


New insights into the origin and relationships of blastoid echinoderms
CHRISTOPHER R.C. PAUL
Paul, C.R.C. 2021. New insights into the origin and relationships of blastoid echinoderms. Acta Palaeontologica Polonica 66 (1): 41–62.
“Pan-dichoporites” (new informal term) is proposed to unite Cambrian blastozoans, such as Cambrocrinus, Ridersia, and Sanducystis, glyptocystitoid and hemicosmitoid rhombiferans, coronates, blastoids, and Lysocystites. Pan-dichoporite ambulacra have double biserial main axes with brachiole facets shared by pairs of floor (glyptocystitoids), side (blastoid) or trunk (hemicosmitoids, coronates) plates. These axial plates are the first two brachiolar plates modified to form the ambulacral axes. In glyptocystitoids the first brachiole facet in each ambulacrum is shared by an oral and another plate. Hence, these are also two modified brachiolar plates and part of the axial skeleton under the Extraxial Axial Theory (EAT). Pan-dichoporites are also characterized by thecae composed of homologous plate circlets. The unique glyptocystitoid genus Rhombifera bears ambulacral facets on five radial plates, which alternate with five orals. The oral area of Lysocystites (blastoid sensu lato) is very similar, which suggests that rhombiferan radials are homologous with “ambulacrals” of Lysocystites and hence with blastoid lancet plates. This implies derivation of blastoids from glyptocystitoids and suggests that blastoid and coronate radials and deltoids are homologous with rhombiferan infralaterals and laterals. Thus, homologous plate circlets occur in all pan-dichoporites, which strengthens the validity of a pan-dichoporite clade. Under Universal Elemental Homology (UEH), deltoids were homologized with rhombiferan orals, but this is inconsistent with the EAT. Deltoids bear respiratory pore structures and so are perforate extraxial skeletal plates, whereas rhombiferan orals are axial skeleton. The new plate homologies also explain why only five plates form the oral frames of coronates, blastoids and Lysocystites, whereas glyptocystitoids (except Rhombifera) have six oral frame plates; all glyptocystitoids have only five laterals. Hemicosmitoids arose by paedomorphic ambulacral reduction, but the paedomorphosis also affected the thecal plates and stem. Paedomorphosis poses special problems for cladistic character analysis, since the new characters often appear to be plesiomorphic.
Key words: Blastozoa, Glyptocystitoida, Hemicosmitoida, Coronata, Blastoidea, homology, phylogeny, ontogeny.
Christopher R.C. Paul [glcrcp@bristol.ac.uk], School of Earth Sciences, University of Bristol, BS8 1RJ, UK.
Received 26 September 2020, accepted 9 December 2020, available online 2 March 2021.
Copyright © 2021 C.R.C. Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Understanding the homologies of fossil structures is essential in reconstructing fossil phylogenies or producing natural, evolutionary classifications. Clues to homologies can be derived from morphological or developmental features. This paper adopts a novel approach to the phylogeny of some blastozoan echinoderms. Instead of attempting to recognize distinctive circlets of plates (morphology) and assign them consistent, homologous terms, as under Universal Elemental Homology (Sumrall 2010), it extends the logic of the Extraxial Axial Theory (Mooi and David 1997; David and Mooi 1998) to consider the precise order of development of the ambulacral plates. The ambulacral plates are chosen because they form part of the axial skeleton, are added terminally during growth according to the Ocular Plate Rule (David and Mooi 1998), and their relative positions do not change subsequently. Thus, the ambulacral plates in an adult sea urchin test, or brachioles along a mature blastozoan ambulacrum, can be numbered from mouth to ambulacral tip and we can be sure that the numbering reflects the order in which the plates or brachioles developed. This approach, which is very similar to that advocated by Wright (2015) as “phylogenetic paleo-ontogeny”, proved extremely successful in investigating Lovén’s Law (Paul and Hotchkiss 2020). Not only did that study suggest a plausible mechanism for the origin of Lovén’s Law, but it also confirmed that Lovén’s Law provides the best means of recognizing ambulacral homologies across all classes of pentaradiate echinoderms. The study also provided a solution to the origin of the puzzling “BD different” pattern of primary brachioles in pentaradiate glyptocystitoid rhombiferans. The pattern of plate addition can also provide another test of proposed homologies, in addition to the conjunction test (Patterson 1982, 1988; Sumrall 1997). Plates that are added in a different sequence cannot be homologous (Wright 2015: 573). The approach adopted here is first to consider the basic assumptions behind the method, then to discuss ambulacral growth in glyptocystitoids and then in all other blastozoans with double biserial ambulacra, hereafter pan-dichoporites. The results suggest a novel interpretation of the homologies of thecal plates in blastoids and their relatives, which confirms their affinities with dichoporite rhombiferans.
Institutional abbreviations.—BMNH, Natural History Museum, London, UK; GIT Department of Geology, TalTech, Tallin, Estonia; MPZ, Museo de Ciencias Naturales, Universidad de Zaragoza, Spain; NMW, National Museum of Wales, Cardiff, UK; ROM Royal Ontario Museum, Ottawa, Canada; SM, Sedgwick Museum, Cambridge, UK; USNM, United States National Museum, Washington DC, USA.
Other abbreviations.—A–E, ambulacra A–E; B, BB, basal plate(s); EAT, Extraxial Axial Theory; D, DD, deltoid(s); IL, ILL, infra-lateral(s); L, LL, lateral(s); OPR, Ocular Plate Rule; R, RR, radial(s); RWV, Radial Water Vessel; UEH, Universal Elemental Homology.
The Extraxial Axial Theory and other basic assumptions
Extraxial Axial Theory.—The EAT (Mooi and David 1997; David and Mooi 1998) derives from the embryology of echinoderms and distinguishes two major body wall components. The axial skeleton is associated with the water vascular system and derived from the larval rudiment. During growth axial plates are added according to the Ocular Plate Rule. Extraxial plates derive from the non-rudiment parts of the larval body and may be added anywhere in the skeleton. The extraxial skeleton is further subdivided into an adoral perforate portion, which in many blastozoans represents the part of the theca with respiratory pore-structures, and an imperforate extraxial skeleton, which always includes the stem and may include parts of the theca as well.
Ocular Plate Rule.—The OPR (Mooi et al. 1994) refers to the pattern of plate addition in the corona of echinoids. All coronal plates are added adjacent to the ocular plates and once formed do not change their positions relative to each other. Thus, each ocular adds four columns of plates, the central pair of alternating ambulacral plates plus a single column of interambulacral plates on either side of the ambulacra. Mooi et al. (1994: fig. 1) referred to these four columns of plates as a growth zone (Fig. 1). The first major assumption of this paper is that growth zones are a fundamental feature of the axial skeletons of echinoderms (Figs. 1, 2).
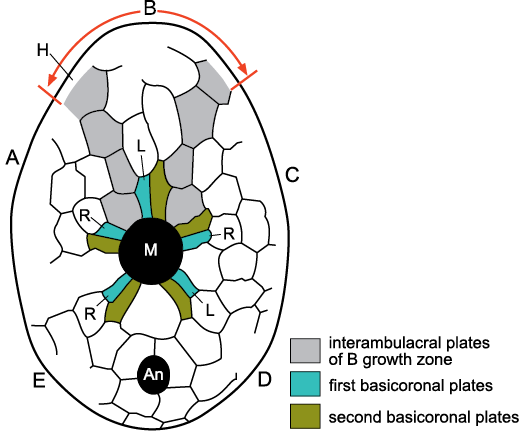
Fig. 1. Growth zone of ambulacrum B in the irregular echinoid Echinocyamus bisexus Kier, 1968, Eocene, Georgia, USA. An echinoid growth zone consists of four columns of plates, two central ambulacral columns the plates of which alternate, flanked by single columns of interambulacral plates (light grey shading). All the plates of a growth zone are added adjacent to the ocular plate at the aboral tip of each ambulacrum (on the opposite surface of the echinoid). The first two ambulacral plates to form during growth, the basicoronal plates adjacent to the mouth (M), express Lovén’s Law. A, B, C, D, E, Carpenter’s (1884, 1891) ambulacra; An, anus; H, hydropore (madreporite) on the opposite surface of the echinoid; L, left; R, right, designate ambulacral columns; M, mouth. Modified from Paul and Hotchkiss 2020: fig. 3.
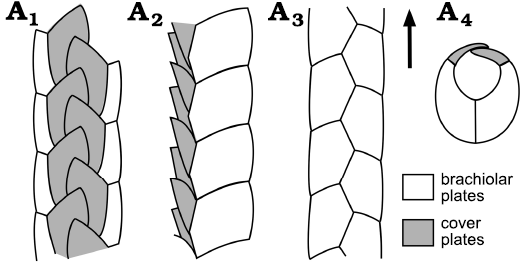
Fig. 2. Growth zone as seen in a brachiole of the early Cambrian blastozoan, Kinzercystis durhami Sprinkle, 1973, early Cambrian, Pennsylvania, USA. The brachiole consists of a central alternating pair of columns of floor (brachiolar) plates, with a single series of cover plates along each lateral margin. Plates are added at the distal tip according to the ocular plate rule. Ventral (A1), right lateral (A2), and dorsal (A3) views; cross section (A4). Arrow indicates growth direction. Redrawn and simplified from Sprinkle 1973: 16, fig. 5A.
In echinoids the addition of ambulacral plates during growth is intimately associated with the branching of the radial water vessel (RWV), since each ambulacral plate contains a pore pair for the tube feet (e.g., Mooi et al. 2005: 545, fig. 2). Branching of the RWV is sometimes regarded as terminal, but in echinoids the very tip of the RWV emerges through the ocular pore. Thus, although the ambulacral plates are added terminally branching of the RWV is subterminal (Fig. 3). This makes no difference to the order of appearance of axial skeletal elements, but has profound consequences for the pattern of branching seen in echinoderm classes with branched ambulacra (Paul and Hotchkiss 2020: fig. 8). A second fundamental assumption of this paper is that RWVs were present in all echinoderm ambulacra and that in all but crinozoans the RWVs branched subterminally.
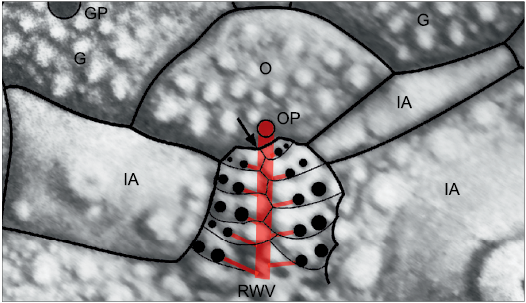
Fig. 3. Subterminal branching of the radial water vessel in a Recent cidarid echinoid Eucidaris metularia (Lamarck, 1816). Black arrow indicates the point where the next branch of the radial water vessel would arise, whereas the tip of the radial water vessel emerges through the ocular pore. Thus, although ambulacral and interambulacral plates are added to the corona terminally, branches of the radial water vessel to the tube feet are subterminal. G, genital plate; GP, genital pore; IA, interambulacral plate; O, ocular plate; OP, ocular pore; RWV, radial water vessel (internal).
Lovén’s Law.—The OPR provides a basis for reconstructing the order of plate addition in the axial skeleton of fossil echinoderms. Application of this approach to the recognition of Lovén’s Law (Lovén 1874) has recently convinced me that Lovén’s Law provides the best basis for recognizing ambulacral homologies in fossil echinoderms (Paul and Hotchkiss 2020). Lovén (1874) first noticed that the basicoronal plates of irregular echinoids differed in size and that the larger plate occurred on opposite sides of individual ambulacra around the mouth. He used an ambulacral notation using Roman numerals clockwise in oral view starting immediately clockwise of the anus. He also assigned letters a and b to the left and right columns of plates in each ambulacrum viewed in the growth direction. Thus, a shorthand summary of Lovén’s Law in echinoids is that the larger basicoronal plates occur in columns: Ia, IIa, IIIb, IVa, and Vb. Later, David et al. (1995) showed that “Lovén’s Law” results from the sequence of addition of ambulacral plates. In particular, they showed that the smaller basicoronal plate was always the first plate to develop in each ambulacrum. Paul and Hotchkiss (2020) thought that a more generally applicable and unambiguous statement of Lovén’s Law could be derived by selecting the first ambulacral plates, using Carpenter’s notation for the ambulacra (letters A–E; Carpenter 1884, 1891) and describing the positions as either left (L) or right (R), again as viewed in the growth direction. Thus, an alternative shorthand statement of Lovén’s Law is that the first plates to form in each ambulacrum occur:
AR, BL, CR, DL, and ER (Figs. 1, 4), with E and A the unique pair of ambulacra that are both adjacent and identical. Paul and Hotchkiss (2020: 5 fig. 4) went on to show that Lovén’s Law arose during the transition from a triradiate echinoderm with a 1-1-1 pattern of ambulacra, to a pentaradiate echinoderm with a 2-1-2 ambulacral pattern (Paul and Hotchkiss 2020: fig. 4; reproduced here as Fig. 4). Thus, it appears that Lovén’s Law is an inevitable consequence of an evolutionary transition early in the history of the echinoderms. Another consequence is that Lovén’s Law has a constant relationship to the 2-1-2 pattern of ambulacra seen in many early echinoderms, which always equates to DE-A-BC of Carpenter (1884, 1891).
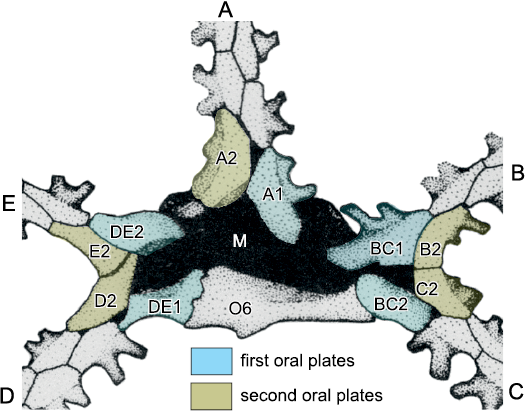
Fig. 4. Origin of Lovén’s Law in echinoderms illustrated by the oral plating in edrioasteroid Walcottidiscus Bassler, 1935 (USNM 376690), early Cambrian, locality unknown. A–E, five ambulacra arranged in a 2-1-2 pattern so that three radiate from the mouth, shared DE, A, and shared BC. A1, BC1, and DE1 represent the first ambulacral plates in the three primary ambulacra. A2, BC2, and DE2 represent the second ambulacral plates. When the shared ambulacra bifurcated to give five there was no other position in which the new ambulacral plates could be added than in the acute angle between the dividing primary rays. Thus, the new plates were B2, C2, D2, and E2. Once all five ambulacra reached the stage of having two floor plates, the positions of the first plates obey Lovén’s Law as restated by Paul and Hotchkiss (2020), i.e., AR, BL, CR, DL, ER, that is the plates labelled A1, BC1, BC2, DE1, and DE2. Redrawn and modified from Paul and Smith 1984: 454, fig. 7A.
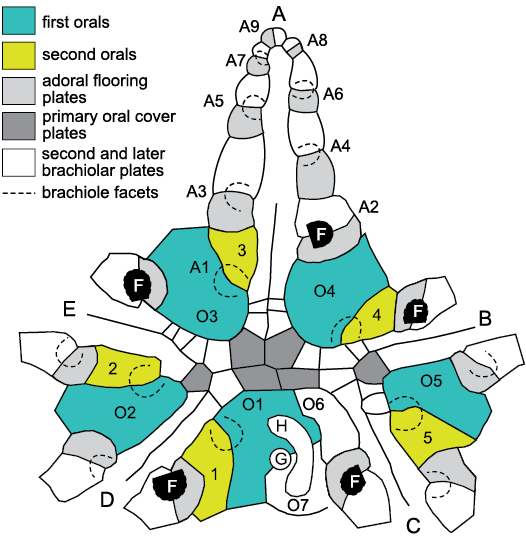
Fig. 5. Plating in the oral area and in ambulacrum A of the glyptocystitoid Lepadocystis moorei (Meek, 1871), Late Ordovician, Ohio, USA, showing interpretation of ambulacral growth and plate homologies. A–E, Carpenter (1884, 1891) ambulacra. Brachiole facets (F) are numbered (A1–A9) in ambulacrum A in the order in which they formed during growth, which cannot be changed after initial development. Each brachiole facet is developed on a pair of floor plates, the adoral of which is shaded pale grey. These are the first brachiolar plates to form during growth and become modified as floor plates. O1–O5, first and 1–5, second oral plates sharing the very first brachiole facet in each ambulacrum. Thus, these are also first brachiolar plates modified as orals. The first facets in each ambulacrum branch to the left as viewed in the growth direction (away from the mouth), but the second (black, F) obey Lovén’s Law as restated by Paul and Hotchkiss (2020), AR, BL, CR, DL, ER, where A–E represent Carpenter’s ambulacra and L and R represent left and right of the ambulacrum. This is because the ambulacra exhibit the “BD different” pattern of primary brachioles in which ambulacra B and D have the first two brachioles on the left, whereas ambulacra A, C and E have only the first brachiole left. Six primary oral cover plates (dark grey) and the two additional orals, O6 and O7, are identified. O1 and O7 always share the gonopore (G) and hydropore (H) in glyptocystitoid rhombiferans. Redrawn from Paul and Hotchkiss 2020: fig. 6.1 and Kesling and Mintz 1961: fig. 1.
The basic method of this paper is to apply the logic of both the Ocular Plate Rule and the pattern of Lovén’s Law to the interpretation of oral and ambulacral plating in glyptocystitoid and other advanced blastozoans to establish basic principles. Lovén’s Law provides evidence for the homology of ambulacra, while the Ocular Plate Rule provides evidence for the homology of individual plates within ambulacra (Fig. 5).
Rival hypotheses.—In evaluating rival hypotheses their relative “explanatory power” is considered significant. For example, both the triradiate Helicoplacus Durham and Caster, 1963, and the pentaradiate Helicocystis Smith and Zamora, 2013, have spiral ambulacra and are closely related (Smith and Zamora 2013: 5, fig. 4). Thus, either Helicocystis is derived and acquired two ambulacra (Smith and Zamora 2013) or Helicoplacus is derived by the loss of two ambulacra (Sprinkle and Wilbur 2005; Wilbur 2005). There is no a priori reason to favour either hypothesis. Nevertheless, if Helicoplacus is derived, the helicoplacoids become an early, specialized offshoot from the main evolutionary history of echinoderms and nothing else is explained. Alternatively, if Helicocystis is derived, this provides a plausible mechanism to explain the 2-1-2 pattern of ambulacra seen in many early pentaradiate echinoderms (Sprinkle 1973: 43, fig. 16A). Furthermore, considering the precise order of plate addition in biserial ambulacra through the transition from a 1-1-1 to a 2-1-2 ambulacral pattern provides an explanation for the development of Lovén’s Law (Paul and Hotchkiss 2020: 5, fig. 4). Equally, the order of addition of brachioles in the ambulacra of pentaradiate glyptocystitoids during the transition from three to five ambulacra results inevitably in the otherwise puzzling “BD different” pattern of primary brachioles (Paul 2015a; Paul and Hotchkiss 2020: 8, fig. 8). Finally, during the ontogeny of living crinoids at the cystidean stage the vestibule, which overlies the position of the juvenile mouth, migrates from a lateral to a terminal position opposite the stem (Hyman 1955: 79, fig. 32). This is analogous to the change in position of the mouth from about mid-lateral in Helicoplacus to terminal opposite the stem in Helicocystis. I need go no further. The hypothesis that the pentaradiate Helicocystis is derived from the triradiate Helicoplacus explains many additional observations for which there is no alternative explanation. It is both the preferable hypothesis and another basic assumption of this paper.
I accept that there is no embryological evidence for the triradiate stage in echinoderm evolution (Richard Mooi, personal communication 2020), yet early Cambrian helicoplacoids were triradiate and ancestral to the pentaradiate spiral Helicocystis according to Smith and Zamora (2013: fig. 4). Furthermore, the supposed transition from triradiate to pentaradiate echinoderms produced the “BD different” pattern of primary brachioles in pentaradiate glyptocystitoids, yet it is the second brachioles that exhibit Lovén’s Law. In echinoids Lovén’s Law is evident in the first pair of basicoronal plates. The simplest explanation of this difference is that the homologues of the blastozoan first brachioles and associated structures are missing in echinoids. Perhaps during this process evidence for a triradiate stage was also lost. Something similar happened in callocystitids, where many Wenlock (Silurian) and later genera lose the “BD different” pattern and have four ambulacra (B–E) all with only the first brachiole to the left.
Summary of basic assumptions.—According to the EAT, axial skeletal elements are added terminally and their relative positions are fixed throughout life. Radial water vessels were present in blastozoan ambulacra, they branched subterminally, and again their relative positions were subsequently fixed. Thecal plates which bear pore-structures form part of the perforate extraxial skeleton.
Early evolution of echinoderms involved a transition from a triradiate 1-1-1 ambulacral pattern to a pentaradiate 2-1-2 pattern. This resulted in both Lovén’s Law and the 2-1-2 = DE-A-BC interpretation of ambulacral homologies, using Carpenter’s alphabetical notation for ambulacra. It also explains the “BD different” pattern of primary brachioles in glyptocystitoids. An evolutionary tree similar to that presented by Smith and Zamora (2013: fig. 4) is accepted for early Cambrian echinoderms.
Key taxa
This paper considers only early blastozoan echinoderms with brachioles arranged alternately such that the main ambulacral axis consists of a double biserial set of floor, side or trunk plates (Fig. 6E). The main taxa involved were discussed by Sumrall and Waters (2012) and considered to form a clade, referred to here as pan-dichoporites. The paper does not attempt to identify all possible pan-dichoporites, but does consider a few Cambrian genera that appear to be stem pan-dichoporites. The principal taxa are glyptocystitoid and hemicosmitoid (= dichoporite) rhombiferans, coronates and blastoids. Key genera are listed in Table 1 and some examples shown in Fig. 6. A brief introduction to their basic morphology is essential before discussing specifics of their axial skeleton and other homologies. This is done in terms of the major body wall components under the EAT.
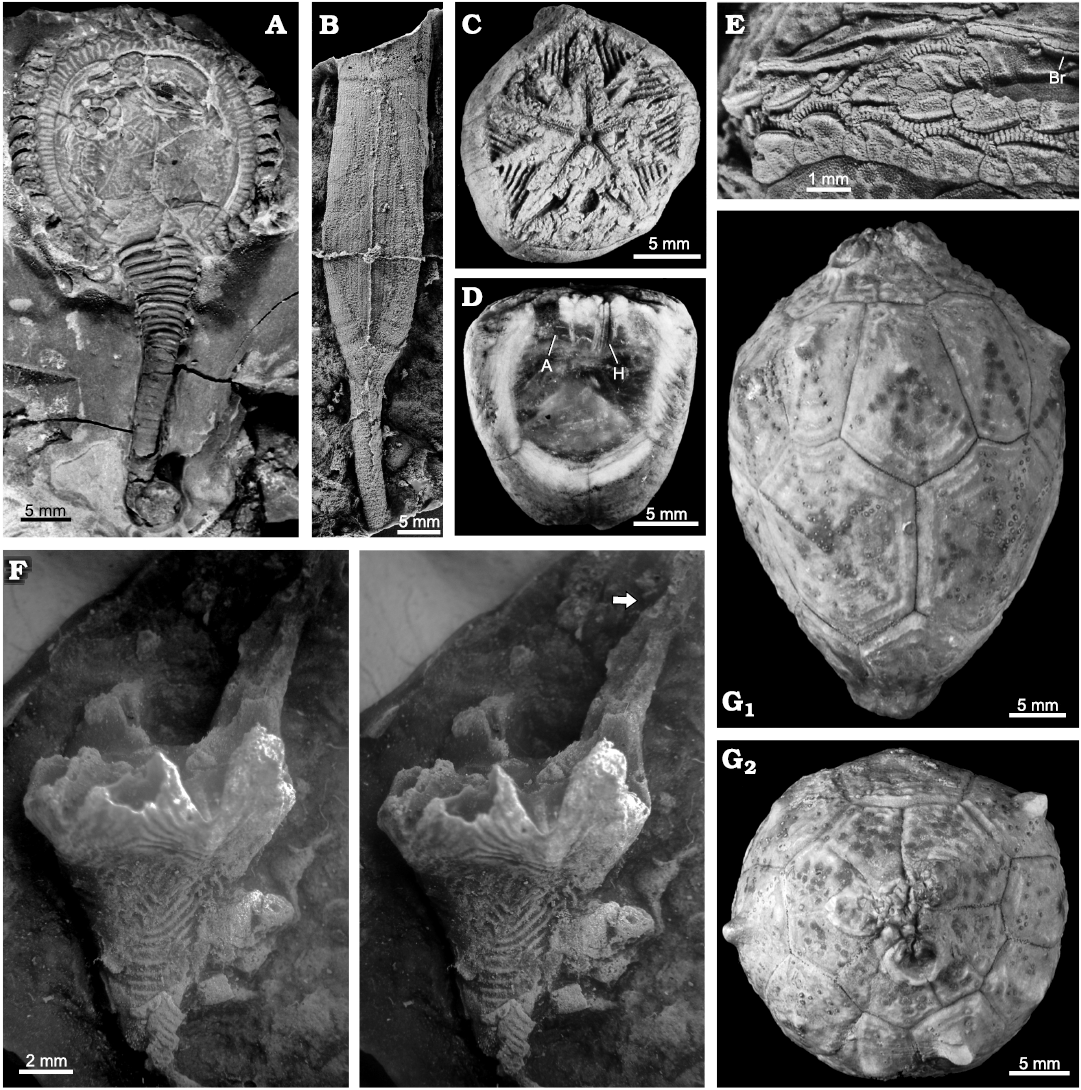
Fig. 6. Examples of pan-dichoporite
blastozoans. A. The
callocystitid Pseudocrinites
bifasciatus Pearce, 1843 (BMNH 40189),
Wenlock (Silurian), West Midlands, UK, showing the
lenticular theca (above) with peripheral ambulacra, the rapidly
tapering proximal stem and more cylindrical distal stem (below)
terminating in a root structure. B.
The rhombiferid Rhombifera
sp. (MPZ 2020/590), Late Ordovician, Zaragoza, Spain, showing the
stem attached to the theca, which includes the basal, infralateral
and base of the lateral plates (above) and one extensive
pectinirhomb. C, D. The fissiculate blastoid Codaster acutus McCoy, 1849, early
Carboniferous, Yorkshire, UK. C.
Oral view of SM E5400 showing the central mouth, five radiating
ambulacra, pectinirhomb-like hydrospires, and circular anus (below).
D. Polished lateral view of SM
E5398 showing basal and radial plates, anus (A) and very thin
hydrospire folds (H). E. One
branched ambulacrum of the callocystitid Callocystites
jewetti Hall, 1852 (ROM 991N), Wenlock
(Silurian), Ontario, Canada, showing the ambulacral
structure. The mouth is to the left. Brachiole facets (lower branch)
are each on a smaller adoral and larger aboral floor plate. Biserial
brachioles alternate along the ambulacrum (Br), so main
ambulacral axis has double biserial floor plates. F.
Stereo images of the coronate Cupulocorona
rugosa Donovan and Paul, 1985 (NMW 2013.8G.65i), Late
Ordovician, Carmarthen, Wales, showing theca shape and extensive
coronal processes (arrow). Top of the small basal plate is
displaced. The rest of the theca up to the tip of the coronal
process is formed by the “radial” plates. Erect ambulacra arose
within the valleys between the coronal processes. G.
The hemicosmitoid Hemicosmites
extraneus Eichwald, 1840 (GIT 398–898), Late
Ordovician, Madise, Estonia. Lateral view (G1)
showing, from below, basal, infralateral, lateral, and radial plate
circlets. Cryptorhomb pores can be seen in the infralateral and
lateral plates. Oral view (G2)
showing three large facets for erect ambulacra.
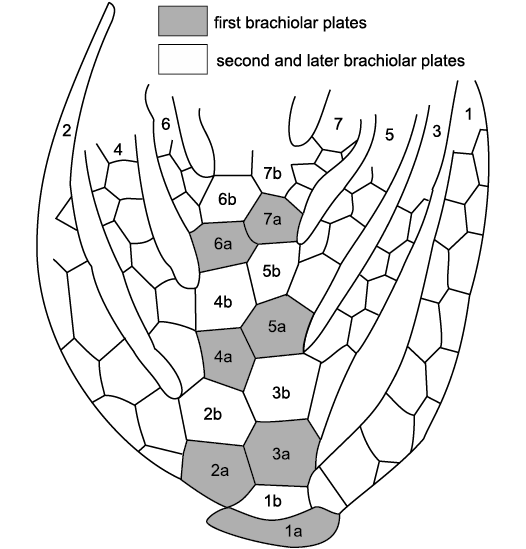
Fig. 7. Structure of the erect ambulacra of the coronate Stephanocrinus angulatus Conrad, 1842, Wenlock (Silurian), New York, USA. Biserial brachioles (1–7) are numbered in order of formation and the first two plates (1a, 1b, and so on) are modified to form the main ambulacral trunk. Note that because this is an aboral view, brachiole 1 is anatomically left but on the right of the figure. Redrawn from Brett et al. 1983: 640, fig. 8.
Table 1. Key genera discussed in the text.
|
Higher taxon |
Family |
Genus |
Age |
References |
|
“outgroup” |
Eocrinidae |
Akadocrinus |
middle Cambrian |
|
|
pan-dichoporites |
Cambrocrinidae |
Cambrocrinus |
late Cambrian |
|
|
Ridersia |
late Cambrian |
|||
|
Sanducystis |
late Cambrian |
|||
|
glyptocystitoids |
Macrocystellidae |
Macrocystella |
Tremadocian |
|
|
Rhombiferidae |
Rhombifera |
Late Ordovician |
||
|
Callocystitidae |
Lepadocystis |
Katian |
||
|
hemicosmitoids |
Caryocrinitidae |
Caryocrinites |
Katian–Silurian |
|
|
Hemicosmitidae |
Hemicosmites |
Middle–Late Odovician |
||
|
Thomacystidae |
Thomacystis |
Katian |
||
|
blastoids sensu lato |
Lysocystitidae |
Lysocystites |
Wenlock |
|
|
coronates |
Stephanocrinidae |
Stephanocrinus |
Katian–Silurian |
|
|
eublastoids |
Macurdablastus |
Katian |
||
|
Codasteridae |
Codaster |
late Carboniferous |
Axial skeleton.—Two basic types of axial skeleton occur in pan-dichoporites: erect pinnate ambulacra, such as those of coronates (Fig. 7) or the hemicosmitoid Caryocrinites Say, 1825, and recumbent pinnate ambulacra such as those of blastoids and the glyptocystitoid Lepadocystis Carpenter, 1891 (Fig. 5). The ambulacra of Caryocrinites were described thoroughly by Sprinkle (1975) and those of the coronate Stephanocrinus by Fay (1962) and Brett et al. (1983). Similar ambulacra may be inferred to have occurred in all other hemicosmitoids and in Lysocystites Miller, 1889, from the occurrence of similar facets to those found in Caryocrinites. Recumbent ambulacra are typical of blastoids (Fig. 6C) and glyptocystitoids (Figs. 6A, E, 8). In the latter they occur in two forms. Plesiomorphic ambulacra form part of the body wall; the “mural ambulacra” of Paul (2017: 594). In the genus Glyptocystites Billings, 1854, and in virtually all genera of the family Callocystitidae, including Lepadocystis, the ambulacra are recumbent on thecal plates (Figs. 5, 6A, E, 8). Recumbent ambulacra are much better known as they are more frequently preserved. In all branched recumbent ambulacra, brachioles arise from facets shared by two floor or side plates (Fig. 6E). During growth the first two plates to form were modified to form the floor or side plates and thereafter normal brachiolar plates were added terminally. Thus, the floor, side or trunk plates are all homologous with each other and may be regarded as the first two brachiolar plates. In glyptocystitoids the very first brachiole facets are shared by an oral plate and a plate Sumrall and Waters (2012: 598, fig. 1) labelled “L” plates (second orals 1–5 of Fig. 5). Extending the interpretation of the other glyptocystitoid floor plates, five of the orals and the corresponding “L” plates are the first two brachiolar plates of each ambulacrum and therefore part of the axial skeleton (Fig. 5).
Perforate extraxial skeleton.—The thecae of all pan-dichoporites are composed of a relatively small number of plates arranged in definite circlets and which are largely homologous. Overall, there is an evolutionary reduction in the number of plate circlets as well as the number of plates in each circlet. The middle Cambrian blastozoan Akadocrinus Prokop, 1962, has a holomeric stem composed of annular columnals, with distinct proximal and distal regions in juveniles. It is possibly the oldest and most primitive pan-dichoporite known, although the Cambrian genus Ubaghsicystis Gil Cid and Dominguez, 2002, is slightly older and may also have differentiated proximal and distal portions to the stem (Samuel Zamora, personal communication 2020). The number of circlets in mature thecae reduced from over ten to just four in blastoids, and the total number of plates from about 100 in mature Akadocrinus (Nohejlová and Fatka 2016: 146) to just 19 in coronates and blastoids. Nohejlová and Fatka’s (2016) study also established that during growth of Akadocrinus new plate circlets were added in an adoral direction, with at least three generations of plates. Thus, the basals are regarded as the oldest plates in the theca. So, pan-dichoporites are discussed on the assumption that taxa with higher numbers of plate circlets and of plates per circlet are likely to be plesiomorphic.
Glyptocystitoids are characterized by five plate circlets, referred to as basals (symbol B, plural BB), infralaterals (IL, ILL), laterals (L, LL), radials (R, RR) plus the orals (O, OO) shown above to be part of the axial skeleton (Figs. 5, 9). Macrocystella Callaway, 1877, the first true glyptocystitoid (Paul 1968a) and many other Ordovician genera had a plate formula of 4BB, 5ILL, 5LL, 6RR, and 7OO. The unique genus Cuniculocystis Sprinkle and Wahlman, 1994, had ten radials, but members of the families Callocystitidae, Cystoblastidae, Echinoencrinitidae, and Rhombiferidae, have only five. Juvenile Lepadocystis (Fig. 9) are known to have added the radial circlet last (Sumrall and Sprinkle 1999).
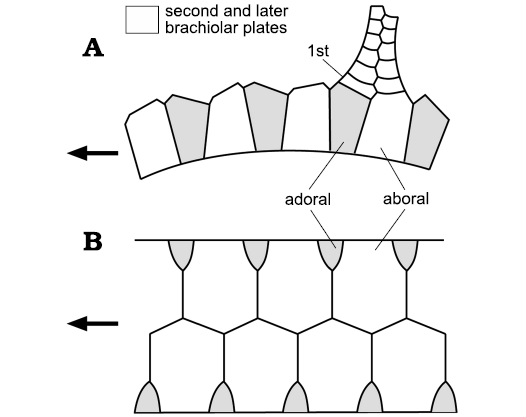
Fig. 8. Structure of the ambulacra and brachioles in the callocystitid Pseudocrinites bifasciatus Pearce, 1843 (SM A.10192), Wenlock (Silurian), Dudley, UK. First plate (1st) in a brachiole lies directly above the adoral floor plates (grey). This suggests floor plates are the first pair of brachiolar plates modified to form the ambulacral axis. Second and subsequent brachiolar plates white. In callocystitids, floor plates are developed on the thecal surface. A. Side view showing the high profile of the ambulacral axis and rapidly tapering brachiole. B. Impressions of ambulacral floor plates on the thecal plates. Arrows point to the mouth. Redrawn and relabelled from Paul 1967a: 326, figs. 17, 18.
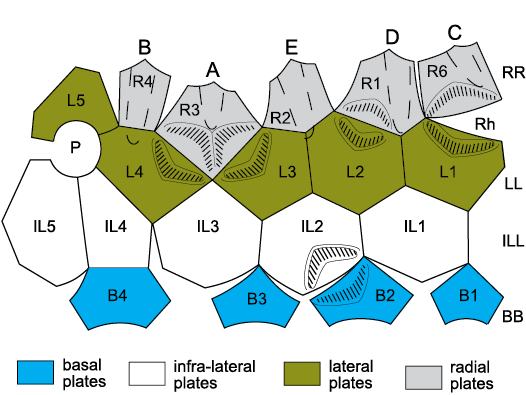
Fig. 9. Thecal plating in the callocystitid Lepadocystis moorei (Meek, 1871), Late Ordovician, Ohio, USA, illustrating typical extraxial plate arrangement in callocystitid and glyptocystitoid rhombiferans. Plates are arranged in four circlets, basals (B, BB), infra-laterals (IL, ILL), laterals (L, LL), and radials (R, RR), plus the orals which are largely axial in origin (see Fig. 5). All glyptocystitoids have four BB, five ILL and LL, and originally six RR. In all callocystitids R5 is absent. In Lepadocystis L5 interrupts the radial circlet and the periproct (P) is surrounded by four plates, IL4, IL5, L4, and L5. Lepadocystis has five, short ambulacra (dashed outlines below letters A–E) on the thecal plates and five pectinirhombs (Rh) across plate sutures B2:IL2, R1:L2, R3:L3, R3:L4, and R6:L1. Currents entered slits in the first mentioned plate and exited from the other plate. For plate homologies see Table 2. Redrawn and relabelled from Kesling 1967: S207, fig. 106.2c.
Hemicosmitoids are assigned to three families, the Hemicosmitidae, Caryocrinitidae, and Thomacystidae, which are also characterized by three or four plate circlets, traditionally referred to by the same names as those of glyptocystitoids. Generally, hemicosmitoids tend to have more plates per circlet. Hemicosmites Buch, 1840, has all four circlets with a formula of 4BB, 6ILL, 9LL, and 9RR (Fig. 10). Caryocrinitids and thomacystids have only three plate circlets, BB, ILL, and LL, and only 8LL. Thomacystis Paul, 1969, has only three BB.
Blastoid thecae have four plate circlets usually referred to as basals, radials, deltoids (D, DD), and lancet plates; the latter are unique to blastoids (Fig. 10). The conventional plating formula is 3BB, 5RR, 6–9DD, and 5 lancets. Two basals are large, the third small, and generally the small basal lies in the AB interray. Exceptionally, in genera such as Diploblastus Fay, 1961, the small basal lies in the DE interray (Sevastopulo 2005: 355). The mouth frame is always composed of five deltoids, but up to four other deltoid plates may occur in the CD inter-ray. The five elongate lancet plates lie radially within each ambulacrum and the side plates that form the axis of the recumbent ambulacrum lie on the lancets.
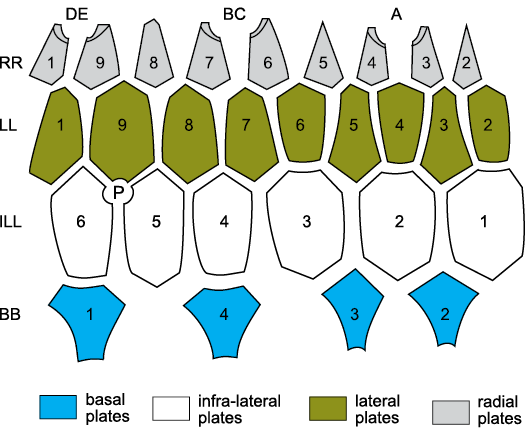
Fig. 10. Standard thecal plate diagram for the
hemicosmitoid Hemicosmites
spp. The theca consists of four circlets, basals (B, BB),
infra-laterals (ILL), laterals (LL), and radials (RR). In Hemicosmites spp. there are four BB, six
ILL, nine LL and nine RR. Hemicosmites
spp. have only three erect ambulacra (A, shared BC, and shared DE)
on facets developed on plates R4:R5, R7:R8, and R1:R2, respectively.
P, periproct (surrounding anus). Compare with Figs. 6G2
and 9. For plate homologies see Table 2. Redrawn and relabelled from
Bockelie 1979: 371, fig. 4a.
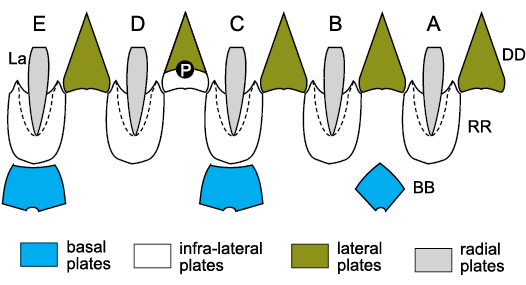
Fig. 11. Thecal plates of blastoids. A–E, Carpenter’s (1884, 1891) ambulacra; BB, basals; DD deltoids; La, lancets; P, periproct; RR, radials; white plate below periproct in CD interambulacrum is one of the extra deltoids. Dashed lines are radial sinuses for ambulacra. For plate homologies see Table 2.
Table 2. Plate homologies in blastozoans with tetraserial ambulacra; in brackets number of plates.
|
Extraxial- axial theory |
Hemicosmitoids |
Thomacystis |
Glyptocystitoids |
Rhombifera |
Lysocystites |
Coronates |
Eublastoids |
|
axial |
brachioles |
? |
brachioles |
? |
? |
brachioles |
brachioles |
|
trunk plates |
? |
floor plates |
? |
? |
brachiolar trunk |
side plates |
|
|
? |
adorals (5) |
periorals (6) |
? |
? |
first ambulacral plates |
first side plates |
|
|
extraxial perforate |
radials (9) |
|
radials (4–6, 10) |
radials (5) |
ambulacrals (5) |
trunk mounting plates (5) |
lancets (5) |
|
laterals (8 or 9) |
laterals (8) |
laterals (5) |
laterals (5) |
orals (5) |
deltoids (5) |
deltoids (5) |
|
|
infralaterals (6) |
infralaterals (6) |
infralaterals (5) |
infralaterals (5) |
radials (5) |
radials (5) |
radials (5) |
|
|
basals (4) |
basals (3) |
basals (4) |
basals (4) |
basals (3) |
basals (3) |
basals (3) |
|
|
extraxial imperforate |
stem |
stem |
stem |
stem |
stem |
stem |
stem |
The affinities of blastoids long remained obscure because the lancet plates were so distinctive, although Etheridge and Carpenter (1886: footnote on p. 118) thought that the coronate Stephanocrinus was a close relative. Eventually, Donovan and Paul (1985: 532, fig. 5) showed that coronates had an identical thecal plate arrangement to eublastoids and the “trunk mounting plates” of Brett et al. (1983: 640, fig. 7) were homologues of the lancets (see Fig. 12). Similarly, the “ambulacral plates” (Sprinkle 1973: 140, fig. 34) of Lysocystites were also lancet homologues (Fig. 13). Since then, it has been widely accepted that Lysocystites, coronates and eublastoids form a monophyletic clade (Sumrall and Waters 2012), but the relationship of blastoids s.l. to other blastozoans has remained obscure. Here, I suggest they are most closely related to the glyptocystitoid genus Rhombifera Barrande, 1867 (Fig. 6B; see below).
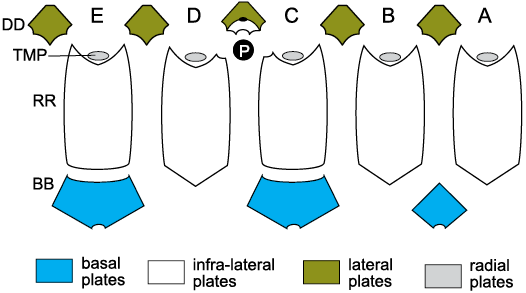
Fig. 12. Thecal plates of coronates showing homologies with blastoid plates. A–E, Carpenter’s (1884, 1891) ambulacra; BB, basal plates; DD, deltoid plates; P, periproct; RR, radial plates; TMP, “trunk mounting plates”, which bear facets for erect ambulacra and are homologues of blastoid lancet plates and glyptocystitoid radial plates. White plate above the periproct is the extra deltoid plate and small black oval above that is the gonopore. For plate homologies see Table 2.
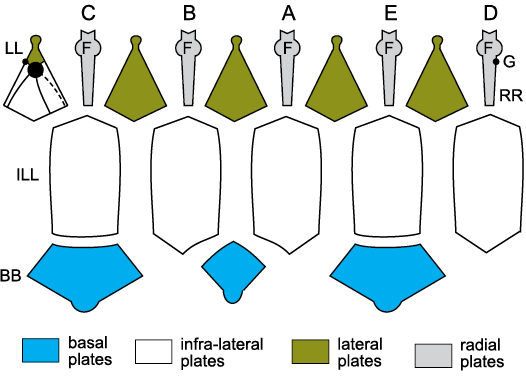
Fig. 13. Thecal plates of the Silurian blastozoan Lysocystites sculptus Miller, 1889, Indiana, USA, showing homologies with blastoids, coronates, and glyptocystitoids. A–E, Carpenter’s (1884, 1891) ambulacra. Unknown erect ambulacra arose from facets (F) on ambulacral plates (RR), homologues of blastoid lancet plates and the radial plates of Rhombifera Barrande, 1867 (Fig. 19A3). The mouth frame is composed of five deltoids (LL, homologues of the lateral plates of Rhombifera). The periproct (P) lies in the CD interambulacrum above additional deltoid plates (white) and shares the gonopore (G). The main body of the cup is formed of five infralateral plates (ILL, homologues of blastoid radials) and three basal plates (BB). Compare with the oral view, Fig. 20B. For plate homologies see Table 2. Redrawn from Sprinkle 1973:140, fig. 34.
Pore structures.—The Cambrian genera listed in Table 1, either lack visible pore-structures or possess simple epispires, small oval sutural pores, as in Akadocrinus. The Ordovician glyptocystitoid Cuniculocystis has specialized, covered epispires. Coronates have coronal canals in their coronal processes (Brett et al. 1983; McDermott and Paul 2015) and Lysocystites has peculiar, tri-radiate “exospires” (Sprinkle 1973: 140). Otherwise, glyptocystitoids, hemicosmitoids and eublastoids have internal canals, through which seawater flowed in life, and which are usually arranged in sets across the sutures between two plates. In glyptocystitoids and hemicosmitoids, the canals are parallel-sided, called dichopores and are arranged in rhombic sets (Paul 1968b). The canals of glyptocystitoids open in slits and the rhombs are called pectinirhombs (Paul 1968b), those of hemicosmitoids open in pores (Fig. 6G1), and the rhombs are called cryptorhombs (Paul 1968b; Bockelie 1979). In hemicosmitoids cryptorhombs occur across most plate sutures of all plate circlets (Bockelie 1979). In glyptocystitoids, pectinirhombs may occur across sutures between all plates except the orals. The number of rhombs per theca was progressively reduced during the Ordovician and many post-Ordovician glyptocystitoids have just three.
Blastoid pore-structures are also formed of canals shared by two thecal plates, but only across the radial:deltoid sutures. The pore-structures are called hydrospires and each may consist of a single canal, or a series of canals (Fig. 6C, D). Two types are generally recognized, fissiculate blastoids have exposed hydrospire slits (Fig. 6C); spiraculates have a series of fine pores along the margins of the hydrospires and large exits (spiracles) adorally. Plates that bear respiratory pore-structures, including the deltoids of blastoids and coronates, are part of the perforate extraxial skeleton.
Finally, another group of blastozoans, the parablastoids, also possess endothecal canals that resemble blastoid hydrospires. Parablastoids have recumbent ambulacra with main axes composed of a single biseries of alternating floor plates, not a double biseries (see Sprinkle 1973: 142–170; Sprinkle and Sumrall 2008) and so are not discussed further here. However, Paul and Cope (1982) described a Welsh, Early Ordovician species, which they interpreted as having a double biseries of floor plates. Thorough re-examination of the specimens, which were not well preserved, is required to confirm this interpretation. Nevertheless, it is possible that the earliest known parablastoid also had double biserial main ambulacral axes and therefore that parablastoids originally shared both similar endothecal canals and similar axial skeletons to other pan-dichoporites.
Imperforate extraxial skeleton.—The imperforate extraxial skeleton of blastozoans is largely confined to the stem, which is significant in pan-dichoporites and is remarkably distinctive (Fig. 6A; Paul 1968a). The stem of Akadocrinus is holomeric, composed of annular columnals with a relatively large lumen, tapers distally and terminates in a polyplated holdfast structure. In juveniles it has recognizable proximal and distal parts (Nohejlová and Fatka 2016: 148, fig. 6). The other three Cambrian genera listed in Table 1 also show evidence of the characteristic glyptocystitoid stem, which is divisible into proximal and distal portions. In Macrocystella and all later glyptocystitoids the proximal portion is composed of alternating inner and outer annular columnals and tapers rapidly. The distal stem is composed of alternating taller and shorter cylindrical columnals with a narrow lumen. The inner proximal columnals fit inside the outer proximals and bear articulation fulcra that define an axis perpendicular to the long axis of the stem about which the pair of columnals could rotate slightly. Each fulcral axis is set at an angle to the one above and below. Thus, the articulation axes spiral down the stem from the base of the theca and enable the proximal stem to bend in any direction (see Paul 1968a, 1984: 80, fig. 51).
Cambrocrinus Orłowski, 1968, has the proximal and distal stem (Dzik and Orłowski 1993: figs. 2j, k, l). Furthermore, the undulations on the external flanges of the outer proximal columnals spiral down the stem. Other details of the stem are unknown, but it certainly resembles a typical glyptocystitoid stem. Ridersia Jell, Burrett, and Banks, 1985, and Sanducystis Zamora, Sumrall, Zhu, and Lefebvre, 2017, both have typical glyptocystitoid stems, although the precise details of the proximal articulations are unknown. Nevertheless, apparently the typical stem preceded either the characteristic plate arrangement or the respiratory rhombs of later Ordovician glyptocystitoids.
To summarize: when they consist of more than one or two brachioles, pan-dichoporite ambulacra, whether erect or recumbent, possess a main axis composed of a double biseries of floor or trunk plates that are the first two brachiolar plates modified to form the ambulacral axis. Brachioles arise from a pair of axial plates, the adoral of which is secreted first and is usually smaller. Since glyptocystitoid orals bear half a brachiole facet, they are also axial skeletal elements.
The theca is composed of a relatively small number of plate circlets. When fully grown, Akadocrinus had about ten circlets and 100 plates of at least three generations. Cambrocrinus had seven circlets plus any orals. All other pan-dichoporites had no more than four circlets, which in the rhombiferans are called basals, infralaterals, laterals, and radials. In glyptocystitoid ontogeny, radials were the last plates to develop. Homologues of these four plate circlets can be recognized in all pan-dichoporites (Table 2).
The glyptocystitoid stem is composed of alternating larger and smaller columnals and possesses a distinctive proximal portion, which by analogy with the growth of living crinoid stems was the last part to form in ontogeny. It is characteristic of the Cambrian pan-dichoporite genera and appears to have been the first key character of pan-dichoporites. All more advanced pan-dichoporite groups possess a simpler, holomeric stem in which a distinct proximal section cannot be recognized.
Homologies within ambulacra
During the larval development of Recent crinoids five primary podia develop, which eventually become the adult radial water vessels (Hyman 1955: 85). Thus, a single primary podium can develop into all parts of the RWV. It is assumed here that all lateral branches are homologous and so a branch to a tube foot is not significantly different from a branch to a brachiole. Lateral branches of the RWV in modern echinoids form subterminally because the very tip of the radial water vessel emerges from the ocular pore (Fig. 3). Similarly, lateral branching of the RWV in brachiole-bearing blastozoans must also have been subterminal, because the existing tip of the RWV lay at the tip of the last brachiole (Fig. 14).
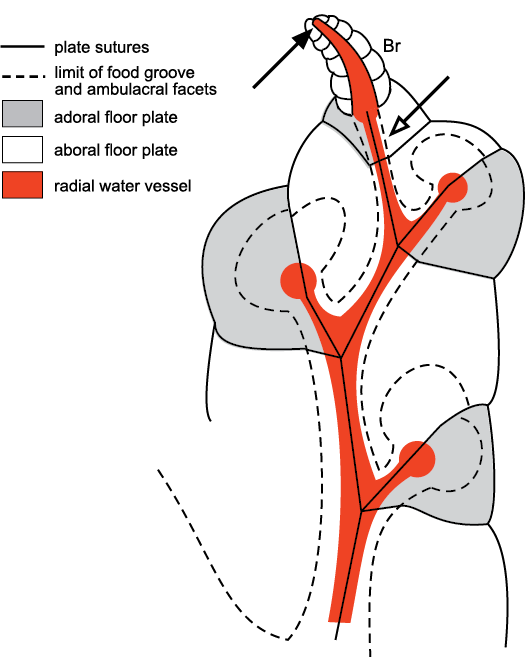
Fig. 14. Subterminal branching in the ambulacra of the callocystitid Callocystites fresti Paul, 2015a (SM A.85652), Wenlock (Silurian), Indiana, USA. Open arrow points to the place where the next lateral branch of the radial water vessel would have taken place, but the tip of the radial water vessel was at the top of the brachiole (Br) that arose from the final facet (solid arrow). Thus, branching of the radial water vessel in brachiole-bearing echinoderms was always subterminal. Modified from Paul and Hotchkiss 2020: fig. 7.
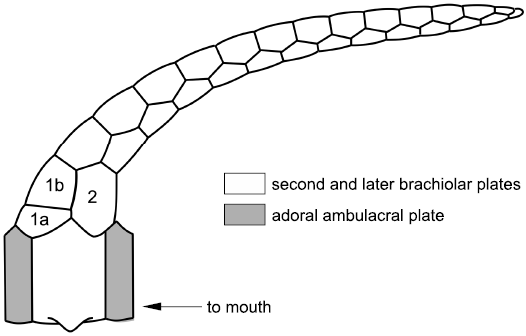
Fig. 15. Brachiole and main ambulacral plating in hemicosmitoid Caryocrinites ornatus Say, 1825, Wenlock (Silurian), New York, USA. The ambulacra are erect. Each brachiole arises from a pair of main ambulacral plates, the adoral of which are shaded. The aboral plates are more than twice as wide. The first brachiolar plate (1a) is adoral to the second (2) and supports the idea that main ambulacral plates are first brachiolar plates modified to form the ambulacral axis. Redrawn from Sprinkle 1975: 1067, fig. 2.
Tetraserial ambulacra.—Ambulacra in callocystitids (Figs. 5, 6E, 8, 14) are recumbent on the thecal plates. Each consists of a double biseries of floor plates that alternate along a zigzag radial suture. Each pair of floor plates shares a brachiole facet and the brachioles consist of an alternating series of brachiolar plates with a single row of cover plates on either side. That is, brachioles are a homologue of the standard axial growth zone modified for feeding (Mooi et al. 2005: 547, fig. 3F; Fig. 2 herein). When a new lateral branch of the RWV developed the first two plates to form were modified as the floor plates. The first floor plate to form was the adoral plate (shaded in Figs. 5, 8, and 14). The second floor plate was the larger, aboral plate. Thereafter, brachiolar plates alternated along the brachiole to its tip. Thus, floor plates are the first pair of brachiolar plates modified to form the main ambulacral axis. This idea can be tested because it predicts that the first true brachiolar plate will always be adoral in position. This prediction is true in the Silurian callocystitids Staurocystis quadrifasciatus (Pearce, 1843), Pseudocrinites bifasciatus Pearce, 1843 (Figs. 6A, 8) and Pseudocrinites pyriformis Paul, 1967a, as well as in the hemicosmitoid rhombiferan Caryocrinites ornatus Say, 1825 (Fig. 15), but should be confirmed in as many other pan-dichoporites as possible.
Extending the logic that floor plates are modified brachiolar plates right back to the mouth, the traditional “orals” and the “L” plates are simply the first and second ambulacral plates in the axial skeleton and are equally homologues of brachiolar plates. Again, this idea can be tested by examining the arrangement of brachiolar plates in the first brachioles of each ambulacrum. Here, I regard the oral and “L” plates as first and second orals, which support the first brachiole (e.g., Fig. 5: A1). The hypothesis that there are potentially two different orals in each ambulacrum can be used to interpret the oral plating of almost all brachiole-bearing blastozoans.
So, Fig. 5 confirms the new interpretation of the oral plating found in pentaradiate glyptocystitoids. Five of the traditional orals become first orals, the five “L” plates become second orals. The ambulacral axes are composed of pairs of floor plates, which share the brachiole facets and the adoral of which was formed first. Under the conventional terminology, six plates form the oral frame. Five are now considered as first ambulacral plates. The origin of the sixth, O6, is unknown, largely because the oral plating in helicoplacoids and Camptostroma has not been described, yet the difference between them might indicate how O6 originated. At present, all I can suggest is that the CD interray of helicoplacoids is the narrowest of the three, whereas the CD interray of pentaradiate echinoderms is the widest of five. The inclusion of an extra plate into the mouth frame in the CD interray during the transition from a triradiate to a pentaradiate echinoderm is, therefore, not surprising. Finally, a seventh plate is present in the “glyptocystitoid” oral area, O7, which shares the gonopore and hydropore with O1 (Fig. 5). The orifices open across a plate suture for convenience in growth. It is possible to enlarge such orifices without resorption. Nevertheless, O1 appears to be the critical plate, not O7. The hydropore frequently opens across the O1:O6 suture in diploporites. In the glyptocystitoid family Pleurocystitidae, where the hydropore and gonopore migrate aborally, it is plate O1 that lengthens and eventually shares both orifices with plate L5 (Paul 1967b: 113, fig. 7).
To summarize, Lovén’s Law and the 2-1-2 pattern of ambulacral branching reveal homologous ambulacra in blastozoans. Then, the timing of plate formation can be used to recognize homologous brachioles and individual plates within each ambulacrum. The combination allows recognition of homologous ambulacra and plates even when not all ambulacra are developed. In particular, the structure of the oral area in glyptocystitoids suggests that the conventional oral and “L” plates are the first two plates in each ambulacrum and form part of the axial skeleton.
Growth of ambulacra.—Growth of ambulacra may be considered in the light of four basic “instructions”: (i) add plates to the ambulacra; (ii) branch soft tissue structures such as the radial water vessels (RWV); (iii) cease further addition and branching; (iv) develop existing plates and branches by enlargement or modification.
Application to the family Callocystitidae.—Lepadocystis is a genus of callocystids with the oldest known species, with the possible exception of the species of Maennilocystis Paul and Rozhnov, 2016 (see below) and may be used as a standard pentaradiate callocystitid. Sumrall and Sprinkle (1999) described its ontogeny. The earliest growth stage found had only three ambulacra, which were accepted as A, shared BC and shared DE. In a tri-radiate Lepadocystis the first growth stage would have consisted of three primary brachioles (Fig. 16A) each supported on two “floor” plates, the oral and “L” plate. Here these are regarded as first and second ambulacral plates, respectively (Fig. 16A). The arrows in Fig. 16A indicate the positions where the new ambulacral branches would have started growing and Fig. 16B indicates the full set of oral and “L” plates for a pentaradiate callocystitid. Then, the arrows in Fig. 16B indicate the positions where the second branch to a brachiole would have occurred in each ambulacrum. Finally, Fig. 16C shows the next stage in which each of the five ambulacra has two brachioles. Note that this has produced the “BD different” pattern of primary brachioles (Paul 2015b), which is characteristic of pentaradiate glyptocystitoids with >2 brachioles in all ambulacra (Fig. 5). This pattern only arises if the lateral shared ambulacra branch after the first two floor plates develop in each ambulacrum. In effect, the first facet (e.g., BC1) branches to the left, the second to the right and becomes C1, then the third branches to the left to become B1 (Fig. 16C).
The next major step in the evolution of the Callocystitidae was that genera with only four ambulacra evolved in the Wenlock. This involved failure to develop the A radial water vessel, but the first ambulacral plate (Fig. 16: A1a) still developed. This suggests that at least in callocystitids the first and second ambulacral plates developed in sequence and not simultaneously. Equally, it suggests that the prime function of the first ambulacral plates was to form the oral frame; supporting the RWV was a less vital function. In the simplest case tetraradiate genera (which always lack ambulacrum A) retained the BD different pattern of primary brachioles (Fig. 5). So, basically loss of ambulacrum A arose by invoking the “Cease” instruction immediately after formation of the first ambulacral plate (A1a) in ambulacrum A. Lepocrinites Conrad, 1840, Sphaerocystites Hall, 1859, and Salirocystis Paul, 2015a, are examples of tetraradiate callocystitid genera that retain the BD different pattern of primary brachioles.
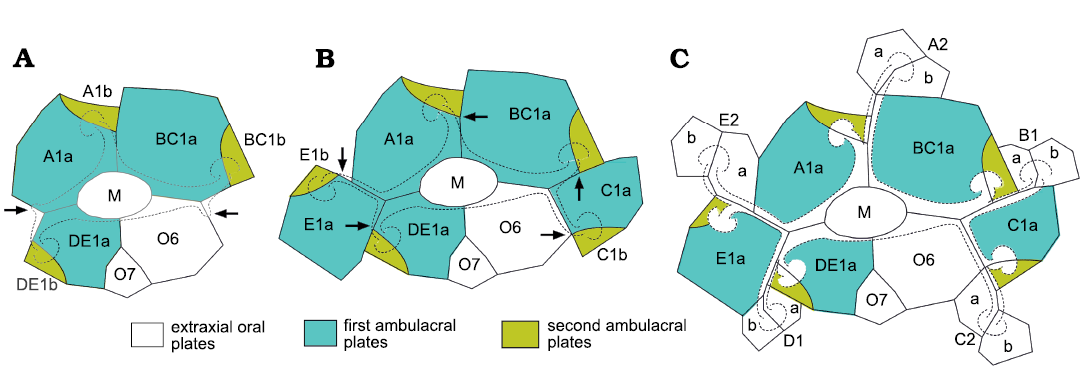
Fig. 16. Ontogenetic development of ambulacra in the callocystitid Lepadocystis moorei (Meek, 1871), Late Ordovician, Ohio, USA, showing addition of oral and ambulacral plates and development of the “BD different” pattern of primary brachioles. A. Triradiate stage with the first pair of ambulacral plates (A1a, A1b, and so on) interpreted as A, shared BC and shared DE. Arrows indicate the positions where the radial water vessels of the shared ambulacra branch to produce a pentaradiate rhombiferan, assuming subterminal branching. B. Pentaradiate stage with a single brachiole per ambulacrum. The two new brachioles are interpreted as the first brachioles in separate ambulacra C and E. Arrows indicate positions where the radial water vessels of all five ambulacra branch to produce second brachioles. C. Pentaradiate stage with two brachioles per ambulacrum. The second brachioles in the shared ambulacra are interpreted as B1 and D1. Note that the subterminal branching has produced the “BD different” pattern of primary brachioles in which the first two brachioles in ambulacra B and D branch to the left as viewed in the growth direction, whereas in ambulacra A, C and E only the first brachiole is on the left. In addition, the second brachioles exhibit a pattern which is A right, B left, C right, D left, and E right, which equals Lovén’s Law as restated by Paul and Hotchkiss (2020). M, mouth; O6 and O7, orals 6 and 7 in the conventional interpretation of the oral circlet. Solid lines, plate sutures; dashed lines, outline of ambulacra and brachiole facets. Redrawn from Paul and Hotchkiss 2020: 1096, fig. 8.
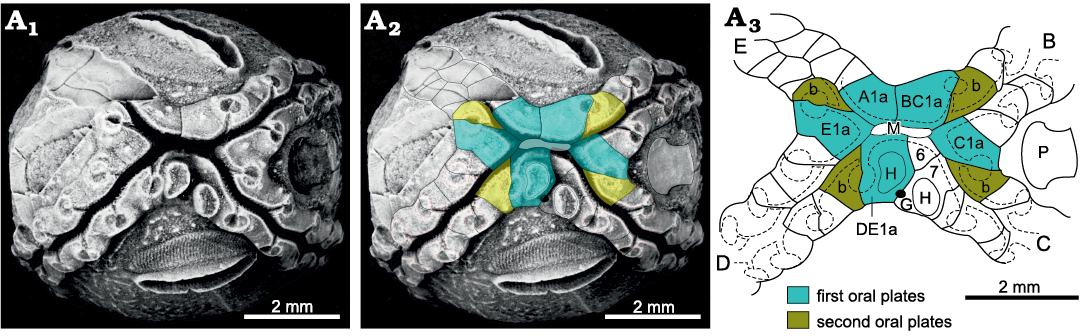
Fig. 17. Oral and ambulacral plating of the callocystitid Lipsanocystis traversensis Ehlers and Leighley, 1922 (UMMP 33311), Middle Devonian, Michigan, USA, illustrating the alternative “B–E the same” pattern of primary brachioles in which all four ambulacra have the first brachiole facet to the left. A1, oral view of showing the four ambulacra and double hydropore; A2, the same with interpretation of oral plates superimposed; A3, interpretation of oral and ambulacral plating. Ambulacrum A stopped developing after the first plate formed. In ambulacra B–E the first brachiole is to the left and the second to the right. B–E, ambulacra; A1a–E1a, first plates in corresponding ambulacra; b second plates in ambulacra B–E; G, gonopore; H, hydropore; M, mouth; P, periproct; 6, 7, orals 6 and 7 under the conventional interpretation. Dashed line outlines of ambulacra and brachiole facets. Only the first two ambulacral plates are shaded.
An alternative pattern of primary brachioles occurs in more derived genera, the “B–E the same pattern” (Paul 2015b), in which only the first brachiole branches to the left in all four ambulacra and the second to the right (Fig. 17). Thereafter, brachioles alternate throughout the ambulacra. Here, it is assumed that the plates of the oral frame are homologous with those in pentaradiate callocystitids with the BD different pattern (Figs. 5, 16). In which case the change involved the second branch along the B and D ambulacra (B2 and D2) giving rise to a brachiole on the right of the main food groove rather than the left. An alternative interpretation is that the BC1 and DE1 brachioles of the BD different pattern failed to develop. At present I see no way to distinguish between these two alternative interpretations.
Finally, while dealing with the Callocystitidae, the genus Pseudocrinites Pearce, 1843, has only two ambulacra (Fig. 18), both of which start with two brachioles on their left sides. In callocystitids with four or five ambulacra, ambulacrum C passes between the periproct and the hydropore and gonopore. One of the ambulacra in Pseudocrinites also does this and was interpreted as ambulacrum C by Kesling (1961). The ambulacrum opposite C is E and so the second ambulacrum in Pseudocrinites was taken as E. Paul (1967a: 325, fig. 16) interpreted the first brachiole in each ambulacrum as all that was left of ambulacra B and D (= IV and II of Jaekel’s 1899 system of denoting ambulacra). However, with the new growth model these first facets are interpreted here as shared BC1 and shared DE1 (Fig. 18). Thus, during the growth of the ambulacra the first brachioles to form would have been BC1 and DE1. Then, the next brachioles would have been C1 and E1, followed by C2 and E2, and so on.
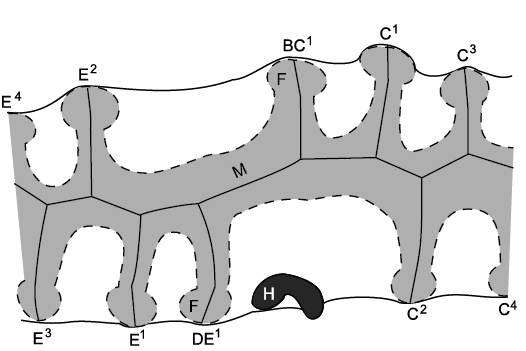
Fig. 18. Ambulacra and brachioles in the callocystitid Pseudocrinites pyriformis Paul, 1967a, Ludlow (Silurian), England, UK. The mouth and food grooves are hidden by cover plates (shaded). Both ambulacra start with two brachiole facets on the left. These are interpreted as BC1 and C1 in the C ambulacrum and DE1 and E1 in the E ambulacrum (left). F, facet; H, hydropore; M, mouth. Redrawn from Paul 1967a: 329, fig. 21.
Previously, an alternative interpretation of the homologies of the ambulacra of Pseudocrinites was possible (see Paul 2017: 587). In pentaradiate callocystitids with the BD different pattern, the two ambulacra with the first two facets to the left are B and D. Thus, it is possible that the two ambulacra in Pseudocrinites are also B and D. However, that interpretation implies that ambulacrum B has rotated on the theca to a position normally occupied by ambulacrum C. This unlikely rotation becomes unnecessary under the new interpretation, which is consistent both with the origin of the BD different pattern of primary brachioles and the traditional positional arguments about ambulacrum C. It also demonstrates that Pseudocrinites evolved directly from an ancestral callocystitid that retained the BD different pattern of primary brachioles. The arrangement of oral plates in Pseudocrinites is still not known in detail. The only illustration published so far (Paul 1967a: 325, fig. 16) is manifestly wrong; the orals surround the mouth, they cannot all be present on one side of it. This lack of knowledge of Pseudocrinites orals does not invalidate the interpretation of the ambulacra and primary brachioles.
Variants and exceptions.—The BD different pattern of primary brachioles is characteristic of all pentaradiate glyptocystitoids with more than two brachioles per ambulacrum with very few exceptions. Bockelie (1981) showed that the number of brachiole facets exceeds the number of ambulacral plates in Echinoencrinites Meyer, 1826, whereas the interpretation of floor plates accepted here predicts there should be twice as many ambulacral plates as brachiole facets. Equally, Paul and Rozhnov (2016: 267, fig. 8) have illustrated the brachioles in ambulacrum D of the pentaradiate Maennilocystis heckeri Paul and Rozhnov, 2016, and the five brachioles alternate left and right, whereas the “BD different” pattern would have the first two brachioles to the left in ambulacrum D. This was the only example of M. heckeri that showed the arrangement of brachioles in either ambulacrum B or D, so it is uncertain whether the pattern is typical of Maennilocystis heckeri or that specimen was anomalous. Nevertheless, all other known examples of glyptocystitoids which lack the “BD different” pattern of primary brachioles have fewer than five ambulacra. This includes a few tetraradiate callocystitid genera from Wenlockian (Silurian) or younger strata and Pseudocrinites with only two ambulacra. In addition, pleurocystitids have just two large brachioles (or one in the Devonian genus Hillocystis Jell, 1983). The two brachioles are interpreted as representing ambulacra C and E (Paul 2017: 593, fig. 15.2).
The oral area of Rhombifera Barrande, 1867, has facets (for unknown erect feeding structures) on its radial plates, not on the orals (Fig. 19). The oral area of Rhombifera (Fig. 20A) is remarkably similar to that of the unusual Silurian genus Lysocystites Miller, 1889. Lysocystites, in turn, shares an identical thecal plate arrangement with all coronates and blastoids (Donovan and Paul 1985; Sumrall and Waters 2012). Here, I suggest that the facet-bearing “radial” plates of Rhombifera are homologous with the facet-bearing “ambulacral” plates of Lysocystites (Fig. 20B) and hence with the lancet plates of eublastoids (see below section on blastoids, coronates, and Lysocystites).
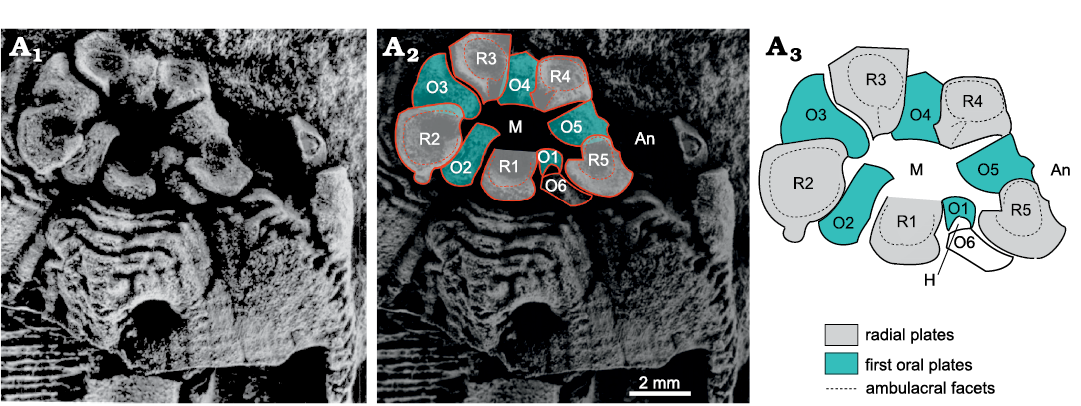
Fig. 19. Oral area of the rhombiferid Rhombifera bohemica Barrande, 1867, repository unknown, Late Ordovician, Czech Republic, showing interpretation of oral plating. A1, latex cast of oral area; A2, the same with interpretation of oral plating superimposed; A3, interpretation of oral plating. An, position of anus; H, position of supposed hydropore; M, mouth; O1–O6, first oral plates; R1–R5, radial plates, which bear the ambulacral facets. R3 supports facet for ambulacrum A, R4 ambulacrum B, etc. clockwise around the mouth. Early glyptocystitoids had six radials. In the Rhombiferidae Kesling, 1962, there are only five; R6 is missing. Photograph from Kesling 1962: pl. 2, fig. 1, right.
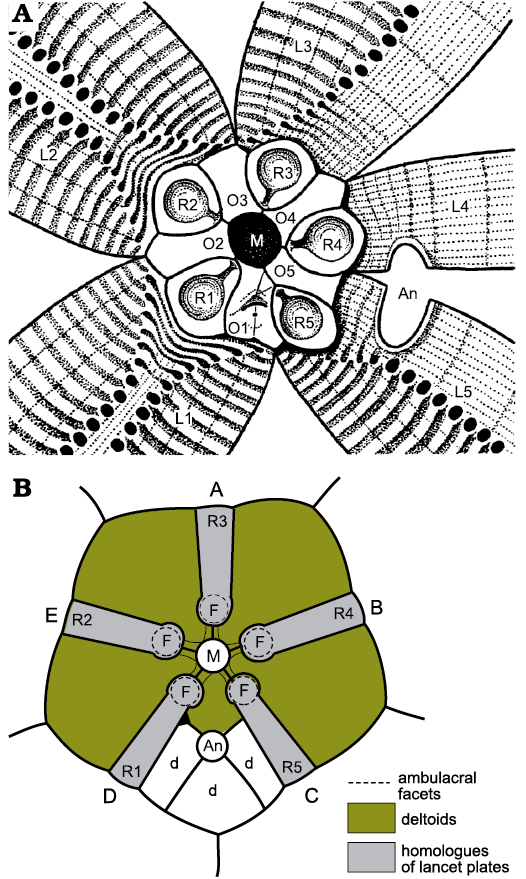
Fig. 20. Oral plating of two blastozoans showing similarities in arrangement of facet-bearing plates. A. Rhombifera bohemica Barrande, 1867, Late Ordovician, Czech Republic. B. Lysocystites sculptus Miller, 1889, early Silurian, Indiana, USA. In both the mouth (M) is encircled by only five plates (O1–O5 in A), which alternate with five radial plates (R1–R5) that bear ambulacral facets (F) and are connected to the mouth by short food grooves. In Lysocystites (B) the facet-bearing plates are the homologues of eublastoid lancet plates. A–E, ambulacra A–E; An, anus; d, additional anal deltoids; L1–L5, lateral plates. For plate homologies see Table 2. A after Kesling 1962: 283, fig. 2; B after Sprinkle 1973: 141, fig. 35.
Hemicosmitoids
Hemicosmitoids include three families, the Hemicosmitidae, Caryocrinitidae, and Thomacystidae, all three of which had erect ambulacra (Figs. 21, 22) and thecae composed of three or four plate circlets, with up to nine plates each (Fig. 10). In Caryocrinites Say, 1825, the ambulacra were composed of a double biseries of trunk plates, off which biserial brachioles arose alternately. Sprinkle (1975) described the structure in detail for the Silurian type species C. ornatus Say, 1825 and Lanc et al. (2015) confirmed the basic details for the Ordovician species C. rugatus (Salter, 1866). Hemicosmitids (Fig. 21) and caryocrinitids undoubtedly have triradiate symmetry, but the details of oral and ambulacral plating are hidden by a well-developed tegmen in Caryocrinites. The Thomacystidae are known only from the unique Welsh, Late Ordovician type species Thomacystis tuberculata Paul, 1969, which had four ambulacra (Fig. 22). The C ambulacrum had a single ambulacral facet; ambulacra B, D and E had a pair of equal-sized facets (Fig. 22). An unusual feature of hemicosmitids described by Bockelie (1979: 375, fig. 8C) is the occurrence of a modified ambulacral facet at the beginning of each ambulacrum (Fig. 21). These first facets were developed on a ‘wedge’ plate and a radial plate and thought to bear reproductive tissues (Bockelie 1979: 375). They are often shallow pits and may have nothing to do with ambulacral growth.
Sumrall (2005, 2008) interpreted the three ambulacra of hemicosmitids as A, shared BC, and shared DE, which arose by a paedomorphic failure to branch the lateral ambulacra and hence produce a pentaradiate echinoderm. He also interpreted the modified ambulacral facets as indicating the first brachioles of the three primary ambulacra (Sumrall 2008: 236, fig. 11.4). This is an ingenious and original interpretation that apparently accounts for all the plates in the former radial circlet. Unfortunately, several points argue against this interpretation. First, the three oral plates (O1, O3, and O4) that are supposed to contribute to the modified ambulacral facets do not do so. The facets are supported by the wedge plates on the left and the left facet plates on the right. Secondly, this means that the left facet plates contribute to both the modified ambulacral facets and the main ambulacral facets, which is incompatible with hemicosmitoid orals being modified brachiolar plates. Finally, in all species of Hemicosmites except the type species, the radial plates bear the adoral halves of cryptorhombs in Bockelie’s system 3 (Bockelie 1979: 373, fig. 7). Thus, in terms of the EAT, these plates form part of the perforate extraxial theca and so cannot be part of the axial skeleton. This probably accounts for the appearance of the wedge plates from beneath the radials (Bockelie 1979: 377, fig. 9), which is inconsistent with the Ocular Plate Rule and terminal addition of axial plates.
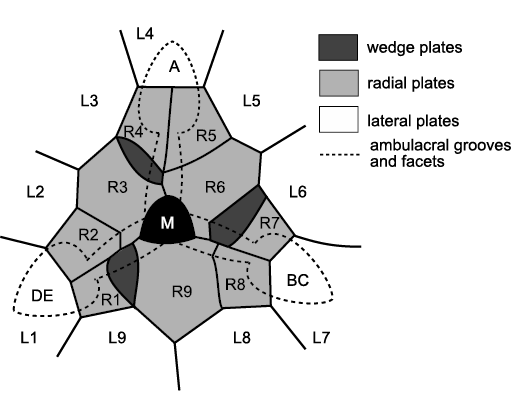
Fig. 21. Oral area of the hemicosmitoid Hemicosmites pyriformis Buch, 1846, Middle Ordovician, Russia. Three ambulacra, interpreted as A, shared BC, and shared DE, converge on the mouth (M) and are surrounded by a circlet of nine radial plates (R1–R9) outside which is another circlet of nine lateral plates (L1–L9). Three wedge plates occur immediately clockwise of radial plates R3, R6, and R9. The hydropore (not shown) occurs in the adoral part of radial 9. In life, biserial cover plates would have roofed over the food grooves. Redrawn and relabelled from Bockelie 1979: 375, fig. 8b.
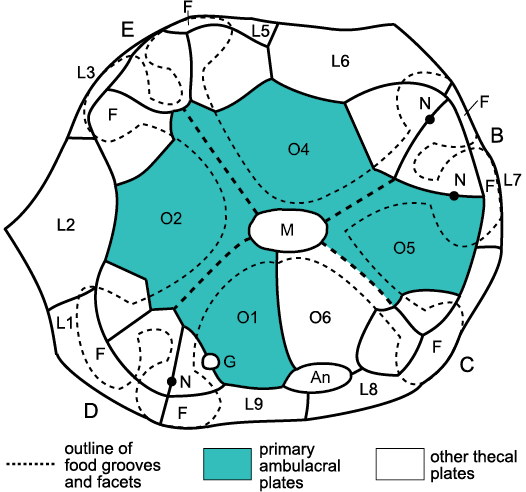
Fig. 22. Oral area of the hemicosmitoid Thomacystis tuberculata Paul, 1969, Late Ordovician, Wales, UK. Ambulacra are interpreted as B, C, D, and E and neither ambulacrum A nor plate O3, the oral associated with ambulacrum A, is developed. Facets (F) are shared by ambulacral plates the homologies of which are uncertain, and lateral plates (L1–L9). Primary ambulacral plates (O1, O2, O4, O5). Plate L4 of hemicosmitids is missing in Thomacystis and caryocrinitids (Paul 1984: 144, fig. 87). An, anus; G, gonopore; M, mouth; N, pore for presumed nerves. Redrawn and relabelled from Paul 1969: 193, fig. 2.
Altogether, it is best to regard these plates as “radial” plates that form part of the perforate extraxial skeleton and upon which the developing ambulacra have encroached. Bockelie showed several plates that did not bear lateral facets for brachioles at the base of the ambulacra in Hemicosmites spp. (e.g., Bockelie 1979: 375–398, figs. 8c, 10, 20a) and it seems likely that the primary ambulacral plates lie among these plates. Unfortunately, very few specimens of Hemicosmites preserve even these remnants of the ambulacra.
The ambulacra of Caryocrinites were thoroughly described by Sprinkle (1975). They are effectively identical to the ambulacra of glyptocystitoids. The principal difference is that hemicosmitoid ambulacra were erect, whereas glyptocystitoid ambulacra either formed part of the thecal wall (the “mural ambulacra” of Paul 2017: 594), or were recumbent on extraxial thecal plates (as in Glyptocystites Billings, 1854, and the family Callocystitidae). Thus, the floor plates of glyptocystitoids are homologous with the trunk plates of hemicosmitoids and represent the first pair of brachiolar plates modified to form the axis of the ambulacra. In both superfamilies the first plate is adoral and smaller, the second the larger plate (Figs. 6E, 8, 15, 17). In both the first true brachiolar plate is adoral compared with the second. The only significant difference is that in Caryocrinites ornatus the first true brachiolar plates (Fig. 15: 1a, 1b, 2) do not alternate regularly. A similar arrangement occurs in C. rugatus (Lanc et al. 2015: figs. 8d, 11).
One final thing concerning the oral and ambulacral plates of hemicosmitoids is the unique genus Thomacystis Paul, 1969, which has four ambulacra and a mouth surrounded by five oral plates (Fig. 22). The ambulacra are interpreted as B, C, D, and E, three of which have two ambulacral facets and C only one. Since ambulacrum A is not developed, it is assumed that the associated oral plate, O3, is also not present. In addition to the orals, smaller ambulacral plates support the proximal parts of the ambulacral facets, but without a systematic association. There are three such plates in ambulacra D and E, but only two in B and C. Three of the four orals do not contribute to ambulacral facets at all, but O5 supports the right half of the right facet in ambulacrum B. As the number of facets increased in ontogeny, additional ambulacral plates developed, but without any fixed relationship between the two. The ambulacra of Thomacystis are unknown, but were probably like those of Caryocrinites. Three of the facets have an obvious pore for nerves, as in Hemicosmites and Caryocrinites. Thomacystis is an exceptional hemicosmitoid rhombiferan.
Blastoids, coronates, and Lysocystites
As mentioned above, the oral area of the Late Ordovician glyptocystitoid genus Rhombifera (Fig. 20A) is remarkably similar to that of the Wenlock (Silurian) genus Lysocystites (Fig. 20B). Rhombifera is the only glyptocystitoid with ambulacral facets (for unknown erect feeding structures) on its radial plates. In Lysocystites similar facets for equally unknown erect feeding structures, lie at the adoral ends of elongate thecal plates that alternate with the five plates that form the mouth frame. Thus, it is reasonable to suggest that the facet-bearing plates in Rhombifera and Lysocystites are homologous, which provides a link between the glyptocystitoid rhombiferans on the one hand and blastoids, coronates and Lysocystites on the other. The oral frame of blastoids is composed of five deltoid plates, one in each interambulacrum (Fig. 23: D). The CD interambulacrum also contains the anus and at least one other deltoid. Coronates have an identical thecal plate arrangement to blastoids, including a mouth frame composed of five deltoids (Fig. 24: L1–L5). A second posterior deltoid lies aboral to deltoid L1 and shares the gonopore (Fig. 24: G). Both posterior deltoids are adoral to the anus.
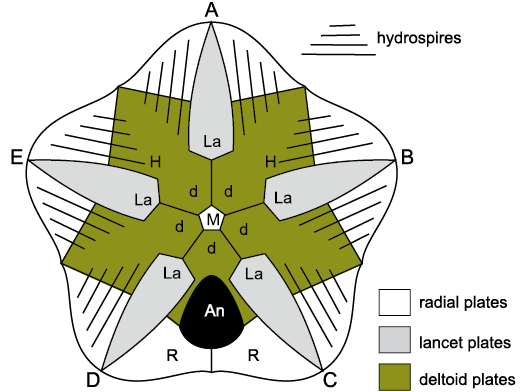
Fig. 23. Oral surface of the blastoid Codaster acutus McCoy, 1849, early Carboniferous, England, UK, showing interpretation of oral plating. Five ambulacra (A–E) are recumbent on the lancet plates (La) with the floor plates omitted for clarity. Five deltoid plates (d) form the mouth (M) frame and share the adoral parts of the hydrospire slits (H), the aboral parts of which are in the radial plates (R). An, anus. For plate homologies see Table 2.
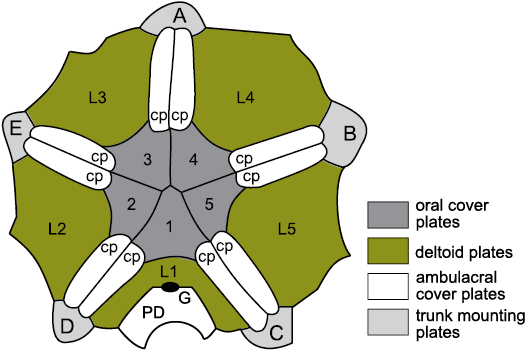
Fig. 24. Oral surface of the coronate Stephanocrinus angulatus Conrad, 1842, early Silurian (Wenlock), New York, USA, showing similarity to blastoid oral surface. Five erect ambulacra (A–E) arise from the trunk mounting plates, which are homologues of the lancets of blastoids. Food grooves are covered by paired ambulacral cover plates (cp). The mouth frame is composed of five homologues of blastoid deltoids and dichoporite lateral plates (L1–L5), and covered by primary oral cover plates (1–5). G, gonopore; PD, posterior deltoid. For plate homologies see Table 2. Redrawn and relabelled from Brett et al. 1983: 638, fig. 4A.
The thecal plate arrangement in blastoids, coronates and Lysocystites is basically identical and consists of four plate circlets. All three taxa have three basals, two large and one small, with the smaller in the AB interradius. They all have five “radials”, five “deltoids” and blastoids have five lancet plates. In coronates the so-called “trunk-mounting plates” (Brett et al. 1983: 629) and in Lysocystites the “ambulacral plates” (Sprinkle 1973: 140, fig. 34) are homologues of the lancet plates in blastoids (Donovan and Paul 1985: 532, fig. 5).
Blastoids, coronates and Lysocystites have very different respiratory pore structures. Blastoids possess hydrospires (Fig. 23), which are similar in basic construction to the pectinirhombs and cryptorhombs of glyptocystitoids and hemicosmitoids, respectively. All three structures are composed of endothecal canals that are shared between two plates and through which seawater flowed in life. Blastoid hydrospires only cross radial:deltoid sutures, but all four plate circlets may bear rhombs in dichoporites. Coronates have coronal canals in their coronal processes through which body fluids flowed (Brett et al. 1983; Donovan and Paul 1985: 537, fig. 8; McDermott and Paul 2015: 176, fig. 3). Like blastoid hydrospires, coronal canals only cross the radial:deltoid sutures. Lysocystites has triradiate “exospires” at the corners of the radial plates, each one shared between three plates, either two basals and a radial, or two radials and a basal or one oral and two radials (Sprinkle 1973: 140, fig. 34). When complete, the triangular channels within the plates were covered externally by a thin calcified roof. Beneath the roof was a large, circular pore connecting to the interior at the junction of the three plates, plus two smaller openings in the paired plates, also connected to the interior. Exospires had body fluids flowing through them in life. Finally, blastoids had recumbent ambulacra composed of alternating outer side plates and side plates, each pair of which shared a brachiole facet and also alternated across the food groove. As with the recumbent ambulacra of callocystitid glyptocystitoids, the smaller, adoral outer side plate was the first to form during growth of blastoid ambulacra. Coronates had erect, pinnate ambulacra, giving rise to biserial brachioles alternately and the first pair of brachiolar plates were modified to form the axis of each ambulacrum (Fig. 7). The ambulacra of Lysocystites are unknown.
Discussion
Nohejlová and Fatka (2016) described the growth of the middle Cambrian Akadocrinus. The proximal stem of juveniles tapered and was composed of thin columnals. A longer distal portion had taller columnals. The theca was made of 20–25 plates that lacked epispires and had 3–4 short brachioles. Mature specimens have a much longer, cylindrical stem composed of thin columnals, a theca with ten or more circlets of plates and 100 or more in total. Most plates in the upper three quarters of the cup bear small sutural epispires and up to 14 brachioles reaching 20 mm long occur adorally. Thus, a stem resembling the typical glyptocystitoid stem, a small number of plate circlets with thecal plates lacking pore-structures, and a small number of brachioles are all juvenile characters. The late Cambrian Cambrocrinus has an extensive proximal stem and a theca of seven plate circlets, with plates added during growth, but the lower two plate circlets and the orals are said to have a fixed arrangement (Dzik and Orłowski 1993: 26). Both Ridersia and Sanducystis have only three plate circlets, which Zamora et al. (2017) interpreted as BB, ILL, and LL. The oral surface of Ridersia seems to have been composed of many small plates (Jell et al. 1985: 202, fig. 12C), but recognizable orals are present in Sanducystis (Zamora et al. 2017: 471, fig. 5O), which is also more derived in having only four ambulacra; A is undeveloped. Neither genus has obvious pore-structures, but Sanducystis has unusual ridges and grooves on the inner surfaces of its thecal plates that may have facilitated gas exchange (Zamora et al. 2017: fig. 5f), while not weakening the theca. Ridersia apparently had similar, but less extensive, internal ridges on its lateral plates (Jell et al. 1985: 203, fig. 13D). Both genera have a typical glyptocystitoid stem with a distinct, tapering, proximal portion and a longer, less derived distal portion. Thus, from the limited number of known Cambrian genera with glyptocystitoid-like stems it would seem that the cup evolved from a primitive condition in which circlets of plates and individual plates were added continuously throughout growth and predominantly in an adoral direction. These plates only developed pore-structures relatively late in ontogeny. As with modern crinoids, stem ossicles were added at the base of the cup, so the proximal stem was the last part of the stem to develop.
By the Tremadoc (Ordovician) the typical plate arrangement of glyptocystitoids sensu stricto had developed, with 4BB, 5ILL, 5LL, 6RR, and 7OO, and with a large, lateral periproct bordered by five thecal plates, IL4, IL5, L1, L4, and L5, as in Macrocystella. With the exception of Cuniculocystis, which has 10RR, most glyptocystitoids retain the pattern seen in Macrocystella, although the large families Callocystitidae and Echinoencrinitidae both lose plate R5, whereas the monotypic families Rhombiferidae and Cystoblastidae lose R6 and R4, respectively. Furthermore, in ontogeny, Lepadocystis, developed the radial plates last (Sumrall and Sprinkle 1999: 410, figs. 1A, B). To summarize the evolutionary trends in thecal plating, primitive pan-dichoporites possessed thecae that added plates throughout growth in an adoral direction. Later genera show evidence of organized plate circlets with a fixed number of plates, again starting with the aboralmost circlet, the basals, and developing adorally. Finally, the theca became composed entirely of a fixed number of plates arranged in definite circlets. The number of circlets also reduced, although Ridersia and Sanducystis already had only three, plus possible orals, by the late Cambrian.
Sumrall (2008) and Sumrall and Waters (2012) interpreted the hemicosmitid oral area in terms of paedomorphic ambulacral reduction (Sumrall and Wray 2007). Hemicosmites had only three ambulacra, which were interpreted as A, shared BC, and shared DE. Other features of hemicosmitoid morphology may also be paedomorphic if the hemicosmitoids were derived from glyptocystitoids. The infralateral, lateral and radial circlets include more plates than corresponding circlets in glyptocystitoids. The hemicosmitoid stem lacks the distinct proximal portion characteristic of glyptocystitoids, but again the proximal stem is the last part to develop in living crinoids. New nodal ossicles are added at the base of the theca (Breimer 1978: T22). So, it may be inferred that the glyptocystitoid proximal stem was the last part to develop in ontogeny.
Both superfamilies are characterized by typical pore structures composed of parallel-sided, internal canals, called dichopores, arranged in rhombic sets that are shared between two adjacent plates. In glyptocystitoids the canals open in slits and the rhombs are pectinirhombs; in hemicosmitoids the canals open in pores and the rhombs are cryptorhombs. In ontogeny the thecal plates develop before the pore structures and there may be a distinct central area lacking any trace of dichopores. So, it is distinctly possible that when the two superfamilies diverged, they did so at an ontogenetic stage before any dichopores had formed. In conventional cladistic analysis the two types of rhombs are considered to have evolved independently and therefore cannot be homologous structures. However, if the two superfamilies diverged at an ontogenetic stage before rhombs began to develop, the genetic code necessary to develop rhombs composed of homologous dichopores need not have been lost. Furthermore, in conventional cladistic analysis two sister groups are considered to have arisen at the same time. Thus, if Akadocrinus already had the typical glyptocystitoid stem by the middle Cambrian, hemicosmitoids would be considered to have diverged by the same time at the latest. Indeed, Zamora et al. (2017: 473, fig. 7) produced a cladogram in which both the coronate Stephanocrinus and Hemicosmites diverged before the late Cambrian Ridersia and Sanducystis evolved. Their cladogram did not consider Akadocrinus, but by the same logic both Stephanocrinus and Hemicosmites would have ghost ranges extending back to the middle Cambrian if Akadocrinus had been included. Thus, I think evolution that involved paedomorphosis poses special problems in cladistic character analysis. At the time when two groups diverged, the daughter clade would be the latest evolutionary development. If, however, it arose by paedomorphosis and retained juvenile characters into its adult morphology, those characters would appear to be plesiomorphic. Thus, it would be natural to assume that the clade arose when the plesiomorphic character state was the most derived character state known, thus creating an unjustified ghost range.
Furthermore, I suspect that at least in some cases paedomorphosis must include the reordering of developmental genes, rather than their loss or development of new ones. Again, the hemicosmitoids offer a potential example. In their ambulacral development one can imagine three instructions: (i) develop ambulacrum A; (ii) develop the paired ambulacra; (iii) branch the paired ambulacra. The order of the first two is not material, but the third cannot occur before two whereas one is immaterial to three.
Hemicosmites and the caryocrinitid genus Stribalocystites Miller, 1891, obey the first two instructions, develop a single facet in each of the three ambulacra, and do not reach instruction three. The unique genus Thomacystis lacks ambulacrum A, but has all four other ambulacra B–E. It must have omitted one, but then followed two and three. Interestingly, unlike glyptocystitoids and diploporites with only four ambulacra, which also always lack ambulacrum A, Thomacystis apparently lacks the oral plate (O3) on which ambulacrum A starts to develop. Thus, I think that in the ontogeny of hemicosmitoids it is more likely that the paired ambulacra developed first, then ambulacrum A, and finally the paired ambulacra divided, as suggested by Sumrall and Wray (2007: 150, fig. 1). Finally, Lanc et al. (2015: 7) distinguished the genera Caryocrinites and Stribalocystites on the basis that the former had multiple facets per ambulacrum, whereas Stribalocystites had only a single facet. Thus, in the development of Caryocrinites not only were instructions one and two invoked, but instruction three applied to all three ambulacra.
A similar situation arises with the origin of the clade that includes the coronates, blastoids and Lysocystites. Rhombifera has isolated radial plates. In the ontogeny of Lepadocystis when the first radial plates develop, they are small, diamond-shaped plates entirely isolated from each other (Sumrall and Sprinkle 1999: 410, fig. 1b). Thus, the adult condition of the radials in Rhombifera is paedomorphic with respect to their normal condition in all other glyptocystitoids. The so-called “ambulacrals” of Lysocystites are almost identical to the radials of Rhombifera, except that they are elongate, whereas those of Rhombifera are short and pear-shaped. Nevertheless, all five bear ambulacral facets and are isolated from each other by the so-called “orals” (Sprinkle 1973: 140, fig. 34; Fig. 20B herein).
Phylogeny
Pan-dichoporites is an informal term to describe a potentially monophyletic lineage that includes glyptocystitoids and hemicosmitoids (= dichoporite rhombiferans), coronates, and blastoids, which have previously been regarded as echinoderm classes. The precise composition of this clade is not yet established; it may include another minor “class”, the parablastoids. Furthermore, the phylogenetic positions of other small but distinctive, high-level echinoderm taxa, such as the cryptocrinoids and paracrinoids need evaluating. Thus, it is premature to erect a new formal taxon that cannot yet be defined. The concept of pan-dichoporites is also radical and needs rigorous evaluation before acceptance. Nevertheless, a preliminary phylogenetic analysis of the 14 genera listed in Table 1 was undertaken using PAUP (version 4.b168, Swofford 2003). Table 3 lists the characters on which the analysis was based. All were unordered and equally weighted. The analysis was parsimony-based, used a heuristic search and was rooted on Akadocrinus, because initially it was not realised that juvenile Akadocrinus possessed the characteristic pan-dichoporite stem and therefore it seemed a suitable outgroup. Results produced six equally parsimonious cladograms of 50 steps, with a consistency index of 0.680 and a retention index of 0.742. The strict consensus tree is shown in Fig. 25. A 50% majority rule consensus tree was identical and all branches above Cambrocrinus had 100% support. The strict consensus tree has two trichotomies. The first involves Macrocystella, Lepadocystis, and all their descendants. The second involves Rhombifera, Hemicosmites, and all their descendants. It seems that as coded the chosen characters do not unequivocally distinguish the glyptocystitoids (Macrocystella, Lepadocystis, and Rhombifera) from the hemicosmitoids (Hemicosmites, Caryocrinites, and Thomacystis), but they do distinguish the blastoids sensu lato.
Table 3. Characters and coding of character states.
|
1 |
stem divisible into proximal and distal sections |
no (0), yes (1) |
|
2 |
proximal stem heteromorphic |
no (0), yes (1) |
|
3 |
distal stem heteromorphic |
no (0), yes (1) |
|
4 |
stem facet triangular |
no (0), yes (1) |
|
5 |
thecal plate circlets |
>10 (0), 7 (1), 5 (2), 4 (3) |
|
6 |
basals |
5 (0), 4(1), 3(2) |
|
7 |
infralaterals |
6 (0), 5 (1) |
|
8 |
laterals |
9 (0), 8 (1), 6 (2), 5 (3) |
|
9 |
laterals bear ambulacra |
no (0), yes (1) |
|
10 |
ambulacra erect |
no (0), yes (1) |
|
11 |
radials absent |
yes (0), no (1) |
|
12 |
radials |
9 (0), 6 (1), 5 (2) |
|
13 |
radial circlet |
closed (0), open (1), radials isolated (2) |
|
14 |
periorals present |
yes (0), no (1) |
|
15 |
oral frame plates |
6 (0), 5 (1) |
|
16 |
primary peristomal cover plates |
6 (0), 5 (1) |
|
17 |
endothecal canals present |
no (0), yes (1) |
|
18 |
endothecal respiratory structures |
cryptorhombs (0), pectinirhombs (1), hydrospires (2) |
|
19 |
exothecal structures present |
no (0), yes (1) |
|
20 |
exothecal respiratory structures |
epispires (0), coronal canals (1), exospires (2) |
|
21 |
pore structures confined to ILL and LL |
no (0), yes (1) |
|
22 |
anus in CD interray |
yes (0), no (1) |
|
23 |
anus |
on oral surface (0), lateral (1) |
|
24 |
periproctal membrane |
yes (0), no (1) |
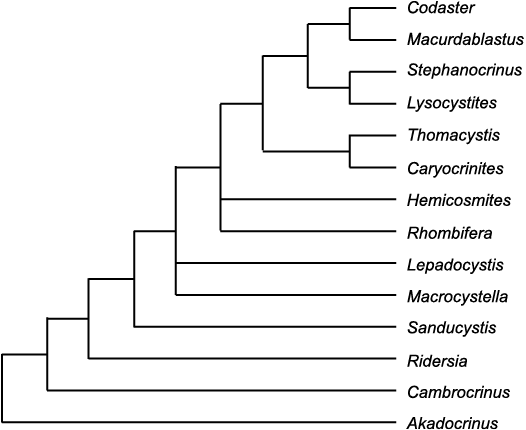
Fig. 25. Strict consensus tree for taxa listed in Table 1.
Figure 25 may be compared with the cladogram presented by Sumrall and Waters (2012: 970, fig. 8). They used the parablastoid genus Eurekablastus Sprinkle and Sumrall, 2008, as an outgroup, and only considered three rhombiferan genera, Hemicosmites, Cheirocystis Paul, 1972, and Lepadocystis. The cladogram grouped the two rhombiferan genera with Hemicosmites as sister group to them, and the three as sister group to the blastoids sensu lato. It also had the three eublastoid genera as the most derived monophyletic clade. The two coronate genera were sister group to the eublastoids, and Lysocystites the sister group to both coronates and eublastoids. Both the suggestions that Hemicosmites preceded the glyptocystitoids and that Lysocystites preceded the remaining blastoids sensu lato are counter to known stratigraphy of occurrences. Even ignoring the supposed Cambrian pan-dichoporite genera discussed here, Macrocystella is known from the Tremadocian (earliest Ordovician), but the earliest reported hemicosmitoids are Floian (late Early Ordovician; Paul et al. 2016). Equally, coronates and the blastoid Macurdablastus Broadhead, 1984, are known from low in the Late Ordovician, whereas Lysocystites is known from high in the Wenlock (early Silurian). Thus, both suggestions involve extensive ghost ranges. It has become fashionable to ignore stratigraphy and the grounds of the incompleteness of the fossil record. I would point out, however, that first appearances of fossils can only be preserved in the wrong order with respect to the order in which they evolved if the fossils coexisted. This fact is not affected by the incompleteness of the fossil record. If the fossil record consisted of just two different individual fossils they would be preserved in the correct evolutionary sequence (Paul 1982). Thus, the longer a ghost range and the more taxa involved in a ghost range, the less likely it is that the ghost range is genuine and the more likely it is an artifact of cladistic methodology. Here, I suggest that both pan-dichoporite ghost ranges result from evolution by paedomorphosis.
Conclusions
Blastozoans with pinnate ambulacra with a main axis that consists of a double biseries of floor or trunk plates form a natural clade. The main axis of their ambulacra is composed of pairs of modified brachiolar plates. This clade includes the traditional taxa glyptocystitoid and hemicosmitoid rhombiferans, coronates, blastoids, the unique genus Lysocystites and several Cambrian “eocrinoids” and requires a relatively high taxonomic rank. The glyptocystitoid genus Rhombifera links the traditional rhombiferan taxa to the blastoids sensu lato. The radial plates of Rhombifera are isolated by the orals and homologous with the “ambulacral” plates of Lysocystites and hence with the lancet plates of blastoids. That homology implies that the two plate circlets between the basals and radials of glyptocystitoids (the infralaterals and laterals) are homologous with the two plates circlets of blastoids and coronates that intervene between their basals and lancets (the radials and deltoids). Thus, homologies can be suggested between all plate circlets in the thecae of all pan-dichoporites (Table 2) except the two most primitive Cambrian genera, Akadocrinus and Cambrocrinus. The suggestion that blastoid and coronate deltoid plates are not homologous with the orals of glyptocystitoids is novel (see Sumrall and Waters 2012: 957, table 1), but it accounts for the fact that only five deltoids form the blastoid mouth frame because no glyptocystitoid has more than five laterals. Apart from Rhombifera, all glyptocystitoid mouth frames have six plates. The novel interpretation is also consistent with the presence of pore structures in all deltoids, which suggests they are part of the perforate extraxial skeleton, as rhombiferan lateral plates are. Five of the glyptocystitoid orals form part of the axial skeleton. Rhombifera, plus all coronates and blastoids have pore structures confined to the same two homologous plate circlets, the infralateral and lateral circlets of Rhombifera, the radial and deltoid circlets of blastoids sensu lato.
The pan-dichoporite clade includes almost all blastozoans with delicate internal respiratory structures; pectinirhombs and cryptorhombs of the dichoporite rhombiferans and the hydrospires of eublastoids. Only the cataspires of parablastoids are excluded. At present this is because most parablastoid genera have pinnate ambulacra in which the main axis is composed of a single biseries of alternating plates. However, the earliest known parablastoid was claimed to have a double biseries of floor plates. That needs further investigation, but is beyond the scope of this paper. Nevertheless, it is possible that parablastoids may also be included within the new taxon, although cataspires differ significantly from dichopores and hydrospire canals.
Hemicosmitoid rhombiferans probably arose from glyptocystitoids by paedomorphosis, as suggested by Sumrall (2008) and Sumrall and Waters (2012). Paedomorphosis poses special problems for cladistic analysis. Although a new paedomorphic taxon will be apomorphic when it first arose, the retention of juvenile characters will appear plesiomorphic. Hemicosmitoids not only retain a plesiomorphic triradiate oral area, but an apparently plesiomorphic theca in having more plates per circlet than glyptocystitoids and a plesiomorphic stem, which lacks the apomorphic proximal portion characteristic of glyptocystitoids. All these factors suggest the hemicosmitoids must have arisen before any typically glyptocystitoid characters, that is by the middle Cambrian at the latest. The earliest hemicosmitoids known are Floian (late Early Ordovician) and appear soon after the earliest known typical glyptocystitoid.
A cladistic analysis of the genera listed in Table 1 produced six equally parsimonious cladograms with 50 steps. A strict consensus tree identified the Cambrian genera as a stem group and a crown group starting with a trichotomy between the glyptocystitoids Macrocystella and Lepadocystis. It identified Rhombifera as the most crownward glyptocystitoid, but involved in a second trichotomy with Hemicosmites. The remaining hemicosmitoids, coronates plus Lysocystites and blastoid genera appear as monophyletic crown groups.
Acknowledgements
I am grateful to the following for access to specimens in their care: Richard Jefferies and Andrew Smith (both Natural History Museum, London, UK), Caroline Buttler (National Museum of Wales, Cardiff, UK), John Monteith (Royal Ontario Museum, Toronto, Canada), Albert Brighton and Barrie Rickards (both Sedgwick Museum, Cambridge, UK), Donald Macurda Jr. (University of Michigan, Museum of Paleontology, Ann Arbor, Mich., USA), Porter Kier (United States National Museum of Natural History, Washington, DC, USA). Samuel Zamora (Instituto Geológico y Minero de España, Madrid, Spain) provided the photograph of Rhombifera (Fig. 6B), the original specimen of which is in the collections of the Museo de Ciencias Naturales, Univeridad de Zaragoza, Spain (MPZ 202/590). Figures 6G1, G2 are from the “Geocollections of Estonia” website. Samuel Zamora and Bertrand Lefebvre (Université Lyon 1, France) provided very useful reviews, which improved the manuscript, but any remaining errors are my own.
References
Barrande, J. 1867. Systême Silurien du centre de la Bohême. Première Partie: Recherches paléontologiques. Vol. 3, Classe des Mollusques. Ordre des Ptéropodes. xiv+179 pp. W. Waagen, Prague.
Bassler, R.S. 1935. The classification of the Edrioasteroidea. Smithsonian Miscellaneous Collections 93 (8): 1–11.
Bauer, J., Waters, J.A., and Sumrall, C.D. 2019. Redescription of Macurdablastus and redefinition of Eublastoidea as a clade of Blastoidea (Echinodermata). Palaeontology 62: 1003–1013. Crossref
Billings, E. 1854. On some new genera and species of Cystidea from the Trenton Limestone. Canadian Journal 2: 250–253.
Bockelie, J.F. 1979. Taxonomy, functional morphology and palaeoecology of the Ordovician cystoid family Hemicosmitidae. Palaeontology 22: 363–406.
Bockelie, J.F. 1981. The oral area of Echinoencrinites von Meyer 1826. Norsk Geologisk Tidskrift 61: 79–82.
Breimer, A. 1978. General morphology: Recent crinoids. In: R.C. Moore and C. Teichert (eds.), Treatise on Invertebrate Paleontology, Part T, Echinodermata 2, T9–T58. University of Kansas Press and Geological Society of America, Lawrence, Kansas.
Breimer, A. and Macurda, D.B. Jr 1972. The phylogeny of the fissiculate blastoids. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, afd. Natuurkunde, Eerster Reeks 26 (3): 1–390.
Brett, C.E., Frest, T.J. Sprinkle, J., and Clement, C.R. 1983. Coronoidea: a new class of blastozoan echinoderms based on taxonomic reevaluation of Stephanocrinus. Journal of Paleontology 57: 627–651.
Broadhead, T.W. 1984. Macurdablastus, a Middle Ordovician blastoid from the southern Appalachians. Univerity of Kansas Paleontological Contributions Paper 110: 1–9.
Buch, L. von 1840. Über Sphaeroniten und einige andere Geschlechter, aus welchen Crinoideen entstehen. Verhandlungen Königlich Preussische Akademie der Wissenschaften zu Berlin 1840: 56–60.
Buch, L. von 1846. Über Cystideen eingeleitet durch die Entwicklung der Eigenthumlichkeiten von Caryocrinites ornatus Say. Abhandlungen Königlichen Preussichen Akademie von Wissenschaften 1844: 89–116.
Callaway, C. 1877. On a new area of upper Cambrian rocks in south Shropshire, with a description of a new fauna. Quarterly Journal of the Geological Society of London 33: 652–672. Crossref
Carpenter, P.H. 1884. Report upon the Crinoidea collected during the voyage of HMS Challenger during the years 1873–76, part 1. General morphology with descriptions of the stalked crinoids. Reports of the Scientific Results of the Voyage of HMS Challenger, Zoology 11: 1–442. Crossref
Carpenter, P.H. 1891. On certain points of the morphology of the Cystidea. Journal of the Linnean Society (Zoology) 34: 1–52. Crossref
Conrad, T.A. 1840. Third annual report of T.A. Conrad, on the palaeontological department of the survey [of New York]. Report of the State Geologist of New York 2: 199–207.
Conrad, T.A. 1842. Observations on the Silurian and Devonian systems of the United States, with descriptions of new organic remains. Journal of the Academy of Natural Sciences, Philadelphia 8: 228–280.
David, B. and Mooi, R. 1998. Major events in the evolution of echinoderms viewed in the light of embryology. In: R. Mooi and M. Telford (eds.), Echinoderms, San Francisco, 21–28. Balkema, Rotterdam.
David, B., Mooi, R., and Telford, M. 1995. The ontogenetic basis of Lovén’s Rule clarifies homologies of the echinoid peristome. In: R.H. Emson, A.B. Smith, and A. Campbell (eds.), Echinoderm Research, 155–164. Balkema, Rotterdam.
Donovan, S.K. and Paul, C.R.C. 1985. Coronate echinoderms from the Lower Palaeozoic of Britain. Palaeontology 28: 527–543.
Durham, J.W. and Caster, K.E. 1963. Helicoplacoidea: a new class of echinoderms. Science 140: 820–822. Crossref
Dzik, J. and Orłowski, S. 1993. The late Cambrian eocrinoid Cambrocrinus. Acta Palaeontologica Polonica 38: 21–34.
Ehlers, G.M. and Leighley, J.B. 1922. Lipsanocystis traversensis, a new cystid from the Devonian of Michigan. Papers of the Michigan Academy of Science, Arts and Letters 2: 155–160.
Eichwald, C.E. von 1840. Ueber das Silurische schichtensystem in Esthland. 210 pp. Medizinischer Akademie, St Petersburg. Crossref
Etheridge, R. Jr and Carpenter, P.H. 1886. Catalogue of the Blastoidea in the Geological Department of the British Museum (Natural History). xv + 322 pp. Trustees of the British Museum, London.
Fay, R.O. 1961. Blastoid studies. University of Kansas Paleontological Contributions, Echinodermata Article 3: 1–147.
Fay, R.O. 1962. Ventral structures in Stephanocrinus angulatus Conrad. Journal of Paleontology 36: 206–210.
Frest, T.J. 1975. Caryocrinitidae (Echinodermata: Rhombifera) of the Laurel Limestone of southeastern Indiana. Fieldiana: Geology 30: 81–106. Crossref
Gil Cid, M.D. and Dominguez Alonso, P. 2002. Ubaghsicystis segurae nov. gen. y sp. nuevo eocrinoide (Echinodermata) del Cámbrico del Norte de España. Coloquios de Paleontología 53: 21–32.
Hall, J. 1852. The Natural History of New York. 6. Palaeontology of New York, 2, Containing Descriptions of the Organic Remains of the Lower Middle Division of the New York System. 362 pp. D. Appleton, and Wiley and Putnam, Albany.
Hall, J. 1859. Natural History of New York. 6. Palaeontology of New York. 3. Containing Descriptions and Figures of the Organic Remains of the Lower Helderberg Group and the Oriskany Sandstone. 532 pp. D. Appleton, and Wiley and Putnam, Albany.
Hyman, L.H. 1955. The Invertebrates, Vol. 4, Echinodermata. v +763 pp. McGraw-Hill, New York.
Jaekel, O. 1899. Stammesgeschichte der Pelmatozoen. 1. Thecoidea und Cystoidea. 442 p. Julius Springer, Berlin. Crossref
Jell, P.A. 1983. Early Devonian echinoderms from Victoria (Rhombifera, Blastoidea and Ophiocistioidea). Memoirs of the Association of Australasian Palaeontologists 1: 209–235.
Jell, P.A., Burrett, C.F., and Banks, M.R. 1985. Cambrian and Ordovician echinoderms from eastern Australia. Alcherringa 9: 183–208. Crossref
Kesling, R.V. 1961. Notes on Jaekelocystis hartleyi and Pseudocrinites gordoni, two rhombiferan cystoids described by Charles Schuchert in 1903. Contributions from the Museum of Paleontology, University of Michigan 16: 245–273.
Kesling, R.V. 1962. An interpretation of Rhombifera bohemica Barrande, 1867, an unusual hydrophoridean cystoid. Contributions from the Museum of Paleontology, University of Michigan 17: 277–289.
Kesling, R.V. 1967. Cystoids. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part S, Echinodermata 1 (1), S85–S267. Geological Society of America and University of Kansas Press, Boulder and Lawrence.
Kesling, R.V. and Mintz, L.W. 1961. Notes on Lepadocystis moorei (Meek) and Upper Ordovician callocystitid cystoid. Contributions from the Museum of Paleontology, University of Michigan 17: 123–148.
Kier, P.M. 1968. Echinoids from the middle Eocene Lake City Formation of Georgia. Smithsonian Miscellaneous Collections 153 (2): 1–45.
Lanc, F.A., McDermott, P.D., and Paul, C.R.C. 2015. The identity of the British Ordovician cystoid “Hemicosmites rugatus Forbes”. Geological Journal 50: 1–16. Crossref
Lovén, S. 1874. Études sur les échinoidées: Kongelige Svenska Vetenskapsakademie Handlingar (new series) 11: 1–91.
Macurda, D.B. Jr 1983. Systematics of the fossilulate Blastoidea. Museum of Paleontology, University of Michigan, Papers on Paleontology 22: 1–290.
McCoy, F. 1849. On some new Palaeozoic Echinodermata. Annals and Magazine of Natural History, Series 2, 3: 244–254. Crossref
McDermott, P.D. and Paul, C.R.C. 2015. Coronate echinoderms from the Ordovician of the Llanddowror area, South Wales. Geological Journal 50: 173–188. Crossref
Meek, F.B. 1871. On some new Silurian crinoids and shells. American Journal of Science, Series 3, 2: 295–299. Crossref
Meyer, H. von 1826. Beschreibung des Echino-Encrinites Senckenbergii, einer neu entdeckten Versteinerung. Archiv für der gesammte Naturlehre 7: 185–192.
Miller, S.A. 1889. North American Geology and Palaeontology. 664 pp. Western Methodist Book Concern, Cincinnati.
Miller, S.A. 1891. Advanced Sheets from the Seventeenth Report of the Geological Survey of Indiana, Palaeontology. 94 pp. Published by author, Indianapolis.
Mooi, R. and David, B. 1997. Skeletal homologies of echinoderms. In: J.A. Waters and C.G. Maples (eds.), Geobiology of Echinoderms. Paleontological Society Papers 3: 305–335. Crossref
Mooi, R. David, B., and Marchand, D. 1994. Echinoderm skeletal homologies: classical morphology meets modern phylogenetics. In: B. David, F. Guille, and M. Roux (eds.), Echinoderms Through Time, 87–95. Balkema, Rotterdam. Crossref
Mooi, R., David, B., and Wray, G.A. 2005. Arrays in rays: terminal addition in echinoderms and its correlation with gene expression. Evolution & Development 7: 542–555. Crossref
Nardin, E., Lefebvre, B., David, B., and Mooi, R. 2009. La radiation des échinodermes au Paléozoïque inférieur, l’exemple des blastozoaires. Comptes Rendus Paleovol 8: 179–188. Crossref
Nohejlová, M. and Fatka, O. 2016. Ontogeny and morphology of Cambrian eocrinoid Akadocrinus (Barrandian area, Czech Republic). Bulletin of Geosciences 91: 141–153. Crossref
Orłowski, S. 1968. Upper Cambrian fauna of the Holy Cross Mountains. Acta Geologica Polonica 18: 257–291.
Patterson, C. 1982. Morphological characters and homology. In: K.A. Joysey and K.E. Friday (eds.), Problems of Phylogenetic Reconstruction. Systematics Association Special Volume 21: 21–74.
Patterson, C. 1988. Homology in classical and molecular biology. Molecular Biology and Evolution 5: 603–625.
Paul, C.R.C. 1967a. The British Silurian cystoids. Bulletin of the British Museum (Natural History), Geology 13: 299–355.
Paul, C.R.C. 1967b. The functional morphology and mode of life of the cystoid Pleurocystites E. Billings, 1854. In: N. Millott (ed.), Echinoderm Biology. Symposia of the Zoological Society of London 20: 105–123.
Paul, C.R.C. 1968a. Macrocystella Callaway, the oldest glyptocystitid cystoid. Palaeontology 11: 580–600.
Paul, C.R.C. 1968b. The morphology and function of dichoporite pore structures in cystoids. Palaeontology 11: 697–730.
Paul, C.R.C. 1969. Thomacystis, a unique new hemicosmitid cystoid from Wales. Geological Magazine 106: 190–196. Crossref
Paul, C.R.C. 1972. Cheirocystella antiqua gen. et sp. nov. from the Lower Ordovician of western Utah, and its bearing on the evolution of the Cheirocrinidae (Rhombifera: Glyptocystitida). Brigham Young University Geology Studies 19: 15–63.
Paul, C.R.C. 1982. The adequacy of the fossil record. In: K.A. Joysey and A.E Friday (eds.), Problems of phylogenetic reconstruction. Systematics Association Special Volume 21: 75–117.
Paul, C.R.C. 1984. British Ordovician Cystoids, part 2. Monographs of the Palaeontographical Society 136 (563): 65–152.
Paul, C.R.C. 2015a. A cystoid with two left facets: the significance of Tetracystis in the evolution of the Callocystitidae (Echinodermata: Glyptocystitoida). Geological Journal 50: 607–633. Crossref
Paul, C.R.C. 2015b. Callocystites fresti sp. nov., and the significance of ambulacral branching in the Callocystitidae (Echinodermata, Glyptocystitoida). Geological Journal 50: 189–209. Crossref
Paul, C.R.C. 2017. Testing for homologies in the axial skeleton of primitive echinoderms. Journal of Paleontology 91: 582–603. Crossref
Paul, C.R.C. and Cope, J.C.W. 1982. A parablastoid from the Arenig of South Wales. Palaeontology 25: 499–507.
Paul, C.R.C. and Hotchkiss, F.H.C. 2020. Origin and significance of Lovén’s Law in echinoderms. Journal of Paleontology 94: 1089–1102. Crossref
Paul, C.R.C. and Rozhnov, S.V. 2016. Revision of Scoliocystis (Rhombifera; Echinoencrinitidae) and related cystoid genera. Paleontological Journal 50: 255–275. Crossref
Paul, C.R.C. and Smith, A.B. 1984. The early radiation and phylogeny of the echinoderms. Biological Reviews 59: 443–481. Crossref
Paul, C.R.C., Donovan, S.K., Muir, L.A., Botting, J.P., Lin, J.-P., and Zhang, Y. 2016. Primitive Ordovician (Floian) echinoderms from Sandu, Guizhou Province, South China, and their significance. Geological Journal 51: 143–156. Crossref
Pearce, J.C. 1843. On an entirely new form of encrinite from the Dudley Limestone. Proceedings of the Geological Society, London 4: 160.
Prokop, R.J. 1962. Akadocrinus nov. gen., a new crinoid from the Cambrian of the Jince area. Sbornik Ūstredniho ústavu geologického, Oddil paleontologicky 27: 31–39.
Salter, J.W. 1866. On the fossils of North Wales. Appendix to Ramsey, A.C., The Geology of North Wales. Memoir of the Geological Survey of the United Kingdom 3: 483–538.
Say, T. 1825. On two genera and several species of Crinoidea. Journal of the Academy of Natural Sciences, Philadelphia 4: 289–296.
Sevastopulo, G.D. 2005. The early ontogeny of blastoids. Geological Journal 40: 351–362. Crossref
Smith, A.B. and Zamora, S. 2013. Cambrian spiral-plated echinoderms from Gondwana reveal the earliest pentaradiate body plan. Proceeding of the Royal Society of London B 280: 1–6. Crossref
Sprinkle, J. 1973. Morphology and Evolution of Blastozoan Echinoderms. 284 pp. Special Publication, Museum of Comparative Zoology, Harvard University, Cambridge. Crossref
Sprinkle, J. 1975. The “arms” of Caryocrinites, a rhombiferan cystoid convergent on crinoids. Journal of Paleontology 49: 1062–1073.
Sprinkle, J. and Sumrall, C.D. 2008. New parablastoids from the western United States. University of Kansas Paleontological Contributions 16: 1–14.
Sprinkle, J. and Wahlman, G.P. 1994. New echinoderms from the Early Ordovician of West Texas. Journal of Palaeontology 68: 324–338. Crossref
Sprinkle, J. and Wilbur, B.C. 2005. Deconstructing helicoplacoids: reinterpreting the most enigmatic Cambrian echinoderms. Geological Journal 40: 281–293. Crossref
Sumrall, C.D. 1997. The role of fossils in the phylogenetic reconstruction of Echinodermata. In: N.G. Lane (ed.), The Geobiology of Echinoderms, Paleontological Society Papers 3, 267–288. Paleontological Society, Washington, D.C. Crossref
Sumrall, C.D. 2005. The origin of Lovén’s law in glyptocystitoid rhombiferans and its bearing on the hemicosmitoid peristomal border. Geological Society of America, Abstracts with Programs 37 (7): 63.
Sumrall, C.D. 2008. The origin of Lovén’s law in glyptocystitoid rhombiferans and its bearing on the plate homology and heterochronic evolution of the hemicosmitoid peristomial border. In: W.I. Ausich and G.D. Webster (eds.), Echinoderm Paleobiology, 228–241. Indiana University Press, Bloomington.
Sumrall, C.D. 2010. A model for elemental homology for the peristome and ambulacra in blastozoan echinoderms. In: L.G. Harris, S.A. Böttger, C.W. Walker, and M.P. Lesser (eds.), Echinoderms: Durham, 269–276. Taylor & Francis, London. Crossref
Sumrall, C.D. and Carlson, D.T. 2000. Suture modification by pectinirhomb growth in Lepadocystis decorus, a new species of callocystitid glyptocystitid rhombiferan (Echinodermata) from Illinois. Journal of Paleontology 74: 487–491. Crossref
Sumrall, C.D. and Sprinkle, J. 1999. Early ontogeny of the glyptocystitid rhombiferan Lepadocystis moorei. In: M.D.C. Carnevalli and F. Bonasoro (eds.), Echinoderm Research 1998. Proceedings of the Fifth European Conference on Echinoderms, 409–414. A.A. Balkema, Rotterdam.
Sumrall, C.D. and Waters, J.A. 2012. Universal elemental homology in glyptocystitoids, hemicosmitoids, coronoids and blastoids: steps towards echinoderm phylogenetic reconstruction in derived Blastozoa. Journal of Paleontology 86: 956–972. Crossref
Sumrall, C.D. and Wray, G.A. 2007. Ontogeny in the fossil record: diversification of body plans and the evolution of “aberrant” symmetry in Paleozoic echinoderms. Paleobiology 33: 149–163. Crossref
Swofford, D.L. 2003. PAUP* Version 4.0.b10: phylogenetic analysis using parsimony and other methods. Sinauer Associates, Sunderland.
Wilbur, B.C. 2005. A Revision of Helicoplacoids and Other Early Cambrian Echinoderms of North America. 365 pp. Unpublished Ph.D. Thesis, The University of Texas, Austin.
Wright, D.F. 2015. Fossils, homology, and “Phylogenetic Paleo-ontogeny”: a reassessment of primary posterior plate homologies among fossil and living crinoids with insights from developmental biology. Paleobiology 41: 570–591. Crossref
Zamora, S. 2012. The first Furongian (late Cambrian) echinoderm from the British Isles. Geological Magazine 149: 940–943. Crossref
Zamora, S., Sumrall, C.D., Zhu, X.-J., and Lefebvre, B. 2017. A new stemmed echinoderm from the Furongian of China and the origin of the Glyptocystitida (Blastozoa, Echinodermata). Geological Magazine 154: 465–475. Crossref
Acta Palaeontol. Pol. 66 (1): 41–62, 2021
https://doi.org/10.4202/app.00825.2020