


The glyptodont Eleutherocercus solidus from the late Neogene of north-western Argentina: Morphology, chronology, and phylogeny
ALIZIA NÚÑEZ-BLASCO, ALFREDO E. ZURITA, ÁNGEL R. MIÑO-BOILINI, RICARDO A. BONINI, and FRANCISCO CUADRELLI
Núñez-Blasco, A., Zurita, A.E., Miño-Boilini, A.R., Bonini, R.A., and Cuadrelli, F. 2021. The glyptodont Eleutherocercus solidus from the late Neogene of North-Western Argentina: Morphology, chronology, and phylogeny. Acta Palaeontologica Polonica XX (Supplement to 3): 79–99.
Glyptodonts (Mammalia, Xenarthra, Glyptodontidae) represent a diversified radiation of large armored herbivores, mainly related to open biomes in South America, with an extensive fossil history since the late Eocene (ca. 33 Ma) until their extinction in the latest Pleistocene–earliest Holocene. During the Pliocene and Pleistocene, glyptodonts arrived in Central and North America as part of the Great American Biotic Interchange. Within glyptodont diversity, one of the most enigmatic groups (and also one of the least known) are the Doedicurinae, mainly recognized by the enormous Pleistocene Doedicurus, with some specimens reaching ca. two tons. Almost nothing is known about the Neogene evolutionary history of this lineage. Some very complete specimens of the previously scarcely known Eleutherocercus solidus, which in turn becomes the most complete Neogene Doedicurinae, are here described in detail and compared to related taxa. The materials come from the Andalhuala and Corral Quemado formations (north-western Argentina), specifically from stratigraphic levels correlated to the Messinian–Piacenzian interval (latest Miocene–Pliocene). The comparative study and the cladistic analysis support the hypothesis that Doedicurinae forms a well supported monophyletic group, located within a large and diversified clade mostly restricted to southern South America. Within Doedicurinae, the genus Eleutherocercus (E. antiquus + E. solidus) is the sister group of the Pleistocene Doedicurus. Unlike most of the late Neogene and Pleistocene lineages of glyptodonts, doedicurins show along its evolutionary history a latitudinal retraction since the Pleistocene, ending with the giant Doedicurus restricted to the Pampean region of Argentina, southernmost Brazil, and southern Uruguay. This hypothetic relationship between body mass and latitudinal distribution suggests that climate could have played an active role in the evolution of the subfamily.
Key words: Mammalia, Xenarthra, Doedicurinae, anatomy, taxonomy, phylogeny, Pliocene, South America.
Alizia Núñez-Blasco [alizia_zgz12@hotmail.com], Alfredo E. Zurita [aezurita74@yahoo.com.ar], Ángel R. Miño-Boilini [angelmioboilini@yahoo.com.ar], and Francisco Cuadrelli [f.cuadrelli@gmail.com], Laboratorio de Evolución de Vertebrados y Ambientes Cenozoicos; Centro de Ecología Aplicada del Litoral (CECOAL-CONICET) Ruta 5, Km 2,5 cc 128 (3400) y Universidad Nacional del Nordeste (UNNE), Corrientes Capital, Argentina.
Ricardo A. Bonini [rbonini@fcnym.unlp.edu.ar], Instituto de investigaciones Arqueológicas y paleontológicas del Cuaternario Pampeano (INCUAPA-CONICET), Facultad de Ciencias Sociales, Universidad Nacional del Centro de la Provincia de Buenos Aires, Olavarría, Argentina. Av. Del Valle 5737, 7400, Olavarría, Buenos Aires, Argentina.
Received 24 September 2020, accepted 15 December 2020, available online 23 August 2021.
Copyright © 2021 A. Núñez-Blasco et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Xenarthra is a peculiar clade of placental mammals characteristic for the Neotropical region (Gaudin and Croft 2015; Gibb et al. 2015; Delsuc et al. 2016), with a long fossil history since the early Eocene (Bergqvist et al. 2004; Lindsey et al. 2020). Their records are remarkably abundant in various Cenozoic sites mainly in South America (particularly in Argentina, see Scillato-Yané 1986), but also in Central America and North America (McDonald 2005; Brandoni et al. 2016; Gillette et al. 2016). Within Xenarthra, two large clades can be recognized, Cingulata and Pilosa, the latter containing the anteaters Vermilingua and the sloths Folivora (or Tardigrada or Phyllophaga, see Delsuc et al. 2001; Lindsey et al. 2020).
Historically, Pilosa (especially Folivora) is much better known from several viewpoints, including ecology (e.g., Bargo et al. 2006), evolutionary history (Gaudin 2004), diet (Hofreiter et al. 2000), and habitat (Bargo et al. 2006) compared to the other large clade, Cingulata (except for the “armadillos” Dasypodidae) (Gaudin and Lyon 2017), although Cingulata represents the most diversified clade among Xenarthra (Abba et al. 2012).
Cingulata (early Eocene–Recent) is morphologically characterized mainly by the presence of a cephalic shield, dorsal carapace, and caudal armor formed by hundreds of osteoderms that cover and protect the body (Gilette and Ray 1981; Gaudin and Wible 2006; Soibelzon et al. 2010). Six families are included in Cingulata: Chlamyphoridae, Dasypodidae, Pampatheriidae, Pachyarmatheriidae, Peltephilidae, and Glyptodontidae (Delsuc et al. 2016; Mitchell et al. 2016; Fernicola et al. 2018).
Within this diversity, glyptodonts (late Eocene–latest Pleistocene/earliest Holocene) are a clade composed of large to very large grazing armored herbivores, with body masses ranging between 100 kg to ca. 2000 kg (Vizcaíno et al. 2011; Soibelzon et al. 2012; Quiñones et al. 2020). In a phylogenetic framewok, there is consensus that pampatheres (Pampatheriidae) is the sister group of glyptodonts (Gaudin and Wible 2006; Gaudin and Lyon 2017; Fernicola et al. 2018; but see Delsuc et al. 2016; Mitchell et al. 2016 for an alternative view).
The early evolutionary history of glyptodonts, during the Paleogene, is poorly known, but the records increase markedly during the Neogene and Pleistocene (Gaudin and Croft 2015; Zurita et al. 2016), especially in southern South America (Zurita et al. 2016; Toriño and Perea 2018).
Though the diversity and phylogenetic relationships of glyptodonts are under study with promising results (see, among others, Fernicola 2008; Fernicola and Porpino 2012; Zurita et al. 2013; Gillette et al. 2016; Cuadrelli et al. 2019, 2020), the subfamily Doedicurinae (late Neogene–late Pleistocene) remains one of the most enigmatic groups. It is mostly known by the Pleistocene species Doedicurus clavicaudatus (Owen, 1847), one of the largest and most bizarre Quaternary forms, with some specimens having body masses of ca. 2000 kg (see Soibelzon et al. 2012). The most conspicuous characters of this clade include a caudal tube in which the distal part is dorso-ventrally compressed and laterally expanded, with terminal rugose concave areas, probably for the insertion of corneous “spines” (see Lydekker 1895: pl. 27). The fossil record (e.g., MLP 16-25) and recent biomechanical analyses show that this caudal tube would make a formidable weapon against predators or to be used in intraspecific combats (see Alexander et al. 1999; Blanco et al. 2009). Another intriguing character of Doedicurinae is the exposed surface of the osteoderms of the dorsal carapace, where large foramina cross the entire thickness of the osteoderms, a unique feature among glyptodonts, and even among Cingulates (see Zurita et al. 2014, 2016), the most remarkable example being the late Pleistocene terminal species D. clavicaudatus. Finally, the geographic distribution along doedicurine evolutionary history is also peculiar, since a latitudinal retraction is especially evident in the Pleistocene genus Doedicurus Burmeister, 1874, with most records being restricted to southern South America (i.e., Argentina, Uruguay, and southernmost Brazil; Zurita et al. 2009, 2014; Varela et al. 2018).
Although the anatomy of Doedicurus is relatively well known, almost nothing is known about the Neogene diversity achieved by the Doedicurinae in southern South America, with a fossil record mostly limited to one dorsal carapace, several fragments of associated osteoderms, and some caudal tubes (see Ameghino 1887, 1889, 1920; Moreno 1888; Lydekker, 1895; Rovereto 1914; Castellanos 1927, 1940; Cabrera 1944). In a historical framework, and despite a large number of very poorly characterized species, only the species currently named Eleutherocercus antiquus (Ameghino, 1887) is known by a relatively complete dorsal carapace associated with a caudal tube (MLP 16-25, holotype of E. copei (Moreno, 1888) (see Ameghino 1887, 1889, 1920; Moreno 1888; Lydekker 1895) originating from the early Pliocene of the Atlantic coast of Argentina (sensu Tomassini et al. 2013). In this context, Zurita et al. (2014) first described and included in a phylogenetic framework the two only known late Neogene Doedicurinae skulls associated with some osteoderms of the dorsal carapace coming from the late Neogene–earliest Pleistocene of the Pampean region of Argentina (ca. 4.5–2.8 Ma). The results indicate that the group including the genera Doedicurus and Eleutherocercus Koken, 1888 (Doedicurinae) is monophyletic, supported mainly by cranial and dorsal carapace synapomorphies (Zurita et al. 2014).
However, and despite the advance in the knowledge of these late Neogene Doedicurinae of the Pampean region of Argentina, other records of the subfamily from North-western Argentina, where one of the most complete late Neogene continental sequences is exposed, are very scarce (see Quiñones et al. 2019). In fact, the last revision of glyptodonts from this area was carried out by Cabrera (1944), and Doedicurinae were among the least addressed. According to this revision, only one species of Doedicurinae was recognized in the Neogene sequences, Eleutherocercus solidus (Rovereto, 1914), the type material (MACN 8335) being represented by osteoderms of the dorsal carapace, and coming from Santa María Valley (SMV).
During many years, the Santa María Valley was a valuable source of palaeontological data, and palaeontologists studied the abundant fossil remains discovered there (e.g., Moreno and Mercerat 1891; Ameghino 1891; Lydekker 1895; Rovereto 1914, among others). On the other hand, the nearby outcrops of the Villavil–Quillay Basin (VQB; Catamarca Province, North-western Argentina; see Fig. 1A) have not been prospected until 1926, during the expedition led by Elmer S. Riggs of the Field Museum of Natural History, Chicago, and immediately followed by Ángel Cabrera and collaborators of the Museo de La Plata in 1927, 1929, and 1930 (see Bonini 2014). During Riggs’ field expedition, several remains of mammals including the most complete specimens of Glyptodontidae were obtained in the VQB, with precise data for their stratigraphic provenance.
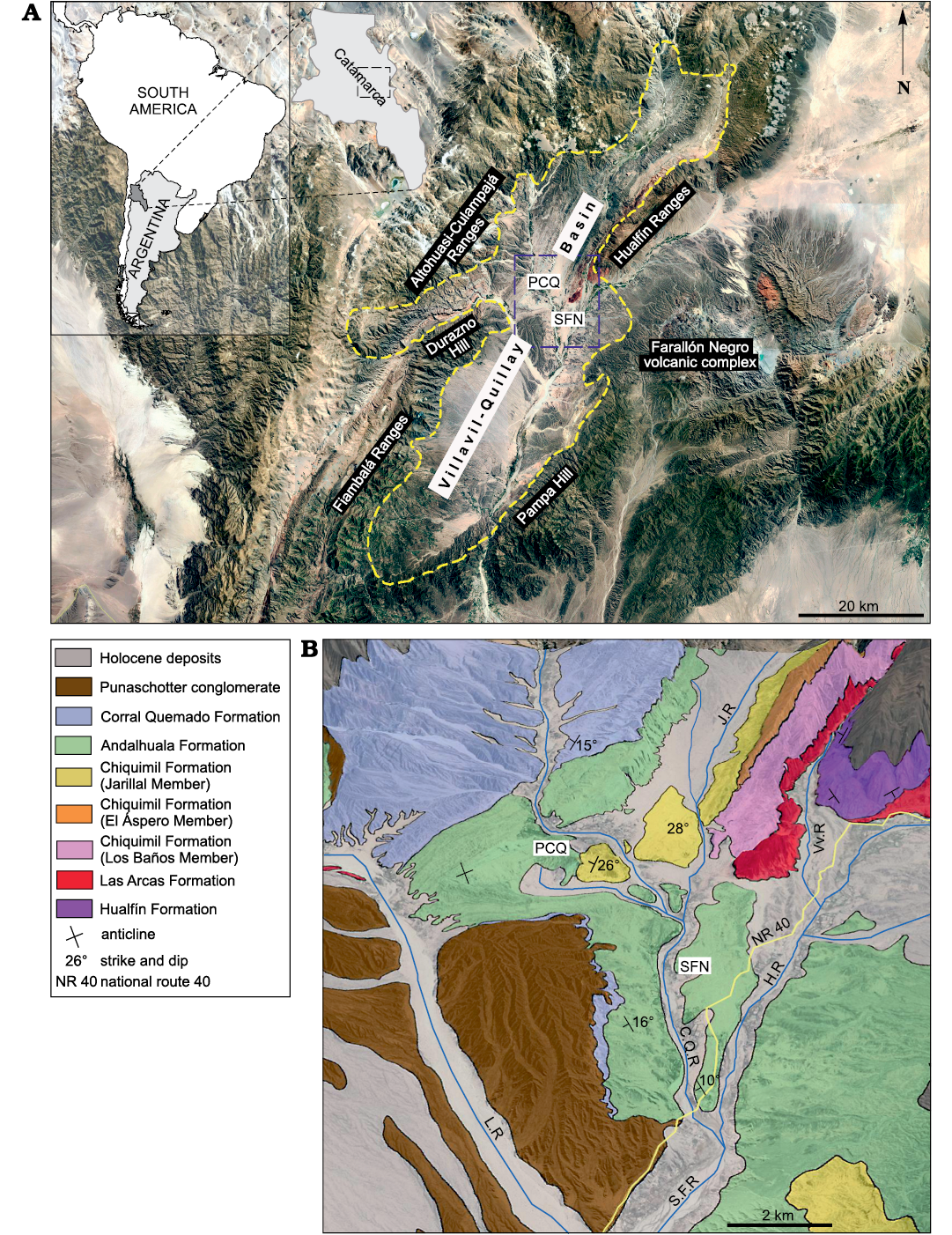
Fig. 1. Geographic and geological maps of the Villavil–Quillay Basin, North-western Argentina showing location of studied localities in Catamarca Province (A) and exposed lithostratigraphic units in the Villavil–Quillay Basin (B). Abbreviations: CQR, Corral Quemado River; HR, Hualfín River; JR, Jarillal River; LR, Loconte River; PCQ, Puerta de Corral Quemado; SFN, San Fernando Norte; VvR, Villavil River.
In this context, and as a result of fieldwork carried out in the upper Neogene from the Villavil–Quillay Basin, in addition to a careful revision of paleontological collections from the USA and Argentina, new and more complete specimens of Doedicurinae are described here, representing the most complete late Neogene glyptodonts ever known. This sample offers the opportunity to carry out a detailed morphological and phylogenetic study of these enigmatic cingulates, as well as to perform a comparative analysis with the relatively well known Doedicurinae from the Pampean region of Argentina, and to test their biostratigraphic importance.
The aims of this paper are: (i) to carry out a detailed description and comparison of new and more complete materials referred to E. solidus; (ii) to provide a taxonomic revision of this species and assess its biostratigraphic value; (iii) to test its relationships within Doedicurinae in a cladistic framework; and (iv) to discuss some aspects concerning the evolutionary history of Doedicurinae in high and middle latitudes of South America.
Institutional abbreviations.—FMNH-P, Paleontological collection, Field Museum of Natural History, Chicago, USA; MACN, Sección Paleontología Vertebrados, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MCH P, Sección Paleontología, Museo Arqueológico Condor Huasi, Belén, Catamarca, Argentina; MLP, División Paleontología Vertebrados, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Buenos Aires, Argentina; MMP, Museo Municipal de Ciencias Naturales “Lorenzo Scaglia”, Mar del Plata, Buenos Aires, Argentina; Xen, Collection “Cementos Avellaneda”, Olavarría, Buenos Aires, Argentina.
Other abbreviations.—FAD, First Appearance Datum; Mf/mf, upper/lower molariforms; PCQ, Puerta de Corral Quemado; SFN, San Fernando Norte; VQB, Villavil–Quillay Basin.
Material and methods
The analysis is mainly based on specimens FMNH-P 14437 and FMNH-P 14446, housed in the Paleontological collection of the Field Museum of Natural History, and MLP 29-X-10-29, housed in the Vertebrate Paleontological collection of Museo de La Plata.
Systematics partially follow Hoffstetter (1958), Paula-Couto (1979), McKenna and Bell (1997), and Fernicola (2008). All the values included in tables are expressed in millimeters, with an error range of 0.5 mm. The description and terminology for osteoderms and molariforms follow Zurita (2007), Krmpotic et al. (2009), and González-Ruiz et al. (2015).
The description of the units of the Santa María Group exposed in the Villavil–Quillay Basin (VQB; i.e., Puerta de Corral Quemado and San Fernando Norte localities) was based on different stratigraphical and geochronological proposals (Fig. 1B). Therefore, we perform here a theoretical model using this information, including stratigraphic, geochronological, and biostratigraphic data of the specimens of Doedicurinae found in VQB, in order to provide the chronological context of the fossils studied here. The provenance of the fossils collected by the expedition of Riggs in 1926 is provided in Marshall and Patterson (1981: appendix 5), while the correlation of the volcanic tuffaceous levels was made from the contributions of Latorre et al. (1997) and Bonini et al. (2017) (see Fig. 2).
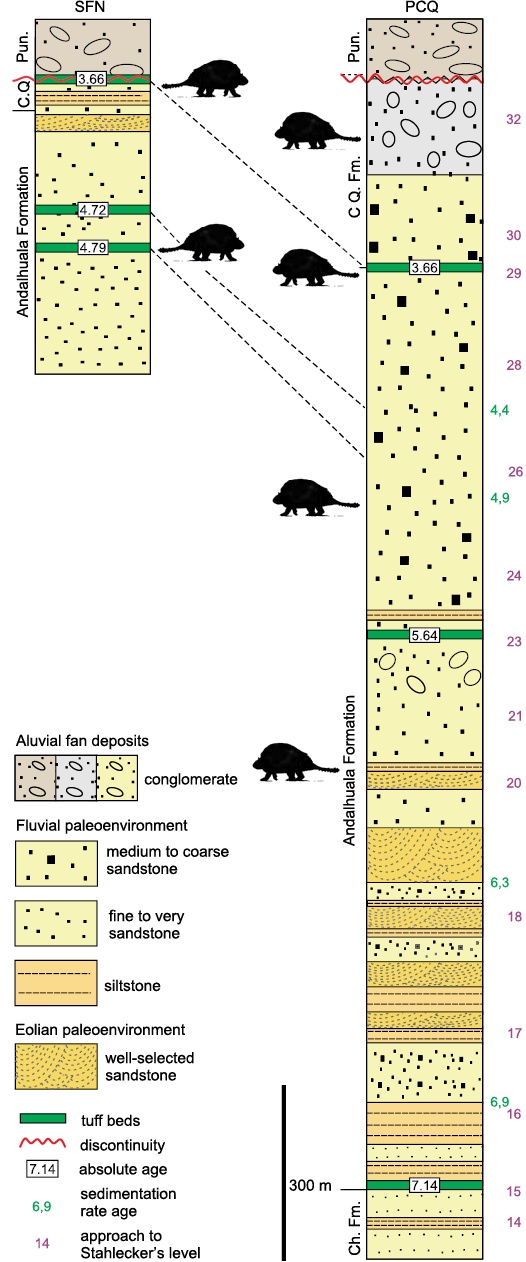
Fig. 2. Lithostratigraphic, chronological, and paleoenvironmental synthesis of San Fernando Norte and Puerta de Corral Quemado sections showing the stratigraphical and chronological distribution of Eleutherocercus solidus Rovereto, 1914 (modified from Georgieff et al. 2017; Bonini et al. 2020). Abbreviations: Ch. Fm., Chiquimil Formation; CQ. Fm., Corral Quemado Formation; Pun., Punaschotter.
In order to test the relationships of Eleutherocercus solidus within Doedicurinae, as well as the monophyly of Doedicurinae, we performed a cladistic analysis. The matrix includes 23 taxa and 57 morphological characters (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Nunez_etal_SOM.pdf). Most characters are based on previous analyses (Fernicola 2008; Porpino et al. 2010; Zamorano and Brandoni 2013; Zurita et al. 2013, 2014, 2017; Cuadrelli et al. 2020), with the addition of six new characters. The characters include five characters from teeth, thirteen from the skull, two from the mandible, three from the appendicular skeleton, two from the cephalic shield, twenty one from the dorsal carapace, and eleven from the caudal armor. The new characters are: angle between naso-frontal and parietal regions located at orbital notch level (in lateral view) (char. 5); angle between posterior margin of the orbital notch and the plane of the palate, near 90° (char. 7); small foramina with nearly homogenous distribution along the exposed surface of the osteoderms without ornamentation pattern (char. 34); posterior region of the dorsal carapace with a hypertrophied dome-shaped glandular structure (char. 38); transverse contour of the distal third of the caudal tube (char. 49); and ornamentation pattern of the dorsal osteoderms of the caudal tube (char. 51). There are 43 binary characters and 14 non-binary. All the characters considered in this study were scored via direct observation of the specimens and from photographs taken by the authors, and they were treated with the same weight (1.0). Character states that were not preserved were coded as “?”. The matrix was obtained with Mesquite 3.04 (Maddison and Maddison 2018). The character-taxon matrix was analyzed via “Implicit enumeration” using TNT (Goloboff et al. 2008), under the criterion of maximum parsimony. Clade support was assessed via Relative and Absolute Bremer support (retained trees suboptimal by 4 steps; see Bremer 1994; Goloboff and Farris 2001); in addition to the Jackknife analysis, we used the option “Implicit enumeration” with 100 replicates.
In the phylogenetic analysis the in-group includes the following taxa: Eleutherocercus solidus, E. antiquus, and Doedicurus clavicaudatus. The remaining genera of Doedicurinae (Castellanosia Kraglievich, 1932, Comaphorus Ameghino, 1886, Daedicuroides Castellanos, 1941, Prodaedicurus Castellanos, 1927, Xiphuroides Castellanos, 1927, and Plaxhaplous Ameghino, 1884) were excluded from this analysis due to the scarcity of characters present in the type materials (mostly limited to very fragmentary osteoderms of the dorsal carapace and caudal tube) and their dubious specific validity. More specifically, a detailed taxonomic revision of these genera is needed before including them in a phylogenetic analysis. The extant dasypodid Euphractus sexcintus Linnaeus, 1758, and the pampathere Pampatherium humboldtii (Lund, 1839) were used to root the tree. In addition, the out-group is composed of Boreostemma venezolensis Simpson, 1947 (Boreostema acostae (Villarroel, 1983), Glyptodon jatunkhirkhi Cuadrelli, Zurita, Toriño, Miño-Boilini, Perea, Luna, Gillette, and Medina, 2020, Glyptodon munizi Ameghino, 1881, Glyptodon reticulatus Owen, 1845, Glyptotherium texanum Osborn, 1903, Glyptotherium cylindricum (Brown, 1912) (Glyptodontinae), Propalaehoplophorus australis Ameghino, 1887, Eucinepeltus petestatus Ameghino, 1891, Cochlops muricatus Ameghino, 1889 (“Propalaehoplophorinae”), Plohophorus figuratus Ameghino, 1887, Pseudoplohophorus absolutus Perea, 2005 (“Plohophorini”), Neosclerocalyptus ornatus (Owen, 1845), Glyptotherium paskoensis (Zurita, 2002) (Neosclerocalyptini), Hoplophorus euphractus Lund, 1839, Propanochthus bullifer (Burmeister, 1874), Panochthus intermedius Lydekker, 1895, and P. tuberculatus (Owen, 1845) (Hoplophorini, Hoplophorinae).
Geological setting
The specimens described here come from late Neogene sediments cropping out in Puerta de Corral Quemado (PCQ) (27°13’S/66°55’ W) and San Fernando Norte (SFN) (27°16’ S/66°54’ W) in the Villavil–Quillay Basin (VQB), Belén Department, Catamarca Province, north-western Argentina. These localities are included in the geological province known as north-western Pampean Ranges, and they are bounded to the NW by Sierra de Altohuasi and Culampajá, to the W by Cerro Durazno, to the SW by Sierra de Fiambalá, to the NE by Sierra de Hualfín, to the E by the Farallón Negro volcanic complex, and to the SE by the Cerro Pampa and Sierra de Belén (Fig. 1).
The glyptodonts studied here come from levels of the “Araucanense” and Corral Quemado horizons (sensu Riggs and Patterson 1939), cropping out in PCQ, which, with some differences at the boundary of units, were correlated with the Andalhuala and Corral Quemado formations, respectively (for more details about the correlations see Bossi et al. 1987; Bossi and Muruaga 2009; Bonini 2014; Esteban et al. 2014; Georgieff et al. 2017). Several authors reported radiometric datings obtained from the tuff levels interbedded in the Andalhuala Formation exposed in both localities (Marshall et al. 1979; Butler et al. 1984; Latorre et al. 1997; Sasso 1997; Bonini et al. 2017). These absolute ages enabled the correlation of both localities and constrained chronologically the fossiliferous levels, which highlighted their biostratigraphic value, as was indicated by Nuñez-Blasco et al. (2020). The specimens studied here and collected in SFN were exhumed from levels of the Andalhuala and Corral Quemado formations that span from ca. 4.8 to 3.6 Ma in this locality, according to the absolute ages proposed by Bonini et al. (2017). On the other hand, those coming from PCQ were found in levels ranging from ca. 5.64 to 3.66 Ma, following the absolute ages proposed by Latorre et al. 1997 (Fig. 2).
Historical background
The species currently known as Eleutherocercus solidus was first recognized and described by Rovereto (1914) as Neuryurus solidus. The holotype (MACN 8335) consists of a small fragment of dorsal carapace, exhumed from the Andalhuala locality, Catamarca Province. Some years later, Castellanos (1927) included the species in the genus Eleutherocercus Koken, 1888, proposing a new combination, Eleutherocercus solidus (Rovereto, 1914). In the same contribution, Castellanos (1927) included another species in Eleutherocercus, namely E. tucumanus, originally Plaxhaplus tucumanus Castellanos, 1927, and designated as holotype the specimen MACN 2893, which consists of the distal part of a caudal tube, discovered in the vicinity of Tiopunco, Tucumán Province. Finally, Cabrera (1944) established Eleutherocercus tucumanus (Castellanos, 1927) as a junior synonym of Eleutherocercus solidus. Our observations are in agreement with the synonymy proposed by Cabrera (1944) since no significant differences are seen when comparing the caudal tubes of both species (see description and comparisons).
Systematic palaeontology
Xenarthra Cope, 1889
Cingulata Illiger, 1811
Glyptodontia Ameghino, 1889
Glyptodontoidea Gray, 1869
Glyptodontidae Gray, 1869
Doedicurinae Trouessart, 1897
Genus Eleutherocercus Koken, 1888
Type species: Eleutherocercus setifer Koken, 1888; Messinian–Zanclean of Uruguay.
Emended diagnosis.—Medium sized glyptodont, and smaller compared to the giant Doedicurus clavicaudatus. Skull with the naso-frontal area ventrally inclined with respect to the parieto-occipital region, forming an angle of ca. 140° and showing some similitude with the genus Panochthus Burmeister, 1866. Orbital notch with vertical posterior margin, delimiting an angle with respect to the palatal plane of ca. 90°. Palate transversally expanded at the level of Mf1, but not as evident as in Doedicurus. Mf1 and mf1 simple and subcircular in outline, somewhat similar to those of Doedicurus; the remaining upper molariforms almost identical to Doedicurus. Dorsal carapace with the posterior region forming a “dome”, perhaps of glandular origin. Exposed surface of the osteoderms of the dorsal carapace rugose and uniformly perforated by numerous and small foramina, a few of them crossing the entire thickness of the osteoderms; in Doedicurus instead, the foramina are larger and mainly concentrated in the central area of the osteoderms, with most of them crossing the entire thickness. Caudal tube similar to Doedicurus, but with its distal part not as laterally expanded; dorsal area of the caudal tube with numerous small foramina (as in the dorsal carapace), but preserving in some restricted areas a “rosette” ornamentation pattern (i.e., a central figure surrounded by several small figures).
Eleutherocercus solidus (Rovereto, 1914)
1927 Eleutherocercus tucumanus; Castellanos 1927: 282.
Holotype: MACN 8335, fragment of carapace composed of three anatomically connected osteoderms, and one isolated osteoderm.
Type locality: Andalhuala, Santa María Department, Catamarca Province, Argentina.
Type horizon: “Araucanian”, late Neogene.
Material.—FMNH-P 14437, almost complete skull, mandible, carapace fragment (postero-dorsal region), complete caudal tube, left and right femora, left and right tibio-fibula, from Puerta de Corral Quemado, Belén Department, Catamarca Province, Argentina, levels 23–28 (sensu Marshall and Patterson 1981: appendix 5), Andalhuala Formation (sensu Bossi et al. 1987 ), ~5.64 Ma, Zanclean, early Pliocene; FMNH-P 14446, highly deformed dorso-ventral skull, carapace fragment (middle or cephalic region), left hemimandible, incomplete caudal tube, vertebra, foot bones, and scapula, from Puerta de Corral Quemado, Belén Department, Catamarca Province, Argentina, level 32 (sensu Marshall and Patterson 1981: appendix 5), Corral Quemado Formation (sensu Bossi et al. 1987), above ca. 3.66 Ma, latest Zanclean–Piacenzian, Pliocene; FMNH-P 14475, complete skull without molariforms, rib, fragment of humerus, vertebrae, and foot bones, from Puerta de Corral Quemado, Belén Department, Catamarca Province, Argentina, level 20 (sensu Marshall and Patterson 1981: appendix 5), Andalhuala Formation (sensu Esteban et al. 2014), Messinian, late Miocene; MLP 29-X-10-9, highly deformed skull, from Puerta de Corral Quemado, Belén Department, Catamarca Province, Argentina, unknown stratigraphic level, probably upper levels of Andalhuala Formation or lower levels of Corral Quemado Formation, Zanclean–Piacenzian, Pliocene; MCH-P 188, small fragment of carapace, San Fernando Norte, Belén Department, Catamarca Province, Argentina, located between tuffs dated at ca. 4.78–4.72 Ma, Andalhuala Formation, Zanclean, Pliocene; MCH-P 253, isolated osteoderm from San Fernando Norte, Belén Department, Catamarca Province, Argentina, 5 m below the tuff dated at 3.66 Ma, Corral Quemado Formation, Zanclean, early Pliocene; MCH-P 325, small fragment of carapace, from San Fernando Norte (SE), Belén Department, Catamarca Province, Argentina, found between tuffs dated at ca. 3.6 and ca. 4.72 Ma, Zanclean, early Pliocene; MLP 29-X-10-21, caudal tube from Puerta de Corral Quemado (near the road to Loconte), Department of Belén, Catamarca Province, Argentina, unknown stratigraphic level, near the limit between Andalhuala and Corral Quemado formations, Pliocene; MACN 2893, medial-distal portion of caudal tube, holotype of Eleutherocercus tucumanus (Castellanos, 1927), from Tiopunco, Deparment of Tafi del Valle, Tucumán Province, Argentina, unknown stratigraphic level.
Emended diagnosis.—Glyptodont similar in size to E. antiquus (Table 1). Skull with an evident convex surface delimiting the rostral and parieto-occipital areas, more developed than in E. antiquus. Nasal openings sub-rectangular in contour, and somewhat different from the more curved lateral margins of E. antiquus and Doedicurus. In dorsal view, rostral area more elongated and subrectangular, different from the more subtriangular rostral area observed in E. antiquus and Doedicurus. Zygomatic arches with a great antero-posterior diameter. In lateral view, posterior margin of the orbital notch with an angle of ca. 90° to the palatal plane. In occlusal view, widening of the palate at the level of the Mf1 less developed compared to E. antiquus and Doedicurus. Very high horizontal ramus, almost identical to that of Doedicurus. Exposed surface of the osteoderms of the dorsal carapace very similar to E. antiquus and very different from Doedicurus. Caudal tube similar to E. antiquus in general morphology and preserving in some restricted areas a “rosette” ornamentation pattern, different from the completely perforated surface observed in Doedicurus.
Table 1. Skull linear measurements (in mm) of Eleutherocercus solidus (FMNH-P 14437) and cf. Eleutherocercus antiquus (MMP 4860).
| |
FMNH-P 14437 |
MMP 4860 |
|
Total length |
285 |
230.74 |
|
Maximum transverse diameter between zygomatic arches |
218 |
235.18 |
|
Height of narial aperture |
55.27 |
71.70 |
|
Transverse diameter of narial aperture |
71.46 |
98.82 |
|
Transverse diameter of post-orbital region |
115.49 |
108.14 |
|
Length of the tooth series |
182 |
144.44 (M1–M6) |
|
Length of the palate |
221 |
157.28 |
|
Transverse diameter of the palate at the level of Mf1 |
47 |
115.20 |
Description.—Skull and dentition: The skull FMNH-P 14437 is almost complete and is the best preserved skull of Neogene doedicurine, since the two other coming from the upper Neogene of the Atlantic coast in the Pampean region do not preserve most of the parieto-occipital region (see Zurita et al. 2014) and since no cranial remains belonging to Doedicurinae are known outside Argentina.
In lateral view (Fig. 3A1), it can be observed that the dorsal profile of the skull resembles mainly that of cf. Eleutherocercus antiquus (MMP 4860) and, to a lesser extent, that of Doedicurus (MACN 2762, MMP 4251, MLP 16-24) (Zurita et al. 2014). The naso-frontal area is ventrally inclined to the parieto-occipital region, forming an angle of ca. 140°. This particular morphology is similar to that of the Panochthus, but in E. solidus this character appears behind the orbital notch, while in Panochthus it is just at the level of the orbital notch. As in cf. E. antiquus, a conspicuous convex surface separates both regions of the skull, a potential synapomorphy for the genus. In turn, the orbital notch is slightly sub-elliptical, with the main axis dorso-ventrally oriented, but less developed compared to cf. E. antiquus and some specimens of Doedicurus (MMP 4251). In fact, the morphology of the orbital notch seems to be more similar between cf. E. antiquus and Doedicurus, compared to E. solidus. However, this morphology is different from that of Glyptodon and Glyptotherium, in which the orbital notch is more circular in outline. The ventral margin of the orbital notch shows a bony crest, very similar to that in cf. E. antiquus and Doedicurus but less developed than in E. solidus. In turn, the posterior margin of the orbital notch is remarkably vertical, drawing an angle with respect to the palatal plane of ca. 90°, as in cf. E. antiquus and Doedicurus. The descending process of the maxillae is more dorso-ventrally elongated than in cf. E. antiquus and much more elongated compared to Doedicurus, curving towards the posterior region of the skull, which is especially evident in the ventral third of this process. The transverse diameter of the zygomatic arch is constant from the region behind the orbital notch to the contact with the temporal bone, but the curved end is less developed than in Doedicurus. The zygomatic arch, remarkably antero-posteriorly developed, is very different from that of cf. E. antiquus and Doedicurus, in which it is clearly less antero-posteriorly developed but dorso-ventrally higher. The dorsal margin is straight and ventrally inclined towards the orbital notch, unlike cf. E. antiquus in which this dorsal margin delimits a more concave surface. In general, this results in a very different morphology for both species (i.e., E. solidus and E. antiquus).
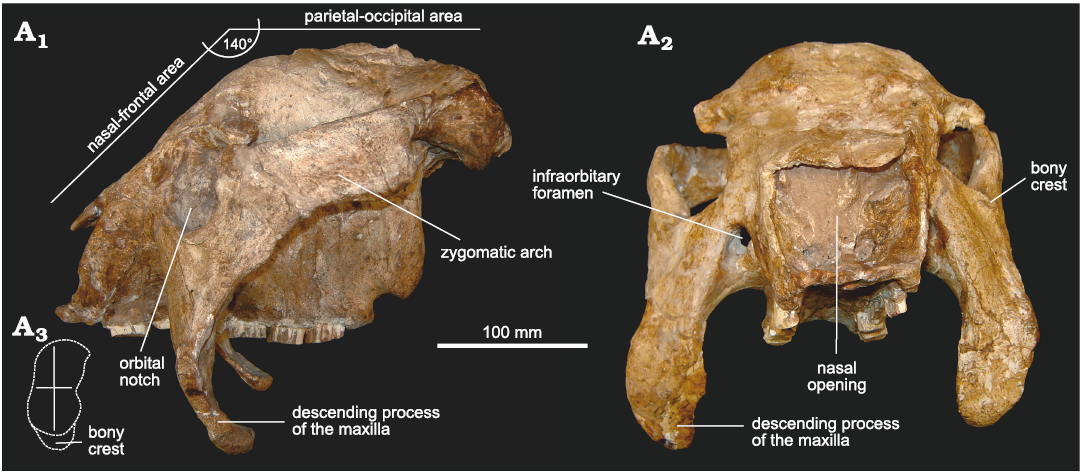
Fig. 3. Skull of doedicurine glyptodont Eleutherocercus solidus (Rovereto, 1914), FMNH-P 14437, from upper Miocene–Pliocene, Puerta de Corral Quemado, Andalhuala Formation, levels 23–28, Argentina; in left lateral (A1) and frontal (A2) views; drawing of detail of orbit notch (A3).
In frontal view (Fig. 3A2), the nasal openings are sub-rectangular, as in D. clavicaudatus (MLP 16-24), but different from cf. E. antiquus and Doedicurus sp. (MACN 2762), in which the lateral margins are somewhat convex, as in Plohophorus figuratus and Eosclerocalyptus tapinocephalus (Cabrera, 1939). In general morphology, the outline of the nasal openings is very different from Glyptodon (G. reticulatus and G. munizi), which shows a clear and inverted subtriangular outline (see Cuadrelli et al. 2019). In turn, the frontal bone shows a great vertical development, similar to cf. E. antiquus, and very different from D. clavicaudatus, Plohophorus figuratus, and G. munizi, in which it is clearly less developed. As observed in lateral view, the ventral margin of the orbital notch has a bony crest, similar in morphology to that of Doedicurus and much less developed compared to cf. E. antiquus. The infraorbitary foramina are sub-circular, and in the same relative position as in cf. E. antiquus and Doedicurus. In G. munizi and G. reticulatus, these foramina are circular, and developing a ventral channel (see Zurita et al. 2013; Cuadrelli et al. 2019) not present in Doedicurinae. The descending processes of the maxillae are in this frontal view very particular and different from those of cf. E. antiquus and Doedicurus. In E. solidus, this structure is much more massive, with a great transverse diameter (especially in its ventral half), also resembling D. clavicaudatus. In Glyptodon, these processes have a similar transverse diameter, but in their ventral-most part, they become clearly pointed (see Cuadrelli et al. 2019, 2020).
In dorsal view (Fig. 4A1), the general morphology of the skull is similar to that of cf. E. antiquus. It is also somewhat similar to D. clavicaudatus. but some differences can be noted. In E. solidus, the rostral area ahead of the orbital notches is more antero-posteriorly elongated than in cf. E. antiquus and Doedicurus, delimiting a sub-rectangular contour. On the contrary, in D. clavicaudatus and Doedicurus sp., the rostral area is more sub-triangular, as in Panochthus tuberculatus and Plohophorus figuratus. As in cf. E. antiquus and Doedicurus, the orbital notches are posteriorly closed by a well-developed post-orbital bar. In E. solidus, this post-orbital bar draws an angle of ca. 90° with the sagittal plane of the skull, while in the species of Doedicurus this angle ranges between 40–50°. The zygomatic arches of E. solidus are straight in their antero-posterior length, while in Doedicurus they are more laterally expanded, making a semicircle. The parietal and frontal bones are remarkably wide, especially between the orbital notches and the posterior-most region of the zygomatic arches. The maximum diameter of the fronto-parietal region coincides with the post-orbital bar. In cf. E. antiquus this morphology is similar, although this lateral expansion is not so evident compared to E. solidus. On the contrary, in other taxa such us D. clavicaudatus, Doedicurus sp., G. munizi, Panochthus tuberculatus, and Plohophorus figuratus, there is a well-developed post-orbital narrowing, weekly developed in E. solidus.
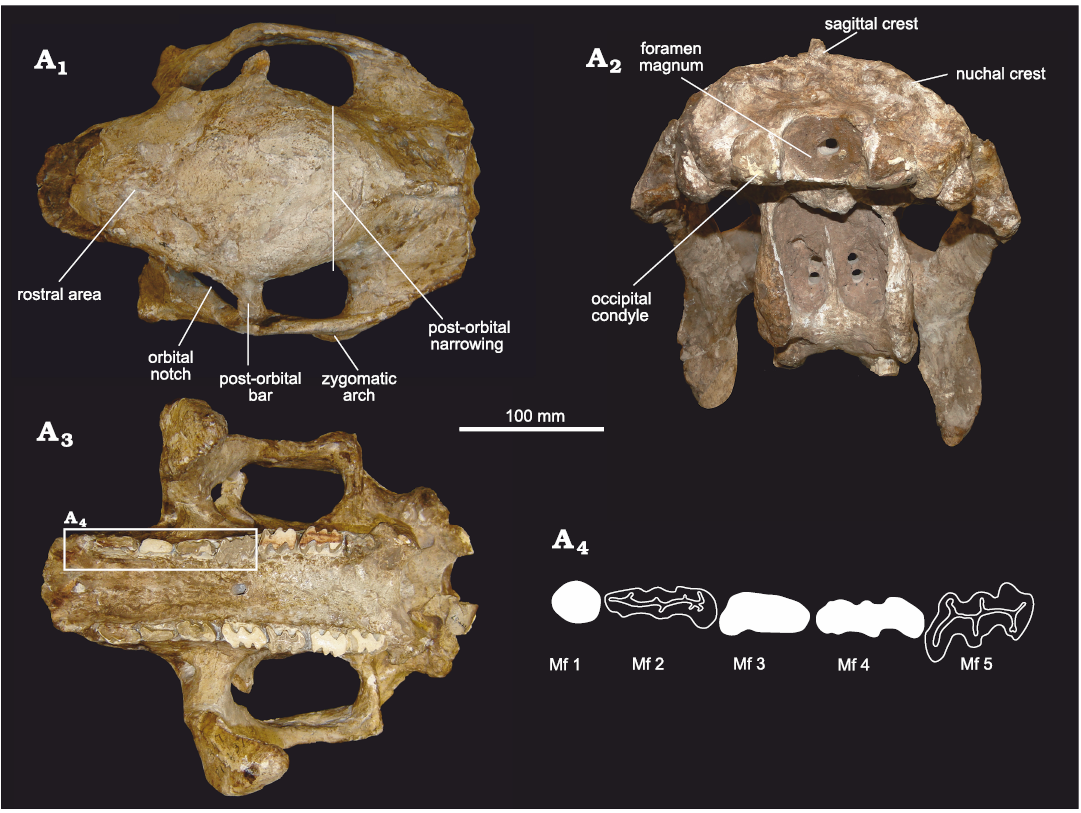
Fig. 4. Skull of doedicurine glyptodont Eleutherocercus solidus (Rovereto, 1914), FMNH-P 14437, from the late Miocene–early Pliocene of Argentina, Puerta de Corral Quemado, Andalhuala Formation, levels 23–28, Argentina; in dorsal (A1) and occipital (A2), and (A3) occlusal views; drawing of detail of the first five molariforms (A4). In white teeth the occlusal surface is covered by sediment.
In occlusal view (Fig. 4A3, A4), some of the most remarkable characters for E. solidus involve the morphology of the molariforms, the difference in size and shape between the Mf1 and the rest of the tooth series, together with the expansion of the palate at the level of Mf1. This set of characters is also seen in cf. E. antiquus, D. clavicaudatus, and Doedicurus sp., constituting potential synapomorphies for Doedicurinae (see Zurita et al. 2014). Mf1 are not lobed, as in Doedicurus and cf. E. antiquus. However, in E. solidus and Doedicurus it has a circular or sub-circular cross section, unlike cf. E. antiquus, in which Mf1 is more elliptical. Although there is a clear widening of the palate at the level of Mf1, this seems to be less obvious than in cf. E. antiquus and species of Doedicurus. The pattern of Mf2 tends to trilobation, while Mf3 is clearly trilobated. This feature is close to the morphology seen in Doedicurus, in which Mf2 already shows a high degree of lobulation, unlike in cf. E. antiquus where Mf2 is bilobated. The Mf3 of cf. E. antiquus and species of Doedicurus are similar, but in E. solidus and cf. E. antiquus the anterior margin of the first lobe is more rounded. In E. solidus, from Mf4 onwards, the trilobation is very marked and there are no significant differences between the other molariforms in the series, except that the anterior margin of the first lobe tends to be convex in Mf3–Mf4, flat in Mf5, and concave in the last three molariforms (Mf6–Mf8). In comparative terms, the Mf4–Mf6 of cf. E. antiquus, D. clavicaudatus, and Doedicurus sp. are very similar to each other, showing a concavity in the labial half of the posterior margin of the third lobe, a small detail also seen in other species of more distantly related genera, such as Plohophorus and Glyptodon. This character is also present in E. solidus, but beginning at the level of Mf5 instead of Mf4. In E. solidus, the minimum width of the palate is observed at the Mf3 and Mf4 level, while this narrowing is observed at the Mf4 and Mf5 level in cf. E. antiquus, and at the Mf4 level in Doedicurus. In cf. E. antiquus and Doedicurus, two large foramina with an anterior canal are present on the palate at the Mf3–Mf4 border, unlike in E. solidus in which those could not be detected. Likewise, in E. solidus the main axis of Mf1–Mf4 is parallel to the longitudinal axis of the tooth series, whereas in cf. E. antiquus only the main axis of Mf2 is parallel to the longitudinal axis, and in Doedicurus sp. only in the last molariforms of the series (Mf6–Mf8) are parallel. In other groups, such as Plohophorus and Glyptodon, this character is observed from Mf4–Mf8. It is worth noting that the skull MLP 29-X-10-9 presents supernumerary teeth, thus having a total of nine molariforms due to the duplication of Mf8. This feature was already described on this specimen by Cabrera (1944), making the first record of hyperdontia in Eleutherocercus and the second record for Glyptodontidae (see González-Ruiz et al. 2015).
In occipital view (Fig. 4A2), the specimen FMNH-P 14437 is slightly deformed due to compaction, mainly affecting the maxillary bone. The dorsal outline of the supraoccipital is rounded and convex, similar to cf. E. antiquus, and a marked sagittal crest extends to the nuchal crest. In D. clavicaudatus and Doedicurus sp. the dorsal profile of the supraoccipital is slightly quadrangular and concave, and the sagittal crest cannot be seen in this view. In G. reticulatus the dorsal profile of the supraoccipital is convex and continues laterally with the nuchal crest. In E. solidus, the foramen magnum is sub-elliptical, with its main axis transversally oriented. In D. clavicaudatus and Doedicurus sp. it is circular, while in G. reticulatus it is distinctly rhomboidal.
Mandible: The material includes FMNH-P 14437 (Fig. 5), represented by a complete and very well-preserved mandible, in addition to another hemi-mandible (FMNH-P 14446). The general morphology is almost identical to that of Doedicurus, although both taxa are temporally separated by more than 4 My. This highlights the conservative dentary morphology observed in this lineage, coinciding with the observations carried out in other glyptodont clades, such as the Neosclerocalyptini Neosclerocalyptus (see Quiñones et al. 2020).
In lateral view (Fig. 5A1), the mandible is very robust, at the level of both the ascending and horizontal rami, as in Doedicurus. The ventral margin of the horizontal ramus is notably concave, much more than that of Glyptodon, Panochthus, and Neosclerocalyptus, reaching its maximum height at the level of the mf4 and mf5. The angle between the symphysial region and the middle and posterior border of the mandibular body is ca. 140°, as in Doedicurus, somewhat different from Glyptodon, in which this angle reaches 150°. Like the mandibular body, the ascending ramus is very robust, with a great antero-posterior diameter, which represents the total length of the tooth series from mf1 to mf6, as in Doedicurus. As in Doedicurus, Neosclerocalyptus, and Glyptodon, the ascending ramus is inclined forwards, drawing an angle of 65° between its anterior margin and the alveolar margin; this anterior margin is at the level of mf5, as in the genera Doedicurus and Glyptodon, and different from Neosclerocalyptus, in which it is located at the level of mf6. The sigmoid notch is very developed and similar to the notch in Doedicurus, while the posteroventral margin of the ascending ramus shows a very conspicuous angular process at the level of the posterior margin of the third lobe of mf8. In turn, the posterior margin of the ascending ramus is concave, especially in its dorsal-most third, as in Doedicurus, but different from Neosclerocalyptus, Panochthus, and Glyptodon, which show a straighter edge.
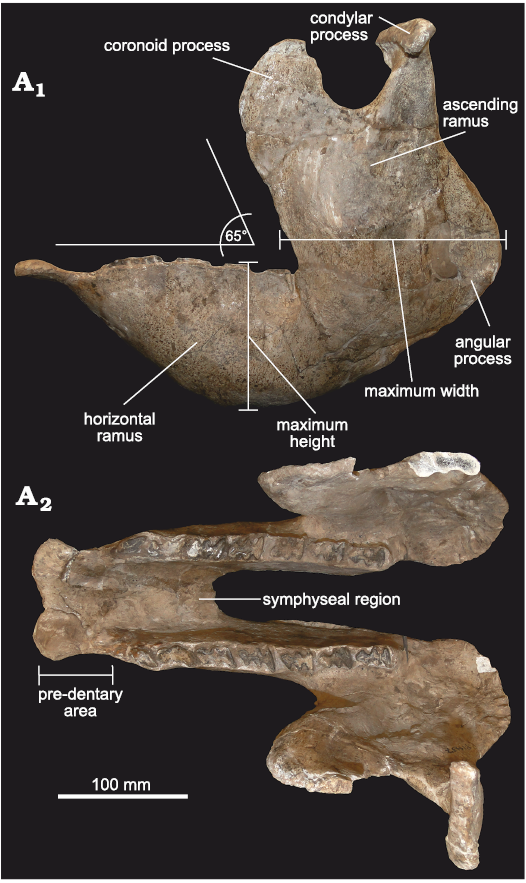
Fig. 5. Mandible of doedicurine glyptodont Eleutherocercus solidus (Rovereto, 1914), FMNH-P 14437, from, upper Miocene–Pliocene, Puerta de Corral Quemado, Andalhuala Formation, levels 23–28, Argentina; in left lateral external (A1), and occlusal (A2) views.
In occlusal view (Fig. 5A2), the pre-dental region of the symphysis is 76 mm long proximo-distally, widening in the most distal portion, where two large mental foramina are seen on the ventral side. The mandibular symphysis ends at the level of the second lobe of mf4, as in Neosclerocalyptus, Glyptodon, and Panochthus.
Molariforms: (Fig. 5A2). The mf1 is sub-circular in section, and the smallest compared to the rest of the molariforms (mf2–mf8). In Doedicurus sp. and cf. E. antiquus this molariform is subcircular to subtriangular and similarly smaller than other molariforms. The mf2 of E. solidus is elliptical in outline, with irregular edges, lacking a defined pattern, so that no clear tendency towards bilobulation or trilobulation is observed. On the contrary, in Doedicurus sp., mf2 is completely trilobulated. This particular morphology observed in the mf1, mf2 of Doedicurinae markedly contrasts with that of Glyptodon, in which a very conspicuous trilobulation is seen from the mf1 (this character being a synapomorphy of the genus; see Zurita et al. 2013; Cuadrelli et al. 2020), together with a limited variation of size along the molariform series. The mf3 shows some tendency towards trilobulation, but not as marked as in the rest of the posterior molariforms. The mf4–mf8 are completely trilobulated, as in cf. E. antiquus; in addition, the general morphology of the last molariforms of E. antiquus and E. solidus is very similar to those of D. clavicaudatus and Doedicurus sp.
Osteoderms: Associated with the material described above, some fragments of dorsal carapace were found. This allows for careful comparisons with the holotype of E. solidus (MACN 8335; see Fig. 6A) and, consequently, to refer the specimens here analyzed to this species, greatly improving its morphological characterization. Other remains were found in nearby localities, but mostly corresponding to isolated osteoderms and small fragments of carapace (MCH-P 188, 253, and 325; Fig. 6D).
The fragment of dorsal carapace of FMNH-P 14446 (Fig. 6B) is composed of 11 associated osteoderms, of which six are complete. The osteoderms are hexagonal and probably correspond to the middle or cephalic part of the carapace, as observed in other glyptodonts. The exposed surface is rugose and has several small foramina irregularly distributed. Most of these foramina do not penetrate the entire thickness of the osteoderms, as in cf. E. antiquus (see Zurita et al. 2014) and the type material of E. solidus. This clearly differs from the morphology observed in Doedicurus, in which the osteoderms are much thicker, and the foramina tend to be, in small number, located in the middle region of the osteoderm, piercing its entire thickness. The other fragment (FMNH-P 14437; see Fig. 6C) belongs to the postero-dorsal region. The high degree of fusion between osteoderms prevents from inferring their exact number in this fragment. As in FMNH-P 14446 and the holotype of E. solidus, the exposed surface shows several small foramina irregularly distributed (see Rovereto 1914). The most marginal osteoderms are extremely irregular in shape and have an anomalous fibrous texture. On the left lateral half of this fragment, part of an irregular dome-like structure can be seen. The high degree of fusion indicates that this is the anterior region of the “dome”. This particular structure (of glandular origin?) has been reported by other authors as characterizing the genus Eleutherocercus (see Moreno 1888; Lydekker 1895; Ameghino 1920; Chimento et al. 2010). We therefore suggest, agreeing with the proposal of Chimento et al. (2010), that it could represent part of a glandular system located at the level of the posterior part of the pelvic girdle. Supporting this hypothesis, several specimens of the Glyptodontinae Glyptodon show very large foramina at the intersection between the annular and radial sulci, in the same region of the dorsal carapace (see Zurita et al. 2016; Cuadrelli et al. 2019).
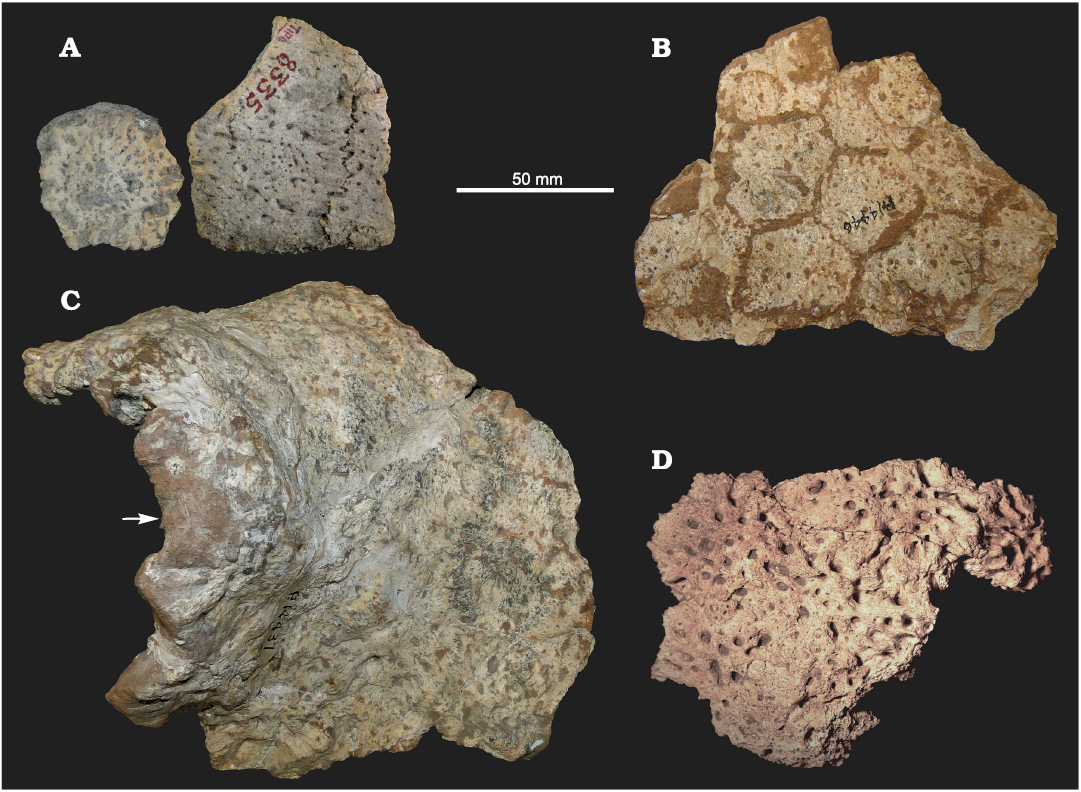
Fig. 6. Osteoderms of doedicurine glyptodont Eleutherocercus solidus (Rovereto, 1914) from Pliocene of Argentina. A. MACN 8335 (holotype), small carapace fragment, from Andalhuala, “Araucanian” (late Neogene). B. FMNH-P 14446, carapace fragment (middle or cephalic region), from Puerta de Corral Quemado, Corral Quemado Formation, level 32. C. FMNH-P 14437, carapace fragment (the arrow is pointing the postero-dorsal region showing the modified osteoderms that constitute the “dome” structure), from Puerta de Corral Quemado, Andalhuala Formation, levels 23–28. D. MCH-P 188, small fragment of carapace, from San Fernando Norte, Andalhuala Formation.
Caudal tube: FMNH-P 14437 includes an almost complete caudal tube, but its exposed surface is not well preserved, precluding the observation of the ornamentation pattern. However, its general morphology can be inferred. Although its transverse diameter is quite similar along its antero-posterior length, the caudal tube is slightly more laterally expanded at the proximal end, whereas the distal-most part is clearly rounded and blunt. In lateral view, the caudal tube shows a greater dorso-ventral diameter (ca. 30%) in its proximal part, being more flattened towards the distal end. MLP 29-X-10-21 (Fig. 7A) and MACN 2893 (holotype of E. tucumanus, Fig. 7B) preserve the exposed surface, with a clear ornamentation pattern. In dorsal view, the ornamentation displays a “rosette” pattern. In each osteoderm the central figure is slightly concave, surrounded by 7–8 smaller and angular peripheral figures. The principal sulcus surrounding the central figure bears several small foramina. In some specimens (e.g., MLP 29-X-10-21) the foramina are more developed in the proximal half of the caudal tube, while in others (e.g., MACN 2893) they are present along the antero-posterior diameter, but this could be probably due to taphonomic factors. On the lateral sides, near both margins, there is a row of large figures with a very rugose surface, which become more numerous towards the distal end of the tube. In lateral view, six or seven lateral figures are present, increasing in size towards the distal end, as in Panochthus and Doedicurus. Unlike in Panochthus, in Eleutherocercus (as well as in Doedicurus) the surface of these figures is concave, very rugose and striated. Instead, in Panochthus and Hoplophorus these figures display a “spine-like” morphology. In E. solidus, lateral figures are separated from each other by a single row of foramina, but the proximal ones also show some peripheral figures. On the distal end of MACN 2893, there are two large figures on each margin, one being more dorsal and the other being more ventral and somewhat larger. The contact area between both figures (dorsal and ventral) coincides with the midline of the lateral sides of the tube. Despite some similarities with Doedicurus, the morphology of the caudal tube of Eleutherocercus shows some differences. In Doedicurus, the distal end of the caudal tube is very laterally expanded, similar to a club. The rosette ornamentation pattern of Eleutherocercus is not observed in Doedicurus; in the latter, the surface is smooth with numerous foramina similar in morphology to those of the dorsal carapace.
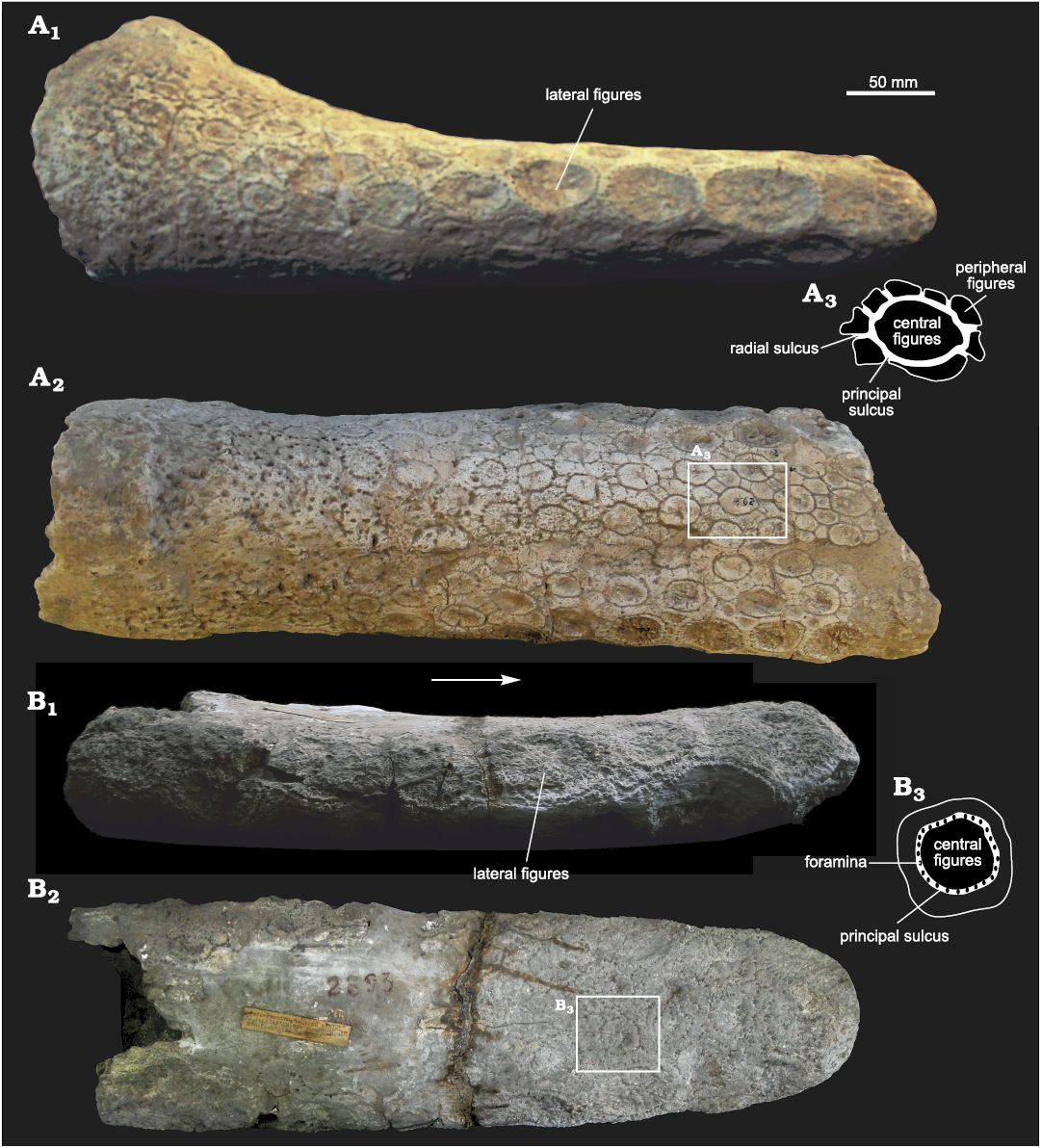
Fig. 7. Caudal tubes of doedicurine glyptodont Eleutherocercus solidus (Rovereto, 1914), from upper Miocene–Pliocene of Argentina. A. MLP 29-X-10-21, from Puerta de Corral Quemado (near the road to Loconte), unknown stratigraphic level; in lateral (A1) and dorsal (A2) views; drawing of detail of dorsal osteoderm (A3). B. MACN 2893 (holotype of E. tucumanus Castellanos) from Tiopunco, unknown stratigraphic level; in lateral (B1) and dorsal (B2) views; drawing of detail of dorsal osteoderm (B3).
However, the most evident differences between the genera Doedicurus and Eleutherocercus are observed at the distal end of the caudal tube, specifically in the location and number of the large rugose figures. In Eleutherocercus these figures are numerous, ranging 10–14 depending on the specimen. From the most distal end of the tube to a proximal position they are located as follows: four at the most distal end, grouped into two pairs, one pair placed in the dorsal surface, and the other two in the ventral surface, the latter being a larger pair; in the dorsal area of the distal end there is a total of up to five figures, grouped into a distal row of three and another more proximal composed of two figures. In turn, the ventral area shows the same ornamentation pattern to that observed in the dorsal surface. On the other hand, in Doedicururs these figures are reduced in number to only 8. From the most distal end of the tube to a proximal position they are located as follows: a pair of symmetrical figures at the most distal end of the caudal tube; four immediately behind, in a more proximal position and grouped in two pairs, one pair in a dorsal position and the other in a ventral position; and finally two more symmetrical figures in a mid-lateral position, the latter being the largest.
Phylogenetic affinities of Eleutherocercus solidus
The cladistic analysis resulted in one most parsimonious tree (MPT) (RI = 0.91 and CI = 0.79; length = 97 steps), see Fig. 8.
Two major radiations are observed in the topology. In the first one, supported by synapomorphies 20:0 and 40:0, Propalaehoplophorus australis is the most basal taxon. However, “Propalaehoplophorinae” is not a monophyletic group, since the three species generally placed in that group (P. australis, Eucinepeltus petestatus, and Cochlops muricatus) branch sequentially, with Cochlops muricatus being recovered as the sister group of the remaining diversity, recognized as valid in this analysis (Plohophorini, Neosclerocalyptini + Hoplophorini [Hoplophorinae] and Doedicurinae sensu Zurita et al. 2014; synapomorphy 48:2 and the ambiguous synapomorphies 24:1; 42:0; 52:1). This is in agreement with the topologies reported by Zurita et al. (2013, 2014), Quiñones et al. (2020), and Cuadrelli et al. (2020).
The clade formed by Pseudoplohophorus absolutus and Plohophorus figuratus (ambiguous synapomorphy 29:1) is recovered as the sister group of the remaining members of this radiation (Doedicurinae and Hoplophorinae [Hoplophorini + Neosclerocalyptini] sensu Zurita et al. 2014; synapomorphy 17:2). In turn, the subfamily Doedicurinae appears as a well supported clade, condition based on several cranial and postcranial characters (synapomorphies 7:1; 12:1; 16:1; 26:0; 27:2; 30:2; 35:3; 50:2; 54:1 and ambiguous synapomorphies 11:1; 42:3; 49:1). It includes the genera Doedicurus and Eleutherocercus, and shows a similar topology to that obtained by Zurita et al. (2014). Within Doedicurinae, the genus Eleutherocercus includes the two species (E. antiquus + E. solidus) and several cranial and postcranial characters support its condition of natural group (synapomorphies 34:1; 38:1; 56:1 and ambiguous synapomorphies 4:0; 45:0). On the other hand, within Hoplophorinae sensu Zurita et al. 2014 (synapomorphies 2;1 14:1; 21:1) a sister group relationship between the tribes Neosclerocalyptini (N. ornatus + N. paskoensis; synapomorphies 3:1; 9:3; 37:0) and Hoplophorini (synapomorphies 8:1; 41:1; 53:1 and ambiguous synapomorphy 49:1) is recovered, with the genus Hoplophorus as sister taxon of Propanochthus + Panochthus (26:3; 33:1; 51:2 and ambiguous synapomorphy 29:1). The position of Propanochthus bullifer as sister group of Panochthus spp. suggests that the former may be interpreted as belonging to the latter genus.
The second radiation of Glyptodontidae is that of Glyptodontinae (synapomorphies 17:3; 22:0; 40:1 and ambiguous synapomorphy 15:0). This subfamily includes the tribes Boreostemmini (sensu Cuadrelli 2020), represented by Boreostemma (synapomorphies 43:1 and the ambiguous synapomorphy 31:1) and Glyptodontini (sensu Cuadrelli 2020; synapomorphies 0:1; 6:1; 9:1; 23:0; 26:1; 28:1; 42:2 and the ambiguous synapomorphy 24:1). In turn, Glyptodontini is divided into two subtribes, Glyptodontina (synapomorphies 1:1; 10:1; 14:0; 32:1; 55:0) that gathers species of the Glyptodon, and Glyptotheriina (synapomorphies 35:1; 36:1; 55:1) including species of the Glyptotherium.
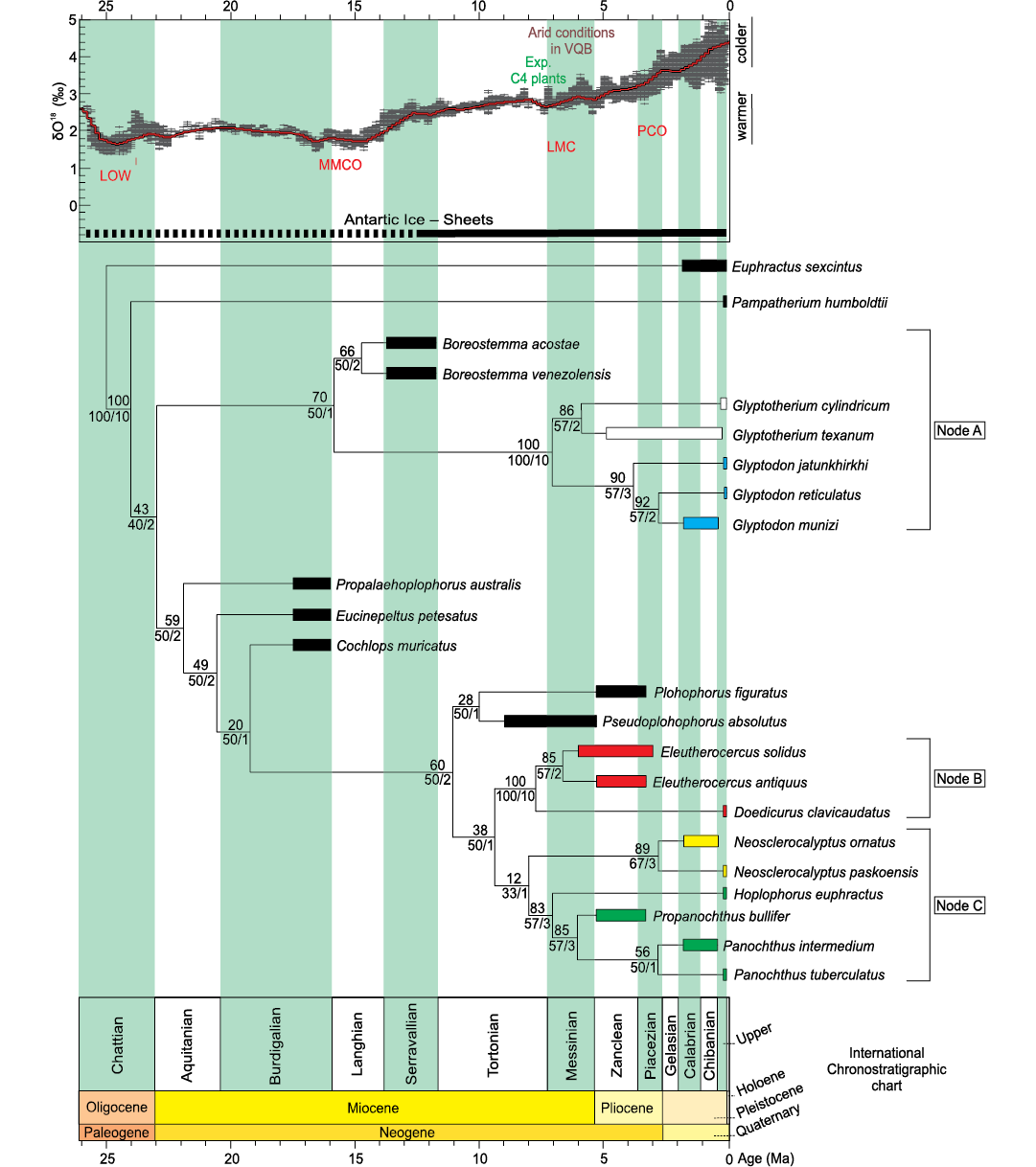
Fig. 8. Phylogeny of Glyptodontidae based on TNT parsimony analysis of 57 osteological characters from 23 taxa (tree length 97 steps; Consistency Index 0.79; Retention Index 0.91). The numbers above each node represent Jackknife values; numbers under each node show relative Bremer support (right) and absolute Bremer support (left). Node A: Glyptodontinae (taxa in white correspond to Glyptotheriini; taxa in blue correspond to Glyptodontini); Node B: Doedicurinae (taxa in red); Node C: Hoplophorinae (taxa in yellow correspond to Neosclerocalyptini; taxa in green correspond to Hoplophorini). Chronostratigraphic calibration of the phylogenetic relationships of Neogene Glyptodontidae is compared to benthic foraminifera δ18O values from Zachos et al. (2001) to visualize the evolution of the Doedicurinae clade in the context of paleoenvironment and climate changes throughout the Neogene in north-western Argentina. Abbreviations: LOW, Late Oligocene Warming; MMCO, Middle Miocene Climatic Optimun; LMC, Late Miocene Cooling; PCO, Pliocene Climatic Optimun; Exp. C4 plants, expansion of C4 plants; VQB, Villavil-Quillay Basin.
Discussion
Glyptodonts constitute one of the most spectacular cingulate radiations in America (Zurita et al. 2016), and their evolutionary history appears to be mostly related to the appearance and development of open biomes since the late Eocene–Oligocene (Carlini et al. 2010; Mitchell et al. 2016; Zachos et al. 2001, 2008; Fig. 8). The biomechanical evidence interpreted glyptodonts as grazers, although some kind of niche partitioning was suggested by some authors (Vizcaíno et al. 2012).
Although the Paleogene evidence is still very scarce and mostly limited to osteoderms of the dorsal carapace, an interesting diversification is observed, represented by two morphotypes, one included in the subfamily “Propalaehoplophorinae” and the other in the poorly known “Glyptatelinae”. This early diversification could be related to the progressive aridization and development of open areas since the latest Eocene–early Oligocene, and is mostly restricted to the Patagonian region of South America (Cuadrelli et al. 2020).
Since the early–middle Miocene (ca. 19–17 Ma) a marked increase in the records of glyptodonts is observed, with the first well-documented Neogene radiation represented by the “Propalaeoplophorinae” and the enigmatic Parapropalaehoplohorus septentrionalis in southern South America (Croft et al. 2007; Vizcaíno et al. 2010; González Ruiz et al. 2020). This evolutionary process may have coincided, at least in part, with the Middle Miocene Climatic Optimum (MMCO) (Zachos et al. 2001; Croft et al. 2016).
From an evolutionary standpoint, the “Propalaehoplophorinae” (i.e., Propalaehoplophorus, Eucinepeltus, and Cochlops) are the most basal taxa of a large southern South American radiation that includes most of the known diversity of glyptodonts, the other large radiation being represented by the Glyptodontinae (Carlini et al. 2008; Zurita et al. 2013; Cuadrelli et al. 2020). In this framework, this first large southern lineage that begins with Propalaehoplophorus australis, underwent, according to the fossil record, a diversification process since the late Miocene–earliest Pliocene, with the first record of several lineages, some of them with long biochrons that reached the latest Pleistocene (Zurita et al. 2016). Interestingly, this major diversification seems to have mainly occurred in high latitudes instead of tropical and intertropical regions of South America, where the diversification was mostly limited to the subfamily Glyptodontinae during the Miocene and Pliocene (Zurita et al. 2013; Cuadrelli et al. 2020).
In this context, in our phylogenetic analysis, Doedicurinae appears as a well supported clade, including until now the genera Eleutherocercus and Doedicurus as sister groups. In turn, Doedicurinae is included in a large southern South American clade, which is in agreement with several previous analyses (see Zurita et al. 2013; Cuadrelli et al. 2020; but see Fernicola 2008; Fernicola and Porpino 2012 for another interpretation). As occurs with most of the taxa composing this large clade, the evolutionary history of Doedicurinae was restricted to high and middle latitudes.
From a chronostratigraphic view point, the records of E. solidus come from the Corral Quemado and Andalhuala formations, specifically, the stratigraphic levels chronologically located in a latest Messinian to Piacenzian interval (ca. 6–3 Ma, latest Miocene–Pliocene). FMNH-P 14475 is considered here as the First Appearance Datum (FAD) of Eleutherocercus solidus in VQB, recorded in level 20 (sensu Stahlecker in Marshall and Patterson 1981: appendix 5), which is located in the stratigraphic section 1100–1200 m at ca. 6 Ma (see Fig. 2). This inferred interval is similar to the one postulated for the geological units in the Pampean region of Argentina where remains of E. antiquus (Monte Hermoso Formation) and cf. E. antiquus (Chapadmalal and El Polvorin formations) are recorded, which span ca. 5.2–2.8 Ma (Zanclean to Piacenzian) (Zárate et al. 2007; Beilinson et al. 2017; Quiñones et al. 2020). This implies that both species of the genus partially overlap in time (Pliocene), but in different regions of southern South America (Zurita 2007; Cione et al. 2000).
During the late Miocene–early Pliocene, a series of paleoenvironmental changes have been documented in the fossiliferous sediments of northwestern Argentina. These changes have been related to floristic (i.e., C3/C4 ratio), tectonic (i.e., rising of mountain ranges), and climatic events (i.e., generalized aridification, seasonality) (Hynek et al. 2012; Georgieff et al. 2017; Armella and Bonini 2020), which probably affected the development and evolution of herbivorous faunas (Cerling et al. 1998; MacFadden et al. 1999; MacFadden 2005). The FAD of E. solidus in VQB is synchronous to large eolian deposits representing arid conditions, which are identified in different locations along the basin (see Muruaga 2001; Hynek et al. 2012; Esteban et al. 2014; Georgieff et al. 2017). Likewise, these deposits coincide with the period in which C4 becomes an important component in the diet of large herbivores (Hynek 2011), before becoming dominant after 4 Ma (Latorre et al. 1997). As stated above, the general morphology of the mandible and teeth of E. solidus is almost identical to that of Doedicurus, which has the highest hypsodonty index among glyptodonts (Vizcaíno et al. 2011). These anatomical features can be interpreted as a way of increasing the mechanical capacity in relation to accidental consumption of abrasive particles (i.e., sand, dust, volcanic glass) adhering to the surface of plants (Janis 1988; Candela and Bonini 2017) or the ingestion of large amounts of food with low nutritive value (Vizcaíno et al. 2011; see Fig. 9).
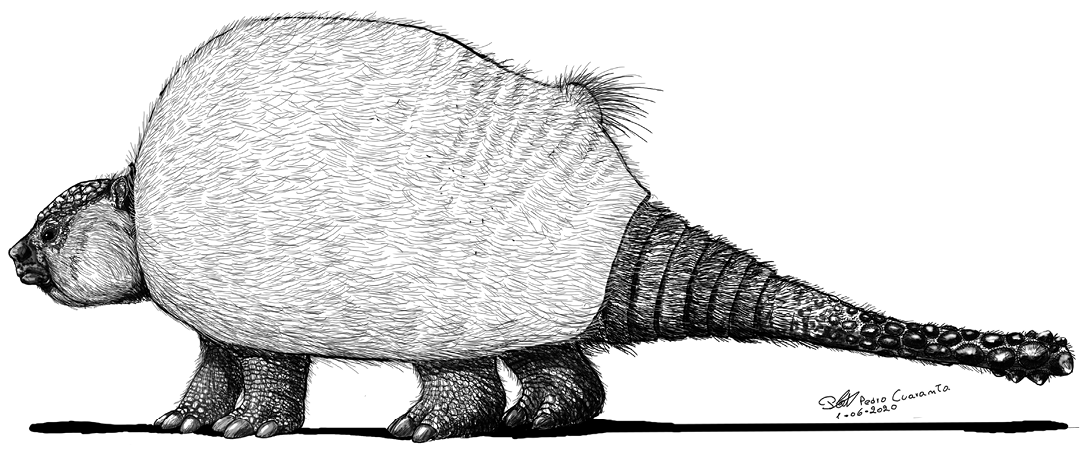
Fig. 9. Hypothetical life reconstruction of the extinct Eleutherocercus solidus (Rovereto, 1914), based on the new specimens described here. Credit: Pedro Cuaranta.
The high frequency of records suggests that Doedicurinae were dominant elements in the late Neogene palaeofauna in both the Pampean and northwestern regions. However, preliminary studies of the El Polvorín Formation (Pliocene–earliest Pleistocene) reveal a decrease in the frequency of records of Doedicurinae towards the Pleistocene (Quiñones et al. 2020). In this framework, and taking into account that most of the glyptodonts present a rich fossil record in South America (see Zurita et al. 2009), we suggest that this very low frequency of records of Pleistocene Doedicurinae in most of South America (perhaps except for some areas of Uruguay) is reflecting a genuine process in which Pleistocene Doedicurinae were very reduced in the number of specimens compared to the other genera (ie., Glyptodon, Panochthus, and Neosclerocalyptus). It is interesting to note that it is quite common to find some associated specimens of Pleistocene glyptodonts in southern South America, suggesting some kind of gregarious habit. However, this was not reported for Doedicurus since all the records correspond to single specimens. Although more evidence is necessary, this could suggest that Doedicurus developed solitary habits.
Outside Argentina, Neogene Doedicurinae is also recorded in the Pliocene of Inchasi, Bolivia (ca. 3.3–4 Ma; see MacFadden et al. 1993; Cione and Tonni 1996), and Uruguay (Koken 1888; Toriño and Perea 2008), at high and middle latitudes. Doedicurinae (together with Glyptodontinae) were some of the most frequently recorded glyptodonts in the Pliocene sequences of Inchasi, Bolivia (AEZ, personal observations). The northernmost record of a Doedicurinae comes from the Pliocene of Ayo Ayo, near La Paz (Bolivia), at a latitude of ca. 16°30´. Based on the high frequency of Neogene records of Doedicurinae in Uruguay, some authors recognized several endemic taxa, among them Prodaedicurus and Castellanosia (Toriño and Perea 2008). However, it cannot be ruled out that some (if not all) could belong to the same taxon. More complete remains are needed to test this taxonomic hypothesis. Indeed, unlike north-western and Pampean regions of Argentina, where Doedicurinae is represented by several relatively complete specimens, the remains from Bolivia and Uruguay are mostly represented by osteoderms of the dorsal carapace and some caudal tubes, a situation that hinders their taxonomic identification. No remains of Doedicurinae are known from the Pleistocene of Bolivia and Peru. However, it seems that during the late Neogene (latest Miocene–Pliocene) Doedicurinae reached a wide latitudinal distribution from ca. 16°30´ (La Paz, Bolivia) to ca. 38°58´ (Monte Hermoso, Argentina), being a frequent component within glyptodont assemblages.
During the Pleistocene (ca. 2.6–0.011 Ma), Doedicurinae underwent a marked latitudinal retraction as the Doedicurus is geographically restricted to the Pampean region of Argentina (Ameghino 1889; Carlini and Scillato-Yané 1999), Uruguay (Ubilla et al. 2004; Zurita et al. 2009) and southernmost Brazil (Oliveira 1992; Pereira et al. 2012). In this scenario, the paleontological evidence agrees with the geographic distribution predicted by Varela et al. (2018; species distribution models).
It is worth noting that the largest (Doedicurus) and the smallest (Neosclerocalyptus) glyptodonts were the most latitudinally restricted during the Pleistocene, with Doedicurus being the most geographically constrained (Zurita et al. 2009; Quiñones et al. 2020). This progressive latitudinal retraction of Doedicurinae since the Pleistocene is quite intriguing because it is exactly opposite to that of the other Pleistocene lineages (e.g., Glyptodon and Panochthus) of South American glyptodonts. In the latter, an increase in body mass is related to an increase in the latitudinal distribution (see Cuarelli et al. 2020), as is currently observed in some African megamammals (i.e., Hippopotamus amphibius) that have a body mass similar to that inferred for Doedicurus (ca. two tons; Soibelzon et al. 2012; Vizcaíno et al. 2011). The co-ocurrence of Doedicurus and at least three other genera of glyptodonts (i.e., Neosclerocalyptus, Panochthus, and Glyptodon) in the Pampean region of Argentina, Uruguay, and southern Brazil, suggests niche partitioning, in agreement with evidence from stable isotope analyses (see Domingo et al. 2012; Melo-Franca et al. 2015) and biomechanical studies (see Vizcaíno et al. 2011; Pomi 2008). According to Cione et al. (2015), the hypothetically low primary productivity of these glacial ecosystems could have led to a lower number of individuals for each species, also stimulating a partition of resources as a key strategy to survive in these areas, especially during glacial stages (see Domingo et al. 2020).
Accordingly, our observations reveal that the frequency of records of Doedicurus is one of the lowest within Pleistocene glyptodont diversity in southern South America. Therefore, it is possible that the number of individuals of Doedicurus living in the same area at the same time was low, as suggested by the fossil record.
This geographic retraction, which probably started in the latest Neogene or early Pleistocene, could be related to the progressive cooling and aridization observed since the Pliocene, which intensified during the Pleistocene in southern South America. Despite this phenomenon, Doedicurus is one of the last survivors of the South American Pleistocene megafauna, as it is recorded until the latest Pleistocene in the Pampean region, a pattern that suggests that this area acted as a refuge for the last member of the megafauna before its extinction (see Politis et al. 2019).
Conclusions
(i) Eleutherocercus solidus is the only well characterized Doedicurinae in North-western Argentina, with a biochron that spans from the latest Miocene to Pliocene; (ii) the mandibular and dental morphology of E. solidus (almost identical to Doedicurus, the most hypsodont glyptodont ever known) reveals that the temporal distribution of this species coincides with a spread of C4 plants in northwestern of Argentina; (iii) the other species of the genus, E. antiquus, shows a similar temporal distribution (Pliocene), but living in a different biogeographical area (i.e., the Pampean region of Argentina); (iv) the Eleutherocercus is a well supported clade, sister group of the giant late Pleistocene Doedicurus clavicaudatus; (v) Eleutherocercus spp. + Doedicurus constitutes the subfamily Doedicurinae, which in turn forms part of a large southern South American glyptodont radiation starting, at least, in the early Miocene, in the Patagonian region of Argentina; (vi) contrary to what was observed for the diversity of the other Pleistocene glyptodonts (i.e., Neosclerocalyptus, Panochthus, and Glyptodon), Doedicurinae underwent a latitudinal retraction ending with the giant Doedicurus being limited to the Pampean region of Argentina, Uruguay, and southernmost Brazil during the late Pleistocene; (vii) the hypothetic relationship between body mass (with some specimens of Doedicurus achieving ca. two tons) and latitudinal retraction suggests that the cyclic climate changes during the Pleistocene could have played an active role in the final steps of the evolutionary history of glyptodonts in South America.
Acknowledgements
We thank Marcelo Reguero (MLP), Laura Cruz (MACN), William Simpson and Keneth Angielczyk (both FMNH), and the Museo “Condor Huasi” Belén for granting us access to their collections, and Dirección de Antropología de Catamarca for giving us the permission to work in Catamarca Province. We also thank Pedro Cuaranta (Universidad Nacional del Nordeste, Corrientes, Argentina) for drawing the reconstruction of Eleutherocercus solidus and Sergio Miguel Georgieff (Universidad Nacional de Tucumán, Tucumán, Argentina) who helped in the field trip and provided comments on the stratigraphic context, and we thank Emilia Sferco and Federico Degrange (both CICTERRA-CONICET-UNC, Córdoba, Argentina), Eugenia García (Dirección Provincial de Antropología de Catamarca, San Fernando del Valle de Catamarca, Argentina), Agustín Scanferla (CONICET-Instituto de Bio y Geociencias del NOA (IBIGEO), Salta, Argentina), Javier Ochoa (Museo Arqueológico e Histórico Regional “Florentino Ameghino”, Int De Buono y San Pedro, Río Tercero, Córdoba, Argentina), Damián Voglino (Museo de Ciencias Naturales “Rvdo. P. Antonio Scasso”, San Nicolás de los Arroyos, Argentina), Miguel Lugo (Museo de historia Hércules Rabagliati, Ramallo, Argentina), and Matias A. Armella (Universidad Nacional de Tucumán y Museo Miguel Lillo, Tucumán, Argentina) for the help during fieldwork. Finally, special thanks are due to the reviewers David Gillette (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium) and Daniel Perea (Universidad de La República, Montevideo, Uruguay) and the editor Olivier Lambert (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium) for their critical reading of the manuscript and their suggestions wich helped to improve the manuscript. This contribution was partially supported by grants PI Q002/17, PICT 2017-0765, and PICT 2015-0724.
References
Abba, A.M., Tognelli, M.F. Seitz, V.P., Bender, J. B., and Vizcaíno, S.F. 2012. Distribution of extant xenarthrans (Mammalia: Xenarthra) in Argentina using species distribution models. Mammalia 76: 123–136. Crossref
Alexander, R.M. Fariña, R., and Vizcaíno, S.F. 1999. Tail blow energy and carapace fractures in a large glyptodont (Mammalia, Xenarthra). Zoological Journal of the Linnean Society 126: 41–49. Crossref
Ameghino, F. 1881. La antigüedad del hombre en el Plata. Vol. 2. 557 pp. Masson-Igon Hermanos, París.
Ameghino, F. 1884. Excursiones geológicas y paleontológicas en la provincia de Buenos Aires. Boletín de la Academia Nacional de Ciencias en Córdoba 6: 1–257.
Ameghino, F. 1886. Contribuciones al conocimiento de los mamíferos fósiles de los terrenos terciarios antiguos del Paraná. Boletín de la Academia Nacional de Ciencias en Córdoba 9: 5–228.
Ameghino, F. 1887. Apuntes preliminares sobre algunos mamíferos extinguidos del yacimiento de “Monte Hermoso” existentes en el “Museo La Plata”. Boletín del Museo de La Plata 1: 1–18.
Ameghino, F. 1889. Contribuciones al conocimiento de los mamíferos fósiles de la República Argentina. Actas de la Academia Nacional de Ciencias de Córdoba 6: 1–1027. Crossref
Ameghino, F. 1891. Sobre algunos restos de mamíferos fósiles, recogidos por el Señor Manuel B. Zavaleta en la formación miocena de Tucumán y Catamarca. Revista Argentina de Historia Natural 1: 88–101.
Ameghino, F. 1920. Sur les Edentés fossiles de l’Argentine; Examen critique, revisión et correction de l’ouvrage de M.R. Lydekker. Obras Completas y Correspondencia Científica 11: 448–907.
Armella, M.A. and Bonini, R.A. 2020. Biostratigraphic significance of the presence of Protypotherium cf. P. antiquum Ameghino 1885 (Interatheriidae, Notoungulata) in the late Miocene of Northwestern Argentina. Journal of South American Earth Sciences 102: 102676. Crossref
Bargo, M.S., De Iuliis, G., and Vizcaíno, S.F. 2006. Hypsodonty in Pleistocene ground sloths. Acta Palaeontologica Polonica 51: 53–61.
Beilinson, E., Gasparini, G.M., Tomassini, R.L., Zárate, M.A., Deschamps, C.M., Barendregt, R.W., and Rabassa, J. 2017. The Quequén Salado River Basin: geology and biochronostratigraphy of the Mio-Pliocene boundary in the southern Pampean plain, Argentina. Journal of South American Earth Sciences 76: 362–374. Crossref
Bergqvist, L.P., Abrantes, E.A.L., and Avilla, L. dos S. 2004. The Xenarthra (Mammalia) of São José de Itabora Basin (upper Paleocene, Itaboraian), Rio de Janeiro, Brazil. Geodiversitas 26: 323–337.
Blanco, R.E., Jones, W.W., and Rinderknecht, A. 2009. The sweet spot of a biological hammer: the centre of percussion of glyptodont (Mammalia: Xenarthra) tail clubs. Proceedings of the Royal Society B: Biological Sciences 276 (1675): 3971–3978. Crossref
Bonini, R.A. 2014. Bioestratigrafía y Diversidad de los Mamíferos del Neógeno de San Fernando y Puerta de Corral Quemado, Catamarca, Argentina. 337 pp. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata.
Bonini, R.A., Candela, A.M., Georgieff, S.M., and Reguero, M.A. 2016. Bioestratigrafía, geocronología y diversidad de los mamíferos del Neógeno de San Fernando, Departamento Belén, Catamarca. Ameghiniana 53 (3 Supplement): R5–R6. Crossref
Bonini, R.A., Georgieff, S.M., and Candela, A.M. 2017. Stratigraphy, geochronology, and paleoenvironments of Miocene–Pliocene boundary of San Fernando, Belén (Catamarca, northwest of Argentina). Journal of South American Earth Sciences 79: 459–471.
Bonini, R.A., Miño-Boilini, A.R., Brandoni, D., and Georgieff, S.M. 2020. New data on the diversity and chronology of late Neogene sloths (Xenarthra, Folivora) from the Villavil–Quillay Basin, Catamarca, Argentina. Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1826470]. Crossref
Bossi, G.E. and Muruaga, C. 2009. Estratigrafía e inversión tectónica del rift neógeno en el Campo del Arenal, Catamarca, NO Argentina. Andean Geology 36: 311–340. Crossref
Bossi, G.E., Ovejero, R., and Strecker, M. 1987. Correlación entre los perfiles del Terciario superior en la Puerta de Corral Quemado-Hualfín y Entre Ríos (Chiquimil). Provincia de Catamarca, Argentina. En Actas del X Congreso Geológico Argentino 2: 117–120.
Brandoni, D., Sclillato-Yané, G.J., Miño-Boilini, A.R., and E. Favotti. 2016. Los Tardigrada (Mammalia, Xenarthra) de Argentina: diversidad, evolución y biogeografía. Contribuciones del MACN 6: 261–272.
Bremer, K. 1994. Branch support and tree stability. Cladistics 10: 295–304. Crossref
Brown, B. 1912. Brachyostracon, a new genus of glyptodonts from México. American Museum of Natural History Bulletin 31: 167–177.
Burmeister, G. 1866. Lista de los mamíferos fósiles del terreno diluviano. Anales del Museo Público de Buenos Aires 1: 121–232.
Burmeister, G. 1870–1874. Monografía de los Glyptodontes en el Museo Público de Buenos Aires. Anales del Museo Publico de Buenos Aires 2: 1–412.
Butler, R.F., Marshall, L. G., Drake, R.E., and Curtis, G.H. 1984. Magnetic polarity stratigraphy and 40K-40Ar dating of late Miocene and early Pliocene continental deposits, Catamarca province, NW Argentina. Journal of Geology 92: 623–636. Crossref
Cabrera A. 1939. Sobre vertebrados fósiles del Plioceno de Adolfo Alsina. Revista del Museo de La Plata 2 (6): 3–35.
Cabrera, A. 1944. Los Gliptodontoideos del Araucaniano de Catamarca. Revista del museo de La Plata (Nueva Serie) 3: 5–76.
Candela, A.M. and Bonini, R.A. 2017. A new guinea pig (Rodentia, Caviomorpha) from northwestern Argentina: implications for the origin of the genus Cavia. Journal of Vertebrate Paleontology 37 (4): e1352591. Crossref
Carlini, A.A. and Scillato-Yané, G.J. 1999. Evolution of quaternary xenarthrans (Mammalia) of Argentina. Quaternary of South America and Antarctic Peninsula 12: 149–175.
Carlini, A.A., Ciancio, M.R., and Scillato-Yané, G.J. 2010. Middle Eocene–early Miocene Dasypodidae (Xenarthra) of southern South America, successive faunas in Gran Barranca; biostratigraphy and palaeoecology. In: R.H. Madden, A.A. Carlini, M.G. Vucetich, and R.F. Kay (eds.), The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia, 106–129. Cambridge University Press, Cambridge.
Carlini, A.A., Zurita, A.E., and Scillato-Yane, G.J. 2008. A new glyptodont species from Codore Formation (Pliocene), Estado Falcón (Venezuela), and the Asterostemma problem. Paläontologische Zeitschritt 82: 125–138. Crossref
Castellanos, A. 1927. Descripción de un fragmento de tubo caudal de un nuevo Doedicurino en relación con sus géneros afines. Anales del Museo de Historia Natural de Montevideo 2: 265–300.
Castellanos, A. 1940. A propósito de los géneros Plohophorus, Nopachthus y Panochthus (2º parte). Publicaciones del Instituto de Fisiografía y Geología. Facultad de Ciencias Matemáticas, Fisico-Químicas y Naturales aplicadas a la Industria de la Universidad Nacional del Litoral 1 (6): 1–418.
Castellanos, A. 1941. A propósito de los géneros Plohophorus, Nopachthus y Panochthus. Publicaciones del Instituto de Fisiografía y Geología. Facultad de Ciencias Matemáticas, Fisico-Químicas y Naturales aplicadas a la Industria de la Universidad Nacional del Litoral 2 (8): 279–418.
Cerling, T.E., Ehleringer, J.R., and Harris, J.M. 1998. Carbon dioxide starvation, the development of C4 ecosystems, and mammalian evolution. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 353: 159–171. Crossref
Chimento, N.R., Ciancio, M.R., Cornero, S., and Carlini, A.A., 2010. La coraza de Eleutherocercus (Xenarthra, Glyptodontidae): presencia de una estructura posiblemente vinculada a una gran masa glandular. In: E.B. Casanave (ed.), Libro de Actas de XXIII Jornadas argentinas de Mastozoologia Resúmenes 15. Bahía Blanca.
Cione, A.L. and Tonni, E.P. 1996. Reassesment of the Pliocene–Pleistocene continental time scale of South America. Correlation of the type Chapadmalalan with Bolivian sections. Journal of South American Earth Sciences 9: 221–236. Crossref
Cione, A.L., Azpelicueta, M.D.L.M, Bond, M., Carlini, A.A., Casciotta, J.R., Cozzuol, M.A., De La Fuente, M., Gasparini, Z., Goin, F.L., Noriega, J.I., Scillato-Yané, G.J., Soibelzon, L.H., Tonni, E.P., Verzi, D., and Vucetich, M.G. 2000. Miocene vertebrates from Entre Ríos province, Eastern Argentina. El Neógeno de Argentina. Serie Correlación Geológica 14: 191–237.
Cione, A.L., Gasparini, G.M., Soibelzon, E., Soibelzon, L.H., and Tonni, E.P. 2015. The Great American Biotic Interchange: A South American Perspective. 97 pp. Springer Briefs in Earth System Sciences, Springer, Dordrecht. Crossref
Cope, E.D. 1889. The edentate of North America. American Naturalist 23: 657–664. Crossref
Croft, D.A., Carlini, A.A., Ciancio, M.R., Brandoni, D., Drew, N.E., Engelman, R.K., and Anaya, F. 2016. New mammal faunal data from Cerdas, Bolivia, a middle-latitude Neotropical site that chronicles the end of the Middle Miocene Climatic Optimum in South America. Journal of Vertebrate Paleontology 36 (5): e1163574. Crossref
Croft, D.A., Flynn, J.J., and Wyss, A.R. 2007. A new basal glyptodontoid and other Xenarthra of the early Miocene Chucal fauna, northern Chile. Journal of Vertebrate Paleontology 27: 781–797. Crossref
Cuadrelli, F. 2020. Los Glyptodontidae Glyptodontinae (Mammalia, Xenarthra): morfología, sistemática y filogenia de los taxones de la radiación austral. 254 pp. Ph.D. Thesis, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba.
Cuadrelli, F., Zurita, A.E. Toriño. P. Miño-Boilini, A.R., Perea, D., Luna, C.A., Gillette. D.D., and Medina, O. 2020. A new species of glyptodontine (Mammalia, Xenarthra, Glyptodontidae) from the Quaternary of the Eastern Cordillera, Bolivia: phylogeny and palaeobiogeography, Journal of Systematic Palaeontology [published online, https://doi.org/10.1080/14772019.2020.1784300]. Crossref
Cuadrelli, F., Zurita, A.E., Toriño, P., Miño-Boilini, A.R., Rodríguez-Bualó, S., Perea, D., and Acuña Suárez, G.E. 2019. Late Pleistocene Glyptodontinae (Mammalia, Xenarthra, Glyptodontidae) from southern South America: a comprehensive review. Journal of Vertebrate Paleontology 38 (5): e1525390. Crossref
Delsuc, F., Catzeflis, F.M., Stanhope, M.J. and Douzery, E.J. P. 2001. The evolution of armadillos, anteaters and sloths depicted by nuclear and mitocondrial phylogenies: implications for the status of the enigmatic fossil Eurotamandua. Proceedings of the Royal Society of London series B 268: 1605–1615. Crossref
Delsuc, F., Gibb, G.C., Kuch, M., Billet, G., Hautier, L., Southon, J., Rouillard, J.M., Fernicola, J.C., Vizcaíno, S. F., MacPhee, R. D., and Poinar, H.N. 2016. The phylogenetic affinities of the extinct glyptodonts. Current Biology 26: 155–156. Crossref
Domingo, L., Prado, J.L., and Alberdi, M.T. 2012. The effect of paleoecology and paleobiogeography on stable isotopes of Quaternary mammals from South America. Quaternary Science Review 55: 102–112. Crossref
Domingo, L., Tomassini, R.L, Montalvo, C.I., Sanz-Pérez, D., and Alberdi, M.T. 2020. The Great American Biotic Interchange revisited: a new perspective from the stable isotope record of Argentine Pampas fossil mammals. Scientific Reports 10 (1): 1–10. Crossref
Esteban, G., Nasif, N., and Georgieff, S. M. 2014. Cronobioestratigrafía del Mioceno tardío-Plioceno temprano, Puerta de Corral Quemado y Villavil, provincia de Catamarca, Argentina. Acta geológica lilloana 26: 165–192
Fernicola, J.C. 2008. Nuevos aportes para la sistemática de los Glyptodontia Ameghino, 1889 (Mammalia, Xenarthra, Cingulata). Ameghiniana 45: 553–574.
Fernicola, J.C. and Porpino, K.O. 2012. Exoskeleton and Systematics: A Historical Problem in the Classification of Glyptodonts. Journal of Mammal Evolution 19 (3): 171–183. Crossref
Fernicola, J.C., Rinderknecht, A., Washington, J., Vizcaíno, S.F., and Porpino, K.O. 2018. A new species of Neoglyptatelus (Mammalia, Xenarthra, Cingulata) from the late Miocene of Uruguay provides new insights on the evolution of the dorsal armor in cingulates. Ameghiniana 55: 233–252. Crossref
Gaudin, T.J. 2004. Phylogenetic relationship among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zoological Journal of the Society 140: 255–305. Crossref
Gaudin, T.J. and Croft, D.A. 2015. Paleogene Xenarthra and the evolution of South American mammals. Journal of Mammalogy 96: 622–634. Crossref
Gaudin, T.J. and Lyon, L.M. 2017. Cranial osteology of the pampathere Holmesina floridanus (Xenarthra: Cingulata; Blancan NALMA), including a description of an isolated petrosal bone. PeerJ 5: e4022. Crossref
Gaudin, T.J. and Wible, J.R. 2006. The phylogeny of living and extinct armadillos (Mammalia, Xenarthra, Cingulata): a craniodental analysis. In: M.T. Carrano, T.J. Gaudin, R.W. Blob, and J.R. Wible (eds.), Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds and Reptiles, 153–198. University of Chicago Press, Chicago.
Georgieff, S.M., Muruaga, C., Ibañez, L.M., Spagnuolo, M.C., Bonini, R.A., Esteban, G., Nasif, N.L., and Del Pero, M. 2017. Estilos de deformación, cronoestratigrafía y evolución paleoambiental de las unidades neógenas de las Sierras Pampeanas Noroccidentales de Catamarca y Tucumán, Argentina. In: C. Muruaga and P. Grosse (eds.), Relatorio XX Congreso Geológico Argentino. 34 pp. Universidad Nacional de Tucumán, San Miguel de Tucumán.
Gibb, G.C., Condamine, F.L., Kuch, M., Enk, J., Moraes-Barros, N., Superina, M., Poinar, H.N., and Delsuc, F. 2015. Shotgun mitogenomics provides a reference phylogenetic framework and timescale for living xenarthrans. Molecular Biology and Evolution 33: 621–642. Crossref
Gillette, D.D. and Ray, C.E. 1981. Glyptodonts of North America. Smithsonian Contributions to Paleobiology 40:1–251. Crossref
Gillette, D.D., Carraza-Castañeda, O., White, R.S., Morgan, G.S., Thrasher, L.C., McCord, R., and McCullough, G. 2016. Ontogeny and sexual dimorphism of Glyptotherium texanum (Xenarthra, Cingulata) from the Pliocene and Pleistocene (Blancan and Irvingtonian NALMA) of Arizona, New Mexico, and Mexico. Journal of Mammalian Evolution 23: 133–154. Crossref
Goloboff, P.A. and Farris, J.S. 2001. Methods for quick consensus estimation. Cladistics 17: 26–34. Crossref
Goloboff, P., Farris, J., and Nixon, K. 2008. TNT, a free program for phylogeny analysis. Cladistics 24: 774–786. Crossref
González-Ruiz, L.R., Brandoni, D., Zurita, A.E., Green, J.L., Novo, N.M., Tauber, A.A., and Tejedor, M.F. 2020. Juvenile glyptodont (Mammalia, Cingulata) from the Miocene of Patagonia, Argentina: Insights into mandibular and dental characters. Journal of Vertebrate Paleontology 40 (1): e1768398. Crossref
González-Ruiz, L.R., Ciancio, M.R. Martin, G.M., and Zurita, A.E. 2015. First record of supernumerary teeth in Glyptodontidae (Mammalia, Xenarthra, Cingulata). Journal of Vertebrate Paleontology 35: e885033. Crossref
Gray, J.E. 1869. Catalogue of Carnivorous, Pachydermatous and Edentate Mammalia in the British Museum. 398 pp. British Museum (Natural History), London.
Hoffstetter, R. 1958. Xenarthra. In: J. Piveteau (ed.), Traité de paléontologie. Vol. 6, 535–636. Masson & Co, Paris.
Hofreiter, M., Poinar, H.N., Spaulding, W.G., Bauer, K., Martin, P.S., Possnert, G., and Paabo, S. 2000. A molecular analysis of ground sloth diet through the last glaciation. Molecular Ecology 9: 1975–1984. Crossref
Hynek, S.A. 2011. Mio-Pliocene Geology of the Southem Puna Plateau Margin, Argentina. 329 pp. Ph.D. Thesis. University of Utah, Salt Lake City.
Hynek, S.A., Passey, B.H., Prado, J.L., Brown, F.H., Cerling, T.E., and Quade, J. 2012. Small mammal carbon isotope ecology across the Miocene–Pliocene boundary, northwestern Argentina. Earth and Planetary Science Letters 321: 177–188. Crossref
Illiger, C. 1811. Prodromus systematis mammalium et avium; additis terminis zoographicis utriusque classis, eurumque versione germanica. 301 pp. C. Safeld, Berlin. Crossref
Janis, C.M. 1988. Estimation of tooth volume and hypsodonty indices in ungulate mammals, and the correlation of these factors with dictary preference. Mémoires du Muséum National d’Histoire Naturelle, série C 53: 367–387.
Koken E. 1888. Eleutherocercus, ein neuer Glyptodont aus Uruguay. Anhang zu den Abhandlungen Königlichen Akademie der Wissenschaften zu Berlin 1888: 1–28.
Kraglievich, L. 1932. Nuevos apuntes para la geología y paleontología uruguayas. Anales del Museo de Historia Natural de Montevideo 3: 257–320.
Krmpotic, C.M., Ciancio, M.R., Barbeito, C., Mario, R.C. and Carlini, A.A. 2009. Osteoderm morphology in recent and fossil euphractine xenarthrans. Acta Zoologica 90: 339–351. Crossref
Latorre, C., Quade, J., and Mclntosh, W.C. 1997. The expansion of C4 grasses and global change in the table Miocene: stable isotope evidence from the Americas. Earth and Planetary Science Letters 146: 83–96. Crossref
Lindsey, E.L., López Reyes, E.X., Matzke, G.E., Rice, K.A., and McDonald, G.H. 2020. A monodominant late-Pleistocene megafauna locality from Santa Elena, Ecuador: Insight on the biology and behavior of gigant ground sloths. Palaeogeography, Palaeoclimatology, Palaeoecology 544: 109599. Crossref
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 824 pp. Laurentii Salvii, Holmiae. Crossref
Lydekker, R. 1895. Contributions to a knowledge of the fossil vertebrates of Argentina. The extinct edentates of Argentina. Anales del Museo de La Plata, Paleontología Argentina 3: 1–118.
Lund, P.W. 1839. Blik paa Brasiliens dyreverden för sidste jordomvaeltning. Anden afhandling: Pattedyrene (Lagoa Santa d. 16de novbr. 1837). Det kongelige Danske Videnskabernes Selskabs naturvidenskabelige og mathematiske Afhandlinger 8: 61–144.
MacFadden, B.J. 2005. Diet and habitat of toxodont megaherbivores (Mammalia, Notoungulata) from the late Quaternary of South and Central America. Quaternary Research 64: 113–124. Crossref
MacFadden, B.J., Anaya, F., and Argollo, J. 1993. Magnetic stratigraphy of Inchasi: a Pliocene mammal-bearing locality from the Bolivian Andes deposited just before the Great American Interchange. Earth and Planetary Science Letters 114: 229–241. Crossref
MacFadden, B.J., Cerling, T.E., Harris, J.M., and Prado, J. 1999. Ancient latitudinal gradients of C3/C4 grasses interpreted from stable isotopes of New Word Pleistocene horse (Equus) teeth. Global Ecology and Biogeography 8: 137–149. Crossref
Maddison, W.P. and Maddison, D.R. 2018. Mesquite: a Modular System for Evolutionary Analysis. Version 3.04. Available at http://mesquiteproject.org
Marshall, L.G. and Patterson, B. 1981. Geology and geochronology of the mammal-bearing Tertiary of the Valle de Santa María and Río Corral Quemado, Catamarca Province, Argentina. Fieldiana Geology 9: 1–80. Crossref
Marshall, L.G., Butler, R.F., Drake, R.E., Curtis, G.H., and Tedford, R.H. 1979. Calibration of the Great American Interchange. Science 204: 272–279. Crossref
McDonald, G.H. 2005. Paleoecology of extinct Xenarthrans and the Great American Biotic Interchange. Bulletin of the Florida Museum of Natural History 45: 319–340.
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals Above the Species Level. 631 pp. Columbia University Press, New York.
Melo-Franca, L., Asevedo, L., Dantas, M.A.T., Bocchiglieri, A., Dos Santos Avilla, L., Lopes, R.P., and Da Silva, J.L.L. 2015. Review of feeding ecology data of late Pleistocene mammalian herbivores from South America and discussion on niche differentiation. Earth-Science Reviews 140: 158–165. Crossref
Mitchell, K. J., Scanferla, A., Soibelzon, E., Bonini, R., Ochoa, J., and Cooper, A. 2016. Ancient DNA from the extinct South America giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontiae) reveals that glyptodonts evolved from Eocene armadillos. Molecular Ecology 25: 3499–3508. Crossref
Moreno, F.P. 1888. Informe preliminar de los progresos del Museo La Plata, durante el primer semestre de 1888. Boletín del Museo de La Plata 2: 1–35.
Moreno, F.P. and Mercerat, A. 1891. Exploración arqueológica de la Provincia de Catamarca: Paleontología. Revista del Museo de La Plata 1: 1–71.
Muruaga, C.M., 2001. Estratigrafía y desarrollo tectosedimentario de los sedimentos terciarios en los alrededores de la Sierra de Hualfín, borde suroriental de la Puna, Catamarca, Argentina. Latin American Journal of Sedimentology and Basin Analysis 8: 27–50.
Nuñez-Blasco, A., Zurita, A.E., Miño-Boilini, A.R., and Bonini, R.A. 2020. Chronologic distribution of the Glyptodontidae (Mammalia, Cingulata) recorded in the Villavil–Quillay Basin, Catamarca, Argentina. In: V. Crespo and P. Citton (eds.), 2nd Palaeontological Virtual Congress, 187. Valencia.
Oliveira, E.V. 1992. Mamíferos fósseis do Quaterário do Estado do Rio Grande do Sul, Brasil. 118 pp. M.Sc. Thesis, Programa de Pós-Graduação em Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre.
Osborn, H.F., 1903. Glyptotherium texanum, a new glyptodont, from the lower Pleistocene of Texas. Bulletin of the American Museum of Natural History 19: 491–494.
Owen, R. 1845. Descriptive and illustrated catalogue of the fossil organic remains of Mammalia and Aves contained in the Museum of the Royal College of Surgeons of London, England. 391 pp. Richard and John E. Taylor, London.
Paula-Couto, C. 1979. Tratado de paleomastozoologia. 590 pp. Academia Brasileira de Ciências. Rio de Janeiro.
Perea, D. 2005. Pseudoplohophorus absolutus n. sp. (Xenarthra, Glyptodontidae), variabilidad en Sclerocalyptinae y redefinición de una biozona del Mioceno Superior de Uruguay. Ameghiniana 42: 175–190.
Pereira, J.C., Lopes, R.P., and Kerber, L. 2012. New remains of late Pleistocene mammals from the Chuí Creek, Southern Brazil. Revista Brasileira de Paleontologia 15: 228–239. Crossref
Politis, G.G., Messineo, P.G., Stafford, T.W., and Lindsey, E.L. 2019. Campo Laborde: a late Pleistocene gigant ground sloth kill and butchering site in the Pampas. Science Advances 5 (3): 2–10. Crossref
Pomi, L.H. 2008. Tafonomía y paleoecología de los Glyptodontidae (Mammalia) pleistocénicos de la provincia de Buenos Aires, Argentina. In: Congreso Latinoamericano de Paleontología de Vertebrados 3, 2008. Resumenes, 200. Neuquén,
Porpino, K.O., Fernicola, J.C., and Bergqvist, L.P. 2010. Revisiting the intertropical Brazilian species Hoplophorus euphractus (Cingulata, Glyptodontoidea) and the phylogenetic affinities of Hoplophorus. Journal of Vertebrate Paleontology 30: 911–927. Crossref
Quiñones, S.I., de Los Reyes, M., Zurita, A.E., Cuadrelli, F., Miño-Boilini, A.R., and Poiré, D. 2020. Neosclerocalyptus Paula Couto (Xenarthra, Glyptodontidae) in the late Pliocene–earliest Pleistocene of the Pampean region (Argentina): its contribution to the understanding of evolutionary history of Pleistocene glyptodonts. Journal of South American Earth Sciences 103: 102701. Crossref
Quiñones, S.I., Miño-Boilini, A.R. Zurita, A.E. Contreras, S., Luna, C.A. Candela, A.M. Camacho, M., Ercoli, M.D., Solís, N., and Brandoni, D. 2019. New records of Neogene Xenarthra (Mammalia) from eastern Puna (Argentina): diversity and biochronology. Journal of Paleontology 93: 1258–1275. Crossref
Riggs, E.S. and Patterson, B. 1939. Stratigraphy of Late Miocene and Pliocene deposits of the Province of Catamarca (Argentina) with notes on the faunae. Physis 14: 143–162.
Rovereto, C. 1914. Los estratos araucanianos y sus fósiles. Anales del Museo Nacional de Historia Natural de Buenos Aires 25: 1–247.
Sasso, A.M. 1997. Geological Evolution and Metallogenic Relationships of the Farallón Negro Volcanic Complex, NW Argentina. 268 pp. Ph.D. Thesis, Queens University, Kingston.
Scillato-Yané, G.J. 1986. Los Xenarthra fósiles de Argentina (Mammalia, Edentata). Actas del IV Congreso Argentino de Paleontología y Bioestratigrafía 2: 151–155.
Simpson, G.G. 1947 A Miocene glyptodont from Venezuela. American Museum Novitates 1368: 1–10
Soibelzon, E., Miño-Boilini, A.R., Zurita, A.E., and Krmpotic, C.M. 2010. Los Xenarthra (Mammalia) del Ensenadense (Pleistoceno inferior a medio) de la Región Pampeana (Argentina). Revista Mexicana de Ciencias Geológicas 27: 449–469.
Soibelzon, L.H., Zamorano, M., Scillato-Yané, G. J., Piazza, D., Rodríguez, S., Soibelzon, E., and Tonni, E.P. 2012. Un Glyptodontidae de gran tamaño en el Holoceno temprano de la región Pampeana, Argentina. Revista Brasileira de Paleontología 15: 105–112. Crossref
Tomassini, R.L., Montalvo, C.I., Deschamps, C.M., and Manera, T. 2013. Biostratigraphy and biochronology of the Monte Hermoso Formation (early Pliocene) at its type locality, Buenos Aires Province, Argentina. Journal of South American Earth Sciences 48: 31–42. Crossref
Toriño, P. and Perea, D. 2008. Tertiary Glyptodontids from Uruguay. I: Glyptatelinae, Doedicurinae and Glyptodontinae. In: III Congreso Latinoamericano de Paleontología de Vertebrados. Libro de resúmenes 250: 22–25. Neuquén.
Toriño, P. and Perea, D. 2018. New contributions to the systematic of the “Plohophorini” (Mammalia, Cingulata, Glyptodontidae) from Uruguay. Journal of South American Earth Sciences 86:410–430. Crossref
Ubilla, M., Perea, D., Aguilar, C.G., and Lorenzo, N. 2004. Late Pleistocene vertebrate from northern Uruguay: tools for biostratigraphic, climatic and environmental reconstruction. Quaternary International 114: 129–142. Crossref
Varela, L., Tambusso, P.S., Patiño, S.J., Di Giacomo, M., and Fariñaa, R.A. 2018. Potential distribution of fossil xenarthrans in South America during the late Pleistocene: co-occurrence and provincialism. Journal of Mammalian Evolution 25: 539–550. Crossref
Villarroel, C. 1983. Descripción de Asterostemma? acostae, nueva especie de propalaehoplophorino (Glyptodontidae, Mammalia) del Mioceno de La Venta, Colombia. Geologia Norandina 7: 29–34.
Vizcaíno, S.F., Bargo, M.S., Kay, R.F., Fariña, R.A., Di Giacomo, M., Perry, J.M., Precosti, F.J., Toledo, N., Cassini, G.H., and Fernicola, J.C. 2010. A baseline paleoecological study for the Santa Cruz Formation (late–early Miocene) at the Atlantic coast of Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 292: 507–519. Crossref
Vizcaíno, S.F., Cassini, G.H., Fernicola, J.C., and Bargo, M.S. 2011. Evaluating hábitats and feeding habits through ecomorphological features in glyptodonts (Mammalia, Xenarthra). Ameghiniana 48: 305–319. Crossref
Vizcaíno, S.F., Cassini, G.H., Toledo, N., and Bargo, M.S. 2012. On the evolution of large size in mammalian herbivores of Cenozoic. In: D. Bruce, B.D. Patterson, and L.P. Costa (eds.), Bones, Clones, and Biomes: the History and Geography of Recent Neotropical Mammals. 432 pp. The University of Chicago Press, Chicago.
Zachos, J.C., Shackleton, N.J., Revenaugh, J.S., Pälike, H., and Flower, B.P. 2001. Climate response to orbital forcing across the Oligocene–Miocene boundary. Science 292: 274–278. Crossref
Zachos, J.C., Dickens, G.R., and Zeebe, R.E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. Crossref
Zamorano, M. and Brandoni, D. 2013. Phylogenetic analysis of the Panochthini (Xenarthra, Glyptodontidae), with remarks on their temporal distribution. Alcheringa 37: 442–45. Crossref
Zárate, M.A., Schultz, P.H., Blasi, A., Heil, C., King, J., and Hames, W. 2007. Geology and geochronology of type Chasicoan (late Miocene) mammal-bearing deposits of Buenos Aires (Argentina). Journal of South American Earth Sciences 23: 81–90. Crossref
Zurita, A.E. 2002. Nuevo gliptodonte (Mammalia, Glyptodontoidea) del Cuaternario de la provincia de Chaco, Argentina. Ameghiniana 39: 175–182.
Zurita, A.E. 2007. Sistemática y evolución de los Hoplophorini (Xenarthra, Glyptodontidae, Hoplophorinae. Mioceno tardío-Holoceno temprano). Importancia bioestratigráfica, paleobiogeográfica y paleoambiental. 376 pp. Ph.D. Thesis, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata.
Zurita, A.E., González-Ruiz, L.R., Gómez-Cruz, A.J., and Arenas-Mosquera, J.E. 2013. The most complete known Neogene Glyptodontidae (Mammalia, Xenarthra, Cingulata) from northern South America: taxonomic, paleobiogeographic, and phylogenetic implications. Journal of Vertebrate Paleontology 33: 696–708. Crossref
Zurita, A.E, Miño-Boilini A.R., Soibelzon, E., Carlini, A.A., and Paredes Ríos, F. 2009. The diversity of Glyptodontidae (Xenarthra, Cingulata) in the Tarija Valley (Bolivia): systematic, biostratigraphic and paleobiogeographic aspects of a particular assemblage. Neues Jahrbuch fur Geologie und Paläontologie Abhandlungen 251: 225–237. Crossref
Zurita, A.E., Taglioretti, M., De los Reyes, M., Cuadrelli, F., and Poire, D. 2016. Regarding the real diversity of Glyptodontidae (Mammalia, Xenarthra) in the late Pliocene (Chapadmalalan age/stage) of Argentina. Anais da Academia Brasileira de Ciências 88: 809–827. Crossref
Zurita, A.E., Taglioretti, M., De los Reyes, M., Oliva, C., and Scaglia, F. 2014. First Neogene skull of Doedicurinae (Xenarthra, Glyptodontidae): morphology and phylogenetic implications. Historical Biology 28: 423–432. Crossref
Zurita, A.E., Zamorano, M., Scillato-Yané, G.J., Iriondo, S.F.M, and Gillette, D.D. 2017. A new species of Panochthus Burmeister (Xenarthra, Cingulata, Glyptodontidae) from the Pleistocene of the Eastern Cordillera, Bolivia. Historical Biology 29: 1076–1088. Crossref
Acta Palaeontol. Pol. 66 (Supplement to 3): 79–99, 2021
https://doi.org/10.4202/app.00824.2020