

A new late Miocene elasmotheriine rhinoceros from Morocco
DENIS GERAADS and SAMIR ZOUHRI
Geraads, D. and Zouhri, S. 2021. A new late Miocene elasmotheriine rhinoceros from Morocco. Acta Palaeontologica Polonica 66 (4): 753–765.
We describe here the first definite representative of the subfamily Elasmotheriinae in North Africa. It comes from the upper Miocene site of Skoura near Ouarzazate, on the southern slope of the Central High Atlas in Morocco. It consists of a virtually complete skull with articulated mandible and a few fragmentary postcranial remains, making it by far the best known elasmotheriine from the African late Miocene. We assign it to a new taxon, Eoazara xerrii gen. et sp. nov. The skull is characterized by long nasal bones indicating a strong horn and long, anteriorly expanded, edentulous premaxillae. Compared to other Rhinocerotidae, the face is moderately elongated; the lower incisors are of medium size; and the premolar row is short. The upper molars have a strongly pinched protocone, a long antecrochet, and an unexpanded central valley. Eoazara xerrii gen. et sp. nov. is at a lower evolutionary grade than the Chinese species of Ningxiatherium and Parelasmotherium, but probably comparable to the very incomplete remains from the East African late Miocene forms. We regard Eoazara as a member of a chiefly Eurasian clade, rather than as a survivor of a hypothetical African elasmotheriine branch. Parsimony analysis confirms the monophyly of the Elasmotheriinae, but that of the remaining Rhinocerotidae is questionable.
Key words: Mammalia, Rhinocerotidae, Elasmotheriinae, Miocene, Morocco.
Denis Geraads [denis.geraads@mnhn.fr, ORCID ID: https://orcid.org/0000-0003-2475-8011], Centre de Recherche en Paléontologie- Paris, Muséum National d’Histoire Naturelle-CNRS-Sorbonne Université, CP 38, 8 rue Buffon, 75231 Paris Cedex 05, France.
Samir Zouhri [samirzouhri@yahoo.fr, ORCID ID: https://orcid.org/0000-0002-3811-9977], Department of Geology, Aïn Chock Faculty of Sciences, Hassan II University of Casablanca, Km 8, route d’El Jadida, BP 5366 Maârif, 20100 Casablanca, Morocco.
Received 10 May 2021, accepted 26 June 2021, available online 19 October 2021.
Copyright © 2021 D. Geraads and S. Zouhri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The five living species of Rhinocerotidae are the highly endangered last survivors of a successful group, of which about 50 genera are currently recognized in the Neogene of the Old World. Even if this number is probably exaggerated (as many genera are based upon very incomplete remains), they were clearly among the most diverse families of large mammals. Following recent discoveries and numerous phylogenetic analyses, the view that this Old World Neogene record can be split in two distinct clades, Rhinocerotinae and Elasmotheriinae, is generally favored (Antoine 1997, 2002, 2003; Antoine and Welcomme 2000; Antoine et al. 2002, 2003; Deng 2005, 2007, 2008; Geraads et al. 2012b, 2016). Early and middle Miocene forms may not always be easy to assign to either subfamily but, by the late Miocene, the Elasmotheriinae have acquired enough derived features to be easily diagnosed to subfamily. We describe here one such member, the first representative of this group in North Africa.
Zouhri et al. (2012) provided a short description of a late Miocene mammal fauna collected north of the road between Skoura and Tizi N’Tadderht, about 50 km ENE of Ouarzazate, Morocco (around 6.50° W, 31.1 to 31.2° N). Remains of Rhinocerotidae were not numerous, but included two genera, cf. Ceratotherium sp., based upon a mandible, some teeth and post-cranials, and aff. Chilotherium sp., based upon an upper cheek tooth that they regarded as a P4, but which is in fact a M1. In 2013, the present authors discovered a complete cranium of a derived species of the proboscidean Tetralophodon (Geraads et al. 2019), and re-identified the upper rhinoceros tooth as Elasmotheriinae gen. et sp. indet. Some other complete vertebrate skulls, such as the holotype of the caprin bovid Skouraia helicoides (Geraads et al. 2012a), were discovered by fossil hunters. The rhino skull described below was purchased from one of them by Serge Xerri in Rabat, who generously presented it to the Aïn Chock Faculty of Sciences, Casablanca. It was skillfully prepared by Philippe Richir and colleagues at the Centre de Recherche en Paléontologie-Paris, Muséum National d’Histoire Naturelle, before its return to the Aïn Chock Faculty of Sciences.
The geological context of the Skoura area, together with the updated faunal list, have recently been summarized by Geraads et al. (2019), while Benvenuti et al. (2020) provided a detailed description of the local stratigraphy. The latter authors assigned the alluvial fossiliferous deposits (sandstone and especially conglomerate) to the AK3 sub-unit of the Aït Kandoula Formation, but their correlations are biased by the belief that Zouhri et al. (2012) had assigned the fauna to the MN (Mammalian Neogene) 13 zone, which is incorrect. A few kilometers to the west, a micromammal assemblage should probably be referred to the early Turolian (Tesón et al. 2010), but cannot positively be correlated with the Skoura deposits. Given the depositional context, it is likely that sedimentation was rapid and that the whole sequence does not cover a long time-span, although all fossils may not be strictly contemporaneous.
The Skoura assemblage (Geraads et al. 2019; Cirilli et al. 2020) includes: Aves: cf. Struthio sp.; Reptilia: cf. Centrochelys sp., Crocodylus cf. niloticus; Mammalia, Carnivora: Felidae gen. et sp. indet.; Perissodactyla: Hippotherium sp., Eurygnathohippus cf. feibeli, aff. Cremohipparion periafricanum, cf. Ceratotherium sp.; Artiodactyla: Suidae gen. et sp. indet., Giraffidae gen. et sp. indet., cf. Prostrepsiceros sp., and Skouraia helicoides. The biochronological significance of this relatively short faunal list is limited; it was regarded as probably of Turolian-equivalent age by Zouhri et al. (2012); the resemblances of Skouraia with the middle Miocene Benicerus Heintz, 1973 speak against a latest Miocene age. Because African mammalian faunas of this age are rare, and because the best-preserved elements of the fauna bear no special resemblance to those of Europe, the age of the Skoura fauna cannot be precisely established, but we favor a relatively early age within the late Miocene, perhaps equivalent to European MN 10 or MN 11.
Institutional abbreviations.—FSC, Aïn Chock Faculty of Sciences, Hassan II University of Casablanca, Morocco; NHMUK, Natural History Museum, London, UK.
Other abbreviations.—Mc, metacarpus; Mt, metatarsus. We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:0BB38E8A-FADE-4424-B06E-E35DB998B0AD
Systematic paleontology
Class Mammalia Linnaeus, 1758
Order Perissodactyla Owen, 1848
Family Rhinocerotidae Gray, 1821
Subfamily Elasmotheriinae Bonaparte, 1845
Genus Eoazara nov.
ZooBank LSID: urn:lsid:zoobank.org:act:0E1A2850-B2AE-48DE-87 E1-7516735A7B8B
Type species: Eoazara xerrii sp. nov., monotypic, see below.
Etymology: From Greek eo, early and azara, Amazigh for rhinoceros.
Diagnosis.—As for the type species.
Eoazara xerrii sp. nov.
Figs. 1, 2.
ZooBank LSID: urn:lsid:zoobank.org:act:FC79A491-7E05-465B-AB FF-4C989ACE8672
Etymology: Named after Serge Xerri, who donated the holotype to the University of Casablanca.
Holotype: FSC-Sk-250, skull with associated mandible, partial humerus, lunar, and trapezoid.
Type locality: Deposits NE of Skoura in Ouarzazate Basin, Morocco (c. 6.50° W, 31.15° N).
Type horizon: Upper Miocene.
Material.—Holotype and FSC-Sk-33, isolated M1; FSC-Sk-45, Mc II; FSC-Sk-53, Mt II (Fig. 3). All from the type locality and horizon.
Diagnosis.—A large-size member of the Elasmotheriinae, comparable in size to the largest living rhinos, but more slenderly built. Cranium long, with concave dorsal profile; strong horn on moderately broad nasals lacking lateral processes and nasal septum; long, edentulous premaxillae slightly expanded rostrally; face of medium length, orbital rim prominent, anterior orbital border above M2; zygomatic arch slender, elevated caudally, no ventral postorbital process; paroccipital and posttympanic processes small, the latter loosely contacting the postglenoid process. Mandible with the second lower incisor (i2) of moderate size; long diastema; front border of ascending ramus far behind the third lower molar (m3), and much inclined posteriorly, so that m3 is very far from the posterior border of the mandible. Premolars much smaller than the molars; cement present; no labial cingulum on cheek teeth; upper molars with strongly constricted protocone, long antecrochet connected with the hypocone on worn teeth, closed postfossette; crochet and crista present but small; central valley unexpanded, with some enamel folding; labial wall weakly undulated. Slender metapodials.
Differs from “Hispanotherium” tungurense Cerdeño, 1996, in its fused, broader nasals, shallower nasal notch, M2 broader than long with flatter buccal wall, larger lower incisors, inclined mandibular ramus; from Iranotherium morgani (Mecquenem, 1908) in its shorter face, more anterior orbit, longer premaxillae and nasal notch, normal-size second upper molar (M2) with thicker lophs, and larger lower incisors; from Parelasmotherium linxiaense Deng, 2001, in its more anterior orbit, less anterior dental row, larger premaxillae, and shorter M2 with unexpanded central valley; from the two species of Ningxiatherium in its smaller size, complete absence of nasal septum, longer and less square nasals, orbit located distinctly more anteriorly, and unexpanded central valley of the upper molars.
Description.—Cranium: The holotype skull FSC-Sk-250 (Fig. 1) is relatively complete; most of the occiput is missing, but the right occipital condyle and basicranium are preserved. Part of the left mandible and most of the right one are also preserved, but they could not be separated from the cranium, because the jaws are closed and because of the numerous cracks that affect both the cranium and mandible. The cranium is somewhat dorsoventrally crushed but, besides this, its proportions are not seriously altered by distortion. Although the right mandibular condyle is correctly articulated with the cranium, the anterior part of the lower jaw is shifted anteriorly relative to its normal position by about 30 mm, p2 being distinctly forward of P2. All right lower teeth are preserved, although only partly visible, but of the upper teeth, only M2 and M3 are preserved, although much worn, with most of their crown visible. Overall, it is the most complete rhino skull from the late Miocene of Africa.
The cranium (see measurements in Table 1) is long relative to its width × height components, which are hard to disentangle because of distortion, but it is likely that it was high rather than broad. Because of the advanced ontogenic age of the specimen, the cranial sutures are no longer traceable. The fused nasal bones are long and inflated, but terminate anteriorly in a long point extending towards the premaxillae (Fig. 1A2, A3). Their dorsal part is strongly rugose, showing that they carried a single large horn. Because of the long nasal bones, the nasal notch is deep, but it does not reach very far posteriorly, about the level of the middle of the missing P2. There is no evidence of a frontal horn, but its presence cannot be excluded, as it sometimes leaves only faint traces on bone in rhinos. The premaxillae are exceptionally well preserved. They are quite long and slender, and fuse rostrally, but bear no tooth. This fused part extends dorsorostrally for about 40 mm, towards the tips of the nasals (Fig. 1A3), from which they remain distant by only 40 mm (a distance that may have been slightly longer in life), thus much closer than usual in, e.g., both living species of Rhinoceros. This premaxillary expansion is now located above the lower incisors but, because of the post-mortem forward shift of the lower jaw, it was in fact originally more rostral, and the snout must have formed a pointed rostrum in life; except for the lack of nasal septum, it resembles that of the Pleistocene woolly rhino “Coelodonta” antiquitatis (Blumenbach, 1799).
Table 1. Measurements (in mm) of skull FSC-Sk-250. Numbers of Guérin (1980) in brackets.
|
Length |
condyle to tip of premaxillae [1] |
780 |
|
condyle to tip of nasals [2] |
750 |
|
|
condyle to front of orbit |
440 |
|
|
condylo-P2 |
620 |
|
|
Depth of nasal notch [4] |
180 |
|
|
Length |
orbit to nasal notch [9] |
203 |
|
infra-orbital foramen to nasal notch |
67 |
|
|
infra-orbital foramen to orbit |
145 |
|
|
condyle to rear of M3 [13] |
390 |
|
|
Minimum width between temporal lines [17] |
50? |
|
|
Maximum bi-orbital width [19] |
ca. 250 |
|
|
Width over nasals |
113 |
|
|
Width of nasal constriction [22] |
110 |
|
|
Width over premaxillae, rostrally |
ca. 53 |
|
|
Length |
p2‒p4 |
ca. 91 |
|
m1‒m3 |
ca. 140 |
|
|
M2–M3 |
114 |
|
|
Length × width of M2 |
50.5 × ca. 70 |
|
|
Length |
tip of i2 to rear of mandible |
575 |
|
diastema |
95 |
|
|
Width over i2s |
ca. 95 |
|
|
Transverse × dorso-ventral diameters of i2 |
33 × 23 |
|
The cranial profile is but slightly concave; it might have been more concave in life but, even accounting for distortion, it was certainly not deeply concave. The infraorbital foramen was located above the back of P3 or above P4. The orbit is located about mid-length of the skull, far from the nasal notch, its anterior border being above the posterior third of M2. It has a distinctly salient margin, especially ventrally. There is no evidence of a supraorbital process, but the anterodorsal orbital margin is imperfectly preserved. The blunt temporal lines are not preserved for their full length, but remained certainly widely separated caudally. The zygomatic arches are long and of regular dorsoventral depth, without postorbital process, inserted rather high on the face, and they are raising high posteriorly, but this part is not fully preserved. They are laterally slightly concave for most of their length, and the maximum bizygomatic width is located very caudally, at the level of the glenoid fossae which are transversally long. The postglenoid process is not particularly large, but the paroccipital and posttympanic processes are distinctly small (Fig. 1A4). The latter is closely applied onto the postglenoid process, thus closing ventrally the pseudo-auditory meatus, but this might be due to post-mortem distortion. The caudal, triangular part of the stylohyal is preserved, but it shows little variation among rhinos. Details of the cranial base are not observable, except that the hypoglossal foramen is located in the middle of the hypoglossal fossa. Most of the occipital is missing, but the occipital plane was certainly inclined posterodorsally.
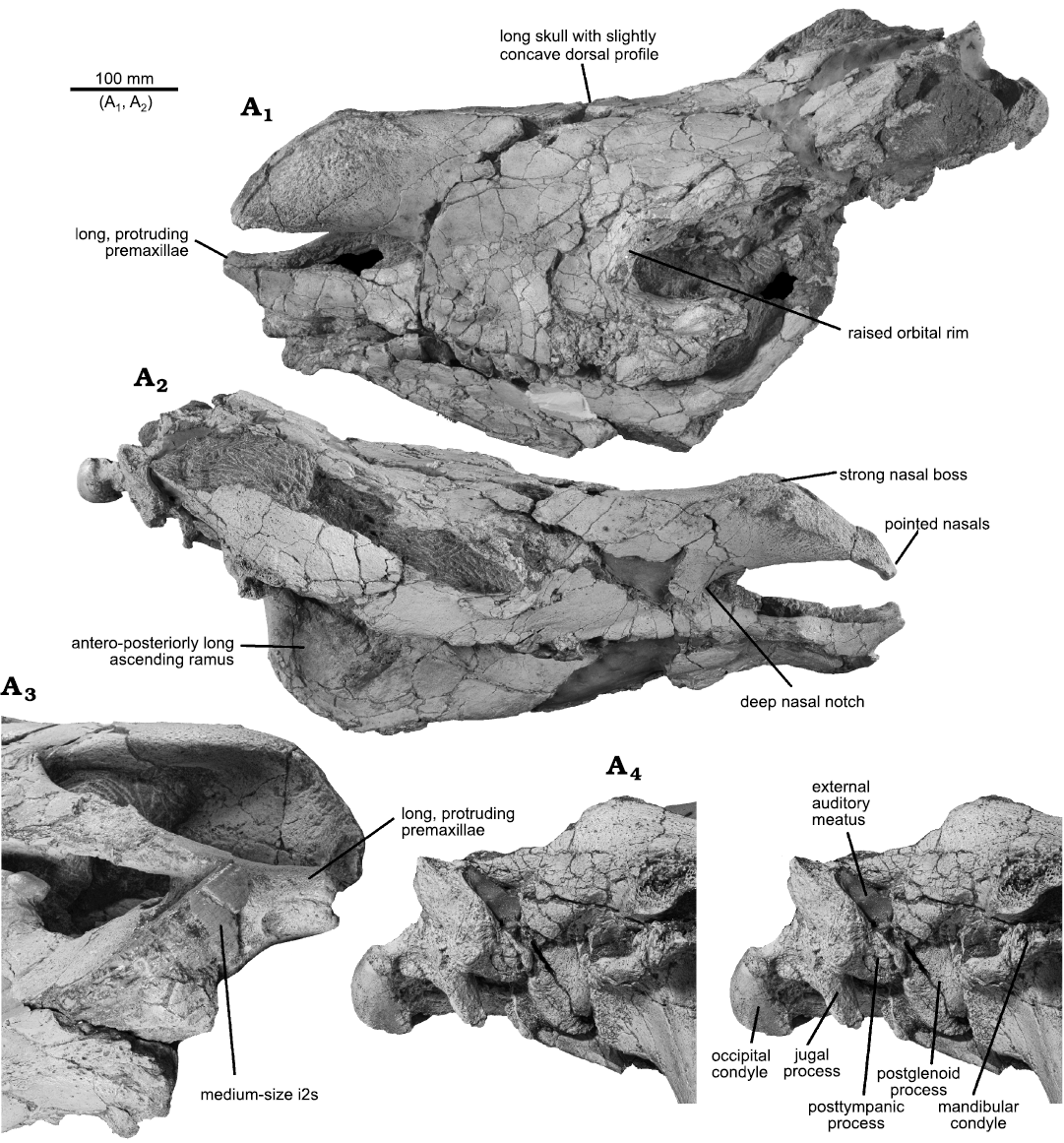
Fig. 1. Elasmotheriine rhinoceros Eoazara xerrii gen. et sp. nov. from the upper Miocene of Skoura, Morocco. Skull (FSC-Sk-250) in left latero-dorsal (A1) and right lateral (A2) views. Antero-ventral view of the front part, showing the right lower incisor, premaxillae, and nasals (A3). Stereo view of the right auditory area (A4). A3, A4, in oblique view, not to scale.
Mandible: The ventral border of the mandibular corpus is almost straight. The posterior border of the ascending ramus is slightly inclined posteriorly, but its anterior border ascends very slowly behind m3, so that the distance between the back of this tooth and the posterior border of the ramus, is about as long as p2–m3, a unique instance among rhinos. The posterior border of the symphysis is at the level of the middle of p2, and the mental foramen is just anterior to this tooth. Like the premaxillae, the diastema is long, about as long as the premolar series, and the diverging mandibular branches are constricted at its level.
Teeth: Nothing can be said about the missing upper premolars and M1. The heavily worn M2 (Fig. 2) is much broader than long as preserved; it has buccally convex paracone and metacone, but weak styles. Wear has created three closed enamel islands. The central valley is closed lingually by the fusion between antecrochet and hypocone; its enamel is wrinkled, and a crochet and a crista of small size are also present, but they do not enclose a medifossette. A postfossette is formed between the posterior cingulum and the metaloph, which is strongly constricted between the medi- and postfossettes. The protoloph is extremely constricted, almost isolating the protocone. The M3 is longer than M2; as usual for rhino M3s, the metaloph is not distinct from the ectoloph but, whereas it is more or less triangular in most rhinos, the outline of this tooth is more pentagonal, the mesial half of the buccal wall being almost perpendicular to the mesial border. The ectometaloph is expanded distally, and is supported by a supplementary root. The labial cingulum is absent on M2 and M3.
The much-worn right i2 is present in situ; it is a moderate-size tooth, obliquely inserted, slightly divergent from its counterpart, much broader (33 mm) than dorsoventrally thick (ca. 23 mm), with a rounded lateral border; the medial edge is still concealed in sediment but was probably sharper, as usual for rhinos. There is enough room between the i2s for small i1s, but there is no evidence of these teeth, which were probably absent.
As usual in rhinos, lower cheek teeth are not very distinctive. The lateral lobes are well rounded, and the ectoflexids are deep. The main feature is the shortness of the premolar row, and the steadily increasing length of the teeth, from p2 to m3. The length of the paralophid cannot be estimated, because most of the occlusal surfaces cannot be observed, except that of p2; its paralophid is constricted. Hypsodonty cannot be estimated, but there are remnants of cement on several teeth; all teeth lack a labial cingulum.
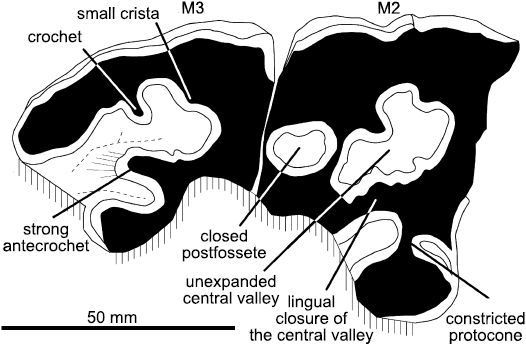
Fig. 2. Elasmotheriine rhinoceros Eoazara xerrii gen. et sp. nov. from the upper Miocene of Skoura, Morocco. Skull FSC-Sk-250, occlusal surface of right M2–M3; buccal is to the top, anterior to the right.
The much-worn isolated tooth FSC-Sk-33 that was regarded as a P4 by Zouhri et al. (2012) is in fact a M1 that has a strongly constricted protocone with an incipient vertical lingual furrow, a transversely elongated postfossette with a posterior wall, and a very long antecrochet whose lingual end reaches the entrance of the median valley (Fig. 3C). There is no cingulum. It is 39.5 mm long and 61 mm broad.
Postcranials: A few postcranial bones were found in the same block as the skull, and are certainly of the same individual, because no accumulations of bones from different individuals were found at Skoura. An incomplete distal humerus has a low olecranon fossa (Antoine 2002: character 193 state 1). On the lunar, the only observable character is the raised area for the insertion of the digit ligaments (Antoine 2002: character 213 state 0). A trapezoid has an asymmetrical antero-proximal edge (Antoine 2002: character 216 state 1).
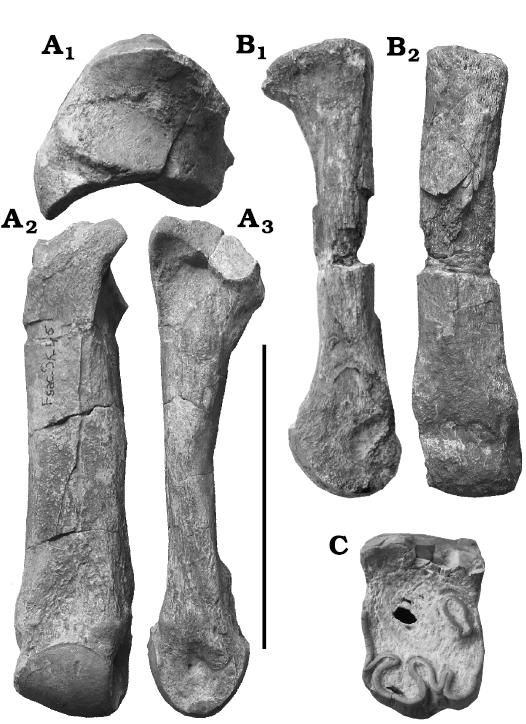
Fig. 3. Elasmotheriine rhinoceros Eoazara xerrii gen. et sp. nov. from the upper Miocene of Skoura, Morocco. A. Second left metacarpal (FSC-Sk-45) in proximal (A1), anterior (A2), and lateral (A3) views. B. Imperfectly preserved right second metatarsal (FSC-Sk-53) in anterior (B1) and lateral (B2) views. C. Left first upper molar (FSC-Sk-33) in occlusal view. Scale bars: A, 50 mm; B, C, 100 mm.
In addition, some postcranials were found during our own field seasons but their imperfect preservation, and the co-existence at Skoura of two rhinos of similar size render identification difficult, except for two metapodials, a Mc II FSC-Sk-45 and a Mt II FSC-Sk 53 (Fig. 3A, B; see measurements in Table 2). These bones are remarkably straight, and distinctly more slender than those of the Dicerotini, and must therefore belong to Eoazara xerrii gen. et sp. nov. On the Mc II, there is a large anterior facet for Mc III, but no posterior one; the trapezium facet is small, and the magnum facet is long and curved. The Mt II resembles NHMUK M32780 from Maboko, Kenya, which Geraads et al. (2012b) regarded as too slender to belong to Victoriaceros kenyensis Geraads, McCrossin, and Benefit, 2012b.
Table 2. Measurements (in mm) of Eoazara xerrii gen. et sp. nov. postcranials.
|
Measurement |
Mc II FSC-Sk-45 |
Mt II FSC-Sk-53 |
|
|
Length |
169 |
154 |
|
|
Proximal |
width |
42 |
– |
|
depth |
37 |
– |
|
|
Distal |
width |
39 |
– |
|
depth |
38 |
38 |
|
Remarks.—Africa: The Rhinocerotidae are poorly represented in the North African Miocene fossil record. Those from the middle Miocene of Beni Mellal in Morocco (Guérin 1976), lower upper Miocene of Bou Hanifia (Geraads 1986), Oued Mya in Algeria (Sudre and Hartenberger 1992), and Djebel Krechem el Artsouma in Tunisia (Geraads 1989), and uppermost Miocene of Lissasfa in Morocco (Raynal et al. 1999) all probably belong to the Dicerotini and are certainly unrelated to Eoazara xerrii gen. et sp. nov. Published material from the upper Miocene of Sahabi in Libya include only two much worn upper cheek teeth, only one being identifiable as Brachypotherium sp. (d’Erasmo 1954; Heissig 1982; Pandolfi and Rook 2019). What Guérin (1966) called Diceros douariensis, from the uppermost Miocene of Douaria in Tunisia, consists of two taxa. One is certainly a Dicerotini; by contrast, the deciduous premolars and the molars of the “paratype” juvenile cranium have a pinched protocone and strong antecrochet and crochet, and the upper orbital border is at the level of the transversally flat cranial roof. Yans et al. (2021) probably correctly assigned it to the Elasmotheriinae, but its incomplete and juvenile nature prevent in-depth comparisons.
Other African rhinos have been reviewed by Geraads (2010), but additional discoveries have been made since then, and are briefly reviewed below.
From the lower Miocene of Langental in Namibia, ca. 20 Ma old, Guérin (2008: pl. 1A) identified as Brachypotherium cf. heinzelini Hooijer, 1963, a P4 that is unlike this genus in the connection of the lingual cusps. Instead, its tooth features are reminiscent of early potential elasmotheres, such as Bugtirhinus praecursor Antoine and Welcomme, 2000, which is roughly contemporaneous. Brachypotherium (various species) has been reported from several other sites (Geraads 2010; Geraads and Miller 2013) and is sharply distinct from Eoazara xerrii gen. et sp. nov.
The poorly known Ougandatherium napakense Guérin and Pickford, 2003, from Napak, Uganda, also of earliest Miocene age, is totally unlike Eoazara xerrii gen. et sp. nov. in its short and hornless nasals, and upper premolars that are not much modified compared to species of the European Oligocene Ronzotherium.
In Rusingaceros leakeyi (Hooijer, 1966) from the lower Miocene of Rusinga, Kenya, the nasals are shifted forwards, as the nasal notch does not even reach the level of P2; the premaxillae bear strong incisors, the premolars are large; and the molars are simple (Hooijer 1966; Geraads 2010). All these features are unlike Eoazara xerrii gen. et sp. nov.
Several other African genera might be related to the Elasmotheriinae, a subfamily best known from Eurasia, where they have been especially studied by Antoine and colleagues (Antoine 1997, 2002, 2003; Antoine and Welcomme 2000; Antoine et al. 2002, 2003). The Victoriaceros includes two species from Kenya, both illustrated by almost complete cranial material, Victoriaceros kenyensis Geraads, McCrossin, and Benefit, 2012b, from Maboko, ca. 16 Ma, and Victoriaceros hooijeri Geraads, Lehmann, Peppe, and McNulty, 2016, from Karungu, ca. 18 Ma. The crania of both species distinctly differ from Eoazara xerrii gen. et sp. nov. in their very short face, probable presence of upper incisors, and simpler molars. Geraads et al. (2016) regarded the genus as close to, if not a member of the Elasmotheriinae.
Turkanatherium acutirostratum Deraniyagala, 1951, from the lower middle Miocene of Moruorot in Kenya, is known by a single skull (Geraads et al. 2016; Sanisidro et al. 2019). The orbit is located much more anteriorly than in Eoazara xerrii gen. et sp. nov., so that the face is much shorter, although not as short as in Victoriaceros kenyensis and Victoriaceros hooijeri Geraads, Lehmann, Peppe, and McNulty, 2016, and the nasals are short and unexpanded. The upper molars have constricted protoloph and metaloph but the antecrochet is short. Sanisidro et al. (2019) regarded Turkanatherium as part of a lineage distinct from Ougandatherium but details of their analysis have yet to appear. In any case, it shares no significant derived feature with Eoazara xerrii gen. et sp. nov.
Chilotheridium pattersoni Hooijer, 1971, from the roughly contemporaneous site of Loperot in Kenya, is known by partial crania and numerous postcranials, but the material is heavily broken and distorted. The orbit reaches farther anteriorly, and the nasal notch farther posteriorly than in the Skoura cranium, and the nasals carried a much smaller horn. The upper premolar lophs have no lingual connection, and the molars have only a short antecrochet and no strong pinching of the lophs. The affinities of Chilotheridium remain unclear, but it shows no clear elasmothere feature and no special resemblance with Eoazara xerrii gen. et sp. nov. Pending publication of detailed evidence, we regard reports of this genus at other sites, including Bukwa, Uganda (Cote et al. 2018), as doubtful. Following Tsujikawa (2005), Handa et al. (2015) assigned to this species a few teeth from Nakali and the Namurungule Formation of the Samburu Hills in Kenya, ca. 9.5–10 Ma, thus at least 5 My younger than the type-locality (and ca. 10 Myr younger than Bukwa). Such a difference in age alone makes species identity unlikely. In addition, the Nakali and Samburu Hills teeth lack diagnostic features, except for a P3 that differs from that of Chilotheridium pattersoni in the presence of a closed medifossette, completely absent at Loperot. Thus, we reject Handa et al.’s (2015) identification. The P3 from the Samburu Hills differs from the holotype P4 of Kenyatherium bishopi Aguirre and Guérin, 1974, from Nakali in much the same way as a P3 differs from a P4, e.g., in Begertherium borissiaki Beliajeva, 1971 (Beliajeva 1971: fig. 4), and we can see no reason for not referring this P3 at least to Kenyatherium bishopi (a species that Nakaya [1994] had identified in the Namurungule Formation).
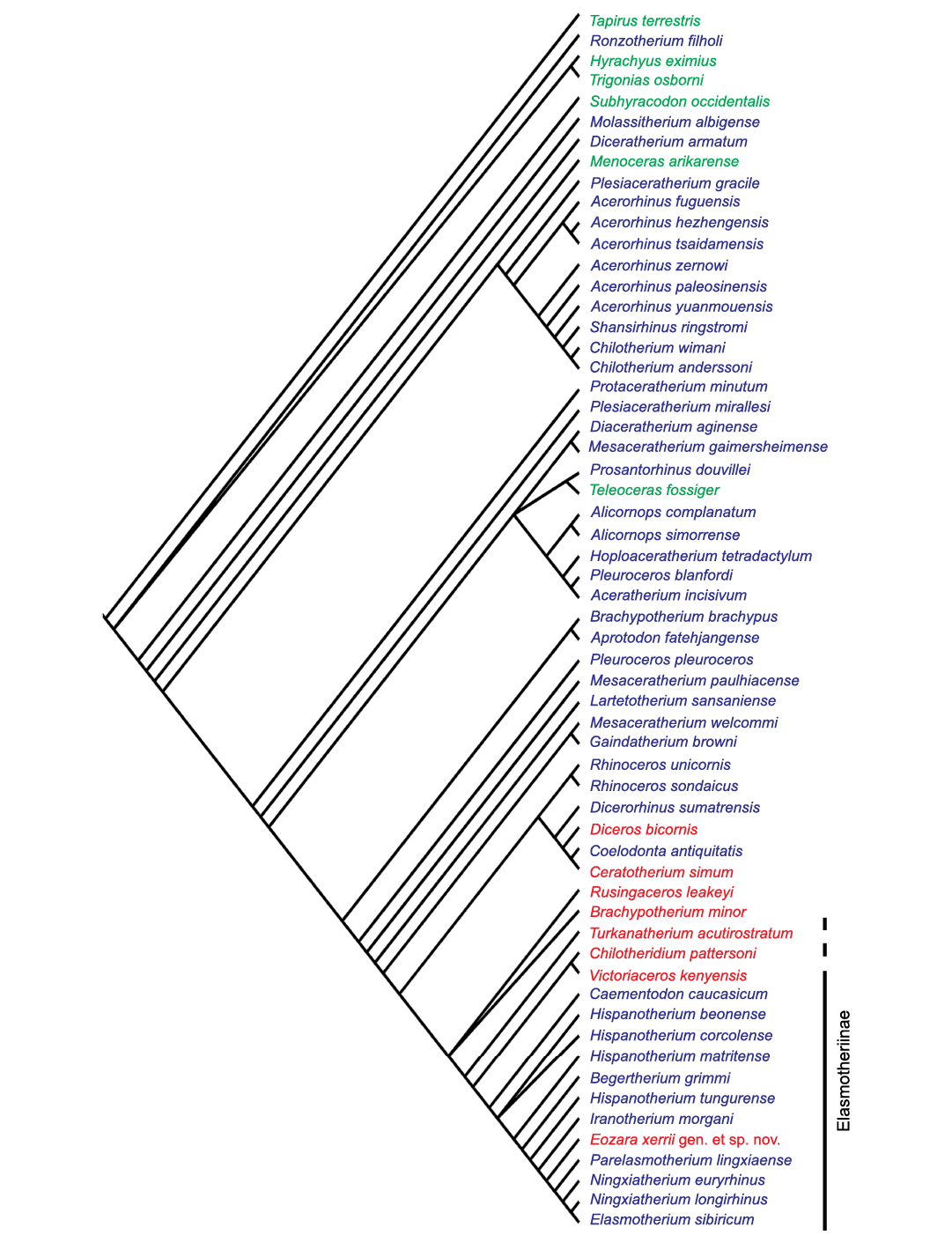
Fig. 4. Majority rule consensus tree of the 16 most parsimonious trees obtained by TNT on the data matrix of the 59 taxa that have at least 50% of the characters scored. Length = 1644; CI = 17; RI = 55. American taxa in green, Eurasian taxa in blue, African taxa in red. The dashed line extends to the taxa that might belong to the Elasmotheriinae as well.
Handa et al. (2017) described a few upper molars (but no upper premolar), again from Nakali and the Namurungule Formation, and deemed it necessary to erect a new binomen for them, Samburuceros ishidai Handa, Nakatsukasa, Kunimatsu, Tsubamoto, and Nakaya, 2017. These teeth have a strongly pinched protocone, a strong antecrochet, a mesiolingually directed hypocone limited anteriorly by a deep groove, and a trapezoidal M3. This morphology fits a member of the Elasmotheriinae, and also fits what would be expected for the molars of Kenyatherium bishopi or of the so-called Chilotheridium pattersoni from these localities. Thus, we regard Samburuceros ishidai as a synonym of Kenyatherium bishopi. Taking into account the wear stages, differences between these teeth and the Skoura ones are not great: less constricted protocone seemingly lacking lingual furrow on M1 (Nakaya et al. 1987: pl. 8: 1) and, on other molars, shorter antecrochet, less expanded central valley with a thicker protoloph, no enamel folding, and perhaps smaller crochet; all these differences reflect a lower evolutionary grade than that of Eoazara xerrii gen. et sp. nov. Most importantly, the complete absence of cranial remains other than teeth at Nakali and the Samburu Hills precludes further comparisons between Kenyatherium, “Samburuceros”, and any other genus so that, pending discovery of cranial material in these localities, these unwisely erected genus names must be restricted to the material from the type-localities.
Eurasia: The overall shape of the Eoazara xerrii gen. et sp. nov. holotype cranium is not unlike that of various other horned rhinos, but all Miocene forms, besides the Elasmotheriinae, have much simpler molars. According to Antoine and Welcomme (2000), the earliest representative of this subfamily is Bugtirhinus praecursor from the lower Miocene of Pakistan, but its skull is fully unknown; the M2 already shows a strongly pinched protocone, but the occlusal pattern is much less complex than in Eoazara xerrii gen. et sp. nov., the tooth is much narrower, and the M3 is triangular. The slightly younger Hispanotherium beonense (Antoine, 1997) from France (Antoine 1997; Antoine et al. 2000, 2002) has a more trapezoidal M3, but the M2 remains rather simple; the premolar row is not shortened, and large I1s are present; the cranium is more brachycephalic, with a short zygomatic arch; the dorsal cranial profile is much more concave; and the orbit distinctly more anterior.
Later middle Miocene forms from Europe and Asia have been revised, and their phylogenetic relationships assessed by Antoine (2003), but many species are mostly known by teeth and some postcranial remains. Procoelodonta mongoliense (Osborn, 1924) from the lower middle Miocene has extremely long nasals like the Skoura form, with incipient lateral flanges; the zygomatic arch is strongly upturned; the face is shorter; the orbits have no salient ridges; and the M2s are much less complex, with an open central valley and no enamel folding. Thus, it is clearly at a lower evolutionary grade than the Skoura form.
Hispanotherium matritense (Prado, 1864) from the middle Miocene of Spain and perhaps France (Ginsburg et al. 1981) is known through relatively complete but strongly distorted cranial remains (Sanisidro et al. 2012). The cheek teeth are only slightly less advanced in their weaker enamel folding, less isolated protocone and hypocone, but it has a thicker cement coating; the cranium seemingly shares a similar long neurocranium and small posttympanic and paroccipital processes, but differs much in being hornless, with short, narrow, pointed nasal bones, in addition to a vertical mandibular ramus (Cerdeño 1992; Cerdeño and Iñigo 1997).
From the middle Miocene of Paşalar and Çandır in Turkey, Heissig (1974, 1976) described Beliajevina tekkayai Heissig, 1974, and Hispanotherium grimmi Heissig, 1974. The former is characterized by the lack of postfossette on P4 but other permanent teeth are poorly known (Fortelius 1990). The latter has short nasals and at most a small horn. The posteriorly inclined mandibular ramus suggests a long skull, but the rostrum is short; the M2 is not broader than long; the antecrochet is moderate; and there are no lower incisors (Geraads and Saraç 2003).
In “Hispanotherium” tungurense Cerdeño, 1996, from the middle Miocene of Tung Gur in Inner Mongolia, the nasals are narrower and unfused; the nasal notch is deeper; the ascending ramus of the mandible is vertical; i1/i2 “appear to be very reduced” (Cerdeño 1996: 19); the external wall of M2 is strongly folded; and the antecrochet is absent on M2.
Several other species are very poorly known, or very incompletely described and illustrated: Begertherium borissiaki, from the middle Miocene of Beger Nur is mostly known by upper teeth (Antoine 2003: fig. 5C); the molars much differ from those of Eoazara xerrii gen. et sp. nov. in the short antecrochet, less constricted protocone, and absence of enamel folding. Caementodon oettingenae Heissig, 1972, from the Chinji of Pakistan, has primitive molars with short antecrochet, poorly constricted protoloph and metaloph, and no closed median valley. Hispanotherium lintungense Zhai, 1978, from the middle Miocene of Shensi, China, seems to have very short horn-bearing nasals, reaching not much farther rostrally than the first deciduous premolar but the figure (Zhai 1978: fig. 46) might be inaccurate; the M2 is broader than long, with no enamel folding, weak antecrochet and small hypocone. “Tesselodon” fangxianensis Yan, 1979, from the middle Miocene of Fangxian, China, known by upper teeth only, seemingly has a poorly constricted M2 protocone and a short antecrochet. Shennongtherium hyposodontus Huang and Yan, 1983, of unknown age, is known by three premolars only (Huang and Yan 1983). Caementodon tongxinensis Guan, 1988, from the middle Miocene of Tongxin, Ningxia, is known by a few cheek teeth only; M3 is trapezoidal; M2 is primitive in its short antecrochet, absence of folding, and open central valley (Guan 1988: pl. 2: 4; Guan et al. 1998: pl. 1: 1, 2). Huaqingtherium qiui Guan and Zhang, 1993, is only known by a poorly preserved and poorly illustrated partial maxilla and some lower teeth (Guan and Zhang 1993).
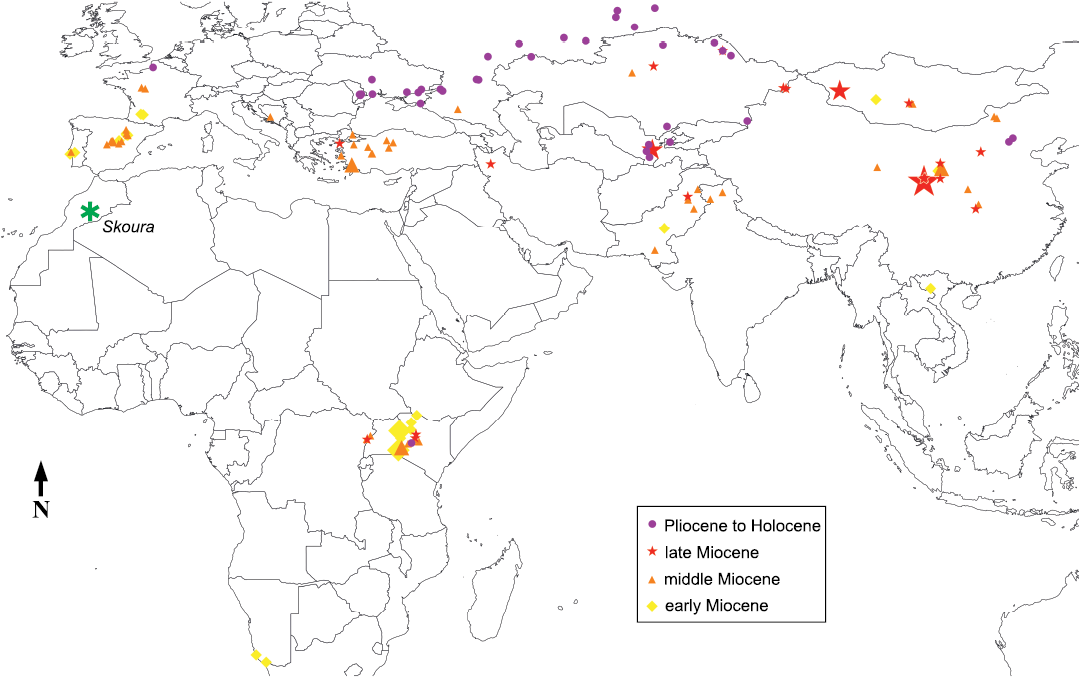
Fig. 5. Distribution map of the Elasmotheriinae. The size of the symbols reflects the number of species in each site (one species for the smallest symbols, up to four for the largest one: Guonigou in China). Eoazara xerrii gen. et sp. nov. is from the area called “Skoura”. Data from Geraads et al. 2021.
Iranotherium morgani from the upper Miocene of Maragheh in Iran, has a long cranium, but differs in its long face with an orbit located behind M3, its shallow nasal notch, the likely presence of a small frontal horn, the short premaxillae, and the posteriorly inflated, elevated, and roughened zygomatic arches (Mecquenem 1908). It further differs in that he second molar is by far the largest cheek tooth, and is longer than broad in its little-worn state; proto- and metaloph are curved backwards, and the central valley is enlarged, with lophs that remain narrow with wear. Deng (2005) reported the same species from the late Miocene of the Linxia Basin, China. The younger ontogenic age of a male cranium may explain its less rugose and inflated nasal and zygoma; together with a female cranium, they confirm the shortness of the rostrum; and a mandible has only a vestigial i2.
Parelasmotherium schansiense Killgus, 1923, is based upon a few upper cheek teeth from the upper Miocene of Shanxi, unusual in the presence of a very strong crista on M1 (Killgus 1923). The upper molars of Parelasmotherium linxiaense Deng, 2001, from the same levels in the Linxia Basin that yielded Iranotherium, are almost identical with those of the type-species, and Parelasmotherium simplum (Chow, 1958) Qiu and Xie, 1998, known by a few teeth only, is also certainly closely related, if not synonym. Deng (2007) described as Parelasmotherium linxiaense a somewhat distorted cranium characterized by its dolichocephaly, very large size, narrowness over the glenoid fossae, long nasals certainly carrying a large horn, small premaxillae lacking incisors, upper cheek teeth highly derived for grazing, with small, highly derived premolars in which the central valley is circled by a continuous “proto-metaloph”, molars with a rather flat labial wall, enlarged central valley, narrow lophs, strongly pinched protocone, long antecrochet, and large and long M3. Compared to the Skoura cranium, the tooth-row is shifted anteriorly, with the orbit located behind M3 and the nasal notch almost reaching M1; the short, slender premaxillae lack rostral expansion; the M2 is much longer than broad; and the cheek teeth are distinctly more derived.
Chen (1977) erected the genus Ningxiatherium (she also spelled it Ninxiatherium, and both names have been used since then, but we hereby act as first revisers to select the former name, see ICZN, art. 24.2) for her new species Ningxiatherium longirhinus Chen, 1977, based upon a complete, very large cranium from the upper Miocene of Zhongning, Ningxia, China. It differs from Parelasmotherium linxiaense in its stronger premaxillae connected to the tips of the nasals by an incipient nasal septum, and a M2 that is broader than long (even accounting for heavier wear); both features bring it slightly closer to Eoazara xerrii gen. et sp. nov., but it much more resembles Parelasmotherium linxiaense in overall cranial shape and enlarged central valley of the molars.
Deng (2008) added another species, Ningxiatherium euryrhinus Deng, 2008, again from the same levels in the Linxia Basin, about 11 Ma, which yielded Parelasmotherium linxiaense and Iranotherium morgani. Dental differences between the two species of Ningxiatherium are subtle, and cranial ones probably owe much to distortion (transverse in the type cranium of Ningxiatherium longirhinus, dorsoventral in that of Ningxiatherium euryrhinus); the nasal notch is said to be deeper and the nasal septum less complete in Ningxiatherium longirhinus. As we have not seen the material, we will keep the species as distinct. Ningxiatherium longirhinus was referred by Antoine (2002) to Parelasmotherium schansiense, but we agree with Deng (2008) that this is certainly incorrect, as the latter taxon has a much stronger post-crista and no expansion of the central valley on the molars.
On the whole, the characters of Ningxiatherium Chen, 1977, resemble those of the Skoura cranium, but the Chinese genus differs in that: (i) size is somewhat larger; (ii) the nasals are longer and broader, even in Ningxiatherium longirhinus, and are more rounded anteriorly, whereas those of the Skoura skull are pointed anteriorly; (iii) there is at least an incipient nasal septum, fully absent on the Skoura cranium, although the raising rostral end of its premaxillae might foreshadow the nasal septum of Ningxiatherium; (iv) the orbit is located fully behind M3, whereas its anterior border is above M2 in the Skoura cranium; (v) the temporal fossa and zygomatic arch are longer, and the latter is more slender; (vi) the posttympanic and paroccipital processes are longer and more robust; and (vii) on the molars, the central valley is more expanded. Most, if not all characters of the Chinese Ningxiatherium are more derived than those of Eoazara, but the latter, being associated with hipparions, is certainly not earlier in age, implying that they must be on different evolutionary lines.
Later Elasmotheriinae are mostly known from Asia. Sinotherium Ringström, 1923, from the Baodean (higher part of the upper Miocene) of China, Mongolia, and Central Asia (Ringström 1924; Deng et al. 2013) is a highly specialized form with a short, deep face, a huge nasofrontal horn, and large, hypsodont, and complex molars. It is clearly a forerunner of the Pleistocene Elasmotherium, from roughly the same areas (Kosintsev et al. 2019), which reached the climax of dental complexity.
We conclude that no previously described member of the Elasmotheriinae is sufficiently similar to the Skoura material to assign the latter to the same genus, especially as this would imply biogeographic connections that cannot safely be assumed. This is confirmed by the parsimony analysis.
Stratigraphic and geographic range.—Type locality and horizon only.
Parsimony analysis
Using TNT (Goloboff and Catalano 2016), we conducted a parsimony analysis on 74 taxa of Old World rhinos (plus Tapirus terrestris as an outgroup), using the data matrix of Geraads et al. (2016), with some corrections, deletions of characters whose coding is too subjective, and addition of the few post-cranial characters that can be observed in Eoazara xerrii gen. et sp. nov. at Skoura (SOM 1, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Geraads_Zouhri_SOM.pdf). Still, the results must be regarded with caution because; while most researchers would probably agree on the scoring of some characters, that of many others is far from straightforward. In addition, as already pointed out (Heissig 1981; Geraads et al. 2016) homoplastic evolution is widespread in rhinocerotids. For instance, within the Elasmotheriinae, features like cement apposition, hypsodonty, decrease of premolar/molar index, protocone constriction, lengthening of antecrochet, closure of fossettes, etc., were probably general trends. In addition, the complexity of upper teeth leads to a great diversity in the detail of their morphology, which can be described by many characters (about a third of all characters deal with them), resulting in an over-emphasis of their importance.
Using the default options of the “Traditional search”, followed by a second round of TBR, TNT yields 87 trees with a length of 1782 steps; the strict consensus tree has 1836 steps and is poorly resolved, but the majority rule consensus tree (cut-off 50) has 1784 steps only (SOM 3). Another analysis was run on the 59 taxa that have at least 50% of their characters scored (SOM 2). It yields 16 trees of 1630 steps, with a majority rule consensus tree (cut-off 50), of 1644 steps (Fig. 4). In-depth discussion of these trees is beyond the scope of this paper, but these two consensus trees do not differ much, showing that the poorly sampled taxa do not add much noise, and both consensus trees can be discussed together. Apomorphies at each node are listed in SOM 3.
The most obvious result is that all taxa commonly included in the Elasmotheriinae form a clade. However, and this is one of the few significant differences between the trees, only in the analysis run on all taxa is the American Menoceras arikarense (Barbour, 1906), a member of this clade. The Eoazara xerrii gen. et sp. nov. branch is deeply nested within this clade, intermediate between Iranotherium morgani and a group consisting of both species of Ningxiatherium, Parelasmotherium linxiaense, and Elasmotherium sibiricum. The African middle Miocene Chilotheridium + Victoriaceros are the sister group of this Elasmotheriinae clade, followed, in this order, by the other African taxa Turkanatherium, Rusingaceros, and Brachypotherium minor (which does not branch close to Brachypotherium brachypus). Sister to all these taxa is a clade including all extant forms plus “Coelodonta” antiquitatis. These results are in general good agreement with previous works (Geraads 2012b, 2016; Sanisidro et al. 2019), which regarded Victoriaceros and Turkanatherium as possible Elasmotheriinae, as it seems that they are at least closely related to them. We also conclude that the Elasmotheriinae (or Elasmotheriini), although quite diverse, are not a major branch of the family, and certainly not the sister-group of all, or most, other Rhinocerotidae.
Our results differ significantly from several previous analyses. In the pioneering analysis of Cerdeño (1995), the Elasmotheriinae do not appear as a distinct clade, and the taxa that form this subfamily in the present work are assigned to either the Rhinocerotinae or Aceratheriinae. The analysis of Deng (2008) is closer to the present one, as it recognizes a clade Elasmotheriinae, but most of the Rhinocerotinae also form a clade. Antoine et al. (2010) also recognize the two subfamilies, but their analysis is based upon much fewer taxa than here (27 instead of 74). Obviously, character choice and coding of their states greatly affect the results, but we believe that the high number of taxa analyzed here makes our results more reliable.
The elasmotheriine branch of the cladograms is in good agreement with chronology. Although precise age correlations are difficult to establish over widely distant sites of the Old World, there is no doubt that all members of the clade including Iranotherium and higher branches are of late Miocene age or younger, while all members below it are of early to middle Miocene age. The position of Eoazara xerrii gen. et sp. nov., just above Iranotherium, fits quite well its probable late Miocene age.
Concluding remarks
Biogeography.—A clade containing all commonly recognized Elasmotheriini, whose earliest representative is Bugtirhinus praecursor, is mostly Eurasian (with the exception of the poorly known Ougandatherium napakense and Kenyatherium bishopi, and of Menoceras arikarense whose position is uncertain) in the complete parsimony analysis (SOM 1), and entirely Eurasian in the restricted parsimony analysis (Fig. 3). Eoazara xerrii gen. et sp. nov. is clearly nested within this group, and its Eurasian origin is not doubtful.
The status of African forms is uncertain. In their phylogeographic reconstruction, Sanisidro and López Cantalapiedra (2010) posit the existence of an African elasmotheriine lineage that probably became independent in the earliest Miocene, starting with Ougandatherium and ending in Kenyatherium. This hypothesis is conceivable; it might be that the middle Miocene Kenyan species of Turkanatherium, Chilotheridium, and Victoriaceros belong to this side branch ending in Kenyatherium bishopi (including “Samburuceros ishidai”). Alternatively, Kenyatherium might not be so deeply rooted in Africa, being in fact more closely related to Eoazara xerrii gen. et sp. nov. than shown by the cladograms, but it is too poorly known to be fruitfully discussed at length.
Another point of biogeographic interest is that the elasmotheriine crania that most resemble that of Eoazara xerrii gen. et sp. nov. are those of the Chinese Ningxiatherium euryrhinus; by contrast, that of the Iberian Hispanotherium matritense is not particularly similar, and the Elasmotheriinae are seemingly fully absent from European upper Miocene deposits (Fig. 5). This confirms the clear differences in the composition of large mammal faunas on both sides of the western Tethys during the first part of the late Miocene, as evidenced by the presence, in the poor North African fauna of this time, of several taxa unknown in Europe, in addition to Eoazara xerrii gen. et sp. nov., such as the bovid Skouraia (Geraads et al. 2012a), the suid Nyanzachoerus (Bishop 2010)and the Anthracotheriidae (Lihoreau et al. 2021). It may be that stronger biogeographic relationships link the Maghreb to similar latitudes in Central and Eastern Asia, as was recently suggested for the Anthracotheriidae (Lihoreau et al. 2021); geographically intermediate faunas (such as those from the middle Miocene of Arabia; Gentry 1987) yielded no member of the Elasmotheriinae, but they are quite poor.
Ecology.—The chief dental characters of Eoazara xerrii gen. et sp. nov., namely the cement cover, complex occlusal morphology with developed accessory formations, folded enamel, shortening of the premolar row, and enlarged M3, are probably adaptations to grazing (in rhinos as well as in other mammals); the slender metapodials denote a cursorial form, so that Eoazara xerrii gen. et sp. nov. can be regarded as an open-country form feeding mostly on grasses. This is not contradicted by its associated fauna, consisting of hipparions, antelopes, giraffes, and Tetralophodon (Zouhri et al. 2012; Geraads et al. 2012a, 2019; Cirilli et al. 2020). Ecologically this faunal association resembles the savannah-like biome which is already present in the Vallesian in the late Miocene Balkano-Iranian Province, but appears only later in Spain (Bonis et al. 1992). Kaya et al. (2018) date its spread to Morocco to the latest Miocene, but the Skoura fauna shows that it occurred certainly earlier.
Acknowledgements
Special thanks to Serge Xerri (Rabat, Morocco), whose generousness allowed Eoazara xerrii gen. et sp. nov. to be known to science, and to David Lefèvre (Université de Montpellier, France) and Jean-Paul Raynal (Université de Bordeaux, France) for support to DG in Morocco. Thanks to Madelaine Böhme (Eberhard-Karls-University of Tübingen, Germany), Emma Mbua and Fredrick Kyalo Manthi (both National Museums of Kenya, Nairobi), and Emmanuel Robert (Université de Lyon, France) for granting access to collections, and to Lilian Cazes and Philippe Loubry (both Centre de Recherche en Paléontologie-Paris, France) for the photos. We thank the editor Olivier Lambert (Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium), Oscar Sanisidro (Universidad de Alcalá de Henares, Madrid, Spain), and an anonymous reviewer for their constructive comments; Naoto Handa (Museum of Osaka University, Osaka, Japan) and two anonymous reviewers also provided helpful comments on a previous version of this manuscript.
References
Aguirre, E., and Guérin, C. 1974. Première découverte d’un Iranotheriinae (Mammalia, Perissodactyla, Rhinocerotidae) en Afrique: Kenyatherium bishopi nov. gen. sp. de la formation vallésienne (Miocène supérieur) de Nakali (Kenya). Estudios geológicos 30: 229–233.
Antoine, P.-O. 1997. Aegyrcitherium beonensis n. g. n. sp., nouvel élasmothère (Mammalia, Rhinocerotidae) du gisement miocène (MN4b) de Montréal du Gers (Gers, France). Position phylogénétique au sein des Elasmotheriini. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 204: 399–414. Crossref
Antoine, P.-O. 2002. Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoires du Muséum National d’Histoire Naturelle 188: 1–359.
Antoine, P.-O. 2003. Middle Miocene elasmotheriine Rhinocerotidae from China and Mongolia: taxonomic revision and phylogenetic relationships. Zoologica Scripta 32: 95–118. Crossref
Antoine, P.-O. and Welcomme, J.-L. 2000. A new rhinoceros from the lower Miocene of the Bugti Hills, Baluchistan, Pakistan: the earliest elasmotheriine. Palaeontology 43: 795–816. Crossref
Antoine, P.-O., Alférez, F., and Iñigo, C. 2002. A new elasmotheriine (Mammalia, Rhinocerotidae) from the early Miocene of Spain. Comptes Rendus Palevol 1: 19–26. Crossref
Antoine, P.-O., Bulot, C., and Ginsburg, L. 2000. Une faune rare de Rhinocerotidés (Mammalia, Perissodactyla) dans le Miocène inferieur de Pellecahus (Gers, France). Geobios 33: 249–255. Crossref
Antoine, P.-O., Downing, K.F, Crochet, J.-Y., Duranthon, F., Flynn, L.J., Marivaux, L., Métais, G., Rajpar, A.R., and Roohi, G. 2010. A revision of Aceratherium blanfordi Lydekker, 1884 (Mammalia: Rhinocerotidae) from the early Miocene of Pakistan: postcranials as a key. Zoological Journal of the Linnean Society 160: 139–194. Crossref
Antoine, P.-O., Duranthon, F., and Welcomme, J.-L. 2003. Alicornops (Mammalia, Rhinocerotidae) dans le Miocène supérieur des Collines Bugti (Balouchistan, Pakistan): implications phylogénétiques. Geodiversitas 25: 575–603.
Barbour, E.H. 1906. Notice on a new Miocene rhinoceros, Diaceratherium arikarense. Science 24: 780–781. Crossref
Beliajeva, E.I. [Belâeva, E.I.] 1971. On some rhinoceroses, family Rhinocerotidae, from the Neogene of Western Mongolia. Mesozoic and Cenozoic fauna of Western Mongolia [in Russian]. Trudy / Sovmestnaâ Sovetsko-Mongolʹskaâ naučno-issledovatelʹskaâ geologičeskaâ èkspediciâ 3: 78–97.
Benvenuti, M., Moratti, G., and Rook, L. 2020. Tectono-stratigraphic revision of the Aït Kandoula Formation (middle Miocene–Pliocene), western Ouarzazate Basin (Southern Morocco), in the frame of fossil vertebrate record. Rivista Italiana di Paleontologia e Stratigrafia 126: 51–64.
Bishop, L.C. 2010. Suoidea. In: L. Werdelin and W. J. Sanders (eds.), Cenozoic Mammals of Africa, 821–842. University of California Press, Berkeley. Crossref
Blumenbach, J.F. 1799. Handbuch der Naturgeschichte. 708 pp. Dieterich, Göttingen.
Bonaparte, C.L. 1845. Systema vertebratorum. Transactions of the Linnean Society of London 18: 31–41.
Bonis, L. de, Bouvrain, G., Geraads, D., and Koufos, G. 1992. Multivariate study of late Cenozoic mammalian faunal compositions and palaeoecology. Paleontología í Evolució 24–25: 93–101.
Cerdeño, E. 1992. Spanish Neogene rhinoceroses. Paleontology 35: 297–308.
Cerdeño, E. 1995. Cladistic analysis of the family Rhinocerotidae (Perissodactyla). American Museum Novitates 3143: 1–25.
Cerdeño, E. 1996. Rhinocerotidae from the middle Miocene of the Tung-Gur Formation, Inner Mongolia (China). American Museum Novitates 3184: 1–43.
Cerdeño, E. and Iñigo, C. 1997. Hispanotherium matritense (Rhinocerotidae) de la ciudad de Madrid. Revista Española de Paleontología 12: 80–90.
Chen, G. 1977. A new genus of Iranotheriinae of Ningxia. Vertebrata PalAsiatica 15: 143–147.
Chow, M.C. 1958. New elasmotheriine rhinoceroses from Shansi. Vertebrata PalAsiatica 2: 131–142.
Cirilli, O., Zouhri, S., El Boughabi, S., Benvenuti, M.G., Papini, M., Bernor, R.L., and Rook, L. 2020. The hipparionine horses (Perissodactyla: Mammalia) from the late Miocene of Tizi N’Tadderht (Southern Ouarzazate Basin; Central High Atlas; Morocco). Rivista Italiana di Paleontologia e Stratigrafia 126: 1–12.
Cote, S., Kingston, J., Deino, A., Winkler, A., Kityo, R., and MacLatchy, L. 2018. Evidence for rapid faunal change in the early Miocene of East Africa based on revised biostratigraphic and radiometric dating of Bukwa, Uganda. Journal of Human Evolution 116: 95–107. Crossref
Deng, T. 2001. New remains of Parelasmotherium (Perissodactyla, Rhinocerotidae) from the late Miocene in Dongxiang, Gansu, China. Vertebrata PalAsiatica 39: 306–311.
Deng, T. 2005. New discovery of Iranotherium morgani (Perissodactyla, Rhinocerotidae) from the late Miocene of the Linxia Basin in Gansu, China, and its sexual dimorphism. Journal of Vertebrate Paleontology 25: 442–450. Crossref
Deng, T. 2007. Skull of Parelasmotherium (Perissodactyla, Rhinocerotidae) from the upper Miocene in the Linxia Basin (Gansu, China). Journal of Vertebrate Paleontology 27: 467–475. Crossref
Deng, T. 2008. A new elasmothere (Perissodactyla, Rhinocerotidae) from the late Miocene of the Linxia Basin in Gansu, China. Geobios 41: 719–728. Crossref
Deng, T, Wang, S.Q., and Hou, S.K. 2013. A bizarre tandem-horned elasmothere rhino from the late Miocene of northwestern China and origin of the true elasmothere. China Science Bulletin 58: 1811–1817. Crossref
Deraniyagala, P.E.P. 1951. A hornless rhinoceros from the Mio-Pliocene deposits of East Africa. Spolia Zeylanica 26: 133–135.
D’Erasmo, G. 1954. Paleontologia di Sahabi (Cirenaica). Sopra un molare di Teleoceras del giacimento fossilifero di Sahabi in Cirenaica. Rendiconti dell’Accademia Nazionale dei 40 (4): 89–102.
Fortelius, M. 1990. Rhinocerotidae from Paşalar, middle Miocene of Anatolia, Turkey. Journal of Human Evolution 19: 489–508. Crossref
Gentry, A.W. 1987. Rhinoceroses from the Miocene of Saudi Arabia. Bulletin of the British Museum (Natural History) Geology 41: 409–432.
Geraads, D. 1986. Sur les relations phylétiques de Dicerorhinus primaevus Arambourg, 1959, rhinocéros du Vallésien d’Algérie. Comptes Rendus de l’Académie des Sciences, sér. II, 302: 835–837. Crossref
Geraads, D. 1989. Vertébrés du Miocène supérieur du Djebel Krechem el Artsouma (Tunisie centrale). Comparaisons biostratigraphiques. Geobios 22: 777–801.
Geraads, D. 2010. Rhinocerotidae. In: L. Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 675–689. University of California Press, Berkeley. Crossref
Geraads, D. and Miller, E. 2013. Brachypotherium minor, a new rhinoceros from the early Miocene of Buluk, Northern Kenya. Geodiversitas 35: 359–375. Crossref
Geraads, D. and Saraç, G. 2003. Rhinocerotidae from the middle Miocene Hominoid locality of Çandir (Turkey). Courier Forschungsinstitut Senckenberg 240: 217–231.
Geraads, D., Cerdeño, E., García Fernandez, D., Pandolfi, L., Billia, E., Athanassiou, A., Albayrak, E., Codrea, V., Obada, T., Deng, T., Tong, H., Lu, X., Pícha, S., Marciszak, A., Jovanovic, G., Becker, D., Zervanova, J., Chaïd Saoudi, Y., Bacon, A.-M., Sévêque, N., Patnaik, R., Brezina, J., Spassov, N., and Uzunidis, A. 2021. A Database of Old World Neogene and Quaternary Rhino-bearing Localities. Available at www.rhinoresourcecenter.com/about/fossil-rhino-database.php.
Geraads, D., El Boughabi, S., and Zouhri, S. 2012a. A new caprin bovid (Mammalia) from the late Miocene of Morocco. Palaeontologia Africana 47: 19–24.
Geraads, D., Lehmann, T., Peppe, D.J., and McNulty, K.P. 2016. New Rhinocerotidae from the Kisingiri localities (lower Miocene of western Kenya). Journal of Vertebrate Paleontology 36 (3): e1103247. Crossref
Geraads, D., McCrossin, M., and Benefit, B. 2012b. A new rhinoceros, Victoriaceros kenyensis gen. et sp. nov., and other Perissodactyla from the middle Miocene of Maboko, Kenya. Journal of Mammalian Evolution 19: 57–75. Crossref
Geraads, D., Zouhri, S., and Markov, G.N. 2019. The first Tetralophodon (Mammalia, Proboscidea) cranium from Africa. Journal of Vertebrate Paleontology 39 (3): e1632321. Crossref
Ginsburg, L., Maubert, F., and Antunes, M.T. 1981. Découverte d’Hispanotherium et de Gaindatherium (Rhinocerotidae, Mammalia) dans le Miocène de France. Bulletin du Muséum National d’Histoire Naturelle sér. 4, C 9: 303–311.
Goloboff, P. and Catalano, S. 2016. TNT, version 1.5, with a full implementation of phylogenetics morphometrics. Cladistics 32 (3): 221–238. Crossref
Gray, J.E. 1821. On the natural arrangment of vertebrose animals. London Medical Repository 15: 296–310.
Guan, J. 1988. The Miocene strata and mammals from Tongxin, Ningxia and Guanghe, Gansu. Memoirs of Beijing Natural History Museum 42: 1–21.
Guan, J. and Zhang, X. 1993. The middle Miocene mammals from Guanghe and Hezheng in northwestern China. Memoirs of Beijing Natural History Museum 53: 237–251.
Guan, J., Hu, S., and Yang, X. 1998. Rhinocerotidae from middle Miocene of Tongxin, Ningxia. Bulletin of Beijing Natural History Museum 56: 147–151.
Guérin, C. 1966. Diceros douariensis nov. sp., un Rhinocéros du Mio-Pliocène de Tunisie du Nord. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon 16: 1–50.
Guérin, C. 1976. Les restes de rhinocéros du gisement miocène de Béni-Mellal, Maroc. Géologie méditerranéenne 3: 105–108. Crossref
Guérin, C. 1980. Les Rhinocéros (Mammalia, Perissodactyla) du Miocène terminal au Pléistocène supérieur en Europe occidentale. Comparaison avec les espèces actuelles. Documents des Laboratoires de Géologie de la Faculté des Sciences de Lyon 79: 1–1185.
Guérin, C. 2008. The Miocene Rhinocerotidae (Mammalia) of the Northern Sperrgebiet, Namibia. Memoir of the Geological Survey of Namibia 20: 331–341.
Guérin, C. and Pickford, M. 2003. Ougandatherium napakense nov. gen. nov. sp., le plus ancien Rhinocerotidae Iranotheriinae d’Afrique. Annales de Paléontologie 89: 1–35. Crossref
Handa, N., Nakatsukasa, M., Kunimatsu, Y., Tsubamoto, T., and Nakaya, H. 2015. New specimens of Chilotheridium (Perissodactyla, Rhinocerotidae) from the upper Miocene Namurungule and Nakali Formations, northern Kenya. Palaeontological Research 19: 181–194. Crossref
Handa, N., Nakatsukasa, M., Kunimatsu, Y., Tsubamoto, T., and Nakaya, H. 2017. A new Elasmotheriini (Perissodactyla, Rhinocerotidae) from the upper Miocene of Samburu Hills and Nakali, northern Kenya. Geobios 50: 197–209. Crossref
Heissig, K. 1972. Paläontologische und geologische Untersuchungen im Tertiär von Pakistan. 5. Rhinocerotidae (Mamm.) aus den unteren und mittleren Siwalik-Schichten. Abhandlungen der Bayerische Akademie der Wissenschaften. Mathematisch Naturwissenschaftliche Klasse 152: 1–112.
Heissig, K. 1974. Neue Elasmotherini (Rhinocerotidae, Mammalia) aus dem Obermiozän Anatoliens. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und historische Geologie 14: 21–35.
Heissig, K. 1976. Rhinocerotidae (Mammalia) aus der Anchitherium-Fauna Anatoliens. Geologisches Jahrbuch B 19: 1–121.
Heissig, K. 1981. Probleme bei der cladistischen Analyse einer Gruppe mit wenigen eindeutigen Apomorphien: Rhinocerotidae. Paläontologische Zeitschrift 55: 117–123. Crossref
Heissig, K. 1982. Note on Sahabi Rhinocerotidae. Garyounis Scientific Bulletin Special Issue 4: 85.
Hooijer, D.A. 1963. Miocene Mammalia of Congo. Annales des Musée Royal de l’Afrique Centrale, Tervuren 46: 1–77.
Hooijer, D.A. 1966. Miocene rhinoceroses of East Africa. Bulletin of the British Museum (Natural History) Geology 13: 119–190.
Hooijer, D.A. 1971. A new rhinoceros from the late Miocene of Loperot, Turkana district, Kenya. Bulletin of the Museum of Comparative Zoology 142: 339–392.
Huang, W. and Yan, D. 1983. New material of Elasmotherini [sic] from Shennongjia, Hubei. Vertebrata PalAsiatica 21: 223–229.
Kaya, F., Bibi, F., Žliobaitė, I., Eronen, J.T., Hui, T., and Fortelius, M. 2018. The rise and fall of the Old World savannah fauna and the origins of the African savannah biome. Nature Ecology & Evolution 2: 241–246. Crossref
Killgus, H. 1923. Unterpliozäne Säuger aus China. Paläontologische Zeitschrift 5: 251–257. Crossref
Kosintsev, P., Mitchell, K.J., Devièse, T., van der Plicht, J., Kuitems, M., Petrova, E., Tikhonov, A., Higham, T., Comeskey, D., Turney, C., Cooper, A., van Kolfschoten, T., Stuart, A.J., and Lister, A.M. 2019. Evolution and extinction of the giant rhinoceros Elasmotherium sibiricum sheds light on late Quaternary megafaunal extinctions. Nature Ecology and Evolution 3: 31–38. Crossref
Lihoreau, F., Essid, E.M., Ammar, H.K., Marivaux, L., Marzougui, W., Tabuce, R., Temani, R., Vianey-Liaud, M., and Merzeraud, G. 2021. The Libycosaurus (Hippopotamoidea, Artiodactyla) intercontinental dispersal event at the early late Miocene revealed by new fossil remains from Kasserine area, Tunisia. Historical Biology 33:146–158. Crossref
Mecquenem, R. de 1908. Contribution à l’étude du gisement de vertébrés de Maragha et de ses environs. Annales d’ Histoire Naturelle 1: 27–79.
Nakaya, H. 1994. Faunal change of late Miocene Africa and Eurasia: mammalian fauna from the Namurungule Formation, Samburu Hills, Northern Kenya. African Study Monographs. Supplementary Issue 20: 1–112.
Nakaya, H., Pickford, M., Yasui, K., and Nakano, Y. 1987. Additional large mammalian fauna from the Namurungule Formation, Samburu Hills, northern Kenya. African Study Monographs. Supplementary Issue 5: 79–129.
Osborn, H.F. 1924. Serridentinus and Baluchitherium, Loh Formation, Mongolia. American Museum Novitates 148: 1–5.
Owen, R. 1848. Contributions to the History of British Fossil Mammals. 71 pp. Taylor, London.
Pandolfi, L. and Rook, L. 2019. The latest Miocene Rhinocerotidae from Sahabi (Libya). Comptes Rendus Palevol 18: 442–448. Crossref
Prado, C. de 1864. Descripción Física y Geológica de la Provincia de Madrid. 219 pp. Junta General de Estadística. Imprenta Nacional, Madrid.
Raynal J.-P., Lefèvre, D., Geraads, D., and El Graoui, M. 1999. Contribution du site paléontologique de Lissasfa (Casablanca, Maroc) à une nouvelle interprétation du Mio-Pliocène de la Meseta. Comptes-Rendus de l’Académie des Sciences, Sciences de la Terre et des Planètes 329: 617–622. Crossref
Ringström, T. 1923. Sinotherium lagrelii, a new fossil rhinocerotid from Shansi. Bulletin of the Geological Survey of China 5: 91–93.
Ringström, T. 1924. Nashörner der Hipparion-Fauna Nord-Chinas. Palaeontologia Sinica C 1 (4): 1–156.
Sanisidro, O. and López Cantalapiedra, J. 2010. Nuevas técnicas paleobiogeográficas aplicadas a la Familia Rhinocerotidae (Perissodactyla). Cidaris 30: 293–298.
Sanisidro, O., Alberdi, M.T., and Morales, J. 2012. The first complete skull of Hispanotherium matritense (Prado, 1864) (Perissodactyla, Rhinocerotidae) from the middle Miocene of the Iberian Peninsula. Journal of Vertebrate Paleontology 32: 446–455. Crossref
Sanisidro, O., López Cantalapiedra, J., and Cote, S. 2019. A review of African Elasmotheres (Mammalia, Rhinocerotidae) and their role on early Miocene migration events into East Africa. In: A. Farke, A. MacKenzie, and J. Miller-Camp (eds.), 79th SVP Annual Meeting Abstracts Volume, Brisbane, 9–12 October 2019, 186. Society of Vertebrate Paleontology, Brisbane.
Sudre, J. and Hartenberger, J.-L. 1992. Oued Mya 1, nouveau gisement de mammifères du Miocène supérieur dans le sud Algérien. Geobios 25: 553–565. Crossref
Tesón, E., Pueyo, E.L., Teixell, A., Barnolas, A., Agustí, J., and Furió, M. 2010. Magnetostratigraphy of the Ouarzazate Basin: Implications for the timing of deformation and mountain building in the High Atlas Mountains of Morocco., Geodinamica Acta 23: 151–165. Crossref
Tsujikawa, H. 2005. The updated late Miocene large Mammal fauna from Samburu Hills, northern Kenya. African Study Monographs Supplement 32: 1–50.
Yan, D. 1979. Einige der Fossilen Miozänen Säugetiere der Kreis von Fangxian in der Provinz Hupei. Vertebrata PalAsiatica 17: 189–199.
Yans, J., Verhaert, M., Gautheron, C., Antoine, P.-O., Moussi, B., Dekoninck, A., Decrée, S., Chaftar, H.-R., Hatira, N., Dupuis, C., Pinna-Jamme, R., and Jamoussi, F. 2021. (U-Th)/He dating of supergene iron (oxyhydr-)oxides of the Nefza-Sejnane District (Tunisia): New insights into mineralization and mammalian biostratigraphy. Minerals 11 (3): 260. [published online, http://doi.org/10.3390/min11030260] Crossref
Zhai, R.J. 1978. A primitive elasmothere from the Miocene of Lintung, Shensi. Professional Papers of Stratigraphy and Palaeontology 7: 122–127.
Zouhri, S., Geraads, D., El Boughabi, S., and El Harfi, A. 2012. Discovery of an upper Miocene Vertebrate fauna near Tizi N’Tadderht, Skoura, Ouarzazate Basin (Central High Atlas, Morocco). Comptes Rendus Palevol 11: 455–461. Crossref
Acta Palaeontol. Pol. 66 (4): 753–765, 2021
https://doi.org/10.4202/app.00904.2021