

Systematic revision of a Miocene sperm whale from Patagonia, Argentina, and the phylogenetic signal of tympano-periotic bones in Physeteroidea
FLORENCIA PAOLUCCI, MÓNICA R. BUONO, MARTA S. FERNÁNDEZ, and JOSÉ CUITIÑO
Paolucci, F., Buono, M.R., Fernández, M.S., and Cuitiño, J. 2021. Systematic revision of a Miocene sperm whale from Patagonia, Argentina, and the phylogenetic signal of tympano-periotic bones in Physeteroidea. Acta Palaeontologica Polonica 66 (1): 63–76.
Sperm whales (Physeteroidea) include today only two genera of morphologically disparate odontocetes: the largest toothed whale known (Physeter macrocephalus) and small sized forms (Kogia spp.). In contrast, their fossil record indicates a high diversity for the group during the Miocene, with over 20 species recognized. Miocene marine sediments from Patagonia (Argentina) record this diversity, including at least five species. Among them, Preaulophyseter gualichensis, from the Miocene of Gran Bajo del Gualicho Formation, has been one of the most enigmatic. Despite the fragmentary nature of the type and referred materials (isolated teeth and periotics), which casts some doubts on its validity, this species has not been revised since its original description. In this contribution, we re-describe the materials referred to P. gualichensis, revise the taxonomic status of the species and evaluate the phylogenetic signal of ear bones among Physeteroidea. Our results indicate that the physeteroid tympano-periotic complex morphology is poorly diagnostic at the species level. Intraspecific variation (including ontogeny and sexual dimorphism) and/or taphonomic processes cannot be ruled out as the causes of the minor differences observed among specimens. We suggest that sperm whale tympano-periotics retain many plesiomorphic characters and are diagnostic only between kogiids and non-kogiid physeteroids. Based on the fragmentary and isolated state of the studied specimens, and the lack of diagnostic characters in both teeth and periotics, we consider P. gualichensis as nomen dubium and we re-assign the referred specimens as Physeteroidea indet. A conservative morphology of the tympano-periotic and, to a lesser extent, the nasal complex in sperm whales, might result from the morpho-functional constraints imposed by a highly specialized but successful echolocation system.
Key words: Mammalia, Physeteroidea, Preaulophyseter gualichensis, Miocene, Patagonia, Argentina.
Florencia Paolucci [paolucciflorencia@fcnym.unlp.edu.ar, paolucciflor@gmail.com] and Marta S. Fernández [martafer@fcnym.unlp.edu.ar], CONICET-División Paleontología Vertebrados, Unidades de Investigación, Anexo Museo, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina.
Mónica R. Buono [buono@cenpat-conicet.gob.ar] and José Cuitiño [jcuitino@cenpat-conicet.gob.ar], Instituto Patagónico de Geología y Paleontología, CCT CONICET-CENPAT, Puerto Madryn, Chubut, Argentina.
Received 29 April 2020, accepted 21 August 2020, available online 12 January 2021.
Copyright © 2021 F. Paolucci et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Physeteroids or sperm whales are one of the living odontocete lineages represented by both small and large forms with a unique cranial morphology. At present, the superfamily includes three extant species divided in two families: the giant sperm whale (Physeter macrocephalus Linnaeus, 1758) as the only representative of Physeteridae; and the dwarf and pygmy sperm whales (Kogia sima Owen, 1866 and Kogia breviceps de Blainville, 1838) comprising Kogiidae. Sperm whales’ evolutionary history dates back to the late Oligocene with Ferecetotherium kelloggi from Azerbaijan (Mchedlidze 1970), and over 20 species are recognized worldwide from the Miocene (e.g., Moreno 1892; Lydekker 1893; Kellogg 1925, 1927, 1965; Gondar 1975; Hirota and Barnes 1994; Bianucci and Landini 2006; Lambert et al. 2008, 2010, 2016; Boersma and Pyenson 2015; Velez Juarbe et al. 2015, 2016; Collareta et al. 2017, 2019; Benites-Palomino et al. 2020). In particular, in the southern Atlantic Ocean the Miocene outcrops from Patagonia (Argentina) hold one of the most important cetacean fossil records, including members of this group (Lydekker 1893; Cabrera 1926; Cozzuol 1996; Buono and Cozzuol 2013; Buono et al. 2014, 2017; Viglino et al. 2018a, b; Gaetán et al. 2019; Paolucci et al. 2019). There are at least five physeteroid species known from there: Diaphorocetus poucheti (Moreno 1892), Idiorophus patagonicus (Lydekker 1893), “Aulophyseter” rionegrensis (Gondar 1975), Livyatan sp. (Piazza et al. 2018); and Preaulophyseter gualichensis (Caviglia and Jorge 1980).
Preaulophyseter gualichensis was originally described by Caviglia and Jorge (1980), based on two isolated, fragmentary teeth and a left periotic (MLP 76-IX-5-1) collected from the Miocene of the Gran Bajo del Gualicho Formation (Rio Negro province, Patagonia). In the same contribution, the authors referred to this species two right periotics (MLP 76-IX-2-3 and MLP 76-IX-2-4) and emphasized the lack of geographical and geological information associated to these specimens. Preaulophyseter was initially included in the subfamily Hoplocetinae (Cabrera 1926), which comprised Miocene forms retaining upper teeth, such as Scaldicetus, Hoplocetus, Idiorophus, Aulophyseter, and Diaphorocetus. The subfamily was further characterized by having teeth with enameled crowns (Muizon 1991; Fordyce and Muizon 2001). Posteriorly, Hampe (2006) remarked the unclear status of Preaulophyseter, which has contributed to its exclusion from many modern phylogenetic analyses. Moreover, the diagnosis of Preaulophyseter is based on scarce and isolated specimens, and knowledge on the evolution of sperm whale tympano-periotic morphology is scarce. Overall, tympano-periotic characters represent a low proportion of the morphological characters in most of the physeteroid matrices (about 11%), and their potential in the resolution of phylogenetic relationships has never been deeply explored. Therefore, the aim of this contribution is to re-describe the type and referred materials of Preaulophyseter, as well as to analyze other isolated physeteroid periotics deposited in the Paleovertebrate Collection of the Museo de La Plata. Finally, considering that tympano-periotics bear a strong phylogenetic signal in odontocetes (Kasuya 1973; Luo and Marsh 1996; Geisler and Sanders 2003; Geisler et al. 2011; Ekdale et al. 2011; Tanaka and Fordyce 2016; Viglino et al. 2018b) and that Argentina holds one of the largest fossil records of physeteroid periotics worldwide, we also analyzed the phylogenetic signal of ear bones and their potential to resolve phylogenetic relationships among species of Physeteroidea.
Institutional abbreviations.—IRSNB, Institut royal des Sciences naturelles de Belgique, Brussels, Belgium; MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MAUL, Museo dell’Ambiente, Università di Lecce, Italy; MLP: Museo de La Plata, Buenos Aires, Argentina; MNHN, Museum National d’Histoire Naturelle, París, France; MPEF, Museo Paleontológico Egidio Feruglio, Trelew, Chubut, Argentina; MUSM, Museo de Historia Natural, Universidad Nacional Mayor de San Marco, Lima, Peru; USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Other abbreviations.—IAM, internal acoustic meatus.
Material and methods
Specimens and terminology.—The present study of Preaulophyseter gualichensis was based on three specimens (MLP 76-IX-5-1, MLP 76-IX-2-3, and MLP 76-IX-2-4), and other isolated and indeterminate physeteroid periotics (MPEF-PV-605, MPEF-PV-651, MPEF-PV-6098, MLP 80-VIII-30-133a and b, MLP 76-IX-2-7, and MLP 52-X-2-8). However, due to preservation and completeness, the anatomical descriptions were based mainly on specimen MLP 76-IX-5-1, unless otherwise indicated. The orientation of the periotic and tympanic bulla used both in the descriptions and comparisons were based on the anatomical standard views used for physeteroids (e.g., Lambert et al. 2016). The anatomical terminology follows Mead and Fordyce (2009) and the measurements were taken according to Kasuya (1973). Photos of the specimens were taken with a Nikon D3200 camera and an 18–55 mm lens. The physeteroid specimens used for anatomical comparisons are listed as follows: Acrophyseter deinodon MNHN SAS 1626 (type), MNHN F-PPI 272; Zygophyseter varolai MAUL 229/1 (type); “Aulophyseter” rionegrensis MLP 62-XII-19-1 (type); Orycterocetus crocodilinus USNM 22953, USNM 22952, USNM 22926, USNM 11234; Aulophyseter morricei USNM 11230 (type), UNSM 10853, USNM 2794, USNM 2795, USNM 2796, USNM 2797; Physeterula dubusi IRSNB M.527 (type); Idiorophus patagonicus MLP 5-2 (type); Eudelphis mortezelensis IRSNB M.523 (type); Physeter macrocephalus MACN 29.768; Livyatan melvillei MUSM 1676 (type).
Phylogenetic analyses.—The phylogenetic analyses were based on a modified version of the published morphological matrix of Paolucci et al. (2019). Codings for Preaulophyseter gualichensis were based on MLP 76-IX-5-1, which exhibits the best preservation state among the sample. The resulting matrix has 32 taxa and 55 characters (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Pauloucci_etal_SOM.pdf). Heuristic parsimony analyses were carried out with the software TNT version 1.5 (Goloboff et al. 2008a, b) using a traditional search (2000 replicates, with random addition sequences -RAS- followed by tree bisection reconnection -TBR- branch-swapping, holding 10 trees per replicate) under implied (k = 3, 10, 20) and equal weights. We performed the analyses in two ways: (i) treating part of the characters as ordered (following the proposal of Lambert et al. 2010) and (ii) treating all characters as unordered. The resulting most parsimonious trees (MPTs) were summarized in a strict consensus with zero-length branches collapsed (“rule 1” of Coddington and Scharff 1994). Branch support was assessed using Bremer decay values (see SOM: fig. S1).
Phylogenetic signal: To determine the phylogenetic signal of the tympano-periotic bones, we used the phylosignal package (Keck et al. 2016) in R Core Team (version 3.4.1, 2017). The input dataset consists of tympano-periotic morphological characters of the morphological matrix (characters 33, 34, 35, 36, 49, 50; see details of matrix in SOM), a MPT and the biochron of each taxa (see SOM). We chose the first MPT of each phylogenetic analysis (equal weights and implied weights with k=3, using either ordered or unordered characters; see above and SOM). Thus, ear bones’ phylogenetic signal was evaluated using four different phylogenetic hypotheses. The phylosignal package uses an algorithm that assumes an evolutionary model of Brownian motion (i.e., randomly) and calculates a statistic number “K”. If K takes values above 1 it means that the evaluated characters have a high phylogenetic signal and thus these characters are dependent on the phylogeny, whereas if K takes values below 1 the characters have a low phylogenetic signal and are independent from the phylogeny.
Geological setting
Specimens described herein were collected from two main regions of northeastern Patagonia (Fig. 1). MLP 76-IX-5-1 (Caviglia and Jorge 1980) was collected near Puesto Echávez, 38 km north of San Antonio Oeste city at the Gran Bajo del Gualicho depression (Fig. 1: location 1). This depression and the San Antonio Oeste city area comprise outcrops from the Gran Bajo del Gualicho Formation (Lizuaín and Sepúlveda 1978). Considering the lack of precise age determinations for this unit (Reichler 2010), as well as the absence of information regarding the stratigraphic provenance of the specimen in this region, we propose a conservative Miocene age for this specimen.
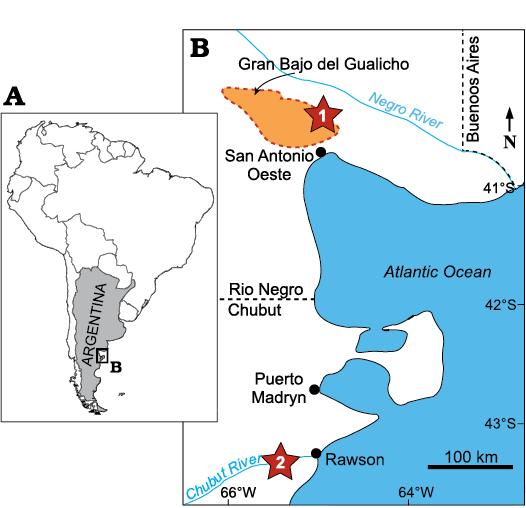
Fig. 1. Geographic location of studied area in Patagonia, southern Argentina (A) and location of the marine Miocene outcrops (B, stars) where the specimens included in this study were collected: Gran Bajo del Gualicho Formation (1) and Gaiman Formation (2).
Other isolated periotics (MPEF-PV-605, MPEF-PV-651, MPEF-PV-6098, and MLP 80-VIII-30-133a and b) were recovered from sediments of the early Miocene (Burdigalian) Gaiman Formation at Bryn Gwyn (= Loma Blanca), within the southern margin of the Lower Valley of the Chubut River, northeast of Chubut province (Fig. 1: location 2). The Gaiman Formation is composed of shallow marine sediments that are well exposed at Bryn Gwyn. The depositional settings, age, and fossil cetacean content of this unit are discussed in Cuitiño et al. (2017, 2019).
A final set of isolated periotics (MLP 76-IX-2-3, MLP 76-IX-2-4, MLP 76-IX-2-7, and MLP 52-X-2-8) belonging to the historical collection of the Museo de La Plata lacks detailed geographical provenance. The only stratigraphic information of these specimens came from their label, which indicates “Patagoniense” age. It refers to the “Patagoniense transgression”, one of the major marine flooding events recorded in South America during the early Miocene, with outcrops recognized in Patagonia from Río Negro up to Tierra del Fuego (Malumián and Náñez 2011). However, considering the unknown specific geographical provenance of the specimens and the lack of precise age of the Patagoniense deposits in regions outside the Chubut province, we also assign them a conservative Miocene age.
Systematic palaeontology
Cetacea Brisson, 1762
Pelagiceti Uhen, 2008
Neoceti Fordyce and Muizon, 2001
Odontoceti Flower, 1868
Physeteroidea Gray, 1821
Physeteroidea indet.
Figs. 2–4.
1980 Preaulophyseter gualichensis, Caviglia and Jorge 1980: 363–368, pls. I, II.
Material.—MLP 76-IX-5-1, a left periotic and two isolated fragmentary teeth, from near Puesto Echávez, 38 km north of San Antonio Oeste city within the Gran Bajo del Gualicho depression, Río Negro province, Argentina; Gran Bajo del Gualicho Formation; Miocene (Caviglia and Jorge 1980); MLP 76-IX-2-3 and MLP 76-IX-2-4, isolated right periotics, MLP 52-X-2-8, an isolated right periotic, and MLP 76-IX-2-7, a fragmentary periotic, from Patagonia, without a precise geographic provenance, Argentina; Miocene (Caviglia and Jorge 1980). MPEF-PV-605, an isolated right periotic and MPEF-PV-651, an isolated right periotic, from Bryn Gwyn (= Loma Blanca); MPEF-PV-6098, an isolated left periotic from Cerro Castillo; MLP 80-VIII-30-133 (right -a and left -b), two isolated periotics from Gaiman, Lower Valley of Chubut River; all Chubut province, Argentina; Gaiman Formation; lower Miocene.
Description.—Teeth: The two isolated teeth of MLP 76-IX-5-1 are broken and eroded. They are conical with an oval transverse section, and a thin layer of crenulated enamel on their crowns. Given that these teeth are isolated, we could neither determine their anteroposterior position along the toothrow nor their location on the skull (lower versus upper jaw). The better-preserved tooth (Fig. 2: I) is long (150 mm) and slender (maximum diameter about 21 mm). There is a shallow crest in both mesial and distal margins of the crown, being more conspicuous on the lingual side (Fig. 2A4). Over this side, and ventral to the crest, there is a dorsoventrally long and shallow occlusal facet, extending for 47 mm on the crown and the root. The occlusal facet runs along the longitudinal axis of the tooth, being obliquely oriented in its proximal region. The presence of this occlusal facet reinforces the hypothesis that both the crown and part of the root were erupted above the gums in most physeteroids (e.g., Bianucci and Landini 2006). Besides, the presence of a long occlusal facet allows us to infer that both upper and lower teeth were present in this specimen. The crown is short, about one fourth of the total length (31 mm), and there is a shallow cingulum at the base, which is clearer on the labial side. The long (83 mm) and fusiform root displays cement rings in the proximal region. The pulp cavity is obscured by sediment and occupies one third of the total length of the tooth (38 mm). In both teeth, some oblique layers of dentine (Growth Layer Groups, GLGs) are observed along break surfaces.
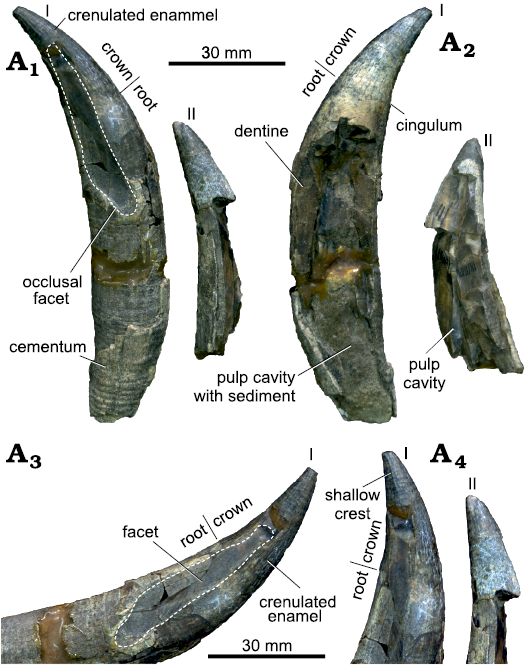
Fig. 2. Teeth of a sperm whale Physeteroidea indet. previously described as “Preaulophyseter gualichensis” Caviglia and Jorge, 1980, MLP 76-IX-5-1, from the Miocene of Gran Bajo del Gualicho Formation, Patagonia, Argentina; in labial (A1) and lingual (A2) views, and detailed view of the crown (A3) and enamel (A4). I and II refer to the two fragmentary teeth of the MLP 76-IX-5-1 (the best and worst preserved tooth, respectively).
Periotics: MLP 76-IX-5-1 includes a left periotic almost completely preserved (Fig. 3). The fenestra rotunda, fenestra ovalis, some parts of the ventral surface of the periotic, as well as the fossa for the stapedial muscle are obscured by sediment. In dorsal view (Fig. 3A1), the circular aperture for the cochlear aqueduct is smaller than the anteroposteriorly compressed aperture for the vestibular aqueduct, and these two openings are not anteroposteriorly aligned. The lateromedially pyriform internal acoustic meatus (IAM) has a well-developed transverse crest and a tear drop-shaped foramen singulare. The IAM also has a large and tear drop-shaped spiral cribriform tract. The proximal opening of the facial canal is a large foramen that is also tear drop-shaped, with its rounded part posteriorly oriented. Some prominences lateral to the IAM give the periotic an irregular dorsolateral surface. In ventral view (Fig. 3A3), the pars cochlearis is mediolaterally wide with a well-defined convex medial surface; it is weakly tilted towards the anterior process. The oval-shaped accessory ossicle is large (Table 1), and, as in most other physeteroid periotics, together with part of the outer lip of the tympanic bulla it is fused with the anterior process of the periotic, leaving visible only a small and circular part of the anterior bullar facet. The mallear fossa is deep and circular, whereas the fenestra ovalis is oval. The conspicuous lateral tuberosity is anterior to the wide and deep epitympanic hiatus. Posteriorly, the posterior bullar facet is ventrally concave and has grooves and fracture lines. In medial and dorsal views, there is a circular prominence on the anterior process dorsal to the accessory ossicle. The facial sulcus is wide and deep. In medial view (Fig. 3A5), the pars cochlearis is subcircular. The fenestra rotunda has a dorsoventrally oval outline, and it is connected with the aperture for the cochlear aqueduct by a shallow groove. The anterior process of the periotic is small and pointed. In lateral view (Fig. 3A7), the body of the periotic is perpendicular to the posterior process, resulting in an L-shaped profile of the periotic. The posterior process has a deep groove at the posteroventral apex.
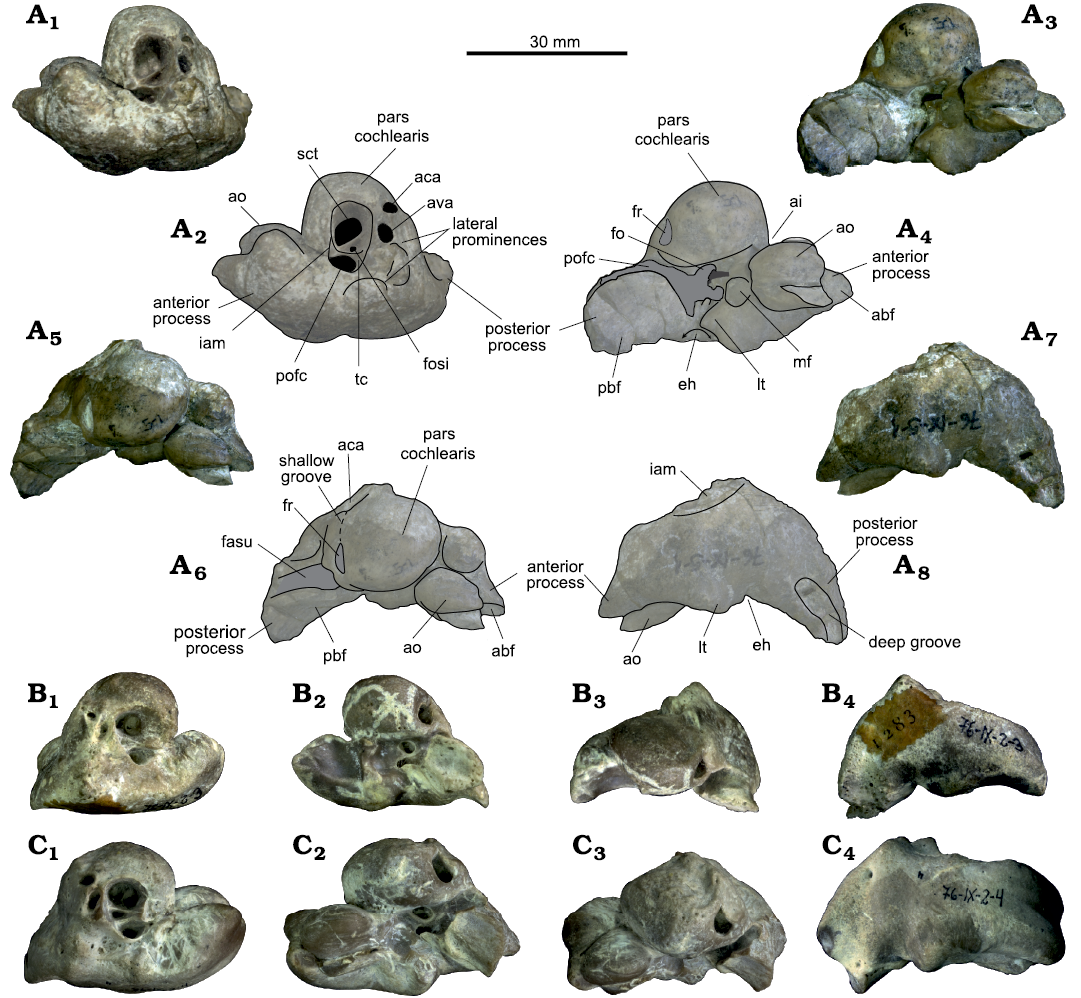
Fig. 3. Sperm whale Physeteroidea indet. A. Left periotic of nomen dubium “Preaulophyseter gualichensis” Caviglia and Jorge, 1980, MLP 76-IX-5-1, from the Miocene of Gran Bajo del Gualicho Formation, Patagonia, Argentina, in dorsal (A1, A2), ventral (A3, A4), medial (A5, A6), and lateral (A7, A8) views. B, C. Two isolated right periotics from the Miocene of Patagonia, MLP 76-IX-2-3 (B) and MLP 76-IX-2-4 (C), in dorsal (B1, C1), ventral (B2, C2), medial (B3, C3), and lateral (B4, C4) views. Photographs (A1, A3, A5, A7, B, C) and explanatory drawings (A2, A4, A6, A8). Abbreviations: abf, anterior bullar facet; aca, aperture for cochlear aqueduct; ai, anterior incisure; ao, accessory ossicle; ava, aperture for the vestibular aqueduct; eh, epitympanic hiatus; fasu, facial sulcus; fo, fenestra ovalis; fosi, foramen singulare; fr, fenestra rotunda; iam, internal acoustic meatus; lt, lateral tuberosity; mf, mallear fossa; pbf, posterior bulla facet; pofc, proximal opening of facial canal (VII); sct, spiral cribiform tract (VIII).
The periotics MLP 76-IX-2-3 and MLP 76-IX-2-4 have the same general characteristics as MLP 76-IX-5-1. When comparing all the periotics, MLP 76-IX-2-3 is smaller, while MLP 76-IX-2-4 and MLP 76-IX-5-1 are similar in size (Table 1). In MLP 76-IX-2-3, the posterior process is broken and the accessory ossicle is missing, making the oval fovea epitubaria visible. In medial view, the periotic has a triangular outline due to the erosion of the dorsal region of the pars cochlearis. In ventral view, the pars cochlearis of MLP 76-IX-2-3 and MLP 76-IX-2-4 (Fig. 3C) is shorter than in MLP 76-IX-5-1, and leaning towards the anterior process more than in MLP 76-IX-5-1. Finally, the fenestra rotunda of MLP 76-IX-2-3 and MLP 76-IX-2-4 is D-shaped, whereas it is oval in MLP 76-IX-5-1.
Table 1. Measurements (in mm) of the periotics of the Patagonian Miocene Physeteroidea indet. included in our study.
|
Specimens Measurements |
MLP 76-IX-5-1 |
MLP 76-IX-2-3 |
MLP 76-IX-2-4 |
MLP 80-VIII-30-133 |
MLP 52-X-2-8 |
MPEF-PV-651 |
MPEF-PV-6098 |
MPEF-PV-605 |
|
Anteroposterior length of pars cochlearis |
18 |
17 |
18 |
17 |
18 |
18 |
18 |
19 |
|
Standard length of periotic |
38 |
31 |
37 |
35 |
- |
34 |
37 |
31 |
|
Mediolateral height of posterior bulla facet |
10 |
14 |
10 |
11 |
15 |
11 |
14 |
13 |
|
Width of periotic across pars cochlearis and superior process |
27 |
25 |
25 |
24 |
25 |
26 |
27 |
24 |
|
Least distance between the margins of internal acoustic meatus and aperture of cochlear aqueduct |
3 |
3 |
3 |
4 |
4 |
4 |
5 |
3 |
|
Least distance between the margins of internal acoustic meatus and aperture of vestibular aqueduct |
1 |
1 |
1 |
1 |
2 |
1 |
2 |
1 |
|
Anteroposterior length of accessory ossicle |
12 |
- |
11 |
- |
- |
- |
- |
9 |
|
Maximun dorsoventral height of periotic |
21 |
19 |
22 |
18 |
21 |
20 |
23 |
16 |
MPEF-PV-605 (Fig. 4A) corresponds to an almost complete right periotic with a certain degree of erosion and of similar size than MLP 76-IX-2-3 (Table 1). The general morphology is in concordance with MLP 76-IX-5-1, so we only provide here the main differences. In medial view, the pars cochlearis has a D-shape profile, with a straight dorsal and a convex ventral margin. The D-shaped fenestra rotunda is positioned on the posterior margin of the pars cochlearis. In ventral view, the fenestra ovalis is circular whereas the fossa incudis is oval. The posterior bullar facet is laterally oriented, concave and has grooves and ridges. The pars cochlearis leans towards the anterior process. In dorsal view, the circular aperture for the cochlear aqueduct is larger than the anteroposteriorly compressed aperture for the vestibular aqueduct. The spiral cribriform tract is large and circular, medial to the oval proximal opening of the facial canal. The foramen singulare is small and mediolaterally narrow, and the transverse crest is less conspicuous than in MLP 76-IX-5-1.
The anterior process and the ventral part of the pars cochlearis of the right periotic MPEF-PV-651 (Fig. 4B) are highly eroded, so the margins of the fovea epitubaria are lost. The pars cochlearis’ profile, fenestra rotunda, aperture for the vestibular and cochlear aqueducts are similar to MPEF-PV-605. In ventral view, the fenestra ovalis has broken edges.
The left periotic MPEF-PV-6098 (Fig. 4C) has a size similar to MLP 76-IX-5-1. Even though it is eroded, most of the features are visible. In dorsal view, the apertures for the cochlear and vestibular aqueducts have approximately the same size. In ventral view, the fovea epitubaria is visible and oval-shaped. The anterior bullar facet is rounded due to erosion. The posterior bullar facet is laterally bended and has crests and grooves as in MPEF-PV-605. In medial view, the pars cochlearis is oval and the fenestra rotunda is D-shaped.
The two periotics MLP 80-VIII-30-133a and b (Fig. 4D, E) have an eroded and rounded surface. The better-preserved (MLP 80-VIII-30-133a) individual (Fig. 4D) lacks the posterior process. In medial view, the pars cochlearis is anteroposteriorly oval, and the fenestra rotunda has a D-shape profile as in MPEF-PV-605, MPEF-PV-6098, and MPEF-PV-651. In dorsal view, the aperture for the vestibular aqueduct is larger than the aperture for cochlear aqueduct, as in MLP 76-IX-5-1. The other left periotic (MLP 80-VIII-30-133b) is poorly preserved (Fig. 4E), lacking most of the pars cochlearis. The posterior process is reduced to a thin process, and the margins of the anterior process are rounded due to erosion. Both processes are at a right angle, resulting in an L-shaped profile of the periotic. Posterior to the anterior process is a prominent lateral tuberosity.
MLP 52-X-2-8 is an eroded and fragmentary right periotic (Fig. 4F), with the anterior process missing. In medial view, the fenestra rotunda is D-shaped and the pars cochlearis is oval as in the previous specimens. In dorsal view, the aperture for the vestibular aqueduct is anteroposteriorly compressed and larger than the aperture for the cochlear aqueduct, as in MLP 76-IX-5-1.

Fig. 4. Isolated periotics of a sperm whale Physeteroidea indet. from the Miocene of Patagonia. A. MPEF-PV-605, right periotic. B. MPEF-PV-651, right periotic. C. MPEF-PV-6098, left periotic. D. MLP 80-VIII-30-133a, right periotic. E MLP 80-VIII-30-133b, left periotic. F. MLP 52-X-2-8, right periotic. In dorsal (A1–F1), ventral (A2–F2), medial (A3–F3), and lateral (A4–F4) views. G. MLP 56-IX-2-7, fragmentary periotic in dorsal (G1) and medial (G2) views.
MLP 76-IX-2-7 is represented by an eroded portion of pars cochlearis, in which the spiral cribriform tract and the medial edge of the foramen singulare are preserved. The spiral cribriform tract is circular and delimited by conspicuous edges.
The periotics analyzed above present the diagnostic characters of Physeteroidea described in the previous section. Cozzuol (1993: 22) referred MPEF-PV-605 to “Aulophyseter” rionegrensis, the only Patagonian physeteroid species that is currently known with associated ear bones. However, we cannot confidently assign any of these isolated periotics to any known or new taxa, and therefore they are here identified as Physeteroidea indet. (see Discussion).
The morphology of the periotics and teeth referred to Preaulophyseter by Caviglia and Jorge (1980) is consistent with that of Physeteroidea. The physeteroid periotic is characterized by a large accessory ossicle often fused with the anterior process of the periotic, contact surface between posterior process of tympanic bulla and periotic with keels and grooves, a pointed anterior process, posteroventrally recurved posterior process at a right angle with the body of periotic, anteriorly inclined pars cochlearis, a thick superior process, concave posterior bullar facet, and a volcano-shaped IAM (Kasuya 1973; Luo and Marsh 1996). However, there are no characteristics that differentiate these specimens from other known non kogiid-physeteroid species. The observed minor differences could possibly be explained by intraspecific (including ontogenetic variation and sexual dimorphism, although the latter has not yet been demonstrated at the level of ear bones in physeteroids; e.g., de Buffrenil et al. 2004; Lancaster et al. 2015) or interspecific variations (e.g., Kasuya 1973; Oelschläger 1986; Gutstein et al. 2014). Therefore, we assign MLP 76-IX-5-1, MLP 76-IX-2-3 and MLP 76-IX-2-4 as Physeteroidea indet. (see Discussion).
The main differences observed between the periotics described above are related to: (i) size; (ii) shape of the pars cochlearis; and (iii) size of the apertures for the cochlear and vestibular aqueducts. Below, we provide detailed comparisons between the specimens analyzed in this contribution and with other non-kogiid physeteroid species.
The periotic MLP 76-IX-5-1 (Fig. 5A) differs from that of “Aulophyseter” rionegrensis in being larger, mediolaterally wide, with a more circular pars cochlearis that is less inclined towards the anterior process. It further differs in having an oval fenestra rotunda. “A.” rionegrensis (Fig. 5B) and MLP 76-IX-2-3 differ only in the size of the pars cochlearis in medial view. Both MLP 76-IX-2-4 and MLP 52-X-2-8 differ from “A.” rionegrensis in being larger. On the other hand, there are no significant differences between “A.” rionegrensis, MPEF-PV-605 and MLP 80-VIII-30-133a and b. MPEF-PV-651 differs from “A.” rionegrensis in having a higher pars cochlearis in ventral view, whilst MPEF-PV-6098 differs in being larger and in having a small prominence along the posteromedial outline on the pars cochlearis, above the fenestra rotunda. The periotic of Acrophyseter deinodon (Lambert et al. 2016; Fig. 5C) differs from all remaining physeteroids in having a quadrangular pars cochlearis in medial view. However, as in MLP-76-IX-5-1, A. deinodon has an oval-shaped fenestra rotunda, whereas in the other studied specimens it is D-shaped. MLP-76-IX-5-1 differs from Zygophyseter varolai (Bianucci and Landini 2006; Fig. 5D) in having a smaller pars cochlearis. Zygophyseter differs from all other studied specimens in having a rectangular fovea epitubaria. MLP-76-IX-5-1 differs from Aulophyseter morricei (Kellogg 1927; Fig. 5E) in having a higher pars cochlearis in ventral view, whilst this genus differs from the other specimens analyzed here in having more circular IAM and apertures for cochlear and vestibular aqueducts. Orycterocetus crocodilinus (Kellogg 1965; Fig. 5F) differs from all the specimens analyzed here in having a larger and circular spiral cribriform tract. In ventral view, O. crocodilinus presents the same small prominence along the posteromedial outline as MPEF-PV 6098. Finally, the main difference between Physeter (Fig. 5G) and the physeteroid periotics studied here is the acute angle formed by the posterior process and the body of the periotic in the former, whereas our specimens display a right angle.

Fig. 5. Schematic comparisons of the periotic of MLP 76-IX-5-1, “Preaulophyseter gualichensis” Caviglia and Jorge, 1980 (A) with “Aulophyseter” rionegrensis (B), Acrophyseter deinodon (C, modified from Lambert et al. 2016), Zygophyseter varolai (D, modified from Bianucci and Landini 2006), Aulophyseter morricei (E, modified from Kellogg 1927), Orycterocetus crocodilinus (F, modified from Kellogg 1965), and Physeter macrocephalus (G, modified from Kasuya 1973). In dorsal (A1–G1), ventral (A2–G2), medial (A3–G3), and lateral (A4–C4, E4–G4) views. Black areas indicate anatomical foramina. Not to scale.
Phylogenetic analyses and signal
The phylogenetic analyses recovered MLP-76-IX-5-1 in an unresolved polytomy with several other physeteroid taxa (see SOM: fig. S1). These results suggest that, in this case, the inclusion of an isolated periotic specimen (even if associated to detached teeth), often considered as a highly informative bone in odontocetes (e.g., Tsai and Fordyce 2016; Tanaka and Fordyce 2016; Viglino et al. 2018b), does not result in a good resolution of its phylogenetic relationships.
On the other hand, the phylogenetic signal analyses of the tympano-periotic morphological characters resulted in K values lower than 1 for all of them (e.g., with ordered characters and implied weights we obtain: character 33, K = 0.043; ch. 34, K = 0.076; ch. 35, K = 0.039; ch. 36, K = 0.059; ch. 49, K = 0.051; ch. 50, K = 0.044; for further details see SOM), reflecting the independence of these characters with phylogenetic relationships among physeteroids, and thus suggesting that the observed morphological variation in the tympano-periotic morphology is not related to phylogeny.
Discussion
Over the last decade and a half, our understanding of the taxonomic diversity of physeteroids has been improved by the description of new and/or historically known species (e.g., Bianucci and Landini 2006; Lambert 2008; Lambert et al. 2008, 2010, 2016; Boersma and Pyenson 2015; Velez Juarbe et al. 2015, 2016; Collareta et al. 2017, 2019; Paolucci et al. 2019; Benites-Palomino et al. 2020). Historically, the physeteroid skull has been considered as the main source to diagnose taxa and resolve phylogenetic relationships among species. However, the diagnostic value of teeth and tympano-periotic bones has been questioned by some authors (Kellogg 1925, 1927; Caviglia and Jorge 1980; Hirota and Barnes 1994; Luo and Marsh 1996; Bianucci and Landini 2006; Kimura et al. 2006; Hampe 2006; Reumer et al. 2017), especially in the case of isolated materials or incomplete skulls.
Physeteroid teeth.—Tooth morphology has been historically used as a diagnostic feature among extinct sperm whales, and many species and even genera have been defined based on one or more isolated teeth (e.g., Hoplocetus Gervais, 1848, Scaldicetus Du Bus, 1867). However, the power of dental characters to diagnose sperm whale species has been questioned by many authors (e.g., Kellogg 1925; Bianucci and Landini 2006; Hampe 2006; Reumer et al. 2017). Nowadays, there is a broad consensus about the fact that dental morphology is not sufficient to identify and differentiate taxa neither at genus nor at species level. This is mainly related to the difficulty to evaluate homodont dentition in odontocetes, but also because there is scarce information on ontogenetic and sexual dimorphism variation in sperm whale teeth. Moreover, most of the dental characters used in physeteroid phylogenetic analyses are based on the size of teeth with respect to skull size, tooth position, or the number of mandibular teeth, with only few characters that could be scored for isolated teeth (e.g., Bianucci and Landini 2006; Lambert et al. 2016; Collareta et al. 2019; Paolucci et al. 2019).
In this study, a careful comparative analysis between the teeth of MLP 76-IX-5-1 and other Patagonian sperm whales (i.e., “A.” rionegrensis and Idiorophus patagonicus) shows that it is the only Patagonian physeteroid in having teeth with enameled crowns. This condition is only shared with macroraptorial forms (e.g., Acrophyseter spp., Brygmophyseter, Livyatan, and Zygophyseter) probably representing a plesiomorphic condition retained by non-obligate suction feeding forms. The general morphology of MLP 76-IX-5-1 resembles other Patagonian taxa (“Aulophyseter” rionegrensis, Idiorophus) in the elongated and conical teeth with no clear boundary between the crown and the root, with smooth-surfaced crown and root, but with the presence of some longitudinal grooves on the root. These conditions are also shared with extinct crown physeteroid morphotypes (such as Aulophyseter, Orycterocetus, and Physeterula; Fig. 6). However, MLP 76-IX-5-1 differs from Orycterocetus, Physeterula, Pliokogia, Scaphokogia cochlearis, and extant physeteroids (Physeter and Kogia) in having enamel on the crown; it further differs from Physeter in having more slender teeth and from Kogia in having more robust teeth. MLP 76-IX-5-1 differs from the macroraptorial morphotype in having a thinner enamel layer, in the absence of an abrupt step between crown and root (such as a constriction or expansion just below the crown; Fig. 6). Thus, MLP 76-IX-5-1 could probably correspond to a stem physeteroid species, retaining primitive traits such as the presence of enamel. However, and as explained above, based on tooth morphology there is not enough information to keep the original assignation of Caviglia and Jorge (1980) as a separate taxon. Associated cranial remains would be needed to finally confirm the taxonomic status of this specimen.
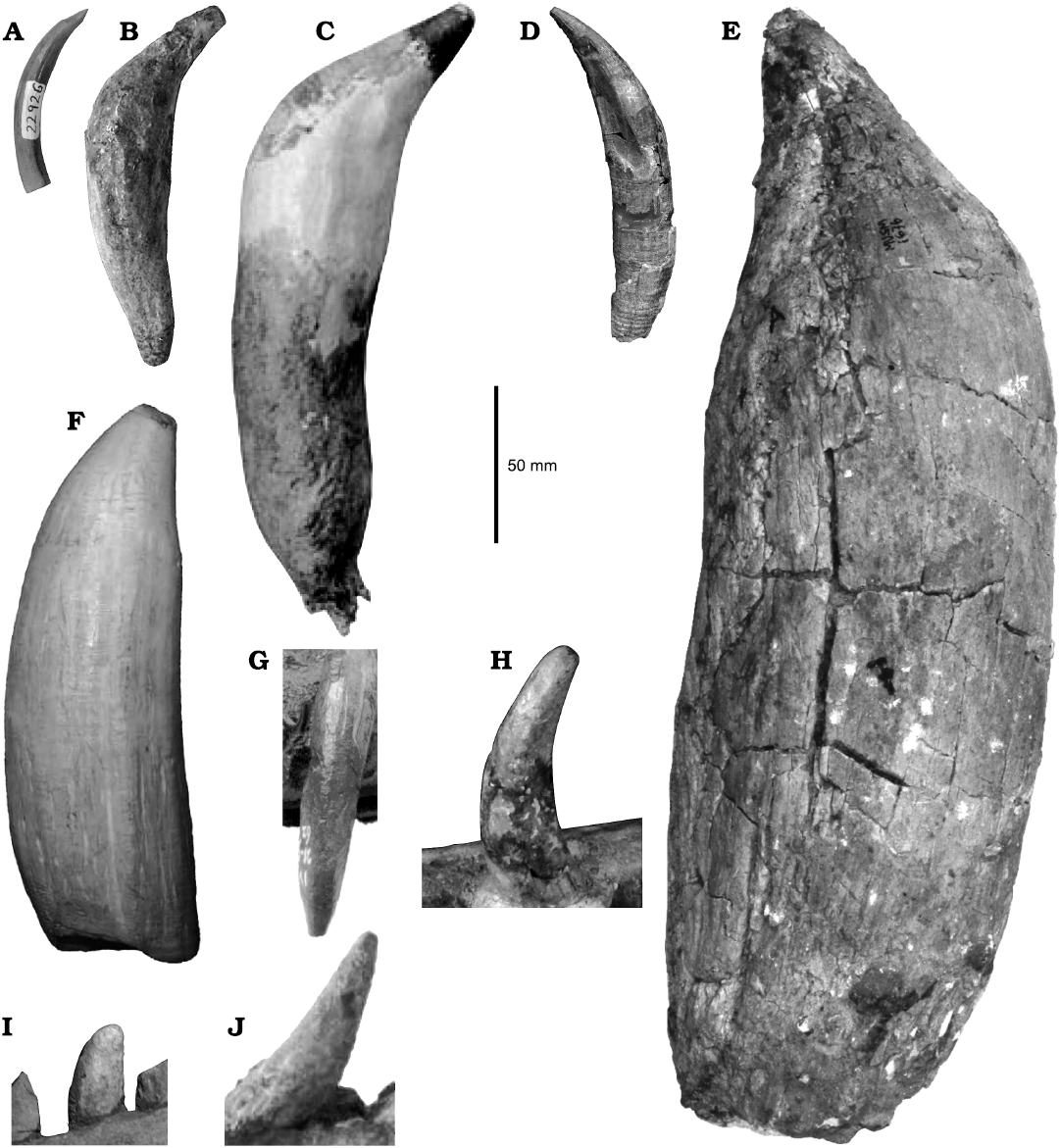
Fig. 6. Comparisons of non-kogiid physeteroid teeth from the Miocene. A–F. Isolated teeth of: Orycterocetus crocodilinus (Cope, 1867), Calvert Formation, between Maryland and Virginia, USA (A, USNM 22926), Acrophyseter sp., Pisco Formation, Cerro los Quesos, Peru (B, MUSM 2182; modified from Lambert et al. 2016), Zygophyseter varolai (Bianucci and Landini, 2006), Pietra Leccese Formation, Cisterna Quarry, Italy (C, MAUL 229/1; modified from Bianucci and Landini 2006), Physeteroidea indet., Gran Bajo del Gualicho Formation, Rio Negro, Argentina (D, MLP 76-IX-5-1, “Preaulophyseter” sensu Caviglia and Jorge 1980), Livyatan melvillei (Lambert, Bianucci, Post, de Muizon, Salas-Gismondi, Urbina, and Reumer, 2010), Pisco Formation, Cerro Colorado, Peru (E, MUSM 1676; modified from Lambert et al. 2016), the extant Physeter macrocephalus (Linnaeus, 1758) (F, MACN 29.768; modified from Perez et al. 2011). G. Maxillary tooth of Eudelphis mortezelensis (Du Bus, 1872), Berchem Formation, Antwerp, Belgium (IRSNB M.523; modified from Lambert 2008). H–J. Mandibular tooth of: Idiorophus patagonicus (Lydekker, 1893), Gaiman Formation, Chubut, Argentina (H, MLP 5-2), “Aulophyseter” rionegrensis (Gondar, 1975), Gran Bajo del Gualicho Formation, Rio Negro, Argentina (I, MLP 62-XII-19-1), and Physeterula dubusi (Van Beneden, 1877), Antwerp, Belgium (J, IRSNB M.527; modified from Lambert 2008).
Physeteroid periotics.—The petrosal and tympanic bones have been an important source of phylogenetic information within mammals (MacPhee 1981; Novacek 1993). In cetaceans, the tympano-periotic bones are a morphologically and functionally complex structure, differing markedly from that of other eutherians (Fordyce 1994), with the potential to contribute to phylogenetic (e.g., Kasuya 1973; Ekdale et al. 2011; Tanaka and Fordyce 2016) and morphofunctional analyses (e.g., Ketten 1984; Oelschläger 1986; Gutstein et al. 2014; Park et al. 2016; Mourlam and Orliac 2017; Ary 2017). Among odontocetes, tympano-periotic morphology is extensively used in the definition of families or, more occasionally, even in the naming of genera and species (e.g., Kasuya 1973; Tanaka and Fordyce 2016; Viglino et al. 2018b), and provides an important source of anatomical data for phylogenetic analyses (e.g., Luo and Marsh 1996; Geisler and Sanders 2003; Geisler et al. 2011; Tanaka and Fordyce 2016; Viglino et al. 2018a, b). However, not all tympano-periotic characters are taxonomically informative in all the cetacean lineages (Luo and Marsh 1996).
Kasuya (1973) provided the first attempt to analyze the potential of the tympano-periotic morphology to differentiate between extant odontocete species. In the case of physeteroids, Kasuya (1973) and Luo and Marsh (1996) provided some diagnostic features for non-kogiid specimens, such as: massiveness, a large accessory ossicle often fused with the anterior process of periotic, suture surface between posterior process of the tympanic bulla and periotic with keels and grooves, pars cochlearis sloping anteriorly, posterior process cylindrical and tapered distally, a pointed anterior process, concave posterior bullar facet, volcano-shaped IAM, and posterior process bent posteroventrally at a right angle with the thick superior process. These authors also found notable differences between Physeter macrocephalus and Kogia spp., but few differences between Kogia sima and Kogia breviceps.
In the case of extinct species, previous studies have pointed out the lack of interspecific variation for the physeteroid tympano-periotic morphology. Kellogg (1927:18) was particularly clear on this topic: “It is remarkable how closely, except for minor modifications in the anterior process, this periotic resembles those of the living sperm whale, for the relative proportions and peculiarities of the various structures are essentially the same. (…) Minor variations in the contour of the articular facet on the posterior process are observable in the eleven periotics under consideration, but most of these may be attributed to the effects of erosion. (…) There is a very little variation in the general outlines of the pars cochlearis as seen from a ventral view in these eight periotics. Whatever differences are observable are of a minor nature”. Kasuya (1973) made a brief observation on the periotics of Orycterocetus and Aulophyseter morricei, and proposed that they differed only in the size of the posterior process. Modern studies also casted doubts on the use of isolated periotics for the identification of extinct physeteroid species (Caviglia and Jorge 1980; Cozzuol 1996; Bianucci and Landini 2006) and it was noted that the major morphological differences are found at the family level (i.e., Physeteridae and Kogiidae; Luo and Marsh 1996; Velez-Juarbe et al. 2016). In addition, the limited interspecific variation of ear bones among physeteroids is reflected in the small number of tympano-periotic characters available in morphological matrices (representing about 11% of the total characters; e.g., Lambert et al. 2016; Collareta et al. 2019; Paolucci et al. 2019). However, the discovery of new specimens with tympano-periotic-skull associations for currently more fragmentarily known species could potentially lead to the addition of new morphological characters of these complex bones in future physeteroid matrices.
Our analysis of the phylogenetic signal of tympano-periotic characters showed that they are independent of the phylogenetic relationships, reinforcing the idea that in physeteroids ear bones are non-diagnostic at the species level. We suggest that, in this group, tympano-periotics retain many plesiomorphic characters, and appear to be diagnostic only at higher taxonomic levels (e.g., Kogiidae vs. Physeteridae, or even between kogiids and non-kogiid physeteroids). For example, the periotics of Zygophyseter and Acrophyseter, both genera phylogenetically recovered outside of Physeteridae (e.g., Lambert 2008; Velez-Juarbe et al. 2015; Lambert et al. 2016; Collareta et al. 2017; Collareta et al. 2019; Paolucci et al. 2019) have far more anatomical differences with kogiids than with physeterids. All non-kogiid physeteroids (e.g., “A.” rionegrensis, Acrophyseter deinodon, Orycterocetus, and Zygophyseter) share a more similar periotic morphology: pyriform IAM, globose pars cochlearis, large accessory ossicle, circular and deep mallear fossa, a right angle between the posterior process and the body of the periotic, and contact surface between posterior processes of the tympanic bulla and the periotic with keels and grooves. These observations suggest that, as in the case of teeth, the diagnosis of isolated physeteroid periotics to a specific genus or species is hazardous. As mentioned above, only a small number of extinct physeteroids are known with tympano-periotic bones associated to the skull, which might explain the low number of diagnostic characters currently available. Pending the discovery of new specimens with tympano-periotic-skull associations, which will allow further testing this hypothesis, we consider all isolated periotics from the Miocene of Patagonia as Physeteroidea indet. Nevertheless, thorough anatomical and quantitative analyses are required to shed light on both inter and intraspecific morphological variation in the tympano-periotic complex of Physeteroidea.
The highly distinct, hypertrophied nasal complex of sperm whales was acquired early in the evolutionary history of the group, as suggested by the occurrence of the main osteological correlate (i.e., supracranial basin) in the early Miocene species. Nasal complex structures, in association with ear bones, are closely related to a sophisticated underwater sound system in sperm whales (e.g., Cranford 1999; Huggenberger et al. 2014), related to deep diving (e.g., Watwood et al. 2006) and other biological aspects of the group (e.g., reproduction; Cranford 1999). Therefore, the conservative tympano-periotic morphology identified along the evolutionary history of non-kogiid sperm whales might be the result of morpho-functional constraints imposed by a successful but highly specialized echolocation system.
Conclusions
In this study, we performed for the first time a complete revision of one of the largest collections of fossil physeteroid periotics worldwide and investigated the phylogenetic signal of ear bones to resolve taxonomical issues within Physeteroidea. Based on the lack of diagnostic characters in both the teeth and the periotic of the holotype of “Preaulophyseter gualicheneses”, we consider this species and the materials previously referred to it as nomen dubium, and assign it, as well as other isolated periotics from the Miocene of Patagonia, as Physeteroidea indet. Our analysis of tympano-periotic morphology suggests that, contrary to most odontocetes, it does not have a diagnostic value at the generic level within physeteroids; thus, the use of isolated periotics for naming new species should be avoided. Morphological differences observed between periotics could be related to intraspecific variation (including sexual dimorphism and/or ontogenetic variations), and even possibly diagenetic processes. Finally, the discovery of new specimens with tympano-periotic-skull associations will help to further test the phylogenetic signal of these bones within Physeteroidea.
Acknowledgments
We thank Eduardo Ruigomez (MEF), Marcelo A. Reguero, Alejo Scarano, and Martin L. de los Reyes (all MLP), and Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for access to the collections under their care. We also would like to thank Olivier Lambert and Jorge Velez-Juarbe (Natural History Museum of Los Angeles County, Los Angeles, California, USA) for providing photographs that allowed anatomical comparisons between specimens. Special thanks to Mariana Viglino (Instituto Patagónico de Geología y Paleontología-CENPAT, Puerto Madryn, Argentina) for useful advice on the phylogenetic signal analysis and for the English revision. This contribution used TNT version 1.5, a program made freely available thanks to a subsidy by the Willi Hennig Society. Finally, thanks to Jorge Velez-Juarbe, Rachel Racicot (Senckenberg Museum of Natural History, Frankfurt am Main, Germany), and O. Lambert for their constructive comments and suggestions. We would like to thank the following organizations for financial support: Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015-0792) to MB and JC, (PICT 2016-1039) to MF; The Society of Marine Mammalogy to FP.
References
Ary, W.J. 2017. Form, Function and Phylogeny in the Cetacean Ear Complex. 132pp. Unpublished Doctoral Dissertation, San Diego State University.
Benites-Palomino, A., Vélez-Juarbe, J., Salas-Gismond, R., and Urbina, M. 2020. Scaphokogia totajpe, sp. nov., a new bulky-faced pygmy sperm whale (Kogiidae) from the late Miocene of Peru. Journal of Vertebrate Paleontology 39 (6): e1728538. Crossref
Bianucci, G. and Landini, W. 2006. Killer sperm whale: a new basal physeteroid (Mammalia, Cetacea) from the Late Miocene of Italy. Zoological Journal of the Linnean Society 148: 103–131. Crossref
Boersma, A.T. and Pyenson, N.D. 2015. Albicetus oxymycterus, a new generic name and redescription of a basal physeteroid (Mammalia, Cetacea) from the Miocene of California, and the evolution of body size in sperm whales. PloS One 10: e0135551. Crossref
Buffrenil V. de, Dabin W., and Zylberberg L. 2004. Histology and growth of the cetacean petro-tympanic bone complex. Journal of the Zoological Society of London 262: 371–381. Crossref
Buono, M.R. and Cozzuol, M.A. 2013. A new beaked whale (Cetacea: Odontoceti) from the late Miocene of Patagonia, Argentina. Journal of Vertebrate Paleontology 33: 986–997. Crossref
Buono, M.R., Dozo, M.T., Marx, F.G., and Fordyce, R.E. 2014. A late Miocene potential neobalaenine mandible from Argentina sheds light on the origins of the living pygmy right whale. Acta Paleontologica Polonica 59: 787–793. Crossref
Buono, M.R., Fernandez, M.S., Cozzuol, M.A., Cuitiño, J.I., and Fitzgerald, E.M. 2017. The early Miocene balaenid Morenocetus parvus from Patagonia (Argentina) and the evolution of right whales. PeerJ 5: e4148. Crossref
Cabrera, A. 1926. Cetáceos fósiles del Museo de La Plata. Revista del Museo de La Plata 29: 363–411.
Caviglia, S.E., and Jorge, R.E. 1980. Preaulophyseter gualichensis General. et sp. Nov. (Cetacea; Physeteridae) en el Terciario marino de Río Negro, República Argentina. Actas del Segundo Congreso Argentino de Paleontología y Bioestratigrafia y Primer Congreso Latinoamericano de Paleontología 2: 363–368.
Coddington, J. and Scharff, N. 1994. Problems with zero length branches. Cladistics 10: 415–423. Crossref
Collareta, A., Fulgosi, F.C., and Bianucci G. 2019. A new kogiid sperm whale from northern Italy supports psychrospheric conditions in the early Pliocene Mediterranean Sea. Acta Palaeontologica Polonica 64: 609–626. Crossref
Collareta, A., Lambert, O., De Muizon, C., Urbina, M., and Bianucci G. 2017. Koristocetus pescei gen. et sp. nov., a diminutive sperm whale (Cetacea: Odontoceti: Kogiidae) from the late Miocene of Peru. Fossil Record 20: 259–278. Crossref
Cozzuol, M.A. 1993. Mamíferos acuáticos del mioceno medio y tardío en Argentina: sistemática, evolución y biogeografía. 175 pp. Unpublished Doctoral Dissertation, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata.
Cozzuol, M.A. 1996. The record of the aquatic mammals in southern South America. Munchner Geowissenschaftliche Abhandlungen 30: 321–342.
Cranford, T.W. 1999. The sperm whale’s nose: sexual selection on a grand scale? Marine Mammal Science 15: 1133–1157. Crossref
Cuitiño, J.I., Buono, M.R., Viglino, M., Farroni, N.D., and Bessone, S. 2019. Factors affecting the preservation and distribution of cetaceans in the lower Miocene Gaiman Formation of Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 526: 110–125. Crossref
Cuitiño, J.I., Dozo, M.T., del Rıo, C.J., Buono, M.R., Palazzesi, L., Fuentes, S., and Scasso, R.A. 2017. Miocene marine transgressions: paleoenvironments and paleobiodiversity In: P. Bouza and A. Bilmes (eds.), Late Cenozoic of Penınsula de Valdes, Patagonia, Argentina: An Interdisciplinary Approach, 47–84. Springer Earth System Sciences, Springer International Publishing AG, Cham. Crossref
Ekdale, E.G., Berta, A., and Deméré, T.A. 2011. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PloS One 6: e21311. Crossref
Fordyce, R.E. 1994. Waipatia maerewhenua, new genus and new species (Waipatiidae, new family), an archaic Late Oligocene dolphin (Cetacea: Odontoceti: Platanistoidea) from New Zealand. Proceedings of the San Diego Society of Natural History 29: 147–176. Crossref
Fordyce, R.E. and Muizon, C. de 2001. Evolutionary history of cetaceans: a review. In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 169–233. Verlag Dr. Friederich Pfeil, München.
Gaetán, C.M., Buono, M.R., and Gaetano, L.C. 2019. Prosqualodon australis (Cetacea: Odontoceti) from the early Miocene of Patagonia, Argentina: Redescription and phylogenetic analysis. Ameghiniana 56: 1–27. Crossref
Geisler, J.H. and Sanders, A.E. 2003. Morphological evidence for the phylogeny of Cetacea. Journal of Mammalian Evolution 10: 23–129. Crossref
Geisler, J.H., McGowen, M.R., Yang, G., and Gatesy, J. 2011. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evolutionary Biology 11: 1–112. Crossref
Goloboff, P.A., Carpenter, J.M., Arias, J.S., and Esquivel, D.R.M. 2008a. Weighting against homoplasy improves phylogenetic analysis of morphological data sets. Cladistics 24: 758–773. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008b. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Gondar, D. 1975. La presencia de cetáceos Physeteridae en el Terciario Superior (‘Rionegrense’) de la Provincia de Rio Negro. Actas del Primer Congreso Argentino de Paleontología y Biostratigrafía Tucumán 2: 349–354.
Gutstein, C.S., Figueroa-Bravo, C.P., Pyenson, N.D., Yury-Yañez, R.E., Cozzuol, M.A., and Canals, M. 2014. High frequency echolocation, ear morphology, and the marine-freshwater transition: A comparative study of extant and extinct toothed whales. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 62–74. Crossref
Hampe, O. 2006. Middle/late Miocene hoplocetine sperm whale remains (Odontoceti: Physeteridae) of North Germany with an emended classification of the Hoplocetinae. Fossil Record 9: 61–86. Crossref
Hirota, K. and Barnes, L.G. 1994. A new species of Middle Miocene sperm whale of the genus Scaldicetus (Cetacea; Physeteridae) from Shiga-Mura, Japan. The Island Arc 3: 453–472. Crossref
Huggenberger, S., Andre, M., and Oelschläger, H.H. 2016. The nose of the sperm whale: overviews of functional design, structural homologies and evolution. Journal of the Marine Biological Association of the United Kingdom 96: 783–806. Crossref
Kasuya, T. 1973. Systematic consideration of recent toothed whales based on the morphology of tympano-periotic bone. Scientific Reports of the Whales Research Institute 25: 1–103.
Keck, F., Rimet, F., Bouchez, A., and Franc, A. 2016. phylosignal: an R package to measure, test, and explore the phylogenetic signal. Ecology and Evolution 6: 2774–2780. Crossref
Kellogg, R. 1925. Two fossil physeteroid whales from California. Contributions to Palaeontology from the Carnegie Institution of Washington 348: 1–34. Crossref
Kellogg, R. 1927. Study of the skull of a fossil sperm-whale from the Temblor Miocene of southern California. Publications of the Carnegie Institution of Washington 346: 1–23.
Kellogg, R. 1965. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia. The Miocene Calvert sperm whale Orycterocetus. Bulletin of the United States National Museum 247: 47–63.
Ketten, D.R. 1984. Correlations of Morphology with Frequency for Odontocete Cochlea: Systematics and Topology. Unpublished Doctoral Dissertation, The Johns Hopkins University, Baltimore.
Kimura, T., Hasegawa, Y., and Barnes, L.G. 2006. Fossil sperm whales (Cetacea, Physeteridae) from Gunma and Ibaraki prefectures, Japan; with observations on the Miocene fossil sperm whale Scaldicetus shigensis. Bulletin Gunma Museum Natural History 10: 1–23.
Lambert, O. 2008. Sperm whales from the Miocene of the North Sea: a re-appraisal. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre 78: 277–316.
Lambert, O., Bianucci, G., and Muizon, C. de 2008. A new stem-sperm whale (Cetacea, Odontoceti, Physeteroidea) from the latest Miocene of Peru. Comptes Rendus Palevol 7: 361–369. Crossref
Lambert, O., Bianucci, G., and Muizon C. de 2016. Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zoological Journal of the Linnean Society 179: 404–474. Crossref
Lambert, O., Bianucci, G., Post, K., Muizon, C. de, Salas-Gismondi, R., Urbina, M., and Reumer, J. 2010. The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru. Nature 466: 105–108. Crossref
Lancaster, W.C., Ary, W.J., Krysl, P., and Cranford, T.W. 2015. Precocial development within the tympanoperiotic complex in cetaceans. Marine Mammal Science 31: 369–375. Crossref
Lizuaín, A. and Sepúlveda, E. 1978. Geología del Gran Bajo del Gualicho (Provincia de Río Negro). Actas 7º Congreso Geológico Argentino 1: 407–422.
Luo, Z. and Marsh, K. 1996. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): implications on relationships and hearing evolution of toothed whales. Journal of Vertebrate Paleontology 16: 328–348. Crossref
Lydekker, R. 1893. Cetacean skulls from Patagonia. Anales del Museo de la Plata 2: 1–13.
Malumian, N. and Nanez, C. 2011. The Late Cretaceous–Cenozoic transgressions in Patagonia and the Fuegian Andes: foraminifera, palaeoecology, and palaeogeography. Biological Journal of the Linnean Society 103: 269–288. Crossref
Mchedlidze, G.A. [Mčedlidze, G.A.] 1970. Nekotorye obŝie čerty istorii kitoobraznyh. Akademiâ Nauk Gruzinskoj S.S.R., Institut Paleobiologii, Metsniereba Press, Tbilisi.
MacPhee, R.D. 1981. Auditory regions of primates and eutherian insectivores. Morphology, ontogeny and character analysis. Contributions to Primatology 18: 1–282
Mead, J.G. and Fordyce, R.E. 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology 627: 1–248. Crossref
Moreno, F.P. 1892. Lijeros apuntes sobre dos géneros de cetáceos fósiles de la República Argentina. Museo La Plata, Revista, 3: 393–400.
Mourlam, M.J. and Orliac, M.J. 2017. Infrasonic and ultrasonic hearing evolved after the emergence of modern whales. Current Biology 27: 1776–1781. Crossref
Muizon, C. de 1991. A new Ziphiidae (Cetacea) from the Early Miocene of Washington State (USA) and phylogenetic analysis of the major groups of odontocetes. Bulletin du Muséum national d’histoire naturelle. Section C, Sciences de la terre, paléontologie, géologie, minéralogie 12: 279–326.
Novacek, M.J. 1993. Patterns of diversity in the mammalian skull. The skull 2: 438–545.
Oelschläger, H.A. 1986. Comparative morphology and evolution of the otic region in toothed whales (Cetacea, Mammalia). American Journal of Anatomy 177: 353–368. Crossref
Paolucci, F., Buono, M.R., Fernández, M.S., Marx, F.G., and Cuitiño, J.I. 2020. Diaphorocetus poucheti (Cetacea, Odontoceti, Physeteroidea) from Patagonia, Argentina: one of the earliest sperm whales. Journal of Systematic Paleontology 18: 335–355 [published online, https://doi.org/10.1080/14772019.2019.1605544]. Crossref
Park, T., Fitzgerald, E.M., and Evans, A.R. 2016. Ultrasonic hearing and echolocation in the earliest toothed whales. Biology Letters 12: 20160060. Crossref
Piazza, D.S., Agnolin, F.L., and Lucero, S. 2018. First record of a macroraptorial sperm whale (Cetacea, Hyseteroidea) from the Miocene of Argentina. The Journal of the Brazilian Society of Paleontology 21: 276–280. Crossref
Pérez, L.M., Cione, A.L., Cozzuol, M. and Varela, A.N. 2011. A sperm whale (Cetacea: Physeteroidea) from the Paraná Formation (late Miocene) of Entre Ríos, Argentina. Environment and Taphonomy. Ameghiniana 48: 648–654. Crossref
R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Reichler, V.A. 2010. Estratigrafía y paleontología del Cenozoico marino del Gran Bajo y Salinas del Gualicho, Argentina, y descripción de 17 especies nuevas. Andean geology 37: 177–219. Crossref
Reumer, J.W., Mens, T.H., and Post, K. 2017. New finds of giant raptorial sperm whale teeth (Cetacea, Physeteroidea) from the Westerschelde Estuary (province of Zeeland, the Netherlands). Deinsea 17: 32–38.
Tanaka, Y. and Fordyce, R.E. 2016. Papahu-like fossil dolphin from Kaikoura, New Zealand, helps to fill the Early Miocene gap in the history of Odontoceti. New Zealand Journal of Geology and Geophysics 59: 551–567. Crossref
Tsai, C.H. and Fordyce, R.E. 2016. Archaic baleen whale from the Kokoamu Greensand: earbones distinguish a new late Oligocene mysticete (Cetacea: Mysticeti) from New Zealand. Journal of the Royal Society of New Zealand 46: 117–138. Crossref
Uhen, M.D. 2008. New protocetid whales from Alabama and Mississippi, and a new cetacean clade, Pelagiceti. Journal of Vertebrate Paleontology 28: 589–593. Crossref
Velez-Juarbe, J., Wood, A.R., and Pimiento, C. 2016. Pygmy sperm whales (Odontoceti, Kogiidae) from the Pliocene of Florida and North Carolina. Journal of Vertebrate Paleontology 36: e1135806. Crossref
Velez-Juarbe, J., Wood, A.R., De Gracia, C., and Hendy, A.J. 2015. Evolutionary patterns among living and fossil kogiid sperm whales: evidence from the Neogene of Central America. PloS One 10: e0123909. Crossref
Viglino, M., Buono, M.R., Fordyce, R.E., Cuitiño, J.I., and Fitzgerald, E.M. 2018a. Anatomy and phylogeny of the large shark-toothed dolphin Phoberodon arctirostris Cabrera, 1926 (Cetacea: Odontoceti) from the early Miocene of Patagonia (Argentina). Zoological Journal of the Linnean Society 185: 511–542. Crossref
Viglino, M., Buono, M.R., Gutstein, C.S., Cozzuol, M.A., and Cuitiño, J.I. 2018b. A new dolphin from the early Miocene of Patagonia, Argentina: insights into the evolution of Platanistoidea in the Southern Hemisphere. Acta Palaeontologica Polonica 63: 261–277. Crossref
Watwood, S.L., Miller P.J.O., Johnson M., Madsen P.T., and Tyack P.L. 2006. Deep‐diving foraging behaviour of sperm whales (Physeter macrocephalus). Journal of Animal Ecology 75: 814–825. Crossref
Acta Palaeontol. Pol. 66 (1): 63–76, 2021
https://doi.org/10.4202/app.00763.2020