).—The village is situated ca. 5 km SE from Bolesławiec; a few quarries, exploited since the Middle Ages, are located south from the village (Staffa et al. 2002: 613). Several plant species are known from the Wartowice beds at the type locality (Williger 1882: 84). Assemblage 2.
Włodzice (Walditz).—Two specimens of Geinitzia (MGUWr, coll. Goeppert) are labelled “Walditz”; it is uncertain whether this refers to Włodzice Małe (Wenig-Walditz) ca. 2 km N from Rakowice Małe on the left bank of the Bóbr, or to Włodzice Wielkie (Gross-Walditz) directly opposite on the right bank of the Bóbr. Based on lithology, these specimens are tentatively included into the Assemblage 6.
Zbylutów (Deutmannsdorf).—An outcrop of the Wartowice beds situated on Karczmisko (Kretschamberg) hill NE from Zbylutów yielded a few plant fossils. Assemblage 2.
Żeliszów (Giersdorf).—An old quarry situated ca. 1 km SE from the village was re-activated about 2017. The environs of the quarry were erroneously interpreted as entirely belonging to the Rakowice Wielkie Formation by Baraniecki et al. (1955), but in 2017 a previously unnoticed occurrence of coaly layers (Nowogrodziec Member) with mesoflora was discovered (first signalled by Halamski et al. 2018b). Assemblage 5. The trunk of the tree fern Protopteris singeri described by Goeppert (1836) from Żeliszów was interpreted as coming from either Turonian or Coniacian sandstone (Greguš et al. 2013). According to the data in Williger (1882: 87), the Coniacian age (Assemblage 3) should be preferred. According to Milewicz (1997: 31), Debeya serrata was listed from this locality by Roemer (1886); however, such an information is not given in the referred paper; no other reference on the presence of Debeya or Dryophyllum at Żeliszów could be traced.
Żerkowice (Sirgwitz).—A quarry exploiting Coniacian sandstone is located north from the village at 51°9’42”N 15°34’17”E. Milewicz (1997) was still able to observe coaly sediments (analogous to those cropping out in Rakowice Małe) in this quarry, but as of 2017 this part of the succession was completely covered (ATH and P. Raczyński, personal observations). Rich collections of fossil plants belonging to to Assemblages 4, 5, and 6 are kept in MB.
German-Polish dictionary of toponyms.
The following is provided to facilitate interpreting pre-1945 literature. Hydronyms are listed for reference purposes only. For the names of coal mines, see the entry on Rakowice Małe in the Appendix 1.
Bober – Bóbr (river)
Bunzlau – Bolesławiec
Deutmannsdorf – Zbylutów
Doberau – Dobra
Gähnsdorf (Gehnsdorf) – Gaszów
Giersdorf – Żeliszów
Hockenau – Czaple
Klitschdorf – Kliczków (see Osiecznica)
Kretschamberg – Karczmisko hill (see under Zbylutów); not to be confused with Karczmarka (Kretschamberg) near Trzebień (Kittlitztreben).
Löwenberg (in Schlesien) – Lwówek Śląski
Naumburg am Queis – Nowogrodziec
Neu-Jäschwitz – Nowe Jaroszowice
Neuen – Nowe
Neu-Warthau – Wartowice
Ottersdorf – Ocice
Queis – Kwisa (river)
Ullersdorf am Bober – Ulina
Ullersdorf am Queis – Ołdrzychów
Sirgwitz – Żerkowice
Uttig – Otok
Walditz – Włodzice
Wehrau – Osiecznica
Wenig-Rackwitz – Rakowice Małe
Palynoassemblage A—list of taxa.
Dinocysts and acritarchs
Alterbia sp.
Chatangiella verrucosa (Manum, 1963) Lentin and Williams, 1976
Dinogymnium cretaceum (Deflandre, 1936) Evitt, Clarke, and Verdier, 1967
Dinogymnium sp.
Fromea amphora Cookson and Eisenack, 1958
Isabelidinium sp.
Micrhystridium sp.
Spinidinium sp.
Spiniferites ramosus var. ramosus (Ehrenberg, 1838) Davey and Williams, 1966
Subtilisphaera sp.
Surculosphaeridium longifurcatum (Firtion, 1952) Davey, Downie, Sarjeant, and Williams, 1966
Fungal spores
Dicellaesporites sp.
Pluricellaesporites psilatus Clarke, 1965
Prasinophytes
Crassosphaera sp.
Leiosphaeridia sp.
Pterospermella helios (Cookson and Eisenack, 1958) Eisenack, 1972
Tasmanites sp.
Non-marine algae
Ovoidites parvus Nakoman, 1966
Paralecaniella sp.
Tetraporina sp.
Schizocystia sp.
Bryophytes s.l.
Cingutriletes clavus Dettmann, 1963
Stereisporites antiquasporites Dettmann, 1963
Stereisporites psilatus Detmann, 1963
Stereisporites sp.Zlivisporis blanensis Pacltová, 1961
Zlivisporis simplex (Cookson and Dettmann, 1958) Braman, 2001
Zlivisporis sp.
Lycophytes
Camarozonosporites ambigens (Fradkina, 1967) Playford, 1971
Camarozonosporites insignis Norris, 1967
Echinatisporis varispinosus Srivastava, 1975
Retitriletes austroclavatidites (Cookson, 1953) Döring, Krutzsch, Mai, and Schulz in Krutzsch, 1963
Pteridophytes
Aequtriradites spinulosus (Delcourt and Sprumont, 1955) Cookson and Dettmann 1961
Apiculatisporites asymmetricus Cookson and Dettmann, 1958
Appendicisporites foveolatus (Deák, 1962) Wingate, 1980
Appendicisporites cristatus (Markova in Ivanova and Markova, 1961) Pocock, 1964
Appendicisporites sp.
Biretisporites cf. deltoideus (Rouse, 1949) Dettmann, 1963
Cicatricosisporites potomacensis Brenner, 1963
Cicatricosisporites venustus Deák, 1963
Cicatricosisporites sp.
Cicatricososporites cf. phaseolus (Delcourt and Sprumont, 1955) Krutzsch, 1959
Clavifera triplex (Bolkhovitina, 1966) Bolkhovitina, 1968
Concavissimisporites punctatus Brenner, 1962
Concavissimisporites sp.
Converrucosisporites proxigranulatus Brenner, 1962
Converrucosisporites sp.
Corniculatisporites alekhinii (Bolchovitina, 1953) Kuvaeva, 1972
Coronatispora sp.
Corrugatisporites sp.
Costatoperforosporites cf. foveolatus Deák, 1962
Cyathidites minor Couper, 1953
Deltoidospora juncta (Kara-Murza, 1954) Singh, 1964
Deltoidospora sp.
Dictyophyllidites harrisii Couper, 1958
Foveogleicheniidites confossus (Hedlund, 1966) Burger in Norvick and Burger, 1976
Gleicheniidites apilobatus Brenner, 1963
Gleicheniidites circiniidites (Cookson, 1953) Brenner, 1963
Gleicheniidites senonicus Ross, 1949
Ischyosporites pseudoreticulatus (Couper, 1958) Srivastava, 1975
Ischyosporites sp.
Klukisporites pseudoreticulatus Couper, 1958
Laevigatosporites ovatus (Wilson and Webster, 1946) Thomson and Pflug, 1953
Leptolepidites sp.
Osmundacidites cf. wellmanii Couper, 1953
Osmundacidites sp.
Ornamentifera baculata Singh, 1971
Pilosisporites sp.
Polypodiaceoisporites sp.
Matonisporites sp.
Plicifera delicata Bolchovitina, 1968
Polypodiidites sp.
Reticulosporis gallicus Deák, 1967
Toroiosporis sp.
Undulatosporites sp.
Varirugosisporites verrucosus (Deák, 1964) Juhász, 1979
Verrucatosporites sp.
Gymnosperms
Cerebropollenites mesozoicus (Couper, 1958) Nilsson, 1958
Classopollis classoides (Pflug, 1953) Pocock and Jansonius, 1961
Classopollis martinotii Reyre, 1970
Classopollis major Groot and Groot, 1962
Classopollis sp.
Cycadopites nitidus (Balme, 1957) Pocock, 1970
Cycadopites sp.
Ephedripites sp.
Monosulcites minimus Cookson, 1947
Monosulcites sp.
Parvisaccites radiatus Couper, 1958
Pinuspollenites sp.
Podocarpidites sp.
Sequoiapollenites sp.
Taxodiaceaepollenites hiatus (Potonié, 1931) Kremp, 1949
Taxodiaceaepollenites sp.
Taxodiaceaepollenites vacuipites (Wodehouse, 1933) Wingate, 1980
Angiosperms
Bohemiapollis sp.
Bohemiperiporis zaklinskai Pacltová, 1968
Clavatipollenites minutus Couper, 1958
Clavatipollenites sp.
Complexiopollis complicatus Góczán, 1964
Complexiopollis sp.
Emscheripollis sp.
Interporopollenites cf. turgidus (Weyland and Krieger, 1953) Tschudy, 1975
Interporopollenites sp.
Liliacidites sp.
Minorpollis minimus Krutzsch, 1959
Neotriangulipollis sp.
Neotriangulipollis cf. piolencipollis Groot and Krutzsch, 1967
Nyssapollenites sp.
Oculopollis minimus Siegl-Farkas, 1982
Oculopollis spp.
Pecakipollis sernoensis Pacltová, 1970
Pecakipollis sp.
Pflugipollis sp.
Piolencipollis sp.
Plicapollis serta Pflug, 1953
Plicapollis sp.
Pseudoplicapollis peneserta Krutzsch in Góczán et al., 1967
Pseudoplicapollis spp.
Psilatricolpites subtilis (Groot, Penny, and Groot, 1963) Singh, 1983
Retitricolporites sp.
Semioculopollis minimus (Góczán, 1964) Goczán, Groot, Krutzsch, and Pacltová, 1967
Striatopollis paraneus (Norris, 1967) Singh, 1971
Tricolporoidites subtilis Pacltová, 1971
Tricolpites vulgaris Brideaux, 1968
Tricolpites minutus (Brenner, 1963) Dettmann, 1973
Tricolpites sp.
Tricolporites sp.
Trudopollis fossulotrudens (Pflug in Thomson and Pflug, 1953) Pflug, 1953
Trudopollis spp.
Vacuopollis sp.
New combinations.
Dryophyllum westerhausianum (Richter, 1904) Halamski and Kvaček comb. nov.—Basionym: Bignonia westerhausiana Richter, 1904 [Richter, Über Kreidepfl. Umgeb. Quedlinburgs: 20; pl. 2: 1–5; 1904].
Dewalquea paulinae (Halamski, 2013) Halamski comb. nov.—Basionym: Debeya (Dewalquea) paulinae Halamski, 2013 [Acta Palaeontologica Polonica 58: 419, fig. 2B].
Acta Palaeontol. Pol. 65 (4): 811–878,
2020 https://doi.org/10.4202/app.00744.2020