

A unique dentary suggests a third genus of batrachosauroidid salamander existed during the latest Cretaceous in the western USA
JAMES D. GARDNER
Gardner, J.D. 2022. A unique dentary suggests a third genus of batrachosauroidid salamander existed during the latest Cretaceous in the western USA. Acta Palaeontologica Polonica 67 (1): 35–50.
An incomplete salamander dentary (AMNH FARB 22965) described herein from the upper Maastrichtian Lance Formation, Wyoming, USA, exhibits a puzzling suite of features. Four features—a prominent bony trough extending anteriorly and curving upwards along the lingual surface of the ramus, lack of an obvious Meckelian fossa or groove, an apparent gap in the tooth row, and a symphysial-like first tooth—are likely anomalies. However, the remaining features are interpreted as normal structures and suggest that AMNH FARB 22965 represents a new genus and species of batrachosauroidid, an extinct family of neotenic salamanders that were prominent components of Cretaceous to Neogene freshwater and floodplain paleocommunities in North America and Europe. The new taxon differs from other batrachosauroidids in a unique suite of dentary and dental features, most notably in having a lingual bony flange paralleling the posterior two-thirds of the dentary tooth row, a prominent and robust coronoid process bearing a grooved anterior face, and the anterior portion of the corpus dentalis behind the symphysis is broadly expanded ventrolingually. The presence of a third batrachosauroidid taxon in the Lance Formation was unexpected, considering that the formation has been well sampled and that its two previously recognized batrachosauroidids, namely Opisthotriton kayi and Prodesmodon copei, are known by abundant isolated bones, including dozens of dentaries, from numerous localities in the unit and elsewhere in the North American Western Interior. Known by a unique dentary from the Bushy Tailed Blowout locality, the taxon represented by AMNH FARB 22965 evidently was uncommon within the Lance Formation paleoenvironment.
Key words: Lissamphibia, Caudata, Batrachosauroididae, Cretaceous, Maastrichtian, Lance Formation, North America.
James D. Gardner [james.gardner@gov.ab.ca], Royal Tyrrell Museum of Palaeontology, Box 7500, Drumheller, Alberta, Canada, T0J 0Y0.
Received 15 July 2021, accepted 27 August 2021, available online 30 March 2022.
Copyright © 2022 J.D. Gardner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Salamanders are a clade of lissamphibians characterized by a generalized tetrapod body plan (i.e., moderately elongate trunk and tail, relatively distinct head, and, primitively, two pairs of similar sized limbs), with a largely Northern Hemisphere distribution and a fossil record extending back to the Middle/Upper Triassic (e.g., Ivachnenko 1978; Estes 1981; Duellman and Trueb 1986; Milner 2000; Vitt and Caldwell 2009; Schoch et al. 2020). Isolated and rare articulated fossil bones from numerous localities in the North American Western Interior (i.e., region to the east of the Rocky Mountains, extending from the foothills eastwards into the Great Plains and stretching from western Canada, southwards through the western USA, and into northern Mexico) demonstrate that salamanders were ubiquitous components of lowland, riparian- and floodplain-dominated ecosystems in the region during the Late Cretaceous (e.g., Estes 1964; Estes et al. 1969; Estes and Berberian 1970; Sahni 1972; Brinkman 1990; Rowe et al. 1992; Peng et al. 2001; Pearson et al. 2002; DeMar and Breithaupt 2008; Aguillon Martinez 2010; Gardner and DeMar 2013; Gardner et al. 2013; Wilson et al. 2014). Late Cretaceous salamander assemblages in the Western Interior consist of neotenic taxa and, by the late Maastrichtian, a mixture of extinct (Batrachosauroididae and Scapherpetidae) and extant (Amphiumidae, Proteidae, and Sirenidae) families were present (e.g., Goin and Auffenberg 1958; Auffenberg 1961; Estes 1964, 1965, 1969a, b, 1981; Estes et al. 1969; Carpenter 1979; Naylor 1979, 1983; Breithaupt 1982; Gardner 2000b, 2003a, b, 2012; Holman 2006; Bonett et al. 2013; DeMar 2013; Gardner and DeMar 2013; Wilson et al. 2014). In the upper Maastrichtian in the Western Interior, two named species of batrachosauroidids have been recognized on the basis of abundant isolated vertebrae, jaws, and other skull bones (e.g., Auffenberg 1961; Estes 1964, 1981; Naylor 1979; Holman 2006; Gardner and DeMar 2013; Wilson et al. 2014): Opisthotriton kayi and Prodesmodon copei. Here I describe a unique dentary from Wyoming, USA, that suggests a third and less common batrachosauroidid taxon was contemporaneous during the late Maastrichtian with O. kayi and Pro. copei.
Institutional abbreviations.—AMNH FARB, American Museum of Natural History, Fossil Amphibians, Reptiles, and Birds collection, New York, USA; OMNH, Oklahoma Museum of Natural History, University of Oklahoma, Norman, USA; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Canada; UALVP, University of Alberta Laboratory for Vertebrate Palaeontology, Edmonton, Canada; UCMP, University of California Museum of Paleontology, Berkeley, USA; USNM, National Museum of Natural History, Washington, D.C., USA; UW, University of Wyoming Collection of Fossil Vertebrates, Laramie, USA.
Material and methods
In this paper, I describe and interpret an incomplete salamander dentary (AMNH FARB 22965) recovered from the Bushy Tailed Blowout locality (UCMP locality V-5711; see Clemens 1963), within the type area of the Lance Formation, in Niobrara County, eastern Wyoming, USA. The Lance Formation consists largely of mudstone, shale, and sandstone beds deposited in a subtropical, fluvial and floodplain setting during the late Maastrichtian and it contains a diverse vertebrate assemblage, including multiple lissamphibian species (e.g., Clemens 1963; Estes 1964; Clemens et al. 1979; Breithaupt 1982; Kielan-Jaworowska et al. 2004; Weishampel et al. 2004; Gardner and DeMar 2013). For comparative purposes, I examined additional dentaries from the Bushy Tailed Blowout and Robber’s Roost localities (for the latter, see Breithaupt 1982) in the Lance Formation, a dentary from the paracontemporaneous Wounded Knee locality (Fox 1989; Redman et al. 2015) in the Frenchman Formation of southwestern Saskatchewan, Canada, a dentary from the slightly older (upper Campanian) OMNH locality V9 (Eaton and Cifelli 1988; Cifelli 1990) in the Kaiparowits Formation of southern Utah, USA, and mandibles from an extant, captive bred salamander.
My use of anatomical terms for salamander dentaries generally follows Vasilyan et al. (2013) where homologies are certain; where homologies are uncertain, I have coined informal, descriptive terms. I generally follow Smith and Dodson’s (2003) terms for anatomical surfaces and directions for teeth and jaws, except instead of their directional terms “rostral” and “caudal” for jaws I use, respectively, “anterior” and “posterior”. For descriptive purposes, dentaries are oriented in labial or lingual aspect with the anteroposteriorly longest and straightest portion of the ramus (typically most of the ramus behind the symphysial region) perpendicular to the viewer, the dorsal margin along the tooth-bearing portion of the ramus approximately horizontal, and the tooth row facing dorsally. Unless indicated otherwise, tooth positions are counted from the anterior end of the tooth row. Linear measurements are straight line distances.
I follow Milner (1988; see also Evans and Milner 1996) in using the name “Urodela” for crown-clade salamanders (i.e., all living salamanders and fossil taxa descended from the same common ancestor) and the name “Caudata” for the more inclusive group of crown-clade salamanders plus stem salamanders, such as the Late Jurassic Karaurus, the Middle Jurassic Kokartus and Marmorerpeton, and the Middle/Late Triassic Triassurus. My family level classification generally follows Estes (1981), with the exception that I follow Martin et al. (2012) in using the spelling “Scapherpetidae”, rather than “Scapherpetonidae” or “Scapherpetontidae”, for the family of extinct, neotenic salamanders that were endemic to North America and co-existed with batrachosauroidids.
Systematic palaeontology
Subclass Lissamphibia Haeckel, 1866
Order Caudata Scopoli, 1777
Crown-order Urodela Duméril, 1806
Family Batrachosauroididae Auffenberg, 1958
Remarks.—Batrachosauroididae are an extinct family of neotenic salamanders reliably known by isolated bones and rare skeletons from the Aptian/Albian–late Miocene of North America, the Campanian–late Miocene of Europe, and, potentially, back into the Bathonian–Berriasian of Europe (e.g., Auffenberg 1958; Estes 1969a, 1981; Naylor 1981: table 1; Duffaud 1995; Milner 2000; Evans and McGowan 2002; Holman 2006; Oreska et al. 2013; Vasilyan and Yanenko 2020). The family contains six named genera (e.g., Estes 1981; Naylor 1981; Denton and O’Neill 1998; Milner 2000; Vasilyan and Yanenko 2020): Batrachosauroides Taylor and Hesse, 1943 (two species: early Eocene–middle Miocene, southern and western USA); Opisthotriton Auffenberg, 1961 (one species: middle/late Santonian–late Paleocene) and Prodesmodon Estes, 1964 (one species: middle/late Campanian–early Paleocene), both in the North America Western Interior; Peratosauroides Naylor in Estes, 1981 (one species: late Miocene, California, USA); Parrisia Denton and O’Neill, 1998 (one species: Campanian, New Jersey, USA); and Palaeoproteus Herre, 1935 (three species: late Paleocene–early Miocene, Austria, France, Germany, and Ukraine).
Batrachosauroididae indet. (probable new genus and species)
Figs. 1–3.
Material.—AMNH FARB 22965, incomplete left dentary from upper part of Lance Formation; upper Maastrichtian; Bushy Tailed Blowout locality (UCMP locality V-5711), type area of Lance Formation, Niobrara County, eastern Wyoming, USA.
Description.—AMNH FARB 22965 (Figs. 1–3) is a posteriorly incomplete left dentary that is broken through the posterior portion of its coronoid process and preserves 17 tooth positions. The dentary is relatively robust in construction and moderately elongate, measuring 10.0 mm in total preserved length (maximum horizontal distance between anterolabial corner of symphysis and posteriormost preserved end of coronoid process).
In lingual or labial outline (Figs. 1A3, A7, 2A1, A2, 3A1, A2), AMNH FARB 22965 resembles a horizontally elongate, irregular quadrilateral. The dentary is moderately deep, expanding from a maximum anterior depth of 1.5 mm (vertical distance between dorsal and ventral edges of symphysis) to a maximum posterior depth of 3.8 mm (vertical distance between apex of coronoid process and broken posteroventral corner of bone). Compared to the shallower anterior end, the posterior portion of the dentary is expanded both dorsally and ventrally. The anteriormost end of the bone is formed by the labial rim of the symphysis. That margin is blunt in labial or lingual outline, with its lower two-thirds straight and slightly tilted posteriorly, whereas its upper one-third is broadly convex and tilted more posteriorly. Proceeding posteriorly from the low and anteroposteriorly short bulge that represents the dorsal rim of the symphysis, the dorsal margin along much of the pars dentalis is shallowly sinuous in labial or lingual outline. A short stretch of the dorsal margin adjacent to the second tooth position is shallowly concave. Beginning adjacent to the third tooth position, the dorsal edge ascends posteriorly in a low and nearly straight line to the apex of a low rise located about one-third of the distance along the ramus or adjacent to the seventh tooth position. From that low apex, the dorsal edge continues posteriorly as a shallowly concave arc to a point about two-thirds of the distance along the ramus or adjacent to the thirteenth tooth position. From there, the dorsal margin continues posteriorly and curves upwards at a steeper angle of about 20° in a gently concave arc and terminates behind the tooth row in a pronounced coronoid process. The ventral edge of the dentary descends at a shallow angle posteriorly from the symphysis and is weakly sinuous in lingual and, especially, labial outline. As best seen in labial view (Figs. 1A7, 2A1, 3A1), two ventral bulges are present behind the symphysis: the shallow and more anterior bulge is the ventral rim of the symphysis, whereas the larger, more posterior, and lingually displaced bulge is the ventral portion of the linguoventrally-directed, post-symphysial expansion (see below). Behind those bulges, the ventral edge of the dentary descends in a shallow, ventrally concave arc at about 10° below horizontal, before abruptly terminating where the posterior end of the dentary is broken. Extrapolating from the ventral profile along the preserved portion of the bone suggests that the missing, more posterior portion was even deeper when the dentary was intact. The posterior end of the specimen is broken in a jagged line that dorsally begins along the posterior base of the coronoid process and extends anteroventrally through the upper two-thirds of the bone, then continues ventrally through the lower one-third of the bone as a vertical break approximately in line with the posterior end of the tooth row.
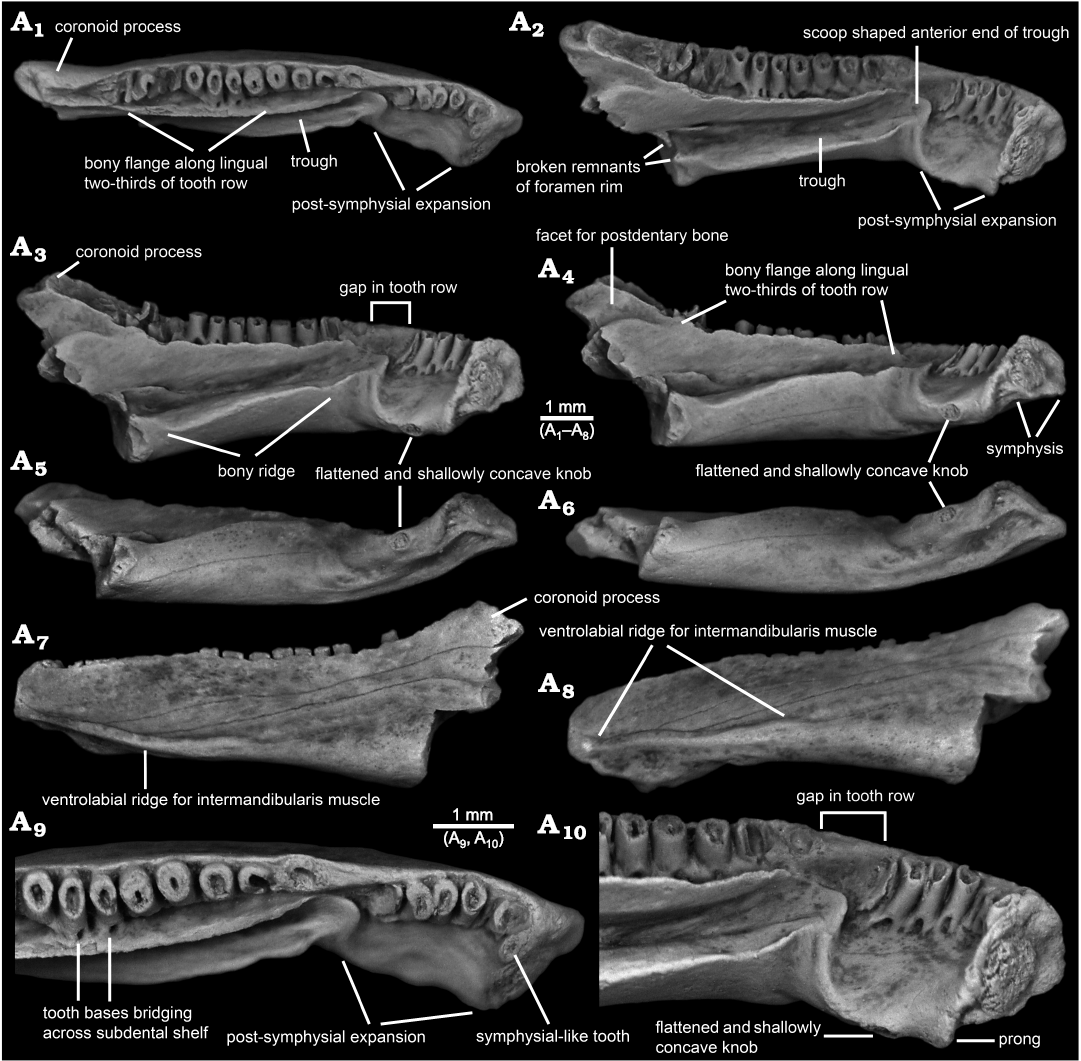
Fig. 1. Photographs of indeterminate batrachosauroidid (probable new genus and species) left dentary, AMNH FARB 22965, from the uppermost Cretaceous (upper Maastrichtian) Lance Formation, Bushy Tailed Blowout, Wyoming, USA. Entire specimen in dorsal (A1), lingual-dorsal (A2), lingual (A3), lingual-ventral (A4), ventral and slightly lingual (A5), ventral (A6), labial (A7), and labial and slightly ventral (A8) views. Detail of anterior end in dorsal (A9) and lingual-dorsal (A10) views. Specimen lightly dusted with ammonium chloride to enhance surface details.
In dorsal or ventral view (Figs. 1A1, A6, 3A3), AMNH FARB 22965 is shallowly arcuate or convex labially and is shallowly bent at the level of the third tooth position. Posterior from the symphysis and relative to the sagittal plane (as demarcated by the symphysial surface), the long axis of the ramus initially extends labioposteriorly at about 60° for a short distance. At the level of the third tooth position, the orientation changes and the remainder of the ramus extends posteriorly at a shallower angle of about 30° relative to the sagittal plane. The dentary is moderately wide labiolingually, having a maximum width of 1.7 mm (measured horizontally and perpendicular to the long axis) at approximately the anteroposterior mid-point of the specimen or in line with the eleventh tooth position.
The lingual surface of AMNH FARB 22965 (Figs. 1A2–A4, A9, A10, 2A2–A6, 3A2) is complex and exhibits several unusual features for a salamander, most notably (i) a pronounced post-symphysial expansion occupying about the anterior one-third of the tooth-bearing portion of the dentary, (ii) a prominent trough extending along the posterior two-thirds of the ramus and curving upwards along its anterior portion towards the marginal tooth row, (iii) approximately at the boundary between those first two features, an apparent gap in the marginal tooth row, and (iv) lack of an obvious Meckelian fossa or groove. Approximately in line with the gap in the tooth row and most obvious in dorsal view (Figs. 1A1, A9, 3A3), the lingual surface of the dentary is labially constricted or pinched and, at that point, is just 1.2 mm wide labiolingually (measured horizontally and perpendicular to the long axis of the ramus). Between this constricted zone and the symphysis, the subdental shelf and its shallow, underlying corpus dentalis are broadly expanded lingually and ventrally to form a prominent shelf that I informally call the “post-symphysial expansion”. This post-symphysial expansion has a maximum labiolingual width of 1.5 mm (measured horizontally and perpendicular to the long axis of the ramus at the level of the fourth tooth) and it extends ventrolingually at about 40° degrees below horizontal. The post-symphysial expansion is dorsoventrally deepest labially and shallowest lingually. Its dorsal surface is broadly convex labiolingually, essentially flat anteroposteriorly, and shallowly pitted, and its labialmost portion grades into the pars dentalis below the bases of the anteriormost six teeth. The anterolingual corner of the post-symphysial expansion extends slightly beyond the ventroposterior rim of the symphysis, as a short, blunt, and prong-like projection (best seen in dorsolingual view: Figs. 1A2, A10, 2A6). The lingual rim of the post-symphysial expansion is broadly convex ventrolingually in transverse view and, midway along its length, bears a small, knob-like structure (“flattened and shallowly concave knob” in Figs. 1A3–A6, A10, 2A5, 3A2) consisting of a raised rim that is elliptical in outline, with its long axis extending anteroposteriorly. The surface enclosed by that raised rim is shallowly bowl-shaped and has a slightly roughened texture. The posterior rim of the post-symphysial expansion curves dorsally and labially, grading into the anterior end of the prominent trough described below.
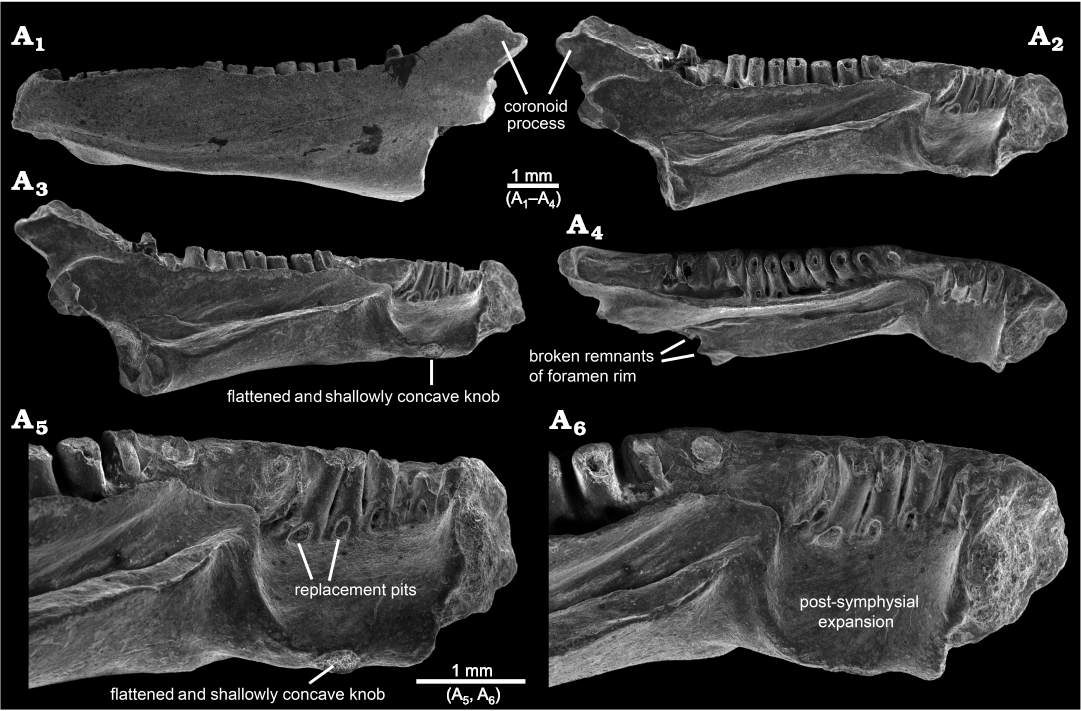
Fig. 2. SEM micrographs of indeterminate batrachosauroidid (probable new genus and species) left dentary, AMNH FARB 22965, from the uppermost Cretaceous (upper Maastrichtian) Lance Formation, Bushy Tailed Blowout, Wyoming, USA. Entire specimen in labial (A1), lingual and slightly dorsal (A2), lingual (A3), and dorsal and slightly lingual (A4) views. Detail of anterior end in lingual (A5) and lingual-dorsal (A6) views.
Behind the post-symphysial expansion is a prominent bony ridge (Figs. 1A2–A4, 2A2–A4, 3A2) that is moderately deep and wide, and extends anteroposteriorly along the ventral part of the lingual face of the ramus. This bony ridge is potentially homologous with the “corpus dentalis” of Vasilyan et al. (2013), but for the purposes of this description I refer to it informally as the “bony ridge”. Regardless of its identity, this bony ridge is deepest anteriorly and becomes shallower posteriorly, before abruptly ending where the bone is broken. The lingual surface of the bony ridge is broadly convex in transverse view and tilted slightly ventrally, with its lower edge grading into the ventral surface of the ramus. The dorsal surface of the bony ridge is developed as a lingually broad shelf that is broadest midway along its preserved length and its surface is shallowly convex from side-to-side. Its dorsolingual edge is developed as a keel-like and low rim anteriorly, rendering the shelf more gutter-like along that portion, whereas posteriorly the dorsolingual edge becomes lower and blunter, rendering the shelf flatter along that portion. The junction between the labial portion of the shelf and adjacent lingual wall of the ramus is shallowest and nearly perpendicular along its central portion, but deepens to form a narrow trench more posteriorly and, especially, anteriorly. Dorsal from that junction, the lingual wall of the ramus rises dorsally and curves slightly lingually, with its dorsalmost portion developed as a labiolingually narrow flange (Figs. 1A1–A4, A9, A10, 2A2–A4, 3A2, A3) that approximately parallels the posterior two-thirds of the tooth row. This narrow flange becomes shallower both anteriorly and posteriorly; the flange anteriorly terminates just behind the gap in the tooth row, whereas posteriorly it extends posterodorsally as a low rim along the lingual edge of the anterior face of the coronoid process. Collectively, the shelf-like dorsal surface of the bony ridge and adjacent lingual wall of the ramus form an elongate, lingually broad trough (Figs. 1A1–A4, A9, A10, 2A2–A6, 3A2, A3) that extends anterodorsally and slightly lingually in a shallow arc, to just past the constricted zone, at which point the trough terminates in a well-defined, scoop-shaped structure below, and lingual to, the gap in the tooth row. The anterior rim of this scoop, in turn, ventrally gives rise to the above-mentioned low ridge that curves ventrolingually and anteriorly, before grading into the posterior rim of the post-symphysial expansion. At its broken posterior end and adjacent to the trough, the dentary bears a pair of small, hook-like projections, one extending dorsally from the dorsolingual corner of the bony ridge and one extending lingually from the lingual wall of the ramus just above the dorsal surface of the bony ridge, that partially enclose a small, semi-circular opening (Figs. 1A2, 2A4). These projections may be the broken rim of a foramen or canal. No obvious Meckelian fossa or groove is present below or immediately behind the tooth-bearing portion of the ramus.
As best seen in lingual and dorsal views (Figs. 1A1–A3, A9, A10, 2A2–A6, 3A2, A3), the pars dentalis is moderately high and 17 tooth positions are preserved on AMNH FARB 22965. Suspected damage to the dorsal surface of the bone at the junction between the coronoid process (see below) and posterior end of the tooth row raises the possibility that an additional tooth position may have been present there in life. The preserved tooth positions (numbered in Fig. 3A2, A3) consist of ten relatively complete pedicels (i.e., preserving intact or nearly intact dorsal rims, at positions 1, 3–5, 9–14), six fragmentary pedicles (at positions 2, 6, 8, 15–17), and one empty slot preserved as a shallowly bowl-shaped divot (at position 7). No tooth crowns or replacement teeth are preserved. As argued below (see “Remarks”) I interpret the teeth as having been pedicellate. Tooth pedicels vary in their position, attachment, and form. In general, the pedicles are marginal in position, moderately pleurodont in attachment (i.e., attached along most of their height to the adjacent bony wall of the dentary), closely spaced, perforated at their base by a lingual replacement pit, and have smooth walls that are subcircular to mesiodistally compressed. The first six teeth along the post-symphysial expansion are separated from the remaining 11 tooth positions by a short gap, located about one-third of the distance along the tooth row and equivalent in length to the diameters of two or three pedicles. When examined visually using a binocular microscope (e.g., Fig. 1A10), this toothless interval does not seem to be an artefact, because the exposed lingual wall of the pars dentalis appears smooth and lacks any trace of tooth bases or slots. However, scanning electron micrographs (Fig. 2A5, A6) indicate that same surface is slightly roughened, which leaves open the possibility that weakly attached teeth may have been present in life, but were detached post-mortem. The second to seventeenth teeth are clearly marginal, in that their pedicels are aligned with each other in a gently curved row that parallels the pars dentalis and each pedicel is labially attached to the lingual face of the pars dentalis. By contrast, when viewed from above, the first tooth pedicel (Fig. 1A9) is displaced or shifted slightly lingually, by about one-half the diameter of its pedicel, relative to the second tooth, and even more so relative to the arc described by the remainder of the tooth row. Also, the attachment of the first tooth differs in being approximately perpendicular to the others, with its pedicel attached mesially to the posterior face of the dorsal rim of the symphysis. Finally, the first tooth pedicel is slightly smaller and more noticeably compressed mesiodistally relative to the other pedicels. Based on the position, attachment, and form of its pedicel, the anteriormost tooth potentially may be a symphysial tooth. Tooth pedicels in front of the gap in AMNH FARB 22965 are completely exposed lingually, whereas those behind the gap are set into a deep gutter (Figs. 1A1, A2, 2A4, 3A2, A3). The labial wall of that gutter is formed by the pars dentalis, whereas the lingual wall is formed by the flange of bone described in the preceding paragraph that extends dorsolingually from the lingual wall of the ramus above the bony ridge. Bases of the adjacent twelfth and thirteenth tooth pedicels bridge the dental gutter and attach along the inner surface of the lingual wall of the gutter (Fig. 1A9), thereby roofing an anteroposteriorly short canal below. Pedicels at positions 1–6 and 12–14 have walls that are relatively thinner and more compressed mesiodistally, whereas the remaining pedicels are stouter and more subcircular in cross section. Pedicels along the post-symphysial expansion also are slightly recurved in lingual view, whereas those behind the gap are more nearly vertical.
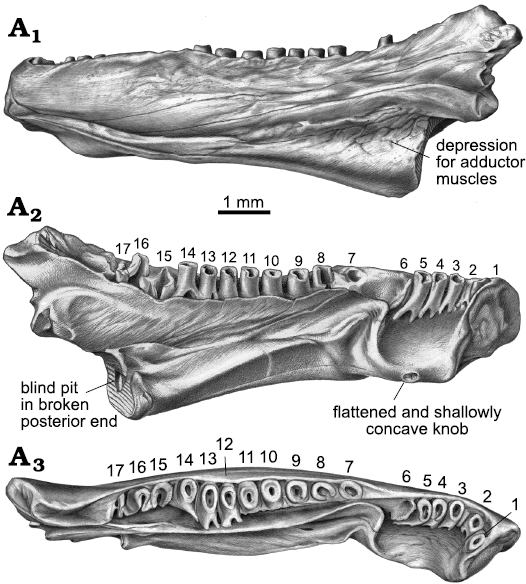
Fig. 3. Drawings of indeterminate batrachosauroidid (probable new genus and species) left dentary, AMNH FARB 22965, from the uppermost Cretaceous (upper Maastrichtian) Lance Formation, Bushy Tailed Blowout, Wyoming, USA. Entire specimen in labial (A1), lingual (A2), and dorsal (A3) views. Tooth positions are numbered anterior-to-posterior with Arabic numbers.
AMNH FARB 22965 bears a pronounced coronoid process (Figs. 1A1–A4, A7, 2A1–A4, 3) that is triangular in labial or lingual view and relatively high (1.0 mm high, measured as vertical height between the apex of the process downwards to the level of the thirteenth tooth position or where the dorsal edge of the ramus begins to curve dorsally and grades into the coronoid process). The coronoid process also is relatively robust, measuring 0.8 mm in maximum labiolingual width just below its apex. The coronoid process has the same roughened labial texture described below for the remainder of the bone. By contrast, the lingual surface of the coronoid process is smooth and indented by a broad, shallow facet that is demarcated ventrally by a dorsally convex rim; this facet presumably was for overlapping contact with a postdentary bone, most likely the prearticular. The inclined, anterior face of the coronoid process is indented by an anteroventrally-posterodorsally elongate groove that is shallowly concave from side-to-side and is bordered both labially and lingually by a low and narrow, yet distinct, bony rim. The posterior two-thirds of this groove has a slightly roughened texture and, thus, appears to preserve its natural surface. More anteriorly, however, the groove has a slightly jagged and shiny texture, suggesting its original external surface has been broken away along this portion of the coronoid process and, perhaps, the posteriormost end of the tooth row. Along its preserved length, the declined, posterior face of the coronoid process is smooth and shallowly concave from side-to-side, but lacks the distinct labial and lingual rims seen along the anterior face. Posterior breakage means the full posterior extent and form of the coronoid process are unknown.
The labial surface of AMNH FARB 22965 (Figs. 1A7, A8, 2A1, 3A1) is broadly convex, almost flat, in transverse profile and has a weakly roughened texture created by scattered pits that are small, extremely shallow, and irregular in outline. A few hairline cracks or grooves extend anteroventrally-posterodorsally at a shallow angle across the labial surface. Foramina mentalia (= external foramina) are lacking. A low, yet distinct ridge extends anteroposteriorly along the ventrolabial margin of the anterior one-half of the ramus. This ridge is labially convex in transverse profile along its anterior and posterior portions, but more keel-like along its central portion. The dorsal margin of the ridge is paralleled by a narrow, shallow groove. More posteriorly, the ventral portion of the labial face of the dentary is indented by a broad, shallow, and posteriorly deepening, anteroposterior depression. Comparisons with extant salamanders (e.g., Francis 1934; Özeti and Wake 1969; Larsen and Guthrie 1975; Erdman and Cundall 1984; Duellman and Trueb 1986; Lorenz Elwood and Cundall 1994; Kleinteich et al. 2014) suggest that the more anterior ridge and groove complex and the more posterior depression served as attachments for, respectively, intermandibularis and adductor muscles.
In ventral view (Fig. 1A5, A6), the above-described knob-like structure on the ventrolingual portion of the post-symphysial expansion and the elongate ventrolabial ridge for attachment of intermandibularis muscles are evident along the anterior portion of AMNH FARB 22965. Otherwise, the ventral surface is relatively smooth and lacks foramina, pits, or a ventral keel. In transverse profile, the ventral surface of the post-symphysial expansion is shallowly concave across the anterior portion and flatter across the posterior portion. Behind that region, the preserved ventral surface of the ramus is broadly convex in transverse profile.
The symphysis of AMNH FARB 22965 (Figs. 1A2–A4, A10, 2A2–A6, 3A2) is preserved intact. It is subtriangular in outline (best seen in Fig. 1A4), being deepest anteriorly and with a broadly convex anterior margin, and tapering to a blunt point linguoventrally. Its articular face is relatively unelaborated and nearly flat. An outer rim of relatively smooth bone encloses a central portion that is subcircular in outline and has a roughened and shallowly convex surface, presumably for ligamentous contact with the opposite mandible.
The posterior end behind and below the coronoid process is missing from AMNH FARB 22965, meaning little can be said about the form and extent of the area for attachment of postdentary bones, aside from suggesting that it was deep and robust. In keeping with the robust build of the specimen, its broken posterior surface (Figs. 1A4–A6, 2A3, A4, 3A2) is relatively wide labiolingually, with a maximum width of 1.4 mm (measured horizontally across the broken dorsal edge of the bony ridge). As described above, what appear to be the broken remnants of the rim of a foramen are preserved adjacent to the broken posterior end of the bony ridge. The broken posterior surface exposes a divot in the central portion of the bony ridge—this appears to be a blind pit (i.e., having a solid interior surface and not penetrating anteriorly into the bone). Otherwise, the broken posterior surface of the dentary appears solid.
Remarks.—AMNH FARB 22965 is a unique dentary that exhibits a puzzling mix of features, including the following: along its posterior two-thirds, the lingual surface bears a prominent bony trough that extends anteriorly and curves upwards towards the marginal tooth row, below the trough is a well-developed bony ridge that may be homologous with the corpus dentalis, above the trough is a tall and narrow bony flange lingually paralleling the posterior two-thirds of the tooth row, and no obvious Meckelian fossa or groove is present; more anteriorly, the lingual surface is developed into a ventrolingually projecting shelf (here called the “post-symphysial expansion”) whose ventrolingual face bears a flattened and shallowly concave knob; the labial surface lacks foramina mentalia and anteriorly bears a prominent ventrolabial ridge; the symphysis is subtriangular in outline and its face is flattened; a prominent and robust coronoid process is present and bears a grooved anterior face; teeth are highly pleurodont and evidently pedicellate, the anteriormost tooth potentially is a symphysial tooth, and there is an apparent gap in the tooth row. To my knowledge, this suite of features has never been reported in a single dentary.
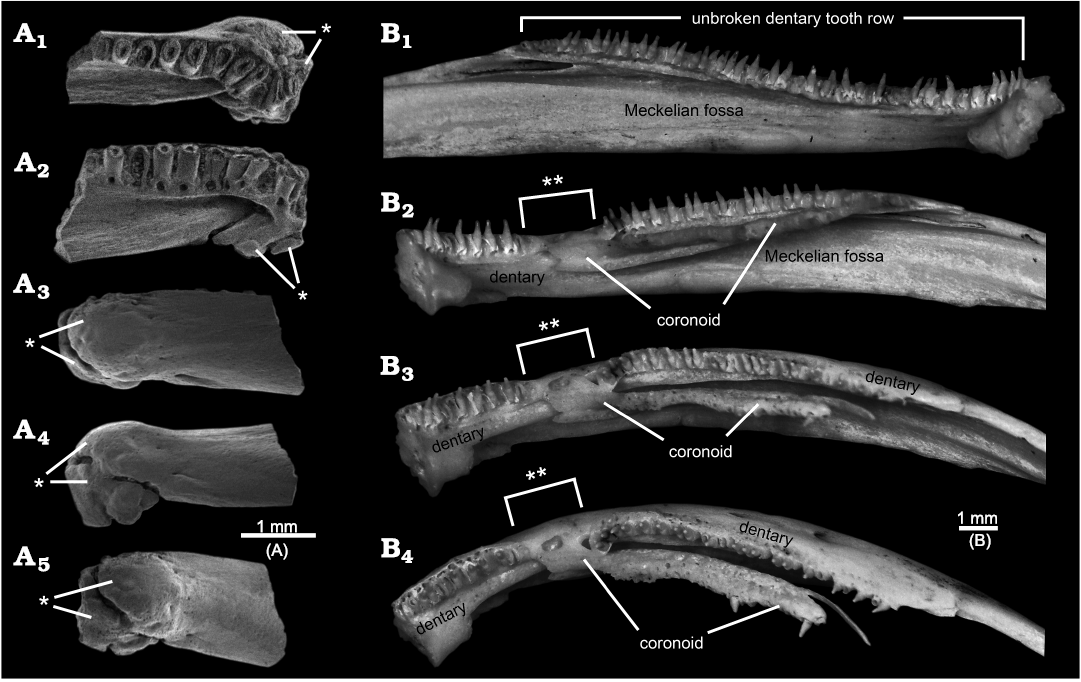
Fig. 4. Examples of pathological salamander mandibles. A. ?Opisthotriton kayi Auffenberg, 1961, anterior portion of left dentary, OMNH 67080, from the Upper Cretaceous (upper Campanian) Kaiparowits Formation, OMNH locality V9, Utah, USA, in dorsal (A1), lingual and slightly dorsal (A2), labial (A3), ventral (A4), and anterior-labial and slightly ventral (A5) views. Note symphysial end distorted by a bony swelling or callus (indicated by single asterisk), presumably formed by healing after an unknown injury. B. Ambystoma mexicanum (Shaw and Nodder, 1798), entire tooth-bearing ramus of left and right dentaries and anterior portion of areas for attachment of post-dentary bones, both from TMP 2010.30.09, an extant and captive bred individual: normal left dentary in lingual view (B1), pathological right dentary + coronoid in lingual (B2), lingual-dorsal (B3), and dorsal (B4) views. Note continuous dentary tooth row in normal left dentary (B1) vs. anterior end of coronoid overlaps and causes gap (indicated by double asterisks) in dentary tooth row in pathological right mandible (B2–B4). Images SEM micrographs (A) and photographs of specimens lightly dusted with ammonium chloride to enhance surface details (B).
Some of the above-listed features may be anomalies or taphonomic artefacts. The bony trough along the lingual surface, the apparent gap in the tooth row, and lack of a Meckelian fossa or groove in AMNH FARB 22965 are strikingly peculiar and, to my knowledge, without precedent among amphibians. None of those features or any other portion of AMNH FARB 22965 shows gross distortions that imply trauma, unlike, for example, rare salamander dentaries from the Upper Cretaceous of Uzbekistan (Skutschas et al. 2018: fig. 2) and Utah, USA (Fig. 4A) preserved with bony calluses resulting from healing of traumatic injuries. The observation that the dorsally curved anterior end of the trough coincides with the gap in the tooth row, suggests those two features may be linked. A potentially instructive skeleton in the TMP collections of the extant, neotenic salamander Ambystoma mexicanum (Shaw and Nodder, 1798), has a normal left dentary bearing an unbroken tooth row and a pathological right dentary in which the anterior end of the lingually placed coronoid anomalously overlaps onto the tooth-bearing region of the dentary, thereby creating a gap in the dentary tooth row (Fig. 4B1 and B2–B4, respectively). It is unclear whether a similar explanation might explain the gap in the tooth row for AMNH FARB 22965 or whether the adjacent, anterior end of the bony trough may have squeezed out the teeth. As noted in the preceding descriptive section, faint hints of a roughened lingual wall for the portion of the pars dentalis along the gap raise the possibility that teeth may have been loosely attached there, but were lost post-mortem. The identity and function of the lingual trough in AMNH FARB 22965 are unclear. The position of the trough is consistent with it being associated with the Meckelian fossa or groove, despite the discrepancies that (i) the anterior end of the trough opens dorsally, rather than extending anteriorly into the interior of the ramus or opening lingually and (ii) the trough projects lingually outwards from the ramus and for much of its preserved length runs along the dorsal surface of what might be the corpus dentalis (here called the “bony ridge”), rather than being a labiolingually shallow fossa or groove within the subdental portion of the ramus (see “Meckelian fossa” labelled in Fig. 5). What appear to be the broken remnants of a foramen at the posterior end of the bony trough suggest that a non-osseus structure (e.g., artery, vein, nerve, or cartilage) passed through that opening and extended along the floor of the trough. Alternatively or, perhaps, additionally, the trough may have either articulated with or been lingually covered by one or more mandibular bones. Conceivably the peculiar lingual trough and gap in the tooth row might be the result of a postdentary bone—the prearticular is a strong candidate, considering its proximity to the affected area—having abnormally overgrown the dentary, thereby both distorting the Meckelian fossa or groove and interrupting the tooth row. That scenario also would explain the striking absence of an obvious (i.e., normal) Meckelian fossa or groove, which in salamanders is developed within the lingual surface of the dentary, below and immediately behind the tooth-bearing portion of the ramus, as a labiolingually shallow depression that is deepest posteriorly and shallows anteriorly (see Figs. 4B, 5). Given the likelihood that the bony trough, gap in the tooth row, and lack of a Meckelian fossa or groove are anomalies, those are best set aside as potentially informative features.
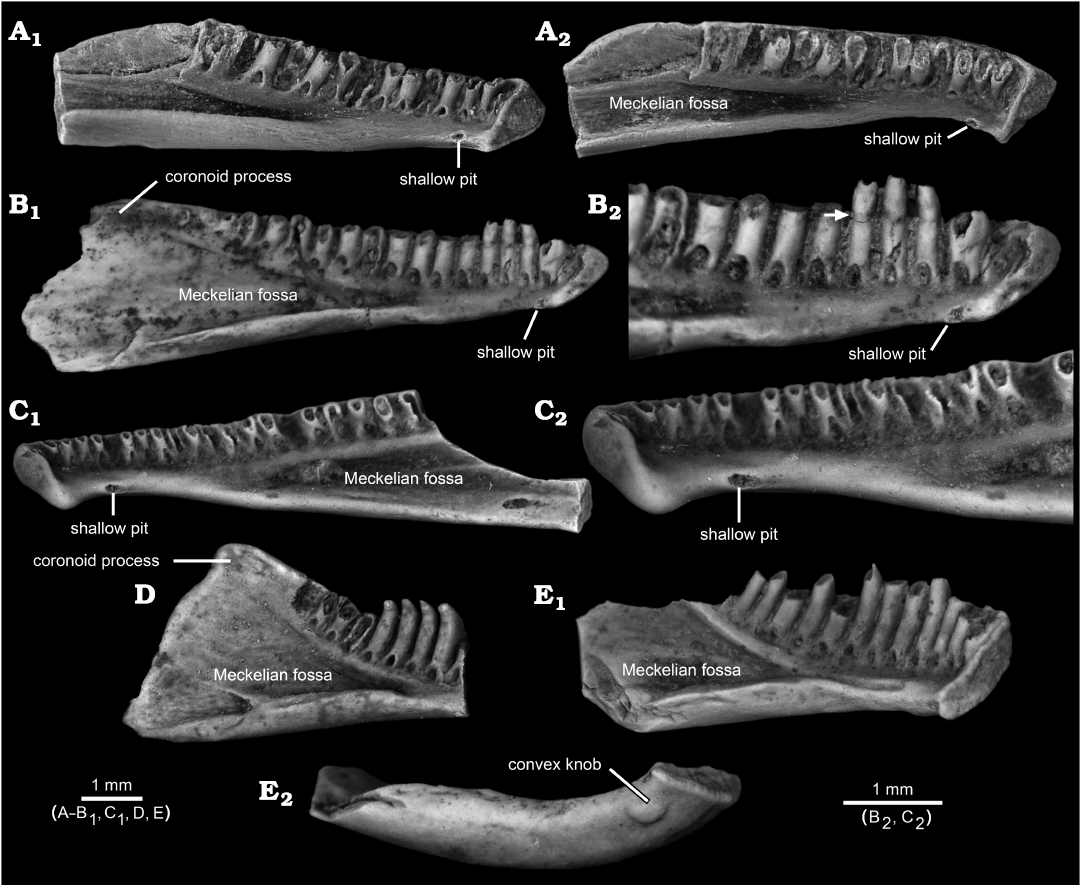
Fig. 5. Referred dentaries of other, paracontemporaneous (upper Maastrichtian) batrachosauroidid salamanders from the North American Western Interior. A–C. Opisthotriton kayi Auffenberg, 1961. A. Posteriorly incomplete left dentary, UW 14575, from Robber’s Roost, Lance Formation, Wyoming, USA, in lingual (A1) and lingual and slightly dorsal (A2) views. B. Nearly complete left dentary, UALVP 12093, from Bushy Tailed Blowout, Lance Formation, Wyoming, USA, lingual views of complete specimen (B1) and detail of anterior end (B2), the latter with arrow denoting slightly constricted zone of weakness between pedicel and crown typical of subpedicellate teeth. C. Posteriorly incomplete right dentary, UALVP 60835, from Wounded Knee, Frenchman Formation, Saskatchewan, Canada, lingual views of complete specimen (C1) and detail of anterior end (C2). D, E. Prodesmodon copei Estes, 1964. D. Anteriorly incomplete left dentary, UALVP 12092, from Bushy Tailed Blowout, Lance Formation, Wyoming, USA, in lingual view. E. Posteriorly incomplete left dentary, UALVP 39928, from Bushy Tailed Blowout, Lance Formation, Wyoming, USA, in lingual (E1) and ventral (E2) views. The three O. kayi dentaries (A–C) each bear a shallow pit in approximately the same position as the flattened and shallowly concave knob in AMNH FARB 22965, whereas the more anteriorly complete Pro. copei dentary (E) bears a convex knob positioned more ventrolabially relative to the potentially homologous pit in O. kayi and the flattened and shallowly concave knob in AMNH FARB 22965. Also note how many of the incomplete teeth in the three O. kayi dentaries (A–C) are broken at the same level, along the plane of weakness between the crown and pedicel, whereas nonpedicellate teeth in the two Pro. copei dentaries (D, E) are broken at various positions along the basal-apical lengths of the teeth. All images are photographs, with specimens lightly dusted with ammonium chloride to enhance surface details.
As noted in the preceding descriptive section, the peculiar anteriormost tooth in AMNH FARB 22965 is reminiscent of a symphysial tooth. I qualify that identification, because unequivocal symphysial teeth that characterize many non-lissamphibian amphibians (e.g., the Permian amphibamid Doleserpeton annectens Bolt, 1969; Sigurdsen and Bolt 2010: fig. 6A) and the enigmatic African Late Cretaceous salamander Kababisha sudanensis Evans, Milner, and Werner, 1996 (Evans et al. 1996: text-figs. 3A, 4A, B), as well as the similarly-placed splenial teeth of many caecilians (e.g., Nussbaum 1977: fig. 2; Taylor 1977), differ in being completely separated lingually from the marginal tooth row and occur within a discrete pocket or groove. By contrast, the anteriormost tooth in AMNH FARB 22965 is only partially shifted lingually relative to the succeeding teeth (i.e., about one-half the diameter of its pedicel, relative to the second tooth) and it is not placed within a discrete depression. Considering its intermediate position, the anteriormost tooth in AMNH FARB 22965 seems better regarded as a marginal first tooth that has been anomalously displaced lingually. That displacement conceivably could have happened if the anterior portion of the tooth row was compressed by the anomalous overgrowth of the prearticular, which left insufficient space for the first tooth to develop in its normal position. Interestingly, in the pathological axolotl dentary with a somewhat similarly interrupted tooth row depicted in Fig. 4B, its anteriormost teeth retain their normal marginal positions. The remaining features in AMNH FARB 22965 are seen to varying degrees in other amphibian dentaries, meaning they appear to be normal structures and, thus, are potentially informative for assessing the taxonomic affinities of this unique dentary.
AMNH FARB 22965 originally was tentatively identified in the AMNH records as a dentary belonging to the albanerpetontid lissamphibian Albanerpeton nexuosum Estes, 1981. That identification is understandable, considering that A. nexuosum is common within the Lance Formation (e.g., Estes 1964 [as jaws then referred to Prodesmodon copei Estes, 1964]; Gardner 2000a; Gardner and DeMar 2013) and that AMNH FARB 22965 superficially resembles dentaries of A. nexuosum (cf., Estes 1981: fig. 3H; Gardner 2000a: fig. 2) in size and robustness, in having moderate sized and highly pleurodont teeth, and in bearing a prominent ventrolabial ridge. However, AMNH FARB 22965 differs from all known albanerpetontid dentaries in lacking the stout and lingually projecting symphysial prongs that are autapomorphic for albanerpetontids, in lacking foramina mentalia labially and a distinctly gutter-like subdental shelf anteriorly, and in having teeth that are pedicellate, rather than being nonpedicellate as in albanerpetontids (e.g., Fox and Naylor 1982; Gardner 2001; Daza et al. 2020).
The inferred presence of pedicellate teeth in AMNH FARB 22965 is based on the observations that (i) many of its preserved tooth pedicels are similar in height, with their dorsal rims approximately in line with the dorsal edge of the pars dentalis, and (ii) where those dorsal rims are largely or completely intact, they are in an essentially horizontal plane and have a relatively smooth dorsal surface. That combination of features is indicative of teeth that exhibit some degree of pedicely (i.e., a poorly mineralized or fibrous zone of weakness between the shaft and crown: e.g., Parsons and Williams 1962; Duellman and Trueb 1986; Davit-Béal et al. 2007; Schoch 2014) and is routinely seen in extant and fossil amphibian jaws that have lost some or all of their crowns post-mortem, but retain their pedicels (e.g., Evans and Sigogneau-Russell 2001: text-fig. 1A, D; Evans and McGowan 2002: pl. 1: 5–7; Gardner 2003b: fig. 6D–F; Sigurdsen and Bolt 2010: fig. 6A). By contrast, breakage of nonpedicellate teeth can occur at any point along the basal-apical length of a given tooth, and those broken surfaces typically are irregular or jagged (e.g., Fig. 5D, E1). Teeth having some amount of pedicely are considered derived within gnathostomes and are characteristic for many lissamphibians and some amphibamids (e.g., Parsons and Williams 1962; Bolt 1969; Milner 1993; Sigurdsen and Bolt 2010; Schoch 2014; Schoch and Milner 2014). AMNH FARB 22965 shows some resemblance to dentaries of amphibamids, such as Doleserpeton annectens (e.g., Bolt 1991; Sigurdsen and Bolt 2010: fig. 6A), in having pedicellate teeth, a symphysial-like (but probably anomalous) tooth, and expansion of the subdental shelf and corpus dentalis behind the symphysis, yet AMNH FARB 22965 differs in having relatively larger and far fewer marginal teeth (as few as 17 vs. at least 60 in D. annectens), in lacking the multiple and more lingually placed symphysial teeth within a distinct depression or pocket and often arranged in a row in D. annectens, in being relatively more robust, and in lacking denticles and pits along the lingual surface below the pars dentalis. A further argument against regarding AMNH FARB 22965 as an amphibamid is that some 200 million years, according to the time scale of Ogg et al. (2016), separate the geologically youngest amphibamids (early Permian: see review by Schoch and Milner 2014) from AMNH FARB 22965 (latest Cretaceous).
Compared to non-albanerpetontid lissamphibians, AMNH FARB 22965 differs from dentaries of anurans (frogs) and resembles pseudodentaries of gymnophionans (caecilians) and dentaries of most caudates (salamanders) in having a relatively robust and complex ramus that bears teeth. The dentary of anurans is substantially different in being a slender, simple, and splint-like bone (e.g., Ecker 1889: fig. 21; Trueb 1993: fig. 6.16D, E) that primitively is toothless, with the sole exception of the extant Gastrotheca guentheri Fitzinger, 1843, which appears to have re-evolved dentary teeth (Wiens 2011; Paluh et al. 2021). The bony, single, and sometimes tusk-like odontoid developed near the anterior end of the mandible in some neobatrachian anurans (e.g., Lynch 1971: fig 15) is not a true tooth. Compared to AMNH FARB 22965, pseudodentaries of gymnophionans differ in having numerous foramina mentalia and, except for the Early Jurassic stem caecilian Eocaecilia micropodia Jenkins and Walsh, 1993, the labial surface is roughened with ridges and grooves and a prominent coronoid process is absent; additionally, many caecilian taxa (including E. micropodia and the Early Cretaceous Rubricaecilia monbaroni Evans and Sigogneau-Russell, 2001) have one or more splenial teeth located within a distinct depression lingual to, and often paralleling, the anterior portion of the marginal tooth row (e.g., Nussbaum 1977: fig. 2; Taylor 1977; Trueb 1993; Evans and Sigogneau-Russell 2001: text-fig. 1; Jenkins et al. 2007: figs. 24–27). AMNH FARB 22965 compares most favourably to caudate dentaries in lacking the above-listed features typical of amphibamids, caecilians, and anurans and in exhibiting a combination of features considered distinctive for salamanders (Estes et al. 1969; Naylor 1979; Fox and Naylor 1982), including: lack of a well defined Meckelian groove; poorly developed subdental shelf; symphysial surface dorsoventrally expanded and flattened; moderate sized and inverted U-shaped replacement pits in lingual bases of teeth; no foramina mentalia (present in some salamanders); and deep and, potentially, elongate region behind the tooth row (see Figs. 4B1, 5). The relatively smooth labial surface lacking ornament or a roughened texture and the presence of teeth in AMNH FARB 22965 also are typical for salamanders, although extant sirenids are unique among salamanders in having edentulous dentaries and upper jaws (e.g., Estes 1981; Duellman and Trueb 1986; Gardner 2003a: text-fig. 2F, 3D, H). Expansion of the anterior portion of the corpus dentalis behind the symphysis (= “post-symphysial expansion” here) is seen to varying degrees in some salamander dentaries (e.g., extant Amphiuma means Garden in Smith, 1821, and Amphiuma tridactylum Cuvier, 1827; Gardner 2003b: fig. 6D and F, respectively), although not as extreme as in AMNH FARB 22965.
The flattened and shallowly concave knob on the ventrolingual surface of the post-symphysial expansion in AMNH FARB 22965 has not, to my knowledge, previously been reported for salamander dentaries. Among comparative specimens available to me, I have identified similar structures only in referred, fossil dentaries belonging to two batrachosauroidid species: Opisthotriton kayi Auffenberg, 1961, dentaries bear a shallow pit (Fig. 5A–C), with a roughened and shallowly concave interior surface enclosed by a slightly raised rim, located on the ventrolingual surface of the corpus dentalis behind the symphysis (i.e., approximately equivalent to the position of the flattened and shallowly concave knob on AMNH FARB 22965) and Prodesmodon copei dentaries bear a convex and smooth knob (Fig. 5E), located at approximately the same anteroposterior position as the knob on AMNH FARB 22965 and the pit in O. kayi, but positioned slightly more ventrolabially relative to both on the underside of the ramus. Although differing in details, overall similarities in the form and position of the post-symphysial knob/pit structures in AMNH FARB 22965, O. kayi, and Pro. copei suggest those are homologous. The presence of a similar feature in other batrachosauroidids is uncertain. Dentaries are unknown (Naylor 1981) for Peratosauroides problematica (Naylor in Estes, 1981), the sole dentary described for Parrisia neocesariensis Denton and O’Neill, 1998, lacks its symphysial end (Denton and O’Neill 1998), and descriptions of dentaries for both species of Batrachosauroides (Taylor and Hesse 1943; Estes 1969a, 1981, 1988; Hinderstein and Boyce 1977) and the three species of Palaeoproteus (Herre 1935; Estes et al. 1967; Estes 1981; Vasilyan and Yanenko 2020) make no mention of a comparable structure. Considering that previous descriptions for O. kayi and Pro. copei dentaries made no mention of the symphysial knob/pit structure (e.g., Estes 1964, 1969a, 1975, 1981; Estes et al. 1969; Naylor 1979; Sullivan 1991; Tokaryk and Bryant 2004), more detailed examination of dentaries referable to other batrachosauroidids is needed to better assess the distribution of that feature within the family. The position and form of the symphysial knob/pit structure and comparisons with dissections of extant salamanders (e.g., Francis 1934; Özeti and Wake 1969; Larsen and Guthrie 1975; Erdman and Cundall 1984; Duellman and Trueb 1986; Lorenz Elwood and Cundall 1994; Kleinteich et al. 2014), suggest that bony structure may have served for attachment of the geniohyoideus or genioglossus muscles, which are hyobranchial levators that serve to pull the hyobranchial apparatus and tongue forward, actions that in aquatic feeding salamanders appear to help expel water out of the mouth after prey capture. Such a function is in keeping with the interpretation that batrachosauroidids were neotenic and aquatic salamanders (e.g., Estes 1969a, 1981; Milner 2000; Holman 2006).
Compared to named batrachosauroidids known by dentaries (i.e., all except for Peratosauroides problematica), AMNH FARB 22965 shares four additional similarities with at least half of the recognized species, as follows: (i) anteroposterior depression present along posterior labial surface (relatively shallow depression shared with Opisthotriton kayi, Palaeoproteus miocenicus Vasilyn and Yanenko, 2020, Parrisia neocesariensis, and Prodesmodon copei vs. relatively deeper depression in both species of Batrachosauroides and in Palaeoproteus gallicus Estes, Hecht, and Hoffstetter, 1967, and Palaeoproteus klatti Herre, 1935); (ii) coronoid process present (well developed process shared with O. kayi, Pal. gallicus, Pal. klatti, and Pro. copei vs. process relatively lower and anteroposteriorly shorter in Batrachosauroides dissimulans Taylor and Hesse, 1945, and Pal. miocenicus; condition unknown for other batrachosauroidids); (iii) pedicellate or subpedicellate teeth (shared with O. kayi, Par. neocesariensis, Pal. gallicus, and Pal. klatti vs. nonpedicellate teeth in Pal. miocenicus, Pro. copei, and both species of Batrachosauroides); and (iv) lacks pronounced ventral projection of the symphysis (shared with O. kayi, Pro. copei, and all species of Palaeoproteus vs. projection present in both species of Batrachosauroides; condition unknown for Par. neocesariensis). AMNH FARB 22965 further resembles O. kayi, Par. neocesariensis, and Pro. copei in lacking foramina mentalia (vs. one or two foramina present in both species of Batrachosauroides and all three species of Palaeoproteus, except for some individuals of Pal. klatti). The above suite of features, coupled with the observation that AMNH FARB 22965 shows no compelling resemblances to dentaries belonging to other salamander families (Amphiumidae, Proteidae, Scapherpetidae, and Sirenidae) known from the latest Cretaceous of the North American Western Interior (e.g., Estes 1964, 1981; Gardner 2003a, b), support assigning AMNH FARB 22965 to Batrachosauroididae.
AMNH FARB 22965 differs from all other batrachosauroidids in having a lingual bony flange paralleling the posterior two-thirds of its tooth row and a prominent post-symphysial expansion. AMNH FARB 22965 may also be unique among batrachosauroidids in having the anterior face of its prominent and robust coronoid process excavated by an anteroposterior groove that is bracketed labially and lingually by a narrow ridge vs. same surface is narrow and keel like in Opisthotriton kayi and Prodesmodon copei, whereas in a referred dentary of Palaeoproteus gallicus depicted by Estes et al. (1967: fig. 3) that surface is similarly wide, but smooth and somewhat bulbous or inflated; the form of the same surface in other batrachosauroidids is uncertain.
Compared to the two named batrachosauroidids in the Lance Formation (Fig. 5), AMNH FARB 22965 more closely resembles dentaries of Opisthotriton kayi in being relatively elongate and shallow (vs. relatively shorter anteroposteriorly and deeper in Prodesmodon copei), in bearing a ventrolabial ridge anteriorly (less prominent in O. kayi, see Estes 1964: fig. 39d, and unknown for Pro. copei), in having its symphysial surface subtriangular in outline (deepest anteriorly and narrowing ventroposteriorly in both vs. narrower and more rectangular in Pro. copei), and teeth pedicellate or subpedicellate (vs. consistently nonpedicellate in Pro. copei). By contrast, AMNH FARB 22965 more closely resembles dentaries of Pro. copei in having a similarly tall coronoid process (vs. relatively lower in O. kayi). Compared to O. kayi and Pro. copei, AMNH FARB 22965 exhibits two intermediate conditions involving teeth. First, its tooth count is 17 (perhaps a few more if an additional tooth was present at the end of the tooth row and if the gap in the tooth row was infilled with teeth) vs. tooth counts of about 25 in O. kayi and 12–14 in Pro. copei. Second, the anteriormost six tooth pedicels in AMNH FARB 22965 are slightly recurved, similar to teeth along the entire tooth row in Pro. copei, yet the more posterior tooth pedicels in AMNH FARB 22965 are straighter, like those along the entire tooth row in O. kayi.
Based on the suite of features listed above, AMNH FARB 22965 is sufficiently unique that it almost certainly represents a new genus and species of batrachosauroidid. At this time, I defer formally naming that new taxon because only one incomplete dentary is available and certain of its features (i.e., bony lingual trough, apparent gap in the tooth row, no obvious Meckelian fossa or groove, and symphysial-like first tooth) are so peculiar that those likely are anomalies and, potentially, may have unduly compromised the structure of AMNH FARB 22965. Additional dentaries or complementary upper jaws are needed to corroborate the features described above for AMNH FARB 22965 and to confirm that it pertains to a new batrachosauroidid taxon.
Discussion
My contention that the dentary AMNH FARB 22965 represents a third batrachosauroidid taxon in the Lance Formation is not the first time a comparable claim has been made. In the largely unpublished batrachosauroidid and scapherpetid chapter from my 2000 PhD dissertation on Late Cretaceous lissamphibians from western North America, I described and figured an atlas (USNM 482352; Gardner 2000b: fig. 11-7A–F) from the Lance Formation, which at the time, I considered distinct enough to warrant informally naming a new batrachosauroidid genus and species. That taxon was never formally published; however, it was mentioned as batrachosauroidid “New gen. and sp. A” (Gardner 2005: table 10.2) and as batrachosauroidid “Gen. et sp. nov. 4” (Gardner and DeMar 2013: table 6, appendix 6). Earlier this year, while starting to finally update my 2000 Ph.D. chapter for publication, and with the benefit of having seen many more batrachosauroidid atlantes over the intervening two decades, I revised my assessment of the atlas USNM 482352—I now regard it as simply an extreme example of one of two atlantal morphs that I originally recognized (and still do) for Opisthotriton kayi. That revised identification means three batrachosauroidid taxa can still be recognized in the Lance Formation: the previously named O. kayi and Pro. copei, both known by vertebrae and jaws, plus the probable new and unnamed taxon represented by the dentary AMNH FARB 22965.
Opisthotriton kayi and Prodesmodon copei are well represented in the Lance Formation by dozens of distinctive dentaries, as well as numerous other skull bones and vertebrae, collected from Bushy Tailed Blowout and other localities (e.g., Estes 1964; Gardner and DeMar 2013: appendix 6). The abundance of their remains indicates both species were common within the fluvial and floodplain depositional system that formed the Lance Formation, during the late Maastrichtian in present-day Wyoming, USA. Both species also are known from outside of the Lance Formation elsewhere in the Western Interior: O. kayi from the early/middle Santonian–late Paleocene and Pro. copei from the middle/late Campanian–early Paleocene (see summary by Gardner and DeMar 2013). By contrast, the probable new batrachosauroidid taxon represented by AMNH FARB 22965 is known by a single dentary from Bushy Tailed Blowout, which suggests the taxon was either locally rare or lived outside the depositional area. The latter explanation seems unlikely, because although AMNH FARB 22965 is incomplete, it shows little evidence of abrasion to suggest it was transported post-mortem any significant distance from outside the Bushy Tailed Blowout source area. More likely, the taxon was uncommon within the Lance Formation paleoenvironment.
By analogy with extant neotenic salamanders of comparable form and size (such as proteids, amphiumids, and sirenids: see species accounts by Petranka 1998), batrachosauroidids were aquatic and largely bottom dwelling predators. The overall form of AMNH FARB 22965 implies the snout of the living animal was robust, moderately elongate and narrow, and sub-pointed. That inferred snout form is similar to those seen in skulls reported for the batrachosauroidids Opisthotriton kayi and Batrachosauroides dissimulans (see Estes 1969a: fig. 4A, B and C, D, respectively), but differs from the less tapered snout in skulls of Palaeoproteus klatti (Herre 1935: figs. 1–4 and pl. 1: 6). Overall similarities in dentary shape (e.g., relatively deeper, anteroposteriorly shorter, and more triangular) between all three species of Palaeoproteus and Prodesmodon copei suggest the latter probably also had a less tapered and shorter snout compared to O. kayi and the taxon represented by AMNH FARB 22965. Considering that other batrachosauroidids for which intact dentary teeth are known (i.e., all except Peratosauroides problematica) have conical and monocuspid crowns, it is reasonable to predict that AMNH FARB 22965 also had similar tooth crowns. Whereas other batrachosauroidid dentaries have teeth that are either recurved (Pro. copei and all species of Palaeoproteus) or relatively straight or peg-like (both species of Batrachosauroides and O. kayi) along the entire ramus, judging by its preserved pedicels AMNH FARB 22965 appears to have borne slightly recurved teeth anteriorly and relatively straighter or peg-like teeth along the posterior two-thirds of its ramus. The recurved teeth in Pro. copei and all species of Palaeoproteus recall the recurved teeth described by Erdman and Cundall (1984) in the neotenic extant salamander Amphiuma tridactylum and by Pedersen (1991) for larval cannibal morphs of the extant salamander Ambystoma tigrinum, both of which are voracious predators capable of subduing and consuming relatively large bodied prey. AMNH FARB 22965 is relatively more robust and absolutely slightly bigger than large dentaries of O. kayi and Pro. copei. In life, all three taxa would have been moderate in body size, perhaps in the range of 30 cm total body length, especially compared to the substantially larger sirenids and scapherpetids with which they co-existed during the latest Cretaceous (Bonett et al. 2013). Differences in dentary sizes, proportions, and relative robustness, and structure of the bone and teeth among the trio of batrachosauroidids in the Lance Formation point towards ecological partitioning in habitats and food, probably comparable to that among similar sized species in modern, aquatic salamander communities (see summaries by Petranka 1998; Wells 2007; Bonett et al. 2013). If the taxon represented by AMNH FARB 22965 was restricted to a less common habitat within the broader Lance floodplain and fluvial paleoecosystem, that might explain its scarcity within the assemblage.
Concluding remarks
The unique dentary AMNH FARB 22965 reported here, from Bushy Tailed Blowout in the uppermost Cretaceous Lance Formation, exhibits a puzzling mix of features. Some of those features are so peculiar that they likely are anomalies (i.e., elongate and dorsally curved bony trough along its lingual surface, lack of an obvious Meckelian fossa or groove, apparent gap in the tooth row, and symphysial-like first tooth), but others indicate the dentary belongs to a previously unrecognized genus and species of batrachosauroidid salamander. The recognition of such a specimen was unexpected, considering that the Lance Formation has been extensively sampled for small vertebrae fossils, including those of lissamphibians, since the late 1800s and that many institutions (e.g., AMNH, UALVP, UCMP, USNM, and UW) hold substantial and relatively well studied collections from Bushy Tailed Blowout and other similarly productive localities in the formation. The discovery of AMNH FARB 22965 is a reminder that even well sampled localities and formations can continue to yield significant new fossils. Many of the named salamander taxa known from the Lance Formation also occur in paracontemporaneous formations elsewhere in the North American Western Interior (see review by Gardner and DeMar 2013), which raises the possibility that the taxon represented by AMNH FARB 22965 may also have lived in those depositional areas. I hope that my detailed description of AMNH FARB 22965 may spur the discovery of additional examples of comparable dentaries or complementary upper jaws, which could be useful for corroborating or disproving the identification and ideas I have presented here.
Acknowledgements
AMNH FARB 22965 has been an on again-off again preoccupation of mine for over a quarter century, ever since I received it as part of a loan for my Ph.D. research. Over the years, I have repeatedly pulled it out to ponder and pester colleagues, and then put it away again to let it percolate further in the dark corners of my mind. I am relieved to have the opportunity to finally describe this peculiar fossil and, for better or worse, present my thoughts on it. This paper was composed in my home office during the covid spring and summer of 2021—I thank my wife, N. Joan Marklund, for tolerating my sometimes erratic work-at-home routine and our dog, Pepper, for keeping me company by dozing on her pillow beside my desk and forcing me to take much-needed breaks in our back garden. I am grateful to the late Charlotte Holton (former AMNH collection manager) for sending me AMNH FARB 22965 in the mid 1990s and to her successor, Carl Mehling (also AMNH), for repeatedly extending the loan. For access to comparative specimens and information, I thank Becky Sanchez and Brandon Strilisky (both TMP), Rich Cifelli and Jennifer Larsen (both OMNH), Frannie Blondheim, Jennifer Bowser, Michael Caldwell, Phil Currie, and Howard Gibbins (all UALVP), and Laura Vietti (UW). George Braybrook (retired from University of Alberta, Edmonton, Canada) took the SEMs used in Fig. 2, Donna Sloan (TMP) made the drawings in Fig. 3, and, even though I did not use them, Craig Scott (TMP) took photographs of AMNH FARB 22965. I am grateful to all my colleagues who offered opinions on the identity of AMNH FARB 22965. I appreciate the invitation from Brian Davis (University of Louisville, Kentucky, USA) to contribute to this volume, the reviewers Dave DeMar, Jr. (USNM) and Pavel Skutschas (Saint Petersburg State University, Saint Petersburg, Russian Federation) for their helpful comments on the submitted version, the efforts of the Acta Palaeontologica Polonica editorial team, and Patty Ralrick and the Royal Tyrrell Museum Cooperating Society for covering page charges. Finally, I thank Rich Cifelli for the many kindnesses and opportunities he has extended to me over the past three decades. May your retirement be dressed all over and zesty mordant, Rich!
References
Aguillon Martinez, M.C. 2010. Fossil Vertebrates From the Cerro del Pueblo Formation, Coahuila, Mexico, and the Distribution of Late Campanian (Cretaceous) Terrestrial Vertebrate Faunas. 135 pp. PhD dissertation, Southern Methodist University, Dallas.
Auffenberg, W. 1958. A new family of Miocene salamanders from the Texas Coastal Plain. Quarterly Journal of the Florida Academy of Sciences 21: 169–176.
Auffenberg, W. 1961. A new genus of fossil salamander from North America. American Midland Naturalist 66: 456–465. Crossref
Bolt, J.R. 1969. Lissamphibian origins: possible protolissamphibian from the Lower Permian of Oklahoma. Science 166: 888–891. Crossref
Bolt, J.R. 1991. Lissamphibian origins. In: H.-P. Schultze and L. Trueb (eds.), Origins of the Higher Groups of Tetrapods: Controversy and Consensus, 194–222. Comstock Publishing Associates, Ithaca.
Bonett, R.M., Trujano-Alvarez, A.L., William, M.J., and Timpe, E.K. 2013. Biogeography and body size shuffling of aquatic salamander communities on a shifting refuge. Proceedings of the Royal Society B 280: 20130200. Crossref
Breithaupt, B.H. 1982. Paleontology and paleoecology of the Lance Formation (Maastrichtian), east flank of Rock Springs Uplift, Sweetwater County, Wyoming. University of Wyoming Contributions to Geology 21: 123–151.
Brinkman, D.B. 1990. Paleoecology of the Judith River Formation (Campanian) of Dinosaur Provincial Park, Alberta, Canada: evidence from vertebrate microfossil localities. Palaeogeography, Palaeoclimatology, Palaeoecology 78: 37–54. Crossref
Carpenter, K. 1979. Vertebrate fauna of the Laramie Formation (Maestrichtian), Weld County, Colorado. University of Wyoming Contributions to Geology 17: 37–49.
Cifelli, R.L. 1990. Cretaceous mammals of southern Utah. IV. Eutherian mammals from the Wahweap Formation (Aquilan) and Kaiparowits (Judithian) formations. Journal of Vertebrate Paleontology 10: 346–360. Crossref
Clemens, W.A. 1963. Fossil mammals of the type Lance Formation, Wyoming. Part I. Introduction and Multituberculata. University of California Publications in Geological Sciences 48: 1–105.
Clemens, W.A., Lillegraven, J.A., Lindsay, E.H., and Simpson, G.G. 1979. Where, when, and what—a survey of known Mesozoic mammal distribution. In: J.A. Lillegraven, Z. Kielan-Jaworowska, and W.A. Clemens (eds.), Mesozoic Mammals: The First Two-thirds of Mammalian History, 7–58. University of California Press, Berkeley.
Cuvier, G.L.C.F.D. 1827. Sur le genre de reptiles batraciens, nommé Amphiuma, et sur une nouvelle espèce de ce genre (Amphiuma tridactylum). Mémoires du Muséum d’Histoire Naturelle 14: 1–14.
Davit-Béal, T., Chisaka, H., Delgado, S., and Sire, J.-Y. 2007. Amphibian teeth: current knowledge, unanswered questions, and some directions for future research. Biological Reviews 82: 49–81. Crossref
Daza, J.D., Stanley, E.L., Bolet, A., Bauer, A.M., Arias, J.S., Čerňanský, A., Bevitt, J.J., Wagner, P., and Evans, S.E. 2020. Enigmatic amphibians in mid-Cretaceous amber were chameleon-like ballistic feeders. Science 370: 687–691. Crossref
DeMar, D.G., Jr. 2013. A new fossil salamander (Caudata, Proteidae) from the Upper Cretaceous (Maastrichtian) Hell Creek Formation, Montana, U.S.A. Journal of Vertebrate Paleontology 33: 588–598. Crossref
DeMar, D.G., Jr. and Breithaupt, B.H. 2008. Terrestrial and aquatic vertebrate paleocommunities of the Mesaverde Formation (Upper Cretaceous, Campanian) of the Wind River and Bighorn basins, Wyoming. In: J.T. Sankey and S. Baszio (eds.), Vertebrate Microfossil Assemblages: Their Role in Paleoecology and Paleobiogeography, 78–103. Indiana University Press, Bloomington.
Denton, R.K., Jr. and O’Neill, R.C. 1998. Parrisia neocesariensis, a new batrachosauroidid salamander and other amphibians from the Campanian of eastern North America. Journal of Vertebrate Paleontology 18: 484–494. Crossref
Duellman, W.E. and Trueb, L. 1986. Biology of Amphibians. 670 pp. McGraw-Hill, New York. Crossref
Duffaud, S. 1995. A Batrachosauroididae (Amphibia, Caudata) from the late Cretaceous of Champ-Garimond (Southern France). In: First European Workshop on Vertebrate Paleontology. Geological Society of Denmark, DGF on Line Series 1. Available at https://drive.google.com/file/d/1glk7ZZB3xYTzChQxNA5LCdvGdcGP6gng/view and https://2dgf.dk/dgf-online-series/a-batrachosauroididae-amphibia-caudata-from-the-late-cretaceous-of-champ-garimond-southern- france/. Both accessed 14 January 2021.
Duméril, A.M.C. 1806. Zoologie Analytique ou Methode Naturelle de Classification des Animaux. 344 pp. Allais Libraire, Paris.
Eaton, J.G. and Cifelli, R.L. 1988. Preliminary report on Late Cretaceous mammals of the Kaiparowits Plateau, southern Utah. University of Wyoming Contributions to Geology 26: 45–55.
Ecker, A.1889. The Anatomy of the Frog. Translated, with Numerous Annotations and Additions by G. Haslam. 449 pp. Clarendon Press, Oxford. [Reprint edition. 1971. A. Asher & Co N.V., Amsterdam.] Crossref
Erdman, S. and Cundall, D. 1984. The feeding apparatus of the salamander Amphiuma tridactylum: morphology and behavior. Journal of Morphology 181: 175–204. Crossref
Estes, R. 1964. Fossil vertebrates from the Late Cretaceous Lance Formation, eastern Wyoming. University of California Publications in Geological Sciences 49: 1–180.
Estes, R. 1965. A new fossil salamander from Montana and Wyoming. Copeia 1965: 90–95. Crossref
Estes, R. 1969a. The Batrachosauroididae and Scapherpetontidae, Late Cretaceous and Early Cenozoic salamanders. Copeia 1969: 225–234. Crossref
Estes, R. 1969b. The fossil record of amphiumid salamanders. Breviora 322: 1–11.
Estes, R. 1975. Lower vertebrates from the Fort Union Formation, late Paleocene, Big Horn Basin, Wyoming. Herpetologica 31: 365–385.
Estes, R. 1981. Gymnophiona, Caudata. In: P. Wellnhofer (ed.), Encyclopedia of Paleoherpetology, Part 2, 1–115. Gustav Fischer Verlag, Stuttgart.
Estes, R. 1988. Lower vertebrates from the Golden Valley Formation, Early Eocene of North Dakota. Acta Zoologica Cracoviensia 31: 541–562.
Estes, R. and Berberian, P. 1970. Paleoecology of a Late Cretaceous vertebrate community from Montana. Breviora 343: 1–35.
Estes, R., Berberian, P., and Meszoely, C.A.M. 1969. Lower vertebrates from the Late Cretaceous Hell Creek Formation, McCone County, Montana. Breviora 337: 1–33.
Estes, R., Hecht, M., and Hoffstetter R. 1967. Paleocene amphibians from Cernay, France. American Museum Novitates 2295: 1–25.
Evans, S.E. and McGowan, G.J. 2002. Lissamphibian remains from the Purbeck Limestone Group, southern England. Special Papers in Palaeontology 68: 103–119.
Evans, S.E. and Milner, A.R. 1996. A metamorphosed salamander from the early Cretaceous of Las Hoyas, Spain. Philosophical Transactions of the Royal Society, London B 351: 627–646. Crossref
Evans, S.E. and Sigogneau-Russell, D. 2001. A stem-group caecilian (Lissamphibia: Gymnophiona) from the Lower Cretaceous of North Africa. Palaeontology 44: 259–273. Crossref
Evans, S.E., Milner, A.R., and Werner, C. 1996. Sirenid salamanders and a gymnophionan amphibian from the Cretaceous of the Sudan. Palaeontology 39: 77–95.
Fitzinger, L.J.F.J. 1843. Systema Reptilium. Fasciculus Primus Ambyglossae. 106 pp. Braumüller et Seidel, Wien.
Fox, R.C. 1989. The Wounded Knee local fauna and mammalian evolution near the Cretaceous–Tertiary boundary, Saskatchewan, Canada. Palaeontographica Abteilung A 208: 11–59.
Fox, R.C. and Naylor, B.G. 1982. A reconsideration of the relationships of the fossil amphibian Albanerpeton. Canadian Journal of Earth Sciences 19: 118–128. Crossref
Francis, E.T. 1934. The Anatomy of the Salamander. 381 pp. Oxford University Press, Oxford.
Gardner, J.D. 2000a. Albanerpetontid amphibians from the Upper Cretaceous (Campanian and Maastrichtian) of North America. Geodiversitas 22: 349–388.
Gardner, J.D. 2000b. Systematics of Albanerpetontids and Other Lissamphibians From the Late Cretaceous of Western North America. 577 pp. Ph.D. Dissertation, University of Alberta, Edmonton.
Gardner, J.D. 2001. Monophyly and the affinities of albanerpetontid amphibians (Temnospondyli; Lissamphibia). Zoological Journal of the Linnean Society 131: 309–352. Crossref
Gardner, J.D. 2003a. Revision of Habrosaurus Gilmore (Caudata; Sirenidae) and relationships among sirenid salamanders. Palaeontology 46: 1089–1122. Crossref
Gardner, J.D. 2003b. The fossil salamander Proamphiuma cretacea Estes (Caudata; Amphiumidae) and relationships within the Amphiumidae. Journal of Vertebrate Paleontology 23: 769–782. Crossref
Gardner, J.D. 2005. Lissamphibians. In: P.J. Currie and E.B. Koppelhus (eds.), Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed, 186–201. Indiana University Press, Indianapolis.
Gardner, J.D. 2012. Revision of Piceoerpeton Meszoely (Caudata: Scapherpetontidae) and description of a new species from the late Maastrichtian and ?early Paleocene of western North America. Bulletin de la Société Géologique de France 183: 611–620. Crossref
Gardner, J.D. and DeMar, D.G., Jr. 2013. Mesozoic and Paleocene lissamphibian assemblages of North America: a comprehensive review. In: J.D. Gardner and R.L. Nydam (eds.), Mesozoic and Cenozoic Lissamphibian and Squamate Assemblages of Laurasia. Palaeobiodiversity and Palaeoenvironments 93: 459–515.
Gardner, J.D., Eaton, J.G., and Cifelli, R.L. 2013. Preliminary report on salamanders (Lissamphibia; Caudata) from the Late Cretaceous (late Cenomanian–late Campanian) of southern Utah, U.S.A. In: A.L. Titus and M.A. Lowen (eds.), At the Top of the Grand Staircase: The Late Cretaceous of Southern Utah, 237–272. Indiana University Press, Bloomington.
Goin, C.J. and Auffenberg, W. 1958 New salamanders of the family Sirenidae from the Cretaceous of North America. Fieldiana Geology 10: 449–459. Crossref
Haeckel, E. 1866. Generelle Morphologie der Organismen. 574 pp. (Vol. 1) 462 pp. (Vol. 2). Reimer, Berlin. Crossref
Herre, W. 1935. Die Schwanzlurche der mitteleocänen (oberlutetischen) Braunkohle des Geiseltales und die Phylogenie der Urodelen unter Einschluß der fossilen Formen. Zoologica – Original-Abhandlungen aus dem Gesamtgebiete der Zoologie 87: 1–85.
Hinderstein, B. and Boyce, J. 1977. The Miocene salamander Batrachosauroides dissimulans (Amphibia, Urodela) from East Texas. Journal of Herpetology 11: 369–372. Crossref
Holman, J.A. 2006. Fossil Salamanders of North America. 232 pp. Indiana University Press, Bloomington.
Ivachnenko, M.F. [Ivačnenko, M.F.] 1978. Urodelans from the Triassic and Jurassic of Soviet Central Asia. Paleontologičeskij žurnal 1978: 84–89 [in Russian] and Paleontological Journal 1978: 362–368 [in English].
Jenkins, F.A., Jr., and Walsh, D.M. 1993. An Early Jurassic caecilian with limbs. Nature 365: 246–250. Crossref
Jenkins, F.A., Jr., Walsh, D.M., and Carroll, R.L. 2007. Anatomy of Eocaecilia micropodia, a limbed caecilian from the Early Jurassic. Bulletin of the Museum of Comparative Zoology 158: 285–366. Crossref
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. 630 pp. Columbia University Press, New York. Crossref
Kleinteich, T., Herzen, J., Beckmann, F., Matsui, M., and Haas, A. 2014. Anatomy, function, and evolution of jaw and hyobranchial muscles in cryptobranchoid salamander larvae. Journal of Morphology 275: 230–246. Crossref
Larsen, J.H., Jr. and Guthrie, D.L. 1975. The feeding system of terrestrial salamanders (Ambystoma tigrinum melanostictum Baird). Journal of Morphology 147: 137–154. Crossref
Lorenz Elwood, J.R. and Cundall, D. 1994. Morphology and behavior of the feeding apparatus in Cryptobranchus alleganiensis (Amphibia: Caudata). Journal of Morphology 220: 47–70. Crossref
Lynch, J.D. 1971. Evolutionary relationships, osteology, and zoogeography of leptodactyloid frogs. University of Kansas Museum of Natural History Miscellaneous Publication 53: 1–238.
Martin, C., Alonso-Zarazaga, M.A., and Sanchiz, B. 2012. Nomenclatural notes on living and fossil amphibians. Graellsia 68: 159–180. Crossref
Milner, A.R. 1988. The relationships and origin of living amphibians. In: M.J. Benton (ed.), The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds. Systematics Association Special Volume 35A, 59–102. Clarendon Press, Oxford.
Milner, A.R. 1993. The Paleozoic relatives of lissamphibians. Herpetological Monographs 7: 8–27. Crossref
Milner, A.R. 2000. Mesozoic and Tertiary Caudata and Albanerpetontidae. In: H. Heatwole and R.L. Carroll (eds.), Amphibian Biology. Volume 4. Paleontology: The Evolutionary History of Amphibians, 1412–1444. Surrey Beatty and Sons, Chipping Norton.
Naylor, B.G. 1979. The Cretaceous salamander Prodesmodon (Amphibia: Caudata). Herpetologica 35: 11–20.
Naylor, B.G. 1981. A new salamander of the family Batrachosauroididae from the late Miocene of North America, with notes on other batrachosauroidids. PaleoBios 39: 1–14.
Naylor, B.G. 1983. New salamander (Amphibia: Caudata) atlantes from the Upper Cretaceous of North America. Journal of Paleontology 57: 48–52.
Nussbaum, R.A. 1977. Rhinatrematidae: a new family of caecilians (Amphibia: Gymnophiona). Occasional Papers of the Museum of Zoology University of Michigan 682: 1–30.
Ogg, J.G., Ogg, G.M., and Gradstein, F.M. 2016. A Concise Geologic Time Scale 2016. 234 pp. Elsevier, Amsterdam.
Oreska, M.P.J., Carrano, M.T., and Dzikiewicz, K.M. 2013. Vertebrate paleontology of the Cloverly Formation (Lower Cretaceous). Faunal composition, biogeographic relationships, and sampling. Journal of Vertebrate Paleontology 33: 264–292. Crossref
Özeti, N. and Wake, D.B. 1969. The morphology and evolution of the tongue and associated structures in salamanders and newts (family Salamandridae). Copeia 1969: 91–123. Crossref
Paluh, D.J., Riddell, K., Early, C.M., Hantak, M.M., Jongsma, G.F.M., Keeffe, R.M., Silva, F.M., Nielsen, S.V., Vallejo-Pareja, M.C., Stanley, E.L., and Blackburn, D.C. 2021. Rampant tooth loss across 200 million years of frog evolution. eLife 10: e66926. Crossref
Parsons, T. and Williams, E. 1962. The teeth of Amphibia and their relation to amphibian phylogeny. Journal of Morphology 110: 375–389. Crossref
Pearson, D.A., Schaefer, T., Johnson, K.R., Nichols, D.J., and Hunter, J.P. 2002. Vertebrate biostratigraphy of the Hell Creek Formation in southwestern North Dakota and northwestern South Dakota. In: J.H. Hartman, K.K. Johnson, and D.J. Nichols (eds.), The Hell Creek Formation and the Cretaceous–Tertiary Boundary in the Northern Great Plains: An Integrated Continental Record of the end of the Cretaceous. Geological Society of America Special Paper 361: 145–167. Crossref
Pedersen, S.C. 1991. Dental morphology of the cannibal morph in the tiger salamander, Ambystoma tigrinum. Amphibia-Reptilia 1991: 1–14. Crossref
Peng, J., Russell, A.P., and Brinkman, D.B. 2001. Vertebrate microsite assemblages (exclusive of mammals) from the Foremost and Oldman formations of the Judith River Group (Campanian) of southeastern Alberta: an illustrated guide. Provincial Museum of Alberta Natural History Occasional Paper 25: 1–54. Crossref
Petranka, J.W. 1998. Salamanders of the United States and Canada. 587 pp. Smithsonian Institution Press, Washington.
Redman, C.M., Gardner, J.D., Scott, S.C., and Braman, D.R. 2015. Geological setting of vertebrate microfossil localities across the Cretaceous–Paleogene boundary in southwestern Saskatchewan, Canada. Canadian Journal of Earth Sciences 52: 846–862. Crossref
Rowe, T., Cifelli, R.L., Lehman, T.M., and Weil, A. 1992. The Campanian Terlingua local fauna, with a summary of other vertebrates from the Aguja Formation, Trans-Pecos Texas. Journal of Vertebrate Paleontology 12: 472–493. Crossref
Sahni, A. 1972. The vertebrate fauna of the Judith River Formation, Montana. Bulletin of the American Museum of Natural History 147: 321–412.
Schoch, R.R. 2014. Amphibian Evolution: The Life of Early Land Vertebrates. 264 pp. Wiley Blackwell, Chichester. Crossref
Schoch, R.R. and Milner, A.R. 2014. Temnospondyli I. In: H.-D. Sues (ed.), Handbook of Paleoherpetology, Part 3A2, 1–150. Verlag Dr. Friedrich Pfeil, München.
Schoch, R.R., Werneburg, R., and Voigt, S. 2020. A Triassic stem-salamander from Kyrgyzstan and the origin of salamanders. Proceedings of the National Academy of Sciences of the United States of America 117: 11584–11588. Crossref
Scopoli, G.A. 1777. Introductio ad Historiam Naturalem, sistens genera Lapidium, Plantarum, et Animalium: hactenus detecta, caracteribus essentialibus donata, in tribus divisia, subinde ad leges naturae. 506 pp. Apud Wolfgangum Gerle, Prague. Crossref
Shaw, G. and Nodder, F.P. 1798. Vivarium Naturae or The Naturalist’s Miscellany. Volume 9. Pages not numbered. Nodder & Co., London.
Sigurdsen, T. and Bolt, J.R. 2010. The Lower Permian amphibamid Doleserpeton (Temnospondyli: Dissorophoidea), the interrelationships of amphibamids, and the origin of modern amphibians. Journal of Vertebrate Paleontology 30: 1360–1377. Crossref
Skutschas, P., Kolchanov, V., Boitsova, E., and Kuzmin, I. 2018. Osseous anomalies of the cryptobranchid Eoscapherpeton asiaticum (Amphibia: Caudata) from the Late Cretaceous of Uzbekistan. Fossil Record 21: 159–169. Crossref
Smith, J.B. and Dodson, P. 2003. A proposal for a standard terminology of anatomical notation and orientation in fossil vertebrate dentitions. Journal of Vertebrate Paleontology 23: 1–12. Crossref
Smith, J.E. 1821. A Selection of the Correspondence of Linnaeus and Other Naturalists from the Original Manuscripts. Volume 1. 630 pp. Longman, Hurst, Rees, Orme, and Brown, London. [Taxonomic authority for Amphiuma means is a letter, reprinted on pp. 594–599, from A. Garden to J. Ellis, dated May 15, 1773.]
Sullivan, R.M. 1991. Paleocene Caudata and Squamata from Gidley and Silberling Quarries, Montana. Journal of Vertebrate Paleontology 11: 293–301. Crossref
Taylor, E.H. 1977. The comparative anatomy of caecilian mandibles and their teeth. The University of Kansas Science Bulletin 51: 261–282. Crossref
Taylor, E.H. and Hesse, C.J. 1943. A new salamander from the Upper Miocene beds of San Jacinto County, Texas. American Journal of Science 241: 185–193. Crossref
Tokaryk, T.T. and Bryant, H.N. 2004. The fauna from the Tyrannosaurus rex excavation, Frenchman Formation (Late Maastrichtian), Saskatchewan. In: Summary of Investigations 2004, Volume 1, Saskatchewan Geological Survey, Saskatchewan Industry Resources, Miscellaneous Report 2004-4.1, CD-ROM, Paper A-18: 1–12. Government of Saskatchewan, Regina.
Trueb, L. 1993. Patterns of structural diversity among the Lissamphibia. In: J. Hanken and B.K. Hall (eds.), The Skull, Volume 2: Patterns of Structural and Systematic Diversity, 255–343. University of Chicago Press, Chicago.
Vasilyan, D. and Yanenko, V. 2020. The last Palaeoproteus (Urodela: Batrachosauroididae) of Europe. Scientific Reports 10: 2733. Crossref
Vasilyan, D., Böhme, M., Chkhikvadze, V.M., Semenov, Y.A., and Joyce, W.G. 2013. A new giant salamander (Urodela, Pancryptobrancha) from the Miocene of Eastern Europe. Journal of Vertebrate Paleontology 33: 301–318. Crossref
Vitt, L.J. and Caldwell, J.P. 2009. Herpetology: An Introductory Biology of Amphibians and Reptiles. Third Edition, 697 pp. Academic Press, San Diego. Crossref
Weishampel, D.B., Barrett, P.M., Coria, R.A., Le Loeuff, J., Xu X., Zhao X., Sahni, A., Gomani, E.M.P., and Noto, C.R. 2004. Dinosaur distribution. In: D.B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria. Second Edition, 517–606. University of California Press, Berkeley. Crossref
Wells, K.D. 2007. The Ecology and Behavior of Amphibians. 1148 pp. University of Chicago Press, Chicago. Crossref
Wiens, J.J. 2011. Re-evolution of mandibular teeth in frogs after more than 200 million years, and re-evaluating Dollo’s law. Evolution 65: 1283–1296. Crossref
Wilson, G.P., DeMar, D.G., Jr., and Carter, G. 2014. Extinction and survival of salamander and salamander-like amphibians across the Cretaceous–Paleogene boundary in northeastern Montana, U.S.A. In: G.P. Wilson, W.A. Clemens, J.R. Horner, and J.H. Hartman (eds.), Through the End of the Cretaceous in the Type Locality of the Hell Creek Formation in Montana and Adjacent Areas. Geological Society of America Special Paper 503: 271–297. Crossref
Acta Palaeontol. Pol. 67 (1): 35–50, 2022
https://doi.org/10.4202/app.00926.2021