

The most complete amiid fish from the Coal Creek Member of the Eocene Kishenehn Formation in northwestern Montana
JACOB D. GARDNER and MARK V.H. WILSON
Gardner, J.D. and Wilson, M.V.H. 2022. The most complete amiid fish from the Coal Creek Member of the Eocene Kishenehn Formation in northwestern Montana. Acta Palaeontologica Polonica 67 (2): 493–508.
The larger-bodied fish fauna of the Kishenehn Formation’s Coal Creek Member (Eocene, 43.5 Ma), northwestern Montana, is understudied because of a sampling bias towards small specimens. Small specimens (<10 cm length) of taxa are usually found as mostly to fully complete compression fossils. Relatively larger-bodied fishes, such as amiids (the bowfin Amia calva and close relatives), are only known from fragmentary remains for which taxonomic resolution is only possible to the family level. Here we describe the most complete amiid fossil (USNM 618000) from the Kishenehn Formation. We assign this specimen to the genus Amia based on the presence of pointed coronoid teeth and a long preural region (81 preural centra). The specimen exhibits a combination of features from multiple species, including a total of 89 centra (like Amia calva and Amia scutata), eight ural centra (like Amia scutata and Amia pattersoni), and a concave anteroventral margin on the first postinfraorbital (like Amia hesperia). The lack of more complete specimens of amiids and other larger-bodied taxa is most often attributed to a preservation bias; however, this could also reflect a rarity of amiids in the ecosystem overall or a partitioning of habitat preference away from the shallow, near-shore regions of the ancient lake. This new specimen enhances the known biodiversity of relatively larger-bodied fishes from this region during the Eocene epoch.
Key words: Actinopterygii, Amiidae, Amia, lacustrine, paleoecology, taphonomy, Eocene, Kishenehn, Montana, USA.
Jacob D. Gardner [jacob.gardner2@montana.edu], Department of Earth Sciences, Montana State University, Bozeman, Montana 59717, USA.
Mark V.H. Wilson [mvwilson@ualberta.ca], Department of Biological Sciences, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; Department of Biology, Loyola University Chicago, Chicago, Illinois 60660, USA.
Received 29 January 2020, accepted 8 November 2021, available online 2 February 2022.
Copyright © 2021 J.D. Gardner and M.V.H. Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Coal Creek Member of the Kishenehn Formation (Eocene, 43.5 Ma) in northwestern Montana is known for its mammal and gastropod fossils (Constenius et al. 1989; Pierce and Constenius 2014) and more recently for its exceptionally preserved insect fossils from the oil shale horizons of the member’s middle sequence (Greenwalt et al. 2013, 2014, 2016; Greenwalt and Labandeira 2013). The large fish fauna, however, is under-studied in comparison to the Eocene and Oligocene faunas of Colorado, Utah, Wyoming, and Washington (USA) and British Columbia (Canada). The description and discussion of the known taxa in the Kishenehn Formation were presented as a short section within a geological and paleontological survey of the formation (Constenius et al. 1989). The recorded fish fauna included rare hiodontids (mooneyes) and clupeids (herrings), catostomids (suckers) of the species Amyzon kishenehnicum Liu, Wilson, and Murray, 2016, and the fragmentary remains of an amiid (the bowfin Amia calva and close relatives). Small-bodied fishes, such as Hiodon consteniorum Li and Wilson, 1994, are usually collected as mostly to fully complete compression fossils (Constenius et al. 1989). The small-bodied fishes are, on average, less than 10 cm in length. The catostomid Amyzon kishenehnicum is an exception, being known from very few larger specimens (up to about 40 cm standard length) and hundreds of small juvenile specimens (Liu et al. 2016). The large-bodied amiids, until now, were only known from fragmentary remains where taxonomic resolution was only possible to the family level.
The insect-containing shale horizons of the middle sequence are hypothesized to be shallow and near-shore in origin due to the presence of small aquatic insects, their intactness, and the presence of Daphnia eggs and larvae (Greenwalt et al. 2014). Their preservation is suggested to be aided by the seasonal growth of microbial mats that often preserve remnants of the insects’ original organic components (Greenwalt et al. 2013, 2014, 2016). Larger-bodied taxa, including fishes and large insects greater than 10 cm and 5 mm, respectively, do not preserve well in these microbial mats and are uncommon in the insect-producing horizons. Remains of larger taxa are usually represented as disarticulated elements, such as cranial elements of fishes and the wings of large insects. Greenwalt et al. (2014) showed that the mineralogy of the horizons containing more fish fossils, and fewer well-preserved insect fossils, is more sapropelic in nature and contains small clastic and carbonized inclusions. These differences could represent slightly different depositional conditions. However, taphonomic studies of comparable and contemporaneous formations have hypothesized that amiid and small-bodied catostomid-containing shale horizons are also representative of shallow and near-shore lake settings (Wilson 1980, 1988; Wilson and Barton 1996; Barton and Wilson 2005).
Until this study, no published update of the Kishenehn Formation has been made on the amiid taxon. Here, we report the description of the most complete amiid fossil (USNM 618000) from the middle sequence of the Kishenehn Formation’s Coal Creek Member (Eocene, 43.5 Ma). This description will allow for the taxonomic resolution of the amiid taxon that was previously reported (Constenius et al. 1989; Liu et al. 2016). The biodiversity, biogeographical, and taphonomic implications of the specimen are discussed and a phylogenetic analysis is implemented to demonstrate its taxonomic placement within Amiinae. Although a unique combination of characters distinguishes this specimen from other known amiid taxa, the fragmentary state of the skull and the lack of preserved dorsal fin rays makes species identification difficult.
Institutional abbreviations.—DMNH, Denver Museum of Natural History, Denver, USA; FMNH, Field Museum of Natural History, Chicago, USA; PU, Princeton University collection, Peabody Museum of Natural History at Yale University, New Haven, USA; UALVP, University of Alberta Laboratory for Vertebrate Paleontology, Edmonton, Canada; UMMP, Museum of Paleontology at the University of Michigan, Ann Arbor, USA; USNM, National Museum of Natural History, Washington, D.C., USA.
Other abbreviations.—BS, bootstrap support; NACD, North American Continental Divide.
Geological setting
The Kishenehn Formation is divided into multiple members depending on the region within the Kishenehn Basin. The Middle Fork region of the basin is underlain by a lower Coal Creek Member and an upper Pinchot Member. The Coal Creek Member is composed mostly of fine-grained clastic rocks and is divided into three sequences. The middle sequence, from which USNM 618000 was discovered, is lithologically very heterogeneous, consisting of oil shale, marlstone, sandstone, and siltstone along with sapropelic coal, claystone, and mudstone. In total, the middle sequence is about 500 m thick and is dated to approximately 46.2 ± 0.4 and 43.5 ± 4.9 Ma using 40Ar/39Ar and fission-track analyses, respectively (Constenius 1996; Pierce and Constenius 2014). The diverse, interstratified lithologies are interpreted as alternating lacustrine, fan-delta, and marsh environments. The site of discovery, referred to as the “Spring Site”, is located within a series of oil shale near the lower end of the middle sequence, stratigraphically equivalent to the Tunnel Creek locality section (Greenwalt et al. 2014; Pierce and Constenius 2014). The shale horizon where the specimen was found is in the upper layers of the Spring Site (Fig. 1), which were found to yield less well-preserved insect fossils than the lower layers of the site.
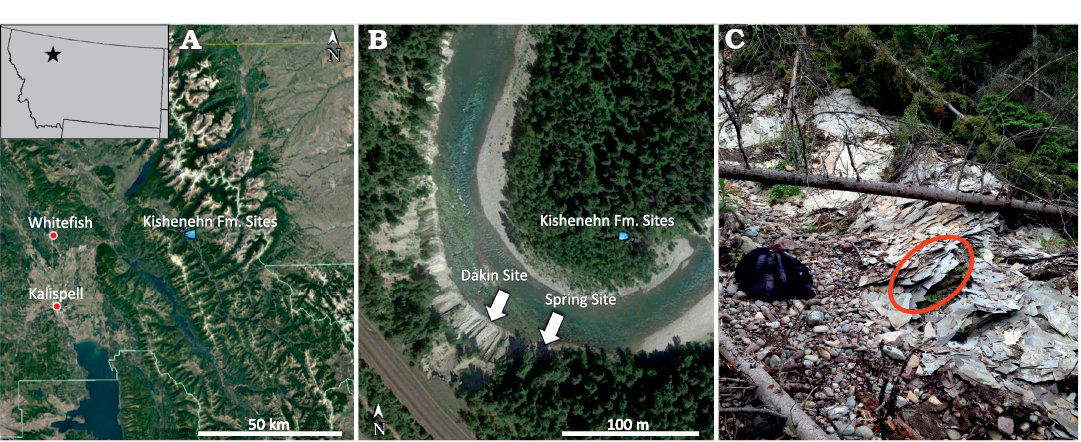
Fig. 1. Google Earth images showing the location of the USNM 618000 discovery site (“Spring Site”) within Montana (A) and among other shale-bearing middle sequence localities (B). The Spring Site is located on the south bend of the river. Picture of the upper Spring Site showing where USNM 618000 was discovered (C).
Previous studies suggest a tropical climate for the Middle Fork region of the Eocene Kishenehn Basin with little temperature seasonality (Wolfe 1995; Zachos et al. 2001; Archibald et al. 2010; Huber and Caballero 2011). Molluscan fossil assemblages from a mixture of climatic groups suggest that the Kishenehn paleolake was surrounded by highlands that were as much as 2000 m above the valley floor (Pierce and Constenius 2014). Some mollusk groups were transported by rivers from the adjacent highlands and deposited into the valley-floor lake where mollusks representative of tropical climatic groups naturally resided. Other mollusk groups representative of subarid to subtropical climatic groups were also transported into the lake from the valley’s elevated interfluvial areas or flanks of the surrounding highland elevations.
Material and methods
Description and comparison.—The USNM 618000 specimen was described using the anatomical nomenclature of Grande and Bemis (1998) and Wilson (1982). USNM 618000 was diagnosed using a set of synapomorphic characters provided by Grande and Bemis (1998) and by comparing with the diagnoses of other North American amiid species. The specimens used by Grande and Bemis (1998) that are representative of the species Amia calva, A. scutata, A. pattersoni, A. hesperia, and Cyclurus gurleyi were compared with USNM 618000. The measurements and counts taken were also modeled after those of Grande and Bemis (1998). Except for the total and standard lengths of the body as well as the length of the skull, difficulties visualizing the other elements on the fossil itself necessitated taking the remaining measurements from the resulting three-dimensional models using ImageJ (Abramoff et al. 2004). A table of measurements and counts is provided in Appendix 1.
Character coding and phylogenetic analysis.—The 69 synapomorphies used in our phylogenetic inferences were taken from Grande and Bemis (1998). The primary phylogenetic inference of Grande and Bemis (1998), with the same 38 taxa, was reanalyzed using parsimony and maximum likelihood methods in RAxML (Stamatakis 2014). The USNM 618000 specimen was then added in a secondary phylogenetic analysis to determine its placement within the subfamily Amiinae. The maximum likelihood trees for these two analyses both used an Mkv model—a Markov model that allows for all sites to vary. A rapid bootstrap analysis was then conducted after both analyses to assess the consistency of the resulting tree topology. We ran each analysis for 100 runs and chose the tree with the greatest likelihood. The topology of the chosen tree was compared with the parsimony tree from the same run. For the rapid bootstrap analysis, we specified four random seeds prior to bootstrapping 100 replicates. Pholidophorus bechei (reclassified as Dorsetichthys bechei by Arratia 2013) and Pholidophorus macrocephalus (reclassified as Siemensichthys macrocephalus by Arratia 2000) were our outgroup taxa for all analyses. The program Mesquite was used to manage the phylogenetic character matrix and FigTree was used to make the figures (Maddison and Maddison 2019; Rambaut 2017). The character matrix for all 38 taxa and USNM 618000 is included in the Supplementary Materials along with the RaxML code. Only 11.22% of the entire character matrix consists of missing character states.
Photography and illustrations.—Photographs were taken using a Nikon D90 digital SLR, and an AF-S Micro Nikkor 60mm f/2.8G ED lens. Illustrations were drawn by the lead author based on the resulting three-dimensional models.
CT scanning and three-dimensional imaging.—Due to the fragmentary and carbonized nature of the bones, we decided against manual preparation. The specimen (USNM 618000) was scanned using computed tomography (CT) at the Smithsonian National Museum of Natural History (Washington, D.C.) using a Siemens SOMATOM Emotion 6 scanner at 70,321 slices of 300 microns and natively 625 microns (pixel size = display field of view/matrix = 381/512 mm = 0.74; rotation time = 0.6 seconds; kilovoltage = 130; milliamps = 80). Although we present the specimen in separate parts (skull and body/tail), both parts were articulated and scanned together. Three-dimensional models were made from the image slices by using the thresholding segmentation feature in OsiriX MD version 7.0.2.
Systematic paleontology
Subclass Actinopterygii Cope, 1887 sensu Rosen et al. 1981
Division Halecostomi Regan, 1923 sensu Rosen et al. 1981
Subdivision Halecomorphi Regan, 1923 sensu Patterson 1973
Order Amiiformes Hay, 1929 sensu Grande and Bemis 1998
Family Amiidae Bonaparte, 1838
Subfamily Amiinae Bonaparte, 1838
Genus Amia Linnaeus, 1766
Type species: Amia calva Linnaeus, 1766; Recent, eastern North America.
Amia sp.
Fig. 2.
Material.—USNM 618000, nearly complete skeleton embedded in shale matrix from upper layers of the Spring Site, Middle Fork region of the Coal Creek Member’s middle sequence in the Kishenehn Formation (Eocene), northwestern Montana, USA (Fig. 1). The middle sequence is dated to approximately 46.2 ± 0.4 and 43.5 ± 4.9 Ma using 40Ar/39Ar and fission track analyses, respectively (Constenius 1996; Pierce and Constenius 2014).
Description.—Preservation and general body form: USNM 618000 is a nearly complete specimen, represented by mostly carbonized remains and impressions, preserved in multiple layers of oil shale (Fig. 2). The carbonized replacement is likely to be a major source of taphonomic alteration in which many elements were modified into abnormal shapes with multiple indeterminable features as revealed by the CT scan. Some sections of the vertebral column and tail are represented only by negative impressions and were, therefore, not registered by the CT scan. Counts, such as the number of centra and hypurals, were partly based on their impressions in the rock along with the three-dimensional models resulting from the CT scan.
The total length of USNM 618000 is 590 mm with a standard length of 522 mm. It is longer than the longest Amia scutata Cope, 1875 and Cyclurus gurleyi Romer and Fryxell, 1928 specimens, as well as the estimated length for the single Amia hesperia Wilson, 1982, but it is not longer than the longest A. calva and Amia pattersoni Grande and Bemis, 1998. The body depth of the specimen is impossible to determine since the dorsal fin is not preserved. The length of the skull is 145 mm from the posterior-most extent of the opercle to the anterior-most extent of the snout. This is greater than the longest known skull of A. scutata, but smaller than the largest known specimens of other amiine species. The width of the skull is approximately 57.4 mm; however, there may be distortion related to measuring from a slightly left-lateral angle rather than a true dorsal position. The length of the mandible is greater than 107.4 mm. The length of the snout is 36.6 mm and the length of the postorbital is at least 38.6 mm. The ornamentation of the specimen’s cranial elements is not well illustrated in the three-dimensional models; however, the first postinfraorbital on the left side of the skull is well ornamented as indicated by an image taken from one of the CT slices (SOM: fig. S1: po1, in Supplementary Online Material available at http://app.pan.pl/SOM/app67-Gardner_Wilson_SOM.pdf). This dermal ornamentation is comparable to those seen on the postinfraorbitals of Amia calva (AMNH 90970 SD; Grande and Bemis 1998) and A. scutata (PU 10172b; Grande and Bemis 1998), which exhibit multiple ridges radiating toward the posterior end.

Fig. 2. Amiid fish Amia sp., USNM 618000, from the Spring Site, Montana, USA; the Kishenehn Formation’s Coal Creek Member, 43.5 Ma (Eocene). A. Shale-imbedded skull in right ventrolateral view. B. Post-crania with the anterior end on the left. C. Three-dimensional model of the entire skeleton from left dorsolateral view. Abbreviations: cop, coprolite; mtg, metapterygium; pb, pelvic bone; pcfr, pectoral fin rays; pfr, principal fin rays; pp, parapophyses; ps, first pelvic fin ray; pvfr-l, left pelvic fin rays; rfr, rudimentary fin rays.
Skull roof and dorsal ethmoid region: The posterior portion of the skull is heavily disarticulated and mostly incomplete, making the interpretation of the posterior skull difficult. The left extrascapular is missing, but a fraction of the right extrascapular (Fig. 3: es-r) is visible. The fragment of the right extrascapular steeply widens laterally toward the right opercle (Fig. 3: op-r). The steep transition in width differs greatly from the gentler transition of other amiid taxa. However, this may be a product of taphonomy. The parietals (Fig. 3: pa) appear to have shifted anterolaterally to the right side of the skull and underneath the frontals (Fig. 3: fr), which makes it impossible to measure their dimensions. A suture line is present right-laterally to the left parietal illustrating the presence of two paired parietal bones, which is a cladistically significant character (character 48, state 0). The posterior margin of the left parietal is very straight and comparable to that observed in other amiid specimens. A potential anterior parietal pit-line (Fig. 3: pla) can be seen extending out from the midline. Parietal pit-lines also occur in Amia calva (AMNH 90970 SD; Grande and Bemis 1998) and A. scutata (PU 10172b; Grande and Bemis 1998).
The left frontal (Fig. 3: fr-l) is present, but anteriorly incomplete, and has an estimated length and measured width of 41 mm and 18.5 mm, respectively. This results in an approximate width-to-length ratio of about 0.45, which falls within the range of Amia calva and A. scutata (Grande and Bemis 1998: tables 2 and 21), but is greater than measured specimens of A. hesperia and A. pattersoni (Grande and Bemis 1998: tables 31 and 41). The width was measured from the middle of the orbital excavation to the medial edge, perpendicular to the length of the frontal. The length was estimated by measuring from the posterior-most preserved end, just medial to the left dermosphenotic (Fig. 3: dsp-l), to the posterior end of the medial projection that sits posterior to the rostral bone (Fig. 3: ro). Although the anterior-most portion of the frontal is missing, its medial edge is still visible and extends to the posterior end of that medial projection. The orbital excavation of the left frontal is shallow and long but is deeper and longer than that of most specimens of A. pattersoni and the holotype specimen of A. hesperia. Its depth falls in the range of A. calva but is much longer than that of most specimens. The orbital excavation is most like that of A. scutata in both depth and length. It has a depth of 2 mm and an estimated length of 21.6 mm. The right frontal (Fig. 3: fr-r) is present but incomplete.
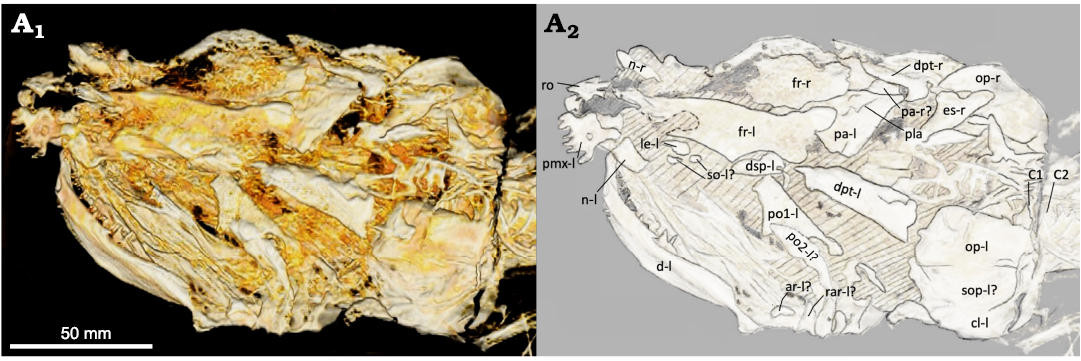
Fig. 3. Amiid fish Amia sp., USNM 618000, from the Spring Site, Montana, USA; the Kishenehn Formation’s Coal Creek Member, 43.5 Ma (Eocene). Skull in left dorsolateral view; three-dimensional model (A1), illustration with labeled elements (A2). The letter following the anatomical abbreviation denotes the left (-l) or right (-r) element. Abbreviations: ar, posterior articular element; cl, cleithrum; d, dentary; dpt, dermopterotic; dsp, dermosphenotic; es, extrascapular; fr, frontal; le, lateral ethmoid; n, nasal; op, opercle; pa, parietals; pla, anterior parietal pit line; pmx, premaxilla; po1, po2, postinfraorbitals 1, 2; rar, retroarticular; ro, rostral; so, subinfraorbitals; sop, subopercle; C1, C2, vertebral centra 1, 2.
The amiine nasal tends to be wider in shape with a small indentation on the anterolateral margin of each nasal for the nostril pathway. This indentation is seen in an element anterior to the right lacrimal (Fig. 4: l-r). The right nasal (Fig. 4: n-r) is more like the nasals of A. hesperia and A. pattersoni in having a lesser width than the nasals of A. calva and A. scutata, which also have a more rounded shape. The left nasal is not immediately apparent. It could be the narrow, oval-shaped element just posterior to the left premaxilla (Fig. 3: pmx-l), but it does not exhibit the characteristic anterolateral notch.
The rostral bone in all amiids shares a very similar V-shape. There are no V-shaped bones present in the anterior portion of the skull. A spade-shaped bone is present in the right location for a rostral bone (Fig. 3: ro). It is likely that this is the rostral bone but rotated 180°. There are multiple cranial bones that are taphonomically altered and shifted. It is least parsimonious to conclude that this spade-shaped bone is shaped differently in life until, at least, new specimens verify this morphology.
Both the left and right dermopterotic bones (Figs. 3, 4: dpt-l, dpt-r) are present in this specimen. In left dorsolateral view, the left dermopterotic appears to be consistent with the dermopterotic bones seen in other amiines. Its width is greatest at its posterior end and converges in the anterior direction. The rounded posterior end of the right dermopterotic is likely a taphonomic artifact as it differs from the squared posterior edge of the left dermopterotic and those of other amiines (e.g., Amia calva, AMNH 90970 SD; Grande and Bemis 1998). The right dermopterotic may have shifted posteriorly. Although there is some variation in the length of the dermopterotics, dermosphenotics, and postinfraorbitals, part of the dermopterotic usually overlies the postinfraorbitals dorsally in most Amia species (Amia calva, AMNH 92586, 92587, and 92588; A. scutata, DMNH 2136a, b and PU 10172a, b; Grande and Bemis 1998); however, the right dermopterotic barely overlaps with the first right postinfraorbital in the A. pattersoni holotype specimen (FMNH PF14091; Grande and Bemis 1998). Only the left dermosphenotic (Fig. 3: dsp-l) is present in this specimen and is found in partial articulation with the posterolateral indentation of the left frontal. The dermosphenotics of other amiines are often wider anteriorly (Amia calva, AMNH 92586, 92587, and 92588; A. scutata, DMNH 2136b and PU 10172a; A. hesperia, UALVP 14758a; A. pattersoni, FMNH PF14091 and PF10235; Grande and Bemis 1998). The dermosphenotic of USNM 618000 is wider towards its posterior end while being more elongate and slenderer towards its anterior end. It is possible that the left dermosphenotic is rotated 180°. If rotated, the kink in the middle would resemble that seen in one A. pattersoni specimen (FMNH PF10235; Grande and Bemis 1998). The sphenotic bone, along with the rest of the interior skull, is not visible in this specimen.
Infraorbital, suborbital, and supraorbital bones, and sclerotic ring: The modal number of infraorbitals is most likely six as in Amia calva, A. scutata, and A. hesperia (Grande and Bemis 1998). There’s the possibility for seven or eight, as in A. pattersoni and Cyclurus gurleyi (Grande and Bemis 1998), with the potential for additional subinfraorbitals on the right side of the skull (Fig. 4: so-r). The last infraorbital, the dermosphenotic, is typically fused to the frontals in most adults. As in other amiine species, there is great individual variation in the shape and number of these elements. The first and anterior-most infraorbital, the lacrimal, appears to only be present on the right side (Fig. 4: l-r). This bone, located dorsal to the anterior tip of the right maxilla, is a good candidate for a right lacrimal given its location and the presence of a notch that often articulates with the anterior-most subinfraorbital in other amiines (e.g., A. calva, AMNH 92586, 92587, and 92588; A. scutata, DMNH 2136b and PU 10172b; Grande and Bemis 1998). The right lacrimal in USNM 618000 is wider at its anterior end, as in some specimens of A. scutata and A. pattersoni (PU 10172b and FMNH PF10235; Grande and Bemis 1998). At least two subinfraorbitals are clearly visible on the right side of the skull (Fig. 4: so-r). They are shorter than those of most specimens of A. calva and A. scutata and more similar in size and shape to those of A. pattersoni and Cyclurus gurleyi (SMMP 78.5.1, FMNH PF10235, PF14071, 14091, and 14095; Grande and Bemis 1998). Their similarity to those of A. pattersoni and C. gurleyi, along with their lack of articulation, raises the potential for additional subinfraorbitals. Two other small and round elements lie in the same region; however, as in many specimens of A. pattersoni, the number of subinfraorbitals could be obscured by disarticulation and poor preservation. In USNM 618000, the left side possesses a few fragments of possibly the broken remains of the left subinfraorbitals (Fig. 3: so-l?).
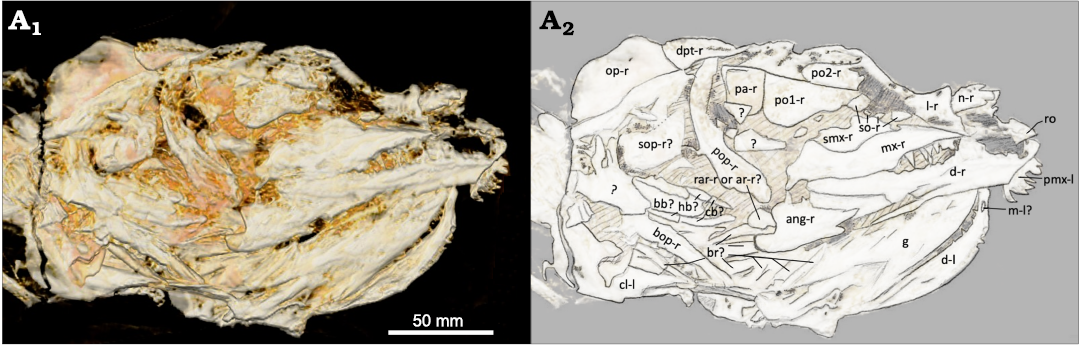
Fig. 4. Amiid fish Amia sp., USNM 618000, from the Spring Site, Montana, USA; the Kishenehn Formation’s Coal Creek Member, 43.5 Ma (Eocene). Skull in right ventrolateral view; three-dimensional model (A1), illustration with labeled elements (A2). The letter following the anatomical abbreviation denotes the left (-l) or right (-r) element. Abbreviations: ang, angular; ar, posterior articular element; bb, basibranchial; bop, branchiopercle; br, branchiostegal rays; cb, ceratobranchials; cl, cleithrum; d, dentary; dpt, dermopterotic; g, gular; hb, hypobranchials; l, lacrimal; m, mentomeckelian; mx, maxilla; n, nasal; op, opercle; pa, parietals; pmx, premaxilla; po1, po2, postinfraorbitals 1, 2; pop, preopercle; rar, retroarticular; ro, rostral; smx, supramaxilla; so, subinfraorbitals; sop, subopercle.
Two postinfraorbitals are present on both sides of the skull. The first and ventral-most postinfraorbital is more triangular-shaped, like those of A. hesperia (UALVP 14758a) and A. pattersoni (FMNH PF 14091). The anterior portion of the first postinfraorbital on the specimen’s right side is relatively flat and articulates with the posterior-most subinfraorbital (Fig. 4: po1-r). Both the dorsal and posterior sides of the first postinfraorbital are also relatively flat, while the ventral side rapidly expands ventrally into a rounded margin. This posteroventral expansion is more exaggerated on the left side (Fig. 3: po1-l), which possesses a concave anteroventral margin; this condition is likely not informative taxonomically as the postinfraorbitals are highly variable in A. calva (AMNH 92586, 92587, and 92588; Grande and Bemis 1998) and A. scutata (FMNH PF 14313 vs. DMNH 2136a). The dorsal and anterior sides of the first postinfraorbital (USNM 618000) are also relatively flat on the left side, with their confluence forming a nearly squared edge (Fig. 3: po1-l).
An unidentifiable bone overlaps the posterior end of the first postinfraorbital on the left side of the skull. Potential candidate elements include the supramaxilla or the second postinfraorbital (Fig. 3: po2-l?). The lack of a left maxilla makes it less likely to be a supramaxilla along with its resemblance in both size and shape to the second postinfraorbital on the right side (Fig. 4: po2-r); they are both relatively small and rectangular. The characteristic of the first postinfraorbital being larger than the second postinfraorbital is observed in many amiine species, except in A. calva. No suborbitals and supraorbitals are present in any amiine species, including this specimen. The lack of a bony sclerotic ring in this specimen is also consistent with the loss of this feature within the group.
Posterior, ventral and lateral braincase, and the ventral ethmoid region: Most of the braincase is not preserved or visible in this specimen. As in other species of Amia, there is no evidence of an opisthotic, a pterotic, or a supraoccipital. The parasphenoid does not appear to be preserved. The extent of the parasphenoid tooth patch is a key character distinguishing Amia from Cyclurus. Parasphenoid tooth patches in Cyclurus are described as being shortened, with the absence of teeth anterior to the arms of the parasphenoid (character 17, state 1 in Cyclurus). All described Amia parasphenoids possess small conical teeth beyond the arms, but the lengths of these tooth patches do vary (Grande and Bemis 1998). Future and more complete amiid specimens from the Kishenehn Formation will help verify the generic assignment. There is an obscured oval-shaped element articulated to the anterior portion of the left frontal’s orbital excavation that is likely to be a left lateral ethmoid (Fig. 3: le-l). The right lateral ethmoid is not preserved.
Otoliths: No otoliths were preserved in the USNM 618000 specimen.
Jaws, palatal bones, suspensorium, and jaw articulation: The jaw teeth of USNM 618000 are sharp, recurved, and conical as in other amiid species. Many of the teeth on various parts of the dentary and maxilla are not preserved, making it difficult to determine tooth count. As far as we can tell, the position of the teeth on the upper and lower jaw and surface of the mouth is like that of other Amia species. The left premaxilla is preserved with six teeth and is structurally like that of other amiids (Fig. 3: pmx-l). Its anterior portion is thick and laterally elongate, and the laterally confined posterior nasal process is not completely preserved. The olfactory foramen is not present either. The left-lateral side of the left premaxilla possesses a foramen that seems too small for the olfactory foramen and too large to be the foramen for the palatine ramus of the facial nerve, as seen in Amia calva (AMNH 90970 SD; Grande and Bemis 1980). It is likely to be one of these foramina but was partially filled with carbonaceous material during the fossilization process. As in Amia calva, the teeth of the maxilla are smaller than those of the premaxilla. The maxilla of USNM 618000 has a deep posterior end that is much shorter than those of other Amia species (Fig. 4: mx-r). The maxillary notch on the posterior edge is broader and more dorsally positioned than in other Amia species; this condition is more likely a taphonomic artifact. The supramaxilla-maxilla contact on the dorsal edge is deeply excavated. The anterior end of the maxilla, after the supramaxilla-maxilla contact, is gently sloped. The anterior end is highly variable in Amia species. It can be flat as in A. calva and A. pattersoni, downward sloping as in A. hesperia, or curved as in some specimens of A. scutata and Cyclurus kehreri Andreae, 1893 (DMNH 2136b and FMNH PF14378b; Grande and Bemis 1998). However, anterior maxilla shape can be highly variable within the same species as well, demonstrated by the kinked anterior end in the A. scutata, DMNH 2136a, and the procurved end in PU 10172a (Grande and Bemis 1998). The right supramaxilla is short and deep (Fig. 4: smx-r). It extends anteriorly beyond a deep dorsal excavation in the maxilla. This excavation is deeper and more uneven in its margin than that of most amiine maxillae (see A. calva, AMNH 90970 SD; A. scutata, DMNH 2136b, PU 10172a, b; A. hesperia, UALVP 14758a; A. pattersoni, FMNH PF14091, 10235; Cyclurus gurleyi, FMNH PF14071, 14095, and UC2201; Grande and Bemis 1998). This likely indicates that both the maxilla and supramaxilla are taphonomically distorted. The supramaxillary notch can also be seen anterior to the supramaxilla.
The amiid lower jaw is composed of two dermal layers or sheets: the dentary, angular, and supraangular on the outside and the coronoid and prearticular on the inside, with the five ossifications of Meckel’s cartilage (retroarticular, anterior and posterior articulars, coronomeckelian, and mentomeckalian) posteriorly in between the two layers. The fragmentary nature and positioning of the skull makes it difficult to identify some of the elements of the inner layer. The retroarticular is the most posterior element and may be visible on both sides of the specimen. The posterior articular element is the second most posterior element and is positioned on the inside of the lower jaw. The small blocky element posterior to the angular on the right side may be the retroarticular or posterior articular element (Fig. 4: rar-r or ar-r?). The numerous small bones posterior to the dentary on the left side may be either of these elements as well (Fig. 3: ar-l? and rar-l?). The anterior articular element and coronomeckelian are also positioned on the inside of the posterior lower jaw but are not visible in this specimen. The mentomeckelian is positioned on the inside of the anterior dentary and may be visible inside of the left dentary from the right side of the skull (Fig. 4: m-l?). It is small and conical as in other species of Amia. The prearticular is the most posterior element of the inner sheet, but it is not preserved in this specimen.
The coronoid is the anterior-most element of the inner sheet, but the most anterior segments of the left lower jaw are not preserved from what can be seen from the right side of the skull. A set of small teeth can be seen on the inside of the left dentary. These teeth are less than half the size of the posterior dentary teeth, suggesting that they are anterior coronoid teeth (Fig. 5). Some of these teeth appear sharp and conical as in other species of Amia, but others appear more rounded. The genus Cyclurus has the derived character of squared and styliform coronoid teeth (character 16, state 1). The apparently rounded coronoid teeth are most likely deformed, given the poor preservation of many skull bones, or the shapes of their tips are artifacts of the CT scan. It is unlikely that the conical teeth were deformed from styliform teeth; therefore, we code USNM 618000 as having sharp and conical coronoid teeth (character 16, state 0).
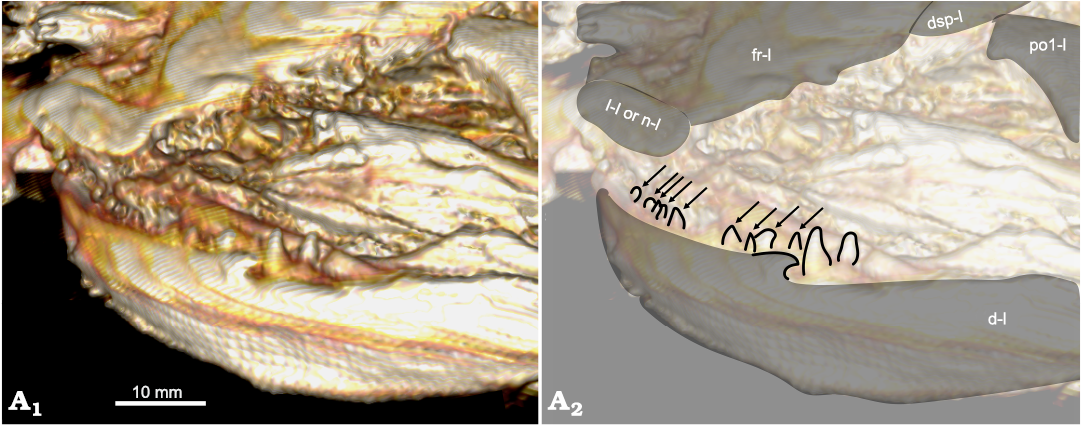
Fig. 5. Amiid fish Amia sp., USNM 618000, from the Spring Site, Montana, USA; the Kishenehn Formation’s Coal Creek Member, 43.5 Ma (Eocene). Dentary in left anterolateral view; three-dimensional model (A1), illustration with elements labeled, coronoid teeth outlined and indicated by arrows (A2). The letter following the anatomical abbreviation denotes the left (-l) element. Abbreviations: d, dentary; dsp, dermosphenotic; fr, frontal; l, lacrimal; n, nasal; po1, postinfraorbital.
The angular is the most posterior element of the outer sheet. It is present on the right side and similar in shape to that of other amiine species, but most like that of A. hesperia with its elongate anterior extension (Fig. 4: ang-r). The supraangular is not preserved in this specimen. The entire dentary of USNM 618000 is shallower than in other amiine species. The tapering transition from the deeper posterior end is relatively short before it flattens into the anterior part of the dentary (Fig. 4: d-r). The mandibular sensory canals are not seen on this specimen. The bones of the pterygoid and palate are not preserved or visible. The three bones of the suspensorium (hyomandibula, quadrate, and symplectic) are not well preserved either. There are a couple of elements present anterior to the right preopercle and posterior to the first postinfraorbital that may represent fragments of two of these elements (Fig. 4: ?).
Opercular series, branchiostegal rays, and gular: The preopercle can be seen on the right side of the skull and is comparable to those of other amiine species (Fig. 4: pop-r). The opercles can be seen on both sides. The width of the left and right opercles are a maximum of about 41.6 mm (Fig. 3: op-l) and 46.8 mm (Fig. 4: op-r). The height of the left opercle is unclear given its obscure ventral boundary, but, unlike those of other amiines, the right opercle as preserved is relatively short (approximately 23.8 mm). This is likely to be a taphonomic effect. The interopercle and branchiopercle are difficult to distinguish on the left side, but the subopercle may be present (Fig. 3: sop-l?). The opercular series on the right side is fragmentary, but most of the elements may be represented. The right subopercle is shallower on its more anterior end and is shorter and square-shaped on its deeper posterior end (Fig. 4: sop-r?); this is reminiscent of the subopercle of some A. scutata specimens (DMNH 2136b and PU 10172a; Grande and Bemis 1998). The branchiopercle on the right side is thinner and more elongate compared to that of other amiine species (Fig. 4: bop-r), but like that of some A. scutata specimens (PU 10172a and UMMP V-57431; Grande and Bemis 1998). The branchiostegal rays are not easily apparent in this specimen (Fig. 4: br?). The gular is well preserved in this specimen, but its complete shape is obscured by the positioning of the fossil (Fig. 4: g). It tapers anteriorly to a point as in other amiine species. The shape of the posterior end is not well defined and appears to be jagged with a depression in the middle, unlike the smooth, round edge of other Amia species and the squared, truncated edge of all Cyclurus species. It has a length of 60 mm, a width of 20.7 mm, and a width-to-length ratio of about 0.35. It is longer than it is wide, like that of A. pattersoni.
Gill arches: The gill arches are not very well preserved or visible in this specimen. A blocky, elongate basibranchial and some segmented hypo- and ceratobranchials may be seen on the right side of the skull (Fig. 4: bb?, hb?, cb?); however, the specific branchial number cannot be determined. The ceratohyals, the hypohyals, or the various tooth patches are not preserved. The dorsal gill arches are also not distinguishable or preserved in this specimen.
Vertebral column: The overall structure of USNM 618000’s vertebral column is like that of other amiid species, including amphicoelous centra (concave anteriorly and posteriorly) and diplospondylous preural vertebrae. The abdominal and caudal regions are well represented with only one section of considerable disarticulation and two regions that are only represented as impressions (SOM: figs. S2 and S3). The total vertebral centra count of at least 89 and total preural centra count of at least 81 falls within the upper range of Amia calva and A. scutata (note: we exclude the first two centra that articulate with the occipital condyle and call them C1 and C2, as per the protocol of Grande and Bemis 1998). These vertebral counts are greater than those of A. pattersoni and Cyclurus gurleyi, as reported by Grande and Bemis (1998). The vertebral column of A. hesperia is too incomplete to be compared.
The first neural arches are not visible or preserved and the first rib-bearing vertebra is difficult to identify. The parapophyses are preserved on several vertebrae and are fused to the vertebral centra (Fig. 2: pp), which is a derived character for the Amiinae (character 10, state 1; Grande and Bemis 1998). The last rib is either not preserved or is disarticulated amongst the cluster of rib fragments. The neural arches and spines are only preserved in the preural caudal region (Fig. 6: na, nsp). The supraneurals are not preserved.
The first visible haemal arch can be seen coming off the impression of the 61st or 63rd centrum (Fig. 6: ha); however, due to disarticulation anterior to this centrum, it is unclear whether this is the first preural caudal centrum. The anterior-most haemal arches and their infrahaemals are not well preserved, and the haemal arch process present on the second haemal arch of A. calva is not clearly visible. The haemal arches and spines of the posterior preural centra are better preserved (Fig. 6: hs). The last preural centrum, bearing the parhypural (or last haemal arch), is present and is followed by eight ural centra (u1-8), which falls within the upper range for A. calva (2–10; Grande and Bemis 1998: table 11) and A. pattersoni (6–8; Grande and Bemis 1998: table 45) and is not much higher than those of A. scutata and Cyculurus gurleyi (7; Grande and Bemis 1998: tables 25 and 65). The first ural centrum bears the first hypural and is followed by the distinctive opening between the first and second hypurals that is seen in other amiine species (Fig. 6: hyp1, hyp2).
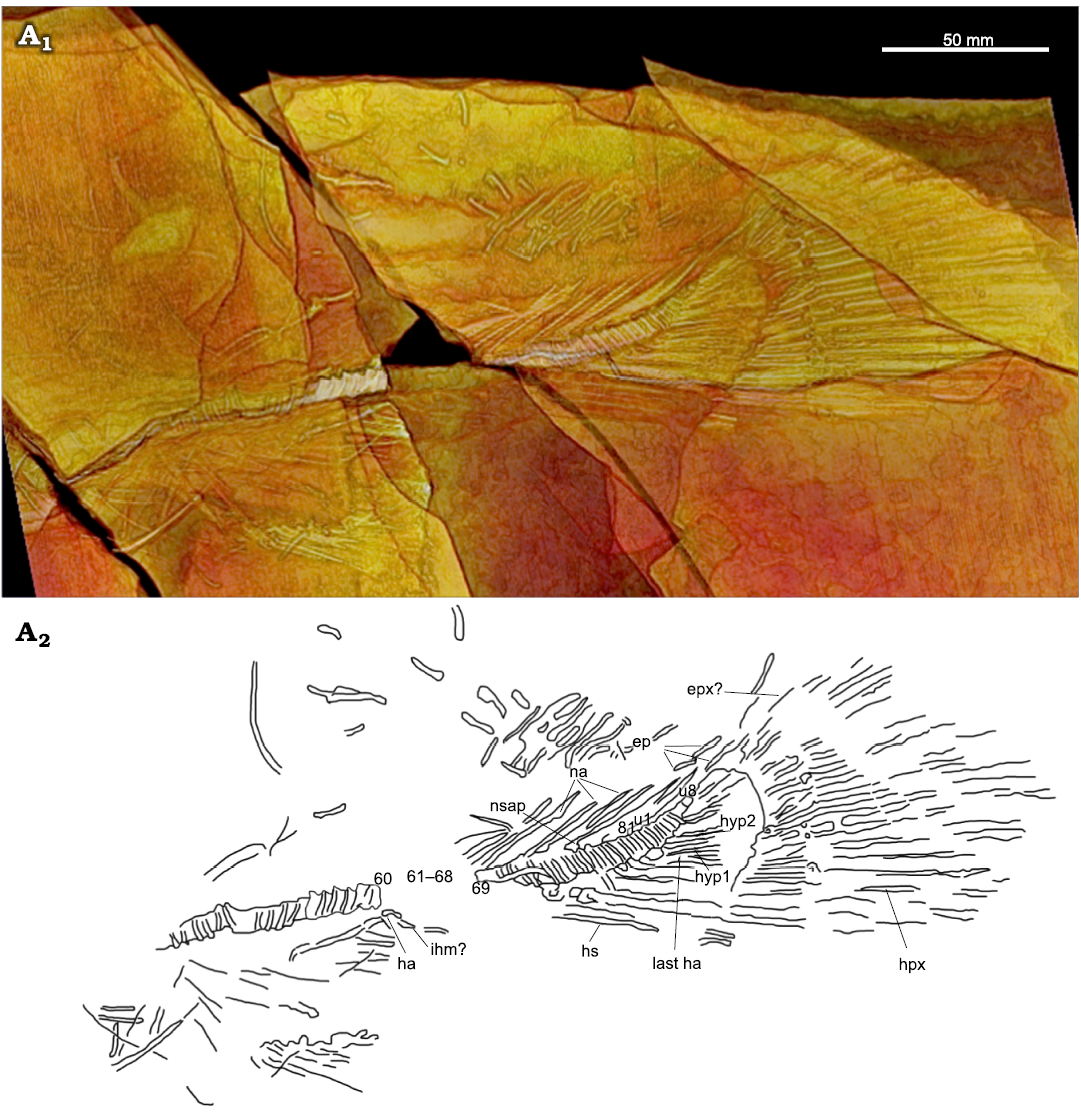
Fig. 6. Amiid fish Amia sp., USNM 618000, from the Spring Site, Montana, USA; the Kishenehn Formation’s Coal Creek Member, 43.5 Ma (Eocene). Tail in left-lateral view; three-dimensional threshold model (A1), explanatory drawing with labeled elements (A2). Vertebral centra 61–68 are represented as impressions in the shale (see SOM: fig. S3). Abbreviations: ep, epurals; epx, epaxial; ha, haemal arch; hpx, hypaxial; hs, haemal spines; hyp1, hyp2, hypurals 1, 2; ihm, infrahaemal; na, neural arches; nsap, anterior process of neural spine; u1, u8, ural centra 1, 8.
Caudal fin and supports: There are 10 hypurals total, a number that compares favorably to Amia calva (9–12; Grande and Bemis 1998: table 15), but they can only be seen in the three-dimensional threshold model produced in OsiriX MD (Fig. 6). The fusion between the hypurals and their corresponding ural centra is a distinguishing character for Amiinae (character 9, state 1; Grande and Bemis 1998). The first hypural (Fig. 6: hyp1) appears to show some separation with centrum 81; however, given that all subsequent hypurals appear to be fused with their corresponding centra, we argue that the separation of the first hypural is due to disarticulation or distortion. The epaxial caudal rays are not well preserved, but the 20 hypaxial rays are well represented except for their distal and ventral ends (Fig. 6: epx?, hpx). Three epurals can only be seen in Fig. 6 (ep). The length of the caudal fin is 137 mm. This is greater than in any reported specimens of A. scutata and the only known specimen of A. hesperia but falls well within the range for known specimens of A. pattersoni and Cyclurus gurleyi. The caudal peduncle length and depth are 134 and 47 mm, respectively. This is similar to that of known specimens of A. scutata, in contrast to the longer and deeper peduncle of A. pattersoni; however, this length may be inaccurate if the anal fin was displaced or is incomplete. The length of the dorsal margin between the dorsal and caudal fins is indeterminable because the dorsal fin is not articulated.
Dorsal and anal fins and supports: The dorsal fin is heavily disarticulated making description and measurement impossible. The anal fin is only partially preserved with its pterygiophores absent. There are approximately 13 principal fin rays preserved with one rudimentary fin ray (Fig. 2: pfr, rfr). The base of the anal fin is 38 mm, which is shorter than that of most known specimens of A. pattersoni and Cyclurus gurleyi and slightly longer than that of all known specimens of A. scutata. The specimen of A. hesperia does not have a preserved anal fin. The USNM 618000 specimen also has a preanal length of 357 mm.
Pectoral girdle and fin: The pectoral girdle is not visible or preserved except for the left cleithrum, which does not differ in structure from other amiines (Fig. 4: cl-l). One of the pectoral fin rays is present, but the number of rays is indeterminable (Fig. 2: pcfr). The prepectoral length is 166 mm, which is shorter than most A. pattersoni specimens (29–400 mm; Grande and Bemis 1998: table 42), longer than all known A. scutata (105–123 mm; Grande and Bemis 1998: table 22) and most Cyclurus gurleyi specimens (37–183 mm; Grande and Bemis 1998: table 62), and longer than the only known A. hesperia specimen (150 mm; Grande and Bemis 1998: table 32).
Pelvic girdle and fin: The pelvic girdle is partially preserved with only one pelvic bone and a possible left metapterygium present (Fig. 2: pb, mtg). The pelvic bone is hourglass-shaped as in other amiine species and the metapterygium is rectangular and runs along the base of the left pelvic fin rays (Fig. 2: pvfr-l). The small, first pelvic fin ray may also be present (Fig. 2: ps). Both pelvic fins are preserved, but the number of fin rays is indeterminable due to poor preservation and their cluttered configuration. The prepelvic length is 268 mm, shorter than in most specimens of A. pattersoni (47–557 mm; Grande and Bemis 1998: table 42) and longer than in all known specimens of A. scutata (178–200 mm; Grande and Bemis 1998: table 22) and in most specimens of Cyclurus gurleyi (20–302 mm; Grande and Bemis 1998: table 62). The specimen of A. hesperia does not have a preserved pelvic fin.
Scales: No scales were preserved on the USNM 618000 specimen.
Traces, stomach contents, and associated specimens: No stomach contents were preserved in the USNM 618000 specimen; however, a coprolite was preserved just dorsal to the specimen’s vertebral column outside of the body (Fig. 2: cop). It exhibits a spiral shape and decreases in circumference towards its tip. Two insect specimens were also found on the reverse sides of two of the shale fragments. Both were identified as Penthetria sp., a genus of March fly (Diptera: Bibionidae).
Remarks.—USNM 618000 was assigned to the genus Amia based on presence of pointed coronoid teeth and a long preural region (81 preural centra).
Phylogenetic results
Run 66 was the best-scoring tree for the reanalysis of the Grande and Bemis (1998) data. Both the parsimony and maximum likelihood trees were comparable in topology to Grande and Bemis (1998)’s primary analysis (SOM: fig. S4). Describing the sister relationships amongst Halecomorphi is outside the scope of this project and most of the differences between our analyses and those of Grande and Bemis (1998) can be explained by weak bootstrap support, due to a lack of characters, and the change in outgroup taxa. The nodes marking Amiinae and Cyclurus are well supported (BS = 100 and 94, respectively), but the interrelationships within Cyclurus and Amia are not and are unstable (SOM: fig. S5). Amia hesperia plotted just outside of A. pattersoni in our best-scoring maximum likelihood tree, but outside the rest of Amiinae in the parsimony analysis and outside Cyclurus in the rapid boostrap analyses with low support (BS = 45). This instability is due to the lack of characters distinguishing the Cyclurus and Amia species.
Run 34 was the best-scoring tree when including USNM 618000 (SOM: fig. S6). In our best-scoring tree, USNM 618000 plots in between Amia pattersoni and a clade uniting A. calva and A. scutata. The rapid bootstrap analysis found that this position for USNM 618000 was poorly supported (Fig. 7; BS = 45). However, our parsimony tree from the same run placed USNM 618000 as a sister taxon to A. scutata (SOM: fig. S6). Although the placement of USNM 618000 differs among analyses, it consistently plots within Amia confirming our diagnosis of the specimen. The change in placement within Amia is expected given the weak support and lack of characters within the genus.
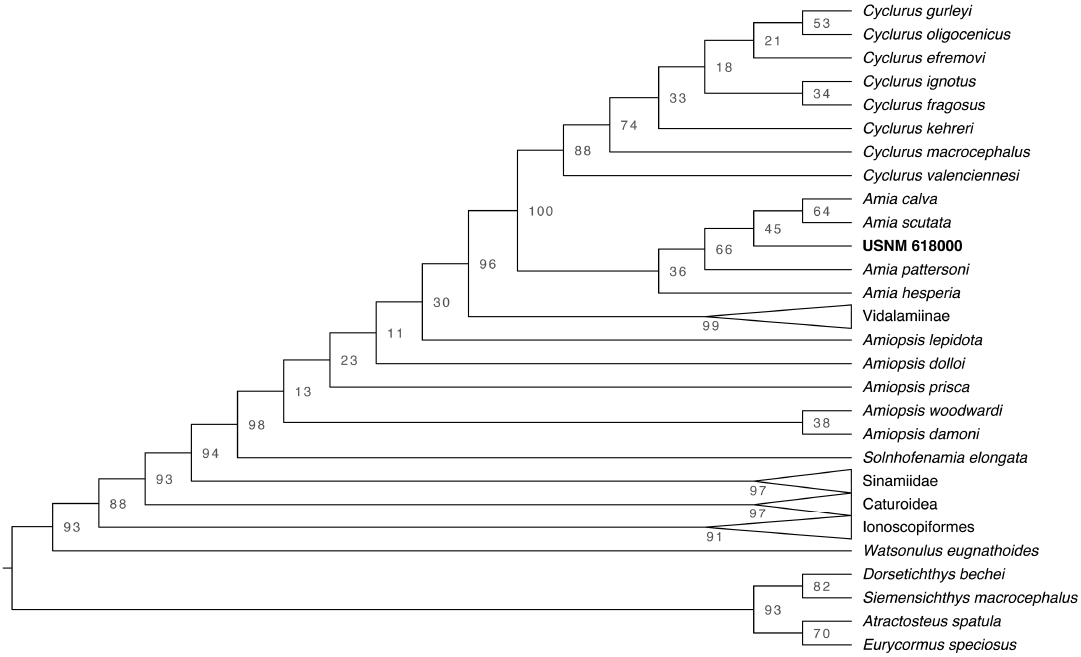
Fig. 7. Cladogram from the maximum likelihood rapid bootstrap analysis with bootstrap support values for each node.
Discussion
Phylogenetic relationships.—The USNM 618000 specimen is shown to be an amiine by the fusion between the hypurals and its corresponding centra (character 9, state 1); the presence of parapophyses fused to the abdominal centra (character 10, state 1); the lack of supraorbital bones (character 12, state 1), urodermals (character 13, state 1), and scapulocoracoid and sclerotic ring ossification (characters 11 and 14, state 1); and the length of the uppermost postinfraorbital being longer or equivalent to the lowermost postinfraorbital (character 66, state 1). The dorsal fin, dimensions of the parietals, and length of the parasphenoid tooth patch are diagnostic features for amiines but are poorly preserved or not at all. However, the presence of pointed coronoid teeth (character 16, state 0) and a long preurual region (83 preural centra) in USNM 618000 allows us to assign this specimen to the genus Amia instead of Cyclurus (character 40, state 1). Despite deformation in the shape of some of the coronoid teeth, the derived state for the length of the preural region unambiguously groups USNM 618000 with Amia instead of Cyclurus. These morphological observations are further verified by our phylogenetic analyses, which consistently placed USNM 618000 within Amia using both maximum likelihood and parsimony methods.
The preservation and orientation of USNM 618000 makes an exact species identification difficult. Amia calva and A. scutata are united by their extremely long dorsal fin, longer than those of A. pattersoni and the other Amiinae, permitting a third character state for those two taxa (Grande and Bemis 1998). The lack of a preserved dorsal fin in USNM 618000 prevents us from clarifying its placement relative to A. calva and A. scutata versus A. pattersoni and is the likely reason for its frequent changes in position in different phylogenetic analyses. In terms of character states, USNM 618000 is about 64% complete. It is missing much of the posterior end and the left side of the skull, most of the bones that make up the gill arches, the dorsal fin, and the pectoral, pelvic, and anal fins are not well preserved and missing most of their elements as well. Most of the opercular series is either not preserved, taphonomically altered, or indistinguishable from the opercle and other elements nearby. Lastly, the bones of the skull roof, braincase, and ethmoid region are either not preserved or not visible. The lack of these elements, their taphonomic alteration, and the orientation of the specimen add to the difficulty of assigning it to species.
The specimen also exhibits a combination of features that are diagnostic of multiple known species. It possesses a total of 89 centra (excluding C1 and C2), which is within the upper range for A. calva and A. scutata and greater than any other amiine species (Grande and Bemis 1998). It has eight ural centra, which is within the range of A. calva and A. pattersoni and is greater than A. scutata (Grande and Bemis 1998). The frontal width-to-length ratio compares favorably to A. calva and A. scutata, but it is greater than that of all measured specimens of A. pattersoni and A. hesperia (Grande and Bemis 1998). Given the lack of additional specimens, we do not name a new species or assign this specimen to an existing species at present.
Kishenehn lake diversity, taphonomy, and paleoenvironment.—The presence of an amiid from the Kishenehn Formation’s Coal Creek Member has been known since 1989, but the fragmentary nature of a small number of specimens in collections prevented a more precise identification. The USNM 618000 specimen demonstrates the presence of at least one species of the genus Amia, increasing the number of known fish genera in the Kishenehn Formation. Four fish families have been reported from this formation (Amiidae, Hiodontidae, Clupeidae, and Catostomidae), but Hiodon consteniorum and Amyzon kishenehnicum were the only named to species (Constenius et al. 1989; Li and Wilson 1994; Liu et al. 2016). The rarity of relatively larger-bodied fish specimens collected from this formation has been discussed multiple times in the literature since the original description of the fauna in 1989 (Constenius et al. 1989; Greenwalt et al. 2014; Liu et al. 2016). Both the fish and the insect fauna are collected from the lacustrine shale deposits of the middle sequence, but they do not seem to co-occur often (Greenwalt et al. 2014). Greenwalt et al. (2014) describe the fish-producing shales as poor yielders of insect fossils and lithologically distinct from the insect-producing shale. They found these fish (juvenile Amyzon kishenehnicum) to be less than 10 cm in length and fully articulated (n = 64). The presence of these small fish in addition to small aquatic insects and Daphnia eggs and larvae suggest that these shale deposits are shallow and near-shore in origin, but deep enough to be protected from storm-induced wave turbulence. They also present evidence for microbial mats that may have preserved the small-bodied organisms and attribute the lack of large-bodied taxa in these shale horizons to a taphonomic bias toward small organisms (i.e., the larger organisms did not stick to the mats or broke them up). The discovery of a relatively complete and larger-bodied fish suggests that this taphonomic bias toward small organisms is not universal among all the shale-producing layers of the Coal Creek Member.
Most of the small fish reported by Greenwalt et al. (2014) were collected from the Park Site. Liu et al. (2016) described the Amyzon kishenehnicum collected from the Disbrow and Tunnel Creek sites and found that they were also primarily small-bodied (less than 10 cm) and juvenile except for two larger specimens (23 and 37 cm). Liu et al. (2016) suggest that A. kishenehnicum partitioned its habitat according to size due to the greater abundance of juvenile specimens and the rarity of larger-bodied specimens. There’s no evidence that this is the case for this Amia species due to the lack of more complete, smaller-bodied specimens, as seen for A. kishenehnicum; however, the presence of habitat partitioning in many fishes, including Amia calva, makes this a reasonable explanation for their rarity in the formation (Becker 1983). The rarity of Amia fossils could, alternatively, reflect their genuine rarity in the ecosystem as a predator compared to prey species, such as A. kishenehnicum. The presence of a more complete Amia sp. specimen, and a couple of larger-bodied A. kishenehnicum specimens, demonstrates that the preservation of larger-bodied fish taxa is possible in the Kishenehn Formation.
The upper layers of the Spring site, where USNM 618000 was recovered, yield fewer insect fossils in comparison to the lower layers of the site where insects are commonly found. This might represent a stratigraphic gradation of different depositional environments between those that are conducive for preserving insects and those that are conducive for preserving fish, such as a change in lake water conditions (e.g., anoxia, pH change, seasonality, temperature, etc.). Temperature and oxygen content, in particular, are thought to be two of the primary factors in determining the preservation of fish skeletons (Elder and Smith 1988). A change to low oxygen content in lake water can prevent scavengers from preying on fish carcasses. Decreasing temperatures can limit bacterial gas production and, therefore, prevent a carcass from floating to the water surface and disarticulating (Elder and Smith 1988). A complete fish skeleton, therefore, suggests either cold, deep water or rapid burial (Elder and Smith 1998; Mancuso 2003). The shale horizons described in Greenwalt et al. (2014) are hypothesized to represent anoxic conditions based on the high total organic content and high hydrogen index of the rocks. The sapropelic nature of the fish-bearing rocks are also consistent with deposition during a winter season, like those observed in the Eocene lacustrine sediments at Horsefly River, British Columbia (Wilson 1977).
The rarity of bowfin fishes in the Kishenehn paleolake is further demonstrated by the small number of known amiid fossils from the formation. Prior to this study, only two potential amiid specimens were collected from the Coal Creek Member of the Kishenehn Formation (UALVP 38959 and an uncatalogued specimen at the UALVP). The UALVP 38959 specimen was recovered from Tunnel Creek, which, as described previously, is stratigraphically equivalent to the Spring site. This specimen consists of scattered bone fragments, mostly fin rays or ribs, and three articulated vertebral centra that remain unprepared. The uncatalogued specimen preserves the anterior third of a dentary with a few large, marginal, conical teeth and 10 articulated vertebral centra. Based on the short antero-posterior width of the vertebrae, both specimens may be identifiable to Amiidae, but they lack the diagnostic traits for further identification.
Diversification of Amiinae.—The only extant species of amiid, Amia calva, is currently restricted to eastern North America. However, starting in the Late Jurassic and through the Eocene, amiids were much more diverse and geographically extensive; totaling at least 22 recognized species from all continents, except Australia and Antarctica (Grande and Bemis 1998). It is unclear when or where the two recognized amiine genera, Amia and Cyclurus, diverged. The earliest occurrence of the Amiinae is from the Upper Cretaceous of Uzbekistan (Grande and Bemis 1998). This taxon was initially described as a possible Amia species but later described as a nomen dubium by Grande and Bemis (1998). The earliest amiine species from North America, Cyclurus fragosus, dates to the Late Cretaceous of Alberta’s Edmonton Group, demonstrating the divergence of the two genera by this time (Grande and Bemis 1998). An Amia species is also known from the late Paleocene Paskapoo Formation of south-central Alberta (Grande and Bemis 1998). The other four widely recognized fossil amiine species from North America all date to the Eocene. The earliest among them are Amia pattersoni and Cyclurus gurleyi from the early Eocene Fossil Butte Member (~50 Ma) of the Green River Formation in southwestern Wyoming (Grande and Bemis 1998). Amiine remains, numerous isolated scales (Cavender 1968) and an undescribed disarticulated skeleton (Grande and Bemis 1998: 337), were also reported from the early to middle Eocene Clarno Formation (~54–39 Ma) of central Oregon (Bestland et al. 1999). Amia hesperia, from the early middle Eocene Allenby Formation (~52–46 Ma) of British Columbia (Wilson 1982; Mustoe 2011), is the next earliest taxon, and the youngest of the North American fossil amiines is Amia scutata from the late Eocene Florissant Formation (~35 Ma) of Colorado (Grande and Bemis 1998).
The North American Continental Divide (NACD), as we know it today, likely formed as a major drainage divide during the Laramide and Sevier orogenies with the uplift of the Rocky Mountains between the Late Jurassic and Paleogene (Yonkee and Weil 2015). Drainage divides are, of course, always changing and controlled by bedrock structure and the positioning of relief terrain, with the latter being controlled by regional tectonics. However, North American drainage was not as simple as an east-west divide. In the Early Cretaceous, much of North America would not have drained into the Gulf of Mexico but, rather, northwestward into the Boreal Sea (Bentley et al. 2016; Wang et al. 2020). Through the Paleocene and Eocene, late-phase Laramide tectonics remained a considerable control on the distribution of drainage basins. Paleocurrent analyses indicate that a drainage divide extended through southern Colorado during the early Paleocene, in which rivers north of the divide drained northeastward into the Cannonball Embayment, a remnant of the Cretaceous Western Interior Seaway (Galloway et al. 2011). The earliest amiine populations in the northern Rockies were likely part of this drainage system or the one farther north that drained into the Hudson Bay. In the late Paleocene, the divide between the Cannonball and Gulf drainage basins shifted northward into southern Wyoming (Galloway et al. 2011). Coincident with the appearance of Amia pattersoni and Cyclurus gurleyi in the early Eocene, a system of closed basins had formed, including the Green River and Uinta basins, which decreased sediment deposition into the Gulf (Galloway et al. 2011). This closed system persisted through the Eocene and Oligocene (Galloway et al. 2011), which would have isolated the Kishenehn paleolake to the north from those in Colorado, Utah, and Wyoming. The degree of connectivity among the basins within this closed system is uncertain.
How changes in drainage distribution affected the diversification of North American amiines is underexplored. The formation of drainage divides has been argued as an isolating mechanism for the divergence of fishes (Bishop 1995; Smith and Bermingham 2005; Unmack 2001). Whether changing drainage distributions influenced the diversification of Amia in North America is difficult to assess, mainly because of poor phylogenetic resolution. Amiines certainly crossed the NACD given their presence in the early to middle Eocene Clarno Formation of Oregon (Cavender 1968). However, the most complete specimen reported from this formation (Grande and Bemis 1998: 337) does not preserve features that allow for a more specific diagnosis. The drainage evolution trends described above predict that the Kishenehn Formation amiine shares a common ancestor with Amia hesperia to the exclusion of A. pattersoni, A. scutata, and possibly A. calva. Our analyses including USNM 618000 are inconsistent with this prediction. Although A. calva and A. scutata form a sister relationship in the maximum likelihood tree, it excludes A. pattersoni (Figs. 6, SOM: fig. S6). The parsimony tree from this run also did not recover a monophyletic A. pattersoni, A. scutata, and A. calva (SOM: fig. S6). In addition, these analyses failed to recover a sister relationship between USNM 618000 and A. hesperia. The difference between the likelihood and parsimony results and lack of node support within Amia owes to the lack of characters distinguishing each taxon and lack of preservation of key characters in USNM 618000. The unique combination of characteristics makes it difficult to conclude which species is most closely related to USNM 618000. In addition, the lack of ancestral A. calva fossils makes it difficult to assess how it became restricted to eastern North America. Currently, the evidence for drainage formation driving the diversification of North American amiines is lacking. A study on fish populations in two neighboring catchments in southern New Zealand showed that dispersal across a young drainage divide is possible (Burridge et al. 2008). Therefore, interdrainage dispersal may have been possible for fish populations in the early formation of the drainage divides described above. These dispersal events would have been especially feasible for amiines since they all lived in freshwater environments (Grande and Bemis 1998).
Conclusions
The large-bodied fish fauna of the Eocene Kishenehn Formation is understudied due to the lack of well-preserved, large-bodied specimens in collections. The USNM 618000 specimen is the most complete amiid specimen from the formation allowing for the taxonomic clarification of the formation’s amiid fossils. We assign this specimen to Amia, a genus that is known from multiple Cenozoic formations in western North America and persists today as one species in eastern North America. Its incompleteness, preservation, orientation, and unique combination of characters makes species level classification difficult and, therefore, remains indeterminate. Finding more complete specimens and clarifying its phylogenetic placement is also crucial for testing hypotheses on the biogeography and diversification of North American amiines.
The discovery of USNM 618000 further demonstrates that, despite a potential taphonomic bias, the preservation of relatively large-bodied fishes in the Kishenehn Formation is possible. It is likely that the lack of well-preserved, relatively large-bodied fish specimens in collections could be due to their genuine rarity in the ancient lake system, as seen in modern ecosystems, or due to habitat partitioning by size and age, as seen for Amia calva, Amyzon kishenehnicum, and many other fishes; though, more complete juvenile specimens are needed to verify this hypothesis. Further discoveries and a more detailed stratigraphic and sedimentological analysis of these shale units are needed to test these hypotheses and to shed light on the paleoenvironment and paleoecology of the Kishenehn lake system.
Acknowledgements
We thank, first and foremost, Dale Greenwalt (Smithsonian National Museum of Natural History, Washington, D.C., USA), and the Undergraduate Scholars Program for providing JDG the funding and opportunity that led to the discovery and description of USNM 618000. We thank Kurt Constenius (University of Arizona, Tucson, USA) for his resources, references, and remarks on the specimen as well as John Bruner (University of Alberta, Alberta, Canada), Janine Hinton, Holly Little, Anna Ragni, and Sabrina Sholts (all Smithsonian National Museum of Natural History), and Lawrence Witmer (Ohio University, Athens, USA) for their assistance with specimen photos, computed tomography, and three-dimensional imaging. We also thank Terry Grande and the members of the Grande Lab at Loyola University Chicago (Chicago, USA) for their hospitality, facilities, and accommodation during JDG’s study visit. This paper was improved by the comments and recommendations from Daniel Barta (Oklahoma State University, Tahlequah, USA), Michael Newbrey (Columbus State University, Columbus, USA), and an anonymous reviewer. This study also benefited from discussions with Helen Dailey (Northern Arizona University, Flagstaff, USA), Ashley Ferguson (Idaho State University, Pocatello, USA), Holley Flora (Oklahoma State University), Daniel Lawver (Stony Brook University, Stony Brook, USA), Juan Liu (University of California Berkeley, Berkeley, USA), David Lageson, Andrew Laskowski, James Schmitt, and John Wilson (all Montana State University, Bozeman, USA) as well as from Richard Carr (Fort Hays State University, Hays, USA) and Elaina Juedeman, Giulio Panascì, Kelli Roemer, and Kevin Surya (all Montana State University) who reviewed early drafts of the manuscript.
References
Abramoff, M.D., Magalhaes, P.J., and Ram, S.J. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42.
Andreae, A. 1893. Vorlaufige Mittheilung uber die Ganoiden (Lepidosteus und Amia) des Mainzer Beckens. Verhandlungen des Naturhistorisch-medizinischen Vereins zu Heidelberg 2: 7–15.
Archibald, S.B., Bossert, W.H., Greenwood, D.R., and Farrell, B.D. 2010. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36: 374–398. Crossref
Arratia, G. 2000. New teleostean fishes from the Jurassic of southern Germany and the systematic problems concerning the “pholidophoriforms”. Paläontologische Zeitschrift 74: 113–143. Crossref
Arratia, G. 2013. Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei). Society of Vertebrate Paleontology Memoir 13. Journal of Vertebrate Paleontology 13 (Supplement to 6): 1–138. Crossref
Barton, D.G. and Wilson, M.V.H. 2005. Taphonomic variations in Eocene fish-bearing varves at Horsefly, British Columbia, reveal 10,000 years of environmental change. Canadian Journal of Earth Sciences 42: 137–149. Crossref
Becker, G.C. 1983. Bowfin family—Amiidae. In: G.C. Becker (ed.), Fishes of Wisconsin, 249–254. University of Wisconsin Press, Madison.
Bentley, S.J., Blum, M.D., Maloney, J., Pond, L., and Paulsell, R. 2016. The Mississippi River source-to-sink system: Perspectives on tectonic, climatic, and anthropogenic influences, Miocene to Anthropocene. Earth-Science Reviews 153: 139–174. Crossref
Bestland, E.A., Hammond, P.E., Blackwell, D.L.S., Kays, M.A., Retallack, G.J., and Stimac, J. 1999. Geologic framework of the Clarno Unit, John Day Fossil Beds National Monument, central Oregon. Oregon Geology 61: 3–19.
Bishop, P. 1995. Drainage rearrangement by river capture, beheading and diversion. Progress in Physical Geography: Earth and Environment 19: 449–473. Crossref
Bonaparte, C.L. 1838. Selachorum tabula analytica. Nouvelles Annales des Sciences Naturelles 2: 195–214.
Burridge, C.P., Craw, D., Jack, D.C., King, T.M., and Waters, J.M. 2008. Does fish ecology predict dispersal across a river drainage divide? Evolution 62: 1484–1499. Crossref
Cavender, T.M. 1968. Freshwater fish remains from the Clarno Formation Ochoco Mountains of north-central Oregon. The Ore Bin 30: 125–141.
Constenius, K. 1996. Late Paleogene extensional collapse of the Cordilleran foreland fold and thrust belt. Geological Society of America Bulletin 108: 20–39. Crossref
Constenius, K., Dawson, M., Pierce, H.G., Walter, R., and Wilson, M. 1989. Reconnaissance paleontologic study of the Kishenehn Formation, northwestern Montana and southeastern British Columbia. In: D.E. French and R.F. Grabb (eds.), Montana Geological Society 1989 Field Conference Guidebook, Montana Centennial Edition, 189–203. Montana Geological Society, Billings.
Cope, E.D. 1875. On the fishes of the Tertiary shales of the South Park. Bulletin of the United States Geological Survey of the Territories 1: 3–5. Crossref
Cope, E.D. 1887. Zittel’s manual of palaeontology. American Naturalist 21: 1014–1019. Crossref
Elder, R.L. and Smith, G.R. 1988. Fish taphonomy and environmental inference in paleoliminology. Palaeogeography, Palaeoclimatology, Palaeoecology 62: 577–592. Crossref
Galloway, W.E., Whiteaker, T.L., and Ganey-Curry, P. 2011. History of Cenozoic North American drainage basin evolution, sediment yield, and accumulation in the Gulf of Mexico basin. Geosphere 7: 938–973. Crossref
Grande, L. and Bemis, W. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. Society of Vertebrate Paleontology Memoir 4, Journal of Vertebrate Paleontology 18: 1–690. Crossref
Greenwalt, D. and Labandeira, C. 2013. The amazing fossil insects of the Eocene Kishenehn Formation in Northwestern Montana. Rocks & Minerals 88: 434–441. Crossref
Greenwalt, D., Goreva, Y., Siljeström, S., Rose, T., and Harbach, R.E. 2013. Hemoglobin-derived porphyrins preserved in a middle Eocene blood-engorged mosquito. Proceedings of the National Academy of Sciences 110: 18496–18500. Crossref
Greenwalt, D., Rose, T., and Chatzimanolis, S. 2016. Preservation of mandibular zinc in a beetle from the Eocene Kishenehn Formation of Montana, USA 1. Canadian Journal of Earth Science 53: 614–621. Crossref
Greenwalt, D., Rose, T., Siljestrom, S., Goreva, Y., Constenius, K., and Wingerath, J.2014. Taphonomic studies of the fossil insects of the middle Eocene Kishenehn Formation. Acta Palaeontologica Polonica 60: 931–947. Crossref
Hay, O.P. 1929. Second Bibliography and Catalogue of the Fossil Vertebrata of North America, Bibliography of Fossil Vertebrates (1901–1927). 932 pp. Carnegie Institution of Washington, Washington. Crossref
Huber, M. and Caballero, R. 2011. The early Eocene equable climate problem revisited. Climate of the Past 7: 603–633. Crossref
Linnaeus, C. 1766. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 532 pp. Laurentii Salvii, Holmiae. Crossref
Li, G.-Q. and Wilson, M.V.H. 1994. An Eocene species of Hiodon from Montana, its phylogenetic relationships, and the evolution of the postcranial skeleton in the Hiodontidae (Teleostei). Journal of Vertebrate Paleontology 14: 153–167. Crossref
Liu, J., Wilson, M., and Murray, A. 2016. A new catostomid fish (Ostariophysi, Cypriniformes) from the Eocene Kishenehn Formation and remarks on the North American species of †Amyzon Cope, 1872. Journal of Paleontology 1: 1–17. Crossref
Maddison, W.P. and Maddison, D.R. 2019. Mesquite: a Modular System for Evolutionary Analysis. Version 3.61.http://www.mesquiteproject.org.
Mancuso, A.C. 2003. Continental fish taphonomy: a case study in the Triassic of Argentina. Journal of South American Earth Sciences 16: 275–286. Crossref
Mustoe, G.E. 2011. Cyclic sedimentation in the Eocene Allenby Formation of south-central British Columbia and the origin of the Princeton Chert fossil beds. Canadian Journal of Earth Sciences 48: 25–43. Crossref
Patterson, C. 1973. Interrelationships of holosteans. In: P.H. Greenwood, R.S. Miles, and C. Patterson (eds.), Interrelationships of Fishes, 233–305. Academic Press, London.
Pierce, H.G. and Constenius, K.N. 2014. Terrestrial and aquatic mollusks of the Eocene Kishenehn Formation, Middle Fork Flathead River, Montana. Annals of the Carnegie Museum 82: 305–329. Crossref
Rambaut, A. 2017. FigTree, a Graphical Viewer of Phylogenetic Trees. Version 1.4.4. http://tree.bio.ed.ac.uk/software/figtree/.
Regan, C.T. 1923. The skeleton of Lepidosteus, with remarks on the origin and evolution of the lower Neopterygian fishes. Proceedings of the Zoological Society of London 93: 445–461. Crossref
Romer, A.S. and Fryxell, F.M. 1928. Paramiatus gurleyi, a deep-bodied amiid fish from the Eocene of Wyoming. American Journal of Science 16: 519–527. Crossref
Rosen, D.E., Forey, P.L., Gardiner, B.G., and Patterson, C. 1981. Lungfishes, tetrapods, paleontology, and plesiomorphy. Bulletin of the American Museum of Natural History 167: 159–276.
Smith, S.A. and Bermingham, E. 2005. The biogeography of lower Mesoamerican freshwater fishes. Journal of Biogeography 32: 1835–1854. Crossref
Stamatakis, A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. Crossref
Unmack, P.J. 2001. Biogeography of Australian freshwater fishes. Journal of Biogeography 28: 1053–1089. Crossref
Wang, H., Gurnis, M., and Skogseid, J. 2020. Continent-wide drainage reorganization in North America driven by mantle flow. Earth and Planetary Science Letters 530: 115910. Crossref
Wilson, M.V.H. 1977. Paleoecology of Eocene lacustrine varves at Horsefly, British Colombia. Canadian Journal of Earth Sciences 14: 953–962. Crossref
Wilson, M.V.H. 1980. Eocene lake environments: Depth and distance-from-shore variation in fish, insect, and plant assemblages. Palaeogeography, Palaeoclimatology, Palaeoecology 32: 21–44. Crossref
Wilson, M.V.H. 1982. A new species of the fish Amia from the middle Eocene of British Columbia. Palaeontology 25: 413–424.
Wilson, M.V.H. 1988. Reconstruction of ancient lake environments using both autochthonous and allochthonous fossils. Palaeogeography, Palaeoclimatology, Palaeoecology 62: 609–623. Crossref
Wilson, M.V.H. and Barton, D.G. 1996. Seven centuries of taphonomic variation in Eocene freshwater fishes preserved in varves: paleoenvironments and temporal averaging. Paleobiology 22: 535–542. Crossref
Wolfe, J.A. 1995. Paleoclimatic estimates from Tertiary leaf assemblages. Annual Review of Earth and Planetary Sciences 23: 119–142. Crossref
Yonkee, W.A. and Weil, A.B. 2015. Tectonic evolution of the Sevier and Laramide belts within the North American Cordillera orogenic system. Earth-Science Reviews 150: 531–593. Crossref
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, Rhythms, and Aberrations in Global Climate. Science 292: 686–693. Crossref
Acta Palaeontol. Pol. 67 (2): 493–508, 2022
https://doi.org/10.4202/app.00733.2020
Table of measurements (in mm) and counts for USNM 618000. * estimated due to positioning of fossil or poor preservation; ** centra count exclude the first two vertebral centra (C1 and C2); ? unknown.
|
Head length |
145 |
Left and right maxillary teeth |
? |
|
Head width |
57.4* |
Left and right branchiostegals |
? |
|
Mandibular length |
>107.4 |
Total centra |
89** |
|
Gular length |
60* |
Total preural centra |
81** |
|
Frontal length |
41* |
Preural caudal centra (ural caudal) |
>20 (8) |
|
Snout length |
36.6 |
Abdominal centra |
<61 |
|
Postorbital length |
>38.6 |
Total diplospondylous vertebrae |
? |
|
Gular width/length |
0.35* |
Ossified supraneurals |
? |
|
Frontal width/length |
0.45* |
Ossified median neural spines in caudal region |
? |
|
Parietal width/length |
? |
Ossified paried neural spines of caudal region |
? |
|
Parietal length/frontal length |
? |
Total ossified neural arches |
? |
|
Snout length/postorbital length |
<0.94 |
Ossified infrahaemals |
? |
|
Right opercle width/height |
1.97* |
Ossified median preural haemal spines |
? |
|
Total length |
590 |
Ossified hypurals |
10 |
|
Standard length |
522 |
Ossified hypochordal elements supporting caudal rays |
? |
|
Body depth |
? |
Ossified epurals |
3 |
|
Prepectoral length |
166 |
Total caudal rays |
20 |
|
Prepelvic length |
268 |
Epaxial caudal rays |
? |
|
Predorsal length |
? |
Hypaxial caudal rays |
20* |
|
Preanal length |
357 |
Dorsal fin rays |
? |
|
Dorsal fin base |
? |
Branched dorsal rays |
? |
|
Anal fin base |
38 |
Ossified dorsal proximal radials |
? |
|
Dorsal/anal fin base |
? |
Anal fin rays (rudimentary) |
14 (1)* |
|
Caudal fin length |
137 |
Branched anal rays |
? |
|
Caudal preduncle length |
134 |
Ossified anal proximal radials |
? |
|
Caudal preduncle depth |
47 |
Pectoral fin rays |
9* |
|
Left and right coronoids |
? |
Branched pectoral fin rays |
? |
|
Left and right dentary teeth |
? |
Pelvic fin rays |
? |
|
Left premaxillary teeth |
6 |
Scales |
? |