

A new buzzard from the late Pliocene of Argentina
FEDERICO J. DEGRANGE, CLAUDIA P. TAMBUSSI, MATÍAS L. TAGLIORETTI, and FERNANDO A. SCAGLIA
Degrange, F.J., Tambussi, C.P., Taglioretti, M.L., and Scaglia, F.A. 2021. A new buzzard from the late Pliocene of Argentina. Acta Palaeontologica Polonica 66 (4): 779–787.
We describe a new species of a large buzzard (Accipitridae), Buteo dondasi sp. nov. from the late Pliocene of Buenos Aires Province, Argentina represented by an incomplete left hind limb, including a distal fragment of tibiotarsus, tarsometatarsus, fragment of os metatarsale I, and toes I and II. The new taxon exhibits characteristics of the crown group Accipitridae and the shape of the tarsometarsus allows its assignment to the genus Buteo. The new species represents the very first record of a representative of a non-scavenging diurnal bird of prey for the Chapadmalalan and one of the largest accipitrids (~ 2.3 kg) known for Argentina.
Key words: Aves, Accipitriformes, Buteo, zoophagy, Pliocene, Atlantic coast, Argentina.
Federico J. Degrange [fjdino@gmail.com]and Claudia P. Tambussi [tambussi.claudia@conicet.gov.ar], Centro de Investigaciones en Ciencias de la Tierra (CICTERRA), Universidad Nacional de Córdoba, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Avenida Vélez Sársfield 1611, X5016GCA, Córdoba, Argentina.
Matías L. Taglioretti [paleomat@gmail.com], Museo Municipal de Ciencias Naturales Lorenzo Scaglia, Instituto de Geología de Costas y del Cuaternario (CIC-UNMdP), Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Deán Funes 3250, B7602AYJ, Mar del Plata, Argentina.
Fernando A. Scaglia [feroscaglia@gmail.com], Museo Municipal de Ciencias Naturales Lorenzo Scaglia, Av. Libertad 3099, B7600HJB, Mar del Plata, Argentina.
Received 11 August 2021, accepted 21 October 2021, available online 24 November 2021.
Copyright © 2021 F.J. Degrange et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Buzzards, hawks, kites, Old-world vultures and eagles are powerful diurnal predators, members of the almost cosmopolitan Accipitridae family living in most habitats throughout the world. Their similarities include hooked beaks, strong legs endowed with strongly curved claws, and forward-facing eyes for stereoscopic vision. They tend to kill their prey using their strong talons, with which they squeeze their victims, suffocating them by compression or piercing vital organs (Sustaita 2008).
Their fossil record begins in the Eocene of Belgium (Mayr and Smith 2019) and although scarce, it includes only remains from Cenozoic deposits (30–50 million years). At the end of the Miocene (~2.5 million years), many of the modern forms of these raptors had already occurred. The fossil record is abundant in North America (e.g., Cracraft 1969; Mayr and Perner 2020) but comparatively poor in South America, where the gap of the knowledge of the evolution of this group stands out.
The oldest record of an accipitrid from Argentina coming from Oligocene beds (Deseadan) from the Río Chico locality in Patagonia (eroded ungual phalanx, ACM 3809). Other early fossil records referable to Accipitridae come from the lower Miocene of Chubut Province (a Buteoninae similar to Geranoaetus, Picasso et al. 2009); the Miocene of Toro Negro Formation (Vinchinavis paka, Tambussi et al. 2021); the late Miocene of the Arroyo Chasicó Formation (Buteoninae indet., Mosto et al. 2007), the Mio-Pliocene Andalhualá Formation (Geranoaetus cf. ales) and the Pleistocene of Miramar Formation (G. melanoleucus, Agnolín 2006).
The Chapadmalalan Formation shows one of the richest fossil bird assemblages in South America, including Rheidae, Tinamidae, Phorusrhacidae, Cathartidae, Charadriidae, Scolopacidae and Passeriformes Furnariidae (Tambussi 2011; Tambussi and Degrange 2013), with the last group being very well documented (Tonni and Noriega 2001; Stefanini et al. 2016). This association is dominated by carnivores or scavengers, most of which shows large or gigantic body-sizes. Various species of Phorusrhacidae such as Llallawavis scagliai Degrange, Tambussi, Taglioretti, Dondas, and Scaglia, 2015, of 18 kg, Mesembriornis milneedwardsi Moreno, 1889, of about 70 kg and a Mesembriornithinae indet. (Psilopterinae indet. according to Tambussi and Noriega 1996) of ~21 kg are avian ground predator representatives. Among scavengers, Dryornis pampeanus Moreno and Mercerat, 1891, a cathartiform that reached 26 kg, being one of the largest bird that ever lived (Degrange et al. 2021); and by a putative Teratornithidae (Agnolín 2016) are included.
New absolute ages information for the Chapadmalal Formation (Prevosti et al. 2021) allow us to interpret that the lower allomember of the Playa Los Lobos Alloformation (Zárate and Fasano 1989) developed under the mid-Piacenzian warm period 3.3–3.0 mya. including the M2 to KM5 marine isotopic stage transition (de la Vega et al. 2020), where the bulk of the Chapadmalalan Formation avian fauna was collected. On the other hand, the avian record from the Chapadmalal Formation indicates an arid or semiarid palaeoclimate and a palaeohabitat consisting of grasslands and forest patches, next to freshwater environments (Tambussi 2011).
Here we present a new species of a large buzzard, Buteo dondasi sp. nov. from the late Pliocene of Chapadmalal, Atlantic coast of Buenos Aires Province, Argentina, and evaluate its role in the paleocommunity of which this buzzard was a member.
Institutional abbreviations.—ACM, Beneski Museum of Natural History, Massachusetts USA; AMNH, American Museum of Natural History, Ornithology Collection, New York, USA; CIT-O, Colección de Aves Actuales of CICTERRA, Córdoba, Argentina; MMP, Museo Municipal de Ciencias Naturales Lorenzo Scaglia, Mar del Plata, Buenos Aires, Argentina; OUVC, Ohio University Vertebrate Collections, Athens, USA; YPM-ORN.O, Yale Peabody Museum, Ornithology Collection, New Haven, USA.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: pub:B1006830-A9FB-42A3-94DD-7BCD0A97FA37
Material and methods
If not indicated otherwise, osteological terminology follows Baumel and Witmer (1993).
Accipitriformes examined.—Accipiter cirrhocephalus AMNH 30840; Accipiter cooperii AMNH 20741, 23723, 26653, OUVC 9804, 10312, 10772, 10834, 10757; Accipiter fasciatus AMNH 20743, 29882; Accipiter gentilis AMNH 20740, 23722, 31661; Accipiter gundlachi AMNH 31642; Accipiter imitator AMNH 23402; Accipiter meyerianus AMNH 16504; Accipiter nisus AMNH 27048, 27049; Accipiter novaehollandiae AMNH 28019; Accipiter striatus AMNH 16440, 24218, 28383, OUVC 10651; Aegypius monachus AMNH 1939, 28556; Aquila chrysaetos AMNH 11, 50, 2039, 3581, 13968, 13969; Aquila pomarina AMNH 3917; Aquila rapax AMNH 495, 1339, 4052; Aquila verreauxi AMNH 1375; Aquila wahlbergi AMNH 3906; Aviceda cuculoides AMNH 8968; Busarellus nigricollis AMNH 11736; Buteo albononatus AMNH 11736, 12646, 16562; Buteo augularis AMNH 4196; Buteo buteo AMNH 2021, 23691, 23692, 31167; Buteo jamaicensis AMNH 19748, 21940, 24514, OUVC 9788, 9790, 9791, 10229, 10315, 10506, 10507, 10907, 10986; Buteo lagopus AMNH 26561; Buteo lineatus AMNH 26691, 26692, 26694, OUVC 10773; Buteo magnirostris AMNH 12647; Buteo platypterus AMNH 24515, 26557, 26558; Buteo polyosoma AMNH 8511; Buteo regalis AMNH 1946, 19629, 25319, OUVC 10313; Buteo rufofuscus AMNH 4054; Buteo swansoni AMNH 25317, 27921; Buteogallus coronatus CIT-O272; Buteogallus urubitinga AMNH 12066; Circus aeruginosus AMNH 24085, 29825; Circus cyaneus AMNH 22598, 24516, 26039, 26040, 26554, OUVC 9654; Circus pygargus AMNH 4209; Elanoides forficatus AMNH 22823, OUVC 10997; Elanus axillaris AMNH 29879; Elanus caeruleus AMNH 22920; Elanus leucurus CIT-O9; Gampsonyx swansonii AMNH 4198; Geranoaetus melanoleucus AMNH 2667, 3914, 8827, CIT-O1, 641, 696; Gypaetus barbatus AMNH 2738, 2870, 5299; Gypohierax angolensis AMNH 2006, 2042, 2320, 3905; Gyps africanus AMNH 28658; Gyps fulvus AMNH 504, 2030, 5378; Haliaeetus albicilla AMNH 27329; Haliaeetus leucocephalus AMNH 616, 975, 18813, 24193; Haliaeetus leucogaster AMNH 2024; Haliaeetus pelagicus AMNH 6277; Haliaeetus vocifer AMNH 27389, individual without collection number; Haliastur indus AMNH 29708; Haliastur sphenurus AMNH 1943, 29880; Harpia harpyja AMNH 496, 1383, 2041, 9579; Heterospizias meridionalis AMNH 2043, 8600, 15003; Hieraaetus morphnoides AMNH 29883; Ictinia plumbea AMNH 4342, 22854; Kaupifalco monogrammicus AMNH 4155; Leucopternis albicollis AMNH 5200, 20577, 20578; Lophoictinia isura AMNH 3857, 29881; Machaeramphus andersoni AMNH 3911; Milvus migrans AMNH 9593, 30796; Morphnus guianensis AMNH 1376; Necrosyrtes monachus AMNH 4136, 31377; Neophron percnopterus AMNH 2733; Pandion haliaetus AMNH 1029, 24588, 26217, 26364, 26365, 27190, 27225; Parabuteo unicinctus AMNH 19590, 26504, 30229; Pernis apivorus AMNH 31665; Rostrhamus sociabilis AMNH 4341, 25873; Sagittarius serpentarius AMNH 1306, 4006, 4253, 9165, 22684, YPM-ORN.O 102276, 105490, 111125; Sarcogyps calvus AMNH 623, 5277; Spilornis cheela AMNH 5063; Stephanoaetus coronatus AMNH 3909, 4256; Terathopius ecaudatus AMNH 4306; Torgos tracheliotus AMNH 2990; Trigonoceps occipitalis AMNH 3908.
Systematic palaeontology
Class Aves Linnaeus, 1758
Subclass Neognathae Pycraft, 1900
Order Accipitriformes Voous, 1973
Family Accipitridae Vieillot, 1816
Genus Buteo Lacépède, 1799
Type species: Buteo buteo (Linnaeus, 1758); Recent, Europe.
Buteo dondasi sp. nov.
Figs. 1–3.
Zoobank LSID: urn:lsid:zoobank.org:pub:B1006830-A9FB-42A3-94 DD-7BCD0A97FA37
Etymology: Named after Alejandro Dondas (1948–2016) who was the curator of the Paleontology Collection housed in the Museo Municipal de Ciencias Naturales Lorenzo Scaglia. At the age of 18, Alejandro established close relationships with Galileo Scaglia, director of the Scaglia Museum, and quickly began his first steps in curating his paleontological collection, until his death in 2016. He left a profound legacy on the responsibility of expanding, caring for and improving one of the most important paleontological collections in South America. The specific epithet is formed from the surname “Dondas,” taken as a noun in the genitive case, plus the Latin suffix “i” (according to the ICZN, 1999, article 31.1.2)
Holotype: MMP 5730, an incomplete left hind limb consisting in a distal fragment of tibiotarsus, tarsometatarsus, fragmentary os metatarsale I, and toes I and II.
Type locality: La Estafeta Beach, Mar del Plata, Buenos Aires Province, Argentina (Degrange et al. 2021: fig. 1); S38°10’1.21”, W57°38’3.83”.
Type horizon: Chapadmalal Formation, lowest part of Playa Los Lobos Alloformation (Paleosoil 6 of Zárate and Fasano 1989; levels 9–10 of Kraglievich 1952), 3.0 million years (Prevosti et al. 2021), Piacenzian, late Pliocene.
Material.—Holotype only.
Diagnosis.—A large buzzard with the following combination of characters (exclusive features are indicated with an *): tibiotarsus with a very marked elliptical scar on the medial side of the diaphysis, corresponding to one of the two scars of the tuberositas retinaculi extensoris, located on the medial side of the shaft; tarsometarsus with the lateral impressio retinaculi extensorii located more distally than the medial impression*, the medial foramen vasculare proximale, on ventral aspect, opens laterally to the crista medialis hypotarsi*, shallower notch for the nervus peroneus than other Buteoninae; the trochlea metatarsi III is more extended distally than the trochlea II; in the phalanx ungualis II both foramina opens into deep pits*.
The new species Buteo dondasi sp. nov. differs from Buteogallus, a genus commonly registered by several fossil species of large body size in the northern Neotropical region (Campbell 1979; Emslie and Czaplewski 1999; Suárez 2004; Suárez and Olson 2007; Olson 2007, 2008; Steadman et al. 2019) in that the crista medialis hypotarsi forms an angle of 65° regarding the shaft, and it is not perpendicular as in the latter. Also, Buteo dondasi sp. nov. shares with species of Amplibuteo, the presence of a lateral impressio retinaculi extensorii located more distally than the medial, an uncommon feature among Accipitridae (FJD personal observations). However, it differs from species of Amplibuteo in having a less proximally extended eminentia intercotylatris, a markedly smaller foramen vasculare distale, and the trochlea metatarsi IV more extended distally. Buteo dondasi sp. nov. differs from species of Geranoaetus in having the medial impressio retinaculi extensorii located more proximally than the lateral, a shallower notch for the nervus peroneus, a wider furrow that continues proximally to the foramen vasculare distale, and by the presence of a foramen at the base of the middle prominence of the phalanx ungualis II. Parabuteo differs from Buteo dondasi sp. nov. in having the cotyla lateralis much narrower, the trochlea metatarsorum III and IV equally extended distally, the crista medialis hypotarsi more elongated proximodistally and by the presence of a narrow and elongated fossa, located medially to the fossa infracotylaris dorsalis. Species of Leucopternis possess an ossified retinaculi extensorii, the tuberositas m. tibialis cranialis is more medially located, the crista medialis hypotarsi is wider and poorly extended ventrally, the sulcus hypotarsis is narrower, and the trochlea metatarsi IV is more extended distally than the III. The holotype of Vinchinavis paka Tambussi, Degrange, Ciccioli and Prevosti, 2021, does not have an overlap of materials with those of Buteo dondasi sp. nov., therefore, comparisons are not possible. Vinchinavis was considered similar to Buteogallus in the original description, which makes it unlikely the assignment of MMP 5730 to this fossil taxon.
Description.—Tibiotarsus: Only the left extremitas distalis tibiotarsi is preserved (Fig. 1). The sulcus extensorius is narrow and deep (wider in species of Aquila, Gyps, Gypaetos, Gypohierax, and Buteo). The canalis extensorius is narrow, opening medially, over the condylus medialis. This opening has a triangular shape as in species of Geranoaetus, Haliaeetus (except for Haliaeetus vocifer Daudin, 1800), Buteo and Harpia (rounded in species of Spilornis, Buteogallus, Gyps, Gypaetos, Torgos, Elanus, and Circus). The pons supratendineus is stout (slender in species of Elanus, Gypaetos, Spilornis, Terathopius, and Gampsonax; stouter in Gyps and Gypohierax), strongly slanted proximomedially, endowed with a small distal lip (more developed in species of Circus). Medially to the sulcus extensorius, there is an ovalated and well-marked scar (as in species of Geranoaetus and Buteogallus, variable in Haliaeetus, more distally located in Sarcogyps and Torgos, proportionally longer in species of Gypohierax, rounded in species of Aquila, Buteo, Gyps, and Aegypius, placed on the medial margin of the shaft in species of Harpia, poorly marked in species of Gypaetus and Milvus). This corresponds to one of the two scars of the tuberositas retinaculi extensoris. The second scar is broad and located on the base of the pons, laterodistally to it. The trochlea cartilaginis tibialis is wide and shallow (deeper in species of Aquila, Buteo, and Harpia, almost flat in species of Gyps). The crista trochleae medialis is smooth, proximomedially directed, while the crista trochleae lateralis is vertically disposed, forming a proximally abruptly flange medially directed. The depressio epicondylaris lateralis is wide and deep. Proximally to this depressio, a laterally facing furrow is observed (more cranially facing in species of Buteo), marked by a sharp cranial edge (strongly projected laterally in species of Stephanoaetus) and a blunt caudal edge (strongly projected laterally in species of Leucopternis). The latter corresponds to the tuberculum retinaculi m. fibularis, which is vertically disposed. The sulcus m. fibularis is shallow and poorly marked as in species of Buteogallus and Buteo (deeper in species of Trigonoceps and Haliaeetus, shallower in species of Aquila). The epicondylus medialis is elliptical and well developed (poorly developed in species of Gypohierax, Kaupifalco, and Ictinia), distally located (more proximally located in species of Aquila and Gyps, more cranially located in species of Buteo and Gypaetos), strongly projected medially as is typical of Accipitridae. The condylus lateralis is subrounded when viewed laterally (more rounded in species of Accipiter and Aegypius). The condylus medialis is low (taller in species of Aquila), medially projected (strongly medially projected in species of Necrosyrtes), and is more extended cranially than the condylus lateralis (almost equally extended in species of Morphnus and Torgos). Both condyli are quite wide when viewed cranially and are delimited by stout and smooth edges. The incisura intercondylaris is wide and deep.
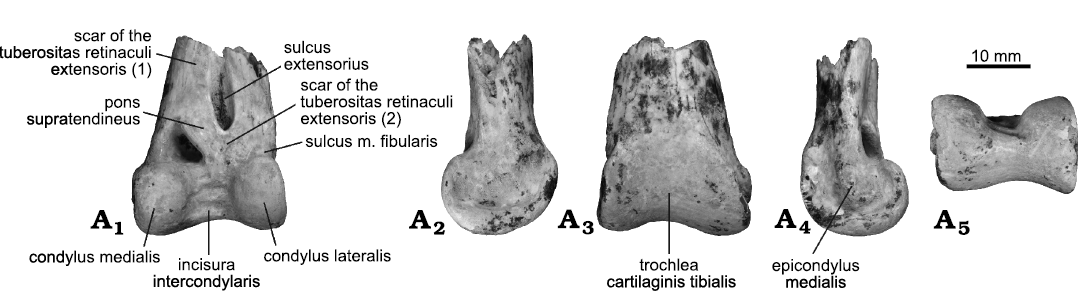
Fig. 1. Left fragmentary tibiotarsus of the buzzard Buteo dondasi sp. nov. (MMP 5730) from Chapadmalal Formation (late Pliocene), La Estafeta Beach, Mar del Plata, Argentina; in cranial (A1), lateral (A2), caudal (A3), medial (A4), and distal (A5) views.
Tarsometatarsus: The tarsometatarsus resembles that of species of Buteo and Buteogallus, being proportionally sturdier than the tarsometatarsus of the latter two taxa, but not that much as in species of Aegypius, Gypaetus, Stephanoaetus, and Harpia or other Buteoninae such as Haliaeetus. The eminentia intercotylaris is poorly developed (Fig. 2, more developed proximally in species of Accipiter and Aquila), as is typical of Accipitridae (Mayr and Perner 2020). The cotyla medialis is a shallow concave depression that has a subcircular contour, medially bordered by a small flange proximo-medially directed. When viewed medially, this cotyla shows on its base a small conic tubercle, which separates it from the crista medialis hypotarsi. The cotyla lateralis is almost flat, subrectangular (deeper in species of Buteo). It has a small flange directed ventro-laterally. This corresponds to the tuberculum m. fibularis brevis. The fossa infracotylaris dorsalis is shallow. The foramina vascularia proximalia are small (large in species of Aquila), and open in this fossa at the same level (as in species of Accipiter, Buteo, and Buteogallus). The impressiones retinaculi extensorii are paired, ridge-like and well-marked (this retinaculi is ossified in species of Pandion and Leucopternis). The medial impressio is located more proximally than the lateral as in species of Buteo (and contrary to other Buteoninae species such as species of Buteogallus and Geranoaetus in which both are located proximally) and the fossil species of Amplibuteo (see Campbell 1979; Suárez 2004; Suárez and Olson 2007). The impressio lig. collateralis lateralis are partially preserved, but it is evident that they are very well marked and located on the lateral surface of the corpus tarsometatarsi. The corpus is elongated and stout as in species of Geranoaetus, Buteo regalis (Gray, 1844), B. jamaicensis (Gmelin, 1788), and species of Buteogallus (more elongated and slender in Accipitrinae and Buteo lineatus (Gmelin, 1788). The sulcus extensorius is concave, narrow and bordered laterally by the tuberositas m. tibialis cranialis, which is elliptical and prominent (more distomedially located in species of Accipiter). The facies subcutanea lateralis is a stout and robust crest. The sulcus hypotarsi is deep, with a rounded dorsal margin. The crista lateralis hypotarsi is robust, with a stout laterally directed tubercle. This crista also presents a small sulcus ligamentosus on its base, poorly extended laterally. As is typical of Buteoninae, the notch for the nervus peroneus is very marked (see Jollie 1977; Mayr and Perner 2020), bounded by a stout and elongated tuberculum m. fibularis brevis (although it is also developed independently in fossil Gypaetinae, see Zhang et al. 2012). This notch, however, is shallower than in species of Buteogallus, Geranoaetus, and Buteo. The crista medialis hypotarsi is strongly projected ventrally, disposed at an angle of 65° posteriad in relation to the shaft, and endowed with a ventral expansion that also extends distally (more than in species of Buteo and Geranoaetus, not extended distally in species of Buteogallus). However, this crista is less extended proximodistally than that species of Buteo, and similar to the condition observed in species of Buteogallus and Geranoaetus. The fossa parahypotarsalis lateralis is narrow, meanwhile the fossa parahypotarsalis medialis is wider. The foramina vascularia proximalia opens ventrally at the same level, but the medial foramen vasculare proximale opens laterally to the crista medialis hypotarsi as in the species of Buteo, Parabuteo, and Haliaeetus (this foramen opens medially in most accipitrids). The sulcus m. fibularis longus is shallow, medially delimited by a small crest. The sulcus flexorius is very deep, laterally bordered by a sharp crista plantaris lateralis strongly projected ventrally, and medially by a sturdier crista plantaris medialis less projected ventrally. The fossa metatarsi I is elliptical and deep. The fossa supratrochlearis plantaris is flat. In the facies dorsalis, the foramen vasculare distale is elliptical and proximally continued on the facies by a conspicuous, wide and deep furrow (shallow and narrow in species of Buteogallus, deep and narrow in Geranoaetus), largely extended proximally as in species of Buteo. The incisurae intertrochlearis lateralis et medialis are narrow. The trochlea metatarsi II has a smooth and curved surface, not furrowed. It is expanded medially through a robust processus medioventrally directed, located ventrally to the conspicuous fovea lig. collateralium. The trochlea metatarsi III shows a shallow furrow (deeper in species of Buteo), of subparallel edges, is slightly directed laterally and is more extended distally than the second as in Buteo regalis, but contrary to Buteo jamaicensis, B. lineatus, species of Buteogallus, and of Geranoaetus. The distal edge of the trochlea metatarsi IV is eroded, but the poorly marked fovea lig. collateralium can be appreciated. This trochlea is ventrally extended through a narrow ventral processus.
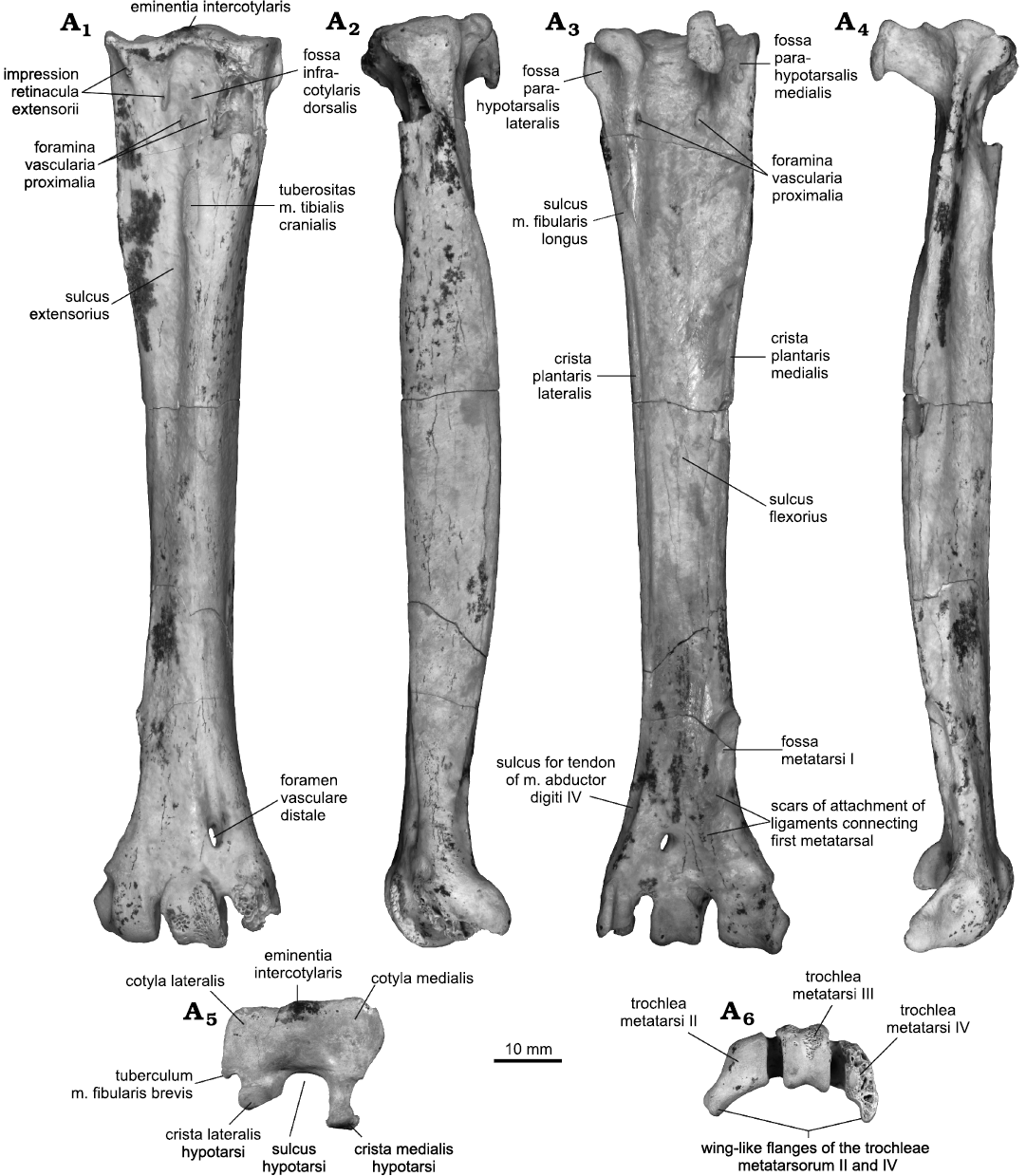
Fig. 2. Left tarsometatarsus of the buzzard Buteo dondasi sp. nov. (MMP 5730) from Chapadmalal Formation (late Pliocene), La Estafeta Beach, Mar del Plata, Argentina; in dorsal (A1), lateral (A2), ventral (A3), medial (A4), proximal (A5), and distal (A6) views.
Os metatarsale I: The processus articularis tarsometatarsalis is lacking (Fig. 3A). It is a short and stout bone with a robust rounded trochlea metatarsi I (similar to Stephanoaetus), strongly extended proximally (poorly extended in species of Aquila, Buteogallus, and of Harpia), ventrally projected and bordered lateroproximally by a deep furrow. Both the tuberculum laterale et mediale are stout. On the dorsal surface, medially located, a conspicuous furrow (shallower in species of Geranoaetus) delimited by two marked crest (stouter in species of Buteogallus) is observed. Laterally to this furrow, the surface is completely concave.
Proximal phalanx I: The phalanx is stout, with a wide and depressed corpus phalangis, slightly curved dorsoventrally (Fig. 3C). The cotyla articularis is flat, wide, with a rounded dorsal edge, flexed distally. The facies dorsalis is convex, whereas the facies plantaris is slightly concave, with a shallow furrow located in the mid-plane. To both sides of the cotyla articularis, two stout tubercles can be identified, being the lateral one more extended distally (also projected distally through a stout accessory processus in species of Haliaeetus). The trochlea articularis is narrow (wide in species of Aquila, Stephanoaetus, Harpia, and Haliaeetus), deep, endowed with a small pit caudoventrally located (deeper in species of Geranoaetus and Buteogallus). In dorsal view, the edges of the trochlea are equally divergent, but when viewed distally, the lateral margin is more directed ventrolaterally than the medial. In lateral view, the trochlea presents a dorsal straight margin and a ventral markedly rounded, projected ventrally. Both fovea lig. collateralium are deep and well-marked.
Phalanx ungualis I: The proximal articulation facet is slightly asymmetric, strongly concave (much more than in species of Harpia), and deep, with a trapezoidal-shape contour (Fig. 3E). The medial cotyla is slightly narrower and less extended distally than the lateral cotyla. Separating both cotyla, a stout dorsoventral ridge can be appreciated. In the ventral area of this ridge, a middle prominence is located. The tuberculum extensorium is very well developed. Anteriorly and on the dorsal and lateral surfaces of the corpus, a strong scar of the position of the keratinous claw case is located. As is typical of Accipitridae (Mosto and Tambussi 2013), the tuberculum flexorium is very stout, ventrocranially directed (more ventrally directed in species of Haliaeetus and Gypaetus). In B. dondasi sp. nov. it is, also, medially directed when viewed ventrally, being narrower than that of species of Aquila. On both sides of the tuberculum, conspicuous oval foramina are located at the same level both ventrally as distally. They are delimited dorsally by a thin bony bridge (thicker in species of Stephanoaetus, Harpia, and Aquila, absent in species of Haliaeetus) that connects the cotylae articularis with the tuberculum flexorium, joining both at the midpoint, on the tuberculum (delimiting also the ventroproximal portion of the corneal case scar). The corpus phalangis is markedly curved ventrally, with a section markedly convex dorsally and straight ventrally. Ventral and dorsal surfaces of the corpus are separated by very sharp edges located on both sides. The sulci neurovasculares are absent. The apex phalangis is pointed.
Proximal phalanx II: It is a short and robust bone (Fig. 3B). The cotyla articularis is dorsoventrally convex and lateromedially concave. The cotyla projects laterally through a long lateral tubercle (more caudally directed in Buteogallus), and medially through a flat distal end tubercle. The trochlea articularis is deep, with divergent margins, delimited dorsally and mediolaterally by a small sharp ridge (poorly marked in species of Buteogallus).
Second phalanx II: It is a robust phalanx (markedly stouter in species of Stephanoaetus and Harpia), with a straighter, narrower and taller corpus than the previous phalanx (Fig. 3D). It has a robust tuberculum extensorium, caudally directed. The facet of articulation is strongly concave and deep, with a sub-triangular outline. The cotyla medialis is more extended dorsally than the lateralis, while the lateralis is slightly more extended ventrally. Small tubercles are located on both sides of the articulation facet, on the ventral surface. Separating both cotylae there is a robust and blunt dorsoventral ridge. The facies dorsalis is convex, whereas the facies plantaris is flat. The trochlea articularis is deep and a small fossa is located both ventrocaudally and dorsocaudally to it. In dorsal view, the margins of the trochlea are divergent, while in distal view they are subparallel. The trochlea presents a straight dorsal margin and a rounded ventral margin, projected ventrally. Both fovea lig. collateralis are deep and well-marked.
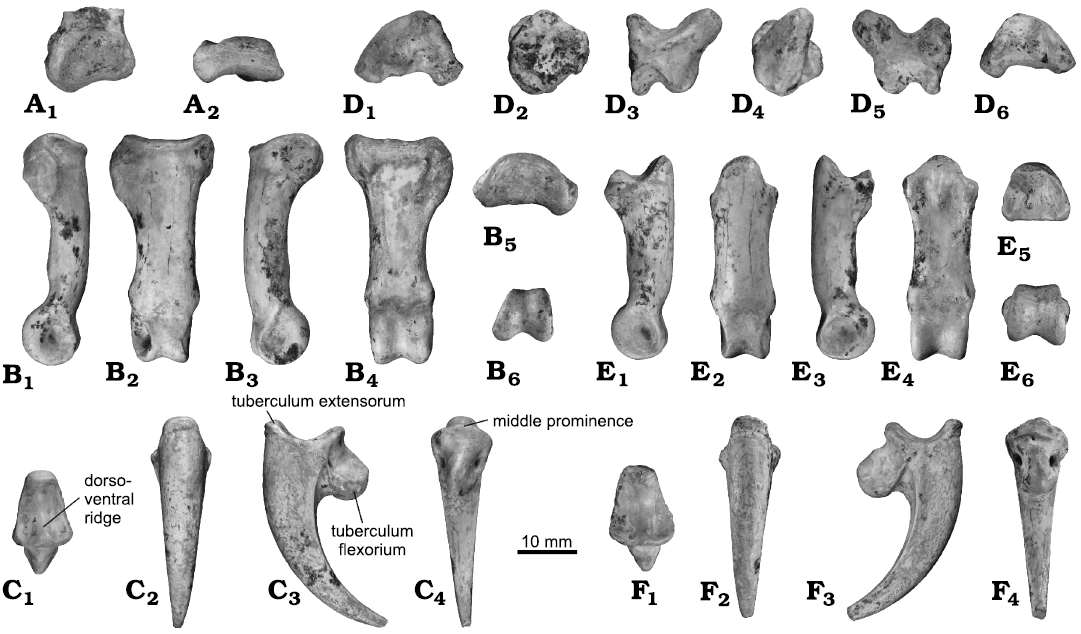
Fig. 3. Partial left pes of the buzzard Buteo dondasi sp. nov. (MMP 5730) from Chapadmalal Formation (late Pliocene), La Estafeta Beach, Mar del Plata, Argentina. A. Left os metatarsale I in dorsal (A1) and distal (A2) views. B. First phalanx of toe I, in lateral (B1), dorsal (B2), medial (B3), ventral (B4), proximal (B5), and distal (B6), views. C. Ungual phalanx of toe I, in proximal (C1), dorsal (C2), medial (C3), and ventral (C4) views. D. First phalanx of toe II, in proximal (D1), lateral (D2), dorsal (D3), medial (D4), ventral (D5), and distal (D6) views. E. Second phalanx of toe II in medial (E1), dorsal (E2), lateral (E3), ventral (E4), proximal (E5), and distal (E6) views. F. Ungual phalanx of toe II, in proximal (F1), dorsal (F2), medial (F3), and ventral (F4) views.
Phalanx ungualis II: The articulation facet is asymmetrical, strongly concave and deep, with a trapezoidal outline (Fig. 3F). Both cotylae are located at the same level, separated by a blunt vertical dorsoventral ridge, located in the middle of the facet. A middle prominence is developed and a small foramen is located cranially to it, on the ventral surface of the phalanx (as in species of Stephanoaetus, two small foramina in species of Harpia, absent in species of Aquila, Geranoaetus, Buteogallus, and Haliaeetus). The tuberculum extensorium is strongly developed. The tuberculum flexorium is very robust, strongly projected cranioventrally (more ventrally directed in species of Gypaetus; more elongated distoproximally in species of Harpia and Stephanoaetus), and medially directed in ventral view. The oval foramina are located at the same level in ventral view on both sides of the tuberculum, although the lateral one is more dorsally. Both foramina open into deep pits (less marked in species of Stephanoaetus), an exclusive feature of this taxon. They are delimited dorsally by a slender bony bridge (thick in species of Stephanoaetus, Harpia, and Aquila, absent in species of Halieetus) that connects the cotylae and borders the tuberculum flexorium dorsally, joining at the midpoint, on top of the tuberculum. The corpus phalangis is markedly curved, with a section markedly convex dorsally and slightly convex ventrally, separated by sharp edges projected to the sides. The sulci neurovasculares are absent.
Stratigraphic and geographic range.—Type locality and horizon only.
Discussion
Together with the general resemblance, the presence of a very marked notch for the nervus peroneus, bounded by a stout and elongated tuberculum m. fibularis brevis, allow us to assign MMP 5730 to Buteoninae, the sister clade of Accipitrinae (Mindell et al. 2018). This last group is characterized by having a more specialized tarsometatarsus, with a slender and usually markedly elongated shaft. Moreover, Accipitrinae possess tuberositas m. tibialis cranialis located on the midline of the shaft, and the foramen vasculare distale is proportionally larger. Among Buteoninae, the new material MMP 5730 can be assigned to Buteo by the following combination of characters: (i) a poorly developed eminentia intercotylaris, (ii) an elongated tuberositas m. tibialis cranialis, (iii) crista hypotarsi medialis disposed at an angle of 65° posteriad in relation to the shaft (following Campbell 1979) and endowed with a ventral expansion that also extends distally, (iv) small foramina vascularia proximalia located equally far proximally, (v) elongated and well-marked paired impressio retinaculi extensorii with the lateral impressio located more distally, (vi) the medial foramen vasculare proximale, on ventral aspect, opening laterally to the crista medialis hypotarsi, (vii) the foramen vasculare distale is continued by a wide and deep furrow largely extended proximally, and (viii) a stout corpus.
Buteo is considered to have originated in South America, with subsequent dispersals to North America (where several fossil species show affinities with living African species, see Zhang et al. 2012; Steadman and MacFadden 2016), and the Old World (Riesing et al. 2003), where the taxon underwent a more recent radiation (Haring et al. 1999). However, it seems that Buteo dondasi sp. nov. would be part of a radiation experienced by the genus in the American continent.
We estimate the body mass of B. dondasi sp. nov. at ~2.3 kg by comparing the length of the tarsometatarsus of the new species (131.3 mm) with B. regalis (101.6 mm, Table 1), the largest taxon of Buteo, whose females reach the 1.776 kg (Dunning 2008). Within the Buteonine, several taxa exceed these sizes, for example, Geranoaetus melanoleucus (3.2 kg), Buteogallus coronatus (2.9 kg), and species of Haliaeetus that can reach 7 kg; or other larger eagles such as Harpia harpyja (4.8 kg), Pitecophaga jefferyi (6 kg), and species of Aquila that can exceed 3 kg. Based on these data, it is inferred that B. dondasi sp. nov. is the largest species of Buteo known to date.
Buteo dondasi sp. nov. represents the first record for a flying diurnal predator for the Chapadmalal Formation is assemblage. An eagle or buzzard-type tarsometatarsus is characteristic of diurnal birds of prey that predate on small rodents and other small mammal (avivorous raptors tend to have longer tarsometarsus) (Mayr and Perner 2020). So, based on its large overall size combined with stout, powerful leg and foot, it is plausible to think that Buteo dondasi sp. nov. preyed on rodents and small ungulates, that are so common in the Chapadmalal Formation.
Table 1. Measurements (in mm) of Buteo dondasi sp. nov., MMP 5730, compared with other extant Buteoninae. * measured between the trochlea metatarsorum II and IV, without the wing-like flanges; ** measured between the prominentia intercotylaris and the trochlea metatarsi III.
|
Taxa |
Tibiotarsus |
Tarsometatarsus |
||||
|
distal width |
condylus |
condylus |
proximal width |
distal width* |
total length** |
|
|
Buteo dondasi sp. nov. MMP 5730 |
22.7 |
16.7 |
15.9 |
24.4 |
24.1 |
131.3 |
|
Buteo albononatus AMNH 12646 |
– |
– |
– |
13.3 |
14.3 |
86.9 |
|
Buteo buteo AMNH 31167 |
– |
– |
– |
14.2 |
13.6 |
77.7 |
|
Buteo jamaicensis AMNH 24514 |
14.8 |
10.5 |
10.2 |
15.1 |
15.5 |
82.6 |
|
Buteo lagopus AMNH 26561 |
– |
– |
– |
13.6 |
13.4 |
70.7 |
|
Buteo lineatus OUVC 10773 |
– |
– |
– |
11.8 |
12.0 |
79.7 |
|
Buteo platypterus AMNH 24515 |
– |
– |
– |
9.8 |
9.3 |
59.6 |
|
Buteo regalis AMNH 1946 |
21.9 |
15.2 |
14.3 |
22.7 |
21.2 |
101.6 |
|
Buteo rufofuscus AMNH 4054 |
– |
– |
– |
14.8 |
15.2 |
91.9 |
|
Buteo swansoni AMNH 27921 |
– |
– |
– |
13.9 |
13.9 |
70.9 |
|
Buteogallus coronatus CIT-O272 |
19.7 |
13.4 |
12.8 |
20.4 |
21.8 |
121.6 |
|
Geranoaetus melanoleucus CIT-O641 |
19.4 |
12.8 |
12.3 |
19.5 |
20.1 |
109.4 |
|
Geranoaetus polyosoma AMNH 8511 |
– |
– |
– |
12.6 |
12.8 |
79.4 |
|
Haliaeetus leucocephalus AMNH 18813 |
24.3 |
18.1 |
17.6 |
24.3 |
25.9 |
98.2 |
|
Haliaeetus leucogaster AMNH 2024 |
19.1 |
13.3 |
13.6 |
18.5 |
20.1 |
95.5 |
|
Haliaeetus vocifer AMNH 27389 |
20.6 |
14.9 |
14.3 |
20.2 |
21.5 |
91.7 |
|
Leucopternis albicollis AMNH 20578 |
12.8 |
9.2 |
8.5 |
13.9 |
13.4 |
80.8 |
|
Parabuteo unicinctus AMNH 26504 |
– |
– |
– |
15.5 |
14.4 |
91.7 |
|
Rupornis magnirostris AMNH 12647 |
– |
– |
– |
10.2 |
9.2 |
65.6 |
Conclusions
Buteo dondasi sp. nov. constitutes the first record of a buzzard for the Chapadmalalan Formation, being one of the largest accipitrid records for Argentina. The Chapadmalalan Formation biome has been claimed to have a great diversity of herbivores but a very low variety of predators and scavengers (Prevosti et al. 2011). The increasing knowledge of its fossil assemblage seems to indicate that birds occupied the role of animal or meat eaters. Within birds, the phorusracids would prey on the ground, chasing their prey with their cursorial mode of locomotion. The scavengers would be represented by the gigantic Cathartidae Dryornis pampeanus and possibly by the teratorns. Buteo dondasi sp. nov. would be an active flying daytime predator that attacked its prey from the sky and killed them by squeezing them with its claws, as buzzards and eagles do today. In this sense, this new knowledge completes the gap in the knowledge of the avian predator and scavenger guilds for the late Pliocene of the Pampean region.
Acknowledgements
AMNH ornithological collection staff, and Larry Witmer (Ohio University Heritage College of Osteopathic Medicine, Athens, USA)are thanked for allowing access to Accipitriformes specimens. Ivana Tapia (CICTERRA) repaired and photographed the fossil with her customary skill. Editor D. Barta (Oklahoma State University Center for Health Sciences, Tahlequah, USA), G. Mayr (Senckenberg Research Institute and Natural History Museum Frankfurt, Germany) and an anonymous reviewer are thanked for providing helpful feedback on the manuscript. This is a contribution to ANPCyT PICT 2019-0771 and PUE 2016, CICTERRA-CONICET.
References
Agnolín, F. 2006. Notas sobre el registro de Accipitridae (Aves, Accipitriformes) fósiles argentinos. Studia Geologica Salmanticensia 42: 67–80.
Agnolín, F.L. 2016. A brief history of South American birds. Contribuciones del MACN 6: 157–172.
Baumel, J. and Witmer, L. 1993. Osteologia. In: J. Baumel, A.S. King, and J.E. Breazile (eds.), Handbook of Avian Anatomy: Nomina Anatomica Avium, 45–132. Cambridge University Press, Cambridge.
Campbell, K. 1979. The non-passerine Pleistocene avifauna of the Talara Tar Seeps, Northwestern Peru. Life Science Contribution, Royal Ontario Museum 118: 1–203. Crossref
Cracraft, J. 1969. Notes of fossil hawks. Auk 86: 353–354. Crossref
Daudin, F.M. 1800. Traité élémentaire et complet d’ornithologie, ou, Histoire naturelle des oiseaux. 474 pp. Bertrandet, Paris. Crossref
De la Vega, E., Chalk, T.B., Wilson, P.A., Bysani, R.P., and Foster, G.L. 2020. Atmospheric CO2 during the Mid-Piacenzian Warm Period and the M2 glaciation. Scientific Reports 10: 1–8. Crossref
Degrange, F.J., Tambussi, C.P., Taglioretti, M.L., and Scaglia, F.A. 2021. Phylogenetic affinities and morphology of the Pliocene cathartiform Dryornis pampeanus Moreno & Mercerat. Papers in Palaeontology [published online, https://doi.org/10.1002/spp2.1361]. Crossref
Dunning, J.B. 2008. CRC Handbook of Avian Body Masses. 655 pp. CRC Press, Boca Raton. Crossref
Emslie, S.D. and Czaplewski, N.J. 1999. Two new fossil eagles from the late Pliocene (late Blancan) of Florida and Arizona and their biogeographic implications. In: S.L. Olson (ed.), Avian Paleontology at the Close of the 20th Century: Proceedings of the Fourth International Meeting of the Society of Avian Paleontology and Evolution. Smithsonian Contributions to Paleobiology 89: 185–198.
Gmelin, J.F. 1788. Systema Naturae. 500 pp. Impensis Georg Emanuel Beer, Leipzig.
Gray, G.R. 1844. The Genera of Birds: Comprising Their Generic Characters, a Notice of the Habits of Each Genus, and an Extensive List of Species Referred to Their Several Genera. 301 pp. Longman, London. Crossref
Haring, E., Riesing, M.J., Pinsker, W., and Gamauf, A. 1999. Evolution of a pseudo-control region in the mitochondrial genome of Palearctic buzzards (genus Buteo). Journal of Zoological Systematics and Evolutionary Research 37: 185–194. Crossref
Jollie, M. 1977. A contribution to the morphology and phylogeny of the Falconiformes. Part 3. Evolutionary Theory 2: 209–300.
Kraglievich, J.L. 1952. El perfil geológico de Chapadmalal y Miramar, Provincia de Buenos Aires. Revista Museo Municipal Ciencias Naturales y Tradicional Mar del Plata 1: 8–37.
Mayr, G. and Perner, T. 2020. A new species of diurnal birds of prey from the late Eocene of Wyoming (USA)—one of the earliest New World records of the Accipitridae (hawks, eagles, and allies). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 297: 205–215. Crossref
Mayr, G. and Smith, T. 2019. New Paleocene bird fossils from the North Sea Basin in Belgium and France. Geologica Belgica 22: 35–46. Crossref
Mindell, D.P., Fuchs, J., and Johnson, J.A. 2018. Phylogeny, taxonomy, and geographic diversity of diurnal raptors: Falconiformes, Accipitriformes, and Cathartiformes. In: J.H. Sarasola, J.M. Grande, and J.J. Negro (eds.), Birds of Prey: Biology and Conservation in the XXI century, 3–32. Springer, Basel. Crossref
Mosto, M.C. and Tambussi, C.P. 2013. Qualitative and quantitative analysis of talons of diurnal bird of prey. Anatomia, Histologia, Embryologia 43: 6–15. Crossref
Mosto, M.M., Degrange, F.J., and Tambussi, C.P. 2007. Falanges ungueales de Accipitridae (Aves Falconiformes) de la Formación Arroyo Chasicó (Mioceno tardío), Argentina. Ameghiniana 44: 30R.
Olson, S.L. 2007. The ‘‘walking eagle’’ Wetmoregyps daggetti Miller: a scaled-up version of the Savanna Hawk Buteogallus meridionalis. Ornithological Monographs 63: 110–113. Crossref
Olson, S.L. 2008. A new genus and species of buteonine hawk from Quaternary deposits in Bermuda (Aves: Accipitridae). Proceedings of the Biological Society of Washington 121: 130–141. Crossref
Picasso, M.B.J., Tambussi, C.P., and Dozo, M.T. 2009. Neurocranial and brain anatomy of a Late Miocene eagle (Aves, Accipitridae) from Patagonia. Journal of Vertebrate Paleontology 29: 831–836. Crossref
Prevosti, F.J., Romano, C.O., Forasiepi, A.M., Hemming, S., Bonini, R., Candela, A.M., Cerdeño, E., Madozzo Jaén, M.C., Ortiz, P.E., Pujos, F., Rasia, L., Schmidt, G.I., Taglioretti, M., MacPhee, R.D.E., and Pardiñas, U.F.J. 2021. New radiometric 40Ar–39Ar dates and faunistic analyses refine evolutionary dynamics of Neogene vertebrate assemblages in southern South America. Scientific Reports 11: 9830. Crossref
Riesing, M.J., Kruckenhauser, L., Gamauf, A., and Haring, E. 2003. Molecular phylogeny of the genus Buteo (Aves: Accipitridae) based on mitochondrial marker sequences. Molecular Phylogenetics and Evolution 27: 328–342. Crossref
Steadman, D.W., Almonte Milan, J.N., and Mychajliw, A.M. 2019. An extinct eagle (Aves: Accipitridae) from the Quaternary of Hispaniola. Journal of Raptor Research 53: 319–333. Crossref
Steadman, D.W. and MacFadden, B.J. 2016. An early Miocene eagle (Aves: Accipitridae) from Panama. Journal of Paleontology 90: 1012–1015. Crossref
Stefanini, M.I., Gómez, R.O., and Tambussi, C.P. 2016. A new species of the Pleistocene furnariid Pseudoseisuropsis (Aves, Passeriformes). Journal of Vertebrate Paleontology 36: e1100630. Crossref
Suárez, W. 2004. The identity of the fossil raptor of the genus Amplibuteo (Aves: Accipitridae) from the Quaternary of Cuba. Caribbean Journal of Science 40: 120–125.
Suárez, W. and Olson S.L. 2007. The Cuban fossil eagle Aquila borrasi Arredondo: A scaled-up version of the Great Black-Hawk Buteogallus urubitinga (Gmelin). Journal of Raptor Research 41: 288–298. Crossref
Sustaita, D. 2008. Musculoskeletal underpinnings to differences in killing behavior between North American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). Journal of Morphology 269: 283–301. Crossref
Tambussi, C.P. 2011. Palaeoenvironmental and faunal inferences based on the avian fossil record of Patagonia and Pampa: what works and what does not. Biological Journal of the Linnean Society 103: 458–474. Crossref
Tambussi, C.P. and Degrange, F.J. 2013. South American and Antarctic Continental Cenozoic Birds: Paleobiogeographic Affinities and Disparities. 113 pp. Springer, Dordrecht. Crossref
Tambussi, C.P. and Noriega, J.I. 1996. Summary of the avian fossil record from the Southern South America. In: G. Arratia (ed.), Contributions of Southern South America to Vertebrate Paleontology. Münchner Geowissenschaftliche Abhandlungen, 254–264. Verlag Dr. Pfeil, Munich.
Tambussi, C.P., Degrange, F.J., Ciccioli, P.L., and Prevosti, F. 2021. Avian remains from the Toro Negro Formation (Neogene), Central Andes of Argentina. Journal of South American Earth Sciences 105: 102988. Crossref
Tonni, E.P. and Noriega, J.I. 2001. Una especie extinta de Pseudoseisura Reichenbach, 1853 (Passeriformes: Furnariidae) del Pleistoceno de la Argentina: comentarios filogenéticos. Ornitologia Neotropical 12: 29–44.
Zárate, M.A. and Fasano, J.L. 1989. The Plio-Pleistocene record of the central eastern Pampas, Buenos Aires province, Argentina: The Chapadmalal case study. Palaeogeography, Palaeoclimatology, Palaeoecology 72: 27–52. Crossref
Zhang, Z., Feduccia, A., and James, H.F. 2012. A Late Miocene Accipitrid (Aves: Accipitriformes) from Nebraska and its implications for the divergence of Old World vultures. PLoS ONE 7: e48842. Crossref
Acta Palaeontol. Pol. 66 (4): 779–787, 2021
https://doi.org/10.4202/app.00933.2021