

Rare evidence of shark-on-shark trophic interactions in the fossil record
VICTOR J. PEREZ, STEPHEN J. GODFREY, and PHILLIP F. CHAPMAN
Perez, V.J., Godfrey, S.J., and Chapman, P.F. 2021. Rare evidenc.e of shark-on-shark trophic interactions in the fossil record. Acta Palaeontologica Polonica 66 (4): 847–856.
Direct evidence of chondrichthyan trophic interactions in the fossil record is largely limited to bite traces on prey items but may also be found within the gut contents of exceptionally well-preserved individuals or as inclusions within coprolites. Shark bite traces are typically observed on durable, bony skeletal elements. Previous publications have shown shark bite traces on skeletal elements of fossil fishes, marine mammals, marine reptiles, and even a pterosaur, offering direct evidence of active predation, failed predation, and/or scavenging. Herein, we describe the first evidence of shark bite traces preserved on cartilaginous vertebral centra of other sharks. Four carcharhiniform centra have been identified from the Neogene Atlantic Coastal Plain, bearing chondrichthyan bite traces, of which two have partial teeth still embedded within them. In one specimen, CMM-V-2700, CT scans showed remodeling of the tissue around two partial teeth embedded in the centrum, indicating that the bitten individual survived the encounter. While shark-on-shark predation is common among living taxa, capturing evidence of these interactions in the fossil record is exceptionally rare.
Key words: Chondrichthyes, Carcharhinidae, trophic interaction, shark predation, shark-on-shark, bite traces, trace fossils, Neogene.
Victor J. Perez [Victor.Perez@calvertcountymd.gov], Department of Paleontology, Calvert Marine Museum, P.O. Box 97, Solomons, 20688, Maryland, USA.
Stephen J. Godfrey [Stephen.Godfrey@calvertcountymd.gov], Department of Paleontology, Calvert Marine Museum, P.O. Box 97, Solomons, 20688, Maryland, USA; Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20013-7012 USA.
Phillip F. Chapman [pchapma2@jhu.edu], Materials Science and Engineering, Johns Hopkins University, Baltimore, 21218, Maryland, USA.
Received 28 May 2021, accepted 8 July 2021, available online 7 December 2021.
Copyright © 2021 V.J. Perez et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Amongst extant chondrichthyans, shark-on-shark predation and cannibalism have been well-documented (Gudger 1932; Springer 1967; Bass et al. 1973, 1975; Dodrill 1977; Van der Elst 1979; Snelson et al. 1984; Stevens 1984; Gruber 1988). These instances of shark-on-shark predation are not uncommon and may actually reflect a dietary preference for some species. Van der Elst (1979) found that captive individuals of Carcharhinus obscurus and Carcharias taurus preferentially fed on smaller sharks, rather than the hundreds of teleosts present in the same tanks. Further, Springer (1967) speculated that many species of sharks utilize nursery habitats in specific shallow water areas to avoid predation pressure from larger sharks. Shark-on-shark predation plays an important role in marine ecosystems, yet these trophic interactions are seldomly mentioned or described in paleontological studies.
For many lamniform sharks, this predisposition towards preying on other sharks begins before birth, otherwise known as oophagy and embryophagy (Gilmore 1993; Uchida et al. 1996; Hamlett and Hysell 1998; Mollet et al. 2000; Gilmore et al. 2005; Hamlett 2011; Chapman et al. 2013; Shimada et al. 2021). Among squaliform sharks, parasitic predation on other sharks has been documented. Specifically, evidence of a trophic interaction between the cookiecutter shark, Isistius brasiliensis, and the great white shark, Carcharodon carcharias, was observed (Hoyos-Padilla et al. 2013). While shark-on-shark predation is evident in present-day marine ecosystems, these trophic interactions are scarce in the fossil record, largely due to a taphonomic filter.
Shark trophic interactions typically preserve in the fossil record as bite traces on vertebrate bone, offering evidence of active predation, failed predation, and/or scavenging. In the published literature, there are now numerous examples of shark bite traces on fossil cetaceans (Deméré and Cerutti 1982; Cigala Fulgosi 1990; Purdy 1996; Bianucci et al. 2000, 2010; Renz 2002; Godfrey 2003; Godfrey and Altman 2005; Noriega et al. 2007; Aguilera et al. 2008; Cozzuol and Aguilera 2008; Cicimurri and Knight 2009; Ehret et al. 2009; Bianucci and Gingerich 2011; Kallal et al. 2012; Govender and Chinsamy 2013; Takakuwa 2014; Govender 2015; Collareta et al. 2017; Godfrey et al. 2018, 2021; Kent 2018; Cortés et al. 2019; Mierzwiak and Godfrey 2019). Fossil shark bite traces have also been documented on other marine mammals, namely dugongids and pinnipeds (Renz 2002; Cozzoul and Aguilera 2008; Collareta et al. 2017; Feichtinger et al. 2021). Likewise, shark bite traces have been documented on teleost bones (Stewart 1993; Schwimmer et al. 1997; Shimada et al. 2002; Shimada and Everhart 2004). From the Mesozoic, shark bite traces have been documented on marine, terrestrial, and avian reptiles (Rothschild and Martin 1993; Schwimmer et al. 1997; Shimada 1997a, b; Corral et al. 2004; Hanks and Shimada 2002; Shimada et al. 2002; Shimada and Hooks 2004; Everhart 2004, 2005; Rothschild et al. 2005; Konuki 2008; Ehret and Harrell 2018; Hone et al. 2018).
There are a few exceptional examples of shark bite traces preserved on material other than bone. Bite traces observed on the calcified mandibular cartilage of a skate, cf. Raja binoculata, may have been produced by a shark (Boessenecker et al. 2014). Some bite traces observed on Carboniferous ammonoids have been attributed to sharks (Mapes and Hansen 1984; Mapes et al. 1995; Mapes and Chaffin 2003). Bite traces preserved on Cretaceous sea star ossicles were tentatively attributed to Squalicorax (Neumann 2000). A failed shark predation event was preserved on the carbonate test of a Cretaceous echinoid (Donovan and Jagt 2020). Perhaps the most unique are examples of Neogene shark-bitten crocodyliform coprolites (Godfrey and Smith 2010).
Shark-on-shark trophic interactions have existed for hundreds of millions of years; however, capturing those interactions in the fossil record requires exceptionally unique preservation. Shark remains present within the gut contents of the Devonian shark Cladoselache represent the oldest documented evidence of shark-on-shark predation (Williams 1990). Pseudocorax and Squalicorax teeth found in association with semi-articulated skeletal and dental elements of Cretoxyrhina mantelli were thought to be shed during scavenging events (Shimada 1997b). Shark-bitten shark teeth have also been documented in the fossil record, but these are usually attributed to self-bitten teeth (Purdy et al. 2001; Renz 2002; Perez 2020). Herein we describe four carcharhiniform shark centra that have been bitten by other sharks, offering direct evidence of shark-on-shark predation and/or scavenging in the fossil record.
Institutional abbreviations.—CMM-V-, Calvert Marine Museum vertebrate paleontology collection, Solomons, MD, USA.
Other abbreviations.—CT, computed tomography; Ma, mega annum.
Geological setting
The four specimens described in this study all originated from Neogene sediments of the Atlantic Coastal Plain along the east coast of North America. Three specimens, CMM-V-2700, CMM-V-6761, and CMM-V-7012 were found along the Calvert Cliffs in Calvert County, Maryland, USA. The fourth specimen, CMM-V-10336, was found at the Lee Creek Mine (currently named the Nutrien Phosphate Mine) in Aurora, North Carolina, USA. The Calvert Cliffs stratigraphy comprises sediments deposited within the Salisbury Embayment, whereas the Lee Creek Mine comprises sediments deposited within the Albemarle Embayment.
CMM-V-2700 (Figs. 1, 2) was collected by William (Bill) Counterman as float on the beach north of the Willows, a private residential community along Calvert Cliffs. Although it was not found in situ, it is presumed to be locally derived from the Miocene-age sediments that crop out immediately adjacent to where it was found. At this point along the naturally eroding sea cliffs, only Beds 4–16a of the Calvert Formation outcrop (Shattuck 1904; Ward and Andrews 2008; Kidwell et al. 2015; Vogt et al. 2018). Therefore, it most likely derived from the early to middle Miocene Plum Point Member of the Calvert Formation.
CMM-V-7012 (Fig. 3) was found in situ by John Nance within Bed 12 of the Calvert Formation near Parkers Creek, which is also part of the Plum Point Member. The Plum Point Member was deposited ~17.5 to 14 Ma, which corresponds to a peak in global temperature known as the Middle Miocene Climatic Optimum (Zachos et al. 2008; Vogt and Parrish 2012; Perez et al. 2018). The Plum Point Member consists of a series of unconformity-bounded transgressive-regressive cycles, each representing approximately 1 Ma (Kidwell 1984, 1988, 1989, 1997; Kidwell et al. 2015). They represent fully marine sediments deposited on an open shelf. Most of the shark teeth found in situ along Calvert Cliffs derive from the Plum Point Member (specifically, Beds [aka Shattuck Zones] 10 and 12) of the Calvert Formation (Visaggi and Godfrey 2010).
CMM-V-6761 (Fig. 4) was collected by John Nance as float north of Driftwood Beach within the Chesapeake Ranch Estates, Lusby, MD, USA, a private community further south along the Calvert Cliffs. The adjacent cliffs expose Beds 21–23, which comprise the Little Cove Point Member of the St. Marys Formation (Shattuck 1904; Ward and Andrews 2008; Kidwell et al. 2015). The late Miocene Little Cove Point Member has been interpreted as a brackish to fully marine paleoenvironment, indicating lower water depth and more variable salinity relative to the older Calvert Formation (Gernant et al. 1971; Kidwell et al. 2015).
CMM-V-10336 (Fig. 5) was found by Norm Riker in the Neogene sediments of the Lee Creek phosphate mine in Aurora, North Carolina. Unfortunately, the field notes for this specimen do not specify whether it originated from the early Miocene Pungo River Formation or the early Pliocene Yorktown Formation. The Pungo River Formation has been interpreted as a neritic paleoenvironment deposited in sub-tropical climatic conditions (Gibson 1967; Purdy et al. 2001). The Yorktown Formation also represents a shallow marine paleoenvironment but is thought to have been deposited in warm temperate climatic conditions (Gibson 1967; Purdy et al. 2001).
Material and methods
Herein, we describe four carcharhiniform centra, bearing shark bite traces. All specimens are reposited in the Calvert Marine Museum (CMM). Three specimens were found as float via surface collecting and one specimen was found in situ. Descriptions of the centra incorporate terminology from Kozuch and Fitzgerald (1989), Purdy et al. (2001), and Burris (2004). Although shark feeding traces are frequently referred to as “bite marks,” the more appropriate term is a bite trace (Jacobsen and Bromley 2009; Vallon et al. 2015). These bite traces correspond with the ichnotaxonomy described by Mikuláš et al. (2006), Jacobsen and Bromley (2009), and Muñiz et al. (2020).
The specimens were photographed on black velvet under flourescent light with a Nikon Coolpix P510 camera. The individual images were edited in Adobe Photoshop and assembled using Adobe Illustrator. All specimens were then digitally measured using ImageJ.
Micro-Computed Tomography (CT) was performed at the Johns Hopkins University Materials Characterization and Processing facility. The 2D projection images or radiographs were acquired with a RX Solutions Easy Tom 150/160 Micro-CT, using a fixed X-ray system and a rotating sample stage. The X-ray tube voltage was 50KV, and 1120 projections were collected during 360 degrees of rotation. Each projection was produced by averaging 4 frames to reduce random noise. Voxel size was 30.38 µm. The projection images were reconstructed using RX Solutions software. Post reconstruction images were generated with ORS Dragonfly software version 2020.2 (Dragonfly 2020).
Results
CMM-V-2700 consists of nearly half of a carcharhiniform centrum (Fig. 1). The articular surface appears to be circular; however, the partial preservation inhibits a precise description. Maximum diameter of the articular surface on the preserved portion of the centrum is 26.8 mm. Maximum anteroposterior thickness is 10.9 mm. The lateral face is slightly concave. None of the foramina are entirely preserved, but the portion present indicates a rectangular or square shape. The foramina do not extend to the articular rim. Dispersed nutrient pores are heavily concentrated around the foramina, with fewer pores scattered around the lateral face.
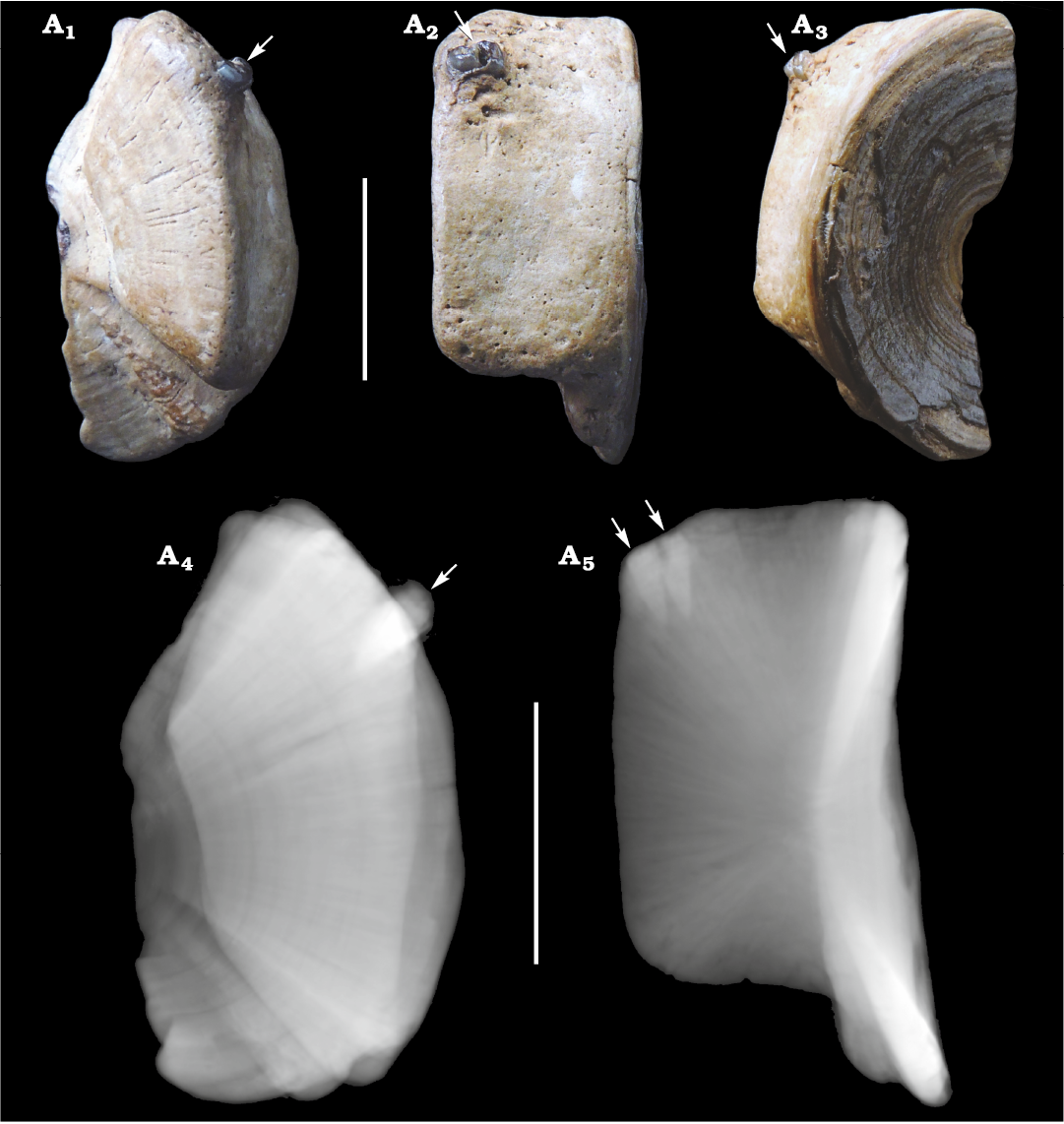
Fig. 1. Centrum of Carcharhinidae indet. (CMM-V-2700) from the Miocene Calvert Formation in Chesapeake Beach, MD, USA. Centrum in articular cross-sectional (A1), lateral (A2), and articular (A3) views; CT-scan in articular view (A4); CT-scan in lateral view (A5). Arrows indicate the two shark teeth embedded in and protruding from the upper quadrant of the centrum. Scale bars 10 mm.
Two adjacent teeth are firmly embedded into the lateral face, presumably on the dorsolateral quadrant of the centrum (Figs. 1 and 2). The two teeth penetrated approximately 3.6 and 3.9 mm, respectively, into the peripheral tissue of the centrum adjacent to the articular rim (Fig. 2). Although, it is not known if the teeth are embedded near the anterior or the posterior articular surface. The tissue is raised around the teeth forming a slight callus, which indicates partial healing and survival of the bitten shark. Interestingly, large nutrient pores are present around the embedded teeth, which may have formed to aid in this healing process. If the teeth were not preserved, the puncture trace would correspond with the ichnotaxon Nihilichnus nihilicus (Mikuláš et al. 2006).
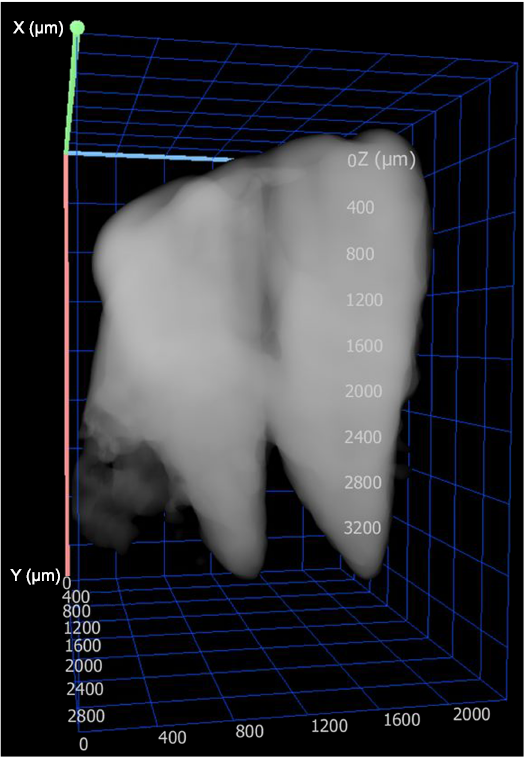
Fig. 2. CT-scan isolating two teeth of Carcharhinidae indet. that are embedded in the carcharhinid centrum (CMM-V-2700) from the Miocene Calvert Formation in Chesapeake Beach, MD, USA.
CMM-V-7012 is an entirely intact carcharhiniform centrum (Fig. 3). The articular surface is circular with a closed notochordal canal (Fig. 3A1). Maximum diameter of the articular surface is 40.5 mm. Maximum anterolateral thickness is 29.3 mm. The lateral face is slightly concave, with elongate foramina that extend to the articular rim. The dorsal foramina are rectangular (Fig. 3A2), whereas the ventral foramina are ovoid (Fig. 3A3). Nutrient pores are faintly scattered across the lateral face and surrounding the foramina. The nutrient pores are not as heavily concentrated as they are in the other centra described in this study.
Multiple sets of bite traces are present in different orientations along the lateral face, as well as the dorsal and ventral sides, of the centra (Fig. 3). The bite traces vary in length from approximately 2.5 to 9.5 mm. The spacing between bite traces range from approximately 0.6 to 1.4 mm, suggesting they may have been produced by different individuals or from different regions of the jaw. At least three unique sets of bite traces can be observed. The bite traces on CMM-V-7012 are much shallower than the bite traces observed on the other centra. The tooth traces seem to lack serrations, which corresponds with the ichnotaxon Linichnus bromleyi (Muñez et al. 2020), rather than the serrated tooth traces Linichnus serratus or Knethichnus parallelum (Jacobsen and Bromley 2009).
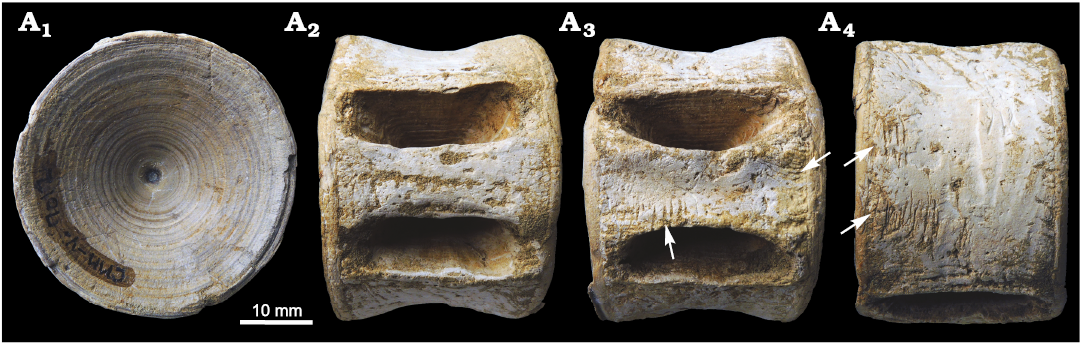
Fig. 3. Centrum of Carcharhinidae indet. (CMM-V-7012) from the Miocene Calvert Formation near Prince Frederick, MD, USA, in articular (A1), dorsal (A2), ventral (A3), and lateral (A4) views. Arrows mark the location of bite traces.
CMM-V-6761 is an entirely intact carcharhiniform centrum (Fig. 4). The articular surface is circular with a closed notochordal canal (Fig. 4A1). Maximum diameter of the articular surface is 30.2 mm. Maximum anterolateral thickness is 19.0 mm. The lateral face is strongly concave, with rectangular foramina that extend to the articular rim (Fig. 4A2, A3). Large, dispersed nutrient pores encompass the entire lateral face (Fig. 4A4). The pores closer to the articular rim are smaller and more densely packed.
There are two obliquely-oriented, parallel bite traces on the lateral surface adjacent to the articular rim (Fig. 4A4). It is unknown whether these bite traces are positioned near the anterior or posterior articular surface. The two bite traces are 4.9 and 6.3 mm long, respectively, and spaced 5.2 mm apart from one another. A small tooth fragment is embedded in the more dorsally positioned bite trace. The presence or absence of serrations cannot be confirmed on the tooth fragment; however, the rough edges of the bite traces offer some evidence that the teeth were serrated. The ichnotaxon Linichnus serratus is used to define bite traces produced by serrated teeth (Jacobsen and Bromley 2009).

Fig. 4. Centrum of a carcharhinid shark, cf. Galeocerdo aduncus (Agassiz, 1843) (CMM-V-6761), from the Miocene St. Marys Formation in Lusby, MD, USA. Centrum in aricular (A1), dorsal (A2), ventral (A3), and lateral (A4) views. Parallel bite traces are visible in A4 (white arrows), broken tooth is indicated by black arrow. The adhered barnacle and bryozoan are Recent taxa not contemporaneous with the centrum.
CMM-V-10336 is an entirely intact carcharhiniform centrum (Fig. 5). The articular surface is circular with a closed notochordal canal (Fig. 5A1). Maximum diameter of the articular surface is 47.9 mm. Maximum anterolateral thickness is 46.6 mm. The lateral face is slightly concave, with elongate, ovoid foramina that nearly extend to the articular rim. Dispersed nutrient pores are most heavily concentrated near the foramina and articular rim, with faint small pores present across the lateral face. However, the weathered lateral walls may obscure the true size and distribution of the pores.
Two obliquely-oriented, sub-parallel bite traces are present on the dorsolateral surface adjacent to the articular rim (Fig. 5A4). Again, it is unknown if the bite traces are positioned near the anterior or posterior articular surface. The more dorsal bite trace is 5 mm long and the more ventral bite trace is 15.8 mm long. The bite traces are spaced approximately 3.3 mm apart. The bite traces were produced by two non-serrated teeth, obliquely slicing into the centrum. The ichnotaxon Linichnus bromleyi describes bite traces produced by non-serrated teeth perpendicular to the substrate (Muñiz et al. 2020). Alternatively, Jacobsen and Bromley (2009) used the term “dental hack” to describe oblique tooth traces on bone produced by serrated teeth and assigned the ichnotaxon Knethichnus parallelum. These serrated bone scraping traces are colloquially referred to as raking marks. A unique ichnotaxon may be needed to define oblique bite traces produced by non-serrated teeth, so for now these traces are referred to as Knethichnus sp.
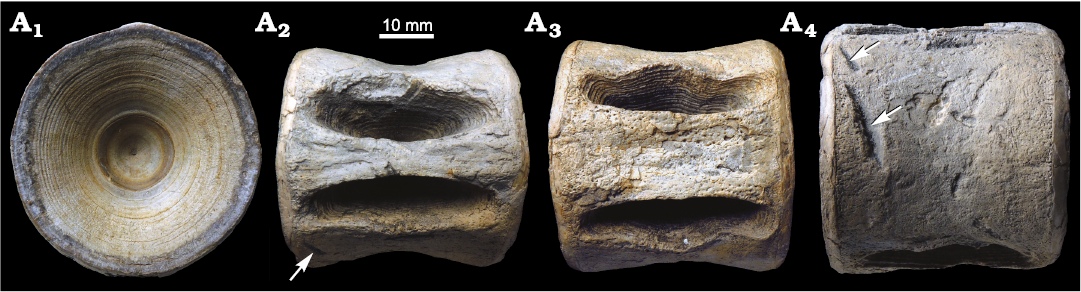
Fig. 5. Centrum of a carcharhinid shark, cf. Galeocerdo cuvier (Péron and Lesueur, 1822) (CMM-V-10336), from the Neogene of Aurora, NC, USA. Centrum in articular (A1), dorsal (A2), ventral (A3), and lateral (A4) views. Sub-parallel bite traces are indicated by arrows.
Concluding remarks
While these specimens provide unique evidence of shark-on-shark trophic interactions in the fossil record, it is difficult to determine the exact species involved and the context of the interaction. Some authors have been able to determine the species of shark responsible for biting other fossil skeletal elements, either by finding shark teeth embedded in the fossil of the prey/scavenged carcass, or by comparing the morphology of the tooth traces to shark teeth found in the same formation (Everhart et al. 1995; Schwimmer et al. 1997; Shimada 1997b; Shimada and Everhart 2004; Shimada and Hooks 2004; Rothschild et al. 2005; Noriega et al. 2007; Cicimurri and Knight 2009; Ehret et al. 2009; Bianucci et al. 2010; Govender and Chinsamy 2013; Godfrey et al. 2018, 2021; Kent 2018). Despite the presence of partial teeth embedded within two of the three centra described herein, the fragmentary nature of the teeth precludes species-level identification.
Identifying the victims of these encounters is also met with challenges. Chondrichthyan taxonomy in the fossil record is largely defined by dental records; however, some researchers have attempted to identify relevant characters on shark centra (e.g., Hasse 1879–1885; Ridewood 1921; Applegate 1967; Compagno 1988; Kozuch and Fitzgerald 1989; Purdy et al. 2001; Burris 2004). Fossil selachian centra are most commonly identified as either belonging to the order Carcharhiniformes or the order Lamniformes. Carcharhiniform centra have a smooth lateral face, whereas the lateral faces of lamniform centra are divided by numerous septa (Kent 1994). The septate morphology of lamniform centra makes them less likely to preserve bite traces, which is yet another taphonomic filter that may obscure evidence of shark-on-shark trophic interactions. As a good example of this, note that Shimada (1997b) provided evidence of sharks scavenging on the carcass of the lamniform shark Cretoxyrhina mantelli based on the co-occurrence of shed teeth from Squalicorax and Pseudocorax, and yet no bite marks were observed on the preserved skeletal elements.
Based on the smooth lateral faces of the centra described herein, they undoubtedly belong to the order Carcharhiniformes. Along the Miocene Calvert Cliffs, five families of carcharhiniform shark (i.e., Scyliorhinidae, Triakidae, Hemigaleidae, Carcharhinidae, and Sphyrnidae) have been documented, representing 16 species (Kent 2018). Within the Mio-Pliocene Lee Creek mine, the same five families of carcharhiniform sharks have been identified, representing 22 species (Purdy et al. 2001).
Among these five families of carcharhiniform sharks, Scyliorhinidae, Triakidae, and Hemigaleidae can be ruled out based on their external morphology. The centra described in this study are too large to have come from the family Scyliorhinidae. Centra from the family Triakidae lack nutrient pores (Burris 2004); whereas the four centra from this study all have nutrient pores present. The foramina in Hemigaleidae centra are bisected by diagonal laminae (Purdy et al. 2001; Burris 2004), which are not present in the four centra described herein. This leaves the families Carcharhinidae and Sphyrnidae as potential contenders.
Burris (2004) found that there are few characteristics that are consistently present across all species within the family Sphyrnidae. The most consistent features observed within sphyrnids were a modified cylindrical shape, with strongly recurved edges, a relatively elongate body, and dispersed nutrient pores. CMM-V-2700 has a cylindrical shape, lacking significant recurvature at the articular rim, and a relatively short body (Fig. 1A2). This suggests that CMM-V-2700 does not belong to the family Sphyrnidae and, thus, by process of elimination belongs to the family Carcharhinidae. Given the size and relatively straight lateral faces of CMM-V-2700, this centrum most likely represents either Carcharhinus or Negaprion. Burris (2004) found that centra of Carcharhinus and Negaprion were indistinguishable, so further identification is not possible. The two teeth embedded in CMM-V-2700 seem to lack serrations and have a relatively rounded cross section. Based on these features and the close spacing of the teeth, they most likely represent lower teeth from either Carcharhinus or Negaprion.
The faint scattered nutrient pores and weak concavity on the lateral walls of CMM-V-7012 indicate that it most likely belongs to the family Carcharhinidae as well. The multiple bite traces present across the centrum were most likely produced by multiple individuals, possibly representing different taxa. While the traces all appear to have been produced by evenly spaced non-serrated teeth, it is difficult to definitively determine if the traces were produced by chondrichthyan or osteichthyan taxa. The tooth spacing observed between traces certainly falls within the range of many extant carcharhinids (e.g., Carcharhinus and Negaprion) and lamnids (e.g., Carcharias and Alopias). However, many bony fish, such as Lepisosteus and Amia, also have tooth spacing within this range (Carnavale and Godfrey 2018) and are capable of producing a similar bite trace (Godfrey and Palmer 2015).
Among carcharhinids, Galeocerdo centra share many of the distinctive features present in Sphyrna (Burris 2004). The biggest differences are that Galeocerdo centra have much larger nutrient pores, tend to have greater concavity on their lateral walls, and typically have a smaller length to width ratio than Sphyrna centra. Although, Burris (2004) also noted that larger individuals of Galeocerdo tend to have weaker concavity on the lateral face. CMM-V-6761 matches well with this description and thus, likely belongs to the genus Galeocerdo. The most common species of Galeocerdo found along the Calvert Cliffs is G. aduncus. Although, we cannot rule out similar, contemporaneous extinct taxa, such as Galeocerdo mayumbensis or Physogaleus contortus (Türtscher et al. 2021). The ragged edges of the bite traces resemble the ichnotaxon Linichnus serratus, suggesting they were produced by a serrated tooth. The cross section of the embedded tooth is somewhat semi-circular, possibly suggesting a flattened labial face and convex lingual face, like the upper teeth of Carcharhinus.
CMM-V-10336 also has comparable features with Galeocerdo. The nutrient pores surrounding the foramina are relatively large, but the pores along the lateral faces are much smaller. Kozuch and Fitzgerald (1989) described Galeocerdo centra as typically having ovoid shaped foramina; however, Burris (2004) disagreed and stated that Galeocerdo tend to have more rectangular foramina. As such, it is unclear if the foramen shape can be used as a diagnostic feature. Burris (2004) noted that Galeocerdo centra are always wider than they are long (i.e., the maximum diameter of the articular surface is greater than the antero-posterior length). Since this specimen originated from the Mio-Pliocene Lee Creek mine, it could belong to either Galeocerdo aduncus, G. mayumbensis, or G. cuvier (Türtscher et al. 2021). Although, given its large size, G. cuvier seems more likely. The smooth bite traces on CMM-V-10336 were produced by a relatively large non-serrated tooth, such as those of Carcharodon hastalis.
Interpreting the context of these shark-on-shark interactions requires a combination of logical reasoning and speculation. The immediate assumption is that these bite traces represent evidence of some form of feeding behavior (i.e., active predation, failed predation, or scavenging). Although, sharks also frequently bite one another during reproduction, leaving their mates scarred (Parsons et al. 2008). Mating scars have been documented on extant individuals of Galeocerdo cuvier (Whitney and Crow 2007). Presumably, this type of biting would not be so aggressive as to make contact with the cartilaginous skeleton, but it is difficult to entirely rule out that possibility.
If our immediate assumption is correct, and these bite traces do represent feeding behavior, then factors such as the location of the bite on the body, depth of the bite, and signs of healing may be utilized to infer the context of the trophic interaction. It is difficult to determine the exact location of these centra within the vertebral column, beyond that they all appear to be pre-caudal centra. The presence of partial teeth embedded in CMM-V-2700 and CMM-V-6761, as well as the deep gouge trace in CMM-V-10336, suggest that these centra were all bitten very forcefully. This type of behavior is more indicative of active predation than scavenging but cannot be considered definitive evidence, as other factors such as body size and jaw musculature also effect bite force.
In contrast, CMM-V-7012 (Fig. 3) exhibits multiple sets of more gracile bite traces, suggestive of scavenging. The variable spacing between the bite traces may have been produced either by multiple individuals or from different regions of the jaw. CMM-V-2700 (Figs. 1 and 2) is the only specimen that has clear evidence of healing around the embedded teeth, which implies the bite was the result of a failed, active predation event. Many extant species of Carcharhinus have lower jaws with two functional rows of narrow pointed teeth that aid in grasping prey. It seems plausible that CMM-V-2700 represents a direct trophic interaction between two individuals of Carcharhinus, in which the lower teeth of the predator were lodged into the vertebral column of the prey (Fig. 6). Although much is still unknown regarding the specifics of these ancient trophic interactions, these specimens offer a unique insight into marine ecosystems during the Neogene.

Fig. 6. One possible way in which the shark centra (CMM-V-2700) could have been bitten. This illustration depicts an active predatory encounter between two requiem sharks (aff. Carcharhinus sp.). Original drawing by Tim Scheirer (formerly CMM). Coloration added by Clarence Schumaker (CMM).
Acknowledgements
We would like to express our thanks to William (Bill) Counterman (formerly CMM), John Nance (CMM), and the late Norm Riker for finding and donating the shark centra described herein. Thank you to artists Tim Scheirer (formerly CMM) and Clarence Schumaker (CMM) for their skillful depiction of the ancient trophic interaction (Fig. 6). We also greatly appreciate the valuable feedback provided by reviewers, Bobby Boessenecker (Mace Brown Museum in Charleston, SC, USA) and Dana Ehret (New Jersey State Museum in Trenton, NJ, USA), and the APP editorial team. This research was funded in part by the citizens of Calvert County Maryland, the County Board of Calvert County Commissioners, and the Clarissa and Lincoln Dryden Endowment for paleontology at the Calvert Marine Museum.
References
Aguilera, O.A, García, L., and Cozzuol, M.A. 2008. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontologische Zeitschrift 82: 204–208. Crossref
Applegate, S.P. 1967. A survey of shark hard parts. In: P.W. Gilbert, R.F. Mathewson, and D.P. Rall (eds.), Sharks, Skates and Rays, 37–67. Johns Hopkins University Press, Baltimore, MD.
Bass, A.J., D’Aubrey, J.D., and Kistnasamy, N. 1973. Sharks of the east coast of southern Africa. The genus Carcharhinus (Carcharhinidae). Oceanographic Research Institute 33: 1–168.
Bass, A.J., D’Aubrey, J.D., and Kistnasamy, N. 1975. Sharks of the east coast of southern Africa. III. The families Carcharhinidae (excluding Mustelus and Carcharhinus) and Sphyrnidae. Oceanographic Research Institute 38: 1–100.
Bianucci, G. and Gingerich, P.D. 2011. Aegyptocetus tarfa, n. gen. et sp. (Mammalia, Cetacea), from the middle Eocene of Egypt: clinorhynchy, olfaction, and hearing in a protocetid whale. Journal of Vertebrate Paleontology 31: 1173–1188. Crossref
Bianucci, G., Bisconti, M., Landini, W., Storai, T., Zuffa, M., Giuliani, S., and Mojetta, A. 2000. Trophic interactions between white sharks (Carcharodon carcharias) and cetaceans: a comparison between Pliocene and recent data. In: M. Vacchi, G. La Mesa, F. Serena, and B. Sèret (eds.), Proceedings 4th Meeting of the European Elasmobranc Association, Livorno (Italy). 27–30 September 2000, 33–48. Imprimerie F. Paillart, Abbeville.
Bianucci, G., Sorce, B., Storai, T., and Landini, W. 2010. Killing in the Pliocene: shark attack on a dolphin from Italy. Palaeontology 53: 457–470. Crossref
Boessenecker, R.W., Perry, F.A., and Schmitt, J.G. 2014. Comparative taphonomy, taphofacies, and bonebeds of the Mio-Pliocene Purisima Formation, Central California: strong physical control on marine vertebrate preservation in shallow marine settings. PLoS One 9 (3): p.e91419. Crossref
Burris, J.H. 2004. Morphology and Phylogenetic Implications of Recent and Fossil Carcharhiniform Shark Vertebral Centra. 219 pp. Ph.D. Dissertation, Michigan State University, East Lansing, MI.
Carnevale, G. and Godfrey, S.J. 2018. Miocene bony fishes of the Calvert, Choptank, St. Marys and Eastover Formations, Chesapeake Group, Maryland and Virginia. In: S.J. Godfrey (ed.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA. Smithsonian Contributions to Paleobiology 100: 161–212.
Chapman, D.D., Wintner, S.P., Abercrombie, D.L., Ashe, J., Bernard, A.M., Shivji, M.S., and Feldheim, K.A. 2013. The behavioural and genetic mating system of the sand tiger shark, Carcharias taurus, an intrauterine cannibal. Biology Letters 9 (3): 20130003. Crossref
Cicimurri, D.J. and Knight, J.L. 2009. Two shark-bitten whale skeletons from Coastal Plain deposits of South Carolina. Southeastern Naturalist 8 (1): 71–82. Crossref
Cigala Fulgosi, F. 1990 Predation (or possible scavenging) by a great white shark on an extinct species of bottlenosed dolphin in the Italian Pliocene. Tertiary Research 12: 17–36.
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology 469: 84–91. Crossref
Compagno, L.J.V. 1988. Sharks of the Order Carcharhiniformes. 486 pp. Princeton University Press, Princeton.
Corral, J.C., Pereda Suberbiola, X., and Bardet, N. 2004. Shark-bite marks in a mosasaur vertebra from the Late Cretaceaous of Álava (Basque–Cantabrian Region). Revista Española de Paleontología 19: 23–32. Crossref
Cortés, D., De Gracia, C., Carrillo-Briceño, J.D., Aguirre-Fernández, G., Jaramillo, C., Benites-Palomino, A., and Atencio-Araúz, J.E., 2019. Shark-cetacean trophic interactions during the late Pliocene in the Central Eastern Pacific (Panama). Palaeontologia Electronica 22 (2): 1–13. Crossref
Cozzuol, M.A. and Aguilera, O.A. 2008. Cetacean remains from the Neogene of northwestern Venezuela. Paläontologische Zeitschrift 82: 196–203. Crossref
Deméré, T.A. and Cerutti, R.A. 1982. A Pliocene shark attack on a cethotheriid whale. Journal of Paleontology 56: 1480–1482.
Dodrill, J.W. 1977. A Hook and Line Survey of the Sharks of Melbourne Beach, Brevard County, Florida. 304 pp. M.Sc. Thesis, Florida Institute of Technology, Melbourne.
Donovan, S. and Jagt, J. 2020. Ichnology of Late Cretaceous echinoids from the Maastrichtian Type area (The Netherlands, Belgium). Shark versus echinoid: failed predation on the holasteroid Hemipneustes. Bulletin of the Mizunami Fossil Museum 47: 49–57.
Dragonfly 2020. Object Research Systems (ORS) Inc, Montreal, Canada, 2020; software available at http://www.theobjects.com/dragonfly.
Ehret, D.J. and Harrell Jr, T.L. 2018. Feeding traces on a Pteranodon (Reptilia: Pterosauria) bone from the Late Cretaceous (Campanian) Mooreville Chalk in Alabama, USA. Palaios 33: 414–418. Crossref
Ehret, D.J., MacFadden, B.J., Jones, D.S., DeVries, T.J., and Salas-Gismondi, R. 2009. Caught in the act: trophic interactions between a 4-million-year-old white shark (Carcharodon) and mysticete whale from Peru. Palaios 24: 329–333. Crossref
Everhart, M.J. 2004. Late Cretaceous interaction between predators and prey. Evidence of feeding by two species of shark on a mosasaur. PalArch, Vertebrate Palaeontology 1 (1): 1–7.
Everhart, M.J. 2005. Bite marks on an elasmosaur (Sauropterygia; Plesiosauria) paddle from the Niobrara Chalk (Upper Cretaceous) as probable evidence of feeding by the lamniform shark, Cretoxyrhina mantelli. PalArch, Vertebrate Palaeontology 2 (2): 14–24.
Everhart, M.J., Everhart, P.A., and Shimada, K. 1995. A new specimen of shark bitten mosasaur vertebrae from the Smoky Hill Chalk (Upper Cretaceous) in western Kansas. In: 127th Annual Meeting, Abstracts. Kansas Academy of Science 14: 19.
Feichtinger, I., Fritz, I., and Göhlich, U.B. 2021. Tiger shark feeding on sirenian—first fossil evidence from the middle Miocene of the Styrian Basin (Austria). Historical Biology [published online, https://doi.org/10.1080/08912963.2021.1906665]. Crossref
Gernant, R.E., Gibson, T.G., and Whitmore, F.C. Jr. 1971. Environmental history of Maryland Miocene. In: Maryland Geological Survey Guidebook 3, 1–58 pp. The Johns Hopkins University, Baltimore.
Gibson, T.G. 1967. Stratigraphy and paleoenvironment of the phosphatic Miocene strata of North Carolina. Geological Society of America Bulletin 78: 631–650. Crossref
Gilmore, R.G. 1993. Reproductive biology of lamnoid sharks. Environmental Biology of Fishes 38 (1): 95–114. Crossref
Gilmore, R.G., Putz, O., and Dodrill, J.W. 2005. Oophagy, intrauterine cannibalism and reproductive strategy in lamnoid sharks. Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras 3: 435–462.
Godfrey, S.J. 2003. Miocene sharks and smoking guns… Bugeye Times 28 (1): 1, 5–7.
Godfrey, S.J. and Altman, J. 2005. A Miocene cetacean vertebra showing a partially healed compression fracture, the result of convulsions or failed predation by the Giant White Shark, Carcharodon megalodon. Jeffersoniana 16: 1–12.
Godfrey, S.J. and Palmer, B.T. 2015. Gar-bitten coprolite from South Carolina, USA. Ichnos 22 (2): 103–108. Crossref
Godfrey, S.J. and Smith, J.B. 2010. Shark-bitten vertebrate coprolites from the Miocene of Maryland. Naturwissenschaften 97: 461–467. Crossref
Godfrey, S.J., Ellwood, M., Groff, S., and Verdin, M.S. 2018. Carcharocles-bitten odontocete caudal vertebrae from the coastal Eastern United States. Acta Palaeontologica Polonica 63: 463–468. Crossref
Godfrey, S.J., Nance, J.R., and Riker, N.L. 2021. Otodus-bitten sperm whale tooth from the coastal Eastern United States. Acta Palaeontologica Polonica 66: 599–603. Crossref
Govender, R. 2015. Shark-cetacean trophic interaction, Duinefontein, Koeberg, (5 Ma), South Africa. South African Journal of Science 111 (11–12): 1–7. Crossref
Govender, R. and Chinsamy, A. 2013. Early Pliocene (5 Ma) shark-cetacean trophic interaction from Langebaanweg, Western Coast of South Africa. Palaios 28: 270–277. Crossref
Gruber, S.H. 1988. Sharks of the shallows. Natural History 97: 50–59.
Gudger, E.W. 1932. Cannibalism among the sharks and rays. The Scientific Monthly 34: 403–419.
Hamlett, W.C. 2011. Oviducal glands in chondrichthyans. In: W.C. Hamlett (ed.), Reproductive Biology and Phylogeny of Chondrichthyes 3, 311–346. CRC Press, Boca Raton, Flroida. Crossref
Hamlett, W.C. and Hysell, M.K. 1998. Uterine specializations in elasmobranchs. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 282: 438–459. Crossref
Hanks, H.D. and Shimada, K. 2002. Vertebrate fossils, including non-avian dinosaur remains and the first shark-bitten bird bone, from a Late Cretaceous (Turonian) marine deposit of northeastern South Dakota. Journal of Vertebrate Paleontology 22 (Supplement to Number 3): 62A.
Hasse, J.C.F. 1879–1885. Das natürliche System der Elasmobranchier auf Grundlage des Baues und der Entwicklung ihrer Wirbelsäule: eine morphologische und paläontologische Studie 1. 382 pp. G. Fischer, Jena. Crossref
Hone, D.W., Witton, M.P., and Habib, M.B. 2018. Evidence for the Cretaceous shark Cretoxyrhina mantelli feeding on the pterosaur Pteranodon from the Niobrara Formation. PeerJ 6: e6031. Crossref
Hoyos-Padilla, M., Papastamatiou, Y.P., O’Sullivan, J., and Lowe, C.G. 2013. Observation of an attack by a cookiecutter shark (Isistius brasiliensis) on a white shark (Carcharodon carcharias). Pacific Science 67 (1): 129–134. Crossref
Jacobsen, A.R. and Bromley, R.G. 2009. New ichnotaxa based on tooth impressions on dinosaur and whale bones. Geological Quarterly 53: 373–382.
Kallal, R.J., Godfrey, S.J., and Ortner, D.J. 2012. Bone reactions on a Pliocene cetacean rib indicate short‐term survival of predation event. International Journal of Osteoarchaeology 22: 253–260. Crossref
Kent, B.W. 1994. Fossil Sharks of the Chesapeake Bay Region. 146 pp. Egan Rees & Boyer, Inc. Columbia.
Kent, B.W. 2018. The cartilaginous fishes (chimaeras, sharks and rays) of Calvert Cliffs, Maryland, USA. In: S.J. Godfrey (ed.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA. Smithsonian Contributions to Paleobiology 100: 45–160.
Kidwell, S.M. 1984. Outcrop features and origin of basin margin unconformities in the lower Chesapeake Group (Miocene), Atlantic Coastal Plain. In: J.S. Schlee (ed.), Interregional Unconformities and Hydrocarbon Accumulation. American Association of Petroleum Geologists Memoir 36: 37–58. Crossref
Kidwell, S.M. 1988. Reciprocal sedimentation and noncorrelative hiatuses in marine-paralic siliciclastics: Miocene outcrop evidence. Geology 16: 609–612. Crossref
Kidwell, S.M. 1989. Stratigraphic condensation of marine transgressive records: Origin of major shell deposits in the Miocene of Maryland. The Journal of Geology 97: 1–24. Crossref
Kidwell, S.M. 1997. Anatomy of extremely thin marine sequences landward of a passive-margin hinge zone: Neogene Calvert Cliffs succession, Maryland, U.S.A. Journal of Sedimentary Research 67: 222–240. Crossref
Kidwell, S.M., Powars, D.S., Edwards, L.E., and Vogt, P.R. 2015. Miocene stratigraphy and paleoenvironments of the Calvert Cliffs, Maryland. The Geological Society of America Field Guide 40: 231–279. Crossref
Konuki, R. 2008. Biostratigraphy of Sea Turtles and Possible Bite Marks on a Toxochelys (Testudine, Chelonioidea) From the Niobrara Formation. M.Sc. Thesis, Forth Hays State University, Hays, Kansas. Available at https://scholars.fhsu.edu/theses/3064
Kozuch, L. and Fitzgerald, C. 1989. A guide to identifying shark centra from southeastern archaeological sites. Southeastern Archaeology 8: 146–157.
Mapes, R.H. and Chaffin, D.T. 2003. Predation on cephalopods. In: P.H. Kelley, M. Kowalewski, and T.A. Hansen (eds.), Predator—Prey Interactions in the Fossil Record, 177–213. Springer, Boston. Crossref
Mapes, R.H. and Hansen, M.C. 1984. Pennsylvanian shark‐cephalopod predation: a case study. Lethaia 17: 175–183. Crossref
Mapes, R.H., Sims, M.S., and Boardman, D.R. 1995. Predation on the Pennsylvanian ammonoid Gonioloboceras and its implications for allochthonous vs. autochthonous accumulations of goniatites and other ammonoids. Journal of Paleontology 69: 441–446. Crossref
Mierzwiak, J.S. and Godfrey, S.J. 2019. Megalodon-bitten whale rib from South Carolina. The Ecphora 34 (2): 15–20.
Mikuláš, R., Kadlecová, E., Fejfar, O., and Dvořák, Z. 2006. Three new ichnogenera of biting and gnawing traces on reptilian and mammalian bones: a case study from the Miocene of the Czech Republic. Ichnos 13 (3): 113–127. Crossref
Mollet, H.F., Cliff, G., Pratt Jr., H.L., and Stevens, J. 2000. Reproductive biology of the female shortfin mako, Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of lamnoids. Fishery Bulletin 98: 299–318.
Muñiz, F., Belaústegui, Z., Toscano, A., Ramirez-Cruzado, S., and Gámez Vintaned, J.A. 2020. New ichnospecies of Linichnus Jacobsen & Bromley, 2009. Ichnos 27 (3): 344–351. Crossref
Neumann, C. 2000. Evidence of predation on Cretaceous sea stars from north‐west Germany. Lethaia 33: 65–70. Crossref
Noriega, J.I., Cione, A.L., and Aceñolaza, F.G. 2007. Shark tooth marks on Miocene balaenopterid cetacean bones from Argentina. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 245: 185–192. Crossref
Parsons, G.R., Hoffmayer, E.R., Hendon, J.M., Bet-Sayad, W.V., Rocha, M.J. Arukwe, A., and Kapoor, B.G. 2008. A review of shark reproductive ecology: life history and evolutionary implications. In: M.A. Rocha, A. Arukwe, and B.G. Kapoor (eds.), Fish Reproduction, 435–469. Science Publishers Inc., Enfield.
Perez, V.J., Godfrey, S.J., Kent, B.W., Weems, R.E., and Nance, J.R. 2018. The transition between Carcharocles chubutensis and Carcharocles megalodon (Otodontidae, Chondrichthyes): lateral cusplet loss through time. Journal of Vertebrate Paleontology 38 (6): e1546732. Crossref
Perez, V.J. 2020. Self-bitten snaggletooth. Ecphora 35: 12.
Purdy, R.W. 1996. Paleoecology of fossil white sharks. In: A.P. Klimley and D.G. Ainley (eds.), Great White Sharks: The Biology of Carcharodon carcharias, 67–78. Academic Press, San Diego. Crossref
Purdy, R.W., Schneider, V.P., Applegate, S.P., McLellan, J.H., Meyer, R.L., and Slaughter, B.H. 2001. The Neogene sharks, rays, and bony fishes from Lee Creek Mine, Aurora, North Carolina. In: C.E. Ray and D.J. Bohaska (eds.), Geology and Paleontology of the Lee Creek Mine, North Carolina. Smithsonian Contributions to Paleobiology 90: 71–202.
Renz, M. 2002. Megalodon: Hunting the Hunter. 159 pp. PaleoPress, Lehigh Acres.
Ridewood, W.G. 1921. On the calcification of the vertebral centra in sharks and rays. Philosophical Transactions of the Royal Society of London, Series B 210 (372–381): 311–407. Crossref
Rothschild, B.M. and Martin, L.D. 1993. Paleopathology Disease in the Fossil Record. 386 pp. CRC Press, Boca Raton, Florida.
Rothschild, B.M., Martin, L.D., and Schulp, A.S. 2005. Sharks eating mosasaurs, dead or alive? Netherlands Journal of Geosciences 84: 335–340. Crossref
Schwimmer, D.R., Stewart, J.D., and Williams, G.D. 1997. Scavenging by sharks of the genus Squalicorax in the Late Cretaceous of North America. Palaios 12: 71–83. Crossref
Shattuck, G.B. 1904. Geological and paleontological relations, with a review of earlier investigations. In: W.B. Clark, G.B. Shattuck, and W.H. Dall (eds.), The Miocene Deposits of Maryland, 33–87. Maryland Geological Survey, Baltimore.
Shimada, K. 1997a. Dentition of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli, from the Niobrara Chalk of Kansas. Journal of Vertebrate Paleontology 17: 269–279. Crossref
Shimada, K. 1997b. Paleoecological relationships of the Late Cretaceous lamniform shark, Cretoxyrhina mantelli (Agassiz). Journal of Paleontology 71: 926–933. Crossref
Shimada, K. and Everhart, M.J. 2004. Shark-bitten Xiphactinus audax (Teleostei: Ichthyodectiformes) from the Niobrara Chalk (Upper Cretaceous) of Kansas. The Mosasaur 7: 35–39.
Shimada, K. and Hooks, G.E. III. 2004. Shark-bitten protostegid turtles from the Upper Cretaceous Mooreville Chalk, Alabama. Journal of Paleontology 78: 205–210. Crossref
Shimada, K., Bonnan, M.F., Becker, M.A., and Griffiths, M.L. 2021. Ontogenetic growth pattern of the extinct megatooth shark Otodus megalodon—implications for its reproductive biology, development, and life expectancy. Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1861608] Crossref
Shimada, K., Everhart, M.J., and Hooks, G.E. III. 2002. Ichthyodectid fish and protostegid turtle bitten by the Late Cretaceous lamniform shark, Cretoxyrhina mantelli. Journal of Vertebrate Paleontology 22 (Supplement to 3): 106A.
Snelson Jr., F.W., Mulligan, T.J., and Williams, S.E. 1984. Food habits, occurrence, and population structure of the bull shark, Carcharhinus leucas, in Florida coastal lagoons. Bulletin of Marine Science 34: 71–80.
Springer, S. 1967. Social organization of shark population. In: P.W. Gilbert, R.F. Matheson, and D.P. Rall (eds.), Sharks, Skates, and Rays, 149–174. John Hopkins Press, Baltimore.
Stevens, J.D. 1984. Biological observations on sharks caught by sport fisherman of New South Wales. Marine and Freshwater Research 35: 573–590. Crossref
Stewart, J.D. 1993. The case of the sword-swallowing shark. Terra 31: 42–43.
Takakuwa, Y. 2014. A dense occurrence of teeth of fossil “mako” shark (“Isurus” hastalis: Chondrichthyes, Lamniformes), associated with a balaenopterid-whale skeleton of the Late Miocene Pisco Formation, Peru, South America. Bulletin of the Gunma Museum of Natural History 18: 77–86.
Türtscher, J., López-Romero, F.A., Jambura, P.L., Kindlimann, R., Ward, D.J., and Kriwet, J. 2021. Evolution, diversity, and disparity of the tiger shark lineage Galeocerdo in deep time. Paleobiology 47: 574–590. Crossref
Uchida, S., Toda, M., Teshima, K., and Yano, K. 1996. Pregnant white sharks and full-term embryos from Japan. In: A.P. Klimley and D.G Ainley (eds.), Great White Sharks: The Biology of Carcharodon carcharias, 139–155. Academic Press, San Diego, California. Crossref
Vallon, L.H., Rindsberg, A.K., and Martin, A.J. 2015. The use of the terms trace, mark and structure. Annales Societatis Geologorum Poloniae 85: 527–528. Crossref
Van der Elst, R.P. 1979. A proliferation of small sharks in the shore-based Natal sport fishery. Environmental Biology of Fishes 4 (4): 349–362. Crossref
Visaggi, C.C. and Godfrey, S.J. 2010. Variation in composition and abundance of Miocene shark teeth from Calvert Cliffs, Maryland. Journal of Vertebrate Paleontology 30: 26–35. Crossref
Vogt, P.R. and Parrish, M. 2012. Driftwood dropstones in Middle Miocene Climate Optimum shallow marine strata (Calvert Cliffs, Maryland Coastal Plain): Erratic pebbles no certain proxy for cold climate. Palaeogeography, Palaeoclimatology, Palaeoecology 323: 100–109. Crossref
Vogt, P.R., Eshelman, R.E., and Godfrey, S.J. 2018. Calvert Cliffs: eroding mural escarpment, fossil dispensary, and paleoenvironmental archive in space and time. Smithsonian Contributions to Paleobiology 100: 3–44.
Ward, L.W. and Andrews, G.W. 2008. Stratigraphy of the Calvert, Choptank, and St. Marys Formations (Miocene) in the Chesapeake Bay area, Maryland and Virginia. Virginia Museum of Natural History, Memoir Number 9: 1–170.
Whitney, N.M. and Crow, G.L. 2007. Reproductive biology of the tiger shark (Galeocerdo cuvier) in Hawaii. Marine Biology 151 (1): 63–70. Crossref
Williams, M.E. 1990. Feeding behavior in Cleveland shale fishes. In: A.J. Boucot (ed.), Evolutionary Paleobiology of Behavior and Coevolution, 273–287. Elsevier, Amsterdam.
Zachos, J.C., Dickens, G.R., and Zeebe, R.E. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451: 279–283. Crossref
Acta Palaeontol. Pol. 66 (4): 847–856, 2021
https://doi.org/10.4202/app.00911.2021