Bone histology of eosauropterygian diapsid Proneusticosaurus silesiacus from the Middle Triassic of Poland reveals new insights into taxonomic affinities
NICOLE KLEIN and DAWID SURMIK
Klein, N. and Surmik, D. 2021. Bone histology of eosauropterygian diapsid Proneusticosaurus silesiacus from the Middle Triassic of Poland reveals new insights into taxonomic affinities. Acta Palaeontologica Polonica 66 (3): 585–598.
The status of Proneusticosaurus silesiacus from the Lower Muschelkalk (lower Anisian) of Poland is controversially discussed. Its femur was histologically sampled to learn more about its taxonomic affinities and life style. It shows a reduced central medullary cavity surrounded by a narrow medullary region and followed by a thick compact cortex, displaying strong osteosclerosis. The tissue type can be summarized as lamellar-zonal. The inner third of the cortex consists of well vascularized parallel-fibred bone and is interpreted as a phase of juvenile growth, whereas the middle and outer cortex is made of highly organized parallel-fibred to lamellar bone. Except for the inner cortex and local accumulations of longitudinally primary osteons in the middle cortex, the femur is widely avascular. The cortex of P. silesiacus is regularly stratified by rest lines. Altogether seven annual growth cycles are counted. The very low growth rate of P. silesiacus, as deduced from tissue type, makes taxonomical affinities to Nothosaurus spp. or basal pistosauroids (i.e., humeri of aff. Cymatosaurus sp.) very unlikely. Proneusticosaurus silesiacus femoral histology does also not match any of the here studied femora of Eosauropterygia indet. Proneusticosaurus silesiacus shares similar low growth rates, the osteosclerotic femur, as well as pachyostotic vertebrae and ribs with the pachypleurosaurs Dactylosaurus gracilis and Neusticosaurus spp. and with the nothosaur Lariosaurus sp. These features are, however, most likely convergent and reflect the degree of secondary aquatic adaptation in shallow marine inhabitants. The high organized and low vascularized tissue, the implied low growth rate, the plesiomorphic femur morphology, and the strongly inclined zygapophyses of the vertebrae (contra roughly horizontal zygapophyses in other Eosauropterygia), makes P. silesiacus unique and evidence that this genus represents a valid genus within early Eosauropterygia. Proneusticosaurus silesiacus might represent one of the most basal members of Eosauropterygia so far known.
Key words: Sauropsida, aquatic adaptation, bone histology, marine reptiles, morphology, aquatic adaptation, Lower Muschelkalk, Poland.
Nicole Klein [nklein@posteo.de], Institute of Geosciences, Palaeontology, University of Bonn, Nußallee 8, 53115 Bonn, Germany.
Dawid Surmik [dawid.surmik@us.edu.pl], Institute of Earth Sciences, Faculty of Natural Sciences, University of Silesia, Ul. Bedzinska 60, 41-200 Sosnowiec.
Received 27 October 2020, accepted 15 December 2020, available online 9 September 2021.
Copyright © 2021 N. Klein and D. Surmik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Sauropterygia was a diverse group of secondarily marine reptiles, which appeared during the recovery after the Permo-Triassic extinction event. They are first documented by different taxa in the Olenekian (upper Lower Triassic) of China (Majiashanosaurus discocoracoidis, Jiang et al. 2014; Lariosaurus sanxianensis, Li and Liu 2020) and Canada (Nothosauroidea indet., Scheyer et al. 2019), in the Olenekian to lower Anisian (upper Lower to lower Middle Triassic) of North America (Corosaurus alcovensis, Case 1936; see Lovelace and Doebbert 2015 for a recent evaluation of the stratigraphical age of the Alcove limestone), and in the lower Anisian (lower Middle Triassic) of Poland (SE Germanic Basin; Dactylosaurus gracilis, Nothosaurus spp., Placodontia indet., Cymatosaurus spp. e.g., Gürich 1884; Schrammen 1899; Kowal-Linka 2015).
Middle Triassic Sauropterygia consists of Pachypleurosauria, Nothosauria, Pistosauria, and Placodontia (Rieppel 2000). The first three groups form the Eosauropterygia, of which at least Nothosauria and Pachypleurosauria occur in high individual numbers throughout the Muschelkalk bonebeds (e.g., Meyer 1847–1855; summarized in Rieppel 2000; Klein et al. 2015b).
One character that uniforms Eosauropterygia is the “butterfly”-shaped platform on vertebral centra resulting from the articulation with the pedicels of the neural arch (Rieppel 2000). This character is easy to identify because usually the centra and neural arches occur disarticulated. Due to the rarity of complete skeletons from Muschelkalk bonebeds, alpha taxonomy of these Eosauropterygia is largely based on cranial characters (Rieppel 2000). Taxonomic assignment of isolated postcranial bones is additionally hampered because the postcranium of Eosauropterygia is very uniform overall, which is also a result of secondary aquatic adaptation (e.g., long neck and tail, flat girdle bones, partial or fully-pachyostotic ribs and vertebrae). Further on, morphological changes during ontogeny, sexual dimorphism, and intraspecific variation in these taxa obscures correct identification.
In 1902, Volz described two partial skeletons from the Gogolin Beds (Lower Muschelkalk) of Zakrzów (formerly Sacrau) and Gogolin (Upper Silesia, Poland). Each specimen was assigned to a different species of a newly-erected genus Proneusticosaurus: Proneusticosaurus madelungi Volz, 1902, and Proneusticosaurus silesiacus Volz, 1902. The generic name was chosen due to overall similarities of the two new specimens with Neusticosaurus pusillus Fraas, 1881, from the Ladinian of Hoheneck (Volz 1902). The main differences with Neusticosaurus Volz (1902) found are the larger body size of Proneusticosaurus and the presence of 6 sacral ribs (compared to 3 in Neusticosaurus) and different ratios of lower to upper limb bones. He established the two species of Proneusticosaurus based on minor morphological differences between the two specimens (Volz 1902). However, most of these differences Volz (1902) listed are likely referable to intraspecific variation and/or are the result of preservation. Of these two specimens of Proneusticosaurus, only the posterior part of the holotype of P. silesiacus (MGU Wr. 4438s; Fig. 1) can nowadays be located. The holotype of P. madelungi and the rest of the skeleton of P. silesiacus were lost during World War II (Rieppel and Hagdorn 1997).
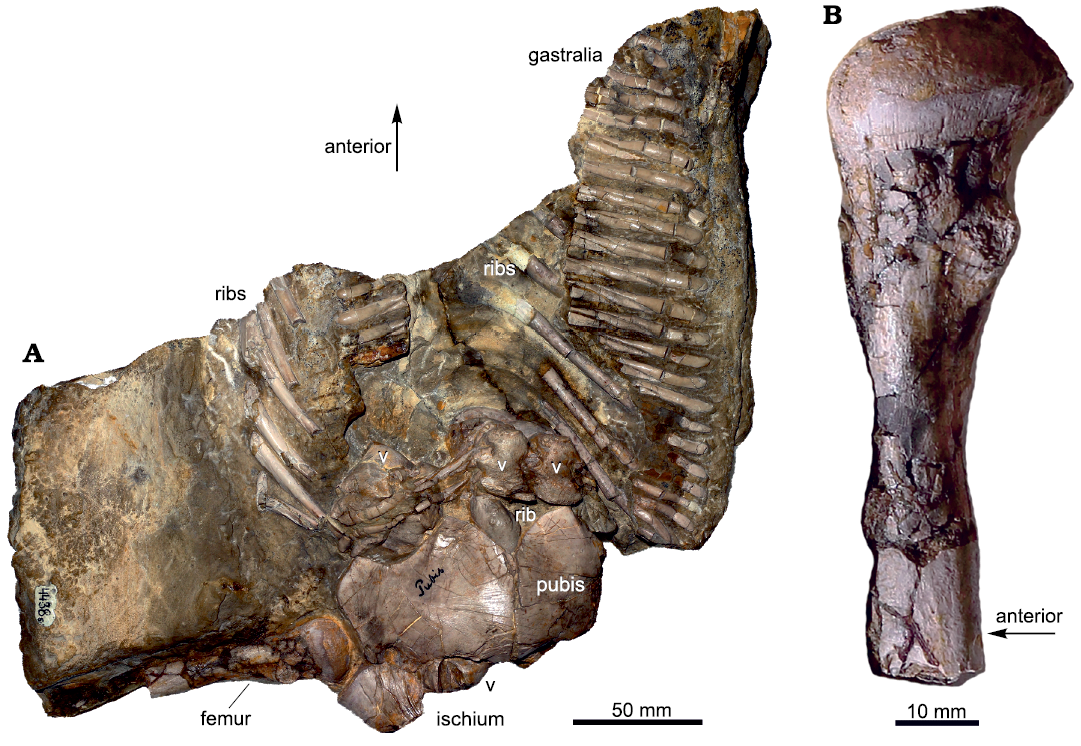
Fig. 1. Remaining specimen of the eosauropterygian diapsid Proneusticosaurus silesiacus Volz, 1902 (MGU Wr. 4438s), from the Lower Muschelkalk of Poland. A. Photograph of the remaining part of the holotype. B. Distally incomplete femur. Sampling location is indicated by the black arrow, the grey arrow marks the position of the femur at the slab. Arrows indicating the anterior orientation is only an estimate because the specimen is partially disarticulated. Abbreviations: v, vertebra. For more details see Volz (1902) and Rieppel and Hagdorn (1997) who both provided outline sketches of the bones preserved with the slab.
Besides Proneusticosaurus spp., the Lower Muschelkalk of Upper Silesia, Poland, had yielded representatives of at least four genera of Eosauropterygia: the pachypleurosaur Dactylosaurus and the eusauropterygians Nothosaurus, Germanosaurus and Cymatosaurus (Rieppel 2000). The latter two taxa are known exclusively from skull material.
Proneusticosaurus spp. was unequivocally identified as a member of Eosauropterygia due to the expanded butterfly-shaped platform on the centra (Rieppel and Hagdorn 1997). In addition, the morphology of the preserved bones fits general eosauropterygian morphology (Rieppel and Hagdorn 1997; this study). Sues (1987) suggested an assignment of the two postcrania of Proneusticosaurus to Cymatosaurus. Cymatosaurus is interpreted as a basal pistosauroid, which is among others known from the same beds as Proneusticosaurus spp. However, Cymatosaurus spp. is so far only known from cranial material (summarized in Rieppel 2000 and Klein 2019). Over the years, different attempts have been made to assign postcranial material to that genus (Sues 1987; Rieppel 1994; Rieppel and Hagdorn 1997; Klein 2010; Sander et al. 2014), which all remain rather hypothetical due to the lack of a skull articulated to or at least associated with any postcranial material (except for gastralia, see Schrammen 1899).
Rieppel and Hagdorn (1997) gave a detailed morphological comparison of Proneusticosaurus silesiacus with Nothosaurus spp. and found some shared characters but also listed a number of morphological differences, concluding that an “unequivocal identification” of the taxonomical affinities of P. silesiacus is impossible. However, they found synonymy of P. silesiacus with Cymatosaurus spp. likely (Rieppel and Hagdorn 1997). They described vertebrae similar to those of Proneusticosaurus sp. from the Vicentinian Alps and “from Lower Muschelkalk localities in the central part of the Germanic Basin” (Rieppel and Hagdorn 1997: 126).
Lately, the holotype of P. silesiacus was studied by Surmik et al. (2018) who described a tuberculosis-like respiratory infection in that specimen. In the course of that study, also the histology of dorsal ribs of P. silesiacus was described.
Another attempt to refer postcranial material to Cymatosaurus spp. was made by Rieppel (1994). Rieppel (1994) suggested a certain, in Lower to Middle Muschelkalk localities, quite common humerus morphotype to assign to cf. Cymatosaurus spp. This assignment was supported by Klein (2010) who studied humerus histology of Triassic Sauropterygia. The argument of Klein (2010) to assign the humerus morphotype described by Rieppel (1994) to the pistosauroid line was its characteristic bone tissue type that indicates high growth rates (Klein 2010), which are also typical for the pistosaur and plesiosaur line (Krahl et al. 2013; Wintrich et al. 2017).
The allocation of a skeleton of a possible basal pistosauroid from the Lower Muschelkalk of Winterswijk (The Netherlands) largely based on its humerus histology (Klein 2010; Sander et al. 2014), too. However, the morphology of this postcranial material differs from P. silesiacus (Sander et al. 2014). Voeten et al. (2014) discussed large isolated eosauropterygian bones from Winterswijk and among this material is one large pubis and one ischium from Winterswijk that both show a striking resemblance to P. silesiacus.
The current study aims to shed more light on the possible taxonomic affinities of P. silesiacus with the help of bone histology. The microanatomy, bone histology and growth pattern of the femur of P. silesiacus are described in detail and compared with femora of other Eosauropterygia. In addition, some morphological characters of P. silesiacus are revisited.
Institutional abbreviations.—IGPB, Institute of Geosciences, Paleontology, Bonn, Germany; IGWH, Institute of Geosciences of the Martin-Luther-University Halle-Wittenberg, Germany; MfN (MB.R.), Museum of Natural History, Leibniz Institute for Research on Evolution and Biodiversity at the Humboldt University Berlin, Germany; MHI, Muschelkalkmuseum Ingelfingen, Germany; MGU Wr., Institute of Geological Sciences, University of Wrocław, Poland; SMNS, State Museum of Natural History, Stuttgart, Germany; Wijk (NMNHL RGM), National Museum of Natural History Naturalis, Leiden, The Netherlands; TWE, Museum TwentseWelle, Enschede, The Netherlands.
Other abbreviations.—BC, bone compactness; LAG, line of arrested growth.
Material and methods
The midshaft of the right femur of the holotype of Proneusticosaurus silesiacus (MGU Wr. 4438s) was sampled (Fig. 1). For comparison, eosauropterygian femora from different stratigraphical horizons (middle Anisian to upper Ladinian) and localities (central and southern Germany, Poland, and The Netherlands) had been studied as well (SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Klein_Surmik_SOM.pdf). Because of a relatively uniform morphology of eosauropterygian femora, a taxonomical assignment based on morphology and size is possible only for small femora of the pachypleurosaurian aff. Neusticosaurus sp. from the Ladinian of Kirchheim and for large femora of Nothosaurus spp. from the upper Anisian to upper Ladinian of southern Germany (SOM: table 1). Some femora had been assigned to Anarosaurus heterodontus Rieppel and Lin, 1995, because they were found associated with other skeletal material (Klein 2012) or because of their characteristic histology (see below; SOM: table 1; Klein 2010). However, due to preservation, unknown ontogenetic, sexual, and interspecific variability as well as due to the incomplete nature of most of these femora available for destructive sampling, a taxonomical assignment was not possible for other samples (SOM: table 1). A former assignment of some of these femora (Klein 2010) could not be unequivocally confirmed.
All samples had been processed into petrographic thin sections following standard methods (Klein and Sander 2007). Histological terminology follows Francillon-Vieillot et al. (1990). The thin sections were studied under a Leica DM2500LP polarizing microscope. Digital photomicrographs were taken with a Leica DFC420 mounted colour camera and edited using the 2007 Leica Image Access EasyLab 7 software. The surface ratio between the medullary and cortical region (SOM: table 1) and bone compactness were measured with a pixel-counting computer program (© Peter Göddertz, IGPB). Microtomographic scans of the entire slab containing the specimen were collected by using GE Phoenix v|tome|x s microCT scanner, installed in the Microtomography Laboratory at the Faculty of Computer Science and Material Science, University of Silesia in Chorzów. The scan was based on 200 images. Kilovolt and microampere were set to 210 kV and 300 μA, respectively, with a voxel size of 123.382 μm.
Results
Microanatomical description.—A small central medullary cavity (in fact more a large, central irregularly shaped erosion cavity) covers only 0.4% of the entire area of the femoral cross section of Proneusticosaurus silesiacus; that of the surrounding medullary region, which is separated from the periosteal cortex by a sharp line (Figs. 2A2, 3A2) 5.5% (SOM: table 1). Vascular density is moderate in the inner cortex and low to avascular throughout the rest of the cortex (Fig. 2, 3). Bone compactness (medullary cavity and vascularity vs. bone tissue) of the entire section is 97.3%. The vascularized inner cortex has a BC of 92.5% and the low to nearly avascular outer cortex has a BC of 98%. Thus, the sample shows a very compact inner structure and is osteosclerotic (Figs. 2A1, A2, 3; SOM: fig. S1). Osteosclerosis is achieved by the reduction of the medullary cavity, an overall thick and low vascularised cortex, and by the inhibition of endosteal and periosteal resorption (see below).
Histological description.—The small medullary region (endosteal domain) of the femur of P. silesiacus surrounding the reduced medullary cavity is separated by a sharp line (Klein and Griebeler 2018) from the periosteal domain (Figs. 2A1, A2, 3A2). The medullary region contains endosteal bone, few small erosion cavities and remains of calcified cartilage (Fig. 2A2). Periosteal bone tissue consists in the inner cortex of parallel-fibred tissue and in the middle and outer half of the section of highly organized parallel-fibred to lamellar bone (Fig. 2). The inner third of the cortex shows a moderate vascular density, consisting of a mixture of simple vascular canals, incompletely lined primary osteons, and fully developed primary osteons, as well as very few secondary osteons (Fig. 2A1–A4). Orientation of canals is primarily longitudinal with a few radial and reticular ones (Fig. 2A3, A4). The rest of the cortex is largely avascular except for some areas where mainly longitudinal primary osteons have locally accumulated (Fig. 2A5). These local accumulations occur in the middle and outer cortex. The longitudinal primary osteons are intermixed with few short radial and reticular simple vascular canals, as well as primary osteons. Periosteal remodelling is restricted to the innermost cortex and occurs in form of few secondary osteons (Fig. 2A3, A4).
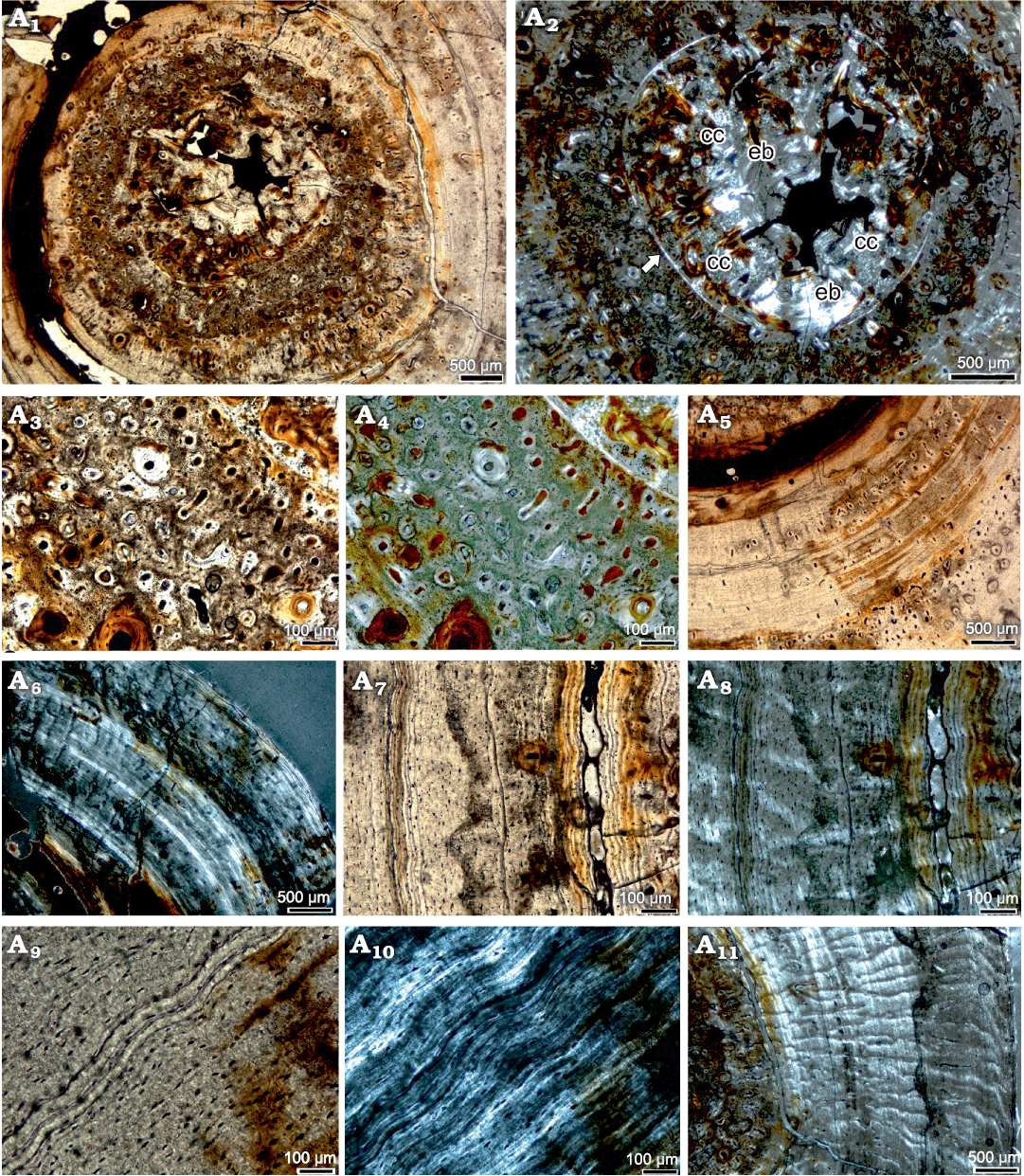
Fig. 2. Bone histological details of the femur of the eosauropterygian diapsid Proneusticosaurus silesiacus Volz, 1902 (MGU Wr. 4438s), from the Lower Muschelkalk of Poland. Detail of inner cortex, i.e., the medullary region and juvenile bone tissue in normal (A1) and polarized (A2) light. Note the remains of calcified cartilage and small erosion cavities in the medullary region as well as the well vascularized surrounding periosteal tissue; the white arrow marks the sharp line, which separates the endosteal from the periosteal region. Detail of low organized and well vascularized parallel-fibred tissue, here interpreted as juvenile tissue in normal (A3) and polarized (A4) light. Note the mixture of primary osteons, simple vascular canals and a secondary osteon. Highly organized parallel-fibred tissue in the middle cortex showing local accumulation of primary osteons in normal light (A5). Middle and outer cortex in polarized light (A6). Note the highly organized parallel-fibred tissue, which is stratified by thin bright layers formed by multiple rest lines. Detail of inner and middle cortex in normal (A7) and polarized (A8) light. Both cycles end in multiple rest lines with the inner cycle being three times broader than the outer cycle. Note that the inner cycle is additionally broadened by a wide crack. Detail of such an accumulation of rest lines in normal (A9) and polarized (A10) light. Note that they appear in this figure not as bright layer due to a different fibre orientation under the crossed nicolls. Note the lamellar tissue. Radial funnel shaped arrangement of crystallites in polarized light in the middle and outer cortex (A11). Abbreviations: cc, calcified cartilage; eb, endosteal bone.
In polarized light, a funnel-shaped arrangement of crystallites is obvious, resulting in a radial pattern and giving the cortex in polarized light an appearance as if traversed by small canyons (Fig. 2A11). A funnel-shaped radial arrangement of crystallites of the highly organized tissue is also documented for the pachypleurosaurs Dactylosaurus sp., aff. Neusticosaurus sp., and Neusticosaurus spp. (Klein and Griebeler 2018), as well as for Nothosaurus spp. (Klein 2010; Klein et al. 2016).
Comparison of rib histology.—Both, the petrographic thin section as well as the X-ray microtomographic images figured by Surmik et al. (2018) show a very compact (= osteosclerotic), low vascularized and high organized tissue, regularly stratified by growth marks (Surmik et al. 2018: figs. 2, 3). Vascularization consists solely of longitudinal primary osteons, which accumulate in mediolateral growth direction underneath rest lines in an otherwise nearly avascular tissue. This accumulation of vascular canals in certain regions is also evident in the femur sample of P. silesiacus (see above). Histology and microanatomy of the rib of MGU Wr. 4438s reflects what is here described for the femur. Growth mark count of the rib revealed a similar age in years as gained by the femur (Surmik et al. 2018).
Growth pattern of Proneusticosaurus.—The entire cortex of the femur (and rib) of P. silesiacus is regularly stratified by growth marks in form of rest lines (Fig. 3). Due to the highly organized tissue throughout and the only locally occurring accumulation of vascular canals, zones and annuli cannot be distinguished. The inner cortex shows a lower organized and better vascularized parallel-fibred tissue, which is easy to distinguish from the outer 2/3 of the cortex, also due to the higher vascular density here (Figs. 2A1, A2, 3). This inner tissue is interpreted as remains of the tissue of the faster growing juvenile individual (Klein and Griebeler 2018). This juvenile tissue is divided by a clear LAG and by an increase of tissue organisation and a decrease in vascular density into two parts (Fig. 3). Thus, the inner juvenile tissue represents two annual growth cycles (Fig. 3). The inner juvenile tissue makes up roughly 1/3 of the entire cortex. It is separated from the rest of the cortex by another distinct LAG (visible LAG 2; Fig. 3A1: grey arrows) that is followed by a nearly avascular highly organized parallel-fibred to lamellar tissue. Due to the presence of this juvenile tissue, it can be concluded that the growth record is fairly complete and no inner cycles are lost by expansion of the medullary region or by periosteal remodelling. The change in tissue from the juvenile to the following tissue (after LAG 2) marks an important change in life history. Conceivable are a change in food preferences and/or lifestyle as is known for many modern squamates such as for varanids. It is also possible that with this tissue change the onset of sexual maturity is marked, which would have happened then after the 2nd year of life but at a very small size (1/3 of size at death). The following growth cycles had been deposited at much lower growth rates due to high tissue organization and low vascularization. Accumulations of rest lines (Fig. 2A7–A10) throughout the high organized tissue are interpreted as representing the end of annual growth cycles. In polarized light, these rest lines appear as a bright thick line (Fig. 2A6, A10). Annual growth cycles are identified by their presence all around the cross section whereas subcycles are defined as rest lines or layers of highly organized avascular tissue that cannot be followed all around the cross section (summarized in Klein et al. 2015a). In polarized light, subcycles are visible in the cortex of P. silesiacus dividing the annual growth cycles (Fig. 3A2). Altogether, seven annual growth cycles can be counted.

Fig. 3. Growth pattern in the femoral cross section of the eosauropterygian diapsid Proneusticosaurus silesiacus Volz, 1902 (MGU Wr. 4438s), from the Lower Muschelkalk of Poland; in normal (A1) and polarized (A2) light. Arrows indicate the end of annual growth cycles. Altogether seven annual cycles are counted. The individual thus died in its 8 year of life. The innermost juvenile tissue is also divided by a clear growth mark, accompanied by a distinct change in tissue organization and vascularization. In polarized light subcycles are obvious, marked by grey arrows.
Comparison
Pachypleurosauria.—The parallel-fibred tissue of femora of the pachypleurosaur Anarosaurus heterodontus is less organized and higher vascularized (partially grading into incipient fibro-lamellar bone) when compared to other pachypleurosaurs, nothosaurs and Proneusticosaurus silesiacus, indicating higher growth rates in Anarosaurus heterodontus (Klein 2010; Klein and Griebeler 2018; SOM: text and fig. S2). The growth pattern in Anarosaurus heterodontus is also different when compared to other Eosauropterygia and P. silesiacus because most of the cortex of Anarosaurus heterodontus does not show distinct growth marks and a clear juvenile tissue cannot be identified. The latter is likely due to the relatively large medullary cavity that has resorbed inner tissue during its expansion. In addition, most of the cortex of Anarosaurus heterodontus looks like juvenile tissue and due to the lack of distinct growth marks an inner juvenile tissue is hard to separate from the rest. The microanatomy of Anarosaurus heterodontus is characterized by the presence of a moderately sized medullary cavity, which results in less osteosclerotic bones when compared to other pachypleurosaurs, some Nothosaurus spp. (Fig. 4; but not all, see Klein et al. 2016; SOM: text and fig. S1), and P. silesiacus. All these histological features are also evident in the humeri of Anarosaurus heterodontus (Klein 2010; Klein and Griebeler 2018). Bone histology and microanatomy as well as growth pattern of Anarosaurus heterodontus thus, clearly differs from P. silesiacus.
Histology, microanatomy and growth pattern of femora of aff. Neusticosaurus sp. from the Ladinian of Kirchheim (Fig. 4A; SOM: text and fig. S2) resembles what was described before for humeri of aff. Neusticosaurus pusillus from the Ladinian of southern Germany (Klein and Griebeler 2018) but differ slightly from Neusticosaurus spp. from the Alpine Triassic (Sander 1990; Hugi et al. 2011; Klein and Griebeler 2018). Differences are related to generally higher vascular density throughout the entire cortex, by the retainment of high amounts of calcified cartilage in the medullary region, and by the lack of juvenile tissue in the innermost cortex in most samples of Neusticosaurus spp. from the Alpine Triassic (Sander 1990; Hugi et al. 2011; Klein and Griebeler 2018). Humeri of the pachypleurosaur Dactylosaurus sp. from the Lower Muschelkalk of Poland (Klein and Griebeler 2018) also show generally higher vascular density when compared to aff. Neusticosaurus sp. from the Ladinian of Kirchheim. Both taxa share the endosteal filling of inner cavities and the presence of a juvenile tissue in the innermost cortex in some samples. The femur of P. silesiacus shares with the humeri of the pachypleurosaur Dactylosaurus sp., for which no femur samples are available for comparison, and with humeri and femora of the pachypleurosaur Neusticosaurus spp. the highly organized tissue and the high degree of osteosclerosis (Hugi et al. 2011; Klein and Griebeler 2018; SOM: text, table 1, fig. S2). However, vascular density in Dactylosaurus sp. from Poland and in Neusticosaurus spp. from the Alpine Triassic is higher and shows a mainly radial orientation of simple vascular canals (Hugi et al. 2011; Klein and Griebeler 2018), compared to the nearly avascular tissue with only few local accumulations of longitudinal primary osteons in Proneusticosaurus. The samples of aff. Neusticosaurus sp. from Kirchheim (SOM: text and fig. S2) and from southern Germany (aff. Neusticosaurus pusillus, Klein and Griebeler 2018) however, show a similar low to avascular tissue in the middle and outer cortex as P. silesiacus does.
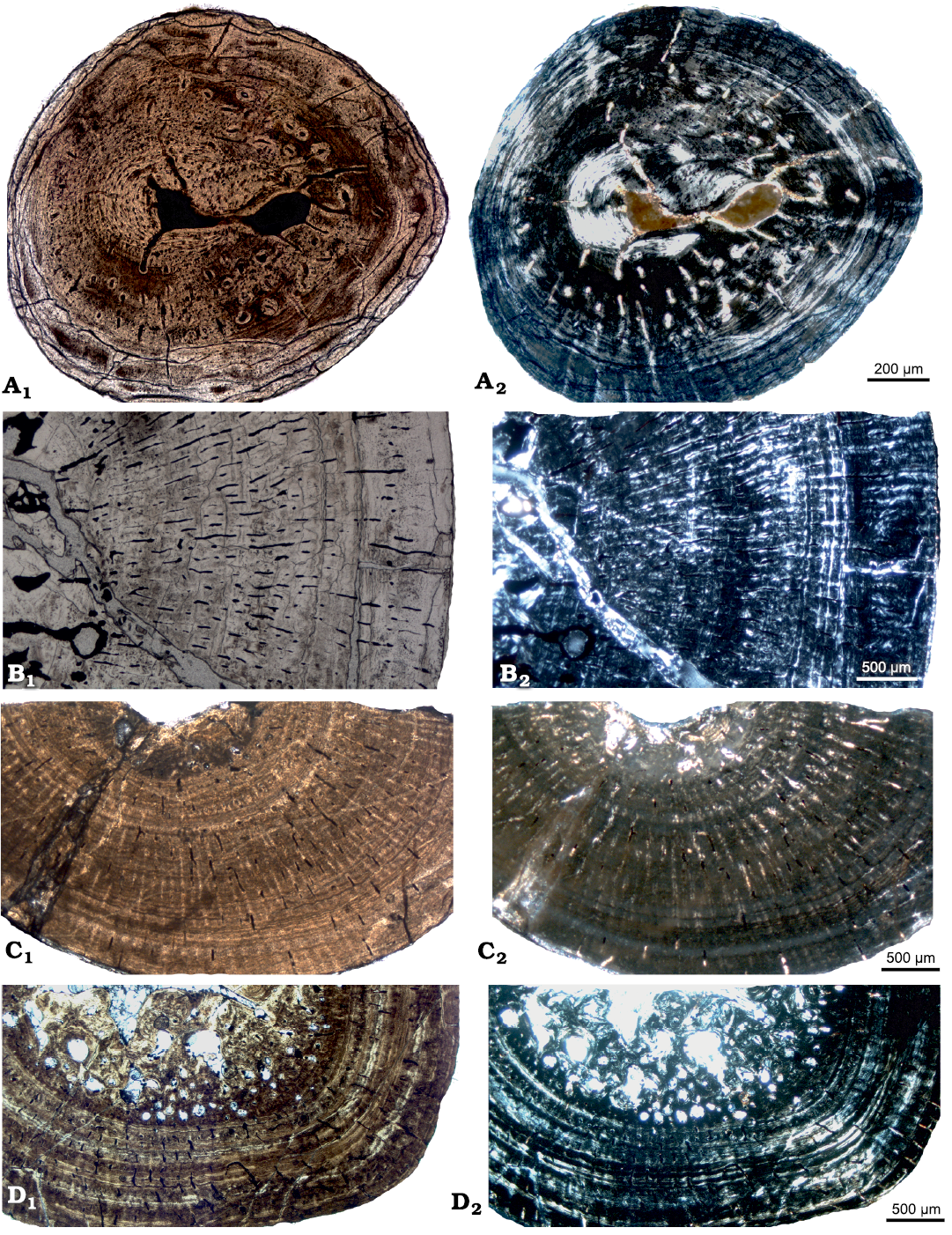
Fig. 4. Femoral histology of various eosauropterygians for comparison. See also SOM. A. aff. Neusticosaurus sp. (IGPB fe IV), Kirchheim near Würzburg, southern Germany, Muschelkalk/Keuper Grenzbonebed, early Ladinian. Note the well vascularized and low organized parallel-fibred tissue in the inner half of the cortex (i.e., juvenile tissue) and the high organized lamellar bone in the outer cortex with numerous rest lines. B. Large Nothosaurus sp. (SMNS 59373), Saarlouis at the highway A8, ?Ladinian. Note the moderately vascularized parallel-fibred tissue with clear growth marks that are not regularly spaced. C. Eosauropterygia indet. (?small Nothosaurus sp., Wijk06-14), Winterswijk, early Anisian. Note the low vascularized and high organized parallel-fibred tissue and the distinct growth marks. D. Eosauropterygia indet. (Wijk05-10), Winterswijk, early Anisian. Note the regularly spaced growth marks and a high organized and moderately vascularized tissue. In normal (A1–D1) and polarized (A2–D2) light.
Bone mass increase in all these pachypleurosaur taxa is achieved by a thick-walled, low, or nearly avascular compact cortex and endosteal remodeling, i.e., by filling of inner cavities by endosteal bone (Dactylosaurus sp., aff. Neusticosaurus sp. from the Germanic Basin) or by the presence of a dense medullary region made of calcified cartilage, endosteal bone, and small erosion cavities (Neusticosaurus spp. from the Alpine Triassic, P. silesiacus).
Growth rate in these pachypleurosaurs is comparably low to that of P. silesiacus. The growth pattern differs in humeri of Dactylosaurus sp. and in humeri and femora of aff. Neusticosaurus sp. and Neusticosaurus spp., when compared to the femur of P. silesiacus, due to the presence of an alternating sequence of zones, annuli and LAGs in the first three taxa. They all share the presence of juvenile tissue in the innermost cortex.
Except for the differences in the sequences of the growth marks and the higher vascular density, Dactylosaurus sp. and Neusticosaurus spp. share most histological and microanatomical features (Sander 1990; Hugi et al. 2011; Klein and Griebeler 2018, this study) with P. silesiacus. The highly organized tissue observed in femora of aff. Neusticosaurus sp. is closest to what is observed in the femur of P. silesiacus.
Nothosauroidea.—Femora of of Nothosaurus spp. and Simosaurus gaillardoti Meyer, 1842, show a similarly wide spectrum of differently organized parallel-fibred tissue (in some grading into lamellar tissue), vascular pattern (i.e., density and organization), and microanatomy (spanning from strongly osteosclerotic to a reduced bird-like cortex, i.e., bone mass decrease) (SOM: text and fig. S3) as their humeri do (Krahl et al. 2013; Klein et al. 2016). Tissue type (i.e., vascular pattern and tissue organization) of nothosaurs (Fig. 4) indicates a generally lower growth rate when compared to Anarosaurus heterodontus but it is clearly higher when compared to Dactylosaurus sp. and Neusticosaurus spp., and to most small to medium sized eosauropterygian femora (SOM: text and figs. S4, S5) and to P. silesiacus. Growth marks in Nothosaurus sp. and Simosaurus gaillardoti usually appear in a sequence of zones, annuli and LAGs, which are not to distinguish in P. silesiacus. An inner juvenile tissue is not identified in nothosaurs. The strong osteosclerosis of the femur of P. silesiacus is within the range of some femora of some large Nothosaurus spp. (SOM: table 1 and fig. S1). Microanatomy of small taxa of Nothosaurus spp. and Simosaurus gaillardoti indicates a moderate osteosclerosis (Klein 2010; Klein et al. 2016). Femora and humeri of Nothosaurus spp. (Klein et al. 2016) and Simosaurus gaillardoti (Klein and Griebeler 2016) display similar histological and microanatomical signals. As for the humeri (Klein et al. 2016), it cannot be clarified if the variety in tissue type, microanatomy and growth patterns is the result of ontogenetic and/or intraspecific variability, sexual dimorphism and/or taxonomical differences or if it is related to an overall high developmental plasticity. The bone histology, microanatomy and growth pattern of Nothosaurus spp. and Simosaurus gaillardoti are quite different from what is observed in P. silesiacus.
Hugi (2012) described femoral and rib histology of Ceresiosaurus spp. from the early Ladinian of the southern Alps. Ceresiosaurus spp. is an eosauropterygian, which was treated by Rieppel (2000) as junior synonym of the nothosaur Lariosaurus but was regarded as a valid taxon by Hänni (2004). The femur of Ceresiosaurus calagnii Peyer, 1931, is pachyosteosclerotic with a medullary region made of calcified cartilage, erosion cavities and endosteal bone (Hugi 2012). The thick periosteal cortex consists of lamellar and parallel-fibred bone, displaying a low to moderate vascular density. The vascular pattern consists of simple radial and longitudinal vascular canals, as well as primary osteons of the same orientation. LAGs and subcycles are regularly distributed throughout the cortex and the inner cortex shows juvenile tissue as indicated by the presence of woven bone (Hugi 2012: fig. 2). As Hugi (2012) already pointed out, the bone histology and microanatomy of Ceresiosaurus calagnii differs largely from that of closely related Nothosaurus spp. but is most similar to what is observed in Neusticosaurus edwardsii (Hugi et al. 2011). The highly organized and low vascularized tissue, the degree of osteosclerosis, and the growth pattern of Ceresiosaurus calagnii are similar to P. silesiacus.
Eosauropterygia indet.—All these femora (Fig. 4; SOM: table 1) grew with parallel-fibred tissue with mainly longitudinal and radial vascular canals, that can occur as simple vascular canals and/or as primary osteons (SOM: text and figs. S4, S5). In all femora of Eosauropterygia indet. periosteal remodelling is inhibited as is the case in P. silesiacus. Endosteal remodelling occurs in some of these eosauropterygian femora, but it is negligible in the femur of P. silesiacus in spite of its advanced ontogenetic stage. The size of the medullary cavities or regions and the degree of osteosclerosis varies (SOM: fig. S1) but none resembles the strong reduction of the medullary cavity that is observed in P. silesiacus. This applies also for the identified growth patterns for Eosauropterygia indet. femora that can usually be separated into zones and annuli but have no juvenile tissue preserved (SOM: text and figs. S4, S5). Thus, none of these femora is in tissue type, microanatomy or growth pattern similar to P. silesiacus.
Pistosauroidea.—Humeri assigned to aff. Cymatosaurus sp. show a characteristic tissue type (Klein 2010) that consists of poorly organized and moderately vascularized parallel-fibred tissue (annuli) alternating with incipient fibro-lamellar bone (zones) having a high vascular density, with mainly radially organized vascular canals. The microanatomy of humeri of aff. Cymatosaurus sp. is also distinct and differs from other Eosauropterygia by the presence of a small medullary cavity, which is filled by endosteal bone during ontogeny. The growth pattern in humeri of aff. Cymatosaurus sp. reveals juvenile tissue in the innermost cortex, followed by an alternating sequence of zones made of incipient fibro-lamellar bone and annuli made of parallel-fibred tissue ending in LAGs. However, none of the sampled femora clearly show these criteria as described by Klein (2010) and thus could not unequivocally be assigned to aff. Cymatosaurus sp. Based on what is known for other taxa (Anarosaurus heterodontus, Neusticosaurus spp., Nothosaurus spp.), it is not to be expected that femora of aff. Cymatosaurus sp. depict a completely different tissue type when compared to their humeri. In spite of a similar tendency towards strong osteosclerosis and the presence of juvenile tissue, the tissue type of aff. Cymatosaurus sp. is very different from that of P. silesiacus.
Comparison of rib histology.—The tissue, vascularization, vascular density, and/microanatomy of the dorsal ribs of P. silesiacus (Surmik et al. 2018; see above) resemble those of the femur as is the case in other Sauropterygia (Klein et al. 2019). Proneusticosaurus silesiacus rib histology and microanatomy differ distinctly when compared to ribs of Nothosaurus spp. and the pachypleurosaur Anarosaurus heterodontus (Klein et al. 2019) but is similar to what is described for Ceresiosaurus calagnii (Hugi 2012).
In conclusion, none of the eosauropterygian humeri, femora, or ribs studied so far resemble exactly the combination of osteosclerosis (microanatomy), highly organized, nearly avascular tissue type (histology) and growth pattern (juvenile tissue preserved in the inner cortex [i.e., no remodelling and resorption], followed by a cortex that is regularly stratified by rest lines but without a further separation into zones and annuli) that the femur and rib of P. silesiacus show. Although none of the samples studied so far displays an exact agreement, bone tissue type and the microanatomy of femora of the pachypleurosaurs Dactylosaurus sp. and aff. Neusticosaurus sp. from the Germanic Basin and of the nothosaur Ceresiosaurus calagnii from the Alpine Triassic seem closest to P. silesiacus due to the shared strong osteosclerosis, the highly organized and poorly vascularized tissue, and a similar growth pattern.
Another result of this study is that the phylogenetic signal in the histology of eosauropterygian femora is less pronounced than in their humeri (Klein 2010). Isolated femora are thus less suited to address taxonomical affinities.
Discussion
Histology.—We attempt to clarify the unclear and controversially discussed (Volz 1902 vs. later authors such as Sues 1987 and Rieppel and Hagdorn 1997) taxonomical affinities of Proneusticosaurus silesiacus via bone histology. However, in our large sample of Triassic Eosauropterygia we found no exact match, i.e., the combination of histological and microanatomical features as well as the growth pattern is so far unique for P. silesiacus. Only humeri of Dactylosaurus sp., femora of aff. Neusticosaurus pusillus and Neusticosaurus sp. from the Ladinian of Kirchheim and southern Germany and femora of Ceresiosaurus calagnii from the Ladinian of the Alpine Triassic show comparable features such as a nearly avascular highly organized tissue and osteosclerosis. This, however, does not necessarily imply phylogenetic relationships. Besides phylogeny, the tissue and growth of vertebrates is also influenced by exogenous (e.g., climate, environmental conditions, food availability) and endogenous factors (e.g., individual’s fitness, inter and/or intraspecific stress, diseases). In addition, tissue types and microanatomy are influenced by functional needs (Ricqlès et al. 2003). Bone histology is thus more correlated to “functional” characters than to phylogeny. However, as Ricqlès et al. (2008: 2) stated, “bone tissue phenotypes can reflect a phylogenetic signal at supra-specific levels if homologous elements are studied, and if ontogenetic trajectories and size-dependent differences are taken into consideration”. This was documented for humeri of the four major groups of Triassic Sauropterygia that could be well distinguished based on their histological features (Klein 2010; Krahl et al. 2013; Klein et al. 2015a, 2016; Klein and Griebeler 2016, 2018). The current study, however, demonstrates that femora of small to moderately sized Triassic Eosauropterygia (i.e., the pachypleurosaur Anarosaurus heterodontus, the nothosaur Nothosaurus spp., the pistosauroid aff. Cymatosaurus sp.) are less useful in this regard because of a relatively uniform histology. Taxonomic assignment of eosauropterygian femora is additionally hampered due to a uniform morphology. Further on, nearly nothing is known about ontogenetic, intraspecific, or sexual variability and/or taxonomic differences in these animals. This makes an unequivocal assignment of isolated femora very difficult if not impossible, contrary to the possibilities for humeri.
Solely environmental reasons for the special tissue type and low growth rate in P. silesiacus can be excluded. This is because bones of Nothosaurus spp. and Eosauropterygia indet. from the Lower Muschelkalk of Poland (Klein et al. 2016; this study) show different tissue types when compared to P. silesiacus. In the case of Nothosaurus spp. and Eosauropterygia indet., femoral growth rates are distinctly higher (Klein et al. 2016; this study) when compared to the femur of P. silesiacus and the humeri of Dactylosaurus sp. from the same locality.
The formerly hypothesized affinities of P. silesiacus to the pistosauroid lineage (i.e., Cymatosaurus sp.; Suess 1987; Rieppel and Hagdorn 1997) are found extremely unlikely based on bone histology. This is because of the very highly organized and poorly vascularized tissue and implied low growth rate documented in the femur and rib of P. silesiacus, when compared to the highly vascularized and low organized tissue type implying increased growth rate, documented in humeri assigned to aff. Cymatosaurus sp. (Klein 2010) and Pistosaurus longaevus (Krahl et al. 2013). As discussed above, based on histology, we can further exclude affinities of P. silesiacus to Nothosaurus spp.
Body size.—Voeten et al. (2014) calculated for an eosauropterygian individual with a femur length of 14 cm, a total body length of around 150–160 cm. For Proneusticosaurus silesiacus with a femur length of 11.9 cm (Volz 1902) we can thus roughly estimate a body length of 130–140 cm, with a corresponding skull length (based on nothosaur morphology!) of around 19 cm. Giving the advanced ontogenetic stage and low growth rate of P. silesiacus, it is unlikely that it had reached a similar body size as the nothosaur Germanosaurus spp., which was found in the same horizon, and of which only two large skulls are known (skull length is 24.4 cm and 27 cm, Rieppel 2000). Thus, taxonomic affinities to Germanosaurus spp. can be rendered unlikely, too. The nothosaur Ceresiosaurus calagnii, which shows a comparable combination of histological and microanatomical characters could have reached a nearly double sized body length of about 3 m. As Volz (1902) already had pointed out, there is a huge size difference between pachypleurosaurs and P. silesiacus. The pachypleurosaur Dactylosaurus sp. reached about 30 cm and Neusticosaurus spp. from the Ladinian of southern Germany and the Alpine Triassic are likewise as small as Dactylosaurus sp. Neusticosaurus edwardssii, however, could have reached up to 120 cm (Sander 1989) but differs by its less organized and more highly vascularized tissue (Hugi et al. 2011) when compared to P. silesiacus.
Morphology.—Volz (1902) and Rieppel and Hagdorn (1997) already gave a detailed description of the morphology of Proneusticosaurus silesiacus. Most characteristic are the strongly pachyostotic vertebrae and proximal halves of dorsal and sacral ribs of P. silesiacus. Centra are distinctly barrel-shaped and carry massive neural arches (Fig. 5A2). The transverse processes are short, the articular facets of the zygapophyses are heavily inclined dorsally (Fig. 5A2; see also Volz 1902 and Rieppel and Hagdorn 1997: fig. 7), and the neural spines are low. We agree with Rieppel and Hagdorn (1997: 129) who found the preserved posterior dorsal vertebrae of P. silesiacus different from Nothosaurus. This is mainly due to the barrel-shaped form of centra in P. silesiacus but also due to the differences in the orientation of the zygapophyses, which in P. silesiacus are 45° or less inclined dorsally, whereas they are nearly horizontal (~90°) in Nothosaurus spp. These inclined articular facets of the vertebrae allow only restricted movements of the vertebral column (Romer 1956) and did presumably not facilitate anguilliform swimming.
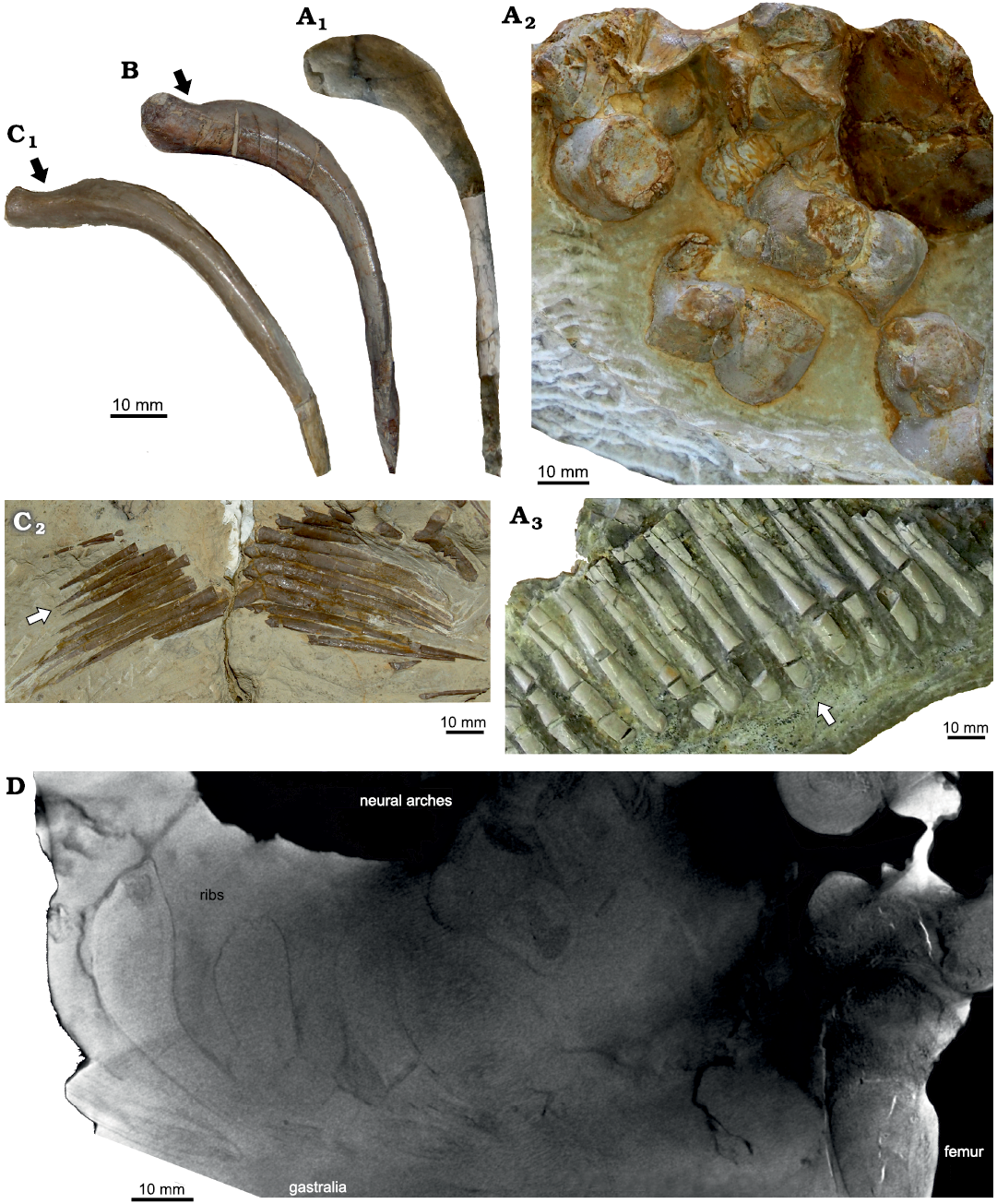
Fig. 5. Morphology of some eosauropterygian diapsids. A. Proneusticosaurus silesiacus Volz, 1902 (MBU Wr 4438s), from the Lower Muschelkalk of Zakrzów, Poland; A1, dorsal rib, note the very massive proximal part of the rib; A2, vertebrae, note the barrel-shaped centra with convex lateral margins; A3, distal gastral elements, which are in general much more massive and distally broad (arrow). These vertebrae are on the opposite on the slab figured in Fig. 1A. B. Dorsal rib of Nothosaurus sp. (MGU Wr. 3934s) from the middle Anisian of Zakrzów, Poland; note the proximally more massive appearance of the rib although the proximal constriction is still present (arrow). C. Nothosaurus sp. (MB R. 150) from the middle Anisian of Oberdorla/Thuringia, Germany; C1, dorsal rib, note the in general slender appearance of the rib and the proximal constriction (arrow); C2, distal gastral elements, which are distally pointed (arrow). D. Micro-Ct-image of P. silesiacus (MBU Wr 4438s) displaying ribs, vertebrae, neural arches, and the proximal half of the femur.
The proximal halves of the dorsal and sacral ribs are extremely pachyostotic in P. silesiacus and not constricted as in Nothosaurus spp. (Figs. 1, 5A1, B, C1, D; SOM: fig. S6). Similar swollen vertebrae and proximal halves of ribs occur only in the pachypleurosaur Dactylosaurus gracilis (Gürich 1884; Sues and Caroll 1984) and in pachypleurosaurs of the Neusticosaurus–Serpianosaurus group (Sander 1989; Rieppel 1989) or in some taxa and/or specimens of Lariosaurus/Ceresiosaurus (summarized in Rieppel 1998). Strong pachyostosis of the proximal half of the ribs is also obvious in one of the stratigraphically oldest Eosauropterygia, Lariosaurus sanxianensis, from the Olenekian of China (Li and Liu 2020). Pachyostosis is a clear feature in the beginning of the course of secondary aquatic adaptation that usually starts in shallow water, making heavy bones (i.e., ballast) necessary to deal with buoyancy (Ricqlès and Buffrénil 2000). However, variability in the degree of pachyostosis is obvious when comparing different individuals of the same taxa (NK personal observation). It is likely related to environmental differences resulting in developmental plasticity. For example, a nothosaur specimen (MGU Wr 3934s) from the same beds as P. silesiacus displays a higher degree of pachyostosis in its ribs when compared to a similar sized nothosaur individual from the Middle Muschelkalk of Oberdorla/Thuringia (MB. R 150) (SOM: fig. S6). Olenekian aged Majiashanosaurus discocoracoidis does only show slight (if at all) pachyostosis of the proximal ribs (Jiang et al. 2014) contrary to the condition in Lariosaurus sanxianensis (Li and Liu 2020). Interpreting Volz’s (1902: 155) comparison, the centra and ribs in Proneusticosaurus madelungi are also less pachyostotic than that of P. silesiacus, which could well be related to environmental differences (please note that the Lower Muschelkalk deposits of Poland represent bonebeds where fossils had accumulated over time and do not represent the actual habitat; Kowal-Linka and Bodzioch 2017).
The massiveness of the gastralia of P.silesiacus is also unique among Eosauropterygia. They are pachyostotic and do not taper as in Nothosaurus (Fig. 5A3, C2) or in the basal pistosauroid from Winterswijk (Sander et al. 2014).
The pubes and ischia of P. silesiacus both display a characteristic shape. The pubis of P. silesiacus is nearly rectangular and very short/stout (SOM: fig. S7B). The pubes of Proneusticosaurus form a closed medial suture and lack a prepubis, which is characteristic for Nothosaurus marchicus (e.g., N. raabi, MB.I.007-18). The ischium (7.2 cm; Volz 1902) of P. silesiacus has a straight medial margin and a convex posterolaterally protruding margin giving the posterior half (the “fan”) an asymmetrical hatchet-shape (SOM: fig. S7A). Comparable pubes and ischia had been identified in public (e.g., BGR, MFN, University of Leipzig, IGWH, TWE) and private collections (Voeten et al. 2014) providing material from various Lower and Middle Muschelkalk localities such as from Poland (Gogolin, Zakrzów), The Netherlands (Winterswijk), and central Germany (Rüdersdorf, Esperstädt/Farnstädt, Freyburg/Unstrut, Oberdorla) (SOM: fig. S7C–H). This indicates a wide distribution of P. silesiacus during that time. A wide distribution and common occurrence of P. silesiacus was already pointed out by Rieppel and Hagdorn (1997) based on vertebrae finds.
The femur of P. silesiacus is relatively long (Volz 1902) and shows a well-developed trochanter, which is a plesiomorphic (terrestrial) condition. The trochanter is reduced in other Eosauropterygia. The femur of P. silesiacus is less curved, distally less broadened, and the proximal head of the femur of P. silesiacus forms a distinct protruding bullet when compared to femora of other Eosauropterygia. Contrary to the highly pachyostotic vertebrae, proximal ribs and gastralia, the femur does not show pachyostosis.
Conclusions
Proneusticosaurus silesiacus femur and rib histology imply an extremely slow growth rate, high degree of osteosclerosis (i.e., nearly no central cavity left), and a distinct growth pattern with juvenile tissue preserved in the innermost cortex. Proneusticosaurus silesiacus bone histology is most comparable to that of the pachypleurosaurs Dactylosaurus sp. and small sized Neusticosaurus spp. as well as with the nothosaur Ceresiosaurus calagnii, whereas it clearly differs from that of Anarosaurus heterodontus, aff. Cymatosaurus sp., Nothosaurus spp., and Simosaurus gaillardoti. However, besides overall morphological differences, the size discrepancy between P. silesiacus and Dactylosaurus sp. as well as the size and stratigraphical discrepancy between P. silesiacus, Neusticosaurus spp., and Ceresiosaurus calagnii, which are both much younger, renders taxonomical affinities unlikely. Shared pachyostotic barrel-shaped centra and the proximally swollen dorsal ribs, as well as femoral osteosclerosis and low growth rates in P. silesiacus, Dactylosaurus sp., Neusticosaurus spp., and Ceresiosaurus calganii are likely convergent and the result of a similar aquatic adaptation to the shallow marine environment and life-style. Based on the distribution of vertebrae, pubes and ischia that resemble the morphology of P. silesiacus (Rieppel and Hagdorn 1997; this study), P. silesiacus was relatively widespread and occurred in early to middle Anisian localities of the central Germanic Basin and southern Alps (i.e., Vicentinian Alps; Rieppel and Hagdorn 1997). Proneusticosaurus silesiacus is the only Triassic marine reptile taxon showing such highly organized lamellar tissue in combination with barrel-shaped (i.e., highly pachyostotic) centra. Because of its unique histology and morphology we concluded that P. silesiacus represents a valid taxon within Eosauropterygia. Whether, the skulls assigned to Cymatosaurus spp. and the postcrania of P. silesiacus belong to the same taxon cannot be clarified but is very unlikley. If so, Cymatosaurus spp. either is not a basal pistosauroid as discussed by Maisch (2014), or does not yet show the characteristic bone tissue type (i.e., indicating high growth rates), known in other members of that clade (Klein 2010; Krahl et al. 2013; Wintrich et al. 2017). Due to its bone tissue type and inferred low growth rates, plesiomorphic femur morphology, strongly dorsally inclined articular facets and early stage of secondary aquatic adaptation, P. silesiacus might be one of the most basal eosauropterygians known so far.
Acknowledgements
We are grateful to Paweł Raczyński (MGU Wr.) who made the sampling of Proneusticosaurus possible. Urszula Kosarewicz (MGU Wr.) is thanked for hosting us during a collection visit. Further thanks go to Hans Hagdorn (MHI), Norbert Hauschke (IGWH), Rainer Schoch (SMNS), and Daniela Schwarz-Wings (MfN) who kindly gave permission for the histological sampling of eosauropterygian femora under their care. Piotr Duda (University of Silesia, Katowice, Poland) is acknowledged for performing micro-CT-scans of the specimen. Roland Plesker (Egelsbach, Germany), made femora and humeri of aff. Neusticosaurus from Kirchheim locality available for study. Olaf Dülfer (IGPB) is acknowledged for the production of thin sections. We would like to thank Tania Wintrich (IGPB) and an anonymous reviewer for their constructive comments. This research project is supported by the National Science Centre, Poland (www.ncn.gov.pl), grant no. 2019/32/C/NZ4/00150 (DS).
References
Case, E.C. 1936. A nothosaur from the Triassic of Wyoming. University of Michigan Contributions from the Museum of Paleontology 5: 1–36.
Francillon-Vieillot, H., Buffrénil, V. de, Castanet, J., Géradudie, J., Sire, F.J., Zylberberg, I., and Ricqlés, A. de 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, 471–530. Van Norstrand Reinhold, New York. Crossref
Gürich, G.J.E. 1884. Über einige Saurier des oberschlesischen Muschelkalkes. Zeitschrift der Deutschen Geologischen Gesellschaft 36: 125–144.
Hänni, K. 2004. Die Gattung Ceresiosaurus—Ceresiosaurus calcagnii Peyer und Ceresiosaurus lanzi n. sp. (Lariosauridae, Sauropterygia). 147 pp. Ph.D. Thesis, vdf Hochschulverlag AG an der ETH, Zürich.
Hugi, J. 2011. The long bone histology of Ceresiosaurus (Sauropterygia, Reptilia) in comparison to other eosauropterygians from the Middle Triassic of Monte San Giorgio (Switzerland/Italy). Swiss Journal of Palaeontology 130: 297–306. Crossref
Hugi, J. 2012. The long bone histology of Ceresiosaurus (Sauropterygia, Reptilia) in comparison to other eosauropterygians from the Middle Triassic of Monte San Giorgio (Switzerland/Italy). Swiss Journal of Palaeontology 130 (2): 297–306. Crossref
Hugi, J., Scheyer, T.M., Klein, N., Sander, P.M., and Sànchez-Villagra, M.R. 2011. Long bone microstructure and life history data of pachypleurosaurids from the Middle Triassic of Monte San Giorgio, Switzerland/Italy. Comptes Rendus Palevol 10: 413–426. Crossref
Jiang, D., Motani, R., Tintori, A., Rieppel, O., Chen, G.-B., Huang J.-D., Zhang, R., Sun, Z.-Y., and Ji, C. 2014. The Early Triassic eosauropterygian Majiashanosaurus discocoracoidis, gen. et sp. nov. (Reptilia, Sauropterygia), from Chaohu, Anhui Province, People’s Republic of China. Journal of Vertebrate Paleontology 34: 1044–1052. Crossref
Klein, N. 2010. Long bone histology of Sauropterygia from the Lower Muschelkalk of the Germanic Basin provides unexpected implications for phylogeny. PLoS One 5 (7): e11613. Crossref
Klein, N. 2012. Postcranial morphology and growth of the pachypleurosaur Anarosaurus heterodontus (Sauropterygia) from the Lower Muschelkalk of Winterswijk, The Netherlands. Paläontologische Zeitschrift 86: 389–408.
Klein, N. 2019. Ancestors to plesiosaurs ? On Triassic Pistosauroidea and the potential presence of cymatosaurs in Winterswijk. In: Voeten, D.F.A.E., During, M., Lankamp, J., and Smit, J. (eds.), The Middle Triassic Vossenfeld Formation in Winterswijk. Grondboor & Hamer, Staringia 16, Volume Jahrgang 73, 1–300. Nederlandse Geologische Vereniging, Arnhem.
Klein, N. and Griebeler, E.-M. 2016. Bone histology, microanatomy, and growth of the nothosauroid Simosaurus gaillardorti (Sauropterygia)from the Upper Muschelkalk of southern Germany/Baden-Württemberg. Comptes Rendus Palevol 15: 142–162. Crossref
Klein, N. and Sander, P.M. 2007. Bone histology and growth of the prosauropod Plateosaurus engelhardti Meyer, 1837 from the Norian bonebed of Trossingen (Germany) and Frick (Switzerland). Special Papers in Paleontology 77: 169–206.
Klein, N. and Griebeler, E.-M. 2018. Growth patterns, sexual dimorphism, and maturation modelled in Pachypleurosauria from Middle Triassic of central Europe (Diapsida: Sauropterygia). Fossil Record 21: 137–157. Crossref
Klein, N., Canoville, A., and Houssaye, A. 2019. Microstructure of vertebrae, ribs, and gastralia of Triassic sauropterygians—new insights into the microanatomical processes involved in aquatic adaptations of marine reptiles. The Anatomical Record 302: 1770–1791. Crossref
Klein, N., Houssaye, A., Neenan, J.M., and Scheyer, T.M. 2015a. Long bone histology and microanatomy of Placodontia (Diapsida: Sauropterygia). Contributions to Zoology 84: 59–84. Crossref
Klein N., Sander P.M., Krahl A., Scheyer T.M., and Houssaye A. 2016. Diverse aquatic adaptations in Nothosaurus spp. (Sauropterygia)—inferences from humeral histology and microanatomy. PLoS One 11: e0158448. Crossref
Klein, N., Voeten, D.F.A.E., Lankamp, J., Bleeker, R., Sichelschmidt, O.J., Liebrand, M., Nieweg, D.C., and Sander, P.M. 2015b. Postcranial material of Nothosaurus marchicus from the Lower Muschelkalk (Anisian) of Winterswijk, The Netherlands, with remarks on swimming styles and taphonomy. Paläontologische Zeitschrift 89: 961–981. Crossref
Kowal-Linka, M. 2015. Analysis of marrow cavity fillings as a tool to recognise diverse taphonomic histories of fossil reptile bones: implications for the genesis of the lower Muschelkalk marine bone-bearing bed (Middle Triassic, Żyglin, S Poland). Palaeogeography, Palaeoclimatology, Palaeoecology 436: 64–76. Crossref
Kowal-Linka, M. and Bodzioch, A. 2017. Genesis of the Lower Triassic bonebeds from Gogolin (S Poland): The impact of microbial mats on trapping of vertebrate remains. Palaeogeography, Palaeoclimatology, Palaeoecology 466: 38–58. Crossref
Krahl, A., Klein, N., and Sander, P.M. 2013. Evolutionary implications of the divergent long bone histologies of Nothosaurus and Pistosaurus (Sauropterygia, Triassic). BMC Evolutionary Biology 13: 1–23. Crossref
Li, Q. and Liu, J. 2020. An Early Triassic sauropterygian and associated fauna from South China provide insights into Triassic ecosystem health. Communications Biology 3: 63. [published online, https://doi.org/10.1038/s42003-020-0778-7] Crossref
Lovelace, D.M. and Doebbert, A.C. 2015. A new age constraint for the Early Triassic Alcova Limestone (Chugwater Group), Wyoming. Palaeogeography, Palaeoclimatology, Palaeoecology 424: 1–5. Crossref
Maisch, M.W. 2014. A well preserved skull of Cymatosaurus (Reptilia: Sauropterygia) from the uppermost Buntsandstein (Middle Triassic) of Germany. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 272: 213–224. Crossref
Meyer, H. von 1847–1855. Zur Fauna der Vorwelt. Die Saurier des Muschelkalkes mit Rücksicht auf die Saurier aus buntem Sandstein und Keuper. 167 pp. Heinrich Keller, Frankfurt a. M.
Ricqlès, A. de and Buffrénil, V. de 2000. Bone histology, heterochronies and the return of tetrapods to life in water: where are we? In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptations of Tetrapods to Life in Water, 289–310. Verlag Dr. Friedrich Pfeil, München.
Ricqlès, A. de, Padian, K., and Horner, J.A. 2003. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Annales de Paléontologie 89: 67–101. Crossref
Ricqlès, A. de, Padian, K., Knoll, F., and Horner, J.A. 2008. On the origin of high growth rates in archosaurs and their ancient relatives: complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology. Annales de Paléontologie 94: 57–76. Crossref
Rieppel, O. 1989. A new pachypleurosaur (Reptilia: Sauropterygia) from the Middle Triassic of Monte San Giorgio, Switzerland. Philosophical Transactions of the Royal Society of London B 323: 1–73. Crossref
Rieppel, O. 1994. Osteology of Simosaurus gaillardoti and the relationships of stem-group Sauropterygia. Fieldiana Geology New Series 28: 1–85. Crossref
Rieppel, O. 1998. The status of the sauropterygian reptile genera Ceresiosaurus, Lariosaurus and Silvestrosaurus from the Middle Triassic of Europe. Fieldiana Geology New Series 38: 1–46. Crossref
Rieppel, O. 2000. Sauropterygia I. In: P. Wellnhofer (ed.), Encyclopedia of Paleoherpetology, Volume 12A. 134 pp. Friedrich Pfeil, München.
Rieppel, O. and Hagdorn, H. 1997. Paleobiogeography of Middle Triassic Sauropterygia in Central and Western Europe. In: J.M. Callaway and E.L. Nicholls (eds.), Ancient Marine Reptiles, 121–144. Academic Press, San Diego. Crossref
Romer, A.S. 1956. Osteology of the Reptiles. 772 pp. The University of Chicago Press, Chicago.
Sander, P.M. 1989. The pachypleurosaurids (Reptilia: Nothosauria) from the Middle Triassic of Monte San Giorgio, (Switzerland), with the description of a new species. Philosophical Transactions of the Royal Society of London B 325: 561–670. Crossref
Sander, P.M. 1990. Skeletochronology in the small Triassic reptile Neusticosaurus. Annales des Sciences Naturelles, Zoologie (13) 11: 213–217.
Sander, P.M., Klein, N., Albers, P.C.H., Bickelmann, C., and Winkelhorst, H. 2014. Postcranial morphology of a basal Pistosauroidae (Sauropterygia) from the Lower Muschelkalk of Winterswijk, The Netherlands. Paläontologische Zeitschrift 88: 55–71. Crossref
Scheyer, T.M., Neuman, A.G., and Brinkman, D.B. 2019. A large marine eosauropterygian reptile with affinities to nothosauroid diapsids from the Early Triassic of British Columbia, Canada. Acta Palaeontologica Polonica 64: 745–755. Crossref
Schrammen, A. 1899. Beitrag zur Kenntnis der Nothosauriden des unteren Muschelkalkes in Oberschlesien. Zeitschrift der Deutschen Geologischen Gesellschaft 51: 388–408.
Sues, H.-D. 1987. Postcranial skeleton of Pistosaurus and interrelationships of the Sauropterygia (Diapsida). Zoological Journal of the Linnean Society 90: 109–131. Crossref
Sues, H.-D. and Carroll, R.L.1985. The pachypleurosaurid Dactylosaurus schroederi (Diapsida: Sauropterygia). Canadian Journal of Earth Sciences 22: 1062–1608. Crossref
Surmik, D., Szczygielski, T., Janiszewska, K., and Rothschild, B.M. 2018. Tuberculosis-like respiratory infection in 245-million-year-old marine reptile suggested by bone pathologies. Royal Society Open Sciences 5: 180225. Crossref
Voeten, D., Sander, P.M., and Klein, N. 2014. Skeletal material from larger Eusauropterygia (Reptilia: Eosauropterygia) with nothosaurian and cymatosaurian affinities from the Lower Muschelkalk of Winterswijk. The Netherlands. Paläontologische Zeitschrift 89: 943–960. Crossref
Volz, W. 1902. Proneusticosaurus, eine neue Sauropterygia Gattung aus dem untersten Muschelkalk Oberschlesiens. Paläontographica 49: 121–164.
Wintrich, T., Hayashi, S., Houssaye, A., Nakajima, Y., and Sander, M. 2017. A Triassic plesiosaurian skeleton and bone histology inform on evolution of a unique body plan. Science Advances 3 (12): e1701144. Crossref