


The endemic radiodonts of the Cambrian Stage 4 Guanshan Biota of South China
DE-GUANG JIAO, STEPHEN PATES, RUDY LEROSEY-AUBRIL, JAVIER ORTEGA-HERNÁNDEZ, JIE YANG, TIAN LAN, and XI-GUANG ZHANG
Jiao, D.-G., Pates, S., Lerosey-Aubril, R., Ortega-Hernández, J., Yang, J., Lan, T., and Zhang, X.-G. 2021. The endemic radiodionts of the Cambrian Stage 4 Guanshan Biota of South China. Acta Palaeontologica Polonica 66 (2): 255–274.
The Guanshan Biota (South China, Cambrian, Stage 4) contains a diverse assemblage of biomineralizing and non-biomineralizing animals. Sitting temporally between the Stage 3 Chengjiang and Wuliuan Kaili Biotas, the Guanshan Biota contains numerous fossil organisms that are exclusive to this exceptional deposit. The Guanshan Konservat-Lagerstätte is also unusual amongst Cambrian strata that preserve non-biomineralized material, as it was deposited in a relatively shallow water setting. In this contribution we double the diversity of radiodonts known from the Guanshan Biota from two to four, and describe the second species of Paranomalocaris. In addition, we report the first tamisiocaridid from South China, and confirm the presence of a tetraradial oral cone bearing small and large plates in “Anomalocaris” kunmingensis, the most abundant radiodont from the deposit. All four radiodont species, and three genera, are apparently endemic to the Guanshan Biota. When considered in the wider context of geographically and temporally comparable radiodont faunas, endemism in Guanshan radiodonts is most likely a consequence of the shallower and more proximal environment in which they lived. The strong coupling of free-swimming radiodonts and benthic communities underlines the complex relationship between the palaeobiogeographic and environmental distributions of prey and predators. This local adaptation of radiodonts to their prey is highlighted by the frontal appendage morphology of the two species of Paranomalocaris, apparently specialised to different feeding modes, while the recognition of the limited geographic range of some radiodont faunas highlights the importance of exploring as many deposits as possible to fully understand this group.
Key words: Amplectobeluidae, Anomalocarididae, Tamisiocarididae, Paranomalocaris, Burgess Shale-type exceptional preservation, shallow water, Cambrian, China.
De-Guang Jiao [103256437@qq.com], Yuxi Normal University, 134 Phoenix Road, Yuxi, Yunnan, 653100, China: Key Laboratory for Palaeobiology, Yunnan University, Kunming, 650091, China; Yunnan Land and Resources Vocational College, Kunming, 650091, China.
Stephen Pates [sp587@cam.ac.uk], Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA; Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ, UK.
Rudy Lerosey-Aubril [rudy_lerosey@fas.harvard.edu], Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA.
Javier Ortega-Hernández [jortegahernandez@fas.harvard.edu] (corresponding author), Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA.
Jie Yang [yangjie@ynu.edu.cn] and Xi-Guang Zhang [xzhang@ynu.edu] (corresponding author), Key Laboratory for Palaeobiology and MEC International Joint Laboratory for Palaeoenvironment, Yunnan University, Kunming, 650091, China; Yunnan Land and Resources Vocational College, Kunming, 650091, China.
Tian Lan [lantianing@sina.com] College of Resources and Environmental Engineering, Guizhou University, Guiyang 550003, China.
Received 22 December 2020, accepted 10 February 2021, available online 2 June 2021.
Copyright © 2021 D.-G. Jiao et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
South China is crucial for our understanding of the early evolution of animal life, as it is home to numerous Cambrian deposits that preserve non-biomineralized fossils (Steiner et al. 2005; Zhang et al. 2008; Gaines 2014; Muscente et al. 2017). The most prolific of these Burgess Shale-type (BST) deposits (e.g., Chengjiang, Fandian, Qingjiang, Xiaoshiba) belong to the Cambrian Stage 3 (Zhang et al. 2001, 2008; Hu et al. 2010b; Hou et al. 2017; Fu et al. 2019; Du et al. 2020). The Cambrian (Stage 4) Wulongqing Formation, which hosts the Guanshan Biota, falls stratigraphically between these fossil sites and another prolific Cambrian BST deposit, the Wuliuan Kaili Formation (Zhao et al. 2011). As such, it complements the South Chinese fossil record of Cambrian non-biomineralizing organisms, and allows the evolution of marine palaeocommunities to be examined at a high temporal resolution (Hu et al. 2010b, 2013; Hopkins et al. 2017; Chen et al. 2019; Zhang et al. 2008; Zhao et al. 2020; Du et al. 2020). In South China, the Guanshan Biota is sub-contemporaneous to the less diverse Balang (Peng et al. 2005) and Shipai faunas (Zhang and Hua 2005). It is also roughly temporally equivalent to several assemblages of exceptionally preserved fossils from around the world (Hu et al. 2013), such as the Emu Bay Shale Biota of East Gondwana (Paterson et al. 2016), the Sinsk Biota of Siberia (Ivantsov et al. 2005), or the Indian Spring (English and Babcock 2010) and Kinzers (Skinner 2005) biotas of Laurentia. This facilitates intercontinental faunal comparisons. For instance, like other Cambrian BST biotas in South China, the Guanshan fauna shows palaeobiogeographic affinities with the Emu Bay Shale fauna in East Gondwana. These similarities are interpreted as evidence for close proximity between these two palaeocontinents during the early Cambrian (Paterson et al. 2015; Holmes et al. 2018; Zhao et al. 2020).
The Guanshan Konservat-Lagerstätte is also important in its own right. It is a Tier 2 BST deposit (sensu Gaines 2014), which has yielded nearly 100 species from over 10 different sites (Liu et al. 2012, 2016; Hu et al. 2013; Chen et al. 2019; Zhao et al. 2020). Importantly, the abundant trace fossils and sedimentological evidence both suggest deposition in a relatively shallow-water shelf environment (Hu et al. 2013; Chen et al. 2019, 2020a; Ding et al. 2018, 2020), in striking contrast with the quiet, deep-water depositional settings inferred for most Cambrian Konservat-Lagerstätten (Gaines 2014). Non-biomineralizing components of the Guanshan fauna include ctenophores, eldoniids, poriferans, scalidophorans, vetulicolians, and a great diversity of panarthropods, such as lobopodians, radiodonts, artiopods, euarthropods with bivalved or folded carapaces, fuxianhuiids, and megacheirans (e.g., Luo et al. 2007; Hu et al. 2010b, 2012, 2013; Wang et al. 2013; Huang and Wang 2014; Li et al. 2015, 2017; Liu et al. 2016; Zeng et al. 2018a; Wu and Liu 2019; Chen et al. 2019, 2020b; Zhao et al. 2020). Exceptional preservation of non-biomineralized structures has also been documented in brachiopods (e.g., pedicle, setae, Hu et al. 2010a; Zhang et al. 2011; Hu et al. 2013; Chen et al. 2019) and trilobites (e.g., appendages, gut, Hu et al. 2013; Hopkins et al. 2017).
The Radiodonta from Guanshan Biota were represented so far by two species, Anomalocaris kunmingensis Wang, Huang, and Hu, 2013, and Paranomalocaris multisegmentalis Wang, Huang, and Hu, 2013, that were first described from a handful of isolated frontal appendages (Wang et al. 2013). The holotype of A. kunmingensis was subsequently re-illustrated by Liu et al. (2018: sup. fig. 3C), while Zeng et al. (2018a) described a well-preserved isolated oral cone that they tentatively assigned to the same species. This assignment was based on the much greater abundance of this taxon in the Wulongqing Formation compared to P. multisegmentalis, the authors noting that more than 100 frontal appendages of A. kunmingensis have been collected (Zeng et al. 2018a). More recently Zhao et al. (2020: fig. 4D, table 1) illustrated an incomplete frontal appendage and mentioned another radiodont fossil, both collected from a new Guanshan section, assigning both to an undetermined anomalocaridid species. In summary, despite the abundance of radiodont fossils in the Guanshan Konservat-Lagerstätte, only a handful of them have been illustrated to date: the five specimens composing the type material of A. kunmingensis, three additional frontal appendages figured by Hu et al. (2013), and the single oral cone tentatively assigned to A. kunmingensis by Zeng et al. (2018a), the incomplete holotype of P. multisegmentalis, and the recently illustrated frontal appendage from the Changchunshan section.
In the present contribution we describe new radiodont fossils from the Guanshan Konservat-Lagerstätte. This new material allows the description of two new taxa, including the first representative of the family Tamisiocarididae in South China, and confirms the presence of an oral cone in Anomalocaris kunmingensis. This newly documented diversity of the radiodont fauna from Guanshan is compared to that reported from other Cambrian Stage 4 BST deposits globally. The completely endemic fauna of Guanshan radiodonts suggests a strong influence of the benthic environment and its communities on the composition of these presumed free-swimming predators.
Institutional abbreviations.—YKLP, Key Laboratory for Palaeobiology, Yunnan University, Kunming, China.
Other abbreviations.—aux, auxiliary spine; BST, Burgess Shale-type; co, central opening of oral cone; ds, dorsal spine; en, endite; la, left appendage; lp, left large plate; oc, oral cone; om, organic matter; pd, podomere; pp, posterior plate; ra, right appendage; rp, right large plate; s, shaft podomere; sp, small plate; ts, terminal spine; w, ecdysozoan worm.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:5FCBD9DE-C9BE-4A8F-9C68-0232D9829401
Overview of radiodont anatomy
Radiodonts are among the latest diverging members of the lower stem-group euarthropods (Daley et al. 2009; Ortega-Hernández 2016). Their body consists of a head region (typically equipped with dorsal and lateral sclerites, a pair of compound eyes, a circlet of sclerotized plates surrounding the mouth, a pair of protocerebral frontal appendages) and a trunk region made of poorly-differentiated segments (Whittington and Briggs 1985; Daley et al. 2009; Paterson et al. 2011, 2020; Cong et al. 2014; Ortega-Hernández et al. 2017). The trunk bears one or two sets of paired swimming flaps laterally, which are considered as homologous to the biramous appendages of the more crown-ward deuteropods (Van Roy et al. 2015). The frontal appendages attached adjacent to the ventral oral cone anteriorly, and were used for feeding following different strategies. These strategies include prey capture (e.g., Daley and Budd 2010; Daley and Edgecombe 2014; Liu et al. 2018), sediment sifting (e.g., Daley et al. 2013a; Moysiuk and Caron 2019) and even suspension feeding (e.g., Vinther et al. 2014; Van Roy et al. 2015; Lerosey-Aubril and Pates 2018). The frontal appendages consist of a series of well-sclerotized podomeres, separated from one another by arthrodial membranes, and carrying endites of varied morphologies. Typically centimetric, these appendages were larger and more heavily sclerotized than the remains of most co-occurring non-biomineralizing organisms, which may partly explain the ubiquity of radiodonts in Cambrian BST deposits. Radiodont frontal appendages also provide a multitude of characters for phylogenetic analyses and systematics, which usually allow assignment of taxa up to the family level. Four families are currently recognized: Amplectobeluidae, Anomalocarididae, Hurdiidae, and Tamisiocarididae. Amplectobeluids, anomalocaridids, and tamisiocaridids have paired endites on their podomeres, differentiating them from hurdiids, which possess a single endite per podomere (with the sole exception of Ursulinacaris, see Pates et al. 2019a). Tamisiocaridids bear slender endites that are typically longer than the height of their corresponding podomere, and are subequal in length along the appendage. In amplectobeluids and anomalocaridids, the endites are approximately the same length or usually shorter than the height of their corresponding podomere, and they alternate long/short on adjacent podomeres. Most amplectobeluids bear a distinctive hypertrophied endite in the proximal part of the appendage. Amplectobeluids may also differ from anomalocaridids in the presence of gnathobase-like structures posterior to the mouth, as-yet only documented in Amplectobelua and Ramskoeldia (Cong et al. 2017, 2018), and a possibly tetraradial, rather than triradial organization of the oral cone (Liu et al. 2018).
Geological setting
The Guanshan Biota is known from over 10 localities (Zhao et al. 2020) in the Wulongqing Formation in East Yunnan (Hu et al. 2013). This lithostratigraphic unit is comprised of sandstone, siltstone, and mudstone beds, with the lower part of the sequence deposited in a deeper-water environment than the higher levels (Hu et al. 2013; Chen et al. 2020). Exceptionally preserved fossils are most often retrieved from mudstone layers with minimal bioturbation, which probably represent rapid burial event beds according to sedimentological and taphonomic analyses (Hu et al. 2013; Forchielli et al. 2014). Sedimentological characteristics and ichnofossils both suggest deposition of the Guanshan Konservat-Lagerstätte in shallower settings compared to most other Cambrian BSTs, with the possible exception of the Emu Bay Shale, Australia (Hu et al. 2013; Paterson et al. 2016; Ding et al. 2018; Chen et al. 2019, 2020a).
The fossils described in this contribution originate from a locality c. 2.5 km southeast of the village of Lihua (Fig. 1). The Cambrian succession is well exposed along a valley, with the exception of the oldest part that is truncated by a fault (Fig. 1C). The succession exposed at the Lihuazhuang section is composed of brownish siltstone and quartzose sandstone beds with subordinate thin-bedded muddy shale, which belong to the Cambrian (Stage 4; Canglangpuan regional stage, Palaeolenus–Megapalaeolenus trilobite zones) Wulongqing Formation (Hu et al. 2013; Jiao et al. 2016). The diverse radiodonts reported here are found alongside numerous brachiopod valves (Zhang et al. 2011).
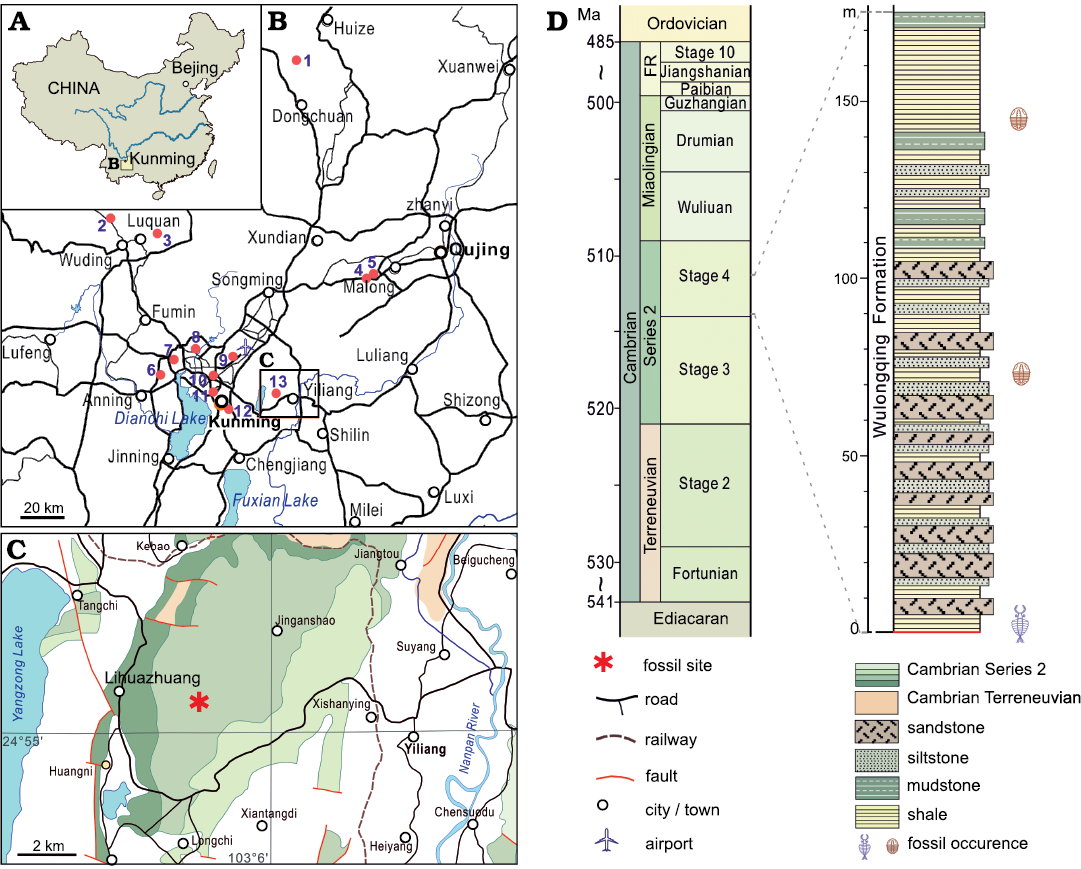
Fig. 1. A. Map of China showing location of the studied area. B. Map showing thirteen sites where fossil assemblages assigned to the Cambrian (Stage 4) Guanshan biota have been found: 1, Dahai (Liu et al. 2012); 2, Shijiangjun (Luo et al. 2008); 3, Xinlongcun (Liu et al. 2016); 4, Wulongqing (Luo et al. 2008); 5, Kanfuqing (Chen et al. 2019); 6, Baimei (Liu et al. 2016); 7, Qiongzhusi (Luo et al. 2008); 9, Dabanqiao (Luo et al. 2008); 10, Changchunshan (Zhao et al. 2020); 11, Gaoloufang (Luo et al. 2008; Hu et al. 2010); 12, Chenggongxincheng (Hu et al. 2013); 13, Lihuazhuang (Jiao et al. 2016; this study). C. The location of the Lihuazhuang section in (asterisk). D. Stratigraphic column showing the occurrence of the fossils reported in this study.
Material and methods
Digital images for all specimens were captured under bright-field illustration using a Leica M205-C stereomicroscope fitted with a Leica DFC 500 digital camera. Figured images were processed with Adobe Photoshop CS 4. Figures were constructed using Inkscape 1.0. Digital measurements were taken from photographs using ImageJ2 (Schneider et al. 2012; Rueden et al. 2017).
All studied fossils are deposited at the Key Laboratory for Palaeobiology, Yunnan University, Kunming, China.
The terminology for isolated radiodont appendages follows the terms used by Daley and Edgecombe (2014) and Guo et al. (2019). The proximal-most podomeres of the frontal appendage are referred to as the “shaft”. Articulations between podomeres in the shaft are more weakly defined than those of the “distal articulated region”, the more distal part of the appendage bearing most of the endites, the outgrowths projecting from the ventral margin, and associated with articulating membranes. In addition, a change in slope on the dorsal margin, or “dorsal kink”, often separates these two regions. The “height” of a podomere refers to the distance between its ventral and dorsal margins, while its “length” describes its extent along the proximo-distal axis of the appendage. The term “auxiliary spines” describes the small spines that project from the endite margins either distally or proximally (i.e., towards the distal or proximal end of the appendage). The position of auxiliary spines on endites is described in reference to the base or the tip of the endite to which they are attached (i.e., towards the insertion site of the endite on the podomere, or opposite to it). The arrangement of these spines can either be pectinate or radiating, depending on whether they are parallel to or diverging from one another, respectively.
Systematic palaeontology
Clade Panarthropoda Nielsen, 1995
Order Radiodonta Collins, 1996
Family Amplectobeluidae Pates, Daley, Edgecombe, Cong, and Lieberman, 2019b
Type genus: Amplectobelua Hou, Bergström, and Ahlberg, 1995.
Genera included: Type genus, Lyrarapax Cong, Ma, Hou, Edgecombe, and Strausfeld, 2014, and Ramskoeldia Cong, Edgecombe, Daley, Guo, Pates, and Hou, 2018, and “Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013.
Remarks.—“Anomalocaris” kunmingensis was one of only a handful of radiodont taxa at the time when it was formally described (Wang et al. 2013). It was assigned to Anomalocaris based on the number of podomeres in the frontal appendage and the alternation of long and short endites on subsequent podomeres (Wang et al. 2013), characters shared with species of Anomalocaris, such as A. canadensis, A. pennsylvanica, and A. saron (Briggs 1979; Whittington and Briggs 1985; Chen et al. 1994; Hou et al. 1995). Our understanding of radiodont diversity and their internal relationships has substantially increased in recent years, and all phylogenetic analyses including this taxon aimed at understanding the interrelationships of radiodont taxa have retrieved “Anomalocaris” kunmingensis as a member of Amplectobeluidae rather than Anomalocarididae (Vinther et al. 2014; Cong et al. 2014; Van Roy et al. 2015; Lerosey-Aubril and Pates 2018; Liu et al. 2018). Additional support for the assignment of this taxon to Amplectobeluidae rather than Anomalocarididae comes from the relative lengths of the endites on the fifth and third podomeres in the distal articulated region, which has been proposed as diagnostic for this family (Cong et al. 2018). In most amplectobeluids, the endite is distinctively longer on the fifth podomere than the third—as seen in a previously described specimen of this taxon (Wang et al. 2013: fig. 1g) – while in Anomalocarididae the endite is longer on the third than the fifth (e.g., Daley and Edgecombe 2014).
Vinther et al. (2014) referred “Anomalocaris” kunmingensis as Amplectobelua kunmingensis, but more recent studies have simply treated this taxon as either Anomalocaris kunmingensis or “Anomalocaris” kunmingensis (e.g., Cong et al. 2014; Van Roy et al. 2015; Lerosey-Aubril and Pates 2018; Liu et al. 2018; Zeng et al. 2018a; Guo et al. 2019; Lerosey-Aubril et al. 2020). The Guanshan taxon comprises 13 podomeres in the distal articulated region, whereas the two species currently assigned to Amplectobelua have 12 podomeres. The recently described amplectobeluid Ramskoeldia has 13 podomeres in its distal articulated region, a less hypertrophied endite than Amplectobelua or Lyrarapax, and endites with multiple auxiliary spines which face both proximally and distally (Cong et al. 2018), all characters exhibited by “Anomalocaris” kunmingensis. However, the amplectobeluid appendage from Guanshan also exhibits notable differences compared to Ramskoeldia, such as a particularly weak distal tapering or the presence of an unusually well-developed shaft endite projecting ventro-proximally. The latter character could suggest closer affinities with Laminacaris (Guo et al. 2019), another possible amplectobeluid from Chengjiang (Lerosey-Aubril and Pates 2018). “Anomalocaris” kunmingensis also differs from Ramskoeldia species in the presence of an oral cone (sensu Liu et al. 2018; Zeng et al. 2018a; this study) and the absence of gnathobase-like structures (Cong et al. 2018). Thus, if “Anomalocaris” kunmingensis can be confidently re-assigned to the family Amplectobeluidae, it remains unclear whether it would warrant the creation of a new genus or could be accommodated within an existing one. Study of the abundant material mentioned by Zeng et al. (2018a) will allow a formal revision of this species.
Genus uncertain
“Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013
Figs. 2–4.
2013 Anomalocaris kunmingensis sp. nov.; Wang et al. 2013: 3938–3939, fig. 1.
2013 Anomalocaris kunmingensis Wang et al. 2013; Hu et al. 2013: 137, fig. 181.
2014 Anomalocaris kunmingensis Wang et al. 2013; Cong et al. 2014: ext. data fig. 4.
2014 Amplectobelua kunmingensis Wang et al. 2013; Vinther et al. 2014: fig. 3
2015 Anomalocaris kunmingensis Wang et al. 2013; Van Roy et al. 2015: ext. data fig. 10.
2018 Anomalocaris kunmingensis Wang et al. 2013; Cong et al. 2018: 619.
2018 Anomalocaris kunmingensis Wang et al. 2013: Lerosey-Aubril and Pates 2018: fig. 2, sup. figs. 4, 5.
2018 “Anomalocaris” kunmingensis Wang et al. 2013; Liu et al. 2018: sup. figs. 2, 3C.
2018 Anomalocaris kunmingensis Wang et al. 2013; Zeng et al. 2018a: 41, 42, 46.
2018 Radiodontan gen. indet. sp. indet.; Zeng et al. 2018a: figs. 2, 3.2.
2020 “Anomalocaris” kunmingensis Wang et al. 2013; Lerosey-Aubril et al. 2020: 506.
Material.—A pair of frontal appendages associated with an oral cone (YKLP 12414), and tentatively one partial frontal appendage (YKLP 12415) from Lihuazhuang section, locality c. 2.5 km southeast of the Lihuazhuang village, China (Fig. 1). Cambrian (Stage 4), lower part of Wulongqing Formation, Palaeolenus Biozone (Hu et al. 2010b).
Description.—The first specimen considered here (YKLP 12414) represents a pair of frontal appendages (Fig. 2: la, ra) anterior to an oral cone (Fig. 2: oc), surrounded by disarticulated material, including an ecdysozoan worm (Fig. 2: w). At least 13 podomeres can be tentatively identified in the left appendage (11 mm along dorsal margin, Fig. 2: la) by counting podomere boundaries and/or paired endites (Fig. 3A2: pd). A break in the rock separates a poorly preserved area, which could represent the proximal part of the distal articulated region or part of the shaft (Fig. 3A2: s?), from the oral cone and the right appendage. Endites are approximately the same length as the height of the podomere to which they attach (Fig. 3A2: en). Up to two small protrusions, which may represent auxiliary spines, are seldom visible on some proximal endites. The distalmost part of the appendage is more crudely preserved ventrally, but a long dorsal spine that curves to follow the dorsal margin of the appendage is visible in this region (Fig. 3A2: ds). Two protrusions of a similar size in more proximal parts of the appendage may represent dorsal spines, or the remains of the dorsal margin that is otherwise missing in this part of the appendage (Fig. 3A2: ds?). The number of podomeres in the preserved part of the right appendage (Fig. 2: ra; 7 mm along the dorsal margin) cannot be counted with certainty due to poor preservation distally, but it seems to be close to 11. Boundaries can be clearly seen in the more proximal region, and the podomeres have a shape similar to those of the left appendage. Each podomere bears a robust, but incomplete, endite.

Fig. 2. Amplectobeluid radiodont “Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013, from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. YKLP 12414, a pair of frontal appendages and associated oral cone. Abbreviations: la, left appendage; oc, oral cone; om, organic matter; ra, right appendage; w, ecdysozoan worm.
Both appendages converge proximally toward a partially preserved oral cone (Fig. 3A3, A4). The latter structure can be orientated anterior-posterior and left-right by comparing to the location of the associated frontal appendages, which sit antero-lateral to it in in situ position. A large area between the right appendage and the partial oral cone is covered with orange/red material, where no discernible features can be observed (Fig. 2: om). The oral cone consists of plates of apparently three different sizes arranged around an approximately rectangular opening. Most of the plates are thin and wedge shaped, wider at the outer margin of the cone than the inner. Some display furrows parallel to the slightly curved plate boundaries, but otherwise lack ornamentation (Fig. 3A4: sp). At the midpoint of the posterior margin of the central opening, one plate extends further into the opening than the other ones; this longer plate is tentatively identified as the posterior plate (Zeng et al. 2018a; Fig. 3A4: pp?). One node-bearing larger plate occurs on each side of the oral cone (Fig. 3A4: lp?, rp). Both face one another and their long axes are orientated normal to that of the posterior plate, which allows their identification as the left (incomplete) and right large plates of Zeng et al. (2018a). Both lateral margins of the right plate are clearly delineated by straight margins, and it extends further into the central opening than the plates to either side. The anterior part of the oral cone is missing.
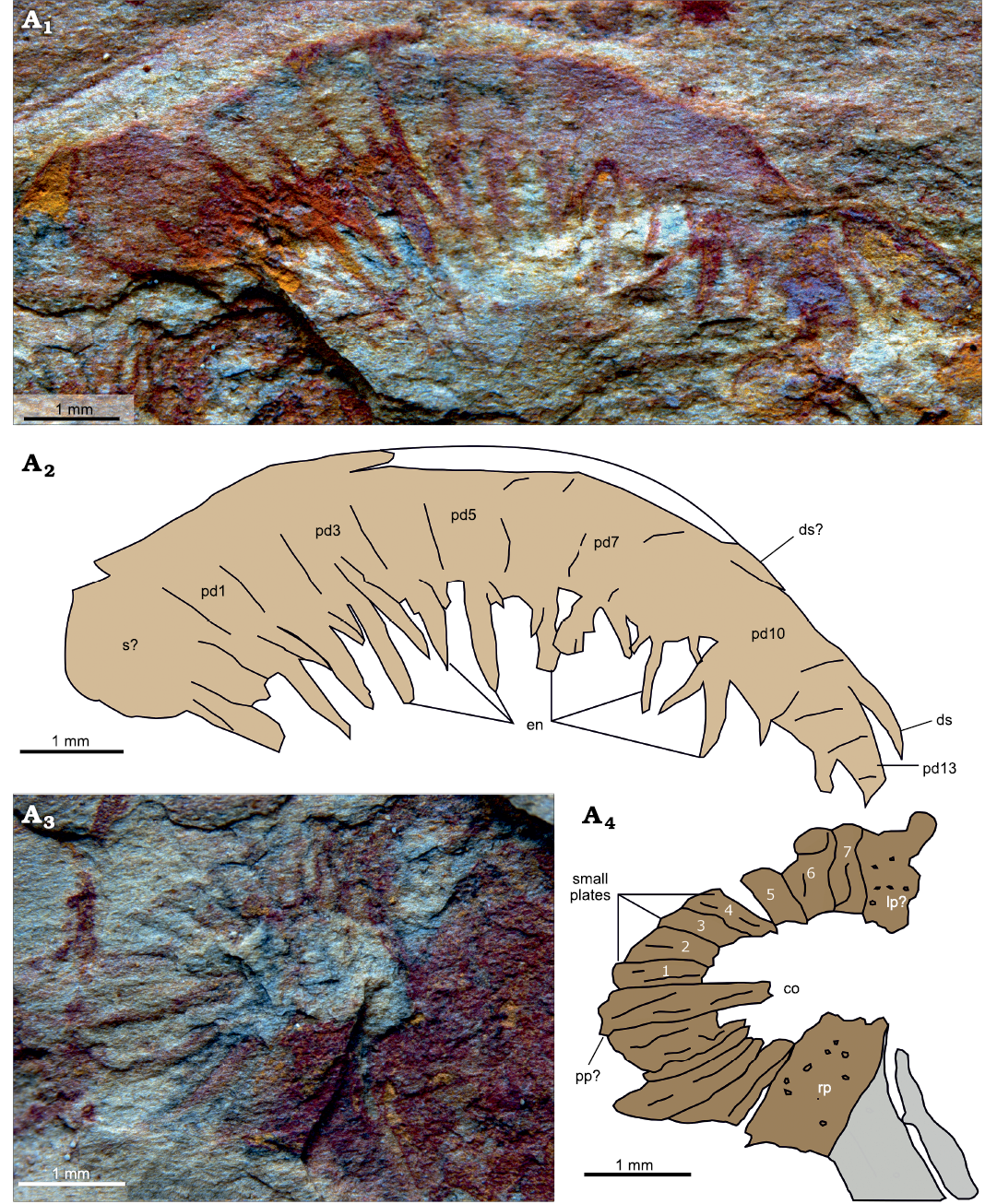
Fig. 3. Amplectobeluid radiodont “Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013, from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. YKLP 12414, details of left frontal appendage (A1, A2), details of oral cone (A3, A4). Photographs (A1, A3) and explanatory drawing (A2, A4). Abbreviations: co, central opening; ds, dorsal spine; en, endite; lp?, putative left large plate; pd, podomere; pp? putative posterior plate; rp, right large plate; s?, shaft podomere; sp, small plate. Numbers indicate individual small plates between posterior and left plates.
A second specimen (YKLP 12415), a partial frontal appendage of at least nine podomeres that measures 15 mm along the dorsal margin, is tentatively assigned to the same taxon (Fig. 4). Podomere proportions slightly change along the appendage, from noticeably taller than long to roughly as tall as long distally (Fig. 4: pd). The proximal-most six podomeres bear large elongate spiniform endites, which are longer than the height of the podomere to which they attach. One auxiliary spine is present on the proximal margin and up to two can be observed with confidence on the distal margin of these endites (Fig. 4: aux). Endites alternate long/short on subsequent podomeres and reduce in length along the appendage. The distalmost three podomeres bear shorter spiniform endites apparently devoid of auxiliary spines. Elongate dorsal spines curved to follow the outline of the appendage are present on the distalmost four podomeres (Fig. 4: pd). These dorsal spines look paired at least on the distalmost two podomeres. The appendage terminates in a short straight terminal spine (Fig. 4: ts).
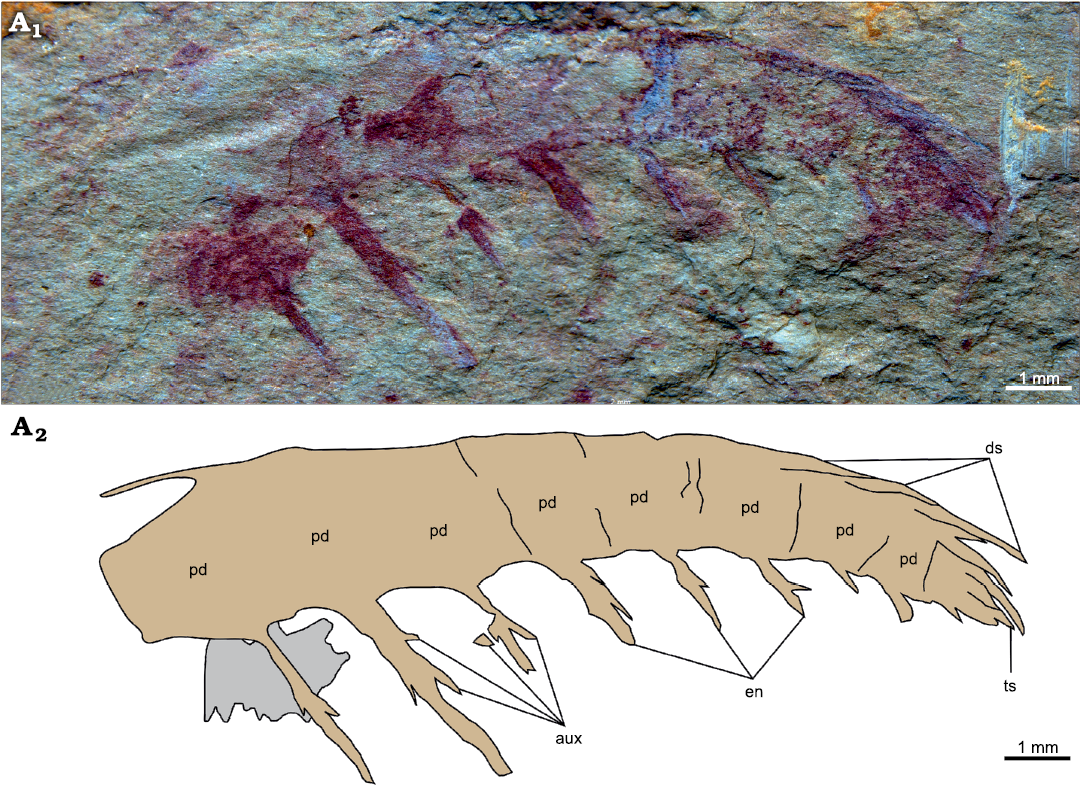
Fig. 4. Amplectobeluid radiodont “Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013, from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. YKLP 12415, a partial frontal appendage. Photograph (A1) and explanatory drawing (A2). Abbreviations: aux, auxiliary spine; ds, dorsal spine; en, endite; pd, podomere; ts, terminal spine.
Remarks.—The morphology of the endites with multiple auxiliary spines, shape of proximal podomeres, curvature of dorsal spines, and number of podomeres in the distal articulated region are all consistent with interpretation of these three appendages as belonging to “Anomalocaris” kunmingensis, which is reported to be by far the most abundant radiodont from the Guanshan Biota (Wang et al. 2013; Zeng et al. 2018a). The oral cone and associated frontal appendages are considered to belong to the same animal. Similar examples of these three structures found together and dissociated with the rest of the body have been reported in several taxa (e.g., Cambroraster, Hurdia, and Stanleycaris; Caron et al. 2010: sup. mat. 5, pl. 1; Daley et al. 2013a: figs. 22D, 24A; Moysiuk and Caron 2019: fig. 2j), although the anatomical or taphonomic reasons for this recurrent association remain unclear. The oral cone shows multiple similarities to the single isolated Guanshan oral cone tentatively assigned to this taxon by Zeng et al. (2018a). Both specimens share a combination of features unique amongst radiodonts: a tetraradial organization, in which the posterior plate is the smallest of the four large plates; the presence of both nodes and furrows; and a central opening elongate rectangular in outline and apparently devoid of inner tooth rows. Despite the absence of the anterior plate in the new specimen, the alignment of the two lateral plates strongly suggests a tetraradial organization for the larger plates. Likewise, slightly more than the posterior half of the central opening is preserved; when complete the outline of this opening was likely closer to an antero-posteriorly elongate rectangle, rather than a square as in some hurdiids, although deformation cannot be entirely ruled out in this case. An association of furrows and nodes has as-yet only been observed in the Guanshan oral cone described by Zeng et al. (2018a), and triradially arranged oral cones of Anomalocaris. Nodes were also described in a juvenile of the amplectobeluid Lyrarapax (Liu et al. 2018), but the preservation did not allow the observation of folds (if originally present). Nodes have also been reported in some hurdiid oral cones (Pates et al. 2018: fig. 2.6; Sun et al. 2020a), but not in association with folds. The nodes in the new specimen are only visible on the large lateral plates, not all plates as in the other oral cone from Guanshan (Zeng et al. 2018a), but this may be the result of poorer preservation. Lastly, the larger plates are separated from one another by sets of seven small plates in all previously described, tetraradially arranged oral cones, which is the case for this new specimen between the posterior plate and left plate. The number of plates between the posterior plate and right plate cannot be counted with certainty.
The presence of an oral cone associated with frontal appendages that are both consistent with material assigned to “Anomalocaris” kunmingensis, supports the conclusions of Zeng et al. (2018a) that this taxon possesses an oral cone. The position of the oral cone relative to the appendages supports the interpretation of Zeng et al. (2018a), that the smallest of the larger plates was at the posterior of the oral cone, and the largest plates were positioned laterally. This is the second report of a tetraradial oral cone in an amplectobeluid radiodont, following a single juvenile specimen of Lyrarapax unguispinus (Liu et al. 2018). Poor preservation and small size prevented the observation of folds (if present) in the latter specimen. Accordingly, it remains unclear whether the combination of a tetraradial organization, nodes, and folds is unique to “Anomalocaris” kunmingensis or could represent an additional diagnostic character for Amplectobeluidae.
Stratigraphic and geographic range.—Gaoloufang village and Lihuazhuang sections, Yunnan, China, Wulongqing Formation (Palaeolenus and Megapalaeolenus zones), Cambrian (Stage 4).
Family Anomalocarididae Raymond, 1935
Type genus: Anomalocaris Whiteaves, 1892.
Genera included: Type genus and Paranomalocaris Wang, Huang, and Hu, 2013.
Remarks.—Paranomalocaris was originally described as a monospecific genus within the family Anomalocarididae. All phylogenetic analyses attempting to resolve the internal relationships of radiodonts to date have failed to resolve the family Anomalocarididae or even genus Anomalocaris (even excluding “Anomalocaris” briggsi and “Anomalocaris” kunmingensis which are not considered members of the genus) as a monophyletic group. Analyses either returned a paraphyletic grade towards monophyletic Amplectobeluidae, or a polytomy with the latter group (e.g., Cong et al. 2014; Vinther et al. 2014; Van Roy et al. 2015; Lerosey-Aubril and Pates 2018; Moysiuk and Caron 2019). Only Liu et al. (2018) retrieved a clade Anomalocarididae (A. kunmingensis and A. briggsi excluded) in one of their analyses, which recovered Paranomalocaris as sister to a clade uniting Anomalocarididae to Amplectobeluidae. In summary, there is no strong phylogenetic support for assigning Paranomalocaris to Anomalocarididae, but this is also the case of all the species of Anomalocaris. Future work may support the erection of a distinct family for Paranomalocaris, but for now we retain this genus within the family as originally described.
Genus Paranomalocaris Wang, Huang, and Hu, 2013
Type species: Paranomalocaris multisegmentalis Wang, Huang, and Hu, 2013; Gaoloufang Village, Kunming, Yunnan, China, Wulongqing Formation (Palaeolenus and Megapalaeolenus zones), Cambrian (Stage 4).
Diagnosis (emended from Wang et al. 2013).—Radiodont with slender frontal appendages, which comprise at least 17 podomeres; proximal podomeres have a tall rectangular lateral outline, which changes to approximately square distally; endites alternate long/short on subsequent podomeres, and reduce in length along the appendage; small triangular terminal spine on distalmost podomere.
Paranomalocaris multisegmentalis Wang, Huang, and Hu, 2013
Fig. 5.
2013 Paranomalocaris multisegmentalis sp. nov.; Wang et al. 2013: 3940, figs. 2, 3.
2013 Paranomalocaris multisegmentalis Wang et al. 2013; Hu et al. 2013: 137, 192, fig. 181.
2014 Paranomalocaris multisegmentalis Wang et al. 2013; Cong et al. 2014: ext. data fig. 4.
2014 Paranomalocaris multisegmentalis Wang et al. 2013; Vinther et al. 2014: fig. 3.
2015 Paranomalocaris multisegmentalis Wang et al. 2013; Van Roy et al. 2015: ext. data fig. 10.
2018 Paranomalocaris multisegmentalis Wang et al. 2013; Lerosey-Aubril and Pates 2018: fig. 2, sup. figs. 4, 5.
2018 Paranomalocaris multisegmentalis Wang et al. 2013; Liu et al. 2018: sup. fig. 2.
2018 Paranomalocaris multisegmentalis Wang et al. 2013; Zeng et al. 2018a: 41, 42, 46.
Holotype: NIGP154564, an incomplete frontal appendage compressed laterally (Wang et al. 2013).
Type locality: Gaoloufang village, Kunming, East Yunnan, China (Wang et al. 2013).
Type horizon: Wulongqing Formation (Palaeolenus and Megapalaeolenus Zone), Cambrian (Series 2, Stage 4) (Wang et al. 2013).
Material.—Type material and YKLP 12416, a partial frontal appendage compressed laterally from Lihuazhuang section, near Lihua village, about seven kilometres northwest of the Yiliang county in Yunnan, China (Fig. 1), Cambrian (Stage 4), lower part of Wulongqing Formation, Palaeolenus Biozone (Hu et al. 2010b).
Diagnosis (emended from Wang et al. 2013).—Paranomalocaris with short, plate-like proximal endites, each bearing a spiniform termination and at least three distally facing auxiliary spines; serrated dorsal spines.
Description.—YKLP 12416 is incomplete proximally, consists of 15 podomeres (Fig. 5: pd1–15) and a terminal spine, and measures 45 mm along the dorsal margin. The appendage curves ventrally with a slight dorsal kink visible at the midpoint of the second podomere (Fig. 5A1). Most podomeres are approximately square shaped, being slightly taller than long in the proximal part and slightly longer than tall towards the middle, except for the four distal ones that are notably taller than long. Endites are present on every podomere (Fig. 5A2: en). No second row of endites is observed. On pd1, the endites are not well preserved. A single outgrowth from the ventral surface close to the proximal margin might represent a broken endite base (Fig. 5A2: en1) orientated towards the proximal part of the appendage. The endite on pd2 is broadly rectangular in outline (3 × 1 mm), with an incomplete spiniform termination, and bears three auxiliary spines on its distal margin (Fig. 5A3). En3, the longest endite (Fig. 5A2), measures c. 4.5 mm from base to tip, and is also rectangular in outline, except for a robust spiniform termination that slightly curves towards the distal portion of the appendage. Three robust and elongate auxiliary spines radiate ventro-distally from the middle third of the distal margin of en3, and a single, much smaller one project from its proximal margin (Fig. 5A3). En4 is much smaller than En3, but exhibits similar shape and distribution pattern of auxiliary spines. En5–11 bear three to six auxiliary spines on both margins, which follow a mostly pectinate arrangement and project at an angle of c. 130° (distal ones) or c. 150° (proximal ones) with the long axes of the endites (Fig. 5A3). The size of the spiniform termination relative to the rest of the endite tends to decrease from en4–11. The preservation of en12–15 is poorer, but en13–15 appear to be short, project distally, and devoid of auxiliary spines. A small dorsal spine may occur on pd14, and a single, robust terminal spine is visible on pd15 (Fig. 5A2: ds, ts).
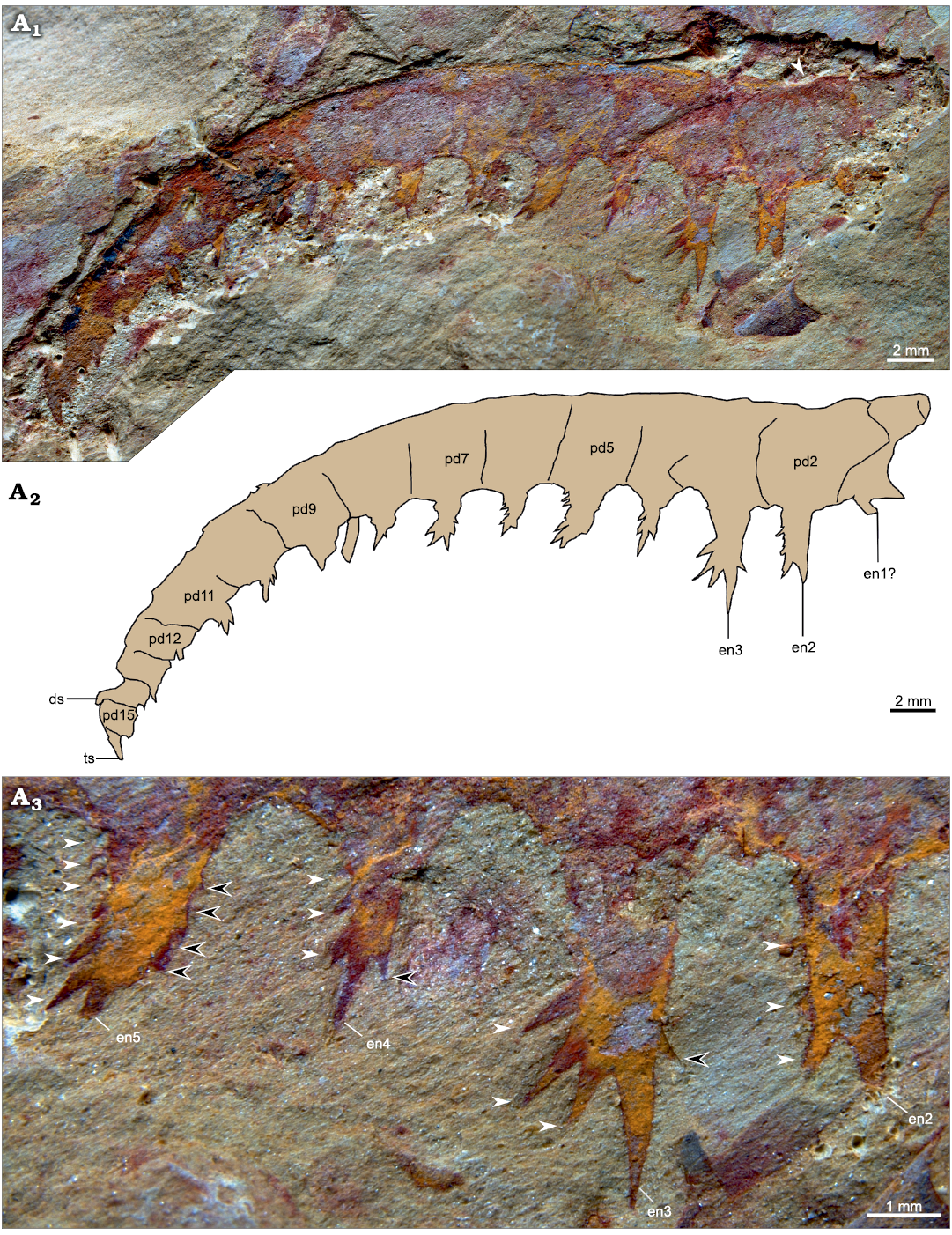
Fig. 5. Anomalocaridid radiodont Paranomalocaris multisegmentalis Wang, Huang, and Hu, 2013, from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. YKLP 12416, incomplete frontal appendage (proximalmost region missing); general view (A1), white arrow indicates fold in dorsal margin; details of the proximal preserved endites (A2, A3), white arrows, distally facing auxiliary spines; black arrows, proximally facing auxiliary spines. Photographs (A1, A3) and explanatory drawing (A2). Abbreviations: ds, dorsal spine; en, endite; pd, podomere; ts, terminal spine.
Remarks.—While the broad shape of en3–11 and their pattern of numerous distal and proximal facing auxiliary spines bear similarity to both Paranomalocaris multisegmentalis and Ramskoeldia platyacantha, the relative length and morphology of en2–7 provide strong support for the interpretation of this specimen as the distal 15 podomeres of a P. multisegmentalis appendage.
The alternation in size of the endites of adjacent podomeres and their overall reduction in length along the appendage are characters known in all anomalocaridids. While members of the Anomalocaris have 13 podomeres in the distal articulated region, all 15 podomeres of the new specimen are considered to belong to the distal articulated region, a number only known in the Paranomalocaris and Tamisiocaris (Daley and Peel 2010; Wang et al. 2013; Vinther et al. 2014). Affinities with the latter genus can be discarded, as endites of Tamisiocaris are all of subequal length, a character that currently distinguishes Tamisiocarididae from Amplectobeluidae and Anomalocarididae.
Under a hypothesis that would interpret this specimen as Ramskoeldia platyacantha, the distal 13 podomeres of YKLP 12416 would represent the distal articulated region, with pd1 and pd2 comprising the shaft. The slight dorsal kink in pd2 could be interpreted as supporting this arrangement, however its presence within a podomere, rather than at the boundary of two adjacent podomeres, suggests that this feature is due to a slight twist of the appendage during compression and does not represent a biological feature. Two further observations allow us to reject the assignment of this specimen to R. platyacantha, in favour of Paranomalocaris. Firstly, the endites decrease in length along the whole appendage with no exception, while an en7 longer than en5 characterizes the amplectobeluid genera Amplectobelua and Ramskoeldia. Secondly, the distalmost shaft podomere bears a small and simple spiniform endite at its distal margin in R. platyacantha, while the specimen described herein would exhibit an elongate endite with auxiliary spines at this position (Fig. 5: en2), if interpreted as a sub-complete appendage of this species. For the specimen under consideration, en2 is smaller than, but morphologically similar to en3, as would be expected from the endites of adjacent podomeres in the distal articulated region of Paranomalocaris.
Some characters diagnostic for Paranomalocaris multisegmentalis cannot be observed in YKLP 12416. The serrated appearance of the distal dorsal margin of the holotype appendage, resulting from the presence of half a dozen dorsal spines, is not observed in the new specimen due to the poor preservation/incomplete exposition of this part of the fossil. Likewise, the proximal part is missing in the new specimen, which therefore only preserves 15 podomeres, whereas Wang et al. (2013) described the holotype specimen to possess “at least 22 podomeres”. We can confirm at least 17 podomeres from the figured specimen (Wang et al. 2013: figs. 2, 3), but since this previously illustrated specimen is clearly incomplete, a higher podomere number must characterize the genus Paranomalocaris. Of note, YKLP 12416 shows that the proximal endites (Fig. 5: en2, en3) differ from the more distal ones in having a single proximally facing auxiliary spine each (rather than multiple ones), a detail that could not be observed in the holotype due to preservation.
Stratigraphic and geographic range.—Gaoloufang village and Lihuazhuang section, Yunnan, China, Wulongqing Formation (Palaeolenus and Megapalaeolenus zones), Cambrian (Stage 4).
Paranomalocaris simplex sp. nov.
Fig. 6.
Zoobank LCID: urn:lsid:zoobank.org:act:F79695F0-AF05-4A3A-B 326-A6516936F3B2
Etymology: From Latin simplex, simple; emphasising the simple morphology of endites and dorsal spines in the new species, which contrast to the complex endites and serrated dorsal spines of the type species.
Type material: Holotype: YKLP 12417, a laterally compressed frontal appendage; paratype: YKLP 12418a, a partial laterally compressed frontal appendage from the type locality.
Type locality: Lihuazhuang section, locality c. 2.5 km southeast of the Lihua village, China (Fig. 1).
Type horizon: Lower part of Wulongqing Formation, Palaeolenus Biozone, Cambrian (Stage 4) (Hu et al. 2010b).
Material.—Type material only.
Diagnosis.—Paranomalocaris with long needle-like endites, some exceeding in length the height of the podomere to which they attach; non-serrated dorsal spines present on every podomere.
Description.—Two isolated near-complete frontal appendages laterally compressed (YKLP 12417, 12418a) measuring 32 mm and 33 mm along the dorsal margin, respectively (Fig. 6). At least 20 podomeres can be identified for each appendage, changing in lateral outline from tall rectangles proximally to square shapes distally (Fig. 6: pd). Podomeres can be identified from their boundaries, visible as simple lines on the surface of the appendage, and/or from the pair of endites projecting ventrally.
Both specimens bear needle-like endites, one pair per podomere, which reduce in length toward the distal tip of the appendage, and alternate long/short on adjacent podomeres (Fig. 6: en). In the holotype (YKLP 12417; Fig. 6A), thin and short auxiliary spines might occur on the distal margin, at an angle of c. 140° with the long axis, on some endites. Other endites appear to lack auxiliary spines. No auxiliary spines are visible on any endites of the paratype (Fig. 6B). Dorsal spines are visible on every podomere in the holotype, either as spines in its distal third (Fig. 6A2: ds) or as circular attachment points in its more proximal region (Fig. 6A2: c). A single spine in the distal region of YKLP 12418a may be a dorsal spine, but most of the dorsal surface of this specimen is poorly preserved. One small and curved terminal spine is visible on each specimen (Fig. 6: ts).
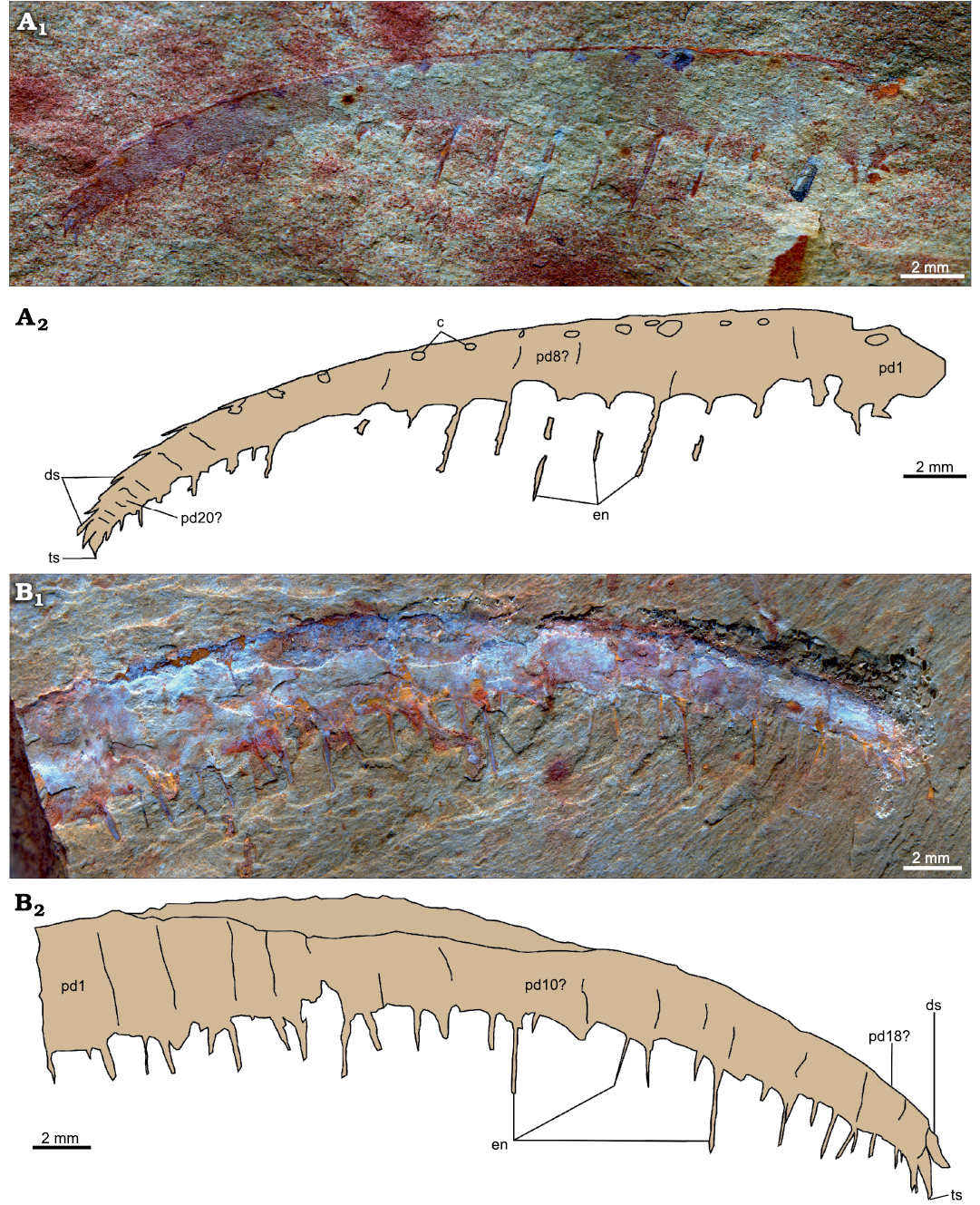
Fig. 6. Anomalocaridid radiodont Paranomalocaris simplex sp. nov. from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. A. YKLP 12417, a near complete frontal appendage. B. YKLP 12418, distal portion of a frontal appendage. Photographs (A1, B1) and explanatory drawings (A2, B2). Abbreviations: c, circular attachment point for dorsal spine; ds, dorsal spine; en, endite; pd, podomere; ts, terminal spine.
Remarks.—The type species and hitherto only species of Paranomalocaris, namely Paranomalocaris multisegmentalis, was distinguished from other members of Anomalocarididae by its high number of podomeres, the morphology of its proximal endites (plate-like and bearing at least five auxiliary spines on both proximal and distal margins), and the presence of serrated dorsal spines.
Among radiodonts only Paranomalocaris and Tamisiocaris possess such a high number of podomeres in the distal articulated region. As discussed above, this feature is combined with an alternation in endite length along the appendage only in Paranomalocaris, allowing a confident assignment of the new taxon to this genus. Paranomalocaris simplex can be easily differentiated from P. multisegmentalis by the slender morphology of its endites, and the absence of serration on the dorsal spines.
Stratigraphic and geographic range.—Lihuazhuang section, Yiliang county, Yunnan, China, Wulongqing Formation (Palaeolenus Biozone), Cambrian (Stage 4).
Family Tamisiocarididae Pates and Daley, 2019
Type genus: Tamisiocaris Daley and Peel, 2010.
Genera included: Type genus and “Anomalocaris” briggsi Nedin, 1995.
Tamisiocarididae indet.
Fig. 7.
Material.—A single partial frontal appendage (YKLP 12419) from Lihuazhuang section, locality c. 2.5 km southeast of Lihua village, China (Fig. 1), Cambrian (Stage 4), lower part of Wulongqing Formation, Palaeolenus Biozone (Hu et al. 2010).
Description.—This near complete appendage is composed of 15 approximately square podomeres (counted from the number of endites and rarely observed podomere boundaries), and measures c. 50 mm along the dorsal margin (Fig. 7). The appendage lacks both proximal and distal ends, but the preserved portion tapers distally (Fig. 7A3, A4). Each podomere bears an elongate and slender endite, subequal in size along the appendage (Fig. 7: en). The best preserved of these endites are longer than the height of the podomere to which they attach and are slightly curved towards the proximal end of the appendage (e.g., Fig. 7A4: en9). Endites bear short and delicate auxiliary spines, c. 0.5–1 mm apart, that project at an angle of 120–150° relative to the long axis, from both the proximal and distal margins following a pectinate arrangement (Fig. 7: aux). The presence of dorsal spines cannot be determined, as the dorsal margin of the appendage is not well preserved, especially distally.
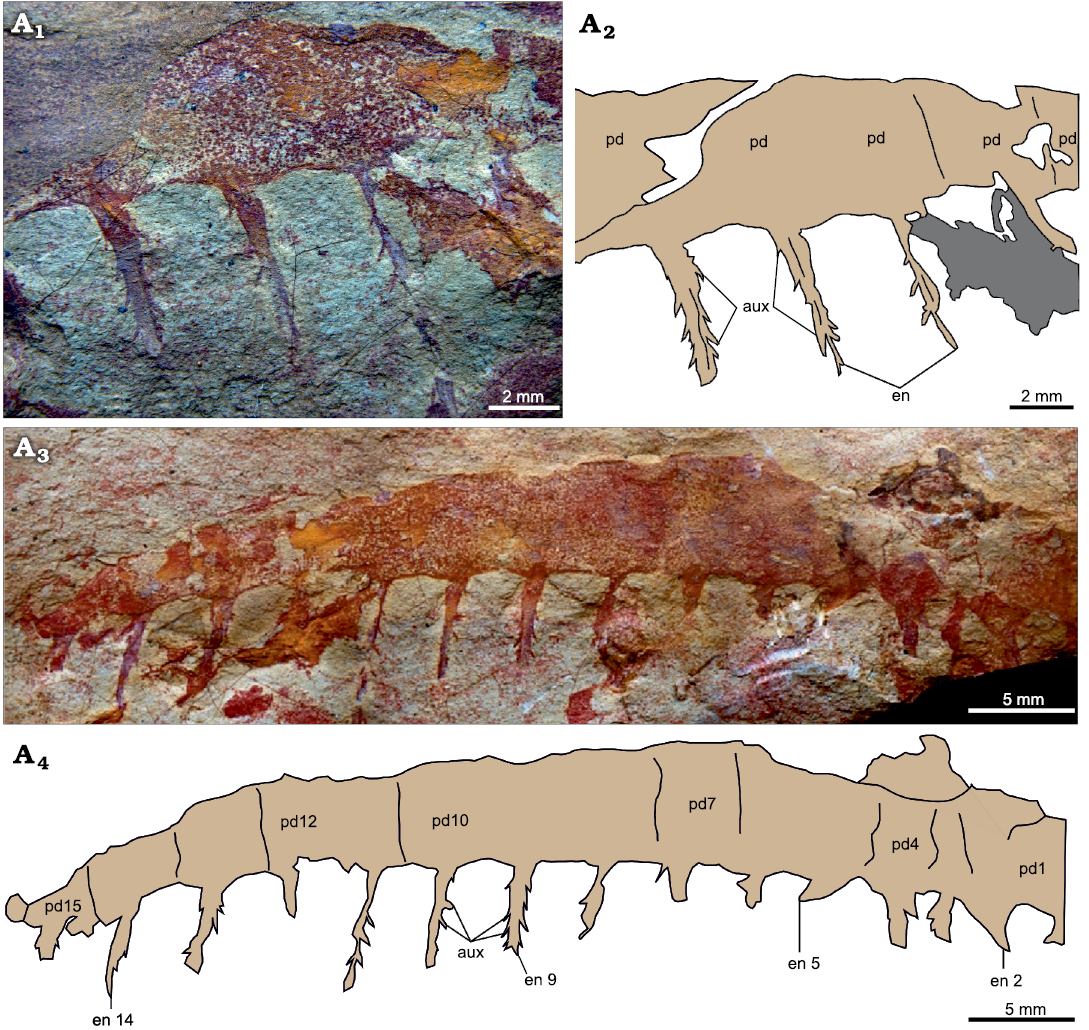
Fig. 7. Tamisiocarididae indet. from the Cambrian (Stage 4) Guanshan Biota, Lihuazhuang section, Yunnan, China. YKLP 12419, a partial frontal appendage. Counterpart (A1, A2), part (A3, A4). Photographs (A1, A3) and explanatory drawings (A2, A4). Abbreviations: aux, auxiliary spine; en, endite; pd, podomere.
Remarks.—YKLP 12419 possesses well-differentiated podomeres bearing endites with auxiliary spines, which is a combination of characters solely known in radiodonts and the recently described deuteropod Kylinxia (Zeng et al. 2020). The presence of numerous elongate endites, similar in size and morphology in the entire distal articulated region allows a confident assignment of this fossil to the radiodont family Tamisiocarididae. Indeed, endites alternate long/short on adjacent podomeres in amplectobeluids, anomalocaridids, and Kylinxia, while there is a marked difference in size and morphology between the proximal and distal (when present) endites of the distal articulated region in hurdiids. A lack of functional articulation and therefore of distal articulated region characterizes the atypical radiodont genus Caryosyntrips.
As only a single row of endites can be observed, the pairing of endites cannot be ascertained in this new taxon. However, it is well-documented from other radiodonts known from many more frontal appendages, such as Anomalocaris canadensis, that the second series of endites often remains concealed within the sediment (Daley and Edgecombe 2014). Moreover, frontal appendages of hurdiids, the one family with unpaired endites, bear auxiliary spines on the distal margin of endites only, and typically have fewer than 14 podomeres. The presence of auxiliary spines on both distal and proximal faces of endites, and the high podomere count, allow a confident assignment of this new material to the family Tamisiocarididae.
The presence of fine auxiliary spines on both proximal and distal margins of endites is known in the two formally described tamisiocaridid taxa, “Anomalocaris” briggsi and Tamisiocaris borealis (Daley et al. 2013b; Vinther et al. 2014). However, the Guanshan specimen notably differs from both these taxa in the outline of its podomeres, which are square in lateral view, rather than tall and rectangular. We leave this incomplete appendage in open nomenclature as important features, such as the total number of podomeres or the presence of dorsal and terminal spines, cannot be determined. However, we recognize that it represents a new species and probably a new genus of tamisiocaridid radiodont. As such, this specimen extends the palaeogeographic range of the family to the South China palaeocontinent. Other tamisiocaridids are known from the Cambrian, Stage 3 of Laurentia (Tamisiocaris borealis, Buen Formation; Daley and Peel 2010; Vinther et al. 2014) and Stage 4 of Gondwana (“Anomalocaris” briggsi, Emu Bay Shale; Daley et al. 2013b) and Laurentia (Tamisiocaris aff. borealis, Kinzers Formation; Pates and Daley 2019).
Stratigraphic and geographic range.—Lihuazhuang section, Yiliang county, Yunnan, China. Wulongqing Formation (Palaeolenus Biozone), Cambrian (Stage 4).
Discussion
Endemism in Guanshan radiodonts: geographic and environmental considerations.—The new material from the Lihuazhuang section increases our knowledge of the diversity of these stem-group euarthropods in the Wulongqing Formation, the Cambrian of South China, and even to some extent the Cambrian Stage 4 worldwide. This study doubles the known diversity of radiodont species in the Guanshan Biota (2–4 or 3–5, if the new material illustrated in Zhao et al. 2020 represents a distinct species) and extends the known palaeogeographic range of the family Tamisiocarididae to South China. All four recognized radiodont families are now known from this palaeocontinent, as amplectobeluids, anomalocaridids, and hurdiids have all been previously reported from multiple BST deposits in the region (Chen et al. 1994; Hou et al. 1995; Liu 2013; Wang et al. 2013; Cong et al. 2014, 2016; Zeng et al. 2018b; Liu et al. 2020). Caryosyntrips, an atypical radiodont genus that may belong to its own family, remains solely known from Gondwana and Laurentia (Daley and Budd 2010; Pates and Daley 2017; Lerosey-Aubril et al. 2020; Pates et al. 2021).
The global distribution of radiodont families contrasts with the apparent endemism of the Guanshan radiodont fauna at the genus and species levels. For example, all four Guanshan radiodont species, and the genus Paranomalocaris Wang, Huang, and Hu, 2013, as a whole, are only known from the Wulongqing Formation. Indeed, if “Anomalocaris” kunmingensis Wang, Huang, and Hu, 2013, represents a new genus, then all four genera are endemic. The absence of any of the nine, possibly eleven radiodont species present in the Chengjiang Lagerstätte is striking. Several of these species have been reported in other, less prolific (i.e., Tier 3 of Gaines 2014) Cambrian BST deposits in South China (Table 1) and the genera Amplectobelua, Anomalocaris, Cambroraster, and Ramskoeldia, also occur in Cambrian Stage 3–Miaolingian BST deposits of North China (Sun et al. 2020a) and Laurentia (e.g., Daley and Budd 2010; Daley and Edgecombe 2014; Moysiuk and Caron 2019; Lerosey-Aubril et al. 2020; Pates et al. 2019b, 2021). This indicates that the marked compositional dissimilarity between the Chengjiang and Guanshan radiodont faunas cannot simply be explained by differences in the palaeogeographical distribution or stratigraphic age. We propose that the endemic nature of the Guanshan radiodonts is best explained by the different environment in which they lived. Both sedimentological and ichnological evidence support a more proximal, possibly more energetic depositional setting above storm wave-base for the Wulongqing Formation, although the reconstructed palaeoenvironment varies from anywhere between the shoreface and a tidal flat (Ding et al. 2018; Chen et al. 2019; see also Chen et al. 2020a for possible variations from a section to another). This contrasts with the quiet, relatively deep-water environment near the shelf break and a fluctuating oxycline proposed for most BST deposits (Gaines 2014). Considering that most radiodonts were capable swimmers (see Moysiuk and Caron 2019 and Liu et al. 2020 for a possible exception), it might be expected that their geographic range was less constrained by the spatial variation of environmental conditions on the seafloor than that of most benthic organisms. This assumption finds support in the intercontinental palaeogeographic distributions of many radiodont genera. However, the high endemicity of the Guanshan radiodont fauna at the genus level illustrates that these animals could also be tightly connected to the benthic environments, and the prey animals that lived in them. In this regard, suspension feeding radiodonts (Vinther et al. 2014; Lerosey-Aubril and Pates 2018; Paterson et al. 2020) and a few other metazoans (e.g., some ctenophores and cnidarians) likely represented rare exceptions of truly pelagic animals, at a time when macroscopic life is thought to have remained largely confined to the hyperbenthic realm 1–10 meters off the bottom of the oceans (Vannier 2009). The ecological coupling of radiodonts and benthic communities might have been more pronounced in proximal shelf environments, hence its clearer expression in the Guanshan Lagerstätte. The combination of this ecological coupling with challenges posed by life in physically (e.g., energetic regime, temperature, turbidity) and chemically (e.g., oxygenation) different environments may explain not only the endemism of the Guanshan radiodont fauna, but also contribute to the apparently complex palaeobiogeographic patterns exhibited by the members of this group, even at regional or intracontinental scales (Pates et al. 2021).
Table 1. Taxonomic diversity of the radiodont faunas from the Cambrian Konservat-Lagerstätten of South China. Radiodonts have also been reported from the Qingjiang Biota, South China (Shuijingtuo Formation, Stage 3), but specimens are yet to be formally described (Fu et al. 2019). An isolated hurdiid lateral carapace element has also been reported from this formation (Daley et al. 2013a). * The radiodont material from the Kaili Formation is in need of revision; the specimens illustrated by Zhao et al. (2005, 2011) might be better regarded as belonging to an amplectobeluid, possibly the Amplectobelua. ** Cucumericrus and Zhenghecaris may not represent true radiodonts.
|
Stage |
Stage 3 |
Stage 4 |
Wuliuan |
Furongian |
|||
|
Lagerstätte |
Chengjiang |
Malong |
Zunyi |
Balang |
Guanshan |
Kaili |
Guole |
|
Amplectobelua |
Amplectobelua
symbrachiata |
– |
Amplectobelua symbrachiata (Steiner et al. 2005) |
– |
– |
– |
– |
|
Anomalocaris |
Anomalocaris
saron |
Anomalocaris saron (Zhang et al. 2001; Luo et al. 2008) |
– |
Anomalocaris sp. (Liu 2013) |
– |
Anomalocaris saron* (Zhao et al. 2005; Zhao et al. 2011) |
– |
|
“Anomalocaris” Amplectobeluidae |
– |
– |
– |
– |
“Anomalocaris”
|
– |
– |
|
Cambroraster |
Cambroraster
|
– |
– |
– |
– |
– |
– |
|
Lenisicaris |
Lenisicaris lupata (Wu et al. 2021) |
– |
– |
– |
– |
– |
– |
|
Lyrarapax |
Lyrarapax trilobus (Cong et al. 2016), Lyrarapax unguispinus (Cong et al. 2014) |
– |
– |
– |
– |
– |
– |
|
Peytoia |
– |
– |
– |
cf. Peytoia nathorsti (Liu, 2013) |
– |
– |
– |
|
Paranomalocaris |
– |
– |
– |
– |
P. multisegmentalis (Wang et al. 2013), P. simplex (this study) |
– |
– |
|
Ramskoeldia |
Ramskoeldia consimilis, Ramskoeldia platyacantha (Cong et al. 2018) |
– |
– |
– |
– |
– |
– |
|
Anomalocarididae |
– |
– |
– |
– |
Anomalocarididae |
– |
– |
|
Hurdiidae |
– |
– |
– |
– |
– |
– |
Hurdiidae indet. (unpublished data) |
|
Tamisiocarididae |
– |
– |
– |
– |
Tamisiocarididae indet. (this study) |
– |
– |
|
Cucumericrus** |
Cucumericrus
decorates |
– |
– |
– |
– |
– |
– |
|
Zhenghecaris** |
Zhenghecaris shankouensis (Zeng et al. 2018) |
– |
– |
– |
– |
– |
– |
Diversity of feeding strategies in radiodont assemblages.—Co-occurring radiodont taxa display adaptations in their frontal appendages and other feeding structures (e.g., gnathobase-like structures and oral cones) that suggest different ecological specializations (Daley and Budd 2010; Cong et al. 2017, 2018). Taxa with prominent triangular membrane between podomeres, relatively short endites with robust auxiliary spines, and prominent dorsal spines in the distal region, are generally considered raptorial predators (e.g., Anomalocaris canadensis, Lyrarapax unguispinus; Daley and Edgecombe 2014; Cong et al. 2014; Liu et al. 2018), while those with more elongate endites tend to be interpreted as sweep feeders (e.g., Hurdia victoria; Daley et al. 2013a) or sediment sifters (e.g., Cambroraster falcatus; Moysiuk and Caron 2019). A small number of radiodonts with microscopic distances between auxiliary spines or setae on their endites were most likely suspension feeders (e.g., Pahvantia hastata, Tamisiocaris borealis, and probably “Anomalocaris” briggsi; Vinther et al. 2014; Lerosey-Aubril and Pates 2018).
Two of the four radiodonts in the Guanshan Biota appear suited for raptorial predation, a hypothesis supported by the relatively short and spinose endites of Paranomalocaris multisegmentalis and “Anomalocaris” kunmingensis. The distinct form of their endites, broad, stubby, and spinose in the former, trident-like in the latter, suggests a specialization for different prey types. The other two Guanshan radiodonts appear more suited for sweep feeding, the elongate and slender endites of Paranomalocaris simplex sp. nov. and the new tamisiocaridid taxon. Parallels can be drawn between the inferred feeding ecologies of components of diverse radiodont assemblages. This is exemplified by comparison to other Cambrian Stage 4 deposits with their different taxonomic makeup (Tables 1, 2). Two taxa in the Kinzers Formation, Amplectobelua aff. symbrachiata and Anomalocaris pennsylvanica , show adaptations for raptorial predation, while Laminacaris? sp. and Tamisiocaris aff. borealis both display long endites more indicative of sweep feeding (Pates and Daley 2019). Again, in the Emu Bay Shale, Anomalocaris cf. canadensis shows adaptations for raptorial predation, and “Anomalocaris” briggsi to suspension feeding (Daley et al. 2013b), and in the Pioche Formation the raptorial predator Anomalocaris magnabasis and likely sweep-feeder Hurdia sp. co-occur (Pates et al. 2019b). Closer geographically to the Guanshan Biota, the two radiodont taxa from the Balang Formation, one species of Anomalocaris and Peytoia, both left in open nomenclature, also appear specialized for distinct feeding ecologies (Liu 2013). A similarly important diversity of feeding ecologies has been inferred for younger radiodont assemblages, such as those of the Burgess Shale and Marjum Lagerstätten (Daley and Budd 2010; Pates et al. 2021). More subtle differences in the endite morphology within radiodont genera, for example, Anomalocaris, could indicate specializations of individual species to different types of local prey (Pates et al. 2019b). The numerous morphological differences exhibited by radiodont feeding appendages, even in taxa from a given locality, evidences the great ability of these organisms to adapt to new food sources in each area they colonized, and further illustrates their strong connection to local, mostly benthic faunas.
Table 2. Taxonomic diversity of radiodont faunas from Cambrian: Stage 4 Konservat-Lagerstätten. Radiodont material has also been recovered from the Cambrian: Stage 4 Poleta Formation (Laurentia), but these specimens remain unidentified beyond the family level (English and Babcock 2010). *Anomalocaris magnabasis is known from both the Comet Shale Member and Combined Metals Member of the Pioche Formation, Hurdia sp. is only known from the Combined Metals Member. ** note that we do not follow Wu et al.’s (2021) reassignment of this species to their new genus Lenisicaris.
|
Palaeocontinents |
Gondwana |
Laurentia |
South China |
||||||
|
Lagerstätte |
Emu Bay Shale |
Valdemiedes Formation |
Carrara Formation, Pyramid Shale Member |
Eager |
Kinzers |
Latham Shale |
Pioche |
Guanshan |
Balang |
|
Amplectobelua |
– |
– |
– |
– |
aff.
Amplectobelua symbrachiata (Pates and |
– |
– |
– |
– |
|
Anomalocaris |
cf. Anomalocaris canadensis (Daley et al. 2013b) |
– |
Anomalocaris magnabasis? (Pates et al. 2019b) |
Anomalocaris
canadensis (Briggs 1979; Daley
and |
Anomalocaris pennsylvanica (Briggs 1979; Pates and Daley 2019)** |
– |
Anomalocaris magnabasis (Pates et al. 2019b) |
– |
Anomalocaris sp. (Liu 2013) |
|
“Anomalocaris” Amplectobeluidae |
– |
– |
– |
– |
– |
– |
– |
“A.” kunmingensis (Wang et al. 2013; Zeng et al. 2018; this study) |
– |
|
“Anomalocaris” Tamisiocarididae |
“Anomalocaris” briggsi (Daley et al. 2013b) |
– |
– |
– |
– |
– |
– |
– |
– |
|
Caryosyntrips |
– |
cf. Caryosyntrips camurus (Pates and Daley 2017) |
– |
– |
– |
– |
– |
– |
– |
|
Hurdia |
– |
– |
– |
– |
– |
– |
Hurdia sp. (Pates et al. 2019b) |
– |
|
|
Laminacaris? |
– |
– |
– |
– |
Laminacaris? sp. (Pates and Daley 2019) |
– |
– |
– |
– |
|
Lenisicaris |
|
|
|
|
Lenisicaris pennsylvanica (Briggs 1979; Pates and Daley 2019; Wu et al. 2021) |
|
|
|
|
|
Paranomalocaris |
– |
– |
– |
– |
– |
– |
– |
Paranomalocaris
multisegmentalis (Wang et al. 2013),
|
– |
|
Peytoia |
– |
– |
– |
– |
– |
– |
– |
|
cf. Peytoia nathorsti (Liu 2013) |
|
Ramskoeldia |
– |
– |
– |
– |
– |
Ramskoeldia consimilis? (Pates et al. 2019b) |
– |
– |
– |
|
Tamisiocaris |
– |
– |
– |
– |
Tamisiocaris sp. (Pates and Daley 2019) |
– |
– |
– |
– |
|
Tamisiocarididae |
– |
– |
– |
– |
– |
– |
– |
Tamisiocarididae indet. (this study) |
– |
Conclusions
Recent collecting efforts and redescriptions placing accessioned material in a modern systematic context have revealed that radiodonts in the Cambrian Stage 4 were more diverse than previously appreciated. In this study we report two new radiodont taxa, doubling the known diversity of this group from the Guanshan Biota. The new radiodont data includes the first tamisiocaridid from South China, a second species of Paranomalocaris, and confirms the presence of a tetraradial oral cone in “Anomalocaris” kunmingensis. All four radiodont taxa are only known from the Guanshan Biota, which further illustrates the differences between its taxonomic composition compared to the Chengjiang Biota, and other Cambrian exceptionally preserved faunas of South China. This unique taxonomic composition is likely a consequence of the Guanshan radiodonts inhabiting a more proximal, shallow-water environment compared to other radiodonts. This in turn implies a strong coupling between the free-swimming radiodont fauna, and the local benthic communities. The importance of these trophic relationships is further illustrated by the numerous morphological adaptations that radiodonts evolved in response to access to different prey locally. In the Guanshan Biota, this local evolution is particularly well exemplified by the endemic genus Paranomalocaris, which is represented by two species specialized for different feeding modes. The endemism of Guanshan radiodonts underscores the importance of exploring as many deposits as possible to gain as complete an understanding of Cambrian total-group euarthropod biodiversity as possible.
Authors’ contributions
JY, TL, and XGZ designed the project. DGJ and XGZ photographed material. DGJ, XGZ, and SP prepared figures with input from RL-A and JO-H. DGJ, JY, TL, and XGZ performed fieldwork, collected and prepared the material. SP and RL-A interpreted the data, and wrote the manuscript with input of all co-authors. All authors performed research, discussed, and approved the final manuscript.
Acknowledgements
We thank Peter Van Roy (Ghent University, Belgium) and an anonymous referee for their constructive reviews. This study was supported by the National Natural Science Foundation of China (41730318). SP acknowledges funding through an Alexander Agassiz Postdoctoral Fellowship awarded by the Museum of Comparative Zoology, Harvard University.
References
Briggs, D.E.G. 1979. Anomalocaris, the largest known Cambrian arthropod. Palaeontology 22: 631–664.
Caron, J.B., Gaines, R.R., Mángano, M.G., Streng, M., and Daley, A.C. 2010. A new Burgess Shale–type assemblage from the “thin” Stephen Formation of the southern Canadian Rockies. Geology 38: 811–814. Crossref
Chen, F., Brock, G.A., Zhang, Z., Laing, B., Ren, X., and Zhang, Z. 2020a. Brachiopod-dominated communities and depositional environment of the Guanshan Konservat-Lagerstätte, eastern Yunnan, China. Journal of the Geological Society 178: jsg2020-043. Crossref
Chen, F., Zhang, Z., Betts, M.J., Zhang, Z., and Liu, F. 2019. First report on Guanshan Biota (Cambrian Stage 4) at the stratotype area of Wulongqing Formation in Malong County, Eastern Yunnan, China. Geoscience Frontiers 10: 1459–1476. Crossref
Chen, H., Legg, D.A., Zhai, D.Y., Liu, Y., and Hou, X.G. 2020b. New data on the anatomy of fuxianhuiid arthropod Guangweicaris spinatus from the lower Cambrian Guanshan Biota, Yunnan, China. Acta Palaeontologica Polonica 65: 139–148. Crossref
Chen, J.Y., Ramsköld, L., and Zhou, G.Q. 1994. Evidence for monophyly and arthropod affinity of Cambrian giant predators. Science 264: 1304–1308. Crossref
Collins, D. 1996. The “evolution” of Anomalocaris and its classification in the arthropod class Dinocarida (nov.) and order Radiodonta (nov.). Journal of Paleontology 70: 280–293. Crossref
Cong, P., Daley, A.C., Edgecombe, G.D., and Hou, X. 2017. The functional head of the Cambrian radiodontan (stem-group Euarthropoda) Amplectobelua symbrachiata. BMC Evolutionary Biology 17: 1–23. Crossref
Cong, P., Daley, A.C., Edgecombe, G.D., Hou, X., and Chen, A. 2016. Morphology of the radiodontan Lyrarapax from the early Cambrian Chengjiang biota. Journal of Paleontology 90: 663–671. Crossref
Cong, P.Y., Edgecombe, G.D., Daley, A.C., Guo, J., Pates, S., and Hou, X.G. 2018. New radiodonts with gnathobase‐like structures from the Cambrian Chengjiang Biota and implications for the systematics of Radiodonta. Papers in Palaeontology 4: 605–621. Crossref
Cong, P., Ma, X., Hou, X., Edgecombe, G.D., and Strausfeld, N.J. 2014. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513: 538–542. Crossref
Daley, A.C. and Budd, G.E. 2010. New anomalocaridid appendages from the Burgess Shale, Canada. Palaeontology 53: 721–738. Crossref
Daley, A.C. and Edgecombe, G.D. 2014. Morphology of Anomalocaris canadensis from the Burgess Shale. Journal of Paleontology 88: 68–91. Crossref
Daley, A.C. and Peel, J.S. 2010. A possible anomalocaridid from the Cambrian Sirius Passet Lagerstatte, north Greenland. Journal of Paleontology 84: 352–355. Crossref
Daley, A.C., Budd, G.E. and Caron, J.B. 2013a. Morphology and systematics of the anomalocaridid arthropod Hurdia from the Middle Cambrian of British Columbia and Utah. Journal of Systematic Palaeontology 11: 743–787. Crossref
Daley, A.C., Budd, G.E., Caron, J.B., Edgecombe, G.D., and Collins, D. 2009. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323: 1597–1600. Crossref
Daley, A.C., Paterson, J.R., Edgecombe, G.D., García‐Bellido, D.C., and Jago, J.B. 2013b. New anatomical information on Anomalocaris from the Cambrian Emu Bay Shale of South Australia and a reassessment of its inferred predatory habits. Palaeontology 56: 971–990. Crossref
Ding, Y., Liu, J., and Chen, F. 2018. Ichnology, palaeoenvironment, and ecosystem dynamics of the Early Cambrian (Stage 4, Series 2) Guanshan Biota, South China. Geological Journal 55: 77–94. Crossref
Ding, Y., Liu, J., and Chen, F. 2020. Ichnology, palaeoenvironment, and ecosystem dynamics of the Early Cambrian (Stage 4, Series 2) Guanshan Biota, South China. Geological Journal 55: 77–94. Crossref
Du, K.S., Ortega-Hernández, J., Yang, J., Yang, X.Y., Guo, Q.H., Li, W., He, J.F., Li, K.R., Du, J.L., Hou, J.B., and Zhang, X.G. 2020. A new early Cambrian Konservat-Lagerstätte expands the occurrence of Burges Shale-type deposits on the Yangtze Platform. Earth Science Reviews 211: 103409. Crossref
English, A.M. and Babcock, L.E. 2010. Census of the Indian Springs Lagerstätte, Poleta Formation (Cambrian), western Nevada, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 295: 236–244. Crossref
Forchielli, A., Steiner, M., Kasbohm, J., Hu, S. and Keupp, H. 2014. Taphonomic traits of clay-hosted early Cambrian Burgess Shale-type fossil Lagerstätten in South China. Palaeogeography, Palaeoclimatology, Palaeoecology 398: 59–85. Crossref
Fu, D., Tong, G., Dai, T., Liu, W., Yang, Y., Zhang, Y., Cui, L., Li, L., Yun, H., Wu, Y., and Sun, A. 2019. The Qingjiang biota—a Burgess Shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363: 1338–1342. Crossref
Gaines, R.R. 2014. Burgess Shale-type preservation and its distribution in space and time. The Paleontological Society Papers 20: 123–146. Crossref
Guo, J., Pates, S., Cong, P., Daley, A.C., Edgecombe, G.D., Chen, T., and Hou, X. 2019. A new radiodont (stem Euarthropoda) frontal appendage with a mosaic of characters from the Cambrian (Series 2 Stage 3) Chengjiang biota. Papers in Palaeontology 5: 99–110. Crossref
Holmes, J.D., García-Bellido, D.C., and Lee, M.S. 2018. Comparisons between Cambrian Lagerstätten assemblages using multivariate, parsimony and Bayesian methods. Gondwana Research 55: 30–41. Crossref
Hopkins, M.J., Chen, F., Hu, S., and Zhang, Z. 2017. The oldest known digestive system consisting of both paired digestive glands and a crop from exceptionally preserved trilobites of the Guanshan Biota (Early Cambrian, China). PLoS ONE 12: e0184982. Crossref
Hou, X.G., Bergström, J., and Ahlberg, P. 1995. Anomalocaris and other large animals in the Lower Cambrian Chengjiang fauna of southwest China. GFF 117: 163–183. Crossref
Hou X.G., David J.S., Siveter, D.J., Aldridge, R.J., Cong P., Gabbott, S.E., Ma X., Purnell, M.A., and Williams, M. 2017. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal life. 2nd edition. John Wiley & Sons, Hoboken. Crossref
Hu, S., Zhang, Z., Holmer, L.E., and Skovsted, C.B. 2010a. Soft-part preservation in a linguliform brachiopod from the lower Cambrian Wulongqing Formation (Guanshan fauna) of Yunnan, South China. Acta Palaeontologica Polonica 55: 495–505. Crossref
Hu, S.X., Steiner, M., Zhu, M.Y., Luo, H.L., Forchielli, A., Keupp, H., Zhao, F.C., and Liu, Q. 2012. A new priapulid assemblage from the early Cambrian Guanshan fossil Lagerstätte of SW China. Bulletin of Geosciences 87: 93–106.
Hu, S.X., Zhu, M.Y., Luo, H.L., Steiner, M., Zhao, F.C., Li, G.X., Liu, Q., and Zhang, Z.F. 2013. The Guanshan Biota [in Chinese with English summary]. 204 pp. Yunnan Science and Technology Press, Kunming.
Hu, S.X., Zhu, M.Y., Steiner, M., Luo, H.L., Zhao, F.C., and Liu, Q. 2010b. Biodiversity and taphonomy of the Early Cambrian Guanshan Biota, eastern Yunnan. Science China Earth Sciences 53: 1765–1773. Crossref
Huang, D.Y. and Wang, Y.N. 2014. The soft anatomy of Isoxys minor from the Guanshan fauna, lower Cambrian of Southwest China. Palaeoworld 23: 225–228. Crossref
Ivantsov, A.Y., Zhuravlev, A.Y., Leguta, A.V., Krassilov, V.A., Melnikova, L.M., and Ushatinskaya, G.T. 2005. Palaeoecology of the early Cambrian Sinsk biota from the Siberian platform. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 69–88. Crossref
Jiao, D.G., Yang, J., and Zhang, X.G. 2016. A superarmoured lobopodian from the Cambrian Stage 4 of southern China. Science Bulletin 61: 1372–1376. Crossref
Lerosey-Aubril, R. and Pates, S. 2018. New suspension-feeding radiodont suggests evolution of microplanktivory in Cambrian macronekton. Nature Communications 9: 1–9. Crossref
Lerosey-Aubril, R., Kimmig, J., Pates, S., Skabelund, J., Weug, A., and Ortega‐Hernández, J. 2020. New exceptionally preserved panarthropods from the Drumian Wheeler Konservat‐Lagerstätte of the House Range of Utah. Papers in Palaeontology 6: 501–531. Crossref
Li, J., Liu, J., and Ou, Q. 2017. New observations on Vetulicola longbaoshanensis from the lower Cambrian Guanshan Biota (series 2, stage 4), South China. Science China Earth Sciences 60: 1795–1804. Crossref
Li, Y.J., Cong, P.Y., Zhao, J., and Hou, X.G. 2015. New observations on morphological variation of genus Vetulicola with quadrate carapace from the Cambrian Chengjiang and Guanshan biotas, South China. Palaeoworld 24: 36–45. Crossref
Liu, J., Han, J., Li, J., Wu, Y., Peng, J., Qi, N., Yang, Y., and Li, J. 2016. New localities and palaeoscolecid worms from the Cambrian (Stage 4, Series 2) Guanshan Biota in Kunming, Yunnan, South China. Acta GeologicaSinica‐English Edition 90: 1939–1945. Crossref
Liu, J., Lerosey-Aubril, R., Steiner, M., Dunlop, J.A., Shu, D., and Paterson, J.R. 2018. Origin of raptorial feeding in juvenile euarthropods revealed by a Cambrian radiodontan. National Science Review 5: 863–869. Crossref
Liu, J.N., Ou, Q., Han, J., Zhang, Z.F., He, T.J., Yao, X.Y., Fu, D.J., and Shu, D.G. 2012. New occurrence of the Cambrian (Stage 4, Series 2) Guanshan Biota in Huize, Yunnan, South China. Bulletin of Geosciences 87: 125–132. Crossref
Liu, Q. 2013. The first discovery of anomalocaridid appendages from the Balang Formation (Cambrian Series 2) in Hunan, China. Alcheringa: An Australasian Journal of Palaeontology 37: 338–343. Crossref
Liu, Y., Lerosey-Aubril, R., Audo, D., Zhai, D., Mai, H., and Ortega-Hernández, J. 2020. Occurrence of the eudemersal radiodont Cambroraster in the early Cambrian Chengjiang Lagerstätte and the diversity of hurdiid ecomorphotypes. Geological Magazine 157: 1200–1206. Crossref
Luo, H., Fu, X., Hu, S., Li, Y., Hou, S., You, T., Pang, J., and Liu, Q. 2007. A new arthropod, Guangweicaris Luo, Fu et Hu gen. nov. from the Early Cambrian Guanshan fauna, Kunming, China. Acta Geologica Sinica 81: 1–7.
Luo, H.L., Li, Y., Hu, S.X., Fu, X.P., Hou, S.G., Liu, X.Y., Chen, L.Z., Li, F.J., Pang, J.Y., and Liu, Q. 2008. Early Cambrian Malong Fauna and Guanshan Fauna from Eastern Yunnan, China. 134 pp. Yunnan Science and Technology Press, Kunming.
Moysiuk, J. and Caron, J.B. 2019. A new hurdiid radiodont from the Burgess Shale evinces the exploitation of Cambrian infaunal food sources. Proceedings of the Royal Society B 286: 20191079. Crossref
Muscente, A.D., Schiffbauer, J.D., Broce, J., Laflamme, M., O’Donnell, K., Boag, T.H., Meyer, M., Hawkins, A.D., Huntley, J.W., McNamara, M., and MacKenzie, L.A. 2017. Exceptionally preserved fossil assemblages through geologic time and space. Gondwana Research 48: 164–188. Crossref
Nedin, C. 1995. The Emu Bay Shale, a Lower Cambrian fossil Lagerstatten, Kangaroo Island, South Australia. Memoirs of the Association of Australasian Palaeontologists 18: 31–40.
Nielsen, C. 1995. Animal Evolution: Interrelationships of the Living Phyla. 467 pp. Oxford University Press, Oxford.
Ortega‐Hernández, J. 2016. Making sense of “lower” and “upper” stem‐group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biological Reviews 91: 255–273. Crossref
Ortega-Hernández, J., Janssen, R., and Budd, G.E. 2017. Origin and evolution of the panarthropod head–a palaeobiological and developmental perspective. Arthropod Structure & Development 46: 354–379. Crossref
Paterson, J.R., Edgecombe, G.D., and García-Bellido, D.C. 2020. Disparate compound eyes of Cambrian radiodonts reveal their developmental growth mode and diverse visual ecology. Science Advances 6: eabc6721. Crossref
Paterson, J.R., Edgecombe, G.D., and Jago, J.B. 2015. The “great appendage” arthropod Tanglangia: biogeographic connections between early Cambrian biotas of Australia and South China. Gondwana Research 27: 1667–1672. Crossref
Paterson, J.R., García-Bellido, D.C., Jago, J.B., Gehling, J.G., Lee, M.S., and Edgecombe, G.D. 2016. The Emu Bay Shale Konservat-Lagerstätte: a view of Cambrian life from East Gondwana. Journal of the Geological Society 173: 1–11. Crossref
Paterson, J.R., García-Bellido, D.C., Lee, M.S., Brock, G.A., Jago, J.B., and Edgecombe, G.D. 2011. Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480: 237–240. Crossref
Pates, S. and Daley, A.C. 2017. Caryosyntrips: a radiodontan from the Cambrian of Spain, USA and Canada. Papers in Palaeontology 3: 461–470. Crossref
Pates, S. and Daley, A.C. 2019. The Kinzers Formation (Pennsylvania, USA): the most diverse assemblage of Cambrian Stage 4 radiodonts. Geological Magazine 156: 1233–1246. Crossref
Pates, S., Daley, A.C., and Butterfield, N.J. 2019a. First report of paired ventral endites in a hurdiid radiodont. Zoological Letters 5: 1–11. Crossref
Pates, S., Daley, A.C., and Lieberman, B.S. 2018. Hurdiid radiodontans from the middle Cambrian (Series 3) of Utah. Journal of Paleontology 92: 99–113. Crossref
Pates, S., Daley, A.C., Edgecombe, G.D., Cong, P., and Lieberman, B.S. 2019b. Systematics, preservation and biogeography of radiodonts from the southern Great Basin, USA, during the upper Dyeran (Cambrian Series 2, Stage 4). Papers in Palaeontology 7 (1): 235–262; paper version published in 2021. Crossref
Pates, S., Lerosey-Aubril, R., Daley, A.C., Kier, C., Bonino, E., and OrtegaHernández, J. 2021. The diverse radiodont fauna from the Marjum Formation of Utah, USA (Cambrian: Drumian. PeerJ 9: e10509. Crossref
Peng, J., Zhao, Y.L., Wu, Y., Yuan, J., and Tai, T. 2005. The Balang Fauna—a new early Cambrian Fauna from Kaili City, Guizhou Province. Chinese Science Bulletin 50: 1159–1162. Crossref
Raymond, P.E. 1935. Leanchoilia and other mid-Cambrian Arthropoda. Bulletin of the Museum of Comparative Zoology at Harvard College 76: 205–230.
Rueden, C.T., Schindelin, J., Hiner, M.C., DeZonia, B.E., Walter, A.E., Arena, E.T., and Eliceiri, K.W., 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529. Crossref
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. Crossref
Skinner, E.S. 2005. Taphonomy and depositional circumstances of exceptionally preserved fossils from the Kinzers Formation (Cambrian), southeastern Pennsylvania. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 167–192. Crossref
Steiner, M., Zhu, M., Zhao, Y., and Erdtmann, B.D. 2005. Lower Cambrian Burgess Shale-type fossil associations of south China. Palaeogeography, Palaeoclimatology, Palaeoecology 220: 129–152. Crossref
Sun, Z., Zeng, H., and Zhao, F. 2020a. A new middle Cambrian radiodont from North China: Implications for morphological disparity and spatial distribution of hurdiids. Palaeogeography, Palaeoclimatology, Palaeoecology 558: 109947. Crossref
Van Roy, P., Daley, A.C., and Briggs, D.E.G. 2015. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522: 77–80. Crossref
Vannier, J. 2009. L’Explosion cambrienne ou l’émergence des écosystemes modernes. Comptes Rendus Palevol 8: 133–154. Crossref
Vinther, J., Stein, M., Longrich, N.R., and Harper, D.A. 2014. A suspension-feeding anomalocarid from the Early Cambrian. Nature 507: 496–499. Crossref
Wang, Y., Huang, D., and Hu, S. 2013. New anomalocardid frontal appendages from the Guanshan Biota, eastern Yunnan. Chinese Science Bulletin 58: 3937–3942. Crossref
Whiteaves, J.F. 1892. Description of a new genus and species of phyllocarid Crustacea from the middle Cambrian of Mount Stephen, B.C. Canadian Record of Science 5: 205–208.
Whittington, H.B. and Briggs, D.E.G. 1985. The largest Cambrian animal, Anomalocaris, Burgess Shale, British Columbia. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 309: 569–609. Crossref
Wu, Y. and Liu, J. 2019. Anatomy and relationships of the fuxianhuiid euarthropod Guangweicaris from the early Cambrian Guanshan Biota in Kunming, Yunnan, Southwest China revisited. Acta Palaeontologica Polonica 64: 543–548. Crossref
Wu, Y., Ma, J., Lin, W., Sun, A., Zhang, X., and Fu, D. 2021. New anomalocaridids (Panarthropoda: Radiodonta) from the lower Cambrian Chengjiang Lagerstätte: Biostratigraphic and paleobiogeographic implications. Palaeogeography, Palaeoclimatology, Palaeoecology 569: 110333. Crossref
Zeng, H., Zhao, F., Niu, K., Zhu, M., and Huang, D. 2020. An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588: 101–105. Crossref
Zeng, H., Zhao, F., Yin, Z., and Zhu, M. 2018a. A new radiodontan oral cone with a unique combination of anatomical features from the early Cambrian Guanshan Lagerstätte, eastern Yunnan, South China. Journal of Paleontology 92: 40–48. Crossref
Zeng, H., Zhao, F., Yin, Z., and Zhu, M. 2018b. Morphology of diverse radiodontan head sclerites from the early Cambrian Chengjiang Lagerstätte, south-west China. Journal of Systematic Palaeontology 16: 1–37. Crossref
Zhang, X. and Hua, H. 2005. Soft-bodied fossils from the Shipai Formation, lower Cambrian of the Three Gorge area, South China. Geological Magazine 142: 699–709. Crossref
Zhang, X., Liu, W., and Zhao, Y. 2008. Cambrian Burgess Shale-type Lagerstätten in south China: distribution and significance. Gondwana Research 14: 255–262. Crossref
Zhang, X., Shu, D., Li, Y., and Han, J. 2001. New sites of Chengjiang fossils: crucial windows on the Cambrian explosion. Journal of the Geological Society 158: 211–218. Crossref
Zhang, Z., Holmer, L.E., Robson, S.P., Hu, S., Wang, X., and Wang, H. 2011. First record of repaired durophagous shell damages in Early Cambrian lingulate brachiopods with preserved pedicles. Palaeogeography, Palaeoclimatology, Palaeoecology 302: 206–212. Crossref
Zhao, J., Li, Y., Selden, P.A., and Cong, P. 2020. New occurrence of the Guanshan Lagerstätte (Cambrian Series 2, Stage 4) in the Kunming area, Yunnan, southwest China, with records of new taxa. Alcheringa 44: 343–355. Crossref
Zhao, Y., Zhu, M., Babcock, L.E., Yuan, J., Parsley, R.L., Peng, J., Yang, X., and Wang, Y. 2005. Kaili Biota: A taphonomic window on diversification of metazoans from the basal Middle Cambrian: Guizhou, China. Acta Geologica Sinica 79: 751–765. Crossref
Zhao, Y.L., Zhu, M.Y., Babcock, L.E., and Peng, J. 2011. The Kaili Biota: Marine Organisms from 508 Million Years Ago. 249 pp. Guizhou Science and Technology Press, Guiyang.
Acta Palaeontol. Pol. 66 (2): 255–274, 2021
https://doi.org/10.4202/app.00870.2020