

Facultative monogamy in an early Eocene brooding oyster and its evolutionary implications
KALYAN HALDER and ANIKET MITRA
Halder, K. and Mitra, A. 2021. Facultative monogamy in an early Eocene brooding oyster and its evolutionary implications. Acta Palaeontologica Polonica 66 (3): 647–662.
Dwarf males of Ostrea jibananandai sp. nov. from the lower Eocene rocks of the Cambay Basin, western India, are found attached inside the anterior end of the hinge of large females. Commonly, one male is found inside a female shell. Equivalent associations are known in the extant oyster Ostrea puelchana and the Neogene “Cubitostrea” alvarezii from Argentina. This association increases successful fertilization of eggs by reducing sperm loss in these spermcasting/brooding oysters. The sperms, released into water, are normally brought in with the inhalant water current before fertilization inside the body of the female in brooding oysters. This male-female association reduces the uncertainty involved in fertilization because sperms are released directly inside the female shell. The phenomenon is christened here as facultative monogamy. With this discovery, its evolution in oysters is pushed back more than 40 myr to over 54 Ma. Facultative monogamy evolved only in these three species over its long history in spite of its obvious advantages. Facultative monogamy reduces evolutionary flexibility by decreasing phenotypic variability. It is argued here that the phenomenon evolved by trading off morphological variability in favour of successful fertilization in response to environmental perturbations that tend to disrupt sperm transport in open water. Rapid global warming is hypothesized to potentially cause environmental perturbation, because two of the three cases of facultative monogamy in oysters, Eocene O. jibananandai sp. nov. and Recent O. puelchana, occurred at the early stages of hyperthermal events.
Key words: Bivalvia, Ostrea, internal fertilization, global warming, spermcasting, Eocene, Cambay Basin, India.
Kalyan Halder [kalyan.geol@presiuniv.ac.in] and Aniket Mitra [ani.vastuni@gmail.com], Department of Geology, Presidency University, 86/1 College Street, Kolkata 700073, West Bengal, India.
Received 26 November 2020, accepted 22 April 2021, available online 10 September 2021.
Copyright © 2021 K. Halder and A. Mitra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
A curious reproductive behaviour is known in an extant oyster Ostrea puelchana d’Orbigny, 1842, from the coastal waters of Patagonia, Argentina. The large females of this species carry dwarf males at the anterior edge of their shells (Calvo and Morriconi 1978; Pascual et al. 1989; Shilts et al. 2007).
Reproduction in oysters involves the release of sperms into water. There are two types of fertilization in the group. In broadcast spawning oysters, fertilization is external. In spermcasting/brooding oysters, fertilization takes place internally and the females rear the hatchlings for some time before releasing them. In such forms, sperms enter the female body with the inhalant water. Ostrea Linnaeus, 1758, belongs to the spermcasting category. Dwarf males of O. puelchana, unlike other members of the genus, release sperms directly inside the female shell. This alternative mating system of O. puelchana (i) provides greater success in fertilization of eggs; (ii) increases male fitness by reducing sperm loss; and (iii) provides protection to the small settling males (Pascual et al. 1989; Zampatti and Pascual 1989; Pascual 1997).
Iribarne et al. (1990) reported an equivalent association of male and female individuals in a fossil oyster, Ostrea alvarezii d’Orbigny, 1842, from the upper Miocene–upper Pliocene of Patagonia, Argentina (see also Bayne 2017). Ostrea alvarezii resembles O. puelchana in having dwarf males attached to large females. The type specimen of O. alvarezii was later ascribed to Cubitostrea Sacco, 1897 (Griffin and Nielsen 2008) belonging to the subfamily Crassostreinae Scarlato and Starobogatov, 1979. Living members of Crassostreinae are broadcast spawners. Cubitostrea alvarezii, however, is still being referred to by some authors as an Ostrea (e.g., Romero et al. 2018). Later studies seem to support a late Miocene age (~10 Ma) for this species (Del Rio et al. 2013; Romero et al. 2018 and references therein).
Here, we report a similar phenomenon, observed in a new Ostrea species, Ostrea jibananandai sp. nov., from the lower Eocene (Ypresian, 56–47.8 Ma) of the Cambay Basin, western India. This report extends the antiquity of this phenomenon by more than 40 myr.
The Cenozoic Cambay Basin was developed over the Upper Cretaceous Deccan Trap volcanics. The basin accumulated dominantly clay sediments in a paralic environment, which also received abundant plant matter from the tropical forest in its vicinity (Prasad et al. 2013a, b; Paul et al. 2015). A succession of claystone and shale beds, alternating with lignite, is a characteristic lithological association of the Ypresian Cambay Basin. This is punctuated by several mollusc-bearing marine beds indicating transient marine transgressions (Prasad et al. 2013b). Ostrea jibananandai sp. nov. thrived in this coastal transitional environment for a short period of time. In contrast, O. puelchana and C. alvarezii are known from shallow intertidal to subtidal waters of the open ocean from the temperate Argentine Basin.
Earlier researchers emphasized the advantages of this intimate association between the male and the female (Pascual et al. 1989; Zampatti and Pascual 1989; Pascual 1997). However, in spite of its long history and wide environmental tolerances the phenomenon does not occur in other oysters. It is pertinent to investigate the cause for its rarity. Here, we discuss the possible causes of the evolution of this phenomenon.
Institutional abbreviation.—PG/CB/Os, Department of Geology, Presidency University, Kolkata, India (CB, bivalves from the Cambay Basin; Os, Ostreidae).
Other abbreviations.—♀, carrying female oyster; ♂, dwarf male oyster; CIE, carbon isotope excursion; ETM2, Eocene Thermal Maximum 2; EEH, Early Eocene Hyperthermal; H, height; L, length; LV, left valve; O, other non-carrying oyster with indeterminate sex; PETM, Paleocene–Eocene Thermal Maximum; RV, right valve; SBZ, shallow benthic zones.
Nomenclatural acts.—This study and the nomenclatural act it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:D2EA6225-6565-41FA-B231-C43B29568A76
Geological setting
The Cambay Basin is an intracratonic rift that accommodates a thick pile of Paleocene–Eocene sedimentary rocks including lignite seams in the lower part of the succession, which was developed on the Upper Cretaceous Deccan Traps (Prasad et al. 2013b; Sahni et al. 2006). A major portion of the succession occurs as subcrop. Lignite of the Cambay Basin is mined in open-cast mines and exposed in the pit walls. The major lignite bearing succession belongs to the lower Eocene Cambay Shale, which overlies the Paleocene–lower Eocene Vagadkhol Formation (Chandra and Chowdhary 1969; Sudhakar and Basu 1973; Figs. 1, 2). Locally, the Cambay Shale directly rests on the Deccan Traps. The Vagadkhol Formation is mostly constituted of variegated claystone, conglomerate, and minor sandstone beds of terrestrial derivation, and contains plant fossils at certain levels (Singh et al. 2011). The Cambay Shale is characterized by claystone, shale, and lignite beds, with intercalations of marine sediments bearing marine fossils (Sudhakar and Basu 1973; Sahni et al. 2006). The Cambay Shale is overlain by the Amravati Formation, which comprises foraminiferal limestone, marl, and sandstone beds. This formation is commonly referred to the upper Eocene (Merh 1995; Sahni et al. 2006; Singh et al. 2011). All these formations are largely covered under soil leaving only small patches of outcrop.
There are several operative mines such as Vastan south pit, Mangrol, Valia, and Tadkeshwar. Another nearby mine, the Vastan north pit (Fig. 1), is no longer operative. However, the latter has yielded numerous well-preserved vertebrate fossils (e.g., Bajpai and Kapur 2004, 2008; Bajpai et al. 2005, 2007; Rose et al. 2009a, b), which have attracted international attention from palaeontologists. This mine has been studied in detail with regard to various aspects such as age-diagnostic microfossils (Garg et al. 2008; Punekar and Saraswati 2010), plant remains and palynostratigraphy (e.g., Rao et al. 2013; Prasad et al. 2013a, b), amber and a diverse arthropod community contained in it (e.g., Mallick et al. 2009; Rust et al. 2010), and isotope geochemistry (Clementz et al. 2011; Samanta et al. 2013). The Cambay Shale encountered in the mines comprises claystone and shale beds of brown, grey and green colours with interlayered lignite seams. The nature, thickness and number of shale and lignite bands vary laterally to a large extent. The sedimentary units are poorly indurated. There are frequent lateral facies variations. There is no effect of large-scale tectonic activity, but the sedimentary pile is disturbed by several small-scale faults. Fossil plant remains, such as leaves, stems and amber, are common at many levels. Molluscan shell beds, primarily constituted of benthic bivalve and gastropod shells, are interlayered in this succession in different numbers in different mines.
The oysters of the present study were collected from the west and north faces of the Mangrol open pit mine (21°26´50˝ N, 73°07´48˝ E; Fig. 1). The lithological succession of the mine is characterized by two main producing lignite seams. The succession below the lower lignite seam is not exposed in the mine. Between these seams, variegated shale and claystone beds are interlayered with several shell concentration units of varied thicknesses. A bright white limestone layer of approximately 0.5 m thickness, composed mostly of shell fragments, occurs near the centre of this interval (Fig. 2). Other shell concentrations mostly consist of entire shells in a clay matrix. The proportion of clay varies in different units. Some of these shell concentrations are monospecific in nature (Fig. 2). The claystone beds that interlayer with these shell concentrations also contain dispersed, well-preserved benthic molluscs. The studied oysters were collected from a thin grey-brown claystone layer 1 m below the white shell limestone layer (Fig. 2). This layer yields abundant shells of Ostrea jibananandai sp. nov. along with small unidentifiable shell fragments and a single individual of an anomiid bivalve attached to an oyster.
The upper lignite seam in the Mangrol open pit mine is overlain by an alternation of claystone and fine-grained sandstone (Fig. 2). The claystone beds vary between grey to white in colour in this part. The lignite-bearing rock succession from the Mangrol open pit mine is comparable to that of the adjacent Vastan north pit (21°26´29˝N, 73°07´19˝E; Fig. 1). The latter is also characterized by two main lignite seams, and an alternation of variegated shale and claystone beds with intercalations of shell concentrations bearing benthic molluscs in between. This interval, characterized by marine molluscs, has been mentioned by all researchers who studied the section there (e.g., Clementz et al. 2011; Prasad et al. 2013b).
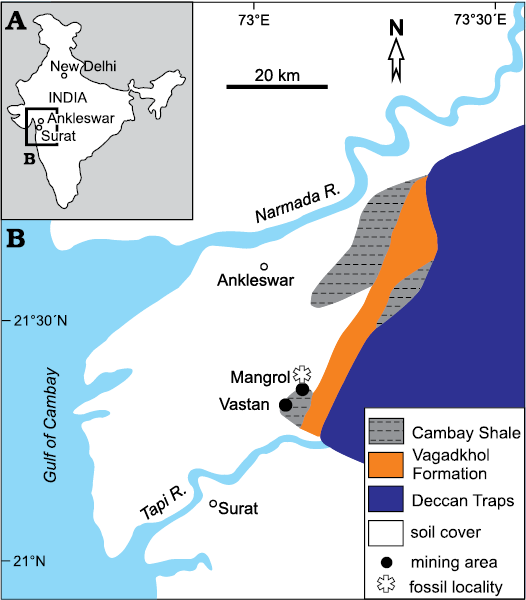
Fig. 1. A. Geographic location of the studied area. B. Geological map of a part of the Cambay Basin with locations of the Mangrol and the Vastan (now abandoned) open pit mines (modified after Sahni et al. 2006).
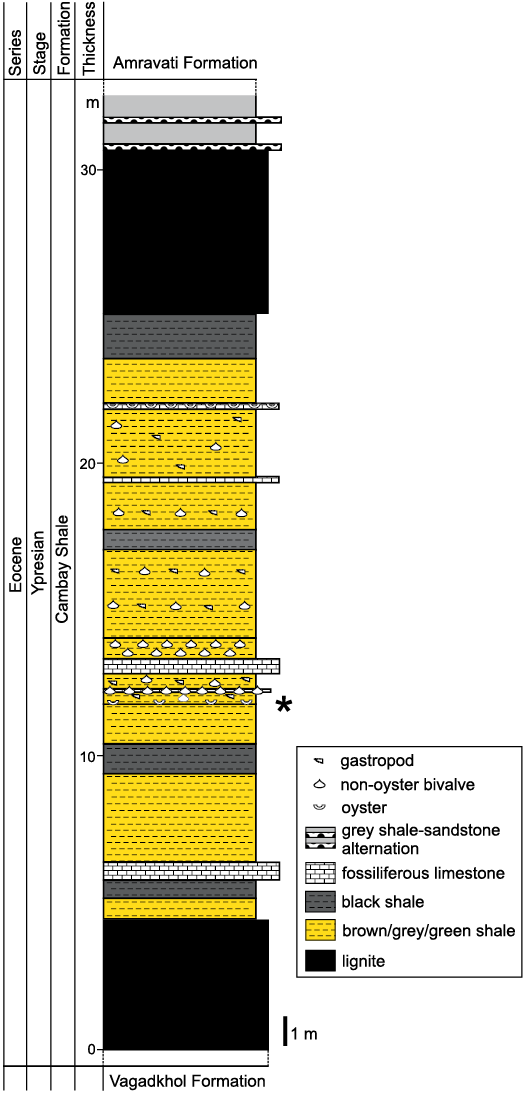
Fig. 2. Lithostratigraphic section of the lower part of the Mangrol open pit mine. The type and relative proportion of fossils in different levels are indicated. The star demarcates the level from which the Ostrea jibananandai sp. nov. specimens were collected.
The age of the Cambay Shale exposed in the open pit mines has been established as Ypresian, based on dinoflagellate cysts (Garg et al. 2008), larger benthic foraminifers (Punekar and Saraswati 2010), strontium isotope ratios (Clementz et al. 2011; Samanta et al. 2013) and argon isotope ratios from glauconite grains (Samanta et al. 2013). These studies were mostly performed on the section exposed at the Vastan north pit. Dinoflagellates and foraminifers are abundant in the part of the section that exhibits maximum marine influence. On the basis of age-diagnostic dinoflagellates, Garg et al. (2008) determined that the lignite-bearing portion of the Cambay Shale is early to middle Ypresian in age (55–52 Ma), corresponding to the larger benthic foraminiferal zones from the upper part of SBZ 7 to the lower part of SBZ 10. On the basis of the larger benthic foraminifer Nummulites burdigalensis, Punekar and Saraswati (2010) placed the marine unit below the upper lignite seam in the SBZ 10, which corresponds to the middle Ypresian. Strontium isotopes were analysed from marine bivalve shells, foraminifers and other fossils. Clementz et al. (2011) found for a ratio of 87Sr/86Sr indicating around 54 Ma for the marine unit below the upper lignite seam. Samanta et al. (2013) dated for the lower part of the Vastan sequence as 56.5–54.5 Ma based on 87Sr/86Sr from bivalve shells. The calcareous nannofossil assemblage from the lower part also suggested an age around the Paleocene–Eocene boundary (Samanta et al. 2013). 40Ar/39Ar ratios from glauconite grains obtained from this part of the succession yielded a comparable age of 56.6±0.7 Ma (Samanta et al. 2013).
The Cambay Shale encountered in the mines bordering the exposed Deccan Traps represents a transitional environment, where terrestrial sedimentation was punctuated by transient marine incursions. The latter resulted in deposition of the shell beds and centimetre-thick shell concentrations. However, the terrigenous influx into this part of the basin never ceased, and clay and plant matter are very common (Samanta et al. 2013; Prasad et al. 2013b). Whereas the underlying Vagadkhol Formation is an entirely continental deposit, the overlying Amravati Formation, comprising foraminiferal limestone, marl, and sandstone beds is a product of a relatively open oceanic phase of the basin.
The Indian Plate was at the equator during the early Eocene phase of its northward movement (Smith et al. 1994; Scotese 2016). The study area has been documented to host typical warm, humid, dense tropical rain forest dominated by coastal plants (Rao et al. 2013; Prasad at al. 2013a; Paul et al. 2015). Arecaceans (palms) were the most common plants and dominated the lower part of the succession. Mangroves, common throughout the succession, became dominant in the upper part. Dinoflagellate cyst and marine plant matter were prevalent in the middle part associated with the shell beds, indicating marine incursions (Garg et al. 2008; Rao et al. 2013; Paul et al. 2015).
The early Eocene “greenhouse world” featured an elevated average temperature, which was punctuated by brief intervals of hyperthermal events. The most well-known hyperthermal of this time, the Paleocene–Eocene Thermal Maximum (PETM), occurred at the beginning of the epoch. There are at least four more Early Eocene Hyperthermal (EEH) events (Cramer et al. 2003; Westerhold et al. 2018). In consequence, this tropical area was receiving high precipitation (Samanta et al. 2013).
Material and methods
Eighty-five specimens of Ostrea jibananandai sp. nov., belonging to different ontogenetic stages, were studied. They include spat and dwarf males attached to other individuals. Several specimens are preserved with both the valves in place. However, the interior of all except five articulated specimens could be studied.
The specimens were studied macroscopically. A Bausch and Lomb hand lens with 20× magnification was used wherever required to study minute features. Measurements of all these specimens including the dwarf males residing inside the large female shells were taken using a digital slide calliper of Mitutoyo brand. The ratio between length (L) and height (H) was plotted in box and jitter plots for different size categories of shells. The specimens were divided into nine categories at 20 mm intervals, based on height. Dwarf males (♂), carrying females (♀), and all other non-carrying individuals (O) including attached spat and articulated shells were separately treated within the size categories. In most cases, the left valve of the male is still attached inside the left valve of the female. In the disarticulated right valves of females, the phenomenon can easily be inferred from the characteristic depression at the anterior end of their hinge. However, the sex of type O cannot be determined based on any specific morphological character. The plots were obtained using PAST 3.14 software (Hammer et al. 2001).
An analysis involving the attached specimens and the attachment scars on the umbonal area of the left valves was performed to get an idea of the substrate preference of the settling larvae. The males were left out of this account. Several attached juveniles that could not be measured because of their extremely small size and indistinct margins, and a few broken specimens attached on other oyster shells, were counted separately. However, their identity was confirmed from their characteristic features. It may be noted that no other oyster species was seen attached on shells of this species or found as mature individuals from the stratigraphic level of the Mangrol mine that yielded all the specimens of Ostrea jibananandai sp. nov. We have counted individuals that settled on three types of substrates: plant, the same oyster species, and indeterminate substrate. In the case of conspecific settlement, we determined whether it occurred on the (i) external surface of the right valve; (ii) internal surface of the right valve; (iii) external surface of the left valve; or (iv) internal surface of the left valve.
The specimens were photographed with a Nikon D7000 DSLR camera using a 60 mm macro lens.
Systematic palaeontology
Class Bivalvia Linnaeus, 1758
Subclass Autobranchia Grobben, 1894
Infraclass Pteriomorphia Beurlen, 1944
Order Ostreida Férussac, 1822
Superfamily Ostreoidea Rafinesque, 1815
Family Ostreidae Rafinesque, 1815
Subfamily Ostreinae Rafinesque, 1815
Genus Ostrea Linnaeus, 1758
Type species: Ostrea edulis Linnaeus, 1758, by subsequent designation (ICZN opinion 94, 1926); Recent, northeastern Atlantic.
Ostrea jibananandai sp. nov.
Figs. 3–5.
Zoobank LCID: urn:lsid:zoobank.org:act:0E347615-3E71-4D17-A357-935B8C930BB4
Etymology: In honour of the late Jibanananda Das (1899–1954), one of the most prominent Bengali poets.
Type material: Holotype: PG/CB/Os 47, well preserved left valve of a carrying female. Paratypes: PG/CB/Os 1, a female with both valves preserved; PG/CB/Os 1a, LV of a female attached on RV of PG/CB/Os 1; PG/CB/Os 3, a female shell with both valves preserved; PG/CB/Os 5, RV of a female; PG/CB/Os 25, LV of a specimen with indeterminate sex; PG/CB/Os 30, RV of a female; PG/CB/Os 36, LV of a specimen with indeterminate sex; PG/CB/Os 41, RV of a specimen with indeterminate sex; PG/CB/Os 45, LV of a male; PG/CB/Os 46, LV of a female. All with preserved shell material. All from the type locality and horizon.
Type locality: Mangrol open pit mine, Surat, Gujarat, western India.
Type horizon: 1 m below the most prominent white shell bed, Mangrol open pit mine section, Cambay Shale, Ypresian (early Eocene), Cambay Basin.
Material.—Eighty five specimens, including the holotype, the paratypes, and 19 attached males from the Mangrol open pit mine section. Several juveniles attached on large shells are also assigned to this species.
Diagnosis.—Shells showing pronounced sexual dimorphism. Female shells large, attaining approximately 85 mm in height, relatively thin; distinctly higher than long; oblique. Strong shape modification occurring during ontogeny, from rounded in early juvenile stage to rather high in adulthood. Prominent umbonal cavity present in both valves. Left valve with anterior flange-like extension in dorsal part of the shell, providing settling space for dwarf male(s). Right valve with corresponding extension overarching dwarf male(s). Shell surface almost smooth, sometimes with blunt radial ridges on left valve and radial gashes on right valve. Dwarf male shells stunted, attaining approximately 20 mm in height, distinctly higher than long, attaching on inner surface of flange-like extension of left valves of females.
Measurements (in mm).—The largest specimen (♀), H = 86.5, L = 72.3; the smallest specimen (♂), H = 4.9, L = 3.87. For details see table S1 in SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Halder_Mitra_SOM.pdf).
Description.—Female shell large, maximum observed height 86.5 mm, higher than long, shape variable from subquadrate to subtrigonal; umbo opisthogyrous, incurved; juvenile shell up to a height approximately 5 mm almost circular, bulged in right valve, often rotated towards posterior nearly at right angles with respect to adult shell; left valve weakly convex, relatively thin; right valve thinner and flat to concave, except for convex umbonal region; anterior shell margin broadly rounded, posterior margin concave, ventral margin slightly rounded; surface of left valve commonly with large attachment scar, remaining part almost smooth, only with comarginal growth threads, sometimes with low blunt radial ridges; outside of right valve smooth with fine radial gashes in some specimens; hinge short, sometimes with auricles; ligamental area longer than high, base of ligament more or less straight, resilifer shallow, wider than flat bourrelets, surface of ligament longitudinally striated; chomata simple, observed mainly in juvenile stage, restricted near hinge; depression housing male(s) in flange-like anterior extension of hinge area in left valve narrow and high, with growth striae on its surface; similarly-shaped low striated platform at corresponding position in right valve; umbonal cavity prominent in left valve, relatively weak in right valve; adductor muscle scar large, high, reniform, oblique, both ends rounded, positioned in posterior half of the shell, slightly above mid-height, position variable to a certain extent with respect to shell height; muscle scar in juveniles suboval and narrow; Quenstedt muscle scar small, rounded, almost central and slightly below resilifer; commissural shelf narrow, slightly concave, proximal margin obscured on ventral side, prominent only in dorso-lateral part.
Male shell small, largest height observed 23.18 mm; commonly quite slender and flat, shape variable; left valve relatively thick, right valve thin; umbo low, opisthogyrous; entire left valve attached to depressed flange-like anterior extension of female shell, surface smooth; posterior margin slightly concave, anterior margin slightly convex, ventral margin nearly straight; hinge narrow, ligamental area large compared to shell size, length greater than height, resilifer shallow, in some specimens relatively deep, wider than bourrelets, sometimes only to a small extent; umbonal cavity shallow, sometimes relatively pronounced; adductor muscle scar relatively large, reniform with rounded ends, placed at mid-height; commissural shelf slightly concave, demarcated by proximal margin only in dorsal part.
Remarks.—The species bears characteristics typical of the genus, such as a relatively flat and higher than long shell, a reniform adductor muscle impression, an elongated ligamental area, weakly developed chomata and a large attachment scar. The genus, however, is commonly characterized by no or only a shallow umbonal cavity, which is well-developed in this species. The representatives of Ostrea are also often characterized by discrepant surface ornamentation of the valves, having a radially ornamented left valve and a more or less smooth right valve (Stenzel 1971). Both valves of the new Eocene species from India are commonly smooth. The species exhibits considerable variation in several morphological features such as the shell shape, the shape of the ligamental area, and the shape and position of the adductor muscle impression. However, a large intraspecific variability is common in oysters (e.g., Amaral and Simone 2014), and is linked to their gregarious habit and varied substrates (Del Rio et al. 2001 and references therein).
The new species, having a relatively thin, flat, quite high and almost smooth shell does not resemble any known Paleogene species from the Indian subcontinent. Most Paleogene ostreid species from this part of the world belong to other genera, such as Crassostrea Sacco, 1897 and Cubitostrea Sacco, 1897 (e.g., Cox 1931; Eames 1951; Iqbal 1969a, b, 1972). This also holds true for the basins from western part of the Tethys Realm such as the Paris Basin (Cossmann and Pissarro 1906; Le Renard and Pacaud 1995). Most of the contemporaneous Tethyan species are characterized by an ornamented left valve, a smooth right valve and by a less slender shell. For instance, Ostrea (Turkostrea) multicostata Deshayes, 1832, well-known from the Eocene of both the western and eastern Tethyan areas, is characterized by strong radial costae on the left valve. It is also very variable and bears a prominent umbonal cavity (Pacaud and Lebrun 2019). Ostrea rakhiensis Eames, 1951, from the lower Eocene of Pakistan is known only by a left valve. It is very slender, and has an almost circular and strongly rotated juvenile shell portion like O. jibananandai sp. nov. but has a radial carina in the middle of the valve, the anterior part of which is characterized by blunt commarginal undulations whereas the posterior part is smooth (see Eames 1951: pl. 11: 50).
Cubitostrea alvarezii (d’Orbigny, 1842), the only fossil oyster species known so far to exhibit the carriage of dwarf males by large females, is not slender and bears strong radial costae on the surface of the left valve, whereas the right valve is smooth (Romero et al. 2018). The female specimen carrying a dwarf male, illustrated by Iribarne et al. (1990), is characterized by a pronounced umbonal cavity. It is smaller and thicker than O. jibananandai sp. nov., and has a high and narrow ligamental area with deeper resilifer. Ostrea puelchana d’Orbigny, 1842, the living oyster with similar male–female association as in O. jibananandai sp. nov., is also less slender than the latter, and has a thicker and radially ornamented left valve (Romero et al. 2013; Pascual et al. 1989). Its right valve bears only feeble radial ornaments (Doldan et al. 2018). It has a similar adductor muscle scar and ligamental area to those of O. jibananandai sp. nov. However, its ligamental area is not as strongly rotated towards posterior as in O. jibananandai sp. nov. The adductor muscle impression in the male of O. puelchana is below the mid-height of the shell interior (Pascual et al. 1989), in contrast to the mid-height position in O. jibananandai sp. nov.. In all the three oyster species with dwarf male and large host female, the males are generally very slender and bear an adult-looking, large ligamental area with numerous growth increments.
Stratigraphic and geographic range.—Type locality and horizon only.
Discussion
Ostrea jibananandai sp. nov. predates the previously known first occurrence of the carrying phenomenon in the Ostreidae Rafinesque, 1815, by more than 40 myr. The previous oldest report was in Cubitostrea alvarezii (d’Orbigny, 1842) from rocks dated as potentially upper Pliocene (2.5–3 Ma) in Argentina (Iribarne et al. 1990; Bayne 2017). More recent literature placed the stratigraphic unit yielding C. alvarezii in the upper Miocene, at approximately 10 Ma age (Del Rio et al. 2013; Romero et al. 2018 and references therein), although confusion still persists (Fuentes et al. 2016). The present report in O. jibananandai sp. nov. is dated as Ypresian, early Eocene (56–47.8 Ma). The layer that yielded all the specimens of O. jibananandai sp. nov. lies at the base of the main marine interval at the Mangrol open pit mine. The equivalent stratigraphic interval at the adjacent Vastan north pit has been deposited around 54 Ma (Clementz et al. 2011). Clementz et al. (2011) demonstrated the presence of the hyperthermal event Eocene Thermal Maximum 2 (ETM2) in the upper part of this interval. The most precise current age of the peak of ETM2 is 54.09 Ma (Westerhold et al. 2007, 2018; see also Lauretano et al. 2015; Cui and Schubert 2017). Samanta et al. (2013) established an age range between 56.5 and 54.5 Ma for the lower deposits cropping out in the Vastan open pit mine below this marine interval. They also demonstrated the presence of the PETM in the lower part of this mine, current consensus regarding the age of which is 55.93 Ma (Westerhold et al. 2018). Hence, the oyster bearing level can be dated as slightly older than 54.09 Ma by inference.
Facultative monogamy.—The normal reproductive style of Ostrea Linnaeus, 1758, and other spermcasting genera, where sperms are brought inside the female shell by the inhalant water current allows (i) sperms from several males to fertilize the eggs of one female; and (ii) sperms from one male to fertilize eggs of several females. Lallias et al. (2010) found in a paternity analysis in a wild Ostrea edulis Linnaeus, 1758 population that 2–40 males contributed to the parentage of larvae from 13 brooding females. On the other hand, in the Ostrea species characterized by the females carrying dwarf males at their hinges, sperms from only these males would fertilize all eggs of the host females. This increases the success of fertilization by reducing uncertainty factors involved in the spermcasting process. Most of the females of O. jibananandai sp. nov. from the Cambay Basin carry one male. This also holds true for the extant Ostrea puelchana d’Orbigny, 1842, from Argentina (Pascual et al. 1989). Hence, this phenomenon is named here as “facultative monogamy”.
The features of facultative monogamy as expressed in O. jibananandai sp. nov. and O. puelchana are similar. Large shells in both species are females (Morriconi and Calvo 1979). Most of the shells of O. puelchana above a height of 50–55 mm are carrying females whereas the individuals below 30 mm are males (Pascual et al. 2001). All the shells of O. jibananandai sp. nov. at a height greater than 60 mm, in which the valve interior could be studied, carry dwarf males (Figs. 3, 4, Table 1; see also SOM). There are 18 such specimens. Four out of 11 specimens with heights between 40 and 60 mm are also carrying. None below 40 mm height is seen to carry males (Table 1; see also SOM). The dwarf males of this species are commonly below 20 mm in height (Table 1; see also SOM).
Table 1. Frequency of different size categories of specimens.
|
Height interval (in mm) |
Female (carrying) (♀) |
Other (sex indeterminate) (O) |
Male (♂) |
|
>80–100 |
4 |
0 |
0 |
|
>60–80 |
14 |
2 |
0 |
|
>40–60 |
4 |
7 |
0 |
|
>20–40 |
0 |
17 |
1 (free) |
|
>0–20 |
0 |
17 |
19 (attached) |
The two Ostrea species also show similarities with regard to the position and the orientation of males within the female shells at their anterior extremity. The dwarf males of O. jibananandai sp. nov. bear a large, adult-looking ligamental area with numerous growth increments and a relatively thick shell with respect to their shell size (Figs. 3A2, B2, 4A, E2). At comparable size, juvenile non-carrying shells belonging to the category O, are thinner and have a much smaller ligamental area (Fig. 5B, E1). A free small individual with a very slender shell (L/H 0.47714) is identified as a male based on its large ligamental area (Fig. 4D1; see also SOM) and relatively thick shell. This is also the largest of the males in our collection. The dwarf males of the Argentine extant oyster O. puelchana, like that of O. jibananandai sp. nov., have a strongly developed ligament, and a short and thick shell.
Both the species commonly have one dwarf male within a female (Calvo and Morriconi 1978; Pascual et al. 1989; Figs. 3A, B, 4B, E). In two cases, two males were found to reside simultaneously inside a female of O. jibananandai sp. nov. (Figs. 3C, 4A). However, in one population of O. puelchana, a relatively high proportion of carrying females have been noted to carry more than one male (Pascual et al. 2001). The extant species also shows variation with regard to the proportion of carrying females between populations (Morriconi and Calvo 1979; Pascual et al. 2001). Such features cannot be compared in the Indian species, because all the specimens come from a single locality.
The non-carrying shells of different growth stages, including spat of O. jibananandai sp. nov., exhibit a gradual modification in shape during the ontogeny from a near equant shell to a high and slender shell (Figs. 5, 6). This ontogenetic shape modification is also clear in individual sub-adult to adult shells (Fig. 5D1, D3). Such shells often exhibit a rounded juvenile growth stage, sharply rotated posteriorly and demarcated by a depression with respect to the rest of the shell (Fig. 5D3, F). This feature is commonly observable in the right valve. The equivalent portion is often not clearly discernible in the left valve because of attachment. The carrying females have a shape comparable to that of the large non-carrying shells at heights between 40 and 60 mm (Fig. 6). It may be noted that the category O at heights >60–80 mm, comprise only two articulated specimens, whose shell interior could not be revealed (Table 1; see also SOM). Similarly, the category ♀ at heights >40–60 mm, which shows slight deviation from average adult L/H, also comprises only three specimens (Fig. 6, Table 1; see also SOM). Attached dwarf males are comparable in this ratio to the adult females (Fig. 6). The only male that has a longer shell (L/H 1.30) is accompanied by another male within the same female shell (Fig. 3C4; see also SOM). Apparently, this shape modification was due to a problem of accommodation of the two individuals within the small space. This attests to the habit-linked phenotypic plasticity in oysters. There is no available observation on O. puelchana to confirm whether a similar shape modification is involved in its ontogeny. However, Ostrea ontogeny commonly involves a similar shape modification starting with an almost circular prodissoconch and growing into a relatively high shell (Hu et al. 1993). Nevertheless, the figured stunted males of O. puelchana are more slender than the host females (Pascual et al. 1989: figs. 2, 3).
The majority of the shells of O. jibananandai sp. nov. (carrying, non-carrying and articulated) are attached to conspecific shells (e.g., Figs. 4B1, 5A). Twenty-six out of 39 measured specimens, in which attachment substrate could be recognized from attachment scars (i.e., plant + conspecific substrate), are attached to conspecific shells; approximately 90% of these settled on the external surface of the right valves (Table 2; see also SOM). The remaining one-third of the shells settled on plants (Table 2; see also SOM) (e.g., Fig. 3A3, B3). The shells attached to conspecific individuals represent different ontogenetic stages ranging from very small settled spat (e.g., Fig. 5A) to adults forming clusters (e.g., Fig. 4B1). Such conspecific settlers that could not be measured also clearly exhibit a preference towards the external surface of the right valve for settlement (Table 2; see also SOM). The shell interior and the outside of the left valve are much less infested (Table 2; see also SOM). Apparently, the surface of the upper right valve was used as substrate for settlement while the host was alive, leading to the formation of clusters of living individuals. Settlement on the shell interior and the external surface of the left valve took place post-mortem. Negligible presence of other fossils, very good preservation, presence of many joined valves, and these settlement characteristics indicate biogenic accumulation of autochthonous to parautochthonous shells in a low-energy depositional regime. It is not known whether O. puelchana has similar substrate preferences and taphonomy. However, a tendency towards cluster formation and settlement on conspecific shells were also observed in this extant species (Pascual et al. 2001; Romero et al. 2013). Conspecific settlement of spat was reported to occur more commonly on the external surface than on the interior (Romero et al. 2013). All juvenile oysters on the shells of O. puelchana are conspecific (Romero et al. 2013), an observation similar to that in the Indian fossil species O. jibananandai sp. nov. However, while varied epibionts were reported attached on the shells of O. puelchana (Romero et al. 2013) bio-infestation on O. jibananandai sp. nov. was not diverse.
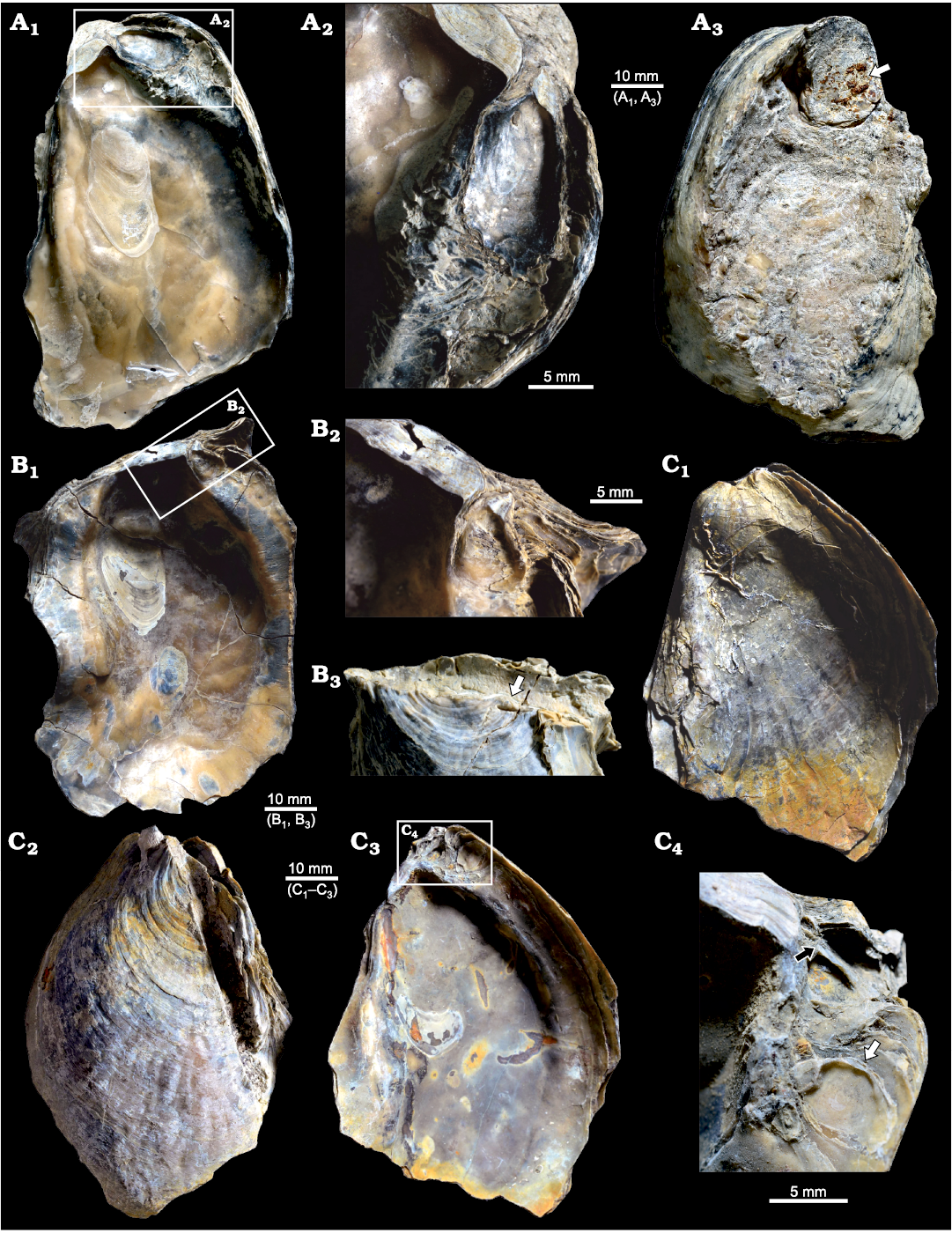
Fig. 3. Ostreid bivalve Ostrea jibananandai sp. nov. from Mangrol open pit mine, Surat, western India, Ypresian Cambay Basin. A. PG/CB/Os 47, holotype, left valve of a carrying female, internal view (A1); detail showing left valve of an attached male (A2); external view showing large attachment scar on plant matter demarcated by arrow (A3). B. PG/CB/Os 46, paratype, left valve of a carrying female with auricles, internal view (B1); detail showing left valve of an attached male (B2); external view of dorsal part showing attachment on plant matter indicated by arrow (B3). C. PG/CB/Os 3, paratype, both valves in place, external view of right valve (C1); external view of left valve (C2); internal view of left valve (C3), inset marks two attached males; detail showing left valve of two males (C4), arrows point to their hinges, the specimen indicated by white arrow is with its length greater than height.
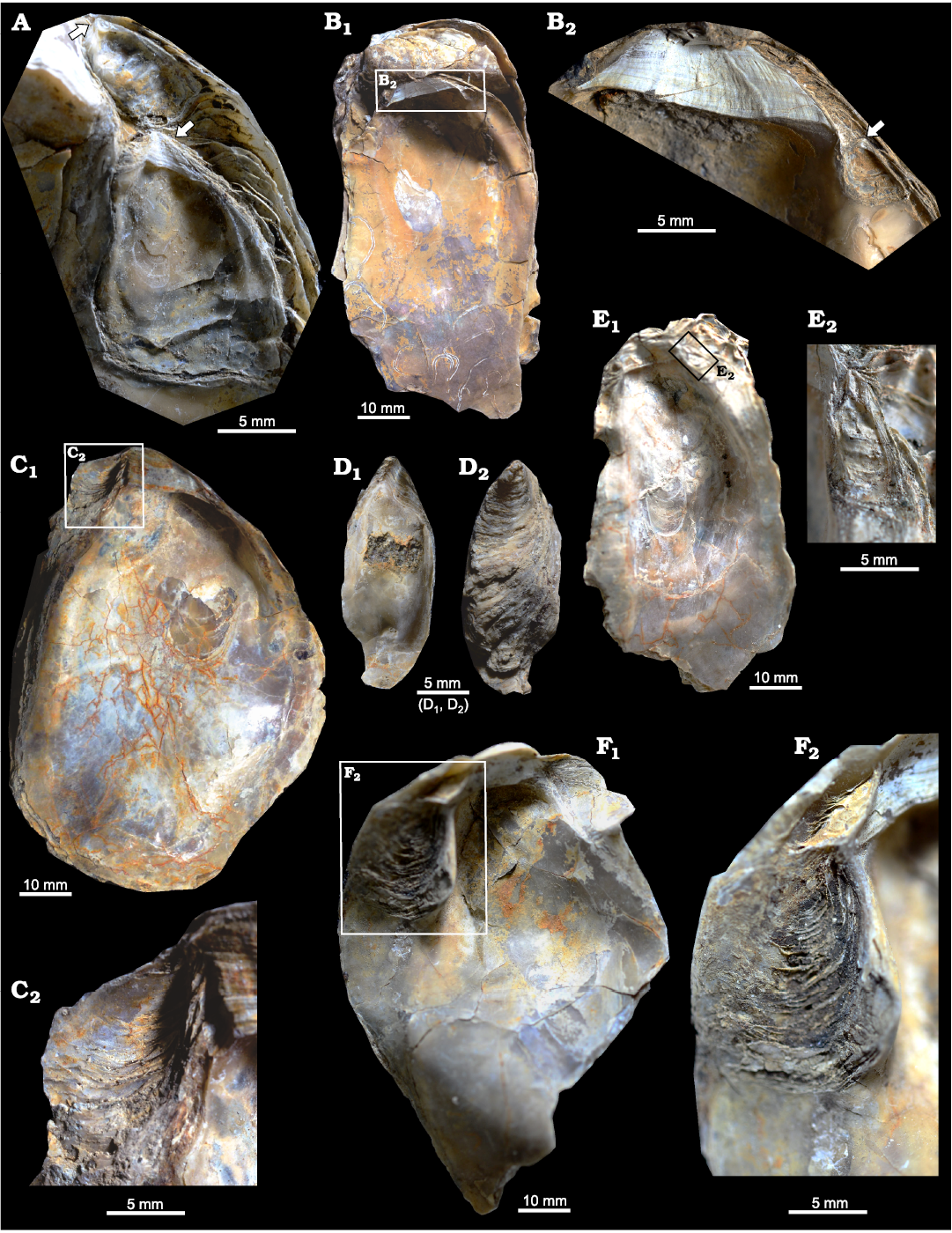
Fig. 4. Ostreid bivalve Ostrea jibananandai sp. nov. from Mangrol open pit mine, Surat, western India, Ypresian Cambay Basin. A. PG/CB/Os 27, a large female (not shown in full) carrying left valve of two dwarf males together, arrows point to their hinges. B. PG/CB/Os 1a, paratype, a left valve attached on the right valve of an articulated specimen, internal view (B1); close-up showing details of the ligamental area and an attached male (B2), arrow indicating its hinge. C. PG/CB/Os 30, paratype, adult right valve of a female, internal view (C1); detail showing depressed platform for accommodation of dwarf male (C2). D. PG/CB/Os 45, paratype, left valve of a detached male, internal (D1) and external (D2) views. E. PG/CB/Os 31, left valve of a large, slender female carrying a dwarf male at its anterior flange, internal view (E1); detail showing left valve of the attached male of similar shape (E2). F. PG/CB/Os 32, right valve of an adult female, internal view (F1); detail showing depressed platform for accommodation of dwarf male (F2).
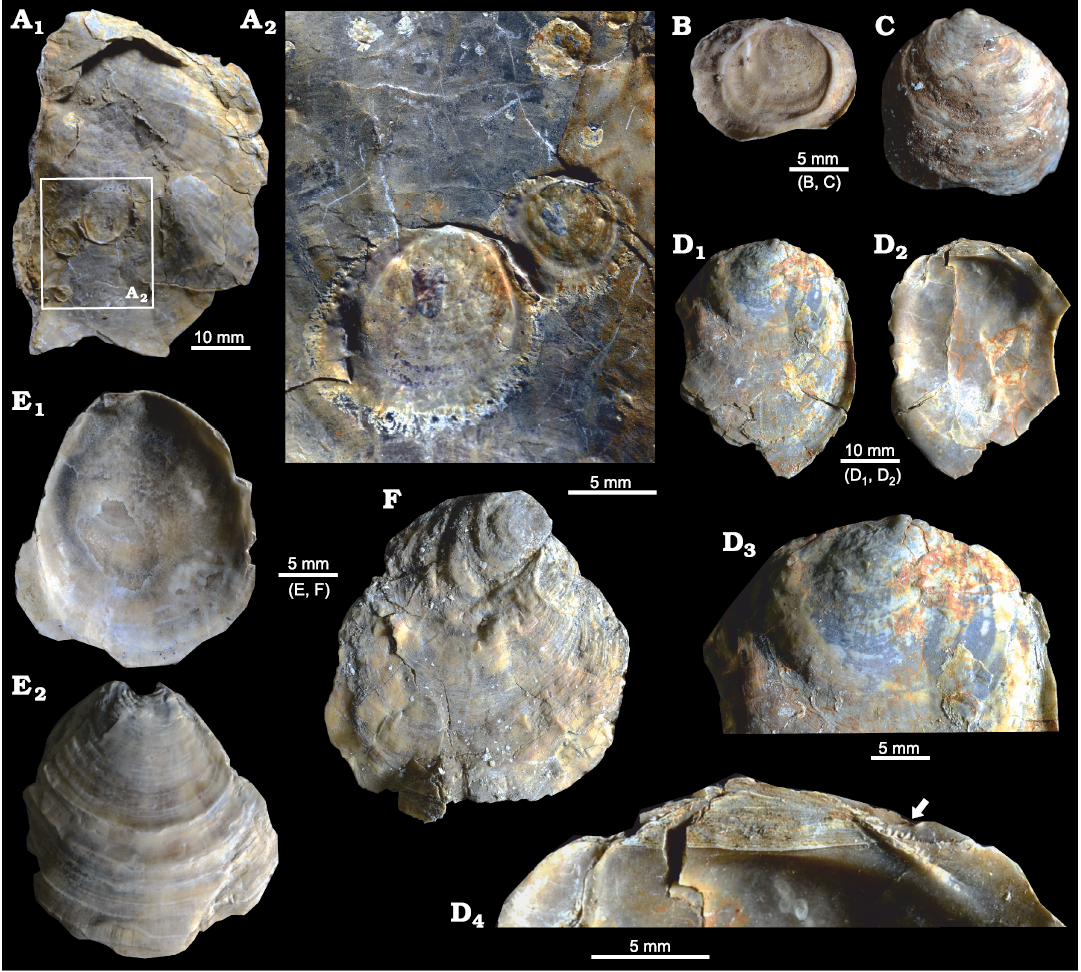
Fig. 5. Ostreid bivalve Ostrea jibananandai sp. nov. from Mangrol open pit mine, Surat, western India, Ypresian Cambay Basin. A. PG/CB/Os 5, paratype, right valve, external view (A1); detail showing 3 attached spat, all more or less rounded (A2, rotated 180° with respect to A1 to make the spat upright). B. PG/CB/Os 41a, paratype, juvenile, left valve, attached on a small right valve (not shown), internal view. C. PG/CB/Os 39, right valve, juvenile, external view showing rounded and convex nature. D. PG/CB/Os 34, right valve of a specimen at mid-ontogeny, external (D1) and internal (D2) views; close-up of dorsal area showing rounded form in early ontogeny (D3); close-up of hinge area (D4) with arrow pointing to chomata. E. PG/CB/Os 36, paratype, juvenile, left valve, internal (E1) and external (E2) views. F. PG/CB/Os 12, juvenile, left valve, external view showing rounded and rotated early growth stage.
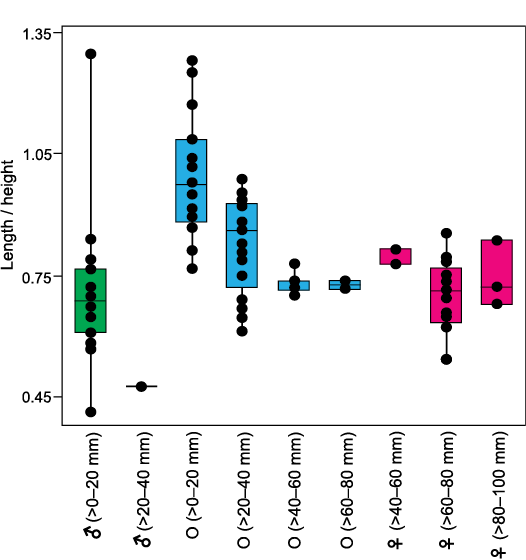
Fig. 6. Box and jitter plots of L/H ratios of different categories of Ostrea jibananandai sp. nov. specimens. Categories as defined in text and Table 1. Boxes represent 25–75 per cent quartiles and horizontal lines inside the boxes indicate median values. Abbreviations: ♀, female; ♂, male; O, other with indeterminate sex.
Neogene Cubitostrea alvarezii from Patagonia, the only other fossil ostreid species characterized by facultative monogamy, was reported in a brief note (Iribarne et al. 1990). The phenomenon was recorded only in three females of the species. However, some major features of the phenomenon can be compared. For example, the nature of the large sexual size dimorphism in this species is similar to that of the other two. The height of the female is approximately 80 mm and that of the male is less than 20 mm. The position and orientation of the male within the female are also similar. Iribarne et al. (1990) noticed the difference in shape of the slender male compared to the rounded and symmetric other small oysters settled on the carrying shell’s surface. The species formed widespread and almost monotypic shell beds, where cluster formation was noticed (Romero et al. 2018). However, the relatively poor information on facultative monogamy in this fossil species is somewhat taken into question by its uncertain generic assignment. If the phenomenon is linked to the advantages of fertilization, as suggested by earlier authors, then it should occur only in internally fertilizing oysters. The extinct genus Cubitostrea is presently assigned to the broadcast-spawning subfamily Crassostreinae Scarlato and Starobogatov, 1979 (Pacaud and Le Renard 1995; Squires 2018). This suggests that either the generic assignment of the species or the subfamily assignment of the genus is erroneous. Alternatively, although less likely, the phenomenon evolved in the species as a special adaptation to internal fertilization.
Ostrea jibananandai sp. nov. thrived in a low energy brackish setting, transitional between shallow marine and freshwater environments. The basin received siliciclastic sediments derived from the weathering Deccan Traps, along with considerable amounts of plant matter from the tropical rainforest prevailing in the area under the influence of an intensified hydrological cycle, due to elevated temperatures of the time (Prasad et al. 2013a, b; Rao et al. 2013; Samanta et al. 2013; Paul et al. 2015). Overall, the restricted basin offered an unfavourable environment for organisms under the influence of fluctuating sea level and high surface run-off, which possibly also led to eutrophication. The extensive presence of undecomposed plant matter and pyrite in the clays indicates prevalent hypoxia in the nutrient-rich environment. Suboxic conditions have also been inferred from biomarker analysis of shale, lignite and amber from neighbouring Surkha mine, yielding contemporaneous lignite seams (Paul et al. 2015). The newly described oyster species, O. jibananandai sp. nov., like many extant oysters (Christo et al. 2010; Amaral and Simone 2014), appears to have lead an “arboreal” life, attached to submerged roots and stems of mangrove and other plants (Fig. 3A3, B3, Table 2; see also SOM). Oysters are eurytopic and known to flourish in marginal marine environments as opportunists, where competition from co-occurring organisms is low because of unfavourable conditions (Bayne 2017). The scarcity of other molluscs and the absence of other oyster species in the stratigraphic unit that yielded O. jibananandai sp. nov. reflect the proliferation of this species as an opportunist taxon under these stressed living conditions. Unlike O. jibananandai sp. nov., O. puelchana lives in shallow marine low intertidal to subtidal environments with full marine salinity (Pascual et al. 1989, 2001; Zampatti and Pascual 1989; Pascual 2000; Bayne 2017). Cubitostrea alvarezii perhaps also lived in a similar environment to that of O. puelchana, i.e., in shallow subtidal channels within a low-energy tidal flat (Romero et al. 2018).
Table 2. Attachment frequency of Ostrea jibananandai sp. nov. on different types of substrate. Abbreviations: LV, left valve; RV, right valve.
|
Type of substrate |
Number of specimens |
||
|
measured |
not measured |
||
|
Conspecific |
LV external surface |
3 |
15 |
|
LV internal surface |
|
8 |
|
|
RV external surface |
23 |
25 |
|
|
RV internal surface |
|
2 |
|
|
Plant |
13 |
|
|
|
Indeterminate |
10 |
|
|
Possible causes of facultative monogamy.—Ostrea and other ostreid genera are common, diverse and geographically very widespread taxa of the Cenozoic (Bayne 2017; Guo et al. 2018). The phenomenon of facultative monogamy is known only in three species of this lineage. The rarity of this phenomenon in spite of its evolution more than 54 myr ago and the advantages associated with it, as stated by earlier authors, is baffling. It is worthwhile to reflect on the possible causes of evolution of this phenomenon. The evolution of a biological phenomenon may be constrained by either phylogeny or adaptation. Alternatively, it may evolve by chance, often as a by-product of another phenomenon (e.g., Seilacher 1970). In this case, the phenomenon can occur sporadically. However, it is difficult to establish a fortuitous evolution without elimination of the other two possibilities.
To some extent, the rarity and geographically restricted nature of facultative monogamy may reflect a lack of adequate documentation. There may be other unreported cases. However, if this rarity is a fact, then the phenomenon is unlikely to have been constrained by phylogeny. A phylogenetically constrained phenomenon should have occurred more frequently in the long history of the clade (see also Iribarne et al. 1990).
The normal spermcasting behaviour of the Ostrea produces greater genotypic variability than facultative monogamy, as it allows sperms from multiple males to fertilize eggs of a single female, as well as sperms from a single male to fertilize eggs of different females. Higher genotypic variability encourages development of phenotypic variability. Natural selection acts upon phenotypic variability. So, this reproductive behaviour provides greater evolutionary flexibility than facultative monogamy. The restrictive nature of the latter behaviour suggests a stressful condition that tends to suppress successful fertilization. Successful reproduction is a necessity, whereas phenotypic plasticity is a luxury in a stressed environment. Facultative monogamy is a possible response to environmental stresses by trading off phenotypic variability in favour of successful fertilization. The nature of facultative monogamy involving sheltering of male(s) inside the female shell suggests difficulty in sperm transport was involved in the evolution of this phenomenon. The restricted occurrence of this phenomenon in time and space also suggests that its manifestation is triggered by sudden environmental perturbations leading to a stressful situation that makes sperm transport difficult. Hence, the possibility of the evolution of facultative monogamy due to adaptive causes cannot be refuted.
The presence of facultative monogamy in O. jibananandai sp. nov. from a sheltered environment in the tropical zone and O. puelchana from an open shallow marine setting in the temperate belt indicates that the physical, chemical and biological factors that differ between these two environments are not important controls on the evolution of this phenomenon. Instead, it indicates a change in global condition that affects both of these environments in similar fashion. One abrupt change that characterises both the early Eocene and the present-day is the rapid increase in global temperature. The early Eocene was a relatively warm period of time with a higher background temperature than today, on which several transient hyperthermal events were superimposed, starting with the PETM at the end of the Paleocene. Elevated levels of 13C-depleted CO2 characterize these early Eocene hyperthermal events. Hence, they are demarcated by carbon isotope excursions (CIE) (Cramer et al. 2003; Westerhold et al. 2018). The current global warming is perhaps the most debated topic within and outside of the scientific community. This warming trend, while unique because of its anthropogenic contribution, is comparable to the PETM and other EEHs in being triggered by the emission of large amounts of CO2 into the atmosphere. The present-day pCO2 is already the highest the Earth has experienced since the Miocene (Cui et al. 2020). The current trend, if continued unabated, will cause one of the most rapid rises in pCO2 of the Cenozoic (IPCC 2013). The PETM is often regarded as the closest analogue for modelling possible effects of the ongoing warming (Zachos et al. 2008; Martin et al. 2013; Burke et al. 2018), in spite of the obvious differences between the climates of the early Eocene and the Recent. For instance, the Eocene is characterized as a greenhouse epoch, where no polar ice caps are recorded. However, the scale, pace and effects of climatic changes are comparable between these two time intervals. Rapid warming often brings about changes in other environmental factors such as ocean acidification, hypoxia etc., which have been noticed in the Recent and are also recorded from the early Eocene (e.g., Zachos et al. 2005; Sluijs et al. 2009; Hönisch et al. 2012; Ivany et al. 2018; Willard et al. 2019). The differences in climatic conditions between the present-day high-latitude Patagonia, Argentina and the tropical western India of the Ypresian are significant. However, transient global warming events, like the PETM and the other EEHs, perturbed the environment at both low and high latitudes in similar fashion. For instance, the high latitude lower Eocene sites from the Arctic Ocean and New Zealand record unambiguous evidence of short-lived warming. This warming led to intensification of the hydrological cycle, enhanced surface run-off, eutrophication and hypoxia, causing considerable changes in the oceanic and terrestrial biota (Sluijs et al. 2009; Slotnick et al. 2012; Willard et al. 2019). Similar effects of EEHs were recorded from tropical sites (e.g., Samanta et al. 2013; D’Onofrio et al. 2016). Warming and its associated environmental modifications are known to influence reproductive processes of many organisms including oysters. It can affect a range of factors linked to reproduction in oysters from gametogenesis to sperm motility and fertilization (e.g., Bayne 2017; Spencer et al. 2020; Boulais et al. 2017, 2018; Byrne and Przeslawski 2013).
Ostrea jibananandai sp. nov. comes from the stratigraphic level that represents the early phase of the marine interval in the Mangrol open pit mine (Fig. 2). Clementz et al. (2011) reported a CIE associated with the ETM2 from the upper part of the marine interval in the adjoining Vastan north pit. Samanta et al. (2013) recorded a similar CIE from a slightly higher stratigraphic level in the same mine. Transgressions concomitant with EEHs including the ETM2 have been documented from tropical as well as temperate regions (e.g., Speijer and Morsi 2002; Sluijs et al. 2008; Pujalte et al. 2014). It is reasonable to assume that O. jibananandai sp. nov. dispersed to the coastal Cambay Basin during the initial incursion of the sea in response to the ETM2. The present-day climatic condition also represents an early stage of a huge and rapid crisis related to global warming (IPCC 2013). Thus, both time intervals from which oysters with convincing facultative monogamy are known represent early phases of rapid environmental perturbations related to warming. Hence, a potential link of the evolution of the phenomenon of facultative monogamy in Ostrea spp. with global warming can be hypothesized. However, the establishment of a causal link between the perturbations related to warming and facultative monogamy requires elaborate multi-proxy analyses that are beyond the scope of this study. It would be interesting to see how O. puelchana responds to perturbations associated with warming, such as ocean acidification and hypoxia, in controlled laboratory experiments. It would also be worth investigating subfossil material to see whether the appearance of facultative monogamy in this species coincides with the major anthropogenic increase in atmospheric pCO2 of the last several decades.
Conclusions
Ostrea jibananandai sp. nov. from the lower Eocene (Ypresian) of the Cambay Basin, western India is represented in our collection by different ontogenetic stages. All individuals in the largest size category, above 60 mm height, carry dwarf individuals at the anterior end of their hinge. The dwarf shells have a similar shape and ligament to the large hosts. The dwarf individuals are males, whereas the large hosts are females. Sexual size dimorphism is a feature associated with gonochoristic organisms. Ostrea Linnaeus, 1758, a protandric consecutive hermaphrodite, exhibits such a strong sexual size dimorphism in the case of this species because of facultative monogamy, where sperms from the dwarf male(s) are likely to fertilize only the eggs of its female host. An equivalent association has been found in the living oyster Ostrea puelchana d’Orbigny, 1842, and the possible upper Miocene Cubitostrea alvarezii (d’Orbigny, 1842), both from Patagonia, Argentina. This discovery pushes back the evolution of the phenomenon in oysters by more than 40 myr.
Earlier authors emphasized the advantages of this intimate male–female association for optimization of successful fertilization (Morriconi and Calvo 1979; Castro and Lucas 1987; Pascual et al. 1989). In spite of the advantages, the phenomenon is known so far only in these three species; presumably because it reduces phenotypic variability and evolutionary flexibility. It is argued here that the phenomenon is a trade-off between phenotypic plasticity and successful reproduction. It perhaps evolved in response to environmental stressors, which tend to disrupt successful fertilization by reducing sperm motility in this internally fertilizing group of animals. Facultative monogamy ensures fertilization in times of crisis, especially when sperm transport is at stake.
The times of appearance of this phenomenon are coincident with global warming events. The phenomenon appeared at the beginning of a warming event on two occasions. It seems that the rapidly changing conditions in response to increasing temperature triggered the development of facultative monogamy in the ostreid lineage. In one other relatively poorly known case, however, it cannot be linked to global warming.
Future studies involving geochemical analyses are needed to test the hypothesis put forward here, regarding the link between this phenomenon and global warming. The extant species Ostrea puelchana and its environment need special attention and detailed analyses, involving experiments in a controlled laboratory set-up together with investigation of the history of the species.
Acknowledgements
We are grateful to the authority of Gujarat Industries Power Company Limited for providing logistics support and giving us access to the mines. Simon Schneider (CASP, Cambridge, UK) and Andrew L.A. Johnson (University of Derby, UK) provided valuable suggestions on the manuscript. This research was undertaken as part of the SERB-DST Core Research Grant scheme (Reference number EMR/2016/002583), India.
References
Amaral, V.S.D. and Simone, L.R.L. 2014. Revision of genus Crassostrea (Bivalvia: Ostreidae) of Brazil. Journal of the Marine Biological Association of the United Kingdom 94: 811–836. Crossref
Bajpai, S. and Kapur, V.V. 2004. Oldest known gobiids from Vastan lignite mine (early Eocene), Surat District, Gujarat. Current Science 87: 433–435.
Bajpai, S. and Kapur, V.V. 2008. Earliest Cenozoic frogs from the Indian subcontinent: implications for out-of-India hypothesis. Journal of the Palaeontological Society of India 53: 65–71.
Bajpai, S., Kapur, V.V., Das, D.P., and Tiwari, B.N. 2007. New early Eocene primate (Mammalia) from Vastan lignite mine, district Surat (Gujarat), western India. Journal of the Palaeontological Society of India 52: 231–234.
Bajpai, S., Kapur, V.V., Das, D.P., Tiwari, B.N., Saravanan, N., and Sharma, R. 2005. Early Eocene land mammals from Vastan lignite mine, district Surat (Gujarat), western India. Journal of the Palaeontological Society of India 50: 101–113.
Bayne, B.L. 2017. Biology of Oysters. 1st Edition. 862 pp. Academic Press, London. Crossref
Beurlen, K. 1944. Beitrage zur Stammesgeschichte der Muscheln. Sitzungsberichte der Bayerischen Akademie der Wissenschaften 1: 133–145.
Boulais, M., Chenevert, K.J., Demey, A.T., Darrow, E.S., Robison, M.R., Roberts, J.P., and Volety, A. 2017. Oyster reproduction is compromised by acidification experienced seasonally in coastal regions. Scientific Reports 7: 1–9. Crossref
Boulais, M., Vignier, J., Loh, A.N., Chu, F.L.E., Lay, C.R., Morris, J.M., Krasnec, M.O., and Volety, A. 2018. Sublethal effects of oil-contaminated sediment to early life stages of the eastern oyster, Crassostrea virginica. Environmental Pollution 243: 743–751. Crossref
Burke, K.D., Williams, J.W., Chandler, M.A., Haywood, A.M., Lunt, D.J., and Otto-Bliesner, B.L. 2018. Pliocene and Eocene provide best analogs for near-future climates. Proceedings of the National Academy of Sciences 115: 13288–13293. Crossref
Byrne, M. and Przeslawski, R. 2013. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integrative and Comparative Biology 53: 582–596. Crossref
Calvo, J. and Morriconi, E.R. 1978. Epibiontie et protandrie chez Ostrea puelchana. Haliotis 9: 85–88.
Castro, N.F. and Lucas, A. 1987. Variability of the frequency of male neoteny in Ostrea puelchana (Mollusca: Bivalvia). Marine Biology 96: 359–365. Crossref
Chandra, P.K. and Chowdhary, L.R. 1969. Stratigraphy of the Cambay Basin. Bulletin Oil and Natural Gas Commission 6: 37–50.
Christo, S.W., Absher, T.M., and Boehs, G. 2010. Morphology of the larval shell of three oyster species of the genus Crassostrea Sacco, 1897 (Bivalvia: Ostreidae). Brazilian Journal of Biology 70: 645–650. Crossref
Clementz, M., Bajpai, S., Ravikant, V., Thewissen, J.G.M., Saravanan, N., Singh, I.B., and Prasad, V. 2011. Early Eocene warming events and the timing of terrestrial faunal exchange between India and Asia. Geology 39: 15–18. Crossref
Cossmann, M. and Pissarro, G. 1906. Iconographie Complète des Coquilles Fossiles de l’Éocène des Environs de Paris, t. 1. pl. 39–45. Hermann, Paris.
Cox, L.R. 1931. A contribution to the molluscan fauna of the Laki and basal Kirthar Groups of the Indian Eocene. Transactions of the Royal Society of Edinburg 57: 25–781. Crossref
Cramer, B.S., Wright, J.D., Kent, D.V., and Aubry, M.P. 2003. Orbital climate forcing of δ13C excursions in the late Paleocene–early Eocene (Chrons C24n–C25n). Paleoceanography 18: 1097. Crossref
Cui, Y. and Schubert, B.A. 2017. Atmospheric pCO2 reconstructed across five early Eocene global warming events. Earth and Planetary Science Letters 478: 225–233. Crossref
Cui, Y., Schubert, B.A., and Jahren, A.H. 2020. A 23 m.y. record of low atmospheric CO2. Geology 48: 888–892. Crossref
Del Rio, C.J., Gallardo, A., Martinez, S.A., and Scasso, R.A. 2001. Nature and origin of spectacular marine Miocene shell beds of northeastern Patagoni (Argentina): Paleoecological and bathymetric significance. Palaios 16: 3–25. Crossref
Del Rio, C.J., Griffin, M., Mcarthur, J.M., Martínez, S., and Thirlwall, M.F. 2013. Evidence for early Pliocene and late Miocene transgressions in southern Patagonia (Argentina): 87Sr/86Sr ages of the pectinid “Chlamys” actinodes (Sowerby). Journal of South American Earth Sciences 47: 220–229. Crossref
Deshayes, G.P. 1832. Description des coquilles fossiles des environs de Paris. Tome 1. 392 pp. Levrault, Paris.
Doldan, M.S., de Rafélis, M., Kroeck, M.A., Pascual, M.S., and Morsan, E.M. 2018. Age estimation of the oyster Ostrea puelchana determined from the hinge internal growth pattern. Marine Biology 165: 119. Crossref
D’Onofrio, R., Luciani, V., Fornaciari, E., Giusberti, L., Boscolo Galazzo, F., Dallanave, E., Westerhold, T., Sprovieri, M., and Telch, S. 2016. Environmental perturbations at the early Eocene ETM2, H2, and I1 events as inferred by Tethyan calcareous plankton (Terche section, northeastern Italy). Paleoceanography 31: 1225–1247. Crossref
d’Orbigny, A. D. 1842. Voyage dans l‘Amérique Méridionale (le Brésil, la République Orientale de l’Uruguay, la République Argentine, la Patagonie, la République du Chili, la République de Bolivia, la République du Perou), exécuté pendant les années 1826, 1827, 1828, 1829, 1830, 1831, 1832 et 1833, Vol. 3, Part 4, Paléontologie. 188 pp. P. Bertrand, Paris.
Eames, F.E. 1951. A contribution to the study of the Eocene in western Pakistan and western India. B. The description of the lamellibranchia from standard sections in the Rakhi Nala and Zinda Pir areas of the western Punjab and in the Kohat district. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 235: 311–482. Crossref
Férussac, A.E.De. 1822. Tableaux Systématiques des Animaux Mollusques. 111 pp. A. Bertrand, Paris.
Fuentes, S.N., Guler, M.V., Cuitiño, J.I., Palazzesi, L., Scasso, R.A., and Barreda, V.D. 2016. Bioestratigrafía basada en quistes de dinoflagelados del Neógeno en el noreste de la Patagonia, Argentina. Revista Brasileira de Paleontología 19: 303–314. Crossref
Garg, R., Ateequzzaman, K., Prasad, V., Tripathi, S.K.M., Singh, I.B., Jauhri, A.K., and Bajpai, S. 2008. Age-diagnostic dinoflagellate cysts from the lignite-bearing sediments of the Vastan lignite mine, Surat District, Gujarat, western India. Journal of the Palaeontological Society of India 53: 99–105.
Griffin, M. and Nielsen, S.N. 2008. A revision of the type specimens of Tertiary molluscs from Chile and Argentina described by d’Orbigny (1842), Sowerby (1846) and Hupé (1854). Journal of Systematic Palaeontology 6: 251–316. Crossref
Grobben, K. 1894. Zur Kenntniss der Morphologie, der Verwandtschaftsverhältnisse und des Systems der Mollusken. Sitzungsberichte der Mathematisch-Naturwissenschaftlichen Klasse der Kaiserlichen Akademie der Wissenschaften 103: 61–86.
Guo, X., Li, C., Wang, H., and Xu, Z. 2018. Diversity and evolution of living oysters. Journal of Shellfish Research 37: 755–771. Crossref
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4 (4): 1–9.
Hönisch, B., Ridgwell, A., Schmidt, D.N., Thomas, E., Gibbs, S. J., Sluijs, A., Zeebe, R., Kump, L., Martindale, R.C., Greene, S.E., and Kiessling, W. 2012. The geological record of ocean acidification. Science 335: 1058–1063. Crossref
Hu, Y.P., Fuller, C., Castagna, M., Vrijenhoek, R.C., and Lutz, R.A. 1993. Shell morphology and identification of early life history stages of congeneric species of Crassostrea and Ostrea. Journal of Marine Biological Association of the United Kingdom 73: 471–496. Crossref
IPCC 2013. Summary for Policymakers. In: T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P.M. Midgley (eds.), Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 3–29. Cambridge University Press, Cambridge.
Iqbal, M.W.A. 1969a. Mega-fauna from the Ghazij Formation (Lower Eocene) Quetta Shahrig area, West Pakistan. Memoirs of the Geological Survey of Pakistan, Palaeontologia Pakistanica 5: 1–41.
Iqbal, M.W.A. 1969b. The Tertiary pelecypod and gastropod fauna from Drug, Zindapir, Vidor (district D.G. Khan), Jhalar and Chharat (district Campbellpore), West Pakistan. Memoirs of the Geological Survey of Pakistan, Palaeontologia Pakistanica 6: 1–95.
Iqbal, M.W.A. 1972. Palaeocene bivalve and gastropod fauna from Jherruk-Lakhra-Bara Nai (Sind), Salt Range (Punjab) and Samana Range (N.W.F.P.), Pakistan. Memoirs of the Geological Survey of Pakistan, Palaeontologia Pakistanica 9: 1–105.
Iribarne, O.O., Pascual, M.S., and Zampatti, E.A. 1990. An uncommon oyster breeding system in a Late Tertiary Patagonian species. Lethaia 23: 153–156. Crossref
Ivany, L.C., Pietsch, C., Handley, J.C., Lockwood, R., Allmon, W.D., and Sessa, J.A. 2018. Little lasting impact of the Paleocene–Eocene Thermal Maximum on shallow marine molluscan faunas. Science Advances 4 (9): eaat5528. Crossref
Lallias, D., Boudry, P., Lapegue, S., King, J.W., and Beaumont, A.R. 2010. Strategies for the retention of high genetic variability in European flat oyster (Ostrea edulis) restoration programmes. Conservation Genetics 11: 1899–1910. Crossref
Lauretano, V., Littler, K., Polling, M., Zachos, J.C., and Lourens, L.J. 2015. Frequency, magnitude and character of hyperthermal events at the onset of the Early Eocene Climatic Optimum. Climate of the Past 11: 1313–1324. Crossref
Le Renard, J. and Pacaud, J.M. 1995. Révision des Mollusques paléogènes du Bassin de Paris. 2. Liste des références primaires des espèces. Cossmanniana 3 (3): 65–132.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum
classes, ordines, genera, species, cum characteribus, differentiis,
synonymis, locis. Tomus 1.
10th
Edition. 824 pp. Laurentii Salvii, Holmiæ, Stockholm. Crossref
Mallick, M., Dutta, S., Greenwood, P.F., and Bertram, N. 2009. Pyrolytic and spectroscopic studies of Eocene resin from Vastan lignite mine, Cambay Basin, western India. Journal Geological Society of India 74: 16–22. Crossref
Martin, P., Grimes, S., Manners, H., and Hart, M. 2013. A critical evaluation of the Paleocene–Eocene Thermal Maximum: an example of things to come? The Plymouth Student Scientist 6: 386–397.
Merh, S.S. 1995. Geology of Gujarat. 222 pp. Geological Society of India, Bangalore.
Morriconi, E. and Calvo, J. 1979. Ciclo reproductivo y alternancia de sexos en Ostrea puelchana. Physis 38: 1–17.
Pacaud, J.M. and Lebrun, P. 2019. Les phosphates du Maroc (2): des faunes d’invertébrés dominées par les bivalves et les gastéropodes. Fossiles. Revue Française de Paléontologie, 40: 17–37.
Pacaud, J.M. and Le Renard, J. 1995. Révision des mollusques Paléogènes du bassin de Paris. IV – Liste systématique actualisée. Cossmanniana 3: 151–187.
Pascual, M.S. 1997. Carriage of dwarf males by adult female puelche oysters: the role of chitons. Journal of Experimental Marine Biology and Ecology 212: 173–185. Crossref
Pascual, M.S. 2000. Dwarf males in the puelche oyster (Ostrea puelchana, d’orb): differential mortality or selective settlement? Journal of Shellfish Research 19: 815–820.
Pascual, M.S., Iribarne, O.O., Zampatti, E.A., and Bocca, A.H. 1989. Female-male interaction in the breeding system of the puelche oyster Ostrea puelchana d’Orbigny. Journal of Experimental Marine Biology and Ecology 132: 209–219. Crossref
Pascual, M.S., Zampatti, E.A., and Iribarne, O.O. 2001. Population structure and demography of the puelche oyster (Ostrea puelchana d’Orbigny, 1841) grounds in northern Patagonia, Argentina. Journal of Shellfish Research 20: 1003–1010.
Paul, S., Sharma, J., Singh, B.D., Saraswati, P.K., and Dutta, S. 2015. Early Eocene equatorial vegetation and depositional environment: biomarker and palynological evidences from a lignite-bearing sequence of Cambay Basin, western India. International Journal of Coal Geology 149: 77–92. Crossref
Prasad, M., Singh, H., Singh, S.K., Mukherjee, D., and Ruiz, E.E. 2013. Early Eocene arecoid palm wood, Palmoxylon vastanensis n. sp. from Vastan Lignite, Gujarat, India: its palaeoenvironmental implications. Journal of the Palaeontological Society of India 58: 115–123. Crossref
Prasad, V., Singh, I.B., Bajpai, S., Garg, R., Thakur, B., Singh, A., Saravanan, N., and Kapur, V.V. 2013. Palynofacies and sedimentology-based high-resolution sequence stratigraphy of the lignite-bearing muddy coastal deposits (early Eocene) in the Vastan lignite mine, Gulf of Cambay, India. Facies 59: 737–761.
Pujalte, V., Schmitz, B., and Baceta, J.I. 2014. Sea-level changes across the Paleocene–Eocene interval in the Spanish Pyrenees, and their possible relationship with North Atlantic magmatism. Palaeogeography, Palaeoclimatology, Palaeoecology 393: 45–60. Crossref
Punekar, J and Saraswati, P.K. 2010. Age of the Vastan lignite in context of some oldest Cenozoic fossil mammals from India. Journal Geological Society of India 76: 63–68. Crossref
Rafinesque, C.S. 1815. Analyse de la Nature ou Tableau de l’Univers et des Corps Organisés. 224 pp. Privately published, Palermo. Crossref
Rao, M.R., Sahni, A., Rana, R.S., and Verma, P. 2013. Palynostratigraphy and depositional environment of Vastan lignite mine (early Eocene), Gujarat, western India. Journal of Earth System Science 122: 289–307. Crossref
Romero, M.V., Brezina, S.S., Bremec, C., and Casadío, S. 2018. Sclerobionts on Patagonian oysters from the Puerto Madryn Formation (early–late Miocene, Argentina). Ameghiniana 55: 179–196. Crossref
Romero, M.V., Brezina, S.S., Hernández, D., Casadío, S., and Bremec, C. 2013. Differential settlement of associated species on Ostrea puelchana d’Orbigny, 1842 (Ostreidae) in Patagonia (Argentina). American Malacological Bulletin 31: 311–323. Crossref
Rose, K.D., Rana, R.S., Sahni, A., Kumar, K., Missiaen, P., Singh, L., and Smith, T. 2009a. Early Eocene primates from Gujarat, India. Journal of Human Evolution 56: 366–404. Crossref
Rose, K.D., Rana, R.S., Sahni, A., Kumar, K., Singh, L., and Smith, T. 2009b. First tillodont from India: additional evidence for an early Eocene faunal connection between Europe and India? Acta Palaeontologica Polonica 54: 351–355. Crossref
Rust, J., Singh, H., Rana, R.S., McCann, T., Singh, L., Anderson, K., Sarkar, N., Nascimbene, P.C., Stebner, F., Thomas, J.C., Kraemer, M.S., Williams, C.J., Engel, M.S., Sahni, A., and Grimaldi, D. 2010. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. PNAS 107: 43. Crossref
Sacco, F. 1897. I Molluschi dei terreni terziarii del Piemonte e della Liguria, Parte 23 (Ostreidae, Anomiidae, Dimyidae). Bollettino dei Musei di Zoologia ed Anatomia Comparata della R. Università di Torino 12 (298): 99–100.
Sahni, A., Saraswati, P.K., Rana, R.S., Kumar, K., Singh, H., Alimohammadian, H., Sahni, N., Rose, K.D., Singh, L., and Smith, T. 2006. Temporal constraints and depositional palaeoenvironments of the Vastan lignite sequence, Gujarat: Analogy for the Cambay Shale hydrocarbon source rock. Indian Journal of Petroleum Geology 15: 1–20.
Samanta, A., Bera, M.K., Ghosh, R., Bera, S., Filley, T., Pande, K., Rathore, S.S., Rai, J., and Sarkar, A. 2013. Do the large carbon isotopic excursions in terrestrial organic matter across Paleocene–Eocene boundary in India indicate intensification of tropical precipitation? Palaeogeography, Palaeoclimatology, Palaeoecology 387: 91–103. Crossref
Scarlato, O.A. and Starobogatov, Y.I. 1979. General evolutionary patterns and the system of the class Bivalvia [in Russian]. In: Y.I. Starobogatov (ed.), Morfologiâ, sistematika i filogeniâ mollûskov. Trudy Zoologičeskogo Instituta Akademii Nauk SSSR 80: 5–38.
Scotese, C.R. 2016. PALEOMAP PaleoAtlas for GPlates and the PaleoData Plotter Program, PALEOMAP 738 Project. Published online, http://www.earthbyte.org/paleomap-paleoatlas-for-gplates. Accessed 15 April 2020. Crossref
Seilacher, A. 1970. Arbeitskonzept zur Konstruktions-Morphologie. Lethaia 3: 393–396. Crossref
Shilts, M.H., Pascual, M.S., and Foighil, D.Ó. 2007. Systematic, taxonomic and biogeographic relationships of Argentine flat oysters. Molecular Phylogenetics and Evolution 44: 467–473. Crossref
Singh H., Prasad M., Kumar, K., and Singh, S.K. 2011. Paleobotanical remains from the Paleocene–lower Eocene Vagadkhol Formation, western India and their paleoclimatic and phytogeographic implications. Palaeoworld 20: 332–356. Crossref
Slotnick, B.S., Dickens, G.R., Nicolo, M.J., Hollis, C.J., Crampton, J.S., Zachos, J.C., and Sluijs, A. 2012. Large-amplitude variations in carbon cycling and terrestrial weathering during the latest Paleocene and earliest Eocene: The record at Mead Stream, New Zealand. The Journal of Geology 120: 487–505. Crossref
Sluijs, A., Brinkhuis, H., Crouch, E.M., John, C.M., Handley, L., Munsterman, D., Bohaty, S.M., Zachos, J.C., Reichart, G.J., Schouten, S., and Pancost, R.D. 2008. Eustatic variations during the Paleocene–Eocene greenhouse world. Paleoceanography 23: PA4216. Crossref
Sluijs, A., Schouten, S., Donders, T.H., Schoon, P.L., Röhl, U., Reichart, G., Sangiorgi, F., Kim, J., Damsté, J.S.S., and Brinkhuis, H. 2009. Warm and wet conditions in the Arctic region during Eocene Thermal Maximum 2. Nature Geoscience 2: 777–780. Crossref
Smith, A.G., Smith, D.G., and Funnel, D.M. 1994. Atlas of Mesozoic and Cenozoic Coastlines. 754 pp. Cambridge University Press, Cambridge.
Speijer, R.P. and Morsi, A.M.M. 2002. Ostracode turnover and sea-level changes associated with the Paleocene–Eocene thermal maximum. Geology 30: 23–26. Crossref
Spencer, L.H., Venkataraman, Y.R., Crim, R., Ryan, S., Horwith, M.J., and Roberts, S.B. 2020. Carryover effects of temperature and pCO2 across multiple Olympia oyster populations. Ecological Applications 30: e02060. Crossref
Squires, R.L. 2018. Paleocene and Eocene oysters from the West Coast of the United States: revision of named species and recognition of new species. Contributions in Science 526: 1–29.
Stenzel, H.B. 1971. Oysters. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Pt. N, Vol. 3, Mollusca 6, Bivalvia, N953–N1224. Geological Society of America and University of Kansas Press, Lawrence.
Sudhakar, R. and Basu, D.N. 1973. A reappraisal of the Paleogene stratigraphy of southern Cambay Basin. Bulletin Oil and Natural Gas Commission 10: 55–76.
Westerhold, T., Röhl, U., Donner, B., and Zachos, J.C. 2018. Global extent of early Eocene hyperthermal events: a new Pacific benthic foraminiferal isotope record from Shatsky Rise (ODP Site 1209). Paleoceanography and Paleoclimatology 33: 626–642. Crossref
Westerhold, T., Röhl, U., Laskar, J., Raffi, I., Bowles, J., Lourens, L.J., and Zachos, J.C. 2007. On the duration of magnetochrons C24r and C25n and the timing of early Eocene global warming events: implications from the Ocean Drilling Program Leg 208 Walvis Ridge depth transect. Paleoceanography 22 (2): PA2201. Crossref
Willard, D.A., Donders, T.H., Reichgelt, T., Greenwood, D.R., Sangiorgi, F., Peterse, F., Nierop, K.G., Frieling, J., Schouten, S., and Sluijs, A. 2019. Arctic vegetation, temperature, and hydrology during early Eocene transient global warming events. Global and Planetary Change 178: 139–152. Crossref
Zachos, J.C., Dickens, G.R., and Zeebe, R.E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. Crossref
Zachos, J.C., Röhl, U., Schellenberg, S.A., Sluijs, A., Hodell, D.A., Kelly, D.C., Thomas, E., Nicolo, M., Raffi, I., Lourens, L.J., and McCarren, H. 2005. Rapid acidification of the ocean during the Paleocene–Eocene thermal maximum. Science 308: 1611–1615. Crossref
Zampatti, E. and Pascual, M. 1989. Larval Rearing, Nursery Growing and Implantation at Oyster Parks of the Argentinian Oyster, Ostrea puelchana d’Orb. 13 pp. Laboratorio de Recursos Bentonicos y Cultivos Marinos Instituto de Biologia Marina y Pesquera, San Antonio Oeste, Rio Negro.
Acta Palaeontol. Pol. 66 (3): 647–662, 2021
https://doi.org/10.4202/app.00863.2020